Abstract
Many sensitizers have not only photodynamic effects, but also sonodynamic effects. Therefore, the combination of sonodynamic therapy (SDT) and photodynamic therapy (PDT) using sensitizers for sono-photodynamic therapy (SPDT) provides alternative opportunities for clinical cancer therapy. Although significant advances have been made in synthesizing new sensitizers for SPDT, few of them are successfully applied in clinical settings. The anti-tumor effects of the sensitizers are restricted by the lack of tumor-targeting specificity, incapability in deep intratumoral delivery, and the deteriorating tumor microenvironment. The application of nanotechnology-based drug delivery systems (NDDSs) can solve the above shortcomings, thereby improving the SPDT efficacy. This review summarizes various sensitizers as sono/photosensitizers that can be further used in SPDT, and describes different strategies for enhancing tumor treatment by NDDSs, such as overcoming biological barriers, improving tumor-targeted delivery and intratumoral delivery, providing stimuli-responsive controlled-release characteristics, stimulating anti-tumor immunity, increasing oxygen supply, employing different therapeutic modalities, and combining diagnosis and treatment. The challenges and prospects for further development of intelligent sensitizers and translational NDDSs for SPDT are also discussed.
Key words: Sensitizers, Sonodynamic therapy, Photodynamic therapy, Sono-photodynamic therapy, Nanoparticles, Drug delivery, Cancer, Efficacy-enhancing strategies
Graphical abstract
This review summarizes various sensitizers as sono/photosensitizers that can be further used in sono-photodynamic therapy (SPDT), and describes different strategies for enhancing sono-photodynamic therapeutic effects with the assistance of nanotechnology-based drug delivery systems (NDDSs).
1. Introduction
Cancer is one of the leading causes of death in developed and developing regions of the world1. As a complex disease, the uncontrolled cancer cells grow within different tissues, causing local damage and inflammation. Surgery, radiotherapy, and chemotherapy are traditional therapeutic approaches to treat and control the processes of this disease2. However, they have some limitations, such as systemic toxicity, low selectivity, drug resistance, and potential long-term side effects. In order to overcome these shortcomings, various different types of therapies were developed, such as phototherapy, immunotherapy3, 4, 5, 6, 7, gene therapy8, sonodynamic therapy (SDT)9, and the combination of photodynamic therapy (PDT) and SDT (also known as sono-photodynamic therapy, SPDT)10.
As one of the available, safe, and minimally invasive treatment modalities based on the synergistic interactions of low-energy light and a photosensitizer, PDT has received widespread attention for the treatment of superficial tumors. Photosensitizers-mediated PDT activated by light can generate reactive oxygen species (ROS) to cause cell death. Over the past two decades, PDT has been developed as a promising tool to detect and treat cancer11,12. However, one major disadvantage of PDT is the limited penetration of laser light into the deep tissues. This could be mitigated by shifting wavelengths into second near infrared (NIR-II) window. Sonosensitizers-mediated SDT with low-intensity ultrasound has better penetrability compared with NIR-II laser and could easily act on the tumor cells deep inside the biological tissues. The focused ultrasound energy to target deep tissue sites and the locally activation of the sonosensitizers-made SDT have improved treatment efficacy.
With the development of PDT and SDT in the past decade, many sensitizers were found to have not only photodynamic effects, but also sonodynamic anti-cancer effects. Therefore, SPDT has attracted tremendous interest for its enabling combination of SDT and PDT to obtain better therapeutic effects with reduced dose of both ultrasound/light energy and sensitizers, thus reducing the toxic side effects. Current sensitizers can be classified into organic, inorganic, and hybrid sensitizers for cancer therapy, but few of them are successfully applied in clinical settings. Most of the clinically used sensitizers lack tumor-targeting specificity, are incapable of deep intratumoral delivery, have skin phototoxicity, and tend to form aggregates in solution resulting in singlet oxygen (1O2) production quenching. Besides, because of the oxygen (O2) requirement during the PDT/SDT processes, the O2 consumption might aggravate tumor hypoxia, thereby reducing the therapeutic effects.
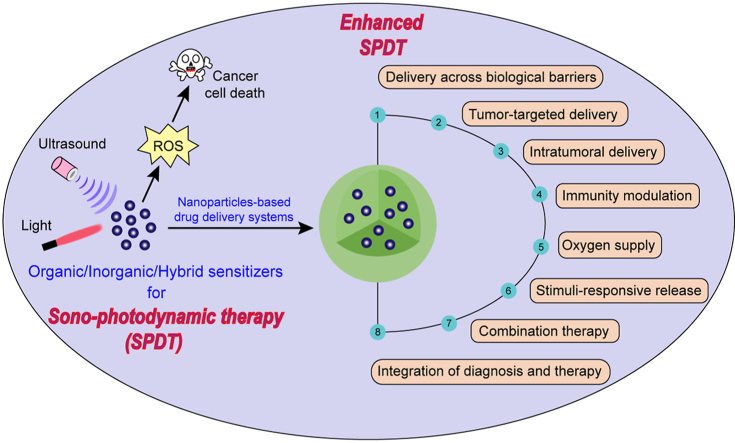
Nanotechnology is the understanding of materials within the 1–100 nm size range and has been used in the design and development of drug delivery systems. Nanotechnology-based drug delivery systems (NDDSs) have achieved good effects in cancer treatment and can be used to improve the anti-tumor effect of drugs13, 14, 15, 16, 17, 18. NDDSs can improve targeting ability of sensitizers through targeted delivery to specific tumor cells or even specific organelles and enhance tumor penetration to increase intratumoral delivery, thus increasing the intracellular drug concentrations in cancer cells while reducing the toxic side effects on normal cells for effective SPDT. Some sensitizers loaded into NDDSs can achieve controlled drug release, prevent aggregation-caused 1O2 quenching, and obtain triggered photoactivities. NDDSs could relieve tumor hypoxic microenvironment by increasing O2 content in tumor tissues, and overcome the limitations of monotherapy by integrating different therapies in a single formulation, thus improving the therapeutic effect of SPDT.
This review summarizes various sensitizers as sono/photosensitizers that can be further used in SPDT, and describes different strategies for enhanced SPDT by NDDSs. The challenges and prospects for further development of intelligent sensitizers and translational NDDSs for SPDT are also discussed.
2. Mechanisms of SPDT
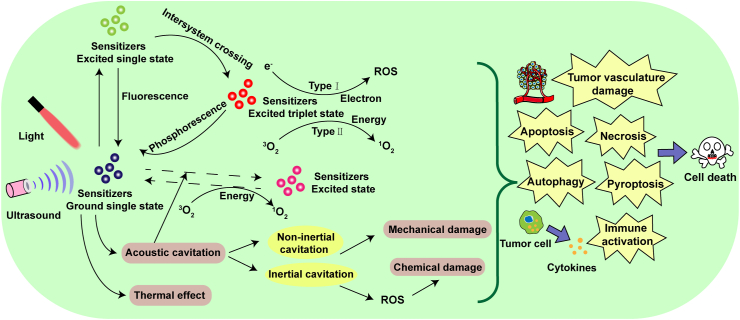
PDT requires three basic conditions, namely light, O2, and photosensitizers. In the presence of light and O2, the photosensitizers that preferentially accumulate in the tumor site are activated to generate ROS (such as superoxide anion radicals O2⋅−, hydroxyl radicals ·OH, hydrogen peroxides H2O2, and 1O2), resulting in cell death15. The photosensitizer under light irradiation can transit from the ground state to the single excited state, which then can transit to the excited triplet state through the intersystem crossing (ISC) process. The photosensitizer in excited triplet state may have two types of reactions19. It can directly react with substrate molecules to form free radicals, and then interact with O2 to generate ROS (type I reaction), or can directly transfer its energy to 3O2 (ground-state molecular O2) to form 1O2 (type II reaction)20. The activated photosensitizer at the tumor site can damage tumor cells, resulting in necrosis, apoptosis, or autophagy21. In the blood vessel, the activated photosensitizer can disrupt the vascular walls and hinder the blood flow to the tumor to cause tumor hypoxia. PDT can also cause the release of some toxic substances to destruct tumor cells and activate the immune response22.
Similar to PDT, SDT also requires three basic conditions, including ultrasound, O2, and sonosensitizers. In the presence of ultrasound and O2, the generation of ROS through the stimulated sonosensitizer and the ultrasound-activated cavitation effects can induce apoptosis, necrosis, and autophagy, thus ultimately contributing to tumor destruction23, 24, 25. Acoustic cavitation can be divided into inertial cavitation and non-inertial cavitation, which is mainly composed of three stages including nucleation, growth, and collapse of bubbles9,26. Inertial cavitation and non-inertial cavitation produce mechanical effects, while inertial cavitation leads to the formation of sonochemical species, including 1O2 and free radicals9. The free radicals generated by ultrasound-activated sonosensitizers can react with the O2 to form peroxyl and alkoxyl radicals. These radicals can induce lipid peroxidation (LPO) and apoptosis, which eventually cause cell death27.
Sensitizers can be activated by ultrasound and light to generate more ROS for SPDT against cancer28. The combination of PDT and SDT can cause tumor necrosis from the surface to the base29. Combined therapy can also inhibit cell migration, decrease mitochondrial membrane potential, and induce apoptosis and autophagy30,31. Inhibition of cell migration capacity was observed in the combined group, accompanied by the declined cell adhesion, severe microfilament network collapse, and decreased expression of matrix metalloproteinase-9 (MMP-9)32. However, the detailed mechanism of SPDT remains unclear. Possible mechanisms of SPDT in cancer therapy are shown in Fig. 1.
Figure 1.
Possible mechanisms of SPDT in cancer therapy.
3. Sensitizers for cancer therapy
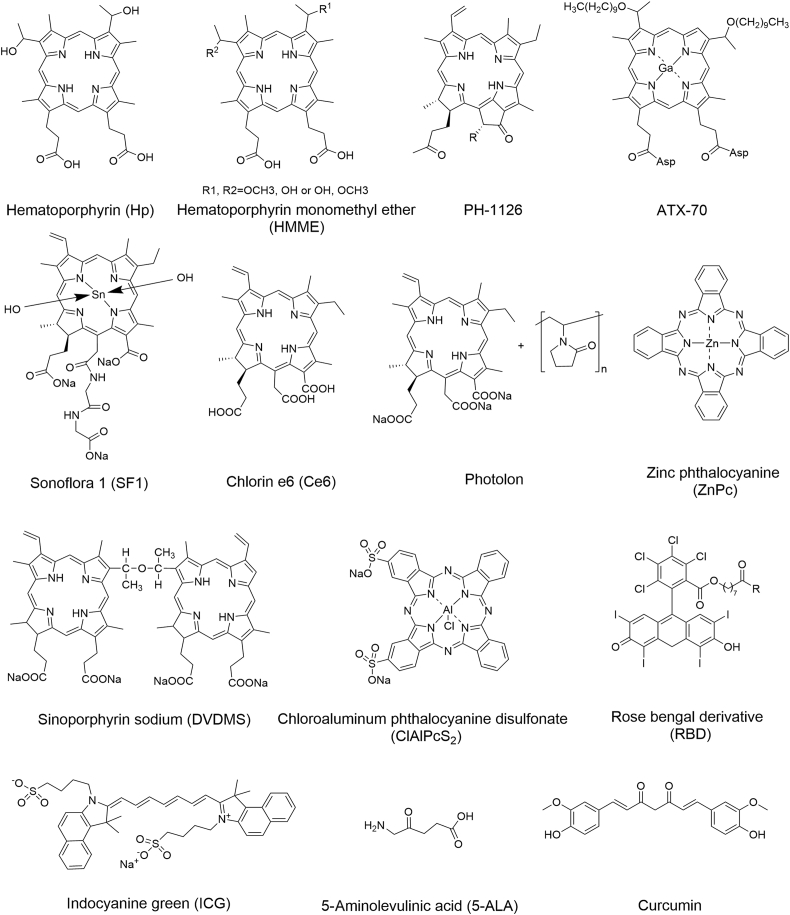
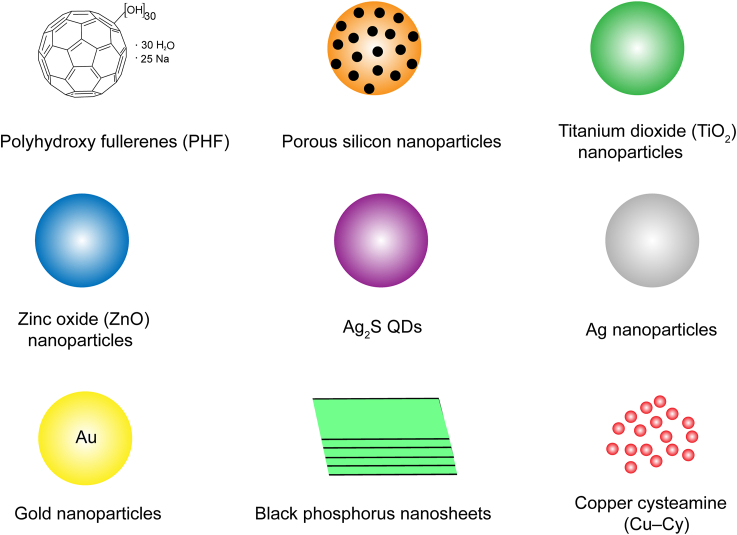
Sono/photo sensitizers can exert therapeutic effects of SDT/PDT and may be further used in SPDT. The chemical structures of organic sensitizers and schematic illustration of inorganic and hybrid sensitizers used in cancer therapy are shown in Figure 2, Figure 3. The characteristic parameters of the sensitizers used in different tumor cells or animal models are summarized in Table 128, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67.
Figure 2.
The chemical structures of organic sensitizers used in cancer therapy.
Figure 3.
Schematic illustration of inorganic and hybrid sensitizers that have been synthesized for SPDT.
Table 1.
The characteristic parameters of sensitizers used in different cancer cell or animal models.
| Sensitizer | Ultrasound |
Laser light |
Cancer cell type | Species | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Frequency (MHz) | Intensity (W/cm2) | Time (min) | Laser wavelength (nm) | Light dose (J/cm2) | ||||
| Hp | 2.2 | 5 | 3 | – | – | S180 | ICR mice | 33 |
| – | – | – | 635 | 4.05 | Fadu | – | 34 | |
| Pheophorbide a | 1.92 | 3 | 15 | – | – | S180 | Male ICR mice | 36 |
| – | – | – | 675 ± 3 | 90 | MCF-7 | Female BALB/c nude mice | 35 | |
| Photofrin | 1.0 | 0.5 | 2 | – | – | U251 | – | 37 |
| – | – | – | 630 | 50–350 | RIF | Female C3H mice | 38 | |
| HMME | 1 | 0.5 | 1.5 | 630 | 20–240 | C6 | – | 39 |
| PH-1126 | 1.0 | 0.51 | 10 | 650 | 44 | SCC | C3H/HeN mice | 29 |
| ATX-70 | 1.0 | 0.51 | 10 | 575 | 88 | SCC | C3H/HeN mice | 29 |
| Ce6 | 1.0 | 0.36 | 1 | 650 | 1.2 | 4T1 | – | 30,31 |
| 1.0 | 0.36 | 1 | 650 | 1.2/2.5 | MDA-MB-231 | – | 32,40 | |
| 1.90 | 1.6 | 3 | 650 | 120 | 4T1 | Female BALB/c mice | 41 | |
| Photolon | 1 | 0.4/0.7/1.0 | 10 | 661 | 50–100 | C6 | White random-bred rats | 42 |
| DVDMS | 1.90 | 1.6 | 3 | 635 | 50 | 4T1 | Female BALB/c mice | 43 |
| ZnPc | 1.1 | 1 | 10 | 670 ± 20 | 300 | CT26 | BALB/c mice | 44 |
| ClAlPcS2 | 1 | 2 | 10 | 660 | 15 | B16F0, NIH3T3 |
– | 45 |
| 1 | 2 | 10 | 635 | 10 | MCF-7 | – | 28 | |
| 1 | 2 | 10 | 635 | 15 | A549 | – | 46 | |
| RBDs | 1.0 | 2.0 | 3 | >500 | 27 | HepG2 | – | 47 |
| ICG | 1 | 3.5 | 3 | 830 | 56.7 | RIF-1 | C3H/HeN mice | 48 |
| 5-ALA | 1 | 3.0 | 10 | – | – | EMT6 | Female BALB/c mice | 50 |
| – | – | – | 630 ± 15 | 40 | A431 | Female SCID mice | 49 | |
| Curcumin | 0.86 | 2 | 5–15 | – | – | THP-1 | – | 52 |
| – | – | – | 445 | 100 | Me180 | Female BALB/c nude mice | 51 | |
| MB | 2 | 0.24 | 0.5 | – | – | S180 | – | 54 |
| – | – | – | 660 | 1–6 | W256 | Female Wistar rats | 53 | |
| HB | 0.84 | 0.25 | 1 | – | – | SGC7901, SGC7901/ADR |
– | 56 |
| – | – | – | 463 | 9 | HepG2 | – | 55 | |
| Porous silicon | 0.88 | 0.5 | 10 | – | – | Hep2 | – | 58 |
| – | – | – | 458 | 15–60 | HeLa, NIH 3T3 |
– | 57 | |
| TiO2 nanoparticles | 1 | 1.0 | 2 | – | – | C32 | BALB/c athymic nude mice | 60 |
| – | – | – | 365 | 5 | U87-MG | Female BALB/c nude mice | 59 | |
| Ag2S QDs | 1.0 | 1.5 | 5 | – | – | C26 | Male BALB/c mice | 61 |
| – | – | – | 808 | 600 | 4T1 | Female BALB/c mice | 62 | |
| Ag nanoparticles | 1 | 0.5–2 | 10 | – | – | A2780 | – | 63 |
| – | – | – | – | 5 × 10−4 | MCF-7 | – | 64 | |
| Au nanoparticles | 1.1 | 2 | 3 | 560 | 35 | CT26 | Male BALB/c mice | 65 |
| Black phosphorus | 1 | 1.5 | 10 | – | – | 4T1 | BALB/c nude mice | 67 |
| – | – | – | 660 | 0.6 | MDA-MB-231 | BALB/nu–nu nude mice | 66 | |
Hp, hematoporphyrin; HMME, hematoporphyrin monomethyl ether; Ce6, chlorin e6; DVDMS, sinoporphyrin sodium; ZnPc, zinc (II) phthalocyanine; ClAlPcS2, chloroaluminum phthalocyanine disulfonate; RBDs, rose bengal derivatives; ICG, indocyanine green; 5-ALA, 5-aminolevulinic acid; MB, methylene blue; HB, hypocrellin B; TiO2, titanium dioxide; S180, mouse sarcoma; ICR, Institute of Cancer Research; Fadu, human oral cancer; MCF-7, human breast cancer; U251, human glioma; RIF, mouse radiation-induced fibrosarcoma; C6, rat glioma; SCC, hunan squamous cell carcinoma; 4T1, mouse breast cancer; MDA-MB-231, human breast cancer; CT26, mouse colon cancer; B16F0, mouse melanoma; NIH3T3, mouse fibroblast; A549, human non-small cell lung cancer; HepG2, human hepatocellular carcinoma; SCID, severe combined immunodeficient; EMT6, mouse mammary carcinoma; A431, human squamous cell carcinoma; THP-1, human monocytes; Me180, human cervical epidermoid carcinoma; W256, walker 256 carcinosarcoma; SGC7901, human gastric adenocarcinoma; Hep2, human laryngeal cancer; HeLa, human cervical cancer; C32, human melanoma; U87-MG, human glioma; A2780, human ovarian carcinoma; ‒, not applicable.
3.1. Organic sensitizers
3.1.1. Hematoporphyrin (Hp)
Hp as a first-generation photosensitizer, has poor water solubility, poor light absorption, and long-enduring skin photosensitivity, which limit its application in PDT68,69. Hp could be used as a sonosensitizer for effective cancer treatment33, solving the problem of poor light tissue permeability. Pheophorbide a35,36, photofrin37,38, tetra-(4-aminophenyl) porphyrin (TAPP)70,71, and meso-tetra(4-carboxyphenyl) porphine (TCPP)72,73 have been proven effective in PDT or SDT.
3.1.2. Hematoporphyrin monomethyl ether (HMME)
HMME as a second-generation porphyrin-related sensitizer, has been used for PDT and SDT with significant anti-cancer effects74,75. Compared with the first-generation sensitizers, HMME has several advantages including better solubility, rapid clearance in the body, and low toxicity76. HMME-mediated SPDT achieved a significant better synergetic effect on rat C6 glioma cells than SDT or PDT alone. Further mechanism investigation showed that HMME-mediated SPDT induced Caspases 3, 8, and 9 activation through ROS generation39. Despite the lack of in vivo study to verify its potential treatment effectiveness, recent results suggested that HMME-mediated SPDT could be a promising approach for human cancer therapy.
3.1.3. PH-1126
PH-1126, a pheophorbide a derivative, has been developed as a photosensitizer by Hamari Chemical Company. PH-1126 could generate more yield of 1O2 than photofrin77. In transplantable mouse squamous cell carcmoma (SCC) model, PH-1126-mediated photo-sonodynamic therapy (PSDT) produced stronger inhibition in tumor growth (98%) than PDT (76%) or SDT (43%) treatment alone. The combined approach could significantly improve the survival of mice as well as reduce drug dosage, resulting in reduced risk of potential skin photosensitivity. Notably, the depth of necrosis increased more than 2-fold by adding of SDT, showing the promise for destruction of non-superficial or nodular tumors29.
3.1.4. ATX-70
ATX-70 is a gallium-porphyrin derivative commonly used as a sonosensitizer78. After intravenous administration of ATX-70 to mouse bearing colon 26 tumor at a dose of 2.5 mg/kg followed by ultrasound irradiation (3 W/cm2), the tumor size decreased within three days after the treatment. Another study reported that using ATX-70 as a sensitizer for PSDT significantly inhibited tumor growth (92%)29.
3.1.5. Sonoflora 1 (SF1)
Chlorophyll is a group of fat-soluble magnesium porphyrin complex, and its derivatives have been used as photosensitizers in PDT79,80. SF1 with molecular weight of 861.48 is a chlorophyll derivative. Using SF1 for SPDT in advanced breast carcinoma has been reported81.
3.1.6. Sonnelux-1
Sonnelux-1 with an average molecular weight of 942 is one of the chlorophyll analogs in that their backbone is porphyrin macrocyclic ring, and the center of the porphyrin ring is populated with a metal ion. This agent displayed high sonodynamic activity without obvious toxicity even in embryonic cells82. Initial in vivo studies showed that sonnelux-1-mediated SDT significantly suppressed the tumor growth of S-180 sarcoma xenograft83. Another work reported that sonnelux-1 has both sonodynamic and photodynamic activities for sono-photodynamic cancer treatment84.
3.1.7. Chlorin e6 (Ce6)
Ce6 is a naturally hydrophilic chlorin derivative, which is also considered to be a second-generation photosensitizer. Several studies demonstrated that Ce6 displayed significant photodynamic and sonodynamic anti-cancer effects. Recently, the combination of PDT with SDT by using Ce6 has been developed for cancer therapy. Ce6 was mainly localized in the mitochondria of the 4T1 murine breast cancer cells, and the enhanced cell death after SDT and PDT revealed the therapeutic potential of Ce6-mediated SPDT. Further investigations showed that Ce6-mediated SPDT could induce significant DNA damage and clonogenicity suppression30. Additionally, ultrasound could enhance cell permeability, which in turn increases the uptake of Ce6 for subsequent laser irradiation, thus improving the therapeutic effect of SPDT30,40. Ce6-mediated SPDT/PSDT decreased the cell viabilities in various breast cancer cell lines (MDA-MB-231, MCF-7, and 4T1) and Ce6-mediated SPDT markedly suppressed tumor growth and metastasis in 4T1 mouse breast cancer xenograft model. Besides, Ce6-mediated SPDT could cause mitochondrial membrane potential loss, induce tumor cell apoptosis, and decrease the expression levels of vascular endothelial growth factor (VEGF) and MMP-941. The inhibition of the adhesion and migration was observed in MDA-MB-231 cells via Ce6-mediated SPDT or PSDT32. Another study investigated the underlying mechanisms of Ce6-mediated SPDT on apoptosis and autophagy in 4T1 cancer cells. SPDT could increase apoptosis-related protein Cleaved-Caspase-3 and PARP, decrease BCL-2 level, and maintain a stable BAX expression level. The conversion of LC3-I to LC3-II indicated the occurrence of autophagy, which was accompanied by the increased BECLIN-1 expression31.
Photolon is one of the most promising photosensitizer, which has been approved for clinical use. It is formed by the combination of Ce6 with hydrophilic polyvinylpyrrolidone (PVP) for improvement of aqueous solubility85. Photolon is mainly distributed in the cytoplasmic organelles and nucleus after administration in the murine colon carcinoma CT-26 cells. Under the light dose of 1 J/cm2, cell apoptosis reached 80%86. Photolon can also increase the cytotoxic effect against glioma C6 cells under ultrasound irradiation87. After intravenous administration of photolon to mouse bearing glioma C6 brain tumor at a dose of 2.5 mg/kg followed by ultrasound and laser irradiation treatment, tumor necrosis could be found in almost the entire tumor area42. These encouraging results demonstrated the potential clinical application of photolon-mediated SPDT.
3.1.8. Sinoporphyrin sodium (DVDMS)
DVDMS as a newly discovered photosensitizer is a conjugate of two porphyrin monomers with enhanced effects and low side effects compared to clinically used photofrin88. Both in vitro and in vivo phototoxicity and sonotoxicity studies revealed that the DVDMS-mediated SPDT had stronger therapeutic effects on breast cancer over SDT or PDT alone43. After the SPDT treatment, the cell viability losses in 4T1, MDA-MB-231, and MCF-7 cells were 77.48%–86.13%, and the tumor growth was significantly suppressed in a mouse 4T1 xenograft model. Investigations also revealed that DVDMS-mediated PSDT had equal anti-tumor effects when the order of SDT and PDT was exchange.
3.1.9. Zinc (II) phthalocyanine (ZnPc)
Phthalocyanines as second generation photosensitizers, are aromatic heterocycles that consist of four isoindole rings bridged by nitrogen atoms, which have long absorption wavelength and high extinction coefficients89. ZnPc is a phthalocyanine in which Zn metal ions are in the coordination center of the phthalocyanine. The Zn metal ions can strongly affect the photochemical properties of ZnPc and make ZnPc an efficient 1O2 generator. ZnPc has the advantages of high therapeutic efficacy and minimal skin photosensitivity, and the generated ROS under light irradiation can damage tumor cells90. However, ZnPc as a photosensitizer has not been approved for clinical use. ZnPc can be used as a sonosensitizer for SDT to produce radicals and damage cell membrane91. Studies on colon carcinoma tumor in BALB/c mice revealed that liposomal ZnPc could enhance ultrasound- and light-caused tumor shrinkage after light (160 mW/cm2, 300 J/cm2) and ultrasound (1.1 MHz, 1 W/cm2, 10 min) treatments44. It is worth mentioning that the arrangement of PDT and SDT was an important factor affecting the efficacy of ZnPc-mediated SPDT. The findings suggested the potency of development of phthalocyanines for SPDT in future.
3.1.10. Chloroaluminum phthalocyanine disulfonate (ClAlPcS2)
ClAlPcS2 as a photosensitizer demonstrated more phototoxic effects on G361 melanoma cells and MCF-7 breast adenocarcinoma cells than on NIH3T3 mouse fibroblasts and B16 mouse melanoma cells92. The number of necrosis cells treated with ClAlPcS2 at 100 μmol/L is higher than that of apoptotic cells after PDT45. MCF-7 cells treated with 100 μmol/L ClAlPcS2 and ultrasound followed by light irradiation at an intensity of 2 mW/cm2 could produce higher ROS, which indicated that ultrasound could enhance the photodynamic effect in breast cancer cells28. A549 human lung cancer cells treated with ClAlPcS2-mediated SDT after PDT could generate more ROS compared with those treated with SDT followed by PDT, demonstrating the great effects of the irradiation sequence on ClAlPcS2-mediated SPDT46.
3.1.11. Rose bengal derivatives (RBDs)
Rose bengal (RB) as a hydrophilic anionic sensitizer has attracted considerable attention in many years. Previous studies have shown that RB can be used as a photosensitizer or sonosensitizer for the treatment of non-melanoma and melanoma skin cancer49, but poor tumor accumulation limited its further clinical application93. The amphiphilic derivatives of RB (RBDs) were synthesized to improve the accumulation ability of RB in tumors with high partition coefficient93,94. One of RBDs linked to a lipid microbubble showed increased 1O2 quantum yield and enhanced cytotoxicity in vitro, and could suppress tumor growth in vivo upon SDT95. Recently, a series of amphiphilic RBDs with enhanced SPDT effects have been developed. In vitro studies revealed that RBDs exhibited significant anti-cancer effects in HepG2 cells which were treated with 0.5 μmol/L of RBDs for 2 h and then exposed to ultrasound (1.0 MHz, 2 W/cm2, 3 min) and light (λ > 500 nm) for 30 min at a dose of 27 J/cm247. These findings implied the potential use of amphiphilic RBDs as sensitizers for SPDT in future.
3.1.12. Indocyanine green (ICG)
ICG as a medical diagnostic agent has been approved by the U.S. Food and Drug Administration (FDA)96. Many studies employed ICG derivatives as useful platforms for design of NIR fluorescent probes97. Human gingival fibroblast (HGF) cells treated with ICG under NIR irradiation could induce significant expression of apoptosis-related gene BAX98, indicating ICG a promising photosensitizer for PDT. ICG-mediated SPDT could lead to a 75% decrease in RIF-1 (radiation-induced fibrosarcoma) cells and the RIF-1 tumor volumes in C3H/HeN mice regressed to nearly immeasurable sizes at earlier stages. In vivo studies also demonstrated that even at Day 25 the RIF-1 tumor sizes had not fully recovered to their initial volume. Although the precise mechanism of this combination treatment is unclear, the ICG-mediated SPDT might be beneficial for cancer therapy48.
3.1.13. 5-Aminolevulinic acid (5-ALA)
Protoporphyrin IX (PpIX) can be used as a photosensitizer or sonosensitizer for cancer therapy99,100. 5-ALA is a metabolic precursor of the endogenously formed sensitizer PpIX, and has significant anti-tumor effects toward mouse mammary EMT6 tumor cells in vitro and in vivo under ultrasound via causing mitochondrial oxidative damage50. 5-ALA-mediated SDT could cause certain killing effects on pancreatic cancer cells through mitochondrial-dependent apoptosis101. 5-ALA-mediated PDT/SDT can induce a significant overexpression of pro-apoptotic gene APAF1 in human fibrosarcoma (HT-1080) cells102. Another study demonstrated that 5-ALA-mediated PDT or SDT had good therapeutic effects in squamous cell carcinoma (A431) cells and A431 ectopic tumors in mice49.
3.1.14. Curcumin
Curcumin is the main active ingredient of turmeric, and has various bioactivity including anti-tumor, anti-oxidation and anti-inflammatory effects103. Recently, studies have found that curcumin can be used as a sensitizer in PDT and SDT51,52. Curcumin has the characteristics of poor absorption, low toxicity, and rapid clearance with reduced skin photosensitivity and poor bioavailability104. Human keratinocyte HaCaT cells treated with curcumin under the ultraviolet radiation b (UVB) irradiation could induce caspase-3 activation, thus leading to cell apoptosis105. Curcumin-mediated SDT could efficiently inhibit cell growth, and induce apoptosis and mitochondrial autophagy to cause cell death106,107.
Other sensitizers also have been proved to be effective in PDT or SDT including acridine orange (AO)108,109, methylene blue (MB)53,54, IR780110,111, and hypocrellin B (HB)55,56.
3.2. Inorganic sensitizers
3.2.1. Polyhydroxy fullerenes (PHF)
Fullerenes with large molar absorption coefficients and high triplet yields can be used as photosensitizers for cancer therapy112. Many functionalized fullerenes (such as hydroxyl, carboxyl, and amino functional groups) were synthesized to increase hydrophilicity113. PHF with water solubility, biocompatibility, and biodegradability could generate 1O2 for PDT under light114. PHF can also be used as a potential sonosensitizer for SDT to treat tumors. Sarcoma 180 cells treated with PHF under ultrasound (2 MHz, 6 W/cm2) could induce cell damage and lipid peroxidation115. In another study, PHF combined with ultrasound could inhibit colon 26 tumor growth in male BALB/c mice116.
3.2.2. Porous silicon
Porous silicon is made of a network of intersecting silicon nanocrystals separated by nanometer-sized pores, and the visible photoluminescence could be observed at room temperature117,118. Previous studies have shown that porous silicon can be used as a photosensitizer to generate 1O2 through energy transfer at room temperature using light of the entire visible range119. Human cervical cancer HeLa cells treated with porous silicon nanoparticles under white light irradiation (60 J/cm2) can cause more cell death than the control group57. Porous silicon nanoparticles can also be used as a sonosensitizer for SDT. Treatment of laryngeal cancer Hep-2 cells with porous silicon nanoparticles upon ultrasound could effectively inhibit cell proliferation58. The dextran-coated porous silicon nanoparticles showed significant anti-tumor effects in vitro and in vivo under ultrasound irradiation with frequencies of 1–3 MHz and intensities of 1–2 W/cm2120.
3.2.3. Titanium dioxide (TiO2)
TiO2 with photocatalytic properties can be used as a photosensitizer for cancer therapy. TiO2 nanoparticles could induce apoptosis and necrosis under UV irradiation, and significantly reduce the growth of gliomas59. In addition to the use of TiO2 nanoparticles for PDT, TiO2 nanoparticles with sonocatalytic properties can also be used as sonosensitizers for SDT. Ultrasound irradiation of TiO2 nanoparticles at different frequencies and intensities had different sonodynamic therapeutic effects, and the generated ·OH could effectively inhibit the growth of HepG2 cancer cells121. Preliminary researches of TiO2 nanoparticles-mediated SDT against melanoma tumors60, hepatoma122, and oral squamous cell carcinoma123 were investigated. To prolong in vivo circulation time, hydrophilic TiO2 was developed124.
3.2.4. Zinc oxide (ZnO)
ZnO nanoparticles with high stability, wide band gaps, and inherent photoluminescence properties could be used for biomedical and cancer applications125. ZnO nanoparticles as photosensitizers could produce ROS under UV irradiation and induce caspase-dependent apoptosis to reduce cell viability of SMMC-7721 hepatocarcinoma cells126. ZnO as a semiconductor sonosensitizer has a potential in sonodynamic cancer therapy. Amino-propyl group-functionalized ZnO nanocrystals could produce ROS under the pulsed ultrasound exposure127. In another study, a defect-rich gadolinium (Gd) doped ZnO (D-ZnOx:Gd) was developed for effective deep tumor sonodynamic eradication. D-ZnOx:Gd can produce more ROS than other sonosensitizers TCPP, TiO2, and ZnO under ultrasound128.
3.2.5. Ag2S quantum dots (QDs)
Semiconductor QDs have attracted increasing attention due to tunable band gaps, high molar extinction coefficients, sufficient photostability, and the ability to generate multiple electron–hole pairs129. Ag2S QDs could be used as either sonosensitizer or photosensitizer to generate ROS for PDT and SDT61,62. Biocompatible Ag2S QDs prepared using high-temperature pyrolysis method were modified with PEGylated phospholipids to form nanoparticles, which had photodynamic behavior under 808 nm NIR irradiation. In addition, the Ag2S QDs could be covalently attached on the polydopamine (PDA) surface to produce stronger PDT effects by generating more ROS62.
3.2.6. Silver (Ag) nanoparticles
Ag nanoparticles have anti-inflammatory and anti-cancer activities, which can be applicable for biomedicine130. Ag nanoparticles could inhibit cancer cells under either ultrasound or light irradiation. A significant decrease of cell viability of human ovarian carcinoma A2780 cells was observed after the treatment of Ag nanoparticles and ultrasound, indicating that Ag nanoparticles have potential to be used as sonosensitizers for SDT63. Biosynthesized Ag nanoparticles could produce ROS under light irradiation from a solar simulator to induce cell apoptosis. Further investigations revealed that Ag nanoparticles-mediated PDT could dramatically increase the ratio of BAX/BCL-2 protein expression in MCF-7 cells64.
3.2.7. Gold (Au) nanomaterials
Au nanomaterials including Au nanocages, Au nanorods, and Au nanoparticles have been used for PDT or SDT131,132. Lipid-coated Au nanocages could efficiently kill HeLa cells in vitro and inhibit B16F0 melanoma tumor growth upon NIR laser131. In another study, significant inhibition of tumor growth was observed in BALB/c mice bearing colon carcinoma tumors treated with Au nanoparticles under intense pulsed light and ultrasound65. The findings suggested that Au nanomaterials can be used for SPDT in future.
3.2.8. Black phosphorus
Black phosphorus is a metal-free layered semiconductor that has the advantages of tunable layer-dependent bandgap, wide light absorption, good biodegradability and biocompatibility, which can be used for PDT and SDT to treat cancer66,67. Ultrathin black phosphorus nanosheets with high quantum yield of 1O2 generation under the entire visible light region could induce cell apoptosis, inhibit cell proliferation, and suppress tumor growth under light irradiation66. Recently, black phosphorus nanosheets which were stable within the timeframe of ultrasound-exposure before degradation were developed for SDT67. Under ultrasound, black phosphorus nanosheets could produce ROS to effectively inhibit cell proliferation, and inhibit tumor growth and metastasis in 4T1 tumor-bearing BALB/c mice.
3.3. Hybrid sensitizers
Copper cysteamine (Cu–Cy) complex as a new type of sensitizer could be activated by light to produce 1O2133. Cu–Cy nanoparticles could generate ROS upon UV light (360 nm) and microwave (2, 5, and 10 W), and were effective in killing KYSE-30 cancer cells at low concentration of Cu–Cy and low microwave power in a relatively short time134. Cu–Cy nanoparticles could inhibit the proliferation of human colorectal cancer cells, induce apoptotic cell death, and decrease mitochondrial membrane potential under X-ray-induced PDT for deep cancer treatment135. In another study, breast cancer cells (MCF-7, 4T1, and MDA-MB-231 cells) treated with Cu–Cy nanoparticles and ultrasound exposure showed cell destruction and cell apoptosis due to ROS generation. Cu–Cy nanoparticles under ultrasound could significantly inhibit tumor growth in 4T1 tumor-bearing mice, suggesting the potential use of Cu–Cy as a sonosensitizer for SDT136.
4. Different strategies for enhanced SPDT by NDDSs
Despite SPDT can induce tumor cell death, the severe skin photosensitization caused by the long-enduring photoactivities of sensitizers and the limited penetration depth of light therapy in tumor tissues restrict the application of SPDT. In addition, sensitizers with short blood circulation time, poor tumor-targeting specificity, and incapability of deep intratumoral penetration significantly affect the therapeutic effect of SPDT. Moreover, due to the O2 dependence, the therapeutic efficacy of PDT/SDT in tumor hypoxic microenvironment is limited.
The fluorescence of sensitizer could be quenched inside the NDDSs and be recovered in response to the stimuli, thus reducing the skin photosensitization137. The blood circulation time of sensitizers could be prolonged when entrapped in NDDSs, achieving high drug accumulation in tumor sites138. NDDSs with targeting ligand-modification can improve tumor targeting and increase cellular uptake of sensitizers139. NDDSs can also be designed to enhance intratumoral penetration of sensitizers and to relieve tumor hypoxia by increasing intracellular O2 content140.
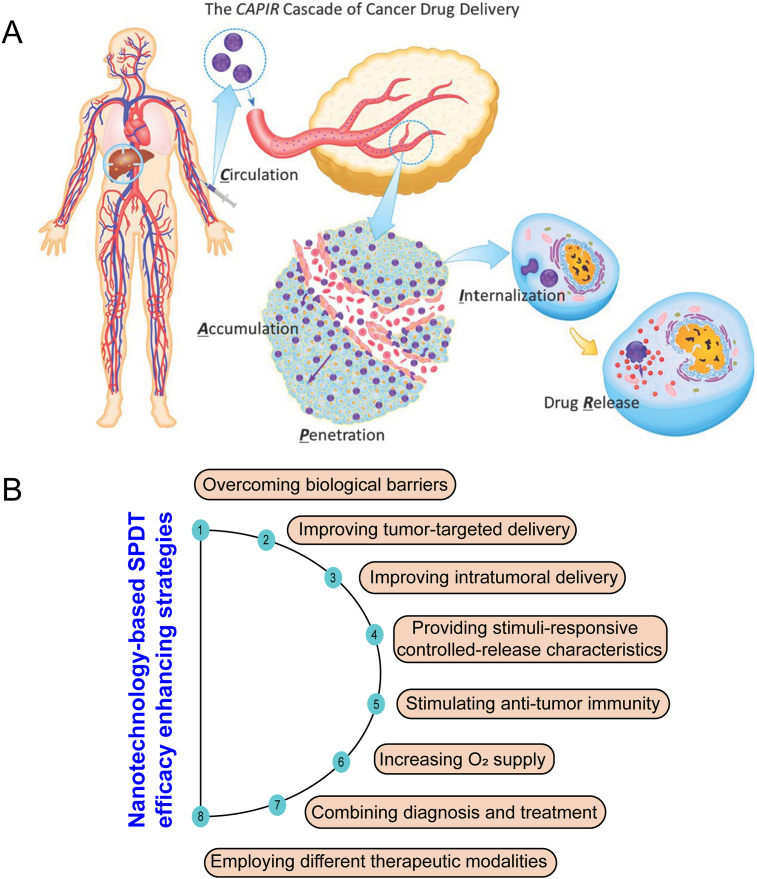
Various NDDSs, including lipid-based nanoparticles, polymer-based nanoparticles, protein-based nanoparticles, and inorganic-based nanoparticles can be used to deliver sensitizers for SPDT to treat tumors. Some inorganic sensitizers can also be used as NDDSs for drug delivery and exert SDT/PDT effects at the same time. The processes of drug delivery to solid tumors can be summed into five critical steps, termed the CAPIR cascade: blood circulation, accumulation and penetration into the tumor, cellular internalization, and intracellular drug release141 (Fig. 4). NDDSs can improve the drug delivery in one or two steps in CAPIR cascade. Different strategies with the assistance of NDDSs, such as overcoming biological barriers, improving the targeting of sensitizers, increasing intratumoral delivery, providing stimulus-responsive controlled-release function, stimulating anti-tumor immunity, increasing O2 supply, employing different therapeutic modalities, and combining diagnosis and treatment have been explored to enhance the therapeutic effect of SPDT (Table 271,138,140,142, 143, 144, 145, 146, 147 and Supporting Information Table S1).
Figure 4.
(A) Schematic diagram of the CAPIR cascade in cancer drug delivery: blood circulation, tumor accumulation and penetration, and subsequent cellular internalization and intracellular drug release. Reprinted with the permission from Ref. 141. Copyright © 2014, Wiley (B) Schematic illustration of different strategies to enhance SPDT by NDDSs for cancer therapy.
Table 2.
Different NDDSs for enhanced SPDT.
| NP Platform | Carrier material | Sensitizer | Light dose | Ultrasonic dose | Cancer cell type | Species | Function | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polymeric NPs | PLGA/PFP/PTX | ICG | 808 nm 1.5W/cm2, 5 min |
1 MHz, 1 W/cm2, 1 min |
SKOV3 | Female BALB/c athymic nude mice | O2 supply Combination therapy Integration of diagnosis and therapy |
147 |
| Micelles | C18GR7RGDS/ICG | ICG | 808 nm 1.5 W/cm2, 3 min |
1 MHz, 2.4 W/cm2, 5 min, 50% duty cycle |
MDA-MB-231 | Male nude mice | Intratumor delivery Combination therapy |
144 |
| Lipid-based NPs | DPPC/DPPG/DSPE-PEG-FA/Cholesterol/PFH | ICG | 808 nm 1.5 W/cm2, 5 min |
300 kHz, 1 W/cm2, 30 s |
SKOV3 | Female BALB/c nude mice | Tumor-targeted delivery Combination therapy Integration of diagnosis and therapy |
142 |
| Protein-based NPs | HSA-Ce6/TAM | Ce6 | 660 nm 5 mW/cm2, 30 min |
– | 4T1 | Female nude mice | Intratumor delivery O2 supply |
140 |
| Peptide-based NPs | C18GR7RGDS/RB | RB | 808 nm 1.5 W/cm2, 3 min |
1.0 MHz, 1.0 W/cm2, 50% |
HeLa | Male nude mice | Intratumoral delivery | 143 |
| Biomimetic NPs | RBC membrane/CAuNCs/HA/PXTK/dPPA | Pba | 650 nm 270 mW/cm2, 4 min |
– | 4T1 | Female BALB/c mice | Intratumoral delivery Combination therapy |
138 |
| Inorganic NPs | HA-Mesoporous CaCO3 | HMME | – | 1 MHz, 1 W/cm2, 1 min |
MCF-7 | BALB/c nude mice | Tumor-targeted delivery Stimuli-responsive release Integration of diagnosis and therapy |
145 |
| Metal-organic frameworks | LMWHA-PEI-MPB | HMME | – | 3 MHz, 1.0 W/cm2, 1 min |
4T1 | Female BALB/c mice | Immunity modulation O2 supply |
146 |
| Hybrid NPs | p-(OEOMA-co-MEMA)/Pt–CuS | TAPP | – | 1 MHz, 1.0 W/cm2, 5 min, 60% duty cycle |
CT26 | Female BALB/c mice | O2 supply Combination therapy Integration of diagnosis and therapy |
71 |
NPs, nanoparticles; PLGA, poly(lactic-co-glycolic) acid; PFP, perfluoropentane; PTX, paclitaxel; ICG, indocyanine green; DPPC, dipalmitoylphosphatidylcholine; DPPG, 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol); DSPE-PEG-FA, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate(polyethyleneglycol)]; PFH, perfluorohexane; HSA, human serum albumin; TAM, tamoxifen; Ce6, chlorin e6; RB, rose bengal; RBC, red blood cell; CAuNCs, cationized gold nanoclusters; HA, hyaluronic acid; PXTK, paclitaxel dimer prodrug; dPPA, anti-PD-L1 peptide; Pba, pheophorbide a; CaCO3, calcium carbonate; HMME, hematoporphyrin monomethyl ether; LMWHA, low molecular weight hyaluronic acid; MPB, mesoporous Prussian blue; p-(OEOMA-co-MEMA), poly(oligo(ethylene oxide)methacrylate-co-2-(2-methoxyethoxy) ethyl methacrylate; CuS, copper sulfide; TAPP, tetra-(4-aminophenyl) porphyrin; SKOV3, human ovarian cancer; MDA-MB-231, human breast cancer; 4T1, mouse breast cancer; HeLa, human cervical cancer; MCF-7, human breast cancer; CT26, mouse colon cancer; ‒, not applicable.
4.1. Overcoming biological barriers
Various biological barriers, such as mucosal, blood–brain barrier (BBB), blood vessel–tumor barrier can hamper the drug delivery, thus limiting the effectiveness of cancer therapy. Some carrier materials were introduced to prepare NDDSs to overcome biological barriers to improve drug transport and delivery. Chitosan can significantly increase the mucosal lipid fluidity to enhance drug delivery across the mucosal layer. Ce6 was incorporated into ursodeoxycholic acid-conjugated chitosan through hydrophobic interaction to fabricate nanoparticles. The nanoparticles could increase the transport of drugs through the mucosal layer for enhanced PDT148. Mechanical action can be used to temporarily open biological barriers. Gas-filled microbubbles can be used as effective adjuvants to enhance SDT by increasing membrane permeability through ultrasound targeted microbubble destruction (UTMD)149. Microbubbles can expand and push/disrupt the endothelial lining in the brain under ultrasound, thus temporarily opening the BBB. UTMD could open the BBB to improve the delivery of iRGD-modified DVDMS liposomes to the brain, and the nanosized DVDMS could significantly suppress the orthotopically implanted C6 gliomas via ROS generation under low intensity ultrasound150. RBD with self-assembling nature can encapsulate a fluorinated gas to fabricate RBD-microbubbles which could in situ convert into RBD-nanoparticles by UTMD. The temporarily induced high permeability of the capillary wall facilitated the cellular drug uptake and resulted in about 7.5 times higher drug accumulation at the tumor tissue than that of other treatment groups to produce high yields of 1O2 for enhanced SDT151. Acoustic droplet vaporization (ADV) can also generate microbubbles, making the blood vessel–tumor barrier more permeable. IR780-based nanodroplets (IR780-NDs) could penetrate deeper tumor tissues due to the disruption of blood vessels and tissue erosion caused by ADV, and produce ROS to induce cell apoptosis under ultrasound irradiation for enhanced deep-penetration SDT152.
4.2. Improving tumor-targeted delivery
After free drugs entered the body, only a small part of them distributed in the lesion site, and some of them metabolized before they reached the lesion site, resulting in reduced efficacy and serious toxic side-effects. NDDSs can selectively deliver drugs to tumor site through negative and active targeting, and thus effectively increase the concentration of drugs in tumor. According to the different target sites, targeting can be divided into tissue/organ level, cellular level, and subcellular level.
4.2.1. Tumor tissue targeting
NDDSs can passively accumulate in tumor tissues due to the enhanced permeability and retention (EPR) effect. Chitosan/RBD composite nanoparticles with high drug accumulation in the tumor site were observed in CT-26 colon cancer transplanted BALB/c mice, which could be an efficient delivery system for targeted SDT94. Some NDDSs with longer blood circulation time can also increase the distribution of sensitizers in tumor tissues. IPH@RBC composed of red blood cell (RBC) membranes and albumin nanoparticles (IPH) was used to encapsulate ICG and perfluorotributylamine (PFTBA). The elimination half-life (t1/2) of IPH@RBC was about 15.71 h, which was nearly 14-fold higher than that of other treatment groups. IPH@RBC could significantly prolong blood circulation time and achieve 5.6-fold higher fluorescence of ICG in tumor site than that of ICG-HSA and IPH, thus effectively inhibiting tumor growth under NIR laser153.
There are various factors and enzymes in tumor microenvironment. NDDSs can mediate the targeted delivery of the encapsulated sensitizers to the tumor site by recognizing these factors and enzymes in the microenvironment. Matrix metalloproteinase-2 (MMP-2) is overexpressed in the tumor microenvironment. NDDSs modified by MMP-2-cleavable polypeptide could effectively reach the tumor site and responsively release cargoes inside the tumor tissue, thus improving the therapeutic effect. Au nanoparticles were co-modified with thiolated peptide HS-R8-PLGLAG-EK10 and 5-ALA to construct prodrug nanocarriers, which could effectively reach the tumor site by the mediation of MMP-2 for targeted PDT154. In another work, MMP-2-cleavable polypeptide modified PEGylated Ce6 could self-assemble into nanoparticles to target tumor site with improved PDT155.
4.2.2. Tumor cell targeting
Employing targeting ligands including folate (FA), transferrin, antibodies, aptamers which recognize receptors overexpressed on cancer cells could allow NDDSs achieving selective cancer cell targeting156. NDDSs can be modified with targeting ligands to improve tumor cell targeting and drug delivery efficiency. FA-modified poly (lactic-co-glycolic) acid (PLGA) nanoplatforms could efficiently accumulate in tumor through FA receptor-binding and the loaded HMME could exert ultrasound-triggered SDT to suppress tumor growth in MDA-MB-231 tumor-bearing mice157. AS1411 aptamer-modified upconversion nanoplatform could promote cellular uptake through nucleolin-binding and achieve NIR-triggered PDT to treat deep-seated tumors158. TiO2-coated upconversion nanoparticles (UCNPs) modified with PEGylated epithelial growth factor receptor (EGFR) affibody could specifically target EGFR expressing cancer cells and were internalized much more rapidly and efficiently (∼3.8-fold) than unmodified TiO2-UCNPs, significantly delaying tumor growth under 980 nm NIR-II laser irradiation159.
FA-targeted perfluorohexane (PFH)/ICG-loaded lipid nanoparticles could specifically target SKOV3 ovarian cancer cells and be endocytosed with a remarkable efficiency for PSDT/photothermal therapy (PTT) treatment in ovarian tumors. The photoacoustic (PA) signals in the tumor area could reach the maximal intensity at 6 h after injection of FA-modified nanoparticles, which is stronger than the non-FA-targeted group at 12 h post-injection142.
4.2.3. Cellular organelle targeting
Various intracellular organelles, including lysosomes, mitochondria, Golgi complex, endoplasmic reticulum, and nuclei, involved in the pathogenesis of cancer and sensitizers can exert their desired therapeutic effects in these organelles. Modification of NDDSs with specific moieties can deliver sensitizers to specific organelles for better efficacy. NDDSs modified with triphenylphosphonium (TPP) have been developed to actively target mitochondria. Mitochondria-targeted mesoporous silica nanoparticles (MSN) could generate a large amount of ROS under laser irradiation and boost the Ce6-mediated PDT, thus causing the mitochondrial dysfunction and irreversible cell death160. Mitochondria-targeted HMME/Cu2+ ion-doped mesoporous silica nanosystems could generate 1O2 under ultrasound, and the released Cu2+ ions could convert endogenous H2O2 to ·OH, thus effectively inducing mitochondrial disintegration and damage161. A conjugate TAT-IR780 and doxorubicin (DOX) were self-assembled into TID nanoparticles for perinuclear region targeting. The TID nanoparticles-mediated PDT could generate ROS and induce cell apoptosis under 785 nm laser irradiation162.
4.2.4. Multi-targeting
NDDSs target more than one site could minimize off-target effects with high target specificity and selectivity. NDDSs designed with multiple-targeting effects can better accumulate in tumors, achieving better therapeutic effects. Receptor integrin not only overexpresses in tumor cells, but also participates in tumor angiogenesis. iRGD (CRGDKGPDC)-modified liposomes could target tumor vessels and tumor cells through integrin αvβ3-binding, and could efficiently ablate laryngeal carcinoma Hep-2 cells upon phototherapy163. In addition to iRGD peptide, F3 peptide that specifically targets nucleolin can also be used for mediating dual-targeting delivery. F3 peptide-modified nanoparticles were developed for targeted delivery to both tumor cells and tumor angiogenic endothelial cells to exert SDT effects in vitro under ultrasound164. Ce6-conjugated β-cyclodextrin and a designed peptide Ad-CGKRK-GFLG-EE-HAIYPRH (T7) could self-assemble into supramolecular micelles with dual targeting ability for enhanced PDT. The micelles could enhance intracellular internalization via a transferrin receptor (TfR)-mediated pathway, and could accumulate in the mitochondria due to the exposure of CGKRK to prompt ROS generation upon irradiation165.
4.3. Improving intratumoral delivery
Although some drugs can reach the tumor sites, they cannot get into deep tumor tissues to obtain good therapeutic effects. Solid tumors are characterized by abnormal tumor vasculature, increased interstitial fluid pressure, as well as dense extracellular matrix (ECM). The deep tumor penetration depends on the physiology of tumors and the properties of NDDSs. Particle size, surface charge, and particle shape of NDDSs have impacts on tumor penetration14. NDDSs with suitable physiochemical properties can penetrate into the internal area of the tumor and reach intratumoral cancer cells to enhance the anti-tumor effect.
4.3.1. Size transition
Nanoparticles with large size have a good retention ability in tumor tissues, while nanoparticles with smaller sizes are apt to penetrate deeply into tumor sites166. Size-switchable NDDSs with self-destructive and tumor penetration characteristics have been developed for enhanced cancer therapy. Biomimetic nanoparticles with optimal size composed of RBC membrane, hyaluronic acid (HA), and cationized Au nanoclusters could degrade into small cores in the presence of hyaluronidase to enhance tumor penetration, and exhibit high tumor accumulation for chemotherapy/PDT/immunotherapy. RBC membrane-coated nanoparticles with the sizes of 300, 200, and 150 nm, showed significantly much higher internalization than the uncoated groups at 4 h (increased by 2.02-, 1.55- and 1.95-fold)138. The self-assembled human serum albumin (HSA)-Ce6/tamoxifen nanocomplexes could change particle size from about 130 to 10 nm owing to the protonation of tamoxifen to induce pH-responsive dissociation of HSA-Ce6. The size change of the nanocomplexes could significantly improve intratumoral penetration for enhanced PDT140. A hypoxia-responsive human serum albumin (HSA)-based nanosystem was developed with a size of 100–150 nm under normoxic condition. The nanosystem could quickly dissociate into ultrasmall therapeutic nanoparticles (below 10 nm) under the hypoxic tumor microenvironment to enhance intratumoral penetration and improve PDT effects167. ICG-conjugated poly (amidoamine) dendrimer (PAMAM-ICG) was conjugated to PEG-b-poly (ε-caprolactone) (PEG-b-PCL) through a 1O2-responsive thioketal bond. The drug-conjugated copolymer/Ce6 nanoparticles could accumulate in the blood vessel extravasation sites due to their large size. Upon 660 nm irradiation, the activated Ce6 could generate 1O2 to kill cancer cells in the perivasculature and small-sized PAMAM-ICG was simultaneously released due to the cleavage of the thioketal bond to penetrate into the internal area of the tumor. The released PAMAM-ICG could efficiently ablate cancer cells in the hypoxic microenvironment after 808 nm irradiation168.
4.3.2. Charge reversal
Besides particle size, surface charge also has great effects on the tumor permeability and treatment efficacy of NDDSs. Because positively charged NDDSs can easily enter negatively charged cells, nanoparticles can be designed to be transformed into positively charged particles via pH-induced surface charge switching to penetrate into tumor cells169,170. Hollow silicon nanoparticles with catalase within their inner cavities and Ce6 doped in the silica lattice structure were modified with (3-carboxypropyl) triphenylphosphonium bromide and a pH-responsive charge-convertible polymer. The nanoparticles could convert into positively charged nanoparticles under acidic condition (pH 6.8) for enhanced tumor penetration and improved PDT170.
4.3.3. Membrane transport
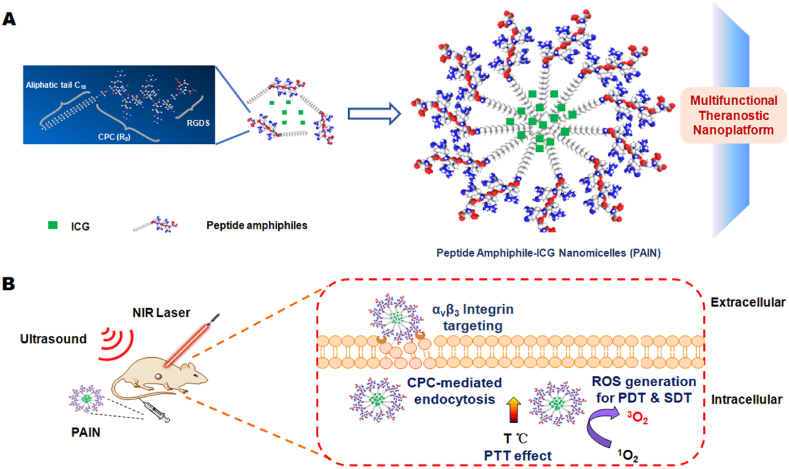
Some membrane transport peptides can traverse the cell membrane for drug delivery. A peptide amphiphile (C18GR7RGDS) was synthesized by introducing a hydrophilic (RGDS) terminal and a hydrophobic (C18) terminal into the spacer ends of a cell-penetrating chain of R8. The mixture of RB and C18GR7RGDS could self-assemble into nanocapsules with cell-penetrating properties to inhibit HeLa tumor growth upon PSDT treatments143. Peptide amphiphile C18GR7RGDS and ICG could self-assemble into functional nanomicelles with efficient cell-penetrating capabilities, which could significantly inhibit MDA-MB-231 tumor growth after PSDT/PTT treatments144 (Fig. 5).
Figure 5.
A schematic illustration for the chemical structure of peptide amphiphile-ICG nanomicelles (PAIN) to treat breast cancer. PAIN with efficient cell-penetrating capabilities could penetrate into tumor cells for synergistic SDT/PDT/PTT. Reprinted with the permission from Ref. 144. Copyright © 2020, Elsevier, Ltd.
4.4. Providing stimuli-responsive controlled-release characteristics
NDDSs can be designed to be responsive to external or intracellular environment to achieve controlled release of sensitizers. Stimuli-responsive release of NDDSs can avoid premature drug release and enhance the intracellular concentration of drugs in cancer cells. The multiple stimuli-responsive NDDSs may enable precise drug release, thus ultimately improving the therapeutic effect. In addition, NDDSs with stimuli-responsive release character can overcome the limitations of sensitizers including phototoxicity and aggregation-caused quenching.
4.4.1. External environment-responsive release
NDDSs can be designed to trigger drug release in responsive to external stimuli, including ultrasound, light, and light-induced heat. DVDMS-encapsulated liposome–microbubble complexes could be triggered to release DVDMS through ultrasound-induced cavitation, and enhance SDT against breast cancer171. ICG was loaded into reconstituted high density lipoproteins (rHDL) to achieve controlled release of ICG and produce 1O2 for PDT under 808 nm laser irradiation172. ZnPc and Au nanoparticles were incorporated into liposomes. Light-induced heat could enhance the liquidity of liposomal membrane to promote the instantaneous release of ZnPc (80% after 72 h) for phototherapy173. DOX and Ce6-loaded hollow mesoporous copper sulfide (CuS) nanoparticles were co-loaded with a phase change material 1-tetradecanol. Under 808 nm light irradiation, 1-tetradecanol was melted to trigger Ce6 release and the PDT effects were then activated under 660 nm174.
4.4.2. Intracellular environment-responsive release
The low pH, hypoxia, and redox condition in tumor microenvironment, and the various enzymes in tumor-associated cells can be used to stimulate drug release. Some NDDSs containing glutathione (GSH)-sensitive materials could achieve the controlled release of sensitizers in redox environment. An amphiphilic branched copolymer with pendant vinyl groups were synthesized using polyethylene glycol (PEG) and ethylene glycol dimethacrylate (EGDMA). The copolymer/Ce6 self-assembled micelles could react with GSH to release Ce6 through swelling of the micelles and decrease the level of GSH for enhanced PDT175. NDDSs containing redox-cleavable disulfide bonds can be designed to trigger sensitizer release in GSH rich tumor-specific environment. The conjugation of pheophorbide a and alginate with redox-sensitive disulfide linkages was used to load DOX to fabricate a nanosystem, which could accelerate approximately 90% pheophorbide a release at high GSH level (10 mmol/L) for PDT against B16 tumor cells176.
Sensitizers in NDDSs could be triggered to be released by breaking pH-sensitive covalent linkages under acidic environment. d-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) was conjugated to acid-sensitive cis-aconitic anhydride-modified DOX to form prodrug nanoparticles. The prodrug nanoparticles encapsulated with Ce6 could be triggered to release Ce6 at pH 5.5 due to the hydrolysis of the acid-sensitive amide linker for PDT177. In another work, 5-ALA was encapsulated in a core–shell structured nanoparticle containing a pH-sensitive hydrazone bond for pH-responsive release for PDT178. Besides, the fabrication of NDDSs through pH-sensitive noncovalent interactions (such as hydrogen bonding, host-guest and electrostatic interactions) has been developed for controlled drug release under acidic condition. Chitosan and catalase can fabricate micelles through electrostatic interaction. The micelles loading Ce6 were disassembled in acidic environment to trigger the release of Ce6 for effective PDT179. Furthermore, some pH-sensitive materials can be employed to prepare NDDSs to facilitate controlled release of drugs under acidic tumor microenvironment. A lipid bilayer-coated calcium carbonate (CaCO3) nanoparticles as a pH-responsive nanoplatform could be decomposed into Ca2+ and carbon dioxide (CO2) under acidic condition to trigger the release of Mn2+-chelated Ce6 for PDT180. Chitosan-capped biodegradable hollow mesoporous silica nanoparticle were developed to trigger the release of pheophorbide a (increased to 68.9% of drug release at 48 h) for PDT under low pH via pH-dependent swelling effect of the coating layer181.
4.4.3. Multiple stimuli-responsive release
NDDSs can be designed to release sensitizers in response to double stimuli or triple stimuli to obtain precise drug release. A pH/ultrasound dual-responsive nanoplatform HMME/CaCO3-HA, which was HMME loaded HA-modified mesoporous CaCO3 nanoparticles, could be decomposed under low pH and ultrasound. The release of HMME at pH 5.8 with ultrasound increased by 56.4% compare to that at pH 5.8 without ultrasound, which is 27.3% higher than that at pH 7.4 without ultrasound145. A pH/light responsive nanoplatform composed of PEG, Hp, and DOX has been developed to trigger the release of Hp at pH 5.8 along with laser irradiation for PDT182. Curcumin-loaded mesoporous magnetic carbon nitride nanohybrids could be triggered to release curcumin for PDT at lysosomal pH 5.2 in the presence of an alternating current magnetic field183. Enzyme/redox, pH/temperature, and pH/GSH dual stimuli-responsive nanoplatforms have been developed for controlled release of Ce6 to exert PDT effects184, 185, 186. The mixture of nitroimidazole-modified chitosan and RBD could self-assemble into nanoparticles, which could be triggered to release drugs in response to intratumoral hypoxia and acidic environment for enhanced PDT187. In another study, multi-triggered tumor-responsive drug delivery vehicles have been developed to promote the release of Ce6 under pH, GSH, and protease triple stimuli for enhanced PDT188.
4.5. Stimulating anti-tumor immunity
Immunotherapy orchestrates the immune system to find and kill the residual tumor cells, thus reducing the risk of cancer metastasis and recurrence189. Some nanomaterials, such as MnO2, UCNPs, and CaCO3 can elicit immunogenic cell death (ICD) or activate macrophages for cancer treatment190. PDT can also induce anti-tumor immune response by stimulating the immune system, further enhancing the anti-tumor effect191. NDDSs containing carrier materials which have the function of activating the immune system could work with the sensitizers to achieve a synergistic effect. HA polysaccharose with different molecular weights can produce pro-inflammatory mediators and modulate macrophage phenotype. Low molecular weight HA-modified mesoporous Prussian blue nanoparticles loaded with HMME could not only remodel tumor-associated macrophages (TAMs) phenotype from pro-tumor M2 to anti-tumor M1, but also exert SDT effects under ultrasound irradiation, thus inhibiting the proliferation and metastasis of 4T1 tumors146. PLGA was used to load perfluoropentane (PFP), ICG, and oxaliplatin (OXP) for combined PSDT/chemotherapy. The nanocomplexes could induce ICD accompanied by the release of damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), adenosine-5ʹ-triphosphate (ATP), and high-mobility group box 1 (HMGB1), and elicit stronger activity of cytotoxic T lymphocyte (CTL)192.
4.6. Increasing O2 supply
The tumor microenvironment is a local homeostatic environment composed of different kinds of cells, such as cancer cells, endothelial cells, cancer-associated fibroblasts (CAFs), cancer stem cells (CSC), immune cells as well as ECM, which is often accompanied by hypoxia and low pH193. Tumor hypoxic microenvironment can reduce the anti-tumor effects, impede immune cell infiltration of tumors, and accelerate tumor recurrence and metastasis194. The continuous O2 consumption in PDT/SDT might aggravate tumor hypoxia. NDDSs can be designed to supply O2, which can alleviate tumor hypoxia, thereby effectively enhancing the therapeutic effects.
4.6.1. Catalase catalyzed reaction
NDDSs containing catalase could decompose the endogenous H2O2 to generate O2 for relieving hypoxia. TCPP-conjugated catalase through amide coupling was mixed with fluorinated chitosan (FCs) to form catalase-TCPP/FCs nanoparticles. The nanoparticles could greatly improve transmucosal adsorption and intratumoral penetration, and generate O2 to relieve tumor hypoxia, thus achieving effective SDT tumor suppression73. The mixture of fluorinated polyethylenimine (F-PEI) and Ce6-conjugated catalase was able to form self-assembled catalase-Ce6/F-PEI nanoparticles to effectively relieve tumor hypoxia and improve PDT to destruct orthotopic bladder tumors195. RBC vesicles were used to encapsulate Pluronic F-127-modified Ag2S QDs to form biomimetic nanoparticles. Oral administration of anti-tumor drug phenethyl isothiocyanate (PEITC) in mice increased the H2O2 concentration, and the enzyme in RBC membranes could catalyze H2O2 in tumor cells to alleviate hypoxia for enhanced SDT61.
4.6.2. Metal-based catalytic reactions
NDDSs containing Cu-based nanoagents196, Prussian blue nanoparticles146, Fe(OH)3 nanocolloids197, hollow iron oxide nanoparticles (HIONs)198, manganese dioxide (MnO2) nanoparticles199, platinum (Pt) nanoparticles71,200, and Au2Pt nanozymes201 with catalase-like activity could degrade H2O2 to O2 to overcome tumor hypoxia. The mixture of HSA, potassium permanganate (KMnO4), and Ce6 could self-assemble into HSA-MnO2-Ce6 nanoparticles to produce O2 to improve the efficacy of PDT for orthotopic bladder cancer202. The nanosystems Hp-HIONs@PDA-PEG composed of HIONs, Fe3O4, Hp, PDA, and PEG could produce O2 to dramatically enhance SDT efficacy, which could effectively suppress tumor growth (85.58%) after SDT/magnetic hyperthermal therapy treatments198. Hollow semiconductor CuS, noble metallic Pt, a temperature-sensitive polymer (poly (oligo (ethylene oxide)methacrylate-co-2-(2-methoxyethoxy) ethyl methacrylate) [p-(OEOMA-co-MEMA)], and TAPP were combined together to form Pt–CuS–P-TAPP nanoparticles. The nanoparticles could accelerate the catalytic activity of Pt to elevate the O2 level under NIR irradiation-induced heat, and produce ROS to induce cell apoptosis under ultrasound71. Au2Pt nanozymes were covalently linked with Ce6 through a PEG linker to form Au2Pt-PEG-Ce6 nanoformulation. The nanoformulation possessed catalase-like activity and peroxidase-like activity to generate O2 and ·OH for synergistic PTT, PDT, and chemodynamic therapy201.
4.6.3. Employing O2 carriers
Some O2 carriers, such as PFTBA153, perfluorooctyl bromide (PFOB)203, PFP147, and PFH204 were entrapped into NDDSs to generate O2 for relieving hypoxia. Fluorocarbon (FC)-chain-functionalized hollow mesoporous organosilica nanoparticles carrying IR780 could supply enough O2 to solve the problem of hypoxia-induced resistance to SDT and produce higher ROS to kill contractile hypoxia pancreatic cancer205. A nanoparticles consisting of methoxy-PEG-PCL (mPEG-PCL), IR780, PFOB, and CRGDK peptide-modified PEG-PCL could continuously supply O2 to augment the sensitivity of tumor cells to PDT203. Zeolitic imidazolate framework-90 (ZIF-90) as an O2 reservoir in nanoparticles could be degraded to quickly release O2 at low pH, and the loaded RB could generate ROS under 808 nm, thus remarkably enhancing anti-tumor effects206. Biomimetic aggressive pseudo-RBCs composed of RBC membranes, hemoglobin (Hb), PDA, and MB could increase O2 supply from the Hb-carried O2 to overcome tumor hypoxia and impose strong PDT efficacy207.
Paclitaxel (PTX)/ICG and O2-carrying liquid PFP co-loaded PLGA nanoparticles have been developed for PSDT/chemotherapy, which could supply O2 to improve tumor hypoxia and exert anti-tumor effects against SKOV3 tumor147.
4.6.4. Reducing endogenous O2 consumption
NDDSs could be designed to reduce the endogenous O2 consumption, thus efficiently attenuating the intratumoral hypoxia status. Tamoxifen in the nanocomplexes HSA-Ce6/tamoxifen could reduce the endogenous O2 consumption under PDT process by inhibiting the activity of NADH dehydrogenase in the mitochondrial electron transport chain, thus greatly improving PDT treatment140. Metformin can inhibit mitochondria-mediated respiration. PEG-PCL co-loaded with IR780 and metformin was developed to overcome tumor hypoxia and achieve superior synergistic PDT/PTT efficacy with reduced O2 consumption. The tumor volume was inhibited by about 2.4-fold when the nanoparticles were exposed to laser irradiation208.
4.6.5. Promoting tumor blood flow
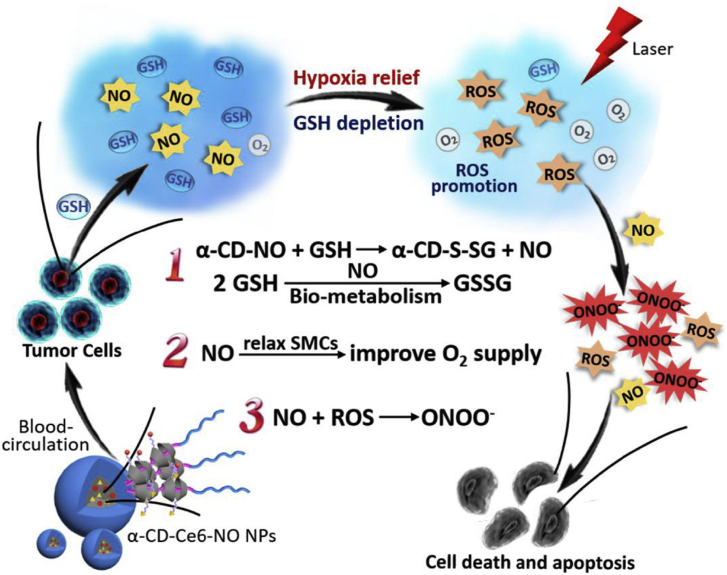
NDDSs can relieve tumor hypoxia through the promotion of tumor blood flow. Nitrosoglutathione (GSNO)- and Ce6-loaded zeolite imidazole framework-8 (ZIF-8) was coated with 4T1 cell membrane to fabricate biomimetic nanoplatform for SDT/gas therapy. Due to the thermal effect caused by ultrasound, the nanoplatform can promote tumor blood flow, thereby alleviating tumor hypoxia209. Poly (2-metharcryloyloxyethyl phophorylcholine) (PEG-b-PMPC) block copolymer, α-cyclodextrin (α-CD)-conjugated S-nitrosothiol (α-CD-NO), and α-CD-conjugated Ce6 were mixed together to form supramolecular nanoparticles α-CD-Ce6-NO. The α-CD-Ce6-NO could not only deplete intracellular GSH, but also relieve hypoxia at tumor sites through NO-mediated relaxation of smooth muscle cells (SMCs) and promotion of tumor blood flow. The subsequent generation of NO and ROS could react with each other to generate reactive peroxynitrite (ONOO−) for enhanced PDT210 (Fig. 6).
Figure 6.
The schematic illustration of how the supramolecular nanoparticles α-CD-Ce6-NO improved the therapeutic efficacy. The α-CD-Ce6-NO could relieve hypoxia at tumor sites through NO-mediated relaxation of smooth muscle cells (SMCs) and promotion of tumor blood flow. Reprinted with the permission from Ref. 210. Copyright © 2018, Elsevier, Ltd.
4.7. Employing different therapeutic modalities
Single-modality treatment is generally unable to obtain satisfactory effects due to the physiological complexity of tumors. Combination therapy can be introduced to target different mechanisms and inhibit different pathways, thereby improving the anti-cancer efficacy. PDT/SDT can be used in combination with other therapies for cancer treatment, such as chemotherapy, immunotherapy, and gene therapy25,211. NDDSs can simultaneously deliver sensitizers and other drugs to achieve the synergistic SPDT and other therapies. Hollow mesoporous organosilica based nanosystems were used to carry PpIX and DOX to exert synergistic SDT/chemotherapeutic effects for hepatocellular carcinoma treatment212. TiO2 nanocrystals coated with an O2-deficient TiO2-x layer were decorated with PEG in the outer layer. The nanocomposites demonstrated high photothermal conversion efficiency and high therapeutic biosafety for enhanced synergistic photothermal hyperthermia/SDT. The tumor suppression rate reached 100% in the combination group, which was higher than that in the group treated with only laser (54.2%) or only ultrasound (74.6%)213. Bovine serum albumin (BSA) co-loaded with DOX and ICG can efficiently inhibit tumor growth through the integration of PDT and PTT with chemotherapy214.
Peptide amphiphile C18GR7RGDS corporated with ICG could self-assemble into functional nanomicelles for in vivo PSDT/PTT combination therapy144. PLGA loaded with PFP, ICG, and OXP have been developed for PSDT/chemotherapy192. PLGA carrying PTX and ICG could induce apoptosis of SKOV3 cells and inhibit SKOV3 tumor growth after PSDT/chemotherapy treatments147.
4.8. Combining diagnosis and treatment
Cancer nanotheranostics involve the integration of diagnosis and treatment in a single plateform, which can monitor drug distribution and evaluate drug efficacy to adjust drug dosage and dosing regimen in time for precise cancer therapy215. With the development of molecular imaging technology, various imaging modalities with different resolutions and sensitivities have been developed, including optical imaging, magnetic resonance imaging (MRI), computed topography (CT), positron emission tomography (PET), PA imaging, optical imaging, and ultrasound imaging216. Nanoparticles carrying both sensitizers and imaging agents in one formulation could be used for simultaneous disease diagnosis and therapy. In addition, inorganic sensitizers themselves could be used as theranostic agents, such as noble metal nanoparticles (Au or Ag) for optical imaging, semiconductor nanoparticles (QD) for fluorescence imaging, and PHF for photoacoustic imaging217.
ICG-loaded MSN was lidded with ZnO QDs and wrapped with erlotinib-modified chitosan to form a nanotheranostic system, which could activate the fluorescence recovery of ICG to identify different molecular subtypes of non-small cell lung cancer (NSCLC) cells though NIR fluorescence imaging and obtain PDT effects to reverse the resistance of NSCLC cells to molecular targeted drugs218. Mn2+-chelated DVDMS was encapsulated into liposomes to form DVDMS-Mn-Liposomes for SDT. The nanoplatform could be used for in vivo monitoring of the drug biodistribution and the tumor-growth suppression by fluorescence imaging and T1-weighted MRI219.
FA-targeted PFH/ICG-loaded lipid nanoparticles have been developed for synergistic PSDT/PTT in ovarian cancer. The lipid nanoparticles could monitor the accumulation of drugs at the tumor region and be capable of enhancing the ultrasound/PA imaging with laser irradiation142. FA-conjugated lipid–polymer hybrid nanoparticles with core–shell structures were used to encapsulate ICG and perfuorocarbon (PFC)-carrying O2 to construct a nanotheranostic agent. The nanotheranostic agent could not only generate large amounts of microbubbles to provide an excellent contrast for both PA and ultrasound imaging, but also generate ROS to achieve therapeutic effects upon PSDT treatments220.
5. Clinical application against cancer
In clinical research, PDT has been widely used to treat cancers. Because of the limited penetrability of light, PDT is usually used to treat superficial skin cancers. With the development of light source equipment, laser light can be transfer through fiber optic cables which could reach internal organs or cavities. Therefore, PDT has been used to treat cancer in the lungs or esophagus inside the body. According to the characteristics and types of tumors, PDT can exert its therapeutic effect alone, or can combine with surgery or other therapies221,222. Patients treated with PDT could effectively improve clinical symptoms, reduce complications and improve quality of life. Adverse reactions in patients including skin solar sensitivity, pain in the irradiation area, and vocal cord edema and adhesion were reported223,224. Some studies have shown that sensitizers can also be used for clinical diagnosis and treatment225,226. PDT is usually used in combination with other therapies in clinic. Compared with surgery alone, patients with extramammary Paget's disease receiving 20% 5-ALA gel-mediated PDT combined with surgery could reduce recurrence rate from 25% to 9.1%227. No recurrence was observed in two patients with recurrent and wide spread extramammary Paget disease underwent 20% 5-ALA gel and imiquimod combination treatment at 24- and 36-month follow-up228. Due to the restriction of the light, PDT is difficult to treat large tumors or cancer that has spread.
There are few studies on the clinical application of SDT and SPDT, and most of them are in the stage of cell or animal experiments. A 55-year-old patient with advanced breast cancer treated with immunotherapy (Gc protein-derived macrophage activating factor, GcMAF), SDT (Ce6 and 5-ALA) and hormone therapy (exemestane) showed dramatic improvement of symptoms (such as cough, back pain, and edema of the right hand) and the axillary tumors decreased and disappeared completely229. Preliminary clinical data revealed that sublingual administration of SF1 in 3 advanced refractory breast cancer patients treated with SPDT had significant partial or complete responses81. Another clinical outcome showed that numerous cases of the 115 patients treated with sonnelux-1-mediated SPDT had a significantly longer predicted median survival230. The main side effect of SPDT is pain or visible inflammatory reaction.
Although SPDT has been used in clinic and is available in several countries including England, Mexico, Israel, China, and Cape Town, there are still many problems that restricted its broad clinical applications. It still lacks sensitizers that are more effective, more selective, and are activated by both light and ultrasound. In addition, the deteriorating tumor microenvironment caused by SPDT can reduce the therapeutic effects. Moreover, there is few work on the development of systemic equipments for the delivery of light and generation of ultrasound that are convenient for clinical use. Furthermore, it is difficult to develop SPDT protocols that are suitable for clinical use.
6. Conclusions and outlook
SPDT provides a highly promising approach to overcome the challenges and offer alternative opportunities in current anti-cancer fields. The development of new potential sensitizers is one of the most essential factors in SPDT. In this review, we attempted to provide an overview of sono/photosensitizers that can be developed into suitable sono-photosensitizers for SPDT and guide the design of the sensitizers. Sensitizers can be classified into organic, inorganic, and hybrid sensitizers. Each class has its own strengths and disadvantages (Table 3). Organic sensitizers generally have low toxicity, but poor water solubility and the chemical/biological instability limit their applications. Inorganic sensitizers are more stable than organic sensitizers, and some of the inorganic sensitizers can be designed for NIR-II phototherapy. However, they show relatively high toxicity and lack in-depth toxicological assessment. Although hybrid sensitizers can combine the advantages of organic and inorganic sensitizers, they are short of detailed and comprehensive researches231. In addition, different types of sensitizers have different mechanisms of action. For example, organic sensitizers, such as ICG and Ce6 can generate 1O2 through energy transfer mechanism, while inorganic sensitizers, such as Ag2S and Au nanoparticles are activated by light to generate electron–hole (e−–h+) pairs, and then generate ROS for PDT. However, there is no clear distinction among the mechanisms of different types of sensitizers activated by ultrasound.
Table 3.
The advantages and disadvantages of organic, inorganic, and hybrid sensitizers.
| Category | Advantage | Disadvantage |
|---|---|---|
| Organic sensitizers |
|
|
| Inorganic sensitizers |
|
|
| Hybrid sensitizers |
|
|
Ideal SPDT agents should exhibit strong sensitive, nontoxic, and high tumor-homing ability without rendering toxic side effects to improve therapeutic efficacy. Recently, some amphiphilic sensitizers, such as ICG, RBDs have been found to have favorable pharmacological and pharmaceutical properties for cancers in SPDT. Therefore, they might be the promising starting points for further rational drug design and molecular structure optimization in many aspects. Today, many institutes adopt the design of sensitizers as their main endeavor, with the aim of executing effective strategies toward cancers. Because some of the most typical sensitizers for SPDT were found from photosensitizers or sonosensitizers, sensitizers for PDT or SDT could be developed into suitable sono-photosensitizers. Another strategy is applying the existing synthesis techniques for the efficient functionalization of current sensitizers. Hybrid sensitizers that combine the distinct advantages of organic and inorganic sensitizers can be developed. Because the limited penetration of light can affect the therapeutic effect of SPDT, sensitizers with innovative structures and some more advanced stimulation tools should be found to increase the depth of light penetration into tissues. Fluorescence resonance energy transfer (FRET) or X-ray excitation source can be beneficial for deep tissue therapy232,233. NIR-II excitable sensitizers are highly desirable for SPDT to improve light penetration depth in biotissues234, 235, 236. In addition, sensitizers with both imaging capabilities and therapeutic functions can be designed for better use in individualized cancer treatment.
Although significant progress has been made in developing SPDT, further improvements of this program through a series of critical preclinical steps are needed prior to their ultimate clinical use. More work is needed to be done before SPDT is accepted as an adjuvant or replacement method for traditional cancer treatment. The efficacy of SPDT is closely related to the sensitizer dose, light dose, ultrasound intensity, and the order of PDT and SDT. For example, different irradiation times were used for the same sensitizers under the same wavelength and power due to the different doses administered in the body140,167. It requires a lot of in-depth and comprehensive research to develop an appropriate standard operation procedure. In addition, the selection of appropriate dosage form, as well as the precise dose and medication method should be considered to increase drug compliance. As SPDT is usually used in combination with other therapies, there is an urgent need to clarify the biological mechanisms of SPDT and the synergistic effects of SPDT with other therapies. At present, a large number of clinical trials have been carried out using PDT for the treatment of brain, skin, prostate, cervix, and peritoneal cavity tumors237. Additionally, extracorporeal photopheresis (ECP) in which blood is treated with PDT ex vivo has been approved by FDA for patients with cutaneous T cell lymphoma (CTCL)238. Based on the results and the considerable experience gained from the clinical PDT studies, SPDT can be developed further and is expected to be a powerful tool used alone or in combination with other therapies against local or metastasis cancers.
NDDSs have achieved good results in cancer treatment and can be used to improve the anti-tumor effects of sensitizers. Some NDDSs with rational design are capable of crossing biological barriers into tumor sites. Ligands-modified NDDSs can actively target tumor blood vessels, tumor tissues/cells, and even cellular organelles, increasing cellular uptake and intracellular drugs concentration. Through size transition, charge reversal, or membrane transport, sensitizers in NDDSs can penetrate into the internal area of the tumor and reach intratumoral cancer cells, thus improving the intratumoral delivery of sensitizers. NDDSs with stimuli-responsive release pattern could prevent premature leakage of sensitizers before reaching cancer cells, thus increasing the intracellular drugs concentration. Sensitizers-loaded NDDSs can be designed to stimulate anti-tumor immunity to prevent tumor metastasis and recurrence and to supply O2 to alleviate tumor hypoxia, thereby enhancing the therapeutic effects. Combination therapies based on NDDSs can inhibit multiple pathways for enhanced SPDT. NDDSs that combine anti-cancer therapeutics with imaging modalities can be used for monitoring drug action sites and therapeutic response to change treatment strategies in time.
Besides the above-mentioned strategies that can be used to improve the anti-tumor SPDT effects of sensitizers, NDDSs can be further designed to provide more functionalities. Some sensitizers have short half-life and retention time in the body, and many sensitizers form aggregates in aqueous media due to π–π stacking, which can decrease or inhibit 1O2 generation, thus reducing the efficacy. NDDSs can be designed to prolong blood circulation time and prevent the aggregation of sensitizers for enhanced SPDT. The controlled release and activated imaging characteristics based on NDDSs can be designed for more precise SPDT. The combination of SPDT with PTT or chemotherapy has shown enhanced anti-tumor effects. NDDSs could provide a platform for combination of SPDT with other therapies, such as molecular targeted therapy, hyperthermia, gas therapy, gene therapy, and immunotherapy for more efficient cancer therapy. As mentioned above, the process of tumor drug delivery is very complicated, including at least five steps. Existing strategies can only improve one or two steps in CAPIR cascade, and cannot improve the overall transportation efficiency. NDDSs that could efficiently accomplish the full CAPIR cascade could be used to deliver sensitizers for SPDT to ensure the overall therapeutic efficiency.
Although NDDSs have made great progress in preclinical research, only a few have gone through feasible clinical trials. Excipientability and scale-up ability of NDDSs are two important factors restrict their clinical transformation. Design of NDDSs which could be prepared by a simple and mass-produced method is an essential prerequisite for further advancement of clinical research. There are few studies on the long-term toxicity and genotoxicity of various nanomaterials. Introducing interdisciplinary science to systematically study the biological safety of nanomaterials might accelerate the clinical conversion of NDDSs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81871481 and 81571802), the Fujian Provincial Youth Top-notch Talent Support program (China), and National Key R&D Program of China (2020YFA0210800).
Author contributions
Yilin Zheng and Jinxiang Ye searched references and wrote the manuscript. Ziying Li was in charge of drawing figures and sorting tables. Yu Gao and Haijun Chen revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.12.016.
Contributor Information
Haijun Chen, Email: chenhaij@gmail.com.
Yu Gao, Email: hellogaoyu@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Dy G.K., Adjei A.A. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63:249–279. doi: 10.3322/caac.21184. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y.Y., Li J.C., Zeng Z.L., Xie C., Lyu Y., Pu K.Y. Organic photodynamic nanoinhibitor for synergistic cancer therapy. Angew Chem Int Ed Engl. 2019;58:8161–8165. doi: 10.1002/anie.201903968. [DOI] [PubMed] [Google Scholar]
- 4.Li J.C., Huang J.G., Lyu Y., Huang J.S., Jiang Y.Y., Xie C. Photoactivatable organic semiconducting pro-nanoenzymes. J Am Chem Soc. 2019;141:4073–4079. doi: 10.1021/jacs.8b13507. [DOI] [PubMed] [Google Scholar]
- 5.Ng C.W., Li J.C., Pu K.Y. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv Funct Mater. 2018;28:1804688. [Google Scholar]
- 6.Li J.C., Cui D., Huang J.G., He S.S., Yang Z.B., Zhang Y. Organic semiconducting pro-nanostimulants for near-infrared photoactivatable cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58:12680–12687. doi: 10.1002/anie.201906288. [DOI] [PubMed] [Google Scholar]
- 7.Li J.C., Luo Y., Pu K.Y. Electromagnetic nanomedicines for combinational cancer immunotherapy. Angew Chem Int Ed Engl. 2020;59:11717. doi: 10.1002/anie.202008386. [DOI] [PubMed] [Google Scholar]
- 8.Yin H., Kauffman K.J., Anderson D.G. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.J., Zhou X.B., Gao Y., Zheng B.Y., Tang F.X., Huang J.D. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today. 2014;19:502–509. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y.Y., Tu J., Yang D.X., Raymond J.L., Roy R.A., Zhang D. Photo- and sono-dynamic therapy: a review of mechanisms and considerations for pharmacological agents used in therapy incorporating light and sound. Curr Pharmaceut Des. 2019;25:401–412. doi: 10.2174/1381612825666190123114107. [DOI] [PubMed] [Google Scholar]
- 11.Rai P., Mallidi S., Zheng X., Rahmanzadeh R., Mir Y., Elrington S. Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucky S.S., Soo K.C., Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y., Xie J.J., Chen H.J., Gu S.G., Zhao R.L., Shao J.W. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv. 2014;32:761–777. doi: 10.1016/j.biotechadv.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Peng F.F., Li R.R., Zhang F., Qin L., Ling G.X., Zhang P. Potential drug delivery nanosystems for improving tumor penetration. Eur J Pharm Biopharm. 2020;151:220–238. doi: 10.1016/j.ejpb.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y.L., Li Z.Y., Chen H.J., Gao Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur J Pharmaceut Sci. 2020;144:105213. doi: 10.1016/j.ejps.2020.105213. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Wang J.C., Wang Y.G., Gao H.L., Wei G., Huang Y.Z. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Z.J., Dai Y., Gao H.L. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;9:1099–1112. doi: 10.1016/j.apsb.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B., Hu Y., Pang Z.Q. Modulating the tumor microenvironment to enhance tumor nanomedicine delivery. Front Pharmacol. 2017;8:952. doi: 10.3389/fphar.2017.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foote C.S. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.Y., Liu Y.C., Sun H.W., Guo D.S. Type I photodynamic therapy by organic–inorganic hybrid materials: from strategies to applications. Coord Chem Rev. 2019;395:46–62. [Google Scholar]
- 21.Zhang J., Jiang C.S., Figueiro Longo J.P., Azevedo R.B., Zhang H., Muehlmann L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm Sin B. 2018;8:137–146. doi: 10.1016/j.apsb.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison R.R., Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clin Endosc. 2013;46:24–29. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal I., Sostaric J.Z., Riesz P. Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Liu X.H., Li S., Wang M., Dai Z.J. Current status and future perspectives of sonodynamic therapy and sonosensitiers. Asian Pac J Cancer Prev. 2015;16:4489–4492. doi: 10.7314/apjcp.2015.16.11.4489. [DOI] [PubMed] [Google Scholar]
- 25.Pan X.T., Wang H.Y., Wang S., Sun X., Wang L.J., Wang W.W. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61:415–426. doi: 10.1007/s11427-017-9262-x. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahiminia A., Mokhtari-Dizaji M., Toliyat T. Dual frequency cavitation event sensor with iodide dosimeter. Ultrason Sonochem. 2016;28:276–282. doi: 10.1016/j.ultsonch.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Pang X., Xu C.S., Jiang Y., Xiao Q.C., Leung A.W. Natural products in the discovery of novel sonosensitizers. Pharmacol Ther. 2016;162:144–151. doi: 10.1016/j.pharmthera.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Kolarova H., Bajgar R., Tomankova K., Krestyn E., Dolezal L., Halek J. In vitro study of reactive oxygen species production during photodynamic therapy in ultrasound-pretreated cancer cells. Physiol Res. 2007;56:S27–S32. doi: 10.33549/physiolres.931298. [DOI] [PubMed] [Google Scholar]
- 29.Jin Z.H., Miyoshi N., Ishiguro K., Umemura S., Kawabata K., Yumita N. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J Dermatol. 2000;27:294–306. doi: 10.1111/j.1346-8138.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 30.Li Q., Wang X.B., Wang P., Zhang K., Wang H.P., Feng X.L. Efficacy of chlorin e6-mediated sono-photodynamic therapy on 4T1 cells. Cancer Biother Radiopharm. 2014;29:42–52. doi: 10.1089/cbr.2013.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q., Liu Q.H., Wang P., Feng X.L., Wang H.P., Wang X.B. The effects of Ce6-mediated sono-photodynamic therapy on cell migration, apoptosis and autophagy in mouse mammary 4T1 cell line. Ultrasonics. 2014;54:981–989. doi: 10.1016/j.ultras.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Wang H.P., Wang P., Zhang K., Wang X.B., Liu Q.H. Changes in cell migration due to the combined effects of sonodynamic therapy and photodynamic therapy on MDA-MB-231 cells. Laser Phys Lett. 2015;12 [Google Scholar]
- 33.Liu Q.H., Wang X.B., Wang P., Xiao L.N., Hao Q. Comparison between sonodynamic effect with protoporphyrin IX and hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol. 2007;60:671–680. doi: 10.1007/s00280-006-0413-4. [DOI] [PubMed] [Google Scholar]
- 34.Kim J., Lim W., Kim S., Jeon S., Hui Z., Ni K. Photodynamic therapy (PDT) resistance by PARP1 regulation on PDT-induced apoptosis with autophagy in head and neck cancer cells. J Oral Pathol Med. 2014;43:675–684. doi: 10.1111/jop.12195. [DOI] [PubMed] [Google Scholar]
- 35.Hoi S.W., Wong H.M., Chan J.Y., Yue G.G., Tse G.M., Law B.K. Photodynamic therapy of pheophorbide a inhibits the proliferation of human breast tumour via both caspase-dependent and -independent apoptotic pathways in in vitro and in vivo models. Phytother Res. 2012;26:734–742. doi: 10.1002/ptr.3607. [DOI] [PubMed] [Google Scholar]
- 36.Umemura K., Yumita N., Nishigaki R., Umemura S.I. Sonodynamically induced antitumor effect of pheophorbide a. Cancer Lett. 1996;102:151–157. doi: 10.1016/0304-3835(96)04174-2. [DOI] [PubMed] [Google Scholar]
- 37.Xu Z.Y., Wang K., Li X.Q., Chen S., Deng J.M., Cheng Y. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with photofrin in glioma stem-like cells. Ultrasonics. 2013;53:232–238. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Qiu H., Kim M.M., Penjweini R., Zhu T.C. Macroscopic singlet oxygen modeling for dosimetry of photofrin-mediated photodynamic therapy: an in-vivo study. J Biomed Optic. 2016;21:88002. doi: 10.1117/1.JBO.21.8.088002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J.H., Chen Z.Q., Huang Z., Zhan Q., Ren F.B., Liu J.Y. In vitro study of low intensity ultrasound combined with different doses of PDT: effects on C6 glioma cells. Oncol Lett. 2013;5:702–706. doi: 10.3892/ol.2012.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Wang X.B., Wang P., Zhang K., Yang S., Liu Q.H. Ultrasound enhances the efficacy of chlorin e6-mediated photodynamic therapy in MDA-MB-231 cells. Ultrasound Med Biol. 2013;39:1713–1724. doi: 10.1016/j.ultrasmedbio.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Wang P., Li C.F., Wang X.B., Xiong W.L., Feng X.L., Liu Q.H. Anti-metastatic and pro-apoptotic effects elicited by combination photodynamic therapy with sonodynamic therapy on breast cancer both in vitro and in vivo. Ultrason Sonochem. 2015;23:116–127. doi: 10.1016/j.ultsonch.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 42.Tserkovsky D.A., Alexandrova E.N., Chalau V.N., Istomin Y.P. Effects of combined sonodynamic and photodynamic therapies with photolon on a glioma C6 tumor model. Exp Oncol. 2012;34:332–335. [PubMed] [Google Scholar]
- 43.Liu Y.C., Wang P., Liu Q.H., Wang X.B. Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo. Ultrason Sonochem. 2016;31:437–448. doi: 10.1016/j.ultsonch.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 44.Bakhshizadeh M., Moshirian T., Esmaily H., Rajabi O., Nassirli H., Sazgarnia A. Sonophotodynamic therapy mediated by liposomal zinc phthalocyanine in a colon carcinoma tumor model: role of irradiating arrangement. Iran J Basic Med Sci. 2017;20:1088–1092. doi: 10.22038/IJBMS.2017.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomankova K., Kolarova H., Kolar P., Kejlova K., Jirova D. Study of cytotoxic effect of photodynamically and sonodynamically activated sensitizers in vitro. Toxicol Vitro. 2009;23:1465–1471. doi: 10.1016/j.tiv.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Tomankova K., Kolarova H., Bajgar R. Study of photodynamic and sonodynamic effect on A549 cell line by AFM and measurement of ROS production. Phys Status Solidi. 2008;205:1472–1477. [Google Scholar]
- 47.Chen H.J., Zhou X.B., Wang A.L., Zheng B.Y., Yeh C.K., Huang J.D. Synthesis and biological characterization of novel rose bengal derivatives with improved amphiphilicity for sono-photodynamic therapy. Eur J Med Chem. 2018;145:86–95. doi: 10.1016/j.ejmech.2017.12.091. [DOI] [PubMed] [Google Scholar]
- 48.Nomikou N., Sterrett C., Arthur C., McCaughan B., Callan J.F., McHale A.P. The effects of ultrasound and light on indocyanine-green-treated tumour cells and tissues. ChemMedChem. 2012;7:1465–1471. doi: 10.1002/cmdc.201200233. [DOI] [PubMed] [Google Scholar]
- 49.McEwan C., Nesbitt H., Nicholas D., Kavanagh O.N., McKenna K., Loan P. Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer. Bioorg Med Chem. 2016;24:3023–3028. doi: 10.1016/j.bmc.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Shimamura Y., Tamatani D., Kuniyasu S., Mizuki Y., Suzuki T., Katsura H. 5-aminolevulinic acid enhances ultrasound-mediated antitumor activity via mitochondrial oxidative damage in breast cancer. Anticancer Res. 2016;36:3607–3612. [PubMed] [Google Scholar]
- 51.He G.F., Mu T.L., Yuan Y.L., Yang W.Y., Zhang Y., Chen Q.Y. Effects of notch signaling pathway in cervical cancer by curcumin mediated photodynamic therapy and its possible mechanisms in vitro and in vivo. J Cancer. 2019;10:4114–4122. doi: 10.7150/jca.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F.P., Gao Q.P., Guo S.Y., Cheng J.L., Sun X., Li Q.N. The sonodynamic effect of curcumin on THP-1 cell-derived macrophages. BioMed Res Int. 2013;2013:737264. doi: 10.1155/2013/737264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrellis M.C., Frigo L., Ribeiro W., Leal-Junior E.C., Oliveira F.R., Maria D.A. Proinflammatory effects of photoactivated methylene blue on rat model of Walker 256 carcinosarcoma. Exp Oncol. 2019;41:112–122. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-2.13047. [DOI] [PubMed] [Google Scholar]
- 54.Komori C., Okada K., Kawamura K., Chida S., Suzuki T. The sonodynamic antitumor effect of methylene blue on sarcoma180 cells in vitro. Anticancer Res. 2009;29:2411–2415. [PubMed] [Google Scholar]
- 55.Ji Y.Y., Ma Y.J., Wang J.W. Cytoprotective role of nitric oxide in HepG2 cell apoptosis induced by hypocrellin B photodynamic treatment. J Photochem Photobiol, B. 2016;163:366–373. doi: 10.1016/j.jphotobiol.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y.C., Bai H., Wang H.P., Wang X.B., Liu Q.H., Zhang K. Comparison of hypocrellin B-mediated sonodynamic responsiveness between sensitive and multidrug-resistant human gastric cancer cell lines. J Med Ultrason. 2019;46:15–26. doi: 10.1007/s10396-018-0899-5. [DOI] [PubMed] [Google Scholar]
- 57.Xiao L., Gu L., Howell S.B., Sailor M.J. Porous silicon nanoparticle photosensitizers for singlet oxygen and their phototoxicity against cancer cells. ACS Nano. 2011;5:3651–3659. doi: 10.1021/nn1035262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sviridov A.P., Osminkina L.A., Nikolaev A.L., Kudryavtsev A.A., Vasiliev A.N., Timoshenko V.Y. Lowering of the cavitation threshold in aqueous suspensions of porous silicon nanoparticles for sonodynamic therapy applications. Appl Phys Lett. 2015;107:123107. [Google Scholar]
- 59.Wang C., Cao S.Q., Tie X.X., Qiu B., Wu A.H., Zheng Z.H. Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol Biol Rep. 2011;38:523–530. doi: 10.1007/s11033-010-0136-9. [DOI] [PubMed] [Google Scholar]
- 60.Harada Y., Ogawa K., Irie Y., Endo H., Feril L.B., Jr., Uemura T. Ultrasound activation of TiO2 in melanoma tumors. J Control Release. 2011;149:190–195. doi: 10.1016/j.jconrel.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Li C., Yang X.Q., An J., Cheng K., Hou X.L., Zhang X.S. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy. Theranostics. 2020;10:867–869. doi: 10.7150/thno.37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng K., Zhang X.S., An J., Li C., Zhang R.Y., Ye R. Hitherto-unexplored photodynamic therapy of Ag2S and enhanced regulation based on polydopamine in vitro and vivo. Chemistry. 2019;25:7553–7560. doi: 10.1002/chem.201900718. [DOI] [PubMed] [Google Scholar]
- 63.Bernard V., Mornstein V., Jaros J., Sedlackova M., Skorpikova J. Combined effect of silver nanoparticles and therapeutical ultrasound on ovarian carcinoma cells A2780. J Appl Biomed. 2014;12:137–145. [Google Scholar]
- 64.Erdogan O., Abbak M., Demirbolat G.M., Birtekocak F., Aksel M., Pasa S. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: the characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sazgarnia A., Shanei A., Taheri A.R., Meibodi N.T., Eshghi H., Attaran N. Therapeutic effects of acoustic cavitation in the presence of gold nanoparticles on a colon tumor model. J Ultrasound Med. 2013;32:475–483. doi: 10.7863/jum.2013.32.3.475. [DOI] [PubMed] [Google Scholar]
- 66.Wang H., Yang X.Z., Shao W., Chen S.C., Xie J.F., Zhang X.D. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J Am Chem Soc. 2015;137:11376–11382. doi: 10.1021/jacs.5b06025. [DOI] [PubMed] [Google Scholar]
- 67.Li Z.Y., Zhang T.M., Fan F., Gao F., Ji H.X., Yang L.H. Piezoelectric materials as sonodynamic sensitizers to safely ablate tumors: a case study using black phosphorus. J Phys Chem Lett. 2020;11:1228–1238. doi: 10.1021/acs.jpclett.9b03769. [DOI] [PubMed] [Google Scholar]
- 68.Berg K., Selbo P.K., Weyergang A., Dietze A., Prasmickaite L., Bonsted A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218:133–147. doi: 10.1111/j.1365-2818.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 69.O'Connor A.E., Gallagher W.M., Byrne A.T. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 70.Yao Y.H., Li J., Yuan L.F., Zhang Z.Q., Zhang F.X. Novel porphyrin-Schiff base conjugates: synthesis, characterization and in vitro photodynamic activities. RSC Adv. 2016;6:45681–45688. [Google Scholar]
- 71.Liang S., Deng X.R., Chang Y., Sun C.Q., Shao S., Xie Z.X. Intelligent hollow Pt–CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19:4134–4145. doi: 10.1021/acs.nanolett.9b01595. [DOI] [PubMed] [Google Scholar]
- 72.Musser D.A., Datta-Gupta N. Inability to elicit rapid cytocidal effects on L1210 cells derived from porphyrin-injected mice following in vitro photoirradiation. J Natl Cancer Inst. 1984;72:427–434. [PubMed] [Google Scholar]
- 73.Li G.Z., Wang S.P., Deng D.S., Xiao Z.S., Dong Z.L., Wang Z.P. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano. 2020;14:1586–1599. doi: 10.1021/acsnano.9b06689. [DOI] [PubMed] [Google Scholar]
- 74.Song K., Kong B.H., Li L., Yang Q.F., Wei Y.Q., Qu X. Intraperitoneal photodynamic therapy for an ovarian cancer ascite model in Fischer 344 rat using hematoporphyrin monomethyl ether. Cancer Sci. 2007;98:1959–1964. doi: 10.1111/j.1349-7006.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J.H., Song D.Y., Xu Y.G., Huang Z., Yue W. In vitro study of haematoporphyrin monomethyl ether-mediated sonodynamic effects on C6 glioma cells. Neurol Sci. 2008;29:229–235. doi: 10.1007/s10072-008-0972-8. [DOI] [PubMed] [Google Scholar]
- 76.Li H.T., Song X.Y., Yang C., Li Q., Tang D., Tian W.R. Effect of hematoporphyrin monomethyl ether-mediated PDT on the mitochondria of canine breast cancer cells. Photodiagnosis Photodyn Ther. 2013;10:414–421. doi: 10.1016/j.pdpdt.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Miyoshi N., Ishihara M., Ono K. In: Oxygen radicals. Yagi K., Kondo M., Niki E., Yoshikawa T., editors. Elsevier Science Publishers; Amsterdam: 1992. Relative yield of active oxygens produced by various sensitizers in vitro; pp. 15–18. [Google Scholar]
- 78.Yumita N., Sasaki K., Umemura S., Yukawa A., Nishigaki R. Sonodynamically induced antitumor effect of gallium–porphyrin complex by focused ultrasound on experimental kidney tumor. Cancer Lett. 1997;112:79–86. doi: 10.1016/s0304-3835(96)04548-x. [DOI] [PubMed] [Google Scholar]
- 79.Sadanala K.C., Chaturvedi P.K., Seo Y.M., Kim J.M., Jo Y.S., Lee Y.K. Sono-photodynamic combination therapy: a review on sensitizers. Anticancer Res. 2014;34:4657–4664. [PubMed] [Google Scholar]
- 80.Gomaa I., Ali S.E., El-Tayeb T.A., Abdel-kader M.H. Chlorophyll derivative mediated PDT versus methotrexate: an in vitro study using MCF-7 cells. Photodiagnosis Photodyn Ther. 2012;9:362–368. doi: 10.1016/j.pdpdt.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Wang X.H., Zhang W.M., Xu Z.Y., Luo Y.F., Mitchell D., Moss R.W. Sonodynamic and photodynamic therapy in advanced breast carcinoma: a report of 3 cases. Integr Cancer Ther. 2009;8:283–287. doi: 10.1177/1534735409343693. [DOI] [PubMed] [Google Scholar]
- 82.Lewis T.J. Toxicity and cytopathogenic properties toward human melanoma cells of activated cancer therapeutics in zebra fish. Integr Cancer Ther. 2010;9:84–92. doi: 10.1177/1534735409355171. [DOI] [PubMed] [Google Scholar]
- 83.Wang X.H., Lewis T.J., Mitchell D. The tumoricidal effect of sonodynamic therapy (SDT) on S-180 sarcoma in mice. Integr Cancer Ther. 2008;7:96–102. doi: 10.1177/1534735408319065. [DOI] [PubMed] [Google Scholar]
- 84.Kenyon J.N., Fuller R.J. Outcome measures following sonodynamic photodynamic therapy—a case series. Curr Drug Ther. 2011;6:12–16. [Google Scholar]
- 85.Chin W.W., Heng P.W., Thong P.S., Bhuvaneswari R., Hirt W., Kuenzel S. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur J Pharm Biopharm. 2008;69:1083–1093. doi: 10.1016/j.ejpb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Ali-Seyed M., Bhuvaneswari R., Soo K.C., Olivo M. Photolon™—photosensitization induces apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes. Int J Oncol. 2011;39:821–831. doi: 10.3892/ijo.2011.1109. [DOI] [PubMed] [Google Scholar]
- 87.Tserkovsky D.A., Alexandrova E.N., Istomin Y.P. Photolon enhancement of ultrasound cytotoxicity. Exp Oncol. 2011;33:107–109. [PubMed] [Google Scholar]
- 88.Wang X.B., Hu J.M., Wang P., Zhang S.L., Liu Y.C., Xiong W.L. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics. 2015;5:772–786. doi: 10.7150/thno.10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bottari G., de la Torre G., Torres T. Phthalocyanine-nanocarbon ensembles: from discrete molecular and supramolecular systems to hybrid nanomaterials. Acc Chem Res. 2015;48:900–910. doi: 10.1021/ar5004384. [DOI] [PubMed] [Google Scholar]
- 90.Roguin L.P., Chiarante N., Vior M.C.G., Marino J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int J Biochem Cell Biol. 2019;114:105575. doi: 10.1016/j.biocel.2019.105575. [DOI] [PubMed] [Google Scholar]
- 91.Milowska K., Gabryelak T. Synergistic effect of ultrasound and phthalocyanines on nucleated erythrocytes in vitro. Ultrasound Med Biol. 2005;31:1707–1712. doi: 10.1016/j.ultrasmedbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 92.Kolarova H., Lenobel R., Kolar P., Strnad M. Sensitivity of different cell lines to phototoxic effect of disulfonated chloroaluminium phthalocyanine. Toxicol Vitro. 2007;21:1304–1306. doi: 10.1016/j.tiv.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 93.Sugita N., Kawabata K.I., Sasaki K., Sakata I., Umemura S. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjugate Chem. 2007;18:866–873. doi: 10.1021/bc060189p. [DOI] [PubMed] [Google Scholar]
- 94.Gao Y., Li Z.H., Wang C.Q., You J.L., Jin B.Y., Mo F. Self-assembled chitosan/rose bengal derivative nanoparticles for targeted sonodynamic therapy: preparation and tumor accumulation. RSC Adv. 2015;5:17915–17923. [Google Scholar]
- 95.Nomikou N., Fowley C., Byrne N.M., McCaughan B., McHale A.P., Callan J.F. Microbubble-sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chem Commun. 2012;48:8332–8334. doi: 10.1039/c2cc33913g. [DOI] [PubMed] [Google Scholar]
- 96.Newton A.D., Predina J.D., Corbett C.J., Frenzel-Sulyok L.G., Xia L., Petersson E.J. Optimization of second window indocyanine green for intraoperative near-infrared imaging of thoracic malignancy. J Am Coll Surg. 2019;228:188–197. doi: 10.1016/j.jamcollsurg.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan L., Lin W.Y., Zheng K.B., He L.W., Huang W.M. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev. 2013;42:622–661. doi: 10.1039/c2cs35313j. [DOI] [PubMed] [Google Scholar]
- 98.Gharesi S., Pourhajibagher M., Chiniforush N., Raoofian R., Hashemi M., Shahabi S. Effect of photodynamic therapy based on indocyanine green on expression of apoptosis-related genes in human gingival fibroblast cells. Photodiagnosis Photodyn Ther. 2017;19:33–36. doi: 10.1016/j.pdpdt.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Sagir T., Gencer S., Kemikli N., Abasiyanik M.F., Isik S., Ozturk R. Photodynamic activities of protoporphyrin IX and its dopamine conjugate against cancer and bacterial cell viability. Med Chem Res. 2012;21:4499–4505. [Google Scholar]
- 100.Su X.M., Wang X.B., Zhang K., Yang S., Liu Q.H., Leung A.W. Sonodynamic therapy induces apoptosis of human leukemia HL-60 cells in the presence of protoporphyrin IX. Gen Physiol Biophys. 2016;35:155–164. doi: 10.4149/gpb_2015051. [DOI] [PubMed] [Google Scholar]
- 101.Li Y.J., Huang P., Jiang C.L., Jia de X., Du X.X., Zhou J.H. Sonodynamically induced anti-tumor effect of 5-aminolevulinic acid on pancreatic cancer cells. Ultrasound Med Biol. 2014;40:2671–2679. doi: 10.1016/j.ultrasmedbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Foglietta F., Canaparo R., Francovich A., Civera P., Serpe L. In vitro study of sonodynamic and photodynamic treatment on human cancer cell lines. Clin Therapeut. 2013;35:51–52. [Google Scholar]
- 103.Xu X.Y., Meng X., Li S., Gan R.Y., Li Y., Li H.B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10:1553. doi: 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 105.Park K., Lee J.H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol Rep. 2007;17:537–540. [PubMed] [Google Scholar]
- 106.Wang X.N., Xia X.S., Leung A.W., Xiang J.Y., Jiang Y., Wang P. Ultrasound induces cellular destruction of nasopharyngeal carcinoma cells in the presence of curcumin. Ultrasonics. 2011;51:165–170. doi: 10.1016/j.ultras.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 107.Wang X.N., Leung A.W., Luo J.M., Xu C.S. TEM observation of ultrasound-induced mitophagy in nasopharyngeal carcinoma cells in the presence of curcumin. Exp Ther Med. 2012;3:146–148. doi: 10.3892/etm.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suzuki N., Okada K., Chida S., Komori C., Shimada Y., Suzuki T. Antitumor effect of acridine orange under ultrasonic irradiation in vitro. Anticancer Res. 2007;27:4179–4184. [PubMed] [Google Scholar]
- 109.Osman H., Elsahy D., Saadatzadeh M.R., Pollok K.E., Yocom S., Hattab E.M. Acridine orange as a novel photosensitizer for photodynamic therapy in glioblastoma. World Neurosurgery. 2018;114:e1310–e1315. doi: 10.1016/j.wneu.2018.03.207. [DOI] [PubMed] [Google Scholar]
- 110.Wang K.K., Zhang Y.F., Wang J., Yuan A.H., Sun M.J., Wu J.H. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci Rep. 2016;6:27421. doi: 10.1038/srep27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y.K., Zhou Q.F., Deng Z.T., Pan M., Liu X., Wu J.R. IR-780 dye as a sonosensitizer for sonodynamic therapy of breast tumor. Sci Rep. 2016;6:25968. doi: 10.1038/srep25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mroz P., Pawlak A., Satti M., Lee H., Wharton T., Gali H. Functionalized fullerenes mediate photodynamic killing of cancer cells: type I versus Type II photochemical mechanism. Free Radic Biol Med. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bosi S., Da Ros T., Spalluto G., Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem. 2003;38:913–923. doi: 10.1016/j.ejmech.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Vileno B., Lekka M., Sienkiewicz A., Marcoux P., Kulik A.J., Kasas S. Singlet oxygen (1Δg)-mediated oxidation of cellular and subcellular components: ESR and AFM assays. J Phys-condens Mat. 2005;17:S1471–S1482. [Google Scholar]
- 115.Yumita N., Iwase Y., Watanabe T., Nishi K., Kuwahara H., Shigeyama M. Involvement of reactive oxygen species in the enhancement of membrane lipid peroxidation by sonodynamic therapy with functionalized fullerenes. Anticancer Res. 2014;34:6481–6487. [PubMed] [Google Scholar]
- 116.Yumita N., Iwase Y., Imaizumi T., Sakurazawa A., Kaya Y., Nishi K. Sonodynamically-induced anticancer effects by functionalized fullerenes. Anticancer Res. 2013;33:3145–3151. [PubMed] [Google Scholar]
- 117.Cullis A.G., Canham L.T., Calcott P.D. The structural and luminescence properties of porous silicon. J Appl Phys. 1997;82:909–965. [Google Scholar]
- 118.Canham L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl Phys Lett. 1990;57:1046–1048. [Google Scholar]
- 119.Fujii M., Minobe S., Usui M., Hayashi S., Gross E., Diener J. Generation of singlet oxygen at room temperature mediated by energy transfer from photoexcited porousSi. Phys Rev B. 2004;70:85311–85315. [Google Scholar]
- 120.Osminkina L.A., Nikolaev A.L., Sviridov A.P., Andronova N.V., Tamarov K.P., Gongalsky M.B. Porous silicon nanoparticles as efficient sensitizers for sonodynamic therapy of cancer. Microporous Mesoporous Mater. 2015;210:169–175. [Google Scholar]
- 121.Ninomiya K., Noda K., Ogino C., Kuroda S.I., Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014;21:289–294. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Ninomiya K., Ogino C., Oshima S., Sonoke S., Kuroda S.I., Shimizu N. Targeted sonodynamic therapy using protein-modified TiO2 nanoparticles. Ultrason Sonochem. 2012;19:607–614. doi: 10.1016/j.ultsonch.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Moosavi Nejad S., Takahashi H., Hosseini H., Watanabe A., Endo H., Narihira K. Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrason Sonochem. 2016;32:95–101. doi: 10.1016/j.ultsonch.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 124.You D.G., Deepagan V.G., Um W., Jeon S., Son S., Chang H. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci Rep. 2016;6:23200. doi: 10.1038/srep23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rasmussen J.W., Martinez E., Louka P., Wingett D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expet Opin Drug Deliv. 2010;7:1063–1077. doi: 10.1517/17425247.2010.502560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang H.J., Shan Y.F., Dong L.J. A comparison of TiO2 and ZnO nanoparticles as photosensitizers in photodynamic therapy for cancer. J Biomed Nanotechnol. 2014;10:1450–1457. doi: 10.1166/jbn.2014.1961. [DOI] [PubMed] [Google Scholar]
- 127.Vighetto V., Ancona A., Racca L., Limongi T., Troia A., Canavese G. The synergistic effect of nanocrystals combined with ultrasound in the generation of reactive oxygen species for biomedical applications. Front Bioeng Biotechnol. 2019;7:374. doi: 10.3389/fbioe.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Y., Wang Y., Zhen W.Y., Wang Y.H., Zhang S.T., Zhao Y. Defect modified zinc oxide with augmenting sonodynamic reactive oxygen species generation. Biomaterials. 2020;251:120075. doi: 10.1016/j.biomaterials.2020.120075. [DOI] [PubMed] [Google Scholar]
- 129.Kouhnavard M., Ikeda S., Ludin N.A., Ahmad Khairudin N.B., Ghaffari B.V., Mat-Teridi M.A. A review of semiconductor materials as sensitizers for quantum dot-sensitized solar cells. Renew Sustain Energy Rev. 2014;37:397–407. [Google Scholar]
- 130.Shanmuganathan R., Karuppusamy I., Saravanan M., Muthukumar H., Ponnuchamy K., Ramkumar V.S. Synthesis of silver nanoparticles and their biomedical applications—a comprehensive review. Curr Pharmaceut Des. 2019;25:2650–2660. doi: 10.2174/1381612825666190708185506. [DOI] [PubMed] [Google Scholar]
- 131.Vankayala R., Lin C.C., Kalluru P., Chiang C.S., Hwang K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials. 2014;35:5527–5538. doi: 10.1016/j.biomaterials.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 132.Shanei A., Akbari-Zadeh H. Investigating the sonodynamic-radiosensitivity effect of gold nanoparticles on HeLa cervical cancer cells. J Kor Med Sci. 2019;34 doi: 10.3346/jkms.2019.34.e243. e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma L., Chen W., Schatte G., Wang W., Joly A.G., Huang Y.N. A new Cu–cysteamine complex: structure and optical properties. J Mater Chem C. 2014;2:4239–4246. [Google Scholar]
- 134.Pandey N.K., Chudal L., Phan J., Lin L.W., Johnson O., Xing M.Y. A facile method for the synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment. J Mater Chem B. 2019;7:6630–6642. doi: 10.1039/c9tb01566c. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z.P., Xiong L., Ouyang G.Q., Ma L., Sahi S., Wang K.P. Investigation of copper cysteamine nanoparticles as a new type of radiosensitiers for colorectal carcinoma treatment. Sci Rep. 2017;7:9290. doi: 10.1038/s41598-017-09375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang P., Wang X., Ma L., Sahi S., Li L., Wang X.B. Nanosonosensitization by using copper-cysteamine nanoparticles augmented sonodynamic cancer treatment. Part Part Syst Char. 2018;35:1700378. [Google Scholar]
- 137.Zhang Y.F., He L.Y., Wu J., Wang K.K., Wang J., Dai W.M. Switchable PDT for reducing skin photosensitization by a NIR dye inducing self-assembled and photo-disassembled nanoparticles. Biomaterials. 2016;107:23–32. doi: 10.1016/j.biomaterials.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 138.Yu W.Q., He X.Q., Yang Z.H., Yang X.T., Xiao W., Liu R. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 139.Bidkar A.P., Sanpui P., Ghosh S.S. Transferrin-conjugated red blood cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for the delivery of doxorubicin and methylene blue. Acs Applied Nano Materials. 2020;3:3807–3819. [Google Scholar]
- 140.Yang Z.J., Chen Q., Chen J.W., Dong Z.L., Zhang R., Liu J.J. Tumor-pH-responsive dissociable albumin–tamoxifen nanocomplexes enabling efficient tumor penetration and hypoxia relief for enhanced cancer photodynamic therapy. Small. 2018;14 doi: 10.1002/smll.201803262. [DOI] [PubMed] [Google Scholar]
- 141.Sun Q.H., Sun X.R., Ma X.P., Zhou Z.X., Jin E.L., Zhang B. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv Mater. 2014;26:7615–7621. doi: 10.1002/adma.201401554. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y.J., Chen S.N., Sun J.C., Zhu S.Y., Chen C.Y., Xie W. Folate-targeted and oxygen/indocyanine green-loaded lipid nanoparticles for dual-mode imaging and photo-sonodynamic/photothermal therapy of ovarian cancer in vitro and in vivo. Mol Pharm. 2019;16:4104–4120. doi: 10.1021/acs.molpharmaceut.9b00339. [DOI] [PubMed] [Google Scholar]
- 143.Liu Z., Li J.P., Jiang Y., Wang D.D. Multifunctional nanocapsules on a seesaw balancing sonodynamic and photodynamic therapies against superficial malignant tumors by effective immune-enhancement. Biomaterials. 2019;218:119251. doi: 10.1016/j.biomaterials.2019.119251. [DOI] [PubMed] [Google Scholar]
- 144.Liu Z., Li J.P., Chen W.N., Liu L., Yu F.F. Light and sound to trigger the Pandora's box against breast cancer: a combination strategy of sonodynamic, photodynamic and photothermal therapies. Biomaterials. 2020;232:119685. doi: 10.1016/j.biomaterials.2019.119685. [DOI] [PubMed] [Google Scholar]
- 145.Feng Q.H., Zhang W.X., Yang X.M., Li Y.Z., Hao Y.W., Zhang H.L. pH/ultrasound dual-responsive gas generator for ultrasound imaging-guided therapeutic inertial cavitation and sonodynamic therapy. Adv Healthc Mater. 2018;7:2192–2659. doi: 10.1002/adhm.201700957. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H.J., Zhang X.G., Ren Y.P., Cao F., Hou L., Zhang Z.Z. An in situ microenvironmental nano-regulator to inhibit the proliferation and metastasis of 4T1 tumor. Theranostics. 2019;9:3580–3594. doi: 10.7150/thno.33141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen S.N., Liu Y.J., Zhu S.Y., Chen C.Y., Xie W., Xiao L.L. Dual-mode imaging and therapeutic effects of drug-loaded phase-transition nanoparticles combined with near-infrared laser and low-intensity ultrasound on ovarian cancer. Drug Deliv. 2018;25:1683–1693. doi: 10.1080/10717544.2018.1507062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee H.M., Jeong Y.I., Kim D.H., Kwak T.W., Chung C.W., Kim C.H. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int J Pharm. 2013;454:74–81. doi: 10.1016/j.ijpharm.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 149.Wang H.P., Wang P., Li L., Zhang K., Wang X.B., Liu Q.H. Microbubbles enhance the antitumor effects of sinoporphyrin sodium mediated sonodynamic therapy both in vitro and in vivo. Int J Biol Sci. 2015;11:1401–1409. doi: 10.7150/ijbs.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Y., Wang H.P., Wang P., Zhang K., Geng X.R., Liu Q.H. Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater Sci. 2019;7:985–994. doi: 10.1039/c8bm01187g. [DOI] [PubMed] [Google Scholar]
- 151.Hou R., Liang X.L., Li X.D., Zhang X., Ma X.T., Wang F. In situ conversion of rose bengal microbubbles into nanoparticles for ultrasound imaging guided sonodynamic therapy with enhanced antitumor efficacy. Biomater Sci. 2020;8:2526–2536. doi: 10.1039/c9bm02046b. [DOI] [PubMed] [Google Scholar]
- 152.Zhang L., Yi H.J., Song J., Huang J., Yang K., Tan B. Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl Mater Interfaces. 2019;11:9355–9366. doi: 10.1021/acsami.8b21968. [DOI] [PubMed] [Google Scholar]
- 153.Ren H., Liu J.Q., Li Y.Q., Wang H.R., Ge S.Z., Yuan A.H. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy. Acta Biomater. 2017;59:269–282. doi: 10.1016/j.actbio.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 154.Wu J.N., Han H.J., Jin Q., Li Z.H., Li H., Ji J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9:14596–14605. doi: 10.1021/acsami.6b15853. [DOI] [PubMed] [Google Scholar]
- 155.Hou W.X., Xia F.F., Alves C.S., Qian X.Q., Yang Y.M., Cui D.X. MMP2-targeting and redox-responsive PEGylated chlorin e6 nanoparticles for cancer near-infrared imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2016;8:1447–1457. doi: 10.1021/acsami.5b10772. [DOI] [PubMed] [Google Scholar]
- 156.He J.Y., Li C.C., Ding L., Huang Y.N., Yin X.L., Zhang J.F. Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Adv Mater. 2019;31 doi: 10.1002/adma.201902409. [DOI] [PubMed] [Google Scholar]
- 157.Huang J., Liu F.Q., Han X.X., Zhang L., Hu Z.Q., Jiang Q.Q. Nanosonosensitizers for highly efficient sonodynamic cancer theranostics. Theranostics. 2018;8:6178–6194. doi: 10.7150/thno.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin H.C., Li W.T., Madanayake T.W., Tao C., Niu Q., Yan S.Q. Aptamer-guided upconversion nanoplatform for targeted drug delivery and near-infrared light-triggered photodynamic therapy. J Biomater Appl. 2020;34:875–888. doi: 10.1177/0885328219882152. [DOI] [PubMed] [Google Scholar]
- 159.Lucky S.S., Idris N.M., Huang K., Kim J., Li Z., Thong P.S. In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers. Theranostics. 2016;6:1844–1865. doi: 10.7150/thno.15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang L.M., Gao P., Huang Y.L., Lu X., Chang Q., Pan W. Boosting the photodynamic therapy efficiency with a mitochondria-targeted nanophotosensitizer. Chin Chem Lett. 2019;30:1293–1296. [Google Scholar]
- 161.Lin K.C., Lin Z.G., Li Y.J., Zheng Y.S., Zhang D. Ultrasound-induced reactive oxygen species generation and mitochondria-specific damage by sonodynamic agent/metal ion-doped mesoporous silica. RSC Adv. 2019;9:39924–39931. doi: 10.1039/c9ra08142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wan G.Y., Cheng Y.Y., Song J., Chen Q., Chen B.W., Liu Y.Y. Nucleus-targeting near-infrared nanoparticles based on TAT peptide-conjugated IR780 for photo-chemotherapy of breast cancer. Chem Eng J. 2020;380:122458. [Google Scholar]
- 163.Wu D., Zhao Z., Wang N., Zhang X.R., Yan H.H., Chen X.Q. Fluorescence imaging-guided multifunctional liposomes for tumor-specific phototherapy for laryngeal carcinoma. Biomater Sci. 2020;8:3443–3453. doi: 10.1039/d0bm00249f. [DOI] [PubMed] [Google Scholar]
- 164.Li Y.Z., Hao L.Q., Liu F., Yin L.X., Yan S.J., Zhao H.Y. Cell penetrating peptide-modified nanoparticles for tumor targeted imaging and synergistic effect of sonodynamic/HIFU therapy. Int J Nanomed. 2019;14:5875–5894. doi: 10.2147/IJN.S212184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tong H.X., Du J.W., Li H., Jin Q., Wang Y.X., Ji J. Programmed photosensitizer conjugated supramolecular nanocarriers with dual targeting ability for enhanced photodynamic therapy. Chem Commun. 2016;52:11935–11938. doi: 10.1039/c6cc06439f. (Camb) [DOI] [PubMed] [Google Scholar]
- 166.Yu W.Q., Liu R., Zhou Y., Gao H.L. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang G.B., Phua S.Z.F., Lim W.Q., Zhang R., Feng L.Z., Liu G.F. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv Mater. 2019;31 doi: 10.1002/adma.201901513. [DOI] [PubMed] [Google Scholar]
- 168.Wang K.W., Tu Y.L., Yao W., Zong Q.Y., Xiao X., Yang R.M. Size-switchable nanoparticles with self-destructive and tumor penetration characteristics for site-specific phototherapy of cancer. ACS Appl Mater Interfaces. 2020;12:6933–6943. doi: 10.1021/acsami.9b21525. [DOI] [PubMed] [Google Scholar]
- 169.Yang H.Y., Jang M.S., Li Y., Fu Y., Wu T.P., Lee J.H. Hierarchical tumor acidity-responsive self-assembled magnetic nanotheranostics for bimodal bioimaging and photodynamic therapy. J Control Release. 2019;301:157–165. doi: 10.1016/j.jconrel.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 170.Yang G.B., Xu L.G., Xu J., Zhang R., Song G.S., Chao Y. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018;18:2475–2484. doi: 10.1021/acs.nanolett.8b00040. [DOI] [PubMed] [Google Scholar]
- 171.Li Y.X., An H.X., Wang X.B., Wang P., Qu F., Jiao Y. Ultrasound-triggered release of sinoporphyrin sodium from liposome-microbubble complexes and its enhanced sonodynamic toxicity in breast cancer. Nano Research. 2017;11:1038–1056. [Google Scholar]
- 172.Wang Y.Z., Wang C., Ding Y., Li J., Li M., Liang X. Biomimetic HDL nanoparticle mediated tumor targeted delivery of indocyanine green for enhanced photodynamic therapy. Colloids Surf B Biointerfaces. 2016;148:533–540. doi: 10.1016/j.colsurfb.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 173.Thakur N.S., Patel G., Kushwah V., Jain S., Banerjee U.C. Self-assembled gold nanoparticle–lipid nanocomposites for on-demand delivery, tumor accumulation, and combined photothermal–photodynamic therapy. ACS Applied Bio Materials. 2018;2:349–361. doi: 10.1021/acsabm.8b00618. [DOI] [PubMed] [Google Scholar]
- 174.Li Q., Sun L.H., Hou M.M., Chen Q.B., Yang R.H., Zhang L. A phase-change material packaged within hollow copper sulfide nanoparticles carrying doxorubicin and chlorin e6 for fluorescence-guided trimodal therapy of cancer. ACS Appl Mater Interfaces. 2019;11:417–429. doi: 10.1021/acsami.8b19667. [DOI] [PubMed] [Google Scholar]
- 175.Cao H.L., Zhong S., Wang Q.S., Chen C., Tian J., Zhang W.A. Enhanced photodynamic therapy based on an amphiphilic branched copolymer with pendant vinyl groups for simultaneous GSH depletion and Ce6 release. J Mater Chem B. 2020;8:478–483. doi: 10.1039/c9tb02120e. [DOI] [PubMed] [Google Scholar]
- 176.Zhang C.N., Shi G.N., Zhang J.F., Niu J., Huang P.S., Wang Z.H. Redox- and light-responsive alginate nanoparticles as effective drug carriers for combinational anticancer therapy. Nanoscale. 2017;9:3304–3314. doi: 10.1039/c7nr00005g. [DOI] [PubMed] [Google Scholar]
- 177.Hou W.X., Zhao X., Qian X.Q., Pan F., Zhang C.L., Yang Y.M. pH-sensitive Self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale. 2016;8:104–116. doi: 10.1039/c5nr06842h. [DOI] [PubMed] [Google Scholar]
- 178.Wang L., Hu Y.J., Hao Y.W., Li L., Zheng C.X., Zhao H.J. Tumor-targeting core–shell structured nanoparticles for drug procedural controlled release and cancer sonodynamic combined therapy. J Control Release. 2018;286:74–84. doi: 10.1016/j.jconrel.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 179.Shen L.Y., Huang Y., Chen D., Qiu F., Ma C., Jin X. pH-Responsive aerobic nanoparticles for effective photodynamic therapy. Theranostics. 2017;7:4537–4550. doi: 10.7150/thno.19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dong Z.L., Feng L.Z., Zhu W.W., Sun X.Q., Gao M., Zhao H. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70. doi: 10.1016/j.biomaterials.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 181.Yan T.S., He J.M., Liu R.M., Liu Z.H., Cheng J.J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr Polym. 2020;231:115706. doi: 10.1016/j.carbpol.2019.115706. [DOI] [PubMed] [Google Scholar]
- 182.Ren Y., Wang R.R., Liu Y., Guo H., Zhou X., Yuan X.B. A hematoporphyrin-based delivery system for drug resistance reversal and tumor ablation. Biomaterials. 2014;35:2462–2470. doi: 10.1016/j.biomaterials.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 183.Yadav P., Zhang C., Whittaker A.K., Kailasam K., Shanavas A. Magnetic and photocatalytic curcumin bound carbon nitride nanohybrids for enhanced glioma cell death. ACS Biomater Sci Eng. 2019;5:6590–6601. doi: 10.1021/acsbiomaterials.9b01224. [DOI] [PubMed] [Google Scholar]
- 184.John J.V., Chung C.W., Johnson R.P., Jeong Y.I., Chung K.D., Kang D.H. Dual stimuli-responsive vesicular nanospheres fabricated by lipopolymer hybrids for tumor-targeted photodynamic therapy. Biomacromolecules. 2016;17:20–31. doi: 10.1021/acs.biomac.5b01474. [DOI] [PubMed] [Google Scholar]
- 185.Jing X.N., Zhi Z., Jin L.M., Wang F., Wu Y.S., Wang D.Q. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale. 2019;11:9457–9467. doi: 10.1039/c9nr01194c. [DOI] [PubMed] [Google Scholar]
- 186.Chu C.W., Ryu J.H., Jeong Y.I.L., Kwak T.W., Lee H.L., Kim H.Y. Redox-responsive nanophotosensitizer composed of chlorin e6-conjugated dextran for photodynamic treatment of colon cancer cells. J Nanomater. 2016;2016:1–12. [Google Scholar]
- 187.Li X.D., Wang J., Cui R.R., Xu D.K., Zhu L.S., Li Z.Y. Hypoxia/pH dual-responsive nitroimidazole-modified chitosan/rose bengal derivative nanoparticles for enhanced photodynamic anticancer therapy. Dyes Pigments. 2020;179:108395. [Google Scholar]
- 188.Zhang N., Zhao F.F., Zou Q.L., Li Y.X., Ma G.H., Yan X.H. Multitriggered tumor-responsive drug delivery vehicles based on protein and polypeptide coassembly for enhanced photodynamic tumor ablation. Small. 2016;12:5936–5943. doi: 10.1002/smll.201602339. [DOI] [PubMed] [Google Scholar]
- 189.Song W.T., Musetti S.N., Huang L. Nanomaterials for cancer immunotherapy. Biomaterials. 2017;148:16–30. doi: 10.1016/j.biomaterials.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ding B.B., Zheng P., Ma P.A., Lin J. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv Mater. 2020;32 doi: 10.1002/adma.201905823. [DOI] [PubMed] [Google Scholar]
- 191.Mroz P., Hashmi J.T., Huang Y.Y., Lange N., Hamblin M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expet Rev Clin Immunol. 2011;7:75–91. doi: 10.1586/eci.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie W., Zhu S., Yang B.Y., Chen C.Y., Chen S.N., Liu Y.J. The destruction of laser-induced phase-transition nanoparticles triggered by low-intensity ultrasound: an innovative modality to enhance the immunological treatment of ovarian cancer cells. Int J Nanomed. 2019;14:9377–9393. doi: 10.2147/IJN.S208404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Senthebane D.A., Rowe A., Thomford N.E., Shipanga H., Munro D., Mazeedi M. The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. 2017;18:1586. doi: 10.3390/ijms18071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kumari R., Sunil D., Ningthoujam R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Control Release. 2020;319:135–156. doi: 10.1016/j.jconrel.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 195.Li G., Yuan S., Deng D., Ou T., Li Y., Sun R. Fluorinated polyethylenimine to enable transmucosal delivery of photosensitizer-conjugated catalase for photodynamic therapy of orthotopic bladder tumors postintravesical instillation. Adv Funct Mater. 2019;29:1901932. [Google Scholar]
- 196.Wang T.T., Zhang H., Han Y.B., Liu H.H., Ren F., Zeng J.F. Light-enhanced O2-evolving nanoparticles boost photodynamic therapy to elicit antitumor immunity. ACS Appl Mater Interfaces. 2019;11:16367–16379. doi: 10.1021/acsami.9b03541. [DOI] [PubMed] [Google Scholar]
- 197.Wu X., Yan P.J., Ren Z.H., Wang Y.F., Cai X.J., Li X. Ferric hydroxide-modified upconversion nanoparticles for 808 nm NIR-triggered synergetic tumor therapy with hypoxia modulation. ACS Appl Mater Interfaces. 2019;11:385–393. doi: 10.1021/acsami.8b18427. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Y., Xu Y.J., Sun D., Meng Z.Y., Ying W.W., Gao W. Hollow magnetic nanosystem-boosting synergistic effect between magnetic hyperthermia and sonodynamic therapy via modulating reactive oxygen species and heat shock proteins. Chem Eng J. 2020;390:124521. [Google Scholar]
- 199.Liu X.M., Tian K., Zhang J.H., Zhao M.L., Liu S.J., Zhao Q. Smart NIR-light-mediated nanotherapeutic agents for enhancing tumor accumulation and overcoming hypoxia in synergistic cancer therapy. ACS Applied Bio Materials. 2019;2:1225–1232. doi: 10.1021/acsabm.8b00790. [DOI] [PubMed] [Google Scholar]
- 200.Liang S., Deng X.R., Xu G.Y., Xiao X., Wang M.F., Guo X.S. A novel Pt-TiO2 heterostructure with oxygen-deficient layer as bilaterally enhanced sonosensitizer for synergistic chemo-sonodynamic cancer therapy. Adv Funct Mater. 2020;30:1908598. [Google Scholar]
- 201.Wang M., Chang M.Y., Chen Q., Wang D.M., Li C.X., Hou Z.Y. Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomaterials. 2020;252:120093. doi: 10.1016/j.biomaterials.2020.120093. [DOI] [PubMed] [Google Scholar]
- 202.Lin T.S., Zhao X.Z., Zhao S., Yu H., Cao W.M., Chen W. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics. 2018;8:990–1004. doi: 10.7150/thno.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao C.Y., Tong Y.J., Li X.L., Shao L.H., Chen L., Lu J.Q. Photosensitive nanoparticles combining vascular-independent intratumor distribution and on-demand oxygen-depot delivery for enhanced cancer photodynamic therapy. Small. 2018;14 doi: 10.1002/smll.201703045. [DOI] [PubMed] [Google Scholar]
- 204.Wang H.Y., Hou L., Li H.L., Wang X., Cao Y., Zhang B.Y. A nanosystem loaded with perfluorohexane and rose bengal coupled upconversion nanoparticles for multimodal imaging and synergetic chemo-photodynamic therapy of cancer. Biomater Sci. 2020;8:2488–2506. doi: 10.1039/c9bm02081k. [DOI] [PubMed] [Google Scholar]
- 205.Chen J., Luo H.L., Liu Y., Zhang W., Li H.X., Luo T. Oxygen-self-produced nanoplatform for relieving hypoxia and breaking resistance to sonodynamic treatment of pancreatic cancer. ACS Nano. 2017;11:12849–12862. doi: 10.1021/acsnano.7b08225. [DOI] [PubMed] [Google Scholar]
- 206.Xie Z.X., Cai X.C., Sun C.Q., Liang S., Shao S., Huang S.S. O2-loaded pH-responsive multifunctional nanodrug carrier for overcoming hypoxia and highly efficient chemo-photodynamic cancer therapy. Chem Mater. 2018;31:483–490. [Google Scholar]
- 207.Liu W.L., Liu T., Zou M.Z., Yu W.Y., Li C.X., He Z.Y. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 2018;30 doi: 10.1002/adma.201802006. [DOI] [PubMed] [Google Scholar]
- 208.Yang Z.Y., Wang J.F., Liu S., Li X.H., Miao L.Y., Yang B. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway. Biomaterials. 2020;229:119580. doi: 10.1016/j.biomaterials.2019.119580. [DOI] [PubMed] [Google Scholar]
- 209.An J., Hu Y.G., Li C., Hou X.L., Cheng K., Zhang B. A pH/ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials. 2020;230:119636. doi: 10.1016/j.biomaterials.2019.119636. [DOI] [PubMed] [Google Scholar]
- 210.Deng Y.Y., Jia F., Chen S.Y., Shen Z.D., Jin Q., Fu G.S. Nitric oxide as an all-rounder for enhanced photodynamic therapy: hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials. 2018;187:55–65. doi: 10.1016/j.biomaterials.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 211.Brodin N.P., Guha C., Tome W.A. Photodynamic therapy and its role in combined modality anticancer treatment. Technol Cancer Res Treat. 2015;14:355–368. doi: 10.1177/1533034614556192. [DOI] [PubMed] [Google Scholar]
- 212.Li Z.L., Han J., Yu L.D., Qian X.Q., Xing H., Lin H. Synergistic sonodynamic/chemotherapeutic suppression of hepatocellular carcinoma by targeted biodegradable mesoporous nanosonosensitizers. Adv Funct Mater. 2018;28:1800145. [Google Scholar]
- 213.Han X.X., Huang J., Jing X.X., Yang D.Y., Lin H., Wang Z.G. Oxygen-deficient black titania for synergistic/enhanced sonodynamic and photoinduced cancer therapy at near infrared-II biowindow. ACS Nano. 2018;12:4545–4555. doi: 10.1021/acsnano.8b00899. [DOI] [PubMed] [Google Scholar]
- 214.Xu L., Wang S.B., Xu C., Han D., Ren X.H., Zhang X.Z. Multifunctional albumin-based delivery system generated by programmed assembly for tumor-targeted multimodal therapy and imaging. ACS Appl Mater Interfaces. 2019;11:38385–38394. doi: 10.1021/acsami.9b11263. [DOI] [PubMed] [Google Scholar]
- 215.Zheng Y.L., Gao Y. Molecular targeted nanotheranostics for future individualized cancer treatment. Expet Opin Drug Deliv. 2020;17:1059–1062. doi: 10.1080/17425247.2020.1772748. [DOI] [PubMed] [Google Scholar]
- 216.Chen Z.Y., Wang Y.X., Lin Y., Zhang J.S., Yang F., Zhou Q.L. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. BioMed Res Int. 2014;2014:819324. doi: 10.1155/2014/819324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kievit F.M., Zhang M.Q. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv Mater. 2011;23:H217–H247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang Y.Y., Zhang L., Lin X.W., Ke L.J., Li B.F., Xu L. Dual-responsive nanosystem for precise molecular subtyping and resistant reversal of EGFR targeted therapy. Chem Eng J. 2019;372:483–495. [Google Scholar]
- 219.Liu H.M., Zhou M.J., Sheng Z.H., Chen Y.K., Yeh C.K., Chen W.T. Theranostic nanosensitizers for highly efficient MR/fluorescence imaging-guided sonodynamic therapy of gliomas. J Cell Mol Med. 2018;22:5394–5405. doi: 10.1111/jcmm.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen C.Y., Sun J.C., Chen S.N., Liu Y.J., Zhu S.Y., Wang Z.G. A multifunctional-targeted nanoagent for dual-mode image-guided therapeutic effects on ovarian cancer cells. Int J Nanomed. 2019;14:753–769. doi: 10.2147/IJN.S187929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yano S., Hirohara S., Obata M., Hagiya Y., Ogura S.I., Ikeda A. Current states and future views in photodynamic therapy. J Photochem Photobiol C Photochem Rev. 2011;12:46–67. [Google Scholar]
- 222.Lucena S.R., Salazar N., Gracia-Cazana T., Zamarron A., Gonzalez S., Juarranz A. Combined treatments with photodynamic therapy for non-melanoma skin cancer. Int J Mol Sci. 2015;16:25912–25933. doi: 10.3390/ijms161025912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sun B.O., Li W., Liu N. Curative effect of the recent photofrin photodynamic adjuvant treatment on young patients with advanced colorectal cancer. Oncol Lett. 2016;11:2071–2074. doi: 10.3892/ol.2016.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hosokawa S., Takahashi G., Sugiyama K.I., Takebayashi S., Okamura J., Takizawa Y. Porfimer sodium-mediated photodynamic therapy in patients with head and neck squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2020;29:101627. doi: 10.1016/j.pdpdt.2019.101627. [DOI] [PubMed] [Google Scholar]
- 225.Papayan G., Goncharov S., Kazakov N., Strui A., Akopov A. Clinical potential of photodynamic diagnosis and therapy of tracheobronchial malignancies in the visible and infrared spectral ranges. Translational Biophotonics. 2020;2 [Google Scholar]
- 226.Farrakhova D., Shiryaev A., Yakovlev D., Efendiev K., Maklygina Y., Borodkin A. Trials of a fluorescent endoscopic video system for diagnosis and treatment of the head and neck cancer. J Clin Med. 2019;8:2229. doi: 10.3390/jcm8122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang H.W., Lv T., Zhang L.L., Lai Y.X., Tang L., Tang Y.C. A prospective pilot study to evaluate combined topical photodynamic therapy and surgery for extramammary Paget's disease. Laser Surg Med. 2013;45:296–301. doi: 10.1002/lsm.22142. [DOI] [PubMed] [Google Scholar]
- 228.Jing W., Juan X., Li X., Chen J.Y., Qin H., Qing L. Complete remission of two patients with recurrent and wide spread extramammary Paget disease obtained from 5-aminolevulinic acid-based photodynamic therapy and imiquimod combination treatment. Photodiagnosis Photodyn Ther. 2014;11:434–440. doi: 10.1016/j.pdpdt.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 229.Inui T., Makita K., Miura H., Matsuda A., Kuchiike D., Kubo K. Case report: a breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy. Anticancer Res. 2014;34:4589–4593. [PubMed] [Google Scholar]
- 230.Kenyon J.N., Fulle R.J., Lewis T.J. Activated cancer therapy using light and ultrasound—a case series of sonodynamic photodynamic therapy in 115 patients over a 4 year period. Curr Drug Ther. 2009;4:179–193. [Google Scholar]
- 231.Lin X.H., Song J.B., Chen X.Y., Yang H.H. Ultrasound activated sensitizers and applications. Angew Chem Int Ed Engl. 2020;59:2–24. doi: 10.1002/anie.201906823. [DOI] [PubMed] [Google Scholar]
- 232.Niu N., Zhang Z., Gao X., Chen Z.J., Li S.J., Li J. Photodynamic therapy in hypoxia: near-infrared-sensitive, self-supported, oxygen generation nano-platform enabled by upconverting nanoparticles. Chem Eng J. 2018;352:818–827. [Google Scholar]
- 233.Hsu C.C., Lin S.L., Chang C.A. Lanthanide-doped core–shell–shell nanocomposite for dual photodynamic therapy and luminescence imaging by a single X-ray excitation source. ACS Appl Mater Interfaces. 2018;10:7859–7870. doi: 10.1021/acsami.8b00015. [DOI] [PubMed] [Google Scholar]
- 234.Jiang Y.Y., Li J.C., Zhen X., Xie C., Pu K.Y. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Adv Mater. 2018;30 doi: 10.1002/adma.201705980. [DOI] [PubMed] [Google Scholar]
- 235.Jiang Y.Y., Upputuri P.K., Xie C., Zeng Z.L., Sharma A., Zhen X. Metabolizable semiconducting polymer nanoparticles for second near-infrared photoacoustic imaging. Adv Mater. 2019;31 doi: 10.1002/adma.201808166. [DOI] [PubMed] [Google Scholar]
- 236.Jiang Y.Y., Zhao X.H., Huang J.G., Li J.C., Upputuri P.K., Sun H. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat Commun. 2020;11:1857. doi: 10.1038/s41467-020-15730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dos Santos AlF., De Almeida D.R.Q., Terra L.F., Baptista M.S., Labriola L. Photodynamic therapy in cancer treatment—an update review. J Cancer Metastasis and Treatment. 2019;5:25. [Google Scholar]
- 238.Hannani D. Extracorporeal photopheresis: tolerogenic or immunogenic cell death? Beyond current dogma. Front Immunol. 2015;6:349. doi: 10.3389/fimmu.2015.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.