Abstract
We prospectively studied whether left atrial (LA) fibrosis is a determinant of atrial fibrillation (AF) in mitral stenosis in patients who underwent balloon mitral valvotomy. There were 2 groups: Group A (n = 16), with AF and Group B (n = 27), without AF. Fibrosis was assessed by MRI. Patients underwent cardioversion before MRI. There were 27 females and 16 males, aged 29 ± 6 years. The LA areas in Groups A and B were 54.3 ± 4.4 mm2 and 39.4 ± 2.3 mm2 (p < 0.05) and the LA volume index was 46.2 ± 2.9 ml/m2 vs 33 ± 3 ml/m2 respectively (p < 0.0001). The presence of LA scarring was not statistically different in the two groups.
Keywords: Arrhythmias, Echocardiography, MRI
1. Introduction
AF is the most common sustained arrhythmia worldwide, and in India, rheumatic mitral stenosis (MS) constitutes an important cause. There is a complex interplay of structural remodeling and electrophysiological abnormalities that promote the onset and perpetuation of AF. It has been hypothesized that initiation and sustenance of AF are due to a combination of both focal firing and reentry, facilitated by LA fibrosis.1
2. Method
This was a single-centre, prospective, observational study, after Institutional review board approval and informed consent. We included patients aged 12–40 years, who had undergone balloon mitral valvotomy (BMV). The patients were divided into 2 groups: Group A, with AF (n = 16) and Group B, in sinus rhythm (n = 27). Every patient was subjected to cardiac MRI. All patients who continued to be in AF after BMV were successfully cardioverted with 100–200 J of synchronous DC cardioversion before the MRI. Variables such as pulmonary hypertension, LA area, LA Volume Index and LA fibrosis were documented. Echocardiographically the LA area was measured using the biplane area-length method at the point of atrial end diastole, in the parasternal long axis view. All measurements were reviewed independently by two cardiologists.
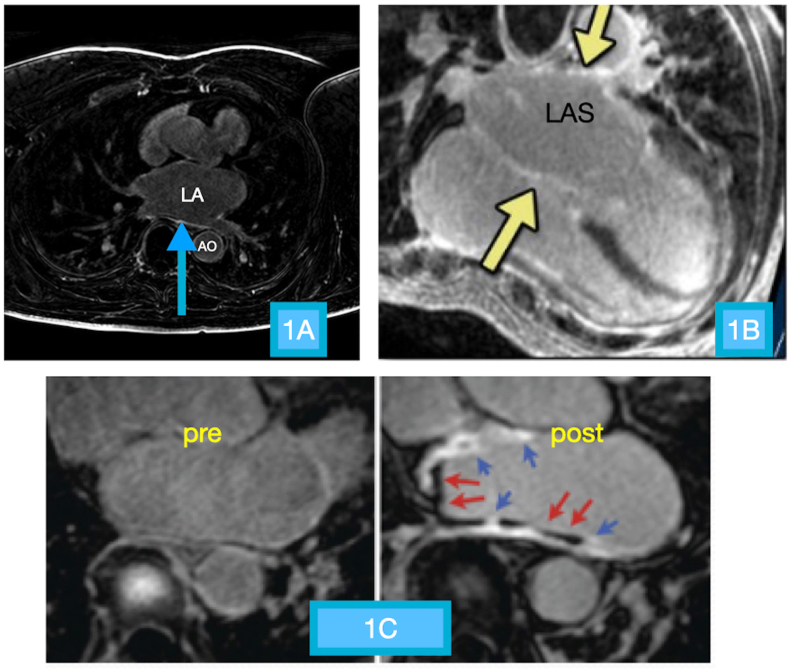
The cardiac MRI (Fig. 1) was performed with a 3 T MRI unit (Philips Achieva) with dedicated 32 channel coil. The protocol also used three dimensional inversion recovery, fast spoiled gradient recall sequence, respiration navigation and ECG gating for image acquisition. Delayed gadolinium enhancement (DGE) imaging was acquired typically 12–15 min after contrast injection. The MRI images were analysed by a blinded experienced investigator. For LA analysis, all parameters were measured at end systole using the frame immediately before the opening of the mitral leaflets. The maximum LA volume was measured using the area-length method. DGE images were obtained in 8-to-14 matching short-axis (8-mm thick, no gap) and 3 radial long–axis planes (2, 3 and 4 chamber views). The degree of fibrosis (%) of the LA was determined by using eyeballing method through expert opinion. Currently no study demonstrating the extent of correlation or dissociation between subjective and object quantification for atrial fibrosis is available and the same is a matter of further investigation.Visual LGE scoring in left ventricle is a viable alternative to semi-automated quantification methods and has been shown have strong association for major adverse cardiac events when compared to them.2 While it appears attractive, automated LGE quantification has its own pitfalls and limitations.The newer techniques of native T1 mapping have also been restricted to LV because of issues with partial volume related to relatively limited spatial resolution, thin LA myocardial wall compared to thicker LV wall and motion issues and near wall flow artifacts. It is challenging to reliably measure T1 signals in the thin wall without being subject to partial volume effects.3 Margulesco A4 et al showed that location of scar was precise for scar areas >5 cm2 for single observers and >15 cm2 for multiple observers, irrespective of experience of operator, demonstrating that interobserver variability for eyeballing method was not significant.
Fig. 1.
Cardiac MRI images. A: Focal area of hyperintense signal along the posterior wall of the LA (blue arrow) suggestive of areas of scarring. B: Diffuse scarring is seen as distinct hyperintense signal diffusely involving the left atrial wall (yellow arrows). C: Baseline and post-contrast images of the left atrium in the axial plane shows scarring identified as hyperintense signal of the left atrial wall (blue arrows) along with underlying hypo-intense lamellar thrombus (red arrows).
All continuous data were presented as mean ± standard deviations. Continuous data were compared by unpaired T-test. The proportion of patients with LA scarring in the AF group and sinus rhythm group was compared by chi-square test. All analyses were performed using SPSS 20 software.
3. Results (Table 1)
Table 1.
Echocardiographic parameters and Cardiac MRI data of the two groups before and after balloon mitral valvotomy.
| Parameters | Cases (n = 16) |
Control (n = 27) |
P value | ||
|---|---|---|---|---|---|
| n(%) | mean ± SD | n(%) | mean ± SD | ||
| Cardiac MRI | |||||
| LA area (mm2) | 34.9 ± 2.61 | 28.8 ± 3.2 | <0.0001 | ||
| LA Volume Index(ml/m2) | 46.2 ± 2.9 | 33.03 ± 2.96 | <0.0001 | ||
| LA scarring | 9 (56.25) | 10 (37.03) | 0.220 | ||
| LA scarring percentage (%) | 15.55 ± 3.4 | 9.7 ± 2.1 | 0.002 | ||
| Transthoracic Echocardiography | |||||
| PRE BMV PARANETERS | |||||
| LA area (mm2) | 54.3 ± 4.4 | 39.4 ± 2.3 | <0.05 | ||
| MV mean PG (mm Hg) | 11.2 ± 1.2 | 8 ± 1.1 | <0.05 | ||
| PASP (mm Hg) | 58.4 ± 4.5 | 46.2 ± 3.2 | <0.05 | ||
| POST BMV PARAMETERS | |||||
| LA area (mm2) | 53.1 ± 4.8 | 37.4 ± 3.5 | <0.05 | ||
| MV mean PG (mm Hg) | 6.2 ± 1.1 | 4.2 ± 1.0 | <0.05 | ||
| PASP (mm Hg) | 34.2 ± 2.1 | 29.3 ± 2.0 | <0.05 | ||
There were 27 females and 16 males, aged 29 ± 6 years (range 14–40 years). The echocardiographic LA area and LA volume were both higher in Group A. The mean trans-mitral gradients before BMV patients were 11.2 ± 1.2 mm Hg and 8 ± 1.1 mm Hg in Groups A and B respectively (p < 0.05). The PASP before BMV was also higher in Group A (58.4 ± 4.5 vs 46.2 ± 3.2 mm Hg, p < 0.05, Table 1).
Cardiac MRI showed that the LA area was higher in Group A (34.9 ± 2.61 mm2 vs 28.8 ± 3.2 mm2, p < 0–0001). The indexed LA volume was also higher in Group A (46.2 ± 2.9 ml/m2 vs 33 ± 3 ml/m2, p < 0.0001). Surprisingly, the presence of LA scarring was not statistically different in the two groups: seen in 9/16 in Group A, and in 10/27 in Group B (p = 0.22). However, the percentage of LA scarred was higher in Group A-15.6 ± 3.4% vs Group B, 9.7 ± 2.1%, p = 0.002.
4. Discussion
As expected, the LA diameter and volume were higher in Group A, as were the mean transmitral gradient and pulmonary artery systolic pressure. We surprisingly found however that there was no difference in patients of AF and sinus rhythm, as far as presence of LA fibrosis was concerned. Nonetheless, the amount of LA scarring was significantly higher in Group A. Mitral valve disease is associated with enlarged LA, elevated atrial pressures, myocardial stretch resulting in slow conduction velocities, increased dispersion of refractoriness and increased automaticity, all of which serve to initiate and perpetuate AF. Vaziri et al5 in the Framingham heart study observed that incidence of AF rises from 3% when the LA diameter is < 40 mm to 54% if the LA diameter is > 40 mm. Kim and coworkers6 reported that in patients with rheumatic MS in sinus rhythm, the annual rate of development of AF was 3.5% per year and increased according to LA size and MS severity.
The role of LA fibrosis as a causative agent for atrial fibrillation is not clear. Studies have implicated leucocyte infiltration, effect of decreased LA local conduction velocity and myocytolysis seen on histopathology. Whether LA fibrosis is a cause or an effect of AF is up for debate. It is hypothesised that AF and continuous electrical remodelling in atrial myocytes ultimately can cause cell apoptosis -fibrotic tissue could then replace the dead atrial myocytes, thus explaining the fibrosis. It is important to recognize that AF alters atrial electrophysiological properties and can promote induction and maintenance that is, “AF begets AF”. Atrial fibrillation induces electrical remodelling primarily due to a very rapid atrial rate and consequent tachycardia-induced atrial remodelling.1
Zhu et al7 found that DGE showed moderate agreement with LA pathology in patients with rheumatic persistent AF. Fukumoto et al8 found that regions of the LA myocardium which had increased gadolinium uptake also had lower local conduction velocity. Lee et al9 performed MRI in 195 patients with chronic AF and found fibrosis in persistent than in paroxysmal AF patients. However, there have also been several studies whose findings are contrary to the above. Bois et al10 in their study of 149 consecutive patients with AF, found that DGE within LA walls was uncommon and when present, did not correlate with AF type or risk of AF recurrence. Shenthar et al 11conducted histological studies of left and right atria in rheumatic MS. They found that sinus rhythm was associated with myocyte hypertrophy whereas AF was associated with myocytolysis. Interstitial fibrosis was seen in >90% of patients but was found to be independent of the rhythm. As per our study, we have not found any statistical differences in the presence or absence of atrial scarring and occurrence of AF in RHD. However, the amount of scarring was greater in patients with AF. This finding may be analogous to findings similar to the presence or absence of LGE in patients with HCM, where presence or absence of scarring <15% did not alter the risk of SCD in low-intermediate risk patients but more than 15% scarring was independently associated with a higher risk of SCD.12 Similarly, the presence or absence of atrial scarring may not contribute to AF up to a certain percentage of scarring, but beyond a certain percentage it may become a significant risk factor. We thus feel that at this preliminary evaluation, the conclusion that the proportion of patients with atrial fibrosis was not different between patients with AF and sinus rhythm but the extent of scarring was greater in those with AF than in sinus rhythm holds good. The latter needs to be studied in detail by more quantitative methods and would be an interesting subject for evaluation.
In patients without manifest scar, AF could be due to chronically elevated LA pressures and volumes, and hence, following relief of MS by surgery or BMV, can maintain sinus rhythm in the long term.13Among the patients with LA scar, the scar load was significantly greater in Group A.
5. Conclusions
We found that proportion of patients with atrial fibrosis were not significantly different between patients with AF and sinus rhythm. The amount of fibrosis though was higher in patients with AF than in those with sinus rhythm. There was an association between LA size and LA volume index with AF.
Funding
Nil.
Declaration of competing interest
Nil.
Acknowledgement
Dr. Gopi Panicker, MBA, for help with the statistical analysis.
References
- 1.Higuchi K., Akkaya M., Akoum N., Marrouche N. Cardiac MRI assessment of atrial fibrosis in atrial fibrillation: implications for diagnosis and therapy. Heart. 2013;100(7):590–596. doi: 10.1136/heartjnl-2013-303884. [DOI] [PubMed] [Google Scholar]
- 2.Gräni C., Eichhorn C., Bière L. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J Cardiovasc Magn Reson. 2019;21(1) doi: 10.1186/s12968-019-0520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouazizi K., Rahhal A., Kusmia S. Differentiation and quantification of fibrosis, fat and fatty fibrosis in human left atrial myocardium using ex vivo MRI. PloS One. 2018;13(10) doi: 10.1371/journal.pone.0205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mărgulescu A., Nuñez-Garcia M., Alarcón F. Reproducibility and accuracy of late gadolinium enhancement cardiac magnetic resonance measurements for the detection of left atrial fibrosis in patients undergoing atrial fibrillation ablation procedures. EP Europace. 2019;21(5):724–731. doi: 10.1093/europace/euy314. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri S., Larson M., Benjamin E., Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89(2):724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 6.Kim H., Cho G., Kim Y. Development of atrial fibrillation in patients with rheumatic mitral valve disease in sinus rhythm. Int J Cardiovasc Imag. 2015;31(4):735–742. doi: 10.1007/s10554-015-0613-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhu D., Wu Z., van der Geest R. Accuracy of late gadolinium enhancement - magnetic resonance imaging in the measurement of left atrial substrate remodeling in patients with rheumatic mitral valve disease and persistent atrial fibrillation - Int Heart J. 2015;56(5):505–510. doi: 10.1536/ihj.15-098. [DOI] [PubMed] [Google Scholar]
- 8.Fukumoto K., Habibi M., Ipek E. Association of left atrial local conduction velocity with late gadolinium enhancement on cardiac magnetic resonance in patients with atrial fibrillation. Circulation: Arrhythmia Electrophysiol. 2016;9(3) doi: 10.1161/CIRCEP.115.002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D., Shim J., Choi J., Kim Y., Oh Y., Hwang S. Left atrial fibrosis assessed with cardiac MRI in patients with paroxysmal and those with persistent atrial fibrillation. Radiology. 2019;292(3):575–582. doi: 10.1148/radiol.2019182629. [DOI] [PubMed] [Google Scholar]
- 10.Bois J., Glockner J., Young P. Low incidence of left atrial delayed enhancement with MRI in patients with AF: a single-centre experience. Open Heart. 2017;4(1) doi: 10.1136/openhrt-2016-000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenthar J., Kalpana S., Prabhu M., Rai M., Nagashetty R., Kamlapurkar G. Histopathological study of left and right atria in isolated rheumatic mitral stenosis with and without atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27(9):1047–1054. doi: 10.1111/jce.13024. [DOI] [PubMed] [Google Scholar]
- 12.Chan R., Maron B., Olivotto I. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 13.Vora A., Karnad D., Goyal V. Control of heart rate versus rhythm in rheumatic atrial fibrillation: a randomized study. J Cardiovasc Pharmacol Therapeut. 2004;9(2):65–73. doi: 10.1177/107424840400900201. [DOI] [PubMed] [Google Scholar]