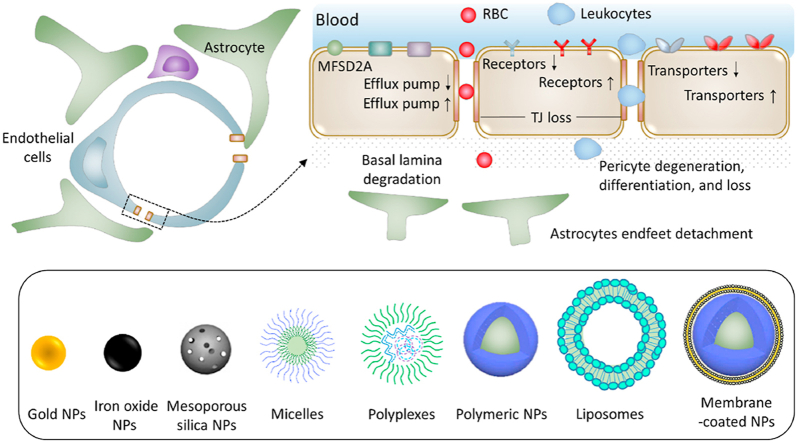
Abstract
Blood–brain barrier (BBB) strictly controls matter exchange between blood and brain, and severely limits brain penetration of systemically administered drugs, resulting in ineffective drug therapy of brain diseases. However, during the onset and progression of brain diseases, BBB alterations evolve inevitably. In this review, we focus on nanoscale brain-targeting drug delivery strategies designed based on BBB evolutions and related applications in various brain diseases including Alzheimer's disease, Parkinson's disease, epilepsy, stroke, traumatic brain injury and brain tumor. The advances on optimization of small molecules for BBB crossing and non-systemic administration routes (e.g., intranasal treatment) for BBB bypassing are not included in this review.
KEY WORDS: Blood–brain barrier, Brain diseases, Brain-targeting, Drug delivery systems, Nanoparticles
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; AMT, alpha-methyl-l-tryptophan; BACE1, β-secretase 1; BBB, blood–brain barrier; BDNF, brain derived neurotrophic factor; BTB, blood–brain tumor barrier; CMT, carrier-mediated transportation; DTPA-Gd, Gd-diethyltriaminepentaacetic acid; EPR, enhanced permeability and retention; Gd, gadolinium; GLUT1, glucose transporter-1; ICAM-1, intercellular adhesion molecule-1; KATP, ATP-sensitive potassium channels; KCa, calcium-dependent potassium channels; LAT1, L-type amino acid transporter 1; LDL, low density lipoprotein; LDLR, LDL receptor; LFA-1, lymphocyte function associated antigen-1; LRP1, LDLR-related protein 1; MFSD2A, major facilitator superfamily domain-containing protein 2a; MMP9, metalloproteinase-9; MRI, magnetic resonance imaging; NPs, nanoparticles; PD, Parkinson's disease; PEG, polyethyleneglycol; PEG-PLGA, polyethyleneglycol-poly(lactic-co-glycolic acid); P-gp, P-glycoprotein; PLGA, poly(lactic-co-glycolic acid); PSMA, prostate-specific membrane antigen; RAGE, receptor for advanced glycosylation end products; RBC, red blood cell; RMT, receptor-mediated transcytosis; ROS, reactive oxygen species; siRNA, short interfering RNA; TBI, traumatic brain injury; TfR, transferrin receptor; TJ, tight junction; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor; ZO1, zona occludens 1
Graphical abstract
Blood–brain barrier (BBB) is evolving during the onset and progression of brain diseases. BBB evolution-based nanoscale brain-targeting drug delivery strategies are summarized in this review for various brain diseases.
1. Introduction
The blood–brain barrier (BBB) strictly controls the transport of various substances from blood to brain1,2. Most drugs are ineffective for brain diseases because their extremely low BBB penetration efficiency is hard to reach therapeutic concentration in brain parenchyma for effective treatment. Various kinds of highly-expressed receptors and transporters on the BBB play the critical roles in supplying brain with essential energy and nutrients3, 4, 5, 6, 7. This physiologic function endows receptor-mediated transcytosis (RMT) and carrier-mediated transportation (CMT) with the potential promising role in brain-targeting drug delivery7,8. Nanotechnology has been widely and extensively applied in drug delivery because of the exceptional features in increasing drug loading and stability and reducing side effects and so on9, 10, 11. Combining nanotechnology with RMT and CMT holds the potential to boost BBB penetration for systemic brain-targeting drug delivery. In the past two decades, specific ligands and substrates of these receptors and transporters have been extensively used to enhance brain-targeting delivery efficiency3,4.
Pathological BBB breakdown with structural and functional changes, happens in brain regions affected by almost all brain diseases, such as chronic Alzheimer's disease (AD) and Parkinson's disease (PD), acute ischemic stroke and traumatic brain injury (TBI), epileptic seizures and brain tumor12,13. These vascular changes often include up-regulation of luminal adhesion molecules, increased adhesion and transmigration of leukocytes, reduced expression of tight junction (TJ), increased endothelial transcytosis, disrupted expression of receptor and transporter, endothelial degeneration, pericyte degeneration, perivascular accumulation of toxic products, inflammation and immune response12,14. It's remarkable that BBB evolutions are often throughout the onset and progression of brain diseases15. However, it remains uncertain whether BBB dysfunction is one of the initial events that lead to the brain diseases, or a downstream consequence13. For example, because the BBB plays a critical role in maintaining brain homeostasis, the disturbance of proper BBB functioning is increasingly recognized as a potential contributor in pathogeneses of neurodegenerative neurological diseases, including AD and PD16. On the one hand, BBB disruption allows influx into the brain of neurotoxic blood-derived debris, cells and microbial pathogens and is associated with inflammatory and immune responses, which can initiate multiple pathways of neurodegeneration12. On the other hand, inflammatory cytokines and chemokines are involved in cleavage, downregulation and spatial redistribution of junction proteins, leading to the function loss of TJ. The BBB evolutions or alterations affect most drug's brain accumulation and intracranial distribution. Disease-initiated BBB breakdown might present an opportunity to deliver treatments to affected neurons. Therefore, drug delivery through the diseased BBB for brain diseases are different and more complex than through the normal BBB. An attempt has been made in this review to briefly introduce the BBB evolutions in various brain diseases and the related strategies for designing nanoscale brain-targeting drug delivery systems.
2. Normal BBB and diseased BBB
2.1. Normal BBB
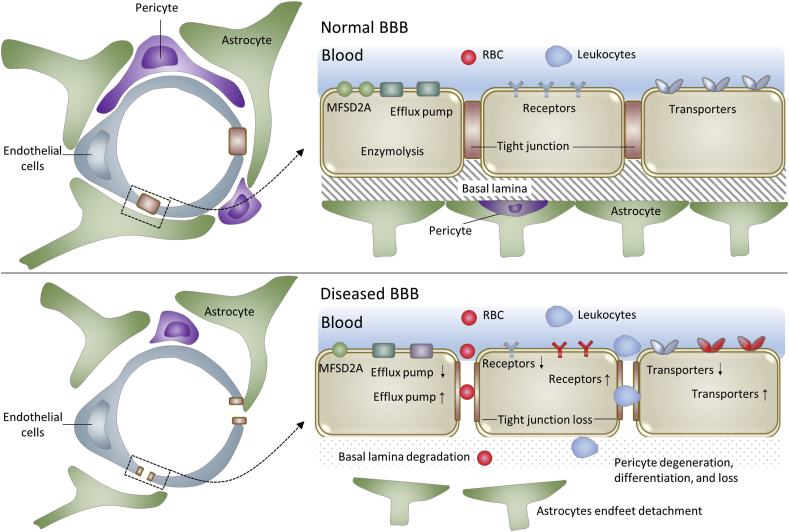
The BBB is a selective barrier between blood circulation and brain and formed by a monolayer of tightly sealed endothelial cells, together with pericytes and astrocytic perivascular endfeet17, 18, 19 (Fig. 1). In contrast to the highly permeable peripheral capillaries, brain capillary endothelial cells or BBB endothelial cells express various TJ proteins and major facilitator superfamily domain-containing protein 2a (MFSD2A). On the one hand, TJ proteins impede the paracellular diffusion of most molecules, creating a paracellular physical barrier and extremely low paracellular permeability17. On the other hand, MFSD2A-regulated low transcytosis rate20, 21, 22, 23, 24, endows the BBB with the transcellular transcytosis barrier. Through both paracellular and transcellular barrier, the BBB strictly regulates both paracellular and transcellular transfer of various substances from blood to brain1,2. Transport systems at the BBB include free diffusion, absorptive transcytosis, RMT and CMT. Taking free diffusion as an example, BBB is only permeable to small molecules with strictly specified structure, e.g., MW < 400 Da threshold and high lipid solubility25,26. Through these precisely controlled transport pathways together with intra-endothelial enzymolysis and active efflux27,28, BBB tightly controls the chemical composition of brain interstitial fluid for proper synaptic functioning, information processing and neuronal connectivity12. Besides neurotoxic plasma components, blood cells and pathogens18, BBB also prevents the entry of most drug molecules to protect the brain25,29. Therefore, most drug treatments are ineffective for brain diseases because of the unavailability of efficient BBB penetration to reach therapeutic concentration in brain parenchyma.
Figure 1.
Schematic drawings of the blood–brain barrier (BBB) in normal brain and the diseased BBB in most brain diseases.
Nevertheless, brain is the most energy-consuming organ in the body. The human brain receives 20% of cardiac output and consumes 20% of total oxygen, glucose and energy under physiological conditions for proper functioning of the brain30, 31, 32, although representing only 2% of total body mass. The human brain contains ∼644 km of blood vessels in total12. Capillaries account for approximately 85% of cerebral vessel length and provide ∼12 m2 of the endothelial surface area available for transport exchanges. The mean intercapillary distance in human brain is about 40 μm33, making the equilibration of solutes almost instantaneous throughout the brain interstitial space once they cross the BBB. BBB plays the lion's share in providing the energy and nutrients including amino acids, peptides, sugars, vitamins, ferric ion, nicotinic acetylcholine and low-density lipoprotein (LDL) to the brain7,34,35. Various kinds of highly expressed receptors and transporters on the BBB play the critical roles in supplying brain with these essential nutrients3, 4, 5, 6, 7. These highly enriched receptors and transporters at the BBB include transferrin receptor (TfR), LDL receptor (LDLR) and glucose transporter-1 (GLUT1) and so on. These receptors and transporters use active transport and facilitative transport to transfer nutrients and energy34,36. Therefore, these receptors and transporters are promising for brain-targeting drug delivery7,8.
2.2. Diseased BBB
Generally, the BBB dysfunctions in various brain diseases, include increased permeability from loss of TJ and enhanced transcytosis rate from loss of MFSD2A and/or the increase of caveolin-1 expression (Fig. 1)20,37,38, weaken or strengthened CMT and RMT functions [e.g., impaired glucose transport and active efflux from reduction of GLUT1 and P-glycoprotein (P-gp) and dysregulated blood-to-brain transport of amyloid beta (Aβ) from increased receptor for advanced glycosylation end products (RAGE) in AD], microbleeds, perivascular deposits of blood-derived products, cellular infiltration and degeneration of pericytes and endothelial cells12.
Drug delivery across the diseased BBB is different and more complex than across the normal BBB. BBB alterations affect drugs’ brain accumulation and intracranial distribution in both stimulative and suppressive ways. From the stimulative perspective, BBB alterations, especially increased paracellular permeability from loss of junction proteins, present an opportunity to deliver drugs, which would normally be excluded by healthy BBB, to the diseased brain regions and affected neurons12,15. However, from the suppressive perspective, influx of blood-borne molecules and cells interferes with the normal solute diffusion in brain interstitial fluid, resulting in impaired drug distribution throughout the brain. Therapeutic agents are likely to get trapped in pathologically altered and enlarged perivascular spaces, preventing them from reaching a little distant neuronal targets12. Solute diffusion in regions with BBB disruptions would become poor, preventing drugs from reaching areas of the brain more than a few hundred microns from the lesion39. Paradoxically, BBB disruptions even retard brain retention of some drugs39, because the efflux transporter for potassium is so robust that BBB disruption does not alter its concentration in the cerebrospinal fluid40. Therefore, BBB dysfunction can improve or impede drug delivery across the BBB to brain.
2.2.1. AD
AD is the most common neurodegenerative disorder and contributes to ∼70% of dementia cases15,41. Cortical and cerebrovascular extracellular deposits of Aβ and the accumulation of intracellular neurofibrillary tangles which are formed by the aggregation of hyperphosphorylated microtubule-associated tau protein are the main histopathological features of AD42. In behavior, AD patients display loss of spatial and temporal orientation and verbal fluency.
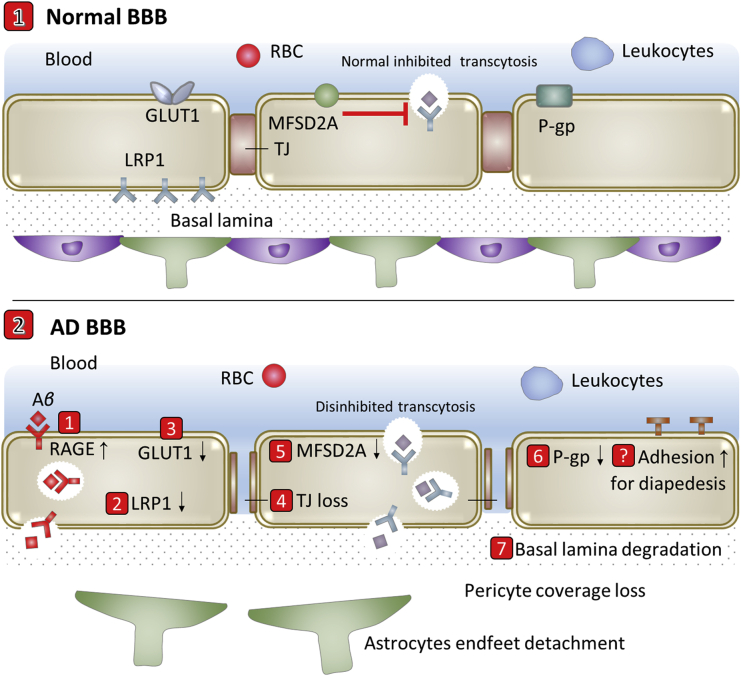
Under physiological conditions, endogenous Aβ is transported bidirectionally across the BBB and has the ability to promote memory39. Influx receptor RAGE, efflux receptor LRP1 and transporter P-gp act in tandem to maintain the brain levels of Aβ at its most optimal concentration43, 44, 45, 46, 47. Aβ accumulation in brain with AD is implicated in BBB evolutions. Under AD conditions, both increased blood-to-brain transport by up-regulated RAGE and decreased efflux by reduced LRP1 and P-gp enhance Aβ accumulation in brain with AD39. It has been proved in animal models that BBB evolutions occur predominantly in microvessels that are surrounded by plaques48. In addition, reduced GLUT1 at the BBB leads to GLUT1 deficiency, early cerebral microvascular degeneration, blood flow reductions and further BBB breakdown, which can worsen AD cerebrovascular degeneration, neuropathology and cognitive function49. And the transcytosis rate increases in AD by the loss of pericyte coverage12,14, through the possible MFSD2A decrease or caveolin-1 increase on BBB endothelial cells. Therefore, BBB evolutions are involved with the pathogenesis and pathology of AD12,15,16,18,39,50, 51, 52. The transport-related BBB evolutions include but are not limited to 1) RAGE up-regulation, 2) LRP1 reduction, 3) GLUT1 reduction, 4) TJ loss, 5) possible MFSD2A decrease or caveolin-1 increase, 6) P-gp reduction and 7) basal lamina degradation by matrix metalloproteinase-9 (MMP9)12. In addition, the expression of adhesion molecules may be increased for diapedesis of leukocytes (Fig. 2).
Figure 2.
Scheme of transport-related BBB evolutions in Alzheimer's disease (AD).
2.2.2. PD
PD is the second most common neurodegenerative disorder. PD is characterized by accumulation of α-synuclein and degeneration of dopaminergic neurons12. The α-synuclein is pathologically aggregated into Lewy bodies or cytoplasmic eosinophilic inclusions53. The formation of Lewy bodies is firstly at the brain stem and olfactory bulb and later at cortex and nigra and can be observed in most neuronal populations in PD patients. This ultimately leads to the dysfunctioning of neurotransmitter systems such as adrenergic, cholinergic and serotonergic. The loss of dopamine happens mainly in regions from the substantia nigra pars compacta to the corpus striatum41,50, resulting in bradykinesia, rigidity and resting tremor, as dopamine is involved in the transmission of electric signals to the normal physical motion.
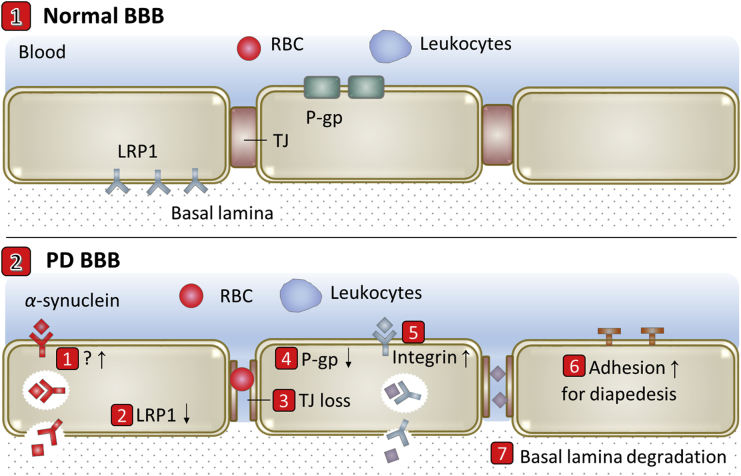
BBB evolutions are a critical feature of PD pathology and occur throughout the basal ganglia of PD patients18. Vascular dysfunction consisting of BBB breakdown and dysfunction, leads to neurotoxic accumulation of fibrin (ogen), thrombin, plasmin (ogen) and red blood cell (RBC) extravasation, and release of hemoglobin and iron causing reactive oxygen species (ROS), which all can injure dopaminergic neurons18. Lee et al.54 reviewed how BBB evolutions promote the onset and development of PD and how PD compromise BBB. The pathway for mediating the BBB crossing of the α-synuclein and for contributing the α-synuclein pool in the brain may be increased18. Endothelial LRP1 is a potential efflux transporter for α-synuclein and may be similarly downregulated in PD as in AD55, 56, 57. Oxidative stress has been identified as a putative molecular contributor to PD pathogenesis and implicated as harmful to BBB by apoptosis inducing and MMP9 activity increasing54. Chen et al.58 revealed reduced TJ proteins zona occludens 1 (ZO1) and occludin in the striatum in PD mouse model. Kortekaas et al.59 found higher accumulation of verapamil by reduced P-gp in PD brain. Several other studies reported angiogenic activity in PD-affected regions (e.g., basal ganglia)54. By summarizing current existing reports, transport-related BBB evolutions in PD include but are not limited to 1) up-regulation of unknown pathway for BBB crossing of α-synuclein, 2) LRP1 reduction, 3) TJ loss, 4) P-gp reduction, 5) possible integrin increase for angiogenesis, 6) the possible increased expression of adhesion molecules for diapedesis of leukocytes and 7) basal lamina degradation by MMP9 (Fig. 3).
Figure 3.
Scheme of transport-related BBB evolutions in Parkinson's disease (PD).
2.2.3. Epilepsy
Epilepsy is a chronic neurological disorder manifesting as spontaneous, recurrent and unpredictable seizures resulting from transient abnormal electrical activity in the brain15,60. The main causes of epilepsy include infection progression in the brain, reduced oxygen supply, head trauma, arteriovenous malformations, cerebrovascular diseases and perinatal injuries50. The impact of epilepsy is multidimensional and extends far beyond the harm induced by seizures themselves61. The unpredictability of seizures imposes severe lifestyle restrictions on patients with epilepsy, resulting in significant impairments in psychological, emotional, economical and/or social spheres61.
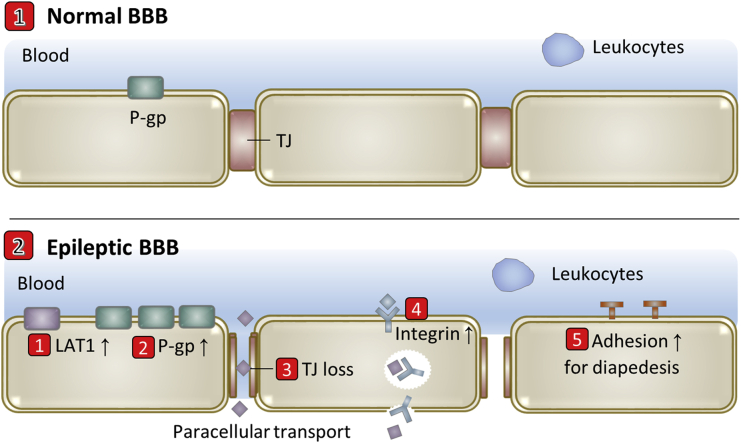
L-Type amino acid transporter 1 (LAT1) is up-regulated due to the huge consumption for tryptophan in epileptogenic focus for the aberrant activation of kynurenine pathway to synthesize kynurenic acid and quinolinic acid62. Specific up-regulation of multidrug efflux transporter P-gp is another important BBB evolution in epilepsy61, 62, 63, 64. Loss of TJ and increased microvascular density were also observed in temporal lobe epilepsy18. These BBB dysfunctions are localized to epileptic regions and positively correlates with seizure frequency, suggesting that BBB plays a contributory role in epileptic disorders. Neuroinflammation is also a hallmark of epileptogenic brain tissue65, during which leukocytes are extensively recruited and trafficked into the brain parenchyma by chemotaxis and diapedesis66. By summarizing current existing reports, transport-related BBB evolutions in epilepsy include but are not limited to 1) LAT1 up-regulation, 2) P-gp up-regulation, 3) TJ loss, 4) possible integrin increase for angiogenesis and 5) increased expression of adhesion molecules for diapedesis of leukocytes (Fig. 4).
Figure 4.
Scheme of transport-related BBB evolutions in epilepsy.
2.2.4. Stroke
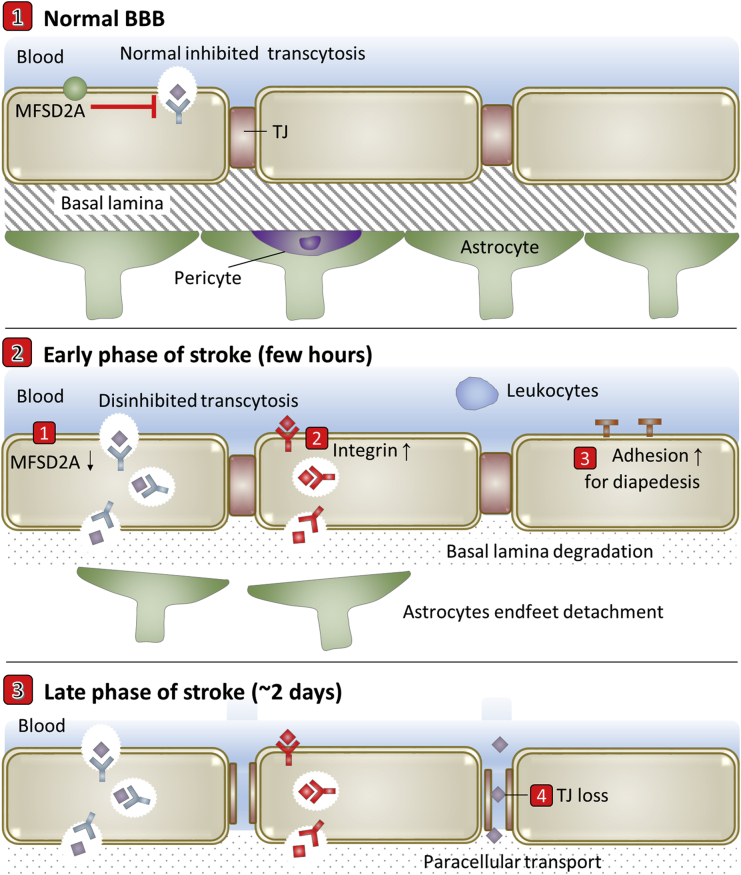
Stroke, secondary to occlusion of cerebral vessels, is a leading cause of death and disability worldwide67. The ischemic cascade involves necrotic cell death, apoptotic cell death and inflammation because neurons are very vulnerable to reduction in supply of oxygen and glucose from reduced cerebral blood flow. Reperfusion of blood to ischemic brain is the only option to reverse brain damage after a stroke. At present, restoration of blood flow via fibrinolytic tissue plasminogen activator (tPA) is the only approved clinical treatment for stroke. However, besides the short therapeutic window of 3–4.5 h post-onset68, tPA activates different pathways for detachment of astrocytes endfeet, MMP9-mediated degradation of basal lamina15, and TJ loss, contributing to BBB alterations.
Biphasic BBB breakdown before neurological damage has been proved in both human and animal ischemia models69. As shown in Fig. 5, the most accepted model of this biphasic BBB hyperpermeability is characterized by (a) an early phase (occurring a few hours poststroke) of enhanced transcellular transport mediated by caveolin-1 increase20 and possible MFSD2A decrease, followed by (b) a delayed phase of hyperpermeability (∼2 days poststroke) in which both enhanced transcellular transport and TJ protein disassembly contribute to the loss of BBB integrity20,67. The ischemia-induced BBB degradation leads to blood shift to the ischemic brain, with the risk of developing edema and hemorrhagic transformation70,71. The reperfusion also causes neuroinflammation response72, during which the up-regulated adhesion molecules at brain capillary endothelial cells strongly interact with activated leukocytes, leading their entry into brain15. The processes involve capture, rolling, activation, adhesion, crawling and trans- or para-cellular diapedesis. In addition, integrin αvβ3 is up-regulated on reactive brain capillary endothelial cells for angiogenesis after ischemia73.
Figure 5.
Scheme of transport-related BBB evolutions in stroke.
2.2.5. TBI
TBI produces primary physical damage to neurons and vessels, leading to loss of neuron function and hemorrhage. The release of intracellular contents from disrupted neurons and the influx of blood components cause secondary injury including ionic imbalance, cytotoxicity, free radical production, oxidative stress, inflammation and further BBB breakdown. These responses lead to vasogenic/osmotic edema for hours and days, resulting in lifelong physical, cognitive and psychosocial impairments74,75. The primary injury appears to be irreversible, making the focus of therapeutic strategies on neuroprotective approaches that suppress progressive cell death and tissue damages during the secondary injury74.
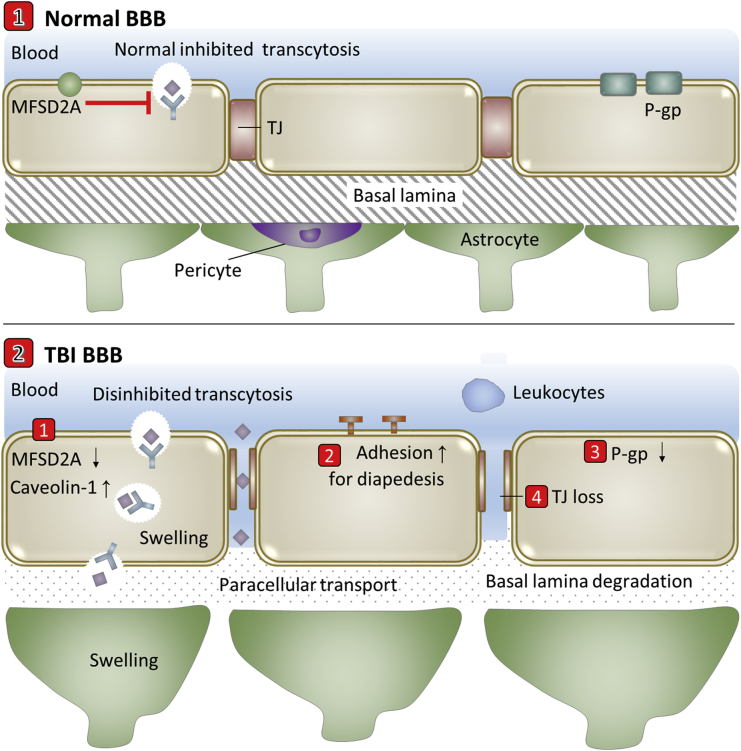
Besides the direct physical damage to vessels by TBI, the hemorrhage and edema lead to further damages to BBB at the lesion site and neighboring regions74. The transcytosis rate increases after TBI, through caveolin-1 increase and possible MFSD2A decrease from pericyte deficiency (Fig. 6). Neuroinflammation involves release of cytokines and chemokines, which activate endothelial cells to up-regulate adhesion molecules to recruit neutrophils through the decreased TJ and degraded extracellular matrix. Changes of molecular expression undergone on neurovascular cells (e.g., down-regulation of P-gp) have been extensively reviewed37,38,76. Oxidative stress and neuroinflammation induce expression of MMPs, which degrade extracellular matrix and TJ37. Neuroinflammation promotes the upregulation of the water channel aquaporin 4 and contributes to swelling of endothelial cells and astrocytic end-feet37,74. It is noteworthy that most of these processes are interconnected and happen simultaneously.
Figure 6.
Scheme of transport-related BBB evolutions in traumatic brain injury (TBI).
It is noteworthy that endothelial cells recover vascular integrity to brain and angiogenesis occurs to support the newly formed scar and connective tissues after astrocytes reestablish glial limitans surrounding the injury tissue. The recovery reestablishes the BBB in the injured brain that revives the inherent problems of drug delivery in the chronic phase following TBI74. Studies in rats with controlled cortical impact have shown that claudin-5 was decreased early at three days after injury but increased when P-gp was upregulated one week after injury38. In addition, the recovery of disrupted BBB appears to be highly variable depending on injury types, severity and complexity.
2.2.6. Brain tumor
Brain tumor is a life-threatening brain disease with a relatively low survival rate77. Glioblastoma, the most invasive brain tumor, is characterized by the highest mortality rate, short lifetime and poor prognosis with a high tendency of recurrence. Surgery is often applied to excise the tumor bulk, but the infiltrating tumor cells inside the normal brain parenchyma cannot be completely removed. On the other hand, these residual tumor cells are protected against drug therapy by the BBB, leading to the tumor recurrence78.
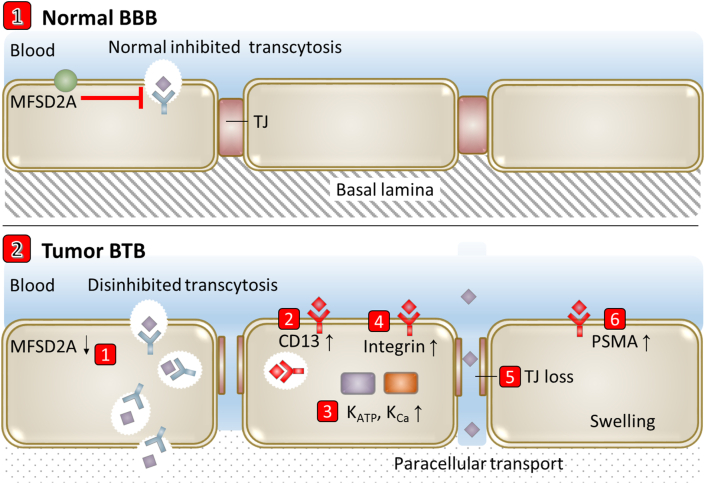
The progression of brain tumor leads to BBB changes including astrocyte endfeet displacement and heterogenous subpopulation of pericyte and astrocyte, forming highly heterogeneous and compromised BBB79. The BBB in brain tumor is often termed as blood–brain tumor barrier (BTB), with different structural and functional characteristics to the BBB (Fig. 7). The transcytosis rate increases on BTB through the decrease expression of MFSD2A80. Permeability of the BTB to drugs or contrast agents was higher but more heterogeneous than that of the BBB81. ATP-sensitive potassium channels (KATP) and calcium-dependent potassium channels (KCa) are specially up-regulated on BTB endothelial cells to regulate BTB permeability82. As a transmembrane protein with internalization function, prostate-specific membrane antigen (PSMA) is specifically expressed on "breast cancer brain metastases-associated" BTB endothelial cells83, 84, 85, 86. Downregulation of ZO1 and vascular-endothelial cell adhesion molecule (VE-CAM) leads to the loss of continuous endothelial cell adhesions, creating abnormal molecular permeation channels81. The BTB was also found to differ from the BBB by: the presence of swollen capillary endothelial cells; abundant vascular endothelial growth factor (VEGF); a prominent local neuroinflammatory milieu consisting of activated microglia, astrocytes and immune cells; an altered basement membrane composition; shifts in subpopulations of pericytes; and loss of astrocyte endfoot polarization of aquaporin channels81. Other molecular alterations reported in BTB endothelial cells that contribute to increased permeability include altered expression of membrane transporters (GLUT1 and breast cancer resistance protein87), cytokine receptors such as the receptor for tumor necrosis factor and membrane proteins and growth factors including claudin-5 and angiopoietin 281.
Figure 7.
Scheme of transport-related BBB evolutions in brain tumor.
2.2.7. MFSD2A decrease in different brain diseases
In the brain, MFSD2A is exclusively expressed at luminal side of BBB21,31. Pericytes are required for MFSD2A expression88. MFSD2A transports lysophosphatidylcholine esterified docosahexaenoic acid to supply the brain with the essential omega-3 fatty acid22. Of particular note is that MFSD2A is required for proper BBB development and functional integrity21,31,32. MFSD2A suppresses caveolae-mediated transcytosis to regulate BBB permeability via controlling lipid composition of BBB endothelial cells21,31,32. In addition, pericytes can regulate vesicular transport at the BBB via regulating MFSD2A expression31.
MFSD2A decrease leads to increase of vesicular transport in the BBB79,89. The response of MFSD2A to pathological processes has been revealed in some brain diseases90, for example, MFSD2A decrease in the perihematomal tissue on mice model of intracerebral hemorrhage and subarachnoid hemorrhage89,91. MFSD2A decrease was also shown on disrupted brain vascular endothelium in brain metastatic tumors79,80. In addition, increased transcytosis in the BBB of AD, ischemic stroke and TBI12,20,37,38, suggests the possible MFSD2A decrease at the BBB besides the increase of caveolin-1 expression. For now, there is no report on directly comparing the degree of MFSD2A decrease in different brain diseases. The involvement and decrease extent of MFSD2A in different neurological diseases deserve further investigation92.
2.2.8. TJ loss in different brain diseases
Almost all brain diseases are involved with loss of TJ. However, for TJ loss, difference of cause, loss extent, time, and location exists between different brain diseases, especially between acute and chronic diseases of the brain. AD and PD belong to chronic neurodegenerative disorders while TBI and stroke are acute brain insults93,94. For AD, endothelial GLUT1 deficiency initiates early BBB disruption as represented by the reduction in TJ proteins in mice16,49. RAGE-mediated Aβ cytotoxicity contributes to disruption of TJ via Ca2+-calcineurin signaling52. The length of TJ in mice with 5 familial AD mutations is significantly shorter than that in littermate control mice16,18. In addition, the TJ loss in AD is mainly located in the cortex and hippocampus12. For PD, α-synuclein accumulation may be an important contributory event in the pathogenesis18. The reduction of TJ proteins in PD is located in the basal ganglia12,18,54.
Acute brain diseases often display relatively instant loss of TJ from the angle of time. For epilepsy, TJ loss was observed early after status epilepticus as well as during latent and chronic periods, and the permeability increase is positively correlated to seizure frequency95. Both similarities and differences on loss of TJ exist between TBI and ischemic stroke96. For TBI, the BBB permeability to macromolecules are biphasic on rat TBI models, peaking at 4–6 h and Day 2–3 after injury76. The expression of claudin-5 rises again 1–2 weeks after injury and remain elevated as much as 4–8 weeks after initial injury. For stroke, TJ remains normal even after 25 h of cerebral ischemia after embolic middle cerebral artery occlusion and display profound structural defects 2 days after ischemia20. Active remodeling of TJ may occur in the late phase of reperfusion when angiogenesis of brain vessels begins. For brain tumor, tumor cells associate with blood vessels and insert themselves between the endfeet and the endothelial wall of the preexisting blood vessel to alter astrocyte-vascular interactions at the preexisting vasculature. The loss of contact between endfeet and blood vessels leads to loss of TJ. Importantly, single glioma cells are sufficient to locally open the BBB97. In addition, VEGF produced in brain tumors, can promote endocytosis of the endothelial cell adhesion molecule VE-cadherin, leading to BBB disruption and increase in endothelial permeability98.
The optimized size of nanocarriers based on the opening of BBB gaps for different brain diseases was discussed in “Section 4.2. TJ loss based strategy”. For now, the comprehensive characterization and quantitative comparison of “dynamic” TJ loss between different brain diseases depend on the disease progression stage, the established animal model and the individual difference. Liu et al.13 previously compared BBB permeability between ischemic stroke and TBI on animal models and found that the BBB dysfunction in cold-injury-induced TBI was more serious than that in ischemic stroke. However, the related studies are still in their infancy and thus need to be extensively and intensively researched. The design of a kind of effective, acceptable and quantitative index will be helpful.
3. Nanoscale drug delivery systems
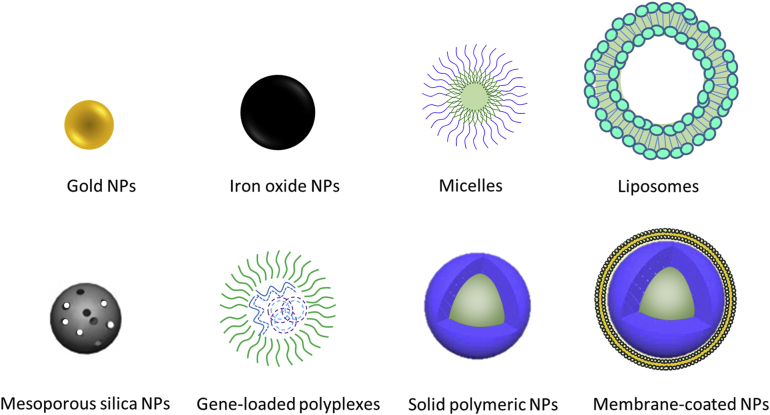
Over the past few decades, the rapid expansion of nanotechnology has begun to be developed for biological and medical applications. In drug delivery field, nanoscale drug delivery systems have already started to exhibit immense potential due to many incomparable advantages. Such advantages include but are not limited to decreased side effects, prolonged blood circulation, and increased drug stability, bioavailability and targeting efficiency10,11. Besides, versatile nanocarriers could simultaneously carry imaging agents, therapeutic drugs and targeting ligands, which is beneficial for tracing drug distribution, activating drug release from an external stimulus (e.g., laser light, temperature, or ultrasound), and theoretically detecting potentially dangerous drug accumulation. Based on all these advantages, nanoscale drug delivery systems have found renowned attention in tumor-targeting imaging and drug therapy99. Due to the rapid expansion of nanotechnology, various types of nanocarriers have been developed, for example, liposomes, micelles and various nanoparticles (NPs, Fig. 8).
Figure 8.
Various types of NPs developed for drug delivery.
Nanoscale brain-targeting drug delivery systems have great perspectives for drug management of brain diseases. For this application, the nanocarriers should be capable of overcoming the BBB, and improving diagnostics and drug therapy efficiency. With subtle design, the functionalization could assist the translocation of nanocarriers across the BBB and provide satisfactory BBB permeation100. Endogenous and exogenous specific ligands and substrates have been extensively used to enhance the efficiency of BBB crossing and brain-targeting delivery via binding with specific CMT or RMT on the BBB3,4,101,102. For example, transferrin, lactoferrin, glucose, polysorbate 80 and anti-TfR antibodies have been developed as brain targeting ligands and have shown high efficiency in amplifying BBB penetration and brain delivery of nanocarriers41,103, 104, 105, 106, 107, 108, 109, 110. Nanocarriers can also be functionalized by loading BBB permeability modulators (e.g., lexiscan) and P-gp inhibitors and conjugating with cell penetrating ligands, to regulate drug diffusion through paracellular barrier, inhibit active efflux, and utilize adsorptive-mediated transcytosis, respectively111, 112, 113. The capacity to cross the BBB offers new ways for drug delivery to brain, which remain much needed in brain diseases. Nazem and Mansoori reviewed the promise of nanotechnology on the diagnosis and therapy of AD and other neurodegenerative diseases114. Kubinova and Sykova115 summarized applications of nanocarriers for therapy of brain stroke and spinal cord injury.
4. Diseased BBB-based brain-targeting delivery strategies
Because of BBB evolutions in brain diseases, drug delivery through the diseased BBB is different and more complex than through the normal BBB. However, BBB crossing strategies can be correspondingly designed against the altered BBB. According to the general BBB alterations in brain diseases, the following strategies have been reported or remain to be explored to specially deliver drug across the diseased BBB.
4.1. Evolutionary RMT and CMT based strategy
In the brain, MFSD2A selectively transports the omega-3 fatty acid docosahexaenoic acid across the BBB21,22,80. More importantly for drug delivery, MFSD2A suppresses caveolin-dependent transcytosis on the BBB, with genetic deletion of murine MFSD2A leading to enhanced transcellular transport and breakdown of the vascular endothelial barrier in the brain23,116. It has been reported that the expression of MFSD2A on brain capillary endothelial cells is significantly reduced in brain regions affected by intracerebral hemorrhage, subarachnoid hemorrhage and metastatic brain tumors79,80,89,91. Therefore, RMT-based ligand-modified brain-targeting delivery strategies should have enhanced crossing efficiency for diseased BBB than normal BBB. In addition, transcytosis of ligand-modified nanocarriers via RMT is likely to be trapped within brain capillary endothelial cells due to the high binding affinity of ligand with receptors, which greatly reduces the amount of nanocarriers across BBB117,118. Acid-responsive dissociation of brain-targeting ligands in endo/lysosomes was previously reported with high efficiency in improving escape from endo/lysosome and transcytosis across the BBB117,118. The increased transcytosis by MFSD2A decrease holds the potential to combine with the promising acid-responsive ligand dissociation strategy for obtaining significantly improved brain-targeting efficiency.
As listed in Table 1, the up-regulated RAGE in AD endows the decoration of RAGE-specific ligand on nanocarriers with the ability of mediating crossing of BBB associated with AD. Integrin receptors for angiogenesis are increased on reactive brain capillary endothelial cells in PD, ischemia and brain tumors54,73,119, which makes it feasible to use related ligands to mediate the crossing of the diseased BBB in these lesions. Epilepsy-specific overexpression of multidrug efflux transporters, such as P-gp, reduces intracranial concentrations of antiepileptic drugs62, 63, 64, and should be emphasized when designing drug delivery systems for epilepsy. The reduced GLUT1 and LDLR-related protein 1 (LRP1) on the BBB in AD may need to be reversed to strengthen the BBB crossing efficiency of glucose-modified nanocarriers or angiopep-2-modified nanocarriers. BTB in brain tumors is often induced by tumor to express PSMA, CD13, integrin receptors, KATP and KCa119, 120, 121, 122, 123, which can be utilized to mediate the crossing of the barrier in brain tumor regions. Some typical brain-targeting drug delivery systems based on up-regulated targets in different brain diseases are listed in Table 265,73,82,120,124, 125, 126.
Table 1.
Transport-related molecular changes in various brain diseases.
| Type of brain diseases | Transport-related BBB molecular change |
|---|---|
| AD | ↓ MFSD2A (possible), ↑ Caveolin-1 (possible), ↑ Adhesion molecules, ↑ RAGE, ↓ LRP1, ↓ GLUT1, ↓ P-gp |
| PD | ↑ α-Synuclein pathway, ↓ LRP1, ↑ Integrin, ↓ P-gp |
| Epilepsy | ↑ LAT1, ↑ P-gp, ↑ Integrin |
| Stroke | ↓MFSD2A (hemorrhagic), ↑ Caveolin-1, ↑ Integrin |
| TBI | ↓MFSD2A (possible), ↑ Caveolin-1, ↑ Adhesion molecules, ↓ P-gp |
| Brain tumor | ↓MFSD2A, ↑ PSMA, ↑ CD13, ↑ Integrin, ↑ KATP, ↑ KCa |
Table 2.
Typical brain-targeting drug delivery systems based on diseased BBB in different brain diseases.
| Brain disease | Up-regulated target on diseased BBB | Ligand | Example | Ref. |
|---|---|---|---|---|
| AD | RAGE | KLVFFAED | A reactive oxygen species (ROS)-responsive curcumin-loaded polymeric micelle to normalize the oxidative and inflammatory microenvironment and reeducate microglia through ROS scavenging and Aβ inhibition. | 124 |
| Epilepsy | Adhesion molecules | Monocytes | Monocytes for delivery of magnetite-laden NPs to epileptogenic brain tissue to delineate inflammation in epilepsy. | 65 |
| Stroke | Integrin αvβ3 | Cyclo(Arg-Gly-Asp-d-Tyr-Lys) c(RGDyK) | The engineered c(RGDyk)-conjugated exosomes to deliver curcumin to ischemic region to suppress inflammatory response and cellular apoptosis in the lesion region. | 73 |
| Stroke | Adhesion molecules (e.g., P-selectin & ICAM-1) | Neutrophils | Neutrophil membrane-derived nanovesicles loaded with resolvin D2 to enhance resolution of inflammation to protect brain damage during ischemic stroke. | 125 |
| Glioma | CD13 | Cyclo(CRNGRGPDC) iNGR | The modified complexes between polycation pOEI and siRNA nanospheres for delivery of RNA interference oncotherapy to glioma. | 120 |
| Glioma | Integrin αvβ3 | c(RGDyK) | The modified red blood cell membrane-coated drug nanocrystals for effective drug delivery to glioma. | 126 |
| Brain metastases | KATP | Loaded minoxidil | The minoxidil-loaded hyaluronic acid-tethered NPs can boost transcytosis across the BTB through strengthening activation of up-regulated KATP on BTB to deliver doxorubicin to brain metastases. | 82 |
4.2. TJ loss-based strategy
If impaired drug diffusion inside the brain was not a consideration, any loss of TJ in the BBB would improve delivery of drugs, especially nanocarriers, to the brain. Nanocarriers allow rapid accumulation in damaged brain regions with TJ loss, while still being large enough to be retained in these regions, not diffusing away as quickly as smaller molecules127. This phenomenon is much similar to the enhanced permeability and retention (EPR) effect seen in tumors128,129. More importantly, the size of nanocarriers should be optimized for different brain diseases and diseases at different stages because of the different leaky gaps at the diseased BBB. Based on the TJ loss in different brain diseases, the size of nanocarriers should be between the normal BBB pore size (1.4–1.8 nm) and the opening of TJ in different brain diseases66,130. Generally, the space between TJ is narrower than that between adherent junctions (∼20 nm)88. There is limited quantitative characterization on the extent of BBB opening at different stages in AD, PD and epilepsy. However, it is estimated that even under severe pathological conditions, only nanocarriers with size smaller than 20 nm can penetrate the BBB66. Amyloid oligomer-specific scFv antibody-modified polyethyleneglycol (PEG)ylated superparamagnetic iron oxide NPs with size of 11.8 nm were shown to be able to cross the BBB and specifically bind to the PD area131. Similar iron oxide NPs with size of 10–20 nm were shown to be able to penetrate the BBB on acute temporal lobe epilepsy model132,133. There exists some controversy about the opening extent of BBB gaps after stroke. On the one hand, up to 30% of TJ were reported to be open at 48–58 h after transient middle cerebral artery occlusion, and the opening of these gaps can increase progressively, reaching gaps of 0.2–1.2 μm20,67. On the other hand, the pore size upper limit of ischemic vasculatures in mice was determined to be in the range of 10–11 nm134. The pore size upper limit in brain tumor vasculatures was reported to be ∼12 nm134,135. However, nanocarriers with size in the range of 5–40 nm were demonstrated to be able to permeate through the BTB136,137. The size of BBB opening after TBI on animal models depends on the impact types (focal and diffuse) and different stages. On blast-induced TBI mouse model, BBB opening in the acute period was ∼70 kDa (hydrodynamic diameters ∼10 nm), followed by recovery of BBB integrity by 1 day post-injury138. On controlled cortical impact model, selective influx of 82 nm PEG-liposomes was found at 0–8 h after injury128, and both 20 and 40 nm PEGylated polystyrene NPs accumulated significantly in the injury penumbra up to 13 h after injury75,139. On diffuse TBI model, PEGylated polystyrene NPs failed to accumulate in the brain tissue after mild closed-head injury140. On cryo-lesion TBI model, 100 nm NPs were shown to have greater penetration onto the brain lesion than larger NPs141.
4.3. Neuroinflammation based strategy
The influx of blood-borne molecules and cells into brain due to the BBB disruption in brain diseases, leads to inflammatory and immune responses, making most of brain diseases accompanying with neuroinflammation. In the whole process including initiation, maintenance and duration, blood-borne cells including RBCs, platelets and leukocytes are extensively recruited and trafficked into the brain parenchyma by chemotaxis, diapedesis and precise molecular interactions66, which is crucial for the development of inflammatory15. The up-regulated adhesion molecules at the surface of brain capillary endothelial cells lead to leukocyte entry to brain. Leukocytes infiltrate across the BBB in a multistep sequential process involving capture, rolling, activation, adhesion, crawling and trans- or para-cellular diapedesis. The recruitment of leukocytes to intracranial lesions offers a unique opportunity for crossing of the diseased BBB and the leukocytes could serve as carriers to deliver drugs across the BBB to inflammation sites inside brain142. The designed drug-bearing nanocarrier-phagocytosed leukocytes could directly target pathologically affected regions in the brain66.
Cell-membrane-coating nanotechnology has attracted much attention to constructing biomimetic drug delivery systems with synthetic NPs as the core and a layer of natural cell membrane as the shell143. The biomimetic nanocarriers inherit the properties of the source cells, possessing a series of favorable functions such as prolonged circulation and disease-relevant targeting144. Some cell membrane camouflaged nanocarriers have been applied for imaging and photoactivatable therapy in various cancer models126,145, 146, 147, 148, 149, 150, 151, 152. Therefore, biomimetic nanocarriers coated by membrane from blood-borne cells can be designed and should be capable of crossing the diseased BBB through the neuroinflammation process.
4.4. Selecting the most favorable transport-related BBB molecular changes for different brain diseases
Different brain diseases display various BBB evolutions throughout the onset and progression. Various BBB crossing strategies can be designed based on these BBB evolutions for brain-targeting drug delivery. For example, for designing AD-targeting nanocarriers, favorable transport-related BBB molecular changes in AD include TJ loss, RAGE up-regulation, adhesion molecule up-regulation and increased transcytosis related possible MFSD2A decrease or caveolin-1 increase. For other brain diseases, transport-related BBB molecular changes can be found in Table 1 and Section 2.2. Diseased BBB.
The most favorable transport-related molecular changes for designing targeted delivery systems for specific brain disease remain to be investigated. TJ loss allows nanocarriers with size less than 20 nm to penetrate the diseased BBB via an EPR-like effect. MFSD2A decrease, caveolin-1 increase and receptor up-regulation could improve transcytosis of ligand-modified nanocarriers through the diseased BBB. Based on increased transcytosis, ligand-modified nanocarriers can be several hundred nanometers with relatively high drug loading. It is complicated to determine which transport-related BBB molecular change is most favorable for designing brain-targeting nanocarriers for specific brain diseases. On the one hand, the degree of transport-related BBB molecular changes determines the efficiency of related strategies. Unfortunately, the quantitative characterization on the extent of many transport-related molecular changes remains to be deciphered. On the other hand, physicochemical properties of nanocarrier (e.g., chemical composition, size and zeta potential) may impact the efficiency. It is remarkable that the efficiency of BBB crossing and brain targeting drug delivery may be significantly boosted by engineered strategies integrating different BBB molecular changes and impacted by BBB recovery or deterioration after drug intervention of the disease progression.
5. Applications
5.1. AD
5.1.1. RAGE up-regulation
RAGE expression in brain microvasculature is increased in AD parallel to advanced stages of the disease153,154. The up-regulated RAGE and RAGE-mediated transcytosis pathway can be employed for brain drug delivery. Lu et al.124 reported a RAGE-targeting ROS-responsive polymeric micelle system to normalize the oxidative and inflammatory microenvironment and reeducate microglia from an early phase of AD based on the crosstalk between microglia and brain microenvironment. Through the RAGE-mediated Aβ transportation pathway, the micelles accumulated into the diseased regions and exert synergistic effects of polymer-based ROS scavenging and cargo-based Aβ inhibition upon microenvironment stimuli. Wu et al.155 found that the nanocarrier assembled by hierarchial forms of a brain specific phage-derived peptide can target brain capillary endothelial cells through both RAGE and TfR, cross the BBB and reach neurons and microglial cells. The brain accumulation of the nanocarrier can reach 5.7% of the injected dose on normal mice. With up-regulated RAGE on BBB in AD brain, the brain delivery efficiency of the nanocarrier may be able to be further enhanced on AD mice. Through intravenous injection, effective down-regulation of β-secretase 1 (BACE1) in the brain was achieved by the delivered short interfering RNA (siRNA) against BACE1 to inhibit Aβ production in AD155.
5.1.2. Reduction of both LRP1 and GLUT1
Under physiological conditions, LRP1, at the abluminal side of the BBB, binds Aβ and initiates Aβ clearance from brain to blood via transcytosis across the BBB. Considering the LRP1 reduction in AD, statins had been used to upregulate LRP1 at the BBB to promote Aβ clearance44,154,156. The strategy of up-regulating LRP1 via statins can be employed for brain drug delivery. Guo et al.157 developed simvastatin-loaded angiopep-2-anchored NPs for raising expression of LRP1 at brain capillary endothelial cells to surmount the low transcytosis of BBB. The developed NPs also heightened LRP1 on brain metastatic tumor cells with improved chemotherapy of brain metastases. However, the strategy of up-regulating LRP1 via statins has not been yet applied in brain-targeting drug delivery and therapy for AD.
AD is also characterized by reduced GLUT1 at the BBB and reduction of glucose transport49. Anraku et al.158 designed rapid glycaemic increase after fasting and self-assembled supramolecular nanocarriers with surface featuring properly configured glucose to boost BBB crossing and brain accumulation of glucose-functionalized nanocarrier. The putative up-regulation of luminal GLUT1 by hypoglycemia and the migration of GLUT1 from the luminal BBB to the abluminal BBB by the rapid glycaemic increase after fasting boosted the BBB crossing and brain accumulation of the developed nanocarriers. The brain accumulation of glucose-properly-configured nanocarrier can reach at 6% dose/g-brain on normal mice with glycaemic control. Although it has not been applied for drug delivery and therapy for AD, this strategy holds the potential to overcome the reduced GLUT1 in AD for drug delivery to brain with AD.
5.1.3. TJ loss
It seems that TJ loss would improve paracellular drug delivery to the brain39. However, BBB with TJ loss is still very restrictive in comparison to peripheral tissue beds39. Cheng et al.159 found that TJ loss in AD animal model was not sufficient to influence brain influx of small molecule drugs. Nanocarriers with prolonged blood circulation possess the potential to improve the leaky vessel-based drug delivery efficiency. Tanifum et al.160 designed Aβ-targeting lipid conjugate-incorporated stealth liposomes to treat amyloid plaque deposition in AD. The liposomes successfully extravasated into brain via the compromised BBB and bound with Aβ plaque deposits161. In addition, lexiscan and minoxidil as BBB modulators were respectively loaded into NPs to further enhance paracellular BBB permeability82,111,157,162. The strategy of co-delivery of BBB modulators can be applied to improve drug delivery to AD brain.
5.2. PD
5.2.1. Crossing of diseased BBB in PD
Although TJ loss and possible increased expression of integrin and adhesion molecules for angiogenesis and leukocyte diapedesis have been identified as critical BBB evolutions in PD, very few diseased BBB-based BBB crossing strategies have been reported for systemic brain-targeting drug delivery. Chen et al.163 reported polyethyleneglycol-poly(lactic-co-glycolic acid) (PEG-PLGA) NPs for delivery of schisantherin A and proved the effective brain delivery and strong neuroprotective effects in PD zebrafish model. Although transcytosis was thought as the underlying BBB crossing mechanism, TJ loss may also contribute to brain uptake of NPs. The α-synuclein, which forms Lewy bodies in brain with PD, holds the potential to be engineered as safe functionalization for BBB crossing and PD targeting. However, neither α-synuclein nor derived peptide has been used for brain drug delivery and PD therapy. Divalent cation Ca2+ is the α-synuclein-specific pathophysiological ligand. Lee et al.164 designed gold NPs which were cross-linked by coated cysteine-bearing α-synuclein, to coat mesoporous silica NPs for intracellular Ca2+-responsive cargo release. In another study, the α-synuclein was used for the mutual connection of gold NPs for protease-sensitive and light-responsive drug release165. It can be postulated that α-synuclein-functionalized delivery systems hold the potential for brain drug delivery.
5.3. Epilepsy
5.3.1. P-gp up-regulation
Based on the specific up-regulation of P-gp on the BBB in epilepsy, about 35%–40% of epilepsy patients are resistant to drug therapy. Therefore, development of formulation that can modulate P-gp function as well as facilitate brain delivery of antiepileptic drug represents a promising strategy for epilepsy intervention61. Zybina et al.63 found that P-gp inhibitor verapamil increased the anticonvulsant effect of carbamazepine and reduced its effective dose by ∼30% in isoniazid-induced rat epilepsy model.
Fang et al.64 designed pluronic P85-coated poly(butylcyanoacrylate) NPs for brain delivery of phenytoin while overcoming the up-regulated P-gp in rats with chronic temporal lobe epilepsy induced by lithium-pilocarpine. The NPs successfully overcame the drug resistance in epilepsy and efficiently delivered phenytoin to rat hippocampus with P-gp overexpression. Liu et al.62 designed tryptophan-functionalized pluronic P123/F127 mixed micelles for delivery of antiepileptic lamotrigine to epileptogenic focus. The delivery was based on the combination of transporter-mediated endocytosis and pluronic block copolymer-mediated overcoming of multidrug resistance. The micelles were efficient in delivering lamotrigine to the brain, especially the hippocampus via the tryptophan-mediated active targeting as well as P-gp modulation at the epileptogenic focus.
5.3.2. TJ loss
Alpha-methyl-l-tryptophan (AMT) is a synthetic amino acid and is recognized as a surrogate marker for epilepsy, characterized by high uptake in the epileptic focus. Based on TJ loss in epilepsy, Wang et al.132 engineered AMT-modified iron oxide NPs for delivery IL-1β monoclonal antibody across the BBB in the acute temporal lobe epilepsy for epilepsy treatment. Akhtari et al.133 developed AMT-attached magnetic NPs for magnetic resonance imaging of acute and chronic temporal lobe epilepsy. The magnetic NPs crossed the BBB, entered into brain parenchyma, and localized to epileptogenicity location in both acute and chronic conditions. In another study, magnetic field was used to improve the BBB crossing efficiency of AMT-coated NPs166. TJ loss was thought as the BBB crossing mechanism of AMT-coated NPs for passive epilepsy targeting.
5.3.3. Neuroinflammation
Neuroinflammation in epilepsy can be targeted by biomimetic delivery systems for BBB crossing through the infiltration mechanisms of leukocytes into the epileptic brain. Han et al.65 designed NPs-loaded monocytes for drug delivery to epileptic brain. The distribution of NPs-loaded monocytes in hippocampal CA1 subregion and dentate gyrus in rats with spontaneous seizures was 176- and 380-fold higher than unloaded NPs.
5.4. Stroke
5.4.1. Integrin up-regulation
Integrin receptors, especially αvβ3, are up-regulated at the BBB in stroke. Tian et al.73 designed c(RGDyK)-conjugated exosomes for delivery of curcumin to brain ischemic lesions in transient middle cerebral artery occlusion mice model. The curcumin-loaded exosomes showed strong suppression of the inflammatory response and cellular apoptosis in the lesion region. In another study, PEGylated ceria NPs was conjugated with biotinylated-LXW7 which is a ligand of integrin αvβ3. The modified ceria NPs could cross the BBB through the interaction between LXW7 and αvβ3 which is upregulated on BBB after stroke, and exhibited better effects in reducing infarction size and the degree of BBB breakdown167.
5.4.2. Neuroinflammation
The recruitment of activated leukocytes to intracranial lesions, a typical feature of neuroinflammation response which occurs in cerebral ischemia, offers a unique opportunity for drug delivery across the BBB to inflammation sites inside brain142. The expression of P-selectin and intercellular adhesion molecule-1 (ICAM-1) on brain capillary endothelial cells are up-regulated during stroke. The interaction between P-selectin and P-selectin glycoprotein ligand-1 which are expressed on leukocytes promotes the binding of leukocytes to BBB in stroke. Leukocytes realized firmer adhesion on the vascular wall through the specific binding between the ICAM-1 and lymphocyte function associated antigen-1 (LFA-1), leukocytes subsequently infiltrate into the ischemic brain parenchyma through the damaged BBB. Dong et al.125 designed neutrophil membrane-coated NPs for brain delivery of anti-inflammation agent to prevent brain damage led by ischemia/reperfusion. Zhang et al.142 developed neutrophil-targeting PGP peptide-modified catalase-loaded cross-linked dendrigraft poly-l-lysine NPs. The NPs can be internalized into neutrophils in blood through the binding of PGP with CXCR2 receptor on neutrophils and subsequent receptor-mediated endocytosis. Neutrophils enhanced catalase delivery to ischemic regions and reduced infarct volume in middle cerebral artery occlusion mice. The neutrophil-based strategy is also promising for treatment of other inflammation-related brain diseases because neuroinflammation occurs in many neurological disorders.
5.4.3. TJ loss
TJ loss in stroke allows drug accumulation in stroke region39,168,169. NPs showed further enhanced delivery of therapeutics via the leaky BBB74,170. Various NPs have been reported to passively cross the disrupted BBB in stroke. Al-Ahmady et al.67 showed selective recruitment of liposomes into ischemic region in brain in mouse model. The brain liposomal level in ipsilateral side was significantly increased from 0.04% dose/g-brain under healthy condition to 0.24% and 0.1% dose/g-brain when given to mice at 0.5 and 48 h after transient middle cerebral artery occlusion. The recruitment of intravenously injected liposomes was correlated with the biphasic BBB breakdown67. The biphasic BBB breakdown precedes neurological damage, endowing NPs with the great potential for neuroprotection. Uptake of liposomes by glial cells in the ischemic region was selectively enhanced, highlighting the potential for blocking inflammatory responses or shifting the polarization of microglia/macrophages toward brain repair.
Based on the ischemia targeting ability of platelet membrane lipids and proteins, Li et al.171 designed platelet membrane-derived nanobubbles for ultrasound imaging of acute ischemic stroke and timely perfusion intervention. The nanobubbles exhibited preferentially accumulation in ischemic region for real-time contrast-enhanced ultrasound imaging to indicate the severity and dynamic development of the stroke and critical microvascular bio-remodeling for recanalization of the obstructed vessels to protect the neural cells around the ischemic region. Adenosine with neuropharmacological activity is often inefficient upon systemic administration because of its fast metabolization and blood clearance. Gaudin et al.172 conjugated adenosine to lipid squalene for formation of nanoassemblies. The nanoassemblies prolonged circulation of nucleoside for extended interaction of adenosine with the neurovascular unit and provided neuroprotection against stroke in mouse model. Animals with cerebral ischemia showed improved neurologic deficit score after receiving systemic squalenoyl adenosine nanoassemblies.
After passively crossing the disrupted BBB in stroke, various NPs have been designed to actively target specific components, e.g., SDF-1, in the ischemic microenvironment. Through various expressed chemokines, e.g., SDF-1 receptor CXCR4, neural stem cells migrate to the injured brain area. Ma et al.173 designed poly(lactic-co-glycolic acid) (PLGA) NPs coated with engineered CXCR4-overexpressed neural stem cell membrane for delivery of anti-edema glyburide to ischemic brain for stroke treatment. RAGE is up-regulated on neurons and glial cells in ischemic brain to induce inflammation174. Oh et al.174 developed RAGE-targeting peptide modified NPs for delivery of heme oxygenase-1 plasmid for ischemic stroke gene therapy. The NPs inhibited RAGE-mediated signal transduction and functioned as cytoprotective reagent to decrease inflammation, apoptosis and ROS in hypoxic cells. In light of upregulated ROS in ischemic neurons, Lv et al.175 designed stroke homing peptide-modified RBC membrane-coated ROS-responsive dextran nanocarrier for delivery of neuroprotective peptide NR2B9C to treat ischemic brain damage. After homing to ischemic brain, the NPs released NR2B9C upon responding to intracellular ROS in ischemic neurons. The NPs showed prolonged systemic circulation, active targeting, and reduced ischemic brain damage by protective effects in the middle cerebral artery occlusion rats175, 176, 177.
5.5. TBI
5.5.1. BBB disruption during the early phase after injury
Although the recruitment of neutrophils and the increase of the transcytosis rate are two important characteristics of early stage TBI, the two characteristics were very rarely used to design brain drug delivery systems for treatment of TBI.
Loss of TJ during the early phase after TBI provides an opportunity for passive accumulation of intravenously administered NPs through an enhanced permeation and retention-like effect75,178. Through the leaky BBB, NPs successfully delivered therapeutics such as cerebrolysin and antioxidants to TBI region in brain74,140,179,180. Bharadwaj et al.139 showed that, when systemically administered within the critical time window (<12 h) after TBI, PEGylated NPs with different size were significantly accumulated in brain in rodent with moderate/severe TBI. Many papers researched the effect of the size of NPs on the efficiency of crossing the compromised BBB to TBI regions75,139, 140, 141. Miller et al.75 found that 80 nm PLGA NPs had maximal permeability coefficient in TBI mouse model when intravenously injected 3 h post-injury. Lin et al.181 synthesized poly(n-butyl-2-cyanoacrylate) NPs for delivery of large molecules through the BBB in injured brains to treat TBI. NPs were distributed widely near injured sites and hardly detected in normal regions, demonstrating the compromised BBB-based brain-targeting.
TBI can lead to internal bleeding in brain and long-term neurological deficits. Currently, there are no treatments for internal bleeding beyond fluid resuscitation and surgery. Hubbard et al.182 designed anti-inflammatory dexamethasone-loaded hemostatic PLGA NPs to stop the bleeding and reduce inflammation after TBI. The NPs reduced apoptosis and restored BBB disruption in the amygdala, ameliorated anxiety parameters, improved survival and long-term functional outcomes in rats with induced TBI. Using nucleic acid therapeutics to modulate destructive pathways is promising for intervention during the secondary injury. Kwon et al.183 engineered a neuron-targeting NP for intracellular trafficking of siRNA. The engineered NPs infiltrated brain tissue with compromised BBB in TBI animal model after systemic administration, to downregulate potential therapeutic target. As a promising neuroprotective agent, NR2B9c peptide showed extremely low brain penetrability. CAQK was reported to have high affinity with extracellular matrix of injured brain site184. Wu et al.185 designed CAQK-modified thrombin-responsive protein NPs for brain delivery of NR2B9c and demonstrated the enhanced targeted delivery of NR2B9c to the injured brain lesion with TBI and improved therapeutic benefits.
5.6. Brain tumor
5.6.1. CD13 up-regulation
Based on widely overexpressed endothelial CD13 on the surface of glioma neovascular endothelial cells, An et al.120 synthesized iNGR-modified polycation with redox-sensitive disulfides to condense siRNA nanospheres for brain siRNA delivery for glioma therapy. The NPs demonstrated stability in circulation, enhanced accumulation in glioma, glutathione-responsive release of siRNA nanospheres in glioma cells, digestion of siRNA nanospheres, and remarkable luciferase knockdown.
5.6.2. KATP and KCa up-regulation
KATP and KCa are specially up-regulated on BTB endothelial cells to regulate BTB permeability82. Minoxidil sulfate, as a molecular targeted KATP modulator, can selectively activate KATP channels present in BTB endothelial cells to up-regulate caveolin-1 and down-regulate TJ proteins, to specially raise BTB permeability in both transcellular and paracellular pathways. Minoxidil sulfate-loaded hyaluronic acid-tethered NPs were designed to surmount the BTB and target brain metastases. The NPs can boost transcytosis and downregulate TJ proteins in brain capillary endothelial cells at brain tumor for promoted BTB penetration82.
5.6.3. Integrin up-regulation
Integrin receptor αvβ3 is upregulated on neovasculature of brain tumor119, and holds the potential for BTB crossing. Chai et al.126 reported c(RGDyK)-modified RBC membrane-coated docetaxel nanocrystals for brain delivery of docetaxel to treat glioma. The nanocrystals exhibited high drug loading, long-term stability, excellent biocompatibility, prolonged retention time, superior tumor accumulation and therapeutic efficacy in mice bearing subcutaneous tumor or orthotropic glioma. Zhu et al.186 designed cRGD-functionalized intracellularly shell-sheddable micelles for enhanced doxorubicin delivery to glioma and improved tumor growth inhibition.
5.6.4. TJ loss
With prolonged circulation time, NPs can access brain tumor through the leaky BBB and the enhanced permeability and retention effect82,111,157,187, 188, 189, 190, 191, 192, 193. There have been many papers using this strategy for targeted delivery of anti-cancer drugs to brain tumor120,194. The use of celastrol for the treatment of cancer is impeded by its low solubility, poor bioavailability and systemic toxicity194. Huang et al.194 prepared liposomal celastrol and assessed the effects in SHG-44 glioma xenografts in mice. Treatment of subcutaneous xenografts with liposomal celastrol induced greater antitumor activity than free celastrol with fewer severe side effects.
Gadolinium (Gd) chelate contrast-enhanced magnetic resonance imaging (MRI) is a preferred method of glioma detection and preoperative localization because it offers high spatial resolution and non-invasive deep tissue penetration195. However, currently used contrast agents, such as Gd-diethyltriaminepentaacetic acid (DTPA-Gd, Magnevist), suffer from rapid renal clearance, non-specificity and low contrast efficiency. Based on the BTB disruption and MMP2 overexpression in glioma, Huang et al.196 designed chlorotoxin-modified poly-l-lysines for brain delivery of DTPA-Gd to image glioma. Increased glioma accumulation of chlorotoxin-modified conjugate, enhanced and prolonged MRI signal in glioma in nude mice. Huang et al.197 also designed chlorotoxin-modified polyamidoamine for brain delivery of gene therapy to treat glioma. Based on the BTB disruption and the highly expressed glucose transporter GLUT1 on tumor cells, Shao et al.198 developed dehydroascorbic acid-decorated intracellular microenvironment-responsive release nanodevice for high integrity in the bloodstream, special binding with tumor cells and intracellular glutathione-triggered drug release in tumor cells.
Based on the BTB disruption and adenosine receptor on brain endothelial cells, an “autocatalytic” approach was designed via loading lexiscan (specific agonist of adenosine receptor) inside chlorotoxin-modified NPs, to boost BTB disruption-based brain tumor targeting efficiency111. The accumulated NPs in brain tumor through the BTB disruption, release lexiscan to modulate the BBB to allow more NPs to enter brain, especially brain tumor regions. The preferential accumulation in brain tumor can be 4.3- and 94.0-fold greater than that in the liver and in brain regions without tumors, respectively.
6. Conclusions and future directions
Brain diseases are difficult to treat pharmacologically due to the existence of the BBB, which is a formidable barrier for drug delivery to brain. However, many aspects of the BBB evolve during the onset and progression of many brain diseases. Substantial proof-of-concept studies tested the combined use of BBB evolutions (e.g., BBB disruption and regulation of transport systems) and nanocarriers for selectively enhanced drug delivery to diseased brain regions for drug therapy of brain diseases. Although many BBB evolution-based brain-targeting strategies have shown significant efficiency, quantitative brain delivery efficiency data (e.g., percentage of dose/g-brain) on disease animal models are missing in some studies and remain to be further investigated. Some BBB evolutions haven't been tested for mediating brain drug delivery, e.g., increased integrin and adhesion molecules and leukocyte diapedesis in PD. Related studies are still in their infancy and thus need to be further extensively and intensively researched. There are still many issues remain to be solved, e.g., to accurately determine the transition time, the degree, the location and the heterogeneity of various BBB evolutions, and to reveal the connection of BBB evolutions with age and restoration after drug treatment. Real-time quantitative monitoring of various BBB evolutions will also be instructive to choose suitable BBB evolution-based BBB crossing strategies and brain-targeting drug delivery systems and to optimize the therapeutic regimen, especially for personalized medicine. In addition, more thorough discovery and understanding of BBB evolutions will be beneficial for the formation of a drug delivery system with extremely high brain-targeting efficiency, which will be very promising for clinical translation. Cellular neurovascular unit models using patient-derived endothelial cells may contribute to a better understanding of the heterogeneity of the BBB evolutions199. Such studies with improved BBB models will also help shed light on the cellular interactions and signaling that occur in pathophysiological conditions. Additional studies must be carried out to overcome the safety and toxicity issues against the use of nanocarriers. Overall, tremendous developments have occurred in diseased-based brain targeting drug delivery during past two decades. Although it is still in development, BBB evolution-based BBB crossing strategies will play an increasingly important role in treating brain diseases.
Acknowledgments
This work was funded by the international cooperative project of the National Key R&D Program of China (No. 2017YFE0126900), the National Natural Science Foundation of China (No. 81703428 and No. 81973254), the Natural Science Foundation of Jiangsu Province (No. BK20191421, China), the Suzhou Science and Technology Development Project (No. SYS2019033, China) and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD, China).
Author contributions
Liang Han and Chen Jiang designed the review, wrote the manuscript, revised the manuscript and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Abbott N.J., Patabendige A.A., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal M., Ajazuddin, Tripathi D.K., Saraf S., Saraf S., Antimisiaris S.G. Recent advancements in liposomes targeting strategies to cross blood–brain barrier (BBB) for the treatment of Alzheimer's disease. J Control Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Mittapalli R.K., Manda V.K., Adkins C.E., Geldenhuys W.J., Lockman P.R. Exploiting nutrient transporters at the blood–brain barrier to improve brain distribution of small molecules. Ther Deliv. 2010;1:775–784. doi: 10.4155/tde.10.76. [DOI] [PubMed] [Google Scholar]
- 4.Bhutia Y.D., Babu E., Prasad P.D., Ganapathy V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J Pharm Sci. 2014;9:293–303. [Google Scholar]
- 5.Geldenhuys W.J., Mohammad A.S., Adkins C.E., Lockman P.R. Molecular determinants of blood–brain barrier permeation. Ther Deliv. 2015;6:961–971. doi: 10.4155/tde.15.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardridge W.M. Blood–brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19:1059–1072. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- 7.Uchida Y., Ohtsuki S., Katsukura Y., Ikeda C., Suzuki T., Kamiie J. Quantitative targeted absolute proteomics of human blood–brain barrier transporters and receptors. J Neurochem. 2011;117:333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 8.Geier E.G., Chen E.C., Webb A., Papp A.C., Yee S.W., Sadee W. Profiling solute carrier transporters in the human blood–brain barrier. Clin Pharmacol Ther. 2013;94:636–639. doi: 10.1038/clpt.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh I., Swami R., Jeengar M.K., Khan W., Sistla R. p-Aminophenyl-α-d-mannopyranoside engineered lipidic nanoparticles for effective delivery of docetaxel to brain. Chem Phys Lipids. 2015;188:1–9. doi: 10.1016/j.chemphyslip.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.J., Guo W.L., Kuang X., Hou S.S., Liu H.Z. Nanopreparations for mitochondria targeting drug delivery system: current strategies and future prospective. Asian J Pharm Sci. 2017;12:498–508. doi: 10.1016/j.ajps.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J.J., Zhang H.L., Chen Z.Y., Xu L.H., Zhang Z.Z. A multi-functional nanoplatform for efficacy tumor theranostic applications. Asian J Pharm Sci. 2017;12:235–249. doi: 10.1016/j.ajps.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W.Y., Wang Z.B., Wang Y., Tong L.C., Li Y., Wei X. Increasing the permeability of the blood–brain barrier in three different models in vivo. CNS Neurosci Ther. 2015;21:568–574. doi: 10.1111/cns.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krol S., Macrez R., Docagne F., Defer G., Laurent S., Rahman M. Therapeutic benefits from nanoparticles: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem Rev. 2013;113:1877–1903. doi: 10.1021/cr200472g. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki Y., Kanekiyo T. Blood–brain barrier dysfunction and the pathogenesis of Alzheimer's disease. Int J Mol Sci. 2017;18:1965. doi: 10.3390/ijms18091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichler A.F., Chung E., Kodack D.P., Loeffler J.S., Fukumura D., Jain R.K. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat Rev Microbiol. 2008;6:625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowland D., Arac A., Sekiguchi K.J., Hsu M., Lutz S.E., Perrino J. Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L.N., Ma D.L., Shui G.H., Wong P.Y., Cazenave-Gassiot A., Zhang X.D. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 23.Andreone B.J., Chow B.W., Tata A., Lacoste B., Ben-Zvi A., Bullock K. Blood–brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94:581–594. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z., Zlokovic B.V. Blood–brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardridge W.M. Blood–brain barrier biology and methodology. J Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 26.Pardridge W.M. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji A., Tamai I. Organic anion transporters. Pharm Biotechnol. 1999;12:471–491. doi: 10.1007/0-306-46812-3_16. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji A., Tamai I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 1999;36:277–290. doi: 10.1016/s0169-409x(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso F.L., Brites D., Brito M.A. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandit R., Chen L.Y., Gotz J. The blood–brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2019;165–166:1–14. doi: 10.1016/j.addr.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Kou L.F., Hou Y.X., Yao Q., Guo W.L., Wang G., Wang M.L. L-Carnitine-conjugated nanoparticles to promote permeation across blood–brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif Cells Nanomed Biotechnol. 2018;46:1605–1616. doi: 10.1080/21691401.2017.1384385. [DOI] [PubMed] [Google Scholar]
- 33.Pardridge W.M. Targeted delivery of protein and gene medicines through the blood–brain barrier. Clin Pharmacol Ther. 2015;97:347–361. doi: 10.1002/cpt.18. [DOI] [PubMed] [Google Scholar]
- 34.Krizbai I.A., Nyul-Toth A., Bauer H.C., Farkas A.E., Traweger A., Hasko J. Pharmaceutical targeting of the brain. Curr Pharm Des. 2016;22:5442–5462. doi: 10.2174/1381612822666160726144203. [DOI] [PubMed] [Google Scholar]
- 35.Xu D., Wu D., Qin M., Nih L.R., Liu C.Y., Cao Z. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv Mater. 2019;31:1900727. doi: 10.1002/adma.201900727. [DOI] [PubMed] [Google Scholar]
- 36.Khan A.R., Yang X.Y., Fu M.F., Zhai G.X. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64. doi: 10.1016/j.jconrel.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Grande B., Ichkova A., Lemarchant S., Badaut J. Early to long-term alterations of CNS barriers after traumatic brain injury: considerations for drug development. AAPS J. 2017;19:1615–1625. doi: 10.1208/s12248-017-0123-3. [DOI] [PubMed] [Google Scholar]
- 38.Badaut J., Ajao D.O., Sorensen D.W., Fukuda A.M., Pellerin L. Caveolin expression changes in the neurovascular unit after juvenile traumatic brain injury: signs of blood–brain barrier healing? Neuroscience. 2015;285:215–226. doi: 10.1016/j.neuroscience.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banks W.A. Drug delivery to the brain in Alzheimer's disease: consideration of the blood–brain barrier. Adv Drug Deliv Rev. 2012;64:629–639. doi: 10.1016/j.addr.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somjen G.G., Segal M.B., Herreras O. Osmotic-hypertensive opening of the blood–brain barrier in rats does not necessarily provide access for potassium to cerebral interstitial fluid. Exp Physiol. 1991;76:507–514. doi: 10.1113/expphysiol.1991.sp003516. [DOI] [PubMed] [Google Scholar]
- 41.Niu X.Q., Chen J.J., Gao J.Q. Nanocarriers as a powerful vehicle to overcome blood–brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci. 2019;14:480–496. doi: 10.1016/j.ajps.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker S.K., Chen Z.L., Norris E.H., Revenko A.S., MacLeod A.R., Strickland S. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2018;115:9687–9696. doi: 10.1073/pnas.1811172115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deane R., Yan S.D., Submamaryan R.K., LaRue B., Jovanovic S., Hogg E. RAGE mediates amyloid-beta peptide transport across the blood–brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 44.Deane R., Wu Z.H., Zlokovic B.V. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood–brain barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 45.Vogelgesang S., Cascorbi I., Schroeder E., Pahnke J., Kroemer H.K., Siegmund W. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Vogelgesang S., Jedlitschky G., Brenn A., Walker L.C. The role of the ATP-binding cassette transporter P-glycoprotein in the transport of β-amyloid across the blood–brain barrier. Curr Pharm Des. 2011;17:2778–2786. doi: 10.2174/138161211797440168. [DOI] [PubMed] [Google Scholar]
- 47.Cirrito J.R., Deane R., Fagan A.M., Spinner M.L., Parsadanian M., Finn M.B. P-glycoprotein deficiency at the blood–brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wisniewski H.M., Vorbrodt A.W., Wegiel J. Amyloid angiopathy and blood–brain barrier changes in Alzheimer's disease. Ann N Y Acad Sci. 1997;826:161–172. doi: 10.1111/j.1749-6632.1997.tb48468.x. [DOI] [PubMed] [Google Scholar]
- 49.Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramanathan S., Archunan G., Sivakumar M., Selvan S.T., Fred A.L., Kumar S. Theranostic applications of nanoparticles in neurodegenerative disorders. Int J Nanomedicine. 2018;13:5561–5576. doi: 10.2147/IJN.S149022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zenaro E., Piacentino G., Constantin G. The blood–brain barrier in Alzheimer's disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Z.Y., Qiao P.F., Wan C.Q., Cai M., Zhou N.K., Li Q. Role of blood–brain barrier in Alzheimer's disease. J Alzheimers Dis. 2018;63:1223–1234. doi: 10.3233/JAD-180098. [DOI] [PubMed] [Google Scholar]
- 53.Braak H., Tredici K.D., Rub U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 54.Lee H., Pienaar I.S. Disruption of the blood–brain barrier in Parkinson's disease: curse or route to a cure? Front Biosci. 2014;19:272–280. doi: 10.2741/4206. [DOI] [PubMed] [Google Scholar]
- 55.Sui Y.T., Bullock K.M., Erickson M.A., Zhang J., Banks W.A. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Storck S.E., Meister S., Nahrath J., Meissner J.N., Schubert N., Di Spiezio A. Endothelial LRP1 transports amyloid-β(1-42) across the blood–brain barrier. J Clin Invest. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deane R., Wu Z.H., Sagare A., Davis J., Yan S.D., Hamm K. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Chen X.S., Lan X., Roche I., Liu R.G., Geiger J.D. Caffeine protects against MPTP-induced blood–brain barrier dysfunction in mouse striatum. J Neurochem. 2008;107:1147–1157. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kortekaas R., Leenders K.L., van Oostrom J.C.H., Vaalburg W., Bart J., Willemsen A.T.M. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 60.Meenu M., Reeta K.H., Dinda A.K., Kottarath S.K., Gupta Y.K. Evaluation of sodium valproate loaded nanoparticles in acute and chronic pentylenetetrazole induced seizure models. Epilepsy Res. 2019;158:106219. doi: 10.1016/j.eplepsyres.2019.106219. [DOI] [PubMed] [Google Scholar]
- 61.Rosillo-de la Torre A., Luna-Barcenas G., Orozco-Suarez S., Salgado-Ceballos H., Garcia P., Lazarowski A. Pharmacoresistant epilepsy and nanotechnology. Front Biosci. 2014;6:329–340. doi: 10.2741/709. [DOI] [PubMed] [Google Scholar]
- 62.Liu J.S., He Y.J., Zhang J., Li J.J., Yu X.R., Cao Z.L. Functionalized nanocarrier combined seizure-specific vector with P-glycoprotein modulation property for antiepileptic drug delivery. Biomaterials. 2015;74:64–76. doi: 10.1016/j.biomaterials.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Zybina A., Anshakova A., Malinovskaya J., Melnikov P., Baklaushev V., Chekhonin V. Nanoparticle-based delivery of carbamazepine: a promising approach for the treatment of refractory epilepsy. Int J Pharm. 2018;547:10–23. doi: 10.1016/j.ijpharm.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Fang Z.Y., Chen S.D., Qin J.M., Chen B., Ni G.Z., Chen Z.Y. Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials. 2016;97:110–121. doi: 10.1016/j.biomaterials.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 65.Han H., Eyal S., Portnoy E., Mann A., Shmuel M., Benifla M. Monocytes as carriers of magnetic nanoparticles for tracking inflammation in the epileptic rat brain. Curr Drug Deliv. 2019;16:637–644. doi: 10.2174/1567201816666190619122456. [DOI] [PubMed] [Google Scholar]
- 66.Furtado D., Björnmalm M., Ayton S., Bush A.I., Kempe K., Caruso F. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30:1801362. doi: 10.1002/adma.201801362. [DOI] [PubMed] [Google Scholar]
- 67.Al-Ahmady Z.S., Jasim D., Ahmad S.S., Wong R., Haley M., Coutts G. Selective liposomal transport through blood brain barrier disruption in ischemic stroke reveals two distinct therapeutic opportunities. ACS Nano. 2019;13:12470–12486. doi: 10.1021/acsnano.9b01808. [DOI] [PubMed] [Google Scholar]
- 68.Hacke W., Kaste M., Bluhmki E., Brozman M., Davalos A., Guidetti D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham L.A., Wetzel M., Rosenberg G.A. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 70.del Zoppo G.J., Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda S., Fini C.A., Mabuchi T., Koziol J.A., Eggleston L.L., Jr., del Zoppo G.J. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao J., Dong X.Y., Wang Z.J. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods. 2020;177:114–125. doi: 10.1016/j.ymeth.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Lee H.J., Ryu J.S., Vig P.J. Current strategies for therapeutic drug delivery after traumatic CNS injury. Ther Deliv. 2019;10:251–263. doi: 10.4155/tde-2019-0006. [DOI] [PubMed] [Google Scholar]
- 75.Miller H.A., Magsam A.W., Tarudji A.W., Romanova S., Weber L., Gee C.C. Evaluating differential nanoparticle accumulation and retention kinetics in a mouse model of traumatic brain injury via Ktrans mapping with MRI. Sci Rep. 2019;9:16099. doi: 10.1038/s41598-019-52622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price L., Wilson C., Grant G. In: Translational research in traumatic brain injury. Laskowitz D., Grant G., editors. CRC Press/Taylor and Francis Group; Boca Raton (FL): 2016. Blood–brain barrier pathophysiology following traumatic brain injury. Chapter 4. [PubMed] [Google Scholar]
- 77.Chen Y.X., Wei C.X., Lyu Y.Q., Chen H.Z., Jiang G., Gao X.L. Biomimetic drug-delivery systems for the management of brain diseases. Biomater Sci. 2020;8:1073–1088. doi: 10.1039/c9bm01395d. [DOI] [PubMed] [Google Scholar]
- 78.Xue J.W., Zhao Z.K., Zhang L., Xue L.J., Shen S.Y., Wen Y.J. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 79.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiwary S., Morales J.E., Kwiatkowski S.C., Lang F.F., Rao G., McCarty J.H. Metastatic brain tumors disrupt the blood–brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter mfsd2a. Sci Rep. 2018;8:8267. doi: 10.1038/s41598-018-26636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Achrol A.S., Rennert R.C., Anders C., Soffietti R., Ahluwalia M.S., Nayak L. Brain metastases. Nat Rev Dis Primers. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 82.Miao T.T., Ju X.F., Zhu Q.N., Wang Y.M., Guo Q., Sun T. Nanoparticles surmounting blood–brain tumor barrier through both transcellular and paracellular pathways to target brain metastases. Adv Funct Mater. 2019;29:1900259. [Google Scholar]
- 83.Kasoha M., Unger C., Solomayer E.F., Bohle R.M., Zaharia C., Khreich F. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis. 2017;34:479–490. doi: 10.1007/s10585-018-9878-x. [DOI] [PubMed] [Google Scholar]
- 84.Wernicke A.G., Varma S., Greenwood E.A., Christos P.J., Chao K.S.C., Liu H. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS. 2014;122:482–489. doi: 10.1111/apm.12195. [DOI] [PubMed] [Google Scholar]
- 85.Nomura N., Pastorino S., Jiang P.F., Lambert G., Crawford J.R., Gymnopoulos M. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14:26. doi: 10.1186/1475-2867-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 87.Yonemori K., Tsuta K., Ono M., Shimizu C., Hirakawa A., Hasegawa T. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116:302–308. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood–brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao C.S., Ma J.W., Wang Z., Li H.Y., Shen H.T., Li X. Mfsd2a attenuates blood–brain barrier disruption after sub-arachnoid hemorrhage by inhibiting caveolae-mediated transcellular transport in rats. Transl Stroke Res. 2020;11:1012–1027. doi: 10.1007/s12975-019-00775-y. [DOI] [PubMed] [Google Scholar]
- 90.Eser Ocak P., Ocak U., Sherchan P., Zhang J.H., Tang J.P. Insights into major facilitator superfamily domain-containing protein-2a (Mfsd2a) in physiology and pathophysiology. What do we know so far? J Neurosci Res. 2020;98:29–41. doi: 10.1002/jnr.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y.R., Xiong X.Y., Liu J., Wu L.R., Zhong Q., Zhou K. Mfsd2a (major facilitator superfamily domain containing 2a) attenuates intracerebral hemorrhage-induced blood–brain barrier disruption by inhibiting vesicular transcytosis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W.T., Chen R.Y., Yang T., Xu N., Chen J., Gao Y.Q. Fatty acid transporting proteins: roles in brain development, aging, and stroke. Prostaglandins Leukot Essent Fatty Acids. 2018;136:35–45. doi: 10.1016/j.plefa.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zlokovic B.V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Yuan X.N., Fu Z.S., Ji P.F., Guo L.B., Al-Ghamdy A.O., Alkandiri A. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int J Nanomedicine. 2020;15:6339–6353. doi: 10.2147/IJN.S259134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lochhead J.J., Yang J.Z., Ronaldson P.T., Davis T.P. Structure, function, and regulation of the blood–brain barrier tight junction in central nervous system disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lo E.H., Singhal A.B., Torchilin V.P., Abbott N.J. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38:140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 97.Watkins S., Robel S., Kimbrough I.F., Robert S.M., Ellis-Davies G., Sontheimer H. Disruption of astrocyte-vascular coupling and the blood–brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss N., Miller F., Cazaubon S., Couraud P.O. The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 99.Singh I., Swami R., Khan W., Sistla R. Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deliv. 2014;11:211–229. doi: 10.1517/17425247.2014.866088. [DOI] [PubMed] [Google Scholar]
- 100.Zhan C.Y., Lu W.Y. The blood–brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13:2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 101.Petri B., Bootz A., Khalansky A., Hekmatara T., Muller R., Uhl R. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J Control Release. 2007;117:51–58. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 102.Gao H.L. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ji B., Maeda J., Higuchi M., Inoue K., Akita H., Harashima H. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006;78:851–855. doi: 10.1016/j.lfs.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 104.Pardridge W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 105.Olivier J.C. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J., Cai P., Shalviri A., Henderson J.T., He C.S., Foltz W.D. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood–brain barrier targeting brain metastases of breast cancer. ACS Nano. 2014;8:9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 107.He C.S., Ping C., Li J., Tian Z., Lin L., Abbasi A.Z. Blood–brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Control Release. 2017;246:98–109. doi: 10.1016/j.jconrel.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 108.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 109.Deeken J.F., Loscher W. The blood–brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 110.Liu G., Men P., Harris P.L.R., Rolston R.K., Perry G., Smith M.A. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett. 2006;406:189–193. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 111.Han L., Kong D.K., Zheng M.Q., Murikinati S., Ma C., Yuan P. Increased nanoparticle delivery to brain tumors by autocatalytic priming for improved treatment and imaging. ACS Nano. 2016;10:4209–4218. doi: 10.1021/acsnano.5b07573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang T.Z., Ferrill L., Gallant L., McGillicuddy S., Fernandes T., Schields N. Verapamil and riluzole cocktail liposomes overcome pharmacoresistance by inhibiting P-glycoprotein in brain endothelial and astrocyte cells: a potent approach to treat amyotrophic lateral sclerosis. Eur J Pharm Sci. 2018;120:30–39. doi: 10.1016/j.ejps.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 113.Pinzon-Daza M.L., Garzon R., Couraud P.O., Romero I.A., Weksler B., Ghigo D. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood–brain barrier cells. Br J Pharmacol. 2012;167:1431–1447. doi: 10.1111/j.1476-5381.2012.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nazem A., Mansoori G.A. Nanotechnology solutions for Alzheimer's disease: advances in research tools, diagnostic methods and therapeutic agents. J Alzheimers Dis. 2008;13:199–223. doi: 10.3233/jad-2008-13210. [DOI] [PubMed] [Google Scholar]
- 115.Kubinova S., Sykova E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine. 2010;5:99–108. doi: 10.2217/nnm.09.93. (Lond) [DOI] [PubMed] [Google Scholar]
- 116.Chow B.W., Gu C.H. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron. 2017;93:1325–1333. doi: 10.1016/j.neuron.2017.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruan S.B., Qin L., Xiao W., Hu C., Zhou Y., Wang R.R. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood–brain barrier transcytosis and programmed glioma targeting delivery. Adv Funct Mater. 2018;28:1802227. [Google Scholar]
- 118.Cai L.L., Yang C.Y., Jia W.F., Liu Y.W., Xie R., Lei T. Endo/lysosome-escapable delivery depot for improving BBB transcytosis and neuron targeted therapy of Alzheimer's disease. Adv Funct Mater. 2020;30:1909999. [Google Scholar]
- 119.Zhang T., Lip H., He C.S., Cai P., Wang Z.G., Henderson J.T. Multitargeted nanoparticles deliver synergistic drugs across the blood–brain barrier to brain metastases of triple negative breast cancer cells and tumor-associated macrophages. Adv Healthc Mater. 2019;8:1900543. doi: 10.1002/adhm.201900543. [DOI] [PubMed] [Google Scholar]
- 120.An S., Jiang X.T., Shi J.S., He X., Li J.F., Guo Y.B. Single-component self-assembled RNAi nanoparticles functionalized with tumor-targeting iNGR delivering abundant siRNA for efficient glioma therapy. Biomaterials. 2015;53:330–340. doi: 10.1016/j.biomaterials.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 121.Ningaraj N.S., Rao M., Black K.L. Calcium-dependent potassium channels as a target protein for modulation of the blood–brain tumor barrier. Drug News Perspect. 2003;16:291–298. doi: 10.1358/dnp.2003.16.5.878815. [DOI] [PubMed] [Google Scholar]
- 122.Ningaraj N.S., Rao M., Hashizume K., Asotra K., Black K.L. Regulation of blood–brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301:838–851. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- 123.Ningaraj N.S., Rao M.K., Black K.L. Adenosine 5′-triphosphate-sensitive potassium channel-mediated blood–brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003;63:8899–8911. [PubMed] [Google Scholar]
- 124.Lu Y.F., Guo Z.Y., Zhang Y.J., Li C., Zhang Y., Guo Q. Microenvironment remodeling micelles for Alzheimer's disease therapy by early modulation of activated microglia. Adv Sci. 2018;6:1801586. doi: 10.1002/advs.201801586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong X.Y., Gao J., Zhang C.Y., Hayworth C., Frank M., Wang Z.J. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13:1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chai Z.L., Ran D.N., Lu L.W., Zhan C.Y., Ruan H.T., Hu X.F. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13:5591–5601. doi: 10.1021/acsnano.9b00661. [DOI] [PubMed] [Google Scholar]
- 127.Nance E., Zhang F., Mishra M.K., Zhang Z., Kambhampati S.P., Kannan R.M. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials. 2016;101:96–107. doi: 10.1016/j.biomaterials.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boyd B.J., Galle A., Daglas M., Rosenfeld J.V., Medcalf R. Traumatic brain injury opens blood–brain barrier to stealth liposomes via an enhanced permeability and retention (EPR)-like effect. J Drug Target. 2015;23:847–853. doi: 10.3109/1061186X.2015.1034280. [DOI] [PubMed] [Google Scholar]
- 129.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 130.Zhang T.T., Li W., Meng G.M., Wang P., Liao W.Z. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci. 2016;4:219–229. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]
- 131.Liu X.G., Lu S., Liu D.Q., Zhang L., Zhang L.X., Yu X.L. ScFv-conjugated superparamagnetic iron oxide nanoparticles for MRI-based diagnosis in transgenic mouse models of Parkinson's and Huntington's diseases. Brain Res. 2019;1707:141–153. doi: 10.1016/j.brainres.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y.L., Wang Y.L., Sun R., Wu X.R., Chu X., Zhou S.H. The treatment value of IL-1β monoclonal antibody under the targeting location of alpha-methyl-l-tryptophan and superparamagnetic iron oxide nanoparticles in an acute temporal lobe epilepsy model. J Transl Med. 2018;16:337. doi: 10.1186/s12967-018-1712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Akhtari M., Bragin A., Cohen M., Moats R., Brenker F., Lynch M.D. Functionalized magnetonanoparticles for MRI diagnosis and localization in epilepsy. Epilepsia. 2008;49:1419–1430. doi: 10.1111/j.1528-1167.2008.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng S.Y., Bai Y.Y., Changyi Y.Z., Gao X.H., Zhang W.Q., Wang Y.C. Multimodal nanoprobes evaluating physiological pore size of brain vasculatures in ischemic stroke models. Adv Healthc Mater. 2014;3:1909–1918. doi: 10.1002/adhm.201400159. [DOI] [PubMed] [Google Scholar]
- 135.Sarin H., Kanevsky A.S., Wu H.T., Brimacombe K.R., Fung S.H., Sousa A.A. Effective transvascular delivery of nanoparticles across the blood–brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang J.H., Cho J., Ko Y.T. Investigation on the effect of nanoparticle size on the blood–brain tumour barrier permeability by in situ perfusion via internal carotid artery in mice. J Drug Target. 2019;27:103–110. doi: 10.1080/1061186X.2018.1497037. [DOI] [PubMed] [Google Scholar]
- 137.Su B.X., Wang R.F., Xie Z.X., Ruan H.T., Li J.C., Xie C. Effect of retro-inverso isomer of bradykinin on size-dependent penetration of blood–brain tumor barrier. Small. 2018;14:1702331. doi: 10.1002/smll.201702331. [DOI] [PubMed] [Google Scholar]
- 138.Hue C.D., Cho F.S., Cao S.Q., Nicholls R.E., Vogel E.W., Iii, Sibindi C. Time course and size of blood–brain barrier opening in a mouse model of blast-induced traumatic brain injury. J Neurotrauma. 2016;33:1202–1211. doi: 10.1089/neu.2015.4067. [DOI] [PubMed] [Google Scholar]
- 139.Bharadwaj V.N., Lifshitz J., Adelson P.D., Kodibagkar V.D., Stabenfeldt S.E. Temporal assessment of nanoparticle accumulation after experimental brain injury: effect of particle size. Sci Rep. 2016;6:29988. doi: 10.1038/srep29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bharadwaj V.N., Rowe R.K., Harrison J., Wu C., Anderson T.R., Lifshitz J. Blood–brain barrier disruption dictates nanoparticle accumulation following experimental brain injury. Nanomedicine. 2018;14:2155–2166. doi: 10.1016/j.nano.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cruz L.J., Stammes M.A., Que I., van Beek E.R., Knol-Blankevoort V.T., Snoeks T.J. Effect of PLGA NP size on efficiency to target traumatic brain injury. J Control Release. 2016;223:31–41. doi: 10.1016/j.jconrel.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 142.Zhang C., Ling C.L., Pang L., Wang Q., Liu J.X., Wang B.S. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics. 2017;7:3260–3275. doi: 10.7150/thno.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fang R.H., Kroll A.V., Gao W.W., Zhang L.F. Cell membrane coating nanotechnology. Adv Mater. 2018;30:1706759. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang C.X., Wu B., Wu Y.T., Song X.Y., Zhang S.H., Liu Z.H. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv Funct Mater. 2020;30:1909369. [Google Scholar]
- 145.Wang D.D., Dong H.F., Li M., Cao Y., Yang F., Zhang K. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12:5241–5252. doi: 10.1021/acsnano.7b08355. [DOI] [PubMed] [Google Scholar]
- 146.Chai Z.L., Hu X.F., Wei X.L., Zhan C.Y., Lu L.W., Jiang K. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Control Release. 2017;264:102–111. doi: 10.1016/j.jconrel.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 147.Sun H.P., Su J.H., Meng Q.S., Yin Q., Chen L.L., Gu W.W. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater. 2017;27:1604300. [Google Scholar]
- 148.Rao L., Bu L.L., Cai B., Xu J.H., Li A., Zhang W.F. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28:3460–3466. doi: 10.1002/adma.201506086. [DOI] [PubMed] [Google Scholar]
- 149.Li S.Y., Cheng H., Qiu W.X., Zhang L., Wan S.S., Zeng J.Y. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149–161. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 150.Yang R., Xu J., Xu L.G., Sun X.Q., Chen Q., Zhao Y.H. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 151.Fang R.H., Hu C.M.J., Luk B.T., Gao W.W., Copp J.A., Tai Y.Y. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Y., Cai K.M., Li C., Guo Q., Chen Q.J., He X. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18:1908–1915. doi: 10.1021/acs.nanolett.7b05263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller M.C., Tavares R., Johanson C.E., Hovanesian V., Donahue J.E., Gonzalez L. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.András I.E., Eum S.Y., Huang W., Zhong Y., Hennig B., Toborek M. HIV-1-induced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol Cell Neurosci. 2010;43:232–243. doi: 10.1016/j.mcn.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu L.P., Ahmadvand D., Su J.A., Hall A., Tan X.L., Farhangrazi Z.S. Crossing the blood–brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide. Nat Commun. 2019;10:4635. doi: 10.1038/s41467-019-12554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zandl-Lang M., Fanaee-Danesh E., Sun Y.D., Albrecher N.M., Gali C.C., Čančar I. Regulatory effects of simvastatin and apoJ on APP processing and amyloid-β clearance in blood–brain barrier endothelial cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:40–60. doi: 10.1016/j.bbalip.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 157.Guo Q., Zhu Q.N., Miao T.T., Tao J., Ju X.F., Sun Z.L. LRP1-upregulated nanoparticles for efficiently conquering the blood–brain barrier and targetedly suppressing multifocal and infiltrative brain metastases. J Control Release. 2019;303:117–129. doi: 10.1016/j.jconrel.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 158.Anraku Y., Kuwahara H., Fukusato Y., Mizoguchi A., Ishii T., Nitta K. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 2017;8:1001. doi: 10.1038/s41467-017-00952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng Z.Q., Zhang J.Q., Liu H.F., Li Y., Zhao Y.G., Yang E. Central nervous system penetration for small molecule therapeutic agents does not increase in multiple sclerosis- and Alzheimer's disease-related animal models despite reported blood–brain barrier disruption. Drug Metab Dispos. 2010;38:1355–1361. doi: 10.1124/dmd.110.033324. [DOI] [PubMed] [Google Scholar]
- 160.Tanifum E.A., Dasgupta I., Srivastava M., Bhavane R.C., Sun L., Berridge J. Intravenous delivery of targeted liposomes to amyloid-β pathology in APP/PSEN1 transgenic mice. PLoS One. 2012;7:48515. doi: 10.1371/journal.pone.0048515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hettiarachchi S.D., Zhou Y.Q., Seven E., Lakshmana M.K., Kaushik A.K., Chand H.S. Nanoparticle-mediated approaches for Alzheimer's disease pathogenesis, diagnosis, and therapeutics. J Control Release. 2019;314:125–140. doi: 10.1016/j.jconrel.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 162.Han L., Cai Q., Tian D.F., Kong D.K., Gou X.C., Chen Z.M. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine. 2016;12:1833–1842. doi: 10.1016/j.nano.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen T.K., Li C.W., Li Y., Yi X., Wang R.B., Lee S.M.Y. Small-sized mPEG-PLGA nanoparticles of schisantherin A with sustained release for enhanced brain uptake and anti-parkinsonian activity. ACS Appl Mater Interfaces. 2017;9:9516–9527. doi: 10.1021/acsami.7b01171. [DOI] [PubMed] [Google Scholar]
- 164.Lee D., Hong J.W., Park C., Lee H., Lee J.E., Hyeon T. Ca2+-dependent intracellular drug delivery system developed with "raspberry-type" particles-on-a-particle comprising mesoporous silica core and α-synuclein-coated gold nanoparticles. ACS Nano. 2014;8:8887–8895. doi: 10.1021/nn5034955. [DOI] [PubMed] [Google Scholar]
- 165.Hong C.S., Park J.H., Lee S., Rhoo K.Y., Lee J.T., Paik S.R. Fabrication of protease-sensitive and light-responsive microcapsules encompassed with single layer of gold nanoparticles by using self-assembly protein of α-synuclein. ACS Appl Mater Interfaces. 2018;10:26628–26640. doi: 10.1021/acsami.8b07661. [DOI] [PubMed] [Google Scholar]
- 166.Pedram M.Z., Shamloo A., Alasty A., Ghafar-Zadeh E. MRI-guided epilepsy detection. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4001–4004. doi: 10.1109/EMBC.2015.7319271. [DOI] [PubMed] [Google Scholar]
- 167.Zhang T., Li C.Y., Jia J.J., Chi J.S., Zhou D., Li J.Z. Combination therapy with LXW7 and ceria nanoparticles protects against acute cerebral ischemia/reperfusion injury in rats. Curr Med Sci. 2018;38:144–152. doi: 10.1007/s11596-018-1858-5. [DOI] [PubMed] [Google Scholar]
- 168.Kroli R.A., Neuwelt E.A. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 169.Greig N.H., Fredericks W.R., Holloway H.W., Soncrant T.T., Rapoport S.I. Delivery of human interferon-alpha to brain by transient osmotic blood–brain barrier modification in the rat. J Pharmacol Exp Ther. 1988;245:581–586. [PubMed] [Google Scholar]
- 170.Ishii T., Asai T., Oyama D., Fukuta T., Yasuda N., Shimizu K. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81–87. doi: 10.1016/j.jconrel.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 171.Li M.X., Liu Y., Chen J.P., Liu T.T., Gu Z.X., Zhang J.Q. Platelet bio-nanobubbles as microvascular recanalization nanoformulation for acute ischemic stroke lesion theranostics. Theranostics. 2018;8:4870–4883. doi: 10.7150/thno.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gaudin A., Yemisci M., Eroglu H., Lepetre-Mouelhi S., Turkoglu O.F., Donmez-Demir B. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol. 2014;9:1054–1062. doi: 10.1038/nnano.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma J.N., Zhang S.Q., Liu J., Liu F.Y., Du F.Y., Li M. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15:1902011. doi: 10.1002/smll.201902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oh J., Lee J., Piao C.X., Jeong J.H., Lee M. A self-assembled DNA-nanoparticle with a targeting peptide for hypoxia-inducible gene therapy of ischemic stroke. Biomater Sci. 2019;7:2174–2190. doi: 10.1039/c8bm01621f. [DOI] [PubMed] [Google Scholar]
- 175.Lv W., Xu J.P., Wang X.Q., Li X.R., Xu Q.W., Xin H.L. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12:5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- 176.Zhao Y., Jiang Y., Lv W., Wang Z.Y., Lv L.Y., Wang B.Y. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 177.Xu J.P., Wang X.Q., Yin H.Y., Cao X., Hu Q.Y., Lv W. Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano. 2019;13:8577–8588. doi: 10.1021/acsnano.9b01798. [DOI] [PubMed] [Google Scholar]
- 178.Smith N.M., Gachulincova I., Ho D., Bailey C., Bartlett C.A., Norret M. An unexpected transient breakdown of the blood brain barrier triggers passage of large intravenously administered nanoparticles. Sci Rep. 2016;6:22595. doi: 10.1038/srep22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruozi B., Belletti D., Sharma H.S., Sharma A., Muresanu D.F., Mossler H. PLGA nanoparticles loaded cerebrolysin: studies on their preparation and investigation of the effect of storage and serum stability with reference to traumatic brain injury. Mol Neurobiol. 2015;52:899–912. doi: 10.1007/s12035-015-9235-x. [DOI] [PubMed] [Google Scholar]
- 180.Bailey Z.S., Nilson E., Bates J.A., Oyalowo A., Hockey K.S., Sajja V.S.S.S. Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J Neurotrauma. 2020;37:1452–1462. doi: 10.1089/neu.2016.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin Y., Pan Y.H., Shi Y.F., Huang X.J., Jia N.Q., Jiang J.Y. Delivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brain. Nanotechnology. 2012;23:165101. doi: 10.1088/0957-4484/23/16/165101. [DOI] [PubMed] [Google Scholar]
- 182.Hubbard W.B., Lashof-Sullivan M., Greenberg S., Norris C., Eck J., Lavik E. Hemostatic nanoparticles increase survival, mitigate neuropathology and alleviate anxiety in a rodent blast trauma model. Sci Rep. 2018;8:10622. doi: 10.1038/s41598-018-28848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kwon E.J., Skalak M., Lo Bu R., Bhatia S.N. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano. 2016;10:7926–7933. doi: 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mann A.P., Scodeller P., Hussain S., Joo J., Kwon E., Braun G.B. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat Commun. 2016;7:11980. doi: 10.1038/ncomms11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wu P., Zhao H.T., Gou X.C., Wu X.W., Zhang S.Q., Deng G. Targeted delivery of polypeptide nanoparticle for treatment of traumatic brain injury. Int J Nanomedicine. 2019;14:4059–4069. doi: 10.2147/IJN.S202353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu Y.Q., Zhang J., Meng F.H., Deng C., Cheng R., Feijen J. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J Control Release. 2016;233:29–38. doi: 10.1016/j.jconrel.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 187.Huang S.X., Li J.F., Han L., Liu S.H., Ma H.J., Huang R.Q. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials. 2011;32:6832–6838. doi: 10.1016/j.biomaterials.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 188.Yu X., Gou X.C., Wu P., Han L., Tian D.F., Du F.Y. Activatable protein nanoparticles for targeted delivery of therapeutic peptides. Adv Mater. 2018;30:1705383. doi: 10.1002/adma.201705383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo Q., Chang Z.Y., Khan N.U., Miao T.T., Ju X.F., Feng H.X. Nanosizing noncrystalline and porous silica material-naturally occurring opal shale for systemic tumor targeting drug delivery. ACS Appl Mater Interfaces. 2018;10:25994–26004. doi: 10.1021/acsami.8b06275. [DOI] [PubMed] [Google Scholar]
- 190.Han L., Li J.F., Huang S.X., Huang R.Q., Liu S.H., Hu X. Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent. Biomaterials. 2011;32:2989–2998. doi: 10.1016/j.biomaterials.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 191.Han L., Huang R.Q., Li J.F., Liu S.H., Huang S.X., Jiang C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials. 2011;32:1242–1252. doi: 10.1016/j.biomaterials.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 192.Han L., Guo Y.B., Ma H.J., He X., Kuang Y.Y., Zhang N. Acid active receptor-specific peptide ligand for in vivo tumor-targeted delivery. Small. 2013;9:3647–3658. doi: 10.1002/smll.201300279. [DOI] [PubMed] [Google Scholar]
- 193.Han L., Ma H.J., Guo Y.B., Kuang Y.Y., He X., Jiang C. pH-controlled delivery of nanoparticles into tumor cells. Adv Healthc Mater. 2013;2:1435–1439. doi: 10.1002/adhm.201300013. [DOI] [PubMed] [Google Scholar]
- 194.Huang Y.L., Zhou D., Hang T.J., Wu Z.H., Liu J.G., Xu Q.N. Preparation, characterization, and assessment of the antiglioma effects of liposomal celastrol. Anticancer Drugs. 2012;23:515–524. doi: 10.1097/CAD.0b013e3283514b68. [DOI] [PubMed] [Google Scholar]
- 195.Li J.F., Huang S.X., Shao K., Liu Y., An S., Kuang Y.Y. A choline derivate-modified nanoprobe for glioma diagnosis using MRI. Sci Rep. 2013;3:1623. doi: 10.1038/srep01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huang R.Q., Han L., Li J.F., Liu S.H., Shao K., Kuang Y.Y. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32:5177–5186. doi: 10.1016/j.biomaterials.2011.03.075. [DOI] [PubMed] [Google Scholar]
- 197.Huang R.Q., Ke W.L., Han L., Li J.F., Liu S.H., Jiang C. Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials. 2011;32:2399–2406. doi: 10.1016/j.biomaterials.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 198.Shao K., Ding N., Huang S.X., Ren S.M., Zhang Y., Kuang Y.Y. Smart nanodevice combined tumor-specific vector with cellular microenvironment-triggered property for highly effective antiglioma therapy. ACS Nano. 2014;8:1191–1203. doi: 10.1021/nn406285x. [DOI] [PubMed] [Google Scholar]
- 199.Kaisar M.A., Sajja R.K., Prasad S., Abhyankar V.V., Liles T., Cucullo L. New experimental models of the blood–brain barrier for CNS drug discovery. Expert Opin Drug Discov. 2017;12:89–103. doi: 10.1080/17460441.2017.1253676. [DOI] [PMC free article] [PubMed] [Google Scholar]