Abstract
Signal-induced proliferation, differentiation, or stress responses of cells depend on mitogen-activated protein kinase (MAPK) cascades, the core modules of which consist of members of three successively acting kinase families (MAPK kinase kinase [MAP3K], MAPK kinase, and MAPK). It is demonstrated here that the MEKK3 kinase inhibits cell proliferation, a biologic response not commonly associated with members of the MAP3K family of kinases. A conditionally activated form of MEKK3 stably expressed in fibroblasts arrests these cells in early G1. MEKK3 critically blocks mitogen-driven expression of cyclin D1, a cyclin which is essential for progression of fibroblasts through G1. The MEKK3-induced block of cyclin D1 expression and of cell cycle progression may be mediated via p38 MAPK, a downstream effector of MEKK3. The MEKK3-mediated block of proliferation also reverses Ras-induced cellular transformation, suggesting possible tumor-suppressing functions for this kinase. Together, these results suggest an involvement of the MEKK3 kinase in negative regulation of cell cycle progression, and they provide the first insights into biologic activities of this kinase.
Mitogen-activated protein kinase (MAPK) cascades are protein kinase signal transduction pathways that have been remarkably conserved in evolution. They are differentially used to relay numerous extracellular signals within cells (reviewed in references 5, 9, 12, 18, and 25). Accordingly, these MAPK cascades have been found to be involved in such diverse cellular functions as proliferation, differentiation, stress responses, and apoptosis. The core of these kinase cascades is a three-tiered module consisting of a MAPK kinase kinase (MAP3K; also called MEKK), a MAPK kinase (MAP2K), and a MAPK. Activation is brought about via sequential phosphorylation reactions. Currently four major MAPK groups have been defined: (i) stress-activated protein kinases (SAPK)/Jun N-terminal kinases (JNK); (ii) p38 kinases; (iii) extracellular signal-regulated kinases (ERK1 and ERK2); and (iv) a more recently discovered distinct MAPK, ERK5 (5).
The SAPK and p38 pathways are primarily activated by proinflammatory cytokines, such as tumor necrosis factor alpha and interleukin-1, and various stress signals, such as UV treatment or osmotic stress (reviewed in references 9 and 18). A number of MAP2Ks (SEK1 and MKK7 for SAPKs; MKK3 and MKK6 for p38) and MAP3Ks (MEKK1 to 4, Ask1, TAK1, Tpl2, Mos, and possibly mixed-lineage kinases [MLKs]) have been demonstrated to regulate the SAPK and/or p38 pathway (9, 13, 53).
In contrast to the stress-responsive MAPKs, ERK1 and -2 are largely activated by mitogenic or differentiation-inducing stimuli. Activation of ERKs may occur via the MAP3K Raf-1 and the MAP2Ks MEK1 and MEK2, and whether proliferation or differentiation is the eventual outcome depends on the signal, the cell type, and the duration of activation (25, 37, 43). One major activator of this important pathway is the small GTPase Ras. Accumulated evidence suggests that Ras functions as a molecular switch for reentry of quiescent cells into the cell cycle, which is at least partly dependent on the Raf-MEK1/2-ERK pathway (16, 22, 33, 52). When very strongly activated, however, the Raf pathway may instead elicit a premature senescence in primary cells, possibly acting to limit the transforming potential of excessive Ras mitogenic signaling (21, 60).
In addition to ample documentation for roles of Ras and Raf in G1 progression and proliferation (16, 22, 35), several reports have also addressed the role of specific MAPKs in the passage through the cell cycle. In line with the notion that the ERK MAPK is a primary target of Ras and Raf signaling, ERK has been demonstrated to be essential in triggering proliferation in response to growth factors (33) and appears to be critical for proliferation in response to heterotrimeric G proteins (30, 46). Furthermore, ERK is able to activate or is required for cyclin D1 expression in various cell lines, with D-type cyclins playing an essential role in G1 progression (1, 19, 55). In contrast, p38 appears to negatively influence cell cycle progression, at least in fibroblasts (19, 31).
The major transitions of the eukaryotic cell cycle (G0/G1, G1/S, and G2/M) are triggered by different cyclin-dependent kinases (cdk’s) in conjunction with cyclins as the activating partners (reviewed in references 26 and 32). Ultimately, cdk activity is controlled by a number of transcriptional and posttranscriptional mechanisms to ensure proper timing and coordination of cell cycle events. The mammalian G1 cyclins include the three D-type cyclins D1, D2, and D3, which assemble predominantly with cdk4 in fibroblasts and macrophages or cdk6 in peripheral blood T cells, for example, and cyclin E, which associates with cdk2 (27, 47). Expression of the D-type cyclins is dependent on mitogenic stimulation, and growth factor withdrawal leads to rapid cyclin D destruction (7, 44, 47). For cells to reenter the cell cycle from a quiescent state, D-type cyclins, and to some extent their cdk4/6 partners, need to be upregulated (24, 27). In contrast, continually cycling cells contain relatively constant levels of the cdk subunits, and the amount of the inherently unstable cyclin D determines the level of cyclin D-dependent kinase activity; any reduction in cyclin D synthesis will rapidly contribute to G1 delay or even arrest. The major function of cyclin D-cdk complexes appears to be inactivation of the retinoblastoma gene product (pRb) by hyperphosphorylation, thereby liberating and thus activating the E2F transcription factor (38). E2F then activates the expression of genes that promote cell cycle progression, including the S-phase cyclin A (48). Cyclin E, which is expressed in late G1 to form an active kinase complex with cdk2 (47), contributes to the G1/S transition by controlling a rate-limiting step different from that of cyclin D, one independent of the pRb phosphorylation state (23, 38, 41).
Whereas cyclin D expression and associated kinase activity remain relatively constant throughout the cell cycle in continually cycling cells (as opposed to cells entering the cycle from a resting state), the kinase activities associated with cyclin E-cdk2, cyclin A-cdk2, and cyclin B1-cdc2 periodically rise and fall during cell cycle progression, with cyclin E-cdk2, cyclin A-cdk2, and cyclin B1-cdc2 being maximally active in late G1, S, and G2/M, respectively (47). This behavior is controlled by periodic synthesis and degradation of cyclins (17).
To elucidate the possible physiological function of a more recently cloned MAP3K termed MEKK3, we made use of fibroblast cell lines stably expressing an estrogen-activatable derivative of MEKK3, MEKK3-ER. Induction of MEKK3 activity with estrogen (E2) dramatically inhibits serum-induced proliferation of quiescent cells as well as proliferation of continuously cycling cells, without causing cell death. Our results indicate that MEKK3 blocks early cell cycle progression and that it does so by inhibiting serum-induced expression of cyclin D1. Ectopic expression of cyclin D1 overcomes this early MEKK3-induced cell cycle block. Inhibition of cyclin D1 expression may be mediated via the p38 MAPK. Most interestingly, the inhibitory influence of MEKK3 on cell cycle progression is dominant even over the activity of constitutively active Ras: MEKK3 strongly represses Ras-induced cyclin D1 expression and anchorage-independent growth. MEKK3’s ability to block cell proliferation may reflect a direct or indirect involvement of this kinase in cell cycle check point controls.
MATERIALS AND METHODS
Plasmids.
The following plasmids have been described previously (8): pCEV MEKK3CD-hERLBD (encoding a fusion protein of the catalytic domain of MEKK3 and the ligand binding domain of the human estrogen receptor [MEKK3-ER]); PMT2T HAp54β, pcDNAI HA-ERK2, and pCEV HAp38 (encoding hemagglutinin [HA]-tagged SAPK, ERK2, and p38, respectively); and expression plasmids for glutathione S-transferase (GST)–c-Jun and GST-ATF2. pcDNA3 MEKK3-ER was cloned as follows. The ligand binding domain of the human estrogen receptor (hERLBD) was excised from pCEV MEKK3CD-hERLBD as an a XhoI/EcoRI fragment and was then ligated into XhoI/EcoRI-cut PMT2TXSE (8) to yield PMT2TXSE hERLBD. To obtain PMT2T MEKK3-ER, the MEKK3 catalytic domain (an XhoI fragment excised from pCEV MEKK3CD-hERLBD) was ligated into the XhoI site of PMT2TXSE hERLBD. Finally, a BamHI/EcoRI fragment encoding MEKK3-ER was excised from PMT2T MEKK3-ER and inserted into BamHI/EcoRI-cut pcDNA3 (Invitrogen) to yield pcDNA3 MEKK3-ER. pRc/RSV cyclin D1 and pRc/RSV cdk4 were kindly provided by C. Sherr. pEGFPC1 was purchased from Clontech (Palo Alto, Calif.). pZip-ras(Q61L) was kindly provided by C. J. Der. Expression vectors for kinase-negative MKK6 (MKK6[KN]) and constitutively active MKK6 (MKK6[2E]) were obtained from E. Nishida.
Cell culture and transfections.
293 embryonic kidney cells and NIH 3T3 fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 1 mM glutamine, 10% fetal calf serum (FCS), and antibiotics. PC12 rat pheochromocytoma cells were grown in DMEM supplemented with 1 mM glutamine, 7% FCS, 7% donor horse serum, and antibiotics. To generate stable lines expressing MEKK3-ER, the cells were transfected with pCEV MEKK3D-hERLBD via calcium phosphate coprecipitation (NIH 3T3) or Lipofectamine (293 and PC12) as instructed by the manufacturer (Life Technologies, Inc., Gaithersburg, Md.). Two days later the cells were plated at various dilutions onto 10-cm-diameter plates, and the stably transfected cells were selected by addition of G418 at 0.5 (NIH 3T3 and PC12) or 0.25 (293) mg/ml. After 2 to 3 weeks, well-separated clones were picked with cloning rings, expanded, and analyzed for expression of the fusion protein by Western blot analysis with an antibody against the human estrogen receptor. To render cells quiescent, they were incubated in DMEM supplemented with 25 mM HEPES (pH 7.3) and 0.1% bovine serum albumin (BSA) for the times indicated in the figure legends. Unless otherwise indicated, cells were treated with 1 μM E2 (1,000× stock solution in ethanol) and with 10 μM SB203580 (2,000× stock solution in dimethyl sulfoxide). Control cells received the same amount of solvent only.
To evaluate the activity of transiently transfected SAPK, cells were transfected in 10-cm-diameter plates with a construct expressing HA-tagged SAPK as previously described (8). For the experiment shown in Fig. 5, NIH 3T3 cells stably expressing MEKK3-ER (NIH 3T3 [MEKK3-ER] cells) were plated at 2 × 104 onto poly-l-lysine-coated coverslips in 24-well plates and then transfected the following day with 0.25 μg of pEGFPC1 and either 0.75 μg of pRc/RSV or 0.6 μg of pRc/RSV cyclin D1 plus 0.15 μg of pRc/RSV cdk4 by using 6 μl of Superfect reagent as instructed by the manufacturer (Qiagen, Inc., Chatsworth, Calif.). For the experiment shown in Fig. 7C and D, NIH 3T3 cells were plated at 2.5 × 104 onto poly-l-lysine-coated coverslips in 12-well plates and transfected the following day, using Lipofectamine Plus (Life Technologies), with 0.1 μg of pEGFPC1 and a total of 0.4 μg of other expression plasmids as indicated in the figure legends.
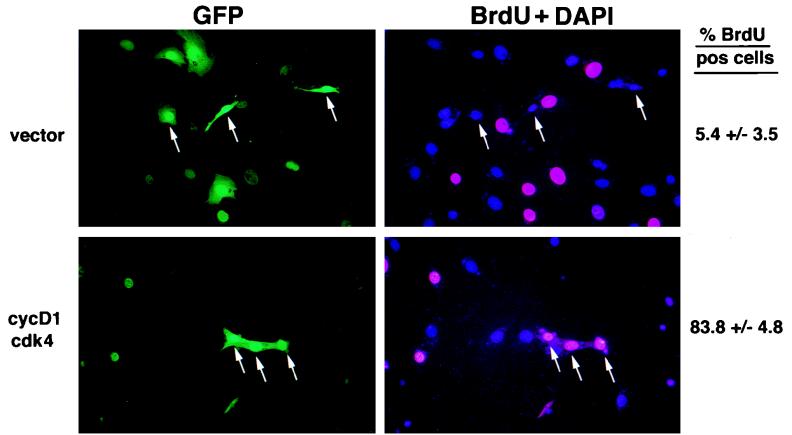
FIG. 5.
Importance of cyclin D1 downregulation in MEKK3-mediated inhibition of cell cycle progression. NIH 3T3 [MEKK3-ER] cells were transfected with GFP and either vector control or cyclin D1 (cycD1) and cdk4 expression vectors. One day after transfection, cells were kept without serum for 30 h in the presence of E2 and then incubated in complete medium with E2 and 10 μM BrdU for 15 h to label cells actively synthesizing DNA. Incorporated BrdU was detected by indirect immunofluorescence (right panel, red nuclei), and nuclei were identified with the general DAPI stain (right panel, blue nuclei). Transfected cells are identified by the green fluorescence of GFP (left panels). On the right the percentage of BrdU-positive cells in the transfected population is shown as the average (±standard deviation) after counting about 800 cells each from either vector- or cyclin D1-plus-cdk4-transfected cells from three different experiments. The arrows mark three examples of transfected cells for each of the two sets of transfections; most of the vector-only transfected cells (top left panel; GFP) were BrdU negative (top right panel), while cells transfected with cyclin D1 and cdk4 (bottom left panel) were mostly BrdU positive (bottom right panel). In this transient transfection experiment, more of the E2-activated NIH 3T3 [MEKK3-ER] cells were BrdU positive than what was observed for the E2-activated NIH 3T3 [MEKK3-ER] cells in the experiment shown in Fig. 3, because the transiently transfected cells could not be starved long enough to make all of them quiescent prior to serum stimulation.
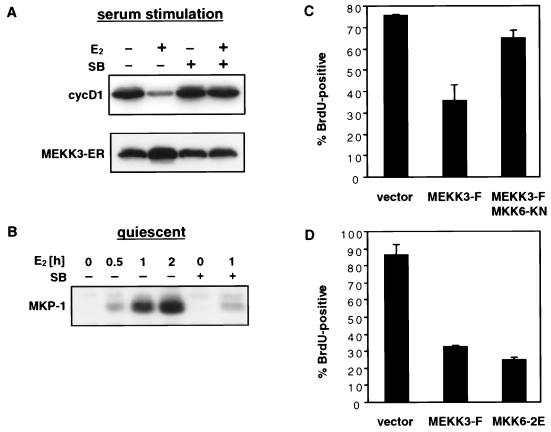
FIG. 7.
MEKK3 inhibits cyclin D1 expression and G1 progression via p38. (A) p38 activity is necessary for inhibition of cyclin D1 induction by MEKK3-ER. NIH 3T3 [MEKK3-ER] cells were kept without serum for 4 days, preincubated for 30 min with or without 10 μM SB203580 (SB), incubated in the presence of E2 for another 2 h, and then stimulated with 10% FCS in the presence or absence of SB203580 and/or E2 for 12 h. Equal amounts of protein (100 μg) were separated by SDS-PAGE and then sequentially immunoblotted with an anti-cyclin D1 antibody (cycD1), followed by an anti-estrogen receptor antibody to detect the stably expressed fusion protein (MEKK3-ER). (B) MEKK3-induced expression of MKP-1 is largely dependent on p38 activity. NIH 3T3 [MEKK3-ER] cells were rendered quiescent by being cultured for 2 days without serum and then were stimulated with E2 for various times, as indicated, without serum. Before addition of E2, some cells were preincubated with 10 μM SB203580 (SB) as well. MKP-1 expression was detected by Western analysis (100 μg/lane) with anti-Pac1 antibody, which cross-reacts with MKP-1. (C) Inhibition of G1/S progression by wild-type MEKK3 but relief of inhibition in the presence of MKK6[KN]. NIH 3T3 cells were transfected with GFP expression plasmid alone or together with 0.1 μg of an expression plasmid expressing full-length MEKK3 (MEKK3-F), in the absence or presence of 0.3 μg of an expression vector expressing MKK6[KN], as indicated. After transfection, the cells were incubated overnight, serum starved for 30 h, and then stimulated with 10% FCS in the presence of BrdU. The bars represent percentages of BrdU-positive cells in the transfected, GFP-positive population (±mean standard deviation of duplicate sets). More than 100 cells were counted for each sample, and similar results were obtained in two independent experiments. (D) Wild-type MEKK3 and a constitutively active form of MKK6 inhibit G1/S progression to similar extents. NIH 3T3 cells were transfected with 0.1 μg of GFP expression plasmid and 0.2 μg of an expression vector encoding MEKK3-F or MKK6[2E], a constitutively active form of MKK6. The cells were treated and evaluated as for panel C. In all experiments, total amounts of transfected DNA were kept constant with the addition of empty vectors as needed.
Cell extract preparation and Western blot analysis.
Cell extracts were prepared as described elsewhere (8), with some modifications. After treatments as indicated, cells were lysed directly on the plate or in 4 to 5 cell pellet volumes after scraping in cold phosphate-buffered saline (PBS). To evaluate the activity of cyclin-cdk complexes, Triton lysis buffer (8) containing only 0.5% Triton X-100 was used. For cdk4 complexes, cells were lysed in 0.1% Tween lysis buffer (50 mM HEPES [pH 7.5], 10% glycerol, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM dithiothreitol, 0.1% Tween 20, 25 mM β-glycerophosphate, 5 mM NaF, 0.5 mM Na3VO4, 7 μg of pepstatin per ml, and a protease inhibitor cocktail contained in Complete tablets from Boehringer Mannheim (Indianapolis, Ind.). For Western blot analyses, equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10 or 12.5% polyacrylamide gel, blotted onto Immobilon (Millipore Corp., Bedford, Mass.), and incubated with the following primary antibodies: anti-human ER, rabbit polyclonal sc-543 (Santa Cruz Biotechnology, Santa Cruz, Calif.), 1:1,500; anti-HA, mouse monoclonal 12CA5 (Boehringer Mannheim), 1 μg/ml; anti-cyclin A, rabbit polyclonal sc-596 (Santa Cruz), 1:1,000; anti-cyclin B1, mouse monoclonal sc-245 or rabbit polyclonal sc-752 (Santa Cruz), 1:1,000; anti-cyclin D1, mouse monoclonal sc-450 or sc-246 (Santa Cruz), 1:1,000; anti-cyclin D3, rat monoclonal sc-453 (Santa Cruz), 1:1,000; anti-cyclin E, rabbit polyclonal sc-481 (Santa Cruz), 1:1,000; anti-cdk2, rabbit polyclonal sc-163 (Santa Cruz), 1:1,000; anti-cdk4, rabbit polyclonal sc-260 (Santa Cruz), 1:1,000; anti-cdk7, rabbit polyclonal sc-529 (Santa Cruz), 1:1,000; anti-p21, rabbit polyclonal sc-397 (Santa Cruz), 1:1,000; anti-p27, rabbit polyclonal sc-776 (Santa Cruz), 1:1,000; anti-ERK1, rabbit polyclonal sc-094 (Santa Cruz) 1:1,000; anti-ERK2, goat polyclonal sc-145 (Santa Cruz), 1:1,000; anti-p38, rabbit polyclonal sc-535 (Santa Cruz), 1:1,000; anti-SAPK, rabbit polyclonal, prepared against a histidine-tagged fusion protein encoding full-length rat SAPKα (kindly provided by E. Nishida); anti-phospho-Y15 cdc2, rabbit polyclonal (New England Biolabs [NEB], Beverly, Mass.). 1:1,000; anti-active p38, rabbit polyclonal (NEB), 1:500; anti-active SAPK, rabbit polyclonal (NEB), 1:1,000; anti-human MEKK3, rabbit polyclonal, prepared against a GST fusion protein encoding amino acids 1 to 333 of human MEKK3, 1:3,000; anti-Pac-1(279–291) (40), which cross-reacts with MAPK phosphatase 1 (MKP-1), 1:250; anti-MKP-1, rabbit polyclonal sc-1199 (Santa Cruz), 1:1,000. Except for the MKP antibody 249 and the anti-active kinase antibodies, which were incubated at 4°C overnight in 5% bovine serum albumin (BSA) in TBST (Tris-buffered saline with 0.1% Tween 20), the primary antibodies were diluted in TBST with 5% nonfat dry milk and incubated with the membranes for 2 h at room temperature (RT). Immunoreactive bands were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL detection kit; Amersham Life Sciences, Inc., Arlington Heights, Ill.) except for the detection of MKP-1 and active MAPKs, for which a peroxidase-linked anti-rabbit antibody (NEB) was used.
Kinase assays.
The kinase activity of transfected HA-tagged SAPK was determined as previously described (8), using equal amounts of protein. Endogenous ERK activity was determined in the same way except that the indicated amount of lysate was immunoprecipitated with a mixture of anti-ERK1 (sc-094; Santa Cruz) and anti-ERK2 (sc-154; Santa Cruz) antibodies (Fig. 6A). To determine kinase activities associated with cyclins or cdks, immunocomplex kinase assays were performed after immunoprecipitation with appropriate antibodies in 0.5% Triton lysis buffer, except for cdk4, which was immunoprecipitated in 0.1% Tween lysis buffer. The immunoprecipitates were washed three times with immunoprecipitation buffer and two times with kinase buffer (8) supplemented with 5 mM MnCl2 and then incubated for 30 min at 30°C in 20 μl of kinase buffer containing 25 μM ATP, 10 μCi of [γ-32P]ATP, and 2 μg of histone H1 or 0.5 μg of GST-pRb (sc-4112; Santa-Cruz) as the substrate.
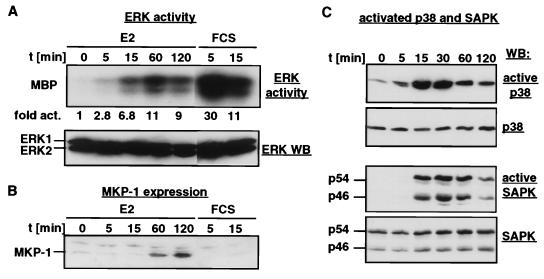
FIG. 6.
Transient activation of MAPKs by MEKK3. NIH 3T3 [MEKK3-ER] cells were serum starved for 2 days and then stimulated with E2 or serum for the indicated times. (A) Endogenous ERK activity (top panel) was measured in an immunocomplex kinase assay after immunoprecipitation with a mixture of antibodies recognizing ERK1 and ERK2, with MBP as the substrate. For comparison, the cells were also stimulated with serum. Kinase activity was determined with a PhosphorImager by measuring the amount of radioactivity that was incorporated into MBP; the data are presented as fold activation relative to the activity seen in unstimulated cells. The amount of extract used after serum stimulation was only 20% (30 μg) of that used after E2 treatment (150 μg). Thus, the maximal ERK activation achieved by MEKK3-ER is only about 7% of that obtained with serum stimulation. Western blot (WB) analysis with an anti-ERK2 antibody (bottom panel) which cross-reacts with ERK1 shows that equal amounts of ERK1 and ERK2 are present in the extracts. (B) MKP-1 expression is induced by MEKK3-ER. The Western blot shown in panel A was stripped and restained with an anti-MKP-1 antibody. (C) The activation state of endogenous p38 and endogenous SAPK was determined by Western blotting with antibodies recognizing only the active forms of the kinases (active p38 and active SAPK). An identical Western blot stained with anti-p38 and anti-SAPK revealed that the total amounts of these kinases do not vary among the samples.
Immunocytochemistry.
Cells grown on poly-l-lysine-coated coverslips were fixed with 3.7% formaldehyde in PBS for 15 min at RT, permeabilized with 3.7% formaldehyde in PBS supplemented with 0.5% Triton X-100 for 5 min at RT, and incubated in blocking solution (PBS with 2% BSA and 10% gamma globulinfree horse serum) for 30 min at RT. For tubulin staining, the fixed cells were incubated with a monoclonal antitubulin antibody (T5168; Sigma, St. Louis, Mo.) diluted 1:1,000 in blocking solution, washed three times with PBS, incubated with fluorescein isothiocyanate (FITC)-labeled anti-mouse secondary antibody (BioSource, Camarillo, Calif.) for 30 min at RT, washed four times with PBS, including 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; 1 μg/ml) in the last wash, dipped in water, and mounted in a glycerol-based mounting solution.
Cell cycle analysis and BrdU incorporation.
For flow cytometric analysis of the cell cycle, quiescent cells were stimulated with serum for 15 h in the presence of 10 μM bromodeoxyuridine (BrdU). Then cells were harvested by trypsinization, washed with PBS containing 1% BSA (PBS-BSA), fixed with 70% ethanol for 30 min on ice, and stored at −20°C. Between all steps, the cells were centrifuged for 5 min at 1,800 rpm in conical 14-ml centrifuge tubes in a swing-out rotor. For staining, the cells were washed with PBS-BSA, and the DNA was denatured with 2 N HCl–0.5% Triton X-100 for 30 min at RT. After neutralization with 0.1 M sodium borate (pH 8.5), the cells of one 10-cm-diameter plate were incubated with 125 μl of PBS–BSA–0.5% Tween 20 and 50 μl of FITC-labeled anti-BrdU antibody (BD 347583; Becton Dickinson, San Jose, Calif.) for 30 min at RT, washed twice with PBS-BSA, and treated with 3 μl of RNase A (Worthington, Lakewood, N.J.) for 30 min at 37°C. Just before fluorescence-activated cell sorting (FACS) analysis, propidium iodide was added to 20 μg/ml. Replicative DNA synthesis and DNA content were analyzed by using bivariate flow cytometry and Cell Fit software (Becton Dickinson).
To examine BrdU incorporation by indirect immunofluorescence, formaldehyde-fixed cells on coverslips were denatured and neutralized as described above, washed with PBS-BSA-Tween 20 and incubated with 100 μl of PBS-BSA-Tween 20 and 40 μl of anti-BrdU antibody (BD 347580; Becton Dickinson) per coverslip for 30 to 60 min at RT. After two washes with PBS and incubation in blocking solution (see Immunocytochemistry, above), bound primary antibody was detected with Cy3-labeled donkey anti-mouse antibody (Jackson ImmunoResearch, Westgrove, Pa.). Then the staining was completed as described above for immunocytochemistry.
Retrovirus-mediated gene transfer.
Retrovirus-mediated gene transfer into NIH 3T3 [MEKK3-ER] cells was done as described by Pear et al. (34), using Bosc23 as the packaging cell line and pZip-rasH(Q61L), which encodes constitutively active Ras, as the retroviral vector. Drug selection for neomycin resistance could not be used for retrovirally infected cells, because the original cell line already carried the neomycin resistance marker; however, selection was not necessary since viral infection was highly efficient and since constitutively active Ras provided a strong growth advantage (see Results and Fig. 8). The soft agar growth assay was done essentially as described elsewhere (6) except that Noble agar (Difco, Detroit, Mich.) was used instead of Bacto Agar.
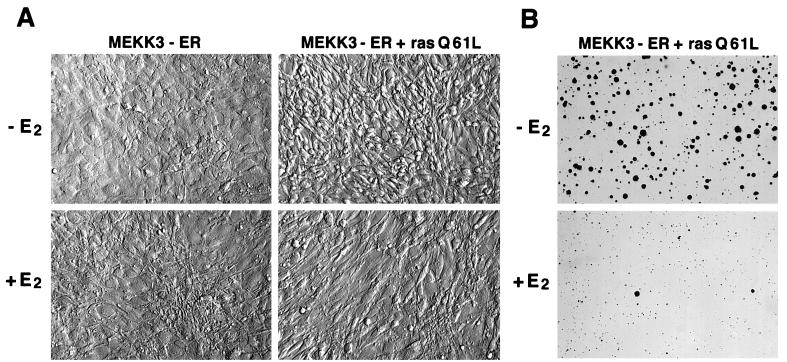
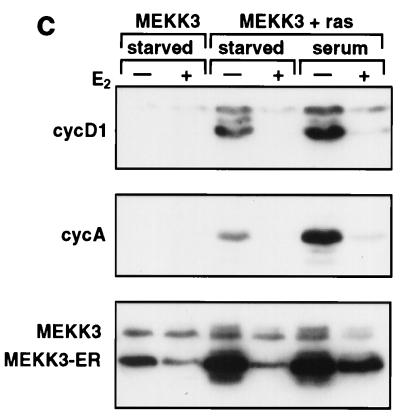
FIG. 8.
MEKK3 reverses cell-transforming activities of oncogenic Ras. NIH 3T3 [MEKK3-ER] cells were infected with a recombinant retrovirus expressing constitutively active RasQ61L and used as a pool of infected cells for all experiments. (A) Uninfected (left panels) or RasQ61L-infected (right panels) NIH 3T3 [MEKK3-ER] cells were seeded at low density in the absence of E2 (top panels) or when confluent in the presence of E2 (bottom panels). After 4 days when cells minus E2 had reached confluency as well, photographs were taken with Hoffmann optics, which gives a three-dimensional image of the cell surface. Ras-transformed cells show the characteristic rounded and spindle-shaped morphology (top right panel) which is largely reverted to a flat morphology in the presence of E2 (bottom panel right). (B) NIH 3T3 [MEKK3-ER] cells transformed with RasQ61L were tested for anchorage-independent growth in the presence (bottom panel) or absence (top panel) of E2, corresponding to active or inactive MEKK3-ER, respectively. Two weeks after seeding photographs were taken and the percentage of colonies consisting of at least five cells was determined in a total of 20 different fields from four different dishes and in two independent experiments, each with or without E2. (C) Untransformed (MEKK3) or RasQ61L-transformed (MEKK3 + ras) NIH 3T3 [MEKK3-ER] cells were starved in the absence of serum for 3.5 days with or without E2 and then either stimulated or not stimulated with 10% FCS for 15 h, with or without the continued presence E2, as indicated. Detergent lysates (100 μg) were separated by SDS-PAGE and immunoblotted with antibodies against cyclin D1 (cycD1), cyclin A, endogenous MEKK3, or the MEKK3-ER fusion protein, as indicated on the left. Ras-transformed cells express the G1-phase cyclin D1 even in the absence of serum, which is suppressed in the presence of E2 and active MEKK3-ER. Serum stimulation further increases cyclin D1 expression, but only in the absence of E2.
RESULTS
Activation of MEKK3 inhibits cell proliferation.
To investigate which role MEKK3 may play in cell physiology, we attempted to establish cell lines stably expressing wild-type, full-length MEKK3 but were unable to obtain such lines, although we readily obtained stable lines expressing a kinase-inactive mutant of MEKK3. These data suggest that functional MEKK3 may have an adverse effect on cell growth or may induce cell death, thus preventing selection of cells expressing wild-type MEKK3. As shown previously with transient expression systems, ectopically expressed MEKK3 was quite active, regardless of whether a full-length version or just the kinase domain (an N-terminal deletion of the full-length clone) (8) was expressed. To circumvent these problems, we generated NIH 3T3 cell lines which stably expressed an estrogen-inducible form of MEKK3, MEKK3-ER (see Materials and Methods). We have demonstrated previously in transient transfection assays that the kinase activity of MEKK3-ER is negligible in the absence of E2 but is rapidly and strongly inducible by treatment with estrogen (8). An analogous approach involving the Raf kinase has been successfully used previously: a stably expressed fusion of the Raf kinase domain and the estrogen ligand-binding domain (Raf-ER) was conditionally activated with E2 in NIH 3T3 cells to characterize the physiologic functions of the Raf kinase (16, 42, 57).
We obtained numerous NIH 3T3 cell clones which stably expressed the MEKK3-ER fusion, consistent with the fact that this fusion is inactive without E2 and thus unlikely to adversely effect cells. All data reported here were obtained with one cell clone which expressed only low levels of the MEKK3-ER fusion, but all major results were confirmed with several independently derived clones. In addition, we transiently transfected these clones with SAPK and ERK to demonstrate that activation of these kinases by MEKK-ER was entirely dependent on addition of E2 (data not shown; see also Fig. 2B).
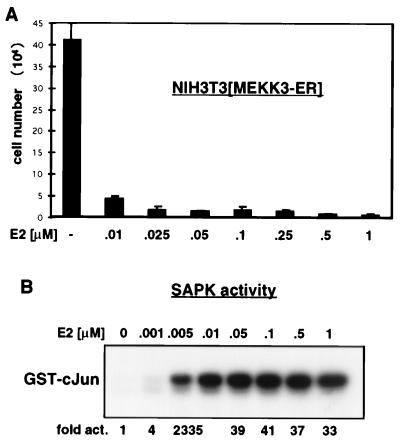
FIG. 2.
Activation of MEKK3 inhibits proliferation. (A) MEKK3-ER prevents cell division. NIH 3T3 [MEKK3-ER] cells were seeded in triplicate at 2 × 104 per well of a six-well plate in complete medium in the absence or presence of increasing concentrations of E2, as indicated. After 4 days, the attached cells were harvested by trypsinization, pooled with the detached cells, and counted in the presence of trypan blue to distinguish between live and dead cells. The percentage of dead cells was marginal, even with the highest concentration of E2. The total number of cells counted is indicated on the y axis (±mean standard deviation). As discussed in Results, no significant cell death could be measured. (B) Activation of SAPK by MEKK3-ER in the presence of increasing concentrations of E2. NIH 3T3 [MEKK3-ER] cells were transiently transfected with HA-tagged SAPK, serum starved overnight, and stimulated for 15 min with the indicated concentrations of E2. The activity of SAPK was measured in an immunocomplex kinase assay with GST–c-Jun as the substrate. After SDS-PAGE, phosphorylated GST–c-Jun was quantified with a Phosphorimager; data are expressed as fold activation relative to the signal obtained in the absence of E2. Western blot analysis confirmed equal expression of HA-SAPK in the different extracts (data not shown). Activation of SAPK and growth inhibition appear to occur at roughly the same low doses of E2.
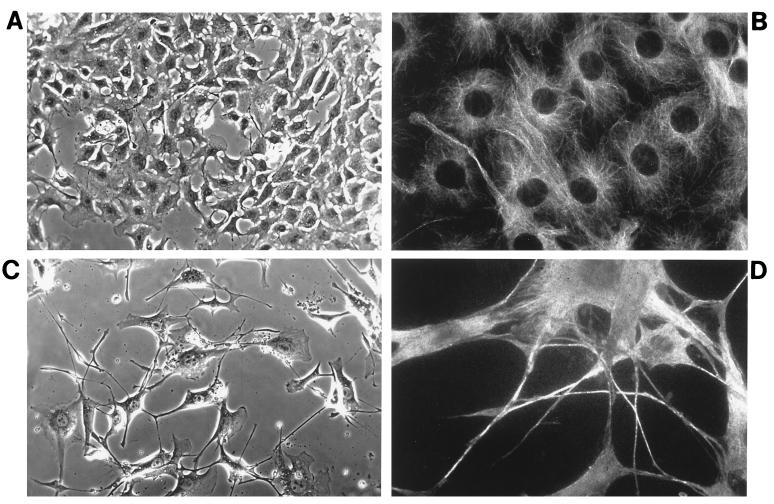
E2 treatment of NIH 3T3 [MEKK3-ER] fibroblasts led to inhibition of growth and to morphological changes (Fig. 1). Despite starting with the same number of cells, cultures kept with E2 for several days contained substantially fewer cells than those kept without E2 (Fig. 1A and C, respectively). The observed growth inhibition provides an explanation as to why transfection of wild-type MEKK3 (which is active) did not permit stably expressing cell lines to emerge. E2-treated cells had increased in size and possessed long processes which were clearly visualized by immunofluorescence staining for microtubules (Fig. 1D). Most likely these processes were stabilized, at least in part, by microtubule bundles. These features superficially resemble those of some terminally differentiated or senescent cells (50). Cell death was not a primary reason for the lower number of cells observed in the presence of E2, since none of the phenotypic changes typically associated with apoptosis were observed (confirmed with terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays [data not shown]), nor were significant numbers of dead or dying cells noted (as judged by trypan blue staining [data not shown]). E2 treatment of fibroblasts not expressing MEKK-ER did not induce any phenotypic changes, as expected and as documented previously (42) (data not shown).
FIG. 1.
Activation of MEKK3 induces morphological changes and formation of cell processes. NIH 3T3 [MEKK3-ER] fibroblasts were seeded onto poly-l-lysine-coated coverslips and incubated in complete medium in the absence (A and B) or presence (C and D) of 1 μM E2. After 4 days, the cells were fixed and either photographed under phase-contrast optics (A and C) or stained with antitubulin antibodies by indirect immunofluorescence (B and D). Magnifications of corresponding top and bottom panels are identical. The number of cells in the presence of E2 (activated MEKK3-ER) is much lower than that observed in the absence of E2 (A and B). Activated MEKK3-ER induces cells to increase in size and to send out long processes. These processes appear to be stabilized by microtubule bundles (D).
Quantitative evaluation of the number of NIH 3T3 [MEKK3-ER] cells grown in the absence or presence of increasing concentrations of E2 confirmed that activation of MEKK3 inhibits cell proliferation (Fig. 2A). Furthermore, the E2 dose-dependent profile of growth inhibition correlated quite well with the E2 dose-dependent MEKK3-ER kinase activity, as measured by the activation of ectopically expressed SAPK (compare Fig. 2B with Fig. 2A). Even the lowest levels of E2 with a measurable effect in the kinase assay significantly inhibited cell proliferation, which suggests that inhibition of cell growth is a primary biologic consequence of the activity of this kinase. Growth of NIH 3T3 [MEKK3-ER] cells was blocked over a wide range of concentrations of E2 ligand. This phenomenon was not unique to NIH 3T3 [MEKK3-ER] cells, since human 293 embryonic kidney cells and rat PC12 cells stably expressing MEKK3-ER did not divide in the presence of E2 either (data not shown). In contrast to MEKK3-ER-induced growth arrest, which occurs in the presence of both high and low doses of E2, Raf-ER-induced effects differ with high and low doses of E2 (45, 57).
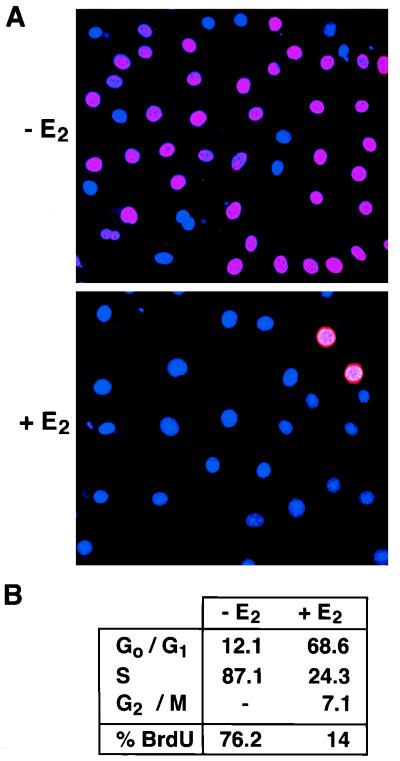
To investigate more closely how MEKK3-ER inhibits proliferation in NIH 3T3 cells, we performed cell cycle analysis and monitored DNA synthesis by incorporation of BrdU into DNA. Cells were starved with or without E2 and then stimulated with serum in the presence of BrdU (with continued addition of E2); 15 h after stimulation, cells were fixed and processed for indirect immunofluorescence (Fig. 3A) or cell cycle analysis (Fig. 3B). Activated MEKK3 profoundly inhibited DNA synthesis, as the number of BrdU-positive nuclei was drastically reduced in the presence of E2 (Fig. 3A). As judged by cell counting, the percentage of BrdU-positive nuclei was decreased from 72 to 5 by treatment with E2 (from data such as shown in Fig. 3A). Flow cytometric analysis independently confirmed that the number of cells actively synthesizing DNA was significantly lower in the presence of E2 (Fig. 3B, % BrdU). In addition, the percentage of cells in S-phase (DNA content between 2N and 4N) was reduced from 87 to 24 upon treatment with E2, whereas the percentage of cells in G0/G1 was increased from 12 to 69. These data suggest that MEKK3 exerts its inhibitory function on growth primarily by arresting cells in the G0/G1 phase of the cell cycle, preventing entry into S phase.
FIG. 3.
Activation of MEKK3 inhibits DNA synthesis. NIH 3T3 [MEKK3-ER] cells were starved in the absence of serum for 3 days with or without E2 and then stimulated with 10% FCS in the presence of 10 μM BrdU (with or without E2). (A) Cells were fixed and analyzed by indirect immunofluorescence for BrdU incorporation, shown in red. Nuclei were visualized with DAPI (blue). (B) Cells were processed for FACS analysis to quantitatively measure BrdU incorporation (% BrdU) and DNA content, the latter to determine the percentage of cells in different phases of the cell cycle. Similar results were obtained in two independent experiments. As demonstrated by both indirect immunofluorescence and FACS analysis, the number of cells actively synthesizing DNA is greatly reduced in the presence of active MEKK3.
Because NIH 3T3 cells usually start synthesizing DNA 12 to 14 h after serum stimulation, none of the cells cultured in the absence of E2 had yet reached a 4N DNA content (G2/M) at the time of harvest (15-h stimulation). Interestingly, some cells with a 4N DNA content could be detected in the E2-treated population. A possible explanation may be derived from results of a separate line of investigation which suggested that activated MEKK3-ER could also interfere with G2/M progression, in addition to blocking G0/G1 progression (8a). Therefore, in the above experiment, those cells which had not yet completed the cell cycle by the end of serum starvation (59) may be trapped at the 4N stage by the activation of MEKK3-ER, even when serum was added subsequently.
Downregulation of cyclin D1 and cdk activities upon activation of MEKK3.
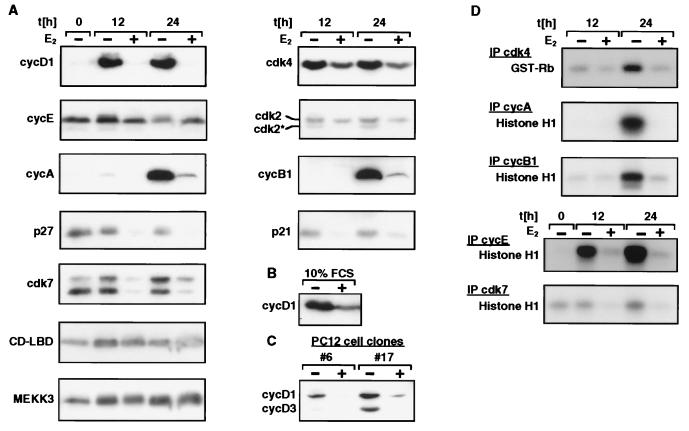
To obtain a more detailed picture of the MEKK3-ER-induced cell cycle arrest, we examined the expression of several cell cycle regulatory proteins by Western blotting (Fig. 4A to C). Cells were made quiescent and then released into complete medium for 12 or 24 h in the presence or absence of E2. MEKK3-ER profoundly inhibited the induction of cyclin D1, the critical D-type cyclin for progression of fibroblasts through G1 (27). In addition, and most likely as a consequence of D1 inhibition, the induced expression of cyclin A and cyclin B1 normally observed by 24 h after stimulation was blocked and the periodic phosphorylation and degradation of cyclin E was prevented (Fig. 4A). Finally, the levels of the kinase subunits cdk2 and cdk4 were reduced after MEKK3-ER activation. As would be expected then, the kinase activities associated with immunoprecipitated cdk4, cyclin E, cyclin A, and cyclin B1 were dramatically reduced (Fig. 4D). Interestingly, the level of cdk7 protein (Fig. 4A) and its associated kinase activity (cdk-activating kinase [CAK]) (Fig. 4D) were also decreased in the presence of active MEKK3-ER. CAK activates cdks via phosphorylation of a conserved threonine residue. Although its activity is not known to be highly regulated, its downmodulation by MEKK3 could contribute to cell cycle arrest. Consistent with this notion, the appearance of the faster-migrating form of cdk2 was prevented in the presence of E2, a form indicative of activating phosphorylation by CAK at T160 (10) (Fig. 4A). We conclude therefore that MEKK3-ER inhibits activation of cdks, downregulates CAK activity, and, most strikingly, inhibits early induction of D1.
FIG. 4.
Activation of MEKK3 inhibits various molecular parameters of cell cycle progression, as determined by analysis of inhibition of cyclin expression and CAK activities. (A and D) NIH 3T3 [MEKK3-ER] cells were kept without serum for 4 days in the presence or absence of E2 and then stimulated with 10% FCS for 12 or 24 h in the presence or absence of E2, as indicated. For the Western blot shown in panel B, cells were continuously kept in medium containing 10% FCS in the presence or absence of E2. cycD1, cyclin D1. (C) Two different clones of PC12 cells stably expressing MEKK3-ER (#6 and #17) were starved in the absence of FCS for 1 day with or without E2 and then released into medium containing 10% FCS for 2 days (with or without E2), as indicated. (A to C) Detergent lysates (100 μg) were electrophoretically separated on denaturing polyacrylamide gels and immunoblotted with antibodies against the proteins indicated on the left. CD-LBD designates the MEKK3-ER fusion protein, and cdk2* designates the T160 phosphorylated form of cdk2. (D) An aliquot of 100 μg (for cdk4 kinase assay) or 50 μg (all others) of extract was immunoprecipitated (IP) with the indicated antibodies and then assayed for associated kinase activity in an immunocomplex kinase assay with histone H1 or GST-pRb as the substrate.
While cell cycle arrest can be mediated by increased expression of cdk-inhibitory proteins (CKIs) (39, 49), MEKK3-ER does not appear to use this mechanism. The amounts of several CKIs tested, including p21, p27 (Fig. 4A), and p15 (data not shown), were not increased by MEKK3-ER activation; rather, the amounts of p21 and p27 appeared to be decreased. E2 treatment did not nonspecifically reduce protein expression, as judged by monitoring the levels of several other proteins, including MEKK3-ER and endogenous MEKK3 (Fig. 4A; equal amounts of protein were loaded in all lanes).
The inhibition of serum-induced cyclin D1 expression by activated MEKK3-ER was also observed in PC12 cells stably expressing MEKK3-ER (Fig. 4C; results for two independent clones are shown). Furthermore and consistent with the observed growth inhibition of continuously cycling NIH 3T3 [MEKK3-ER] cultures treated with E2, cyclin D1 levels were reduced in these cells as well, despite the continuous presence of serum (Fig. 4B). Reverse transcription-PCR analysis indicated that this was due, at least in part, to lower levels of cyclin D1 gene transcripts (data not shown).
Early cell cycle arrest depends on cyclin D1 suppression.
Since cyclin D1 expression is rate limiting for G1-phase progression of fibroblasts, it may represent a major target for MEKK3 to induce cell cycle arrest. To directly test the importance of reduced levels of cyclin D1 in MEKK3-ER-mediated arrest, we transfected NIH 3T3 [MEKK3-ER] cells with cyclin D1 plus cdk4 and green fluorescent protein (GFP) as a transfection marker (Fig. 5). The transfected cells were made quiescent in the presence of E2 and then stimulated with serum in the presence of BrdU and E2; cells were subsequently stained with an anti-BrdU antibody to assess the number of the transfected, GFP-positive cells that had actively synthesized DNA. Cells which ectopically expressed cyclin D1 and cdk4 had largely overcome the block imposed by E2-activated MEKK3-ER: nearly 84% of these cells were now positive for BrdU, compared to only 5.4% of the cells which did not express exogeneous cyclin D1-cdk (vector control) (examples of transfected, GFP-positive cells are indicated by arrows). This result strongly suggests that MEKK3-ER-mediated cell cycle arrest depends in large part on downregulation of cyclin D1; in addition, partial downregulation of cdk4 may contribute to the block as well.
MEKK3-induced suppression of cyclin D1 may be mediated by the p38 MAPK.
To further dissect the mechanisms by which MEKK3 may block cyclin D1 expression, we looked more closely at the signaling paths activated by MEKK3. Using a transient cotransfection system, we and others previously observed that constitutively active MEKK3 could activate ectopically expressed SAPK and ERK but apparently not p38 (3, 8). Given the present cell lines which stably express conditionally active MEKK3 [MEKK3-ER], we reexamined this issue and assessed the influence of MEKK3 on the endogenous MAPK pathways.
Endogenous ERK activity was transiently activated by E2 treatment of NIH 3T3 [MEKK3-ER] cells, as shown by a kinase assay of immunoprecipitated ERK with myelin basic protein (MBP) as the substrate (Fig. 6A). However, the level of ERK activation by MEKK3 was only about 7% of that obtained with serum; moreover, the kinetics were slightly slower. MEKK3 strongly activated endogenous SAPK and endogenous p38 in these cells. Activation of both kinases was rapid and transient (Fig. 6C). Activation of p38 and SAPK was demonstrated here with recently developed antibodies specific to the activated (phosphorylated) forms of these kinases; control Western analyses to visualize all forms of p38, SAPK, and ERK revealed that protein levels of these kinases did not change during the observation period (Fig. 6A and C). The kinetics and levels of activation of all three MAPKs were confirmed in independent experiments (data not shown). It is likely that activation of p38 was not readily observed in transient transfection experiments previously because the then commonly used p38 kinase assay appears to be relatively insensitive. When assayed with the antibodies specific to the activated form only, it was now possible to see E2/MEKK3-ER-dependent activation of p38 in transient cotransfection experiments as well. Importantly, wild-type MEKK3 behaved similar to E2-activated MEKK3-ER in such transient transfection experiments, further supporting the notion that the conditionally activated form of MEKK3 (MEKK3-ER) reflects the activity of wild-type MEKK3. Thus, both forms of this kinase similarly activated cotransfected SAPK, p38, and ERK (data not shown).
The transient nature of MAPK activation suggested that MEKK3 may induce the expression or activity of an MKP(s), which then rapidly inactivates MAPKs by dephosphorylation. Indeed, Western blot analysis revealed the expression of the MKP-1 by 30 to 60 min after stimulation with E2 (Fig. 6B). Thus, the rapid E2-MEKK3-ER-mediated induction of MKP-1 expression (and possibly other MKPs) could be responsible for the observed transience in activation of MAPKs. The data indicate that all three major MAPK pathways are targeted for activation by MEKK3, albeit to different degrees, and that inhibitory feedback mechanisms are most likely activated to then downmodulate MAPK activity.
Given previously reported negative effects of p38 (19, 31) and of ectopically expressed MKP-1 (4) on cyclin D1 expression and on cell cycle progression, we investigated their possible involvement in MEKK3-induced growth arrest. In support of an essential role of p38 in the MEKK3-induced block, we observed that addition of the p38 inhibitor SB203580 prior to activation of MEKK3-ER restored serum-induced cyclin D1 expression (Fig. 7A) and significantly reversed MEKK3-ER-imposed inhibition of cell proliferation (data not shown). This inhibitor of p38 also significantly interfered with MEKK3-ER-dependent induction of MKP-1, suggesting that p38 is a major contributor to MEKK3-ER-induced expression of MKP-1 in the absence of serum (Fig. 7B). Even in the presence of serum, activation of MEKK3-ER resulted in expression of MKP-1 much more prolonged than that observed with serum alone, and the prolonged expression was eliminated by SB203580 (data not shown). This finding suggests the possibility that prolonged MEKK3-induced expression of MKP-1-like phosphatases via p38 may contribute to inhibition of cyclin D1, possibly by keeping ERK activity below a necessary threshold over an extended period of time (15, 28, 56).
While the data implicate the only known highly sensitive target of SB203580, the p38 MAPK, as the essential mediator of the MEKK3-induced cell cycle block, it remains possible that this inhibitor has other, as yet unknown targets. This caveat was raised by the observation that SB203580’s selective inhibition of p38 appears to be based on a few critical amino acids in the ATP-binding region. When the analogous regions of several other kinases, including JNK, were mutated in only a few critical residues to better align them with the p38 sequence, then these mutant kinases acquired a weak sensitivity to this drug (11). However, even a 10-fold reduction in the amount of SB203580 in our experiments still significantly reversed the E2/MEKK3-ER-mediated inhibition of cyclin D1 (data not shown), ruling out an involvement of other only weakly inhibited kinases in the cell cycle block. In addition, we obtained independent evidence for a critical role of p38 in the MEKK3-induced cell cycle block. MKK6 is one of two known direct activators of p38 and we used an inactive mutant of this kinase (MKK6[KN]) to selectively interfere with the p38 pathway. MKK6[KN] largely reversed the MEKK3-induced inhibition of the cell cycle (Fig. 7C); conversely, a constitutively active mutant of MKK6 (MKK6[2E]) inhibited S-phase entry to about the same extent as did MEKK3 (Fig. 7D). In these experiments, we transiently cotransfected wild-type MEKK3 and GFP with or without MKK6[KN] and evaluated cell cycle progression/S-phase entry by counting GFP-positive (transfected) cells which also had incorporated BrdU into their DNA. MKK6[KN] alone had no effect on cell cycle progression. Together, these data support the notion that the p38 MAPK plays an essential role in MEKK3-induced cell cycle arrest. Furthermore, the results validate the use of MEKK3-ER as a model system to explore the physiologic roles of wild-type MEKK3, since transfection of the latter protein significantly interfered with cell cycle progression in a manner analogous to that of E2-induced MEKK3-ER.
MEKK3 is dominant over transformation by oncogenic Ras.
Overexpression of oncogenic Ras in NIH 3T3 cells has been shown to increase cyclin D1 expression, which is necessary for Ras-induced G1 progression (22). To examine whether MEKK3 can also block the stimulation of cell cycle progression by Ras, NIH 3T3 [MEKK3-ER] cells were infected with a recombinant retrovirus expressing constitutively active Ras (RasQ61L). Infection was very efficient, as judged by the appearance of exclusively rounded and spindle-shaped cells, a characteristic of transformed cells (Fig. 8A, top right panel). A further indication of the transformed phenotype of these cells was their refractile appearance under bright-field optics (data not shown). When incubated with E2, however, the morphology of the Ras-infected cells reverted to that seen with parental E2-activated MEKK3-ER cells (Fig. 8A, bottom panels); the E2-treated cells now formed long cell processes and, importantly, assumed a much flatter appearance.
This phenotypic analysis indicated that MEKK3 may be able to inhibit the activity of oncogenic Ras. To more rigorously test for a dominance of MEKK3 over oncogenic Ras, we also analyzed the soft agar cloning efficiency of RasQ61L-expressing NIH 3T3 [MEKK3-ER] cells in the presence or absence of E2 (Fig. 8B). Strikingly, anchorage-independent growth, another feature characteristic of transformed cells, was nearly completely blocked by activated MEKK3-ER. The cloning efficiency was reduced at least 50-fold upon addition of E2 (from about 46% to less than 1%).
In agreement with a previous report (22), we observed that Ras-transformed cells grew (data not shown) and continued to express relatively high levels of cyclin D1 (Fig. 8C), even in the absence serum. However, activated MEKK3-ER was able to prevent cyclin D1 expression in Ras-transformed cells, both in the presence and in the absence of serum. MEKK3-ER also interfered with Ras- and serum-induced cyclin A expression (Fig. 8C) and with growth of Ras-transformed cells in the presence of serum (data not shown). Although Ras transformation somehow caused an increase in the amount of the MEKK3-ER polypeptide (Fig. 8C; compare Ras-transformed and parental cells in the absence of E2), the growth-inhibitory activity of MEKK3-ER was still totally dependent on E2, consistent with the tight regulation of this fusion protein (see above).
DISCUSSION
We describe dramatic biologic consequences in cells conditionally activated for a single kinase, MEKK3. MEKK3 is a member of a larger family of MEK kinases (MEKKs and MAP3Ks) which include the structurally more closely related kinases MEKK1, -2, and -4, as well as the more distantly related kinases Raf, Ask1, TAK1, Tpl-2, Mos, MLKs, MUK, and NIK, among others. These kinases are thought to function as part of prearranged, core signaling modules, exemplified by the classical mammalian Raf→MEK→ERK-MAPK module (MEKK/MAP3K→MEK/MAP2K→MAPK). We now show that conditionally activated, stably expressed MEKK3 causes a profound growth arrest in early G1 and that it induces cytoskeletal changes reminiscent of some differentiated cell phenotypes. These are the first functions to be associated with MEKK3, a kinase for which no functions or pathways have been described before. The activities revealed in our studies here portend likely roles for this kinase in cell cycle checkpoint controls and possibly during differentiation and senescence. Thus, MEKKs are now clearly dedicated not only in proliferation, differentiation, stress responses, and apoptosis but also in growth arrest, as shown here for MEKK3. We demonstrate that the MEKK3-induced cell cycle arrest in early G1 is accomplished, at least in part, via suppression of cyclin D1 protein levels, and we furthermore provide evidence which suggests the suppression may be mediated via p38 MAPK. This mechanism for growth arrest is distinct from that set in motion by several growth-arresting extracellular signals which have been reported to exert their effects via induction of CKIs. Remarkably, MEKK3-induced growth arrest reverses even Ras-mediated cell transformation and is thus dominant over it, suggesting a possible tumor suppressor-like role of this kinase.
MEKK3 suppresses synthesis of cyclin D1 and arrests cells in G1. We have demonstrated the growth-inhibitory role of MEKK3 through the use of NIH 3T3 cell lines that stably express low levels of a tightly controlled, E2-inducible derivative of MEKK3, MEKK3-ER. Previously, an analogous, E2-activatable derivative of the Raf kinase (Raf-ER) has been used in stably transfected fibroblasts to characterize the physiologic targets and biologic responses to activation of the MEKK Raf, which is primarily involved in growth stimulation and cell transformation (42, 57). The MEKK3 derivative used in our studies appears to behave similarly to the wild-type version in transient transfection experiments, including activation of the same signaling pathways and inhibition of cell cycle progression, apparently via p38. Furthermore, the failure to generate stable cell lines with wild-type MEKK3, while a kinase-inactive mutant readily yielded such lines, also supports the notion that the growth inhibition seen with the E2-activated MEKK3-ER derivative in stable lines reflects a wild-type activity of this kinase. Finally, we note that growth inhibition of stably transfected NIH 3T3 [MEKK3-ER] lines occurs even at the lowest levels of E2 which can be demonstrated to measurably activate the MEKK3-ER kinase. Thus, we conclude that the conditional activation of MEKK3-ER in stably transfected NIH 3T3 cells represents a model system to identify and dissect functions of MEKK3.
Serum-induced expression of cyclin D1 is profoundly suppressed by MEKK3-ER, and this suppression appears to be an essential component of the MEKK3-induced growth arrest in the early G1 phase of the cell cycle. D-type cyclins have been critically implicated in progression through G1 (38, 41, 47), and cyclin D1 is the principal D-type cyclin in NIH 3T3 fibroblasts (27). MEKK3 suppresses serum-induced expression of cyclin D1 in quiescent cells as well as its expression in asynchronously growing cells kept in complete growth medium. The strong effect on cyclin D1 protein levels (Fig. 4A to C) is at least partially mediated by inhibition of cyclin D1 transcription (data not shown), although additional effects on translation and protein stability can not presently be excluded. To provide direct evidence that MEKK3 arrests cells in G1 by inhibiting synthesis of cyclin D1, we show that reintroduction of cyclin D1-cdk4 via transfection can largely overcome the MEKK3-ER-induced early G1 arrest, allowing serum-stimulated (previously quiescent) cells to enter into S phase, despite E2-mediated activation of MEKK3-ER.
In addition to the critical suppression of cyclin D1, MEKK3 may also target other activities to interfere with cell cycle progression. We note a reduced expression level of the G1 phase kinases cdk2 and cdk4, as well as of cdk7, the catalytic subunit of CAK. In a separate set of experiments, we have also developed evidence which suggests an inhibitory effect of MEKK3-ER during G2/M progression (8a). Nevertheless, the primary effect on cells in our experiments is a G1 arrest that depends in large part on suppression of cyclin D1 expression.
MEKK3-mediated suppression of cyclin D1 may occur via MAPK p38.
MEKK3 may mediate its growth-arresting functions via the p38 MAPK, since the p38-specific inhibitor SB203580 largely reversed this arrest: addition of this drug allowed for significant cell proliferation and reemergence of cyclin D1 in response to serum, despite the continuous presence of E2-activated MEKK3-ER.
Two reports clearly implicate p38 pathways in inhibition of cell cycle progression. By microinjection studies, Molnar et al. (31) were able to show that several components of p38 pathways can inhibit serum-stimulated cell cycle progression at the G1/S transition, including p38 itself, the p38-activating MAP2Ks, MKK3 (distinct from MEKK3), and MKK6, and cdc42Hs, a possible upstream activator of p38. Using a cyclin D1 promoter-dependent reporter construct, Lavoie et al. (19) demonstrated that the cyclin D1 promoter is regulated positively by the ERK pathway and negatively by the p38 pathway. Because the SB203580 inhibitor could theoretically target unknown proteins relevant to the MEKK3-induced cell cycle arrest, use of this inhibitor does not strictly prove an involvement of p38. However, we were also able to reverse the MEKK3-induced cell cycle arrest by inhibiting the p38 pathway in an independent manner. Cotransfection of a kinase-inactive form of a major direct activator of p38, MKK6[KN], allowed for entry of cells into S phase which were otherwise inhibited from doing so in the presence of active MEKK3. We were also able to rule out an involvement of proteins whose activity may be less specifically inhibited by SB203580, since even lower concentrations were still effective in relieving the arrest.
It is not known precisely how MEKK3 might utilize p38 to block cyclin D1 expression, but present data show a correlation of p38 activation with strong and sustained induction of MKP-1. MEKK3 also leads to delayed induction of MKP-3 (data not shown), and these and possibly other MEKK3-induced phosphatases may then critically attenuate ERK activity for an extended period of time after serum stimulation (33). In line with this hypothesis, expression of cyclin D1 and progression through G1 have been reported to depend on sustained ERK activity, well past the initial serum-induced peak of ERK activity (15, 28, 56). Future research is required to fully elucidate the molecular mechanisms by which MEKK3 suppresses cyclin D1 expression and arrests the cell cycle. A model for cell signaling emerges in which the precise relative levels and kinetics of activation of the various MAPKs are integrated through cross talk over time to determine the final physiologic response of cells. In this scenario, MKPs could be critical components in the quantitative regulation and cross talk.
MEKK3 reverts Ras-induced cellular transformation.
Oncogenic Ras induces the expression of cyclin D1 in NIH 3T3 cells, and this is necessary for its transforming activity (22). The strong growth-inhibitory effect of MEKK3-ER was evident even in Ras-transformed NIH 3T3 cells, indicating a dominance of MEKK3 over Ras and suggesting a tumor suppressor-like role for this kinase. The dominant negative effect of MEKK3-ER on both serum- and Ras-induced expression of cyclin D1 and on proliferation lends further support to the idea that the mechanism by which MEKK3 arrests cells may involve its negative effect on ERK, at least on prolonged activation of this kinase throughout G1. This is because Ras is known to induce cyclin D1 expression via the Raf-MEK-ERK pathway, and this pathway has been shown to be necessary for the transforming activity of oncogenic Ras (22, 25). Despite these correlations, however, mechanisms of cyclin D1 inhibition by MEKK3 which do not involve ERK modulation cannot yet be ruled out. It will be of interest to determine if mutational inactivation or modulation of function of MEKK3 occurs in association with tumor formation in vivo, since that may present a way for cells to bypass a growth-inhibitory signal mediated via MEKK3.
Possible biological functions of MEKK3.
A critical question which remains to be answered in future experiments is which signaling cascades flow through MEKK3. Immunocomplex kinase assays of endogenous MEKK3 revealed only high basal activity with several substrates, a level of activity which was only minimally modulated by any number of extracellular signals given to cells (data not shown). It is possible that regulated activity is lost with cell homogenization. We have observed endogenous MEKK3 to migrate as one to three bands on denaturing protein gels, with the slower-migrating species representing hyperphosphorylated forms (data not shown). Some extracellular stimuli can increase the amount of the slower-migrating forms, which suggests signal-dependent phosphorylation and thus possibly modulation of function. Ras transformation also caused increased phosphorylation of MEKK3 (Fig. 8). However, the relevance of these observations remains unclear since changes in the phosphorylation status of endogenous MEKK3 could not be correlated with significant changes in kinase activity as measured with immunocomplex assays.
The effects of conditional activation of the MEKK3 kinase described in this report, including cell cycle arrest in early G1, changes in cell morphology, and inhibition of Ras-induced transformation, suggest an involvement of MEKK3 in cell cycle checkpoint controls. It is conceivable, for example, that MEKK3 temporarily halts the cell cycle in G1 to allow cells time to deal with stress or damage. MEKK3 may have other functions as well, given the demonstrated activation of various MAPK signaling paths. Activation of p38 by MEKK3 may be important not only to arrest or slow down cells but also to coordinate a differentiated response to an environmental challenge, since the induced expression of various cytokines has been shown to involve p38 (20).
Another role of MEKK3 may be participation in cell differentiation. It is well established that cell cycle withdrawal and terminal differentiation are tightly coupled processes in many cell types. One mechanism for cell cycle withdrawal appears to involve upregulation of the cdk inhibitor p21, which has been described for various differentiation models (14, 29, 51, 54), including the nerve growth factor-induced neuronal differentiation of PC12 cells (2, 36). In this system, p21 is thought to critically inhibit cyclin D1-associated kinase activity (58). Although MEKK3 uses a different mechanism to downregulate cdk4-cyclin D1-associated kinase activity (MEKK3-ER prevents induction of cyclin D1), the final growth arrested phenotype appears to be similar to that seen during terminal differentiation processes. Indeed, MEKK3’s ability to arrest the cell cycle in early G1 may be part of its ability to induce a differentiation-like phenotype in PC12 cells, including the formation of neurite-like processes (data not shown). Finally, the phenotypic changes induced in E2-treated NIH 3T3 [MEKK3-ER] cells also superficially resemble those of senescent cells, which adopt a flat, enlarged morphology and cease proliferation at subconfluent densities, despite the presence of serum (reviewed in reference 50). The results described here reveal the first insights into functions of MEKK3, which include a profound cell cycle arrest in the absence of apoptosis or necrosis.
ACKNOWLEDGMENTS
We are most appreciative of technical assistance given by S. Lizarraga. We thank J. Der, C. Sherr, S. Gutkind, and E. Nishida for kindly providing plasmids. We are grateful to K. Holmes and D. Stephany of the NIAID Flow Cytometry Facility for assistance with flow cytometric analysis, to Ricardo Dreyfuss for the preparation of figures, and to M. Rust for help with preparation of the manuscript. We are indebted to Y. Ward and K. Takenaka for expert help and advice, E. Nishida for support during the final stages of this work, and K. Brown and A. Leonardi for discussions. We thank A. Fauci for continuous support and encouragement.
Footnotes
Present address: Department of Biophysics, Graduate School of Science, Kyoto University, Kitashirakawa-Oiwake, Sakyo-ku, Kyoto 606-8502, Japan.
REFERENCES
- 1.Albanese C, Johnson J, Watanabe G, Eklund N, Dzuy V, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 2.Billon N, van Grunsven L A, Rudkin B B. The cdk inhibitor p21WAF1/CIP1 is induced through a p300-dependent mechanism during NGF-induced neuronal differentiation of PC12 cells. Oncogene. 1996;13:2047–2054. [PubMed] [Google Scholar]
- 3.Blank J L, Gerwins P, Elliott E M, Sather S, Johnson G L. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. J Biol Chem. 1996;271:5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 4.Brondello J M, McKenzie F R, Sun H, Tonks N K, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- 5.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 6.Cox A D, Der C J. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. doi: 10.1016/0076-6879(94)38026-0. [DOI] [PubMed] [Google Scholar]
- 7.Diehl J A, Zindy F, Sherr C J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 8.Ellinger-Ziegelbauer H, Brown K, Kelly K, Siebenlist U. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J Biol Chem. 1997;272:2668–2674. doi: 10.1074/jbc.272.5.2668. [DOI] [PubMed] [Google Scholar]
- 8a.Ellinger-Ziegelbauer, H., and U. Siebenlist. Unpublished observations.
- 9.Fanger G R, Gerwins P, Widmann C, Jarpe M B, Johnson G L. MEKKs, GCKs, MLKs, PAKs, TAKs, and Tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- 10.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of cdk2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gum R J, McLaughlin M M, Kumar S, Wang Z, Bower M J, Lee J C, Adams J L, Livi G P, Goldsmith E J, Young P R. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB203580, by alteration of one or more amino acids within the ATP binding pocket. J Biol Chem. 1998;273:15605–15610. doi: 10.1074/jbc.273.25.15605. [DOI] [PubMed] [Google Scholar]
- 12.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 13.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Lin J, Su Z Z, Collart F R, Huberman E, Fisher P B. Induction of differentiation in human promyelocytic HL60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994;9:3397–3406. [PubMed] [Google Scholar]
- 15.Kahan C, Seuwen K, Meloche S, Poussegur J. Coordinate, biphasic activation of p44 mitogen-activated protein kinase and S6 kinase by growth factors in hamster fibroblasts. Evidence for thrombin-induced signals different from phosphoinositide turnover and adenylylcyclase inhibition. J Biol Chem. 1992;267:13369–13375. [PubMed] [Google Scholar]
- 16.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King R W, Deshaies R J, Peters J-M, Kirschner M W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie J N, L’Allemain G, Brunet A, Mueller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 20.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 21.Lin A W, Barradas M, Stone J C, van Aelst L, Serrano M, Lowe S W. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J-J, Chao J-R, Jiang M-C, Ng S-Y, Yen J J-Y, Yang-Yen H-F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995;5:3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 24.Lucas J J, Szepesi A, Modiano J F, Domenico J, Gelfand E W. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin D-associated cdk4 homologue in normal human T lymphocytes. J Immunol. 1995;14:6275–6284. [PubMed] [Google Scholar]
- 25.Marshall C J. Taking the Rap. Nature. 1998;392:553–554. doi: 10.1038/33293. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Castellanos C, Moreno S. Recent advances on cyclins, cdks and cdk inhibitors. Trends Cell Biol. 1997;7:95–98. doi: 10.1016/S0962-8924(96)10055-6. [DOI] [PubMed] [Google Scholar]
- 27.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meloche S, Seuwen K, Pages G, Poussegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- 29.Missero C, Calautti E, Eckner R, Chin J, Tsai L H, Livingston D M, Dotto G P. Involvement of the cell cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsui H, Takuwa N, Kurikawa K, Exton J H, Takuwa Y. Dependence of activated Gα12-induced G1 to S phase cell cycle progression on both ras/mitogen-activated protein kinase and ras/rac1/Jun N-terminal kinase cascades in NIH3T3 fibroblasts. J Biol Chem. 1997;272:4904–4910. doi: 10.1074/jbc.272.8.4904. [DOI] [PubMed] [Google Scholar]
- 31.Molnar A, Theodoras A M, Zon L I, Kyriakis J M. Cd42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 32.Morgan D O. Principles of cdk regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 33.Pages G, Kenormana P, L’Allemain G, Chambard J-C, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer, helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J A, Ewen M E. Ras signalling linked to the cell cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 36.Poluha W, Schonhoff C M, Harrington K S, Lachyankar M B, Crosbie N E, Bulseco D A, Ross A H. A novel nerve growth factor-activated pathway involving nitric oxide, p53, and p21WAF1 regulates neuronal differentiation of PC12 cells. J Biol Chem. 1997;271:24002–24007. doi: 10.1074/jbc.272.38.24002. [DOI] [PubMed] [Google Scholar]
- 37.Racke F K, Lewandowska K, Goueli S, Goldfarb A. Sustained activation of the extracellular signal-regulated/mitogen-activated protein kinase is required for megakaryocyte differentiation of K562 cells. J Biol Chem. 1997;271:23366–23370. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 38.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reysnidottir I, Massague J. The subcellular locations of p15INK4b and p27KIP1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 40.Rohan P J, Davis P, Moskaluk C A, Kearns M, Krutzsch H, Siebenlist U, Kelly K. Pac-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science. 1993;259:1763–1766. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- 41.Roussel M F, Theodoras A M, Pagano M, Sherr C J. Rescue of defective mitogenic signaling by D-type cyclins. Proc Natl Acad Sci USA. 1995;92:6837–6841. doi: 10.1073/pnas.92.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuels M L, Weber M J, Bishop J M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 44.Sewing A, Buerger C, Bruesselbach S, Schalk C, Lucibello F C, Mueller R. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci. 1993;104:545–554. doi: 10.1242/jcs.104.2.545. [DOI] [PubMed] [Google Scholar]
- 45.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shapiro P S, Evans J N, Davis R J, Posada J A. The seven-transmembrane-spanning receptors for endothelin and thrombin cause proliferation of airway smooth muscle cells and activation of the extracellular regulated kinase and c-Jun NH2-terminal kinase groups of mitogen-activated protein kinases. J Biol Chem. 1996;271:5750–5754. doi: 10.1074/jbc.271.10.5750. [DOI] [PubMed] [Google Scholar]
- 47.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 48.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1652. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 49.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 50.Stein G H, Dulic V. Origins of G1 arrest in sensecent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 51.Steinman R A, Hoffman B, Iro A, Guillouf C, Liebermann D A, el-Houseini M E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994;9:3389–3396. [PubMed] [Google Scholar]
- 52.Takuwa N, Takuwa Y. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:5348–5358. doi: 10.1128/mcb.17.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tibbles L A, Ing Y L, Kiefer F, Chan J, Iscove N, Woodgett J R, and Lassam N J. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe G, Lee R J, Albanese C, Rainey W E, Batle D, Pestell R G. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- 56.Weber J D, Raben D M, Phillips P J, Baldassare J. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan G Z, Ziff E B. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zetterberg A, Larsson O. Kinetic analysis of the regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365–5469. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu J, Woods D, McMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]