Over evolution, the human species has acquired higher cognitive function, which helps to distinguish Homo sapiens from other mammals. Particularly, humans have a large neocortex through the evolutionary expansion of the brain, which is believed to be instrumental in the establishment of comprehensive neuronal circuits [1, 2]. A hallmark of the human brain is the highly convoluted brain surface structure, named gyrification or cortical folding, consisting of gyri and sulci, which significantly increase the cortical surface area. However, the mechanisms underlying the cortical expansion remain elusive.
Previous studies have suggested that the emergence of cortical folding is related to extensive neuronal production, that induces neocortical surface folding to fit the confined cranium. In turn, the augmented neuronal production is reflected by the strengthened proliferative ability of neuronal progenitor cells (NPCs) as well as by an extended neurogenic period [3]. In the developing human neocortex, there are two major classes of NPCs: radial glial cells (RGCs) and intermediate progenitors (IPs), which give rise to neurons or glial cells directly or indirectly [4]. Unlike apical RGCs located in the ventricular zone (VZ), outer RGCs (oRGs) proliferate and divide in the outer subventricular zone (OSVZ), away from the apical surface. Accumulating evidence suggests that expansion of the progenitor pool, including oRGs within the OSVZ, is highly correlated with gyrification [1]. However, the evolutionary mechanisms underlying the control of proliferation of progenitor cells during human brain development are largely unknown. Efforts have been made using comparative analyses to identify the genomic diversity that potentially illuminates human-specific neurodevelopment characteristics. One approach is to identify human-specific genes that endow neocortical expansion properties. Indeed, studies have identified several human-specific genes that originated from genomic segmental duplication. These genes have pivotal functional impact on cortical evolution [5]. However, the underlying genetic mechanisms remain to be elucidated. Recently, Hou and colleagues reported that hominoid-specific gene TBC1D3 functions through an interaction with euchromatic histone lysine N-methyltransferase G9a to de-repress genes involved in the proliferation of neural progenitors in the developing brain, bridging the epigenetic modification of genomic changes with enhanced neocortical expansion [6]. The authors utilized the three-dimensional system derived from human embryonic stem cells (hESCs) in vitro, human cerebral organoids, to determine the roles of TBC1D3 in human brain development.
Interestingly, the time-point of TBC1D3 gene duplications coincides with evolutionary branching in primates, suggesting that TBC1D3 is related to the evolution of the human lineage and thereby human-specific cortical features [7]. Previous studies have shown that TBC1D3 is strongly expressed in the ventricular and subventricular zones of the human fetal brain, where neural stem cells are harbored [8]. Importantly, overexpression of TBC1D3 in neural progenitors of the mouse brain leads to cortical folding [8], suggesting an important role for TBC1D3 in promoting gyrification. Here, the authors generated human cerebral organoids overexpressing TBC1D3. Intriguingly, up-regulation of TBC1D3 led to a significant expansion of neural progenitors and thereby an increase in organoid diameter, similar to cerebral expansion. In contrast, loss of TBC1D3 in human cerebral organoids resulted in a remarkable reduction of oRGs, and untimely, neuronal production.
Using the yeast two-hybrid system, the authors identified the histone methyltransferases G9a as a key target of TBC1D3. Unlike TBC1D3, G9a, along with its binding partner G9a-like protein (GLP), is highly conserved among species and regulates histone post-translational modifications [9]. Mutation of G9a has been suggested in Kleefstra syndrome, characterized by deficiency in cognition, further underlining its crucial role in brain development [10]. The interaction of TBC1D3 and G9a was later confirmed by co-immunoprecipitation using human brain lysates. Particularly, they have shown that a fragment containing amino-acids 465–481 in TBC1D3 is indispensable for the direct interaction with G9a, specifically the SET [Su(var)3-9, Enhancer-of-zeste and Trithorax] domain.
To investigate whether the interaction with TBC1D3 is required for G9a activity, the authors performed a histone methylation assay in vitro and showed that the level of H3K9me2 was significantly reduced by the up-regulation of TBC1D3, indicating a possible inhibitory effect on the function of G9a by TBC1D3. Conversely, down-regulation of TBC1D3 led to an augmented H3K9me2 level, suggesting that TBC1D3 is a direct repressor of G9a.
Fascinatingly, when examining the level of H3K9me2 in their previously-generated TBC1D3 transgenic mouse, which was characterized by cortical expansion and folding [8], they found that the level of H3K9me2 decreased, indicating that epigenetic regulation may play an essential role in cortical expansion. To test this hypothesis, they treated cerebral organoids with UNC0638, a specific and competitive inhibitor of G9a [11]. Similar to the phenotype of TBC1D3 up-regulation, they observed that the neural progenitor pool expanded and thereby the size of cerebral organoids increased upon the treatment, suggesting that the level of H3K9me2 mediated by G9a is the key effector of TBC1D3 during neurogenesis in human brain development.
To uncover downstream signaling pathways that control neural progenitor proliferation through G9a-mediated epigenetic regulation, the authors performed transcriptomic analysis of organoids with or without TBC1D3 blockade. The differentially-expressed genes (DEGs) were selected and further analyzed by gene ontology. They found that the up-regulated genes were enriched in apoptotic signaling pathways. However, the down-regulated genes were related to forebrain development and neuronal differentiation. Particularly, these genes were enriched in signaling pathways involved in cell proliferation, such as the AKT, WNT, or MAPK pathways. Furthermore, genome-wide H3K9me2 chromatin immunoprecipitation sequencing (ChIP-seq) in human cerebral organoids revealed an increase of H3K9me2 signals at transcriptional start sites after impeding the interaction between TBC1D3 and G9a. Interestingly, 253 DEGs with H3K9me2 peaks were mainly related to proliferative pathways, such as PI3K-Akt and MAPK signaling. Conclusively, the hominoid-specific protein TBC1D3 functions through an epigenetic mechanism to modulate the stemness of neural progenitors, thus promoting the expansion of human neocortex.
Overall, this study reveals an important intrinsic mechanism underlying neural progenitor proliferation and provides novel insights into the understanding of cortical expansion (Fig. 1). In addition to the cytosolic role of TBC1D3 in EGFR signaling [12], the authors have now identified several pathways that are involved in progenitor proliferation regulated by nuclear TBC1D3, such as the WNT and MAPK pathways. However, these pathways also play important roles in regulating neural progenitor proliferation in mouse brain with no characteristic gyrification. A previous study has shown that continuous activation of Wnt signaling induces gyrification-like convolutions in mice [13]. However, this is due to the exaggerated proliferation of neural progenitors in the ventricular zone upon the activation of Wnt signaling. Therefore, the gyrification-like structure in the mouse model is different from the cortical folding of gyrencephalic brains in which the ventricular zone has less expansion than the gray matter. Therefore, expansion of oRGs may play an essential role in the formation of gyrification. Interestingly, recent studies have shown that NOTCH2NL, a hominoid-specific paralog of NOTCH2, promotes Notch signaling to control the expansion of human cortical progenitors, including oRGs, and their neuronal output [14, 15]. It is possible that the expression level of these hominoid-specific genes is regulated through epigenetic modification in different cell types. For example, TBC1D3-mediated histone methylation, which provides a dynamic expression of these genes to control progenitor proliferation during human brain development. Therefore, it would be interesting to examine how the downstream targets, particularly the human-specific genes, regulated by TBC1D3 contribute to the human-specific traits in neurodevelopment.
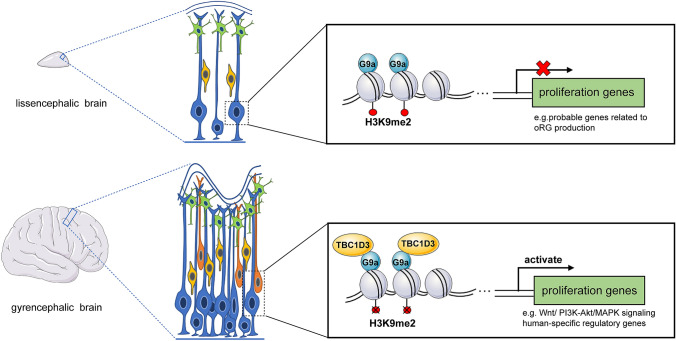
Fig. 1.
TBC1D3 promotes neural progenitor proliferation in an epigenetic pattern. In lissencephalic mammals, such as mouse, the proliferative potency of neural progenitors is impeded due to the repression of proliferation genes, especially those probably related to the production of oRGs. However, in gyrencephalic mammals like humans, TBC1D3 interacts with G9a to de-repress proliferation genes, like those involved in WNT, PI3K-Akt, MAPK signaling or human-specific genes, thus expanding the progenitor pool to generate gyrification. Blue, RGCs; yellow, IPCs; orange, oRGs; green, neurons.
In addition, despite the high similarity between organoids and human brains, the simplified structure of cerebral organoids without fully-developed cortical layers fails to mimic neural progenitors and neurons at the right positions [16]. Therefore, to investigate roles of TBC1D3 in gyrencephalic mammals, in vivo models, such as ferrets and non-human primates, may be better than organoids to capture the unique and dynamic features of brain development in vivo and related brain disorders. In general, Hou and colleagues have discovered that TBC1D3 coordinates epigenetic regulation through G9a, which possibly induces specific integrated proliferative signaling pathways during human brain development.
Acknowledgements
This Research Highlight was supported by the National Key Research and Development Program of China (2018YFA0108000), the National Natural Science Foundation of China (31872763), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01, 19JC1411003) and ZJLab.
Conflict of interest
The authors declare no competing interests.
References
- 1.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li QW, Wang LF, Ma YL, Yue WH, Zhang D, Li J. P-Rex1 overexpression results in aberrant neuronal polarity and psychosis-related behaviors. Neurosci Bull. 2019;35:1011–1023. doi: 10.1007/s12264-019-00408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namba T, Huttner WB. Neural progenitor cells and their role in the development and evolutionary expansion of the neocortex. Wiley Interdiscip Rev Dev Biol. 2017 doi: 10.1002/wdev.256. [DOI] [PubMed] [Google Scholar]
- 4.Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: From molecules to malformations. Nat Rev Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cárdenas A, Borrell V. Molecular and cellular evolution of corticogenesis in amniotes. Cell Mol Life Sci. 2020;77:1435–1460. doi: 10.1007/s00018-019-03315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou QQ, Xiao Q, Sun XY, Ju XC, Luo ZG. TBC1D3 promotes neural progenitor proliferation by suppressing the histone methyltransferase G9a. Sci Adv. 2021;7:eaba8053. doi: 10.1126/sciadv.aba8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl PD, Wainszelbaum MJ. Human-specific genes may offer a unique window into human cell signaling. Sci Signal. 2009;2:pe59. doi: 10.1126/scisignal.289pe59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju XC, Hou QQ, Sheng AL, Wu KY, Zhou Y, Jin Y, et al. The hominoid-specific gene TBC1D3 promotes generation of basal neural progenitors and induces cortical folding in mice. Elife. 2016;5:e18197. doi: 10.7554/eLife.18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benevento M, van de Molengraft M, van Westen R, van Bokhoven H, Kasri NN. The role of chromatin repressive marks in cognition and disease: A focus on the repressive complex GLP/G9a. Neurobiol Learn Mem. 2015;124:88–96. doi: 10.1016/j.nlm.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Kleefstra T, Smidt M, Banning MJ, Oudakker AR, van Esch H, de Brouwer AP, et al. Disruption of the gene Euchromatin Histone Methyl Transferase1 (Eu-HMTase1) is associated with the 9q34 subtelomeric deletion syndrome. J Med Genet. 2005;42:299–306. doi: 10.1136/jmg.2004.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedadi M, Barsyte-Lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, et al. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat Chem Biol. 2011;7:566–574. doi: 10.1038/nchembio.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainszelbaum MJ, Charron AJ, Kong C, Kirkpatrick DS, Srikanth P, Barbieri MA, et al. The hominoid-specific oncogene TBC1D3 activates Ras and modulates epidermal growth factor receptor signaling and trafficking. J Biol Chem. 2008;283:13233–13242. doi: 10.1074/jbc.M800234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 14.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, et al. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell. 2018;173:1356–1369.e22. doi: 10.1016/j.cell.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki IK, Gacquer D, Van Heurck R, Kumar D, Wojno M, Bilheu A, et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through delta/notch regulation. Cell. 2018;173:1370–1384.e16. doi: 10.1016/j.cell.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]