Abstract
Objective
To determine whether the Mayo Cardiac Intensive Care Unit (CICU) Admission Risk Score (M-CARS) is associated with CICU resource utilization.
Patients and Methods
Adult patients admitted to our CICU from 2007 to 2018 were retrospectively reviewed, and M-CARS was calculated from admission data. Groups were compared using Wilcoxon test for continuous variables and χ2 test for categorical variables.
Results
We included 12,428 patients with a mean age of 67±15 years (37% female patients). The mean M-CARS was 2.1±2.1, including 5890 (47.4%) patients with M-CARS less than 2 and 644 (5.2%) patients with M-CARS greater than 6. Critical care restricted therapies were frequently used, including mechanical ventilation in 28.0%, vasoactive medications in 25.5%, and dialysis in 4.8%. A higher M-CARS was associated with greater use of critical-care therapies and longer CICU and hospital length of stay. The low-risk cohort with M-CARS less than 2 was less likely to require critical-care–restricted therapies, including invasive or noninvasive mechanical ventilation (8.0% vs 46.1%), vasoactive medications (10.1% vs 38.8%), or dialysis (1.0% vs 8.2%), compared with patients with M-CARS greater than or equal to 2 (all P<.001).
Conclusion
Patients with M-CARS less than 2 infrequently require critical-care resources and have extremely low mortality, suggesting that the M-CARS could be used to facilitate the triage of critically ill cardiac patients.
Abbreviations and Acronyms: ACS, acute coronary syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; BUN, blood urea nitrogen; CA, cardiac arrest; CCI, Charlson Comorbidity Index; CCCTN, Critical Care Cardiology Trials Network; CICU, cardiac intensive care unit; CRRT, continuous renal replacement therapy; CS, cardiogenic shock; CVC, central venous catheter; ECMO, extracorporeal membrane oxygenation; HF, heart failure; IABP, intra-aortic balloon pump; ICU, intensive care unit; IMCU, intermediate care unit; LOS, length of stay; M-CARS, Mayo Cardiac Intensive Care Unit Admission Risk Score; PAC, pulmonary arterial catheter; PCI, percutaneous coronary intervention; RBC, red blood cell; RDW, red blood cell distribution width; SOFA, Sequential Organ Failure Assessment; VF, ventricular fibrillation
The first coronary care units were developed in the 1960s to provide early defibrillation for patients with acute myocardial infarction.1 With progressive improvements in care for patients with acute coronary syndrome (ACS), coronary care units have evolved into cardiac intensive care units (CICUs) that provide critical care across a broad spectrum of acute and chronic cardiovascular pathology.2 Over the years, there has been a reduction in the prevalence of uncomplicated ACS in tertiary-care CICU populations and an increase in the diversity of critical care conditions including sepsis, respiratory failure, shock, and organ failure.2,3
Recent studies have suggested that patients with uncomplicated ACS admitted to CICUs have higher hospital costs without necessarily having improved outcomes.4 Furthermore, many patients admitted to contemporary tertiary-care CICUs have no specific critical-care needs.5 These studies raise important questions about which patients are likely to benefit from admission to CICUs, and whether there are low-risk CICU admission candidates who could safely be cared for in a lower-intensity setting. This is a particularly important issue, given the dramatically rising costs of critical care in the United States, coupled with the increasing occupancy of critical care beds that can adversely affect the availability of critical-care services in some settings.6, 7, 8
Standard indications for CICU admission include the need for critical-care–restricted therapies or a high risk of deterioration that could require urgent intervention. Approaches to patient selection for admission to CICUs are likely to differ among providers and institutions, but few, if any, studies exist to guide these triage decisions outside of patients presenting with ST-elevation myocardial infarction.9 Although patient triage must necessarily depend heavily on clinician judgment, the availability of decision-support tools could facilitate more consistent practices within and across institutions. To blunt the rising health care costs and improve resource allocation, previous studies have focused on identifying patients who are too ill to benefit from intensive care unit (ICU) admission; this approach is controversial insofar as criteria for futility are not widely agreed upon.10 In contrast, we believe that a reciprocal approach involving selection of the lowest-risk potential CICU admissions to be cared for in a non-CICU setting might be of value, provided that these patients are indeed at low risk of adverse outcomes or subsequent deterioration and are not anticipated to require critical-care–restricted therapies.
We recently reported a novel CICU-specific scoring system called the Mayo CICU Admission Risk Score (M-CARS), which predicts hospital mortality using 7 variables available at the time of CICU admission, with greater discrimination for mortality than conventional ICU mortality risk-stratification scoring systems among CICU patients.11 Patients with admission M-CARS less than 2 were indeed low risk, with less than 1% observed hospital mortality in both the derivation and validation cohorts. We hypothesized that these low-risk patients with M-CARS less than 2 would also be unlikely to require critical-care–restricted therapies during hospitalization.
Methods
Study Population
This study was approved by the Institutional Review Board of Mayo Clinic as minimal risk and was conducted under a waiver of informed consent. We analyzed a previously constructed database of consecutive unique adult (aged ≥18 years) patients admitted to the CICU at Mayo Clinic Hospital St. Mary’s Campus, whose entire CICU admission occurred between January 1, 2007, and April 30, 2018.2,11 The Mayo Clinic CICU is a closed 16-bed unit serving critically ill cardiac medical patients; postoperative cardiac surgery patients and patients receiving extracorporeal membrane oxygenator (ECMO) support are cared for in a separate ICU. Indications for CICU admission at Mayo Clinic include initiation and titration of inotrope and vasopressor medication; initiation of antiarrhythmic therapy for ventricular arrhythmias; use of pulmonary artery catheter (PAC), arterial line, and mechanical circulatory support; high-flow oxygen therapy, invasive and noninvasive ventilation; continuous renal-replacement therapy (CRRT); and targeted temperature management. The analyzed data were limited to the first CICU admission during the study period to avoid potential bias due to readmissions. As per Minnesota state law, we excluded patients not providing Minnesota Research Authorization.
Data Sources
Demographic, vital sign, laboratory, clinical, and outcome data including CICU and hospital length of stay (LOS) were extracted electronically from the medical record using the Multidisciplinary Epidemiology and Translational Research in Intensive Care (METRIC) DataMart.2,11, 12, 13, 14, 15, 16 Data were collected regarding therapies and procedures provided in the CICU as well as the performance of coronary angiography and percutaneous coronary intervention (PCI) during hospitalization. Critical-care–restricted therapies were defined as invasive and noninvasive ventilation, vasoactive drugs (vasopressors and inotropes), intra-aortic balloon pump (IABP), PAC, Impella (Abiomed, Danvers, MA), and CRRT. Central venous catheters (CVC) and arterial lines were not included as critical-care–restricted therapies, as we were unable to separate the different types of invasive lines.
We calculated the M-CARS for all patients using data from the time of CICU admission, with scoring criteria shown in Table 1.11 Patients with M-CARS less than 2 were considered low risk, given their low risk of mortality in our previous study. The following established ICU risk scores were calculated automatically for all patients using data from the first 24 hours of CICU admission, with missing variables imputed as normal as the default: Acute Physiology and Chronic Health Evaluation (APACHE)-III score, APACHE-IV predicted hospital mortality, and Day 1 Sequential Organ Failure Assessment (SOFA) score.13, 14, 15,17, 18, 19, 20, 21 The mean and maximum daily SOFA scores during CICU admission (up to the first 7 days) were recorded. The Charlson Comorbidity Index (CCI) and individual comorbidities were determined from diagnoses in the medical record, based on a previously validated electronic algorithm.22
Table 1.
Criteria for Calculating M-CARS Score
| Variable | Value | Points assigned |
|---|---|---|
| Admission value of BUN | >23 mg/dL | 1 |
| ≤23 mg/dL | 0 | |
| Admission value of anion gap | >14 | 1 |
| ≤14 | 0 | |
| Admission Braden skin score | ≤12 | 2 |
| 13-15 | 1 | |
| >15 | 0 | |
| Admission value of RDW | >14.3 | 1 |
| ≤14.3 | 0 | |
| Admission diagnosis of cardiac arrest | Yes | 2 |
| No | 0 | |
| Admission diagnosis of shock | Yes | 2 |
| No | 0 | |
| Admission diagnosis of respiratory failure | Yes | 1 |
| No | 0 |
Missing data are assumed to be normal (score 0). The score ranges from 0 to 10.
BUN, blood urea nitrogen; M-CARS, Mayo Clinic Intensive Care Unit Admission Risk Score; RDW, red blood cell distribution width.
Admission diagnoses were defined as all International Classification of Diseases (ICD)-9 and ICD-10 diagnostic codes recorded on the day of CICU admission and the day before or after CICU admission; these admission diagnoses were not mutually exclusive, and the primary admission diagnosis could not be determined. Admission diagnoses of interest included ACS, including all forms of acute myocardial infarction and unstable angina; heart failure (HF); shock, cardiogenic shock (CS); cardiac arrest (CA); respiratory failure; and sepsis (including septic shock). Patients with CA were subdivided based on the presence of a concomitant admission diagnosis of ventricular fibrillation (VF) into VF CA and non-VF CA.
Statistical Methods
We assessed CICU and hospital mortality and LOS, duration of mechanical ventilation, and use of critical-care therapies during the CICU stay as a function of the M-CARS at the time of CICU admission. Data are reported as mean ± standard deviation (SD) for continuous variables and number (%) for categorical variables. Patients with M-CARS less than 2 and more than or equal to 2 were compared using Wilcoxon test for continuous variables and χ2 test for categorical variables. Trends across M-CARS groups were analyzed using Cochran-Armitage trend tests for categorical variables and linear regression for continuous variables. Statistical analyses were performed using JMP version 13.0 Pro (SAS Institute, Cary, NC). No extramural funding was used to support this research.
Results
Baseline Characteristics
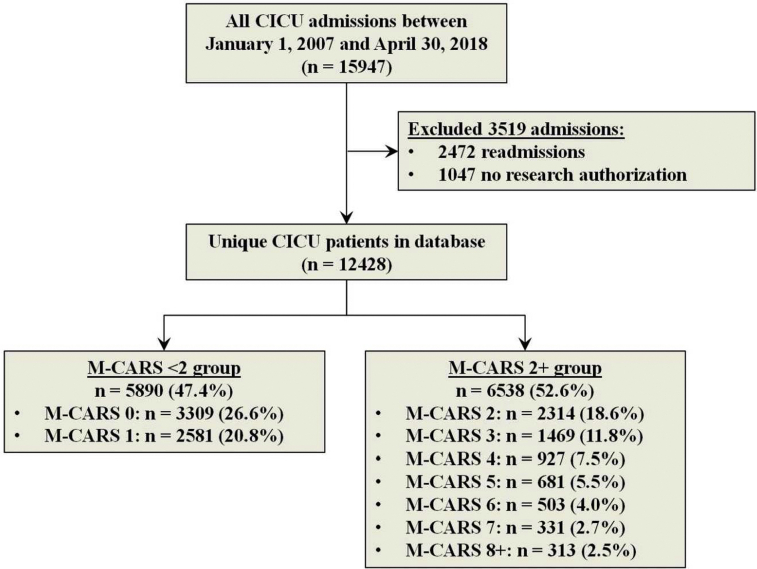
Among the 15,947 adult patients admitted to the CICU within the study period, 3519 were excluded (2472 readmissions and 1047 without Minnesota Research Authorization), leaving 12,428 unique CICU patients in the final study population (Supplemental Figure 1, available online at http://mcpiqojournal.org). The final study population had a mean age of 67.6±15.2 years, including 4686 (37.7%) female patients (Table 2). Admission diagnoses included ACS in 5238 (42.5%) patients, HF in 6008 (48.8%) patients, coronary artery disease in 1479 (12.0%), shock in 1859 (15.1%), sepsis in 780 (6.3%), and respiratory failure in 2986 (24.2%). The mean M-CARS was 2.1±2.1, including 5890 (47.4%) patients with M-CARS less than 2 and 644 (5.2%) patients with M-CARS greater than 6. The low-risk cohort of patients with M-CARS less than 2 differed substantially from the remaining patients with M-CARS greater than or equal to 2 (Table 2), with a lower age, fewer comorbidities, and lower severity of illness. Notably, all patients with cardiac arrest or shock had M-CARS greater than or equal to 2, by definition.
Table 2.
Baseline Characteristics of the Final Study Population, Patients With M-CARS Less Than 2 and M-CARS Greater Than or Equal to 2
| Variable | Final study population (n=12,428) | M-CARS <2 (n=5890) | M-CARS ≥2 (n=6538) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years, mean) | 67.6±15.2 | 65.2±15.3 | 69.8±14.7 | <.001 |
| Female, n (%) | 4686 (37.7) | 2134 (36.2) | 2552 (39.0) | .0013 |
| Caucasian, n (%) | 11,466 (92.2) | 5436 (92.3) | 6030 (92.2) | .89 |
| Hospital days before CICU (mean) | 0.7±2.7 | 0.4±1.5 | 1.0±3.4 | <.001 |
| Comorbidities | ||||
| Charlson comorbidity index | 2.4±2.6 | 1.6±2.2 | 3.1±2.8 | <.001 |
| Previous myocardial infarction, n (%) | 2300 (18.5) | 962 (16.4) | 1338 (20.5) | <.001 |
| Previous heart failure, n (%) | 2521 (20.3) | 604 (10.3) | 1917 (29.4) | <.001 |
| Previous stroke, n (%) | 1483 (11.9) | 519 (8.83) | 964 (14.8) | <.001 |
| Previous diabetes mellitus, n (%) | 3544 (28.6) | 1247 (21.2) | 2297 (35.2) | <.001 |
| Previous cancer, n (%) | 2623 (21.1) | 1042 (17.7) | 1581 (24.2) | <.001 |
| Previous lung disease, n (%) | 2410 (19.4) | 881 (15.0) | 1529 (23.4) | <.001 |
| Previous chronic kidney disease, n (%) | 2561 (20.7) | 538 (9.15) | 2023 (31.0) | <.001 |
| Previous dialysis, n (%) | 620 (4.9) | 73 (1.2) | 547 (8.4) | <.001 |
| Admission diagnosesa | ||||
| Acute coronary syndrome | 5238 (42.5) | 2780 (47.8) | 2458 (37.8) | <.001 |
| Heart failure | 6008 (48.8) | 1659 (28.5) | 4349 (66.8) | <.001 |
| Shock | 1859 (15.9) | 0 (0) | 1859 (28.6) | <.001 |
| Cardiogenic shock | 1498 (12.2) | 0 (0) | 1498 (23.0) | <.001 |
| Cardiac arrest | 1479 (12.0) | 0 (0) | 1479 (22.7) | <.001 |
| VF arrest | 753 (6.1) | 0 (0) | 753 (11.6) | <.001 |
| Non-VF arrest | 726 (5.9) | 0 (0) | 726 (11.2) | <.001 |
| Respiratory failure | 2986 (24.2) | 137 (2.4) | 2849 (43.8) | <.001 |
| Sepsis | 780 (6.3) | 59 (1.0) | 721 (11.1) | <.001 |
| Severity of illness | ||||
| APACHE-III score (mean) | 60.7±25.1 | 48.0±16.2 | 72.2±26.2 | <.001 |
| APACHE-IV predicted hospital mortality (%) | 16.8±19.7 | 7.5±8.0 | 25.2±23.1 | <.001 |
| Day 1 SOFA score (mean) | 3.5±3.2 | 1.8±1.5 | 5.1±3.5 | <.001 |
| Maximum week 1 SOFA score | 21 | 16 | 21 | <.001 |
| Mean week 1 SOFA score | 3.0 | 1.7 | 4.2 | <.001 |
| Procedures and therapies | ||||
| Any critical care therapy | 5260 (42.3%) | 1129 (19.2%) | 4131 (63.2%) | <.001 |
| Inpatient coronary angiogram, n (%) | 7254 (58.4) | 3859 (65.5) | 3395 (51.9) | <.001 |
| Inpatient PCI, n (%) | 4320 (34.8) | 2567 (43.6) | 1753 (26.8) | <.001 |
| Any invasive line, n (%) | 5503 (44.3) | 2038 (34.6) | 3465 (53.0) | <.001 |
| Central venous line, n (%) | 2507 (20.2) | 584 (9.9) | 1923 (29.4) | <.001 |
| Arterial line, n (%) | 3985 (32.1) | 1513 (25.7) | 2472 (37.8) | <.001 |
| Pulmonary artery catheter, n (%) | 1198 (9.6) | 236 (4.0) | 962 (14.7) | <.001 |
| IABP in CICU, n (%) | 1051 (8.5) | 242 (4.1) | 809 (12.4) | <.001 |
| Impella (Abiomed, Danvers, MA) in hospital, n (%) | 73 (0.6) | 9 (0.2) | 64 (1.0) | <.001 |
| ECMO in CICU, n (%) | 30 (0.2) | 2 (0.0) | 28 (0.4) | <.001 |
| Any mechanical ventilation, n (%) | 3483 (28.0) | 470 (8.0) | 3013 (46.1) | <.001 |
| Duration of mechanical ventilation, days | 2.1±3.0 | 1.0±1.9 | 2.1±2.9 | <.001 |
| Invasive ventilator use, n (%) | 2034 (16.4) | 108 (1.8) | 1924 (29.4) | <.001 |
| Duration of invasive ventilation, days | 1.9±2.8 | 0.9±1.8 | 2.2±3.0 | <.001 |
| Duration of invasive ventilation <1 day, n (%) | 948 (46.6) | 90 (83.3) | 858 (44.6) | <.001 |
| Noninvasive ventilator use, n (%) | 1923 (15.5) | 377 (6.4) | 1546 (23.7) | <.001 |
| Duration of noninvasive ventilation, days | 1.2±1.8 | 1.0±1.9 | 1.3±1.8 | <.001 |
| Vasoactive drug use, n (%) | 3131 (25.5) | 592 (10.1) | 2539 (38.8) | <.001 |
| Vasopressor use, n (%) | 2672 (21.5) | 443 (7.5%) | 2229 (34.1) | <.001 |
| Inotrope use, n (%) | 1150 (9.3) | 223 (3.8) | 927 (14.2) | <.001 |
| Blood transfusion, n (%) | 1393 (11.2) | 258 (4.4) | 1135 (17.4) | <.001 |
| Dialysis, n (%) | 594 (4.8) | 59 (1.0) | 535 (8.2) | <.001 |
| New dialysis start, n (%) | 393 (3.2) | 50 (0.9) | 343 (5.3) | <.001 |
| CRRT, n (%) | 244 (2.0) | 1 (0.0) | 243 (3.7) | <.001 |
| In-hospital CPR, n (%) | 317 (2.6) | 17 (0.3) | 300 (4.6) | <.001 |
| Length of stay | ||||
| CICU length of stay (days) | 2.5±4.3 | 1.8±3.8 | 3.1±4.6 | <.001 |
| CICU length of stay <1 day, n (%) | 3515 (28.3) | 1997 (33.9) | 1518 (23.2) | <.001 |
| CICU length of stay >7 days, n (%) | 593 (4.8) | 53 (0.9) | 540 (8.3) | <.001 |
| Hospital length of stay (days, mean) | 8.0±13.4 | 4.9±6.9 | 10.7±16.8 | <.001 |
| Hospital length of stay <3 days, n (%) | 3844 (30.9) | 2575 (43.7) | 1269 (19.4) | <.001 |
| Hospital length of stay >14 days, n (%) | 1565 (12.6) | 238 (4.0) | 1327 (20.3) | <.001 |
APACHE, Acute Physiology and Chronic Health Evaluation; CICU, cardiac intensive care unit; CPR, cardiopulmonary resuscitation; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; M-CARS, Mayo Clinic Intensive Care Unit Admission Risk Score; PCI, percutaneous coronary intervention; SOFA, Sequential Organ Failure Assessment; VF, ventricular fibrillation.
Data displayed as n (%) for categorical variables or mean ± standard deviation for continuous variables. Reported P value is for between-group comparison of patients with M-CARS less than 2 and M-CARS greater than or equal to 2, using χ2 test (categorical variables) or Wilcoxon rank-sum test (continuous variables).
Admission diagnoses are not mutually exclusive and may add up to more than 100%.
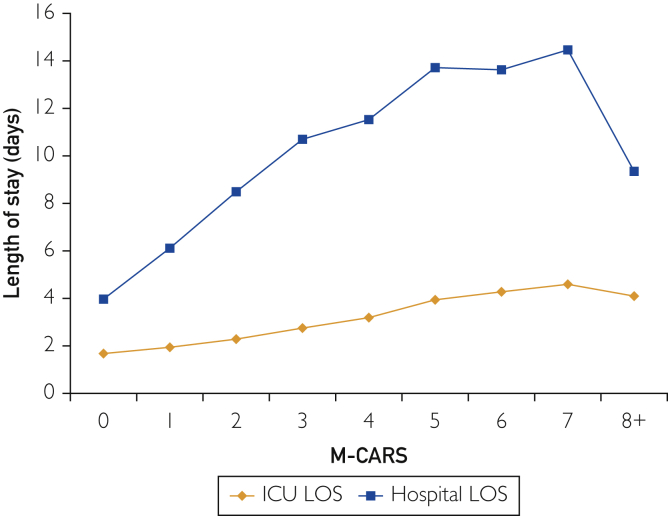
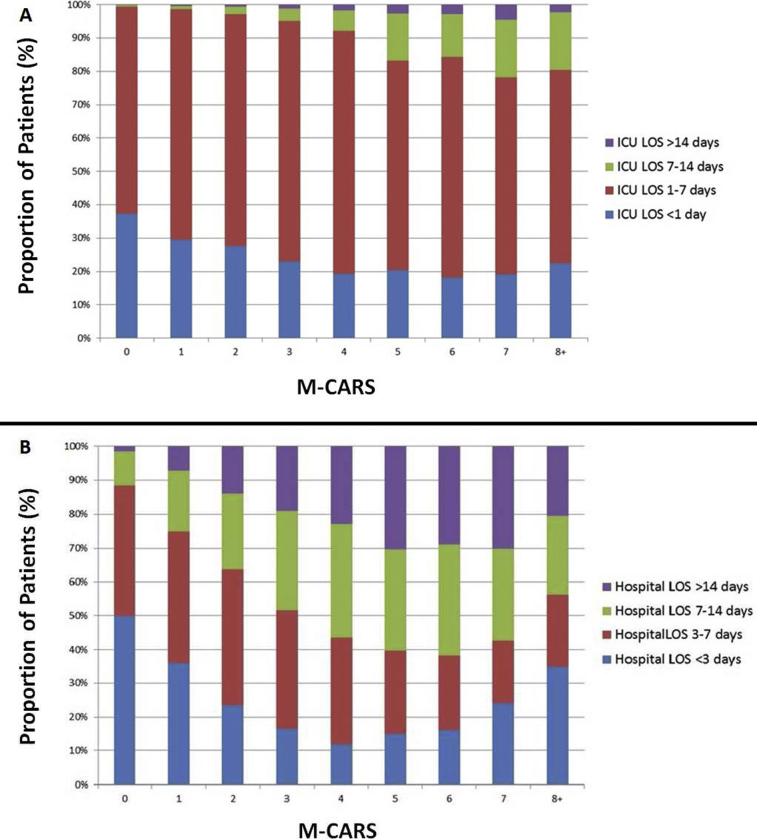
Overall mean CICU LOS was 2.5±4.3 days, and overall mean hospital LOS was 8.0±13.4 days. Both CICU and hospital LOS increased significantly as a function of rising M-CARS (P<.001; Figure 1 and Supplemental Figure 2, available online at http://mcpiqojournal.org); however, M-CARS only explained 3.8% and 5.1% of the variability in CICU and hospital LOS, respectively. The strength of association between M-CARS and the use of each specific therapy varied substantially, being strongest for invasive ventilator and weakest for coronary angiography (Table 3). Patients with M-CARS less than 2 had shorter CICU and hospital LOS than patients with M-CARS greater than or equal to 2 (P<.001). Overall, 5260 patients received critical-care–restricted therapies, with 1129 (19.2%) of patients with M-CARS less than 2 receiving any critical-care–restricted therapy compared with 4131 (63.2%) of patients with M-CARS greater than or equal to 2 (P<.001). A total of 1685 (28.6%) patients had M-CARS less than 2, CICU LOS lest than 1 day, and did not receive any critical-care–restricted therapies.
Figure 1.
Cardiac intensive care unit (CICU) and hospital length of stay (LOS) as a function of Mayo Cardiac Intensive Care Unit Admission Risk Score (M-CARS).
Table 3.
Association Between the M-CARS and Resource Utilization on Univariate Logistic Regression
| Beta estimate (standard error) | Unit OR per 1-point increase in M-CARS (95% CI) | R2 | AUC | P value | |
|---|---|---|---|---|---|
| Respiratory support therapies | |||||
| Any ventilator | 0.707 (0.014) | 2.027 (1.973-2.082) | 0.279 | 0.833 | <.0001 |
| Invasive ventilator | 0.836 (0.017) | 2.308 (2.233-2.385) | 0.383 | 0.891 | <.0001 |
| Noninvasive ventilator | 0.270 (0.011) | 1.310 (1.283-1.339) | 0.057 | 0.703 | <.0001 |
| Hemodynamic support therapies | |||||
| Vasopressors | 0.559 (0.012) | 1.750 (1.708-1.792) | 0.212 | 0.792 | <.0001 |
| Inotropes | 0.290 (0.013) | 1.336 (1.303-1.371) | 0.062 | 0.701 | <.0001 |
| Any vasoactive drug | 0.520 (0.012) | 1.682 (1.644-1.720) | 0.184 | 0.773 | <.0001 |
| IABP | 0.314 (0.014) | 1.369 (1.333-1.406) | 0.073 | 0.695 | <.0001 |
| Coronary angiography | –0.107 (0.009) | 0.898 (0.883-.914) | 0.009 | 0.580 | <.0001 |
| PCI | –0.166 (0.010) | 0.847 (0.831-.864) | 0.019 | 0.609 | <.0001 |
| Invasive lines | |||||
| Any invasive line | 0.287 (0.010) | 1.332 (1.307-1.357) | 0.059 | 0.641 | <.0001 |
| Central line | 0.377 (0.011) | 1.458 (1.428-1.489) | 0.108 | 0.718 | <.0001 |
| Arterial line | 0.247 (0.009) | 1.280 (1.257-1.304) | 0.047 | 0.620 | <.0001 |
| PA catheter | 0.298 (0.013) | 1.347 (1.313-1.381) | 0.066 | 0.702 | <.0001 |
| Other support therapies | |||||
| Any dialysis | 0.377 (0.017) | 1.458 (1.410-1.508) | 0.099 | 0.768 | <.0001 |
| CRRT | 0.515 (0.027) | 1.673 (1.587-1.765) | 0.161 | 0.855 | <.0001 |
| Blood t ransfusion | 0.316 (0.012) | 1.372 (1.339-1.405) | 0.076 | 0.718 | <.0001 |
| Outcomes | |||||
| CICU death | 0.633 (0.019) | 1.884 (1.816-1.954) | 0.263 | 0.871 | <.0001 |
| Hospital death | 0.634 (0.016) | 1.885 (1.827-1.944) | 0.270 | 0.868 | <.0001 |
AUC, area under the curve; CI, confidence interval; CICU, cardiac intensive care unit; CRRT, continuous renal replacement therapy; IABP, intra-aortic balloon pump; M-CARS, Mayo Clinic Intensive Care Unit Admission Risk Score; OR, operating room; PA, pulmonary artery; PCI, percutaneous coronary intervention.
Mechanical Ventilation
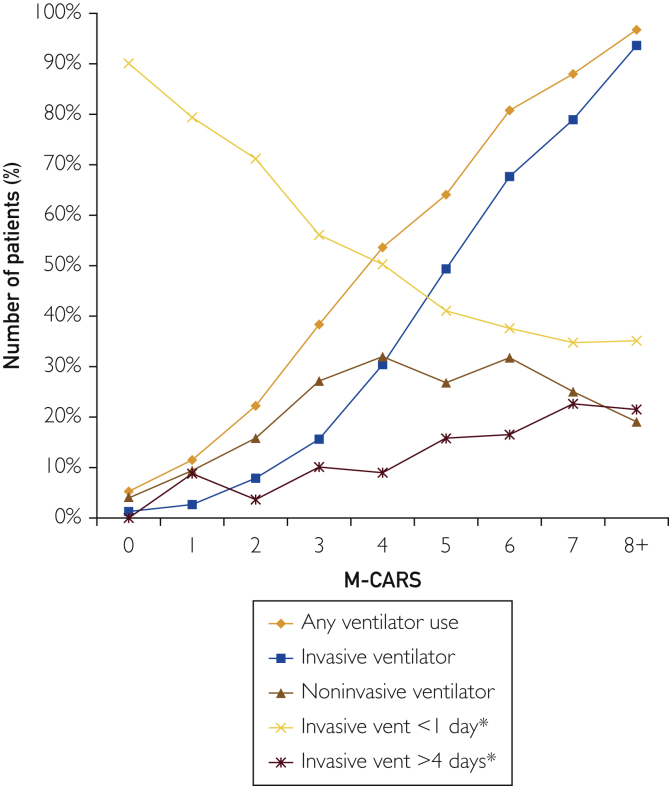
Mechanical ventilation was used in 3483 (28.0%) patients, including invasive ventilation in 2034 (16.4%) and noninvasive ventilation in 1923 (15.5%) patients. The use of any mechanical ventilation (including both invasive and noninvasive ventilation) increased as a function of increasing M-CARS (P<.001; Figure 2). There was an inverted U-shaped relationship between M-CARS and the use of noninvasive ventilation, with the highest rates at intermediate M-CARS; at lower M-CARS, noninvasive ventilation was used more commonly, whereas invasive ventilation predominated at higher M-CARS (Figure 3). The duration of ventilation increased with rising M-CARS (P<0.001), with a decreasing proportion of ventilated patients with duration of ventilation <1 day (Figure 2). Patients with M-CARS less than 2 infrequently required any type of mechanical ventilation compared with those with M-CARS greater than or equal to 2 (8.0% vs 46.1%), and 83.3% of patients with M-CARS less than 2 who required invasive ventilation had duration of ventilation less than 1 day.
Figure 2.
Use of mechanical ventilation as a function of Mayo Cardiac Intensive Care Unit Admission Risk Score (M-CARS). Mechanical ventilation is categorized by any ventilator use, invasive ventilator, noninvasive ventilator, invasive ventilation less than1 day, and invasive ventilation greater than 4 days. ∗Denotes among only those patients receiving invasive ventilation.
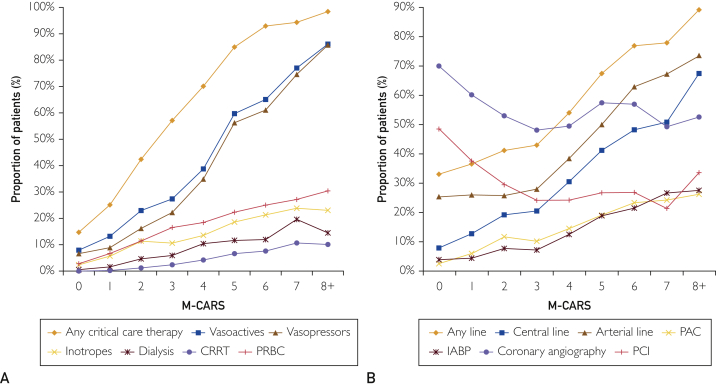
Figure 3.
Proportion of patients (%) needing (A) any critical care therapy, vasoactive drugs, vasopressors, inotropes, dialysis, continuous renal replacement therapy (CRRT), and packed red blood cell (pRBC) transfusion or (B) invasive lines, central lines, arterial lines, pulmonary arterial catheter (PAC), intra-aortic balloon pump (IABP), coronary angiography, or percutaneous intervention (PCI) (B) as a function of Mayo Cardiac Intensive Care Unit Admission Risk Score (M-CARS).
Vasoactive Drugs and Other Therapies
A total of 3131 (25.2%) patients received vasoactive drugs, including 2672 (21.5%) who received vasopressors and 1150 (9.3%) who received inotropes. The use of vasoactive drugs, including vasopressors and inotropes, increased progressively with rising M-CARS (P<.001; Figure 3A). Among patients with M-CARS less than 2, only 592 (10.0%) required use of vasoactive drugs during the CICU stay, including vasopressors in 443 (7.5%) and inotropes in 223 (3.8%). A total of 594 (4.8%) patients required dialysis during the CICU stay, including 244 (2.0%) who received CRRT; red blood cell (RBC) transfusion was required in 1393 (11.2%) patients. Dialysis, CRRT, and RBC transfusion were all more prevalent with rising M-CARS (P<.001; Figure 3A). Few patients with M-CARS less than 2 required dialysis, CRRT, or RBC transfusion during their admissions.
Invasive Lines and Other Procedures
A total of 5503 (44.3%) patients had any invasive line during the CICU stay, including arterial line in 3985 (32.1%), central venous line in 2507 (20.2%), PAC in 1198 (9.6%), and IABP in 1051 (8.5%). Use of all invasive lines increased as a function of rising M-CARS (P<.001; Figure 3B). Use of invasive lines was less common among patients with M-CARS less than 2 (34.6% vs 53.0%), especially for central venous lines (9.9% vs 29.4%), PAC (4.0% vs 14.7%) and IABP (4.1% vs 12.4%). The use of coronary angiography and PCI had a curvilinear relationship with M-CARS (Figure 3B), with higher use of coronary angiography and PCI among patients with M-CARS less than 2 consistent with the higher prevalence of patients with ACS and lower prevalence of patients with HF in this group.
Hospital Mortality and Resource Utilization in Low-Risk Patients
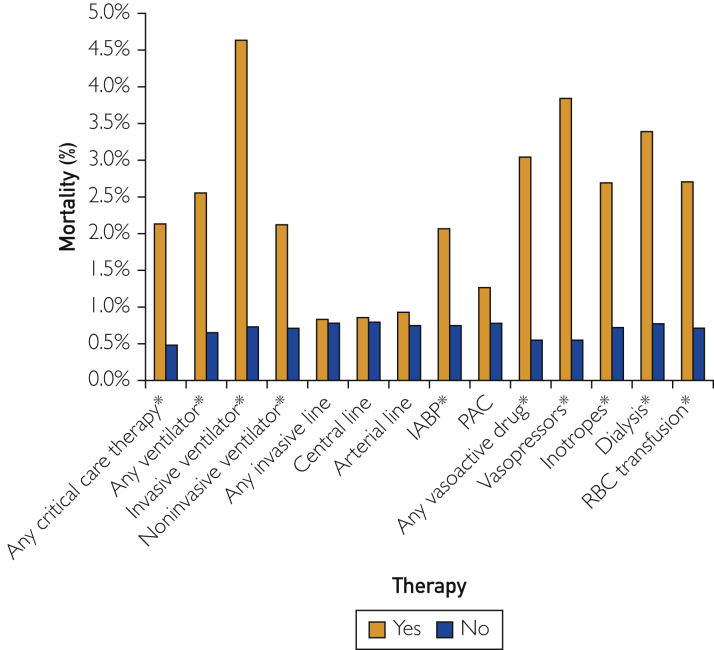
Among low-risk patients with M-CARS less than 2, hospital mortality occurred in only 47 (0.8%) patients, including 28 (0.5%) who died in the CICU. Patients with M-CARS less than 2 who did not receive any critical-care therapies had lower hospital mortality (0.5% vs 2.1%; P<.001). Similarly, patients with M-CARS greater than or equal to 2 who did not receive any critical-care therapies had lower hospital mortality (6.8% vs 22.7%; P<.001). Hospital mortality among patients with M-CARS less than 2 who did and did not receive specific therapies while in the CICU is shown in Figure 4. Patients with M-CARS less than 2 who received invasive or noninvasive ventilation, vasopressors, or inotropes, dialysis, RBC transfusion, or IABP had higher observed hospital mortality than those who did not (all P<.05), whereas we did not observe a statistically significant difference in hospital mortality for patients who did and did not receive central venous lines, arterial lines, or PAC (all P>.1).
Figure 4.
Hospital mortality in low-risk patients with M-CARS less than 2 who did and did not receive specific therapies in the CICU. ∗Denotes P<.05 between groups.
Subgroup Analysis: Patients With Acute Coronary Syndrome
The major findings of the main analysis were consistent in the subgroup of patients with an admission diagnosis of ACS. Among those with ACS, there were 2780 (53.1%) patients with M-CARS less than 2 and 2458 (46.9%) patients with M-CARS greater than or equal to 2. Only 23 (0.8%) of patients with ACS and M-CARS less than 2 died in the hospital, and hospital mortality increased incrementally with increasing M-CARS (P<.0001). Hospital and CICU LOS similarly increased with rising M-CARS (both P<.0001).
Patients with ACS and M-CARS less than 2 were less likely to require mechanical ventilation, CRRT, hemodialysis, vasoactive medications, CVC, arterial line, packed red blood cells (pRBC), IABP, ECMO, or PAC (all P<.0001). Only 428 (15.4%) of patients with ACS and M-CARS less than 2 required any critical-care therapies compared with 1593 (64.8%) of those with M-CARS greater than or equal to 2.
Discussion
To our knowledge, this is the first comprehensive study assessing the use of a prognostic score to determine the need for critical-care resources among patients admitted to a contemporary CICU. Among 12,428 patients admitted to our tertiary-care CICU, a higher M-CARS was associated with increased CICU and hospital LOS and greater use of all critical-care–restricted therapies. Patients with M-CARS less than 2 were less likely to require critical-care resources despite comprising 47.4% of all admissions, and hospital mortality was rare in this group (less than 1% of patients). Among this low-risk cohort, more than 25% required no critical-care therapies and had CICU LOS less than 1 day, comprising a group that likely could have been cared for outside the CICU setting. Our data suggest that many CICU patients with admission M-CARS less than 2 who do not have immediate critical-care needs could, in theory, be cared for safely in a non-ICU setting and could be candidates for initial triage to a non-ICU setting or early transfer out of the CICU. This opens a potential opportunity to supplement clinician judgment with an evidence-based risk-stratification tool such as M-CARS for the purposes of CICU admission triage.
The decision to admit a patient to the CICU vs a non-ICU setting can be challenging and is typically based on multiple patient and institutional factors. The admitting clinician must consider objective physiological parameters, prognosis, disease reversibility, and the actual or potential need for critical-care–restricted therapies while also considering logistic factors such as the availability of beds and resources. Common indications for CICU admission include need for vasoactive medications, invasive lines, mechanical ventilation, CRRT, or the need for close monitoring.5 Although several guidelines have been constructed to help with the triage of critically ill patients,23, 24, 25 these are rarely used in clinical practice, as exemplified by the wide variability in ICU triaging practices among different institutions, with refusal rates ranging from 16% to 51%.10,26 The CICU triage process can be even more challenging because of historical trends, which may no longer be reflective of the modern CICU environment.
The effective use of CICU beds is imperative, as critical care is expensive and resources are often limited. Previous studies have estimated that the daily cost of care in an ICU is 3 to 5 times more than a general medical or surgical ward,27, 28, 29 with particularly high expenses during the first few days of admission.30,31 The use of critical care beds has continued to increase in the United States, with a 19.3% increase in ICU days between 2000 and 2010.6 This was accompanied by a 92.9% increase ($56 to $108 billion) in annual critical care costs, accounting for $14,964 trillion (0.72% of the gross domestic product). This increased use of critical-care beds has led to a scarcity of available resources in many institutions. Resource availability has been shown to affect triage decisions, as patients are less likely to be admitted to the ICU and more likely to have their goals of care changed in times of ICU bed scarcity.7,8 Some institutions have created intermediate-care units (IMCUs) to provide for patients requiring higher levels of care than a general ward but not requiring the aggressiveness of an ICU; IMCUs include heterogeneous populations with large institutional variability, making it difficult to assess their benefits in cost and resource allocations.32 The relationship between the IMCU and CICU has not been studied, but IMCUs have been shown to improve outcomes in postcardiac surgery patients.33 Although our institution does not have an IMCU, the M-CARS could, theoretically, apply to the decision between admission to CICUs and IMCUs in institutions with this option.
The Critical Care Cardiology Trials Network (CCCTN) multicenter study of 3049 CICU patients demonstrated an increasing proportion of primary noncardiac diagnoses as well as wide variability of triage practices among different institutions.5 The overall CICU LOS and use of critical-care therapies in the CCCTN database parallels our CICU practices, with small differences in the use of certain specific therapies. Notably, 36.2% of patients were triaged to the CICU solely for the perceived need of monitoring, ICU-level nursing, frequent laboratory testing, or postprocedural monitoring in the absence of any other specific critical care needs; these patients had the lowest mortality among common diagnosis groups. Remarkably, 41.8% of admissions were treated without any CICU-level therapies or procedures, suggesting a significant subset of patients who could be cared for in a lower-intensity setting. Our study mirrors these findings, particularly among patients with low M-CARS, suggesting that there is substantial room for improvement in the CICU patient-triage process.
Prognostic scoring systems, including SOFA, APACHE, and Oxford Acute Severity of Illness Score (OASIS) have all been validated for prediction of mortality risk in the CICU population.13,14 APACHE-IV provides additional clinical information regarding LOS among ICU populations.34 Zimmerman et al recently reported that a model based on the APACHE scoring system could be used to identify low-risk patients who were unlikely to require general ICU resources.35 A major limitation of these scoring systems is the need for 24 hours of data for accurate risk stratification, which limits the ability to help triage patients at the time of presentation, unlike the M-CARS, which uses variables available at the time of admission.11 The M-CARS is a CICU-specific tool that combines admission laboratory values (blood urea nitrogen [BUN], anion gap, red blood cell distribution width [RDW]), a marker of frailty (Braden skin score), and admission diagnoses (CA, CS, respiratory failure) to derive a prognostic score that was superior to the established risk scores in predicting hospital mortality in CICU patients.
The current study extends the potential usefulness of the M-CARS to identify patients with a low probability of needing critical-care resources, to facilitate triage at the time of CICU admission using a criterion other than mortality risk. An M-CARS less than 2 identified a sizeable group (47.4% of all admissions) of low-risk patients who were unlikely to require CICU therapies, in addition to being unlikely to die during hospitalization. Patients with M-CARS less than 2 had extremely low in-hospital mortality rates (below 1%), and fewer than 10% of these patients received vasoactive medications, mechanical ventilation, PAC, or IABP. Although the rates of utilization of these critical-care therapies are not negligible, the vast majority of these low-risk patients never received any critical-care therapy. The frequent use of arterial lines in this low-risk group may reflect our inability to differentiate arterial lines placed in the CICU for monitoring from access sheaths placed in the procedural laboratory and subsequently removed; furthermore, use of arterial lines for monitoring of critically ill patients has not been shown to improve outcomes, so many of these patients may have done well without this intervention.36 If even a fraction of patients with M-CARS less than 2 could have been safely cared for outside of the CICU, this could have translated into substantial cost savings.
Limitations
In common with most retrospective observational studies, this study has important limitations that preclude drawing strong causal inferences. The referral population reflected in this single-center CICU cohort likely differs significantly from other CICU populations in terms of demographics and other important factors. Furthermore, our institution does not have an intermediate care unit, which may be available at other facilities, and could affect the triage decisions. Nonetheless, the use of critical-care therapies and case-mix in our cohort are consistent with recently reported CICU studies.5,37, 38, 39, 40 Although the assumption that patients who do not require critical care therapies do not need CICU care is implicit in our analysis, this is certainly not always the case, and there are numerous potential indications for CICU admission of lower-acuity patients who require frequent monitoring or pose substantial risks of deterioration. It is crucial to note that the observed low mortality and limited critical-care therapy after CICU admission among low-risk patients does not rule out an important role of the CICU in patient stabilization and does not imply that all such patients would have done equally well outside of a CICU setting. In addition, patients who were high risk but successfully rescued in the CICU are virtually indistinguishable in our database from patients who never needed CICU care in the first place; this is essential, considering the growing trend toward earlier ICU transfer for high-risk patients. Importantly, we did not have data on the timing of the use critical-care therapies, so we could not differentiate among patients admitted because of the immediate requirements for these therapies vs patients who subsequently deteriorated and required these therapies later. Furthermore, any analysis of LOS must be interpreted in the light of the competing risk of mortality, which shortens LOS (particularly in high-risk groups). We could not determine resuscitation status or the presence of any care restrictions: important factors that could further influence CICU triage, resource utilization, and outcomes. Overall, our hypothesis-generating suggestion that patients with low M-CARS could be triaged to a non-CICU setting could be assessed directly by comparing patients with low M-CARS who were and were not admitted to the CICU, but our database only includes CICU patients, precluding this analysis.
Conclusion
Our study demonstrates that higher M-CARS is associated with increased critical-care resource utilization during CICU admission and could potentially be used in the triage of critically ill cardiac patients by identifying those with higher or lower likelihoods of needing critical care resources. Patients with M-CARS less than 2 are unlikely to die or require critical-care–restricted therapies, despite accounting for nearly one-half of all CICU admissions, highlighting the need to reconsider standard CICU admission indications and offering an area in which substantial improvement can be made. Our data suggest that using M-CARS at the time of admission could be valuable in identifying low-risk patients who may not require CICU resources. Prospective studies are needed to validate the strong negative predictive value of M-CARS for hospital mortality and resource utilization and will be necessary before M-CARS can be integrated into practice for triage of potential CICU admissions.
Acknowledgments
We would like to thank the dedicated physicians, nurses, and allied health staff members who provide tireless and exemplary care for our CICU patients every day.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
Supplemental Figure 1.
Flow diagram demonstrating inclusion/exclusion criteria for the final study population.
Supplemental Figure 2.
Proportion of patients (%) with length of stay (LOS) <1 day, 1-7 days, 7-14 days, and >14 days with in the (A) Cardiac intensive care unit (CICU) and (B) hospital.
References
- 1.Brown K.W.G., Macmillan R.L., Forbath N., Mel’Grano F., Scott J.W. Coronary unit: an intensive-care centre for acute myocardial infarction. Lancet. 1963;282(7303):349–352. doi: 10.1016/s0140-6736(63)93011-3. [DOI] [PubMed] [Google Scholar]
- 2.Jentzer J.C., van Diepen S., Barsness G.W. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J. 2019;215:12–19. doi: 10.1016/j.ahj.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Katz J.N., Shah B.R., Volz E.M. Evolution of the coronary care unit: clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit Care Med. 2010;38(2):375–381. doi: 10.1097/CCM.0b013e3181cb0a63. [DOI] [PubMed] [Google Scholar]
- 4.van Diepen S., Bakal J.A., Lin M., Kaul P., McAlister F.A., Ezekowitz J.A. Variation in critical care unit admission rates and outcomes for patients with acute coronary syndromes or heart failure among high- and low-volume cardiac hospitals. J Am Heart Assoc. 2015;4(3):001708. doi: 10.1161/JAHA.114.001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohula E.A., Katz J.N., Van Diepen S. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019;4(9):928–935. doi: 10.1001/jamacardio.2019.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern N.A., Goldman D.A., Tan K.S., Pastores S.M. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000-2010. Crit Care Med. 2016;44(8):1490–1499. doi: 10.1097/CCM.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert R., Coudroy R., Ragot S. Influence of ICU-bed availability on ICU admission decisions. Ann Intensive Care. 2015;5(1):55. doi: 10.1186/s13613-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mery E., Kahn J.M. Does space make waste? The influence of ICU bed capacity on admission decisions. Crit Care. 2013;17(315) doi: 10.1186/cc12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca G., Suryapranata H., Van’t Hof A.W.J. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109(22):2737–2743. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 10.Sinuff T., Kahnamoui K., Cook D.J., Luce J.M., Levy M.M. Rationing critical care beds: a systematic review. Crit Care Med. 2004;32(7):1588–1597. doi: 10.1097/01.ccm.0000130175.38521.9f. [DOI] [PubMed] [Google Scholar]
- 11.Jentzer J.C., Anavekar N.S., Bennett C. Derivation and validation of a novel cardiac intensive care unit admission risk score for mortality. J Am Heart Assoc. 2019;8(17):013675. doi: 10.1161/JAHA.119.013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herasevich V., Pickering B.W., Dong Y., Peters S.G., Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett C.E., Wright R.S., Jentzer J. Severity of illness assessment with application of the APACHE IV predicted mortality and outcome trends analysis in an academic cardiac intensive care unit. J Crit Care. 2019;50:242–246. doi: 10.1016/j.jcrc.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jentzer J.C., Bennett C., Wiley B.M. Predictive value of the Sequential Organ Failure Assessment score for mortality in a contemporary cardiac intensive care unit population. J Am Heart Assoc. 2018;7(6):008169. doi: 10.1161/JAHA.117.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jentzer J.C., Murphree D.H., Wiley B. Comparison of mortality risk prediction among patients ≥70 versus <70 years of age in a cardiac intensive care unit. Am J Cardiol. 2018;122(10):1773–1778. doi: 10.1016/j.amjcard.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Jentzer J.C., Wiley B., Bennett C. Temporal trends and clinical outcomes associated with vasopressor and inotrope use in the cardiac intensive care unit. SHOCK. 2019;53(4):452–459. doi: 10.1097/SHK.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 17.Chandra S., Kashyap R., Trillo-Alvarez C.A. Mapping physicians’ admission diagnoses to structured concepts towards fully automatic calculation of Acute Physiology and Chronic Health Evaluation score. BMJ Open. 2011;1:000216. doi: 10.1136/bmjopen-2011-000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus W.A., Wagner D.P., Draper E.A. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1296–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 20.Aakre C., Franco P.M., Ferreyra M., Kitson J., Li M., Herasevich V. Prospective validation of a near real-time EHR-integrated automated SOFA score calculator. Int J Med Inform. 2017;103:1–6. doi: 10.1016/j.ijmedinf.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Singh B., Singh A., Ahmed A. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egol A., Fromm R., Guntupalli K.K. Guidelines for intensive care unit admission, discharge, and triage: Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633–638. [PubMed] [Google Scholar]
- 24.Blanch L., Abillama F.F., Amin P. Triage decisions for ICU admission: report from the Task Force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2016;36:301–305. doi: 10.1016/j.jcrc.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Nates J.L., Nunnally M., Kleinpell R. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med. 2016;44(8):1553–1602. doi: 10.1097/CCM.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 26.Walter K.L., Siegler M., Hall J.B. How decisions are made to admit patients to medical intensive care units (MICUs): a survey of MICU directors at academic medical centers across the United States. Crit Care Med. 2008;36(2):414–420. doi: 10.1097/01.CCM.0000299738.26888.37. [DOI] [PubMed] [Google Scholar]
- 27.Kalb P.E., Miller D.H. Utilization strategies for intensive care units. JAMA. 1989;261(16):2389–2395. [PubMed] [Google Scholar]
- 28.Sirio C.A., Angus D.C., Rosenthal G.E. Cleveland Health Quality Choice (CHQC): an ongoing collaborative, community-based outcomes assessment program. New Horiz. 1994;2(3):321–325. [PubMed] [Google Scholar]
- 29.Shorr A.F. An update on cost-effectiveness analysis in critical care. Curr Opin Crit Care. 2002;8(4):337–343. doi: 10.1097/00075198-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport J., Teres D., Zhao Y., Lemeshow S. Length of stay data as a guide to hospital economic performance for ICU patients. Med Care. 2003;41(3):386–397. doi: 10.1097/01.MLR.0000053021.93198.96. [DOI] [PubMed] [Google Scholar]
- 31.Dasta J.F., McLaughlin T.P., Mody S.H., Piech C.T. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 32.Vincent J.L., Rubenfeld G.D. Does intermediate care improve patient outcomes or reduce costs? Crit Care. 2015;19(1):89. doi: 10.1186/s13054-015-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labata C., Oliveras T., Berastegui E. Intermediate care unit after cardiac surgery: impact on length of stay and outcomes. Rev Esp Cardiol (Engl Ed) 2018;71(8):638–642. doi: 10.1016/j.rec.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M., Shaffer V.L. Intensive care unit length of stay: benchmarking based on Acute Physiology and Chronic Health Evaluation (APACHE) IV. Crit Care Med. 2006;34(10):2517–2529. doi: 10.1097/01.CCM.0000240233.01711.D9. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman J.E., Kramer A.A. A model for identifying patients who may not need intensive care unit admission. J Crit Care. 2010;25(2):205–213. doi: 10.1016/j.jcrc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Gershengorn H.B., Wunsch H., Scales D.C., Zarychanski R., Rubenfeld G., Garland A. Association between arterial catheter use and hospital mortality in intensive care units. JAMA Intern Med. 2014;177(3):388–396. doi: 10.1001/jamainternmed.2014.3297. [DOI] [PubMed] [Google Scholar]
- 37.Goldfarb M., van Diepen S., Liszkowski M., Jentzer J.C., Pedraza I., Cercek B. Noncardiovascular disease and critical care delivery in a contemporary cardiac and medical intensive care unit. J Intensive Care Med. 2019;34(7):537–543. doi: 10.1177/0885066617741873. [DOI] [PubMed] [Google Scholar]
- 38.Holland E.M., Moss T.J. Acute noncardiovascular illness in the cardiac intensive care unit. J Am Coll Cardiol. 2017;69(16):1999–2007. doi: 10.1016/j.jacc.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Watson R.A., Bohula E.A., Gilliland T.C., Sanchez P.A., Berg D.D., Morrow D.A. Editor's Choice: Prospective registry of cardiac critical illness in a modern tertiary care cardiac intensive care unit. Eur Heart J Acute Cardiovasc Care. 2018;8(8):755–761. doi: 10.1177/2048872618789053. [DOI] [PubMed] [Google Scholar]
- 40.Berg D.D., Bohula E.A., van Diepen S. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12(3):e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]