Abstract
Purpose:
We aimed to evaluate the acute physiological effects of high-load deadlift exercise on the lumbar intervertebral discs using MR diffusion-weighted imaging (DWI).
Methods:
Fifteen volunteers (11 men and 4 women; 23.2 ± 3.3 years) without lumbar intervertebral disc degeneration performed deadlift exercise (70% of 1 repetition maximum, 6 repetitions, 5 sets, 90 s rest between sets) using a Smith machine. Sagittal MR diffusion-weighted images of the lumbar intervertebral discs were obtained using a 1.5-Tesla MR system with a spine coil before and immediately after the exercise. We calculated apparent diffusion coefficient (ADC; an index of water movement) of the nucleus pulposus from diffusion weighted images at all lumbar intervertebral discs (L1/2 through L5/S1).
Results:
All lumbar intervertebral discs showed significantly decreased ADC values immediately after deadlift exercise (L1/2, −2.8%; L2/3, −2.1%; L3/4, −2.8%; L4/5, −4.9%; L5/S1, −6.2%; P < 0.01). In addition, the rate of ADC decrease of the L5/S1 disc was significantly greater than those of the L1/2 (P = 0.017), L2/3 (P < 0.01), and L3/4 (P = 0.02) discs.
Conclusion:
The movement of water molecules within the lumbar intervertebral discs is suppressed by high-load deadlift exercise, which would be attributed to mechanical stress on the lumbar intervertebral discs during deadlift exercise. In particular, the L5/S1 disc is subjected to greater mechanical stress than the other lumbar intervertebral discs.
Keywords: magnetic resonance diffusion-weighted image, intradiscal water movement, lumbar spine, lifting, mechanical stress
Introduction
A deadlift is a popular exercise that is frequently incorporated into athletic training programs. This exercise is effective in strengthening the back and lower extremity muscles, but the lumbar spine is subject to mechanical stress such as shear and compression forces during deadlift.1–4 The stress is expected to become greater with increasing exercise weight. Therefore, the lumbar region is most susceptible to injury during high-load deadlift.5,6 In addition, the repeated lifting of heavy weights has been identified as a risk factor for lumbar intervertebral disc degeneration/herniation.5 The lumbar intervertebral discs are thought to gradually degenerate through high-load deadlift training. However, to the best of our knowledge, little is known about the acute physiological changes of the lumbar intervertebral discs resulting from high-load deadlift exercise.
MRI has been used as a noninvasive method to investigate the status of the intervertebral discs in both clinical and experimental settings. In particular, diffusion-weighted imaging (DWI) can quantitatively evaluate the movement of water molecules (water diffusion) within the intervertebral disc by calculating an apparent diffusion coefficient (ADC). The intradiscal water movement is partially associated with intervertebral disc composition. Degenerated intervertebral discs show significantly lower ADC values than normal discs mainly due to reduced absolute intradiscal water content.7–10 In addition, the intradiscal water movement is sensitive to mechanical stress placed on the intervertebral disc. It was confirmed using ovine lumbar intervertebral discs11 or cadaveric human lumbar intervertebral discs12 that the ADC value of the lumbar intervertebral disc decreases under compressive loads. Thus, the ADC value is thought to be a useful parameter for noninvasively evaluating the acute stress responses of the lumbar intervertebral discs to high-load deadlift exercise.
The purpose of this study was to investigate the acute physiological effects of high-load deadlift exercise on the lumbar intervertebral discs using MR DWI. The findings of this study would help to deepen our understanding of the mechanism by which the repeated lifting of heavy weights leads to lumbar intervertebral disc degeneration/herniation. We expected that the water movement within the lumbar intervertebral discs would decrease after high-load deadlift exercise as an acute stress response to repetitive mechanical stress.
Materials and Methods
Subjects
A total of 15 healthy volunteers (11 men and 4 women; mean age, 23.2 ± 3.3 years; age range, 19–30 years) with normal lumbar intervertebral discs participated in this study. The state of the lumbar intervertebral discs was assessed a few days before the measurement, according to the Pfirrmann scale13 for disc degeneration based on the signal intensity (SI) on a midsagittal T2-weighted MR image. Those with bulging, degenerated, and/or herniated discs were not included in this study. All of the participants were not regularly engaged in any sports activities at the time of measurement. No participants reported any pain and discomfort at the time of measurement and there was no previous history of surgery or injury in the lumbar region or lower extremities. Participants were instructed to refrain from any physical exercise beginning 48 h prior to the measurement.
This study was approved by the ethical committee of Waseda University and followed the ethical guidelines of the Declaration of Helsinki. Prior to the measurement, all participants were given a brief description of the study, the examination procedures, and the potential risks. Written informed consent was obtained from each participant.
Deadlift
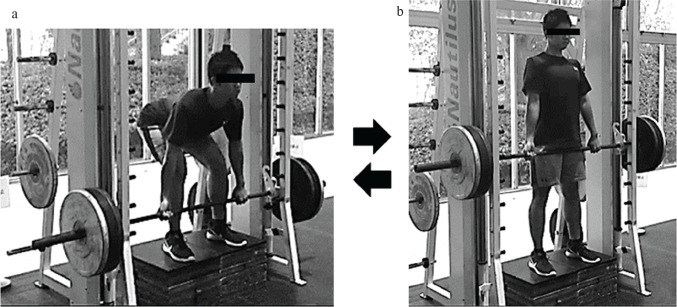
After pre-exercise MRI measurements of the lumbar intervertebral discs, the participants performed a non-standardized warm-up including self-stretching and submaximal deadlift repetitions. The deadlift was performed without a weight belt using a Smith machine (Nautilus, Vancouver, WA, USA) (Fig. 1). The participants stood near the bar with a shoulder width stance on a stool placed in the Smith machine and squatted down to grasp the bar with an alternated grip, slightly wider than shoulder width with the arms straight and the hips lower than the shoulders. From the start position, they lifted a weight corresponding to 70% (69.2 ± 18.7 kg) of their 1 repetition maximum by extending the hips and knees and keeping the posture of the spinal column in a neutral position (a straight back), until a fully erect body position was established. Then, they lowered the bar maintaining the neutral spinal position. We instructed them to lift and lower the bar as near to the body as possible. Verbal feedback regarding their deadlift technique was provided during the exercise. Each repetition was performed in a controlled manner by using a metronome with 2 s lifting and lowering phases. This 4 count action was repeated for 5 sets of 6 repetitions with a 90 s interval between sets, for a total of 30 repetitions.
Fig. 1.
The start (a) and finish (b) positions of high-load deadlift exercise. The participants lifted and lowered a weight corresponding to 70% of their 1 repetition maximum using a Smith machine.
MR imaging
Midsagittal DW images of the lumbar intervertebral discs were obtained in a supine position before and immediately after the deadlift exercise using a 1.5-Tesla MR system (Signa HDxt, GE Healthcare UK, Little Chalfont, Buckinghamshire, England) with a spine coil at Waseda University. We could start the scan of a post-exercise DW image within 5 min after deadlift exercise because the exercise was performed near a MRI room. The DWI sequence (spin-echo type single-shot echo-planar imaging without parallel imaging) was as follows: TR, 6000 ms; TE, 76.4 ms; 256 × 256 matrix; number of excitations, 4; field of view (FOV), 300 mm; rectangular (phase) FOV, 0.5; slice thickness, 10 mm; b-value, 500 s/mm2; acquisition time, 1 min 42 s; and water excitation of a single slice. The choice of a rectangular FOV minimized geometric distortions from susceptibility differences. We applied a motion-probing gradient with a b-value of 500 s/mm2 sequentially in each of three main orthogonal orientations (x-, y-, and z-axes). DW image acquisition produced one baseline echo-planar T2-weighted image (b-value, 0 s/mm2) without a motion-probing gradient and one isotropic DW image (b-value, 500 s/mm2) that was generated by averaging the ADC value from each DW image in the three orthogonal orientations.
To calculate the ADC values of 5 lumbar intervertebral discs (L1/2 through L5/S1) before and immediately after the exercise, we constructed pre- and post-exercise ADC maps using the FuncTool 2 software program (GE Healthcare UK) built into the MR device. We obtained the ADC map using one baseline echo-planar T2-weighted image and one isotropic DW image. The intensity of the pixels on the map corresponded to the absolute ADC values of tissue. A ROI was drawn so that it completely surrounded the nucleus pulposus of each lumbar intervertebral disc on the echo planar T2-weighted image. The ROI was then copied to the ADC map. The ADC value of the nucleus pulposus was calculated using the equation: ADC = ln(SI0/SI500)/(b500 − b0), where SI0 is the SI in the ROI without a motion-probing gradient (b-value, 0 s/mm2; b0), and SI500 is the SI in the ROI with a b-value of 500 s/mm2 (b500). In addition, the percentage change in ADC value post-exercise was defined as a % change in ADC value = (post-exercise ADC value − pre-exercise ADC value)/pre-exercise ADC value × 100.
Statistical analysis
We calculated the mean and standard deviations for pre- and post-exercise ADC values, and the percentage change in ADC values of fie lumbar intervertebral discs. The Shapiro–Wilk test was used to check for normal distributions of all ADC values. Then, the ADC values of 5 lumbar intervertebral discs were compared before and after the deadlift exercise using a paired t-test. In addition, significant differences between the percentage changes in ADC values of five lumbar intervertebral discs were evaluated by one-way analysis of variance with a Tukey’s post-hoc test. Moreover, effect sizes (Cohen’s d) were calculated and evaluated as trivial (0–0.19), small (0.20–0.49), medium (0.50–0.79), and large (0.80 and greater). Statistical analysis was performed using SPSS Statistics 23 software (IBM, Armonk, NY, USA). Statistical significance was set at P < 0.05.
Results
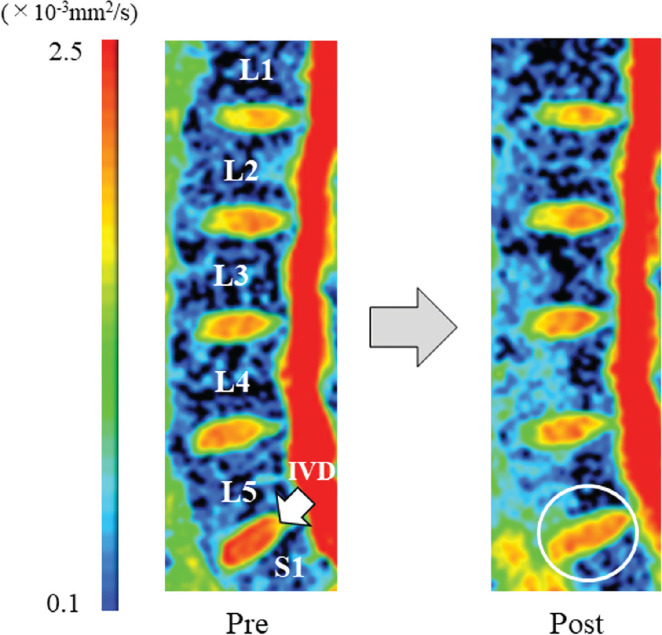
Table 1 displays the ADC values of five lumbar intervertebral discs before and immediately after high-load deadlift exercise. All lumbar intervertebral discs showed significantly decreased ADC values after the exercise (P < 0.01). In addition, ADC decrease of the L5/S1 disc was significantly greater than those of the L1/2 (P = 0.017), L2/3 (P < 0.01), and L3/4 (P = 0.02) discs (Fig. 2). Figure 3 shows the decreased ADC value of the L5/S1 disc on an ADC map after high-load deadlift exercise for a representative subject.
Table 1.
ADC values of 5 lumbar intervertebral discs before and after high-load deadlift exercise
| Disc level | ADC (×10−3 mm2/s) | Effect size (d) | |
|---|---|---|---|
|
| |||
| Pre | Post | ||
| L1/2 | 1.77 (0.09) | 1.72 (0.09)* | 0.56 |
| L2/3 | 1.79 (0.06) | 1.75 (0.05)* | 0.72 |
| L3/4 | 1.82 (0.04) | 1.77 (0.05)* | 1.1 |
| L4/5 | 1.83 (0.06) | 1.74 (0.07)* | 1.38 |
| L5/S1 | 1.85 (0.13) | 1.73 (0.1)* | 1.04 |
P < 0.01, Pre vs. Post. Mean (± SD).
ADC, apparent diffusion coefficient.
Fig. 2.
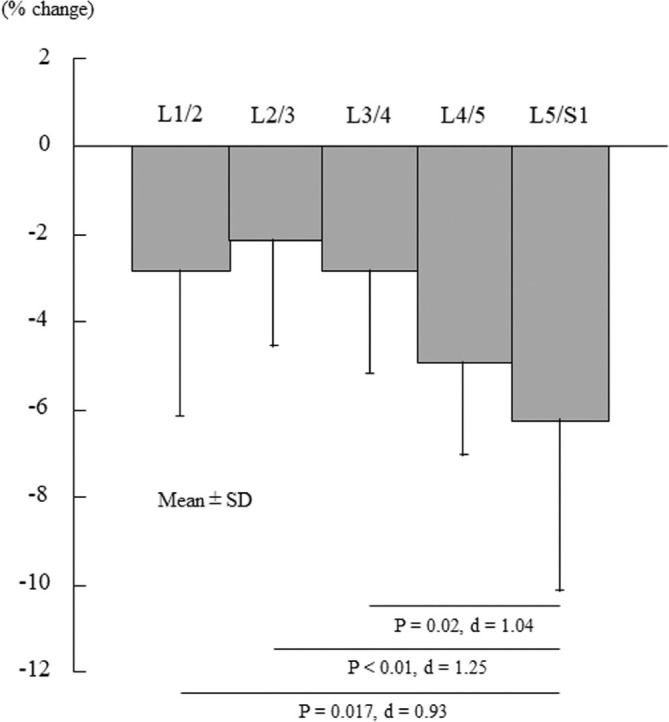
The percentage change in the apparent diffusion coefficient values of 5 lumbar intervertebral discs before and immediately after high-load deadlift exercise.
Fig. 3.
Sagittal ADC maps of the lumbar spine of a representative subject (23-year-old male) before and immediately after high-load deadlift exercise. The color-coded pixels on the ADC map correspond to the absolute values of ADC in the tissues. Compared with the pre-exercise ADC map, the post-exercise ADC map shows a decrease in the red region within the L5/S1 IVD (circle). ADC, apparent diffusion coefficient; IVD, intervertebral disc.
Discussion
In the present study, all 5 lumbar intervertebral discs showed significantly decreased ADC values after high-load deadlift exercise. ADC value reflects both the water diffusion and capillary perfusion within tissues.14 However, the effect of perfusion is thought to be negligible in the intervertebral discs because of their avascularity,15 although we used relatively low b-value (500 s/mm2). Thus, the findings of this study imply that repeated lifting of heavy weights decreased the movement of water molecules within the nucleus pulposus of each lumbar intervertebral disc. Intradiscal water movement is suppressed by applying compression force to the lumbar intervertebral disc.11,12 Eltoukhy et al.1 calculated, using a biomechanical spine model based on motion capturing, that the lumbar vertebrae are subject to axial compressive force (6488–7963N), shear force (1220–1903N), and bending moment (685–747 Nm) during the deadlift with a weight corresponding to approximately 75% of the one repetition maximum. They also found that the axial compressive force reaches a maximum at up-right standing position during the deadlift. Thus, we infer that the decreased ADC values reflect repetitive mechanical stress on the lumbar intervertebral discs that occurred during high-load deadlift exercise.
In addition, the present study revealed that the L5/S1 disc shows a significantly greater ADC decrease than the L1/2, L2/3, and L3/4 discs after high-load deadlift exercise. This finding suggests that the L5/S1 disc are more subject to mechanical stress resulting from high-load deadlift exercise among the lumbar intervertebral discs. Eltoukhy et al.1 reported that maximum compressive and shear forces occurred at the L5 vertebra during high-load deadlift. Thus, larger mechanical forces might occur at the L5/S1 disc during high-load deadlift exercise in the present study. Considering that the L5/S1 disc is a high incidence site of lumbar intervertebral degeneration/herniation,16 special clinically attention should be paid to this region during high-load exercise to prevent lumbar injuries.
Degenerated intervertebral discs show significantly lower ADC values than normal discs.7–10 The decreased ADC values would be associated with losses of water and/or proteoglycan contents in the nucleus pulposus.17 It is likely that repetitive mechanical stress gradually changes the composition of the intervertebral disc, resulting in intervertebral disc degeneration.7,18,19 In the present study, acute ADC decreases as a result of high-load deadlift exercise were relatively small, but the accumulation of the small responses may lead to lumbar intervertebral disc degeneration in the future. Furthermore, although the findings of the present study do not imply that the lumbar intervertebral discs will necessarily be degenerated by repetitively performing high-load deadlifts, we hope that these findings will contribute to a better understanding of the mechanisms of lumbar injuries during deadlift exercise.
There are some limitations in the present study. First, since we only performed MRI measurements before and immediately after the deadlift exercise, the recovery time of decreased ADC values remains unclear. Second, the participants could not completely keep the bar in contact with their body during the deadlift by using the Smith machine. Thus, it is possible that the flexion moment at the lumbar spine became greater than what would have occurred in a free-weight deadlift where the bar is kept close to the body throughout, resulting in greater mechanical stress on the lumbar intervertebral discs when using the Smith machine. Third, although we evaluated the status of the lumbar intervertebral discs in a midsagittal image, additional axial images might provide useful physiological information on the intervertebral discs. However, the number of post-exercise measurements should be limited to adequately identify the acute responses of the tissues to exercise. Finally, although we did not measure the sagittal alignment of the lumbar spine before exercise, it might influence on the magnitude of mechanical stress on the lumbar intervertebral discs during deadlift exercise.20
Conclusion
High-load deadlift exercise places mechanical stress on all of the lumbar intervertebral discs. In particular, the L5/S1 disc is subjected to greater mechanical stress compared with the upper lumbar discs during high-load deadlift exercise.
Acknowledgment
This study was funded by a research grant from the Nakatomi Foundation. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Conflicts of Interest
We have no conflicts of interest to declare.
References
- 1.Eltoukhy M, Travascio F, Asfour S, Elmasry S, Heredia-Vargas H, Signorile J. Examination of a lumbar spine biomechanical model for assessing axial compression, shear, and bending moment using selected Olympic lifts. J Orthop 2016; 13:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholewicki J, McGill SM, Norman RW. Lumbar spine loads during the lifting of extremely heavy weights. Med Sci Sports Exerc 1991; 23:1179–1186. [PubMed] [Google Scholar]
- 3.Swinton PA, Stewart A, Agouris I, Keogh JW, Lloyd R. A biomechanical analysis of straight and hexagonal barbell deadlifts using submaximal loads. J Strength Cond Res 2011; 25:2000–2009. [DOI] [PubMed] [Google Scholar]
- 4.Edington C, Greening C, Kmet N, et al. The effect of set up position on EMG amplitude, lumbar spine kinetics, and total force output during maximal isometric conventional-stance deadlifts. Sports (Basel) 2018; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siewe J, Rudat J, Röllinghoff M, Schlegel UJ, Eysel P, Michael JW. Injuries and overuse syndromes in powerlifting. Int J Sports Med 2011; 32:703–711. [DOI] [PubMed] [Google Scholar]
- 6.Winwood PW, Hume PA, Cronin JB, Keogh JW. Retrospective injury epidemiology of strongman athletes. J Strength Cond Res 2014;28:28–42. [DOI] [PubMed] [Google Scholar]
- 7.Kealey SM, Aho T, Delong D, Barboriak DP, Provenzale JM, Eastwood JD. Assessment of apparent diffusion coefficient in normal and degenerated intervertebral lumbar disks: initial experience. Radiology 2005; 235:569–574. [DOI] [PubMed] [Google Scholar]
- 8.Beattie PF, Morgan PS, Peters D. Diffusion-weighted magnetic resonance imaging of normal and degenerative lumbar intervertebral discs: a new method to potentially quantify the physiologic effect of physical therapy intervention. J Orthop Sports Phys Ther 2008; 38:42–49. [DOI] [PubMed] [Google Scholar]
- 9.Niu G, Yang J, Wang R, Dang S, Wu EX, Guo Y. MR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: apparent diffusion coefficient versus T2 quantitation. AJNR Am J Neuroradiol 2011; 32:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu G, Yu X, Yang J, Wang R, Zhang S, Guo Y. Apparent diffusion coefficient in normal and abnormal pattern of intervertebral lumbar discs: initial experience. J Biomed Res 2011; 25:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drew SC, Silva P, Crozier S, Pearcy MJ. A diffusion and T2 relaxation MRI study of the ovine lumbar intervertebral disc under compression in vitro. Phys Med Biol 2004; 49:3585–3592. [DOI] [PubMed] [Google Scholar]
- 12.Alkalay RN, Burstein D, Westin CF, Meier D, Hackney DB. MR diffusion is sensitive to mechanical loading in human intervertebral disks ex vivo. J Magn Reson Imaging 2015; 41:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001; 26:1873–1878. [DOI] [PubMed] [Google Scholar]
- 14.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168:497–505. [DOI] [PubMed] [Google Scholar]
- 15.Kerttula L, Kurunlahti M, Jauhiainen J, Koivula A, Oikarinen J, Tervonen O. Apparent diffusion coefficients and T2 relaxation time measurements to evaluate disc degeneration. A quantitative MR study of young patients with previous vertebral fracture. Acta Radiol 2001; 42:585–591. [DOI] [PubMed] [Google Scholar]
- 16.Hangai M, Kaneoka K, Hinotsu S, et al. Lumbar intervertebral disk degeneration in athletes. Am J Sports Med 2009; 37:149–155. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou J, Demers CN, Beaudoin G, et al. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging 2004; 22:963–972. [DOI] [PubMed] [Google Scholar]
- 18.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995; 20:1307–1314. [DOI] [PubMed] [Google Scholar]
- 19.Horner HA, Urban JP. Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976) 2001; 26:2543–2549. [DOI] [PubMed] [Google Scholar]
- 20.Bassani T, Casaroli G, Galbusera F. Dependence of lumbar loads on spinopelvic sagittal alignment: an evaluation based on musculoskeletal modeling. PLoS ONE 2019; 14:e0207997. [DOI] [PMC free article] [PubMed] [Google Scholar]