Dear Editor,
Neuropathic pain (NeuP) is known as a common and notorious neurological disease, characterized by spontaneous pain and evoked abnormal pain that includes hypersensitivity to external innocuous (allodynia) and noxious stimulation (hyperalgesia). Allodynia is especially torturous because gentle touch may trigger pain. The high incidence of resistance to common analgesics makes the clinical management of NeuP challenging. More research is urgently needed to better understand the mechanisms of NeuP and to develop new therapeutic strategies.
The lateral habenula (LHb) is a small, evolutionarily conserved epithalamic area occupying a key position in the communication between forebrain and midbrain structures. Preclinical data have implicated the LHb in an astonishing variety of biological functions and behaviors [1]. The LHb is mainly composed of projecting glutamatergic neurons (LHbGlu). Early studies found that about two-thirds of LHb neurons respond to peripheral noxious stimulation, the majority being excited [2]. These findings imply that the LHb is involved in pain processing. As an integration center for negative emotions, the excitation of the LHb has also long been linked to encoding the aversive aspect of pain. On the other hand, LHb neurons send dense projection fibers to pain-modulating nuclei in the midbrain and brain stem. Studies have shown that manipulation of LHb activity by chemical compounds results in anti-nociception [3], indicating an involvement of the LHb in pain modulation.
Although a contribution of the LHb to NeuP has been proposed because NeuP involves profound sensory and emotional distress and imaging studies have found that Hb activity changes in several types of NeuP [4], direct evidence is still lacking. A recent study reported that LHbGlu are activated after injury to the infraorbital nerve, and selective inhibition of these neurons using a chemogenetic approach alleviates postoperative anxiety, but not orofacial allodynia [5]. These results imply that LHb activation contributes to mood comorbidity in trigeminal neuropathic pain, but not to pain hypersensitivity. However, studies by us and others have revealed that the mechanisms underlying NeuP may have peculiarities at cephalic versus extra-cephalic levels [6]. Whether the LHb plays a role in somatic NeuP, and thus could serve as a potential therapeutic target, remain unknown.
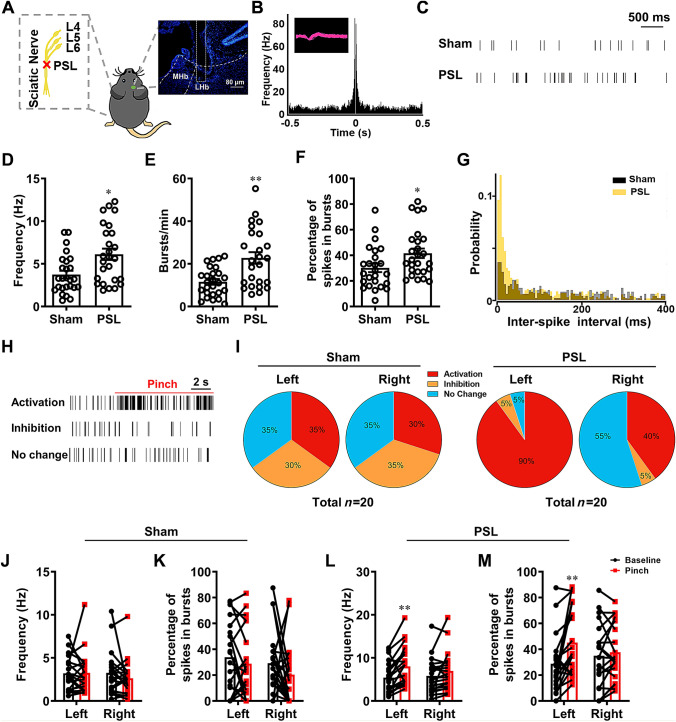
In the present study, we first assessed the neuronal activity of LHb neurons in mice by measuring the spontaneous and evoked firing using in vivo single-unit recordings in the right LHb on day 14 after left partial sciatic nerve ligation (PSL) injury (Fig. 1A). The putative glutamatergic nature of neurons was identified by half pulse width and autocorrelation analysis of firing (Fig. 1B). We found that these neurons showed spontaneous and burst-like firing. The firing frequency in the PSL group was significantly higher than that in the sham group (Fig. 1C, D). The increased burst-like firing numbers/min and percentage of spikes in burst-like firing (Fig. 1E, F) as well as the smaller inter-spike intervals (Fig. 1G) together demonstrated that burst-like firing also increased in the PSL group. These electrophysiological data indicate that under somatic NeuP conditions, the overall and burst-like firing of LHb neurons are elevated, i.e., they are hyperactive.
Fig. 1.
LHb neurons are hyperactive after PSL. A Schematic of PSL surgery on the left side and a representative microphotograph of the electrode placement in the right LHb. B Autocorrelation and waveform (inset) of action potentials from a representative LHb neuron. C Example firing traces of LHb neurons from the sham and PSL groups (scale bar, 500 ms). D–G Firing frequency (D), number of burst-like firings/min (E), percentage of spikes in burst-like firing (F), and inter-spike interval analysis of firing (G) in LHb neurons in the sham and PSL groups (n = 26/group from 12 and 22 mice in the sham and PSL groups, respectively; *P <0.05, **P <0.01, sham vs PSL). H Example firing traces of LHb neurons showing activation, inhibition, and no change of firing in response to pinch stimulation to the left hind paw (scale bar, 2 s). I Proportions of the three response patterns in LHb neurons to pinch stimulation of bilateral hind paws in the sham and PSL groups. J, K Changes in firing frequency (J) and percentage of spikes in burst-like firing (K) in the sham group. L, M Changes in firing frequency (L) and percentage of spikes in burst-like firing (M) in the PSL group (n = 20/group from 14 and 22 mice in the sham and PSL group, respectively; **P < 0.01, baseline vs pinch).
To determine whether the characteristics of LHb activity in response to noxious stimulation change under somatic neuropathic pain conditions, pinch stimulation was applied to bilateral hind paws during single-unit recordings. In the sham group, the firing of LHb neurons was either activated, inhibited, or unchanged upon pinch stimulation (Fig. 1H). The proportions of neurons with the three responsive patterns were similar on both sides (Fig. 1I), resulting in an unchanged average firing frequency by stimulation on either side (Fig. 1J). Meanwhile, the percentage of spikes in burst-like firing did not change either (Fig. 1K). However, in the PSL group, 90% (18 of 20) of neurons were activated by the pinch stimulation of the left hind paw (the nerve-injured side, contralateral to the recording) (Fig. 1I), supported by a higher firing frequency (Fig. 1L) and a greater percentage of spikes with burst-like firing (Fig. 1M). When stimulating the intact hind paw (right, ipsilateral to the recording), fewer neurons were inhibited (5% vs 35%) and more neurons retained their activity (55% vs 35%), compared with the sham group (Fig. 1I), although the average firing frequency and percentage of spikes with burst-like firing did not show significant changes (Fig. 1L, M). These results indicate that LHb neurons contralateral to the somatic nerve injury are prone to fire more actively and in bursts spontaneously or upon noxious stimulation.
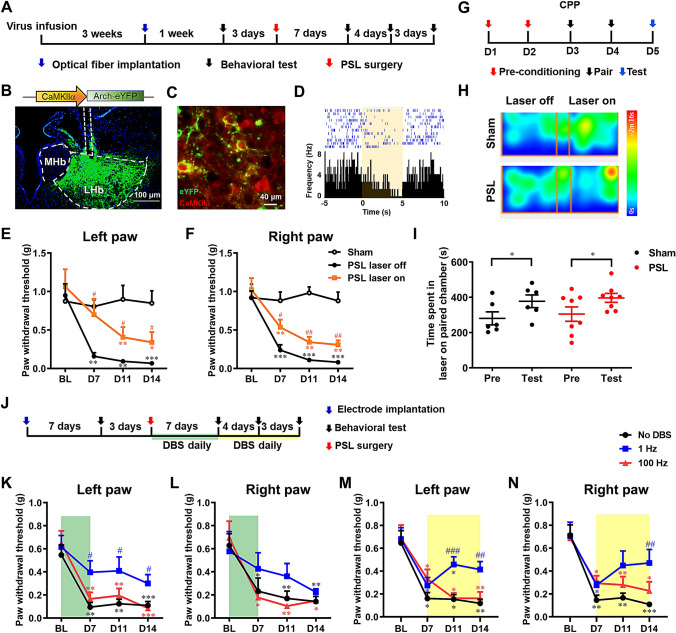
The role of hyperactivity of LHb neurons after PSL was then investigated by selectively inhibiting LHbGlu using an optogenetic approach (Fig. 2A). pAAV-CaMKIIα-eArchT-eYFP was infused into the right LHb and the precise and functional transduction was confirmed by the co-localization of eYFP and CaMKIIα, a marker for glutamatergic neurons (Fig. 2B, C), and the inhibition of neuronal firing upon yellow laser illumination (Fig. 2D). In the intact condition, unilateral photoinhibition of LHbGlu did not affect bilateral paw withdrawal threshold (PWT), indicating that these neurons are not tonically involved in the modulation of mechanical nociception. However, on days 7, 11, and 14 after PSL when mechanical allodynia had been established bilaterally, illumination with yellow light elevated the PWT on both sides, indicating the alleviation of neuropathic allodynia (laser ON vs laser OFF, two-way ANOVA with repeated measures (RM), for the left paw, treatment, F1,14 = 4.822, P = 0.045; time, F3,42 = 30.23, P <0.001; interaction, F3,42 = 1.731, P = 0.175; for the right paw, F1, 14 = 10.790, P = 0.005; time, F3,42 = 43.350, P <0.001; interaction, F3,42 = 0.560, P = 0.644; t-test, P <0.05 and 0.01, respectively; Fig. 2E, F). In the conditioned place preference (CPP) test, inhibition of LHbGlu in the sham group induced place preference, since animals spent more time in the chamber paired with laser ON treatment (Fig. 2G, H), indicating that suppression of LHbGlu is rewarding. This rewarding effect remained in neuropathic mice, because they also showed a preference for the illuminated chamber (Fig. 2I). These results together demonstrate that selective inhibition of LHbGlu alleviates mechanical allodynia and negative affect after PSL, indicating a contributory role of LHbGlu in somatic neuropathic pain.
Fig. 2.
Selective inhibition of LHbGlu and low-frequency electrical stimulation of LHb alleviate somatic neuropathic pain after PSL. A Schedule of experimental procedures. B Construct of pAAV-CaMKIIa-eArchT-eYFP and an example photomicrograph of the site of viral injection and optic fiber implantation. C Example photomicrograph showing the co-localization (yellow) of eYFP (green) and CaMKIIα (red) in the LHb. D Example putative glutamatergic LHb neuron that responded to yellow laser stimulation by decreasing its firing frequency. E, F Optical inhibition of unilateral (right) LHbGlu alleviates mechanical allodynia in the left (E) and (F) right paws (n = 8/group; *P <0.05, **P <0.01, ***P <0.001 vs baseline (BL) in the respective group; #P <0.05, ##P <0.01, laser ON vs laser OFF at the same time points; the sham group received optic fiber implantation without laser stimulation). G Schedule of the CPP test. H Representative heatmaps of the CPP test. I Optical inhibition of unilateral (right) LHbGlu induces place preference in the sham and PSL groups (n = 6/group. *P <0.05, pre vs test). J Schedule of experimental procedures. K, L Electrical stimulation of the unilateral (right) LHb during the early phase alleviates mechanical allodynia in the left (K, the side injury), but less so in the right (L) paw (n = 9 for both No DBS and 1-Hz groups, n = 10 for the 100-Hz group). M, N Electrical stimulation of the unilateral (right) LHb during the late phase alleviates mechanical allodynia in the left (M) and right paw (N) (n = 8 for the No DBS group, n = 7 for both the 1-Hz and 100-Hz groups; *P <0.05, **P <0.01, ***P <0.001, vs BL in the respective group; #P< 0.05, ##P <0.01, ###P <0.001, vs No DBS group at the same time points).
Several lines of evidence indicate that electrical stimulation modulates the neuronal activity of brain nuclei and deep brain stimulation (DBS) has been emerging as an important option for the management of a variety of neurological and psychiatric disorders [7]. To test whether the LHb could serve as a target of DBS for the relief of somatic NeuP, 15 min of high (100 Hz) or low (1 Hz) frequency electrical stimulation was delivered to the contralateral (right) LHb daily during the early or late phase after PSL on the left side (Fig. 2J). We found that DBS of the LHb at 1 Hz through the first 7 days after PSL significantly elevated the PWT on the left side, not only within the period of DBS treatment, but also for the following week (two-way ANOVA with RM, treatment, F2,25 = 4.212, P = 0.027; time, F3,75 = 29.190, P <0.001; interaction, F6,75 = 1.228, P = 0.302; one-way ANOVA with Dunnett’s post-hoc, P <0.05 on days 7, 11, and 14, compared with the No DBS group). The postoperative PWT on the right side in the 1-Hz group was not significantly different from the No DBS and 100-Hz groups (two-way ANOVA with RM, treatment, F2,25 = 0.959, P = 0.397; time, F3,75 = 18.730, P <0.001; interaction, F6,75 = 1.153, P = 0.340), neither was it different from the baseline (one-way ANOVA with Dunnett’s post-hoc, P >0.05), indicating that the preventive and lasting effect of 1-Hz DBS on the ipsilateral neuropathic allodynia was not as evident as that on the contralateral allodynia. In contrast, 100-Hz stimulation had no preventive effect (Fig. 2K, L). When the 1-Hz stimulation was applied through postoperative days 7 to 14, the established mechanical allodynia was alleviated. This effect was stronger for the contralateral hind paw, because the alleviation of allodynia was recorded on days 11 and 14 in the contralateral, but only on day 14 in the ipsilateral hind paw (two-way ANOVA with RM, for the left paw, treatment, F2,20 = 5.441, P = 0.013; time, F3,60 = 25.980, P <0.001; interaction, F6,60 = 1.777, P = 0.119; one-way ANOVA with Dunnett’s post-hoc, P <0.001 and P < 0.01 on days 11 and 14, respectively; for the right paw, treatment, F2,20 = 3.849, P = 0.039; time, F3,60 = 22.090, P <0.001; interaction, F6,60 = 1.283, P = 0.279; one-way ANOVA with Dunnett’s post-hoc, P <0.01 on day14; Fig. 2M, N). However, the 100-Hz stimulation had no effect on either side (Fig. 2M, N). These results together demonstrate that low-frequency DBS of the LHb has both preventive and therapeutic effects on somatic neuropathic pain when applied in the early and late phase, respectively.
Here, we found that under physiological conditions, LHb neurons exhibited spontaneous activity. These neurons responded to noxious mechanical stimulation of the bilateral hind paws by either increasing, decreasing, or continuing their activity, with each response pattern constituting about one third. These findings indicate the LHb is involved in pain processing with cellular heterogeneity. On the other hand, neither optogenetic inhibition nor activation of LHbGlu affected nociceptive sensitivity. Few previous studies have reported that pharmacological inhibition of LHb activity in rats reduces nociceptive sensitivity [3]. However, it has also been reported that lesions of the habenula do not affect nociception [8]. Given that the habenula comprises many types of neuron [9], we deduce that these conflicting results may be due to discrepancies in experimental methodology, especially the approaches to manipulating neuronal activity. Moreover, non-selective manipulation hardly differentiates the functions of different neuron subgroups, when no subgroups dominate. Although the optogenetic approach is much more selective than pharmacological treatment and lesioning, the current methodology was still unable to preferentially manipulate certain subgroups of LHbGlu. Therefore, more precise research is still needed to specifically elucidate the role of LHbGlu in modulating nociception when novel tools become available in the future.
We found that LHb neurons fired at a higher frequency, generated more burst-like spikes, and more neurons were activated by noxious mechanical stimulation of the hind paw after sciatic nerve injury. This neuronal hyperactivity was particularly vigorous in the LHb contralateral to the injury. These results indicate that, contrary to that under physiological conditions, LHb activity and responses to peripheral stimulation are enhanced under somatic neuropathic conditions. The finding that optogenetic inhibition of LHbGlu alleviated neuropathic allodynia provided direct evidence for the contribution of LHbGlu hyperexcitability to NeuP. Moreover, inhibition of LHbGlu induced place preference in both sham-operated and neuropathic mice, indicating that LHbGlu activity is associated with aversion under both physiological and neuropathic conditions. Because psychosocial variables play key roles in the development of chronic pain and can modulate the outcomes of treatment, relief of emotional distress should be taken into consideration to achieve optimal effectiveness [10]. Our results suggest that the inhibition of LHbGlu possesses the advantage of relieving both the abnormal pain and negative emotion under the conditions of somatic neuropathic pain. Cui et al. [5] recently reported that LHbGlu are activated after infraorbital nerve injury, and optogenetic inhibition of these neurons does not affect mechanical and cold allodynia in the orofacial area. Combining our results, we propose that the hyperexcitability of LHbGlu contribute to somatic rather than orofacial NeuP. Therefore, inhibition of LHb neuronal activity may be an effective therapeutic approach for somatic but not orofacial NeuP.
The safety and feasibility of the LHb as a target of DBS for the treatment of neuropsychiatric diseases have been demonstrated by clinical studies. To further verify the role of the LHb in NeuP and to examine the possibility of the LHb as a DBS target for NeuP treatment, we evaluated the effect of low (1 Hz) and high (100 Hz) frequency stimulation of the LHb on somatic neuropathic allodynia. We found that 1-Hz stimulation prevented the development of neuropathic allodynia contralaterally when applied during the early phase (days 1–7 post-injury), and attenuated established neuropathic allodynia bilaterally when applied during the late phase (days 7–14 post-injury), while-100 Hz had no effect regardless of the timing of application. Although the exact mechanisms of DBS are still elusive, it has been reported that low-frequency stimulation inhibits neuronal discharge, induces long-term depression, and has a cumulative effect on diseases [11, 12]. Moreover, the firing patterns of LHb neurons are very sensitive to slight alterations in membrane potential [13]. Therefore, the modulation of neuronal firing and plasticity may underlie the inhibition of neuropathic allodynia by 1-Hz DBS of the LHb. Interestingly, studies have also shown that 100-Hz stimulation can decrease LHb activity [14], and is effective on other diseases [15]. The absence of analgesic effect on somatic neuropathic allodynia indicates that different stimulation parameters may modulate LHb activity differentially, and hence are required for different diseases when using LHb as the target of DBS.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81872843, 81673404, and 81821091).
Conflict of interest
All authors claim that there are no conflict of interest.
Footnotes
Yu Du, Yu-Xing Wu and Fang Guo have contributed equally to this work.
Contributor Information
Zhong Chen, Email: chenzhong@zju.edu.cn.
Shi-Hong Zhang, Email: shzhang713@zju.edu.cn.
References
- 1.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benabid AL, Jeaugey L. Cells of the rat lateral habenula respond to high-threshold somatosensory inputs. Neurosci Lett. 1989;96:289–294. doi: 10.1016/0304-3940(89)90393-5. [DOI] [PubMed] [Google Scholar]
- 3.Khalilzadeh E, Saiah GV. The possible mechanisms of analgesia produced by microinjection of morphine into the lateral habenula in the acute model of trigeminal pain in rats. Res Pharm Sci. 2017;12:241–248. doi: 10.4103/1735-5362.207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol. 2012;96:208–219. doi: 10.1016/j.pneurobio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui WQ, Zhang WW, Chen T, Li Q, Xu F, Mao-Ying QL, et al. Tacr3 in the lateral habenula differentially regulates orofacial allodynia and anxiety-like behaviors in a mouse model of trigeminal neuralgia. Acta Neuropathol Commun. 2020;8:44. doi: 10.1186/s40478-020-00922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu TT, Wang RR, Tang YY, Wu YX, Yu J, Hou WW, et al. TLR4 deficiency abrogated widespread tactile allodynia, but not widespread thermal hyperalgesia and trigeminal neuropathic pain after partial infraorbital nerve transection. Pain. 2018;159:273–283. doi: 10.1097/j.pain.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 7.Lee DJ, Lozano CS, Dallapiazza RF, Lozano AM. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 2019;131:333–342. doi: 10.3171/2019.4.JNS181761. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs P, Cox VC. Habenula lesions attenuate lateral hypothalamic analgesia in the formalin test. Neuroreport. 1993;4:121–124. doi: 10.1097/00001756-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Quina LA, Walker A, Morton G, Han V, Turner EE. GAD2 expression defines a class of excitatory lateral habenula neurons in mice that project to the raphe and pontine tegmentum. eNeuro 2020, 7: ENEURO.0527–19.2020. [DOI] [PMC free article] [PubMed]
- 10.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17:T70–T92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi Z, Naderi N, Shojaei A, Raoufy MR, Ahmadirad N, Barkley V, et al. The inhibitory effect of different patterns of low frequency stimulation on neuronal firing following epileptiform activity in rat hippocampal slices. Brain Res. 2019;1706:184–195. doi: 10.1016/j.brainres.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Ruan Y, Xu C, Lan J, Nao J, Zhang S, Fan F, et al. Low-frequency stimulation at the subiculum is anti-convulsant and anti-drug-resistant in a mouse model of lamotrigine-resistant temporal lobe epilepsy. Neurosci Bull. 2020;36:654–658. doi: 10.1007/s12264-020-00482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox KS, Gutnick MJ, Christoph GR. Electrophysiological properties of neurons in the lateral habenula nucleus: an in vitro study. J Neurophysiol. 1988;59:212–225. doi: 10.1152/jn.1988.59.1.212. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23:1094–1112. doi: 10.1038/mp.2018.2. [DOI] [PubMed] [Google Scholar]