Abstract
Many species face extinction risks owing to climate change, and there is an urgent need to identify which species' populations will be most vulnerable. Plasticity in heat tolerance, which includes acclimation or hardening, occurs when prior exposure to a warmer temperature changes an organism's upper thermal limit. The capacity for thermal acclimation could provide protection against warming, but prior work has found few generalizable patterns to explain variation in this trait. Here, we report the results of, to our knowledge, the first meta-analysis to examine within-species variation in thermal plasticity, using results from 20 studies (19 species) that quantified thermal acclimation capacities across 78 populations. We used meta-regression to evaluate two leading hypotheses. The climate variability hypothesis predicts that populations from more thermally variable habitats will have greater plasticity, while the trade-off hypothesis predicts that populations with the lowest heat tolerance will have the greatest plasticity. Our analysis indicates strong support for the trade-off hypothesis because populations with greater thermal tolerance had reduced plasticity. These results advance our understanding of variation in populations' susceptibility to climate change and imply that populations with the highest thermal tolerance may have limited phenotypic plasticity to adjust to ongoing climate warming.
Keywords: thermal acclimation, phenotypic plasticity, heat tolerance, trade-off hypothesis, climate change, local adaptation
1. Introduction
Climate change is increasing the frequency and severity of extreme weather events across both aquatic and terrestrial environments [1,2]. In particular, heatwaves have caused mass mortalities of habitat-forming species and may reshape entire ecosystems over the coming century [3,4]. There is an urgent need to understand how thermal limits vary within and across species in order to predict vulnerability to changing temperature regimes [5]. Most of this work has focused on measurements of individual species’ thermal tolerances made in single populations at single points in time. However, several factors may contribute to intraspecific variation in thermal limits, including both phenotypic plasticity and variation among individuals in the magnitude of that plasticity. Phenotypic plasticity describes the ability of a single genotype to produce two or more phenotypes in response to variation in the environment [6–8]. Thermal plasticity, also known as acclimation, or hardening, occurs when prior exposure to different temperatures changes an organism's upper or lower thermal limit [9–12].
In recent years, there has been great interest in quantifying and predicting phenotypic plasticity in wild populations of plants and animals, because it may act as a buffer against extinction risk from climate change, allowing vulnerable populations to adjust their physiology in order to maintain homeostasis [12–20]. Species and populations may vary substantially in their capacity for thermal acclimation, so there is a need to understand what factors contribute to variation in this trait [14,21–23]. Considering intraspecific variation in thermal plasticity is important both because of the implications for a species' evolutionary potential, and because it implies that some populations are more vulnerable to climate warming than others.
There are two competing hypotheses to explain why organisms might vary in their capacity for thermal tolerance acclimation (hereafter, thermal acclimation). One possibility is that variation in this trait is shaped largely by variation in the environment (hereafter, the variability hypothesis), with organisms from more thermally variable and/or seasonal habitats having greater plasticity, as variable climates would select for greater thermal niche breadths [24–28]. In this case, organisms from more thermally stable environments would have the lowest acclimation capacity and therefore are expected to be the most vulnerable to warming [29–32]. An alternate hypothesis suggests that variation in thermal plasticity is governed largely by trade-offs (hereafter, the trade-off hypothesis) [12,21,26,33,34]. If high upper thermal limits are costly (for example, the energetic cost of making heat shock proteins [35]), then organisms might invest either in the ability to maintain a high upper thermal limit, or greater plasticity in these limits, so that those with the highest upper thermal limits would be expected to have the lowest plasticity. In these cases, we might expect organisms from the warmest habitats to be the least able to adjust to further warming [12].
Gunderson & Stillman [36] tested the ability of these hypotheses to explain variation in thermal plasticity among 232 species of ectotherms. They found little support for either scenario, suggesting that variation in the capacity for thermal acclimation may be too idiosyncratic to be explained by any generalizable patterns across ecosystems. However, it may be difficult to identify forces that shape trait variation using interspecific comparisons because correlations among traits can be produced either by selection or physiological trade-offs. Selection may generate associations among traits when correlated environmental conditions produce correlated selection pressures [37–40]. Alternatively, physiological trade-offs can produce negative associations among traits if allocation of resources to one trait results in fewer resources available for another. It is often difficult to untangle the effects of correlated selection and physiological trade-offs. For example, if warmer habitats also tend to be less seasonal, correlated selection can generate a negative correlation between upper thermal limits and plasticity. However, physiological trade-offs can also produce negative correlations between heat tolerance and plasticity, making it difficult to determine the cause of observed correlations. This problem is compounded over longer periods of evolutionary time, and when one population is used as a proxy for an entire species.
By comparing variation in thermal tolerance among populations of the same species, we increase the chances of observing evidence for true trade-offs among traits. This is because populations are subject to fewer obscuring effects of correlated selection pressures as there has been less evolutionary time between populations than between species. [41]. In addition, measurements of traits from individual populations are matched with environmental conditions for that population, rather than the range of conditions experienced by the species as a whole. To date, most macroecological studies of thermal physiology have focused on interspecific variation, giving an incomplete picture of not only the mechanisms, but also the impacts and extent of variation in the plasticity of thermal tolerance. Considering the intraspecific variation in thermal plasticity is important both because of the implications for a species’ evolutionary potential, and because it means that some populations could be more vulnerable to climate warming than others. Finally, there is a growing appreciation that the ecological effects of intraspecific trait variation may equal or even exceed those of interspecific variation for some important traits [42,43].
Here, we report the results of a meta-analysis examining intraspecific patterns of thermal plasticity. We synthesize the results of 20 empirical studies that use common garden laboratory experiments to quantify thermal limit acclimation capacities across multiple populations of 19 species. We observed strong support for the trade-off hypothesis, with the most heat-tolerant populations having the lowest acclimation capacity. Our analysis advances our understanding of forces shaping variation in species' responses to climate change and points to the need for future work that examines among-population variation in thermal plasticity.
2. Methods
(a) . Literature search and criteria for inclusion
Studies that measure thermal tolerance plasticity typically collect organisms from nature and acclimatize them to different temperatures for defined periods of time in the laboratory before measuring thermal tolerance. Important methodological considerations for these types of studies include: (i) for how long and at what temperature organisms are acclimated, (ii) how fast the temperature is changed, and (iii) whether measurements are made on field collected or F1 individuals, given that parental conditions are known to influence thermal plasticity. To identify publications for use in our study, we followed preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (electronic supplementary material, figure S1) [44]. We searched titles, abstracts and keywords in Web of Science (Clarivate Analytics, Philadelphia, USA) using the following search string: (Thermal OR temperatures) AND (Lethal OR ‘Thermal tolerance’ OR ‘Thermal limit’ OR CTmax OR CTmin OR LT50 OR ‘freezing tolerance’) AND (‘Local* Adapt*’ OR ‘Latitud* Var’ OR Intraspecific). We conducted an initial literature search on 24 August 2019 and updated the search on 28 July 2020. We also added studies that we were aware of but were not returned in the literature search. We used the following criteria for inclusion, where each study must have: (1) reported new results of whole-organism upper thermal limit in degrees Celsius for at least two populations of the same species, (2) experimentally measured thermal tolerance after acclimating all individuals to at least two temperatures, (3) reported a measure of error for the thermal tolerance estimate, and (4) not measured tolerance of introduced species, hybrid lines, cultivars, domesticated species or later generations of experimental laboratory populations (greater than F2). Although we initially included both cold and heat tolerance studies, we later excluded cold tolerance studies from our analysis owing to insufficient data. Studies of cold tolerance and additional studies that met criteria (1) and (2) but were excluded based on (3) and/or (4) are listed in the electronic supplementary material, table S1. We screened 400 publications that measured thermal tolerances across populations and identified 20 studies to include in our meta-analysis, representing 19 species. The studies that were accepted after looking through titles and abstracts but were later excluded from our analysis are listed in the electronic supplementary material, table S1. We extracted data from each publication using relevant figures, tables, text or electronic supplementary material. In the case of figures, we used WebPlotDigitizer to extract means and error estimates [45].
Although our data initially included studies that used several different thermal tolerance metrics, in the end, we included only the studies that measured the maximum critical thermal limit (hereafter, CTmax) in our weighted meta-analytic model because of a lack of comparability in thermal tolerance metrics. This method weights data points by sample size and error associated with data collected. Typically, studies in our dataset using the temperature at which 50% of individuals die (LT50) lacked error estimates, an important input in meta-analytic models needed for effect size weighting in our dataset. In addition, plant thermal tolerance metrics were all based on LT50 estimates and therefore our dataset is restricted to animals. Because CTmax was the most commonly used metric, limiting our weighted analysis to studies that used CTmax returned the most robust dataset. We also completed an unweighted analysis using all the available data that fitted our search criteria for upper thermal limits, which is described more in detail below.
(b) . Calculation of effect sizes
To quantify plasticity across studies, we used Hedges' g, the standardized mean difference between CTmax at two or more acclimation temperatures for each population [46]. Hedges' g simply quantifies the change in CTmax between acclimation treatments for each population. For studies that used more than two acclimation temperatures, we used a ‘common control’ approach [47], such that the CTmax of the lowest acclimation temperature was the control for all other acclimation temperature treatments. This approach powerfully uses the most data available but induces correlation between estimates by reusing the control groups, which is addressed in the statistics below. A positive Hedges' g value indicates that the thermal tolerance increased with greater acclimation temperature, whereas a negative value indicates the thermal tolerance decreased. To account for the variation in acclimation temperatures across studies, we included the difference between acclimation temperatures as a predictor in our model. Other studies have used acclimation response ratio (hereafter, ARR; [36]) as a measurement of plasticity. ARR allows for more data to be used because this metric standardizes plasticity across thermal tolerance metrics. In our main analysis, we use meta-analytic methods that require a sampling variance to be included, which allows for variation within and between publications to be modelled directly. For comparability, we also calculated ARR values for all included studies which are included in the electronic supplementary material files. We also performed an unweighted analysis to test whether our results were robust to the inclusion of data that could not be included in the main analysis.
(c) . Modelling approach
We used a weighted inverse-variance random effect meta-regression [48] to evaluate intraspecific variation in the plasticity of thermal tolerance. This approach gives greater weight to the effect sizes calculated from observations with lower error (greater precision) and is preferred over unweighted or vote counting approaches [49]. We included four moderators in our meta-regression: (i) difference in acclimation temperature, (ii) annual range of temperatures experienced at any given location, (iii) mean thermal limit of the lower acclimation temperature, and (iv) ecosystem (marine, terrestrial and freshwater). We included the difference in acclimation temperature as a covariate because it is well known that plasticity can be influenced by the difference in acclimation temperatures used in each experiment. To evaluate the variability hypothesis, we used the annual range of temperatures experienced at every collection location. To compile these temperature records, we extracted remote-sensed temperature data from Bio-ORACLE (sea surface temperature) [50,51] and CHELSA (land and freshwater surface temperature) [52–54]. We calculated the annual temperature range as the difference in the maximum temperature of the warmest month minus the minimum temperature of the coldest month from annual trends for each population. To evaluate the trade-off hypothesis, we included the mean thermal limit of the lowest acclimation temperature as reported by the study. To account for differences in plasticity by habitat, we included ecosystem as a predictor because evidence suggests that thermal tolerance plasticity might be influenced by habitat type [36]. To account for non-independence among observations, we modelled the crossed random effects of study and phylum. To account for the common control approach, we computed the variance-covariance matrix for effect size estimates within each study and used this as the weighting in our meta-regression [47]. To enable comparison among moderators, we centred and scaled all continuous predictors prior to analysis. To evaluate our global model for collinearity between moderators, we calculated variance inflation factors to be less than two for all predictors, showing that our moderators are not collinear [55]. We constructed all possible candidate models with additive predictors and conducted model comparison using corrected Akaike's information criterion (AICc) to evaluate support for competing models [56]. Limited sample size did not allow us to include interaction terms in our modelling. We then used model averaging to average results from candidate models with weights greater than one per cent.
To confirm that the results from our inverse-weighted meta-analytic model would hold if LT50 data were included, we conducted an unweighted analysis. We calculated the ARR for the comparison of the lowest and highest acclimation temperatures from each study. We then constructed a linear mixed model with ARR as the response and thermal limit of the lower acclimation temperature, annual temperature range and ecosystem as predictors. As in the weighted analysis, we included study and phylum as random effects. We again constructed all possible candidate models and conducted model comparison using AICc to evaluate competing models. We used model averaging to average results from candidate models with weights greater than one per cent.
To test the effects that publication bias would have on our results, we calculated Rosenberg's fail-safe number [57], which is the number of hypothetical unpublished studies with a null result that would have to be included in our analysis to change our results (also known as the ‘file drawer effect’). We calculated our threshold as 5n + 10 (where n is the number of independent publications) [57]. We conducted all analyses with R (v. 3.6.3) [58] using the packages ‘metafor’, [59] ‘raster’, [60] ‘tidyverse’, [61], ‘MuMIn’ [62] and ‘glmmTMB’ [63].
3. Results
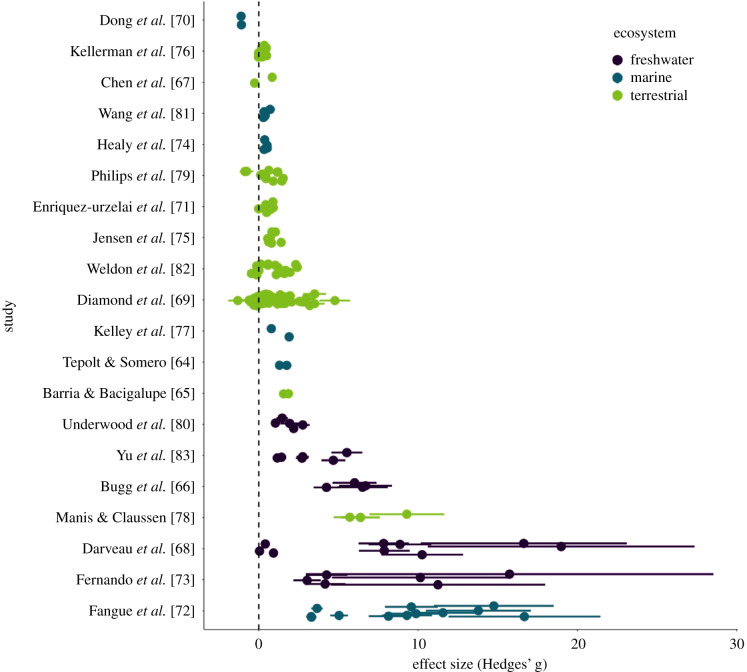
Our literature search identified 400 publications, of which 20 studies met our inclusion criteria. All 20 included studies that used ectotherms, including seven arthropods, 11 chordates and two molluscs (table 1). Species studied included nine from terrestrial, six from marine and five from freshwater environments. Studies quantified plasticity in 4.94 populations on average, with a median of three populations (s.d. = 3.27). Overall, studies reported positive values of Hedges' g with confidence intervals for most studies that did not cross zero, indicating that acclimation resulted in increased thermal limits (figure 1). The sum of model Akaike weights was highest for models including the thermal limit of the lowest acclimation temperature and difference in acclimation temperature as covariates, and these two estimates were the only parameters to deviate from zero. Our meta-analytic regression indicated that there is evidence for a strong negative effect of thermal tolerance on plasticity (figures 2a and 3, estimate = −1.98154, s.e. = 0.23090, p < 0.001). However, we observed no effect of thermal variability on plasticity (figures 2c and 3, estimate = −0.03316, s.e. = 0.04630, p = 0.474). We also did not observe an overall effect of the ecosystem on plasticity (figure 3; electronic supplementary material, table S1). The difference in acclimation temperature covariate had a highly positive effect (figures 2b and 3; estimate = 0.65522, s.e. = 0.05657, p < 0.001). Our unweighted analysis also supports the results from the weighted analysis (electronic supplementary material, table S3). Thermal limit of the lower acclimation temperature was a strong predictor of plasticity (estimate = −0.064, s.e. = 0.010, p < 0.001). By contrast, this model did not reveal an effect of annual temperature range (estimate = 0.001, s.e. = 0.004546, p = 0.8069).
Table 1.
List of publications and associated meta-data used in our weighted analysis. (Note: Reference [64] collected organisms from seven populations, but only two were used in our analysis because five populations were in the species’ introduced range.)
| study | species | number of populations | number of temperatures | ecosystem |
|---|---|---|---|---|
| Barria & Bacigalupe [65] | Pleurodema thaul (frog) | 2 | 2 | terrestrial |
| Bugg et al. [66] | Acipenser fulvescens (fish) | 2 | 3 | freshwater |
| Chen et al. [67] | Buergeria japonica (frog) | 2 | 2 | terrestrial |
| Darveau et al. [68] | Couesius plumbeus (fish) | 3 | 4 | freshwater |
| Diamond et al. [69] | Temnothorax curvispinosus (ant) | 3 | 5 | terrestrial |
| Dong et al. [70] | Cellana toreuma (limpet) | 3 | 2 | marine |
| Enriquez-Urzelai et al. [71] | Rana temporaria (frog) | 7 | 2 | terrestrial |
| Fangue et al. [72] | Fundulus heteroclitus (fish) | 2 | 7 | marine |
| Fernando et al. [73] | Atractosteus spatula (fish) | 3 | 3 | freshwater |
| Healy et al. [74] | Tigriopus californicus (copepod) | 10 | 2 | marine |
| Jensen et al. [75] | Orchesella cincta (insect) | 7 | 2 | terrestrial |
| Kellermann et al. [76] | Drosophila melanogaster (fruit fly) | 2 | 6 | terrestrial |
| Kelley et al. [77] | Carcinus maenas (crab) | 2 | 2 | marine |
| Manis & Claussen [78] | Rana sylvatica (frog) | 5 | 2 | terrestrial |
| Philips et al. [79] | Lampropholis coggeri (skink) | 13 | 2 | terrestrial |
| Tepolt & Somero [64] | Carcinus maenas (crab) | 7 | 2 | marine |
| Underwood et al. [80] | Oncorhynchus clarkii pleuriticus (fish) | 3 | 3 | freshwater |
| Wang et al. [81] | Lottia limatula (limpet) | 2 | 3 | marine |
| Weldon et al. [82] | Ceratitis capitata (fruit fly) | 8 | 3 | terrestrial |
| Yu et al. [83] | Rhynchocypris oxycephalus (fish) | 2 | 4 | freshwater |
Figure 1.
Forest plot showing effect sizes (Hedges' g) for each included publication in the weighted analysis ordered in increasing mean effect size. Each point refers to a pairwise contrast between acclimation temperatures within a population. Error bars denote ± s.d. A positive value refers to an increase in upper thermal limits with acclimation at higher temperature whereas negative values refer to a decrease in thermal limits. The standardized magnitude of change in thermal limits is the measure of plasticity used in this study. (Online version in colour.)
Figure 2.
Graphs showing outputs from our meta-analytic regression model. Meta-analytic scatter plots show plasticity (standardized change in upper thermal limits) as a function of (a) standardized mean thermal limit of the lower acclimation temperature, (b) standardized difference in acclimation temperature and (c) standardized range in annual temperature. All variables were standardized so that the mean is zero. Solid line denotes model predictions, varying terrestrial ecosystems while holding other predictors at the mean. Dotted line shows 95% prediction intervals. (Online version in colour.)
Figure 3.
Plot showing the parameter estimates for each predictor in the model average in the weighted analysis. Error bars represent 95% confidence intervals.
As a measure of the effect that publication bias could have on our results, we calculated Rosenberg's fail-safe number as 61 862, suggesting that a very large number of studies would need to be added to change our results. This is well above our calculated threshold of 110 individual studies, showing that publication bias probably does not influence our analysis. Our model did not show any support for phylum-level random variation (electronic supplementary material, figure S3).
4. Discussion
Phenotypic plasticity, including the capacity for temperature acclimation, plays a major role in species' responses to climate change [84]. We found that the capacity for thermal acclimation varies substantially within species, adding to previous work illustrating among-species variation [36]. Our analysis provides evidence that variation in thermal acclimation capacity in ectotherms is shaped by trade-offs: within species, the populations with the highest upper thermal limit also had the lowest capacity for acclimation. This pattern of intraspecific variation is consistent with limited data from laboratory selection studies that revealed decreased plasticity in populations that were experimentally selected for increased heat tolerance [85–87]. One possible explanation for the observed trade-off is that increased thermal limits may evolve through genetic assimilation of plastic responses [88]. If increased thermal tolerance evolves by converting acclimation responses into fixed thermal tolerance limits, we would expect the evolution of increased heat tolerance to be accompanied by a loss of plasticity. Transcriptomic studies have suggested a similar physiological basis for evolved heat tolerance and plasticity, with heat-tolerant populations having a higher baseline expression of heat stress response genes but a smaller change in gene expression in response to heat stress [89–94].
Many taxa show a strong phylogenetic signal and little variation in upper thermal limits among closely related species. This suggests constraint on the evolution of heat tolerance, and possibly that substantial molecular changes are required to increase upper thermal limits [28,95–97]. As a result, the fixing of a plastic response to temperature may represent evolutionary ‘low hanging fruit’, requiring relatively few evolutionary changes to increase tolerance. For example, in Tigriopus copepods, intraspecific variation in the plastic upregulation of heat shock protein maps to a single locus, indicating a simple genetic basis for this response [98]. By contrast, larger changes in heat tolerance in this species required changes at many loci, suggesting that fixed thermal tolerances were more complex [99].
In contrast with the strong support we observed for the trade-off hypothesis, we observed no correlation between acclimation capacity and environmental variability. This finding is surprising, given the long-standing prediction in macroecology that species from thermally variable environments should tend to have broader thermal niches [22,24,27]. This prediction is borne out across a range of taxa and ecosystems [28,100,101]. However, there are several possible reasons why plasticity may not consistently contribute to this niche breadth. Theory predicts that plasticity will be favoured only when the change in the environment is accompanied by a reliable cue and sufficient lag time to produce the new optimal phenotype [6]. As a result, whether or not the capacity for thermal acclimation is favoured by natural selection depends fundamentally on the timescale and predictability of thermal variation [102–104]. Thus, thermally variable environments with high levels of temporal autocorrelation (‘red noise,’ sensu [105]) might favour plasticity while stochastic daily and hourly variation might favour broad thermal niches that are fixed [106]. Our analysis was only able to incorporate the range of thermal variability, not its predictability. Future studies could attempt to correlate plasticity with the predictability of temperature variation. Also, because each species' thermal plasticity has evolved in the context of its specific thermal regime, laboratory acclimation experiments that reflect natural timescales of thermal variability may be more likely to elicit a plastic response [107]. Future thermal tolerance studies should seek to place laboratory acclimation conditions in the context of natural timescales of thermal variation.
Selection on heat tolerance may also be moderated by the Bogert effect, where behavioural thermoregulation buffers against selection on physiological traits [108–110]. The importance of the Bogert effect is supported by the fact that upper thermal limits and acclimation capacity vary relatively little with latitude in terrestrial ectotherms, but do vary with latitude in marine taxa which have fewer options for behavioural thermoregulation (i.e. owing to sessile life-history stages and/or the spatial grain size of thermal variation in the sea) [36,100].
The strong support we observed for the trade-off hypothesis is surprising, because a much larger meta-analysis examining interspecific patterns of acclimation capacity did not find similar support [36]. One possible explanation for this discrepancy is that the longer periods of evolutionary time between species increases the likelihood that evidence for trade-offs will be obscured by selection induced by environmental conditions that are correlated with temperature [37–40]. It is possible, therefore, that the trade-off hypothesis is universally true but only studies of intraspecific variation like ours can identify these patterns. A meta-analysis restricted to studies that compare acclimation capacity across closely related species (e.g. within a genus) may be better able to detect the patterns found in our study. Another possible reason for the discrepancy between our results and those of Gunderson and Stillman is the different sample sizes between the two studies (78 populations across 19 species versus 232 species). As a result, it is possible that publication bias contributed to the strength of our results, if authors were more likely to publish data that demonstrated plasticity differences between populations, or more likely to test for differences in plasticity in species where they had some reason to suspect that these differences existed, a priori. However, despite our small sample size, the support we observed for the trade-off hypothesis was extremely strong, borne out in the fail-safe number of 61 862, the number of unpublished null results that would have to exist in order to invalidate our results.
Our results are robust across methodologies for measuring upper thermal tolerance, given that the inclusion of LT50 data in the unweighted analysis matches the conclusions of the weighted meta-regression. The electronic supplementary material, table S1 summarizes data from 13 studies that were excluded from our meta-analysis. Across studies that measured heat tolerance, five species showed the same patterns observed here, with the lowest plasticity in the most tolerant population. Three other species either lacked plasticity or exhibited indeterminate relationships where the most tolerant population was neither the most plastic or the least plastic. Only one species (the introduced Nile perch) showed the opposite pattern, where the most tolerant population was also the most plastic. The electronic supplementary material, table S1 also summarizes results for five studies of intraspecific variation in cold tolerance acclimation, which we were unable to include in our analysis owing to the small number of studies on this trait. Intriguingly, four out of five of these studies report that the most cold-tolerant population had the greatest capacity for acclimation, the exact opposite from the pattern we observed for heat tolerance. This suggests that the capacity for cold acclimation does not evolve according to the same trade-offs as heat acclimation and highlights the need for more studies of intraspecific variation in this trait.
Our results highlight the importance of intraspecific variation when forecasting species’ vulnerability to climate change. Most models of climate change responses assume that thermal tolerance is fixed within species (niche conservatism); however, there is a growing recognition that incorporating intraspecific variation can lead to more accurate predictions of extinction risk [111]. The studies incorporated in our analysis highlight three important sources of intraspecific variation: evolved differences, differences produced by acclimation, and evolved differences in the capacity for acclimation.
Our results also have important implications for the joint contributions of plasticity and adaptive evolution to species' vulnerability to climate change. It is generally recognized that both plasticity and adaptive evolution may contribute to climate change responses; but how plasticity and adaptive evolution will interact to influence extinction risk is less clear [112,113]. Most theoretical models assume that the buffering effects of plasticity and adaptation will be additive [114]. However, if, as our study suggests, the evolution of increased heat tolerance incurs a loss of thermal acclimation capacity, it will not tend to confer additional protections above and beyond those already conferred by plasticity. Our results are based on a limited number of studies, but the compelling patterns in our dataset suggest that we may be underestimating the effects of rising temperatures on species persistence. Our work highlights the urgent need for more research on intraspecific variation in thermal plasticity.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation OCE-1764316, ‘Research Coordination Network: Evolution in the Changing Seas' to M.K. and OCE-2023571 to B.S.C. This project was also supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Center for Agriculture, Food and the Environment and the Department of Environmental Conservation at the University of Massachusetts Amherst, under project number MAS00558 to B.S.C. This manuscript is the outcome of a synthesis workshop organized by Kathleen Lotterhos, Molly Albecker, Geoffrey Trussell, Daniel Bolnick, Joanna Kelley, and Morgan Kelly, and hosted by Shoals Marine Laboratory. Finally, we thank the authors of the primary studies.
Data accessibility
Data and code are available at https://github.com/jmbarley1/plasticity and from the Dryad Digital Repository: https://doi.org/10.5061/dryad.zs7h44j8z [115].
Authors' contributions
J.M.B.: conceptualization, data curation, formal analysis, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing; B.S.C.: conceptualization, formal analysis, writing—review and editing; M.S.: conceptualization, data curation, writing—review and editing; S.G.-W.: data curation, writing—review and editing; C.G.H.: data curation, writing—review and editing; A.B.P.: data curation, writing—review and editing; S.S.: data curation, writing—review and editing; A.R.V.: data curation, writing—review and editing; M.K.: conceptualization, data curation, supervision, writing—original draft, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no conflicts of competing interests.
Funding
This study was funded by National Science Foundation OCE-1764316 and OCE-2023571. This project was also supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Center for Agriculture, Food and the Environment and the Department of Environmental Conservation at the University of Massachusetts Amherst, under project number MAS00558. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIFA.
References
- 1.Easterling DR. 2000Climate extremes: observations, modeling, and impacts. Science 289, 2068-2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 2.Ummenhofer CC, Meehl GA. 2017Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135. ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smale DA, et al. 2019Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 9, 306-312. ( 10.1038/s41558-019-0412-1) [DOI] [Google Scholar]
- 4.Stillman JH. 2019Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86-100. ( 10.1152/physiol.00040.2018) [DOI] [PubMed] [Google Scholar]
- 5.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. 2008Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, e325. ( 10.1371/journal.pbio.0060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeWitt TJ, Sih A, Wilson DS. 1998Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77-81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 7.West-Eberhard MJ. 2003Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 8.Scheiner SM. 1993Genetics and evolution of phenotypic plasticity. Ann. Rev. Ecol. Evol. Syst. 24, 35-68. ( 10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 9.Somero GN. 2010The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912-920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 10.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665-1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. 2014Mechanisms of reef coral resistance to future climate change. Science 344, 895-898. ( 10.1126/science.1251336) [DOI] [PubMed] [Google Scholar]
- 12.Stillman JH. 2003Acclimation capacity underlies susceptibility to climate change. Science 301, 65. ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 13.Helmuth B. 2009From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J. Exp. Biol. 212, 753-760. ( 10.1242/jeb.023861) [DOI] [PubMed] [Google Scholar]
- 14.Calosi P, Bilton DT, Spicer JI. 2008Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 4, 99-102. ( 10.1098/rsbl.2007.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seebacher F, White CR, Franklin CE. 2015Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 5, 61-66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 16.Moller AP, Rubolini D, Lehikoinen E. 2008Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195-16 200. ( 10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevin LM, Hoffmann AA. 2017Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138. ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391-3400. ( 10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendry AP. 2016Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 107, 25-41. ( 10.1093/jhered/esv060) [DOI] [PubMed] [Google Scholar]
- 20.Tomanek L. 2010Variation in the heat shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 213, 971-979. ( 10.1242/jeb.038034) [DOI] [PubMed] [Google Scholar]
- 21.van Heerwaarden B, Kellermann V. 2020Does plasticity trade off with basal heat tolerance? Trends Ecol. Evol. 35, 874-885. ( 10.1016/j.tree.2020.05.006) [DOI] [PubMed] [Google Scholar]
- 22.Gaston KJ, et al. 2009Macrophysiology: a conceptual reunification. Am. Nat. 174, 595-612. ( 10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 23.Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI. 2018The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425-1439. ( 10.1111/ele.13107) [DOI] [PubMed] [Google Scholar]
- 24.Janzen DH. 1967Why mountain passes are higher in the tropics. Am. Nat. 101, 233-249. ( 10.1086/282487) [DOI] [Google Scholar]
- 25.Bozinovic F, Calosi P, Spicer JI. 2011Physiological correlates of geographic range in animals. Ann. Rev. Ecol. Evol. Syst. 42, 155-179. ( 10.1146/annurev-ecolsys-102710-145055) [DOI] [Google Scholar]
- 26.Chown SL, Gaston KJ, Robinson D. 2004Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct. Ecol. 18, 159-167. ( 10.1111/j.0269-8463.2004.00825.x) [DOI] [Google Scholar]
- 27.Ghalambor CK. 2006Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5-17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 28.Addo-Bediako A, Chown SL, Gaston KJ. 2000Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739-745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann GE. 2000Heat-shock protein expression is absent in the antarctic fish Trematomus bernacchii (Family Nototheniidae). J. Exp. Biol. 203, 2331-2339. ( 10.1242/jeb.203.15.2331) [DOI] [PubMed] [Google Scholar]
- 30.Somero GN. 2005Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2, 1. ( 10.1186/1742-9994-2-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez Pérez HJ, Garland T. 2009Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939-1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gause GF. 1942The relation of adaptability to adaptation. Q. Rev. Biol. 17, 99-114. ( 10.1086/394649) [DOI] [Google Scholar]
- 34.Chown SL. 2001Physiological variation in insects: hierarchical levels and implications. J. Insect. Physiol. 47, 649-660. ( 10.1016/S0022-1910(00)00163-3) [DOI] [PubMed] [Google Scholar]
- 35.Hoekstra LA, Montooth KL. 2013Inducing extra copies of the Hsp70 gene in Drosophila melanogaster increases energetic demand. BMC Evol. Biol. 13, 68. ( 10.1186/1471-2148-13-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunderson AR, Stillman JH. 2015Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401. ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal AA, Conner JK, Rasmann S. 2010Tradeoffs and negative correlations in evolutionary ecology. In Evolution after Darwin: the first 150 years (eds Bell MA, Eanes WF, Futuyma DJ, Levinton JS), pp. 243-268. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 38.Roff DA, Fairbairn DJ. 2007The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433-447. ( 10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 39.Armbruster WS, Schwaegerle KE. 1996Causes of covariation of phenotypic traits among populations. J. Evol. Biol. 9, 261-276. ( 10.1046/j.1420-9101.1996.9030261.x) [DOI] [Google Scholar]
- 40.Stebbins G. 1950Variation and evolution in plants. New York, NY: Columbia University Press. [Google Scholar]
- 41.Shaw FH, Shaw RG, Wilkinson GS, Turelli M. 1995Changes in genetic variances and covariances: G Whiz! Evolution 49, 1260-1267. ( 10.2307/2410450) [DOI] [PubMed] [Google Scholar]
- 42.Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA, Palkovacs EP. 2018The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2, 57-64. ( 10.1038/s41559-017-0402-5) [DOI] [PubMed] [Google Scholar]
- 43.Bennett S, Duarte CM, Marbà N, Wernberg T. 2019Integrating within-species variation in thermal physiology into climate change ecology. Phil. Trans. R. Soc. B 374, 20180550. ( 10.1098/rstb.2018.0550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. 2009Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097-6. ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohatgi A.2020WebPlotDigitizer - extract data from plots, images, and maps. See https://automeris.io/WebPlotDigitizer (accessed on 3 March 2021).
- 46.Hedges LV. 1981Distribution theory for Glass's estimator of effect size and related estimators. J. Edu. Stat. 6, 107-128. ( 10.3102/10769986006002107) [DOI] [Google Scholar]
- 47.Gleser LJ, Olkin I. 2009Stochastically dependent effect sizes. In The handbook of research synthesis and meta-analysis (eds Cooper H, Hedges LV, Valentine JC), pp. 357-376. New York, NY: Russell Sage Foundation. [Google Scholar]
- 48.Cooper H, Hedges LV, Valentine JC. 2009The handbook of research synthesis and meta-analysis. New York, NY: Russel Sage Foundation. [Google Scholar]
- 49.Koricheva J, Gurevitch J, Mengersen K. 2013Handbook of meta-analysis in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 50.Assis J, Tyberghein L, Bosch S, Verbruggen H, Serrão EA, De Clerck O. 2018Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277-284. ( 10.1111/geb.12693) [DOI] [Google Scholar]
- 51.Tyberghein L, Verbruggen H, Pauly K, Troupin C, Mineur F, De Clerck O. 2011Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272-281. ( 10.1111/j.1466-8238.2011.00656.x) [DOI] [Google Scholar]
- 52.Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M. 2017Climatologies at high resolution for the Earth's land surface areas. Sci. Data 4, 170122. ( 10.1038/sdata.2017.122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck HE, Wood EF, Mcvicar TR, Zambrano-Bigiarini M, Alvarez-Garreton C, Baez-Villanueva OM, Sheffield J, Karger DN. 2020Bias correction of global high-resolution precipitation climatologies using streamflow observations from 9372 catchments. J. Clim. 33, 17. ( 10.5194/egusphere-egu2020-10699) [DOI] [Google Scholar]
- 54.Karger DN, Schmatz DR, Dettling G, Zimmermann NE. 2020High-resolution monthly precipitation and temperature time series from 2006 to 2100. Sci. Data 7, 248. ( 10.1038/s41597-020-00587-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graham MH. 2003Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809-2815. ( 10.1890/02-3114) [DOI] [Google Scholar]
- 56.Burnham KP, Anderson DR. 2004Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261-304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 57.Rosenberg MS. 2005The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464-468. ( 10.1111/j.0014-3820.2005.tb01004.x) [DOI] [PubMed] [Google Scholar]
- 58.R Core Team. In press. — European Environment Agency. See https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 3 March 2021).
- 59.Viechtbauer W. 2010Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1-48. [Google Scholar]
- 60.Hijmans RJ.2021raster: geographic data analysis and modeling. R package version 3.0-7. See https://CRAN.R-project.org/package=raster.
- 61.Wickham H, et al. 2019Welcome to the tidyverse. J. Open Sour. Softw. 4, 1686. ( 10.21105/joss.01686) [DOI] [Google Scholar]
- 62.Barton K.2019MuMIn: multi-model inference. R package version 1.43.15. See https://CRAN.R-project.org/package=MuMIn.
- 63.Brooks ME, Kristensen K, van Benthemm KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017{glmmTMB} balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/rj-2017-066) [DOI] [Google Scholar]
- 64.Tepolt CK, Somero GN. 2014Master of all trades: thermal acclimation and adaptation of cardiac function in a broadly distributed marine invasive species, the European green crab, Carcinus maenas. J. Exp. Biol. 217, 1129-1138. ( 10.1242/jeb.093849) [DOI] [PubMed] [Google Scholar]
- 65.Barria AM, Bacigalupe LD. 2017Intraspecific geographic variation in thermal limits and acclimatory capacity in a wide distributed endemic frog. J. Therm. Biol. 69, 254-260. ( 10.1016/j.jtherbio.2017.08.010) [DOI] [PubMed] [Google Scholar]
- 66.Bugg WS, Yoon GR, Schoen AN, Laluk A, Brandt C, Anderson WG, Jeffries KM. 2020Effects of acclimation temperature on the thermal physiology in two geographically distinct populations of lake sturgeon (Acipenser fulvescens). Conserv. Physiol. 8, coaa087. ( 10.1093/conphys/coaa087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen TC, Kam YC, Lin YS. 2001Thermal physiology and reproductive phenology of Buergeria japonica (Rhacophoridae) breeding in a stream and a geothermal hotspring in Taiwan. Zool. Sci. 18, 7. ( 10.2108/zsj.18.591) [DOI] [Google Scholar]
- 68.Darveau C-A, Taylor EB, Schulte PM. 2012Thermal physiology of warm-spring colonists: variation among lake chub (Cyprinidae: Couesius plumbeus) populations. Physiol. Biochem. Zool. 85, 607-617. ( 10.1086/665539) [DOI] [PubMed] [Google Scholar]
- 69.Diamond SE, Chick LD, Perez A, Strickler SA, Martin RA. 2018Evolution of thermal tolerance and its fitness consequences: parallel and non-parallel responses to urban heat islands across three cities. Proc. R. Soc. B 285, 20180036. ( 10.1098/rspb.2018.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong Y, Han G, Ganmanee M, Wang J. 2015Latitudinal variability of physiological responses to heat stress of the intertidal limpet Cellana toreuma along the Asian coast. Mar. Ecol. Prog. Ser. 529, 107-119. ( 10.3354/meps11303) [DOI] [Google Scholar]
- 71.Enriquez-Urzelai U, Tingley R, Kearney MR, Sacco M, Palacio AS, Tejedo M, Nicieza AG. 2020The roles of acclimation and behaviour in buffering climate change impacts along elevational gradients. J. Anim. Ecol. 89, 1722-1734. ( 10.1111/1365-2656.13222) [DOI] [PubMed] [Google Scholar]
- 72.Fangue NA, Hofmeister M, Schulte PM. 2006Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859-2872. ( 10.1242/jeb.02260) [DOI] [PubMed] [Google Scholar]
- 73.Fernando AV, Lochmann SE, Haukenes AH. 2016Critical thermal maxima of juvenile alligator gar (Atractosteus spatula, Lacépède, 1803) from three Mississippi-drainage populations acclimated to three temperatures. J. Appl. Ichthyol. 32, 701-705. ( 10.1111/jai.13047) [DOI] [Google Scholar]
- 74.Healy TM, Bock AK, Burton RS. 2019Variation in developmental temperature alters adulthood plasticity of thermal tolerance in Tigriopus californicus. J. Exp. Biol. 222, jeb213405. ( 10.1242/jeb.213405) [DOI] [PubMed] [Google Scholar]
- 75.Jensen A, Alemu T, Alemneh T, Pertoldi C, Bahrndorff S. 2019Thermal acclimation and adaptation across populations in a broadly distributed soil arthropod. Funct. Ecol. 33, 833-845. ( 10.1111/1365-2435.13291) [DOI] [Google Scholar]
- 76.Kellermann V, van Heerwaarden B, Sgrò CM. 2017How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proc. R. Soc. B 284, 20170447. ( 10.1098/rspb.2017.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelley AL, de Rivera CE, Buckley BA. 2011Intraspecific variation in thermotolerance and morphology of the invasive European green crab, Carcinus maenas, on the west coast of North America. J. Exp. Mar. Biol. Ecol. 409, 70-78. ( 10.1016/j.jembe.2011.08.005) [DOI] [Google Scholar]
- 78.Manis ML, Claussen DL. 1986Environmental and genetic influences on the thermal physiology of Rana sylvatica. J. Therm. Biol. 11, 31-36. ( 10.1016/0306-4565(86)90014-8) [DOI] [Google Scholar]
- 79.Phillips BL, Muñoz MM, Hatcher A, Macdonald SL, Llewelyn J, Lucy V, Moritz C. 2016Heat hardening in a tropical lizard: geographic variation explained by the predictability and variance in environmental temperatures. Funct. Ecol. 30, 1161-1168. ( 10.1111/1365-2435.12609) [DOI] [Google Scholar]
- 80.Underwood ZE, Myrick CA, Rogers KB. 2012Effect of acclimation temperature on the upper thermal tolerance of Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus: thermal limits of a North American salmonid. J. Fish Biol. 80, 2420-2433. ( 10.1111/j.1095-8649.2012.03287.x) [DOI] [PubMed] [Google Scholar]
- 81.Wang T, Tanner RL, Armstrong EJ, Lindberg DR, Stillman JH. 2019Plasticity of foot muscle and cardiac thermal limits in the limpet Lottia limatula from locations with differing temperatures. Aquat. Biol. 28, 113-125. ( 10.3354/ab00714) [DOI] [Google Scholar]
- 82.Weldon CW, Nyamukondiwa C, Karsten M, Chown SL, Terblanche JS. 2018Geographic variation and plasticity in climate stress resistance among southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Sci. Rep. 8, 9849. ( 10.1038/s41598-018-28259-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu D, Zhang Z, Shen Z, Zhang C, Liu H. 2018Regional differences in thermal adaptation of a cold-water fish Rhynchocypris oxycephalus revealed by thermal tolerance and transcriptomic responses. Sci. Rep. 8, 11703. ( 10.1038/s41598-018-30074-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167-178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 85.Sikkink KL, Reynolds RM, Ituarte CM, Cresko WA, Phillips PC. 2014Rapid evolution of phenotypic plasticity and shifting thresholds of genetic assimilation in the nematode Caenorhabditis remanei. G3 4, 1103-1112. ( 10.1534/g3.114.010553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelly MW, Pankey MS, DeBiasse MB, Plachetzki DC. 2017Adaptation to heat stress reduces phenotypic and transcriptional plasticity in a marine copepod. Funct. Ecol. 31, 398-406. ( 10.1111/1365-2435.12725) [DOI] [Google Scholar]
- 87.Sasaki MC, Dam HG. In press.Negative relationship between thermal tolerance and plasticity in tolerance emerges during experimental evolution in a widespread marine invertebrate. Evol. Appl. eva.13270. ( 10.1111/eva.13270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kelly M. 2019Adaptation to climate change through genetic accommodation and assimilation of plastic phenotypes. Phil. Trans. R. Soc. B 374, 20180176. ( 10.1098/rstb.2018.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. 2013Genomic basis for coral resilience to climate change. Proc. Natl Acad. Sci. USA 110, 1387-1392. ( 10.1073/pnas.1210224110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gleason LU, Burton RS. 2015RNA-seq reveals regional differences in transcriptome response to heat stress in the marine snail Chlorostoma funebralis. Mol. Ecol. 24, 610-627. ( 10.1111/mec.13047) [DOI] [PubMed] [Google Scholar]
- 91.Kim B-M, Kim K, Choi IY, Rhee J-S. 2017Transcriptome response of the Pacific oyster, Crassostrea gigas susceptible to thermal stress: a comparison with the response of tolerant oyster. Mol. Cell Toxicol. 13, 105-113. ( 10.1007/s13273-017-0011-z) [DOI] [Google Scholar]
- 92.Kenkel CD, Meyer E, Matz MV. 2013Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol. Ecol. 22, 4322-4334. ( 10.1111/mec.12390) [DOI] [PubMed] [Google Scholar]
- 93.Franssen SU, Gu J, Bergmann N, Winters G, Klostermeier UC, Rosenstiel P, Bornberg-Bauer E, Reusch TBH. 2011Transcriptomic resilience to global warming in the seagrass Zostera marina, a marine foundation species. Proc. Natl Acad. Sci. USA 108, 19 276-19 281. ( 10.1073/pnas.1107680108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomanek L, Somero GN. 1999Variation in the heat-shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 213, 971-979. ( 10.1242/jeb.038034) [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann AA, Chown SL, Clusella-Trullas S. 2013Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934-949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 96.Bennett JM, et al. 2021The evolution of critical thermal limits of life on Earth. Nat. Commun. 12, 1198. ( 10.1038/s41467-021-21263-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013Heat freezes niche evolution. Ecol. Lett. 16, 1206-1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 98.Tangwancharoen S, Semmens BX, Burton RS. 2020Allele-specific expression and evolution of gene regulation underlying acute heat stress response and local adaptation in the Copepod Tigriopus californicus. J. Hered. 111, 539-547. ( 10.1093/jhered/esaa044) [DOI] [PubMed] [Google Scholar]
- 99.Griffiths JS, Kawji Y, Kelly MW. 2021An experimental test of adaptive introgression in locally adapted populations of splash pool Copepods. Mol. Biol. Evol. 38, 1306-1316. ( 10.1093/molbev/msaa289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sunday JM, Bates AE, Dulvy NK. 2011Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823-1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clusella-Trullas S, Chown SL. 2014Lizard thermal trait variation at multiple scales: a review. J. Comp. Physiol. B 184, 5-21. ( 10.1007/s00360-013-0776-x) [DOI] [PubMed] [Google Scholar]
- 102.Dong Y, Li X, Choi FMP, Williams GA, Somero GN, Helmuth B. 2017Untangling the roles of microclimate, behaviour and physiological polymorphism in governing vulnerability of intertidal snails to heat stress. Proc. R. Soc. B 284, 20162367. ( 10.1098/rspb.2016.2367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernhardt JR, O'Connor MI, Sunday JM, Gonzalez A. 2020Life in fluctuating environments. Phil. Trans. R. Soc. B 375, 20190444. ( 10.1098/rstb.2019.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ashander J, Chevin LM, Baskett ML. 2016Predicting evolutionary rescue via evolving plasticity in stochastic environments. Proc. R. Soc. B 283, 20161690. ( 10.1098/rspb.2016.1690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall DJ, Burgess SC. 2015Deconstructing environmental predictability: seasonality, environmental colour and the biogeography of marine life histories. Ecol. Lett. 18, 174-181. ( 10.1111/ele.12402) [DOI] [PubMed] [Google Scholar]
- 106.Kingsolver JG, Huey RB. 1998Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 38, 545-560. ( 10.1093/icb/38.3.545) [DOI] [Google Scholar]
- 107.Harada AE, Burton RS. 2019Ecologically relevant temperature ramping rates enhance the protective heat shock response in an intertidal ectotherm. Physiol. Biochem. Zool. 92, 152-162. ( 10.1086/702339) [DOI] [PubMed] [Google Scholar]
- 108.Muñoz MM, Losos JB. 2018Thermoregulatory behavior simultaneously promotes and forestalls evolution in a tropical lizard. Am. Nat. 191, E15-E26. ( 10.1086/694779) [DOI] [PubMed] [Google Scholar]
- 109.Muñoz MM, Bodensteiner BL. 2019Janzen's hypothesis meets the Bogert effect: connecting climate variation, thermoregulatory behavior, and rates of physiological evolution. Integr. Org. Biol. 1, oby002. ( 10.1093/iob/oby002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huey RB, Hertz PE, Sinervo B. 2003Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357-366. ( 10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 111.Moran EV, Hartig F, Bell DM. 2016Intraspecific trait variation across scales: implications for understanding global change responses. Glob. Change Biol. 22, 137-150. ( 10.1111/gcb.13000) [DOI] [PubMed] [Google Scholar]
- 112.Chevin L-M, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. 2013Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089. ( 10.1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Samani P, Bell G. 2016The ghosts of selection past reduces the probability of plastic rescue but increases the likelihood of evolutionary rescue to novel stressors in experimental populations of wild yeast. Ecol. Lett. 19, 289-298. ( 10.1111/ele.12566) [DOI] [PubMed] [Google Scholar]
- 114.Chevin LM, Lande R, Mace GM. 2010Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barley JM, Cheng BS, Sasaki M, Gignoux-Wolfsohn S, Hays CG, Putnam AB, Sheth S, Villeneuve AR, Kelly M. 2021Data from: Limited plasticity in thermally tolerant ectotherm populations: evidence for a trade-off. Dryad Digital Repository. ( 10.5061/dryad.zs7h44j8z) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Barley JM, Cheng BS, Sasaki M, Gignoux-Wolfsohn S, Hays CG, Putnam AB, Sheth S, Villeneuve AR, Kelly M. 2021Data from: Limited plasticity in thermally tolerant ectotherm populations: evidence for a trade-off. Dryad Digital Repository. ( 10.5061/dryad.zs7h44j8z) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and code are available at https://github.com/jmbarley1/plasticity and from the Dryad Digital Repository: https://doi.org/10.5061/dryad.zs7h44j8z [115].