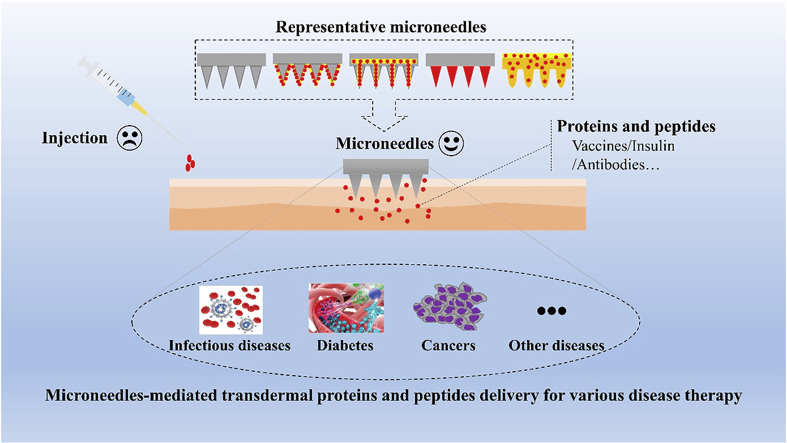
Abstract
Proteins and peptides have become a significant therapeutic modality for various diseases because of their high potency and specificity. However, the inherent properties of these drugs, such as large molecular weight, poor stability, and conformational flexibility, make them difficult to be formulated and delivered. Injection is the primary route for clinical administration of protein and peptide drugs, which usually leads to poor patient's compliance. As a portable, minimally invasive device, microneedles (MNs) can overcome the skin barrier and generate reversible microchannels for effective macromolecule permeation. In this review, we highlighted the recent advances in MNs-mediated transdermal delivery of protein and peptide drugs. Emphasis was given to the latest development in representative MNs design and fabrication. We also summarize the current application status of MNs-mediated transdermal protein and peptide delivery, especially in the field of infectious disease, diabetes, cancer, and other disease therapy. Finally, the current status of clinical translation and a perspective on future development are also provided.
Keywords: Microneedles, Transdermal drug delivery, Proteins, Peptides, Infectious diseases, Diabetes, Cancer, Clinic
Graphical abstract
Proteins and peptides have become a significant therapeutic modality for various diseases, and microneedles provide a great prospect for the transdermal delivery of proteins and peptides.
1. Introduction
Proteins and peptides exhibit the most prominent effects in human body, such as molecular transportation, biological scaffold, cellular regulation, and enzymatic catalysis, which have played an important role in almost every medical field1, 2, 3, 4. Insulin was the first therapeutic protein approved in 1982 and since then remarkable progress has been achieved in the clinical application of numerous protein and peptide therapeutics5,6. However, the application of protein and peptide drugs is commonly restrained by certain limitations. The large molecular weight of these drugs substantially decreases their permeability capacity across biological barriers such as skin and mucous membranes. Besides, loss of biological activity in response to external conditions (moisture and temperature) and endogenous proteolytic enzyme put a high difficulty on formulation and delivery technologies7.
Currently, injection is the primary route for clinical administration of protein and peptide drugs. Intravenous, subcutaneous, and intramuscular injection are the most widely used ways for delivering protein and peptide drugs2,8, 9, 10, 11, 12. Regardless of the injection method, most protein and peptide drugs are easily degraded by various metabolic enzymes in the body, resulting in short half-life in vivo, which means frequent injections are required. Furthermore, injection therapy is inconvenient and unfriendly, especially for patients with chronic diseases such as rheumatoid arthritis and diabetes. Injection safety should also be considered, since contamination of needles during administration can lead to transmission of some infectious diseases such as Hepatitis B and C. Therefore, for the delivery of protein and peptide drugs, there is a great need for an alternative drug delivery system that can be readily administrated with improved therapeutic efficacy, good patient compliance, and safety.
Transdermal drug delivery is a choice that delivers biologically active agents through skin portals for local or systemic effects, which is noninvasive and can be self-administered13. There are some requirements for the drugs suitable for transdermal administration, such as a maximum molecular weight of 1000 Da, and a balance between hydrophobicity and polarity due to the stratum corneum barrier14. Most protein and peptide drugs are hydrophilic and macromolecular in nature, and therefore they cannot easily penetrate into the skin. Over the past a few decades, various chemical and physical methods such as penetration enhancers15, microjet16, laser17, electroporation18, sonophoresis19, and iontophoresis20 have been developed as feasible strategies to improve transdermal drug permeation. But these techniques are usually expensive and cumbersome to use, and still exhibit limited efficiency for successful transdermal delivery of macromolecular drugs.
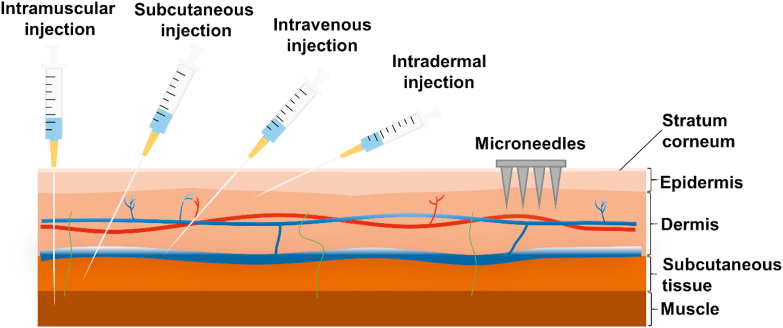
Recently, microneedles (MNs) have become a new type of drug delivery technique, and the applications of MNs have been extended to various aspects, including small chemical molecules21,22, vaccines23,24, genes25, proteins4,26, and nanoparticles27. Particularly, MNs provide a great prospect for the transdermal delivery of proteins and peptides28,29. MNs are minimally invasive device with needles (<1 mm) arranged orderly on the base. They can directly penetrate the stratum corneum by generating reversible microchannels in the skin. These microchannels can grant access of drugs to the dermal microcirculation located in the interior layers of the skin (Fig. 1). Compared with injection, MNs will not contact with blood vessels and nerves in the deep dermis, which provide better patient compliance and favorable safety profile. Moreover, the mild fabrication condition of MNs will not impact the biological activity of proteins and peptides.
Figure 1.
Schematic illustration of protein and peptide drug delivery by conventional injections and microneedles.
This review provides comprehensive updates on MNs-mediated transdermal delivery of protein and peptide drugs. Emphasis was given to the latest development and advance in representative MNs design and fabrication. Additionally, we summarized the recent studies about the applications of MNs-mediated protein and peptide delivery, particularly focusing in the field of infectious disease, diabetes, cancer, and other disease therapy. Finally, the current status of clinical translation and a perspective on future development were also provided.
2. Representative types of MNs
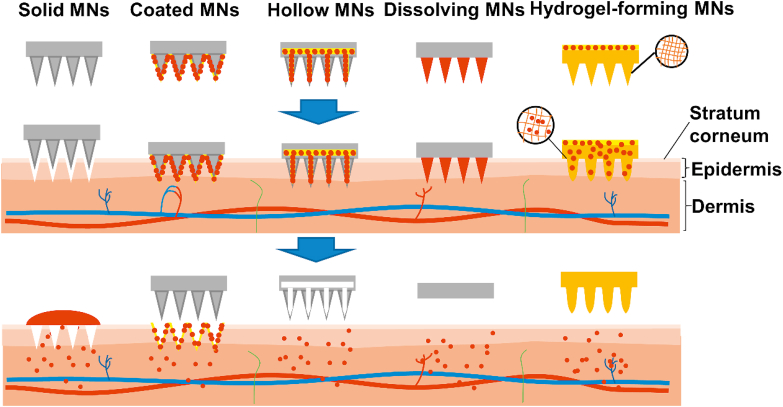
Gerstel et al. proposed the concept of MNs in 1971, and Henry et al.30 firstly reported the utilization of MNs for transdermal drug delivery in vivo in 199830,31. Since then, various types of MNs have been successfully developed32. Based on different drug delivery strategies, MNs can be generally classified into five categories, including solid MNs, coated MNs, hollow MNs, dissolving MNs, and hydrogel-forming MNs (Fig. 2). Each type of MNs has been extensively studied for transdermal drug delivery. However, the protein and peptide drugs are usually sensitive to high temperature, pH value, and organic solvents compared with inert small molecules33. To avoid the damage of their biological activity, it is necessary to understand the properties of each type of MNs, and then select reasonable MNs types to formulate them. In this section, the typical applications associated with different MNs-mediated delivery approaches are described in detail.
Figure 2.
Representative types of MNs for transdermal drug delivery.
2.1. Solid MNs
Solid MNs usually need a two-step operation for drug delivery. Briefly, solid MNs are first inserted into the skin and subsequently removed to form temporary microchannels. Then, a suitable pharmaceutical dosage form (such as gel, cream, or ointment) is applied to the previously formed microchannels23,34.
Solid MNs should offer sufficient mechanical strength for successful skin pretreatment by selecting the materials of MNs23. Typically, solid MNs are fabricated from silicon35 and metal36,37. It is worth noting that silicon and metal have good properties for solid MNs fabrication, but they may be unsuitable for transdermal drug delivery. Their non-biodegradable nature may cause safety issues after being inserted into the skin. In contrast, polymeric materials usually have good biocompatibility. Various polymeric materials, such as polylactic acid (PLA), polymethylmethacrylate, polycarbonate, and carboxymethylcellulose (CMC) have been developed to prepare solid MNs as an alternative to non-biodegradable metal or silicon38, 39, 40.
Solid MNs deliver drugs by passive diffusion through the generated microchannels in the skin. Therefore, the length and density design of solid MNs used for skin pretreatment will affect drug penetration41,42. Moreover, the properties of the drugs also affect delivery efficiency. Contrary to the traditional transdermal delivery, the microchannels formed by the pretreatment of solid MNs will increase the penetration of hydrophilic compounds43. McAllister et al.44 demonstrated that the permeation of bovine serum albumin (BSA) and insulin was increased after skin pretreatment using solid silicon MNs. The molecular weight of drugs can also affect passive transport by using solid MNs45,46. Verbaan et al.46 observed that the transport rate of the larger molecular weight (72 kDa) compound was much lower than the compounds with molecular weight of 10 kDa and 538 Da.
Solid MNs have some inherent drawbacks. A two-step administration process including pretreatment with MNs array and then application of pharmaceutical preparations is considered inconvenient, and it may cause imprecise dosage47. Due to the negative impact on patient compliance, drug delivery strategies based on other MNs have now become more prevalent.
2.2. Coated MNs
To avoid a two-step application process, solid MNs are coated with drugs on the surface of the needles to obtain coated MNs. Coated MNs provide a more convenient and controllable way for transdermal drug delivery. When coated MNs are inserted, the drug coating layer will dissolve and further deposit the active pharmaceutical ingredients into the skin, then the MNs can be removed48.
Coated MNs are typically prepared from metal or silicon. To avoid the use of less biocompatible materials, polymeric coated MNs have also been widely investigated. The solid microstructure transdermal system (sMTS) is prepared by a strong polymer, which can retain its structural integrity upon insertion into the skin49, 50, 51. Kapoor et al.51 developed the coated sMTS for Peptide A delivery. Two hundred and fifty micrograms of Peptide A were coated on a patch containing 316 needles. The successful transdermal delivery was achieved with the bioavailability being similar to the subcutaneous injection. Besides, the stability of peptide A was significantly improved when coated on the sMTS51.
Several techniques, such as spray coating, dip coating, and piezoelectric inkjet printing, were applied for the coating of MNs52. The spray coating and dip coating are the most common methods using an aqueous drug solution with high viscosity to retain more drugs on MNs surface. The main challenge is how to ensure sufficient therapeutic agents are uniformly coated. Therefore, it is important to optimize the coating process and formulation composition. Surfactants, viscosity enhancers, and peptide stabilizers are usually required in the formulations, to ensure coating stability and uniformity of the drugs53. Since most biomolecules are hydrophilic, the coating solution is usually aqueous. Zhao et al.54 developed a coating formulation, which contained ternary co-solvents and polyvinyl alcohol 2000, for both hydrophilic and hydrophobic peptide loading with maintained bioactivity. Other methods such as layer-by-layer technique are also effective in MNs coating. In this approach, drug molecules can be coated onto MNs by alternately dipping into two solutions containing oppositely charged solutes to form a polyelectrolyte multilayers55.
Although the mechanical strength of coated MNs is usually retained, their tip sharpness is reduced with the drug loading, which may influence the skin penetration ability56. Therefore, the drug loading amount of coated MNs is compromised, which indicates that proteins and peptides with high potency are suitable for this strategy, such as desmopressin56, human growth hormone57, and interferon alpha58.
2.3. Hollow MNs
Hollow MNs are sub-millimeter devices acted like micron-scale syringes, which can penetrate the stratum corneum to allow the flow of liquid formulation into the epidermis or dermis59. In the simplest form, drug delivery using hollow MNs is achieved through passive diffusion. Since the passive diffusion rate in dense tissues is relatively low, faster transport rate through pressure-driven flow or diffusion has been successfully achieved21,47. Consequently, compared with solid MNs, hollow MNs can allow the administration of larger doses, and simultaneously provide an exact transport rate21,60, 61, 62.
The digitally controlled hollow MNs injection system (DC-hMN-iSystem) can provide accurate amount of therapeutic vaccine. Immunization study in mice showed that HPV peptide vaccine delivered through the DC-hMN-iSystem induced powerful cytotoxic and T helper response63. Hollow MNs-mediated intradermal delivery of nanoparticles is also an effective strategy to improve the effectiveness of vaccine. Antigen-loaded poly(d,l-lactide-glycolide) nanoparticles delivered via hollow MNs elicited a remarkably higher antibody response and more lymphocytes than intramuscular injection and soluble antigen delivered via hollow MNs64.
Hollow MNs usually need a more complicated fabrication technology. In addition to preparing a needle with suitable inner holes, hollow MNs should also be combined with some form of drug reservoir. Hollow MNs are usually prepared from metal or silicon with different inner hole diameters, which are inherently weaker than solid MNs and have a greater risk of breakage65.
2.4. Dissolving MNs
Dissolving MNs are usually prepared from dissolvable materials with therapeutic agents incorporated into the needles, which can effectively deliver drugs into the skin by the dissolution of needle matrix66, 67, 68. Many materials have been used to prepare dissolving MNs, from low molecular weight carbohydrates to high molecular biodegradable polymers, including dextran, CMC sodium, hyaluronic acid (HA), chondroitin sulfate, polyvinylpyrrolidone (PVP), and polyvinylalcohol (PVA). The use of dissolving MNs is also a one-step administration that is pretty compliant for patients. Dissolving MNs have the unique advantages that they leave no harmful material and do not generate biohazardous sharp waste after application69, 70, 71. In addition, the mild preparation condition of dissolving MNs makes industrialization easier to achieve, which is quite beneficial to protein and peptide drugs. The solid state of the encapsulated biomolecules can also protect them from cold chain storage and transport72.
Various methods such as micromolding73, drawing lithography74, droplet-borne air blowing75, electro-drawing76, and photolithography77 have been developed for fabricating dissolving MNs. Micromolding method is most widely adopted. Briefly, micromolds are filled by polymer melt or solvent casting, sometimes with the additional use of vacuum and/or centrifugal force. Then the molds are allowed to solidify or in situ polymerize of liquid in the microcavities23. It should be noted that the abovementioned methods are usually only suitable for the small-scale preparation of MNs in academic field. For scale-up fabrication, several novel techniques have been designed to manufacture dissolving MNs in a highly effective, controllable, and scalable way78. The double-penetration female mold-based positive-pressure microperfusion technique was also developed by our group79 for scale-up fabrication of dissolving MNs79.
Heat-sensitive proteins and peptides should be encapsulated in micromolds and solidified at mild conditions that will not destroy their activity. Park et al.80 fabricated poly-lactide-co-glycolide (PLGA) MNs using the micromolding method to encapsulate microparticles containing BSA and calcein. They proved the feasibility of the controlled release of calcein and BSA using polymeric MNs80. However, due to the use of elevated temperature in processing, protein activity had a slight loss. To address this issue, Lee et al.69 employed milder preparation condition to fabricate dissolving MNs from ultra-low viscosity CMC with the full enzymatic activity. Similarly, erythropoietin loaded dissolving MNs were prepared using a thread-forming polymer as a base at room temperature81.
Although dissolving MNs have significant advantages in transdermal drug delivery, it is hard to control the amount and localization of drugs within needles due to the drug diffusion from needles to base during the micromolding process, which may lead to imprecise dose and limited drug delivery efficiency82. To deal with this issue, Prausnitz's group83,84 concentrated drugs in tips by incorporating an air bubble at the base of the MNs, which effectively prevented drug diffusion. The multilayered dissolving MNs are also useful to achieve controlled drug delivery85, 86, 87. Li et al.88 developed a multilayered MNs patch containing an effervescent backing to facilitate rapid separation. Our group85 also developed a rapidly separating dissolving MNs to realize precise drug delivery as well as rapid separation property. In this approach, the drugs were concentrated in the needle tip, while the blank separating part allowed rapid separation within 30 s in mimic skin85.
The materials used as matrix for dissolving MNs should be concerned, which may affect the preparation process and the efficacy of the drug. Moreover, it should be noted that long-term use of dissolving MNs may lead to safety problems of polymer accumulation in the skin89.
2.5. Hydrogel-forming MNs
Hydrogel-forming MNs are usually fabricated from crosslinked polymeric materials, which can pierce the stratum corneum and absorb interstitial fluid to cause the polymeric matrix swell. The drug diffusion through the swollen matrix allows for the delivery to the dermal tissue. Hydrogel-forming MNs can be removed from the skin, leaving almost no polymeric residue behind22. Besides, the hydrogel-forming MNs also involve a one-step application, and its drug diffusion will not be blocked by compressed skin tissue like hollow MNs22.
Hydrogel-forming MNs usually does not contain the drug, and instead, drugs are loaded into a matching reservoir, such as a polymeric film90. Therefore, it is not limited by the amount of drug that can be loaded into the needle or needle surface, which significantly increases the drug amount that can permeate into the skin. Recently, other forms of hydrogel-forming MNs have also appeared, in which the drug has not been loaded separately from the needles73,91. Novel in situ hydrogel-forming MNs were also developed using biocompatible thermosensitive copolymer. Sivaraman et al.92 utilized the transition property of poloxamer from solution at room temperature to gel at skin temperature (32 °C) to prepare in situ hydrogel-forming MNs. No matter where the drug is located, the swelling degree of the hydrogel matrix plays a key role in drug delivery, and altering the crosslink density of the matrix can control release rate93. Hydrogel-forming MNs can also be used for diagnostic purpose through the analysis of interstitial fluid absorbed by the MNs upon insertion into the skin94.
Hydrogel-forming MNs are fabricated by swellable materials formed by chemically or physically cross-linking polymers95, such as crosslinked poly (methylvinylether/maleic acid) (PMVE/MA)-poly(ethylene glycol) (PEG) 10,00096, and PVA-dextran73. Hydrogel-forming MNs can be regarded as a subtype of polymeric MNs where the polymers display physicochemical properties of the hydrogel97. Typically, micromolding method is widely employed to prepare hydrogel-forming MNs. According to the research conducted by Donnelly et al.96, an aqueous blend containing PMVE/MA and PEG10,000 was used to produce hydrogel-forming MNs by using silicone micromold. The adhesive drug reservoir patch was prepared in advance and then attached to the needles with moderate pressure, thereby forming an integrated hydrogel MNs system. This system successfully delivered various drugs with different molecular weights, including large molecular weight proteins and peptides (insulin and BSA)96. Yang et al.73 designed a phase-transition MNs system which enabled highly efficient transdermal delivery of insulin by utilizing polyvinyl alcohol as the microneedle material via microcrystalline cross-linking strategy. Lutton et al.98 also designed a scalable manufacturing process for hydrogel-forming MNs, which was conducted at ambient condition utilizing a combination of injection moulding and roller casting.
Since the hydrogel-forming MNs are commonly fabricated from polymeric materials, it should be noted that their mechanical strength and physical stability are possible concerns during the application and storage process.
3. Application of MNs-mediated protein and peptide delivery
Proteins and peptides have become significant therapeutic modalities for various diseases, which continue to enter the market at a steady pace99, 100, 101. This can be attributed to their target specificity, high potency, and favorable safety compared with traditional small-molecule drugs. As a minimally invasive device, MNs can improve the patient's compliance and offer a multifunctional platform to overcome the skin barrier for hydrophilic and macromolecular drugs32. Moreover, the mild fabrication condition and solid state nature are a major advantage of MNs compared to traditional injection of the aqueous solution, which can improve drug stability and reduce the use of cold chain80.
With the progress of material science and microfabrication technology, many MNs-mediated protein and peptide delivery strategies have been developed. Typically, MNs have been utilized to deliver various forms of cargoes, from native drugs to the nanoparticle or microparticle-based formulations27. In this section, we summarized the recent advances in MNs-mediated protein and peptide delivery, especially focused on their application for infectious disease therapy, diabetes therapy, and cancer therapy.
3.1. Infectious disease therapy
Infectious diseases such as influenza, measles, and hepatitis B are one of the main causes of human deaths, which is a major public health concern worldwide. Vaccination has been recognized as the most successful, and cost-effective public health intervention strategy to combat infectious diseases47,102. Compared with other antigen molecules, only proteins can induce both cellular and humoral immunity103. In addition, the versatility and customizability of proteins make protein-based vaccines one of the most effective strategies for artificially immunity induction103.
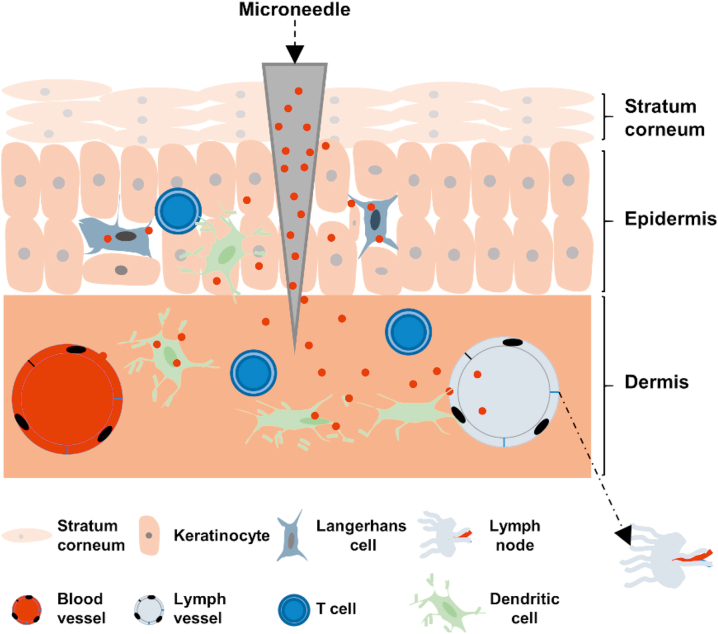
Most vaccines are administered by subcutaneous or intramuscular injection, which is relatively painful, resulting in poor patient compliance104. There are a large number of antigen presenting cell populations in the skin, such as macrophages, dermal dendritic cells (DCs), and Langerhans cells, making the skin a unique target for immunomodulation59,105, 106, 107. MNs are easy to use with minimal pain, which provide a promising platform for transcutaneous immunization with improved efficacy108, 109, 110 (Fig. 3). Over the past few decades, MNs have been developed successfully as an experimental delivery system for various protein and peptide vaccines (Table 1).
Figure 3.
Mechanisms of MNs-mediated transdermal immunomodulation.
Table 1.
MNs-mediated transdermal delivery of proteins and peptides for prophylaxis of infectious diseases.
| Disease | Protein/peptide drug | MNs type | MNs material | Ref. |
|---|---|---|---|---|
| Model | OVA | Hollow MNs | Silica | 111,112 |
| OVA | Coated MNs | Titanium | 37,113 | |
| OVA | Coated MNs | PLLA | 114 | |
| OVA | Coated MNs | PLGA | 115 | |
| OVA | Coated and hydrogel-forming MNs | Zein | 116 | |
| OVA | Dissolving MNs | PMVE/MA | 117,118 | |
| OVA and platycodin | Dissolving MNs | HA | 119 | |
| OVA and Poly(I:C) | Dissolving MNs | CMC, trehalose | 120,121 | |
| OVA and Poly(I:C) | Dissolving MNs | PLGA and poly(acrylic acid) | 122 | |
| OVA and Poly(I:C) | Dissolving MNs | Silk and poly(acrylic acid) | 123 | |
| OVA | Dissolving and hydrogel-forming MNs | PMVE/MA, PEG, sodium carbonate/Gantrez S-97 | 124 | |
| BSA and recombinant protective antigen | MicroCor™ (dissolving MNs) | PVA, trehalose, maltitol, HP-β-CD | 125 | |
| Influenza | Inactivated influenza virus proteins | Coated MNs | Stainless steel | 126 |
| Adenoviral serotype | Coated MNs | PLA | 127 | |
| Envelope protein Domain III subunit antigen | Coated MNs | Poly(l-lactic acid) | 128 | |
| Virus vaccine antigens | Dissolving MNs | PVP | 72 | |
| Virus vaccine antigens | Dissolving MNs | Trehalose and sodium CMC | 129 | |
| Influenza antigens | Dissolving MNs | Trehalose/sucrose, sucrose/arginine, and arginine/heptagluconate | 130 | |
| Hemagglutinin | Dissolving MNs | CMC sodium, ammonium acetate buffer, PVA, sucrose | 131 | |
| 4M2e-tFliC fusion protein | Dissolving MNs | CMC sodium, arginine/heptagluconate, sucrose | 132 | |
| Influenza subunit vaccine and GM-CSF | Dissolving MNs | PVA, BSA, CMC, trehalose | 133 | |
| HIV | Recombinant HIV-1 CN54gp140 | Dissolving MNs | Gantrez® AN-139 | 134 |
| Trimer immunogen and adjuvant | Dissolving MNs | Poly(acrylic acid) and silk | 135 | |
| Pneumonia | Recombinant protein subunit | Dissolving MNs | CMC | 136 |
| Diarrhea | Rotavirus vaccine | Coated MNs | Stainless steel | 137 |
| Hepatitis B | Surface antigen | Coated MNs | Titanium | 138 |
| Plague | F1 antigen | Microchannel Skin System | Plastic | 139 |
| Tuberculosis | Protein derivative | Dissolving MNs | HA | 140 |
| Measles | 1000 TCID50 | Dissolving MNs | Sucrose, threonine, and CMC | 141 |
| Leishmaniasis | Recombinant protein LiHyp1 | Dissolving MNs | Sugar | 142 |
The MNs-mediated transcutaneous vaccination can effectively present antigens to skin-resident immunocyte which often enables lower dose and stronger topical immunization143,144. Matriano et al.37 compared different routes of OVA (model antigen) administration, and when the protein antigen was delivered, the immune response was most efficient by using coated MNs and intradermal administration as compared to subcutaneous or intramuscular administration. Similarly, the titers of IgG in mice that received 0.5 μg of antigen with MNs were comparable or higher than those received 5 μg of antigen by intramuscular administration137. Dissolving MNs for influenza vaccine delivery could also improve the efficiency of virus clearance and enhance cellular recall response, compared with conventional intramuscular injection72,129.
The key parameter of protein and peptide vaccine formulation is to maintain the stability of the vaccine component, which is crucial during the fabrication, transportation, and storage process. Appropriate formulation techniques using MNs can retain the long-term antigen immunogenicity and allow flexible storage conditions145,146. DeMuth et al.127 found that the sucrose-coated MNs effectively delivered adenovirus into the skin and allowed storage at room temperature for several months without losing the biological activity of adenovirus vectors. Mistilis et al.130 screened different dissolving MNs formulation combinations to stabilize a trivalent subunit influenza vaccine. After being stored at 25 °C for 24 months, dissolving MNs formulated by combinations of arginine/heptagluconate, sucrose/arginine, and trehalose/sucrose still retained the vaccine immunogenicity. The mice immunization experiment also proved that the antibody titer was equivalent to the fresh liquid vaccine provided by intradermal injection130.
Many available vaccines are formulated with adjuvant112,119,121, 122, 123. Balmert et al.121 used dissolving MNs to deliver OVA and Poly(I:C) adjuvant. Although the addition of Poly(I:C) showed little effect on the IgG1 response, it promoted a moderate increase in IgG2c response. Specifically, many MNs polymeric matrix materials can also be adapted as adjuvants to enhance the immune response ascribed to their intrinsic immunogenicity. For example, poly[di(carboxylatophenoxy)phosphazene] can serve as both vaccine adjuvant and fabrication material. When used in coated MNs for antigen delivery, it exhibited superior activity in pigs and significant antigen sparing potential compared to intramuscular administration138. It can be predicted that this will further promote the application of polymeric MNs in immunity.
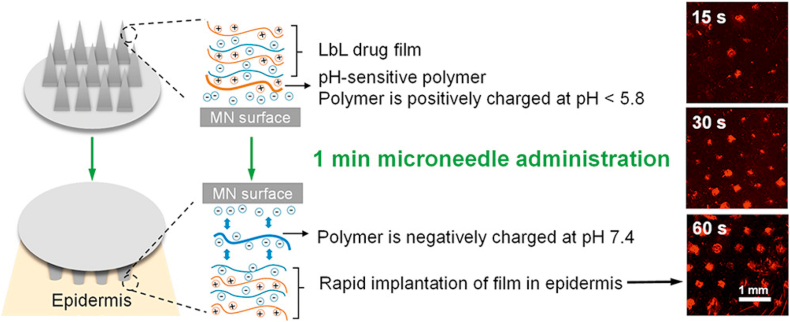
OVA, a model protein with unique lymph node-targeting ability, is commonly used to assess the performance of MNs for immunization37,111, 112, 113, 114, 115, 116, 117, 118, 119,121,124. Zaric et al.118 encapsulated OVA into PLGA nanoparticles which were then delivered to the skin by the dissolving MNs. Skin-derived DCs could deliver nanoparticles to skin draining lymph nodes through afferent lymphatic vessels, thereby inducing a potent antigen-specific immune response. Besides, PLGA nanoencapsulation maintained the stability of antigen in the dissolving MNs which further facilitated antigen retention into the skin118. He et al.114 prepared a layer-by-layer coated MNs based on a synthetic pH-induced charge-invertible polymer to shorten the implantation time, which only required 60 s to implant layer by layer films in vivo during the insertion process (Fig. 4). The coated MNs triggered a strong immune response, and the serum OVA-specific IgG1 levels of the coated MNs group were 160 times and 9 times higher than that of the subcutaneous and intramuscular injection groups, respectively114.
Figure 4.
The implantation of layer-by-layer drug films using coated MNs for enhanced transdermal vaccination. Reprinted with permission from Ref. 114. Copyright © 2018, American Chemical Society.
With the rapid development of nanotechnology, recently the MNs have been employed to efficiently deliver macromolecules along with nanoparticle-based therapies. The advantages of both nanoparticles and MNs can be leveraged to improve the transdermal delivery efficiency of proteins and peptides. Du et al.112 compared intradermal delivery efficiency of four nanoparticulate vaccines using hollow MNs. Although both nanoparticles and solution aroused strong total IgG and IgG1 responses, the nanoparticles significantly increased the IgG2a response112.
MNs-mediated transdermal immunomodulation has been mostly studied for influenza72,126, 127, 128, 129, 130, 131, 132, 133. Zhu et al.126 coated virus protein on stainless MNs and then used them to immunize mice. Four weeks after immunization, all mice immunized with virus-coated MNs survived as well as intramuscular injection, while the mice in the control group died on Day 5–8 after challenge126. Littauer et al.133 demonstrated that incorporation of the thermolabile granulocyte-macrophage colony stimulating factor into the H1N1 vaccine-loaded dissolving MNs could result in improved vaccine-induced immunity, which provides a pipeline to other active recombinant molecules as adjuvants for maximized vaccination efficacy to combat with influenza. MNs-mediated transdermal immunomodulation has also been widely investigated to combat other infectious diseases, such as HIV134,135, diarrhea137, hepatitis B138, plague139, tuberculosis140, measles141, and leishmaniasis142, with representative examples listed in Table 1. Especially, the recent prevalent COVID-19 coronaviruses have caused a serious threat to public health. Coronaviruses-S1 subunit vaccines are a promising immunization modality against coronaviruses infection. Kim et al.136,147 incorporated the protein into carboxymethyl cellulose to fabricate the dissolving MNs at room temperature. All dissolving MNs vaccines elicited higher levels of neutralizing antibody, even beyond those induced by subcutaneous injection of monophosphoryl lipid A adjuvanted vaccine.
Although its efficacy and safety need further research, transdermal delivery of proteins and peptides based on MNs represents a promising strategy for combating various infectious diseases. In particular, for vaccines that require multiple administrations, transdermal MNs vaccination provides a much more convenient option.
3.2. Diabetes therapy
Diabetes is a chronic disease of glucose metabolism disorder characterized by abnormal accumulation of glucose in the blood148. Diabetes is commonly induced by the reduced insulin secretion (type 1) or the defective responsiveness of the body to insulin (type 2)149,150. Exogenous insulin administration is indispensable for the treatment of diabetes151,152. Insulin, a 51-amino-acid peptide, is one of the hormones for modulating blood glucose level. However, the great pain caused by frequent and repeated subcutaneous injections adversely affects compliance with treatment153. In contrast, transdermal delivery of insulin is an attractive delivery method105,154. Introducing MNs into insulin delivery will benefit a large number of diabetic patients because it is minimal pain and easy to administer26,155,156.
The solid MNs fabricated by different materials, such as silicon157, metal158, and polymer44, have successfully reduced the blood glucose level by improving the insulin permeability through skin pretreatment. Zhou et al.158 used stainless steel MNs with different needle lengths to evaluate the delivery efficacy of insulin to diabetic rats. The results showed that the skin's permeability to insulin increased, and blood glucose levels decreased rapidly within 1 h158. Besides, the integration of solid MNs with other techniques such as iontophoresis can further enhance the transdermal delivery efficiency of insulin159,160.
Hollow MNs-mediated intradermal insulin delivery results in faster insulin onset, which can be driven by passive diffusion161, pressure44, or electricity162. McAllister et al.44 found that hollow MNs allowed microliter of solutions to enter the skin, and a larger pressure triggered a faster decrease in blood glucose levels. Roxhed et al.162 designed a patch system based on MNs with an electronically controlled liquid dispenser. The plasma insulin concentration of the electrically driven active administration was about 5 times higher than that of the passive diffusion group at 3 h post dosing162.
Insulin delivery using drug-free MNs (solid MNs, hollow MNs) generally requires two or more steps, which is inconvenient for patients. The drug-loaded MNs (coated MNs, dissolving MNs, hydrogel-forming MNs) can overcome these issues26. Ross et al.163 developed insulin polymeric layers coated metal MNs. The thin and homogeneous layers could retain insulin intact, and rapid insulin release was realized within 20 min, indicating that solid-state insulin delivery by coated MNs is feasible. However, further studies about insulin coated MNs are limited, which probably due to the insufficient dose of coated insulin.
Dissolving MNs encapsulated insulin in the MNs matrix are more promising due to their favorable biocompatibility, relatively simple manufacturing method, and low cost22. Since insulin is heat-sensitive, it is important to incorporate insulin in dissolving MNs at mild temperature. Various water-soluble polymers such as HA164, chondroitin sulfate12, poly-gamma-glutamic acid165, and a mixture of starch and gelatin166 had been employed to prepare insulin loaded dissolving MNs at room temperature by using micromold casting method. Liu et al.164 evaluated the ability of dissolving MNs prepared by HA to deliver insulin to diabetic rats in vivo. The results showed that insulin administered through dissolving MNs could effectively enter the systemic circulation, and the hypoglycemic effect was almost similar to subcutaneous injection164.
Conventional diabetes treatment based on subcutaneous injection is usually associated with poor blood glucose control. The closed-loop drug delivery strategy can delicately control the insulin release profile in response to fluctuations in blood glucose levels, which shows great promise in the diabetes treatment. Hence, glucose-responsive MNs have been developed based on the glucose-sensing elements, such as glucose oxidase (GOx)167, 168, 169, 170, 171, 172, 173 and phenylboronic acid174,175. Yu et al.174 designed an MNs patch loaded with insulin by using a non-degradable glucose-responsive polymer. Under the hyperglycaemic condition, the polymeric matrix swelled and weakened the electrostatic interaction between the negatively charged polymers and insulin, thereby promoting the release of insulin. When exposed to euglycemic condition, the inhibited volume change and the restoration of electrostatic interaction slowed down the insulin release rate174.
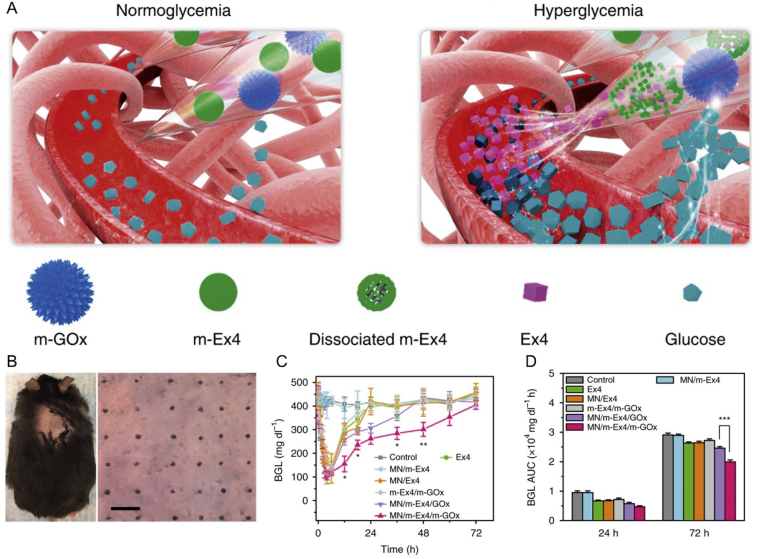
Another potential approach to combat diabetes is using glucagon-like peptide-1 receptor agonists173,176,177. Chen et al.173 constructed a smart exendin-4 (Ex4), a synthetic 39-amino acid peptide, delivery platform based on MNs incorporated with dual mineralized microparticles separately containing GOx and exendin-4 (Fig. 5). The closed-loop MNs system showed excellent glucose regulation ability by the rapid specific response to hyperglycemia state, thereby significantly improving the therapeutic performance of exendin-4173.
Figure 5.
The MNs patch incorporated with dual mineralized microparticles for diabetes therapy. (A) Schematic of glucose-responsive Ex4 delivery mediated by MNs patch. (B) Photograph of the mouse after inserted by an MNs patch. Scale bar, 500 μm. (C) Long-term blood glucose level of mice after different treatments (mean ± SD, n = 3). (D) The area under the curve of blood glucose level (mean ± SD, n = 3). Reprinted with permission from Ref. 173. Copyright © 2017, Springer Nature.
3.3. Cancer therapy
Cancer is the main concern of public health due to the widespread prevalence, high morbidity and mortality178. Apart from surgery, radiotherapy, and chemotherapy, immunotherapy has become an effective strategy for cancer treatment. Instead of directly killing the tumor cells, immunotherapeutic drugs are utilized to activate the body's immune system to attack the cancer cells, many of which are evaded when cancer occurs179. Therefore, immunotherapy is considered a promising strategy to treat or even cure certain types of cancer. The number of approved immunotherapeutic drugs has been increasing, and there are many treatments in preclinical and clinical stages. Generally, immunotherapeutic agents are mainly divided into five categories: cancer vaccines, checkpoint inhibitors, engineered T cells, lymphocyte-promoting cytokines, agonistic antibodies against co-stimulatory receptors180, many of which are composed of proteins and peptides. In the preclinical studies, many MNs-mediated transdermal deliveries of proteins and peptides have shown promising efficacy in cancer immunotherapy (Table 2).
Table 2.
MNs-mediated transdermal delivery of proteins and peptides for cancer immunotherapy.
| Therapy | Protein/peptide drug | MNs type | MNs material | Ref. |
|---|---|---|---|---|
| Cancer vaccine | OVA | Dissolving MNs | PMVE/MA | 118 |
| OVA and resiquimod (R848) | Dissolving MNs | Pluronic F127/PEG | 181 | |
| Human melanoma antigens (Trp2) and adjuvant (CpG) | Coated MNs | PLLA | 182 | |
| Microparticulate ovarian cancer vaccine | AdminPen™ (Hollow MNs) | Stainless steel | 183 | |
| Whole cell lysate of B16F10 cancer cells, GM-CSF | Dissolving MNs | HA | 184 | |
| Murine breast cancer whole cell lysate | Solid MNs | Metal | 185 | |
| S-91 melanoma cancer cells vaccine antigen | Solid MNs | Dermaroller | 186 | |
| HPV E743–63 synthetic long peptide | Hollow MNs | Silica capillaries | 63 | |
| Gene therapy | Octaarginine/BRAF siRNA | Coated MNs | Stainless steel | 187 |
| Plasmid OVA and poly(I:C) | Dissolving MNs | A cationic polypeptide and PEG | 188 | |
| Checkpoint inhibitors | aPD-1 | Dissolving MNs | HA | 189 |
| aPD-1 | Hollow MNs | PVP/PVA | 190 | |
| aCTLA-4 | Dissolving MNs | PVP | 191 | |
| aPD-1 and 1-methyl-d,l-tryptophan | Dissolving MNs | HA | 192 | |
| aCTLA-4 and zinc phthalocyanine | Dissolving MNs | HA | 193 | |
| 1-Methyl-d,l-tryptophan and ICG | Dissolving MNs | HA, PVP, PVA | 194 |
aPD-1: anti-programmed cell death protein 1 antibodies; aPD-L1: anti-programmed death-ligand 1 antibodies; aCTLA-4: anti-cytotoxic T-lymphocyte-associated protein 4 antibodies; ICG: indocyanine green.
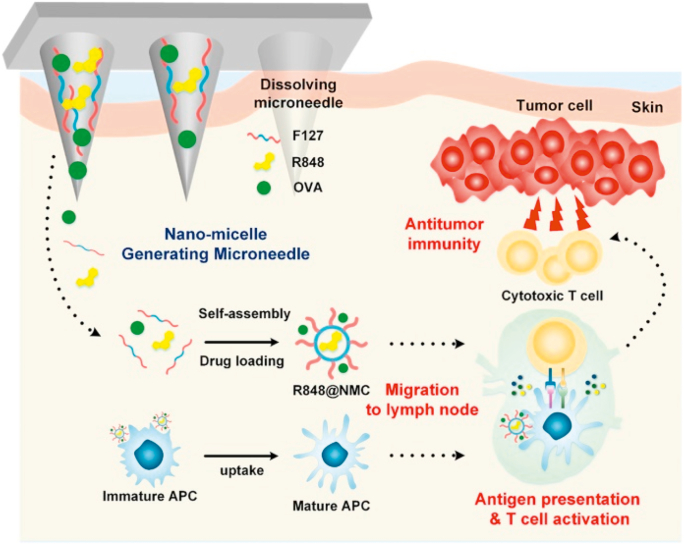
Therapeutic cancer vaccines represent a viable option for active immunotherapy of cancers by using a patient's own immune system, which include cell vaccines (tumor or immune cell), genetic (DNA, RNA, and viral) vaccines, and protein/peptide-based vaccines195. Vaccination with antigens by MNs can generate a robust antigen-specific cellular immune response. By activating antigen-specific CD8 cytotoxic T-lymphocytes, it can effectively eliminate tumors, just like the complete vaccination protection of the body in infectious diseases118. The immune adjuvant can be used simultaneously with the antigen or in advance, which can non-specifically enhance the body's immune response to the antigen. Kim et al.181 utilized dissolving MNs to deliver model antigen (OVA) and immunostimulatory adjuvant (resiquimod) into lymph nodes to mature and activate antigen-presenting cells (Fig. 6). The dissolving MNs based on amphiphilic triblock copolymer could generate nanomicelles in situ after being dissolved in the skin, which facilitated the delivery of poorly water-soluble resiquimod. The results of antitumor immune response showed that the application of the dissolving MNs containing OVA and resiquimod to tumor-bearing mice induced a significant level of antigen-specific cellular and humoral immunity181.
Figure 6.
Enhanced cancer vaccination by in situ nanomicelle-generating dissolving MNs containing OVA and resiquimod (R848). Reprinted with permission from Ref. 181. Copyright © 2018, American Chemical Society.
Proteins and peptides with catalytic abilities can be used as adjuvant agents for other therapeutic modalities or as anticancer drugs themselves196. Meanwhile, certain proteins and peptides can also work as drug delivery carriers, due to their biocompatibility and bioresorbable ability. Some cell-penetrating peptides can be combined with vaccines for immunotherapy. Ruan et al.187 developed an siBraf delivery system based on cell-penetrating peptide octaarginine nanocomplexes combined with coated MNs for targeted anti-melanoma treatment. The results showed that octaarginine presented lower cytotoxicity than polyethyleneimine, while exhibited comparable gene transfection and silencing efficacy. The octaarginine/siBraf coated MNs could successfully pierce into the melanoma site and effectively inhibit tumor growth187. Duong et al.188 developed a dissolving MNs-based polypeptide cocktail to augment cancer immunotherapy. Compared with subcutaneous vaccination, the dissolving MNs induced higher OVA-specific antibody titer and significantly inhibited OVA-expressing metastatic tumor.
The immunomodulatory antibodies can induce a powerful antitumor immune response. However, they usually generate substantial autoimmunity, leading to adverse effects197. Targeted and controlled release of antibodies in the desired cell types can achieve minimal off-target effects and reduce toxicity. MNs can directly accumulate sufficient immunotherapies within the topical disease site to effectively target the desired tumor and immune cells. Therefore, integrating MNs with immunomodulatory antibody is promising for fighting against malignant tumors. In particular, nanoparticles-encapsulated MNs have been designed to enable controlled release of immune checkpoint inhibitors, including aPD-1/aPD-L1189,190, aCTLA-4191,194, and 1-methyl-D,L-tryptophan192,194. Wang et al.189 developed a self-degradable MNs for the sustained delivery of aPD-1. Hyaluronic acid integrated with pH-sensitive dextran nanoparticles containing aPD-1 and GOx were formulated into MNs. The tumor acidic microenvironment promoted the sustained release of aPD-1. In vivo antitumor study in mice melanoma model showed that application of the self-degradable MNs induced strong immune response compared to the MNs without degradation trigger or intratumor injection of free aPD-1189. The MNs co-loaded with different checkpoint inhibitors resulted in the synergistic treatment of tumors189,192. Ye et al.192 constructed the MNs platform to co-deliver aPD-1 and 1-methyl-D,L-tryptophan. The results demonstrated that the synergistic treatment enhanced effective T cell immunity in a B16F10 melanoma model192.
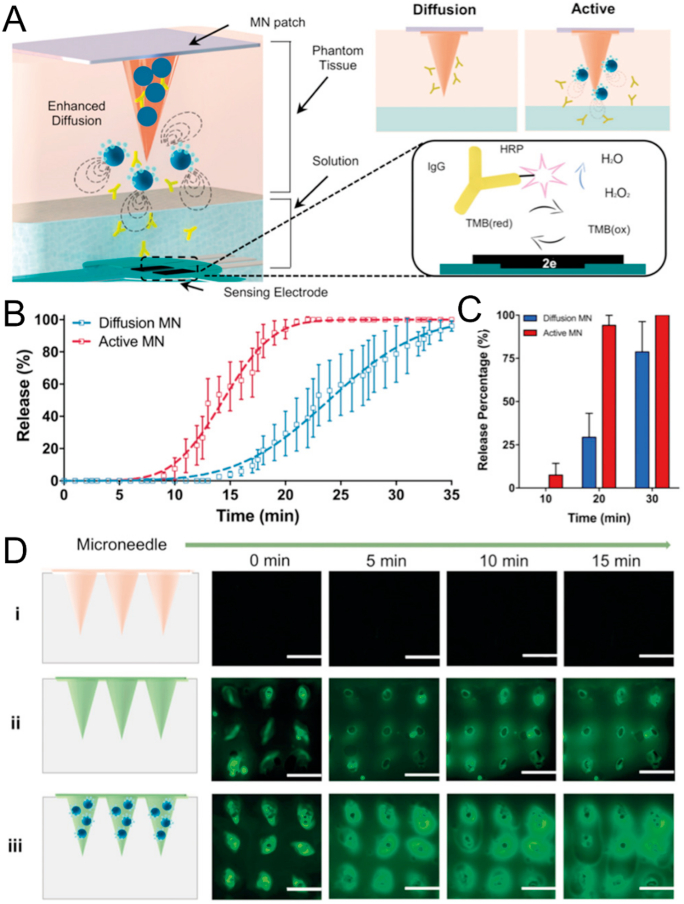
Drug delivery based on MNs usually relies on passive diffusion, which may limit the distribution and penetration depth of the therapeutic agents. Lopez-Ramirez et al.191 loaded magnesium particles into the MNs as a built-in engine to achieve faster and deeper intradermal drug delivery (Fig. 7). The magnesium particles could react with the interstitial fluid to quickly generate H2 bubbles, thereby providing extremely local high fluid flow to break through the dermal barrier and enhance local payload delivery191. In vivo antitumor experiments showed that the passive MNs delivering the therapeutic aCTLA-4 initially delayed tumor growth of B16F10 melanoma. However, by day 46, all mice in this group showed exceeding tumor burden of 1500 mm3. In sharp contrast, 60% of the mice treated with the active MNs exhibited a completely tumor-free state191.
Figure 7.
Built-in active MNs patch with enhanced drug delivery. (A) Schematic illustration of the design and mechanism of the active MNs patch. (B) Drug release kinetics of different MNs at pH 6.0. (C) Corresponding release percentage of aCTLA-4. (D) The fluorescence images of MNs patch obtained from top view. (i) Blank MNs, (ii) FITC-loaded MNs, and (iii) FITC-loaded active MNs. Scale bar, 1 mm. Reprinted with permission from Ref. 191. Copyright © 2019, John Wiley and Sons.
Immune checkpoint blockade therapy based on MNs can be combined with other cancer therapies. Besides, the activation of the skin immune system can enhance anti-cancer immunity both locally and systemically190,194. Chen et al.190 developed hollow MNs that combined checkpoint inhibitor and cold atmospheric plasma. Cold atmospheric plasma induced tumor cell death, and the released tumor-associated antigens then initiated immune response. Meanwhile, aPD-L1 released from the hollow MNs patch further augmented the antitumor immunity. Immunotherapy combined with phototherapy is also used to further enhance the anti-cancer effect190. Chen et al.193 designed a MNs-assisted platform for synergistic photodynamic and immunotherapy, which simultaneously encapsulated hydrophobic zinc phthalocyanine and hydrophilic aCTLA-4. In this approach, photodynamic therapy worked firstly to kill tumor and triggered the immune response, subsequently facilitated robust immunotherapy with aCTLA-4193. Our group194 also designed a core‒shell structure MNs to boost the immune response by combining photothermal therapy and immunotherapy. The obtained system could effectively eradicate primary melanoma tumor and inhibit metastasized tumor194.
In addition to immunotherapy, proteins can also exert an anti-cancer effect through other therapies. For example, bevacizumab can be used to treat a variety of cancers by inhibiting tumor angiogenesis. Courtenay et al.198 provided high dose transdermal delivery of bevacizumab using MNs, which highlighted the potential of MNs to provide sustained drug delivery to the systemic and lymph circulation. Collectively, the delivery of proteins and peptides assisted by MNs for cancer treatment is a useful strategy.
3.4. Other disease therapy
MNs-mediated transdermal protein and peptide delivery can also be used in other disease therapy, such as hypoglycemia199, osteoporosis200, cosmeceuticals45, and wound healing201.
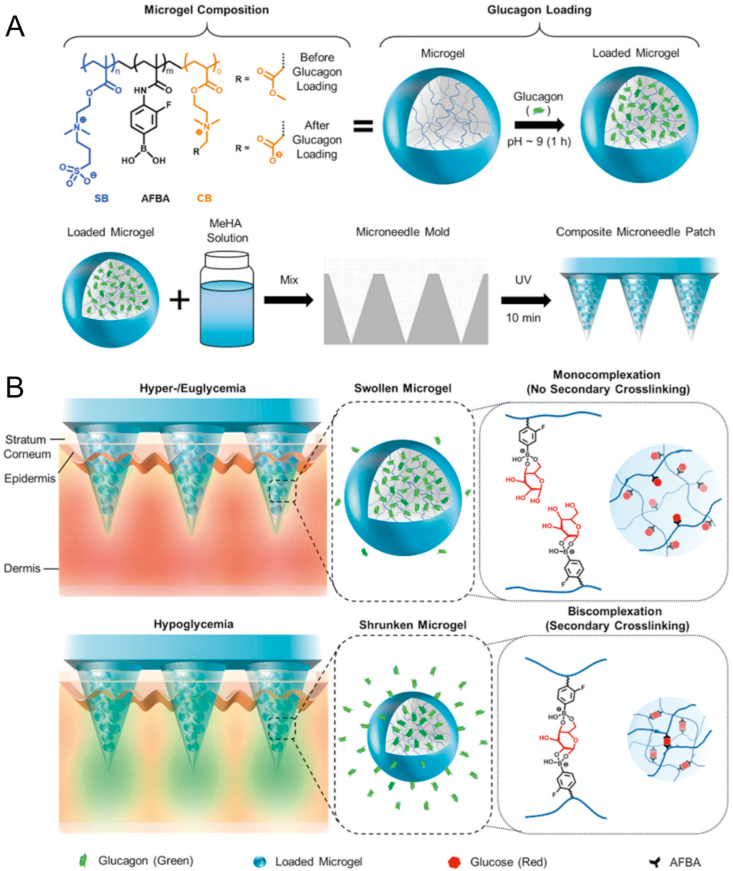
The administration of insulin may cause hypoglycemia, a life-threatening condition characterized by abnormally low blood glucose level202. To address this issue, GhavamiNejad et al.199 designed a smart MNs patch to specifically release glucagon at the hypoglycemia condition. The MNs patch was prepared by a photo-crosslinked methacrylated hyaluronic acid embedded multifunctional microgels, which enabled hypoglycemia triggered release property (Fig. 8). In the type 1 diabetes rat model, the MNs patch successfully prevented hypoglycemia caused by insulin overdose199.
Figure 8.
Schematic illustration of the controlled glucagon release from the MNs patch. (A) The fabrication process of MNs patch. (B) The mechanism of glucagon release from the MNs patch. Reprinted with permission from Ref. 199. Copyright © 2019, John Wiley and Sons.
Naito et al.200 designed a dissolving MNs patch loaded with human parathyroid hormone to treat osteoporosis. The MNs obviously improved the stability of parathyroid hormone compared to solution. The in vivo study showed that the bioavailability of parathyroid hormone-loaded MNs was 100 ± 4% relative to subcutaneous injection. In a rat model of osteoporosis, parathyroid hormone-loaded MNs successfully inhibited the decrease in bone density.
Proteins and peptides play an important role in cosmetic applications. Mohammed et al.45 investigated the effect of stainless steel MNs on the skin penetration of different chain length peptides, including melanostatin, rigin, and palmitoyl-pentapeptide. They observed that peptides with smaller molecular weight were associated with local delivery enhancement45.
Chi et al.201 developed vascular endothelial growth factor encapsulated chitosan MNs to promote wound healing. The drug release could be controlled via the temperature rise induced by the inflammation response at the wound site. The in vitro antibacterial test and in vivo wound healing study suggested that the MNs patch could promote collagen deposition, inflammatory inhibition, and tissue regeneration during wound closure201.
4. MNs-mediated protein and peptide delivery in the clinic
As mentioned above, the fundamental research has proved the advantages and feasibility of MNs-mediated protein and peptide delivery. At present, many therapies based on MNs-mediated transdermal delivery of protein and peptide drugs have entered clinical use. As shown in Table 3, most currently active clinical trials focus on the vaccination of infectious diseases and insulin delivery for diabetes treatment. These clinical trials mainly utilized the hollow MNs infusion system, and a few investigated dissolving or coated MNs. This is mainly because the research on coated MNs, dissolving MNs or hydrogel-forming MNs started later. And they usually require more sophisticated MNs design and manufacturing techniques. The interdisciplinary divide between microfabrication and pharmaceutical research also delayed the development of drug delivery23. At this stage, the field is at an important transitional point. More MNs products will be translated into clinical and medical practice in the near future.
Table 3.
Currently active clinical trials with MNs for therapeutic protein and peptide delivery.
| Condition or disease | Therapeutic agent | MNs type | CT phase | NCT identifier |
|---|---|---|---|---|
| Influenza | Inactivated influenza vaccine (IIV) | Dissolving MNs | 1 | NCT02438423 |
| Influenza | Trivalent influenza vaccine | Hollow MNs | 1/2 | NCT01707602 |
| Influenza | Intanza ® | A micro-needle injection system | 4 | NCT01368796 |
| Influenza | S-OIV H1N1 vaccine | MicronJet 600 (hollow MNs) | Not applicable | NCT01049490 |
| Influenza | Influenza vaccine (TIV 2010/2011) | Microneedle device (hollow MNs) | Not applicable | NCT01304563 |
| Influenza | Flu vaccine | Microneedle injectors (hollow MNs) | Not applicable | NCT00558649 |
| Healthy | H1N1 pandemic influenza | Microneedle device | Not applicable | NCT01039623 |
| Measles and Rubella | Measles rubella vaccine | Dissolving MNs | 1/2 | NCT04394689 |
| Renal Failure | HBV vaccine | A novel intradermal microneedle | 2/3 | NCT02621112 |
| Varicella Zoster infection | Zostavax | A novel intradermal microneedle | 2/3 | NCT02329457 |
| Atopic dermatitis | Fluzone® intradermal | An ultra-fine micro-needle | 1 | NCT01518478 |
| Atopic dermatitis | Fluzone® intradermal | An ultra-fine micro-needle | Not applicable | NCT01737710 |
| Intradermal injections | Insulin | MicronJet (hollow MNs) | 1 | NCT00602914 |
| Diabetes | Insulin | Hollow MNs | 1/2 | NCT01061216 |
| Diabetes | Insulin | Hollow MNs | 2/3 | NCT00837512 |
| Diabetes | C19-A3 GNP peptide | Nanopass microneedles | 1 | NCT02837094 |
| Diabetes | Insulin and glucagon | MicronJet (hollow MNs) | 2 | NCT01684956 |
| Hypoglycemia | Glucagon | Microneedle patch system | 1 | NCT02459938 |
| Postmenopausal osteoporosis | Abaloparatide | Solid microstructured transdermal system | 3 | NCT04064411 |
| Postmenopausal osteoporosis | Abaloparatide | Coated transdermal microarray | 2 | NCT01674621 |
| Postmenopausal osteoporosis | Zosano Pharma parathyroid hormone | Coated MNs | 1 | NCT02478879 |
| Primary axillary hyperhidrosis | Botulinum toxin type A | Fractional micro-needle radiofrequency | Not applicable | NCT03054480 |
| Poliomyelitis | Fractional IPV | MicronJet600 (hollow MNs) | 3 | NCT01813604 |
| Auto-immune/auto-inflammatory diseases | Adalimumab | MicronJet600 (hollow MNs) | 1/2 | NCT03607903 |
5. Conclusions and prospects
Proteins and peptides have high specificity and potency compared to small molecules, which have been demonstrated to be effective for the treatment of various diseases. Nonetheless, because of the inherent properties of proteins and peptides, such as large molecular weight, poor stability, and conformational flexibility, they are usually administered by injection, which is inconvenient and unfriendly. MNs can improve the patient's compliance and overcome the skin barrier for protein and peptide drugs. MNs have been developed in several designs with different delivery strategies, which can be generally classified into solid MNs, coated MNs, hollow MNs, dissolving MNs, and hydrogel-forming MNs. Skin plays a unique role in biology and immunomodulation. The active immune environment in the skin can synergize with the MNs-mediated vaccine delivery to fight infectious diseases and treat cancers. It is also an important application for MNs in diabetes treatment, and MNs also make safer closed-loop glucose-responsive therapies possible. MNs-mediated transdermal delivery of checkpoint inhibitors has reduced their off-target effect and achieved local targeted delivery to treat superficial cancers. In short, MNs are a very promising strategy for protein and peptide delivery to treat various diseases.
The successful formulation of proteins and peptides depends on a thorough understanding of their physicochemical and biological characteristics. Notably, the formulation and handling of proteins and peptides need special attention in optimizing their stability and efficacy. The researches for addressing fundamental issues including drug loading, pharmacokinetic and pharmacodynamic profile, safety, and storage of MNs will promote transdermal protein and peptide drug delivery. With the advancement already achieved in the area of microfabrication technologies available in designing MNs, more intelligent MNs systems will gradually emerge. Proteins and peptides are potent active pharmaceutical ingredients, which may break the limit of low drug loading of MNs. The comprehensive characterization methodologies, including both in vitro and in vivo, have been used to evaluate the ability of MNs to deliver drugs safely and effectively into the skin. The approaches currently used in the field will pave way to the development of standardized protocols for MNs evaluation in the future97. It is optimistically expected that extensive academic research in combination with the pharmaceutical industry will further accelerate the clinical translation of MNs-mediated transdermal delivery of protein and peptide drugs.
Acknowledgments
This work was funded by the National Natural Science Foundation (Project No. 81803466, China), Guangdong Macao joint innovation funding project (Project No. 2020A050515009, China), the Research and Development Plan for Key Areas in Guangdong Province (Project No. 2019B020204002, China), and the Foundation of Traditional Chinese Medicine Bureau of Guangdong Province (Project No. 20191057, China).
Author contributions
Guilan Quan, Xin Pan and Chuanbin Wu conceived the review. Ting Liu wrote the manuscript with assistance of Minglong Chen, Jingtao Fu, Ying Sun and Chao Lu. Minglong Chen, Guilan Quan and Xin Pan revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Guilan Quan, Email: xiaoplanet@163.com.
Xin Pan, Email: panxin2@mail.sysu.edu.cn.
References
- 1.Agyei D., Ahmed I., Akram Z., Iqbal H.M., Danquah M.K. Protein and peptide biopharmaceuticals: an overview. Protein Pept Lett. 2017;24:94–101. doi: 10.2174/0929866523666161222150444. [DOI] [PubMed] [Google Scholar]
- 2.Jain D., Mahammad S.S., Singh P.P., Kodipyaka R. A review on parenteral delivery of peptides and proteins. Drug Dev Ind Pharm. 2019;45:1403–1420. doi: 10.1080/03639045.2019.1628770. [DOI] [PubMed] [Google Scholar]
- 3.Leader B., Baca Q.J., Golan D.E. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 4.Ye Y.Q., Yu J.C., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv Drug Deliv Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichert J.M. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov. 2003;2:695–702. doi: 10.1038/nrd1178. [DOI] [PubMed] [Google Scholar]
- 6.Pavlou A.K., Reichert J.M. Recombinant protein therapeutics—success rates, market trends and values to 2010. Nat Biotechnol. 2004;22:1513–1519. doi: 10.1038/nbt1204-1513. [DOI] [PubMed] [Google Scholar]
- 7.Zhu G., Mallery S.R., Schwendeman S.P. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 8.Tanner T., Marks R. Delivering drugs by the transdermal route: review and comment. Skin Res Technol. 2008;14:249–260. doi: 10.1111/j.1600-0846.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- 9.Zalevsky J., Chamberlain A.K., Horton H.M., Karki S., Leung I.W., Sproule T.J. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y., Hasegawa R., Fukushima K., Sugioka N., Takada K. Self-dissolving micropile array chip as percutaneous delivery system of protein drug. Biol Pharm Bull. 2010;33:683–690. doi: 10.1248/bpb.33.683. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima K., Yamazaki T., Hasegawa R., Ito Y., Sugioka N., Takada K. Pharmacokinetic and pharmacodynamic evaluation of insulin dissolving microneedles in dogs. Diabetes Technol Therapeut. 2010;12:465–474. doi: 10.1089/dia.2009.0176. [DOI] [PubMed] [Google Scholar]
- 13.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 14.Munch S., Wohlrab J., Neubert R.H.H. Dermal and transdermal delivery of pharmaceutically relevant macromolecules. Eur J Pharm Biopharm. 2017;119:235–242. doi: 10.1016/j.ejpb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Karande P., Jain A., Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22:192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- 16.Arora A., Hakim I., Baxter J., Rathnasingham R., Srinivasan R., Fletcher D.A. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc Natl Acad Sci U S A. 2007;104:4255. doi: 10.1073/pnas.0700182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee W.R., Shen S.C., Al-Suwayeh S.A., Yang H.H., Li Y.C., Fang J.Y. Skin permeation of small-molecule drugs, macromolecules, and nanoparticles mediated by a fractional carbon dioxide laser: the role of hair follicles. Pharm Res-dordr. 2013;30:792–802. doi: 10.1007/s11095-012-0920-4. [DOI] [PubMed] [Google Scholar]
- 18.Becker S., Zorec B., Miklavčič D., Pavšelj N. Transdermal transport pathway creation: electroporation pulse order. Math Biosci. 2014;257:60–68. doi: 10.1016/j.mbs.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Masterson J., Kluge B., Burdette A., Sr G.L. Sustained acoustic medicine; sonophoresis for nonsteroidal anti-inflammatory drug delivery in arthritis. Ther Deliv. 2020;11:363–372. doi: 10.4155/tde-2020-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawat S., Vengurlekar S., Rakesh B., Jain S., Srikarti G. Transdermal delivery by iontophoresis. Indian J Pharm Sci. 2008;70:5–10. doi: 10.4103/0250-474X.40324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prausnitz M.R. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Tuan-Mahmood T.-M., McCrudden M.T.C., Torrisi B.M., McAlister E., Garland M.J., Singh T.R.R. Microneedles for intradermal and transdermal drug delivery. J Pharm Sci. 2013;50:623–637. doi: 10.1016/j.ejps.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arya J., Prausnitz M.R. Microneedle patches for vaccination in developing countries. J Control Release. 2016;240:135–141. doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., Li H., Shi D., Liu Z.G., Yuan W.E. Microneedles as a delivery system for gene therapy. Front Pharmacol. 2016;7:137. doi: 10.3389/fphar.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X., Zhu D.D., Chen B.Z., Ashfaq M., Guo X.D. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Chen M.L., Quan G.L., Sun Y., Yang D., Pan X., Wu C.B. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J Control Release. 2020;325:163–175. doi: 10.1016/j.jconrel.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Banga A.K. CRC press; 2011. Transdermal and intradermal delivery of therapeutic agents: application of physical technologies. [Google Scholar]
- 29.Schoellhammer C.M., Blankschtein D., Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expet Opin Drug Deliv. 2014;11:393–407. doi: 10.1517/17425247.2014.875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar S., Dave K., Venuganti V.V.K. Microneedles in the clinic. J Control Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Chandrasekhar S., Iyer L.K., Panchal J.P., Topp E.M., Cannon J.B., Ranade V.V. Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expet Opin Drug Deliv. 2013;10:1155–1170. doi: 10.1517/17425247.2013.797405. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Hu L.Z., Xu C.J. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17:1373–1387. doi: 10.1039/c7lc00016b. [DOI] [PubMed] [Google Scholar]
- 34.Williams A.C. Pharmaceutical Press; London: 2003. Transdermal & topical drug delivery. [Google Scholar]
- 35.Wilke N., Mulcahy A., Ye S.R., Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J. 2005;36:650–656. [Google Scholar]
- 36.Tham H.P., Xu K., Lim W.Q., Chen H., Zheng M., Thng T.G.S. Microneedle-assisted topical delivery of photodynamically active mesoporous formulation for combination therapy of deep-seated melanoma. ACS Nano. 2018;12:11936–11948. doi: 10.1021/acsnano.8b03007. [DOI] [PubMed] [Google Scholar]
- 37.Matriano J.A., Cormier M., Johnson J., Young W.A., Buttery M., Nyam K. Macroflux® microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 38.Jin C.Y., Han M.H., Lee S.S., Choi Y.H. Mass producible and biocompatible microneedle patch and functional verification of its usefulness for transdermal drug delivery. Biomed Microdevices. 2009;11:1195. doi: 10.1007/s10544-009-9337-1. [DOI] [PubMed] [Google Scholar]
- 39.Moon S.J., Lee S.S., Lee H., Kwon T. Fabrication of microneedle array using liga and hot embossing process. Microsyst Technol. 2005;11:311–318. [Google Scholar]
- 40.Park J.-H., Choi S.-O., Seo S., Choy Y.B., Prausnitz M.R. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76:282–289. doi: 10.1016/j.ejpb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Yan G., Warner K.S., Zhang J., Sharma S., Gale B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int J Pharm. 2010;391:7–12. doi: 10.1016/j.ijpharm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Cheung K., Han T., Das D.B. Effect of force of microneedle insertion on the permeability of insulin in skin. J Diabetes Sci Technol. 2014;8:444–452. doi: 10.1177/1932296813519720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks S.L., Pinninti R.R., Gill H.S., Crooks P.A., Prausnitz M.R., Stinchcomb A.L. Flux across [corrected] microneedle-treated skin is increased by increasing charge of naltrexone and naltrexol in vitro. Pharm Res. 2008;25:1677–1685. doi: 10.1007/s11095-008-9578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAllister D.V., Wang P.M., Davis S.P., Park J.H., Canatella P.J., Allen M.G. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohammed Y.H., Yamada M., Lin L.L., Grice J.E., Roberts M.S., Raphael A.P. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbaan F.J., Bal S.M., van den Berg D.J., Groenink W.H.H., Verpoorten H., Lüttge R. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117:238–245. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly R.F., Singh T.R.R., Larrañeta E., McCrudden M.T. John Wiley & Sons; New Jersey: 2018. Microneedles for drug and vaccine delivery and patient monitoring. [Google Scholar]
- 48.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Brown K., Siebenaler K., Determan A., Dohmeier D., Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29:170–177. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Siebenaler K., Brown K., Dohmeier D., Hansen K. Adjuvants to prolong the local anesthetic effects of coated microneedle products. Int J Pharm. 2012;439:187–192. doi: 10.1016/j.ijpharm.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 51.Kapoor Y., Milewski M., Dick L., Zhang J., Bothe J.R., Gehrt M. Coated microneedles for transdermal delivery of a potent pharmaceutical peptide. Biomed Microdevices. 2019;22:7. doi: 10.1007/s10544-019-0462-1. [DOI] [PubMed] [Google Scholar]
- 52.Haj-Ahmad R., Khan H., Arshad M.S., Rasekh M., Hussain A., Walsh S. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics. 2015;7:486–502. doi: 10.3390/pharmaceutics7040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gill H.S., Prausnitz M.R. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X., Coulman S.A., Hanna S.J., Wong F.S., Dayan C.M., Birchall J.C. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release. 2017;265:2–13. doi: 10.1016/j.jconrel.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Tang T., Weng T.J., Jia H.X., Luo S.D., Xu Y., Li L.H. Harnessing the layer-by-layer assembly technique to design biomaterials vaccines for immune modulation in translational applications. Biomat Sci. 2019;7:715–732. doi: 10.1039/c8bm01219a. [DOI] [PubMed] [Google Scholar]
- 56.Cormier M., Johnson B., Ameri M., Nyam K., Libiran L., Zhang D.D. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Ameri M., Kadkhodayan M., Nguyen J., Bravo J.A., Su R., Chan K. Human growth hormone delivery with a microneedle transdermal system: preclinical formulation, stability, delivery and pK of therapeutically relevant doses. Pharmaceutics. 2014;6:220–234. doi: 10.3390/pharmaceutics6020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusamori K., Katsumi H., Sakai R., Hayashi R., Hirai Y., Tanaka Y. Development of a drug-coated microneedle array and its application for transdermal delivery of interferon alpha. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/1/015006. [DOI] [PubMed] [Google Scholar]
- 59.Shrestha P., Stoeber B. Fluid absorption by skin tissue during intradermal injections through hollow microneedles. Sci Rep. 2018;8:13749. doi: 10.1038/s41598-018-32026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahlam A., Mccrudden M.T., Ryan D. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sušić A., Hrnjica Z., Kajgana I., Mujezinović M., Hasanbegović A., Brčkalo J. Springer International Publishing; New York: 2019. Use of hollow microneedle drug delivery systems in treatment of diabetes mellitus, CMBEBIH; pp. 575–580. 2020. [Google Scholar]
- 62.Terashima S., Tatsukawa C., Takahashi T., Suzuki M., Aoyagi S. Fabrication of hyaluronic acid hollow microneedle array. Jpn J Appl Phys. 2020;59:SIIJ03. [Google Scholar]
- 63.van der Maaden K., Heuts J., Camps M., Pontier M., Terwisscha van Scheltinga A., Jiskoot W. Hollow microneedle-mediated micro-injections of a liposomal hpv e743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and t-helper responses. J Control Release. 2018;269:347–354. doi: 10.1016/j.jconrel.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Niu L., Chu L.Y., Burton S.A., Hansen K.J., Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Davis S.P., Landis B.J., Adams Z.H., Allen M.G., Prausnitz M.R. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37:1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Ita K. Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother. 2017;93:1116–1127. doi: 10.1016/j.biopha.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Chen W., Wang C., Yan L., Huang L.B., Zhu X.Y., Chen B. Improved polyvinylpyrrolidone microneedle arrays with non-stoichiometric cyclodextrin. J Mater Chem B. 2014;2:1699–1705. doi: 10.1039/c3tb21698e. [DOI] [PubMed] [Google Scholar]
- 68.Thakur R.R.S., Tekko I.A., Al-Shammari F., Ali A.A., McCarthy H., Donnelly R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv Transl Res. 2016;6:800–815. doi: 10.1007/s13346-016-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J.W., Park J.-H., Prausnitz M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7:90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Y.Q., Li C., Zhang S.H., Yang G.Z., He M.L., Gao Y.H. Systemic delivery of artemether by dissolving microneedles. Int J Pharm. 2016;508:1–9. doi: 10.1016/j.ijpharm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan S.P., Koutsonanos D.G., del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.-O. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang S.X., Wu F., Liu J.G., Fan G.R., Welsh W., Zhu H. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv Funct Mater. 2015;25:4633–4641. [Google Scholar]
- 74.Lee K., Jung H. Drawing lithography for microneedles: a review of fundamentals and biomedical applications. Biomaterials. 2012;33:7309–7326. doi: 10.1016/j.biomaterials.2012.06.065. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.D., Kim M., Yang H., Lee K., Jung H. Droplet-born air blowing: novel dissolving microneedle fabrication. J Control Release. 2013;170:430–436. doi: 10.1016/j.jconrel.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 76.Vecchione R., Coppola S., Esposito E., Casale C., Vespini V., Grilli S. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv Funct Mater. 2014;24:3515–3523. [Google Scholar]
- 77.Dardano P., Caliò A., Di Palma V., Bevilacqua M.F., Di Matteo A., De Stefano L. A photolithographic approach to polymeric microneedles array fabrication. Materials. 2015;8:8661–8673. doi: 10.3390/ma8125484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang S.X., Feng Y., Zhang L.J., Chen N.X., Yuan W.E., Jin T. A scalable fabrication process of polymer microneedles. Int J Nanomed. 2012;7:1415–1422. doi: 10.2147/IJN.S28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H.P., Wu B.Y., Zhang M.M., Yang P.P., Yang B.B., Qin W.B. A novel scalable fabrication process for the production of dissolving microneedle arrays. Drug Deliv Transl Res. 2019;9:240–248. doi: 10.1007/s13346-018-00593-z. [DOI] [PubMed] [Google Scholar]
- 80.Park J.-H., Allen M.G., Prausnitz M.R. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 81.Ito Y., Yoshimitsu J.I., Shiroyama K., Sugioka N., Takada K. Self-dissolving microneedles for the percutaneous absorption of epo in mice. J Drug Target. 2006;14:255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- 82.Wang Q.Q., Yao G.T., Dong P., Gong Z.H., Li G., Zhang K.J. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. J Pharm Sci. 2015;66:148–156. doi: 10.1016/j.ejps.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Chu L.Y., Choi S.-O., Prausnitz M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99:4228–4238. doi: 10.1002/jps.22140. [DOI] [PubMed] [Google Scholar]
- 84.Li W., Terry R.N., Tang J., Feng M.R., Schwendeman S.P., Prausnitz M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat Biomed Eng. 2019;3:220–229. doi: 10.1038/s41551-018-0337-4. [DOI] [PubMed] [Google Scholar]
- 85.Hou A.L., Quan G.L., Yang B.B., Lu C., Chen M.L., Yang D. Rational design of rapidly separating dissolving microneedles for precise drug delivery by balancing the mechanical performance and disintegration rate. Adv Healthc Mater. 2019;8:1900898. doi: 10.1002/adhm.201900898. [DOI] [PubMed] [Google Scholar]
- 86.Fukushima K., Ise A., Morita H., Hasegawa R., Ito Y., Sugioka N. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm Res-dordr. 2011;28:7–21. doi: 10.1007/s11095-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 87.Raphael A.P., Prow T.W., Crichton M.L., Chen X., GJP Fernando, Kendall M.A.F. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6:1785–1793. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 88.Li W., Tang J., Terry R.N., Li S., Brunie A., Callahan R.L. Long-acting reversible contraception by effervescent microneedle patch. Sci Adv. 2019;5:eaaw8145. doi: 10.1126/sciadv.aaw8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donnelly R.F., Woolfson A.D. Patient safety and beyond: what should we expect from microneedle arrays in the transdermal delivery arena? Ther Deliv. 2014;5:653–662. doi: 10.4155/tde.14.29. [DOI] [PubMed] [Google Scholar]
- 90.Donnelly R.F., McCrudden M.T.C., Zaid Alkilani A., Larrañeta E., McAlister E., Courtenay A.J. Hydrogel-forming microneedles prepared from "super swelling" polymers combined with lyophilised wafers for transdermal drug delivery. PLoS One. 2014;9:e111547. doi: 10.1371/journal.pone.0111547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardy J.G., Larrañeta E., Donnelly R.F., McGoldrick N., Migalska K., McCrudden M.T.C. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol Pharmaceut. 2016;13:907–914. doi: 10.1021/acs.molpharmaceut.5b00807. [DOI] [PubMed] [Google Scholar]
- 92.Sivaraman A., Banga A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv Transl Res. 2017;7:16–26. doi: 10.1007/s13346-016-0328-5. [DOI] [PubMed] [Google Scholar]
- 93.Dimatteo R., Darling N.J., Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev. 2018;127:167–184. doi: 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiang T.K., Ranamukhaarachchi S.A., Ensom M.H. Revolutionizing therapeutic drug monitoring with the use of interstitial fluid and microneedles technology. Pharmaceutics. 2017;9:43. doi: 10.3390/pharmaceutics9040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Demir Y.K., Akan Z., Kerimoglu O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donnelly R.F., Singh T.R.R., Garland M.J., Migalska K., Majithiya R., McCrudden C.M. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabri A.H., Kim Y., Marlow M., Scurr D.J., Segal J., Banga A.K. Intradermal and transdermal drug delivery using microneedles—fabrication, performance evaluation and application to lymphatic delivery. Adv Drug Deliv Rev. 2019;153:195–215. doi: 10.1016/j.addr.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Lutton R.E., Larraneta E., Kearney M.C., Boyd P., Woolfson A.D., Donnelly R.F. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int J Pharm. 2015;494:417–429. doi: 10.1016/j.ijpharm.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lau J.L., Dunn M.K. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorgan Med Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 100.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Market P.T. Infinium Global Research; 2018. Global industry analysis, trends, market size and forecasts up to 2024. [Google Scholar]
- 102.Bloom D.E., Black S., Rappuoli R. Emerging infectious diseases: a proactive approach. P Natl Acad Sci USA. 2017;114:4055. doi: 10.1073/pnas.1701410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saylor K., Gillam F., Lohneis T., Zhang C. Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He X.X., Sun J.Y., Zhuang J., Xu H., Liu Y., Wu D.M. Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-response. 2019;17 doi: 10.1177/1559325819878585. 1559325819878585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prausnitz M.R., Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lambert P.H., Laurent P.E. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 107.Peachman K.K., Rao M., Alving C.R. Immunization with DNA through the skin. Methods. 2003;31:232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- 108.Chen Z.J., Lv Y.J., Qi J.P., Zhu Q.G., Lu Y., Wu W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov Today. 2018;23:181–186. doi: 10.1016/j.drudis.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Chen Z.J., He J.J., Qi J.P., Zhu Q.G., Wu W., Lu Y. Long-acting microneedles: a progress report of the state-of-the-art techniques. Drug Discov Today. 2020;25:1462–1468. doi: 10.1016/j.drudis.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 110.Li J.W., Zeng M.T., Shan H., Tong C.Y. Microneedle patches as drug and vaccine delivery platform. Curr Med Chem. 2017;24:2413–2422. doi: 10.2174/0929867324666170526124053. [DOI] [PubMed] [Google Scholar]
- 111.de Groot A.M., Du G., Mönkäre J., Platteel A.C.M., Broere F., Bouwstra J.A. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J Control Release. 2017;266:27–35. doi: 10.1016/j.jconrel.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 112.Du G., Hathout R.M., Nasr M., Nejadnik M.R., Tu J., Koning R.I. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 113.Widera G., Johnson J., Kim L., Libiran L., Nyam K., Daddona P.E. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 114.He Y.P., Hong C., Li J.H., Howard M.T., Li Y.Z., Turvey M.E. Synthetic charge-invertible polymer for rapid and complete implantation of layer-by-layer microneedle drug films for enhanced transdermal vaccination. ACS Nano. 2018;12:10272–10280. doi: 10.1021/acsnano.8b05373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeMuth P.C., Moon J.J., Suh H., Hammond P.T., Irvine D.J. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhatnagar S., Chawla S.R., Kulkarni O.P., Venuganti V.V.K. Zein microneedles for transcutaneous vaccine delivery: fabrication, characterization, and in vivo evaluation using ovalbumin as the model antigen. ACS Omega. 2017;2:1321–1332. doi: 10.1021/acsomega.7b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCrudden M.T.C., Torrisi B.M., Al-Zahrani S., McCrudden C.M., Zaric M., Scott C.J. Laser-engineered dissolving microneedle arrays for protein delivery: potential for enhanced intradermal vaccination. J Pharm Pharmacol. 2015;67:409–425. doi: 10.1111/jphp.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zaric M., Lyubomska O., Touzelet O., Poux C., Al-Zahrani S., Fay F. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-d,l-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7:2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao J.H., Zhang Q.B., Liu B., Piao X.H., Yan Y.L., Hu X.G. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomed. 2017;12:4763–4772. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erdos G., Balmert S.C., Carey C.D., Falo G.D., Patel N.A., Zhang J. Improved cutaneous genetic immunization by microneedle array delivery of an adjuvanted adenovirus vaccine. J Invest Dermatol. 2020;140:2528–2531.e2. doi: 10.1016/j.jid.2020.03.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balmert S.C., Carey C.D., Falo G.D., Sethi S.K., Erdos G., Korkmaz E. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J Control Release. 2020;317:336–346. doi: 10.1016/j.jconrel.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DeMuth P.C., Garcia-Beltran W.F., Ai-Ling M.L., Hammond P.T., Irvine D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv Funct Mater. 2013;23:161–172. doi: 10.1002/adfm.201201512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeMuth P.C., Min Y., Irvine D.J., Hammond P.T. Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Adv Healthc Mater. 2014;3:47–58. doi: 10.1002/adhm.201300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Courtenay A.J., Rodgers A.M., McCrudden M.T., McCarthy H.O., Donnelly R.F. Novel hydrogel-forming microneedle array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol Pharmaceut. 2018;16:118–127. doi: 10.1021/acs.molpharmaceut.8b00895. [DOI] [PubMed] [Google Scholar]
- 125.Wendorf J.R., Ghartey-Tagoe E.B., Williams S.C., Enioutina E., Singh P., Cleary G.W. Transdermal delivery of macromolecules using solid-state biodegradable microstructures. Pharm Res. 2011;28:22–30. doi: 10.1007/s11095-010-0174-y. [DOI] [PubMed] [Google Scholar]
- 126.Zhu Q., Zarnitsyn V.G., Ye L., Wen Z., Gao Y., Pan L. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DeMuth P.C., Li A.V., Abbink P., Liu J., Li H., Stanley K.A. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol. 2013;31:1082–1085. doi: 10.1038/nbt.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uppu D.S.S.M., Turvey M.E., Sharif A.R.M., Bidet K., He Y., Ho V. Temporal release of a three-component protein subunit vaccine from polymer multilayers. J Control Release. 2020;317:130–141. doi: 10.1016/j.jconrel.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 129.Kommareddy S., Baudner B.C., Oh S., Kwon S.-Y., Singh M., O'Hagan D.T. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101:1021–1027. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 130.Mistilis M.J., Joyce J.C., Esser E.S., Skountzou I., Compans R.W., Bommarius A.S. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 2017;7:195–205. doi: 10.1007/s13346-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mistilis M.J., Bommarius A.S., Prausnitz M.R. Development of a thermostable microneedle patch for influenza vaccination. J Pharm Sci. 2015;104:740–749. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu W., Pewin W., Wang C., Luo Y., Gonzalez G.X., Mohan T. A boosting skin vaccination with dissolving microneedle patch encapsulating m2e vaccine broadens the protective efficacy of conventional influenza vaccines. J Control Release. 2017;261:1–9. doi: 10.1016/j.jconrel.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Littauer E.Q., Mills L.K., Brock N., Esser E.S., Romanyuk A., Pulit-Penaloza J.A. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J Control Release. 2018;276:1–16. doi: 10.1016/j.jconrel.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pattani A., McKay P.F., Garland M.J., Curran R.M., Migalska K., Cassidy C.M. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162:529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boopathy A.V., Mandal A., Kulp D.W., Menis S., Bennett N.R., Watkins H.C. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc Natl Acad Sci U S A. 2019;116:16473–16478. doi: 10.1073/pnas.1902179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moon S., Wang Y., Edens C., Gentsch J.R., Prausnitz M.R., Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Andrianov A.K., DeCollibus D.P., Gillis H.A., Henry H.K., Marin A., Prausnitz M.R. Poly[di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci U S A. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y.C., Chen S.J., Cheng H.-F., Yeh M.K. Development of Yersinia pestis F1 antigen-loaded liposome vaccine against plague using microneedles as a delivery system. J Drug Deliv Sci Technol. 2020;55:101443. [Google Scholar]
- 140.Wang W., Liu H.M., Zhou J., Wang Y.G., Feng X., Tang H. Skin test of tuberculin purified protein derivatives with a dissolving microneedle-array patch. Drug Deliv Transl Res. 2019;9:795–801. doi: 10.1007/s13346-019-00629-y. [DOI] [PubMed] [Google Scholar]
- 141.Edens C., Collins M.L., Goodson J.L., Rota P.A., Prausnitz M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33:4712–4718. doi: 10.1016/j.vaccine.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lanza J.S., Vucen S., Flynn O., Donadei A., Cojean S., Loiseau P.M. A tlr9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int J Pharm. 2020;586:119390. doi: 10.1016/j.ijpharm.2020.119390. [DOI] [PubMed] [Google Scholar]
- 143.Glenn G.M., Taylor D.N., Li X., Frankel S., Montemarano A., Alving C.R. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6:1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 144.Kenney R.T., Frech S.A., Muenz L.R., Villar C.P., Glenn G.M. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 145.Choi H.J., Yoo D.G., Bondy B.J., Quan F.S., Compans R.W., Kang S.M. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33:3756–3769. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin C.J., Allender C.J., Brain K.R., Morrissey A., Birchall J.C. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release. 2012;158:93–101. doi: 10.1016/j.jconrel.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 147.Kim E., Okada K., Kenniston T., Raj V.S., AlHajri M.M., Farag E.A. Immunogenicity of an adenoviral-based middle east respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32:5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di A.E. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Atkinson M.A., Eisenbarth G.S. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 150.Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 151.Owens D.R., Zinman B., Bolli G.B. Insulins today and beyond. Lancet. 2001;358:739–746. doi: 10.1016/S0140-6736(01)05842-1. [DOI] [PubMed] [Google Scholar]
- 152.Zaykov A.N., Mayer J.P., DiMarchi R.D. Pursuit of a perfect insulin. Nat Rev Drug Discov. 2016;15:425–439. doi: 10.1038/nrd.2015.36. [DOI] [PubMed] [Google Scholar]
- 153.Guo X.H., Wang W. Challenges and recent advances in the subcutaneous delivery of insulin. Expet Opin Drug Deliv. 2017;14:727–734. doi: 10.1080/17425247.2016.1232247. [DOI] [PubMed] [Google Scholar]
- 154.Prausnitz M.R., Mitragotri S., Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y.Q., Yu J., Kahkoska A.R., Wang J.Q., Buse J.B., Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. doi: 10.1016/j.addr.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X., Wang L., Yu H.J., Li C.J., Feng J.Y., Haq F. Preparation, properties and challenges of the microneedles-based insulin delivery system. J Control Release. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 157.Xie Y., Xu B., Gao Y.H. Controlled transdermal delivery of model drug compounds by mems microneedle array. Nanomed-Nanotechnol. 2005;1:184–190. doi: 10.1016/j.nano.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 158.Zhou C.P., Liu Y.L., Wang H.L., Zhang P.X., Zhang J.L. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;392:127–133. doi: 10.1016/j.ijpharm.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 159.Chen H.B., Zhu H.D., Zheng J.N., Mou D.S., Wan J.L., Zhang J.Y. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release. 2009;139:63–72. doi: 10.1016/j.jconrel.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 160.Qin G.J., Gao Y.H., Wu Y., Zhang S.H., Qiu Y.Q., Li F. Simultaneous basal-bolus delivery of fast-acting insulin and its significance in diabetes management. Nanomed-Nanotechnol. 2012;8:221–227. doi: 10.1016/j.nano.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 161.Davis S.P., Martanto W., Allen M.G., Prausnitz M.R. Hollow metal microneedles for insulin delivery to diabetic rats. Ieee T Bio-med Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 162.Roxhed N., Samel B., Nordquist L., Griss P., Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. Ieee T Bio-med Eng. 2008;55:1063–1071. doi: 10.1109/TBME.2007.906492. [DOI] [PubMed] [Google Scholar]
- 163.Ross S., Scoutaris N., Lamprou D., Mallinson D., Douroumis D. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv Transl Res. 2015;5:451–461. doi: 10.1007/s13346-015-0251-1. [DOI] [PubMed] [Google Scholar]
- 164.Liu S., Jin M.N., Quan Y.S., Kamiyama F., Katsumi H., Sakane T. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release. 2012;161:933–941. doi: 10.1016/j.jconrel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 165.Chen M.C., Ling M.H., Kusuma S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015;24:106–116. doi: 10.1016/j.actbio.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 166.Ling M.H., Chen M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9:8952–8961. doi: 10.1016/j.actbio.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 167.Yu J.C., Zhang Y.Q., Ye Y.Q., DiSanto R., Sun W.J., Ranson D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu J., Qian C., Zhang Y., Cui Z., Zhu Y., Shen Q. Hypoxia and h2o2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17:733–739. doi: 10.1021/acs.nanolett.6b03848. [DOI] [PubMed] [Google Scholar]
- 169.Ye Y., Yu J., Wang C., Nguyen N.Y., Walker G.M., Buse J.B. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28:3115–3121. doi: 10.1002/adma.201506025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu X.L., Yu J.C., Qian C.G., Lu Y., Kahkoska A.R., Xie Z.G. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11:613–620. doi: 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang J.Q., Ye Y.Q., Yu J.C., Kahkoska A.R., Zhang X.D., Wang C. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12:2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Y.Q., Wang J.Q., Yu J.C., Wen D., Kahkoska A.R., Lu Y. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small. 2018;14 doi: 10.1002/smll.201704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen W., Tian R., Xu C., Yung B.C., Wang G., Liu Y. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat Commun. 2017;8:1777. doi: 10.1038/s41467-017-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu J.C., Wang J.Q., Zhang Y.Q., Chen G.J., Mao W.W., Ye Y.Q. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng. 2020;4:499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen G.J., Yu J.C., Gu Z. Glucose-responsive microneedle patches for diabetes treatment. J Diabetes Sci Technol. 2019;13:41–48. doi: 10.1177/1932296818778607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu S., Wu D., Quan Y.S., Kamiyama F., Kusamori K., Katsumi H. Improvement of transdermal delivery of exendin-4 using novel tip-loaded microneedle arrays fabricated from hyaluronic acid. Mol Pharmaceut. 2016;13:272–279. doi: 10.1021/acs.molpharmaceut.5b00765. [DOI] [PubMed] [Google Scholar]
- 177.Fakhraei Lahiji S., Jang Y., Huh I., Yang H., Jang M., Jung H. Exendin-4-encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci Rep. 2018;8:1170. doi: 10.1038/s41598-018-19789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. 2019. [DOI] [PubMed] [Google Scholar]
- 179.Klevorn L.E., Teague R.M. Adapting cancer immunotherapy models for the real world. Trends Immunol. 2016;37:354–363. doi: 10.1016/j.it.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim N.W., Kim S.Y., Lee J.E., Yin Y., Lee J.H., Lim S.Y. Enhanced cancer vaccination by in situ nanomicelle-generating dissolving microneedles. ACS Nano. 2018;12:9702–9713. doi: 10.1021/acsnano.8b04146. [DOI] [PubMed] [Google Scholar]
- 182.Zeng Q., Gammon J.M., Tostanoski L.H., Chiu Y.C., Jewell C.M. In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater Sci Eng. 2017;3:195–205. doi: 10.1021/acsbiomaterials.6b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tawde S.A., Chablani L., Akalkotkar A., D'Souza M.J. Evaluation of microparticulate ovarian cancer vaccine via transdermal route of delivery. J Control Release. 2016;235:147–154. doi: 10.1016/j.jconrel.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 184.Ye Y.Q., Wang C., Zhang X.D., Hu Q.Y., Zhang Y.Q., Liu Q. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5692. [DOI] [PubMed] [Google Scholar]
- 185.Chablani L., Tawde S.A., Akalkotkar A., D'Souza M.J. Evaluation of a particulate breast cancer vaccine delivered via skin. AAPS J. 2019;21:12. doi: 10.1208/s12248-018-0285-7. [DOI] [PubMed] [Google Scholar]
- 186.Bhowmik T., D'Souza B., Shashidharamurthy R., Oettinger C., Selvaraj P., D'Souza M.J. A novel microparticulate vaccine for melanoma cancer using transdermal delivery. J Microencapsul. 2011;28:294–300. doi: 10.3109/02652048.2011.559287. [DOI] [PubMed] [Google Scholar]
- 187.Ruan W.Y., Zhai Y.H., Yu K.Y., Wu C.B., Xu Y.H. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553:298–309. doi: 10.1016/j.ijpharm.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 188.Duong H.T.T., Yin Y., Thambi T., Kim B.S., Jeong J.H., Lee D.S. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J Mater Chem B. 2020;8:1171–1181. doi: 10.1039/c9tb02175b. [DOI] [PubMed] [Google Scholar]
- 189.Wang C., Ye Y., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 190.Chen G.J., Chen Z.T., Wen D., Wang Z.J., Li H.J., Zeng Y. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc Natl Acad Sci U S A. 2020;117:3687. doi: 10.1073/pnas.1917891117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lopez-Ramirez M.A., Soto F., Wang C., Rueda R., Shukla S., Silva-Lopez C. Built-in active microneedle patch with enhanced autonomous drug delivery. Adv Mater. 2020;32:1905740. doi: 10.1002/adma.201905740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ye Y.Q., Wang J.Q., Hu Q.Y., Hochu G.M., Xin H.L., Wang C. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 193.Chen S.X., Ma M., Xue F.F., Shen S., Chen Q., Kuang Y. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J Control Release. 2020;324:218–227. doi: 10.1016/j.jconrel.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 194.Chen M.L., Quan G.L., Wen T., Yang P.P., Qin W.B., Mai H. Cold to hot: binary cooperative microneedle array-amplified photoimmunotherapy for eliciting antitumor immunity and the abscopal effect. ACS Appl Mater Interfaces. 2020;12:32259–32269. doi: 10.1021/acsami.0c05090. [DOI] [PubMed] [Google Scholar]
- 195.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang N., Mei K., Guan P., Hu X., Zhao Y. Protein-based artificial nanosystems in cancer therapy. Small. 2020:1907256. doi: 10.1002/smll.201907256. [DOI] [PubMed] [Google Scholar]
- 197.June C.H., Warshauer J.T., Bluestone J.A. Is autoimmunity the achilles' heel of cancer immunotherapy? Nat Med. 2017;23:540–547. doi: 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 198.Courtenay A.J., McCrudden M.T.C., McAvoy K.J., McCarthy H.O., Donnelly R.F. Microneedle-mediated transdermal delivery of bevacizumab. Mol Pharmaceut. 2018;15:3545–3556. doi: 10.1021/acs.molpharmaceut.8b00544. [DOI] [PubMed] [Google Scholar]
- 199.GhavamiNejad A., Li J., Lu B., Zhou L.W., Lam L., Giacca A. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon. Adv Mater. 2019;31:1901051. doi: 10.1002/adma.201901051. [DOI] [PubMed] [Google Scholar]
- 200.Naito C., Katsumi H., Suzuki T., Quan Y.S., Kamiyama F., Sakane T. Self-dissolving microneedle arrays for transdermal absorption enhancement of human parathyroid hormone. Pharmaceutics. 2018;10:215. doi: 10.3390/pharmaceutics10040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chi J.J., Zhang X.X., Chen C.W., Shao C.M., Zhao Y.J., Wang Y.G. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact Mat. 2020;5:253–259. doi: 10.1016/j.bioactmat.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu J.C., Zhang Y.Q., Sun W.J., Kahkoska A.R., Wang J.Q., Buse J.B. Insulin-responsive glucagon delivery for prevention of hypoglycemia. Small. 2017;13:1603028. doi: 10.1002/smll.201603028. [DOI] [PMC free article] [PubMed] [Google Scholar]