Abstract
Proteases have a fundamental role in maintaining physiological homeostasis, but their dysregulation results in severe activity imbalance and pathological conditions, including cancer onset, progression, invasion, and metastasis. This striking importance plus superior biological recognition and catalytic performance of proteases, combining with the excellent physicochemical characteristics of nanomaterials, results in enzyme-activated nano-drug delivery systems (nanoDDS) that perform theranostic functions in highly specific response to the tumor phenotype stimulus. In the tutorial review, the key advances of protease-responsive nanoDDS in the specific diagnosis and targeted treatment for malignancies are emphatically classified according to the effector biomolecule types, on the premise of summarizing the structure and function of each protease. Subsequently, the incomplete matching and recognition between enzyme and substrate, structural design complexity, volume production, and toxicological issues related to the nanocomposites are highlighted to clarify the direction of efforts in nanotheranostics. This will facilitate the promotion of nanotechnology in the management of malignant tumors.
KEY WORDS: Protease-responsive, Drug delivery, Nanomedicine, Multifunctional construction, Precise diagnosis, Targeted therapy, Synergistic theranostic, Malignancy
Graphical abstract
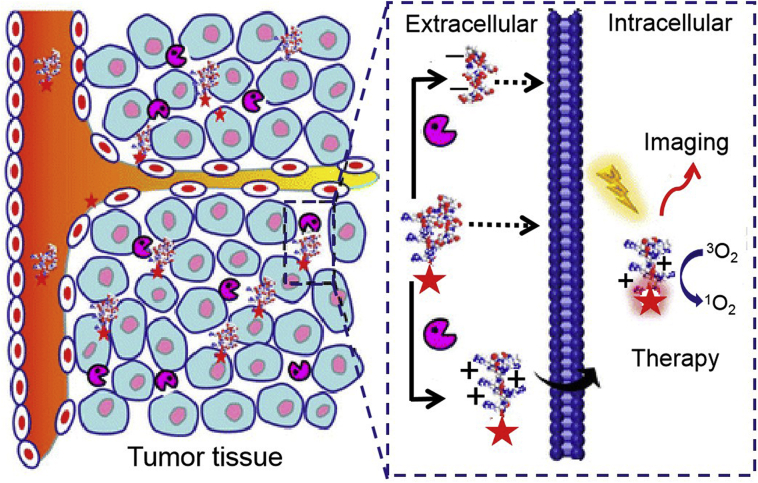
Excellent protease properties combined with thriving nanotechnology generate versatile enzyme-responsive nanoplatforms, which demonstrate outstanding superiority in cancer theranostics. These nanoarchitectures will realize universal clinical potency for the precise cancer management.
1. Introduction
Despite remarkable progress in the prevention, diagnosis, treatment, and surveillance over the years, cancer remains a serious threat to human health and quality of life. Its morbidity and mortality are increasing year by year worldwide1, especially in China2. Thus, more challenging theranostic methods are required to address this human public health impact. Currently, surgery remains the preferred option for the vast majority of solid tumors. Even so, limitations such as tumor accessibility, pathological complexity, preoperative spread, and metastasis make surgical resection inapplicable to all solid tumors3. Fittingly, chemotherapy interventions have emerged as stand-alone regimens or as an adjunct to other focal treatments, like surgery or radiotherapy4. Although a series of cytotoxic chemotherapeutics have become the first choice in clinical treatment against many kinds of cancer and have achieved significant progress, the toxicity to normal tissues, non-targeting, and the occurrence of drug resistance limit their clinical efficacy and application.
With research intensifying on the biological and pharmacological mechanisms of cancer at molecular level, a variety of molecular targeted drugs, gene remediation molecules, and immune microenvironment regulators have emerged to direct tumor signal transduction disturbances and metabolic abnormalities5, 6, 7, 8. These novel drug models exhibit greater selectivity, targeting, histocompatibility, and therapeutic index than traditional toxic therapeutants, and they further improve the host's immune strength to remove tumor cells and resist foreign carcinogens. Despite some success, the identification complexity of carcinogenic and immunomodulatory targets, anchored by drugs, the instability, diversity, and mutation susceptibility of proto-oncogenes, greatly limit the progress and application of targeted treatment due to scanty drug targeting and concentration enrichment at the lesion9, 10, 11.
To address this limitation, an alternative strategy is to integrate nanotechnology and stimulus responsiveness of tumor phenotype rather than intracellular signaling pathways, such as differential tumor-normal tissue physicochemical characteristics12, 13, 14, 15, distinct vascular structures16, and altered enzyme expression which is highly active in tumors and less active in normal organs17. Among these, proteases, with intrinsic high specificity, strong catalytic activity, mild reaction conditions, significant expression and activity distribution in tumors, have been developed not merely as detective/prognostic biomarkers18,19 or targets of molecular targeted therapy20, 21, 22, but also be used in the intelligent presentation of bioactive substances by constructing nano-drug delivery systems (nanoDDS)23, 24, 25, 26, 27, 28.
The expanding understanding of cancer pathology and nanomedicine has outlined the prosperity of enzyme-stimulated drug delivery in cancer management. This review begins with a classified introduction to the structure and function of various tumor-related proteases based on the catalytic mechanism for substrate cleavage. Emphatically, the latest advances in protease-triggered nanoDDS in the targeted diagnosis and treatment of malignancies are discussed in terms of their classification criteria. In addition, some key obstacles such as the construction, scale production, and toxicity effect of nanocomposites, as well as the efficient matching of enzyme/substrate have been concerned to prospect the clinical potency of protease-responsive nanotheranostics.
2. Proteases in cancer
As proteolytic enzymes, proteases irreversibly catalyze the hydrolysis of peptide bonds, leading to protein disintegration in microseconds. About 588 proteases in humans are encoded by 7% of the protein-coding genome and are classified into five major categories according to their catalytic mechanism and decreasing order of abundance in the human body: the metallo-, serine, cysteine, threonine, and aspartic family of proteases29,30. These proteases cleave peptide bonds in different ways. Serine, cysteine, and threonine proteases covalently catalyze the lysing of peptide bonds through active side chains in the cleft. By comparison, metallo- and aspartic proteases hydrolyze peptide bonds by noncovalently forming highly active water molecules. Serving as natural “Swiss Army Knife”, they can degrade proteins both spectrally and specifically to activate or passivate the substrates, thus playing an important role in multiple physiological events such as cell differentiation, cycle continuation, cell migration, hemostasis, tissue remodeling, immune response, wound healing, and programmed cell death31. However, their dysregulation can also lead to a wide range of diseases such as Alzheimer's disease32, inflammation33, and especially cancer34. Therefore, clarifying the structure and function of proteases and their hydrolysis mechanism is a prerequisite for analyzing them as therapeutic targets and theranostic responders, which is summarized in Table 1.
Table 1.
Summary of tumor-related proteases and their characteristics.
| Protease | Classification | Property | Function |
|---|---|---|---|
| MMPs | Gelatinases Matrilysins Archetypal MMPs Furin-activatable MMPs |
The enzymatic potency of MMPs undergoes three processes: the synthesis of inactive pre-proenzymes, the removal of signal peptides to produce proenzymes, and the cleavage of the bridge structure leading to enzyme activation | Physiological activities: angiogenesis, wound healing, embryonic development, and tissue remodeling Pathological features: tumorigenesis, migration, invasion and metastasis |
| Serine proteases | uPA | uPA is secreted in a non-activated pro-uPA form and activated by binding to uPAR, and then it cleaves plasminogen into plasmin that exerts serine protease activity directly or reciprocally activates pro-uPA to uPA in a positive feedback loop | Induce ECM degradation and tissue remodeling Promote tumor growth, progression, and migration |
| PSA | Active PSA is only localized near the prostate cells. It specifically recognizes and rapidly cleaves heptapeptide sequence based on semenogelin |
Induce ECM degradation and tissue remodeling Promote tumor development |
|
| Thrombin | It converts soluble fibrinogen into insoluble fibrin and increases malignant cell adhesion | Promote tumorigenesis, progression, and metastasis | |
| Cysteine proteases | Cathepsins | They are lysosomal proteases sharing a common papain-like fold containing three α-helix domains and a β-barrel domain except for cathepsin C, as well as they show a tissue-specific distribution | Participate in antigen presentation, thyroid hormone liberation, epidermal homeostasis, precursor activation, keratinocyte differentiation, cell apoptosis, and bone remodelling Promote ECM degradation and tumor progression and metastasis |
| Legumain | Legumain is synthesized as an inactive zymogen and then be catalytically activated in an acidic lysosome | Stimulate angiogenesis, enhance tumor progression and metastasis | |
| Threonine proteases | Testes-specific protease 50 | Its catalytic triplet is located near the opening of the pocket consisting of two sets of β sheets, which facilitates the substrate peptides to approach the threonine catalytic site | Participate in multiple cellular physiological processes Promote cell proliferation, stimulate tumor formation and progression |
| Threonine aspartase 1 | It is a “non-oncogene addiction” protease to crack the main regulator of mixed leukemic proteins and other human regulatory proteins | Be involved in head morphogenesis, segment recognition, spermatogenesis, and proliferation Affect cell proliferation, transformation, cycle progression, and tumor development |
|
| Aspartic proteases | Cathepsin D Cathepsin E Memapsin |
They are synthesized as inactive zymogen progenitors and convert to mature active proteases under acidic pH without the assistance of other catalytic molecules. | Perform a series of physiological functions Promote cancer initiation and progression |
MMPs, matrix metalloproteinases; uPA, urokinase plasminogen activator; uPAR, uPA receptor; ECM, extracellular matrix; PSA, prostate specific antigen.
3. Protease-responsive nanoDDS for the targeted theranostics of malignancy
As the name implies, nanomedicine is the application of nanotechnology in the medical arena, that is, nanomaterials or devices are used in the diagnosis, treatment, monitoring, and prognosis assessment of diseases35. As the cornerstone of nanomedicine, varied nanomaterials have been developed as carriers to support diverse bioactive compounds such as hydrophilic or hydrophobic drugs, peptides, nucleic acids, proteins and so on36, 37, 38, 39, 40. Synergistic utilization of the passive targeting EPR effect (enhanced permeability and retention) determined by the irregular reticular structure of tumor vasculature and active targeting induced by highly expressed specific receptors/antigens on the tumor cell surface, physically encapsulated or chemically coupled drug-carrying nanosystems have shown significant advantages in cancer theranostics41, 42, 43, 44. NanoDDS have multiple superiorities such as matrix diversity, flexibility of surface modification, improved stability and solubility, reduced drug leakage and immature biological effects, enhanced lesion/tissue absorption ratio, controlled pharmacodynamics and pharmacokinetics, minimized DDS-derived immunogenicity, and reasonable multicomponent co-loading capacity, which are closely related to the multicomponent dosing regimens, avoidance of drug resistance, enhanced therapeutic effect, improved patient compliance and life quality45, 46, 47, 48.
Considering the important roles of proteases in cancer pathology, a range of enzyme-responsive nanopreparations with multiple functional modules have been developed to provide advantages in optimizing the in vivo distribution of bioactive agents, improving diagnostic sensitivity, broadening the therapeutic window, and enhancing the synergy between diagnosis and treatment. In the following sections, progress in the construction of protease-induced nanovectors and their applications in targeted cancer theranostics in recent five years will be systematically discussed, along with the aforementioned classification criteria for proteases.
3.1. Matrix metalloproteinases-responsive nanoDDS for cancer theranostics
Matrix metalloproteinases (MMPs) represent a family of Zn2+- and Ca2+-dependent endopeptidases responsible for the degradation of extracellular matrix (ECM) components including collagen, laminin, fibronectin, elastin, and the basal lamina49. The enzyme potency of MMPs originates from a non-activated bridge structure between an unpaired cysteine in the highly conserved “Pro-Arg-Cys-Gly-X-Pro-Asp” sequence of the protease prodomain and the catalytic zinc. After breaking this bridge through proteolysis or chemical disruption, MMPs are activated for enzyme functions50. MMPs are associated with a variety of cellular physiological pathways and are defined as important regulators of tumorigenesis, progression, invasion, and metastasis. Among the 24 known MMPs, MMP-2, MMP-9, and MMP-14 are typically overexpressed in almost all types of cancer and are correlated to tumor invasion through degradation of collagen IV, laminin 5, and elastin51, 52, 53. Therefore, developing smart nanoDDS using MMP-2, MMP-9 or MMP-14 as stimulus has huge potential in cancer treatment and will be intensively evaluated below.
3.1.1. MMP-2-triggered drug delivery
The strategy of functionalizing nanoDDS with specific substrates for the recognition of MMP-2 that are disorderly upregulated in tumor tissues has been recognized and approved to activate the bioactive components to improve theranostic performance. These intelligent systems are classified as follows in terms of their diagnostic and therapeutic modalities.
3.1.1.1. MMP-2-triggered cancer chemotherapy
Fay et al.54 explored the cell-targeting specificity of MMP-2 sensitive surface-converting polyethylene glycol (PEG) coatings using a variety of detection methods such as optical, nuclear, and magnetic resonance imaging (MRI) techniques. Chakroun et al.55 designed asymmetric reverse bolaamphiphile (RBA) supramolecular self-assemblies identified by tumor-specific MMP-2 for hydrogel degradation, to release anticarcinogens. Thereafter, considerable basic and clinical breakthroughs have been made in enzyme-responsive cancer chemotherapy, based on various favored chemotherapeutic agents.
Mesoporous silica nanoparticles (MSNs) are promising candidates for a new generation of nano delivery carriers. A PEG decorated folic acid (FA)-targeted MMP-2-triggered MSN nanocarrier, PGFMSN, was obtained for programmed doxorubicin (DOX) release by sequential encounters with MMP-2 recognition, FA targeting, and gelatin layer disengagement56. A gold (Au) nanoparticles (Au NPs)-biotin conjugate with an MMP-2-cleavable linker capped MSN was designed as a pH- and enzyme-responsive nanocarrier for controlled DOX delivery with excellent tumor destruction and minimal side effects57. Zhang et al.58 proposed an envelope-type MSN to achieve hyaluronic acid (HA) receptor-mediated cell targeting and MMP-2/hyaluronidases bienzyme-responsive DOX release. Further, a pH/redox/MMP-2 triple sensitive nanohybrids based on DOX preloaded and responders assembled MSN exhibited enhanced intracellular DOX delivery and improved anticancer cytotoxicity59.
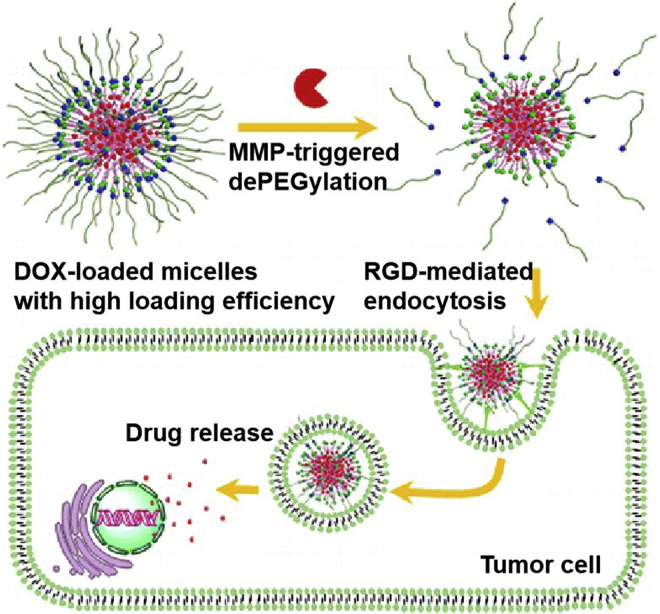
NanoDDS loading DOX with other carriers have also emerged in recent years. Representatively, Chen et al.60 prepared a biotin-PEG-b-poly(l-lysine, PLL)-peptide-DOX polymeric micelles to realize receptor-enhanced cell uptake and MMP-2-stimulated drug release for targeted tumor clearance. An MMP-2/ROS (reactive oxygen species) dual-sensitive DOX-encapsulated poly(l-methionine-b-l-lysine)-PLGLAG-PEG (MLMP) micelle was designed to exert MMP-2-urged bond breaks and ROS-driven DOX release, causing enhanced tumor inhibition with ideal targetability and in vivo circulation61. By coupling small dendrimer poly-l-lysine (DGL) with PEG-poly(caprolactone) micelles via an MMP-2-activated peptide, a size-adjustable nanosystem, DGL/DOX@PP was obtained to perform protease-induced thorough tumor permeability and tumor microenvironment (TME) regulation62. A well-defined block copolymer, PEG-GPLGVRGDG-P(BLA-co-Asp, PBLA: poly(β-benzyl l-aspartate)), was obtained via click reaction to encapsulate DOX for MMP-2-induced peptide cleavage, the dePEGylation of polymer carriers, and the exposure of RGD on the surface of nanoDDS, bringing about ligand-mediated endocytosis, increased drug release and deep penetration for effective therapeutic potency (Fig. 1)63. Another size-variable enzyme-responsive poly(amidoamine, PAMAM) dendritic molecules conjugated with HA were synthesized by click chemical reaction. When MMP-2 was encountered, a significant reduction in size from ∼200 nm to ∼10 nm promoted nanoconjugates extravasation, accumulation, diffusion, and penetration into solid tumors for excellent anticancer efficacy64. In another construction, DOX was covalently linked with a 12-amino-acid anti-HER2 peptide mimetic YCDGFYACYMDV-NH2 (AHNP) to form an MMP-2 sensitive nanoconjugate for synergistic chemotherapy and biotherapy of HER2-positive breast cancer65. Apart from these, DOX-based nanoscale multidrug systems have also been developed to enable coordinated treatment of cancer, such as chemophototherapy66,67.
Figure 1.
Schematic illustration of MMP-2-responsive dePEGylation and RGD-enhanced endocytosis of DOX-carried block copolymer micelles for targeted drug delivery and cell killing. Reprinted with the permission from Ref. 63. Copyright © 2016 American Chemical Society.
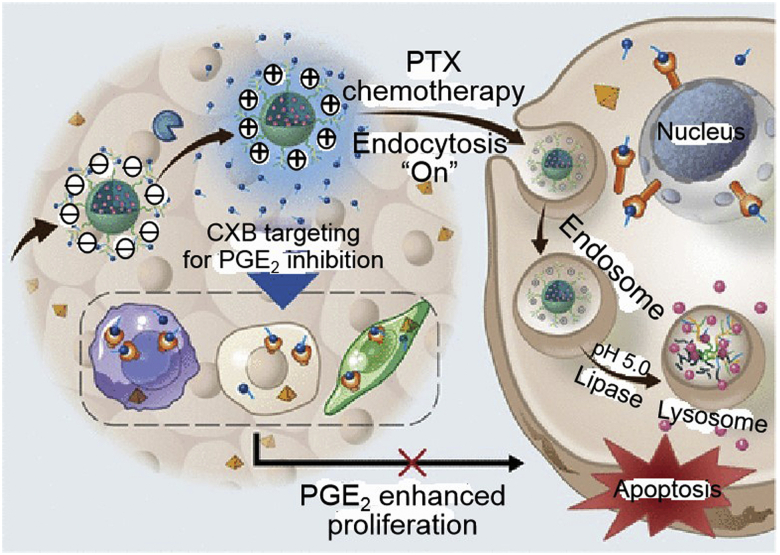
Paclitaxel (PTX) is a recognized first-line anticancer star, but poor solubility, severe off-target toxicity, and annoying drug resistance still limit its clinical application. Nanotechnology plays an important role in drug delivery, especially introducing specific markers for tumor-activated therapeutic release. As a typical example, trilayer nanocapsules cross-linked by an oil-core based on a monodisperse oil-in-water nanoemulsion preloaded with PTX and an MMP-2-sensitive shell, were proposed for protease-triggered and tumor-selective drug delivery68. An enzyme-responsive triblock copolymer vesicle, PEG-GPLGVRG-b-poly(ε-caprolactone)-b-poly(3-guanidinopropyl methacrylamide, PEG-GPLGVRG-PCL-PGPMA), was demonstrated with asymmetrically distributed cell-penetrating PGPMA segments (91% inside and 9% outside). Upon entry into the MMP-2 environment, peptide linker breakage led to the dePEGylation of nanocarriers and the redistribution of PGPMA with 76% exposed to the carrier surface for enhanced cytotoxicity69. MMP-2-sensitive copolymers based on poly(lactic-co-glycolic acid, PLGA) and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) were formed to present PTX for sensitive MMP-2 induction and enhanced antitumor potency70. Clinically approved PEG-b-poly(d,l-lactide, PEG-PDLLA), an amphiphilic block copolymer, was attached to MMP-2-cleavable peptide that facilitated the vector dePEGylation and cellular absorption, prolonged blood circulation, and improved antitumor activity71. Other multifunctional collaborative nanosystems, such as “all-in-one” polymer-lipid conjugate micelle72, PTX@celecoxib core-shell nanoarchitecture (Fig. 2)73, pH-sensitive PTX@sunitinib core-shell micelle74, PTX/losartan in an amphiphilic gelatin matrix75, PTX/2-acetylpyridine-4,4-dimethyl-3-thiosemicarbazone-copper(II) combination76 and so on, have also been exploited for MMP-2-dependent on-demand programmed site-specific drug delivery and synergistic tumor-targeted treatment.
Figure 2.
In vivo behavior of MMP-2-responsive nanoparticles loading PTX and anti-inflammatory celecoxib (CXB). Reprinted with the permission from Ref. 73. Copyright © 2019 American Chemical Society.
In addition, many other chemotherapeutics-derived nanoDDS, for example gemcitabine (GEM)77,78, coumarin 627, trichosanthin79, methotrexate (MTX)80, pirfenidone81, pirarubicin82, cabazitaxel83, and SN3884, have also made extensive biomedical advances as stand-alone or multidrug combinations.
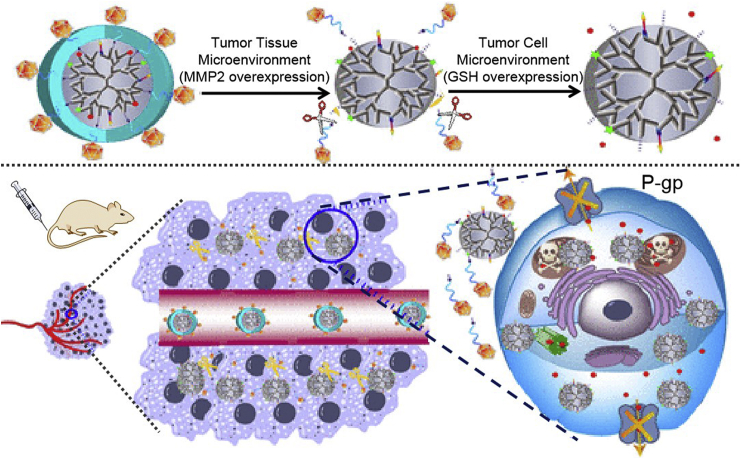
Multidrug resistance is a key stumbling block to the clinical progress of chemotherapeutic drugs. To solve this problem, a series of targeted measures have emerged, which mainly include synthesizing nanomaterials that can reverse drug resistance85, 86, 87, 88, encapsulating drugs into enzyme-responsive organelle-directed nanocarriers (Fig. 3)89, or other size-controllable nanoconjugates90. Furthermore, some actively targeted and intelligently size-shrinking nanoplatforms have been constructed for accomplishing high accumulation, deep penetration, strong targeting, and improved efficiency in a variety of tumor types91, 92, 93, 94, 95. In order to overcome the premature release of physically encapsulated nanodrugs before they reach the tumor site, polymeric carrier-based prodrugs were created by conjugating therapeutics with the targeted group or protease-responsive peptide sequence to prolong blood circulation, improve tumor targetability, enhance cell internalization and intracellular distribution, and potentiate anticancer chemotherapy23,96, 97, 98.
Figure 3.
The mechanism of nanoconjugates targeting organelles with MMP-2/GSH dual responsiveness and overcoming drug resistance. Reprinted with the permission from Ref. 89. Copyright © 2018 American Chemical Society.
3.1.1.2. MMP-2-triggered cancer phototherapy
Photodynamic therapy (PDT) is a safe, effective and low damage clinical regimen, while the dark toxicity, poor water solubility, unsatisfactory half-life (<40 ns) and diffusion depth (<20 nm) of ROS in the living environment greatly limit its antitumor efficiency. To improve drug efficacy, multifarious intelligently responsive vehicles have been proposed for targeted deep delivery of photosensitizers, especially nanoDDS that can be sensitized by tumor-specific proteases. Representatively, a conjugate composed of MMP-2-activated peptide, cell penetrating peptide (CPP) and photosensitizer protoporphyrin (PpIX, Fig. 4)99, and MMP-2/pH dual-responsive photosensitizer nano-prodrugs100, were designed for purposeful drug release and tumor-targeted treatment.
Figure 4.
Action pathway of MMP-2-activated ACPP-PpIX nanocomposites for tumor imaging-synergized photodynamic therapy. Reprinted with the permission from Ref. 100. Copyright © 2015 American Chemical Society.
3.1.1.3. MMP-2-triggered cancer gene therapy
Gene therapy has great anticancer potential by inserting therapeutic genes into plasmid vectors and delivering them to tumor lesions. Unsatisfactory in vivo efficacy limits the clinical transformation of widely used gene carriers such as lipoplex and polyplex. Against this disadvantage, nanotechnology has been skillfully used to construct various therapeutic gene delivery systems, typically such as PEG shielded MMP sensitive CPPs101, envelope-type non-viral nanodevice for liver-targeting siRNA delivery102, polyethylenimine (PEI)-based chlorotoxin-targeted melittin gene delivery to MMP-2 positive prostate cancer cells103, MMP-2-cleavable PEG-β-cyclodextrin-PEI nanoconjugates for tumor suppressor microRNA miR-34a-induced breast cancer treatment104, MMP-2-cleavable motif-included short amphiphilic peptide hydrogels for cell-demanded remedial peptide release into cervical cancer cells105, MMP-2-sensitive cytotoxic peptide‒dendrimer conjugates for enhanced intracellular enrichment, deep tumor penetration, promoted cell apoptosis and anti-glioblastoma (GBM) performance106, multifunctional fusion proteins for targeting the release of interferon gamma107, pro-apoptotic peptide108, or cytotoxic enediyne109 into MMP-2-dysregulated tumor tissues. Moreover, some promising nanocomplexes were also developed for the co-delivery of heat shock protein 70-specific siRNA (siHSP70) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)110, sequentially responsive dual-targeted peptide presentation111, MMP-2/glutathione (GSH) dual-stimulated DOX/miRNA-34a co-delivery to tumor mass112, and MMP-2-triggered on-demand submission of neurotrophic growth factors (NTFs) to induce neural differentiation113.
3.1.1.4. MMP-2-triggered cancer theranostics
Multipurpose all-in-one nanocomposites have attracted great enthusiasm for their opportunities to synergistically achieve sensitive diagnosis, targeted therapy, efficacy monitoring, and prognosis assessment of cancer. Many types of MMP-2-responsive nanotheranostics emerge at the right moment. For instance, DOX/AuNPs/quantum dots (QDs) nanoclusters for fluorescence imaging (FI)-mediated anticancer treatment114, DOX-gelatin/epigallocatechin gallate/AuNPs nanoconjugates for FI-cooperated prostate cancer inhibition115, pH-responsive Au nanoclusters for synergistic near-infrared (NIR) FI and chemo-PDT of MMP-2 positive lung cancer116, MSN loaded with fluorescent and targeting peptides for FI-triggered tumor therapy117, dual-modal photoacoustic (PA)/NIR FI-enhanced photothermal therapy (PTT)/PDT for lung cancer118, and other theranostic nanosystems driven by additional optical principles including aggregation-induced emission (AIE)119 and fluorescence resonance energy transfer (FRET)120. MSN- or 3D-printed biodegradable microswimmer-based magnetic nanoagents were designed for in vivo MMP-2-induced drug release and magnetic resonance imaging (MRI)121,122. DOX-encapsulated gold nanoclusters were presented for computed tomography (CT) imaging-visualized cancer chemotherapy123. The above MMP-2-encouraged representative achievements in cancer theranostics are summarized in Table 223,56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123. In another work, chlorotoxin-conjugated radionuclide 131I-labeled dendrimers were reported for CT-mediated cancer radiotherapy124.
Table 2.
Illustrative achievements of MMP-2-responsive nanoDDS and their applications in the targeted theranostics of malignancies.
| Nanocarrier | Substrate | Drug | Diagnosis | Therapy | Tumor | Ref. |
|---|---|---|---|---|---|---|
| MSN-FA@Gelatin-PEG | Gelatin | DOX | – | Chemotherapy | HT29 human CRC cells | 56 |
| Au-biotin-peptide-MSN | KDPLGVC | DOX | FI | Chemotherapy | 4T1 mouse breast cancer cells T47D human breast cancer cells |
57 |
| MSN@HA-gelatin-PEG | Gelatin, HA | DOX | – | Chemotherapy | MDA-MB-231 human breast cancer cells | 58 |
| MSN-gelatin-BAC | Gelatin | DOX | – | Chemotherapy | A549 human non-small cell lung cancer cells | 59 |
| Biotin-PEG-b-PLL-peptide-DOX polymeric micelles | CPLGLAGG | DOX | FI | Chemotherapy | SCC-7 mouse squamous cell cancer cells | 60 |
| poly(l-methionine-block-l-lysine)-PLGLAG-PEG | PLGLAG | DOX | – | Chemotherapy | NCI-H460 human lung cancer cells HT1080 human fibrosarcoma cells |
61 |
| PEG-GPLGVRGDG-P(BLA-co-Asp) | GPLGVRGDG | DOX | – | Chemotherapy | 4T1 mouse breast cancer cells HT1080 human fibrosarcoma cells |
63 |
| Oil-in-water nanoemulsion | GPLGIAGQ | PTX | – | Chemotherapy | U87 human glioblastoma cells | 68 |
| PEG-GPLGVRG-PCL-PGPMA | GPLGVRG | PTX | – | Chemotherapy | HT1080 human fibrosarcoma cells | 69 |
| TPGS3350-pp-PLGA | GPLGIAGQ | PTX | – | Chemotherapy | MCF-7 human breast cancer cells | 70 |
| PEG-GPLGVRGDG-PDLLA | GPLGVRGDG | PTX | – | Chemotherapy | 4T1 mouse breast cancer cells H22 mouse hepatoma cells |
71 |
| R9GPLGLAGE8 | GPLGLAG | PpIX | FI | PDT | SCC-7 mouse squamous cell carcinoma cells HT1080 human fibrosarcoma cells |
99 |
| Au-R8-PLGLAG-EK10 | PLGLAG | ALA | FI | PDT | SCC-7 mouse squamous cell carcinoma cells | 100 |
| PEI-SPDP-chlorotoxin | Chlorotoxin | melittin gene | – | Gene therapy | PC-3 human prostate cancer cells NIH3T3 mouse embryonic fibroblasts |
103 |
| CD-PEI-GPLGIAGQ-PEG2000 | GPLGIAGQ | miR-34a | – | Gene therapy | 4T1 mouse breast cancer cells | 104 |
| Ac-I3SLKG-NH2 hydrogel | I3SLKG | G(IIKK)3I-NH2 | – | Gene therapy | HeLa human cervical cancer cells | 105 |
| PKT-S-PEG | GPLGIAGQ | KLAK | – | Gene therapy | U87 human glioblastoma cells | 106 |
| Nanoclusters containing DOX/azide@AuNP and Alkyne-MMP@QDs | CVGLPGD | DOX | FI | Chemotherapy | A549 human non-small cell lung cancer cells NIH3T3 mouse embryonic fibroblasts |
114 |
| Ce6-DOX-Au nanoclusters-MMP-2 polypeptide NPs | CPLGVRGRGDS | DOX, Ce6 | NIR FI | Chemotherapy, PDT | A549 human non-small cell lung cancer cells | 116 |
| Au nanostars@BSA/I-MMP-2 | GPLGIAGQ | IR-780 iodide | PA, NIR FI | PTT, PDT | A549 human non-small cell lung cancer cells | 118 |
| peptide-Fe3O4@MSN | PLGVR | Fe3O4, DOX | FI, MRI | Chemotherapy | HT1080 human fibrosarcoma cells NIH3T3 mouse embryonic fibroblasts |
121 |
| DOX@AuNCs | CRVGLPDC | DOX | μCT | Chemotherapy | A549 human non-small cell lung cancer cells | 123 |
MSN, mesoporous silica nanoparticle; PEG, polyethylene glycol; PLL, poly(l-lysine); PBLA, poly(β-benzyl l-aspartate); PCL, poly(ε-caprolactone); PGPMA, poly(3-guanidinopropyl methacrylamide); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate; PLGA, poly (lactic-co-glycolic acid); PDLLA, poly(d,l-lactide); R9, polycationic cell-penetrating peptide (CPP); E8, polyanionic peptide; R8, cationic CPP RRRRRRRR; EK10, zwitterionic stealth peptide EKEKEKEKEKEKEKEKEKEK; PEI, polyethylenimine; SPDP, N-succinimidyl 3-(2-pyridyldithio) propionate; CD, β-cyclodextrin; PKT-S-PEG, KLAK, TAT (CPP) and MMP-2-sensitive peptide-PEG were conjugated onto dendrimers; BSA, bovine serum albumin; AuNCs, gold nanoparticle clusters; DOX, doxorubicin; PTX, paclitaxel; PpIX, protoporphyrin; ALA, 5-aminolevulinic acid; G(IIKK)3I-NH2, anticancer peptide; KLAK, cytotoxic peptide; Ce6, chlorin e6; Au, gold; NP, nanoparticle; QDs, quantum dots; FA, folic acid; HA, hyaluronic acid; BAC, N,N′-bis(acryloyl)cystamine; MMP-2, matrix metalloproteinase-2; PDT, photodynamic therapy; PTT, photothermal therapy; FI, fluorescence imaging; NIR, near-infrared; PA, photoacoustic imaging; MRI, magnetic resonance imaging; μCT, micro computerized tomography; CRC, colorectal cancer; ‒, not applicable.
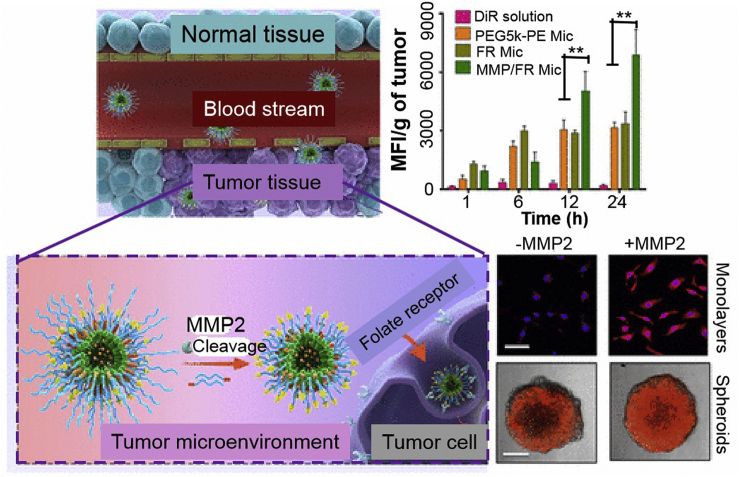
In addition, multiple MMP-2-activated nanocarriers have also been explored for other diagnostic and therapeutic applications, representative examples including the dual-targeting of the blood brain barrier (BBB) and glioma using MMP-2-responsive micelles that act as gene presentation vectors, to enhance the radiotherapy sensitivity of glioma125, genetically-engineered attenuated Salmonella Typhimurium as anti-invasive vectors for the targeted therapy of orthotopic glioma126, MMP-2/folate receptor (FR) dual-targeted polymeric micelles to improve tumor targeting, enhance anticancer efficacy, and overcome the resistance to molecular targeted therapeutics (Fig. 5)127, and finally MMP-2-sensitive PEG hydrogel loaded with quinacrine sensitizing GBM cells to TRAIL for improved combination treatment efficiency128.
Figure 5.
MMP-2-sensitive FR-targeted polymeric micelles directing dasatinib to tumor sites to increase anticancer potency and overcome multidrug resistance. Reprinted with the permission from Ref. 128. Copyright © 2017 American Chemical Society.
3.1.2. MMP-9-triggered drug delivery
MMP-9, another gelatinase, is overexpressed in almost all known tumor entities. Its expression is directly related to the malignancy of tumors, thus opportunities for MMP-9 as a therapeutic target to inhibit its activity through specific siRNA or shRNA have been explored. Zhou et al.129 designed an amphiphilic star copolymer based on dendritic poly(l-lysine) and hyperbranched polyglycerol via click reaction to co-load MMP-9 siRNA and docetaxel (DTX) for breast cancer therapy. A GSH-responsive chemotherapeutic self-sensitized polymeric prodrug consisting of hyperbranched poly(amido amine) (PAA) and MTX was synthesized to carry shRNA plasmid of MMP-9 for chemo/gene dual therapy of breast carcinoma130. Further, a similar transferrin (Tf)-targeted polymeric prodrug Tf-PAA-MTX was exploited to present MMP-9 shRNA plasmid and showed considerable expectations in chemotherapy gene therapy for nasopharyngeal carcinoma131.
Except as a therapeutic target, the possibility of MMP-9 as an intelligent responder by using its proteolytic activity for drug release to tumor target has been confirmed and a variety of MMP-9-stimulated nanoDDS have been developed for cancer theranostics. The successful construction of various nanocarriers containing MMP-9-cleavable motifs, such as PLGA-b-PEG-based polymeric NPs132 and PEG-derived polymeric nanogels133, have played a key role in the enzyme-triggered chemotherapeutics delivery. Typically, MMP-9 catalyzed the hydrolysis of peptide micelles into nanofiber depots to facilitate DOX release134. MMP-9-susceptive tetrapeptide vesicles were established for targeting DOX to tumor lesion135. Combretastatin A4 nanodrug-upregulated MMP-9 expression promoted the selective release of DOX prodrug into human breast cancer cells136. A pH/MMP-9 sequentially responsive DOX-conjugated peptide was employed for structure transformation (from spherical to rod-like)-induced drug action process optimization and efficacy improvement137. Porta et al.138 modified poly-(dimethyl siloxane)-poly-b-(methyloxazoline, PDMS-PMOXA) polymersome with an MMP-9-cleavable peptide and carried PTX for enzymatic digestion-enhanced pharmacological activity. Leukocyte-mimicking pluronic P123-lipid nanohybrids loading PTX were established to suppress the growth and metastasis of breast cancer, with 80.84% tumor inhibition and 10.62% metastatic foci in lung tissue139. Functionally similar, a reduction/MMP-9 dual-sensitive shrapnel methoxy PEG-peptide-vitamin E succinate NPs loading DTX was developed to inhibit breast cancer growth (81% of inhibitory rate) and metastasis (92% of inhibitory rate)140. MMP-9/cathepsin B dual-enzyme-cleavable GEM nanovectors and MMP-9-sensitive curcumin/p53 DNA co-delivery nanoDDS were designed for programmed pancreatic cancer suppression141 and reversal of cisplatin resistance in ovarian cancer142, respectively. Furthermore, MMP-9-activated chemotherapeutic-based combinations have also appeared, such as DTX/quercetin/imatinib synergy was proved to be promising for metastatic breast cancer therapy143, PTX/thioridazine/PD-1 inhibitor were integrated into a cocktail strategy to improve breast cancer treatment144.
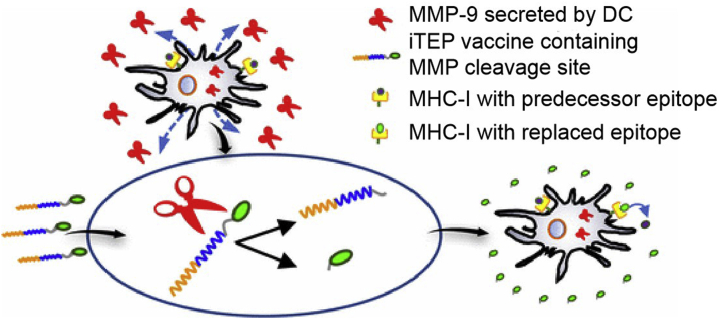
An MMP-9-responsive cytotoxic T lymphocyte (CTL) vaccine delivering immune-tolerant elastin-like polypeptide (iTEP) was developed to enhance CTL vaccines and was considered an alternative to traditional dendritic cell-based vaccines (Fig. 6)145,146. Amphiphilic diblock copolymers containing Toll-like receptor 7 (TLR7) agonists and MMP-9 substrate peptide were prepared to enhance the immunotherapeutic efficacy and reduce immunotoxicity147.
Figure 6.
MMP-9 sensitized iTEP vaccine presentation for the direct exposure of CTL epitope onto MHC class I complexes located on the surface of dendritic cells. Reprinted with the permission from Ref. 146. Copyright © 2017 American Chemical Society.
To attenuate the nonspecific cytotoxicity and hemolytic activity of melittin, its MMP-9-sensitive prodrug was connected to perfluorocarbon NPs, which greatly enhanced the enrichment of nanoDDS in tumor sites and the prodrug decomposition restored pharmacological activity148. In addition to its important role in cancer therapy, MMP-9-specific fluorescent-labeled substrate peptides were introduced into nanodiamonds to enhance peptide stability and quantitatively respond to the efficacy of MMP-9 in early detection of tumor metastasis149. The construction and anticancer applications of MMP-9-responsive nanocomplexes are illustrated in Table 3135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149.
Table 3.
Representative development of MMP-9-sensitive nanoDDS and their applications in the targeted theranostics of malignancies.
| Nanocarrier | Substrate | Drug | Diagnosis | Therapy | Tumor | Ref. |
|---|---|---|---|---|---|---|
| HWGF | HWGF | DOX | FI | Chemotherapy | MCF7 human breast cancer cells | 135 |
| CA4-NPs, MMP-9-DOX-NPs | GPLGL | DOX | – | Chemotherapy | 4T1 mouse breast cancer cells | 136 |
| 2-(Nap)-FFKTPA–DOXAGLDDRGD | AGLDD | DOX | FI | Chemotherapy | 4T1 mouse breast cancer cells HepG2 human hepatoma cells MCF7 human breast cancer cells H22 mouse hepatoma cells |
137 |
| PDMS-PMOXA polymersomes | SRLSLPGC | PTX | FI | Chemotherapy | MCF7 human breast cancer cells | 138 |
| Leukocyte-mimicking pluronic-lipid nanovesicle hybrids | Pluronic P123 | PTX | FI | Chemotherapy | 4T1 mouse breast cancer cells | 139 |
| mPEG-peptide-VES copolymers | PLGLAG | DTX | – | Chemotherapy | 4T1 mouse breast cancer cells | 140 |
| PEG-CycloRGD-CdSe/ZnS QDs | GGPLGVRGK, GFLG | GEM | FI | Chemotherapy | BxPC-3 human pancreatic cancer cells | 141 |
| Cationic nanostructured lipid carriers | Gelatin | DTX, Qu, IMA | FI | Chemotherapy, molecular targeted therapy | 4T1 mouse breast cancer cells | 143 |
| PDB-PPV | PLGLAG | PTX, THZ, HY19991 | FI | Chemotherapy, stem cell therapy, immunotherapy | MCF7 human breast cancer cells | 144 |
| Amphiphilic diblock copolymers | GPLGLAGGERDG | 1V209 | FI | Immunotherapy | 4T1 mouse breast cancer cells | 147 |
| Perfluorocarbon NPs | GPQGIAGQ | Melittin | Ultrasonic imaging | Gene therapy | B16F10 mouse melanoma cells | 148 |
| Nanodiamonds | LGRMGLPGK | FITC, 5-TAMRA | FI | – | Huh7 human hepatoma cells SNU398 human hepatoma cells |
149 |
Abbreviations: HWGF, His-Trp-Gly-Phe; NPs, nanoparticles; 2-(Nap)-FFK, 2-naphthylacetic acid-Phe-Phe-Lys; PDMS, poly(dimethyl siloxane); PMOXA, poly-b-(methyloxazoline); mPEG, methoxy polyethylene glycol; VES, vitamin E succinate; PDB, PEG-block-poly[(1,4-butanediol)-diacrylate-β-N,N-diisopropylethylenediamine]; PPV, mPEG-peptide-VES; DOX, doxorubicin; CA4, combretastatin A4; PTX, paclitaxel; DTX, docetaxel; GEM, gemcitabine; Qu, quercetin; IMA, imatinib; THZ, thioridazine; 1V209, 2-methoxyethoxy-8-oxo-9-(4-carboxy benzyl)adenine; QDs, quantum dots; FITC, fluorescein isothiocyanate; 5-TAMRA, 5-carboxytetramethylrhodamine; MMP-9, matrix metalloproteinase-9; RGD, Arg-Gly-Asp; TPA, 4-formylbenzoic acid; FI, fluorescence imaging; ‒, not applicable.
On the basis of exciting achievements in cancer theranostics using MMP-2 or MMP-9 as a single responder, a series of multipurpose polymeric nanoplatforms have also been exploited for the accurate theranostics of cancer with MMP-2/MMP-9 dual-sensitivity by intelligently loading and on-demand presenting various cytotoxic therapeutics such as DOX150, 151, 152, 153, 154, PTX155, DTX156, camptothecin157, bFGF158, MMP inhibitors159, immune modulators160, and so on.
3.1.3. MMP-14-triggered drug delivery
MMP-14 is a primary endopeptidase highly expressed on the surface of many tumor cells and is closely related to cancer development. In recent years, it has been widely used as a responder to stimulate tumor theranostic events. Typically, Gao et al.161 developed a graphene oxide (GO)/Au nanocomplex conjugated with NIR dye (Cy5.5) labeled MMP-14 substrate. After encountering MMP-14, this theranostic system exhibited sharp FI and PA signals, as well as excellent tumor inhibition against mouse squamous cell carcinoma. In another multifunctional nanosystem, ferumoxytol was conjugated with azademethylcolchicine (ICT) by an MMP-14-recognizable peptide (Ala-Cys-Arg-Ser-Cit-Gly-HPhe-Tyr-Leu-Tyr). The created nanotemplate selectively accumulated at breast cancer lesions and emerged acute MRI contrast and image-mediated efficacy monitoring162. Further, the therapeutic effect and cardiovascular toxicity of this protease-responsive ICT nanoprodrug were systematically evaluated in human breast, lung, prostate, and colorectal cancer. It concluded the remarkable clinical transformation potency of the MMP-14-sensitive nanotherapeutics163.
3.2. Serine proteases-responsive nanoDDS for cancer theranostics
Serine proteases are the second most important family of proteases, accounting for approximately one-third of all proteases. Their catalysis is realized by a coordinated triad structure formed by the close arrangement of three amino acids of histidine (His57), serine (Ser195), and aspartic acid (Asp102)164. The serine proteases closely related to tumor progression and widely studied are urokinase plasminogen activator (uPA) and prostate specific antigen (PSA). Their utility in tumor-targeted drug presentation and disease treatment are discussed below, with a focus on the representative progress in the past five years.
3.2.1. uPA-triggered drug delivery
Overexpression of uPA is an important hallmark of many cancers. Its substrate specificity, that is, exclusively breaking the Arg-Ser bond in the Ser-Gly-Arg-Ser-Ala sequence of substrate peptides165, contributes to the successful development of uPA-controlled tumor penetrating peptide166 and fusion protein-based NPs167 for effective drug loading and protease-driven payload delivery in tumor lesion. In order to achieve the super-early detection and good prognosis of pancreatic ductal adenocarcinoma (PDAC), a Gd3+-based MRI contrast agent and cyanine dye Cy5.5 were combined with U11 (a pancreatic cancer-specific peptide) to form a dual-modal MRI/NIR FI nanoprobe for highly sensitive and spatially resolved detection of pancreatic precancerosis168. Matsumura et al.169 prepared a clever PEGylated four-arm 64Cu-bombesin analog tetramer connected by a substrate linker to achieve the desired positron emission tomography (PET) contrast and clearance enhancement in less time.
Some uPAR-driven smart nanosystems are also being explored for cancer treatment. Typically, a Wnt/uPAR co-targeting ultra-small magnetic iron oxide nanoparticle (IONP) was designed to inhibit cancer stem cell phenotype and deliver DOX to chemo-resistant breast cancer170. DOX nanocomplex coated with human serum albumin (HSA) and attached to the amino-terminal fragment (ATF) of urokinase was proposed to improve the anticancer efficiency and minimize the cardiotoxicity of DOX171. A pH-responsive uPAR-targeted DOX/curcumin nanosynergy was synthesized to combat the resistance and toxicity of DOX in the treatment of lung cancer172. A delicate fusion protein was developed to guide uPA-triggered scorpion toxin delivery for combating the growth and invasion of breast cancer173.
Besides, uPA-stimulated theranostic nanocompositions have also played an important role in cancer management such as luteinizing hormone releasing hormone receptor/uPAR dual-targeting IONPs for the diagnosis and treatment of prostate cancer174, uPAR-targeted PEGylated IONPs for MRI-guided drug delivery into peritoneal tumors175, milk protein-protected uPA-targeted IONPs loading cisplatin for MRI-monitored effective therapy of pancreatic cancer with minimal side effects176, pH responsive, uPAR-targeted MSNs for the identification of pancreatic cancer using multispectral optoacoustic tomography177, iridium (Ir) complex-loaded Au nanostars functionalized with uPAR targeting module for photothermal/X-ray CT/PA three type diagnosis-synergized PTT/chemotherapy combination treatment for triple negative breast cancer (TNBC)178. Typical uPA-mediated cancer diagnosis and treatment events are briefly summarized in Table 4168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178.
Table 4.
The latest advances of uPA-cleavable nanoDDS and their applications in the targeted theranostics of malignancies.
| Nanocarrier | Substrate | Drug | Diagnosis | Therapy | Tumor | Ref. |
|---|---|---|---|---|---|---|
| DGL-U11 | U11 | Gd3+-DTPA, Cy5.5 | MRI, NIR FI | – | PANC1 human pancreatic cancer cells | 168 |
| Four-arm PEGylated64Cu-bombesin analog tetramer | CGSGRSAG | 64Cu | PET | – | PC-3 human prostate cancer cells | 169 |
| Ultra-small magnetic IONPs | DKK1, ATF24 | DOX | NIR FI | Chemotherapy | MDA-MB-231 human breast cancer cells | 170 |
| ATF-HSA | ATF | DOX | FI | Chemotherapy | H1299 human non-small cell lung cancer cells H22 mouse hepatoma cells |
171 |
| U11-DOX/curcumin NPs | U11 | DOX, curcumin | – | Chemotherapy | A549 human non-small cell lung cancer cells | 172 |
| ALA fusion protein | ATF | AGAP | – | Gene therapy | MDA-MB-231 human breast cancer cells | 173 |
| LHRH-AE105-IONPs | Modified LHRH peptide, AE105 peptide | PTX | MRI | Chemotherapy | PC-3 human prostate cancer cells | 174 |
| PEGylated IONPs | ATF | Cisplatin, DOX | NIR FI, MRI | Chemotherapy | PANC02 mouse pancreatic cancer cells | 175 |
| Milk protein-coated IONPs | ATF | Cisplatin | MRI | Chemotherapy | MIA PaCa-2 human pancreatic cancer cells | 176 |
| MSN-uPA NPs | Chitosan, uPA | GEM | MSOT | Chemotherapy | ASPC1, PANC1, and MIA PaCa-2 human pancreatic cancer cells | 177 |
| GNS@Ir@P-AE105 | P-AE105 | GNS, Ir complex | PT, PA, X-ray CT | PTT, chemotherapy | MDA-MB-231 human breast cancer cells | 178 |
DGL, dendron-grafted poly-l-lysine; PEG, polyethylene glycol; IONPs, iron oxide nanoparticles; HSA, human serum albumin; ALA, ATF and AGAP linked with the uPA-cleavable linker gene; MSN, mesoporous silica nanoparticle; GNS, gold nanostars; DOX, doxorubicin; AGAP, one of the scorpion toxin polypeptides; PTX, paclitaxel; GEM, gemcitabine; Gd3+-DTPA, Gd3+-diethylene triamine pentaacetic acid; Cy5.5, cyanine dye; Ir, iridium; uPA, urokinase plasminogen activator; U11, tumor-targeting peptide; DKK1, Dickkopf-related protein-1; ATF, amino-terminal fragment of urokinase; LHRH, luteinizing hormone-releasing hormone; AE105, a potent nine-mer peptide antagonist of uPAR binding; P-AE105, a polyetherimide-AE105 peptide conjugate; PTT, photothermal therapy; MRI, magnetic resonance imaging; NIR, near-infrared; FI, fluorescence imaging; PET, positron emission tomograph; MSOT, multispectral optoacoustic tomography; PT, photothermal; PA, photoacoustic; CT, computed tomography; ‒, not applicable.
3.2.2. PSA-triggered drug delivery
PSA, also known as glutamate carboxypeptidase II (GCPII), is the most prominent biomarker of prostate cancer for diagnostic and therapeutic targeting and is overexpressed in other solid tumors to promote their neovasculature and angiogenesis. Some first-line therapeutics have shown achievements in preclinical or clinical trials but have been associated with obvious side effects. Considering this, alternative targeted delivery schemes have been actively developed to enrich bioactive constituents in tumors for improved diagnostic and therapeutic outcomes by introducing PSA-recognizable ligands, aptamers, or antibodies into the nanoDDS.
To improve diagnostic performance, Machulkin et al.179 synthesized a fluorescence-conjugated magnetite-Au NPs carrying PSA ligand for tissue-specific MRI. A distearoyl phosphatidyl ethanolamine (DSPE)-PEG-maleimide copolymer that bonded the anti-PSA single chain was labeled with 64Cu for enhanced tumor targeting and PET imaging visibility180.
Considerable achievements have also been made in PSA-mediated drug accumulation and efficacy improvement. Concretely, tumor lesions targeted delivery of PSA-specific motif-conjugated nanocomplexes carrying a variety of therapeutics either alone or in combination, mainly included DOX181, 182, 183, 184, 185, PTX186, DTX187, SN38188, camptothecin189, thapsigargin190, boron-containing reagents191, zinc chelator192, antibody-drug conjugates193,194, kinase inhibitors195, siRNA (Fig. 7)196, 197, 198, 199, 200, miRNA201, oligonucleotide202, therapeutic proteins203, and chemotherapy-based combination regimens with molecular targeted therapy204, antibiotic therapy205, gene therapy206,207, and anti-inflammatory therapy208.
Figure 7.
Schematic diagram displays the multifunctional envelope-type nanoplatform for PSA-targeted siRNA delivery and prostate cancer therapy. Reprinted with the permission from Ref. 200. Copyright © 2017 American Chemical Society.
PSA-targeted nanocomposites have also shown high levels of activity in imaging-synergized cancer treatment, typically such as FI-mediated chemotherapy209 or PDT210, PET-mediated chemotherapy211, MRI/NIR FI-induced hyperthermia212, ultrasound-guided gene therapy213 or chemotherapy214, and MRI-induced combination therapy215. Furthermore, some PSA-targeted nanoDDS, particularly DTX-derived nanotheranostics, have already hinted valuable prospects in the clinical potential development216, phase I217, phase II and further clinical stages218.
3.3. Cysteine proteases-responsive nanoDDS for cancer theranostics
Lysosomal proteases, mainly the cathepsin family and legumain, play a key role in the malignant evolution of diseases219. Cathepsins can be classified into three categories, namely cysteine (cathepsins B, C, F, H, K, L, O, S, V, W and X), serine (cathepsins A and G), and aspartic cathepsins (cathepsins D and E). Of these, cysteine cathepsins account for the majority. They share a common papain-like fold containing three α-helix domains and a β-barrel domain except for cathepsin C, in which the catalytic dyad Cys-His is localized and two separate domains define the substrate binding cleft220. The recognized function of cysteine cathepsins is their proteolysis of damaged or unwanted proteins in lysosomes for recycling221. Cathepsins B, L and S are key members of the cysteine protease group and play indispensable roles in many physiological events and pathological regulations of cancer. They have been widely used as responders in the intelligent delivery of imaging contrast agents and/or active therapeutics. Cathepsin B-induced drug delivery has been systematically described in our previous report28. Thus, the importance of cathepsin L, cathepsin S, and legumain, caspase 3 (the other two cysteine proteases) in motivating cancer management will be then highlighted.
3.3.1. Cathepsin L-triggered drug delivery
Cathepsin L is another well-established cysteine cathepsin upregulated in a multitude of human cancers222. Its expression level is closely related to tumor malignancy, invasiveness, grading and staging, and tumor occurrence. Upon glycosaminoglycan-mediated activation, the secreted cathepsin L degrades cell adhesion components directly or indirectly thus promotes tumor progression and metastasis223,224. Downregulation of cathepsin L can induce apoptosis and inhibit metastasis in some tumor models including pancreatic cancer, lung cancer, liver cancer, etc. While in other tumors such as squamous cell carcinoma, cathepsin L exhibits significant expression positively dependent antitumor activity225,226. Hence, the importance of cathepsin L in stimulating drug migration and cancer management is outlined below.
For the past few years, considerable attention has been paid to the importance of cathepsin L in tumorigenesis and malignant progression. Meanwhile, its feasibility as a drug delivery target has also been developed. It is worth mentioning that Wang et al.227 reported a cathepsin L-responsive acetylated azidomannosamine to mediate tumor-specific labeling in vivo that further enhanced DOX prodrug aggregation in tumor lesions and facilitated ablation of colorectal cancer (CRC), TNBC, and metastatic breast cancer in mice. In view of the significance of cathepsin L in promoting the proteolytic disassembly of reovirus which is critical for its antitumor activity, pretreatment with cathepsin L inhibitor greatly enhanced the sensitivity of reovirus-resistant tumor cells to reovirus-cationic liposome complex, thereby targeting reovirus to the cytosol and causing apoptosis228.
3.3.2. Cathepsin S-triggered drug delivery
In the cysteine cathepsin family, cathepsin S has previously been detected in multiple tumor types, which often implies poor disease status and clinical outcomes. It is closely related to tumorigenesis and progression by stimulating angiogenic factors to promote angiogenesis229. In view of this, except as a therapeutic target, the properties of cathepsin S have been integrated into nanocomplexes for protease-susceptible oncologic imaging or targeted therapy.
In a typical manner, Fan et al.230 synthesized a cathepsin S-degradable multi-block copolymers based on a water-soluble and biofriendly polymer, poly[N-(2-hydroxypropyl)methacrylamide] (HPMA), as the backbone. By coupling cathepsin S-sensitive peptide substrate (PMGLP) and radiolabeling with lutetium-177 (177Lu), the nanocomposites showed highly sensitive single-photon emission computed tomography (SPECT)/CT imaging in PDAC. While capturing early lesions, the untargeted scavenging associated with mononuclear phagocyte system (MPS) tissues was enhanced without discounting the tumor localization and retention capability of the nanoagents. To further clarify the effect of block size on the in vivo bioefficacy of cathepsin S-fractured multi-block HPMA copolymer, dual-isotope labeling technique combined multiple cellular and in vivo strategies were implemented in PDAC cancer models. It is concluded that copolymers with smaller blocks exhibited faster enzyme cleavage kinetics than their larger counterparts. Moreover, the smaller ones enjoyed the superior MPS-related clearance and improvement in untargeted retention. However, this tendency did not affect the targeting and nanoenrichment at tumor sites. This discovery revealed the implication of nanoprofile for their internal functioning and biological performance, and provided a basis for designing nanotheranostics with a reasonable appearance231. Considering the overexpression and co-promotion of cathepsins S and L in tumor progression, they were focused as co-targets for enhanced diagnostic events and drug release. An endogenous small molecule protease inhibitor, stefin A, was embedded in the liposome matrix for bright tumor diagnosis in combination with effective treatment232.
3.3.3. Legumain-triggered drug delivery
Legumain, with specific catalytic sequence His-Gly-Spacer-Ala-Cys, is another lysosomal cysteine protease from the legumain family (C13) and CD clan233. It is also a caspase and specifically cleaves the C-terminal substrate peptide of asparagines234. Legumain is mainly found in antigen presenting cells (APCs) and is upregulated in many human solid tumors, such as breast235, ovarian236, colorectal237, prostate238, and gastric239 cancers. In addition, it can be expressed by tumor-associated macrophages (TAMs) and intratumoral blood vessels. Importantly, legumain mediates tumor development and stimulates angiogenesis, tumor progression and metastasis in a number of ways. All of these provide references for the design of legumain-targeted prodrug and legumain-triggered nanoDDS.
As a representative, Ruan et al.240 synthesized a bifunctional Au nanocomplex by synergistic amplification of receptor-mediated endocytosis and EPR effect. The NPs contained an legumain-activated AK peptide (Ala-Ala-Asn-Cys-Asp) and a integrin αvβ3 receptor binding peptide (R8-RGD). After touching the glioblastoma site, the nanocomponents underwent a legumain-induced click cycloaddition to precisely locate and concentrate in lesion. This synergistic model greatly improved the anti-glioma efficacy of the loaded DOX. Similarly, modifying Au NPs with AK peptide and 2-cyano-6-aminobenzothiazole (CABT), the resulting nanocomposites were highly concentrated in the glioma site. After connecting DOX with pH-responsive groups, the obtained Au nanohybrids exhibited legumain-induced FI and acidity-enhanced antineoplastic effect241. To further suppress DOX-caused cytoprotective autophagy and enhance the immune response, hydroxychloroquine and anti-PD-L1 (programmed cell death ligand 1) antibody were simultaneously introduced into the above legumain-activatable Au NPs. This exquisite nanocombination effectively alleviated the immunosuppressive microenvironment and achieved exciting anti-glioma outcome without recurrence242. By covalently attaching the alanine-alanine-asparagine to the cyclic iRGD, the multitargeted tadpole-like peptide could transport DOX directionally. DOX-loaded multi-target liposomes specifically attacked tumor vascular endothelial cells to achieve unobstructed permeability. They substantially eliminated breast cancer without recurrence or metastasis due to exhausted tumor associated macrophage-related TME regulation243. This phenomenon was confirmed by another iRGD-mediated legumain-susceptible Au NPs for FI-guided ablation of breast cancer244. Beyond that, other strategies have also been used for legumain-enhanced cancer theranostics, such as an N-benzyloxycarbonyl-Ala-Asn-DOX prodrug for anti-cervical cancer treatment245, a pH-triggered and legumain-digestible DOX-carbon dots prodrug for FI-monitored drug release246, a substrate-bridged HA-DOX polygonal nanogel for CD44-targeted and legumain-responsive lung cancer killing247, a legumain-cleavable tetrapeptide Ala-Ala-Asn-Leu conjugated 4-arm PEG-DOX polymeric prodrug for breast cancer-targeted drug delivery and enhanced antitumor index248, a clever DOX-loaded liposome modified by fibronectin-binding peptide and legumain-cleavable peptide in tandem for tumor ecological microenvironment-disturbed breast cancer inhibition249, and charge-driven self-assembled DOX-chitosan-PEG-PGA (poly(glutamic acid)) nanocomposites containing legumain substrate peptide for enhanced melanoma therapy250.
In addition to DOX, other chemotherapeutics, targeted drugs or traditional Chinese medicine have also been successively exploited for legumain-induced cancer treatment. Jin et al.251 co-coupled simvastatin and PTX using legumain-specific peptides to obtain TME-activatable liposomes. This multifunctional system effectively lowered cholesterol and reversed epithelial-mesenchymal transition (EMT)-related PTX resistance in non-small cell lung cancer (NSCLC). Cisplatin and indocyanine green (ICG) co-loaded nanopolymers, with legumain-responsive heptapeptide (Gly-Cys-Gly-Ala-Ala-Asn-Leu) upon their surface, showed excellent photothermal transformation-enhanced chemotherapeutic efficacy for gastric cancer252. On this basis, glutamic cisplatin-loaded Au@MSN core-shell NPs were functionalized with Gly-Cys-Gly-Ala-Ala-Asp-Leu to achieve protease-induced synergistic chemotherapy/PTT against malignant gastric cancer253. In order to overcome the drug delivery difficulty of metastatic breast cancer, inflammatory monocytes254 or bioengineered macrophages255 were exploited to anchor legumain-specific peptides and cytotoxic mertansine or soravtansine, respectively. The nanocomplexes could deliver therapeutics to preinvasive breast cancer and its lung metastase to achieve controlled anti-metastasis therapy. Further, legumain-sensitive melittin-mounted nanopolymers were designed to co-encapsulate IR-780 and sorafenib for oral available combinational chemotherapy and PTT of malignant gastric carcinoma256. Other perishable drugs, typically such as active protein trichosanthin, were cleverly genetically fused into an endogenous albumin scaffold via a legumain-cleavable substrate peptide. The dual-protein hitchhike prodrug was enriched at the tumor site by protease actuation and albumin mediation to remove orthotopic breast cancer257.
3.3.4. Caspase-3-triggered drug delivery
Caspases are a 15-member family of cysteine proteases and are widely known for their important roles in programmed cell death and inflammation258. Among them, caspase 3 is the most typical executor of apoptosis. Under the initiation of caspase-8 or -9, the non-activated pro-caspase-3 is proteolytically cleaved to the active caspase-3, which migrates into the nucleus to cleave a variety of important intracellular functional proteins259. Commonly used therapies, such as chemotherapy, radiotherapy and immunotherapy, generally induce apoptosis by activating caspase 3. Therefore, up-regulated caspase-3 is considered by numerous researchers to be a positive indicator for evaluating the effectiveness of cancer treatments.
However, with further research, caspase-3 is depicted in a more refined functional spectrum in cancer development and treatment. It plays important roles in promoting the carcinogenesis after cytotoxic drug therapy260, inducing tumor regeneration following radiotherapy through paracrine pathway261, stimulating tumor growth by creating a pro-angiogenic microenvironment262, and other such non-apoptotic effects. In a recent representative study, Zhou et al.263 revealed the molecular mechanism by which caspase-3 stimulated the chemoradiotherapy resistance and tumor metastasis in CRC by regulating EMT, and predicted the potential of caspase-3 inhibition synergized with cytotoxic regimen in enhancing the CRC treatment. Further, Bernard et al.264 discovered and dissected the cleaved caspase-3's identity as a transcription factor. The transcriptional activity is determined mainly by a DNA-binding domain in the subunit of caspase-3 and an activated configuration. Caspase-3 acted directly on the promoter of multiple pro-angiogenic genes, especially vascular endothelial growth factor A (VEGFA), thereby inducing oncogenic angiogenesis and tumorigenesis by transcriptional regulation. Simultaneously, caspase-3 activation indirectly reduced a series of pro-apoptotic genes and caused resistance to cytotoxic therapeutics. Targeted inhibition of caspase-3 effectively sensitized chemotherapeutic agents and suppressed cancer progression and metastasis.
For years now, scientists have focused on clarifying the routine pro-apoptotic effect of caspase-3 and exploring its non-classical cancer-promoting mechanism. This “double-edged sword” effect makes caspase-3 a crucial target for tumor-specific treatment and reversal of drug resistance, as well as a personalized strategy to conquer cancer invasion and metastasis. However, relatively few studies have used it only as a responsive switch for protease-induced anticancer therapeutics release.
3.4. Threonine proteases-responsive nanoDDS for cancer theranostics
Testes-specific protease 50 (TSP50) and threonine aspartase 1 are representatives of threonine proteases. Their importance is overwhelmingly reflected in their involvement in the whole process of tumor development and as effective anticancer targets. TSP50 was detected in human breast cancer cell-derived hypomethylated DNA fragments and abnormally activated in most breast and colorectal cancers265,266. Its transcripts are exclusively distributed in human testes. The catalytic triplet of TSP50 is composed of histidine at 153, aspartic acid at 206, and threonine at 310267. It is involved in a variety of cellular physiological behaviors including blood coagulation, body immunity, and protein processing. However, its dysregulation greatly promotes cell proliferation and tumor development268. TSP50 participated in multiple malignant processes of cancer, including stimulating cell proliferation via inhibiting activin signaling269, promoting invasion and metastasis by inducing EMT270. It was also discovered as a potential predictor of early recurrence and poor prognosis in malignancies271, and as a unique biomarker or treatment target for a variety of cancer therapies272, 273, 274, 275.
Threonine aspartase 1 is a strongly conserved developmental protease that is overexpressed in a variety of liquid and solid tumors. It is similar to type 2 asparaginases and N-terminal nucleophile (Ntn) proteases in structure, but shows unique biological functions including head morphogenesis, segment recognition, spermatogenesis, and proliferation276. Although the pathobiological mechanism has not yet been revealed, clarifying the structure-function relationship of threonine aspartase 1 is essential and has been widely revealed to explore its potential in diagnostic and therapeutic regimens277,278.
All of the above threonine proteases, whether intrinsic in humans, derived from other organisms in nature, or converted by other proteases, are prominent candidates for the exploitation of their predictive, diagnostic, and therapeutic values, as well as their potential for protease-driven delivery of bioactive theranostic agents, which is currently less reported.
3.5. Aspartic proteases-responsive nanoDDS for cancer theranostics
Cathepsins D and E are widely popular aspartic proteases that are ubiquitous in the progression of many types of cancers. Even with inevitable extracellular secretion, they are largely confined to lysosomes for functions279. Cathepsin D is competent in a variety of physiological processes and is highly activated in a variety of tumors. Its upregulation, caused by altered trafficking-induced proenzyme increase, leads directly to poorer prognosis and shorter recurrence-free survival280. Cathepsin E belongs to the pepsin family and the endolysosomal pathway, which is significantly expressed in the immune cells, lymphatic system, gastrointestinal mucosa, epidermal keratinocytes, red blood cells, and tumor cells281. It exhibits regular cell-type specific localization and is also secreted into the ECM of activated phagocytes and cancer cells282. This differentiated cellular distribution of cathepsin E determines its cellular and tissue-specific biological efficacy, as well as overactivation-caused tumor development283.
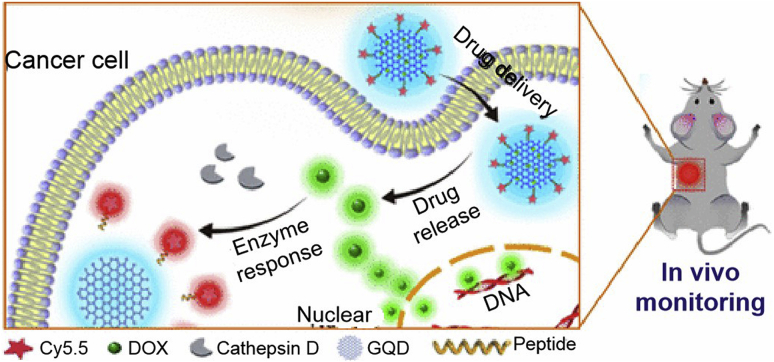
For the past few years, nanostrategies incorporating the responsiveness of these two proteases have been developed for the sensitive presentation of active therapeutics. Exemplarily, graphene QDs loading DOX was conjugated with Cy5.5 by a peptide that specifically responds to cathepsin D. The resulting nanoprobes programmatically released DOX and fluorescently tracked their internal processes (Fig. 8)284. Bhattacharya et al.285 conjugated an N-terminal CPP, dexamethasone, and cathepsin D-activated peptide linker via hydrazone bond. This chemically stable conjugate could be efficiently ingested by retinal cells and exhibited cathepsin D-accelerated drug release and targeted therapy for ocular diseases. Another approach focused on the vectorization of cathepsin D inhibitors and the interpretation of their structure-function relevance286,287. For cathepsin E, recent studies emphasized exploring its feasibility as a molecular target for cancer theranostics and developing its clinical application potency288.
Figure 8.
Graphene quantum dots (GQDs) loaded with DOX and conjugated with Cy5.5 for cathepsin D-responsive drug delivery and internal process visualization. Reprinted with the permission from Ref. 285. Copyright © 2017 American Chemical Society.
3.6. Other proteases-responsive nanoDDS for cancer theranostics
Other proteases, such as furin, also play an indispensable role in the regulation of cancer processes and the promotion of theranostic programs. Furin is a proprotein convertase and mainly distributed in the cell surface or trans-Golgi body. It is dysregulated in microbial infections, fibrosis, Alzheimer's disease, and a variety of cancers such as breast cancer, head and neck cancer, NSCLC and rhabdomyosarcoma289. Furin performs a series of important physiological and pathological functions by cleaving the specific sequence (Arg-X-Arg/Lys−Arg↓) of paired basic amino acids in the substrates, or stimulating the activation of some protein precursors such as α- and β-secretases290.
Based on the proteolytic properties of furin, a series of intelligent nanosystems have been developed for furin-responsive cancer detection and treatment. Li et al.291 constructed an amphiphilic peptide, an Arg-Val-Arg-Arg-Phe-Phe-Phe-NBD (a nitrobenzoxadiazole) sequence. Among which, NBD was a hydrophobic environment-enhanced fluorescent chromophore. The Phe-Phe-Phe triplet provided hydrophobicity-promoted nano-assembly and cellular permeability. The hydrophilic Arg-Val-Arg-Arg sequence improved the membrane penetration and acted as a substrate for the specific detection of furin. The reasonably designed peptide self-assembled into a stable micelle and the fluorescence of NBD was lit to quickly detect furin in vitro and in vivo. This rapid response provided the possibility for furin-associated tumor recognition and progression evaluation. The substrates of furin are rich in basic amino acids, which give them the CPP potential. In view of this, furin substrates with different repeats[(Arg-Val-Arg-Arg)m] were developed to deliver the cell-impermeable pro-apoptotic peptide KLAKLAKKLAKLAK and chemotherapeutic chlorambucil. After approaching the tumor lesion, the drug-carrying nanocomposites exhibited magnified cell permeability and enrichment, as well as furin-enhanced breast cancer elimination292. In addition, various other furin-responsive nanoplatforms were also rapidly evolving. As an example, furin substrate peptide-modified liposomes were designed to load vincristine for protease-specific rhabdomyosarcom treatment293.
Summarizing the above analysis, the types of proteases and their structure-activity relationships have been gradually revealed. The clarity of protease function provides considerable application space for the development of enzyme-specific probes and inhibitors for cancer surveillance and treatment. Protease-mediated tumor-targeted enrichment of bioactive agents has shown promising progress. Moreover, this strategy will present an overwhelming prospect if the following considerations can be considered comprehensively. (1) Designing simple and clinically acceptable nanoplatform, and preparing them by easier, more environmentally friendly, and more economical methods. (2) Exploring new proteases and synchronously analyzing the pathophysiological diversity of proteases reported. (3) Exploiting intelligent nanocomposites based on the heterogeneity of protease function. (4) Updating the tumor models according to the improvement of protease function.
4. Concluding remarks and future perspectives
Proteases underpin important physiological functions but dysregulate in a variety of disease-associated microenvironments and pathological cellular processes. It is extremely promising to exploit differentiated activity or expression of tumor-specific proteases as therapeutic targets or triggers. The thriving nanobiotechnology boosted enzyme-responsive DDS to be an overwhelming booster in targeted cancer management. In the present review, the origin, structure-function correlation, and pivotal physiological/pathological activities of cancer-related proteases are classified according to proteolytic mechanisms. Emphatically, recent advances in protease-stimulated cargo delivery and cancer theranostics are systematically explored.
Despite the encouraging achievements, several obstacles remain in the way towards the clinical transformation of enzyme-responsive nanoDDS: (1) the diversity, complexity, and patient variability of tumor-associated proteases; (2) substrate overlap and partial enzyme-substrate incompatibility; (3) cumbersome design and extreme construction of some nanocomplexes; (4) potential toxicity and biocompatibility consideration of nanoformulations. Therefore, further intensive and in-depth investigations such as refining the structure of enzymes and matching them to diseases, exacting the design and screening of substrate sequences, tailoring individual materials and integrating them into a delicate nanocomposite with negligible nanotoxicity and desired biocompatibility, are urgently needed to improve the theranostic efficiency of protease-responsive nanoDDS and facilitate their clinical transformation.
In conclusion, the significance of proteases in cancer ecology has made it necessary to elucidate the structure-activity relationship of proteases and their roles in the pathophysiological processes of cancer. A variety of specific substrates have been developed and engineered into multiple nanoprobes. These nanocomplexes have attracted considerable attention and achieved exciting efficiency in disease treatment. However, some limitations remain, such as the functional diversity of proteases, substrate sequence overlap, the construction complexity of nanocomposites, the difficulty of tumor modeling, and possible nanotoxicity. These obstacles can be addressed specifically through multidisciplinary approaches including oncology, biology, and materials chemistry. These advances will provide architectural and mechanistic insights for the new generation of nanotheranostics and ultimately promote their clinical applications in the targeted management of malignant tumors.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81903662, 81860630 and 51903201), China Postdoctoral Science Foundation (2019M661057 and 2019M653660), Natural Science Foundation of Shaanxi Province (2020JQ-086, China), the Natural Science Foundation of Jiangxi (20181BAB205087, China), the Key Project of Jiangxi (20192ACB70012, China).
Author contributions
Yanan Li, Cangang Zhang, Guo Li and Guowei Deng were responsible for reviewing relevant studies and writing the paper. Hui Zhang, Yongbing Sun and Feifei An provided instructional advice polished the contents of the article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hui Zhang, Email: zhanghui_mr@163.com.
Yongbing Sun, Email: yongbing_sun@hotmail.com.
Feifei An, Email: anfeifei@xjtu.edu.cn.
References
- 1.Jabir N.R., Anwar K., Firoz C.K., Oves M., Kamal M.A., Tabrez S. An overview on the current status of cancer nanomedicines. Curr Med Res Opin. 2018;34:911–921. doi: 10.1080/03007995.2017.1421528. [DOI] [PubMed] [Google Scholar]
- 2.Wu C.C., Li M.N., Meng H.B., Liu Y.K., Niu W.H., Zhou Y. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 3.Wyld L., Audisio R.A., Poston G.J. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12:115–124. doi: 10.1038/nrclinonc.2014.191. [DOI] [PubMed] [Google Scholar]
- 4.Galmarini D., Galmarini C.M., Galmarini F.C. Cancer chemotherapy: a critical analysis of its 60 years of history. Crit Rev Oncol Hematol. 2012;84:181–199. doi: 10.1016/j.critrevonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 6.Sun W.M., Shi Q.L., Zhang H.Y., Yang K.X., Ke Y.X., Wang Y.P. Advances in the techniques and methodologies of cancer gene therapy. Discov Med. 2019;27:45–55. [PubMed] [Google Scholar]
- 7.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y.N., Cheng Y., Zhang M.Q., He X.L., Kong L., Zhou K.X. A new compound with increased antitumor activity by cotargeting MEK and Pim-1. iScience. 2020;23:101254. doi: 10.1016/j.isci.2020.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan L.Y., Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med. 2018;26:219–229. [PubMed] [Google Scholar]
- 10.Mayekar M.K., Bivona T.G. Current landscape of targeted therapy in lung cancer. Clin Pharmacol Ther. 2017;102:757–764. doi: 10.1002/cpt.810. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.N., Dong Q.R., Cui Y.K. Synergistic inhibition of MEK and reciprocal feedback networks for targeted intervention in malignancy. Cancer Biol Med. 2019;16:415–434. doi: 10.20892/j.issn.2095-3941.2019.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari A.P., Hwang T.I., Oh J.M., Maharjan B., Chun S., Kim B.S. pH/NIR-responsive polypyrrole-functionalized fibrous localized drug-delivery platform for synergistic cancer therapy. ACS Appl Mater Interfaces. 2018;10:20256–20270. doi: 10.1021/acsami.7b17664. [DOI] [PubMed] [Google Scholar]
- 13.Guo X.S., Cheng Y., Zhao X.T., Luo Y.L., Chen J.J., Yuan W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnol. 2018;16:74. doi: 10.1186/s12951-018-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R.R., Peng F.F., Cai J., Yang D.D., Zhang P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J Pharm Sci. 2020;15:311–325. doi: 10.1016/j.ajps.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W., Xing X.Y., Wang X.L., Wu D., Wu W., Guo J.L. Nanocarrier-mediated cytosolic delivery of biopharmaceuticals. Adv Funct Mater. 2020;30:1910566. [Google Scholar]
- 16.Tahmasbi Rad A., Chen C.W., Aresh W., Xia Y., Lai P.S., Nieh M.P. Combinational effects of active targeting, shape, and enhanced permeability and retention for cancer theranostic nanocarriers. ACS Appl Mater Interfaces. 2019;11:10505–10519. doi: 10.1021/acsami.8b21609. [DOI] [PubMed] [Google Scholar]
- 17.Li Y.N., Du L.P., Wu C.S., Yu B., Zhang H., An F.F. Peptide sequence-dominated enzyme-responsive nanoplatform for anticancer drug delivery. Curr Top Med Chem. 2019;19:74–97. doi: 10.2174/1568026619666190125144621. [DOI] [PubMed] [Google Scholar]
- 18.Thomas H. Biliary tract: MMP7-a diagnostic biomarker for biliary atresia. Nat Rev Gastroenterol Hepatol. 2018;15:68. doi: 10.1038/nrgastro.2017.175. [DOI] [PubMed] [Google Scholar]
- 19.Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol. 2016;39:97–106. doi: 10.1007/s13402-016-0268-6. (Dordr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Y.X., Chen G.Z., Xi X.P., Ma C.B., Wang L., Burrows J.F. Discovery and rational design of a novel bowman-birk related protease inhibitor. Biomolecules. 2019;9:280. doi: 10.3390/biom9070280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis M.I., Pragani R., Fox J.T., Shen M., Parmar K., Gaudiano E.F. Small molecule inhibition of the ubiquitin-specific protease USP2 accelerates cyclin D1 degradation and leads to cell cycle arrest in colorectal cancer and mantle cell lymphoma models. J Biol Chem. 2016;291:24628–24640. doi: 10.1074/jbc.M116.738567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henschke L., Frese M., Hellmuth S., Marx A., Stemmann O., Mayer T.U. Identification of bioactive small molecule inhibitors of separase. ACS Chem Biol. 2019;14:2155–2159. doi: 10.1021/acschembio.9b00661. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y., Huang F.J., Min X.H., Gao P.C., Zhang T.C., Li X.C. Protease-responsive prodrug with aggregation-induced emission probe for controlled drug delivery and drug release tracking in living cells. Anal Chem. 2016;88:8913–8919. doi: 10.1021/acs.analchem.6b02833. [DOI] [PubMed] [Google Scholar]
- 24.Li S.P., Jiang Q., Liu S.L., Zhang Y.L., Tian Y.H., Song C. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018;36:258–264. doi: 10.1038/nbt.4071. [DOI] [PubMed] [Google Scholar]
- 25.Hu Q.Y., Katti P.S., Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273–12286. doi: 10.1039/c4nr04249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrawati R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp Biol Med. 2016;241:972–979. doi: 10.1177/1535370216647186. (Maywood) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi Y., Wolfram J., Mu C.F., Liu X.W., Blanco E., Shen H. Enzyme-responsive multistage vector for drug delivery to tumor tissue. Pharmacol Res. 2016;113:92–99. doi: 10.1016/j.phrs.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.N., Mei T., Han S.P., Han T., Sun Y.B., Zhang H. Cathepsin B-responsive nanodrug delivery systems for precise diagnosis and targeted therapy of malignant tumors. Chin Chem Lett. 2020;31:3027–3040. [Google Scholar]
- 29.Rawlings N.D., Waller M., Barrett A.J., Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42:D503–D509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Otín C., Overall C.M. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–519. doi: 10.1038/nrm858. [DOI] [PubMed] [Google Scholar]
- 31.Seife C. Blunting nature's Swiss army knife. Science. 1997;277:1602–1603. doi: 10.1126/science.277.5332.1602. [DOI] [PubMed] [Google Scholar]
- 32.Park H., Oh J., Shim G., Cho B., Chang Y., Kim S. In vivo neuronal gene editing via CRISPR–Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer's disease. Nat Neurosci. 2019;22:524–528. doi: 10.1038/s41593-019-0352-0. [DOI] [PubMed] [Google Scholar]
- 33.Wertz I.E., Newton K., Seshasayee D., Kusam S., Lam C., Zhang J. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- 34.Young M.J., Hsu K.C., Lin T.E., Chang W.C., Hung J.J. The role of ubiquitin-specific peptidases in cancer progression. J Biomed Sci. 2019;26:42. doi: 10.1186/s12929-019-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q.H., Zhou Z.X., Qiu N.S., Shen Y.Q. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 36.Li Y.N., Xin J.Q., Sun Y.B., Han T., Zhang H., An F.F. Magnetic resonance imaging-guided and targeted theranostics of colorectal cancer. Cancer Biol Med. 2020;17:307–327. doi: 10.20892/j.issn.2095-3941.2020.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y.N., Chang Y.T., Lian X.F., Zhou L.Q., Yu Z.Q., Wang H.X. Silver nanoparticles for enhanced cancer theranostics: In vitro and in vivo perspectives. J Biomed Nanotechnol. 2018;14:1515–1542. doi: 10.1166/jbn.2018.2614. [DOI] [PubMed] [Google Scholar]
- 38.Li Y.N., Yang Y.L., An F.F., Liu Z., Zhang X.J., Zhang X.H. Carrier-free, functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/1/015103. 015103. [DOI] [PubMed] [Google Scholar]
- 39.Teng C., Lin C.S., Huang F.F., Xing X.Y., Chen S.Y., Ye L. Intracellular codelivery of anti-inflammatory drug and anti-miR 155 to treat inflammatory disease. Acta Pharm Sin B. 2020;10:1521–1533. doi: 10.1016/j.apsb.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He W., Kapate N., Shields C.W., Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2020;165–166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 41.An F.F., Yang Z., Zheng M.C., Mei T., Deng G.W., Guo P. Rationally assembled albumin/indocyanine green nanocomplex for enhanced tumor imaging to guide photothermal therapy. J Nanobiotechnol. 2020;18:49. doi: 10.1186/s12951-020-00603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y.N., Zhang H. Fe3O4-based nanotheranostics for magnetic resonance imaging-synergized multifunctional cancer management. Nanomedicine. 2019;14:1493–1512. doi: 10.2217/nnm-2018-0346. (Lond) [DOI] [PubMed] [Google Scholar]
- 43.Xiao Q.Q., Zhu X., Yuan Y.T., Yin L.F., He W. A drug-delivering-drug strategy for combined treatment of metastatic breast cancer. Nanomedicine. 2018;14:2678–2688. doi: 10.1016/j.nano.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 44.An F.F., Chen N.D., Conlon W.J., Hachey J.S., Xin J.Q., Aras O. Small ultra-red fluorescent protein nanoparticles as exogenous probes for noninvasive tumor imaging in vivo. Int J Biol Macromol. 2020;153:100–106. doi: 10.1016/j.ijbiomac.2020.02.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y.N., Dong Q.R., Mei T., Zheng M.C., Kumar R.R., Yu B. Nanosized modification strategies for improving the antitumor efficacy of MEK inhibitors. Curr Drug Targets. 2020;21:228–251. doi: 10.2174/1389450120666190807143245. [DOI] [PubMed] [Google Scholar]
- 46.Li Y.N., Zhang H. Nanoparticle-based drug delivery systems for enhanced tumor-targeting treatment. J Biomed Nanotechnol. 2019;15:1–27. doi: 10.1166/jbn.2019.2670. [DOI] [PubMed] [Google Scholar]
- 47.Xin X.F., Du X.Q., Xiao Q.Q., Azevedo H.S., He W., Yin L.F. Drug nanorod-mediated intracellular delivery of microRNA-101 for self-sensitization via autophagy inhibition. Nano-Micro Lett. 2019;11:453–468. doi: 10.1007/s40820-019-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y.B., Ma W., Yang Y.Y., He M.X., Li A.M., Bai L. Cancer nanotechnology: enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy. Asian J Pharm Sci. 2019;14:581–594. doi: 10.1016/j.ajps.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Xu D.Q., Zhou J.L., Lou X.D., He J.H., Ran T.T., Wang W.W. Myroilysin is a new bacterial member of the M12A family of metzincin metallopeptidases and is activated by a cysteine switch mechanism. J Biol Chem. 2017;292:5195–5206. doi: 10.1074/jbc.M116.758110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polette M., Nawrocki-Raby B., Gilles C., Clavel C., Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49:179–186. doi: 10.1016/j.critrevonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 54.Fay F., Hansen L., Hectors S.J.C.G., Sanchez-Gaytan B.L., Zhao Y.M., Tang J. Investigating the cellular specificity in tumors of a surface-converting nanoparticle by multimodal imaging. Bioconjug Chem. 2017;28:1413–1421. doi: 10.1021/acs.bioconjchem.7b00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakroun R.W., Sneider A., Anderson C.F., Wang F.H., Wu P.H., Wirtz D. Supramolecular design of unsymmetric reverse bolaamphiphiles for cell-sensitive hydrogel degradation and drug release. Angew Chem Int Ed Engl. 2020;59:4434–4442. doi: 10.1002/anie.201913087. [DOI] [PubMed] [Google Scholar]
- 56.Zou Z., He X.X., He D.G., Wang K.M., Qing Z.H., Yang X. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials. 2015;58:35–45. doi: 10.1016/j.biomaterials.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 57.Eskandari P., Bigdeli B., Porgham Daryasari M., Baharifar H., Bazri B., Shourian M. Gold-capped mesoporous silica nanoparticles as an excellent enzyme-responsive nanocarrier for controlled doxorubicin delivery. J Drug Target. 2019;27:1084–1093. doi: 10.1080/1061186X.2019.1599379. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Xu J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R Soc Open Sci. 2018;5:170986. doi: 10.1098/rsos.170986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo W., Xu X., Zhou B.J., He P.X., Li Y.L., Liu C.S. Formation of enzymatic/redox-switching nanogates on mesoporous silica nanoparticles for anticancer drug delivery. Mater Sci Eng C Mater Biol Appl. 2019;100:855–861. doi: 10.1016/j.msec.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Chen W.H., Luo G.F., Lei Q., Jia H.Z., Hong S., Wang Q.R. MMP-2 responsive polymeric micelles for cancer-targeted intracellular drug delivery. Chem Commun. 2015;51:465–468. doi: 10.1039/c4cc07563c. (Camb) [DOI] [PubMed] [Google Scholar]
- 61.Yoo J., Sanoj Rejinold N.S., Lee D., Jon S., Kim Y.C. Protease-activatable cell-penetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy. J Control Release. 2017;264:89–101. doi: 10.1016/j.jconrel.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 62.Cun X.L., Li M., Wang S.Y., Wang Y.F., Wang J.L., Lu Z.Z. A size switchable nanoplatform for targeting the tumor microenvironment and deep tumor penetration. Nanoscale. 2018;10:9935–9948. doi: 10.1039/c8nr00640g. [DOI] [PubMed] [Google Scholar]
- 63.Ke W.D., Li J.J., Zhao K.J., Zha Z.S., Han Y., Wang Y.H. Modular design and facile synthesis of enzyme-responsive peptide-linked block copolymers for efficient delivery of doxorubicin. Biomacromolecules. 2016;17:3268–3276. doi: 10.1021/acs.biomac.6b00997. [DOI] [PubMed] [Google Scholar]
- 64.Han M., Huang-Fu M.Y., Guo W.W., Guo N.N., Chen J.J., Liu H.N. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl Mater Interfaces. 2017;9:42459–42470. doi: 10.1021/acsami.7b10098. [DOI] [PubMed] [Google Scholar]
- 65.You Y.W., Xu Z.Y., Chen Y. Doxorubicin conjugated with a trastuzumab epitope and an MMP-2 sensitive peptide linker for the treatment of HER2-positive breast cancer. Drug Deliv. 2018;25:448–460. doi: 10.1080/10717544.2018.1435746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H.H., Fu Z.G., Li W., Li Y.X., Zhao L.S., Wen L. The synthesis and application of nano doxorubicin-indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int J Nanomedicine. 2019;14:623–638. doi: 10.2147/IJN.S191069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y., Zhang J.N., Liu X.Y., Huo P.C., Zhang Y., Chen H. MMP-2-responsive gelatin nanoparticles for synergistic tumor therapy. Pharm Dev Technol. 2019;24:1002–1013. doi: 10.1080/10837450.2019.1621899. [DOI] [PubMed] [Google Scholar]
- 68.Iaccarino G., Profeta M., Vecchione R., Netti P.A. Matrix metalloproteinase-cleavable nanocapsules for tumor-activated drug release. Acta Biomater. 2019;89:265–278. doi: 10.1016/j.actbio.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 69.Li J.J., Xiao S.Y., Xu Y.X., Zuo S., Zha Z.S., Ke W.D. Smart asymmetric vesicles with triggered availability of inner cell-penetrating shells for specific intracellular drug delivery. ACS Appl Mater Interfaces. 2017;9:17727–17735. doi: 10.1021/acsami.7b02808. [DOI] [PubMed] [Google Scholar]
- 70.Pan J., Li P.J., Wang Y., Chang L., Wan D., Wang H. Active targeted drug delivery of MMP-2 sensitive polymeric nanoparticles. Chem Commun. 2018;54:11092–11095. doi: 10.1039/c8cc05504a. (Camb) [DOI] [PubMed] [Google Scholar]
- 71.Ke W.D., Zha Z.S., Mukerabigwi J.F., Chen W.J., Wang Y.H., He C.X. Matrix metalloproteinase-responsive multifunctional peptide-linked amphiphilic block copolymers for intelligent systemic anticancer drug delivery. Bioconjug Chem. 2017;28:2190–2198. doi: 10.1021/acs.bioconjchem.7b00330. [DOI] [PubMed] [Google Scholar]
- 72.Yao Q., Dai Z., Hoon Choi J.H., Kim D., Zhu L. Building stable MMP2-responsive multifunctional polymeric micelles by an all-in-one polymer–lipid conjugate for tumor-targeted intracellular drug delivery. ACS Appl Mater Interfaces. 2017;9:32520–32533. doi: 10.1021/acsami.7b09511. [DOI] [PubMed] [Google Scholar]
- 73.Huang J.S., Xu Y.M., Xiao H., Xiao Z.C., Guo Y., Cheng D. Core–shell distinct nanodrug showing on-demand sequential drug release to act on multiple cell types for synergistic anticancer therapy. ACS Nano. 2019;13:7036–7049. doi: 10.1021/acsnano.9b02149. [DOI] [PubMed] [Google Scholar]
- 74.He J., Xiao H., Li B., Peng Y., Li X.X., Wang Y. The programmed site-specific delivery of the angiostatin sunitinib and chemotherapeutic paclitaxel for highly efficient tumor treatment. J Mater Chem B. 2019;7:4953–4962. doi: 10.1039/c9tb01159e. [DOI] [PubMed] [Google Scholar]
- 75.Lai Y.H., Chiang C.S., Kao T.H., Chen S.Y. Dual-drug nanomedicine with hydrophilic F127-modified magnetic nanocarriers assembled in amphiphilic gelatin for enhanced penetration and drug delivery in deep tumor tissue. Int J Nanomedicine. 2018;13:3011–3026. doi: 10.2147/IJN.S161314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z.L., Zhang J.Z., Jiang M., Zhao L., Li S.H., Sun H.B. Human serum albumin-based dual-agent delivery systems for combination therapy: acting against cancer cells and inhibiting neovascularization in the tumor microenvironment. Mol Pharm. 2020;17:1405–1414. doi: 10.1021/acs.molpharmaceut.0c00133. [DOI] [PubMed] [Google Scholar]
- 77.Ji T.J., Li S.P., Zhang Y.L., Lang J.Y., Ding Y.P., Zhao X. An MMP-2 responsive liposome integrating antifibrosis and chemotherapeutic drugs for enhanced drug perfusion and efficacy in pancreatic cancer. ACS Appl Mater Interfaces. 2016;8:3438–3445. doi: 10.1021/acsami.5b11619. [DOI] [PubMed] [Google Scholar]
- 78.Cun X.L., Chen J.T., Li M.M., He X., Tang X., Guo R. Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size-switchable nanoparticle. ACS Appl Mater Interfaces. 2019;11:39545–39559. doi: 10.1021/acsami.9b13957. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y.Z., Zhang M., Jin H.Y., Li D.D., Xu F., Wu A.H. Glioma dual-targeting nanohybrid protein toxin constructed by intein-mediated site-specific ligation for multistage booster delivery. Theranostics. 2017;7:3489–3503. doi: 10.7150/thno.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Y.C., Yuan S.X., Huo M.M., Chaudhuri A.S., Zhao M.H., Wu Z.H. Spatial controlled multistage nanocarriers through hybridization of dendrimers and gelatin nanoparticles for deep penetration and therapy into tumor tissue. Nanomedicine. 2017;13:1399–1410. doi: 10.1016/j.nano.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Ji T.J., Lang J.Y., Wang J., Cai R., Zhang Y.L., Qi F.F. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano. 2017;11:8668–8678. doi: 10.1021/acsnano.7b01026. [DOI] [PubMed] [Google Scholar]
- 82.Jiang X.L., Fan X.B., Xu W., Zhao C.G., Wu H.L., Zhang R. Self-assembled peptide nanoparticles responsive to multiple tumor microenvironment triggers provide highly efficient targeted delivery and release of antitumor drug. J Control Release. 2019;316:196–207. doi: 10.1016/j.jconrel.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 83.Barve A., Jain A., Liu H., Zhao Z., Cheng K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020;113:501–511. doi: 10.1016/j.actbio.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramezani P., Abnous K., Taghdisi S.M., Zahiri M., Ramezani M., Alibolandi M. Targeted MMP-2 responsive chimeric polymersomes for therapy against colorectal cancer. Colloids Surf B Biointerfaces. 2020;193:111135. doi: 10.1016/j.colsurfb.2020.111135. [DOI] [PubMed] [Google Scholar]
- 85.Dai Z., Tu Y., Zhu L. Multifunctional micellar nanocarriers for tumor-targeted delivery of hydrophobic drugs. J Biomed Nanotechnol. 2016;12:1199–1210. doi: 10.1166/jbn.2016.2249. [DOI] [PubMed] [Google Scholar]
- 86.Dai Z., Yao Q., Zhu L. MMP2-sensitive PEG–lipid copolymers: a new type of tumor-targeted P-Glycoprotein inhibitor. ACS Appl Mater Interfaces. 2016;8:12661–12673. doi: 10.1021/acsami.6b03064. [DOI] [PubMed] [Google Scholar]
- 87.Yao Q., Liu Y., Kou L.F., Tu Y., Tang X., Zhu L. Tumor-targeted drug delivery and sensitization by MMP2-responsive polymeric micelles. Nanomedicine. 2019;19:71–80. doi: 10.1016/j.nano.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y.Z., Zhang M., Jin H.Y., Tang Y.S., Wu A.H., Xu Q. Prodrug-like, PEGylated protein toxin trichosanthin for reversal of chemoresistance. Mol Pharm. 2017;14:1429–1438. doi: 10.1021/acs.molpharmaceut.6b00987. [DOI] [PubMed] [Google Scholar]
- 89.Ma P.K., Chen J.H., Bi X.N., Li Z.H., Gao X., Li H.P. Overcoming multidrug resistance through the GLUT1-mediated and enzyme-triggered mitochondrial targeting conjugate with redox-sensitive paclitaxel release. ACS Appl Mater Interfaces. 2018;10:12351–12363. doi: 10.1021/acsami.7b18437. [DOI] [PubMed] [Google Scholar]
- 90.Mo L.T., Zhao Z.L., Hu X.X., Yu X., Peng Y.B., Liu H. Smart nanodrug with nuclear localization sequences in the presence of MMP-2 to overcome biobarriers and drug resistance. Chem Eur J. 2019;25:1895–1900. doi: 10.1002/chem.201805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu G.L., Chun X.L., Wang Y., He Q., Gao H.L. Peptide mediated active targeting and intelligent particle size reduction-mediated enhanced penetrating of fabricated nanoparticles for triple-negative breast cancer treatment. Oncotarget. 2015;6:41258–41274. doi: 10.18632/oncotarget.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu G.L., Zhang H.Q., Zhang L., Ruan S.B., He Q., Gao H.L. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment. Int J Pharm. 2015;496:1057–1068. doi: 10.1016/j.ijpharm.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 93.Ruan S.B., Cao X., Cun X.L., Hu G.L., Zhou Y., Zhang Y.J. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100–110. doi: 10.1016/j.biomaterials.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Cun X.L., Ruan S.B., Chen J.T., Zhang L., Li J.P., He Q. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater. 2016;31:186–196. doi: 10.1016/j.actbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y.Y., Li L., Li L.J., Zhou Z., Wang F.L., Xiong X.F. Programmed drug delivery system based on optimized "size decrease and hydrophilicity/hydrophobicity transformation" for enhanced hepatocellular carcinoma therapy of doxorubicin. Nanomedicine. 2018;14:1111–1122. doi: 10.1016/j.nano.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Tu Y., Zhu L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J Control Release. 2015;212:94–102. doi: 10.1016/j.jconrel.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 97.Huang C.J., Yi X.L., Kong D.X., Chen L.G., Min G. Controlled release strategy of paclitaxel by conjugating to matrix metalloproteinases-2 sensitive peptide. Oncotarget. 2016;7:52230–52238. doi: 10.18632/oncotarget.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Shen N., Sakurai K., Tang Z.H. Multi-stimuli-responsive polymeric prodrug for enhanced cancer treatment. Macromol Biosci. 2019;19:1900329. doi: 10.1002/mabi.201900329. [DOI] [PubMed] [Google Scholar]
- 99.Li S.Y., Cheng H., Qiu W.X., Liu L.H., Chen S., Hu Y. Protease-activable cell-penetrating peptide–protoporphyrin conjugate for targeted photodynamic therapy in vivo. ACS Appl Mater Interfaces. 2015;7:28319–28329. doi: 10.1021/acsami.5b08637. [DOI] [PubMed] [Google Scholar]
- 100.Wu J.N., Han H.J., Jin Q., Li Z.H., Li H., Ji J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9:14596–14605. doi: 10.1021/acsami.6b15853. [DOI] [PubMed] [Google Scholar]
- 101.Veiman K.L., Künnapuu K., Lehto T., Kiisholts K., Pärn K., Langel Ü. PEG shielded MMP sensitive CPPs for efficient and tumor specific gene delivery in vivo. J Control Release. 2015;209:238–247. doi: 10.1016/j.jconrel.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Wei H.T., Xu L.S., Yan G.W., Ma C., Yu M. Preparation and evaluation of a non-viral gene vector for SiRNA: multifunctional envelope-type nano device. Artif Cells Nanomed Biotechnol. 2016;44:1259–1265. doi: 10.3109/21691401.2015.1024840. [DOI] [PubMed] [Google Scholar]
- 103.Tarokh Z., Naderi-Manesh H., Nazari M. Towards prostate cancer gene therapy: development of a chlorotoxin-targeted nanovector for toxic (melittin) gene delivery. Eur J Pharm Sci. 2017;99:209–218. doi: 10.1016/j.ejps.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Zeng Y., Zhou Z.X., Fan M.M., Gong T., Zhang Z.R., Sun X. PEGylated cationic vectors containing a protease-sensitive peptide as a miRNA delivery system for treating breast cancer. Mol Pharm. 2017;14:81–92. doi: 10.1021/acs.molpharmaceut.6b00726. [DOI] [PubMed] [Google Scholar]
- 105.Chen C.X., Zhang Y., Hou Z., Cui X.J., Zhao Y.R., Xu H. Rational design of short peptide-based hydrogels with MMP-2 responsiveness for controlled anticancer peptide delivery. Biomacromolecules. 2017;18:3563–3571. doi: 10.1021/acs.biomac.7b00911. [DOI] [PubMed] [Google Scholar]
- 106.Liu F.H., Hou C.Y., Zhang D., Zhao W.J., Cong Y., Duan Z.Y. Enzyme-sensitive cytotoxic peptide-dendrimer conjugates enhance cell apoptosis and deep tumor penetration. Biomater Sci. 2018;6:604–613. doi: 10.1039/c7bm01182b. [DOI] [PubMed] [Google Scholar]
- 107.Ando M., Fujimoto M., Takahashi Y., Nishikawa M., Hamana A., Takakura Y. Targeted delivery of interferon gamma using a recombinant fusion protein of a fibrin clot-binding peptide with interferon gamma for cancer gene therapy. J Pharm Sci. 2017;106:892–897. doi: 10.1016/j.xphs.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 108.Yin J., Liu D.K., Bao L.C., Wang Q., Chen Y., Hou S. Tumor targeting and microenvironment-responsive multifunctional fusion protein for pro-apoptotic peptide delivery. Cancer Lett. 2019;452:38–50. doi: 10.1016/j.canlet.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 109.Xu J., Du Y., Liu X.J., Zhu B.Y., Zhang S.H., Li L. Recombinant EGFR/MMP-2 bi-targeted fusion protein markedly binding to non-small-cell lung carcinoma and exerting potent therapeutic efficacy. Pharmacol Res. 2017;126:66–76. doi: 10.1016/j.phrs.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Zhou A.W., Du J.J., Jiao M.Y., Xie D.P., Wang Q.Q., Xue L.J. Co-delivery of TRAIL and siHSP70 using hierarchically modular assembly formulations achieves enhanced TRAIL-resistant cancer therapy. J Control Release. 2019;304:111–124. doi: 10.1016/j.jconrel.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 111.Cheng K.M., Ding Y.P., Zhao Y., Ye S.F., Zhao X., Zhang Y.L. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18:3250–3258. doi: 10.1021/acs.nanolett.8b01071. [DOI] [PubMed] [Google Scholar]
- 112.Salzano G., Costa D.F., Sarisozen C., Luther E., Mattheolabakis G., Dhargalkar P.P. Mixed nanosized polymeric micelles as promoter of doxorubicin and miRNA-34a co-delivery triggered by dual stimuli in tumor tissue. Small. 2016;12:4837–4848. doi: 10.1002/smll.201600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koss K., Tsui C., Unsworth L.D. Induced neural differentiation of MMP-2 cleaved (RADA)4 drug delivery systems. J Control Release. 2016;243:204–213. doi: 10.1016/j.jconrel.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 114.Kim H.S., Yoon S., Son Y.J., Park Y., Jung Y.M., Yoo H.S. High-yield clicking and dissociation of doxorubicin nanoclusters exhibiting differential cellular uptakes and imaging. J Control Release. 2015;217:64–73. doi: 10.1016/j.jconrel.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 115.Tsai L.C., Hsieh H.Y., Lu K.Y., Wang S.Y., Mi F.L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine. 2016;11:9–30. doi: 10.2217/nnm.15.183. (Lond) [DOI] [PubMed] [Google Scholar]
- 116.Xia F.F., Hou W.X., Zhang C.L., Zhi X., Cheng J., de la Fuente J.M. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018;68:308–319. doi: 10.1016/j.actbio.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 117.Hu J.J., Liu L.H., Li Z.Y., Zhuo R.X., Zhang X.Z. MMP-responsive theranostic nanoplatform based on mesoporous silica nanoparticles for tumor imaging and targeted drug delivery. J Mater Chem B. 2016;4:1932–1940. doi: 10.1039/c5tb02490k. [DOI] [PubMed] [Google Scholar]
- 118.Xia F.F., Niu J.Q., Hong Y.P., Li C.L., Cao W., Wang L.R. Matrix metallopeptidase 2 targeted delivery of gold nanostars decorated with IR-780 iodide for dual-modal imaging and enhanced photothermal/photodynamic therapy. Acta Biomater. 2019;89:289–299. doi: 10.1016/j.actbio.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Chen X.H., Gao H.Q., Deng Y.Y., Jin Q., Ji J., Ding D. Supramolecular aggregation-induced emission nanodots with programmed tumor microenvironment responsiveness for image-guided orthotopic pancreatic cancer therapy. ACS Nano. 2020;14:5121–5134. doi: 10.1021/acsnano.0c02197. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z.H., Wang Y.H., Jia X.Q., Han Q.J., Qian Y.X., Li Q. MMP-2-controlled transforming micelles for heterogeneic targeting and programmable cancer therapy. Theranostics. 2019;9:1728–1740. doi: 10.7150/thno.30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li E.D., Yang Y.M., Hao G.Y., Yi X., Zhang S.H., Pan Y. Multifunctional magnetic mesoporous silica nanoagents for in vivo enzyme-responsive drug delivery and MR imaging. Nanotheranostics. 2018;2:233–242. doi: 10.7150/ntno.25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceylan H., Yasa I.C., Yasa O., Tabak A.F., Giltinan J., Sitti M. 3D-Printed biodegradable microswimmer for theranostic cargo delivery and release. ACS Nano. 2019;13:3353–3362. doi: 10.1021/acsnano.8b09233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mao W., Kim H.S., Son Y.J., Kim S.R., Yoo H.S. Doxorubicin encapsulated clicked gold nanoparticle clusters exhibiting tumor-specific disassembly for enhanced tumor localization and computerized tomographic imaging. J Control Release. 2018;269:52–62. doi: 10.1016/j.jconrel.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 124.Zhao L.Z., Zhu J.Y., Cheng Y.J., Xiong Z.J., Tang Y.Q., Guo L.L. Chlorotoxin-conjugated multifunctional dendrimers labeled with radionuclide 131I for single photon emission computed tomography imaging and radiotherapy of gliomas. ACS Appl Mater Interfaces. 2015;7:19798–19808. doi: 10.1021/acsami.5b05836. [DOI] [PubMed] [Google Scholar]
- 125.Jiao X.X., Yu Y., Meng J.X., He M., Zhang C.J., Geng W.Q. Dual-targeting and microenvironment-responsive micelles as a gene delivery system to improve the sensitivity of glioma to radiotherapy. Acta Pharm Sin B. 2019;9:381–396. doi: 10.1016/j.apsb.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen M., Zheng J.H., Choi J.M., Pei J., Li C.H., Li S.Y. Genetically-engineered salmonella typhimurium expressing TIMP-2 as a therapeutic intervention in an orthotopic glioma mouse model. Cancer Lett. 2018;433:140–146. doi: 10.1016/j.canlet.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 127.Yao Q., Choi J.H., Dai Z., Wang J., Kim D., Tang X. Improving tumor specificity and anticancer activity of dasatinib by dual-targeted polymeric micelles. ACS Appl Mater Interfaces. 2017;9:36642–36654. doi: 10.1021/acsami.7b12233. [DOI] [PubMed] [Google Scholar]
- 128.Erkoc P., Cingöz A., Bagci-Onder T., Kizilel S. Quinacrine mediated sensitization of glioblastoma (GBM) cells to TRAIL through MMP-sensitive PEG hydrogel carriers. Macromol Biosci. 2017;17:1600267. doi: 10.1002/mabi.201600267. [DOI] [PubMed] [Google Scholar]
- 129.Zhou X.Y., Zheng Q.Q., Wang C.Y., Xu J.K., Wu J.P., Kirk T.B. Star-shaped amphiphilic hyperbranched polyglycerol conjugated with dendritic poly(L-lysine) for the codelivery of docetaxel and MMP-9 siRNA in cancer therapy. ACS Appl Mater Interfaces. 2016;8:12609–12619. doi: 10.1021/acsami.6b01611. [DOI] [PubMed] [Google Scholar]
- 130.Tang Q., Ma X., Zhang Y., Cai X., Xue W., Ma D. Self-sensibilized polymeric prodrug co-delivering MMP-9 shRNA plasmid for combined treatment of tumors. Acta Biomater. 2018;69:277–289. doi: 10.1016/j.actbio.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 131.Liu T., Wu X.D., Chen S.H., Wu P.N., Han H., Zhang H.B. A cationic polymeric prodrug with chemotherapeutic self-sensibilization co-delivering MMP-9 shRNA plasmid for a combined therapy to nasopharyngeal carcinoma. Drug Deliv. 2019;26:1280–1291. doi: 10.1080/10717544.2019.1698674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grünwald B., Vandooren J., Locatelli E., Fiten P., Opdenakker G., Proost P. Matrix metalloproteinase-9 (MMP-9) as an activator of nanosystems for targeted drug delivery in pancreatic cancer. J Control Release. 2016;239:39–48. doi: 10.1016/j.jconrel.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 133.Gordon M.R., Zhao B., Anson F., Fernandez A., Singh K., Homyak C. Matrix metalloproteinase-9-responsive nanogels for proximal surface conversion and activated cellular uptake. Biomacromolecules. 2018;19:860–871. doi: 10.1021/acs.biomac.7b01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalafatovic D., Nobis M., Son J., Anderson K.I., Ulijn R.V. MMP-9 triggered self-assembly of doxorubicin nanofiber depots halts tumor growth. Biomaterials. 2016;98:192–202. doi: 10.1016/j.biomaterials.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 135.Bhunia D., Pradhan K., Das G., Ghosh S., Mondal P., Ghosh S. Matrix metalloproteinase targeted peptide vesicles for delivering anticancer drugs. Chem Commun. 2018;54:9309–9312. doi: 10.1039/c8cc05687k. (Camb) [DOI] [PubMed] [Google Scholar]
- 136.Jiang J., Shen N., Ci T.Y., Tang Z.H., Gu Z., Li G. Combretastatin A4 nanodrug-induced MMP9 amplification boosts tumor-selective release of doxorubicin prodrug. Adv Mater. 2019;31:1904278. doi: 10.1002/adma.201904278. [DOI] [PubMed] [Google Scholar]
- 137.Xu C.F., Sun Y., Yu Y.L., Hu M., Yang C.L., Zhang Z.P. A sequentially responsive and structure-transformable nanoparticle with a comprehensively improved 'CAPIR cascade' for enhanced antitumor effect. Nanoscale. 2019;11:1177–1194. doi: 10.1039/c8nr08781d. [DOI] [PubMed] [Google Scholar]
- 138.Porta F., Ehrsam D., Lengerke C., Meyer zu Schwabedissen H.E. Synthesis and characterization of PDMS–PMOXA-based polymersomes sensitive to MMP-9 for application in breast cancer. Mol Pharm. 2018;15:4884–4897. doi: 10.1021/acs.molpharmaceut.8b00521. [DOI] [PubMed] [Google Scholar]
- 139.Chen Q.Y., Chen Y.T., Sun Y.L., He W.X., Han X.L., Lu E.H. Leukocyte-mimicking pluronic-lipid nanovesicle hybrids inhibit the growth and metastasis of breast cancer. Nanoscale. 2019;11:5377–5394. doi: 10.1039/c8nr08936a. [DOI] [PubMed] [Google Scholar]
- 140.Xu P.F., Meng Q.S., Sun H.P., Yin Q., Yu H.J., Zhang Z.W. Shrapnel nanoparticles loading docetaxel inhibit metastasis and growth of breast cancer. Biomaterials. 2015;64:10–20. doi: 10.1016/j.biomaterials.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 141.Han H.J., Valdepérez D., Jin Q., Yang B., Li Z.H., Wu Y.L. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281–1291. doi: 10.1021/acsnano.6b05541. [DOI] [PubMed] [Google Scholar]
- 142.Guo X.L., Fang Z., Zhang M., Yang D.Y., Wang S.Y., Liu K.H. A co-delivery system of curcumin and p53 for enhancing the sensitivity of drug-resistant ovarian cancer cells to cisplatin. Molecules. 2020;25:2621. doi: 10.3390/molecules25112621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao X., Zhang J., Huang Z., Zuo T.T., Lu Q., Wu G.Y. Reducing interstitial fluid pressure and inhibiting pulmonary metastasis of breast cancer by gelatin modified cationic lipid nanoparticles. ACS Appl Mater Interfaces. 2017;9:29457–29468. doi: 10.1021/acsami.7b05119. [DOI] [PubMed] [Google Scholar]
- 144.Lang T.Q., Liu Y.R., Zheng Z., Ran W., Zhai Y.H., Yin Q. Cocktail strategy based on spatio-temporally controlled nano device improves therapy of breast cancer. Adv Mater. 2019;31:1806202. doi: 10.1002/adma.201806202. [DOI] [PubMed] [Google Scholar]
- 145.Dong S.Y., Wang P., Zhao P., Chen M.N. Direct loading of iTEP-delivered CTL epitope onto MHC class I complexes on the dendritic cell surface. Mol Pharm. 2017;14:3312–3321. doi: 10.1021/acs.molpharmaceut.7b00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang P., Dong S.Y., Zhao P., He X., Chen M.N. Direct loading of CTL epitopes onto MHC class I complexes on dendritic cell surface in vivo. Biomaterials. 2018;182:92–103. doi: 10.1016/j.biomaterials.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Battistella C., Callmann C.E., Thompson M.P., Yao S.Y., Yeldandi A.V., Hayashi T. Delivery of immunotherapeutic nanoparticles to tumors via enzyme-directed assembly. Adv Healthc Mater. 2019;8:1901105. doi: 10.1002/adhm.201901105. [DOI] [PubMed] [Google Scholar]
- 148.Jallouk A.P., Palekar R.U., Marsh J.N., Pan H., Pham C.T.N., Schlesinger P.H. Delivery of a protease-activated cytolytic peptide prodrug by perfluorocarbon nanoparticles. Bioconjug Chem. 2015;26:1640–1650. doi: 10.1021/acs.bioconjchem.5b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X., Gu M.J., Toh T.B., Abdullah N.L.B., Chow E.K. Stimuli-responsive nanodiamond-based biosensor for enhanced metastatic tumor site detection. SLAS Technol. 2018;23:44–56. doi: 10.1177/2472630317735497. [DOI] [PubMed] [Google Scholar]
- 150.Li N., Guo C.H., Duan Z.Y., Yu L.Z., Luo K., Lu J. A stimuli-responsive Janus peptide dendron-drug conjugate as a safe and nanoscale drug delivery vehicle for breast cancer therapy. J Mater Chem B. 2016;4:3760–3769. doi: 10.1039/c6tb00688d. [DOI] [PubMed] [Google Scholar]
- 151.Shi N.Q., Li Y., Zhang Y., Shen N., Qi L., Wang S.R. Intelligent “peptide-gathering mechanical arm” tames wild “trojan-horse” peptides for the controlled delivery of cancer nanotherapeutics. ACS Appl Mater Interfaces. 2017;9:41767–41781. doi: 10.1021/acsami.7b15523. [DOI] [PubMed] [Google Scholar]
- 152.Shi N.Q., Qi X.R. Taming the wildness of “trojan-horse” peptides by charge-guided masking and protease-triggered demasking for the controlled delivery of antitumor agents. ACS Appl Mater Interfaces. 2017;9:10519–10529. doi: 10.1021/acsami.7b01056. [DOI] [PubMed] [Google Scholar]
- 153.Sivaraman B., Swaminathan G., Moore L., Fox J., Seshadri D., Dahal S. Magnetically-responsive, multifunctional drug delivery nanoparticles for elastic matrix regenerative repair. Acta Biomater. 2017;52:171–186. doi: 10.1016/j.actbio.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 154.Ji Y.J., Xiao Y.Y., Xu L., He J.Y., Qian C., Li W.D. Drug-bearing supramolecular MMP inhibitor nanofibers for inhibition of metastasis and growth of liver cancer. Adv Sci. 2018;5:1700867. doi: 10.1002/advs.201700867. (Weinh) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X.F., Chen Q.Y., Zhang X.Y., Ren X.Q., Zhang X.L., Meng L. Matrix metalloproteinase 2/9-triggered-release micelles for inhaled drug delivery to treat lung cancer: preparation and in vitro/in vivo studies. Int J Nanomedicine. 2018;13:4641–4659. doi: 10.2147/IJN.S166584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang X.Y., Wang X.F., Zhong W.T., Ren X.Q., Sha X.Y., Fang X.L. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation. Int J Nanomedicine. 2016;11:1643–1661. doi: 10.2147/IJN.S101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu H.L., Chen J., Liu S., Lu Q., He J., Zhou Z.Y. Enzyme sensitive, surface engineered nanoparticles for enhanced delivery of camptothecin. J Control Release. 2015;216:111–120. doi: 10.1016/j.jconrel.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 158.Fan C.X., Shi J.J., Zhuang Y., Zhang L.L., Huang L., Yang W. Myocardial-infarction-responsive smart hydrogels targeting matrix metalloproteinase for on-demand growth factor delivery. Adv Mater. 2019;31:1902900. doi: 10.1002/adma.201902900. [DOI] [PubMed] [Google Scholar]
- 159.Lyu Y.Q., Xiao Q.Q., Yin L.F., Yang L., He W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduct Target Ther. 2019;4:26. doi: 10.1038/s41392-019-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hou B., Zhou L., Wang H., Saeed M., Wang D.G., Xu Z.A. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32:1907210. doi: 10.1002/adma.201907210. [DOI] [PubMed] [Google Scholar]
- 161.Gao S., Zhang L.W., Wang G.H., Yang K., Chen M.L., Tian R. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials. 2016;79:36–45. doi: 10.1016/j.biomaterials.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 162.Ansari C., Tikhomirov G.A., Hong S.H., Falconer R.A., Loadman P.M., Gill J.H. Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small. 2014;10:566–575. doi: 10.1002/smll.201301456. 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gill J.H., Loadman P.M., Shnyder S.D., Cooper P., Atkinson J.M., Ribeiro Morais G. Tumor-targeted prodrug ICT2588 demonstrates therapeutic activity against solid tumors and reduced potential for cardiovascular toxicity. Mol Pharm. 2014;11:1294–1300. doi: 10.1021/mp400760b. [DOI] [PubMed] [Google Scholar]
- 164.Agback P., Agback T. Direct evidence of a low barrier hydrogen bond in the catalytic triad of a serine protease. Sci Rep. 2018;8:10078. doi: 10.1038/s41598-018-28441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu S., Bugge T.H., Leppla S.H. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J Biol Chem. 2001;276:17976–17984. doi: 10.1074/jbc.M011085200. [DOI] [PubMed] [Google Scholar]
- 166.Braun G.B., Sugahara K.N., Yu O.M., Kotamraju V.R., Mölder T., Lowy A.M. Urokinase-controlled tumor penetrating peptide. J Control Release. 2016;232:188–195. doi: 10.1016/j.jconrel.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li S.J., Yuan C., Chen J.C., Chen D., Chen Z., Chen W.L. Nanoparticle binding to urokinase receptor on cancer cell surface triggers nanoparticle disintegration and cargo release. Theranostics. 2019;9:884–899. doi: 10.7150/thno.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li H., Wang P., Gong W.Y., Wang Q., Zhou J., Zhu W.H. Dendron-grafted polylysine-based dual-modal nanoprobe for ultra-early diagnosis of pancreatic precancerosis via targeting a urokinase-type plasminogen activator receptor. Adv Healthc Mater. 2018;7:1700912. doi: 10.1002/adhm.201700912. [DOI] [PubMed] [Google Scholar]
- 169.Matsumura K., Zouda M., Wada Y., Yamashita F., Hashida M., Watanabe Y. Urokinase injection-triggered clearance enhancement of a 4-arm PEG-conjugated 64Cu-bombesin analog tetramer: a novel approach for the improvement of PET imaging contrast. Int J Pharm. 2018;545:206–214. doi: 10.1016/j.ijpharm.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 170.Miller-Kleinhenz J., Guo X.X., Qian W.P., Zhou H.Y., Bozeman E.N., Zhu L. Dual-targeting wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 2018;152:47–62. doi: 10.1016/j.biomaterials.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zheng K., Li R., Zhou X.L., Hu P., Zhang Y.X., Huang Y.M. Dual actions of albumin packaging and tumor targeting enhance the antitumor efficacy and reduce the cardiotoxicity of doxorubicin in vivo. Int J Nanomedicine. 2015;10:5327–5342. doi: 10.2147/IJN.S84478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hong Y., Che S.M., Hui B.N., Yang Y.Y., Wang X.L., Zhang X.Z. Lung cancer therapy using doxorubicin and curcumin combination: targeted prodrug based, pH sensitive nanomedicine. Biomed Pharmacother. 2019;112:108614. doi: 10.1016/j.biopha.2019.108614. [DOI] [PubMed] [Google Scholar]
- 173.Liu X.F., Liu X.T., Sunchen S., Liu M.X., Shen C., Wu J.J. A novel tumor-activated ALA fusion protein for specific inhibition on the growth and invasion of breast cancer cells MDA-MB-231. Drug Deliv. 2017;24:1811–1817. doi: 10.1080/10717544.2017.1406560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahmed M.S.U., Salam A.B., Yates C., Willian K., Jaynes J., Turner T. Double-receptor-targeting multifunctional iron oxide nanoparticles drug delivery system for the treatment and imaging of prostate cancer. Int J Nanomedicine. 2017;12:6973–6984. doi: 10.2147/IJN.S139011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao N., Bozeman E.N., Qian W.P., Wang L.Y., Chen H.Y., Lipowska M. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics. 2017;7:1689–1704. doi: 10.7150/thno.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang J., Qian W.P., Wang L.Y., Wu H., Zhou H.Y., Wang A.Y. Functionalized milk-protein-coated magnetic nanoparticles for MRI-monitored targeted therapy of pancreatic cancer. Int J Nanomedicine. 2016;11:3087–3099. doi: 10.2147/IJN.S92722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gurka M.K., Pender D., Chuong P., Fouts B.L., Sobelov A., McNally M.W. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J Control Release. 2016;231:60–67. doi: 10.1016/j.jconrel.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu S.M., Huang G.N., Yuan R.M., Chen T.F. A uPAR targeted nanoplatform with an NIR laser-responsive drug release property for tri-modal imaging and synergistic photothermal-chemotherapy of triple-negative breast cancer. Biomater Sci. 2020;8:720–738. doi: 10.1039/c9bm01495k. [DOI] [PubMed] [Google Scholar]
- 179.Machulkin A.E., Garanina A.S., Zhironkina O.A., Beloglazkina E.K., Zyk N.V., Savchenko A.G. Nanohybride materials based on magnetite-gold nanoparticles for diagnostics of prostate cancer: synthesis and in vitro testing. Bull Exp Biol Med. 2016;161:706–710. doi: 10.1007/s10517-016-3490-3. [DOI] [PubMed] [Google Scholar]
- 180.Wong P., Li L., Chea J., Delgado M.K., Crow D., Poku E. PET imaging of 64Cu-DOTA-scFv-anti-PSMA lipid nanoparticles (LNPs): enhanced tumor targeting over anti-PSMA scFv or untargeted LNPs. Nucl Med Biol. 2017;47:62–68. doi: 10.1016/j.nucmedbio.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y.F., Zhang Y.Q., Ru Z.X., Song W., Chen L., Ma H. A ROS-responsive polymeric prodrug nanosystem with self-amplified drug release for PSMA (‒) prostate cancer specific therapy. J Nanobiotechnol. 2019;17:91. doi: 10.1186/s12951-019-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dostalova S., Cerna T., Hynek D., Koudelkova Z., Vaculovic T., Kopel P. Site-directed conjugation of antibodies to apoferritin nanocarrier for targeted drug delivery to prostate cancer cells. ACS Appl Mater Interfaces. 2016;8:14430–14441. doi: 10.1021/acsami.6b04286. [DOI] [PubMed] [Google Scholar]
- 183.Leach J.C., Wang A., Ye K.M., Jin S. A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int J Mol Sci. 2016;17:380. doi: 10.3390/ijms17030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ge Z.L., Guo L.J., Wu G.Q., Li J., Sun Y.L., Hou Y.Q. DNA origami-enabled engineering of ligand–drug conjugates for targeted drug delivery. Small. 2020;16:1904857. doi: 10.1002/smll.201904857. [DOI] [PubMed] [Google Scholar]
- 185.Ivanenkov Y.A., Machulkin A.E., Garanina A.S., Skvortsov D.A., Uspenskaya A.A., Deyneka E.V. Synthesis and biological evaluation of doxorubicin-containing conjugate targeting PSMA. Bioorg Med Chem Lett. 2019;29:1246–1255. doi: 10.1016/j.bmcl.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 186.Lv Q.Z., Yang J.C., Zhang R.S., Yang Z.M., Yang Z.T., Wang Y.J. Prostate-specific membrane antigen targeted therapy of prostate cancer using a DUPA–paclitaxel conjugate. Mol Pharm. 2018;15:1842–1852. doi: 10.1021/acs.molpharmaceut.8b00026. [DOI] [PubMed] [Google Scholar]
- 187.Feng X., Zhou Y.F., Xie X., Li M., Huang H., Wang L.L. Development of PSMA-targeted and core-crosslinked glycol chitosan micelles for docetaxel delivery in prostate cancer therapy. Mater Sci Eng C Mater Biol Appl. 2019;96:436–445. doi: 10.1016/j.msec.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 188.Lee S., Lee Y., Kim H., Lee D.Y., Jon S. Bilirubin nanoparticle-assisted delivery of a small molecule-drug conjugate for targeted cancer therapy. Biomacromolecules. 2018;19:2270–2277. doi: 10.1021/acs.biomac.8b00189. [DOI] [PubMed] [Google Scholar]
- 189.Xu B., Zhou F., Yan M.M., Cai D.S., Guo W.B., Yang Y.Q. PSMA-oriented target delivery of novel anticancer prodrugs: design, synthesis, and biological evaluations of oligopeptide-camptothecin conjugates. Int J Mol Sci. 2018;19:3251. doi: 10.3390/ijms19103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Levy O., Brennen W.N., Han E., Rosen D.M., Musabeyezu J., Safaee H. A prodrug-doped cellular trojan horse for the potential treatment of prostate cancer. Biomaterials. 2016;91:140–150. doi: 10.1016/j.biomaterials.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang S.N., Blaha C., Santos R., Huynh T., Hayes T.R., Beckford-Vera D.R. Synthesis and initial biological evaluation of boron-containing prostate-specific membrane antigen ligands for treatment of prostate cancer using boron neutron capture therapy. Mol Pharm. 2019;16:3831–3841. doi: 10.1021/acs.molpharmaceut.9b00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stuart C.H., Singh R., Smith T.L., D'Agostino R., Jr., Caudell D., Balaji K.C. Prostate-specific membrane antigen-targeted liposomes specifically deliver the Zn2+ chelator TPEN inducing oxidative stress in prostate cancer cells. Nanomedicine. 2016;11:1207–1222. doi: 10.2217/nnm-2015-0017. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Niaz M.O., Sun M., Ramirez-Fort M.K., Niaz M.J. Prostate-specific membrane antigen based antibody-drug conjugates for metastatic castration-resistance prostate cancer. Cureus. 2020;12 doi: 10.7759/cureus.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nessler I., Khera E., Vance S., Kopp A., Qiu Q.F., Keating T.A. Increased tumor penetration of single-domain antibody-drug conjugates improves in vivo efficacy in prostate cancer models. Cancer Res. 2020;80:1268–1278. doi: 10.1158/0008-5472.CAN-19-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Barve A., Jain A., Liu H., Jin W., Cheng K. An enzyme-responsive conjugate improves the delivery of a PI3K inhibitor to prostate cancer. Nanomedicine. 2016;12:2373–2381. doi: 10.1016/j.nano.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 196.Rahme K., Guo J.F., Holmes J.D. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. Methods Mol Biol. 2019;1974:291–301. doi: 10.1007/978-1-4939-9220-1_21. [DOI] [PubMed] [Google Scholar]
- 197.Shi S.J., Wang L.J., Han D.H., Wu J.H., Jiao D., Zhang K.L. Therapeutic effects of human monoclonal PSMA antibody-mediated TRIM24 siRNA delivery in PSMA-positive castration-resistant prostate cancer. Theranostics. 2019;9:1247–1263. doi: 10.7150/thno.29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pi F.M., Binzel D.W., Lee T.J., Li Z.F., Sun M.Y., Rychahou P. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13:82–89. doi: 10.1038/s41565-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu X.D., Wu J., Liu Y.L., Saw P.E., Tao W., Yu M. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11:2618–2627. doi: 10.1021/acsnano.6b07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tai W.Y., Li J.W., Corey E., Gao X.H. A ribonucleoprotein octamer for targeted siRNA delivery. Nat Biomed Eng. 2018;2:326–337. doi: 10.1038/s41551-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hao Z., Fan W., Hao J., Wu X., Zeng G.Q., Zhang L.J. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Deliv. 2016;23:874–881. doi: 10.3109/10717544.2014.920059. [DOI] [PubMed] [Google Scholar]
- 202.Jiao J., Zou Q., Zou M.H., Guo R.M., Zhu S., Zhang Y. Aptamer-modified PLGA nanoparticle delivery of triplex forming oligonucleotide for targeted prostate cancer therapy. Neoplasma. 2016;63:569–575. doi: 10.4149/neo_2016_410. [DOI] [PubMed] [Google Scholar]
- 203.Leconet W., Liu H., Guo M., Le Lamer-Déchamps S., Molinier C., Kim S. Anti-PSMA/CD3 bispecific antibody delivery and antitumor activity using a polymeric depot formulation. Mol Cancer Ther. 2018;17:1927–1940. doi: 10.1158/1535-7163.MCT-17-1138. [DOI] [PubMed] [Google Scholar]
- 204.Karandish F., Haldar M.K., You S., Brooks A.E., Brooks B.D., Guo B. Prostate-specific membrane antigen targeted polymersomes for delivering mocetinostat and docetaxel to prostate cancer cell spheroids. ACS Omega. 2016;1:952–962. doi: 10.1021/acsomega.6b00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Patil Y., Shmeeda H., Amitay Y., Ohana P., Kumar S., Gabizon A. Targeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA) Nanomedicine. 2018;14:1407–1416. doi: 10.1016/j.nano.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 206.Jing P., Cao S.S., Xiao S.L., Zhang X.Q., Ke S.Y., Ke F.M. Enhanced growth inhibition of prostate cancer in vitro and in vivo by a recombinant adenovirus-mediated dual-aptamer modified drug delivery system. Cancer Lett. 2016;383:230–242. doi: 10.1016/j.canlet.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 207.Chen Z.H., Penet M.F., Krishnamachary B., Banerjee S.R., Pomper M.G., Bhujwalla Z.M. PSMA-specific theranostic nanoplex for combination of TRAIL gene and 5-FC prodrug therapy of prostate cancer. Biomaterials. 2016;80:57–67. doi: 10.1016/j.biomaterials.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pathak R.K., Basu U., Ahmad A., Sarkar S., Kumar A., Surnar B. A designer bow-tie combination therapeutic platform: an approach to resistant cancer treatment by simultaneous delivery of cytotoxic and anti-inflammatory agents and radiation. Biomaterials. 2018;187:117–129. doi: 10.1016/j.biomaterials.2018.08.062. [DOI] [PubMed] [Google Scholar]
- 209.Rosenfeld L., Sananes A., Zur Y., Cohen S., Dhara K., Gelkop S. Nanobodies targeting prostate-specific membrane antigen for the imaging and therapy of prostate cancer. J Med Chem. 2020;63:7601–7615. doi: 10.1021/acs.jmedchem.0c00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mangadlao J.D., Wang X.N., McCleese C., Escamilla M., Ramamurthy G., Wang Z.Y. Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano. 2018;12:3714–3725. doi: 10.1021/acsnano.8b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kumar A., Mastren T., Wang B., Hsieh J.T., Hao G.Y., Sun X.K. Design of a small-molecule drug conjugate for prostate cancer targeted theranostics. Bioconjug Chem. 2016;27:1681–1689. doi: 10.1021/acs.bioconjchem.6b00222. [DOI] [PubMed] [Google Scholar]
- 212.Ngen E.J., Benham Azad B., Boinapally S., Lisok A., Brummet M., Jacob D. MRI assessment of prostate-specific membrane antigen (PSMA) targeting by a PSMA-targeted magnetic nanoparticle: potential for image-guided therapy. Mol Pharm. 2019;16:2060–2068. doi: 10.1021/acs.molpharmaceut.9b00036. [DOI] [PubMed] [Google Scholar]
- 213.Wu M., Zhao H.Y., Guo L., Wang Y.R., Song J., Zhao X.L. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 2018;25:226–240. doi: 10.1080/10717544.2017.1422300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wu M., Wang Y., Wang Y.R., Zhang M.B., Luo Y.K., Tang J. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int J Nanomedicine. 2017;12:5313–5330. doi: 10.2147/IJN.S136032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kaittanis C., Bolaender A., Yoo B., Shah N., Ouerfelli O., Grimm J. Targetable clinical nanoparticles for precision cancer therapy based on disease-specific molecular inflection points. Nano Lett. 2017;17:7160–7168. doi: 10.1021/acs.nanolett.7b04209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pooja D., Kulhari H., Adams D.J., Sistla R. Formulation and dosage of therapeutic nanosuspension for active targeting of docetaxel (WO 2014210485A1) Expert Opin Ther Pat. 2016;26:745–749. doi: 10.1080/13543776.2016.1180365. [DOI] [PubMed] [Google Scholar]
- 217.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., LoRusso P.M. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 218.Autio K.A., Dreicer R., Anderson J., Garcia J.A., Alva A., Hart L.L. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4:1344–1351. doi: 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pišlar A., Perišić Nanut M., Kos J. Lysosomal cysteine peptidases-molecules signaling tumor cell death and survival. Semin Cancer Biol. 2015;35:168–179. doi: 10.1016/j.semcancer.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 220.Rudzińska M., Parodi A., Soond S.M., Vinarov A.Z., Korolev D.O., Morozov A.O. The role of cysteine cathepsins in cancer progression and drug resistance. Int J Mol Sci. 2019;20:3602. doi: 10.3390/ijms20143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Turk B., Turk D., Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 222.Chauhan S.S., Goldstein L.J., Gottesman M.M. Expression of cathepsin L in human tumors. Cancer Res. 1991;51:1478–1481. [PubMed] [Google Scholar]
- 223.Novinec M., Lenarčič B., Turk B. Cysteine cathepsin activity regulation by glycosaminoglycans. Biomed Res Int. 2014;2014:309718. doi: 10.1155/2014/309718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Goretzki L., Schmitt M., Mann K., Calvete J., Chucholowski N., Kramer M. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Lett. 1992;297:112–118. doi: 10.1016/0014-5793(92)80339-i. [DOI] [PubMed] [Google Scholar]
- 225.Navab R., Pedraza C., Fallavollita L., Wang N., Chevet E., Auguste P. Loss of responsiveness to IGF-I in cells with reduced cathepsin L expression levels. Oncogene. 2008;27:4973–4985. doi: 10.1038/onc.2008.144. [DOI] [PubMed] [Google Scholar]
- 226.Dennemärker J., Lohmüller T., Mayerle J., Tacke M., Lerch M.M., Coussens L.M. Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene. 2010;29:1611–1621. doi: 10.1038/onc.2009.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang H., Wang R.B., Cai K.M., He H., Liu Y., Yen J. Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat Chem Biol. 2017;13:415–424. doi: 10.1038/nchembio.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sakurai F., Inoue S., Kaminade T., Hotani T., Katayama Y., Hosoyamada E. Cationic liposome-mediated delivery of reovirus enhances the tumor cell-killing efficiencies of reovirus in reovirus-resistant tumor cells. Int J Pharm. 2017;524:238–247. doi: 10.1016/j.ijpharm.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 229.Wilkinson R., Burden R., McDowell S., McArt D., McQuaid S., Bingham V. A novel role for cathepsin S as a potential biomarker in triple negative breast cancer. J Oncol. 2019;2019:3980273. doi: 10.1155/2019/3980273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fan W., Shi W., Zhang W.T., Jia Y.N., Zhou Z.Y., Brusnahan S.K. Cathepsin S-cleavable, multi-block HPMA copolymers for improved SPECT/CT imaging of pancreatic cancer. Biomaterials. 2016;103:101–115. doi: 10.1016/j.biomaterials.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fan W., Zhang W.T., Jia Y.N., Brusnahan S.K., Garrison J.C. Investigation into the biological impact of block size on cathepsin S-degradable HPMA copolymers. Mol Pharm. 2017;14:1405–1417. doi: 10.1021/acs.molpharmaceut.6b01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bratovš A., Kramer L., Mikhaylov G., Vasiljeva O., Turk B. Stefin A-functionalized liposomes as a system for cathepsins S and L-targeted drug delivery. Biochimie. 2019;166:94–102. doi: 10.1016/j.biochi.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 233.Dall E., Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. doi: 10.1016/j.biochi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 234.Ishii S. Legumain: asparaginyl endopeptidase. Methods Enzymol. 1994;244:604–615. doi: 10.1016/0076-6879(94)44044-1. [DOI] [PubMed] [Google Scholar]
- 235.Lewēn S., Zhou H., Hu H.D., Cheng T.M., Markowitz D., Reisfeld R.A. A legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang L.N., Chen S., Zhang M.N., Li N., Chen Y.N., Su W.J. Legumain: a biomarker for diagnosis and prognosis of human ovarian cancer. J Cell Biochem. 2012;113:2679–2686. doi: 10.1002/jcb.24143. [DOI] [PubMed] [Google Scholar]
- 237.Haugen M.H., Johansen H.T., Pettersen S.J., Solberg R., Brix K., Flatmark K. Nuclear legumain activity in colorectal cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0052980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ohno Y., Nakashima J., Izumi M., Ohori M., Hashimoto T., Tachibana M. Association of legumain expression pattern with prostate cancer invasiveness and aggressiveness. World J Urol. 2013;31:359–364. doi: 10.1007/s00345-012-0977-z. [DOI] [PubMed] [Google Scholar]
- 239.Li N., Liu Q.L., Su Q., Wei C.Y., Lan B., Wang J.Y. Effects of legumain as a potential prognostic factor on gastric cancers. Med Oncol. 2013;30:621. doi: 10.1007/s12032-013-0621-9. [DOI] [PubMed] [Google Scholar]
- 240.Ruan S.B., Xiao W., Hu C., Zhang H.J., Rao J.D., Wang S.H. Ligand-mediated and enzyme-directed precise targeting and retention for the enhanced treatment of glioblastoma. ACS Appl Mater Interfaces. 2017;9:20348–20360. doi: 10.1021/acsami.7b02303. [DOI] [PubMed] [Google Scholar]
- 241.Ruan S.B., Hu C., Tang X., Cun X.L., Xiao W., Shi K.R. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano. 2016;10:10086–10098. doi: 10.1021/acsnano.6b05070. [DOI] [PubMed] [Google Scholar]
- 242.Ruan S.B., Xie R., Qin L., Yu M.N., Xiao W., Hu C. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019;19:8318–8332. doi: 10.1021/acs.nanolett.9b03968. [DOI] [PubMed] [Google Scholar]
- 243.Song X., Wan Z.Y., Chen T.J., Fu Y., Jiang K.J., Yi X.L. Development of a multi-target peptide for potentiating chemotherapy by modulating tumor microenvironment. Biomaterials. 2016;108:44–56. doi: 10.1016/j.biomaterials.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 244.Yang Y.Y., Chen Q.L., Li S.Y., Ma W., Yao G.Y., Ren F. iRGD-mediated and enzyme-induced precise targeting and retention of gold nanoparticles for the enhanced imaging and treatment of breast cancer. J Biomed Nanotechnol. 2018;14:1396–1408. doi: 10.1166/jbn.2018.2592. [DOI] [PubMed] [Google Scholar]
- 245.Chen H.C., Rui W., You S.Y., Liu X.W., Huang J., Chen H.Y. Evaluation of the anti-cervical cancer effect of a prodrug: CBZ-AAN-DOX with hypoxic cell culture and tumor-bearing zebrafish models. Exp Cell Res. 2020;391:111980. doi: 10.1016/j.yexcr.2020.111980. [DOI] [PubMed] [Google Scholar]
- 246.Li Y., Niu Y.M., Zhu J.H., Gao C.C., Xu Q.W., He Z.Y. Tailor-made legumain/pH dual-responsive doxorubicin prodrug-embedded nanoparticles for efficient anticancer drug delivery and in situ monitoring of drug release. Nanoscale. 2020;12:2673–2685. doi: 10.1039/c9nr08558k. [DOI] [PubMed] [Google Scholar]
- 247.Lin S., Li T., Xie P.L., Li Q., Wang B.L., Wang L. Targeted delivery of doxorubicin to tumour tissues by a novel legumain sensitive polygonal nanogel. Nanoscale. 2016;8:18400–18411. doi: 10.1039/c6nr05870a. [DOI] [PubMed] [Google Scholar]
- 248.Zhou H.C., Sun H.J., Lv S.X., Zhang D.W., Zhang X.F., Tang Z.H. Legumain-cleavable 4-arm poly(ethylene glycol)-doxorubicin conjugate for tumor specific delivery and release. Acta Biomater. 2017;54:227–238. doi: 10.1016/j.actbio.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 249.Zhao T., Zhou H.L., Lei L., Guo C.Q., Yang Q., Gong T. A new tandem peptide modified liposomal doxorubicin for tumor “ecological therapy”. Nanoscale. 2020;12:3359–3369. doi: 10.1039/c9nr09585c. [DOI] [PubMed] [Google Scholar]
- 250.Luo M.M., Li Q., Wang D.M., Ge C.X., Wang J.J., Nan K.H. Fabrication of chitosan based nanocomposite with legumain sensitive properties using charge driven self-assembly strategy. J Mater Sci Mater Med. 2018;29:142. doi: 10.1007/s10856-018-6149-y. [DOI] [PubMed] [Google Scholar]
- 251.Jin H.Y., He Y., Zhao P.F., Hu Y., Tao J., Chen J. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics. 2019;9:265–278. doi: 10.7150/thno.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shi T.Y., Gu L.S., Sun Y., Wang S.L., You C.Q., Zhang X.Y. Enhanced legumain-recognition and NIR controlled released of cisplatin-indocyanine nanosphere against gastric carcinoma. Eur J Pharmacol. 2017;794:184–192. doi: 10.1016/j.ejphar.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 253.Gao Z.G., Shi T.Y., Li Y.J., You C.Q., Cheng K.W., Sun B.W. Mesoporous silica-coated gold nanoframes as drug delivery system for remotely controllable chemo-photothermal combination therapy. Colloids Surf B Biointerfaces. 2019;176:230–238. doi: 10.1016/j.colsurfb.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 254.He X.Y., Cao H.Q., Wang H., Tan T., Yu H.J., Zhang P.C. Inflammatory monocytes loading protease-sensitive nanoparticles enable lung metastasis targeting and intelligent drug release for anti-metastasis therapy. Nano Lett. 2017;17:5546–5554. doi: 10.1021/acs.nanolett.7b02330. [DOI] [PubMed] [Google Scholar]
- 255.Cao H.Q., Wang H., He X.Y., Tan T., Hu H.Y., Wang Z.W. Bioengineered macrophages can responsively transform into nanovesicles to target lung metastasis. Nano Lett. 2018;18:4762–4770. doi: 10.1021/acs.nanolett.8b01236. [DOI] [PubMed] [Google Scholar]
- 256.Wang J., Wang Y.Q., Cao H.Q., Wang H., Li J., Li Y.X. Orally delivered legumain-activated nanovehicles improve tumor accumulation and penetration for combinational photothermal-chemotherapy. J Control Release. 2020;323:59–70. doi: 10.1016/j.jconrel.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 257.Chang Y., Yao S., Chen Y.F., Huang J.J., Wu A.H., Zhang M. Genetically-engineered protein prodrug-like nanoconjugates for tumor-targeting biomimetic delivery via a SHEATH strategy. Nanoscale. 2019;11:611–621. doi: 10.1039/c8nr08951e. [DOI] [PubMed] [Google Scholar]
- 258.Eckhart L., Ballaun C., Uthman A., Kittel C., Stichenwirth M., Buchberger M. Identification and characterization of a novel mammalian caspase with proapoptotic activity. J Biol Chem. 2005;280:35077–35080. doi: 10.1074/jbc.C500282200. [DOI] [PubMed] [Google Scholar]
- 259.Galluzzi L., López-Soto A., Kumar S., Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–231. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 260.Liu X.J., He Y.J., Li F., Huang Q., Kato T.A., Hall R.P. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell. 2015;58:284–296. doi: 10.1016/j.molcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Huang Q., Li F., Liu X.J., Li W.R., Shi W., Liu F.F. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Feng X., Yu Y., He S.J., Cheng J., Gong Y.P., Zhang Z.X. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2017;385:12–20. doi: 10.1016/j.canlet.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhou M., Liu X.J., Li Z.H., Huang Q., Li F., Li C.Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143:921–930. doi: 10.1002/ijc.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bernard A., Chevrier S., Beltjens F., Dosset M., Viltard E., Lagrange A. Cleaved caspase-3 transcriptionally regulates angiogenesis-promoting chemotherapy resistance. Cancer Res. 2019;79:5958–5970. doi: 10.1158/0008-5472.CAN-19-0840. [DOI] [PubMed] [Google Scholar]
- 265.Shan J.D., Yuan L.M., Xiao Q.X., Chiorazzi N., Budman D., Teichberg S. TSP50, a possible protease in human testes, is activated in breast cancer epithelial cells. Cancer Res. 2002;62:290–294. [PubMed] [Google Scholar]
- 266.Zheng L., Xie G.F., Duan G.J., Yan X.C., Li Q.W. High expression of testes-specific protease 50 is associated with poor prognosis in colorectal carcinoma. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xu H.P., Shan J.D., Jurukovski V., Yuan L.M., Li J.H., Tian K.G. TSP50 encodes a testis-specific protease and is negatively regulated by p53. Cancer Res. 2007;67:1239–1245. doi: 10.1158/0008-5472.CAN-06-3688. [DOI] [PubMed] [Google Scholar]
- 268.Song Z.B., Bao Y.L., Zhang Y., Mi X.G., Wu P., Wu Y. Testes-specific protease 50 (TSP50) promotes cell proliferation through the activation of the nuclear factor κB (NF-κB) signalling pathway. Biochem J. 2011;436:457–467. doi: 10.1042/BJ20101780. [DOI] [PubMed] [Google Scholar]
- 269.Song Z.B., Wu P., Ni J.S., Liu T., Fan C., Bao Y.L. Testes-specific protease 50 promotes cell proliferation via inhibiting activin signaling. Oncogene. 2017;36:5948–5957. doi: 10.1038/onc.2017.198. [DOI] [PubMed] [Google Scholar]
- 270.Cao Q.H., Liu F., Li C.Z., Liu N., Shu M., Lin Y. Testes-specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer. BMC Cancer. 2018;18:94. doi: 10.1186/s12885-018-4000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Qiao W.L., Shi B.W., Han Y.D., Tang H.M., Lin J., Hu H.Y. Testes-specific protease 50 as an independent risk factor for poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 2018;15:8796–8804. doi: 10.3892/ol.2018.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang D.F., Zhao Y.Q., Wang Y.M., Rong Y., Qin H.S., Bao Y.L. 25-methoxyl-dammarane-3β,12β,20-triol and artemisinin synergistically inhibit MDA-MB-231 cell proliferation through downregulation of testes-specific protease 50 (TSP50) expression. Tumour Biol. 2016;37:11805–11813. doi: 10.1007/s13277-016-5037-7. [DOI] [PubMed] [Google Scholar]
- 273.Mi X.G., Song Z.B., Sun L.G., Bao Y.L., Yu C.L., Wu Y. Cardamonin inhibited cell viability and tumorigenesis partially through blockade of testes-specific protease 50-mediated nuclear factor-kappaB signaling pathway activation. Int J Biochem Cell Biol. 2016;73:63–71. doi: 10.1016/j.biocel.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 274.Lu S., Lin C.Z., Cheng X.B., Hua H.J., Xiang T., Huang Y. Cardamonin reduces chemotherapy resistance of colon cancer cells via the TSP50/NF-κB pathway in vitro. Oncol Lett. 2018;15:9641–9646. doi: 10.3892/ol.2018.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Qiao W.L., Hu H.Y., Shi B.W., Zang L.J., Jin W., Lin Q. Lentivirus-mediated knockdown of TSP50 suppresses the growth of non-small cell lung cancer cells via G0/G1 phase arrest. Oncol Rep. 2016;35:3409–3418. doi: 10.3892/or.2016.4763. [DOI] [PubMed] [Google Scholar]
- 276.Stauber R.H., Hahlbrock A., Knauer S.K., Wünsch D. Cleaving for growth: threonine aspartase 1—a protease relevant for development and disease. FASEB J. 2016;30:1012–1022. doi: 10.1096/fj.15-270611. [DOI] [PubMed] [Google Scholar]
- 277.Zhao Z.B., Wang L., Volk A.G., Birch N.W., Stoltz K.L., Bartom E.T. Regulation of MLL/COMPASS stability through its proteolytic cleavage by taspase1 as a possible approach for clinical therapy of leukemia. Genes Dev. 2019;33:61–74. doi: 10.1101/gad.319830.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Balkin D.M., Poranki M., Forester C.M., Dorsey M.J., Slavotinek A., Pomerantz J.H. TASP1 mutation in a female with craniofacial anomalies, anterior segment dysgenesis, congenital immunodeficiency and macrocytic anemia. Mol Genet Genomic Med. 2019;7:e818. doi: 10.1002/mgg3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Szecsi P.B. The aspartic proteases. Scand J Clin Lab Invest Suppl. 1992;210:5–22. [PubMed] [Google Scholar]
- 280.Tandon A.K., Clark G.M., Chamness G.C., Chirgwin J.M., McGuire W.L. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990;322:297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- 281.Pontious C., Kaul S., Hong M., Hart P.A., Krishna S.G., Lara L.F. Cathepsin E expression and activity: role in the detection and treatment of pancreatic cancer. Pancreatology. 2019;19:951–956. doi: 10.1016/j.pan.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yamamoto K., Kawakubo T., Yasukochi A., Tsukuba T. Emerging roles of cathepsin E in host defense mechanisms. Biochim Biophys Acta. 2012;1824:105–112. doi: 10.1016/j.bbapap.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 283.Zaidi N., Hermann C., Herrmann T., Kalbacher H. Emerging functional roles of cathepsin E. Biochem Biophys Res Commun. 2008;377:327–330. doi: 10.1016/j.bbrc.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 284.Ding H., Zhang F., Zhao C.C., Lv Y.L., Ma G.H., Wei W. Beyond a carrier: graphene quantum dots as a probe for programmatically monitoring anti-cancer drug delivery, release, and response. ACS Appl Mater Interfaces. 2017;9:27396–27401. doi: 10.1021/acsami.7b08824. [DOI] [PubMed] [Google Scholar]
- 285.Bhattacharya M., Sadeghi A., Sarkhel S., Hagström M., Bahrpeyma S., Toropainen E. Release of functional dexamethasone by intracellular enzymes: a modular peptide-based strategy for ocular drug delivery. J Control Release. 2020;327:584–594. doi: 10.1016/j.jconrel.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 286.Vezenkov L.L., Sanchez C.A., Bellet V., Martin V., Maynadier M., Bettache N. Structure-activity relationships of JMV4463, a vectorized cathepsinD inhibitor with antiproliferative properties: the unique role of the AMPA-based vector. ChemMedChem. 2016;11:302–308. doi: 10.1002/cmdc.201500457. [DOI] [PubMed] [Google Scholar]
- 287.Rahmani S., Budimir J., Sejalon M., Daurat M., Aggad D., Vives E. Large pore mesoporous silica and organosilica nanoparticles for pepstatin A delivery in breast cancer cells. Molecules. 2019;24:332. doi: 10.3390/molecules24020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Koller M., Hartmans E., de Groot D.J.A., Zhao X.J., van Dam G.M., Nagengast W.B. Data-driven prioritization and review of targets for molecular-based theranostic approaches in pancreatic cancer. J Nucl Med. 2017;58:1899–1903. doi: 10.2967/jnumed.117.198440. [DOI] [PubMed] [Google Scholar]
- 289.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Park B.H., Kim H.G., Jin S.W., Song S.G., Jeong H.G. Metallothionein-III increases ADAM10 activity in association with furin, PC7, and PKCα during non-amyloidogenic processing. FEBS Lett. 2014;588:2294–2300. doi: 10.1016/j.febslet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 291.Li X., Cao C.Y., Wei P., Xu M.Y., Liu Z.K., Liu L.Y. Self-assembly of amphiphilic peptides for recognizing high furin-expressing cancer cells. ACS Appl Mater Interfaces. 2019;11:12327–12334. doi: 10.1021/acsami.9b01281. [DOI] [PubMed] [Google Scholar]
- 292.Cao C.Y., Sheng D.L., Li X., Xue F.F., Liu L.Y., Zhong Y.P. Furin substrate as a novel cell-penetrating peptide: combining a delivery vector and an inducer of cargo release. Chem Commun. 2019;55:11872–11875. doi: 10.1039/c9cc02353d. [DOI] [PubMed] [Google Scholar]
- 293.Roveri M., Pfohl A., Jaaks P., Alijaj N., Leroux J.C., Luciani P. Prolonged circulation and increased tumor accumulation of liposomal vincristine in a mouse model of rhabdomyosarcoma. Nanomedicine. 2017;12:1135–1151. doi: 10.2217/nnm-2017-0430. (Lond) [DOI] [PubMed] [Google Scholar]