Abstract
Purpose
Prediction of high flow nasal cannula (HFNC) failure in COVID-19 patients with acute hypoxemic respiratory failure (AHRF) may improve clinical management and stratification of patients for optimal treatment. We performed a systematic review and meta-analysis to determine performance of ROX index as a predictor of HFNC failure.
Materials and methods
Systematic search was performed in electronic databases (PubMed, Google Scholar, Web of Science and Cochrane Library) for articles published till 15 June 2021 investigating ROX index as a predictor for HFNC failure. Quality In Prognosis Studies (QUIPS) tool was used to analyze risk of bias for prognostic factors, by two independent authors.
Results
Eight retrospective or prospective cohort studies involving 1301 patients showed a good discriminatory value, summary area under the curve (sAUC) 0.81 (95% CI, 0.77–0.84) with sensitivity of 0.70 (95% CI, 0.59–0.80) and specificity of 0.79 (95% CI, 0.67–0.88) for predicting HNFC failure. The positive and negative likelihood ratio were 3.0 (95% CI, 2.2–5.3) and 0.37 (95% CI, 0.28–0.50) respectively, and was strongly associated with a promising predictive accuracy (Diagnostic odds ratio (DOR) 9, 95% CI, 5–16).
Conclusion
This meta-analysis suggests ROX index has good discriminating power for prediction of HFNC failure in COVID-19 patients with AHRF.
Keywords: High flow nasal cannula, ROX index, Acute hypoxemic respiratory failure, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) has so far led to a huge disruption in socio-economic conditions and death of more than 3.8 million people worldwide [1]. Treatment of acute hypoxemic respiratory failure (AHRF) in COVID-19 patients is critical for saving lives. High flow nasal cannula (HFNC) oxygen therapy has now been successfully used as a non-invasive procedure in the management of AHRF in COVID-19 patients [2]. However, many patients have suffered from HFNC failure in the management of AHRF and lead to worsening of conditions [3].
Thus the early prediction of HFNC failure at the time of acute period of AHRF may improve clinical management and stratification of patients for optimal treatment. Recently some studies have evaluated prognostic significance of Sequential Organ Failure Assessment (SOFA) score [4,5] and acute physiology and chronic health evaluation (APACHE II) score [4,6] for predicting HFNC failure.
The ROX index, a score that has been accepted in the management of pneumonia and acute respiratory distress syndrome (ARDS) [7,8], could have the potential to predict HFNC outcomes in COVID-19 patients.
Roca et al. were the first to use ROX index to predict HFNC failure in ICU patients suffering from pneumonia [8]. ROX index is described as a combination of the ratio of oxygen saturation to the fraction of inspired oxygen [SPO2/FiO2] and respiratory rate. The use of the ROX index could improve the management and treatment of patients with COVID-19 during the current pandemic and recently describe in a variety of observational studies. As it takes only a few data sets and is easy to measure at the bedside and may have great clinical utility.
Several studies during the COVID-19 pandemic have been reported to assess the predictive accuracy of the ROX index for predicting HFNC failure, but the findings are inconsistent due to differences in the clinical setting, cut-off used and heterogeneous population [4,7,[9], [10], [11], [12], [13], [14]]. Computing the pooled predictive power of the ROX index in predicting HNFC failure would provide key information for its evidence-based use in clinical settings. Therefore, we aimed to conduct a systemic review and meta-analysis to determine the predictive accuracy of the ROX index for predicting HFNC failure in COVID-19 patients with AHRF.
2. Materials and methods
The protocol for our systemic review was registered on PROSPERO (CRD42021236603). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) declaration [15] and the Cochrane Handbook for Systematic Reviews of Interventions [16] were used to carry out this study.
2.1. Study selection criteria
All citations were screened in duplicate, with any discrepancies settled through conversation and, if necessary, third-party arbitration. Two authors (JP, PKB) independently and repeatedly screened all possibly important citations and references in two phases, first reviewing titles and abstracts and thereafter complete manuscripts for those which qualified the parameters. Disagreements were settled by a third author (AK). We recorded the criteria for exclusion during the full manuscript review stage.
2.2. Types of studies
We included retrospective or prospective cohort studies to predict the HFNC failure in patients with COVID-19 with AHRF. Case reports, case series (describing only phenomenology without outcome ascertainment and those with sample size less than 10), review articles, abstract publications, and conference presentations were excluded.
2.3. Types of participants
We included COVID-19 patients (>18 years), diagnosed with reverse transcription-polymerase chain reaction (RT-PCR) testing, with AHRF who required HFNC in the hospital or intensive care unit (ICU). We accepted AHRF definition used by the study authors.
2.4. Exposure
ROX index score using any cut off value.
2.5. Comparison
HFNC success versus HFNC failure.
2.6. Types of outcome measures
HFNC failure, was defined as use of either invasive or non-invasive mechanical ventilation.
2.7. Search methods for identification of studies
We searched electronic databases such as PubMed, Google Scholar, Web of Science, and the Cochrane Library for articles published between the inception of the database and 15 June 2021. There was no language barrier; however, the filter was only applied to COVID-19 patients. We also checked the references of related journals to make sure we didn't skip any studies.
2.8. Data extraction and quality assessment
Data were extracted independently by two authors (JP and AK) using predefined data abstraction forms. We used two tier approach to resolve conflicts between two authors performing data extraction; first through discussion between them; but if the issues remained unresolved we invited a third author (AKY) to do independent data extraction followed by disccuson to resolve the conflict. The following data were abstracted: study characteristics, demographic data, outcomes, and individual study risk of bias. HFNC failure was described as patients who needed non-invasive ventilation (NIV) or invasive mechanical ventilation (IMV) for the context of this research. The following data were collected for each eligible study: authors, publication year, country, study design, study group, proportion of HFNC failure, sensitivity, specificity, true positive, true negative and receiver operating characteristic (ROC) curve alongwith demographic and baseline characteristics such as sample size, a cut-off value of ROX, age, sex, body mass index (BMI), diabetes mellitus (DM), hypertension, lymphocyte count, CRP, D-dimer, length of HFNC, SOFA score, HFNC delivery device, humidifier, flow rate and FiO2.
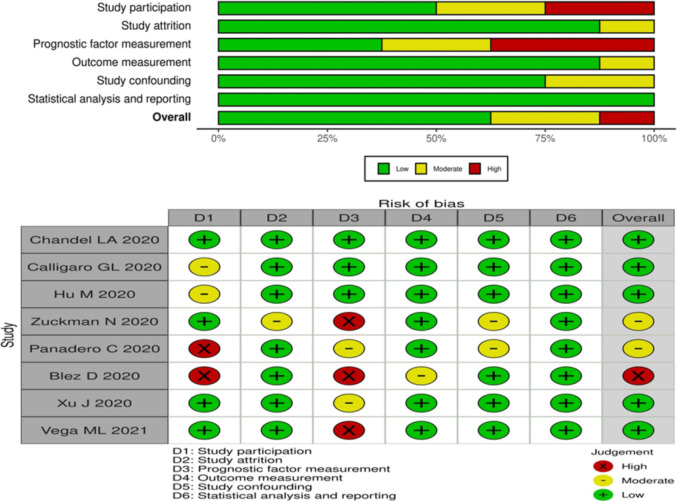
We used the Quality In Prognosis Studies (QUIPS) tool [17] to assess the risk of bias (RoB) independently and in duplicate in studies of prognostic factors. This tool summarizes the six bias domains, including prompting items and considerations for each one, as well as overall rating assessments. For each of the following domains, QUIPS tool classifies RoB as “low”, “moderate” or “high”: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting.
2.9. Statistical analysis
This meta-analysis, which was carried on purpose to predict HFNC failure, included all patients who have been allocated to the current study. Data were obtained through direct extraction or indirect calculation. In our meta-analyses, DerSimonian and Laird random-effects model was used. The inverse variance approach was used to construct study weights. The Cochran Q test for heterogeneity and the I2 statistic [18], were used to determine heterogeneity between studies. We also looked at the funnel plot visually to see if there was any publication bias.
We conducted meta-regression analyses to explore potential sources of heterogeneity among studies. We examined potential sources of heterogeneity keeping following variables as covariate/moderator variables; mean age (continuous variable), percent of hypertensive subjects (continuous variable), percent with diabetes (continuous variable), mean D-dimer level (continuous variable), percent of male gender (continuous variable), percent of cardiac disease (continuous variable), mean CRP (continuous variable) and time of ROX index (continuous variable), Cut-off value (continuous variable). Considering the clinical relevance, we further conducted a sub-group analysis for ROX index examined within 6 h/all studies and cut-off value of ROX index ≤5/ >5. We considered a normal distribution for continuous variables and converted interquartile ranges to standard deviations (SD) using Cochrane Collaboration guidelines [19]. Finally, the findings were depicted in forest plots. All the statistical analysis was conducted STATA version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
3. Results
3.1. Search results and study characteristics
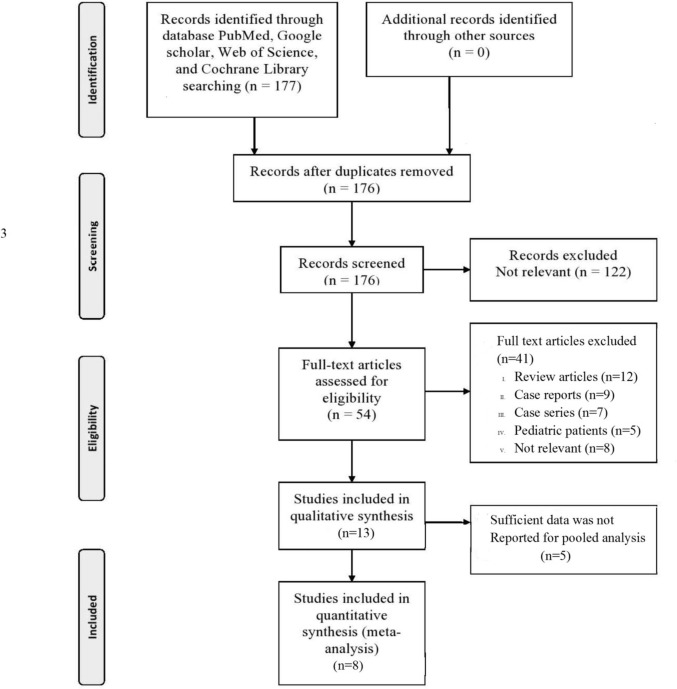
Initially, a total of 176 potentially eligible studies were identified. 54 full-text studies were extracted for screening after duplicate results were removed and titles and abstracts were screened. We contacted through e-mail the authors of relevant articles and we got data for our meta-analysis from two authors, however, five authors did not respond. Finally, eight retrospective or prospective cohort studies [4,7,[9], [10], [11], [12], [13], [14]] including 1301 patients were considered for pooled analysis [Fig. 1 ] to determine the predictive accuracy of the ROX index for HFNC failure. Table 1 shows the study characteristics of each study included in the present study. Table 2 show the demographic parameters and characteristics of the patients involved in the study. Five of the included studies had a low risk of bias [4,7,9,11,13], one trial had a high risk of bias [12], and two trials had a moderate risk of bias [10,14]. Five trials took place in the ICU setting [[9], [10], [11], [12], [13]] while three trials were conducted in the respiratory care unit [4,7,14]. The risk of bias in the individual study included in the present meta-analysis is shown in Fig. 2 .
Fig. 1.
PRISMA flow diagram.
Table 1.
Basic characteristics of the included studies.
| Study | Study type | Country | Settings | Patients | Delivery device | Humidifier | Flow rate (L/min) | FiO2 |
|---|---|---|---|---|---|---|---|---|
| Chandel [9] | Multi-centered observational cohort study | USA | ICU | COVID-19 | Fisher & Paykel Optiflow™ system | MR810 heated humidifier | N/a | N/a |
| Calligaro [11] | Multi-centered observational study | South Africa | ICU | COVID-19 | Hamilton C1 Ventilator, AIRVO™ (Fisher & Paykel) or Inspire O2 FLO | N/a | 50–60 L/min | 0.8–1.0 |
| Hu [4] | Retrospective observational study | China | Respiratory wards | COVID-19 | AIRVO2, Fisher & Paykel | N/a | 30 L/min | 1.0 |
| Panadero [14] | Retrospective observational study | Spain | Intermediate Respiratory Care Unit (IRCU) | COVID-19 | AIRVO2, Fisher & Paykel | N/a | 50–60 L/min | N/a |
| Xu [13] | Mulitcenter retrospective observational study | China | ICU | COVID-19 | Fisher & Paykel | N/a | 30 L/min | N/a |
| Vega ML [7] | Retrospective observational study | Italy | Respiratory wards | COVID-19 | N/a | N/a | 50–60 L/min | N/a |
| Blez [12] | Prospective observational study | France | ICU | COVID-19 | Optiflow® | MR810 heated humidified | 60 L/min | 1.0 |
| Zucman [10] | Retrospective observational study | France | ICU | COVID-19 | Fisher & Paykel | N/a | 50 L/min | 0.8 |
ICU: intensive care unit; COVID-19: coronavirus disease 2019; FiO2: fraction of inspired oxygen; N/a- not available.
Table 2.
Demographic parameters and characteristics of the patients included in studies.
| Study | HFNC status | Sample size | Cut–off value of ROX with time (h) after HFNC initiation | Age (Yr) | Sex (M/F) | BMI (kg/m2) | DM | Hypertension | Lymphocyte count (109/L) | CRP (mg/L) | D–dimer (μg/ml) | SOFA Score | Length of HFNC (days) | AHRF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chandel [9] | HFNC Success | 164 | 3.67 (at 12 h) | 54 ± 14 | 104/60 | 28.6 ± 5.7 [IQR 25.5–33.2] | 56 | 64 | N/a | 16.7 ± 10.2 [IQR 9.8–23.6] | 1.3 ± 1.6 [IQR 0.8–2.2] | 2 ± 2.2 [IQR 1–4] | 4 ± 3.7 [IQR 2–7] | SpO2 <88% RR > 35 breath/min |
| HFNC Failure | 108 | 60 ± 13 | 76/32 | 28.7 ± 6.4 [IQR 24.9–33.6] | 45 | 52 | 17.2 ± 11.5 [IQR 10.8–26.3] | 1.3 ± 1.3 [IQR 0.9–2.7] | 4 ± 3.7 [IQR 2–7] | 2 ± 2.2 [IQR 1–4] | ||||
| Calligaro [11] | HFNC Success | 134 | 2.7 (at 6 h) | 50 ± 9.6 [IQR 44–57] | 79/58 | N/a | 76 | 59 | 1.23 ± 0.6 [IQR 0.83–1.62] | 173 ± 125.2 [IQR 105–274] | 0.56 ± 1.5 [IQR 0.36–1.78] | N/a | N/a | SpO2 <92% RR > 30 breath/min O2 supply– 15L/min |
| HFNC Failure | 145 | 53 ± 10.4 [IQR 44–58] | 84/72 | 82 | 72 | 1.15 ± 0.5 [IQR 0.92–1.57] | 235 ± 149.6 [IQR 142–344] | 1.03 ± 4.1 [IQR 0.49–4.44] | ||||||
| Hu [4] | HFNC Success | 65 | 5.55 (at 6 h) | 59.5 ± 10.9 | 26/39 | N/a | N/a | N/a | 0.62 ± 0.2 [IQR 0.49–0.79] | 45.6 ± 39.3 [IQR 30.4–83.5] | 0.62 ± 1.5 [IQR 0.42–1.78] | 3 ± 0 [IQR 3–3] | 6 ± 3.7 [IQR 3.5–8.5] | SpO2≤92% RR ≥25 breath/min |
| HFNC Failure | 40 | 71.3 ± 7.6 | 25/15 | 0.7 ± 0.30 [IQR 0.36–0.80] | 39.3 ± 45.9 [IQR 23.4–85.4] | 1.04 ± 4.7 [IQR 0.46–5] | 4 ± 1.5 [IQR 3–5] | 3 ± 6.7 [IQR 2–11] | ||||||
| Panadero [14] | HFNC Success | 19 | 4.94 (2 to 6 h) | 56.6 ± 12.8 | 14/5 | 28.1 ± 3.2 | 3 | 9 | N/a | 1283 ± 1006 | 6.2 ± 14.4 | 4.5 ± 0.8 | 6 ± 2.22 [IQR 5–8] | N/a |
| HFNC Failure | 21 | 60.9 ± 10.8 | 14/7 | 30.5 ± 5.1 | 5 | 7 | 1118 ± 1006 | 5.1 ± 6 | 4.2 ± 0.6 | 2 ± 2.2 [IQR 1–4] | ||||
| Xu [13] | HFNC Success | 173 | 5.31 (within 4 h) | 60.6 ± 15.5 | 119/58 | N/a | 34 | 78 | 0.6 ± 0.37 [IQR 0.4–0.9] | N/a | 2.6 ± 5.9 [IQR 0.8–8.8] | 2.0 ± 1.1 [IQR 2–3.5] | 10 ± 5.9 [IQR 7–15] | SpO2 <90% RR> 30 breath/min O2 supply– 10L/min |
| HFNC Failure | 220 | 66.3 ± 12.5 | 100/47 | 26 | 69 | 0.6 ± 0.3 [IQR 0.4–0.8] | 4.8 ± 16.9 [IQR 1.1–17.7] | 4 ± 1.5 [IQR 3–5] | 3 ± 2.2 [IQR 1–4] | |||||
| Vega [7] | HFNC Success | 85 | 5.99 (at 12 h) | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a |
| HFNC Failure | 35 | |||||||||||||
| Blez [12] | HFNC Success | 14 | 4.88 (at 0.5 h) | 64 ± 11.1 [IQR 57.5–72.5] | 11/3 | 25.6 ± 2.6 [IQR 25–28.5] | 2 | 6 | N/a | N/a | N/a | N/a | N/a | RR≥ 30 breath/min O2 supply– 10L/min |
| HFNC Failure | 16 | 64 ± 5.4 [IQR 59–66.3] | 10/6 | 30.5 ± 3.5 [IQR 28.4–33.1] | 5 | 10 | ||||||||
| Zucman [10] | HFNC Success | 21 | 5.37 (within 4 h) | 55 ± 11.11 [IQR 48–63] | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a |
| HFNC Failure | 41 |
HFNC, high–flow nasal cannula; BMI: body mass index; DM: diabetes mellitus; CRP: C–reactive protein; SOFA: sequential organ failure assessment; AHRF– acute hypoxemic respiratory failure, RR– respiratory rate, IQR– interquartile range, N/a– not available, Yr– year.
Fig. 2.
Risk of bias summary.
3.2. Outcomes
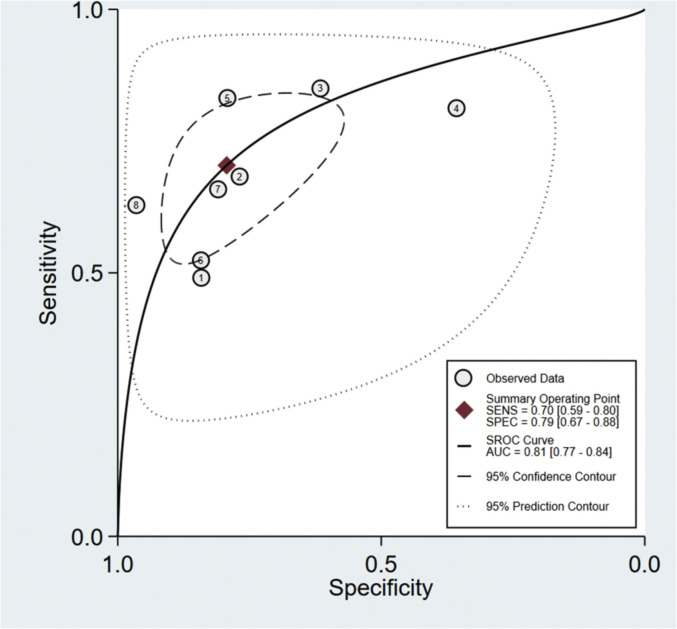
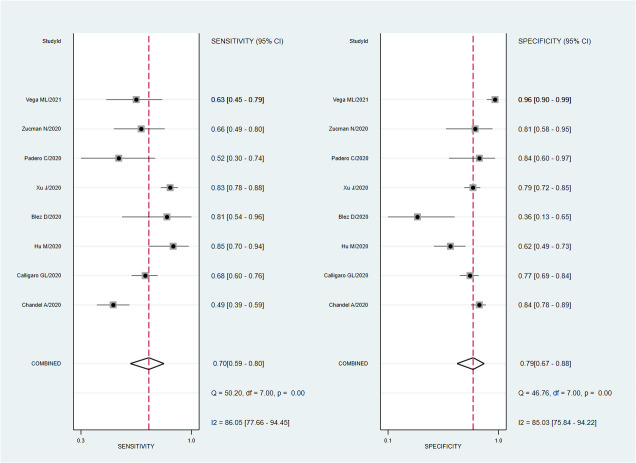
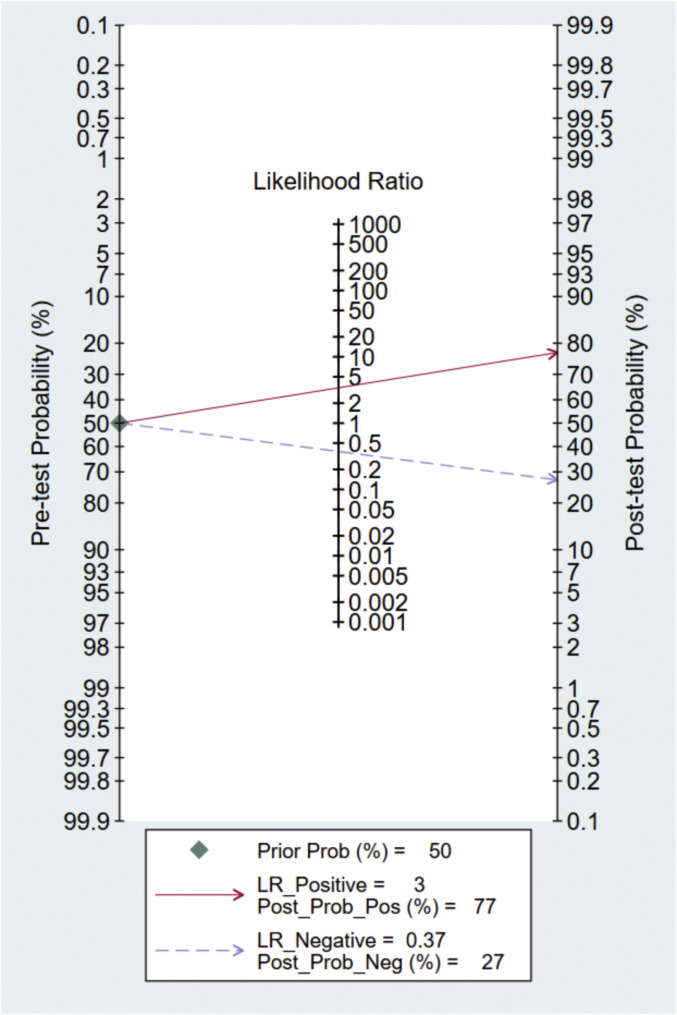
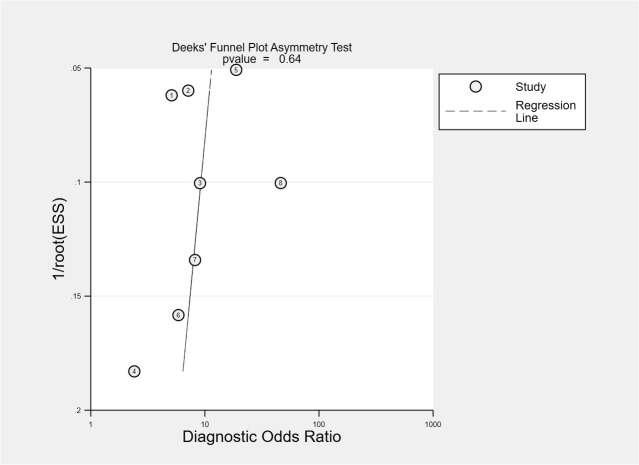
A total of eight studies involving 1301 subjects met the inclusion criteria of the present meta-analysis. We observed that the ROX index score show good discrimination with summary area under the curve (sAUC) of 0.81 (95% CI, 0.77–0.84) [Fig. 3 ]. The pooled sensitivity and specificity were 0.70 (95% CI, 0.59–0.80) and 0.79 (95% CI, 0.67–0.88), respectively for predicting HNFC failure in COVID-19 patients [Fig. 4 ]. Inconsistency measured by I2 statistics were significant (86% for sensitivity and 85% for specificity) [Fig. 4]. The positive and negative likelihood ratio were 3.0 (95% CI, 2.2–5.3) and 0.37 (95% CI, 0.28–0.50) respectively, and had a substantially good diagnostic odds ratio (OR 9, 95% CI, 5–16) for predicting HNFC failure outcome in COVID-19 patients [Fig. 5 ]. We did not observe the significant publication bias of the funnel plot (P = 0.64) suggesting the reliability of the study findings [Fig. 6 ]. Considering the pre-test probability of 50%, a ROX index may be linked with a positive likelihood ratio of 3.0 and a post-test HNFC failure probability of 77%. The negative likelihood ratio was 0.37 associated with a post-test negative predictive value of 27%. We explored the source of heterogeneity using the clinically important variables (hypertension, diabetes, cardiac disease, mean age, gender, D-dimer, CRP, time of ROX index) on the effect size, however, we did not observe anyone the variables significantly explain the source of variation on pooled sensitivity and pooled specificity [Fig. 7 ].
Fig. 3.
Summary receiver operating characteristic graph for the included studies. The AUC of ROX-index for probability in predicting HNFC failure was 0.81 (95% CI, 0.77–0.84).
Fig. 4.
Forest plot of the sensitivity and specificity of ROX-index for predicting HNFC failure in patients with COVID-19. The pooled sensitivity and specificity were 0.70 (95% CI, 0.59–0.80) and 0.79 (95% CI, 0.67–0.88), respectively.
Fig. 5.
Fagan nomogram showing pre-test probability and post-test probability using ROX index for predicting HNFC failure.
Fig. 6.
Deek funnel plot showing publication bias for studies included in the meta-analysis.
Fig. 7.
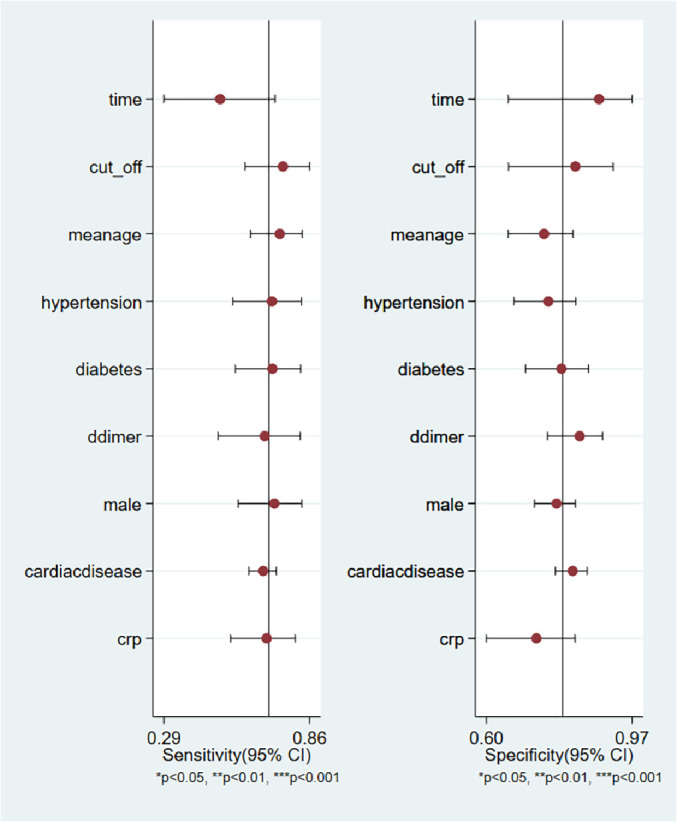
Forest plot showing pooled sensitivity and pooled specificity of clinical variables for predicting HNFC Failure.
Subgroup analysis: We conducted a subgroup analysis based on the timing of ROX- index assessment and cut-off value reported in the included studies. Our subgroup analysis did not observe the significant difference in the predictive accuracy of ROX-index including only those studies which examined ROX-index ≤6 h compared to overall studies. The sAUC was 0.81 (95% CI, 0.77 to 0.84) and 0.81 (95% CI 0.78 to 0.84) respectively. Similarly, eight studies reporting the predictive accuracy of ROX-index divided into cut-off value ≤5 (four studies) and > 5 (four studies). Our subgroup analysis demonstrated higher discriminatory accuracy including studies used cut-off value >5 [sAUC, 0.87 (95% CI, 0.83 to 0.89)] compared to ≤5 cut-off value [sAUC, 0.76 (95% CI 0.72 to 0.80)], respectively with P value = 0.002 [Table 3 ].
Table 3.
Results of subgroup analysis using ROX index for predicting HNFC failure.
| Categories | Sensitivity (95% CI) | Specificity (95% CI) | sAUC (95% CI) | DOR (95% CI) | I2 |
|---|---|---|---|---|---|
| Time from onset to ROX- index assessment | |||||
| All studies | 0.70 (0.59 – 0.80) | 0.79 (0.67 – 0.88) | 0.81 (0.77 – 0.84) | 9 (5 – 16) | Sensitivity: 86.05% Specificity: 85.03% |
| Within 6 h | 76 (0.65 – 0.84) | 0.74 (0.62 – 0.83) | 0.81 (0.78 – 0.84) | 9 (5 –15) | Sensitivity: 77.15% Specificity: 75.79% |
| Cut-off value | |||||
| Cut-off ≤5 | 0.65 (0.48 – 0.79) | 0.75 (0.59 – 0.87) | 0.76 (0.72 – 0.80) | 6 (4 – 9) | Sensitivity: 77.01% Specificity: 84.38% |
| Cut-off >5 | 0.77 (0.65 – 0.86) | 0.85 (0.67 – 0.94) | 0.87 (0.83 – 0.89) | 19 (11 – 35) | Sensitivity: 76.53 Specificity: 89.15% |
sAUC – summary area under the curve, DOR- diagnostic odds ratio, CI- confidence interval.
4. Discussion
This systematic review and meta-analysis which included an extensive literature search, pre-registered protocol, a focus on only COVID-19 patients with AHRF, the use of the QUIPS tool to determine study bias, and the inclusion of recent trials suggests that ROX index is a good predictor of HFNC failure in COVID-19 patients with AHRF. Up to the best of our knowledge, this would be the first meta-analysis on ROX index for the prediction of HFNC outcomes in COVID-19 patients.
HFNC failure has been linked to a poor clinical outcome, predicting the failure of HFNC has remained a focus of research. In the clinical practice of treating AHRF in patients with COVID-19, studies have observed that the ROX index has a good predictive value in HFNC failure. Studies have reported various thresholds to ROX for predicting HFNC outcomes. Clinicians are therefore unclear regarding the optimal thresholds of ROX that should be applied to know the HFNC outcomes.
Previous data from AHRF patients treated with HFNC revealed that the set flow rate has a significant impact on oxygenation and RR; it was then investigated whether increasing the set flow rate would affect the ROX index.
In the current meta-analysis, we observed that the ROX index could be used for risk stratification in determining whether or not a patient requires mechanical ventilation at an early hour of admission. It was demonstrated that the ROX index is a convenient tool that can distinguish patients with COVID-19 infection who need hospitalization (ROX index less than 25.7) from those who can be safely discharged at the time of admission. Also, in COVID-19 patients with AHRF, the ROX index has high sensitivity, confirming that a lower ROX index predicts higher mortality risk [20]. Our meta-analysis showed that ROX index might discriminate with a value of sAUC of 0.81 (95% CI, 0.77–0.84) with sensitivity of 0.70 (95% CI, 0.59–0.80) and specificity of 0.79 (95% CI, 0.67–0.88) for predicting HFNC failure in COVID-19 patients. The heterogeneity (I2 or inconsistency) was significant (86% for sensitivity and 85%, for specificity). We explored the source of factors that may influence variation in the studies using meta-regression analysis, although the potential clinical conditions e.g. proportion of hypertension, the proportion of diabetes, mean age, D-dimer level, the proportion of male subjects, presence of cardiac disease, CRP level, and lymphocyte count did not influence the pooled prognostic value of ROX-index for prediction of worse outcome except lymphocyte count for specificity which was significant. The absence of publication bias further confirms the validity of the findings observed in the current meta-analysis.
The cut-off value used in the included studies varied from 2.7 to 5.9 in obtaining the homogenous and clinically acceptable cut-off value. We excluded the extreme outlier cut-off value of 25.26 in the paper published by Suliman et al. [21]. Based on the studies included in the meta-analysis, the optimal cut-off value may fall close to 5 of ROX index within the 24 h of admission for predicting HNFC failure.
Timing of the measurement of ROX index among the included studies ranged from 2 h to 12 h. Only two studies reported data for prognostic accuracy of ROX index at 12 h. Our meta-regression analysis did not observe significant moderator effect of differences in the timing of ROX index examination on discriminatory power of ROX index.Still we conducted a subgroup analysis also, and observed that discriminatory ability of ROX index based on studies that examined ROX-index within 6 h which was comparable to finding when all studies were included in the analysis. Early prediction of outcome is needed to provide optimal care to patients and stratification at the earliest hours to predict HFNC failure. A study published by Lemiale et al. [22] also observed that maximum diagnostic accuracy and static measurement of the ROX index was at 6 h.
The finding of the present study indicate that the ROX index could help in identifying subjects at more risk for worse outcomes therefore, early invasive mechanical ventilation may be used to prevent worse outcomes in patients with COVID-19-associated AHRF.
4.1. Limitations
The limitations of our study were that none of the studies included in the meta-analysis have shown the calibration and validation of the model which limits the validity of the prediction accuracy of the ROX index. We also observe high heterogeneity among the studies as indicated by I2, indicating the need to conduct well-designed prospective studies. The cut-off value for the ROX index was not uniform across the studies included in the meta-analysis which may be due to different clinical conditions of patients and settings. However to obtain the uniform results we have excluded the studies used extreme cut-off value. We were also not able to obtain data from five studies which could have decreased the power of the study. Meta-regression analysis does not have adequate power due to limited number of studies to examine the sources of heterogeneity.
5. Conclusion
Our meta-analysis demonstrated that the ROX index has good discriminating power for the prediction of HFNC failure in COVID-19 patients with AHRF. Further large-scale, multicenter studies with uniform cut-offs and at specific time intervals are needed to strengthen the current findings.
Conflicts of interest
NIL
Funding
NIL
Author's statements
Jay Prakash developed the initial idea of this study and conducted a comprehensive search of four databases. Jay Prakash and Pradip Kumar Bhattacharya took responsibility for selecting the study. Jay Prakash, Amit Kumar and Arun Kumar Yadav extracted data. All authors have made their contributions to research design, interpretation of results, and ideas for writing articles. Jay Prakash and Amit Kumar synthesized and analyzed the data and drafted the article. Kameshwar Prasad, Arun Kumar Yadav and Lal Chand Tudu reviewed this article and provided suggestion for it. All of the authors have carefully examined this manuscript and agreed with the ideas presented in the article.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int
- 2.Shoukri A.M. High flow nasal cannula oxygen and non-invasive mechanical ventilation in management of COVID-19 patients with acute respiratory failure: a retrospective observational study. Egypt J Bronchol. 2021;15:17. doi: 10.1186/s43168-021-00063-0. [DOI] [Google Scholar]
- 3.Frat J.P., Coudroy R., Marjanovic N., Thille A.W. High-flow nasal oxygen therapy and noninvasive ventilation in the management of acute hypoxemic respiratory failure. Ann Transl Med. 2017;5:297. doi: 10.21037/atm.2017.06.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu M., Zhou Q., Zheng R., Li X., Ling J., Chen Y., et al. Application of high-flow nasal cannula in hypoxemic patients with COVID-19: a retrospective cohort study. BMC Pulm Med. 2020;20:324. doi: 10.1186/s12890-020-01354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beduneau G., Boyer D., Guitard P.-G., Gouin P., Carpentier D., Grangé S., et al. Covid-19 severe hypoxemic pneumonia: a clinical experience using high-flow nasal oxygen therapy as first-line management. Respir Med Res. 2021;80:100834. doi: 10.1016/j.resmer.2021.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q., Shen J., Chen L., Li S., Zhang W., Jiang C., et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. 2020;89:1092–1098. doi: 10.1097/TA.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega M.L., Dongilli R., Olaizola G., et al. COVID-19 Pneumonia and ROX index: Time to set a new threshold for patients admitted outside the ICU. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2021.04.003. S2531–0437(21)00092–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roca O., Messika J., Caralt B., et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Chandel A., Patolia S., Brown A.W., et al. High-flow nasal cannula therapy in COVID-19: using the ROX index to predict success. Respir Care. 2021;66:909–919. doi: 10.4187/respcare.08631. [DOI] [PubMed] [Google Scholar]
- 10.Zucman N., Mullaert J., Roux D., Roca O., Ricard J.D., Contributors Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46:1924–1926. doi: 10.1007/s00134-020-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calligaro G.L., Lalla U., Audley G., et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-Centre prospective observational study. EClinicalMedicine. 2020;28:100570. doi: 10.1016/j.eclinm.2020.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blez D., Soulier A., Bonnet F., Gayat E., Garnier M. Monitoring of high-flow nasal cannula for SARS-CoV-2 severe pneumonia: less is more, better look at respiratory rate. Intensive Care Med. 2020;46:2094–2095. doi: 10.1007/s00134-020-06199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J., Yang X., Huang C., et al. A novel risk-stratification models of the high-flow nasal cannula therapy in COVID-19 patients with hypoxemic respiratory failure. Front Med (Lausanne) 2020;7:607821. doi: 10.3389/fmed.2020.607821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panadero C., Abad-Fernández A., Rio-Ramirez M.T., et al. High-flow nasal cannula for acute respiratory distress syndrome (ARDS) due to COVID-19. Multidiscip Respir Med. 2020;15:693. doi: 10.4081/mrm.2020.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Green S. The Cochrane Collaboration; Oxford: 2011. Cochrane handbook for systematic reviews of interventions. Version 5.1.0.http://handbook-5-1.cochrane.org [Google Scholar]
- 17.Hayden J.A., van der Windt D.A., Cartwright J.L., Côté P., Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges (htm)
- 20.Gianstefani A., Farina G., Salvatore V., et al. Role of ROX index in the first assessment of COVID-19 patients in the emergency department. Intern Emerg Med. 2021:1–7. doi: 10.1007/s11739-021-02675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suliman L.A., Abdelgawad T.T., Farrag N.S., Abdelwahab H.W. Validity of ROX index in prediction of risk of intubation in patients with COVID-19 pneumonia. Adv Respir Med. 2021;89:1–7. doi: 10.5603/ARM.a2020.0176. [DOI] [PubMed] [Google Scholar]
- 22.Lemiale V., Dumas G., Demoule A., et al. Performance of the ROX index to predict intubation in immunocompromised patients receiving high-flow nasal cannula for acute respiratory failure. Ann Intensive Care. 2021;11:17. doi: 10.1186/s13613-021-00801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]