Abstract
Lipid-based formulations (LBFs) have demonstrated a great potential in enhancing the oral absorption of poorly water-soluble drugs. However, construction of in vitro and in vivo correlations (IVIVCs) for LBFs is quite challenging, owing to a complex in vivo processing of these formulations. In this paper, we start with a brief introduction on the gastrointestinal digestion of lipid/LBFs and its relation to enhanced oral drug absorption; based on the concept of IVIVCs, the current status of in vitro models to establish IVIVCs for LBFs is reviewed, while future perspectives in this field are discussed. In vitro tests, which facilitate the understanding and prediction of the in vivo performance of solid dosage forms, frequently fail to mimic the in vivo processing of LBFs, leading to inconsistent results. In vitro digestion models, which more closely simulate gastrointestinal physiology, are a more promising option. Despite some successes in IVIVC modeling, the accuracy and consistency of these models are yet to be validated, particularly for human data. A reliable IVIVC model can not only reduce the risk, time, and cost of formulation development but can also contribute to the formulation design and optimization, thus promoting the clinical translation of LBFs.
KEY WORDS: Lipid-based formulation, In vitro and in vivo correlations, Lipolysis, Absorption, Oral delivery, Model, In silico prediction, Perspectives
Abbreviations: ANN, artificial neural network; AUC, area under the curve; BE, bioequivalence; BCS, biopharmaceutics classification system; CETP, cholesterol ester transfer protein; Cmax, peak plasma concentration; DDS, drug delivery system; FDA, US Food and Drug Administration; GI, gastrointestinal; HLB, hydrophilic–lipophilic balance; IVIVC, in vitro and in vivo correlation; IVIVR, in vitro and in vivo relationship; LBF, lipid-based formulation; LCT, long-chain triglyceride; MCT, medium-chain triglyceride; PBPK, physiologically based pharmacokinetic; PK, pharmacokinetic; SCT, short-chain triglyceride; SEDDS, self-emulsifying drug delivery system; SGF, simulated gastric fluid; SIF, simulated intestinal fluid; SLS, sodium lauryl sulfate; SMEDDS, self-microemulsifying drug delivery system; SNEDDS, self-nanoemulsifying drug delivery system; TIM, TNO gastrointestinal model; TNO, Netherlands Organization for Applied Scientific Research; Tmax, time to reach the peak plasma concentration
Graphical abstract
In vitro and in vivo correlations (IVIVCs) are powerful tools for preparation development. Constructing IVIVCs for lipid-based formulations is rather challenging. This article reviews the current status and future perspectives in this field.
1. Introduction
Oral route is the most popular way for drug administration. Currently, more than 50% of marketed drugs and 90% of drug candidates are poorly water soluble, and these proportions continue to grow because of the rapid progress in drug discovery1, 2, 3, 4, 5. Since dissolution is a prerequisite for drug absorption, poor solubility always leads to retarded dissolution rate and, thereby, poor bioavailability. The situation is even worse for drug candidates with poor solubility and poor permeability6,7. Great efforts have been made in the past to improve poor bioavailability of such compounds in an attempt to unlock their therapeutic potential as oral medicines and achieve some success8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18. The enhancement of dissolution and absorption is one of the enduring research topics in pharmaceutical researches19, 20, 21.
Inspired by the positive “pharmaceutical food effect”22, lipid-based formulations (LBFs) have been developed and demonstrated a great potential in enhancing the oral bioavailability of poorly water-soluble drugs23,24. Based on their components and contents, four main classes of LBFs have been evolved25. Type I LBFs are lipid solutions, which are non-dispersible in aqueous media but release co-formulated drugs upon digestion. Type II LBFs are self-emulsifying drug delivery systems (SEDDSs) comprising lipids and surfactants. The surfactants bear a hydrophilic–lipophilic balance (HLB) value of less than 12, and the type II LBFs generally form emulsions in aqueous media. Type III LBFs consist of lipids, hydrophilic surfactants with a HLB value larger than 12, and hydrophilic cosolvents. They are subdivided into types IIIa (SEDDSs) and IIIb [self-microemulsifying DDSs (SMEDDSs) and self-nanoemulsifying DDSs (SNEDDSs)], based on the size of the formed emulsions. Type IV LBFs only contain surfactants and hydrophilic cosolvents, without lipids, and form micelles when dispersed in water. All of the types of LBFs have been available in market, being shown in Table 126. The first approval for each types of LBFs by the US Food and Drug Administration (FDA) is in 1941 (Drisdol®, type I), 1983 (Sandimmune®, type II), 1995 (Neoral®, type III), and 1999 (Agenerase®, type IV), respectively23. Numerous discoveries and substantial improvements have been achieved in the field of LBFs in the last 5 years, bringing this old technology back to the limelight27. Nonetheless, very few LBFs are available as commercial products on the market, while some have been discontinued (Table 1)26,28. On the one side, the problem is due to the scale-up and stability challenges. The majority of LBFs are filled in soft gelatin capsules for clinical application. However, in-house manufacturing capabilities of soft gelatin may be missing in a few countries, while soft gelatin capsules are not acceptable in all countries. In addition, incompatibility of the excipients with the shells of the soft gelatin as well as precipitation of the active ingredients during storage at a lower temperature are common stability issues for LBFs, which requires solidification of the formulation29, 30, 31. On the other side, the lack of in vitro tests that are able to predict the in vivo behavior of LBFs with much accuracy, is the crucial reason for the limited number of products10,32.
Table 1.
FDA-approved drugs utilizing lipid systems.
| Molecule (trade name) | New drug application year | Biopharmaceutic classification system | Type of lipid-based formulation | Oil | Surfactant (HLB <12) | Surfactant (HLB >12) | Hydrophilic cosolvent |
|---|---|---|---|---|---|---|---|
| Ergocalciferol (Drisdol®) | 1941 | 3 | I | Soybean oil | ‒ | ‒ | ‒ |
| Calcitriol (Rocaltrol®) | 1978 | 2/4 | I | Fractionated triglycerides of coconut oil | ‒ | ‒ | ‒ |
| Valproic acid (Depakene®) | 1978 | 1 | I | Corn oil | ‒ | ‒ | ‒ |
| Isotretinoin (Accutane®) Discontinued | 1982 | 2 | I | Beeswax, hydrogenated soybean oil flakes, hydrogenated vegetable oil, soybean oil | ‒ | ‒ | ‒ |
| Cyclosporin A (Sandimmune®) | 1983 | 2 | II | Olive oil | ‒ | Polyoxyethylated oleic glycerides | Ethanol 12.5% |
| Dronabinol (Marinol®) | 1985 | 2/4 | I | Sesame oil | ‒ | ‒ | ‒ |
| Clofazimine (Lamprene®) Discontinued | 1986 | 2 | I | Beeswax | ‒ | ‒ | ‒ |
| Cyclosporin A (Sandimmune®) | 1990 | 2 | II | Corn oil | Linoleic macroglycerides | ‒ | Ethanol 12.7% |
| Ranitidine (Zantac®) Discontinued | 1994 | 3 | ‒ | Medium-chain triglycerides | Gelucire 33/01 | ‒ | ‒ |
| Cyclosporin A (Neoral®) | 1995 | 2 | III A/III B | Corn oil mono-di-triglycerides | ‒ | Polyoxyl 40 hydrogenated castor oil | Ethanol 11.9%, glycerol, propylene glycol |
| Tretinoin (Vesanoid®) Discontinued | 1995 | ‒ | I | Beeswax, hydrogenated soybean oil flakes, hydrogenated vegetable oil, soybean oil | ‒ | ‒ | ‒ |
| Ritonavir (Norvir®) | 1996 | 4 | III A | ‒ | Oleic acid | Polyoxyl 35 castor oil | Ethanol |
| Saquinavir (Fortovase®) Discontinued | 1997 | 4 | ‒ | Medium-chain mono- and di-glycerides | ‒ | ‒ | ‒ |
| Progesterone (Prometrium®) | 1998 | 2 | I | Peanut oil | ‒ | ‒ | ‒ |
| Amprenavir (Agenerase®) Discontinued | 1999 | 2 | IV | ‒ | ‒ | Vitamin E TPGS | PEG400, propylene glycol |
| Bexarotene (Targretin®) | 1999 | ‒ | IV | ‒ | ‒ | Polysorbate 20 | PEG400 |
| Doxercalciferol (Hectorol®) | 1999 | 2/4 | I | Coconut oil | ‒ | ‒ | Alcohol |
| Sirolimus (Rapamune®) | 1999 | ‒ | III | Phosphatidylcholine, mono- and di-glycerides, soy fatty acids, ascorbyl palmitate | ‒ | Polysorbate 80 | 1.5%–2.5% ethanol, propylene glycol |
| Cyclosporin A (Gengraf®) | 2000 | 2 | IV | ‒ | ‒ | Polysorbate 80, Polyoxyl 35 castor oil | Propylene glycol, alcohol 12.8% v/v |
| Cyclosporin A (Gengraf®) | 2000 | 2 | IV | ‒ | ‒ | Polyoxyl 40 hydrogenated castor oil, polysorbate 80 | Propylene glycol |
| Ritonavir/lopinavir (Kaletra®) Discontinued | 2000 | 4 | III | ‒ | Oleic acid | Polyoxyl 35 castor oil | Propylene glycol |
| Dutasteride (Avodart®) | 2001 | 2/4 | I | Mono-di-glycerides of caprylic/capric acid | ‒ | ‒ | ‒ |
| Isotretinoin (Claravis®) | 2003 (ANDA) | 2 | ‒ | Hydrogenated vegetable oil, soybean oil, white wax | ‒ | Polysorbate 80 | ‒ |
| Omega-3-acid ethyl esters (Lovaza®) | 2004 | ‒ | I | Soybean oil | ‒ | ‒ | ‒ |
| Tipranavir (Aptivus®) | 2005 | 4 | III A | Mono-/di-glycerides of caprylic/capric acids | ‒ | Polyoxyl 35 castor oil | Ethanol, propylene glycol |
| Tipranavir (Aptivus®) | 2005 | 4 | IV | ‒ | ‒ | Vitamin E TPGS | PEG 400, propylene glycol, water |
| Paricalcitol (Zemplar®) | 2005 | 4 | I | Medium-chain triglycerides fractionated from coconut oil or palm kernel oil | ‒ | ‒ | Alcohol |
| Lubiprostone (Amitiza®) | 2006 | 2/4 | I | Medium-chain triglycerides | ‒ | ‒ | ‒ |
| Fenofibrate (Lipofen®) | 2006 | 2 | III | ‒ | ‒ | Gelucire 44/14 (lauroyl macrogol glyceride type 1500) | ‒ |
| Topotecan HCl (Hycamtin®) | 2007 | 1 | I | Hydrogenated vegetable oil | Glyceryl monostearate | ‒ | ‒ |
| Loratadine (Claritin®) | 2008 | 2 | ‒ | Caprylic/capric glycerides | ‒ | Polysorbate 80 | ‒ |
| Isotretinoin (Absorica®) | 2012 | 2 | ‒ | Soybean oil, stearoyl polyoxylglycerides | Sorbitan monooleate | ‒ | ‒ |
| Enzalutamide (Xtandi®) | 2012 | 2 | I | Caprylocaproyl polyoxyglycerides | ‒ | ‒ | ‒ |
| Nintedanib (Ofev®) | 2014 | 2/4 | II | Medium-chain triglycerides, hard fat | Lecithin | ‒ | ‒ |
| Calcifediol (Rayaldee®) | 2016 | 2/4 | II/III | Mixture of lipophilic emulsifier with a HLB <7 and an absorption enhancer with HLB of 13–18 Oily vehicle-mineral oil, liquid paraffins, or squalene |
|||
‒, not applicable; HLB, hydrophilic–lipophilic balance.
The table is adapted from Ref. 26 complying with the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
In vitro and in vivo correlations (IVIVCs) are powerful tools for optimizing the formulation and dosage, setting dissolution limits, and reducing bioequivalence (BE) studies19,33, 34, 35, 36, 37, 38, 39. By definition, an IVIVC is a mathematical model bridging in vitro properties and an in vivo response of a preparation40. Dissolution is the most commonly used in vitro property, while the fraction of drug absorbed is the popular in vivo response. In vitro dissolution can be a surrogate for BE studies upon availability of an established IVIVC. Considerable interest in IVIVCs has been elicited in the pharmaceutical industry, academia, and regulatory sectors20,38,41, while dosage forms have been extended from oral extended-release to oral immediate-release forms19,35,42, 43, 44, 45, 46, 47, modified-release parenteral dosage forms36,47, 48, 49, 50, 51, and transdermal DDSs33,34,52, 53, 54, 55, 56. Similarly, a reliable IVIVC model could promote the development of LBFs. However, it is a significant challenge to establish IVIVCs for LBFs because of the complex in vivo process. Unlike normal dosage forms, lipid components in LBFs undergo extensive lipolysis in the gastrointestinal (GI) tract, while co-formulated drugs may precipitate or be dissolved during the intermediate phase of lipolysis24,57, 58, 59, 60, 61, 62, 63, 64, 65, 66. The lack of mechanistic understanding of the in vivo behavior of LBFs hampers the possibility of obtaining an IVIVC67.
This review briefly introduces the relationship between GI digestion of lipid/LBFs and enhanced oral drug absorption, as well as the concept of IVIVC. On this basis, the current status of establishing IVIVCs for LBFs is reviewed, and future perspectives in this field are discussed.
2. Lipid digestion and enhanced drug absorption
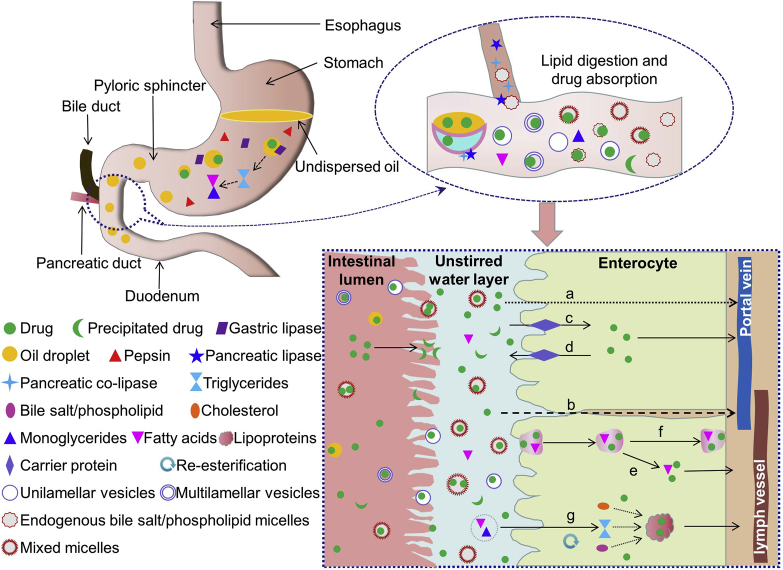
The development of LBFs was inspired by the phenomenon that a high-fat diet enhances the bioavailability of poorly water-soluble drugs. The underlying mechanisms are correlated with the digestion and absorption of lipids (Fig. 1), i.e., the “pharmaceutical food effect” promotes physiological changes, assisting drug absorption. Lipid ingestion stimulates the secretion of gastric lipase, which partly breaks down triglycerides into diglycerides and fatty acids in the stomach. The process contributes to ∼15% of the overall lipid digestion in the GI tract68. In the meantime, dietary fat is converted into an emulsion of fine oil droplets. The transfer of these lipidic substances into the duodenum stimulates the secretion of pancreatic lipase and bile. Bile salts, phospholipids, and cholesterol coat and stabilize the emulsion droplets, which become more accessible to the action of pancreatic enzymes. The remaining lipids are completely digested in the small intestine via breakdown of triglycerides into a 2-monoglyceride and two fatty acid molecules. The lipolysis proceeds from the outside with a continuously changing interface. Multilamellar liquid crystals are formed at the interface during hydrolysis and are further converted into diverse colloidal structures in combination with bile salts68. The identified structures include multilamellar and unilamellar vesicles, mixed micelles, and micelles. The lipophilic products of the breakdown of dietary fats (fatty acids and monoglycerides), as well as co-administered poorly soluble drugs, are solubilized in colloidal structures, which deliver the cargos across the unstirred water layer and reach the brush-border membranes of intestinal cells. The loaded drug may either leave the structures to diffuse across the epithelium or be absorbed as the cargo of the intact vehicles or micelles. The transepithelial pathways include passive diffusion of free drugs via either transcellular (Fig. 1a) or paracellular (Fig. 1b) way, facilitated drug influx by membrane proteins (Fig. 1c), and endocytosis (Fig. 1e) or transcytosis (Fig. 1f) of the colloidal structures. Efflux of ingested drugs is also possible (Fig. 1d). The intracellular monoglycerides and fatty acids are re-esterified to form triglycerides, which are further packed into chylomicrons and exocytosed to enter the central lacteal lymph vessels (Fig. 1g). Drugs that have a high affinity to chylomicrons may have a high potential to be transported via the lymph route, while others are mainly absorbed via the hepatic portal vein32,59,63,69, 70, 71, 72.
Figure 1.
Illustration of gastrointestinal lipid digestion and enhanced absorption of co-administered drugs. Digestion of triglycerides in gastrointestinal tract liberates monoglycerides and fatty acids, which form unilamellar/multilamellar vesicles, mixed micelles, and micelles in combination with endogenous bile salts and phospholipids. Co-administrated drugs are solubilized in these colloidal structures, delivered across the unstirred water layer, and reach the enterocytes. The drug molecules may be released from the structures and diffuse to the basolateral side via either (a) transcellular or (b) paracellular pathway. Facilitated drug (c) influx by membrane proteins and (d) efflux of ingested drugs are also possible. In addition, the drug loaded vehicles or micelles may be absorbed via (e) endocytosis and (f) transcytosis pathways. The intracellular monoglycerides and fatty acids are re-esterified to form triglycerides, which are further (g) packed into chylomicrons. Drugs with high affinity to chylomicrons are then transported via the lymph route with chylomicrons.
Similarly, LBFs play a beneficial role in solubilization and absorption of co-administered poorly soluble drugs. The presence of LBFs in the GI tract also stimulates the secretion of endogenous lipases and bile73. The biliary lipids are combined with the exogenous lipids and lipid digestion products to form complex colloidal structures74,75. During this process, co-formulated drugs may either be solubilized in the intermediate colloidal phases or precipitate. It is reasonable to expect good IVIVCs for LBFs that keep solubilization of co-delivered drugs during lipolysis. However, recent studies on halofantrine and cinnarizine SNEDDSs have shown controversial results for general cognition67,76. The formulations that underwent rapid drug precipitation during in vitro lipolysis had similar bioavailability to those that did not show any precipitation. Although the reason was attributed to ready redissolution of the precipitated drugs, due to their amorphous state, the situation complicates the establishment of IVIVCs for LBFs. Nonetheless, the dispersion and digestion of formulation-derived lipids as well as the solubilization of co-administered drugs in the GI tract should be systematically considered in vitro models to obtain a more accurate prediction of the in vivo performance of LBFs.
3. A brief introduction to IVIVCs
In 1997, the FDA published guidelines concerning the construction of IVIVCs for development of extended-release oral preparations40. Four levels (A, B, C, and multiple C) of IVIVCs were proposed in the guidance based on the correlating relationships between in vitro data and the plasma drug concentration–time curve77. Level A is a point-to-point correlation between in vitro dissolution and in vivo drug absorption78, wherein a straight line through the origin with a slope of one is obtained79. As the highest degree of correlation, level A is the only one that is recognized by FDA to grant a biowaiver from in vitro dissolution tests80. In addition, the level A correlation helps control the quality of the formula and choose an appropriate formula39,81,82. The principles of statistical moment analysis are adopted in the construction of level B IVIVC, wherein the mean in vitro dissolution time correlates with the mean in vivo residence time. Due to the absence of a point-to-point correlation, level B IVIVC is unable to predict the in vivo performance of preparations. The level C is the lowest level of IVIVC, which shows a single-point correlation between in vitro parameters (e.g., the time for 50% of the drug being dissolved or a dissolution percentage at 4 h) and pharmacokinetic (PK) parameters [e.g., area under the curve (AUC), peak plasma concentration (Cmax), and time to reach Cmax (Tmax)]. Level C IVIVC is mainly adopted for formulation screening and development of quality standards. Multiple level C is a multiple correlation between drug dissolution in vitro at different time points (at least three points) and one or several PK parameters.
4. In vitro release/dispersion and IVIVC
Since the release of drug substance from dosage forms and the subsequent solubilization of the released drugs under physiological conditions are critical steps for drug absorption via oral route, in vitro dissolution is the main test for the prediction of the in vivo performance of oral solid preparations83. Similarly, in vitro release from SMEDDSs in enzyme-free aqueous media was first used to establish the IVIVCs for LBFs84,85. The process, which uses a USP type II dissolution apparatus, is rather simple. The rationale for this test is based on the recognition that the solubilized drug, instead of the precipitated one, is available for absorption. Hence, an IVIVC may be achieved using this in vitro release/dispersion test86, 87, 88, 89. Inspired by a level A IVIVC for a cyclosporine SMEDDS, a biowaiver extension for a poorly water-soluble drug was claimed using a SMEDDS formulation84. However, during the in vitro release process, co-formulated drugs are not released to the media in the molecular form because SMEDDSs spontaneously form drug-loaded microemulsions, resulting in a dispersion process rather than drug release. The situation is completely different from that of solid dosage forms, wherein released drugs are solubilized in the media and available for absorption. In addition, this test ignores possible in vivo precipitation of dispersed drugs, due to the lipolysis of formulations in the GI tract, which leads to inconsistent results in terms of obtaining an IVIVC90.
As a result of an insufficiently accurate simulation of the physiological environment in the GI tract by compendial media, biorelevant dissolution media were developed to achieve a better IVIVC for poorly water-soluble drugs91, 92, 93, 94, 95, 96. Simulated gastric fluid (SGF) containing 0.5% (w/v) sodium lauryl sulfate (SLS) was adopted for in vitro drug release from an olmesartan medoxomil-loaded SMEDDS using a USP type II dissolution apparatus97. A high predictive power of the in vitro dissolution performance for the in vivo absorption was revealed by obtaining a level A IVIVC. Furthermore, a dialysis bag method was developed to understand the drug release profile, which was performed in SGF containing 0.5% (w/v) SLS for 1 h and simulated intestinal fluid (SIF) for another 2 h69. In a membrane with a cutoff of 12 kDa, >90% of the drug was released within 1 h, of which nearly 80% was released within 30 min. In a membrane with a cutoff of 1 kDa, only 13%–22% of the drug was released within 30 min, and a maximum of 54%–61% of the drug was released within 3 h. The reduced drug release profile of the 1 kDa membrane was due to the small cutoff, which only allowed a passage of free drug molecules. Nonetheless, in addition to a level A and a level B IVIVC, a level C correlation was achieved between in vitro drug release parameters (t30%, t50%, and t90%) and Cmax, Tmax, and AUC.
In some cases, an in vitro release in biorelevant media failed to produce an IVIVC98. The in vitro release of fenofibrate from LBFs was shown to be dependent on both biorelevant media and the LBF composition (Tween 80 with different lipids). In contrast to the in vitro results, the tested LBFs exhibited similar in vivo performance in rats in both fasted and fed states. The authors attributed these inconsistencies to incessant excretion of bile in rats, leading to the enhanced solubility of fenofibrate in vivo. Therefore, animal model may be crucial in the establishment of IVIVC.
The dissolution apparatus may also affect the construction of an IVIVC. The paddle (USP Apparatus 2) and Bio-Dis (USP Apparatus 3) methods were used to study the release of RZ-50 from lipid suspensions in compendial and biorelevant media, respectively99. The paddle method led to a very low drug release due to the poor dispersibility of the formulation, whereas the Bio-Dis method enhanced drug release by facilitating emulsification of the formulation. A level A IVIVC was obtained under fed gastric conditions using the Bio-Dis method.
5. In vitro digestion models and IVIVC
Despite attractive and simple, in vitro release/dispersion is not suitable to predict the in vivo performance of LBFs because of the inconsistency in achieving IVIVCs66. The primary drawback of the test is the lack of mimicking the complex in vivo digestion of LBFs and micellar solubilization100. Accordingly, in vitro lipolysis is more suitable for assessing the fate of LBFs by mimicking the intestinal lipid digestion process101, 102, 103. To obtain a strong IVIVC, it is crucial to simulate the complex physiological conditions that present in the human GI tract, such as pH, enzymes, transit times, and mixing104,105. However, none of the currently available models can simulate all of these complex multistage processes owing to technical challenges. Only simplified digestion models have been developed by capturing one or more key elements in human GI digestion. The pH-stat lipolysis model and the TNO (Netherlands Organization for Applied Scientific Research) GI model (TIM-1), which differ in the complexity, compartmental numbers, and physiological effects considered, are the most commonly used models for the evaluation of LBFs.
5.1. pH-stat lipolysis model
The pH-stat lipolysis model, which mainly simulates enzymatic digestion, is the most frequently used model in the evaluation of LBFs. Since retention of administered LBFs is negligible in the oral cavity, the model typically mimics the enzymatic conditions in the intestinal (one-compartment) or GI (one- or two-compartment) phase of digestion, while studies are all performed at a fixed pH.
5.1.1. One-compartment intestinal digestion model
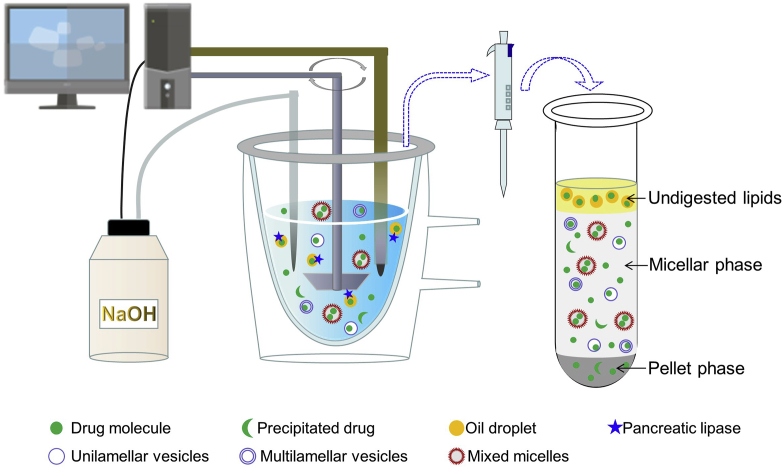
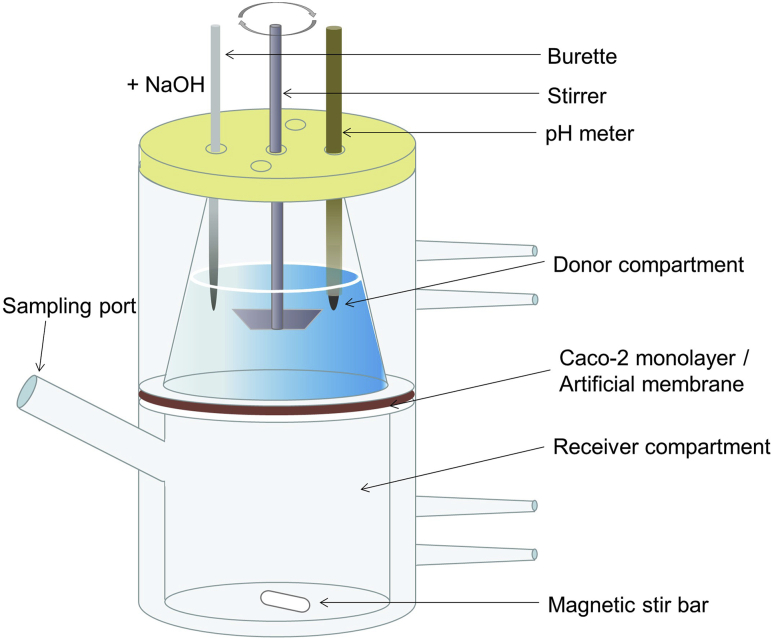
The experimental setup mainly comprises a thermostated vessel (generally, at 37 °C), an overhead stirrer, a pH electrode, and a titrator (Fig. 2). LBFs are dispersed in a medium mimicking fasted- or fed-state intestinal digestive fluid. Initiation of lipid digestion by addition of lipase and colipase leads to the liberation of fatty acids, causing a drop in the pH consequently. The pH variation is measured by the electrode, while the released fatty acids are automatically titrated with sodium hydroxide using the titrator. The extent of the digestion can be indirectly quantified using the rate of the addition of sodium hydroxide based on its stoichiometric reaction with fatty acids. Samples can be taken during the digestion process and ultracentrifuged to obtain three distinct phases, namely, an oil phase containing undigested lipids, a micellar phase containing a solubilized drug in colloidal structures, and a pellet phase comprising the precipitated drug. Quantification of the drug amounts in each phase enables prediction of the solubilizing capability of the formulation to co-formulated drugs in the GI tract. Furthermore, the solubilized amount of the drug in the micellar phase can be correlated with the in vivo PK parameters to construct an IVIVC. At the least, a rank order of the likely in vivo performances may be established for a series of LBFs, based on the hypothesis that the high percentage solubilized in the micellar phase results in a high bioavailability.
Figure 2.
Schematic representation of the one-compartment pH-stat lipolysis model.
5.1.2. GI digestion model
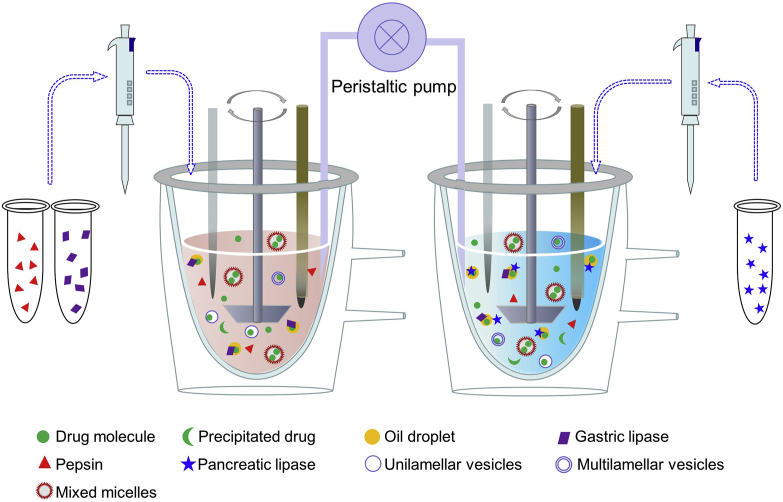
The one-compartment intestinal digestion model is simple and has been widely adopted in the evaluation of LBFs. The rationale of the model is that the intestine is the main site for lipid digestion and drug absorption. However, the model is inadequate for simulating GI physiology because it does not consider processes and conditions in the stomach. As mentioned above, lipid digestion in stomach contributes to ∼15% of the overall lipid digestion in the GI tract. In addition, the effects of gastric emptying and sudden pH changes on the solubilization of co-formulated drugs are ignored61. Therefore, GI digestion pH-stat models, either two-step one compartment or two-step two compartments, were developed to simulate both gastric and intestinal digestion106,107. In the one-compartment model, the simulated gastric and intestinal digestion is performed in two sequential steps, respectively. LBFs are first dispersed in SGF, and gastric digestion is initiated by adding gastric lipases. After a period of time, the SGF was transferred to a medium similar to the intestinal fluid by addition of a concentrated SIF and pancreatic lipases. During both steps, automatic titration with sodium hydroxide maintains a constant pH, corresponding to the gastric and intestinal pH, respectively106,107. Two individual setups of the pH-stat model are used in the two-compartment model to simulate the stomach and small intestine, respectively (Fig. 3). SGF and SIF, as well as the corresponding lipases, are respectively added to the two reaction vessels, which are connected by a peristaltic pump. During the digestion process, the medium in the gastric compartment is continuously pumped to the intestinal one at a rate mimicking gastric emptying107, 108, 109. In this regard, the two-compartment model more closely mimics the in vivo conditions than does the one-compartment model.
Figure 3.
Simulation of the digestion process in the stomach and small intestine by a two-step two-compartment digestion model.
5.1.3. IVIVCs and the pH-stat lipolysis model
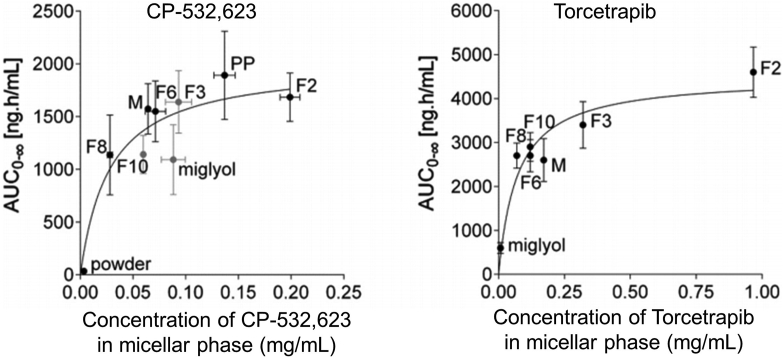
The pH-stat lipolysis model is more reliable in the rank ordering of LBFs than in the construction of level A IVIVCs. The absolute bioavailability of danazol was found to increase with the dose of Labrafil® M2125CS, while the same rank order was obtained based on the percentage of solubilized danazol in the micellar phase following in vitro lipolysis of the formulations110. However, the release profile of danazol failed to correlate with the absorption profile in the in vivo study. Similar results were obtained for a lipid solution and suspension of halofantrine102, supporting the potential utility of the model to evaluate and rank the in vivo performances of LBFs. Moreover, in vitro solubilization data for two cholesterol ester transfer protein (CETP) inhibitors, obtained using in vitro lipolysis of a series of SEDDSs, were plotted against in vivo drug exposure (AUC) with the same formulations (Fig. 4111). Although the plots were not linear, good rank orders between the in vitro and in vivo data were obvious.
Figure 4.
In vitro and in vivo correlations for two CETP inhibitors using diverse self-emulsifying drug delivery systems. The areas under the curves are plotted vs. the drug concentrations in the micellar phase during in vitro lipolysis. Reprinted with the permission from Ref. 111. Copyright © 2014 Elsevier B.V.
In addition to typical LBFs, the rank ordering capability of the pH-stat lipolysis models was demonstrated in fenofibrate-loaded lipid particles64. Nanoparticles (100 nm) showed increased absorption than did microparticles and a crystalline suspension. The data correlated well with those of in vitro lipolysis, wherein a higher level of fenofibrate in the micellar phase was obtained from the 100-nm nanoparticles than from the microparticles and suspension. Consequently, the same rank order was observed between release and absorption, that is, 100-nm nanoparticle > microparticle > suspension.
Compared with a cell model of intestinal drug permeability, the pH-stat lipolysis model provided a superior simulation of oral absorption of LBFs, facilitating the establishment of a correlation with an in vivo output63. A SNEDDS significantly increased the solubility of four Biopharmaceutics Classification System (BCS) II drugs (griseofulvin, phenytoin, indomethacin, and ketoprofen), while their permeation through MDCK cell monolayers was lower than that of saturated water solutions. These results were attributed to differences in the drug states in the formulations. In saturated aqueous solutions, drugs are dissolved and transported in a molecular form, while in SNEDDSs, drugs are trapped inside oil cores and are transported as particles. The large size of the particles, relative to that of a molecule, hinders intestinal membrane permeability of drugs. However, in vivo absorption from the SNEDDS was significantly higher than that of free drug molecules, while an in vitro and in vivo relationship (IVIVR) was demonstrated between the drug content in the lipid phase and its oral bioavailability. Similar results were obtained for dexamethasone, griseofulvin, and progesterone solubilization from long (LCT)-, medium (MCT)-, and short (SCT)-chain triglyceride formulations103,112. Good correlations between the bioavailability and the drug contents in the micellar phase of in vitro lipolysis were obtained. The rank orders were LCT = MCT = SCT for dexamethasone, MCT > LCT > SCT > H2O for griseofulvin, and MCT > LCT > SCT for progesterone. In addition, permeation of the drugs through the gut wall was tested using a modified Ussing chamber system following completion of the lipolysis. However, permeability did not correlate with the oral bioavailability. Even though the SCT formulation doubled the permeability coefficients of the drugs, the oral bioavailability of the formulation was more related to the solubilizing capability during lipolysis. More interestingly, a strong correlation with a correlation coefficient >0.99 was obtained between the griseofulvin concentrations in the micellar phase following in vitro lipolysis and the AUC values of the corresponding formulations via oral administration.
In vitro lipolysis data may fail to construct IVIVC. In some cases, bioavailability from formulations that presented rapid drug precipitation following in vitro lipolysis was similar to that from formulations that did not show any drug precipitation67,76,113. Studies on halofantrine and cinnarizine SNEDDSs revealed that the precipitates were in an amorphous form, with a rather high dissolution rate, which may explain the enhanced absorption67,76. Therefore, the authors suggested that solid-state characterization of the pellet phase is essential in validating the predictive power of the in vitro lipolysis test. However, it is also possible that in vitro lipolysis failed to mimic physiological conditions. In addition, the theory would not work for BCS IV drugs, which are poorly permeable even in a solubilized form.
Of note, variations in the data obtained across different laboratories may be due to variable experimental conditions114. The complexity of the in vivo processing of LBFs has long interfered with the establishment of robust IVIVCs for LBFs. Variations of experimental conditions in the pH and the volume of the digestive medium, the employed concentrations of bile salts and calcium, and buffering capacity, may strongly affect the establishment of an IVIVC. To obtain consistent data across different laboratories, the Lipid Formulation Classification System Consortium was established to standardize the protocols of the in vitro digestion tests for the assessment of LBFs. The Consortium has published a series of papers, to which interested readers are referred, reporting the results of systematic studies of the factors affecting IVIVCs, including method parameters, effects of bile salt concentrations and drug loading, supersaturation versus precipitation potential, lipolysis by gastric lipase, and effects of varying pancreatin and calcium levels114, 115, 116, 117, 118, 119.
5.2. TIM and IVIVC
The TIM was developed to study food products under conditions close to GI physiology of human120. The dynamic process of the transit and digestion of a meal in the GI tract was simulated in the TIM. The simulated parameters include mixing, transit, pH variation, input of digestive media, and output of water and digestive products. A computer program was utilized to control and reproduce a specific digestive setting. Protocols have been developed to simulate physiologies of different species (e.g., human, dog, pig, and calve) and different populations (e.g., the young, the adult, and the aged).
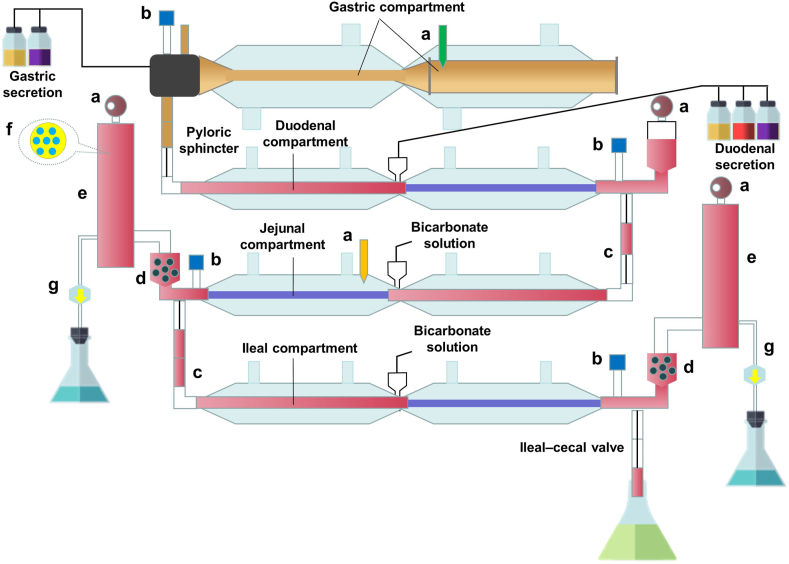
TIM-1 (Fig. 5) is the most popular configuration of the TIM platform, which consists of four tubular compartments, i.e., the gastric, the duodenal, the jejunal, and the ileal compartment, respectively. Peristaltic valve pumps connect the compartments for the passage of chyme in a controlled way. An alternating pressure is put on the flexible walls of the compartments to mix the contents. A water jacket outside the walls is used to control the temperature in the compartment. Gastric and duodenal secretions, containing bile salts, electrolytes, and digestive enzymes (pepsin, a fungal lipase as an alternative to gastric lipase, and pancreatin), are pumped into the individual compartments. The flow of all secretions is programmed in time as shown in Table 2121. The pH in the compartment is measured by an individual pH meter and is controlled via titration of hydrochloric acid or sodium bicarbonate to follow a physiologically relevant pH profile. The model also incorporates a hollow fiber membrane (cutoff size: 50 nm) on the jejunal and ileal compartments to mimic the absorption of dissolved/solubilized drugs. The pore size of the membrane has been verified to allow the passage of intermediate colloidal structures122. Approximately 80% of a nonprecipitating solute is recovered by filtration, at an aspiration rate of 3.9 mL/min, within 5 h123. The filtrates from the jejunum and ileum compartments can be collected to estimate the bioaccessibility of the formulation, which is defined as the percentage of the solubilized drug in both the oil and the micellar phases123. It is reasonable to predict the bioavailability of formulations using the bioaccessibility because solubilized drugs are readily absorbed.
Figure 5.
Schematic representation of the TNO gastrointestinal model (TIM-1). (a) Sensors; (b) pH meters; (c) Peristaltic valve; (d) Prefilter; (e) Filtration system; (f) Cross-set of the filtration system; (g) Filtrate.
Table 2.
Typical parameter settings in the TNO gastrointestinal model (TIM-1) in response to the digestion of a high-fat meal.
| Parameter | Setting |
|---|---|
| Volume (mL) | Stomach: 300, duodenum: 55, jejunum: 130, ileum: 130 |
| Meal size (g) | 300 |
| Gastric secretion (mL/min) | 1 |
| Gastric emptying curve | t1/2 = 80 min, β = 2 |
| Gastric pH curve (time, pH) | (0, 5.2) (30, 3.2) (60, 2.2) (120, 1.7) |
| Bile secretion (mL/min) | 0.5 |
| Pancreatin/electrolytes (mL/min) | 0.5 |
| Ileal emptying curve | t1/2 = 220 min, β = 2.2 |
| Small intestinal pH | Duodenum: 6.2, jejunum: 6.5, ileum: 7.4 |
The table is adapted from Ref. 121 complying with the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
By close mimicking the GI physiology, TIM-1 offers a promising tool to predict the oral bioavailability of most pharmaceutical compositions under one standardized experimental setting124,125. A systematic evaluation of the predictive power of TIM-1 was performed by researchers from AstraZeneca on nine model drugs of different BCS types and six formulations126. TIM-1 correctly predicted the in vivo rank order in 84% and 79% of cases for the AUC and Cmax, respectively. A linear relationship with a correlation coefficient of 0.78 was observed between the bioaccessibility obtained in TIM-1 and the AUC. Owing to its strong predictive capability, TIM-1 has been deployed by AstraZeneca in the drug development for predicting the oral absorption of drug candidates and their formulations.
Until recently, TIM-1 has been used for the evaluation of Pickering emulsions because of a limited availability of the instrument127. The bioaccessibility obtained using TIM-1 showed a great potential for the rank ordering of Pickering emulsions in terms of their in vivo performance128, 129, 130. Compared with TIM-1, the pH-stat lipolysis model may overestimate the bioaccessibility of the formulations128, 129, 130. The difference was attributed to the differences in the designs and simulations of the models. In the pH-stat model, the formulations are fully exposed to the digestion media under continuous stirring until the digestion ends. By contrast, the transit of formulations in the GI tract is a peristalsis movement, which is mimicked by TIM-1, while the absorption of the formulation is concurrent with the lipolysis under realistic circumstances. Consequently, the unrealistic conditions in the pH-stat model lead to overestimation of bioaccessibility. However, it has also been noted that the adsorption of model drugs on the walls of the compartments in the TIM-1 digestive system causes loss of bioaccessibility.
Despite the superiority to the pH-stat lipolysis model, the TIM-1 shows obvious disadvantages. On the one hand, the setup of the model is rather complex, hindering its popularization and application. In addition, the complex process may greatly affect the accuracy and consistency of the data because one mistake may fail the process. A TinyTIM was designed to increase the throughput by simplifying TIM-1121. The simplified version retains the gastric compartment but only has one small intestinal compartment and no ileal efflux. On the other hand, the filter system is unable to mimic the active transport, efflux, and gut wall metabolism. A valid correlation between bioaccessibility and bioavailability cannot be obtained unless the transepithelial transport is not a limiting step. The combination of TIM-1 with a Caco-2 cell culture model or in silico modeling provides a solution to bridge the gap131.
5.3. Combined models and IVIVC
As mentioned above, in vitro lipolysis studies may fail to accurately predict the oral bioavailability of LBFs because the model does not fully represent in vivo conditions. As a closed system, this model lacks the absorption sink that is present in vivo and may therefore overestimate the precipitation potential98,113,132, 133, 134. The intraluminal solvation capacity may be damaged because of the altered composition of GI fluids in the process of intestinal digestion, leading to supersaturation and consequent drug precipitation32,135. Meanwhile, in vivo absorption may lead to a rapid and sufficient drop in the luminal drug concentration to avoid precipitation. The absorption sink effect works even when the initial supersaturation is high, provided that the absorption is fast136. In addition to the absorption issue, absorbed drugs may undergo first-pass metabolism. In this case, the in vitro lipolysis model may overestimate the solubilization potential. Therefore, combined lipolysis–permeation and digestion–microsomal metabolism models were developed, respectively, to obtain a better IVIVC.
5.3.1. In vitro lipolysis–permeation models
In addition to the solubilization, supersaturation, and precipitation of co-formulated drugs during digestion of LBFs, permeation of model drugs is included in the lipolysis–permeation models. The original setup of the model consisted of two separate single compartments (Fig. 6). The lipolysis and permeation were performed in a consecutive way. Dispersion and digestion of LBFs were performed in a single compartment, utilizing the regular pH-stat lipolysis model. At predetermined intervals, samples were withdrawn and transferred to another compartment for the permeation study. A normal setup of the Transwell system (top to bottom) or Ussing chambers (side by side) can be adopted in this step. However, the absorptive membrane should resemble the intestinal epithelia and withstand the harsh lipolysis conditions, including pancreatic enzymes, diverse surfactants, excipients of LBFs, and digestion. Permeability through the Caco-2 cell (a human colon carcinoma cell line) monolayer represents the gold standard for the evaluation of oral drug absorption133,137, 138, 139, 140, 141. Differentiated Caco-2 cells resemble the epithelium of human intestine, which enables the assessment of drug transport mediated via different pathways, e.g., passive versus active transport and paracellular versus transcellular routes142, 143, 144. Due to the intolerability of Caco-2 cells to the pancreatic enzymes, immobilized lipase was used in the digestion step and was shown to successfully digest LBFs and be tolerated by cell monolayers133. An artificial membrane (PermeaPad®)145 and intestinal rat tissue112 are used as alternative membranes for Caco-2 cell monolayer. However, the model fails to establish the IVIVC for LBFs because of the lack of concurrence of the digestion and permeation132,133,145, 146, 147. As illustrated using griseofulvin LCT, MCT, and SCT LBFs, the consecutive lipolysis–permeation model failed to establish the IVIVC. Instead, the single lipolysis model was found to be useful112.
Figure 6.
Consecutive use of combined in vitro lipolysis–permeation models.
To capture the simultaneous occurring of drug release and permeation during digestion, an in situ single-pass intestinal perfusion in rats was coupled with the in vitro lipolysis148. For in situ intestinal perfusion, the small intestine of an anesthetized rat was exposure by a midline incision in the abdomen; the jejunum (10 cm) was cannulated, while the intestinal contents were removed with saline flush. The mesenteric vein that drained the isolated region of the jejunum was cannulated to measure the drug absorption; donor blood was infused via the cannula to the jugular vein to maintain a consistent blood supply. The coupled model successfully predicted the in vivo performances of three fenofibrate LBFs, while the single in vitro lipolysis model failed148. In addition, the coupled model provided valuable mechanistical insights into the interplay among drug solubilization, supersaturation, precipitation, and absorption of LBFs during controlled digestion. However, due to the high technical threshold, the model is not a viable option.
Recently, a simple device consisting of two chambers, which are separated by a Caco-2 cell monolayer or an artificial membrane, was developed to simultaneously study lipolysis and permeation of LBFs132,133,145, 146, 147 (Fig. 7). The upper chamber is used for digestion studies, while the lower one is for assessment of drug permeation. The presence of the absorptive monolayer allows reduction of drug concentration in the digestion chamber and thus maintains sink conditions, which facilitates improving the in vitro predictions24,149. Similarly, immobilized lipase was used in the digestion chamber for compatibility with the Caco-2 cell monolayer132,146. The accuracy of the prediction for in vivo drug exposure, based on drug amount in the acceptor chamber, has been validated with different fenofibrate- or carvedilol-loaded LBFs132,146. Conversely, absence of the absorption membrane led to fail of predicting the in vivo exposure of the formulations. It was intriguing to find that the mixture of lipids and carvedilol was as efficient as the carvedilol loaded LBF in oral bioavailability146. Alternatively, artificial membranes can be used to tolerate a porcine pancreatic extract. Screening with the membrane integrity marker Lucifer Yellow indicated that n-dodecane-coated polyvinylidene difluoride membrane supports (0.45 μm pore size, thickness 100–145 μm) were able to withstand the lipolysis with porcine pancreatin over a sufficient assay period147. The rank order of apparent permeability coefficients for different fenofibrate-loaded LBFs was similar to that obtained using the Caco-2 cell-based model. However, the IVIVR of the cell-free model is yet to be improved using alternative digestive agents.
Figure 7.
Simultaneous use of a combined lipolysis and permeation model.
In addition to the absorption membrane, an everted gut sac was recently combined with the pH-stat lipolysis model to better evaluate and predict the in vivo absorption of LBFs150. The everted gut sac model is efficient to study the mechanisms and kinetics of drug absorption151, but it fails to evaluate LBFs because of the absence of lipolytic conditions. The issue was solved by incubating an everted gut sac in the medium of the pH-stat lipolysis model. The performance is similar to that of the original pH-stat model, except that samples are collected from the gut sac. Simultaneous lipolysis and absorption of LBFs are well simulated in this model. With optimized pH and concentrations of d-glucose and pancreatic lipase, the combined model showed a superior IVIVC (r = 0.9772) between the in vitro absorption percentages of an indomethacin LBF and the in vivo absorption fraction compared with that obtained using the single everted gut sac model150. However, the combined model has some drawbacks. Tissue viability represents one limiting factor. Another disadvantage is the presence of the muscularis mucosa, which may lead to underestimation of the absorption of compounds with a tendency to bind to muscle cells151.
5.3.2. Lipolysis–microsomal metabolism model
As far as the oral bioavailability of BCS II drugs is concerned, solubilization in GI tract as well as metabolism in enterocytes and liver, instead of permeability, are the main obstacles. Microsomal metabolism was thus coupled with an in vitro lipolysis to allow prediction of oral bioavailability of LBFs in human152. Marinol® (sesame oil solution of dronabinol) and Neoral® (SMEDDS of cyclosporine A) were used as model preparations. The in vitro lipolysis model enables an estimation of intraluminal solubility of delivered drugs, while microsomal stability assays provide the information on the first-pass metabolism ratio. The LBFs were digested in two separate lipolysis buffers, with different concentrations of sodium taurocholate and phosphatidylcholine. The absorption fraction (Fabs) was predicted by the drug concentration in the micellar phase following in vitro lipolysis, seeing that all solubilized drugs would be completely absorbed. Metabolism occurs both in the liver and within enterocytes. The fractions of the nonmetabolized drug dose in the liver (Fh) and in the gut (Fg) were determined by metabolism studies using human hepatic and intestinal microsomes, respectively. Subsequently, the predicted oral bioavailability (Fpredicted) was estimated as shown in Eq. (1).
| Fpredicted = Fabs × Fh × Fg | (1) |
A strong correlation between the observed and predicted oral bioavailabilities was verified by Pearson's correlation for both drugs at different doses. The composition of the digestion buffer affected the accuracy. More accurate predictions were obtained using the media with composition closer to physiological conditions. However, it should be noted that the predicted values disregard the effects of gastric metabolism and lymphatic transport, which facilitate the bypassing of hepatic metabolism.
6. In silico prediction of IVIVCs
The complex in vivo processing of LBFs hinders the predictability of the in vitro lipolysis model. Even a fairly complex model such as TIM-1 cannot simulate all of the complex, multistage in vivo processes, which involve the dispersion, digestion, solubilization, precipitation, absorption, and metabolism of LBFs and co-formulated drugs. However, in silico physiologically based PK (PBPK) modeling provides a possibility to predict the complex in vivo behavior via computational calculation based on the available in vitro data. Several commercial programs are now available for model generation, such as Gastroplus™, STELLA®, Simcyp™, and PK-Sim®153. Although the combination of in vitro solubility, dissolution, and precipitation testing with in silico modeling is still in its infancy, it has shown a great potential to predict the oral bioavailability of solid preparations154,155.
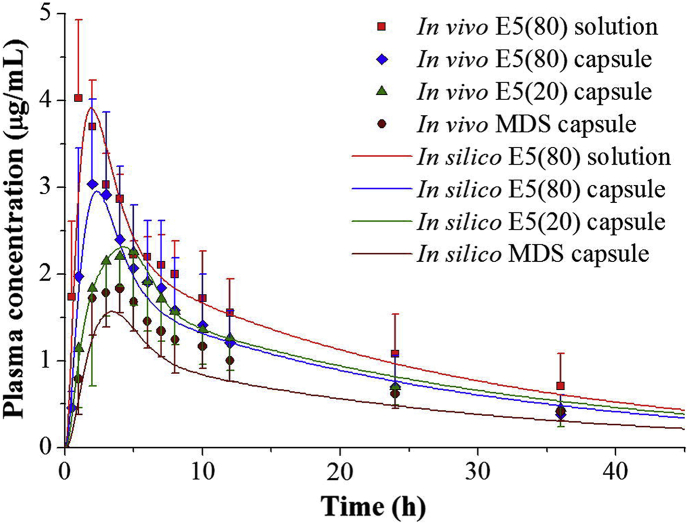
An in silico approach was proposed to establish the IVIVC of fenofibrate LBFs156. Lipid excipients significantly enhanced the solubility and dissolution of fenofibrate in gastric and intestinal media, producing a high supersaturated state. Precipitation of the drug after dissolution in the GI media was detected and depended on the composition of the LBFs. The in vitro dissolution behavior of the formulations and the in vivo PK parameters were incorporated in a STELLA® software to set up the PBPK model. In silico simulation enables taking into account the possible precipitation and redissolution of co-formulated drugs during digestion of LBFs. Consequently, the simulated plasma concentration profiles were accurately fitted with the observed ones for all of the LBFs (Fig. 8156).
Figure 8.
In silico approach facilitated establishment of in vitro and in vivo correlations of fenofibrate lipid-based formulations (LBFs). Simulated (solid lines) and observed (symbols with error bars) plasma fenofibric acid concentration profiles for the LBFs. Reprinted with the permission from Ref. 156. Copyright © 2013 Elsevier B.V.
In addition to PBPK modeling, artificial intelligence, such as artificial neural networks (ANNs), has been adopted to deal with nonlinear in vitro and in vivo relationships and intrinsic variable parameters that may be faced during IVIVC modeling157,158. Recently, neuro-fuzzy modeling, a combination of ANNs and a fuzzy logic with a capability to treat nonlinear complex problems, has been introduced for IVIVC modeling of probucol LBFs159. In the study, the release of probucol from an oil solution, a SMEDDS, and a SNEDDS was tested using a lipolysis model159. The rank order of the rate and extent of probucol release (SMEDDS > SNEDDS > oil solution) was similar to that of the bioavailability in an in vivo study. A significantly high prediction ability was achieved using the neuro-fuzzy model for different data formations, without employing complex configurations.
Both the in vitro and the in vivo data should be mathematically treated by either compartmental or linear methods to establish IVIVC, which can be facilitated by different modules affiliated to GastroPlus™. The PKPlus™ module provides the relevant PK parameters by analyzing the plasma concentration profiles using compartment methods. The IVIVCPlus™ module implements deconvolution using the Wager–Nelson (one-compartment), Loo–Riegelman (two- and three-compartment), and numerical deconvolution single and double Weibull methods to calculate the fraction of the drug absorbed for establishing a correlation (linear, power function, and second- and third-order polynomial). Based on the advantage of the powerful in silico GastroPlus™ simulation, good IVIVCs have been established for furosemide-loaded solid lipid nanoparticles160, fenofibrate lipidic dispersions161, and a rifampicin-loaded solidified SMEDDS162.
7. Summary and future perspectives
Several in vitro models have been developed to construct IVIVCs of LBFs, which are summarized in Table 3. The pH-stat lipolysis model is the most popular one and forms the basis for the development of advanced models. Although a few early studies reported successful IVIVCs, a growing number of studies have demonstrated the inability of the pH-stat lipolysis model to generate level A IVIVCs. The absence of the absorption process is the main drawback in the design of the model. However, the pH-stat model is efficient in the rank ordering of formulations, which makes it an excellent tool in formulation screening. A simplified pH-stat lipolysis model adopted for 96-well plates may greatly increase the throughput and cost effectiveness of screening163, 164, 165, 166. TIM-1 is preferable to the pH-stat model because of a closer simulation of the GI physiology in dealing with lipid digestion and removal of water and metabolites. Although pharmaceutical companies such as AstraZeneca have recognized the value of TIM-1, its application is limited by the high price and complex setup. However, TinyTIM may provide a practical option. The initial application of TIM-1 for the evaluation of Pickering emulsions shows a good potential in the rank ordering of formulations. The capability of the model to construct a level A IVIVC is yet to be confirmed. Meanwhile, the combined digestion–permeation model shows promise in constructing IVIVCs of LBFs. A model combined with a Caco-2 cell monolayer or everted gut sac is particularly promising for fulfilling level A IVIVC modeling because of the involvement of active transportation of solubilized drugs and metabolism inside the epithelia. None of the present models are able to provide full and consistent IVIVCs due to the inability to mimic fully the overall processes occurring in vivo. Yet some physiological and physicochemical parameters have not been touched, such as the hormonal and nervous control, feedback mechanisms, mucosal cell activity, realistic shape and motility of GI tract, mechanical forces from physiological contractions, and involvement of the local immune system104. It is also crucial to mimic the dynamic secretion of digestive enzymes/bile salts and changes in gastric emptying and GI transit time. The future perspective on the setup of the in vitro model is to closely simulate the physiological and physicochemical environments in the GI tract to increase the predictive capability.
Table 3.
Summary of the current in vitro models.
| Model | Component | Simulated parameter | Advantage | Disadvantage |
|---|---|---|---|---|
| In vitro release/dispersion model | USP type II or type III dissolution apparatus | Drug release from formulation; dispersion of formulation | Simple | Absence of the gastrointestinal situation. |
| One-compartment intestinal digestion model | A thermostatic vessel, an overhead stirrer, a pH electrode, and a titrator | Lipid digestion in intestinal track, solubilizing or precipitation of drugs during lipolysis | Simple, most widely adopted model in evaluation of lipid-based formulations | Ignoring lipolysis in stomach, gastric emptying, and pH changes in gastrointestinal tract; Absence of dynamic secretion of digestive enzymes and bile salts; Absence of the absorption process. |
| Gastrointestinal digestion model | Similar to the intestinal digestion model | Both gastric and intestinal digestion, pH changes in gastrointestinal tract, and gastric emptying | Mimicking both the gastric and the intestinal conditions; Gastrointestinal transit and pH changes are included. |
More complex than one-compartment intestinal digestion model; Absence of dynamic secretion of digestive enzymes and bile salts; Absence of the absorption process. |
| TNO gastrointestinal model | Four tubular compartments (i.e., the gastric, the duodenal, the jejunal, and the ileal compartment), peristaltic valve pumps connecting the compartments, gastric and duodenal secretions, pH meter, titration, filtration system | Lipid digestion in both gastric and intestinal tract, gastric emptying, pH changes in gastrointestinal tract, absorption of solubilized drugs | Closely mimicking the dynamic process of the transit, digestion, and absorption of formulations in gastrointestinal tract | Extremely complex setup, high price, poor reproducibility; The filtration system cannot provide active and facilitated transport processes and brush border enzyme activities. |
| In vitro lipolysis–permeation models | The lipolysis setup is similar to the one-compartment intestinal digestion model; The permeation study utilizes Transwell system, Ussing chamber, or diffusion cell; Caco-2 cell monolayer, artificial membrane, or everted gut sac is adopted as absorptive monolayer | Lipid digestion and permeation of model drugs in a consecutive or in a simultaneous way | Providing the absorption sink effect. | Absence of dynamic secretion of digestive enzymes and bile salts; Absence of transit in gastrointestinal tract. |
| Lipolysis–microsomal metabolism model | The lipolysis setup is similar to the one-compartment intestinal digestion model; microsomal stability assays | Solubilization of co-formulated drug following digestion; metabolism of the drug in enterocytes and liver | The first-pass metabolism is included in the model. | The model is limited to drugs with high first-pass metabolism; Absence of dynamic secretion of digestive enzymes and bile salts; Absence of absorption process. |
| In silico prediction | Physiologically based pharmacokinetic modeling | The dispersion, digestion, solubilization, precipitation, absorption, and metabolism of formulations and co-formulated drugs. | Computational calculation of the complex in vivo behavior based on the available in vitro data. Prediction of the in vivo performance in human is possible. |
Accuracy of the model is yet to be validated. |
In addition to the setup of an in vitro model, other issues should be considered in the construction of IVIVCs. Model drugs adopted in present studies, such as fenofibrate, griseofulvin, phenytoin, indomethacin, and ketoprofen, are typical BCS II drugs. They have poor water solubility but good permeability, which indicates a good probability of obtaining level A IVIVCs for BCS II drugs if they are solubilized during the lipolysis of LBFs. However, LBFs are overqualified for oral delivery of BCS II drugs and are more applicable to BCS IV drugs by increasing both their solubility and permeability. In this regard, the feasibility of an in vitro model for constructing an IVIVC should be determined for BCS IV drugs. Moreover, it should be noted that the ultimate goal of an IVIVC is to predict the in vivo behavior of LBFs in humans. The majority of the present studies are performed in rats, while the GI physiology of animals is different from that of humans. For example, bile is continuously secreted in rats, while bile secretion in humans is stimulated by food. It is crucial to verify the predictability of in vitro models using data obtained in humans. PBPK modeling may be promising in this regard. The PBPK platform provides equations describing the whole processes of administrated formulations in different compartments (e.g., the gastric lumen, the intestinal lumen, the plasma, the liver, the glomerular filtration, and the periphery tissues) based on human physiological parameters. Combining with drug dependent parameters (e.g., physicochemical properties, permeability, protein binding, and metabolism by hepatic enzymes) enables building a PBPK model to predict in vivo performance of formulations in human. For detailed concept of PBPK, please refer to recent reviews167,168. Lastly, the in vitro model should be conducive to understanding the mechanisms of action of LBFs. Present studies only measure the total drug amount for the construction of IVIVCs but do not discriminate between free drug molecules and those solubilized in formulations. It is unknown whether and to what extent the LBFs contribute to the absorption of drug molecules, particularly BCS IV drugs. Environment-responsive fluorescent probes, such as aggregation-caused quenching and Förster resonance energy transfer probes, may provide a powerful tool to answer this question. The environment-responsive fluorescent probes enable self-discrimination of LBFs via the fluorescent quenching (aggregation-caused quenching) or switching to different wavelengths (Förster resonance energy transfer) when the probes are released from the vehicles upon lipolysis. Theoretically, the fluorescent intensity can be utilized to quantify the intact LBFs. Since the hydrophobic cargos are not leaked from the LBFs unless the formulation is broken down upon lipolysis, the quantity of the intact LBFs may be converted to the drug amount still in formulation. Although these probes have been widely used for qualitative analysis, quantification is yet to be realized. Breakthrough in this technique will bring about critically important information for design of LBFs.
8. Conclusions
The feasibility of LBF use in oral drug delivery has been fully recognized by both academia and industry. Construction of IVIVCs is a prioritized research which provides a powerful tool to promote the development of LBFs. A variety of in vitro models have been developed to understand and predict the in vivo performance of LBFs. However, none of the present models are able to mimic fully the overall processes of LBFs occurring in vivo, leading to frequent failure in obtaining level A IVIVCs. Great efforts have been made to improve the predictive power of in vitro models by closely simulating the gastrointestinal physiology. A substantial improvement in this field will definitely promote the clinical translation of LBFs.
Acknowledgments
This work was supported by Science and Technology Commission of Shanghai Municipality (Nos. 19430741400 and 19410761200, China) and National Natural Science Foundation of China (Nos. 81973247 and 81703434).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Quangang Zhu, Email: qgzhu@126.com.
Yi Lu, Email: fd_luyi@fudan.edu.cn.
Author contributions
Wei Wu, Quangang Zhu, and Yi Lu proposed the conception of the review. Yanping Huang and Qin Yu wrote the original manuscript. Zhongjian Chen, Quangang Zhu, and Yi Lu revised the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Maleki A., Kettiger H., Schoubben A., Rosenholm J.M., Ambrogi V., Hamidi M. Mesoporous silica materials: from physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J Control Release. 2017;262:329–347. doi: 10.1016/j.jconrel.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Patel P., Patel M. Nanostructured lipid carriers—a versatile carrier for oral delivery of lipophilic drugs. Recent Pat Nanotechnol. 2021;15:154–164. doi: 10.2174/1872210514666200909154959. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Taleb H.A., Fathalla Z., Abdelkader H. Comparative studies of the effects of novel excipients amino acids with cyclodextrins on enhancement of dissolution and oral bioavailability of the non-ionizable drug carbamazepine. Eur J Pharmaceut Sci. 2020;155:105562. doi: 10.1016/j.ejps.2020.105562. [DOI] [PubMed] [Google Scholar]
- 4.Hibino M., Yamada Y., Fujishita N., Sato Y., Maeki M., Tokeshi M. The use of a microfluidic device to encapsulate a poorly water-soluble drug CoQ in lipid nanoparticles and an attempt to regulate intracellular trafficking to reach mitochondria. J Pharmaceut Sci. 2019;108:2668–2676. doi: 10.1016/j.xphs.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Blaabjerg L.I., Grohganz H., Lindenberg E., Löbmann K., Müllertz A., Rades T. The influence of polymers on the supersaturation potential of poor and good glass formers. Pharmaceutics. 2018;10:164. doi: 10.3390/pharmaceutics10040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu W., Lu Y., Qi J.P. Editorial: persistent endeavors for the enhancement of dissolution and oral bioavailability. Acta Pharm Sin B. 2019;9:2–3. doi: 10.1016/j.apsb.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekhawat P.B., Pokharkar V.B. Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm Sin B. 2017;7:260–280. doi: 10.1016/j.apsb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paudwal G., Rawat N., Gupta R., Baldi A., Singh G., Gupta P.N. Recent advances in solid dispersion technology for efficient delivery of poorly water-soluble drugs. Curr Pharmaceut Des. 2019;25:1524–1535. doi: 10.2174/1381612825666190618121553. [DOI] [PubMed] [Google Scholar]
- 9.Padrela L., Rodrigues M.A., Duarte A., Dias A.M.A., Braga E.M.E., de Sousa H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—a comprehensive review. Adv Drug Deliv Rev. 2018;131:22–78. doi: 10.1016/j.addr.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Kazi M., Al Amri R., Alanazi F.K., Hussain M.D. In vitro methods for in vitro‒in vivo correlation (IVIVC) for poorly water soluble drugs: lipid based formulation perspective. Curr Drug Deliv. 2018;15:918–929. doi: 10.2174/1567201815666180116090910. [DOI] [PubMed] [Google Scholar]
- 11.Li C., Zhou K.X., Chen D.M., Xu W., Tao Y.F., Pan Y.H. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int J Nanomed. 2019;14:1619–1631. doi: 10.2147/IJN.S183479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X.W., Xing H.J., Zhao Y., Ma Z.G. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics. 2018;10:74. doi: 10.3390/pharmaceutics10030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das T., Mehta C.H., Nayak U.Y. Multiple approaches for achieving drug solubility: an in silico perspective. Drug Discov Today. 2020;25:1206–1212. doi: 10.1016/j.drudis.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Korani S., Korani M., Bahrami S., Johnston T.P., Butler A.E., Banach M. Application of nanotechnology to improve the therapeutic benefits of statins. Drug Discov Today. 2019;24:567–574. doi: 10.1016/j.drudis.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Dengale S.J., Grohganz H., Rades T., Löbmann K. Recent advances in co-amorphous drug formulations. Adv Drug Deliv Rev. 2016;100:116–125. doi: 10.1016/j.addr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Han J.W., Wei Y.F., Lu Y., Wang R.Z., Zhang J.J., Gao Y. Co-amorphous systems for the delivery of poorly water-soluble drugs: recent advances and an update. Expet Opin Drug Deliv. 2020;17:1411–1435. doi: 10.1080/17425247.2020.1796631. [DOI] [PubMed] [Google Scholar]
- 17.Sun M., Hu H.K., Sun L.M., Fan Z. The application of biomacromolecules to improve oral absorption by enhanced intestinal permeability: a mini-review. Chin Chem Lett. 2020;31:1729–1736. [Google Scholar]
- 18.Zhao S.N., Li J.H., Wang F.Z., Yu T., Zhou Y., He L.L. Semi-elastic core-shell nanoparticles enhanced the oral bioavailability of peptide drugs. Chin Chem Lett. 2020;31:1147–1152. [Google Scholar]
- 19.Figueroa-Campos A., Sánchez-Dengra B., Merino V., Dahan A., González-Álvarez I., García-Arieta A. Candesartan cilexetil in vitro–in vivo correlation: predictive dissolution as a development tool. Pharmaceutics. 2020;12:633. doi: 10.3390/pharmaceutics12070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolskee A., Darwich A.S., Galetin A., Rostami-Hodjegan A., Aarons L. Deconvolution and IVIVC: exploring the role of rate-limiting conditions. AAPS J. 2016;18:321–332. doi: 10.1208/s12248-015-9849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-García I., Mangas-Sanjuán V., Merino-Sanjuán M., Bermejo M. In vitro–in vivo correlations: general concepts, methodologies and regulatory applications. Drug Dev Ind Pharm. 2015;41:1935–1947. doi: 10.3109/03639045.2015.1054833. [DOI] [PubMed] [Google Scholar]
- 22.Qi J.P., Zhuang J., Lu Y., Dong X.C., Zhao W.L., Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov Today. 2017;22:166–172. doi: 10.1016/j.drudis.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Holm R. Bridging the gaps between academic research and industrial product developments of lipid-based formulations. Adv Drug Deliv Rev. 2019;142:118–127. doi: 10.1016/j.addr.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Feeney O.M., Crum M.F., McEvoy C.L., Trevaskis N.L., Williams H.D., Pouton C.W. 50 years of oral lipid-based formulations: provenance, progress and future perspectives. Adv Drug Deliv Rev. 2016;101:167–194. doi: 10.1016/j.addr.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Pouton C.W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharmaceut Sci. 2006;29:278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Savla R., Browne J., Plassat V., Wasan K.M., Wasan E.K. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm. 2017;43:1743–1758. doi: 10.1080/03639045.2017.1342654. [DOI] [PubMed] [Google Scholar]
- 27.Bernkop-Schnürch A., Müllertz A., Rades T. Self-emulsifying drug delivery systems (SEDDS)—the splendid comeback of an old technology. Adv Drug Deliv Rev. 2019;142:1–2. doi: 10.1016/j.addr.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Mullertz A., Ogbonna A., Ren S., Rades T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J Pharm Pharmacol. 2010;62:1622–1636. doi: 10.1111/j.2042-7158.2010.01107.x. [DOI] [PubMed] [Google Scholar]
- 29.Tao C., Yu Y., Chen Z.Z., Zhang M.X., Liu L.L., Liu Z.H. Effect of mesopores on solidification of sirolimus self-microemulsifying drug delivery system. Chin Chem Lett. 2018;29:1849–1852. [Google Scholar]
- 30.Lei Y., Lu Y., Qi J.P., Nie S.F., Hu F.Q., Pan W.S. Solid self-nanoemulsifying cyclosporin A pellets prepared by fluid-bed coating: preparation, characterization and in vitro redispersibility. Int J Nanomed. 2011;6:795–805. doi: 10.2147/IJN.S17711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei Y., Qi J.P., Nie S.F., Hu F.Q., Pan W.S., Lu Y. Solid Self-nanoemulsifying cyclosporine a pellets prepared by fluid-bed coating: stability and bioavailability study. J Biomed Nanotechnol. 2012;8:515–521. doi: 10.1166/jbn.2012.1400. [DOI] [PubMed] [Google Scholar]
- 32.Kollipara S., Gandhi R.K. Pharmacokinetic aspects and in vitro‒in vivo correlation potential for lipid-based formulations. Acta Pharm Sin B. 2014;4:333–349. doi: 10.1016/j.apsb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondamudi P.K., Tirumalasetty P.P., Malayandi R., Mutalik S., Pillai R. Lidocaine transdermal patch: pharmacokinetic modeling and in vitro–in vivo correlation (IVIVC) AAPS PharmSciTech. 2016;17:588–596. doi: 10.1208/s12249-015-0390-1. [DOI] [PubMed] [Google Scholar]
- 34.González-García I., Mangas-Sanjuan V., Merino-Sanjuán M., Álvarez-Álvarez C., Díaz-Garzón Marco J., Rodríguez-Bonnín M.A. IVIVC approach based on carbamazepine bioequivalence studies combination. Pharmazie. 2017;72:449–455. doi: 10.1691/ph.2017.7011. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz Picazo A., Martinez-Martinez M.T., Colón-Useche S., Iriarte R., Sánchez-Dengra B., González-Álvarez M. In vitro dissolution as a tool for formulation selection: telmisartan two-step IVIVC. Mol Pharm. 2018;15:2307–2315. doi: 10.1021/acs.molpharmaceut.8b00153. [DOI] [PubMed] [Google Scholar]
- 36.Somayaji M.R., Das D., Przekwas A. A new level A type ivivc for the rational design of clinical trials toward regulatory approval of generic polymeric long-acting injectables. Clin Pharmacokinet. 2016;55:1179–1190. doi: 10.1007/s40262-016-0388-1. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed M.F., Trueman S., Othman A.A., Han J.H., Ju T.R., Marroum P. Development of in vitro‒in vivo correlation for upadacitinib extended-release tablet formulation. AAPS J. 2019;21:108. doi: 10.1208/s12248-019-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stillhart C., Pepin X., Tistaert C., Good D., Van Den Bergh A., Parrott N. PBPK absorption modeling: establishing the in vitro‒in vivo link-industry perspective. AAPS J. 2019;21:19. doi: 10.1208/s12248-019-0292-3. [DOI] [PubMed] [Google Scholar]
- 39.Davanço M.G., Campos D.R., Carvalho P.O. In vitro‒in vivo correlation in the development of oral drug formulation: a screenshot of the last two decades. Int J Pharm. 2020;580:119210. doi: 10.1016/j.ijpharm.2020.119210. [DOI] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration. Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/extended-release-oral-dosage-forms-development-evaluation-and-application-vitroin-vivo-correlations. [Accessed on January 18, 2021].
- 41.Nguyen M.A., Flanagan T., Brewster M., Kesisoglou F., Beato S., Biewenga J. A survey on IVIVC/IVIVR development in the pharmaceutical industry—past experience and current perspectives. Eur J Pharmaceut Sci. 2017;102:1–13. doi: 10.1016/j.ejps.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 42.Hu X.Q., Zhang J.W., Tang X.M., Li M.Y., Ma S.Y., Liu C. An accelerated release method of risperidone loaded PLGA microspheres with good IVIVC. Curr Drug Deliv. 2018;15:87–96. doi: 10.2174/1567201814666170516113406. [DOI] [PubMed] [Google Scholar]
- 43.Hirota K., Doty A.C., Ackermann R., Zhou J., Olsen K.F., Feng M.R. Characterizing release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: in vitro evaluation. J Control Release. 2016;244:302–313. doi: 10.1016/j.jconrel.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Bermejo M., Hens B., Dickens J., Mudie D., Paixão P., Tsume Y. A mechanistic physiologically-based biopharmaceutics modeling (PBBM) approach to assess the in vivo performance of an orally administered drug product: from IVIVC to IVIVP. Pharmaceutics. 2020;12:74. doi: 10.3390/pharmaceutics12010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porwal A., Dwivedi H., Pathak K. Gastroretentive bilayer film for sustained release of atorvastatin calcium and immediate release of amlodipine besylate: pharmaceutical, pharmacokinetic evaluation, and IVIVC. Pharmaceut Dev Technol. 2020;25:416–431. doi: 10.1080/10837450.2019.1705486. [DOI] [PubMed] [Google Scholar]
- 46.Jereb R., Opara J., Legen I., Petek B., Grabnar-Peklar D. In vitro–in vivo relationship and bioequivalence prediction for modified-release capsules based on a PBPK absorption model. AAPS Pharm Sci Tech. 2019;21:18. doi: 10.1208/s12249-019-1566-x. [DOI] [PubMed] [Google Scholar]
- 47.Beyer S., Xie L., Schmidt M., de Bruin N., Ashtikar M., Rüschenbaum S. Optimizing novel implant formulations for the prolonged release of biopharmaceuticals using in vitro and in vivo imaging techniques. J Control Release. 2016;235:352–364. doi: 10.1016/j.jconrel.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Q., Wei Y.D., Li C.H., Mao S.R. Inner layer-embedded contact lenses for ion-triggered controlled drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;93:36–48. doi: 10.1016/j.msec.2018.07.065. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q., Liu C., Sun Z., Zhang X.F., Liang N., Mao S.R. Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery. Eur J Pharm Biopharm. 2018;128:220–229. doi: 10.1016/j.ejpb.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Li J.Q., Zheng H.L., Qin L., Xu E.Y., Yang L.L., Zhang L. In vitro‒in vivo correlation of inhalable budesonide-loaded large porous particles for sustained treatment regimen of asthma. Acta Biomater. 2019;96:505–516. doi: 10.1016/j.actbio.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 51.Shen J., Burgess D.J. In vitro–in vivo correlation for complex non-oral drug products: where do we stand? J Control Release. 2015;219:644–651. doi: 10.1016/j.jconrel.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y., Manda P., Pavurala N., Khan M.A., Krishnaiah Y.S. Development and validation of in vitro‒in vivo correlation (IVIVC) for estradiol transdermal drug delivery systems. J Control Release. 2015;210:58–66. doi: 10.1016/j.jconrel.2015.05.263. [DOI] [PubMed] [Google Scholar]
- 53.Patel H., Joshi A., Joshi A., Stagni G. Transdermal delivery of etoposide phosphate II: in vitro in vivo correlations (IVIVC) J Pharmaceut Sci. 2016;105:2139–2145. doi: 10.1016/j.xphs.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Mittapelly N., Pandey G., Tulsankar S.L., Arfi S., Bhatta R.S., Mishra P.R. In depth analysis of pressure-sensitive adhesive patch-assisted delivery of memantine and donepezil using physiologically based pharmacokinetic modeling and in vitro/in vivo correlations. Mol Pharm. 2018;15:2646–2655. doi: 10.1021/acs.molpharmaceut.8b00176. [DOI] [PubMed] [Google Scholar]
- 55.Shin S.H., Thomas S., Raney S.G., Ghosh P., Hammell D.C., El-Kamary S.S. In vitro‒in vivo correlations for nicotine transdermal delivery systems evaluated by both in vitro skin permeation (IVPT) and in vivo serum pharmacokinetics under the influence of transient heat application. J Control Release. 2018;270:76–88. doi: 10.1016/j.jconrel.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 56.Simon A., Amaro M.I., Healy A.M., Cabral L.M., de Sousa V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo‒in vitro correlation. Int J Pharm. 2016;512:234–241. doi: 10.1016/j.ijpharm.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 57.Kuentz M. Drug supersaturation during formulation digestion, including real-time analytical approaches. Adv Drug Deliv Rev. 2019;142:50–61. doi: 10.1016/j.addr.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Alskär L.C., Keemink J., Johannesson J., Porter C.J., Bergström C.A. Impact of drug physicochemical properties on lipolysis-triggered drug supersaturation and precipitation from lipid-based formulations. Mol Pharm. 2018;15:4733–4744. doi: 10.1021/acs.molpharmaceut.8b00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams H.D., Trevaskis N.L., Yeap Y.Y., Anby M.U., Pouton C.W., Porter C.J. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30:2976–2992. doi: 10.1007/s11095-013-1126-0. [DOI] [PubMed] [Google Scholar]
- 60.Sassene P.J., Michaelsen M.H., Mosgaard M.D., Jensen M.K., Van Den Broek E., Wasan K.M. In vivo precipitation of poorly soluble drugs from lipid-based drug delivery systems. Mol Pharm. 2016;13:3417–3426. doi: 10.1021/acs.molpharmaceut.6b00413. [DOI] [PubMed] [Google Scholar]
- 61.Carriere F. Impact of gastrointestinal lipolysis on oral lipid-based formulations and bioavailability of lipophilic drugs. Biochimie. 2016;125:297–305. doi: 10.1016/j.biochi.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 62.O'Dwyer P.J., Box K.J., Koehl N.J., Bennett-Lenane H., Reppas C., Holm R. In vivo novel biphasic lipolysis method to predict performance of lipid-based formulations. Mol Pharm. 2020;17:3342–3352. doi: 10.1021/acs.molpharmaceut.0c00427. [DOI] [PubMed] [Google Scholar]
- 63.Ye J.Y., Wu H.Y., Huang C.L., Lin W.T., Zhang C.F., Huang B. Comparisons of in vitro Fick's first law, lipolysis, and in vivo rat models for oral absorption on BCS II drugs in SNEDDS. Int J Nanomed. 2019;14:5623–5636. doi: 10.2147/IJN.S203911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borkar N., Xia D., Holm R., Gan Y., Mullertz A., Yang M. Investigating the correlation between in vivo absorption and in vitro release of fenofibrate from lipid matrix particles in biorelevant medium. Eur J Pharmaceut Sci. 2014;51:204–210. doi: 10.1016/j.ejps.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Khan J., Rades T., Boyd B.J. Lipid-based formulations can enable the model poorly water-soluble weakly basic drug cinnarizine to precipitate in an amorphous-salt form during in vitro digestion. Mol Pharm. 2016;13:3783–3793. doi: 10.1021/acs.molpharmaceut.6b00594. [DOI] [PubMed] [Google Scholar]
- 66.Berthelsen R., Klitgaard M., Rades T., Mullertz A. In vitro digestion models to evaluate lipid based drug delivery systems: present status and current trends. Adv Drug Deliv Rev. 2019;142:35–49. doi: 10.1016/j.addr.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Larsen A.T., Ohlsson A.G., Polentarutti B., Barker R.A., Phillips A.R., Abu-Rmaileh R. Oral bioavailability of cinnarizine in dogs: relation to SNEDDS droplet size, drug solubility and in vitro precipitation. Eur J Pharmaceut Sci. 2013;48:339–350. doi: 10.1016/j.ejps.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Binder H.J., Reuben A. In: Medical physiology: a cellular and molecular approach. Boron W.F., Boulpaep E.L., editors. Elsevier Saunders; Philadelphia: 2009. Nutrient digestion and absorption; pp. 949–979. [Google Scholar]
- 69.Beg S., Sharma G., Thanki K., Jain S., Katare O.P., Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm. 2015;493:466–482. doi: 10.1016/j.ijpharm.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 70.Yáñez J.A., Wang S.W., Knemeyer I.W., Wirth M.A., Alton K.B. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63:923–942. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trevaskis N.L., Charman W.N., Porter C.J. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trevaskis N.L., Kaminskas L.M., Porter C.J. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 73.Kossena G.A., Charman W.N., Wilson C.G., O'Mahony B., Lindsay B., Hempenstall J.M. Low dose lipid formulations: effects on gastric emptying and biliary secretion. Pharm Res. 2007;24:2084–2096. doi: 10.1007/s11095-007-9363-8. [DOI] [PubMed] [Google Scholar]
- 74.Kossena G.A., Charman W.N., Boyd B.J., Dunstan D.E., Porter C.J. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: a phase diagram approach. J Pharmaceut Sci. 2004;93:332–348. doi: 10.1002/jps.10554. [DOI] [PubMed] [Google Scholar]
- 75.Kossena G.A., Charman W.N., Boyd B.J., Porter C.J. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J Pharmaceut Sci. 2005;94:481–492. doi: 10.1002/jps.20260. [DOI] [PubMed] [Google Scholar]
- 76.Thomas N., Holm R., Mullertz A., Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) J Control Release. 2012;160:25–32. doi: 10.1016/j.jconrel.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 77.Shafiq S., Shakeel F., Talegaonkar S., Ahmad F.J., Khar R.K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Tiwari R., Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415:232–243. doi: 10.1016/j.ijpharm.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 79.Patere S.N., Desai N.S., Jain A.S., Kadam P.P., Thatte U.M., Gogtay N. Compritol® 888 ATO a lipid excipient for sustained release of highly water soluble active: formulation, scale-up and IVIVC study. Curr Drug Deliv. 2013;10:548–556. doi: 10.2174/1567201811310050006. [DOI] [PubMed] [Google Scholar]
- 80.Shafiq S., Shakeel F., Khar R.K. Enhanced stability of ramipril in nanoemulsion containing cremophor-EL: a technical note. AAPS PharmSciTech. 2008;9:1097–1101. doi: 10.1208/s12249-008-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kesisoglou F., Hermans A., Neu C., Yee K.L., Palcza J., Miller J. Development of in vitro‒in vivo correlation for amorphous solid dispersion immediate-release suvorexant tablets and application to clinically relevant dissolution specifications and in-process controls. J Pharmaceut Sci. 2015;104:2913–2922. doi: 10.1002/jps.24362. [DOI] [PubMed] [Google Scholar]
- 82.Duan J.Z. A biopharmetrics approach for drug product quality control with clinical relevance. J Pharmaceut Sci. 2021;110:478–488. doi: 10.1016/j.xphs.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 83.McCarthy C.A., Faisal W., O'Shea J.P., Murphy C., Ahern R.J., Ryan K.B. In vitro dissolution models for the prediction of in vivo performance of an oral mesoporous silica formulation. J Control Release. 2017;250:86–95. doi: 10.1016/j.jconrel.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 84.Yang S.G. Biowaiver extension potential and IVIVC for BCS Class II drugs by formulation design: case study for cyclosporine self-microemulsifying formulation. Arch Pharm Res. 2010;33:1835–1842. doi: 10.1007/s12272-010-1116-2. [DOI] [PubMed] [Google Scholar]
- 85.Singh B., Singh R., Bandyopadhyay S., Kapil R., Garg B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf B Biointerfaces. 2013;101:465–474. doi: 10.1016/j.colsurfb.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Cheng X., Gao J.L., Li J., Cheng G., Zou M.J., Piao H.Y. In vitro‒in vivo correlation for solid dispersion of a poorly water-soluble drug efonidipine hydrochloride. AAPS PharmSciTech. 2020;21:160. doi: 10.1208/s12249-020-01685-1. [DOI] [PubMed] [Google Scholar]
- 87.Cheng L.Z., Gai X.M., Wen H.Y., Liu D.D., Tang X., Wang Y.Y. Aqueous polymer dispersion coating used for osmotic pump tablets: membrane property investigation and IVIVC evaluation. AAPS PharmSciTech. 2018;19:242–250. doi: 10.1208/s12249-017-0837-7. [DOI] [PubMed] [Google Scholar]
- 88.Jablonka L., Ashtikar M., Gao G., Jung F., Thurn M., Preuß A. Advanced in silico modeling explains pharmacokinetics and biodistribution of temoporfin nanocrystals in humans. J Control Release. 2019;308:57–70. doi: 10.1016/j.jconrel.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 89.Jablonka L., Ashtikar M., Gao G.F., Thurn M., Modh H., Wang J.W. Predicting human pharmacokinetics of liposomal temoporfin using a hybrid in silico model. Eur J Pharm Biopharm. 2020;149:121–134. doi: 10.1016/j.ejpb.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Vithani K., Jannin V., Pouton C.W., Boyd B.J. Colloidal aspects of dispersion and digestion of self-dispersing lipid-based formulations for poorly water-soluble drugs. Adv Drug Deliv Rev. 2019;142:16–34. doi: 10.1016/j.addr.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Galia E., Nicolaides E., Hörter D., Löbenberg R., Reppas C., Dressman J.B. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15:698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 92.Nicolaides E., Galia E., Efthymiopoulos C., Dressman J.B., Reppas C. Forecasting the in vivo performance of four low solubility drugs from their in vitro dissolution data. Pharm Res. 1999;16:1876–1882. doi: 10.1023/a:1018959511323. [DOI] [PubMed] [Google Scholar]
- 93.Dressman J.B., Reppas C. In vitro‒in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharmaceut Sci. 2000;11(Suppl 2):S73–S80. doi: 10.1016/s0928-0987(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 94.Sunesen V.H., Pedersen B.L., Kristensen H.G., Müllertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur J Pharmaceut Sci. 2005;24:305–313. doi: 10.1016/j.ejps.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Wei H., Löbenberg R. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur J Pharmaceut Sci. 2006;29:45–52. doi: 10.1016/j.ejps.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Nicolaides E., Symillides M., Dressman J.B., Reppas C. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm Res. 2001;18:380–388. doi: 10.1023/a:1011071401306. [DOI] [PubMed] [Google Scholar]
- 97.Beg S., Katare O.P., Saini S., Garg B., Khurana R.K., Singh B. Solid self-nanoemulsifying systems of olmesartan medoxomil: formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol. 2016;294:93–104. [Google Scholar]
- 98.Do T.T., Van Speybroeck M., Mols R., Annaert P., Martens J., Van Humbeeck J. The conflict between in vitro release studies in human biorelevant media and the in vivo exposure in rats of the lipophilic compound fenofibrate. Int J Pharm. 2011;414:118–124. doi: 10.1016/j.ijpharm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Jantratid E., Janssen N., Chokshi H., Tang K., Dressman J.B. Designing biorelevant dissolution tests for lipid formulations: case example—lipid suspension of RZ-50. Eur J Pharm Biopharm. 2008;69:776–785. doi: 10.1016/j.ejpb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Porter C.J., Charman W.N. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev. 2001;50(Suppl 1):S127–S147. doi: 10.1016/s0169-409x(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 101.Zangenberg N.H., Müllertz A., Kristensen H.G., Hovgaard L. A dynamic in vitro lipolysis model. I. Controlling the rate of lipolysis by continuous addition of calcium. Eur J Pharmaceut Sci. 2001;14:115–122. doi: 10.1016/s0928-0987(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 102.Porter C.J., Kaukonen A.M., Taillardat-Bertschinger A., Boyd B.J., O'Connor J.M., Edwards G.A. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J Pharmaceut Sci. 2004;93:1110–1121. doi: 10.1002/jps.20039. [DOI] [PubMed] [Google Scholar]
- 103.Dahan A., Hoffman A. Use of a dynamic in vitro lipolysis model to rationalize oral formulation development for poor water soluble drugs: correlation with in vivo data and the relationship to intra-enterocyte processes in rats. Pharm Res. 2006;23:2165–2174. doi: 10.1007/s11095-006-9054-x. [DOI] [PubMed] [Google Scholar]
- 104.Guerra A., Etienne-Mesmin L., Livrelli V., Denis S., Blanquet-Diot S., Alric M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012;30:591–600. doi: 10.1016/j.tibtech.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Barroso E., Cueva C., Pelaez C., Martinez-Cuesta M.C., Requena T. In: The Impact of Food Bioactives on Health: in vitro and ex vivo models. Verhoeckx K., Cotter P., Lopez-Exposito I., editors. Springer, Cham; Switzerland: 2015. The computer-controlled multicompartmental dynamic model of the gastrointestinal system SIMGI; pp. 319–327. [Google Scholar]
- 106.Christophersen P.C., Christiansen M.L., Holm R., Kristensen J., Jacobsen J., Abrahamsson B. Fed and fasted state gastro-intestinal in vitro lipolysis: in vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur J Pharmaceut Sci. 2014;57:232–239. doi: 10.1016/j.ejps.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Klitgaard M., Sassene P.J., Selen A., Müllertz A., Berthelsen R. Studying furosemide solubilization using an in vitro model simulating gastrointestinal digestion and drug solubilization in neonates and young infants. Eur J Pharmaceut Sci. 2017;109:191–199. doi: 10.1016/j.ejps.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Kamstrup D., Berthelsen R., Sassene P.J., Selen A., Müllertz A. In vitro model simulating gastro-intestinal digestion in the pediatric population (neonates and young infants) AAPS PharmSciTech. 2017;18:317–329. doi: 10.1208/s12249-016-0649-1. [DOI] [PubMed] [Google Scholar]
- 109.Jorgensen S.D.S., Al Sawaf M., Graeser K., Mu H.L., Mullertz A., Rades T. The ability of two in vitro lipolysis models reflecting the human and rat gastro-intestinal conditions to predict the in vivo performance of SNEDDS dosing regimens. Eur J Pharm Biopharm. 2018;124:116–124. doi: 10.1016/j.ejpb.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 110.Larsen A., Holm R., Pedersen M.L., Müllertz A. Lipid-based formulations for danazol containing a digestible surfactant, Labrafil M2125CS: in vivo bioavailability and dynamic in vitro lipolysis. Pharm Res. 2008;25:2769–2777. doi: 10.1007/s11095-008-9641-0. [DOI] [PubMed] [Google Scholar]
- 111.McEvoy C.L., Trevaskis N.L., Edwards G.A., Perlman M.E., Ambler C.M., Mack M.C. In vitro‒in vivo evaluation of lipid based formulations of the CETP inhibitors CP-529,414 (torcetrapib) and CP-532,623. Eur J Pharm Biopharm. 2014;88:973–985. doi: 10.1016/j.ejpb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Dahan A., Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67:96–105. doi: 10.1016/j.ejpb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 113.Griffin B.T., Kuentz M., Vertzoni M., Kostewicz E.S., Fei Y., Faisal W. Comparison of in vitro tests at various levels of complexity for the prediction of in vivo performance of lipid-based formulations: case studies with fenofibrate. Eur J Pharm Biopharm. 2014;86:427–437. doi: 10.1016/j.ejpb.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Williams H.D., Sassene P., Kleberg K., Bakala-N'Goma J.C., Calderone M., Jannin V. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharmaceut Sci. 2012;101:3360–3380. doi: 10.1002/jps.23205. [DOI] [PubMed] [Google Scholar]
- 115.Bakala-N'Goma J.C., Williams H.D., Sassene P.J., Kleberg K., Calderone M., Jannin V. Toward the establishment of standardized in vitro tests for lipid-based formulations. 5. Lipolysis of representative formulations by gastric lipase. Pharm Res. 2015;32:1279–1287. doi: 10.1007/s11095-014-1532-y. [DOI] [PubMed] [Google Scholar]
- 116.Sassene P., Kleberg K., Williams H.D., Bakala-N'Goma J.C., Carriere F., Calderone M. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 6: effects of varying pancreatin and calcium levels. AAPS J. 2014;16:1344–1357. doi: 10.1208/s12248-014-9672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams H.D., Sassene P., Kleberg K., Calderone M., Igonin A., Jule E. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 4: proposing a new lipid formulation performance classification system. J Pharmaceut Sci. 2014;103:2441–2455. doi: 10.1002/jps.24067. [DOI] [PubMed] [Google Scholar]
- 118.Williams H.D., Sassene P., Kleberg K., Calderone M., Igonin A., Jule E. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 3: understanding supersaturation versus precipitation potential during the in vitro digestion of type I, II, IIIa, IIIb and IV lipid-based formulations. Pharm Res. 2013;30:3059–3076. doi: 10.1007/s11095-013-1038-z. [DOI] [PubMed] [Google Scholar]
- 119.Williams H.D., Anby M.U., Sassene P., Kleberg K., Bakala-N'Goma J.C., Calderone M. Toward the establishment of standardized in vitro tests for lipid-based formulations. 2. The effect of bile salt concentration and drug loading on the performance of type I, II, IIIa, IIIb, and IV formulations during in vitro digestion. Mol Pharm. 2012;9:3286–3300. doi: 10.1021/mp300331z. [DOI] [PubMed] [Google Scholar]
- 120.Minekus M., Marteau P., Havenaar R., Huis in't Veld J.H.H. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern Lab Anim. 1995;23:197–209. [Google Scholar]
- 121.Minekus M. In: The Impact of Food Bioactives on Health: in vitro and ex vivo models. Verhoeckx K., Cotter P., López-Expósito I., editors. Springer; Cham: 2015. The TNO gastro-intestinal model (TIM) pp. 37–46. [PubMed] [Google Scholar]
- 122.Reis P.M., Raab T.W., Chuat J.Y., Leser M.E., Miller R., Watzke H.J. Influence of surfactants on lipase fat digestion in a model gastro-intestinal system. Food Biophys. 2008;3:370–381. doi: 10.1007/s11483-008-9091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dickinson P.A., Abu Rmaileh R., Ashworth L., Barker R.A., Burke W.M., Patterson C.M. An investigation into the utility of a multi-compartmental, dynamic, system of the upper gastrointestinal tract to support formulation development and establish bioequivalence of poorly soluble drugs. AAPS J. 2012;14:196–205. doi: 10.1208/s12248-012-9333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Souliman S., Blanquet S., Beyssac E., Cardot J.M. A level A in vitro/in vivo correlation in fasted and fed states using different methods: applied to solid immediate release oral dosage form. Eur J Pharmaceut Sci. 2006;27:72–79. doi: 10.1016/j.ejps.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Cardot J.M., Beyssac E., Alric M. In vitro‒in vivo correlation: importance of dissolution in IVIVC. Dissolution Technol. 2007;14:15–19. [Google Scholar]
- 126.Barker R., Abrahamsson B., Kruusmägi M. Application and validation of an advanced gastrointestinal in vitro model for the evaluation of drug product performance in pharmaceutical development. J Pharmaceut Sci. 2014;103:3704–3712. doi: 10.1002/jps.24177. [DOI] [PubMed] [Google Scholar]
- 127.Tai Z.G., Huang Y.P., Zhu Q.G., Wu W., Yi T., Chen Z.J. Utility of Pickering emulsions in improved oral drug delivery. Drug Discov Today. 2020;25:2038–2045. doi: 10.1016/j.drudis.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 128.Lu X.X., Zhu J.Y., Pan Y.J., Huang Q.R. Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO's gastrointestinal model. Food Funct. 2019;10:2583–2594. doi: 10.1039/c8fo02495b. [DOI] [PubMed] [Google Scholar]
- 129.Lu X.X., Zhang H.W., Zheng T., Liu Q.R., Zhu J.Y., Huang Q.R. Evaluation of oral bioaccessibility of aged citrus peel extracts encapsulated in different lipid-based systems: a comparison study using different in vitro digestion models. J Agric Food Chem. 2020;68:97–105. doi: 10.1021/acs.jafc.9b05372. [DOI] [PubMed] [Google Scholar]
- 130.Wei Z.H., Zhu J.Y., Cheng Y.J., Huang Q.R. Ovotransferrin fibril-stabilized Pickering emulsions improve protection and bioaccessibility of curcumin. Food Res Int. 2019;125:108602. doi: 10.1016/j.foodres.2019.108602. [DOI] [PubMed] [Google Scholar]
- 131.Déat E., Blanquet-Diot S., Jarrige J.F., Denis S., Beyssac E., Alric M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: application to the assessment of lycopene and alpha-tocopherol bioavailability from a whole food. J Agric Food Chem. 2009;57:11314–11320. doi: 10.1021/jf902392a. [DOI] [PubMed] [Google Scholar]
- 132.Keemink J., Mårtensson E., Bergstrom C.A.S. Lipolysis-permeation setup for simultaneous study of digestion and absorption in vitro. Mol Pharm. 2019;16:921–930. doi: 10.1021/acs.molpharmaceut.8b00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keemink J., Bergström C.A.S. Caco-2 cell conditions enabling studies of drug absorption from digestible lipid-based formulations. Pharm Res. 2018;35:74. doi: 10.1007/s11095-017-2327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomas N., Richter K., Pedersen T.B., Holm R., Müllertz A., Rades T. In vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014;16:539–549. doi: 10.1208/s12248-014-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fong S.Y., Bauer-Brandl A., Brandl M. Oral bioavailability enhancement through supersaturation: an update and meta-analysis. Expet Opin Drug Deliv. 2017;14:403–426. doi: 10.1080/17425247.2016.1218465. [DOI] [PubMed] [Google Scholar]
- 136.Bevernage J., Brouwers J., Annaert P., Augustijns P. Drug precipitation–permeation interplay: supersaturation in an absorptive environment. Eur J Pharm Biopharm. 2012;82:424–428. doi: 10.1016/j.ejpb.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 137.Gleeson J.P., McCartney F. Striving towards the perfect in vitro oral drug absorption model. Trends Pharmacol Sci. 2019;40:720–724. doi: 10.1016/j.tips.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 138.Dubray O., Jannin V., Demarne F., Pellequer Y., Lamprecht A., Béduneau A. In-vitro investigation regarding the effects of Gelucire 44/14 and Labrasol® ALF on the secretory intestinal transport of P-gp substrates. Int J Pharm. 2016;515:293–299. doi: 10.1016/j.ijpharm.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 139.Wuyts B., Riethorst D., Brouwers J., Tack J., Annaert P., Augustijns P. Evaluation of fasted state human intestinal fluid as apical solvent system in the Caco-2 absorption model and comparison with FaSSIF. Eur J Pharmaceut Sci. 2015;67:126–135. doi: 10.1016/j.ejps.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 140.Araújo F., Sarmento B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int J Pharm. 2013;458:128–134. doi: 10.1016/j.ijpharm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Zhuang J., Wang D.D., Li D., Yang Y.Q., Lu Y., Wu W. The influence of nanoparticle shape on bilateral exocytosis from Caco-2 cells. Chin Chem Lett. 2018;29:1815–1818. [Google Scholar]
- 142.Neves A.R., Queiroz J.F., Costa Lima S.A., Figueiredo F., Fernandes R., Reis S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: relevance for oral drug delivery. J Colloid Interface Sci. 2016;463:258–265. doi: 10.1016/j.jcis.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 143.Reinholz J., Diesler C., Schöttler S., Kokkinopoulou M., Ritz S., Landfester K. Protein machineries defining pathways of nanocarrier exocytosis and transcytosis. Acta Biomater. 2018;71:432–443. doi: 10.1016/j.actbio.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 144.Alama T., Kusamori K., Katsumi H., Sakane T., Yamamoto A. Absorption-enhancing effects of gemini surfactant on the intestinal absorption of poorly absorbed hydrophilic drugs including peptide and protein drugs in rats. Int J Pharm. 2016;499:58–66. doi: 10.1016/j.ijpharm.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 145.Bibi H.A., Holm R., Bauer-Brandl A. Simultaneous lipolysis/permeation in vitro model, for the estimation of bioavailability of lipid based drug delivery systems. Eur J Pharm Biopharm. 2017;117:300–307. doi: 10.1016/j.ejpb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 146.Alskär L.C., Parrow A., Keemink J., Johansson P., Abrahamsson B., Bergström C.A.S. Effect of lipids on absorption of carvedilol in dogs: is coadministration of lipids as efficient as a lipid-based formulation? J Control Release. 2019;304:90–100. doi: 10.1016/j.jconrel.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 147.Hedge O.J., Bergström C.A.S. Suitability of artificial membranes in lipolysis-permeation assays of oral lipid-based formulations. Pharm Res. 2020;37:99. doi: 10.1007/s11095-020-02833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crum M.F., Trevaskis N.L., Williams H.D., Pouton C.W., Porter C.J. A new in vitro lipid digestion‒in vivo absorption model to evaluate the mechanisms of drug absorption from lipid-based formulations. Pharm Res. 2016;33:970–982. doi: 10.1007/s11095-015-1843-7. [DOI] [PubMed] [Google Scholar]
- 149.Gautschi N., Bergström C.A., Kuentz M. Rapid determination of drug solubilization versus supersaturation in natural and digested lipids. Int J Pharm. 2016;513:164–174. doi: 10.1016/j.ijpharm.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 150.Xiao L., Liu Y., Yi T. Development of a new ex vivo lipolysis-absorption model for nanoemulsions. Pharmaceutics. 2019;11:164. doi: 10.3390/pharmaceutics11040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alam M.A., Al-Jenoobi F.I., Al-mohizea A.M. Everted gut sac model as a tool in pharmaceutical research: limitations and applications. J Pharm Pharmacol. 2012;64:326–336. doi: 10.1111/j.2042-7158.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- 152.Benito-Gallo P., Marlow M., Zann V., Scholes P., Gershkovich P. Linking in vitro lipolysis and microsomal metabolism for the quantitative prediction of oral bioavailability of BCS II drugs administered in lipidic formulations. Mol Pharm. 2016;13:3526–3540. doi: 10.1021/acs.molpharmaceut.6b00597. [DOI] [PubMed] [Google Scholar]
- 153.Kostewicz E.S., Aarons L., Bergstrand M., Bolger M.B., Galetin A., Hatley O. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharmaceut Sci. 2014;57:300–321. doi: 10.1016/j.ejps.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 154.Juenemann D., Jantratid E., Wagner C., Reppas C., Vertzoni M., Dressman J.B. Biorelevant in vitro dissolution testing of products containing micronized or nanosized fenofibrate with a view to predicting plasma profiles. Eur J Pharm Biopharm. 2011;77:257–264. doi: 10.1016/j.ejpb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 155.Stillhart C., Imanidis G., Griffin B.T., Kuentz M. Biopharmaceutical modeling of drug supersaturation during lipid-based formulation digestion considering an absorption sink. Pharm Res. 2014;31:3426–3444. doi: 10.1007/s11095-014-1432-1. [DOI] [PubMed] [Google Scholar]
- 156.Fei Y., Kostewicz E.S., Sheu M.T., Dressman J.B. Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro‒in silico‒in vivo approach. Eur J Pharm Biopharm. 2013;85:1274–1284. doi: 10.1016/j.ejpb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Dowell J.A., Hussain A., Devane J., Young D. Artificial neural networks applied to the in vitro‒in vivo correlation of an extended-release formulation: initial trials and experience. J Pharmaceut Sci. 1999;88:154–160. doi: 10.1021/js970148p. [DOI] [PubMed] [Google Scholar]
- 158.Kortejärvi H., Malkki J., Marvola M., Urtti A., Yliperttula M., Pajunen P. Level A in vitro‒in vivo correlation (IVIVC) model with Bayesian approach to formulation series. J Pharmaceut Sci. 2006;95:1595–1605. doi: 10.1002/jps.20592. [DOI] [PubMed] [Google Scholar]
- 159.Fatouros D.G., Nielsen F.S., Douroumis D., Hadjileontiadis L.J., Mullertz A. In vitro‒in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks. Eur J Pharm Biopharm. 2008;69:887–898. doi: 10.1016/j.ejpb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 160.Ali H., Prasad Verma P.R., Dubey S.K., Venkatesan J., Seo Y., Kim S.-K. In vitro–in vivo and pharmacokinetic evaluation of solid lipid nanoparticles of furosemide using Gastroplus™. RSC Adv. 2017;7:33314–33326. [Google Scholar]
- 161.O'Shea J.P., Faisal W., Ruane-O'Hora T., Devine K.J., Kostewicz E.S., O'Driscoll C.M. Lipidic dispersion to reduce food dependent oral bioavailability of fenofibrate: in vitro, in vivo and in silico assessments. Eur J Pharm Biopharm. 2015;96:207–216. doi: 10.1016/j.ejpb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 162.Hussain A., Shakeel F., Singh S.K., Alsarra I.A., Faruk A., Alanazi F.K. Solidified SNEDDS for the oral delivery of rifampicin: evaluation, proof of concept, in vivo kinetics, and in silico GastroPlus(TM) simulation. Int J Pharm. 2019;566:203–217. doi: 10.1016/j.ijpharm.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 163.Mosgaard M.D., Sassene P.J., Mu H.L., Rades T., Müllertz A. Development of a high-throughput in vitro intestinal lipolysis model for rapid screening of lipid-based drug delivery systems. Eur J Pharm Biopharm. 2015;94:493–500. doi: 10.1016/j.ejpb.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 164.Ülker S., Placidi C., Point V., Gadenne B., Serveau-Avesque C., Canaan S. New lipase assay using pomegranate oil coating in microtiter plates. Biochimie. 2016;120:110–118. doi: 10.1016/j.biochi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 165.Verma R., Kaushik D. In vitro lipolysis as a tool for the establishment of IVIVC for lipid-based drug delivery systems. Curr Drug Deliv. 2019;16:688–697. doi: 10.2174/1567201816666190620115716. [DOI] [PubMed] [Google Scholar]
- 166.Mosgaard M.D., Sassene P.J., Mu H.L., Rades T., Müllertz A. High-throughput lipolysis in 96-well plates for rapid screening of lipid-based drug delivery systems. J Pharmaceut Sci. 2017;106:1183–1186. doi: 10.1016/j.xphs.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 167.Zhuang X.M., Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016;6:430–440. doi: 10.1016/j.apsb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Utembe W., Clewell H., Sanabria N., Doganis P., Gulumian M. Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials. Nanomaterials (Basel) 2020;10:1267. doi: 10.3390/nano10071267. [DOI] [PMC free article] [PubMed] [Google Scholar]