Abstract
Orally administered drug entities have to survive the harsh gastrointestinal environment, penetrate the enteric epithelia and circumvent hepatic metabolism before reaching the systemic circulation. Whereas the gastrointestinal stability can be well maintained by taking proper measures, hepatic metabolism presents as a formidable barrier to drugs suffering from first-pass metabolism. The pharmaceutical academia and industries are seeking alternative pathways for drug transport to circumvent problems associated with the portal pathway. Intestinal lymphatic transport is emerging as a promising pathway to this end. In this review, we intend to provide an updated overview on the rationale, strategies, factors and applications involved in intestinal lymphatic transport. There are mainly two pathways for peroral lymphatic transport—the chylomicron and the microfold cell pathways. The underlying mechanisms are being unraveled gradually and nowadays witness increasing research input and applications.
KEY WORDS: Drug delivery, Oral, Lymphatic transport, Drug absorption, Chylomicron, Microfold cell, Drug carriers, Nanoparticles
Abbreviations: ACQ, aggregation-caused quenching; ASRT, apical sodium-dependent bile acid transporter; AUC, area under curve; BCS, biopharmaceutics classification system; CM, chylomicron; DC, dendritic cell; DDT, dichlorodiphenyltrichloroethane; DTX, docetaxel; FA, fatty acid; FAE, follicle-associated epithelia; FRET, Föster resonance energy transfer; GIT, gastrointestinal tract; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; LDV, Leu-Asp-Val; LDVp, LDV peptidomimetic; M cell, microfold cells; MG, monoglyceride; MPA, mycophenolic acid; MPS, mononuclear phagocyte system; OA, oleate; PCL, polycaprolactone; PEG-PLA, polyethylene glycol-poly(lactic acid); PEI, polyethyleneimine; PLGA, poly(lactic-co-glycolic acid); PVA, poly(vinyl alcohol); RGD, Arg-Gly-Asp; RGDp, RGD peptidomimetic; SNEDDS, self-nanoemulsifying drug delivery system; SEDDS, self-emulsifying drug delivery system; SLN, solid lipid nanoparticles; TEM, transmission electron microscopy; TG, triglyceride; TPGS, D-α-tocopherol polyethylene glycol 1000 succinate; TU, testosterone undecanoate; WGA, wheat germ agglutinin; YCW, yeast cell wall
Graphical abstract
Orally administered molecular drugs and particulates could be absorbed alternatively via lymphatic transport. There are two main pathways—the chylomicron and M cell pathway—for efficient lymphatic transport.
1. Introduction
The oral route is usually preferable to other routes for drug delivery owing to safety considerations, convenience of administration, patient compliance, flexibility in dosage adjustment, suitability for long-term use and less strict requirements for oral dosage forms1,2. Orally administered drug formulations should go across the stomach, small intestine and colon in sequence. Drug absorption begins in the stomach but the very short gastric emptying time (0.5–2 h) limits the overall drug absorption extent and only weakly acidic or neutral drugs that present in absorbable molecular form are absorbed in fair amount1. The small intestine is generally regarded as the major absorption site for a majority of orally administered drug entities. The presence of villi and microvilli lining the intestinal walls enlarges the absorptive surface area to approximately 300–400 m2, thus greatly increasing the chances of absorption of numerous molecules3,4. A majority of drug entities entering the gastrointestinal tract (GIT) are absorbed by a passive diffusion or active transport mechanism in common via the enterocytes—the main type of cells dwelling on the apical surfaces of the enteric epithelia—and delivered through the portal vein to the liver and finally to the systemic circulation5,6. Commonly, drugs with suitable properties—for example, classification I drugs according to the Biopharmaceutics Classification System (BCS) that possess high solubility and high permeability—are able to achieve high bioavailability and satisfactory therapeutic efficacy7,8.
Despite the above-mentioned advantages, oral drug delivery is confronted with multiple challenges. The harsh gastrointestinal environment owing to secretion of gastric acid, various surfactants and enzymes tends to degrade or inactivate certain labile entities including both small molecular drugs and biomacromolecules9, 10, 11, 12. Some therapeutic compounds are stable and absorbable in the GIT, but are susceptible to first-pass hepatic metabolism and suffer from extremely low bioavailability13,14. Moreover, the enteric epithelia may also present as a formidable barrier to the entry of entities of less favorable properties15,16. BCS III (high solubility, poor permeability) and IV (poor solubility, poor permeability) drugs and biomacromolecules fall within this category. Despite advances in formulation and delivery system designs in recent years, oral delivery of labile and poorly permeable therapeutics remains a challenge4,17, 18, 19.
In this review, we will address an alternative pathway—the lymphatic pathway—of high promise for oral drug delivery. In contrast with the portal vein-to-liver pathway, lymphatic transport directs drugs first to the lymphatics and finally to the systemic circulation. There are so far two major targets—the chylomicrons (CMs) in enterocytes and the microfold cells (M cells) in Peyer's patches—clearly identified to attain efficient lymphatic transport20,21. Compared with portal transport, lymphatic transport has several advantages: 1) entities absorbed are delivered directly to the systemic circulation via the lymphatics and thus circumvent hepatic first-past metabolism; 2) the leaky capillaries of the lymphatics allow transport of macromolecules and particles that have relatively large sizes; 3) lymphatic delivery holds promise for the treatment of diseases afflicting the lymphatic systems such as human immunodeficiency virus (HIV) infection22, 23, 24. Intestinal lymphatic transport has been established as a new alternative platform for oral drug development.
2. Physiology related to enteric lymphatic transport
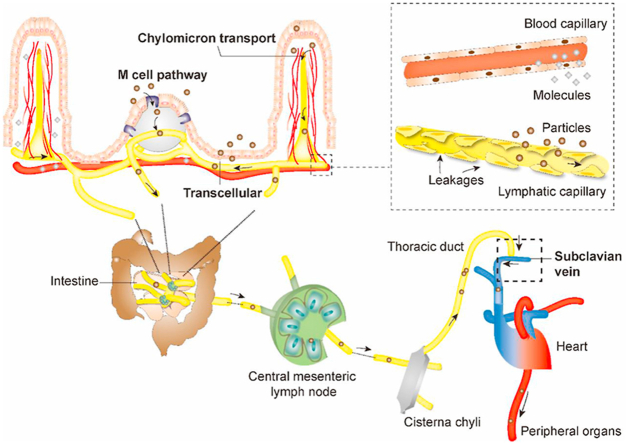
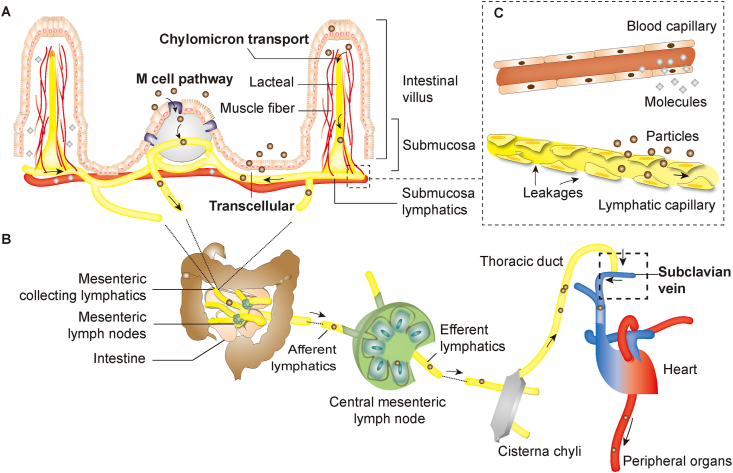
The intestinal lymphatic system is virtually a unidirectional system that drains interstitial fluids25,26. It is mainly comprised of lymphatics—sophisticated networks of lymph vessels, lymphatic nodes, and lymphoid organs (Fig. 1)27. The enteric lymphatics are generally blind-ended capillaries originating from organs or tissues and then proceed into less branched and more integrated pre-collecting lymphatics, collecting lymphatics, lymphatic trunks and lymphatic ducts in sequence (Fig. 1B)27,28. The collecting lymphatics, termed as afferent and efferent lymphatics for pre-nodal and post-nodal collecting lymphatics respectively, pass through the lymph nodes and converge into the thoracic duct or right lymph duct, and eventually reach the systemic circulation through the subclavian veins (Fig. 1B)29.
Figure 1.
Schematic presentation of the structures of the lymphatic systems involved in transport of particulates and macromolecules. (A) Intestinal lymphatics; (B) Lymphatic circulation; (C) Comparison of blood and lymphatic capillaries.
The organization of intestinal lymphatic vasculatures is of critical importance concerning drug and particle absorption. It contains mainly two independent subdivisions—one consisting of lacteals and submucosal lymphatics and the other muscular-layered lymphatics30. Each subdivision drains independently and converges concurrently into mesenteric collecting lymphatics30,31. Lacteals and submucosal lymphatics are at the forefront of intestinal lymphatic transport32,33. Lacteals are blind-ended lymphatic capillaries located in the center of each intestinal villus's lamina propria and surrounded by contractible smooth muscle fibers as shown in Fig. 1A,34. Lacteals connect with submucosal lymphatics, which successively converge into mesenteric collecting vessels, lymphatic nodes, cisterna chyli and thoracic duct and finally reach the systemic circulation through jugular and left subclavian vein27,30,35,36. CMs secreted in enterocytes to facilitate lipid absorption are transported through this pathway21,37, 38, 39. In comparison with peripheral blood capillaries, which have tight vessel walls, the leaky capillaries of lymphatics with fenestrations commonly of a few tens to hundreds of nanometers allow drainage of macromolecules and particulates of suitable sizes despite the much faster blood flow than lymph flow (500 vs.1)40, 41, 42 (Fig. 1C).
The M cells-to-lymphatics pathway is another important passage for lymphatic transport. Peyer's patches are a secondary lymphoid tissue mainly distributed in ileum24,43 (Fig. 1A). The surfaces of Peyer's patches are covered by follicle-associated epithelia (FAE), and the M cells comprise approximately 10% of the cell population of FAE in murine and less than 5% in human44. The function of M cells is to capture particles such as pathogens from the intestinal lumen and transport them to sub-FAE lymphoid tissues, where they are retained and destroyed45, 46, 47. Both less coated mucus layers and lower intracellular enzyme activities associated with M cells are beneficial to transport of particles. The particles entrapped in the ‘dome trap’—according to Qi et al.48—have the opportunities to escape and migrate via the lymphatics to the systemic circulation. The lymphatics surrounding the Peyer's patches play an important role in particle transport. Generally, they originate from the lacteals and submucosal lymphatic networks and develop into inter-follicular regions, forming basket-like shape by encircling the medium-basal part of each Peyer's patch49,50. The lymphatics always run along with blood vessels; both of them are abundant in peri-follicular and inter-follicular regions but rare in germinal centers except for a few tiny branches51,52. Each Peyer's patch has a drainage pathway with distinct pre-collectors reaching the same destination as the lacteals52. Muscular lymphatics distributing around the superior proportion of Peyer's patches may also contribute to drug delivery53. They drain independently and eventually converge into the mesenteric lymph for further transport. Peyer's patches open gates for the entry of particulates, though we still do not know the exact contribution to oral absorption54.
Pre-collecting vessels are the initial destinations for lymphatic drainage55. Their surface structures are also irregular, discontinuous and similar to small capillaries56. Nevertheless, larger lymphatics like lymphatic collecting vessels are not as permeable as these smaller absorptive vessels57. They generally have continuous zipper junctions and are covered by complete basement membranes, pumping fluid together with its content—absorbed particles or drug molecules—with valves and smooth muscles lined on the outer side of the vessels57,58. These large lymphatics further converge, to form important immune tissues—lymph nodes. Approximately 100–200 lymph nodes are found in the mesenteric system for proceeding lymphatics into more branchless ones. Central mesenteric lymph nodes are responsible for uniting trunks from different digestive organs to the gastrointestinal trunk59. And the gastrointestinal trunk along with lumbar trunk gathers in cisterna chyli, forming the root of thoracic chyle duct59. From the thoracic duct, the lymph flows into the systemic circulation via the subclavian veins, which commonly involve a single or multiple channels—that is, through the internal jugular vein, through the jugulovenous angle, directly into the subclavian veins or through multiple channels combined60.
It should also be noted that the behaviors of particulates in the gastrointestinal lumen may affect later stages of lymphatic drug transport. In the case of lipid-based delivery systems, the vehicles are transformed into secondary lyotropic vesicular and finally micellar vehicles with reinforced mucus-penetrating ability upon lipolysis by lipases of the constituting lipids in the GIT. The encapsulated drugs should be transferred efficiently without premature release through the sequential structural transformation. In the case of particulates that can be delivered across the mucus and the enteric epithelia intact, the drugs are meant to be secured with the vehicles throughout the whole transportation process.
3. The chylomicron pathway
3.1. Chylomicron structure and transport mechanism
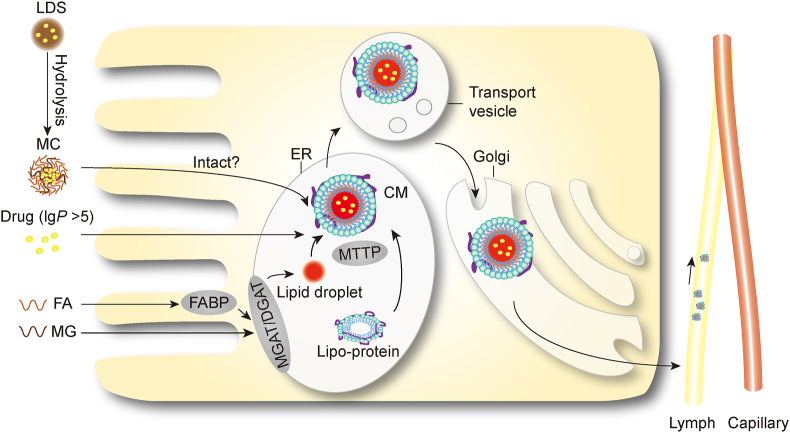
CMs are lipid spheroids formed in enterocytes in response to stimulation of lipid ingestion and digestion to facilitate transport of lipids, especially entities of high lipophilicity such as triglycerides (TG). The common structure of CM is comprised of TG and cholesterol esters in the cores and phospholipids, cholesterol and wrapping proteins on the surfaces (Fig. 2)61.
Figure 2.
Schematic presentation of the formation of chylomicrons (MC: micelle; LDS: lipid-based delivery system; FA: fatty acid; MG: monoglyceride; FABP: fatty acid binding protein; MGAT/DGAT: monoacylglycerol acyltransferase/diacylglycerol acyltransferase; MTTP: microsomal triglyceride transfer protein; ER: endoplasmic reticulum; CM: chylomicron). It is still unclear whether micelles could be taken up into the enterocytes intact.
Following lipolysis in the intestinal lumen, fatty acids (FA) and monoglycerides (MG), mainly 2-MG, are liberated and subsequently absorbed by enterocytes via a mechanism mediated by FA-binding membrane proteins and FA-related transporters dwelling on the apical membrane surfaces20,62, 63, 64. Long-chain FAs are able to be re-esterified and bind with lipoprotein—mainly apolipoprotein B—to form pre-CM65. After a series of endocellular processes, pre-CMs mature into CMs which are secreted into the mesenteric lymph and thereafter transferred via the lymphatics into the systemic circulation66. In the blood, with the aid of lipoprotein lipases, TGs constituting the CM cores are partially hydrolyzed while CMs evolve to form CM remnants which finally turn to the liver, bind with low-density lipoprotein (LDL) receptors or LDL receptor-related proteins, and are removed by lipoprotein lipases and hepatic lipase E66,67.
3.1.1. Prerequisites for drug transport via CM
It is envisioned that entities having CM-binding capacity may take advantage of the CM pathway to be delivered via the lymphatic route. Empirically, only a few highly lipophilic molecules with lgP values more than 5 and long-chain TG solubility more than 50 mg/g are transported via the CM pathway20,68. When lipid-based vehicles are involved, the vehicles are usually degraded first through lipolysis, transformed into micellar vehicles, absorbed by enterocytes and re-assembled with CM69.
LgP and TG solubility are the most frequently reported key factors for predicting drug lymphatic transport. Numerous drugs have been screened to testify these critical parameters. The lymphatic absorption of dichlorodiphenyltrichloroethane (DDT) with a lgP of 6.19 and TG solubility of 80 mg/g and halofantrine with a lgP of 8.5 and TG solubility >50 mg/mL was found to be 15% and 20% of the initial dose, respectively70. Vitamin D3 and E, which meet the lgP and TG solubility standards, exhibited high CM binding percentage (>50% of the original dose) and lymphatic delivery efficiency (15%–20% of the original dose)71. However, it is inaccurate to conclude that lgP and TG solubility are the sole parameters for prediction of lymphatic transport72. Some studies give contradictory results. For example, both penclomedine (lgP 5.48, TG solubility 175 mg/g) and CI-976 (lgP 5.83, TG solubility >100 mg/g) match the lgP and TG solubility criteria but indicate low lymphatic exposure70. A retrospective study revealed poor correlation between lymphatic bioavailability and lgP or lipid solubility but high correlation with drug–CM affinity71. In addition to lgP and TG solubility, factors such as hydrogen binding acceptors, polar surface area and pKa, among others, all play an indispensable role in CM binding and lymphatic drug transport73,74.
3.1.2. Critical influencing factors
Lipid type (e.g., chain lengths and saturation degree) is reported to be one of the essential factors that influence CM transport. The lipophilicity of short-, medium- and long-chain fatty acids is positively correlated to chain lengths, so is the CM binding capacity. It is reported that short-chain fatty acid prefers transport via the portal vein, whereas medium- or long-chain fatty acids are prone to be transported via the lymph75. Recovery of more self-nanoemulsifying drug delivery systems (SNEDDS) consisting of long-chain than medium-chain fatty acids in lymph adds evidence to support the chain length dependency76. An investigation on lipid-based vehicles with extraordinarily lipophilic halofantrine as a model drug indicated that the lymphatic affinity of the vehicles followed the order of C18 (15.8%)>C8–10 (5.5%)>C4 (2.22%)>C0 (0.34%)77. Increase in FA chain length correlates well with the higher drug transport efficiency. This could be attributed to the higher lipophilicity of long-chain FAs which have higher affinity to intracellular CMs and lipoproteins. Administered in an oil solution of 1,3-dioctanoyl-2-linoleyl-sn-glycerol, encoded as MLM to indicate the position of “medium–long–medium” chains, leads to increased portal absorption of halofantrine but similar levels of lymphatic transport as compared with the sunflower oil solution78. Nevertheless, following formulation of halofantrine into SNEDDS based on MLM and 1,3-dilinoyl-2-octanoyl-sn-glycerol, encoded as LML to indicate “long–medium–long” chain lengths, the lymphatic transport was found to be 17.9% and 27.4%, whereas the plasma availability was 56.9% (MLM) and 37.2% (LML), respectively79. It is implied that proper design of chain lengths and proportions may be a possible measure to alter the drug distribution between different pathways.
The food effect is another factor that may influence lymphatic transport and thereby plasma bioavailability. The oral bioavailability of a radio-labeled cannabinoid receptor agonist CRA13 is approximately 72%–75% in fed dogs vs. 8%–20% in fasted ones, with 43.7% through lymphatic transport80. Administration after a meal increases the total amount of lymphatic transport of halofantrine from 1.3% (administered before a meal) to 54% of the administered dose81. Sometimes, food intake may delay the absorption process but not necessarily alter the AUC and systemic bioavailability82. It should be noted that there are exceptions with regard to the food effect. For example, after ingestion of a high-fat meal, the AUC of DDT with high CM binding efficiency increases by 1.5-fold, whereas no significant difference is observed for diazepam with low CM binding efficiency under the same conditions; the lymphatic transport is higher for DDT but not for diazepam83. The food effect may be partly attributed to stimulation of CMs84. Ingestion of not only fat but also high contents of carbohydrates contribute to higher CM production84. Moreover, lipid ingestion induces secretion of bile salts which take part in formation of mixed micelles together with MGs and drugs, thus facilitating drug uptake and CM assembling within the enterocytes85,86. The observation of significantly reduced halofantrine output in lymph in bile duct-cannulated rats highlights the important role of multiple endogenous bile salts87,88.
3.2. Approaches for enhancement of chylomicron transport
3.2.1. Prodrug approaches
Designing of lipophilic prodrugs is an efficient approach to improve drug solubility in fat and thereby oral bioavailability owing to enhanced lymphatic transport89, 90, 91. The basic principle is to impart drug molecules with lipophilicity by conjugating to FAs, MGs or phospholipids21,92. The designed prodrugs enter the enterocytes, associate with lipoproteins, transport via the lymphatics and finally reach the systemic circulation in CM-encapsulated form93. In the case of testosterone, a hormonic drug with significant first-pass metabolism, its glyceride-mimicking prodrug bridged with a glyceride through self-immolative spacers remarkably enhances the plasma exposure of testosterone up to 90-fold that of testosterone undecanoate (TU), a commercial testosterone product39. By collecting the mesenteric fluid from lymph-cannulated rats, prodrugs could be recovered in as high as 28% of the administered dose in comparison with 0% for pure testosterone and 1.9% for TU39. This verifies the high lymphatic transport efficiency of the triglyceride-mimetic prodrug strategy. The significantly enhanced plasma concentration and AUC of free testosterone are indicative of the contribution of lymphatic transport.
3.2.2. Oil induction to stimulate chylomicron production
Oil ingestion has been known to induce the formation and secretion of CMs, which may be employed to facilitate lymphatic transport of co-administered or pre-administered drugs, provided that the drugs readily associate with CMs upon contact83,94. An immunomodulator tacrolimus, for example, could achieve 15 times higher absorption rate constant in lymphatics vs. blood following delivery with cacao or sunflower butter together95. Moxidectin, if co-administrated with sunflower oil, could realize 98% increase in bioavailability as a result of enhanced intestinal lymphatic transport96. Moreover, both lymphatic output and transport efficiency of lutein could be significantly enhanced when administered with safflower oil and certain monoglycerides or diglycerides as vehicles97. The oil type may influence the CM production and lymphatic drug transport process. Among the above mentioned oil types, safflower oil performed the worst for lymphatic transport of lutein, while the others separately achieved 70%–211% improvement in comparison with the safflower group94. Induction of CM by oil ingestion could be reinforced by increasing the oil dosage, but not necessarily in a linear correlation and with possible saturation98. Fat saturation affects the number and size of CMs produced84,94. The numbers may eventually influence the output of cargoes, while the size may affect CM metabolism in the systemic circulation99,100. For soybean oil, its dose effect on carotenoid absorption is only predominant from 0 to 32 g; beyond that, no significant enhancement effect can be observed. For more lipophilic drug halofantrine, the absorption may be limited when the oil dose exceeds 250 mg/kg and reaches a plateau when the oil dose reaches 1000 mg/kg101.
It is of note that not all types of oils benefit lymphatic transport of the payloads. For instance, sesame oil limits the absorption of cholesterol by inhibiting the micellar solubilization process102,103. Corn oil and poly-unsaturated fatty acid such as linoleic acid or linolenic acid disfavor absorption of some carotenoids probably due to the poor lipolysis capability and lower extent of micellarization104,105. Therefore, it is important to select a proper type of oil for higher lymphatic transport efficiency.
3.2.3. Lipid-based delivery system
Though oil ingestion promotes lymphatic absorption of drugs to some extent, in vivo dispersity may still pose a hurdle for more efficient drug absorption106. Hence, lipid-based delivery systems are designed and several lipid-based carriers such as nano/micro emulsions, self-emulsifying drug delivery system (SEDDS), solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) and liposomes all have shown increased bioavailability for various drug compounds by promoting lymphatic transport15,107,108. Upon lipid hydrolysis, FA, MG and bile salts form drug-loaded mixed micelles together. The formed micellar structures can be taken up intact by enterocytes or disassociate to release the payloads. Then free molecules are able to be transported via the portal vein, while the particles may associate with CMs and then be transported via the lymphatics109,110. These vehicles are trafficked to endoplasmic reticulum and Golgi complexes via the apical recycling endosome or common recycling endosome routes after internalization, subsequently stimulating the formation of CM and exocytosis of the carriers111,112.
Despite numerous results in support of lipid-based carriers for enhancement of lymphatic transport and systemic bioavailability, controversies exist with regard to transport mechanism because of the complexity of gastrointestinal physiology and presence of multiple transport pathways113,114. CM flow suppressors such as cycloheximide which inhibit the synthesis of proteins and specifically block the transport of CM have been utilized to evaluate the contribution of CM transport115. For example, the peak plasma concentration (Cmax) and AUC of raloxifene hydrochloric acid, as encapsulated in SLNs, decrease by 34% and 29%, respectively after pre-treatment with cycloheximide116. For docetaxel lipid-based nanocapsules, nearly no drugs could be detected in plasma with cycloheximide pre-treatment117. Significant decrease in Cmax and AUC was also observed for carvedilol liposomes as a result of cycloheximide pretreatment, confirming the contribution of the CM pathway to overall bioavailability118.
Additionally, other methods have been adopted for assessment of the CM pathway. Electron microscopy was utilized to visualize the formation of CM in a Caco-2 cell model119. Formation of CMs of sizes ranging from 70 to 150 nm on the basolateral side was observed following oral administration of lipid-based polymethoxyflavones particles with three-fold enhancement of absorption119. Lymphatic ligation is an alternative method, but it should be noted that lymph collected this way may be a mix that includes the lymph derived from the M cell pathway120. The monitoring of TGs and drugs in lymph provides indirect information to help explain the transport mechanisms. The fact that saquinavir delivered with Cremophor/oleic acid mixed micelles, D-α-tocopherol polyethylene glycol 1000 succinate (TPGS)/oleic acid mixed micelles and oleic acid microemulsions has the same transporting rate as TG may work to support that the drug was delivered in association with CMs121. However, the fraction of lymphatic transport only accounts to 0.025%–0.05% bespeaks the limited or negligible contribution of lymphatic transport. The finding was echoed by Guan et al.‘s results122 that the cumulative percentage of lymphatic transport was only 0.25% and 0.73% for cyclosporine A delivered by liposomes and bilosomes, respectively. It is implied that though lipid-based vehicles facilitate lymphatic drug transport, the contribution of lymphatic transport to overall bioavailability may be negligible for some drugs. Overall, there are controversies regarding the underlying mechanisms of facilitated lymphatic transport by lipid-based vehicles. It seems that multiple mechanisms may be involved.
4. M cell pathway
4.1. Transport mechanism
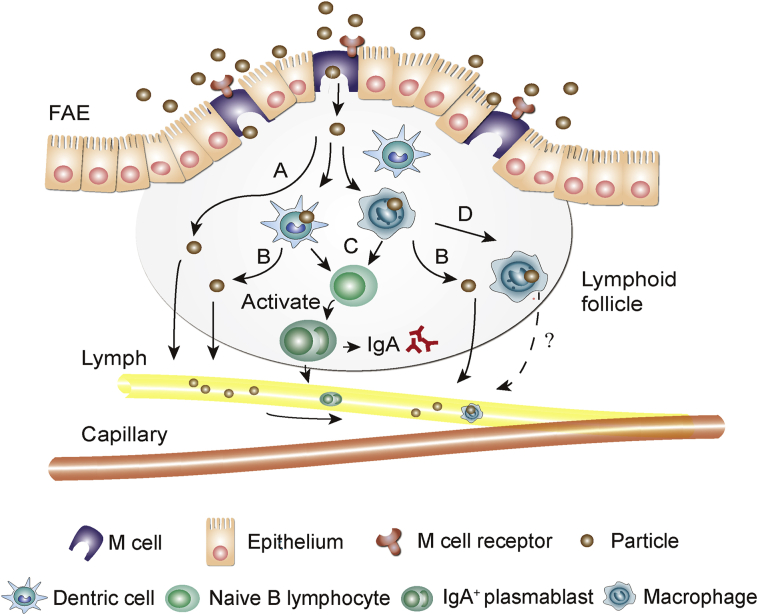
Peyer's patches are important lymphoid tissues in the gut. The epithelia on the follicle surfaces, termed as FAE, takes a “dome shape”23,123 (Fig. 3). The M cells located in FAE are capable of taking up and transporting antigens from the lumen side to the underlying lymphoid follicles where they are eliminated through induction of immune responses43,124. The FAE are also responsible for uptake of a small fraction of orally ingested particulates; this is always regarded as a potential portal for the entry of particulates into the systemic circulation125. Although M cells merely occupy approximately 10% of the cell population of FAE in murine and FAE is mainly located to the ileum, the high transcytosis capacity of M cells compensates for the limited cell population126,127.
Figure 3.
Schematic presentation of cross-section structure of the Peyer's patch and the M cell transport pathway. (A) Particles bypassing immune cells; (B) Particles escaping from immune cells; (C) Particles entrapped in dendritic cells or macrophages inducing immune response; (D) Particles being entrapped within macrophages.
Following uptake by M cells, antigens or pathogens are transported to the pockets located to the other side of the M cells, where antigen-presenting dendritic cells dwell and keep alert to take over128,129. After antigen sampling and presenting, immune responses are initiated. With regard to the fate of non-antigenic particulates, there are several possibilities: 1) bypassing immunocytes; 2) escaping immunocytes while eliciting immune responses; 3) escaping immunocytes without eliciting immune responses; 4) encampment and ending up in immunocytes23 (Fig. 3). It is not hard to make a judgment on the fate of internalized particulates—ending up in the immune system while eliciting immune responses simultaneously, or being transported to distant organs and tissues. Researches have been done to track the translocation of particles. For instance, bioengineered glucan microparticles, or yeast cell wall (YCW) microparticles, are able to remain intact for extended times after uptake by macrophages and distribute in organs of the mononuclear phagocyte systems (MPS) such as the liver, lungs and spleen130. Another study131 reported that microparticles could migrate to MPS organs or macrophage-enriched inflammatory tissues via lymph with the aid of macrophages as delivery vehicles. However, factors that influence transport via the M cell pathway are still inexplicit, except for the observation that larger particle size favors accumulation, rather than escape, of particulates in Peyer's patches132. The M cell pathway presents as a potential portal for particulates to enter the systemic circulation and be subsequently delivered to distant organs or tissues with targeting promises. Importantly, lymphatic transport is also of high interest for oral vaccination or treatment of diseases inflicting the lymphatic systems133.
4.2. Strategies for the M cell pathway
As discussed above, the M cells readily recognize pathogens through recognizing the specific ligands presenting on the surfaces of pathogen particles. Therefore, particles can be engineered to mimic specific ligands to reinforce uptake and transport through this pathway. M cells also take up unadorned particles but probably in less amount and a non-specific way. Active targeting to M cells based on decoration with ligands, such as peptide/protein, non-peptide/protein and microbe-derived ligands, proves to be an efficient strategy for enhancement of lymphatic transport of particulates134, 135, 136, 137. The mostly investigated ligands include lectins, peptidic ligands such as RGD (Arg-Gly-Asp) and glucans.
Lectins, mainly extracted from plants, bind specifically to the carbohydrate residues of proteins or lipids on M cell surfaces138. Ulex europaeus 1 (UEA1), for instance, specifically recognizes α-linked fucose residues and binds nearly exclusively to the apical surfaces of M cells139. Wheat germ agglutinin lectin (WGA), another member of the lectin family that has better stability and lower immunogenicity, facilitates nanoparticle uptake and transport with more efficiency and safety140,141. Decoration of liposomes with WGA stabilizes the vehicles, which brings about extra benefits for labile payloads142. However, it is of note that WGA does not target M cells only—it also binds and facilitates penetration of particulates across the biomembranes via enterocytes143. Tomato lectins bind M cells too, but it is not a good candidate for M cell targeting owing to its overly high affinity with villi144.
Peptidic ligand RGD recognizes α5β1 integrin overexpressed on M cells136,145,146 but undergoes gradual degradation in the GIT, thus calling for synthesis of more stable non-peptidic analogues such as RGD peptidomimetic (RGDp)147. RGDp-decorated poly (lactic-co-glycolic acid) (PLGA) nanoparticles was prepared to testify the efficacy of non-peptidic ligands148. It is observed that RGDp has the same affinity with M cells as RGD but reinforced therapeutic efficacy owing to improved stability in the GIT147. The pair of LDV (Arg-Gly-Asp) and LDV peptidomimetic (LDVp) is another similar example22,149.
YCW is primarily composed of β-1,3-d-glucan that binds with dectin-1 expressed on M cells and its submucosal macrophages150. Using YCW microparticles to devise nano-in-micro carriers achieves dual targeting to M cells on FAE surfaces and macrophages in sub-FAE pockets, leading to high drug accumulation in Peyer's patches151. Interestingly, bioimaging to reveal the M cell transport pathway could be achieved by electronically loading quantum dots or organic fluorescent nanoparticles into YCW microparticles131. Flagellin from Salmonella enteritidis was also selected as superior ligands to decorate nanoparticles to achieve higher immune responses152.
Other M cell specific markers such as glycoprotein 2, claudin 4, C5aR (C5a receptor), PrPC (cellular prion protein) and annexin A5 have shown potential to be applied in M cell targeting as well153, 154, 155, 156.
4.3. Factors influencing M cell uptake
Except for active targeting strategy, some physicochemical properties including particle size, surface hydrophobicity, surface charge and particle shape significantly influence M cell uptake157. These factors must be taken into account when designing delivery systems.
4.3.1. Particle size
Particle size is one of the critical characteristics that affect the in vivo fate of nanoparticles. The size effect was investigated by tracking the translocation of polycaprolactone (PCL) nanoparticles labeled by near-infrared fluorescent probes with aggregation-caused quenching (ACQ) properties48,158. After oral administration, more particles of the 600 and 2000 nm groups were transported via the lymphatics than the smaller size groups (50 and 200 nm), evidenced by quantification of particles in lymph collected through mesenteric lymphatic duct cannulation48,158. The particle size effect of nanocrystals was investigated by employing similar ACQ-based bioimaging strategy. Following oral administration of nanocrystals hybridized with ACQ probes, nanocrystals with larger sizes (550 and 1100 nm) showed higher retention in Peyer's patches probably due to the slower dissolution rate and more integral structures as compared with the smaller ones (280 nm)159. Similar results were observed for cyclosporine A ultrafine particles with 550 nm particles being taken up by M cells in more amount than 250 nm particles160. More findings concerning glucan, latex and organosilica particles add more evidence to support size dependency in lymphatic transport161, 162, 163. It seems that within a certain limit—for example, less than 3 μm, larger particles tend to be stuck in Peyer's patches due to various reasons164. After uptake by M cells, particles below 1 μm can be transported to lymphatics and systemic circulation, while particles larger than 5 μm are mainly trapped in Peyer's patches165,166.
Notwithstanding numerous researches on this topic, there are still controversies with regard to the effect of particle size and distribution167,168. A study on organosilica particles concluded that 95–200 nm nanoparticles were more optimal for Peyer's patches163. It is hard to compare the findings reported in different articles because of utilization of different animal models, material diversity and different methods employed169.
4.3.2. Surface hydrophobicity
PEGylation is an efficient measure to promote mucus-penetrating ability of nanoparticles and thus enhance oral absorption170. However, M cells with thinner mucus coating are more preferable for particles with hydrophobic surfaces3. Coating rifampicin-gantrezAN-119 nanoparticles with a hydrophobic polymer ethyl cellulose leads to enhanced absorption of the particles, as evidenced by histological images171. Similar results of enhanced bioavailability and distant organ accumulation were observed with rifampicin gantrez nanoparticles coated with another hydrophobic polymer—polyethylene sebacate172. Another explanation for higher M cell transport efficiency is that the relatively hydrophobic surfaces favor protein binding in the intestinal fluid, which in turn affects its transcytosis process173.
4.3.3. Surface charge
It is reported that positively charged particles are easier to be taken up than negatively charged ones owing to the electrostatic affinity with intestinal mucus or cell membranes174. The systemic and lymphatic exposure of positively charged polyethylene glycol-poly (lactic acid) (PEG-PLA) nanoparticles is higher than neutral and negatively charged particles, though the lymphatic pathway is not predominant175. Decoration of SLNs with positively charged hydroxypropyl trimethylammonium chloride chitosan enhances M cell uptake and accumulation in Peyer's patches176. The same conclusion was drawn for positively charged chitosan-coated liposomes as vehicles for DNA vaccine delivery as well177,178. Notably, there are also controversial reports on the effect of surface charges. For instance, it was reported that neutral surfaces performed better for accumulation of 130 nm and 950 nm polystyrene particles in Peyer's patches3, while negatively charged poly (vinyl alcohol) (PVA)-coated PLGA nanoparticles showed higher accumulation in Peyer's patches compared to polyethyleneimine (PEI)-coated positively charged ones164. Negatively charged gold nanoparticles had higher absorption than positively charged ones127. All these results imply that there may be interplay between surface charges and other factors such as hydrophobicity, stability, and lipolysis rate179.
4.3.4. Particle shape
Particle shape is an important physicochemical factor that influences multiple biological behaviors of drug delivery system in vivo. Regarding oral delivery, variation in particle shape leads to deviated biological interactions, which result in deviated dissolution, permeation, cellular translocation, distribution and ultimately drug bioavailability and therapeutic effect180. For instance, the M cell uptake efficiency is subjected to particle shape variations, as evidenced by cellular uptake and trans-monolayer transport in Caco-2/Raji-B cell models157. Results showed that rod- and disc-shape particles achieved 15% and 18% transport of total particles, respectively, while the spherical ones only reached a maximum of approximately 11%. The shape effect was also proved by in vivo investigation181. Compared to the spherical-shaped particles (0.98%), rod-shaped particles attained higher extent of lymphatic transport (1.75%), as evidenced by semi-quantification based on Föster resonance energy transfer (FRET) bioimaging. Nevertheless, the underlying mechanisms behind the shape effect remain elusive and it is difficult to evaluate this sole parameter without altering other factors182.
5. Paracellular and transcellular pathways
In addition, both the paracellular and transcellular pathways whose role in lymphatic drug transport has not been clearly identified also hold promise for further investigation. Intercellular tight junctions that are built of multiple protein complexes constitute the main barriers to drug transport via the paracellular route183. Many absorption enhancers like ethylenediaminetetraacetic acid (EDTA), chitosan, bile salts, sodium caprate and some synthetic peptides are able to open tight junctions, thus creating tentative apertures for the entry of macromolecules and particles of appropriate sizes184,185. By co-delivery of insulin with an absorption enhancer sodium caprate in microcontainers, the oral bioavailability of insulin could be enhanced partially owing to the contribution of the paracellular route186. Generally, the paracellular apertures only allow the transport of macromolecules with suitable particle sizes, such as insulin with a hydrodynamic radius of approximately 2 nm at pH 7.4 and fluorescently labeled dextran with a Stokes radius of approximately 1.3–2 nm187,188. However, it is controversial whether particles with larger sizes are able to penetrate the paracellular passages. A report suggested that 5 nm gold nanoparticles could transport through cellular intervals189. Even polyisobutyl cyanoacrylate nanocapsules with a size of approximately 150 nm could be observed in intercellular spaces of the intestinal villi tip by SEM190,191. However, evidence showed that the maximum pore size could only be expanded to approximately 20 nm even with efficient permeation enhancers192. It is highly probable that intact particles could hardly pass across the intercellular barriers.
The fate of macromolecules or particles beyond enterocyte uptake may be different. It is reported that macromolecules such as insulin and GLP-1 are preferably transported through the portal vein, thus being able to imitate the secretion under physiological conditions193, while particles with larger sizes are prone to be transported further via the lymphatic capillaries owing to the obstruction by blood vessel walls194. Fluorescently labeled dextrans of a series of molecular weight provide a useful tool to test this hypothesis. The transport via lymphatics overwhelms that via blood vessels when the molecular weight of dextrans increases to above a threshold195. However, it should be attributed to reduced uptake by blood capillaries, rather than elevated transport via lymphatics195.
Another important absorption pathway is the transcellular pathway. Though it is a general route for oral absorption of small molecules, the possibility of absorption of particles through this pathway should not be excluded. If nanoparticles are able to be absorbed intact, there are chances for them to be transported into the systemic circulation, and they may preferably choose the lymphatic pathway because of the fenestrated walls of the lymphatics196. This is especially the case for small-sized PCL nanoparticles (50 nm) which are taken up more profoundly by in vitro Caco-2 cell models and transported more efficiently via the lymphatics158. Except for this leakage-aided process, receptor-mediated transport via lymphatics was also proposed197. High lymphatic absorption of exenatide achieved by dextran-coated particles may have taken advantage of the specific binding between dextran and receptors expressed on the surfaces of lymphatics198,199.
Notably, all the transport pathways mentioned in this part are difficult to verify because of a lack of dynamic monitoring and reliable quantification measures. Nevertheless, all of them may play a role in oral absorption of biomacromolecules and particulates because of the large absorption area and preliminary observation of intracellular behaviors.
6. Application of lymphatic transport in drug delivery
Based on increasing understanding of involved mechanisms, lymphatic transport has become an important approach in drug discovery. Table 139,171,200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229 summarizes examples of application of lymphatic transport in oral drug delivery for reduced first-pass effect, facilitated oral absorption, distant organ targeting and lymphatic associated treatment.
Table 1.
Summary of application of intestinal lymphatic transport.
| Model drug | Prodrug/vehicle | Result | Ref. |
|---|---|---|---|
| Circumvent first-pass metabolism | |||
| Testosterone | Testosterone undecanoate | Absolute oral bioavailability (% lymphatic transport) of two TU products: 3.25% (91.5%); 2.88% (99.7%) | 200 |
| Testosterone | TST-TG | AUC: 1.8- and 2.6-fold higher than TU at 8 and 4 mg/kg oral dose | 201 |
| Testosterone | TST-acetal self-immolative group-TG; TST-trimethyl group-TG | AUC: 10–90-fold higher than TU | 39 |
| Docetaxel | DTX-S-OA | Bioavailability: 6.2- and 2.0-fold higher than DTX solution and DTX SNEDDS | 202 |
| Docetaxel | DTX-S-S-TG | Absolute bioavailability: 44.3%; relative bioavailability: 470.7% vs. DTX solution) | 203 |
| Quercetin | Emulsions | Higher lymphatic delivery and higher systemic exposure of both quercetin and its metabolites | 204 |
| Nitrendipine | Solid lipid nanoparticles | Bioavailability: 3.09–3.93 times higher than its suspension | 205 |
| Berberine chloride | Cremochylomicron (Cremophor EL, Tween 80) | 43% reduction in absorption after pre-treatment with cycloheximide | 206 |
| Carvedilol | Microemulsions | 2.84–4.91-fold enhanced in bioavailability | 207 |
| Olanzapine | Nanostructured lipid carriers | 5.5-fold increase in bioavailability vs. suspension | 171 |
| Lopinavir | TG-mimetic 1,3-dipalmitoyl glycerol-decorated mesoporous silica nanocarriers | Cmax: 1.69-fold increase; AUC: 5.97-fold increase (vs. free lopinavir) | 208 |
| Facilitated oral absorption by particulates | |||
| Probucol | SNEDDS | 10.2-fold improvement in bioavailability | 209 |
| Baicalin | Nanoemulsions | AUC: 14.56-fold increase; 26.8% decrease after pre-treatment with cycloheximide | 210 |
| Solvent Green 3 | SNEDDS | Absorption was totally inhibited after pre-treatment with cycloheximide | 211 |
| curcumin | N-Carboxymethyl chitosan coated solid lipid nanoparticles | Higher lymphatic transport and bioavailability | 212 |
| Silybin | Silybin-phospholipid; SB-PC SNEDDS | Relative bioavailability: 1265.9% (SB-PC); 1802.5% (SB-PC SNEDDS). Lymphatic exposure:12.2 (SB-PC) and 22.7 (SB-PC SNEDDS)-fold higher than pure SB | 213 |
| Candesartan cilexetil | Solid lipid nanoparticle | Bioavailability: 12-fold increase vs. CC suspension; AUC: 30% decrease after cycloheximide treatment | 214 |
| Morin | Phospholipid complex-based SNEDDS | Multiple absorption pathways were observed including the M cell and CM pathways | 215 |
| Lutein | Solid dispersions and SMEDDS | Enhanced lymphatic transport efficiency was achieved; different absorption rates between two formulations were observed | 216 |
| Insulin | Polymeric nanoparticles | Relative bioavailability: 13.21%; high Peyer's patch accumulation | 217 |
| Insulin | Glucan microparticles | Pharmacological bioavailability: 9%–10%; good correlation between lymphatic transport and pharmacological bioavailability | 218 |
| Insulin | Chondroitin sulfate-taurocholic acid conjugate-decorated liposomes | Chylomicron transport was observed | 219 |
| Exenatide | Phase-changeable nanoemulsions | Higher drug accumulations were observed in pancreas; drug absorption and lymphatic transport were inhibited by cycloheximide | 220 |
| Distant site targeting | |||
| Paclitaxel | WGA-decorated SLNs | Lung targeting | 221 |
| Cisplatin | YCW microcapsules | Lung tumor targeting | 222 |
| Sorafenib | Sugar-grafted SLNs | Liver tumor targeting | 223 |
| Vinpocetine | Mixed micelles | Brain targeting | 224 |
| Treatment involving the lymphatic system | |||
| Ovalbumin | Mannose- and flagellin-decorated Polyanhydride nanoparticles | Enhanced and balanced Th1/Th2 response; high intestinal IgA level | 225 |
| HBsAg | Lectin-decorated PLGA nanoparticles | Higher anti-HBsAg antibody levels and enhancing mucosal immunity | 226 |
| HBsAg | UAE 1-decorated liposomes | Comparable IgG level with intra-muscular HBsAg following immunization for three consecutive days | 227 |
| Mycophenolic acid | Triglyceride mimetic prodrug | 103-fold higher concentration in lymphocytes for prodrugs and 28-fold higher in lymph nodes for mycophenolic acid | 228 |
| Bexarotene and retinoic acid | Ester prodrug | 17- and 2.4-fold higher concentration in lymph nodes vs. free drug | 229 |
TU, testosterone undecanoate; TST, testosterone; TG, triglyceride; DTX, docetaxel; DTX-S-OA, docetaxel-sulfur-oleate; DTX-S-S-TG, docetaxel -sulfur-sulfur-triglyceride; SB, silybin; SB-PC, silybin-phospholipid; CC, candesartan cilexetil; EXT, exenatide; WGA, wheat germ agglutinin lectin; SNEDDS, self-nanoemulsifying drug delivery systems; SMEDDS, self-microemulsifying drug delivery system; SLNs, solid lipid nanoparticles; CM, chylomicron; YCM, yeast cell wall microparticles; PLGA, poly(lactic-co-glycolic acid); HBsAg, hepatitis B surface antigen.
6.1. Reduction of first-pass effect
As the mesenteric lymph flow circumvents the portal vein, drugs transported via the lymphatic route do not subject to first-pass hepatic metabolism. Therefore, structural modification to render them amenable for lymphatic transport has become an attractive option for entities with severe first-pass metabolism—for example, testosterone and docetaxel. The oral bioavailability of testosterone is too low to elicit any therapeutic effect. Hence, substituted, long-chain ester TU is marketed for clinical use as an oral dosage form due to significantly enhanced lymphatic transport and reduced liver metabolism. The absolute oral bioavailability of two commercial TU products was increased to approximately 3.25% and 2.88%, for which the contribution of lymphatic transport was approximately 91.5% and 99.7%, respectively, as revealed in a study in thoracic lymph duct-cannulated dogs200. However, the long-chain ester prodrug strategy is not without concerns—the balance between stability of the ester derivatives in vitro and cleavage rate in vivo sometimes poses a challenge. To address this, testosterone-triglyceride prodrugs were designed to improve the stability of testosterone and elevate its systemic exposure in rabbits with AUCs enhanced by 1.8 and 2.6 times as compared with TU at a dose of 8 and 4 mg/kg, respectively201. Further insertion of self-immolative spacers—acetal self-immolative group and trimethyl group for “locking”—into the prodrug structure ensures fast cleavage of the ester bonds and instant release of testosterone in the blood stream with 10–90-fold increase in AUC after oral administration39. Conjugation of docetaxel (DTX), an anti-cancer drug with pronounced hepatic metabolism, with oleate through a thioether bond (DTX-S-OA) increases the lipophilicity of DTX and thereby drug loading in SNEDDS202. The oral bioavailability of DTX-S-OA is 6.2- and 2.0-fold higher than DTX solution and SNEDDS, respectively202. Additionally, by conjugating DTX with triglyceride through a reduction-sensitive disulfide bond, the absolute bioavailability of DTX could be increased to as high as 44.3%203.
When a prodrug approach is unlikely, lymphatic transport via particulates creates new opportunities for facilitated oral absorption of drug entities with severe first-pass metabolism. Emulsions consisting of fatty acids of different chain lengths were designed to deliver quercetin204. Significantly increased lymphatic exposure was observed for quercetin and its active metabolites, especially by long-chain fatty acid-based nanoemulsions204. Nitrendipine was delivered as SLNs using three different lipid matrices—tripalmitin, cetyl palmitate and glyceryl monostearate205. All formulations displayed increased oral bioavailability by 3.09–3.93-fold as compared with the suspension counterpart205. By constructing berberine-loaded cremochylomicrons—particles composed of Cremophor EL and Tween 80 to imitate CM, two times higher absorption than the pure drug was detected206. After treating with cycloheximide, a reduction of approximately 43% in oral absorption was observed206, which stands for the same amount of lymphatic transport. More examples with carvedilol and olanzapine prove the partial contribution of lymphatic transport to enhanced oral bioavailability174,207. Inorganic carriers were also exploited with high drug loading and lymphatic targeting property. In a study on triglyceride-mimetic 1,3-dipalmitoyl glycerol-decorated silica nanocarriers, the Cmax and AUC of the model drug lopinavir were increased by 1.69- and 5.97-fold as compared with free lopinavir208. Pre-treatment with cyclohexamide reduced the plasma Cmax and AUC by 62.12% and 85.98%, respectively, highlighting the role of lymphatic transport208.
6.2. Facilitated oral absorption by particulates
Lymphatic transport of particulates can be also utilized to enhance the oral absorption of labile or poorly permeable entities such as biomacromolecules, extraordinarily lipophilic drugs, and BCS III and IV drugs. Probucol, with a lgP of 10.3, is a model drug of choice for the study of lymphatic transport73. The oral absorption of probucol is extremely limited because of not only its poor solubility but also retention in enterocytes that line the apical surfaces of microvilli. Formulating probucol into SNEDDS enhances lymphatic transport and overall oral bioavailability by 10.22-fold as compared with probucol suspension209,230. Similarly, formulating baicalin into nanoemulsions promotes its AUC by 14.56-fold, and CM-based lymphatic transport contributes 26.8% to the systemic exposure of the drug as observed in a CM blocking model by using cycloheximide210. The oral bioavailability of a SNEDDS formulation of Solvent Green 3, a poorly water-soluble drug was 1.7-fold higher than the soybean oil emulsion, while nearly no absorption was observed after pre-treatment with another CM blocker, colchicine211. On the other hand, surface modification with polymers or ligands sometimes results in altered oral absorption and transport behaviors. N-carboxymethyl chitosan-coated SLNs showed more than 4-fold and near 2-fold increase in lymphatic transport of curcumin in comparison with curcumin solution and uncoated SLNs, respectively, echoing the capacity of opening tight junction and lymphatic transport via the paracellular pathway212. It is acknowledged that besides improved dissolution and permeability, lymphatic transport also plays a role in enhanced oral absorption of several successfully marketed lipid-based products69,213,214,231. Multiple mechanisms, though difficult to clarify, may act concurrently in lymphatic transport. For instance, not only the CM but also the M cell pathway participates in lymphatic transport of morin phospholipid complex-loaded SNEDDS, as visualized by fluorescence bioimaging after labeling the vehicles with a near infrared fluorescent dye, Nile red215. The enhanced lymphatic transport of lutein by a solid dispersion and SMEDDS formulation in different ratios is ascribed to the particles size effect and different transport mechanisms216.
As for biomacromolecules like insulin or poorly permeable drugs with virtually no oral absorption, particulate-facilitated lymphatic transport seems to be more attractive. Furthermore, the relatively high availability of particulates—for example, approximately 13.21% for PCL/Eudragit RS50/50 nanoparticles—makes oral delivery of the most challengeable drug entities possible217. In a study on oral delivery of insulin by thermosensitive gel-thickened glucan microparticles218, cumulative lymphatic transport was determined by mesenteric lymphatic cannulation together with visualization by live imaging to reveal the translocation of the particles. The pharmacological bioavailability of insulin could reach 9%–10%, and positive correlation was established between mesenteric accumulation and pharmacological bioavailability, which highlighted the contribution of M cell-based lymphatic transport218. Another study with insulin-loaded liposomes decorated by chondroitin sulfate-taurocholic acid confirmed the role of the CM pathway in lymphatic transport219. After binding with apical sodium-dependent bile acid transporter and being internalized intact, nanoparticles could be transported into the lymph, as evidenced by cannulation and visualized by transmission electron microscopy (TEM)219. Similarly, a hydrophilic peptide exenatide was loaded in phase-changeable nanoemulsions that exhibit effective lymphatic transport and higher pancreas accumulation. Evidence collected by both confocal laser scanning microscopy and cycloheximide inhibition helps identify the contribution of the M cell-based lymphatic transport pathway220.
6.3. Distant site targeting
In view of probable oral absorption of intact particulates as discussed above, peroral targeting of particulates together with payloads to distant sites beyond the gastrointestinal tract is highly expectable. After uptake by M cells, particulates that reach the lymphatic system will be transported from the right lymphatic duct to the subclavian vein and pulmonary artery successively, and finally reach different MPS organs and tissues including the liver, spleen, lungs and kidneys, as well as tumors and inflammatory sites232. A pioneer study on yeast whole glucan particles revealed apparent accumulation of the particles in organs and tissues, which are abundant in macrophages, ranging from Peyer's patches to spleen, lymph nodes and marrow233. Similar results with curdlan, a large particulate β-1.3-glucan, also supports oral absorption of glucan particles and subsequent targeting to distant sites234.
Distant targeting through the lymphatic pathway for therapeutic purposes has already been reported. Orally delivered WGA-conjugated SLNs loading paclitaxel tend to accumulate in the lung, with 6.2-fold higher local drug levels than pure paclitaxel solution221. By tracking the signals of the fluorescent dye Cy7.5, orally administered YCW microcapsules could be found migrating from intestinal Peyer's patches to mesenteric lymph nodes and then to MPS organs and tissues and finally reaching human lung carcinoma xenografts in mice222. Chitosan-binding peptide-decorated PLGA nanoparticles loading rifampicin/gantrez attained more than 10 times of drug levels in lung tissues and realized effective antifungal treatment171,235. Although the underlying transport mechanisms are unclear, the scavenging capacity of monocytes in Peyer's patches may play an essential role in particle transport151. The lung, as the first location where lymph originated from the mesentery converges with blood, is the first site for particle retention232. After leaving the lung, the particles enter the systemic circulation and distribute in other MPS organs such as the liver achieving high targeting efficiency, as evidenced by sugar-conjugated sorafenib nanoparticles for the treatment of hepatocellular carcinoma223. Orally administered particles are able to reach distant inflammatory sites or lesions such as in atherosclerotic vessels131,236 and even in the brain as well224,237.
Though the efficiency of peroral targeting is very low in comparison with the intravenous route, multiple dosing may be employed to attain therapeutic-level accumulation in specific organs or tissues238.
6.4. Treatment involving the immune system
As oral delivery via lymphatic transport takes advantages of the intestinal and systemic immune system, it is reasonable to propose that oral lymphatic delivery may be better suited for oral immunization and treatment of diseases inflicting the immune systems. With respect to particle delivery, the protective mechanisms of the human body block the entry of a majority of particulates exposed in the GIT. The limited population of M cells imposes certain challenges for efficient drug delivery239.
Oral vaccination is the most widely investigated area for particle delivery. Compared to systemic vaccination, oral mucosal vaccination is able to induce both systemic and mucosal immunity and is more preferable than the parenteral routes in terms of safety and convenience in administration240, 241, 242. However, the harsh gastrointestinal conditions and presence of multiple barriers limit the efficiency of oral immunization243. Therefore, researches resort to particulate delivery systems and make use of the immunogenic capacity of follicles in Peyer's patches244. Enhanced and more balanced systemic Th1 and Th2 responses were elicited following oral administration of ovalbumin-loaded polyanhydride nanoparticles coated with mannose and flagellin225. The high levels of intestinal IgA titers elicited demonstrate enhanced mucosal immune response. It is also worth noting that a balance between Th1 and Th2 is beneficial to immune responses owing to the Th1/Th2 mutual adjustment effect245,246. Although this study did not give detailed information about drug transport, the possible contribution of M cell was highlighted. In another study on oral immunization with hepatitis B surface antigen (HBsAg) by utilizing lectin-conjugated PLGA nanoparticles, higher levels of serum anti-HBsAg antibodies and mucosa-secreted IgA were detected and efficient M cell uptake was recorded by confocal laser scanning microscopy226. Consecutive dosing of HBsAg-loaded liposomes decorated with UEA 1 for 3 days elicits maximum serum levels of anti-HBsAg IgG comparable to muscular delivery after three weeks227. In addition, mucosal IgA secreted locally is able to migrate to systemic mucosal systems, thus possibly enabling distant mucosal immunity via oral delivery247. The vaccines may be released from the vehicles after uptake165, but some particles such as glucan microparticles may remain intact in macrophages for a long time. Exploration of particle behaviors in vivo is essential in understanding the underlying mechanisms of immunization.
Besides oral vaccination, lymphatic drug delivery is beneficial for the treatment of diseases inflicting the immune systems. Conjugation of mycophenolic acid (MPA), an immunomodulator, to triglycerides (2-MPA-TG) enhances the drug concentration in lymphocytes by 103-fold and MPA level in lymphatic nodes by 28-fold228. The bexarotene and retinoic acid carboxylic ester prodrug produces similar enhanced drug levels in lymphocytes by 17-fold and lymph nodes by 2.4-fold229. Although no pharmacological results are reported for these two studies, the lymphatic drug enrichment is believed to benefit the efficacy of treatment.
7. Models for evaluation of lymphatic transport
Several models have been developed for the evaluation of lymphatic transport. The in vitro models aim at mimicking the function of either M cells or CMs, while the in vivo models mainly aim at collection and estimation of lymphatic drug transport in total via lymphatic cannulation.
7.1. In vitro model
7.1.1. M-like cell model
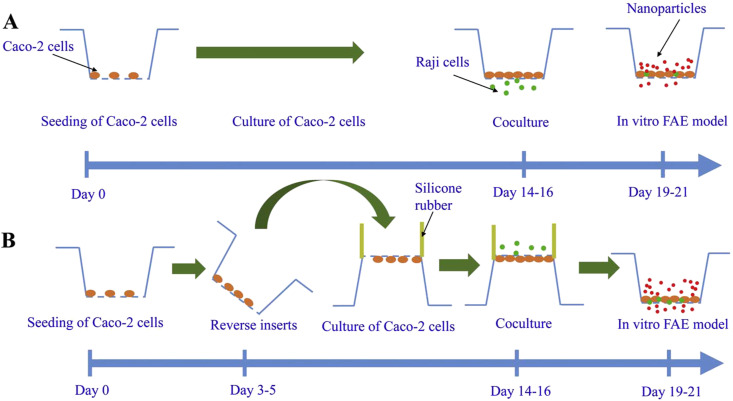
Caco-2 cell lines have been established as an in vitro model for the evaluation of drug permeation efficiency by cellular uptake and trans-monolayer transport experiments. Addition of lymphocytes imparts Caco-2 cell lines (monolayers) with M cell-mimicking capacities126. This is commonly done by co-culturing Caco-2 cells with lymphoid cells like Raji cells or murine derived lymphocytes248,249. Among different culture protocols, filter-grown co-culture Caco-2/Raji system was the most investigated one250. The first proposed model was practiced by seeding Caco-2 cells on the inverted insert and incubating with lymphoid cells on the downside of the insert251. However, this method suffers from multiple variations and low reproducibility250. Hence, modified methods, namely the “non-inverted” and “inverted” method, have been developed252,253. In a “non-inverted” model, Raji cells are added directly to the basolateral side of an insert, while in an “inverted” model, Raji cells are added after inversing the insert (Fig. 4)23,253. In a common M-like model, the lymphoid cells are allowed to permeate through the gaps on the filters and co-culture with Caco-2 cells without altering the function of each cell lines251. The “inverted” model has higher reproducibility and more robustness, though more difficult to conduct than the “non-converted” one250. Successful M cell transformation is usually verified by observation of increased attachment of particles such as bacteria or viruses, altered expression of certain genes, changed morphology with irregular microvilli by TEM and scanning electron microscopy44,254,255. M-like models have been applied to study of basic physiology, selection of ligands and simulation of pathogen transport234,248,256. In recent years, the models have been well adapted for prediction of enteric uptake of biomacromolecules and particulates160,257,258. Nevertheless, in vitro models should not be utilized to replace in vivo models because of the simplified cell monolayer structures and presence of excessively high content of M-like cells which may lead to overestimation of experimental results259.
Figure 4.
Schematic of in vitro M cell-like model. (A) “Non-inverted” method;(B) “Inverted” method. Reprinted with the permission from Ref. 253. Copyright © 2007 Elsevier.
7.1.2. Chylomicron association model
As virtual association of drug molecules with CMs in enterocytes governs lymphatic drug transport efficiency, in vitro CM association model may provide crucial information for the prediction of in vivo mesenteric transport. DDT with greater CM affinity showed significantly higher lymphatic transport compared to diazepam with limited binding efficiency83. Linear correlation between CM binding capability and lymphatic transport was established with nine high fat-soluble components including halofantrine, probucol, vitamins, etc 71. Physicochemical properties concerning this behavior were further testified by an in silico model68. Although natural CMs may work better in prediction, biomimetic CMs are preferred due to lower inter-group variations, reduced number of animals sacrificed and avoidance of tedious operations for separation of natural CMs73. The insignificant difference observed between natural and biomimetic CM models justifies the utilization of the latter for drug association study247. In addition to basic physicochemical properties of drugs, a series of factors such as addition of surfactants may also matter due to possible alternation of drug solubility74. Therefore, the contribution of excipients should also be considered.
7.2. In vivo model
7.2.1. Lymphatic cannulation model
As lacteals that collect particles or drug-associated CMs absorbed from the apical side coverage at the mesenteric lymphatics, mesenteric lymphatic cannulation is a good option to collect and measure total lymphatic drug transport. Therefore, the mesenteric lymphatic cannulation model has been established and utilized for evaluation of lymphatic transport79,260,261. Detailed surgery procedures can be found in literature262. The drug content in the collected lymphatic fluid can be quantified by assaying measures such as HPLC and LC–MS/MS, whereas the content of particles can be quantified based on specific physicochemical signals associated with the particles or constituting materials263, 264, 265. Big animals (pig, dog) are preferred to smaller ones (rat and mouse) because of physiological and anatomical keenness (e.g., biliary secretion) to human266,267. Moreover, it is easy to simulate pre- and post-prandial states in big animals than in small ones which display constant bile flow regardless of dietary81. Nevertheless, large animals are more costly for preliminary drug screening and more difficult to procure and handle. Taking together all factors, the rat model is the most popular model available. Thoracic cannulation is an alternative option reported with operational convenience, especially in mice268, but it may be less accurate in estimation of lymphatic transport because the thoracic lymphatic duct collects lymph from lymphatics other than the mesenteric lymphatic duct42.
Despite the easiness associated with sample collection and quantification, lymphatic cannulation is invasive and thus associated with certain limitations269. Cannulation involves complex surgery and may alter the lymph flow and vessel pressure gradient, which make consecutive sampling difficult after several sampling treatments. Owing to the influence of multiple factors, the success rate of lymphatic cannulation is generally very low263,270. Therefore, further improvements or substitutes are highly expected.
7.2.2. Lymph blocking model
In order to avoid operational stress involved in cannulation, lymph blocking models have been established by utilizing blocking reagents. Cycloheximide, a protein synthesis inhibitor, selectively suppresses the secretion of CMs but does not affect other absorption pathways. Though the blockage is irreversible, no obvious adverse effects have been recorded in literature271. The high correlation of the CM blocking model with lymphatic cannulation model validates its reliability for the prediction of lymphatic absorption272,273. However, whether cycloheximide specifically blocks CMs remains arguable. Researches indicate that cycloheximide may have inhibitory effect on the M cells too, which thus compromises the estimation accuracy of the CM pathway274. Other inhibitors with different blocking mechanisms such as Pluronic L-81, diacylglycerol acyl transferase inhibitor and colchicine have also been reported263. Similarly, the specificity of these blocking agents is yet to be tested too.
Several M cell blocking models have been utilized to assess the contribution of the M cells. NF-κB ligand (RANKL) expressed on the epithelium is essential for M cell differentiation. After treatment with anti-RANKL antibody, oral pathogenesis study was successfully done275. B cell knocking is another model established to characterize M cell depletion276. The pathogen translocation mechanism is identified involving both Peyer's patches and epithelia. Furthermore, recombination-activating gene 2 (Rag 2) and gamma chain (γc) deficient BALB/c mice (Rag-γc–/–) were also applied to investigate oral norovirus infection277. The lack of Peyer's patches and mature M cells demonstrates successful establishment of the models. Though numerous studies have showed the feasibility of M cell deficient model for disease investigation, there are still no research reported in drug absorption evaluation.
8. Conclusions and perspectives
Intestinal lymphatic transport is emerging as an attractive strategy for oral drug delivery with precious advantages that other strategies cannot accommodate, such as evading first-pass metabolism, enhancing overall oral bioavailability, and benefiting treatment of lymphatic diseases. By now, there have been fundamentally two pathways confirmed for intestinal lymphatic transport—that is, the chylomicron and M cell pathways. Though there are reports that transcellular and paracellular penetration across enterocytes may be involved, the potential contribution of these pathways is yet to be proved. The chylomicron pathway takes advantage of the lipolysis and absorption mechanisms of fat or oil which is primarily comprised of triglycerides. Both triglyceride-mimetic and long-chain fatty acid prodrugs are able to achieve greatly enhanced oral bioavailability with the contribution of lymphatic transport being as high as more than a half of the original dose. The M cell pathway presents as the main portal in the GIT for the entry of particulates into the body. However, the efficiency of this pathway is restricted to less than approximately 10% in general, probably owing to the limited population of M cells in enteric epithelia and the retention in sub-FAE follicles but limited escape and transport to the lymphatics. For better evaluation of lymphatic transport efficiency, several in vitro and in vivo models have been established for different lymphatic transport pathways, though none of them provide adequate information to verify the actual amount transported via the lymphatics.
Lymphatic delivery provides an alternative option for drug discovery and development, especially for those with poor stability, low solubility and permeability. With appropriate molecular and/or formulation designs, therapeutic levels of active pharmaceutical ingredients can be attained in both lymphatic and systemic circulation by exploiting enteric lymphatic transport. Currently, several relevant drug products have been marketed with sound therapeutic effects as well as commercial success. In the near future, more lymphatic transport-oriented products are foreseeable in view of the ever-growing challenges and financial burden associated with conventional roadmap of drug discovery. On the other hand, there are as well various unsolved problems involved in lymphatic drug delivery despite the promises. The influencing factors have not been fully unraveled and there is a lack of effective quantification measures and both in vitro and in vivo models for accurate estimation of the contribution of lymphatic transport, thus hindering the product and clinical translation greatly. However, it is envisioned that with the advancement of technologies like bioimaging and a better knowledge of the in vivo behaviors of drug molecules and the delivery vehicles, lymphatic transport efficiency can be enhanced substantially to fulfill not only therapeutic but also unidentified commitment such as targeting to remote sites beyond the GIT.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81872815, 82030107, and 81690263) and Science and Technology commission of Shanghai Municipality (No. 19XD1400300, China).
Author contributions
Zichen Zhang wrote the manuscript. Wei Wu, Zicen Zhang, Yi Lu and Jianping Qi revised the manuscript. All of the authors have read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Ansel H.C., Popovich N.G. 5th ed. Lea and Febiger; Philadelphia: 1990. Pharmaceutical dosage forms and drug delivery systems. [Google Scholar]
- 2.Date T., Paul K., Singh N., Jain S. Drug-lipid conjugates for enhanced oral drug delivery. AAPS PharmSciTech. 2019;20:41. doi: 10.1208/s12249-018-1272-0. [DOI] [PubMed] [Google Scholar]
- 3.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He H., Lu Y., Qi J., Zhu Q., Chen Z., Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9:36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pridgen E.M., Alexis F., Farokhzad O.C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expet Opin Drug Deliv. 2015;12:1459–1473. doi: 10.1517/17425247.2015.1018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shugarts S., Benet L.Z. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abuhelwa A.Y., Williams D.B., Upton R.N., Foster D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm. 2017;112:234–248. doi: 10.1016/j.ejpb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Wu W., Lu Y., Qi J. Editorial: persistent endeavors for the enhancement of dissolution and oral bioavailability. Acta Pharm Sin B. 2019;9:2–3. doi: 10.1016/j.apsb.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright L., Barnes T.J., Prestidge C.A. Oral delivery of protein-based therapeutics: gastroprotective strategies, physiological barriers and in vitro permeability prediction. Int J Pharm. 2020;585:119488. doi: 10.1016/j.ijpharm.2020.119488. [DOI] [PubMed] [Google Scholar]
- 10.Han Y., Gao Z., Chen L., Kang L., Huang W., Jin M. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm Sin B. 2019;9:902–922. doi: 10.1016/j.apsb.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Shrestha N., Préat V., Beloqui A. Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J Control Release. 2020;322:486–508. doi: 10.1016/j.jconrel.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Drucker D.J. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19:277–289. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 13.Ye J.Y., Chen Z.Y., Huang C.L., Huang B., Zheng Y.R., Zhang Y.F. A non-lipolysis nanoemulsion improved oral bioavailability by reducing the first-pass metabolism of raloxifene, and related absorption mechanisms being studied. Int J Nanomed. 2020;15:6503–6518. doi: 10.2147/IJN.S259993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui W., Zhang S., Zhao H., Luo C., Sun B., Li Z. Formulating a single thioether- bridged oleate prodrug into a self-nanoemulsifying drug delivery system to facilitate oral absorption of docetaxel. Biomater Sci. 2019;7:1117–1131. doi: 10.1039/c8bm00947c. [DOI] [PubMed] [Google Scholar]
- 15.Porter C.J., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 16.Schultz H.B., Meola T.R., Thomas N., Prestidge C.A. Oral formulation strategies to improve the bioavailability and mitigate the food effect of abiraterone acetate. Int J Pharm. 2020;577:119069. doi: 10.1016/j.ijpharm.2020.119069. [DOI] [PubMed] [Google Scholar]
- 17.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Yang J., Xie Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int J Pharm. 2019;570:118642. doi: 10.1016/j.ijpharm.2019.118642. [DOI] [PubMed] [Google Scholar]
- 19.Talegaonkar S., Bhattacharyya A. Potential of lipid nanoparticles (SLNS and NLCS) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech. 2019;20:121. doi: 10.1208/s12249-019-1337-8. [DOI] [PubMed] [Google Scholar]
- 20.Vishwakarma N., Jain A., Sharma R., Mody N., Vyas S., Vyas S.P. Lipid-based nanocarriers for lymphatic transportation. AAPS PharmSciTech. 2019;20:83. doi: 10.1208/s12249-019-1293-3. [DOI] [PubMed] [Google Scholar]
- 21.Markovic M., Ben-Shabat S., Keinan S., Aponick A., Zimmermann E.M., Dahan A. Lipidic prodrug approach for improved oral drug delivery and therapy. Med Res Rev. 2019;39:579–607. doi: 10.1002/med.21533. [DOI] [PubMed] [Google Scholar]
- 22.Managuli R.S., Raut S.Y., Reddy M.S., Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expet Opin Drug Deliv. 2018;15:787–804. doi: 10.1080/17425247.2018.1503249. [DOI] [PubMed] [Google Scholar]
- 23.Qi J., Zhuang J., Lv Y., Lu Y., Wu W. Exploiting or overcoming the dome trap for enhanced oral immunization and drug delivery. J Control Release. 2018;275:92–106. doi: 10.1016/j.jconrel.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Hussain N., Jaitley V., Florence A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50:107–142. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 25.Swartz M.A. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 26.Nune S.K., Gunda P., Majeti B.K., Thallapally P.K., Forrest M.L. Advances in lymphatic imaging and drug delivery. Adv Drug Deliv Rev. 2011;63:876–885. doi: 10.1016/j.addr.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margaris K.N., Black R.A. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface. 2012;9:601–612. doi: 10.1098/rsif.2011.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cifarelli V., Eichmann A. The intestinal lymphatic system: functions and metabolic implications. Cell Mol Gastroenterol Hepatol. 2019;7:503–513. doi: 10.1016/j.jcmgh.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslin J.W., Yang Y., Scallan J.P., Sweat R.S., Adderley S.P., Murfee W.L. Lymphatic vessel network structure and physiology. Comp Physiol. 2018;9:207–299. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrova T.V., Koh G.Y. Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med. 2018;215:35–49. doi: 10.1084/jem.20171868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulvmar M.H., Makinen T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res. 2016;111:310–321. doi: 10.1093/cvr/cvw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong B.W., Zecchin A., Garcia-Caballero M., Carmeliet P. Emerging concepts in organ-specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell. 2018;45:289–301. doi: 10.1016/j.devcel.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Trevaskis N.L., Kaminskas L.M., Porter C.J.H. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 34.Choe K., Jang J.Y., Park I., Kim Y., Ahn S., Park D.Y. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125:4042–4052. doi: 10.1172/JCI76509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernier-Latmani J., Petrova T.V. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510–526. doi: 10.1038/nrgastro.2017.79. [DOI] [PubMed] [Google Scholar]
- 36.Johnson O.W., Chick J.F.B., Chauhan N.R., Fairchild A.H., Fan C.M., Stecker M.S. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26:2482–2493. doi: 10.1007/s00330-015-4112-6. [DOI] [PubMed] [Google Scholar]
- 37.Hokkanen K., Tirronen A., Ylae-Herttuala S. Intestinal lymphatic vessels and their role in chylomicron absorption and lipid homeostasis. Curr Opin Lipidol. 2019;30:370–376. doi: 10.1097/MOL.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 38.Zhou A., Qu J., Liu M., Tso P. The role of interstitial matrix and the lymphatic system in gastrointestinal lipid and lipoprotein metabolism. Front Physiol. 2020;11:4. doi: 10.3389/fphys.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu L., Quach T., Han S., Lim S.F., Yadav P., Senyschyn D. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance oral bioavailability. Angew Chem Int Ed Engl. 2016;55:13700–13705. doi: 10.1002/anie.201604207. [DOI] [PubMed] [Google Scholar]
- 40.Dixon J.B. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab. 2010;21:480–487. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishioka Y., Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47:55–64. doi: 10.1016/s0169-409x(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 42.O'Driscoll C.M. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–415. doi: 10.1016/s0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 43.van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 44.Brayden D.J., Jepson M.A., Baird A.W. Keynote review: intestinal Peyer's patch M cells and oral vaccine targeting. Drug Discov Today. 2005;10:1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- 45.Shakweh M., Ponchel G., Fattal E. Particle uptake by Peyer's patches: a pathway for drug and vaccine delivery. Expet Opin Drug Deliv. 2004;1:141–163. doi: 10.1517/17425247.1.1.141. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Hernandez M.B., Liu T., Payne H.C., Stencel-Baerenwald J.E., Ikizler M., Yagita H. Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells. J Virol. 2014;88:6934–6943. doi: 10.1128/JVI.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolesnikov M., Curato C., Zupancic E., Florindo H., Shakhar G., Jung S. Intravital visualization of interactions of murine Peyer's patch-resident dendritic cells with M cells. Eur J Immunol. 2020;50:537–547. doi: 10.1002/eji.201948332. [DOI] [PubMed] [Google Scholar]
- 48.Qi J., Hu X., Dong X., Lu Y., Lu H., Zhao W. Towards more accurate bioimaging of drug nanocarriers: turning aggregation-caused quenching into a useful tool. Adv Drug Deliv Rev. 2019;143:206–225. doi: 10.1016/j.addr.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Azzali G., Arcari M.L. Ultrastructural and three dimensional aspects of the lymphatic vessels of the absorbing peripheral lymphatic apparatus in Peyer's patches of the rabbit. Anat Rec. 2000;258:71–79. doi: 10.1002/(SICI)1097-0185(20000101)258:1<71::AID-AR8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Ma B., von Wasielewski R., Lindenmaier W., Dittmar K.E. Immunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol. 2007;36:62–74. doi: 10.1111/j.1439-0264.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 51.Goswami A.K., Khaja M.S., Downing T., Kokabi N., Saad W.E., Majdalany B.S. Lymphatic anatomy and physiology. Semin Intervent Rad. 2020;37:227–236. doi: 10.1055/s-0040-1713440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzali G. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol Rev. 2003;195:178–189. doi: 10.1034/j.1600-065x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 53.Azzali G., Vitale M., Arcari M.L. Ultrastructure of absorbing peripheral lymphatic vessel (Alpa) in Guinea pig Peyer's patches. Microvasc Res. 2002;64:289–301. doi: 10.1006/mvre.2002.2428. [DOI] [PubMed] [Google Scholar]
- 54.Florence A.T. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 2004;12:65–70. doi: 10.1080/10611860410001693706. [DOI] [PubMed] [Google Scholar]
- 55.Miller M.J., McDole J.R., Newberry R.D. Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci. 2010;1207 doi: 10.1111/j.1749-6632.2010.05708.x. 21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asano K., Nakajima Y., Mukai K., Urai T., Okuwa M., Sugama J. Pre-collecting lymphatic vessels form detours following obstruction of lymphatic flow and function as collecting lymphatic vessels. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson A.R., Choi H., Lee E. Blood and lymphatic vasculatures on-chip platforms and their applications for organ-specific in vitro modeling. Micromachines. 2020;11:147. doi: 10.3390/mi11020147. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballard M., Wolf K.T., Nepiyushchikh Z., Dixon J.B., Alexeev A. Probing the effect of morphology on lymphatic valve dynamic function. Biomech Model Mechanobiol. 2018;17:1343–1356. doi: 10.1007/s10237-018-1030-y. [DOI] [PubMed] [Google Scholar]
- 59.Suy R., Thomis S., Fourneau I. The discovery of lymphatic system in the seventeenth century. Part i: the early history. Acta Chir Belg. 2016;116:260–266. doi: 10.1080/00015458.2016.1176792. [DOI] [PubMed] [Google Scholar]
- 60.Phang K., Bowman M., Phillips A., Windsor J. Review of thoracic duct anatomical variations and clinical implications. Clin Anat. 2014;27:637–644. doi: 10.1002/ca.22337. [DOI] [PubMed] [Google Scholar]
- 61.Beilstein F., Carriere V., Leturque A., Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res. 2016;340:172–179. doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Berthelsen R., Klitgaard M., Rades T., Mullertz A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv Drug Deliv Rev. 2019;142:35–49. doi: 10.1016/j.addr.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Amara S., Bourlieu C., Humbert L., Rainteau D., Carriere F. Variations in gastrointestinal lipases, pH and bile acid levels with food intake, age and diseases: possible impact on oral lipid-based drug delivery systems. Adv Drug Deliv Rev. 2019;142:3–15. doi: 10.1016/j.addr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Mu H.L., Porsgaard T. The metabolism of structured triacylglycerols. Prog Lipid Res. 2005;44:430–448. doi: 10.1016/j.plipres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Julve J., Martin-Campos J.M., Caries Escola-Gil J., Blanco-Vaca F. Chylomicrons: advances in biology, pathology, laboratory testing, and therapeutics. Clin Chim Acta. 2016;455:134–148. doi: 10.1016/j.cca.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Xiao C.T., Stahel P., Lewis G.F. Regulation of chylomicron secretion: focus on post-assembly mechanisms. Cell Mol Gastroenterol Hepatol. 2019;7:487–501. doi: 10.1016/j.jcmgh.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakajima K., Tokita Y., Tanaka A., Takahashi S. The VLDL receptor plays a key role in the metabolism of postprandial remnant lipoproteins. Clin Chim Acta. 2019;495:382–393. doi: 10.1016/j.cca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Gershkovich P., Fanous J., Qadri B., Yacovan A., Amselem S., Hoffman A. The role of molecular physicochemical properties and apolipoproteins in association of drugs with triglyceride-rich lipoproteins: in- silico prediction of uptake by chylomicrons. J Pharm Pharmacol. 2009;61:31–39. doi: 10.1211/jpp/61.01.0005. [DOI] [PubMed] [Google Scholar]
- 69.Chakraborty S., Shukla D., Mishra B., Singh S. Lipid-An emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73:1–15. doi: 10.1016/j.ejpb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Lawless E., Griffin B.T., O'Mahony A., O'Driscoll C.M. Exploring the impact of drug properties on the extent of intestinal lymphatic transport—In vitro and in vivo studies. Pharm Res. 2015;32:1817–1829. doi: 10.1007/s11095-014-1578-x. [DOI] [PubMed] [Google Scholar]
- 71.Gershkovich P., Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci. 2005;26:394–404. doi: 10.1016/j.ejps.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Rysanek P., Grus T., Sima M., Slanar O. Lymphatic transport of drugs after intestinal absorption: impact of drug formulation and physicochemical properties. Pharm Res. 2020;37:166. doi: 10.1007/s11095-020-02858-0. [DOI] [PubMed] [Google Scholar]
- 73.Lu Y., Qiu Y., Qi J., Feng M., Ju D., Wu W. Biomimetic reassembled chylomicrons as novel association model for the prediction of lymphatic transportation of highly lipophilic drugs via the oral route. Int J Pharm. 2015;483:69–76. doi: 10.1016/j.ijpharm.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Kim H., Seong I., Ro J., Hwang S.-H., Yun G., Lee J. Enhanced association of probucol with chylomicron by pharmaceutical excipients: an in vitro study. Drug Dev Ind Pharm. 2015;41:1073–1079. doi: 10.3109/03639045.2014.927479. [DOI] [PubMed] [Google Scholar]
- 75.Mu H. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 76.Imada C., Takahashi T., Kuramoto M., Masuda K., Ogawara K., Sato A. Improvement of oral bioavailability of N-251, a novel antimalarial drug, by increasing lymphatic transport with long-chain fatty acid-based self-nanoemulsifying drug delivery system. Pharm Res. 2015;32:2595–2608. doi: 10.1007/s11095-015-1646-x. [DOI] [PubMed] [Google Scholar]
- 77.Caliph S.M., Charman W.N., Porter C.J.H. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 2000;89:1073–1084. doi: 10.1002/1520-6017(200008)89:8<1073::aid-jps12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 78.Holm R., Porter C.J.H., Mullertz A., Kristensen H.G., Charman W.N. Structured triglyceride vehicles for oral delivery of halofantrine: examination of intestinal lymphatic transport and bioavailability in conscious rats. Pharm Res. 2002;19:1354–1361. doi: 10.1023/a:1020311127328. [DOI] [PubMed] [Google Scholar]
- 79.Holm R. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci. 2003;20:91–97. doi: 10.1016/s0928-0987(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 80.Trevaskis N.L., Shackleford D.M., Charman W.N., Edwards G.A., Gardin A., Appel-Dingemanse S. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res. 2009;26:1486–1495. doi: 10.1007/s11095-009-9860-z. [DOI] [PubMed] [Google Scholar]
- 81.Khoo S.M., Edwards G.A., Porter C.J.H., Charman W.N. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J Pharm Sci. 2001;90:1599–1607. doi: 10.1002/jps.1110. [DOI] [PubMed] [Google Scholar]
- 82.Amekyeh H., Billa N., Yuen K.H., Lim S.C. Effect of food status on the gastrointestinal transit of amphotericin B-containing solid lipid nanoparticles in rats. AAPS PharmSciTech. 2016;17:1060–1066. doi: 10.1208/s12249-015-0438-2. [DOI] [PubMed] [Google Scholar]
- 83.Gershkovich P., Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci. 2007;32:24–32. doi: 10.1016/j.ejps.2007.05.109. [DOI] [PubMed] [Google Scholar]
- 84.Desmarchelier C., Borel P., Lairon D., Maraninchi M., Valero R. Effect of nutrient and micronutrient intake on chylomicron production and postprandial lipemia. Nutrients. 2019;11:30. doi: 10.3390/nu11061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maldonado-Valderrama J., Wilde P., Macierzanka A., Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci. 2011;165:36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Trevaskis N.L., Porter C.J.H., Charman W.N. Bile increases intestinal lymphatic drug transport in the fasted rat. Pharm Res. 2005;22:1863–1870. doi: 10.1007/s11095-005-6808-9. [DOI] [PubMed] [Google Scholar]
- 87.Tonsberg H., Holm R., Mu H., Boll J.B., Jacobsen J., Mullertz A. Effect of bile on the oral absorption of halofantrine in polyethylene glycol 400 and polysorbate 80 formulations dosed to bile duct cannulated rats. J Pharm Pharmacol. 2011;63:817–824. doi: 10.1111/j.2042-7158.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 88.Holm R., Tonsberg H., Jorgensen E.B., Abedinpour P., Farsad S., Mullertz A. Influence of bile on the absorption of halofantrine from lipid-based formulations. Eur J Pharm Biopharm. 2012;81:281–287. doi: 10.1016/j.ejpb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Bala V., Rao S., Bateman E., Keefe D., Wang S., Prestidge C.A. Enabling oral SN38-based chemotherapy with a combined lipophilic prodrug and self-microemulsifying drug delivery system. Mol Pharm. 2016;13:3518–3525. doi: 10.1021/acs.molpharmaceut.6b00591. [DOI] [PubMed] [Google Scholar]
- 90.Han S., Hu L., Quach T., Simpson J.S., Trevaskis N.L., Porter C.J.H. Constitutive triglyceride turnover into the mesenteric lymph is unable to support efficient lymphatic transport of a biomimetic triglyceride prodrug. J Pharm Sci. 2016;105:786–796. doi: 10.1002/jps.24670. [DOI] [PubMed] [Google Scholar]
- 91.Markovic M., Ben-Shabat S., Aponick A., Zimmermann E.M., Dahan A. Lipids and lipid-processing pathways in drug delivery and therapeutics. Int J Mol Sci. 2020;21:3248. doi: 10.3390/ijms21093248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yanez J.A., Wang S.W., Knemeyer I.W., Wirth M.A., Alton K.B. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63:923–942. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dahan A., Zimmermann E.M., Ben-Shabat S. Modern prodrug design for targeted oral drug delivery. Molecules. 2014;19:16489–16505. doi: 10.3390/molecules191016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams C.M., Bateman P.A., Jackson K.G., Yaqoob P. Dietary fatty acids and chylomicron synthesis and secretion. Biochem Soc Trans. 2004;32:55–58. doi: 10.1042/bst0320055. [DOI] [PubMed] [Google Scholar]
- 95.Yoshida T., Nakanishi K., Yoshioka T., Tsutsui Y., Maeda A., Kondo H. Oral tacrolimus oil formulations for enhanced lymphatic delivery and efficient inhibition of T-cell’s interleukin-2 production. Eur J Pharm Biopharm. 2016;100:58–65. doi: 10.1016/j.ejpb.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Bassissi M.F., Lespine A., Alvinerie M. Enhancement of oral moxidectin bioavailability in rabbits by lipid co-administration. Parasitol Res. 2004;94:188–192. doi: 10.1007/s00436-004-1192-7. [DOI] [PubMed] [Google Scholar]
- 97.Tso P., Vurma M., Ko C.W., Lee D., DeMichele S. Effect of mono- and diglycerides on the digestion and absorption of lutein in lymph fistula rats. Am J Physiol Gastrointest Liver Physiol. 2018;315:95–103. doi: 10.1152/ajpgi.00236.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.White W.A.-O., Zhou Y., Crane A., Dixon P., Quadt F., Flendrig L.M. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am J Clin Nutr. 2017;106:1041–1051. doi: 10.3945/ajcn.117.153635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buttet M., Traynard V., Thi Thu Trang T., Besnard P., Poirier H., Niot I. From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins. Biochimie. 2014;96:37–47. doi: 10.1016/j.biochi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 100.Irawati D., Mamo J.C.L., Slivkoff-Clark K.M., Soares M.J., James A.P. Dietary fat and physiological determinants of plasma chylomicron remnant homoeostasis in normolipidaemic subjects: insight into atherogenic risk. Br J Nutr. 2017;117:403–412. doi: 10.1017/S0007114517000150. [DOI] [PubMed] [Google Scholar]
- 101.Trevaskis N.L., Caliph S.M., Nguyen G., Tso P., Charman W.N., Porter C.J. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm Res. 2013;30:3254–3270. doi: 10.1007/s11095-013-1000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahman M.H., Yeasmin K., Rana A.Y.K.M.M., Rahman S.S., Ali M., Meherunnahar A study on biochemical properties and effects of different vegetable oils on blood indices in wistar rats. J Pharm Res Int. 2019;25:1–10. [Google Scholar]
- 103.Tateishi N., Morita S., Yamazaki I., Okumura H., Kominami M., Akazawa S. Administration timing and duration-dependent effects of sesamin isomers on lipid metabolism in rats. Chronobiol Int. 2020;37:493–509. doi: 10.1080/07420528.2019.1700998. [DOI] [PubMed] [Google Scholar]
- 104.Salvia-Trujillo L., Verkempinck S.H.E., Zhang X., Van Loey A.M., Grauwet T., Hendrickx M.E. Comparative study on lipid digestion and carotenoid bioaccessibility of emulsions, nanoemulsions and vegetable-based in situ emulsions. Food Hydrocoll. 2019;87:119–128. [Google Scholar]
- 105.Mashurabad P.C., Palika R., Jyrwa Y.W., Bhaskarachary K., Pullakhandam R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J Food Sci Tech Mys. 2017;54:333–341. doi: 10.1007/s13197-016-2466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nielsen F.S., Petersen K.B., Müllertz A. Bioavailability of probucol from lipid and surfactant based formulations in minipigs: influence of droplet size and dietary state. Eur J Pharm Biopharm. 2008;69:553–562. doi: 10.1016/j.ejpb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 107.Feeney O.M., Crum M.F., McEvoy C.L., Trevaskis N.L., Williams H.D., Pouton C.W. 50 years of oral lipid-based formulations: provenance, progress and future perspectives. Adv Drug Deliv Rev. 2016;101:167–194. doi: 10.1016/j.addr.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Qi J.P., Lu Y., Wu W. Absorption, disposition and pharmacokinetics of solid lipid nanoparticles. Curr Drug Metab. 2012;13:418–428. doi: 10.2174/138920012800166526. [DOI] [PubMed] [Google Scholar]
- 109.Trevaskis N.L., Charman W.N., Porter C.J. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yao M., Xiao H., McClements D.J. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Annu Rev Food Sci Technol. 2014;5:53–81. doi: 10.1146/annurev-food-072913-100350. [DOI] [PubMed] [Google Scholar]
- 111.Fang G., Tang B., Chao Y., Zhang Y., Xu H., Tang X. Improved oral bioavailability of docetaxel by nanostructured lipid carriers: In vitro characteristics, in vivo evaluation and intestinal transport studies. RSC Adv. 2015;5:96437–96447. [Google Scholar]
- 112.Chai G.H., Hu F.Q., Sun J., Du Y.Z., You J., Yuan H. Transport pathways of solid lipid nanoparticles across madin-darby canine kidney epithelial cell monolayer. Mol Pharm. 2014;11:3716–3726. doi: 10.1021/mp5004674. [DOI] [PubMed] [Google Scholar]
- 113.Kim K.S., Suzuki K., Cho H., Youn Y.S., Bae Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano. 2018;12:8893–8900. doi: 10.1021/acsnano.8b04315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu M., Yang Y., Zhu C., Guo S., Gan Y. Advances in the transepithelial transport of nanoparticles. Drug Discov Today. 2016;21:1155–1161. doi: 10.1016/j.drudis.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 115.Zhao B., Gu S., Du Y., Shen M., Liu X., Shen Y. Solid lipid nanoparticles as carriers for oral delivery of hydroxysafflor yellow A. Int J Pharm. 2018;535:164–171. doi: 10.1016/j.ijpharm.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 116.Ravi P.R., Aditya N., Kathuria H., Malekar S., Vats R. Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur J Pharm Biopharm. 2014;87:114–124. doi: 10.1016/j.ejpb.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 117.Attili-Qadri S., Karra N., Nemirovski A., Schwob O., Talmon Y., Nassar T. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci U S A. 2013;110:17498–17503. doi: 10.1073/pnas.1313839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghassemi S., Haeri A., Shahhosseini S., Dadashzadeh S. Labrasol-enriched nanoliposomal formulation: novel approach to improve oral absorption of water-insoluble drug, carvedilol. AAPS PharmSciTech. 2018;19:2961–2970. doi: 10.1208/s12249-018-1118-9. [DOI] [PubMed] [Google Scholar]
- 119.Yao M., Chen J., Zheng J., Song M., McClements D.J., Xiao H. Enhanced lymphatic transport of bioactive lipids: cell culture study of polymethoxyflavones incorporation into chylomicrons. Food Funct. 2013;4:1662–1667. doi: 10.1039/c3fo60335k. [DOI] [PubMed] [Google Scholar]
- 120.Wu H., Zhou A., Lu C., Wang L. Examination of lymphatic transport of puerarin in unconscious lymph duct-cannulated rats after administration in microemulsion drug delivery systems. Eur J Pharm Sci. 2011;42:348–353. doi: 10.1016/j.ejps.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Griffin B.T., O'Driscoll C.M. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol. 2006;58:917–925. doi: 10.1211/jpp.58.7.0006. [DOI] [PubMed] [Google Scholar]
- 122.Guan P., Lu Y., Qi J., Wu W. Readily restoring freeze-dried probilosomes as potential nanocarriers for enhancing oral delivery of cyclosporine A. Colloids Surf B Biointerfaces. 2016;144:143–151. doi: 10.1016/j.colsurfb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 123.Wagner C., Bonnardel J., Da Silva C., Martens L., Gorvel J.-P., Lelouard H. Some news from the unknown soldier, the Peyer's patch macrophage. Cell Immunol. 2018;330:159–167. doi: 10.1016/j.cellimm.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi N., Takahashi D., Takano S., Kimura S., Hase K. The roles of Peyer's patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lundquist P., Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 126.Fasciano A.C., Blutt S.E., Estes M.K., Mecsas J. Induced differentiation of M cell-like cells in human stem cell-derived ileal enteroid monolayers. Jove-J Vis Exp. 2019 doi: 10.3791/59894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schleh C., Semmler-Behnke M., Lipka J., Wenk A., Hirn S., Schaeffler M. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;6:36–46. doi: 10.3109/17435390.2011.552811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohno H. Intestinal M cells. J Biochem. 2016;159:151–160. doi: 10.1093/jb/mvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie Y., Hu X., He H., Xia F., Ma Y., Qi J. Tracking translocation of glucan microparticles targeting M cells: implications for oral drug delivery. J Mater Chem B. 2016;4:2864–2873. doi: 10.1039/c5tb02706c. [DOI] [PubMed] [Google Scholar]
- 131.Zhou X., Zhang X., Han S., Dou Y., Liu M., Zhang L. Yeast microcapsule-mediated targeted delivery of diverse nanoparticles for imaging and therapy via the oral route. Nano Lett. 2017;17:1056–1064. doi: 10.1021/acs.nanolett.6b04523. [DOI] [PubMed] [Google Scholar]
- 132.Clark M.A., Jepson M.A., Hirst B.H. Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev. 2001;50:81–106. doi: 10.1016/s0169-409x(01)00149-1. [DOI] [PubMed] [Google Scholar]
- 133.Brayden D.J., Baird A.W. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Adv Drug Deliv Rev. 2004;56:721–726. doi: 10.1016/j.addr.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 134.Li X., Yu M., Fan W., Gan Y., Hovgaard L., Yang M. Orally active-targeted drug delivery systems for proteins and peptides. Expet Opin Drug Deliv. 2014;11:1435–1447. doi: 10.1517/17425247.2014.924500. [DOI] [PubMed] [Google Scholar]
- 135.Kaklotar D., Agrawal P., Abdulla A., Singh R.P., Sonali, Mehata A.K. Transition from passive to active targeting of oral insulin nanomedicines: enhancement in bioavailability and glycemic control in diabetes. Nanomedicine. 2016;11:1465–1486. doi: 10.2217/nnm.16.43. [DOI] [PubMed] [Google Scholar]
- 136.des Rieux A., Pourcelle V., Cani P.D., Marchand-Brynaert J., Preat V. Targeted nanoparticles with novel non-peptidic ligands for oral delivery. Adv Drug Deliv Rev. 2013;65:833–844. doi: 10.1016/j.addr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 137.Man A.L., Prieto-Garcia M.E., Nicoletti C. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology. 2004;113:15–22. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Devi R.V., Basil-Rose M.R. Lectins as ligands for directing nanostructured systems. Curr Drug Deliv. 2018;15:448–452. doi: 10.2174/1567201815666180108101246. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X., Wu W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov Today. 2014;19:898–904. doi: 10.1016/j.drudis.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 140.Zhang N., Ping Q.N., Huang G.H., Xu W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 141.Yin Y., Chen D., Qiao M., Wei X., Hu H. Lectin-conjugated PLGA nanoparticles loaded with thymopentin: Ex vivo bioadhesion and in vivo biodistribution. J Control Release. 2007;123:27–38. doi: 10.1016/j.jconrel.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 142.Zhang N., Ping Q., Huang G., Xu W., Cheng Y., Han X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327:153–159. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 143.Jepson M.A., Clark M.A., Hirst B.H. M cell targeting by lectins: a strategy for mucosal vaccination and drug delivery. Adv Drug Deliv Rev. 2004;56:511–525. doi: 10.1016/j.addr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 144.Florence A.T. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm Res. 1997;14:613–618. doi: 10.1023/a:1012153011884. [DOI] [PubMed] [Google Scholar]
- 145.Shreya A.B., Raut S.Y., Managuli R.S., Udupa N., Mutalik S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: recent advances. AAPS PharmSciTech. 2018;20:15. doi: 10.1208/s12249-018-1262-2. [DOI] [PubMed] [Google Scholar]
- 146.Managuli R.S., Wang J.T., Faruqu F.M., Pandey A., Jain S., Al-Jamal K.T. Surface engineered nanoliposomal platform for selective lymphatic uptake of asenapine maleate: In vitro and in vivo studies. Mater Sci Eng C Mater Biol Appl. 2020;109:110620. doi: 10.1016/j.msec.2019.110620. [DOI] [PubMed] [Google Scholar]
- 147.Wang W., Camenisch G., Sane D.C., Zhang H., Hugger E., Wheeler G.L. A coumarin-based prodrug strategy to improve the oral absorption of RGD peptidomimetics. J Control Release. 2000;65:245–251. doi: 10.1016/s0168-3659(99)00241-2. [DOI] [PubMed] [Google Scholar]
- 148.Fievez V., Plapied L., des Rieux A., Pourcelle V., Freichels H., Wascotte V. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73:16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Momtaz M., Rerat V., Gharbi S., Gerard E., Pourcelle V., Marchand-Brynaert J. A graftable LDV peptidomimetic: design, synthesis and application to a blood filtration membrane. Bioorg Med Chem Lett. 2008;18:1084–1090. doi: 10.1016/j.bmcl.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 150.De Smet R., Allais L., Cuvelier C.A. Recent advances in oral vaccine development: yeast-derived beta-glucan particles. Hum Vaccines Immunother. 2014;10:1309–1318. doi: 10.4161/hv.28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren T., Gou J., Sun W., Tao X., Tan X., Wang P. Entrapping of nanoparticles in yeast cell wall microparticles for macrophage-targeted oral delivery of cabazitaxel. Mol Pharm. 2018;15:2870–2882. doi: 10.1021/acs.molpharmaceut.8b00357. [DOI] [PubMed] [Google Scholar]
- 152.Salman H.H., Gómez S., Gamazo C., Costa Martins R., Zabaleta V., Irache J.M. Micro-organism-like nanoparticles for oral antigen delivery. J Drug Deliv Sci Tech. 2008;18:31–39. [Google Scholar]
- 153.Wang M., Gao Z., Zhang Z., Pan L., Zhang Y. Roles of M cells in infection and mucosal vaccines. Hum Vaccines Immunother. 2014;10:3544–3551. doi: 10.4161/hv.36174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kimura S., Nio-Kobayashi J., Kishimoto A., Iwanaga T. The broad distribution of GP2 in mucous glands and secretory products. Biomed Res-Tokyo. 2016;37:351–358. doi: 10.2220/biomedres.37.351. [DOI] [PubMed] [Google Scholar]
- 155.Marshall A., Bradford B.M., Clarke A.R., Manson J.C., Mabbott N.A. Oral prion neuroinvasion occurs independently of PrPC expression in the gut epithelium. J Virol. 2018;92:e01010–e01018. doi: 10.1128/JVI.01010-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim S.H., Jung D.I., Yang I.Y., Kim J., Lee K.Y., Nochi T. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol. 2011;41:3219–3229. doi: 10.1002/eji.201141592. [DOI] [PubMed] [Google Scholar]
- 157.Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He H., Xie Y., Lv Y., Qi J., Dong X., Zhao W. Bioimaging of intact polycaprolactone nanoparticles using aggregation-caused quenching probes: size-dependent translocation via oral delivery. Adv Healthc Mater. 2018;7:1800711. doi: 10.1002/adhm.201800711. [DOI] [PubMed] [Google Scholar]
- 159.Shen C., Yang Y., Shen B., Xie Y., Qi J., Dong X. Self-discriminating fluorescent hybrid nanocrystals: efficient and accurate tracking of translocation via oral delivery. Nanoscale. 2017;10:436–450. doi: 10.1039/c7nr06052a. [DOI] [PubMed] [Google Scholar]
- 160.Xie Y., Shi B., Xia F., Qi J., Dong X., Zhao W. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J Control Release. 2018;270:65–75. doi: 10.1016/j.jconrel.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 161.De Jesus M., Ostroff G.R., Levitz S.M., Bartling T.R., Mantis N.J. A population of langerin-positive dendritic cells in murine Peyer's patches involved in sampling beta-glucan microparticles. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lwin S., Inoshima Y., Ueno H., Ishiguro N. Uptake and transport of foreign particles in Peyer's patches of both distal ileum and jejunum of calves. Cell Tissue Res. 2009;337:125–135. doi: 10.1007/s00441-009-0793-y. [DOI] [PubMed] [Google Scholar]
- 163.Awaad A., Nakamura M., Ishimura K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer's patches using fluorescent organosilica particles. Nanomedicine. 2012;8:627–636. doi: 10.1016/j.nano.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 164.Shakweh M., Besnard M., Nicolas V., Fattal E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer's patches in mice. Eur J Pharm Biopharm. 2005;61:1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 165.Islam M.A., Firdous J., Badruddoza A.Z.M., Reesor E., Azad M., Hasan A. M cell targeting engineered biomaterials for effective vaccination. Biomaterials. 2019;192:75–94. doi: 10.1016/j.biomaterials.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 166.des Rieux A., Fievez V., Garinot M., Schneider Y.J., Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 167.He C., Yin L., Tang C., Yin C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials. 2012;33:8569–8578. doi: 10.1016/j.biomaterials.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 168.Desai M.P., Labhasetwar V., Amidon G.L., Levy R.J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 169.Choi S.W., Kim W.S., Kim J.H. Surface modification of functional nanoparticles for controlled drug delivery. J Disper Sci Technol. 2007;24:475–487. [Google Scholar]
- 170.Huckaby J.T., Lai S.K. Pegylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. doi: 10.1016/j.addr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 171.Bachhav S.S., Dighe V.D., Devarajan P.V. Exploring Peyer's patch uptake as a strategy for targeted lung delivery of polymeric rifampicin nanoparticles. Mol Pharm. 2018;15:4434–4445. doi: 10.1021/acs.molpharmaceut.8b00382. [DOI] [PubMed] [Google Scholar]
- 172.Patel M.D., Date P.V., Gaikwad R.V., Samad A., Malshe V.C., Devarajan P.V. Comparative evaluation of polymeric nanoparticles of rifampicin comprising gantrez and poly(ethylene sebacate) on pharmacokinetics, biodistribution and lung uptake following oral administration. J Biomed Nanotechnol. 2014;10:687–694. doi: 10.1166/jbn.2014.1739. [DOI] [PubMed] [Google Scholar]
- 173.des Rieux A., Ragnarsson E.G., Gullberg E., Preat V., Schneider Y.J., Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur J Pharm Sci. 2005;25:455–465. doi: 10.1016/j.ejps.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 174.Jawahar N., Hingarh P.K., Arun R., Selvaraj J., Anbarasan A., S S. Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int J Biol Macromol. 2018;110:269–275. doi: 10.1016/j.ijbiomac.2018.01.121. [DOI] [PubMed] [Google Scholar]
- 175.Du X.J., Wang J.L., Iqbal S., Li H.J., Cao Z.T., Wang Y.C. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater Sci. 2018;6:642–650. doi: 10.1039/c7bm01096f. [DOI] [PubMed] [Google Scholar]
- 176.Shi L.L., Xie H., Lu J., Cao Y., Liu J.Y., Zhang X.X. Positively charged surface-modified solid lipid nanoparticles promote the intestinal transport of docetaxel through multifunctional mechanisms in rats. Mol Pharm. 2016;13:2667–2676. doi: 10.1021/acs.molpharmaceut.6b00226. [DOI] [PubMed] [Google Scholar]
- 177.Channarong S., Chaicumpa W., Sinchaipanid N., Mitrevej A. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: the improvement of Peyer's patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech. 2011;12:192–200. doi: 10.1208/s12249-010-9559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jung T., Kamm W., Breitenbach A., Kaiserling E., Xiao J.X., Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147–160. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 179.Yu Z., Fan W., Wang L., Qi J., Lu Y., Wu W. Effect of surface charges on oral absorption of intact solid lipid nanoparticles. Mol Pharm. 2019;16:5013–5024. doi: 10.1021/acs.molpharmaceut.9b00861. [DOI] [PubMed] [Google Scholar]
- 180.Zhu X., Vo C., Taylor M., Smith B.R. Non-spherical micro- and nanoparticles in nanomedicine. Mater Horizons. 2019;6:1094–1121. [Google Scholar]
- 181.Li D., Zhuang J., He H., Jiang S., Banerjee A., Lu Y. Influence of particle geometry on gastrointestinal transit and absorption following oral administration. ACS Appl Mater Interfaces. 2017;9:42492–42502. doi: 10.1021/acsami.7b11821. [DOI] [PubMed] [Google Scholar]
- 182.Rivera-Gil P., De Aberasturi D.J., Wulf V., Pelaz B., Del Pino P., Zhao Y.Y. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Chem Res. 2013;46:743–749. doi: 10.1021/ar300039j. [DOI] [PubMed] [Google Scholar]
- 183.Krug S.M., Hayaishi T., Iguchi D., Watari A., Takahashi A., Fromm M. Angubindin-1, a novel paracellular absorption enhancer acting at the tricellulare tight junction. J Control Release. 2017;260:1–11. doi: 10.1016/j.jconrel.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 184.Lemmer H.J., Hamman J.H. Paracellular drug absorption enhancement through tight junction modulation. Expet Opin Drug Deliv. 2013;10:103–114. doi: 10.1517/17425247.2013.745509. [DOI] [PubMed] [Google Scholar]
- 185.Luo Y.Y., Xiong X.Y., Tian Y., Li Z.L., Gong Y.C., Li Y.P. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2016;23:1882–1891. doi: 10.3109/10717544.2015.1052863. [DOI] [PubMed] [Google Scholar]
- 186.Jorgensen J.R., Jepsen M.L., Nielsen L.H., Dufva M., Nielsen H.M., Rades T. Microcontainers for oral insulin delivery—in vitro studies of permeation enhancement. Eur J Pharm Biopharm. 2019;143:98–105. doi: 10.1016/j.ejpb.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 187.Jensen S.S., Jensen H., Cornett C., Moller E.H., Ostergaard J. Insulin diffusion and self-association characterized by real-time UV imaging and taylor dispersion analysis. J Pharm Biomed Anal. 2014;92:203–210. doi: 10.1016/j.jpba.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 188.Pearce S.C., Al-Jawadi A., Kishida K., Yu S., Hu M., Fritzky L.F. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018;16:19. doi: 10.1186/s12915-018-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin I.C., Liang M., Liu T.Y., Monteiro M.J., Toth I. Cellular transport pathways of polymer coated gold nanoparticles. Nanomed-Nanotechnol. 2012;8:8–11. doi: 10.1016/j.nano.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 190.Borges O., Lebre F., Bento D., Borchard G., Junginger H.E. Mucosal vaccines: recent progress in understanding the natural barriers. Pharm Res. 2010;27:211–223. doi: 10.1007/s11095-009-0011-3. [DOI] [PubMed] [Google Scholar]
- 191.Damge C., Reis C.P., Maincent P. Nanoparticle strategies for the oral delivery of insulin. Expet Opin Drug Deliv. 2008;5:45–68. doi: 10.1517/17425247.5.1.45. [DOI] [PubMed] [Google Scholar]
- 192.Lopes M.A., Abrahim B.A., Cabral L.M., Rodrigues C.R., Seica R.M., de Baptista Veiga F.J. Intestinal absorption of insulin nanoparticles: contribution of M cells. Nanomedicine. 2014;10:1139–1151. doi: 10.1016/j.nano.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 193.Maher S., Mrsny R.J., Brayden D.J. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319. doi: 10.1016/j.addr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 194.Sheue Nee Ling S., Magosso E., Abdul Karim Khan N., Hay Yuen K., Anne Barker S. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2008;32:335–345. doi: 10.1080/03639040500519102. [DOI] [PubMed] [Google Scholar]
- 195.Yoshikawa H. Lymphatic delivery in ‘rectal drug delivery systems’. Adv Drug Deliv Rev. 1997;28:239–251. [Google Scholar]
- 196.Ma B.L., Yang Y., Dai Y., Li Q., Lin G., Ma Y.M. Polyethylene glycol 400 (PEG400) affects the systemic exposure of oral drugs based on multiple mechanisms: taking berberine as an example. RSC Adv. 2017;7:2435–2442. [Google Scholar]
- 197.Takakura Y., Matsumoto S., Hashida M., Sezaki H. Enhanced lymphatic delivery of mitomycin-C conjugated with dextran. Cancer Res. 1984;44:2505–2510. [PubMed] [Google Scholar]
- 198.Soudry-Kochavi L., Naraykin N., Nassar T., Benita S. Improved oral absorption of exenatide using an original nanoencapsulation and microencapsulation approach. J Control Release. 2015;217:202–210. doi: 10.1016/j.jconrel.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 199.Pustylnikov S., Sagar D., Jain P., Khan Z.K. Targeting the c-type lectins-mediated host-pathogen interactions with dextran. J Pharm Pharm Sci. 2014;17:371–392. doi: 10.18433/j3n590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shackleford D.M., Faassen W.A., Houwing N., Lass H., Edwards G.A., Porter C.J. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther. 2003;306:925–933. doi: 10.1124/jpet.103.052522. [DOI] [PubMed] [Google Scholar]
- 201.Amory J.K., Scriba G.K.E., Amory D.W., Bremner W.J. Oral testosterone-triglyceride conjugate in rabbits: single-dose pharmacokinetics and comparison with oral testosterone undecanoate. J Androl. 2003;24:716–720. doi: 10.1002/j.1939-4640.2003.tb02732.x. [DOI] [PubMed] [Google Scholar]
- 202.Cui W.P., Zhang S.W., Zhao H.Q., Luo C., Sun B.J., Li Z.B. Formulating a single thioether-bridged oleate prodrug into a self-nanoemulsifying drug delivery system to facilitate oral absorption of docetaxel. Biomater Sci. 2019;7:1117–1131. doi: 10.1039/c8bm00947c. [DOI] [PubMed] [Google Scholar]
- 203.Tian C., Guo J., Wang G., Sun B., Na K., Zhang X. Efficient intestinal digestion and on site tumor-bioactivation are the two important determinants for chylomicron-mediated lymph-targeting triglyceride-mimetic docetaxel oral prodrugs. Adv Sci. 2019;6:1901810. doi: 10.1002/advs.201901810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Murota K., Cermak R., Terao J., Wolffram S. Influence of fatty acid patterns on the intestinal absorption pathway of quercetin in thoracic lymph duct-cannulated rats. Br J Nutr. 2013;109:2147–2153. doi: 10.1017/S0007114512004564. [DOI] [PubMed] [Google Scholar]
- 205.Kumar V.V., Chandrasekar D., Ramakrishna S., Kishan V., Rao Y.M., Diwan P.V. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335:167–175. doi: 10.1016/j.ijpharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 206.Elsheikh M.A., Elnaggar Y.S.R., Hamdy D.A., Abdallah O.Y. Novel cremochylomicrons for improved oral bioavailability of the antineoplastic phytomedicine berberine chloride: optimization and pharmacokinetics. Int J Pharm. 2018;535:316–324. doi: 10.1016/j.ijpharm.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 207.Sanjula B., Shah F.M., Javed A., Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17:249–256. doi: 10.1080/10611860902718672. [DOI] [PubMed] [Google Scholar]
- 208.Mao Y., Feng S., Li S., Zhao Q., Di D., Liu Y. Chylomicron-pretended nano-bio self-assembling vehicle to promote lymphatic transport and galts target of oral drugs. Biomaterials. 2019;188:173–186. doi: 10.1016/j.biomaterials.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 209.Sha X., Wu J., Chen Y., Fang X. Self-microemulsifying drug-delivery system for improved oral bioavailability of probucol: preparation and evaluation. Int J Nanomed. 2012;7:705–712. doi: 10.2147/IJN.S28052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wu L., Bi Y., Wu H. Formulation optimization and the absorption mechanisms of nanoemulsion in improving baicalin oral exposure. Drug Dev Ind Pharm. 2017;44:266–275. doi: 10.1080/03639045.2017.1391831. [DOI] [PubMed] [Google Scholar]
- 211.Iwanaga K., Kushibiki T., Miyazaki M., Kakemi M. Disposition of lipid-based formulation in the intestinal tract affects the absorption of poorly water-soluble drugs. Bio Pharm Bull. 2006;29:508–512. doi: 10.1248/bpb.29.508. [DOI] [PubMed] [Google Scholar]
- 212.Baek J.-S., Cho C.-W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur J Pharm Biopharm. 2017;117:132–140. doi: 10.1016/j.ejpb.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 213.Tong Y., Zhang Q., Shi W., Wang J. Mechanisms of oral absorption improvement for insoluble drugs by the combination of phospholipid complex and SNEDDS. Drug Deliv. 2019;26:1155–1166. doi: 10.1080/10717544.2019.1686086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang Z., Gao F., Bu H., Xiao J., Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine. 2012;8:740–747. doi: 10.1016/j.nano.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 215.Zhang J., Li J., Ju Y., Fu Y., Gong T., Zhang Z. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12:504–513. doi: 10.1021/mp5005806. [DOI] [PubMed] [Google Scholar]
- 216.Sato Y., Joumura T., Nashimoto S., Yokoyama S., Takekuma Y., Yoshida H. Enhancement of lymphatic transport of lutein by oral administration of a solid dispersion and a self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2018;127:171–176. doi: 10.1016/j.ejpb.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 217.Damge C., Maincent P., Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release. 2007;117:163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 218.Xie Y., Jiang S., Xia F., Hu X., He H., Yin Z. Glucan microparticles thickened with thermosensitive gels as potential carriers for oral delivery of insulin. J Mater Chem B. 2016;4:4040–4048. doi: 10.1039/c6tb00237d. [DOI] [PubMed] [Google Scholar]
- 219.Kim K.S., Kwag D.S., Hwang H.S., Lee E.S., Bae Y.H. Immense insulin intestinal uptake and lymphatic transport using bile acid conjugated partially uncapped liposome. Mol Pharm. 2018;15:4756–4763. doi: 10.1021/acs.molpharmaceut.8b00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lin P.Y., Chen K.H., Miao Y.B., Chen H.L., Lin K.J., Chen C.T. Phase-changeable nanoemulsions for oral delivery of a therapeutic peptide: toward targeting the pancreas for antidiabetic treatments using lymphatic transport. Adv Funct Mater. 2019;29:1809015. [Google Scholar]
- 221.Pooja D., Kulhari H., Kuncha M., Rachamalla S.S., Adams D.J., Bansal V. Improving efficacy, oral bioavailability, and delivery of paclitaxel using protein-grafted solid lipid nanoparticles. Mol Pharm. 2016;13:3903–3912. doi: 10.1021/acs.molpharmaceut.6b00691. [DOI] [PubMed] [Google Scholar]
- 222.Zhou X., Ling K., Liu M., Zhang X., Ding J., Dong Y. Targeted delivery of cisplatin-derived nanoprecursors via a biomimetic yeast microcapsule for tumor therapy by the oral route. Theranostics. 2019;9:6568–6586. doi: 10.7150/thno.35353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tunki L., Kulhari H., Vadithe L.N., Kuncha M., Bhargava S., Pooja D. Modulating the site-specific oral delivery of sorafenib using sugar-grafted nanoparticles for hepatocellular carcinoma treatment. Eur J Pharm Sci. 2019;137:104978. doi: 10.1016/j.ejps.2019.104978. [DOI] [PubMed] [Google Scholar]
- 224.Ding J., Sun Y., Li J., Wang H., Mao S. Enhanced blood–brain barrier transport of vinpocetine by oral delivery of mixed micelles in combination with a message guider. J Drug Target. 2017;25:532–540. doi: 10.1080/1061186X.2017.1289541. [DOI] [PubMed] [Google Scholar]
- 225.Salman H.H., Irache J.M., Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27:4784–4790. doi: 10.1016/j.vaccine.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 226.Mishra N., Tiwari S., Vaidya B., Agrawal G.P., Vyas S.P. Lectin anchored PLGA nanoparticles for oral mucosal immunization against hepatitis B. J Drug Target. 2011;19:67–78. doi: 10.3109/10611861003733946. [DOI] [PubMed] [Google Scholar]
- 227.Gupta P.N., Vyas S.P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf B Biointerfaces. 2011;82:118–125. doi: 10.1016/j.colsurfb.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 228.Han S., Quach T., Hu L., Wahab A., Charman W.N., Stella V.J. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J Control Release. 2014;177:1–10. doi: 10.1016/j.jconrel.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 229.Lee J.B., Zgair A., Malec J., Kim T.H., Kim M.G., Ali J. Lipophilic activated ester prodrug approach for drug delivery to the intestinal lymphatic system. J Control Release. 2018;286:10–19. doi: 10.1016/j.jconrel.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zaghloul A., Lila A., Abd-Allah F., Nada A. Probucol self-emulsified drug delivery system: stability testing and bioavailability assessment in human volunteers. Curr Drug Deliv. 2019;16:325–330. doi: 10.2174/1567201816666181227111912. [DOI] [PubMed] [Google Scholar]
- 231.Khan S., Baboota S., Ali J., Khan S., Narang R.S., Narang J.K. Nanostructured lipid carriers: an emerging platform for improving oral bioavailability of lipophilic drugs. Int J Pharm Investig. 2015;5:182–191. doi: 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Parayath N.N., Nehoff H., Muller P., Taurin S., Greish K. Styrene maleic acid micelles as a nanocarrier system for oral anticancer drug delivery—dual uptake through enterocytes and M-cells. Int J Nanomed. 2015;10:4653–4667. doi: 10.2147/IJN.S87681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hong F., Yan J., Baran J.T., Allendorf D.J., Hansen R.D., Ostroff G.R. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 234.Masuda Y., Inoue H., Ohta H., Miyake A., Konishi M., Nanba H. Oral administration of soluble beta-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int J Canc. 2013;133:108–119. doi: 10.1002/ijc.27999. [DOI] [PubMed] [Google Scholar]
- 235.Tang Y., Wu S., Lin J., Cheng L., Zhou J., Xie J. Nanoparticles targeted against cryptococcal pneumonia by interactions between chitosan and its peptide ligand. Nano Lett. 2018;18:6207–6213. doi: 10.1021/acs.nanolett.8b02229. [DOI] [PubMed] [Google Scholar]
- 236.Zhang X., Xu X., Chen Y., Dou Y., Zhou X., Li L. Bioinspired yeast microcapsules loaded with self-assembled nanotherapies for targeted treatment of cardiovascular disease. Mater Today. 2017;20:301–313. [Google Scholar]
- 237.Tosi G., Ruozi B., Belletti D., Vilella A., Zoli M., Vandelli M.A. Brain-targeted polymeric nanoparticles: in vivo evidence of different routes of administration in rodents. Nanomedicine. 2013;8:1373–1383. doi: 10.2217/nnm.12.172. [DOI] [PubMed] [Google Scholar]
- 238.Serrano D.R., Lalatsa A., Dea-Ayuela M.A., Bilbao-Ramos P.E., Garrett N.L., Moger J. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol Pharm. 2015;12:420–431. doi: 10.1021/mp500527x. [DOI] [PubMed] [Google Scholar]
- 239.Kim S.H., Seo K.W., Kim J., Lee K.Y., Jang Y.S. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010;185:5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 240.Shakya A.K., Chowdhury M.Y.E., Tao W., Gill H.S. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang L., Yang W., Hu C., Wang Q., Wu Y. Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination. Int J Nanomed. 2018;13:2973–2987. doi: 10.2147/IJN.S154743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang L., Hu C., Yang W., Liu X., Wu Y. Chemical synthesis, versatile structures and functions of tailorable adjuvants for optimizing oral vaccination. ACS Appl Mater Interfaces. 2016;8:34933–34950. doi: 10.1021/acsami.6b10470. [DOI] [PubMed] [Google Scholar]
- 243.Shima H., Watanabe T., Fukuda S., Fukuoka S., Ohara O., Ohno H. A novel mucosal vaccine targeting Peyer's patch M cells induces protective antigen-specific IgA responses. Int Immunol. 2014;26:619–625. doi: 10.1093/intimm/dxu061. [DOI] [PubMed] [Google Scholar]
- 244.Mahapatro A., Singh D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gandhi G.R., Neta M., Sathiyabama R.G., Quintans J.S.S., de Oliveira E.S.A.M., Araujo A.A.S. Flavonoids as Th1/Th2 cytokines immunomodulators: a systematic review of studies on animal models. Phytomedicine. 2018;44:74–84. doi: 10.1016/j.phymed.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 246.Jones S.W., Roberts R.A., Robbins G.R., Perry J.L., Kai M.P., Chen K. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest. 2013;123:3061–3073. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yeboah K.G., Akande J., Addo R.T., Siwale R.C., Aninkorah-Yeboah K., Siddig A. In vitro and ex vivo characterization of lectin-labeled mycobacterium tuberculosis antigen-containing microspheres for enhanced oral delivery. J Drug Target. 2014;22:34–47. doi: 10.3109/1061186X.2013.833206. [DOI] [PubMed] [Google Scholar]
- 248.Finn R., Ahmad T., Coffey E.T., Brayden D.J., Baird A.W., Boyd A. Translocation of vibrio parahaemolyticus across an in vitro M cell model. FEMS Microbiol Lett. 2014;350:65–71. doi: 10.1111/1574-6968.12323. [DOI] [PubMed] [Google Scholar]
- 249.Ude V.C., Brown D.M., Maciaszek K., Stone V., Johnston H.J. Comparing the sensitivity of different intestinal Caco-2 in vitro monocultures and co-cultures to amorphous silicon dioxide nanomaterials and the clay montmorillonite. NanoImpact. 2019;15:100165. [Google Scholar]
- 250.Ahmad T., Gogarty M., Walsh E.G., Brayden D.J. A comparison of three Peyer's patch "M-like" cell culture models: particle uptake, bacterial interaction, and epithelial histology. Eur J Pharm Biopharm. 2017;119:426–436. doi: 10.1016/j.ejpb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 251.Kerneis S., Bogdanova A., Kraehenbuhl J.P., Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 252.Gullberg E., Leonard M., Karlsson J., Hopkins A.M., Brayden D., Baird A.W. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Bioph Res Co. 2000;279:808–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 253.des Rieux A., Fievez V., Théate I., Mast J., Préat V., Schneider Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30:380–391. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 254.Pielage J.F., Cichon C., Greune L., Hirashima M., Kucharzik T., Schrnidt M.A. Reversible differentiation of Caco-2 cells reveals galectin-9 as a surface marker molecule for human follicle-associated epithelia and M cell-like cells. Int J Biochem Cell Biol. 2007;39:1886–1901. doi: 10.1016/j.biocel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 255.Lo D., Tynan W., Dickerson J., Scharf M., Cooper J., Byrne D. Cell culture modeling of specialized tissue: identification of genes expressed specifically by follicle-associated epithelium of Peyer's patch by expression profiling of Caco-2/Raji co-cultures. Int Immunol. 2004;16:91–99. doi: 10.1093/intimm/dxh011. [DOI] [PubMed] [Google Scholar]
- 256.Tonry J.H., Popov S.G., Narayanan A., Kashanchi F., Hakami R.M., Carpenter C. In vivo murine and in vitro M-like cell models of gastrointestinal anthrax. Microb Infect. 2013;15:37–44. doi: 10.1016/j.micinf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 257.Kenngott E.E., Kiefer R., Schneider-Daum N., Hamann A., Schneider M., Schmitt M.J. Surface-modified yeast cells: a novel eukaryotic carrier for oral application. J Control Release. 2016;224:1–7. doi: 10.1016/j.jconrel.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 258.Liang E., Kabcenell A.K., Coleman J.R., Robson J., Ruffles R., Yazdanian M. Permeability measurement of macromolecules and assessment of mucosal antigen sampling using in vitro converted M cells. J Pharmacol Toxicol. 2001;46:93–101. doi: 10.1016/s1056-8719(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 259.Beloqui A., Brayden D.J., Artursson P., Preat V., des Rieux A. A human intestinal M-cell-like model for investigating particle, antigen and microorganism translocation. Nat Protoc. 2017;12:1387–1399. doi: 10.1038/nprot.2017.041. [DOI] [PubMed] [Google Scholar]
- 260.Holm R., Mullertz A., Christensen E., Hoy C.E., Kristensen H.G. Comparison of total oral bioavailability and the lymphatic transport of halofantrine from three different unsaturated triglycerides in lymph-cannulated conscious rats. Eur J Pharm Sci. 2001;14:331–337. doi: 10.1016/s0928-0987(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 261.Michaelsen M.H., Wasan K.M., Sivak O., Mullertz A., Rades T. The effect of digestion and drug load on halofantrine absorption from self-nanoemulsifying drug delivery system (SNEDDS) AAPS J. 2016;18:180–186. doi: 10.1208/s12248-015-9832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chaudhary S., Garg T., Murthy R.S., Rath G., Goyal A.K. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 2014;22:871–882. doi: 10.3109/1061186X.2014.950664. [DOI] [PubMed] [Google Scholar]
- 263.Yu Z., Fan W., Wang L., He H., Lv Y., Qi J. Slowing down lipolysis significantly enhances the oral absorption of intact solid lipid nanoparticles. Biomater Sci. 2019;7:4273–4282. doi: 10.1039/c9bm00873j. [DOI] [PubMed] [Google Scholar]
- 264.Krishnan Y., Mukundan S., Akhil S., Gupta S., Viswanad V. Enhanced lymphatic uptake of leflunomide loaded nanolipid carrier via chylomicron formation for the treatment of rheumatoid arthritis. Adv Pharm Bull. 2018;8:257–265. doi: 10.15171/apb.2018.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Han S., Hu L., Gracia Quach T., Simpson J.S., Edwards G.A. Lymphatic transport and lymphocyte targeting of a triglyceride mimetic prodrug is enhanced in a large animal model: studies in greyhound dogs. Mol Pharm. 2016;13:3351–3361. doi: 10.1021/acs.molpharmaceut.6b00195. [DOI] [PubMed] [Google Scholar]
- 266.Edwards G.A., Porter C.J.H., Caliph S.M., Khoo S.M., Charman W.N. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 2001;50:45–60. doi: 10.1016/s0169-409x(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 267.Uwiera R.R., Mangat R., Kelly S., Uwiera T.C., Proctor S.D. Long-term catheterization of the intestinal lymph trunk and collection of lymph in neonatal pigs. J Vis Exp. 2016;109:53457. doi: 10.3791/53457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nguyen T.M., Sawyer J.K., Kelley K.L., Davis M.A., Kent C.R., Rudel L.L. Acat2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J Lipid Res. 2012;53:1598–1609. doi: 10.1194/jlr.M026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Trevaskis N.L., Hu L., Caliph S.M., Han S., Porter C.J. The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. Jove-J Vis Exp. 2015 doi: 10.3791/52389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Patel M.H., Sawant K.K. Self microemulsifying drug delivery system of lurasidone hydrochloride for enhanced oral bioavailability by lymphatic targeting: in vitro, Caco-2 cell line and in vivo evaluation. Eur J Pharm Sci. 2019;138:105027. doi: 10.1016/j.ejps.2019.105027. [DOI] [PubMed] [Google Scholar]
- 271.Dahan A., Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24:381–388. doi: 10.1016/j.ejps.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 272.Lind M.L., Jacobsen J., Holm R., Mullertz A. Intestinal lymphatic transport of halofantrine in rats assessed using a chylomicron flow blocking approach: the influence of polysorbate 60 and 80. Eur J Pharm Sci. 2008;35:211–218. doi: 10.1016/j.ejps.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 273.Li F., Hu R., Wang B., Gui Y., Cheng G., Gao S. Self-microemulsifying drug delivery system for improving the bioavailability of huperzine a by lymphatic uptake. Acta Pharm Sin B. 2017;7:353–360. doi: 10.1016/j.apsb.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sun M., Zhai X., Xue K., Hu L., Yang X., Li G. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci. 2011;43:132–140. doi: 10.1016/j.ejps.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 275.Donaldson D.S., Kobayashi A., Ohno H., Yagita H., Williams I.R., Mabbott N.A. M cell-depletion blocks oral prion disease pathogenesis. Mucosal Immunol. 2012;5:216–225. doi: 10.1038/mi.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bermudez L.E., Petrofsky M., Sommer S., Barletta R.G. Peyer's patch-deficient mice demonstrate that mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination. Infect Immun. 2010;78:3570–3577. doi: 10.1128/IAI.01411-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kolawole A.O., Gonzalez-Hernandez M.B., Turula H., Yu C., Elftman M.D., Wobus C.E. Oral norovirus infection is blocked in mice lacking Peyer's patches and mature M cells. J Virol. 2016;90:1499–1506. doi: 10.1128/JVI.02872-15. [DOI] [PMC free article] [PubMed] [Google Scholar]