Abstract
Background
Continuous positive airway pressure (CPAP) therapy is commonly used for respiratory failure due to severe COVID-19 pneumonitis, including in patients deemed not likely to benefit from invasive mechanical ventilation (nIMV). Little evidence exists demonstrating superiority over conventional oxygen therapy, whilst ward-level delivery of CPAP presents practical challenges. We sought to compare clinical outcomes of oxygen therapy versus CPAP therapy in patients with COVID-19 who were nIMV.
Methods
This retrospective multi-centre cohort evaluation included patients diagnosed with COVID-19 who were nIMV, had a treatment escalation plan of ward-level care and clinical frailty scale ≤ 6. Recruitment occurred during the first two waves of the UK COVID-19 pandemic in 2020; from 1st March to May 31st, and from 1st September to 31st December. Patients given CPAP were compared to patients receiving oxygen therapy that required FiO2 ≥0.4 for more than 12 hours at hospitals not providing ward-level CPAP. Logistic regression modelling was performed to compare 30-day mortality between treatment groups, accounting for important confounders and within-hospital clustering.
Findings
Seven hospitals provided data for 479 patients during the UK COVID-19 pandemic in 2020. Overall 30-day mortality was 75.6% in the oxygen group (186/246 patients) and 77.7% in the CPAP group (181/233 patients). A lack of evidence for a treatment effect persisted in the adjusted model (adjusted odds ratio 0.84 95% CI 0.57-1.23, p=0.37). 49.8% of patients receiving CPAP-therapy (118/237) chose to discontinue it.
Interpretation
No survival difference was found between using oxygen alone or CPAP to treat patients with severe COVID-19 who were nIMV. A high patient-initiated discontinuation rate for CPAP suggests a significant treatment burden. Further reflection is warranted on the current treatment guidance and widespread application of CPAP in this setting.
Funding
L Pearmain is supported by the MRC (MR/R00191X/1). TW Felton is supported by the NIHR Manchester Biomedical Research Centre.
Keywords: COVID-19, Non-invasive ventilation, Oxygen, nIMV, Invasive mechanical ventilation, Ceiling of care
Research in context.
Evidence before this study
We searched PubMed for English language articles up to 27/04/2021 with the terms “continuous positive airway pressure,” “CPAP” or “non-invasive respiratory support” and “COVID-19” or “SARS-COV-2”, and searched the ISRCTN clinicaltrials.gov, and clinicaltrialsregister.eu clinical trial registries. We also searched for relevant clinical guidelines from national and international organisations. One randomised controlled trial comparing continuous positive airway pressure (CPAP) to usual care (RECOVERY-RS) in the critical care setting is still recruiting. We did not find any evidence comparing CPAP to conventional oxygen therapy. Clinical guidelines differ in their view on the role of CPAP in COVID-19, and they acknowledge a paucity of evidence on which to base recommendations.
Added value of this study
This multicentre cohort evaluation is the first, to our knowledge, to compare CPAP to conventional oxygen therapy among patients who are ineligible for invasive mechanical ventilation (nIMV). Mortality rates amongst hypoxic nIMV patients were high and there was no difference between treatment groups, even though baseline patient characteristics were comparable between the groups. We also identified a high rate of CPAP discontinuation according to patient choice, suggesting an associated treatment burden.
Implications of all the available evidence
While CPAP can be delivered outside the critical care setting, its effectiveness amongst nIMV patients with respiratory failure due to COVID-19 is called into question. Further comparative evidence would be of value, including amongst patients who are candidates for invasive mechanical ventilation. Further work to understand and improve patient tolerance of CPAP is also needed.
Alt-text: Unlabelled box
1. Introduction
During the early months of the COVID-19 pandemic unprecedented numbers of patients presented to hospitals with acute respiratory failure. With intensive care services at risk of being overwhelmed, strategies were sought to reduce invasive mechanical ventilation (IMV). Whilst not previously a standard treatment for viral pneumonitis, early anecdotal accounts and preliminary data highlighted the use of continuous positive airway pressure (CPAP) in COVID-19 [1,2]. Subsequently it gained traction as an intervention that could be delivered outside of the intensive therapy unit (ITU) and high-dependency unit (HDU) [3], [4], [5].
Over a year later there remains an ongoing lack of high-quality evidence for the role of CPAP in respiratory failure due to COVID-19. Results from RECOVERY Respiratory Support (RECOVERY RS, international standardised control trial number 1691207) are eagerly anticipated, but will only address the role of CPAP and high-flow nasal oxygen (HFNO) in patients who are considered suitable for IMV [6]. Patients considered unlikely to benefit from IMV with a ward-level treatment escalation plan (hereafter termed nIMV) comprise the majority of hospitalised patients with COVID-19 and have greatest mortality and morbidity [7]. At the time of writing no registered trials of CPAP were found investigating its use in patients who are nIMV. Moreover, the current evidence base, upon which the use of CPAP is predicated, focuses on its role in patients suitable for IMV [8], [9], [10].To date, evidence generated during this pandemic comprises single-arm observational studies, or where early IMV, HFNO and CPAP were delivered simultaneously at study hospitals based solely upon clinician judgement, obscuring treatment effect [1,[8], [9], [10], [11], [12]]. Evidence prior to COVID-19 consists of small observational studies in viral pneumonitis or is extrapolated from trials of CPAP in surgery-associated acute respiratory distress syndrome [13], [14], [15]. Of the few studies to report mortality in patients who are nIMV the largest cohort in Italy (n=140) shows high mortality (73% 60-day mortality) [8]. No study that focuses on patients who are nIMV has a control comparison group [11,12,16].
In the UK, and several other countries, expert consensus was used to formulate national guidelines on the use of CPAP in COVID-19 pneumonitis patients [[3], [4], [5],17]. Practice has varied within the North-West region of England; some hospitals did not offer CPAP to patients who were nIMV, whilst others did. This was due to clinical equipoise, given the weakness of the quality of available evidence for CPAP benefit, and practical considerations regarding aerosol generation and infection control principles, nursing availability, availability of CPAP machines and oxygen pipeline capacity. Additional to the resource burden of delivering CPAP is the potential burden on individual patients.
Data on CPAP efficacy compared to ward-level oxygen delivery for patients who are nIMV with severe COVID-19 pneumonitis are urgently needed, both in countries with established consensus guidelines and resource-restricted countries with critical decisions on resource allocation. This multi-centre retrospective cohort evaluation had the objective of describing, evaluating and comparing the effect of treating patients who are nIMV with conventional ward-based oxygen or CPAP treatment. Primary outcomes of 30-day mortality and secondary outcomes of survival to discharge and survivor length of stay are compared between treatment groups. This study provides outcomes for the largest ward-level (not treated in ITU or HDU) cohort of patients who received CPAP therapy and considered unlikely to benefit from IMV. It is, as far as we are aware, the first comparison with an independent control group.
2. Methods
2.1. Study design
A retrospective multi-centre service evaluation was conducted to inform service delivery. We compared the outcomes of patients who received two different forms of respiratory support: ward-level oxygen therapy (excluding HFNO) and CPAP. The North West Collaborative Organisation for Respiratory Research (NWCORR) facilitated multi-centre collaboration. Institutional approval was gained at each hospital. Full ethics committee approval was not required, as confirmed by the NHS Health Research Authority decision tool [18]. Informed patient consent was not obtained as the study was observational in nature and no patient identifiable information was used. The following authors had access to the complete anonymised dataset: P Bradley, J Wilson, R Taylor and L Pearmain. All other authors had access to the dataset of patients within their institution.
Seven hospitals participated across the North West of England, representing varying local practice in the management of hypoxia in COVID-19. Two hospitals did not offer CPAP or HFNO to patients who were nIMV, while the other hospitals provided CPAP or CPAP with HFNO as second line therapy. The time periods captured were during ‘wave 1’ and ‘wave 2’ of the COVID-19 pandemic in the UK, taken as 1st March to 31st May 2020 and 1st September to 31st December 2020 respectively. The primary outcome measure was all-cause 30-day mortality. Secondary outcome measures were time to death and time from inclusion to discharge (termed time to discharge) in survivors.
All patients admitted within the defined time-period were included if they had (i) a primary diagnosis of COVID-19, (ii) hypoxia requiring treatment as below, and (iii) they were considered unlikely to benefit from intubation and invasive mechanical ventilation and had a ward-level ceiling of care (were nIMV). Patients were split into oxygen or CPAP groups based upon the treatment they received. Patients in the oxygen group were recruited from hospitals not offering CPAP or HFNO outside ITU and HDU. Patients in the CPAP group were recruited from hospitals offering this treatment at the ward-level. In the CPAP group, patients were included if they received CPAP treatment according to the five local hospital protocols; these protocols aligned closely with contemporaneous national CPAP guidance (Supplementary 1). Criteria for inclusion of patients in the oxygen group were selected to mirror consensus criteria for selecting patients to initiate CPAP therapy, meaning that in addition to criteria (i-iii) above they needed: (iv) hypoxia sufficient to have been managed with supplemental oxygen therapy with a fraction of inspired oxygen (FiO2) ≥ 0.4 for ≥ 12 hours, (v) clinical frailty scale score (CFS) ≤ 6 recorded in admission documentation or as determined by review of clinical notes [3,4].
Diagnosis of COVID-19 was either proven with polymerase chain reaction (PCR) testing for SARS-CoV-2 or considered likely according to clinical judgement, which was based upon radiological changes seen on chest radiographs and occasionally computed tomography imaging, consistent with COVID-19 pneumonitis, combined with typical signs or symptoms of COVID-19. All COVID-19 inpatients at the seven hospitals within the study inclusion dates were screened to see if they met inclusion criteria.
2.2. Data collection
Data collection was performed retrospectively by interrogation of clinical records. Records were reviewed at least 30 days from the date each patient met inclusion criteria to capture mortality. Baseline data collected includes patient age, ethnicity, obesity (body mass index (BMI) ≥ 30kg/m2) and comorbidities. Blood results for urea and C-reactive protein (CRP) were recorded at the time-point patients met the inclusion criteria. All patients had the vital signs of pulse oximetry (SpO2), FiO2 and respiratory rate (RR) recorded from observations captured prior to inclusion. Additionally, initial and maximum CPAP pressures, and maximum FiO2, were recorded. CPAP machines included Respironics A40 (Phillips, USA), ResMed Airsense (ResMed, Aus) and Sleepcube (DeVilbiss, UK) that utilised entrained oxygen across three hospitals and Trilogy 202 (Phillips, USA) that utilised blended oxygen across two hospitals. It was recorded when CPAP treatment was discontinued due to patient wishes; when this was not the case, CPAP discontinuation is categorised as clinician-decided, whether due to successful weaning or futility. Provision of palliative care interventions and/or specialist consultation was recorded.
2.3. Statistical analysis
Unadjusted associations between 30-day mortality and explanatory variables, including treatment, were assessed with univariable logistic regression modelling. A Kaplan-Meier curve was created to illustrate the overall survival by treatment group and the survival difference tested using a log-rank test. Within the CPAP-group descriptive analysis was performed and then stratified by (i) 30-day mortality, and (ii) decision to discontinue CPAP treatment.
The independent relationship between treatment group and 30-day mortality was assessed using a Generalised Estimating Equations (GEE) approach with exchangeable working correlation structure to account for potential clustering by hospital [19]. This method assumes that patients within a hospital are equally correlated with each other, and as a marginal model it provides a population average interpretation: the effect on mortality rate of treating the whole eligible cohort with CPAP. A saturated multivariable model was fitted and then refined using the following principles: retention of key demographics (age, sex); retention of study inclusion criteria dependent variables (FiO2, CFS); retention of any unadjusted variable identified as significant (p < 0.1); the principle of parsimony. The Quasi-likelihood under Independence Model Criterion (QIC), as a quasi-likelihood alternative to Akaike's Information Criterion, is also used to guide decisions [19,20]. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) are reported.
Sensitivity analyses are conducted to test the stability and robustness of the final candidate explanatory model. This included: (i) excluding patients in the oxygen-group with maximum FiO2 < 0.60, (ii) excluding patients in the CPAP-group with duration of use < 24 hours, (iii) excluding any patients with prolonged time from admission to inclusion, (iv) including an obesity-age interaction term (Supplementary 8 a-e).
Selection bias was minimised by (i) aligning inclusion criteria at oxygen-group sites with CPAP-group sites and national guidelines, (ii) selecting oxygen-group and CPAP-group patients from sites only offering that treatment (avoiding clinician bias) (iii) multiple study sites, (iv) employing a modelling approach that adjusts for important predictors of mortality, and recognises the clustering by hospital site, and (v) performing sensitivity analyses to account for differences in baseline characteristics and model selection.
Patients were assigned a co-morbidity score based upon their number of co-morbidities. CFS and co-morbidity scores were modelled as ordinal variables, with previously utilised groupings explored in sensitivity model analysis (sensitivity analyses (v) and (vi) respectively) (Supplementary 8 e-f) [7,21,22]. Variables are assessed for collinearity using correlation matrices (Supplementary 3). Statistical tests were two-tailed with statistical significance defined at 5%. Data analysis was performed in R (version 4.0.3) using R Studio (version 1.4.1103) with the ‘geepack’ package [23], [24], [25].
3. Role of the funding source
No funding source had any role in the design, collection, analysis, interpretation or preparation of the manuscript.
4. Results
Across the seven hospitals 492 patients were identified for inclusion in the study, with 13 (2.6%) excluded from the CPAP group due to pre-treatment FiO2 < 35% (9/13) and CFS > 6 (4/13) (Supplementary 2). The final study population (N = 479) was 64% male, mean age 77 years (IQR 71-83), 93% of white ethnicity and median CFS 5 (4-5 IQR) (Table 1). There were 246 patients included in the oxygen group (51.4%) and 233 in the CPAP group (48.6%). All patients were followed up for 30 days or until death occurred, with median follow-up time of 5 days (IQR 2-23.5 days). No patients were lost to follow-up.
Table 1.
Study population demographics stratified by oxygen treatment and CPAP treatment. 1n (%); Median (IQR). 2Fisher's exact test; Wilcoxon rank sum test. Abbreviations used: HIV, human immunodeficiency virus; CFS, clinical frailty scale; CRP, C-reactive protein; FiO2, fraction of inspired oxygen; RR, respiratory rate; SpO2, pulse oximetry.
| Variable | Overall, N = 4791 | Treatment group |
p-value [2] | |
|---|---|---|---|---|
| Oxygen N = 2461 | CPAP N = 2331 | |||
| Ethnicity | 0.3 | |||
| Black | 8 (1.7%) | 6 (2.4%) | 2 (0.9%) | |
| Chinese | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| South Asian | 26 (5.4%) | 15 (6.1%) | 11 (4.7%) | |
| White | 443 (93%) | 223 (91%) | 220 (94%) | |
| Age | 77 (71, 83) | 78 (72, 85) | 77 (70, 82) | 0.004 |
| Sex (male) | 307 (64%) | 151 (61%) | 156 (67%) | 0.2 |
| Comorbidities | ||||
| Cardiac | 334 (70%) | 186 (76%) | 148 (64%) | 0.004 |
| Respiratory | 171 (36%) | 87 (35%) | 84 (36%) | 0.9 |
| Chronic kidney disease | 74 (15%) | 34 (14%) | 40 (17%) | 0.3 |
| Liver | 28 (5.8%) | 12 (4.9%) | 16 (6.9%) | 0.4 |
| Dementia | 19 (4.0%) | 11 (4.5%) | 8 (3.4%) | 0.6 |
| Neurological | 74 (15%) | 35 (14%) | 39 (17%) | 0.4 |
| Obesity | 138 (29%) | 79 (32%) | 59 (25%) | 0.10 |
| HIV | 3 (0.6%) | 0 (0%) | 3 (1.3%) | 0.11 |
| Diabetes | 158 (33%) | 92 (37%) | 66 (28%) | 0.035 |
| Cancer | 92 (19%) | 40 (16%) | 52 (22%) | 0.093 |
| Comorbidity score (grouped) | 0.3 | |||
| A (0,1) | 150 (31%) | 70 (28%) | 80 (34%) | |
| B (2) | 167 (35%) | 93 (38%) | 74 (32%) | |
| C (3-5) | 162 (34%) | 83 (34%) | 79 (34%) | |
| CFS | 0.4 | |||
| 2-3 | 116 (24%) | 59 (24%) | 57 (24%) | |
| 4 | 113 (24%) | 60 (24%) | 53 (23%) | |
| 5 | 152 (32%) | 84 (34%) | 68 (29%) | |
| 6 | 98 (20%) | 43 (17%) | 55 (24%) | |
| Urea (mmol/L) | 9 (6, 13) | 10 (6, 14) | 8 (6, 13) | 0.13 |
| CRP (mg/L) | 131 (78, 196) | 118 (66, 189) | 146 (89, 206) | 0.008 |
| Inclusion FiO2 (grouped) | <0.001 | |||
| 0.4-0.59 | 154 (32%) | 103 (42%) | 51 (22%) | |
| 0.6-0.79 | 123 (26%) | 58 (24%) | 65 (28%) | |
| 0.8 | 202 (42%) | 85 (35%) | 117 (50%) | |
| Inclusion RR (breaths/minute) | 24 (20, 28) | 22 (20, 26) | 26 (22, 30) | <0.001 |
| Inclusion SpO2 (%) | 92 (88-94) | 92 (90, 94) | 92 (88, 95) | 0.2 |
| Duration: admission to inclusion (days) | 2 (0, 5) | 1 (0, 5) | 2 (0, 4) | 0.5 |
4.1. Baseline characteristics
Baseline characteristics between the two groups are described in Table 1, with statistically significant increases in median age in the oxygen group (78y (IQR 72-85) vs CPAP group (77y (70-82), p = 0.004), and prevalence of cardiovascular morbidity (76% in oxygen group vs 64% in CPAP group, p = 0.004). The median time from admission to treatment, defined as requiring FiO 2 ≥ 0.4 or CPAP initiation, was 1 day (IQR 0-5) and 2 days (IQR 0-4) respectively (p = 0.5). Precedent RR 22/minute (IQR 20-26) vs 26/minute (IQR 20-30), (p < 0.001) and FiO2 (see Table 1, p < 0.001) differed between oxygen group and CPAP group respectively.
4.2. Analysis of survival: unadjusted model
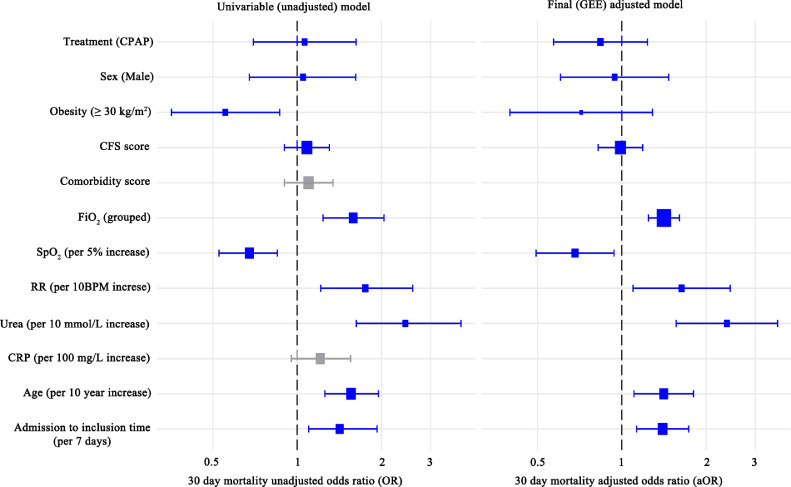
Overall 30-day mortality was 76.2%. Treatment had no unadjusted association with mortality (OR 1.06 CI 0.7-1.62 p = 0.78, Figure 1). Advancing age (OR 1.56 per 10-year increase in age, CI 1.26-1.95, p < 0.001) was significantly associated with increased 30-day mortality, as was increasing urea (OR 2.44 per 10 mmol/L-increase in urea, CI 1.63-3.85, p < 0.001) and lower SpO2 (OR 0.68 per 5%-SpO2 increase, CI 0.53-0.85, p = 0.001). Conversely patients with obesity had significantly lower unadjusted mortality (OR 0.55, CI 0.36-0.87, p = 0.009). Worsened 30-day mortality was also associated with: longer time to inclusion (OR 1.42 per week increase CI 1.10-1.93, p = 0.014); higher pre-inclusion RR (OR 1.75 per 10-breath per minute increase, CI 1.21-2.59, p = 0.004); higher inclusion FiO2 (OR 1.59 per group increase (see Table 1), CI 1.24-2.04, p = 0.003). Patient characteristics stratified by survival are shown in Supplementary 7.
Figure 1.
30 day mortality unadjusted odds ratios (OR, left) of variables and final Generalised Estimating Equations (GEE) model with estimated 30-day mortality adjusted odds ratios (aOR, right). Variables coloured grey were not included in the final GEE model. An OR>1 represents increased occurrence of 30 day mortality. Abbreviations used: FiO2, fraction of inspired oxygen; RR, respiratory rate; SpO2, pulse oximetry; CRP, C-reactive protein; BMI, body mass index; CFS, clinical frailty scale. Blue variables were subsequently used in the final adjusted OR model.
We included 171 patients from wave 1 and 308 from wave 2. There was no significant difference in 30-day mortality (OR 1.04, 95% CI 0.68-1.58, p=0.87) or in survival time (p=0.55, Supplementary 4) between the first two waves of the UK pandemic. Hospital site conferred no significant association with 30-day mortality, time to discharge or time to death (p=0.18, Supplementary 5).
4.3. Effect of treatment on survival
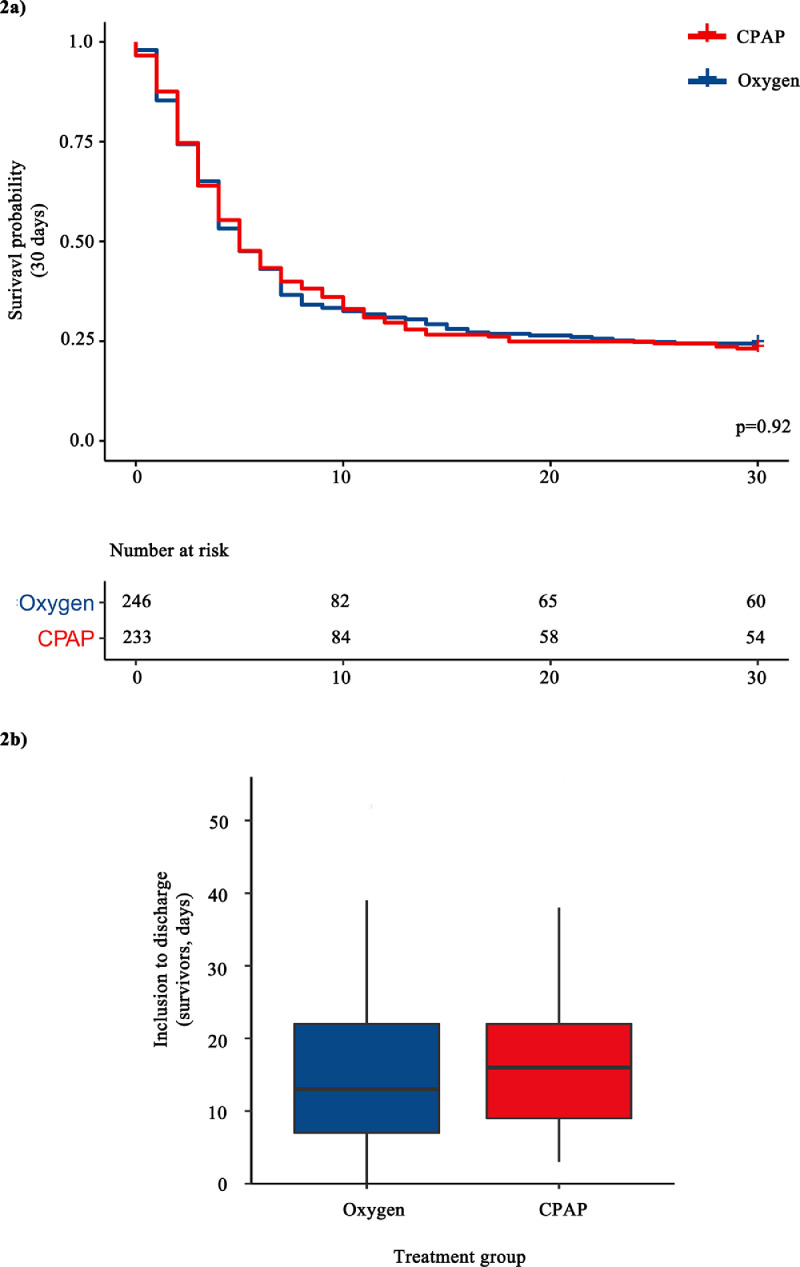
30-day mortality was 75.6% (186/246) in the oxygen group and 77.7% (181/233) in the CPAP group (Pearson's χ2, p = 0.8). No significant difference was seen between treatment groups in the secondary outcomes of survival time (log-rank p = 0.92) or length of stay of survivors from inclusion (log-rank p = 0.39), as shown in Figure 2 a-b.
Figure 2.
a) 30-day survival curve analysis stratified by treatment (unadjusted) with log-rank test p-value. There was no censored data at any time-point, b) Box and whisker plot showing time from inclusion to discharge of survivors (days). Boxes are bound by the upper and lower quartiles, whilst whiskers are bounded by these ± 1.5x the interquartile range.
The final explanatory model is presented in Figure 1. The model refinement process is presented in Supplementary 6. Adjusted treatment OR (aOR 0.84 CI 0.57-1.23, p = 0.37) was not significantly different to unadjusted OR (OR 1.06 CI 0.70-1.62 p = 0.78). Increasing age (aOR 1.41 per 10-year increase CI 1.11-1.80, p < 0.01), time to treatment initiation (aOR per 7 days 1.41 CI 1.11-1.73, p = 0.0021), RR pre-treatment (aOR 1.64 per 10-breath per minute increase CI 1.10-2.44, p = 0.016), SpO2 pre-treatment (aOR 0.68 per 5% increase CI 0.49-0.94, p=0.019), and urea (aOR 2.37 per 10 mmol/L-increase CI 1.56-3.60, p < 0.0001) all remained significantly associated with worsened survival. There was no significant survival association with obesity (p = 0.26), which was weakened when analysed in the context of other variables. The presence of comorbidities was not significantly associated with mortality (ungrouped p = 0.91, grouped p = 0.57).
In sensitivity analyses using the final model (Supplementary figure 8 a-d) there was no treatment association with 30-day mortality when: i) patients with maximum FiO2 < 0.60 were excluded (p = 0.12); ii) patients with CPAP duration < 24 hours were excluded (p = 0.79); iii) patients with time to inclusion > 5 days (upper quartile of CPAP group) were excluded (p = 0.42); iv) when the age-obesity interaction term was included in the final model (p = 0.37) (nor was there a significant interaction between the two variables, p = 0.35). Finally grouping of (v) baseline co-morbidities and (vi) CFS were explored (Supplementary figure 8 e-f) [21,22]. Findings did not change in any sensitivity analysis conducted (Supplementary figure 8 a-f).
4.4. CPAP treatment: characterisation and experience
The baseline characteristics of patients treated with CPAP are seen in Table 1. Discontinuation decisions were classified as clinician-initiated or patient-initiated (see Table 2): 49.8% (116/233) of CPAP discontinuation was initiated by clinicians and 50.2% (117/233) by patients (range across hospitals 44-60%). Palliative care was accessed for 48.9% (114/233) of CPAP patients and 49.6% (122/246) of oxygen group patients.
Table 2.
Patient Characteristics of CPAP treatment and subgroups. 1n (%); Median (IQR). 2Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test. Abbreviations used: FiO2, fraction of inspired oxygen; RR, respiratory rate; SpO2, pulse oximetry; CFS, clinical frailty scale.
| Variable | Overall CPAP group N = 2331 |
CPAP Treatment sub-group |
p-value [2] | |
|---|---|---|---|---|
| CPAP, clinician discontinued N =116 [1] |
CPAP, patient discontinued N =117 [1] |
|||
| Death within 30 days | 179 (77%) | 83 (72%) | 96 (82%) | 0.058 |
| Time from inclusion to discharge (of survivors, days) | 16 (9, 22) | 16 (12, 23) | 13 (6, 18) | 0.12 |
| Palliative care accessed | 111 (49%) | 51 (44%) | 60 (53%) | 0.2 |
| Initial CPAP settings | ||||
| Pressure (cm H2O) | 10 (5, 10) | 10 (5, 10) | 10 (5, 10) | 0.5 |
| FiO2 | 50 (38, 53) | 53 (38, 53) | 49 (38, 53) | 0.8 |
| Maximum CPAP settings | ||||
| Pressure | 10 (10, 12) | 10 (10, 14.2) | 10 (10, 12) | 0.011 |
| FiO2 | 53 (46, 56) | 53 (48, 56) | 53 (46, 60) | 0.4 |
| Duration of CPAP use (days) | 3 (1, 5) | 3 (1, 7) | 2 (1, 4) | 0.014 |
The 30-day mortality of clinician-discontinuation and patient-discontinuation subgroups was 71.6% and 82.1% respectively, p = 0.058 (Table 2). Survival time from inclusion was not significantly different when stratified by decision to discontinue treatment: clinician-discontinued 4 days (median, IQR 2-7), patient-discontinued 4 days (median, IQR 2-7), p = 0.9. Additional demographics are presented in Supplementary 9.
5. Discussion
This multi-centre evaluation is the first to report outcomes in patients not likely to benefit from invasive mechanical ventilation (nIMV) treated with conventional ward-level oxygen compared to CPAP therapy for COVID-19 respiratory failure. We found no significant association between 30-day mortality and treatment group, even after adjustment for important confounders. Given the resources required to provide CPAP it raises the question as to whether it should be provided to patients who are nIMV, which has been commonplace during the COVID-19 pandemic. Importantly, however, we found no evidence of worsened outcomes in patients treated with CPAP.
Patient-initiated CPAP discontinuation rate was high across all study hospitals (50.2% range 44-60%) despite being delivered by respiratory specialist teams experienced in providing CPAP, alongside readily available palliative care interventions. Patients who discontinued their CPAP treatment did so after a reasonable duration of use (median 2 days, IQR 1-4) and reached therapeutic maximal pressures. Sensitivity analyses were further conducted to exclude patients with a short CPAP treatment duration (< 24 hours): there was still no association between treatment and mortality. This suggests that patient discontinuation of CPAP is unlikely to be the reason for a lack of superiority. Furthermore, the high patient-discontinuation rate implies a high treatment burden which requires further research.
Our mortality in patients treated with CPAP therapy is remarkably similar to smaller studies in Italy, as well as in the UK [8,26]. Our study population demographics are broadly similar to those reported in other large UK studies [7]. Additional reassurance in the study design and model used is provided by finding that several variables predictive of mortality, included in the ISARIC 4C study and mortality risk score, had their association with mortality replicated here [22].
Although treatments were provided at separate hospitals, patient demographics were similar between treatment groups, and measures have been taken to account for within-hospital clustering (GEE model). Furthermore, for any variables that had significantly different distribution between treatment groups (p < 0.1), analysis was undertaken both with and without these variables in the adjusted OR model, with no substantive differences in findings. Using a pragmatic study design, data were obtained from routinely recorded clinical information; consequently our findings are reflective of real-world practice.
Whilst the lack of significant association between CFS and mortality is intuitively surprising, our study protocol (patients were nIMV and CFS ≤ 6) selected for a narrow range of clinical frailty (median CFS 5, IQR 4-5). Moreover, no significant association with mortality has been found when comparing fitter patients (CFS 1-3) with mildly frail patients (CFS 4-5) under 65 years of age [21]. This is mirrored in other acute illnesses where over-65 year-old patient cohorts with CFS 4-5 have been shown to have poor predictive value in 6- and 12-month mortality [27]. This has raised concern regarding the use of CFS to weight treatment decisions in this patient group during the COVID-19 pandemic [27].
The difference in RR and FiO2 between treatment groups at inclusion is likely to represent pragmatic differences in the initiation of CPAP and oxygen. Conventional oxygen therapy is generally escalated reflexively in response to desaturation below a predetermined target SpO2. In practice, CPAP initiation does not happen as quickly: the decision to initiate CPAP is made by a senior physician after clinical review following deterioration; at times there may be an inclination to delay CPAP once FiO2 0.4 is reached, giving them a period of further observation in the hope of improvement; and setting up CPAP requires the practicalities of preparing equipment and often moving a patient to a suitable bedspace where it can be delivered. We observed only 9/246 patients in the oxygen treatment group with a maximal FiO2 < 0.60, indicating that this group did not disproportionately capture patients with mild disease. Sensitivity analysis, when excluding patients in the oxygen group with maximal FiO2 < 0.60, showed no significant difference in treatment effect to that presented in the final model.
To ensure the rigour of the study findings, the stability and robustness of the final candidate explanatory model was tested with a sensitivity analysis that excludes any important patient groups that may result in underestimating the effect of CPAP therapy. This included: (i) excluding patients in the oxygen group with maximum FiO2 < 0.60, a proportion of whom may not have been included in the CPAP treatment group if they did not exhibit increased respiratory effort; (ii) excluding patients with a short duration of CPAP therapy, as they may not have achieved any potential CPAP efficacy; (iii) excluding any patients with time from admission to inclusion > 7 days, as prolonged admission to inclusion time may indicate hospital acquired infection; (iv) the authors noted the presence of a relatively younger and more obese group of patients in the CPAP group with favourable survival. To account for any potentially significant impact of obesity on the relationship between treatment group and 30-day mortality, an obesity-age interaction term is included. The lack of change in treatment effect aOR in all the sensitivity analyses conducted indicates the model is robust when stressed.
A limitation of this evaluation is that it is observational. However, a large randomised control trial including this population may not be performed, particularly as vaccination begins to reduce the prevalence of severe COVID-19. Treatment groups were determined by treatment policies at each hospital rather than case-by-case clinician judgement, so physician-level selection bias is minimised. Nonetheless, individual clinician (or departmental) ITU selection may affect the characteristics of the nIMV study groups presented here. However, the similar demographics seen between treatment groups and the multi-centre design of the study greatly reduces the likelihood major study findings are affected. Whilst we do not report specifically upon the severity of radiological changes in patients, it does not have a role in CPAP treatment decisions. Furthermore, radiology was reviewed as part of the diagnostic process by treating clinicians. The assessment of inflammatory markers was limited to CRP as procalcitonin was not routinely used at study hospitals. Patients underwent blood gas analysis to confirm acute hypoxaemic respiratory failure, however this was not done through standardised methods (capillary or arterial sampling) or timing, meaning results could not be reliably compared in our evaluation. Serial arterial blood gas analyses are not routine practice in the UK in nIMV patients outside critical care settings, and for this reason it was not possible to consistently report, or incorporate into the inclusion criteria, the presence of acute respiratory distress syndrome. Other non-invasive physiological parameters (for example, ROX scores) are utilised in our nIMV cohort, as recommended in national and international guidelines [3,4,17]. Devices used to deliver CPAP include those that provide entrained oxygen, as detailed in the methods, which are capable of delivering a lower theoretical maximal FiO2 than others within the study that provide blended oxygen. This represents the machines available at the time in this real-world study and did not appear to have a large impact upon mortality at sites that predominantly used one type of machine.
Some caution should be applied to these findings, as there may be patient sub-groups who benefit from CPAP in the setting of COVID-19. There have been no studies looking at predictors of CPAP efficacy in this patient cohort to date, and our study was not designed to address this question. Furthermore, the scope of this study is to assess the efficacy of CPAP compared to conventional oxygen therapy according to current treatment guidelines in the COVID-19 patient population who are nIMV. Whilst sequential administration of CPAP after failure of oxygen therapy has been described elsewhere in an nIMV cohort with similar mortality (71.5%) we are unable to draw conclusions about whether other CPAP treatment regimens may have a mortality effect (e.g. sequential or earlier CPAP use) [28].
It has been suggested in very small studies that increased BMI may be positively associated with CPAP response, with large studies suggesting an obesity survival paradox in the general COVID-19 hospital population [29,30]. Whilst obesity is a significant factor in improved unadjusted mortality in our data (OR 0.54, CI 0.36-0.87, p < 0.01) this was seen across treatment groups (oxygen group OR 0.52, CI 0.29-0.96 vs CPAP OR 0.60, CI 0.31-1.18). In the final model we find that obesity is no longer significantly associated with mortality. This is likely due to negative confounding from age, with age positively correlated with mortality, and negatively correlated with obesity. Obesity predisposes to more severe COVID-19 infection, however the current lack of robust evidence for a difference in mortality outcome for hospitalised obese patients receiving CPAP or IMV compared to non-obese counterparts mean it should not be used as the basis for formulating treatment decisions [30,31].
In summary, this study shows no evidence of a survival advantage from CPAP treatment as a ceiling of care in severe COVID-19 pneumonitis when compared to conventional oxygen therapy amongst patients who were nIMV. CPAP administration in the ward setting has been demonstrated to be feasible, but it has high mortality and may bring a significant treatment burden to patients. This invites pause for reflection on whether the current treatment guidance and widespread application of CPAP in this setting is appropriate.
Funding
L Pearmain is supported by the MRC (MR/R00191X/1). TW Felton is supported by the NIHR Manchester Biomedical Research Centre.
Data sharing statement
Data requests will be considered and should be addressed to the corresponding author. Any request would be subject to institutional approval.
Author contributions
PB, LP & JN conceived and designed the study. PB, JW, JN, JR, PW, LP, MG, KK, AH, PD, CW, RCR, CL, ND, DSHM, JC, MSD, SR, MM, HA, KS, MH, DG acquired data. PB, JW, LP, PW, TG, AA, JN,TB verified underlying data. JW, PB, LP, JN, PB TB, TG, TWF, NC, AA, EN, AB contributed to data interpretation. JW, LP, PB & RT designed and conducted the analysis. LP, JW and PB drafted the manuscript and LP, PB, JW, NC, TWF, TG, TB and AB edited the manuscript. All authors reviewed and approved the final manuscript.
Declaration of Competing Interest
AB reports fees for speaking/session chair for Fisher and Paykel webinars (high nasal flow cannulae). AB is an advisory board member for Sanofi Genzyme (respiratory management of Pompe disease). All other authors have nothing to declare.
Acknowledgments
The authors would like to acknowledge the NWCORR for providing the platform enabling this collaboration. Additionally, we would like to thank the staff in all study hospitals for their dedication and care towards our patients. Finally, we would like to take the opportunity to remember our patients included in this work, many of whom are sadly no longer with us.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101122.
Appendix. Supplementary materials
References
- 1.Wang D, Hu B, Hu C. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - Journal of the American Medical Association. 2020;323 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitacca M, Nava S, Santus P, Harari S. Early consensus management for non-ICU acute respiratory failure SARS-CoV-2 emergency in Italy: From ward to trenches. European Respiratory Journal. 2020;55 doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network (SIGN). COVID-19 position statement: CPAP for COVID-19 related respiratory failure [Internet]. 2020 [updated 2020 Sept, cited 2021 July]. Available from http://www.sign.ac.uk.
- 4.NHS England and NHS Improvement. Guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected) [Discontinued] [Internet]. 2020 [updated 2020 Apr, cited 2021 Jul]. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-NIV-respiratory-support-and-coronavirus-v3.pdf.
- 5.World Health Organisation (WHO). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance [Internet]. 2019 [updated 2020 Mar, cited 2021 Jul]. Available from: https://apps.who.int/iris/handle/10665/331446.
- 6.Perkins GD, Couper K, Connolly B. RECOVERY- Respiratory Support: Respiratory Strategies for patients with suspected or proven COVID-19 respiratory failure; Continuous Positive Airway Pressure, High-flow Nasal Oxygen, and standard care: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:687. doi: 10.1186/s13063-020-04617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. The BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaschetto R, Barone-Adesi F, Racca F. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Research. 2021;7 doi: 10.1183/23120541.00541-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aliberti S, Radovanovic D, Billi F. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. European Respiratory Journal. 2020;56 doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco C, Facciolongo N, Tonelli R. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. European Respiratory Journal. 2020;56 doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashish A, Unsworth A, Martindale J. CPAP management of COVID-19 respiratory failure: A first quantitative analysis from an inpatient service evaluation. BMJ Open Respiratory Research. 2020;7 doi: 10.1136/bmjresp-2020-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns GP, Lane ND, Tedd HM. Improved survival following ward-based non-invasive pressure support for severe hypoxia in a cohort of frail patients with COVID-19: Retrospective analysis from a UK teaching hospital. BMJ Open Respiratory Research. 2020;7 doi: 10.1136/bmjresp-2020-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faria DAS, da Silva EMK, Atallah ÁN, Vital FMR. Noninvasive positive pressure ventilation for acute respiratory failure following upper abdominal surgery. Cochrane Database of Systematic Reviews. 2015;2015 doi: 10.1002/14651858.CD009134.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Hua S, Peng L. The application of bi-level positive airway pressure in patients with severe pneumonia and acute respiratory failure caused by influenza A (H1N1) virus. Journal of Thoracic Disease. 2010;2 doi: 10.3978/j.issn.2072-1439.2010.02.03.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vdoushkina E, Chernogayeva G, Povalyaeva L, Borodulina E. European Respiratory Journal Conference: European Respiratory Society Annual Congress. 2014. Noninvasive ventilation support in treatment of pneumonia A (H1N1) p. 44. [Google Scholar]
- 16.Bradley P, Nixon J, Wilson J. Continuous positive airway pressure (CPAP) as a ceiling of care treatment for hypoxemic respiratory failure due to COVID-19. Journal of the Intensive Care Society. 2021 doi: 10.1177/1751143721996538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Australian and New Zealand Intensive Care Society (ANZICS) ANZICS; Melbourne: 2020. ANZICS COVID-19 Guidelines.https://www.anzics.com.au/coronavirus-guidelines/ Version 3.[updated 2020 Oct, cited 2021 Jul]. Available from: [Google Scholar]
- 18.NHS Health Research Authority. Decision tool: do I need NHS Research Ethics Committee review? [cited 2021 Jul]. Available from: http://www.hra-decisiontools.org.uk/ethics/.
- 19.Zeger SL, Liang K-Y, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics. 1988;44 doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- 20.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57 doi: 10.1111/j.0006-341X.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 21.Sablerolles RSG, Lafeber M, van Kempen JAL. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. The Lancet Healthy Longevity. 2021;2 doi: 10.1016/s2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight SR, Ho A, Pius R. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. The BMJ. 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team . R Foundation for Statistical Computing; 2019. R: A language and environment for statistical computing. [Google Scholar]
- 24.Højsgaard S, Halekoh U, Yan J. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2006 CRAN. [Google Scholar]
- 25.Allaire JJ. RStudio: Integrated development environment for R. The Journal of Wildlife Management. 2015;75 [Google Scholar]
- 26.John C, Crickett R, Owen W, et al. P58 Reviewing the role of continuous positive airway pressure (CPAP) in patients with severe COVID-19: a multi-site observational study. 2021. DOI:10.1136/thorax-2020-btsabstracts.203.
- 27.Chong E, Chan M, Tan HN, Lim WS. COVID-19: Use of the Clinical Frailty Scale for Critical Care Decisions. Journal of the American Geriatrics Society. 2020;68 doi: 10.1111/jgs.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppadoro A, Benini A, Fruscio R. Helmet CPAP to treat hypoxic pneumonia outside the ICU: an observational study during the COVID-19 outbreak. Critical Care. 2021;25 doi: 10.1186/s13054-021-03502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah A, Naidu S, Saigal A, Hurst J, Lipman M, Mandal S. P56 Higher body mass index (BMI) is associated with improved continuous positive airway pressure (CPAP) outcomes in patients with hypoxic respiratory failure secondary to COVID-19. 2021. DOI:10.1136/thorax-2020-btsabstracts.201.
- 30.Helvaci N, Eyupoglu ND, Karabulut E, Yildiz BO. Prevalence of Obesity and Its Impact on Outcome in Patients With COVID-19: A Systematic Review and Meta-Analysis. Frontiers in Endocrinology. 2021;12 doi: 10.3389/fendo.2021.598249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh R, Garcia MA, Rajendran I, et al. ICU outcomes in Covid-19 patients with obesity. Therapeutic Advances in Respiratory Disease. 2020; 14. DOI:10.1177/1753466620971146. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.