Dear Editor,
Itch (pruritus) is an unpleasant somatic sensation that is accompanied by the desire to scratch. While itch provides a warning signal that protects us from potential threats in normal conditions, severe or chronic itch associated with dermatosis or systemic diseases is often difficult to alleviate and causes severe skin and tissue damage. In recent years, considerable advances have been made in the molecular and cellular mechanisms underlying itch at the peripheral and spinal levels. For example, Mas-related G-protein-coupled receptor A3+ neurons in the dorsal root ganglion (DRG) specifically carry information about itch [1]. In addition, natriuretic polypeptide b (NPPB), which has been identified as an itch-selective neuropeptide, can be released from the primary afferents and act on the NPPB receptor NPRA in the spinal dorsal horn [2]. Meanwhile, neuropeptide gastrin-releasing peptide (GRP)+ interneurons in lamina II receive signals from NPPB+ neurons and relay itch signals to another group of interneurons in laminae I-II expressing GRP receptors (GRPRs) [3]. Thus, itch is at least partially represented by a ‘‘labeled line’’ in the DRG and spinal cord. However, the circuit mechanisms of supraspinal areas in itch modulation are poorly understood.
The primary somatosensory cortex (S1) is the main receptive area for the senses of itch, pain, touch, and temperature. Functional imaging studies in humans have revealed that during itch S1 is activated, which is thought to be associated with localization and the intensity rating [4]. Anatomical studies in animals have shown that S1 has descending projections to subcortical areas such as the thalamus, trigeminal nuclei, and dorsal column nuclei to modulate the sensory information ascending to S1 itself. Recent studies have shown that S1 even sends long-distance projections to the contralateral lumbar dorsal horn to control touch or tactile neuropathic pain [5]. The spinal dorsal horn (SDH) plays an important role in relaying itch information from peripheral tissue to the brain [6]. Whether S1 regulates spinal itch transmission via the direct descending projection is unclear.
To determine whether S1-SDH projection neurons are involved in acute itch, we injected Compound 48/80 (C48/80) or chloroquine (CQ) into the calf to induce itch (Fig. S1A, B). We then injected AAVretro-hSyn-EGFP-2A-Cre into the left lumbar dorsal horn to retrogradely label S1-SDH projection neurons. One week later, AAV2/9-flex-taCasp3-TEVp (or AAV2/9-flex-EGFP) was injected into the right S1 (Fig. S1C–E). This manipulation caused a significant reduction (decreased by 94.7% ± 0.3%) in the number of EGFP+ S1-SDH projection neurons (Fig. 1A–C). Surprisingly, behavioral experiments showed that C48/80-induced itch behaviors were significantly increased (1.9 ± 0.1-fold) in mice injected with AAV2/9-flex-taCasp3-TEVp (Fig. 1D). Consistently, CQ-induced itch behaviors were also increased (1.7 ± 0.2-fold) in AAV2/9-flex-taCasp3-TEVp-treated mice (Fig. 1E). In addition, AAV2/9-flex-taCasp3-TEVp treatment did not affect the motor function of mice (Fig. 1F). These results suggest that S1-SDH projection neurons tonically inhibit spinal itch transmission.
Fig. 1.
S1-SDH projection neurons have a tonic inhibitory effect on itch behaviors. A Representative image showing the distribution of S1-SDH projection neurons labeled by EGFP without ablation. B Representative image showing the distribution of S1-SDH projection neurons labeled by EGFP after ablation. C Summary of the number of EGFP+ neurons in S1 (n = 3). D, E Effects of ablation of S1-SDH projection neurons on itch behaviors induced by C48/80 (D) or CQ (E) (n = 8). F Effects of ablation of S1-SDH projection neurons on motor function of mice (n = 8). G, H Pharmacogenetic inhibition of S1-SDH projection neurons enhances itch behaviors in response to C48/80 (G, n = 7) or CQ (H, n = 7). I Injection of hM4Di viruses has no effect on the motor function of mice (n = 7). J, K Pharmacogenetic activation of S1-SDH projection neurons reduces itch behaviors in response to C48/80 (J, n = 7) or CQ (K, n = 7). L Injection of hM3Dq viruses has no effect on the motor function of mice (n = 7).
To further confirm the role of S1-SDH projection neurons in itch processing, we employed chemogenetic methods to manipulate the activity of these neurons. We injected AAVretro-hSyn-EGFP-2A-Cre into the left lumbar dorsal horn and AAV2/9-Ef1a-DIO-hM4D(Gi)-mCherry (hM4Di) [or AAV2/9-Ef1a-DIO-hM3D(Gq)-mCherry (hM3Dq) or AAV2/9-Ef1a-DIO-mCherry (mCherry)] into the right S1 1 week later (Fig. S2A-C). The EGFP and mCherry were expressed and co-localized in the S1 (Fig. S3A). Electrophysiological recording confirmed the effect of CNO on the decrease or increase of the excitation of S1 pyramidal neurons of mice injected with hM4Di or hM3Dq (Fig. S3B). Furthermore, CNO injection into the mice infused with hM4Di to pharmacogenetically inhibit S1-SDH projection neurons significantly increased the itch behaviors evoked by C48/80 or CQ, compared to the CNO + mCherry group (C48/80: 1.7 ± 0.1-fold, Fig. 1G; CQ: 1.3 ± 0.1-fold, Fig. 1H). In contrast, the AAV treatment did not affect motor function of mice (Fig. 1I). Consistently, pharmacogenetic activation of S1-SDH projection neurons by CNO injection into the mice infused with hM3Dq decreased C48/80- or CQ-evoked itch behaviors, compared to the CNO + mCherry group (C48/80: decreased by 42.8% ± 3.0%, Fig. 1J; CQ: decreased by 54.2% ± 9.5%, Fig. 1K). The motor function did not differ between the mCherry group and hM3Dq group (Fig. 1L). These results indicate that S1-SDH projection neurons negatively regulate spinal itch transmission.
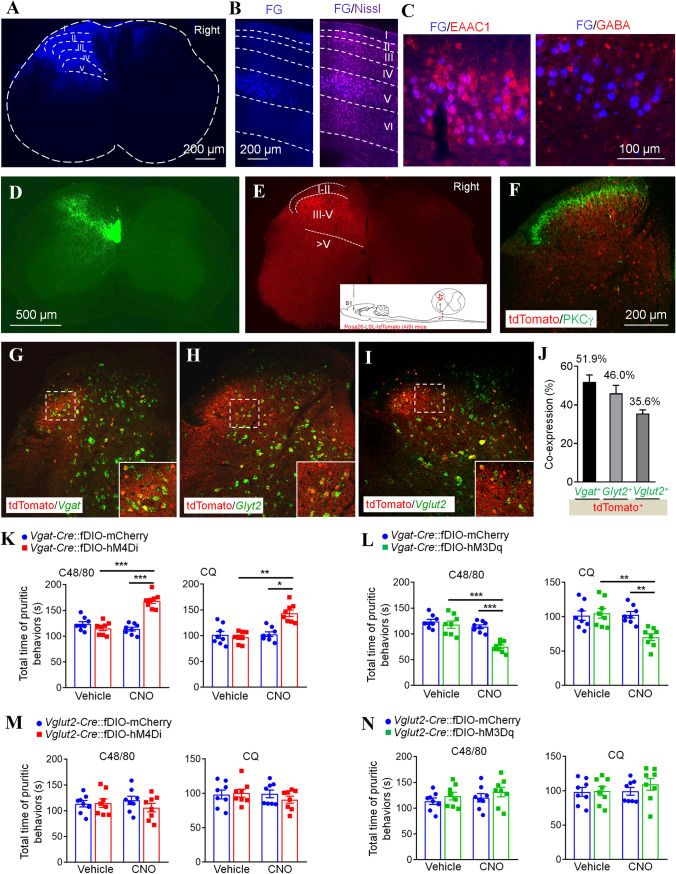
To explore the mechanism of action of the S1-SDH descending projection on itch transmission, we first examined the type of S1-SDH projection neurons. Fluorogold (FG) was injected into the left lumbar dorsal horn (Laminae I–V, Fig. 2A). Ten days later, the distribution of FG in the S1 was checked. Nissl staining showed that FG+ cells were distributed in layer V of the contralateral S1 (Fig. 2B). Further staining with the excitatory neuronal marker excitatory amino-acid carrier 1 (EAAC1) and the inhibitory neuronal marker γ-aminobutyric acid (GABA) showed that FG+ cells were largely co-localized with EAAC1 (> 95%) and very few with GABA (Fig. 2C), suggesting that S1-SDH projection neurons are mainly excitatory. We further checked the spatial distribution of S1-SDH projection neurons in S1 labeled by AAVretro-hSyn-EGFP which was intraspinally injected into the left lumbar dorsal horn. The statistical data showed that the number and percentage of EGFP+ neurons peaked at bregma − 1.06 mm (35.3% ± 1.6% of layer V neurons), and then decreased rostrally and caudally in the contralateral S1 (Fig. S4A-E).
Fig. 2.
Inhibitory interneurons of the spinal dorsal horn mediate the inhibitory effect of S1-SDH projection on itch transmission. A Fluorescence image showing a site of FG injection in the spinal dorsal horn. B Coronal brain section showing the location of FG+ neurons in the S1. C Immunostaining against EAAC1 or GABA with FG in the S1. D Image showing the S1-SDH tract terminates in the deep laminae of the spinal dorsal horn. E Representative image showing tdTomato+ post-synaptic neurons of the S1-SDH projection in the spinal dorsal horn. The insert shows the strategy for identifying spinal post-synaptic neurons of the S1-SDH projection. F Representative image showing the staining of PKCγ with tdTomato in the spinal dorsal horn. G–I In situ hybridization showing Vgat (G), Gylt2 (H), and Vglut2 (I) with tdTomato in spinal sections, and statistical data showing the percentages of tdTomato+ neuronal co-expression with Vgat, Glyt2, and Vglut2 (J). K Pharmacogenetic inhibition of spinal post-synaptic Vgat+ neurons enhances itch behaviors in response to C48/80 or CQ (n = 8). L Pharmacogenetic activation of spinal post-synaptic Vgat+ neurons alleviates itch behaviors in response to C48/80 or CQ (n = 8). M Pharmacogenetic inhibition of spinal post-synaptic Vglut2+ neurons has no effect on itch behaviors in response to C48/80 or CQ (n = 8). N Pharmacogenetic activation of spinal post-synaptic Vglut2+ neurons has no effect on itch behaviors in response to C48/80 or CQ (n = 8).
We then examined the spinal distribution of S1 projection terminals and the spinal post-synaptic neurons receiving the descending input from S1. First, we injected the anterograde tracing virus AAV2/9-hSyn-EGFP into S1 (bregma − 1.06 mm). Three weeks later, the axons were shown in laminae III–VII, with high densities in laminae III–V of the contralateral spinal dorsal horn (Fig. 2D). Next, we injected anterograde trans-monosynaptic AAV2/1-hSyn-Cre into S1 of Ai9 (Rosa26-LSL-tdTomato) mice. Consistently, tdTomato+ spinal post-synaptic neurons appeared in the contralateral spinal dorsal horn (Fig. 2E). Statistical data further showed that approximately 79.9% of tdTomato+ neurons were distributed in laminae III–V of the spinal dorsal horn, approximately 9.9% in laminae I–II, and 10.1% in laminae V–VII. The immunostaining with PKCγ showed that most of the tdTomato+ neurons were distributed ventrally to PKCγ+ neurons (Fig. 2F).
To characterize these spinal post-synaptic neurons, we then examined the co-localization of tdTomato with inhibitory neuronal probes against Vesicular GABA transporter (Vgat) or Glycine transporter 2 (Glyt2) and excitatory neuronal probes against Vesicular glutamate transporter 2 (Vglut2). In situ hybridization showed that approximately 51.9% of tdTomato+ neurons expressed the GABAergic inhibitory neuronal marker Vgat (Fig. 2G, J), 46.0% expressed the glycinergic inhibitory neuronal marker Glyt2 (Fig. 2H, J), and 35.6% expressed the glutamatergic excitatory neuronal marker Vglut2 (Fig. 2I, J). Thus, these data suggest that the majority of tdTomato+ neurons are inhibitory.
To determine whether S1 modulates itch transmission through spinal inhibitory interneurons innervated directly by S1-SDH projection neurons, we injected anterograde trans-monosynaptic AAV2/1-hSyn-flex-Flpo into the right S1 of Vgat-Cre mice, then injected AAV2/9-Efla-fDIO-hM4D(Gi)-mCherry (fDIO-hM4Di) or AAV2/9-Efla-fDIO-hM3D(Gq)-mCherry (fDIO-hM3Dq) or AAV2/9-Efla-fDIO-mCherry (fDIO-mCherry) into the left lumbar dorsal horn 1 week later (Fig. S5A–C). Pharmacogenetic inhibition of these spinal post-synaptic inhibitory interneurons by CNO application significantly enhanced the itch behaviors evoked by C48/80, compared to CNO + fDIO-mCherry (1.5 ± 0.04-fold) or CQ (1.4 ± 0.06-fold, Fig. 2K). These AAVs had no effect on the motor function in these mice (Fig. S6A). Consistently, when these spinal post-synaptic inhibitory interneurons were activated by CNO, the C48/80- or CQ-evoked itch behaviors were significantly reduced (C48/80: decreased by 34.3 ± 3.2%; CQ: decreased by 31.6 ± 4.8%, Fig. 2L). The AAV injection had no effect on the motor function in these mice (Fig. S6B). These data suggest that spinal post-synaptic VGAT+ inhibitory interneurons of S1-SDH projection neurons mediate the descending inhibition of S1 on spinal itch transmission.
To check the role of spinal post-synaptic excitatory interneurons of S1-SDH projection neurons in itch transmission, we injected anterograde trans-monosynaptic AAV2/1-hSyn-flex-Flpo into the right S1 of Vglut2-Cre mice, then injected fDIO-hM4Di or fDIO-hM3Dq or fDIO-mCherry into the left lumbar dorsal horn 1 week later (Fig. S5A-C). Surprisingly, chemogenetic activation of S1-innervated spinal VGluT2+ neurons did not affect C48/80- or CQ-induced itch behaviors, and neither did chemogenetic inhibition of S1-innervated spinal VGluT2+ neurons (Fig. 2M, N). In addition, the motor function was not defected by these chemogenetic viruses (Fig. S6C, D). These data suggest that spinal post-synaptic VGluT2+ excitatory interneurons of S1-SDH projection neurons may not be involved in the inhibition of spinal itch transmission by S1.
Recent studies have demonstrated that several areas, such as the rostral ventromedial medulla, periaqueductal gray (PAG), and anterior cingulate cortex (ACC) gate the processing of itch by top-down modulation [7]. In the present study, ablation or functional inhibition of S1-SDH projection neurons enhanced the CQ- and C48/80-induced itch behaviors. On the contrary, activation of S1-SDH projection neurons alleviated these itch behaviors, indicating the tonic inhibitory effect of S1 on spinal itch transmission. The data also suggest that the descending S1-SDH projection plays the same role in the regulation of histamine-dependent and histamine-independent itch.
Liu et al. demonstrated that transection of the corticospinal tract or ablation of hindlimb somatosensory corticospinal neurons selectively impair the behavioral responses to light touch in mice [5]. In addition, such manipulation attenuated tactile allodynia in a model of peripheral neuropathic pain, suggesting a facilitatory role of the descending S1 projection on touch or mechanical allodynia. Recent studies have also indicated a distinct role of the descending regulation on itch and pain. For example, manipulation of the Tac1+ neurons in the PAG affect itch processing, but has no effect on pain sensitivity [8]. Optogenetic inhibition of ACC inputs to the dorsal medial striatum reduces histamine-induced itch responses, but has no effect on acute pain-related behaviors. Combined with the report by Liu et al. [5], the present data indicate different roles of the descending S1-SDH projection on itch and pain.
S1-SDH projection neurons were distributed in layer V and predominantly expressed EAAC1, a glutamatergic neuronal marker, suggesting that these neurons are excitatory, which is consistent with the previous findings showing that corticospinal terminals in laminae III–IV of the spinal cord are mainly glutamate-immunopositive in rats [9]. In the spinal cord, 90% of GABAergic interneurons co-express glycine in the deep dorsal horn. Consistently, our data showed that approximately 52% of tdTomato+ neurons expressed VGAT (GABAergic neurons) and 46% of tdTomato+ neurons expressed GlyT2 (glycinergic neurons). It has been reported that chemogenetic activation of spinal GABAergic neurons or pharmacological activation of spinal α2 and α3 GABAA receptors suppresses pruritogen-induced scratching behavior [10]. In addition, activation of glycinergic neurons in spinal deep lamina also suppresses CQ- and histamine-induced scratching behavior [11]. As descending S1 neurons are excitatory, we speculate that S1 may tonically excite spinal inhibitory interneurons to release GABA or glycine to inhibit itch transmission. It has been shown that GlyT2-positive neuropil and glycine receptor α3 are densely expressed in the superficial dorsal horn, and GRPR+ itch-specific neurons are distributed in laminae I–II [12]. Thus, spinal inhibitory neurons innervated by S1 may inhibit itch signal processing via inhibiting itch-specific GRPR+ neurons. It is also possible that inhibitory neurons innervated by S1 directly regulate the function of projection neurons in the deep dorsal horn. Further studies are needed to decipher the spinal circuits involving the descending S1 regulation of spinal itch transmission.
The present study also showed that activation or inhibition of S1-innervated glutamatergic neurons in the spinal cord did not affect C48/80- or CQ-induced itch behaviors. Previous studies have shown that the excitatory interneurons contributing to itch modulation are mainly distributed in laminae I–II [12]. In the deep dorsal horn, the corticospinal tract from somatosensory cortex facilitates mechanical allodynia via excitatory cholecystokinin+ interneurons [5]. A recent study showed that S1-SDH projection neurons connect to spinal Vglut3+ interneurons to control skilled movements. In addition, activation of Vglut3+ neurons in the deep dorsal horn conveys mechanical hypersensitivity [13]. Thus, the excitatory postsynaptic neurons of the S1-SDH tract may specifically regulate pain and fine movement, but not itch.
In summary, our results demonstrate that the descending S1 projection to the spinal cord has a tonic inhibitory effect on itch transmission, probably via exciting inhibitory interneurons in the spinal cord. This descending modulation may represent an endogenous inhibitory pathway for itch transmission. In addition, it is known that itch can be modulated by psychological factors or even be induced mentally. Our results may provide a plausible explanation for this phenomenon and also suggest that activation of the S1-SDH pathway may be an effective strategy for the treatment of chronic itch.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32030048, 31970938, and 31871064) and the Natural Science Foundation of Jiangsu Province (BK20191448).
Conflict of interests
The authors declare that they have no competing financial interests.
Footnotes
Zi-Han Wu and Han-Yu Shao have contributed equally to this work.
Contributor Information
Yong-Jing Gao, Email: gaoyongjing@ntu.edu.cn.
Zhi-Jun Zhang, Email: zhzhj@ntu.edu.cn.
References
- 1.Han L, Ma C, Liu Q, Weng HJ, Cui YY, Tang ZX, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong XT, Dong XZ. Peripheral and central mechanisms of itch. Neuron. 2018;98:482–494. doi: 10.1016/j.neuron.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mochizuki H, Hernandez LE, Yosipovitch G, Sadato N, Kakigi R. The functional network processing acute electrical itch stimuli in humans. Front Physiol. 2019;10:555. doi: 10.3389/fphys.2019.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YY, Latremoliere A, Li XJ, Zhang ZC, Chen MY, Wang XH, et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature. 2018;561:547–550. doi: 10.1038/s41586-018-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry DM, Munanairi A, Chen ZF. Spinal mechanisms of itch transmission. Neurosci Bull. 2018;34:156–164. doi: 10.1007/s12264-017-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen XJ, Sun YG. Central circuit mechanisms of itch. Nat Commun. 2020;11:3052. doi: 10.1038/s41467-020-16859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao ZR, Chen WZ, Liu MZ, Chen XJ, Wan L, Zhang XY, et al. Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron. 2019;101:45–59.e9. doi: 10.1016/j.neuron.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Valtschanoff JG, Weinberg RJ, Rustioni A. Amino acid immunoreactivity in corticospinal terminals. Exp Brain Res. 1993;93:95–103. doi: 10.1007/BF00227784. [DOI] [PubMed] [Google Scholar]
- 10.Ralvenius WT, Neumann E, Pagani M, Acuña MA, Wildner H, Benke D, et al. Itch suppression in mice and dogs by modulation of spinal α2 and α3GABAA receptors. Nat Commun. 2018;9:3230. doi: 10.1038/s41467-018-05709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peirs C, Williams SP, Zhao XY, Walsh CE, Gedeon JY, Cagle NE, et al. Dorsal horn circuits for persistent mechanical pain. Neuron. 2015;87:797–812. doi: 10.1016/j.neuron.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.