Abstract
Overexpression of the human epidermal growth factor 2 (HER2)/neu glycoprotein receptor in breast cancer is associated with increased risk of brain metastases, especially in patients with advanced disease. Improvements in the treatment of HER2-positive breast cancer has led to prolonged survival of patients with advanced disease, but the prevention and management of central nervous system metastases still poses unique clinical challenges given the associated morbidity and mortality of this site of disease. HER2-positive brain metastases are treated with surgery, radiation (stereotactic radiosurgery or whole brain radiotherapy), and systemic therapies, and are best managed by an experienced multidisciplinary team. The present article aims to provide an overview to our approach to treatment of HER2-positive brain metastases, including a review of agents with central nervous system activity, as well as management suggestions for several nuanced clinical scenarios.
Key words: HER2, HER2-positive, breast cancer, brain metastases, CNS metastases, treatment
Highlights
-
•
HER2-positive subtype of breast cancer is a risk factor for development of intracranial metastases in advanced disease.
-
•
Treatment paradigms for HER2-positive brain metastases include both local and systemic approaches.
-
•
Several anti-HER2-directed therapies that show intracranial activity should be used for systemic treatment of brain metastases.
Introduction
The incidence of brain metastases varies by breast cancer tumor subtype and stage. Overexpression of the human epidermal growth factor 2 (HER-2)/neu glycoprotein receptor is associated with increased risk of brain metastases, especially in patients with advanced disease. The increased incidence of brain metastases in the targeted anti-HER2 therapy era likely reflects prolonged survival, biologic predilection for the central nervous system (CNS), and a sanctuary site for metastasis in the CNS. For patients with HER2-positive breast cancer who undergo breast conservation treatment and receive systemic therapy, the risk of brain metastases at 10 years is around 12%.1 In contrast, ∼50% of patients with metastatic HER2-positive disease develop brain metastases during their illness.2 After a median follow up of 4 years in the HERA trial, the risk of a CNS event as initial site of recurrence was low (2% in both the trastuzumab and the observation groups, P = 0.55).3 Among the patients in the HERA trial who died, however, there was a trend towards decreased CNS events among patients originally treated with trastuzumab (47% versus 57%), though this difference did not meet statistical significance (P = 0.06), and event rates were very high in both arms. Unfortunately, no adjuvant approaches (including trastuzumab, pertuzumab, lapatinib, or ado-trastuzumab emtansine) have demonstrated an ability to prevent CNS recurrence. For example, in the ALLTO trial, no differences in incidence were observed for CNS as the first site of relapse (2% in both the lapatinib plus trastuzumab and the trastuzumab alone groups).4 Likewise, in the KATHERINE trial, 1486 patients with residual disease after neoadjuvant anti-HER2 therapy were randomized to continue trastuzumab or to switch to ado-trastuzumab emtansine (T-DM1). There was a significant reduction in metastasis events in the T-DM1 arm, however, there was no reduction in the risk of CNS as the first site of relapse, which occurred in ∼5% of patients in both arms, and comprised more than half of distant relapse events in the T-DM1 arm.5 The CNS was the only site of recurrence in 4.8% of patients in the T-DM1 arm versus 2.8% with trastuzumab. Median time to recurrence in the CNS was 17.5 months with T-DM1 and 11.9 months with trastuzumab.6 After 8 years of follow-up on the ExteNET trial of adjuvant neratinib for early-stage HER2-positive disease, the incidence of CNS disease was 1.3% in the neratinib group and 1.8% in those treated with placebo.7 In this article we will discuss our approach to the treatment of HER2-positive brain metastases.
Approach to treatment
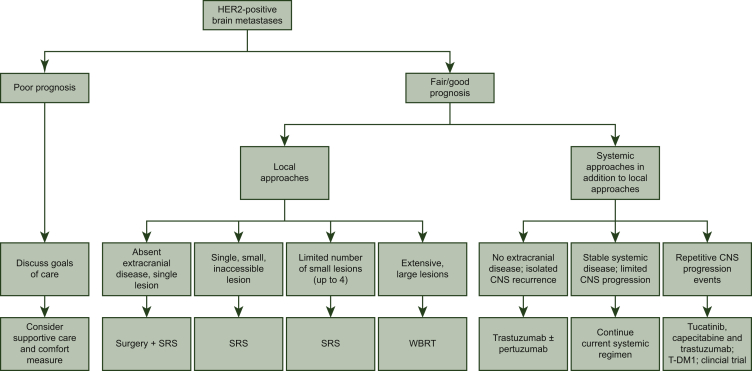
The first step in the treatment of HER2-positive brain metastases is to estimate the patient's overall prognosis to help inform the goals of care. As a result of the advances in anti-HER2-directed therapy, patients with HER2-positive brain metastases tend to have longer mean survival compared with patients with other subtypes of breast cancer with brain metastases, but survival times can still be highly variable.8
There are several treatment options for local control of HER2-positive brain metastases, namely surgery, radiation [stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT)], and systemic therapy. Decisions regarding these therapies and their sequence should be made with an experienced multidisciplinary team. Considerations for which local therapy to use upfront include the number, size, and location of the lesions, as well as the patient's symptoms, performance status, overall prognosis, and the status of systemic disease. In general, surgery is favored for patients with absent or controlled extracranial disease who have a single brain metastasis that is large or is associated with edema and mass effect. In patients who have not previously been diagnosed with metastatic disease, surgery also provides tissue confirmation of metastatic disease. We generally recommend postoperative radiation to the surgical cavity to improve local control. Upfront SRS is an alternative to surgery for single, small, or inaccessible tumors. For patients presenting with multiple brain metastases, we strongly favor SRS for those with a limited number of tumors. Randomized data support the use of SRS over WBRT in patients with up to four lesions, although as some patients with HER2-positive brain metastases can survive many years, we also consider SRS (versus systemic therapy) in patients presenting with more than four lesions. A randomized trial comparing hippocampal-sparing WBRT versus SRS in patients with 5 to 20 brain metastases is ongoing (NCT03075072). Although our preference is to avoid WBRT, given its acute and chronic toxicities (especially its adverse neurocognitive effects), it may be indicated in patients with an extensive number of intracranial tumors or multiple large tumors. For newly diagnosed patients with HER2-positive disease who have limited and asymptomatic or minimally symptomatic brain metastases, systemic therapy for upfront management can be considered. We also consider systemic therapy in patients with CNS disease progression despite prior local therapy.
When systemic therapy is offered for the management of HER2-positive brain metastases, there are several treatment options and combinations depending on the clinical scenario (see Table 1 and Figure 1).
Table 1.
Systemic therapy options for HER2-positive brain metastases
| Regimen | Reference | Trial population | Number of patients with CNS metastases | Key outcomes |
|---|---|---|---|---|
| Lapatinib plus capecitabine (EGF105084) | Lin et al., Clin Cancer Res. 20099 | HER2-positive breast advanced cancer, progressive brain metastases, prior trastuzumab, and cranial radiotherapy | 242 (lapatinib monotherapy); 50 (lapatinib plus capecitabine) | Lapatinib monotherapy: CNS ORR (≥50% volumetric reduction in 6% of patients, primary endpoint) ≥20% volumetric reduction in 21% of patients Lapatinib plus capecitabine: CNS ORR 20%, ≥20% volumetric reduction in 40% of patients |
| Lapatinib plus capecitabine | Pivot et al., J Clin Oncol. 201510 | HER2-positive metastatic breast cancer assigned to lapatinib plus capecitabine or trastuzumab-capecitabine | NA | Incidence of CNS metastases as first site of relapse 3% (8/251) for lapatinib + capecitabine versus 5% (12/250) |
| Neratinib plus capecitabine (TBCRC 022) | Freedman et al., J Clin Oncol. 201911 | HER2-positive advanced breast cancer, measurable, progressive brain metastases (92% after receiving CNS surgery and/or radiotherapy), lapatinib-naive and lapatinib-treated cohorts | 49 (37 lapatinib-naive, 12 lapatinib-treated) | CNS ORR 49% (95% CI, 32%-66%) in lapatinib-naive patients; CNS ORR 33% (95% CI, 10%-65%) in lapatinib-treated patients |
| Neratinib plus capecitabine (NALA) | Saura et al., J Clin Oncol. 202012 | Comparison of neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer with ≥2 HER2-directed regimens, including those with asymptomatic or stable (treated or untreated) CNS metastases | 101 (51 in neratinib plus capecitabine group) | Intervention for CNS disease, cumulative incidence 22.8% (15.5%-30.9%) versus 29.2% (22.5%-36.1%) |
| Tucatinib plus Trastuzumab plus Capecitabine (HER2CLIMB) | Lin et al., J Clin Oncol. 202013 | HER2-positive advanced breast cancer previously treated with trastuzumab, pertuzumab, and T-DM1, active or stable brain metastases (including untreated and previously treated) | 291 | Median CNS-PFS 9.9 months versus 4.2 months; median OS 18.1 months versus 12.0 months; ORR-IC 47.3% versus 20.0% |
| Pyrotinib plus capecitabine (PERMEATE) | Yan et al., J Clin Oncol. 202114 | HER2-positive metastatic breast cancer with brain metastases. Cohort A included patients with radiotherapy-naive BM and cohort B included radiotherapy treated BM | 78 | CNS ORR 74.6% (95% CI 61.6%-85.0%) in cohort A and 42.1% (95% CI 20.3%-66.5%) in cohort B |
| Trastuzumab Emtasine (KAMILLA) | Montemurro et al., Ann Oncol. 202015 | HER2-positive advanced breast cancer, prior HER2-targeted therapy, and chemotherapy, progressed on or after most recent treatment or within 6 months of adjuvant therapy. Untreated, asymptomatic BM or controlled brain disease treated with radiotherapy |
398 Total with baseline BM, 126 with measurable BM | CNS ORR 21.4% (95% CI, 14.6%-29.6%); clinical benefit rate 42.9% (95% CI, 34.1%-52.0%) |
| High dose trastuzumab and pertuzumab (PATRICIA) | Lin et al., J Clin Oncol. 202116 | HER2-positive advanced breast cancer, CNS metastases, and CNS progression despite prior radiotherapy | 39 | CNS ORR 11% (95% CI, 3%-25%); clinical benefit rate at 4 months 68%; clinical benefit rate at 6 months 51% |
| Trastuzumab deruxtecan (DESTINY-Breast01, CNS subgroup) | Jerusalem et al., J Clin Oncol. 202117 | HER-positive unresectable or metastatic breast cancer with baseline brain metastases | 24 | ORR 58.3%, mPFS 18.1 months, CNS response rate per investigators 50% (7 of 14 patients) |
BM, brain metastases; CI, confidence interval; CNS, central nervous system; HER2, human epidermal growth factor 2; mPFS, median progression free survival; ORR, overall response rate; PFS, progression-free survival; OS, overall survival; ORR-IC, intracranial objective response rate; T-DM1, ado-trastuzumab emtansine.
Figure 1.
Treatment pathway for HER2-positive brain metastases.
HER2, human epidermal growth factor 2; CNS, central nervous system; SRS, stereotactic radiosurgery; T-DM1, ado-trastuzumab emtansine; WBRT, whole brain radiation therapy.
For patients without any evidence of systemic disease but who have recurrence in the CNS
When there is evidence of metastasis in the CNS without evidence of extracranial disease, we treat the CNS progression with local therapy, surgery, and/or radiation. For systemic treatment, we acknowledge the lack of well-conducted prospective studies to guide the choice of treatment in addition to local therapy. In the absence of data, we often offer trastuzumab or trastuzumab plus pertuzumab (and endocrine therapy in patients with estrogen receptor-positive disease) after local treatment of the CNS disease (surgery and/or radiation) given their generally favorable toxicity profile; however, observation would also be reasonable. There are no studies that have examined the duration of systemic treatment in this setting, so the optimal duration of treatment is also individualized.
For patients with evidence of stable systemic disease who develop limited CNS progression amenable to SRS
When there is stable or responding extracranial disease with limited progression in the CNS, we will typically treat the progressive CNS lesions with SRS and continue the existing line of systemic therapy. Anti-HER2 monoclonal antibody treatment can be continued throughout radiation treatment. If a patient is receiving chemotherapy, this is held and resumed 1-2 weeks after completion of radiation treatment.
If patients develop repetitive CNS progression events over a short time interval (i.e. rapid disease trajectory), we will tend to defer SRS and treat with a switch of systemic therapy in hopes of both treating the index lesions and having potential efficacy on micrometastatic disease. In general, our approach to systemic therapy is relatively straightforward. First, for patients who have not yet received T-DM1, we use this agent. Second, for patients who have previously progressed on TDM-1, we favor the combination of tucatinib, capecitabine, and trastuzumab given the randomized data supporting increased CNS response rates, progression-free survival (PFS), and overall survival (OS) with use of this triplet in patients with brain metastases.18 In HER2CLIMB, among the 291 patients with brain metastases, the 1-year PFS was 24.9% in the tucatinib arm compared with 0% in the placebo-containing arm (hazard ratio 0.48, 95% confidence interval 0.34-0.69, P < 0.001).18 In an exploratory analysis among the patients with brain metastases, the median intracranial confirmed objective response rate was 47% versus 20%, P = 0.03, with a median duration of intracranial response of 6.8 months versus 3.0 months, and median OS of 18.1 versus 12.0 months, respectively.19 Where tucatinib is not available, lapatinib plus capecitabine20 or neratinib plus capecitabine12 can be substituted. Given the evidence of CNS efficacy of tucatinib in the HER2CLIMB trial, the ongoing COMPASS-RD trial will test tucatinib plus T-DM1 in the high-risk residual disease setting (NCT03975647) with the hope that it will improve invasive disease-free survival and, more specifically, CNS progression. In patients who have progressed in the CNS despite T-DM1 and HER2 tyrosine kinase inhibitors (TKIs), other systemic therapies can be considered (e.g. trastuzumab in combination with carboplatin or liposomal doxorubicin, or high dose trastuzumab with pertuzumab), though the evidence base is typically small non-randomized studies or case series. The efficacy of switching HER2 TKI is not well described in the literature, though responses have been described with both tucatinib and neratinib in HER2 TKI-pretreated patients.11,21
For patients with evidence of stable systemic disease but who develop more than minimal CNS progression (example, many small CNS lesions)
When patients have stable or responding systemic disease but progress in the CNS with numerous small CNS lesions not amenable to SRS, we offer WBRT (if not already given) versus systemic treatment. If the patient has not yet received tucatinib-capecitabine-trastuzumab, this is a clinical scenario in which we would consider its use with close follow-up, to try to delay the need for WBRT and its associated toxicities. For patients who have already received WBRT, we consider systemic therapy (tucatinib if not already received, a clinical trial, or other regimens as listed above and in Table 1).
For patients with extensive CNS progression
Although we try to avoid WBRT in most clinical situations, when a patient has extensive progression in the brain, its use is appropriate to prevent morbidity and mortality, especially given the high likelihood of response.
For patients with simultaneous CNS and extracranial progression
For patients who experience CNS and systemic progression at the same time, we follow guideline-directed treatment paradigms for advanced HER2-positive disease, but preferentially select regimens with reported CNS activity.
Other considerations
Clinicians should be mindful that in some instances, what appears radiographically to be progressive CNS disease after prior SRS may be radiation necrosis. If radiation necrosis is suspected, we will follow clinically with interval imaging. If radiation necrosis is symptomatic, we will typically begin with corticosteroids, and reserve bevacizumab for patients with chronic or refractory symptoms as a steroid-sparing measure.22
Investigational approaches
There are many clinical trials investigating new approaches to the management of HER2-positive CNS metastases, and where available, we encourage participation in such trials. Fundamental to these trials is a greater understanding of the biology of HER2-positive brain metastases. The blood brain barrier presents some drug delivery challenges, but in the setting of a disrupted barrier it appears that most agents penetrate the CNS, albeit sometimes in lower concentrations. Genetic divergence in breast cancer brain metastases and differences in the CNS tumor microenvironment, contributing to disease resistance, are areas of ongoing investigation.23
Conclusion
The treatment of HER2-positive brain metastasis is complex and requires input from an experienced multidisciplinary team. Our approach to the various clinical scenarios outlined above is meant to serve as a guideline, but is in no way exhaustive, and patient preference must always be considered. Research is underway to study existing treatments' activity in the CNS, as well as for the development of novel agents with CNS efficacy. As our patients with advanced HER2-positive disease live longer, it is of utmost importance to be able to develop more effective strategies to manage, and ultimately to prevent, CNS disease.
Acknowledgments
Funding
None declared.
Disclosure
ES declares consulting honoraria from Veracyte; EPW declares grant support from Genentech and consulting honoraria from Athenex, Carrick Therapeutics, Genentech, Genomic Health, Gilead, GlaxoSmithKline, Jounce, Leap, Lilly, Novartis, Seattle Genetics; NUL declares grant support from Genentech, Novartis, Merck, Pfizer, and SeaGen, royalties from Up-to-Date and consulting honoraria from Prelude Therapeutics, Denali Therapeutics, Olema Therapeutics, Aleta BioPharma, Affinia Therapeutics.
References
- 1.Arvold N.D., Oh K.S., Niemierko A. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–160. doi: 10.1007/s10549-012-2243-x. [DOI] [PubMed] [Google Scholar]
- 2.Pestalozzi B.C., Zahrieh D., Price K.N. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17(6):935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 3.Pestalozzi B.C., Holmes E., de Azambuja E. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14(3):244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart M., Holmes E., Baselga J. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34(10):1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Minckwitz G., Huang C.S., Mano M.S. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 6.Untch M., Geyer C.E., Jr., Huang C. Peripheral neuropathy, thrombocytopenia and central nervous system recurrence: an update of the phase III KATHERINE trial of post-neoadjuvant trastuzumab emtansine (T-DM1) or trastuzumab (H) in patients with residual invasive HER2-positive breast cancer. Ann Oncol. 2019;30:ix183–ix202. [Google Scholar]
- 7.Holmes F.A., Moy B., Delaloge S. AACR; 2021. Abstract PD3-03: Continued efficacy of neratinib in patients with HER2-positive early-stage breast cancer: final overall survival analysis from the randomized phase 3 ExteNET trial. [Google Scholar]
- 8.Sperduto P.W., Mesko S., Li J. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin N.U., Diéras V., Paul D. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 10.Pivot X., Manikhas A., Żurawski B. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 11.Freedman R.A., Gelman R.S., Anders C.K. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saura C., Oliveira M., Feng Y.H. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin N.U., Borges V., Anders C. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-Positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan M., Ouyang Q., Sun T. Pyrotinib plus capecitabine for HER2-positive metastatic breast cancer patients with brain metastases (PERMEATE): a multicenter, single-arm phase II study. J Clin Oncol. 2021;39(suppl 15):1037. [Google Scholar]
- 15.Montemurro F., Delaloge S., Barrios C.H. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350–1358. doi: 10.1016/j.annonc.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Lin N.U., Pegram M., Sahebjam S. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J Clin Oncol. 2021;39(24):2667–2675. doi: 10.1200/JCO.20.02822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerusalem G.H.M., Park Y.H., Yamashita T. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol. 2021;39(suppl 15):526. [Google Scholar]
- 18.Murthy R.K., Loi S., Okines A. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 19.Lin N.U., Krishnamurthy R., Anders C.K. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with previously treated HER2+ metastatic breast cancer with brain metastases (HER2CLIMB) J Clin Oncol. 2020;38(suppl 15):1005. [Google Scholar]
- 20.Petrelli F., Ghidini M., Lonati V. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer. 2017;84:141–148. doi: 10.1016/j.ejca.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Metzger Filho O., Leone J.P., Li T. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann Oncol. 2020;31(9):1231–1239. doi: 10.1016/j.annonc.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Boothe D., Young R., Yamada Y. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15(9):1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brastianos P.K., Carter S.L., Santagata S. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]