Abstract
Proteins and peptides (PPs) have gradually become more attractive therapeutic molecules than small molecular drugs due to their high selectivity and efficacy, but fewer side effects. Owing to the poor stability and limited permeability through gastrointestinal (GI) tract and epithelia, the therapeutic PPs are usually administered by parenteral route. Given the big demand for oral administration in clinical use, a variety of researches focused on developing new technologies to overcome GI barriers of PPs, such as enteric coating, enzyme inhibitors, permeation enhancers, nanoparticles, as well as intestinal microdevices. Some new technologies have been developed under clinical trials and even on the market. This review summarizes the history, the physiological barriers and the overcoming approaches, current clinical and preclinical technologies, and future prospects of oral delivery of PPs.
Key words: Proteins, Peptides, Oral delivery, Permeation enhancer, Enzyme inhibitor, Stability, Clinical
Abbreviations: ASBT, apical sodium-dependent bile acid transporter; BSA, bovine serum albumin; CAGR, compound annual growth; CaP, calcium phosphate; CD, Crohn's disease; COPD, chronic obstructive pulmonary disease; CPP, cell penetrating peptide; DCs, dendritic cells; DDVAP, desmopressin acetate; DTPA, diethylene triamine pentaacetic acid; EDTA, ethylene diamine tetraacetic acid; EPD, empirical phase diagrams; EPR, electron paramagnetic resonance; FA, folic acid; FcRn, Fc receptor; FDA, U.S. Food and Drug Administration; GALT, gut-associated lymphoid tissue; GI, gastrointestinal; GIPET, gastrointestinal permeation enhancement technology; GLP-1, glucagon-like peptide 1; GRAS, generally recognized as safe; HBsAg, hepatitis B surface antigen; HPMCP, hydroxypropyl methylcellulose phthalate; IBD, inflammatory bowel disease; ILs, ionic liquids; LBNs, lipid-based nanoparticles; LMWP, low molecular weight protamine; MCT-1, monocarborxylate transporter 1; MSNs, mesoporous silica nanoparticles; NAC, N-acetyl-l-cysteine; NLCs, nanostructured lipid carriers; PAA, polyacrylic acid; PBPK, physiologically based pharmacokinetics; PCA, principal component analysis; PCL, polycarprolacton; PGA, poly-γ-glutamic acid; pHPMA, N-(2-hydroxypropyl)methacrylamide; pI, isoelectric point; PLA, poly(latic acid); PLGA, poly(lactic-co-glycolic acid); PPs, proteins and peptides; PVA, poly vinyl alcohol; RGD, Arg-Gly-Asp; RTILs, room temperature ionic liquids; SAR, structure–activity relationship; sc, subcutaneous; sCT, salmon calcitonin; SDC, sodium deoxycholate; SGF, simulated gastric fluids; SGC, sodium glycocholate; STC, sodium taurocholate; SIF, simulated intestinal fluids; SLNs, solid lipid nanoparticles; SNAC, sodium N-[8-(2-hydroxybenzoyl)amino]caprylate; SNEDDS, self-nanoemulsifying drug delivery systems; TAT, trans-activating transcriptional peptide; Tf, transferrin; TfR, transferrin receptors; TMC, N-trimethyl chitosan; UC, ulcerative colitis; UEA1, ulex europaeus agglutinin 1; VB12, vitamin B12; WGA, wheat germ agglutinin
Graphical abstract
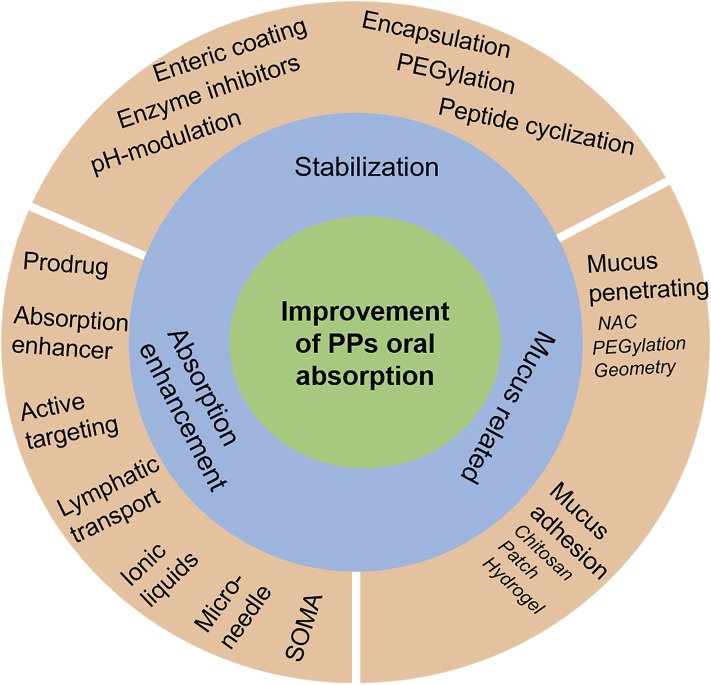
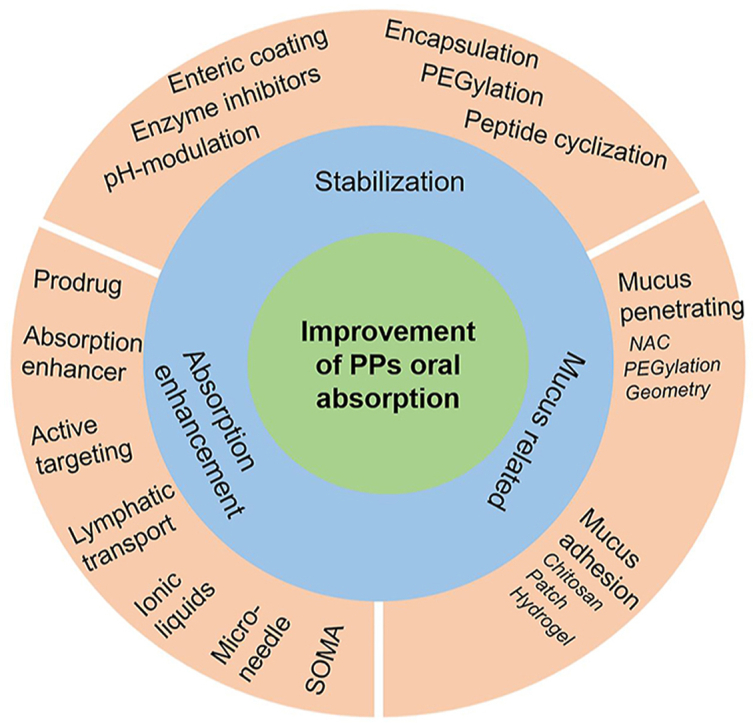
The current strategies for improving oral absorption of proteins and peptides (PPs) include stabilization, absorption enhancement and mucus-related technologies. Most of marketed oral PP products employ two or more strategies.
1. Introduction
With rapid advancement of biotechnology, more and more proteins and peptides (PPs) have been developed for treatment of various diseases1. The PPs have become one of alternatives of small molecular drugs because they are highly selective and effective, but low toxicity2, which stimulates interests of pharmaceutical industry. The statistical data of Coherent Market Insights revealed the global biologics market was approximately US $ 255.19 billion in 2019 and was expected to be increasing over the forecast period (2019–2027) with a compound annual growth rate (CAGR) of 7.6%3. Similarly, biologics, including nucleotides and PPs, account for nearly 30% of all drugs approved by the U.S. Food and Drug Administration (FDA) between 2015 and 20184. In addition, more than 90% of the recently approved biologics were monoclonal antibodies (mAbs) based drugs4.
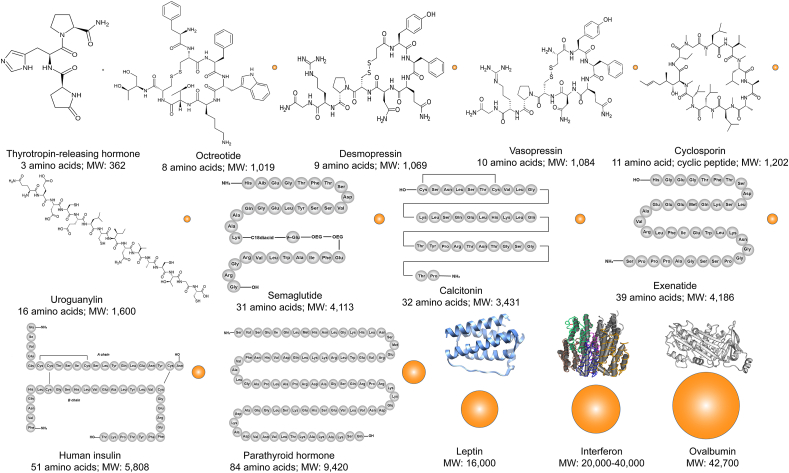
PPs are constituted of lots of amino acids linked by peptide bonds. Generally, the short chains between two and fifty amino acids are defined as peptides. There are oligopeptides which have less than ten or fifteen amino acids, and polypeptides which have more than fifteen amino acids. It is known as a protein when the chains longer than fifty amino acids5. However, there is still controversy with respect to the use of proteins or peptides, for example, mature human insulin with 51 amino acid is confused to define as proteins or peptides6. Some references have also regarded the peptides as the smaller proteins with molecular mass less than 9000 Da7,8. Therefore, PPs have large variations in molecular size and structure (Fig. 1). Besides, PPs have big differences in physicochemical characteristics with chemical drugs. Most of PPs are highly hydrophilic9, but some cyclic peptides exert hydrophobic properties, such as cyclosporine10. Owing to the ionization of amino and carboxyl groups, PPs have isoelectric point (pI) which leads to different charges under different pHs11. The largest difference with chemical drugs is that the conformation is able to affect the pharmacological activity of PPs absolutely12. Hence, unlike conventional small molecular drugs, it is impossible to develop clinical use of PPs without some sort of sophisticated pharmaceutical technology13.
Figure 1.
Schematic illustration of the molecular size and chemical structures of typical proteins and peptides (the conformational structures of leptin, interferon and ovalbumin are from Wikipedia). The spheres represent the relative size of PPs molecules.
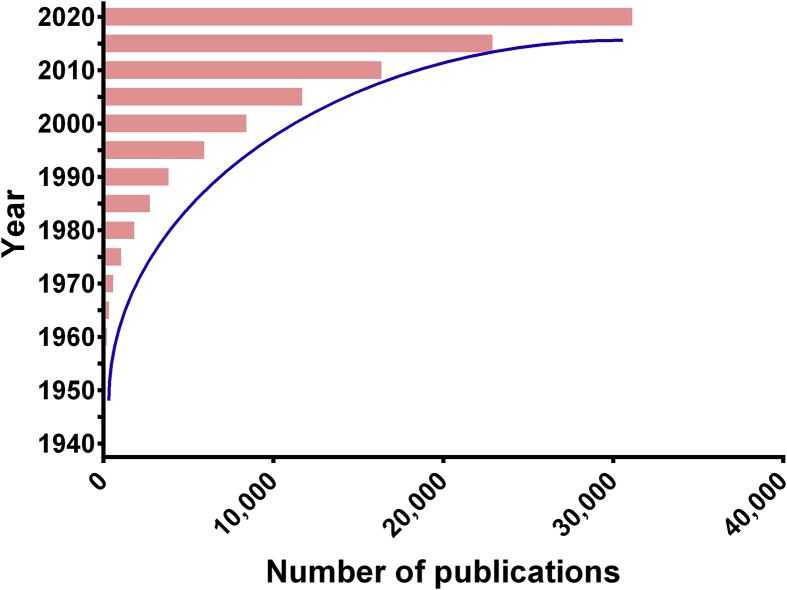
Appropriate administration routes enable not only the therapeutic efficacy of drugs but also patient compliance. However, the administration route of PPs is usually parenteral injection due to their poor oral bioavailability14. The long-term continuous injection could pose a big challenge of medication adherence, including pain, aversion to injections, concerns about needle size and local irritation. Consequently, many scientific groups attempted to develop the alternative routes to deliver the PPs, such as oral, nasal, ophthalmic, buccal and transdermal administration4,15, among which oral route is the most attractive alternative due to the higher safety and compliance16, 17, 18. Furthermore, the oral route is able to mimic the physiological fate of the endogenous insulin which could achieve better glucose homeostasis than subcutaneous (sc) injection6. The oral route would enhance the health outcomes for the treatment of certain chronic conditions by improving the living conditions of patients. According to the recent report from allied market research, the global market of oral PPs is expected to grow from US$ 643 million in 2016 to 8.23 billion in 202819. In addition, academic efforts are also focused on develop some novel technologies to improve the oral absorption of PPs20,21. Since 1995, the number of publication about oral delivery of PPs was increasing exponentially (Fig. 2). However, the commercial products of oral proteins and peptides are very limited to some special peptides, such as Neoral® for cyclosporin A and Rybelsus® for semaglutide. The main obstacles to develop the oral delivery systems of PPs include the harsh environments of gastrointestinal (GI) tract, large molecular size, high hydrophilicity, and poor transmembrane permeability22, 23, 24.
Figure 2.
Chronological number of publications about oral delivery of PPs by searching Web of Science to Nov. 9th, 2020 using the phrases of “oral AND (absorption OR delivery) AND (protein∗ OR peptide∗)”.
To be honest, there are still numerous excellent reviews about oral delivery of PPs, which however have different viewpoints. For instance, there are lots of reviews focused on oral delivery strategies of peptides7,17, while more reviews focused on how nanoparticles improve the oral delivery of PPs25,26. Some big reviews were written from the biologics which include a large amount of irrelative contents with PPs4,23. This review aims to offer a comprehensive overview of the developing history of oral delivery of PPs, the major delivery challenges and the strategies of improving oral absorption, the current technologies in clinical and preclinical phases, as well as the future prospects.
2. The history
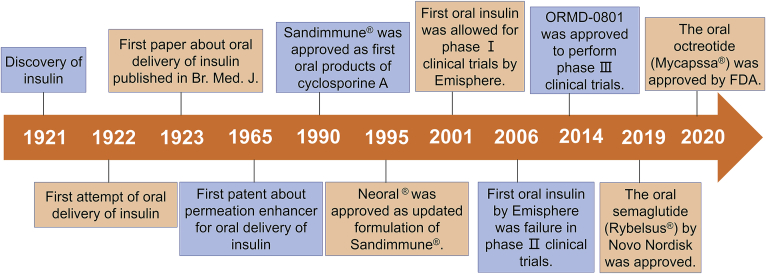
Although it is really tough to develop the oral delivery systems of PPs, various attempts have ever not ceased since the discovery of insulin (Fig. 3). Insulin was discovered by Dr. Frederick Banting and Dr. Charles Best in Canada in 1921 and developed by collaborators in the United States and Europe18. Only after one year, the first attempt of oral delivery of insulin was conducted in 192227, which opened preclude to develop oral formulations of PPs. Unfortunately, the results of the first attempt were negative, which makes the critical challenges of oral protein delivery become apparent. Therefore, it is necessary to employ novel delivery technologies to facilitate the oral absorption of PPs. The first paper about oral delivery of insulin was published in 192328, in which alcohol was used to improve the oral absorption of insulin. Edgar A. Ferguson firstly tried to mix anhydroformaldehyde-aniline with insulin as oral absorption enhancer and won the first patent of oral formulation of insulin in 196529. With the deep research of oral insulin, more and more scientists and companies kept eyes on other PPs drugs. In 1990, Sandimmune® approved by FDA was the first oral formulation of cyclosporin A which is a cyclic peptide with molecular weight of 1202 and also recognized as the first oral dosage form of peptides though it is usually sorted into poorly soluble drugs. After 5 years, the improved formulation of cyclosporin A, Neoral®, was developed by Novartis and approved. Henceforth, self-nanoemulsifying drug delivery systems (SNEDDS) were regarded as an important strategy for improving oral absorption of drug molecules20. However, SNEDDS was not able to increase the oral bioavailability of hydrophilic PPs to large extent. Lots of companies have claimed to develop new delivery technologies to overcome the barriers of oral PPs. Nevertheless, some companies have vanished or been not interested in this field currently, such as AutoImmune, Biosante, Coremed, Coretecs, Eligen, Nobex and Protein Delivery. Five companies are always working on oral insulin for many years and have established some platforms, including Emishphere in USA, Biocon in India, Diabetology in UK, Diasome in USA and Oramed in Israel27. The first oral insulin formulation by Emisphere was allowed to conduct phase I clinical trials by FDA in 2001. In 2006, a 90-day double-blind phase II clinical study in India performed by Emisphere showed no significant differences from placebo. But the pace of development of oral insulin does not stop. In 2014, ORMD-0801 developed by Oramed was approved to perform phase III clinical trials by FDA30. Emisphere developed an enhancer, SNAC (sodium N-[8-(2-hydroxybenzoyl)amino]caprylate)31, for improving oral delivery of insulin, which has been finally used to improve the oral absorption of sermaglutide. In 2019, Rybelsus®, an oral formulation of semaglutide developed by Novo Nordisk, was approved by FDA for treatment of type II diabetes. Subsequently, the sustained release capsule of octreotide (Mycapssa®) developed through transient permeability enhancer (TPE®) technology was also approved by FDA in 2020. These two successful oral products of peptides would bring about revolutionary changes in clinical administration of PPs and accelerate the development of oral delivery systems of PPs.
Figure 3.
The historical major events in the development of oral delivery systems of PPs.
3. The barriers to the oral absorption of PPs
Though the oral delivery of PPs has attracted enormous interests of the pharmaceutical companies and the funding agencies, there are lots of factors impeding the development of oral PPs, such as instability in GI tract, poor permeability across intestinal epithelia and difficulty in development of formulation. The physiological barriers are the major obstacles to hinder the oral absorption of PPs due to the innate nature of GI tract which is not only the major position of food digestion and nutrient uptake but also is the first line defense against toxins and pathogens. Thus, it is necessary to fully understand the physiological and formulation factors for overcoming barriers of oral delivery of PPs.
3.1. The physiological barriers
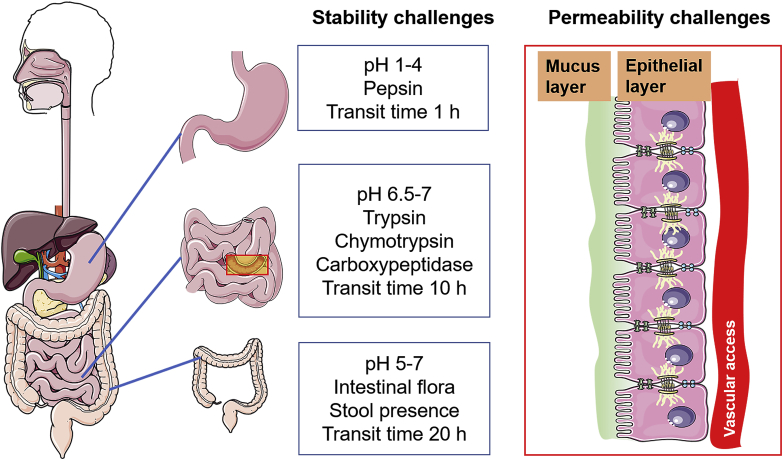
After oral administration, drugs suffer from gastric fluids firstly in stomach and then move into small intestine where most of drugs are absorbed. However, it is absolutely different of environments between stomach and intestines, including pH, enzymes, mucus and even epithelial permeability (Fig. 4), all of which influence the stability and absorption of PPs32.
Figure 4.
Schematic representation of physiological barriers in GI tract. PPs are prone to be degraded in harsh environments of gastrointestinal tract including pH gradient and various enzymes in lumen, which is stability challenge for oral delivery of PPs. In addition, most of PPs are hardly to transport across mucus layer and epithelial layer, which is permeability challenge.
3.1.1. pH gradient
The GI pH is absolutely different in each region of GI tract and influenced by various factors including presence of food, pathological conditions, even age and gender. Generally, in the healthy adult, the pH of gastric fluids is acidic (pH 1.5–3.5), and rises to around pH 5–6 in the duodenum due to neutralization of carbonate and bile juices, and then increases to pH 7–8 in the distal jejunum and ileum, while the colonic pH could be more than 8 or drop to pH 6 with high interindividual variability33,34. The age growth is almost no effect on GI pH35, which indicates the GI pH condition could maintain relative stable in whole life. However, The gastric pH is high shortly after birth, and rapidly reduces to pH 1 to 336. The intake of food would influence the GI pH transiently, such as elevation of gastric pH37. In addition, the personal diet custom could be an important reason of individual variability in colonic pH. The GI pH could be changed by diseases significantly, such as inflammatory bowel disease (IBD) and GI cancers. The colonic pH in IBD patients differs from healthy adults and is characterized with big individual variability, but the general trend of pH was becoming more acidic. The average colonic pH was detected to be 5.3 in patients with Crohn's disease (CD)38, while could even drop to 2–3 in patients under active ulcerative colitis (UC)39. The GI local cancers could change the pH environment to a large extent. For example, the gastric pH was detected to be around 6–7 in 89 gastric cancer patients40, which could be caused by reduction of secretion of gastric acid. The complicated pH environments in GI tract could lead to conformational alteration or enzymatic degradation of PPs, resulting in the loss of therapeutic efficacy. The extreme pHs could result in unfolding due to increase of electrostatic repulsions. Generally, proteins are stable in a narrow pH range which is not far away from their pI, such as pH 6.5–7.0 for recombinant factor VIII SQ (FVIII SQ)41. Thus, some proteins could be inactive in gastric fluids due to pH induced unfolding. What's more, the activity of enzymes is dependent on pH, for example, pepsin can exert the strongest ability of degradation in pH 2–3, but be inactive completely in pH above 532. Most PPs are degraded very fast in stomach of healthy adult42.
3.1.2. Enzymes
PPs are highly susceptible to various proteolytic enzymes including luminal enzymes from gastrointestinal and pancreatic secretions, bacterial enzymes in the colon and mucosal enzymes43. They are primarily degraded by luminal enzymes (Table 144,45) before penetration across mucus. The entry of the protein could stimulate the gastric mucosa to secret pepsins by the cells lining the stomach. Pepsin is able to degrade proteins into smaller fragments of peptides by hydrolyzing the peptide bonds46. A great deal of proteolytic enzymes in the upper part of the small intestine are secreted by pancreas, such as trypsin, chymotrypsin, carboxypeptidase and elastase47. Moreover, the remaining parts of the proteins are finally digested by various peptidases (e.g., aminopeptidase and dipeptidase) in brush border membrane into tripeptides, dipeptides and respective amino acids that are able to be absorbed into the blood capillaries from epithelium48. However, most degradation data of PPs were obtained by in vitro simulated gastric or intestinal fluids with specific enzymes which are hard to be same activity as in vivo condition. For example, the pH 1.2 hydrochloride solution with 0.32% pepsin and the pH 6.8 phosphate buffer with 1% trypsin were often used to evaluate the stability of PPs in vitro47. Most proteins can be degraded very fast in simulated gastric fluids (SGF), such as no insulin detected after 30 min incubation with SGF49,50. Through comparison of human or pig GI fluids and simulated GI fluids, Wang et al.51 found there were good correlation between SGF and both human and pig gastric fluids for the stability of peptides, while the rate of peptide degradation in simulated intestinal fluids (SIF) was more rapid than that in human or pig intestinal fluids. What's more, there is a very interesting result that only 3 of 17 peptides left in human intestinal fluids after 30 min incubation. These 3 peptides are respectively cyclosporin (99%), desmopressin (25%) and octreotide (22%) which are all developed as oral therapeutic products named as Neoral®, Minrin® and Mycapssa®51. Therefore, it is the one of important prerequisites for successful development of oral PPs to protect the stability of PPs in GI tract.
Table 1.
| Secretion site | Enzyme | Specificity |
|---|---|---|
| Stomach | Pepsin | Asp, hydrophobic amino acids |
| Pancreas | Trypsin | Arg, Lys |
| Chymotrypsin | Aliphatic amino acids (Phe, Tyr) | |
| Carboxypeptidase A | Aromatic amino acids in C-terminal (Tyr, Phe, Ile, Thr, Glu, His, Ala) | |
| Carboxypeptidase B | Arg, Lys in C-terminal | |
| Elastase | Ala, Gly, Ser | |
| Small intestine | Aminopeptidase A | Asp, Glu in N-terminal |
| Aminopeptidase N | Ala, Leu in N-terminal | |
| Aminopeptidase P | Pro in N-terminal | |
| Aminopeptidase W | Typ, Tyr, Phe in N-terminal | |
| γ-Glutamyl transpeptidase | γ-Glutamic acid in N-terminal | |
| Dipeptidyl peptidase IV | Pro, Ala | |
| Peptidylpeptidase A | His–Leu | |
| Carboxypeptidase M | Lys, Arg in C-terminal | |
| Carboxypeptidase P | Pro, Gly, Ala in C-terminal | |
| γ-Glutamyl carboxypeptidase | γ-Glutamic acid | |
| Endopeptidase-24.11 | Hydrophobic amino acids | |
| Endopeptidase-24.18 | Aromatic amino acids | |
| Enteropeptidase | (Asp)4-Lys |
3.1.3. Mucus
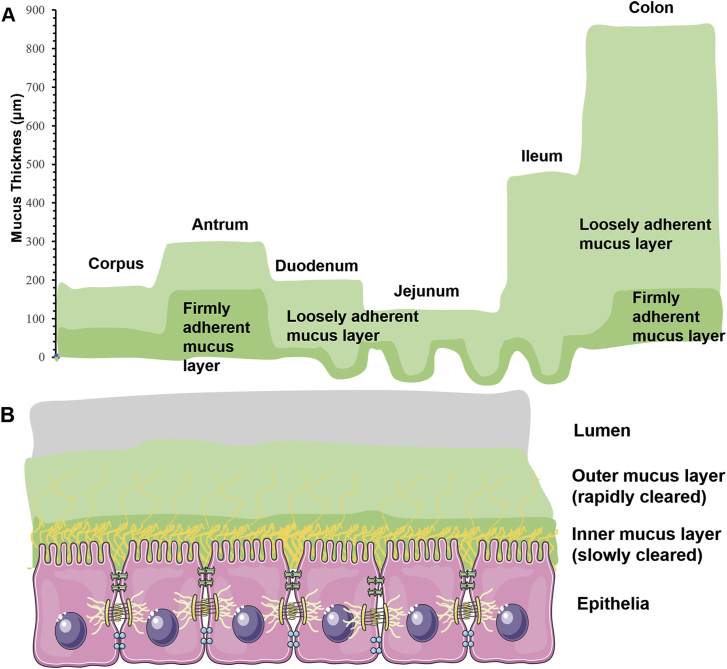
Mucus is a sticky and viscoelastic gel layer covering the entire GI tract. It is secreted by the goblet cells. Mucus can capture the foreign moieties and protect epithelia from the attack of exogenous pathogens52. The mucus in whole GI tract is composed of two layers including loosely and firmly adherent mucus layer from lumen to epithelia (Fig. 5). The thickness of mucus layer varies significantly in different GI regions. Taking rat GI tract as an example, it ranges from 200 μm in upper part to 800 μm in lower part of GI tract53. In humans, the thickest mucus layers are also located on the stomach (180 μm) and the colon (110–160 μm)54. The components of mucus are very complex. The mucin glycoprotein is the major functional constituent and the other components include carbohydrates, proteins, lipids, salts, immunoglobulins, bacteria and cellular remnants55. Mucins, including secreted and cell-bound types, are at least twenty subtypes encoded by the MUC genes. There are three secreted mucin types found in the GI tract, such as MUC2, MUC5AC and MUC656. The interactions between mucins make mucus gel layer viscoelastic, however, the viscoelasticity could be influenced by water, lipid or ion content57,58. What's more, there is a pH gradient across whole mucus layer, especially gastric mucosa. The gastric mucus pH on the luminal surface is about 1–2 which is similar as gastric pH, but increases to neutral pH at the epithelial surface59. This pH gradient could be main mechanism to protect gastric epithelial cell against digestion of pepsin.
Figure 5.
Schematic illustration of the mucus layers covering GI tract. Mucus layer is composed of two layers including outer mucus layer which is loosely adherent and inner mucus layer which is firmly adherent on epithelia. The mucus depth varies with GI sites (A). Outer mucus layer is cleared more rapidly than inner mucus layer (B). Reprinted with the permission from Ref. 54. Copyright © 2012 Elsevier.
The mucus exerts multiple barriers against the transport of drugs into the submucosal tissue. The high viscosity decreases the diffusivity of PPs through mucus, which directly affects the residence time of PPs in the small intestine21. In the intestine, the average mucus turnover time is around 50–270 min resulting in the removal of trapped particles in the mucus layer thereby, limiting the adhesion and holding time of the particles or PPs60. The continuous secretion and replacement of mucus make it quite challenging for the PPs passing through the unstirred mucous layer by infiltration before reaching the surface of the epithelium. Greater interaction through electrostatic force may exist between mucin and drug particles which may be attributed to the fact that mucin is highly negatively charged due to the glycosylation of serine, and the presence of threonine and proline domain54. Furthermore, mucins may function as a size-exclusion filter lowering the mobility of large compounds like proteins due to their brush-like scaffold structure61,62. What's more, structural modification of proteins or entrapment of particles might occur due to the fact that mucin fibers interact non-covalently with proteins or particles via van der Waals and electrostatic forces, hydrogen bonding, and hydrophobic interactions64, 65, 66, thus hindering their absorption.
3.1.4. Epithelial barriers
The epithelial cells lying beneath the mucus also act as another predominant restrictions towards oral protein drug delivery. The intestinal epithelia include various types of cells with specific functions, such as enterocytes for absorption, goblet cells for secretion of mucus, paneth cells for secretion of enzymes and M cells for transporting foreign particles63. The enterocytes are the major absorptive cells and also comprise around 90% of intestinal epithelium64. A continuous monolayer is formed by these polarized epithelial cells, separating the intestinal lumen from the underlying lamina propria. The tight junctions (TJs), found between two neighboring epithelial cells, make the intestinal epithelium impermeable and a gatekeeper to macromolecules65,66. TJs are elaborate networks formed by multiprotein junctional complexes, which is composed of peripheral membrane proteins like zonula occludens (ZO-1, ZO-2), transmembrane integral proteins like claudins, junctional adhesion molecules and regulatory proteins as well67. Except for normal intestinal epithelium, there are some discontinuous follicle-associated epithelia (FAE) which is featured by few mucus, and the location of M cells, numerous intra-epithelial lymphocytes and macrophages68. M cells are the most important epithelial cell types involved in the uptake and transport of a wide variety of particulates including intestinal antigens and large proteins, and thus recognized as immune cells of intestinal lumen69.
The intestinal absorption of drugs is primarily dependent on transcellular pathway, while paracellular pathway is the main route of some small hydrophilic molecules22. According to Lipinksi “Rule of 5”70, PPs are predicted to be extremely low transcellular permeability because LogP of PPs is likely to be below −1 that is far lower than 5, and PPs have a great number of hydrogen bond donors or acceptors, and molecular weight is far more than 500 Da. Thus, PPs are hard to be absorbed into portal vein by transcellular pathway. Moreover, the paracellular route refers to the passage of drugs through water-filled pores of TJs, the pore sizes of which usually range between 3 and 10 Å71. The molecules larger than 500 Da are generally not recognized to be able to move through these small pores72. TJs can be regulated by some permeation enhancers, which makes pores larger73. However, the width is still less than 20 nm even in fully opened state and the total surface of water filled pores only account for 0.01%–0.1% of entire intestinal epithelia74. Therefore, the oral bioavailability of PPs is still extremely low even though the intestinal permeation enhancers have been added in formulation, such as transient permeability enhancer (TPE®) and SNAC18,75. Compared with normal epithelia, lumen antigens, macromolecules and pathogenic particles are transported effectively and rapidly by M cells from the lumen to the underlying gut-associated lymphoid tissue (GALT) via pinocytosis and phagocytosis76, which looks a favorable route for oral delivery of PPs. However, the numbers of M cell are very limited in human intestines, accounting for less than 1%77. In addition, some endogenous PPs transported by M cells may stimulate the immune responses78.
3.1.5. Inter-individual variability
The tremendous inter-individual variability is also a barrier to limit the development of oral PPs. Inter-individual variability in the anatomical and physiological properties of humans and animals is a common sense. For oral delivery, the inter-individual variability in the physiology of GI tract has significantly affected the bioavailability of oral PPs, such as the condition of mucus, the secretion of enzymes and gut motility79. Especially in some disease states, the inter-individual variability is more evident. For example, gastric emptying and oesophageal motility have shown large variability in type 2 diabetic patients with different stages80. The variability in gut mobility could be particularly relevant to the difference in absorption rate of PPs, such as insulin for diabetes80. Moreover, the pH and the expression of digestive enzymes in GI tract vary with individuals significantly, which leads to the inter-individual variability of degradation of PPs in GI tract. The relevant transporters potentially contribute to the extent and rate of transmucosal absorption of PPs, but the expression of transporters in the intestinal epithelia is dependent on individual genes81. In addition, most of PPs are endogenous substances for regulating the physiological factors, but some physiological factors can also be influenced by other endogenous PPs. For example, glucose level can be regulated by insulin and glucagon simultaneously. The inter-individual variability of glucagon secretion also cause the differential hypoglycemic effect of oral insulin in different patients or animals82. Therefore, it is necessary to establish some models to evaluate the inter-individual variability of oral PPs for clinical development, such as physiologically based pharmacokinetics (PBPK) modelling83.
3.2. Formulation factors
Except for physiological barriers, formulation is also a great challenge during the development of oral PPs commercial products. The chemical and physical stability of PPs are the most important considerations in formulation development, which aims to enable stability of PPs in manufacturing processes, transportation, storage and administration. There have been some excellent reviews to summarize the formulation factors influencing stability of PPs84, 85, 86, 87, especially for parenteral formulations. Unlike small molecular drugs, the major stability of PPs is generally referred to as their conformational integrity which is dominated by hydrophobic interaction, hydrogen bonding and electrostatic interaction88,89. The formulation pH could change the protein's surface charge, density and distribution, which could cause the alteration of conformation of PPs90. Meanwhile, the pH can also influence the colloidal stability of PPs and then changes the rate of protein aggregation and degradation91,92. Ionic strength also affects the physical stability of PPs in solution as pH93. For example, the increase of salts could improve the aggregation of proteins due to enhanced hydrophobic interactions94. Hence, buffer solutions are usually employed to stabilize PPs in solution formulation. In addition, some excipients are very necessary to be added in formulation to improve solubility or suppress aggregation of proteins, such as arginine, histidine, glycine and so on95. Arginine can reduce aggregation of proteins to stabilize the proteins' structure96. The surfactant is also generally used to stabilize proteins through reducing molecular interactions in different interfaces, such as polysorbate97. Chemical instability of PPs involves in product development, manufacturing and even post-administration. Oxidation is the most common factor inducing instability of PPs with residues of methionine, tryptophan, histidine, cysteine, phenylalanine or tyrosine98, which could be hindered by anti-oxidants including methionine and ascorbic acid. However, enzymatic degradation is the biggest challenge in protecting PPs in GI environments after oral administration as described in Section 3.1.2. Most oral formulations are primarily to protect stability of PPs in GI tract against various digestive enzymes, such as enteric coating, encapsulation and enzymatic inhibitors, which will be described in detail in Section 4.1. In order to enhance epithelial permeability, some permeation enhancers are added in oral formulations, such as SNAC, bile salts and non-ionic surfactants (Section 4.3.2).
Excipients can reduce the molecular interactions between PPs to avoid aggregations, but the interactions between PPs and excipients can also not be ignored. Understanding the protein–excipient interactions is indispensable to better design stable formulations of PPs. There is an excellent review to fully sum up the protein‒excipient interactions in liquid formulations including mechanism and characterization99. Owing to the complexity of PPs molecules, multiple interactions involved between PPs and excipients, such as electrostatic interactions, hydrogen bonding, preferential hydration and dispersive forces, which can be characterized by various technologies including Raman spectroscopy, circular dichroism, fluorescence, nuclear magnetic resonance, scanning probe microscopy and electron paramagnetic resonance (EPR) spectroscopy, and some advance numerical analysis methods including principal component analysis (PCA) and empirical phase diagrams (EPD). For example, most of amino acids are able to stabilize proteins in liquid formulation through preferential hydration or direct binding100, while sugars and carbohydrates can stabilize protein in solid state with the combining effect of specific interactions and formation of highly viscous glassy matrices101. In PPs formulations, some polymers and non-ionic surfactants are usually used to increase the stability. The non-ionic surfactants can compete the hydrophobic surface with protein molecules to avoid adsorbing-induced denaturation102. Some polymers are employed to encapsulate PPs for improving the stability or controlling the release. But the hydrophobic domain and charges of polymers can influence the stability of PPs, such as aggregation or adsorption103,104.
4. Current strategies towards enhancement of the oral absorption of PPs
Despite multiple strategies to increase the oral absorption of PPs, the primary principles are based on three aspects including stabilization, mucus penetration or adhesion, and permeation enhancer (Fig. 6). These approaches are commonly integrated into one delivery system together.
Figure 6.
Flow chart of the general considerations in enhancement of oral bioavailability of PPs. There are various technologies based on three rationales including stabilization, absorption enhancement and mucus-related technologies.
4.1. Stabilization
Based on physiological and formulation factors, the stability of PPs after oral administration is primarily affected by pH and enzymes in GI tract. In addition, the structure of peptides influences their stability significantly. This section explores the stabilization strategies for oral PPs which have been widely used in formulation development.
4.1.1. pH modulation
The GI enzymes are the main sources to degrade oral PPs, but they need optimal pH to exert their effect. For example, pepsin can cleaves multiple proteins or peptides readily in the acidic environment, however, pepsin starts to lose their effect when the pH is over 3105. Therefore, if we can modify the pH of microenvironment to 5, PPs can be protected against degradation in stomach. Nevertheless, enteric coating is generally used to overcome the degradation of PPs in stomach rather than pH modulation due to simpler formulation106. Unfortunately, the proteolytic enzymes in the small intestine are also proficient at degradation of PPs. Similarly, these enzymes are also dependent on pH environment. Luminal proteases, such as trypsin and chymotrypsin, exhibit maximum activity at pH ≥ 6.5107. Therefore, adjusting the pH of the intestinal contents has become an efficient approach to protect PPs in intestine. Some organic acids, such as citric acid, have been generally used as pH-lowering agents to inhibit the activity of intestinal enzymes108. It has been proven that co-administration of citric acid and salmon calcitonin (sCT) is able to enhance the oral absorption of sCT in beagle dogs by reducing the activity of pancreatic serine protease trypsin109. In addition, Tarsa Therapeutics (Philadelphia, USA) has successfully completed a phase III trial for oral delivery of sCT (ORACAL®) which comprises of an enteric coated capsule to bypass the stomach and citric acid to modulate the pH microenvironment in intestine110.
4.1.2. Enzymatic inhibitors
Except for pH modulation, the most important approach for inhibiting enzymes is using enzyme inhibitors. Enzyme inhibitors inactivate the target enzymes by binding to the specific site of the enzyme reversibly or irreversibly111. There are multiple categories of enzyme inhibitors including non-amino acids, amino acids and modified amino acids, peptides and modified peptides. Many chemical molecules can inhibit the activity of enzymes, such as cholic acids and their derivatives, diisopropyl fluorophosphates22,112. However, these chemical molecules are rarely used due to their high toxicity. Besides, they could be absorbed faster than PPs itself due to low mass, leading to systemic side effects and loss of inhibition capacity. Amino acids and modified amino acids have the same problems as chemical inhibitors22. Hence, peptides and modified peptides derived enzymatic inhibitors have been extensively studied, such as aprotinin inhibiting trypsin and chymotrypsin and soybean trypsin inhibiting pancreatic endopeptidases. However, it is noteworthy that long duration administration of such enzymatic inhibitors could result in the deficiency of these enzymes in humans. The chicken and duck ovomucoids are recently developed and recognized as safer. They can efficiently inhibit the activity of α-chymotrypsin and trypsin and offer 100% protection for insulin113.
The enzymatic inhibitors have been extensively used in clinically developing products. For instance, soybean trypsin inhibitor and chelating agent which is a cofactor for many proteases have been used in ORMD-0801 (developed by Oramed) as a formulation component for oral delivery of insulin114. The Chronotropic™ platform technologies developed by Dexcel Pharma Technologies, Ltd. (Jerusalem, Israel) combine the protease inhibitor (camostat mesilate) and absorption enhancer (sodium glycocholate) to improve the oral bioavailability of insulin115. However, we have to consider their toxicities which are caused by high concentration and long duration.
4.1.3. Enteric coating and colon-specific delivery
The enteric coating can prevent the drug release in stomach but permit the drug release in the small intestine due to the dissolution of coating materials in higher pH116. Thus, the enteric coating is able to protect PPs against degradation by low pH and pepsin in stomach completely. Some pH-responsive polymers are usually used to coat the tablet, capsule or even micro-/nano-particles. Polyacrylic polymers have been widely used for enteric coating and shown to release insulin at different rates and different pH, such as Eudragit S100 or L100117. Most of oral PPs products have adopted enteric coating technology to bypass the stomach, such as enteric coating capsule for oral insulin by Oramed (ORMD 0801) and Diabetology (Capsulin™ OAD)118. However, the oral bioavailability of PPs can't be improved significantly if only enteric coating is used because there are still a great amount of digestive enzymes in intestine. Therefore, enteric coating is usually employed to improve the oral absorption of PPs by combination with protease inhibitors or permeation technologies119. Nanoparticles coated by enteric materials have shown significant enhanced effect for oral absorption of PPs. The relative bioavailability of insulin was found to be approximately 20% after oral administration of enteric coated capsules filled with chitosan/poly(γ-glutamic acid) nanoparticles120. In addition, pH-responsive polymers can fabricate nanoparticles directly with other polymers which can enhance the permeability for oral delivery of PPs. Nanoparticles composed of hydroxypropyl methylcellulose phthalate (HPMCP) and chitosan increased the hypoglycemic effect of insulin by more than 9.8 and 2.8-fold as compared to oral insulin solution and chitosan nanoparticles without enteric materials121.
The colon acts as a more suitable absorption site for PPs compared to stomach and small intestine due to its decrease of enzyme activity and neutral pH value. Moreover, longer residence time and higher responsiveness to absorption enhancers make colon as ideal administration site of oral PPs122. There are a number of examples to develop colon targeted delivery systems for PPs, such as vasopressin, insulin, calcitonin, glucagon and so on123. However, proper care must be taken to enable the release of the PPs at the target site. Among the various pH responsive polymers, Eudragit® enteric release polymers have been extensively exploited for oral delivery of PPs. It was reported that the oral bioavailability of insulin was enhanced by 1.73-fold via Eudragit S100®-coated chitosan nanoparticles loaded with insulin and trans-activating transcriptional peptide (TAT), compared to nanoparticles without enteric coating124. Other carbohydrate polymers that are used to specifically deliver oral PPs to the colon include anionic carboxymethyl starch, cationic quaternary ammonium starch, gellan gum, retrograded starch and pectin etc125,126. However, there are some challenges hindering the colon-specific delivery systems of PPs including lower surface area and tight junctions in colonic absorption site. What's more, alteration of enzymatic activity induced by some certain colonic diseases could affect drug release or stability127.
4.1.4. Micro/nano-encapsulation
Therapeutic drugs or PPs can be protected from hydrolytic and enzymatic degradation in the harsh gastric milieu of the GI tract via encapsulation, by which a drug or protein of interest is encaged inside polymeric carriers, so as to improving their intestinal absorption128. Particles can also enhance transport across epithelia except for protecting PPs against degradation25. Therefore, the therapeutic PPs could be encapsulated in nanoparticles to improve the blood concentration after oral administration, while vaccines encapsulated in microparticles can be taken up by Peyer's patches for enhancing mucosal immunity129. The particle transport is also affected by the stability in GI tract, surface properties, morphology and specific ligands130,131. Both natural and synthetic materials can be used to encapsulate PPs, such as natural materials including chitosan, dextran, alginate, hyaluronic acid, and lipidic materials, and synthetic polymers including poly(latic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), polycarprolacton (PCL), and so on128. The generally recognized as safe (GRAS) ingredients are highly recommended to prepare edible micro/nano-particles. So far, nanoparticles have been extensively developed for oral macromolecular drug delivery, such as polymeric nanoparticles, lipid nanoparticles, liposomes, nanoemulsions and inorganic nanoparticles, which were described in detail in Section 4.3.8.
4.1.5. PEGylation and peptide cyclization
The stability of PPs can be also improved by chemical approaches, such as PEGylation and peptide cyclization. PEGylation is generally used to reduce plasma clearance rate by increase the stability of PPs in the systemic circulation132. Several injectable PEGylated proteins have been launched to the market, such as growth hormone antagonist (Somavert®, Pfizer, USA), erythropoietin (Mircera®, Roche, Switzerland), and anti-TNF-α Fab (Cimzia®, UCB, Belgium)133. Similarly, PEGylation can increase pH and thermal stability of PPs, and also resistance to intestinal proteolytic digestion134. In addition, the branched chain PEGs demonstrate better than the linear PEGs135. However, it is important to realize that PEGylation could lead to risk of different efficacy and side effect profiles with parent protein.
Cyclization makes peptide non-susceptible to enzymes by removing exposed N and C terminal from peptide molecules136,137. It is inspired by many natural small cyclic proteins, such as cyclosporine and desmopressin138. Desmopressin which is a cyclic analogue of vasopressin displays greater resistance to enzymatic degradation than vasopressin139. Ring closure of a peptide can be attained by four different ways: head-to-tail (C-terminus to N-terminus), head-to-side chain, side chain-to-tail or side chain-to-side chain, depending on its functional groups140. The typical example is Arg-Gly-Asp (RGD) which is highly susceptible to chemical degradation due to presence an aspartic acid residue in its structure, while the rigidity can prevent the Asp side chain carboxylic acid from positioning itself in the right position for attack on the peptide backbone after cyclization141. Furthermore, cyclization can also decrease the exposure of polar atoms to surroundings by folding peptides into bioactive conformations, leading to the increase of oral bioavailability142.
4.2. Mucus penetrating and mucoadhesive systems
As mentioned before, mucus lining along the intestinal membrane of the GIT serves major hurdle for protein absorption by presenting multiple barriers. However, mucus is a double-edge sword in design of drug delivery systems. There are two opposing approaches to improve the delivery efficiency, including mucus-penetrating and muco-adhesive systems. Mucus penetrating systems are able to pass through the unstirred layer rapidly to reach intestinal epithelium for absorption. In contrast, muco-adhesive systems can prolong drug residence time for absorption at the intestinal tract by avoiding mucociliary clearance. There have been lots of excellent reviews to clarify the mucus-penetrating and mucoadhesive systems143, 144, 145.
4.2.1. Mucus penetrating systems
For mucus-penetrating systems, the mucolytics have been firstly used to disrupt mucus barrier. The mucolytics are generally used to remove abnormal mucus in pulmonary disease, such as chronic obstructive pulmonary disease (COPD), while able to diminish the mucus barrier transiently for healthy mucosa146. For example, N-acetyl-l-cysteine (NAC) is a commonly used mucolytic and can cause a 6-fold increase in the absorption of 3.2 μm polystyrene particles in Peyer's patches147. Although mucolytics can facilitate the attachment of particles to intestinal absorptive cells by removing mucus covering surface of epithelium and further enhance the oral absorption, the depletion of mucus barrier could lead to the injury of intestinal epithelium due to direct contact with proteolytic enzymes and acid. Therefore, it is necessary to employ the particles with specific properties to penetrate through mucus for drug delivery.
Inspiring from viruses, scientists deduced some possible characteristics of mucus-penetrating particles, including small size, highly hydrophilic and net-neural surfaces54,148. A study has demonstrated that polymeric particle less than 230 nm could pass through mouse colorectal mucus rapidly149, which is similar as the size of some viruses. In order to increase the hydrophilicity of particle surface, the particles are commonly modified by PEG to enhance mucus penetration150. In addition to PEG, poly vinyl alcohol (PVA)151 and N-(2-hydroxypropyl)methacrylamide copolymer (pHPMA)152 can also engineer the mucus-inert particles to improve the oral absorption. The nanocomplex of insulin and cell penetrating peptide (CPP) demonstrated no evident hypoglycemic effect after oral administration to diabetic rats, while the blood glucose level can decline to around 50% by nanocomplex coated by pHPMA. Meanwhile, nanocomplex coated by pHPMA exhibited 20-fold higher transport than free insulin on mucus-secreting epithelium cells152. Recently, protein corona liposomes are also able to facilitate the penetration of mucus and transepithelial transport153.
Another important factor influencing the mucus-penetrating is surface charge of nanoparticles. Both positive and negative charge are not good for mucus-penetrating, but nanoparticles with densely charges coated net-neutral surfaces which is like virus surface exhibited higher diffusion through mucus layer. A biomimetic virus-like or charge reversible nanoparticles are able to improve the oral insulin delivery by overcoming mucus barriers154. In addition, particle geometry can affect the mucus-penetrating ability significantly by micromovement155. It has been revealed that the nanorods diffused across mucus layer rapidly by rotation156. Owing to the strong mucus-penetrating capacity, the rod shaped nanoparticles can penetrate into deep mucus and reside there to prolong the residence time in GI tract130. What's more, SNEDDS produces droplets (≤50 nm) with hydrophilic surfaces and their shape deformability facilitates them suitable for diffusion through mucus157. Better mucus diffusion was achieved by medium chain lipids (MC)-SNEDDS compared to lipids with short or long chains. For example, MC-SNEDDS produced 2-fold increase of oral bioavailability of enoxaparin158.
4.2.2. Mucoadhesive systems
Mucoadhesion is a common phenomenon for particles, which was found by Florey in 1962 from India ink particles adhering intestinal mucus159. Most of microparticles or nanoparticles exerted non-specific mucoadhesion with intestinal mucus. However, mucoadhesive polymers have to be used for improving residence time significantly. For example, mucoadhesive microspheres with a diameter of 680–850 μm fabricated by copolymers of fumaric acid and sebacic acid were able to significantly prolong retention time in the rat GI tract compared to that of non-adhesive polymers160. The hydrophobicity, surface charge and chemistry influence the mucoadhesive properties of polymers significantly. The hydrophobic particles were absorbed 100-fold more than particles composed of hydrophilic cellulose161, which indicated that hydrophobic interactions was also an important aspect for mucoadhesion. Due to the negative charge of mucus layer, the positively charged particles are strongly mucoadhesive. Chitosan, especially N-trimethyl chitosan (TMC), was commonly used to engineer or coat nanoparticles for improving the drug absorption through electrostatic mucoadhesive with mucins. TMC nanoparticles produced much higher antibody titers of IgG and secretory IgA after oral delivery of urease than the solution by increasing mucoadhesion and epithelial permeability162. Thiolation on the surface of polymer is a common strategy to increase the mucoadhesive ability owing to formation of disulfide between thiol group of polymer or cysteine-rich subdomains of mucus glycoproteins163. The mucoadhesive properties of polymers could be enhanced by up to 100-fold after thiolation164. TMC nanoparticles modified by cysteine increased insulin transport by 1.7–2.6-fold compared to TMC nanoparticles165. Compared with the mucoadhesive polymers, the another category of molecules exert the bioadhesion on epithelial cells rather than mucus gel layer, such as lectins. They are able to specifically recognize receptor-like structures of the cell membrane and therefore bind directly to epithelium and hence called as the second generation of bioadhesives166. The lectin modified nanoparticles are able to not only bind to intestinal epithelium for prolonging the residence time but also probably triggering the active transport by receptor mediated uptake. Many studies employed lectin modified nanoparticles to target M cell for enhancing transport of large molecules167,168. The absorption enhancement of lectin was described in Section 4.3.3. in detail.
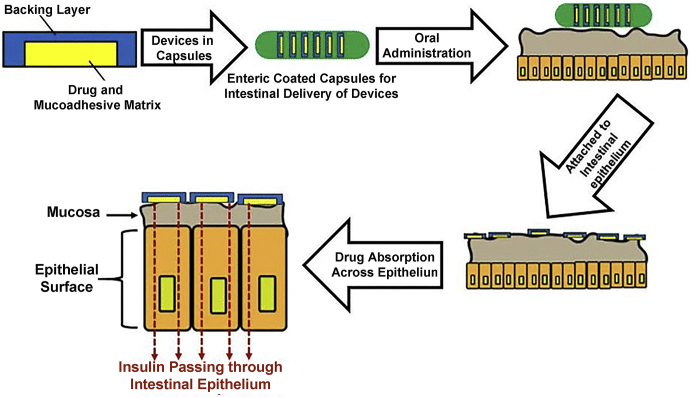
In addition to mucoadhesive micro-/nano-particles, intestinal patches have also been attempted to improve the oral delivery of PPs169,170. Intestinal patches are like transdermal patches and millimeter sized patches composed of a pH sensitive layer, mucoadhesive drug reservoir layer and a backing layer171, which are suitable to deliver PPs orally because they can release PPs locally near the mucosa and protect it from proteolytic degradation172,173. Insulin-loaded intestinal patches can significantly reduce the blood glucose level at the dose of 10 IU/kg after jejunal administration. The author attributed it that the intestinal micropatches can be put into enteric capsules and strongly adhesive to the intestinal mucosa after entering small intestine (Fig. 7), which also facilitate oral absorption of insulin significantly174. Similar as transdermal patches, intestinal patches can load various formulations to modify the drug loading, release or absorption, such as solid-in-oil formulation as a drug reservoir in intestinal patch for oral delivery of insulin175.
Figure 7.
Schematic illustration of structure of intestinal patch and administration device, and in vivo mechanism of adhesion, drug release and absorption across intestinal epithelium. Reprinted with the permission from Ref. 176. Copyright © 2016 Springer.
Hydrogels have also been extensively explored for enhancing oral absorption of PPs177. Hydrogels can enable PPs reside within specific gut regions for a prolonged residence time due to their mucoadhesive properties and resist enzymatic degradation simultaneously. Complexation hydrogels are the optimal choice for oral delivery of PPs due to their environment responsiveness. For example, complexation hydrogels composed of poly(methacrylic acid) grafted with poly(ethylene glycol) do not swell in acidic environment due to strong hydrogen bonds and hence prevent insulin release in stomach, while dissociate in small intestine, resulting in rapid swelling and release178. The complexation hydrogels led to a drastic reduction of plasma calcium concentration by improving intestinal absorption of sCT. Moreover, the oral bioavailability of insulin-loaded complexation hydrogels reached up to 7.2%179. In addition, superporous hydrogels have also been used for enhancing intestinal absorption of PPs180. They can swell to several hundred times within a few minutes and exhibit enhanced mucoadhesive force181. Oral administration of insulin-loaded superporous hydrogels leads to notable insulin absorption and hypoglycemic effect, which could attribute the prolonged residence time in high concentration of insulin within specific intestinal region and reversible opening of tight junctions182.
4.3. Absorption enhancement
In addition to instability in GI tract, another important factor limiting oral bioavailability of PPs is their extremely poor permeability across epithelial membrane due to large molecular weight and high hydrophilicity. It is indispensable to enhance the intestinal permeability of PPs by chemical or pharmaceutical approaches for development of oral products. So far, there are various strategies to enhance oral absorption of PPs, among which absorption enhancers could be the most commonly used in clinical or preclinical products.
4.3.1. Prodrugs
The prodrugs strategy is the most common approach to modulate physicochemical properties of drugs via chemical derivatization, such as improving stability, solubility or permeability. The prodrugs molecules are able to overcome barriers and then converted to be active form by in vivo degradation reactions11. The bioreversible cyclization of peptide backbone has been recognized as a promising prodrug methodology for PPs, which increases the intramolecular hydrogen bonding interactions, but decreases the intermolecular hydrogen bonding interactions with aqueous solvent183. Borchardt et al.184 used this method to develop the pheylpropionic acid based cyclic prodrugs of (Leu5)-enkephaline which have shown around 1680 folds higher permeability across Caco-2 cell monolayer than parent peptide. In the same study, coumarinic acid-based prodrugs of (Leu5)-enkephaline exhibited both high permeability and good stability. Lipidization is another promising approach to create prodrugs of PPs. Lipidization can increase hydrophobicity of peptides, leading to improved permeability. For example, two palmitoylated insulins lipidized by B1-monopalmitoyl and B29-dipalmityl showed higher lipophilicity and greater stability, which leads to increased intestinal absorption185. However, lipidization could reduce the biological activity of a peptide, which have been overcome by a reversible lipidization technique186. This method can be carried out in an aqueous solution for conjugation of fatty acid and polypeptide, and can regenerate the original active polypeptides after oral absorption187. The oral absorption of reversible lipidic prodrugs of salmon calcitonin was improved by at least 19 times compared to parent peptide188. In addition, the prodrug design combining with lipid raft can generate site specific delivery by conjugation with targeting moiety. The combination of lipid and receptor targeting exhibited synergistic effect, leading to rapid transport through the cell membrane, which could be an alternative technology for enhancing absorption of hydrophilic biomacromolecules including PPs189. However, the prodrug strategy is currently limited in modification of peptides. Proteins are hard to optimize their characteristics by chemical modification due to huge molecule, and conformational instability during chemical reaction.
4.3.2. Absorption enhancers
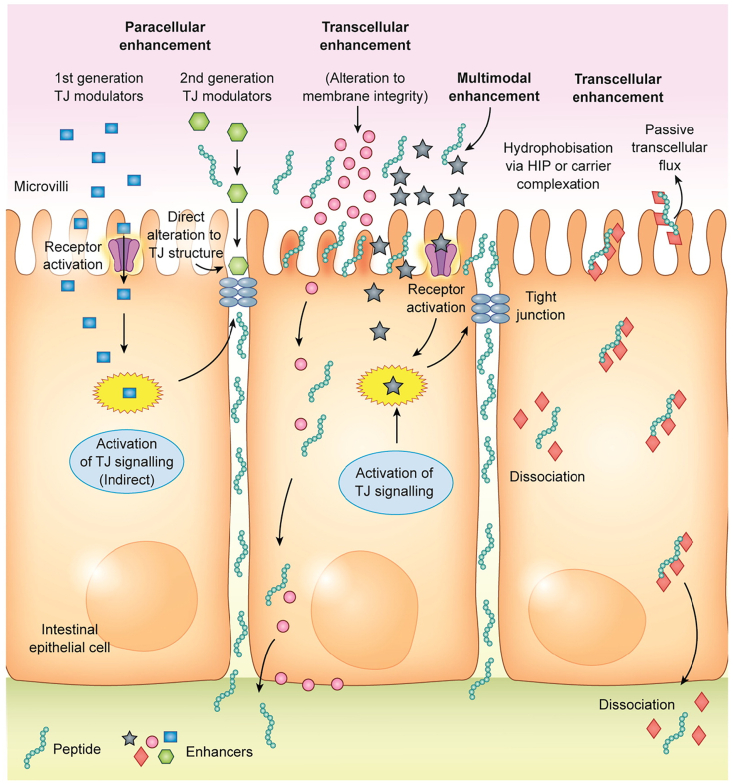
The largest obstruct for oral delivery of PPs is poor permeability across intestinal epithelium. The absorption enhancers are recognized to improve the intestinal permeability by altering the epithelial structure transiently, therefore extensively used in oral formulations of PPs. The possible mechanisms involved in current absorption enhancers are shown as Fig. 8. There are over 250 substances that have been used in preclinical studies as absorption enhancers for oral delivery of PPs according to an excellent review about intestinal permeation enhancers21, some of typical which have been listed in Table 2. Absorption enhancers have attracted more attention from pharmaceutical and biomaterial scientists since 1961 when a study found that sodium ethylene diamine tetraacetic acid (EDTA) was able to improve the oral absorption of heparin at dose of 50 mg/kg in dogs190. Some semi-synthetic and synthetic substances have been developed as absorption enhancers including chelating agents, surfactants, polymers and bacterial toxins. These absorption enhancers can facilitate oral absorption of PPs either paracellularly via the opening of tight junctions or transcellularly through increasing membrane permeability, or a combination of both.
Figure 8.
The schematic illustration of mechanisms of absorption enhancers including transcellular and paracellular pathways. Reprinted with the permission from Ref. 21. Copyright © 2016 Elsevier.
Table 2.
List of some typical permeation enhancers for oral absorption of PPs.
| Enhancer | Mechanism | Application |
|---|---|---|
| EDTA | Chelating agents; paracellular | ORMD-0801; ORMD-0901 (Oramed Pharma, USA) |
| Citric acid | Chelating agents; paracellular | Peptelligence™ (Tarsa, USA) |
| Bile salts | Multimodal | IN-105 (Biocon, India) |
| Sodium caprate (C10) | Multimodal | GIPET® (Merrion Pharma, Ireland) |
| Sodium carprylate (C8) | Multimodal | TPE® (Chiasma, Israel) |
| SNAC/5-CNAC | Transcellular | Eligen® (Emisphere, USA) |
| Chitosan | Multimodal | Oral insulin (NanoMega, USA) |
| Penetratin/PenetraMax | Transcellular | Reported for various peptides |
Chelating agents, like EDTA and citric acid, can generally enhance paracellular absorption by opening tight junctions which is caused by reduction of intracellular calcium due to chelating properties108. Diethylene triamine pentaacetic acid (DTPA), a novel chelator, has been approved to improve the oral insulin absorption with relative bioavailability of 20% by integrating into chitosan nanoparticles191.
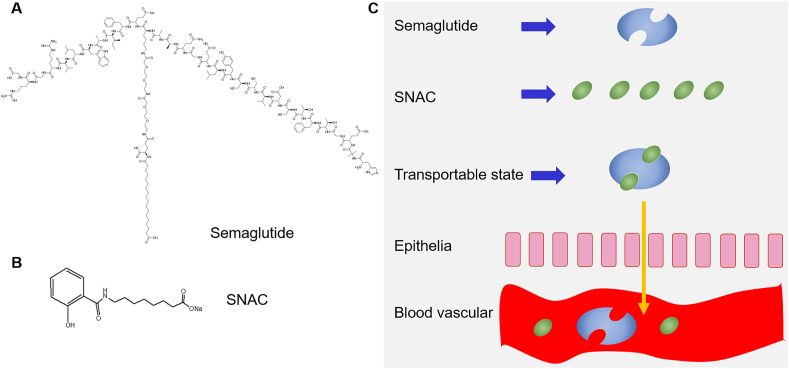
Surfactants are main absorption enhancers in clinical studies, such as sodium caprylate/caprate and their derivatives, and endogenous bile salts. Endogenous bile salts and their derivatives have been investigated to increase oral relative bioavailability of insulin by protecting stability of PPs and enhancing intestinal permeability47,192,193. The advantages of endogenous bile salts and their derivatives include good biocompatibility and high drug loading for PPs when they are used in fabrication of liposomes194. Sodium caprylate/caprate and their derivatives are the most promising absorption enhancers and have been marketed for oral delivery of PPs, such as sodium caprylate in oral octreotide (Mycappssa®, Chiasma Pharma, USA/Israel) and SNAC in oral semaglutide (Rybelsus®, Novo Nordisk, Denmark). The SNAC was firstly approved using in Eligen® carrier for improving oral delivery of vitamin B12 developed by Emisphere (USA). It is a derivative of sodium caprylate whose structure and mechanism of action are presented in Fig. 9. It was reported that SNAC were capable of enhancing permeation of heparin, sCT and insulin significantly195, 196, 197. Most of studies regarded hydrophobic SNAC non-covalently associated with peptides improves their absorption across the intestinal epithelium. After transported, the peptide disassociated from the SNAC carrier and passed into the circulation freely198. The success of Rybelsus® is related to its strong association with semaglutide199. However, some studies also thought the SNAC enhanced transport of peptides through opening tight junctions because they caused significant decline of TEER and a 36-fold increase in mannitol permeability across Caco-2 monolayers200.
Figure 9.
The chemical structure of semaglutide (A) and SNAC (B), and the rationale of permeation enhancing effect of SNAC on semaglutide (C). Reprinted with the permission from Ref. 201. Copyright © 2016 Springer.
Chitosan and its derivatives are the most common polymers for enhancing oral delivery of PPs depending on the positive charge density and bioadhesive ability. They are generally fabricated as nano/micro particles to encapsulate PPs for improving oral absorption, which described in Section 4.4 in detail.
Some absorption enhancers emerging from toxins have gradually been used in improving oral delivery of PPs by altering paracellular or transcellular permeability202. Due to safety consideration of native toxin, the common approach is that the short peptide sequence is developed by structure activity relationships (SAR) studies. For example, native ZoT (45 kDa) can enhance small intestine permeability via PKC-dependent cytoskeletal contraction which is exerted by its first six amino acids203,204. Alba Therapeutics (USA) developed a short peptide sequence AT1002 (H-FCIGRL-OH) which can lead to 40-fold increment of lucifer yellow in Caco-2 monolayer205. The larazotide acetate, an 8-mer peptide that promotes tight junctions assembly, has been used in clinical development by Alba therapeutics (USA)206. In addition, some short peptides emerging from toxins could target tight junctions related proteins, such as claudins or occludins, to increase paracellular permeability207,208. CPP are a sort of peptides derived from the transactivator of transcription (HIV-1 TAT) protein of the human immunodeficiency virus and can increase the membrane permeability of PPs209. The first CPP, Penetratin® (RQIKIWFQNRRMKWKK) consisting of 16 amino acids, was discovered in 1994210. The therapeutic PPs are linked with the CPPs by chemical conjugation or complexed with the CPPs by non-covalent bonds211. The possible mechanism of CPPs on enhancing cellular uptake is that they can increase paracellular and transcellular transport through endocytic pathway212. The low molecular weight protamine (LMWP) with a sequence of VSRRRRRRGGRRRRC is the one of CPPs, which can increase the intestinal cell membrane permeability and oral relative bioavailability of exenatide-Zn2+ by 29-fold213. Both Penetratin® and its analog PenetraMax® exerted absorption enhanced ability for oral insulin in D-form by non-covalent approach; but there is no synergistic effect observed when using the combination of these two CPPs214. Although CPPs have exerted excellent capacity in improving membrane permeability, they have not yet been validated in the clinic studies of oral delivery of peptides due to complex GI environment.
Absorption enhancers have been evidenced in improving oral absorption of PPs, even some enhancers have been used in marketed products, such as SNAC and EDTA. However, safety and regulation are the main concerns in the application of absorption enhancers in oral delivery. Toxicity has been considered as a potential drawback impeding the application of enhancers21. Fortunately, there have no significant adverse events reported for any absorption enhancers tested in clinical trials to date.
4.3.3. Active targeting
Increasing active transport has also become a promising approach to facilitate the oral absorption of PPs by targeting receptors, transporters and specialized cells in intestinal epithelia215. The colloidal carriers decorated with a specific ligand (Table 3168,216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228) have emerged as a promising technology to increase interaction with the epithelium by active targeting followed by higher transport.
Table 3.
List of ligands for actively targeting the enteric epithelia.
| Ligand | Target | Cell | Carrier | Biologic | Ref. |
|---|---|---|---|---|---|
| Folate | Folate receptor | Enterocyte | PLGA nanoparticles | Insulin | 216 |
| Biotin | Avidin | Enterocyte | Liposomes | Insulin | 217 |
| Vitamin B12 | IF-CbI receptor∗ | Enterocyte | Dextran nanoparticles; calcium phosphate nanoparticles | Insulin | 218,219 |
| Galactose | Galactose receptor | Macrophage | PLGA nanoparticles | siRNA | 220 |
| Mannose | Mannose receptor (DEC-205) | Enterocyte | Proliposome | Glutathione | 221,222 |
| Hyaluronate | CD44 receptor | Enterocyte | CaCO3 nanoparticles | Insulin | 223 |
| Transferrin | Transferrin receptor | Enterocyte | Fuse protein | Human growth hormone | 224 |
| Fc fragments | Neonatal Fc receptor | Enterocyte | PLA-PEG nanoparticles | Insulin | 225 |
| lectin | Integrin receptor | M-cell | Liposome; solid lipid nanoparticles | Insulin | 168,226 |
| RGD | Integrin αvβ3 receptor | Enterocyte; M-cell | PLGA-mPEG nanoparticles | Insulin | 227 |
| CSKSSDYQC (CSK) | Goblet cell | Chitosan nanoparticles | Insulin | 228 |
IF-CBI: combination of gastric intrinsic factor (IF) and cobalamin (CbI, vitamin B12).
Receptor-mediated endocytosis can take up extracellular substances efficiently by internalization triggered by binding ligand molecules with receptors, which is a critical pathway to acquire sufficient essential nutrients for human body, such as vitamins, transferrin and hormones229. Therefore, some nutritional components including vitamins, saccharides and fatty acids have been extensively explored to decorate drug carriers for enhancing active transport. Some nutritional vitamins have to be transported by receptor mediated mechanisms from diet and other exogenous sources, such as vitamin B12 (VB12) and folate. VB12 was used as a ligand to modify dextran-g-poly-ethyleneoxide cetyl ether micelles for oral delivery of cyclosporine A, which demonstrated increased permeability of cyclosporine A on Caco-2 monolayer. Moreover, VB12 modified nanoparticles loading insulin produced 70%–75% blood glucose reductions218. However, the limited absorption site of VB12 in the distal ileum leads to slow uptake230, compromising its potential application. Folate and biotin are also the aqueous vitamin B family members and there are a large number of receptors in whole intestinal tract. A folic acid (FA)-pluronic 85-poly(lactide-co-glycolide) polymersome exhibited higher cellular uptake than unmodified polymersome and showed better enhanced absorption effect of insulin. The folate receptor mediated endocytosis pathway was also validated by cellular uptake mechanisms study231. Insulin loaded liposomes modified by biotin showed significantly higher hypoglycemic effect with almost 2-fold relative bioavailability compared to the conventional liposomes217. Like vitamins, there are a variety of saccharide receptors located on the intestinal epithelia for active transport, such as mannose, galactose and hyaluronic acid receptors232. For example, the galactose-modified nanoparticles exhibited higher cellular uptake and ex vivo intestinal epithelial permeability compared with galactose free nanoparticles233. However, most saccharide receptors locate in M cells, by which oral delivery of vaccines can be enhanced234. Transferrin receptors (TfR) has recently become a promising target for oral delivery of PPs because they are distributed throughout the small intestinal epithelium235. In a recent study, Yong et al.236 found that nanoparticles modified by transferrin (Tf) were taken up by Caco-2 cells via TfR-mediated transcytosis more than the unmodified counterparts significantly. However, the increased number of endogenous Tf reduces the specificity of Tf-functionalized nanocarriers237. To address this issue, Liu et al. developed a nanosystem modified by cycle peptide CRTIGPSVC (CRT) to target the Tf–TfR complex for circumventing the competitive inhibition, which significantly increased the Caco-2 cellular uptake via a non-canonical allosteric directed mechanism238. The Fc receptor (FcRn) is the most promising ligand candidate to actively transport biomacromolecules into circulation from small intestines due to its high efficiency in transporting immunoglobulin G antibodies across epithelial barriers239. FcRn targeted nanoparticles were able to increase a mean absorption efficiency up to approximately 13-fold and oral insulin-loaded FcRn targeted nanoparticles could leads to similar hypoglycemic effect as s.c. insulin at the same dose225. More recently, Martins et al.240 developed porous silica nanoparticles conjugated with Fc fragment of immunoglobulin G by microfluidics technology which exerted higher cytocompatibility and greater interaction with the intestinal cells, as well as ensued better absorption of glucagon-like peptide 1 (GLP-1).
Transporters located on the epithelia surface can selectively transport some specific molecules into the cytoplasm but rarely cause cell membrane active deformation to engulf the particles unlike receptor mediated endocytosis241. Therefore, most transporters are used for improving oral bioavailability of small molecular drugs through development of prodrugs, but rarely for biomacromolecules. For example, the prodrug of zanamivir with amino acid groups increased intestinal jejunal permeation by transportation of amino acid transporter242. However, some studies also demonstrated ligand modified nanoparticles were also transported by transporters. For example, the nanoparticles functionalized with deoxycholic acid are able to overcome multiple obstacles and enhance oral absorption of insulin by targeting the apical sodium-dependent bile acid transporter (ASBT)243. Moreover, the insulin-loaded butyrate-PEG nanoparticles produced approximately 3.0-fold improvement of relative pharmacological bioavailability of oral insulin by targeting monocarboxylate transporter 1 (MCT-1) compared to the unmodified nanoparticles244.
Recently, targeting specialized cells in intestinal epithelia attracted lots of interests as an approach of improving oral delivery, such as M cells in peyer's patches, goblet cells and some immune cells. M cells are the most common target cell for oral drug delivery of antigens or proteins due to their special physiological functions245. Particles in intestinal gut can be transported by M cells very fast from apical side to basolateral side and then captured by immune cells in “dome trap” or further enter into lymphatic vessels63. There are a variety of receptors expressed on the surface of M cells for targeting, such as intercellular adhesion molecule (ICAM)-1, l-fucose, β1 integrin and glycoprotein 2 (GP2)246. Lectins are the most common used ligand binding reversibly to receptors of M cells, such as wheat germ agglutinin (WGA) and ulex europaeus agglutinin 1 (UEA1)226,247. Lectin conjugated microparticles and lipid nanoparticles loading insulin resulted in larger glucose level reduction and increment of residence time at intestinal membrane168,248. The tripeptide RGD are extensively used for enhancing the transport of nanoparticles across M cells through targeting β1 integrin234. Besides, some new ligands for targeting M cells were obtained by the phage display technique, such as CKS9249. Goblet cells, a mucus secretion cells, are rarely used to be as target sites for oral delivery. However, recent studies started to pay attention to drug delivery system based on targeting goblet cells. Nanoparticles modified with a peptide of CSKSSDYQC (CSK) which can target to goblet cells can facilitate the uptake in villi and higher internalization via calthrin and caveolae mediated endocytosis on HT29-MTX cells (goblet cell like model)250. Moreover, this nanoparticles loading insulin showed 1.5-fold improvement of relative bioavailability compared to the unmodified ones228. Besides, targeting dendritic cells (DCs) located on apical side of intestinal epithelia has been attempted to improve delivery of vaccines. Several DCs targeting peptides have been validated to increase oral delivery efficiency of antigen and enhance immunization, such as DC-pep that was screened out by phage display251,252.
Although active targeting is able to increase the uptake in specific intestinal cell group, the insufficient absorption area limits the absorption extent of PPs, which is difficult to increase the oral bioavailability to a large extent. More targets which distribute more extensively in intestinal epithelia need to be explored for oral delivery.
4.3.4. Lymphatic transport
Lymphatic route is also an important way for oral absorption. There are various accesses to lymph depending on characteristic of drugs (Fig. 10). After transported across intestinal epithelia, small hydrophilic drug molecules or macromolecules that are smaller than 10 nm (or 16–20 kDa for proteins) are transported primarily into the blood capillaries253,254. Nevertheless, the highly lipophilic drugs could be assembled into chylomicron with lipoproteins and subsequently transported into lymph. Particles or macromolecules (antigens or proteins) that are larger than 10 nm are hard to enter into blood capillaries due to small interstitial space of blood capillaries, but able to drain into lymph vessels. However, particles larger than 100 nm are poorly transported into lymph due to reduced diffusion and convection through the interstitium255,256. Both lymphoid (Peyer's patches) and non-lymphoid tissue (villous) in intestinal lumen contribute to the lymphatic transport. Transferring into lymph vessels via non-lymphoid tissue depends upon the lipid pathway, vehicle effects, sieving mechanisms of the blood vessels and the application site. The proximal small intestine is the best lymphatic transport site, while the presence of lymphatic transport has also been proven in rectal administration. M cell in Peyer's patches can take up intestinal particles by phagocytosis and complete transcytosis very fast, which is the main route for highly potent compounds such as lymphokines and antigens. Some excellent reviews have showed various approaches for improving oral lymphatic delivery257,258.
Figure 10.
The schematic illustration of the intestinal lymphatic transport of chemical drugs or antigens (proteins) after oral administration. (A) Dietary lipids and some highly lipophilic drugs are taken up by enterocytes and then assembled as chylomicron with lipoproteins to be drained into mesenteric lymph. (B) Soluble antigens (proteins) access the mesenteric lymphatics directly or via phagocytosis by dendritic cells after transport by various routes including paracellular diffusion (①), uptake into endosome and then exocytosis by exosomes (②), transcytosis by enterocytes (③), transport by M cells (④) or dendritic cells (⑤). (C) Particulate antigens (proteins) are primarily transported by M cells and then processed by a large amount of immune cells under subepithelial dome. Reprinted with the permission from Ref. 259. Copyright © 2016 Nature.
In order to increase absorption of lipid pathway, some lipid formulations were used to mimic the absorption process of dietary fats for improving oral lymphatic transport of PPs. For example, insulin-loaded solid lipid nanoparticles (SLNs) demonstrated significant drug accumulation within intestinal lymphatic system260. In addition, lipidization of peptides via chemical modification with fatty acids is an important approach to increase the lymphatic transport by improving association with chylomicrons, which have been applied in oral delivery of several peptides, such as sCT, encephalin, tetragastrin and insulin261. However, the flow rate of lymph through the intestinal lymphatic system is approximately 500-fold slower than that of blood through intestinal blood capillaries and portal vein, which leads to not sufficient quantity absorbed in systemic circulation for therapeutics262. Therefore, it is very limited to elevate oral bioavailability of therapeutic PPs by targeting lymphatic systems. So far, there are no clinical and commercial products developed by lymphatic targeting technology. However, M cells route has been regarded as an effective pathway to oral deliver vaccine and protein therapeutics. The glucan microparticles incorporating with thermosensitive poloxamer 407 gel improved the oral absorption of insulin, hence producing mild reduction in blood glucose level for over 20 h in diabetic rats263. Meanwhile, the lymphatic transportation is highly correlated with pharmacological bioavailability, which indicates the lymphatic route plays critical role in oral absorption of insulin264. The M cell transport is likely related to the physicochemical characteristics of the particles, such as physical and chemical stability, size, surface charge, shape and elasticity. For example, the polystyrene particles with a range of 50 nm and 3 μm have 6%–34% absorption ratios after oral administration265. But particles larger than 10 μm are rarely able to be transported by M cell266. Meanwhile, the targeting ligands may influence the adhesive to M cell or uptake of M cell significantly, such as lectins and RGD peptides, which has been described in section 4.3.3 in detail. However, the GALT comprises less than 10% of whole intestinal epithelial surface, which limits the absorption extent of therapeutic PPs. In addition, the particles could be captured in dome trap after transcytosis by M cells to inhibit the entry of therapeutic PPs into systemic circulation via lymph vessels63. Due to high potency of vaccines, M cell uptake of particulate oral vaccines demonstrated promising potential in clinical trials. The PLGA microspheres containing the Escherichia coli colonization factor antigen II as potential vaccine for enterotoxigenic E. coli can generate antibody responses in 5 out of 10 human subjects267. The PLGA microspheres encapsulating CS6 antigen also demonstrated effective vaccination in phase I clinical trials268. Nevertheless, these clinical trials have not been continued because of variability in immune response generation. Except for the immunology issues, the formulation design may be important hurdles for antigen-loaded particles, including stability of antigen, and release in intestinal lumen or Peyer's patches.
4.3.5. Ionic liquids
Ionic liquids (ILs) are a category of ionic compounds with melting point below 100 °C, while are called as room temperature ILs (RTILs) if the melting point declines to room temperature269. ILs have been extensively used in chemical engineering as solvents, catalysts, reagents and so on. ILs have good capacity for solubilizing poorly soluble drugs270 and strong permeation enhanced ability for biomacromolecules271,272. However, most of ILs in chemistry are not biocompatible for drug delivery or other biological use273. Recently, a class of RTILs based on natural components were developed for improving drug delivery, such as choline and organic acids274. They demonstrated good biocompatibility and permeation enhanced capacity. They are firstly employed to improve transdermal delivery of PPs, such as insulin and bovine serum albumin (BSA)275. Recent study indicated that choline and geranate (CAGE) ILs were able to enhance oral absorption of insulin and insulin-loaded CAGE ILs (3–10 IU/kg) produced a significant hypoglycemic effect after intrajejunal administration or oral intake of enteric capsules276. Meanwhile, insulin can maintain conformational and chemical stability in CAGE ILs. ILs could form a self-assembled nanostructure with gastrointestinal fluids spontaneously, which probably contribute to oral absorption or biodistribution in vivo277. Angsantikul et al.278 found that choline and glycolate ILs were also able to reduce the viscosity of the intestinal mucus and enhance the paracellular transport. Therefore, they can effectively deliver TNFα antibodies into the intestinal mucosa as well as systemic circulation. ILs also act as permeation enhancer for biomacromolecules in other mucosal barrier, such as nasal delivery279. ILs can also combine with other formulations as a permeation enhancer. For example, Peng et al.280 fabricated a mucoadhesive ionic liquid gel patches that can improve the oral transport of insulin. However, the interaction between ILs and water has to be highly valued because water is the most common substances in biological body. It is not clear whether water can attenuate the effect of ILs or not281.
4.3.6. Intestinal microneedles
Microneedle-based technology has been broadly used in transdermal delivery in pharmaceutical and cosmetics products. Microneedles are able to overcome the main barriers hindering drug absorption, such as stratum corneum in transdermal delivery282. In addition, microneedles can penetrate the physical barrier to improve drug penetration but bring about no damage to the tissue or nerves by tuning the needle length to appropriate size. Therefore, microneedle is a pain-free administration technology283. Recently, microneedles have gradually used in other mucosal delivery routes, such as ocular, oral and vaginal284.
Mucosal and epithelial barriers are the main factors influencing the oral absorption of PPs. Traverso et al.285 firstly demonstrated proof-of-concept experiments in swine that microneedles were capable to completely overcome the gastrointestinal mucosa and epithelia, and promote oral bioavailability of a biologically active molecule (Fig. 11A‒C). This device was 2 cm in length and 1 cm in diameter, and the microneedles were made of metals. The drug can be loaded in hollow or solid microneedles for release (Fig. 11D). In spite of good safety and tolerability of this device indicating in experimental period, the biocompatibility is still a significant concern if it would be developed to be a clinical product. In order to avoid the toxicity caused by metals, biodegradable or dissolvable microneedles are fabricated for biomedical use. Abramson et al.286 employed polymers to fabricate a unfolding microneedle injector (LUMI) (Fig. 11E). This injector is composed of three flexible arms, each of which has a 0.5 cm2 microneedle patch on the far end. The arms are initially bundled together and then the injector is filled in a capsule. The LUMI arms unfold outward after the injector is pushed out of capsule when the capsule reaches the intestine via intragastric administration. Then the arms press the microneedle patches against the intestinal wall to penetrate the epithelia barriers. A company (Rani Therapeutics, San Jose, CA, USA) is developing a related technology to deliver oral biologics, which has been studied in clinical trials287. However, future studies have to be determined whether the microneedle cause distension of small intestine. In addition, the small damage of intestine caused by microneedle could bring about large risk of systemic infection due to presence of a large amount of microorganisms in intestines.
Figure 11.
Schematic illustration of the rationale of orally administered microneedles. (A) Computer-aided design of the radial prototype; (B) The produced microdevice with metal endcap and pin; (C) Radiography of the microneedle; (D) Therapeutic use concept of both hollow and solid microneedles; (E) The concept of oral delivery of PPs via microneedle patch. Reprinted with the permission from Refs. 286 and 288. Copyright © 2015 Elsevier and 2019 Nature.
4.3.7. Self-orienting millimeter-scale applicator (SOMA)
In order to efficiently deliver the biomacromolecules by oral routes, various administration devices were attempted. A self-orienting millimeter scale applicator (SOMA) was inspired by the leopard tortoise's ability to passively reorient (Fig. 12) and could deliver biologic drugs by penetrating gastric mucosa rather than intestinal gut288. The thickness of stomach's wall is approximately 4- to 6-mm which provides more space to design the length of needles for safety and efficacy. The hypoglycemic effect of insulin delivered by SOMA is similar as that of sc insulin. However, this oral device is intrinsically an injectable administration and the difference from the common injection is only injection site. Moreover, the injection in GI tract could cause larger risk than sc or intramuscular injection. Recently, a microjet vaccination system was developed to avoid the use of needles. It is a three-dimensional microelectromechanical systems-based drug delivery technology and can produce a high-pressure liquid jet of vaccine to penetrate the buccal mucosal layer289.
Figure 12.
The schematic diagram of SOMA. (A) The mechanism of localization, orientation and injection of SOMA in stomach; (B) The fabricated product of SOMA; (C) The shape comparison between SOMA and the leopard tortoise (S. pardalis); (D) The injection device in SOMA. Reprinted with the permission from Ref. 289. Copyright © 2019 Science.
4.3.8. Nanocarrier-facilitated oral delivery of PPs
The largest barriers in oral delivery of PPs are mainly enzymatic degradation and poor permeability across intestinal epithelium. Almost most of strategies are based on overcoming these two barriers to improve oral bioavailability of PPs. Nanocarriers can entrap the active biomacromolecules into the matrix or the core to protect against enzyme degradation through GI lumen. In addition, nanocarriers can be taken up by epithelial cells or M cells in Peyer's patches. Nanocarriers are also able to entrap penetration enhancers together with PPs or be decorated by ligands to further enhance the oral absorption. Hence, various nanocarrier systems have been extensively investigated in oral delivery of biologics including PPs, vaccines and nucleic acids. According to materials of nanocarriers, there are primarily three categories of nanoparticles, such as polymeric, lipid-based and inorganic nanoparticles, some of which have demonstrated good delivery effect for PPs by oral routes (Table 4106,120,217,219,225,290, 291, 292, 293, 294, 295, 296, 297, 298, 299, 300, 301, 302, 303, 304, 305, 306, 307, 308, 309, 310, 311).
Table 4.
Representative examples of nanotechnology-based strategies used for enhancing oral absorption of PPs.
| Nanotechnology | Material | Modification | Size (nm) | PPs | Absorption | Ref. |
|---|---|---|---|---|---|---|
| Polymeric nanoparticles | PLGA | TMC | 247.6 | Insulin | 2-Fold higher relative bioavailability for insulin | 290 |
| WGA | 231.9 | Thymopentin | Enhancing the interaction with M cells by 1.8–4.2-fold and increasing the values of CD4+/CD8+ ratios | 291 | ||
| RGD-PEG | 200 | Ovalbumin | Concentrating in M cells particularly | 292 | ||
| No | 302–328 | β-LGDP | Increasing mucosal immunity and milk allergy prevention | 293 | ||
| PLA-PEG | Fc fragments | 55 | Insulin | Producing a prolonged hypoglycemic effect in wild-type mice at a clinically relevant insulin dose of 1.1 U/kg | 225 | |
| Chitosan | PGA | 232.9 | Insulin | Approximately 20% of relative oral bioavailability | 120 | |
| PEG | 200–250 | PTH1-34 | Improving oral bioavailability of PTH remarkably | 294 | ||
| Dextran/TMC | 200–300 | Lipoprotein | Increasing the humoral immunity and the level of antibody | 295 | ||
| Eudragit L100/mannosylated | 558.2 | BSA | Eliciting strong systemic IgG antibody and mucosal IgA responses | 106 | ||
| Dendrimer | PEG | 1100 | Insulin | Controlling the blood glucose levels | 296 | |
| Lipid-based nanoparticles | Liposomes | WGA-carbopol | 100 | sCT | Enhancing oral absorption of sCT by more than 20-fold | 297 |
| Biotinylated | 150 | Insulin | The relative oral bioavailability achieved to 12.09% | 217 | ||
| Sodium caprate | 200–250 | hGH | Leading to a relative bioavailability of hGH of 3.4% | 298 | ||
| PLFE | 500 | OVA | Improving the immune response of OVA | 299 | ||
| MPC | 300 | BSA | Higher levels of IgG in the sera and IgA in the mucosa | 300 | ||
| UEA-1 | 400–500 | BSM | Higher sIgA level and cytokines level | 301 | ||
| SLN | Cationic lipids | 300 | Insulin | Protecting insulin from the enzymatic degradation | 302 | |
| CSK/IRQ | 244/410 | sCT | Leading to the absolute bioavailability of 12.41% and 10.05%, respectively | 303 | ||
| Nanoemulsions | Alginate/chitosan | 500 | Insulin | The oral relative bioavailability was 8.42% | 304 | |
| Oil-structured nanoemulsion | 250.8 | Exenatide | Improving the bioavailability of exenatide via intestinal lymphatic transport | 305 | ||
| Inorganic nanoparticles | Silica | Chitosan | 226 | Exenatide | Enhancing transport across Caco-2 monolayer by 1.7-fold | 306 |
| CPP-PEG | 173.2 | hGH | Leading to 4.91-fold increase in pharmacodynamics | 307 | ||
| No | 200 | Insulin | Showing sustaining hypoglycemic effect. | 308 | ||
| No | 175–321 | OVA | Enhancing the phagocytosis by dendritic cells and the production of specific antibodies | 309 | ||
| Calcium phosphate | VB12 | <250 | Insulin | Enhancing oral bioavailability by 4-fold and showing sustained hypoglycemic effects up to 12 h | 219 | |
| Polysaccharide | 14–35 | OVA | Enhancing the mucosal IgA and serum IgG responses | 310 | ||
| Gold | Chondroitin sulfate | 123 | Insulin | The oral absorption was enhanced with 6.61-fold | 311 |
Abbreviations: PLGA, poly(d,l-lactide-co-glycolide); TMC, N-trimethyl chitosan chloride; WGA, wheat germ agglutinin; RGD, arginylglycylaspartic acid; PLA-PEG, poly(lactic acid)-poly(ethylene oxide); PGA, poly(γ-glutamic acid); PTH1–34, parathyroid hormone 1–34; β-LGDP, beta-lactoglobulin-derived peptides; BSA, bovine serum albumin; sCT, salmon calcitonin; hGH, human growth hormone; PLFE, polar lipid fraction E; UEA-1, ulex europaeus agglutinin 1; BSM, bovine submaxillary mucin; MPC, mannose-PEG-cholesterol conjugate; CPP, cell-penetrating peptide; OVA, ovalbumin; VB12, vitamin B12.
4.3.8.1. Polymeric nanoparticles
Polymeric nanoparticles have been widely explored to circumvent multiple barriers hindering oral delivery of PPs. Except for protecting PPs from degradation in harsh GI environments, polymeric nanoparticles can increase the epithelial transport by improving cellular uptake and inhibiting efflux of P-gp312. In addition, some polymers can reversibly open tight junctions, allowing transport of PPs through paracellular pathway, such as chitosan and its derivatives313. The active transport through enterocytes, goblet and M cells can be enhanced by decorating ligands on the surface of nanoparticles215. Polymeric nanoparticles are generally composed of synthetic, semi-synthetic or natural polymers with diameter ranging from 10 to 1000 nm. Natural polymers are abundantly present in nature and have good compatibility, hence, have gained extensive interests in oral delivery of PPs, such as chitosan, gelatin, alginate and hyaluronic acid1. Chitosan is the most common materials as nanoparticulate delivery system for PPs due to its mucoadhesive and permeation enhancement characteristics. In addition, chitosan is also easily modified to achieve various aims, such as pH-responsive release, increasing hydrophilicity and positive charges or improving mucoadhesive ability314, 315, 316. A pH-responsive nanoparticles developed by conjugating chitosan and poly-γ-glutamic acid (PGA) can enhance the paracellular transport of insulin by opening the tight junctions between adjacent cells317. Chitosan/alginate nanoparticles can increase pharmacological availability of insulin to 6.8% and 3.4% for the 50 and 100 IU/kg dose respectively318. The N-trimethyl chitosan (TMC) can significantly enhance the permeability of peptides or proteins in comparison of normal chitosan319, hence, has been used as permeation enhancer to coat other nanocarriers320 or engineer nanocarriers together with other materials321. Recently, chitosan oligomers were found to reduce the coulombic repulsion between anionic calcitonin and negatively charged intestinal epithelial cells, which facilitates the oral absorption of calcitonin322. In terms of synthetic polymers, the PLGA, PCL, PLA and polyacrylic acid (PAA) are frequently studied for PPs delivery due to good biocompatibility. These polymeric nanoparticles exhibited better capacity to protect against GI digestion, but it is necessary to combine with other permeation enhancers for improving intestinal epithelial permeability of PPs323. However, lack of self-regulating release of insulin is still a big issue for oral delivery. Recently, Paul et al.324,325 developed a biomimetic imprinted nanoparticles to increase self-regulating adhesion and release of insulin by molecular imprinting technique. The imprinted nanoparticles significantly prolonged hypoglycemic effect for up to 24 h and increased insulin transport via transcellular pathway. Yet, the oral bioavailability of PPs delivered by polymeric nanoparticles is still very limited and not sufficient for clinical therapy. Meanwhile, long-term exposure of these nanoparticles in GI tract is still a concern.
4.3.8.2. Lipid-based nanocarriers
Lipid-based nanocarriers (LBNs) are composed of natural lipids or phospholipids to form emulsion, solid particles or vesicles326. Due to high biocompatibility, LBNs have received much attention as oral delivery systems. However, LBNs are difficult to entrap hydrophilic macromolecules with high efficiency, which is a primary hurdle to be developed for oral delivery of PPs327. In addition, LBNs can be degraded by lipolysis in GI tract, leading to poor protecting ability for entrapped PPs328. Therefore, SLNs appear to be better than emulsions or liposomes. Nevertheless, nanoemulsions and liposomes have also exerted certain superiority for oral delivery of some PPs by regulating formulation.
The nanoemulsions are generally used to incorporate lipophilic drugs into the oil droplets for improving oral absorption. Although the incorporation of hydrophilic PPs in the internal phase of o/w nanoemulsions is difficult to achieve, some poorly water soluble peptides have been successfully developed to be oral products by SNEDDS, such as Sandimmum Neoral® for cyclosporine A329. In order to effectively entrap hydrophilic PPs in nanoemulsions, the w/o nanoemulsion was employed for oral delivery of PPs. The PPs can be completely encapsulated in the core of w/o nanoemulsions to protect against degradation in the harsh GI environments330. A insulin-loaded w/o nanoemulsions with the diameter of 161.7 ± 24.7 nm enhanced oral bioavailability of insulin by 10-fold compared with plain insulin solution331. Moreover, the w/o microemulsion exhibited more significant effect in oral delivery of other proteins, such as earthworm fibrinolytic enzyme which are increased to 208-fold higher bioavailability than that of control solution by intraduodenal administration332. However, the w/o nanoemulsions definitely occur phase conversion due to a large amount of water in GI tract, which could cause the leakage of encapsulated PPs. Therefore, Li et al.304 employed chitosan and alginate to coat the nanoemulsion to avoid fast leakage of insulin from the core of nanoemulsion.
Liposomes are simulated natural cell membrane structure and have been successfully used in delivery of anticancer drugs to decrease the toxicity, such as Doxil® for doxorubicin. Likewise, multiple remarkable advantages of liposomes received much interests in oral drug delivery, such as good biocompatibility, flexible encapsulation and tunable characteristics333. Liposomes have been attempted to be investigated in oral drug delivery of insulin as early as the late 1970s334,335. Unfortunately, the results are always indistinct. For example, a study indicated that oral insulin-loaded liposomes were able to exhibit hypoglycemic effect in only 54% of the normal rats and 67% of the diabetic rabbits336, which could be ascribed to poor stability of conventional liposomes in GI tract112,337. Recent modification technologies facilitate the development of liposomes in oral delivery by addition of polymer coating and modulating liposomal compositions. Liposomes containing bile salts have revealed better stability in GI tract than conventional liposomes192,193 by avoiding the destructive effect of physiological bile salts. In addition, bile salts, such as sodium glycocholate (SGC), sodium taurocholate (STC) and sodium deoxycholate (SDC), are capable of inhibiting the activity of GI enzymes including pepsin, trypsin and α-chymotrypsin112. Moreover, liposomes containing bile salts can improve the trans-enterocytic internalization compared with conventional liposomes, which could be an important mechanism for enhanced oral absorption of PPs192. Besides, cholesterols in conventional liposomes replaced by other sterols (i.e., ergosterol) is also a promising strategy for improving stability and transmembrane ability of liposomes in GI tract47. Liposomes containing bile salts, also named as bilosomes, have been widely used in the fields of vaccine delivery338. The general sense of bilosomes refers to bilayer vesicles fabricated by surfactants with the incorporation of bile salts which is to stabilize the vesicles in GI tract by preventing membrane destabilization. For example, bilosomes constructed from monopalmitoylglycerol, cholesterol, dicetyl phosphate and SDC showed promoted effect in reduction of viral cell load in an influenza challenge study by increasing uptake within the Peyer's patches339.
Lipid nanoparticles are composed of solid lipids or mixtures of solid and liquid lipids with a diameter size smaller than 1000 nm, including SLNs and nanostructured lipid carriers (NLCs)340. Due to highly hydrophilic nature of PPs, they are poorly encapsulated in the matrix of SLNs and could distributed in the water phase or interface between oil and water owing to the presence of surfactants341. In addition, the drug expulsion usually occurs in SLNs after polymorphic transition during storage. NLCs can improve the loading capacity and avoid drug expulsion due to the involvement of liquid lipids compared with SLNs342. Since the mid 1990's, many groups have explored the encapsulation of various PPs in lipid nanoparticles through optimization of formulation and preparation, such as BSA, insulin, sCT, LHRH and protein antigens341. Although entrapment efficiency can be improved significantly, the loading capacity for most of PPs is still not more than 10%. Sarmento et al.343 engineered an insulin-loaded SLNs with entrapment efficiency of over 43% demonstrated a considerable hypoglycemic effect during 24 h after oral administration to diabetic rats. Various surface modification technologies were employed to further increase the delivery efficiency of SLNs, such as chitosan coating344, lectin168 and octaarginine modification345. In addition, surface charge of lipid nanoparticles is a crucial factor determining the in vivo behaviors after oral administration. Anionic charges can slow down the lipolysis of SLNs, while cationic charge can accelerate this process. The absorption of intact SLNs into blood circulation with the fastest and largest absorption was observed for net neutral SLNs346. The absorption amount of intact SLNs is highly significant for therapeutic PPs.
4.3.8.3. Inorganic nanoparticles
Inorganic nanoparticles have attracted increasing attention in drug delivery systems and also been used in improving oral delivery of PPs, such as silica nanoparticles26, gold nanoparticles311, calcium carbonate or phosphate nanoparticles347.
Silica nanoparticles are the most commonly used drug carrier in inorganic nanoparticles due to good biocompatibility and tunability in size or morphology. Mesoporous silica nanoparticles (MSNs) have been widely used in improving oral bioavailability of poorly water soluble drugs348. PPs can be loaded in MSNs by physical absorption and covalent conjugation. Physical absorption is difficult to achieve higher loading capacity which is generally lower than 15%. Whereas, covalent conjugation can bring about environmental sensitive release for PPs26. MSNs can protect PPs against degradation by harsh GI environments. In addition, the surface of MSNs can also be coated or modified for improving stability or intestinal transport. PEGylated MSNs are able to protect the conformation of insulin under simulated gastric and intestinal condition349 and polymethacrylates coating enables pH responsive release of insulin from MSNs350. The SBA-15, a kind of MSNs, were used to deliver hepatitis B orally, indicating that they were able to protect and release the hepatitis B surface antigen (HBsAg) and offer better antibody response than intravenous delivery351. Calcium based nanoparticles have attracted more interests in nanocarrier for PPs, such as calcium phosphate (CaP) and calcium carbonate (CaCO3)352. Similarly as MSNs, calcium based nanoparticles were generally coated using some polymers to improve the oral absorption and biocompatibility, for instance, hyaluronic acid coated CaCO3 nanoparticles exerted satisfied hypoglycemic effect for oral insulin delivery223 and PEGylated CaP nanoparticles were found to be able to protect the insulin and sustain the release at the physiological pH353. Further, vitamin B12 was grafted to chitosan as a ligand to coat CaP nanoparticles for improving oral insulin absorption by 4.3-fold compared with naked nanoparticles219. Gold nanoparticles capped with chondroitin sulfate or apple polysaccharide are able to increase plasma insulin concentration and anti-diabetic capability311,354. The composites of insulin/zirconium phosphate (ZrP) coated with titanium dioxide (TiO2) were found to be non-toxic to biological environment and used for oral insulin delivery, which indicates promising in vitro drug release for a long time355.
However, biosafety is a big concern for inorganic nanoparticles in drug delivery since they are non-degradable in biological environments356. Most of PPs are needed to be administered for a long time due to short half-life, which could lead to accumulation of inorganic nanoparticles in human body357. Thus, inorganic nanoparticles could be not ideal carriers for oral delivery of PPs.
5. Oral delivery systems of PPs commercially available and under clinical and preclinical studies
5.1. Current oral PPs on the market
Since the approval of first recombinant insulin in 1981, a great number of PPs have been developed in the recent years as new therapeutics. However, few PPs are administered via oral route due to great challenges in oral delivery358. Moreover, current oral PPs on the market include both systemic delivery and local retention in the GI tract. For instance, cyclosporine A is one of the marketed oral peptides and formulated as SNEDDS359. While, numerous oral enzyme products are delivered to local GI tract to treat metabolic disorders. Some oral PPs commercially available are listed in Table 5.
Table 5.
Oral products of PPs on markets.
| PPs | Trade name | Technology | Indication | Company |
|---|---|---|---|---|
| Cyclosporin A | Neoral®/Sandimmune® | SNEDDS | Immunosuppression; systemic delivery | Novatis AG (Switzerland) |
| Desmopressin acetate | DDAVP® | Chemical modification | Central diabetes insipidus; systemic delivery | Ferring Pharmaceuticals (Switzerland) |
| Octreotide | Mycapssa® | Enteric coating; permeation enhancer | Long-term maintenance treatment in acromegaly patients; systemic delivery | Chiasma (USA) |
| Semaglutide | Rybelsus® | Permeation enhancer | Type 2 diabetes mellitus; systemic delivery | Novo Nordisk (Denmark) |
| Taltirelin hydrate | Ceredist®/Ceredist OD® | Chemical modification | Spinocerebellar degeneration; systemic delivery | Mitsubishi Tanabe Pharma Co. (Japan) |
| Linaclotide | Linzess® | Acts locally | Irritable bowel syndrome, chronic idiopathic constipation; local delivery | Actavis, Inc. (USA) |
| Vancomycin | Vancocin® | Acts locally | Infection | ANI Pharmaceuticals, Inc (USA) |
| Colistin sulfate | Koolistin® | Acts locally | Infection | Biocon Ltd. (India) |
| Tyrothricin | Lozenges® | Acts locally on the throat | Pharyngitis | The Boots Company PLC (UK) |
| Pancrelipase | Creon® | Delayed release; acts locally | Exocrine pancreatic insufficiency | AbbVie Inc. (USA) |
| Tilactase | Lacteeze® | Chewable tablets; acts locally | Lactose intolerance | Lacteeze (USA) |
| Sacrosidase | Sucraid® | Oral solutions; acts locally | Congenital sucrase-isomaltase deficiency | QOL Medical, LLC (USA) |
| Diamine oxidase | DAOSiN® | Acts locally | Histamine intolerance | SCiOTEC (Austria) |
Cyclosporine A is the one of the most successful oral peptide products in market. The combination of the cyclic lipophilic undecapeptide with SNEDDS technologies enables the oral bioavailability achieve 19%–40%360. Meanwhile, SNEDDS formulation (Neoral®) overcome the high intra- and inter-patients pharmacokinetics variability in a high percentage of patients by better control of droplet size, increasing intestinal permeability and inhibiting P-glycoprotein efflux and P450 metabolism360. Desmopressin acetate (DDVAP) is also a cyclic peptide and an analog of arginine vasopressin. Its stability is improved by chemical modifications including deamination of the first amino acid and substitution of the eighth amino acid l-arginine by d-arginine361. It was developed as an oral tablet by Ferring Pharmaceuticals (Denmark) as early as 1995 and subsequently a variety of generic products were also approved by FDA. However, the oral bioavailability of DDVAP is only around 0.1% because there is no any permeation enhanced technology used in these oral tablet362. Octreotide is another cyclic peptide which has been commercially available oral products on the market. It is a synthetic analog of the endogenous hormone somatostatin and exerts higher stability than somatostatin in SGF with pepsin due to cyclic structure49,363. The oral enteric capsule of octreotide developed by an oily suspension containing the permeation enhancer sodium caprylate was approved in June 2020 by FDA. However, the phase I and II clinical pharmacokinetics showed the oral bioavailability was only 0.5%364. As a peptide drug treating diabetes, semaglutide was successfully developed as oral formulation by Novo Nordisk. Semaglutide is a GLP-1 analog consisting of 31 amino acid residues and far larger than DDVAP and octreotide. The oral semaglutide (Rybelsus®) was formulated as a tablet combining with permeation enhancer SNAC developed by Emisphere technologies75. The clinical trials demonstrated that 40 mg oral dose was comparable with the 1 mg sc dose365. In addition, taltirelin and reduced l-glutathione can also be administered orally to exert therapeutic efficacy.
Some proteins are not needed to be delivered systemically, but needed to retain in GI tract to treat some local diseases, such as linaclotide for irritable bowel syndrome and oral enzyme products for GI metabolic disorders. Linaclotide acts as a guanylyl cyclase C agonist locally in the small intestine and was developed as an oral hard capsules to treat chronic idiopathic constipation and irritable bowel syndrome with constipation366. The oral linaclotide is almost not absorbed into systemic circulation. Vancomycin is a glycosylated tricyclic heptapeptide antibiotics and poorly absorbed due to high hydrophilicity and large cyclic structure367. Thus, oral vancomycin capsule was approved for treatment of pseudomembranous colitis by FDA. Some enzymes related with GI function prefer to be administered by oral route. There are a variety of oral enzyme products on the market for GI metabolic disorders such as Creon®, Lacteeze®, Sucraid® and DAOSiN®368. However, most of them are approved as dietary supplements and only pancreatin was approved as drug by FDA.
5.2. Overview of the current clinical studies
In the past decades, the non-invasive routes have attracted more attention for delivery of PPs. A number of oral PPs developed by various new technologies are being clinically evaluated (Table 6) for both systemic and local delivery. The majority of the technologies for systemic delivery of PPs under clinical trials are for oral insulin133.
Table 6.
Oral PP products under clinical trials (clinical trials.gov).
| PPs | Phase | Technology | Company |
|---|---|---|---|
| Insulin | I | Enteric coating; bioadhesive calcium phosphate nanoparticles (Nodlin™) | NOD Pharmaceuticals, Inc. (China) |
| Insulin | I | Gastrointestinal permeation enhancement technology (GIPET™) | Merrion Pharmaceuticals Ltd. (Ireland) with Novo Nordisk (Denmark) |
| Insulin | I | Permeation enhancer (Eligen®) | Emishphere (USA) with Novo Nordisk (Denmark) |
| GLP-1 analog | I | Permeation enhancer (sodium caparate) | Merrion Pharmaceuticals Ltd. (Ireland) with Novo Nordisk (Denmark) |
| Insulin | II | Silica-based nanoparticles | Oshadi (Israel) |
| Insulin | II | Enteric coating; enzyme inhibitor; permeation enhancer | Oramed (Israel) |
| Insulin | II | Permeation enhancers (Axess™, Capsulin™) | Proxima Concepts Ltd/Diabetology (UK) |
| PTH | II | Permeation enhancers (Axess™, CaPTHymone™) | Proxima Concepts Ltd./Diabetology (UK) |
| rhPTH(1–31) | II | Permeation enhancers; enzyme inhibitor (Peptelligence TM) | Enteris Biopharma, Inc. (USA) |
| CsA | II | Oil in water emulsion | Sigmoid Pharma (Ireland) |
| Dolcanatide | II | Chemical modification | Synergy Pharmaceuticals Inc. (USA) |
| Acyline | II | Gastrointestinal permeation enhancement technology (GIPET™) | Merrion Pharmaceuticals Ltd. (Ireland) |
| Leuprolide | II | Permeation enhancer; pH modulator; enzyme inhibitor | Enteris Biopharm (USA) |
| Insulin | III | Liver-targeted liposomes | Diasome Pharma (USA) |
| Insulin | III | Chemical modification (PEGylated insulin) | Biocon (India) |
| sCT | III | Permeation enhancers (Axess™, Capsitonin™) | Proxima Concepts Ltd./Diabetology (UK) |
| sCT | III | pH modulator (Peptelligence TM) | Tarsa therapeutics, Inc. (USA) |
| sCT | III | Permeation enhancer (5-CNC) | Emisphere (USA) |
| Plenacatide | III | Chemical modification; local delivery | Chiasma (Israel) |
There are two typical oral insulin products under phase I clinical trials which are developed by Novo Nordisk (Denmark) and NOD Pharmaceuticals Inc. (China). Novo Nordisk employed gastrointestinal permeation enhancement technology (GIPET) to formulate an oral insulin tablet with Merrion Pharmaceuticals (Ireland)369. The formulation is comprised of micelles with absorption enhancers (sodium caprate). Moreover, NOD encapsulated insulin into enteric coated bioadhesive calcium phosphate nanoparticles which were filled into a capsule (Nodlin™). Nodlin™ has completed phase I clinical trials (ChiCTR-TRC-12001872) on healthy volunteers, which exhibited similar hypoglycemic effect compared with sc insulin370.
An oral insulin enteric capsule containing protease inhibitors and permeation enhancers developed by Oramed Pharmaceuticals Inc. (Israel) has completed phase II clinical evaluation for the treatment of T1DM and T2DM. In phase II trial (NCT00867594), the ORME-0801 capsule is able to reduce blood glucose level on eight T1DM patients significantly371. The capsules showed good tolerance and no hypoglycemic phenomenon on all patients ever though a high dose of insulin was administered. Inorganic silica nanoparticles were firstly used for oral insulin delivery by the Oshadi Drug Administration Ltd. (Israel), which is under phase II clinical trial. The formulation is developed by inert silica nanoparticles (1–100 nm) loading oily suspended insulin together with the branched polysaccharides372. Biocon limited (India) developed an oral insulin named Tregopil based on chemical modification. Tregopil employed chemical modification to conjugate the β29-Lys-amino group of human insulin with a single methoxy triethylene glycol propionyl unit through amide linkage373. Recently, Tregopil has completed phase II clinical trials for two doses (45 mg and 30 mg). Tregopil demonstrated well toleration with the patients, and rapid and prolonged hypoglycemic effect at different dosing interval. Besides, the composition of the observed meal has no influences on pharmacodynamic effect of the insulin374. In addition, there are some other oral peptides under phase II clinical trials, such as PTH for osteoporosis and leuprolide for endometriosis.
It is very interesting that a hepatocyte targeting liposomes of oral insulin developed by Diasome Pharmaceuticals (USA) has completed phase II clinical trials and is preparing for phase III clinical trials. This product is formulated by nanoparticle technology and comprised of liposomes smaller than 150 nm containing insulin conjugated with hepatocyte-targeting moieties (biotin-phosphatidyl-ethanolamine)375. These liposomes are transported by intestinal epithelia into portal vein and then captured by hepatocytes to mimic the physiological insulin delivery. Although recent results of clinical trials have not yet been disclosed, the preliminary results revealed that it showed well toleration with the patients and better control of blood glucose level even after oral administration of a low dose of insulin (5 IU)376. sCT is also one of the most developed peptides for oral delivery except for insulin. Emisphere technologies Inc (NJ, USA) employed their Eligen® technology to develop oral sCT (SMC021) which is under phase III clinical trials. This formulation used permeation enhancer 8-(N-2-hydroxy-5-chlorobenzoyl)-amino-caprylic acid) (5-CNAC) to improve the oral absorption of sCT, which is similar rationale with oral semaglutide377.
For local delivery, most of products primarily employed new technologies to make them resistant against enzymatic degradation in GI tract. For instance, the Vectrix™ platform developed by Protagonist Therapeutics Inc. designed highly constrained and stable peptides by molecular design tools and libraries of scaffolds. Currently, there have been two products in clinical trials based on Vectrix™ platform, including PN-10-943 for UC and PTG-200 for the treatment of CD378. Following a similar rationale, Avaxia Biologics, Inc developed a milk-derived antibody (AVX-470) to treat UC, which is now in phase I379. In addition, biomimetic drug delivery systems are broadly used in local delivery of PPs. The cellulose wall of plant cell is able to protect the recombinant proteins from degradation along the GI tract. The company Protalix Biotherapeutics have developed several candidates in clinical trials based on the ProCellEx delivery platform, such as a recombinant human tumor necrosis factor receptor II fused to an IgG1 Fc domain (TNFRII-Fc)) for UC in phase II and the glycosylated glucocerebrosidase enzyme (prGCD) for the treatment of Gaucher disease also in phase II380. Furthermore, more and more new technologies are emerging for oral delivery of PPs to treat local diseases in GI tract, such as Vorabodies™ or ActoBio Therapeutics™23.
5.3. Recent status of preclinical strategies
As mentioned above, there have been a variety of strategies to improve oral delivery of PPs, some of which have been widely used in marketed products and clinical trials, such as enteric coating, enzyme inhibitors, permeation enhancers, nanotechnology, colonic targeting, as well as chemical modification. There are hundreds of papers published about new oral delivery systems for PPs every year. Table 7 lists partial projects of oral delivery systems for PPs in preclinical phase.
Table 7.
Examples of preclinical projects of oral delivery systems for PPs.
| Company | PPs | Technology |
|---|---|---|
| Emisphere (USA) | hGH PTH(1–34) PYY |
Permeation enhancer (Eligen®) |
| NanoMega Medical Corp. (USA) | Insulin Exendin-4 |
Chitosan nanoparticles |
| Transgene Biotek Ltd. (India) | Insulin | Solid lipid nanoparticles |
| Aegis Therapeutics, LLC. (USA) | Octreotide D-Leu-OB-3 (leptin) PTH GLP-1 analogs |
Permeation enhancer (Intravail®) |
| Rani Therepeutics, LLC (USA) | Insulin | Microneedles; pH modulator; Robotic pill |
| Oramed | Exenatide (ORMD-0901) | Enteric coating; permeation enhancer; enzyme inhibitor |
| I2O therapeutics (USA) | Insulin | Ionic liquids |
Currently, nanotechnology have become as the main research hotspot to improve the oral bioavailability of PPs. The nanoparticle system of NanoMega (CA, USA) is capable of decreasing the blood glucose over 8 h in diabetic rats. What's more, an oral suspension of nanoparticles demonstrates a relative bioavailability of 15%, while an enteric capsule containing freeze-dried nanoparticles shows a relative bioavailability of 20%. This nanoparticles is composed of chitosan and PGA120. Chitosan could act as a permeation enhancer by transient opening of tight junctions. Meanwhile, this technology has also been used for oral delivery of exendin-4381. Nanoparticles are further conjugated with ligands on surface to facilitate intestinal absorption by targeting intestinal receptors or transporters. Transgene Biotek Ltd. (India) conjugated VB12 or Tf on SLNs to establish a TrabiOral™ platform for oral delivery of PPs. A oral insulin project (TBL-1002OI) led by Transgene has revealed prolonged hypoglycemia in rats for 10 h after oral administration382).
Additionally, the intestinal microdevices attract more and more attention in oral delivery of PPs, such as microneedles. A robotic pill was developed by Rani Therapeutics (USA) for oral protein delivery. It is actually a balloon-like structure of sugar microneedles encapsulated by PLGA which is a degradable polymer. Insulin loaded the microneedles exhibited encouraging hypoglycemic effect with oral bioavailability of 50% in preclinical trials383. Likewise, intestinal patches have also been attempted to orally delivery sCT, exenatide, insulin and interferon-α. The patches can be prepared as the size of millimeters or micrometers coated with pH-responsive polymer and contain drug reservoir. This device could also integrate absorption enhancers or enzyme inhibitors to further improve oral absorption of PPs170.
Permeation enhancers are the main components in products of oral PPs in market or clinical trials. Until now, a number of companies are still developing various permeation enhancers. For instance, Emisphere developed a series of derivatives of caprylic acid as permeation enhancers, such as SNAC and 5-CNAC384. Aegis Therapeutics (CA, USA) developed a category of permeation enhanced excipients named Intravail® which are a group of alkylsaccharides composed of disaccharides and alkyl chain substituents with lengths between 10 and 16 carbons. They have been used in oral delivery of various peptides including octreotide and D-Leu-OB3. The results showed high systemic bioavailability after combination of peptides and Intravail® in rodents compared to sc administration385.
6. Conclusions and future perspectives
Oral delivery of PPs has become more attractive in drug research and development since the increasing market share in the last decade. Therefore, various new technologies are emerging for improving oral bioavailability of PPs by overcoming obstacles of PPs in terms of stability and permeability. Some technologies have been successfully used in oral marketed products of PPs, such as enteric coating, enzyme inhibitors, permeation enhancers, as well as chemical modification. Currently, nanotechnology-based approaches have shown potential for developing oral PPs formulation. Moreover, intestinal microdevices were also developed for delivering PPs by oral route, such as intestinal microneedles. However, the safety, efficacy and reproducibility in preparation technique of these new technologies are needed to be improved and evaluated thoroughly. Furthermore, the oral bioavailability of PPs for systemic delivery is still very low, even lower than 1% for some products despite using new technology to improve the stability and permeability.
Nanoparticles are able to facilitate the intestinal transport of PPs, which has been reported by numerous papers. However, it is more important to shed light on well-understanding the detailed mechanism of interaction between these nanocarriers and PPs or the intestinal milieu. Moreover, elucidation of PPs transport in the intestinal epithelium and the influence of physiological factors on absorption of PPs are also indispensable. Therefore, elucidating the in vivo fate of nanoparticles and PPs after oral administration is the necessary prerequisite to development of highly efficient oral systems of PPs.
Author contributions
Quangang Zhu, Zhongjian Chen and Pijush Kumar Paul collected references and drafted the manuscript. Yi Lu collected references. Jianping Qi and Wei Wu proposed the concept and revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (No.s 81872815, 81872826 and 82073801), Science and Technology Commission of Shanghai Municipality (No. 18ZR1404100, China), Shanghai Pujiang Program (No. 18PJD001, China), and Key Subject of Shanghai Skin Disease Hospital (No. 2019ZDXK03, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Wei Wu, Email: wuwei@shmu.edu.cn.
Jianping Qi, Email: qijianping@fudan.edu.cn.
References
- 1.Patel A., Patel M., Yang X., Mitra A.K. Recent advances in protein and peptide drug delivery: a special emphasis on polymeric nanoparticles. Protein Pept Lett. 2014;21:1102–1120. doi: 10.2174/0929866521666140807114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rengasamy K.R.R., Khan H., Ahmad I., Lobine D., Mahomoodally F., Suroowan S. Bioactive peptides and proteins as alternative antiplatelet drugs. Med Res Rev. 2019;39:2153–2171. doi: 10.1002/med.21579. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y.A., Yoon Y.H., Choi K., Kwon M., Goo S.H., Cha J.S. Enhanced oral bioavailability of morin administered in mixed micelle formulation with pluronic F127 and tween 80 in rats. Biol Pharm Bull. 2015;38:208–217. doi: 10.1248/bpb.b14-00508. [DOI] [PubMed] [Google Scholar]
- 4.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Walle C. 1st ed. Academic Press; London: 2011. Peptide and protein delivery. [Google Scholar]
- 6.Ismail R., Csoka I. Novel strategies in the oral delivery of antidiabetic peptide drugs—insulin, GLP 1 and its analogs. Eur J Pharm Biopharm. 2017;115:257–267. doi: 10.1016/j.ejpb.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Rader A.F.B., Weinmuller M., Reichart F., Schumacher-Klinger A., Merzbach S., Gilon C. Orally active peptides: is there a magic bullet? Angew Chem Int Ed. 2018;57:14414–14438. doi: 10.1002/anie.201807298. [DOI] [PubMed] [Google Scholar]
- 8.Drucker D.J. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19:277–289. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 9.Di L. Strategic approaches to optimizing peptide adme properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohley M., Haunberger A., Goepferich A.M. Intracellular availability of poorly soluble drugs from lipid nanocapsules. Eur J Pharm Biopharm. 2019;139:23–32. doi: 10.1016/j.ejpb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg M., Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 13.Morishita M., Peppas N.A. Is the oral route possible for peptide and protein drug delivery?. Drug Discov Today. 2006;11:905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Jain D., Mahammad S.S., Singh P.P., Kodipyaka R. A review on parenteral delivery of peptides and proteins. Drug Dev Ind Pharm. 2019;45:1403–1420. doi: 10.1080/03639045.2019.1628770. [DOI] [PubMed] [Google Scholar]
- 15.Chung S.W., Hil-lal T.A., Byun Y. Strategies for non-invasive delivery of biologics. J Drug Target. 2012;20:481–501. doi: 10.3109/1061186X.2012.693499. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S.R., Byun Y. Advances in oral macromolecular drug delivery. Expet Opin Drug Deliv. 2014;11:1955–1967. doi: 10.1517/17425247.2014.945420. [DOI] [PubMed] [Google Scholar]
- 17.Brayden D.J., Alonso M.J. Oral delivery of peptides: opportunities and issues for translation. Adv Drug Deliv Rev. 2016;106:193–195. doi: 10.1016/j.addr.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Moroz E., Matoori S., Leroux J.C. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. doi: 10.1016/j.addr.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Shaikh S., Jaiswal P. 2018. Oral proteins and peptides market overview.https://www.alliedmarketresearch.com/oral-proteins-peptides-market Available from: [Google Scholar]
- 20.Mahmood A., Bernkop-Schnurch A. SEDDS: a game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv Drug Deliv Rev. 2019;142:91–101. doi: 10.1016/j.addr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Maher S., Mrsny R.J., Brayden D.J. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319. doi: 10.1016/j.addr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Kou Y., Zhang X., Cheng H., Chen X., Mao S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expet Opin Drug Deliv. 2018;15:223–233. doi: 10.1080/17425247.2017.1395853. [DOI] [PubMed] [Google Scholar]
- 23.Duran-Lobato M., Niu Z., Alonso M.J. Oral delivery of biologics for precision medicine. Adv Mater. 2020;32 doi: 10.1002/adma.201901935. [DOI] [PubMed] [Google Scholar]
- 24.Smart A.L., Gaisford S., Basit A.W. Oral peptide and protein delivery: intestinal obstacles and commercial prospects. Expet Opin Drug Deliv. 2014;11:1323–1335. doi: 10.1517/17425247.2014.917077. [DOI] [PubMed] [Google Scholar]
- 25.Cao S.J., Xu S., Wang H.M., Ling Y., Dong J., Xia R.D. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech. 2019;20:190. doi: 10.1208/s12249-019-1325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan X., Liu X., Zhang Y., Zhang H., Lin X., Pu C. Silica nanoparticles on the oral delivery of insulin. Expet Opin Drug Deliv. 2018;15:805–820. doi: 10.1080/17425247.2018.1503250. [DOI] [PubMed] [Google Scholar]
- 27.Heinemann L., Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3:568–584. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison G.A. Insulin in alcoholic solution by the mouth. Br Med J. 1923;2:1204–1205. doi: 10.1136/bmj.2.3286.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson J.E.A. 1965. Oral blood sugar lowering compositions. US3172814 A. [Google Scholar]
- 30.Wong C.Y., Martinez J., Dass C.R. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol. 2016;68:1093–1108. doi: 10.1111/jphp.12607. [DOI] [PubMed] [Google Scholar]
- 31.Muheem A., Shakeel F., Jahangir M.A., Anwar M., Mallick N., Jain G.K. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharmaceut J. 2016;24:413–428. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stillhart C., Vučićević K., Augustijns P., Basit A.W., Batchelor H., Flanagan T.R. Impact of gastrointestinal physiology on drug absorption in special populations––an ungap review. Eur J Pharmaceut Sci. 2020;147:105280. doi: 10.1016/j.ejps.2020.105280. [DOI] [PubMed] [Google Scholar]
- 33.Koziolek M., Grimm M., Becker D., Iordanov V., Zou H., Shimizu J. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J Pharm Sci. 2015;104:2855–2863. doi: 10.1002/jps.24274. [DOI] [PubMed] [Google Scholar]
- 34.Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan M.S., Roberts M.S. Challenges and innovations of drug delivery in older age. Adv Drug Deliv Rev. 2018;135:3–38. doi: 10.1016/j.addr.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Mooij M.G., de Koning B.A.E., Huijsman M.L., de Wildt S.N. Ontogeny of oral drug absorption processes in children. Expet Opin Drug Metabol Toxicol. 2012;8:1293–1303. doi: 10.1517/17425255.2012.698261. [DOI] [PubMed] [Google Scholar]
- 37.Deng J., Zhu X., Chen Z., Fan C.H., Kwan H.S., Wong C.H. A review of food–drug interactions on oral drug absorption. Drugs. 2017;77:1833–1855. doi: 10.1007/s40265-017-0832-z. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki Y., Hada R., Nakajima H., Fukuda S., Munakata A. Improved localizing method of radiopill in measurement of entire gastrointestinal ph profiles: colonic luminal pH in normal subjects and patients with Crohn's disease. Am J Gastroenterol. 1997;92:114–118. [PubMed] [Google Scholar]
- 39.Fallingborg J., Christensen L.A., Jacobsen B.A., Rasmussen SNr. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig Dis Sci. 1993;38:1989–1993. doi: 10.1007/BF01297074. [DOI] [PubMed] [Google Scholar]
- 40.Lu P.J. Gastric juice acidity in upper gastrointestinal diseases. World J Gastroenterol. 2010;16:5496–5501. doi: 10.3748/wjg.v16.i43.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185:129–188. doi: 10.1016/s0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 42.Gracia R., Yus C., Abian O., Mendoza G., Irusta S., Sebastian V. Enzyme structure and function protection from gastrointestinal degradation using enteric coatings. Int J Biol Macromol. 2018;119:413–422. doi: 10.1016/j.ijbiomac.2018.07.143. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Li Y., Zou P., Wu M., Zhang Z., Zhang T. The effects of pharmaceutical excipients on gastrointestinal tract metabolic enzymes and transporters—an update. AAPS J. 2016;18:830–843. doi: 10.1208/s12248-016-9928-8. [DOI] [PubMed] [Google Scholar]
- 44.Bernkop-Schnürch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release. 1998;52:1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 45.Whitcomb D.C., Lowe M.E. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 46.Sanchón J., Fernández-Tomé S., Miralles B., Hernández-Ledesma B., Tomé D., Gaudichon C. Protein degradation and peptide release from milk proteins in human jejunum: comparison with in vitro gastrointestinal simulation. Food Chem. 2018;239:486–494. doi: 10.1016/j.foodchem.2017.06.134. [DOI] [PubMed] [Google Scholar]
- 47.Woodley J.F. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 1994;11:61–95. [PubMed] [Google Scholar]
- 48.Miner-Williams W.M., Stevens B.R., Moughan P.J. Are intact peptides absorbed from the healthy gut in the adult human?. Nutr Res Rev. 2015;27:308–329. doi: 10.1017/S0954422414000225. [DOI] [PubMed] [Google Scholar]
- 49.Cui M., Wu W., Hovgaard L., Lu Y., Chen D., Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int J Pharm. 2015;489:277–284. doi: 10.1016/j.ijpharm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 50.He H., Lu Y., Qi J., Zhao W., Dong X., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sinica B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Yadav V., Smart A.L., Tajiri S., Basit A.W. Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol Pharm. 2015;12:966–973. doi: 10.1021/mp500809f. [DOI] [PubMed] [Google Scholar]
- 52.Wu L., Shan W., Zhang Z., Huang Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv Drug Deliv Rev. 2018;124:150–163. doi: 10.1016/j.addr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Varum F.J., Veiga F., Sousa J.S., Basit A.W. Mucus thickness in the gastrointestinal tract of laboratory animals. J Pharm Pharmacol. 2012;64:218–227. doi: 10.1111/j.2042-7158.2011.01399.x. [DOI] [PubMed] [Google Scholar]
- 54.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles:the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;2012:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bansil R., Turner B.S. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15. doi: 10.1016/j.addr.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Corfield A.P., Carroll D., Myerscough N., Probert C. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 57.Murty V., Sarosiek J., Slomiany A., Slomiany B. Effect of lipids and proteins on the viscosity of gastric mucus glycoprotein. Biochem Biophys Res Commun. 1984;121:521–529. doi: 10.1016/0006-291x(84)90213-4. [DOI] [PubMed] [Google Scholar]
- 58.Demouveaux B., Gouyer V., Gottrand F., Narita T., Desseyn J.L. Gel-forming mucin interactome drives mucus viscoelasticity. Adv Colloid Interface Sci. 2018;252:69–82. doi: 10.1016/j.cis.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Lichtenberger L. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 60.Homayun B., Lin X., Choi H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11:129. doi: 10.3390/pharmaceutics11030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boegh M., Nielsen H.M. Mucus as a barrier to drug delivery–understanding and mimicking the barrier properties. Basic Clin Pharmacol Toxicol. 2015;116:179–186. doi: 10.1111/bcpt.12342. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X., Cheng H., Dong W., Zhang M., Liu Q., Wang X. Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J Control Release. 2018;272:29–38. doi: 10.1016/j.jconrel.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 63.Qi J., Zhuang J., Lv Y., Lu Y., Wu W. Exploiting or overcoming the dome trap for enhanced oral immunization and drug delivery. J Control Release. 2018;275:92–106. doi: 10.1016/j.jconrel.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Cheng H., Leblond C. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine: unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 65.Capaldo C.T., Powell D.N., Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med. 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expet Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target?. Nat Rev Gastroenterol Hepatol. 2017;14:9. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owen R.L., Jones A.L. Epithelial cell specialization within human peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 69.Jung C., Hugot J.P., Barreau F. Peyer's patches: the immune sensors of the intestine. Int J Inflamm. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 71.Anderson J.M., Balda M.S., Fanning A.S. The structure and regulation of tight junctions. Curr Opin Cell Biol. 1993;5:772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- 72.Anderson J., Van Itallie C. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol-gastr L. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antosova Z., Mackova M., Kral V., Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Maher S., Brayden D.J. Overcoming poor permeability: translating permeation enhancers for oral peptide delivery. Drug Discov Today. 2012;9:e113–e119. doi: 10.1016/j.ddtec.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Wolf J.L., Rubin D.H., Finberg R., Kauffman R.S., Sharpe A.H., Trier J.S. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 77.Singh B., Maharjan S., Jiang T., Kang S.K., Choi Y.J., Cho C.S. Combinatorial approach of antigen delivery using M cell-homing peptide and mucoadhesive vehicle to enhance the efficacy of oral vaccine. Mol Pharm. 2015;12:3816–3828. doi: 10.1021/acs.molpharmaceut.5b00265. [DOI] [PubMed] [Google Scholar]
- 78.Ménard S., Lebreton C., Schumann M., Matysiak-Budnik T., Dugave C., Bouhnik Y. Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am J Pathol. 2012;180:608–615. doi: 10.1016/j.ajpath.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 79.Tyagi P., Pechenov S., Subramony J.A. Oral peptide delivery: translational challenges due to physiological effects. J Control Release. 2018;287:167–176. doi: 10.1016/j.jconrel.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 80.Boronikolos G.C., Menge B.A., Schenker N., Breuer T.G., Otte J.M., Heckermann S. Upper gastrointestinal motility and symptoms in individuals with diabetes, prediabetes and normal glucose tolerance. Diabetologia. 2015;58:1175–1182. doi: 10.1007/s00125-015-3538-3. [DOI] [PubMed] [Google Scholar]
- 81.Sugihara M., Takeuchi S., Sugita M., Higaki K., Kataoka M., Yamashita S. Analysis of intra- and inter- subject variability in oral drug absorption in human bioequivalence studies of 113 generic products. Mol Pharm. 2015;12:4405–4413. doi: 10.1021/acs.molpharmaceut.5b00602. [DOI] [PubMed] [Google Scholar]
- 82.Jamal Azam Y., Machavaram K.K., Rostami-Hodjegan A. The modulating effects of endogenous substances on drug metabolising enzymes and implications for inter-individual variability and quantitative prediction. Curr Drug Metabol. 2014;15:599–619. doi: 10.2174/1389200215666140926151642. [DOI] [PubMed] [Google Scholar]
- 83.Bois F.Y., Jamei M., Clewell H.J. PBPK modelling of inter-individual variability in the pharmacokinetics of environmental chemicals. Toxicology. 2010;278:256–267. doi: 10.1016/j.tox.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Gervasi V., Agnol R.D., Cullen S., McCoy T., Vucen S., Crean A. Parenteral protein formulations: an overview of approved products within the European Union. Eur J Pharm Biopharm. 2018;131:8–24. doi: 10.1016/j.ejpb.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 85.Krause M.E., Sahin E. Chemical and physical instabilities in manufacturing and storage of therapeutic proteins. Curr Opin Biotechnol. 2019;60:159–167. doi: 10.1016/j.copbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Duerr C., Friess W. Antibody–drug conjugates—stability and formulation. Eur J Pharm Biopharm. 2019;139:168–176. doi: 10.1016/j.ejpb.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Ohtake S. Science and art of protein formulation development. Int J Pharm. 2019;568:118505. doi: 10.1016/j.ijpharm.2019.118505. [DOI] [PubMed] [Google Scholar]
- 88.Pace C.N., Fu H., Fryar K.L., Landua J., Trevino S.R., Shirley B.A. Contribution of hydrophobic interactions to protein stability. J Mol Biol. 2011;408:514–528. doi: 10.1016/j.jmb.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nick Pace C., Scholtz J.M., Grimsley G.R. Forces stabilizing proteins. FEBS Lett. 2014;588:2177–2184. doi: 10.1016/j.febslet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oyetayo O.O., Méndez-Lucio O., Bender A., Kiefer H. Diversity selection, screening and quantitative structure–activity relationships of osmolyte-like additive effects on the thermal stability of a monoclonal antibody. Eur J Pharmaceut Sci. 2017;97:151–157. doi: 10.1016/j.ejps.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 91.Singh S.N., Kumar S., Bondar V., Wang N., Forcino R., Colandene J. Unexplored benefits of controlled ice nucleation: lyophilization of a highly concentrated monoclonal antibody solution. Int J Pharm. 2018;552:171–179. doi: 10.1016/j.ijpharm.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 92.Qian J., Yearley E., Tian S., Jing L., Balsaraf A., Lo Surdo P. Non-enzymatic and site-specific glycan shedding: a novel protein degradation pathway observed in a stabilized form of RSV prefusion F protein. Anal Chem. 2018;90:10897–10902. doi: 10.1021/acs.analchem.8b02402. [DOI] [PubMed] [Google Scholar]
- 93.Calero-Rubio C., Ghosh R., Saluja A., Roberts C.J. Predicting protein‒protein interactions of concentrated antibody solutions using dilute solution data and coarse-grained molecular models. J Pharm Sci. 2018;107:1269–1281. doi: 10.1016/j.xphs.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi S., Hashemi V., Wang S.C., Yang J., Yang M., Semple A. Overcoming challenges with intravenous administration of an investigational protein therapeutic. J Pharm Sci. 2017;106:3465–3473. doi: 10.1016/j.xphs.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Falconer R.J. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol Adv. 2019;37 doi: 10.1016/j.biotechadv.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Cirkovas A., Sereikaite J. Different effects of L-arginine on the heat-induced unfolding and aggregation of proteins. Biologicals. 2011;39:181–188. doi: 10.1016/j.biologicals.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Geraldes D.C., Beraldo-de-Araújo V.L., Pardo B.O.P., Pessoa Junior A., Stephano M.A., de Oliveira-Nascimento L. Protein drug delivery: current dosage form profile and formulation strategies. J Drug Target. 2020;28:339–355. doi: 10.1080/1061186X.2019.1669043. [DOI] [PubMed] [Google Scholar]
- 98.Bane J., Mozziconacci O., Yi L., Wang Y.J., Sreedhara A., Schöneich C. Photo-oxidation of IGG1 and model peptides: detection and analysis of triply oxidized his and trp side chain cleavage products. Pharm Res. 2017;34:229–242. doi: 10.1007/s11095-016-2058-2. [DOI] [PubMed] [Google Scholar]
- 99.Kamerzell T.J., Esfandiary R., Joshi S.B., Middaugh C.R., Volkin D.B. Protein–excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63:1118–1159. doi: 10.1016/j.addr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Forney-Stevens K.M., Bogner R.H., Pikal M.J. Addition of amino acids to further stabilize lyophilized sucrose-based protein formulations: I. screening of 15 amino acids in two model proteins. J Pharm Sci. 2016;105:697–704. doi: 10.1002/jps.24655. [DOI] [PubMed] [Google Scholar]
- 101.Bhatnagar B.S., Bogner R.H., Pikal M.J. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharmaceut Dev Technol. 2007;12:505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 102.Arsiccio A., Pisano R. Surfactants as stabilizers for biopharmaceuticals: an insight into the molecular mechanisms for inhibition of protein aggregation. Eur J Pharm Biopharm. 2018;128:98–106. doi: 10.1016/j.ejpb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Semenyuk P., Muronetz V. Protein interaction with charged macromolecules: from model polymers to unfolded proteins and post-translational modifications. Int J Mol Sci. 2019;20:1252. doi: 10.3390/ijms20051252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yahyaei M., Mehrnejad F., Naderi-Manesh H., Rezayan A.H. Protein adsorption onto polysaccharides: comparison of chitosan and chitin polymers. Carbohydr Polym. 2018;191:191–197. doi: 10.1016/j.carbpol.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 105.Mat D.J.L., Cattenoz T., Souchon I., Michon C., Le Feunteun S. Monitoring protein hydrolysis by pepsin using pH-stat: in vitro gastric digestions in static and dynamic ph conditions. Food Chem. 2018;239:268–275. doi: 10.1016/j.foodchem.2017.06.115. [DOI] [PubMed] [Google Scholar]
- 106.Xu B., Zhang W., Chen Y., Xu Y., Wang B., Zong L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int J Biol Macromol. 2018;113:534–542. doi: 10.1016/j.ijbiomac.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 107.Schilling R.J., Mitra A.K. Degradation of insulin by trypsin and alpha-chymotrypsin. Pharm Res. 1991;8:721–727. doi: 10.1023/a:1015893832222. [DOI] [PubMed] [Google Scholar]
- 108.Welling S.H., Hubálek F., Jacobsen J., Brayden D.J., Rahbek U.L., Buckley S.T. The role of citric acid in oral peptide and protein formulations: relationship between calcium chelation and proteolysis inhibition. Eur J Pharm Biopharm. 2014;86:544–551. doi: 10.1016/j.ejpb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 109.Lee Y.H., Perry B.A., Labruno S., Lee H.S., Stern W., Falzone L.M. Impact of regional intestinal ph modulation on absorption of peptide drugs: oral absorption studies of salmon calcitonin in beagle dogs. Pharm Res. 1999;16:1233–1239. doi: 10.1023/a:1014849630520. [DOI] [PubMed] [Google Scholar]
- 110.Binkley N., Bolognese M., Sidorowicz-Bialynicka A., Vally T., Trout R., Miller C. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the oral calcitonin in postmenopausal osteoporosis (oracal) trial. J Bone Miner Res. 2012;27:1821–1829. doi: 10.1002/jbmr.1602. [DOI] [PubMed] [Google Scholar]
- 111.Choonara B.F., Choonara Y.E., Kumar P., Bijukumar D., du Toit L.C., Pillay V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol Adv. 2014;32:1269–1282. doi: 10.1016/j.biotechadv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Niu M., Lu Y., Hovgaard L., Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomed. 2011;6:1155. doi: 10.2147/IJN.S19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Agrwal V., Reddy I.K., Khan M.A. Oral delivery of proteins: effect of chicken and duck ovomucoid on the stability of insulin in the presence of α-chymotrypsin and trypsin. Pharm Pharmcol Commun. 2000;6:223–227. [Google Scholar]
- 114.Arbit E., Kidron M. Oral insulin delivery in a physiologic context. J Diabetes Sci Technol. 2017;11:825–832. doi: 10.1177/1932296817691303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Del Curto M.D., Maroni A., Palugan L., Zema L., Gazzaniga A., Sangalli M.E. Oral delivery system for two-pulse colonic release of protein drugs and protease inhibitor/absorption enhancer compounds. J Pharm Sci. 2011;100:3251–3259. doi: 10.1002/jps.22560. [DOI] [PubMed] [Google Scholar]
- 116.Maderuelo C., Lanao J.M., Zarzuelo A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur J Pharmaceut Sci. 2019;138:105019. doi: 10.1016/j.ejps.2019.105019. [DOI] [PubMed] [Google Scholar]
- 117.Nikam V.K., Kotade K., Gaware V., Dolas R., Dhamak K., Somwanshi S. Eudragit a versatile polymer: a review. Pharmacologyonline. 2011;1:152–164. [Google Scholar]
- 118.Werle M., Makhlof A., Takeuchi H. Oral protein delivery: a patent review of academic and industrial approaches. Recent Pat Drug Deliv Formulation. 2009;3:94–104. doi: 10.2174/187221109788452221. [DOI] [PubMed] [Google Scholar]
- 119.Li P., Tan A., Prestidge C.A., Nielsen H.M., Müllertz A. Self-nanoemulsifying drug delivery systems for oral insulin delivery: in vitro and in vivo evaluations of enteric coating and drug loading. Int J Pharm. 2014;477:390–398. doi: 10.1016/j.ijpharm.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 120.Sonaje K., Chen Y.J., Chen H.L., Wey S.P., Juang J.H., Nguyen H.N. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 121.Makhlof A., Tozuka Y., Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharmaceut Sci. 2011;42:445–451. doi: 10.1016/j.ejps.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 122.Sinha V.R., Singh A., Kumar R.V., Singh S., Kumria R., Bhinge J. Oral colon-specific drug delivery of protein and peptide drugs. Crit Rev Ther Drug. 2007;24 doi: 10.1615/critrevtherdrugcarriersyst.v24.i1.30. [DOI] [PubMed] [Google Scholar]
- 123.Patel M.M., Shah T., Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit Rev Ther Drug. 2007;24 doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 124.Chen S., Guo F., Deng T., Zhu S., Liu W., Zhong H. Eudragit S100-coated chitosan nanoparticles co-loading TAT for enhanced oral colon absorption of insulin. AAPS PharmSciTech. 2017;18:1277–1287. doi: 10.1208/s12249-016-0594-z. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Chi C., Huang X., Zou Q., Li X., Chen L. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract. Carbohydr Polym. 2017;171:242–251. doi: 10.1016/j.carbpol.2017.04.090. [DOI] [PubMed] [Google Scholar]
- 126.Meneguin A.B., Beyssac E., Garrait G., Hsein H., Cury B.S. Retrograded starch/pectin coated gellan gum-microparticles for oral administration of insulin: a technological platform for protection against enzymatic degradation and improvement of intestinal permeability. Eur J Pharm Biopharm. 2018;123:84–94. doi: 10.1016/j.ejpb.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 127.Kumar K.V., Sivakumar T., Mani T.T. Colon targeting drug delivery system: a review on recent approaches. Int J Pharma Bio Sci. 2011;2:11–19. [Google Scholar]
- 128.Ye C., Chi H. A review of recent progress in drug and protein encapsulation: approaches, applications and challenges. Mater Sci Eng C. 2018;83:233–246. doi: 10.1016/j.msec.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Onuigbo E., Iseghohimhen J., Chah K., Gyang M., Attama A. Chitosan/alginate microparticles for the oral delivery of fowl typhoid vaccine: innate and acquired immunity. Vaccine. 2018;36:4973–4978. doi: 10.1016/j.vaccine.2018.05.087. [DOI] [PubMed] [Google Scholar]
- 130.Li D., Zhuang J., He H., Jiang S., Banerjee A., Lu Y. Influence of particle geometry on gastrointestinal transit and absorption following oral administration. ACS Appl Mater Interfaces. 2017;9:42492–42502. doi: 10.1021/acsami.7b11821. [DOI] [PubMed] [Google Scholar]
- 131.Banerjee A., Qi J., Gogoi R., Wong J., Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roberts M., Bentley M., Harris J. Chemistry for peptide and protein pegylation. Adv Drug Deliv Rev. 2002;54:459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 133.Aguirre T.A., Teijeiro-Osorio D., Rosa M., Coulter I., Alonso M., Brayden D. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv Drug Deliv Rev. 2016;106:223–241. doi: 10.1016/j.addr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 134.Nojima Y., Suzuki Y., Iguchi K., Shiga T., Iwata A., Fujimoto T. Development of poly(ethylene glycol) conjugated lactoferrin for oral administration. Bioconjugate Chem. 2008;19:2253–2259. doi: 10.1021/bc800258v. [DOI] [PubMed] [Google Scholar]
- 135.Ryan S.M., Wang X., Mantovani G., Sayers C.T., Haddleton D.M., Brayden D.J. Conjugation of salmon calcitonin to a combed-shaped end functionalized poly(poly (ethylene glycol)methyl ether methacrylate) yields a bioactive stable conjugate. J Control Release. 2009;135:51–59. doi: 10.1016/j.jconrel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 136.Gentilucci L., De Marco R., Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharmaceut Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 137.Conibear A.C., Chaousis S., Durek T., Johan Rosengren K., Craik D.J., Schroeder C.I. Approaches to the stabilization of bioactive epitopes by grafting and peptide cyclization. Pept Sci. 2016;106:89–100. doi: 10.1002/bip.22767. [DOI] [PubMed] [Google Scholar]
- 138.Zhang R.Y., Thapa P., Espiritu M.J., Menon V., Bingham J.P. From nature to creation: going around in circles, the art of peptide cyclization. Bioorg Med Chem. 2018;26:1135–1150. doi: 10.1016/j.bmc.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 139.Matsui K., Kimura T., Ota K., Iitake K., Shoji M., Inoue M. Resistance of 1-deamino[8-d-argininei]-vasopressin to in vitro degradation as compared with arginine vasopressin. Endocrinol Jpn. 1985;32:547–557. doi: 10.1507/endocrj1954.32.547. [DOI] [PubMed] [Google Scholar]
- 140.White C.J., Yudin A.K. Contemporary strategies for peptide macrocyclization. Nat Chem. 2011;3:509. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 141.Bogdanowich-Knipp S.J., Chakrabarti S., Siahaan T.J., Williams T.D., Dillman R.K. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 142.Nielsen D.S., Shepherd N.E., Xu W., Lucke A.J., Stoermer M.J., Fairlie D.P. Orally absorbed cyclic peptides. Chem Rev. 2017;117:8094–8128. doi: 10.1021/acs.chemrev.6b00838. [DOI] [PubMed] [Google Scholar]
- 143.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ensign L.M., Schneider C., Suk J.S., Cone R., Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater. 2012;24:3887–3894. doi: 10.1002/adma.201201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu T., Chisholm J., Choi W.J., Anonuevo A., Pulicare S., Zhong W. Mucus-penetrating nanosuspensions for enhanced delivery of poorly soluble drugs to mucosal surfaces. Adv Healthc Mater. 2016;5:2745–2750. doi: 10.1002/adhm.201600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suk J.S., Lai S.K., Boylan N.J., Dawson M.R., Boyle M.P., Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine. 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan J., Iiboshi Y., Cui L., Wasa M., Okada A. Role of intestinal mucus on the uptake of latex beads by peyer's patches and on their transport to mesenteric lymph nodes in rats. Jpen-parenter Enter. 1999;23:19–23. doi: 10.1177/014860719902300119. [DOI] [PubMed] [Google Scholar]
- 148.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maisel K., Ensign L., Reddy M., Cone R., Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release. 2015;197:48–57. doi: 10.1016/j.jconrel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huckaby J.T., Lai S.K. Pegylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. doi: 10.1016/j.addr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 151.Popov A., Enlow E., Bourassa J., Chen H. Mucus-penetrating nanoparticles made with “mucoadhesive” poly(vinyl alcohol) Nanomedicine. 2016;12:1863–1871. doi: 10.1016/j.nano.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 152.Shan W., Zhu X., Liu M., Li L., Zhong J., Sun W. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano. 2015;9:2345–2356. doi: 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- 153.Wang A., Yang T., Fan W., Yang Y., Zhu Q., Guo S. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Adv Healthc Mater. 2019;8:1801123. doi: 10.1002/adhm.201801123. [DOI] [PubMed] [Google Scholar]
- 154.Wu J., Zheng Y., Liu M., Shan W., Zhang Z., Huang Y. Biomimetic virus like and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl Mater Interfaces. 2018;10:9916–9928. doi: 10.1021/acsami.7b16524. [DOI] [PubMed] [Google Scholar]
- 155.Guo M., Wei M., Li W., Guo M., Guo C., Ma M. Impacts of particle shapes on the oral delivery of drug nanocrystals: mucus permeation, transepithelial transport and bioavailability. J Control Release. 2019;307:64–75. doi: 10.1016/j.jconrel.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 156.Yu M., Wang J., Yang Y., Zhu C., Su Q., Guo S. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett. 2016;16:7176–7182. doi: 10.1021/acs.nanolett.6b03515. [DOI] [PubMed] [Google Scholar]
- 157.Dünnhaupt S., Kammona O., Waldner C., Kiparissides C., Bernkop-Schnürch A. Nano-carrier systems: strategies to overcome the mucus gel barrier. Eur J Pharm Biopharm. 2015;96:447–453. doi: 10.1016/j.ejpb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 158.Zupančič O., Grieβinger J.A., Rohrer J., de Sousa I.P., Danninger L., Partenhauser A. Development, in vitro and in vivo evaluation of a self-emulsifying drug delivery system (sedds) for oral enoxaparin administration. Eur J Pharm Biopharm. 2016;109:113–121. doi: 10.1016/j.ejpb.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 159.Florey H.W. Secretion and function of intestinal mucus. Gastroenterology. 1962;43:326–329. [PubMed] [Google Scholar]
- 160.Chickering D., Iii, Jacob J., Desai T., Harrison M., Harris W., Morrell C. Bioadhesive microspheres: III. an in vivo transit and bioavailability study of drug-loaded alginate and poly(fumaric-co-sebacic anhydride) microspheres. J Control Release. 1997;48:35–46. [Google Scholar]
- 161.Eldridge J.H., Hammond C.J., Meulbroek J.A., Staas J.K., Gilley R.M., Tice T.R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer's patches. J Control Release. 1990;11:205–214. [Google Scholar]
- 162.Chen F., Zhang Z.R., Yuan F., Qin X., Wang M., Huang Y. In vitro and in vivo study of n-trimethyl chitosan nanoparticles for oral protein delivery. Int J Pharm. 2008;349:226–233. doi: 10.1016/j.ijpharm.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 163.Bernkop-Schnürch A. Thiomers: a new generation of mucoadhesive polymers. Adv Drug Deliv Rev. 2005;57:1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 164.Bernkop-Schnürch A., Hornof M., Zoidl T. Thiolated polymers—thiomers: synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int J Pharm. 2003;260:229–237. doi: 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 165.Yin L., Ding J., He C., Cui L., Tang C., Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30:5691–5700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 166.Lehr C.M. Lectin-mediated drug delivery: the second generation of bioadhesives. J Control Release. 2000;65:19–29. doi: 10.1016/s0168-3659(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 167.Leong K.H., Chung L.Y., Noordin M.I., Onuki Y., Morishita M., Takayama K. Lectin-functionalized carboxymethylated kappa-carrageenan microparticles for oral insulin delivery. Carbohydr Polym. 2011;86:555–565. [Google Scholar]
- 168.Zhang N., Ping Q., Huang G., Xu W., Cheng Y., Han X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327:153–159. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 169.Teutonico D., Ponchel G. Patches for improving gastrointestinal absorption: an overview. Drug Discov Today. 2011;16:991–997. doi: 10.1016/j.drudis.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 170.Banerjee A., Mitragotri S. Intestinal patch systems for oral drug delivery. Curr Opin Pharmacol. 2017;36:58–65. doi: 10.1016/j.coph.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 171.Shen Z., Mitragotri S. Intestinal patches for oral drug delivery. Pharm Res. 2002;19:391–395. doi: 10.1023/a:1015118923204. [DOI] [PubMed] [Google Scholar]
- 172.Gupta V., Hwang B.H., Lee J., Anselmo A.C., Doshi N., Mitragotri S. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release. 2013;172:753–762. doi: 10.1016/j.jconrel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 173.Whitehead K., Shen Z., Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J Control Release. 2004;98:37–45. doi: 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 174.Banerjee A., Wong J., Gogoi R., Brown T., Mitragotri S. Intestinal micropatches for oral insulin delivery. J Drug Target. 2017;25:608–615. doi: 10.1080/1061186X.2017.1300664. [DOI] [PubMed] [Google Scholar]
- 175.Toorisaka E., Watanabe K., Ono H., Hirata M., Kamiya N., Goto M. Intestinal patches with an immobilized solid-in-oil formulation for oral protein delivery. Acta Biomater. 2012;8:653–658. doi: 10.1016/j.actbio.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 176.Gupta V., Hwang B.H., Doshi N., Banerjee A., Anselmo A.C., Mitragotri S. Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann Biomed Eng. 2016;44:1993–2007. doi: 10.1007/s10439-016-1558-x. [DOI] [PubMed] [Google Scholar]
- 177.Peppas N.A., Wood K.M., Blanchette J.O. Hydrogels for oral delivery of therapeutic proteins. Expet Opin Biol Ther. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 178.Kamei N., Morishita M., Chiba H., Kavimandan N.J., Peppas N.A., Takayama K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J Control Release. 2009;134:98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakamura K., Murray R.J., Joseph J.I., Peppas N.A., Morishita M., Lowman A.M. Oral insulin delivery using p(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release. 2004;95:589–599. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 180.Dorkoosh F., Verhoef J.C., Borchard G., Rafiee-Tehrani M., Verheijden J., Junginger H. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm. 2002;247:47–55. doi: 10.1016/s0378-5173(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 181.Yin L., Fei L., Cui F., Tang C., Yin C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials. 2007;28:1258–1266. doi: 10.1016/j.biomaterials.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 182.Yin L., Ding J., Zhang J., He C., Tang C., Yin C. Polymer integrity related absorption mechanism of superporous hydrogel containing interpenetrating polymer networks for oral delivery of insulin. Biomaterials. 2010;31:3347–3356. doi: 10.1016/j.biomaterials.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 183.Pauletti G.M., Gangwar S., Siahaan T.J., Jeffrey A., Borchardt R T. Improvement of oral peptide bioavailability: peptidomimetics and prodrug strategies. Adv Drug Deliv Rev. 1997;27:235–256. doi: 10.1016/s0169-409x(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 184.Borchardt R.T. Optimizing oral absorption of peptides using prodrug strategies. J Control Release. 1999;62:231–238. doi: 10.1016/s0168-3659(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 185.Hashizume M., Douen T., Murakami M., Yamamoto A., Takada K., Muranishi S. Improvement of large intestinal absorption of insulin by chemical modification with palmitic acid in rats. J Pharm Pharmacol. 1992;44:555–559. doi: 10.1111/j.2042-7158.1992.tb05463.x. [DOI] [PubMed] [Google Scholar]
- 186.Shen W.C. Oral peptide and protein delivery: unfulfilled promises? Drug Discov Today. 2003;8:607–608. doi: 10.1016/s1359-6446(03)02692-8. [DOI] [PubMed] [Google Scholar]
- 187.Ekrami H.M., Kennedy A.R., Shen W.C. Water-soluble fatty acid derivatives as acylating agents for reversible lipidization of polypeptides. FEBS Lett. 1995;371:283–286. doi: 10.1016/0014-5793(95)00910-2. [DOI] [PubMed] [Google Scholar]
- 188.Wang J., Chow D., Heiati H., Shen W.C. Reversible lipidization for the oral delivery of salmon calcitonin. J Control Release. 2003;88:369–380. doi: 10.1016/s0168-3659(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 189.Vadlapudi A.D., Vadlapatla R.K., Kwatra D., Earla R., Samanta S.K., Pal D. Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int J Pharm. 2012;434:315–324. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Windsor E., Cronheim G.E. Gastro-intestinal absorption of heparin and synthetic heparinoids. Nature. 1961;190:263–264. doi: 10.1038/190263a0. [DOI] [PubMed] [Google Scholar]
- 191.Su F.Y., Lin K.J., Sonaje K., Wey S.P., Yen T.C., Ho Y.C. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33:2801–2811. doi: 10.1016/j.biomaterials.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 192.Niu M., Tan Y.N., Guan P., Hovgaard L., Lu Y., Qi J. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int J Pharm. 2014;460:119–130. doi: 10.1016/j.ijpharm.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 193.Hu S., Niu M., Hu F., Lu Y., Qi J., Yin Z., Wu W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int J Pharm. 2013;441:693–700. doi: 10.1016/j.ijpharm.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 194.Niu M., Lu Y., Hovgaard L., Guan P., Tan Y., Lian R. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm. 2012;81:265–272. doi: 10.1016/j.ejpb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 195.Malkov D., Angelo R., Wang H.Z., Flanders E., Tang H., Gomez-Orellana I. Oral delivery of insulin with the eligen® technology: mechanistic studies. Curr Drug Deliv. 2005;2:191–197. doi: 10.2174/1567201053586001. [DOI] [PubMed] [Google Scholar]
- 196.Rivera T.M., Leone-Bay A., Paton D.R., Leipold H.R., Baughman R.A. Oral delivery of heparin in combination with sodium N-[8-(2-hydroxybenzoyl) amino] caprylate: pharmacological considerations. Pharm Res. 1997;14:1830. doi: 10.1023/a:1012160703533. [DOI] [PubMed] [Google Scholar]
- 197.Twarog C., Fattah S., Heade J., Maher S., Fattal E., Brayden D.J. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and sodium caprate (C10) Pharmaceutics. 2019;11:78. doi: 10.3390/pharmaceutics11020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McGavigan A.K., Murphy K.G. Gut hormones: the future of obesity treatment?. Br J Clin Pharmacol. 2012;74:911–919. doi: 10.1111/j.1365-2125.2012.04278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bucheit J.D., Pamulapati L.G., Carter N., Malloy K., Dixon D.L., Sisson E.M. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol Therapeut. 2020;22:10–18. doi: 10.1089/dia.2019.0185. [DOI] [PubMed] [Google Scholar]
- 200.Brayden D., Creed E., O'connell A., Leipold H., Agarwal R., Leone-Bay A. Heparin absorption across the intestine: effects of sodium N-[8-(2-hydroxybenzoyl) amino] caprylate in rat in situ intestinal instillations and in Caco-2 monolayers. Pharm Res. 1997;14:1772–1779. doi: 10.1023/a:1012192115828. [DOI] [PubMed] [Google Scholar]
- 201.Arbit E., Goldberg M., Gomez-Orellana I., Majuru S. Oral heparin: status review. Thromb J. 2006;4:6. doi: 10.1186/1477-9560-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fasano A., Nataro J.P. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv Drug Deliv Rev. 2004;56:795–807. doi: 10.1016/j.addr.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 203.Fasano A., Fiorentini C., Donelli G., Uzzau S., Kaper J.B., Margaretten K. Zonula occludens toxin modulates tight junctions through protein kinase c-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Di Pierro M., Lu R., Uzzau S., Wang W., Margaretten K., Pazzani C. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem. 2001;276:19160–19165. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- 205.Gopalakrishnan S., Pandey N., Tamiz A.P., Vere J., Carrasco R., Somerville R. Mechanism of action of ZOT-derived peptide AT-1002, a tight junction regulator and absorption enhancer. Int J Pharm. 2009;365:121–130. doi: 10.1016/j.ijpharm.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 206.Kelly C.P., Green P.H.R., Murray J.A., DiMarino A., Colatrella A., Leffler D.A. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–262. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- 207.Tavelin S., Hashimoto K., Malkinson J., Lazorova L., Toth I., Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol. 2003;64:1530–1540. doi: 10.1124/mol.64.6.1530. [DOI] [PubMed] [Google Scholar]
- 208.Sonoda N., Furuse M., Sasaki H., Yonemura S., Katahira J., Horiguchi Y. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Böhmová E., Machová D., Pechar M., Pola R., Venclíková K., Janoušková O. Cell-penetrating peptides: a useful tool for the delivery of various cargoes into cells. Physiol Res. 2018;67:S267–S279. doi: 10.33549/physiolres.933975. [DOI] [PubMed] [Google Scholar]
- 210.Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 211.Sánchez-Navarro M., Garcia J., Giralt E., Teixidó M. Using peptides to increase transport across the intestinal barrier. Adv Drug Deliv Rev. 2016;106:355–366. doi: 10.1016/j.addr.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 212.Danielsen E.M., Hansen G.H. Impact of cell-penetrating peptides (CPPs) melittin and HIV-1 TAT on the enterocyte brush border using a mucosal explant system. Biochim Biophys Acta-Biomembranes. 2018;1860:1589–1599. doi: 10.1016/j.bbamem.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 213.Zhang L., Shi Y., Song Y., Sun X., Zhang X., Sun K. The use of low molecular weight protamine to enhance oral absorption of exenatide. Int J Pharm. 2018;547:265–273. doi: 10.1016/j.ijpharm.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 214.Kamei N., Shigei C., Hasegawa R., Takeda-Morishita M. Exploration of the key factors for optimizing the in vivo oral delivery of insulin by using a noncovalent strategy with cell-penetrating peptides. Biol Pharm Bull. 2018;41:239–246. doi: 10.1248/bpb.b17-00798. [DOI] [PubMed] [Google Scholar]
- 215.Zhang X., Wu W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov Today. 2014;19:898–904. doi: 10.1016/j.drudis.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 216.Jain S., Rathi V.V., Jain A.K., Das M., Godugu C. Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine. 2012;7:1311–1337. doi: 10.2217/nnm.12.31. [DOI] [PubMed] [Google Scholar]
- 217.Zhang X., Qi J., Lu Y., He W., Li X., Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 2014;10:167–176. doi: 10.1016/j.nano.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 218.Chalasani K.B., Russell-Jones G.J., Jain A.K., Diwan P.V., Jain S.K. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J Control Release. 2007;122:141–150. doi: 10.1016/j.jconrel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 219.Verma A., Sharma S., Gupta P.K., Singh A., Teja B.V., Dwivedi P. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: a mucoadhesive and ph responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016;31:288–300. doi: 10.1016/j.actbio.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 220.Zhang J., Tang C., Yin C. Galactosylated trimethyl chitosan–cysteine nanoparticles loaded with MAP4k4 siRNA for targeting activated macrophages. Biomaterials. 2013;34:3667–3677. doi: 10.1016/j.biomaterials.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 221.Byeon J.C., Lee S.E., Kim H., Ahn J.B., Kim D.H., Choi J.S. Design of novel proliposome formulation for antioxidant peptide, glutathione with enhanced oral bioavailability and stability. Drug Deliv. 2019;26:216–225. doi: 10.1080/10717544.2018.1551441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.East L., Isacke C.M. The mannose receptor family. Biochim Biophys Acta Gen Subj. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 223.Liu D., Jiang G., Yu W., Li L., Tong Z., Kong X. Oral delivery of insulin using CaCO3-based composite nanocarriers with hyaluronic acid coatings. Mater Lett. 2017;188:263–266. [Google Scholar]
- 224.Amet N., Wang W., Shen W.C. Human growth hormone–transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141:177–182. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R., Blumberg R.S. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang N., Ping Q.N., Huang G.H., Xu W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 227.Liu C., Shan W., Liu M., Zhu X., Xu J., Xu Y. A novel ligand conjugated nanoparticles for oral insulin delivery. Drug Deliv. 2016;23:2015–2025. doi: 10.3109/10717544.2015.1058433. [DOI] [PubMed] [Google Scholar]
- 228.Jin Y., Song Y., Zhu X., Zhou D., Chen C., Zhang Z. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 229.Russell-Jones G. Intestinal receptor targeting for peptide delivery: an expert's personal perspective on reasons for failure and new opportunities. Ther Deliv. 2011;2:1575–1593. doi: 10.4155/tde.11.129. [DOI] [PubMed] [Google Scholar]
- 230.Hamman J.H., Demana P.H., Olivier E.I. Targeting receptors, transporters and site of absorption to improve oral drug delivery. Drug Target Insights. 2007;2:71–81. [PMC free article] [PubMed] [Google Scholar]
- 231.Xie S., Gong Y.C., Xiong X.Y., Li Z.L., Luo Y.Y., Li Y.P. Targeted folate-conjugated pluronic P85/poly(lactide-co-glycolide) polymersome for the oral delivery of insulin. Nanomedicine. 2018;13:2527–2544. doi: 10.2217/nnm-2017-0372. [DOI] [PubMed] [Google Scholar]
- 232.Kokrashvili Z., Mosinger B., Margolskee R.F. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90 doi: 10.3945/ajcn.2009.27462T. 822s-5s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li Y., Yang B., Zhang X. Oral delivery of imatinib through galactosylated polymeric nanoparticles to explore the contribution of a saccharide ligand to absorption. Int J Pharm. 2019;568:118508. doi: 10.1016/j.ijpharm.2019.118508. [DOI] [PubMed] [Google Scholar]
- 234.Fievez V., Plapied L., des Rieux A., Pourcelle V., Freichels H., Wascotte V. Targeting nanoparticles to m cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73:16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 235.Ganugula R., Arora M., Guada M., Saini P., Kumar M.N.V.R. Noncompetitive active transport exploiting intestinal transferrin receptors for oral delivery of proteins by tunable nanoplatform. ACS Macro Lett. 2017;6:161–164. doi: 10.1021/acsmacrolett.7b00035. [DOI] [PubMed] [Google Scholar]
- 236.Yong J.M., Mantaj J., Cheng Y., Vllasaliu D. Delivery of nanoparticles across the intestinal epithelium via the transferrin transport pathway. Pharmaceutics. 2019;11:298. doi: 10.3390/pharmaceutics11070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kang T., Jiang M., Jiang D., Feng X., Yao J., Song Q. Enhancing glioblastoma-specific penetration by functionalization of nanoparticles with an iron-mimic peptide targeting transferrin/transferrin receptor complex. Mol Pharm. 2015;12:2947–2961. doi: 10.1021/acs.molpharmaceut.5b00222. [DOI] [PubMed] [Google Scholar]
- 238.Liu M., Wu L., Shan W., Cui Y., Huang Y. Iron-mimic peptide converts transferrin from foe to friend for orally targeting insulin delivery. J Mater Chem B. 2018;6:593–601. doi: 10.1039/c7tb02450a. [DOI] [PubMed] [Google Scholar]
- 239.Azevedo C., Nilsen J., Grevys A., Nunes R., Andersen J.T., Sarmento B. Engineered albumin-functionalized nanoparticles for improved fcrn binding enhance oral delivery of insulin. J Control Release. 2020;327:161–173. doi: 10.1016/j.jconrel.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 240.Martins J., Liu D., Fontana F., Ferreira M., Correia A., Valentino S. Microfluidic nanoassembly of bioengineered chitosan-modified FcRn-targeted porous silicon nanoparticles @ hypromellose acetate succinate for oral delivery of anti-diabetic peptides. ACS Appl Mater Interfaces. 2018;10:44354–44367. doi: 10.1021/acsami.8b20821. [DOI] [PubMed] [Google Scholar]
- 241.Sun A.Q., Zhu L., Luo Y., Xu S., Suchy F.J. Human organic solute transporter (host): protein interaction and membrane sorting process. Int J Biochem Mol Biol. 2012;3:290–301. [PMC free article] [PubMed] [Google Scholar]
- 242.Varghese Gupta S., Gupta D., Sun J., Dahan A., Tsume Y., Hilfinger J. Enhancing the intestinal membrane permeability of zanamivir: a carrier mediated prodrug approach. Mol Pharm. 2011;8:2358–2367. doi: 10.1021/mp200291x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fan W., Xia D., Zhu Q., Li X., He S., Zhu C. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials. 2017;151:13–22. doi: 10.1016/j.biomaterials.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 244.Wu L., Liu M., Shan W., Zhu X., Li L., Zhang Z. Bioinspired butyrate-functionalized nanovehicles for targeted oral delivery of biomacromolecular drugs. J Control Release. 2017;262:273–283. doi: 10.1016/j.jconrel.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 245.Kunisawa J., Kurashima Y., Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 246.Brayden D.J., Jepson M.A., Baird A.W. Intestinal peyer's patch m cells and oral vaccine targeting. Drug Discov Today. 2005;10:1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- 247.Clark M.A., Blair H., Liang L., Brey R.N., Brayden D., Hirst B.H. Targeting polymerised liposome vaccine carriers to intestinal M cells. Vaccine. 2001;20:208–217. doi: 10.1016/s0264-410x(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 248.Kim B.Y., Jeong J.H., Park K., Kim J.D. Bioadhesive interaction and hypoglycemic effect of insulin-loaded lectin-microparticle conjugates in oral insulin delivery system. J Control Release. 2005;102:525–538. doi: 10.1016/j.jconrel.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 249.Yoo M.K., Kang S.K., Choi J.H., Park I.K., Na H.S., Lee H.C. Targeted delivery of chitosan nanoparticles to peyer's patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31:7738–7747. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 250.Zhang P., Xu Y., Zhu X., Huang Y. Goblet cell targeting nanoparticle containing drug-loaded micelle cores for oral delivery of insulin. Int J Pharm. 2015;496:993–1005. doi: 10.1016/j.ijpharm.2015.10.078. [DOI] [PubMed] [Google Scholar]
- 251.Mohamadzadeh M., Duong T., Sandwick S.J., Hoover T., Klaenhammer T.R. Dendritic cell targeting of bacillus anthracis protective antigen expressed by lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci U S A. 2009;106:4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kathania M., Zadeh M., Lightfoot Y.L., Roman R.M., Sahay B., Abbott J.R. Colonic immune stimulation by targeted oral vaccine. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ryan G.M., Kaminskas L.M., Porter C.J. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Control Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 254.Supersaxo A., Hein W.R., Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7:167–169. doi: 10.1023/a:1015880819328. [DOI] [PubMed] [Google Scholar]
- 255.Oussoren C., Zuidema J., Crommelin D., Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: II. influence of liposomal size, lipid composition and lipid dose. Biochim Biophys Acta Biomembr. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 256.Reddy S.T., Van Der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 257.Ke X., Howard G.P., Tang H., Cheng B., Saung M.T., Santos J.L. Physical and chemical profiles of nanoparticles for lymphatic targeting. Adv Drug Deliv Rev. 2019;151:72–93. doi: 10.1016/j.addr.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 258.Managuli R.S., Raut S.Y., Reddy M.S., Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expet Opin Drug Deliv. 2018;15:787–804. doi: 10.1080/17425247.2018.1503249. [DOI] [PubMed] [Google Scholar]
- 259.Trevaskis N.L., Kaminskas L.M., Porter C.J.H. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 260.Trotta M., Carlotti M.E., Gallarate M., Zara G.P., Muntoni E., Battaglia L. Insulin-loaded SLN prepared with the emulsion dilution technique: in vivo tracking of nanoparticles after oral administration to rats. J Dispersion Sci Technol. 2011;32:1041–1045. [Google Scholar]
- 261.Hackett M.J., Zaro J.L., Shen W.C., Guley P.C., Cho M.J. Fatty acids as therapeutic auxiliaries for oral and parenteral formulations. Adv Drug Deliv Rev. 2013;65:1331–1339. doi: 10.1016/j.addr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Florence A.T. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov Today. 2005;2:75–81. doi: 10.1016/j.ddtec.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 263.Xie Y., Jiang S., Xia F., Hu X., He H., Yin Z. Glucan microparticles thickened with thermosensitive gels as potential carriers for oral delivery of insulin. J Mater Chem B. 2016;4:4040–4048. doi: 10.1039/c6tb00237d. [DOI] [PubMed] [Google Scholar]
- 264.Xie Y., Hu X., He H., Xia F., Ma Y., Qi J. Tracking translocation of glucan microparticles targeting m cells: implications for oral drug delivery. J Mater Chem B. 2016;4:2864–2873. doi: 10.1039/c5tb02706c. [DOI] [PubMed] [Google Scholar]
- 265.Desai M.P., Labhasetwar V., Amidon G.L., Levy R.J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 266.Jani P., Halbert G.W., Langridge J., Florence A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 267.Tacket C.O., Reid R.H., Boedeker E.C., Losonsky G., Nataro J.P., Bhagat H. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine. 1994;12:1270–1274. doi: 10.1016/s0264-410x(94)80038-2. [DOI] [PubMed] [Google Scholar]
- 268.Katz D.E., DeLorimier A.J., Wolf M.K., Hall E.R., Cassels F.J., Van Hamont J.E. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine. 2003;21:341–346. doi: 10.1016/s0264-410x(02)00613-8. [DOI] [PubMed] [Google Scholar]
- 269.Lei Z., Chen B., Koo Y.M., MacFarlane D.R. Introduction: ionic liquids. Chem Rev. 2017;117:6633–6635. doi: 10.1021/acs.chemrev.7b00246. [DOI] [PubMed] [Google Scholar]
- 270.Wu X., Yu Q., Wu J., Li T., Ding N., Wu W. Ionic liquids containing ketoconazole improving topical treatment of T. Interdigitale infection by synergistic action. Int J Pharm. 2020;589:119842. doi: 10.1016/j.ijpharm.2020.119842. [DOI] [PubMed] [Google Scholar]
- 271.Wu X., Chen Z., Li Y., Yu Q., Lu Y., Zhu Q. Improving dermal delivery of hydrophilic macromolecules by biocompatible ionic liquid based on choline and malic acid. Int J Pharm. 2019;558:380–387. doi: 10.1016/j.ijpharm.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 272.Wu X., Zhang H., He S., Yu Q., Lu Y., Wu W. Improving dermal delivery of hyaluronic acid by ionic liquids for attenuating skin dehydration. Int J Biol Macromol. 2020;150:528–535. doi: 10.1016/j.ijbiomac.2020.02.072. [DOI] [PubMed] [Google Scholar]
- 273.Egorova K.S., Gordeev E.G., Ananikov V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117:7132–7189. doi: 10.1021/acs.chemrev.6b00562. [DOI] [PubMed] [Google Scholar]
- 274.Zakrewsky M., Lovejoy K.S., Kern T.L., Miller T.E., Le V., Nagy A. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci U S A. 2014;111:13313–13318. doi: 10.1073/pnas.1403995111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Banerjee A., Ibsen K., Iwao Y., Zakrewsky M., Mitragotri S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv Healthc Mater. 2017;6:1601411. doi: 10.1002/adhm.201601411. [DOI] [PubMed] [Google Scholar]
- 276.Banerjee A., Ibsen K., Brown T., Chen R., Agatemor C., Mitragotri S. Ionic liquids for oral insulin delivery. Proc Natl Acad Sci U S A. 2018;115:7296–7301. doi: 10.1073/pnas.1722338115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shi Y., Zhao Z., Gao Y., Pan D.C., Salinas A.K., Tanner E.E. Oral delivery of sorafenib through spontaneous formation of ionic liquid nanocomplexes. J Control Release. 2020;322:602–609. doi: 10.1016/j.jconrel.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 278.Angsantikul P., Peng K., Curreri A.M., Chua Y., Chen K.Z., Ehondor J. Ionic liquids and deep eutectic solvents for enhanced delivery of antibodies in the gastrointestinal tract. Adv Funct Mater. 2020;n/a:2002912. [Google Scholar]
- 279.Li Y., Wu X., Zhu Q., Chen Z., Lu Y., Qi J. Improving the hypoglycemic effect of insulin via the nasal administration of deep eutectic solvents. Int J Pharm. 2019;569:118584. doi: 10.1016/j.ijpharm.2019.118584. [DOI] [PubMed] [Google Scholar]
- 280.Peng K., Shi Y., LaBarbiera A., Mitragotri S. Mucoadhesive ionic liquid gel patches for oral delivery. ACS Biomater Sci Eng. 2020 doi: 10.1021/acsbiomaterials.0c01024. Available from: [DOI] [PubMed] [Google Scholar]
- 281.Tanner E.E., Piston K.M., Ma H., Ibsen K.N., Nangia S., Mitragotri S. The influence of water on choline-based ionic liquids. ACS Biomater Sci Eng. 2019;5:3645–3653. doi: 10.1021/acsbiomaterials.9b00243. [DOI] [PubMed] [Google Scholar]
- 282.Rzhevskiy A.S., Singh T.R.R., Donnelly R.F., Anissimov Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. 2018;270:184–202. doi: 10.1016/j.jconrel.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 283.Lee K., Goudie M.J., Tebon P., Sun W., Luo Z., Lee J. Non-transdermal microneedles for advanced drug delivery. Adv Drug Deliv Rev. 2019;165–166:41–59. doi: 10.1016/j.addr.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lee J.W., Prausnitz M.R. Drug delivery using microneedle patches: not just for skin. Expet Opin Drug Deliv. 2018;15:541–543. doi: 10.1080/17425247.2018.1471059. [DOI] [PubMed] [Google Scholar]
- 285.Traverso G., Schoellhammer C.M., Schroeder A., Maa R., Lauwers G.Y., Polat B.E. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015;104:362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Abramson A., Caffarel-Salvador E., Soares V., Minahan D., Tian R.Y., Lu X. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat Med. 2019;25:1512–1518. doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Prausnitz M.R., Gomaa Y., Li W. Microneedle patch drug delivery in the gut. Nat Med. 2019;25:1471–1472. doi: 10.1038/s41591-019-0606-0. [DOI] [PubMed] [Google Scholar]
- 288.Abramson A., Caffarel-Salvador E., Khang M., Dellal D., Silverstein D., Gao Y. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611–615. doi: 10.1126/science.aau2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Aran K., Chooljian M., Paredes J., Rafi M., Lee K., Kim A.Y. An oral microjet vaccination system elicits antibody production in rabbits. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf6413. [DOI] [PubMed] [Google Scholar]
- 290.Sheng J., Han L., Qin J., Ru G., Li R., Wu L. N-Trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl Mater Interfaces. 2015;7:15430–15441. doi: 10.1021/acsami.5b03555. [DOI] [PubMed] [Google Scholar]
- 291.Yin Y., Chen D., Qiao M., Lu Z., Hu H. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J Control Release. 2006;116:337–345. doi: 10.1016/j.jconrel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 292.Garinot M., Fiévez V., Pourcelle V., Stoffelbach F., des Rieux A., Plapied L. Pegylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 293.Kostadinova A.I., Middelburg J., Ciulla M., Garssen J., Hennink W.E., Knippels L.M.J. PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow's milk allergy prevention. Eur J Pharmacol. 2018;818:211–220. doi: 10.1016/j.ejphar.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 294.Narayanan D., Anitha A., Jayakumar R., Chennazhi K.P. In vitro and in vivo evaluation of osteoporosis therapeutic peptide PTH 1–34 loaded pegylated chitosan nanoparticles. Mol Pharm. 2013;10:4159–4167. doi: 10.1021/mp400184v. [DOI] [PubMed] [Google Scholar]
- 295.Marasini N., Giddam A.K., Khalil Z.G., Hussein W.M., Capon R.J., Batzloff M.R. Double adjuvanting strategy for peptide-based vaccines: trimethyl chitosan nanoparticles for lipopeptide delivery. Nanomedicine. 2016;11:3223–3235. doi: 10.2217/nnm-2016-0291. [DOI] [PubMed] [Google Scholar]
- 296.Zeng Z., Qi D., Yang L., Liu J., Tang Y., Chen H. Stimuli-responsive self-assembled dendrimers for oral protein delivery. J Control Release. 2019;315:206–213. doi: 10.1016/j.jconrel.2019.10.049. [DOI] [PubMed] [Google Scholar]
- 297.Makhlof A., Fujimoto S., Tozuka Y., Takeuchi H. In vitro and in vivo evaluation of WGA–carbopol modified liposomes as carriers for oral peptide delivery. Eur J Pharm Biopharm. 2011;77:216–224. doi: 10.1016/j.ejpb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 298.Parmentier J., Hofhaus G., Thomas S., Cuesta L.C., Gropp F., Schröder R. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. J Pharm Sci. 2014;103:3985–3993. doi: 10.1002/jps.24215. [DOI] [PubMed] [Google Scholar]
- 299.Li Z., Zhang L., Sun W., Ding Q., Hou Y., Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29:5260–5266. doi: 10.1016/j.vaccine.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 300.Wang N., Wang T., Zhang M., Chen R., Niu R., Deng Y. Mannose derivative and lipid a dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur J Pharm Biopharm. 2014;88:194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 301.Gupta P.N., Vyas S.P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf, B. 2011;82:118–125. doi: 10.1016/j.colsurfb.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 302.Hecq J., Amighi K., Goole J. Development and evaluation of insulin-loaded cationic solid lipid nanoparticles for oral delivery. J Drug Deliv Sci Technol. 2016;36:192–200. [Google Scholar]
- 303.Fan T., Chen C., Guo H., Xu J., Zhang J., Zhu X. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur J Pharm Biopharm. 2014;88:518–528. doi: 10.1016/j.ejpb.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 304.Li X., Qi J., Xie Y., Zhang X., Hu S., Xu Y. Nanoemulsions coated with alginate/chitosan as oral insulin delivery systems: preparation, characterization, and hypoglycemic effect in rats. Int J Nanomed. 2013;8:23–32. doi: 10.2147/IJN.S38507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lin P.Y., Chen K.H., Miao Y.B., Chen H.L., Lin K.J., Chen C.T. Phase-changeable nanoemulsions for oral delivery of a therapeutic peptide: toward targeting the pancreas for antidiabetic treatments using lymphatic transport. Adv Funct Mater. 2019;29:1809015. [Google Scholar]
- 306.Abeer M.M., Meka A.K., Pujara N., Kumeria T., Strounina E., Nunes R. Rationally designed dendritic silica nanoparticles for oral delivery of exenatide. Pharmaceutics. 2019;11:418. doi: 10.3390/pharmaceutics11080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tan X., Zhang Y., Wang Q., Ren T., Gou J., Guo W. Cell-penetrating peptide together with PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides. Biomater Sci. 2019;7:2934–2950. doi: 10.1039/c9bm00274j. [DOI] [PubMed] [Google Scholar]
- 308.Lamson N.G., Berger A., Fein K.C., Whitehead K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020;4:84–96. doi: 10.1038/s41551-019-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Scaramuzzi K., Tanaka G.D., Neto F.M., Garcia P.R.A.F., Gabrili J.J.M., Oliveira D.C.A. Nanostructured SBA-15 silica: an effective protective vehicle to oral hepatitis B vaccine immunization. Nanomedicine. 2016;12:2241–2250. doi: 10.1016/j.nano.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 310.Cao P., Han F.Y., Grøndahl L., Xu Z.P., Li L. Enhanced oral vaccine efficacy of polysaccharide-coated calcium phosphate nanoparticles. ACS Omega. 2020;5:18185–18197. doi: 10.1021/acsomega.0c01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cho H.J., Oh J., Choo M.K., Ha J.I., Park Y., Maeng H.J. Chondroitin sulfate-capped gold nanoparticles for the oral delivery of insulin. Int J Biol Macromol. 2014;63:15–20. doi: 10.1016/j.ijbiomac.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 312.Lundquist P., Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 313.Sonaje K., Chuang E.Y., Lin K.J., Yen T.C., Su F.Y., Tseng M.T. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm. 2012;9:1271–1279. doi: 10.1021/mp200572t. [DOI] [PubMed] [Google Scholar]
- 314.Yu Z., Ma L., Ye S., Li G., Zhang M. Construction of an environmentally friendly octenylsuccinic anhydride modified pH-sensitive chitosan nanoparticle drug delivery system to alleviate inflammation and oxidative stress. Carbohydr Polym. 2020;236:115972. doi: 10.1016/j.carbpol.2020.115972. [DOI] [PubMed] [Google Scholar]
- 315.Thanou M., Nihot M., Jansen M., Verhoef J.C., Junginger H. Mono-n-carboxymethyl chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J Pharm Sci. 2001;90:38–46. doi: 10.1002/1520-6017(200101)90:1<38::aid-jps5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 316.Gradauer K., Barthelmes J., Vonach C., Almer G., Mangge H., Teubl B. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J Control Release. 2013;172:872–878. doi: 10.1016/j.jconrel.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sonaje K., Lin K.J., Wang J.J., Mi F.L., Chen C.T., Juang J.H. Self-assembled pH-sensitive nanoparticles: a platform for oral delivery of protein drugs. Adv Funct Mater. 2010;20:3695–3700. [Google Scholar]
- 318.Sarmento B., Ribeiro A., Veiga F., Sampaio P., Neufeld R., Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24:2198–2206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 319.Thanou M., Florea B.I., Langemeÿer M.W.E., Verhoef J.C., Junginger H.E. N-Trimethylated chitosan chloride (TMC) improves the intestinal permeation of the peptide drug buserelin in vitro (Caco-2 cells) and in vivo (rats) Pharm Res. 2000;17:27–31. doi: 10.1023/a:1007558206506. [DOI] [PubMed] [Google Scholar]
- 320.Moghassemi S., Parnian E., Hakamivala A., Darzianiazizi M., Vardanjani M.M., Kashanian S. Uptake and transport of insulin across intestinal membrane model using trimethyl chitosan coated insulin niosomes. Mater Sci Eng C. 2015;46:333–340. doi: 10.1016/j.msec.2014.10.070. [DOI] [PubMed] [Google Scholar]
- 321.Tsai L.C., Chen C.H., Lin C.W., Ho Y.C., Mi F.L. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int J Biol Macromol. 2019;126:141–150. doi: 10.1016/j.ijbiomac.2018.12.182. [DOI] [PubMed] [Google Scholar]
- 322.Zhang H., Huang X., Sun Y., Xing J., Yamamoto A., Gao Y. Absorption-improving effects of chitosan oligomers based on their mucoadhesive properties: a comparative study on the oral and pulmonary delivery of calcitonin. Drug Deliv. 2016;23:2419–2427. doi: 10.3109/10717544.2014.1002946. [DOI] [PubMed] [Google Scholar]
- 323.Zhang X., Sun M., Zheng A., Cao D., Bi Y., Sun J. Preparation and characterization of insulin-loaded bioadhesive PLGA nanoparticles for oral administration. Eur J Pharmaceut Sci. 2012;45:632–638. doi: 10.1016/j.ejps.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 324.Paul P.K., Nopparat J., Nuanplub M., Treetong A., Suedee R. Improvement in insulin absorption into gastrointestinal epithelial cells by using molecularly imprinted polymer nanoparticles: microscopic evaluation and ultrastructure. Int J Pharm. 2017;530:279–290. doi: 10.1016/j.ijpharm.2017.07.071. [DOI] [PubMed] [Google Scholar]
- 325.Paul P.K., Treetong A., Suedee R. Biomimetic insulin-imprinted polymer nanoparticles as a potential oral drug delivery system. Acta Pharm. 2017;67:149–168. doi: 10.1515/acph-2017-0020. [DOI] [PubMed] [Google Scholar]
- 326.Martins S., Sarmento B., Ferreira D.C., Souto E.B. Lipid-based colloidal carriers for peptide and protein delivery–liposomes versus lipid nanoparticles. Int J Nanomed. 2007;2:595. [PMC free article] [PubMed] [Google Scholar]
- 327.Li P., Nielsen H.M., Müllertz A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expet Opin Drug Deliv. 2012;9:1289–1304. doi: 10.1517/17425247.2012.717068. [DOI] [PubMed] [Google Scholar]
- 328.Qi J., Zhuang J., Lu Y., Dong X., Zhao W., Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov Today. 2017;22:166–172. doi: 10.1016/j.drudis.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 329.Zhang X., Yi Y., Qi J., Lu Y., Tian Z., Xie Y. Controlled release of cyclosporine a self-nanoemulsifying systems from osmotic pump tablets: near zero-order release and pharmacokinetics in dogs. Int J Pharm. 2013;452:233–240. doi: 10.1016/j.ijpharm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 330.Li Y., Yokoyama W., Xu S., Zhu S., Ma J., Zhong F. Formation and stability of w/o microemulsion formed by food grade ingredients and its oral delivery of insulin in mice. J Func Food. 2017;30:134–141. [Google Scholar]
- 331.Sharma G., Wilson K., van der Walle C.F., Sattar N., Petrie J.R., Ravi Kumar M.N.V. Microemulsions for oral delivery of insulin: design, development and evaluation in streptozotocin induced diabetic rats. Eur J Pharm Biopharm. 2010;76:159–169. doi: 10.1016/j.ejpb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 332.Cheng M.B., Wang J.C., Li Y.H., Liu X.Y., Zhang X., Chen D.W. Characterization of water-in-oil microemulsion for oral delivery of earthworm fibrinolytic enzyme. J Control Release. 2008;129:41–48. doi: 10.1016/j.jconrel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 333.He H., Lu Y., Qi J., Zhu Q., Chen Z., Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sinica B. 2019;9:36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Patel H.M., Ryman B.E. Oral administration of insulin by encapsulation within liposomes. FEBS Lett. 1976;62:60–63. doi: 10.1016/0014-5793(76)80016-6. [DOI] [PubMed] [Google Scholar]
- 335.Dapergolas G., Gregoriadis G. Hypoglycaemic effect of liposome-entrapped insulin administered intragastrically into rats. Lancet. 1976;308:824–827. doi: 10.1016/s0140-6736(76)91209-5. [DOI] [PubMed] [Google Scholar]
- 336.Arrieta-Molero J.F., Aleck K., Sinha M.K., Brownscheidle C.M., Shapiro L.J., Sperling M.A. Orally administered liposome-entrapped insulin in diabetic animals. Hormone Res Paedia. 1982;16:249–256. doi: 10.1159/000179509. [DOI] [PubMed] [Google Scholar]
- 337.Liu W., Li D., Dong Z., Liu K., He H., Lu Y. Insight into the in vivo translocation of oral liposomes by fluorescence resonance energy transfer effect. Int J Pharm. 2020;587:119682. doi: 10.1016/j.ijpharm.2020.119682. [DOI] [PubMed] [Google Scholar]
- 338.Shukla A., Khatri K., Gupta P.N., Goyal A.K., Mehta A., Vyas S.P. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes) J Pharm Pharmaceut Sci. 2008;11:59–66. doi: 10.18433/j3k01m. [DOI] [PubMed] [Google Scholar]
- 339.Wilkhu J.S., McNeil S.E., Anderson D.E., Perrie Y. Characterization and optimization of bilosomes for oral vaccine delivery. J Drug Target. 2013;21:291–299. doi: 10.3109/1061186X.2012.747528. [DOI] [PubMed] [Google Scholar]
- 340.Qi J., Lu Y., Wu W. Absorption, disposition and pharmacokinetics of solid lipid nanoparticles. Curr Drug Metabol. 2012;13:418–428. doi: 10.2174/138920012800166526. [DOI] [PubMed] [Google Scholar]
- 341.Almeida A.J., Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 342.Yoon G., Jin W.P., Yoon I.S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharm Invest. 2013;43:353–362. [Google Scholar]
- 343.Sarmento B., Martins S., Ferreira D., Souto E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2:743–749. [PMC free article] [PubMed] [Google Scholar]
- 344.Sarmento B., Mazzaglia D., Bonferoni M.C., Neto A.P., do Céu, Monteiro M., Seabra V. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system. Carbohydr Polym. 2011;84:919–925. [Google Scholar]
- 345.Zhang Z.H., Zhang Y.L., Zhou J.P., Lv H.X. Solid lipid nanoparticles modified with stearic acid–octaarginine for oral administration of insulin. Int J Nanomed. 2012;7:3333. doi: 10.2147/IJN.S31711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yu Z., Fan W., Wang L., Qi J., Lu Y., Wu W. Effect of surface charges on oral absorption of intact solid lipid nanoparticles. Mol Pharm. 2019;16:5013–5024. doi: 10.1021/acs.molpharmaceut.9b00861. [DOI] [PubMed] [Google Scholar]
- 347.Morçöl T., Nagappan P., Nerenbaum L., Mitchell A., Bell S. Calcium phosphate-PEG-insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int J Pharm. 2004;277:91–97. doi: 10.1016/j.ijpharm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 348.Florek J., Caillard R., Kleitz F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—current status and perspective of msns drug carriers. Nanoscale. 2017;9:15252–15277. doi: 10.1039/c7nr05762h. [DOI] [PubMed] [Google Scholar]
- 349.Andreani T., de Souza A.L.R., Kiill C.P., Lorenzón E.N., Fangueiro J.F., Calpena A.C. Preparation and characterization of peg-coated silica nanoparticles for oral insulin delivery. Int J Pharm. 2014;473:627–635. doi: 10.1016/j.ijpharm.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 350.Choi S.R., Jang D.-J., Kim S., An S., Lee J., Oh E. Polymer-coated spherical mesoporous silica for pH-controlled delivery of insulin. J Mater Chem B. 2014;2:616–619. doi: 10.1039/c3tb21494j. [DOI] [PubMed] [Google Scholar]
- 351.Rasmussen M.K., Kardjilov N., Oliveira C.L., Watts B., Villanova J., Botosso V.F. 3D visualisation of hepatitis B vaccine in the oral delivery vehicle SBA-15. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-42645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sharma S., Verma A., Teja B.V., Pandey G., Mittapelly N., Trivedi R. An insight into functionalized calcium based inorganic nanomaterials in biomedicine: trends and transitions. Colloids Surf, B. 2015;133:120–139. doi: 10.1016/j.colsurfb.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 353.Ramachandran R., Paul W., Sharma C.P. Synthesis and characterization of pegylated calcium phosphate nanoparticles for oral insulin delivery. J Biomed Mater Res B. 2009;88:41–48. doi: 10.1002/jbm.b.31241. [DOI] [PubMed] [Google Scholar]
- 354.Kumari Y., Singh S.K., Kumar R., Kumar B., Kaur G., Gulati M. Modified apple polysaccharide capped gold nanoparticles for oral delivery of insulin. Int J Biol Macromol. 2020;149:976–988. doi: 10.1016/j.ijbiomac.2020.01.302. [DOI] [PubMed] [Google Scholar]
- 355.Safari M., Kamari Y., Ghiaci M., Sadeghi-Aliabadi H., Mirian M. Synthesis and characterization of insulin/zirconium phosphate@ TiO2 hybrid composites for enhanced oral insulin delivery applications. Drug Dev Ind Pharm. 2017;43:862–870. doi: 10.1080/03639045.2016.1220573. [DOI] [PubMed] [Google Scholar]
- 356.Hofmann-Amtenbrink M., Grainger D.W., Hofmann H. Nanoparticles in medicine: current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine. 2015;11:1689–1694. doi: 10.1016/j.nano.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 357.Yang G., Phua S.Z.F., Bindra A.K., Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31:1805730. doi: 10.1002/adma.201805730. [DOI] [PubMed] [Google Scholar]
- 358.Lopes M., Simões S., Veiga F., Seiça R., Ribeiro A. Why most oral insulin formulations do not reach clinical trials. Ther Deliv. 2015;6:973–987. doi: 10.4155/TDE.15.47. [DOI] [PubMed] [Google Scholar]
- 359.Hauss D.J. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59:667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 360.Kovarik J.M., Mueller E.A., Kutz K., Van Bree J.B., Tetzloff W. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci. 1994;83:444–446. doi: 10.1002/jps.2600830336. [DOI] [PubMed] [Google Scholar]
- 361.Zaoral M. DDAVP (desmopressin) and solid phase peptide synthesis. Pept Sci. 2008;90:213. doi: 10.1002/bip.20936. [DOI] [PubMed] [Google Scholar]
- 362.F. Pharmaceuticals . 2010. Minirin® Tablets—New Zealand data sheet.http://www.medsafe.govt.nz/profs/datasheet/m/Minirintab.pdf Available from: [Google Scholar]
- 363.Pohl E., Heine A., Sheldrick G.M., Dauter Z., Wilson K.S., Kallen J. Structure of octreotide, a somatostatin analogue. Acta Crystallogr D. 1995;51:48–59. doi: 10.1107/S0907444994006104. [DOI] [PubMed] [Google Scholar]
- 364.Tuvia S., Atsmon J., Teichman S.L., Katz S., Salama P., Pelled D. Oral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J Clin Endocrinol Metab. 2012;97:2362–2369. doi: 10.1210/jc.2012-1179. [DOI] [PubMed] [Google Scholar]
- 365.https://www.clinicaltrials.gov/ct2/show/results/NCT01923181?term=oral+semaglutide&phase=1&draw=2&rank=1.
- 366.Busby R.W., Bryant A.P., Bartolini W.P., Cordero E.A., Hannig G., Kessler M.M. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328–335. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 367.Rao S., Kupfer Y., Pagala M., Chapnick E., Tessler S. Systemic absorption of oral vancomycin in patients with clostridium difficile infection. Scand J Infect Dis. 2011;43:386–388. doi: 10.3109/00365548.2010.544671. [DOI] [PubMed] [Google Scholar]
- 368.Fuhrmann G., Leroux J.-C. Improving the stability and activity of oral therapeutic enzymes—recent advances and perspectives. Pharm Res. 2014;31:1099–1105. doi: 10.1007/s11095-013-1233-y. [DOI] [PubMed] [Google Scholar]
- 369.Company Announcement: financial report for the period 1 January 2015 to 30 June 2015. Novo Nordisk. Available from: https://www.novonordisk.com/content/dam/Denmark/HQ/investors/irmaterial/quarterly_financial_reports/2015/20150806_finanical%20report_Q2%202015_UK.pdf [Accessed August 12, 2019].
- 370.Li J., Wang Y., Han L., Sun X., Yu H., Yu Y. Time–action profile of an oral enteric insulin formulation in healthy Chinese volunteers. Clin Therapeut. 2012;34:2333–2338. doi: 10.1016/j.clinthera.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 371.Eldor R., Arbit E., Corcos A., Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zijlstra E., Heinemann L., Plum-Mörschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8:458–465. doi: 10.1177/1932296814529988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Khedkar A., Lebovitz H., Fleming A., Cherrington A., Jose V., Athalye S.N. Impact of insulin tregopil and its permeation enhancer on pharmacokinetics of metformin in healthy volunteers: randomized, open-label, placebo-controlled, crossover study. Clin Transl Sci. 2019;12:276–282. doi: 10.1111/cts.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Khedkar A., Lebovitz H., Fleming A., Cherrington A., Jose V., Athalye S.N. Pharmacokinetics and pharmacodynamics of insulin tregopil in relation to premeal dosing time, between meal interval, and meal composition in patients with type 2 diabetes mellitus. Clin Pharmacol Drug Develop. 2020;9:74–86. doi: 10.1002/cpdd.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Geho W.B., Geho H.C., Lau J.R., Gana T.J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J Diabetes Sci Technol. 2009;3:1451–1459. doi: 10.1177/193229680900300627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Geho W.B., Rosenberg L.N., Schwartz S.L., Lau J.R., Gana T.J. A single-blind, placebo-controlled, dose-ranging trial of oral hepatic-directed vesicle insulin add-on to oral antidiabetic treatment in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2014;8:551–559. doi: 10.1177/1932296814524871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Karsdal M.A., Byrjalsen I., Henriksen K., Riis B.J., Lau E.M., Arnold M. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthr Cartilage. 2010;18:150–159. doi: 10.1016/j.joca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 378.https://www.protagonist-inc.com/pipeline/product-candidates/.
- 379.http://avaxiabiologics.com/programs/avx-470.php.
- 380.http://protalix.com/pipeline/.
- 381.Sung HW, Liao ZX, Peng SF, Tu H, inventors; GP Medical, Inc., National Tsing Hua University, assignee. Nanoparticles for protein drug delivery. United States patent US No. 8283317B1. 2012 Oct 9.
- 382.Transgene reports excellent results from TrabiOralTM oral insulin studies, Available from: http://www.transgenebiotek.com/images/inner/news/press_release_120521_tbl_trabioral_insulin_efficacy_studies.pdf2012 [Accessed 7 December, 2015].
- 383.Thwala L.N., Préat V., Csaba N.S. Emerging delivery platforms for mucosal administration of biopharmaceuticals: a critical update on nasal, pulmonary and oral routes. Expet Opin Drug Deliv. 2017;14:23–36. doi: 10.1080/17425247.2016.1206074. [DOI] [PubMed] [Google Scholar]
- 384.https://emisphere.com/technology/.
- 385.Maggio E.T. Novel formulations for non-invasive delivery & stabilization of peptides. Biopolymers. 2013;13:68–75. [Google Scholar]