Abstract
Background
The aim of the RESGEX study was to compare the efficacy and safety of the anti-epidermal growth factor receptor (anti-EGFR) antibody tomuzotuximab against cetuximab both in combination with chemotherapy in patients with recurrent and/or metastatic squamous cell cancer of the head and neck in the first-line treatment.
Patients and methods
In this phase II trial 240 patients were equally randomized for six cycles to receive either tomuzotuximab (initial dose 990 mg then 720 mg) weekly and cisplatin 100 mg/m2 and fluorouracil (5-FU; 1000 mg/m2/day, days 1-4) every 3 weeks or cetuximab (400 mg/m2 subsequent 250 mg/m2) weekly with the same chemotherapeutic backbone followed by antibody maintenance treatment. The primary endpoint was progression-free survival.
Results
Median progression-free survival was 6.5 months [95% confidence interval (CI) 5.9-7.9 months] in the tomuzotuximab group and 6.2 months (95% CI 5.8-7.3 months) in the cetuximab group (P = 0.86). The median overall survival (OS) estimate was 11.6 months (95% CI 9.5-17.2 months) in the tomuzotuximab group and 13.8 months (95% CI 12.3-16.4 months) in the cetuximab group (P = 0.96). In an exploratory analysis a small subgroup of p16-positive patients had a significantly longer OS compared with p16-negative patients (hazard ratio 1.860, 95% CI 1.09-3.16, P = 0.02).
Conclusions
The glyco-engineered antibody tomuzotuximab failed to demonstrate improved efficacy with a chemotherapeutic backbone in the first-line treatment of recurrent or metastatic head and neck squamous cell carcinoma. It remains a so far unanswered question whether such antibody would partner better with different drugs such as checkpoint inhibitors.
Key words: tomuzotuximab, cetuximab, HNSCC, HNC, palliative care, head and neck cancer, ADCC
Highlights
-
•
Tomuzotuximab has a potential higher antibody-dependent cell cytotoxicity than other EGFR-directed antibodies.
-
•
Comparison of two anti-EGFR antibodies combined with chemotherapy in patients with squamous cell cancer of head and neck.
-
•
Efficacy, safety, and tolerability of tomuzotuximab and cetuximab in combination with chemotherapy were similar.
Introduction
Head and neck squamous cell carcinomas (HNSCCs) comprise tumors of the upper aerodigestive tract arising from the mucosal epithelia. Smoking, the excessive consumption of alcohol, and human papillomavirus infection in oropharyngeal tumors have been defined as common risk factors.1 Whereas surgery and concomitant radiochemotherapy are the standard approach in the curative setting, systemic treatment is the main pillar for recurrent or metastatic disease.2 In 2008, a new reference treatment termed EXTREME was reported, introducing the addition of the epidermal growth factor receptor (EGFR) antibody cetuximab to a cisplatin and 5-flourouracil chemotherapeutic backbone regimen, resulting in a significant and clinically meaningful prolongation of overall survival (OS).3 Various attempts to improve efficacy and toxicity of this reference treatment had little success.4 Therefore the EXTREME regimen remained the standard of care for patients with sufficient performance status, when patients for this trial were recruited. The recently introduced checkpoint inhibitors have added a new modality to the treatment of HNSCC. However, for patients without PD-L1 expression in the tumor tissue, chemotherapy remains the treatment of choice for the majority of patients.5,6
Optimization of the glycosylation of monoclonal antibodies has resulted in increased antigen-dependent cellular toxicities, a mode of action that has been described for cetuximab.7 Tomuzotuximab is an improved second-generation antibody, designed to fully retain the antigen-binding properties of cetuximab, with fully human glycosylation and reduced fucosylation.8 In the dose escalation single-agent study of tomuzotuximab in patients with advanced cancers, infusion-related reactions (IRRs) linked to cytokine release were observed in the majority of patients (75%) at the first infusion, demonstrating an increased immune activation.9
Fc-gamma receptor IIIa (FcγRIIIa) polymorphism has been associated with differential antibody-dependent cell cytotoxicity (ADCC) in various antibodies. Two allotypes of this receptor are known, which have different affinities to human IgG1 depending on the glycosylation of the Fc tail of IgG1. While antibodies with core fucose such as rituximab, trastuzumab, and cetuximab bind to the V allotype inducing some ADCC activity in homozygous patients, they show a strongly reduced binding to the F allotype with little or no ADCC activity in homozygous (FF) or heterozygous (FV) patients.10,11 In vitro assays showed that glyco-optimization of tomuzotuximab leads to a higher binding affinity of the molecule to the FcγRIIIa on natural killer cells, thereby enhancing ADCC activity.
Clinical data for the role of FcγRIIIa and the association of treatment response with cetuximab in colorectal cancer have been conflicting. Analysis of FcγRIIIa has therefore not been introduced into clinical routine testing to stratify for EGFR-directed treatment.12, 13, 14, 15
Based on improved antitumor potency of tomuzotuximab in preclinical evaluation and promising activity in early clinical application,9 the randomized phase II RESGEX study was designed to evaluate tomuzotuximab with chemotherapy against the standard EXTREME regimen in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck.
Methods
Study design and participants
The RESGEX study was a phase II, randomized, controlled, open-label, multicenter study of first-line treatment in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Treatment consisted of fluorouracil (5-FU; 1000 mg/m2/day, days 1-4) and cisplatin (100 mg/m2, on day 1) in combination with tomuzotuximab (initially 990 mg, subsequently 720 mg weekly) in arm A versus the identical 5-FU/cisplatin regimen in combination with standard cetuximab regimen (initially 400 mg/m2, subsequently 250 mg/m2 weekly) in arm B. Patients aged at least 18 years with histologically confirmed recurrent and/or metastatic HNSCC not eligible for curative treatment were enrolled in this study. Additional inclusion criteria included measurable disease and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients with prior systemic chemotherapy were excluded, except if given as part of a multimodal treatment for locally advanced disease which was completed >6 months prior to randomization. Exclusion criteria included prior treatment with cetuximab or another EGFR targeting agent, surgery within 30 days, concomitant antitumor therapy, immunotherapy or live vaccines, and renal or hepatic impairment. Treatment cycles were scheduled to be repeated every 3 weeks in the absence of prohibiting toxicity or tumor progression for a maximum of six cycles, and followed by a weekly single-agent maintenance therapy of tomuzotuximab (720 mg, weekly) or cetuximab (250 mg/m2, weekly), respectively. Evaluation of tumor size by computed tomography or magnetic resonance imaging was scheduled every 6 weeks after randomization. Toxicity was graded and recorded following National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE version 4). In case of relevant cisplatin-related toxicity switch to carboplatin was possible. The study was conducted at 43 centers in Germany, Belgium, France, Italy, Poland, Romania, Spain, and the United States. The study protocol and all amendments were approved by the appropriate ethics committee at each center. The study was conducted in accordance with the protocol, its amendments, and standards of good clinical practice. All participants provided written informed consent before enrollment.
Objectives, statistical considerations, and randomization
The primary objective of the study was to evaluate the efficacy and safety of tomuzotuximab as compared with cetuximab, both in combination with platinum-based chemotherapy in terms of progression-free survival (PFS). Analysis was planned after 192 events to demonstrate an increase in PFS from 5.6 to 8.4 months in the experimental arm, corresponding to a hazard ratio of 0.67. Based on this assumption 240 patients had to be randomized. Secondary objectives were to evaluate further efficacy criteria, which were best overall response rate, objective response rate, clinical benefit rate, duration of response, OS, time to treatment failure, safety, and quality of life (QoL) assessed by EORTC-QLQ-C30 and EORTC-QLQ-H&N35. QoL was assessed on day 1 of cycle 1, as well as on day 1 of cycles 3 and 5, 18 weeks after randomization and 28 days after last treatment administration (safety visit). Further objectives were to evaluate pharmacokinetic parameters and profiles of tomuzotuximab and to assess efficacy and safety based on genetic markers for immune response (FcγR allotypes).
The primary efficacy analysis for PFS as assessed by the investigator was based on the intent-to-treat (ITT) population. A 2-sided log-rank test was used to test the null hypothesis of equal treatment effects at an overall significance level of .05. The Kaplan–Meier method was used to estimate the survival functions.
Randomization to tomuzotuximab versus cetuximab was performed using a 1 : 1 ratio and was stratified by FcγRIIIa status (FF or FV or VV), oral cavity and oropharynx versus other locations, locally recurrent versus metastatic disease, and EGFR treatment naïve versus EGFR treatment as part of multimodal therapy.
Results
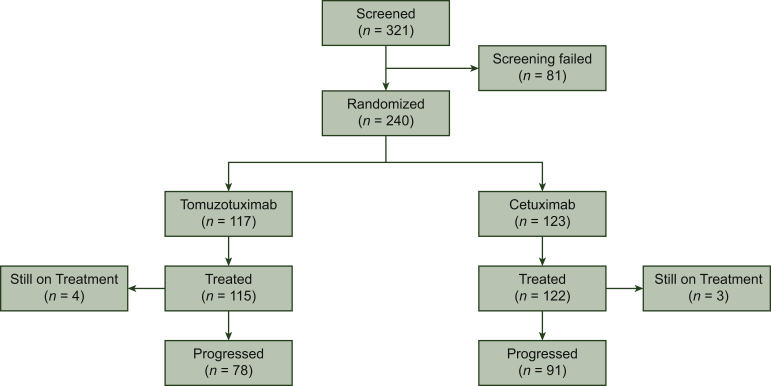
Between 27 February 2014 and 25 January 2016, a total of 321 patients were screened, of whom 81 (25.2%) failed screening. A total of 240 patients were randomly allocated to either arm A (tomuzotuximab 117 patients) or arm B (cetuximab 123 patients), which constituted the ITT population. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Arm A (tomuzotuximab), n = 117 | Arm B (cetuximab), n = 123 | |
|---|---|---|
| Age, years | 59.8 | 59.8 |
| Sex, n (%) | ||
| Female | 18 (15.4) | 17 (13.8) |
| Male | 99 (84.6) | 106 (86.2) |
| Eastern Cooperative Oncology Group, n (%) | ||
| 0 | 22 (18.8) | 26 (22) |
| 1 | 95 (81.2) | 96 (78) |
| Smoking status, n (%) | ||
| Nonsmoker | 20 (17.1) | 19 (15.4) |
| Former smoker | 71 (60.7) | 77 (62.6) |
| Active smoker | 26 (22.2) | 27 (22) |
| Primary tumor localization, n (%) | ||
| Oral cavity | 37 (31.6) | 38 (30.9) |
| Oropharynx | 41 (35) | 46 (37.4) |
| Hypopharynx | 13 (11.1) | 15 (12.2) |
| Larynx | 18 (15.4) | 17 (13.8) |
| Other | 8 (6.8) | 7 (5.7) |
| Disease status, n (%) | ||
| Locally recurrent | 52 (44.4) | 57 (46.3) |
| Metastatic | 65 (55.6) | 66 (53.7) |
| p16 status, n (%) | ||
| Positive | 17 (14.5) | 15 (12.2) |
| Negative | 91 (77.8) | 104 (84.6) |
| Missing | 9 (7.7) | 4 (3.2) |
| FcγRIII allotype, n (%) | ||
| FF | 44 (37.6) | 48 (39) |
| FV | 55 (47) | 56 (45.5) |
| VV | 18 (15.4) | 19 (15.4) |
| Epidermal growth factor receptor, n (%) | ||
| Treatment naïve | 106 (90.6) | 114 (92.7) |
| Pretreated as part of multimodality treatment | 11 (9.4) | 9 (7.3) |
Overall, there were slightly more patients with metastatic disease than locally recurrent disease (54.6% versus 45.4%), and more than two-thirds of the population had their primary tumor in the oral cavity or oropharynx (67.9%) rather than other locations (32.1%). The majority of patients were EGFR treatment naïve (91.7%). The most frequently observed FcγRIIIa allotypes were the FV (46.3%) and FF (38.3%), with relatively few patients having the VV allotype (15.4%).
Overall, 169 patients (78 in arm A and 91 in arm B) experienced disease progression during the study (Figure 1). In both treatment groups, the most frequently reported reason for discontinuation other than progressive disease was an adverse event (AE): 19 and 17 patients in arms A and B, respectively. Furthermore, no statistical difference between the two arms was observed with regard to cumulative platinum dosage or percentage of patients switched to carboplatin.
Figure 1.
Patients' distribution.
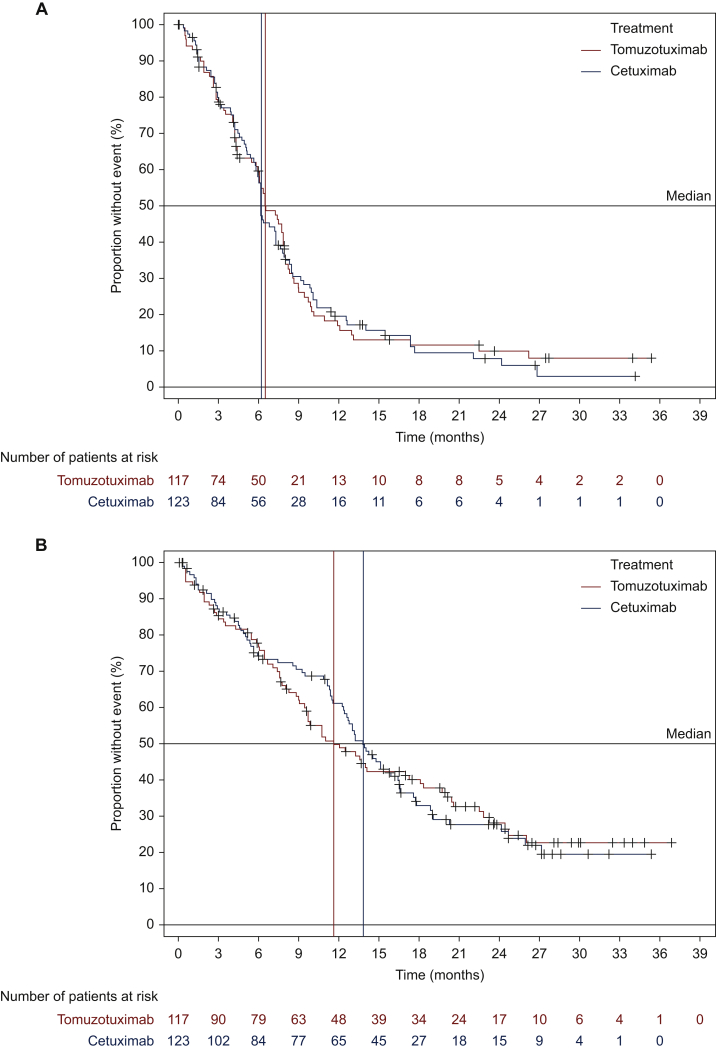
Comparison of the two treatment groups with regard to PFS showed no statistically significant difference between treatments (P = 0.86). Median PFS was 6.5 months [95% confidence interval (CI) 5.9-7.9 months] in arm A and 6.2 months (95% CI 5.8-7.3 months) in arm B (Figure 2A).
Figure 2.
Kaplan–Meier curves according to anti-EGFR treatments (ITT population).
(A) Progression-free survival (PFS): a multivariate Cox proportional hazards model, adjusted for the stratification factors, showed a hazard ratio of 1.000 (95% CI 0.736-1.359) for the comparison of PFS between the tomuzotuximab and cetuximab groups. (B) Overall survival: a multivariate Cox proportional hazards model, adjusted for the stratification factors, showed a hazard ratio of 1.012 (95% CI 0.734-1.396) for the comparison of OS.
CI, confidence interval; EGFR, epidermal growth factor receptor; ITT, intent to treat.
Based on the investigator assessment of response, an objective response was observed in 44.4% of patients (95% CI 35.4%-53.4%) in the tomuzotuximab group and 46.3% of patients (95% CI 37.5%-55.5%) in the cetuximab group (P = 0.77). Furthermore, no statistical difference was observed for clinical benefit rates [75.2% (95% CI 67.4%-83.0%) versus 76.4% (95% CI 68.9%-83.9%)] or duration of response [26.1 weeks (arm A) compared with 30.1 weeks (arm B)] nor for best overall response rate. The median OS estimate was 11.6 months (95% CI 9.5-17.2 months) in the tomuzotuximab group, numerically lower than in the cetuximab group (13.8 months; 95% CI 12.3-16.4 months; P = 0.96; Figure 2B).
Interestingly the subgroup of 32 (13.3%) p16-positive patients showed a significantly longer OS compared with 195 (81.3%) p16-negative patients (hazard ratio 1.86; 95% CI 1.09-3.16; P = 0.02), a difference which, although not formally analyzed, appeared to remain in the 17 patients under treatment with tomuzotuximab but not in the 15 patients under treatment with cetuximab. For patients who were p16 positive, the median OS was 23.2 months in the tomuzotuximab group, compared with 16.4 months in the cetuximab group. By contrast, for p16-negative patients, median OS was 9.8 months in the tomuzotuximab and 13.0 months in the cetuximab group (Figure 3).
Figure 3.
Kaplan–Meier plot of overall survival by p16 status subgroup (ITT population).
For patients who were p16 positive, the median overall survival (OS) was 23.2 months in the tomuzotuximab group, compared with 16.4 months in the cetuximab group. By contrast, for p16-negative patients, median OS was 9.8 months in the tomuzotuximab group and 13.0 months in the cetuximab group. The Cox regression model showed a statistically significant difference in OS between the p16-negative patients and the p16-positive patients (hazard ratio 1.86, 95% confidence interval 1.09-3.16, P = 0.022).
ITT, intent to treat.
The study stratified for FcγRIIIa allotypes based on preclinical data showing an increased ADCC for the V allotype. No difference in efficacy based on the different FcγRIIIa allotypes has been observed (data not shown). As seen with efficacy data no differences were detected for QoL between the two treatment arms (Supplementary material, available at https://doi.org/10.1016/j.esmoop.2021.100242). Concerning safety, no new safety signals were detected. Almost all patients experienced an AE during study treatment (99.2%). An overall summary of the serious adverse events (SAEs) detected in more than three study participants is given in Table 2. Serious AEs were reported in 148 patients overall (62.4%); a total of 164 SAEs were reported in 70 patients (60.9%) in arm A, with 181 SAEs in 78 patients (63.9%) in arm B. Among these, 20 patients (17.4%) in the tomuzotuximab group and 14 patients (11.5%) in the cetuximab group died due to treatment-emergent AE. These were mainly infectious, including sepsis (n = 10), cardiac or vascular events (n = 9), or hemorrhage (n = 8) next to less frequent causes. While the incidence of AEs was generally comparable between the two treatment groups, there was a marked disparity in the incidence of IRRs. The most frequently reported IRR in arm A was chills, reported in 25 patients (21.7%), compared with two patients (1.6%) in arm B. The incidence of patients who remained on cisplatin treatment rather than switching to carboplatin was comparable in arm A (80.0%) and the cetuximab group (81.1%).
Table 2.
Summary of the most frequently reported serious adverse events during the study (in ≥3 of the total patients)
| System organ class/preferred term | Tomuzotuximab (N = 115), n (%) | Cetuximab (N = 122), n (%) | Total (N = 237), n (%) |
|---|---|---|---|
| Any serious adverse event | 70 (60.9) | 78 (63.9) | 148 (62.4) |
| Infections and infestations | 22 (19.1) | 26 (21.3) | 48 (20.3) |
| Pneumonia | 12 (10.4) | 9 (7.4) | 21 (8.9) |
| Sepsis | 3 (2.6) | 7 (5.7) | 10 (4.2) |
| Device-related infection | 5 (4.3) | 4 (3.3) | 9 (3.8) |
| Lung abscess | 0 | 3 (2.5) | 3 (1.3) |
| Blood and lymphatic system disorders | 20 (17.4) | 17 (13.9) | 37 (15.6) |
| Anemia | 9 (7.8) | 9 (7.4) | 18 (7.6) |
| Neutropenia | 8 (7.0) | 7 (5.7) | 15 (6.3) |
| Thrombocytopenia | 2 (1.7) | 4 (3.3) | 6 (2.5) |
| Febrile neutropenia | 3 (2.6) | 2 (1.6) | 5 (2.1) |
| Leukopenia | 1 (0.9) | 4 (3.3) | 5 (2.1) |
| Metabolism and nutrition disorders | 14 (12.2) | 18 (14.8) | 32 (13.5) |
| Dehydration | 4 (3.5) | 5 (4.1) | 9 (3.8) |
| Hypomagnesaemia | 4 (3.5) | 5 (4.1) | 9 (3.8) |
| Hypokalemia | 3 (2.6) | 4 (3.3) | 7 (3.0) |
| Decreased appetite | 2 (1.7) | 3 (2.5) | 5 (2.1) |
| General disorders and administration site conditions | 13 (11.3) | 14 (11.5) | 27 (11.4) |
| General physical health deterioration | 2 (1.7) | 2 (1.6) | 4 (1.7) |
| Device dislocation | 1 (0.9) | 2 (1.6) | 3 (1.3) |
| Fatigue | 2 (1.7) | 1 (0.8) | 3 (1.3) |
| Mucosal inflammation | 3 (2.6) | 0 | 3 (1.3) |
| Gastrointestinal disorders | 11 (9.6) | 9 (7.4) | 20 (8.4) |
| Diarrhea | 3 (2.6) | 2 (1.6) | 5 (2.1) |
| Nausea | 3 (2.6) | 2 (1.6) | 5 (2.1) |
| Vomiting | 2 (1.7) | 2 (1.6) | 4 (1.7) |
| Respiratory, thoracic, and mediastinal disorders | 9 (7.8) | 10 (8.2) | 19 (8.0) |
| Pulmonary embolism | 2 (1.7) | 3 (2.5) | 5 (2.1) |
| Nervous system disorders | 5 (4.3) | 8 (6.6) | 13 (5.5) |
| Cerebrovascular accident | 2 (1.7) | 1 (0.8) | 3 (1.3) |
Discussion
In the RESGEX study, a randomized phase II study, the glyco-optimized monoclonal antibody did not improve PFS compared with cetuximab with a common chemotherapeutic backbone. Furthermore, other parameters of efficacy and subgroup analyses showed no differences between the two treatment arms. Remarkable were the relatively high response and OS rates compared with the previously published data in the EXTREME trial.3 This phenomenon is in line with the results of the recent TPEx trial, where also unexpectedly high response and survival data were observed in both study arms.6 It remains elusive whether this is a phenomenon by chance, as we did not observe any differences in the KEYNOTE-048 trial control group compared with the EXTREME study population, or whether improved management of comorbidities and toxicities has led to improved outcome.5 An additional explanation for the favorable results might be the prerequisite of cisplatin eligibility in the TPEx study, which might have excluded patients with relevant comorbidities. Furthermore, the patient population of the original EXTREME trial was slightly different from the RESGEX study, especially with regard to inclusion of patients with ECOG status 2 in the former trial, which accounted for 12%. Those factors need to be taken into consideration for data interpretation and future trial planning.
p16 positivity has been established as a prognostic marker in the curative setting. In the metastatic setting this role remains less clear.16 Within the RESGEX study an exploratory analysis showed that the small subgroup of p16-positive patients had a significantly longer OS compared with p16-negative patients.
The study stratified for FcγRIIIa allotypes based on preclinical data of increased ADCC for the V allotype. However, there was no difference in efficacy based on the different FcγRIIIa allotypes. Possible reasons are the amelioration of ADCC due to the combination with chemotherapy. Furthermore, diminished immune reactivity in the advanced course of disease might be another possible reason for the lack of expected activity.17
The safety and tolerability of tomuzotuximab in combination with chemotherapy (5-FU and cisplatin) were similar to those of cetuximab in this study, although an expected increase in the incidence of IRRs was observed relative to cetuximab treatment, driven largely by the incidence of chills. No new safety signals or trends were noted.
The RESGEX study therefore failed to demonstrate an improved efficacy with tomuzotuximab. It remains an interesting so far unanswered question whether the chemotherapeutic backbone diminished the suggested accelerated immune response and a glyco-optimized monoclonal antibody will partner better with agents such as a checkpoint inhibitor in an earlier course of the disease.
Acknowledgments
Funding
The study had been designed by senior author of this report together with the sponsor Glycotope, who also provided tomuzotuximab. The sponsor participated in data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and approved the final version for publication.
Disclosure
KK reports advisory board participation, invited speaker or conference honoraria from Merck, Sanofi, Merck Sharp & Dohme, Glycotope, Roche, Novartis, and Bristol-Myers Squibb. JF reports personal fees from MSD, BMS, AstraZeneca, Merck, Innate, Roche, Pfizer, and Rakuten; and nonfinancial support from MSD, BMS, AstraZeneca, and Pfizer. AK reports consulting fees from MSD and BMS, and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from AstraZeneca, BMS, MSD, and Merck; he also participates on a DSMB or Advisory Board of MSD, BMS, and Merck. AD reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events as well as participation on a DSMB or Advisory Board of MSD, Sanofi, BMS, and Nanobiotix. PS reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD, Merck Serono, AstraZeneca, and BMS; and support for attending meetings and/or travel from BMS, MSD, and Merck Serono. He participates on a DSMB or Advisory Board of MSD, BMS, and Merck Serono; and reports stock or stock options from AstraZeneca and BMS. GF reports grants or contracts from Merck Serono; consulting fees from BMS, MSD, Roche, Merck, Amgen, Bayer, Sanofi-Aventis, and Servier; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from BMS, MSD, Merck, Roche, Amgen, Falk, and Servier. PD reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer, LEO Pharma, and VIFOR Pharma; and support for attending meetings and/or travel for Pfizer and LEO Pharma. AJ is employee at Premier Research, the CRO conducting the study. IA-F, BD, AZ, and HB are employees or consultants at Glycotope, the sponsor of the study. In addition, HB owns stock or stock option of Glycotope. SO reports advisory board participation, invited speaker or conference honoraria from Merck, Sanofi, Merck Sharp & Dohme, and Bristol-Myers Squibb. UK reports advisory board participation, invited speaker or conference honoraria from Amgen, Astra Zeneca, Merck, Merck Sharp & Dohme, Glycotope, Novartis, Pfizer, Roche, and Bristol-Myers Squibb; research grants from AstraZeneca, Merck, and Pfizer. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Dhull A.K., Atri R., Dhankhar R., Chauhan A.K., Kaushal V. Major risk factors in head and neck cancer: a retrospective analysis of 12-year experiences. World J Oncol. 2018;9(3):80–84. doi: 10.14740/wjon1104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer J.D., Burtness B., Le Q.T., Ferris R.L. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16(11):669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken J.B., Mesia R., Rivera F. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Klinghammer K., Gauler T., Dietz A. Cetuximab, fluorouracil and cisplatin with or without docetaxel for patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CeFCiD): an open-label phase II randomised trial (AIO/IAG-KHT trial 1108) Eur J Cancer. 2019;122:53–60. doi: 10.1016/j.ejca.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Burtness B., Harrington K.J., Greil R. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 6.Guigay J., Fayette J., Mesia R. TPExtreme randomized trial: TPEx versus extreme regimen in 1st line recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) J Clin Oncol. 2019;37(suppl 15):6002. [Google Scholar]
- 7.Umana P., Jean-Mairet J., Moudry R., Amstutz H., Bailey J.E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 8.Kellner C., Otte A., Cappuzzello E., Klausz K., Peipp M. Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy. Transfus Med Hemother. 2017;44(5):327–336. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiedler W., Cresta S., Schulze-Bergkamen H. Phase I study of tomuzotuximab, a glycoengineered therapeutic antibody against the epidermal growth factor receptor, in patients with advanced carcinomas. ESMO Open. 2018;3(2):e000303. doi: 10.1136/esmoopen-2017-000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields R.L., Lai J., Keck R. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 11.Shinkawa T., Nakamura K., Yamane N. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 12.Bibeau F., Lopez-Crapez E., Di Fiore F. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 13.Park S.J., Hong Y.S., Lee J.L. Genetic polymorphisms of FcgammaRIIa and FcgammaRIIIa are not predictive of clinical outcomes after cetuximab plus irinotecan chemotherapy in patients with metastatic colorectal cancer. Oncology. 2012;82(2):83–89. doi: 10.1159/000335959. [DOI] [PubMed] [Google Scholar]
- 14.Shepshelovich D., Townsend A.R., Espin-Garcia O. Fc-gamma receptor polymorphisms, cetuximab therapy, and overall survival in the CCTG CO.20 trial of metastatic colorectal cancer. Cancer Med. 2018;7(11):5478–5487. doi: 10.1002/cam4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Gordon M., Schultheis A.M. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25(24):3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 16.Argiris A., Li S., Ghebremichael M. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014;25(7):1410–1416. doi: 10.1093/annonc/mdu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bridgewater J., Cervantes A., Markman B. Efficacy and safety analysis of imgatuzumab (GA201), a novel dual-acting monoclonal antibody designed to enhance antibody-dependent cellular cytotoxicity, in combination with FOLFIRI compared to cetuximab plus FOLFIRI in second-line KRAS exon 2 wild type or with FOLFIRI alone in mutated metastatic colorectal cancer. J Clin Oncol. 2015;33:669. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.