Abstract
The mi-iuy croaker Miichthys miiuy has immense commercial value in the Republic of Korea. The red drum Sciaenops ocellatus is widely produced by aquaculture, although its price is approximately 25% that of M. miiuy. S. ocellatus has black spots on its tail, enabling it to be distinguished from M. miiuy based on appearance. However, identifying S. ocellatus after simple processing steps, such as skin removal and dicing, is difficult. Certain traders misrepresent and sell S. ocellatus as M. miiuy or cultured M. miiuy for illegal economical gain. Therefore, an accurate and rapid identification method is required to distinguish between M. miiuy and S. ocellatus in the field. Here, a method for rapid field identification was developed based on species-specific primers using a portable ultra-fast PCR instrument. The ultra-fast real-time PCR method can complete the entire analytical procedure, including DNA isolation, amplification, and detection, within 30 min, thus maintaining the accuracy of identifying M. miiuy and S. ocellatus products on site. Forty-nine commercial products were tested, and all samples were successfully identified. Thus, the developed method is rapid, efficient tool for ensuring consumer protection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00954-4.
Keywords: Economically motivated adulteration, fishery products, Species-specific PCR, Miichthys miiuy, Ultra-fast real-time PCR
Introduction
The mi-iuy croaker Miichthys miiuy and red drum Sciaenops ocellatus are marine fish species in the family Sciaenidae. M. miiuy is mainly distributed in the coastal waters of Korea, China, and Japan, and S. ocellatus naturally inhabits estuaries and coastal waters of the United States and Mexico in the West Atlantic and Gulf of Mexico (Choi et al., 2002; Lin et al., 2020; Wan-shu and Qi-yong, 2002; Zhu et al., 1975). S. ocellatus was introduced into China from the United States for mariculture purposes a few decades ago and has become an important aquaculture fish species along the coastal areas of China (Cheng et al., 2012; Lin et al., 2020).
Miichthys miiuy is one of the most economically important fishery resources of South Korea because of its delectable taste and vitalization-inducing properties; the demand for M. miiuy increases considerably in the hot season as a health food. However, M. miiuy production has drastically declined recently because of over-fishing, pollution, and coastal construction (Cheng et al., 2020; Shan et al., 2008; Xu et al., 2014). To meet the growing demand for M. miiuy, S. ocellatus is being promoted as an attractive alternative among the fishing community. S. ocellatus is mass-produced via aquaculture in China and sold at a quarter of the price of M. miiuy after being imported to South Korea. M. miiuy is called ‘Mineo’ in Korea, whereas S. ocellatus is called ‘Red Mineo’; this similarity in local names of the two species confuses consumers who assume that they are the same fish.
According to the National Institute of Fisheries Science (Busan, Republic of Korea), both M. miiuy and S. ocellatus belong to the family Sciaenidae. However, S. ocellatus is characterized by black spots on the tail, whereas M. miiuy lacks these spots; the caudal fin shape of both species also differs. These two species are typically traded and sold in the form of fillet or sashimi (sashimi is a Japanese delicacy that consists of thinly sliced raw fish meat). The fish scales, skin, and tails are removed during the manufacture of fillets or sashimi, making it difficult to identify the specific species utilized for production. There are frequently reported cases of certain traders who misrepresent and conduct the sale of S. ocellatus as M. miiuy or cultured M. miiuy for illegal economic gain in South Korea. Therefore, it is necessary to develop a rapid and accurate analytical method that can be routinely used to both identify and distinguish between M. miiuy and S. ocellatus in the field for consumer right protection.
Previous studies of M. miiuy and S. ocellatus were mostly related to aquaculture such as artificial propagation and breeding or molecular genetic diversity using DNA barcodes without routine species identification methods (Cheng et al., 2012, 2020; Lin et al., 2020; Shan et al., 2008; Wan-shu and Qi-yong, 2002; Zhu et al., 1975). DNA is generally more stable than protein during processing steps such as heating and grinding, and DNA can be analyzed using only a small quantity of tissue. Furthermore, DNA exists in every cell type, whereas the occurrence of proteins varies with age and tissue types. Additionally, DNA can be detected by an analytical method with high sensitivity and specificity (Lockley and Bardsley, 2000; Teletchea et al., 2005). Polymerase chain reaction (PCR) is a method employed for the amplification of specific gene sequences, and numerous species identification methods are based on this technique such as random amplification of polymorphic DNA and restriction fragment length polymorphism. Among them, species-specific PCR has been regarded as a suitable method for routine analysis of food adulteration. Species-specific primers amplify specific target sequences in samples, including mixed samples without additional complex steps such as enzyme treatments. In addition, species-specific PCR is simple and inexpensive, and the results are easy to analyze (Fajardo et al., 2010; Haunshi et al., 2009; Lockley and Bardsley, 2000; Rasmussen and Morrissey, 2008). Analysis of marine organisms using species-specific primers and real-time PCR has been reported for tuna species, haddock, and whiting, European sole, and common substitute species (Chuang et al., 2012; Herrero et al., 2012; Taylor et al., 2002). Alaska pollock and cod were also identified by species-specific PCR (Namikoshi et al., 2011). In addition to identifying marine resources, species-specific PCR has been used to determine the authenticity of halal products (Man et al., 2007), identify species in commercial products containing poultry/bovine components (Chuah et al., 2016), detect the presence of ostrich meat in processed meat products, and determine the authenticity of meat ingredients in animal feed (Okuma and Hellberg, 2015; Rojas et al., 2011).
Ultra-fast real-time PCR is more efficient than conventional PCR because of the increased temperature control speed, improvement in the thermal conductivity of reaction tubes or chips, and reduction in the volume of reaction reagents. The short analysis time and miniaturization of equipment make it possible to analyze samples in the field as well as in the laboratory (Khandurina et al., 2000; Neuzil et al., 2006). To reduce the reaction time of thermal cycling in PCR, ultra-fast real-time PCR system using microfluidic chips have been recently developed and applied within various fields (Aboud et al., 2013; Furutani et al., 2017; Houssin et al., 2016; Wheeler et al., 2011). The chip-based PCR technique can be distinguished rapidly and directly from real-time PCR which is integrating EvaGreen Fluorescence detection technology that enables users to visually confirm expressed wells through the naked eye within 30 min, including reverse transcription from marine resources.
In the present study, M. miiuy- and S. ocellatus-specific primer sets were designed based on cytochrome oxidase subunit I (COI). Two primer sets were used to develop species identification methods using conventional PCR for routine analysis in the lab and ultra-fast real-time PCR for urgent analysis in the field. Forty-nine commercial products were verified using these two PCR-based methods, and sequence analysis was performed to identify exact species. This is the first study in which rapid and reliable PCR methods have been developed and used to differentiate M. miiuy and S. ocellatus.
Materials and methods
M. miiuy- and S. ocellatus-specific primer sets
Reference samples, one each of M. miiuy and S. ocellatus, were provided by the Ministry of Food and Drug Safety. Forty-nine commercial products were purchased from local markets in Cheongju and Daejeon, Republic of Korea, and from online markets. All samples employed in the study are listed in Table 1. DNA was extracted using a DNA Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were confirmed with a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Genomic DNA samples were stored at − 20 °C.
Table 1.
Results of species identification in M. miiuy products
| No. | Product | Label | Conventional PCRa | Ultra-fast real-time PCR | Species identified by FINS | ||||
|---|---|---|---|---|---|---|---|---|---|
| M.mb | S.oc | M.m | S.o | Accession no | Species | ||||
| R1 | Reference material | + | − | 24.35 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | ||
| R2 | Reference material | − | + | N.D. | 24.24 ± 0.97 | KP641522.1 | Sciaenops ocellatus | ||
| 1 | Seasoned | Mi-iuy croaker | + | − | 27.78 ± 0.49 | N.D. | MF004320.1 | Miichthys miiuy | |
| 2 | Seasoned | Mi-iuy croaker | + | − | 28.28 ± 0.29 | N.D. | MF004320.1 | Miichthys miiuy | |
| 3 | Seasoned | Mi-iuy croaker | + | − | 26.96 ± 0.76 | N.D. | MF004320.1 | Miichthys miiuy | |
| 4 | Seasoned | Mi-iuy croaker | + | − | 28.34 ± 0.46 | N.D. | MF004320.1 | Miichthys miiuy | |
| 5 | Seasoned | Croaker | − | − | N.D. | N.D. | KY802028.1 | Micropogonias furnieri | |
| 6 | Seasoned | Mi-iuy croaker | + | − | 24.89 ± 0.40 | N.D. | MF004320.1 | Miichthys miiuy | |
| 7 | Seasoned | Mi-iuy croaker | + | − | 22.90 ± 0.38 | N.D. | MF004320.1 | Miichthys miiuy | |
| 8 | Seasoned | Mi-iuy croaker | + | − | 22.20 ± 0.55 | N.D. | MF004320.1 | Miichthys miiuy | |
| 9 | Seasoned | Mi-iuy croaker | + | − | 26.34 ± 0.39 | N.D. | MF004320.1 | Miichthys miiuy | |
| 10 | Seasoned | Mi-iuy croaker | + | − | 25.07 ± 0.01 | N.D. | MF004320.1 | Miichthys miiuy | |
| 11 | Seasoned | Croaker | − | − | N.D. | N.D. | KR632719.1 | Micropogonias megalops | |
| 12 | Seasoned | Mi-iuy croaker | + | − | 26.00 ± 0.08 | N.D. | MF004320.1 | Miichthys miiuy | |
| 13 | Seasoned | Mi-iuy croaker | + | − | 23.14 ± 0.75 | N.D. | MF004320.1 | Miichthys miiuy | |
| 14 | Seasoned | Croaker | − | − | N.D. | N.D. | DQ885030.1 | Otolithes ruber | |
| 15 | Seasoned | Croaker | − | − | N.D. | N.D. | KR632719.1 | Micropogonias megalops | |
| 16 | Seasoned | Mi-iuy croaker | + | − | 25.90 ± 0.88 | N.D. | MF004320.1 | Miichthys miiuy | |
| 17 | Seasoned | Mi-iuy croaker | + | − | 29.21 ± 0.48 | N.D. | MF004320.1 | Miichthys miiuy | |
| 18 | Seasoned | Croaker | − | − | N.D. | N.D. | KP722769.1 | Pseudotolithus senegallus | |
| 19 | Seasoned | Mi-iuy croaker | + | − | 22.28 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | |
| 20 | Raw | Mi-iuy croaker | + | − | 19.07 ± 0.06 | N.D. | MF004320.1 | Miichthys miiuy | |
| 21 | Raw | Mi-iuy croaker | + | − | N.D. | N.D. | MF004320.1 | Miichthys miiuy | |
| 22 | Raw | Mi-iuy croaker | + | − | 20.04 ± 0.13 | N.D. | MF004320.1 | Miichthys miiuy | |
| 23 | Raw | Red drum | − | + | N.D. | 26.14 ± 0.77 | KP641522.1 | Sciaenops ocellatus | |
| 24 | Raw | Red drum | − | + | N.D. | 18.32 ± 0.03 | KP641522.1 | Sciaenops ocellatus | |
| 25 | Raw | Red drum | − | + | N.D. | 19.67 ± 0.73 | KP641522.1 | Sciaenops ocellatus | |
| 26 | Raw | Red drum | − | + | N.D. | 21.36 ± 0.86 | KP641522.1 | Sciaenops ocellatus | |
| 27 | Seasoned | Croaker | − | − | N.D. | N.D. | KY802028.1 | Micropogonias furnieri | |
| 28 | Seasoned | Croaker | − | − | N.D. | N.D. | KR632719.1 | Micropogonias megalops | |
| 29 | Seasoned | Croaker | − | − | N.D. | N.D. | KY802028.1 | Micropogonias furnieri | |
| 30 | Seasoned | Mi-iuy croaker | + | − | 19.22 ± 0.06 | N.D. | MF004320.1 | Miichthys miiuy | |
| 31 | Seasoned | Mi-iuy croaker | + | − | 20.31 ± 0.44 | N.D. | MF004320.1 | Miichthys miiuy | |
| 32 | Seasoned | Mi-iuy croaker | + | − | 20.46 ± 0.39 | N.D. | MF004320.1 | Miichthys miiuy | |
| 33 | Seasoned | Mi-iuy croaker | + | − | 19.17 ± 0.13 | N.D. | MF004320.1 | Miichthys miiuy | |
| 34 | Seasoned | Croaker | − | − | N.D. | N.D. | KY802028.1 | Micropogonias furnieri | |
| 35 | Raw | Croaker | − | − | N.D. | N.D. | KT184692.1 | Argyrosomus japonicus | |
| 36 | Seasoned | Mi-iuy croaker | + | − | 19.45 ± 0.03 | N.D. | MF004320.1 | Miichthys miiuy | |
| 37 | Seasoned | Mi-iuy croaker | + | − | 20.12 ± 0.00 | N.D. | MF004320.1 | Miichthys miiuy | |
| 38 | Seasoned | Mi-iuy croaker | + | − | 21.13 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | |
| 39 | Raw | Mi-iuy croaker | + | − | 18.50 ± 0.43 | N.D. | MF004320.1 | Miichthys miiuy | |
| 40 | Seasoned | Mi-iuy croaker | + | − | 19.58 ± 0.35 | N.D. | MF004320.1 | Miichthys miiuy | |
| 41 | Seasoned | Mi-iuy croaker | + | − | 17.18 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | |
| 42 | Seasoned | Croaker | − | − | N.D. | N.D. | KR632719.1 | Micropogonias megalops | |
| 43 | Seasoned | Croaker | − | − | N.D. | N.D. | KR632719.1 | Micropogonias megalops | |
| 44 | Seasoned | Mi-iuy croaker | + | − | 16.02 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | |
| 45 | Seasoned | Mi-iuy croaker | + | − | 17.34 ± 0.04 | N.D. | MF004320.1 | Miichthys miiuy | |
| 46 | Seasoned | Mi-iuy croaker | + | − | 18.25 ± 0.02 | N.D. | MF004320.1 | Miichthys miiuy | |
| 47 | Seasoned | Mi-iuy croaker | + | − | 18.30 ± 0.03 | N.D. | MF004320.1 | Miichthys miiuy | |
| 48 | Seasoned | Mi-iuy croaker | + | − | 18.41 ± 0.28 | N.D. | MF004320.1 | Miichthys miiuy | |
| 49 | Seasoned | Mi-iuy croaker | + | − | 23.92 ± 0.47 | N.D. | MF004320.1 | Miichthys miiuy | |
a+: Detected/−: Not-detected
bM. miiuy
cS. ocellatus
Species-specific PCR using conventional PCR
Species-specific primers for the mitochondrial COI region were designed using BioEdit Sequence Alignment Editor (http://www.mbio.ncsu.edu/Bioedit/bioedit.html) (Table 2). PCR was performed in a total volume of 20 μL containing 1 × PCR buffer, 0.2 mM dNTP mixture, 1.0 mM MgCl2, 1 μM of each primer, 1 unit of recombinant Taq polymerase (Takara, Shiga, Japan), 5 ng of template DNA, and sterilized water. Amplification was performed using a C1000™ Touch Thermal Cycler (Bio-Rad, Hercules, CA, USA) under the following cycling conditions: 94 °C for 5 min followed by 35 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and a final extension step at 72 °C for 5 min. The amplified products were analyzed using the QIAxcel Advanced system with QX Size Marker 50–800 bp (Qiagen) and QX Alignment Marker 15 bp/1.0 kb (Qiagen). Short-length fragments amplified using the species-specific primers under optimal PCR conditions were purified by agarose gel electrophoresis and then cloned into pGEM-T easy vectors (Promega, Madison, WI, USA). Nucleotide sequences were analyzed with BioEdit software, and the data were analyzed using the Basic Local Alignment Search Tool (BLAST) of the NCBI database.
Table 2.
Primer sets used in the present study
| Name | Sequence (5′–3′) | Size (bp) | Gene | Reference |
|---|---|---|---|---|
| MM-F | AGGTGTTTCCTCAATTCTAGGT | 125 | COI | This study |
| MM-R | GAGGACTGCTGTGATCAGG | |||
| SO-F | TTTCGGGAACTGACTCGTACC | 124 | COI | This study |
| SO-R | TACACCTGAGGAGGTAAGGAGA | |||
| FISHF2 | TCGACTAATCATAAAGATATCGGCAC | 655 | COI | Ward et al. (2005) |
| FISHR1 | TAGACTTCTGGGTGGCCAAAGAATCA |
Direct ultra-fast PCR
DNA was extracted from tissue samples by mixing 40 mg of tissue with 200 μL Direct lysis Buffer (Genesystem, Daejeon, South Korea) and homogenizing the sample with micropestles. The homogenized samples were vortexed at 1 min intervals and incubated at 25 °C for 5 min for DNA extraction. A volume of 10 μL of extraction supernatant was mixed with 90 μL sterile distilled water to obtain a tenfold dilution. This step was repeated to obtain a dilution of 100-fold dilution; 2 μL of the final solution was used as template DNA. Cloned plasmid DNA was used as a positive control. PCR was performed in a total volume of 10 μL containing 1 × Rapi qPCR premix buffer (Genesystem), 0.6 μM of M. miiuy-specific primers or 0.8 μM of S. ocellatus-specific primers, respectively, and sterile distilled water. Amplification was performed using a Genechecker® UF-100 (Genesystem) under the following cycling conditions: 98 °C for 30 s followed by 40 cycles at 98 °C for 3 s, 60 °C for 6 s, and 72 °C for 3 s.
FINS identification of samples
Reference and commercial samples were authenticated by FINS (forensically informative nucleotide sequencing) in order to test the reliability of the method developed. DNA from the commercial samples was extracted as described in section “M. miiuy- and S. ocellatus-specific primer sets” and the amplified using the primers described previously by Ward et al. (2005) (Table 2).
PCR was performed in a total volume of 20 μL containing 1 × PCR buffer, 0.2 mM dNTP mixture, 1.0 mM MgCl2, 1 μM of each primer, 1 unit of recombinant Taq polymerase (Takara, Shiga, Japan), 5 ng of template DNA, and sterilized water. Amplification was performed using a C1000™ Touch Thermal Cycler (Bio-Rad, Hercules, CA, USA) under the following cycling conditions: 95 °C for 3 min followed by 35 cycles at 94 °C for 30 s, 52 °C for 40 s, 72 °C for 1 min, and a final extension step at 72 °C for 5 min. The sizes and sequences of the PCR amplicons were analyzed as described in section “Species-specific PCR using conventional PCR”.
Results and discussion
M. miiuy- and S. ocellatus-specific primer sets
Designing species-specific primers can be challenging because the primers must act fast, accurately, and sensitively to detect sequences in both raw materials and processed foods. Mitochondrial regions are widely used as genetic markers for species identification because they are present in higher copy number than nuclear regions and are typically highly conserved, thus enabling identification of close-relative species. The genomic location of each species-specific primer set used in this study is shown in Fig. S1. Primer sets were designed to amplify short amplicons to facilitate the analysis of processed samples, and theoretical cross-reactivity was confirmed using the primer-BLAST NCBI tool.
Species-specific PCR assays
As expected, M. miiuy- and S. ocellatus-specific primer sets produced 124-base pair PCR amplicons. No cross-reactivity was detected between M. miiuy and S. ocellatus (Fig. 1). The sensitivity of conventional PCR assays was analyzed using serially diluted DNA from each species (tenfold serial dilutions from 5 to 0.0005 ng/μL); the limit of detection was 0.005 ng/μL (Fig. 2).
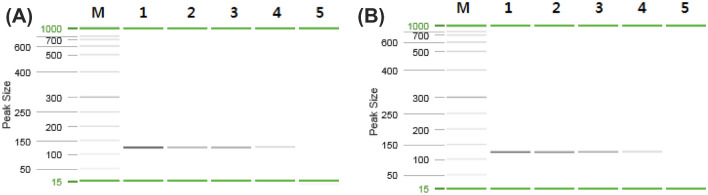
Fig. 1.
Specificity of M. miiuy- and S. ocellatus-specific primer sets. (A) M. miiuy-specific primer set. (B) S. ocellatus-specific primer set. Lane M: 50–800 bp size marker (QIAGEN), lane 1: M. miiuy (124 bp), lane 2: S. ocellatus (124 bp), lane 3: negative control
Fig. 2.
Sensitivity of M. miiuy- and S. ocellatus-specific primer sets. (A) M. miiuy-specific primer set. (B) S. ocellatus-specific primer set. Lane M: 50–800 bp size marker (QIAGEN), lane 1–5: 5 to 0.0005 ng of serial-diluted M. miiuy DNA (A) and S. ocellatus DNA (B)
Forty-nine commercial samples were analyzed to validate species-specific PCR assays and identify M. miiuy species. Thirty-three samples were labeled as Mi-iuy croaker, 4 samples were Red drum, and 12 samples were labeled Croaker without any detailed description of the exact species (Table 1). Thirty-three samples were amplified by M. miiuy primers and four samples were amplified by S. ocellatus primers. Twelve samples were not amplified with either M. miiuy- or S. ocellatus-specific primers (Fig. 3).
Fig. 3.
Species identification for commercial products. (A) M. miiuy-specific primer set. (B) S. ocellatus-specific primer set. Lane M: 50–800 bp size marker (Qiagen), lane R1: M. miiuy DNA, lane R2: S. ocellatus DNA, lane 1–49: template DNA extracted from commercial products listed in Table 2, lane 50: negative control
All samples were verified with FINS for precise species identification. Thirty-three of the 49 samples were identified as M. miiuy and 4 were identified as S. ocellatus. These results agree with the data obtained using species-specific PCR methods. The other 12 samples were identified as Micropogonias megalops, Argyrosomus japonicus, M. furnieri, Otolithes ruber, and Pseudotolithus senegallus belonging to Sciaenidae (Table 1). As M. miiuy is one of the most widely traded croaker species in South Korea, most purchased croaker products were predicted to be M. miiuy. As expected, approximately 67% of collected samples were identified as M. miiuy. The species-specific PCR methods successfully detected M. miiuy and S. ocellatus among the croaker species.
Development of direct ultra-fast real-time PCR assay
The species-specific PCR methods were further improved and optimized to develop a direct ultra-fast real-time PCR assay. Previously, the specificity of the two primer sets was confirmed by conventional PCR assays. To overcome the limitations of conventional PCR in the field, an ultra-fast real-time PCR assay was introduced, which generates real-time results and requires only a short amplification time. Seventeen commercial products were analyzed by the ultra-fast real-time PCR assay in the present study. The results matched the data obtained by conventional PCR (Table 1). The Ct values of the six unamplified samples using species-specific primer sets were not determined. Consequently, the ultra-fast real-time PCR method can complete the entire analytical procedure, including DNA isolation, amplification, and detection, within 30 min, thus maintaining the accuracy of identifying M. miiuy and S. ocellatus products on site.
In the present study, two highly sensitive primer sets were developed and validated by conventional PCR and ultra-fast real-time PCR methods. This species-specific PCR technique does not require costly instruments and reagents, such as enzymatic treatment, and it is easy to interpret the results. The method is accurate, rapid, and cost-effective.
In the present study, M. miiuy- and S. ocellatus-specific primer sets were designed and used to develop two PCR methods for identifying croaker product components. The accuracy of the developed methods was validated by sequence analysis of amplified PCR products from 49 commercial samples. Although the number of samples that can be analyzed at a given time using ultra-fast real-time PCR is limited, this can be overcome with further technical advances. Ultra-fast real-time PCR enabled the completion of the entire analytical procedure within 30 min while maintaining analytical accuracy. Therefore, these newly developed PCR-based methods can be used as efficient tools under varying analytical conditions such as the conversion of raw materials to processed products, transition from the laboratory to the field, and fine-tuning of simple screening procedures to exact species identification.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Ministry of Food and Drug Safety of Korea. We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This study was funded by the Ministry of Food and Drug Safety of Korea (Grant numbers 18161MFDS060).
Declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yewon Hong, Email: yw515@korea.kr.
Jung Ju Kim, Email: jungju90@korea.kr.
Yeon-Cheol Yu, Email: yuyc@korea.kr.
Hyung Soo Kim, Email: jungin98@korea.kr.
Guiim Moon, Email: luna@korea.kr.
Eun Mi Park, Email: empark0731@korea.kr.
References
- Aboud M, Oh HH, McCord B. Rapid direct PCR for forensic genotyping in under 25 min. Electrophoresis. 2013;34:1539–1547. doi: 10.1002/elps.201200570. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Shi G, Xu T, Li H, Sun Y, Wang R. Complete mitochondrial genome of the red drum, Sciaenops ocellatus (Perciformes, Sciaenidae): Absence of the typical conserved motif in the origin of the light-strand replication. Mitochondrial DNA. 2012;23:126–128. doi: 10.3109/19401736.2011.653807. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Xu T, Shi G, Wang R. Complete mitochondrial genome of the miiuy croaker Miichthys miiuy (Perciformes, Sciaenidae) with phylogenetic consideration. Marine Genomics. 2020;3:201–209. doi: 10.1016/j.margen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim JH, Park JY. Marine fishes of Korea. Seoul, Korea: Kyohaksa Publishing Co., Ltd; 2002. [Google Scholar]
- Chuah LO, He XB, Effarizah ME, Syahariza ZA, Shamila-Syuhada AK, Rusul G. Mislabelling of beef and poultry products sold in Malaysia. Food Control. 2016;62:157–164. doi: 10.1016/j.foodcont.2015.10.030. [DOI] [Google Scholar]
- Chuang PS, Chen MI, Shiao JC. Identification of tuna species by a real-time polymerase chain reaction technique. Food Chemistry. 2012;133:1055–1061. doi: 10.1016/j.foodchem.2012.01.076. [DOI] [Google Scholar]
- Fajardo V, Gonzalez I, Rojas M, Garcia T, Martin R. A review of current PCR-based methodologies for the authentication of meats from game animal species. Trends in Food Science & Technology. 2010;21:408–421. doi: 10.1016/j.tifs.2010.06.002. [DOI] [Google Scholar]
- Furutani S, Hagihara Y, Nagai H. On-site identification of meat species in processed foods by a rapid real-time polymerase chain reaction system. Meat Science. 2017;131:56–59. doi: 10.1016/j.meatsci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Herrero B, Lago FC, Vieites JM, Espiñeira M. Real-time PCR method applied to seafood products for authentication of European sole (Solea solea) and differentiation of common substitute species. Food Additives & Contaminants: Part A. 2012;29:12–18. doi: 10.1080/19440049.2011.623682. [DOI] [PubMed] [Google Scholar]
- Haunshi S, Basumatary R, Girish PS, Doley S, Bardoloi RK, Kumar A. Identification of chicken, duck, pigeon and pig meat by species-specific markers of mitochondrial origin. Meat Science. 2009;83:454–459. doi: 10.1016/j.meatsci.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Houssin T, Cramer J, Grojsman R, Bellahsene L, Clas G, Moulet H, Minnella W, Pannetier C, Leberre M, Plecis A, Chen Y. Ultrafast, sensitive and large-volume on-chip real-time PCR for the molecular diagnosis of bacterial and viral infections. Lab on a Chip. 2016;16:1401–1411. doi: 10.1039/C5LC01459J. [DOI] [PubMed] [Google Scholar]
- Khandurina J, McKnight TE, Jacobson SC, Waters LC, Foote RS, Ramsey JM. Integrated system for rapid PCR-based DNA analysis in microfluidic devices. Analytical Chemistry. 2000;72:2995–3000. doi: 10.1021/ac991471a. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang Y, Li J, Kang B, Fang L, Zheng L, Liu M. First records of small juveniles of the red drum Sciaenops ocellatus (Linnaeus, 1766) in a subtropical mangrove habitat of China. BioInvasions Records. 2020;9:96–102. doi: 10.3391/bir.2020.9.1.13. [DOI] [Google Scholar]
- Lockley AK, Bardsley RG. DNA-based methods for food authentication. Trends in Food Science & Technology. 2000;11:67–77. doi: 10.1016/S0924-2244(00)00049-2. [DOI] [Google Scholar]
- Man YC, Aida AA, Raha AR, Son R. Identification of pork derivatives in food products by species-specific polymerase chain reaction (PCR) for halal verification. Food Control. 2007;18:885–889. doi: 10.1016/j.foodcont.2006.05.004. [DOI] [Google Scholar]
- Namikoshi A, Takashima Y, Iguchi J, Yanagimoto T, Yamashita M. Species identification of Alaska pollock, Gadus spp., and Micromesistius spp. in cod roe products using a PCR-based method. Fisheries Science. 2011;77:671–678. doi: 10.1007/s12562-011-0349-4. [DOI] [Google Scholar]
- Neuzil P, Pipper J, Hsieh TM. Disposable real-time microPCR device: lab-on-a-chip at a low cost. Molecular BioSystems. 2006;2:292–298. doi: 10.1039/b605957k. [DOI] [PubMed] [Google Scholar]
- Okuma TA, Hellberg RS. Identification of meat species in pet foods using a real-time polymerase chain reaction (PCR) assay. Food Control. 2015;50:9–17. doi: 10.1016/j.foodcont.2014.08.017. [DOI] [Google Scholar]
- Rasmussen RS, Morrissey MT. DNA-based methods for the identification of commercial fish and seafood species. Comprehensive Reviews in Food Science and Food Safety. 2008;7:280–295. doi: 10.1111/j.1541-4337.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- Rojas M, Gonzalez I, Pavon MA, Pegels N, Hernandez PE, Garcia T, Martin R. Application of a real-time PCR assay for the detection of ostrich (Struthio camelus) mislabelling in meat products from the retail market. Food Control. 2011;22:523–531. doi: 10.1016/j.foodcont.2010.09.039. [DOI] [Google Scholar]
- Shan XJ, Cao L, Huang W, Dou SZ. Feeding, morphological changes and allometric growth during starvation in miiuy croaker larvae. Environmental Biology of Fishes. 2008;28:121–130. [Google Scholar]
- Taylor MI, Fox C, Rico I, Rico C. Species-specific TaqMan probes for simultaneous identification of (Gadus morhua L.), haddock (Melanogrammus aeglefinus L.) and whiting (Merlangius merlangus L.) Molecular Ecology Notes. 2002;2:599–601. doi: 10.1046/j.1471-8286.2002.00269.x. [DOI] [Google Scholar]
- Teletchea F, Maudet C, Hanni C. Food and forensic molecular identification: update and challenges. Trends in Biotechnology. 2005;23:359–366. doi: 10.1016/j.tibtech.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Wan-shu H, Qi-yong Z. Artificial propagation and breeding of marine fish in China. Chinese Journal of Oceanology and Limnology. 2002;20:41–51. doi: 10.1007/BF02846610. [DOI] [Google Scholar]
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia's fish species. Philosophical Transactions of the Royal Society b: Biological Sciences. 2005;360:1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EK, Hara CA, Frank J, Deotte J, Hall SB, Benett W, Spadaccini C, Beer NR. Under-three minute PCR: Probing the limits of fast amplification. Analyst. 2011;136:3707–3712. doi: 10.1039/c1an15365j. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang Y, Xu D, Lou B, Guo Y, Sun X, Guo B. Genetic population structure of miiuy croaker (M. miiuy) in the Yellow and East China Seas base on mitochondrial COI sequences. Biochemical Systematics and Ecology. 2014;54:240–246. doi: 10.1016/j.bse.2014.01.013. [DOI] [Google Scholar]
- Zhu Y, Yun-Ling L, Han-ling W. A study on the classification of the sciaenoid fishes of China, with description of new genera and species. Translation Series. Virginia Institute of Marine Science, College of William and Mary (1975). https://scholarworks.wm.edu/reports/33.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.