Abstract
The discovery of human pluripotent stem cells (PSCs) at the turn of the century opened the door to a new generation of regenerative medicine research. Among PSCs, the donors available for induced pluripotent stem cells (iPSCs) are greatest, providing a potentially universal cell source for all types of cell therapies including cancer immunotherapies using natural killer (NK cells). Unlike primary NK cells, those prepared from iPSCs can be prepared with a homogeneous quality and are easily modified to exert a desired response to tumor cells. There already exist several protocols to genetically modify and differentiate iPSCs into NK cells, and each has its own advantages with regards to immunotherapies. In this short review, we detail the benefits of using iPSCs in NK cell immunotherapies and discuss the challenges that must be overcome before this approach becomes mainstream in the clinic.
Keywords: adoptive immunotherapy, directed differentiation, genome editing, induced pluripotent stem cells, natural killer cells
INTRODUCTION
Adoptive cell transfer (ACT) is the latest arsenal in the battle against cancer. This therapy has primarily focused on T cells because of their responses to tumor cells, including potent and specific cytotoxicity and high expansion capacity (Perica et al., 2015). However, because natural killer (NK) cells too have anti-cancer effects but with milder side effects, they are also under consideration for ACT (Lee, 2019). Gene engineering has added to their virulence. Furthermore, T cells and NK cells complement each other in that certain immune suppression mechanisms taken by tumor cells that are effective against T cells, such as the downregulation of human leukocyte antigens (HLA), have proven to be stimulatory for NK cells.
While initial research on NK cell-based ACT has used primary NK cells from peripheral blood (PB-NK), NK cells in cord blood (CB-NK) and immortalized lines such as NK-92 are respectively more abundant (10% vs 30%) and easier to genetically modify (Myers and Miller, 2021; Shankar et al., 2020). Among all cell types, however, stem cells in many ways have the best advantages. Accordingly, scientists have developed protocols for the expansion, genetic modification and differentiation of stem cells to NK cells.
Among stem cells, induced pluripotent stem cells (iPSCs) have a number of features that make them ideal as the starting source. In this review, we examine the manufacturing and gene engineering of NK cells generated from iPSCs (iPS-NK) for ACT.
NK CELLS
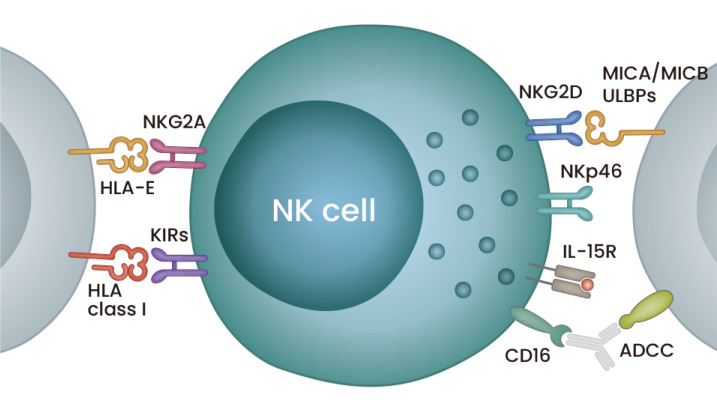
NK cells were the first type of innate lymphoid cells reported (Kiessling et al., 1975a; 1975b). They are recognized by the downregulation of CD3 and upregulation of CD56, which is primarily regulated by IL-15 signaling. Their cytotoxicity depends on the stimulation of multiple activating and inhibitory receptors and the secretion of perforin and granzyme B. These receptors include CD16, which triggers antibody-dependent cellular cytotoxicity (ADCC), along with natural cytotoxicity receptor family members (NKp30, NKp40, NKp44, and NKp46) and NKG2D, which bind directly to ligands of the target cells (Fig. 1). In addition, NK cells secrete a number of factors that activate other immune cells (Shimasaki et al., 2020). On the other hand, other ligands, such as HLA, bind to inhibitory receptors NKG2A and killer immunoglobulin-like receptors (KIR) to inhibit NK cells, providing a form of protection for self-cells from innate immunity. Thus, the overall response of an NK cell depends on the balance of stimulating these different receptors.
Fig. 1. An illustration of an NK cell with major surface receptors.
The NK cell in the middle is shown to express some major inhibitory and activating receptors on the left and right, respectively. Blue cells on the sides are target cells. Details of the receptors are provided in the text. ULBPs, UL16-binding proteins.
Consistently, one mechanism through which cancer cells evade NK cell immunosurveillance is to maintain the expression of their HLA class I molecules to activate KIR (Dianat-Moghadam et al., 2018). Other mechanisms for the evasion include the upregulation of HLA-E to activate NKG2A or the shedding of MICA and MICB to suppress NKG2D. The secretion of the cytokines TGF-β, which blocks IL-15, and IL-10 and the immune checkpoint inhibitor PD-1 also promotes immune evasion. Conversely, therapeutic countermeasures can overcome this evasion. For example, antibodies that prevent the shedding of MICA and MICB maintain the NK cell response to melanomas through NKG2D stimulation (Ferrari de Andrade et al., 2018). Methods that enhance IL-15 expression are especially of interest. IL-15 expression is a possible biomarker for the risk of relapse in cancer patients, and NK cells expressing inactive TGF-β receptors show higher cytotoxic effects in a glioblastoma model (Mlecnik et al., 2014; Yvon et al., 2017). Finally, IL-15 superagonists that enhance NK cell activation, proliferation and expansion are under clinical investigation for both hematopoietic malignancies and solid tumors (Romee et al., 2018; Wrangle et al., 2018).
Overall, the various types of NK cells used in ACT are well tolerated, persist and expand, and in some patient subpopulations they can induce complete remission (Bachanova and Miller, 2014). One reason for the tolerance is that the cells are re-educated in the host body, thus maintaining a lethal response to non-HLA-expressing cells and minimizing the risk of graft versus host disease. Because of this feature, allogeneic NK cells are commonly used in ACT, expanding the number of available donors, a significant plus when compared with T cell immunotherapies (Ruggeri et al., 2002).
At the same time, because the number of PB-NK and CB-NK that can be acquired from donors is insufficient for ACT, ex vivo expansion is required. Partly because it was the first cytokine to have its receptor cloned, IL-2 has long been used in several expansion protocols. However, IL-15 is now preferred, because unlike IL-2, it activates NK cells with high specificity, whereas IL-2 activates an assortment of immune cells, including regulatory T cells, which can suppress the anti-cancer response (Yang and Lundqvist, 2020). Still, the optimal timing and dose of IL-15 need confirmation, as otherwise the expanded NK cells risk developing the exhausted phenotype (Felices et al., 2018). The immortalized cell line NK-92 shows comparable anti-tumor effects as these other NK cell sources and has the added bonus of indefinite proliferation (Zhang et al., 2019). The major disadvantage of NK-92 cells is that, because of their aneuploidy and instability, they must be irradiated prior to the ACT, which suppresses their proliferation in the patient. Further, these cells do not express CD16, which prevents them from executing ADCC.
Another way to enhance NK cell activity is through genetic modifications, but this is not trivial in cell types that have relatively low proliferation capacity such as PB-NK and CB-NK. Stem cells, including not just iPSCs, but also their functional counterpart, embryonic stem cells (ESCs), and hematopoietic stem cells (HSCs), in contrast, are easier to modify. Among them, iPSCs distinguish themselves with superior stemness than HSCs and availability than ESCs.
iPSCs FOR CLINICAL USE
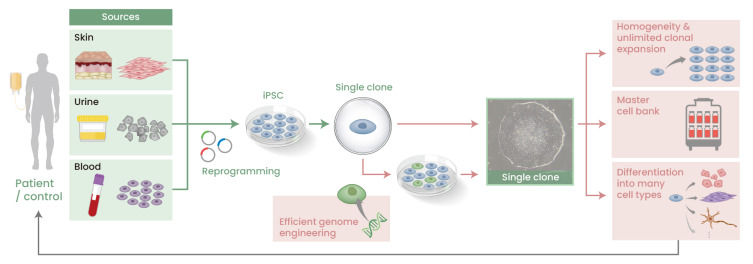
iPSCs describe somatic cells that have been reprogrammed to an embryonic-like cell state. Theoretically, any somatic cell type is susceptible to reprogramming, but for the same reason, iPSCs have almost exclusively been prepared using readily accessible cells such as fibroblasts and blood cells. From the perspective of clinical applications, the appeal of iPSCs is their high expansion capacity, which allows a bountiful number to be prepared from a small sample of somatic cells including from just one single donor, and their pluripotency, which allows them to be differentiated to any desired cell type including NK cells (Karagiannis et al., 2019). These properties are advantageous for safety and quality testing. Furthermore, unlike ESCs, which require embryo donors, a much wider donor population is available for iPSCs, which simplifies donor-patient matching. Finally, when stored as a master cell bank, somatic cells induced by the differentiation of a single iPSC clone offer a robust and reproducible cell source for clinical application (Fig. 2). In addition to these properties, advances in CRISPR/Cas technology and single cell techniques have simplified iPSC genome editing (Woltjen et al., 2016).
Fig. 2. Application of iPSCs to regenerative medicine.
In theory, any somatic cell type can be reprogrammed into an iPSC, but in practice, easily accessible cells, such as those from skin, urine or blood, are commonly used. Upon the acquisition of iPSC clones by transfecting reprogramming factors into the cells, a single clone is selected and expanded indefinitely and homogeneously. As new technologies have simplified the genome editing of iPSCs, iPSCs can be genetically modified precisely at the clonal level (green cells). The expanded cells can be used to establish a master cell bank or differentiated to specific cell types of interest for various purposes including cell therapies.
Clinical trials using iPSCs including iPS-NK are ongoing for a number of diseases (Yamanaka, 2020). The first in-patient trial using an iPSC product treated age-related macular degeneration. Here, autologous fibroblasts were reprogrammed into iPSCs, which were then differentiated into retinal epithelial cell sheets and transplanted into the patient’s eye (Mandai et al., 2017). Autologous cells were used because they were expected to minimize immune reactions to the graft. While that assumption was confirmed by the patient follow-up, the time and cost of preparing the product for transplantation are respectively detrimental to the patient suffering from a progressive degenerative disease and prohibitive to the payer, be it the patient or a national insurance program.
In response, there are concerted efforts to prepare stocks of HLA-homozygous and HLA-edited iPSCs. Both types of iPSCs are designed to maximize the number of patients who can receive a cellular product from the perspective of immune-matching. To collect HLA-homozygous iPSCs, donors are recruited in cooperation with blood and bone marrow banks (Umekage et al., 2019). In Japan, iPSCs prepared from just ten donors homozygous for the most frequent HLA should match half the population. However, the number of lines needed to satisfy a larger percentage grows non-linearly, with estimates of 75 and 140 lines necessary to serve 80% and 90%, respectively (Okita et al., 2011). Moreover, Japan has relative homogenous population, and other countries will require far more lines.
Aside from reducing the number of lines, research to produce an HLA-edited iPSC stock could ironically advance research on preparing more potent NK cells for ACT, since these efforts involve studying the mechanisms that inhibit NK cells. Deleting beta 2-microglobulin (B2M) gene removes all HLA expression, thus eliminating the concern of host T cell rejection of an HLA-mismatched graft. However, for the same reason, the knockout exposes these cells to a reaction by host NK cells. To prevent this response while maintaining the T cell evasion, scientists have forced the expression of HLA-E or retention of one HLA-C allele (Gornalusse et al., 2017; Xu et al., 2019a).
NK CELL DIFFERENTIATION
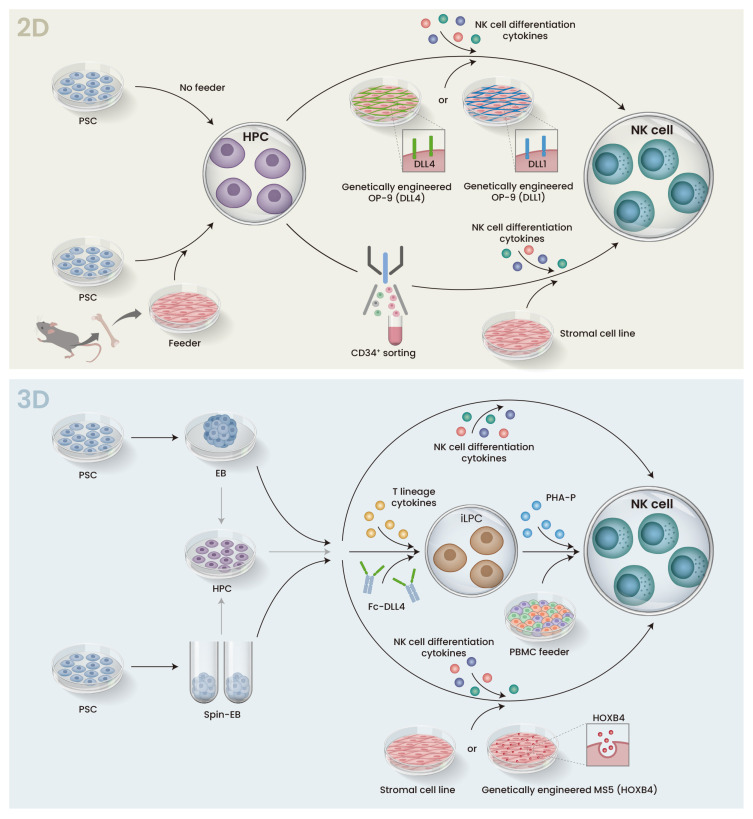
iPS-NK differentiation protocols can be divided into two- and three-dimensional (2D and 3D) culture systems. Both approaches induce hematopoietic differentiation and pass the hematopoietic progenitor cell (HPC) stage to acquire NK cells. Feeder systems are suitable for basic research because of their higher differentiation efficiency. On the other hand, they are disadvantageous when conducting clinical research, because they increase cost, as more feeder cells are needed for large-scale manufacturing and more safety tests are needed for each step of the manufacturing process in which they are involved.
Because human ESCs were reported nearly ten years prior to iPSCs, several protocols used to generate iPS-NK are based on the differentiation of ESCs. In one of the earliest, the Kauffman group used a two-step in vitro 2D differentiation scheme that co-cultured human ESCs with mouse bone marrow stromal cells to promote hematopoietic differentiation and then co-cultured the sorted HPCs with a second stromal cell line and a cytokine cocktail including IL-15, IL-3, IL-7, SCF, and Flt3L to generate ESC-derived NK cells (ESC-NK) (Fig. 3, top). ESC-NK exerted good functional characteristics including cytokine production and cytolytic activity in vitro and in vivo against tumor cells (Woll et al., 2005; 2009). Other groups have replaced the second stromal line with genetically engineered feeder cells expressing Notch ligand DLL1 or DLL4, which promote the expansion of HPCs with a bias for NK lineage (Mesquitta et al., 2019; Zeng et al., 2017).
Fig. 3. Schematic representation of 2D and 3D protocols for iPSC differentiation to NK cells.
Both 2D and 3D protocols are broken into two stages. The first stage differentiates PSCs into HPCs, and the HPCs are then cultured with a cytokine cocktail that induces NK cell differentiation in the second stage. (Top) In the 2D protocol, PSCs are co-cultured with murine bone marrow stromal cells (feeder) to promote hematopoietic differentiation. In the subsequent step, CD34+ HPCs are sorted and transferred to a second stromal cell line. Alternatively, sorting can be avoided, and the cells are transferred onto a stromal cell line such as OP-9 that is engineered to express DLL4 or DLL1. In either case, cytokines such as IL-15 are added to promote NK cell differentiation. (Bottom) In the 3D protocol, HPC differentiation is performed in suspension culture via EB or Spin-EB formation. The EBs are directly transferred to feeder stromal cell lines or feeder-free conditions, or HPCs are dissociated from the EBs and transferred. In either case, cytokines are added to induce NK cell differentiation. iLPC, iPSC-derived lymphocyte progenitor cells; PHA-P, phytohemagglutinin-P; PBMC, peripheral blood mononuclear cells.
When iPSCs are cultured in suspension without feeder layers (low-attachment plate), they spontaneously form aggregates known as embryoid bodies (EB). The cells in EBs grow and differentiate toward specific cell lineages in response to exogenous cytokines. One problem with EBs, however, is that they are of heterogeneous size, which influences the reproducibility of the differentiation and thus makes it difficult to satisfy Good Manufacturing Practice (GMP). Spin-EBs are one solution to this problem. The centrifugation of known numbers of iPSCs produces EBs of uniform size, which allows for synchronized and more efficient differentiation. The Kauffman group applied spin-EBs to generate iPS-NK in feeder-free conditions without cell sorting, an approach that can be applied to a completely defined system amenable to clinical applications (Knorr et al., 2013). Furthermore, these iPS-NK had similar genotype and phenotype as iPS-NK produced using stromal cells. Although elegant, the wide application of their approach is hindered by patent rights, which have motivated alternative 3D approaches. One method that avoids spin-EB was reported by the Kaneko group. After inducing the hematopoietic differentiation of EBs, they cultured the resulting HPCs on FcDLL-4, a recombinant protein fused to the Fc domain of human IgG, without stromal cells in a cytokine cocktail for T cell differentiation to produce lymphocyte progenitor cells that were then expanded with phytohemagglutinin-P (Ueda et al., 2020). Notably, this study did not produce iPS-NK but instead reported NK/innate lymphoid cells that showed efficient cytotoxicity against solid tumors and extended the lifespan of a mouse model. Future work will confirm if modifications to the cytokine cocktail can produce pure iPS-NK. Another factor that could advance EB techniques for the establishment of iPS-NK but has not been explored in detail is the inclusion of the HOXB4 homeoprotein, an important regulator of HSC self-renewal and expansion. Indeed, one study found that culturing dissociated HPCs derived from EB on HOXB4 promotes the expansion (Fig. 3, bottom) (Larbi et al., 2012). More investigation of this mechanism should lead to the identification of small molecules with the same effects and satisfy requirements for GMP. In general, small molecules reduce the cost of product development while maintaining or even increasing the NK cell differentiation efficiency. For example, the Slukvin team showed that UM171, an HSC-agonist pyrimido-indole derivative, selectively promotes the expansion of a lymphoid progenitor population that has a 10-fold propensity for NK cell differentiation (Mesquitta et al., 2019).
One last aspect about NK cells in cancer immunity commented on here is their indirect cytotoxic effects. NK cells can activate several other immune cells including B cells, T cells, macrophages, dendritic cells and neutrophils (Vivier et al., 2011). Demonstrating the same holds for iPS-NK, Cichocki et al. (2020) showed that iPS-NK recruit T cells to the tumor site, where both cell types exert cytotoxicity when combined with an anti–PD-1 antibody. These observations suggest that by recruiting other immune cells to the tumor site, iPS-NK-based ACT could prove effective for heterogeneous tumors.
NK CELL GENE ENGINEERING
Chimeric antigen receptors (CAR) are synthetic receptors that redirect the specificity and function of the modified immune cell. The first approvals of CAR therapies, axicabtagene ciloleuel and tisagenlecleucel, are the biggest clinical milestones to date for this technology. These therapies, which treat hematological malignancies and depend on autologous T cells, have remarkable response rates, reaching as high as 90% (Maude et al., 2014). However, many unresolved issues are preventing CAR T cell therapies from becoming mainstream. Most pressing perhaps is their cost. At several thousand dollars per treatment, they test just how much value society puts on a life. Further, life-threatening cytokine release syndrome (CRS) and neurotoxicities are common. One cause of CRS is IL-6, a cytokine not secreted by NK cells, suggesting that NK cells may be safer (Ueda and Kaneko, 2020). Adding that CAR is compatible with NK cells, nearly 20 clinical trials using CAR-NK are ongoing, including one using CAR-iPS-NK (Imai et al., 2005; Xie et al., 2020).
CAR consists of an extracellular single chain variable fragment (scFv) and an intracellular immune cell activation domain (Sadelain et al., 2013). Later generations have added co-stimulatory domains to the design. Using CAR-T as a paradigm, initial studies on NK cells incorporated the 4-1BB co-stimulatory domain, which endows higher persistence; however, 2B4, a NK cell-specific co-stimulatory domain, improves the cell function in several ways, including enhanced cytotoxicity, proliferation, cytokine secretion and persistence (Xu et al., 2019b). Using iPS-NK, the Kaufman group compared 11 CAR with different NK-cell specific domains. They found that along with 2B4, the inclusion of the NGK2D transmembrane domain resulted in the best response to an ovarian cancer xenograft model (Li et al., 2018). Notably, this was not true for all CAR that included the two domains, reflecting the fact that many questions remain on how to best design CAR. Moreover, CAR experiments using PB-NK have indicated that subpopulations respond differently to CAR stimulation, with terminally differentiated NK cells showing the best response (Oei et al., 2018). However, this observation was only tested for the anti-CD19 ectodomain. Which subpopulations respond best to modifications with other ectodomains requires further investigation. This point reaffirms the benefits of iPSCs, since multiple NK subpopulations can be produced for the optimization.
The inclusion of IL-15 signaling into CAR benefits the anti-tumor response. Taking advantage, the Rezvani group genetically modified CB-NK so that they react to CD19+ cells by secreting IL-15 (Liu et al., 2018). The addition of IL-15 significantly enhanced the persistence of the cells, resulting in more potent anti-tumor activity. The genetic modification also included a caspase gene that could be activated through exposure to a small molecule. These cells are now undergoing clinical trials, and the researchers have switched the source to iPSCs, having initiated a clinical trial with iPS-NK as well (Liu et al., 2020).
Although not CAR, other gene modifications can sustain IL-15 for a stronger iPS-NK anti-tumor effect. Cytokine-inducible SH2-containing protein (CIS) is a negative regulator of IL-15 signaling. In mice, deletion of its encoding gene, Cish, renders NK cells with elevated survival and cytotoxicity and animals resistant to several cancers (Delconte et al., 2016). The Kaufman team confirmed the same properties hold for CISH in humans by deleting the gene in iPSCs prior to NK cell differentiation (Zhu et al., 2020a). The cells showed significantly improved metabolic fitness, including benefits to glycolysis and mitochondria respiration. Metabolic responses to the tumor environment are key determinants of the anti-tumor effect of immune cells (Wegiel et al., 2018). Interestingly, the enhanced sensitivity to IL-15 was only shown at lower concentration levels: CISH-KO iPSC-NK showed higher function than wild-type iPSC-NK at 1 ng/ml IL-15, but no difference if the level was raised to 10 ng/ml. Additionally, the study found that prolonged IL-15 treatment at 10 ng/ml was accompanied by an exhaustion profile and lower mitochondrial respiration in NK cells. The lower dose could have important implications in clinical applications because of the potential exhaustion caused by prolonged IL-15 exposure, as explained above.
As the mediator of ADCC by binding to the Fc domain of IgG, CD16 is the most potent activating receptor of NK cells. Its expression on the cell surface is regulated by ADAM17 (a disintegrin and metalloproteinase-17), which cleaves the receptor in response to NK cell activation or in the tumor environment (Romee et al., 2013). Because NK-92 do not express CD16 and are therefore unable to perform ADCC, several groups have attempted to transduce the CD16 gene with success (Mantesso et al., 2020). Engineering a cleavage-resistant CD16 in iPSCs resulted in iPS-NK with improved ADCC against multiple tumor types (Zhu et al., 2020b). However, much like prolonged IL-15 exposure, simply extending the lifespan of the CD16 expression may actually be deleterious to the NK cell attack, because the cleavage is crucial for allowing NK cells to detach from their target cells and attack other cells. Indeed, prolonging the attachment could lower the anti-tumor response by reducing the secretion of perforin (Srpan et al., 2018).
The final target surface receptor for gene engineering we consider here is CD38. Daratumumab is a monoclonal antibody that significantly improves the outcomes of multiple myeloma, but most patients respond only transiently. One reason is that along with the target cells, NK cells too express CD38, making them susceptible to the antibody. Accordingly, patients receiving daratumumab show a rapid depletion of NK cells and reduced ADCC. To protect the cells, researchers have investigated a CD38-knockout population using CRISPR-Cas9 to delete CD38 in primary NK cells (Naeimi Kararoudi et al., 2020). As hoped, this strategy sustained the NK cell population and recovered ADCC. Because the CRISPR-Cas9 system has higher efficiency in iPSCs, we expect the transition to iPS-NK will advance this approach.
CONCLUSION
With the growing success of CAR- and iPSC-based therapies in the clinic, there is much anticipation about the shift of these technologies to new cancer immunotherapies using NK cells. NK cells offer a potentially safer yet comparably potent therapy than T cells and a wider class of donors, since HLA-mismatch is not of the same concern. With refinement of the differentiation protocols, iPS-NK should eventually equal PB-NK in all phenotypes, including receptor expressions and the corresponding expansion, persistence, and cytotoxicity in response to the tumor environment. Moreover, the relative simplicity of gene engineering in iPSCs will enable the production of CAR iPS-NK that have desired features such as higher persistency and target specificity, robustness to exhaustion, and even the ability to activate other immune cells to enhance the tumor attack. Finally, the development of iPSC stocks will expedite the availability of iPS-NK to a wider population, since they will provide a homogenous product.
ACKNOWLEDGMENTS
The authors thank Misaki Ouchida (Center for iPS Cell Research and Application) and Bokyung Moon (THERABEST, Co., Ltd.) for one of the figure illustrations and the iPS cell microscopy image, respectively. This work was supported by the Core Center for iPS Cell Research (20bm0104001h0008), Research Center Network for Realization of Regenerative Medicine from AMED (Japan Agency for Medical Research and Development) and a 2020 Gibon Yeongu Program from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2020R1F1A106727812 to S.I.K.).
Footnotes
AUTHOR CONTRIBUTIONS
P.K. and S.I.K. wrote the manuscript and S.I.K. secured funding.
CONFLICT OF INTEREST
S.I.K. is currently an employee of THERABEST, Co., Ltd. P.K. has no potential conflicts of interest to disclose.
REFERENCES
- Bachanova V., Miller J.S. NK cells in therapy of cancer. Crit. Rev. Oncog. 2014;19:133–141. doi: 10.1615/critrevoncog.2014011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki F., Bjordahl R., Gaidarova S., Mahmood S., Abujarour R., Wang H., Tuininga K., Felices M., Davis Z.B., Bendzick L., et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl. Med. 2020;12:eaaz5618. doi: 10.1126/scitranslmed.aaz5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delconte R.B., Kolesnik T.B., Dagley L.F., Rautela J., Shi W., Putz E.M., Stannard K., Zhang J.G., Teh C., Firth M., et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 2016;17:816–824. doi: 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- Dianat-Moghadam H., Rokni M., Marofi F., Panahi Y., Yousefi M. Natural killer cell-based immunotherapy: from transplantation toward targeting cancer stem cells. J. Cell. Physiol. 2018;234:259–273. doi: 10.1002/jcp.26878. [DOI] [PubMed] [Google Scholar]
- Felices M., Lenvik A.J., McElmurry R., Chu S., Hinderlie P., Bendzick L., Geller M.A., Tolar J., Blazar B.R., Miller J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:e96219. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, de Andrade L., Tay R.E., Pan D., Luoma A.M., Ito Y., Badrinath S., Tsoucas D., Franz B., May K.F., Jr., Harvey C.J., Jr., et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359:1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornalusse G.G., Hirata R.K., Funk S.E., Riolobos L., Lopes V.S., Manske G., Prunkard D., Colunga A.G., Hanafi L.A., Clegg D.O., et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017;35:765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C., Iwamoto S., Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis P., Takahashi K., Saito M., Yoshida Y., Okita K., Watanabe A., Inoue H., Yamashita J.K., Todani M., Nakagawa M., et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 2019;99:79–114. doi: 10.1152/physrev.00039.2017. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975a;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975b;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Knorr D.A., Ni Z., Hermanson D., Hexum M.K., Bendzick L., Cooper L.J., Lee D.A., Kaufman D.S. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl. Med. 2013;2:274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A., Gombert J.M., Auvray C., l'Homme B., Magniez A., Feraud O., Coulombel L., Chapel A., Mitjavila-Garcia M.T., Turhan A.G., et al. The HOXB4 homeoprotein promotes the ex vivo enrichment of functional human embryonic stem cell-derived NK cells. PLoS One. 2012;7:e39514. doi: 10.1371/journal.pone.0039514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.A. Cellular therapy: adoptive immunotherapy with expanded natural killer cells. Immunol. Rev. 2019;290:85–99. doi: 10.1111/imr.12793. [DOI] [PubMed] [Google Scholar]
- Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Tong Y., Dotti G., Shaim H., Savoldo B., Mukherjee M., Orange J., Wan X., Lu X., Reynolds A., et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Mantesso S., Geerts D., Spanholtz J., Kucerova L. Genetic engineering of natural killer cells for enhanced antitumor function. Front. Immunol. 2020;11:607131. doi: 10.3389/fimmu.2020.607131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquitta W.T., Wandsnider M., Kang H., Thomson J., Moskvin O., Suknuntha K., Slukvin I.I. UM171 expands distinct types of myeloid and NK progenitors from human pluripotent stem cells. Sci. Rep. 2019;9:6622. doi: 10.1038/s41598-019-43054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B., Bindea G., Angell H.K., Sasso M.S., Obenauf A.C., Fredriksen T., Lafontaine L., Bilocq A.M., Kirilovsky A., Tosolini M., et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl. Med. 2014;6:228ra237. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- Myers J.A., Miller J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeimi Kararoudi M., Nagai Y., Elmas E., de Souza Fernandes Pereira M., Ali S.A., Imus P.H., Wethington D., Borrello I.M., Lee D.A., Ghiaur G. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood. 2020;136:2416–2427. doi: 10.1182/blood.2020006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei V.Y.S., Siernicka M., Graczyk-Jarzynka A., Hoel H.J., Yang W., Palacios D., Almasbak H., Bajor M., Clement D., Brandt L., et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol. Res. 2018;6:467–480. doi: 10.1158/2326-6066.CIR-17-0207. [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K., et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Perica K., Varela J.C., Oelke M., Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 2015;6:e0004. doi: 10.5041/RMMJ.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E., Weisdorf D.J., Blazar B.R., Ustun C., DeFor T.E., et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Foley B., Lenvik T., Wang Y., Zhang B., Ankarlo D., Luo X., Cooley S., Verneris M., Walcheck B., et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A., Posati S., Rogaia D., Frassoni F., Aversa F., et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K., Capitini C.M., Saha K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res. Ther. 2020;11:234. doi: 10.1186/s13287-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- Srpan K., Ambrose A., Karampatzakis A., Saeed M., Cartwright A.N.R., Guldevall K., De Matos G., Onfelt B., Davis D.M. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018;217:3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Kaneko S. Induced pluripotent stem cell-derived natural killer cells gene-modified to express chimeric antigen receptor-targeting solid tumors. Int. J. Hematol. 2020 Jul 23; doi: 10.1007/s12185-020-02951-5. [Epub]. https://doi.org/10.1007/s12185-020-02951-5. [DOI] [PubMed] [Google Scholar]
- Ueda T., Kumagai A., Iriguchi S., Yasui Y., Miyasaka T., Nakagoshi K., Nakane K., Saito K., Takahashi M., Sasaki A., et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020;111:1478–1490. doi: 10.1111/cas.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekage M., Sato Y., Takasu N. Overview: an iPS cell stock at CiRA. Inflamm. Regen. 2019;39:17. doi: 10.1186/s41232-019-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B., Vuerich M., Daneshmandi S., Seth P. Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Front. Oncol. 2018;8:284. doi: 10.3389/fonc.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P.S., Grzywacz B., Tian X., Marcus R.K., Knorr D.A., Verneris M.R., Kaufman D.S. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P.S., Martin C.H., Miller J.S., Kaufman D.S. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J. Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- Woltjen K., Oceguera-Yanez F., Kagawa H., Kim S.I. In: At the conflux of human genome engineering and induced pluripotency. Genome Editing, Turksen K., editors. Springer; Cham, Switzerland: 2016. pp. 45–64. [Google Scholar]
- Wrangle J.M., Velcheti V., Patel M.R., Garrett-Mayer E., Hill E.G., Ravenel J.G., Miller J.S., Farhad M., Anderton K., Lindsey K., et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang B., Ono M., Kagita A., Fujii K., Sasakawa N., Ueda T., Gee P., Nishikawa M., Nomura M., et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019a;24:566–578.e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu Q., Zhong M., Wang Z., Chen Z., Zhang Y., Xing H., Tian Z., Tang K., Liao X., et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 2019b;12:49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lundqvist A. Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers (Basel) 2020;12:3586. doi: 10.3390/cancers12123586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon E.S., Burga R., Powell A., Cruz C.R., Fernandes R., Barese C., Nguyen T., Abdel-Baki M.S., Bollard C.M. Cord blood natural killer cells expressing a dominant negative TGF-beta receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19:408–418. doi: 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Zeng J., Tang S.Y., Toh L.L., Wang S. Generation of "off-the-shelf" natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. Stem Cell Reports. 2017;9:1796–1812. doi: 10.1016/j.stemcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zheng H., Diao Y. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int. J. Mol. Sci. 2019;20:317. doi: 10.3390/ijms20020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Blum R.H., Bernareggi D., Ask E.H., Wu Z., Hoel H.J., Meng Z., Wu C., Guan K.L., Malmberg K.J., et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell. 2020a;27:224–237.e6. doi: 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Blum R.H., Bjordahl R., Gaidarova S., Rogers P., Lee T.T., Abujarour R., Bonello G.B., Wu J., Tsai P.F., et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020b;135:399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]