Abstract
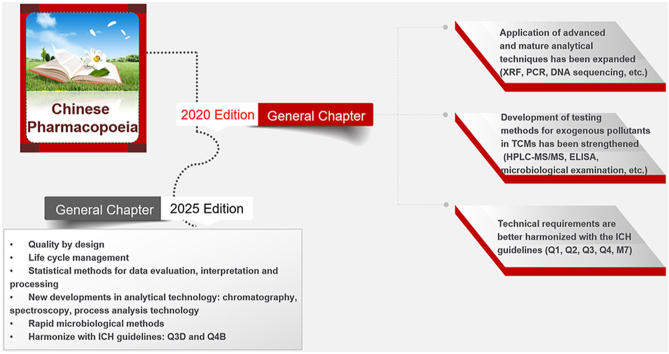
The Chinese Pharmacopoeia 2020 edition was reviewed and approved by the National Medical Products Administration and the National Health Commission of the People's Republic of China in July 2020. The current edition was officially implemented on December 30, 2020. The general chapters of the Chinese Pharmacopoeia discuss the general testing methods and guidelines, which are the common requirements and basis for the implementation of drug standards in the Chinese Pharmacopoeia. Owing to adherence to the principles of scientificity, versatility, operability, and sustainable development, there is an improvement in the general chapters of the 2020 edition over those of the previous editions. Further, the application of advanced and mature analytical techniques has expanded, the development of testing methods for exogenous pollutants in traditional Chinese medicines has been strengthened, and technical requirements are now better harmonized with international standards. The updated edition provides technical and methodological support to ensure safety, effectiveness, and control of pharmaceuticals in China and will play an important and active role in encouraging the application of advanced technologies, improving the quality control of medicines, and strengthening the means of drug regulation in China. This review provides a comprehensive introduction of the main features of and changes to the general chapters in the Chinese Pharmacopoeia 2020 edition and aims to provide reference for its correct understanding and accurate implementation.
Keywords: Chinese pharmacopoeia, 2020 edition, General chapter, Development, Review
Graphical abstract
Highlights
-
•
The additions and revisions of the Chinese Pharmacopoeia 2020 edition general chapters are introduced.
-
•
The applications of advanced techniques have been expanded in the Chinese Pharmacopoeia 2020 edition.
-
•
The technical requirements will be better harmonized with ICH in the Chinese Pharmacopoeia 2025 edition.
1. Introduction
The Chinese Pharmacopoeia 2020 edition was reviewed and approved by the National Medical Products Administration (NMPA) and the National Health Commission of the People's Republic of China in July 2020. This edition was officially implemented on December 30, 2020. The Chinese Pharmacopoeia is a statutory technical specification that must be implemented for drug development, production, use, and regulation in China. The general chapters in the Chinese Pharmacopoeia are the basis for the accurate implementation of the Chinese Pharmacopoeia. The 2020 edition contains 360 general chapters, including 23 new and 83 revised chapters. This updated edition reflects not only the current level of technology used in the pharmaceutical industry in China but also the technologies used for international drug quality control.
2. Application of advanced and mature analytical techniques has been expanded
Method 0451, “X-ray fluorescence spectroscopy”, was added to guide the application of X-ray fluorescence spectroscopy in the qualitative and quantitative analyses of elemental impurities [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. Oscillating transducer density meter application and instrumentation were added in method 0601, “Determination of Relative Density” [10,11]. In method 0713, “Tests of Fat and Fatty Oil”, the melting range, saponification value, and iodine value were revised, and information concerning unsaponifiable matter, fatty acid composition, alkaline impurities, anisidine value, sterols, and trans fatty acids was added [[12], [13], [14], [15], [16]]. Methods 1001, “Polymerase Chain Reactions”; 1021, “Identification of Bacterial DNA Sequences”; and 9108, “DNA Sequencing”, were added. These methods are used to ensure the accurate identification and clinical safety of drugs [[17], [18], [19], [20], [21], [22]]. In vitro methods, which involve the use of an instrument to determine endpoints, have replaced in vivo biological methods, which is in line with the goal of reducing, replacing, and refining laboratory animal use. The anti-factor IIa and anti-factor Xa assays were added to method 1208, “Biological Assay of Heparin” [[23], [24], [25], [26], [27]]. The heparin-binding capacity assay was added to method 1213, “Biological Assay of Protamine Sulfate” [[28], [29], [30], [31], [32]]. New sterilization methods (vapor-phase sterilization and liquid-phase sterilization) were added to method 1421, “Methods of Sterilization”, to guide the sterilization/filtration-based production of drugs to ensure that the sterility level meets the requirements [[33], [34], [35], [36]]. The monocyte activation test was added in guideline 9301, “Application of Safety Tests for Injection” [[37], [38], [39], [40], [41], [42]]. Based on research detailing the technical requirements for the verification and transfer of analytical methods from the United States Pharmacopoeia (USP) and the American Association of Analytical Chemists [[43], [44], [45], [46], [47], [48], [49]] to the Chinese Pharmacopoeia, guidelines 9099, “Verification of Compendial Procedures”, and 9100, “Transfer of Analytical Procedures”, were added.
The applicability requirements of the analytical methods were enhanced. In method 1105, “Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests”, an improved method for the preparation of aerosol test samples was included, and information regarding the quantities of small-dose, low-content, and small-batch samples to be tested was added. In method 1107, “Microbiological Acceptance Criteria of Nonsterile Pharmaceutical Products”, the microbiological acceptance criteria for semisolid preparations were modified to ensure strict control, as is required for liquid preparations. The definition and scope of antimicrobial preservatives in method 1121, “Antimicrobial Effectiveness Testing”, have been revised to enhance accuracy. The recovery rates of the suitability test for the medium and test microorganisms used in the operational suitability test were revised to ensure consistency with method 1105 [50]. Other newly added and revised general testing methods are detailed in Table 1.
Table 1.
Additions and revisions in the Chinese Pharmacopoeia 2020 edition general testing methods.
| General chapter | Additions and revisions |
|---|---|
| 0421, Raman Spectroscopy | Transmittance, tip-enhanced Raman spectroscopy, and imaging techniques were introduced, and their applications in the fields of physics, chemistry, process control, and other analyses was expanded. |
| 0512, High-Performance Liquid Chromatography | The latest HPLC developments and application progress were fully detailed. Information about multidimensional HPLC, charged aerosol detection, adjusted chromatographic conditions, and common qualitative analysis methods was added. |
| 0661, Thermal Analysis | A thermogravimetry-mass spectrometry method was added to realize the qualitative and quantitative analyses of crystallization solvents (aqueous) or other volatile components in a test sample |
| 0981, Crystallinity | Differential scanning calorimetry was added for crystallinity tests of the sharp endothermic peak of crystalline materials or the dispersion (or no endothermic peak) characteristics of amorphous materials. This method can also be used to identify the crystalline form when there is a difference in the endothermic peak position of the solid state of different crystal forms of the same compound. |
| 0991, Determination of Specific Surface Area; 0992, Determination of the Density of Solids |
The basic definitions and terminology of specific surface area and solid density are given. Information about instruments and measuring methods is provided. |
| 1143, Test for Bacterial Endotoxins; 9251, Guideline for the Application of the Bacterial Endotoxin test |
Traceability with international standards and a description of false-positive results and processing methods were added to avoid misinterpretation due to β-glucans. The gel-clot method was revised, and the requirement to initially fill the amoebocyte lysate, followed by the addition of endotoxin, was removed. To standardize the design, procedure, and limit setting of interference experiments, to ensure even quality, and to address a lack of amoebocyte lysate, information about the contents of the bacterial endotoxin for limit setting, choice of methods, and pretreatment methods for test samples were added. The recombinant factor C assay, which is suitable for testing samples containing β-glucans, factor B, and prothrombin, was introduced to address the shortage of amoebocyte lysate resources. |
| 1146, Test for Histamines; 9301, Guideline for the Application of Safety Tests for Injections |
The preparation method for the test histamine solution, the method suitability test, and the determination of the minimum valid concentration test were added. |
| 9015, Guideline for Studies and Quality Control of Drug Polymorphisms | Solid nuclear magnetic resonance spectroscopy method was added. The differences in the chemical environment of the same atomic nucleus of different crystal forms of a test sample causes differences in chemical shifts, coupling constants, and relative intensities during identification of the crystalline states. |
3. Development of testing methods for exogenous pollutants in traditional Chinese medicines (TCMs) has been strengthened
Methods for determining exogenous pollutants in TCMs have been improved in the Chinese Pharmacopoeia 2020 edition. Qualitative screening and quantitative analytical methods, including gas chromatography-tandem mass spectrometry (GC-MS/MS) and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), were added to method 2341, “Determination of Pesticide Residues”. Qualitative screening methods are used for the rapid qualitative testing, risk monitoring, and early warning testing of pesticides. Pesticides with limited requirements can be directly determined using a quantitative analytical method. In the 2020 edition, the number of pesticides tested has increased to 592. Eighty-eight pesticides were identified using GC-MS/MS and 523 pesticides were determined using HPLC-MS/MS. For pesticides that can be determined with GC-MS/MS and HPLC-MS/MS, the preferred method is provided, and the maximum possible number of characteristic ions is recommended [[51], [52], [53], [54], [55], [56]].
Accurate and low-detection-limit methods, including HPLC-MS/MS analysis of aflatoxin and patulin; HPLC and HPLC-MS/MS analyses of ochratoxin A, zearalenone, and vomitoxin; and HPLC-MS/MS analysis of multiple mycotoxins, were added to method 2351, “Determination of Mycotoxins”. Considering that the above-mentioned methods require complex sample pretreatment, specific instruments, and specialized personnel training, they will be subject to limitations for being used in quality control of TCMs. A fast, sensitive, simple, and low-cost aflatoxin ELISA method was added as a new technique for the quality control of TCM in China (Table 2) [[57], [58], [59], [60], [61], [62], [63]].
Table 2.
Comparison of immunological and chemical methods for the determination of aflatoxins.
| Item | Pretreatment | Sensitivity | Type | Percent recovery (%) |
Equipment | Speed | Cost (RMB/test) | Personnel | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hordei Fructus Germinatus | Ziziphi Spinosae Semen | Persicae Semen | Coicis Semen | |||||||||
| ELISA | Direct dilution or extraction, 20–60 min | ng/mL | AFB1 | 82.8–95.9 | 74.7–88.5 | 94.1–101.9 | 84.0–89.1 | Fluorophotometer (10,000–30,000 RMB) | 60 min/test | 30–50 | Ordinary personnel can operate | [58] |
| AFTs | 98.4–110.4 | 87.0–98.6 | 104.0–112.0 | 86.8–103.3 | ||||||||
| HPLC | Immunoaffinity column, 4–8 h | ng/mL | AFB1 | 61.3–70.9 | 57.0–61.8 | 68.1–78.5 | 63.9–69.7 | HPLC (<100,000 RMB) | 3 h/test | 200–300 | Requires trained personnel to operate | [58] |
| AFTs | 62.5–78.5 | 58.1–67.8 | 69.2–84.0 | 65.1–74.5 | ||||||||
AFB1: aflatoxin B1; AFTs: total aflatoxin.
As the development of acceptable microbiological criteria for TCM decoction pieces was a breakthrough, strategies and methods for specific microbial contamination control in TCMs with different uses were introduced. Compared with the medicines produced according to good manufacturing practices, TCM decoction pieces contain microorganisms in high abundance, with a wider variety of species and a more uneven distribution. Further, the microbial analysis requirements are unique to each type of medicinal material. Therefore, method 1108, “Microbiological Examination of Traditional Chinese Medicine Decoction Pieces”, was added. The enumerated microbial parameters include total aerobic microbial counts, total combined yeast/mold counts, and the number of heat-resistant bacteria; the parameters for specified microorganisms include the number of bile-tolerant gram-negative bacteria, Escherichia coli, and Salmonella. The quantity of the product to be tested, the preparation method for the test solution, and the suitability test of the counting method are specified; and the uncertainty in the interpretation of the results can be greater for TCMs than that for other products.
In method 2322, “Determination of Mercury and Arsenic Speciation and Valence States”, the method of test solution preparation was improved to address the difficulties in determining the valence states of arsenic and mercury in marine- and animal-derived TCMs. Notably, information regarding the preparation of the test solution, determination of the sample amount, and principle of the method application, was added.
4. Technical requirements are better harmonized with the International Council for Harmonization (ICH) of technical requirements for pharmaceuticals for human use guidelines
In 2017, the NMPA joined the ICH. In the process of compiling the Chinese Pharmacopoeia 2020 edition, the implementation of international standards was further strengthened (Table 3) [[64], [65], [66], [67]]. Considering the current status of drug production and quality control and the current applicability of products already on the market in China, the newly added general technical requirements are consistent with the ICH guidelines, and the revised general technical requirements are better harmonized with the ICH guidelines as much as possible.
Table 3.
Implementation status of the ICH Q4B in the Chinese Pharmacopoeia 2020 edition.
| ICH No./Chinese Pharmacopoeia No. | Testing method | Implementation status | Main differences | Refs. |
|---|---|---|---|---|
| Annex 1/0841 | Residue on ignition/sulfated ash | In the process of implementation | Sulfuric acid addition amount, ignition temperature, and conditions for the end of the experiment | [[64], [65], [66]] |
| Annex 2/0102, 0942 | Test for extractable volume of parenteral preparations | In the process of implementation | Sampling method, method details, and result interpretation | [[64], [65], [66]] |
| Annex 3/0903 | Test for particulate contamination: subvisible particles | In the process of implementation | Instrument calibration for the light obscuration particle count test, requirements for testing environmental water samples, sampling method, and the evaluation of injections with a labeled volume of 100 mL | [[64], [65], [66]] |
| Annex 4A/1105 | Microbiological examination of nonsterile products: microbial enumeration tests | In the process of implementation | Strains, medium, and method details | [64,66] |
| Annex 4B/1106 | Microbiological examination of nonsterile products: test for specified microorganisms | In the process of implementation | Strains, medium, method details, and result interpretation | [64,66] |
| Annex 4C/1107 | Microbiological examination of nonsterile products: acceptance criteria for pharmaceutical preparations and substances for pharmaceutical use | In the process of implementation | Scope, Salmonella tests, and microbial acceptance criteria for small and microdose preparations such as patches, and standards for traditional Chinese medicines (vegetable medicines) | [64,66] |
| Annex 5/0921 | Disintegration test | In the process of implementation | Apparatus, result interpretation | [[64], [65], [66]] |
| Annex 6/0941 | Uniformity of dosage units | In the process of implementation | Methods, result interpretation | [[64], [65], [66]] |
| Annex 7/0931 | Dissolution test | In the process of implementation | Methods, result interpretation | [[64], [65], [66]] |
| Annex 8/1101 | Sterility test | In the process of implementation | Strains, number of products to be tested, filter times, and quantity of the rinsing fluid | [64,66] |
| Annex 9/0923 | Tablet friability | In the process of implementation | Apparatus, notes | [[64], [65], [66], [67]] |
| Annex 10/0541 | Polyacrylamide gel electrophoresis | In the process of implementation | Method details | [[64], [65], [66]] |
| Annex 11/0542 | Capillary electrophoresis | In the process of implementation | Method details | [[64], [65], [66]] |
| Annex 12/0982 | Analytical sieving | In the process of implementation | Chinese Pharmacopoeia includes the manual sieving method; the ICH guideline includes the sonic-sifter sieving method | [[64], [65], [66]] |
| Annex 13/0993 | Bulk density and tapped density of powders | In the process of implementation | No changes | [[64], [65], [66]] |
| Annex 14/1145 | Bacterial endotoxins test | In the process of implementation | Method description | [64,66] |
Stability is one of the critical factors influencing competivity in the drug market and is an important field of technological innovation. Guideline 9001, “Stability Testing of Drug Substances and Preparations”, was revised according to the ICH Q1A [68]. The definition of “significant changes” in preparation quality was proposed to guide manufacturers to focus on critical quality attributes. Additionally, the requirements for the transportation of temperature-sensitive drugs have been clarified. For special preparations, such as sustained- and controlled-release preparations and inhalations, the important parameters affecting their stability test are listed. Guideline 9101, “Validation of Analytical Methods”, was revised to be consistent with the ICH Q2 [69]; the contents regarding the correction factor were deleted and the methods for accuracy and precision were revised. The reporting, identification, and qualification thresholds for drug impurities and the decision tree of the ICH Q3A and Q3B [70,71] were introduced in guideline 9102, “Analysis of Impurities in Drugs”. To ensure consistency with the ICH Q3C [72], cumene and methyl isobutyl ketone were revised from class 3 solvents to class 2 solvents, and triethylamine, a class 3 solvent, was added to method 0861, “Determination of Residual Solvents”. The flow-through cell and reciprocating cylinder methods were added to method 0931, “Dissolution and Drug Release Test”. The instruments, methods, and interpretations related to the ICH Q4B Annex 7 [[73], [74], [75], [76], [77], [78], [79], [80], [81], [82]] and the research results on specific preparations, such as compound ketoconazole cream and lithium carbonate sustained-release tablets, were introduced. Method 1101, “Sterility Tests”, was improved, based on the ICH Q4B Annex 8, to be more instructive and practical [83]. The scope of environmental monitoring, the storage and use of culture media and strains, the culture time of the medium sensitivity test, the number and quantity of products to be tested, and the requirements of incubation and observation were revised. The bulk density and tapped density are important functionality-related characteristics of pharmaceutical excipients in powder form. These densities are commonly used to calculate the Hausner ratio and compressibility index of the powders. Referring to the ICH Q4B Annex 13 [[84], [85], [86]], method 0993, “Bulk Density and Tapped Density of Powders”, was added. Referring to the ICH M7 [87], guideline 9306, “Genotoxic Impurities Control”, was added, and the general principles, assessment methods, and calculation methods of acceptable intakes and limits were introduced.
However, some general testing methods in the Chinese Pharmacopoeia 2020 edition still differ from those in the ICH Q4B (Table 3). The general testing methods in the Chinese Pharmacopoeia were originally drafted according to the British Pharmacopoeia and the World Health Organization, and these general chapter methods have a long history of use and a wide variety of applications in China. However, the current mainstream drug standard harmonization is based on the Pharmacopoeia Discussion Group and the ICH. Moreover, due to limited information and the complexity of regulatory adjustments, the information in international standards referenced by the Chinese Pharmacopoeia is not comprehensive, and the revisions are not timely. Despite these challenges, harmonization with international standards is still vigorously promoted by the Chinese Pharmacopoeia Commission (ChPC). In October 2018, the ICH Q4 symposium was held in Beijing. More than 20 experts from the ICH Expert Working Group and ChPC discussed strategies for implementing ICH Q4 in China. In 2020, the ICH Q4B implementation status of the Chinese Pharmacopoeia was added to the official ICH website for the first time [64].
5. Summary and prospects
The general chapters of the Chinese Pharmacopoeia 2020 edition are based on science, risk, and applicability, and refer to the ICH guidelines. New technologies and requirements developed in recent years were introduced to provide technical and methodological support to ensure the safety, effectiveness, and controllability of pharmaceuticals in China. The current edition will play an active role in encouraging the application of advanced technologies, improving quality control of drugs, and strengthening the means of drug regulation in China.
As observed from the history of other pharmacopoeias, the development of drug standards is a process of gradual and continuous improvement owing to the limitations of scientific cognition. The concept of quality by design and life cycle management will be further implemented in the general chapters of the 2025 Chinese Pharmacopoeia [[88], [89], [90], [91], [92], [93], [94], [95], [96]]. For example, analytical procedure lifecycle guidelines and process analysis technologies will be introduced, and the roles of statistical methods in data evaluation, interpretation, and processing for the development, validation, transfer, and verification of analytical methods will be strengthened. The system for microbiological control based on risk assessment will also be improved.
Widely used analytical technologies, such as HPLC, GC, and atomic spectroscopy, will be revised. Moreover, additional scientific, objective, and convenient techniques will be introduced in a timely manner. The development of personalized microbial testing methods for specific preparations and research detailing rapid microbiological methods will be further elaborated [[97], [98], [99], [100], [101]]. The testing methods for active and toxic ingredients, exogenous pollutants in crude TCMs, and the microbiological examination requirements for TCM decoction pieces will continue to be improved.
In 2020, the revision of the Q4B guidelines was initiated by the ICH. The ChPC will continue to expand its participation in the harmonization of drug standards and actively promote harmonization/interchangeability with ICH Q4 based on validation. Other ICH guidelines, such as the ICH Q3D, will also be harmonized. Further, the general chapter of “Elemental Impurities Limits and Procedures” in the Chinese Pharmacopoeia will be developed to better assess and control elemental impurities in drugs in China.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors acknowledge the financial support from the Chinese Pharmacopoeia Commission Drug Standard Promoting Funds and Comprehensive Reform of the Chinese Drug and Medical Device Review and Approval System Funds (2015–2020).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Xu X.X., Liu Z., Hong X.X. Application of X-ray fluorescence spectrometry in analysis of drug elemental impurities. Pharm. Clin. Res. 2019;27:368–370. [Google Scholar]
- 2.Resano M., Flórez M.R., Queralt I. Determination of palladium, platinum and rhodium in used automobile catalysts and active pharmaceutical ingredients using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Spectrochim. Acta Part B: At. Spectrosc. 2015;105:38–46. [Google Scholar]
- 3.Lewen N., Soumeillant M., Qiu J. Use of a field-portable XRF instrument to facilitate metal catalyst scavenger screening. Org. Process Res. Dev. 2015;19:2039–2044. [Google Scholar]
- 4.Davis D., Furukawa H. Using XRF as an alternative technique to plasma spectrochemistry for the new USP and ICH directives on elemental impurities in pharmaceutical materials. spectroscopy. 2017;32:12–17. [Google Scholar]
- 5.Balaram V. Recent advances in the determination of elemental impurities in pharmaceuticals-Status, challenges and moving frontiers. Trends Anal. Chem. 2016;80:83–95. [Google Scholar]
- 6.Furukawa H., Ichimaru N., Suzuki K. The comparative verification of calibration curve and background fundamental parameter methods for impurity analysis in drug materials. X Ray Spectrom. 2017;46:382–387. [Google Scholar]
- 7.The United States Pharmacopeial Convention . Vol. 4. United Book Press; Baltimore: 2018. <735> X-ray fluorescence spectrometry; pp. 6486–6491. (United States Pharmacopeia 41). [Google Scholar]
- 8.The European Pharmacopoeia Commission, 2.2.37 . Druckerei C.H. Beck; Nördlingen: 2020. X-ray fluorescence spectrometry; pp. 65–66. (European Pharmacopoeia 10th Edition). [Google Scholar]
- 9.The British Pharmacopoeia Commission . Vol. V. Her/His Majesty’s Stationary Office; London: 2020. Appendix II F: X-ray fluorescence spectrometry. (British Pharmacopoeia 2021). V-A198-V-A199. [Google Scholar]
- 10.The European Pharmacopoeia Commission . Druckerei C.H. Beck; Nördlingen: 2020. 2.2.5 Relative density; pp. 25–26. (European Pharmacopoeia 10th Edition). [Google Scholar]
- 11.Saburou W., Atsusi O., Yasutaka S., Amakara A comparison between the hydrometer method and the oscillating-type density meter method in ethanol concentration measurement. J. Brew. Soc. Jpn. 2007;102:155–159. [Google Scholar]
- 12.The United States Pharmacopeial Convention . Vol. 4. United Book Press; Baltimore: 2018. <401> Fats and fixed oil; pp. 6184–6197. (United States Pharmacopeia 41). [Google Scholar]
- 13.Animal and vegetable fats and oils — gas chromatography of fatty acid methyl esters — Part 1: Guidelines on modern gas chromatography of fatty acid methyl esters. ISO International Standard. 2014;12966–1 [Google Scholar]
- 14.Fat (total, saturated and unsaturated) in foods, hydrolytic extraction gas chromatographic method. AOAC Official Method. 2001;6 996. [Google Scholar]
- 15.P Muniz M.A., Santos M.N.F.D., Costa C.E.F. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Phcog. Mag. 2015;11:147–151. doi: 10.4103/0973-1296.149730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Foster E.N., Adebayo A.S., Justiz-Smith N. Physico-chemical properties of Blighia sapida (ackee) oil extract and its potential application as emulsion base. Afr. J. Pharm. Pharmacol. 2012;6:200–210. [Google Scholar]
- 17.The United States Pharmacopeial Convention . Vol. 5. United Book Press; Baltimore: 2018. <1113> Microbial Characterization, Identification, and Strain Typing; <1125>Nucleic acid-based techniques; <1126> Nucleic acid-based techniques-extraction, detection and sequencing; <1127> Nucleic acid-based techniques-amplification; <1128> Nucleic acid-based techniques-microarray; <1129> Nucleic acid-based techniques-genotyping; <1130> Nucleic acid-based techniques-approaches for detecting trace nucleic acids (residual DNA testing); <1132> Residual Host Cell Protein Measurement in Biopharmaceuticals; pp. 7301–7305. (United States Pharmacopeia 41). 7353-7414. [Google Scholar]
- 18.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. 2.6.21 Nucleic acid amplification techniques, 2.6.7 Mycoplasma; pp. 219–224. 194-199. [Google Scholar]
- 19.Japanese Pharmacopoeia 17th Edition. Ministry of Health, Labour and Welfare; Tokyo: 2016. The Committee on Japanese Pharmacopoeia, G4 Microorganisms: rapid identification of microorganisms based on molecular biological methods, G5 Purity test on crude drugs using genetic information; pp. 2503–2505. 2516-2519. [Google Scholar]
- 20.Xu G., Wang X., Liu C. Authentication of official Da-huang by sequencing and multiplex allele-specific PCR of a short maturase K gene. Genome. 2013;56:109–113. doi: 10.1139/gen-2012-0182. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y., Wang Z.Q., Jiang C. Establishment of polymerase chain reaction method in Chinese Pharmacopoeia (2020 edition) China J. Chin. Mater. Med. 2020;45:4537–4544. doi: 10.19540/j.cnki.cjcmm.20200603.609. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C., Yuan Y., Chen M.F. Molecular authentication of multispecies honeysuckle tablets. Genet. Mol. Res. 2013;12:4827–4835. doi: 10.4238/2013.October.22.2. [DOI] [PubMed] [Google Scholar]
- 23.The United States Pharmacopeial Convention . Vol. 4. United Book Press; Baltimore: 2018. <208> Anti-factor Xa and anti-factor IIa assays for unfractionated and low molecular weight heparins; pp. 6113–6117. (United States Pharmacopeia 41). [Google Scholar]
- 24.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. 2.7.5 Assay of heparin. pp. 269. [Google Scholar]
- 25.Martinez C., Savadogo A., Agut C. Reproducibility of the anti-Factor Xa and anti-Factor IIa assays applied to enoxaparin solution. J. Pharmaceut. Biomed. Anal. 2013;81–82:138–145. doi: 10.1016/j.jpba.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T., Ishii-Watabe A., Hashii N. The establishment and validation of efficient assays for anti-IIa and anti-Xa activities of heparin sodium and heparin calcium. Biologicals. 2013;41:415–423. doi: 10.1016/j.biologicals.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Berkovskii A.L., Sergeeva E.V., Suvorov A.V. Evaluating the activity of low-molecular-weight heparin in preparations and substances. Pharm. Chem. J. 2012;46:249–252. [Google Scholar]
- 28.Zhang Y., Li Z., Tan D.J. Feasibility study of heparin-binding capacity method for testing biological potency of protamine sulfate. Chin. J. Pharm. Anal. 2017;37:1260–1265. [Google Scholar]
- 29.Guo Y.D., Wu B., Hu Y.C. Study on the standard of biological assay of protamine sulfate. Chin. J. Pharm. Anal. 2017;8:1541–1547. [Google Scholar]
- 30.Japanese Pharmacopoeia 17th Edition. Ministry of Health, Labour and Welfare; Tokyo: 2016. The committee on Japanese pharmacopoeia, protamine sulfate; pp. 1483–1484. [Google Scholar]
- 31.The United States Pharmacopeial Convention . Vol. 3. United Book Press; Baltimore: 2018. Protamine sulfate; pp. 3503–3504. (United States Pharmacopeia 41). [Google Scholar]
- 32.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. Protamine sulfate; pp. 3667–3669. [Google Scholar]
- 33.The United States Pharmacopeial Convention . Vol. 5. United Book Press; Baltimore: 2018. <1229.1> Steam sterilization by direct contact, <1229.2> Moist heat sterilization of aqueous liquids, <1229.4> Sterilizing filtration of liquids, <1229.6> Liquid-phase sterilization, <1229.11> Vapor phase sterilization; pp. 7689–7706. (United States Pharmacopeia 41). 7709-7716, 7719-7722, 7733-7734. [Google Scholar]
- 34.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. 5.1.1 Methods of preparation of sterile products; pp. 619–622. [Google Scholar]
- 35.Japanese Pharmacopoeia 17th Edition. Ministry of Health, Labour and Welfare; Tokyo: 2016. The committee on Japanese pharmacopoeia, G4 microorganisms: sterilization and sterilization indicators; pp. 2507–2513. [Google Scholar]
- 36.Sterilization of health care products — radiation — Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices. ISO International Standard. 2006;11137–1 [Google Scholar]
- 37.Koryakina A., Frey E., Bruegger P. Cryopreservation of human monocytes for pharmacopeial monocyte activation test. J. Immunol. Methods. 2014;405:181–191. doi: 10.1016/j.jim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Mattos K.A., Navega E.C.A., Silva V.F. Applicability of the monocyte activation test (MAT) in the quality control of the 17DD yellow fever vaccine. Altern. Lab. Anim. 2018;46:23–37. doi: 10.1177/026119291804600107. [DOI] [PubMed] [Google Scholar]
- 39.Wunderlich C., Schumacher S., Kietzmann M. Pyrogen detection methods: comparison of bovine whole blood assay (bWBA) and monocyte activation test (MAT) BMC Pharmacol. Toxicol. 2014;15:50. doi: 10.1186/2050-6511-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentini S., Santoro G., Baffetta F. Monocyte-activation test to reliably measure the pyrogenic content of a vaccine: an in vitro pyrogen test to overcome in vivo limitations. Vaccine. 2019;37:3754–3760. doi: 10.1016/j.vaccine.2018.10.082. [DOI] [PubMed] [Google Scholar]
- 41.Utescher C.L.A., Buosi K.L., Botosso V.F. Monocyte activation test (MAT) as a possibility of replacement for the rabbit pyrogen test in hyperimmune sera. Braz. J. Pharm. Sci. 2018;54 [Google Scholar]
- 42.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. 2.6.30 monocyte-activation test; pp. 233–239. [Google Scholar]
- 43.The United States Pharmacopeial Convention . Vol. 5. United Book Press; Baltimore: 2018. 1226> Verification of compendial procedures, <1224> Transfer of analytical procedures; pp. 7671–7672. (United States Pharmacopeia 41). 7663-7665. [Google Scholar]
- 44.Eichhorn J., Mentgen-Wolny M. Verification of pharmacopoeial methods in the analytical laboratory: how comprehensive in practice? Pharm. Ind. (Pharmind) 2017;79:274–279. [Google Scholar]
- 45.Zuo T.T., Jin H.Y., Xu M.Z. Transfer, validation and verification of analytical procedures described in General chapter <1224> <1225> <1226> of U.S. Pharmacopoeia (USP37-NF32) and their significances for trace analysis assurance system of traditional Chinese medicines. Chin. J. Pharm. Anal. 2016;36:868–872. [Google Scholar]
- 46.Ermer J., Limberger M., Lis K. The transfer of analytical procedures. J. Pharmaceut. Biomed. Anal. 2013;85:262–276. doi: 10.1016/j.jpba.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Fromke C., Hothorn L.A., Sczesny F. Analytical method transfer: improving interpretability with ratio-based statistical approaches. J. Pharmaceut. Biomed. Anal. 2013;74:186–193. doi: 10.1016/j.jpba.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Kaminski L., Schepers U., Watzig H. Analytical method transfer using equivalence tests with reasonable acceptance criteria and appropriate effort: extension of the ISPE concept. J. Pharmaceut. Biomed. Anal. 2010;53:1124–1129. doi: 10.1016/j.jpba.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Agut C., Caron A., Giordano C. Transfer of analytical procedures: a panel of strategies selected for risk management with emphasis on an integrated equivalence-based comparative testing approach. J. Pharmaceut. Biomed. Anal. 2011;56:293–303. doi: 10.1016/j.jpba.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 50.Vu N.L., Nguyen K., Kupiec T. The essentials of United States Pharmacopeia Chapter antimicrobial effectiveness testing and its application in pharmaceutical compounding. Int. J. Pharm. Compd. 2014;18:123–130. [PubMed] [Google Scholar]
- 51.He H.R., Gao F., Zhang Y.H. Effect of processing on the reduction of pesticide residues in a traditional Chinese medicine (TCM) Food Addit. Contam. 2020;37:1156–1164. doi: 10.1080/19440049.2020.1748725. [DOI] [PubMed] [Google Scholar]
- 52.Huang J.W., Nan T.G., Yuan Y. Basic data investigation for quality of traditional Chinese medicine analysis of pesticide residues. Chin. J. Exp. Tradit. Med. Formulae. 2017;24:56–61. [Google Scholar]
- 53.Xiao J.J., Xu X., Fan W. Analysis of exposure to pesticide residues from traditional Chinese medicine. J. Hazard Mater. 2019;365:857–867. doi: 10.1016/j.jhazmat.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y.Y., Han G.M., Sun C. Research on sample preparation techniques for pesticide residues in Chinese medicinal materials. Res. Pract. Chin. Med. 2015;3:81–84. [Google Scholar]
- 55.Wang L.L., Kong W.J., Yang M.H. Safety issues and new rapid detection methods in traditional Chinese medicinal materials. Acta Pharm. Sin. B. 2015;5:38–46. doi: 10.1016/j.apsb.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z.F., Xu J., Li J.M. Determination of pesticide residues in ganoderma lucidum by GC-MS and GC-MS/MS. Res. Pract. Chin. Med. 2015;5:20–23. [Google Scholar]
- 57.Xie H., Zhang X., Wang X. Preparation of anti-aflatoxin B1 monoclonal antibodies and its use in an indirect competitive ELISA for aflatoxin B1. Microbiology (Beijing, China) 2015;42:2033–2040. [Google Scholar]
- 58.Nan T.G., Hong X.Y., Xu X.Y. Development of the enzyme-linked immunosorbent assay of aflatoxin of Chinese herbal medicines. China J. Chin. Mater. Med. 2020;45:4158–4162. doi: 10.19540/j.cnki.cjcmm.20200608.101. [DOI] [PubMed] [Google Scholar]
- 59.Roman B.E., Driksna D., Abouzied M.M. Validation of MAX aqueous extraction on Veratox® for total aflatoxin ELISA test kit. J. AOAC Int. 2017;100:1131–1133. doi: 10.5740/jaoacint.17-0034. [DOI] [PubMed] [Google Scholar]
- 60.Peng H., Chang Y.W., Baker R.C. Interference of mycotoxin binders with ELISA, HPLC and LC-MS/MS analysis of aflatoxins in maize and maize gluten. Food Addit. Contam. 2020;37:496–506. doi: 10.1080/19440049.2019.1701717. [DOI] [PubMed] [Google Scholar]
- 61.Pereira C.S., Cunha S.C., Fernandes J.O. Validation of an enzyme-linked immunosorbent assay (ELISA) test kit for determination of aflatoxin B1 in corn feed and comparison with liquid-chromatography tandem mass spectrometry (LC-MS/MS) Method. Food Anal. Method. 2020;13:1806–1816. [Google Scholar]
- 62.Beyene A.M., Du X.W., Schrunk D.E. High-performance liquid chromatography and enzyme-linked immunosorbent assay techniques for detection and quantification of aflatoxin B1 in feed samples: a comparative study. BMC Res. Notes. 2019;12:492. doi: 10.1186/s13104-019-4538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamasaki T., Miyake S., Sato N. Development of enzyme-linked immunosorbent assay for analysis of total aflatoxins based on monoclonal antibody reactive with aflatoxins B1, B2, G1 and G2. J. Food Hyg. Soc. Jpn. 2018;59:200–205. doi: 10.3358/shokueishi.59.200. [DOI] [PubMed] [Google Scholar]
- 64.Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions Q4B, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://www.ich.org/page/quality-guidelines. (accessed on 11 December, 2020).
- 65.Xu X.X., Xu H.Y., Jin G.M. Overview and analysis of general chapters (appendices) of physical and chemical testing methods in pharmacopoeias. Chin. Pharmaceut. J. 2018;15:1323–1332. [Google Scholar]
- 66.Zhang Z., Xu X.X., Liu Z. Comparative assessment of the differences between Chinese Pharmacopoeia and ICH Q4B detection methods. Chin. Food & Drug Admin. Mag. 2019;12:24–33. [Google Scholar]
- 67.Xu X.X., Liu Z., Zhang Z. Review of the Chinese Pharmacopoeia tablet friability test revision history and prospects for harmonization with ICH. Chin. Pharm. 2019;22:1138–1140. [Google Scholar]
- 68.Stability testing of new drug substances and products Q1A (R2), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf. (accessed on 11 December, 2020).
- 69.Validation of analytical procedures: text and methodology Q2 (R1), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q2_R1__Guideline.pdf. (accessed on 11 December, 2020).
- 70.Impurities in new drug substances Q3A (R2), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q3A_R2__Guideline.pdf. (accessed on 11 December, 2020).
- 71.Impurities in new drug products Q3B (R2), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q3B_R2__Guideline.pdf. (accessed on 11 December, 2020).
- 72.Impurities for residual solvents Q3C (R6), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q3C-R6_Guideline_ErrorCorrection_2019_0410_0.pdf. (accessed on 11 December, 2020).
- 73.Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on dissolution test general chapter Q4B Annex 7 (R2), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q4B%20Annex%207%20%28R2%29%20Guideline.pdf. (accessed on 11 December, 2020).
- 74.The European Pharmacopoeia Commission . European Pharmacopoeia 10th Edition. Druckerei C.H. Beck; Nördlingen: 2020. 2.9.3 Dissolution test for solid dosage forms; pp. 326–333. [Google Scholar]
- 75.Hori S., Kawada T., Kogure S. Comparative release studies on suppositories using the basket, paddle, dialysis tubing and flow-through cell methods I. Acetaminophen in a lipophilic base suppository. Pharmaceut. Dev. Technol. 2017;22:130–135. doi: 10.1080/10837450.2016.1230132. [DOI] [PubMed] [Google Scholar]
- 76.Medina R., Garcia C.A., Hurtado M. Comparison of USP paddle and flow-through cell dissolution methods for testing ketoprofen and acetaminophen from fixed-dose combination formulation. Lat. Am. J. Pharm. 2016;35:1573–1581. [Google Scholar]
- 77.Paprskářová A., Možná P., Oga E.F. Instrumentation of flow-through USP IV dissolution apparatus to assess poorly soluble basic drug products: a technical note. AAPS PharmSciTech. 2016;17:1261–1266. doi: 10.1208/s12249-015-0444-4. [DOI] [PubMed] [Google Scholar]
- 78.Qiu S., Wang K., Li M.Z. In vitro dissolution studies of immediate-release and extended-release formulations using flow-through cell apparatus 4. Dissolution Technol. 2014;21:6–16. [Google Scholar]
- 79.Emara L.H., Elsayed E.W., El-Ashmawy A.A. The flow-through cell as an in vitro dissolution discriminative tool for evaluation of gliclazide solid dispersions. J. Appl. Pharmaceut. Sci. 2017;7:70–77. [Google Scholar]
- 80.Rudd N.D., Reibarkh M., Fang R. Interpreting in vitro release performance from long-acting parenteral nanosuspensions using USP-4 dissolution and spectroscopic techniques. Mol. Pharm. 2020;17:1734–1747. doi: 10.1021/acs.molpharmaceut.0c00208. [DOI] [PubMed] [Google Scholar]
- 81.Pezzini B.R., Issa M.G., Duque M.D. Applications of USP apparatus 3 in assessing the in vitro release of solid oral dosage forms. Braz. J. Pharm. Sci. 2015;51:265–273. [Google Scholar]
- 82.Perivilli S., Kakhi M., Stippler E. Computational fluid dynamics simulation of hydrodynamics in USP apparatus 3-the influence of dip rate. Pharm. Res. 2015;32:1304–1315. doi: 10.1007/s11095-014-1534-9. [DOI] [PubMed] [Google Scholar]
- 83.Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on sterility test general Chapter Q4B Annex 8 (R1), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q4B%20Annex%208%28R1%29%20Guideline.pdf. (accessed on 11 December, 2020).
- 84.Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on bulk density and tapped density of powders general Chapter Q4B Annex 13, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q4B_Annex_13_Annex.pdf. (accessed on 11 December, 2020).
- 85.Silva J.P.S., Splendor D., Goncalves I.M.B. Note on the measurement of bulk density and tapped density of powders according to the European Pharmacopeia. AAPS PharmSciTech. 2013;14:1098–1100. doi: 10.1208/s12249-013-9994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akseli I., Hilden J., katz J.M. Reproducibility of the measurement of bulk/tapped density of pharmaceutical powders between pharmaceutical laboratories. J. Pharm. Sci. 2019;108:1081–1084. doi: 10.1016/j.xphs.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7 (R1), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf. (accessed on 11 December, 2020).
- 88.Nethercote P., Ermer J. Quality by design for analytical methods: implications for method validation and transfer. Pharmaceut. Technol. 2012;36:74–79. [Google Scholar]
- 89.Pharmaceutical development Q8 (R2), Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf. (accessed on 11 December, 2020).
- 90.Quality risk management Q9, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q9%20Guideline.pdf. (accessed on 11 December, 2020).
- 91.Pharmaceutical quality system Q10, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q10%20Guideline.pdf. (accessed on 11 December, 2020).
- 92.Development and manufacture of drug substances (chemical entities and biotechnological/biological entities) Q11, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q11%20Guideline.pdf. (accessed on 11 December, 2020).
- 93.Technical and regulatory considerations for pharmaceutical product lifecycle management Q12, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf. (accessed on 11 December, 2020).
- 94.Ich Q13: Continuous manufacturing for drug substances and drug products, compilation prepared by international council for harmonisation of technical requirements for pharmaceuticals for human use (ICH). https://database.ich.org/sites/default/files/Q13%20Business%20Plan.pdf. (accessed on 11 December, 2020).
- 95.Ich Q14: Analytical procedure development and revision of Q2 (R1) analytical validation, Compilation prepared by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://database.ich.org/sites/default/files/Q2R2-Q14_EWG_Concept_Paper.pdf. (accessed on 11 December, 2020).
- 96.Parr M.K., Schmidt A.H. Life cycle management of analytical methods. J. Pharmaceut. Biomed. Anal. 2018;147:506–517. doi: 10.1016/j.jpba.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 97.Bugno A., Sanches D.P.S., Almodovar A.A.B. Performance survey and comparison between rapid sterility testing method and pharmacopoeia sterility test. J. Pharm. Inno. 2018;13:27–35. doi: 10.1007/s12247-017-9303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller M.J., Heuvel E.V., Roesti D. The role of statistical analysis in validating rapid microbiological methods. Eur. Biopharm. Rev. 2016;21:46–53. [Google Scholar]
- 99.Chisholm J., Bhatt S., Chaboureau A. Strategy for an abbreviated in-house qualification of a commercially available Rapid Microbiology Method (RMM) for canadian regulatory approval. Cytotherapy. 2017;19:1529–1536. doi: 10.1016/j.jcyt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Suessner S., Hennerbichler S., Schreiberhuber S. Validation of an alternative microbiological method for tissue products. Cell Tissue Bank. 2014;15:277–286. doi: 10.1007/s10561-014-9455-8. [DOI] [PubMed] [Google Scholar]
- 101.Moldenhauer J. The rush to rapid microbiological methods-or not. Eur. Pharm. Rev. 2017;22:28–30. [Google Scholar]