Abstract
To assess myocardial fibrosis associated with muscular dystrophy, T1-mapping and extracellular volume fraction (ECV) quantification was prospectively performed using cardiovascular MR (CMR) imaging in 6 male patients with muscular dystrophy and 5 female putative carriers of Duchenne or Becker muscular dystrophy. Five patients and all putative carriers had an elevated ECV (>29.5% for men and >35.2% for women), suggesting that ECV has a potential to detect diffuse fibrotic changes in patients and putative carriers of muscular dystrophy.
Keywords: cardiovascular magnetic resonance imaging, T1-mapping, extracellular volume fraction, muscular dystrophy, putative carrier
Introduction
Muscular dystrophy is characterized by progressive wasting and weakness of skeletal muscles that result from mutations in many genes affecting striated muscle. For many muscular dystrophies, cardiac complications are a major cause of morbidity and mortality.1 The most common form of cardiac involvement in muscular dystrophy is dilated cardiomyopathy,2 presenting as an age-related progression of left ventricular (LV) dysfunction and myocardial fibrosis detected by late gadolinium enhancement (LGE) cardiovascular MR (CMR) imaging.3 Accurate evaluation of myocardial fibrosis could be useful for risk stratification4 and determining early initiation of cardioprotective treatment in patients with muscular dystrophy.5,6 Cardiomyopathy associated with muscular dystrophy has also been reported in female carriers of Duchenne and Becker muscular dystrophy.7,8 These female carriers are basically asymptomatic, and their myocardial fibrosis are likely to precede the development of LV dysfunction.8 Although LGE is well established for detecting myocardial fibrosis, LGE cannot detect diffuse fibrotic changes in the myocardium. The image contrast in LGE strongly depends on the difference in post-contrast signal intensity between diseased and normal myocardium, which is attenuated by diffuse myocardial fibrosis.9 Previous studies suggested that native T1 values and extracellular volume fraction (ECV) mapping using CMR might be more suitable for detecting diffuse myocardial fibrosis.10 Therefore, this study aimed to detect diffuse myocardial fibrosis in patients and putative carriers of muscular dystrophy by myocardial T1-mapping CMR.
Materials and Methods
Patients with muscular dystrophy and female putative carriers of Duchenne or Becker muscular dystrophy were prospectively recruited at Hokkaido University Hospital, Japan, between August 2019 and March 2020. Inclusion criteria in the patient group were: (1) patients diagnosed with muscular dystrophy based on a clinical examination, dystrophin gene analysis, muscle biopsy, or family history of muscular dystrophy; (2) ≥6 years of age; and (3) referred for clinically indicated CMR. Inclusion criteria in the putative carrier group were women ≥20 years of age who had a first-degree male relative with a confirmed diagnosis of Duchenne or Becker muscular dystrophy. Exclusion criteria were participants with renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2), contraindications to CMR, or limited life expectancy. The study was approved by the Institutional Review Board of Hokkaido University Hospital (IRB No. 018-0287) and registered with the University Hospital Medical Information Network clinical trials registry (UMIN000037608; http://www.umin.ac.jp/ctr/index.htm). All participants ≥18 years of age gave their written informed consent. For participants younger than 18 years, written informed consent was obtained from a parent or guardian of each participant, and an age-appropriate assent form was obtained from the participant.
CMR was performed using a 3T whole-body scanner (Achieva TX; Philips Medical Systems, Best, the Netherlands) with a 32-channel phased-array receiver torso-cardiac coil, including cine, pre- and post-contrast T1-mapping for ECV quantification, and phase sensitive inversion recovery (PSIR) LGE. LV short-axis cine images were obtained using a retrospectively electrocardiogram-gated, balanced steady-state free precession pulse sequence as previously described.11 For T1-mapping, basal, midventricular, and apical short-axis images were obtained using a modified Look-Locker inversion recovery sequence. LGE and post-contrast T1-mapping were obtained 10–15 min after administration of 0.1 mmol/kg of gadobutrol (Gadovist; Bayer Yakuhin, Osaka, Japan). CMR images were analyzed using Ziostation2 (Ziosoft, Tokyo, Japan). LV volume, mass, and ejection fraction were measured semiautomatically from short-axis cine images.11 The LGE images were visually assessed with consensus reading by an expert radiologist (N.O-M. with 20 years of experience in cardiovascular imaging) and a cardiologist (K.K. with 3 years of experience in cardiovascular imaging).12 Both readers were blinded to clinical information. The extent of LGE was expressed as a percentage of LV mass using a 5-standard deviation (SD) threshold above the mean of normal myocardium3 because the extent of LGE on PSIR sequence quantified using the “n”-SD method has been reported to be comparable to that on inversion-recovery balanced steady-state free precession sequence in both ischemic and non-ischemic cardiomyopathies.13 Global ECV was calculated using pre- and post-contrast T1 values of myocardium and blood pool (T1 myo pre, T1 myo post, T1 blood pre, and T1 blood post, respectively) as follows: ECV (%) = (1 − hematocrit) × (1/T1 myo post − 1/T1 myo pre)/(1/T1 blood post − 1/T1 blood pre).14 Venous blood samples for hematocrit and cardiac biomarkers were taken just prior to the CMR study. Given the fact that ECV mapping requires the administration of gadolinium-based contrast material, it was ethically and practically difficult to obtain the reference value of ECV in healthy children.15 For this reason, the cut-off values of ECV for men and women were derived from the previous study in healthy volunteers using 3T CMR,16 in which women (mean ± SD of age, 55 ± 17 years) had a higher ECV as compared to age-matched men (mean ± SD; 28.4 ± 3.4% vs. 25.1 ± 2.2%, P < 0.001). ECV higher than the mean + 2SD of the reference values (>29.5% for men and >35.2% for women) was considered elevated.
Results
Six patients with muscular dystrophy and five female putative carriers of Duchenne or Becker muscular dystrophy participated in this study. Characteristics of study participants are shown in Table 1. All 6 patients were male with a median age of 16 (range, 8.6–34.4) years, while the median age of 5 putative carriers was 46 (range, 43.0–51.7) years. The patient group was included patients with Duchenne muscular dystrophy (n = 2), Becker muscular dystrophy (n = 3), and myotonic dystrophy (n = 1). Four patients were being treated with angiotensin-converting enzyme inhibitor, and 2 of these 4 patients were also treated with β-blocker. All putative carriers were mothers of confirmed male patients with Duchenne or Becker muscular dystrophy without any symptoms and known comorbidities, and all of them had normal levels of cardiac biomarkers including NT-proBNP and troponin T. Of the 11 participants, Case 3 was the brother of Case 4, and Case 9 was the mother of Case 2. Four of the patients and 1 of the putative carriers had a reduced LV ejection fraction (<55%).
Table 1.
Characteristics of study participants
| Characteristics | Patients | Putative carriers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | |
| Age (years) | 8.6 | 13.3 | 13.7 | 18.3 | 20.1 | 34.4 | 43.0 | 44.4 | 46.0 | 46.5 | 51.7 |
| Sex | Male | Male | Male | Male | Male | Male | Female | Female | Female | Female | Female |
| Type of muscular dystrophy | Duchenne | Duchenne | Becker | Becker | Myotonic | Becker | Becker | Duchenne | Duchenne | Becker | Duchenne |
| Body mass index (kg/m2) | 15.5 | 16.3 | 17.8 | 23.8 | 17.9 | 21.7 | 20.8 | 19.5 | 21.8 | 19.9 | 21.3 |
| Wheelchair bound | No | Yes | No | No | No | No | No | No | No | No | No |
| Medications | None | Enalapril 10 mg/day, Steroid | Enalapril 10 mg/day | Enalapril 10 mg/day, Bisoprolol 1.25 mg/day | Enalapril 5 mg/day, Carvedilol 5 mg/day | None | None | None | None | None | None |
| Blood data* | |||||||||||
| Creatine kinase (IU/L) | 6509 | 2739 | 1430 | 2158 | 240 | 328 | 66 | 76 | 192 | 71 | 93 |
| NT-proBNP (pg/mL† ) | 189 | 80 | NA‡ | 302 | 26 | 7 | 99 | 47 | 67 | 39 | 51 |
| Troponin T (ng/mL† ) | 0.059 | 0.115 | 0.024 | 0.073 | 0.046 | 0.005 | ≤0.014 | ≤0.014 | ≤0.014 | ≤0.014 | ≤0.014 |
| Hematocrit (%) | 40.8 | 40.8 | 38.0 | 40.8 | 47.9 | 47.5 | 37.5 | 40.2 | 40.6 | 42.6 | 38.8 |
| CMR findings | |||||||||||
| LVEF (%) | 57.6 | 54.0 | 56.5 | 31.8 | 46.4 | 53.7 | 56.2 | 61.0 | 49.6 | 60.3 | 65.3 |
| LVEDVI (mL/m2) | 53.5 | 50.5 | 49.4 | 115.0 | 68.9 | 53.9 | 51.1 | 65.7 | 62.5 | 58.6 | 54.0 |
| LVESVI (mL/m2) | 22.7 | 23.2 | 21.5 | 78.4 | 37.0 | 25.0 | 22.3 | 25.6 | 31.5 | 23.3 | 18.7 |
| LV mass (g) | 23.7 | 36.8 | 30.9 | 79.3 | 33.4 | 44.8 | 37.6 | 28.7 | 39.9 | 29.8 | 53.0 |
| LVMI (g/m2) | 26.7 | 32.6 | 23.7 | 46.7 | 21.2 | 27.2 | 26.5 | 19.4 | 26.4 | 18.3 | 33.3 |
| LGE extent, % of LV mass§ | NA | 6.8 | 6.2 | 14.6 | 0 | 5.3 | 0 | 2.6 | 19.8 | 0.5 | 1.2 |
| Native T1 value (ms) | |||||||||||
| Basal (mean) | 1292 | 1326 | 1325 | 1316 | 1279 | 1195 | 1269 | 1306 | 1293 | 1267 | 1259 |
| Mid-ventricular (mean) | 1276 | 1377 | 1300 | 1264 | 1240 | 1207 | 1336 | 1341 | 1334 | 1330 | 1269 |
| Apical (mean) | 1354 | 1480 | 1315 | 1452 | 1357 | 1297 | 1312 | 1408 | 1341 | 1407 | 1287 |
| ECV (%) | 32.3 | 39.7 | 37.0 | 43.8 | 32.6 | 27.6 | 38.7 | 39.8 | 36.1 | 37.4 | 36.7 |
Case 3 was the brother of Case 4, and Case 9 was the mother of Case 2.
The normal reference ranges for NT-proBNP and troponin T are ≤55 pg/mL and ≤0.014 ng/mL, respectively.
The plasma level of brain natriuretic peptide (normal reference range, ≤18.4 pg/mL) in Case 3 was 7.3 pg/mL.
Case 1 did not have sufficient image quality for LGE analysis due to an incomplete breath-hold.
BNP, brain natriuretic peptide; CMR, cardiovascular magnetic resonance; ECV, extracellular volume fraction; LGE, late gadolinium enhancement; LV, left ventricular; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LVMI, left ventricular mass index; NA, not available; NT-proBNP, N-terminal pro-brain natriuretic peptide.
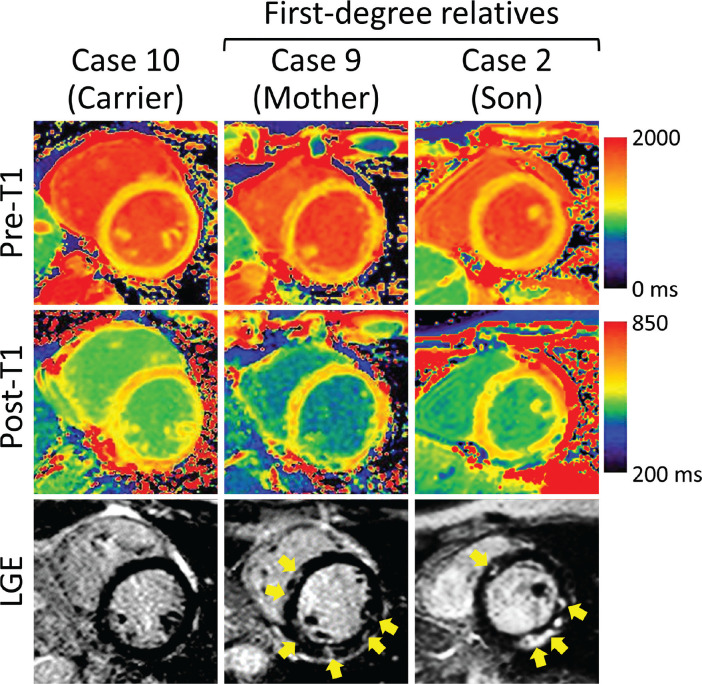
Representative CMR images are shown in Fig. 1. Four patients and 2 putative carriers showed visually detected LGE. The median LGE extent was 6.2% (range, 5.3–14.6%) of LV mass in the patient group. Five patients and all putative carriers had an elevated ECV. The median values of ECV in the patient and putative carrier groups were 34.8% (range, 27.6 – 43.8%) and 37.4% (range, 36.1–39.8%), respectively. When comparing CMR findings between patients and putative carriers in the same family, a similar distribution of LGE was observed in both families: Case 3 and 4 (brothers with Becker muscular dystrophy) showed subepicardial hyperenhancement in the lateral wall; Case 2 and 9 (mother and son with Duchenne muscular dystrophy) showed midwall hyperenhancement in the septal and lateral walls (Fig. 1).
Fig. 1.
Representative images in a putative carrier of Becker muscular dystrophy (Case 10), a patient with Duchenne muscular dystrophy (Case 2), and the mother of Case 2 (Case 9; a putative carrier of Duchenne muscular dystrophy). Case 2 and 9 show nonischemic patchy hyperenhancement (yellow arrows) on the LGE image and abnormal pre- and post-contrast T1 values located in the same areas. Both putative carriers had an elevated extracellular volume fraction (37.4% and 36.1%, respectively). LGE, late gadolinium enhancement.
Discussion
This study showed that diffuse myocardial fibrosis detected by elevated ECV was observed both in patients with muscular dystrophy and female putative carriers. Previous studies have shown the utility of myocardial T1 values and ECV for detecting myocardial fibrosis in patients with Duchenne and Becker muscular dystrophy.10,15,17,18 In the present study, elevated ECV was observed even in female putative carriers without LGE. Furthermore, mild diffuse fibrosis detected ECV appears to precede the development of LV dysfunction or substantial LGE, suggesting that myocardial tissue characterization by T1-mapping and ECV may be more sensitive to detect cardiac involvement in muscular dystrophy compared to conventional CMR techniques.
The similarity of LGE findings between female carriers of Duchenne or Becker muscular dystrophy and their male relatives was in agreement with previous studies.7 Although further studies are still needed to confirm this observation, CMR screening should be considered in putative female carriers who had male relatives with muscular dystrophy-related cardiomyopathy. Since the number of participants is small in this early experience, large-scale studies and long-term follow-up are needed to substantiate clinical utility of myocardial T1-mapping, to adjust genetic background including the parent–child relationship, and to evaluate the long-term effects of myocardial ECV on changes in LV ejection fraction and outcomes in patients and putative carriers of muscular dystrophy.
Acknowledgments
This work was supported by JSPS KAKENHI (grant number 19K17189), the Watanabe Foundation (Magnetic Health Science Foundation), Japan Heart Foundation Research Grant, and Japan Intractable Diseases (Nanbyo) Research Foundation. The funders had no role in the study design, data collection, analysis, interpretation of data, or in the writing of the manuscript. We thank Kinya Ishizaka for technical assistance.
Footnotes
Conflicts of Interest
Dr. Aikawa was supported by postdoctoral fellowships from the Uehara memorial Foundation, the Kanzawa Medical Research Foundation, the Suginome Memorial Foundation, and the Nakayama Foundation for Human Science; and was affiliated with a department with endowments from Medtronic Japan and Win International between April and August 2019. Dr. Anzai has received lecture fees from Daiichi-Sankyo Co. Ltd., Ono Pharmaceutical Co. Ltd., Bayer Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Boehringer-Ingelheim Japan Co., Ltd.; and has received research funding from Daiichi-Sankyo Co., Ltd. All other authors declare that they have no conflicts of interest.
References
- 1.Feingold B, Mahle WT, Auerbach S, et al. Management of cardiac involvement associated with neuromuscular diseases: a scientific statement From the American Heart Association. Circulation 2017; 136:e200–e231. [DOI] [PubMed] [Google Scholar]
- 2.Arbustini E, Di Toro A, Giuliani L, Favalli V, Narula N, Grasso M.Cardiac phenotypes in hereditary muscle disorders: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72:2485–2506. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa T, Takeda A, Oyama-Manabe N, et al. Progressive left ventricular dysfunction and myocardial fibrosis in Duchenne and Becker muscular dystrophy: a longitudinal cardiovascular magnetic resonance study. Pediatr Cardiol 2019; 40:384–392. [DOI] [PubMed] [Google Scholar]
- 4.Menon SC, Etheridge SP, Liesemer KN, et al. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol 2014; 35:1279–1285. [DOI] [PubMed] [Google Scholar]
- 5.Silva MC, Magalhães TA, Meira ZM, et al. Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy: a randomized clinical trial. JAMA Cardiol 2017; 2:190–199. [DOI] [PubMed] [Google Scholar]
- 6.Raman SV, Hor KN, Mazur W, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2015; 14:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florian A, Rösch S, Bietenbeck M, et al. Cardiac involvement in female Duchenne and Becker muscular dystrophy carriers in comparison to their first-degree male relatives: a comparative cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2016; 17:326–333. [DOI] [PubMed] [Google Scholar]
- 8.Wexberg P, Avanzini M, Mascherbauer J, et al. Myocardial late gadolinium enhancement is associated with clinical presentation in Duchenne muscular dystrophy carriers. J Cardiovasc Magn Reson 2016; 18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA.Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011; 57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U, Yilmaz A.Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging 2014; 15:1004–1012. [DOI] [PubMed] [Google Scholar]
- 11.Aikawa T, Naya M, Koyanagawa K, et al. Improved regional myocardial blood flow and flow reserve after coronary revascularization as assessed by serial 15O-water positron emission tomography/computed tomography. Eur Heart J Cardiovasc Imaging 2020; 21:36–46. [DOI] [PubMed] [Google Scholar]
- 12.Aikawa T, Oyama-Manabe N, Naya M, et al. Delayed contrast-enhanced computed tomography in patients with known or suspected cardiac sarcoidosis: a feasibility study. Eur Radiol 2017; 27:4054–4063. [DOI] [PubMed] [Google Scholar]
- 13.Muehlberg F, Arnhold K, Fritschi S, et al. Comparison of fast multi-slice and standard segmented techniques for detection of late gadolinium enhancement in ischemic and non-ischemic cardiomyopathy - a prospective clinical cardiovascular magnetic resonance trial. J Cardiovasc Magn Reson 2018; 20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takagi H, Ota H, Umezawa R, et al. Left ventricular T1 mapping during chemotherapy-radiation therapy: serial assessment of participants with esophageal cancer. Radiology 2018; 289:347–354. [DOI] [PubMed] [Google Scholar]
- 15.Starc JJ, Moore RA, Rattan MS, et al. Elevated myocardial extracellular volume fraction in Duchenne muscular dystrophy. Pediatr Cardiol 2017; 38:1485–1492. [DOI] [PubMed] [Google Scholar]
- 16.Roy C, Slimani A, de Meester C, et al. Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 2017; 19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soslow JH, Damon BM, Saville BR, et al. Evaluation of post-contrast myocardial T1 in Duchenne muscular dystrophy using cardiac magnetic resonance imaging. Pediatr Cardiol 2015; 36:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soslow JH, Damon SM, Crum K, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2016; 18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]