Abstract
The global outbreak of coronavirus-2019 (COVID-19) still claims more lives daily around the world due to the lack of a definitive treatment and the rapid tendency of virus to mutate, which even jeopardizes vaccination efficacy. At the forefront battle against SARS-CoV-2, an effective innate response to the infection has a pivotal role in the initial control and treatment of disease. However, SARS-CoV-2 subtly interrupts the equations of immune responses, disrupting the cytolytic antiviral effects of NK cells, while seriously activating infected macrophages and other immune cells to induce an unleashed “cytokine storm”, a dangerous and uncontrollable inflammatory response causing life-threatening symptoms in patients. Notably, the NK cell exhaustion with ineffective cytolytic function against the sources of exaggerated cytokine release, acts as an Achilles’ heel which exacerbates the severity of COVID-19. Given this, approaches that improve NK cell cytotoxicity may benefit treatment protocols. As a suggestion, adoptive transfer of NK or CAR-NK cells with proper cytotolytic potentials and the lowest capacity of cytokine-release (for example CD56dim NK cells brightly express activating receptors), to severe COVID-19 patients may provide an effective cure especially in cases suffering from cytokine storms. More intriguingly, the ongoing evidence for persistent clonal expansion of NK memory cells characterized by an activating phenotype in response to viral infections, can benefit the future studies on vaccine development and adoptive NK cell therapy in COVID-19. Whether vaccinated volunteers or recovered patients can also be considered as suitable candidates for cell donation could be the subject of future research.
Keywords: Adoptive cell therapy, CD8+ T cell, Coronavirus disease 2019 (COVID-19), Cytokine storm, Innate immune response, Memory cells, NK cell, SARS-CoV-2, Vaccine
Abbreviations: ACE, Angiotensin converting enzyme; ADCC, Antibody-dependent cellular cytotoxicity; ARDS, Acute respiratory distress syndrome; CAR-NK, Chimeric antigen receptor-NK; COVID-19, Corona virus disease 2019; CRS, cytokine release syndrome; DCs, Dendritic cells; DNAM, DNAX Accessory Molecule; G-CSF, Granulocyte-colony stimulating factor; GM-CSF, Granulocyte macrophage colony-stimulating factor; HLA, Human leukocyte antigen; ICAM, Intercellular Adhesion Molecule; ICU, Intensive care unit; IFN, Interferon; IL, Interleukin; IP-10, IFN-γ-inducible protein 10; KIRs, Killer immunoglobulin-like receptors; MCP, Monocyte chemoattractant protein; MDA, Melanoma differentiation-associated protein; MERS, Middle East respiratory syndrome; MHC, Major histocompatibility; MIG, Monokine induced by gamma interferon; MIP, Macrophage inflammatory protein; NCRs, Natural cytotoxicity receptors; NK, Natural killer; NKD, NK cell deficiency; PAMPs, Pathogen associated molecular patterns; PBMCs, Peripheral blood mononuclear cells; PRRs, Pattern recognition receptors; RANTES, Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; RIG, Retinoic acid-inducible gene; SARS-CoV, Severe acute respiratory syndrome-coronavirus; TGF, Transforming growth factor; TLR, Toll-like receptor; TNF, Tumor necrosis factor
1. Introduction:
The recent outbreak of coronavirus disease 2019 (COVID-19) which is rapidly spreading around the globe has been considered as the most devastating pandemic in current decades. Its death toll is daily increasing while no definitive therapeutic drug is available yet. COVID-19 has a diverse clinical manifestation and whilst 80% of infected people are asymptomatic, or mildly symptomatic, the rest experiences severe complication [1]. Although people with older age or comorbidities show more severe disease dominated with strong inflammatory symptom, other factors such as gender or blood groups may affect individuals’ susceptibilities to severe acute respiratory syndrome coronavirus (SARS-CoV)-2. Of note, unlike adults, the vast majority of children with COVID-19 have mild symptoms and are largely safe from severe respiratory symptoms [2], [3]. These are the facts that highlight the variable nature of immune responses against the diseases depending on different factors. In general, main pathologic symptom of coronavirus is manifested by augmented inflammatory responses that cause severe pneumonia, pulmonary oedema, acute respiratory distress syndrome (ARDS) or multiple organ dysfunctions, even leading to death [4]. Overactivation of inflammatory immune response induced by severe infection, drugs or autoimmune diseases may lead to “hyperferritinemic syndrome”. This condition resembles “macrophage activation syndrome” in that an unleashed cytokine storms result in subsequent immune exhaustion leading to severe clinical manifestations of diseases. In severe cases of COVID-19, the poor therapeutic outcome has shown to be correlated with cytokine storm [5] which is associated with the major involvement of macrophages in the lung injury [6]. Therefore, the presence of macrophage activation syndrome is considered as a main risk factor of lung inflammation in these patients [7]. Although from a public perspective, macrophages should act as the most important barrier to infection, they experience an uncontrolled activation which leads to exaggerated cytokines release aggravating inflammatory condition. It may have two important reasons: first, it is due to the direct effect of viral infection which induces stormy cytokine production and release by immune cells mainly macrophages and second, is the malfunction or possible suppression of cytolytic effectors such as natural killer (NK) cells which eradicate infected cells limiting cytokine storms and viral load in the patients. Thereby, shedding more light to complex mechanisms involved in the pathogenesis of COVID-19, this review is started with brief information about antiviral function of NK cell as the key player of innate immunity against viral infection. We then discuss about the ways by which SARS-CoV-2 inhibiting innate immunity response to cause severe symptoms of COVID-19 in the patients.
2. NK cell morphology and distribution
NK cells were initially identified as the cells of the innate immune system with cytolytic activity against different targets such as tumor-derived or virus-infected cells [8]. Morphologically, these cells are large granular lymphocytes with abundant pale cytoplasm [9] surrounding a round or indented nuclei with condensed chromatin and usually containing prominent nucleoli. The presence of circular 50–800 nm membrane-bound granules (primary lysosomes) is a characteristic cytoplasmic feature of these cells. The granules contain different acid hydrolases, such as acid phosphatase, naphthyl acetate-esterase and glucuronidase [9], [10] as well as phospholipids, proteoglycans and the proteins responsible for cytotoxic functions such as serine esterases (granzymes) and pore-forming proteins (perforin) [11]. Mature NK cells constitute approximately 10–15 percent of circulating lymphocytes. NK cells are also found in the spleen, bone marrow, liver (pit cells), lung, intestinal mucosa [8], [12] and the uterus as well as in small numbers in the lymph nodes [13], [14]. Importantly, liver NK cells play a key role against hepatotropic viruses such as HCV [15], [16] while lung-resident NK cells are also in the spotlight due to their pivotal roles in the pathogenesis of bacterial and viral lung infections, including Influenza, SARS-CoV-1 and MERS-CoV infections as well as COVID-19 [17], [18], [19], [20], [21].
3. NK cell receptors
Similar to T cells, NK cells also express CD8, however its expression on NK cells is homodimer rather than being the heterodimer as is presented by T cells. NK cells do not express the CD3 antigen complex or the T cell receptor (TCR) and the genes encoding the TCR chains are not rearranged in these cells [10]. Alternatively, CD16, the Fcγ receptor III (FcRIII) and CD56 are two characteristic markers found on the surface of NK cells [22], [23]. CD16 is a low-affinity Fc receptor which is expressed in most NK cells, neutrophils, monocytes and macrophages. This receptor is responsible for antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells. Another characteristic surface marker of NK cells is CD56 which is usually expressed at a low density on most NK cells [24] while its expression increases to a higher level following activation. Around 10% of circulating NK cells (and approximately 100% in secondary lymphoid tissues) express high levels of CD56 (CD3negCD56bright cells) and can produce large amounts of cytokines and chemokines within minutes of activation. However, these population of blood NK cells have little or no ability to spontaneously kill target cells [25]. The other 90% of NK cells in the blood that show low CD56 expression (dim population) have a relatively lower ability to produce cytokines in response to activation, while most of these CD3negCD56dim NK cells can lyse the target cells effectively in the absence of prior stimulation. This is an antibody-independent mechanism by which NK cells identify and lyse target cells with no or deficient expression of MHC (major histocompatibility complex) class I in a “missing self-recognition scenario” [26]. However, despite this recognition mechanism, the activation of these cells and their subsequent cytotoxic capability also require a precise balance between activating and inhibitory signals simultaneously generated by the interactions between NK cell specific receptors and their ligands [27], [28], [29], [30]. NK cells express two main families of functional receptors, the immunoglobulin superfamily and the C-type lectin-like receptors. The immunoglobulin superfamily includes the killer immunoglobulin like receptors (KIRs), leukocyte immunoglobulin-like receptor (LILR) and natural cytotoxicity receptors (NCRs) [29], [30]. KIRs that bind to classical MHC class Ia ligands human leukocyte antigen (HLA)-A, B, and C are important receptors mediating both inhibitory and activating signals by NK cells. In inhibitory KIRs, including KIR2DL and KIR3DL, the long cytoplasmic tail of receptors signals through the contribution of the immunoreceptor tyrosine-based inhibitory motif (ITIM) [31], [32] whereas, short-tail activatory receptors (KIR2DS, KIR3DS) have been reported to enhance activating signals serving immunoreceptor tyrosine‐based activation motif (ITAM) sequences located on adaptor DNAX-activating protein (DAP)-12 which are paired with the transmembrane portion of receptors [33], [34]. Other important receptors of this family are NCRs which mainly include NKp46 (NCR1), NKp44 (NCR2), NKp30 (NCR3) and NKp80 (also known as killer cell lectin-like subfamily F, member 1) as the major NK cell activatory receptors that enable cell mediated killing through a series of activating signals mediated by ITAM [29] (or an atypical hemi-ITAM–like sequence for NKp80 [35]). It has been reported that following the activation of a single NCR, the signaling pathways of the other NCRs are also activated. This suggests functional cross-talk between different NCRs perhaps to strengthen the activating signals [36], [37]. Contrary to KIRs, these receptors have a variety of ligands that some of them are not fully characterized yet. One of the reported ligands for NKp46 and NKp44 is viral hemagglutinin. NKp30 has shown to interact with heparin sulfate proteoglycans and B7-H6 (a member of the B7 family of immunoreceptors [38]) while NKp80 can bind activation-induced C-type lectin. HLA-B-associated transcript 3 is also another ligand of NCRs, which is released from tumor cells and can bind to NKp30. Taken together, NCRs activity support one of the most important mechanisms of NK cells in destroying tumor targets [29]. The C-type lectin like family also represents members of Natural Killer Group (NKG)2x/CD94 heterodimer receptors and a NKG2D receptor which is structurally different, not forming a heterodimer with CD94 subunit [39]. Similar to KIRs, this family also includes both activatory and inhibitory receptors, with the difference that although NK cells recognize MHC class Ia (classical MHC-I) molecules through KIRs [40], the heterodimeric NKG2x/CD94 [41] and homodimeric NKG2D receptors [42] interact with MHC class Ib (non-classical MHC-I) and MHC-I-like molecules, as their ligand, respectively. HLA-E is a common ligand for NKG2x/CD94 family. It is a member of non-classical MHC class I and interacts with both subunits of the receptor. The subunit of CD94 specifies the interaction with HLA-E ligand whereas NKG2 subunit determines the HLA-E recognition outcome (either is inhibitory or activatory) [43]. In order to be expressed on the cell surface, HLA-E needs to be bound to specific peptides derived from the leader sequences of other MHC class I molecules. Hence, the down-regulation of MHC class I expression can also reduce the cell surface expression of HLA-E [43]. Actually, the binding of MHC class I peptides to HLA-E allows the NK cell, via NKG2A/CD94, to indirectly monitor the cell surface expression of other MHC class I molecules [44], [45]. Proteins other than MHC class I molecules, have been shown to contain peptides that can bind to HLA-E. One example is glycoprotein UL40 of the human CMV which contains peptides in its leader sequence with the same structure as seen in the HLA-Cw03 molecule. These peptides can up-regulate the expression of HLA-E which then interacts with NKG2A/CD94. The interaction leads to the protection of target cells infected by CMV from NK cell lysis [46], [47]. Main receptors from NKG2x/CD94 family include the inhibitory, ITIM-containing CD94–NKG2A receptor and activating, DAP-12-associating CD94–NKG2C receptor, both of which bind to the HLA-E molecule as their ligand. The reason for the existence of a common ligand for the both activatory and inhibitory receptors of this family is not yet known [29]. One suggestion, however, is that, depending on normal and disturbing conditions, HLA-E expression does not necessarily lead to NK cell activation [48]. The activatory NKG2D receptor is another member of C-type lectin-like family, which is expressed by human NK cells, αβ and γδ T cells as well as CD8+ T cells [49], [50]. At least 6 ligands with MHC class I homology has been recognized to interact with this receptor including three transmembrane proteins (MICA, MICB, and ULBP4) [51], [52] and 3 glycophosphatidylinositol (GPI)–anchored proteins (ULBP1-3). None of these ligands are expressed by normal tissue, however, under pathological conditions such as viral infection or malignant deformity, cellular or genotoxic stress can increase their expression, leading to NK cell activation [53]. On the other hand, normal tissues expressing self-MHC class I ligand that correspond to at least one of their NK cell inhibitory receptors do not express these NKG2D ligands and are therefore not prone to autologous NK-mediated cytotoxicity [54]. From the signaling point of view, here, this activating receptor signals through a DAP10 adaptor contains the costimulatory YINM sequence instead of an ITAM one [55]. This compartment is responsible for signal transduction via the recruitment of either the p85α regulatory subunit of phosphatidylinositol 3 kinase (PI3K) or growth factor receptor-bound protein 2 (Grb2), which eventually leads to the NK cell activation and initiation of cytotoxicity [56]. Overall, members of KIR group and NKG2A/CD94 are the most important inhibitory receptors and prevent autoreactivity through the recognition of MHC class I ligands expressed on the surface of healthy cells. On the other hand, NCRs and NKG2D are the important activating receptors on NK cells which are involved in cytolytic effects against infected targets or tumor cells [29].
4. Mechanism of NK cell mediated cytotoxicity
NK cells have cytotoxic capability that leads to target cell death [57]. NK cell cytotoxicity takes place following the activation of the lytic mechanism which usually involves granules release. Amongst the released materials, lytic molecules stored in granules particularly perforin, granzymes and granulysin have been shown to play a pivotal role in inducing apoptosis and death in targets [57]. Perforin, a soluble monomer molecule, is released by exocytosis from granules [58]. The perforin molecules are then polymerized in the presence of Ca2+ to make the pores [59], [60], [61] in the target cell membrane through which the granzymes pass into the cytoplasm [62]. Another important lytic agent, granulysin is also released from the granules of different lymphocytes including NK cells, cytotoxic T cells, T helper cells and NKT cells. This cytolytic molecule is a member of the saposin-like proteins family which can cause membrane damage in target cells [63]. These cytotoxic effects are primarily triggered by the mechanism introduced as “missing self” recognition [26]. By this mechanism, targets including infected or tumor modified cells that actually lack or are deficient in MHC class I, are recognized by NK cells as missing self-targets. Here, missing MHC on target cells reduces inhibitory signals while activating NK cells. However, in the next step, the interactions between specific ligands on infected cells and activatory receptors can stimulate NK cell cytolytic activity [54]. One other hand, in presence of specific antibodies, primed target cells can also be recognized by CD16+ NK cells especially those with CD56dim phenotype which abundantly express CD16 as the low affinity Fcγ receptor III. NK cells bound to constant (Fc) region of immunoglobulin immobilized on a target cell surface are activated by a CD16-mediated signal which leads to cell degranulation and perforin dependent targets lysis, a mechanism called antibody-dependent cellular cytotoxicity (ADCC) [64]. In addition to these granule-dependent killing, cytotoxicity can also be mediated by the interaction of surface molecules mostly through the Fas-FasL intercellular linkage-mediated pathway in which effector T cells and NK cells express FasL (CD178), whereas target cells express Fas (CD95 or Apo-1) [65], [66]. Furthermore, cytotoxicity can also be mediated by the interaction of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) on NK cells with their death-inducing receptors on target cells. These pathways induce another mechanism by which the alteration of membrane permeability in target cells leads to their apoptosis and subsequent lysis [11], [57].
5. Innate immunity against viral infection: the critical role of NK cells
As the first effective defense barrier facing with infections, the innate immune system is crucially involved in the fighting against viruses. Tissue resident macrophages and dendritic cells (DCs) are considered as first responders [67] which recognize viral infected cells expressing pathogen associated molecular patterns (PAMPs) through germline-encoded pattern recognition receptors (PRRs) [68]. NK cells are also other members of innate immune system which play a key role in the host’s immune defense against pathogens preventing the establishment of infection and the viral spreading through the body [69]. So far, several studies in mice models or humans with compromised NK cell activity or lacking functional NK cells have highlighted the crucial role of these cells against viral infection [70]. As evidence, patients with NK cell deficiency (NKD) are quite vulnerable to viral infections [70], [71]. Different cancers also showed to increase patients’ susceptibilities to viral infections [72], which may be due to compromised functional activity of NK cells induced by malignancies [73], [74], [75], [76], [77]. In addition to these cells, some members of adaptive immune systems including cytotoxic T cells, immune conducted macrophages and NKT cells are also required to provide an active immune response against viruses. However, it is only NK cells that have the unique ability to effectively detect and lyse virus-infected target cells without prior recognition. Significant progress has recently been made in understanding the function of NK cells against viral infections. These cells are activated in response to innate cytokines that are released from virus-infected cells. NK cells have the ability to eradicate viruses either by direct cytotoxic effect or by regulating other pathways of the immune system (cellular or humoral immunity) that lead to the neutralization and destruction of the viruses. Airway epithelial cells which are in the first line exposure to RNA viruses play a critical role in introducing pathogens to the immune system. Upon respiratory viral exposure, these infected cells express various arrays of PRRs (pattern recognition receptors) including Toll-like receptors (TLRs 3, 7 and TLR8), melanoma differentiation-associated protein (MDA)-5 (a viral RNA binding protein essential for activating antiviral immune responses), and retinoic acid-inducible gene (RIG)-I (a PRR receptor responsible for the type-1 interferon (IFN1) response). These receptors are important germline-encoded host sensors provided by the responders of innate immunity, which especially contribute to fight against respiratory RNA viruses, such as coronaviruses [78]. After viral infections, cellular sensing of viruses through PRRs results in the transcription of genes involved in the inflammatory response. Induction of type I interferon (IFN-α/β) production by infected cells is a critical part of the antiviral response [79]. Type I IFNs are produced by many immune and non-immune cells [67], [78], [80], and while leading to a reduction in viral replication, they substantially prime innate and adaptive lymphocytes, including NK cells [81]. Activating cytokines including type I interferon (IFN-α/β) and interleukins (ILs) 2, 12, 15 and 18 [82] released by either virus infected targets or activated antigen presenting cells [83], [84] can modulate NK cell function while enhancing their survival, proliferation, cytotoxicity, and cytokine production [82]. Of note, IL-12 increases the generation and secretion of IFN-γ by NK cells while IL-15 induces NK proliferation. IFN-γ generation in NK cells enhances NK cell cytolytic activity against infected targets. On the other hand, this interferon also regulates the function of other key players of immune system against viral infections. Activated NK cells also produce tumor necrosis factor (TNF), another effective cytokine that plays an important role in antiviral and immune regulation processes. Sensing of chemokine gradients induced by infection, NK cells track and distinguish viral-infected targets via the recognition of viral proteins on the surface of infected cells. Followed by the recognition of infected targets, their intimate cross-talk with NK cells can lead them to exert some direct or indirect effects against viruses. In general, this viral induced NK cell activation is achieved in two ways. On the one hand, viral intrusion into cells may upregulate the expression of some self-encoded molecules [85], [86] which interact with activating NK cell receptors including NCRs (NKp30, NKp44, NKp46 and NKp80), NKG2D [56], [87], as well as co-activating receptors such as DNAM-1 [88]. Alternatively, some viruses may also induce NK cell activation through the down-regulation of ligands in infected cells that supposed to inhibit NK cell function in normal condition, via the interaction with their inhibitory counter- receptors including KIRs [89], [90], [91] and the C-type lectin-like receptor CD94-NKG2A [92], [93]. Some NK cell receptors like PAMPS [94] or NKG2C [95] may also directly be engaged in the recognition of viral moieties. Consequently, followed by NK cell activation, they can exert different functional activity against infected cells. The first is the release of IFN-γ by activated NK cells which can up-regulate the antigen presentation in infected targets supporting their further interaction with either NK cells or other key players of innate immune system (especially the adaptive one). In addition, via the above mentioned intimate cellular interactions, activated NK cell can directly attack virus-infected cells, punching them with the release of perforin and granzyme B, a Ca2+-dependent exocytosis of cytolytic granules [96] which results in cytolysis of infected cells. Regardless of release function in NK cells, their close contact with targets can be supported by either interaction between NK cell receptors and specific antigens presented by infected cells leading to cytolytic effects (necrosis), or the interaction of NK cell ligands (FasL or TRAIL) with the death receptors on the surface of targets [97] which may result in apoptosis. On the other hand, followed by the presentation of viral antigens to adaptive immune system and creation of specific antibodies against antigens expressed on infected cells, the recognition of these antibodies by NK cell FC receptor [98], CD16, leads to ADCC [99]. It has been shown that NK cell receptors play an essential role in the antiviral defense mechanisms of these cells [69]. In general, NK cells receive negative “self” signals from the normal tissue expressing class I MHC molecules to their inhibitory receptors. This is a base of self-recognition by NK cells. Most viruses reduce the expression of MHC class I on the surface of infected target cells, a mechanism that helps them escape from the adaptive immune system and recognition by cytotoxic T cells. However, the reduced expressions of these molecules as the major ligands for NK cell inhibitory receptors (KIRs) in infected cells make them vulnerable to the detection by NK cells which now, with the reduction of receiving inhibitory signals can recognize infected cells as unfamiliar targets. Non-specifically, NK cells recognize different ligands on virus-infected cells by a repertoire of their germ-line encoded receptors. Contrary to T and B lymphocytes which generate antigen-specific receptors, NK cell receptors do not require recombination as they can innately interact with ligands on infected target cells. Again unlike T lymphocytes, in NK cells, this is a balance between the two groups of activating and inhibitory receptors, which ultimately determines how these cells respond and function [100]. Here, the inhibitory signals play a critical role, so that reducing the induction of inhibitory receptors has an important effect on the functional activities of NK cells, particularly the role of inhibitory receptor NKG2A is very important [101], [102]. The main ligand of this receptor is HLA-E molecule (a member of non-classical MHC class I which its interaction with NKG2A is crucially involved in the regulating of NK cell function. While viral infection reduces the expression of the MHC class I, since this molecule in turn acts as a supplier of complementary peptides required for the proper expression of HLA-E, thereby reducingexpression of MHC class I has also a negative effect on the level of HLA-E expression and its interaction with NKG2A. As important ligands for NK cell inhibitory receptors (KIRs and NKG2A), such attenuating levels in the expression of MHC class I and HLA-E significantly shifts the balance toward activating signals transduced by NK cell activating receptors, especially those which interact with viral ligands or stress molecules rather than classical or non-classical MHC I class [30], [103]. These activating receptors are mainly included in NCRs (NKPs 30, 44, 46 and 80) and NKG2D [104].
6. NK cell cross-talk with other key players of immune response to control viral infection
NK cells provide a rapid and potent response to viral infection; however an effective and long-lasting antiviral response is generally supported by their cooperation with other key players in immune system, including neutrophils, macrophages and dendritic cells. Actually, these complex cellular interactions can regulate the cytokine milieu, initial viral load and CD4+ T cell mediated cellular immune responses during viral infections. Although, the main response of NK cells to the virus is achieved by the eradication of infected target cells which effectively reduce viral load, they also control the systemic inflammatory response and unwilling cytokine storm called as “hyperferritinemic syndrome” by the lyse and elimination of activated inflammatory cells including neutrophils, dendritic cells, monocytes/ macrophages, and T cells [105], [106], [107].
Evidence has shown that direct cross-talk between NK cell and neutrophil can modulate their mutual maturation, activation and effector functions [108], [109]. In one study, to preserve an immune response, IL-15- or IL-18-activated NK cells were co-cultured with neutrophils which their activation and survival were modulated with IFN-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) [110] released from NK cells. In contrast, another study indicated that how human NK cells can promote neutrophil apoptosis via NKp46 and Fas-dependent mechanisms, to limit inflammatory function and further activation of immune responses [111]. On the other hand, neutrophils are also enable to modulate NK cell licensing, their maturation, activation, function, homeostasis and cytokine generation [112], [113], [114], [115]. NK cells also have intimate cross-talk with the different members of adaptive immune system of which macrophage also acts as a key player at the frontline fighting against infectious agents. Upon viral contact, activated NK cells release cytokines and chemoattractants including macrophage inflammatory protein (MIP)-1α that effectively attract and recruit immune-conducted macrophage to infected tissue. In a reciprocal contribution, as antigen presenting cells, macrophages also release inflammatory mediators and express viral antigens associated with different ligands to NK cells, enhancing their cytolytic effects [116]. Although, macrophages play an important role in virus eradication, they may also act as a double-edged sword during strict viral infection where their stormy cytokine release creates an unwilling destructive manifestation of disease called as macrophage activation syndrome. In a close contact with fully infected macrophages, receiving potent signals, agitated NK cells can lyse these virus-infected cells. Therefore, as a direct evidence that highlights a critical role for NK cells in macrophage modulation, this event not only limits viral burden but also decreases the augmented cytokine release by infected cells while quenching unwilling inflammatory responses and cytokine storms. NK cells also reciprocally interact with dendritic cells (DCs) as potent antigen-presenting cells contributing to adaptive immune response [117], [118], [119], [120]. Similarly, NK cell-mediated lysis of infected DCs reduces viral load while dampening the inflammatory responses. Infected DCs release inflammatory cytokines including IFN-α and TNF-α which induce NK cell degranulation and IFN-γ generation [121], [122]. In addition, ligands of Toll-like receptor up-regulate DCs expression of CD155 and CD112, which bind to their ligands DNAX accessory molecule (DNAM)-1 on NK cells. These interactions can attenuate immune responses through the promotion of NK cell-dependent cytolysis of immature and mature DCs [123]. GM-CSF also showed to increase DCs expression of CD155 and intercellular adhesion molecule (ICAM)-1, which promote NK cell cytotoxicity against DCs [124]. On the other hand, in some conditions, NK cell can support DC maturation that benefits cellular immunity. It has been shown that IL-2-activated NK cells promote DCs maturation and their crosstalk with naϊve CD4+ T cells converting them to polarized CD4+ T cells which induce antigen-specific cytotoxic CD8+ T cell responses [125], [126]. Whilst indirect contribution of NK cells seems to enhance T cell mediated immune responses, direct interaction of NK cells with these cells supports some converse effects. Activated NK cells may lyse CD4+ T cells [127] limiting antiviral T cell responses [128], [129], [130]. Intriguingly, NK cell depletion has shown to promote either cellular or humoral immunity via the enhancement of antibody responses, increasing antigen presentation and CD4+ T cell expansion that improve CD8+ T cell responses. These observations may propose a dual role for NK cells in the modulation of adaptive immune responses against viral infection. It seems that in seriously ill patients with high viral doses, NK cell may limit exaggerated CD4+ and CD8+ T cell responses. In this condition, despite viral persistence, NK cells may prevent fatal pathology and death via the quenching aggressive inflammatory responses caused by unwilling cytokine storm.
7. NK cells and COVID-19
Having no significant complications in the majority of healthy people who are exposed to SARS-CoV-2 highlights the fact that an efficient innate immune response may be able to prevent pathological symptoms of COVID-19 and clear the body from infection. Nevertheless, as an escape and resistance strategy, SARS-CoV-2 showed an unusual ability to deal with the innate immune system by either distorting its main function or disabling the key players against infection such as NK cells. Cytokine storm is a clear sign of immune system deviation in the effective response to infection, which occurs when the virus enters affected cells, including macrophages, forcing them to produce more and more inflammatory mediators. Such an imbalance production of inflammatory cytokines not only does not support an effective fight against the virus, but also ultimately leads to widespread inflammatory damage, especially in the lungs, which is one of the major complications of the disease and one of the leading causes of death in patients. In the face of such a situation, the role of NK cells in the elimination of virus-infected macrophages seems to be very important. With the elimination of the most contaminated zombie-like macrophages that produce unbridled inflammatory mediators, the intensity of the cytokine storm is moderated while the viral load is also reduced. This can also lead to infection control with the proper functioning of other immune cells such as cytotoxic T cells that could be exhausted if the cytokine storm persists. This is what eventually happens in most coronavirus infections to prevent the disease from getting worse. But in the case of COVID-19, the ending may not be as enjoyable as in fairy tales, as the virus inhibits the functional activity of NK cells in different ways. Similar to other coronaviruses, angiotensin converting enzyme 2 (ACE2) is thought to be the main receptor for SARS-CoV-2 spike protein which allowing the viral entrance into the vulnerable cells [131], [132]. This occurs in the first exposure of viruses with pulmonary tissue in which small set of alveolar type 2 epithelial cells (type II pneumocytes) [133] as well as alveolar macrophages express ACE2. Upon viral entrance, the infected cells produce interferons, and their associated responsive genes that induce a robust innate immune response [134], which attracts first line defenders of immune systems including DCs, macrophages, and neutrophils. These facts were evidenced by high infiltrations of ACE2 expressing macrophages with SARS-CoV-2 nucleoprotein antigen in lung bronchopneumonia area, spleen and the lymph nodes of COVID-19 patients [6], [135]. This is typical form of macrophage activation syndrome in which the strong IL-6 production by infiltrating macrophages can be associated with an extreme inflammation in COVID-19 [6], [7]. In the most types of primary or infection-dependent inflammation, this syndrome is showed to be associated with the loss of NK cell functional activity [101], [136], [137]. As an important hint for the pathogenesis of macrophage activation syndrome, the negative impacts of IL-6 elevation on NK cell cytotoxicity has been shown by Cifaldi et al where they also reported improved function of NK cell in the presence of anti-IL-6 monoclonal antibody tocilizumab [138]. Accordingly, severe cases of COVID-19 are accompanied with highest levels of IL-6 production [139] while in these patients the elevated production of IL-6 is shown to be associated with NK cell functional inhibition. It has been suggested that the increasing levels of IL-6 and IL-10 in SARS-CoV2-infected patients [140] can directly attenuate the expression of PERF and granzyme B in NK cells leading to their reduced cytotoxicity [138], [141]. In a recent study, Mazzoni et al. have shown an inverse correlation between NK cell function and serum levels of IL-6 in COVID-19 intensive care unit (ICU) patients. They also showed improved markers of NK cell activation (granzyme A and perforin) in a small group of the patients treated with tocilizumab [142]. Alternatively, IL-6 may also diminish the expression of NKG2D as an important NK cell receptor which plays a crucial role in the elimination of virally infected cells [143]. These findings along with the promising therapeutic achievements in COVID-19 which obtained by IL-1R antagonist Anakinra [144] and Cytosorb [145] suggested that targeting the IL-6 axis may benefit severe COVID-19 patients through the improvement of NK cell functional activity. In addition to IL-6, as an inhibitory factor, transforming growth factor beta (TGF)-β, may be also involved in NK cell hyporesponsiveness in COVID-19 patients. This hypothesis can be supported by an observation that showed significantly higher levels of TGF-β in SARS patients is in direct correlation with their hospitalization length. Since TGF-β is a key suppressor of NK cell functions, the higher levels of this inhibitory molecule associated with other suppressing cytokines in coronavirus patients may play an important role in the inhibition of NK cell antiviral activity [146]. Besides abovementioned cytokine-induced NK cell dysfunction, several line of evidence also indicated an obvious decrement in circulating NK cell in COVID-19 patients especially in those with severe respiratory complications. Studies showed higher neutrophil and lower lymphocyte counts in blood obtained from COVID-19 patients compared to healthy controls while patients with severe infection presented significant reduction in CD8+ lymphocytes and NK cells compared to those with mild infection and healthy controls [147]. Similar patterns are also observed in other coronaviruses. SARS infection is associated with significant reduction in NK cell numbers with the lower levels indicated in severe disease [148] while a strong association between Middle East respiratory syndrome (MERS) infection and lymphopenia has been also reported [149], [150], [151], [152]. The exact mechanisms by which CoVs attenuate circulating NK cells in patients has not been clear yet. One possible reason might be NK cell redistribution into the infected tissues [153], as some circulating lymphocytes including NK cells may migrate into pulmonary tissues in response to inflammatory cytokines that released by infected epithelial cells or engaged macrophages [154]. Actually, within acute phase of disease, the significant accumulation of monocyte/macrophages and neutrophils in lung is associated with a robust release of inflammatory mediators which activate and attract lymphocytes, including NK cells, to the site of infection [155]. However, the level of inflammatory state could be dependent on disease severity where mild cases of disease showed alveolar macrophages infiltration in lung whereas in patients with severe ARDS, lungs were infiltrated by highly inflammatory monocyte-derived macrophages [156]. This confirmed by Liao et al. observations that showed significantly more infiltration of NK cells into the lungs in severe COVID-19 patients compared to those with the mild infection [156]. However, it seems that NK cell cytopenia can be considered as a reliable marker to characterize SARS-CoV-2 infected patients [157]. Regardless of indirect effects on NK cells counts or functions, as these cells also express ACE2 [158], thus, SARS-CoV-2 may directly interact with NK cells and suppress their functions. It has been already shown that some RNA viruses which cause acute pulmonary infections also induce NK cell apoptosis [159], [160]. COVID-19 patients also experience significant increment in expression of NKG2A as one of the most important inhibitory receptors of NK cells. At the early stage of disease, the upregulation of NKG2A results in the exhaustion of cytotoxic T cells and NK cells, which thereby leading to disease progression to severe stage [147]. Severe cases with ARDS have shown reduced proportion of mature NK cells with increased NKG2A, PD-1, and CD39 [161]. In this stage, although NK cells maintained their degranulation capacity, they exhibited a hyporesponsive phenotype with reduced levels of TNF-α, IFN-γ, IL-2 and granzyme B [147]. In a study presented by Wilk et al., all COVID-19 patients had significantly reduced population of CD56bright while of those, patients with severe disease also exhibited depleted population of CD56dim NK cells with the increased expression of the exhaustion markers LAG3 and HAVCR2 [162]. In a study performed on the cells obtained from bronchoalveolar lavage fluid of mild and severe cases of COVID-19, Liao et al. found significantly more NK cell infiltration into the lungs while they showed that patients with severe disease had reduced proportions of NK cells highly expressing NKG2A and CD94 [156]. Accordingly, patients who recovered from COVID-19 exhibited higher numbers of NK cells and lower NKG2A expression in comparison with those with active disease [147]. Since NKG2A overexpression exhausts the functional capabilities of NK cell and thereby severely compromises the innate immune response [163], whether inhibiting NKG2A in severe cases of COVID-19 can be beneficial in patient treatment remains to be determined. However, indirect answers to this question can be found in some studies related to cancer treatment. These studies also indicated that overexpression of NKG2A can subsequently result in functional exhaustion of cytotoxic T and NK cells leading to tumor growth [164], [165]. Thus, it has been suggested that blocking NKG2A by an inhibiting antibody (Monalizumab) can restore the function of CD8+ T and NK cells in cancers, restricting tumor progression, with no significant side effects [166].
8. NK cell role in the pathogenesis of COVID-19
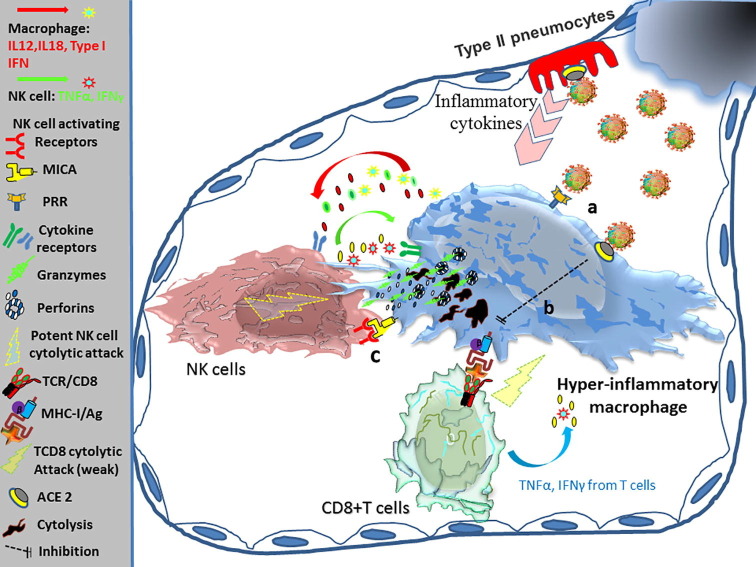
There is a general belief that the cytokine storm induced by hyperactive lung macrophages is the main reason of COVID-19 progression to a very acute and fatal condition. In pneumonia model developed by Chen et al., pulmonary infection of SARS-CoV was associated with a biphasic inflammatory response. The first phase is orchestrated by a contribution between infected airway epithelial cells, alveolar macrophages, and recruited inflammatory monocyte-macrophages and neutrophils. At the early stage of disease, these affected cells release different arrays of cytokines and chemokines including TNF-α, IL-6, IFN-γ -induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1a and Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted (RANTES) [167], [168]. These inflammatory mediators create a gradient that attracts NK cells, plasmacytoid (p), DCs, macrophages, CD4+ T cells and NKT cells into the site of pulmonary infection where they induce a second wave of inflammatory response by the release of cytokines including TNF-α, IL-6, IFN-γ, IL-2, IL-5, and chemokines such as MCP-1, MIP- 1a, RANTES, monokine induced by gamma interferon (MIG) and IP-10. The 2nd wave of inflammatory mediators which occurs within 7 days after infection is associated with the further infiltration of immune cells in lung [169]. It seems that lymphocyte migration, including NK cells, into the lungs, associated with their activation results in the significant production of IFN-γ [155], which may play a pivotal role in macrophage activation and cytokine storm in the acute phase of SARS infection [146]. In this condition, augmented IFN-γ production exhausts T cell responses while resulting in epithelial and endothelial cell apoptosis and vascular leakage which is associated with an additional accumulation of activated macrophages. These are the events that change the homeostasis of lung tissue leading to ARDS [167]. Of note, the observation of a reverse correlation between IFN-γ levels and peripheral lymphocyte counts might be due to either lymphocytes apoptosis or their lung sequestration induced by IFN-γ [146]. Similarly, in COVID-19, it is also thought that following viral invasion to pulmonary tissue, the release of innate cytokines by infected targets leads NK cells to infiltrate into the lungs. According to similar models of inflammation, it is postulated that IFN-γ liberated from NK cells plays an essential role in inducing the stormy production of cytokines by macrophages and other inflammatory cells. However, it is important to note that in COVID-19 patients, NK cells themselves are affected by the inhibitory effect of the virus and have virtually minimal cytokine release activities. This is confirmed by significantly reduced population of CD56bright NK cells during disease. Of note, in severe disease, overexpression of inhibitory NKG2A receptor on circulating NK cells also showed a hyporesponsive phenotype with lower levels of IFN-γ, TNF-α, IL-2, and granzyme B [147]. This is in accordance with a study made by Chen et al. that also showed low production of IFN-γ in severe cases of COVID-19 [170]. Thus, however, NK cells may play a role in inducing inflammatory conditions in the early stages of COVID-19, the presentation of cytokine storm in a later phase of disease cannot be mainly induced with such a phenotype of NK cells. Instead, in these patients cytokine storm is manifested by increasing inflammatory mediators including IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A and TNF-α [171] of those, due to critical role of IL-6 in propagating cytokine release syndrome (CRS) [172], its targeting with anti-IL-6R monoclonal antibodies (e.g., tocilizumab, sarilumab, siltuximab) is of main interest for therapeutic approach. In this regard, according to the cytokine profile in critically ill patients with life threatening respiratory distress, it is clear that the absence of IFN-γ can undermine its critical role in the induction of CRS at this stage of disease. This is also in accordance with observations that highlight the association of high IL‐6/IFN‐γ ratio with severe disease in COVID‐19 patients as higher levels of IL‐6 showed to be correlated with severe condition and patients’ mortality [139], [173], [174]. In contrast, IFN‐γ expression in complicated patients tends to be slightly lower than those observed in moderate cases of disease, possibly due to the decreased population of CD4+, CD8+, and NK lymphocytes in severe cases of COVID-19 [175]. Therefore, despite the prevailing opinions that consider NK cell released effectors to be the main cause of cytokine storm in COVID-19 patients, it seems that this is basically a defect in the lethal function of NK cells, which is the main cause of disease severity. Most importantly, the significant shortage in cytotoxic CD56dim NK cells at this stage of disease highlights the critical role of these cells in the elimination of macrophage-induced cytokine storms. Because under normal conditions, NK cells are expected to effectively destroy infected macrophages and other inflammatory cells that are responsible for causing cytokine storms. On the other hand, it is also important to note that in addition to NK cells, CD4+ T cells can also be a major source of inflammatory mediators including IFN-γ which exacerbate cytokine storm following the involvement of adaptive immune system. In a study on COVID-19 presented by Zhou et al. CD4+ T-cells from ICU patients showed significant levels of GM-CSF, IL-6 and IFN-γ expression and release that contributing to cytokine storms. While CD8+ T cells also express some levels of GM-CSF, neither NK cells nor CD8+ T cells showed to express IL6 in these patients. Therefore, in the presence of exhausted NK cells (non-secretory phenotype), IFN-γ released by other sources may play more prominent role in the induction of cytokine storm. However, before being affected by the virus, normal NK cells maybe primarily involved in potentiating cytokine storms. Fig. 1 summarizes different pathways through which SARS-CoV-2 induce macrophage hyper-activation and CD4+ T cell release whereas suppressing NK and CD8+ T cell functional activity.
Fig. 1.
Modulation of immune response by SARS-CoV-2: a) Virus directly interacts with alveolar and immune cells through expressed ACE2 receptors. b) Innate cytokines released by infected alveolar cells (type II pneumocytes) attract and recruit immune cells to the site of pulmonary infection. c) Viral interaction with alveolar or recruited macrophages induces the stormy release of cytokines (including TNF-α, IL-6, IP-10, MCP-1, MIP-1a and RANTES) leading to strong inflammatory response. d) Virus also enhances CD4+ T cell cytokine release including GM-CSF, IFN-γ and IL-6. Induced T helper activity increases macrophage activation and cytokine storm. e) SARS-CoV-2 causes overexpression of inhibitory receptor NKG2A on NK and CD8+ T cells while diminishing their cytolytic functions against infected cells. f) These exhausted, less functional cytolytic cells could at the most act as decoy cells. ACE; Angiotensin converting enzyme, GM-CSF; Granulocyte macrophage colony-stimulating factor, IFN; Interferon, IL; Interleukin, IP-10; IFN-γ-inducible protein 10, MCP; Monocyte chemoattractant protein, MIP; Macrophage inflammatory protein, SARS-CoV; Severe acute respiratory syndrome-coronavirus, TNF; Tumor necrosis factor.
9. NK cell therapy: Whether it would be effective to prevent the severe condition of COVID-19
Because under normal conditions, NK cells are expected to effectively destroy infected macrophages and other infected cells which are responsible for causing cytokine storms (Fig. 2 ), their lower circulating counts and exhausted phenotype [147], [161], [162] may cause the severe condition of disease in COVID-19 patients. Thereby, NK cell reconstitution in patients or adoptive transfer of properly functioning NK cells might be a possible cure for the patients even those who affected by severe condition. So far, three approaches of NK cell-based therapies have gained most interest to fight against COVID-19, which are included in receiving immune stimulants by patients such as recombinant IL-2 [176] or IL-15 [177], to improve the in vivo activity of patients’ NK cells [178], possible application of drugs that block NK cell inhibitory receptors and therapeutic adoptive NK cells therapies [179]. So far, these approaches have shown promising clinical results with the acceptable safety and efficacy in the treatment of different malignancies; however their potential application in effectiveness of NK cell therapy for the treatment of viral infections has not yet been extensively evaluated in large-scale clinical trials. Despite this, due to the emergency situation created in the global epidemic of COVID-19 and relying on the promising therapeutic results that NK cell therapy has shown in the safe treatment of some cancers, today, few clinical trials are studying the effectiveness of these approaches in the treatments of COVID-19 patients. For NK cell therapies, therapeutic cells can be obtained from two conventional sources including functional NK cells expanded from granulocyte-colony stimulating factor (G-CSF)- mobilized peripheral blood mononuclear cells (G-PBMCs) [180] or stem cells [181] as well as immortalized human NK cell lines which are genetically engineered [182]. A unique advantage of NK cell expansion methods is the ability to provide an optimal cell culture condition in which the scientists can produce NK cells with the maximum levels of cytotoxic potential [30]. Through different treatment strategies, scientists can achieve to shift NK cell production to CD56dim population with the highest levels of cytolytic effect rather than CD56bright one which is usually considered as a phenotype with highest capacity of IFN-γ release [180], [181]. Therefore, if NK cell therapy is to be considered as an elective treatment for COVID-19 patients, the best option is the administration of CD56dim NK cells with the highest levels of cytolytic capacities. On the other hand, it should be noted that due to the destructive role of IFN-γ in the acute exacerbation of patients’ condition with the induction of cytokine storms [146], administration of CD56bright NK cells might be harmful to the patients. In a same way, unlike genetically engineered T cells that produce high levels of IFN-γ, similar NK cell lines do not produce high levels of this interferon and therefore will not induce cytokine storms [147], which also suggests them as a suitable choice for cell therapy in these patients. As the first investigational cell-based drug for COVID-19 patients to be approved by FDA, Celularity has introduced its off-the-shelf, allogeneic cryopreserved NK cell derived from placental CD34+ cells to be used for cellular therapy in COVID-19 (clinical trials phase I/II with gov identifier: NCT04365101) [183]. In phase I, the trial aims to evaluate the adverse effects of NK cell infusion to the mild, non-ICU COVID-19 patients. In another clinical trial being conducted in China (ClinicalTrials.gov identifier: NCT04280224), 30 pneumatic COVID-19 patients are subjected to a conventional treatment plus twice a week of NK cell therapies. More intriguingly, in a clinical trial study conducted in Colombia (clinical trials phase I/II with gov identifier: NCT04344548), allogeneic NK cells from PBMCs of healthy donors are subjected to patients infected with SARS-CoV-2. They collect donors’ PBMCs by apheresis. Then through immunomagnetic removal of several types of unwanted cells such as B, T and CD33+ cells, they enrich NK cells which will be expanded in bioreactors with GMP culture media supplemented with IL-2, and IL-15. This technique is almost similar to the method presented by the authors, except that in ours, the donors were prescribed with G-CSF to mobilize more precursor cells into their circulation (G-CSF mobilized PBMCs) [180]. Similarly, it seems that here, the supplementation of IL-2 and IL-15 provides more NK cell maturation (CD56dim phenotype) with an appropriate cytolytic effect. Besides adoptive cell therapies, with the technologies available to produce chimeric antigen receptor (CAR)-NK cells, it is nowadays also possible to design specific cell line expressing any receptor(s) of interest to virtually enhance NK cell capabilities to eliminate targets through the recognition of their specific antigens [184], [185]. This technique which has already applied for successful treatment of some cancers, not only enhances the function of NK cells but it also provides specificity for more targeted function of these cells against malignancies. Currently, in a phase I/II study (with gov identifier: NCT04324996), the researchers apply two different CAR-NK cells in COVID-19 patients, one is NKG2D-ACE2 CAR-NK cells which can target SARS-CoV-2 virions and infected cells, and another is ACE2 CAR-NK cells. Once these cells get infected with SARS-CoV-2 virions, they may act as decoy cells that trap viruses while indirectly protect lung epithelial cells from the infection. This design is based on the suggestion that ACE2 expressed by NK cell can act as a receptors for the virus. Instead of NK cell therapy in COVID-19 patients, the patients may be alternatively treated with cytokines to enhance the function of their persistence NK cells. The best selected cytokines ever used in clinical trials are IL-2 and IL-15 [176], [177], [178] which are specifically involved in the expansion and maturation of NK cells [180]. Accordingly in a study on RV144 HIV-1 vaccine induced antibodies, Fisher, Leigh, et al suggested that vaccine-induced ADCC-mediating antibodies produce more effective cytotoxicity against infected cells by NK cells incubated with IL-15 [186]. It seems that if the cytokine therapy is successful, their use in the treatment of COVID-19 patients can be less costly and time consuming than NK cell therapies. However, due the fact that these interleukins also act as pro-inflammatory cytokines, their therapeutic use should be associated with extreme caution to prevent the worsening of COVID-19 patients’ inflammatory conditions. In particular, previous studies have shown an association between elevated levels of IL-15 and chronic pulmonary inflammatory diseases [187] and Middle East respiratory syndrome coronavirus (MERS-CoV) infection [188].
Fig. 2.
NK cell mediated cytotoxicity against infected hyper-activated macrophage: a) Macrophage senses SARS-CoV-2 antigens with PRR receptors. Virus can also enter the macrophage through the interaction with ACE2. b) Infected macrophages express reduced levels of MHC-I molecule, helping them to evade from CD8+ T cell recognition and their cytolytic effects. c) Despite diminished expression of MHC-I molecule on macrophage, NK cell still recognizes different ligands on infected cells by its activating receptors where their interactions augment potent cytolytic effects of NK cells against infected macrophages, lowering viral load and cytokine storms. ACE; Angiotensin converting enzyme, MHC; Major histocompatibility, NK; Natural killer, PRR; Pattern recognition receptors, SARS-CoV; Severe acute respiratory syndrome-coronavirus.
10. NK memory cells: new insights from vaccine development to cell therapy against COVID-19
Several lines of evidence have shown that both neutralizing antibodies (which prevent the virus from entering target cells) and antibodies involved in cytotoxicity (ADCC) contribute to effective protection against viruses such as HIV and Ebola during infection or in vaccinated individuals [186], [189]. However, after vaccination, increasing the concentration of vaccine-induced antibodies along with the developing differentiation of NK cells plays a key role in preventing viral infection, the evidence that emphasizes the importance of antibody-mediated activation of NK cells in vaccine-induced immune responses. Here the eminent role of ADCC activity in controlling early replication of virus, which is achieved by the eradication of infected cells can even constrain the possible escape pathways of neutralizing antibodies to ensure efficient immune responses [190]. The clonal proliferation of antigen-specific lymphocytes is also considered as a prominent feature of immunological memory during primary infection. So far, several lines of evidence indicated that similar to adaptive immune cells, some subsets of NK cells can also present features of antigen-specific memory [191]. This was first confirmed by a study on primates (rhesus macaque) that indicated a strong and durable, antigen-specific NK cell memory after viral infection (by some immunodeficiency viruses) or vaccination (with Ad26 vectors expressing specific Env or Gag antigens of viruses) [192]. In this regard, identifying the population of “adaptive” NK cells (NKG2C+CD57+) that have expanded and remained as memory cells in CMV-infected patients is an important evidence for this claim in human studies [193], [194].These observations raise the possibility that NK cell memory may also be generated to pathogens other than CMV including SARS-CoV-2. More interestingly, NK memory cells generated by CMV showed a persistent clonal expansion that mainly express the activating receptor NKG2C whereas they were negative or low for the expression of the inhibitory receptor NKG2A [195]. This fact also attracts much interest to the future studies on vaccine development as well as other putative medical approaches that have so far highlighted the advantages of adoptive transfer of NK cells in the management of severe cases of COVID-19.
11. Conclusion
In the absence of any specific and definitive treatment for SARS-CoV-2, researchers have to devise alternative therapies to some extent to prevent the rapid spread and high mortality of COVID-19 by different strategies. From the point of view of most researchers, cytokine storm is considered to be as the leading cause of patients’ mortality and morbidity in severe cases of COVID-19. Therefore, any treatment that can alleviate these conditions in patients to some extent can be life-saving and effective that should not be ignored at all. Given the importance of strengthening the innate immune system, especially the function of NK cells to halt cytokine storms, it is very likely that this strategy will be effective in preventing either acute conditions of COVID-19 or in treatment of severe forms of this disease. In this regard, different strategies of NK cell therapies will be a choice that may effectively benefit the patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Parts of this paper have been extracted from EH's doctoral thesis at Monash University (as referred). The authors wish to thank Prof. Anthony P. Schwarer from Department of Hematology and Oncology, Eastern School, Monash University, Melbourne, Australia.
Authors’ contributions
EH provided intellectual input, co-wrote the paper as corresponding author.
MG provided intellectual input, and wrote the paper as first authors.
AG provided intellectual input, depicted figures and wrote their legends.
References
- 1.Chan J.-F.-W., Yuan S., Kok K.-H., To K.-K.-W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2019;2020(395):514. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenkel LD, Gomez F, Bellanti JA: COVID-19 in children: Pathogenesis and current status. Allergy & Asthma Proceedings, vol 42, 2021. [DOI] [PubMed]
- 3.Lee B., Raszka W.V. COVID-19 in children: looking forward, not back. Pediatrics. 2021;147 doi: 10.1542/peds.2020-029736. [DOI] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.M.D. Park, Macrophages: a Trojan horse in COVID-19?, Nature Publishing Group, 2020. [DOI] [PMC free article] [PubMed]
- 7.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird S.M. In: Williams Hematology. Lichtman M.A., Beutler E., Kipps T.J., Seligsohn U., Kaushansky K., Prchal J.T., editors. McGraw-Hill Companies Inc; New York: 2006. Morphology of lymphocytes and plasma cells. http://www.accessmedicine.com.ezproxy.lib.monash.edu.au/content.aspx?aID=2146569. [Google Scholar]
- 10.Trinchieri G., Lanier L. In: Williams Hematology. Lichtman M.A., Beutler E., Kipps T.J., Seligsohn U., Kaushansky K., Prchal J.T., editors. McGraw-Hill Companies Inc; New York: 2006. Functions of natural killer cells. http://www.accessmedicine.com.ezproxy.lib.monash.edu.au/content.aspx?aID=2147009. [Google Scholar]
- 11.Young J.D., Cohn Z.A. Cellular and humoral mechanisms of cytotoxicity: structural and functional analogies. Adv. Immunol. 1987;41:269. doi: 10.1016/s0065-2776(08)60033-4. [DOI] [PubMed] [Google Scholar]
- 12.Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J. Immunol. 1983;130:2133. [PubMed] [Google Scholar]
- 13.Yokoyama W.M., Kim S., French A.R. The dynamic life of natural killer cells. Annu. Rev. Immunol. 2004;22:405. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 14.Ferlazzo G., Münz C. NK cell compartments and their activation by dendritic cells. J. Immunol. 2004;172:1333. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad A., Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J. Leukoc. Biol. 2004;76:743. doi: 10.1189/jlb.0304197. [DOI] [PubMed] [Google Scholar]
- 16.Gao B., Radaeva S., Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J. Leukoc. Biol. 2009;86:513. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai H.-C., Chang C.-J., Lin C.-S., Wu T.-R., Hsu Y.-J., Wu T.-S., et al. NK cell-derived IFN-γ protects against nontuberculous mycobacterial lung infection. J. Immunol. 2018;201:1478. doi: 10.4049/jimmunol.1800123. [DOI] [PubMed] [Google Scholar]
- 19.Aguilera E.R., Lenz L.L. Inflammation as a modulator of host susceptibility to pulmonary influenza, pneumococcal, and co-infections. Front. Immunol. 2020;11:105. doi: 10.3389/fimmu.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masselli E., Vaccarezza M., Carubbi C., Pozzi G., Presta V., Mirandola P., et al. NK cells: a double edge sword against SARS-CoV-2. Adv. Biol. Regul. 2020;77 doi: 10.1016/j.jbior.2020.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardain A., Marakalala M.J., Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2020;159:245. doi: 10.1111/imm.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier L.L., Phillips J.H., Hackett J., Jr., Tutt M., Kumar V. Natural killer cells: definition of a cell type rather than a function. J. Immunol. 1986;137:2735. [PubMed] [Google Scholar]
- 23.Hercend T., Griffin J.D., Bensussan A., Schmidt R.E., Edson M.A., Brennan A., et al. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985;75:932. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanier L.L., Le A.M., Phillips J.H., Warner N.L., Babcock G.F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J. Immunol. 1983;131:1789. [PubMed] [Google Scholar]
- 25.Cooper M.A., Fehniger T.A., Turner S.C., Chen K.S., Ghaheri B.A., Ghayur T., et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood, J. Am. Soc. Hematol. 2001;97:3146. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 26.Ljunggren H.-G., Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 27.Bryceson Y.T., March M.E., Ljunggren H.G., Long E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006;214:73. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivier E., Nunès J.A., Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 29.Pegram H.J., Andrews D.M., Smyth M.J., Darcy P.K., Kershaw M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011;89:216. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 30.Hosseini E. Monash University); 2010. HLA-E Polymorphisms and NK Cell Reconstitution After Allogeneic Hematopoietic Stem Cell Transplantation. Doctoral dissertation. [Google Scholar]
- 31.Andre P., Biassoni R., Colonna M., Cosman D., Lanier L.L., Long E.O., et al. New nomenclature for MHC receptors. Nat. Immunol. 2001;2:661. doi: 10.1038/90589. [DOI] [PubMed] [Google Scholar]
- 32.Carrington M, Norman PJ: The KIR gene cluster. National Library of Medicine (U.S.), Vol. 2003. National Center for Biotechnology Information, Bethesda, Md.. 2003 phttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books.
- 33.Biassoni R., Cantoni C., Falco M., Verdiani S., Bottino C., Vitale M., et al. The human leukocyte antigen (HLA)-C-specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J. Exp. Med. 1996;183:645. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biassoni R., Pessino A., Malaspina A., Cantoni C., Bottino C., Sivori S., et al. Role of amino acid position 70 in the binding affinity of p50.1 and p58.1 receptors for HLA-Cw4 molecules. Eur. J. Immunol. 1997;27:3095. doi: 10.1002/eji.1830271203. [DOI] [PubMed] [Google Scholar]
- 35.Dennehy K.M., Klimosch S.N., Steinle A. Cutting edge: NKp80 uses an atypical hemi-ITAM to trigger NK cytotoxicity. J. Immunol. 2011;186:657. doi: 10.4049/jimmunol.0904117. [DOI] [PubMed] [Google Scholar]
- 36.Augugliaro R., Parolini S., Castriconi R., Marcenaro E., Cantoni C., Nanni M., et al. Selective cross-talk among natural cytotoxicity receptors in human natural killer cells. Eur. J. Immunol. 2003;33:1235. doi: 10.1002/eji.200323896. [DOI] [PubMed] [Google Scholar]
- 37.R. Biassoni, Natural killer cell receptors Multichain Immune Recognition Receptor Signaling, Spatiotemporal Organization to Human Disease Series. In : Advances in Experimental Medicine and Biology vol Vol. 640 (A.B. Sigalov, ed.) New York, Springer, 2008, p 35. [PubMed]
- 38.Matta J., Baratin M., Chiche L., Forel J.-M., Cognet C., Thomas G., et al. Induction of B7–H6, a ligand for the natural killer cell–activating receptor NKp30, in inflammatory conditions. Blood, J. Am. Soc. Hematol. 2013;122:394. doi: 10.1182/blood-2013-01-481705. [DOI] [PubMed] [Google Scholar]
- 39.Middleton D., Curran M., Maxwell L. Natural killer cells and their receptors. Transpl. Immunol. 2002;10:147. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 40.Boyton R.J., Altmann D.M. Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin. Exp. Immunol. 2007;149:1. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borrego F., Masilamani M., Kabat J., Sanni T.B., Coligan J.E. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol. Immunol. 2005;42:485. doi: 10.1016/j.molimm.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 42.Gasser S., Raulet D.H. Activation and self-tolerance of natural killer cells. Immunol. Rev. 2006;214:130. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 43.Petrie E.J., Clements C.S., Lin J., Sullivan L.C., Johnson D., Huyton T., et al. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 2008;205:725. doi: 10.1084/jem.20072525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrego F., Ulbrecht M., Weiss E.H., Coligan J.E., Brooks A.G. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J. Exp. Med. 1998;187:813. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braud V.M., Allan D.S., O'Callaghan C.A., Soderstrom K., D'Andrea A., Ogg G.S., et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 46.Ulbrecht M., Martinozzi S., Grzeschik M., Hengel H., Ellwart J.W., Pla M., et al. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J. Immunol. 2000;164:5019. doi: 10.4049/jimmunol.164.10.5019. [DOI] [PubMed] [Google Scholar]
- 47.Tomasec P., Braud V.M., Rickards C., Powell M.B., McSharry B.P., Gadola S., et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 48.Michaëlsson J., Teixeira de Matos C., Achour A., Lanier L.L., Kärre K., Söderström K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med. 2002;196:1403. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watzl C., Long E.O. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J. Exp. Med. 2003;197:77. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez S., Lopez-Soto A., Suarez-Alvarez B., Lopez-Vazquez A., Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 52.Chalupny N.J., Sutherland C.L., Lawrence W.A., Rein-Weston A., Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem. Biophys. Res. Commun. 2003;305:129. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 53.Cosman D., Müllberg J., Sutherland C.L., Chin W., Armitage R., Fanslow W., et al. ULBPs, novel MHC class I–related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 54.Caligiuri M.A. Human natural killer cells. Blood, J. Am. Soc. Hematol. 2008;112:461. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horng T., Bezbradica J.S., Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat. Immunol. 2007;8:1345. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 56.Wensveen F.M., Jelenčić V., Polić B. NKG2D: a master regulator of immune cell responsiveness. Front. Immunol. 2018;9:441. doi: 10.3389/fimmu.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavez-Galan L., Arenas-Del Angel M.C., Zenteno E., Chavez R., Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 2009;6:15. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters P.J., Borst J., Oorschot V., Fukuda M., Krahenbuhl O., Tschopp J., et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991;173:1099. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauer H., Pratsch L., Tschopp J., Bhakdi S., Peters R. Functional size of complement and perforin pores compared by confocal laser scanning microscopy and fluorescence microphotolysis. BBA. 1991;1063:137. doi: 10.1016/0005-2736(91)90363-d. [DOI] [PubMed] [Google Scholar]
- 60.Liu C.C., Walsh C.M., Young J.D. Perforin: structure and function. Immunol. Today. 1995;16:194. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 61.Voskoboinik I., Thia M.C., Fletcher J., Ciccone A., Browne K., Smyth M.J., et al. Calcium-dependent plasma membrane binding and cell lysis by perforin are mediated through its C2 domain: a critical role for aspartate residues 429, 435, 483, and 485 but not 491. J. Biol. Chem. 2005;280:8426. doi: 10.1074/jbc.M413303200. [DOI] [PubMed] [Google Scholar]
- 62.Cullen S.P., Martin S.J. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 63.Latinovic-Golic S., Walch M., Sundstrom H., Dumrese C., Groscurth P., Ziegler U. Expression, processing and transcriptional regulation of granulysin in short-term activated human lymphocytes. BMC Immunol. 2007;8:9. doi: 10.1186/1471-2172-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alderson K.L., Sondel P.M. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z.X., Yang L., Young K.J., DuTemple B., Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat. Med. 2000;6:782. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 66.Chen J.J., Sun Y., Nabel G.J. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janeway C.A., Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 69.Vidal S.M., Khakoo S.I., Biron C.A. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2011;1:497. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orange J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132:515. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biron C.A., Byron K.S., Sullivan J.L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989;320:1731. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 72.Rolston K.V. Infections in cancer patients with solid tumors: a review. Infect Dis Ther. 2017;6:69. doi: 10.1007/s40121-017-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J.-C., Lee K.-M., Kim D.-W., Heo D.S. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J. Immunol. 2004;172:7335. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 74.Tai L.-H., de Souza C.T., Bélanger S., Ly L., Alkayyal A.A., Zhang J., et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97. doi: 10.1158/0008-5472.CAN-12-1993. [DOI] [PubMed] [Google Scholar]
- 75.Ardolino M., Azimi C.S., Iannello A., Trevino T.N., Horan L., Zhang L., et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Investig. 2014;124:4781. doi: 10.1172/JCI74337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angka L., Martel A.B., Kilgour M., Jeong A., Sadiq M., de Souza C.T., et al. Natural killer cell IFNγ secretion is profoundly suppressed following colorectal cancer surgery. Ann. Surg. Oncol. 2018;25:3747. doi: 10.1245/s10434-018-6691-3. [DOI] [PubMed] [Google Scholar]
- 77.Hijano D.R., Maron G., Hayden R.T. Respiratory viral infections in patients with cancer or undergoing hematopoietic cell transplant. Front. Microbiol. 2018;9:3097. doi: 10.3389/fmicb.2018.03097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J. Innate Immun. 2020;12:4. doi: 10.1159/000503030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNab F., Mayer-Barber K., Sher A., Wack A. O'garra A: type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin T.R., Frevert C.W. Innate immunity in the lungs. Proc. Am. Thoracic Soc. 2005;2:403. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paolini R., Bernardini G., Molfetta R., Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Zwirner N.W., Domaica C.I. Cytokine regulation of natural killer cell effector functions. BioFactors. 2010;36:274. doi: 10.1002/biof.107. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen K., Salazar-Mather T.P., Dalod M.Y., Van Deusen J.B., Wei X.Q., Liew F.Y., Caligiuri M.A., Durbin J.E., Biron C.A. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;169:4279. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 84.Wu Y., Tian Z., Wei H. Developmental and functional control of natural killer cells by cytokines. Front. Immunol. 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zucchini N., Crozat K., Baranek T., Robbins S.H., Altfeld M., Dalod M. Natural killer cells in immunodefense against infective agents. Expert Rev. Anti-infective Therapy. 2008;6:867. doi: 10.1586/14787210.6.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu M.M., Mahmoud A.B., Makrigiannis A.P. Licensed and unlicensed NK cells: differential roles in cancer and viral control. Front. Immunol. 2016;7:166. doi: 10.3389/fimmu.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horton N.C., Mathew P.A. NKp44 and natural cytotoxicity receptors as damage-associated molecular pattern recognition receptors. Front. Immunol. 2015;6:31. doi: 10.3389/fimmu.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huntington N.D., Martinet L., Smyth M.J. DNAM-1: would the real natural killer cell please stand up! Oncotarget. 2015;6:28537. doi: 10.18632/oncotarget.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D'andrea A., Chang C., Franz-Bacon K., McClanahan T., Phillips J., Lanier L. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J. Immunol. 1995;155:2306. [PubMed] [Google Scholar]
- 90.Wagtmann N., Biassoni R., Cantoni C., Verdiani S., Malnati M.S., Vitale M., et al. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra-and intracellular domains. Immunity. 1995;2:439. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 91.Colonna M., Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 92.Allan D.S.J., O'Callaghan C.A., Söderström K., D'Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., Lanier L.L., McMichael A.J. HLA-E binds to natural killer receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 93.Vance R.E., Kraft J.R., Altman J.D., Jensen P.E., Raulet D.H. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1b. J. Exp. Med. 1998;188:1841. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adib-Conquy M., Scott-Algara D., Cavaillon J.M., Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol. Cell Biol. 2014;92:256. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 95.Orr M.T., Sun J.C., Hesslein D.G., Arase H., Phillips J.H., Takai T., et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J. Exp. Med. 2009;206:807. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krzewski K., Coligan J.E. Human NK cell lytic granules and regulation of their exocytosis. Front. Immunol. 2012;3:335. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S.-H., Miyagi T., Biron C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Mitra R., Singh S., Khar A. Antitumour immune responses. Expert Rev. Mol. Med. 2003;5:1. doi: 10.1017/S1462399403005623. [DOI] [PubMed] [Google Scholar]
- 99.Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kärre K. NK cells, MHC class I molecules and the missing self. Scand. J. Immunol. 2002;55:221. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 101.Osman M.S., van Eeden C., Tervaert J.W.C. Fatal COVID-19 infections: Is NK cell dysfunction a link with autoimmune HLH? Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toubi E., Vadasz Z. Innate immune-responses and their role in driving autoimmunity. Autoimmun. Rev. 2019;18:306. doi: 10.1016/j.autrev.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Hosseini E., Schwarer A.P., Ghasemzadeh M. Do human leukocyte antigen E polymorphisms influence graft-versus-leukemia after allogeneic hematopoietic stem cell transplantation? Exp. Hematol. 2015;43:149. doi: 10.1016/j.exphem.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., et al. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marcenaro E., Ferranti B., Moretta A. NK–DC interaction: on the usefulness of auto-aggression. Autoimmun. Rev. 2005;4:520. doi: 10.1016/j.autrev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 106.Vitale M., Cantoni C., Della Chiesa M., Ferlazzo G., Carlomagno S., Pende D., et al. An historical overview: the discovery of how NK cells can kill enemies, recruit defense troops, and more. Front. Immunol. 2019;10:1415. doi: 10.3389/fimmu.2019.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lam V.C., Lanier L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017;44:43. doi: 10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013;210:1283. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scapini P., Cassatella M.A. Social networking of human neutrophils within the immune system. Blood, J. Am. Soc. Hematol. 2014;124:710. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 110.Costantini C., Micheletti A., Calzetti F., Perbellini O., Pizzolo G., Cassatella M.A. Neutrophil activation and survival are modulated by interaction with NK cells. Int. Immunol. 2010;22:827. doi: 10.1093/intimm/dxq434. [DOI] [PubMed] [Google Scholar]
- 111.Thorén F.B., Riise R.E., Ousbäck J., Della Chiesa M., Alsterholm M., Marcenaro E., et al. Human NK Cells induce neutrophil apoptosis via an NKp46-and Fas-dependent mechanism. J. Immunol. 2012;188:1668. doi: 10.4049/jimmunol.1102002. [DOI] [PubMed] [Google Scholar]
- 112.Jaeger B.N., Donadieu J., Cognet C., Bernat C., Ordoñez-Rueda D., Barlogis V., et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012;209:565. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riise R.E., Bernson E., Aurelius J., Martner A., Pesce S., Della Chiesa M., et al. TLR-stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses. J. Immunol. 2015;195:1121. doi: 10.4049/jimmunol.1500709. [DOI] [PubMed] [Google Scholar]
- 114.Pliyev B.K., Kalintseva M.V., Abdulaeva S.V., Yarygin K.N., Savchenko V.G. Neutrophil microparticles modulate cytokine production by natural killer cells. Cytokine. 2014;65:126. doi: 10.1016/j.cyto.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Amano K, Hirayama M, Azuma E, Iwamoto S, Keida Y, Komada Y: Neutrophils induced licensing of natural killer cells. Mediators Inflamm. 2015;2015. [DOI] [PMC free article] [PubMed]
- 116.Michel T., Hentges F., Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2013;3:403. doi: 10.3389/fimmu.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferlazzo G., Pack M., Thomas D., Paludan C., Schmid D., Strowig T., et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. 2004;101:16606. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vitale M., Chiesa M.D., Carlomagno S., Romagnani C., Thiel A., Moretta L., et al. The small subset of CD56brightCD16–natural killer cells is selectively responsible for both cell proliferation and interferon-γ production upon interaction with dendritic cells. Eur. J. Immunol. 2004;34:1715. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 119.Mortier E., Woo T., Advincula R., Gozalo S., Ma A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 2008;205:1213. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2002;2:957. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 121.Vogel K., Thomann S., Vogel B., Schuster P., Schmidt B. Both plasmacytoid dendritic cells and monocytes stimulate natural killer cells early during human herpes simplex virus type 1 infections. Immunology. 2014;143:588. doi: 10.1111/imm.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swiecki M., Wang Y., Gilfillan S., Colonna M. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pende D., Castriconi R., Romagnani P., Spaggiari G.M., Marcenaro S., Dondero A., et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 124.Smith L.E., Olszewski M.A., Georgoudaki A.M., Wagner A.K., Hägglöf T., Karlsson M.C., et al. Sensitivity of dendritic cells to NK-mediated lysis depends on the inflammatory environment and is modulated by CD54/CD226-driven interactions. J. Leukoc. Biol. 2016;100:781. doi: 10.1189/jlb.3A0615-271RR. [DOI] [PubMed] [Google Scholar]
- 125.Mailliard R.B., Son Y.-I., Redlinger R., Coates P.T., Giermasz A., Morel P.A., et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 2003;171:2366. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 126.Morandi B., Bougras G., Muller W.A., Ferlazzo G., Münz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-γ secretion. Eur. J. Immunol. 2006;36:2394. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 127.Waggoner S.N., Daniels K.A., Welsh R.M. Therapeutic depletion of natural killer cells controls persistent infection. J. Virol. 2014;88:1953. doi: 10.1128/JVI.03002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su H.C., Nguyen K.B., Salazar-Mather T.P., Ruzek M.C., Dalod M.Y., Biron C.A. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 2001;31:3048. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 129.Krebs P., Barnes M.J., Lampe K., Whitley K., Bahjat K.S., Beutler B., et al. NK cell–mediated killing of target cells triggers robust antigen-specific T cell–mediated and humoral responses. Blood, J. Am. Soc. Hematol. 2009;113:6593. doi: 10.1182/blood-2009-01-201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cook K.D., Waggoner S.N., Whitmire J.K. NK cells and their ability to modulate T cells during virus infections. Crit. Rev.™ in Immunol. 2014;34 doi: 10.1615/critrevimmunol.2014010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respiratory Soc. 2020 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. Covid-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153:725. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vandenhaute J., Wouters C.H., Matthys P. Natural killer cells in systemic autoinflammatory diseases: a focus on systemic juvenile idiopathic arthritis and macrophage activation syndrome. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F., et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67:3037. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 139.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Investig. 2020;130 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lassen M.G., Lukens J.R., Dolina J.S., Brown M.G., Hahn Y.S. Intrahepatic IL-10 maintains NKG2A+ Ly49− liver NK cells in a functionally hyporesponsive state. J. Immunol. 2010;184:2693. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020 doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ge X., Li C.R., Yang J., Wang G.B. Aberrantly decreased levels of NKG 2D expression in children with Kawasaki disease. Scand. J. Immunol. 2013;77:389. doi: 10.1111/sji.12022. [DOI] [PubMed] [Google Scholar]
- 144.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh Y., Chhabra S., Lashkari K., Taneja A., Garg A., Chandra A., et al. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb®) in the management of septic shock: a retrospective observational study. Int. J. Artificial Organs. 2020;43:372. doi: 10.1177/0391398819891739. [DOI] [PubMed] [Google Scholar]
- 146.Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Q.-M. Dong, Z.-P. He, H. Zhuang, S.-J. Song, W.-S. Dai, S.-P. Zhang et al.: Dynamics of peripheral blood B lymphocytes and natural killer cells in patients with severe acute respiratory syndrome. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi 2004;25:695. [PubMed]
- 149.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet. Infect. Dis. 2013;13:752. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 151.Cunha C.B., Opal S.M. Middle East respiratory syndrome (MERS) A new zoonotic viral pneumonia. Virulence. 2014;5:650. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Al-Tawfiq J., Hinedi K., Abbasi S., Babiker M., Sunji A., Eltigani M. Hematologic, hepatic, and renal function changes in hospitalized patients with Middle East respiratory syndrome coronavirus. Int. J. Lab. Hematol. 2017;39:272. doi: 10.1111/ijlh.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rajaram S., Canaday L.M., Ochayon D.E., Rangel K.M., Ali A., Gyurova I.E., et al. The promise and peril of natural killer cell therapies in pulmonary infection. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;1 doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 157.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Y. Zhu, M. Jiang, L. Gao, X. Huang, Single cell analysis of ACE2 expression reveals the potential targets for 2019-nCoV, 2020.
- 159.Mao H., Tu W., Liu Y., Qin G., Zheng J., Chan P.-L., et al. Inhibition of human natural killer cell activity by influenza virions and hemagglutinin. J. Virol. 2010;84:4148. doi: 10.1128/JVI.02340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wong C., Lam C., Wu A., Ip W., Lee N., Chan I., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.J. Carvelli, O. Demaria, F. Vély, L. Batista, N.C. Benmansour, J. Fares, et al.: Identification of immune checkpoints in COVID-19. 2020. [DOI] [PMC free article] [PubMed]
- 162.A.J. Wilk, A. Rustagi, N.Q. Zhao, J. Roque, G.J. Martinez-Colon, J.L. McKechnie, et al., A single-cell atlas of the peripheral immune response to severe COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 163.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T., et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Habif G., Crinier A., André P., Vivier E., Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell. Mol. Immunol. 2019;16:415. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mamessier E., Sylvain A., Thibult M.-L., Houvenaeghel G., Jacquemier J., Castellano R., et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011;121:3609. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van Hall T., André P., Horowitz A., Ruan D.F., Borst L., Zerbib R., et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J. ImmunoTher. Cancer. 2019;7:263. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Sem. Immunopathol. 2017;39:529. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020 doi: 10.1016/j.cell.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84:1289. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Murthy H., Iqbal M., Chavez J.C., Kharfan-Dabaja M.A. Cytokine release syndrome: current perspectives. ImmunoTargets Therapy. 2019;8:43. doi: 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fujisaki H., Kakuda H., Shimasaki N., Imai C., Ma J., Lockey T., et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cooley S., He F., Bachanova V., Vercellotti G.M., DeFor T.E., Curtsinger J.M., et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3:1970. doi: 10.1182/bloodadvances.2018028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yaqinuddin A., Kashir J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med. Hypotheses. 2020 doi: 10.1016/j.mehy.2020.109777. 109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghasemzadeh M., Hosseini E., Schwarer A.P., Pourfathollah A.A. NK cell maturation to CD56(dim) subset associated with high levels of NCRs overrides the inhibitory effect of NKG2A and recovers impaired NK cell cytolytic potential after allogeneic hematopoietic stem cell transplantation. Leuk. Res. 2016;43:58. doi: 10.1016/j.leukres.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 181.Hosseini E., Ghasemzadeh M., Kamalizad M., Schwarer A.P. Ex vivo expansion of CD3(depleted) cord blood-MNCs in the presence of bone marrow stromal cells; an appropriate strategy to provide functional NK cells applicable for cellular therapy. Stem Cell Res. 2017;19:148. doi: 10.1016/j.scr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 182.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Disc. 2020:1. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 183.I. Celularity, Celularity Announces FDA Clearance of IND Application for CYNK-001 in Coronavirus, First in Cellular Therapy. PR Newswire. Available online at: 4. 2020.
- 184.Han J., Chu J., Chan W.K., Zhang J., Wang Y., Cohen J.B., et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382:545. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fisher L., Zinter M., Stanfield-Oakley S., Carpp L.N., Edwards R.W., Denny T., et al. Vaccine-induced antibodies mediate higher antibody-dependent cellular cytotoxicity after interleukin-15 pretreatment of natural killer effector cells. Front. Immunol. 2019;10:2741. doi: 10.3389/fimmu.2019.02741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muro S., Taha R., Tsicopoulos A., Olivenstein R., Tonnel A.-B., Christodoulopoulos P., et al. Expression of IL-15 in inflammatory pulmonary diseases. J. Allergy Clin. Immunol. 2001;108:970. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 188.Noroozi R., Branicki W., Pyrc K., Łabaj P.P., Pośpiech E., Taheri M., et al. Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine. 2020;155143 doi: 10.1016/j.cyto.2020.155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wagstaffe H.R., Clutterbuck E.A., Bockstal V., Stoop J.N., Luhn K., Douoguih M., et al. Antibody-dependent natural killer cell activation after Ebola vaccination. J. Infect. Dis. 2021;223:1171. doi: 10.1093/infdis/jiz657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mielke D., Bandawe G., Pollara J., Abrahams M.-R., Nyanhete T., Moore P.L., et al. Antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies constrain neutralizing antibody escape pathway. Front. Immunol. 2019;10:2875. doi: 10.3389/fimmu.2019.02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sheppard S., Sun J.C. Virus-specific NK cell memory. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reeves R.K., Li H., Jost S., Blass E., Li H., Schafer J.L., et al. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 2015;16:927. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lopez-Vergès S., Milush J.M., Schwartz B.S., Pando M.J., Jarjoura J., York V.A., et al. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. 2011;108:14725. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gumá M., Angulo A., Vilches C., Gómez-Lozano N., Malats N., López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 195.Zhang T., Scott J.M., Hwang I., Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J. Immunol. 2013;190:1402. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]