Abstract
Natural extracellular vesicles (EVs) play important roles in many life processes such as in the intermolecular transfer of substances and genetic information exchanges. Investigating the origins and working mechanisms of natural EVs may provide an understanding of life activities, especially regarding the occurrence and development of diseases. Additionally, due to their vesicular structure, EVs (in small molecules, nucleic acids, proteins, etc.) could act as efficient drug-delivery carriers. Herein, we describe the sources and biological functions of various EVs, summarize the roles of EVs in disease diagnosis and treatment, and review the application of EVs as drug-delivery carriers. We also assess the challenges and perspectives of EVs in biomedical applications.
KEY WORDS: Extracellular vesicles, Exosomes, Biomarkers, Intercellular communications, Drug delivery
Graphical abstract
In this review, we summarize the mechanism of extracellular vesicles in many physical processes and diseases, and describe the biomedical applications of extracellular vesicles (EV) in disease diagnosis and therapy, drug-delivery carriers.
1. Introduction
Extracellular vesicles (EVs) are lipid-bound vesicles that are naturally released from prokaryotes and eukaryotic cells to the extracellular milieu under physiological and pathological conditions1, 2, 3, 4. In 1946, Chargaff and West5 pioneered the search for subcellular activators of blood clotting in mammalian tissues. The activators converting prothrombin to thrombin and triggering blood coagulation were isolated by ultracentrifugation and were determined to be high-molecular-weight lipoproteins5. In 1967, the morphology of these lipoprotein activators in serum was revealed by transmission electron microscopy (TEM) to be lipid-bound vesicles, and these vesicles were named as platelet dust6. The diameter of platelet dust was between 20 and 50 nm and their density varied between 1.020 and 1.025 g/mL. Lipid component analysis and TEM investigations revealed that the platelet dust is rich in phospholipids and selectively released from platelets, rather than from platelet membrane fragments, and contains key protein components to induce the coagulant activity of blood6.
In 1983, Pan and Johnstone7 observed the selective externalization of transferrin receptors of sheep reticulocytes during the maturation process of reticulocytes, i.e., immature red blood cells, into the supernatant of culture medium in the form of nanovesicles. The released nanovesicles, which were termed as exosomes, can be harvested by centrifugation at 100,000×g for 90 min7,8. The phospholipids extracted from these exosomes contain phosphatidylethanolamine, phosphatidylcholine, sphingomyelin, and phosphatidylserine8. The total protein cargos of exosomes include membrane proteins as well as cytosolic proteins: transferrin receptor, hemoglobin, acetylcholinesterase, the nucleoside carrier, the glucose carrier, and Na+/K+-ATPase8. These protein cargoes allow reticulocyte-derived vesicles to implement a series of biological functions, such as shedding of specific membrane proteins (e.g., the transportation of transferrin receptor from the original cells), nitrobenzylthioinosine binding (mediated by the nucleoside carrier), cytochalasin B binding (mediated by the glucose carrier), and ATPase activity (mediated by Na+/K+-ATPase)8.
In parallel, Harding, Heuser, and Stahl9 investigated the endocytosis of transferrin in rat reticulocytes and found that the cellular uptake of transferrin is reversible, i.e., the internal transferrin can be released into the culture medium in an intact form. They further labeled transferrin with colloidal gold particles, which strongly scatter electrons, to achieve image-tracking of the externalization process of the bound transferrin in a reticulocyte by electron microscopy. In the electron microscopy images, the colloidal gold-modified transferrins were bound to the wall of multivesicular bodies (MVBs), densely clustered around the inclusions, and released into the extracellular space as nanovesicles9.
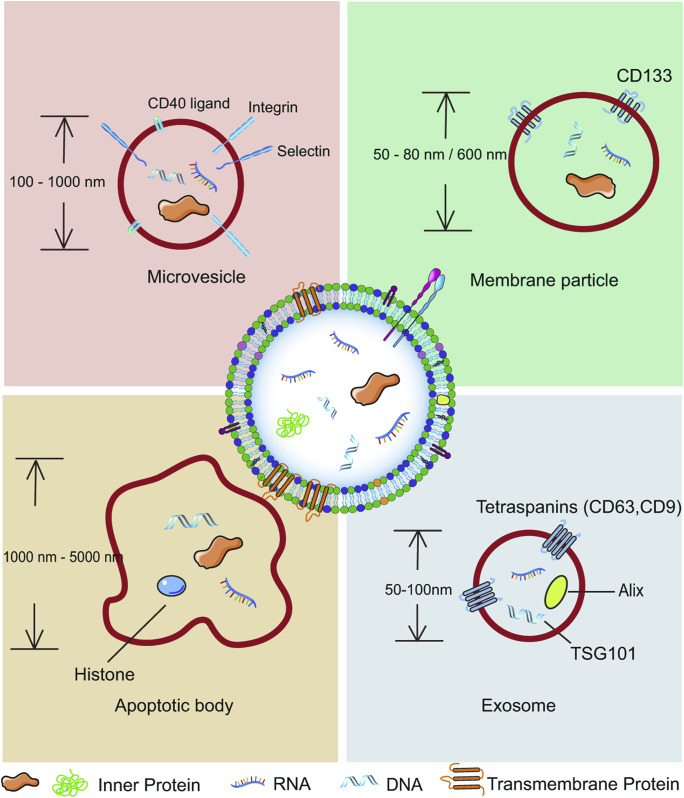
These early discoveries lay the foundations for the emerging research field of EVs. Currently, growing evidence demonstrates that the distribution of EVs is widespread and can be collected from various cell types and extracellular media. Many types of cells have been reported to possess the capacity to secrete EVs, including dendritic cells (DCs)10, B cells11, T cells12, mast cells13, epithelial cells14, and tumor cells15. Additionally, EVs can be found in all body fluids (urine, blood, ascites, and cerebrospinal fluid)3. Their diverse cell sources and biogenesis pathways make EVs highly heterogeneous with regards to their structure and function. Based on the structural features, EVs can be generally divided into four subclasses: exosomes, microvesicles, membrane particles, and apoptotic vesicles3,16 (Fig. 1).
Figure 1.
The discrete structural and compositional characteristics of four types of extracellular vesicles.
Exosomes are mainly formed via an exocytosis pathway, in which intraluminal vesicles (ILVs) form within MVBs and are consequently transported to the extracellular space as exosomes when MVBs fuse with the plasma membrane. The size of cup-shaped exosomes ranges from 50 to 100 nm and their density falls within 1.13–1.19 g/mL. The main protein markers of exosomes are tetraspanins (CD63, CD9), Alix, and tumor susceptibility gene 101 (TSG101)3,16, 17, 18. Microvesicles are directly shed from the plasma surface upon stimulation. They are also cup-shaped but their diameters are between 100 and 1000 nm. Specific protein markers include integrins, selectins, and CD40 ligand3,16,19, 20, 21. Membrane particles, which are specifically released by epithelial cells, can be small round vesicles (50–80 nm in diameter) or large round particles (∼600 nm in diameter), with a density of 1.04–1.07 g/mL. CD133 is the specific protein marker of membrane particles3,16,22. Apoptotic bodies are formed during the execution phase of the apoptotic process. They display striking morphological variability and their size ranges between 1000 and 5000 nm, with a density of 1.16–1.28 g/mL. Histone proteins are the chief markers of apoptotic bodies3,16,23,24. Beyond the main subclasses mentioned above, EVs also include ectosomes, exosome-like vesicles, and oncosomes, among others3,16.
EVs play essential roles in the horizontal transfer of substances and genetic information in intercellular communications by entering and releasing their cargos (lipids, proteins, RNA, DNA, and other cellular components1,25,26) to the neighboring or distant recipient cells1, 2, 3,16. In 2007, Lötvall et al.25 unraveled the presence of substantial and heterogeneous RNA species in the exosomes derived from mouse and human mast-cell lines (MC/9 and HMC-1) as well as from human primary bone marrow-derived mast cells. The exosomal RNAs are resistant to RNase and trypsin treatment, indicating that the RNAs are packed inside of exosomes rather than exposed on the exterior surface. By using microarrays, 1272 messenger RNAs (mRNAs) can be detected in the exosomes. These exosomal mRNAs have been proven to be intact and active, i.e., they carry genetic information and are capable of being translated into proteins in vitro. Intriguingly, the incubation of human mast-cell HMC-1 with mouse MC/9 exosomes leads to the expression of mouse mRNA-encoded proteins in human cells25, indicating that exosomes act as a vehicle to achieve the intercellular transfer of mRNAs and the genetic exchange between cells. Not only limited to mRNA which contains the protein-coding information from DNA, non-protein-coding RNAs, such as microRNA (miRNA), long non-coding RNA, short interfering RNA (siRNA), piwi-interacting RNA, are also identified from EVs1. The presence of enriched RNA cargos suggests the potency of EVs to modulate the gene expression and function of recipient cells. EVs can affect the recipient cells/tissues at distant sites by travelling in biofluids; thus, EV-based cell–cell communication has been recognized as an important step in many physiological and pathological processes such as cell signaling, antigen presentation, and cancer progression1,3.
Due to their unique biological characteristics, EVs have promising potential for clinical translation and have therefore become an emerging research topic27. The abundance of EVs circulating in blood, urine, and other biological fluids make them readily obtained and their biological content can be utilized as biomarkers for the diagnosis and prognosis of diseases28,29. The generation, transport, and internalization pathways of EVs provide a potential target for therapeutic intervention to prevent disease progression30. Furthermore, due to the unique role of EVs in intercellular communications and targeted cargo delivery, diverse natural EVs or synthetic EV-derivatives have been explored as novel nanomedicine or drug-delivery platforms for pharmaceutical purposes31. EVs is a hot research topic as documented in many reviews1,3,4,32, 33, 34, 35, 36, 37, 38. Different from the reviews which focus on the studies of EVs derived from mammalian cells, we will also include the recent advances made in the fields of vesicles extracted from plant and bacterial cells. Our review aims to introduce the recent progress made in the medical applications of EVs relating to the diagnosis and therapy of diseases. We also elucidate the biogenesis, cellular uptake, and the molecular mechanisms underlying the bioactivities conferred by EVs and envision the development of EVs as the next generation of nanomedicines.
2. The biogenesis, secretion, transportation, and uptake of EVs
To date, the mechanisms underlying the intracellular trafficking and intercellular communications of EVs are only partially understood. Different subgroups of EVs implement distinguishable bioactivities that are interdigitated to form a complex interaction network. In this section, we provide a brief introduction to summarize the knowledge of the biogenesis, secretion, intercellular transportation, and internalization processes of EVs.
2.1. EV biogenesis and secretion
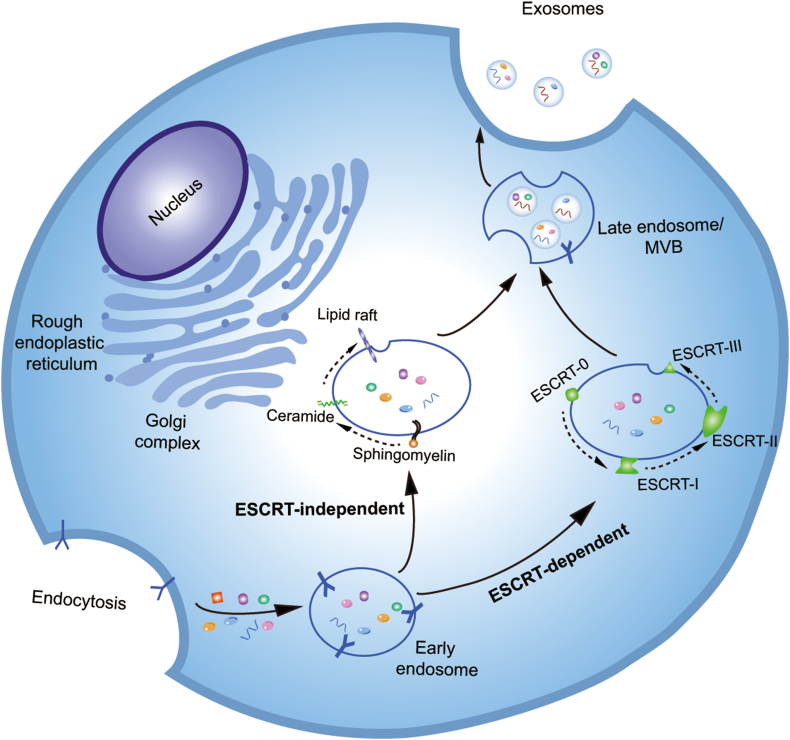
Considering the diverse and heterogeneous nature of EVs, we herein focus on exosomes as a representative EV and introduce the biogenesis, secretion, transportation, and uptake of exosomes. Currently, studies on the biosynthesis mechanisms of exosomes are mainly focused on exosomes derived from mammalian cells. A three-step MVB/ILV model is proposed to understand the biogenesis and secretion processes of exosomes (Fig. 2). In the first step, the inward budding of the plasma membrane packages cytosolic and transmembrane cargos (proteins, nucleic acids, and small molecules) into the small membranous vesicles to form intracellular endosomes39. In the second step, intracellular endosomes with their contents fuse together to associate into early endosomes for further recycling, degradation, or exocytosis40. Finally, the early endosomes further mature and transform into late endosomes, also referred to as MVBs, encapsulating ILVs with a diameter of 30–100 nm. The MVBs can fuse with lysosomes to generate endolysosomes or with the plasma membrane to release ILVs into the extracellular environment as exosomes40. This ILV/MVB model depicts a general scenario for the generation of exosomes. However, there is controversy with regards to the molecular mechanisms underlying the formation of ILV/MVBs and of MVB fusion with the plasma membrane. Two distinct mechanisms, the endosomal sorting complex required for transport (ESCRT) family-dependent pathway and the ESCRT-independent pathway, are proposed to interpret the formation of MVBs. The ESCRT machineries, consisting of four members, i.e., ESCRT-0, I, II, and III, are involved in the endosomal binding, sorting, and clustering of ubiquitinated cargos41,42. In the ESCRT-dependent pathway, the mono-ubiquitinated proteins are initially recognized by the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) domain of ESCRT-0 and recruit ESCRT-I to form an ESCRT-0/ESCRT-I complex. The ESCRT-0/-I complex exploits ESCRT-II to trigger the onset of nascent ILV budding. This leads to the inclusion of cytosolic cargoes by the lumen of ILVs. Subsequently, ESCRT-III converges with the ESCRT-0/-I/-II complex to detach from the membrane and releases the cargo-containing vesicles, i.e., ILVs, into the endosome. Deubiquitinating enzymes switch the mono-ubiquitinated protein to its deubiquitinated state. Vacuolar protein sorting protein-4 (Vps4) disassembles the ESCRT machinery and allows the ESCRT members to be recruited for the next cycle of ILV formation. Finally, when MVBs are fused with the plasma membrane, the ILVs are transported into the extracellular environment and are donated as ‘exosomes’41. In addition to the ESCRT-dependent pathway, several alternative pathways, referred to as the ubiquitin- and ESCRT-independent pathways, have been identified, including the oligomerization of the tetraspanin complexes43, phospholipase D2 and ADP ribosylation factor-6-mediated ILV budding44, and the sphingomyelinase pathway, which catalyzes the transformation of sphingomyelin into ceramide and initiates ILV formation41. Although the biogenesis of exosomes has often been described as either an ESCRT-dependent or ESCRT-independent mechanism, these pathways might not be entirely disconnected as they may work synergistically33.
Figure 2.
The biogenesis and release of exosomes in mammalian cells. The inward budding of the plasma membrane packages cytosolic and transmembrane cargos to form early endosomes, which transform into late endosomes (MVBs) with intraluminal vesicles through ESCRT-dependent pathways or ESCRT-independent pathways. The MVBs fuse with the plasma membrane and release intraluminal vesicles as exosomes.
Multiple factors are responsible for the formation and secretion of exosomes in parental cells and have been implicated in the regulatory network of exosomes. Lipids (e.g., ceramide), cell type, culture conditions, and the genomic health of the cell exhibit a correlation with the biogenesis of exosomes32,45, 46, 47, 48, 49, 50. Diverse proteins are also involved in the regulation of exosome biogenesis and secretion. For example, specific members of the Rab guanosine triphosphatase (GTPase) family, such as Rab27a and Rab27b, are manifested to participate in the translocation of MVBs to the cell periphery and in their fusion with the plasma membrane. The knockdown of Rab27 and/or the effectors (exophilin-5 and synaptotagmin-like protein-4) results in a reduction in the secretion of exosomes in HeLa cells35,51. Additionally, the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex was observed to drive membrane fusion and the secretion of the exosomes52. Baietti et al.53 found that syndecan-syntenin could support the intraluminal budding of endosomal membranes with the help of apoptosis-linked gene-2-interacting protein X (Alix). Yu et al.54 showed that the tumor repressor protein p53 and its downstream effector tumor suppressor-activated pathway-6 (TSAP6) increase the production of exosomes.
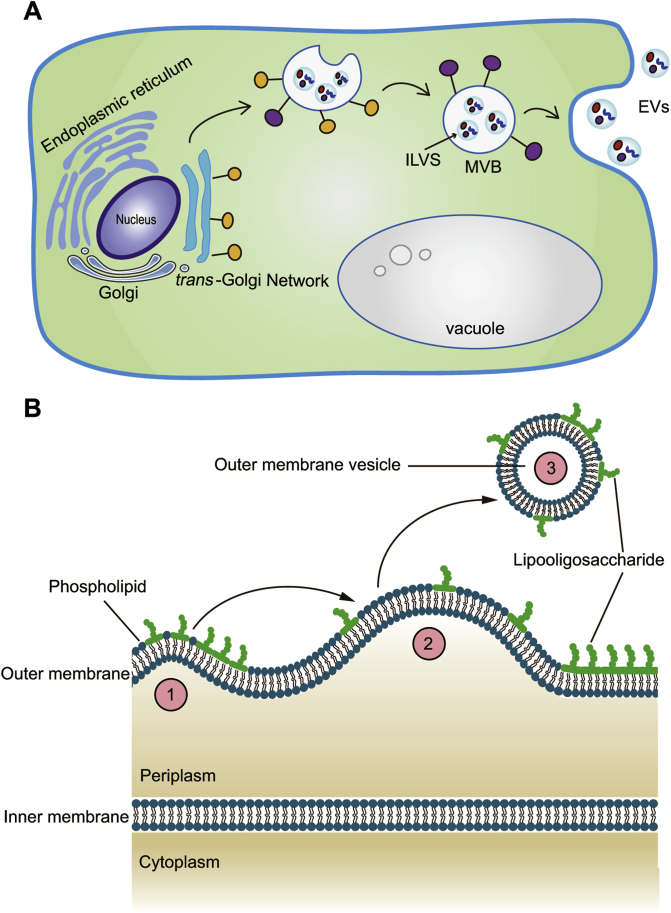
Compared to mammalian cells, in which the biogenesis of exosomes involves the maturation of early endosomes, plant and prokaryotic cells engage different mechanisms to generate exosomes. In plant cells, the trans-Golgi network, in which the freshly synthesized proteins are packaged into transport carriers, is suggested to play similar functions as the early endosome in mammalian cells55. The secretory, recycling, and membrane trafficking of vacuolar protein cargoes by the sorting station trans-Golgi network are followed by the maturation of MVBs and the internalization into ILVs by inward budding of the MVBs56,57. MVBs fuse with either the vacuole, where they release their contents for degradation, or the plasma membrane to release ILVs as plant exosomes58, 59, 60 (Fig. 3A). In prokaryotic cells, the naturally released spherical membrane structures are termed as outer-membrane vesicles (OMVs; roughly 10–300 nm in diameter)61.
Figure 3.
The proposed models for the biogenesis and secretion of extracellular vesicles in plant cells and in Gram-negative bacteria. (A) In plant cells, the trans-Golgi network plays a similar function as the early endosome in mammalian cells. (B) In Gram-negative bacteria, the outer membrane is an asymmetrical bilayer membranous structure with lipopolysaccharide in the outer leaflet and phospholipids in the inner leaflet. The phospholipid layer possesses different molecular packing manners and triggers the budding of the outer membrane (① and ②). Finally, the budding of the outer membrane is enclosed and released as outer-membrane vesicles (③).
The proteins governing the trafficking of lipids play important roles in the biogenesis of OMVs61; for example, the ATP-binding cassette transport system, VacJ/Yrb, is crucial for the generation of OMVs in Gram-negative bacteria. The outer membrane of Gram-negative bacteria is an asymmetrical bilayer membranous structure presenting lipopolysaccharide (LPS) in the outer leaflet and mostly phospholipids in the inner leaflet. The lipid asymmetry of the outer membrane is maintained by the lipid transport system, VacJ/Yrb, which prevents the accumulation of phospholipids in the outer leaflet. Roier et al.61 discovered that a decreased or diminished expression of VacJ with or without Yrb results in an upregulated production of OMVs in two distantly related Gram-negative bacteria, Haemophilus influenzae and Vibrio cholera. The weakened VacJ/Yrb system disrupts the trafficking of phospholipids from the outer leaflet and contributes to an enrichment and segregation of phospholipids in the LPS-rich outer leaflet of the outer membrane. Relative to LPS, phospholipids possess different molecular packing parameters and thus induce a change in the membrane curvature, which triggers the budding of the outer membrane. Finally, the budding of the outer membrane is enclosed and released as OMVs61. Not only limited to the VacJ/Yrb system, the biogenesis of OMVs is also found to be correlated with other types of proteins (e.g., lipoprotein NLPI, outer membrane protein A, peptidoglycan), lipids (e.g., phospholipids, lipopolysaccharide, and 2-heptyl-3-hydroxy-4-quinolone), and external factors (e.g., temperature, the antibiotic concentration in the growth environment)62, 63, 64, 65, 66, 67, 68 (Fig. 3B).
2.2. EV transportation
EVs are not only captured and internalized in situ by cells and extracellular matrix in the proximity of parent cells but also gain access to the systemic circulation for distant transportation. The transfer of EVs shuttles biomolecules affect the functionality of recipient cells. For example, subcutaneously injected melanoma exosomes can travel through the lymphatic system and emerge as mediators of tumorigenesis and influence the lymph node distribution pattern of free melanoma cells69. In vivo imaging and tracing experiments showed that the terminals of intravenously injected melanoma-derived exosomes trafficking in the blood circulation were the preferred sites of metastasis, such as spleen, lung, liver, and bone marrow70,71. EVs derived from the central nervous system are observed to regulate the peripheral cytokine response towards inflammatory brain injury, stimulating the migration of leukocytes into the brain72. EVs have the potential to pass through diverse tissue barriers, such as the tight endothelial cell junction in the blood‒brain barrier (BBB)73. Three types of mechanisms might be involved in the transportation of EVs across the BBB. First, the BBB permeability is upregulated under inflammatory conditions74. For example, the peripheral administration of LPS, a pathogen stimulating the innate immune system, can trigger the transcytosis of α-synuclein-rich erythrocyte-derived EVs across the BBB, promoting the progression of Parkinson’s disease75. Another example is the brain metastatic cancer cells which release microRNA-181c to activate PDKL1 targeted degradation, resulting in endothelial dysfunction and increased BBB permeability76. Second, the transcytosis of EVs is mediated by some membrane proteins in the BBB, such as the receptor for advanced glycation end products (RAGE) protein and the low-density lipoprotein receptor (LDLR)77,78. Third, the neuron-specific proteins or peptides, which are presented on the surface of EVs, endow EVs with the potential to target and penetrate across the BBB. For instance, the EVs fused with neuron-specific rabies viral glycoprotein (RVG)-derived peptides can shuttle EVs from intravenous fluid to brain neurons79.
2.3. The cellular internalization of EVs
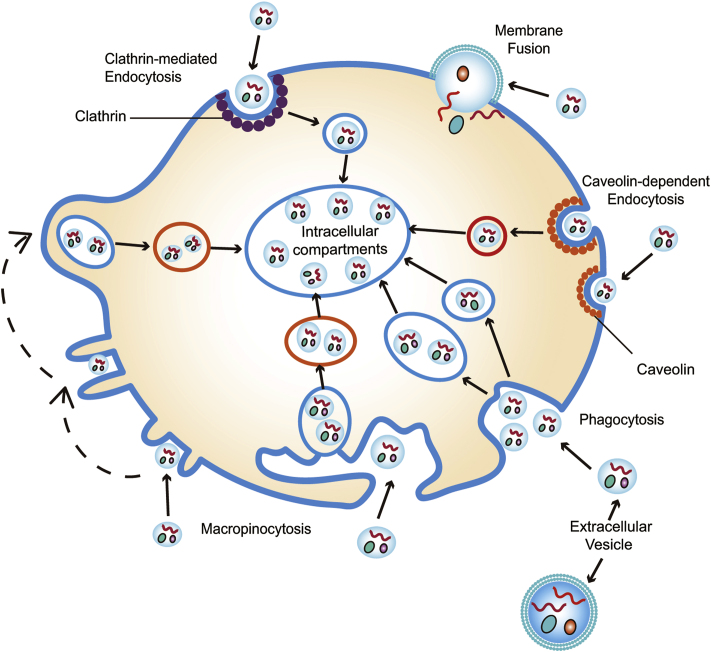
A better understanding of EV uptake is essential to potentiate the capacity of EVs to induce phenotypic changes in recipient cells. Several hypotheses, including plasma membrane fusion, phagocytosis, macropinocytosis, clathrin-mediated endocytosis (CME), and caveolin-dependent endocytosis (CDE), have been proposed, attempting to unmask the molecular mechanism underlying the internalization of EVs80, 81, 82 (Fig. 4). However, our understanding of EV uptake remains limited. Herein, we briefly summarize the current progresses regarding the EV uptake routes and mechanisms.
Figure 4.
The cellular internalization of extracellular vesicles. Membrane fusion, phagocytosis, micropinocytosis, clathrin-mediated endocytosis, and caveolin-dependent endocytosis processes are separately illustrated.
Plasma membrane fusion refers to the direct membrane merge between EVs and the recipient cells83, 84, 85. When the two distinct lipid bilayer membranes come in close contact, a hemi-fusion stalk is formed due to the fusion of the outer leaflets. The stalk spreads and propagates along the interface between the two contacted membranes, producing the hemi-fusion diaphragm bilayer. Finally, the fusion of an EV with the cell membrane opens a pore that releases the EV cargo directly into the cytoplasm83, 84, 85. Proteins such as Rab, Sec1/Munc-18 related proteins, lysosomal-associated membrane protein-1 (LAMP-1), and SNAREs participate in the process of plasma membrane fusion86. Membrane fusion can be modulated by the microenvironment of EVs. For example, the fusion of the melanoma-derived exosomes with the plasma membrane is enhanced under acidic conditions87.
Phagocytosis is a dynamic actin cytoskeleton-mediated internalization process by which a cell uses its plasma membrane to swallow nanoparticles, including EVs88. This process can be regulated by several specific transmembrane receptors (e.g., scavenger, Toll-like receptors, complement receptors) that are essential for the membrane invagination surrounding the nanoparticles88, 89, 90. The phosphatidylserine on the outer leaflet of EV membranes is a crucial checkpoint to manage the phagocytosis of EVs91. For instance, the incubation of mouse macrophages with an antibody that targets the phosphatidylserine receptor, T cell membrane protein-4 (TIM-4), interrupting the phosphatidylserine-dependent phagocytosis pathway, results in a reduced level of internalized exosomes82. Phosphatidylinositol 3-kinase (PI3Ks) can affect phagocytosis by enabling the plasma membrane insertion into phagosomes92. Other proteins, such as actin cytoskeleton and dynamin 2, are also implicated in the process of phagocytosis93.
Macropinocytosis is a means by which cells ingest extracellular liquid and dissolved species depending on actin-driven membrane protrusions94. Different from phagocytosis, direct contact between the internalized species and the recipient cell is not necessary in macropinocytosis, rather the growth and elongation of actin filaments drive the extension of sheet-like protrusions from the cell surface. The closure of membrane protrusions generates endocytic vesicles encompassing EVs, resulting in an intracellular separation of the macropinosomes from the plasma membrane90. Several experiments suggest that the Na+/H+ exchanger, cholesterol, actin, dynamin, PI3K, Ras, Src, Rac1, and GTPase are implicated in the process of macropinocytosis94, 95, 96, 97, 98, 99, 100.
CME is an endocytic process that involves the formation of a mechanistic framework by clathrin101, 102, 103. Clathrin is a 190-kDa triskelion-shaped scaffold protein composed of three heavy chains and three light chains and able to self-assemble or co-assemble with adaptor proteins into rigid cages with pentagonal, hexagonal, and heptagonal faces104. During the internalization of cargoes, adaptor proteins (e.g., AP1, AP2, and AP3) gather to the local membrane surrounding the cargoes and recruit clathrin to interact with plasma membrane. The formation of clathrin and adaptor protein supramolecular lattices renders an invagination of the membrane. During the process of membrane invagination, the intracellular vesicles containing cargoes pitch off from the plasma membrane and transfer cargoes into the cell. The coated clathrin assemblies dissociate, allowing clathrin to be recruited for the next cycle of CME105. Molecules such as chlorpromazine, which inhibit the clathrin association and coating, can affect CME-mediated EV internalization106,107.
CDE is a process mediated by caveolin, a dimeric protein that can oligomerize into a highly condensed domain, i.e., caveolae, in the plasma membrane108. Caveolae is a specific lipid raft composed of cholesterol and sphingolipid-rich microdomains and displays a flask-shaped invagination. With the assistance of dynamin 2, caveolae can assemble and expand in the plasma membrane, achieving endocytic internalization via transcellular shuttling89,109, 110, 111. Thus, dynamin-specific inhibitors (e.g., dynasore) or knockdown of caveolin protein significantly suppress the CDE-mediated cellular uptake of EVs112,113.
Some factors are considered critical, as they affect the endocytotic process of EVs. First, the size of EVs is important. For example, micropinocytosis is a pathway to selectively internalize small EVs instead of large EVs5. Second, the cellular uptake pathway of EVs is also dependent on the surface composition of recipient cells. Different types of recipient cells prefer distinct mechanisms to internalize EVs: melanoma cells (membrane fusion), microglia (macropinocytosis, phagocytosis, CME, and CDE), neurons (CDE, phagocytosis), epithelial cells (CME), DCs (phagocytosis, receptor-mediated endocytosis), and tumor cells (macropinocytosis, lipid raft-/cholesterol-dependent endocytosis)82,87,91,107,113, 114, 115, 116, 117, 118. Inhibitors, antibodies, and small interfering RNAs that target the checkpoints in the endocytotic process of EVs can be used to regulate and control the cellular uptake pathways of EVs5.
3. The application of EVs in the diagnosis and treatment of disease
EVs inherit unique membrane and cytoplasmic components (proteins and lipids) from their parental cells, playing an important role in intercellular communication and producing a variety of biological effects such as signal transduction, coagulation, disease resistance, and immune defense71,119,120. Therefore, EVs have been widely applied in the diagnosis of and treatment against various diseases, including tumors, cardiovascular diseases, diabetes, neurodegenerative diseases, and other pathological states121, 122, 123. In this section, we aim to review the recent efforts made to exploit the clinical applications of EVs with regards to disease biomarkers, immune regulation, modulating cell proliferation and differentiation, and drug delivery. We will also outline the advances made in the application of EVs derived from plants and microbes.
3.1. Application as disease biomarkers
EVs exist in all types of body fluids and reflect the different states of the host cells. Thus, they can be regarded as important biomarkers for the diagnosis and prognosis of disease. For example, exosomes, the major subgroup of EVs, can be identified from the extracellular matrix in almost all types of eukaryotic cells, including immune cells10, 11, 12,124, neurons125, epithelial cells14, endothelial cells126, fibroblast cells127, and tumor cells128. Exosomes can also be found in all types of body fluids, including human saliva, plasma, breast milk129, cerebrospinal fluid130, semen131, urine132, pleural effusion133, amniotic fluid134, and bronchoalveolar lavage fluid135. To date, 9769 proteins, 1116 lipids, 3408 mRNA, and 2838 miRNA have been identified in the cargoes of exosomes (http://www.exocarta.org/)32. In a homeostatic state, a constant basal level of exosomes is preserved to maintain homeostasis. An abnormal level of EV secretion is correlated with a deviation from the normal range of the body’s physiological conditions136. Thus, EVs can serve as disease biomarkers for clinical diagnostics.
Currently, EVs have been implicated as diagnostic markers in the clinical liquid biopsy for cancer, central nervous disorders, cardiovascular diseases, and immune diseases, among others49. Relative to other types of biomarkers in liquid biopsy, e.g., circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs), the main advantages of EVs are as follows: first, the concentration of EVs in body fluids (i.e., 109 vesicles/mL in the peripheral circulation) is high enough for downstream analyses, whereas the concentration of CTCs is extremely low. Second, EVs have a membrane structure to stabilize the encapsulated cargoes, whereas ctDNAs is directly exposed in vivo and susceptible to degradation. Third, EVs are present in the majority of body fluids, whereas the transportation and distribution of CTCs in the human body are restricted by various physiological barriers. Fourth, EVs are secreted by living cells, providing effective information to reflect the status of parental cells. In contrast, ctDNA is released by dying cells via apoptosis, necrosis, and phagocytosis, lacking the potential to reflect the status of parental cells in time. Finally, many methodologies have been developed to extract EVs from samples and therefore the clinical application of EVs in liquid biopsy is feasible137, 138, 139, 140.
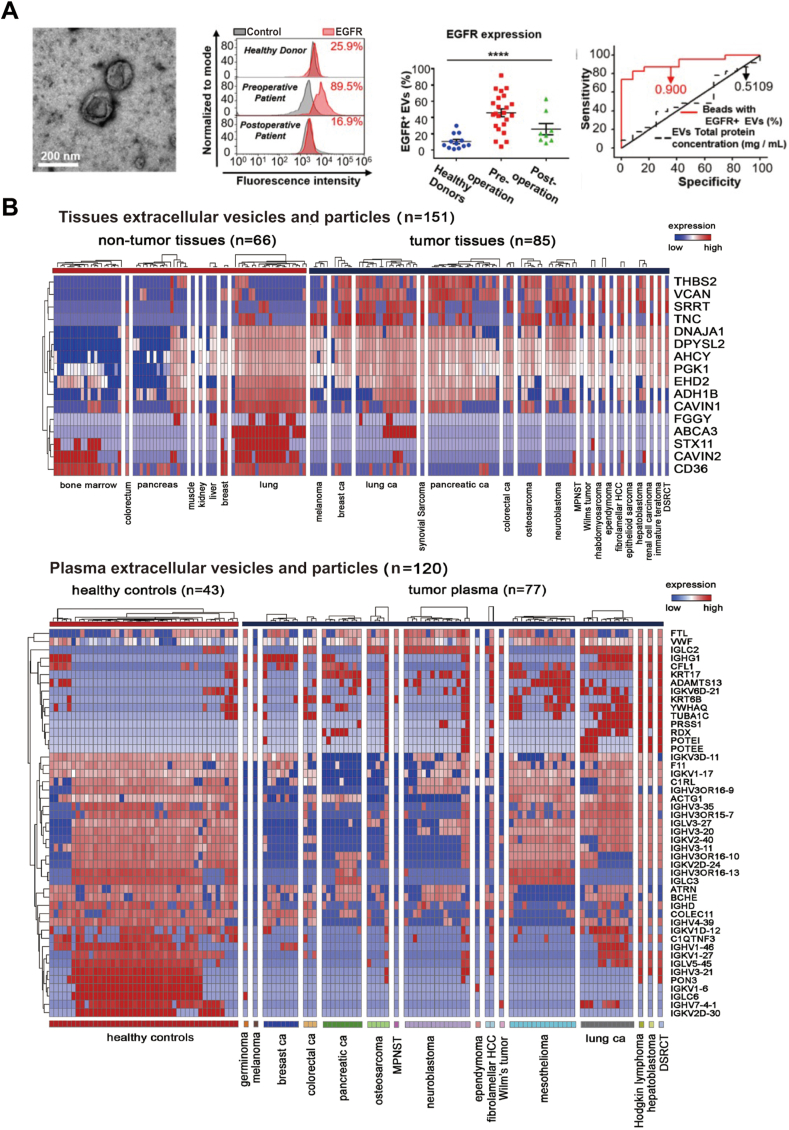
Herein, tumor-derived EVs are used as an example to manifest the potency of exosomes as biomarkers for cancer diagnosis. Glioma is the most common type of malignant brain tumor, has a high morbidity and mortality, and lacks a reliable non-invasive diagnostic method137. Wang et al.137 isolated EVs from the serum of patients with glioma and optimized a microbead-facilitated flow cytometry method to quantify the expression level of epidermal growth factor receptor (EGFR) on the surface of EVs. They found that the expression of EGFR was significantly higher in EVs derived from patients than in those of healthy donors. Following surgical removal of the tumor, the level of EGFR in serum EVs correspondingly decreased. The expression of EGFR in serum EVs was also positively correlated with the grade of glioma based on the World Health Organization classification. Indeed, receiver operating characteristic analysis revealed that EGFR+ EVs are effective diagnostic and prognostic markers of glioma, with a high sensitivity (87%) and specificity (84%). Similarly, EGFRvIII mRNA in the microvesicles isolated from patient serum can be regarded as a potential biomarker for the diagnosis of glioblastoma141. Hoshino et al.140 investigated the EVs and particles in 426 cancer patient samples and healthy donors by proteomic profiling. The EV proteome analysis revealed that the EV cargoes, such as versican, tenascin C, and thrombospondin 2, can be used as reliable diagnostic markers to distinguish cancer tissue from normal tissue with a sensitivity of 90% and a specificity of 94%140 (Fig. 5).
Figure 5.
The application of extracellular vesicles as disease biomarkers. (A) EGFR+ extracellular vesicles (EVs) are non-invasive biomarkers in glioma. The TEM image of EVs isolated from the serum of patients with glioma (left). The flow cytometry data and percentage of EGFR+ EVs showed the expression level of EGFR on the surface of EVs in healthy individuals (n = 12), preoperative glioma patients (n = 23), and postoperative glioma patients (n = 8) (middle). The ROC curve showed the discriminative ability of EGFR+ EVs in differentiating patients with glioma (n = 23) from healthy donors (n = 12) (right). Adapted with permission from Ref. 137. Copyright © 2019 Ivyspring International Publisher. (B) A liquid chromatography tandem-mass spectrometry-based proteomic analysis of EVs and particles identified the tumor-associated EVs and particle signatures in the surgically removed tissues and plasma from patients with multiple tumor types. The predictive values were predicted by random forest algorithm. The number of samples identified is noted in each box. Adapted with permission from Ref. 140. Copyright © 2020 Elsevier Inc.
3.2. Application in immune modulation
The immune system is composed of the innate immune system and the adaptive immune system, both of which play vital roles in protecting the body against pathogens, such as bacteria, virus, and other foreign substances, through numerous pathways. The innate immune system contains macrophages, DCs, mast cells, neutrophils, and natural killer (NK) cells that act as the body’s first line of defense against pathogens, responding quickly and non-specifically142. In contrast, the adaptive immune system, also referred to as the acquired immune system, is composed of highly specialized lymphocytes and responds slowly and specifically142. B cells and T cells are the major types of lymphocytes in the adaptive immune system. The innate and adaptive immune systems are tightly linked and work synergistically. The cross-talk between these two systems is dependent on intercellular communications and the release of soluble cytokines/chemokines, in which EVs are engaged in and play an important function as immunomodulators143,144.
EVs are essential in the cross-talk between antigen-presenting cells (i.e., B cells, DCs, and macrophages) and T cells to initialize an adaptive immune response145. For example, EVs derived from B lymphocytes carrying antigen-specific major histocompatibility complex (MHC) class II stimulate CD4+ T cell responses11, whereas EVs secreted by tumor peptide-pulsed DCs prime tumor-specific cytotoxic T lymphocytes146. Of note, T cells exclusively recognize the antigens bound to MHC molecules and DCs are adept at using MHC I or MHC II to internalize foreign antigens and presenting them to the naïve CD4+ or CD8+ T cells. Thus, among the diverse EVs identified from the immune system, DC-derived EVs lie at the center of the activation of T cells147,148. Tkach et al.149 isolated EVs released by human primary DCs and showed that they carry MHC class II and exhibited a potency to bind to CD4+ T cells, upregulating early T-cell activation marker CD69 in the T cells. The presence of DC-derived EVs also facilitated the proliferation of CD4+ T cells in a dose-dependent manner and activated the CD4+ T cells to release cytokines after 6 days of EV incubation. A similar activation of CD8+ T cells was also observed by the DC-derived EVs. Taken together, DC-derived EVs can activate the CD4+ and CD8+ T cells in an MHC-dependent pathway149. The activation of T cells by DC-derived EVs displays a correlation with the maturation state of the EV donor DCs. Relative to EVs derived from immature DCs, EVs released by mature DCs that experience LPS exposure induce T cell proliferation and activation more efficiently and confer the potency of activating naïve T cells to B cells148.
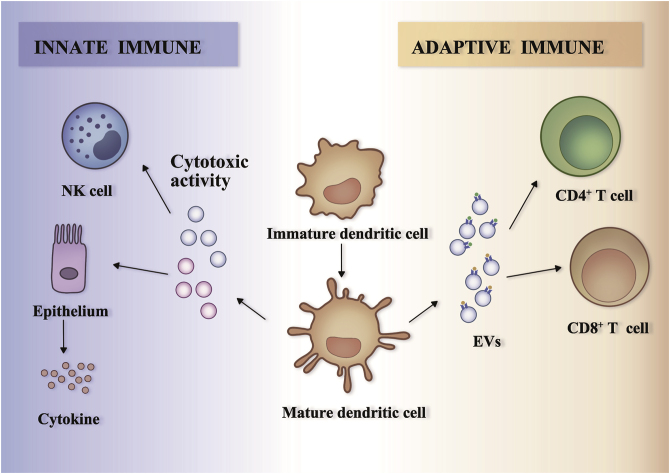
Besides of the adaptive immune system, EVs are also important in the regulation of immune response in the innate immunity system. For example, the surface of DC-derived EVs contains multiple tumor necrosis factor (TNF) superfamily members, such as TNF-α, Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL), which provide a direct binding to the surface receptors of NK cells to enhance their cytotoxic activity150. LPS-treated, mature, DC-derived EVs can activate epithelial cells and induce the release of cytokines and chemokines such as interleukin-8 (IL-8) and C–C motif chemokine ligand 5 (CCL5), both of which are engaged in the pathogenesis of sepsis151. EVs that are released by other types of donor cells are also reported to participate in the modulation of innate immunity response. Eosinophils could increase the release of EVs containing cationic proteins (such as major basic protein) and eosinophil peroxidase upon interferon-γ stimulation152. T cell-derived EVs stimulate mast cells and increase the production of cytokines153. Collectively, these data support the notion that EVs are involved in intercellular communication between multiple cell types among the immune systems (Fig. 6).
Figure 6.
Dendritic cell-derived extracellular vesicles modulate innate and adaptive immune responses. The extracellular vesicles (EVs) released from mature dendritic cells stimulate the innate immune response in immune cells and other cell types. They could induce the cytotoxic activity of natural killer cells or stimulate epithelial cells to secrete cytokines (left). The EVs released from mature dendritic cells present peptide–MHC complexes that could directly target CD4+ T cells or CD8+ T cells, and consequently promote the activation of adaptive immune responses (right).
The above-mentioned impacts of EVs in the immune system indicate that they can be exploited as an immune modulator (i.e., as a vaccine) in medical practice. Vaccines induce humoral and cellular immunity against various disease-causing pathogens. Ideal vaccines should be safe, stable, molecularly defined, and ready-made reagents that can effectively trigger effector and memory antigen-specific T cell-based immune responses. The utilization of EVs as vaccines has received substantial attention due to the potent immunostimulatory functions of EVs146,154,155.
EVs that are secreted by tumor cells presenting tumor antigens can be applied as vaccines to induce anti-tumor immunity for cancer immunotherapy. For example, exosomes derived from DCs carry the tumor-specific antigenic peptide and can initialize an anti-tumor immune response mediated by T cells and NK cells in experimental animals as well as in cancer patients146,156. The potency of DC-derived exosomes is retained in the treatment of malignant tumors with poor response to immunotherapy. In mouse models of hepatocellular carcinoma with antigenic and pathological heterogeneity, EVs derived from the antigen-expressing DCs triggered strong antigen-specific immune responses, showing a significant anti-tumor function and resulting in a prolonged survival rate157. In the past decade, the safety and efficacy of DC-derived EVs as a novel cell-free therapeutic cancer vaccine to induce anti-tumor immunity have been assessed in several clinical trials, including in phase I studies of immunotherapy in the patients with advanced non-small cell lung cancer, metastatic melanoma, or colorectal cancer156,158, 159, 160.
EVs that are released from mammalian cells infected by pathogenic microbes serve as vaccines to induce antigen-specific cellular and humoral immune responses against infection155,161,162. For example, EVs derived from macrophages treated with Mycobacterium tuberculosis culture filtrate proteins provide protection against the invasion of M. tuberculosis by stimulating a T-helper 1 cell and boosting the relevant immune response161. EVs extracted from the peripheral blood of BALB/c mice infected with the reticulocyte-prone non-lethal Plasmodium yoelii 17X strain carry the virulence factors of malaria parasite. The administration of mice with these EVs led to a substantial attenuation in the progression of parasitemia, which works parallel with a protective anti-malaria immune response to achieve a prolonged survival time155. EVs derived from bone marrow-derived cells presenting antigens efficiently initialize an immune response protecting mice from the lethal infection of Leishmaniasis major162,163. Thus, EVs are important immunomodulators to induce a specific, long-lasting immune response against tumors or microbial infections146,155,164. Finally, EVs derived from cancer cells may be involved in cancer progression, metastasis and drug resistance165, and thus a safety evaluation is necessary before their clinical application166.
3.3. Regulating cellular proliferation and differentiation
The intercellular communication mediated by EVs is involved in a wide range of cell expansion and division processes in normal as well as in pathological tissues. The dysregulation in EV leads to an aberration in homeostasis and the occurrence of disease. For example, EVs engaged in the tumor microenvironment and circulation participate in angiogenesis, thrombosis, and tumor cell proliferation167,168. The tumor microenvironment is an interactive cellular environment responsible for tumorigenesis through cellular communication, composed of stromal fibroblast cells, endothelial cells, immune cells, and other cell types169,170. Tumor-derived EVs shuttle nucleic acids and oncogenic proteins to stromal cells, preparing the normal cells to favor tumor growth, invasion, and metastasis.
3.3.1. Angiogenesis
Collective evidence demonstrates that cancer-derived EVs support the growth of cancer vasculature and blood vessels by interacting with endothelial cells and their progenitors168. An in vitro angiogenesis assay found that the tubule length of brain microvascular endothelial cells elongated after 16 h in culture in endothelial basal medium with the glioblastoma-derived EVs141. By evaluating the expression level of angiogenic proteins in cells and EVs, this effect was attributed to the glioblastoma-derived EVs transferring angiogenic proteins to the receipt endothelial cells141. Several key angiogenic proteins and their corresponding mRNAs have been identified in the cargo of tumor-derived EVs; for example, the tetraspanin family contributes to the internalization of EVs by endothelial cells and promotes vessel branching171,172, delta-like four protein suppresses the Notch signaling pathway to drive the formation of filopodia and vessel branching173, and EGFR protein elevates the autocrine secretion of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor-2 (VEGFR-2)168,174. The miR-17-92 cluster and other microRNAs from tumor-derived EVs participate in the genetic exchange between endothelial cells and tumor cells to facilitate vessel formation25,175.
3.3.2. Tumor-associated stroma
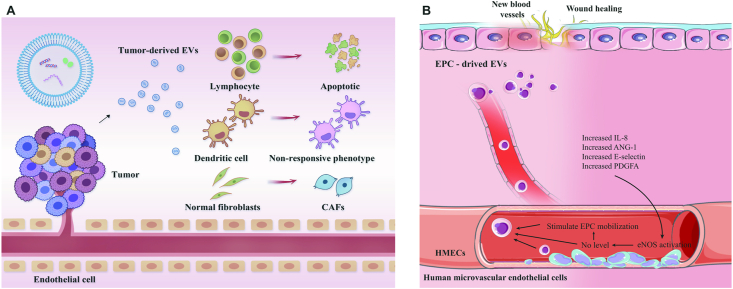
Fibroblasts of the stroma are regulated by tumor-derived EVs to favor the promotion of tumor progression and metastasis, the chemoresistance of cancer cells, and the enhancement of immunosuppression176, 177, 178. Normal fibroblasts in the mesenchymal stroma can be activated by tumor-derived EVs to initialize the differentiation into cancer-associated fibroblasts. Fang et al.179 analyzed EVs isolated from highly metastatic hepatocellular carcinoma (HCC) cells and revealed that EVs released from highly metastatic cancer cells were more abundant in population than those from lowly metastatic cancer cells. HCC-derived EVs exhibited a strong potency to enter into fibroblasts and induced the expression of cytokines and fibroblast markers in vitro and in vivo. The internalization of tumor-derived EVs was positively correlated with the metastatic ability of tumor cells. Subsequently, miR-1247-3p from EVs secreted by highly metastatic HCC cells was shown to mediate fibroblast activation, and B4GALT3 is the target gene of miR-1247-3p. Both the overexpression of miR-1247-3p or knockdown of B4GALT3 can induce cytokine expression and cell migration179. Similarly, exosomes secreted from breast cancer cells differentiate mesenchymal stem cells into myofibroblast-like cells with high expression levels of α-smooth muscle actin (α-SMA), stromal derived factor-1 (SDF-1), and VEGF, among other proteins180. EVs carrying oncogenic stem cell factor receptor tyrosine kinase in the gastrointestinal stromal tumor convert progenitor smooth muscle cells to tumor-promoting stromal cells181 (Fig. 7A).
Figure 7.
The role of extracellular vesicles in regulating cellular proliferation and differentiation. (A) The role of tumor-derived extracellular vesicles (EVs) in the tumor microenvironment. The tumor-derived EVs activate the normal fibroblasts to initialize the differentiation into cancer-associated fibroblasts. They could also induce lymphocyte apoptosis and convert dendritic cells into a non-responsive phenotype. (B) The role of endothelial progenitor cell-derived EVs in the repair of blood vessels and wound healing. EPC-derived EVs increase the level of a series of pro-angiogenic cytokines and improve the proliferation and migration ability of human microvascular endothelial cells, which facilitates tissue repair and regeneration.
3.3.3. Metastasis
The distant dissemination of EVs in the circulation drives tumor spread. For example, melanoma-derived EVs accumulate in sentinel lymph nodes, facilitating the seeding of tumor metastasis69. The exosomes released from melanoma can also establish metastatic sites in brain and bone and train bone marrow progenitor cells to have a pro-metastatic phenotype71.
3.3.4. Immune evasion
Diverse and complex mechanisms are involved in the construction of the tumor microenvironment mediated by EVs to escape from immune surveillance of host, such as the lymphocyte apoptotic death induced by the Fas-ligand bearing EVs119, the down-regulation in the antigen recognizing and responding ability of lymphocytes mediated by the specific protein cargoes in the EVs182, an impaired lymphocyte response to interleukin-2183, and the tolerance of DCs to antigen generated by the conversion of DCs into a non-responsive phenotype by EVs168,184.
EVs also act as modulators to facilitate tissue repair and regeneration by promoting the growth and differentiation of cells.
3.3.5. Angiogenesis
Endothelial progenitor cell (EPC)-derived exosomes can be taken up by human microvascular endothelial cells, contributing to the repair of blood vessels185. The internalization of EPC-derived EVs significantly improved the proliferation and migration ability of human microvascular endothelial cells and upregulated the level of a series of pro-angiogenic cytokines, including endothelial nitric oxide synthase (eNOS), IL-8, angiopoietin-1 (ANG-1), E-selectin, VEGF factor A, VEGFR-2, hypoxia inducible factor-1α (HIF-1α), chemokine (C-X-C motif) ligand-16 (CXCL16), and platelet-derived growth factor-α polypeptide (PDGFA). This result suggests that EPC-derived EVs are responsible for activating the angiogenic process in endothelial cells, which can benefit wound healing in diabetes and the endothelialization of blood vessels after vascular injury185. The exosomes secreted by human induced pluripotent stem cell-derived cardiac cells participate in the recovery from myocardial infarction by promoting endothelial tube and microvessel formation and reducing apoptosis186 (Fig. 7B).
3.3.6. Osteogenesis
A local injection of EPC-derived EVs into the distraction gap between the segments of membranous bone resulted in accelerated bone formation and consolidation187. The bone regeneration therapeutic effect of EVs is attributed to the complex activity exerted by the RNA cargo of EPC-derived EVs, that is, miR-126, via a pathway involving the activation of angiogenetic genes. Furthermore, angiogenesis stimulated by the EPC-derived EVs can accelerate bone regeneration by inducing osteogenesis187. Zhai et al.188 modified the surface of Ti-alloy scaffolds with exosomes extracted from pre-differentiated human mesenchymal stem cells and used this Ti scaffolds as implanted materials for bone regeneration. The human mesenchymal stem cell-derived exosomes carry the osteogenic miRNAs, including Hsa-miR-146a-5p, Hsa-miR-503-5p, Hsa-miR-483a-3p, Hsa-miR-129-5p, and activate the PI3K/Akt and MAPK signaling pathways to facilitate the new bone differentiation on the exosome-loaded Ti scaffold both in vitro and in vivo188.
3.3.7. Neurogenesis
The therapeutic application of EVs can also be leveraged to rescue the deficits in neural development by promoting neurogenesis and circuit assembly189. The exosomes extracted from human induced pluripotent stem cell-derived neurons carry neurodevelopmental signaling proteins to facilitate the proliferation in human primary neurons in vitro. The exosomes derived from neurons also increased the number and intensity of synaptic puncta and initialized a synchronized response among different neurons during the maturation of neural circuits by reversing the deficient phenotypes. A similar neurodevelopmental activity mediated by exosome was displayed in vivo; an injection of exosomes harvested from rodent primary neural cells into the lateral ventricles of mice was observed to significantly improve the density of cells in the granule cell layer of the dentate gyrus189.
3.4. Applications in drug delivery
Extracellular vesicle-based drug delivery systems have attracted considerable attention due to the following reasons: the membrane proteins and signal peptides carried by EVs promise active targeting of specific regions; the membrane structure of EVs can be engineered to encapsulate the external payloads such as synthetic organic compounds and macromolecular drugs; EVs exhibit a strong potential to cross various physiological barriers, including the blood–brain barrier; and EVs are biocompatible and less immunogenic relative to artificial drug carriers (e.g., liposomes or lipid-based nanoparticles)170,187,190, 191, 192.
Substantial advances have been made in the application of EVs as drug delivery carriers for the treatment of a variety of diseases, including cancer, cardiovascular disease, diabetic wound-healing, and neurodegenerative diseases52,79,190,193,194. For example, Sonic hedgehog (Shh) is a secretory protein with angiogenic properties, and Mackie et al.193 functionalized CD34+ cells by transfecting a Shh vector and found that EVs released by the functionalized CD34+ cells act as a shuttle to transfer Shh to other cells. In the mouse model of myocardial infarction, the Shh-contained EVs reversed ventricular dilation and preserved cardiac function against ischemic myocardium by promoting angiogenesis. However, a direct administration of Shh does not induce a protective effect. This result highlights the hitchhiking role of EVs in the delivery of bioactive macromolecules193. More examples can be found in the research field of anti-tumor agent delivery52.
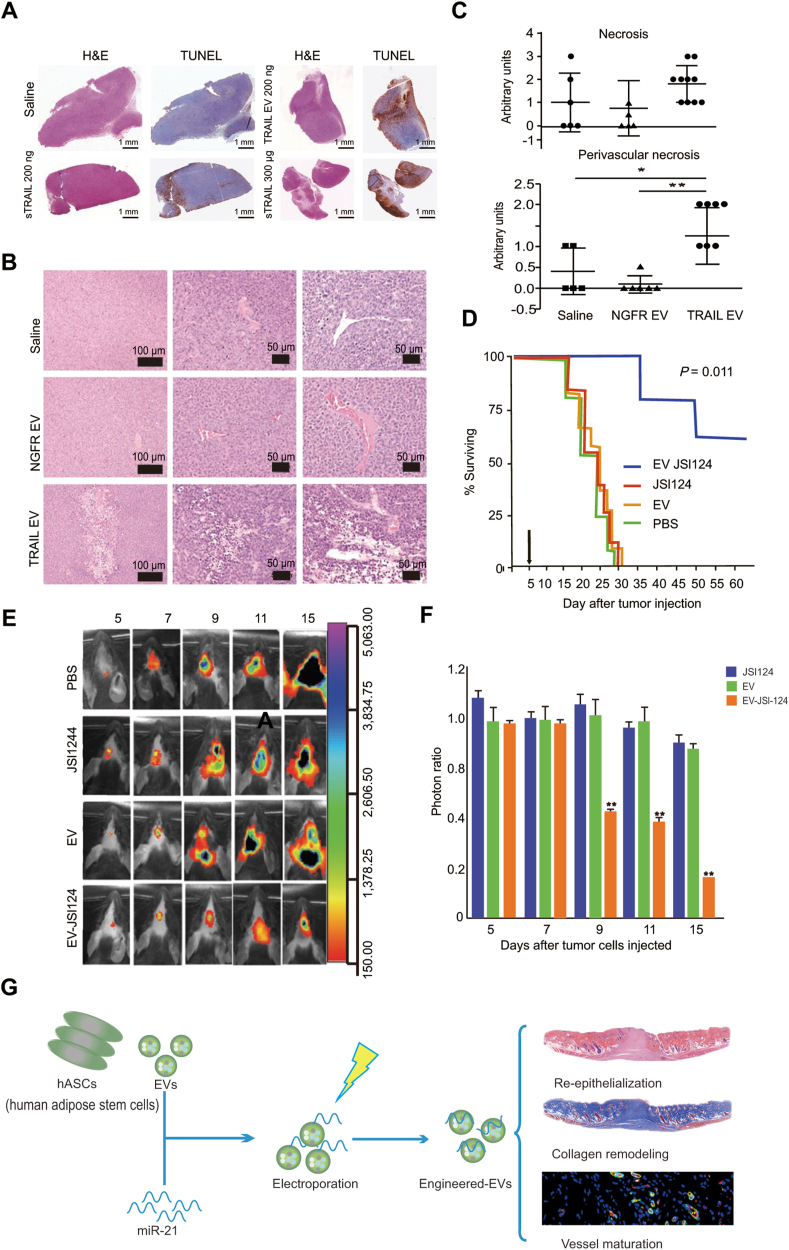
TRAIL is a protein initiating the apoptotic signaling cascade, resulting in programmed cell death. Rivoltini et al.195 engineered K562 cells to release EVs presenting TRAIL. In vitro experiments demonstrated that TRAIL+ EVs induced significant apoptosis of TRAIL receptor-bearing cells. The intratumor injection of TRAIL+ EVs inhibited the progression of tumors that express the TRAIL receptor, such as lymphoma SUDHL4 and melanoma INT12, and led to necrosis in KMS11 tumors195. Chemotherapeutic compounds, such as paclitaxel196, doxorubicin197, curcumin, and JSI124 (a signal transducer and activator of transcription-3 inhibitor), can be packaged into EVs, with the ability to cross the blood–brain barrier to treat brain inflammatory-related diseases such as glioblastoma190. Monocyte- and macrophage-derived EVs loaded with the antioxidant catalase can be intranasally or intravenously administered, accumulating in the brain neurons and microglial cells, providing a neuroprotective therapy effect against Parkinson’s disease198. Recently, the EV-based drug exoIL-12, which bears IL-12 on surface, was tested in a phase I clinical trial in cutaneous T cell lymphoma. Another artificially engineered EV-based drug, exoSTING, which includes a cGAS‒STING pathway activator (cyclic dinucleotide) in the inner space and presents prostaglandin F2 receptor-negative regulator (PTGFRN) on the exosome surface, has entered phase I/II clinical trials in the treatment against head and neck squamous cell carcinoma, triple-negative breast cancer, anaplastic thyroid carcinoma, and squamous cell carcinoma. EVs improve the stability of cargoes, providing a feasible approach to protect RNAs from degradation. They benefit the clinical applications of RNA drugs, i.e., miRNAs and siRNAs, in the treatment of diabetic wound-healing, tumors, and neurodegenerative diseases52,79,194. For instance, human adipose stem cell-derived EVs loaded with miR-21-5p improved diabetic wound-healing by promoting re-epithelialization, angiogenesis, collagen remodeling, and vessel maturation in vivo194. Exosomes derived from the normal fibroblast-like mesenchymal cells expressing CD47 protein, an integrin associated transmembrane protein that protects cells from phagocytosis199, have a high retention time in the circulation system. Such exosomes display a powerful potency to deliver miRNAs to pancreatic tumors by specifically targeting the oncogenic KRAS in pancreatic ductal adenocarcinoma200.
A crucial step in the preparation of EV-based drug carriers is the uptake of the drugs by EVs. Several loading techniques have been developed, such as simple co-incubation, electroporation, chemical-facilitated transfection, and engineering the parental cells to produce cargo-loaded EVs34,201, 202, 203. The methodologies of loading different cargoes, i.e., small molecules, proteins, and nucleic acids, into EVs are distinct. Simple co-incubation can be applied to load small molecules, such as low molecular antioxidant, curcumin190,204, anticancer agents, doxorubicin (Dox)205,206 and paclitaxel (PTX), into EVs. Electroporation and chemical-facilitated transfection have been used to incorporate EVs with nucleic acids, including mRNA, siRNA, miRNA, noncoding RNA, mitochondrial DNA, and genomic DNA79,199,201,207, 208, 209. Specifically, electroporation is a process by which an external electric field is applied to generate pores on the membrane of EVs to promise nucleic acids enter into EVs199. However, electroporation sometimes leads to the aggregation of EVs or the aggregation of siRNAs, decreasing the loading efficiency201,209. An alternative method to prepare siRNA-loaded EVs is chemical-facilitated transfection that uses chemical reagents to create transmembrane pores199,210. For delivering proteins, overexpressing a certain gene in EV-donor cells to produce cargo protein-loaded EVs is as an effective method199,201,211.
EV-based drug delivery platforms can achieve passive targeting to shuttle cargoes. For instance, the exosomes derived from DCs are targeted to activate T cells by the interactions between the intercellular cell adhesion molecule-1 (ICAM-1) carried by the DC-exosomes and the lymphocyte function-associated antigen-1 (LFA-1) expressed on T cells212. The exosomes derived from B cells are capable of specifically recognizing splenic macrophages mediated by the interactions between the α2,3-linked sialic acids on the B cell-derived exosomes and the sialoadhesin (CD169) expressed on macrophages213. However, EVs secreted by most types of cells lack the potency to selectively recognize their target. Thus, bioengineering approaches, such as introducing the target ligand to the outer surfaces of EVs, are needed to endorse EVs with cell- or tissue-targeting specificity36,52,214. For instance, immature DCs were engineered to express a fusion protein of iRGD peptide and lysosome associated membrane glycoprotein-2b (Lamp2b) and secreted iRGD-Lamp2b+ EVs. The iRGD-Lamp2b+ EVs can load doxorubicin via electroporation and selectively target to breast cancer tissues, reducing tumor growth in mice206. EVs isolated from DCs or bone marrow mesenchymal stem cells that are engineered to co-express central nervous system-specific rabies viral glycoprotein (RVG) and Lamp2b, deliver siRNAs or miRNAs specifically to neurons. The administration of RVG-Lamp2b+ EVs induced neural progenitors to differentiate into neuronal phenotypes at the infarct sites in a model of chronic neurodegenerative disorders79,215. Similarly, exosomes released from GATA-4-overexpressing mesenchymal stem cells can increase the survival of cardiomyocytes and reduce cardiomyocyte apoptosis, providing cardioprotection and regenerating the ischemic myocardium216 (Fig. 8).
Figure 8.
Applications of extracellular vesicles in drug delivery. (A)‒(C) The antitumor activity of TRAIL extracellular vesicle treatments against KMS11 multiple myeloma. (A) Intratumor treatment. KMS11-bearing mice were treated by TRAIL EVs every 48 h and then measured by the TUNEL assay. (B) and (C) Systematic treatment. Hematoxylin and eosin staining images (B) and determination of necrotic areas (C) of tumors of KMS11-bearing mice were treated by four intravenous treatments of TRAIL EVs, NGFR EVs, or saline every 48 h (n = 5–6 saline and mice accepting NGFR EVs; n = 10 mice accepting TRAIL EVs). Adapted with permission from Ref. 195. Copyright © 2016 American Association for Cancer Research. (D)–(F) Treatment with JSI124-EVs prevents the in vivo growth of injected brain tumor cells. (D) The surviving rate of mice treated with EV-JSI124, JSI124, EV and control administration. (E) and (F) A representative photo of brain tumor signals (E) and the growth potential of cells (F) of mice treated with JSI124, EV, or EV-JSI124. Adapted with permission from Ref. 190. Copyright © 2011 The American Society of Gene & Cell Therapy. (G) The human adipose stem cell-derived EVs loaded with miR-21-5p improved diabetic wound healing. Adapted with permission from Ref. 194. Copyright © 2020 American Chemical Society.
Evaluation of EV biosafety is critical before clinical application. The immunogenicity of EVs is comprehensive and dependent on the composition of EVs. For example, the immune system of C57BL/6 mice was inert to the EVs derived from human sources. After an intravenous or intraperitoneal administration of the EVs derived from HEK293T cells for 3 weeks, the hematology test, blood chemistry, immune marker analysis with blood and the immunophenotypic analysis with spleen cells revealed that no sign of immunogenicity or toxicity was observed217. A parallel study demonstrated that the intravenously administrated HEK293T-derived EVs can be eliminated from the spleen, liver, kidney, and lung of mice with an elimination half-life of 3 h218. However, a change in the composition of EVs might lead to different immunogenic property. For example, the engineered HEK293T-derived EVs carrying miR-199a-3p displayed low immunogenicity and a minimal evidence of changes in immune markers was observed after the treatment of mice by engineered EVs217. Some strategies have been proposed to reduce the immunogenicity of EVs, such as fabricating heterozygous vesicles by the combination of EVs/liposomes or EVs/enveloped protein nanocage, or generating EVs in immature dendritic cells79,219,220. In the future, more feasible approaches for regulating the immunogenicity of EVs need to be discovered.
3.5. The application of plant-derived vesicles
Plant-derived vesicles are a collection of membrane-bound vesicular structures isolated from dietary plants and plant-based products after manufacturing processes221, 222, 223, 224. Similar to EVs secreted by mammalian cells, plant-derived vesicles contain lipids, proteins, nucleic acids, and secondary metabolites. The origins of plant-derived vesicles are diverse; most are extracted by using fruit and vegetable juices, including cell lysis steps, thus originating from both the intracellular and extracellular space in plants221,225. Alternatively, some of plant-derived vesicles are isolated from the decoction of plants following a heating process and are therefore artificially created221,226. Moreover, the lipids and bioactive molecules identified from plant-derived vesicles can also be utilized as building blocks to co-assemble into functionalized liposomes as mimics of natural vesicles221,226,227.
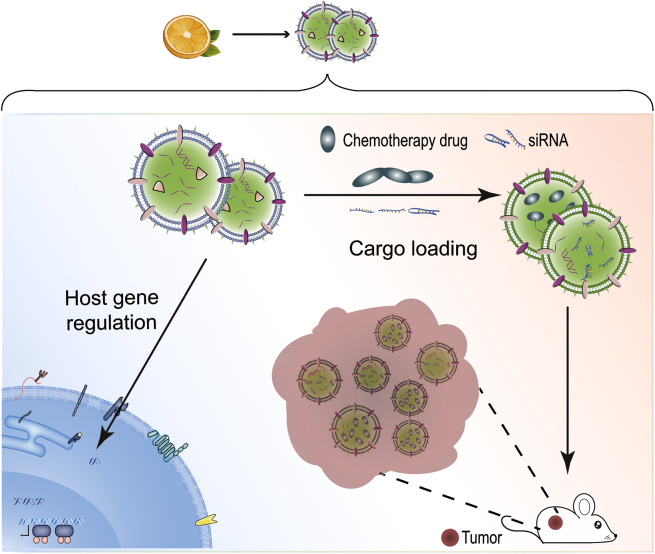
Collective research results have demonstrated that plant-derived vesicles provide a novel therapeutic approach towards the treatment of numerous diseases due to their striking roles in the regulation of mammalian and microbial cellular processes. For instance, ginger-derived nanoparticles were isolated from ginger juice and purified via sucrose-gradient ultracentrifugation225; these ginger-derived nanoparticles displayed a cup-shaped geometry with a diameter of ∼200 nm and were composed of lipids (mainly phosphatidic acids, digalactosyldiacylglycerol, and monogalactosyldiacylglycerol), miRNAs, active small molecules (6-gingerol and 6-shogaol), cytosolic proteins (actin and proteolysis enzymes), and membrane proteins (aquaporin and chloride channels). In vivo experiments revealed that the oral administration of ginger-derived nanovesicles greatly reduced mRNA levels of pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and the proliferation-related cyclin D1225. The therapeutic potential of this plant-derived nanovesicle to regulate the expression of inflammatory factors has been shown in mouse colitis models, in which a decrease in the tumor numbers and tumor loads was parallel with an increase in the survival and proliferation of intestinal epithelial cells225. The biological functions of vesicles isolated from plants can be summarized as regulating host gene expression and acting as an efficient delivery system to reduce drug degradation.
Plenty of functional small RNAs (sRNAs) have been identified from plant-derived vesicles and found to regulate the host gene expression. Following entry into the animal body, plant sRNAs are found to escape from degradation, surviving in the functional form and affecting the genetic expression of the host cell, a term known as cross-kingdom gene regulation225,228,229. For example, PGY-sRNA-6 is a plant-derived sRNA carried by the nanovesicles existing in the decoctions of Pu Gong Ying (PGY, TARAXACI HERBA, Taraxacum mongolicum)226. Following the oral administration of the nanovesicles encapsulating PGY-sRNA-6, PGY-sRNA-6 targets the RELA gene, downregulating the mRNA expressions of the relevant cytokines such as IL-1β, IL-6, and TNF-α226.
Another relevant example is the ginger-derived exosome-like nanoparticle shaping the gut microbiota224. Teng et al.224 found that the exosomal miRNAs carried by the ginger-derived nanoparticles can target the genes in gut bacteria Lactobacillus rhamnosus (LGG) and consequently alter gut microbiome function. Specifically, gma-miR396e reduces the mRNA level of LGG transcription repressor LexA. Mdo-miR7267-3p binds to the LGG ycnE gene, leading to an increase in the level of indole-3-carboxaldehyde (I3A). The upregulated I3A production ameliorates gut barrier function by facilitating the secretion of IL-22. MiR167a-5p decreases the expression of the LGG pilus-specific SpaC gene weakening the migration ability of LGG invading into gut epithelial cells and other tissues224. Commensal microbiota co-exists with human forming an indivisible ecosystem. The profound impacts of plant-derived vesicles on the gut microbiota suggest a valuable strategy to modulate host physiology by targeting the commensal microbiota.
Another promising application of plant-derived vesicles is as carriers for drug delivery due to their low immunogenicity, stability in the gastrointestinal tract, and selective targeting230. For example, methotrexate (MTX) is an anti-cancer chemotherapy drug a high dose of which can lead to kidney toxicity. To reduce the side effects of MTX, grapefruit-derived nanovesicles, extracted from grapefruit juice by a sucrose gradient centrifugation, were used to encapsulate MTX to generate an oral delivery system231. Grapefruit-derived nanovesicles are easily taken up by intestinal macrophages. Consequently, the in vivo biodistribution of MTX was altered by the grapefruit vesicles, resulting in a reduction in toxicity and an improved therapeutic effect in a mouse model of colitis231. Plant-derived vesicles also have promising potential in RNAs delivery for the treatment of diseases. For example, the liposomes made by the grapefruit-derived lipids can deliver miR-18a via intravenous administration to suppress liver metastasis of colon cancer232. The vesicles formed by the co-assembly of the T. mongolicum lipid (sphinganine) and PGY-sRNA-6 can ameliorate bleomycin-induced lung fibrosis and poly (I:C)-induced lung inflammation via oral administration226 (Fig. 9).
Figure 9.
The applications of plant-derived vesicles. Regulation of host gene expression (left) and delivery systems for RNA and chemotherapy drugs (right).
3.6. The application of vesicles derived from bacteria
Vesicles derived from microbes play important roles in the communication between bacteria and their hosts and have been applied to regulate the balance between the host innate immune system and microbes, as described below 233, 234, 235.
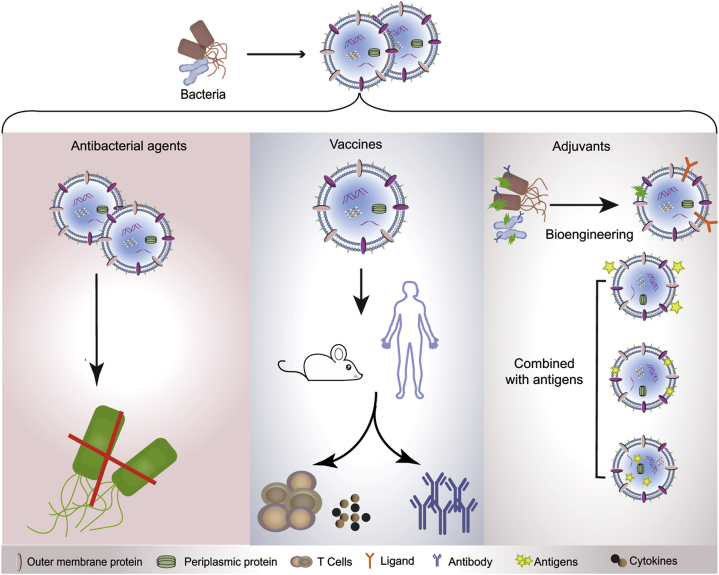
First, bacteria-derived vesicles serve as antibiotics against bacterial infection. During the production of vesicles by bacteria, peptidoglycan hydrolase and other types of cell wall-degrading enzymes in the periplasmic space are packaged into the released vesicles, rendering them capable of lysing different bacteria in the environment236. For example, vesicles isolated from various strains, including Citrobacter, Enterobacter, Escherichia, Klebsiella, Morganella, Proteus, Salmonella, and Shigella, possess the lytic ability to degrade the surrounding Gram-positive bacteria as well as Gram-negative bacteria by shuttling peptidoglycan hydrolase into their recipient bacteria. The OMVs derived from two strains of non-pathogenic soil bacteria, Cystobacter velatus Cbv34 and Sorangiineae SBSr073, exhibit antibiotic activity against Escherichia coli through membrane fusion233.
Second, bacteria-derived vesicles are non-replicative, immunogenic mimics of their parental bacteria and are able to regulate both the innate and adaptive immunity of host237,238. For example, the OMVs derived from Bacteroides fragilis induce a promotion of regulatory T cells and anti-inflammatory cytokine secretion via the activation of the host DCs239. B. fragilis is a symbiotic Gram-negative bacterium identified from the gastrointestinal tract of mammals. Different from the replication of pathogenic bacteria, which typically causes infection and illness in a host, the growth and propagation of commensal bacteria have beneficial impacts on host health. Particularly, B. fragilis secretes an immunomodulatory molecule, polysaccharide A, which ameliorates human inflammatory disorders such as inflammatory bowel disease and multiple sclerosis240,241. The OMVs derived from B. fragilis are rich in polysaccharide A, thus displaying a therapeutic activity comparable to its parental bacterium with regards to immune regulation239. Thus, the vesicles released by the numerous non-pathogenic commensal bacteria provide valuable therapeutic resources for humans.
Additionally, vesicles derived from symbiotic bacteria have a low toxicity and good immunogenicity, allowing them to be used as vaccine adjuvants to promote comprehensive immune responses242,243. Intranasal administration of OMVs extracted from an intestinal Gram-negative bacterium Bacteroides thetaiotaomicron caused the presence of large organized lymphoid follicles in the nasal cavity and lungs of mice, indicative of the activation of an immune response244. When these OMVs were loaded with exogenous protein cargoes, such as the vaccine antigen of Salmonella, OMVs exhibited a potent adjuvant effect generating both systematic and mucosal antibody responses. Specifically, the administration of OMVs presenting the vaccine antigen of Salmonella via either oral or intranasal routines led to a significantly high level of antigen-specific serum IgG and IgA antibodies244.
Vesicles released by pathogenic bacteria can also be used as adjuvants in vaccines. For instance, OMVs isolated from Neisseria meningitidis, a life-threatening bacterium that causes meningococcal disease, can induce potent protective immune responses by the host to prevent meningococci and have been successfully applied as adjuvants in several commercial vaccine formulations238,245, 246, 247, 248. Similarly, the administration of OMVs derived from V. cholera induce a long-lasting immune response against cholera164 that can be transferred from pregnant mice to their offspring164. To expand the potential use of vesicles derived from bacteria in vaccines, genetic engineering approaches have been used to create recombinant vesicles carrying exogenous protein antigens, stimulating a specific and strong immune response by the host242,249. Thus, vesicles derived from bacteria provide a new immunomodulatory strategy to fight against pathogens, serving as a potential candidate for the treatment of diseases and revealing an inter-kingdom communication mechanism between the microbiota and mammals (Fig. 10).
Figure 10.
The applications of vesicles derived from bacteria. Outer-membrane vesicles (OMVs) derived from bacteria serve as antibiotics against bacterial infection (left). OMVs are potential vaccines against bacterial infections (middle). OMVs can also be used as competent adjuvants in vaccines (right).
4. Conclusions, challenges and perspectives
Herein, we have introduced the sources, biological functions, and biomedical applications of various types of EVs, showing that they exhibit great potential in disease diagnosis and treatment as well as drug delivery. However, many challenges need to be overcome before the EVs become ideal biomaterials. For example, the preparation of exosomes is difficult to scale up, especially for mammalian cell-derived exosomes, limiting their application as translational nanomedicines. Thus, plant-derived EVs and bacterial OMVs, which can be obtained in large quantities, have promising potential as drug carriers. Furthermore, it is difficult to guarantee a high purity of specific EVs due to the size distribution is not clear for different subgroups of EVs. Therefore, specific labeling of EVs is supposed helpful to distinguish the type of EVs and to trace the EVs when applied in vivo.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (31901007, 81630023, 81970852, and 82000962), CAMS Innovation Fund for Medical Sciences, China (2018-I2M-3-006 and 2019-I2M-5-022, China), China Postdoctoral Science Foundation (2020T130006ZX), the Open Project Fund provided by Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS (NSKF202019, China), the State Key Laboratory Special Fund 2060204, the National Key R&D Program of China (2016YFC0905200), the program for the Changjiang scholars and innovative research team (IRT13082, China), the Beijing Bai-Qian-Wan talent project (2019A32, China), and the Public Welfare Development and Reform Pilot Project (2019-10, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Tianjiao Ji, Email: jitj@nanoctr.cn.
Luo Zhang, Email: dr.luozhang@139.com.
Chenxuan Wang, Email: wangcx@ibms.pumc.edu.cn.
Author contributions
Chenxuan Wang, Luo Zhang and Tianjiao Ji conceived the project and supervised the project. Yan Zhao, Lu Xiao, Wenbo Zhang and Lanlan Yu summarized the literatures and wrote the manuscript. Zhun Deng, Mingwei Liu. Shanshan Mo, Ruonan Wang, Jinming Zhao and Yun Hao were involved in drawing the figures. Shuli Liu and Xiangdong Wang proofread the structures and figures. Chenxuan Wang, Luo Zhang and Tianjiao Ji revised the manuscript. All authors approved the final manuscript.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.O'Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deatherage B.L., Cookson B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Pol E., Boing A.N., Harrison P., Sturk A., Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 4.Gyorgy B., Szabo T.G., Pasztoi M., Pal Z., Misjak P., Aradi B. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chargaff E., West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–197. [PubMed] [Google Scholar]
- 6.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 7.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 9.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thery C., Regnault A., Garin J., Wolfers J., Zitvogel L., Ricciardi-Castagnoli P. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 13.Raposo G., Tenza D., Mecheri S., Peronet R., Bonnerot C., Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Niel G., Raposo G., Candalh C., Boussac M., Hershberg R., Cerf-Bensussan N. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 15.Mears R., Craven R.A., Hanrahan S., Totty N., Upton C., Young S.L. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 16.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 17.Escola J.M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 18.Heijnen H.F., Schiel A.E., Fijnheer R., Geuze H.J., Sixma J.J. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 19.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Tricarico C., Clancy J., D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dragovic R.A., Gardiner C., Brooks A.S., Tannetta D.S., Ferguson D.J., Hole P. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzesco A.M., Janich P., Wilsch-Brauninger M., Dubreuil V., Langenfeld K., Corbeil D. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 23.Turiak L., Misjak P., Szabo T.G., Aradi B., Paloczi K., Ozohanics O. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteomics. 2011;74:2025–2033. doi: 10.1016/j.jprot.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Hristov M., Erl W., Linder S., Weber P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 25.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 26.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Susa F., Limongi T., Dumontel B., Vighetto V., Cauda V. Engineered extracellular vesicles as a reliable tool in cancer nanomedicine. Cancers. 2019;11:1979. doi: 10.3390/cancers11121979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murillo O.D., Thistlethwaite W., Rozowsky J., Subramanian S.L., Lucero R., Shah N. exRNA Atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell. 2019;177:463–477. doi: 10.1016/j.cell.2019.02.018. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S., Extracellular R.N.A.C.C., Ansel K.M., Bitzer M., Breakefield X.O., Charest A. The Extracellular RNA communication consortium: establishing foundational knowledge and technologies for extracellular RNA research. Cell. 2019;177:231–242. doi: 10.1016/j.cell.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romagnoli G.G., Zelante B.B., Toniolo P.A., Migliori I.K., Barbuto J.A. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol. 2014;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoi A., Yoshioka Y., Yamamoto Y., Ishikawa M., Ikeda S.I., Kato T. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. doi: 10.1038/ncomms14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Familtseva A., Jeremic N., Tyagi S.C. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 2019;459:1–6. doi: 10.1007/s11010-019-03545-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Li S., Li L., Li M., Guo C., Yao J. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev Reprod Biol. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li I., Nabet B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beach A., Zhang H.G., Ratajczak M.Z., Kakar S.S. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro Oncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalra H., Drummen G.P., Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassereau P. Division of labour in ESCRT complexes. Nat Cell Biol. 2010;12:422–423. doi: 10.1038/ncb0510-422. [DOI] [PubMed] [Google Scholar]
- 43.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghossoub R., Lembo F., Rubio A., Gaillard C.B., Bouchet J., Vitale N. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 45.Akerman A.W., Blanding W.M., Stroud R.E., Nadeau E.K., Mukherjee R., Ruddy J.M. Elevated wall tension leads to reduced miR-133a in the thoracic aorta by exosome release. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 47.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 48.Kowal J., Tkach M., Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Mathieu M., Martin-Jaular L., Lavieu G., Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 50.Record M., Silvente-Poirot S., Poirot M., Wakelam M.J.O. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018;59:1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 52.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 54.Yu X., Harris S.L., Levine A.J. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 55.Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richter S., Kientz M., Brumm S., Nielsen M.E., Park M., Gavidia R. Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. Elife. 2014;3 doi: 10.7554/eLife.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen L.L., Nielsen M.E. Plant exosomes: using an unconventional exit to prevent pathogen entry? J Exp Bot. 2017;69:59–68. doi: 10.1093/jxb/erx319. [DOI] [PubMed] [Google Scholar]
- 58.Meyer D., Pajonk S., Micali C., O'Connell R., Schulze-Lefert P. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986–999. doi: 10.1111/j.1365-313X.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen M.E., Thordal-Christensen H. Transcytosis shuts the door for an unwanted guest. Trends Plant Sci. 2013;18:611–616. doi: 10.1016/j.tplants.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Micali C.O., Neumann U., Grunewald D., Panstruga R., O'Connell R. Biogenesis of a specialized plant-fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cell Microbiol. 2011;13:210–226. doi: 10.1111/j.1462-5822.2010.01530.x. [DOI] [PubMed] [Google Scholar]
- 61.Roier S., Zingl F.G., Cakar F., Durakovic S., Kohl P., Eichmann T.O. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwechheimer C., Rodriguez D.L., Kuehn M.J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiologyopen. 2015;4:375–389. doi: 10.1002/mbo3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwechheimer C., Kulp A., Kuehn M.J. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 2014;14:324. doi: 10.1186/s12866-014-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni H.M., Jagannadham M.V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology (Read) 2014;160:2109–2121. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 65.Mashburn L.M., Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 66.McBroom A.J., Kuehn M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMahon K.J., Castelli M.E., Garcia Vescovi E., Feldman M.F. Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J Bacteriol. 2012;194:3241–3249. doi: 10.1128/JB.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadurugamuwa J.L., Beveridge T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hood J.L., San R.S., Wickline S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 71.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dickens A.M., Tovar Y.R.L.B., Yoo S.W., Trout A.L., Bae M., Kanmogne M. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10 doi: 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolburg H., Lippoldt A. Tight junctions of the blood‒brain barrier: development, composition and regulation. Vasc Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 74.Haseloff R.F., Dithmer S., Winkler L., Wolburg H., Blasig I.E. Transmembrane proteins of the tight junctions at the blood−brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2015;38:16–25. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto J., Stewart T., Sheng L., Li N., Bullock K., Song N. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood−brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun. 2017;5:71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood−brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dehouck B., Fenart L., Dehouck M.P., Pierce A., Torpier G., Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood−brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Candela P., Gosselet F., Saint-Pol J., Sevin E., Boucau M.C., Boulanger E. Apical-to-basolateral transport of amyloid-beta peptides through blood−brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis. 2010;22:849–859. doi: 10.3233/JAD-2010-100462. [DOI] [PubMed] [Google Scholar]
- 79.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 80.Simons M., Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Mathivanan S., Lim J.W., Tauro B.J., Ji H., Moritz R.L., Simpson R.J. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010;9:197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng D., Zhao W.L., Ye Y.Y., Bai X.C., Liu R.Q., Chang L.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 83.Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jahn R., Lang T., Sudhof T.C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 85.Chernomordik L.V., Melikyan G.B., Chizmadzhev Y.A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 86.Jahn R., Sudhof T.C. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 87.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller S.R.R.H. In vitro phagocytosis assay of nano- and microparticles by chemiluminescence. III. Uptake of differently sized surface-modified particles, and its correlation to particle properties and in vivo distribution. Eur J Pharmaceut Sci. 1993;1:31–39. [Google Scholar]
- 89.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 90.Swanson J.A. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.L., Stolz D.B., Papworth G.D. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 92.Stephens L., Ellson C., Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 93.Gold E.S., Underhill D.M., Morrissette N.S., Guo J., McNiven M.A., Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kerr M.C., Teasdale R.D. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 95.Grimmer S., van Deurs B., Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–2962. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- 96.Ahram M., Sameni M., Qiu R.G., Linebaugh B., Kirn D., Sloane B.F. Rac1-induced endocytosis is associated with intracellular proteolysis during migration through a three-dimensional matrix. Exp Cell Res. 2000;260:292–303. doi: 10.1006/excr.2000.5031. [DOI] [PubMed] [Google Scholar]
- 97.Gao Y.S., Hubbert C.C., Lu J., Lee Y.S., Lee J.Y., Yao T.P. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007;27:8637–8647. doi: 10.1128/MCB.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amyere M., Payrastre B., Krause U., Van Der Smissen P., Veithen A., Courtoy P.J. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veithen A., Cupers P., Baudhuin P., Courtoy P.J. v-Src induces constitutive macropinocytosis in rat fibroblasts. J Cell Sci. 1996;109(Pt 8):2005–2012. doi: 10.1242/jcs.109.8.2005. [DOI] [PubMed] [Google Scholar]
- 100.Tian T., Zhu Y.L., Zhou Y.Y., Liang G.F., Wang Y.Y., Hu F.H. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 102.DeTulleo L., Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gagnon A.W., Kallal L., Benovic J.L. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the beta2-adrenergic receptor. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 104.Paraan M., Mendez J., Sharum S., Kurtin D., He H., Stagg S.M. The structures of natively assembled clathrin-coated vesicles. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 106.Wang L.H., Rothberg K.G., Anderson R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Escrevente C., Keller S., Altevogt P., Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nichols B.J., Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- 109.Jadli A.S., Ballasy N., Edalat P., Patel V.B. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467:77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- 110.Nabi I.R., Le P.U. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parton R.G., Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 112.Newton A.J., Kirchhausen T., Murthy V.N. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nanbo A., Kawanishi E., Yoshida R., Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuyama K., Sun H., Mitsutake S., Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 116.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fruhbeis C., Frohlich D., Kuo W.P., Amphornrat J., Thilemann S., Saab A.S. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Svensson K.J., Christianson H.C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L.M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andreola G., Rivoltini L., Castelli C., Huber V., Perego P., Deho P. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torben H.J. Springer Science+Business Media; New York: 2010. RNA exosome. [Google Scholar]
- 121.Chaput N., Taieb J., Schartz N., Flament C., Novault S., Andre F. The potential of exosomes in immunotherapy of cancer. Blood Cells Mol Dis. 2005;35:111–115. doi: 10.1016/j.bcmd.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 122.Pitt J.M., Andre F., Amigorena S., Soria J.C., Eggermont A., Kroemer G. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu L., Kalimuthu S., Gangadaran P., Oh J.M., Lee H.W., Baek S.H. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7:2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caby M.P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 125.Chivet M., Hemming F., Pernet-Gallay K., Fraboulet S., Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dozio V., Sanchez J.C. Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J Extracell Vesicles. 2017;6:1302705. doi: 10.1080/20013078.2017.1302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li W., Zhang X., Wang J., Li M., Cao C., Tan J. TGFbeta1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget. 2017;8:96035–96047. doi: 10.18632/oncotarget.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whiteside T.L. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lasser C., Alikhani V.S., Ekstrom K., Eldh M., Paredes P.T., Bossios A. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Street J.M., Barran P.E., Mackay C.L., Weidt S., Balmforth C., Walsh T.S. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Madison M.N., Roller R.J., Okeoma C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 2014;11:102. doi: 10.1186/s12977-014-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gonzales P.A., Pisitkun T., Hoffert J.D., Tchapyjnikov D., Star R.A., Kleta R. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bard M.P., Hegmans J.P., Hemmes A., Luider T.M., Willemsen R., Severijnen L.A. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 134.Keller S., Rupp C., Stoeck A., Runz S., Fogel M., Lugert S. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 135.Kulshreshtha A., Ahmad T., Agrawal A., Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131:1194–1203. doi: 10.1016/j.jaci.2012.12.1565. 203 e1-14. [DOI] [PubMed] [Google Scholar]
- 136.Kosaka N., Yoshioka Y., Fujita Y., Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126:1163–1172. doi: 10.1172/JCI81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang H., Jiang D., Li W., Xiang X., Zhao J., Yu B. Evaluation of serum extracellular vesicles as noninvasive diagnostic markers of glioma. Theranostics. 2019;9:5347–5358. doi: 10.7150/thno.33114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu R., Greening D.W., Zhu H.J., Takahashi N., Simpson R.J. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–1061 e18. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaplin D.D. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kabelitz D., Medzhitov R. Innate immunity—cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Buzas E.I., Gyorgy B., Nagy G., Falus A., Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 145.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 147.Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 148.Segura E., Nicco C., Lombard B., Veron P., Raposo G., Batteux F. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 149.Tkach M., Kowal J., Zucchetti A.E., Enserink L., Jouve M., Lankar D. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012–3028. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Munich S., Sobo-Vujanovic A., Buchser W.J., Beer-Stolz D., Vujanovic N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. OncoImmunology. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Obregon C., Rothen-Rutishauser B., Gerber P., Gehr P., Nicod L.P. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol. 2009;175:696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Canas J.A., Sastre B., Mazzeo C., Fernandez-Nieto M., Rodrigo-Munoz J.M., Gonzalez-Guerra A. Exosomes from eosinophils autoregulate and promote eosinophil functions. J Leukoc Biol. 2017;101:1191–1199. doi: 10.1189/jlb.3AB0516-233RR. [DOI] [PubMed] [Google Scholar]
- 153.Shefler I., Pasmanik-Chor M., Kidron D., Mekori Y.A., Hershko A.Y. T cell-derived microvesicles induce mast cell production of IL-24: relevance to inflammatory skin diseases. J Allergy Clin Immunol. 2014;133:217–224. doi: 10.1016/j.jaci.2013.04.035. e1-3. [DOI] [PubMed] [Google Scholar]
- 154.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 155.Martin-Jaular L., Nakayasu E.S., Ferrer M., Almeida I.C., Del Portillo H.A. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Escudier B., Dorval T., Chaput N., Andre F., Caby M.P., Novault S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu Z., Zuo B., Jing R., Gao X., Rao Q., Liu Z. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 158.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barros F.M., Carneiro F., Machado J.C., Melo S.A. Exosomes and immune response in cancer: friends or foes? Front Immunol. 2018;9:730. doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheng Y., Schorey J.S. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schnitzer J.K., Berzel S., Fajardo-Moser M., Remer K.A., Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010;28:5785–5793. doi: 10.1016/j.vaccine.2010.06.077. [DOI] [PubMed] [Google Scholar]
- 163.von Stebut E., Belkaid Y., Nguyen B.V., Cushing M., Sacks D.L., Udey M.C. Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur J Immunol. 2000;30:3498–3506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 164.Schild S., Nelson E.J., Bishop A.L., Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77:472–484. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vipond C., Sutherland J., Nordgren K., Kemp G., Heath A., Care R. Development and validation of a monocyte activation test for the control/safety testing of an OMV-based meningococcal B vaccine. Vaccine. 2019;37:3747–3753. doi: 10.1016/j.vaccine.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 167.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Webber J., Yeung V., Clayton A. Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell Dev Biol. 2015;40:27–34. doi: 10.1016/j.semcdb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 169.Hill B.S., Sarnella A., D'Avino G., Zannetti A. Recruitment of stromal cells into tumour microenvironment promote the metastatic spread of breast cancer. Semin Cancer Biol. 2020;60:202–213. doi: 10.1016/j.semcancer.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 170.Zhang W., Yu L., Ji T., Wang C. Tumor Microenvironment-responsive peptide-based supramolecular drug delivery system. Front Chem. 2020;8:549. doi: 10.3389/fchem.2020.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gesierich S., Berezovskiy I., Ryschich E., Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 172.Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 173.Sheldon H., Heikamp E., Turley H., Dragovic R., Thomas P., Oon C.E. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 174.Al-Nedawi K., Meehan B., Kerbel R.S., Allison A.C., Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Umezu T., Ohyashiki K., Kuroda M., Ohyashiki J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 176.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 177.Su S., Chen J., Yao H., Liu J., Yu S., Lao L. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856. doi: 10.1016/j.cell.2018.01.009. e16. [DOI] [PubMed] [Google Scholar]
- 178.Costa A., Kieffer Y., Scholer-Dahirel A., Pelon F., Bourachot B., Cardon M. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479. doi: 10.1016/j.ccell.2018.01.011. e10. [DOI] [PubMed] [Google Scholar]
- 179.Fang T., Lv H., Lv G., Li T., Wang C., Han Q. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho J.A., Park H., Lim E.H., Lee K.W. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 181.Atay S., Banskota S., Crow J., Sethi G., Rink L., Godwin A.K. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clayton A., Mitchell J.P., Court J., Linnane S., Mason M.D., Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 183.Clayton A., Mitchell J.P., Court J., Mason M.D., Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 184.Yang C., Kim S.H., Bianco N.R., Robbins P.D. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li X., Chen C., Wei L., Li Q., Niu X., Xu Y. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 2016;18:253–262. doi: 10.1016/j.jcyt.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 186.Gao L., Wang L., Wei Y., Krishnamurthy P., Walcott G.P., Menasche P. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay1318. [DOI] [PubMed] [Google Scholar]
- 187.Jia Y., Zhu Y., Qiu S., Xu J., Chai Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res Ther. 2019;10:12. doi: 10.1186/s13287-018-1115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhai M., Zhu Y., Yang M., Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv Sci. 2020;7:2001334. doi: 10.1002/advs.202001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sharma P., Mesci P., Carromeu C., McClatchy D.R., Schiapparelli L., Yates J.R., 3rd Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci U S A. 2019;116:16086–16094. doi: 10.1073/pnas.1902513116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao H., Yang L., Baddour J., Achreja A., Bernard V., Moss T. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5 doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lai R.C., Yeo R.W., Tan K.H., Lim S.K. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 193.Mackie A.R., Klyachko E., Thorne T., Schultz K.M., Millay M., Ito A. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lv Q., Deng J., Chen Y., Wang Y., Liu B., Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17:1723–1733. doi: 10.1021/acs.molpharmaceut.0c00177. [DOI] [PubMed] [Google Scholar]
- 195.Rivoltini L., Chiodoni C., Squarcina P., Tortoreto M., Villa A., Vergani B. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22:3499–3512. doi: 10.1158/1078-0432.CCR-15-2170. [DOI] [PubMed] [Google Scholar]
- 196.Pascucci L., Cocce V., Bonomi A., Ami D., Ceccarelli P., Ciusani E. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 197.Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R. Exosome delivered anticancer drugs across the blood−brain barrier for brain cancer therapy in Danio rerio. Pharm Res (N Y) 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jaiswal S., Jamieson C.H., Pang W.W., Park C.Y., Chao M.P., Majeti R. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kooijmans S.A.A., Stremersch S., Braeckmans K., de Smedt S.C., Hendrix A., Wood M.J.A. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 202.Clayton A., Turkes A., Dewitt S., Steadman R., Mason M.D., Hallett M.B. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 203.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 207.Faruqu F.N., Xu L., Al-Jamal K.T. Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp. 2018;142:e58814. doi: 10.3791/58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ma T., Chen Y., Chen Y., Meng Q., Sun J., Shao L. MicroRNA-132, Delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cell Int. 2018;2018:3290372. doi: 10.1155/2018/3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 210.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bellavia D., Raimondo S., Calabrese G., Forte S., Cristaldi M., Patinella A. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7:1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nolte-'t Hoen E.N., Buschow S.I., Anderton S.M., Stoorvogel W., Wauben M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 213.Saunderson S.C., Dunn A.C., Crocker P.R., McLellan A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao C., Busch D.J., Vershel C.P., Stachowiak J.C. Multifunctional transmembrane protein ligands for cell-specific targeting of plasma membrane-derived vesicles. Small. 2016;12:3837–3848. doi: 10.1002/smll.201600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang J., Zhang X., Chen X., Wang L., Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yu B., Kim H.W., Gong M., Wang J., Millard R.W., Wang Y. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhu X., Badawi M., Pomeroy S., Sutaria D.S., Xie Z., Baek A. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730. doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lai C.P., Mardini O., Ericsson M., Prabhakar S., Maguire C., Chen J.W. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sato Y.T., Umezaki K., Sawada S., Mukai S.A., Sasaki Y., Harada N. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Votteler J., Ogohara C., Yi S., Hsia Y., Nattermann U., Belnap D.M. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature. 2016;540:292–295. doi: 10.1038/nature20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yu L., Deng Z., Liu L., Zhang W., Wang C. Plant-derived nanovesicles: a novel form of nanomedicine. Front Bioeng Biotechnol. 2020;8:584391. doi: 10.3389/fbioe.2020.584391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang M., Viennois E., Xu C., Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4 doi: 10.1080/21688370.2015.1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cao M., Yan H., Han X., Weng L., Wei Q., Sun X. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li X., Liang Z., Du J., Wang Z., Mei S., Li Z. Herbal decoctosome is a novel form of medicine. Sci China Life Sci. 2019;62:333–348. doi: 10.1007/s11427-018-9508-0. [DOI] [PubMed] [Google Scholar]
- 227.Zhang M., Wang X., Han M.K., Collins J.F., Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huang F., Du J., Liang Z., Xu Z., Xu J., Zhao Y. Large-scale analysis of small RNAs derived from traditional Chinese herbs in human tissues. Sci China Life Sci. 2019;62:321–332. doi: 10.1007/s11427-018-9323-5. [DOI] [PubMed] [Google Scholar]
- 229.Spinler J.K., Karri V., Hirschi K.D. Planting the microbiome. Trends Microbiol. 2019;27:90–93. doi: 10.1016/j.tim.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 230.Yang C., Zhang M., Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6:1312–1321. doi: 10.1039/C7TB03207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang B., Zhuang X., Deng Z.B., Jiang H., Mu J., Wang Q. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Teng Y., Mu J., Hu X., Samykutty A., Zhuang X., Deng Z. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7:25683–25697. doi: 10.18632/oncotarget.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schulz E., Goes A., Garcia R., Panter F., Koch M., Muller R. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J Control Release. 2018;290:46–55. doi: 10.1016/j.jconrel.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 234.Chen L., Valentine J.L., Huang C.J., Endicott C.E., Moeller T.D., Rasmussen J.A. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc Natl Acad Sci U S A. 2016;113:E3609–E3618. doi: 10.1073/pnas.1518311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shehata M.M., Mostafa A., Teubner L., Mahmoud S.H., Kandeil A., Elshesheny R. Bacterial Outer membrane vesicles (OMVs)-based dual vaccine for influenza A H1N1 virus and MERS-CoV. Vaccines (Basel) 2019;7:46. doi: 10.3390/vaccines7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li Z., Clarke A.J., Beveridge T.J. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J Bacteriol. 1998;180:5478–5483. doi: 10.1128/jb.180.20.5478-5483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alaniz R.C., Deatherage B.L., Lara J.C., Cookson B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 238.Sanders H., Feavers I.M. Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines. Expert Rev Vaccines. 2011;10:323–334. doi: 10.1586/erv.11.10. [DOI] [PubMed] [Google Scholar]
- 239.Shen Y., Giardino Torchia M.L., Lawson G.W., Karp C.L., Ashwell J.D., Mazmanian S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 241.Ochoa-Reparaz J., Mielcarz D.W., Ditrio L.E., Burroughs A.R., Begum-Haque S., Dasgupta S. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 242.Chen D.J., Osterrieder N., Metzger S.M., Buckles E., Doody A.M., DeLisa M.P. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci U S A. 2010;107:3099–3104. doi: 10.1073/pnas.0805532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tan K., Li R., Huang X., Liu Q. Outer Membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front Microbiol. 2018;9:783. doi: 10.3389/fmicb.2018.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Carvalho A.L., Fonseca S., Miquel-Clopes A., Cross K., Kok K.S., Wegmann U. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J Extracell Vesicles. 2019;8:1632100. doi: 10.1080/20013078.2019.1632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Holst J., Martin D., Arnold R., Huergo C.C., Oster P., O'Hallahan J. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 246.Gorringe A.R., Pajon R. Bexsero: a multicomponent vaccine for prevention of meningococcal disease. Hum Vaccines Immunother. 2012;8:174–183. doi: 10.4161/hv.18500. [DOI] [PubMed] [Google Scholar]
- 247.Sandbu S., Feiring B., Oster P., Helland O.S., Bakke H.S., Naess L.M. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin Vaccine Immunol. 2007;14:1062–1069. doi: 10.1128/CVI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Matthias K.A., Reveille A., Connolly K.L., Jerse A.E., Gao Y.S., Bash M.C. Deletion of major porins from meningococcal outer membrane vesicle vaccines enhances reactivity against heterologous serogroup B Neisseria meningitidis strains. Vaccine. 2020;38:2396–2405. doi: 10.1016/j.vaccine.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bartolini E., Ianni E., Frigimelica E., Petracca R., Galli G., Berlanda Scorza F. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J Extracell Vesicles. 2013;2:20181. doi: 10.3402/jev.v2i0.20181. [DOI] [PMC free article] [PubMed] [Google Scholar]