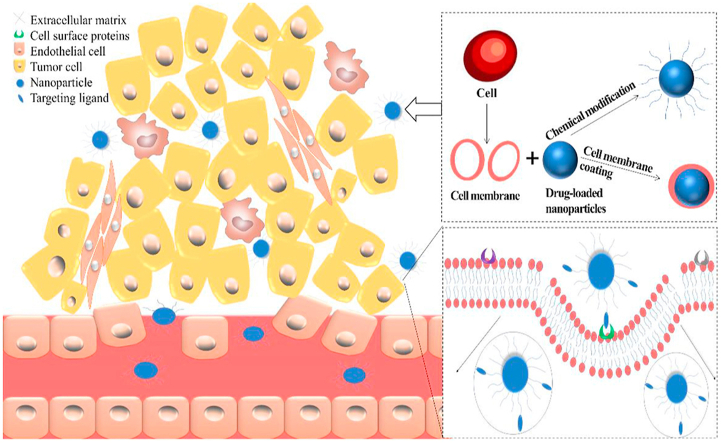
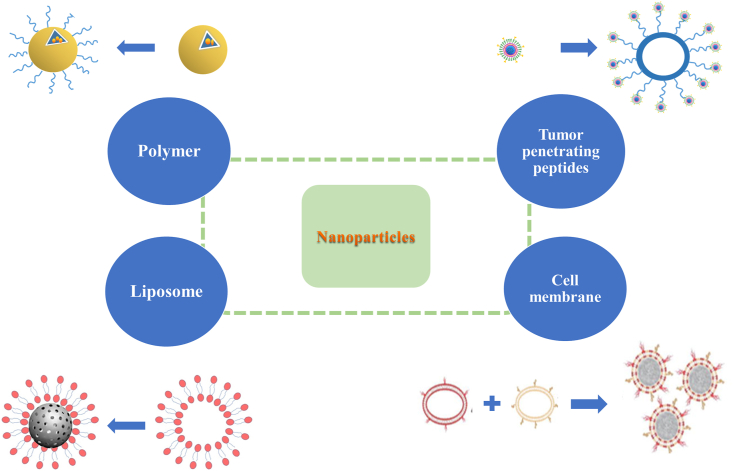
Abstract
The administration of nanoparticles (NPs) first faces the challenges of evading renal filtration and clearance of reticuloendothelial system (RES). After that, NPs infiltrate through the expanded endothelial space and penetrated the dense stroma of tumor microenvironment to tumor cells. As long as possible to prolong the time of NPs remaining in tumor tissue, NPs release active agent and induce pharmacological action. This review provides a comprehensive summary of the physical and chemical properties of NPs and the influence of various biological factors in tumor microenvironment, and discusses how to improve the final efficacy through adjusting the characteristics and structure of NPs. Perspectives and future directions are also provided.
KEY WORDS: Nanoparticles, Drug delivery, Reticuloendothelial system, Tumor microenvironment, Tumor stroma, Cancer-associated fibroblasts, Extracellular matrix, Tumor vascular endothelial cells
Graphical abstract
Nanoparticles, which are specially modified, reach around the tumor site through the blood system, interacting with tumor cells after passing through capillary wall and dense tumor stroma.
1. Introduction
As a new drug delivery system, NPs have many essential advantages in targeted therapy. It improves the bioavailability of insoluble drugs, enhances the anti-tumor therapeutic effect, reduces side effects and enhances patient compliance1, 2, 3, 4, 5, 6. Through advancement of diagnosis techniques, prevention strategies and treatment, nanotechnology has shown great potential in improving cancer treatment. Nowadays, NPs have been widely used in various fields of pharmaceutical research. Combining with the tumor microenvironment (TME), researchers have designed various NPs and modified their surfaces. The nano-drug delivery system has specific physical and chemical properties, thereby improving the treatment efficiency of the corresponding tumors. At present, common nanocarriers for drug delivery, especially for targeted cancer therapy, include liposomes, micelles, polymeric NPs, dendrimers, inorganic carriers7, such as silica8, carbon9, 10, 11, gold12, 13, 14, 15, 16, 17, magnetic carriers18, etc.
In order to design an effective nanoparticulate system for drug delivery, it is necessary to have a comprehensive understanding of the interactions between nanomaterials and biological systems. The nano-drug delivery system needs to go through three stages to be delivered to the tumor. First, they circulate in the body and are partially engulfed by macrophages from the reticuloendothelial system14,19, 20, 21; second, they continue to penetrate and release drugs to the tumor; finally, they accumulate in tumor and exert therapeutic effects. However, before NPs reach the tumor site, they suffer from the problem of foreign body recognition and phagocytosis by macrophages22. Even when they reach the vascular systems surrounding the tumor tissues, complex physiological conditions (such as high interstitial pressure and dense tumor matrix) are another major obstacle for NPs to penetrate into the tumor site23,24. These obstacles increase the difficulty and complexity of using NPs to treat tumors, so that only 0.7% of NPs reach the tumor site25. For example, the changes in pharmacokinetics of NPs in vivo, the poor stability of NPs, the insufficient accumulation of selective tumors, the early release of drugs at non-tumorous sites, etc. In the changeable physiological and pathological environment, NP systems can be adjusted to overcome their main shortcomings, even if they can not overcome all shortcomings, in order to maintain their desired biological effects.
During the in vivo delivery process, the cell membrane presents a huge challenge to the effective localization and drug delivery of NPs. The outer surface of the membrane is decorated with a variety of receptors, which act as the key to signal transduction, mediation, binding and internalization of cells30. Receptors are proteins that respond to specific chemical signals and perform signal transducers. Their structures are usually composed of extracellular, transmembrane and intracellular domains31. Differential expressions of receptors on the surface of normal and malignant cells can be used as a method to identify cancer cells. Folic acid (FA) receptor, integrin, prostate specific membrane antigen, CD44 (a cell surface glycoprotein), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) receptor and so on are overexpressed on the surface of specific cancer cells32, 33, 34, 35, 36, 37. Therefore, in the process of designing nano-drug delivery systems, designers need to take the interaction between NPs and cells into consideration38.
Depending on the internalization mechanism, NPs are mainly transported via endolysosome channels39. In this case, NPs and their loaded drugs will experience harsh environments in lysosomes39. For example, lower pH value and degradation by various enzymes will greatly reduce the therapeutic effect40. It is very significant for NPs to escape the nucleosome before being digested. Lysosomes are composed of intracellular components, which poses a huge challenge to nano-drug delivery systems. Recently reported, metal-phenolic networks (MPNs) uses polycation polymer and cell penetrating peptides (CPPs) to form a nontoxic coating in order to escape from the lysosomal compartment41. Another challenge is the subcellular targeting efficiency of NPs. The necessity of subcellular localization depends on the drug delivered, such as mRNA and peptides. In these cases, NPs need to penetrate the cytoplasm to find the target organelle and induce drug transfer. This transport strategy can further improve the processing efficiency, but it requires careful design of NPs. For example, camptothecin is carried in the trastuzumab-coated nanorods, while doxorubicin (DOX) is wrapped in the trastuzumab corona around the NPs42. The nanorods show cell-specific internalization. The results showed that trastuzumab circulated to the plasma membrane, camptothecin nanorods remained in the perinuclear region, and DOX was introduced into the nucleus, thereby inhibiting tumor growth.
This review summarizes the interactions between nanomaterials and biological systems at different stages in targeted cancer therapy. These stages are divided into several nodes to describe the interactions between NPs and TME, cell membrane and receptor, respectively. In each section, we will discuss the chemical and structural composition of each biological environment and the environmental challenges associated with NPs transportation. At the same time, we will also list methods to ameliorate the composition and structure of NPs, in order to increase the efficiency of NP transmission.
2. Circulation of nanocarriers in blood and their interactions with RES
In the first stage of drug delivery, nanocarriers first enter the humoral circulation and interact with the RES. In this process, macrophages of liver and spleen play primary roles43, 44, 45. On the one hand, the ligands on the surface of NPs cause the recognition of macrophage surface receptors. Macrophages quickly engulf NPs, thus shortening their circulation time in the blood. Scavenger receptor (SR) usually binds to a variety of ligands through phagocytosis, adhesion and signal transduction46 and degrades or removes foreign foreign bodies, such as SR-A, SR-B and SR-D47. On the other hand, NPs often adsorb some recognizable serum proteins (mainly immunoglobulins and complement proteins) in the circulation process, and then they are swallowed by phagocytes22,48,49. NP, as an exogenous substance, can produce corresponding antibodies in the body. After binding, it can promote macrophage recognition and phagocytosis50,51. At the same time, the surface receptors of other hepatocytes (such as sinusoidal endothelial cells and hepatic stellate cells) also affect the metabolism of NPs52,53.
It is necessary to study the pharmacokinetics (PK) of NPs in vivo from the perspective of biological safety and drug delivery system. There are four speed limiting steps in the process of drug delivery to tumor tissue: blood flow limitation, extravasation limitation, diffusion limitation, local binding or metabolic restriction54. The abnormal blood vessels and the disorder of intercellular fluid in tumor tissue greatly weaken the active targeting transport of nanodrugs55,56. Due to the lack of endothelial cell adhesion molecule and tight junction protein, the vascular wall of tumor tissue contains a large number of microporous structures with the size of 100–780 nm57. Tumor blood vessels have stronger permeability than normal blood vessels, and nano drugs overflow faster from blood vessels, which is also called passive targeting effect58. Due to the retention effect (EPR effect) of tumor tissue, nano drug delivery system can show passive targeting effect, thus improving the efficacy and reducing the side effects59.
Nanodrug delivery system mainly enters cells through endocytosis. The main endocytosis pathways include megacytic pinocytosis, reticulin mediated endocytosis, caveolin mediated endocytosis and reticulin/caveolin independent endocytosis. The PK performance and endocytosis pathway of NPs in vivo mainly depend on its chemical and physical properties, such as size, charge and surface properties. Compared with the smaller nanoparticles, the larger nanoparticles have a slower internalization rate60. Liu et al.61 injected radiolabeled 30–400 nm nanoliposomes into mice through vein. They found that 4 h after administration, the distribution of nanoliposomes with particle size of 100–200 nm in tumor tissue and blood was four times higher than that of nanoliposomes larger than 300 nm or less than 50 nm. For small size NPs, although it is easy to enter the tissue through discontinuous epithelial cells in RES tissue, it also has a high probability of returning to the blood through endothelial cells. When the nanoparticles permeate through the blood vessels, they are difficult to continue to deliver due to the hindrance of the interstitial macromolecular network, and can only produce local effects. Therefore, the absorption and distribution of small-sized NPs in tumor tissues can be significantly increased by specific surface modification (Fig. 1). The endocytosis of positively charged NPs is mainly via the reticulin mediated pathway62. It has a stronger interaction with cells and is easier to enter the cell than its negative charged counterpart, and tends to bypass the lysosomal pathway. There are many kinds of surface properties, such as the composition of copolymer monomer, degree of substitution, hydrophilicity and hydrophobicity. Studies have shown that the hydrophobic segment of polymer micelles plays an important role in the transport speed, and the hydrophilic segment determines the localization of intracellular organelles63.
Figure 1.
NPs coated with different materials.
2.1. Strategies to reduce RES uptake of NPs
2.1.1. Chemical modification of NPs
Polyethylene glycol (PEG) is a common method of surface modification that affects the interaction between NPs and RES64. PEG can prevent NPs from binding to serum albumin, and promote NPs to enter cells through matrix29,64, 65, 66, 67, 68, 69. According to reports, 114-nm-diameter NPs tightly packed in PEG could diffuse into the brains of humans and rats, and circulate for a long time70,71. Magnetic nanoparticles (MNPs) were coated with aminated cross-linked starch (DN) and aminosilane (A), and then modified with 5 kDa (A5, D5) or 20 kDa (A20, D20) PEG chains. It was found that the absorption of MNPs by macrophages RAW264.7 was reduced to half of the original level72. In addition to PEG, modification with other chemicals (as shown in Table 173, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89) can also extend the blood circulation time of NPs.
Table 1.
Surface modification materials that can modify NPs effectively.
| Surface modification material | Carrier | Ref. |
|---|---|---|
| PEG | PEG-NPs, PEG-liposome | 73,74 |
| Chitosan | Microswimmer | 75 |
| Hyaluronic acid | CuS NPs, CaP NPs | 76,77 |
| PVMMA | mPEG-b-PLA NPs | 78 |
| Cyclodextrin | Porous silicon NPs | 79 |
| Folic acid, BSA | Bi–Bi2S3 NPs | 80 |
| Tween 80 | SPIONs | 81 |
| PEO | Micelle | 82 |
| Poloxamer | PLA/PLGA NPs | 83,84 |
| HPMA | Liposome | 85 |
| PVP | Liposome | 85 |
| PMOX | Liposome | 85 |
| PDMA | Liposome | 85 |
| PAcM | Liposome | 85 |
| iRGD | Self-assembling nanovesicles | 86 |
| iNGR | Liposome | 87 |
| Angiopep-2 | PEG-PCL NPs | 88 |
| Linear TT1 peptide | Iron oxide NPs | 89 |
NPs, nanoparticles; PEG, polyethylene glycol; PLA, polylactide; PLGA, poly (lactic-co-glycolic acid); PVMMA, poly(vinyl methyl ether/maleic anhydride); BSA, bovine serum albumin; SPIONs, superparamagnetic ironoxide NPs; PEO, poly(ethylene oxide); HPMA, poly[N-(2-hydroxypropyl)methacrylamide]; PVP, poly(vinylpyrrolidone); PMOX, poly(2-methyl-2-oxazoline); PDMA, poly(N,N-dimethyl acrylamide); PAcM, poly(N-acryloyl morpholine).
The surface modification of NPs can endow them with active targeting ability and increase their tumor permeability. There are many tumor specific receptors, such as vascular endothelial growth factor receptor, integrin and endothelin, which are highly expressed on the surface of tumor vessels90,91. Specific ligands of these receptors, such as RGD or VEGF, have been attached to the surface of nanodrug delivery systems to improve tumor targeting efficiency and tumor permeability. iRGD is a circular tumor homing and tissue penetrating peptide. Integrin receptors (αvβ3, αvβ5, and αvβ1) highly expressed in tumor endothelial cells and tumor cells can bind to iRGD closely92. In order to introduce self-assembly properties into iRGD, Jiang et al.93 linked a hydrophilic arginine rich sequence and a hydrophobic alkyl chain sequence to the iRGD motif. This method endows NPs with tumor targeting and deep tumor penetration.
iNGR specifically recognizes the tumor vessels mediated by CD13 receptor, which is overexpressed in glioma neovascularized endothelial cells. At the same time, it can also be cleaved into iNGRt peptide by specific enzymes near the tumor, which specifically binds to NRP-1 overexpressed receptor in tumor blood vessels and glioblastoma cells94. Kang et al.95 demonstrated that functionalized poly(ethyleneglycol)-poly (l-lactic-co-glycolic acid) nanoparticles with an iNGR moiety (iNGR-NPs) can achieve specific deep penetration of tumor tissue. iNGR-NPs can significantly enhance the cell uptake of human umbilical vein endothelial cells, and has good pharmacokinetics and tumor homing characteristics.
LyP-1, as a disulfide cyclized peptide, interacts with cell surface protein P32/gClqR L C (LyP-1) to specifically bind tumor cells and lymphatic vessels96. LyP-1 modified dendrimers showed good cytocompatibility in the concentration range of 0.1–10 μmol/L for 24 h, with satisfactory radiochemical purity (>99%) and stability (>16 h, 90%)97. LinTT1 can be localized in CD31 positive tumor vessels, including transdifferentiation endothelial cells, and co localization with tumor macrophages and lymphatic vessels. LinTT1 targeting strategy can be used to increase the uptake of glioblastoma nanoparticles throughout the body to improve imaging and treatment98. These tumor penetration peptides can specifically recognize tumor cells, promote the development of receptor-mediated endocytosis, and accelerate the penetration of nanomedicine into tumors. It can be concluded that the coated tumor infiltrating peptide can be used to achieve tumor homing and tissue penetration, which provides a new strategy to improve the accumulation and penetration of NPs.
Different molecular weights of PEG have different effects on NPs99. For PEG mesoporous silica nanoparticles (MSNs) of the same size, the phagocytic correlation decreases with the increase of PEG molecular weight (10–40 kDa)100. Although PEG reduces the interaction between NPs and RES, excessive PEG will affect NP instability. For example, when the surface-modified PEG exceeds 8%, ordinary PEGylated particles DSPE-PEG2000 can degrade liposomes. PEG can induce antibody production and neutralize long-term efficacy101,102. In addition, repeated injection of PEGylated liposomes may result in accelerated blood clearance (ABC) by inducing anti-polyethylene glycol antibodies103.
2.1.2. Cell membrane surface coating
Although PEG modification can improve the pharmacokinetic properties of NPs, RES still has significant scavenging effect on NPs. This leads to an unsatisfactory distribution and accumulation of NPs in the body. To avoid removal, covering NPs with cell membranes can disguise NPs as endogenous substances, such as erythrocyte membrane104, leukocyte membrane105, platelet membrane106, mesenchymal stem cell membrane107, tumor cell membrane28,108, and autopeptide109. The bionic method is not to make the preparation invisible directly, but to interact with the body directly. This leads the body system to believe that it is an endogenous substance in the body, thus escaping the clearance of the immune system. As one of the most basic units of biology, cells can interact with various substances in the complex internal environment without rejection, such as proteins, other cells and intercellular substance.
In 2011, Hu et al.110 first reported the cell membrane coating technology, which directly used the whole cell membrane as the material of NP coating. The first cell used to wrap NPs is red blood cell (RBC). After hypotonic treatment, the source cells are repeatedly squeezed through a 100-nm porous membrane to form vesicles derived from the red blood cell membrane. Negatively charged polymer NPs can be wrapped in them for camouflage. The half-life of the NPs encapsulated in RBC membrane is more than twice the half-life of PEG-coated NPs. When gold nanoparticles (AuNPs) are placed on the red blood cell membrane from top to bottom, the phagocytosis of RES can reduce104.
It can also be seen that the nanoporous silicon particles are wrapped in the purified leukocyte membrane105. The fusion of erythrocyte membranes and platelet membranes forms double-coated NPs with two cell origin characteristics111. When coated with platelet membrane, the clearance rate of NPs by macrophages is reduced, and the binding of NPs to platelet adhesion pathogens is increased106. The NPs based on mesenchymal stem cell (MSC) membrane have good biocompatibility, and can maintain the MSC specific tumor targeting in vitro and in vivo107. The NPs composed of 4T1 breast cancer cell membrane and paclitaxel polymer have high cell-specific targeting effect on homologous tumors, whether it is primary or metastatic108. The cancer cell membrane-encapsulated nanoparticles derived from the source cells have immune escape ability and homologous targeting ability. The self-peptide is a kind of polypeptide designed to be similar to human cell membrane protein CD47 (with self recognition function). It can escape macrophage phagocytosis through signal transduction of phagocyte receptor CD172a109. Rodriguez et al.112 pointed out that NPs combined with “self” peptide would greatly reduce the clearance rate. As a method of biomimetic replication of cell membrane characteristics, biomimetic cell membrane combines the advantages of protocell and core nanoparticles, thus greatly improving the biocompatibility. By promoting this bionic strategy, it is possible one day to develop a multifunctional nano-drug delivery system that spans multiple diagnostic and therapeutic modes. We firmly believe that these bionic cell membrane-encapsulated nanomaterials will open up an exciting new field in precise diagnosis and tumor treatment.
2.1.3. Adjusting the particle size, morphology and potential of NPs
In a certain form, the particle size greatly affects the interaction between NPs and RES. And the size of NPs is also a critical element in tumor penetration. Generally speaking, RES is easy to remove NPs with too small particle size, and the kidney can directly remove NPs smaller than 10 nm113,114. However, NPs larger than 150 nm cannot be internalized into cells115. Among gold and silver NPs in the range of 2–100 nm, 40‒50 nm-sized particles can most effectively bind to receptors and induce receptor-mediated endocytosis116. Therefore, particles with a diameter of 40–100 nm can avoid the scavenging effect of RES to the greatest extent. It will make full use of the enhanced tumor permeability and EPR effect, and have the ability of intracellular differentiation117,118.
The morphology of NPs also contributes significantly to the circulatory time and the aggregation in the tumor site. In general, macrophages have a tendency to engulf spherical NPs. Adnan et al.13 compared the DOX release of spherical, rod-shaped and star shaped AuNPs in living cells. Despite the highest graft density of spherical AuNPs, rod-like NPs exhibited better drug delivery performance. Compared with spherical micelles, fiber micelles have demonstrated promising long-term properties119. A series of results show that the cycle life of rod micelles is much higher than that of spherical micelles120,121.
Zeta potential (ξ) indicates the electrostatic charge on the surface of particles. Cells have strong internalization ability of cations, but the weakest absorption of anions122. Since the cell membrane is negatively charged, the positively charged NPs on the surface are easier to contact and take up with the cell. Positively charged NPs enter cells in various forms, such as reticulin-dependent endocytosis123, macropinocytosis124, caveolin-dependent endocytosis or others. Negatively charged NPs enter cells mainly through caveolin-dependent endocytosis. The NPs with negative charge interact with the positive position of membrane proteins and are highly internalized due to membrane repulsion125. However, positive charges tend to adhere to serum proteins in the blood. Therefore, when NPs are transported in the blood, the surface charge should be as close as possible to an intermediate state of −10 to 10 mV. It is not only necessary to avoid adhesion to serum proteins and clearance by macrophages, but also to be easily absorbed by cancer cells60,126. In tumor tissue, AuNPs with mixed charge have a higher accumulation and a slower clearance rate than PEG-AuNPs127.
2.1.4. Optimizing the composition of NPs
When NPs enter the blood, they interact nonspecific with selective plasma proteins to form protein corona134, 135, 136. The pigments and complement proteins in corona layer promote the recognition of NPs by macrophages137. Although surface modification can reduce the binding of additional biomolecules, the binding of some biomolecules may still occur138. From the thermodynamic point of view, non-ionic and hydrophilic polymers are considered to have higher potential to prevent protein adsorption139,140. Recently, Zou et al.141 found that at 30% (w/w), polyglycerol (PG) coated NP almost completely prevented corona formation. Regardless of the size of NP and the core material, PG was more effective than PEG in shielding protein adsorption and completely escaping from being absorbed by macrophages. Protein-bound drugs retain longer in the body than unbound drugs, so the half-life of the drug after protein-bound drugs increases. The protein with high affinity to NP surface can replace the precoated protein through Vroman effect. Peng et al.142 demonstrated that pre-formed albumin corona could inhibit the adsorption of plasma proteins, reduce complement activation, and ultimately prolong blood circulation time. The affinity and quantity of adsorbed proteins depend on the composition and surface chemical properties of nanomaterials. It is also related to the structure of natural protein and the thickness of adsorbed protein layer143. Superparamagnetic iron oxide nanoparticles (SPIONs) have special magnetic properties and good biocompatibility144. Now it has been used as a passive targeting material combining peptides and proteins in clinical applications.
2.2. Reduction of RES vitality and adjustment of individualized dose
Reducing RES activity is also a way to prolong the cycle time of NPs. The real purpose is to increase the accumulation time of NPs at the tumor site. Prolonged contact between drugs and normal tissues will cause more damage to normal tissues. Using polymer materials such as polyethylene glycol to modify NPs reduces the interaction with phagocytes, but also reduces the binding of target cells145. Tang et al.146 designed an anti-enzyme peptide ligand derived from CD47 and placed it in liposomes. CD47 is a glycoprotein expressed on mammalian cell membrane. Its extracellular domain interacts with signal regulated protein alpha (SIRPα) in phagocytes to produce inhibitory signals. Therefore, CD47 is a hypothetical “self” marker that gives phagocytes a “don't eat me” signal. The cycle time of the nanocarrier injected intravenously has been prolonged.
Different doses of NPs will not accumulate in the same location147. During the entire administration period, the perfusion status of different blood vessels may change148. In the course of treatment, we hope to extend the main focus of treatment time and reduce adverse reactions. Firstly, according to the responsiveness of different patients, the dosage should be adjusted separately to reduce side effects. Secondly, considering the bidirectional reaction between NPs and renewable energy, careful planning should be made when formulating multiple dose regimens. Under certain conditions, PEG or PMOX modified liposomes can cause IgM reactions, so the ABC of liposomes during the second injection149. Amphoteric poly (carboxyethylamine) (PCB) modified siRNA can effectively avoid ABC phenomenon in the treatment of drug liposomes150. It has been proved that the liposomes modified with PVP, PDMA, PACM or HA have good invisibility properties and will not cause ABC phenomenon or increase liver uptake74,85.
3. Extravasation of NPs from tumor vessels and their retention in tumor tissues
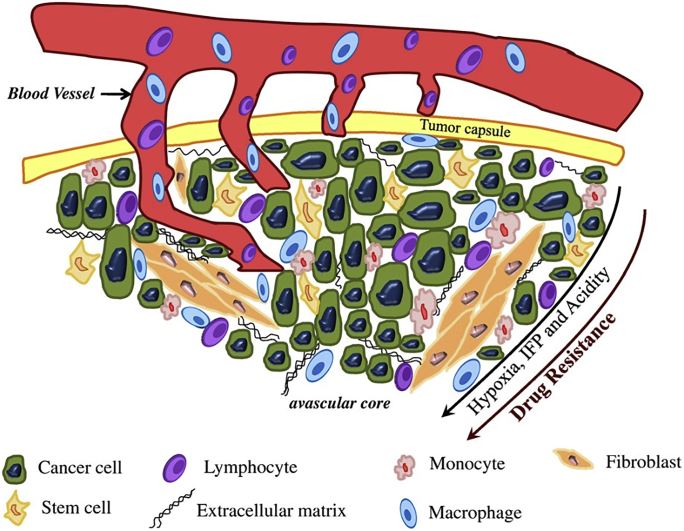
Using the EPR effect, NPs penetrate into the tumor tissue from blood vessels around the tumor site. Cancer cells are embedded in a matrix composed of lymphocytes, monocytes, fibroblasts, macrophages and extracellular matrix (ECM)5,120,151, 152, 153. Hypoxia, interstitial fluid pressure (IFP), acidity and drug resistance ensue, as the distance between the tumor and blood vessel increases (Fig. 2). This is the second stage of drug delivery on nanocarriers.
Figure 2.
Schematic portraying complex microenvironment of tumors. Reprinted with the permission from Ref. 151. Copyright © 2011 Elsevier.
3.1. Tumor vessels
In normal tissues, there is a continuous and complete blood vessel wall between blood and tissue, which can prevent the entry of macromolecules and coarse particles154. However, compared with normal tissues, the microenvironment of solid tumors is highly complex and disordered, often accompanied with vascular abnormalities and unique pathological features155. Tumor tissue has a series of characteristics, including high vascular density, poor maturity, large lumen, branch disorder, highly irregular distribution, including isomerization, uneven perfusion and permeability156. These vascular abnormalities, combined with pressure from rapidly spreading tumors, can affect blood flow and impair tumor perfusion157. At the same time, in order to maintain the rapid proliferation of tumors, tumor cells and stromal cells secrete VEGF and EGF to continuously promote the growth of new blood vessels158,159. This may lead to abnormal angiogenesis or physiological damage to tumors. Due to the excessive angiogenesis catalysts, including VEGF, basic fibroblast growth factor (BFGF) and tumor necrosis factor alpha (TNF-α), endothelial gap junction defects (usually 200–800 nm)160. Therefore, the vascular density gradually decreases from the edge to the center of the tumor, and the drug distribution in solid tumors is uneven161, 162, 163. Moreover, the absorption of NPs is positively correlated with tumor vascular density164. Wang et al.165 found that NPs with a diameter of about 100 nm were mainly concentrated on the edge of the tumor. This indicated that the invasion of NPs mainly came from the tumor margin diffusion.
3.2. Extracellular matrix
Extracellular matrix (ECM) is a complex non-cellular and dynamic structure where cells reside, remodel and interact to allow tissue homeostasis, differentiation and histomorphogenesis166. The ECM is composed of locally sequestering biomacromolecules secreted by epithelial cells and stromal cells, which is typically classified into three categories according to their functions: (1) structural proteins, including collagen and elastin, that are organized into a fibrillar network and provide tensile strength to the skeleton of ECM167. (2) connexins, including fibronectin (FN), laminin (LN) and tenascin (TN), that provide adhesive binding sites for cell binding and thereby facilitating the process of cell adhesion, spreading, migration and even differentiation. (3) Proteoglycan (PG) and its graft glycosaminoglycans (GAG), including HA, chondroitin sulfate, heparin, heparin sulfate (HS) and keratan sulfate, which indirectly sequester water molecules through a cationic intermediary, resulting in a hydrogel-like network, and endows ECM with unique biophysical properties such as high compressive strength, viscoelastic effects, and streaming potentials168. In terms of spatial structure, ECM is typically divided into basement membrane (BM) and interstitial connective tissue (ICT) which are responsible for separating the epithelium from the surrounding stroma. ICT primarily consists of collagen I and FN, which provides a structural scaffold for tissues and controls the differentiation of resident cells through interacting with their surface receptors. In contrast, the BM is denser than ICT and composed of collagen IV, LN, HS and proteoglycan169. In addition, lysyl oxidase (LOX), matrix metalloproteinases (MMPs) and other regulatory enzymes responsible for the posttranslational processing of ECM proteins are often considered as components of ECM.
The cytoskeleton remodeling, structural plasticity and mechanical strength of ECM are increasingly recognized as the key factors determining the penetration and spatial distribution of NPs. The biomechanical properties of ECM are strictly regulated by the specific components in the matrix as well as post-translational modifications, such as glycosylation, transglutamination and crosslinking170. The occurrence of collagen crosslinking is primarily mediated by lysyl oxidase (LOX) and the LOX family of secreted amine oxidases, which catalyze the crosslinking of collagen through the oxidative deamination of lysine residues. Concurrently, components of ECM undergo degradation by matrix-degrading enzymes, including heparanase, cathepsins, hyaluronidases, MMPs, and ADAMs (a disintegrin and metalloproteinases). This tightly regulated ECM homeostasis is sensitive to the altered expression of these proteases, and will lead to excessive ECM remodeling upon abnormal changes occur171.
It is known that the poor diffusion in ECM together with the structural and functional abnormalities in tumor vasculature lead to a reduced oxygen availability in the regions of solid tumor stroma172. As one of the target gene products regulated by hypoxia-inducible factor (HIF), the expression and activity level of LOX enzymes showed remarkable enhancement in response to hypoxia, which in turn led to the accumulation of abundant collagens in the tumor stroma as a result of desmoplasia173. The crosslinking of collagens is primarily initiated by the LOX family of secreted enzymes, which are usually overexpressed in a variety of tumors and negatively correlated with the survival rate of patients. It has been found that collagen crosslinking induced by LOX promoted the invasion of premalignant epithelium into a stiffened, cross-linked ECM, as well as clustering of β1 integrin that facilitated focal adhesions and PI3K signaling enhancement174. More importantly, the excessive collagen deposition and cross-linkage contributes to the stiffening of ECM through extensive post-translational modifications that increase tensile strength, in addition to the building of an interstitial matrix. The elevated hypoxia and metabolic stress caused by poor diffusion in stiff tumor ECM lead to the upregulation of multiple immunosuppressive cytokines such as IL-10, TGF-β, PGE2 and VEGF-A175. Accordingly, abundant and highly compact ECM inevitably attenuates the infiltration of either drugs, resulting in only poor paratumoral tissues being supplied by individual vessels176,177.
4. Strategies to enhance NPs into tumors
4.1. Physicochemical properties of designed NPs
Small size NPs have better tumor penetration ability than large size NPs178. The same trend has been observed in various NPs, such as polymer NPs27, super-small gold NPs26 and silicon dioxide quantum dots (QDs) composite NPs179. 25 nm particles exuded from the blood vessels twice as much as 60 nm particles. This is due to the existence of collagen network, making it difficult for particles larger than 60 nm to infiltrate the tumor180. In order to evenly distribute NPs and their inclusions in the tumor stroma, a smaller size design is required. However, small particles less than 5.5 nm can be quickly cleared by bone marrow cells, and the total accumulation of tumors at 20–30 nm is almost one third of that at 60–100 nm181. When studying the size dependence of particle distribution in tumors, Popovic et al.179 found that 60 nm particles would ooze out, but they would not leave the perivascular space immediately. Therefore, the optimal particle size of NPs is 40–60 nm, which can not only avoid the scavenging effect of liver, spleen and kidney, but also can infiltrate into tumor tissue182,183 (Fig. 3).
Figure 3.
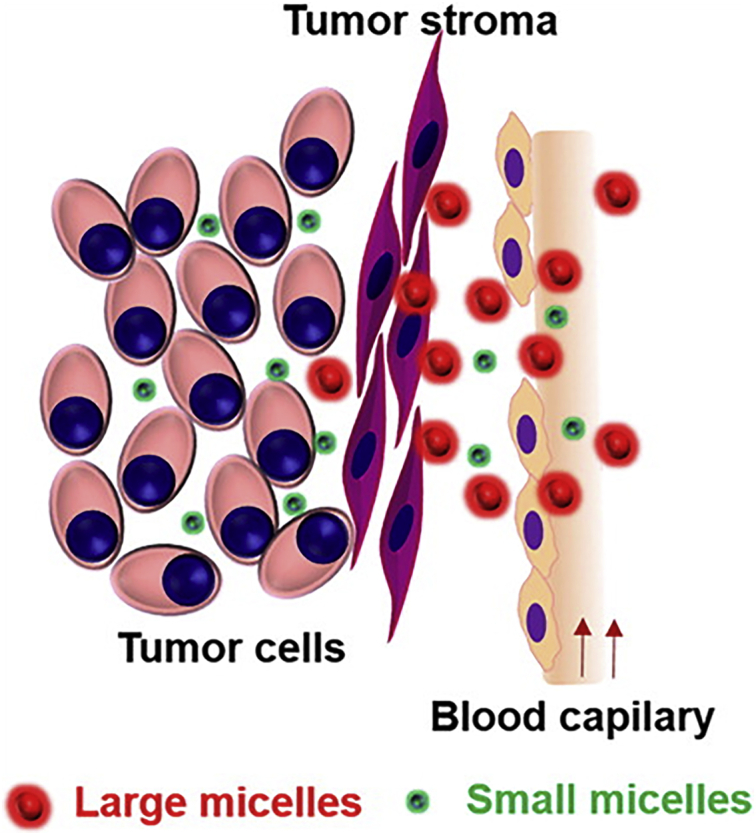
The role of micelle size in tumor accumulation, penetration, and treatment. Reprinted with the permission from Ref. 165. Copyright © 2019 American Chemical Society.
In terms of particle shape, rod-shaped NPs with the same particle size have stronger internal velocity and penetration efficiency184. Black et al.127 compared the ability of AuNPs to penetrate tumors in four forms: gold nanospheres, nanodiscs, nanorods and cubic NPs. The results showed that gold nanospheres and nanodiscs had a high absorption rate to tumors, but mainly remained at the edge of the tumor, while nanorods and NPs penetrated the core of the tumor. Compared with nanospheres with similar plasma half-life, nanorods have better tumor transport and distribution in the body, and the permeability of nanorods is 1.7 times that of nanospheres60.
The distance that neutral particles (±10 mV) penetratie the tumor is several times that of similar charged particles. And the distribution of the neutral particles in the tumor is more even185. This is because cationic or anionic particles can form aggregates with the charge components of the matrix, such as HA reaction and collagen reaction. These reactions will greatly hinder the transport of NPs in tumors. Cells are more capable of absorbing cations than neutral ions, while the weakest anions absorb186. However, anionic NPs have the strongest diffusion in cancer tissues, while cationic NPs have the weakest diffusion122. Therefore, considering many factors (such as penetration and absorption), NPs with a charge of ‒10‒0 mV are the most suitable.
It has been found that the target rigidity played a decisive role in phagocytosis128. The effects of mechanical properties of nano carriers on in vivo transport include cycle time, tumor accumulation, tumor infiltration and cell internalization187. Compared with soft nanocarriers, macrophages give preference to phagocytize rigid counterparts128,129. Hui et al.188 designed liquid filled silica nanocapsules (SNCs, ∼150 nm) with a wide stiffness range (Young's modulus ranging from 704 kpa to 9.7 Gpa). The results showed that the uptake of macrophages in soft SNCs was 3 times less than that in hard SNCs. Soft particles also perform well in terms of physical infiltration and permeability130,131. High deformable NPs are easily trapped in the fenestrations of spleen for a short time, and then released back to the blood circulation, which may be the reason for the formation of extremely long elimination half-life. During the observation period of two and 48 h later, the accumulation of hard (15 kPa) discoid particles in the liver was much higher than that of soft (1.3 kPa) discoid particles131,189. These examples confirm that the softness of nanoparticles regulates their organ accumulation. Liang et al.132 proved that DOX@3D-MPs, softer nanomedicines, are superior to DOX@2D-MPs in blood extravasation and tumor infiltration. These advantages are attributed to their softness. During the preparation of amphiphilic polyethylene glycol-b-(poly (ε-caprolactone-g-butyl acrylate) (PEG-(PCL-g-PBA)), Deng et al.133 increased the content of PBA to increase the softness. The rigidity of core–shell NPs was reduced by 42.5 GPa. At the same time, it has also been proved that the less rigid NPs can pass through the tumor extracellular matrix more easily. Several examples have shown that cancer cells prefer to internalize hard NPs. For example, in HeLa cells, rigid (1.2 GPA) PLGA lipid NPs showed higher cellular uptake than soft (0.76 GPA) counterparts190. These prove once again that the mechanical properties of nanoparticles play key roles in regulating their interactions with biological systems. When the nanostructures involved have variable mechanical properties, the relationship between nanoparticle hardness and passive/active tumor targeting efficiency becomes more complex.
4.2. Tumor stroma
Studies have shown that there is a special interaction between tumors and stromal cells to control tumor growth, invasion and metastasis191,192. The tumor stroma is mainly composed of vasculature, ECM, and fibroblasts, which determines the depth of NP penetration into the tumor120,157,161. Tumor stroma not only provides a physical scaffold for tumor growth, but also acts as a source of various paracrine cytokines and chemokines. It regulates the recruitment, infiltration, polarization and function of immune cells. Therefore, targeting and reconstruction of tumor stroma may provide a better opportunity to assist NP infiltration. The small size, large surface area and unique physicochemical properties of NPs enable them to overcome various biological obstacles and deliver anti-tumor drugs to targeted tumor tissues. Herein we summarize the barriers of matrix components to NP penetration into tumors. More importantly, we emphasize the recent attempts to remodel inhibitory tumor stroma through carefully designed and manufactured NPs, which provides promising strategies for improving the effectiveness of anti-tumor treatments and are of great significance (Table 2).
Table 2.
The paths of targeted reconstruction of tumor stroma.
| Target | Path |
|---|---|
| TVEC | The normalization of tumor vasculature |
| ECM | Inhibiting of collagen synthesis |
| Degradation of stromal collagen | |
| Hyaluronic acid as a therapeutic target | |
| Treatment of hypoxia | |
| CAFs | Paracrine pathway |
| Remodeling ECM | |
| Promoting tumor angiogenesis | |
| Targeting CAFs to normalize tumor stiffness | |
| Reprogramming CAFs to an immunosupportive state |
TVEC, tumor vascular endothelial cells; ECM, extracellular matrix; CAFs, cancer-associated fibroblasts.
Owing to the fast-paced growth of nanomaterials in recent years and a deeper understanding of matrix remodeling in anti-tumor therapy, NPs have been put to extensive use for regulating the tumor stroma and improving tumor therapy. (1) Synthetic and natural NPs, such as lipids, proteins, polymers, and inorganic materials, have unique physicochemical properties, which enable them to serve as drug carriers to meet medical needs. (2) With reference to the unique properties of tumor stroma, NPs with different types of environmental stimuli responses can be designed and developed. Examples include hypoxia, weakly acidic pH and tumor pressure gradient, and the nature of ECM. It is desired to achieve precise delivery of anti-tumor drug components to specific cells or non-cellular components in the matrix193. (3) NPs can easily undergo functional chemical or biological modifications. Tumor stroma and cancer cells typically overexpress or specifically express specific cell surface molecules and secreted factors, thus providing a general idea for the functional design of NPs. Targeting these cell markers has the potential to increase the uptake of NPs by the tumor stroma and reduce the adverse effects on normal cells.
4.2.1. Tumor vascular endothelial cells
Tumor vascular endothelial cells originate from normal vascular endothelial cells surrounding the tumor. Its functional characteristics and cellular phenotypes have undergone significant changes due to the long-term exposure to TME. For example, the expression of adhesion molecules is reduced, the adhesion of white blood cells is weakened, and the expression and secretion of a large number of ECMs are poor. Certain secretory functions of vascular endothelial cells in tumor stroma are changed due to the induction of tumor cells, which facilitates tumor cell growth, metastasis and immune escape. Tumor vascular endothelium not only provides nutrition for tumor growth and progression, but also is the first barrier for NPs to enter tumor tissue.
The abnormality of tumor vascular system is manifested as heterogenous vessel diameter, tortuosity and distribution, and insufficient blood and oxygen supply. This directly leads to hypoxia, pH reduction, interstitial pressure enhancement in the tumor stroma. Therefore, the normalization of tumor vasculature may be a way to improve the penetration of NPs into tumors194. One of the important considerations for the success of anti-tumor therapy is the dose of anti-angiogenic agents. Unlike normal blood vessels, the tumor vasculature is abnormally tortuous and branched, which poses a challenge to the effective diffusion of the therapeutic part within tumor stroma195. In view of the over-expression and secretion of VEGF-A in the tumor stroma can cause abnormalities in tumor vasculature, current anti-angiogenesis therapies predominantly target this molecule or its receptor196. Anti-vascular drugs mainly include tyrosine kinase inhibitors (TKI) that target pro-angiogenesis-related receptors to block their signal transduction pathways, such as sunitinib and sorafenib. There are also monoclonal antibodies that directly target circulating VEGF or VEGFR, such as bevacizumab. Nevertheless, the tumor vascular network exposed to a high dose of VEGF antagonist often suffers destruction, which aggravates the hypoxia in TME and prevents TAM from polarizing towards the inflammatory phenotype. In addition, the absence of vascular system further impaired the diffusion of NPs within the tumor stroma197. Therefore, it is important to normalize the tumor vasculature, rather than completely destroy it. The rational use of anti-vascular drugs to promote tumor blood vessel normalization is expected to improve the penetration of NP into tumor depth.
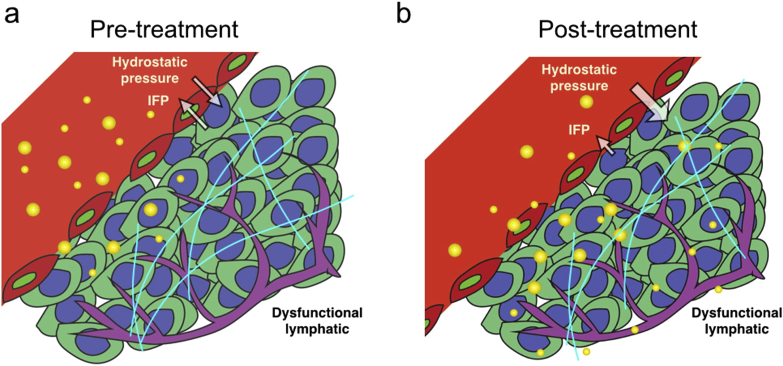
In the process of NP design, it can be considered to add components to reduce IFP. For example, hyaluronidase inhibits tumor angiogenesis, degrade stromal cells and ECM198. And the destruction of VEGF signaling pathway can inhibit the overexpression of vascular growth factor in tumor tissues120. Limiting the expression of VEGF can block the excessive division of vascular endothelial cells, thus increasing blood perfusion and enhancing the delivery capacity of small molecule drugs32,160,199,200. Jiang et al.27 combined the tumor vascular remodeling strategy and adopted the NP design to improve the transmission of tumor vascular remodeling to solid tumors (Fig. 4). Transforming growth factor-β (TGF-β) inhibitors can also increase tumor vascular permeability, thereby improving the delivery of NPs163.
Figure 4.
The mechanism of NPs entering tumor before and after vascular reconstruction. (a) Before treatment, NPs (expressed in the form of gold ring) leakage occurre in tumor blood vessels. (b) After the reconstruction of the pressure gradient, NPs effectively transfer from the vascular cavity to the stroma (light blue line: collagen) and distribute evenly, reaching the depth of the tumor. Reprinted with the permission from Ref. 27. Copyright © 2019 American Chemical Society.
In order to explore the effect of tumor vascular normalization on the delivery of nanomedicine, Chauhan et al.156 carried out VEGFR2 blockade to modulate the penetration rates of quantum dot-based NPs in orthotopic mammary tumors. They showed that blocking VEGFR2 to repair abnormal vessels in breast tumors could reduce the size of pores in the blood vessel walls and alleviate IFP in tumors, thus allowing smaller NPs (∼12 nm) to enter them more rapidly. Concomitantly, the increase in steric hindrance and hydrodynamic disturbances associated with the reduction of vascular wall pores hindered the transport of larger particles (∼125 nm)160. Their results indicated that the restoration of dysfunctional tumor vasculature could reconstruct the pressure gradient between the intravascular and interstitial spaces, which is essential for the delivery of nanomedicine into solid tumors. Although NPs smaller than 10 nm in size maximally benefited from tumor vascular normalization for enhanced nanomedicine delivery, their applicability is severely limited due to the too small particle size. It was later demonstrated that tumor revascularization could enhance the transvascular delivery of medium-sized NPs, up to 40 nm27. Upon entering the tumor stroma, however, smaller NPs will encounter lower diffusional hindrances, resulting in a more homogeneous distribution within the tumor interstitium. The findings suggested that anti-angiogenesis-based NPs can be adjustable in size to meet the multi-stage delivery of nanomedicines in solid tumors.
Copper chelation has been proven to be an effective anti-angiogenic treatment strategy for breast cancer. Zhou et al.201 synthesized a coil-comb block copolymer capable of chelating coppers, in which poly-l-histidine (PHis) and RGD-coupled poly-γ-glutamic acid (γ-PGA) served as hydrophobic and hydrophilic motifs, respectively. In the neutral systemic circulation, the polymers could self-assemble into multifunctional NPs due to aggregation of PHis segments via hydrophobic interaction and therefore load resiquimod (R848), an agonist of Toll-like receptor 7/8 (TLR7/8). Upon intravenous administration, the NPs could reach the targeted tumor tissues via specific binding of RGD peptide with integrin αvβ3 that is overexpressed on tumor neovasculature, thereby exhibiting strong anti-angiogenic activity.
The study of Schmittnaegel et al.202 revealed that a combined blockade of angiopoietin-2 (ANG2) and VEGF-A by a bispecific antibody (A2V) normalized the remaining blood vessels and facilitated the extravasation and perivascular accumulation of activated CTLs. Consequently, the promotion of vascular regression, tumor necrosis, and antigen presentation through intratumoral phagocytes was achieved. As one of the promising nanocarries, AuNPs possess excellent performance for drug delivery and inherent anti-tumor activities. Huang et al.203 synthesized AuNPs targeting folic acid receptor. The NPs were stabilized by an amphiphilic block copolymer of poly (ethylene glycol)-b-poly (diethylaminoethyl acrylate) (PEG-PDEAEA). The studies have found that in addition to inhibiting tumor angiogenesis, AuNPs normalize the tumor vascular system by increasing the expression level of VE-cadherin, as well as pericyte coverage and strengthening tight junctions, thereby attenuating vascular permeability, improving vascular perfusion and relieving tissue hypoxia.
In order to combine vascular normalization therapy and tumor cell metabolism treatment, Jet-lagged NPs with chitosan cationic core were constructed to load apatinib (APA) and lonidamine (LND), respectively. APA acted as an antiangiogenic agent for vascular normalization therapy by specifically blocking the A TP-binding sites of VEGFR2, while LND could be employed for restraining the lactic acid efflux and alleviating acidosis by downregulating the monocarboxylic acid transporter receptor 4 (MCT4)204. Then the secondary assembling of negative hyaluronic acid (HA) and polystyrene sulfonate were following to ensure the stable and safe delivery of NPs in vivo. After treatment, Jet-lagged NPs could remarkably reduce the LA level in TME by limiting the efflux of lactic acid. In addition, the pericytes coverage increased to 69%, which was significantly higher than that of the mono APA group (47%).
4.2.2. Extracellular matrix
4.2.2.1. Inhibiting of collagen synthesis.
As the major structural component of ECM, excessive secretion and deposition of collagen predominantly contribute to the fibrosis of tumor tissues. The dense collagen network in the tumor stroma significantly abbreviates the permeability and efficacy of nanotherapeutics. It is generally believed that the activation of the SMAD-2/3 intracellular pathway via TGF-β ligand is heavily implicated in fibrosis, in which TGF-β1 is considered to be a major driving force of fibrotic pathology205. The blockade of TGF-β improved the recruitment and migration of perivascular cells toward tumor vasculature, as well as the fraction of perfused vessels. In addition, the normalization of the neoplastic ECM was achieved by reducing the content of type I collagen, which allows both conventional chemotherapeutics and nanotherapeutics to enhance the permeability of tumor stroma.
The angiotensin II receptor antagonists such as losartan have been proved to inhibit collagen I synthesis and improve the distribution and efficacy of nanotherapeutics in tumors206. Losartan's anti-fibrotic effect is partly attributed to its antagonism with the angiotensin II type I receptor (AGTR1), which leads to the down-regulation of positive regulator of TGF-β1 such as thrombospondin-1 (TSP-1) and thereby the suppression of TGF-β1 levels. It was found that intraperitoneal administration of losartan led to a dose-dependent reduction in stromal collagen in desmoplastic models of melanoma and sarcoma in mice. Moreover, it also improved the intratumoral distribution and therapeutic efficacy of both oncolytic herpes simplex viruses and liposomal DOX. Later, it was discovered that Losartan depleted the stromal collagen of human ovarian cancer cells through up-regulating the expression of anti-fibrotic miRNAs such as miR-133207.
During the process of tumor progression, the cancer-related fibroblasts (CAFs) are the major factor leading to the imbalance of collagen fiber turnover. This promotes excessive deposition of collagen fibers in the tumor matrix, which leads to tumor fibrosis (desmoplasia). It has been shown that CAFs can be reprogrammed from an active myofibroblast state to a quiescent one, such as through blocking angiotensin II (Ang II) signaling, which promotes myofibroblast activity or regulating the main transcriptional myofibroblast phenotype through vitamin D receptors regulator. Drugs such as angiotensin receptor blocker (ARB) can block Ang II signal transduction, inactivate the fibroblast state of CAFs, and reduce αSMA + CAFs levels. Chauhan et al.208 constructed a compound library based on combinatorial chemistry and high-throughput screening technology to screen polymer carrier materials that are highly sensitive to the pH value (6.7) of TME. They have synthesized a series of polyacetals from polyols and acetals or vinyl ether monomers through modular reactions of acetal exchange or polycondensation. These acetals were expected to be degraded under acidic conditions. The ARB was then chemically conjugated to the screened polymer most sensitive to the slightly acidic TME. And then the generated TME selectively activated polymer (TMA-ARB) was formulated into NPs via nanoprecipitation, which would provide ARBs with enhanced tumor permeability and locally responsive release of active immunoregulators through TME. Compared with free ARB, TMA-ARB reduced the expression of α-SMA and collagen I, as well as the level of solid stress within the tumor stroma of mammary tumor-bearing mice. This reduced tumor blood vessel and increased vascular perfusion, indicating that TMA-ARB treatment was facilitated to the normalization of the tumor stroma driven by CAFs reprogramming. RNA sequencing of the mammary tumors in mice showed that TMA-ARB treatment alleviated the expression of hypoxia and TGF-β in the tumor stroma, which was consistent with the alleviated vascular compression and CAFs activation.
4.2.2.2. Degradation of stromal collagen.
As the most prevalent component of tumor ECM, collagen is increasingly becoming an attractive therapeutic target for cancer immunotherapy. Therefore, collagenase for collagen degradation is employed to improve the penetration of immunomodulators and nanotherapeutics into tumor stroma. Inspired by this context, studies have implemented the synergistic treatment of nanotherapeutics and collagenase.
Oncolytic virus (OV) therapy is a novel and promising therapeutic approach for tumors that involves selective infection and lysis of tumor mass. Nevertheless, the inability to efficiently propagate throughout the tumor stroma and infect target cells distant from the administration site has restricted their capacity to achieve consistent therapeutic responses. This limitation seems to be size-dependent caused by the fibrillar collagen network within the ECM. Because NPs of equivalent size manifest the same extent of intratumoral infiltration, while smaller particles are more widely distributed. Accordingly, simultaneous administration of bacterial collagenase led to an oncolytic herpes simplex virus (HSV) vector that is enhanced and more homogenous distributed within the xenograft model of melanoma209.
In view of the fact that collagen dominates most of the stroma of pancreatic ductal adenocarcinoma (PDAC), it reaches 12.8% of the volume, while the proportion in healthy tissues is 1.4%. Therefore, liposomal collagenase sized 100 nm has been developed to decompose the dense collagen stroma of PDAC and improve drug penetration into the pancreatic tumor. The liposomal encapsulation protected the collagenase from premature inactivation and prolonged its release rate at the target site. Intravenous administration of liposomal collagenase allowed a remarkable reduction of fibrotic tissue (5.6%) as well as tumor burden210.
In addition to exogenous collagenase, an anti-fibrotic hormone, relaxin, can also be used to stimulate collagenase synthesis and down-regulate the secretion of collagen. As a naturally occurring peptide hormone in the human body, relaxin has been well-demonstrated to abrogat the Smad2 phosphorylation via its cognate G protein-coupled receptor, relaxin family peptide receptor 1 (RXFP1). Thus, it inhibits the TGF-β1-induced aberrant myofibroblast differentiation and collagen deposition. Besides, an extracellular signal-regulated kinase 1/2 phosphorylation (pERK1/2) and neuronal nitric oxide synthase (nNOS)/cGMP-dependent pathway are also involved in this signal transduction. In particular, studies have shown that relaxin can exert an anti-hepatic fibrosis effect by reducing the level of α-SMA, collagen and tissue inhibitor of metalloproteinases 1 (TIMP-1) in activated hepatic stellate cells (aHSC). Accordingly, Hu et al.211 hypothesized that this endogenous repair mechanism on hepatic fibrosis may be leveraged for the treatment of liver metastasis via enforced relaxin expression. They developed lipid-calcium-phosphate (LCP) NPs with aminoethylanisamide (AA) as the targeting moiety, which predominately transfected both metastatic tumor cells and aHSCs within the metastatic lesion by the loaded pDNA encoding relaxin (pRLN), thereby transforming them into depots that express relaxin in situ. The local expression of relaxin deactivated aHSCs and remodeled the matrix environment in the metastatic lesion. Compared with PBS control, α-SMA expression and collagen content decreased dramatically. The prometastatic chemokine of CXCL12, the profibrogenic factor of TGF-β, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) were all down-regulated.
4.2.2.3. HA as a therapeutic target to remodel ECM.
As the most abundant non-sulfated glycosaminoglycans in the ECM, HA has emerged as another attractive target for remodeling fibrotic tumor stroma. It is believed that the accumulation of HA predominantly contributes to reducing the elasticity in tumor stroma and increasing the pressure of the hydrogel212. In fact, it has been proved that HA production is related to the plasticizing reaction in PDAC, which generates inordinately high IFP and induces vascular collapse, thereby presenting substantial barriers to the perfusion and diffusion of therapeutics213. Systemic delivery of hyaluronidase to ablate existing stromal HA has been explored for normalizing IFP as well as re-expanding the tumor microvasculature. For example, Zhou et al.214 reported the conjugation of recombinant human hyaluronidase (HAase) on the surfaces of poly (lactic-co-glycolic acid)-b-polyethylene glycol (PLGA-PEG) NPs, which facilitated the diffusion of NPs and quadrupled their accumulation in 4T1 xenograft breast tumors.
At present, a PEGylated HAase has entered the later clinical trial, which endows HAase with reduced immunogenicity and prolonged circulation time215. To further improve its tumor specificity, a dextran biocompatible polymer (DEX) has been employed to couple with HAase via a pH-sensitive traceless linker. Compared to free HAase, the obtained DEX-HAase NPs with enhanced enzyme stability against protease and diminished immunogenicity exhibit greatly prolonged blood circulation half-life post intravenous injection. Upon passive accumulation in tumors, DEX-HAase within the acidic TME would be dissociated to release native HAase. The decomposition of HA is then triggered to loosen the ECM structure, which subsequently leads to enhanced penetration of oxygen and therapeutic NPs. The largely relieved tumor hypoxia would promote the therapeutic effect of photodynamic therapy (PDT), accompanied by the reverse of the immunosuppressive TME to boost immune checkpoint blockade therapy216.
4.2.2.4. Treatment of hypoxia.
The rapid growth of tumor cells consumes a large amount of oxygen, which keeps the part of tumor tissue far away from blood vessels in a state of hypoxia217. Most chemotherapy drugs, photodynamic therapy and radiotherapy have little response to cancer cell proliferation and hypoxia cell218,219. In addition, when cells are anoxic, anaerobic glucose decomposition, lactate production and many other factors lead to tumor microenvironment acidification.
Therefore, the design of NPs should take into account the microacidity in cancer tissues, such as the surface modification of antibodies or small ligands220,221. Tian et al.222 synthesized a selenium-rubyrin (NMe2Se4N2)-loaded NP which can specifically recognize cancer cells through FA–FA receptor. It selectively enters the lysosome to activate its pH sensitivity and produce the spatiotemporal regulation of singlet oxygen. Human ferritin NPs had the ability to preferentially bind to hypoxic tumor tissues and combine with hypoxic inhibitors to restore the efficacy of traditional systemic chemotherapy223. NPs can also be combined with anaerobic agents or oxygen generators to effectively reach hypoxic sites of tumors or generate oxygen224,225. Manganese dioxide (MnO2)/DOX albumin NPs can also improve the microenvironment of hypoxic tumors and promote drug release226. Samanta D227. combined with hypoxia-inducible factor inhibitors to overcome the resistance of breast cancer stem cells to paclitaxel or gemcitabine, thereby eradicating cancer.
4.2.3. Cancer-associated fibroblasts (CAFs)
In most types of cancers, fibroblasts are the predominant cellular component of tumor stroma. CAFs also known as tumor-associated fibroblasts (TAFs), myofibroblasts or active stromal fibroblasts, refer to a population of activated fibroblasts in the tumor stroma228. Compared with resting fibroblasts in normal adult tissues, this cell population has undergone significant changes in morphological characteristics and functional protein expression as well as other biological characteristics. The main manifestations are that the cells are spindle-shaped, larger in size, and the nucleus has obvious depressions or notches. Moreover, a variety of shrinkage filaments and tension filaments were found in the cytoplasm, and rich in the rough endoplasmic reticulum229. Therefore, they are more similar to those transiently present in the developing fetus and wound healing process. Like their wounds or fetal counterparts, CAFs exhibit enhanced motility and proliferation, and provide essential functions to promote tumor survival and growth230.
4.2.3.1. CAFs regulate NP permeability via paracrine pathway.
As the main stromal cells of the TME (up to 90% in some tumor interstitial tissues), CAFs are mainly distributed in the front of tumor invasion, tumor mesenchymal interface or adjacent to the vascular endothelial cells in the tumor stroma and surround the cancer nest231. They maintain interaction with tumor cells and endothelial cells, and thus play an important regulatory role in tumor occurrence, development and metastasis. In recent years, a considerable number of research reports have clarified the relationship between CAFs and tumors, providing a theoretical basis for CAFs as a new target for tumor therapy. CAFs not only promote the growth and metastasis of tumor cells through paracrine, but also regulate angiogenesis and tumor immunity through interaction with other cells232.
Once activated into CAFs, fibroblasts can secrete a considerable amount of soluble cytokines, such as chemokine ligand 12 (CXCL12), chemokine ligand 7 (CCL7), transforming growth factor β (TGF-β), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), etc233. These secreted cytokines can crosstalk with the corresponding receptors or ligands expressed on adjacent tumor cells, and promote the malignant behavior of tumor cells through a complex paracrine signal network. Among them, TGF-β is particularly concerned, and HGF and CXCL12 are also molecules that have been studied more. The TGF-β secreted by CAFs induces epithelial–mesenchymal transition (EMT) of tumor cells through the TGF-β/SMAD signal transduction pathway, which leads to remodeling of the extracellular stroma, thereby creating a permissive microenvironment for tumor invasion and distant metastasis234. Studies have shown that Wnt7a secreted by aggressive breast cancer cells could enhance the activity of the TGF-β receptors, and the activation of TGF-β pathway facilitated the conversion of fibroblasts into CAFs235. HGF secreted by CAFs regulates the growth, proliferation, and invasion of various tumor cells by acting on the c-MET receptor on tumor cells and thereafter activating the tyrosine signaling cascade236. As a chemokine highly expressed in CAFs, CXCL12 binds to the CXCR4 ligand expressed on tumor cells. This interaction induces tumor cells to perform EMT in breast and prostate cancer, thereby promoting breast cancer cell proliferation and prostate cancer metastasis. In addition, CXCL12 also mediates the recruitment of endothelial progenitor cells through CAFs, thus stimulating the formation of new blood vessels237.
4.2.3.2. CAFs regulate NP permeability by remodeling ECM.
The differentiation and function of related immune cells in the TME depend to a large extent on the structure and physicochemical characteristics of the surrounding tissues, especially the hardness, density and plasticity of the ECM. The activated CAFs can secrete a large amount of enzymes and proteins related to ECM remodeling, such as Fas-associated phosphatase (FAP), MMP as well as fibronectin and type I collagen238. As a membrane-bound glycoprotein, FAP can activate related growth factors in the ECM, thereby promoting tumor cell proliferation and angiogenesis. MMP is a group of endopeptidase that can directly decompose the cadherin in the extracellular domain to cause the normal epithelium disintegrate. This in turn leads to the occurrence of EMT and the destruction of the histological barrier against tumor cell invasion, thereby contributing to the infiltration and metastasis of malignant tumors239. As a major regulatory enzyme of collagen secretion, the expression level of lysyl oxidase (LOX) in CAFs was significantly increased, which can promote the synthesis of collagen by CAFs and enhance the hardness of ECM, so that the ECM could facilitate tumor cell metastasis while inhibiting immune cell infiltration240.
Another important consequence of CAF's conversion of ECM in the TME is to further aggravate the hypoxic environment241. The existence of Warburg effect in tumor cells and a large number of abnormal blood vessels cause a decrease in the partial pressure of oxygen in the TME. Studies have shown that decreased oxygen partial pressure can increase the expression levels of collagen related genes in fibroblasts, so it is a positive regulation of CAFs on collagen secretion242. In addition, CAFs-mediated signaling pathways can regulate the cytoskeleton, promote the generation of tension in the tumor stroma, and enhance the stiffness of ECM to support the malignant process243.
4.2.3.3. CAFs regulate NP permeability by promoting tumor angiogenesis.
Tumor vasculatures are the channels for tumor nutrition supply and tumor cell dissemination, which play an important role in tumor growth and metastasis. CAFs induce the formation of vascular endothelial cell networks in the tumor stroma by secreting vascular endothelial cell growth factors such as VEGF and FGF, or recruiting CXCR4 positive endothelial progenitor cells, thereby promoting tumor angiogenesis244. The TGF-β and SDF-1 secreted by CAFs can also elevate the expression of E-cadherin, MMP-2 and laminin-5γ2 through TGF-βR1 and CXCR4 axis of tumor cells, thereby ultimately promoting the formation of vascular mimicry245. Studies have shown that stromal-derived WNT2 promotes the angiogenesis of colorectal cancer (CRC) by increasing EMT molecules related to angiogenesis, such as ANG-2, IL-6, G-CSF, and PGF246. Autocrine CXCL14 can induce CAFs to secrete FGF-2, VEGF and other cytokines, thereby promoting tumor angiogenesis247. The activation of Ets2 (v-ets erythroblastosis virus E26 oncogene homolog 2) in breast stromal fibroblasts can also induce the expression of a large number of tumor-related genes such as MMP9, VEGF-A, etc., and recruit endothelial progenitor cells to jointly induce and promote the process of tumor angiogenesis248
4.2.3.4. Targeting CAFs to normalize tumor stiffness
The ECM, mainly generated from CAFs and accumulated in the TME, can form a dense physical barrier and elevate the hydraulic pressure of the tumor interstitium. This hinders the effective penetration of nano-immunomodulators into deeper tumor tissues. To this end, Ernsting et al.249 synthesized a conjugate of docetaxel (DTX), PEG and acetylated carboxymethyl cellulose, termed as Cellax-DTX, which could self-assemble into an aqueous NP with a size of 120 nm. In the PDA xenografts derived both from highly stromal primary patients and metastatic mouse models, more than 90% of Cellax-DTX NPs passively accumulated in SMA + CAFs, which lead to the depletion of the stromal cellular populations, thereby reducing the stroma density. As a result, the perfusion of NPs to tumor tissues elevated more than ten folds, and effectively alleviated tumor progression and metastasis. Zhen et al.250 used a dense nanoferritin cage as a photosensitizer carrier, and bound it to a specific single-chain variable fragment of fibroblast activation protein on the surface of ferritin. After irradiation, nano-pits could effectively reduce CAF in tumor tissues and enhance T cell infiltration.
In a recent study undertaken by Fang et al., a composite nanoparticulate system with programmable drug release capability, termed G (TM) PPSP, was developed for TME remodeling and treatment of mammary cancer251. In this nanomedicine delivery system, an angiotensin II type one receptor (A T1R) antagonist of telmisartan (TM) was loaded into gelatin NPs, and paclitaxel (PTX) was linked with platinum nanoparticles (PtNPs) via a diselenide bond of dual redox responsiveness. Finally, these two NPs were covalently coupled together to form a composite NP with a size of approximately 214 nm. Gelatin degradation induces a tumor stroma responsive of TM released from gelatin NPs, which is attributed to the MMP-2 secreted by CAFs. Then, due to the high levels of reactive oxygen species (ROS) or glutathione in the tumor cells, the disselenium bond is broken, and PTX can be released in the cells. Therefore, the designed NP delivery system allowed the programmable release of both agents in the correct location of the tumor tissues. The down-regulation of TGF-β by TM leads to ECM depletion, which helps GTMPPSP penetrate deep tumor stroma. Concomitantly, cytotoxic agents-mediated chemotherapy and PtNPs-mediated photothermal therapy jointly suppressed the progression of 4T1 tumors.
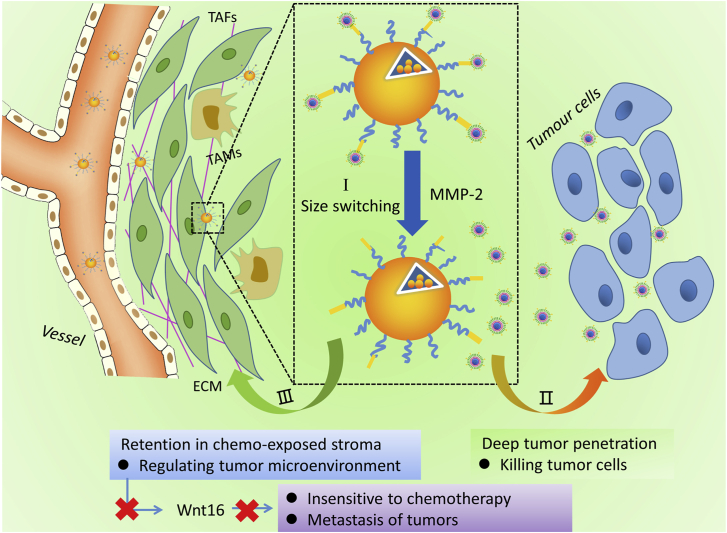
Cun et al.252 also reported a multifunctional NP with a switchable size that could co-deliver gemcitabine (GEM) and 18β-glycyrrhetinic acid (GA) for TME remodeling, thereby promoting deep tumor penetration (Fig. 5). The complex nanocarriers, termed as DGL/GEM@PP/GA, were designed by conjugating GEM with dendritic grafted poly-l-lysine (DGL), which is a kind of dendrimer and is able to self-assembly with a smaller size. Through the connection of the substrate peptide of MMP-2, DGL was coupled with NPs of PEG-PCL pre-loaded with GA. Upon systemic administration in the mice with pancreatic ductal adenocarcinoma and mammary cancer models, GEM-coupled small NPs were released from the complex nanocarriers due to the enzymatic hydrolysis of MMP-2 overexpressed in the tumor stroma. The dissociated DGL particles could penetrate the deep part of the tumor with a smaller size, and then release GEM intracellularly to kill tumor cells. While the residual larger particles (PP/GA) with residual GA could accumulate around tumor stromal vessels and be preferentially taken up by CAFs due to their poor tissue permeability, thereby down-regulating the secretion of WNT16. WNT16 is a signal molecule involved in the DNA damage response program (DDRP) that can promote the proliferation and invasion of adjacent cancer cells, leading to tumor metastasis and gaining resistance to chemo/immunotherapy. Targeting CAFs with GA resulted in effective suppression of GEM-induced CAFs activation, as evidenced by the decrease in expression of α-SMA and collagen in tumor stroma.
Figure 5.
Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size switchable nanoparticle.
4.2.3.5. Reprogramming CAFs to an immunosupportive state.
In view of the research work of Huang's research group, it is shown that genotoxic chemicals can cause DNA damage in CAFs, and thereafter induction of Wnt16 secretion can contribute to the drug resistance in neighboring cancer cells253. Many low-toxic natural anti-fibrotic compounds, such as quercetin, puerarin, α-mangostin254 and fraxinellone255, have the ability to down-regulate the expression of WNT/β-catenin in a variety of cell lines, thereby reversing tumor resistance to cytotoxic chemicals. Hu et al.256 encapsulated quercetin phosphate into targeted lipid calcium phosphate nanoparticles (LCPs) with high loading capacity (26.6%, w/w) and small particle size (about 35 nm). The systemic administration of quercetin-loaded LCP could effectively remold the TME, as manifested in significantly reducing α-SMA + fibroblasts and collagen in the tumor stroma, thereby enhancing the entry of cisplatin-loaded NPs into the tumor after subsequent administration position. When combined with cisplatin NPs, a significant down-regulation of Wnt16 expression could be observed in a matrix-rich urothelial bladder cancer model.
To reverse immunosuppressive TME of desmoplastic melanoma primarily mediated by TGF-β, Hou et al.257 developed a nanoemulsion that can target the anti-fibrotic agent fraxinellone to CAFs. Upon systemic administration, the small particle size of 145 nm enabled the formulation to efficiently accumulate at the tumor site and was internalized into CAFs and tumor cells.
5. Summary and prospect
In collaboration with physiology and materials science, a series of novel NPs are being developed for cancer therapy. They improve their design in terms of uptake of reticuloendothelial system and targeted regulation of tumor stroma, which provides an attractive drug delivery method. It can effectively protect the degradation of anti-tumor drugs, improve the biodistribution and pharmacokinetics in vivo, and increase its accumulation in the tumor site. In addition, they can specifically target various tumor stromal components, thus reducing their own specificity and improving the efficacy of tumor treatment.
An effective drug delivery system must take all factors into account and try to solve all possible delivery problems. At the same time, we should seriously consider various mutually restrictive factors. For example, when designing the size of NPs, we should not only avoid being removed by RES in vivo, but also allow NPs to effectively penetrate into tumor tissues. We should remodel the TME to reduce IFP, but the normalization of blood vessels will affect the effect of EPR, which is not conducive to nano-drug delivery systems. Therefore, we should use nanocomposites flexibly, which is conducive to NPs effectively achieving its goals in the body and minimizing its toxicity and side effects to normal tissues. For example, in combination with pH-sensitive materials, the particle size of NPs at different positions in the body can be changed. Endogenous substances (such as erythrocyte membrane, cancer cell membrane, etc.) are used to modify the surface of NPs and extend its circulation time in the blood.
We should not only focus on killing cancer cells, but also activate immune cells in the body to fight cancer, such as modifying antibodies or enzyme inhibitors on the surface of NPs. The tumor stroma is an extremely complex network in which there are crosstalks among immune cells, stromal cells and tumor cells via various autocrine and paracrine signaling pathway as well as direct action. We currently lack a comprehensive understanding of the immune regulation mechanisms of tumors, which is why we are encountering a considerable of difficulties and confusions when attempting to solve antitumor issues from an immune perspective. An in-depth understanding of the crosstalks and immunological attributes of the infiltrating components within the tumor stroma will help to providing more immunotherapeutic targets for stromal modulation of NPs, and lay the foundation for the construction of intelligent nanomedicine delivery systems with environmentally responsiveness. Further elucidating the dynamic network of stromal signals facilitating immune tolerance and tumor evasion from immune surveillance is expected to help guide the implementation of new stromal immunomodulation strategies by NPs, and more effectively relieve the suppressive effect of tumor stroma milieu on immunotherapy.
In the prospective research work on tumor immunotherapy, NPs with multiple stromal stimuli responsiveness (for instance, extracellular acidity, hypoxia, and matrix enzymes) and external stimulus responsiveness such as photothermal and photoacoustic will emerge as hot research directions, which are expected to overcome a variety of physiological barriers encountered while delivering immunomodulators in vivo. Nevertheless, these environmental stimuli responsive NPs also have issues related to their complicated design and the synthesis of carrier materials, such as difficult in quality control. It is believed that with the continuous development of materials science, oncology and immunology, these issues will be solved effectively, and intelligent NPs will be developed towards direction of more rational design and precise targeting. The combination of cancer immunology and nanotechnology is becoming an emerging field in biomedical science.
Although considerable progress has been made in drug delivery in recent years, there are still many problems and challenges to be solved, such as the poor effect of long-term cycling and the ABC phenomena caused by some commonly used drugs. In addition, the current research on nano-drug delivery systems mainly focuses on the penetration of EPR effects in tumors, but few studies report the retention effects caused by tumor lymphatic drainage. In addition to macrophages, other cells (such as white blood cells) also have an impact on drug delivery system258; because of the limitation of blood‒brain barrier, some methods for improving the pharmacokinetics and permeability of NPs are not suitable for brain tumors; different types of tumors and different stages of tumors have certain differences in TME, which adds more resistance to the strategy of NPs infiltrating the tumors.
Therefore, we should continue to develop more quantitative imaging techniques to study the lymphatic function of tumor sites. The next step is to explore how it affects the infiltration and retention of IFP and NPs, and how to reduce the retention effect caused by lymphatic drainage, for example, the use of photodynamic therapy to reduce lymphatic drainage at the tumor site; the normalization of blood vessels at the tumor site without affecting the effective infiltration of NPs into the tumor tissue; the use of more appropriate nanomaterials and delivery methods in the design of NPs for the particularity of the blood‒brain barrier; and the connection between the therapeutic function of NPs and the immune system. For tumors of different types and stages, we should closely combine their particularity and individuality to improve the composition design of NPs, which has not yet been promoted.
It is believed that with the continuous development of materials science and oncology, these issues will be solved effectively, and the intelligent NPs will be developed towards direction of more rational design and precise targeting. In the future, we should design a more advantageous nano-drug delivery system based on the close collaboration of medical, chemical and material disciplines, in order to achieve the greatest therapeutic effect and realize the transformation from laboratory to clinical.
Acknowledgments
The authors wish to thank the National Natural Science Foundation of China (No. 81971729) for financial support.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Mingming Zhang wrote the manuscript. Shan Gao, Dongjuan Yang, Yan Fang, Xiaojie Lin and Xuechao Jin were responsible for reviewing relevant researches. Yuli Liu, Xiu Liu and Kexin Su provided manuscript polishing. Kai Shi provided guidance and improved the content of the article. All of the authors have read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Chen Y., Su M.F., Li Y.Q., Gao J.B., Zhang C., Cao Z. Enzymatic PEG-poly(amine-co-disulfide ester) nanoparticles as pH-and redox-responsive drug nanocarriers for efficient antitumor treatment. ACS Appl Mater Interfaces. 2017;9:30519–30535. doi: 10.1021/acsami.7b10148. [DOI] [PubMed] [Google Scholar]
- 2.Park W., Na K. Advances in the synthesis and application of nanoparticles for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:494–508. doi: 10.1002/wnan.1325. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Wu M., Pan L., Shi J. Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int J Nanomed. 2016;11:93–105. doi: 10.2147/IJN.S81156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsirikis P., Wilson K., Xiang S., Wei W., Ma G., Selomulya C. Immunogenicity and biodistribution of nanoparticles in vivo. Eur J Immunol. 2016;46 32-32. [Google Scholar]
- 5.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Gaudin A., Song E., Amanda K., Desmaele D., Couvreur P., Saltzman M. PEGylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Cancer Res. 2017;77 doi: 10.1016/j.biomaterials.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yohan D., Cruje C., Lu X., Chithrani D. Elucidating the uptake and distribution of nanoparticles in solid tumors via a multilayered cell culture model. Nano-Micro Lett. 2015;7:127–137. doi: 10.1007/s40820-014-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Z., Ding X., Hu Y., Wu S., Xiang Y., Zeng Y. Engineering a hollow nanocontainer platform with multifunctional molecular machines for tumor-targeted therapy in vitro and in vivo. ACS Nano. 2013;7:10271–10284. doi: 10.1021/nn404676w. [DOI] [PubMed] [Google Scholar]
- 9.Yao H.J., Zhang Y.G., Sun L., Liu Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials. 2014;35:9208–9223. doi: 10.1016/j.biomaterials.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Pekkanen A.M., DeWitt M.R., Rylander M.N. Nanoparticle enhanced optical imaging and phototherapy of cancer. J Biomed Nanotechnol. 2014;10:1677–1712. doi: 10.1166/jbn.2014.1988. [DOI] [PubMed] [Google Scholar]
- 11.Burke A., Ding X., Singh R., Kraft R.A., Levi-Polyachenko N., Rylander M.N. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci U S A. 2009;106:12897–12902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun T.M., Wang Y.C., Wang F., Du J.Z., Mao C.Q., Sun C.Y. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;35:836–845. doi: 10.1016/j.biomaterials.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Adnan N.N.M., Cheng Y.Y., Ong N.M.N., Kamaruddin T.T., Rozlan E., Schmidt T.W. Effect of gold nanoparticle shapes for phototherapy and drug delivery. Polym Chem. 2016;7:2888–2903. [Google Scholar]
- 14.Chen D.D., Monteiro-Riviere N.A., Zhang L.S.W. Intracellular imaging of quantum dots, gold, and iron oxide nanoparticles with associated endocytic pathways. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2017;9:1–19. doi: 10.1002/wnan.1419. [DOI] [PubMed] [Google Scholar]
- 15.Kang S., Ahn S., Lee J., Kim J.Y., Choi M., Gujrati V. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J Control Release. 2017;256:56–67. doi: 10.1016/j.jconrel.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy L.C., Bickford L.R., Lewinski N.A., Coughlin A.J., Hu Y., Day E.S. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 17.Qin Z., Bischof J.C. Thermophysical and biological responses of gold nanoparticle laser heating. Chem Soc Rev. 2012;41:1191–1217. doi: 10.1039/c1cs15184c. [DOI] [PubMed] [Google Scholar]
- 18.Luo Z., Cai K., Hu Y., Li J., Ding X., Zhang B. Redox-responsive molecular nanoreservoirs for controlled intracellular anticancer drug delivery based on magnetic nanoparticles. Adv Mater. 2012;24:431–435. doi: 10.1002/adma.201103458. [DOI] [PubMed] [Google Scholar]
- 19.MacParland S.A., Tsoi K.M., Ouyang B., Ma X.Z., Manuel J., Fawaz A. Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano. 2017;11:2428–2443. doi: 10.1021/acsnano.6b06245. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama M. Macrophage recognition of crystals and nanoparticles. Front Immunol. 2018;9:103. doi: 10.3389/fimmu.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapoport N., Gupta R., Kim Y.S., O'Neill B.E. Polymeric micelles and nanoemulsions as tumor-targeted drug carriers: insight through intravital imaging. J Control Release. 2015;206:153–160. doi: 10.1016/j.jconrel.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W.Z., Wang M.Z., Tang W., Wen R., Zhou S.Y., Lee C. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv Mater. 2018;30 doi: 10.1002/adma.201805557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo S., Lin C.M., Xu Z., Miao L., Wang Y., Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano. 2014;8:4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak B., Ozcelikkale A., Shin C.S., Park K., Han B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J Control Release. 2014;194:157–167. doi: 10.1016/j.jconrel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min Y., Caster J.M., Eblan M.J., Wang A.Z. Clinical translation of nanomedicine. Chem Rev. 2015;115:11147–11190. doi: 10.1021/acs.chemrev.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang K.Y., Ma H.L., Liu J., Huo S.D., Kumar A., Wei T. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W., Huang Y., An Y., Kim B.Y. Remodeling tumor vasculature to enhance delivery of intermediate-sized nanoparticles. ACS Nano. 2015;9:8689–8696. doi: 10.1021/acsnano.5b02028. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J.Y., Zheng D.W., Zhang M.K., Yu W.Y., Qiu W.X., Hu J.J. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–5901. doi: 10.1021/acs.nanolett.6b02786. [DOI] [PubMed] [Google Scholar]
- 29.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen D.M., Rentero C., Magenau A., Abu-Siniyeh A., Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc. 2011;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- 31.Laganowsky A., Reading E., Allison T.M., Ulmschneider M.B., Degiacomi M.T., Baldwin A.J. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto S., Kato A., Sakurai Y., Hada T., Harashima H. Modality of tumor endothelial VEGFR2 silencing-mediated improvement in intratumoral distribution of lipid nanoparticles. J Control Release. 2017;251:1–10. doi: 10.1016/j.jconrel.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Fasolato C., Giantulli S., Capocefalo A., Toumia Y., Notariello D., Mazzarda F. Antifolate SERS-active nanovectors: quantitative drug nanostructuring and selective cell targeting for effective theranostics. Nanoscale. 2019;11:15224–15233. doi: 10.1039/c9nr01075k. [DOI] [PubMed] [Google Scholar]
- 34.Morath I., Hartmann T.N., Orian-Rousseau V. CD44: more than a mere stem cell marker. Int J Biochem Cell Biol. 2016;81:166–173. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Kano M.R., Bae Y., Iwata C., Morishita Y., Yashiro M., Oka M. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts A.B., Wakefield L.M. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurosaki A., Hasegawa K., Kato T., Abe K., Hanaoka T., Miyara A. Serum folate receptor alpha as a biomarker for ovarian cancer: implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138:1994–2002. doi: 10.1002/ijc.29937. [DOI] [PubMed] [Google Scholar]
- 38.Goni F.M. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Pye C.R., Hewitt W.M., Schwochert J., Haddad T.D., Townsend C.E., Etienne L. Nonclassical size dependence of permeation defines bounds for passive adsorption of large drug molecules. J Med Chem. 2017;60:1665–1672. doi: 10.1021/acs.jmedchem.6b01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varkouhi A.K., Scholte M., Storm G., Haisma H.J. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Li J., Zhou J., Lin Z., Cavalieri F., Czuba-Wojnilowicz E. Metal-phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano. 2019;13:11653–11664. doi: 10.1021/acsnano.9b05521. [DOI] [PubMed] [Google Scholar]
- 42.Barua S., Mitragotri S. Synergistic targeting of cell membrane, cytoplasm, and nucleus of cancer cells using rod-shaped nanoparticles. ACS Nano. 2013;7:9558–9570. doi: 10.1021/nn403913k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elsabahy M., Wooley K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keliher E.J., Ye Y.X., Wojtkiewicz G.R., Aguirre A.D., Tricot B., Senders M.L. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun. 2017;8:14064. doi: 10.1038/ncomms14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paluri S.L.A., Ryan J.D., Lam N.H., Nepal D., Sizemore I.E. Analytical-based methodologies for examining the in vitro absorption, distribution, metabolism, and elimination (ADME) of silver nanoparticles. Small. 2017;13:1603093. doi: 10.1002/smll.201603093. [DOI] [PubMed] [Google Scholar]
- 46.Prabhudas M., Bowdish D., Drickamer K., Febbraio M., Herz J., Kobzik L. Standardizing scavenger receptor nomenclature. J Immunol. 2014;192:1997–2006. doi: 10.4049/jimmunol.1490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 48.Cully M. CANCER Re-educating tumour-associated macrophages with nanoparticles. Nat Rev Drug Discov. 2018;17 doi: 10.1038/nrd.2018.102. 468-68. [DOI] [PubMed] [Google Scholar]
- 49.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 50.Chen F., Wang G., Griffin J.I., Brenneman B., Banda N.K., Holers V.M. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vu V.P., Gifford G.B., Chen F., Benasutti H., Wang G., Groman E.V. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260–268. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin C., Evason K.J., Asahina K., Stainier D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Thurber G.M., Weissleder R. A systems approach for tumor pharmacokinetics. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niu Y., Zhu J., Li Y., Shi H., Gong Y., Li R. Size shrinkable drug delivery nanosystems and priming the tumor microenvironment for deep intratumoral penetration of nanoparticles. J Control Release. 2018;277:35–47. doi: 10.1016/j.jconrel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown M., Assen F.P., Leithner A., Abe J., Schachner H., Asfour G. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 58.Sykes E.A., Dai Q., Sarsons C.D., Chen J., Rocheleau J.V., Hwang D.M. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113:E1142–E1151. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 60.Gratton S.E., Ropp P.A., Pohlhaus P.D., Luft J.C., Madden V.J., Napier M.E. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu D., Mori A., Huang L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta. 1992;1104:95–101. doi: 10.1016/0005-2736(92)90136-a. [DOI] [PubMed] [Google Scholar]
- 62.Harush-Frenkel O., Debotton N., Benita S., Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 63.Yu C., He B., Xiong M.H., Zhang H., Yuan L., Ma L. The effect of hydrophilic and hydrophobic structure of amphiphilic polymeric micelles on their transport in epithelial MDCK cells. Biomaterials. 2013;34:6284–6298. doi: 10.1016/j.biomaterials.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Sims L.B., Curtis L.T., Frieboes H.B., Steinbach-Rankins J.M. Enhanced uptake and transport of PLGA-modified nanoparticles in cervical cancer. J Nanobiotechnol. 2016;14:33. doi: 10.1186/s12951-016-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li F., Lu Y., Li W., Miller D.D., Mahato R.I. Synthesis, formulation and in vitro evaluation of a novel microtubule destabilizer, SMART-100. J Control Release. 2010;143:151–158. doi: 10.1016/j.jconrel.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 66.Li P.Y., Lai P.S., Hung W.C., Syu W.J. Poly(L-lactide)-vitamin E TPGS nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant MCF-7 breast cancer cells. Biomacromolecules. 2010;11:2576–2582. doi: 10.1021/bm1005195. [DOI] [PubMed] [Google Scholar]
- 67.Gao W., Xiang B., Meng T.T., Liu F., Qi X.R. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials. 2013;34:4137–4149. doi: 10.1016/j.biomaterials.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Yang M., Lai S.K., Yu T., Wang Y.Y., Happe C., Zhong W. Nanoparticle penetration of human cervicovaginal mucus: the effect of polyvinyl alcohol. J Control Release. 2014;192:202–208. doi: 10.1016/j.jconrel.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., Black K.C., Luehmann H., Li W., Zhang Y., Cai X. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7:2068–2077. doi: 10.1021/nn304332s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nance E., Timbie K., Miller G.W., Song J., Louttit C., Klibanov A.L. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood–brain barrier using MRI-guided focused ultrasound. J Control Release. 2014;189:123–132. doi: 10.1016/j.jconrel.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nance E.A., Woodworth G.F., Sailor K.A., Shih T.Y., Xu Q., Swaminathan G. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra19. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cole A.J., David A.E., Wang J., Galban C.J., Hill H.L., Yang V.C. Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting. Biomaterials. 2011;32:2183–2193. doi: 10.1016/j.biomaterials.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sims L.B., Huss M.K., Frieboes H.B., Steinbach-Rankins J.M. Distribution of PLGA-modified nanoparticles in 3D cell culture models of hypo-vascularized tumor tissue. J Nanobiotechnol. 2017;15:67. doi: 10.1186/s12951-017-0298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q., Deng C., Fu Y., Sun X., Gong T., Zhang Z. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol Pharm. 2016;13:1800–1808. doi: 10.1021/acs.molpharmaceut.5b00952. [DOI] [PubMed] [Google Scholar]
- 75.Bozuyuk U., Yasa O., Yasa I.C., Ceylan H., Kizilel S., Sitti M. Light-triggered drug release from 3D-printed magnetic chitosan microswimmers. ACS Nano. 2018;12:9617–9625. doi: 10.1021/acsnano.8b05997. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L.W., Gao S., Zhang F., Yang K., Ma Q.J., Zhu L. Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano. 2014;8:12250–12258. doi: 10.1021/nn506130t. [DOI] [PubMed] [Google Scholar]
- 77.Shi K., Fang Y., Gao S., Yang D., Bi H., Xue J. Inorganic kernel—supported asymmetric hybrid vesicles for targeting delivery of STAT3-decoy oligonucleotides to overcome anti-HER2 therapeutic resistance of BT474R. J Control Release. 2018;279:53–68. doi: 10.1016/j.jconrel.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q., Li C., Ren T., Chen S., Ye X., Guo H. Poly(vinyl methyl ether/maleic anhydride)-doped PEG-PLA nanoparticles for oral paclitaxel delivery to improve bioadhesive efficiency. Mol Pharm. 2017;14:3598–3608. doi: 10.1021/acs.molpharmaceut.7b00612. [DOI] [PubMed] [Google Scholar]
- 79.Correia A., Shahbazi M.A., Makila E., Almeida S., Salonen J., Hirvonen J. Cyclodextrin-modified porous silicon nanoparticles for efficient sustained drug delivery and proliferation inhibition of breast cancer cells. ACS Appl Mater Interfaces. 2015;7:23197–23204. doi: 10.1021/acsami.5b07033. [DOI] [PubMed] [Google Scholar]
- 80.Dong L., Zhang P., Liu X., Deng R., Du K., Feng J. Renal Clearable Bi-Bi2S3 heterostructure nanoparticles for targeting cancer theranostics. ACS Appl Mater Interfaces. 2019;11:7774–7781. doi: 10.1021/acsami.8b21280. [DOI] [PubMed] [Google Scholar]
- 81.Huang Y.P., Zhang B.L., Xie S.B., Yang B.N., Xu Q., Tan J. Superparamagnetic iron oxide nanoparticles modified with tween 80 pass through the intact blood–brain barrier in rats under magnetic field. ACS Appl Mater Interfaces. 2016;8:11336–11341. doi: 10.1021/acsami.6b02838. [DOI] [PubMed] [Google Scholar]
- 82.Fan B., Gillies E.R. Poly(ethyl glyoxylate)-poly(ethylene oxide) nanoparticles: stimuli-responsive drug release via end-to-end polyglyoxylate depolymerization. Mol Pharm. 2017;14:2548–2559. doi: 10.1021/acs.molpharmaceut.7b00030. [DOI] [PubMed] [Google Scholar]
- 83.Singh Y., Srinivas A., Gangwar M., Meher J.G., Misra-Bhattacharya S., Chourasia M.K. Subcutaneously administered ultrafine PLGA nanoparticles containing doxycycline hydrochloride target lymphatic filarial parasites. Mol Pharm. 2016;13:2084–2094. doi: 10.1021/acs.molpharmaceut.6b00206. [DOI] [PubMed] [Google Scholar]
- 84.Beck-Broichsitter M., Bohr A., Ruge C.A. Poloxamer-decorated polymer nanoparticles for lung surfactant compatibility. Mol Pharm. 2017;14:3464–3472. doi: 10.1021/acs.molpharmaceut.7b00477. [DOI] [PubMed] [Google Scholar]
- 85.Kierstead P.H., Okochi H., Venditto V.J., Chuong T.C., Kivimae S., Frechet J.M.J. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J Control Release. 2015;213:1–9. doi: 10.1016/j.jconrel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D.H., Chu Y.H., Qian H.Q., Qian L.Y., Shao J., Xu Q.P. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomed. 2020;15:735–747. doi: 10.2147/IJN.S231448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J.E., Yu J., Gao L., Sun L., Peng T., Wang J. iNGR-modified liposomes for tumor vascular targeting and tumor tissue penetrating delivery in the treatment of glioblastoma. Mol Pharm. 2017;14:1811–1820. doi: 10.1021/acs.molpharmaceut.7b00101. [DOI] [PubMed] [Google Scholar]
- 88.Xin H., Jiang X., Gu J., Sha X., Chen L., Law K. Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 89.Sharma S., Kotamraju V.R., Molder T., Tobi A., Teesalu T., Ruoslahti E. Tumor-penetrating nanosystem strongly suppresses breast tumor growth. Nano Lett. 2017;17:1356–1364. doi: 10.1021/acs.nanolett.6b03815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shein S.A., Kuznetsov, Abakumova T.O., Chelushkin P.S., Melnikov P.A., Korchagina A.A. VEGF- and VEGFR2-targeted liposomes for cisplatin delivery to glioma cells. Mol Pharm. 2016;13:3712–3723. doi: 10.1021/acs.molpharmaceut.6b00519. [DOI] [PubMed] [Google Scholar]
- 91.Kulhari H., Pooja D., Kota R., Reddy T.S., Tabor R.F., Shukla R. Cyclic RGDfK peptide functionalized polymeric nanocarriers for targeting gemcitabine to ovarian cancer cells. Mol Pharm. 2016;13:1491–1500. doi: 10.1021/acs.molpharmaceut.5b00935. [DOI] [PubMed] [Google Scholar]
- 92.Danhier F., Le Breton A., Preat V. RGD-based strategies to target alphav beta3 integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 93.Jiang Y., Pang X., Liu R., Xiao Q., Wang P., Leung A.W. Design of an amphiphilic irgd peptide and self-assembling nanovesicles for improving tumor accumulation and penetration and the photodynamic efficacy of the photosensitizer. ACS Appl Mater Interfaces. 2018;10:31674–31685. doi: 10.1021/acsami.8b11699. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad A., Mondal S.K., Mukhopadhyay D., Banerjee R., Alkharfy K.M. Development of liposomal formulation for delivering anticancer drug to breast cancer stem-cell-like cells and its pharmacokinetics in an animal model. Mol Pharm. 2016;13:1081–1088. doi: 10.1021/acs.molpharmaceut.5b00900. [DOI] [PubMed] [Google Scholar]
- 95.Kang T., Gao X.L., Hu Q.Y., Jiang D., Feng X.Y., Zhang X. iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials. 2014;35:4319–4332. doi: 10.1016/j.biomaterials.2014.01.082. [DOI] [PubMed] [Google Scholar]
- 96.Zhang X.Y., Wang F., Shen Q., Xie C., Liu Y., Pan J. Structure reconstruction of LyP-1: L c(LyP-1) coupling by amide bond inspires the brain metastatic tumor targeted drug delivery. Mol Pharm. 2018;15:430–436. doi: 10.1021/acs.molpharmaceut.7b00801. [DOI] [PubMed] [Google Scholar]
- 97.Song N.N., Zhao L.Z., Xu X.Y., Zhu M.L., Liu C.C., Sun N. LyP-1-modified multifunctional dendrimers for targeted antitumor and antimetastasis therapy. ACS Appl Mater Interfaces. 2020;12:12395–12406. doi: 10.1021/acsami.9b18881. [DOI] [PubMed] [Google Scholar]
- 98.Saalik P., Lingasamy P., Toome K., Mastandrea I., Rousso-Noori L., Tobi A. Peptide-guided nanoparticles for glioblastoma targeting. J Control Release. 2019;308:109–118. doi: 10.1016/j.jconrel.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 99.Osman G., Rodriguez J., Chan S.Y., Chisholm J., Duncan G., Kim N. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J Control Release. 2018;285:35–45. doi: 10.1016/j.jconrel.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui J., De Rose R., Alt K., Alcantara S., Paterson B.M., Liang K. Engineering poly(ethylene glycol) particles for improved biodistribution. ACS Nano. 2015;9:1571–1580. doi: 10.1021/nn5061578. [DOI] [PubMed] [Google Scholar]
- 101.Hu X., Gao X. Silica-polymer dual layer-encapsulated quantum dots with remarkable stability. ACS Nano. 2010;4:6080–6086. doi: 10.1021/nn1017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia Y.G., Zhu X.X. Thermo- and pH-responsive copolymers bearing cholic acid and oligo(ethylene glycol) pendants: self-assembly and pH-controlled release. ACS Appl Mater Interfaces. 2015;7:24649–24655. doi: 10.1021/acsami.5b06909. [DOI] [PubMed] [Google Scholar]
- 103.Mima Y., Hashimoto Y., Shimizu T., Kiwada H., Ishida T. Anti-PEG IgM is a major contributor to the accelerated blood clearance of polyethylene glycol-conjugated protein. Mol Pharm. 2015;12:2429–2435. doi: 10.1021/acs.molpharmaceut.5b00144. [DOI] [PubMed] [Google Scholar]
- 104.Gao W., Hu C.M., Fang R.H., Luk B.T., Su J., Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25:3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toledano Furman N.E., Lupu-Haber Y., Bronshtein T., Kaneti L., Letko N., Weinstein E. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13:3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 108.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28:9581–9588. doi: 10.1002/adma.201602173. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez P.L., Harada T., Christian D.A., Pantano D.A., Tsai R.K., Discher D.E. Minimal "self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dehaini D., Wei X., Fang R.H., Masson S., Angsantikul P., Luk B.T. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29 doi: 10.1002/adma.201606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodriguez P.L., Harada T., Christian D.A., Pantano D.A., Tsai R.K., Discher D.E. Minimal "Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Itty Ipe B. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi C.H., Zuckerman J.E., Webster P., Davis M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A. 2011;108:6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conner S.D., Schmid S.L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 116.Jiang W., Kim B.Y., Rutka J.T., Chan W.C. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 117.Prabhakar U., Maeda H., Jain R.K., Sevick-Muraca E.M., Zamboni W., Farokhzad O.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maeda H., Nakamura H., Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albanese A., Tang P.S., Chan W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 122.Kim B., Han G., Toley B.J., Kim C.K., Rotello V.M., Forbes N.S. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vina-Vilaseca A., Bender-Sigel J., Sorkina T., Closs E.I., Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286:8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harush-Frenkel O., Rozentur E., Benita S., Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–443. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- 125.Agarwal R., Singh V., Jurney P., Shi L., Sreenivasan S.V., Roy K. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc Natl Acad Sci U S A. 2013;110:17247–17252. doi: 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Black K.C., Wang Y., Luehmann H.P., Cai X., Xing W., Pang B. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8:4385–4394. doi: 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beningo K.A., Wang Y.L. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 129.Anselmo A.C., Zhang M.W., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E. Elasticity of nanopartides influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9:3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 130.Merkel T.J., Jones S.W., Herlihy K.P., Kersey F.R., Shields A.R., Napier M. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108:586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang L., Cao Z.Q., Li Y.T., Ella-Menye J.R., Bai T., Jiang S.Y. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano. 2012;6:6681–6686. doi: 10.1021/nn301159a. [DOI] [PubMed] [Google Scholar]
- 132.Liang Q.L., Bie N.N., Yong T.Y., Tang K., Shi X.L., Wei Z.H. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat Biomed Eng. 2019;3:729–740. doi: 10.1038/s41551-019-0405-4. [DOI] [PubMed] [Google Scholar]
- 133.Deng H.Z., Song K., Zhang J.H., Deng L.D., Dong A.J., Qin Z.H. Modulating the rigidity of nanoparticles for tumor penetration. Chem Commun. 2018;54:3014–3017. doi: 10.1039/c8cc00398j. [DOI] [PubMed] [Google Scholar]
- 134.Monopoli M.P., Aberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 135.Lundqvist M., Stigler J., Cedervall T., Berggard T., Flanagan M.B., Lynch I. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5:7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- 136.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 137.Cai R., Chen C. The Crown and the Scepter: roles of the protein corona in nanomedicine. Adv Mater. 2019;31 doi: 10.1002/adma.201805740. [DOI] [PubMed] [Google Scholar]
- 138.Hamad I., Al-Hanbali O., Hunter A.C., Rutt K.J., Andresen T.L., Moghimi S.M. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering. ACS Nano. 2010;4:6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 139.Yoshino F., Amano T., Zou Y.J., Xu J., Kimura F., Furusho Y. Preferential tumor accumulation of polyglycerol functionalized nanodiamond conjugated with cyanine dye leading to near-infrared fluorescence in vivo tumor imaging. Small. 2019;15 doi: 10.1002/smll.201901930. [DOI] [PubMed] [Google Scholar]
- 140.Garcia K.P., Zarschler K., Barbaro L., Barreto J.A., O'Malley W., Spiccia L. Zwitterionic-coated "stealth" nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10:2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 141.Zou Y., Ito S., Yoshino F., Suzuki Y., Zhao L., Komatsu N. Polyglycerol grafting shields nanoparticles from protein corona formation to avoid macrophage uptake. ACS Nano. 2020;14:7216–7226. doi: 10.1021/acsnano.0c02289. [DOI] [PubMed] [Google Scholar]
- 142.Peng Q., Zhang S., Yang Q., Zhang T., Wei X.Q., Jiang L. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34:8521–8530. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 143.Lacerda S.H., Park J.J., Meuse C., Pristinski D., Becker M.L., Karim A. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2010;4:365–379. doi: 10.1021/nn9011187. [DOI] [PubMed] [Google Scholar]
- 144.Mahmoudi M., Sant S., Wang B., Laurent S., Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 145.Hennig R., Pollinger K., Veser A., Breunig M., Goepferich A. Nanoparticle multivalency counterbalances the ligand affinity loss upon PEGylation. J Control Release. 2014;194:20–27. doi: 10.1016/j.jconrel.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 146.Tang Y., Wang X., Li J., Nie Y., Liao G., Yu Y. Overcoming the reticuloendothelial system barrier to drug delivery with a "don't-eat-us" strategy. ACS Nano. 2019;13:13015–13026. doi: 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- 147.Abdelhalim M.A., Jarrar B.M. Histological alterations in the liver of rats induced by different gold nanoparticle sizes, doses and exposure duration. J Nanobiotechnol. 2012;10:5. doi: 10.1186/1477-3155-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stirland D.L., Matsumoto Y., Toh K., Kataoka K., Bae Y.H. Analyzing spatiotemporal distribution of uniquely fluorescent nanoparticles in xenograft tumors. J Control Release. 2016;227:38–44. doi: 10.1016/j.jconrel.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu H.L., Ramanathan R.K., Zamboni B.A., Strychor S., Ramalingam S., Edwards R.P. Mechanism-based model characterizing bidirectional interaction between PEGylated liposomal CKD-602 (S-CKD602) and monocytes in cancer patients. Int J Nanomed. 2012;7:5555–5564. doi: 10.2147/IJN.S35751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Y., Liu R., Shi Y., Zhang Z., Zhang X. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics. 2015;5:583–596. doi: 10.7150/thno.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Danquah M.K., Zhang X.A., Mahato R.I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv Drug Deliv Rev. 2011;63:623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 152.Kobayashi H., Watanabe R., Choyke P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target?. Theranostics. 2013;4:81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hansen A.E., Petersen A.L., Henriksen J.R., Boerresen B., Rasmussen P., Elema D.R. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano. 2015;9:6985–6995. doi: 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- 154.Barua S., Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miao L., Lin C.M., Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J Control Release. 2015;219:192–204. doi: 10.1016/j.jconrel.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chauhan V.P., Stylianopoulos T., Boucher Y., Jain R.K. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 157.Khawar I.A., Kim J.H., Kuh H.J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 158.Kim S.J., Jung K.H., Son M.K., Park J.H., Yan H.H., Fang Z. Tumor vessel normalization by the PI3K inhibitor HS-173 enhances drug delivery. Cancer Lett. 2017;403:339–353. doi: 10.1016/j.canlet.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 159.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chauhan V.P., Stylianopoulos T., Martin J.D., Popovic Z., Chen O., Kamoun W.S. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Y., Wang J., Wientjes M.G., Au J.L. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64:29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.DeWitt M.R., Rylander M.N. Tunable collagen microfluidic platform to study nanoparticle transport in the tumor microenvironment. Methods Mol Biol. 2018;1831:159–178. doi: 10.1007/978-1-4939-8661-3_12. [DOI] [PubMed] [Google Scholar]
- 163.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 164.Torosean S., Flynn B., Axelsson J., Gunn J., Samkoe K.S., Hasan T. Nanoparticle uptake in tumors is mediated by the interplay of vascular and collagen density with interstitial pressure. Nanomedicine. 2013;9:151–158. doi: 10.1016/j.nano.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J.Q., Mao W.W., Lock L.L., Tang J.B., Sui M.H., Sun W.L. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano. 2015;9:7195–7206. doi: 10.1021/acsnano.5b02017. [DOI] [PubMed] [Google Scholar]
- 166.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brown E., McKee T., diTomaso E., Pluen A., Seed B., Boucher Y. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 168.Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cox T.R., Erler J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Henke E., Nandigama R., Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2019;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Erler J.T., Bennewith K.L., Nicolau M., Dornhofer N., Kong C., Le Q.T. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 174.Levental K.R., Yu H.M., Kass L., Lakins J.N., Egeblad M., Erler J.T. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schaaf M.B., Garg A.D., Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018;9:115. doi: 10.1038/s41419-017-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Raave R., van Kuppevelt T.H., Daamen W.F. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release. 2018;274:1–8. doi: 10.1016/j.jconrel.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 177.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 178.Tang L., Gabrielson N.P., Uckun F.M., Fan T.M., Cheng J.J. Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol Pharm. 2013;10:883–892. doi: 10.1021/mp300684a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Popovic Z., Liu W.H., Chauhan V.P., Lee J., Wong C., Greytak A.B. A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed. 2010;49:8649–8652. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee H., Hoang B., Fonge H., Reilly R.M., Allen C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm Res. 2010;27:2343–2355. doi: 10.1007/s11095-010-0068-z. [DOI] [PubMed] [Google Scholar]
- 181.Lee H., Fonge H., Hoang B., Reilly R.M., Allen C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharm. 2010;7:1195–1208. doi: 10.1021/mp100038h. [DOI] [PubMed] [Google Scholar]
- 182.Jin H., Heller D.A., Sharma R., Strano M.S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano. 2009;3:149–158. doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 183.Lu F., Wu S.H., Hung Y., Mou C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. 2009;5:1408–1413. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- 184.Chauhan V.P., Popovic Z., Chen O., Cui J., Fukumura D., Bawendi M.G. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nomura T., Koreeda N., Yamashita F., Takakura Y., Hashida M. Effect of particle size and charge on the disposition of lipid carriers after intratumoral injection into tissue-isolated tumors. Pharm Res. 1998;15:128–132. doi: 10.1023/a:1011921324952. [DOI] [PubMed] [Google Scholar]
- 186.Li H.J., Du J.Z., Du X.J., Xu C.F., Sun C.Y., Wang H.X. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci U S A. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Z., Xiao C., Yong T.Y., Li Z.F., Gan L., Yang X.L. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem Soc Rev. 2020;49:2273–2290. doi: 10.1039/c9cs00575g. [DOI] [PubMed] [Google Scholar]
- 188.Hui Y., Wibowo D., Liu Y., Ran R., Wang H.F., Seth A. Understanding the effects of nanocapsular mechanical property on passive and active tumor targeting. ACS Nano. 2018;12:2846–2857. doi: 10.1021/acsnano.8b00242. [DOI] [PubMed] [Google Scholar]
- 189.Key J., Palange A.L., Gentile F., Aryal S., Stigliano C., Di Mascolo D. Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano. 2015;9:11628–11641. doi: 10.1021/acsnano.5b04866. [DOI] [PubMed] [Google Scholar]
- 190.Sun J., Zhang L., Wang J., Feng Q., Liu D., Yin Q. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv Mater. 2015;27:1402–1407. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 191.Polyak K., Haviv I., Campbell I.G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 192.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Du J., Lane L.A., Nie S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J Control Release. 2015;219:205–214. doi: 10.1016/j.jconrel.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jain R.K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 195.Cully M. Cancer: tumour vessel normalization takes centre stage. Nat Rev Drug Discov. 2017;16:87. doi: 10.1038/nrd.2017.4. [DOI] [PubMed] [Google Scholar]
- 196.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 197.Goel S., Duda D.G., Xu L., Munn L.L., Boucher Y., Fukumura D. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brekken C., de Lange Davies C. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett. 1998;131:65–70. doi: 10.1016/s0304-3835(98)00202-x. [DOI] [PubMed] [Google Scholar]
- 199.Huang Y., Yuan J., Righi E., Kamoun W.S., Ancukiewicz M., Nezivar J. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang Y., Stylianopoulos T., Duda D.G., Fukumura D., Jain R.K. Benefits of vascular normalization are dose and time dependent—letter. Cancer Res. 2013;73:7144–7146. doi: 10.1158/0008-5472.CAN-13-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou P., Qin J., Zhou C., Wan G., Liu Y., Zhang M. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials. 2019;195:86–99. doi: 10.1016/j.biomaterials.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 202.Schmittnaegel M., Rigamonti N., Kadioglu E., Cassara A., Rmili C.W., Kiialainen A. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
- 203.Huang N., Liu Y.Q., Fang Y.S., Zheng S.T., Wu J.H., Wang M.H. Gold nanoparticles induce tumor vessel normalization and impair metastasis by inhibiting endothelial smad2/3 signaling. ACS Nano. 2020;14:7940–7958. doi: 10.1021/acsnano.9b08460. [DOI] [PubMed] [Google Scholar]
- 204.Jiang Z.J., Xiong H., Yang S., Lu Y., Deng Y.D., Yao J.X. Jet-lagged nanoparticles enhanced immunotherapy efficiency through synergistic reconstruction of tumor microenvironment and normalized tumor vasculature. Adv Healthcare Mater. 2020;9 doi: 10.1002/adhm.202000075. [DOI] [PubMed] [Google Scholar]
- 205.Liu J.Q., Liao S., Diop-Frimpong B., Chen W., Goel S., Naxerova K. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2012;109:16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Diop-Frimpong B., Chauhan V.P., Krane S., Boucher Y., Jain R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao Y.X., Cao J.H., Melamed A., Worley M., Gockley A., Jones D. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2019;116:2210–2219. doi: 10.1073/pnas.1818357116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chauhan V.P., Chen I.X., Tong R., Ng M.R., Martin J.D., Naxerova K. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci U S A. 2019;116:10674–10680. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McKee T.D., Grandi P., Mok W., Alexandrakis G., Insin N., Zimmer J.P. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 210.Zinger A., Koren L., Adir O., Poley M., Alyan M., Yaari Z. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13:11008–11021. doi: 10.1021/acsnano.9b02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu M.Y., Wang Y., Xu L.G., An S., Tang Y., Zhou X.F. Relaxin gene delivery mitigates liver metastasis and synergizes with check point therapy. Nat Commun. 2019;10:2993. doi: 10.1038/s41467-019-10893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wong K.M., Horton K.J., Coveler A.L., Hingorani S.R., Harris W.P. Targeting the tumor stroma: the biology and clinical development of pegylated recombinant human hyaluronidase (PEGPH20) Curr Oncol Rep. 2017;19:47. doi: 10.1007/s11912-017-0608-3. [DOI] [PubMed] [Google Scholar]
- 213.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou H., Fan Z.Y., Deng J.J., Lemons P.K., Arhontoulis D.C., Bowne W.B. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16:3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 215.Gong H., Chao Y., Xiang J., Han X., Song G.S., Feng L.Z. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16:2512–2521. doi: 10.1021/acs.nanolett.6b00068. [DOI] [PubMed] [Google Scholar]
- 216.Wang H.R., Han X., Dong Z.L., Xu J., Wang J., Liu Z. Hyaluronidase with pH-responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic-immunotherapy of cancer. Adv Funct Mater. 2019;29:1902440. [Google Scholar]
- 217.Hielscher A., Gerecht S. Hypoxia and free radicals: role in tumor progression and the use of engineering-based platforms to address these relationships. Free Radic Biol Med. 2015;79:281–291. doi: 10.1016/j.freeradbiomed.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kirtane A.R., Kalscheuer S.M., Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv Drug Deliv Rev. 2013;65:1731–1747. doi: 10.1016/j.addr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Khan K.A., Kerbel R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 220.Liu Z., Chen W., Li Y., Xu Q. Integrin alphavbeta3-targeted c-dot nanocomposites as multifunctional agents for cell targeting and photoacoustic imaging of superficial malignant tumors. Anal Chem. 2016;88:11955–11962. doi: 10.1021/acs.analchem.6b03927. [DOI] [PubMed] [Google Scholar]
- 221.Thambi T., Park J.H., Lee D.S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives. Chem Commun. 2016;52:8492–8500. doi: 10.1039/c6cc02972h. [DOI] [PubMed] [Google Scholar]
- 222.Tian J., Ding L., Xu H.J., Shen Z., Ju H., Jia L. Cell-specific and pH-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J Am Chem Soc. 2013;135:18850–18858. doi: 10.1021/ja408286k. [DOI] [PubMed] [Google Scholar]
- 223.Huang X., Zhuang J., Chung S.W., Huang B., Halpert G., Negron K. Hypoxia-tropic protein nanocages for modulation of tumor- and chemotherapy-associated hypoxia. ACS Nano. 2019;13:236–247. doi: 10.1021/acsnano.8b05399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang W.C., Chiang W.H., Cheng Y.H., Lin W.C., Yu C.F., Yen C.Y. Tumortropic monocyte-mediated delivery of echogenic polymer bubbles and therapeutic vesicles for chemotherapy of tumor hypoxia. Biomaterials. 2015;71:71–83. doi: 10.1016/j.biomaterials.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 225.Song M., Liu T., Shi C., Zhang X., Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10:633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang M., Xing L., Ke H., He Y.J., Cui P.F., Zhu Y. MnO2-based nanoplatform serves as drug vehicle and mri contrast agent for cancer theranostics. ACS Appl Mater Interfaces. 2017;9:11337–11344. doi: 10.1021/acsami.6b15247. [DOI] [PubMed] [Google Scholar]
- 227.Samanta D., Gilkes D.M., Chaturvedi P., Xiang L., Semenza G.L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:E5429–E5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 228.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 229.De Wever O., Demetter P., Mareel M., Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 230.Chen X.M., Song E.W. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 231.Pure E., Lo A. Can targeting stroma pave the way to enhanced antitumor immunity and immunotherapy of solid tumors?. Cancer Immunol Res. 2016;4:269–278. doi: 10.1158/2326-6066.CIR-16-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu T.Y., Han C.C., Wang S.W., Fang P.Q., Ma Z.F., Xu L. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tao L.L., Huang G.C., Song H.Z., Chen Y.T., Chen L.B. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yu Y., Xiao C.H., Tan L.D., Wang Q.S., Li X.Q., Feng Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Avgustinova A., Iravani M., Robertson D., Fearns A., Gao Q., Klingbeil P. Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun. 2016;7:10305. doi: 10.1038/ncomms10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu X.Y., Chen X.H., Zhou Q., Li P., Yu B.Q., Li J.F. Hepatocyte growth factor activates tumor stromal fibroblasts to promote tumorigenesis in gastric cancer. Cancer Lett. 2013;335:128–135. doi: 10.1016/j.canlet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 237.Liang Z., Brooks J., Willard M., Liang K., Yoon Y., Kang S. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Erdogan B., Webb D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Boire A., Covic L., Agarwal A., Jacques S., Sherifl S., Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 240.Barker H.E., Cox T.R., Erler J.T. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 241.Petrova V., Annicchiarico-Petruzzelli M., Melino G., Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Najafi M., Farhood B., Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–2790. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 243.Gilkes D.M., Semenza G.L., Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 245.Yang J.N., Lu Y., Lin Y.Y., Zheng Z.Y., Fang J.H., He S. Vascular mimicry formation is promoted by paracrine TGF-beta and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016;383:18–27. doi: 10.1016/j.canlet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 246.Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–177. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Augsten M., Hagglof C., Olsson E., Stolz C., Tsagozis P., Levchenko T. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci U S A. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wallace J.A., Li F., Balakrishnan S., Cantemir-Stone C.Z., Pecot T., Martin C. Ets2 in tumor fibroblasts promotes angiogenesis in breast cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ernsting M.J., Hoang B., Lohse I., Undzys E., Cao P., Do T. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–130. doi: 10.1016/j.jconrel.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhen Z., Tang W., Wang M., Zhou S., Wang H., Wu Z. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 251.Fang T., Zhang J., Zuo T., Wu G., Xu Y., Yang Y. Chemo-photothermal combination cancer therapy with ROS scavenging, extracellular matrix depletion, and tumor immune activation by telmisartan and diselenide-paclitaxel prodrug loaded nanoparticles. ACS Appl Mater Interfaces. 2020;12:31292–31308. doi: 10.1021/acsami.0c10416. [DOI] [PubMed] [Google Scholar]
- 252.Cun X., Chen J., Li M., He X., Tang X., Guo R. Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size-switchable nanoparticle. ACS Appl Mater Interfaces. 2019;11:39545–39559. doi: 10.1021/acsami.9b13957. [DOI] [PubMed] [Google Scholar]
- 253.Miao L., Wang Y., Lin C.M., Xiong Y., Chen N., Zhang L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J Control Release. 2015;217:27–41. doi: 10.1016/j.jconrel.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Feng J.X., Xu M.J., Wang J.H., Zhou S.L., Liu Y.P., Liu S.S. Sequential delivery of nanoformulated alpha-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907. doi: 10.1016/j.biomaterials.2020.119907. [DOI] [PubMed] [Google Scholar]
- 255.Pei Y.Y., Chen L., Huang Y.K., Wang J.H., Feng J., Xu M.J. Sequential targeting TGF-beta signaling and KRAS mutation increases therapeutic efficacy in pancreatic cancer. Small. 2019;15 doi: 10.1002/smll.201900631. [DOI] [PubMed] [Google Scholar]
- 256.Hu K.L., Miao L., Goodwin T.J., Li J., Liu Q., Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11:4916–4925. doi: 10.1021/acsnano.7b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hou L., Liu Q., Shen L.M., Liu Y., Zhang X.Q., Chen F.Q. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics. 2018;8:3781–3796. doi: 10.7150/thno.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Betker J.L., Jones D., Childs C.R., Helm K.M., Terrell K., Nagel M.A. Nanoparticle uptake by circulating leukocytes: a major barrier to tumor delivery. J Control Release. 2018;286:85–93. doi: 10.1016/j.jconrel.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]