Abstract
To defense harmful stimuli or maintain the immune homeostasis, the body produces and recruits a superfamily of cytokines such as interleukins, interferons, chemokines etc. Among them, chemokines act as crucial regulators in defense systems. CCL5/CCR5 combination is known for facilitating inflammatory responses, as well as inducing the adhesion and migration of different T cell subsets in immune responses. In addition, recent studies have shown that the interaction between CCL5 and CCR5 is involved in various pathological processes including inflammation, chronic diseases, cancers as well as the infection of COVID-19. This review focuses on how CCL5/CCR5 axis participates in the pathological processes of different diseases and their relevant signaling pathways for the regulation of the axis. Moreover, we highlighted the gene therapy and chemotherapy studies for treating CCR5-related diseases, including the ongoing clinical trials. The barriers and perspectives for future application and translational research were also summarized.
Keywords: Cancer, CCL5/CCR5, Infection, Therapy
Introduction
Chemokine is a molecular family about 8–10 kD that attracts different cytokines, cells and substance to specific sites. These small proteins regulate the positioning of cells, and are involved in a wide spectrum of biological processes such as homeostasis, angiogenesis, metastasis, immune response, inflammation and chemotaxis.1, 2, 3 Based on the number of amino acids between the first two cysteine residues, chemokines are classified into four subfamilies, CXC, CC, CX3C and XC.4 CCL5, also called RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted), belongs to CC subfamily of chemokines. Most inflammatory cells can express CCL5, among them, T cells and monocytes are the most common types of CCL5-expressing cells. While CCL5 can bind to CCR1, CCR3, CCR4 and CCR5, it has the highest affinity to CCR5.5, 6, 7 CCR5 (also known as CD195), a G-protein-coupled receptor (GPCR), whose transcription is regulated by CREB-1.8 It expresses on T cells, smooth muscle endothelial cells, epithelial cells, even parenchymal cells etc.6,9 Besides CCL5, CCR5 also combine to CCL3 (MIP-1α), CCL4 (MIP-1β) with a N-terminal extracellular tail.10 Additionally, CCR5 is the most important receptor that allows HIV-1 infection with gp-120 combination, thus it has been considered as a promising target for anti-HIV therapies.11
In different conditions, the function of CCL5/CCR5 axis varies, which might be concerned with HIV infection,12 cell proliferation,13 migration, angiogenesis,14 metastasis15 and survival.1,16 Nowadays, CCL5/CCR5 has been studied in many diseases such as inflammation, cancers,3 virus infections7 and immune responses.15 This review focuses on the downstream and upstream of CCL5/CCR5 and how they are related to the pathological processes of diseases. Furthermore, the strategies to treat cancers or to induce an anti-tumor microenvironment by targeting CCL5/CCR5 axis are discussed. Moreover, the current and classical therapies for CCR5-related diseases are highlighted and summarized.
Downstream and upstream pathway of CCL5/CCR5 axis
Downstream pathway of CCL5/CCR5
It has been reported that the downstream pathway of CCL5/CCR5 including PI3K/AKT, NF-κB, HIF-, RAS-ERK-MEK, JAK-STAT and TGF-β-smad pathways are associated with cell proliferation, angiogenesis, apoptosis, invasion, division, metastasis and inflammation.5,17, 18, 19, 20
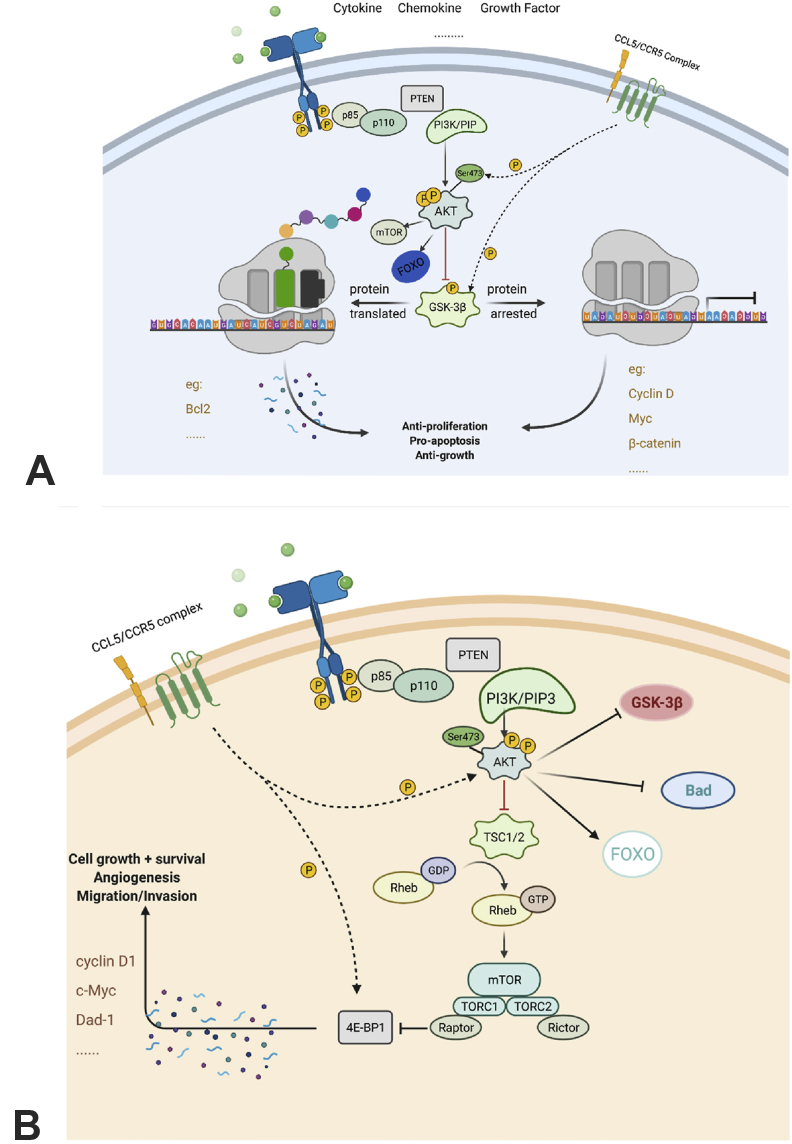
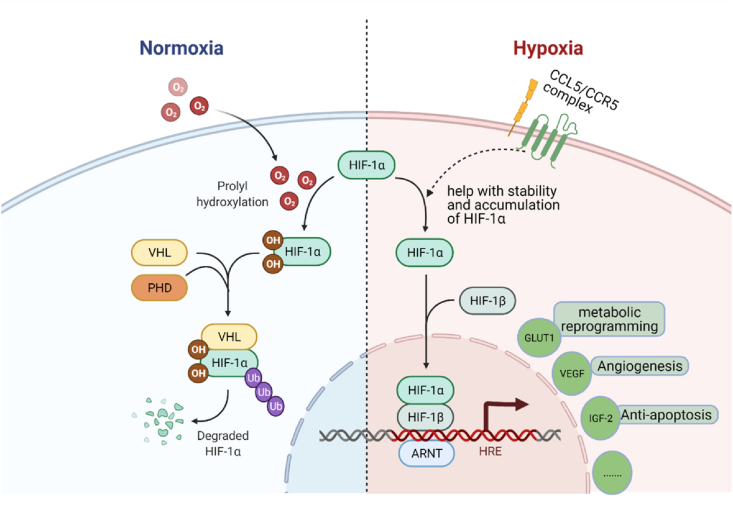
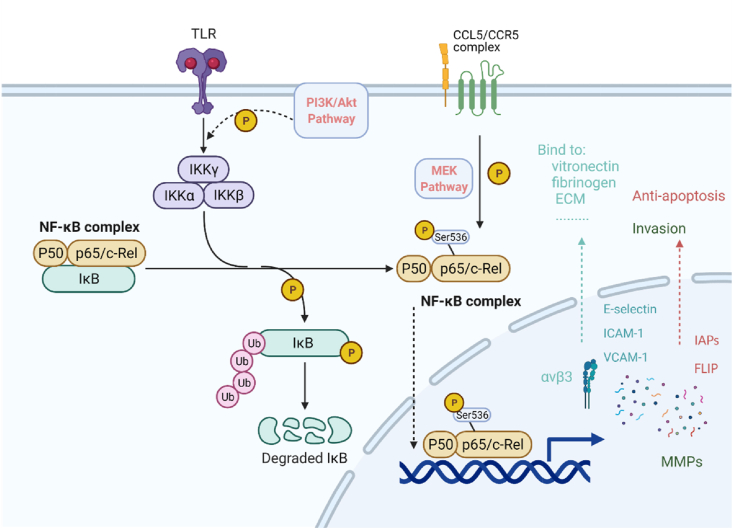
CCL5/CCR5 axis could contribute to the activation of AKT and GSK-3β in PI3K/AKT pathway, thus regulating its downstream. Specifically, PI3K gets phosphorylated after CCL5 binding to CCR5, and contributes to the phosphorylation of the serine 473 of AKT. The AKT/PKB complex then phosphorylates GSK-3 to interrupt its activities. The expression of several downstream proteins is either activated or arrested (Fig. 1A).20 For example, the expression level of Bcl2 is upregulated to promote cell apoptosis;21 -catenin gets phosphorylated and then degraded;22,23 the level of Cyclin D, a crucial protein for the regulation of cell cycle, can be arrested by inactivated GSK-3.23 In the PI3K/AKT/mTOR pathway, CCL5/CCR5 phosphorylates 4E-BP1, which pushes mTOR-4E-BP1/S6K1-eIF4F pathway forwards. As soon as mTOR, a tyrosine kinase, receiving signaling from CCL5/CCR5-PI3K/AKT, it phosphorylates 4E-BP1, S6K1 and eIF4F gradually. After that, activated eIF4F disassociates 5′-untranslated region (UTR) and promotes mRNA translation. Moreover, CCL5/CCR5-mediated-PI3K/AKT phosphorylates Thr1462 of TSC-2, thereby prevent the formation of TSC-1/TSC-2 complex. This mediates the de-inhibition of Rheb, promoting the activation of mTOR and its downstream pathways (Fig. 1B). In reverse, mTOR can directly activate AKT pathway and send signals downstream.24,25 CCL5/CCR5 could also activate hypoxia inducible factor α (HIF-α) pathway by improving the stability and accumulation of HIF-1α.26 At a hypoxia situation, HIF-α initiates angiogenesis processes via a von Hippel Lindau protein (VHL) related ubiquitin degradation or HIF response element (HRE)27,28 (Fig. 2). CCL5/CCR5 phosphorylated p65 serine 536 can modulate signaling at both protein and mRNA levels through NK-κB pathway with a co-activator29 (Fig. 3). Up to date, it has been found that NF-κB pathway is associated with up-regulated expression level of Inhibitors of Apoptosis Proteins (IAPs), FLICE-like inhibitory proteins (FLIPs) and matrix metalloproteinase (MMP) proapoptotic protein as well as lower p53 level.30, 31, 32, 33 CCL5/CCR5 can initiate RAS-ERK-MEK pathway by phosphorylating MEK and enhancing the kinase activity.34,35 For example, while downstream proteins like Msk1, RSK and Mnk136,37 are secreted for contributing to chromatin remodeling or protein synthesis, Ets, ELK-1 and SAP-1 are able to modify transcription.38, 39, 40 In addition, the activation can activate PI3K/AKT pathway to act synergistically.41 JAKs at adjacent receptors approach each other and get trans-phosphorylation after CCL5/CCR5 combination. Src homology 2 domain (SH2) of STAT and SOCS (suppressor of cytokine signaling) family enable receptor binding, which promotes gene transcription.42,43 As evinced by the T cell lymphoma seen in clinical patients' tissues, CCL5/CCR5 upregulate DNA methyltransferase 1 (DNMT1) expression level in malignant T cells.44 Additionally, tumor associate macrophage, especially M2 macrophage, release CCL5 to stimulate DNMT1 expression in ACG and HR cell lines in gastric cancer process.45 Indirect activation of other pathways such as TGF- pathway46 by CCL5/CCR5 are not discussed here.
Figure 1.
CCL5/CCR5 axis in PI3K/AKT pathways. (A) CCL5/CCR5 axis in PI3K/AKT/GSK-3 pathway. CCL5/CCR5 binding contributes to the formation of AKT/PKB complex, the serine 473 of AKT would be phosphorylated, and interrupt activation or arrest of downstream signalings. (B) CCL5/CCR5 axis in PI3K/AKT/mTOR pathway. CCL5/CCR5-mediated-PI3K/AKT prevent the formation of TSC-1/TSC-2 complex and then lead to de-inhibition of Rheb.
Figure 2.
CCL5/CCR5 axis in HIF- pathway. At normoxia, HIF-α could bind to PHD and undergo a VHL related ubiquitin degradation. When the oxygen level is low, HIF-α would accumulate and complex with HIF-to form HRE. Next, HRE would bind to the promoter of gene and amplify genes.
Figure 3.
CCL5/CCR5 axis in NF-κB pathway. PI3K/AKT, phosphorylated p65 serine 536 mediates cascade phosphorylation of IKK and IκB subunits, then NF-κB gets activated. NF-κB enters cell nucleus after the ubiquitin-related degradation of phosphorylated IκB.
Upstream regulators of CCL5/CCR5
Numerous studies have revealed the upstream regulators modulating CCL5/CCR5 signaling, including plasminogen activator inhibitor-1 (PAI-1),47 kruppel-like zinc-finger transcription factor 5 (KLF5),48,49 SOCS-1, Rig1 etc. For instance, PAI-1 could activate the secretion of CCL5, which in turn contributes to PAI-1 production, forming a positive feedback loop. Resultantly, PAI-1 as a regulator of CCL5 could promote cell migration, angiogenesis and inhibit cell apoptosis through MMPs and cytokines secretion at a preferable concentration.50,51 In addition, activated c-Jun N-terminal kinase could bind to the promoter of CCL5 gene and regulate the initiation of the transcription.52 Studies have shown that there is a positive correlation between the level of enhancer of zeste homolog 2 (EZH2) and CCL5. EZH2 regulates epigenetic gene through regulating the methylation of histone H3 lysine 27 (H3K27). EZH2 could also be considered as an key regulator of macrophage-mediated cancer cell progression and migration.53,54 Moreover, HER2 and phosphatase and tensin homolog deleted on chromosome ten (PTEN) are a pair of cancer-related proteins that are closely associated with metastasis. The increased level of HER2 and the decreased level of PTEN will result in more CCL5, IL-6 and IL-8 secretion.55,56
External stimuli could also regulate CCL5/CCR5 signaling. Vascular damage-resulted angiotensin 2 (Ang2) upregulation would lead to the enhanced transcription level of CCL5 mRNA in perivascular adopt tissue (pVAT). As a consequence, the CCR5+ leucocytes would migrate to pVAT and IFN- secretion also prolongs immune response.57,58 When it comes to irradiation, the transcription of CCL5 can be initiated by cGAMP-STING signaling pathway as interferon-stimulated genes (ISG). Alternatively, oncogenic events like Neu-T, H-RAS or c-SRC stimulation also activates CCL5/CCR5 signaling, which may lead to the enhancement of tumor cell aggressiveness.59
Besides of activators of CCL5, researchers have also already found some inhibitors. It has been demonstrated that SOCS-1, Rig1 and abnormal LDH could downregulate the expression of CCL5. However, the underlying mechanism has not been fully uncovered so far.60
CCL5/CCR5 and diseases
As reported by previous studies, the abnormal interaction of CCL5/CCR5 has been found in multiple types of inflammation,61 including hepatitis, atherosclerosis, and microglia inflammation. Some viral infections are also mediated by CCL5/CCR5 axis. More importantly, the elevated level of CCL5/CCR5 have been found in different types of cancers including gastric cancer, breast cancer, lung cancer, osteosarcoma, prostate cancer and pancreas cancer.5,62 Additionally, in several other diseases, such as diabetes, Alzheimer's disease and endometriosis, CCL5/CCR5 was also found to be expressed in an aberrant manner.
Inflammations and infections
When getting damaged, pro-inflammatory factors are released into damaged tissues. Common pro-inflammatory factors are tumor necrosis factor (TNF), interleukin (IL) and TGF-.
Oral lichen planus
First example is oral lichen planus (OLP), which is characterized by the liquefaction degeneration of basal epithelial cells. The lesion is caused by the over proliferation and activation of T cells. Basically, CCL5/CCR5 axis attracts T cells, allowing their proliferation and activation. Once T cells interact with epithelium, CCL5/CCR5 axis would cause the basal keratinocyte damage and initiate inflammation. In addition, Th1 cells secret IFN- to increase CCL5 expression and aggravate epithelium damage.63,64 On the other hand, OLP-related mast cell degranulation could cause upregulation of the cytokines mediating the expression of CCL5, such as TNF-. As a result, the increased CCL5 would strengthen the accumulation of specific T cells, which further prolongs the inflammatory response.65
Atherosclerosis
Previously, atherosclerosis is viewed as a lipid-initiated disease, a gene mutation disease or a receptor deletion disease. But as the scientists delve deeper into the pathology of the disease, it is now widely accepted that atherosclerosis is an inflammatory disease. Moreover, following the development of atherosclerosis, cytokines' level would be upregulated in both peripheral blood and local tissues.66, 67, 68 MMP9 secreted from macrophages increases and helps with the generation of plagues. Besides, there are many co-action factors, such as perforin-1, vascular cell adhesion molecule-1 (VCAM-1), IL-6, selectin, TNF-α, etc., being involved in the whole pathologic process.69, 70, 71 Further, it has been reported that CCL5/CCR5 could attract T cells to release these inflammatory factors and worsen the inflammatory damage. Also, by acting with vascular smooth muscle cells (SMCs), the cell-mediated immune response would be prolonged.72,73 As a factor affecting vascular function, SMCs' behaviors are critical for the metabolism of vascular cells. It has been shown that the CCL5/CCR5 interaction could influence the proliferation rate and migration of SMCs via PI3K/AKT, NF-κB, Ras/MEK pathways.74, 75, 76 Meanwhile, overexpression of CCL5 mediated by Ang2 gives rise to unstable lipid plague and leads to vascular neointimal thickening, which may cause atherosclerosis. Incidentally, CCL5-related atherosclerosis is associated with hypertension and the development of obesity, which are two common risk factors for cardiovascular diseases. Interestingly, people with CCR5Δ32 mutation have low risk of getting cardiovascular diseases such as coronary disease and myocardial ischemia, as the mutation downregulates the level of factors that increase the risk of getting cardiovascular diseases (low density lipoprotein and triglycerides), while upregulate the level of factors that decrease the chance to prevent them (high density lipoprotein, cholesterol).77
Inflammatory bowel diseases
It has been reported that inflammatory bowel diseases (IBDs), a chronic inflammation, may be related with CCL5/CCR5 axis. The CCL5 level was found to be elevated in the damaged tissue and then induced the influx of inflammatory factors. Moreover, CCR5+/+ mice-IBD model has also shown that CCR5 antagonists are effective in the treatment of IBDs.78 Besides, rheumatic disease (RA) is an autoimmune disease caused by the infiltration of Th1 cells. In RA patients, CCR5+ T cells, monocytes, and NK cells significantly thrive and accumulate in the synovial effusions as compared with those in the peripheral blood. Meanwhile, various types of chemokines, including high level of CCL5, were also detected in synovial fluid and synovial tissue of RA. This evidential example may prove the important role of CCL5/CCR5 axis-mediated inflammatory responses in the development of RA.
Hepatic inflammation
Drug-related hepatic inflammation is partially caused by pro-inflammatory factors. Normally, continuous activation of N-acetyl-p-benzoquinone (NAPQI)-related liver macrophages (Kupffer cells [KCs]) results in the secretion of cytokines, neutrophil homing and the migration of macrophages. These cellular and molecular processes could facilitate hepatic inflammation.79,80 New studies have shown that, CCL5/CCR5 interaction can accelerate hepatic inflammation process via NF-κB pathway.81, 82, 83 Notably, both M1 and M2 macrophages participate in this process. M1 phenotype, with the pro-inflammation roles, aggravates inflammation by secreting pro-inflammatory factors IL-1 and prompting the infiltration of CD11b+ Gr-1+ cells. In contrast, M2 phenotype removes the necrotic cell debris to alleviate the inflammation. After cascade phosphorylation of IKK and IκB subunits, NF-κB pushes the polarization balance towards TAM-M1 rather than TAM-M2, accelerating the progression of inflammation.84
COVID-19
Additionally, CCL5/CCR5 axis also participates in virus infections. The unprecedented pandemic disease, COVID-19, is caused by the infection of SARS-CoV-2. Since the outbreak of COVID-19, substantial efforts have been made to find the effective treatment of it. It has been shown that inhibition of CCL5/CCR5 axis by monoclonal antibody, leronlimab, can relieve the symptoms of patients who are critically ill.85 Also, it has been observed that, following the anti-CCR5 treatment, the level of inflammatory molecules such as CCL5, IL-6, TNF were reduced.86 Furthermore, 17 years ago, patients with SARS also had shown elevated CCL5 level.87
Other infections
Besides, Toxoplasma gondii and Staphylococcus aureus are able to kill cells without triggering host immune response by taking advantage of CCL5/CCR5 axis.88 Conversely, CCR5 in West Nile virus (WNV) infection plays an antiviral role. The chemotaxis of CCL5/CCR5 could initiate CD4+/CD8+ T cell-mediated immunity, NK cell migration and the infiltration of macrophages into infected tissues.89
Cancers
Recently, CCL5/CCR5 axis has been extensively studied in the context of tumorigenesis of different types of cancers including chondrosarcoma, gastric cancer, breast cancer, pancreatic cancer, head and neck cancer, etc.5 Among them, CCL5/CCR5 axis always intends to create more suitable microenvironment for tumor cell survival. One the one hand, CCL5/CCR5 triggers cell signaling like PI3K/AKT, NF-κB, ERK/MEK and HIF- to give tumor cell character of uncontrolled proliferation and immortality.5 One the other hand, these signaling pathways regulate MMPs, growth factors and inflammatory factors to remove barriers for tumor metastasis and invasion.90 Moreover, CCL5/CCR5 also recruits Tregs, MDSCs and TAM to induce immunosuppression of the tumor.9
Chondrosarcoma
Chondrosarcoma is a kind of malignant bone tumor due to the chondrocyte or mesenchymal leaf tissue lesions. The invasion and migration of chondrsarcoma are closely related to CCL5/CCR5 axis, with PI3K/AKT, ERK and NK-κB pathways taking part in MMP upregulation. Researchers have shown that the expression level of CCL5 was elevated in cancerous cartilage. Although the levels of various MMPs proteins were increased in human chondrosarcoma cells, only the level of MMP3 were increased along with the elevation of CCL5 level. And it can be reduced by CCR5 inhibitor (Met-CCL5) CCR5 or co-culturing with siRNA.91, 92, 93 A study in Israel has demonstrated that CCR5 engagement by CCL5 could induce the phosphorylation of tyrosine residues on ERK and initiate ERK-MEK signaling pathway, which causes the adhesion of T cells.94 Besides, more studies have proved that CCL5/CCR5-mediated cell invasion and migration are dependent on degradation of extracellular matrix (ECM) by MMP3 via PI3K/AKT, ERK and NK-κB pathways. CCL5/CCR5 axis phosphorylates Akt Ser473 and GSK-3, which are directly or indirectly involved in the regulation the process. Then, the level of MMP3 increases in a time-dependent manner to achieve cell migration. However, opinions on NF-κB-mediated MMP3 expression are controversial. Some hold the view that p65 Ser536 gets phosphorylated by CCL5/CCR5 through PI3K/AKT pathway. Moreover, the activated NF-κB pathway could assist the transcription of MMP3, which is indispensable for expanding the cell invasion depth and the migration range. However, some others hold the opposite views. Borghaei RC et al have found that NF-κB could suppress the expression of MMP3 via a polymorphic site-stromelysin IL-1 responsive element (SIRE) site binding.95 According to the report, the expression of MMP3 could be suppressed by the binding of NF-κB and the polymorphic site in promotor.96
Osteosarcoma
Osteosarcoma is a malignant childhood-bone tumor. Most of the patients with this disease are children. Derived from mesenchymal stromal cells (MSC), osteosarcoma grows rapidly, which requires fast angiogenesis and active cell migration.97 This is for the most part due to the CCL5/CCR5 axis-mediated adhesion production and VEGF secretion in tumor microenvironment (TME). In patients with osteosarcoma, CCL5/CCR5 axis is commonly found to be activated at pathological tissues and tumor microenvironment. Along with elevated expression level of CCL5 in osteosarcoma tumor microenvironment, PKC and c-Src are highly activated, leading to the upregulation of HIF- miRNA level.26 Moreover, VEGF is the main functional factor downstream of PKC-c-Src- HIF- pathway. CCL5/CCR5 axis promotes VEGF production by attracting endothelial progenitor cells (EPCs). The CCL5-overexpressing-osteosarcoma makes EPCs migrate to abnormal sites. Then, the secreted VEGFs accelerate the process of angiogenesis.98,99 As for the migration of tumor cells, NK-κB pathway increases the yields of many adhesion molecules such as αvβ3 integrin, E-selectin etc. at tumor cell surface, which can bind to proteins on ECM. Among them, 3 integrin is one of the major drivers. Previous studies indicated that CCL5/CCR5 axis is responsible for the activation of MEK, which may participate in NK-κB p65 Ser536 phosphorylation process. It has been shown that the activity of CCL5-mediated NF-κB promoter could be reduced by antibodies against CCR5 as well as the inhibitors of Met-RANTES or MEK (PD98059, and U0126). Thus, mediated by NK-κB pathway, the secretion of 3 integrin by tumor cells contributes to the migration of osteosarcoma cells. Similarly, in lung cancer, not only NF-κB, but also PI3K/AKT can up-regulates 3 integrin expression at tumor cell surface, which contributes to the migration. In addition, EZH2 enhances CCR5 expression in lung cancer to strengthen tumor cell migration by increasing macrophages recruitment and infiltration. So that, CCL5 secreted by para-carcinoma tissues contributes to the invasiveness of tumor cells.100
Hodgkin lymphoma
Classical Hodgkin lymphoma (cHL) is a unique hematopoietic neoplasm. The character of cHL is Hodgkin and Reed-Sternberg cells (H-RS cells) infiltration of blood.101 The tumor immunosuppression realized main contributes to chemotactic function of CCL5/CCR5 to mesenchymal stromal cells (MSC). Recent researches demonstrated CCL5/CCR5 signaling is involved in cHL tumor growth and TME formation. There, CCL5 and CCR5 express widely at surface of cHL tumor cells, monocytes, lymphocytes and stromal cells. Within the cH TME, H-RS cells can secret CCL5 to attract inflammatory cells inside and cause FGF2, TGF and TNF explosion. For instance, MSC migration and monocytes recruitment.102 Then MSCs further secret CCL5 to chemotaxis more CCR5+ macrophages to the lesions. By this, cHL gets growth even reprogram large macrophages to achieve immunosuppression.103
Melanoma
As melanoma is used to be seen as neural-crest derived melanocytes, fewer scientists connect it with EMT. However, newly reports pointed that EMT do exist in melanoma process, even is a major determinant of its metastasis.104,105 Liu et al demonstrated CCL5-stimulated CCR5+ tumor cells are more metastatic. There, CCL5/CCR5 recruits Tregs with high TGF1 expression. Differently, TGF1-induced EMT here is found to activated PI3K/AKT/GSK-3 pathway, not smad. Following E-cadherin downregulation and N-cadherin, vimentin, snail and slug upregulation, cancerous melanocytes undergo EMT-like process and metastasis.106 In addition, CCL5 production triggered by lymphocytes in melanoma bind to CCR5, following with release of cytochrome c from mitochondria. The process joins in caspase-9/3 activation that leads to apoptosis of tumor infiltrating lymphocytes.107
Head and neck cancer
Salivary adenoid cystic carcinoma (SACC) and oral squamous cell carcinoma (SCC) are two types of head and neck cancer known so far, which are closely related to CCL5/CCR5 axis. However, their tumor characters and pathogenic mechanism behind are totally differenced. Perineural invasion (PNI) is induced to occur more often at SACC by CCL5/CCR5 axis activation, while, the axis-mediated MMP9 produced enhancing by leukocyte is the key factors in SCC patients for tumor invasion and migration.
SACC is a subtype of salivary gland carcinoma. As the hallmark of SACC, PNI is linked with tumor progression and poor prognosis.100 It has been reported that the activation of CCL5/CCR5 axis is involved in the progression of SACC through participating in the process of PNI. Notably, CCL5 generated from dorsal root ganglia (DRG) could lead to the growth of longer neurite and more branches in DRG. When CCL5 binds to the CCR5 on the surface of SACC cells, the cells would chemotactically migrate towards DRG and induce PNI. Thus, the CCL5/CCR5 axis-mediated PNI is important for the development of SACC.108
SCC is a malignant carcinoma with high rate of metastasis and invasiveness. Addictions to cigarettes and alcohol are two risk factors of SCC due to chronic epithelial irritation.109 Among several cell lines, CCR5+CCL5+ high expression SCC4 cell line shows higher mobility and aggressiveness. Researches demonstrated the signaling behind it depends on CCL5/CCR5 axis initiated MMP9 overexpression. CCL5 in TME stimulates CCR5+ tumor cells, causing PLC3 phosphorylation. Then PKC gets activated, involvement of PI-PLC pathway, and enhances NF-κB activity to bind MMP9 promoter. Thus, MMP9 expression and enzyme activity get increased by CCL5/CCR5-, PLC3-, PKC-, NF-κB-dependent pathway.110
Esophageal cancer
Esophageal cancer, another digestive tract tumor, has been recently proved related to CCL5/CCR5 axis. Researchers cultivated 4 ESCC cell lines and found CCL5 overexpression, especially in TWES-4LN cell line. Furthermore, CCL5 secreted by cells derived from lymph nodes bind to CCR5+ TAM, CCR5+ MDSC and CCR5+ Treg to recruit them to tumor lesion. Thus increase the growth rate, migration and invasive ability of tumor cells.111
Gastric cancer
As a type of malignant tumors with high prevalence, the early diagnose of gastric cancer is of most importance. In the process of oncogenesis, the gastric mucosa epithelial cells undergo uncontrolled proliferation, migration etc. Worthy, CCL5/CCR5 axis focus on influencing the cell cycle of tumor cells through abnormally activating NF-κB, mTOR and PI3K/AKT signaling pathways. At the molecular level, the cell division-related genes like cyclin D1, c-Myc and Dad-1 are overexpressed at tumor cell surface. They cooperate with each other to strengthen the mitogenesis. Furthermore, mTOR-mediated glycolysis and ATP production provide sufficient energy for the growth of tumor cells. In this process, CCL5/CCR5 combination triggers mTOR signaling continuously for cell proliferation.112,113 Also, CCL5/CCR5-mediated DNMT1 over-expression could methylate promotor of gelsolin (GSN) and thereby hamper the expression of GSN. In this way, F-actin becomes abnormal and contributes to tumor survival.114 Additionally, mTOR-4E-BP1/S6K1-eIF4F pathway activated by CCL5/CCR5 complex takes the role to disassociate 5′-untranslated region (UTR) and promote tumor survival-related mRNA translation. GSN suppression and eIF4F activation are all favorable for the progression of gastric cancer.45 H. pylori is a potential cause of gastric cancer. Once infected, chemokines, growth factors and cytokines including CCL5, IL-10, MMPs, VEGF etc. would be secreted. Apart from that, cell chemotactic function of CCL5/CCR5 also play a pivotal role. Stimulated by these cytokines, peripheral blood monocytes are transformed into TAMs and MSDCs, which are recruited to inflammatory sites. Especially, elevated CCL5 level promotes the process of transformation. In addition, the pro-tumor role of TAM is gradually disclosed. Particularly, TAM could influence nearly all tumorigenesis processes, including but not limited to cell proliferation, metabolism, tissue remodeling, immunosuppression, tumor invasion, metastasis and EMT processes.115, 116, 117, 118 TAM could release a variety of growth factors, including CCL5, into the tumor microenvironment. The mutual effects between cytokines and TAMs accelerates tumorigenesis. Meanwhile, TAMs could attract Tregs with some other chemokines like CCL22. In addition to TAM, CCR5+ myeloid-derived suppressor cells (MDSCs) and CCR5+ Treg can also be recruited by CCL5 and initiate immune response by enhancing IL-6 and IL-10.119,120 These cytokines suppress the immune response mediated by effector T cells and achieve tumor cell immunosuppression.121,122 By the way, MDSCs are also characterized with powerful immunosuppress function, resist not only the innate, but also the acquired immune cells functions.123,124 Additionally, mediated by KLF5, CAF generated in tumor sites makes it easy for the effective metastasis. Newest studies have demonstrated that CCL5/CCR5 axis could support the uncontrollable proliferation of tumor cells by affecting their metabolism activities.125
Colorectal carcinoma
In colorectal carcinoma, CD3+ T cell secret CCL5 to recruit macrophage, undergoing EMT and tumor growth. Also, CCL5/CCR5 induced phenotype switch from TAM-M1 to TAM-M2 can be used by tumor cells for further growth.126 In addition, higher expression of CCR5 at tumor cell surface is positive related to tumor metastasis. In colorectal carcinoma, CCL5/CCR5 axis influences cell cycle by upregulating mTOR or downregulation P53, P21, FOXM1 and E2F1. Thus, cyclin D1 and cyclin E1 are in moderate higher activity to activate CDK4/6/2 and promote G1/S phase of cell cycle.127,128
Breast cancer
Nowadays, breast cancer is one of the deadliest cancers.129 CCL5 is closely associated with the tumorigenesis of breast cancer. So as other tumor process, the binding of CCL5 and its receptor could initiate PI3K/AKT/mTOR pathway and enhance the proliferation, progression, transformation and resistance to apoptosis in breast cancer progression.130,131 In the past 10 years, it had been found that insulin-like growth factor 1 (IGF-1) pathway is involved in CCL5 upregulation of breast cancer cells. This process coordinates with αvβ3 integrin, collagen and other extracellular matrix proteins secretion, promoting invasion of tumor cells.132 In tumor microenvironment, the elevated level of CCL5 leads to high GLUT1 expression on the surface of cells. Under anoxic conditions of tumor microenvironment, glucose can only undergo anaerobic glycolysis. Thus, CCL5-mediated upregulation of GLUT1 provides enough energy for the proliferation of tumor cells as well as angiogenesis. Specifically, the upregulation of GLUT-1 and increment of glucose concentration is dependent on mTOR/AKT pathway. CCL5-mediated 4E-BP-1 and GSK-3 phosphorylation initiate mTOR/AKT pathway, which allows the transcription of GLUT-1 gene.133,134 Moreover, the metabolic intermediates of glucose-anaerobic glycolysis, such as glucose-6-phosphate, pyruvate, peptide and lipid, are all enriched. These intermediates could undergo further catabolism through tricarboxylic acid (TCA) cycle and enzymatic hydrolysis, and then provide more energy. In breast cancer, researchers have also found that high concentration of IL-6 could stimulates various pathways, even increase the yield of CCL5. As mentioned, HER2-PTEN balance adjustment contributes to this and accelerates breast cancer process. By contrast, Santos Mañes made a point that CCR5 expression could inhibit the progression of breast cancer through activated p53-mediated antitumor response.135 However, the idea that CCR5 promotes the tumorigenesis is still the current mainstream.
Pancreatic cancer
Different from other organs, the pancreas is hidden between the stomach and the posterior abdominal wall, which means it is difficult to visualize pancreatic lesions by image technologies such as X-ray, CT, MRI, etc. Actin polymerization was reported as a crucial factor in pancreatic cancer migration, which can be largely influenced by CCL5/CCR5 axis through RAS-ERK-MEK, RAS-Ral-Cdc42 and PI3K-AKT pathways activation. A pilot study has demonstrated that FOXP3 protein is overexpressed in human pancreatic ductal adenocarcinoma, which is correlative to patient's poor prognosis. It has been proved that the motif- 4 in the CCL5 promoter could bind to FOXP3 to directly initiate the transcription of CCL5. PDAC cells could recruit foxp3+ Tregs to boost the proliferation and metastasis of tumor cells.136,137
Another report shows that the protein and mRNA level of CCL5 rise up significantly in MACs/PCCs co-cultured samples. The CCL5 transactivated by IGF-1 pathway could initiate downstream signaling cascades and effector molecules via CCL5/CCR5 axis.138 Cell migration relies on actin polymerization. Scientists showed that CCL5/CCR5 axis could induce F-actin polymerization in pancreatic cancer. This process requires the participation of RAS-ERK-MEK, RAS-Ral-Cdc42 and PI3K-AKT pathways. RAS activates Cdc42 and PL3K and then stimulate the downstream second messengers for the gradient signal amplification. With the assists of Arp2/3 complex and WASP/WAVE protein family, pseudopodia are formed and got polymerized, which confer the ability to migrate on cells.139
Prostate cancer
Prostate cancer is one of the most common causes that leads death in males worldwide.129 Previous researches have already proved, during prostate cancer process, there is a high level of both CCR5 and CCL5 expression within intracellular pools.140 The CCL5/CCR5 combination in TME of prostate cancer promote cell proliferation by enhancing cyclin D1, c-myc in mTOR dependent pathway.141 Also, the axis can increase glucose uptake and ATP production for cell proliferation.142 Otherwise, CCL5/CCR5-induced NF-κB/STAT dependent angiogenesis is involved in.143 Apart from that, autophagy becomes another potential reason in prostate cancer process. As demonstrated before, androgen receptor (AR) downregulation by IL-6 directly results in TGF--induced MMP-9 overexpression and cell autophagy.90 Recently, scientists showed CCL5/CCR5 axis also inhibits AR signaling. It can decrease AR expression and transcriptional activity through HIF2 signaling pathway. Then paxillin degradation and focal adhesion damage, allowing autophagy and cell migration.144
Other diseases
Besides, some other chronic diseases such as endometriosis and type 2 diabetes (T2D), are related to CCL5/CCR5 axis. Endometriosis, a benign disease of women, which cause progressively worsen dysmenorrhea, infertility, as well as abnormal menstrual. Many potential factors are associated with the disease, including hormone imbalance, genetic abnormalities etc.145, 146, 147 Recently, it has been reported that the recruitment of CCR5+ MDSCs (especially Mo-CCR5+ MDSCs) could promote the development of endometriosis. Samples from the clinical study have demonstrated that the level of CCL5 was elevated in cell plasma and peritoneal fluid of late endometriosis patients. It has been observed that CCR5+ MDSCs could migrate to the endometriosis lesions148 and contribute to the progression of endometriosis through enhancing the generation of arginase (ARG-1), indoleamine2,3-dioxygenase1 (IDO), inducible nitric oxide synthase (iNOS) and PD-L1.149,150 The production of ARG-1 might be associated with the consumption of the amino acids that is required for the T cell function, thus, inhibiting T cells in several ways. In addition to that, both IDO and PD-L1 could act as suppressors for T cells.151,152 By the way, researches had shown CCL5/CCR5 axis-induced Treg recruitment leads a Fas-FasL-mediated T cell apoptosis with a decrease of T cell reactivity in endometriosis.46,153 In this way, CCL5/CCR5 axis promotes endometriosis progression through recruiting MDSCs into endometriosis lesions and T cell suppression.154
In addition, CCL5/CCR5 axis also has protective functions. T2D is characterized by the insulin resistance due to IRS-1 S302 phosphorylation-mediated disassociation from insulin receptors.155 CCL5/CCR5 makes its way to inactivate AMPK- via dephosphorylation pathway. Next, the inactivated AMPK- fails to stimulate p70S6K, then leads to the suppression of IRS-1 S302 phosphorylation, which consequently erase the insulin resistance.156 Furthermore, CCL5/CCR5 axis could promote the translocation of GLUT4, the glucose channel, on T cell membrane, which increases the uptake of glucose.157
Treatment targeting CCL5/CCR5
Above researches demonstrate how CCL5/CCR5 axis is important in tumorigenesis, migration, metastasis and prognosis.100,106,112,158 In addition, CCR5 secreted by tumor microenvironment is highly sensitive to drug blocking, especially in breast cancer.159 Therefore, the exploration of target therapies to block CCL5/CCR5 axis has broad application prospect in tumor therapy in the future. Nowadays, the development of small molecule inhibitors are the main strategies. Notably, inhibitors such as maraviroc, cenicriviroc, anibamine, MET-CCL5, etc. have been evaluated clinically with anti-inflammatory and anti-cancer effects.
Maraviroc
Some known CCR5 inhibitors are able to slow the progression of CCR5 aberrant expression diseases down. Maraviroc, the first FAD-approved drug for treating HIV-infection, is also being used in some anti-tumor regimens. Directly, maraviroc competes with CCL5 to combine with CCR5, blocking the internalization of CCR5 and inhibiting T-cell chemotaxis.160 Acting as an antagonist of CCR5, maraviroc could inhibit the recruitment of mesenchymal-stromal cells (MSCs), monocytes and some growth factors by tumor cells, which could reduce the progression of cancer.126,161 Studies have shown that maraviroc could effectively prevent the further infiltration of classic Hodgkin lymphoma (cHL) by reducing heterospheroid self-assembling by cHL, HL-MSCs and monocytes;103 in breast cancer, it is able to control tumor cells metastasis, especially in lungs;162 At the same time, the migration of Treg could also be restricted by maraviroc; it also inhibits the accumulation of fibroblasts mediated by colorectal cancer.163 Indirectly, maraviroc possess the function of enhancing the efficacy of chemotherapy drugs. For example, maraviroc strengthen the anti-tumor effects of doxorubicin and bentuximab vedotin in vitro model.103 Recently, it has been reported that maraviroc could be used in the treatment of diseases including hepatic steatosis of non-alcoholic fatty liver disease (NAFLD),164 cancers,159 graft-versus-host disease,165,166 heart diseases,167 lung diseases, type 1 diabetes,168,169 rheumatoid arthritis170 and hemorrhage.108 Clinical trials considering maraviroc intervention are in full swing. Scientists are trying to combine this drug with conventional medical treatments and see if these therapies would be more effective (NCT01736813, NCT01276236, NCT02741323).
Directed by Professor Niels, the clinical trial (NCT01736813) related to human colorectal cancer liver metastasis demonstrates how maraviroc protect metastasis by inhibiting CCL5/CCR5 axis. After analyzing 12 participants' samples, scientists found the blockade of CCR5 by maraviroc can influence AKT and ERK pathways to lower down PIAS3, VEGF, MIF etc. Also, CCL5/CCR5 axis is arrested and results in macrophage repolarization through STAT3 pathway activation. The phaseⅠclinical trial proved the possibility of maraviroc treatment for colorectal cancer especially patients have tumor metastasis.126
Cenicriviroc
Cenicriviroc is an oral antagonist of dual chemokine receptor CCR2/CCR5, which inhibits the migration of monocytes. In comparison to maraviroc, cenicriviroc possesses higher efficiency in disruption of links between cells.171 Nowadays, cenicriviroc-related clinical trials have demonstrated that the drug has both antiviral and anti-fibrosis effects in the condition of infections, non-alcoholic steatohepatitis (NASH), primary sclerosing cholangitis (PSC) (NCT02653625) and many other conditions.172,173
In NASH clinical trial (NCT02217475), patients are divided into three groups randomly, providing CVC, placebo or cross. After two-year cohort survey, Eric Lefebvre et al demonstrated patients with fibrosis response at the beginning maintained it one year later when treating with CVC. Contrarily, natural fluctuations of the disease in placebo group failed to reach such a great effect. Longer and larger clinical benefit may appear if prolong the study. However, side effects also increase like diarrhea, abdominal pain etc.174
Based on the CCL5/CCR5 axis's function on macrophage migration and infiltration, J.P. Nicandro et al initiate an exploratory of CVC on PSC (NCT02653625). As a powerful anti-inflammatory and anti-fibrosis drugs, CVC blokes CCR5 positive leucocyte recruitment and relieve inflammation. Study showed an apparent median ALP absolute reduction at week 24 after treatment, which means less liver damage. Although key secondary efficacy endpoint was not achieved, the encourage data offer a new idea for targeting PSC.175
Anibamine
Anibamine is the first natural CCR5 antagonism. Similarly, after blocking CCR5/CCL5 interactions, Ca2+ flux gets limited and most prostate cancer cell lines decreased the proliferation rate. Meanwhile, abanimine could significantly change the morphology, metastatic and adhesion ability of M12 cancer cells. Additionally, its special side chain of high affinity to CCR5 makes it a favorable therapeutic agent for the treatment of prostate cancer.176 Although hemolysis sometimes happens, concentration below 1 μM is toxicity-free. Besides, in another study, the core ring system and side chains of abanimine were proved to be related to high CCR5 affinity and inhibited proliferation rate of cancer cells.177
MET-CCL5 and others
Different from agents discussed above, MET-CCL5 is composed of amino end of CCL5 and a methionine. MET-CCL5 could prevent binding of CCL5 and CCR5. The special agent is usually used for reducing inflammatory response of peripherally injured neve at late phase. Hyperalgesia can also be partially eased by MET-CCL5. These therapeutic effects are achieved by the reduction of macrophages infiltration and pro-inflammatory cytokine mediated by MET-CCL5-interference.178 Clinical trials on the drug are still absent. Vicriviroc, another CCR5 antagonist, whose role is preventing tumor cells migrate to targeted organs.159 It limits Ca2+ signaling and powerful antiretroviral activity in a phase II trial (NCT00523211). Disappointingly, it failed to further demonstrate its efficacy in another phase III trial 179, 180 (NCT00474370).
Besides, some non-classical agents like TAK779, zoledronic acid and aplaviroc have also been evaluated in clinic studies. TAK799 is a nonpeptide CCR5 antagonist with small molecular weight. The specific binding sites have been discovered to be related to the helices 1, 2, 3 and 7 of CCR5 to inhibit Ca2+ signaling.181 Zoledronic acid treatment can downregulate CCR5 expression on V2-T cells of cancer patients, however, the underlying mechanism is still not sure.182 Aplaviroc also specifically binds to CCR5 and prevents it from other ligands. However, trials were terminated or stagnated because of inevitably serious side effects or unsolvable difficulties.183 Although these therapies have advantages in specific target inhibition, there are still limitations. Targeted drug cannot completely block the CCR5 and tumor cells can escape from it by increasing drug resistance. Side effects also cannot be ignored.184 Clinical trials of anti-CCR5 therapies are summarized in Table 1.
Table 1.
Summary of the anti-CCR5 therapies clinical trials.
| Disease or condition | Intervention/treatment | Phase | NCT number | Allocation | Actual/estimated enrollment | Status | Outcomes |
|---|---|---|---|---|---|---|---|
| Kaposi's sarcoma | Maraviroc | Ⅱ | NCT01276236 | N/A | 13 participants | Completed | Primary/secondary endpoints |
| Hematologic malignancy | Maraviroc | Ⅱ | NCT01785810 | N/A | 37 participants | Completed | Primary endpoint |
| Colorectal cancer | Maraviroc | Ⅰ | NCT01736813 | N/A | 12 participants | Completed | Primary/secondary endpoints |
| Metastatic colorectal cancer MSS | Pembrolizumab/Maraviroc | Ⅰ | NCT03274804 | N/A | 20 participants | Completed | Primary/secondary endpoints |
| Lymphoma | Lentivirus vector rHIV7-shI-TAR-CCR5RZ-transduced hematopoietic -progenitor cells/Carmustine/Cyclophosphamide/Etoposide/Autologous hematopoietic stem cell transplantation | Ⅰ | NCT00569985 | N/A | 5 participants | Completed | Primary endpoint |
| Pancreatic ductal adenocarcinoma | Stereotactic Body Radiation (SBRT)/Nivolumab/CCR2/CCR5 dual antagonist/GVAX | Ⅰ/Ⅱ | NCT03767582 | Randomized | 30 participants | Recruiting | |
| Colorectal neoplasms | Vicriviroc/Pembrolizumab | Ⅱ | NCT03631407 | Randomized | 40 participants | Active, not recruiting | |
| Triple negative breast neoplasms | Leronlimab/AUC 5 Carboplatin | Ⅰ/Ⅱ | NCT03838367 | Non-Randomized | 48 participants | Recruiting | |
| Blood cell neoplasm HIV infection Myelodysplastic syndrome Non-Hodgkin lymphoma Refractory anemia |
Fludarabine/Cyclophosphamide/Thiotepa/Total-Body Irradiation/Umbilical Cord Blood Transplantation/Cellular Therapy | Ⅱ | NCT04083170 | Non-Randomized | 10 participants | Recruiting | |
| Solid tumor, adult | Leronlimab | Ⅲ | NCT04504942 | N/A | 30 participants | Recruiting | |
| Rheumatoid arthritis | AZD5672/Etanercept/Placebo | Ⅱ | NCT00713544 | Randomized | 373 participants | Completed | Primary/secondary endpoints |
| Graft-versus-host disease Hematopoietic stem cell transplantation |
Maraviroc | Ⅰ/Ⅱ | NCT00948753 | Non-Randomized | 38 participants | Completed | Primary/secondary endpoints |
| Hypertriglyceridemia | Maraviroc/placebo | Ⅰ | NCT01133210 | Randomized | 27 participants | Completed | Primary/secondary endpoints |
| Non-alcoholic steatohepatitis | Cenicriviroc/Placebo | Ⅱ | NCT02217475 | Randomized | 289 participants | Completed | Primary/secondary endpoints |
| Liver insufficiency | Cenicriviroc | Ⅰ | NCT02120547 | Non-Randomized | 31 participants | Completed | Primary/secondary endpoints |
| Prediabetic state Non-alcoholic fatty liver disease Type 2 diabetes mellitus |
Cenicriviroc/Placebo | Ⅱ | NCT02330549 | Randomized | 45 participants | Completed | Primary/secondary endpoints |
| Primary sclerosing cholangitis | Cenicriviroc | Ⅱ | NCT02653625 | N/A | 24 participants | Completed | Primary/secondary endpoints |
| Stroke | Maraviroc/Rehabilitation therapy/Placebo | Ⅱ/Ⅲ | NCT03172026 | Randomized | 60 participants | Recruiting | |
| Hepatic impairment | Cenicriviroc | Ⅰ | NCT03376841 | Non-Randomized | 16 participants | Completed | Primary/secondary endpoints |
| Non-alcoholic steatohepatitis (NASH) | Placebos/leronlimab | Ⅱ | NCT04521114 | Randomized | 60 participants | Recruiting | |
| Non-alcoholic steatohepatitis (NASH) | Tropifexor (LJN452) Cenicriviroc (CVC) |
Ⅱ | NCT03517540 | Randomized | 193 participants | Completed | Primary/secondary endpoints |
| COVID-19 | Cenicriviroc (CVC)/Placebo | Ⅱ | NCT04500418 | Randomized | 183 participants | Recruiting | |
| COVID-19 | Maraviroc | Ⅰ | NCT04435522 | N/A | 9 participants | Completed | Primary/secondary endpoints |
| COVID-19 | Leronlimab/Placebos | Ⅱ | NCT04347239 | Randomized | 390 participants | Recruiting |
Note: N/A not applicable.
Gene editing
As mentioned before, CCR5Δ32 population is exempt from the infection of HIV. In the famous case –“Berlin patient”, a patient with AIDs-related acute myeloid leukemia (AML) was transplanted with CCR5Δ32 stem cells. Encouragingly, the HIV infection was successfully cured.74 This phenomenon has elicited the idea that the transplantation of CCR5Δ32 stem cells or positional locus knockout could potentially cure CCL5/CCR5-related diseases. At present, there are lots of techniques that can be implemented into the therapies against such diseases, like zinc finger nuclease (ZFN), TALEN,185 ribozyme,186 shRNA187 and CRISPR/Cas9.188
ZFN, TALEN and CRISPR/Cas9 all take use of the combination of an endonuclease and a specific CCR5 sequence recognition region. In details, ZFN has already been applied on HIV-infected patients. University of Pennsylvania has conducted a clinical trial in 2018. The CCR5 gene of CD4+ T cells from HIV positive patients was edited by ZFN (SB-728mR) (NCT02388594). Among several CCR5-TALEN designs, CCR5-TALEN-515 has been proved to be better than CCR5-ZFN.185 CCR5 modified by CRISPR/Cas9 was successfully applied on both HIV-infected patients and an acute lymphocytic leukemia case. Results show that the patient achieved complete remission for 19 months after the transplantation of CRISPR-edited stem cells.188 Gene silencing technique ribozyme and shRNA do not reprogram the cells to produce modified CCR5. Instead, they block the expression of CCR5.
Although gene therapy has been studied several years, its applications are still limited. Because there are still plenty of unknown risks. The most realistic problem is that patients with gene-modified CCR5 would have a difficult time coping with deficiency of necessary CCL5/CCR5-mediated immune/inflammatory responses. Although no CCR5Δ32-related disadvantages have been found in individuals carrying inherited CCR5Δ32 mutation, the current techniques are still not mature enough to knockout CCR5 gene without affecting the normal functioning of chemokine system.189,190
Perspectives and conclusions
CCL5/CCR5 as a well characterized pathways in inflammation and cancer, considerable efforts have been devoted developing therapeutic strategies. However, several points might be addressed in the translational research concerned with CCL5/CCR5-targeted therapy. (1) When it comes to small inhibitors, the antagonist of dual chemokine receptor CCR2/CCR5 seems more efficient than the inhibitor only targeting CCL5/CCR5 binding.191,192 The investigation of small molecules with dual-/multi-inhibiting properties of CCL5/CCR5 and its related pathways might be a direction in future study. (2) For the treatment of cancer, the combinational treatment of inhibitor and chemotherapy drug synergistically improved the prognosis. With the fast development of immunotherapy and cell therapy platforms for major disease, more possibilities might be found in the combinational treatment with the classic CCL5/CCR5 inhibitor and new techniques. (3) Gene therapy targeting CCL5/CCR5 is a promising field in future, however, which is substantially underpinned by the development of current techniques of gene interfering and gene delivery. The safety issue and efficacy of the gene delivery system (viral carriers or non-viral ones made into nano sizes) might be the main barriers in initiating a clinical trial of CCL5/CCR5-targeted gene therapy. Much remains to be done before the next generation of CCL5/CCR5-targeted gene therapy can bring significant clinical benefits to the patients. In this review, we illustrated the molecular factors and signaling pathways upstream or downstream of CCL5/CCR5 axis, and discussed how the pathogenesis of the diseases related to CCL5/CCR5 axis. Moreover, we highlighted several classic therapies in CCR5-related diseases and focused on the clinical trials. In summary, the CCL5/CCR5 axis is a potential target in the treatment of a wide range of diseases. With the improvement of fields such as immunology, pathology, gene editing etc., more precise therapeutic strategies targeting CCL5/CCR5 might be developed.
Conflict of interests
The authors have no conflicts to declare.
Funding
This work is supported by the Key R&D Project of Sichuan Province (No. 2020YFS0553), the Excellent Youth Foundation of Sichuan Scientific Committee Grant in China (No. 2019JDJQ008) and the National Natural Science Foundation Regional Innovation and Development, China (No. U19A2003).
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, ClinicalTrails, PubMed, and references from relevant articles using the search terms “CCL5”, “CCR5”, “cancer”, “inflammation”, “infection” and “diseases”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 1998 and 2021 were included.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.López-Cotarelo P., Gómez-Moreira C., Criado-García O., Sánchez L., Rodríguez-Fernández J.L. Beyond chemoattraction: multifunctionality of chemokine receptors in leukocytes. Trends Immunol. 2017;38(12):927–941. doi: 10.1016/j.it.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hughes C.E., Nibbs R.J.B. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranjc M.K., Novak M., Pestell R.G., Lah T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol Oncol. 2019;53(4):397–406. doi: 10.2478/raon-2019-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomiyama H., Osada N., Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Gene Cell. 2013;18(1):1–16. doi: 10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldinucci D., Borghese C., Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancer. 2020;12(7):e1765. doi: 10.3390/cancers12071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasco-Velázquez M., Xolalpa W., Pestell R.G. The potential to target CCL5/CCR5 in breast cancer. Expert Opin Ther Targets. 2014;18(11):1265–1275. doi: 10.1517/14728222.2014.949238. [DOI] [PubMed] [Google Scholar]
- 7.Marques R.E., Guabiraba R., Russo R.C., Teixeira M.M. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 2013;17(12):1439–1460. doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wierda R.J., Kuipers H.F., van Eggermond M.C., et al. Epigenetic control of CCR5 transcript levels in immune cells and modulation by small molecules inhibitors. J Cell Mol Med. 2012;16(8):1866–1877. doi: 10.1111/j.1582-4934.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlecker E., Stojanovic A., Eisen C., et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189(12):5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 10.Weitzenfeld P., Ben-Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Canc Lett. 2014;352(1):36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Muñoz L., Barroso R., Dyrhaug S.Y., et al. CCR5/CD4/CXCR4 oligomerization prevents HIV-1 gp120IIIB binding to the cell surface. Proc Natl Acad Sci U S A. 2014;111(19):E1960–E1969. doi: 10.1073/pnas.1322887111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hütter G., Nowak D., Mossner M., et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 13.Abid S., Marcos E., Parpaleix A., et al. CCR2/CCR5-mediated macrophage-smooth muscle cell crosstalk in pulmonary hypertension. Eur Respir J. 2019;54(4):1802308. doi: 10.1183/13993003.02308-2018. [DOI] [PubMed] [Google Scholar]
- 14.Wang S.W., Wu H.H., Liu S.C., et al. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PloS One. 2012;7(4):e35101. doi: 10.1371/journal.pone.0035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao X., Nawab O., Patel T., et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Canc Res. 2019;79(19):4801–4807. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez-Carmona M., Chaorentong P., Kather J.N., et al. CCR5 status and metastatic progression in colorectal cancer. OncoImmunology. 2019;8(9):e1626193. doi: 10.1080/2162402X.2019.1626193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C.Y., Fong Y.C., Lee C.Y., et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2009;77(5):794–803. doi: 10.1016/j.bcp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Ridley A.J., Schwartz M.A., Burridge K., et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 19.Jiao X., Velasco-Velázquez M.A., Wang M., et al. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Canc Res. 2018;78(7):1657–1671. doi: 10.1158/0008-5472.CAN-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermida M.A., Dinesh Kumar J., Leslie N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Deng S., Dai G., Chen S., et al. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother. 2019;110:602–608. doi: 10.1016/j.biopha.2018.11.103. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Hu Y., Yang L., et al. Runx2 alleviates high glucose-suppressed osteogenic differentiation via PI3K/AKT/GSK3β/β-catenin pathway. Cell Biol Int. 2017;41(8):822–832. doi: 10.1002/cbin.10779. [DOI] [PubMed] [Google Scholar]
- 23.Lin J., Song T., Li C., Mao W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta Mol Cell Res. 2020;1867(5):e118659. doi: 10.1016/j.bbamcr.2020.118659. [DOI] [PubMed] [Google Scholar]
- 24.Mossmann D., Park S., Hall M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Canc. 2018;18(12):744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 25.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4(4):64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C., Yang C., Feldman M.J., et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8(34):56110–56125. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schödel J., Grampp S., Maher E.R., et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69(4):646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGettrick A.F., O'Neill L.A.J. The role of HIF in immunity and inflammation. Cell Metabol. 2020;32(4):524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Li Z.H., Zhang L., Lu S.B. ADAM8 promotes chondrosarcoma cell migration and invasion by activating the NF-κB/MMP-13 signaling axis. Anticancer Drugs. 2019;30(7):714–721. doi: 10.1097/CAD.0000000000000790. [DOI] [PubMed] [Google Scholar]
- 31.Ha S.H., Kwon K.M., Park J.Y., et al. Esculentoside H inhibits colon cancer cell migration and growth through suppression of MMP-9 gene expression via NF-kB signaling pathway. J Cell Biochem. 2019;120(6):9810–9819. doi: 10.1002/jcb.28261. [DOI] [PubMed] [Google Scholar]
- 32.Yin Y., Li F., Shi J., Li S., Cai J., Jiang Y. MIR-146a regulates inflammatory infiltration by macrophages in polymyositis/dermatomyositis by targeting TRAF6 and affecting IL-17/ICAM-1 pathway. Cell Physiol Biochem. 2016;40(3–4):486–498. doi: 10.1159/000452563. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira C.E., Oda J.M., Losi Guembarovski R., et al. CC chemokine receptor 5: the interface of host immunity and cancer. Dis Markers. 2014;2014:126954. doi: 10.1155/2014/126954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza M.C., Er E.E., Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida M., Ishida T., Nakashima H., et al. Mnk1 is required for angiotensin II-induced protein synthesis in vascular smooth muscle cells. Circ Res. 2003;93(12):1218–1224. doi: 10.1161/01.RES.0000105570.34585.F2. [DOI] [PubMed] [Google Scholar]
- 37.Romeo Y., Zhang X., Roux P.P. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 38.Slack C., Alic N., Foley A., Cabecinha M., Hoddinott M.P., Partridge L. The Ras-Erk-ETS-signaling pathway is a drug target for longevity. Cell. 2015;162(1):72–83. doi: 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torii S., Kusakabe M., Yamamoto T., Maekawa M., Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7(1):33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Wasylyk C., Criqui-Filipe P., Wasylyk B. Sumoylation of the net inhibitory domain (NID) is stimulated by PIAS1 and has a negative effect on the transcriptional activity of Net. Oncogene. 2005;24(5):820–828. doi: 10.1038/sj.onc.1208226. [DOI] [PubMed] [Google Scholar]
- 41.Aldinucci D., Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. 2018;19(5):1477. doi: 10.3390/ijms19051477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18(4):374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regulation I., Progression C. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. 2019;11(12):2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q., Wang H.Y., Woetmann A., Raghunath P.N., Odum N., Wasik M.A. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108(3):1058–1064. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H.C., Chen C.W., Yang C.L., et al. Tumor-associated macrophages promote epigenetic silencing of gelsolin through DNA methyltransferase 1 in gastric cancer cells. Cancer Immunol Res. 2017;5(10):885–897. doi: 10.1158/2326-6066.CIR-16-0295. [DOI] [PubMed] [Google Scholar]
- 46.Chang L.Y., Lin Y.C., Mahalingam J., et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Canc Res. 2012;72(5):1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- 47.Declerck P.J., Gils A. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost. 2013;39(4):356–364. doi: 10.1055/s-0033-1334487. [DOI] [PubMed] [Google Scholar]
- 48.Mi Z., Bhattacharya S.D., Kim V.M., Guo H., Talbotq L.J., Kuo P.C. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32(4):477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitra A.K., Zillhardt M., Hua Y., et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Canc Discov. 2012;2(12):1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang T., Chen M., Yang X., et al. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Canc Biol Ther. 2017;18(10):806–815. doi: 10.1080/15384047.2017.1373219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danø K., Behrendt N., Høyer-Hansen G., et al. Plasminogen activation and cancer. Thromb Haemostasis. 2005;93(4):676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- 52.Mgrditchian T., Arakelian T., Paggetti J., et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc Natl Acad Sci U S A. 2017;114(44):E9271–E9279. doi: 10.1073/pnas.1703921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia L., Zhu X., Zhang L., Xu Y., Chen G., Luo J. EZH2 enhances expression of CCL5 to promote recruitment of macrophages and invasion in lung cancer. Biotechnol Appl Biochem. 2020;67(6):1011–1019. doi: 10.1002/bab.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K.H., Roberts C.W. Targeting EZH2 in cancer. Nat Med. 2016;22(2):128–134. doi: 10.1038/nm.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin K., Pandey N.B., Popel A.S. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res. 2018;20(1):54. doi: 10.1186/s13058-018-0981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korkaya H., Kim G.I., Davis A., et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mikolajczyk T.P., Nosalski R., Szczepaniak P., et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. Faseb J. 2016;30(5):1987–1999. doi: 10.1096/fj.201500088R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin A.A., Tripathi P.K., Sholl A., Jordan M.B., Hildeman D.A. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol. 2009;83(17):8604–8615. doi: 10.1128/JVI.02477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knobloch M., Braun S.M., Zurkirchen L., et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493(7431):226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramírez-Martínez G., Cruz-Lagunas A., Jiménez-Alvarez L., et al. Seasonal and pandemic influenza H1N1 viruses induce differential expression of SOCS-1 and RIG-I genes and cytokine/chemokine production in macrophages. Cytokine. 2013;62(1):151–159. doi: 10.1016/j.cyto.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan J., Li S., Wang C., et al. Expression and biological functions of the CCL5-CCR5 axis in oral lichen planus. Exp Dermatol. 2019;28(7):816–821. doi: 10.1111/exd.13946. [DOI] [PubMed] [Google Scholar]
- 62.Murooka T.T., Rahbar R., Fish E.N. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387(2):381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Du G., Shi L., Shen X., Shen Z., Liu W. Altered expression of CCN1 in oral lichen planus associated with keratinocyte activation and IL-1β, ICAM1, and CCL5 up-regulation. J Oral Pathol Med. 2020;49(9):920–925. doi: 10.1111/jop.13087. [DOI] [PubMed] [Google Scholar]
- 64.Hu J.Y., Zhang J., Cui J.L., et al. Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine. 2013;62(1):141–145. doi: 10.1016/j.cyto.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Z.Z., Sugerman P.B., Zhou X.J., Walsh L.J., Savage N.W. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral Dis. 2001;7(4):246–251. [PubMed] [Google Scholar]
- 66.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y., Xian X., Wang Z., et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8(3):80. doi: 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geovanini G.R., Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 69.Chistiakov D.A., Melnichenko A.A., Grechko A.V., Myasoedova V.A., Orekhov A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104(2):114–124. doi: 10.1016/j.yexmp.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Ranjit N., Diez-Roux A.V., Shea S., et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167(2):174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 71.Madan M., Bishayi B., Hoge M., Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197(2):504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakogiannis C., Sachse M., Stamatelopoulos K., Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. 2019;122:154157. doi: 10.1016/j.cyto.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Liu X., Duan W., et al. Batf3-dependent CD8α + dendritic cells aggravates atherosclerosis via Th1 cell induction and enhanced CCL5 expression in plaque macrophages. EBioMedicine. 2017;18:188–198. doi: 10.1016/j.ebiom.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vangelista L., Vento S. The expanding therapeutic perspective of CCR5 blockade. Front Immunol. 2018;8:1981. doi: 10.3389/fimmu.2017.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28(11):1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 76.van der Vorst E.P., Vanags L.Z., Dunn L.L., Prosser H.C., Rye K.A., Bursill C.A. High-density lipoproteins suppress chemokine expression and proliferation in human vascular smooth muscle cells. Faseb J. 2013;27(4):1413–1425. doi: 10.1096/fj.12-212753. [DOI] [PubMed] [Google Scholar]
- 77.Jones K.L., Maguire J.J., Davenport A.P. Chemokine receptor CCR5: from AIDS to atherosclerosis. Br J Pharmacol. 2011;162(7):1453–1469. doi: 10.1111/j.1476-5381.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mencarelli A., Cipriani S., Francisci D., et al. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci Rep. 2016;6:30802. doi: 10.1038/srep30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen Y., Lambrecht J., Ju C., Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18(1):45–56. doi: 10.1038/s41423-020-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guillot A., Tacke F. Liver macrophages: old dogmas and new insights. Hepatol Commun. 2019;3(6):730–743. doi: 10.1002/hep4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong Y., Torsoni A.S., Wu F., et al. Hepatic NF-kB-inducing kinase (NIK) suppresses mouse liver regeneration in acute and chronic liver diseases. Elife. 2018;7:e34152. doi: 10.7554/eLife.34152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seki E., De Minicis S., Gwak G.Y., et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luedde T., Schwabe R.F. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2):108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M., Sun X., Zhao J., et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17(7):753–764. doi: 10.1038/s41423-019-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pattterson B.K., Seetthamraju H., Dhody K., et al. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. medRxiv. 2020 [Google Scholar]
- 86.Agresti N., Lalezari J.P., Amodeo P.P., et al. Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: case series of four critically ill patients treated with leronlimab. J Transl Autoimmun. 2021;4:100083. doi: 10.1016/j.jtauto.2021.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Law H.K., Cheung C.Y., Ng H.Y., et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alonzo F., 3rd, Kozhaya L., Rawlings S.A., et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493(7430):51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glass W.G., Lim J.K., Cholera R., Pletnev A.G., Gao J.L., Murphy P.M. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202(8):1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X., Lee S.O., Xia S., et al. Endothelial cells enhance prostate cancer metastasis via IL-6→androgen receptor→TGF-β→MMP-9 signals. Mol Canc Therapeut. 2013;12(6):1026–1037. doi: 10.1158/1535-7163.MCT-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang C.H., Yamamoto A., Lin Y.T., Fong Y.C., Tan T.W. Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol. 2010;79(2):209–217. doi: 10.1016/j.bcp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Fray M.J., Dickinson R.P., Huggins J.P., Occleston N.L. A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J Med Chem. 2003;46(16):3514–3525. doi: 10.1021/jm0308038. [DOI] [PubMed] [Google Scholar]
- 93.Nganvongpanit K., Chaochird P., Siengdee P., et al. In vitro suppression of the MMP-3 gene in normal and cytokine-treated human chondrosarcoma using small interfering RNA. J Orthop Surg Res. 2009;4:45. doi: 10.1186/1749-799X-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brill A., Hershkoviz R., Vaday G.G., Chowers Y., Lider O. Augmentation of RANTES-induced extracellular signal-regulated kinase mediated signaling and T cell adhesion by elastase-treated fibronectin. J Immunol. 2001;166(12):7121–7127. doi: 10.4049/jimmunol.166.12.7121. [DOI] [PubMed] [Google Scholar]
- 95.Borghaei R.C., Gorski G., Javadi M., Chambers M. NF-kappaB and ZBP-89 regulate MMP-3 expression via a polymorphic site in the promoter. Biochem Biophys Res Commun. 2009;382(2):269–273. doi: 10.1016/j.bbrc.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borghaei R.C., Rawlings P.L., Jr., Javadi M., Woloshin J. NF-kappaB binds to a polymorphic repressor element in the MMP-3 promoter. Biochem Biophys Res Commun. 2004;316(1):182–188. doi: 10.1016/j.bbrc.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 97.Kelleher F.C., O'Sullivan H. Monocytes, macrophages, and osteoclasts in osteosarcoma. J Adolesc Young Adult Oncol. 2017;6(3):396–405. doi: 10.1089/jayao.2016.0078. [DOI] [PubMed] [Google Scholar]
- 98.Wang S.W., Liu S.C., Sun H.L., et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis. 2015;36(1):104–114. doi: 10.1093/carcin/bgu218. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Z., Dong J., Lobe C.G., Gong P., Liu J., Liao L. CCR5 facilitates endothelial progenitor cell recruitment and promotes the stabilization of atherosclerotic plaques in ApoE-/- mice. Stem Cell Res Ther. 2015;6(1):36. doi: 10.1186/s13287-015-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Z., Yang X., Bi G., et al. Ligand-receptor interaction atlas within and between tumor cells and T cells in lung adenocarcinoma. Int J Biol Sci. 2020;16(12):2205–2219. doi: 10.7150/ijbs.42080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shanbhag S., Ambinder R.F. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aldinucci D., Lorenzon D., Cattaruzza L., et al. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Canc. 2008;122(4):769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 103.Casagrande N., Borghese C., Visser L., Mongiat M., Colombatti A., Aldinucci D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica. 2019;104(3):564–575. doi: 10.3324/haematol.2018.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alonso S.R., Tracey L., Ortiz P., et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Canc Res. 2007;67(7):3450–3460. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- 105.Fang R., Zhang G., Guo Q., et al. Nodal promotes aggressive phenotype via Snail-mediated epithelial-mesenchymal transition in murine melanoma. Canc Lett. 2013;333(1):66–75. doi: 10.1016/j.canlet.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Liu J., Wang C., Ma X., et al. High expression of CCR5 in melanoma enhances epithelial-mesenchymal transition and metastasis via TGFβ1. J Pathol. 2019;247(4):481–493. doi: 10.1002/path.5207. [DOI] [PubMed] [Google Scholar]
- 107.Mellado M., de Ana A.M., Moreno M.C., Martínez C., Rodríguez-Frade J.M. A potential immune escape mechanism by melanoma cells through the activation of chemokine-induced T cell death. Curr Biol. 2001;11(9):691–696. doi: 10.1016/s0960-9822(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 108.Gao T., Shen Z., Ma C., Li Y., Kang X., Sun M. The CCL5/CCR5 chemotactic pathway promotes perineural invasion in salivary adenoid cystic carcinoma. J Oral Maxillofac Surg. 2018;76(8):1708–1718. doi: 10.1016/j.joms.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 109.Lai C.K., Rao Y.K., Chang K.R., et al. 3,3',4',5'-Tetramethoxychalcone inhibits human oral cancer cell proliferation and migration via p53-mediated mitochondrial-dependent apoptosis. Anticancer Res. 2014;34(4):1811–1819. [PubMed] [Google Scholar]
- 110.Chuang J.Y., Yang W.H., Chen H.T., et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009;220(2):418–426. doi: 10.1002/jcp.21783. [DOI] [PubMed] [Google Scholar]
- 111.Wu Y.C., Shen Y.C., Chang J.W., Hsieh J.J., Chu Y., Wang C.H. Autocrine CCL5 promotes tumor progression in esophageal squamous cell carcinoma in vitro. Cytokine. 2018;110:94–103. doi: 10.1016/j.cyto.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 112.Fattahi S., Amjadi-Moheb F., Tabaripour R., Ashrafi G.H., Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: epigenetics and beyond. Life Sci. 2020;262:118513. doi: 10.1016/j.lfs.2020.118513. [DOI] [PubMed] [Google Scholar]
- 113.Lin J., Chen Z., Huang Z., et al. Effect of T-cadherin on the AKT/mTOR signaling pathway, gastric cancer cell cycle, migration and invasion, and its association with patient survival rate. Exp Ther Med. 2019;17(5):3607–3613. doi: 10.3892/etm.2019.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan G., Martinowich K., Chin M.H., et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132(15):3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 115.Zhang S., Che D., Yang F., et al. Correction: tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget. 2020;11(52):4845–4846. doi: 10.18632/oncotarget.27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim J., Bae J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pathria P., Louis T.L., Varner J.A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40(4):310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y., Song Y., Du W., Gong L., Chang H., Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1) doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shevyrev D., Tereshchenko V. Treg heterogeneity, function, and homeostasis. Front Immunol. 2020;10 doi: 10.3389/fimmu.2019.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takeuchi Y., Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2015;28(8):401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yurchenko E., Tritt M., Hay V., Shevach E.M., Belkaid Y., Piccirillo C.A. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203(11):2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan M.C., Goedegebuure P.S., Belt B.A., et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182(3):1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gabrilovich D.I. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu L., Wang H., Gong Y., et al. Nesfatin-1 regulates the lateral hypothalamic area melanin-concentrating hormone-responsive gastric distension-sensitive neurons and gastric function via arcuate nucleus innervation. Metabolism. 2017;67:14–25. doi: 10.1016/j.metabol.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 126.Halama N., Zoernig I., Berthel A., et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Canc Cell. 2016;29(4):587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 127.Pervaiz A., Ansari S., Berger M.R., Adwan H. CCR5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med Oncol. 2015;32(5) doi: 10.1007/s12032-015-0607-x. [DOI] [PubMed] [Google Scholar]
- 128.Pervaiz A., Zepp M., Georges R., et al. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J Canc Res Clin Oncol. 2021;147(1):73–91. doi: 10.1007/s00432-020-03382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 130.Guerrero-Zotano A., Mayer I.A., Arteaga C.L. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Canc Metastasis Rev. 2016;35(4):515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 131.Isakoff S.J., Engelman J.A., Irie H.Y., et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Canc Res. 2005;65(23):10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 132.Mira E., Lacalle R.A., González M.A., et al. A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep. 2001;2(2):151–156. doi: 10.1093/embo-reports/kve027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chien H.C., Chan P.C., Tu C.C., et al. Importance of PLC-dependent PI3K/AKT and AMPK signaling in RANTES/CCR5 mediated macrophage chemotaxis. Chin J Physiol. 2018;61(5):266–279. doi: 10.4077/CJP.2018.BAG584. [DOI] [PubMed] [Google Scholar]
- 134.Gao D., Cazares L.H., Fish E.N. CCL5-CCR5 interactions modulate metabolic events during tumor onset to promote tumorigenesis. BMC Canc. 2017;17(1) doi: 10.1186/s12885-017-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mañes S., Mira E., Colomer R., et al. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J Exp Med. 2003;198(9):1381–1389. doi: 10.1084/jem.20030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X., Li X., Wei X., et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang X., Lang M., Zhao T., et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+) Treg cells in pancreatic ductal adenocarcinoma. Oncogene. 2017;36(21):3048–3058. doi: 10.1038/onc.2016.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Makinoshima H., Dezawa M. Pancreatic cancer cells activate CCL5 expression in mesenchymal stromal cells through the insulin-like growth factor-I pathway. FEBS Lett. 2009;583(22):3697–3703. doi: 10.1016/j.febslet.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 139.Singh S.K., Mishra M.K., Eltoum I.A., Bae S., Lillard J.W., Jr., Singh R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-19643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaday G.G., Peehl D.M., Kadam P.A., Lawrence D.M. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66(2):124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 141.Aldinucci D., Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang R., Guo L., Gao M., Li J., Xiang S. Research trends and regulation of CCL5 in prostate cancer. OncoTargets Ther. 2021;14:1417–1427. doi: 10.2147/OTT.S279189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katona T.M., Neubauer B.L., Iversen P.W., Zhang S., Baldridge L.A., Cheng L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin Canc Res. 2005;11(23):8358–8363. doi: 10.1158/1078-0432.CCR-05-0962. [DOI] [PubMed] [Google Scholar]
- 144.Zhao R., Bei X., Yang B., et al. Endothelial cells promote metastasis of prostate cancer by enhancing autophagy. J Exp Clin Canc Res. 2018;37(1) doi: 10.1186/s13046-018-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laganà A.S., Garzon S., Götte M., et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci. 2019;20(22) doi: 10.3390/ijms20225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shafrir A.L., Farland L.V., Shah D.K., et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Parazzini F., Viganò P., Candiani M., Fedele L. Diet and endometriosis risk: a literature review. Reprod Biomed Online. 2013;26(4):323–336. doi: 10.1016/j.rbmo.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 148.Guo P., Bi K., Lu Z., et al. CCR5/CCR5 ligand-induced myeloid-derived suppressor cells are related to the progression of endometriosis. Reprod Biomed Online. 2019;39(4):704–711. doi: 10.1016/j.rbmo.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 149.Romano A., Parrinello N.L., La Cava P., et al. PMN-MDSC and arginase are increased in myeloma and may contribute to resistance to therapy. Expert Rev Mol Diagn. 2018;18(7):675–683. doi: 10.1080/14737159.2018.1470929. [DOI] [PubMed] [Google Scholar]
- 150.Yang T., Li J., Li R., et al. Correlation between MDSC and immune tolerance in transplantation: cytokines, pathways and cell-cell interaction. Curr Gene Ther. 2019;19(2):81–92. doi: 10.2174/1566523219666190618093707. [DOI] [PubMed] [Google Scholar]
- 151.Mougiakakos D., Jitschin R., von Bahr L., et al. Immunosuppressive CD14+ HLA-DRlow/neg Ido+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. 2013;27(2):377–388. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 152.Cimino-Mathews A., Thompson E., Taube J.M., et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(1):52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riccio L.D.G.C., Santulli P., Marcellin L., Abrão M.S., Batteux F., Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 154.Dong B., Ding Y., Huang Q., Guan X. Different triple-negative breast cancer tumor cell lysates (TCLs) induce discrepant anti-tumor immunity by PD1/PDL-1 interaction. Med Sci Mon Int Med J Exp Clin Res. 2019;25:500–515. doi: 10.12659/MSM.911689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de la Monte S.M., Longato L., Tong M., Wands J.R. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Invest Drugs. 2009;10(10):1049–1060. [PMC free article] [PubMed] [Google Scholar]
- 156.Chou S.Y., Ajoy R., Changou C.A., Hsieh Y.T., Wang Y.K., Hoffer B. CCL5/RANTES contributes to hypothalamic insulin signaling for systemic insulin responsiveness through CCR5. Sci Rep. 2016;6 doi: 10.1038/srep37659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan O., Burke J.D., Gao D.F., Fish E.N. The chemokine CCL5 regulates glucose uptake and AMP kinase signaling in activated T cells to facilitate chemotaxis. J Biol Chem. 2012;287(35):29406–29416. doi: 10.1074/jbc.M112.348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karnoub A.E., Dash A.B., Vo A.P., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 159.Velasco-Velázquez M., Jiao X., De La Fuente M., et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Canc Res. 2012;72(15):3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 160.Kawana-Tachikawa A., Llibre J.M., Bravo I., et al. Effect of maraviroc intensification on HIV-1-specific T cell immunity in recently HIV-1-infected individuals. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0087334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ban Y., Mai J., Li X., et al. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Canc Res. 2017;77(11):2857–2868. doi: 10.1158/0008-5472.CAN-16-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Halvorsen E.C., Hamilton M.J., Young A., et al. Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. OncoImmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1150398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tanabe Y., Sasaki S., Mukaida N., Baba T. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget. 2016;7(30):48335–48345. doi: 10.18632/oncotarget.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pérez-Martínez L., Pérez-Matute P., Aguilera-Lizarraga J., et al. Maraviroc, a CCR5 antagonist, ameliorates the development of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD) J Antimicrob Chemother. 2014;69(7):1903–1910. doi: 10.1093/jac/dku071. [DOI] [PubMed] [Google Scholar]
- 165.Choi S.W., Hildebrandt G.C., Olkiewicz K.M., et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110(9):3447–3455. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reshef R., Luger S.M., Hexner E.O., et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Braunersreuther V., Steffens S., Arnaud C., et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28(6):1090–1096. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- 168.Tien P.C., Schneider M.F., Cole S.R., et al. Antiretroviral therapy exposure and insulin resistance in the Women's Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49(4):369–376. doi: 10.1097/qai.0b013e318189a780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pfleger C., Kaas A., Hansen L., et al. Relation of circulating concentrations of chemokine receptor CCR5 ligands to C-peptide, proinsulin and HbA1c and disease progression in type 1 diabetes. Clin Immunol. 2008;128(1):57–65. doi: 10.1016/j.clim.2008.03.458. [DOI] [PubMed] [Google Scholar]
- 170.Fleishaker D.L., Garcia Meijide J.A., Petrov A., et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res Ther. 2012;14(1) doi: 10.1186/ar3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.D'Antoni M.L., Mitchell B.I., McCurdy S., et al. Cenicriviroc inhibits trans-endothelial passage of monocytes and is associated with impaired E-selectin expression. J Leukoc Biol. 2018;104(6):1241–1252. doi: 10.1002/JLB.5A0817-328RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li M., Lu C., Zhu H., et al. Cenicriviroc ameliorates the severity of graft-versus-host disease through inhibition of CCR5 in a rat model of liver transplantation. Am J Transl Res. 2019;11(6):3438–3449. [PMC free article] [PubMed] [Google Scholar]
- 173.Lefebvre E., Moyle G., Reshef R., et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ratziu V., Sanyal A., Harrison S.A., et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72(3):892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 175.Eksteen B., Bowlus C.L., Montano-Loza A.J., et al. Efficacy and safety of cenicriviroc in patients with primary sclerosing cholangitis: PERSEUS study. Hepatol Commun. 2020;5(3):478–490. doi: 10.1002/hep4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang X., Haney K.M., Richardson A.C., et al. Anibamine, a natural product CCR5 antagonist, as a novel lead for the development of anti-prostate cancer agents. Bioorg Med Chem Lett. 2010;20(15):4627–4630. doi: 10.1016/j.bmcl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Y., Arnatt C.K., Zhang F., Wang J., Haney K.M., Fang X. The potential role of anibamine, a natural product CCR5 antagonist, and its analogues as leads toward development of anti-ovarian cancer agents. Bioorg Med Chem Lett. 2012;22(15):5093–5097. doi: 10.1016/j.bmcl.2012.05.127. [DOI] [PubMed] [Google Scholar]
- 178.Liou J.T., Mao C.C., Ching-Wah Sum D., et al. Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain. 2013;14(1):24–35. doi: 10.1016/j.jpain.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 179.Caseiro M.M., Nelson M., Diaz R.S., et al. Vicriviroc plus optimized background therapy for treatment-experienced subjects with CCR5 HIV-1 infection: Final results of two randomized phase III trials. J Infect. 2012;65(4):326–335. doi: 10.1016/j.jinf.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 180.Landovitz R.J., Angel J.B., Hoffmann C., et al. Phase II study of vicriviroc versus efavirenz (both with zidovudine/lamivudine) in treatment-naive subjects with HIV-1 infection. J Infect Dis. 2008;198(8):1113–1122. doi: 10.1086/592052. [DOI] [PubMed] [Google Scholar]
- 181.Qi B., Fang Q., Liu S., et al. Advances of CCR5 antagonists: from small molecules to macromolecules. Eur J Med Chem. 2020;208 doi: 10.1016/j.ejmech.2020.112819. [DOI] [PubMed] [Google Scholar]
- 182.Fowler D.W., Copier J., Dalgleish A.G., Bodman-Smith M.D. Zoledronic acid causes γδ T cells to target monocytes and down-modulate inflammatory homing. Immunology. 2014;143(4):539–549. doi: 10.1111/imm.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Caseiro M.M., Nelson M., Diaz R.S., et al. Vicriviroc plus optimized background therapy for treatment-experienced subjects with CCR5 HIV-1 infection: final results of two randomized phase III trials. J Infect. 2012;65(4):326–335. doi: 10.1016/j.jinf.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 184.Maeda K., Das D., Nakata H., Mitsuya H. CCR5 inhibitors: emergence, success, and challenges. Expet Opin Emerg Drugs. 2012;17(2):135–145. doi: 10.1517/14728214.2012.673584. [DOI] [PubMed] [Google Scholar]
- 185.Shi B., Li J., Shi X., et al. TALEN-mediated knockout of CCR5 confers protection against infection of human immunodeficiency virus. J Acquir Immune Defic Syndr. 2017;74(2):229–241. doi: 10.1097/QAI.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 186.Bai J., Gorantla S., Banda N., Cagnon L., Rossi J., Akkina R. Characterization of anti-CCR5 ribozyme-transduced CD34+ hematopoietic progenitor cells in vitro and in a SCID-hu mouse model in vivo. Mol Ther. 2000;1(3):244–254. doi: 10.1006/mthe.2000.0038. [DOI] [PubMed] [Google Scholar]
- 187.Shimizu S., Hong P., Arumugam B., et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu L., Wang J., Liu Y., et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381(13):1240–1247. doi: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
- 189.Telenti A. Safety concerns about CCR5 as an antiviral target. Curr Opin HIV AIDS. 2009;4(2):131–135. doi: 10.1097/COH.0b013e3283223d76. [DOI] [PubMed] [Google Scholar]
- 190.Barmania F., Pepper M.S. C-C chemokine receptor type five (CCR5): an emerging target for the control of HIV infection. Appl Transl Genom. 2013;2:3–16. doi: 10.1016/j.atg.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Okamoto M., Toyama M., Baba M. The chemokine receptor antagonist cenicriviroc inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;182 doi: 10.1016/j.antiviral.2020.104902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miao M., De Clercq E., Li G. Clinical significance of chemokine receptor antagonists. Expet Opin Drug Metabol Toxicol. 2020;16(1):11–30. doi: 10.1080/17425255.2020.1711884. [DOI] [PubMed] [Google Scholar]