Abstract
Parasite range expansions are a direct consequence of globalization and are an increasing threat to biodiversity. Here, we report a recent range expansion of the SGS1 strain of a highly invasive parasite, Plasmodium relictum, to two non-migratory passerines in North America. Plasmodium relictum is considered one of the world's most invasive parasites and causes the disease avian malaria: this is the first reported case of SGS1 in wild non-migratory birds on the continent. Using a long-term database where researchers report avian malaria parasite infections, we summarized our current understanding of the geographical range of SGS1 and its known hosts. We also identified the most likely geographical region of this introduction event using the MSP1 allele. We hypothesize that this introduction resulted from movements of captive birds and subsequent spillover to native bird populations, via the presence of competent vectors and ecological fitting. Further work should be conducted to determine the extent to which SGS1 has spread following its introduction in North America.
Keywords: Plasmodium relictum, SGS1, avian malaria, ecological fitting, chickadee
1. Introduction
Specialization is considered a driving force that shapes parasite diversity [1–4]. The cospeciation of parasites with hosts is considered specialization and results in congruent host–parasite phylogenies, but specialization may still occur when parasites undergo range expansions involving the infection of new hosts, where shared host traits are accessed by parasites [5,6]. This concept, ecological fitting, may explain the occurrence of parasite range expansions and ecological invasions, even when parasites exhibit some form of specialization [6,7]. For example, ecological fitting can explain ecological invasions by parasites such as Philornis downsi, a fly introduced to the Galápagos Islands whose larvae feed on the blood of bird nestlings [8]; the lung fluke Haematoloechus floedae that was likely introduced to Costa Rica by infected bullfrogs and subsequently expanded its range to include native leopard frogs [6]; and Plasmodium relictum, an avian malaria-causing protozoan that was introduced to the Hawaiian Islands and subsequently expanded its range to include endemic birds [9]. Each of these parasite invasions occurred through some form of shared traits and/or resources across host species and their geographical range (e.g. the presence of bird nestlings for Ph. downsi larvae to feed on [8], aquatic environments with a suitable snail and dragonfly nymph for the completion of H. floedae's life cycle [1], and a sustaining population of competent insect vectors to transmit Pl. relictum [10]). While ecological fitting does not imply a lack of coevolution between hosts and parasites, it does help to explain the preponderance of parasite range expansions and invasions.
Avian malaria is a wildlife disease caused by infections with Plasmodium parasites that infect birds [11]. Plasmodium parasites have an obligate relationship with the mosquito vector in which they sexually reproduce, but they infect birds during the asexual portion of their lifecycle (figure 1a). Birds with avian malaria may show signs of lethargy, loss of appetite, reduced haematocrit, and in extreme cases, paralysis, convulsions and death [11]. This disease is often observed in bird species that are infected by Plasmodium parasites from a recent range expansion event [12]. For example, the Pl. relictum strain GRW4 was introduced to the Hawaiian islands in the late nineteenth century, and subsequent avian malaria infections have been described as a driver of extinction for many endemic Hawaiian bird species [10,12,13]. The geographical and host specificity of avian malaria parasites is complex [14,15], but some highly invasive strains of Pl. relictum appear to easily infect new avian hosts and it is now considered one of the world's most invasive species [16–18].
Figure 1.
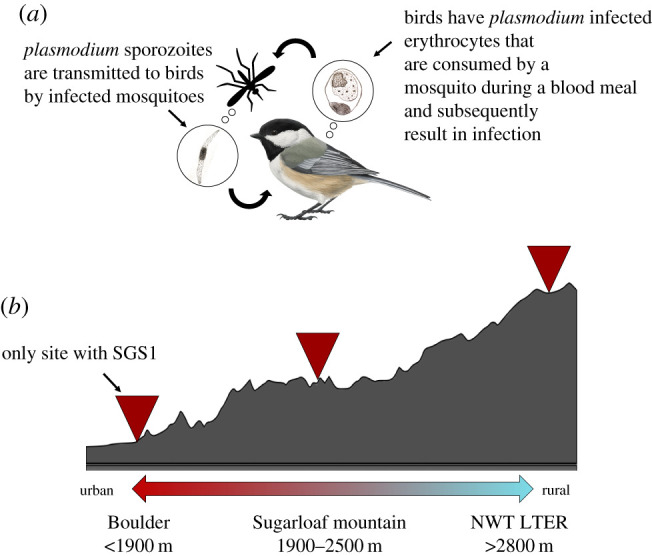
The presence of SGS1 in our focal species is shaped, in part, by the presence of vectors (a). We only observed SGS1 infections in chickadees that we sampled in Boulder, and at elevations below 1700 m (b). While SGS1 is a generalist parasite that can infect multiple avian host species, it is specialized in the sense that it requires a competent insect vector that transmits sporozoites that infect avian hosts. We show a brief schematic of this cycle in (a) but note that we have not confirmed that SGS1 is able to complete its lifecycle in chickadee hosts. Broadly our sampling locations can be divided into three field sites spanning an elevation gain of approximately 1500 m along an urban–rural gradient shown in (b). We hypothesize that SGS1 was found only at our lowest elevation field site because the common SGS1 vector, Culex pipiens, only occurs at elevations below 1700 m in the Colorado Front Range. To our knowledge, C. pipiens is the only mosquito species in the Colorado Front Range that can act as a vector for SGS1. Its presence at lower elevations is shaped by the availability of peridomestic breeding sites. The combination of both competent and accessible vector and avian hosts underlies this range expansion.
We documented SGS1, a strain of Pl. relictum, in two novel host species of North American songbird, the black-capped (Poecile atricapillus) and mountain (Poecile gambeli) chickadee that we sampled in Boulder county, Colorado. SGS1 is a generalist parasite that has been reported infecting numerous bird species of the Eastern Hemisphere and eight mosquito vector species [17–19]. Conversely, SGS1 has rarely been reported infecting Western Hemisphere birds despite a number of studies with large sampling efforts (e.g. [20–23]). To our knowledge, there has been only one previous report of SGS1 in wild birds of North America—an infection documented in a single migratory tree swallow (Tachycineta bicolor) out of 1562 screened birds that were sampled in southern Québec, Canada [23]. Additionally, we conducted a geographical survey of the known range for SGS1 (figure 2) and we compiled reports of SGS1 infections among avian families (table 1). We distinguished between avian families where these infections have been reported in captive hosts and wild hosts because avian malaria outbreaks have been shown to occur among birds in captivity, and these outbreaks correlate with bird mortalities, thereby warranting the need for further documentation [26]. Finally, the merozoite surface protein 1 (MSP1) for Pl. relictum has been shown to exhibit geographical specificity [17]. We, therefore, amplified a region of the MSP1 gene for the SGS1 parasites that we identified in Colorado chickadees to predict its most likely geographical origin.
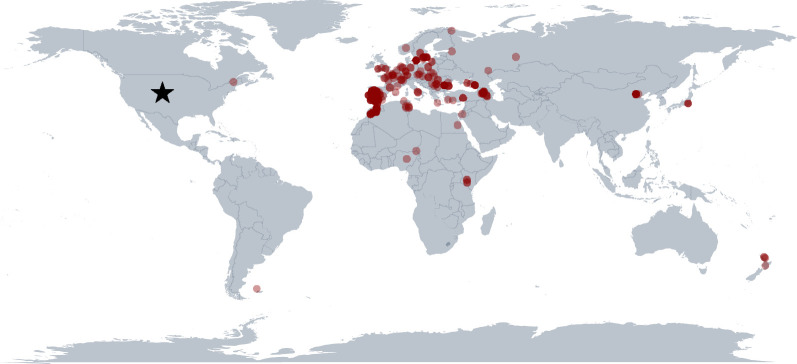
Figure 2.
Our current understanding of the global range of SGS1. This map was generated using 357 SGS1 reports that had corresponding GPS data in addition to our observations of SGS1 infecting black-capped (Po. atricapillus) and mountain (Po. gambeli) chickadees in Boulder, Colorado (indicated by the star). Points are semi-translucent, and they overlap in some locations. This map was generated using the ggplot2 R package [24,25].
Table 1.
Reports of SGS1 infections were retrieved from the MalAvi database and summarized by avian family. ‘Total tested’ indicates the sum of all individuals for each family that has been tested for SGS1 infection and ‘total infected’ refers to the sum of all SGS1 infections per family. We calculated prevalence by dividing entries in the ‘total infected’ column by entries in the ‘total tested’ column for each avian family. The number of reports for each family is indicated in ‘total reports’. We also show the number of reports lacking complete sampling data (given that this will impact our prevalence calculation) and we indicate reports involving captive birds since there are notable disparities of studies involving captive birds between avian families. Chickadees are members of the Paridae family, and this avian family is indicated with an asterisk.
| avian family | total tested | total infected | total prevalence | total reports | reports with incomplete sampling information | reports including captive birds |
|---|---|---|---|---|---|---|
| Alaudidae | 434 | 52 | 12.0 | 2 | 0 | 0 |
| Alcidae | N/A | N/A | N/A | 1 | 1 | 1 |
| Anatidae | 28 | 3 | 10.7 | 3 | 0 | 1 |
| Ardeidae | 13 | 1 | 7.69 | 2 | 1 | 1 |
| Certhiidae | 98 | 19 | 19.4 | 13 | 0 | 0 |
| Ciconiidae | 15 | 1 | 6.67 | 1 | 1 | 0 |
| Columbidae | N/A | N/A | N/A | 1 | 1 | 0 |
| Corvidae | 255 | 73 | 28.6 | 15 | 4 | 0 |
| Cracticidae | 6 | 1 | 16.7 | 1 | 0 | 0 |
| Estrildidae | 56 | 1 | 1.79 | 2 | 1 | 0 |
| Fringillidae | 803 | 89 | 11.1 | 49 | 10 | 2 |
| Gruidae | N/A | N/A | N/A | 5 | 5 | 5 |
| Hirundinidae | 2480 | 65 | 2.62 | 8 | 1 | 0 |
| Laniidae | 9 | 2 | 22.2 | 1 | 0 | 0 |
| Laridae | 148 | 22 | 14.9 | 4 | 0 | 1 |
| Motacillidae | 92 | 1 | 1.09 | 2 | 1 | 0 |
| Muscicapidae | 3955 | 56 | 1.41 | 33 | 5 | 0 |
| Paridae* | 5701 | 1105 | 19.4 | 95 | 11 | 0 |
| Passeridae | 6056 | 1268 | 20.9 | 124 | 5 | 0 |
| Phasianidae | 52 | 7 | 13.5 | 4 | 2 | 4 |
| Ploceidae | 603 | 8 | 1.33 | 5 | 0 | 0 |
| Procellariidae | 28 | 1 | 3.57 | 1 | 0 | 0 |
| Pycnonotidae | 39 | 6 | 15.4 | 3 | 1 | 2 |
| Recurvirostridae | 7 | 1 | 14.3 | 1 | 0 | 0 |
| Scolopacidae | 85 | 1 | 1.18 | 1 | 0 | 0 |
| Sittidae | 19 | 6 | 31.6 | 1 | 0 | 0 |
| Spheniscidae | 64 | 7 | 10.9 | 3 | 2 | 3 |
| Strigidae | 6 | 2 | 33.3 | 4 | 2 | 1 |
| Sturnidae | 216 | 3 | 1.39 | 7 | 4 | 2 |
| Sylviidae | 4046 | 341 | 8.43 | 85 | 19 | 0 |
| Turdidae | 543 | 42 | 7.73 | 21 | 4 | 0 |
2. Methods
Between the years 2017 and 2020, we collected blood samples from 68 mountain and 111 black-capped chickadees that we captured either by hand or using mist-nets in Boulder County, Colorado. Birds were sampled along an approximately 1500 m elevation gradient at three broad sites (figure 1b). All birds were bled from the brachial vein using a 27-gauge needle and samples were stored in a 1% lysis buffer. After conducting a DNA salt-based extraction from chickadee blood samples, we first screened every chickadee for Plasmodium infection using primers HAEMNF1 and HAEMNR3, followed by the nested set of primers HAEMF and HAEMR2 that screen for both Plasmodium and Haemoproteus infections [27]. We ran PCRs in three separate batches based on year. The laboratory space where these screens took place has not been used to screen any European Hemisphere birds for Plasmodium parasites and chickadees were not tested with any other bird species. We screened every chickadee twice for the presence of infection and all samples that tested positive for infection were Sanger sequenced. All chickadees that tested positive for SGS1 infection were further screened twice and subsequently sequenced using the Plasmodium-specific cytb primers 983R and 621F [28]. Sequencing for all resulting PCRs was bidirectional. We aligned all resulting Plasmodium cytb sequences from the HAEMF and HAEMR2 primers to the MalAvi database, a repository for avian malaria data, using the BLAST algorithm where a 100% match indicated infection with SGS1 [19]. We used the same approach for sequences resulting from the 983R and 621F primers but using the NCBI database.
We retrieved all 504 reports of SGS1 that have been submitted to the MalAvi database (retrieved March 2021) in addition to the two reports of SGS1 infection data that we describe here––one for each chickadee species in our study. These data are available in the ‘hosts and sites’ datasheet through the MalAvi website [19]. We did not include eight reports by Marzal et al. [29] that described SGS1 infections in South American birds because these reports still need to be verified to remove doubts of sample contamination [29,30]. In total, we used 498 reports on SGS1 infections for our study. To characterize the geographical distribution of SGS1, we used the available location information for all reports, but we excluded 23 reports as they were focused on captive birds. Of the remaining reports, 118 lacked GPS information, resulting in 357 reports that we used to map our current understanding of the global range of SGS1 (figure 2). We further compiled the reports of SGS1 infection for each avian family using the ‘hosts and sites’ dataset (table 1). We included all 498 reports for both captive and wild-caught birds and we obtained total prevalence data for each family by dividing the total number of reported infections by the total number of birds tested. Some reports lacked information on sample size which will affect prevalence data. We, therefore, listed the number of these reports for each avian family (table 1).
To determine the geographical origin of Colorado SGS1 infections, we used an additional nested PCR protocol to amplify the MSP1 allele for the three SGS1 infected chickadees in our study. We used the outside primers MSP1–3F and MSP1–3R, followed by the nested primers MSP1–3FN and MSP1–3RN, as described by Hellgren et al. [17], to amplify and subsequently Sanger-sequence this region. We then aligned the resulting sequences to the NCBI database using the blast algorithm. The alignment was further compared with all described MSP1 alleles and associated locations by Hellgren et al. [17] (summarized in table 2).
Table 2.
Variation in the MSP1 allele can be used to broadly characterize the phylogeography of Pl. relictum. This table is a summary of findings from Hellgren et al. [17]. Black-capped (Po. atricapillus) and mountain (Po. gambeli) chickadees sampled in Boulder, Colorado tested positive for the MSP1 Pr2 variant of SGS1. Pr2 is most commonly identified in SGS1 strains sampled from European birds.
| MSP1 allele | associated SGS1 population |
|---|---|
| Pr1 | Kenya and Nigeria |
| Pr2 | throughout Europe, but also observed infecting a single bird sampled in South Africa, and Culex pipiens sampled in Japan |
| Pr3 | Japan |
| Pr7 | Japan |
3. Results
We identified two mountain and one black-capped chickadee with SGS1 infections out of 179 birds sampled. Overall, we identified one chickadee infected with SGS1 during each year across a 3-year timespan of our study. Infections with SGS1 only occurred in chickadees sampled in the city of Boulder (figure 1b), which is our lowest elevation and most urban field site, at an overall prevalence of less than 1% (black-capped chickadees) and approximately 13% (mountain chickadees). Additionally, we successfully amplified a region of the MSP1 allele, and comparisons with the previously described geographical distributions of MSP1 alleles indicated a 100% alignment with the Pr2 variant. Broadly, these results suggest that the SGS1 infecting Colorado chickadees have origins in Europe (table 2, [17]).
Building on the previous survey conducted by Hellgren et al. [17], SGS1 is a globally invasive parasite and the highest density of reported cases are in Southern Europe (figure 2). Avian malaria sampling disparities exist, however, particularly when comparing reports in Europe with other continents. Figure 2 represents our best current understanding of the range of SGS1, which will change with additional sampling. Our comparison of infections among avian families indicates that SGS1 has been identified in 132 bird species across 32 avian families. Our findings indicate that, by a large margin, SGS1 is most abundantly reported in the avian families Passeridae and Paridae (table 1). Chickadees are among other bird species in the Paridae family that experience SGS1 infections. Further, of the genus Poecile—SGS1 has also been reported infecting willow tits sampled in China (Poecile montanus) and marsh tits (Poecile palustris) in Sweden [31,32].
4. Discussion
Here, we report a recent range expansion of the SGS1 strain of the avian malaria parasite, Pl. relictum into two wild, non-migratory bird species in North America. We hypothesize that this range expansion resulted from ecological fitting that was promoted in part by the presence of vectors capable of transmitting the parasite. This would explain why we only observed SGS1 infections in Boulder––it is likely our only field site that has vectors capable of transmitting SGS1 (figure 1a; none of the 72 birds that we sampled at elevations exceeding 1700 m tested positive for SGS1). Currently, there are eight mosquito species known to transmit Pl. relictum (see MalAvi database ‘vector table’). One of these vectors, Culex pipiens, occurs commonly along the Colorado Front Range, but a previous survey showed that it does not occur above the foothills (elevation > 1700 m) [33]. None of the other eight vector species are known to have sustaining populations in the Colorado Front Range (Vector and Disease Control International 2021, personal communication). Our other field sites are above the foothills, in montane habitats that exceed the known elevation range of C. pipiens. Because chickadees typically only disperse once during the juvenile phase of their lives, the malaria parasite infections that they harbour are likely indicative of their current surrounding habitat, rather than a carryover from their natal habitat [34].
Ecological fitting in this system could also be facilitated by the presence of introduced house sparrows (Passer domesticus) that overlap in range with native birds throughout North America. House sparrows are native to Europe and Northern Africa and were first released in the USA by the Brooklyn Institute in 1851 in Brooklyn, New York [35]. The ability of house sparrows to succeed in urban environments, followed by subsequent introductions, has resulted in their range extending throughout North America [35,36]. House sparrows are known to commonly exhibit infections with SGS1 in their native range and a recent study by Dadam et al. [37] suggests that SGS1 is correlated with the decline of house sparrow populations in some towns and cities in Europe [37]. Opportunities for spillover of SGS1 to occur in North American bird species might be enhanced by house sparrows that can act as reservoirs for the parasite and serve as bridges to new hosts.
Given that we found a single SGS1 infection each year, from 2018 to 2020 in our study, the strain appears to be infecting chickadees at a low prevalence. Our report of these infections in chickadees is novel, given that previous studies involving chickadees in other North American locations such as Alaska (n = 1770 black-capped chickadees, [38]) and Pennsylvania (n = 960 hybridizing black-capped and Carolina chickadees) did not find SGS1 infections [39]. While we have only screened chickadees for SGS1 infections, we suspect that infections are found in other local bird species. In particular, we hypothesize that house sparrows harbour these infections and may have promoted this range expansion of SGS1. Interestingly, a survey by Marzal et al. [40], published over 10 years ago on malaria parasite infections of house sparrows, included 42 sparrows sampled in the Colorado Front Range and none of these birds exhibited SGS1 infections, supporting that this is a recent range expansion [40]. Importantly, despite only a single report of SGS1 infecting a wild bird in North America prior to our work, competent vectors exist throughout North America that transmit a different strain of Pl. relictum, GRW4, that has been shown to infect non-migratory birds such as house sparrows throughout the southern parts of the USA [40]. Our finding that the MSP1 Pr2 variant aligns completely with the SGS1 strain that we found infecting chickadees further supports a recent range expansion event with origins in Europe. This is because MSP1 alleles are known to rapidly evolve due to diversifying selection [27,41].
Concern surrounding the emergence of infectious diseases has brought about a surge of interest in identifying the ecological factors that shape parasite range expansions [42]. We hypothesize that SGS1 is able to persist in Boulder (the lowest elevation field site where we sampled birds) by means of ecological fitting because of the presence of competent vectors and access to avian hosts that are genetically similar to hosts in its native range (e.g. chickadees and house sparrows). Given the highly invasive nature of SGS1, and the fact that many North American birds lack a coevolutionary history with SGS1, its presence is concerning for North American bird populations. We also lack an understanding of the extent to which SGS1 has expanded into the Colorado Front Range and the specific circumstances underlying this introduction event. However, we speculate that this range expansion may have occurred from the import of captive birds that harboured infections, followed by subsequent spillover to local birds. This hypothesis is supported by findings from Spottiswoode et al. [43], who reported 17 Inca terns, seven penguins and a single eider duck that tested positive for SGS1 at a New York Zoo [43]. Whether birds sampled in Spottiswoode et al. [43] had been imported from other continents is not known, but it is likely that SGS1 came to the zoo and established transmission among these captive birds with its origin being from an exotic bird that was chronically infected with SGS1. Since the house sparrow is a common species that inhabits zoo gardens, it might be a potential bridge for spillover events between captive and wild bird populations.
SGS1 is clearly infecting chickadees but microscopy methods must be conducted to verify that it is completing the asexual portion of its lifecycle (i.e. producing gametocytes) in this novel host. The relatively low infection prevalence that we observed suggests that chickadees might not be the primary host of SGS1 in this novel geographical region. The effects of SGS1 on North American songbirds are also unclear, but given its invasive nature, it is imperative that we take further measures to document its range expansion (including other host species), drivers and consequences.
Acknowledgements
We thank Vanessa Arnold, Cori Carver, Shay Ding, Katherine Feldmann and Morgan Friedman for assistance with field work for this research. This project was made possible by the numerous individuals throughout Boulder County who allowed K.C.G. and A.N.T. to sample chickadees at their properties. We thank members of the Taylor Lab and the Center for the Study of Origins for discussion on this research.
Ethics
We received permission from all landowners whose properties we sampled chickadees at. All fieldwork for this study was conducted with permission from the Animal Care and Use Committee (IACUC number, 2683), Colorado Parks and Wildlife (License number: 1970147338) and US Fish and Wildlife (Permit number: 24169).
Data accessibility
Data for SGS1 infections from this research are available from the Dryad Digital Repository (doi:10.5061/dryad.pk0p2ngp7) [44]. All data pertaining to SGS1 infections in chickadees from our study have been submitted to the MalAvi database. Additional data that were used to describe the geographical presence of SGS1 and its associated hosts are available through the MalAvi database using the link below for the ‘hosts and sites’ table. http://130.235.244.92/bcgi/malaviReport.cgi?report4=Hosts+And+Sites+Table.
Authors' contributions
A.N.T., S.A.T. and S.B. designed this research. Both A.N.T. and K.C.G. collected blood samples and conducted lab work. A.N.T. analysed the data and wrote this manuscript with significant contributions made by K.C.G., S.A.T. and S.B.. All authors have approved the final version of this manuscript for publication and agree to be held accountable for all aspects of this work.
Competing interests
We declare we have no competing interests.
Funding
Support for this research was provided by the CU Boulder Graduate School, a CU Boulder RIO SEED Grant, the CU Boulder EBIO Department, the NWT LTER, the Society of Systematic Biologists Graduate Student Award Program and the Center for the Study of Origins.
References
- 1.Brooks DR, McLennan DA, León-Règagnon V, Hoberg E. 2006Phylogeny, ecological fitting and lung flukes: helping solve the problem of emerging infectious diseases. Rev. Mex. Biodiver. 77, 225-233. [Google Scholar]
- 2.Loiseau C, Harrigan RJ, Robert A, Bowie RCK, Thomassen HA, Smith TB, Sehgal RNM. 2012Host and habitat specialization of avian malaria in Africa. Mol. Ecol. 21, 431-441. ( 10.1111/j.1365-294X.2011.05341.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morand S, Bordes F. 2015Parasite diversity of disease-bearing rodents of Southeast Asia: habitat determinants and effects on sexual size dimorphism and life-traits. Front. Ecol. Evol. 3, 110. ( 10.3389/fevo.2015.00110) [DOI] [Google Scholar]
- 4.McCoy KD, Léger E, Dietrich M. 2013Host specialization in ticks and transmission of tick-borne diseases: a review. Front. Cell. Infect. Microbiol. 3, 57. ( 10.3389/fcimb.2013.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton DH, Bush SE, Johnson KP. 2004Ecology of congruence: past meets present. Syst. Biol. 53, 165-173. ( 10.1080/10635150490265102) [DOI] [PubMed] [Google Scholar]
- 6.Brooks DR, León-Règagnon V, McLennan DA, Zelmer D. 2006Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 87, S76-S85. ( 10.1890/0012-9658(2006)87[76:EFAADO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 7.Ellis VA, et al. 2015Local host specialization, host-switching, and dispersal shape the regional distributions of avian haemosporidian parasites. Proc. Natl Acad. Sci. USA 112, 11 294-11 299. ( 10.1073/pnas.1515309112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNew SM, Clayton DH. 2018Alien invasion: biology of philornis flies highlighting Philornis downsi, an introduced parasite of galápagos birds. Annu. Rev. Entomol. 63, 369-387. ( 10.1146/annurev-ento-020117-043103) [DOI] [PubMed] [Google Scholar]
- 9.Warner RE. 1968The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70, 101-120. ( 10.2307/1365954) [DOI] [Google Scholar]
- 10.van Riper C, van Riper SG, Goff ML, Laird M.. 1986The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327-344. ( 10.2307/1942550) [DOI] [Google Scholar]
- 11.Atkinson CT, Thomas NJ, Hunter DB (eds) 2008. Parasitic diseases of wild birds. Ames, IA: Wiley-Blackwell. [Google Scholar]
- 12.LaPointe DA, Atkinson CT, Samuel MD. 2012Ecology and conservation biology of avian malaria: ecology of avian malaria. Ann. N Y Acad. Sci. 1249, 211-226. ( 10.1111/j.1749-6632.2011.06431.x) [DOI] [PubMed] [Google Scholar]
- 13.Soares L, Marra P, Gray L, Ricklefs RE. 2017The malaria parasite Plasmodium relictum in the endemic avifauna of eastern Cuba: malaria in Cuban avifauna. Conserv. Biol. 31, 1477-1482. ( 10.1111/cobi.12995) [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Vishnudas CK, Ramakrishnan U, Robin VV, Dharmarajan G.. 2019Geographical and host species barriers differentially affect generalist and specialist parasite community structure in a tropical sky-island archipelago. Proc. R. Soc. B 286, 20190439. ( 10.1098/rspb.2019.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doussang D, Sallaberry-Pincheira N, Cabanne GS, Lijtmaer DA, González-Acuña D, Vianna JA. In press. Specialist versus generalist parasites: the interactions between host diversity, environment and geographic barriers in avian malaria. Int. J. Parasitol. S002075192100179X. ( 10.1016/j.ijpara.2021.04.003) [DOI] [PubMed] [Google Scholar]
- 16.Ricklefs RE, Fallon SM. 2002Diversification and host switching in avian malaria parasites. Proc. R. Soc. Lond. B 269, 885-892. ( 10.1098/rspb.2001.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellgren O, et al. 2015Global phylogeography of the avian malaria pathogen Plasmodium relictum based on MSP1 allelic diversity. Ecography 38, 842-850. ( 10.1111/ecog.01158) [DOI] [Google Scholar]
- 18.Martínez-de la Puente J, Santiago-Alarcon D, Palinauskas V, Bensch S.. 2021Plasmodium relictum. Trends Parasitol. 37, 355-356. ( 10.1016/j.pt.2020.06.004) [DOI] [PubMed] [Google Scholar]
- 19.Bensch S, Hellgren O, Pérez-Tris J. 2009MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353-1358. ( 10.1111/j.1755-0998.2009.02692.x) [DOI] [PubMed] [Google Scholar]
- 20.Ricklefs RE, Outlaw DC, Svensson-Coelho M, Medeiros MCI, Ellis VA, Latta S.. 2014Species formation by host shifting in avian malaria parasites. Proc. Natl Acad. Sci. USA 111, 14 816-14 821. ( 10.1073/pnas.1416356111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fecchio A, Wells K, Bell JA, Tkach VV, Lutz HL, Weckstein JD, Clegg SM, Clark NJ. 2019Climate variation influences host specificity in avian malaria parasites. Ecol. Lett. 22, 547-557. ( 10.1111/ele.13215) [DOI] [PubMed] [Google Scholar]
- 22.McNew SM, et al. 2021Contrasting drivers of diversity in hosts and parasites across the tropical Andes. Proc. Natl Acad. Sci. USA 118, e2010714118. ( 10.1073/pnas.2010714118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turcotte A, Bélisle M, Pelletier F, Garant D. 2018Environmental determinants of haemosporidian parasite prevalence in a declining population of tree swallows. Parasitology 145, 961-970. ( 10.1017/S0031182017002128) [DOI] [PubMed] [Google Scholar]
- 24.Wickham H. 2011ggplot2: ggplot2. WIREs Comput. Stat. 3, 180-185. ( 10.1002/wics.147) [DOI] [Google Scholar]
- 25.R Core Team. 2013R: a language and environment for statistical computing. 3.
- 26.Alley M, Fairley R, Martin D, Howe L, Atkinson T. 2008An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala). N Z Vet. J. 56, 247-251. ( 10.1080/00480169.2008.36842) [DOI] [PubMed] [Google Scholar]
- 27.Hellgren O, Waldenström J, Bensch S. 2004A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797-802. ( 10.1645/GE-184R1) [DOI] [PubMed] [Google Scholar]
- 28.Richard FA, Sehgal RNM, Jones HI, Smith TB. 2002A comparative analysis of PCR-based detection methods for avian malaria. J. Parasitol. 88, 819-822. ( 10.1645/0022-3395(2002)088[0819:ACAOPB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 29.Marzal A, García-Longoria L, Cárdenas Callirgos JM, Sehgal RN. 2015Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol. Invasions 17, 39-45. ( 10.1007/s10530-014-0718-x) [DOI] [Google Scholar]
- 30.Bensch S, et al. 2021Contaminations contaminate common databases. Mol. Ecol. Resour. 21, 355-362. ( 10.1111/1755-0998.13272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis VA, et al. 2020Explaining prevalence, diversity and host specificity in a community of avian haemosporidian parasites. Oikos 129, 1314-1329. ( 10.1111/oik.07280) [DOI] [Google Scholar]
- 32.Huang X, Dong L, Zhang C, Zhang Y. 2015Genetic diversity, temporal dynamics, and host specificity in blood parasites of passerines in north China. Parasitol. Res. 114, 4513-4520. ( 10.1007/s00436-015-4695-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisen L, Bolling BG, Blair CD, Beaty BJ, Moore CG. 2008Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado Front Range. J. Med. Entomol. 45, 12. ( 10.1093/jmedent/45.4.800) [DOI] [PubMed] [Google Scholar]
- 34.Branch CL, Kozlovsky DY, Croston R, Pitera A, Pravosudov VV. 2016Mountain chickadees return to their post-natal dispersal settlements following long-term captivity. Behaviour 153, 551-567. ( 10.1163/1568539X-00003363) [DOI] [Google Scholar]
- 35.Robbins CS. 1973Introduction, spread, and present abundance of the house sparrow in North America. Ornithol. Monogr. 14, 3-9. ( 10.2307/40168051) [DOI] [Google Scholar]
- 36.Sullivan BL, Wood CL, Iliff MJ, Bonney RE, Fink D, Kelling S. 2009eBird: a citizen-based bird observation network in the biological sciences. Biol. Conserv. 142, 2282-2292. ( 10.1016/j.biocon.2009.05.006) [DOI] [Google Scholar]
- 37.Dadam D, Robinson RA, Clements A, Peach WJ, Bennett M, Rowcliffe JM, Cunningham AA. 2019Avian malaria-mediated population decline of a widespread iconic bird species. R. Soc. Open Sci. 6, 182197. ( 10.1098/rsos.182197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson LC, Handel CM, Van Hemert C, Loiseau C, Sehgal RNM. 2016Avian malaria in a boreal resident species: long-term temporal variability, and increased prevalence in birds with avian keratin disorder. Int. J. Parasitol. 46, 281-290. ( 10.1016/j.ijpara.2015.12.008) [DOI] [PubMed] [Google Scholar]
- 39.Rice A, Curry RL, Weckstein JD. 2021Haemosporidian prevalence and community composition vary little across a chickadee hybrid zone. Ornithology 138, ukab035. ( 10.1093/ornithology/ukab035) [DOI] [Google Scholar]
- 40.Marzal A, et al. 2011Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 6, e21905. ( 10.1371/journal.pone.0021905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira MU, Ribeiro WL, Tonon AP, Kawamoto F, Rich SM. 2003Sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-1 (MSP-1) of Plasmodium falciparum. Gene 304, 65-75. ( 10.1016/S0378-1119(02)01180-0) [DOI] [PubMed] [Google Scholar]
- 42.Wells K, Clark NJ. 2019Host specificity in variable environments. Trends Parasitol. 35, 452-465. ( 10.1016/j.pt.2019.04.001) [DOI] [PubMed] [Google Scholar]
- 43.Spottiswoode N, Bartlett SL, Conley KJ, Seimon TA, Griffin DO, Sykes JM. 2020Analysis of Plasmodium lineages identified in captive penguins (Sphenisciformes spp.), eiders (Somateria spp.), and inca terns (Larosterna inca) in a North American zoological collection. J. Zoo Wildl. Med. 51, 140. ( 10.1638/2019-0078) [DOI] [PubMed] [Google Scholar]
- 44.Theodosopoulos AN, et al. 2021Data from: A highly invasive malaria parasite has expanded its range to non-migratory birds in North America. Dryad Digital Repository. ( 10.5061/dryad.pk0p2ngp7) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for SGS1 infections from this research are available from the Dryad Digital Repository (doi:10.5061/dryad.pk0p2ngp7) [44]. All data pertaining to SGS1 infections in chickadees from our study have been submitted to the MalAvi database. Additional data that were used to describe the geographical presence of SGS1 and its associated hosts are available through the MalAvi database using the link below for the ‘hosts and sites’ table. http://130.235.244.92/bcgi/malaviReport.cgi?report4=Hosts+And+Sites+Table.