Abstract
Purpose:
The native T1 value at 3T MRI is a sensitive marker for diffuse fibrosis or damage in various organs including the heart, liver, and pancreas. Despite the fact that Fontan-associated liver disease (FALD) is a crucial issue in adults with Fontan circulation, there are only a few studies with liver T1 mapping in children and adolescents. We investigated the potential of the liver native T1 mapping in detecting FALD in adult patients.
Methods:
We prospectively enrolled 16 consecutive adults with Fontan circulation (age 31.3 ± 8.5 years), who were in New York Heart Association Functional class II–IV. Twenty with tetralogy of Fallot (TOF), and 20 age-matched controls also underwent cardiac magnetic resonance (CMR) imaging at 3T. Myocardial T1 mapping with a Modified Look-Locker Inversion recovery sequence was applied to liver T1 mapping. Patients in the Fontan group underwent the right heart catheter and liver function tests, including those for fibrotic markers.
Results:
Liver native T1 values in the Fontan group were significantly higher than that in TOF and controls (P < 0.001). In the Fontan group, the liver native T1 value was significantly correlated with age, γ -glutamyltransferase, model for end-stage liver disease XI score, and albumin-bilirubin score (P = 0.01, 0.01, 0.044, 0.001). However, it demonstrated no correlation with central venous pressure, pulmonary vessel resistance, or fibrotic markers.
Conclusion:
Liver native T1 value derived from CMR may be a non-invasive adjunctive and/or screening marker to detect FALD.
Keywords: cardiac magnetic resonance, T1 mapping, liver dysfunction, Fontan
Introduction
Fontan-associated liver disease (FALD) is a severe complication in adults with Fontan circulation.1 Although multifactorial, FALD etiologies primarily include chronically elevated central venous pressure and diminished cardiac output.1–7 Liver fibrosis and cirrhosis occurs as a universal, long-term sequel of the Fontan operation;1–7 furthermore, it is often accompanied by hepatocellular carcinoma (HCC).8,9 Staging liver fibrosis in FALD requires a multi-modality approach involving clinical assessment, biochemical/haematological parameters, non-invasive fibrosis scores, radiological imaging, elastography, and invasive liver histology.10–14 So far, there has been no golden standard as a non-invasive screening tool for FALD. Abdominal ultrasound is useful and available in many hospitals; however, it is challenging to follow the small temporal changes quantitatively. Furthermore, some Fontan patients, in particular heterotaxy syndrome, show an unusual shaped liver, suggesting difficulty in detection and insufficiency in reproduction.
It is also challenging to evaluate liver complications since their severity is not typically associated with changes in serum biochemistry test parameters, such as transaminase and hyaluronic acid. The model for end-stage liver disease (MELD) XI score is often used in Fontan patients.1 It is repeatable and quantitative, but this score is the combination of the kidney and liver dysfunction, not a genuine marker of liver dysfunction. Not a few Fontan patients have moderate kidney dysfunction, and MELD XI score is not informative for them. A contrast medium for computer tomography scan is also not available in such patients. Although liver biopsy is conventionally considered as the gold standard, it is difficult to repeat such an invasive investigation in unstable patients with poor Fontan circulation, so-called ‘failing Fontan’. Moreover, it cannot accurately predict the prognosis of liver fibrosis in its later stages. Therefore, non-invasive and non-contrast imaging tools are necessary to identify temporal changes in the liver.
T1 mapping is a novel MRI technology to quantify the diffuse damage/fibrosis/congestion/edema/inflammation of parenchyma in organs such as the liver, heart, and pancreas.15,16 Liver data in cases of congenital heart disease (CHD) are particularly lacking, with only a few prior studies in children and adolescents with Fontan circulation that reported the potential value of the liver native T1 values as a screening tool;17,18 however, there is no data for adults with Fontan circulation. Furthermore, the correlations between the liver disease biomarkers and T1 values remain unknown. Here, we focused on the liver native T1 values at 3T in adults with Fontan circulation, and evaluated the potential usefulness of this method in detecting chronic liver damages as a pilot study.
Materials and Methods
We prospectively enrolled 16 consecutive adults with Fontan circulation (10 with an atrio-pulmonary connection and 6 with total cavopulmonary bypass), and 20 consecutive adults with tetralogy of Fallot (TOF) who had undergone intra-cardiac repair, who were in New York Heart Association Functional class (NYHA FC) II–IV. Each patient underwent cardiac and liver MRI at our institute between March 2018 and December 2019. Patients in NYHA I were excluded, because invasive tests should be avoided in such relatively healthy patients. Patients under 18 years old were not enrolled. Patients with fatty liver were also excluded, because the fatty liver has a great influence on T1 mapping values. The reason why we enrolled TOF as well as Fontan was that TOF is a well-known representative CHD with the RV dysfunction and some studies reported liver damages in TOF patients.19 We performed a blood test within 3 months, and screened the patient’s medical records for the outcomes of catheterization that was performed within 2 years of MRI. Fontan patients underwent an abdominal ultrasound examination within 1 year of the MRI.
The MELD XI score = 11.76 × Ln (creatinine) + 5.11 × Ln (total bilirubin) + 9.44, and albumin-bilirubin (ALBI) score = [(log10 bilirubin (μmol/L) × 0.66) + (albumin (g/L) × −0.085)], were determined as objective indicators of hepatic injury.20,21 The MELD score is typically used to assess FALD; however, since adults Fontan patients are often prescribed with anticoagulants, we evaluated both the MELD XI, which excludes international normalized ratio (INR) and ALBI scores.22,23
Twenty age-matched adults were enrolled as a control group. They underwent cardiac MRI to screen for palpitations and non-specific chest pain and showed normal results on all tests including electrocardiography, brain natriuretic peptide (BNP), transthoracic echocardiography, cardiac MR and/or coronary MR and/or computed tomography angiography. None of the controls had hypertension, diabetes mellitus, or ischemic heart disease. Patients with fatty liver were also excluded because the fatty liver has a great influence on T1 mapping values. They underwent an annual health check regularly, and the results of blood test and abdominal ultrasound were available.
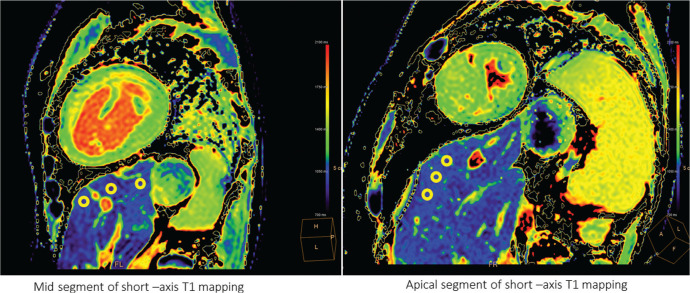
All participants underwent MRI with a 3T whole-body imager (Ingenia 3T; Philips Healthcare, Best, the Netherlands) equipped with dual-source, parallel radiofrequency transmission and 32-element cardiac phased-array coils for radiofrequency reception. The Modified Look-Locker Inversion recovery sequence with acquisitions of eight images at a fixed time of 5s(3s)5s was used for myocardial T1 mapping,24,25 the outcomes of which were subsequently analysed for liver T1 mapping. Other scan parameters were as follows: FOV: 360 mm, matrix size: 128 × 256, sensitivity encoding factor: 2, TR: 2.7 ms, TE: 1.26 ms, slice thickness: 8 mm, flip angle: 10°, turbo field echo factors: 33, and shot mode: single-shot. We selected the slice with the largest section of the liver from the basal, mid, and apical segments of left ventricular short-axis T1 mapping. We used a commercial variable software to draw three elliptical regions of interest (ROI) (approximately 30 mm2) at a depth of 1 cm from the liver surface, avoiding regions with artefacts, biliary, and vascular tree structures (Fig. 1). The average T1 value of the three ROIs was defined as a patient representative. To assess for intra-observer agreement of T1 mapping measurements, 8 randomly selected data were re-analyzed by the first experienced imager after 2 weeks interval, blinded to clinical outcomes and the first set of measurements.
Fig. 1.
Liver native T1 image. We drew three elliptical regions of interest at the depth of 1 cm from the liver surface, avoiding regions with artefacts, biliary and vascular tree structures.
Fontan patients underwent abdominal ultrasound. FALD is difficult to be defined and remains vague by using imaging modalities. In this current study, the definition of the liver congestion was significantly dilated liver veins and the inferior vena cave without respiratory change. Chronic liver damage including fibrosis/cirrhosis was defined as follows: surface nodularity, overall coarse, and/or heterogeneous echotexture.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. A prior approval was received from the institution’s human research committee and our hospital’s ethical committee. All patients provided informed consent for undergoing MRI.
Statistics
The data was evaluated using Student’s t-test, Mann–Whitney U-test, and one-way analysis of variance using SPSS software (ver. 20; SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to examine distribution. Continuous variables were assessed using either Pearson’s or Spearman’s correlation coefficient. Intra- and interobserver variabilities were assessed using intra- and interclass correlation coefficients, absolute values, and 95% confidence intervals.
Results
Basic characteristics in adults with Fontan, TOF, and controls
Patient age was not significantly different between the three groups (Fontan: 31.3 ± 8.5 years, TOF: 35.2 ± 7.5 years, and controls: 40.7 ± 14.9). Serum total bilirubin and gamma-glutamyl transferase (γ -GTP) were the highest, while serum albumin and platelet counts were the lowest in the Fontan group (Table 1).
Table 1.
Basic characteristics
| Fontan | TOF | Controls | Three groups | Fontan vs. TOF | |
|---|---|---|---|---|---|
|
| |||||
| 16 Patients | 20 Patients | 20 Patients | P | P | |
| Age (years) | 31.3 ± 8.5 | 35.2 ± 7.5 | 40.7 ± 14.9 | 0.12 | 0.46 |
| Male | 8 (50%) | 9 (45%) | 10 (50%) | 0.97 | 0.51 |
| NYHA FC | 0/12/4/0 | 0/19/1/0 | – | – | – |
| BNP (pg/mL) | 140 ± 146 | 59.5 ± 36.2 | – | – | 0.17 |
| Heart failure | 5 | 3 | 0 | – | – |
| Systemic ventricular EF (%) | 43.1 ± 14.6 | 52.4 ± 10.0 | – | – | 0.27 |
| Albumin (g/mL) | 4.08 ± 0.87 | 4.59 ± 0.24 | 4.75 ± 0.38 | 0.001 | 0.02 |
| Platelet count (×104 μL) | 16.1 ± 7.0 | 24.1 ± 6.7 | 27.9 ± 8.1 | 0.01 | 0.003 |
| Total bilirubin (mg/dL) | 1.12 ± 0.6 | 0.87 ± 0.41 | 0.79 ± 0.35 | 0.01 | 0.03 |
| γ -GTP (IU) | 92.6 ± 59.1 | 29.4 ± 19.6 | 47.8 ± 42.9 | 0.001 | 0.008 |
| Creatinine (mg/dL) | 0.75 ± 0.12 | 0.75 ± 0.18 | 0.68 ± 0.21 | 0.68 | 0.23 |
| Chronic liver damage | 11 | – | – | ||
| CVP (mmHg) | 10.5 ± 1.6 | – | – | ||
| PVR (wood unit/m2) | 1.61 ± 0.7 | – | – | ||
| Hyaluronic acid (ng/mL) | 48.1 ± 30.4 | – | – | ||
| Procollagen III peptide (U/mL) | 7.8 ± 7.5 | – | – | ||
| IV type collagen (ng/mL) | 7.3 ± 1.5 | – | – | ||
BNP, brain natriuretic peptide; CVP, central venous pressure; EF, Ejection fraction; γ -GTP, gamma-glutamyl transpeptidase; NYHA FC, New York Heart Association functional class; PVR, pulmonary vascular resistance; TOF, tetralogy of Fallot.
Eleven patients in Fontan group were diagnosed with chronic liver damage via ultrasound analysis. No enrolled patients had fatty liver. We used several diagnostic modalities to confirm the absence of HCC in the three groups. Among the 16 Fontan patients, 5 had been hospitalized due to heart failure (HF); additionally, 2 out of 5 presented with protein losing enteropathy.
Liver T1 mapping data at 3T
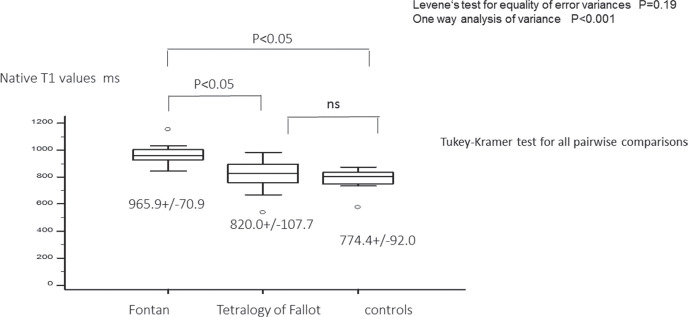
Liver native T1 value was the highest for the three groups (Fontan: 965.9 ± 70.9 ms, TOF: 820.0 ± 107.7 ms, controls: 774.4 ± 92.0 ms; P < 0.001) and the liver native T1 values of the Fontan group was significantly higher than that of TOF. There was no statistically significant difference between TOF and controls (Figs. 2, 3 and Table 2).
Fig. 2.
Liver native T1 in Fontan, tetralogy of Fallot, and controls. The liver T1 values of the Fontan group was significantly higher than that of tetralogy of Fallot. There was no significant difference between tetralogy of Fallot and controls. ns, not significant.
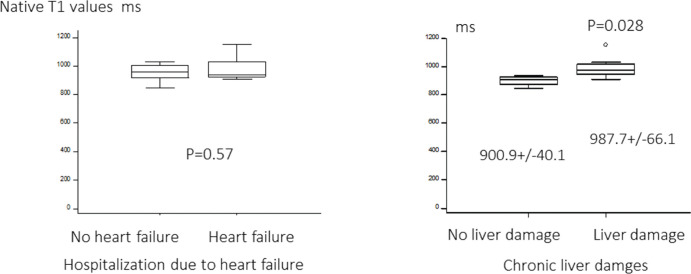
Fig. 3.
Native T1 value with and without heart failure and/or liver damages in Fontan. Ultrasound analysis revealed that the liver native T1 values of Fontan patients with liver damages was higher than those without.
Table 2.
Correlation of liver T1 value in Fontan
| r | P | |
|---|---|---|
| Age (years) | 0.61 | 0.01 |
| NYHA FC | 0.43 | 0.09 |
| BNP (pg/mL) | 0.36 | 0.17 |
| CVP (mmHg) | 0.14 | 0.62 |
| PVR (wood unit/m2) | −0.08 | 0.79 |
| Albumin (g/mL) | −0.45 | 0.07 |
| Platelet count (×104μL) | 0.001 | 0.99 |
| Creatinine (mg/dL) | 0.23 | 0.39 |
| Total bilirubin (mg/dL) | 0.02 | 0.94 |
| γ -GTP (IU) | 0.61 | 0.01 |
| Hyaluronic acid (ng/mL) | 0.32 | 0.26 |
| Procollagen III peptide (U/mL) | −0.37 | 0.15 |
| IV type collagen (ng/mL) | −0.36 | 0.17 |
BNP, brain natriuretic peptide; CVP, central venous pressure; γ -GTP, gamma-glutamyl transpeptidase; NYHA FC, New York Heart Association functional class; PVR, pulmonary vascular resistance.
The liver native T1 values in Fontan patients was significantly correlated with their ages and γ -GTP levels (r = 0.61 and 0.61, P = 0.01 and 0.01). Although albumin showed a similar tendency, it did not show statistically significant correlations (P = 0.07). However, the liver native T1 values were not significant associated with BNP, central venous pressure (CVP), pulmonary vascular resistance (PVR), platelet counts, serum creatinine, total bilirubin, or fibrotic makers (serum hyaluronic acid, Procollagen 3 peptide, and Type 4 collagen level).
Ultrasound analysis revealed that the liver native T1 values of Fontan patients with chronic liver damages was higher than those without (987.7 ± 66.1 ms vs. 900.9 ± 40.1 ms, P = 0.028). There was no significant difference in liver T1 values between Fontan patients with and without a history of hospitalization due to HF.
Correlations of MELD XI and ALBI scores
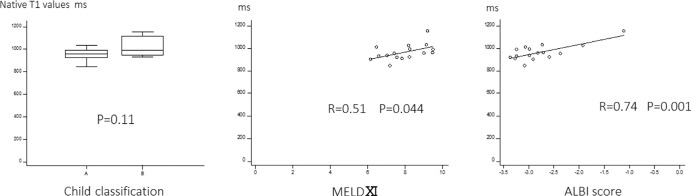
Eight out of the 16 patients had been prescribed with anti-coagulants. The liver native T1 values significantly correlated with MELD XI and ALBI score (r = 0.51 and 0.74, P = 0.04 and 0.001). T1 values of the Child-Pugh A and B classification were not significantly different (Fig. 4).
Fig. 4.
Child classification, MELD XI and ALBI score and liver native T1 values. The liver native T1 values significantly correlated with MELD XI and ALBI score. ALBI, albumin-bilirubin; MELD, model for end-stage liver disease.
Correlation of liver and myocardial native T1 values
In Fontan patients, myocardial T1 value of the mid ventricular free wall was 1240.3 ± 41.4 ms, and liver T1 value was 965.9 ± 70.9 ms. There was no statistical correlations between the two values (P = 0.12).
Reproducibility
The intra- and inter-observer agreements for the liver native T1 values were good (intra/interclass correlation coefficients: 0.925 and 0.90, 95% confidence interval: 0.79–0.97 and 0.68–0.97).
Discussion
Despite being a preliminary investigation, this is possibly the first study on the potential of liver native T1 mapping using 3T MR imaging in adults with Fontan circulation. Our results revealed that (1) the liver native T1 values of adult Fontan patients were significantly higher than that of TOF and controls, suggesting the presence of chronic liver damages in Fontan adults, and (2) there was a strong correlation between liver native T1 values and MELD XI and ALBI score, which are the representative makers of the liver damage and provide a potential risk score for invasive treatments.
FALD and liver native T1 values
FALD is a severe but common complication occurring after reaching adulthood.1 Incidence of liver cirrhosis was reported to be as high as 43%, with the mean follow-up age being 30 years after Fontan surgery.7 Etiologically, FALD is multifactorial and its clinical presentation may range from a benign nodular hyperplasia to liver cirrhosis and failure. The passive systemic venous return circuit in the Fontan circulation can be maintained by ensuring an elevated central venous pressure and chronic passive venous congestion. Furthermore, lymphatic obstruction and low cardiac output reportedly decreased portal venous flow and impaired hepatic venous drainage.26,27 This may subsequently result in hepatic arterialization, sinusoidal dilation, parenchymal atrophy, and progressive collagen deposition in a perivenular distribution. Premalignant and malignant nodules may also be identified occasionally. Liver biopsy is considered to be the gold standard for the diagnosis of fibrosis; however, it is invasive and has several limitations with regard to the biopsy sites and frequency. Ultrasonography or MRI-based elastography may be used as an alternative method to non-invasively evaluate chronic liver disease.14,16 However, the limitations of the liver stiffness assessed by elastography were as follows: (1) indirect measurement of tissue features, (2) inability to distinguish between fibrosis and congestion, (3) requirement of additional hardware, and (4) unknown prognostic value of FALD. Liver T1 mapping has recently been used to as a novel non-invasive method to diagnose non-congestive adult chronic liver disease.16,28,29 Native T1 times can distinguish between histologic fibrotic grades and may predict outcomes, specifically in patients with fatty liver diseases and/or with auto-immune hepatitis.16,28,29 Alternatively, only a few studies have reported the evaluations of T1 mapping for FALD in children and adolescents.17,18 They demonstrated significantly elevated liver native T1 values in Fontan patients than normal controls, and a strong correlation with MR elastography-derived liver stiffness.17,18 However, whether T1 mapping can differentiate congestion from fibrosis remains unclear. Therefore, liver T1 mapping has the following advantages: (1) simultaneous scanning of the heart and liver, (2) availability of retrospective assessments, (3) no need of special hardware, and (4) non-contrast imaging in the case of native T1 mapping. Overall, liver T1 mapping may play a screening role in this population.
A few previous studies reported that both native T1 value and extracellular volume (ECV) were elevated in Fontan, but it is too early to conclude whether liver MR relaxometry should be performed using native T1, ECV or both, due to the technical limitations of ECV measurements. Considering this, we only assessed native T1 values. Theoretically, ECV can better quantify fibrosis than T1, due to which numerous non-CHD studies prioritized ECV measurements. However, the technical difficulties of the ECV measurements are as follows: (1) gadolinium contrast agent with even partial biliary excretion must be avoided as they are not suitable in quantifying ECV, possibly due to the fact that their concentration in the bile system falsely decreases post-contrast T1 and generates erroneously high ECV results, and (2) accurate oxygen saturation of the liver remains unknown. Generally, T1 is lower in less oxygenated blood. The lower levels of oxygen saturations in the hepatic veins are expected to be comparable to that of ventricular blood. Approximately 75% of the blood flow in the liver can be attributed to the portal vein, while the remaining 25% is provided by the hepatic artery. Therefore, performing the ECV calculations using a 75/25% split between hepatic venous blood and arterial cardiac lumen blood may produce a more ‘true’ hepatic ECV value in cyanotic CHDs.
T1 relaxometry does not unequivocally distinguish between fibrosis, congestion or inflammation, as their values are elevated in both native T1 and ECV outcomes; it is possible that all three mechanisms contribute to the abnormal T1 and ECV values. Therefore, adding T2 mapping and T2 weighted imaging may help in distinguishing between fibrosis and edema.28,29
Liver native T1 values, serum parameters, and MELD/ALBI score
Our results showed correlations between parameters such as age, γ -GTP, MELD XI score, and ALBI score. γ -GTP is one of the most sensitive makers of chronic liver congestion caused by right heart failure.30 Alternatively, the CVP and PVR values at rest, along with the fibrotic makers, were not associated with the native T1 values, as previously reported in similar studies on Fontan children and adolescents.17,18 The possible reasons could be (1) elevated CVP and PVR during exercise may significantly influence liver damage, and (2) fibrotic makers are not liver-specific, and do not demonstrate a good degree of correlation. MELD XI and/or ALBI score (if the patient is on anticoagulants) are essential in screening for liver damage.20–23 Additionally, the liver native T1 values in our study showed good correlation with the two scores, suggesting that the liver native T1 mapping may play an adjunct role in screening for FALD in adult Fontan patients. The prognostic significance of liver T1 relaxometry requires further elucidation. Larger prospective studies with a dedicated T1 mapping of the liver with long-term follow-up data may further delineate its role in FALD.
Technical issues of liver T1 mapping derived from cardiac magnetic resonance imaging at 3T
There may be a potential problem of shimming of liver T1 mapping derived from cardiac MRI. We measured the area of the upper liver under the diaphragm, which was inside the shimming area. In fact, we compared the T1 value with and without the shimming area, but there was no significant difference (965.9 ± 70.9 ms vs. 964.1 ± 68.1 ms, P = 0.78). Therefore, we concluded that there was no technical problem of shimming of liver T1 mapping derived from cardiac MRI.
In this current study, we used 3T MRI, not 1.5T, because the evaluation of tissue characterization was better using 3T than 1.5T.31 As mentioned above, previous studies used only 1.5T in FALD. From this perspective, our current results are clinical informative.
Limitations
The limitations of this study include its relatively small population size and the lack of a cox-hazard analysis; therefore, the prognostic values of liver native T1 mapping remained unknown. As mentioned above, T1 relaxometry does not unequivocally distinguish between fibrosis, congestion or inflammation; however, this novel technique is non-contrast and easily reproducible, making it ideal for chronic liver diseases like FALD. Furthermore, T1 value is shortened by fat and iron content in the tissue. In this study, there was no enrolled patients with fatty liver, and no subjects were being treated with iron drugs due to anemia. Therefore, the effect of iron deposition was considered to be small. Although this was a preliminary study of native T1 mapping at 3T in adults with Fontan circulation, we believe that it is clinically informative. We performed a catheterization that was performed within 2 years of MRI and there was a possibility of the status gap between the MRI and a catheterization.
Conclusion
The liver native T1 value at 3T may be a sensitive, contrast-free, and non-invasive screening marker of chronic liver damages in adults with Fontan circulation.
Acknowledgment
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: a review. J Cardiol 2019; 74:223–232. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JA, Cetta F, Graham RP, et al. Identifying predictors of hepatic disease in patients after the Fontan operation: a postmortem analysis. J Thorac Cardiovasc Surg 2013; 146:140–145. [DOI] [PubMed] [Google Scholar]
- 3.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg 2005; 129:1348–1352. [DOI] [PubMed] [Google Scholar]
- 4.Kiesewetter CH, Sheron N, Vettukattill JJ, et al. Hepatic changes in the failing Fontan circulation. Heart 2007; 93:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendall TJ, Stedman B, Hacking N, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol 2008; 61:504–508. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DJ, Surrey LF, Glatz AC, et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc 2017; 6:e004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pundi K, Pundi KN, Kamath PS, et al. Liver disease in patients after the Fontan operation. Am J Cardiol 2016; 117:456–460. [DOI] [PubMed] [Google Scholar]
- 8.Nandwana SB, Olaiya B, Cox K, Sahu A, Mittal P. Abdominal imaging surveillance in adult patients after Fontan procedure: risk of chronic liver disease and hepatocellular carcinoma. Curr Probl Diagn Radiol 2018; 47:19–22. [DOI] [PubMed] [Google Scholar]
- 9.Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med 2013; 368:1756–1757. [DOI] [PubMed] [Google Scholar]
- 10.Bae JM, Jeon TY, Kim JS, et al. Fontan-associated liver disease: spectrum of US findings. Eur J Radiol 2016; 85:850–856. [DOI] [PubMed] [Google Scholar]
- 11.Bulut OP, Romero R, Mahle WT, et al. Magnetic resonance imaging identifies unsuspected liver abnormalities in patients after the Fontan procedure. J Pediatr 2013; 163:201–206. [DOI] [PubMed] [Google Scholar]
- 12.Wolff D, van Melle JP, Dijkstra H, et al. The Fontan circulation and the liver: a magnetic resonance diffusion-weighted imaging study. Int J Cardiol 2016; 202:595–600. [DOI] [PubMed] [Google Scholar]
- 13.Wallihan DB, Podberesky DJ, Marino BS, Sticka JS, Serai S. Relationship of MR elastography determined liver stiffness with cardiac function after Fontan palliation. J Magn Reson Imaging 2014; 40:1328–1335. [DOI] [PubMed] [Google Scholar]
- 14.Poterucha JT, Johnson JN, Qureshi MY, et al. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc 2015; 90:882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Lai Y, Makowski M, et al. Native T1 mapping of autoimmune pancreatitis as a quantitative outcome surrogate. Eur Radiol 2019; 29:4436–4446. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Liu H, Zhang C, et al. Native T1 mapping compared to ultrasound elastography for staging and monitoring liver fibrosis: an animal study of repeatability, reproducibility, and accuracy. Eur Radiol 2020; 30:337–345. [DOI] [PubMed] [Google Scholar]
- 17.de Lange C, Reichert MJE, Pagano JJ, et al. Increased extracellular volume in the liver of pediatric Fontan patients. J Cardiovasc Magn Reson 2019; 21:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran P, Serai SD, Veldtman GR, et al. Assessment of liver T1 mapping in fontan patients and its correlation with magnetic resonance elastography-derived liver stiffness. Abdom Radiol (NY) 2019; 44:2403–2408. [DOI] [PubMed] [Google Scholar]
- 19.Yamamura K, Sakamoto I, Morihana E, et al. Elevated non-invasive liver fibrosis markers and risk of liver carcinoma in adult patients after repair of tetralogy of Fallot. Int J Cardiol 2019; 287:121–126. [DOI] [PubMed] [Google Scholar]
- 20.Fragaki M, Sifaki-Pistolla D, Orfanoudaki E, Kouroumalis E. Comparative evaluation of ALBI, MELD, and Child-Pugh scores in prognosis of cirrhosis: is ALBI the new alternative? Ann Gastroenterol 2019; 32:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng M, Ng SWY, Cheung ST, Chong CCN. Clinical application of Albumin-Bilirubin (ALBI) score: the current status. Surgeon 2020; 18:178–186. [DOI] [PubMed] [Google Scholar]
- 22.Evans WN, Acherman RJ, Ciccolo ML, et al. MELD-XI scores correlate with post-Fontan hepatic biopsy fibrosis scores. Pediatr Cardiol 2016; 37:1274–1277. [DOI] [PubMed] [Google Scholar]
- 23.Assenza GE, Graham DA, Landzberg MJ, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart 2013; 99:491–496. [DOI] [PubMed] [Google Scholar]
- 24.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014; 16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiina Y, Inai K, Taniguchi K, Takahashi T, Nagao M. Potential value of native T1 mapping in symptomatic adults with congenital heart disease: a preliminary study of 3.0 tesla cardiac magnetic resonance imaging. Pedatric Cardiol 2020; 41:94–100. [DOI] [PubMed] [Google Scholar]
- 26.Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol 2013; 19:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Iwakiri Y. The hepatic lymphatic vascular system: structure, function, markers, and lymphangiogenesis. Cell Mol Gastroenterol Hepatol 2016; 2:733–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman DH, Ayoola A, Nickel D, Han F, Chandarana H, Shanbhogue KP. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom Radiol (NY) 2020; 45:692–700. [DOI] [PubMed] [Google Scholar]
- 29.Luetkens JA, Klein S, Träber F, et al. Quantification of liver fibrosis at T1 and T2 mapping with extracellular volume fraction MRI: preclinical results. Radiology 2018; 288:748–754. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu M, Miyamoto K, Nishihara Y, et al. Risk factors and serological markers of liver cirrhosis after Fontan procedure. Heart Vessels 2016; 31:1514–1521. [DOI] [PubMed] [Google Scholar]
- 31.Nakamori S, Dohi K, Ishida M, et al. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 2018; 11:48–59. [DOI] [PubMed] [Google Scholar]