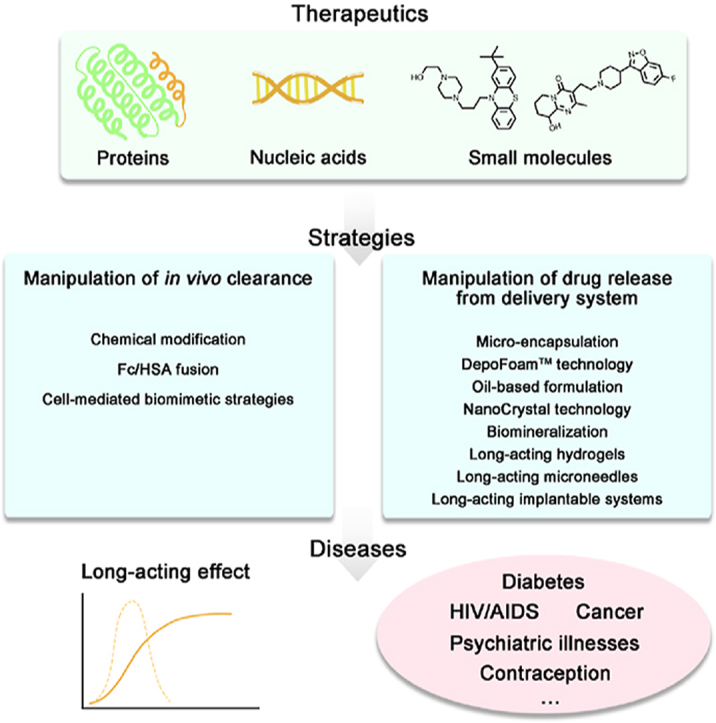
Abstract
The need for long-term treatments of chronic diseases has motivated the widespread development of long-acting parenteral formulations (LAPFs) with the aim of improving drug pharmacokinetics and therapeutic efficacy. LAPFs have been proven to extend the half-life of therapeutics, as well as to improve patient adherence; consequently, this enhances the outcome of therapy positively. Over past decades, considerable progress has been made in designing effective LAPFs in both preclinical and clinical settings. Here we review the latest advances of LAPFs in preclinical and clinical stages, focusing on the strategies and underlying mechanisms for achieving long acting. Existing strategies are classified into manipulation of in vivo clearance and manipulation of drug release from delivery systems, respectively. And the current challenges and prospects of each strategy are discussed. In addition, we also briefly discuss the design principles of LAPFs and provide future perspectives of the rational design of more effective LAPFs for their further clinical translation.
KEY WORDS: Long-acting, Chemical modification, Fc/HSA fusion, Biomimetic strategies, Hydrogels, Nanocrystal suspensions, Microneedles, Implantable systems
Abbreviations: 2′-F, 2′-fluoro; 2′-OMe, 2′-O-methyl; 2′-O-MOE, 2′-O-(2-methoxyethyl); 3D, three-dimensional; ART, antiretroviral therapy; ASO, antisense oligonucleotide; DDS, drug delivery systems; ECM, extracellular matrix; ENA, ethylene-bridged nucleic acid; ESC, enhanced stabilization chemistry; EVA, ethylene vinyl acetate; FcRn, Fc receptor; GLP-1, glucagon like peptide-1; GS, glycine–serine; HA, hyaluronic acid; HES, hydroxy-ethyl-starch; hGH, human growth hormone; HP, hypoparathyroidism; HSA, human serum albumin; IgG, immunoglobulin G; im, intramuscular; ISFI, in situ forming implants; iv, intravenous; LAFs, long-acting formulations; LAPFs, long-acting parenteral formulations; LNA, locked nucleic acid; MNs, microneedles; mPEG, methoxypolyethylene glycol; NDS, nanochannel delivery system; NPs, nanoparticles; OA, osteoarthritis; PCPP-SA, poly(1,3-bis(carboxyphenoxy)propane-co-sebacic-acid); PEG, polyethylene glycol; PM, platelet membrane; PMPC, poly(2-methyacryloyloxyethyl phosphorylcholine); PNAs, peptide nucleic acids; PS, phase separation; PSA, polysialic acid; PTH, parathyroid hormone; PVA, polyvinyl alcohol; RBCs, red blood cells; RES, reticuloendothelial system; RNAi, RNA interference; SAR, structure‒activity relationship; sc, subcutaneous; SCID, severe combined immunodeficiency; SE, solvent extraction; STC, standard template chemistry; TNFR2, tumor necrosis factor receptor 2
Graphical abstract
This review introduces the latest advances of long-acting parenteral formulations in preclinical and clinical stages, focusing on the strategies and underlying mechanisms for achieving long-acting effect.
1. Introduction
Long-acting formulations (LAFs) are used for pharmacotherapy as sustained-release medications over a period of several days, weeks, or even months. Compared to conventional preparations, LAFs have many distinguished advantages related to its long-lasting curative effect, as well as its reduced toxicity, dosage and frequency of administration. These outstanding features of LAFs have encouraged researchers to pursue their further development to fulfill the unmet need for long-term treatments of chronic diseases or other prevalent diseases that threaten human health.
Patients are often forced to take daily prescriptions of medicine for years to treat chronic diseases or other serious diseases such as HIV/AIDS, psychiatric illnesses, cancer, and diabetes. However, most patients often cannot adhere to a frequent and prolonged dosing schedule. For the treatment of these conditions, long-acting parenteral formulations (LAPFs) are preferred over conventional formulations. The release duration of drug delivery conferred by LAPFs may be able to improve patient adherence and consequently improve treatment outcomes. The replacement of daily oral regimens with antiviral LAPFs could successfully improve HIV/AIDS prevention and treatment. A single subcutaneous (sc) implantation of antiviral LAPFs can provide a protective level of drug concentration for months, a year or even longer1. Besides AIDS medication, long-acting innovative anti-infective drugs have proven effective to eliminate the hepatitis B virus and chronic hepatitis C virus2,3. Similarly, long-acting antipsychotics formulations can effectively reduce relapse and re-admission to psychiatric institutions and improve long-term prognosis due to their consistent plasma drug concentration4,5. Furthermore, as the prevalence of diabetes is a growing problem worldwide, reaching the target blood glucose level is critical for the vast majority of diabetic patients, as their improper management of glycemia can lead to life-threatening adverse effects6. Currently, clinicians have many long-acting basal insulins to select from 7, but there are still some unmet needs and challenges. Daily variability, low patient compliance and progressive micro and macrovascular risks require further research to formulate novel LAPFs for diabetic patients6. In addition to the treatment of above-mentioned diseases, LAPFs also have proven effective for contraception. Long-acting reversible contraceptives are recommended as first-line contraceptives for adolescents and young women8. Long-acting hormonal contraceptives with great efficacy and relative ease of use are widely accepted by millions of women with a variety of options9, 10, 11. Although great progress has been made in the development of LAPFs to meet clinical needs, it is still far from ideal. All of these indicate that LAPFs are very promising and have market demand.
Presently, small molecules share almost 90% of the global pharmaceutical market and are still a leading in new drug approvals. However, some small molecular drugs require multiple doses in a day owing to its short biological half-life, which is due to its structure, as well as other characteristics. Therefore, the major issues of these drugs are their large fluctuations in blood concentration and the risk of potential side effects. These factors limit their efficacy and clinical use. So far, many efforts have been made to improve the pharmacokinetics of these drugs and some progress has been made, such as PEGylation of methotrexate12 or making it into albumin-based nanomedicines13,14, and making vincristine into injectable sulfate loaded dextran microspheres amalgamated with chitosan-β-glycerophosphate gel to achieve long-term effectiveness15,16. Furthermore, combining small molecular drugs with ligands (such as long-chain fatty acids) or albumin prolongs the half-life of the drug. Currently, a greater number of researchers are turning their attention to macromolecular drugs that exert specific therapeutic effects and occupy an increasing market share. However, most of these drugs express poor stability, easy enzymes degradation, and rapid kidney clearance. To achieve therapeutic effects, long-term, frequent, or high-dose administrations are required through injection, which are accompanied by injection-associated pain, discomfort, certain psychological and economic burden. Encouragingly, significant attention and effort have been dedicated to the design and development of LAPFs of macromolecular drugs to keep them active for longer time in the body and improve their pharmacokinetics and therapeutic efficacy17,18.
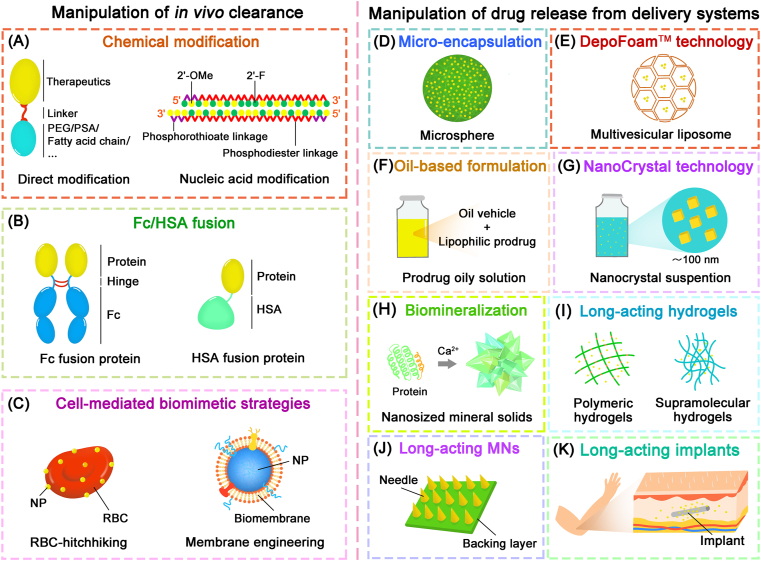
The principles to produce long-acting therapeutics are based on maintaining the drug activity for longer periods of time and to improve their tolerance in the body. Toward this end, a diverse array of strategies (Fig. 1) has been developed to design LAPFs through endowing the therapeutic drugs with features that include slow and controlled release, delayed clearance, resistance to enzymes, increased stability, extended half-life19,20. By rationally designing, LAPFs are safer and more efficient. Many LAPFs have been clinically approved, which has enabled the reduction of dosing frequency to daily, weekly, biweekly, or even monthly. Along with innovation of popular used approach for designing LAPFs and the emergence of novel strategies, LAPFs candidates have been scaled up to preclinical and clinical studies.
Figure 1.
Strategies for long-acting parenteral formulations. (A) Chemical modification. (B) Fc/HSA fusion. (C) Cell-mediated biomimetic strategies. (D) Micro-encapsulation. (E) DepoFoamTM technology. (F) Oil-based formulation. (G) NanoCrystal technology. (H) Biomineralization. (I) Long-acting hydrogels. (J) Long-acting microneedles. (K) Long-acting implantable systems.
In this study, the recent advances of LAPFs and their corresponding feasible design strategies are discussed. According to their underlying mechanisms, various strategies are classified into two categories: manipulation of drug release from delivery systems and manipulation of in vivo clearance. The preclinical and clinical progress of each design strategy together with its current landscape and challenges are discussed respectively. In addition, we also share our perspectives on viable directions for the rational design of LAPFs to improve therapeutic efficacy and further their clinical translation.
2. Existing strategies to manipulate the drug in vivo clearance
2.1. Chemical modification
The strategy of chemical modification, such as PEGylation, PSAylation and lipidation, etc. has been considered to be one of the most efficient approaches and has been recognized in clinical practice because of its definite structure, lack of impurity, and mature methods21. The key of the strategy is to prolong the half-life of drugs with little influence on their bio-activity as much as possible, which requires a comprehensive understanding of the structure–activity relationship (SAR) of drugs, as well as precise site-modification methods.
2.1.1. PEGylation and alternatives to PEGylation
Polyethylene glycol (PEG), a neutral polyether polymer, has become an indispensable part of drug delivery systems (DDS)22,23. The amphiphilic chain of PEG moieties, which is derived from the polymerization of ethylene oxide, increases the size of relatively small therapeutic drugs (e.g., peptides, proteins and nanobodies, etc.) in order to avoid excessive renal clearance and to prolong the residence time in the blood circulation, imparting “stealth” properties24. At the same time, the hydration layer produced by PEG can also play a protective role, thus avoiding enzymatic degradation of bio-macromolecules25. Adagen® (pegademase bovine), the first PEGylated product approved by US Food and Drug Administration (FDA) in 1990, is a modified enzyme used for the treatment of severe combined immunodeficiency (SCID) that is associated with a deficiency of adenosine deaminase. Since then, PEGylation has been considered as a well-tolerated module for half-life extension and has been utilized for 50 years26. It is worth mentioning that PEGylation can be used not only for the direct modification of drugs, but also for the improvement of DDS.
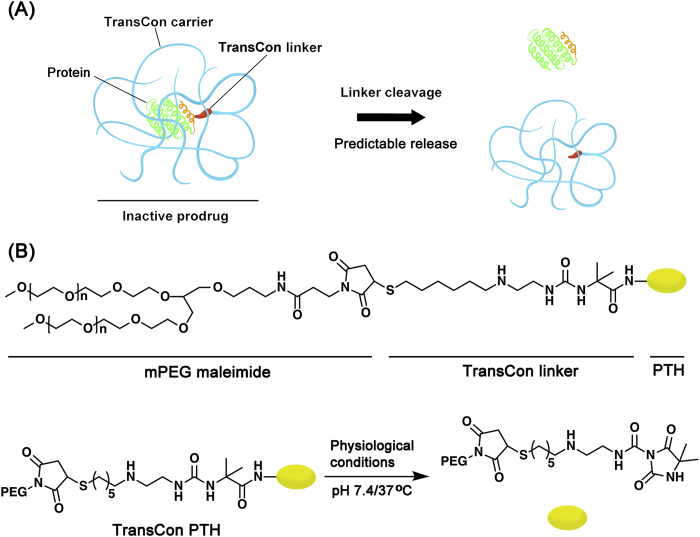
It is noteworthy that the “PEG dilemma” is brought after the PEGylation, which affects the interaction between carriers/drugs and cells, and limits cell uptake and endosomal escape resulting in the inevitably loss of bioactivity27,28. Therefore, cleavable PEG derivatives, which bonds are easy to break under specific physiological and pathological conditions, are designed to overcome the above limitations29,30. The reported sensitive bonds for cleavable PEG derivatives include peptide bonds, disulfide bonds, vinyl ether bonds, hydrazone bonds, ester bonds, as well as other types of bonds31. TransConTM technology platform (Fig. 2A), developed by Ascendis Pharma, provides a new strategy, which preserves the activity of therapeutic drugs and supports dosing frequency from a daily basis up to a monthly basis by combining the benefits of prodrugs and the sustained release mode32. Several candidate drugs (e.g., TransCon PTH, TransCon hGH, TransCon CNP, etc.), based on TransconTM technology, have been developed in clinical trial stages33. For example, TransCon parathyroid hormone (PTH), an inactive prodrug for sc administration, is developed to maintain a sustained release of PTH in the bloodstream, thereby overcoming the limitation about the use of short-acting PTH in the treatment of hypoparathyroidism (HP). As Fig. 2B shows, the PTH is transiently bound to methoxypolyethylene glycol (mPEG) via a TransCon linker to form TransCon PTH. The protective carrier has the ability to inactivate and shield PTH, and prolongs the circulation time of hormones due to its reduced renal clearance. Under physiological conditions, active PTH is sustained released with a half-life of approximately 2.5 days34. Recently, the reported phase I data support TransCon PTH to be a daily replacement therapy for the treatment of HP, providing a physiological level of PTH all day long and advancement into phase II clinical trial35.
Figure 2.
TransConTM technology platform. (A) TransCon molecules consist of three components: a therapeutic drug, a protective carrier and a cleavable linker, which is used to temporarily bind the above two parts. The protective carrier has the ability to inactivate and shield the therapeutic drug and the cleavable linker is designed to achieve the predictable release of active parent drug under a specific physiological environment. (B) The structure of TransCon PTH and the mechanism of drug release. Reproduced with the permission from Ref. 33. Copyright © 2017 Oxford University Press.
It cannot be ignored that PEG is immunogenic and non-degradable36, 37, 38. Therefore, developing alternative polymers to PEG have received great attention in the pharmaceutical industry39, 40, 41. Poly(2-methyacryloyloxyethyl phosphorylcholine) (PMPC), a kind of non-PEG based degradable polymer, was synthesized and coupled to interferon-α-2a to form PMPC-interferon-α-2a, which possesses a longer half-life than PEG modified interferon-α-2a42. In addition, naturally degradable polymers including polysialic acid (PSA), hydroxy-ethyl-starch (HES)43 and hyaluronic acid (HA) have also been applied for prolonging half-life as an alternative material to PEG44. PolyXenTM technology developed by Xenetic Biosciences, Inc. is an enabling platform for peptide and protein drug delivery. PolyXenTM technology utilizes the proprietary technology to conjugate hydrophilic and biodegradable PSA molecules to specific sites of therapeutic drugs for prolonging their half-life and improving their stability in vivo. Both the length and the modification site of the PSA chain can change the apparent hydrodynamic radius of the molecule which influence the properties and biological characteristics of therapeutic17. In addition, a carbohydrate shield called “glycocalyx” around the therapeutic molecule is formed after PSAylation, protecting the drugs less exposed to the immune system and the clearance systems including renal clearance and serum proteases. Several polysialyted candidates, such as polysialylated erythropoietin (PSA-EPO) and polysialylated recombinant factor VIII (PSA-FVIII), have been applied in clinical development45. The former is currently in phase III of clinical trials and the latter is in phase I.
2.1.2. Lipidation
Human serum albumin (HSA), the most abundant protein in plasma, has a long average half-life of 19 days and possesses the function of transporting exogenous and endogenous compounds46,47. On this basis, conjugating a fatty acid chain to the therapeutic protein can enhance its non-covalent combination with albumin in vivo, thus achieving a prolonged half-life. This strategy has been successfully applied in the modification of insulin and glucagon like peptide-1 (GLP-1) for half-life extension. Levemir® (insulin detemir), approved by FDA in 2004, is an insulin analog in which the Lys29 is myristoylated at the amino group of the side chain, allowing reversible combination with albumin for longer action time and more stable efficacy48. After modification, the long distribution phase of insulin detemir of about 8 h after sc administration is sufficient to achieve a once-daily administration49. A similar strategy was utilized to liraglutide which is an insulinotropic GLP-1 analog. The Lys26 of liraglutide is modified with a palmitic acid chain to achieve a half-life of 13 h after sc administration. In addition, the strategy of lipidation is widely applied to optimize drug properties to meet the requirements of various preparations. Although lipidation-based prodrug strategies hold promises for clinical translation of LAPFs, itʹs essential to note that this strategy is not suitable to all drug molecules to be modified, especially in case of lack of hydroxyl group and carboxyl group for esterification.
2.1.3. Nucleic acid modification
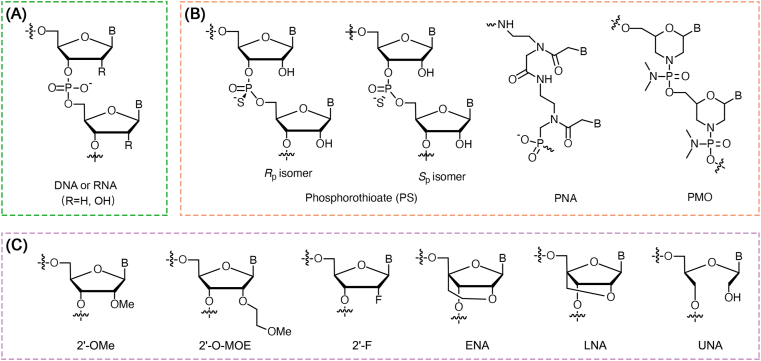
Gene therapy, based on nucleic acid drugs [e.g., antisense oligonucleotide (ASO), siRNA, miRNA, saRNA, aptamer, etc.], is considered as one of the promising strategies for precision medicine through up-regulation or down-regulation of target genes in specific cells. However, native nucleic acid drugs still have many difficulties to overcome, such as poor stability, rapid degradation by RNase, rapid clearance by the kidneys, immunogenicity and low cell uptake18. For improving their properties, the structures of nucleic acid drugs can be optimized through chemical synthesis50. Fig. 3 shows the structures of common chemical modifications of nucleic acid. In general, backbone modifications [e.g., phosphorothioate (PS), peptide nucleic acids (PNAs), etc.] can enhance nuclease resistance, improve pharmacokinetic characteristics and facilitate cell uptake. Moreover, sugar modifications [e.g., 2′-O-methyl (2′-OMe), 2′-O-(2-methoxyethyl) (2′-O-MOE), 2′-fluoro (2′-F), locked nucleic acid (LNA), 4′-C-ethylene-bridged nucleic acid (ENA), etc.] may reduce immunogenicity and toxicity, enhance RNA binding affinity, increase stability and bioavailability51,52. Due to the well development of nucleic acid chemistry, more than ten nucleic acid drugs have been approved on the market. Among them, the recently approved siRNA products, Givlaari® (givosiran) and Oxlumo® (lumasiran) developed by Alnylam Pharmaceuticals, through the combination of the GalNAc conjugation technology and the enhanced stabilization chemistry (ESC) modification technology, represent a surprising long-term effect after initial dosing. At present, dozens of siRNA drugs developed by Alnylam Pharmaceuticals are in clinical trials. Among them, inclisiran (ALN-PCSsc) is utilized in the treatment of hypercholesterolemia and currently in phase III clinical trial. The advantage of treating with inclisiran is that the LDL-C level can be reduced by more than 50% with only two injections per year53. If inclisiran is successful in clinical trials, it will overturn the existing chronic disease treatment model54. Recently, Brown et al.55 investigated the pharmacodynamic durability of GalNAc–siRNA conjugates, and found that the long-term stability of modified siRNA in acidic intracellular compartments plays a key role for long-term activity. The siRNA can sustainably escape from these acidic intracellular compartments, which serve as a long-term depot, and subsequently, over time (possibly even weeks), enable RNA interference (RNAi) activity in the cytoplasm after dosing.
Figure 3.
Structures of common chemical modifications of nucleic acid. (A) The structure of natural nucleic acid. (B) The structures of phosphate backbone modifications. (C) The structures of sugar modifications.
Gene therapy holds broad promises for the treatment of multiple human diseases, such as genetic disorders, cancers, virus infection, etc. Nucleic acid chemistry has been approved as an effective way to achieve increased stability, enhanced tissue specificity and reduced toxicity. Although the long-term mechanism of recently approved gene drug has not been clarified, numerous experiences and excellent progresses associated with gene therapy have been achieved in the past 20 years, and its long-term effect may be subversive to the existing treatment model.
2.2. Fc/HSA fusion
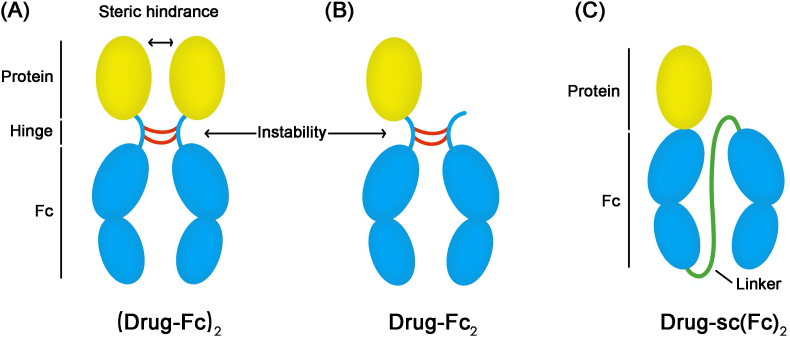
The strategy of Fc fusion provides the therapeutic protein a longer half-life by combining them with the Fc domain of an immunoglobulin G (IgG)56,57. Enbrel® (etanercept), was the first Fc fusion protein approved by FDA in 1998; it comprises of the extracellular region of tumor necrosis factor receptor 2 (TNFR2) and has been used in the treatment of rheumatoid arthritis58,59. To date, more than ten products, based on Fc fusion proteins, have been approved by the FDA. On the one hand, the binding of Fc-domain makes the size of the therapeutic protein larger than the threshold of kidney filtration resulting in a longer circulation time; on the other hand, the added Fc-domain has the ability to further prolong the circulation time through the neonatal Fc receptor (FcRn) mediated recycling procedure60, 61, 62. As shown in Fig. 4A, most Fc-fusion proteins possess the structure of a drug-Fc homo-dimer represented as (drug-Fc)2. Because of the adjacency of two drug-Fc monomers, the Fc-fusion proteins show physical instability and decreased bioactivity. For solving these issues, the drug-Fc2 (Fig. 4B) containing a protein linked two Fc domains has been developed and clinically approved. Eloctate® (recombinant factor VIII Fc fusion protein) and Alprolix® (recombinant factor IX Fc fusion protein) based on drug-Fc2 were both approved by FDA in 201463. However due to the impurity and low production yields, this technology cannot be utilized for all kinds of protein drugs. Besides, several protease cleavage sites, and the reduction of disulfide bonds in the hinge region, introduce potential instability of fusion proteins. In order to overcome above limitations, Zhou et al.64 developed a novel kind of Fc fusion strategy (Fig. 4C) based on a long and flexible glycine–serine (GS) linker between two Fc chains, and removed the hinge sequence to form a single chain Fc-dimer represented as sc(Fc)2. In this study, human growth hormone (hGH) was modified by sc(Fc)2 to create a novel fusion protein, which showed longer half-life and increased bioactivity than traditional Fc fusion-based proteins.
Figure 4.
The structures of a series of Fc fusion proteins. (A) The structure of (drug-Fc)2. (B) The structure of drug-Fc2. (C) The structure of drug-sc(Fc)2. Reproduced with the permission from Ref. 64. Copyright © 2017 Elsevier.
Recently, HSA fusion technology has also been utilized for extending the half-life of therapeutic proteins because of the special kinetic properties of HSA65,66. The mechanism of HSA fusion in prolonging half-life is the same as Fc fusion67,68. Meanwhile, HSA has the features of low immunogenicity and high biocompatibility69. Tanzeum® (albigutide), a GLP-1-HSA fusion protein approved in 2014, is the first product based on HSA fusion for the treatment of type 2 diabetes70. The half-life of native GLP-1 is 1–2 min, and the half-life of albigutide is 4–7 days, which can greatly reduce the frequency of administration in clinic. In 2016, Idelvion® (coagulation factor IX recombinant human), an HSA fusion of coagulation factor IX, was also approved by FDA for the treatment of hemophilia B71. In addition, Lee et al.72 developed a novel drug delivery system through the genetic fusion of an anti-FcRn affibody (AFF) and an albumin binding domain to therapeutic proteins. A synergistic effect on prolonging the plasma residence time can be achieved by the ABD-mediated albumin binding and AFF-mediated FcRn binding. This ABD-AFF fusion strategy is applied to exendin-4 to form EX-ABD-AFF, which revealed a longer half-life and better hypoglycemic effect than control group in mice.
Fc/HSA fusion proteins, as compared to the traditional therapeutic proteins, represent many improved characteristics, and have received extensive attention around the world. With the increasing complexity of fusion proteins, highly analytical and bioanalytical strategies should be established to achieve the comprehensive characterization of fusion proteins. Fusion proteins can not only prolong the circulation half-life of therapeutics, but also achieve superior therapeutic efficacy. Therefore, it has been increasingly recognized as useful and safe therapeutic proteins.
2.3. Cell-mediated biomimetic strategies
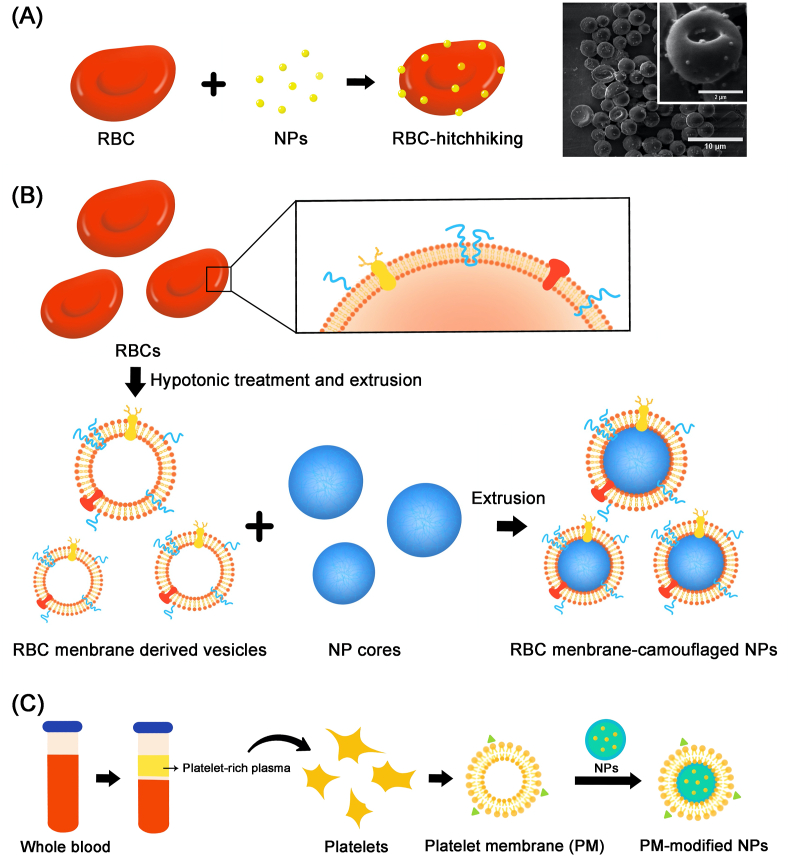
To date, a series of nanoparticles (NPs) have been prepared for the treatment of disorders such as malignant tumor, diabetes and cardiovascular diseases, but few of them have been applied in clinic. These nanomedicines are still recognized as “foreign objects”, resisted out of the physiological barrier or cleared quickly from the body. Biomimetic strategies such as RBC-hitchhiking and membrane engineering endow NPs with new properties in prolonging circulation time, evading host immune response and targeting to specific sites73, 74, 75, 76. Different cells have different functions. According to different design requirements to DDS, appropriate cells are selected to modify NPs77. Various cells, such as platelets, red blood cells (RBCs), tumor cells, lymphocytes, macrophages, have been modified as “chaperones” for NPs78,79. Among them, RBCs and platelets are often used in the study of long-acting preparations due to their special properties80.
RBCs are approximately 7 μm in diameter and possess a long-circulation property (~120 days in humans). Its highly malleable and flexible biconcave shape allow it to squeeze through capillary venules, which ultimately facilitates efficient gaseous exchange. The plasma membrane from an RBC contains more than 300 proteins, among which, CD-47, a surface receptor protein, acts as a “self-recognition” protein that helps the cell to avoid the immune system. Immobilising nanoparticle-based therapeutics on the surface of RBC for transportation, commonly referred to as RBC-hitchhiking (Fig. 5A), is considered to be a promising strategy for increasing the blood circulation lifetime. In 2010, Liu et al.81 first reported the adsorption of amphiphilic polymers onto the surface of RBCs, and proposed the idea that RBC-hitchhiking can potentially extend circulation time. After that, Anselmo et al.82 reported that the noncovalent attachment of NPs to RBCs can significantly increase the circulation time of NPs in the blood and transient accumulation in the lung over a 24 h period, meanwhile decreasing the uptake of NPs by spleen and liver. In this study, RBC-hitchhiking mediated NPs represented a three-fold higher retention in circulation, and a seven-fold increased accumulation in the lung. Moreover, biomembrane engineering is developed to form biomimetic nano-DDS, which retain the functional membrane protein from the origin cells83. In 2011, Hu et al.84 first reported a biomimetic delivery system based on RBC membrane-camouflaged polymetric NPs (Fig. 5B). The biomimetic particles in this study revealed significant retention in the blood circulation 72 h following an intravenous (iv) injection. Recently, Gao et al.85 reported a strategy that combined HSA binding and RBC membrane engineering. RBC membrane-camouflaged HSA NPs were constructed to delivery antioxidants for relieving Alzheimerʹs disease symptoms, providing long-term circulation and improved biocompatibility. Similar to RBCs, platelets also have the ability to escape the uptake by macrophage cells and prolong the circulation time in vivo due to the “self-recognition” proteins like CD47 on their membranes78,86. Platelet-based biomembrane engineering (Fig. 5C) has been widely used in NPs delivery for long-circulation87. Hu et al.88 developed a core–shell nano-carrier coated with a platelet membrane (PM) for targeting delivery of anti-tumor drugs. Besides the enhanced anti-tumor efficacy, this kind of nano-vehicle can achieve prolonged blood circulation and effectively eliminate in-circulation tumor cells in vivo, which ultimately inhibits the development of tumor metastasis.
Figure 5.
The strategies of RBC-hitchhiking and membrane engineering. (A) The strategy of RBC-hitchhiking. Reproduced with the permission from Ref. 82. Copyright © 2013 American Chemical Society. (B) The membrane of RBCs is used to form camouflaged NPs. Reproduced with the permission from Ref. 84. Copyright © 2011 National Academy of Sciences. (C) The platelet membrane (PM) is extracted from the blood and then used to form PM-modified NPs. Reproduced with the permission from Ref. 88. Copyright © 2015 John Wiley and Sons.
The strategy of biomimetic material engineering provides lots of inspiration for the preparation of multifunctional DDS. A large number of long-acting preparations based on bionic strategies have been reported. However, due to the difficulties in obtaining raw materials, complicated preparation process and complex components, biomimetic preparations lack advantages in clinical transformation. Therefore, the design of drug delivery systems involved in biomimetic technologies must keep the principles including scalability and reproducibility in mind for turning scientific advances into clinic.
3. Strategies to manipulate the drug release from delivery systems
3.1. Micro-encapsulation
The process of micro-encapsulation is that solid or liquid drug substances are dispersed or dissolved in polymeric materials to form microspheres, or as a core surrounded by a polymeric shell to form a microcapsule89,90. The first sustained-release injectable microsphere (Lupron Depot®) based on biodegradable poly(lactic-co-glycolic acid) (PLGA) was approved by FDA in 1986 for the treatment of prostatic cancer91,92. The latest approved microsphere-based product (Zilretta®) is used for the therapy of osteoarthritis (OA) pain of the knee. This kind of microsphere can slowly release triamcinolone acetonide in the OA joint for over 3 months93. The polymers used to produce microspheres include natural (e.g., gelatin, alginate chitosan, etc.), seminatural [e.g., cellulose acetate phthalate (CAP), ethyl cellulose (EC), methyl cellulose (MC), etc.] and synthetic materials [e.g., polylactic acid (PLA), PLGA, etc.]. Among these materials, PLGA is the most popularly used biodegradable material to prepare microspheres, accounting for 46% of all markets. The process of drug release can be adjusted by controlling the PLGA molecular weight, the ratio of drug to polymer, the size of microspheres, the ratio of glycolic acid to lactic acid, the end terminus of polymer and the properties of excipients94,95. The major mechanism of drug release from biodegradable microspheres includes diffusion, dissolution as well as polymer erosion and degradation. The possible mechanisms of drug release are following five pathways96. The first is initial release from the surface of microspheres; the second is release through the pores in microspheres; the third is diffusion through the intact polymer barrier; the fourth is diffusion through a water swollen barrier; the last is polymer erosion and degradation. All above mechanisms together play a part to achieve sustained drug release97,98. In addition, some polymers such as poly(1,3-bis(carboxyphenoxy)propane-co-sebacic-acid) (PCPP-SA), HA, tri(ethylene glycol)-poly(ortho ester) (TEG-POE), etc. are potential alternatives to PLGA, and possess the similar biocompatibility and ability to control drug release over prolonged periods. They are developed for loading drug substances that are incompatible with PLGA. Although many challenges of microspheres including uneven particle size, poor injectability, limited drug loading capacity and poor stability, it is still a promising strategy for preparing long-acting preparations and has been successfully translated in the clinic.
3.2. Multivesicular liposomes
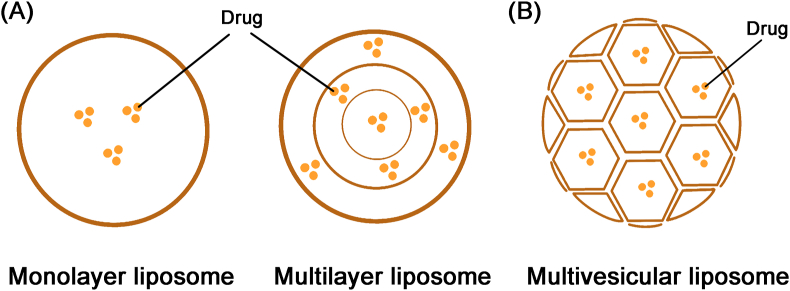
Liposomes are widely used in drug delivery because of its biocompatibility and universality. Different packing modes of lipid vesicles are essential for drug release of liposomes. In general, the packing mode of liposomes is concentric and the lipid membranes are assembled to form monolayer or multilayer structures (Fig. 6A). In this way, the collapse of internal lipid membranes will cause the accumulation of drugs, following the rapid drug release with the breach of external layer99. Doxil® (doxorubicin liposomal), the first FDA-approved PEGylated liposome, represents increased efficiency and decreased toxicity, but does not have a long-term effect100. Therefore, sustained release of therapeutics cannot be achieved through packing drugs in traditional liposomes. DepoFoam™ technology was developed by Pacira Pharmaceuticals, Inc. to produce multivesicular liposomes and has been successfully applied in clinic101. So far, three drugs (Depocyt®, DepoDur® and Exparel®) based on DepoFoam™ technology are approved by FDA and two more DepoFoam drugs including DepoTXA (DepoTranexamic Acid) and DepoMLX (DepoMeloxicam) are under clinical trial stage. Depocyt® (liposomal cytarabine), the first DepoFoam drug approved by FDA in 1999, is a sustained-release formulation for the intrathecal treatment of lymphomatous meningitis with a prolonged drug concentration of cytarabine in cerebrospinal fluid102. As shown in Fig. 6B, multivesicular liposomes with an average diameter of 3–30 μm are composed of hundreds of non-concentric and polyhedral aqueous compartments, and each compartment is separated by a continuous network of lipid membranes103. It achieves sustained drug release through gradual degradation of outermost vesicles. Internal vesicles are protected by outer vesicles to avoid burst drug release and the structure of multivesicular liposomes remains during the rearrangement of internal vesicles104. Multiple factors such as the properties and composition of lipid phase, interactions between the lipid and the encapsulated drug, composition of the aqueous phase and osmolarity of the aqueous phase contribute to the release rate104. The preparation of multivesicular liposomes involves a double emulsification process to form a water-in-oil-in-water emulsion. DepoFoam™ technology is also utilized to tackle pain management before and after surgical procedure. Exparel® (bupivacaine liposome), approved by FDA in 2011, is a longer acting form of traditional bupivacaine105. Day et al.106 reported that the patients who took Exparel® subcutaneously or orally represented extended and better pain control than conventional analgesic regimen in children after pharyngoplasty.
Figure 6.
The structures of a series of liposomes. (A) The structure of monolayer and multilayer liposome. (B) The structure of multivesicular liposome. Reproduced with the permission from Ref. 101. Copyright © 2002 Elsevier.
Multivesicular liposomes provide a pragmatic strategy to achieve longer residence time of drug due to its multi-compartment structure. Currently, DepoFoam™ technology has been applied for the treatment of neurological diseases and cancer, post-surgical pain management as well as reducing surgical bleeding. But the complex manufacturing process of multivesicular liposomes is still a challenge for its clinical translation.
3.3. Oil-based LAPFs
Oil-based LAPFs can be prepared by covalent attachment of a fatty acid chain and the therapeutics to form prodrugs, which are placed in the oil phase107. Decanoate, enanthate and caproate are often used to construct an ester bond with the parent drug to increase its solubility in the oil phase and to enhance the partitioning in the fatty tissues108. Lyogen Depot® (fluphenazine decanoate) and Haldol Depot® (haloperidol decanoate), two oil-based parenteral formulations, are both typical antipsychotics which have been available in US as LAFs109. After intramuscular (im) injection, these formulations can form a reservoir, which slowly releases prodrugs into surrounding blood, following the liberation of active molecules after hydrolysis. The rate of drug release depends not only on the slow release of prodrugs from fatty tissues into the circulation, but also the sustained hydrolysis of ester bonds to release the parent drug. In addition, some parameters such as the drug concentration in the oil phase, the surface area of the oil depot, as well as the partition coefficient between the tissue fluid and the oil depot are essential to control drug release110. Oil-based LAPFs can be used for both systemic and localized drug delivery. Cost-effectiveness and simple manufacturing process are the major advantages of oil-based depot formulations. But this kind of LAPFs is barely employed in recent clinical trials. The cause of the limited number of undergoing clinical trials might be the difficulties in tuning drug release kinetics.
3.4. Nanocrystal suspensions
The strategy of nano-crystallization is widely used to improve the properties of poorly soluble drugs, and the nano-formulation prepared by NanoCrystal technology represents significant long-acting effects, contributing to the treatment of various diseases such as antipsychotic and antiviral therapy111,112. Various technologies such as top-down, bottom-up and combination technologies are developed to prepare these nanocrystals. In this way, the solubility of poorly soluble drugs can be increased by decreasing the particle size of the drug and increasing the surface to volume ratio. Some drugs are modified as hydrophobic prodrugs and then converted into nanocrystals, which are further developed as nanocrystal suspensions. The first monthly injectable atypical antipsychotic Invega Sustenna® (paliperidone) based on NanoCrystal technology was approved by the FDA in 2009, which was developed by Janssen Pharmaceuticals, Inc. for the treatment of schizophrenia113. In 2015, the improvement version Invega Trinza® (paliperidone), a three-month injection, was approved by FDA for the treatment of schizophrenia in patients who have been adequately treated with Invega Sustenna® for more than four months114. The prescription of nano-suspensions includes poorly water-soluble drug crystals, stabilizers and appropriate buffers. Notably, paliperidone palmitate, the active ingredient of Invega Sustenna® and Invega Trinza®, is an insoluble prodrug synthesized by modification of paliperidone with palmitic acid115. The necessity for liberating the water soluble paliperidone prolongs the release time resulting in a long-acting pharmacological action.
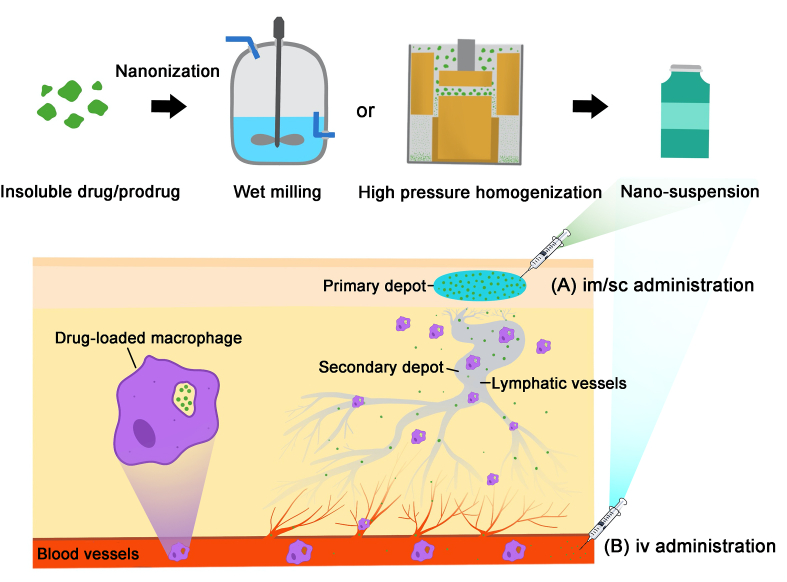
With the successful development of nanosuspension based on nanocrystals of cabotegravir and rilpivirine in phase III clinical trial, long-acting antiretroviral therapy (ART) has the potential to revolutionise current HIV/AIDS treatments116. As shown in Fig. 7, after high-pressure homogenization or wet milling, the nanoscale crystals in range of 50–1000 nm can be obtained, allowing high drug loading capacity, controllable size and long-term storage after lyophilization. It is reported that a drug depot is formed at the injection site after im or sc administration and the drugs are slowly released from the depot. Subsequently, nanocrystals enter the systemic circulation in following possible three pathways. First nanocrystals can be absorbed by blood capillaries, and then directly enter the systemic circulation. Second, after being absorbed by blood capillaries, the nanocrystals are drained into thoracic lymphatic vessels and subsequently released from lymphatic system into systemic circulation. Finally, nanocrystals can also be absorbed by macrophages and then enter lymphatic capillaries to form secondary depot117. In the study of pharmacokinetics of rilpivirine nanosuspension, van't Klooster et al.118 found that the concentration of rilpivirine in the lymph nodes draining the injection site was more than 100-fold higher than plasma concentration of rilpivirine 1 month after im administration in dogs, and decreased to 3- to 6-fold higher than plasma concentration of rilpivirine beyond 3 months. In addition, after direct iv administration, macrophages phagocytize nanocrystals to act as a drug reservoir resulting in sustained drug release in systemic circulation119. The drug dissolution and absorption of nanocrystals are dependent on many factors, such as aqueous solubility, particle size, injection site and depth, phagocytic efficiency of macrophages, degradation rate of prodrugs and the efficiency of lymphatic uptake.
Figure 7.
The preparation process and the mechanism of long-acting nanocrystals. This strategy is suitable for drugs/prodrugs with a low solubility in water. The manufacturing of nanocrystals can be achieved by milling or high-pressure homogenization. The long-term effect of the suspension of nanocrystalline is mainly achieved by the following two ways: (A) after im/sc administration, a primary depot of insoluble drugs/prodrugs can be formed in the site of injection, and then the drugs/prodrugs are slowly drained into the thoracic lymphatic vessels to form secondary depot. Subsequently, the active substance is gradually released from the lymphatic system into systemic circulation for long-acting effect; (B) after iv administration, macrophages phagocytize nanocrystals to act as a drug reservoir resulting in sustained drug release in systemic circulation. Reproduced with the permission from Ref. 116. Copyright © 2020 Elsevier.
In the past 20 years, drug nanocrystals have been gaining popularity, with the attraction of prolonged release characteristics after im injection. However, there are still challenges which need to be overcome in the future, such as large administration volumes, lower patient compliance, risks from tissue irritation and drug residues and safety requirements. Based on above mentioned, combined with clinical demands, nanosuspensions with sustained release characteristics, high drug loading and reduced administration volume have been developed in order to minimize the administration times and improve the compliance of patients. In the future, more and more intramuscularly administered nanocrystal suspensions will be developed and utilized in clinic for the treatment of different kinds of diseases.
3.5. Biomineralization
Direct use of natural biomaterials is a desirable strategy to bypass the foreign body response and maintain drug activity. Besides cell-mediated biomimetic strategies, the natural biomineralization process has attracted the attention of researchers. Biomineralization is an essential process to form hierarchically structured minerals with unique functions in biological organisms120. Interestingly, macromolecule biomineral complexes have the ability to load and release the bioactive molecules in the assembly and disassembly process in vivo121. Inspired by the natural biomineralization, Chen et al.122 reported that nanosized mineral solids, based on exendin-4, were formed through the interaction between calcium ions and acidic amino acid residues from the peptide in a supersaturated environment. This biomimetic system responded to physiological supersaturation, following the effective and long-term exendin-4 delivery. The acidic amino acid residues (one aspartic acid residue and five glutamic acid residues per molecule) from exendin-4 provided a “nucleation site” to chelate calcium ions to increase local supersaturation. Under anionic phosphate participation, tiny crystals were formed from calcium phosphate nucleation around the peptides, and further orderly assembled to macromolecule–biomineral complexes. It is worth noting that this strategy presented negligible influence on the bioactivity of the peptide. The mineralized exendin-4 may be dissociated and sustainably released after being spontaneously absorbed by a living body. The long-acting hypoglycemic effect of mineralized exendin-4 has been demonstrated in diabetic mice. Different from others, the strategy based on biomineralization is cost-effective and facile with negligible influence on the activity of proteins. In addition, the biocompatible biomineralized materials can avoid severe foreign body response, and be completely absorbed in vivo. Although this kind of biomimetic formulation represents promising application prospects in preclinical study, itʹs still uncertain whether it can also achieve long-term effect in the human body.
3.6. Long-acting hydrogels
Hydrogels, which have high water absorbing capacity, are cross-linked hydrophilic three-dimensional (3D) polymer networks123. Due to the close resemblance to extracellular matrix (ECM), hydrogels are considered as biocompatible materials applied to tissue engineering and drug delivery. In addition, the porous structure and aqueous environment of hydrogels are beneficial to drug loading, which makes it a promising candidate for controlled delivery systems for bioactive agents such as gene medicine, chemical drugs and proteins. In general, hydrogels are widely used as long-acting preparations through locally sustained drug release. The mechanism of drug release depends on the swelling of the hydrogels and drug diffusion from the polymer network. However, the phenomenon called “burst release”, which leads to unwanted side effects, limits the clinical application of hydrogels. In addition, the safety of chemical cross-linked hydrogels for long-term use is still unclear because of unidentified biocompatibility, refractory biodegradation and residual trace harmful reagent.
Supramolecular hydrogels are formed by self-assembly of molecular building blocks termed as hydrogelators through noncovalent interactions such as electrostatic interactions, hydrophobic interactions, van der Waals interactions, hydrogen bonding, metal-ligand, π–π stacking and sulfone-sulfone bonding124,125. Although supramolecular hydrogels have similar functions with polymeric hydrogels, the network structures formed by them are different. Supramolecular hydrogels are formed by self-assembly to nanofibers and helices, leading to entangled networks, while polymeric hydrogels are formed generally through chemical cross-linking125,126. In addition, due to the small molecular weights of hydrogelators, supramolecular hydrogels are easier to be cleaned up in vivo than polymeric hydrogels, which have higher molecular weights. Aromatic peptide-based supramolecular hydrogels was successfully employed to co-assemble and slowly release different bioactive factors to respectively enhance the anti-hepatic fibrosis effect of dexamethasone127, enhance the anticancer effect of etoposide128, overcome organ transplant rejection of tacrolimus129, and promote periodontal bone regeneration130. Abundant innovative efforts on directed self-assemble hydrogels formed by active small molecules or their derivatives (e.g., rhein131, raltitrexed132, berberine133, a glycyrrhetinic acid-modified curcumin134, etc.) have been developed from their beneficial high-purity, facile and environmentally friendly nature, void of any chemical synthesis process. For example, Qian et al.132 reported that raltitrexed, as a pure anticancer molecular, self-assembled into a hydrogel via π‒π stacking and hydrogen bond, has the potential to be a long-term auxiliary implement for avoiding postoperative local tumor recurrence.
With the progress of intelligent materials, hydrogels are endowed with various functions in response to environmental stimuli such as temperature, pH, photo, enzyme, ionic concentration and chemical signals135,136. For these stimuli responsive hydrogels or environmentally sensitive hydrogels, as the stimulus appears, the properties of hydrogels such as the structure, swelling behavior, permeability, solubility and mechanical strength can change resulting in the controlled delivery of therapeutics137,138. These changes can be irreversible or reversible. Vong et al.139 reported a temperature-sensitive and NO-releasing redox injectable hydrogel (NO-RIG). Under the temperance change after the intracardiac injection to mice, NO-RIG can convert to gel and retain in the myocardial tissue for at least 10 days. Additionally, situ-forming hydrogels at the tumor site provide a new strategy for long-term anti-tumor and anti-metastasis. Zheng et al.140 constructed a situ-forming artificial ECM based on fibrinogen and thrombin for selectively cutting-off the tumor metabolic flux. The physical barrier can further inhibit the metastasis of tumor cells. Through the combined application with clinical treatment, this strategy also has the long-term effect of preventing tumor recurrence.
In the past decade, the use of hydrogel preparations as a depot for locally sustained release of drugs has developed quickly especially in the case of contained and localized pathology. Rational hydrogel design can provide stimuli-triggered drug release to impart more precise, long-acting, and potent therapeutic effects. However, there remains challenging issues such as inevitable burst release and the inability to control the release kinetics overtime.
3.7. Long-acting microneedles
Microneedles (MNs), an array of micro-scale needles with a length of 0.2–1.5 mm, provide a painless approach for dermal delivery by overcoming the stratum corneum barrier141,142. Polymeric materials adopted to fabricate long-acting MNs can be divided into three categories including swellable polymers, biodegradable polymers and both. Swellable polymers are often used to form hydrogels through absorbing interstitial fluid from the skin. The diffusion of drug molecules is the main release pattern of swellable polymers based MNs, which its release rate is influenced by the cross-linking density of the polymer, the solubility of the drug and the interaction between the drug and the polymeric materials141. Due to the infusibility to skin tissue, swellable polymers based NMs can be completely removed after application without any safety issues of residual polymers. MNs based on biodegradable polymers, such as PLGA and PLA, control the drug release by the degradation of the polymer matrix rather than drug diffusion143,144. Some polymers derived from natural sources such as silk, fibroin and gelatin, are both biodegradable and swellable. Their respective drug release patterns are complex and rely not only on the rate of swelling but also the rate of degradation of the polymer. In brief, drug release control of MNs can be adjusted through modifying the properties of the polymeric materials.
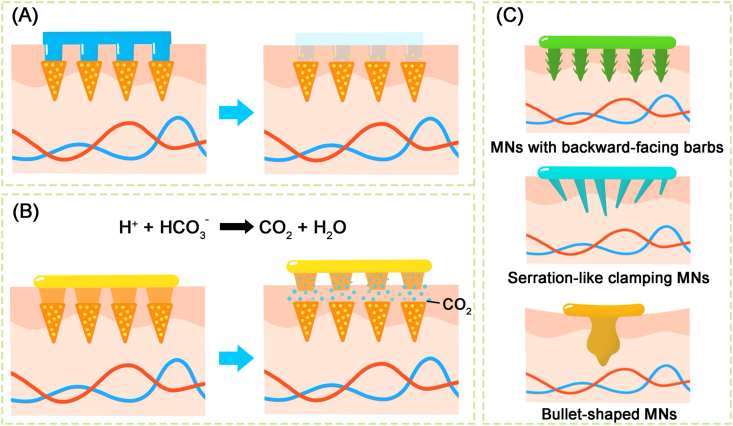
The design of the drug reservoir is the key of MNs preparation. The needle-based reservoir with separable needles or bioinspired needles is the common design of long-acting MNs. Separable needle-based MNs can regulate the drug containing-needles separate from the backing layer by the following different mechanisms: swelling triggered, bubble-aided145 and degradation-based separation for drug release146 (Fig. 8A and B). For example, Li et al.147 developed a maneuverable and safe microneedle patch, which was able to slowly release the contraceptive hormone for more than 1 month. The separate mechanism of MNs from the patch is triggered by effervescence after contacting with skin's interstitial fluid. However, due to the relatively shallow penetration, MNs fail to tightly contact the skin during long-term use. Bioinspired needles (Fig. 8C) inspired by natural animals are designed to construct a firm adhesion between skin tissue and MNs. MNs with backward-facing barbs are inspired by the stinger of honeybees148. Furthermore, the serrated forelegs of praying mantises and the attaching mechanism of endoparasite worms were used to design the shape of MN arrays149,150. A backing layer-based reservoir provides another design of long-acting MNs, which significantly increases the storage capacity to meet the requirement of drug loading for long-term exposure. Dissolvable polymers are used to fabricate the needles at an early stage. Drug diffusion from the backing reservoir is achieved through the micropores created after the needles dissolve completely. However, the micropores formed in this way cannot be retained for a long time. This results in the disruption of drug release. Swellable hydrogel-based needles are applied to overcome the above limitation, which provide a continuous and smooth channel between the drug reservoir and the dermal microcirculation151.
Figure 8.
The common designs of microneedles. (A) The needles separate from the backing layer triggered by degradation-based mechanism. (B) The needles separate from the backing layer triggered by bubble-aided mechanism. (C) Bioinspired needles to achieve firm skin adhesion. Reproduced with the permission from Ref. 141. Copyright © 2020 Elsevier.
MNs, a promising technology, constructs an ideal drug delivery system because of the convenient and painless dermal route of administration, which translates into high patient compliance152. Although MNs-based products in clinical trials are currently only used for bolus delivery, long-acting MNs lying on sustained delivery should be available in the near future.
3.8. Long-acting implantable systems
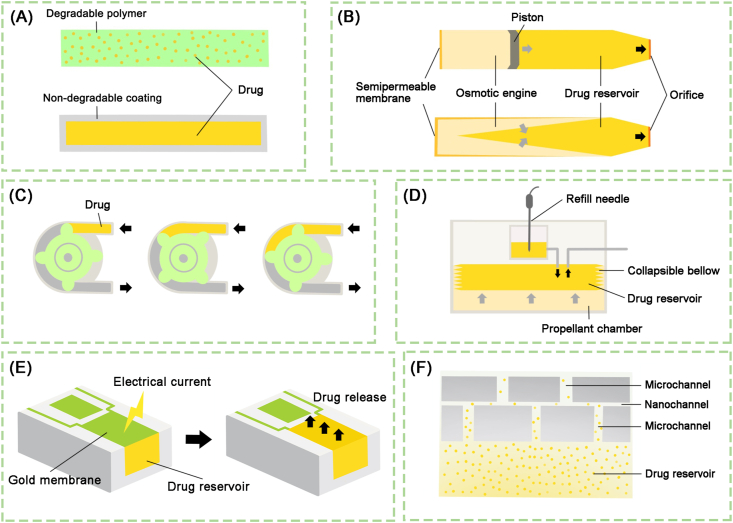
Long-acting implantable systems, which is based on biocompatible materials and intelligent devices, have become a promising strategy for local tissue therapy or systemic delivery. According to different drug release mechanisms, the long-acting implants can be divided into four types including polymer-based implants, implanted pumps, implantable chip and nanochannel based implants153. Fig. 9 illustrates the design and mechanism of the above implantable systems, and the following is their introduction in brief.
Figure 9.
The design of long-acting implantable systems. (A) Polymer-based implants. (B) Osmotic pumps are composed of a drug reservoir, an osmotic engine and a movable piston. A semipermeable membrane is used to separate the osmotic from the outside, and micro-holes are designed to connect the drug reservoir and the outside. The increased hydrostatic pressure in the osmotic engine is the motive force of pumps for drug release. (C) Positive displacement is the motive force of peristaltic pump for transporting the fluid inside the tube. (D) Implantable infusion pumps are divided into three parts including a propellant, a collapsible bellow and drug reservoir. The propellant changes bigger in the volume under body temperature leading to compression of collapsible bellow, following the drug release. (E) The mechanism of implantable chip is that the single reservoir can be opened by applying an electrical potential to the gold membrane resulting in the complete dissolution of the membrane, which subsequently results in the release of the drug. (F) A drug reservoir can be conveniently covered by the nanochannel delivery system (nDS) membrane to achieve zero-order kinetic drug release from the reservoir. Reproduced with the permission from Ref. 153. Copyright © 2019 Springer Nature.
According to properties of polymeric materials, the polymer-based implants include fully degradable polymeric systems as well as non-degradable polymeric systems154. The classical degradable implants (e.g., SinoFuan®, Zoladex®, Propel®, etc.), cylindrical solid rods, are prepared by melt extrusion to from biodegradable polymers. The non-degradable implants (e.g., Implanon®, Nexplanon®, Vantas®, etc.) consist of a drug core and a non-degradable film. These implants often employ the polymers such as silicone, ethylene vinyl acetate (EVA) and polyvinyl alcohol (PVA). The rate of drug release is controlled by the properties of polymeric coating, such as molecular weight, coating thickness and polymer configuration, as well as the physicochemical properties of drug, such as molecular weight, particle size and solubility. In situ forming implants (ISFI) are liquid or semisolid formulations based on biodegradable polymers, which have the ability to achieve spontaneous gelation/solidification at the site of injection155. According to the formulation compositions, ISFI can be formed by in situ precipitation, in situ gelling or cross-linking of the polymer chains. Among them, the solvent extraction (SE)/phase separation (PS) induced in situ precipitation has proved to be a successful approach to form long-acting ISFI (e.g., Perseris®, Eligard®, Atridox®, etc.) in clinical practice156. This kind of ISFI consists of a biodegradable water-insoluble polymer such as PLGA, a water miscible organic solvent such NMP and DMSO as well as therapeutics dissolved or dispersed in the homogeneous solution157. Upon sc injection, this polymer-based biodegradable system, which becomes a solid drug depot in situ after organic solvent extraction, provides drug release for weeks and even months158.
Implantable pumps are categorized into different classes according to the different motive forces responsible for drug release: osmotic pumps, peristaltic pumps and infusion pumps. They can be suitably chosen depending on disease patterns, the site of implantation and the drug release longevity. The following is an introduction to the different types of aforementioned implantable pumps. TARIS® System developed by TARIS Biomedical is based on an osmotic pump, which improves intravesical drug delivery. These intravesical osmotic pumps are currently undergoing clinical trials; its advantages including high loading efficiency, as well as reduced side effects and injection frequency. However, accidental rupture of the implantable pump can lead to drug overdose; moreover, the device might be difficult removed from the body without performing a cystoscopy. SynchroMed™ II pump developed by Medtronic, Inc. is an implantable FDA-approved peristaltic pump system, which has been used for the delivery of morphine sulfate, treprostinil, ziconotide, etc. This kind of pump can be programmed to control the flow rate, leading to personalizing the drug dose for each patient. Nonetheless, it requires relatively large volumes to accommodate the battery, leading to low loading efficiency of therapeutics. The Codman® 3000 pump developed by Codman & Shurtleff Inc. is an approved infusion pump for intrathecal delivery of morphine sulfate as a means of alleviating pain; it is also approved for hepatic arterial infusion of chemotherapy to target sites. Although the pump can achieve a constant rate of drug release under body temperature, and also can be transcutaneously refilled via a self-sealing silicone central port, its large size limits the volumetric loading efficiency. Implantable chips utilize the radio-frequency communication between electronic circuitry and the remote-control unit to trigger the procedural dissolution of covered reservoir membranes for controlled drug release. Each micro-chip has an array of drug reservoirs of 300–600 nL, and can be individually opened over time, following a pulsatile delivery profile159. Chua et al.160 reported a cylindrical intratumoral device with a nanochannel delivery system (nDS) membrane at one end and a silicone cap on the other end, which is developed to release CD40 and OX40 leading to increased immune cell infiltration. This study highlights the nDS as a potential platform for sustained intratumoral immunotherapeutic delivery.
Long-acting implantable systems could manipulate drug release at specific site for up to years without much plasma concentration fluctuation, and thus have irreplaceable superiority. However, their main problem is that surgical removal is required if the material cannot be completely degraded and causes inflammation, and that the very complex and sophisticated system design of implants increases research difficulty and development costs. In the future, use of smarter and fully biodegradable materials, as well as smaller and gentler implant equipment will contribute to their clinical translation.
4. Future perspectives
While we have highlighted many successful examples of LAPFs (Table 120,46,56,161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172), challenges still remain in many areas of their application. Improved technologies still need to solve the potential issues of current strategies. As conventional and popular used long-acting strategies, microspheres and nanocrystals are less difficult to industrialize and will continue to be used to innovate formulations and obtain transformed products. Moreover, LAPFs designed through strategies based on endogenous substances have good biocompatibility and in vivo safety and will be more developed. With the development of PEGylation, endogenous molecule such as HA, fatty acid, PSAylation, GalNAc conjugation modification technology and fusion protein technology have been derived. Fc/HSA fusion and nucleic acid modification technology have already been widely used to fuse or modify macromolecule therapeutics. With Givlaari® and Oxlumo® being approved in recent years, other drug candidates, designed by nucleic acid modification strategies, have now progressed into preclinical and clinical studies. Endogenous macromolecule, as a modifier with higher safety in vivo, has a tendency to gradually replace PEG to modify drugs and manipulate the in vivo clearance. These modified strategies are expected to be a hotpot in LAPFs development in the coming years. Furthermore, the use of endogenous polymers represented by HA, dextran and silk protein as soluble microneedle needle construction materials is a trend in the development of long-acting MNs. RBC-hitchhiking and membrane engineering technology, which are both based on endogenous substances, have encountered a series of problems in their transformation due to their individual differences, damage to cells or cell membranes during drug loading, and preservation problems. With the increase of research in these directions, the above-mentioned problems will be solved one by one, and LAPFs designed by RBC-hitchhiking and membrane engineering strategies will be greatly developed. Besides, with the continuous improvement of functionalized excipients and innovation of drug delivery devices, the long-acting implantable systems integrating medical devices and drugs will be developed. Furthermore, intelligent drug release systems will provide lots of inspirations for designing innovative LAPFs with the development of the electronic technology, precision instrument technology and microchip technology. In these LAPFs, the dynamic release of drugs will respond to changes in human physiological indicators. With the application of more new materials and new formulation technologies, novel LAPFs will be designed to precise control the dose, manage side effects, and avoid drug-drug interactions. In short, researchers will continue to seek new strategies to develop innovative LAPFs to provide the market with more therapeutic options.
Table 1.
List of approved long-acting preparations.
| Product name | Therapeutic drug | Strategy | Route of administration | Medical application | Ref. |
|---|---|---|---|---|---|
| Adagen® | Adenosine deaminase | PEGylation | im | Severe combined immunodeficiency disease | 161 |
| Oncaspar® | Asparagines | PEGylation | iv/im | Acute lymphoblastic leukemia | 161 |
| Peg-Intron® | Interferon alpha-2a | PEGylation | sc | Hepatitis C | 161 |
| Pegasys® | Interferon alpha-2a | PEGylation | sc | Hepatitis C | 161 |
| Neulasta® | G-CSF | PEGylation | sc | Treat neutropenia during chemotherapy | 161 |
| Somavert® | HGH antagonist | PEGylation | sc | Acromegaly | 161 |
| Macugen® | Aptamer | PEGylation | Intravitreal | Age related muscular degeneration | 161 |
| Mircera® | EPO | PEGylation | iv/sc | Anemia associated with chronic kidney disease | 161 |
| Cimzia® | PEG-anti-TNF Fab | PEGylation | sc | Rheumatoid arthritis | 161 |
| Puricase® | Uricase | PEGylation | iv | Gout | 162 |
| Sylatron® | Interferon alpha-2b | PEGylation | sc | Melanoma | 163 |
| Omontys® | PEGinesatide | PEGylation | iv/sc | Anemia with chronic kidney diseases | 161 |
| Jintrolong® | Somatotropin | PEGylation | sc | Growth hormone | 164 |
| Plegridy® | Interferon beta-1a | PEGylation | sc | Multiple sclerosis | 161 |
| Adynovate® | Alphapegol | PEGylation | iv | Haemophilia A | 165 |
| Rebinyn® | Coagulation factor IX | PEGylation | iv | Haemophilia A | 165 |
| Revcovi® | Adenosine deaminase | PEGylation | im | Adenosine deaminase severe combined immunodeficiency | 166 |
| Asparlas® | Asparagines | PEGylation | iv | Acute lymphoblastic leukemia | 167 |
| Ziextenzo® | G-CSF | PEGylation | iv | Infection during chemotherapy | 168 |
| Esperoct® | Coagulation factor IX | PEGylation | iv | Haemophilia A | 169 |
| Levemir® | Insulin | Lipidation | sc | Diabetes | 46 |
| Victoza® | liraglutide | Lipidation | sc | Diabetes | 170 |
| Givlaari® | siALAS1 | Nucleic acid modification | sc | Acute hepatic porphyria | 171 |
| Oxlumo® | siHAO1 | Nucleic acid modification | sc | Primary hyperoxaluria type 1 | 171 |
| Enbrel® | Etanercept | Fc fusion | sc | Juvenile idiopathic arthritis | 56 |
| Orencia® | Abatacept | Fc fusion | iv/sc | Rheumatoid arthritis | 56 |
| Arcalyst® | Rilonacept | Fc fusion | sc | Cryopyrin-associated periodic syndromes | 56 |
| Nplate® | Romiplostim | Fc fusion | sc | Chronic immune thrombocytopenic purpura | 56 |
| Nulojix® | Belatacept | Fc fusion | iv | Prevent transplant rejection in kidney transplant patients | 56 |
| Eylea® | Ablibercept | Fc fusion | iv | Various eye diseases | 56 |
| Zaltrap® | Ziv-aflibercept | Fc fusion | im | Metastatic colorectal cancer | 56 |
| Elocta® | Efmoroctocog α | Fc fusion | Powder and solvent for solution for injection | Haemophilia A (congenital factor VIII deficiency) | 56 |
| Alprolix® | Eftrenonacog α | Fc fusion | Powder and solvent for solution for injection | Haemophilia B (congenital factor IX deficiency) | 56 |
| Strensiq® | Asfotase α | Fc fusion | Solution for injection | Paediatric-onset hypophosphatasia | 56 |
| Trulicity® | Dulaglutide | Fc fusion | Solution for injection | Type 2 diabetes mellitus | 56 |
| Reblozyl® | Luspatercept | Fc fusion | iv | Beta thalassemia | 56 |
| Tanzeum® | Albiglutide | HSA fusion | sc | Diabetes | 46 |
| Idelvion® | Coagulation factor IX | HSA fusion | iv | Hemophilia B | 172 |
| Lupron Depot® | Leuprolide | Micro-encapsulation | im | Breast and prostatic cancer | 20 |
| Sandostatin® LAR | Octreotide acetate | Micro-encapsulation | im | Acromegaly, carcinoid tumors | 20 |
| Nutropin® | Somatotropin | Micro-encapsulation | sc | GH deficiency | 20 |
| Suprecur® MP | Buserelin acetate | Micro-encapsulation | sc | Endometriosis | 20 |
| Signifor® LAR | Pasireotide pamoate | Micro-encapsulation | im | Acromegaly | 20 |
| Somatuline® Depot | Lanreotide | Micro-encapsulation | im | Acromegaly | 20 |
| Trelstar® Depot | Triptorelin | Micro-encapsulation | im | Prostatic cancer | 20 |
| Arestin® | Minocycline hydrochloride | Micro-encapsulation | Subgingival | Gum infection | 20 |
| Risperdal Consta® | Risperidone | Micro-encapsulation | im | Schizophrenia, psychotic disorders | 20 |
| Vivitro® | Naltrexone | Micro-encapsulation | im | Alcohol opioid dependence | 20 |
| Bydureon® | Exenatide | Micro-encapsulation | sc | Type 2 diabetes mellitus | 20 |
| Signifor® LAR | Pasireotide pamoate | Micro-encapsulation | im | Acromegaly | 20 |
| Zilretta® | Triamcinolone acetonide | Micro-encapsulation | Intra-articular | Pain killer | 20 |
| Depocyt® | Cytarabine | Multivesicular liposomes | iv | Lymphomatous meningitis | 103 |
| DepoDur® | Morphine sulfate | Multivesicular liposomes | Epidural injection | Post-surgical pain relief | 103 |
| Exparel® | Bupivacaine | Multivesicular liposomes | Infiltration | Post-surgical pain relief | 103 |
| Androcur Depot® | Cyproterone acetate | Oil-based formulation | im | Cancer, prostate | 20 |
| Clopixol Depot® | Zuclopenthixol decanoate | Oil-based formulation | im | Schizophrenia |
20 |
| Delatestryl® | Testosterone enanthate | Oil-based formulation | im | Breast cancer hypogonadism |
20 |
| Lyogen Depot® | Fluphenazine decanoate | Oil-based formulation | im | Psychotic disorders | 20 |
| Haldol Depot® | Haloperidol decanoate | Oil-based formulation | im | Tourette's syndrome, schizophreni | 20 |
| Fluanxol Depot® | Flupenthixol decanoate | Oil-based formulation | im | Schizophrenia |
20 |
| Makena® | hydroxyprogesterone caproate | Oil-based formulation | im | Preterm Birth | 20 |
| Faslodex® | Fulvestrant | Oil-based formulation | im | Breast cancer | 20 |
| Naldebain ER® | Dinalbuphine sebacate | Oil-based formulation | im | Pain management | 20 |
| Agofollin Depot® | Estradiol benzoate | Nanocrystal suspension | sc | Hypoestrogenism | 20 |
| Aristada® | Aripiprazole lauroxil | Nanocrystal suspension | im | Schizophrenia | 20 |
| Aristada Initio™ | Aristada Initio | Nanocrystal suspension | im | Schizophrenia | 20 |
| Betason L.A® | Betamethasone | Nanocrystal suspension | im, intra-articular, intrabursal or intradermal | Inflammatory & allergic states | 20 |
| Bicillin® L-A | Penicillin G benzathine | Nanocrystal suspension | im | Syphilis, prophylaxis | 20 |
| Depo-Medrol® | Methylprednisolone acetate | Nanocrystal suspension | Intra-/peri-articular and intra-bursal | Epicondylitis, others | 20 |
| Depo-subQ Provera 104® | Medroxyprogesterone acetate | Nanocrystal suspension | im | Contraception & endometriosis | 20 |
| Invega Sustenna® | Paliperidone palmitate | Nanocrystal suspension | im | Schizophrenia | 20 |
| Invega Trinza® | Paliperidone palmitate | Nanocrystal suspension | im | Schizophrenia | 20 |
| Kenalog® | Triamcinolone acetonide | Nanocrystal suspension | im, intravitreal | Arthritis, inflammatory diseases | 20 |
| Zyprexa® | Olanzapine pamoate | Nanocrystal suspension | im | Schizophrenia | 20 |
| Relprevv® | Olanzapine pamoate | Nanocrystal suspension | im | Schizophrenia | 20 |
| Atridox® | Doxycycline hyclate | Implantable system | Subgingival | Periodontal disease | 20 |
| CiproScrew® | CiproScrew | Implantable system | Intra-bone insertion | Bone infection in surgery | 20 |
| Leuprone HEXAL® | Leuprolide acetate | Implantable system | sc | Breast and prostatic cancer | 20 |
| Ozurdex® | Dexamethasone | Implantable system | Intravitreal | Macular edema, non-infectious uveitis | 20 |
| Suprefact® Depot | Buserelin acetate | Implantable system | sc | Prostatic cancer | 20 |
| Sinuva® | Mometasone furoate | Implantable system | Intra-ethmoidal | Sinusitis | 20 |
| SinoFuan® | 5-Fluorouracil | Implantable system | Intraperitoneal | Cancer | 20 |
| Zoladex® | Goserelin acetate | Implantable system | sc | Breast and prostatic cancer | 20 |
| Perseris® | Risperidone | Implantable system | sc | Schizophrenia | 20 |
| Eligard® | Leuprolide acetate | Implantable system | sc | Prostatic cancer | 20 |
| Scenesse® | Afamelanotide | Implantable system | sc | Erythropoietic protoporphyria | 20 |
EPO, erythropoietin; G-CSF, granulocyte colony stimulating factor; GH, growth hormone; im, intramuscular; iv, intravenous; sc, subcutaneous.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81603041).
Author contributions
Yujie Shi, An Lu, Xiangyu Wang and Zakia Belhadj were responsible for original draft and visualization. Jiancheng Wang and Qiang Zhang revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicting interests in connection with this article.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Jiancheng Wang, Email: wang-jc@bjmu.edu.cn.
Qiang Zhang, Email: zqdodo@bjmu.edu.cn.
References
- 1.Weld E.D., Flexner C. Long-acting implants to treat and prevent HIV infection. Curr Opin HIV AIDS. 2020;15:33–41. doi: 10.1097/COH.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollinger R.C., Thio C.L., Sulkowski M.S., McKenzie-White J., Thomas D.L., Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet HIV. 2020;7:e443–e448. doi: 10.1016/S2352-3018(19)30342-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma M., Chu J.N., Salama J.A.F., Faiz M.T., Eweje F., Gwynne D. Development of a long-acting direct-acting antiviral system for hepatitis C virus treatment in swine. Proc Natl Acad Sci U S A. 2020;117:11987–11994. doi: 10.1073/pnas.2004746117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindenmayer J.P., Glick I.D., Talreja H., Underriner M. Persistent barriers to the use of long-acting injectable antipsychotics for the treatment of schizophrenia. J Clin Psychopharmacol. 2020;40:346–349. doi: 10.1097/JCP.0000000000001225. [DOI] [PubMed] [Google Scholar]
- 5.Morris M.T., Tarpada S.P. Long-acting injectable paliperidone palmitate: a review of efficacy and safety. Psychopharmacol Bull. 2017;47:42–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma D., Singh J. Long-term glycemic control and prevention of diabetes complications in vivo using oleic acid-grafted-chitosanzinc-insulin complexes incorporated in thermosensitive copolymer. J Control Release. 2020;323:161–178. doi: 10.1016/j.jconrel.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallegos Aragon K., Elmaoued A.A., Pham N.T., Conklin J.R., Ray G.M. Long-acting basal insulins: a review of the more recently approved agents. Cardiol Rev. 2019;27:260–266. doi: 10.1097/CRD.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 8.Salinas A., Merino P.M., Giraudo F., Codner E. Long-acting contraception in adolescents and young women with type 1 and type 2 diabetes. Pediatr Diabetes. 2020;21:1074–1082. doi: 10.1111/pedi.13069. [DOI] [PubMed] [Google Scholar]
- 9.Benagiano G., Gabelnick H., Brosens I. Long-acting hormonal contraception. Womens Health. 2015;11:749–757. doi: 10.2217/whe.15.68. [DOI] [PubMed] [Google Scholar]
- 10.Mäkäräinen L., van Beek A., Tuomivaara L., Asplund B., Coelingh Bennink H. Ovarian function during the use of a single contraceptive implant: implanon compared with Norplant. Fertil Steril. 1998;69:714–721. doi: 10.1016/s0015-0282(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 11.Winner B., Peipert J.F., Zhao Q., Buckel C., Madden T., Allsworth J.E. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 12.Yousefi G., Shafaati A., Zarghi A., Foroutan S.M. Pharmacokinetics and biodistribution of pegylated methotrexate after iv administration to mice. Iran J Pharm Res. 2018;17:111–123. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L., Hu F., Wang H., Wu X., Eltahan A.S., Stanford S. Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano. 2019;13:5036–5048. doi: 10.1021/acsnano.9b01710. [DOI] [PubMed] [Google Scholar]
- 14.Li X.Y., Li H., Zhang Y., Gao S., Dong C.P., Wu G.F. Development of albumin coupled, cholesterol stabilized, lipid nanoemulsion of methotrexate, and TNF-alpha inhibitor for improved in vivo efficacy against rheumatoid arthritis. AAPS PharmSciTech. 2017;18:2774–2782. doi: 10.1208/s12249-017-0762-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Chen Y., Li X., Liang X., Luo X. The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int J Nanomed. 2016;11:4187–4197. doi: 10.2147/IJN.S109547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur V., Kush P., Pandey R.S., Jain U.K., Chandra R., Madan J. Vincristine sulfate loaded dextran microspheres amalgamated with thermosensitive gel offered sustained release and enhanced cytotoxicity in THP-1, human leukemia cells: in vitro and in vivo study. Mater Sci Eng C Mater Biol Appl. 2016;61:113–122. doi: 10.1016/j.msec.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Schlapschy M., Binder U., Borger C., Theobald I., Wachinger K., Kisling S. PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng Des Sel. 2013;26:489–501. doi: 10.1093/protein/gzt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Therapeut. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlQahtani A.D., OʹConnor D., Domling A., Goda S.K. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed Pharmacother. 2019;113:108750. doi: 10.1016/j.biopha.2019.108750. [DOI] [PubMed] [Google Scholar]
- 20.Nkanga C.I., Fisch A., Rad-Malekshahi M., Romic M.D., Kittel B., Ullrich T. Clinically established biodegradable long acting injectables: an industry perspective. Adv Drug Deliv Rev. 2020;167:19–46. doi: 10.1016/j.addr.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman A.S., Lai J.J. Three significant highlights of controlled drug delivery over the past 55 years: PEGylation, ADCs, and EPR. Adv Drug Deliv Rev. 2020;158:2–3. doi: 10.1016/j.addr.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman A.S. The early days of PEG and PEGylation (1970s‒1990s) Acta Biomater. 2016;40:1–5. doi: 10.1016/j.actbio.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Wu L., Chen J., Wu Y., Zhang B., Cai X., Zhang Z. Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone. J Control Release. 2017;249:84–93. doi: 10.1016/j.jconrel.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence P.B., Price J.L. How PEGylation influences protein conformational stability. Curr Opin Chem Biol. 2016;34:88–94. doi: 10.1016/j.cbpa.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DʹSouza A.A., Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expet Opin Drug Deliv. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Zhang M., Jin H., Tang Y., Wang H., Xu Q. Intein-mediated site-specific synthesis of tumor-targeting protein delivery system: turning PEG dilemma into prodrug-like feature. Biomaterials. 2017;116:57–68. doi: 10.1016/j.biomaterials.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong L., Campbell F., Kros A. DePEGylation strategies to increase cancer nanomedicine efficacy. Nanoscale Horiz. 2019;4:378–387. doi: 10.1039/c8nh00417j. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y., Xue J., Gao S., Lu A., Yang D., Jiang H. Cleavable PEGylation: a strategy for overcoming the "PEG dilemma" in efficient drug delivery. Drug Deliv. 2017;24:22–32. doi: 10.1080/10717544.2017.1388451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M., Huang H., Wang D., Lu H., Chen J., Chai Z. Light-triggered PEGylation/dePEGylation of the nanocarriers for enhanced tumor penetration. Nano Lett. 2019;19:3671–3675. doi: 10.1021/acs.nanolett.9b00737. [DOI] [PubMed] [Google Scholar]
- 31.Kanamala M., Palmer B.D., Wilson W.R., Wu Z. Characterization of a smart pH-cleavable PEG polymer towards the development of dual pH-sensitive liposomes. Int J Pharm. 2018;548:288–296. doi: 10.1016/j.ijpharm.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Hoybye C., Pfeiffer A.F., Ferone D., Christiansen J.S., Gilfoyle D., Christoffersen E.D. A phase 2 trial of long-acting TransCon growth hormone in adult GH deficiency. Endocr Connect. 2017;6:129–138. doi: 10.1530/EC-17-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatelain P., Malievskiy O., Radziuk K., Senatorova G., Abdou M.O., Vlachopapadopoulou E. A randomized phase 2 study of long-acting TransCon GH vs daily GH in childhood GH deficiency. J Clin Endocrinol Metab. 2017;102:1673–1682. doi: 10.1210/jc.2016-3776. [DOI] [PubMed] [Google Scholar]
- 34.Holten-Andersen L., Pihl S., Rasmussen C.E., Zettler J., Maitro G., Baron J. Design and preclinical development of transcon PTH, an investigational sustained-release PTH replacement therapy for hypoparathyroidism. J Bone Miner Res. 2019;34:2075–2086. doi: 10.1002/jbmr.3824. [DOI] [PubMed] [Google Scholar]
- 35.Karpf D.B., Pihl S., Mourya S., Mortensen E., Kovoor E., Markova D. A randomized double-blind placebo-controlled first-in-human phase 1 trial of TransCon PTH in healthy adults. J Bone Miner Res. 2020;35:1430–1440. doi: 10.1002/jbmr.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bleher S., Buck J., Muhl C., Sieber S., Barnert S., Witzigmann D. Poly(sarcosine) surface modification imparts stealth-like properties to liposomes. Small. 2019;15 doi: 10.1002/smll.201904716. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P., Sun F., Liu S., Jiang S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244:184–193. doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiraishi K., Kawano K., Maitani Y., Aoshi T., Ishii K.J., Sanada Y. Exploring the relationship between anti-PEG IgM behaviors and PEGylated nanoparticles and its significance for accelerated blood clearance. J Control Release. 2016;234:59–67. doi: 10.1016/j.jconrel.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Khutoryanskiy V.V. Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev. 2018;124:140–149. doi: 10.1016/j.addr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Hoang Thi TT., Pilkington E.H., Nguyen D.H., Lee J.S., Park K.D., Truong N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers. 2020;12:298. doi: 10.3390/polym12020298. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulati N.M., Stewart P.L., Steinmetz N.F. Bioinspired shielding strategies for nanoparticle drug delivery applications. Mol Pharm. 2018;15:2900–2909. doi: 10.1021/acs.molpharmaceut.8b00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis A., Tang Y., Brocchini S., Choi J.W., Godwin A. Poly(2-methacryloyloxyethyl phosphorylcholine) for protein conjugation. Bioconjugate Chem. 2008;19:2144–2155. doi: 10.1021/bc800242t. [DOI] [PubMed] [Google Scholar]
- 43.Kalyane D., Raval N., Maheshwari R., Tambe V., Kalia K., Tekade R.K. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi: 10.1016/j.msec.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 44.Knop K., Hoogenboom R., Fischer D., Schubert U.S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 45.Meng H., Jain S., Lockshin C., Shaligram U., Martinez J., Genkin D. Clinical application of polysialylated deoxyribonuclease and erythropoietin. Recent Pat Drug Deliv Formulation. 2018;12:212–222. doi: 10.2174/1872211312666180717164758. [DOI] [PubMed] [Google Scholar]
- 46.Larsen M.T., Kuhlmann M., Hvam M.L., Howard K.A. Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther. 2016;4:3. doi: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Sun T., Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8:34–50. doi: 10.1016/j.apsb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heise T., Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metabol. 2017;19:3–12. doi: 10.1111/dom.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnolds S., Kuglin B., Kapitza C., Heise T. How pharmacokinetic and pharmacodynamic principles pave the way for optimal basal insulin therapy in type 2 diabetes. Int J Clin Pract. 2010;64:1415–1424. doi: 10.1111/j.1742-1241.2010.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith C.I.E., Zain R. Therapeutic oligonucleotides: state of the art. Annu Rev Pharmacol Toxicol. 2019;59:605–630. doi: 10.1146/annurev-pharmtox-010818-021050. [DOI] [PubMed] [Google Scholar]
- 51.Chen C., Yang Z., Tang X. Chemical modifications of nucleic acid drugs and their delivery systems for gene-based therapy. Med Res Rev. 2018;38:829–869. doi: 10.1002/med.21479. [DOI] [PubMed] [Google Scholar]
- 52.Shen X., Corey D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46:1584–1600. doi: 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray K.K., Landmesser U., Leiter L.A., Kallend D., Dufour R., Karakas M. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–1440. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 54.Dyrbus K., Gasior M., Penson P., Ray K.K., Banach M. Inclisiran-new hope in the management of lipid disorders? J Clin Lipidol. 2020;14:16–27. doi: 10.1016/j.jacl.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Brown C.R., Gupta S., Qin J., Racie T., He G., Lentini S. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020;48:11827–11844. doi: 10.1093/nar/gkaa670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duivelshof B.L., Murisier A., Camperi J., Fekete S., Beck A., Guillarme D. Therapeutic Fc-fusion proteins: current analytical strategies. J Separ Sci. 2020;44:35–62. doi: 10.1002/jssc.202000765. [DOI] [PubMed] [Google Scholar]
- 57.Liu L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell. 2018;9:15–32. doi: 10.1007/s13238-017-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassett B., Singh E., Mahgoub E., OʹBrien J., Vicik S.M., Fitzpatrick B. Manufacturing history of etanercept (Enbrel®): consistency of product quality through major process revisions. mAbs. 2018;10:159–165. doi: 10.1080/19420862.2017.1388483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott L.J. Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs. 2014;74:1379–1410. doi: 10.1007/s40265-014-0258-9. [DOI] [PubMed] [Google Scholar]
- 60.Levin D., Golding B., Strome S.E., Sauna Z.E. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33:27–34. doi: 10.1016/j.tibtech.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Rath T., Baker K., Dumont J.A., Peters R.T., Jiang H., Qiao S.W. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol. 2015;35:235–254. doi: 10.3109/07388551.2013.834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unverdorben F., Richter F., Hutt M., Seifert O., Malinge P., Fischer N. Pharmacokinetic properties of IgG and various Fc fusion proteins in mice. mAbs. 2016;8:120–128. doi: 10.1080/19420862.2015.1113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemostasis. 2015;13(Suppl 1):S176–S179. doi: 10.1111/jth.12929. [DOI] [PubMed] [Google Scholar]
- 64.Zhou L., Wang H.Y., Tong S., Okamoto C.T., Shen W.C., Zaro J.L. Single chain Fc-dimer-human growth hormone fusion protein for improved drug delivery. Biomaterials. 2017;117:24–31. doi: 10.1016/j.biomaterials.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 65.Tan H., Su W., Zhang W., Zhang J., Sattler M., Zou P. Albumin-binding domain extends half-life of glucagon-like peptide-1. Eur J Pharmacol. 2020;890:173650. doi: 10.1016/j.ejphar.2020.173650. [DOI] [PubMed] [Google Scholar]
- 66.Elsadek B., Kratz F. Impact of albumin on drug delivery—new applications on the horizon. J Control Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 67.Larsen M.T., Rawsthorne H., Schelde K.K., Dagnaes-Hansen F., Cameron J., Howard K.A. Cellular recycling-driven in vivo half-life extension using recombinant albumin fusions tuned for neonatal Fc receptor (FcRn) engagement. J Control Release. 2018;287:132–141. doi: 10.1016/j.jconrel.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 68.Sobieraj A.M., Huemer M., Zinsli L.V., Meile S., Keller A.P., Rohrig C. Engineering of long-circulating peptidoglycan hydrolases enables efficient treatment of systemic Staphylococcus aureus infection. mBio. 2020;11 doi: 10.1128/mBio.01781-20. e01781-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M., Zhang L., Cai Y., Yang Y., Qiu L., Shen Y. Bioengineered human serum albumin fusion protein as target/enzyme/pH three-stage propulsive drug vehicle for tumor therapy. ACS Nano. 2020;14:17405–17418. doi: 10.1021/acsnano.0c07610. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y.M., Lee S.M., Chung H.S. Novel AGLP-1 albumin fusion protein as a long-lasting agent for type 2 diabetes. BMB Rep. 2013;46:606–610. doi: 10.5483/BMBRep.2013.46.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyseng-Williamson K.A. Coagulation factor IX (recombinant), albumin fusion protein (albutrepenonacog alfa; Idelvion®): a review of its use in haemophilia B. Drugs. 2017;77:97–106. doi: 10.1007/s40265-016-0679-8. [DOI] [PubMed] [Google Scholar]
- 72.Lee J.Y., Park T., Hong E., Amatya R., Park K.A., Park Y.H. Genetic engineering of novel super long-acting exendin-4 chimeric protein for effective treatment of metabolic and cognitive complications of obesity. Biomaterials. 2020;257:120250. doi: 10.1016/j.biomaterials.2020.120250. [DOI] [PubMed] [Google Scholar]
- 73.Shields C.W., 4th, Evans M.A., Wang L.L., Baugh N., Iyer S., Wu D. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Z., Ukidve A., Gao Y., Kim J., Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Z., Ukidve A., Kim J., Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181:151–167. doi: 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Z., Ukidve A., Krishnan V., Fehnel A., Pan D.C., Gao Y. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng. 2021;5:441–454. doi: 10.1038/s41551-020-00644-2. [DOI] [PubMed] [Google Scholar]
- 77.Ayer M., Klok H.A. Cell-mediated delivery of synthetic nano- and microparticles. J Control Release. 2017;259:92–104. doi: 10.1016/j.jconrel.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z., Hu Q., Gu Z. Leveraging engineering of cells for drug delivery. Acc Chem Res. 2018;51:668–677. doi: 10.1021/acs.accounts.7b00526. [DOI] [PubMed] [Google Scholar]
- 79.Shao D., Zhang F., Chen F., Zheng X., Hu H., Yang C. Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an X-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv Mater. 2020;32 doi: 10.1002/adma.202004385. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Zhou C., Ding Y., Liu M., Tai Z., Jin Q. Red blood cell-hitchhiking chitosan nanoparticles for prolonged blood circulation time of vitamin K1. Int J Pharm. 2020;592:120084. doi: 10.1016/j.ijpharm.2020.120084. [DOI] [PubMed] [Google Scholar]
- 81.Liu Z., Janzen J., Brooks D.E. Adsorption of amphiphilic hyperbranched polyglycerol derivatives onto human red blood cells. Biomaterials. 2010;31:3364–3373. doi: 10.1016/j.biomaterials.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 82.Anselmo A.C., Gupta V., Zern B.J., Pan D., Zakrewsky M., Muzykantov V. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malhotra S., Dumoga S., Joshi A., Mohanty S., Singh N. Polymeric micelles coated with hybrid nanovesicles enhance the therapeutic potential of the reversible topoisomerase inhibitor camptothecin in a mouse model. Acta Biomater. 2020;121:579–591. doi: 10.1016/j.actbio.2020.11.049. [DOI] [PubMed] [Google Scholar]
- 84.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao C., Wang Y., Sun J., Han Y., Gong W., Li Y. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimerʹs disease mice. Acta Biomater. 2020;108:285–299. doi: 10.1016/j.actbio.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 86.Finley M.J., Rauova L., Alferiev I.S., Weisel J.W., Levy R.J., Stachelek S.J. Diminished adhesion and activation of platelets and neutrophils with CD47 functionalized blood contacting surfaces. Biomaterials. 2012;33:5803–5811. doi: 10.1016/j.biomaterials.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H., Liu Y., He R., Xu D., Zang J., Weeranoppanant N. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8:552–568. doi: 10.1039/c9bm01392j. [DOI] [PubMed] [Google Scholar]
- 88.Hu Q., Sun W., Qian C., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burness C.B., Dhillon S., Keam S.J. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs. 2014;74:1673–1691. doi: 10.1007/s40265-014-0283-8. [DOI] [PubMed] [Google Scholar]
- 90.Park K., Otte A., Sharifi F., Garner J., Skidmore S., Park H. Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles. J Control Release. 2020;329:1150–1161. doi: 10.1016/j.jconrel.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada H., Toguchi H. Biodegradable microspheres in drug delivery. Crit Rev Ther Drug Carrier Syst. 1995;12:1–99. doi: 10.1615/critrevtherdrugcarriersyst.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J., Walker J., Ackermann R., Olsen K., Hong J.K.Y., Wang Y. Effect of manufacturing variables and raw materials on the composition-equivalent PLGA microspheres for 1-month controlled release of leuprolide. Mol Pharm. 2020;17:1502–1515. doi: 10.1021/acs.molpharmaceut.9b01188. [DOI] [PubMed] [Google Scholar]
- 93.Paik J., Duggan S.T., Keam S.J. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79:455–462. doi: 10.1007/s40265-019-01083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sridharan B., Mohan N., Berkland C.J., Detamore M.S. Material characterization of microsphere-based scaffolds with encapsulated raw materials. Mater Sci Eng C Mater Biol Appl. 2016;63:422–428. doi: 10.1016/j.msec.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crotts G., Park T.G. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul. 1998;15:699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 96.Sinha V.R., Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 97.Tamani F., Bassand C., Hamoudi M.C., Siepmann F., Siepmann J. Mechanistic explanation of the (up to) 3 release phases of PLGA microparticles: monolithic dispersions studied at lower temperatures. Int J Pharm. 2021;596:120220. doi: 10.1016/j.ijpharm.2021.120220. [DOI] [PubMed] [Google Scholar]
- 98.Gasmi H., Siepmann F., Hamoudi M.C., Danede F., Verin J., Willart J.F. Towards a better understanding of the different release phases from PLGA microparticles: dexamethasone-loaded systems. Int J Pharm. 2016;514:189–199. doi: 10.1016/j.ijpharm.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 99.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 101.Mantripragada S. A lipid based depot (DepoFoam technology) for sustained release drug delivery. Prog Lipid Res. 2002;41:392–406. doi: 10.1016/s0163-7827(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 102.Benesch M., Urban C. Liposomal cytarabine for leukemic and lymphomatous meningitis: recent developments. Expet Opin Pharmacother. 2008;9:301–309. doi: 10.1517/14656566.9.2.301. [DOI] [PubMed] [Google Scholar]
- 103.Salehi B., Mishra A.P., Nigam M., Kobarfard F., Javed Z., Rajabi S. Multivesicular liposome (Depofoam) in human diseases. Iran J Pharm Res (IJPR) 2020;19:9–21. doi: 10.22037/ijpr.2020.112291.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breitsamer M., Winter G. Vesicular phospholipid gels as drug delivery systems for small molecular weight drugs, peptides and proteins: state of the art review. Int J Pharm. 2019;557:1–8. doi: 10.1016/j.ijpharm.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 105.He Y., Qin L., Huang Y., Ma C. Advances of nano-structured extended-release local anesthetics. Nanoscale Res Lett. 2020;15:13. doi: 10.1186/s11671-019-3241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Day K.M., Nair N.M., Griner D., Sargent L.A. Extended release liposomal bupivacaine injection (Exparel) for early postoperative pain control following pharyngoplasty. J Craniofac Surg. 2018;29:726–730. doi: 10.1097/SCS.0000000000004312. [DOI] [PubMed] [Google Scholar]
- 107.Tien Y.E., Huang W.C., Kuo H.Y., Tai L., Uang Y.S., Chern W.H. Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation. Biopharm Drug Dispos. 2017;38:494–497. doi: 10.1002/bdd.2088. [DOI] [PubMed] [Google Scholar]
- 108.Rahnfeld L., Luciani P. Injectable lipid-based depot formulations: where do we stand? Pharmaceutics. 2020;12:567. doi: 10.3390/pharmaceutics12060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyer J.M. Converting oral to long-acting injectable antipsychotics: a guide for the perplexed—CORRIGENDUM. CNS Spectr. 2018;23:186. doi: 10.1017/S1092852918000895. [DOI] [PubMed] [Google Scholar]
- 110.Weng Larsen S., Larsen C. Critical factors influencing the in vivo performance of long-acting lipophilic solutions—impact on in vitro release method design. AAPS J. 2009;11:762–770. doi: 10.1208/s12248-009-9153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohammad I.S., Hu H., Yin L., He W. Drug nanocrystals: fabrication methods and promising therapeutic applications. Int J Pharm. 2019;562:187–202. doi: 10.1016/j.ijpharm.2019.02.045. [DOI] [PubMed] [Google Scholar]
- 112.Pawar V.K., Singh Y., Meher J.G., Gupta S., Chourasia M.K. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66. doi: 10.1016/j.jconrel.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 113.Chue P., Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383–1397. doi: 10.1586/ern.12.137. [DOI] [PubMed] [Google Scholar]
- 114.Mu Q., Yu J., McConnachie L.A., Kraft J.C., Gao Y., Gulati G.K. Translation of combination nanodrugs into nanomedicines: lessons learned and future outlook. J Drug Target. 2018;26:435–447. doi: 10.1080/1061186X.2017.1419363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bernardo M., Bioque M. Three-month paliperidone palmitate—a new treatment option for schizophrenia. Expet Rev Clin Pharmacol. 2016;9:899–904. doi: 10.1080/17512433.2016.1191945. [DOI] [PubMed] [Google Scholar]
- 116.Surve D.H., Jindal A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J Control Release. 2020;324:379–404. doi: 10.1016/j.jconrel.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 117.Kraft J.C., McConnachie L.A., Koehn J., Kinman L., Sun J., Collier A.C. Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation. J Control Release. 2018;275:229–241. doi: 10.1016/j.jconrel.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van't Klooster G., Hoeben E., Borghys H., Looszova A., Bouche M.P., van Velsen F. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54:2042–2050. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edagwa B.J., Zhou T., McMillan J.M., Liu X.M., Gendelman H.E. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr Med Chem. 2014;21:4186–4198. doi: 10.2174/0929867321666140826114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao S., Jin B., Liu Z., Shao C., Zhao R., Wang X. Biomineralization: from material tactics to biological strategy. Adv Mater. 2017;29 doi: 10.1002/adma.201605903. [DOI] [PubMed] [Google Scholar]
- 121.Grassmann O.M., Müller G., Löbmann P. Organic−inorganic hybrid structure of calcite crystalline assemblies grown in a gelatin hydrogel matrix: relevance to biomineralization. Chem Mater. 2002;14:4530–4535. [Google Scholar]
- 122.Chen W., Wang G., Yung B.C., Liu G., Qian Z., Chen X. Long-acting release formulation of exendin-4 based on biomimetic mineralization for type 2 diabetes therapy. ACS Nano. 2017;11:5062–5069. doi: 10.1021/acsnano.7b01809. [DOI] [PubMed] [Google Scholar]
- 123.Lai W.F., He Z.D. Design and fabrication of hydrogel-based nanoparticulate systems for in vivo drug delivery. J Control Release. 2016;243:269–282. doi: 10.1016/j.jconrel.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 124.Cai Y., Zheng C., Xiong F., Ran W., Zhai Y., Zhu H.H. Recent progress in the design and application of supramolecular peptide hydrogels in cancer therapy. Adv Healthc Mater. 2020;10 doi: 10.1002/adhm.202001239. [DOI] [PubMed] [Google Scholar]
- 125.Dou X.Q., Feng C.L. Amino acids and peptide-based supramolecular hydrogels for three-dimensional cell culture. Adv Mater. 2017;29 doi: 10.1002/adma.201604062. [DOI] [PubMed] [Google Scholar]
- 126.Braun G.A., Ary B.E., Dear A.J., Rohn M.C.H., Payson A.M., Lee D.S.M. On the mechanism of self-assembly by a hydrogel-forming peptide. Biomacromolecules. 2020;21:4781–4794. doi: 10.1021/acs.biomac.0c00989. [DOI] [PubMed] [Google Scholar]
- 127.Tang W., Zhao Z., Chong Y., Wu C., Liu Q., Yang J. Tandem enzymatic self-assembly and slow release of dexamethasone enhances its antihepatic fibrosis effect. ACS Nano. 2018;12:9966–9973. doi: 10.1021/acsnano.8b04143. [DOI] [PubMed] [Google Scholar]
- 128.Kiran S., Hai Z., Ding Z., Wang L., Liu Y., Zhang H. Alkaline phosphatase-triggered assembly of etoposide enhances its anticancer effect. Chem Commun. 2018;54:1853–1856. doi: 10.1039/c7cc09365a. (Camb) [DOI] [PubMed] [Google Scholar]
- 129.Wu J., Zheng Z., Chong Y., Li X., Pu L., Tang Q. Immune responsive release of tacrolimus to overcome organ transplant rejection. Adv Mater. 2018;30 doi: 10.1002/adma.201805018. [DOI] [PubMed] [Google Scholar]
- 130.Tan J., Zhang M., Hai Z., Wu C., Lin J., Kuang W. Sustained release of two bioactive factors from supramolecular hydrogel promotes periodontal bone regeneration. ACS Nano. 2019;13:5616–5622. doi: 10.1021/acsnano.9b00788. [DOI] [PubMed] [Google Scholar]
- 131.Zheng J., Fan R., Wu H., Yao H., Yan Y., Liu J. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2019;10:1604. doi: 10.1038/s41467-019-09601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qian Q., Wang D., Shi L., Zhang Z., Qian J., Shen J. A pure molecular drug hydrogel for post-surgical cancer treatment. Biomaterials. 2021;265:120403. doi: 10.1016/j.biomaterials.2020.120403. [DOI] [PubMed] [Google Scholar]
- 133.Li T., Wang P., Guo W., Huang X., Tian X., Wu G. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano. 2019;13:6770–6781. doi: 10.1021/acsnano.9b01346. [DOI] [PubMed] [Google Scholar]
- 134.Chen G., Li J., Cai Y., Zhan J., Gao J., Song M. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci Rep. 2017;7:44210. doi: 10.1038/srep44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vazquez-Gonzalez M., Willner I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew Chem Int Ed Engl. 2020;59:15342–15377. doi: 10.1002/anie.201907670. [DOI] [PubMed] [Google Scholar]
- 136.Deng H., Dong A., Song J., Chen X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J Control Release. 2019;297:60–70. doi: 10.1016/j.jconrel.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 137.Kasinski A., Zielinska-Pisklak M., Oledzka E., Sobczak M. Smart hydrogels—synthetic stimuli-responsive antitumor drug release systems. Int J Nanomed. 2020;15:4541–4572. doi: 10.2147/IJN.S248987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang D., Hu Y., Liu P., Luo D. Bioresponsive DNA hydrogels: beyond the conventional stimuli responsiveness. Acc Chem Res. 2017;50:733–739. doi: 10.1021/acs.accounts.6b00581. [DOI] [PubMed] [Google Scholar]
- 139.Vong L.B., Bui T.Q., Tomita T., Sakamoto H., Hiramatsu Y., Nagasaki Y. Novel angiogenesis therapeutics by redox injectable hydrogel—regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials. 2018;167:143–152. doi: 10.1016/j.biomaterials.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 140.Zheng D.W., Hong S., Zhang Q.L., Dong X., Pan P., Song W.F. Controllable gelation of artificial extracellular matrix for altering mass transport and improving cancer therapies. Nat Commun. 2020;11:4907. doi: 10.1038/s41467-020-18493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Z., He J., Qi J., Zhu Q., Wu W., Lu Y. Long-acting microneedles: a progress report of the state-of-the-art techniques. Drug Discov Today. 2020;25:1462–1468. doi: 10.1016/j.drudis.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 142.Chen M., Quan G., Sun Y., Yang D., Pan X., Wu C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J Control Release. 2020;325:163–175. doi: 10.1016/j.jconrel.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 143.Di Natale C., De Rosa D., Profeta M., Jamaledin R., Attanasio A., Lagreca E. Design of biodegradable bi-compartmental microneedles for the stabilization and the controlled release of the labile molecule collagenase for skin healthcare. J Mater Chem B. 2020;9:392–403. doi: 10.1039/d0tb02279a. [DOI] [PubMed] [Google Scholar]
- 144.Luo Z., Sun W., Fang J., Lee K., Li S., Gu Z. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li W., Terry R.N., Tang J., Feng M.R., Schwendeman S.P., Prausnitz M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat Biomed Eng. 2019;3:220–229. doi: 10.1038/s41551-018-0337-4. [DOI] [PubMed] [Google Scholar]
- 146.Ke C.J., Lin Y.J., Hu Y.C., Chiang W.L., Chen K.J., Yang W.C. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials. 2012;33:5156–5165. doi: 10.1016/j.biomaterials.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 147.Li W., Tang J., Terry R.N., Li S., Brunie A., Callahan R.L. Long-acting reversible contraception by effervescent microneedle patch. Sci Adv. 2019;5 doi: 10.1126/sciadv.aaw8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X., Wang F., Yu Y., Chen G., Shang L., Sun L. Bio-inspired clamping microneedle arrays from flexible ferrofluid-configured moldings. Sci Bull. 2019;64:1110–1117. doi: 10.1016/j.scib.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 149.Seong K.Y., Seo M.S., Hwang D.Y., OʹCearbhaill E.D., Sreenan S., Karp J.M. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 150.Han D., Morde R.S., Mariani S., La Mattina A.A., Vignali E., Yang C. 4D printing of a bioinspired microneedle array with backward—facing barbs for enhanced tissue adhesion. Adv Funct Mater. 2020;30 [Google Scholar]
- 151.Donnelly R.F., Singh T.R., Garland M.J., Migalska K., Majithiya R., McCrudden C.M. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang D., Chen M.L., Sun Y., Jin Y.P., Lu C., Pan X. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2020;121:119–133. doi: 10.1016/j.actbio.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 153.Pons-Faudoa F.P., Ballerini A., Sakamoto J., Grattoni A. Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases. Biomed Microdevices. 2019;21:47. doi: 10.1007/s10544-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stewart S.A., Dominguez-Robles J., Donnelly R.F., Larraneta E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers. 2018;10:1379. doi: 10.3390/polym10121379. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kempe S., Mader K. In situ forming implants—an attractive formulation principle for parenteral depot formulations. J Control Release. 2012;161:668–679. doi: 10.1016/j.jconrel.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 156.Parent M., Nouvel C., Koerber M., Sapin A., Maincent P., Boudier A. PLGA in situ implants formed by phase inversion: critical physicochemical parameters to modulate drug release. J Control Release. 2013;172:292–304. doi: 10.1016/j.jconrel.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 157.Agarwal P., Rupenthal I.D. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 158.Packhaeuser C.B., Schnieders J., Oster C.G., Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58:445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 159.Farra R., Sheppard N.F., Jr., McCabe L., Neer R.M., Anderson J.M., Santini J.T., Jr. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4:122ra21. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 160.Chua C.Y.X., Jain P., Susnjar A., Rhudy J., Folci M., Ballerini A. Nanofluidic drug-eluting seed for sustained intratumoral immunotherapy in triple negative breast cancer. J Control Release. 2018;285:23–34. doi: 10.1016/j.jconrel.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 161.Turecek P.L., Bossard M.J., Schoetens F., Ivens I.A. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharmacol Sci. 2016;105:460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 162.Schlesinger N. New agents for the treatment of gout and hyperuricemia: Febuxostat, puricase, and beyond. Curr Rheumatol Rep. 2010;12:130–134. doi: 10.1007/s11926-010-0093-2. [DOI] [PubMed] [Google Scholar]
- 163.Herndon T.M., Demko S.G., Jiang X., He K., Gootenberg J.E., Cohen M.H. U.S. Food and Drug Administration approval: peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma. Oncol. 2012;17:1323–1328. doi: 10.1634/theoncologist.2012-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luo X., Hou L., Liang L., Dong G., Shen S., Zhao Z. Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase II and phase III multicenter, randomized studies. Eur J Endocrinol. 2017;177:195–205. doi: 10.1530/EJE-16-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park E.J., Choi J., Lee K.C., Na D.H. Emerging PEGylated non-biologic drugs. Expet Opin Emerg Drugs. 2019;24:107–119. doi: 10.1080/14728214.2019.1604684. [DOI] [PubMed] [Google Scholar]
- 166.Murguia-Favela L., Min W., Loves R., Leon-Ponte M., Grunebaum E. Comparison of elapegademase and pegademase in ADA-deficient patients and mice. Clin Exp Immunol. 2020;200:176–184. doi: 10.1111/cei.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lew G. Space for calaspargase? A new asparaginase for acute lymphoblastic leukemia. Clin Cancer Res. 2020;26:325–327. doi: 10.1158/1078-0432.CCR-19-2975. [DOI] [PubMed] [Google Scholar]
- 168.Jeon Y.W., Lim S.T., Gwak H., Park S.Y., Shin J., Han H.S. Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel–cyclophosphamide chemotherapy. Ann Surg Treat Res. 2021;100:59–66. doi: 10.4174/astr.2021.100.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Doshi B.S., Rana J., Castaman G., Shaheen M.A., Kaczmarek R., Butterfield J.S. B cell activating factor modulates the factor VIII immune response in hemophilia A. J Clin Invest. 2021;131:142906. doi: 10.1172/JCI142906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kontermann R.E. Half-life extended biotherapeutics. Expet Opin Biol Ther. 2016;16:903–915. doi: 10.1517/14712598.2016.1165661. [DOI] [PubMed] [Google Scholar]
- 171.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.B. The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol. 2021;189:114432. doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rodríguez-Merchán E.C. Recent advances in surgery and its perioperative treatment in people with hemophilia. Expet Rev Hematol. 2021;14:271–280. doi: 10.1080/17474086.2021.1893689. [DOI] [PubMed] [Google Scholar]