The severe acute respiratory syndrome coronavirus-2 and related coronavirus disease 2019 (COVID-19) pandemic have deleteriously affected cancer research worldwide, including basic, translational, and clinical fields, because of prolonged shut-down of research laboratories consequent to lockdown restrictions, curtailment of researchers’ laboratory access, decreased availability of patient samples, and clinical trial disruptions.1, 2, 3, 4, 5 In addition, cuts in funding are significantly contributing to the impact and are threatening the viability of cancer research as a whole.6, 7, 8, 9 The consequences of this disruption may cause additional morbidity and mortality in the years to come beyond those directly related to COVID-19, the so-called nonviral casualties.
Currently, no objective data are available on the pandemic’s impact on preclinical projects. Regarding clinical trials, recent reports showed generalized detrimental experience added to a 60% decrease in new trials for cancer therapeutics since the pandemic started.10 , 11 In parallel, loss in research funding has been significant. Prominent philanthropic cancer-focused organizations such as the American Cancer Society, the Canadian Institutes of Health Research, and Cancer Research UK have lost an estimated $200 million each during 2020 as a consequence of a lack of fundraising activities and donations.6, 7, 8, 9 Data from patient organizations indicate widespread economic crises that will further decrease research funding.12
This situation is particularly concerning for pancreatic cancer (PC), the most aggressive and lethal solid tumor for which accelerating research is essential to improve early diagnosis, treatment, and overall outcomes.13, 14, 15, 16 The abrupt slowing of PC cancer research during the COVID-19 pandemic and the uncertainty about its future sustainability are challenging not only for scientific advances made to date, but also for future progress. Given this alarming picture, PC was recently referred to as being in a state of medical emergency.17 The design of collaborative and multidimensional strategies for resuming research for this neglected tumor is therefore an urgent public health priority, especially because PC incidence and mortality are constantly increasing, projected to soon become the second cause of cancer-related death worldwide).15 , 16
In this context the PanCaCOVID-19 Study Group was formed to analyze the pandemic's impact on PC research and to understand the magnitude of the damage to provide useful information to assist policymakers for safeguarding research networks. To this aim, a cross-sectional study was launched in November 2020 on the occasion of PC awareness month. It consisted of a quantitative and qualitative analysis of COVID-19's impact on PC research across the globe, including basic/translational research, clinical trials, and research advocates. Three separate online, open, mixed-methods surveys were used to examine each specific field. The target population consisted of 3 different groups: principal investigators (PIs) focused on basic/translational projects, PIs or subinvestigators of clinical trials for PC, and coordinators of PC patient organizations. The recommended standards for conducting and reporting web-based surveys (Checklist for Reporting Results of Internet E-surveys) were followed.18
The study covered 418 individuals from 37 countries across 5 continents, including 164 laboratory PIs (surveyed from November 1 to November 30, 2020) and 102 clinical PIs (surveyed from November 23 to December 14, 2020). Patient organizations were surveyed twice by the World Pancreatic Cancer Coalition (44 in May and 23 in December 2020).
Preclinical Research
Basic research was impaired substantially due to abrupt and prolonged interruption secondary to the COVID-19 outbreak, as reported by two-thirds of preclinical PIs. Although the pandemic has stimulated alternative approaches to conduct some aspects of cancer research activities and scientific meetings, in vitro and in vivo experiments cannot be performed without a laboratory. The total and/or intermittent closure of laboratories and the forced turnover of staff to reduce interpersonal contact, combined with reduction in clinical trial enrollment and overall access to hospital facilities, stopped projects based on human samples such as patient-derived xenograft and patient-derived organoids establishment. In addition, delays in materials and reagent supply, due to pandemic-related increased demand and prioritization, have negatively impacted the conduct of all types of preclinical non–COVID-19 research projects for most survey responders. Although some of these negative effects will likely be rolled back once the acute phase of the health emergency recedes, other aspects are poised to endure and are likely to cause long-lasting negative impact. Two-thirds of responders estimated it would take up to 1 year to return to prepandemic levels once peaks of the pandemic subside, whereas the persistence of COVID-19 outbreaks would have detrimental effects on the research activity that would take years to reverse.
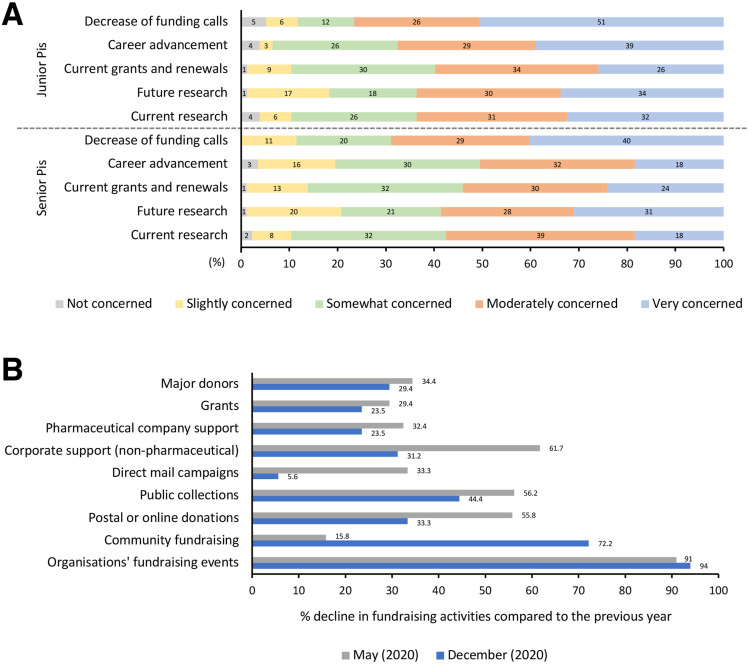
Travel and mobility restrictions have significantly impacted the ability to recruit talented international scientists and trainees. This, along with denied access to education and laboratory training courses for graduates and PhD students, increased teaching load and remote teaching preparation, stress and anxiety, and increased time spent caring for children and/or family members, have moderately to severely affected research projects for two-thirds of preclinical PIs. The overall serious concerns about the future of early career staff expressed by almost the totality of PIs (both senior and junior) is alarming (Figure 1 A). Difficulties in maintaining research productivity, job mobility, networking, and funding will have a detrimental long-term impact on early career scientists and will ultimately undermine the scientific community as a whole.19 , 20 To emphasize the seriousness of the situation, the prospect of a lost generation of cancer researchers, with early career researchers moving to other fields, has been recently suggested.9
Figure 1.
Impact of the COVID-19 pandemic on PC preclinical research and patient organizations. (A) Concerns of preclinical PIs (principal investigators) expressed in percent (%) of responses. (B) Decline in fundraising activities of patient organizations compared to the previous year expressed in %.
Another recurrent concern was the economic sustainability of research, not only regarding the present (two-thirds of participants lost funding, with an average loss of 107.196 US dollars) but also, and particularly, the future (Figure 1 A). On one hand, the prolonged suspension of research activities did not allow the acquisition of preliminary data necessary to write new grant proposals. On the other hand, cuts in cancer research funding are going to decrease the availability of novel grant opportunities. Interestingly, two-thirds of PIs relied on charitable-based funds.
Patient Organizations
Patient organizations are vital to sustain PC research. According to data from the National Cancer Institute, in 1999 total public funding for PC in the United States was only 17.3 million dollars. It increased to 177.9 million dollars by 2017, mainly through the advocacy efforts of the Pancreatic Cancer Action Network. In addition to helping increase federal research funding for PC, the Pancreatic Cancer Action Network funds private research. Many other groups play a key role in funding PC research across the globe. Among those who participated in the World Pancreatic Cancer Coalition's survey, 27 organizations funded research projects.
Our data indicate that PC patient organizations have been brought to their knees by the COVID-19 pandemic. Almost all those included in the current study experienced a reduction in income by half compared with 2019. All forms of fundraising were affected to varying degrees. Fundraising events were the most severely and constantly affected (Figure 1 B). Two-thirds of participants have tried new ways of fundraising, mainly through virtual activities. Half of funding research programs were either reduced or paused. Concerns that reduced research will negatively impact the development of new diagnostic and therapeutic strategies, and significant anxiety about the disruption of clinical trials' activity in a challenging cancer type like PC were common.
Only one-third of research advocates expected to guarantee usual levels of research funding after the end of the pandemic. This indicates that a substantial proportion of PC research is at serious risk.
Clinical Trials
Clinical research is vital to test and validate new therapeutic (or preventative) strategies. Given the current limited efficacy of available therapeutics for improving overall outcomes and the absence of standard early-detection programs,14 every patient with PC should be offered the opportunity to participate in clinical trials.
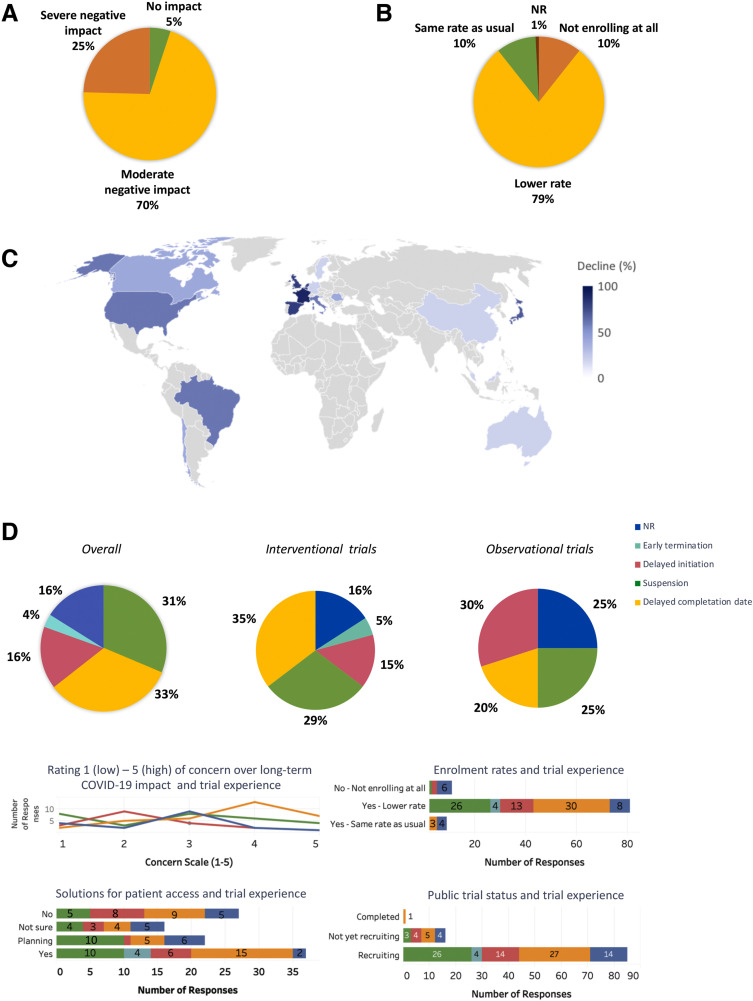
The picture of the COVID-19's impact on 102 clinical trials (from phase I to phase IV) for patients with PC in sites across 40 countries is alarming. In addition to the reorientation of clinical studies toward COVID-19, the disruption of clinical research was due to the inability of patients to return to healthcare centers for study visits, limited availability of ancillary services (including biopsies, scans, blood tests), and difficulties of holding timely meetings between investigators, investors, review boards, and contract research organizations. A moderate to severe negative effect on trials was experienced by nearly all PIs (Figure 2 A). Most investigators were still enrolling patients, albeit at a lower rate (Figure 2 B). The average recruitment rate dropped by almost half since the pandemic began, compared with 2019, with worse trends in central Europe and the United States (Figure 2C). Detailed trial experience is shown in Figure 2 D. Observational studies had a higher proportion of delayed initiation (30%) than interventional studies (15%) (Figure 2 D). Investigator responses regarding trial experiences correlated with their concerns about the long-term impact of COVID-19 (Figure 2 D). When the status of public trials was queried from surveyed investigators, none of the current trial statuses reflected that reported by investigators. This discrepancy indicates a possibly greater damage to clinical trials than reflected in our survey. Figure 2 D also shows recruitment rate trends by trial experience and solutions to allow patient access to ongoing studies, adopted by 37% of the respondents (ie, implementations of virtual platforms, protocol amendments, and shipping oral study drugs directly to patients).
Figure 2.
Impact of the COVID-19 pandemic on clinical trials. (A) Overall impact of the COVID-19 pandemic on clinical trials. (B) Trends in enrollment rates. (C) Global overview of the average decline in enrollment rates by country compared to 2019. (D) Details on trial experience: overall, by study type and sub-analyses.
Learning From Disruption to Recovery
The alarming situation on several inter-related fronts observed in the current study suggests that future sustainability of PC research is at serious risk. In the short term we are likely to expect a detrimental impact on patient outcomes because of the reduced recruitment rates in clinical trials or to the decreased availability of active studies, which compounds the breakdown of cancer care for PC patients.21 This will undoubtedly worsen the already critical situation characterized by poor availability of clinical trials—and pharmaceutical industry investment—for PC compared with other cancers.17 Nevertheless, even more concerning is the long-term impact that might follow the disruption of discovery science aimed at investigating mechanisms of disease susceptibility, initiation, progression, and new drugs and biomarkers for therapy. Impoverishment of governments, universities, and patient organizations in addition to diversion of the public’s attention and funds to the more urgent issues centered on the pandemic will significantly contribute to long-lasting detriment.
It is worth noting that the current study was conducted in November and December 2020, at the beginning of the second wave of the COVID-19 pandemic in many countries. Given the subsequent peaks and associated restrictions, the picture we have presented may be an underestimation of the real damage to PC research.
However, disruptive changes, albeit often detrimental, particularly in the short term, can be positively constructive in the longer term, triggering innovation and progress. What did we learn from the COVID-19 experience and how can we take advantage of it to enhance PC research?
If on one hand the COVID-19 outbreak has dismantled cancer research, on the other it has highlighted the essential social value of the scientific community and its ability to adapt to unfavorable circumstances, innovate, and deliver results with exceptional speed. In addition, the urgent global need for novel treatments and vaccines for the COVID-19 infection has simplified bureaucracy and accelerated traditional regulatory timelines in an unprecedented manner. This, along with enhanced international cooperation, expedited bench to bedside translation, improved public education, and enforced interaction between industry, academia, governments, funders, regulatory agencies, and public health institutions, inspires novel opportunities that can be taken forward to reform and potentially accelerate the way cancer research is conducted.
Some positive strategies adopted in clinical cancer research during the pandemic, such as telehealth consultations, remote consent, and increased flexibility on trial protocols, may help improve trial accessibility in the future.2 In this way the COVID-19 outbreak provided a unique chance to reform, simplify, democratize, and accelerate clinical investigation.22
Nevertheless, a key point remains the uncertainty about economic sustainability. Governments should consider this with urgent priority and direct adequate investments from recovery plans for cancer research and cancer patient organizations.23
Finally, sharing ideas, expertise, and resources; creating networks; and strengthening collaborations between the scientific community and patient organizations will be crucial to mitigate the adverse impact of the pandemic to fund a solid proactive ground for the postpandemic era. The cooperation set in place for the current study is a good example of such a strategy.
In conclusion, research for PC is vital and requires attention from policymakers. Borrowing the slogan of world PC day 2020, “It's about time," we are calling for urgent transdisciplinary measures to protect science, researchers, clinical trials, and advocates during the COVID-19 outbreak and to accelerate the recovery process in the postpandemic era to avoid irreparable regression with consequent harm to PC patients and society.
The following interventions should be considered for restructuring to enhance cancer research in the future:
-
•
Simplification of grant application processes: reduce supplemental documentation, reduce bureaucracy, and accelerate the revision process and the communication of decisions.
-
•
Enhanced support and flexibility from agencies, institutions, and scientific societies: postponing deadlines for current and future grants, extension of funding, flexibility of timelines, no-cost extensions for current grants, and reduce hiring freezes.
-
•
Ensure fiscal support to researchers: economic sustainability in case of grant suspension/funding cut because of the pandemic and implement furlough funds.
-
•
Policies targeted at early investigators and women24: implement funding for junior PIs, extend early investigator status for grant applications, extend tenure for academics, guarantee promotion and career advancement despite decreased productivity, introduction of gender balance in grant applications and awards, and implement childcare policies (onsite facilities, full-day childcare programs).
-
•
Promote clinical research following the American Society of Clinical Oncology recovery recommendations25: ensure that clinical research is accessible, affordable, and equitable; design more pragmatic and efficient clinical trials; minimize administrative and regulatory burdens on research sites; recruit, retain, and support a well-trained clinical research workforce; and appropriate oversight and review of clinical trial conduct and results.
-
•
Implement enhanced networking: sharing ideas, expertise, resources, and strengthening the collaboration between scientists, oncologists, industry, cancer societies, and patient organizations.
-
•
Attract investment: exploit social media to stimulate interest in cancer research and to attract private donations, encourage pharmaceutical companies to invest in cancer research, and promote academic–industrial partnerships in the direction of openness and cooperation.
-
•
Increase governmental funds for cancer research: urge governments to take bold new actions to increase funds for cancer research such as recovery funds and innovative plans for allocation of the income taxes and promote supportive ecosystem that includes government agencies, academia, and research institutions
-
•
Develop recovery plans for patient organizations.
Acknowledgments
We thank the Pancreatic Cancer Europe, Precision-PANC, Pancreatic Cancer UK, the United European Gastroenterology, the European-African Hepato-Pancreato-Biliary Association, the National Institute for Health and Medical Research, The São Paulo Research Foundation, and the European Association of Cancer Research for their precious contribution in disseminating the surveys. We also thank the AIRC Foundation for Cancer Research for their support.
Footnotes
The PanCaCovid-19 Study Group includes Vincenzo Corbo,5,6 Samik Upadhaya,7 Yu Jia Xin,7 Lorena Torroni,8 Chiara Braconi,1,9 Paola Cappello,10,11 Carmine Carbone,12,13 David K. Chang,1,3 Elisa Giovannetti,14 Sara Lovisa,15,16 Miriam Martini,11 Antonio Pea,17 Geny Piro,12,13 Pancreatic Cancer Action Network, Fondazione Nadia Valsecchi Onlus, Italian Pancreatic Cancer Community, Preclinical Dutch Pancreatic Cancer Group, World Pancreatic Cancer Coalition, Michele Milella,18 Aldo Scarpa,5,6 Claudio Bassi,17 Jay Campbell7; from the 1Wolfson Wohl Cancer Research Centre, Institute of Cancer Sciences, University of Glasgow, UK; 3West of Scotland Pancreatic Unit, Glasgow Royal Infirmary, Glasgow, UK; 5Department of Diagnostics and Public Health, Section of Pathology, University and Hospital Trust of Verona, Italy; 6ARC-Net Research Centre, University of Verona, Italy; 7Anna-Maria Kellen Clinical Accelerator, Cancer Research Institute, New York, New York; 8Department of Diagnostics and Public Health, Unit of Epidemiology and Medical Statistics, University and Hospital Trust of Verona, Italy; 9Beatson West of Scotland Cancer Centre, Glasgow, UK; 10Center for Experimental Research and Medical Studies, AOU Città della Salute e della Scienza di Torino, University of Torino, Italy; 11Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Center, University of Torino, Italy; 12Comprehensive Cancer Center-Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; 13Medical Oncology, Department of Medical and Surgical Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; 14Fondazione Pisana per la Scienza, Pisa, Italy; 15Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; 16Humanitas Clinical and Research Center, IRCCS, Rozzano, Italy; 17General and Pancreatic Surgery Unit, Pancreas Institute, University and Hospital Trust of Verona, Italy; 18Section of Oncology, Department of Medicine, University and Hospital Trust of Verona, Italy.
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by the Associazione Italiana Ricerca Cancro (AIRC 5x1000 n. 18718), Associazione Italiana Ricerca Cancro (AIRC 5x1000 n. 12182), Worldwide Cancer Research grant (WWCR, 20-0033), Fondazione Italiana Malattie Pancreas, Italian Ministry of Health (CUP_J38D19000690001), Fondazione Cariverona: Oncology Biobank Project “Antonio Schiavi” (prot. 203885/2017), Cancer Research UK (C29717/A17263, C29717/A18484, C596/A18076, C596/A20921, C29717/A23526), Wellcome Trust Senior Investigator Award (103721/Z/14/Z), Pancreatic Cancer UK Future Research Leaders Fund (FLF2015_04_Glasgow), Scottish Genomes Partnership (SEHHD-CSO 1175759/2158447), MRC/EPSRC Glasgow Molecular Pathology Node MR/N005813/1, and The Howat Foundation.
Contributor Information
The PanCaCovid-19 Study Group:
Vincenzo Corbo, Samik Upadhaya, Yu Jia Xin, Lorena Torroni, Chiara Braconi, Paola Cappello, Carmine Carbone, David K. Chang, Elisa Giovannetti, Sara Lovisa, Miriam Martini, Antonio Pea, Geny Piro, Pancreatic Cancer Action Network, Fondazione Nadia Valsecchi Onlus, Italian Pancreatic Cancer Community, Preclinical Dutch Pancreatic Cancer Group, World Pancreatic Cancer Coalition, Michele Milella, Aldo Scarpa, Claudio Bassi, and Jay Campbell
References
- 1.Unger J.M., Blanke C.D., LeBlanc M., et al. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterhouse D.M., Harvey R.D., Hurley P., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology Survey. JCO Oncol Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 3.Harris A.L. COVID-19 and cancer research. Br J Cancer. 2020;123:689–690. doi: 10.1038/s41416-020-0960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kourie H.R., Eid R., Haddad F., et al. The future of cancer research after COVID-19 pandemic: recession? Future Oncol. 2020;16:1493–1495. doi: 10.2217/fon-2020-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulholland E.J. Impact of COVID-19 on in vivo work and patient sample availability for cancer research. Nat Rev Cancer. 2021;21:139–140. doi: 10.1038/s41568-021-00333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke C.H., Buckley J.A., Fung E.T. SELDI-TOF-MS proteomics of breast cancer. Clin Chem Lab Med. 2005;43:1314–1320. doi: 10.1515/CCLM.2005.225. [DOI] [PubMed] [Google Scholar]
- 7.Webster P. How is biomedical research funding faring during the COVID-19 lockdown? [Published online ahead of print April 16, 2020] Nat Med. 2020 doi: 10.1038/d41591-020-00010-4. [DOI] [PubMed] [Google Scholar]
- 8.Clarke W., Silverman B.C., Zhang Z., et al. Characterization of renal allograft rejection by urinary proteomic analysis. Ann Surg. 2003;237:660–664. doi: 10.1097/01.SLA.0000064293.57770.42. [discussion 664–665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burki T.K. Cuts in cancer research funding due to COVID-19. Lancet Oncol. 2021;22:e6. doi: 10.1016/S1470-2045(20)30749-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhaya S., Yu J.X., Hodge J., et al. COVID-19 impact on oncology clinical trials: a 1-year analysis. Nat Rev Drug Discov. 2021;20:415. doi: 10.1038/d41573-021-00086-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson E. Dramatic drop in new cancer drug trials during the COVID-19 pandemic. Lancet Oncol. 2021;22:305. doi: 10.1016/S1470-2045(21)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Cancer Coalitions Network. COVID-19: Impact on Cancer Patient Organisations Worldwide in 2020. Available at: https://www.abcglobalalliance.org/wp-content/uploads/2021/02/GCCN-COVID19-Impact-Report-2021.pdf. Accessed May 12, 2021.
- 13.Nevala-Plagemann C., Hidalgo M., Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 14.Landman A., Feetham L., Stuckey D. Working together to reduce the burden of pancreatic cancer. Lancet Oncol. 2020;21:334–335. doi: 10.1016/S1470-2045(20)30088-7. [DOI] [PubMed] [Google Scholar]
- 15.Huang J., Lok V., Ngai C.H., et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160:744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Rahib L., Wehner M.R., Matrisian L.M., et al. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Lancet Gastroenterology & Hepatology (Editorial) Pancreatic cancer: a state of emergency? Lancet Gastroenterol Hepatol. 2021;6:81. doi: 10.1016/S2468-1253(20)30397-6. [DOI] [PubMed] [Google Scholar]
- 18.Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed M.A., Behbahani A.H., Bruckner A., et al. The precarious position of postdocs during COVID-19. Science. 2020;368:957–958. doi: 10.1126/science.abc5143. [DOI] [PubMed] [Google Scholar]
- 20.Levine R.L., Rathmell W.K. COVID-19 impact on early career investigators: a call for action. Nat Rev Cancer. 2020;20:357–358. doi: 10.1038/s41568-020-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oba A., Stoop T.F., Lohr M., et al. Global survey on pancreatic surgery during the COVID-19 pandemic. Ann Surg. 2020;272:e87–e93. doi: 10.1097/SLA.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabhan C., Choueiri T.K., Mato A.R. Rethinking clinical trials reform during the COVID-19 pandemic. JAMA Oncol. 2020;6:1327–1329. doi: 10.1001/jamaoncol.2020.3142. [DOI] [PubMed] [Google Scholar]
- 23.Nelson R. Organisations for patients with cancer feel the brunt of COVID-19 pandemic. Lancet Oncol. 2020;21:1020. doi: 10.1016/S1470-2045(20)30389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matulevicius S.A., Kho K.A., Reisch J., et al. Academic medicine faculty perceptions of work-life balance before and since the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennell N.A., Dillmon M., Levit L.A., et al. American Society of Clinical Oncology road to recovery report: learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol. 2021;39:155–169. doi: 10.1200/JCO.20.02953. [DOI] [PubMed] [Google Scholar]