Abstract
Patients with severe asthma have unmet clinical needs for effective and safe therapies. One possibility may be mesenchymal stem cell (MSC) therapy, which can improve asthma in murine models. However, it remains unclear how MSCs exert their beneficial effects in asthma. Here, we examined the effect of human umbilical cord blood-derived MSCs (hUC-MSC) on two mouse models of severe asthma, namely, Alternaria alternata-induced and house dust mite (HDM)/diesel exhaust particle (DEP)-induced asthma. hUC-MSC treatment attenuated lung type 2 (Th2 and type 2 innate lymphoid cell) inflammation in both models. However, these effects were only observed with particular treatment routes and timings. In vitro co-culture showed that hUC-MSC directly downregulated the interleukin (IL)-5 and IL-13 production of differentiated mouse Th2 cells and peripheral blood mononuclear cells from asthma patients. Thus, these results showed that hUC-MSC treatment can ameliorate asthma by suppressing the asthmogenic cytokine production of effector cells. However, the successful clinical application of MSCs in the future is likely to require careful optimization of the route, dosage, and timing.
Keywords: cell therapy, innate lymphoid cells, mesenchymal stem cells, severe asthma, Th2 cells
INTRODUCTION
Asthma is a chronic inflammatory disease with symptoms of shortness of breath, dyspnea, and coughing. It is not only the most common chronic airway disease, its prevalence is still rising in many parts of the world (Lundback et al., 2016). Asthma treatment is based on bronchodilators, which provide short-term symptom relief, and corticosteroids that depress the inflammatory responses (McCracken et al., 2017). However, some patients have difficulty controlling their symptoms even if they adhere closely to such standard treatment regimens. Therefore, new treatment regimens are needed for poorly controlled asthma, and one possibility is mesenchymal stem cell (MSC) therapy.
MSCs (also known as mesenchymal stromal cells) are heterogeneous populations of cells that can be obtained from various sources, including bone marrow, adipose tissue, and umbilical cord blood (Keating, 2012; Ryu et al., 2013). MSCs can replace damaged tissues and regulate immune reactions. The immunoregulatory role of MSCs is particularly promising for inflammation-related conditions such as asthma and has consequently attracted more attention than its regenerative role. The immunomodulatory activities of MSCs are exerted by cell-to-cell contact and paracrine effects through the release of soluble mediators like cytokines or extracellular vesicles (Fan et al., 2020). These features make MSCs an attractive potential therapeutic target for inflammatory diseases. Moreover, many studies show that the transfer of MSCs to human patients and rodent models can have beneficial effects in a variety of autoimmune diseases (Chae et al., 2021; Kim et al., 2016; Munir and McGettrick, 2015). Several studies have shown that MSC transfer also reduces lung inflammation and tissue remodeling in allergic asthma (Hong et al., 2017; Sun et al., 2012). This effect is mediated by several critical mechanisms. First, MSCs reduce interleukin (IL)-4, IL-5, or IL-13 expression, thereby mitigating asthmogenic type 2 inflammation (Castro et al., 2020; Goodwin et al., 2011). Second, MSCs activate regulatory T cells (Treg), which produce anti-inflammatory cytokines such as IL-10 and transforming growth factor beta (TGF-β) that suppress airway inflammation (Kavanagh and Mahon, 2011; Nemeth et al., 2010). Third, MSCs can regulate the functions of dendritic cells (DCs) and macrophages, which play essential roles in asthma. Specifically, Cahill et al. (2015) showed that MSCs inhibit the maturation of DCs in peripheral blood mononuclear cells (PBMCs) from asthma patients; the resulting semi-mature DCs then promoted the expansion of Treg. MSCs also cause macrophages to shift from the M1 phenotype to the M2 phenotype, which attenuates asthma (Braza et al., 2016; Song et al., 2015). Finally, MSCs reduce airway remodeling by inhibiting the mucus production of goblet cells and airway smooth muscle cell proliferation (Lee et al., 2011).
Thus, previous studies have provided important insights into how MSC transfer may ameliorate asthma. However, the precise details of these mechanisms and the roles of immune cells other than T cells, DCs, and macrophages remain to be explored. This is particularly true for type 2 innate lymphoid cells (ILC2s), which have been shown recently to play important roles in asthma (Kim et al., 2016; 2021). We, therefore, explored the effect of transferring human umbilical cord blood-derived MSCs (hUC-MSCs) into two different mouse models of severe asthma; namely, asthma induced by Alternaria alternata and asthma generated by a combination of house dust mite (HDM) and diesel exhaust particles (DEP). Our results showed that MSCs directly reduced the IL-5 and IL-13 production from both Th2 cells and ILC2s. However, the effectiveness of MSCs was largely dependent on treatment dosage, timing, and routes. Co-culture of MSCs with differentiated mouse T cells or PBMCs from asthma patients inhibits cytokine productions, including IL-5 and IL-13. Therefore, our results suggest that MSCs are potent therapy for airway inflammation of severe asthmatic patients by directly modulating both T cells and ILCs.
MATERIALS AND METHODS
Mice
Female BALB/c mice (6-8 weeks old) were purchased from Koatech (Korea) and maintained in the Seoul National University Hospital Biomedical Research Institute specific pathogen-free animal facility (Korea), which is accredited by the AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care). All experiments were approved by the Seoul National University Hospital Institutional Animal Care and Use Committee (No. 17-0150 and 19-0195).
Human umbilical cord blood-derived MSCs
Human umbilical cord samples were obtained from healthy full-term donors delivering at Seoul National University Hospital (Korea). The protocol was approved by the Seoul National University Hospital Institutional Review Board (No. 1708-083-878). All subjects provided written informed consent. hUC-MSCs were collected and cultured as described previously (Jeong et al., 2019). hUC-MSCs that had been cryopreserved at passage 4 were cultured to passage 7 and then used in the experiments described below.
Asthma mouse models and MSC treatment
Allergic asthma was induced by intratracheal instillation of either A. alternata or a combination of HDM and DEP. For the A. alternata mouse model, mice were intratracheally exposed to 10 μg A. alternata extract (Greer, USA) on days 0, 2, 4, and 6 and evaluated on day 8. For the HDM/DEP asthma model, mice were intratracheally sensitized with 10 μg HDM allergen from Dermatophagoides pteronyssinus 1 (Der p 1) extract (Greer) and 150 μg DEP (Standard Reference Material®1650b; National Institute for Standards and Technology, USA) on day 0. These mice were then intratracheally challenged with 10 μg HDM and 75 μg DEP on days 7, 8, and 9 and evaluated on day 10. For MSC treatment, MSCs were washed in phosphate-buffered saline (PBS) and 1.0 × 105 cells were either resuspended in 100 μl PBS and injected via a tail vein or resuspended in 50 μl PBS and intratracheally instilled. MSCs were delivered on day 0 or day 3 (for the A. alternata model) or day 0 and/or day 7 (for the HDM/DEP model). In the case where allergens and MSCs were administered on the same day, the MSCs were introduced 4 h after the allergen administration.
Measurement of airway hyperresponsiveness (AHR)
Mice were anesthetized with 150 mg/kg of pentobarbital sodium, intubated with 18-gauge catheters, and mechanically ventilated at a tidal volume of 0.2 ml and a frequency of 140 breaths per minute. Lung resistance (RL) was measured with BUXCO FinePointe Resistance and Compliance (BUXCO Electronics, USA) in response to aerosolized methacholine (5, 10, 20, 40 mg/ml) (Sigma-Aldrich, USA).
Preparation of mouse bronchoalveolar lavage and lung cells and human PBMCs
The euthanized mice were subjected to bronchoalveolar lavage (BAL) to evaluate the immune cells that had infiltrated the bronchioles. BAL fluid was obtained by flushing the trachea with 1 ml of cold PBS 3 times. Cells from BAL fluids were washed and analyzed by flow cytometry using anti-Siglec-F (E50-2440; BD Biosciences, USA), anti-CD11b (M1/70; Biolegend, USA), anti-CD11c (N418; Biolegend), anti-CD45 (30-F11; Biolegend), and anti-Ly6G (1A8; Biolegend). To obtain single cells from mouse lung, lung tissue was mechanically dissociated into small pieces and incubated with 1 mg/ml collagenase IV (Worthington Biochemical, USA) and 50 μg/ml DNase I (Sigma-Aldrich) in RPMI1640 with 10% fetal bovine serum (FBS) for 90 min at 37°C in a shaking incubator. Lung single cell suspensions were obtained after passing the samples through 40 μm strainers. Red blood cells were lysed with RBC lysis buffer (Sigma-Aldrich) and the remaining cells were washed and prepared for further experiments. For in vitro experiments on human blood cells, human peripheral blood was obtained from asthma patients. All donors provided written informed consent. The study protocol was approved by the Seoul National University Hospital Institutional Review Board (No. 1607-148-778). Human PBMCs were prepared after the centrifugation of PBS-diluted blood with Ficoll-PaqueTM PLUS (GE Healthcare, Sweden) at 2,000 rpm, 20°C with no brakes.
Flow cytometric analysis
To evaluate cytokine production by flow cytometry, 1 × 106 cells were re-stimulated with 100 ng/ml PMA (Sigma-Aldrich), 1 μg/ml ionomycin (Sigma-Aldrich), and 1 μl/ml Monensin (BD Biosciences) in RPMI1640 media with 10% FBS for 4 h. Suspended mouse cells were treated with mouse Fc block (BD Biosciences) for 10 min and stained with anti-CD90.2 (30-H12; Biolegend), anti-CD4 (RM4-5; Biolegend), anti-CD45 (30-F11; Biolegend), and the lineage antibody cocktail containing anti-CD3e (145-2C11; BD Biosciences), anti-CD19 (1D3; BD Biosciences), anti-CD49b (DX5; BD Biosciences), anti-CD11b (M1/70; BD Biosciences), anti-CD11c (HL3; BD Biosciences), anti-F4/80 (BM8; Biolegend), and anti-FcεRIα (MAR-1; Biolegend) on ice for 30 min. The mouse cells were then fixed and permeabilized with BD Cytofix/CytopermTM solution (BD Biosciences) on ice for 20 min and stained with anti-IL-5 (TRFK5; Biolegend) and anti-IL-13 (eBio13A, eBioscience) on ice for an hour. All stained cells were analyzed by flow cytometry, BD LSRFortessa X-20 (BD Biosciences). The data were analyzed using FlowJo v10.6.1 software (BD Biosciences).
In vitro culture of mouse Th1, Th2, and Th17 cells and human PBMCs with hUC-MSCs
Naïve mouse CD4 T cells were differentiated into Th1, Th2, and Th17 cells in vitro by using the CellXVivo Mouse Th1, Th2, and Th17 Cell differentiation kit (R&D Systems, USA) according to the manufacturer’s instructions, respectively. Briefly, naïve splenic CD4 T cells were isolated by using the MojoSort Mouse Naïve CD4 T Cell Isolation Kit (Biolegend) and then cultured in Th differentiation media for 6 days. hUC-MSCs were added on day 3 at a MSC/T cell ratio of 1:10 and the cell preparations were subjected to flow cytometric analysis 3 days later. Human PBMCs were cultured with hUC-MSCs at MSC/PBMC ratio of 1:10 for 24 h. The PBMCs were then subjected to quantitative polymerase chain reaction (qPCR) analysis.
qPCR analysis
RNA from PBMCs was extracted with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. cDNA was synthesized from 1 μg RNA by using the SensiFAST cDNA synthesis kit (Bioline, UK). The cDNA was used as templates for qPCR with SensiFAST SYBR Lo-ROX kit (Bioline). All primers were purchased from Integrated DNA Technologies (USA). The transcripts were normalized to RPLP0 expression and the gene expression was expressed as 2-∆∆Cq-value. The PCR primer sequences were as follows: RPLP0 forward, TGT CTG CTC CCA CAA TGA AAC; RPLP0 reverse, TCG TCT TTA AAC CCT GCG TG; IL4 forward, TGC TTC CCC CTC TGT TCT; IL4 reverse, AGC CCT GCA GAA GGT TTC; IL5 forward, GGA GAG TAA ACC AAT TCC TAG ACT; IL5 reverse, TTG GCC CTC ATT CTC ACT G; IL13 forward, CAT CAT TAT TTG CAG AGA CAG GAC; and IL13 reverse, GCA CAG GCT GAG GTC TAA G.
Statistical analysis
All statistical analyses were performed by using GraphPad Prism 7 (GraphPad Software, USA). The results are expressed as mean ± SEM. Unpaired or paired two-tailed Student’s t-test or one-way ANOVA with a Dunnett post-hoc test was used to compare the groups. Two-way ANOVA test was used for AHR analysis. P values less than 0.05 were considered statistically significant.
RESULTS
Intravenous, but not intratracheal, hUC-MSC treatment activates immune cells in the lungs of naïve mice
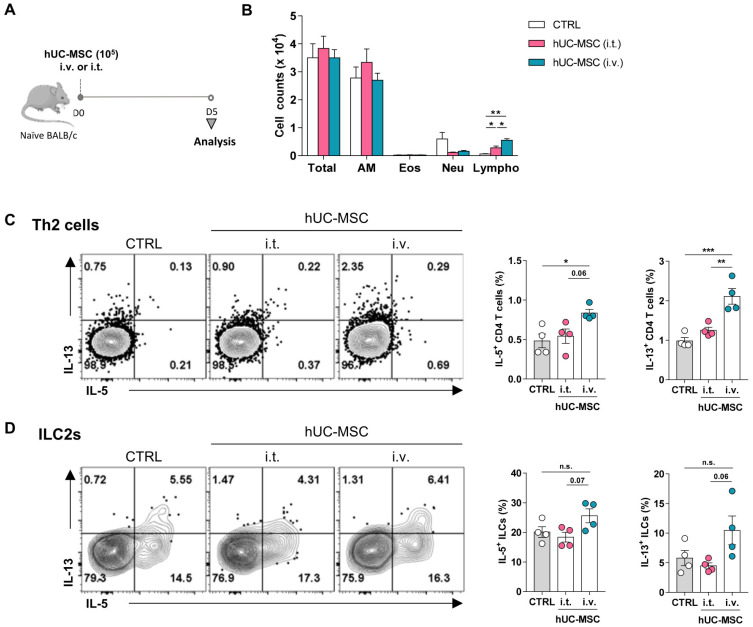
In previous studies, MSCs were transferred into murine models of asthma via the intravenous or intratracheal route (Boldrini-Leite et al., 2020; Sun et al., 2012). However, these studies did not assess the effect of MSC on the immune response of naïve lungs. There are many concerns regarding the activation of host immune cells caused by the transferred foreign cells including allogeneic stem cell transplantation. Although authorities have mandated these tests, few reports study the immunological effects of hUC-MSCs on healthy lungs (Ministry of Food and Drug Safety, 2014a; 2014b). To address this issue, 1 × 105 hUC-MSCs were transferred intravenously or intratracheally into naïve mice and the immune cells in the respiratory system were analyzed (Fig. 1A). Surprisingly, analysis of the immune cells in the BAL fluid showed that MSC transfer significantly increased the lymphocyte numbers in the lung when the intravenous route was used (Fig. 1B). FACS analysis of the Th2 cells and ILC2s in the lymphocytes then showed that intravenous hUC-MSC treatment significantly increased the IL-5 and particularly IL-13 production by the Th2 cells (Fig. 1C). Intravenous hUC-MSC treatment also slightly increased the IL-5 and IL-13 secretion by ILC2s, although these changes were not statistically significant (Fig. 1D). On the other hand, intratracheal administration of hUC-MSC slightly increased the number of lymphocytes in BAL fluids (Fig. 1B) but did not increase cytokine production in Th2 cells or ILC2s (Figs. 1C and 1D). Thus, these data showed unexpectedly that intravenous hUC-MSC treatment increased the numbers and activation of type 2 immune cells in the lungs of naïve mice.
Fig. 1. Association between the route of hUC-MSC administration with the baseline lung lymphocyte profiles.
Naïve mice were treated intravenously (i.v.) or intratracheally (i.t.) with hUC-MSCs. (A) Schematic depiction of the treatment regimen. (B) Counts of the indicated cells in the BAL fluid after treatment. (C and D) IL-5 and IL-13 production of the Th2 cells (C) and ILC2s (D) in the lung after hUC-MSC treatment. The data shown represent 3 independent experiments (n = 4 for each group). The data are expressed as mean ± SEM. Groups were compared by one-way (C and D) or two-way (B) ANOVA with Dunnett’s post-hoc test. *P ˂ 0.05; **P ˂ 0.01; ***P ˂ 0.001; n.s., not significant. AM, alveolar macrophages; Eos, eosinophils; Neu, neutrophils; Lympho, lymphocytes; CTRL, control.
hUC-MSC treatment suppresses lung type 2 immune cell activity in A. alternata-induced severe asthma but this effect depends on the treatment route and timing
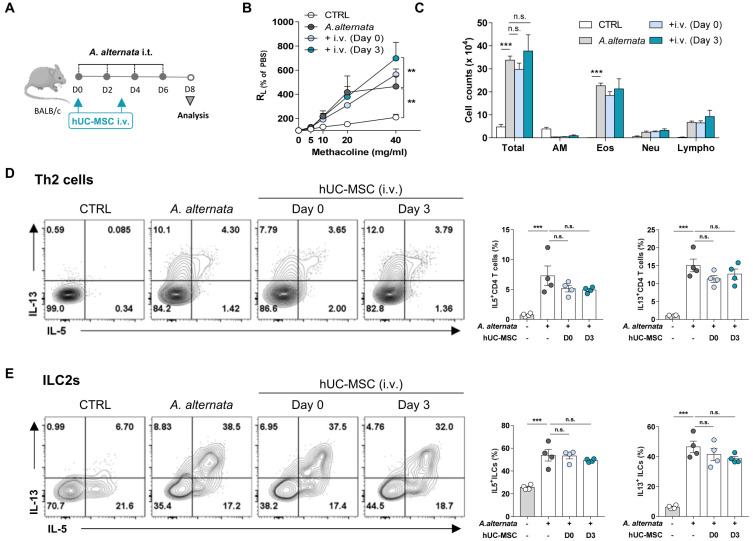
We then assessed whether the intravenous transfer of hUC-MSC ameliorated asthma that was induced by repeated exposure to A. alternata, a fungal allergen linked to the development of type 2 high severe asthma (Snelgrove et al., 2014). After confirming that intravenous hUC-MSCs were delivered to the lungs (Supplementary Fig. S1), asthmatic mice were treated with MSCs on either day 0 or day 3 intravenously (Fig. 2A). As expected, A. alternata exposure increased AHR and the infiltration of immune cells, including eosinophils, into the bronchioles. However, intravenous hUC-MSC treatment had no effect on AHR and the immune cell numbers in the BAL fluid, regardless of whether they were delivered early or later during sensitization (Figs. 2B and 2C). The treatments also had no effect on the type 2 cytokine production of the lung T cells and ILC2s (Figs. 2D and 2E).
Fig. 2. No therapeutic effect of hUC-MSC on A. alternata-induced asthma by intravenous route.
Mice were induced to develop A. alternata-induced asthma and were then injected intravenously (i.v.) with hUC-MSCs on day 0 or 3. (A) Schematic depiction of the treatment protocol. i.t., intratracheally. (B and C) Measurement of AHR (B) and BAL cell counts (C) after treatment. (D and E) IL-5 and IL-13 production of the Th2 cells (D) and ILC2s (E) in the lung after treatment. The data shown represent 3 independent experiments (n = 4 for each group). The data are expressed as mean ± SEM. Groups were compared by one-way (D and E) or two-way (B and C) ANOVA with Dunnett’s post-hoc test. **P ˂ 0.01; ***P ˂ 0.001; n.s., not significant. CTRL, control; AM, alveolar macrophages; Eos, eosinophils; Neu, neutrophils; Lympho, lymphocytes.
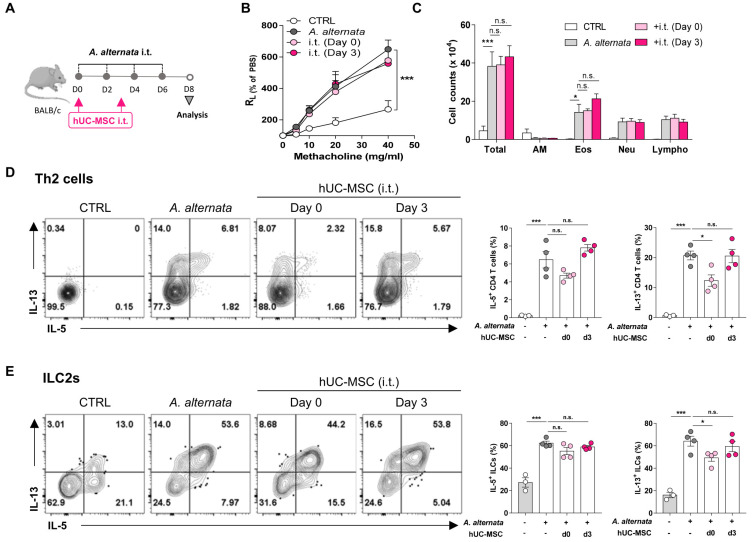
Since intravenous hUC-MSC transfer did not have a therapeutic effect in the A. alternata asthma model and in fact induced lung Th2 cell activation in naïve mice, we examined the effect of directly delivering hUC-MSC into the lungs intratracheally on day 0 or day 3 (Fig. 3A). Notably, although the intratracheal treatments did not affect the development of AHR or immune cell infiltration into the bronchioles (Figs. 3B and 3C), treatment with MSCs during the asthma induction phase (day 0) significantly reduced the IL-13 production by both the Th2 cells and ILC2s in the lungs (Figs. 3D and 3E). The same treatment also tended to reduce the numbers of IL-5-secreting Th2 cells and ILC2s. These results suggest that while hUC-MSC treatment can inhibit the type 2 inflammation in the lung that is mediated by Th2 cells and ILC2s, especially with regard to their IL-13 production, this effect depends profoundly on the timing and route of the treatment.
Fig. 3. Reduction of lung type 2 cytokine production in A. alternata-induced asthma by intratracheal hUC-MSC treatment during the induction period.
Mice were induced to develop A. alternata-induced asthma and were then injected intratracheally (i.t.) with hUC-MSCs on day 0 or 3. (A) Schematic depiction of the treatment protocol. (B and C) Measurement of AHR (B) and BAL cell counts (C) after treatment. (D and E) IL-5 and IL-13 production of Th2 cells (D) and ILC2s (E) in the lung after treatment. The data shown represent 3 independent experiments (n = 4 for each group). The data are expressed as mean ± SEM. Groups were compared by one-way (D and E) or two-way (B and C) ANOVA with Dunnett’s post-hoc test. *P ˂ 0.05; ***P ˂ 0.001; n.s., not significant. CTRL, control; AM, alveolar macrophages; Eos, eosinophils; Neu, neutrophils; Lympho, lymphocytes.
Intratracheal hUC-MSC treatment improves AHR and airway inflammation in HDM/DEP-induced severe asthma but marked effects depend on repeated administration
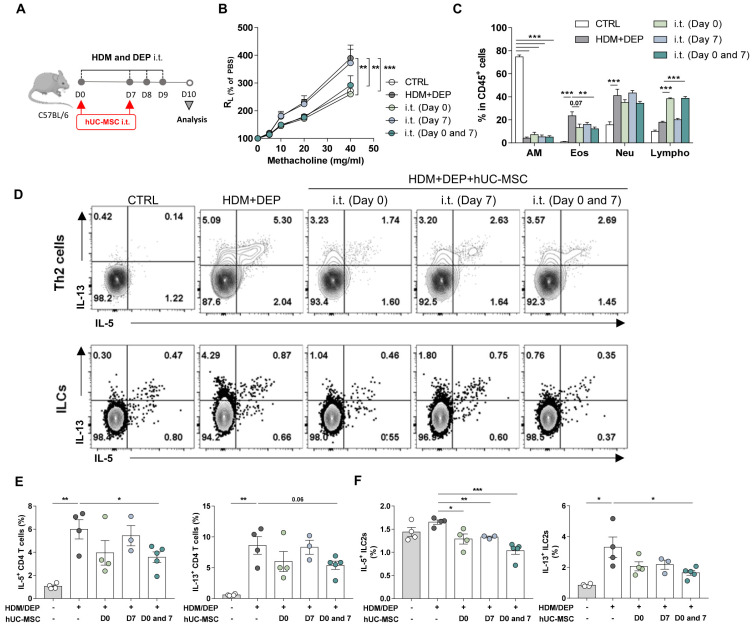
A. alternata-induced asthma is an eosinophilic asthma model. Since MSC transfer had a marginal effect on this model, we assessed the effect of this treatment on a different murine model of severe asthma. This model is induced by simultaneously exposing mice to an allergen (HDM) and air pollutants (diesel exhaust particle, DEP) (Fig. 4A). The mice develop a mixed phenotype of eosinophilic and neutrophilic inflammations that reflects the characteristics of severe asthma (Acciani et al., 2013; Brandt et al., 2015). These HDM/DEP asthma mice were treated intratracheally with hUC-MSCs on day 0 and/or day 7 (Fig. 4A). Treatment in the asthma progression phase (day 7) had no effect on AHR. In addition, while it did significantly reduce lung eosinophils, it did not shape lymphocyte numbers in the lung (Figs. 4B and 4C). By contrast, treatment in the asthma induction phase (day 0), or both the induction and progression phases (days 0 and 7), significantly reduced AHR and eosinophil in the BAL fluids (Figs. 4B and 4C). Analysis of the Th2 cells and ILC2s showed that MSC treatment also reduced their type 2 cytokine secretion, with the least pronounced effects being seen with day 7 treatment and the most marked effects being seen when the mice were repeatedly treated on days 0 and 7 (Figs. 4D-4F). However, hUC-MSC treatment did not alter interferon gamma (IFNγ) production from T cells or ILCs in vivo (Supplementary Fig. S2). These results suggest that repeated treatment with hUC-MSCs can suppress the mixed cellular phenotype of severe asthma.
Fig. 4. The therapeutic effect of repeated intratracheal treatment of hUC-MSCs in the development of HDM/DEP-induced severe asthma.
Mice were induced to develop HDM/DEP-induced asthma and were then injected intratracheally (i.t.) with hUC-MSCs on day 0 and/or 7. (A) Schematic depiction of the treatment protocol. (B and C) Measurement of AHR (B) and BAL cell counts (C) after treatment. (D and E) IL-5 and IL-13 production of Th2 cells (D) and ILC2 (F) in the lung after treatment. The data shown represent 3 independent experiments (n = 3~4 for each group). The data are expressed as mean ± SEM. Groups were compared by one-way (D and E) or two-way (B and C) ANOVA with Dunnett’s post-hoc test. *P ˂ 0.05; **P ˂ 0.01; ***P ˂ 0.001. CTRL, control; AM, alveolar macrophages; Eos, eosinophils; Neu, neutrophils; Lympho, lymphocytes.
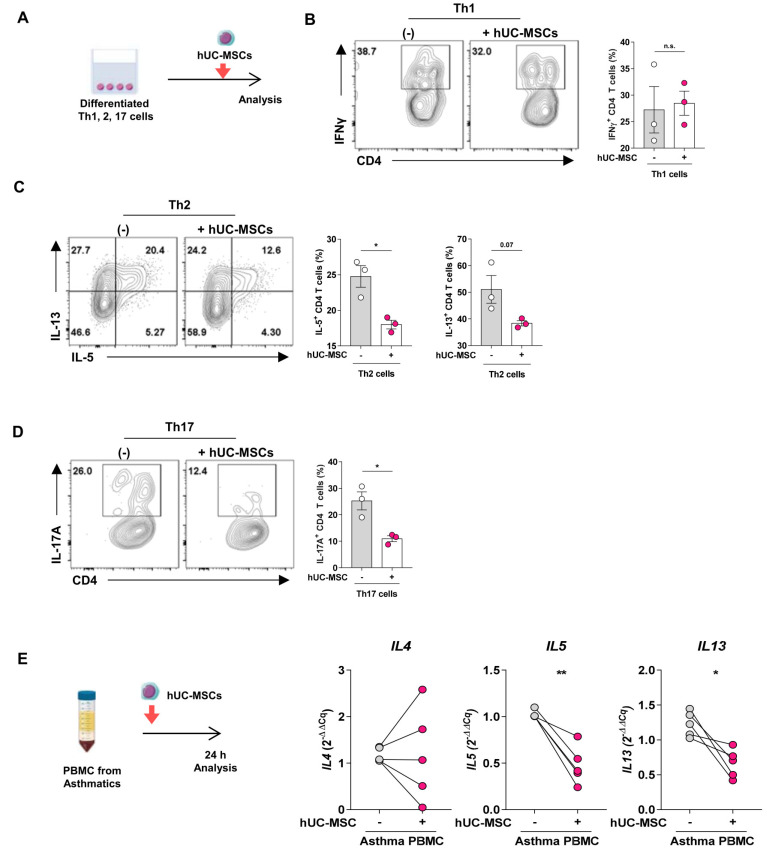
hUC-MSCs directly inhibit the type 2 cytokine production of murine Th2 cells and PBMCs from asthmatics in vitro
To determine whether hUC-MSCs can regulate the cytokine production of Th cells directly, we co-cultured hUC-MSCs with in vitro-differentiated mouse Th1, Th2, and Th17 cells (Fig. 5A). The treatment significantly reduced the hallmark cytokine production of the Th2 and Th17 cells but did not influence the IFNγ secretion of the Th1 cells (Figs. 5B-5D).
Fig. 5. The inhibitory effect of hUC-MSCs on type 2 cytokine production from murine Th2 cells and PBMC from asthmatics.
(A-D) In vitro differentiated Th1, Th2, and Th17 cells were co-cultured with hUC-MSCs (A), after which FACS was used to measure the IFNγ (B), IL-5, IL-13 (C), and IL-17A (D) produced by the Th1, Th2, and Th17 cells, respectively. (E) PBMCs isolated from asthma patients were co-cultured for 24 h with hUC-MSCs (left panel), after which the mRNA expression of IL4, IL5, and IL13 in the PBMCs (right panels) was measured. The data in Figs. 5B-5D represent 3 independent experiments (n = 3 for each group). The data in Fig. 5E are from a single experiment with PBMC from five asthma patients. All data are expressed as mean ± SEM. Groups were compared by unpaired (B-D) or paired two-tailed Student’s t-test (E). *P ˂ 0.05; **P ˂ 0.01; n.s., not significant.
We then examined whether co-culture of hUC-MSCs had a similar anti-inflammatory effect on PBMCs from asthma patients. Indeed, the MSCs significantly decreased their mRNA expression of IL-5 and IL-13, but not IL-4, by hUC-MSCs (Fig. 5E). Thus, hUC-MSCs can modulate activated Th2 and Th17 cell functions.
DISCUSSION
Asthma affects around 339 million people worldwide. Of these, approximately 20% have severe persistent asthma (Backman et al., 2018; GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017). At present, high-dose inhaled corticosteroids with a long-acting β2-agonist treatment is recommended for severe persistent asthma (Partridge, 2007). Biological agents that target specific immune mediators such as type 2 cytokines (IL-4, IL-5, and IL-13) or immunoglobulin E (IgE) have also recently emerged as alternative treatments for severe asthma (Khurana et al., 2020). However, some patients still poorly respond to the costly biological agents. Thus, patients with severe persistent asthma continue to have unmet clinical needs for effective and safe treatments.
Here, we showed that hUC-MSCs not only significantly reduced AHR and lung eosinophil in a murine model of severe asthma, they also directly inhibited Th2 cells and ILC2s. This suggests that stem cells have therapeutic potential for severe asthma. This significantly expands the stem cell research field, which has shown after decades of research that stem cells are a safe and viable alternative treatment for incurable diseases such as neurodegenerative and cardiac disorders, and cancer (Levy et al., 2020). Indeed, several clinical trials on MSC-based therapy are currently being conducted for such diseases (Regmi et al., 2019). With regard to asthma, many studies with different animal models have explored the therapeutic potential of MSCs in asthma (Mirershadi et al., 2020; Srour and Thebaud, 2014). They show that MSCs not only attenuate pathological remodeling in the asthmatic lung, they also suppress the lung inflammatory responses. Specifically, MSCs reduce eosinophilic inflammation, AHR, collagen fiber deposition, and serum IgE levels and regulate the Th1:Th2 ratio (Inamdar and Inamdar, 2013; Nemeth et al., 2010; Zeng et al., 2015; Zhang and He, 2019). However, as described recently by the review of Mirershadi et al. (2020), these studies used different asthma models and their MSC treatment protocols markedly vary in terms of dose, timing, and route. Thus, the optimal MSC treatment regimens for specific types of asthma remain unclear. Since this complicates the design of clinical trials on the efficacy of MSCs for asthma, we here evaluated the efficacy with which MSC transfer protocols that differ in route and timing influenced severe asthma in two animal models.
We initially found that MSCs had little effect on A. alternata asthma when the cells were delivered intravenously, regardless of whether they were administered during the induction or progression phases of asthma. However, intratracheal MSC administration during induction significantly suppressed the IL-13 production of lung Th2 cells and ILC2s, although this effect did not translate to improved AHR. Unexpectedly, we noted that intravenous, but not intratracheal, MSC administration in naïve mice elevated the IL-5 and IL-13 production of lung Th2 cells. Since this cast doubt on the safety of intravenous MSC administration, we then examined the effect of intratracheal MSC treatment in the HDM/DEP mouse model of severe asthma. Treatment of this model during the induction phase of asthma significantly reduced AHR, lung eosinophil and lymphocyte numbers, and the IL-5 and IL-13 production of both Th2 cells and ILC2s. These outcomes were not observed when treatment was conducted during the progression phase of asthma; however, treatment during both phases yielded the strongest effects. Moreover, the Th2 cytokine-suppressing effect of MSCs was recapitulated in vitro: differentiated mouse T cells or PBMCs from asthma patients that were co-cultured with MSCs produced less IL-5 and IL-13. These results suggest that (i) MSC treatment may be effective for some, but not necessarily all, forms of severe asthma; (ii) MSCs can directly modulate both T cells and ILCs; and (iii) in terms of the optimal MSC treatment regimen (i.e., the route, dose, and timing) for severe asthma, intratracheal MSC administration may be safer and effective than intravenous administration, and repeated intratracheal treatment may be more effective than single treatments.
Since asthma is a heterogeneous disease, the immune cells in charge of the pathology differ depending on the asthma endotype (Kim et al., 2010). Therefore, it is essential to test the efficacy of hUC-MSCs using different models of asthma, which represent the different endotypes of asthma (Fallon and Schwartz, 2020). Here, we adopt two asthma models called A. alternata induced-, and HDM/DEP induced-asthma. A. alternata-induced asthma is a model reflecting type 2 high severe asthma. In our settings, intratracheal treatment of hUC-MSCs partially protects the development of A. alternata-induced asthma and type 2 cytokine productions. Instead, we could observe the significant therapeutic effect of hUC-MSCs, when we treated hUC-MSCs intratracheally into HDM/DEP, a mixed type of Th2 and Th17 asthma. This discrepancy implied that the severe asthma patients with the mixed phenotype (Th2 and Th17 high) would benefit from the MSC therapy. Also, these results would be helpful to determine the target patients for the MSC clinical trials. Thus, MSCs therapy may be more suitable for steroid-refractory severe asthmatics who currently have clinical-unmet needs.
A critical element that shapes the efficacy of MSC treatment is the route of delivery. The most common routes of MSC administration are intravenous infusion, intra-arterial infusion, or intra-tissue injection (Kurtz, 2008). When we compared intravenous injection and intratracheal instillation, we found that intratracheal, but not intravenous, MSC treatment significantly reduced the cytokine responses of lung Th2 cells and ILC2s. Thus, the route of administration can significantly affect MSC treatment efficacy in asthma. We also found that intratracheal administration effectively suppressed HDM/DEP-induced severe asthma in mice. Interestingly, and unexpectedly, we also observed that intravenous, but not intratracheal, MSC treatment in naïve mice induced the IL-5 and IL-13 production of lung Th2 cells. Moreover, this change became more pronounced as the number of MSCs increased (data not shown). Notably, intravenous MSC transfer has been reported to cause cases of pulmonary thromboembolism (Moll et al., 2019). This may reflect the presence of small capillaries in the lung and the strong adhesion properties of MSCs. Compared to the intravenous route, the direct delivery of MSCs may accompany the invasive bronchoscopic approach which makes it difficult to apply repetitively. Nevertheless, our experiments showed that intratracheal MSC administration not only induced therapeutic effects in two models of asthma, it did not associate with unexpected and potentially unwelcome baseline immune profile changes in the lung. Thus, the benefits of intratracheal administration of MSCs may outweigh its risks, especially in patients with severe asthma.
Another important element in MSC treatment regimens is when the treatment should be given. We found that in both severe asthma models, MSC treatment just after the first sensitization suppressed airway inflammation better than treatment during the progression stage. In particular, treatment of the HDM/DEP model mice on day 0 alone, but not day 7 alone, potently decreased the AHR, the numbers of inflammatory cells in the BAL fluid, and the levels of type 2 cytokines secreted by lung Th2 cells and ILC2s. However, repeating the treatment on day 7 did associate with greater suppression of lung Th2 cells and ILC2s. Thus, our data suggest that early and multiple treatments with MSCs may have the best results in terms of modulating airway inflammation.
It should be noted that our study is based on xenogeneic transplantation of human MSCs into an animal model of asthma. However, our study regimen and results are supported by several studies that showed that like mouse MSCs, human-derived MSCs have therapeutic effects in acute or chronic mouse asthma models due to their potent immunomodulatory properties (Bonfield et al., 2010; Sun et al., 2012). Previous studies suggest that human-derived MSCs ameliorate asthma by promoting anti-inflammatory M2 macrophage polarization (Song et al., 2015) and directly suppressing effector T cells via exosomes or extracellular vesicles (Cruz et al., 2015; de Castro et al., 2017). This regulatory activity also occurs via cell-to-cell contact through receptor ligation or mitochondrial transmission (Court et al., 2020; Luz-Crawford et al., 2019; Ren et al., 2010; Wang et al., 2013). However, since this mechanism remains very poorly understood, further research is required.
Despite the apparent advantages and benefits of MSC therapy, many questions must be solved before MSCs can be used clinically for asthma. They include: how do MSCs mediate their beneficial effects on asthma? Moreover, how can we ensure MSCs arrive in the target organ? In addition, how can we ensure that MSCs are safe for clinical use? Our present results showed that optimized treatment regimens that are characterized by specific routes, timing, and doses are needed to successfully and safely evoke the desired anti-inflammatory functions of MSCs in the asthma microenvironment. However, more comprehensive studies are needed to elucidate the optimal protocol for MSC isolation and preparation for clinical use. Moreover, although our study suggests that MSCs have beneficial effects in severe asthma, more research that improves our understanding of the mechanisms by which MSCs exert these effects are needed before stem cells can be applied in clinical settings.
Supplemental Materials
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
ACKNOWLEDGMENTS
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. NRF-2017M3A9B4061887 and SRC 2017R1A5A1014560). The authors would like to express their gratitude to Professor In-Gyu Kim of Seoul National University College of Medicine for providing the hUC-MSCs.
Footnotes
AUTHOR CONTRIBUTIONS
J.W.S. and S.R. designed, and performed the experiments. J.W.S., S.R., K.J., and H.Y.K. wrote the manuscript. S.L. and D.H.C. analyzed the data. J.H. and H.R.K. contributed to human sample preparation. H.Y.K. supervised the project.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Acciani T.H., Brandt E.B., Khurana Hershey G.K., Le Cras T.D. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin. Exp. Allergy. 2013;43:1406–1418. doi: 10.1111/cea.12200. [DOI] [PubMed] [Google Scholar]
- Backman H., Jansson S.A., Stridsman C., Eriksson B., Hedman L., Eklund B.M., Sandstrom T., Lindberg A., Lundback B., Ronmark E. Severe asthma among adults: prevalence and clinical characteristics. Eur. Respir. J. 2018;52(Suppl 62):PA3918. doi: 10.1183/13993003.congress-2018.PA3918. [DOI] [Google Scholar]
- Boldrini-Leite L.M., Michelotto P.V., Jr., de Moura S.A.B., Jr., Capriglione L.G.A., Jr., Barussi F.C.M., Jr., Fragoso F.Y.I., Jr., Senegaglia A.C., Jr., Brofman P.R.S., Jr. Lung tissue damage associated with allergic asthma in BALB/c mice could be controlled with a single injection of mesenchymal stem cells from human bone marrow up to 14 d after transplantation. Cell Transplant. 2020;29:963689720913254. doi: 10.1177/0963689720913254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfield T.L., Koloze M., Lennon D.P., Zuchowski B., Yang S.E., Caplan A.I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E.B., Biagini Myers J.M., Acciani T.H., Ryan P.H., Sivaprasad U., Ruff B., LeMasters G.K., Bernstein D.I., Lockey J.E., LeCras T.D., et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J. Allergy Clin. Immunol. 2015;136:295–303.e7. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braza F., Dirou S., Forest V., Sauzeau V., Hassoun D., Chesne J., Cheminant-Muller M.A., Sagan C., Magnan A., Lemarchand P. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34:1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- Cahill E.F., Tobin L.M., Carty F., Mahon B.P., English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res. Ther. 2015;6:19. doi: 10.1186/s13287-015-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L.L., Kitoko J.Z., Xisto D.G., Olsen P.C., Guedes H.L.M., Morales M.M., Lopes-Pacheco M., Cruz F.F., Rocco P.R.M. Multiple doses of adipose tissue-derived mesenchymal stromal cells induce immunosuppression in experimental asthma. Stem Cells Transl. Med. 2020;9:250–260. doi: 10.1002/sctm.19-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae D., Han S., Lee M., Kim S. Genome edited Sirt1-overexpressing human mesenchymal stem cells exhibit therapeutic effects in treating collagen-induced arthritis. Mol. Cells. 2021;44:245–253. doi: 10.14348/molcells.2021.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court A.C., Le-Gatt A., Luz-Crawford P., Parra E., Aliaga-Tobar V., Batiz L.F., Contreras R.A., Ortuzar M.I., Kurte M., Elizondo-Vega R., et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21:e48052. doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F.F., Borg Z.D., Goodwin M., Sokocevic D., Wagner D.E., Coffey A., Antunes M., Robinson K.L., Mitsialis S.A., Kourembanas S., et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates Aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl. Med. 2015;4:1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro L.L., Xisto D.G., Kitoko J.Z., Cruz F.F., Olsen P.C., Redondo P.A.G., Ferreira T.P.T., Weiss D.J., Martins M.A., Morales M.M., et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017;8:151. doi: 10.1186/s13287-017-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon P.G., Schwartz C. The high and lows of type 2 asthma and mouse models. J. Allergy Clin. Immunol. 2020;145:496–498. doi: 10.1016/j.jaci.2019.11.031. [DOI] [PubMed] [Google Scholar]
- Fan X.L., Zhang Y., Li X., Fu Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020;77:2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, author. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M., Sueblinvong V., Eisenhauer P., Ziats N.P., LeClair L., Poynter M.E., Steele C., Rincon M., Weiss D.J. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.H., Kwon H.S., Lee K.Y., Ha E.H., Moon K.A., Kim S.W., Oh W., Kim T.B., Moon H.B., Cho Y.S. hMSCs suppress neutrophil-dominant airway inflammation in a murine model of asthma. Exp. Mol. Med. 2017;49:e288. doi: 10.1038/emm.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A.C., Inamdar A.A. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp. Lung Res. 2013;39:315–327. doi: 10.3109/01902148.2013.816803. [DOI] [PubMed] [Google Scholar]
- Jeong E.M., Shin J.W., Lim J., Kim J.H., Kang H., Yin Y., Kim H.M., Kim Y., Kim S.G., Kang H.S., et al. Monitoring glutathione dynamics and heterogeneity in living stem cells. Int. J. Stem Cells. 2019;12:367–379. doi: 10.15283/ijsc18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh H., Mahon B.P. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015,. [DOI] [PubMed] [Google Scholar]
- Khurana S., Bush A., Holguin F. Management of severe asthma: summary of the European Respiratory Society/American Thoracic Society task force report. Breathe (Sheff.) 2020;16:200058. doi: 10.1183/20734735.0058-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., DeKruyff R.H., Umetsu D.T. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Umetsu D.T., Dekruyff R.H. Innate lymphoid cells in asthma: will they take your breath away? Eur. J. Immunol. 2016;46:795–806. doi: 10.1002/eji.201444557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Ryu S., Kim H.Y. Innate lymphoid cells in tissue homeostasis and disease pathogenesis. Mol. Cells. 2021;44:301–309. doi: 10.14348/molcells.2021.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Kokturk N., Kim J.Y., Lee S.W., Lim J., Choi S.J., Oh W., Oh Y.M. Gene profiles in a smoke-induced COPD mouse lung model following treatment with mesenchymal stem cells. Mol. Cells. 2016;39:728–733. doi: 10.14348/molcells.2016.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells. 2008;1:1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Jang A.S., Kwon J.H., Park S.K., Won J.H., Park C.S. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol. Res. 2011;3:205–211. doi: 10.4168/aair.2011.3.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., De Biasio M., Heinelt M., Reeve B., Abdi R., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundback B., Backman H., Lotvall J., Ronmark E. Is asthma prevalence still increasing? Expert Rev. Respir. Med. 2016;10:39–51. doi: 10.1586/17476348.2016.1114417. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P., Hernandez J., Djouad F., Luque-Campos N., Caicedo A., Carrere-Kremer S., Brondello J.M., Vignais M.L., Pene J., Jorgensen C. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 2019;10:232. doi: 10.1186/s13287-019-1307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken J.L., Veeranki S.P., Ameredes B.T., Calhoun W.J. Diagnosis and management of asthma in adults: a review. JAMA. 2017;318:279–290. doi: 10.1001/jama.2017.8372. [DOI] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety, author. Considerations in Immunotoxicity Assessment of Allogenic Stem Cell Therapy Product. Ministry of Food and Drug Safety; Cheongju: 2014a. [Google Scholar]
- Ministry of Food and Drug Safety, author. Guideline in Quality, Non-clinical and Clinical Assessment of Stem Cell Therapy Product. Ministry of Food and Drug Safety; Cheongju: 2014b. [Google Scholar]
- Mirershadi F., Ahmadi M., Rezabakhsh A., Rajabi H., Rahbarghazi R., Keyhanmanesh R. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res. Ther. 2020;11:400. doi: 10.1186/s13287-020-01921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll G., Ankrum J.A., Kamhieh-Milz J., Bieback K., Ringden O., Volk H.D., Geissler S., Reinke P. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol. Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Munir H., McGettrick H.M. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24:2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- Nemeth K., Keane-Myers A., Brown J.M., Metcalfe D.D., Gorham J.D., Bundoc V.G., Hodges M.G., Jelinek I., Madala S., Karpati S., et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge M.R. Examining the unmet need in adults with severe asthma. Eur. Respir. Rev. 2007;16:67–72. doi: 10.1183/09059180.00010402. [DOI] [Google Scholar]
- Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019;98:151041. doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Ren G., Zhao X., Zhang L., Zhang J., L'Huillier A., Ling W., Roberts A.I., Le A.D., Shi S., Shao C., et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.J., Cho T.J., Lee D.S., Choi J.Y., Cho J. Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol. Cells. 2013;35:557–564. doi: 10.1007/s10059-013-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove R.J., Gregory L.G., Peiro T., Akthar S., Campbell G.A., Walker S.A., Lloyd C.M. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J. Allergy Clin. Immunol. 2014;134:583–592.e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Xie S., Lu K., Wang C. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38:485–492. doi: 10.1007/s10753-014-9954-6. [DOI] [PubMed] [Google Scholar]
- Srour N., Thebaud B. Stem cells in animal asthma models: a systematic review. Cytotherapy. 2014;16:1629–1642. doi: 10.1016/j.jcyt.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Sun Y.Q., Deng M.X., He J., Zeng Q.X., Wen W., Wong D.S., Tse H.F., Xu G., Lian Q., Shi J., et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30:2692–2699. doi: 10.1002/stem.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang S., Wang F., Li G., Zhang L., Luan X. Expression and biological function of programmed death ligands in human placenta mesenchymal stem cells. Cell Biol. Int. 2013;37:137–148. doi: 10.1002/cbin.10024. [DOI] [PubMed] [Google Scholar]
- Zeng S.L., Wang L.H., Li P., Wang W., Yang J. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol. Med. Rep. 2015;12:2511–2520. doi: 10.3892/mmr.2015.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.B., He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis. Pulm. Pharmacol. Ther. 2019;54:39–52. doi: 10.1016/j.pupt.2018.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.