Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a pandemic of respiratory and cardiovascular diseases, known as coronavirus disease 2019 (COVID-19). SARS-CoV-2 encodes the structural proteins spike (S), envelope (E), membrane (M), and nucleocapsid (N). The receptor-binding domain on the surface subunit S1 is responsible for attachment of the virus to angiotensin (Ang)-converting enzyme 2 (ACE2), which is highly expressed in host cells. The cytokine storm observed in patients with COVID-19 contributes to the endothelial vascular dysfunction, which can lead to acute respiratory distress syndrome, multiorgan failure, alteration in iron homeostasis, and death. Growth and differentiation factor 15 (GDF15), which belongs to the transforming growth factor-β (TGF-β) superfamily of proteins, has a pivotal role in the development and progression of diseases because of its role as a metabolic regulator. In COVID-19, GDF15 activity increases in response to tissue damage. GDF15 appears to be a strong predictor of poor outcomes in patients critically ill with COVID-19 and acts as an ‘inflammation-induced central mediator of tissue tolerance’ via its metabolic properties. In this review, we examine the potential properties of GDF15 as an emerging modulator of immunity in COVID-19 in association with iron metabolism. The virus life cycle in host cell provides potential targets for drug therapy.
Keywords: GDF15, COVID-19, metabolism, iron, therapeutic
Introduction
SARS-CoV-2 (see Glossary) is a transmissible, pathogenic coronavirus that has caused a pandemic of acute respiratory diseases, now known as COVID-19. COVID-19 was first reported in Wuhan, China, in late December 2019, spreading rapidly to become a global pandemic. Cardiovascular complications are rapidly emerging as a major threat in COVID-19 in addition to respiratory disease. Compared with SARS-CoV-1, SARS-CoV-2 is more readily transmitted from human to human, spreading to multiple continents and leading to the declaration of a Public Health Emergency of International Concern by the WHO [1]. Whole-genome sequencing identified a novel coronavirus: SARS-CoV-2 [2]. COVID-19 is characterized by two or three stages: ~80% of those infected experience two stages of illness, beginning with an asymptomatic incubation period, followed by symptomatic illness lasting for several weeks. The third phase is characterized by severe respiratory illness, frequently accompanied by organ dysfunction. COVID-19 can lead to acute respiratory distress syndrome (ARDS) and has high morbidity and mortality. COVID-19 also affects the cardiovascular, renal, cerebrovascular, and blood coagulation systems [3]. Between the index case in early December 2019 and July 1, 2021, more than 180 million cases and 4 million deaths due to COVID-19 were reported worldwide.
COVID-19, cellular injury and induction of endothelialitis
Structure of SARS-CoV-2
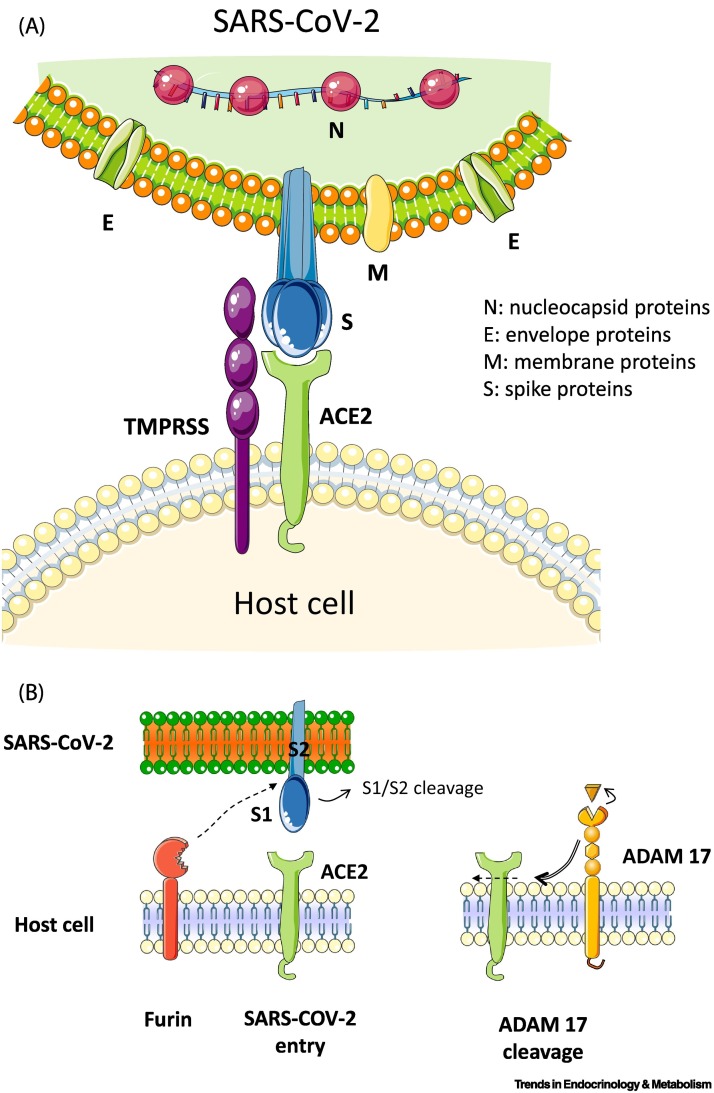
SARS-CoV-2 is a member of the genus Betacoronavirus, similar to the coronaviruses that have caused previous pandemic diseases, such as the Middle East respiratory syndrome coronavirus (MERS-CoV) [3,4]. Coronaviruses, which are a large family of single-stranded enveloped RNA viruses, were not recognized as being particularly pathogenic in humans until the outbreak of SARS caused by SARS-CoV in 2002. Coronaviruses can be divided into four genera: α, β, γ, and δ, of which only α and β-coronaviruses are known to infect humans. Phylogenetic analyses have demonstrated that SARS-CoV-2 and SARS-CoV-1 are closely related, with a genomic percent identity of ~80%. SARS-CoV-2 has a positive-sense RNA genome that encodes 16 nonstructural proteins (NSPs), which are essential for replicative functions, such as RNA polymerization by the RNA-dependent RNA polymerase, and four structural proteins. Coronaviruses have a crown-like morphology, comprising these four structural proteins, known as the S, E, M, and N proteins. The viral genome, surrounded by the N protein, is a single-stranded RNA [5,6]. Coronavirus S proteins comprise three segments: an ectodomain, a single-pass transmembrane anchor, and an intracellular tail. The spike S comprises two subunits, S1 and S2. To engage a host cell receptor, the receptor-binding domain (RBD) of S1 undergoes hinge-like conformational movements that transiently hide or expose the determinants of receptor binding. The S protein contains two functional domains: the first is the RBD, and the second is a domain containing sequences that mediate the fusion of viral and cell membranes. The S protein contains a potential cleavage site for furin protease and a polybasic furin cleavage site (FCS). In COVID-19, FCS is reportedly linked to natural selection, and it appears that FCS is necessary for SARS-CoV-2 to infect human lung cells. Furin is transcriptionally induced by Notch-1, a member of the Notch family of receptors that also includes Notch-2, -3, and -4. The interaction between the Notch receptor on one cell and the ligand on the adjacent cell results in conformational changes in the extracellular domain of the receptor, exposing a motif that is cleaved by A Disintegrin and Metalloprotease 17 (ADAM17, also known as tumor necrosis factor-α converting enzyme; TACE). Thus, Notch modulation can be considered a potential alternative approach to furin inhibition and ADAM17 upregulation. Therapeutics against SARS-CoV-2 should involve furin inhibitors [7] (Figure 1A,B).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus causing coronavirus disease 2019 (COVID-19), enters host cells via binding of its spike (S) protein to angiotensin (Ang)-converting enzyme 2 (ACE2).
(A) Coronaviruses have a crown-like morphology, comprising four structural proteins: S, envelope (E), membrane (M), and nucleocapsid (N) proteins. The receptor-binding domain (RBD) on the surface subunit S1 of the trimeric S protein is responsible for attachment of the virus to ACE2. Priming of S protein by transmembrane serine protease 2 (TMPRSS2), a cell membrane-bound protease, is an essential step for cell entry by the virus. (B) Furin precleaves S proteins at the S1/S2 site and promotes subsequent TMPRSS2-dependent entry into host cells. Cell membrane receptor ACE2 mediates entry of SARS-CoV-2. The spikes interact with ACE2 through a RBD. A disintegrin and metalloproteinase-17 (ADAM17) is a membrane-bound enzyme that cleaves cell surface proteins, such as ACE2. MPRSS2 competes with the metalloprotease ADAM17 for ACE2 processing.
ACE2/TMPRSS2/ADAM17 interplay in COVID-19
Among structural proteins, the trimeric S protein has a pivotal role in virus attachment and entry and disease pathogenesis. In SARS-CoVs, virus entry into the host cell is achieved by the viral S protein binding to ACE2 (Figure 1). The S protein comprises two subunits: the S1 subunit, which contains a RBD that binds to ACE2 on the surface of host cells, and the S2 subunit, which mediates fusion between the membranes of the virus and the host cell. ACE2 is a transmembrane zinc metalloprotease that has 42% homology with ACE. In the classical renin–Ang system (RAS) cascade, the decapeptide Ang I is converted to Ang II by ACE (kininase II; EC 3.4.15.1). There are two other peptidases: cathepsin A (carboxypeptidase A, lysosomal protective protein, deamidase; EC 3.4.16.5) and human homolog of ACE (ACE2). The Ang II type 1 receptor (AT1 receptor) is one of the key players in the RAS. The AT1 receptor promotes various intracellular signaling pathways through NADPH oxidase and reactive oxygen species (ROS) signaling [8] (Figure 2 and see Outstanding questions).
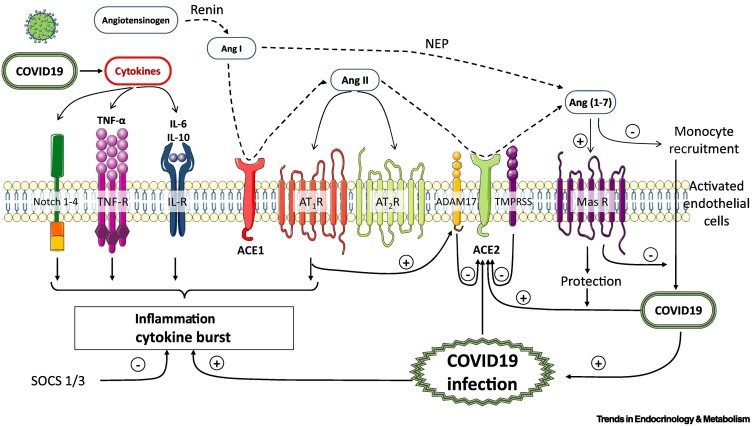
Figure 2.
Role of inflammatory response, cytokine storm, and monocyte recruitment in endothelial vascular dysfunction (endothelialitis) during coronavirus disease 2019 (COVID-19) [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection].
Angiotensin (Ang)-II is formed from the cleavage of Ang-I by angiotensin-converting enzyme (ACE). Ang-II binds to Ang-II type 1 (AT1R), and type 2 (AT2R) receptors. ACE2 is a monocarboxypeptidase homologous to ACE the active site of which is exposed at the extracellular surface. Ang II can be hydrolyzed into Ang(1-7) through the actions of ACE2. The polypeptide Ang I can be converted into Ang(1-7) by the actions of neprilysin (NEP). Ang(1-7) activates the G-protein-coupled mitochondrial assembly (MAS) receptor (MASR). The soluble form of ACE2 (sACE2) circulates in the blood. In COVID-19 (SARS-CoV-2), the proinflammatory response and cytokine storm represent the cornerstone of COVID-19 pathogenesis, inducing critical incidences for the host. A cytokine profile in COVID-19 is characterized by increases in various endogenous compounds, such as interleukin (IL) -6, IL-10, tumor necrosis factor-α (TNF-α), and the Notch 1-4 pathway. Suppressor of the cytokine signaling proteins (SOCS 1/3) regulate the immune response.
Outstanding questions.
GFRAL was identified as the receptor for GDF15, signaling though the RET coreceptor. Is GFRAL expression restricted to the brainstem region?
GDF15 is an informative protein with prognostic importance in patients with COVID-19. What are the molecular mechanisms implicated in these clinical conditions?
GDF15 is expressed and secreted in response to oxidative stress and inflammation, and is prominently induced following ‘injury’: what are the mechanisms involved in such clinical situations?
Is the ACE2/COVID-19 interface a promising therapeutic target? Could recombinant GDF15 be used as a treatment for COVID-19?
Alt-text: Outstanding questions
Similar to ACE, ACE2 is a member of the M2 family of zinc metallopeptidases. It exists in a membrane-bound form and is widely expressed in a variety of mammalian tissues, including the brain. ACE2 is an 805-amino acid-long carboxypeptidase, with a 17-amino acid N-terminal signal peptide and a C-terminal membrane anchor. ACE2 is a well-characterized negative regulator of the RAS system and converts Ang II into the vasodilatory fragment Ang(1-7), simultaneously decreasing the Ang II concentration to further facilitate the antihypertensive effects. Ang(1-7) activates the G-protein-coupled mitochondrial assembly (MAS) receptor (MASR), inducing many beneficial cardiovascular effects, including vasodilation, inhibition of cell growth, and antithrombotic effects.
The ACE2/Ang(1-7)/MASR axis has crucial roles in both the cardiovascular and immune systems, and its dysfunction intensifies inflammation and contributes to impaired function in the inflamed tissue. ACE2 and MASR are highly expressed in the lungs, kidneys, heart, and blood vessels. ACE2 expression is thought to be crucial for the biological mechanism underlying tissue-specific infection, and is expressed primarily in alveolar epithelial type II cells in normal adult lungs. These cells produce surfactant proteins that reduce surface tension, preventing the alveoli from collapsing [9]. The expression of ACE2 in the heart and coronary arteries is even higher than in the lungs. Single-cell RNA-sequencing data revealed that cardiomyocytes (especially those in the right ventricle) express ACE2 at a lower level than in pericytes [10,11]. A second protein that interacts with ACE2 is transmembrane protease serine 2 (TMPRSS2), which, as a transmembrane protease, is expressed in high levels in various organs. TMPRSS2 triggers the cleavage of S protein into S1 and S2 [12]. It is now demonstrated that SARS-CoV-2 uses ACE2 for cell entry, in which TMPRSS2 has a critical role. The steps that lead to virus entry are: (i) priming of S-protein by TMPRSS2, which is necessary for the correct maturation of these proteins; and (ii) formation of an entry route into the cell through ACE2, resulting in virus entry.
The role of ADAM17 in the progression of COVID-19 has recently been demonstrated. ADAMs are a Zn2+-dependent transmembrane protein and members of the secreted metalloprotease superfamily, so-called ‘molecular scissors’. Similar to other ADAMs, ADAM17 comprises an N-terminal signal sequence, a prodomain, a metalloprotease (or catalytic) domain, a disintegrin domain, a cysteine-rich region, a transmembrane region, and a cytoplasmic tail (Figures 1B and 2).
Several lines of evidence suggest that lower expression of the ACE2 in membrane form (mACE2) and higher ADAM17 activity are associated with organ disease. ADAM17 is expressed in both the brain and peripheral organs, including muscles, lungs, and heart. It exists in two forms: pro-ADAM17, which is a full-length protein (~100 KDa), and the mature form, lacking its prodomain (~80 KDa) [13]. Shedding of membrane proteins by ADAM proteases occurs not only at the cell surface, but also in exosomes. Currently, more than 80 substrates have been shown to be cleaved by ADAM17. Neutrophils and endothelial cells constitutively express ADAM17 on their surface. ADAM17 is a membrane-bound enzyme that cleaves cell surface proteins, such as cytokines and adhesion proteins (e.g., L-selectin and ICAM-1). Activated endothelium upregulates the expression of these adhesion molecules and allows leukocytes to attach and migrate. The protease can also be activated in response to pathogen infection through Toll-like receptors (TLRs); pathogens, such as viruses and bacteria, activate the epidermal growth factor receptor (EGFR) signaling pathway through TLRs [14]. ADAM17 is believed to be the molecule responsible for uncontrolled interleukin (IL)-6 trans-signaling, which increases proinflammatory responses during infection. This is because ADAM17 is crucial for membrane-bound IL-6R shedding, an action implicated in the control of pro- and anti-inflammatory responses to viral antigenic stimuli [15] (see Outstanding questions).
COVID-19 and endothelialitis
SARS-CoV-2 infection is triggered by binding of viral S protein to human ACE2, whereas TMPRSS2 induces S protein priming, as previously reported [12]. Endothelial cells are a direct target of SARS-CoV-2 infection, contributing to endothelial dysfunction. In this context, pronounced endothelialitis and the recruitment of inflammatory cells has been demonstrated.
ACE2 counterbalances the vasoconstriction induced by activation of the ACE-Ang II-AT1 axis of the RAS (Figure 2). Overactivation of the ACE-Ang II-AT1 axis of the RAS and the endothelialitis caused by the viral infection promote vasoconstriction, inflammation, and thrombosis in the vascular bed, where it can cause various tissue injuries. This damage contributes to the mortality of a SARS-CoV-2 infection. Thus, viral and inflammatory endothelialitis may have a key role in the pathogenesis of COVID-19. The associated thrombo-inflammation may explain the coagulopathy observed in patients with COVID-19 and the high incidence of micro- and macrothrombosis observed in the lungs and other organs; endothelialitis also contributes to the pathophysiology of these microcirculatory changes. Patients with COVID-19 are at high risk for venous thromboembolism, with varied coagulopathies reported, and are also at high risk for venous thromboembolisms (VTE). Thromboplasminflammation in COVID-19 coagulopathy involves Ang II-induced coagulopathy, activated factor XII (FXIIa) and kallikrein–kinin system-enhanced fibrinolysis, and disseminated intravascular coagulation (DIC) [16]. In addition, induction of apoptosis and pyroptosis might have an important role in endothelial cell injury in patients with COVID-19 [17]. The mechanisms involved in this viral and inflammatory endothelialitis remain incompletely elucidated and direct infection of the endothelium by SARS-CoV2 is open to question. Viral proteins were also found in the endothelia of multiple organs of patients with persistent SARS-CoV2 infection [18]. The organs typically affected are lungs, kidneys, liver, heart, brain, and gastrointestinal tract.
Pathogenetic factors, including innate immunity factors, are involved in the induction of endothelialitis in patients with COVID. The host immune system has multiple innate immune receptors, and innate immunity is a crucial component of preventing virus invasion. The suppressor of the cytokine signaling proteins (SOCS) family is one of the main regulators of the innate immune response. SOCS comprises intracellular proteins, SOCS1–7. Cytokines activate a specific innate immune response against viruses, but dysregulation of host cytokine signaling during disease infection can cause organ dysfunction. The SOCS family is a class of negative regulators induced by cytokines that can block the signal transduction of cytokines [19]. SOCS1 and SOCS3 (SOCS1/3) function as virus-induced intrinsic virulence factors [20]. There is considerable evidence that SOCS1 and SOCS3 have important roles in viral immune evasion involving a range of viruses. SOCS3 is expressed at a higher level in obese patients, who are at greater risk of the disease compared with nonobese individuals.
It is reasonable to assume that the endothelium contributes to COVID-19-associated vascular inflammation, particularly endothelialitis in the lung, heart, and kidney. An inflammatory cascade in endothelial cells promotes leukocyte recruitment and oxidative stress. SARS-CoV-2 could trigger disruption of the balance between pro-oxidant and antioxidant mediators, the magnitude of which could reflect the severity of infection and lung injury [21] (Figure 3 ). Leukocyte transmigration is an important part of the inflammatory response involving oxidative stress, and includes the recruitment of blood leukocytes, adhesion to endothelial cells, and diapedesis. The accumulation of mononuclear cells in small lung vessels is implicated in endothelial injury [17]. Earlier studies showed that neutrophil extracellular traps (NETs) may contribute significantly to virus-associated pathology. Histones are the main protein components of NETs and have cytotoxic effects. The role of NETs is increasingly recognized as an important factor in the pathogenesis of respiratory diseases. NETs comprise intracellular material that neutrophils organize in the cytoplasm and then release when activated. Overactivation of the anaphylatoxin-NET axis has a pathological role in COVID-19 [22] (see Outstanding questions).
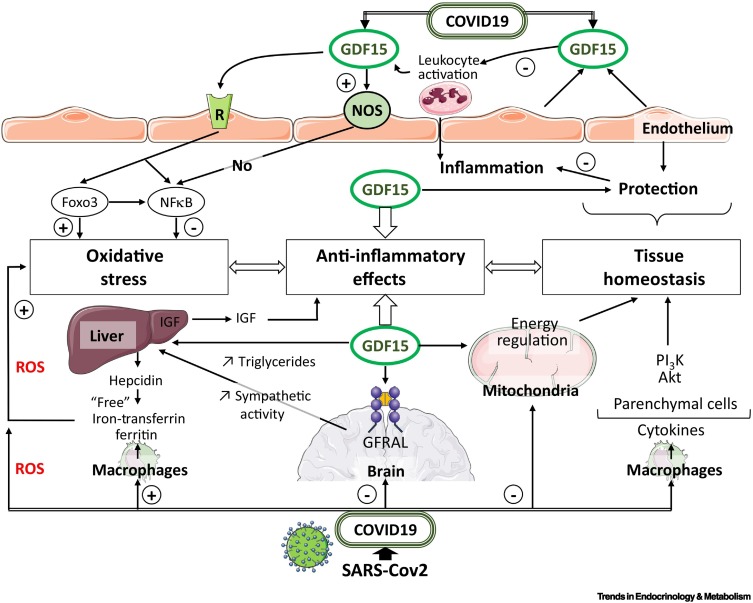
Figure 3.
Pathways contributing to hyperinflammation in coronavirus disease 2019 (COVID-19) and anti-inflammatory effects of growth and differentiation factor 15 (GDF15).
Activated monocyte-derived macrophages contribute to the COVID-19 cytokine storm by releasing massive amounts of proinflammatory cytokines. Macrophage activation and iron metabolism are implicated in COVID-19 infection. The cytokine storm observed in patients with COVID-19 contributes to the endothelial vascular dysfunction inducing endothelialitis. Activated monocytes are recruited to endothelial cells, which are activated by cytokines and produce monocyte chemoattractants and adhesion molecules. During COVID-19, GDF15 is induced and takes into a tissue-protective effect, indicating GDF15 as an inflammation-induced central mediator of tissue tolerance. Cellular stress, and chronic inflammation associated with COVID-19 infection induce the secretion and release of GDF15, which appears in the blood to target specific receptors. GDF15 binds to glial-derived neurotrophic factor (GDNF)-family receptor α-like (GFRAL) in the brain. GDF15 exerts direct effects on immune cells independent of centrally regulated mechanisms and has the potential to affect the outcome of COVID-19 infection as an emerging modulator of immune responses. The GDF15–GFRAL complex mediates the regulation of central and peripheral functions, resulting in activation of nitric oxide synthase (NOS), PI3K/AKT, nuclear factor κB (NF-κB)and the forkhead box (FOX) transcription factors in Foxo3 pathways. These pathways are essential in regulating cellular controls on tissue homeostasis and anti-inflammatory effects. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can trigger disruption of the balance between pro-oxidant and antioxidant mediators; the magnitude of the oxidative stress could reflect the severity of infection and lung injury. Liver injury is common in patients with COVID-19. Interactions occur during the inflammatory process, oxidative stress, iron metabolism, and hepatic functions through ferritin and hepcidin. Moreover, labile iron can promote inflammation by increased production of reactive oxygen species (ROS). Insulin-like growth factor 1 (IGF1), synthesized primarily in the liver, acts as an important modulator of cellular functions. Growth hormone (GH) secreted from the pituitary gland sends signals to the liver to stimulate the production of IGF1. Circulating GDF15 levels influence the liver to inhibit the actions of GH. COVID19-associated multiorgan failure is linked to impaired mitochondrial function. Mitochondria localization may be necessary for SARS-CoV-2 replication.
Finally, in patients with COVID-19, hemodynamic changes associated with systemic inflammatory reactions and the prothrombotic environment contribute to the initiation and development of cardiovascular complications, such as respiratory diseases and myocarditis [23]. However, the incidence of typical viral myocarditis in patients with COVID-19 appears low. In hearts of patients with COVID-19, the presence of an increased number of CD68+ cells suggests that COVID-19 incites a form of myocarditis different from typical viral myocarditis, and associated with diffusely infiltrative cells of the monocyte/macrophage lineage [24].
The early evaluation and continued monitoring of these specific diseases are important through the progression of COVID-19. Biomarkers of the inflammatory process represented by various cytokines and D-dimers may be also used to forecast the outcome of SARS-CoV-2 infection [25,26].
Understanding the profile of specific biomarkers and their variations as a function of different COVID-19 outcomes has been the aim of many studies. Morphological and metabolic disturbances of the heart during COVID-19 infection are associated with elevated concentrations of cardiac blood biomarkers. Elevation of brain natriuretic peptide (BNP) or NT-proBNP is associated with worse outcomes among patients with ARDS. Thus, determination of these biomarkers is useful for diagnosis and prognosis [27] and would allow clinicians to use a stratified approach to the care of patients with COVID-19 disease according to their risk. In such an approach, GDF15 is a highly informative protein (Box 1 ) that may have considerable prognostic importance in patients with COVID-19 (Box 2 ).
Box 1. Background: structure, secretion, and distribution of GDF15.
GDF15 is also known as macrophage inhibitory cytokine-1 (MIC-1), placental transformation growth factor (PTGF-b), prostate-derived factor (PDF), placental bone morphogenetic protein (PLAB), or nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1). It belongs to the TGF-β superfamily of proteins, which comprises more than 30 members, including several ligands such as TGF-βs, activins, bone morphogenetic proteins (BMPs), and GDFs. GDFs belong to the activating/myostatin (MSTN) subclass. The members of this subfamily are named GDF1–15. TGF-β family proteins bind to distinct type I and type II serine/threonine kinase receptors. Signaling induced by the TGF family ligands is necessary for multiple processes during development, tissue homeostasis, and organ functioning [28,29]. GDF15 is synthesized as a precursor protein: proGDF15. The mature protein is secreted as a homodimer linked by disulfide bonds and is released from the propeptide following intracellular cleavage. Mature GDF15 is soluble (sGDF15) and can be evaluated in blood. It is an endogenous compound with a broad normal circulating range of ~0.15–1.15 ng/ml in healthy humans. GDF15 is widely expressed at different levels in human tissues. Various reports have shown the association of GDF15 with aging and longevity and, thus, GDF15 has been investigated for its contribution to aging and age-related cognitive decline.
In humans, GDF15 is widely expressed in skeletal muscle, and circulating GDF15 increases during, and recovery from, exercise. It is possible that GDF15 is secreted in response to cellular stress or injury to act locally [30]. GDF15 expression increases during many pathological conditions and serves as a marker of cellular stress and injury. GDF15 has multiple and even paradoxical roles within a pathological condition. Its effects can be dose and time related, and dependent of the targeted tissues [31]. GDF15 acts as a ‘protective’ cytokine, although, in certain conditions, it causes endothelial dysfunction by impairing vascular contraction and relaxation, potentially inducing microvascular disease and causing organ function to deteriorate [32].
Research groups from different pharmaceutical companies simultaneously identified GDNF-family receptor α-like (GFRAL) as the receptor for GDF15, signaling though the rearranged during transfection (RET) co-receptor. Alternatively, several laboratories have shown that both GDNF and GFRAL signal independently of RET [33., 34., 35., 36.].
Alt-text: Box 1
Box 2. GDF15: a strong predictor of poor outcomes in patients with COVID-19.
It has been suggested that GDF15 has the potential to significantly affect the outcome of a viral infection. The aim of a recent single-center retrospective study was to assess the levels of cytokines (IL-6 and IL-10), GDF15, and immunological markers in patients with confirmed COVID-19. When compared with controls, patients with COVID-19 had higher levels of GDF15 (mean 12.4 ng/ml versus <2 ng/ml) [37]. The recent Framingham Heart Study (FHS) evaluated associations among plasma biomarkers, such as GDF15 and interstitial lung abnormalities (ILA), and mortality in patients with COVID-19. Causal inference analysis showed the association of age with ILA, likely mediated by GDF15. ILA progression was associated with an increased risk of death compared with those without ILA. After adjustment for ILA, age, sex, body mass index, and current smoking, GDF15 remained associated with greater mortality [38].
The aim of the COVID MECH study was to investigate the prognostic value of GDF15 in patients with laboratory-confirmed infection with SARS-CoV-2 and symptoms of COVID-19. GDF15 levels were correlated with other inflammatory and cardiovascular biomarkers. GDF15 concentrations were also associated with older age and higher CRP and D-dimer concentrations. The median baseline concentration of GDF15 in patients with COVID-19 was 2798 (1667–4528) pg/ml, and 79% of patients had GDF15 concentrations above age-specific standard values. GDF15 and ferritin remained associated with the endpoint of intensive care unit admission or death. Overall, GDF15 appeared to be a strong predictor of poor outcomes in patients critically ill with COVID-19 [39]. From two international cohorts of older patients with atrial fibrillation, recent data showed that GDF15 and other cardiovascular biomarkers, such as NT-proBNP and hs-cTnT, had the strongest associations with the sACE2 level. These results could be used to better identify individuals at risk of severe COVID-19 infection [40].
Alt-text: Box 2
GDF15, immunity, and COVID-19
Several epidemiological studies have shown that GDF15 is associated with aging and that changes in GDF15 concentrations over time are predictors of all-cause mortality. Several studies have found that inflammatory markers are elevated in older patients and are associated with senescence. Senescence may be induced by various stimuli, and is a complex stress response associated with modification of the expression of regulators, including GDF15 [41,42].
These findings indicate that, because of its function as a metabolic regulator, GDF15 has a pivotal role in the development and progression of disease. It is expressed and secreted in response to oxidative stress and inflammation. Oxidative stress is closely associated with chronic inflammation and has a key role in the pathogenesis of vascular complications [43]. GDF15 is induced during sepsis and has a role in tissue protection, distinguishing GDF15 as an ‘inflammation-induced central mediator of tissue tolerance’. In certain situations, GDFF15 takes on two opposing roles. The first implies the activation of GDNF-family receptor α-like (GFRAL), inducing β-adrenergic signaling to stimulate the release of triglycerides from the liver. Cardioprotection is then achieved by maintaining triglyceride levels during acute inflammation [44] (Figure 3).
The direct effect of GDF15 on immune cells contributes to its ability to limit inflammation-induced damage. The protective mechanisms displayed by GDF15 through cellular reactions are multifactorial. GDF15 sometimes operates paradoxically in the regulation of immune responses and inflammation, and is thought to have a protective effect on various cardiovascular functions. These actions have been attributed to abnormal inflammation in response to systemic infection. Therefore, under abnormal physiological conditions, GDF15 appears to be protective, preventing the tissue damage that is associated with inflammation [45].
Markers of systemic inflammation implicating C-reactive protein (CRP) and IL-6 are elevated in patients with poor outcomes, and the origin of the dysregulated release of cytokines in COVID-19 has been ascribed to various factors. It is assumed that viral replication that occurs during the onset of infection results in an elevated proinflammatory response. This cytokine storm (Box 3 ) produces an excessive inflammatory and immune response. While inflammation is vital for a healthy immune response, dysregulated inflammation can result in major damage to various organs. With aging, the efficacy of the innate and adaptive immune response declines, and this immunosenescence has a central role in the age-related severity of COVID-19 [53,54].
Box 3. COVID-19 and the cytokine storm.
The cytokine storm is a well-established clinical condition that is characterized by significant proinflammatory cytokine release leading to a dysregulated and hyperactive immune response. In COVID-19, the cytokine profile is characterized by increased IL-2, IL-6, IL-7, granulocyte macrophage colony-stimulating factor (GM-CSF), CC-chemokine ligand 2 (CCL2), interferon-γ (INF-γ) inducible protein 10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1-α (MIP 1-α), tumor necrosis factor-α (TNF-α), and the Notch pathway.
Furthermore, the cytokine storm observed in patients with severe COVID-19 contributes to further destruction of the endothelium, leading to ARDS, multiorgan failure, and death [46,47]. The term ‘cytokine storm’ was first coined in 1993 to describe a graft-versus-host disease. The term has since been extended to describe the similar sudden cytokine releases associated with autoimmune diseases, sepsis, cancer, acute immunotherapy responses, and infectious diseases. The dysfunction and destruction of alveolar epithelia that are a consequence of viral infection of the respiratory epithelium increase the permeability of the capillary endothelium. It is important to underline differences between responses of the superficial subepithelial microcirculation, which is present all along the conducting airways, and the pulmonary microcirculation. An endothelial–epithelial cooperative is described and associated with an humoral defense [48]. These effects are mediated, in part, by the proinflammatory cytokines that are produced during the antiviral innate immune response. It has been reported that markers of systemic inflammation, such as CRP and IL-6, are elevated in patients with poor outcomes. IL-6, via its soluble receptor (sIL-6R), initiates a proinflammatory response, while an anti-inflammatory response is triggered by the membrane-bound IL-6 receptor (IL-6R). In addition, proinflammatory cytokines, such as TNF-α and IFN-γ, are highly upregulated in patients with COVID-19, leading to the cytokine storm and participating in cell death, and tissue and organ damage. TNF-α and IFN-γ cause an increase in nitric oxide (NO) production in endothelial cells via inducible NO synthase (iNOS) activity. This process has been proposed in COVID-19 infection. It has been demonstrated that NOS2 was significantly upregulated in patients with severe and critical COVID-19 [49]. It is not easy to determine the specific role of NO because it can be cytotoxic or cytostatic depending upon the context [50].
In vivo, the protection offered by neutralizing TNF-α and IFN-γ during SARS-CoV-2 infection suggests that inhibition of TNF-α and IFN-γ signaling and reduction of iNOS activity would be beneficial in cytokine storm syndromes, such as COVID-19 [51]. Among the inductors of iNOS, lipopolysaccharides (LPS), a component of the outer cell wall of Gram-negative bacteria, has the ability to elicit the inflammatory response syndrome. There is a link between high LPS levels in the blood and metabolic syndrome that predisposes patients to severe COVID-19. Studies have also demonstrated an interaction between the S protein and LPS that leads to intensified inflammation [52].
Alt-text: Box 3
Studies in humans demonstrated that GDF15 activity increases under stress conditions in response to tissue damage, and that plasma GDF15 levels are higher in older patients, perhaps in relation to the immunological outcome. GDF15 decreased the expression of proinflammatory cytokines and prevented the activation of T cells in the liver of mice with fibrosis, while GDF15 deficiency aggravated hepatic injury [55]. The plasma levels of GDF15 increase with age and are inversely associated with an active lifestyle. In particular, at any age, circulating GDF15 is significantly higher in inactive patients and significantly lower in active individuals, such as cyclists before a race, with respect to control subjects. The data indicate that GDF15 is associated with decreased muscle performance and increased inflammation [56]. GDF15 reduced the activation of proinflammatory factors and prevented lipopolysaccharide (LPS)-induced injury. Serum GDF15 levels are significantly increased in patients who are critically ill, associated with sepsis, organ failure, and disease severity. However, the function of GDF15 in sepsis remains unclear. Further research is required to examine the potential clinical importance of the preventive role of GDF15 against LPS-induced injury and the inflammatory response [57].
Patients with hemorrhagic shock and encephalopathy syndrome (HSES) have a high early mortality rate, which may be caused by a cytokine storm. HSES and severe viral sepsis involved in COVID-19 appear to assign collective characteristics, including increased synthesis of cytokines, endothelial cell dysfunction, and coagulation abnormalities. Cytokine and GDF15 levels were significantly higher in patients with HSES than in controls [58]. Finally, upon immune challenge, GDF15 has the potential to improve immunotherapies through its immune-regulatory functions [59]. In this context, immunomodulatory therapies are now proposed for moderating the cytokine storm caused by COVID-19 [60].
GDF15, iron metabolism, and COVID-19
Throughout the inflammatory process, complex interactions have been observed between GDF15 and enzymatic activities, erythropoiesis, iron metabolism, and hepcidin [61,62]. Hepcidin, which regulates iron homeostasis, is stimulated by iron and inflammation but is suppressed by hypoxia and erythropoiesis. It has been demonstrated that hepcidin expression is suppressed by GDF15, thereby increasing iron availability for hemoglobin synthesis [63]. As a hypoxia effector molecule, GDF15 can be significantly upregulated by anemia and hypoxia; as a signal molecule, it is associated with erythropoiesis and iron regulation. Finally, because it affects iron status, GDF15 might be involved in the pathogenesis of anemia in patients with cardiovascular disease. Considering the relationship between hepcidin metabolism and GDF15, it is reasonable to consider that GDF15 may limit the availability of iron for hematopoiesis and provide an alternative explanation for the link with anemia. In older individuals with anemia, the observed elevation of GDF15 was correlated with kidney function. Recent studies demonstrated the important role of GDF15 in cancer research, in particular for blood cancers. As described herein, GDF15 has a significant role in erythropoiesis and regulates iron homeostasis via the modulation of hepcidin. In individuals with multiple myeloma (MM), GDF15 is abnormally secreted in marrow stromal cells. MM is a malignant condition that manifests with bone disease, renal failure, and anemia. It has been demonstrated that the serum concentration of GDF15 in patients with MM is positively correlated with the established prognostic factors of the disease, and that these concentrations were significantly higher in the studied patients with anemia and inversely correlated with blood hemoglobin and serum iron [64]. A recent report showed that the levels of GDF15 mRNA were increased during erastin-induced ferroptosis. GDF15 has also been investigated to clarify the relationship between GDF15 and iron metabolism, implicating a role in ferroptosis. A recent study using the human gastric cell line MGC803 showed that GDF15 knockdown promotes erastin-induced ferroptosis by attenuating the expression of SLC7A11, decreasing intracellular glutathione (GSH) levels and increasing peroxide levels [65]. Overall, these recent reports suggest that GDF15 has a critical role in regulating ferroptosis and iron metabolism [66].
Within hours of bacterial or viral infections, or other inflammatory stimuli, plasma iron concentrations decrease. In COVID-19, the potential mechanisms behind the systemic clinical findings include dysregulated iron homeostasis, resulting in oxidative stress and an inflammatory response. The dysregulation of iron homeostasis and higher iron levels may support the progression of viral infections. Therefore, evaluating serum ferritin levels in patients with COVID-19 might help to predict outcomes.
Concluding remarks and therapeutic perspectives
Therapeutic approaches could be developed to target various phases of the life cycle of SARS-CoV-2: adhesion and viral entry to host cell, viral protease, inhibition of the cytokine storm, and protection of the organs. Recently, it was reported that 1500 clinical trials related to COVID-19 have been registered, but none have yet found an optimal strategy [67].
As reported here, COVID-19 endotheliitis could explain the systemic impaired microcirculatory function in different vascular beds and their clinical sequelae in patients with COVID-19. This hypothesis provides a rationale for therapies that protect the endothelium, such as common anti-inflammatory anticytokine drugs [68] or cardiovascular protectors, such as ACE inhibitors and statins. However, preventive measures remain the best strategy in COVID-19, and several vaccines and monoclonal antibodies have been developed since the beginning of the pandemic. New clinical trials are exploring therapies that target the immune response and minimize the risk of a cytokine storm in COVID-19 [69].
Thus, endolysosomal function can be considered a target in COVID-19. As supported by recent clinical data, patients who have already taken lysosomotropic drugs for pre-existing conditions likely benefit from this treatment, which prevents SARS-CoV-2 infection and transition to COVID-19 [70]. In these cellular environments, there are obvious changes in iron metabolism during SARS-CoV-2 infection. Increased iron levels and/or dysregulated iron homeostasis occur in several lung diseases, including pulmonary fibrosis. In experimental and clinical studies, fibrosis and lung function decline are associated with pulmonary iron accumulation in bleomycin-induced pulmonary fibrosis. In addition, iron accumulation is increased in lung sections from patients with idiopathic pulmonary fibrosis [71]. Several studies have assessed the potential antiviral effect of iron-chelating therapy, and some trials (NCT04333550 and NCT04361032) have attempted to evaluate the efficacy and safety of deferoxamine, a common iron chelator, in patients with COVID-19 [72]. Various studies have focused on better understanding the impact of antioxidants and the ways in which viral infections, such as COVID-19 infection, disturb REDOX homeostasis. Current antioxidant agents of interest are enzymes (superoxide dismutases: SODs and GPXs), GSH, vitamins C, D, E, and B6, melatonin, minerals, selenium, N-acetylcysteine, quercetin, curcumin, and naturally occurring polyphenols. However, more experiments and larger clinical studies are needed before any of these agents can be considered antiviral agents [73].
Anti-inflammatory and immunosuppressive drugs have been tested as therapeutic regimens for pulmonary fibrosis, but none have been sufficiently effective in prolonging patient survival. Recombinant GDF15 could be developed as a biopharmaceutical for treating pulmonary fibrosis. GDF15 demonstrated therapeutic effects in a mouse model of bleomycin-induced pulmonary fibrosis [74]. An association with an additional antiviral drug might be possible, such as the suggested association with SOCS1/3 antagonists. The properties of SOCS1/3 could be harnessed pharmacologically as prophylactic and/or therapeutic mechanisms against COVID-19 infection [20].
The ACE2/COVID-19 interface is also a promising therapeutic target [75]. Structure-based rational design of binders with enhanced affinities to either ACE2 or the S protein may accelerate development of decoy ligands or neutralizing antibodies to neutralize the viral infection [76]. Preclinical studies of neutralizing-antibody treatments for SARS-CoV-2 infection in several animal models have shown promising results. LY-CoV555 (also known as LY3819253), a potent anti-S neutralizing monoclonal antibody that binds to the RBD of SARS-CoV-2, was derived from convalescent plasma obtained from a patient with COVID-19. LY-CoV555 appeared to accelerate the natural decline in viral load over time [77]. To date, eight SARS-CoV-2-neutralizing antibodies have entered clinical evaluation: LY-CoV555, JS016, REGN-COV2, TY027, BRII-196, BRII-198, CT-P59, and SCTA01 [78].
On the therapeutics side, another approach is activation of the MAS receptor or administration of Ang(1-7) or MAS analogs, which could be important additive measures to control the inflammatory response mediated by SARS-CoV-2 [78]. Protective effects have been elicited in vivo by Ang(1-7) as well as by the MAS-receptor agonist AVE 0991 (C₂₉H₃₂N₄O₅S₂), which can reportedly mimic the effects of Ang(1-7) [79] (Figure 2).
Given that TMPRSS2 is involved in SARS-CoV-2 cell entry, TMPRSS2 inhibitors might constitute a treatment option once they have been tested and approved for clinical use. Camostat mesylate is a synthetic serine protease inhibitor that can inhibit TMPRSS2 in lung cells. Nafamostat mesylate, another TMPRSS2 inhibitor, may also prevent the entry of virus into the host cell [80,81]. Similarly, we previously reported that FCS was necessary for SARS-CoV-2 to infect human lung cells, and that therapeutics against SARS-CoV-2 should involve furin inhibitors. Synthetic furin inhibitors, such as MI-1851, decanoyl-RVKR-chloromethylketone (CMK), and naphthofluorescein, appear to be able to inhibit SARS-CoV-2 replication in human respiratory tract cells [7,82., 83., 84.]. In conclusion, a list of currently available drugs, their possible mechanisms of action, and the strategies that could be used to treat patients with COVID-19 are being investigated in preclinical and clinical trials [85] (Table 1 ). Several biomedical research laboratories are also currently testing a variety of treatments to determine their efficacy and to identify approaches with the most promise [90] (see Outstanding questions).
Table 1.
Proposed drugs for the treatment of COVID-19
| Repurposed drugs [86] | |
| Protease inhibitors | Lopinavir, ritonavir, molnupiravir |
| S glycoprotein inhibitor | Arbidol |
| Anti-inflammatory drugs | Dexamethasone and other steroids, N-3 polyunsaturated fatty acids |
| RAS inhibitors | Irbesartan, losartan |
| Possible therapeutic targets [87] | |
| Anti-IL-6 | Siltuximab |
| Anti-IL-6 receptors | Tocilizumab, sarilumab |
| Anti-IL-1β | Canakinumab |
| Anti-IL-1β receptors | Anakinra |
| Anti-TNF | Infliximab, adalimumab, golimumab, certolizumab |
| Anti-GM-CSF | Lenzilumab |
| Anti-IFNγ | Emapalumab |
| Anti-JAK | Baricitinib, ruxolitinib, tofacitinib |
| Anti-CCRs | Leronlimab |
| Anti-C5 | Eculizumab |
| Immunomodulators | IFNs: IFN beta: SNG001; IFN alpha2b, Vitamin D |
| Immunoglobulins | IgG |
| Emerging therapeutics [88,89] | |
| New protease inhibitors | ASC09 TMC310911 |
| New immunomodulators | CD24Fc (CD24 and Fc fusion protein), recombinant GDF15 (rGDF15), artemisinin, colchicine |
| Neurokinin-1 Inhibitors | Remdesivir, tradipitant |
| CCR antibody | Leronlimab |
| TMPRSS2 inhibitors | Camostat mesilate, nafamostat mesilate |
| Furin inhibitors | MI-1851, naphthofluorescein diminazene |
| RNA polymerase inhibitors | Remdesivir, ribavirin, favipiravir |
| RAS modulators | Ang(1-7) |
| Iron inhibitors | Deferoxamine, deferiprone, deferasirox |
| Ferroptosis inhibitors | Ferrostatin-1, liproxstatin-1 |
| ROS scavengers | Vitamins C ,E, A; glutathione, bilirubin, melatonin |
| Antiparasitic class | Ivermectin |
| Convalescent plasma | From patients recovered from COVID-19 |
Acknowledgments
Acknowledgments
This work was supported by grants from the Agence Nationale pour la Recherche (ANR SMOG-15, ANR-19-CE17-0009), the French Ministry of Research, the Regional Council of Bourgogne - Franche-Comté, FEDER, and Association de Cardiologie de Bourgogne. The authors wish to thank Suzanne Rankin for revising the English.
Authors’ contributions
L.R. and C.V. wrote the manuscript; M.Z. and Y.C. provided significant contributions to the content of the manuscript.
Declaration of interests
The authors declare that there are no conflicts of interest.
Glossary
- A disintegrin and metalloproteinase-17 (ADAM17)
also known as tumor necrosis factor-α-converting enzyme (TACE); a membrane-bound enzyme that cleaves cell surface proteins, such as cytokines and adhesion proteins.
- Acute respiratory distress syndrome (ARDS)
characterized by the acute onset of noncardiogenic pulmonary edema leading to acute hypoxemic respiratory failure. Patients require invasive mechanical ventilation.
- Angiotensin-converting enzyme 2 (ACE2)
in SARS-CoVs, virus entry into the host cell is achieved by the viral S protein binding to ACE2.
- Apoptosis
process of programmed cell death, characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms.
- Coronavirus disease 2019 (COVID-19)
a pandemic of acute respiratory diseases.
- Cytokine storm
life-threatening systemic inflammatory syndrome involving elevated levels of circulating cytokines and immune-cell hyperactivation.
- Endothelialitis
inflammation of the endothelium, contributing to the pathophysiology of the microcirculatory changes.
- Glial-derived neurotrophic factor (GDNF)-family receptor α-like (GFRAL)
receptor for GDF15, signaling through the rearranged during transfection (RET) coreceptor.
- Growth and differentiation factor 15 (GDF15)
also known as macrophage inhibitory cytokine 1 (MIC-1); belongs to the TGF-β superfamily of proteins.
- Pyroptosis
form of inflammatory programed cell death pathway activated by caspases. These caspases are used by the host to control bacterial and viral pathogens.
- Reactive oxygen species (ROS)
utilization of molecular oxygen by aerobic organisms results in the formation of several ROS, with an important role in both the physiology and pathophysiology of aerobic life.
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
a transmissible, pathogenic coronavirus.
- Toll-like receptors (TLRs)
pattern recognition receptors recognized by various immune cells. The discovery of TLRs in humans revolutionized the field of host–pathogen interaction research.
References
- 1.Chan J.F., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiga M., et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J., et al. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., et al. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W. Delving deep into the structural aspects of a furin cleavage site inserted into the spike protein of SARS-CoV-2: a structural biophysical perspective. Biophys. Chem. 2020;264:106420. doi: 10.1016/j.bpc.2020.106420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochette L., et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol. Ther. 2013;140:239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Sungnak W., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvinukova M., et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos R.A.S., et al. The ACE2/Angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H., et al. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur. Urol. 2020;78:296–298. doi: 10.1016/j.eururo.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheller J., et al. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Koff J.L., et al. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 15.Mahmud-Al-Rafat A., et al. Decoding the enigma of antiviral crisis: does one target molecule regulate all? Cytokine. 2019;115:13–23. doi: 10.1016/j.cyto.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gando S., Wada T. Thromboplasminflammation in COVID-19 coagulopathy: three viewpoints for diagnostic and therapeutic strategies. Front. Immunol. 2021;12:649122. doi: 10.3389/fimmu.2021.649122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga Z., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabolcs M., et al. Identification of immunohistochemical reagents for in situ protein expression analysis of coronavirus-associated changes in human tissues. Appl. Immunohistochem. Mol. Morphol. 2021;29:5–12. doi: 10.1097/PAI.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S., et al. SOCS proteins participate in the regulation of innate immune response caused by viruses. Front. Immunol. 2020;11:558341. doi: 10.3389/fimmu.2020.558341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson H.M., et al. SOCS, intrinsic virulence factors, and treatment of COVID-19. Front. Immunol. 2020;11:582102. doi: 10.3389/fimmu.2020.582102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif-Askari N.S., et al. Upregulation of oxidative stress gene markers during SARS-COV-2 viral infection. Free Radic. Biol. Med. 2021;172:688–698. doi: 10.1016/j.freeradbiomed.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englert H., et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. EBioMedicine. 2021;67:103382. doi: 10.1016/j.ebiom.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dou Q., et al. Cardiovascular manifestations and mechanisms in patients with COVID-19. Trends Endocrinol. Metab. 2020;31:893–904. doi: 10.1016/j.tem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox S.E., et al. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc. Pathol. 2021;54:107361. doi: 10.1016/j.carpath.2021.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grobler C., et al. Covid-19: the rollercoaster of fibrin(Ogen), D-dimer, Von Willebrand factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int. J. Mol. Sci. 2020;21:5168. doi: 10.3390/ijms21145168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdeen Y., et al. The prognostic effect of brain natriuretic peptide levels on outcomes of hospitalized patients with COVID-19. Avicenna J. Med. 2021;11:20–26. doi: 10.4103/ajm.ajm_169_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unsicker K., et al. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013;24:373–384. doi: 10.1016/j.cytogfr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Rochette L., et al. Growth and differentiation factor 11 (GDF11): functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol. Ther. 2015;156:26–33. doi: 10.1016/j.pharmthera.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Kleinert M., et al. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018;9:187–191. doi: 10.1016/j.molmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Mudares F., et al. Role of growth differentiation factor 15 in lung disease and senescence: potential role across the lifespan. Front. Med. (Lausanne) 2020;7:594137. doi: 10.3389/fmed.2020.594137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazagova M., et al. Growth differentiation factor 15 impairs aortic contractile and relaxing function through altered caveolar signaling of the endothelium. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H709–H718. doi: 10.1152/ajpheart.00543.2012. [DOI] [PubMed] [Google Scholar]
- 33.Mullican S.E., et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017;23:1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 34.Emmerson P.J., et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017;23:1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 35.Hsu L.A., et al. Growth differentiation factor 15 may predict mortality of peripheral and coronary artery diseases and correlate with their risk factors. Mediat. Inflamm. 2017;2017:9398401. doi: 10.1155/2017/9398401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochette L., et al. Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol. Metab. 2020;31:939–951. doi: 10.1016/j.tem.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Notz Q., et al. Pro- and anti-inflammatory responses in severe COVID-19-induced acute respiratory distress syndrome-an observational pilot study. Front. Immunol. 2020;11:581338. doi: 10.3389/fimmu.2020.581338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders J.L., et al. The association of aging biomarkers, interstitial lung abnormalities, and mortality. Am. J. Respir. Crit. Care Med. 2021;203:1149–1157. doi: 10.1164/rccm.202007-2993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myhre P.L., et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation. 2020;142:2128–2137. doi: 10.1161/CIRCULATIONAHA.120.050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallentin L., et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur. Heart J. 2020;41:4037–4046. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita Y., et al. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr Gerontol Int. 2016;16:17–29. doi: 10.1111/ggi.12724. [DOI] [PubMed] [Google Scholar]
- 43.Rochette L., et al. Redox functions of heme oxygenase-1 and biliverdin reductase in diabetes. Trends Endocrinol. Metab. 2018;29:74–85. doi: 10.1016/j.tem.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Luan H.H., et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eddy A.C., Trask A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021;57:11–18. doi: 10.1016/j.cytogfr.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson L.A., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teuwen L.A., et al. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persson C. Early humoral defence: contributing to confining COVID-19 to conducting airways? Scand. J. Immunol. 2021;93 doi: 10.1111/sji.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan S.P., et al. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res. Rev. 2020;64:101201. doi: 10.1016/j.arr.2020.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke J.P. Inflammation and its role in regeneration and repair. Circ. Res. 2019;124:1166–1168. doi: 10.1161/CIRCRESAHA.118.314669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karki R., et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petruk G., et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2021;12:916–932. doi: 10.1093/jmcb/mjaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bektas A., et al. Age-associated changes in human CD4(+) T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging (Albany NY) 2019;11:9234–9263. doi: 10.18632/aging.102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franceschi C., et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 55.Chung H.K., et al. GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury. Sci. Rep. 2017;7:17238. doi: 10.1038/s41598-017-17574-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conte M., et al. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front. Immunol. 2020;11:915. doi: 10.3389/fimmu.2020.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song H., et al. GDF-15 prevents lipopolysaccharide-mediated acute lung injury via upregulating SIRT1. Biochem. Biophys. Res. Commun. 2020;526:439–446. doi: 10.1016/j.bbrc.2020.03.103. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi H., et al. Elevated cytokine, chemokine, and growth and differentiation factor-15 levels in hemorrhagic shock and encephalopathy syndrome: a retrospective observational study. Cytokine. 2021;137:155324. doi: 10.1016/j.cyto.2020.155324. [DOI] [PubMed] [Google Scholar]
- 59.Wischhusen J., et al. Growth/differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint. Front. Immunol. 2020;11:951. doi: 10.3389/fimmu.2020.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de la Rica R., et al. COVID-19: in the eye of the cytokine storm. Front. Immunol. 2020;11:558898. doi: 10.3389/fimmu.2020.558898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gudjoncik A., et al. Iron, oxidative stress, and redox signaling in the cardiovascular system. Mol. Nutr. Food Res. 2014;58:1721–1738. doi: 10.1002/mnfr.201400036. [DOI] [PubMed] [Google Scholar]
- 62.Rochette L., et al. The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol. Ther. 2015;146:35–52. doi: 10.1016/j.pharmthera.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Wang C., et al. Reciprocal regulation between hepcidin and erythropoiesis and its therapeutic application in erythroid disorders. Exp. Hematol. 2017;52:24–31. doi: 10.1016/j.exphem.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Banaszkiewicz M., et al. Evaluating the relationship of GDF-15 with clinical characteristics, cardinal features, and survival in multiple myeloma. Mediat. Inflamm. 2020;2020:5657864. doi: 10.1155/2020/5657864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., et al. GDF15 knockdown promotes erastin-induced ferroptosis by decreasing SLC7A11 expression. Biochem. Biophys. Res. Commun. 2020;526:293–299. doi: 10.1016/j.bbrc.2020.03.079. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y., et al. The application of ferroptosis in diseases. Pharmacol. Res. 2020;159:104919. doi: 10.1016/j.phrs.2020.104919. [DOI] [PubMed] [Google Scholar]
- 67.Kotta S., et al. Combating the pandemic COVID-19: clinical trials, therapies and perspectives. Front. Mol. Biosci. 2020;7:606393. doi: 10.3389/fmolb.2020.606393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldmann M., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blaess M., et al. COVID-19/SARS-CoV-2 infection: lysosomes and lysosomotropism implicate new treatment strategies and personal risks. Int. J. Mol. Sci. 2020;21:4953. doi: 10.3390/ijms21144953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali M.K., et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020;251:49–62. doi: 10.1002/path.5401. [DOI] [PubMed] [Google Scholar]
- 72.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020;19:102538. doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suhail S., et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39:644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y.I., et al. Epithelial cell-derived cytokines CST3 and GDF15 as potential therapeutics for pulmonary fibrosis. Cell Death Dis. 2018;9:506. doi: 10.1038/s41419-018-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Unni S., et al. Identification of a repurposed drug as an inhibitor of Spike protein of human coronavirus SARS-CoV-2 by computational methods. J. Biosci. 2020;45:130. doi: 10.1007/s12038-020-00102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan R., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen P., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renn A., et al. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-Cov-2. Trends Pharmacol. Sci. 2020;41:815–829. doi: 10.1016/j.tips.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dobrocsyova V., et al. AVE0991, a nonpeptide angiotensin 1-7 receptor agonist, improves glucose metabolism in the skeletal muscle of obese Zucker rats: possible involvement of prooxidant/antioxidant mechanisms. Oxidative Med. Cell. Longev. 2020;2020:6372935. doi: 10.1155/2020/6372935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuentes-Prior P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J. Biol. Chem. 2020;296:100135. doi: 10.1074/jbc.REV120.015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKee D.L., et al. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bestle D., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng Y.W., et al. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33:108254. doi: 10.1016/j.celrep.2020.108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C., et al. Furin: a potential therapeutic target for COVID-19. iScience. 2020;23:101642. doi: 10.1016/j.isci.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cuadrado A., et al. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol. Sci. 2020;41:598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lem F.F., et al. Molecular mechanism of action of repurposed drugs and traditional Chinese medicine used for the treatment of patients infected with COVID-19: a systematic scoping review. Front. Pharmacol. 2020;11:585331. doi: 10.3389/fphar.2020.585331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iovino L., et al. Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heustess A.M., et al. Clinical management of COVID-19: a review of pharmacological treatment options. Pharmaceuticals (Basel) 2021;14:520. doi: 10.3390/ph14060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kern C., et al. Modeling of SARS-CoV-2 treatment effects for informed drug repurposing. Front. Pharmacol. 2021;12:625678. doi: 10.3389/fphar.2021.625678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajaiah R., et al. Evaluation of mechanisms of action of re-purposed drugs for treatment of COVID-19. Cell. Immunol. 2020;358:104240. doi: 10.1016/j.cellimm.2020.104240. [DOI] [PMC free article] [PubMed] [Google Scholar]