Abstract
Frontotemporal dementia encompasses a group of clinical syndromes defined pathologically by degeneration of the frontal and temporal lobes. Historically, these syndromes have been challenging to diagnose, with an average of about three years between the time of symptom onset and the initial evaluation and diagnosis. Research in the field of neuroimaging has revealed numerous biomarkers of the various frontotemporal dementia syndromes, which has provided clinicians with a method of narrowing the differential diagnosis and improving diagnostic accuracy. As such, neuroimaging is considered a core investigative tool in the evaluation of neurodegenerative disorders. Furthermore, patterns of neurodegeneration correlate with the underlying neuropathological substrates of the frontotemporal dementia syndromes, which can aid clinicians in determining the underlying etiology and improve prognostication. This review explores the advancements in neuroimaging and discusses the phenotypic and pathologic features of behavioral variant frontotemporal dementia, semantic variant primary progressive aphasia, and nonfluent variant primary progressive aphasia, as seen on structural magnetic resonance imaging and positron emission tomography.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01101-x.
Keywords: Frontotemporal dementia, Frontotemporal lobar degeneration, Behavioral variant frontotemporal dementia, Semantic variant primary progressive aphasia, Semantic dementia, Nonfluent agrammatic variant primary progressive aphasia, Progressive nonfluent aphasia, Tau, FTLD-tau, TDP-43, FTLD-TDP, Neuroimaging, Magnetic resonance imaging, Positron emission tomography
Introduction
Frontotemporal dementia (FTD) is a group of clinically and pathologically heterogeneous syndromes that, historically, have been challenging to diagnose. This challenge is partly due to the rarity of these conditions and lack of familiarity by physicians, however, diagnostic accuracy is also complicated by the complexity and wide range of clinical manifestations of the various FTD syndromes. The ability to visualize the brain in vivo has been fundamental to understanding the pathophysiology of FTD and other neurodegenerative diseases. Historically, neuroimaging was used to “rule out” other potential pathologies responsible for or contribute to cognitive impairment. However, our understanding of neurodegenerative diseases and the development of new neuroimaging techniques and more sophisticated analysis methods have expanded dramatically over the past decades, ultimately leading to the identification of neuroimaging signatures associated with specific neurodegenerative diseases. These advancements have led to neuroimaging being considered a core clinical investigative tool in evaluating cognitive disorders [1–6].
Frontotemporal Dementia—Core Clinical Syndromes
Frontotemporal dementia is a spectrum of syndromes characterized by progressive deficits in behavior, language, and cognition associated pathologically with frontotemporal lobar degeneration (FTLD). Clinically, FTD is divided into three prototypical clinical syndromes based on the features most prominent early in the disease course. These three syndromes are the behavioral variant of FTD (bvFTD), the semantic variant of primary progressive aphasia (svPPA), and the nonfluent/agrammatic variant of PPA (nfvPPA).
Additionally, several other clinical syndromes with overlapping features with the core FTD symptomatology are included within the FTD clinical spectrum at large, and include FTD with motor neuron disease (FTD-MND), progressive supranuclear palsy syndrome (PSP-S), and corticobasal syndrome (CBS) [7–9]. In the case of PSP and CBS, the terminology can be confusing because of the unperfect clinical-neuropathological correspondence. An important distinction must be made between the clincal syndrome and the underlying neuropathology, as PSP-S and CBS can result from various neuropathological entities. For example, CBS is a syndrome that can result from corticobasal degeneration (CBD), Alzheimer’s disease, or GRN mutations among others, whereas CBD neuropathology can manifest as a CBS, PSP-S, bvFTD, or nfvPPA syndrome. The focus of this review will be primarily on the three prototypical clinical syndromes, bvFTD, svPPA, and nfvPPA, and the underlying pathology associated with these syndromes.
Behavioral Variant Frontotemporal Dementia
Behavioral variant FTD is the most common form of FTD and makes up more than 50% of all FTD cases [10]. It is characterized by early deficits in behavior and executive functioning [4, 11]. The mean age of onset is 58 years with a standard deviation of 11 years [4]. The incidence is 2.7–4.1 per 100,000 person-years, and the prevalence is 15–22 per 100,000 person-years [12]. The time between symptom onset and the initial evaluation is slightly over 3 years, with a standard deviation of about 3 years [4]. The duration of illness is about 8 years from the time of symptom onset, with a standard deviation of 4 years [4, 13].
The behavioral changes typically seen in bvFTD, including disinhibition, apathy, lack of empathy, perseverative or compulsive behavior, and hyperorality, often become severe as the disease progresses [4, 11]. Behavioral disinhibition occurs early in the disease course in about 76% of cases [4]. Disinhibition is often the most salient feature of bvFTD and can have various manifestations, such as impulsivity, socially inappropriate behavior, or loss of manners or decorum [4, 14, 15]. Apathy and inertia are early features in about 84% of cases [4] Apathy is characterized by a reduction in goal-directed behavior, goal-directed cognitive activity, diminished emotional reactivity, or social engagement [16–18], whereas intertia is characterized by a reduction in initiation of behavior [4]. The initial manifestations of apathy and inertia may be subtle; however, motivation and spontaneity is often increasingly apparent as the disease progresses and, in some cases, can significantly limit the patient’s ability to independently perform basic activities of daily living [16–21]. Perseverative, stereotyped, or compulsive/ritualistic behaviors occur early in the disease course in about 71% of cases [4] and may manifest as simple repetitive movements or vocalizations, such as eye blinking, throat clearing, or tapping, or more complex behaviors such as collecting certain objects, hoarding, repetitive storytelling, or frequent unnecessary trips to the restroom [4, 22–27]. Loss of empathy or sympathy is an early symptom in about 73% of cases [4], and often manifests as a lack of concern for the feelings of others or a reduction in social interaction [4, 28–31]. The indifference exhibited by those with bvFTD can lead to a great deal of emotional stress for the caregiver, often the patient’s spouse, due to difficulty connecting with the patient emotionally [21, 32, 33]. Hyperorality and dietary changes occur early in the disease course in about 59% of cases [4]. A range of disordered eating behaviors have been described in bvFTD, including increased appetite, binge eating, and changes in food preferences, often with a preference for carbohydrates. In severe cases, oral exploration and ingestion of inedible objects can occur [34–36].
Semantic Variant Primary Progressive Aphasia
The semantic variant of PPA is one of two FTD language variants and is characterized by a progressive loss of semantic knowledge of words [37]. The age of onset is typically between 55 and 70 years [38]. Information regarding the incidence and prevalence of svPPA is limited, though the available data indicates a prevalence of about 4–8 per 100,000 [38]. The svPPA entity is very close to the previously developed “semantic dementia” diagnostic category [11, 39], and most patients will fulfill both sets of criteria; see Mesulam et al. [40] for more discussion on the nuances between these concepts.
Individuals with svPPA have difficulty recalling the meaning of words, which worsens as the disease progresses over time [4]. Additionally, they often experience severe anomia, impaired comprehension of single words, and surface dyslexia and dysgraphia [4]. Notably, repetition, grammar, and motor speech are typically spared [41].
Nonfluent Variant Primary Progressive Aphasia
The nonfluent/agrammatic variant of PPA is characterized by agrammatic or effortful, halting speech with inconsistent speech sound errors and distortions [41]. The mean age of onset is about 60 years, though the age of onset is broad, ranging from about 40 to 80 years [10]. Like svPPA, incidence and prevalence data are lacking, though extrapolation of FTLD autopsy data suggests an incidence of 0.4–0.7 per 100,000 and a prevalence of 0.5–3 per 100,000 [42].
Agrammatism secondary to difficulty processing the grammatical components of speech is a core feature of nfvPPA and is the most significant contributor to nonfluent speech [43]. Other factors that impact fluency include deficits in executive functioning and impaired motor-speech planning [44, 45]. Impaired comprehension of syntactically complex sentences is also commonly observed. In contrast to svPPA, single-word comprehension and object knowledge are typically spared [41, 44].
Apraxia of speech is another core feature of nfvPPA and is characterized by deficits in motor speech planning. Difficulty with motor planning of speech impacts an individual’s ability to coordinate complex oral movements and leads to phonetic or prosodic speech abnormalities [46, 47]. It is important to note that apraxia of speech may occur as a manifestation of nfvPPA [45, 46], but may also occur in the absence of any other symptoms [46]. If apraxia of speech occurs as the sole manifestation of a neurodegenerative process, this is termed primary progressive apraxia of speech (PPAOS) [46]. PPAOS can be divided into three subtypes, reflecting the dominant clinical characteristics of affected individuals. Sound substitutions and additions characterize the phonetic subtype, and typically worsen with increased utterance length, the number of syllables, or word complexity. The prosodic subtype is characterized by syllable segmentation within multisyllabic words or across words in phrases and lengthened intersegment durations between syllables, words, or phrases [46–48]. Furthermore, nfvPPA without apraxia of speech appears to be a distinct clinical syndrome [49].
FTLD Neuropathology and Genetics
Frontotemporal lobar degeneration is a neuropathological entity characterized by neuronal loss, gliosis, and progressive neurodegeneration predominantly affecting the frontal and temporal lobes [50–52]. The pathologic protein aggregates that form in FTLD are primarily associated with three major molecular classes: TAR-DNA-binding protein 43 (FTLD-TDP), tau (FTLD-tau), and the group of fused in sarcoma protein (FUS), Ewing’s sarcoma protein (EWS), and TATA-binding protein-associated factor 15 protein (TAF15) (collectively known as FTLD-FET). These molecular classes can be further divided into specific molecular subtypes. Molecular subtypes are generally associated with more than one clinical phenotype, which presents a significant challenge when attempting to classify FTLD neuropathology with the various FTD clinical syndromes (Table 1). Furthermore, Alzheimer’s disease accounts for a small percentage of cases in each of the core FTD syndromes. The complexity between the clinical syndromes of FTD and the underlying neuropathology is demonstrated in a 2017 study by Perry et al. [53], which examined the clinical, pathologic, and neuroimaging findings in cases of bvFTD. This study showed numerous pathologic substrates, including Pick’s disease, corticobasal degeneration (CBD), PSP, FTLD-TDP type A, B, C, and D, FTLD-FUS, and Alzheimer’s disease, could result in the bvFTD clinical syndrome. Furthermore, each pathological subtype was found to be associated with partially distinct patterns of atrophy and clinical symptoms.
Table 1.
Frontotemporal dementia core clinical phenotypes
| FTD variant | Clinical features | Neuroanatomy | Most frequent FTLD pathology subtypes | Common genetic mutations |
|---|---|---|---|---|
| bvFTD | Behavioral disinhibition; apathy; loss of empathy; compulsive behavior; dietary changes; executive dysfunction | Bilateral frontal lobes and anterior temporal lobes |
FTLD-TDP type A, B, C FTLD-tau (PiD, CBD, PSP, GGT, MAPT) FTLD-FUS |
C9ORF72, GRN, MAPT, VCP |
| svPPA | Impaired confrontation naming; impaired single-word comprehension; impaired object knowledge; surface dyslexia/dysgraphia | Bilateral anterior temporal lobes, typically left > right |
FTLD-TDP type C FTLD-Tau (PiD, GGT) |
– |
| nfvPPA | Agrammatism; effortful, halting speech with speech sound errors; impaired comprehension of syntactically complex sentences | Left inferior frontal gyrus and insula |
FTLD-TDP type A FTLD-Tau (PiD, CBD, PSP) |
GRN |
bvFTD behavioral variant frontotemporal dementia, CBD corticobasal degeneration, FTLD frontotemporal lobar degeneration, GGT globular glial tauopathy, nfvPPA nonfluent/agrammatic variant primary progressive aphasia, PiD Pick’s disease, PSP progressive supranuclear palsy, svPPA semantic variant primary progressive aphasia
FTLD-TDP
The TAR-DNA-binding protein of 43 kDa (TDP-43) is a protein encoded by the TARDBP gene located on chromosome 1p36.2. TDP-43 is involved in many cellular processes, including RNA splicing, stabilization, transport, transcription, and translation [54, 55]. The majority of TDP-43 is found within the nucleus of neurons; however, it shuttles between the nucleus and cytoplasm under normal physiologic conditions, and up to 30% of TDP-43 can be found in the cytoplasm [51, 56, 57]. In FTLD-TDP, TDP-43 is translocated from the nucleus to the cytoplasm, where it aggregates and forms pathologic intracellular inclusions [51, 57]. The cortical laminar distribution and morphology of the inclusions are variable, resulting in the various FTLD subtypes [51, 58, 59]. The FTLD-TDP subtype terminology can be confusing as multiple classification schemes have been developed and used in the literature. In this review, we use the harmonized classification system proposed in 2011 by Mackenzie et al. [59]. In studies published before 2011, earlier classification systems proposed by Mackenzie et al. [60] and Sampathu et al. [61] were used.
FTLD-TDP type A is characterized pathologically by the presence of numerous short dystrophic neurites and crescentic or oval neuronal cytoplasmic inclusions concentrated primarily in upper cortical layers II/III, as well as moderate numbers of lentiform neuronal intranuclear inclusions [62–64]. The most common clinical phenotypes include nfvPPA, bvFTD, and rarely FTD-MND [62]. FTLD-TDP type B is characterized by a moderate number of neuronal cytoplasmic inclusions and sparse dystrophic neurites throughout all cortical layers [62–64]. The most common clinical phenotypes include bvFTD and FTD-MND [62]. FTLD-TDP type C is characterized by the accumulation of TDP aggregates within elongated dystrophic neurites and rare neuronal cytoplasmic inclusions [62–64]. FTLD-TDP type C is always associated with anterior temporal lobe degeneration, and therefore the most common clinical phenotypes include svPPA and bvFTD [62]. FTLD-TDP type D is characterized by numerous short dystrophic neurites and frequent lentiform neuronal intranuclear inclusions [62–64]. Type D is always associated with a multisystem proteinopathy caused by mutations in the valosin-containing protein (VCP) gene, which cause a variable phenotypical expression of inclusion-body myopathy, Paget’s disease of the bone, and FTD [65]. A fifth FTLD-TDP subtype, type E, was described in 2017 by Lee et al. [62] in which a series of seven cases demonstrated granulofilamentous neuronal inclusions and grain-like deposits in all neocortical layers that did not meet the 2011 classification scheme proposed by Mackenzie et al. [59].
FTLD-Tau
Microtubule-associated protein tau (Tau) is a protein encoded by the MAPT gene located on chromosome 17q21 and is involved in the assembly and stabilization of microtubules [66–68]. Six tau isoforms are generated from MAPT through alternative mRNA splicing of exons 2, 3, and 10. Exon 10 encodes one of the repeat-containing sequences of the microtubule-binding domain, and alternative splicing gives rise to tau isoforms containing either three (3R) or four (4R) microtubule-binding repeats, each of which group contains three isoforms [67, 69–71].
In pathologic states, hyperphosphorylation of tau weakens the interaction between tau and the microtubule, causing tau to dissociate from the microtubule and form into insoluble aggregates [69, 72]. The composition of these aggregates depends on the ratio of tau isoforms, the degree of hyperphosphorylation, and multiple other posttranslational modifications [73]. Morphologically, these aggregates may consist of paired helical filaments, straight filaments, or twisted filaments [74–78]. The neuropathologic changes in Alzheimer’s disease include the formation of neuronal tau aggregates consisting of a mixture of 3R and 4R tau isoforms, which form into paired helical filaments and straight filaments [79, 80]. In non-Alzheimer’s disease tauopathies, tau inclusions are associated with tau protofilaments that have unique, disease-specific atomic structures [81]. As such, the histopathological findings can vary widely between 3 and 4R tauopathies, and between sporadic and inherited tauopathies secondary to MAPT mutations [82, 83]. Although the neuropathology of tauopathies may vary, neuronal and glial (both astrocytic and oligodendroglial) cytoplasmic tau inclusions are the neuropathological hallmarks of disease seen in all cases of tau-related neurodegenerative disorders [68, 70].
FTLD can result from multiple neuropathologically distinct tauopathies associated with 3R, 4R, or mixed 3R/4R tau isoforms. In addition, mutated forms of tau protein aggregate in inherited tauopathies are associated with MAPT mutations that lead to a change in the primary structure of tau. Pick’s disease (PiD) is a sporadic tauopathy characterized pathologically by the pathognomonic rounded intracytoplasmic inclusions (Pick bodies) composed of 3R tau [84, 85]. Tau astrocytic inclusions, named ramified astrocytes, are typical of Pick’s disease. Ballooned neurons (Pick cells) and tau oligodendroglial inclusions (coiled bodies) are also found; however, these are not specific features of Pick’s disease [85]. The most common clinical syndromes associated with Pick’s disease are bvFTD [53], nfvPPA, and svPPA [86].
The 4R tauopathies are comprised of progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), globular glial tauopathy (GGT), and argyrophilic grain disease (AGD). Progressive supranuclear palsy is a pathological entity primarily affecting subcortical regions such as the midbrain, subthalamic nucleus, globus pallidus, and dentate nucleus of the cerebellum, and variable disease spreading across various neocortical regions, particularly within the frontal lobe. The pathognomonic pathological hallmarks are tufted astrocytes. Neuronal cytoplasmic inclusions acquiring a globose shape (globose neurofibrillary tangles of PSP) are also seen in regions particularly vulnerable to degeneration, such as the globus pallidus and subthalamic nucleus. Oligodendroglial coiled bodies are commonly seen in the affected cortical and subcortical white matter [87]. The classic clinical presentation, Richardson syndrome (PSP-RS), manifests as atypical parkinsonism with axial rigidity and postural instability leading to falls, bradykinesia, vertical supranuclear gaze palsy, and a dysexecutive cognitive syndrome [88]. Other FTD syndromes may arise from PSP neuropathology, including bvFTD [53], nfvPPA [86], and CBS [89].
Corticobasal degeneration is a pathological entity often associated with asymmetric cortical degeneration and characterized pathologically by 4R tau-immunoreactive astrocytic plaques and thread-like neuritic pathology in gray and white matter. Neuronal cytoplasmic inclusions, ballooned neurons, and coiled bodies are typically present together with substantia nigra neuronal loss [90, 91]. The classic clinical syndrome associated with CBD, CBS, often presents with limb or axial rigidity, bradykinesia, dystonia, cognitive and behavioral impairment, limb apraxia, cortical sensory loss, and alien limb syndrome [92]. However, it is important to remember that CBS is only associated with CBD pathology in about a third of cases [92], with most cases being caused by other neuropathological entities, mainly AD and PSP neuropathologies. Additionally, CBD can result in other FTD clinical syndromes, including bvFTD [53, 93], nfvPPA [86, 93], and PSP-S [93].
Argyrophilic grain disease (AGD) is tauopathy characterized neuropathologically by 4R tau-positive spindle-shaped inclusions in neuronal dendrites and axons (argyrophilic grains), pre-neurofibrillary tangles in neurons, and coiled bodies in oligodendrocytes [94]. Ballooned neurons may also be found similar to those in Pick’s disease and CBD, however, ballooned neurons are localized predominantly to limbic regions in AGD, whereas in Pick’ disease and CBD, they are also found in the frontal and parietal cortices [95]. GGT is characterized pathologically by 4R tau-positive globular astrocytic and oligodendroglial inclusions [96]. This rare pathological entity is associated with multiple clinical syndromes, though more commonly with bvFTD and bvFTD-MND [96–98].
aFTLD-U
Atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions (aFTLD-U) is a relatively rare cause of FTD characterized by FUS-positive inclusions, and is the underlying pathology in about 5% of all bvFTD cases [99]. In cases of aFTLD-U, the age of onset is very early, often occurring before 40 years. While patients typically meet clinical criteria for bvFTD, the clinical presentation is distinct from other variants of bvFTD, and is characterized by severe progressive psychobehavioural abnormalities, often with severe disinhibition, apathy, compulsions, and aggressive behavior [53, 100]. Cognitive dysfunction, aphasia, and motor features are less common at the time of the initial presentation [100].
Cases of aFTLD-U demonstrate severe degeneration of the frontal and temporal lobes, hippocampal CA1 and subiculum, striatum, globus pallidus, and substantia nigra [99]. It is characterized histopathologically by small, round, neuronal cytoplasmic inclusions that are immunoreactive for FUS [99, 100].
Genetics
Frontotemporal dementia is predominantly a sporadic disease; however, about 30–40% of cases have a strong familial history [101, 102], though heritability varies by the clinical syndrome [102]. About 15% of FTD cases demonstrate an autosomal dominant pattern of inheritance [102], with the majority being due to a pathogenic expansion in chromosome 9 open reading frame 72 (C9ORF72), or a mutation in microtubule-associated protein tau (MAPT), or progranulin (GRN) genes [102, 103]. C9ORF72 pathogenic expansion is associated with a unique form of FTLD, which, together with features of FTLD-TDP type A or B or often unclassifiable as intermediate between type A and B, present unique pathological hallmarks in forms of intranuclear RNA-foci and cytoplasmic ubiquitinated dipeptide repeat (DPR) inclusions [104]. C9ORF72 pathogenic expansion is more commonly associated with bvFTD, MND, or bvFTD-MND. GRN is invariably associated with FTLD-TDP type A neuropathology and can lead to bvFTD, nfvPPA, and CBS [86, 105–107]. Finally, the various pathogenic MAPT mutations are associated with distinct tauopathies that may be predominately characterized by 3R, 4R, or a mixture of 3R and 4R inclusions. With the exception of the intronic mutation and silent mutations, the MAPT mutation that change the primary structure of tau leads to the deposition of mutant tau species. The neuropathology associated with these cases, while often resembling prototypical CBD, PSP, or Pick’s disease, should be regarded as fundamentally different from the one observed in sporadic tauopathies.
Neuroimaging of Frontotemporal Dementia Syndromes
Neuroimaging Modalities
Neuroimaging modalities used in assessing neurodegenerative disorders fall into broad categories, structural, functional, and molecular imaging. Structural imaging modalities commonly used include computed tomography (CT) and structural magnetic resonance imaging (MRI), which allow for the visualization of neuroanatomy. Functional imaging encompasses a broad variety of modalities such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and functional MRI (fMRI), which measure various parameters such as metabolic activity, regional blood flow, or hemodynamic changes while the patient is either at rest or performing a specific task. In addition, molecular imaging refers to techniques that measure molecular and cellular events in living organisms (e.g., specific receptors or protein aggregates). Of all neuroimaging tools, structural MRI and PET are two of the most commonly used in the assessment of FTD, both in the clinical and research setting. As such, this review will focus primarily on these two techniques.
Magnetic Resonance Imaging
MRI works by utilizing a strong magnet that forces protons in the body to align parallel or antiparallel to the magnetic field vector. A radiofrequency pulse is applied, which disrupts the alignment of protons along the magnetic field vector, causing them to spin out of equilibrium. The radiofrequency pulse is then stopped, and the protons realign with the magnetic field, releasing energy in the process. The length of time that it takes for protons to realign with the magnetic field, and the amount of energy released during the process, can be measured and varies between tissue types. These variations can then be displayed and differentiated based on signal intensity [108].
MRI is a widely used imaging modality in both the clinical and research setting. In the clinical setting, MRIs are typically analyzed visually by a trained radiologist. However, in the research setting, automated computational techniques are commonly employed to assess brain structure. One of the most common approaches to assessing the volume of specific brain regions (“regions of interest”), determined a priori. Other approaches, such as voxel-based morphometry [109] or cortical thickness analyses [110], allow running exploratory analyses of the entire brain structure.
Positron Emission Tomography
Positron emission tomography (PET) is a nuclear imaging technique that allows for three-dimensional mapping of physiologic processes in vivo. PET imaging works on the principle of beta plus (β+) decay, a type of radioactive decay in which a proton in an unstable radioisotope undergoes conversion to a neutron, resulting in the emission of a positron and an electron neutrino. The emitted positron collides with an electron within the tissue, resulting in the annihilation of the particles and emission of two photons, which a PET scanner can detect. The PET image is then displayed as a three-dimensional image with different intensity levels corresponding to the radiotracer concentration [111, 112]. Many radiotracers have been developed to target a number of proteins, including neuroreceptors, reuptake transporters, synaptic proteins, enzymes, and aggregated proteins such as amyloid-beta and tau [113].
PET is often utilized to measure the degree of neuronal metabolic activity in vivo through the use of the radiotracer 18F-fluoro-2-deoxyglucose (FDG), a glucose analog labeled with an 18F radioisotope. FDG is readily taken up by metabolically active cells, and the degree of FDG uptake can be assessed by PET to determine the extent of cellular metabolic activity. Decreased levels of FDG uptake suggest hypometabolism of the brain region being assessed, indicating potential dysfunction. Although FDG-PET only characterizes the relative metabolism of brain regions and does not provide direct information regarding the etiology of disease, regional patterns of hypometabolism can help identify specific neurodegenerative diseases and underlying pathology [111, 112].
PET radiotracers have been developed to visualize pathological proteins in vivo. Much of the research regarding neurodegenerative diseases has focused on small-molecule PET radiotracers that bind to amyloid-beta or tau. The first radiotracer selective for Aβ, [11C]Pittsburgh Compound-B (PiB), was developed in the early 2000s at the University of Pittsburgh [114, 115]. The Pittsburgh group demonstrated that small-molecule PET radiotracers were effective in characterizing the burden of amyloid beta in vivo [116], paving the way for the development of additional small-molecule PET radiotracers, including [18F]Florbetapir and [18F]Florbetaben, which have been used extensively in the research setting to characterize Alzheimer’s disease [117].
About a decade after PiB was developed, PET radiotracers targeting tau with a high degree of specificity were demonstrated. To date, most studies have utilized first-generation tau radiotracers, namely [18F]Flortaucipir (formerly known as AV1451 or T807), [18F]THK5117, [18F]THK5351, and [11C]PBB3. These radiotracers readily bind to intracellular and extracellular tau, neuritic plaques, and ghost tangles [118, 119]; however, off-target binding to molecules other than tau is a major issue with first-generation radiotracers [118, 119]. Numerous second-generation tau radiotracers have been developed to improve the binding specificity to tau and have reduced the degree of off-target binding [119].
Typical Neuroimaging Features of the Core FTD Phenotypes
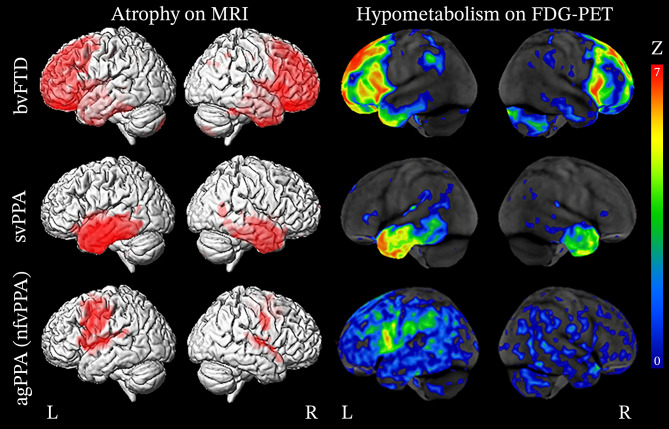
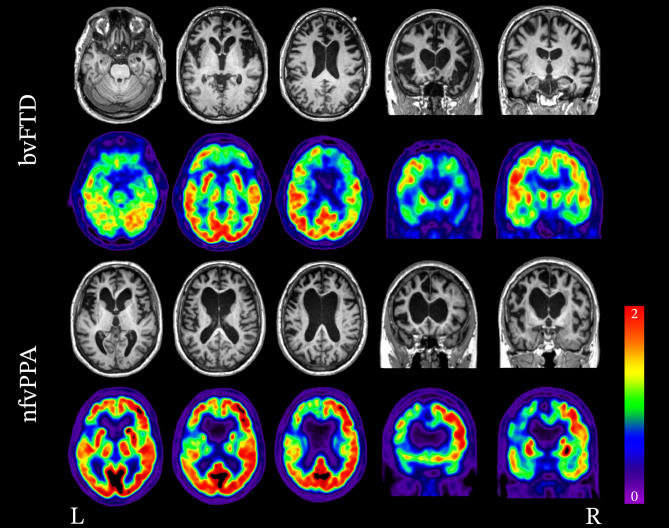
This section describes the general patterns of atrophy and hypometabolism described in patients grouped based on their clinical diagnoses (Fig. 1).
Fig. 1.
Patterns of atrophy and hypometabolism associated with FTD clinical syndromes. Group-level maps depicting gray matter atrophy compared to controls are shown on three-dimensional renders of the brain. Hypometabolism on FDG-PET is shown for individual patients, represented as Z score maps depicting abnormalities compared to controls. Adapted with permission from Whitwell 2019 [258]
Behavioral Variant Frontotemporal Dementia
Behavioral variant FTD is associated with degeneration of the prefrontal cortex (PFC), anterior temporal lobes, limbic, and subcortical regions [120, 121]. Early in the disease course, von Economo neurons in the anterior insula and anterior cingulate cortex are selectively vulnerable [122]. These regions represent the epicenter of pathology in bvFTD and are often the first to demonstrate structural and metabolic neuroimaging abnormalities [123–125]. Pathological protein aggregates are thought to spread to other regions by propagating along brain networks in a prion-like manner, leading to disease progression [123–129]. In general, atrophy is asymmetric and typically involves the right hemisphere more than the left as demonstrated on structural neuroimaging [53, 130]. However, a recent study by Irwin et al. [131] examining the asymmetry of post-mortem neuropathology in bvFTD showed that the while the right orbitofrontal cortex was generally more atrophic than the left, the left ventrolateral temporal lobe was generally more atrophic than the right, particularly in cases of FTLD-tau [131].
Structural MRI may reveal atrophy of the anterior insula and anterior cingulate cortex in the earliest stages of the disease before patients fulfill the criteria for a diagnosis of bvFTD [121, 124, 132–139]. As the disease progresses into the mild stages, additional areas become increasingly involved. The frontal lobes are widely impacted and typically exhibit degeneration of the frontal poles, dorsolateral PFC, medial PFC, orbitofrontal cortex, and premotor cortex [134–137, 140]. The anterior temporal lobes, limbic structures, including the hippocampus and amygdala, as well as the thalamus and striatum, are also commonly impacted [135–138, 140]. In the later stages of the disease, atrophy becomes more pronounced and involves the contralateral hemisphere to a greater degree, widely affecting the frontal and temporal lobes [133, 138, 140–142]. Atrophy may also extend posteriorly to involve the parietal lobes and cerebellum [140, 141].
In cases where structural imaging is equivocal, FDG-PET can potentially help determine if there is underlying neuronal dysfunction. FDG-PET typically demonstrates hypometabolism of the frontal and temporal lobes and is generally spatially consistent with atrophy patterns, with larger effect sizes [143]. Hypometabolism can often be found impacting the frontal lobes, particularly in the medial PFC, dorsolateral PFC, ventrolateral PFC, orbitofrontal cortex, and anterior cingulate cortex [138, 144, 145]. The anterior and inferior aspect of the temporal lobes are commonly impacted and extends to the posterior fusiform gyrus [138, 145]. The medial temporal lobe is also commonly involved and hypometabolism of associated limbic structures, including the hippocampus and amygdala [138, 145], as well as the caudate and thalamus [144, 145]. In the later stages of the disease, further hypometabolism is demonstrated in the regions initially impacted [138, 142, 146], as well as the frontal poles, supplementary motor area, middle temporal gyri, posterior cingulate cortex, precunei, inferior parietal lobes, lateral superior occipital cortex, and cerebellum [138, 146].
Semantic Variant Primary Progressive Aphasia
The hallmark of svPPA on structural MRI is asymmetric degeneration of the anterior temporal lobes, impacting the language-dominant cerebral hemisphere (typically the left) more than the non-language dominant hemisphere. In the early stages of disease, atrophy typically involves the medial temporal lobe, specifically the hippocampus, amygdala, fusiform gyrus, and the inferior and middle temporal gyri, which exhibit a strong anterior–posterior gradient [147]. Atrophy can also be observed in the medial PFC, orbitofrontal cortex, pars opercularis, insula, and striatum [138, 148]. As the disease progresses, brain involvement becomes increasingly diffuse with more widespread atrophy of the frontal lobes, parietal lobes, and to a lesser degree, the occipital lobe. Specific areas of involvement include the superior and middle frontal gyrus, anterior cingulate cortex, posterior cingulate cortex, parietal operculum, precuneus, angular gyrus, supramarginal gyrus, superior parietal lobule, occipital fusiform gyrus, lingual gyrus, and the superior and inferior lateral occipital cortex [138].
Metabolic imaging with FDG-PET reveals hypometabolism in regions where atrophy is typically demonstrated on structural imaging [149, 150]. The most significant degree of hypometabolism is seen in the inferior, middle, and superior regions of the anterior temporal lobes, extending posteriorly to the temporoparietal junction, more so on the left than the right [151–154]. Other affected regions include the orbitofrontal cortex, anterior cingulate cortex, insula, amygdala, subiculum, entorhinal cortex, and hippocampus [154, 155].
In a study by Bejanin et al. [138], FDG-PET revealed diffuse hypometabolism throughout the brain in the mild stages of disease (Clinical Dementia Rating (CDR) 0.5), sparing only the occipital lobes. Specifically, hypometabolism was noted in the frontal lobes (frontal pole, orbitofrontal cortex, medial PFC ventrolateral PFC, dorsolateral PFC, and frontal operculum), temporal lobes (inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus, planum polare and temporale, transverse temporal gyrus, and fusiform gyrus, extending posteriorly to the temporoparietal junction), parietal lobes (angular gyrus, supramarginal gyrus, and parietal operculum), and subcortical structures (anterior cingulate cortex, insula, hippocampal complex, striatum, and thalamus). Interestingly, hypometabolism was present in regions without atrophy, including the frontal poles, dorsolateral PFC, pars triangularis, angular gyrus, supramarginal gyrus, parietal operculum, and the thalamus. In later stages of disease (CDR 1–2), additional areas were increasingly involved, including the precentral and postcentral gyri, supplementary motor area, superior frontal gyrus, posterior cingulate cortex, and superior parietal lobule, all of which demonstrated a significantly greater degree of hypometabolism than would be expected given the degree of atrophy.
Nonfluent/Agrammatic Variant Primary Progressive Aphasia
The region of the earliest involvement in nfvPPA is found in the left inferior frontal gyrus (pars triangularis and pars opercularis) [124, 148, 156]. Additional areas of atrophy observed early in the disease course include the insula, supplementary motor area, premotor cortex, and motor cortex [152, 157]. Other areas of involvement during the disease course include the medial PFC, dorsolateral PFC, orbitofrontal cortex, medial and lateral temporal lobe, striatum, and thalamus [138, 157–159]. Additionally, some studies have demonstrated that compared to individuals with mixed agrammatism and apraxia of speech, those who exhibit predominantly agrammatism have more pronounced atrophy and hypometabolism in the prefrontal and anterior temporal lobes [46–49, 160].
Hypometabolic regions seen with FDG-PET are most significant in the left ventrolateral PFC, including the pars opercularis and pars triangularis, dorsolateral PFC, medial PFC, and supplementary motor area [138, 152]. Other regions where hypometabolism is observed include the orbitofrontal cortex, anterior cingulate cortex, insula, precentral and postcentral gyrus, parietal operculum, striatum, and thalamus [152].
Neuroimaging of FTLD Neuropathologic Subtypes
Numerous studies have demonstrated specific patterns of atrophy correlating with the various neuropathologic subtypes of FTLD-TDP and FTLD-tau. In clinical syndromes associated with multiple neuropathologic subtypes, such as bvFTD, the different atrophy and metabolic patterns of each subtype can help narrow the differential diagnosis and predict the underlying neuropathology. Importantly, genetic mutations can substantially impact the pattern of atrophy across the FTLD subtypes. Genetics will be discussed briefly, however, for a more in-depth discussion regarding the role of genetic mutations in FTLD, we recommend the recent review by Häkkinen et al. [161].
FTLD-TDP
FTLD-TDP Type A
FTLD-TDP type A is associated with a variety of clinical presentations, namely bvFTD, nfvPPA, and to a lesser extent, CBS. In imaging studies of patients with FTLD-TDP type A, atrophy and hypometabolism has been found to be widespread and asymmetric (either left- or right-predominant), involving the frontal, temporal, and parietal lobes [162, 163]. A number of retrospective analyses exploring clinicopathologic and neuroimaging features in patients with FTLD-TDP type A have found similar patterns of atrophy. The most significantly impacted regions include the dorsal frontal lobes, anterior, medial, and posterior temporal lobes, inferior parietal lobes, orbitofrontal cortex, insula, frontal operculum, caudate, and thalamus (Fig. 2) [53, 89, 162, 163]. There is particular involvement of the anterior insula, frontal operculum, and parietal lobes, which can help differentiate FTLD-TDP type A from other FTLD-TDP subtypes [53, 89, 163]. Among the various FTLD pathological entities, FTLD-TDP type A is the strongest association with hippocampal sclerosis.
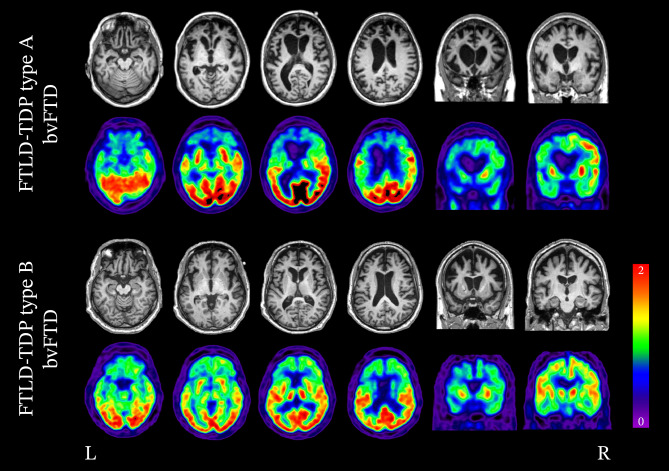
Fig. 2.
Neuroimaging of bvFTD associated with FTLD-TDP type A and B (top row: structural MRI; bottom row: FDG-PET). The variability in patterns of degeneration attributed to FTLD-TDP neuropathology is readily seen in these two cases of bvFTD. In the case of bvFTD associated with FTLD-TDP type A, degeneration and hypometabolism of the bilateral frontal lobes are present; however, the left is significantly more impacted than the right and extends to the left parietal lobe. In bvFTD associated with FTLD-TDP type B, significant degeneration of the bilateral frontal lobes is seen; however, in contrast to type A, the parietal lobes are less affected and atrophy is relatively symmetrical
Although FTLD-TDP type A often occurs sporadically, it is also the prototypical neuropathology of inherited FTLD associated with GRN mutations. Mutations in GRN often result in a haploinsufficiency of the progranulin protein and progressive neurodegeneration [60, 164–167].
Atrophy is typically widespread and asymmetric. A 2015 study by Rohrer et al. [168] involving 45 asymptomatic GRN carriers from the Genetic Frontotemporal Dementia Initiative (GENFI) cohort observed atrophy of the insula 15 years before expected symptom onset, in the temporal and parietal lobes 10 years before expected onset, and the striatum 5 years before expected onset. With progression of disease, atrophy increasingly impacts the dorsolateral PFC, ventromedial PFC, insula, anterior cingulate cortex, superior and lateral temporal lobes, striatum, and lateral and medial parietal lobes [164, 169, 170]. Atrophy of the medial parietal lobes is particularly characteristic of GRN mutations and may help differentiate GRN from other genetic subtypes [107, 164, 169, 170].
FTLD-TDP Type B
FTLD-TDP type B is typically associated with bvFTD and FTD-MND [51, 60, 171–173]. Atrophy and hypometabolism are relatively symmetric and involve the medial and lateral frontal lobes (Fig. 2) [53, 162, 163]. Limbic and subcortical structures are also significantly involved, namely the anterior cingulate, anterior insula, hippocampi, striatum, and thalamus [53, 162, 163]. At a group level, atrophy of the parietal lobes is more pronounced in FTLD-TDP type B when compared to type C. Additionally, type B has the least amount of temporal lobe atrophy when compared to the other FTLD-TDP subtypes [163].
The most common genetic cause of FTD-MND is a hexanucleotide repeat expansion within the C9ORF72 gene, which is also the most common genetic cause of FTD, and of amyotrophic lateral sclerosis (ALS) [174, 175]. C9ORF72 most commonly results in FTLD-TDP type B pathology, though type A pathology also occurs to a lesser degree [176]. The presence of a neuropathology with mixed type A and type B features, or unclassifiable features is also common. The most distinctive feature of FTLD-TDP linked to C9ORF72 remains the presence of DPR cytoplasmic inclusions and intranuclear RNA foci inclusions. These inclusions are thought to precede TDP-43 deposition and are the only pathological hallmarks found in some cases [177]. Among C9ORF72 carriers who develop a neurodegenerative disease, bvFTD occurs in about 48%, FTD-MND in about 36%, and ALS in about 16% [176]. Neuroimaging often reveals early atrophy and hypometabolism of subcortical structures, including the thalamus, hippocampus, amygdala, and striatum. Cortical atrophy and hypometabolism are often mild, but widespread and symmetric. Regions commonly found to be involved include the orbitofrontal cortex, dorsolateral PFC, ventromedial PFC, anterior temporal lobes, anterior and posterior cingulate cortex, superior and inferior parietal lobes, and cerebellum. Impacted regions that help differentiate cases of C9ORF72 from other genetic subtypes include the thalamus, inferior parietal lobe, and superior cerebellum [161, 170, 176]. In a group of 16 patients, including a majority of patients with FTLD-TDP type B pathology and 7 cases with a C9ORF72 mutation, Pasquini et al. showed that the severity of TDP pathology measured in von Economo neurons and fork cells in the right frontal insula was correlated with the severity of antemortem atrophy in the salience network [178].
FTLD-TDP Type C
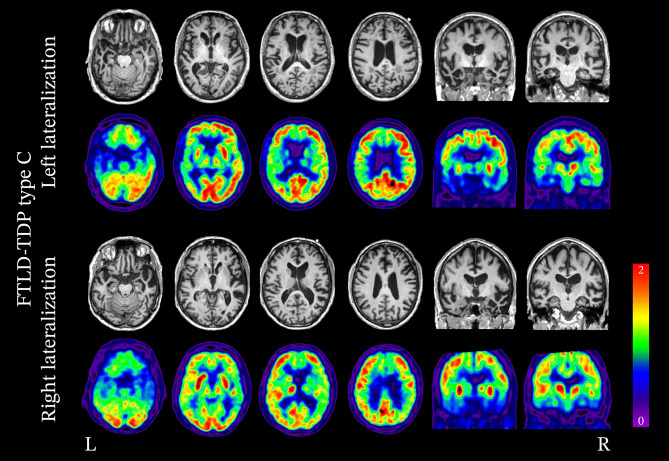
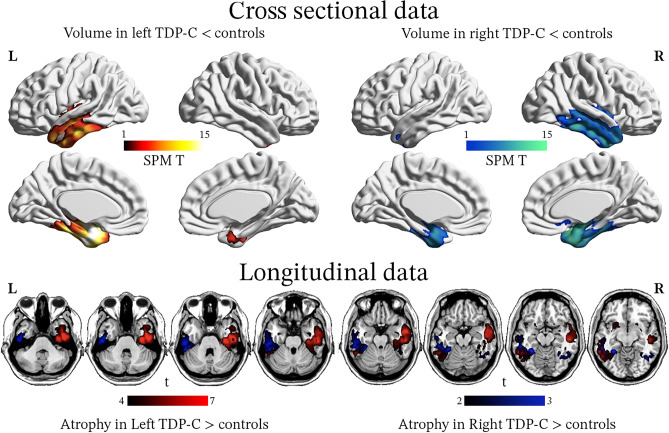
FTLD-TDP type C is strongly associated with asymmetric, degeneration of the medial aspect of the anterior temporal lobes, specifically the temporal pole, fusiform gyrus, lingual gyrus, and amygdalo-hippocampal area (Fig. 3) [179, 180]. In the majority of cases where imaging abnormalities are predominant in the language-dominant (most commonly the left hemisphere) temporal lobe, the clinical diagnosis is svPPA [53, 163]. The retrospective study of 30 patients with FTLD-TDP type C pathology and available ante mortem MRI from Borghesani et al. showed that 12 cases (40%) demonstrated lateralization of atrophy to the non-language dominant hemisphere [179]. This pattern was associated with the onset of behavioral symptoms preceding language impairment [179, 181, 182]. Regardless of whether the disease is initially lateralized to the right or left hemisphere, atrophy progresses in a stereotypical pattern [179]. Initially, it can be detected in one of the anterior temporal lobes, then progresses to both the ipsilateral posterior temporal areas and the contralateral temporal pole (Fig. 4), and later involves the orbitofrontal cortex. However, the greatest degree of atrophy is found in the anterior temporal lobes throughout the disease course [179].
Fig. 3.
Neuroimaging patterns associated with FTLD-TDP type C. FTLD-TDP type C typically exhibits significant anterior temporal lobe degeneration, which strongly lateralizes to either the left or right hemisphere. In cases of left lateralization, the clinical syndrome is typically consistent with svPPA, whereas in cases of right lateralization, there is a greater degree of behavioral symptoms, and language deficits are not typically the initial symptoms
Fig. 4.
Gray matter atrophy associated with FTLD-TDP type C: left and right variants. All patients had pathology-proven TDP-C but were classified as having either a left- (n = 18) or right- (n = 12) predominant pattern of atrophy on ante mortem MRIs. At baseline, both groups show asymmetric but bilateral volume reduction in the temporal lobes, with a strong predominance in anterior and medial areas. In patients with multiple MRIs (n = 13 left- and 4 right-predominant cases), longitudinal analyses revealed that in both subgroups, atrophy progressed to the contralateral hemisphere and to more posterior temporal areas. Adapted with permission from Borghesani 2020 [179]
FTLD-Tau
The Three-Repeat Tauopathy Pick’s Disease
Pick’s disease (PiD) is the only sporadic 3R tauopathy FTLD. It is primarily associated with bvFTD, though nfvPPA, svPPA, and CBS can also occur [183–185]. In general, neuroimaging in PiD demonstrates striking atrophy, often described as “knife-edge” due to its severity (Fig. 5), affecting the frontal and temporal lobes, specifically the dorsolateral PFC, ventromedial PFC, temporal poles, and the anteromedial and anterolateral regions of the temporal lobes, with often remarkable sparing of the peri-Rolandic gyri. Subcortical structures, including the anterior cingulate cortex, anterior insula, posterior insula, and caudate, are also impacted, while the substantia nigra is fairly spared from neurodegeneration compared to the degree observed in 4R tauopathies such as CBD and PSP [186–188].
Fig. 5.
Neuroimaging patterns associated with Pick’s disease (FTLD-tau, 3R). Structural MRI and FDG-PET demonstrating the variability in patterns of atrophy and hypometabolism attributed to Pick’s disease (3R tau) neuropathology. In the case of bvFTD, significant bilateral frontal lobe atrophy and hypometabolism is seen. In the case of nfvPPA, atrophy and hypometabolism is lateralized and is greatly impacting the left frontal lobe more so than the right.
In cases of bvFTD, circumscribed frontotemporal atrophy is present throughout the frontal and anterior temporal lobes [187]. In a recent study by Whitwell et al. [189] of 17 individuals with PiD (8 with bvFTD, 6 with nfvPPA, 1 with svPPA, 1 with unclassified PPA, and 1 with CBS), those with bvFTD showed a striking degree of predominantly right-sided atrophy involving the insula, anterior cingulate cortex, and orbitofrontal cortex. Over time, atrophy increasingly involved the frontal poles, anterior and middle cingulate gyri, medial frontal lobe, gyrus rectus, orbitofrontal cortex, inferior and middle frontal gyri, temporal poles, inferior and middle temporal lobes, fusiform gyrus, parahippocampal gyrus, right anterior hippocampus, precuneus, right angular and supramarginal gyri, and bilateral basal ganglia. Similar findings have been described in other studies [53, 89, 187, 188].
In cases of nfvPPA, the left inferior frontal gyrus, middle frontal gyrus, insula, and supplemental motor area are significantly atrophic. Other regions of atrophy include the precentral gyrus and orbitofrontal gyrus bilaterally [86, 189].
Semantic variant PPA can also be associated with PiD, although very rarely. A study by Spinelli et al. [86] of 29 individuals with svPPA, of which only two had PiD, found that the anterior cingulate cortex and striatum were affected to a greater degree in PiD compared to that seen in FTLD-TDP type C. Additionally, frontal lobe and subcortical atrophy was more substantial when compared to other pathologies associated with svPPA [86]. Specific regions most impacted in those with svPPA due to PiD include the bilateral anterior temporal lobes, orbitofrontal cortex, inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, anterior cingulate cortex, insula, striatum, left middle temporal gyrus, and fusiform gyrus [86].
Four-Repeat Tauopathies
Multiple 4R tauopathies may lead to FTD, including PSP, CBD, GGT, and AGD. PSP and CBD are, by far, the most common 4R tauopathies resulting in FTD syndromes. The epicenters of PSP neuropathology are heterogeneous, leading to a variety of clinical syndromes associated with PSP neuropathology. PSP most commonly presents as Richardson syndrome (PSP-RS), although it may manifest as parkinsonism (PSP-P), pure akinesia with gait freezing (PSP-PAGF), CBS (PSP-CBS), bvFTD (PSP-bvFTD), or nfvPPA (PSP-nfvPPA). The degree of brainstem degeneration is greater in PSP-RS, PSP-P, and PSP-PAGF and is associated with pronounced atrophy of the midbrain and superior cerebellar peduncles, which are characteristic findings on structural MRI [190, 191]. Additionally, several neuroimaging findings have been proposed as suggestive of PSP pathology, including the “hummingbird sign,” “Mickey Mouse sign,” and “morning glory sign.” However, their sensitivity in clinical practice is limited [192, 193]. The hummingbird sign is seen on midline sagittal T1 sequences as atrophy of the midbrain, resulting in a flattening or concavity of the superior aspect of the midbrain, which is normally convex [194, 195]. The Micky Mouse sign refers to a reduction in the anterior–posterior diameter of the midbrain at midline when viewed in the axial plane [195, 196]. The morning glory sign refers to a concavity of the lateral margin of the tegmentum of the midbrain when viewed in the axial plane [197].
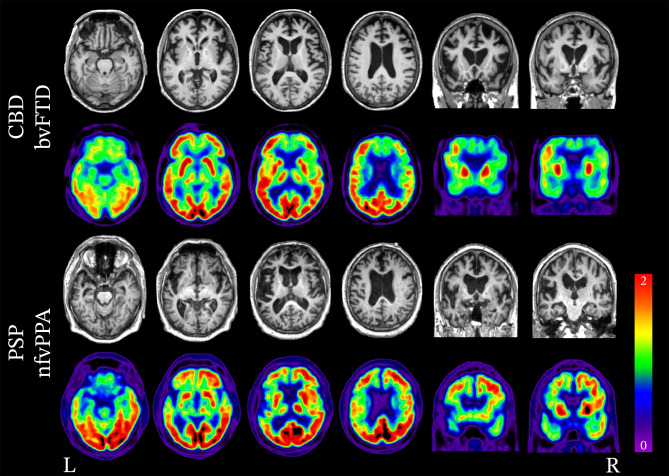
In cases of PSP-bvFTD, frontal atrophy is often relatively mild, and the temporal lobes are almost always spared, whereas posterior cerebellar atrophy is prominent and might help distinguish PSP-bvFTD from other subtypes [53]. Atrophy in CBD-bvFTD (Fig. 6) tends to be more frontal than that seen in PSP. It involves the dorsolateral and medial frontal regions, with involvement of both the precentral and postcentral gyri, distinguishing CBD-bvFTD from other subtypes [53]. In cases of nfvPPA, the left inferior frontal gyrus is the epicenter of disease and is atrophic in both PSP and CBD. PSP-nfvPPA exhibits a greater degree of atrophy in the bilateral precentral and middle frontal gyri, supplemental motor area, dorsal midbrain, and right cerebellar regions compared to CBD-nfvPPA. In CBD-nfvPPA, atrophy is distributed more widely and found in the left insula, putamen, supplemental motor area, and the left precentral, middle, and inferior frontal gyri [198]. In a group of 26 patients with underlying 4R tau pathology (PSP or CBD) and various clinical diagnoses (including bvFTD), Spina et al. [199] showed strong associations between grey matter volumes measured on antemortem MRI and neuropathological measures at autopsy. More specifically, they showed that higher scores of tau inclusion burden and neurodegeneration (a composite measure of neuronal loss, astrogliosis, and microvacuolations) were partly independently predictive of lower grey matter volumes.
Fig. 6.
Neuroimaging patterns associated with 4R tauopathies (FTLD-tau). Structural MRI and FDG-PET demonstrating the variability in patterns of atrophy and hypometabolism attributed to 4R tauopathies, CBD and PSP
FTLD-tau caused by MAPT mutation is overall characterized by early and severe symmetric bilateral temporal lobe degeneration, with early involvement of the anterior cingulate gyrus, orbitofrontal, and fronto-insular regions. Early degeneration of the hippocampal formation is common [83, 200].
Atypical Frontotemporal Lobar Degeneration with Ubiquitin-Positive Inclusions (aFTLD-U)
In cases of aFTLD-U, neuroimaging shows extensive atrophy of the bilateral frontal and temporal lobes [53, 201–203]. A small case series of 2 patients by Rohrer et al. [202] showed atrophy predominantly affecting the orbitofrontal cortex, insula, anteromedial temporal lobe, anterior cingulate, and caudate. An independent analysis of 3 cases with aFTLD-U showed that caudate atrophy was major and exceed the degree of atrophy seen in FTLD-TDP and FTLD-tau subtypes (n = 10 in each group) [203].
Amyloid- and Tau-PET in FTD and FTLD
Amyloid-PET to Detect AD Neuropathology
The emergence of amyloid plaque-binding radiotracers in the mid-2000s has made it possible to detect the defining features of AD neuropathology in living patients. PET-to-autopsy studies have shown that elevated amyloid-PET signal is highly specific to amyloid neuropathology: false-positive scans (i.e., high cortical PET signal in the absence of amyloid deposits) are rare [204–206]. Yet, the presence of amyloidosis is not always equivalent to clinically relevant AD neuropathology, which also requires AD tau tangles [207]: Braak III or above for intermediate level of AD neuropathology changes, Braak V or VI for high level. Moreover, amyloid-PET positivity is not always associated with clinical deficits as it is frequently found in clinically normal individuals, especially at an older age [208].
Amyloid-PET positivity is relatively rare (< 15%) in patients with a clinical diagnosis of bvFTD, svPPA, or nvfPPA [209–212], in line with autopsy studies of FTD showing a low frequency of underlying AD neuropathology (see earlier section). However, as previously mentioned, amyloid-PET positivity should be interpreted with caution, as it does not necessarily indicate that AD neuropathology is the main or sole etiology of a clinical syndrome. In cases with FTD, additional imaging information might help estimate the likelihood of underlying AD neuropathology versus “incidental” amyloidosis. Indeed, multiple groups have reported cases with amyloid-PET positivity in patients with an FTD syndrome who also showed typical FTD-like patterns of atrophy or glucose metabolism (not the posterior, temporo-parietal pattern of degeneration seen in AD). In these cases, autopsy typically showed mixed neuropathology, with the co-occurrence of FTLD and AD [213–215].
Tau-PET in AD, FTLD-Tau, and FTLD-TDP
Radiotracers developed for tau-PET have originally been developed to detect AD-type tau pathology (i.e., paired helical filaments composed of 3R/4R tau). In vivo studies have shown that tau-PET distinguishes amyloid-positive patients with a clinical diagnosis of AD dementia from patients with FTD syndromes with high accuracy [216]. Recent PET-to-autopsy studies [217–219] have consistently reported elevated Flortaucipir-PET signal in patients with advanced AD tau pathology (usually Braak stages IV or V), and the FDA recently approved Flortaucipir for the estimation of aggregated tau neurofibrillary tangles in adult patients with cognitive impairment who are being evaluated for AD [220].
In addition to the ability to distinguish AD from other processes, several groups have used tau-PET in patients with FTD syndromes to assess its usefulness in distinguishing FTLD-tau pathology from other types of neuropathology. Early studies validating Flortaucipir on brain tissues have found little or no binding to straight filaments in non-AD tauopathies, and there is no binding to other abnormal protein deposits such as alpha-synuclein or TDP-43 [221–225]. In vivo studies have reported conflicting findings. On the one hand, Flortaucipir binding has not been observed in patients with pathology-proven FTLD-tau (e.g., Pick’s disease and CBD) [189, 217], or in groups of patients with suspected FTLD-tau (e.g., patients with nfvPPA or some patients with bvFTD) [226–229]. In these patients, Flortaucipir signal is mild (lower than what can be observed in AD) and often found in the subcortical white matter underlying frontal, cingulate, and insula cortices, and in the putamen and globus pallidus. On the other hand, similar mild Flortaucipir PET signal has been extensively reported in most patients with svPPA [227, 228, 230, 231], while the vast majority of patients are expected to harbor FTLD-TDP type C pathology, as described in previous sections. In these patients, Flortaucipir PET signal is seen in the anterior temporal lobes, in areas showing atrophy/hypometabolism. To date, the biological underpinnings of the tracer accumulation in this degenerative tissue is not understood [232, 233], but it should be noted that Flortaucipir is known to have multiple sources of “off-target” binding [119, 223, 228, 234–237].
Recently, Soleimani-Meigooni et al. published a case series of 20 patients who underwent in vivo Flortaucipir PET and autopsy, including 12 with a neuropathological diagnosis of FTLD [217]. The authors showed that a mild increase in Flortaucipir-PET binding was not a reliable indicator of FTLD-tau pathology: such signal was observed in patients with underlying 4R tau pathology (PSP, CBD) but also in a patient with bvFTD and underlying TDP-A pathology due to a GRN mutation. In this patient, tau immunohistochemistry was negative in the areas showing high Flortaucipir signal in vivo.
Finally, a few studies have used tau-PET in patients with MAPT mutations [228, 238–240]. Overall, elevated Flortaucipir-PET signal was observed in a subset of patients with specific mutations that result in a mix of 3R/4R tau pathology, namely V337M and R406W. This observation is not tracer-specific as tau-PET binding has also been observed in R406W mutation carriers with [18F]RO848, a “second generation” tau radiotracer [119]. However, Soleimani-Meigooni et al. [217] reported Flortaucipir binding in a patient carrying an S305I mutation associated with 4R tau pathology, suggesting that tau-PET could not clearly differentiate between FTLD-tau subtypes.
Neuroimaging Heterogeneity Within FTD Clinical Phenotypes: Data-Driven Approaches
In parallel to the aforementioned studies looking at neuroimaging patterns in groups of patients based on their neuropathological subtype, another approach has emerged: using neuroimaging data itself to identify subgroups of patients. This approach has gained much interest in the Alzheimer’s field in recent years [241, 242], and some groups have applied similar data-driven methods to patients within specific FTD syndromes.
bvFTD
In those with a clinical diagnosis of bvFTD, the patterns of neurodegeneration are quite heterogenerous. A 2009 study by Whitwell et al. [130] of 66 subjects diagnosed with bvFTD found four distinct anatomical subtypes based on the pattern of atrophy; all were associated with significant behavioral abnormalities, though memory, executive function, and language deficits were variable across subtypes. The frontal dominant subtype (n = 21) showed atrophy largely restricted to the frontal lobes, though the parietal lobes were also affected to a mild degree. The frontotemporal subtype (n = 12) also demonstrated a substantial degree of frontal atrophy and mild parietal atrophy, but also demonstrated significant atrophy of the temporal lobes, whereas the frontal dominant subtype did not. These two subtypes were associated with similar deficits in executive functioning, but the frontotemporal subtype had greater memory and language deficits compared to the frontal dominant subtype. The temporofrontoparietal subtype (n = 27), as the name suggests, showed frontoparietal atrophy with additional involvement of the medial temporal lobes, though frontal lobe atrophy was not as severe as that seen in the frontal dominant and frontotemporal subtypes. Executive functioning deficits were less severe in the temporofrontoparietal subtype compared to the frontal dominant and frontotemporal subtypes, and mamory and language deficits were similar to that seen in the frontotemporal subtype. The temporal dominant subtype (n = 6) showed atrophy that was entirely localized to the temporal lobes and much more pronounced than the other three subtypes. Memory and language deficits were most prominent in the temporal dominant subtype, whereas executive functioning deficits were mild.
A retrospective observational study by Ranasinghe et al. [243] classified 90 subjects who met criteria for bvFTD based on their patterns of gray matter volume using a principal component analysis using 18 regions of interest. Four subtypes were identified, with atrophy patterns mainly overlapping with the salience network (n = 21 with a predominant frontal/temporal pattern, n = 27 with a predominant frontal pattern), which corresponded to the frontal dominant and frontotemporal subtypes described by Whitwell et al. Other patients had atrophy located in the semantic appraisal network (n = 8), comparable to Whitwell’s temporal dominant subtype or predominant subcortical atrophy (n = 30). In general, the frontotemporal and temporal subtypes had the greatest frequency of most core diagnostic features of bvFTD, though executive dysfunction was much more prominent in the frontal and subcortical subtypes. A subset of 24 patients had available neuropathological data, with limited statistical power to detect differences. Yet, it should be noted that each of the four subtypes included both patients with FTLD-tau and FTLD-TDP43, suggesting that these imaging-based clusters were not simply reflective of neuropathological subtypes.
In 2016, Cerami et al. [244] conducted a retrospective study of 52 subjects who fulfilled Rascovsky [4] criteria for probable bvFTD, demonstrating that clinical phenotypes are correlated with specific FDG-PET patterns at the individual level. In this study, two distinct subtypes were found based on FDG-PET, a frontal (n = 25) and temporolimbic (n = 27) subtype. Both subtypes were associated with hypometabolism of the insula, anterior cingulate cortex, ventromedial PFC, nucleus accumbens, and thalamus. However, the frontal subtype demonstrated more pronounced hypometabolism of the PFC, whereas the temporolimbic subtype was predominantly demonstrated hypometabolism of the medial temporal lobe. The neuropsychological profiles of these two subtypes showed similar degrees of impaired empathy/sympathy, socioemotional deficits, and disinhibition and apathy, though isolated apathy was more prevalent in the frontal subtype and isolated disinhibition was present only in the temporolimbic subtype. Executive dysfunction and immediate recall were significantly more impaired in the frontal subtype, whereas delayed recall was more impaired in the temporal subtype. It is important to note that these subtypes have not been studied in pathology-confirmed cases, so it is unclear whether the differences seen are related to different neuropathological subtypes, or another causes of variability.
svPPA
In cases of svPPA, atrophy is predominantly asymmetric and affects the anterior temporal lobe of the language-dominant cerebral hemisphere (generally the left hemisphere) in most cases. The neuropathological process causing this syndrome affects the non-language dominant cerebral hemisphere in about 40% of cases [132, 245]. When the non-language dominant anterior temporal lobe is affected, patients’ early presentation differs from svPPA. Rather than presenting as language impairment, early symptoms typically include impairment of facial recognition, loss of semantic knowledge regarding specific people, and behavioral changes similar to those seen in bvFTD [132, 181, 245, 246]. Regardless of which hemisphere is primarily affected, symptoms increasingly overlap as the disease progressively involves the contralateral hemisphere [247].
nfvPPA
In cases of nfvPPA, structural and metabolic imaging reveals a left-predominant decline in the frontal lobe involving the pars opercularis, insula, middle frontal gyrus, and supplementary motor area [86]. In a 2019 study by Matias-Guiu et al. [248], it was found that in those with nfvPPA, two subtypes could be differentiated based on clustering of FDG-PET hypometabolism. In the first cluster, involvement of the left frontal lobe was predominant, impacting the left superior and inferior frontal gyri, insula, anterior cingulate, left caudate, and the left middle and medial frontal gyri. The second cluster revealed hypometabolism in the left superior, middle, medial, and inferior frontal gyri as well, but also in the left precentral and right superior frontal gyri. Interestingly, while both subtypes were associated with agrammatic or effortful, halting speech, only the second subtype was associated with apraxia of speech.
Summary and Perspectives
Frontotemporal dementia is an umbrella term for several clinical syndromes, including bvFTD, svPPA, and nfvPPA, that are clinically,pathologically, and radiologically heterogeneous. Neuroimaging plays a key role in evaluating patients with FTD and can help clarify the clinical syndrome and underlying pathology, particularly in cases with distinct patterns of atrophy strongly associated with a specific pathology. However, it should be noted that the patterns of neurodegeneration are indirect markers of pathologic subtypes and are not necessarily diagnostic for a specific clinical syndrome. For example, FTLD-TDP type A is commonly associated with both bvFTD and nfvPPA. As such, the clinical manifestations must be considered in addition to the neuroimaging findings. In addition, the current literature on the association between imaging and neuropathology is based on small patient samples, with most groups being smaller than 20 patients, or even smaller than 5 patients when comparing neuropathological subtypes [249]. Consequently, most published studies had limited statistical power to detect group differences: when comparing two groups of 15 patients each using a two-tailed two sample t-test, one can expect to detect effect sizes ≥ 1.06 (with a power of 80% and α = 0.05). It should therefore be acknowledged that only large effect sizes have been reliably identified and that the absence of a statistically significant difference in a given measure or brain region should be interpreted with caution, recognizing the likelihood of false negative findings.
In FTLD-TDP, the three subtypes responsible for the majority of cases (A, B, and C) show group-level differences in patterns of neurodegeration. Type A is associated with asymmetric degeneration of the frontotemporal lobes as well as involvement of the parietal lobes and manifests most often as bvFTD or nfvPPA. Type B is associated with relatively symmetric medial and lateral frontal lobe atrophy as well as significant involvement of subcortical structures and manifests most often as bvFTD. Type C is associated with severe, asymmetric atrophy of the left or right anterior temporal lobes with a strong anterior–posterior gradient. Type C manifests as svPPA if the language dominant hemisphere is predominantly affected, or as impairment of facial recognition, loss of semantic knowledge regarding specific people, and behavioral changes similar to those seen in bvFTD if the non-language dominant hemisphere is predominantly affected.
In FTLD-tau, Pick’s disease, PSP, and CBD are responsible for the majority of cases. Pick’s disease (3R tau) is associated with a striking degree of frontotemporal atrophy generally beyond that seen in the other FTLD variants and manifests most commonly as bvFTD though nfvPPA, svPPA, and CBS also occur. PSP-bvFTD is most commonly associated with atrophy of the posterior cerebellum with relatively mild frontal lobe atrophy and sparing of the temporal lobes. CBD-bvFTD is associated with widespread atrophy symmetrically involving the prefrontal cortex, peri-Rolandic cortex, and striatum. In PSP-nfvPPA and CBD-nfvPPA, atrophy predominantly affects the left interior frontal gyrus, though atrophy in CBD-nfvPPA is distributed more widely.
In cases of aFTLD-U, there is extensive atrophy of the bilateral frontal and temporal lobes and caudate. Caudate atrophy is typically more severe compared to FTLD-TDP and FTLD-tau variants. Patients commonly develop symptoms before 40 years of age and typically meet clinical criteria for bvFTD.
In spite of these group-level patterns, there is an emerging focus on relatively unexplored factors driving heterogeneity within clinically or pathologically defined groups, or in the association between neuroimaging and clinical symptoms. A recent study by Illán-Gala et al. [250] found that females had a greater degree of atrophy compared to males while having similar clinical characteristics, suggesting that females have a greater degree of cognitive reserve. Another recent study by Lee et al. [251] explored the differences in neuroimaging features between early- and late-onset nfvPPA. The authors found that those with early-onset nfvPPA had a greater reduction of cortical thickness of the left perisylvian, lateral and medial prefrontal, temporal, posterior cingulate, and precuneus regions, despite having the same degree of clinical impairment. This observation might be due to differences in neuropathological subtype between early and late-onset nfvPPA or by a higher cognitive resilience in younger patients.
Despite the recent focus on these previously unexplored factors, there are many areas in which further studies are needed. One area that is significantly understudied is the impact of linguistic, geographic, social, and ethnoracial factors on neurodegenerative diseases, particularly regarding their neuroimaging signature. This is due to the fact that most of the neuroimaging research is being conducted in western countries, where study samples are not reflective of the overall population with an over-representation of white and educated individuals that biases neuroimaging findings [252]. As such, few studies have explored the associations between ethnoracial factors and structural or functional imaging, leaving a glaring gap in our knowledge of how these diseases might affect different groups [253, 254]. Lastly, the development of data-driven methods to directly identify subgroups of patients within a given clinically or even neuropathologically defined subtype [241, 255, 256] together with the constitution of large multicenter cohorts and data sharing opportunities [257] will help us better characterize the heterogeneity of FTLD pathophysiological processes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors thank Amelia Strom for her help in the early stages of the literature review.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Disclosures
Authors report no disclosure. Drs. Spina and La Joie are supported by the National Institute of Health (K08-AG052648, K99-AG065501).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, et al. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. 2012;19(9):1159–1179. doi: 10.1111/j.1468-1331.2012.03784.x. [DOI] [PubMed] [Google Scholar]
- 6.Young PNE, Estarellas M, Coomans E, Srikrishna M, Beaumont H, Maass A, et al. Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res Ther. 2020;12(1):49. doi: 10.1186/s13195-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljubenkov PA, Miller BL. A Clinical Guide to Frontotemporal Dementias. Focus Am Psychiatr Publ. 2016;14(4):448–464. doi: 10.1176/appi.focus.20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olney NT, Spina S, Miller BL. Frontotemporal Dementia. Neurol Clin. 2017;35(2):339–374. doi: 10.1016/j.ncl.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finger EC. Frontotemporal Dementias. Contin Minneap Minn. 2016 Apr;22(2 Dementia):464–89. [DOI] [PMC free article] [PubMed]
- 10.Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62(6):925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 11.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/WNL.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 12.Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci. 2011;45(3):330–335. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal S, Ahmed RM, D’Mello M, Foxe D, Kaizik C, Kiernan MC, et al. Predictors of survival and progression in behavioural variant frontotemporal dementia. Eur J Neurol. 2019;26(5):774–779. doi: 10.1111/ene.13887. [DOI] [PubMed] [Google Scholar]
- 14.Miller B, Llibre Guerra JJ. Frontotemporal dementia. Handb Clin Neurol. 2019;165:33–45. doi: 10.1016/B978-0-444-64012-3.00003-4. [DOI] [PubMed] [Google Scholar]
- 15.Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85(8):865–870. doi: 10.1136/jnnp-2013-306948. [DOI] [PubMed] [Google Scholar]
- 16.Robert P, Onyike CU, Leentjens AFG, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Robert P, Lanctôt KL, Agüera-Ortiz L, Aalten P, Bremond F, Defrancesco M, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71–76. doi: 10.1016/j.eurpsy.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex N Y N 1991. 2006 Jul;16(7):916–28. [DOI] [PubMed]
- 19.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 20.Clarke DE, Ko JY, Lyketsos C, Rebok GW, Eaton WW. Apathy and cognitive and functional decline in community-dwelling older adults: results from the Baltimore ECA longitudinal study. Int Psychogeriatr. 2010;22(5):819–829. doi: 10.1017/S1041610209991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrilees J, Dowling GA, Hubbard E, Mastick J, Ketelle R, Miller BL. Characterization of apathy in persons with frontotemporal dementia and the impact on family caregivers. Alzheimer Dis Assoc Disord. 2013;27(1):62–67. doi: 10.1097/WAD.0b013e3182471c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moheb N, Charuworn K, Ashla MM, Desarzant R, Chavez D, Mendez MF. Repetitive Behaviors in Frontotemporal Dementia: Compulsions or Impulsions? J Neuropsychiatry Clin Neurosci. 2019;31(2):132–136. doi: 10.1176/appi.neuropsych.18060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosso SM, Roks G, Stevens M, de Koning I, Tanghe HLJ null, Kamphorst W, et al. Complex compulsive behaviour in the temporal variant of frontotemporal dementia. J Neurol. 2001 Nov;248(11):965–70. [DOI] [PubMed]
- 24.Mitchell E, Tavares TP, Palaniyappan L, Finger EC. Hoarding and obsessive-compulsive behaviours in frontotemporal dementia: Clinical and neuroanatomic associations. Cortex. 2019;121:443–453. doi: 10.1016/j.cortex.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Ames D, Cummings JL, Wirshing WC, Quinn B, Mahler M. Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci. 1994;6(2):100–113. doi: 10.1176/jnp.6.2.100. [DOI] [PubMed] [Google Scholar]
- 26.Nyatsanza S, Shetty T, Gregory C, Lough S, Dawson K, Hodges JR. A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74(10):1398–1402. doi: 10.1136/jnnp.74.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez MF, Perryman KM, Miller BL, Swartz JR, Cummings JL. Compulsive behaviors as presenting symptoms of frontotemporal dementia. J Geriatr Psychiatry Neurol. 1997;10(4):154–157. doi: 10.1177/089198879701000405. [DOI] [PubMed] [Google Scholar]
- 28.Carr AR, Mendez MF. Affective Empathy in Behavioral Variant Frontotemporal Dementia: A Meta-Analysis. Front Neurol [Internet]. 2018 [cited 2020 Mar 11];9. Available from: 10.3389/fneur.2018.00417/full [DOI] [PMC free article] [PubMed]
- 29.Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, et al. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci [Internet]. 2014 [cited 2020 Mar 11];6. Available from: 10.3389/fnagi.2014.00262/full [DOI] [PMC free article] [PubMed]
- 30.Mendez MF, Carr AR, Jimenez EE, Riedel BC, Thompson PM. Impaired Empathy Versus General Hypoemotionality in Frontotemporal Dementia. J Neuropsychiatry Clin Neurosci. 2019;31(4):378–385. doi: 10.1176/appi.neuropsych.18090202. [DOI] [PubMed] [Google Scholar]
- 31.Rankin KP, Kramer JH, Miller BL. Patterns of Cognitive and Emotional Empathy in Frontotemporal Lobar Degeneration. Cogn Behav Neurol. 2005;18(1):28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol. 2013;26(3):174–184. doi: 10.1177/0891988713495448. [DOI] [PubMed] [Google Scholar]
- 33.Pomponi M, Ricciardi L, La Torre G, Fusco D, Morabito B, Ricciardi D, et al. Patient’s Loss of Empathy Is Associated With Caregiver Burden. J Nerv Ment Dis. 2016;204(9):717–722. doi: 10.1097/NMD.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 34.Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dement Basel Switz. 1995;6(4):195–199. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- 35.Mendez MF, Licht EA, Shapira JS. Changes in Dietary or Eating Behavior in Frontotemporal Dementia Versus Alzheimer’s Disease. Am J Alzheimers Dis Dementiasr. 2008;23(3):280–285. doi: 10.1177/1533317507313140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolley JD, Gorno-Tempini M-L, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 37.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 38.Coyle-Gilchrist ITS, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonner MF, Ash S, Grossman M. The New Classification of Primary Progressive Aphasia into Semantic, Logopenic, or Nonfluent/Agrammatic Variants. Curr Neurol Neurosci Rep. 2010;10(6):484–490. doi: 10.1007/s11910-010-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mesulam M-M, Coventry C, Bigio EH, Geula C, Thompson C, Bonakdarpour B, et al. Nosology of Primary Progressive Aphasia and the Neuropathology of Language. In: Ghetti B, Buratti E, Boeve B, Rademakers R, editors. Frontotemporal Dementias [Internet]. Cham: Springer; 2021 [cited 2021 Jan 18]. p. 33–49. (Advances in Experimental Medicine and Biology; vol. 1281). Available from: 10.1007/978-3-030-51140-1_3 [DOI] [PMC free article] [PubMed]
- 41.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11(6):545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;75(7):588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tee BL, Gorno-Tempini ML. Primary progressive aphasia: a model for neurodegenerative disease. Curr Opin Neurol. 2019;32(2):255–265. doi: 10.1097/WCO.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy JR, Josephs KA. The diagnosis and understanding of apraxia of speech: why including neurodegenerative etiologies may be important. J Speech Lang Hear Res JSLHR. 2012;55(5):S1518–1522. doi: 10.1044/1092-4388(2012/11-0309). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81(4):337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utianski RL, Duffy JR, Clark HM, Strand EA, Botha H, Schwarz CG, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018;184:54–65. doi: 10.1016/j.bandl.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tetzloff KA, Duffy JR, Clark HM, Utianski RL, Strand EA, Machulda MM, et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain. 2019;142(8):2466–2482. doi: 10.1093/brain/awz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brun A, Liu X, Erikson C. Synapse loss and gliosis in the molecular layer of the cerebral cortex in Alzheimer’s disease and in frontal lobe degeneration. Neurodegeneration. 1995;4(2):171–177. doi: 10.1006/neur.1995.0021. [DOI] [PubMed] [Google Scholar]
- 51.Mackenzie IRA, Neumann M. Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem. 2016;138(S1):54–70. doi: 10.1111/jnc.13588. [DOI] [PubMed] [Google Scholar]
- 52.Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017 Jul 5;9(7):a028035. [DOI] [PMC free article] [PubMed]
- 53.Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329–3345. doi: 10.1093/brain/awx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratti A, Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem. 2016;138(Suppl 1):95–111. doi: 10.1111/jnc.13625. [DOI] [PubMed] [Google Scholar]
- 55.Buratti E, Baralle FE. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010;7(4):420–429. doi: 10.4161/rna.7.4.12205. [DOI] [PubMed] [Google Scholar]
- 56.Ederle H, Dormann D. TDP-43 and FUS en route from the nucleus to the cytoplasm. FEBS Lett. 2017;591(11):1489–1507. doi: 10.1002/1873-3468.12646. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami I, Arai T, Hasegawa M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol (Berl). 2019;138(5):751–770. doi: 10.1007/s00401-019-02077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol (Berl). 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol (Berl). 2011;122(1):111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackenzie IRA, Baborie A, Pickering-Brown S, Plessis DD, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl). 2006;112(5):539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, et al. Pathological Heterogeneity of Frontotemporal Lobar Degeneration with Ubiquitin-Positive Inclusions Delineated by Ubiquitin Immunohistochemistry and Novel Monoclonal Antibodies. Am J Pathol. 2006;169(4):1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee EB, Porta S, Baer GM, Xu Y, Suh E, Kwong LK, et al. Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol (Berl). 2017;134(1):65–78. doi: 10.1007/s00401-017-1679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol (Berl). 2009;117(1):15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan RH, Shepherd CE, Kril JJ, McCann H, McGeachie A, McGinley C, et al. Classification of FTLD-TDP cases into pathological subtypes using antibodies against phosphorylated and non-phosphorylated TDP43. Acta Neuropathol Commun. 2013;1(1):33. doi: 10.1186/2051-5960-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Obeidi E, Al-Tahan S, Surampalli A, Goyal N, Wang AK, Hermann A, et al. Genotype-phenotype study in patients with valosin-containing protein mutations associated with multisystem proteinopathy. Clin Genet. 2018;93(1):119–125. doi: 10.1111/cge.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 67.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33(1):95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 68.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol (Berl). 2017;133(5):665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mandelkow E-M, Mandelkow E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb Perspect Med. 2012 Jul 1;2(7):a006247. [DOI] [PMC free article] [PubMed]
- 70.Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol. 2015;41(1):24–46. doi: 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fá M, Puzzo D, Piacentini R, Staniszewski A, Zhang H, Baltrons MA, et al. Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory. Sci Rep. 2016;6(1):19393. doi: 10.1038/srep19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell. 2020;183(6):1699–1713.e13. doi: 10.1016/j.cell.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hernández F, Avila J. Tauopathies. Cell Mol Life Sci CMLS. 2007;64(17):2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chong FP, Ng KY, Koh RY, Chye SM. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell Mol Neurobiol. 2018;38(5):965–980. doi: 10.1007/s10571-017-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray ME, Kouri N, Lin W-L, Jack CR, Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther. 2014;6(1):1. doi: 10.1186/alzrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crowther RA, Goedert M. Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol. 2000;130(2–3):271–279. doi: 10.1006/jsbi.2000.4270. [DOI] [PubMed] [Google Scholar]
- 78.Goedert M, Eisenberg DS, Crowther RA. Propagation of Tau Aggregates and Neurodegeneration. Annu Rev Neurosci. 2017;25(40):189–210. doi: 10.1146/annurev-neuro-072116-031153. [DOI] [PubMed] [Google Scholar]
- 79.Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547(7662):185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goedert M, Falcon B, Zhang W, Ghetti B, Scheres SHW. Distinct Conformers of Assembled Tau in Alzheimer’s and Pick’s Diseases. Cold Spring Harb Symp Quant Biol. 2018;83:163–171. doi: 10.1101/sqb.2018.83.037580. [DOI] [PubMed] [Google Scholar]
- 81.Scheres SH, Zhang W, Falcon B, Goedert M. Cryo-EM structures of tau filaments. Curr Opin Struct Biol. 2020;64:17–25. doi: 10.1016/j.sbi.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Götz J, Halliday G, Nisbet RM. Molecular Pathogenesis of the Tauopathies. Annu Rev Pathol Mech Dis. 2019;14(1):239–261. doi: 10.1146/annurev-pathmechdis-012418-012936. [DOI] [PubMed] [Google Scholar]
- 83.Spina S, Farlow MR, Unverzagt FW, Kareken DA, Murrell JR, Fraser G, et al. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain. 2008;131(Pt 1):72–89. doi: 10.1093/brain/awm280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mann DMA, Snowden JS. Frontotemporal lobar degeneration: Pathogenesis, pathology and pathways to phenotype. Brain Pathol. 2017;27(6):723–736. doi: 10.1111/bpa.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J Mol Neurosci MN. 2011;45(3):384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, et al. Typical and atypical pathology in primary progressive aphasia variants: Pathology in PPA Variants. Ann Neurol. 2017;81(3):430–443. doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44(11):2015–2019. doi: 10.1212/WNL.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 88.Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord Off J Mov Disord Soc. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134(9):2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol. 2011;7(5):263–272. doi: 10.1038/nrneurol.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kouri N, Murray ME, Hassan A, Rademakers R, Uitti RJ, Boeve BF, et al. Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011;134(11):3264–3275. doi: 10.1093/brain/awr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Armstrong RA, Hamilton RL, Mackenzie IRA, Hedreen J, Cairns NJ. Laminar distribution of the pathological changes in sporadic frontotemporal lobar degeneration with transactive response (TAR) DNA-binding protein of 43 kDa (TDP-43) proteinopathy: a quantitative study using polynomial curve fitting. Neuropathol Appl Neurobiol. 2013;39(4):335–347. doi: 10.1111/j.1365-2990.2012.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131(6):1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 95.Yokota O, Miki T, Ikeda C, Nagao S, Takenoshita S, Ishizu H, et al. Neuropathological comorbidity associated with argyrophilic grain disease. Neuropathology. 2018;38(1):82–97. doi: 10.1111/neup.12429. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, et al. Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol (Berl). 2013;126(4):537–544. doi: 10.1007/s00401-013-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rösler TW, Tayaranian Marvian A, Brendel M, Nykänen N-P, Höllerhage M, Schwarz SC, et al. Four-repeat tauopathies. Prog Neurobiol. 2019 Sep;180:101644. [DOI] [PubMed]
- 98.Liu AJ, Chang JE, Naasan G, Boxer AL, Miller BL, Spina S. Progressive supranuclear palsy and primary lateral sclerosis secondary to globular glial tauopathy: a case report and a practical theoretical framework for the clinical prediction of this rare pathological entity. Neurocase. 2020;26(2):91–97. doi: 10.1080/13554794.2020.1732427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132(Pt 11):2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mackenzie IRA, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131(Pt 5):1282–1293. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- 101.Rohrer JD, Guerreiro R, Vandrovcova J, Uphill J, Reiman D, Beck J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73(18):1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood EM, Falcone D, Suh E, Irwin DJ, Chen-Plotkin AS, Lee EB, et al. Development and Validation of Pedigree Classification Criteria for Frontotemporal Lobar Degeneration. JAMA Neurol. 2013;70(11):1411–1417. doi: 10.1001/jamaneurol.2013.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deleon J, Miller BL. Frontotemporal dementia. Handb Clin Neurol. 2018;148:409–430. doi: 10.1016/B978-0-444-64076-5.00027-2. [DOI] [PubMed] [Google Scholar]
- 104.Vatsavayai SC, Nana AL, Yokoyama JS, Seeley WW. C9orf72-FTD/ALS pathogenesis: evidence from human neuropathological studies. Acta Neuropathol (Berl). 2019;137(1):1–26. doi: 10.1007/s00401-018-1921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moreno F, Indakoetxea B, Barandiaran M, Alzualde A, Gabilondo A, Estanga A, et al. “Frontotemporoparietal” dementia: Clinical phenotype associated with the c.709–1G>A PGRN mutation. Neurology. 2009;73(17):1367–1374. doi: 10.1212/WNL.0b013e3181bd82a7. [DOI] [PubMed] [Google Scholar]
- 106.Yu C-E, Bird TD, Bekris LM, Montine TJ, Leverenz JB, Steinbart E, et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol. 2010;67(2):161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Grafman J, et al. Corticobasal syndrome associated with the A9D Progranulin mutation. J Neuropathol Exp Neurol. 2007;66(10):892–900. doi: 10.1097/nen.0b013e3181567873. [DOI] [PubMed] [Google Scholar]
- 108.Dale BM, Brown MA, Semelka RC, editors. MRI: Basic Principles and Applications, 5th Edition [Internet]. 5th edition. Chichester, West Sussex ; Hoboken, NJ: Wiley-Blackwell; 2015. 248 p. Available from: 10.1002/9781119013068
- 109.Whitwell JL. Voxel-Based Morphometry: An Automated Technique for Assessing Structural Changes in the Brain. J Neurosci. 2009;29(31):9661–9664. doi: 10.1523/JNEUROSCI.2160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seiger R, Ganger S, Kranz GS, Hahn A, Lanzenberger R. Cortical Thickness Estimations of FreeSurfer and the CAT12 Toolbox in Patients with Alzheimer’s Disease and Healthy Controls. J Neuroimaging. 2018;28(5):515–523. doi: 10.1111/jon.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loveland WD, Morrissey DJ, Seaborg GT. Modern Nuclear Chemistry [Internet]. 2nd edition. Hoboken, NJ, USA: Wiley; 2017. 800 p. Available from: 10.1002/9781119348450
- 112.Choppin G, Liljenzin J-O, Rydberg J, Ekberg C. Radiochemistry and Nuclear Chemistry [Internet]. 4th edition. Amsterdam; Boston: Elsevier; 2013. 866 p. Available from: 10.1016/C2011-0-07260-5
- 113.NIMH CNS Radiotracer Table [Internet]. National Institute of Mental Health - CNS Radiotracer Table. [cited 2020 Dec 30]. Available from: https://www.nimh.nih.gov/research/research-funded-by-nimh/therapeutics/cns-radiotracer-table.shtml
- 114.Mathis CA, Wang Y, Holt DP, Huang G-F, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 115.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 116.Mathis CA, Lopresti BJ, Ikonomovic MD, Klunk WE. Small-Molecule PET Tracers for Imaging Proteinopathies. Semin Nucl Med. 2017;47(5):553–575. doi: 10.1053/j.semnuclmed.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kobylecki C, Langheinrich T, Hinz R, Vardy ERLC, Brown G, Martino M-E, et al. 18F-florbetapir PET in patients with frontotemporal dementia and Alzheimer disease. J Nucl Med Off Publ Soc Nucl Med. 2015;56(3):386–391. doi: 10.2967/jnumed.114.147454. [DOI] [PubMed] [Google Scholar]
- 118.Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging: present and future directions. Mol Neurodegener. 2017;12(1):19. doi: 10.1186/s13024-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leuzy A, Chiotis K, Lemoine L, Gillberg P-G, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry. 2019;24(8):1112–1134. doi: 10.1038/s41380-018-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schroeter ML, Raczka K, Neumann J, von Cramon DY. Towards a nosology for frontotemporal lobar degenerations—A meta-analysis involving 267 subjects. NeuroImage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 121.Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia–a meta-analysis. Neurobiol Aging. 2008;29(3):418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 122.Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60(6):660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- 123.Brown JA, Deng J, Neuhaus J, Sible IJ, Sias AC, Lee SE, et al. Patient-Tailored, Connectivity-Based Forecasts of Spreading Brain Atrophy. Neuron. 2019;104(5):856–868.e5. doi: 10.1016/j.neuron.2019.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73(6):1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287(23):19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT, et al. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci U S A. 2016;113(50):E8187–E8196. doi: 10.1073/pnas.1616344113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4(1):124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Hock E-M, Polymenidou M. Prion-like propagation as a pathogenic principle in frontotemporal dementia. J Neurochem. 2016;138(Suppl 1):163–183. doi: 10.1111/jnc.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132(11):2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Irwin DJ, McMillan CT, Xie SX, Rascovsky K, Van Deerlin VM, Coslett HB, et al. Asymmetry of post-mortem neuropathology in behavioural-variant frontotemporal dementia. Brain. 2018;141(1):288–301. doi: 10.1093/brain/awx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30(1):103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26(1):37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 134.Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/WNL.58.2.198. [DOI] [PubMed] [Google Scholar]
- 135.Kipps CM, Hodges JR, Fryer TD, Nestor PJ. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132(9):2566–2578. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- 136.Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–542. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(9):2502–2512. doi: 10.1093/brain/awr173. [DOI] [PubMed] [Google Scholar]
- 138.Bejanin A, Tammewar G, Marx G, Cobigo Y, Iaccarino L, Kornak J, et al. Longitudinal structural and metabolic changes in frontotemporal dementia. Neurology. 2020;95(2):e140–e154. doi: 10.1212/WNL.0000000000009760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borroni B, Cosseddu M, Pilotto A, Premi E, Archetti S, Gasparotti R, et al. Early stage of behavioral variant frontotemporal dementia: clinical and neuroimaging correlates. Neurobiol Aging. 2015;36(11):3108–3115. doi: 10.1016/j.neurobiolaging.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 140.Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 142.Kanda T, Ishii K, Uemura T, Miyamoto N, Yoshikawa T, Kono AK, et al. Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging. 2008;35(12):2227–2234. doi: 10.1007/s00259-008-0871-5. [DOI] [PubMed] [Google Scholar]
- 143.Buhour M-S, Doidy F, Laisney M, Pitel AL, de La Sayette V, Viader F, et al. Pathophysiology of the behavioral variant of frontotemporal lobar degeneration: A study combining MRI and FDG-PET. Brain Imaging Behav. 2017;11(1):240–252. doi: 10.1007/s11682-016-9521-x. [DOI] [PubMed] [Google Scholar]
- 144.Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Förstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging. 2007;28(1):42–50. doi: 10.1016/j.neurobiolaging.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 145.Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57(2):216–225. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- 146.Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. 18F-FDG PET Findings in Frontotemporal Dementia: An SPM Analysis of 29 Patients. J Nucl Med. 2005;46(2):233–239. [PubMed] [Google Scholar]
- 147.Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49(4):433–442. doi: 10.1002/ana.92. [DOI] [PubMed] [Google Scholar]
- 148.Brambati SM, Amici S, Racine CA, Neuhaus J, Miller Z, Ogar J, et al. Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser-based morphometry study. NeuroImage Clin. 2015;8:345–355. doi: 10.1016/j.nicl.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bejanin A, La Joie R, Landeau B, Belliard S, de La Sayette V, Eustache F, et al. Distinct Interplay Between Atrophy and Hypometabolism in Alzheimer’s Versus Semantic Dementia. Cereb Cortex. 2019;29(5):1889–1899. doi: 10.1093/cercor/bhy069. [DOI] [PubMed] [Google Scholar]
- 150.Acosta-Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, Williams GB, et al. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134(7):2025–2035. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- 151.Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Förstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging. 2004;25(8):1051–1056. doi: 10.1016/j.neurobiolaging.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 152.Routier A, Habert M-O, Bertrand A, Kas A, Sundqvist M, Mertz J, et al. Structural, Microstructural, and Metabolic Alterations in Primary Progressive Aphasia Variants. Front Neurol. 2018;9:766. doi: 10.3389/fneur.2018.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. NeuroImage. 2008;39(2):619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 154.Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Perneczky R, Forstl H, et al. Longitudinal changes of cerebral glucose metabolism in semantic dementia. Dement Geriatr Cogn Disord. 2006;22(4):346–351. doi: 10.1159/000095624. [DOI] [PubMed] [Google Scholar]
- 155.Iaccarino L, Crespi C, Rosa PAD, Catricalà E, Guidi L, Marcone A, et al. The Semantic Variant of Primary Progressive Aphasia: Clinical and Neuroimaging Evidence in Single Subjects. PLoS One. 2015 Mar 10;10(3):e0120197. [DOI] [PMC free article] [PubMed]
- 156.Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, et al. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain. 2016;139(10):2778–2791. doi: 10.1093/brain/aww195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tetzloff KA, Duffy JR, Clark HM, Strand EA, Machulda MM, Schwarz CG, et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain. 2018 01;141(1):302–17. [DOI] [PMC free article] [PubMed]
- 158.Josephs KA. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72(18):1562–1569. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Utianski RL, Whitwell JL, Schwarz CG, Duffy JR, Botha H, Clark HM, et al. Tau uptake in agrammatic primary progressive aphasia with and without apraxia of speech. Eur J Neurol. 2018;25(11):1352–1357. doi: 10.1111/ene.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Häkkinen S, Chu SA, Lee SE. Neuroimaging in genetic frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol Dis. 2020;1(145):105063. doi: 10.1016/j.nbd.2020.105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75(24):2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whitwell JL, Jack CR, Parisi JE, Senjem ML, Knopman DS, Boeve BF, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010;75(24):2212–2220. doi: 10.1212/WNL.0b013e31820203c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131(Pt 3):706–720. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Baraibar MA, et al. Clinicopathologic features of frontotemporal dementia with progranulin sequence variation. Neurology. 2007;68(11):820–827. doi: 10.1212/01.wnl.0000254460.31273.2d. [DOI] [PubMed] [Google Scholar]
- 166.Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, et al. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006;60(3):374–380. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen-Plotkin AS, Martinez-Lage M, Sleiman PMA, Hu W, Greene R, Wood EM, et al. Genetic and Clinical Features of Progranulin-Associated Frontotemporal Lobar Degeneration. Arch Neurol. 2011;68(4):488. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rohrer JD, Warren JD, Omar R, Mead S, Beck J, Revesz T, et al. Parietal Lobe Deficits in Frontotemporal Lobar Degeneration Caused by a Mutation in the Progranulin Gene. Arch Neurol. 2008;65(4):506. doi: 10.1001/archneur.65.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cash DM, Bocchetta M, Thomas DL, Dick KM, van Swieten JC, Borroni B, et al. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging. 2018;62:191–196. doi: 10.1016/j.neurobiolaging.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol (Berl). 2011;122(2):137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grossman M, Wood EM, Moore P, Neumann M, Kwong L, Forman MS, et al. TDP-43 Pathologic Lesions and Clinical Phenotype in Frontotemporal Lobar Degeneration With Ubiquitin-Positive Inclusions. Arch Neurol. 2007;64(10):1449. doi: 10.1001/archneur.64.10.1449. [DOI] [PubMed] [Google Scholar]
- 173.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol (Berl). 2009;118(3):349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, et al. Frontotemporal dementia due to C9ORF72 mutations: Clinical and imaging features. Neurology. 2012;79(10):1002–1011. doi: 10.1212/WNL.0b013e318268452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vatsavayai SC, Yoon SJ, Gardner RC, Gendron TF, Vargas JNS, Trujillo A, et al. Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain. 2016;139(Pt 12):3202–3216. doi: 10.1093/brain/aww250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pasquini L, Nana AL, Toller G, Brown JA, Deng J, Staffaroni A, et al. Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia. Cereb Cortex N Y N 1991. 2020 Sep 3;30(10):5387–99. [DOI] [PMC free article] [PubMed]
- 179.Borghesani V, Battistella G, Mandelli ML, Welch A, Weis E, Younes K, et al. Regional and hemispheric susceptibility of the temporal lobe to FTLD-TDP type C pathology. NeuroImage Clin. 2020 Jan 1;28:102369. [DOI] [PMC free article] [PubMed]
- 180.Bocchetta M, Iglesias Espinosa M del M, Lashley T, Warren JD, Rohrer JD. In vivo staging of frontotemporal lobar degeneration TDP-43 type C pathology. Alzheimers Res Ther. 2020 Mar 27;12(1):34. [DOI] [PMC free article] [PubMed]
- 181.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.WNL.0000091868.28557.B8. [DOI] [PubMed] [Google Scholar]
- 182.Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73(18):1443–1450. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choudhury P, Scharf EL, Paolini MA, Graff-Radford J, Alden EC, Machulda MM, et al. Pick’s disease: clinicopathologic characterization of 21 cases. J Neurol. 2020;267(9):2697–2704. doi: 10.1007/s00415-020-09927-9. [DOI] [PubMed] [Google Scholar]
- 184.Piguet O, Halliday GM, Reid WGJ, Casey B, Carman R, Huang Y, et al. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology. 2011;76(3):253–259. doi: 10.1212/WNL.0b013e318207b1ce. [DOI] [PubMed] [Google Scholar]
- 185.Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol. 2016;79(2):272–287. doi: 10.1002/ana.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic Resonance Imaging Signatures of Tissue Pathology in Frontotemporal Dementia. Arch Neurol. 2005;62(9):1402. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- 187.Whitwell JL, Jack CR, Parisi JE, Knopman DS, Boeve BF, Petersen RC, et al. Imaging signatures of molecular pathology in behavioral variant frontotemporal dementia. J Mol Neurosci MN. 2011;45(3):372–378. doi: 10.1007/s12031-011-9533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rankin KP, Mayo MC, Seeley WW, Lee S, Rabinovici G, Gorno-Tempini ML, et al. Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick’s disease. J Mol Neurosci MN. 2011;45(3):594–608. doi: 10.1007/s12031-011-9615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whitwell JL, Tosakulwong N, Schwarz CC, Senjem ML, Spychalla AJ, Duffy JR, et al. Longitudinal anatomic, functional, and molecular characterization of Pick disease phenotypes. Neurology. 2020;95(24):e3190–e3202. doi: 10.1212/WNL.0000000000010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Massey LA, Micallef C, Paviour DC, O’Sullivan SS, Ling H, Williams DR, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord Off J Mov Disord Soc. 2012;27(14):1754–1762. doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 191.Massey LA, Jäger HR, Paviour DC, O’Sullivan SS, Ling H, Williams DR, et al. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80(20):1856–1861. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mueller C, Hussl A, Krismer F, Heim B, Mahlknecht P, Nocker M, et al. The diagnostic accuracy of the hummingbird and morning glory sign in patients with neurodegenerative parkinsonism. Parkinsonism Relat Disord. 2018;54:90–94. doi: 10.1016/j.parkreldis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 193.Lee W. Conventional Magnetic Resonance Imaging in the Diagnosis of Parkinsonian Disorders: A Meta-Analysis. Mov Disord Clin Pract. 2021;8(2):217–223. doi: 10.1002/mdc3.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gröschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology. 2006;66(6):949–950. doi: 10.1212/01.wnl.0000203342.77115.bf. [DOI] [PubMed] [Google Scholar]
- 195.Righini A, Antonini A, De Notaris R, Bianchini E, Meucci N, Sacilotto G, et al. MR imaging of the superior profile of the midbrain: differential diagnosis between progressive supranuclear palsy and Parkinson disease. AJNR Am J Neuroradiol. 2004;25(6):927–932. [PMC free article] [PubMed] [Google Scholar]
- 196.Josephs KA. Frontotemporal lobar degeneration. Neurol Clin. 2007 Aug;25(3):683–96, vi. [DOI] [PMC free article] [PubMed]
- 197.Adachi M, Kawanami T, Ohshima H, Sugai Y, Hosoya T. Morning glory sign: a particular MR finding in progressive supranuclear palsy. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 2004;3(3):125–132. doi: 10.2463/mrms.3.125. [DOI] [PubMed] [Google Scholar]
- 198.Santos-Santos MA, Mandelli ML, Binney RJ, Ogar J, Wilson SM, Henry ML, et al. Features of Patients With Nonfluent/Agrammatic Primary Progressive Aphasia With Underlying Progressive Supranuclear Palsy Pathology or Corticobasal Degeneration. JAMA Neurol. 2016;73(6):733–742. doi: 10.1001/jamaneurol.2016.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spina S, Brown JA, Deng J, Gardner RC, Nana AL, Hwang J-HL, et al. Neuropathological correlates of structural and functional imaging biomarkers in 4-repeat tauopathies. Brain. 2019 Jul;142(7):2068–81. [DOI] [PMC free article] [PubMed]
- 200.Chu SA, Flagan TM, Staffaroni AM, Jiskoot LC, Deng J, Spina S, et al. Brain volumetric deficits in MAPT mutation carriers: a multisite study. Ann Clin Transl Neurol. 2020 Nov 28; [DOI] [PMC free article] [PubMed]
- 201.Seelaar H, Klijnsma KY, de Koning I, van der Lugt A, Chiu WZ, Azmani A, et al. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;257(5):747–753. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rohrer JD, Lashley T, Holton J, Revesz T, Urwin H, Isaacs AM, et al. The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2011;82(12):1405–1407. doi: 10.1136/jnnp.2010.214437. [DOI] [PubMed] [Google Scholar]
- 203.Josephs KA, Whitwell JL, Parisi JE, Petersen RC, Boeve BF, Jack CR, Jr, et al. Caudate atrophy on MRI is a characteristic feature of FTLD-FUS. Eur J Neurol. 2010;17(7):969–975. doi: 10.1111/j.1468-1331.2010.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lowe VJ, Lundt ES, Albertson SM, Przybelski SA, Senjem ML, Parisi JE, et al. Neuroimaging correlates with neuropathologic schemes in neurodegenerative disease. Alzheimers Dement J Alzheimers Assoc. 2019;15(7):927–939. doi: 10.1016/j.jalz.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.La Joie R, Ayakta N, Seeley WW, Borys E, Boxer AL, DeCarli C, et al. Multisite study of the relationships between antemortem [11C]PIB-PET Centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimers Dement J Alzheimers Assoc. 2019;15(2):205–216. doi: 10.1016/j.jalz.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 207.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol (Berl). 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tan RH, Kril JJ, Yang Y, Tom N, Hodges JR, Villemagne VL, et al. Assessment of amyloid β in pathologically confirmed frontotemporal dementia syndromes. Alzheimers Dement Diagn Assess Dis Monit. 2017;1(9):10–20. doi: 10.1016/j.dadm.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shi Z, Fu L-P, Zhang N, Zhao X, Liu S, Zuo C, et al. Amyloid PET in Dementia Syndromes: A Chinese Multicenter Study. J Nucl Med Off Publ Soc Nucl Med. 2020;61(12):1814–1819. doi: 10.2967/jnumed.119.240325. [DOI] [PubMed] [Google Scholar]
- 211.Santos-Santos MA, Rabinovici GD, Iaccarino L, Ayakta N, Tammewar G, Lobach I, et al. Rates of Amyloid Imaging Positivity in Patients With Primary Progressive Aphasia. JAMA Neurol. 2018;75(3):342–352. doi: 10.1001/jamaneurol.2017.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BNM, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lesman-Segev OH, La Joie R, Iaccarino L, Lobach I, Rosen HJ, Seo SW, et al. Diagnostic Accuracy of Amyloid versus 18 F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia. Ann Neurol. 2021;89(2):389–401. doi: 10.1002/ana.25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Langheinrich T, Kobylecki C, Jones M, Thompson JC, Snowden JS, Hinz R, et al. Amyloid-PET Positive Patient with bvFTD: Wrong Diagnosis, False Positive Scan, or Co-pathology? Neurol Clin Pract [Internet]. 2021 Jan 18 [cited 2021 Apr 7]; Available from: https://cp.neurology.org/content/early/2021/01/25/CPJ.0000000000001049 [DOI] [PMC free article] [PubMed]
- 215.De Leon J, Mandelli ML, Nolan A, Miller ZA, Mead C, Watson C, et al. Atypical clinical features associated with mixed pathology in a case of non-fluent variant primary progressive aphasia. Neurocase. 2019;25(1–2):39–47. doi: 10.1080/13554794.2019.1609522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Schöll M, Strandberg O, et al. Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2018;320(11):1151–1162. doi: 10.1001/jama.2018.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Soleimani-Meigooni DN, Iaccarino L, La Joie R, Baker S, Bourakova V, Boxer AL, et al. 18F-flortaucipir PET to autopsy comparisons in Alzheimer’s disease and other neurodegenerative diseases. Brain. 2020;143(11):3477–3494. doi: 10.1093/brain/awaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lowe VJ, Lundt ES, Albertson SM, Min H-K, Fang P, Przybelski SA, et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement J Alzheimers Assoc. 2020;16(3):561–571. doi: 10.1016/j.jalz.2019.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fleisher AS, Pontecorvo MJ, Devous MD, Lu M, Arora AK, Truocchio SP, et al. Positron Emission Tomography Imaging With [18F]flortaucipir and Postmortem Assessment of Alzheimer Disease Neuropathologic Changes. JAMA Neurol. 2020;77(7):829–839. doi: 10.1001/jamaneurol.2020.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mattay VS, Fotenos AF, Ganley CJ, Marzella L. Brain Tau Imaging: Food and Drug Administration Approval of 18F-Flortaucipir Injection. J Nucl Med Off Publ Soc Nucl Med. 2020;61(10):1411–1412. doi: 10.2967/jnumed.120.252254. [DOI] [PubMed] [Google Scholar]
- 221.Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue: Validation of PET Tracer. Ann Neurol. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain J Neurol. 2016;139(Pt 5):1539–1550. doi: 10.1093/brain/aww023. [DOI] [PubMed] [Google Scholar]
- 223.Marquié M, Verwer EE, Meltzer AC, Kim SJW, Agüero C, Gonzalez J, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol Commun. 2017;5(1):75. doi: 10.1186/s40478-017-0482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, et al. Characterization of tau positron emission tomography tracer [18 F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12(11):1116–1124. doi: 10.1016/j.jalz.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 226.Cho H, Seo SW, Choi JY, Lee HS, Ryu YH, Lee MS, et al. Predominant subcortical accumulation of 18F-flortaucipir binding in behavioral variant frontotemporal dementia. Neurobiol Aging. 2018;66:112–121. doi: 10.1016/j.neurobiolaging.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 227.Josephs KA, Martin PR, Botha H, Schwarz CG, Duffy JR, Clark HM, et al. [18 F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol. 2018;83(3):599–611. doi: 10.1002/ana.25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tsai RM, Bejanin A, Lesman-Segev O, LaJoie R, Visani A, Bourakova V, et al. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther. 2019 31;11(1):13. [DOI] [PMC free article] [PubMed]
- 229.Cho H, Kim HJ, Choi JY, Ryu YH, Lee MS, Na DL, et al. 18F-flortaucipir uptake patterns in clinical subtypes of primary progressive aphasia. Neurobiol Aging. 2019;75:187–197. doi: 10.1016/j.neurobiolaging.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 230.Makaretz SJ, Quimby M, Collins J, Makris N, McGinnis S, Schultz A, et al. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2018;89(10):1024–1031. doi: 10.1136/jnnp-2017-316409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bevan-Jones WR, Cope TE, Jones PS, Passamonti L, Hong YT, Fryer TD, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2018;89(10):1032–1037. doi: 10.1136/jnnp-2017-316402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schaeverbeke J, Celen S, Cornelis J, Ronisz A, Serdons K, Van Laere K, et al. Binding of [18F]AV1451 in post mortem brain slices of semantic variant primary progressive aphasia patients. Eur J Nucl Med Mol Imaging. 2020;47(8):1949–1960. doi: 10.1007/s00259-019-04631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pascual B, Funk Q, Zanotti-Fregonara P, Cykowski MD, Veronese M, Rockers E, et al. Neuroinflammation is highest in areas of disease progression in semantic dementia. Brain. 2021 Apr 6; [DOI] [PubMed]
- 234.Marquié M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Antón-Fernández A, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017 Jan;81(1):117–28. [DOI] [PMC free article] [PubMed]
- 235.Hansen AK, Knudsen K, Lillethorup TP, Landau AM, Parbo P, Fedorova T, et al. In vivo imaging of neuromelanin in Parkinson’s disease using 18F-AV-1451 PET. Brain J Neurol. 2016;139(Pt 7):2039–2049. doi: 10.1093/brain/aww098. [DOI] [PubMed] [Google Scholar]
- 236.Lockhart SN, Baker SL, Okamura N, Furukawa K, Ishiki A, Furumoto S, et al. Dynamic PET Measures of Tau Accumulation in Cognitively Normal Older Adults and Alzheimer’s Disease Patients Measured Using [18F] THK-5351. PloS One. 2016;11(6):e0158460. doi: 10.1371/journal.pone.0158460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Baker SL, Harrison TM, Maass A, La Joie R, Jagust WJ. Effect of Off-Target Binding on 18F-Flortaucipir Variability in Healthy Controls Across the Life Span. J Nucl Med Off Publ Soc Nucl Med. 2019;60(10):1444–1451. doi: 10.2967/jnumed.118.224113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Spina S, Schonhaut DR, Boeve BF, Seeley WW, Ossenkoppele R, O’Neil JP, et al. Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations. Neurology. 2017;88(8):758–766. doi: 10.1212/WNL.0000000000003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Smith R, Puschmann A, Schöll M, Ohlsson T, van Swieten J, Honer M, et al. 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain. 2016;139(Pt 9):2372–2379. doi: 10.1093/brain/aww163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jones DT, Knopman DS, Graff-Radford J, Syrjanen JA, Senjem ML, Schwarz CG, et al. In vivo 18F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology. 2018;90(11):e947–e954. doi: 10.1212/WNL.0000000000005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Habes M, Grothe MJ, Tunc B, McMillan C, Wolk DA, Davatzikos C. Disentangling Heterogeneity in Alzheimer’s Disease and Related Dementias Using Data-Driven Methods. Biol Psychiatry. 2020;88(1):70–82. doi: 10.1016/j.biopsych.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ferreira D, Nordberg A, Westman E. Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology. 2020;94(10):436–448. doi: 10.1212/WNL.0000000000009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, et al. Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA Neurol. 2016;73(9):1078. doi: 10.1001/jamaneurol.2016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cerami C, Dodich A, Lettieri G, Iannaccone S, Magnani G, Marcone A, et al. Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex. 2016;83:101–112. doi: 10.1016/j.cortex.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 245.Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139(3):986–998. doi: 10.1093/brain/awv387. [DOI] [PubMed] [Google Scholar]
- 246.Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132(5):1287–1298. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- 247.Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Matias-Guiu JA, Díaz-Álvarez J, Cuetos F, Cabrera-Martín MN, Segovia-Ríos I, Pytel V, et al. Machine learning in the clinical and language characterisation of primary progressive aphasia variants. Cortex. 2019;119:312–323. doi: 10.1016/j.cortex.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 249.Harper L, Bouwman F, Burton EJ, et al. Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry. 2017;88:908–916. doi: 10.1136/jnnp-2016-314978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Illán-Gala I, Casaletto KB, Borrego-Écija S, Arenaza-Urquijo EM, Wolf A, Cobigo Y, et al. Sex differences in the behavioral variant of frontotemporal dementia: A new window to executive and behavioral reserve. Alzheimers Dement. 2021 Feb 16;alz.12299. [DOI] [PMC free article] [PubMed]
- 251.Lee JS, Yoo S, Park S, Kim HJ, Park K-C, Seong J-K, et al. Differences in neuroimaging features of early- versus late-onset nonfluent/agrammatic primary progressive aphasia. Neurobiol Aging. 2020;86:92–101. doi: 10.1016/j.neurobiolaging.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 252.LeWinn KZ, Sheridan MA, Keyes KM, Hamilton A, McLaughlin KA. Sample composition alters associations between age and brain structure. Nat Commun. 2017;8(1):874. doi: 10.1038/s41467-017-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on Ethnic and Racial Disparities in Alzheimer’s Disease and Related Dementias: Update and Areas of Immediate Need. Alzheimers Dement J Alzheimers Assoc. 2019;15(2):292–312. doi: 10.1016/j.jalz.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Onyike CU, Shinagawa S, Ellajosyula R. Frontotemporal Dementia: A Cross-Cultural Perspective. Adv Exp Med Biol. 2021;1281:141–150. doi: 10.1007/978-3-030-51140-1_10. [DOI] [PubMed] [Google Scholar]
- 255.Vogel JW, Young AL, Oxtoby NP, et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat Med. 2021;27:871–881. doi: 10.1038/s41591-021-01309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Young AL, Marinescu RV, Oxtoby NP, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018;9:4273. doi: 10.1038/s41467-018-05892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rojas JC, Wang P, Staffaroni AM, et al. Plasma Neurofilament Light for Prediction of Disease Progression in Familial Frontotemporal Lobar Degeneration. Neurology. 2021;96:e2296–e2312. doi: 10.1212/WNL.0000000000011848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Whitwell JL. FTD spectrum: Neuroimaging across the FTD spectrum. Prog Mol Biol Transl Sci. 2019;165:187–223. doi: 10.1016/bs.pmbts.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.