Schizophrenia (SZ) is a chronic, serious, and recurrent mental illness, characterized by cognitive impairment as the core symptom, which can be an important sign before the onset of psychosis [1]. Furthermore, persistent cognitive impairment is specific to the disease and disrupts the cognitive trajectory of patients with SZ [2]. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive intervention for SZ patients with auditory hallucinations or negative symptoms [3], as well as antipsychotic treatment failure [4]. According to recent reports, rTMS might be an effective treatment for cognitive impairment [5–7]. At present, the site of stimulation for rTMS application in clinical practice is often ambiguous. Indeed, patients with SZ usually do not exhibit significant brain lesions, leaving uncertain the accurate localization of most neuropsychiatric symptoms. Yet due to certain differences in brain structure and function among patients [8], this non-individualized coil placement may partly explain why rTMS treatment is still ineffective in some cases. With the development of precision medicine, the introduction of individualized rTMS in the treatment of SZ is a field calling for further research.
Current Viewpoints Between rTMS and Cognitive Dysfunction in SZ
In recent years, rTMS has been evaluated as a novel treatment option for various neuropsychiatric disorders, including SZ. Yet the improvement of symptoms (auditory hallucinations, negative symptoms, cognitive dysfunction) in SZ after rTMS treatment remains to be identified and the therapeutic mechanisms remain unclear. Does rTMS regulate brain neurotransmitters (e.g., serotonin and dopamine), or alter synaptic plasticity or brain-derived neurotrophic factor levels? There is not enough evidence. Although some encouraging results have been reported, most are focused on auditory hallucinations or negative symptoms, rarely involving cognitive dysfunction [9]. Here, we mainly introduce the significance of rTMS in the treatment of SZ from the cognitive perspective.
To our knowledge, the dorsolateral prefrontal cortex (DLPFC), which is involved in the manipulation and temporal encoding of information, namely, working memory, is an attractive target for rTMS studies in SZ, as previous studies have reported that this region is associated with cognitive deficits [5–7]. Regretfully, with traditional stimulation coils, rTMS is largely limited to the cortical surface, so deeper regions cannot be directly or selectively stimulated. rTMS studies have, therefore, focused on the left DLPFC as one accessible node of the cognitive network. Research shows that the DLPFC is anatomically heterogeneous, especially in terms of its intrinsic functional connectivity, which can be used to generate individualized locations for DLPFC studies. Such individual-based targets might be clinically superior to those selected at the group level [10], revealing the importance of individualization. Barr et al. first demonstrated that bilateral 20 Hz rTMS targeting the DLPFC significantly improve patients’ working memory, compared with sham-control subjects [6]. Francis et al. demonstrated that 20 Hz bilateral rTMS improves cognitive dysfunction in the early course of SZ, and frontal cortical thickness may positively predict the response to rTMS [9]. Xiu and colleagues assessed the efficacy of different frequencies of neuro-navigated rTMS in ameliorating cognitive impairment, and their results showed that 20 Hz rTMS treatment had an effective therapeutic benefit on the immediate memory of patients with chronic SZ at week 8, but 10 Hz rTMS did not [7]. Interestingly, both of the studies by Francis et al. and Xiu et al. reported that delayed effects on cognitive function were produced at the follow-up after rTMS treatment, that is to say, some of the cognitive improvement did not appear immediately after the treatment sessions. Therefore, rTMS has potential as a pathophysiologically oriented therapy for cognitive impairment and long-term longitudinal studies are needed.
In spite of several positive findings, i.e., that high-frequency rTMS to the left PFC improves cognitive symptoms, the effects are inconsistent and small. The latest meta-analysis involving nine rTMS studies indicated that there was no association with a reliable improvement of working memory performance in SZ when applying rTMS over the DLPFC and no association between the number of stimulation sessions and treatment effects [11]. Indeed, the therapeutic effect of rTMS on the cognitive symptoms of SZ is still ambiguous, and there are not enough data to discuss whether rTMS can modulate the cognitive function of SZ patients in more detail. Thus, it is important to investigate whether particular stimulation protocols in combination with neuroimaging, e.g., magnetic resonance imaging (MRI), are able to exert a greater effect on cognitive impairment.
The Potential Clinical Value of MRI in Neuromodulation of SZ
The overwhelming heterogeneity of SZ is one of the greatest challenges for the development of cures for the disorder [2]. At present, using symptom subtyping analysis to reduce the heterogeneity is promising and may have a potential influence on research and practice. Objective evidence is more helpful in formulating a treatment plan. Therefore, the application of MRI before treatment is helpful in exploring the biological subtypes and predicting the response of SZ to rTMS on the basis of anatomical structure and functional activation. Soon after the appearance of psychosis, patients whose cognitive performance declines tend to show a smaller brain volume than patients who have a stable cognitive performance, indicating that cognitive decline may identify a biologically distinct subgroup [2]. Nevertheless, another study attempted to identify two biological subtypes based on neuroanatomy using structural MRI: one subtype with lower volumes of gray matter (prominently in the thalamus, nucleus accumbens, medial temporal, medial prefrontal/frontal, and insular cortices), and another subtype with either increased volumes (basal ganglia and internal capsule) or normal volumes [12]. Significantly, the classification approach should be designed to discover patterns associated with the disease rather than normal anatomical variation. Similarly, the neurobiological differentiation of subtypes based on resting-state functional connectivity patterns has been investigated, and the individual subtype was predicted with good accuracy from functional connectivity profiles of the ventro-medial frontal cortex, temporo-parietal junction, and precuneus [13]. Together with subtyping and the demonstrated ability to predict biological subtype from neuroimaging, these findings could further disentangle the heterogeneity in schizophrenia. The studies of subtypes are conducive to the precise detection of a target. Future research will provide a more detailed relationship of these subtypes and the structure and function of the brain and clinical features, including cognitive performance. Fields of particular research value include using connectivity to guide the selection of rTMS targets, even realizing the modulation of deep targets.
Abnormal development of the brain networks appears to be a reliable profile of the neuroanatomical and functional basis of the cognitive changes prior to the onset of SZ [1]. It has been suggested that patients with SZ suffer from disconnection throughout the clinical course [14]. Cognitive impairment in this disorder is associated with connectome damage [15], and are especially relevant to the disrupted hub nodes in the frontal and parietal lobes. As well, activating the DLPFC via rTMS can result in improved cognition [5, 7]. Lesion network mapping is anticipated to identify new symptom-based treatment targets [16]. The functional networks of SZ are more sensitive and disrupted earlier than the network of the healthy population [17]. And using rTMS modulates abnormal network interactions identified with resting state functional connectivity MRI [18]. Cognitive brain networks such as the default-mode, frontoparietal, and salience networks, are the key functional networks of the human brain. Thus, future work is needed to determine if cognitive network modulation can be used to treat SZ.
The Application of MRI-Guided and Navigated rTMS in the Treatment of Cognitive Impairment in SZ
Nevertheless, it is still difficult to locate the stimulation site accurately. To better localize stimulation targets, neuroimaging-based navigation methods are warranted. Navigation procedures are based on the integration of brain imaging data provided by multiple modalities such as structural or functional MRI [19]. Using the infrared tracking system, researchers can evaluate the head position of patients, measure the positions of scalp marks (nose tip, nasion, and invagination of the left and right ears) seen on the MRI, and then place stimulation coils over the target brain area [7]. These technologies have provided the possibility of directly visualizing the anatomical structure being stimulated. It takes into account the inter-subject variability in brain anatomy and cerebral cortex function. Therefore, it allows the development of treatment strategies to address individual structural and functional differences and alleviate cognitive impairment more effectively. Besides increasing the accuracy of target location in regions associated with cognition, navigation systems can also help to keep the coil steady over the desired target throughout the therapeutic session [19].
Future research should focus on specific parameters of rTMS application to SZ (such as different frequencies, stimulation loci and sessions, and biological subtypes of SZ) to establish guidelines for individualized treatment. Based on this guideline, the optimal stimulation protocol will be based on anatomical and functional analyses of each patient’s images, in conjunction with the results of cognitive testing; and precise positioning will be achieved through the navigation system. The effectiveness of the current clinical application of rTMS treatment is insufficient, the protocols vary among existing studies, and the conclusions are controversial for treating cognitive impairment in SZ. The establishment of treatment guidelines would contribute to clinical practice. Remembering that MRI has advantages for the study of brain anatomy and functional connectivity as we note that rTMS has potential therapeutic value for SZ, their combination through a navigation system facilitates the implementation of individualized stimulation according to the patient’s characteristics.
The Prospect of Individualized Treatment Based on MRI: Both Guidance and Neuro-navigation
MRI has added ample valuable insights into the neural pathophysiology of SZ and its relation to clinical presentation. There is great potential for individualized treatment studies by the combination of navigated rTMS and MRI methods. Neuro-navigation is an indispensable tool for precise planning, localization, and monitoring in brain stimulation studies [5]. The use of navigation technology and imaging methods is conducive to the analysis and expression of rTMS treatment results. MRI has indicated that different patients present distinct cerebral patterns and multi-parametric MRI-based features may predict the response to treatment with neurostimulation [20], thus needing individualized stimulation parameters. The clinical use of rTMS calls for the careful identification of individual variation because the location, frequency, and total stimulation received are potential moderators of the effect. Targeting the DLPFC with high-frequency stimulation has advantages in reducing negative symptoms. Similarly, targeting the temporo-parietal junction with low-frequency stimulation is conducive to reducing auditory hallucinations [21]. Interventions such as rTMS are effective only if the stimulated brain is able to produce a significant plasticity response in the target network [21]. Brady et al. [22] combined resting-state functional MRI with multivariate data analysis and showed that disconnection of the right DLPFC from the cerebellar network is directly associated with the severity of negative symptoms, and correcting this disconnection via rTMS improves the severity of negative symptoms. However, the best stimulus point to match a specific cognitive deficit remains a great challenge. The appropriate application of navigation techniques with rTMS not only defines the anatomical structure of the individual cortex, but also defines the “dose” of stimulation.
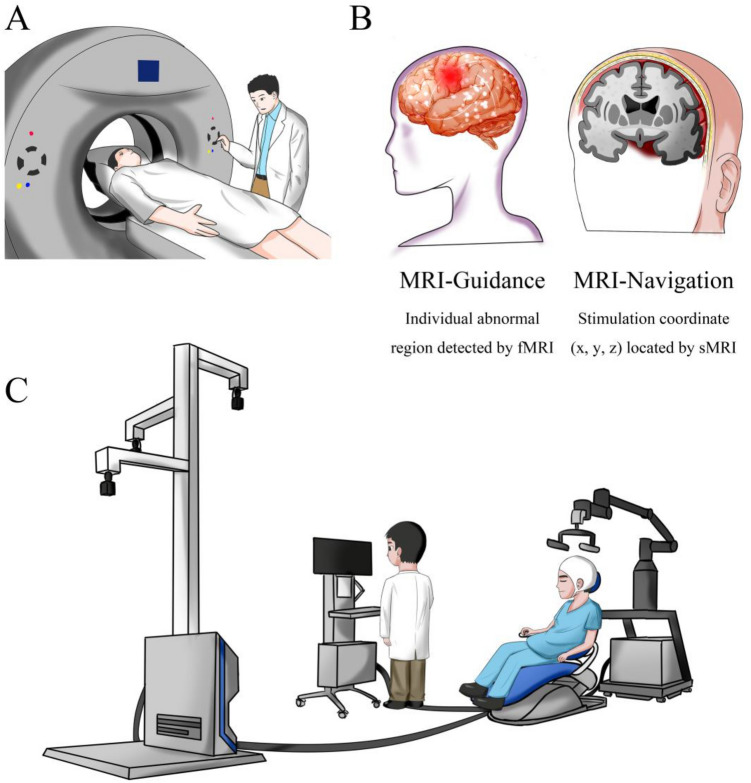
With respect to the “dose”, rTMS should be regulated like psychotropic medications. In this way, each patient should be treated individually and given a different stimulus scheme. In the scatter plots in the literature [7, 21], despite the overall trend relating rTMS treatment and symptom improvement, some points are far from the average value. Similarly, a possible reason for these uncorrelated results may be the lack of an individual “dose”. We propose to establish a detailed parameter range composed of various biomarkers in the future. Based on the abnormal parameters, we can make stimulation plans based on the corresponding brain regions, which vary from person to person (for example, in the DLPFC, cerebellum, temporo-parietal junction, or other more specific anatomical sites). Finally, cognitive impairment in SZ may be improved more effectively by MRI-guided and navigated rTMS (Fig. 1).
Fig. 1.
A proposed MRI-guided and navigated rTMS for cognitive impairment in SZ. A Patients undertake MRI scans. B Functional and structural data are obtained to detect abnormal activations and design a stimulation target at the individual level (guidance and navigation). C Patients receive rTMS using a neuro-navigation system based on MRI. fMRI, functional magnetic resonance imaging; sMRI, structural magnetic resonance imaging.
Conclusions
In conclusion, MRI-guided and navigated individualized rTMS might be a potential tool for cognitive enhancement. However, whether the therapeutic efficacy of rTMS can benefit from more precise targeting using a navigation system and identifying the pathway of clinical response remain to be demonstrated. Furthermore, rTMS has yet to be robustly evaluated in randomized controlled trials, and may present follow-up challenges in terms of accessibility and compliance. Some suggestions for future studies are: first, carrying out double-blind randomized sham-controlled multicenter trials; second, performing more accurate efficacy assessment using standardized neuropsychological measurement tools; third, studying the optimal stimulation site and stimulation protocol corresponding to different biological subtypes of SZ; fourth, combining anatomical structure and functional network studies; fifth, establishing individualized rTMS treatment guidelines; and finally, translating these results as far as possible into meaningful guidance for people with SZ. A multi-disciplinary team is the solution to this key clinical issue. When targeting cognitive impairment, should psychiatrists write the MRI-guided and navigated rTMS prescription for SZ? This will depend on radiologists and computational neuroscientists, as well as patients.
Acknowledgements
This insight was supported by grants from the Fourth Military Medical University (2019CYJH) and the China Postdoctoral Science Foundation (2019TQ0130).
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Xu-Sha Wu and Tian-Cai Yan have contributed equally to this work.
Contributor Information
Hong Yin, Email: yinhong@fmmu.edu.cn.
Long-Biao Cui, Email: lbcui@fmmu.edu.cn.
References
- 1.Kahn RS. On the origins of schizophrenia. Am J Psychiatry. 2020;177:291–297. doi: 10.1176/appi.ajp.2020.20020147. [DOI] [PubMed] [Google Scholar]
- 2.Keefe RSE, Kahn RS. Cognitive decline and disrupted cognitive trajectory in schizophrenia. JAMA Psychiatry. 2017;74:535–536. doi: 10.1001/jamapsychiatry.2017.0312. [DOI] [PubMed] [Google Scholar]
- 3.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Cui LB, Cai M, Wang XR, Zhu YQ, Wang LX, Xi YB, et al. Prediction of early response to overall treatment for schizophrenia: A functional magnetic resonance imaging study. Brain Behav. 2019;9:e01211. doi: 10.1002/brb3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan HY, Zhao JM, Wang KQ, Su XR, Pan YF, Guo JM, et al. High-frequency neuronavigated rTMS effect on clinical symptoms and cognitive dysfunction: A pilot double-blind, randomized controlled study in Veterans with schizophrenia. Transl Psychiatry. 2020;10:79. doi: 10.1038/s41398-020-0745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Xiu MH, Guan HY, Zhao JM, Wang KQ, Pan YF, Su XR, et al. Cognitive enhancing effect of high-frequency neuronavigated rTMS in chronic schizophrenia patients with predominant negative symptoms: A double-blind controlled 32-week follow-up study. Schizophr Bull. 2020;46:1219–1230. doi: 10.1093/schbul/sbaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Y, Lv D, Liang Y, Zhang H, Pei K, Shao R, et al. Abnormal brain structure and function in first-episode childhood- and adolescence-onset schizophrenia: Association with clinical symptoms. Neurosci Bull. 2019;35:522–526. doi: 10.1007/s12264-019-00359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis MM, Hummer TA, Vohs JL, Yung MG, Visco AC, Mehdiyoun NF, et al. Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: A pilot study. Brain Imaging Behav. 2019;13:852–861. doi: 10.1007/s11682-018-9902-4. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan NP, Byrne LK, Enticott PG, Lum JAG. Non-invasive brain stimulation does not improve working memory in schizophrenia: A meta-analysis of randomised controlled trials. Neuropsychol Rev. 2021;31:115–138. doi: 10.1007/s11065-020-09454-4. [DOI] [PubMed] [Google Scholar]
- 12.Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 2020;143:1027–1038. doi: 10.1093/brain/awaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Patil KR, Weis S, Sim K, Nickl-Jockschat T, Zhou J, et al. Neurobiological divergence of the positive and negative schizophrenia subtypes identified on a new factor structure of psychopathology using non-negative factorization: An international machine learning study. Biol Psychiatry. 2020;87:282–293. doi: 10.1016/j.biopsych.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang JB, Cao Y, An NY, Yang Q, Cui LB. Magnetic resonance imaging-based connectomics in first-episode schizophrenia: From preclinical study to clinical translation. Front Psychiatry. 2020;11:565056. doi: 10.3389/fpsyt.2020.565056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collin G, Keshavan MS. Connectome development and a novel extension to the neurodevelopmental model of schizophrenia. Dialogues Clin Neurosci. 2018;20:101–111. doi: 10.31887/DCNS.2018.20.2/gcollin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379:2237–2245. doi: 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]
- 17.Arzouan Y, Moses E, Peled A, Levit-Binnun N. Impaired network stability in schizophrenia revealed by TMS perturbations. Schizophr Res. 2014;152:322–324. doi: 10.1016/j.schres.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefaucheur JP. Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Neurophysiol Clinique/Clinical Neurophysiol. 2010;40:1–5. doi: 10.1016/j.neucli.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Gong J, Cui LB, Xi YB, Zhao YS, Yang XJ, Xu ZL, et al. Predicting response to electroconvulsive therapy combined with antipsychotics in schizophrenia using multi-parametric magnetic resonance imaging. Schizophr Res. 2020;216:262–271. doi: 10.1016/j.schres.2019.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Hasan A, Wobrock T, Guse B, Langguth B, Landgrebe M, Eichhammer P, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22:857–864. doi: 10.1038/mp.2016.161. [DOI] [PubMed] [Google Scholar]
- 22.Brady RO, Gonsalvez I, Lee I, Öngür D, Seidman LJ, Schmahmann JD, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176:512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]