Abstract
Pulmonary administration route has been extensively exploited for the treatment of local lung diseases such as asthma, chronic obstructive pulmonary diseases and respiratory infections, and systemic diseases such as diabetes. Most inhaled medicines could be cleared rapidly from the lungs and their therapeutic effects are transit. The inhaled medicines with extended pulmonary exposure may not only improve the patient compliance by reducing the frequency of drug administration, but also enhance the clinical benefits to the patients with improved therapeutic outcomes. This article systematically reviews the physical and chemical strategies to extend the pulmonary exposure of the inhaled medicines. It starts with an introduction of various physiological and pathophysiological barriers for designing inhaled medicines with extended lung exposure, which is followed by recent advances in various strategies to overcome these barriers. Finally, the applications of the inhaled medicines with extended lung exposure for the treatment of various diseases and the safety concerns associated to various strategies to extend the pulmonary exposure of the inhaled medicines are summarized.
KEY WORDS: Pulmonary drug delivery, Pulmonary clearance pathways, Pulmonary exposure, Pharmaceutical strategies, Inhaled sustained release formulations, Local lung diseases, Systemic diseases, Pulmonary safety
Abbreviations: ALIS, amikacin liposomal inhalation suspension; API, active pharmaceutical ingredient; BALF, bronchoalveolar lavage fluid; COPD, chronic obstructive pulmonary diseases; CS, chitosan; Da, aerodynamic diameters; DPIs, dry powder inhalers; DPPC, dipalmitoylphosphatidylcholine; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; ELF, epithelial lining fluid; FDA, US food and drug administration; FDKP, fumaryl diketopiperazine; HA, hyaluronic acid; IL-4, interleukin-4; IL-5, interleukin-5; LABA, long-acting β2-adrenoceptor agonist; LPPs, large porous particles; MCE, mucociliary escalator; MDIs, metered dose inhalers; Mn, number-average molecular weight; MP, mucoadhesive particles; MPP, mucus-penetrating particles; MW, molecular weight; NLCs, nanostructured lipid carriers; PCL, poly-ε-caprolactone; PDD, pulmonary drug delivery; PEG, polyethylene glycol; PK, pharmacokinetics; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid); PVA, polyvinyl alcohol; SLNs, solid lipid nanoparticles; Tmax, time of maximum concentration
Graphical abstract
Strategies including molecular modification, polymeric conjugation, formulation technologies, etc. are being developed to extend pulmonary drug exposure. Inhaled medicines with extended lung exposure could be potentially favored for therapeutic efficacy.
1. Introduction
The lung with its unique physiological and anatomical features including large absorption area, highly permeable alveolar epithelial membrane, and high vascularization with limited first-pass effects has become an important route for drug administration1. This route has attracted increasing attention as it offers several advantages over other administration routes including rapid onset of the action, targeted delivery, reduced side effects, and improved bioavailability2,3. Pulmonary drug delivery (PDD) systems have been exploited not only for the treatment of some local diseases such as asthma, chronic obstructive pulmonary diseases (COPD) and respiratory tract infections, but also for the achievement of enhanced bioavailability to better treat systemic diseases like diabetes4.
Generally, an inhaler device is indispensable to facilitate the deposition of inhaled medicine at the targeted sites of the lungs5. The inhalation devices current in use are nebulizers, metered dose inhalers (MDIs) and dry powder inhalers (DPIs)6. Different from nebulizers and MDIs, which mainly delivery the drugs in the form of solution or suspension, DPIs are used for the delivery of dry powder formulations which is typically composed of micronized drug powder and various coarse carrier particles (e.g., lactose)7. Among them, the DPIs are gaining popularity as they are easier to handle and more favorable to the stability of drugs8,9. The proper selection of appropriate drug formulations and devices with specific design is extremely crucial for drugs delivery to the lungs efficiently and reproducibly. Once the drug particles are deposited, they could possibly be cleared from the lung via various clearance pathways including mucociliary clearance, phagocytosis by macrophages, dissolution and translocation from the airways to other sites10. Consequently, the local drug concentration in the lungs could decline rapidly to fail to exert optimal therapeutic effects. In order to maintain the effective drug concentration at the action sites, patients have to take the drugs frequently, which may result in poor patient compliance and adherence11,12. Extending the retention of inhaled particles in the lungs is an effective strategy to achieve prolonged pharmacological effect.
Herein, we start with an introduction of various physiological and pathophysiological barriers for designing inhaled medicines with extended lung exposure, which is followed by recent advances in various physical and chemical strategies to overcome these barriers. Finally, the applications of the inhaled medicines with extended lung exposure for the treatment of various diseases and the safety concerns associated to various strategies to extend the pulmonary exposure of the inhaled medicines are summarized.
2. Factors influencing the pulmonary exposure of the inhaled medicines
2.1. Lung physiology and drug delivery
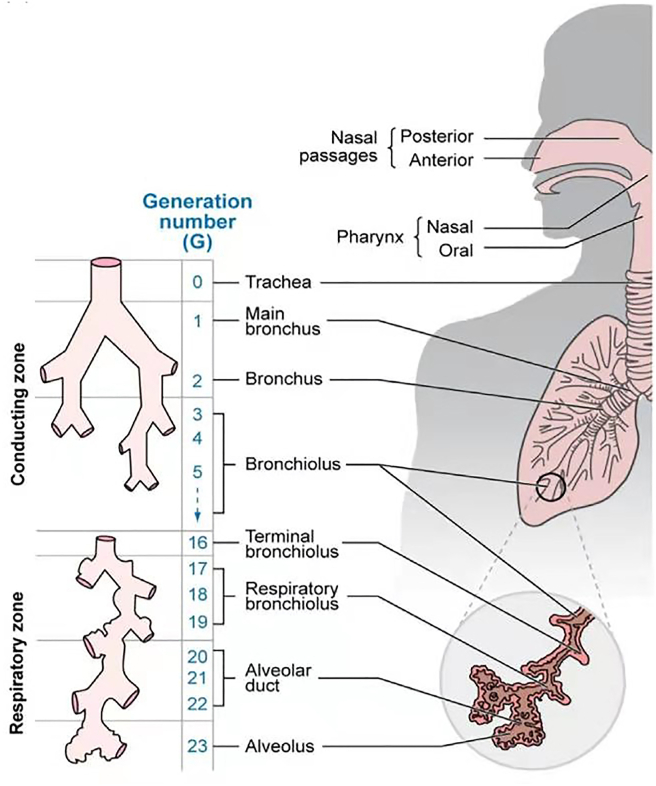
As shown in Fig. 113, the lung is composed of conducing zone and respiratory zone and with the structure resembling an inverted tree. The trachea subdivides into two main bronchi, which successively branch into more and more narrow and short bronchioles and end at sac-like alveoli for the gas-exchange14. From the trachea to the alveolar sacs, there are about 23 bifurcations in total. The proximal conducting airways are constituted with pseudostratified columnar epithelium composed of ciliated cells, goblet or mucus secreting cells and basal or progenitor cells. The lower to the more distal airways are progressively replaced with a simple cuboidal cell layer and there is a very thin epithelial lining in the alveoli5. The fate of the inhaled medicines is highly dependent on the site where they are deposited in the lungs. For example, the large surface area, highly permeable bio-membrane, and good blood supply of the alveolar region are favorable for rapid absorption, while the epithelial cells in the conducting airways constitute a strong barrier for systemic absorption15.
Figure 1.
The physiological structure of the lungs with conducting and respiratory zones. The conducting zone (G0‒G16) comprises of the trachea, bronchi, bronchioles and terminal bronchioles, which is responsible for conducting air to the respiratory regions of the lungs. The respiratory bronchioles, alveolar ducts and alveolar sacs constitute the respiratory zone (G17‒G23), which facilitates the gas exchange between the airspaces and blood capillaries. Reprinted with the permission from Ref. 13. Copyright © 2019 Elsevier.
There are three main mechanisms controlling the deposition of inhaled drug particles including inertial impaction, gravitational sedimentation and Brownian diffusion. The aerodynamic diameter (Da) of the particles is the main factor deciding the site of deposition and which mechanism is followed16.Generally, the large drug particles (Da > 5 μm) are mainly deposited in the upper airways (i.e., mouth, trachea and main bronchi, where the air velocity is relatively high) by inertial impaction mechanism as these particles could not able to follow the change of the airstream flow direction. The drug particles with Da within the range of 1–5 μm could be deposited via the gravitational settling mechanism in the central and distal tract, where the air velocity is quite low. The drug particles with Da < 1 μm could remain suspended in the air and are mostly exhaled. The ultrafine particles (<100 nm)could largely be deposited in the respiratory tract by random Brownian motion. The particles with diameter <100 nm could reach the alveolar region, while the particles with size <10 nm could readily be deposited in the tracheo-bronchial region of the lungs due to their high diffusion coefficients17.
2.2. The barriers—pulmonary clearance pathways
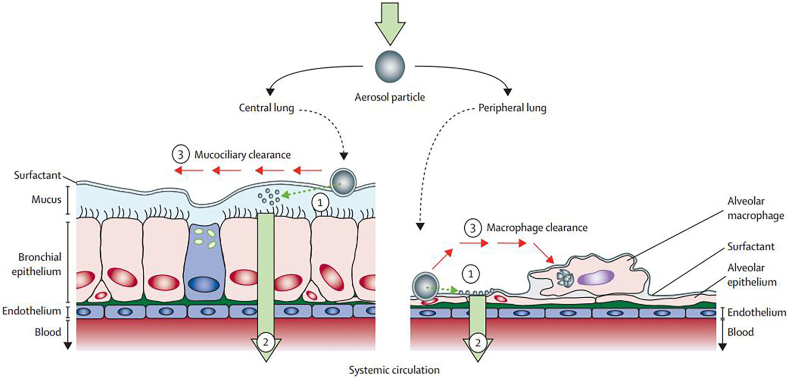
It is challenging to develop inhaled medicines with extended pulmonary exposure to exert prolonged pharmacological effect. It is because there are various elimination pathways, including coughing, mucociliary transport, phagocytosis by macrophages and translocation into the cells, blood and lymph (Fig. 218), through which the inhaled drugs can quickly be removed from the lungs. Some physiological factors, the pathophysiological conditions of the patients and physicochemical properties of the inhaled drugs all have significant influence on the retention of inhaled medicine in lungs by interfering these pathways. In this section, various clearance pathways that influence the duration of pulmonary exposure of the inhaled drugs are reviewed.
Figure 2.
The fate of aerosol drugs after deposition in the lungs. (1) Active pharmaceutical ingredient (API) is released from the deposited aerosol particles when these come in contact with the lung lining fluid. Both physicochemical properties of the drug molecular and the formulation forms could control this process. (2) The particle absorption process via translocation into the cells, blood and lymph. (3) The particle clearance by the mucociliary escalator and phagocytosis of macrophages. Reprinted with the permission from Ref. 18. Copyright © 2013 Elsevier.
2.2.1. Mucociliary escalator
For the inhaled drugs deposited in the upper and middle airways, the mucociliary escalator (MCE) may be the dominant clearance mechanism19. This MCE is a self-clearing mechanism of the airways, which is functioning through the cilia in the mucociliary layers to keep the airways clear of mucus and dirt, and to propel sperm. The mucociliary layer is composed of ciliated columnar cells and goblet cells covered by a lung lining fluid. The lung lining fluid, consisted of a periciliary sol layer and an overlying mucus gel layer, plays an important role in the clearance of the inhaled drugs. The mucus layer is composed of mucin, proteins, lipids, salts, and water. The mucus and cilia work together for the removal of foreign objects from the lungs. When the cilia rhythmically swings, it can expel the particles trapped in the mucus layer out of the lungs20. The MCE works efficiently for eliminating larger particles, while smaller particles can penetrate through mucus and enter the bronchial epithelium, escaping from mucociliary clearance21. The lung lining fluid gets thinner as the diameter of the airways become smaller. Finally, in the alveoli, the thickness of the lung lining fluid can be less than 0.1 μm, where the mucus layer disappears and it is replaced by surfactant layer composed of lipids, cholesterol and proteins22,23.
2.2.2. Phagocytosis by macrophages
The phagocytosis by the pulmonary macrophages is another important mechanism to eliminate the particles from the lungs besides MCE, which is believed to be the dominant clearance mechanism in the deep lungs24. The air side surfaces of each of the 500 million alveoli in the human lungs are usually “patrolled” by 12–14 alveolar macrophages, which engulf and digest any insoluble particles deposited in the alveoli3. The size of particles and molecules are important factors that control the macrophage uptake. In general, the macromolecules with molecular weight (MW) ≤25 kDa are rapidly cleared, while MW ≥ 40 kDa are slowly cleared by the macrophages14. The optimal Da for lung deposition is 1–5 μm, whereas particles within the geometric diameter range 0.5–3 μm are readily endocytosed by macrophages25. For macromolecule drugs, e.g., peptides, proteins and nucleic acids are readily aggregated with the lung surfactants and eventually cleared by macrophages, which is an important cause of drug loss14.
In addition, macrophages could also manipulate the fate of inhaled particles by stimulating the immune response against the inhaled drugs26. Naturally, antigens from the invading pathogens processed by alveolar macrophages are transferred back to regional lymph nodes and come in contact with T lymphocytes and cofactors, leading to the activation or suppression of cellular and humoral immunity27. Such immune responses can largely avoid the harm caused by the inhalation of pathogens that enter into the deep lungs. However, the administration of drugs with immunogenicity such as polysaccharides, proteins or peptides to the lungs may stimulate an immune response in the lungs, and subsequently produce anti-drug antibodies, which can reduce the efficacy of these therapeutic molecules significantly28. Therefore, macrophages play an important role in controlling the residence time and the pharmacological efficiency of the inhaled drugs in deep lung.
2.2.3. Translocation into cells, blood and lymph
The translocation process of the drug molecules includes transcytosis into the epithelial cells and/or across the respiratory epithelia into the interstitium and then to blood and lymph29. Generally, drug molecules are absorbed into the systemic circulation more quickly via the lung than any other non-invasive drug administration routes30,31, which is one of the reasons for the short pulmonary exposure of inhaled medicines. When small, lipophilic drug molecules are deposited from the air into the lung lining fluid, they may firstly contact with the lung surfactants, which could facilitate the dissolution of drugs and thereby enhance their absorption32. For macromolecular drugs, it will take longer time (within an hour) to be absorbed into the systemic circulation33, but relatively high bioavailability could be expected for inhalation compared with other nonparenteral routes.
The thickness of the lining fluid in the airways is about 5–10 μm33 and its main components are water (96%), salt, phospholipids and proteins34, which may improve the dissolution rate of the hydrophilic drugs. The thickness of the lining fluid gradually decreases distally and it is about 0.01–0.08 μm at the alveoli33. The lining fluid at the alveoli is composed of phospholipids and proteins (alveolar surfactant), which would increase the wetting, the solubility, and the dissolution rate of poorly water-soluble drugs35. The absorption rates of the drugs with different solubility could be enhanced in the lungs due to the presence of these physiological substances.
In addition, the huge surface area of the alveoli (more than 100 m2) and the extremely short distance from the gas side to the blood side in alveoli (0.1–0.2 μm) also make big contribution to the rapid drug absorption in the lung14. The thinness of the alveolar region offers great potential for the rapid systemic absorption of drug molecules within seconds or minutes11. Moreover, there are abundant capillaries around the alveoli14. The drugs deposited in the alveoli can quickly pass through the gas-blood barrier and be absorbed into the blood3. In contrast, the epithelial cells in the airways constitute a strong barrier to the absorption of the drug molecules into the bloodstream. The fast absorption of the drugs in the lungs is also related to the presence of high levels of drug transporters expressed in the lungs, such as carrier-mediated and vesicle-mediated transports, which play vital roles in lung absorption36. The vesicle-mediated transport is mainly involved in macromolecular transport across the alveolar epithelium37. These transporters facilitate the transport of their substrates through the epithelial cells, thereby shortening the residence time of drugs in the lungs.
For the purpose of local treatment, rapid clearance will lead to the decline of the local drug concentration in the lungs. Once the drug concentration is too low to maintain the effective therapeutic concentration, the desired therapeutic outcome would not be expected. For example, in the case of bacterial infections to the lungs, the epithelial lining fluid (ELF) is a susceptible site for pathogens38. Maintaining certain concentrations of the inhaled antibacterial agents in the ELF is a key factor to achieve their optimal therapeutic effects and suppress the resistance39,40. If the antibacterial agents were easily adsorbed into the systemic circulation or eliminated from the lungs by various clearance mechanisms, there would not be sufficient antibacterial drug concentration in the ELF and eventually the antibacterial agents would not exert their killing and inhibition effects. Even in a worse case, this may result in the development of serious antibiotic resistance. In short, more and more attention should be paid to the research of inhaled medicines with extended pulmonary exposure.
3. Strategies to extend the pulmonary exposure of the inhaled medicines
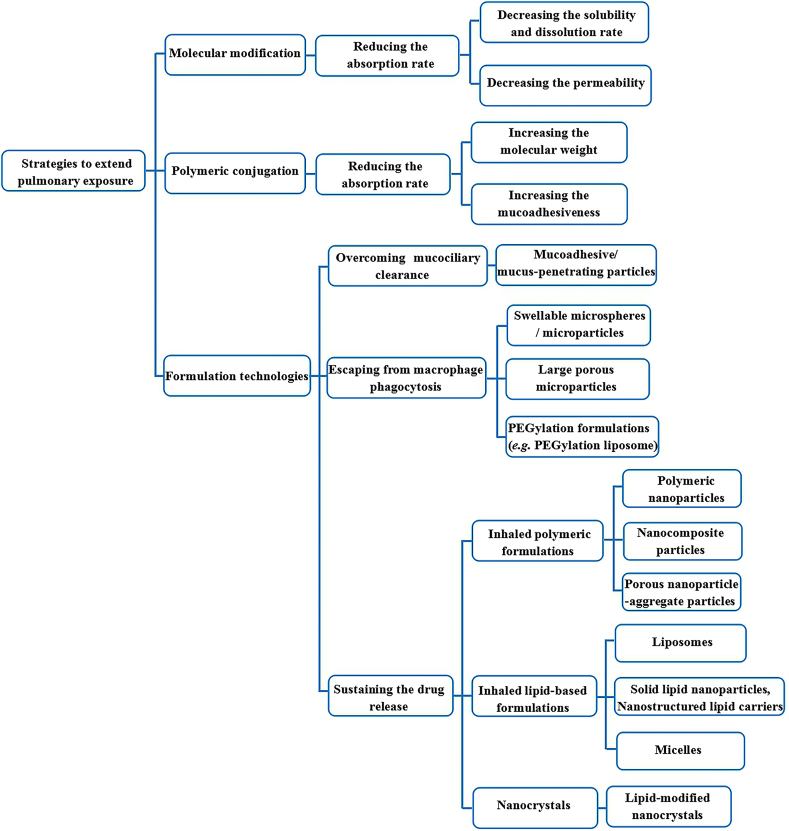
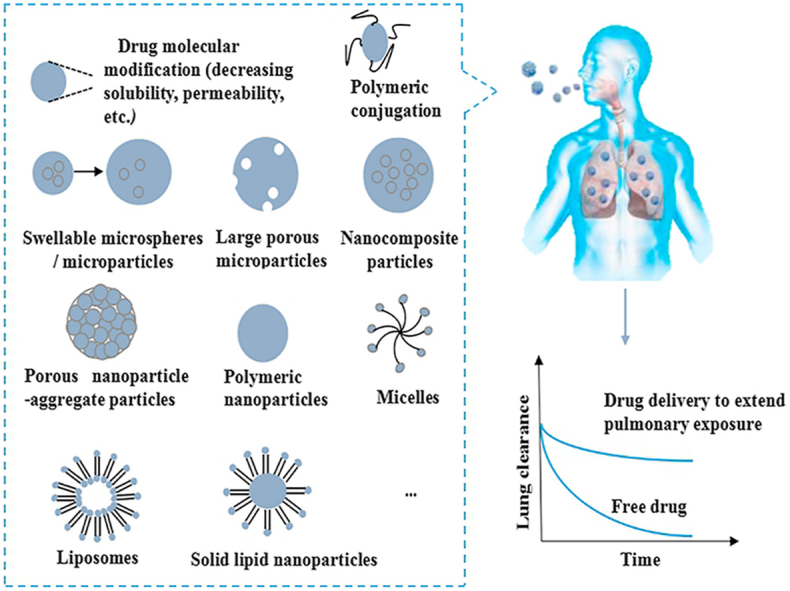
One of the strategies to extend the pulmonary drug exposure may be exploiting sustained release formulations for pulmonary administration41. These sustained release formulations could confer an extended time of the drug action, reduce the frequency of dosing and consequently improve the patient compliance and even reduce their side effects11,43. Besides the inhaled sustained release formulations, there are also some other physical and chemical approaches including particle engineering and chemical synthesis and modification that have been exploited to extend the pulmonary exposure of the inhaled medicines to bring clinical benefits to the patients recently. These strategies have been largely based on our increasing understanding of the physiological and pathophysiological features of the lungs and the pulmonary clearance mechanisms, including mucociliary clearance and phagocytosis by macrophages, systemic absorption and metabolic degradation21. With the suitable structural modification of the drug molecules and delivery carriers, the pulmonary exposure of the inhaled medicines could be extended by minimizing the elimination rate of the drugs through the powerful airway clearance mechanisms1. In the following subsections, several strategies adopted to overcome the clearance of inhaled drugs from the lungs in order to provide extended pulmonary drug exposure are systematically reviewed. A summary of these strategies are presented in Fig. 3.
Figure 3.
A summary of various strategies to extend the pulmonary exposure of the inhaled drugs.
3.1. Molecular modification
3.1.1. Decreasing the solubility and dissolution rate
Inhaled drugs must be dissolved in the lung lining fluid for subsequent diffusion, cellular uptake, absorption, and interaction with the receptors42. The difference in the solubility and the dissolution rate of the drug compounds may result in different clearance pathways. After inhalation, water-soluble drugs may quickly dissolve in the lung lining fluid and diffuse through the airway epithelium, and disappear from the lungs by absorption43. However, in the case of poorly water-soluble drug, sufficient dissolving of the inhaled drug particles might not be reached due to the limited volume of lung lining fluid. If the undissolved fraction of inhaled particles successfully escaped from the MCE or phagocytosis of microphages clearance, the slow dissolution rate may result in prolonged retention time in the lungs. For example, fluticasone propionate, an water-insoluble steroid anti-inflammatory agents used for managing asthma, shows an extended absorption time in pharmacokinetic profiles due to its poor dissolution rate, which leads to a prolonged lung exposure44. Several poorly water-soluble drugs such as sex steroids and antifungal drugs typically exhibit slow absorption and elimination rates from the lungs44,45. These drugs often dissolve slowly in the lung lining fluid, resulting in slow absorption to the blood circulation and extended retention time in the lungs45. The same phenomenon has also been observed in the case of inhaled biologics such as insulin. It is well known that the monomeric or dimeric form of insulin could convert to a hexamer in the presence of zinc ions, affording an insoluble complex. The hexamer of insulin could act as a reservoir and gradually be depolymerized into a monomer, the only absorbable form of insulin. Consequently, the retention time of inhaled insulin and absorption time in the lungs was prolonged significantly. Vanbever et al.46 loaded insoluble insulin complexes into large porous particles and delivered them into the lungs of rats, which resulted in 12 h of plasma insulin concentration. Therefore, lowering the solubility or dissolution rate of inhaled drugs via physical modification could potentially extend the pulmonary exposure and pharmacological effects.
3.1.2. Decreasing the permeability
The permeability of a drug compound is another important physicochemical property that influence the residence time of the drug compound in the lungs. Low permeability of inhaled drugs is generally preferred to extend the residence time of the drug in the lungs by diminishing the diffusion of the drugs through the lung epithelium into the interstitium of the lungs or systemic circulation47. Lipophilic drugs are mainly absorbed via passive diffusion through the cells, whereas the hydrophilic drugs are absorbed by the diffusion through the tight junctions48. The former causes a faster absorption through the respiratory epithelium into the bloodstream than the latter. The drugs with poor membrane permeability are not easily passed through the lung epithelial cells, and thus, a relatively high drug concentration may be achieved in the lungs. Several studies have shown that the drugs with low permeability, such as colistin, aztreonam could retain a high drug concentration in the lungs after inhalation49. This can be critically meaningful for the inhaled antibiotics to maintain sufficient high concentration in the lungs to combat respiratory infection.
For those inhaled drugs with high permeability, scientists have attempted some molecular engineering techniques to alter the polarity of molecules. Ciprofloxacin is a potent antibacterial agent, and widely employed to eradicate the bacterial infections in the respiratory tract. However, the pulmonary delivery of ciprofloxacin causes a rapid drug diffusion through the lung epithelium, offering marginal or no advantage over the systemic administration50. It was reported that the systemic and pulmonary exposures of ciprofloxacin were almost equivalent after intravenous and pulmonary administration of ciprofloxacin solution to rats51. In order to reduce the permeability of ciprofloxacin, Brillault et al.50 formulated a complex of the drug with metal cations, which can limit the diffusion through the epithelium, reduce its absorption, and maintain a high concentration in the lungs. Furthermore, Lamy and colleagues52 showed that the complexes formed between copper ions and ciprofloxacin could slow the absorption rate of ciprofloxacin from the ELF into plasma, resulting in high and sustained drug concentrations in ELF of the lungs. The attachment of the metal ions with the drug molecules could often ionize the molecules structure50, which can increase the polarity of the molecule, can diminish the permeation of the molecules through respiratory epithelium.
Besides the strategy of modifying the polarity of drug molecules, increasing the affinity between drug molecules with the lipophilic group of receptors localized at the surface of lung cells also could extend pulmonary exposure. Salmeterol is one of the examples. It is a long-acting β2-adrenoceptor agonist (LABA) and belongs therapeutically to β2-sympathomimetics. It binds to G-protein coupled β2-receptors in the lungs and inhibits the contraction of the smooth muscle53,54. Compared to another β2-adrenoceptor agonists salbutamol, which duration of action is 4–6 h, the effect of salmeterol lasts 12 h. Although both molecules have the same lead structure, salmeterol has longer nonpolar group, i.e., hydrophobic N-substituents with saligenin ethanolamines, than salbutamol. When the active sites of salmeterol bind to the β2-adrenoceptor, the nonpolar group of salmeterol can bind to nearby nonpolar region of cell membrane or hydrophobic area of the receptor protein55. Thus with this molecular modification, salmeterol can maintain its binding site, extend the lung exposure and activate the β2-adrenoceptor repeatedly to exert prolonged pharmacological responses56.
3.2. Polymeric conjugation
3.2.1. Increasing the molecular weight
The synthesis of polymer–drug conjugates is another approach to diminish the elimination of inhaled drugs from the lungs and extend their lung exposure. By synthesizing polymer–drug conjugates, the molecular weight and hydrophilicity of the molecules can be increased and consequently their lung absorption could be retarded and the residence time of the drugs in the lungs can be prolonged57. In this context, polymers such as polyethylene glycol (PEG) and polyethyleneimine have been utilized58,59. PEG is a water-soluble polymer containing ethylene glycol repeating units and its molecular weight is related to the degree of polymerization. To achieve optimal residence time of the drug conjugates in the lungs, the selection of the PEG with appropriate molecular weight is important. One study showed that if the molecular weight of PEG is less than 2 kDa, it can be cleared from the rat lungs within 2 days, but it can reside into the lungs up to 7 days when its molecular weight is greater than 5 kDa60. As compared to the pure prednisolone, a steroid medication used to treat allergies, inflammation, autoimmune disorders and cancer, its PEG-prednisolone conjugates showed a 7.7-fold of the reduction of the absorption rate of the drugs from the lungs, which could prolong the retention of inhaled drug in the lungs, improve its pharmacokinetics profiles and also reduce its systemic adverse effects61. Apart from a decrease in the permeability by enlarging molecule weights of inhaled drugs via PEGylation, it was also reported that PEGylated insulin may be able to evade phagocytosis of macrophage and resulting in sustained insulin delivery after pulmonary administration62.
3.2.2. Increasing the mucoadhesiveness
Researches also designed mucoadhesive polymer-drug conjugates to extend the residence time of the inhaled drugs in the lungs63, 64, 65. This modification could create an intimate contact of the drug molecules with endogenous substances such as mucin at the surface of respiratory tract and thus sustain their residence time in the lungs. Generally, the binding bond between drug and polymer is designed to be broken at certain time point to release free drug, which might slowly diffuse into the sites of action to exert their effects. Chitosan (CS) is one of the mostly studied polymers used for this purpose. The NH2 groups present in the structure of CS can be conjugated with various functional groups to achieve further conjugation of the drug molecules and other ligands60. Due to the presence of COOH and NH2 groups in its backbone, which can generate hydrogen bonds with the lung mucosa, CS exhibits mucoadhesive property at physiological pH and the capacity to prolong the residence time of the drugs in the lungs65. Thiolized CS has been developed to further improve the mucoadhesion property of the polymer-drug conjugates in the lungs66. The thiol groups of the thiolated glycol CS could form disulfide bonds with the cysteine-rich domains of the mucus67. Compared to pristine CS, the mucoadhesion potential of the thiolated glycol CS was increased by 2 times66. The mucoadhesive polymer-drug conjugates could not only overcome MCE by anchoring to the mucin but also diminish the diffusion of the chemical entities through the epithelial membrane due to the enlarged sizes and the increased hydrophilicity.
3.3. Formulation technologies
Besides the aforementioned modifications of drug molecules, various formulation technologies have been exploited to extend the pulmonary exposure of inhaled drugs, providing improved therapeutic benefits to the patients. In the following subsections, various formulation strategies to overcome the MCE and phagocytosis of microphages are described. Moreover, the recent advances in inhaled sustained-release formulations are also critically reviewed.
3.3.1. Overcoming mucociliary clearance
Inhaled drugs need to firstly pass through the mucus barriers to reach epithelial membrane at the upper airways. To reduce the elimination of inhaled drugs by MCE, researchers proposed the development of mucoadhesive formulations, which could anchor with the mucin moleculesviamultivalent interactions to effectively decrease their mucociliary clearance68,69. The mucoadhesive polymers (e.g.,CS, hyaluronic acid) are commonly used in these formulations. The particle size of the formulations also has a considerable influence. For instance, the MCE could remove the particles with size larger than 6 μm from the human airways within 24 h, and the particles with diameter less than 6 μm could retain in the lungs for more than 24 h69. In a study, salbutamol sulphate was co–spray dried with hyaluronic acid (HA) to produce biomucoadhesive microparticles with negative surface charges and Da around 5 μm. It was found that the resultant microparticles exhibited excellent mucoadhesion property in an in vitro model. The lung retention time of salbutamol was significantly prolonged from 2 to 8 h in a rat model after being incorporated in HA microparticles. In addition, the systemic exposure of salbutamol sulphate in the biomucoadhesive microparticles was significantly reduced, which potentially reduced the side effect of inhaled drugs70.In another study, the similar strategy was used to formulate budesonide, a poorly water-soluble drug, into biomucoadhesive microparticles composed of HA with negative surface charges and Da around 5.3 μm to extend the pulmonary retention71.The results showed that HA did not alterin vitro release of budesonide from the biomucoadhesive microparticles, whereas, the budesonide in the microparticles exhibited a significantly prolonged time of maximum concentration (Tmax) and increased bioavailability as compared to the formulations without HA after intratracheal administration to rats. That study demonstrates HA could provide budesonide with sustained pharmacological effects. This can be attributed to the prolonged retention time of the drugs by overcoming the mucociliary clearance with the polymers.
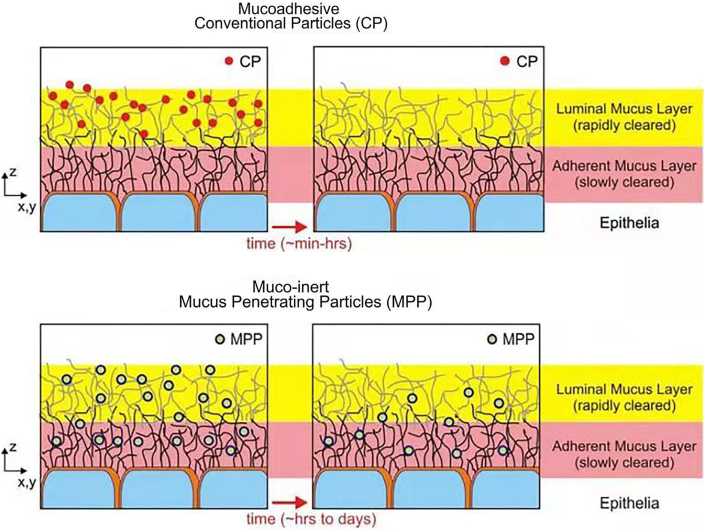
Alternative to using mucoadhesive polymers to interact with the mucus to overcome mucociliary clearance in the upper airways, researchers also attempted to fabricate mucus-penetrating particles (MPP), which could exhibit prolonged lung residence time. This method was developed because of the concern of the potential negative impact of mucoadhesive particles to the airway surfaces. In order to relieve the potential negative impact of mucoadhesive particles, researchers attempted to fabricate MPP to prolonged lung residence time (Fig. 4)72. In addition, it was also reported that mucoadhesive particles (MP) suffered from a rapid clearance mediated via phagocytosis by macrophages73 and the inhaled drugs that could not diffuse through the mucus layers will be cleared quickly by rhythmically swings of cilia to expel the particles trapped in the mucus layer74. Hence the inhaled drug particles have to rapidly diffuse through the mucus lining fluid and enter into the periciliary fluid layer75. The enhanced mucus-penetrating ability of nanoparticles and PEG modified nanoparticles allowed the nanoparticles to escape from mucus and continue their journey in the mucus, which could effectively prolonging the residence time of drugs in the lungs76,77.
Figure 4.
A comparison between the fate of the conventional mucoadhesive particles (CP) and mucus-penetrating particles (MPP) administered to a mucosal surface. The CP are largely immobilized in the luminal mucus layer and removed along with the luminal mucus layer quickly. In contrast, the MPP could enter into the adherent mucus layer and retained, leading to a prolonged residence time for the MPP at the mucosal surfaces. Reprinted with the permission from Ref. 72. Copyright © 2009 Elsevier.
3.3.2. Escaping from macrophage phagocytosis
Macrophage phagocytosis may be the next clearance pathway for those inhaled drug particles that survive from MCE. In the following section, some formulation strategies to evade macrophage phagocytosis are reviewed.
3.3.2.1. Swellable microspheres/microparticles
Most inhalable drug particles are in a micron range of 1–5 μm, but unfortunately the microparticles within this size range could be readily captured by the alveolar macrophages via phagocytosis process78.It was reported that anti-tubercular drugs formulated into this size range microspheres could be exploited to target alveolar macrophages for the treatment of the pulmonary tuberculosis79.However, the inhaled drugs used to treat other lung diseases should avoid the phagocytosis by macrophages. In order to achieve it, researchers80,81accomplished swellable microspheres. Before deposition in the lungs, the sizes of the swellable microspheres are in an inhalable range of 1–5 μm. When landed in the lung lining fluid, these microspheres could swell and their size becomes too large to be engulfed by macrophages. These microparticles are often fabricated using some biopolymers such as CS, sodium alginate1.El-Sherbinyet al.82loaded curcumin into inhalable and expandable CS-PEG microspheres and showed that about 20% of the encapsulated curcumin was rapidly released within 1 h as the microspheres are expanded rapidly. Afterwards, the release was subsequently slowed down and lasted for about 24 h.Some other researchers83also reported that CS based swellable microparticles could potentially be used as nanocrystal carrier to achieve sustained pharmacological effect viapulmonary delivery.
3.3.2.2. Large porous microparticles
The design of large porous microparticles is another formulation approach to bypass the phagocytosis of macrophages. The large porous microparticles with a geometric diameter larger than 5 μm, but a low density could exhibit similar aerodynamic performance as the small but compact microparticles as Da is a combined factor of geometric particle size, density and shape as shown in Eq. (1):
| (1) |
where, Da is the aerodynamic particle diameter, D represents the geometric particle diameter, and symbolize the densities of the particles and water(1 g/cm3), respectively, andxdesignates the dynamic shape factor48.According to Eq.(1), it is possible to enlarge the geometric particle diameterD, but keep the aerodynamic particle diameter Da the same by a decrease in the densities of the particles. Therefore the large porous microparticles could be designed to evade macrophage phagocytosis by the large size, but not compromise aerosol performance by decreasing the density. In addition, some researchers84have proved that macrophages are not able to engulf large particles. Chvatal et al.85compared the performances of the inhalable non-porous fine particles with the large porous particles (LPPs) containing meloxicam. It was found that a higher proportion of LPPs reached the lower respiratory tract than the non-porous fine particles. At the same time, the LPPs could avoid phagocytosis due to their large size and exhibit prolonged residence time in the lungs. Kim et al.86encapsulated doxorubicin in the highly porous and large PLGA microparticles, which were administered through pulmonary route for the treatment of metastatic lung cancer. Their results showed that the large porous PLGA microparticles exhibited sustained drug release characteristics in the lungs and provided excellent therapeutic efficacy.
3.3.2.3. PEGylation
As described in section 3.2.1, the fabrication of PEG–drug conjugates could increase the molecular weight of the drugs and diminish their elimination from the lungs. In fact, apart from the conjugation with drugs, PEGs have also been attached to particulate delivery systems to extend pulmonary exposure of inhaled drugs. Kaneko et al.87 compared the distribution of PEGylated liposomes and unmodified liposomes in ELF and alveoli after pulmonary administration. It was found that the PEGylated liposomal systems are more stable than the unmodified liposomes. In addition, the PEGylated liposomes could escape from the opsonization by the surfactant proteins, which is the process of recognition and removal of foreign substances by the alveolar macrophages in the lungs87. Consequently, the PEGylated liposomes were not obviously engulfed by alveolar macrophages as compared to the unmodified systems in the lungs, which resulted in their extended pulmonary retention. Although PEGylated formulations in general possesses various advantages over unmodified systems in terms of stability, safety, and biocompability, in a few cases, wrapping PEG layer may also result in some unfavorable features to the formulation. Li et al.88 reported that the antibacterial activity of colistin-loaded PEGylated liposomes was lower than that of non-PEGylated liposomes, which can be partially attributed to the fact that the PEG chain hindered the direct contact of liposomes with the bacterial cell walls and eventually the drug delivery efficiency was compromised.
3.3.3. Sustaining the drug release
There are large numbers of literature reporting the design of inhaled sustained release formulations to provide therapeutic benefits to the patients. In the following section, various formulation strategies to design inhaled sustained drug release formulations are reviewed.
3.3.3.1. Inhaled polymeric formulations
As developing other formulations, the selection of suitable excipients is a key step in the development of inhaled polymeric formulations. A large number of polymers including natural polymers such as CS, gelatin, alginate, collagen, and HA, and synthetic polymers such as polylactic acid (PLA), poly(lactic-co-glycolic acid) PLGA, oligo (lactic acid), poly-ε-caprolactone (PCL) and polyvinyl alcohol (PVA) are widely used for the PDD applications89. The type of the polymers used would affect the particle disposition, drug release, and the efficacy of the inhaled drugs90. Polymer-based inhaled formulations are often micro- or nano-particles in either dry powder form or colloidal dispersion form. Depending on the types of the polymers and characteristics of micro-, nano-particles, the inhaled drugs might experience different clearance pathways in the lungs91.
The most studied polymeric formulations for inhalation may be nanoparticle based formulations, which are used to load bioactive substances through encapsulation or adsorption. These systems protect the drugs from the degradation, modulate the dissolution rate or release of the drugs, reduce the side effect, and may facilitate targeting some specific cells or tissues in the lungs92. One of the most investigated inhaled nanoparticles may be for the treatment of lung cancers. For this purpose, the inhaled polymeric nanoparticles have been designed to reduce the side effects of the chemotherapeutic drugs. In addition, the inhaled polymeric nanoparticles are expected to provide targeting features to the tumor sites and extend the pharmacological effects of the anti-tumor drugs. Instead of formulating polymeric nanoparticles into colloidal dispersion forms, many researchers afforded nano-in-microparticles, nanoembedded microparticles, or nanocomposite particles in order to achieve adequate aerosol performances93, 94, 95. The matrices of the microparticles are commonly composed of hydrophilic sugars. After dissolution of the matrix materials in the lung lining fluid, the nanoparticles are reconstituted in the lungs to exert the therapeutic effects96,97. Liu et al.98 used oleic acid-CS as the carrier to load paclitaxel and quercetin in nanoparticles, and then transformed the nanoparticles into inhalable micropartilces using the spray drying process. Their results showed that the microparticles composed of a large number of nanoparticles exhibit adequate aerosol performance. In addition, the in vivo study showed that the microparticles deposited in the lung exhibited sustained drug release characteristics up to 48 h. Other research group99 applied the coating of self-assembled copolymers onto drug nanoparticles and subsequently encapsulated into inhalable/expandable semi-interpenetrating polymeric network microspheres, which exhibited extended pulmonary exposure. Besides these systems, another form of inhaled polymeric particles that were used to extend pulmonary exposure may be LPPs composed of nanoparticles for inhalation. This formulation strategy combined the merits of porous microparticles and nanoparticles, including high lung deposition and extended pharmacological effects100. One of the examples is that, rifampicin, an antibiotic drug were loaded into PLGA nanoparticles and then processed into inhalable porous microparticles for the treatment of pulmonary tuberculosis101.
CS and PLGA may be two most commonly investigated natural polymers and synthetic polymers, respectively, in the inhaled formulations to extend the pulmonary exposure and pharmacological effects because of their biodegradability and biocompatibility. CS is a type of natural-origin polysaccharides produced by the deacetylation of chitin102. Due to its mucoadhesive property, CS is used either to form drug-conjugates or modify the surfaces of micro-, nano-particles to overcome MCE. It is reported that the CS can be dissociated into natural non-toxic metabolites by the lysozymes present in the respiratory secretions, which is an attractive feature for its pulmonary administration103. Besides the controlled-release feature, inhaled particles composed of CS were also reported to swell and evade phagocytosis of macrophages, thereby prolonging the retention of the inhaled drugs in the lungs. Various therapeutic agents such as antibiotics, anti-tuberculosis molecules, anti-tumor drugs, peptides and proteins have been formulated with CS for their pulmonary delivery103,104. In fact, it is also reported that CS itself has some biological activities, which can be used as antioxidants, antimicrobial and antifungal agents105. As for PLGA, it is a co-polymer made from the polymerization of lactic acid and glycolic acid, which can regulate its own biodegradability by varying the composition (lactide/glycolide ratio), molecular weight and chemical structure106. It is not a natural polymer, but its degradation products are lactic acid and glycolic acid, which can be eliminated by the citric acid cycle107, possess excellent biocompatibility and biodegradability. However, to overcome the hydrophobicity of PLGA or modulate the drug release, hydrophilic polymers such as PEG, PVA, HA can be employed to modify PLGA in the inhaled formulations108, 109, 110. Various polymeric excipients used in inhaled sustained-release formulations are summarized in Table 199,111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121.
Table 1.
Examples of polymeric particles based sustained-release formulations for inhalation.
| Polymer | The property of polymer | MW of polymer | API | Aerosol form | Da | Performance in in vitro release profiles/in vivo PK studies | Ref. |
|---|---|---|---|---|---|---|---|
| Natural polymers | |||||||
| Albumin | Naturally present at the lungs, non-immunogenic, biocompatible, biodegradable | ∼66 kDa | Benzothiazinones | Dry powders | 2.2–2.7 μm (geometric diameters) | The drug release was triggered by proteases, causing more than 50% of drug being released within 4 h, which was followed by a slower and sustained release profile lasting for 48 h. | 111 |
| Alginate | Biodegradable, biocompatible, mucoadhesive | – | Isoniazid, rifampicin | Dry powders | 1–2 μm | The formulations exhibited an initial burst release (30%–40% within the first 4 h) followed by a sustained release pattern (90% within 60 h). | 112 |
| CS | Biocompatible, biodegradable, non-immunogenic, antimicrobial activity, mucoadhesive | – | Levofloxacin | Dry powders | Less than 5 μm | A sustained in vitro release profile was observed (cumulative release of 80%–90% over 24 h) | 113 |
| Gelatin | Biodegradable, biocompatible | <300 kDa | Methotrexate | Dry powders | 2.2–2.9 μm | Only 35% of the loaded drug was released from the methotrexate conjugated gelatin particles within 72 h in the presence of trypsin, while the unconjugated entirely released the drug within 36 h. | 114 |
| HA | Naturally present at the lungs, non-immunogenic, biodegradable, mucoadhesive | ∼2000 kDa | Insulin | Dry powders | 1–4 μm | As compared to spray-dried pure insulin powders, the formulations had no immediate blood concentration peak and displayed a slower in vivo clearance phenomenon. | 115 |
| Synthetic polymers | |||||||
| PCL | Biocompatible, biodegradable | 8 kDa (Mn) | Dexamethasone | Dry powders | ∼5 μm | Dexamethasone-loaded PCL microparticles exhibited a sustained in vitro release profile (cumulative release of 45% over 24 h) as compared to the dexamethasone prepared without PCL. | 116 |
| PLGA | Biocompatible, biodegradability could be altered by varying composition | 7–17 kDa | Tobramycin | Dry powders | ∼3.4 μm | A sustained release profile with a cumulative release of more than 90% over one month was achieved. | 117 |
| PVA | Ability to improve the colloidal stability, biocompatible | 85–124 kDa | Ciprofloxacin | Dry powders | ∼5.06 μm | A sustained in vitro release profile with cumulative 90% of drug released over 24 h was achieved. | 118 |
| Copolymers | |||||||
| PLGA-PVA | Biodegradable, amphiphilic | – | siRNA | Nebulized nanoparticulate suspensions | 150–200 nm (hydrodynamic diameters) | A sustained siRNA release profile with cumulative release of 85% within 8 h was acquired. | 119 |
| PLGA-PEG | Biocompatible, amphiphilic, high drug loading capacity | – | Salbutamol | Dry powders | 8.24 μm (geometric diameters) | A sustained drug release profile with cumulative release of 91.1% over 12 h was achieved. | 120 |
| PEG-CS | Hydrophilic, biocompatible, biodegradable, non-toxic | CS: 190–310 kDa PEG: 5 kDa (Mn) |
Bovine serum albumin | Dry powders | 1.02–2.63 μm | A tri-phase drug release pattern with a burst release (25%–45%) within the first 2 h, followed by a slow sustained release up to 4 days and an even slower release in late phase. | 99 |
| PEG-PLA | Biocompatible, biodegradable | ∼100 kDa | Placebo nanoparticles | Nebulized nanoparticulate suspensions | 129–141 nm (hydrodynamic diameters) | – | 121 |
API, active pharmaceutical ingredient; CS, chitosan; Da, aerodynamic diameters; HA, hyaluronic acid; Mn, number-average molecular weight; MW, molecular weight; PCL, poly-ε-caprolactone; PEG, polyethylene glycol; PK, pharmacokinetics; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid); PVA, polyvinyl alcohol;Tmax, time of maximum concentration; ‒, not applicable.
3.3.3.2. Inhaled lipid-based formulations
Lipid is another commonly used group of excipients for pulmonary administration. One of the reasons is due to their biocompatibility and safety especially when unwanted immune response of inhaled formulation can be avoided using endogenous lipids122. Several lipid based systems such as liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), micelles are generally formulated to render sustained-release profiles of inhaled drugs.
In liposomes, drugs are encapsulated in a lipid bilayer structured vesicles, which can be used as drug reservoir to extend the drug release profiles and lung exposure. Furthermore, the liposomes could reduce the toxicity of the encapsulated drugs and improve their tolerability, leading to better patient compliance123. Some clinical studies have demonstrated that inhaled liposomal formulations could be used to reduce the frequency of administration, but maintain high local concentrations in the lungs and reduce systemic side effects98. Liposomal formulations have been exploited to load a variety of drugs and used in the treatment of a broad range of diseases123. At present, the representative sustained release formulation for pulmonary delivery is amikacin liposomal inhalation suspension (ALIS; Arikayce®), which has been approved in the USA for use as part of a combination antibacterial drug regimen against Mycobacterium avium complex lung disease124. In this product, amikacin was encapsulated in liposomal vesicles composed of dipalmitoylphosphatidylcholine (DPPC, an ideal excipient used in inhaled liposomal formulations125) and cholesterol. As compared to pristine amikacin, the inhaled liposomal formulations exhibited higher local concentrations in the lungs and prolonged lung exposure of amikacin for several hours, resulting in a better therapeutic outcome in terms of killing respiratory bacteria126. Researchers87 reported that liposomal formulations were suitable for targeting alveolar epithelial cell lining fluid to treat lung diseases in the deep lungs, where inhaled liposomes exhibited a continuous distribution in alveolar epithelial cell lining fluid. Most inhaled liposomal formulations are administered via nebulization as these are a liquid dosage form, i.e., liposomal suspension or colloidal dispersion. The colloidal stability and also potential leakages of drugs from the liposomes during nebulization may limit their clinical applications127. To overcome these problems, various dehydration methods such as freeze drying, spray drying, spray freeze drying have been attempted to produce liposomal dry powders for inhalation128, 129, 130, 131, 132, 133. As the liposomal formulations are soft as compare to polymeric particulate systems, their reconstitution from the dry powders upon deposition in the lungs is more challenging in terms of particle size distribution128,130.
SLNs are made up of lipid shell and a solid hydrophobic core, in which the drug molecules are dispersed or dissolved in the molten solid lipid matrices127. The size, composition and method of delivery of SLNs determine the drug release rate and deposition in the lungs134. SLNs are used to control the drug release and achieve long-term drug exposure in the lungs, and they are initially used as alternatives to polymer nanoparticles135. It is because SLNs are in general degraded faster than polymeric nanoparticles in an in vivo environment, which may alleviate the burden to the lung tissues caused by slow degradation rate of polymers. Moreover, most lipids are better biocompatible than the polymers127. As compared to liposomes, the SLNs are much more stable under nebulization due to their rigidity1. Vieira et al.136 loaded rifampicin into CS-coated SLNs, which showed high encapsulation efficiency and improved drug delivery efficacy to the alveolar macrophages, and the researchers concluded that the CS-coated SLNs were expected to be a safe and effective against pulmonary tuberculosis. The SLNs were also be used as drug carriers for pulmonary delivery of macromolecular drugs. For example, Bi et al.137 loaded insulin into SLNs, which conferred prolonged hypoglycemic effects and relatively high insulin bioavailability after their administration into the lungs of diabetic rats through intratracheal perfusion. NLCs, which are composed of a solid–liquid mixed lipid core, are a second-generation lipid nanoparticles, have attracted increasing attentions to deliver drugs to the lungs and to extend pulmonary drug exposure. Similar to SLNs, the NLCs are more stable than the liposomes during the nebulization and storage, and are more biocompatible than the polymeric nanoparticles and other forms of nanoformulations138,139. Pastor et al.140 compared the efficacy of NLCs and SLNs for the pulmonary delivery of an antibacterial drugs, i.e., sodium colistimethate. Interestingly, the encapsulation efficiency of NLCs (94.8%) was significantly higher than that of SLNs (79.7%). Both NLCs and SLNs exhibited sustained release profiles, whereas, the release of SLNs were incomplete. In contrast, the drugs release from NLCs were almost complete within the sustained release time.
The micelles are another kind of lipid formulations often used for pulmonary drug administration to extend their pulmonary exposure, reduce the frequency of administrations and improve patient compliance. They are self-assembled systems with a hydrophobic inner core and a hydrophilic outer shell in a certain aqueous medium. The hydrophobic micellar core could improve the drug loading efficiency of the poorly water soluble drugs141. Micelles are small in the size and with the bulky hydrophilic shell, it was found they can evade the uptake of alveolar macrophages and prolong the retention of drugs in the lungs48. In literature, phospholipids and amphiphilic molecules including block copolymers, polymer–lipid conjugates, etc. were used to prepare micelles, and their fate in the lungs depends upon the compositions of the micelles142,143. Block copolymers micelles are composed of hydrophobic and hydrophilic polymer molecules. The degradation rate of the block copolymers based micelles is slow, which can resemble that of polymeric nanoparticles, may be harmful to the lungs144. In contrast, the micelles composed of lipid-polymer conjugates exhibit better biocompatibility in the lungs and illustrate a prolonged residence time in the lungs and improved physical stability145. Various hydrophilic polymers such as PEG and PVA are commonly used to afford the polymer-lipid conjugates. Among others, the PEGylated lipid may be the most studied scaffold to accomplish micelles for PDD127. The PEGylation is recognized as an efficient means to avoid the recognition of phagocytes, leading to a prolonged residence time of drug delivery systems in the lungs. Craparo et al.146 loaded beclomethasone dipropionate into PEGylated phospholipid-polyaspartamide micelles where the hydrophilic shell was based on both polyaspartamide and PEG, and the hydrophobic stearoyl tails attached to phospholipids. The micelles increased ca. 240 times of the solubility of beclomethasone dipropionate, and exhibited excellent biocompatibility, cellular internalization and promising for the treatment of lung diseases.
3.3.3.3. Nanocrystals
Nanocrystals are submicron colloidal dispersions of pure drug particles stabilized by excipients147. They are regarded as a promising formulation strategy for poorly soluble drugs due to their ability to increase the dissolution rate and saturated solubility of the drugs. However, if the drug nanocrystals are directly inhaled into the lungs, these may be absorbed to the systemic circulation as fast as intravenous injection, resulting in a shorter pulmonary exposure71. In order to retain the advantage of nanocrystals and also extend their pulmonary exposure in the lungs, researchers used lipids to modify the nanocrystals148. The lipid-modified nanocrystals could not only take the advantage of improving the dissolution rate of poorly water-soluble drugs, but also prolong the residence time of the drugs in the lung tissues by modulating the drug release rate. The nanocrystals of fluticasone propionate modified with phospholipids exhibited reduced dissolution rate of drug as compared to the unmodified nanocrystals and extended its retention time in the lungs148. In another study, Hu and coworkers11 formulated curcumin acetate nanocrystals, a highly lipophilic and poorly water soluble drug, into respirable particles via spray drying and delivered them into the lungs of rats. The results showed that the absorption time in the lungs was 7.2 times longer than that of curcumin, and a better pharmacological effect was observed in a pulmonary hypertension rat model due to prolonged drug retention in the lungs. In addition, the irregular shape of nanocrystals as compared to the conventional carriers, might also affect their lung exposure time. The recent literature has demonstrated that the rod or fiber-like nanoparticles could usually confer a less-efficient clearance by the macrophages149. Therefore, the irregularly shaped nanocrystals might experience a prolonged retention in the lungs. However, the nanocytotoxicity caused due to the long-term pulmonary retention of this nanomedicine should be seriously considered.
So far, a wide range of active substances including small molecules, proteins, peptides and nucleic acids have been formulated in inhaled sustained-release formulations150. Some of them have been evaluated in preclinical animal models or in clinic trial, and there are also a few approved products for marketing, which have been listed in Table 2126,151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161. The treatment of local diseases in the lungs such as asthma, COPD, respiratory infection and pulmonary hypertension can obviously benefits from the extended pulmonary exposure by using inhaled sustained-release formulations126,151,153,158. For some therapeutic macromolecules used for the treatment of systemic diseases, for example insulin, prolonged pharmacological effects and improved patient compliance also could be expected when they are formulated in inhaled sustained release formulations as long as the formulations could slow down the absorption rate of macromolecules162.
Table 2.
Examples of some inhaled sustained drug release formulations used to treat both local lung diseases and systemic diseases.
| Disease | API | Main excipient used | Type of sustained release formulations | Therapeutic outcome | Stage | Ref. |
|---|---|---|---|---|---|---|
| Local lung diseases | ||||||
| Asthma | Budesonide | PLGA, poly(vinylpyrrolidone) | Large porous particles | The formulation significantly reduced the total number of inflammatory cells and expression of IL-4 and IL-5 in BALF as compared to the physical mixture (budesonide/lactose). | Preclinical | 151 |
| Fluticasone propionate | PLA, PLA-PEG | Mucus-penetrating nanoparticles | It inhibited the accumulation of BALF neutrophils for a longer period of time as compared to the free fluticasone propionate. | Preclinical | 152 | |
| COPD | Fluticasone propionate | Glycerol tripalmitate, glyceryldistearate, CS oligosaccharide lactate | Mucoadhesive solid lipid microparticles | It was more effective than the pure fluticasone propionate in controlling oxidative stress. | Preclinical | 153 |
| Cystic fibrosis | Lumacaftor, ivacaftor | Squalene, PEG, Tween 80, Span 35 | Nanostructured lipid carriers | It showed the superior ability to restore the expression and function of cystic fibrosis transmembrane receptor proteins as compared to the non-encapsulated drugs. | Preclinical | 154 |
| Lung infection | Amikacin | DPPC, cholesterol | Liposomes (Arikace®) | It demonstrated significant activity against Mycobacterium avium complex. | Approved by FDA | 126 |
| Ciprofloxacin | Hydrogenated soy phosphatidylcholine, cholesterol | Liposomes (Pulmaquin®) | It showed antibacterial activity against Pseudomonas aeruginosa. | Phase Ⅲ clinical trails | 155 | |
| Tobramycin | Precirol® ATO 5, Compritol® 888 ATO, Poloxamer 188, Tween 80 | Nanostructured lipid carriers | It exhibited antibacterial potential against isolated Pseudomonas aeruginosa. | Preclinical | 156 | |
| Lung cancer | 9-Nitro-20(S)-camptothecin | Dilauroylphosphatidylc-holine, cholesterol | Liposomes | It was clinically effective in reducing tumor size with minimal toxicity profiles. | Phase Ⅱ clinical trails | 157 |
| Pulmonary hypertension | Bosentan | PLGA, PVA | Polymeric nanoparticles | It exhibited a more persistent vasodilation effect on the pulmonary blood vessels. | Preclinical | 158 |
| Fasudil | DPPC, cholesterol | Liposomes | It provided a sustained vasodilation effect on pulmonary blood vessels. | Preclinical | 159 | |
| Systemic diseases | ||||||
| Bone disorder | Calcitonin | PLGA, PVA, CS | Mucoadhesive polymeric nanospheres | It maintained the blood calcium at low level for a prolonged period of time. | Preclinical | 160 |
| Diabetes | Insulin | CS, mannitol, pentasodium tripolyphosphate | Nanocomposite microparticles | It exhibited a prolonged hypoglycemic effect as compared to the insulin solution. | Preclinical | 161 |
API, active pharmaceutical ingredient; BALF, bronchoalveolar lavage fluid; COPD, chronic obstructive pulmonary diseases; CS, chitosan; DPPC, dipalmitoylphosphatidylcholine; FDA, US Food and Drug Administration; IL-4, interleukin-4; IL-5, interleukin-5; PEG, polyethylene glycol; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid); PVA, polyvinyl alcohol.
4. Safety concerns in designing of inhaled formulations with extended pulmonary exposure
Despite the advantages of inhaled formulations with extended pulmonary exposure, the safety of exploiting these inhaled formulations, especially when large proportions of excipients have to be used in the particulate delivery systems, is always one of the main concerns in the drug development and clinical translation. Currently, the number of excipients approved for inhalation is rather limited, which include some sugars, amino acids, lipids, salt, phospholipid and small size PEGs163. The use of unapproved excipients in any novel inhaled formulations could increase workload, cost, time, and risk of rejection by the regulatory agencies11. This could partially explain the reasons of existence of limited number of inhaled formulations with extended pulmonary exposure on the present market. With the existing limited numbers of US food and drug administration (FDA)-approved excipients, it is extremely challenging to make innovative inhaled formulations122. Nevertheless, the efforts to develop novel excipients and assess the existing excipients for PDD applications have never ceased122. Fumaryl diketopiperazine (FDKP) used in Technosphere® inhaled insulin (Afrezza®) is an example of novel excipient although it is not used for extending pulmonary exposure of the drugs. With continuous innovation, more suitable and safe excipients for inhalation can be developed. In the following sections, the toxicity concerns of the existing excipients, nanocytotoxicity and immunogecity associated to the inhaled formulations with extended pulmonary exposure are reviewed.
4.1. Toxicity of excipients used in the inhaled formulations
4.1.1. Polymers
The safety concerns of the polymers applied for the PDD mainly involve their degradation pathways and the toxicity of the degraded products in the lungs. The uses of non-biodegradable polymers in the inhaled formulations are prohibited even though they could provide sustained release profiles for the loaded drugs. The polymers, which are degraded and/or eliminated slowly, may lead to local accumulation and their degradation products may cause potential pulmonary toxicity. Thus, these should be avoided in inhaled formulations18. In this context, PLGA is one of the most commonly studied polymers as it exhibits excellent biocompatibility and biodegradability, and provides extended pulmonary exposure after inhalation. However, some researchers164 have proved that PLGA particles could retain in mouse lungs for more than 20 days. The chronic toxicological data of the inhaled PLGA particles for such a long period of time in the lungs is still not well investigated165. The potential irritation effects of the acidic degradation products of PLGA (e.g., lactic acid and glycolic acid) to the lungs are expected122. To date, the safety profiles of the post pulmonary administration of the formulations composed of CS has not fully been examined103. Some data suggest that CS could open epithelial tight junctions, which may cause emphysema, and thus, the pulmonary delivery using CS is not often recommended166. Apparently, there are more work to be done to assess the safety profiles of other polymeric excipients prior to successful translation of the inhaled sustained release formulations composed of polymers in clinic.
4.1.2. Lipids
As aforementioned lipid-based particulate delivery systems such as liposomes, SLNs, NLCs have attracted considerable interests as there are comprised of some endogenous lipids and their safety profiles are more promising as compared to inhaled polymeric formulations167. The lung surfactants are composed of many different types of lipids including DPPC (saturated phosphatidylcholine), unsaturated phosphatidylcholine, phosphatidylinositol, phosphatidylglycerol, phosphatidylethanolamine, cholesterol, etc., which can potentially be used in the inhaled formulations to elicit better safety profiles than other lipids163. For examples, phosphatidylcholine was used as one of the components of inhaled solid lipid microparticles, which had been proven to be safe for the lung cells168. In addition, the cytotoxicity of liposomes composed of DPPC was examined by measuring the release of lactate dehydrogenase from lung tissues, and the results showed that liposome composed of DPPC did not damage lung tissues at the dose used in the study169. Other lipids such as phospholipid and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), have been used in TOBI® Podhaler® produced by Novartis Pharmaceuticals, and their long-term safety has been established at high daily doses in cystic fibrosis patients170. The DSPC could be catabolized and ultimately recycled by type II cells in the lungs171. Overall, the safety profiles of the lipids in the pulmonary formulations are more promising as compared to the polymeric excipients.
4.1.3. Metal ions
In some cases, metal ions could be introduced to increase the polarity of molecules such as fluoroquinolones to limit the absorption or form complexes with biologics such as insulin to provide extended lung retention. However, the cytotoxicity concerns of the metal ions to the lungs are inevitable. For example, the exposure of the ferric oxide nanoparticles in the lungs can cause oxidative stress and a variety of inflammatory reactions by recruiting inflammation and immune cells to the lungs172. Studies showed that Zn2+, Al3+ and Cu2+ exhibit higher cytotoxicity than Mg2+ and Ca2+50. In addition, the toxicity of the metal ions is largely related to their concentrations used, which provide some guidance of selection of the ions and dose.
4.2. Nanocytotoxicity
Nanoparticles could provide many benefits to the PDD as described in the previous sections. However, the cytotoxicity of the nano-sized materials is a serious concern for their clinical translation. One of nanoparticle-induced toxicological effects is associated to the production of reactive oxygen species, which can ultimately result in oxidative stress in the biological systems173. The increase in the reactive oxygen species caused by nanoparticles further leads to inflammation174. The toxicity of inhaled nanoparticles seems to be related to the physical and chemical properties of inhalable nanoparticles such as size, specific surface area and surface chemistry175. The clinical and experimental data suggested that the nanoparticles with smaller particle size and larger surface area could cause more lung injury174. This might be explained by the fact that the ultrafine nanoparticles might easily evade phagocytosis of macrophages than the larger nanoparticles. Consequently, these are not easily cleared from the alveolar area, leading to long-term accumulation and subsequent toxicity in the lungs176. For nanoparticles, the aggregation state in physiological situation also needs to be considered. A single gold nanoparticle with size 50 nm elicits a lesser acute hazard than its agglomerates or slightly larger particles when using pulmonary inflammation as a marker to assess the toxicity of nanoparticles177.
The shapes of the nanoparticles also have impacts on their toxicity in the lungs. The spherical nanoparticles are considered to be safer and are more rapidly internalized by the macrophages than the rod- or fibrous-like nanoparticles149, and the spherical nanoparticles seem to be safer delivery vehicles for PDD and also for other administration routes178. Moreover, the surface properties of the nanoparticles play a role in producing toxicity. For example, the nanoparticles with positive surface charges could exhibit a higher cytotoxicity than the negative or neutral charged nanoparticles179. In this aspect, the surface modification of the nanoparticles with PEG could often reduce the production of reactive oxygen species and eliminate cytotoxicity180. In addition, it was reported that the selection of muco-inert or muco-adhesive coating on the surfaces of the nanoparticles has an influences on the toxicity. Schneider et al.73 assessed the preclinical safety of the MP and MPP following inhalation. They found the mice treated with MP showed acute inflammation, while the group receiving MPP showed almost no difference in the toxicity as compared to the mice treated with saline. Lastly, poorly water-soluble or more persistent nanoparticles in the lungs after inhalation could induce more oxidative stress, which might eventually lead to inflammation, fibrosis and cancer173. This implies the importance of the selection of biodegradable and biocompatible materials when designing inhaled formulations with extended pulmonary exposure.
4.3. Immunogenicity
The aforementioned toxicity is mainly referring to the inflammation caused the excipients in the inhaled formulations. Besides inflammation, the immunogenicity of inhaled formulations should not be overlooked, which can be caused not only by some biological active substances such as proteins but also by some excipients when being formulated with biologics. The immunogenicity refers to the ability of foreign substances to induce host immune response181. Immunogenic reactions may cause patients to suffer from cytokine storms or strong allergic reactions, such as anaphylaxis or pure red-cell aplasia representing life-threatening situations182. An immunogenic risk assessment is an ongoing and continuous process throughout the clinical development with a goal of maximizing the safety to the patients183.
The lipid excipients may not provoke an immune response as these are relatively small molecules. However, some protein drugs and some polysaccharides based excipients could display immunogenic properties, inducing humoral immune responses, especially when these are in different aggregation states184. The conjugation of lipids and proteins may increase the immunogenic potency too185. Various proteins and peptide therapeutics have occupied a considerable fraction of current pharmaceutical market, which have drastically changed the landscape of treatment of many diseases. The lungs are recognized as attractive administration route and alternative to injection routs for the therapeutic proteins and peptides186. Currently, several inhaled peptide and protein drugs, such as insulin, calcitonin, cyclosporin A and aldesleukin are under the development and in clinical trials187. The potential immune responses of the therapeutic proteins undermine their clinical utility188. An example is inhaled human insulin. Although inhaled human insulin has been launched on the market, its dry powder or liquid forms, are more immunogenic than subcutaneous insulin injection189.
Protein aggregates have been considered as a potential risk factor for patient immunogenicity190. It would not be surprising to encounter immunogenic reactions while developing inhaled biologic formulations with extended pulmonary exposure, where excipients such as polymers and lipids involved. The aggregation of proteins and some unwanted interactions between proteins and excipients might occur during their preparation (e.g., at gas–liquid or solid–liquid interfaces and filling), storage, transportation, and administration (e.g., vigorous shaking before administration)191, which could raise immunogenicity of the products. In order to avoid protein aggregation, non-ionic surfactants can be added, which could stabilize peptides and proteins and diminish their adsorption induced unfolding and aggregation192. In addition, the coupling of polymeric excipients with proteins and peptides could also produce biological drugs to reduce the immunogenicity. For example, the combination of PEG and albumin with peptides can reduce peptide immunogenicity in vivo193. Moreover, the duration of treatment, administration route, and frequency of administration impact the immunogenicity of the biological drugs194. Data have shown long-term treatment and frequent administration were often increase the immunogenicity of biological drug products195, which should be critically evaluated during the development of the inhaled formulations with extended pulmonary exposure using sustained-release formulations. Besides protein and peptide drugs, the immunogenicity of the nucleic acid-based therapeutics such as DNA, RNA species or analogues and their vectors often hinder their translations into the clinical practice196. The extracellular DNA can be recognized by immune cell receptors as damage-related molecular patterns or pathogen-related molecular patterns, activating pro-inflammatory signaling pathways or immunosuppressive cell functions197. Some specific RNA sequences or structures can also be recognized by the corresponding immune receptors to trigger a series of immune events, including cytokine secretion, immune cell proliferation and survival, or activation of adaptive immunity198.
5. Conclusions and perspectives
The administration of drugs to or via the lungs has been exploited to treat both local lung diseases and systemic diseases. Due to the unique physiological and anatomic features of the respiratory tract, most inhaled medicines can rapidly be eliminated from the lungs, resulting in transit pharmacological effects. The inhaled medicines with extended pulmonary exposure may not only improve the patient adherence and compliance by reducing the frequency of drug administration, but also provide clinical benefits to the patients. To extend pulmonary retention and prolong pharmacological effects of inhaled therapeutics, various physical and chemical principles have been adopted to overcome the inherent clearance mechanisms of the lungs, namely mucociliary escalator, phagocytosis of macrophages, and translocation to cells and systemic circulation. The modification of the inhaled drugs with respect to their size, polarity, permeability and mucoadhesiveness via molecular engineering or conjugation is considered as powerful means to sustain pulmonary retention of the products, as long as the modification would not significantly compromise their pharmacological activities and cause unwanted side effects. The pharmaceutical technologies such as formulating biomucoadhesive, swellable, and large porous particles intended for inhalation could also overcome the clearance mechanisms of the lungs. Design of inhaled sustained release formulations has become a commonly applied strategy to extend the pulmonary exposure and pharmacological effects. However, the number of excipients approved for inhalation is rather limited at this moment. The incorporation of various unapproved excipients in some innovative inhaled formulations and assessment of their safety profiles require additional workload, cost, and risk, which hinder their translations into the clinical practice. From our perspective, the right choice of excipients with satisfactory balance between efficacy and safety is critical for the successful translation of inhaled sustained release formulations. As we learned from the approved product of amikacin loaded inhalable liposomes, endogenous lipids-based formulations and drug-lipid conjugates hold the greatest promising for the clinical applications in the near future.
In conclusion, the future efforts should be placed on exploring innovative strategies to not only overcome the lung clearance mechanisms but also develop innovative and suitable excipients to expedite translation of the inhaled formulations with extended pulmonary exposure to the clinic.
Acknowledgments
We thankfully acknowledge the Liaoning Pan Deng Xue Zhe scholarship for the financial support (China). Dongmei Cun thanks the financial support from the Guiding Project for Science and Technology of Liaoning Province (No. 2019-ZD-0448, China), and Minister of Education Chunhui Program (China). Hriday Bera thanks the National Natural Science Foundation of China (Nos. 81850410554 and 82050410448).
Author contributions
Yi Guo, Dongmei Cun and Mingshi Yang contributed in conception, design, and drafting of the manuscript. Hriday Bera, Changzhi Shi and Li Zhang contributed in manuscript drafting. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Liang Z.L., Ni R., Zhou J.Y., Mao S.R. Recent advances in controlled pulmonary drug delivery. Drug Discov Today. 2015;20:380–389. doi: 10.1016/j.drudis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Courrier H.M., Butz N., Vandamme T.F. Pulmonary drug delivery systems: recent developments and prospects. Crit Rev Ther Drug Carrier Syst. 2002;19:425–498. doi: 10.1615/critrevtherdrugcarriersyst.v19.i45.40. [DOI] [PubMed] [Google Scholar]
- 3.Patton J.S., Byron P.R. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 4.Salama R., Traini D., Chan H.K., Young P.M. Recent advances in controlled release pulmonary therapy. Curr Drug Deliv. 2009;6:404–414. doi: 10.2174/156720109789000546. [DOI] [PubMed] [Google Scholar]
- 5.He Y., Liang Y.M., Han R., Lu W.L., Mak J.C.W., Zheng Y. Rational particle design to overcome pulmonary barriers for obstructive lung diseases therapy. J Control Release. 2019;314:48–61. doi: 10.1016/j.jconrel.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Yang W., Peters J.I., Williams R.O. Inhaled nanoparticles—a current review. Int J Pharm. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Andrade F., Rafael D., Videira M., Ferreira D., Sosnik A., Sarmento B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv Drug Deliv Rev. 2013;65:1816–1827. doi: 10.1016/j.addr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q., Tang P., Leung S.S.Y., Chan J.G.Y., Chan H.K. Emerging inhalation aerosol devices and strategies: where are we headed?. Adv Drug Deliv Rev. 2014;75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q., Leung S.S.Y., Tang P., Parumasivam T., Loh Z.H., Chan H.K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Liang W.L., Pan H.W., Vllasaliu D., Lam J.K.W. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12:1025. doi: 10.3390/pharmaceutics12111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X., Yang F.F., Wei X.L., Yao G.Y., Liu C.Y., Zheng Y. Curcumin acetate nanocrystals for sustained pulmonary delivery: preparation, characterization and in vivo evaluation. J Biomed Nanotechnol. 2017;13:99–109. doi: 10.1166/jbn.2017.2326. [DOI] [PubMed] [Google Scholar]
- 12.Grenha A., Grainger C.I., Dailey L.A., Seijo B., Martin G.P., Remuñán-López C. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur J Pharmaceut Sci. 2007;31:73–84. doi: 10.1016/j.ejps.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Deng Q.H., Deng L.J., Miao Y.F., Guo X.L., Li Y.G. Particle deposition in the human lung: health implications of particulate matter from different sources. Environ Res. 2019;169:237–245. doi: 10.1016/j.envres.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Osman N., Kaneko K., Carini V., Saleem I. Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expet Opin Drug Deliv. 2018;15:821–834. doi: 10.1080/17425247.2018.1502267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patton J.S., Fishburn C.S., Weers J.G. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 16.Todoroff J., Vanbever R. Fate of nanomedicines in the lungs. Curr Opin Colloid Interface Sci. 2011;16:246–254. [Google Scholar]
- 17.Heyder J., Gebhart J., Rudolf G., Schiller C.F., Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci. 1986;17:811–825. [Google Scholar]
- 18.Ruge C.A., Kirch J., Lehr C.M. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1:402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 19.Grainger C.I., Greenwell L.L., Martin G.P., Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur J Pharm Biopharm. 2009;71:318–324. doi: 10.1016/j.ejpb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Antunes M.B., Cohen N.A. Mucociliary clearance—a critical upper airway host defense mechanism and methods of assessment. Curr Opin Allergy Clin Immunol. 2007;7:5–10. doi: 10.1097/ACI.0b013e3280114eef. [DOI] [PubMed] [Google Scholar]
- 21.Hickey A.J. Controlled delivery of inhaled therapeutic agents. J Control Release. 2014;190:182–188. doi: 10.1016/j.jconrel.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid–protein interactions. Biochim Biophys Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Bernhard W., Haslam P.L., Floros J. From birds to humans: new concepts on airways relative to alveolar surfactant. Am J Respir Cell Mol Biol. 2004;30:6–11. doi: 10.1165/rcmb.2003-0158TR. [DOI] [PubMed] [Google Scholar]
- 24.Palecanda A., Kobzik L. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med. 2001;1:589–595. doi: 10.2174/1566524013363384. [DOI] [PubMed] [Google Scholar]
- 25.Chono S., Tanino T., Seki T., Morimoto K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J Drug Target. 2006;14:557–566. doi: 10.1080/10611860600834375. [DOI] [PubMed] [Google Scholar]
- 26.Bivas-Benita M., Ottenhoff T.H.M., Junginger H.E., Borchard G. Pulmonary DNA vaccination: concepts, possibilities and perspectives. J Control Release. 2005;107:1–29. doi: 10.1016/j.jconrel.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt P.G. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc. 2005;2:116–120. doi: 10.1513/pats.200502-017AW. [DOI] [PubMed] [Google Scholar]
- 28.Jiskoot W., Kijanka G., Randolph T.W., Carpenter J.F., Koulov A.V., Mahler H.C. Mouse models for assessing protein immunogenicity: lessons and challenges. J Pharm Sci. 2016;105:1567–1575. doi: 10.1016/j.xphs.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferin J., Oberdörster G. Translocation of particles from pulmonary alveoli into the interstitium. J Aerosol Med. 1992;5:179–187. [Google Scholar]
- 30.Chaurasiya B., Zhao Y.Y. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics. 2020;13:31. doi: 10.3390/pharmaceutics13010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tronde A., Nordén B., Marchner H., Wendel A.K., Lennernäs H., Bengtsson U.H. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: structure‒absorption relationships and physicochemical profiling of inhaled drugs. J Pharm Sci. 2003;92:1216–1233. doi: 10.1002/jps.10386. [DOI] [PubMed] [Google Scholar]
- 32.Wiedmann T.S., Bhatia R., Wattenberg L.W. Drug solubilization in lung surfactant. J Control Release. 2000;65:43–47. doi: 10.1016/s0168-3659(99)00230-8. [DOI] [PubMed] [Google Scholar]
- 33.Patton J.S. Mechanisms of macromolecule absorption by the lungs. Adv Drug Deliv Rev. 1996;19:3–36. [Google Scholar]
- 34.Fischer H., Widdicombe J.H. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham S., Wiedmann T.S. Note: dissolution of aerosol particles of budesonide in Survanta™, a model lung surfactant. J Pharm Sci. 2001;90:98–104. doi: 10.1002/1520-6017(200101)90:1<98::aid-jps11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Kandasamy R., Chandrasekaran K. Sustained release aerosol for pulmonary drug delivery system: a review. Int J Pharm Pharmacoeu Sci. 2013;5:126–130. [Google Scholar]
- 37.Gumbleton M. Caveolae as potential macromolecule trafficking compartments within alveolar epithelium. Adv Drug Deliv Rev. 2001;49:281–300. doi: 10.1016/s0169-409x(01)00142-9. [DOI] [PubMed] [Google Scholar]
- 38.Fish D.N. Bronchoscopic sampling of drug concentrations: penetration to tissue is the issue. Am J Respir Crit Care Med. 2003;168:1263–1265. doi: 10.1164/rccm.2309013. [DOI] [PubMed] [Google Scholar]
- 39.Rodvold K.A., George J.M., Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet. 2011;50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Kiem S., Schentag J.J. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardy J.G., Chadwick T.S. Sustained release drug delivery to the lungs: an option for the future. Clin Pharmacokinet. 2000;39:1–4. doi: 10.2165/00003088-200039010-00001. [DOI] [PubMed] [Google Scholar]
- 42.Edsbäcker S., Johansson C.J. Airway selectivity: an update of pharmacokinetic factors affecting local and systemic disposition of inhaled steroids. Basic Clin Pharmacol Toxicol. 2006;98:523–536. doi: 10.1111/j.1742-7843.2006.pto_355.x. [DOI] [PubMed] [Google Scholar]
- 43.Patton J.S., Brain J.D., Davies L.A., Fiegel J., Gumbleton M., Kim K.J. The particle has landed-characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23:S71–S87. doi: 10.1089/jamp.2010.0836. [DOI] [PubMed] [Google Scholar]
- 44.Winkler J., Hochhaus G., Derendorf H. How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1:356–363. doi: 10.1513/pats.200403-025MS. [DOI] [PubMed] [Google Scholar]
- 45.Tolman J.A., Williams R.O. Advances in the pulmonary delivery of poorly water-soluble drugs: influence of solubilization on pharmacokinetic properties. Drug Dev Ind Pharm. 2010;36:1–30. doi: 10.3109/03639040903092319. [DOI] [PubMed] [Google Scholar]
- 46.Vanbever R., Ben-Jebria A., Mintzes J.D., Langer R., Edwards D.A. Sustained release of insulin from insoluble inhaled particles. Drug Dev Res. 1999;48:178–185. [Google Scholar]
- 47.Eriksson J., Sjögren E., Thörn H., Rubin K., Bäckman P., Lennernäs H. Pulmonary absorption-estimation of effective pulmonary permeability and tissue retention of ten drugs using an ex-vivo rat model and computational analysis. Eur J Pharm Biopharm. 2018;124:1–12. doi: 10.1016/j.ejpb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Loira-Pastoriza C., Todoroff J., Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Nurbaeti S.N., Olivier J.C., Adier C., Marchand S., Couet W., Brillault J. Active mediated transport of chloramphenicol and thiamphenicol in a Calu-3 lung epithelial cell model. J Pharm Sci. 2018;107:1178–1184. doi: 10.1016/j.xphs.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Brillault J., Tewes F., Couet W., Olivier J.C. In vitro biopharmaceutical evaluation of ciprofloxacin/metal cation complexes for pulmonary administration. Eur J Pharmaceut Sci. 2017;97:92–98. doi: 10.1016/j.ejps.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Gontijo A.V.L., Brillault J., Grégoire N., Lamarche I., Gobin P., Couet W. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, moxifloxacin, and grepafloxacin. Antimicrob Agents Chemother. 2014;58:3942–3949. doi: 10.1128/AAC.02818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamy B., Tewes F., Serrano D.R., Lamarche I., Gobin P., Couet W. New aerosol formulation to control ciprofloxacin pulmonary concentration. J Control Release. 2018;271:118–126. doi: 10.1016/j.jconrel.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 53.Isogaya M., Yamagiwa Y., Fujita S., Sugimoto Y., Nagao T., Kurose H. Identification of a key amino acid of the beta 2-adrenergic receptor for high affinity binding of salmeterol. Mol Pharmacol. 1998;54:616–622. [PubMed] [Google Scholar]
- 54.Lötvall J. The long and short of β2-agonists. Pulm Pharmacol Therapeut. 2002;15:497–501. doi: 10.1006/pupt.2002.0400. [DOI] [PubMed] [Google Scholar]
- 55.Brittain R.T. Approaches to a long-acting, selective beta 2-adrenoceptor stimulant. Lung. 1990;168:111–114. doi: 10.1007/BF02718122. [DOI] [PubMed] [Google Scholar]
- 56.Jack D. The 1990 Lilly Prize Lecture. A way of looking at agonism and antagonism: lessons from salbutamol, salmeterol and other beta-adrenoceptor agonists. Br J Clin Pharmacol. 1991;31:501–514. doi: 10.1111/j.1365-2125.1991.tb05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gursahani H., Riggs-Sauthier J., Pfeiffer J., Lechuga-Ballesteros D., Fishburn C.S. Absorption of polyethylene glycol (PEG) polymers: the effect of PEG size on permeability. J Pharm Sci. 2009;98:2847–2856. doi: 10.1002/jps.21635. [DOI] [PubMed] [Google Scholar]
- 58.Alven S., Nqoro X., Buyana B., Aderibigbe B.A. Polymer–drug conjugate, a potential therapeutic to combat breast and lung cancer. Pharmaceutics. 2020;12:406. doi: 10.3390/pharmaceutics12050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rades N., Achazi K., Qiu M., Deng C., Haag R., Zhong Z.Y. Reductively cleavable polymer–drug conjugates based on dendritic polyglycerol sulfate and monomethyl auristatin E as anticancer drugs. J Control Release. 2019;300:13–21. doi: 10.1016/j.jconrel.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 60.Marasini N., Haque S., Kaminskas L.M. Polymer–drug conjugates as inhalable drug delivery systems: a review. Curr Opin Colloid Interface Sci. 2017;31:18–29. [Google Scholar]
- 61.Bayard F.J.C., Thielemans W., Pritchard D.I., Paine S.W., Young S.S., Bäckman P. Polyethylene glycol–drug ester conjugates for prolonged retention of small inhaled drugs in the lung. J Control Releasee. 2013;171:234–240. doi: 10.1016/j.jconrel.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Depreter F., Pilcer G., Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447:251–280. doi: 10.1016/j.ijpharm.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 63.Vigani B., Rossi S., Sandri G., Bonferoni M.C., Caramella C.M., Franca F. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics. 2020;12:859. doi: 10.3390/pharmaceutics12090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassano R., Trapani A., Di Gioia M.L., Mandracchia D., Pellitteri R., Tripodo G. Synthesis and characterization of novel chitosan-dopamine or chitosan-tyrosine conjugates for potential nose-to-brain delivery. Int J Pharm. 2020;589:119829. doi: 10.1016/j.ijpharm.2020.119829. [DOI] [PubMed] [Google Scholar]
- 65.Chopra S., Mahdi S., Kaur J., Iqbal Z., Talegaonkar S., Ahmad F.J. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J Pharm Pharmacol. 2006;58:1021–1032. doi: 10.1211/jpp.58.8.0002. [DOI] [PubMed] [Google Scholar]
- 66.Makhlof A., Werle M., Tozuka Y., Takeuchi H. Nanoparticles of glycol chitosan and its thiolated derivative significantly improved the pulmonary delivery of calcitonin. Int J Pharm. 2010;397:92–95. doi: 10.1016/j.ijpharm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Leitner V.M., Walker G.F., Bernkop-Schnürch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm. 2003;56:207–214. doi: 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 68.Alpar H.O., Somavarapu S., Atuah K.N., Bramwell V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–430. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Henning A., Schneider M., Nafee N., Muijs L., Rytting E., Wang X.Y. Influence of particle size and material properties on mucociliary clearance from the airways. J Aerosol Med Pulm Drug Deliv. 2010;23:233–241. doi: 10.1089/jamp.2009.0806. [DOI] [PubMed] [Google Scholar]
- 70.Li Y., Han M.H., Liu T.T., Cun D.M., Fang L., Yang M.S. Inhaled hyaluronic acid microparticles extended pulmonary retention and suppressed systemic exposure of a short-acting bronchodilator. Carbohydr Polym. 2017;172:197–204. doi: 10.1016/j.carbpol.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 71.Liu T.T., Han M.H., Tian F., Cun D.M., Rantanen J., Yang M.S. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym. 2018;181:1143–1152. doi: 10.1016/j.carbpol.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 72.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider C.S., Xu Q.G., Boylan N.J., Chisholm J., Tang B.C., Schuster B.S. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv. 2017;3 doi: 10.1126/sciadv.1601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuek L.E., Lee R.J. First contact: the role of respiratory cilia in host-pathogen interactions in the airways. Am J Physiol Lung Cell Mol Physiol. 2020;319:603–619. doi: 10.1152/ajplung.00283.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lay J.C., Stang M.R., Fisher P.E., Yankaskas J.R., Bennett W.D. Airway retention of materials of different solubility following local intrabronchial deposition in dogs. J Aerosol Med. 2003;16:153–166. doi: 10.1089/089426803321919915. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y.Y., Lai S.K., Suk J.S., Pace A., Cone R., Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that "slip" through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usmani O.S., Biddiscombe M.F., Barnes P.J. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–1504. doi: 10.1164/rccm.200410-1414OC. [DOI] [PubMed] [Google Scholar]
- 78.Hirota K., Hasegawa T., Hinata H., Ito F., Inagawa H., Kochi C. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release. 2007;119:69–76. doi: 10.1016/j.jconrel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Verma R.K., Kaur J., Kumar K., Yadav A.B., Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob Agents Chemother. 2008;52:3195–3201. doi: 10.1128/AAC.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El-Sherbiny I.M., Mcgill S., Smyth H.D.C. Swellable microparticles as carriers for sustained pulmonary drug delivery. J Pharm Sci. 2010;99:2343–2356. doi: 10.1002/jps.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selvam P., El-Sherbiny I.M., Smyth H.D.C. Swellable hydrogel particles for controlled release pulmonary administration using propellant-driven metered dose inhalers. J Aerosol Med Pulm Drug Deliv. 2011;24:25–34. doi: 10.1089/jamp.2010.0830. [DOI] [PubMed] [Google Scholar]
- 82.El-Sherbiny I.M., Smyth H.D.C. Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol Pharm. 2012;9:269–280. doi: 10.1021/mp200351y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni R., Zhao J., Liu Q.Y., Liang Z.L., Muenster U., Mao S.R. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur J Pharmaceut Sci. 2017;99:137–146. doi: 10.1016/j.ejps.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Edwards D.A., Hanes J., Caponetti G., Hrkach J., Ben-Jebria A., Eskew M.L. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 85.Chvatal A., Ambrus R., Party P., Katona G., Jójárt-Laczkovich O., Szabó-Révész P. Formulation and comparison of spray dried non-porous and large porous particles containing meloxicam for pulmonary drug delivery. Int J Pharm. 2019;559:68–75. doi: 10.1016/j.ijpharm.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 86.Kim I., Byeon H.J., Kim T.H., Lee E.S., Oh K.T., Shin B.S. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer. Biomaterials. 2012;33:5574–5583. doi: 10.1016/j.biomaterials.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 87.Kaneko K., Togami K., Yamamoto E., Wang S.J., Morimoto K., Itagaki S. Sustained distribution of aerosolized PEGylated liposomes in epithelial lining fluids on alveolar surfaces. Drug Deliv Transl Res. 2016;6:565–571. doi: 10.1007/s13346-016-0310-2. [DOI] [PubMed] [Google Scholar]
- 88.Li Y., Tang C.C., Zhang E.B., Yang L. Colistin-entrapped liposomes driven by the electrostatic interaction: mechanism of drug loading and in vivo characterization. Int J Pharm. 2016;515:20–29. doi: 10.1016/j.ijpharm.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 90.Emami F., Mostafavi Yazdi S.J., Na D.H. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J Pharm Invest. 2019;49:427–442. [Google Scholar]
- 91.Shiehzadeh F., Tafaghodi M. Dry powder form of polymeric nanoparticles for pulmonary drug delivery. Curr Pharmaceut Des. 2016;22:2549–2560. doi: 10.2174/1381612822666160128150449. [DOI] [PubMed] [Google Scholar]
- 92.Cun D.M., Wan F., Yang M.S. Formulation strategies and particle engineering technologies for pulmonary delivery of biopharmaceuticals. Curr Pharmaceut Des. 2015;21:2599–2610. doi: 10.2174/1381612821666150416100800. [DOI] [PubMed] [Google Scholar]
- 93.Yang M.S., Yamamoto H., Kurashima H., Takeuchi H., Yokoyama T., Tsujimoto H. Design and evaluation of inhalable chitosan-modified poly (dl-lactic-co-glycolic acid) nanocomposite particles. Eur J Pharmaceut Sci. 2012;47:235–243. doi: 10.1016/j.ejps.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 94.Yang M.S., Yamamoto H., Kurashima H., Takeuchi H., Yokoyama T., Tsujimoto H. Design and evaluation of poly(DL-lactic-co-glycolic acid) nanocomposite particles containing salmon calcitonin for inhalation. Eur J Pharmaceut Sci. 2012;46:374–380. doi: 10.1016/j.ejps.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 95.Agnoletti M., Bohr A., Thanki K., Wan F., Zeng X.H., Boetker J.P. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur J Pharm Biopharm. 2017;120:9–21. doi: 10.1016/j.ejpb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Wan F., Maltesen M.J., Bjerregaard S., Foged C., Rantanen J., Yang M.S. Particle engineering technologies for improving the delivery of peptide and protein drugs. J Drug Deliv Sci Technol. 2013;23:355–363. [Google Scholar]
- 97.Bohr A., Boetker J.P., Rades T., Rantanen J., Yang M.S. Application of spray-drying and electrospraying/electospinning for poorly water-soluble drugs: a particle engineering approach. Curr Pharmaceut Des. 2014;20:325–348. doi: 10.2174/13816128113199990399. [DOI] [PubMed] [Google Scholar]
- 98.Liu K., Chen W.J., Yang T.T., Wen B., Ding D.J., Keidar M. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int J Nanomed. 2017;12:8239–8255. doi: 10.2147/IJN.S147028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Sherbiny I.M., Smyth H.D.C. Biodegradable nano-micro carrier systems for sustained pulmonary drug delivery: (I) self-assembled nanoparticles encapsulated in respirable/swellable semi-IPN microspheres. Int J Pharm. 2010;395:132–141. doi: 10.1016/j.ijpharm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L.K., Luo J., Shi S.J., Zhang Q., Sun X., Zhang Z.R. Development of a pulmonary peptide delivery system using porous nanoparticle-aggregate particles for systemic application. Int J Pharm. 2013;451:104–111. doi: 10.1016/j.ijpharm.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 101.Sung J.C., Padilla D.J., Garcia-Contreras L., Verberkmoes J.L., Durbin D., Peloquin C.A. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res. 2009;26:1847–1855. doi: 10.1007/s11095-009-9894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y.J., Sun T., Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8:34–50. doi: 10.1016/j.apsb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Islam N., Ferro V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale. 2016;8:14341–14358. doi: 10.1039/c6nr03256g. [DOI] [PubMed] [Google Scholar]
- 104.Osman R., Kan P.L., Awad G., Mortada N., EL-Shamy A.E., Alpar O. Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int J Pharm. 2013;449:44–58. doi: 10.1016/j.ijpharm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Barclay T.G., Day C.M., Petrovsky N., Garg S. Review of polysaccharide particle-based functional drug delivery. Carbohydr Polym. 2019;221:94–112. doi: 10.1016/j.carbpol.2019.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mundargi R.C., Babu V.R., Rangaswamy V., Patel P., Aminabhavi T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Rytting E., Nguyen J., Wang X.Y., Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expet Opin Drug Deliv. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 108.Patel B., Gupta V., Ahsan F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release. 2012;162:310–320. doi: 10.1016/j.jconrel.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Pistel K.F., Breitenbach A., Zange-Volland R., Kissel T. Brush-like branched biodegradable polyesters, part III protein release from microspheres of poly(vinyl alcohol)-graft-poly(d,l-lactic-co-glycolic acid) J Control Release. 2001;73:7–20. doi: 10.1016/s0168-3659(01)00231-0. [DOI] [PubMed] [Google Scholar]
- 110.Wu J.T., Deng C., Meng F.H., Zhang J., Sun H.L., Zhong Z.Y. Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J Control Release. 2017;259:76–82. doi: 10.1016/j.jconrel.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 111.Patel A., Redinger N., Richter A., Woods A., Neumann P.R., Keegan G. In vitro and in vivo antitubercular activity of benzothiazinone-loaded human serum albumin nanocarriers designed for inhalation. J Control Release. 2020;328:339–349. doi: 10.1016/j.jconrel.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 112.Garg T., Goyal A.K., Rath G., Murthy R.S.R. Spray-dried particles as pulmonary delivery system of anti-tubercular drugs: design, optimization, in vitro and in vivo evaluation. Pharmaceut Dev Technol. 2016;21:951–960. doi: 10.3109/10837450.2015.1081613. [DOI] [PubMed] [Google Scholar]
- 113.Shah S., Ghetiya R., Soniwala M., Chavda J. Development and optimization of inhalable levofloxacin nanoparticles for the treatment of tuberculosis. Curr Drug Deliv. 2020;17 doi: 10.2174/1567201817999201103194626. [DOI] [PubMed] [Google Scholar]
- 114.Abdelrady H., Hathout R.M., Osman R., Saleem I., Mortada N.D. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur J Pharmaceut Sci. 2019;133:115–126. doi: 10.1016/j.ejps.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 115.Surendrakumar K., Martyn G.P., Hodgers E.C.M., Jansen M., Blair J.A. Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Releasee. 2003;91:385–394. doi: 10.1016/s0168-3659(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 116.Fontana M.C., Durli T.L., Pohlmann A.R., Guterres S.S., Beck R.C.R. Polymeric controlled release inhalable powder produced by vibrational spray-drying: one-step preparation and in vitro lung deposition. Powder Technol. 2014;258:49–59. [Google Scholar]
- 117.Ungaro F., d'Angelo I., Coletta C., Bianca R.D.D.V., Sorrentino R., Perfetto B. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release. 2012;157:149–159. doi: 10.1016/j.jconrel.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 118.Silva D.M., Paleco R., Traini D., Sencadas V. Development of ciprofloxacin-loaded poly(vinyl alcohol) dry powder formulations for lung delivery. Int J Pharm. 2018;547:114–121. doi: 10.1016/j.ijpharm.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 119.Nguyen J., Steele T.W.J., Merkel O., Reul R., Kissel T. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J Control Release. 2008;132:243–251. doi: 10.1016/j.jconrel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.SreeHarsha N., Venugopala K.N., Nair A.B., Roopashree T.S., Attimarad M., Hiremath J.G. An efficient, lung-targeted, drug-delivery system to treat asthma via microparticles. Drug Des Dev Ther. 2019;13:4389–4403. doi: 10.2147/DDDT.S216660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harush-Frenkel O., Bivas-Benita M., Nassar T., Springer C., Sherman Y., Avital A. A safety and tolerability study of differently-charged nanoparticles for local pulmonary drug delivery. Toxicol Appl Pharmacol. 2010;246:83–90. doi: 10.1016/j.taap.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 122.Pilcer G., Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 123.Bassetti M., Vena A., Russo A., Peghin M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs. 2020;80:1309–1318. doi: 10.1007/s40265-020-01359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shirley M. Amikacin liposome inhalation suspension: a review in mycobacterium avium complex lung disease. Drugs. 2019;79:555–562. doi: 10.1007/s40265-019-01095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scherlieβ R. Future of nanomedicines for treating respiratory diseases. Expet Opin Drug Deliv. 2019;16:59–68. doi: 10.1080/17425247.2019.1553955. [DOI] [PubMed] [Google Scholar]
- 126.Li Z.L., Zhang Y.L., Wurtz W., Lee J.K., Malinin V.S., Durwas-Krishnan S. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J Aerosol Med Pulm Drug Deliv. 2008;21:245–254. doi: 10.1089/jamp.2008.0686. [DOI] [PubMed] [Google Scholar]
- 127.Ngan C.L., Asmawi A.A. Lipid-based pulmonary delivery system: a review and future considerations of formulation strategies and limitations. Drug Deliv Transl Res. 2018;8:1527–1544. doi: 10.1007/s13346-018-0550-4. [DOI] [PubMed] [Google Scholar]
- 128.Ingvarsson P.T., Yang M.S., Mulvad H., Nielsen H.M., Rantanen J., Foged C. Engineering of an inhalable DDA/TDB liposomal adjuvant: a quality-by-design approach towards optimization of the spray drying process. Pharm Res. 2013;30:2772–2784. doi: 10.1007/s11095-013-1096-2. [DOI] [PubMed] [Google Scholar]
- 129.Ingvarsson P.T., Schmidt S.T., Christensen D., Larsen N.B., Hinrichs W.L.J., Andersen P. Designing CAF-adjuvanted dry powder vaccines: spray drying preserves the adjuvant activity of CAF01. J Control Release. 2013;167:256–264. doi: 10.1016/j.jconrel.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 130.Ingvarsson P.T., Yang M.S., Nielsen H.M., Rantanen J., Foged C. Stabilization of liposomes during drying. Expet Opin Drug Deliv. 2011;8:375–388. doi: 10.1517/17425247.2011.553219. [DOI] [PubMed] [Google Scholar]
- 131.Ye T.T., Yu J.Q., Luo Q.H., Wang S.J., Chan H.K. Inhalable clarithromycin liposomal dry powders using ultrasonic spray freeze drying. Powder Technol. 2017;305:63–70. [Google Scholar]
- 132.Yu S.H., Yuan H.Y., Chai G.H., Peng K., Zou P.Z., Li X.X. Optimization of inhalable liposomal powder formulations and evaluation of their in vitro drug delivery behavior in Calu-3 human lung epithelial cells. Int J Pharm. 2020;586:119570. doi: 10.1016/j.ijpharm.2020.119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu S.H., Wang S.N., Zou P.Z., Chai G.H., Lin Y.W., Velkov T. Inhalable liposomal powder formulations for co-delivery of synergistic ciprofloxacin and colistin against multi-drug resistant Gram-negative lung infections. Int J Pharm. 2020;575:118915. doi: 10.1016/j.ijpharm.2019.118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nassimi M., Schleh C., Lauenstein H.D., Hussein R., Hoymann H.G., Koch W. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur J Pharm Biopharm. 2010;75:107–116. doi: 10.1016/j.ejpb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 135.Müller R.H., Mäder K., Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 136.Vieira A.C.C., Chaves L.L., Pinheiro S., Pinto S., Pinheiro M., Lima S.C. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int J Pharm. 2018;536:478–485. doi: 10.1016/j.ijpharm.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 137.Bi R., Shao W., Wang Q., Zhang N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J Biomed Nanotechnol. 2009;5:84–92. doi: 10.1166/jbn.2009.036. [DOI] [PubMed] [Google Scholar]
- 138.Sans-Serramitjana E., Fusté E., Martínez-Garriga B., Merlos A., Pastor M., Pedraz J.L. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J Cyst Fibros. 2016;15:611–618. doi: 10.1016/j.jcf.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 139.Nafee N., Makled S., Boraie N. Nanostructured lipid carriers versus solid lipid nanoparticles for the potential treatment of pulmonary hypertension via nebulization. Eur J Pharmaceut Sci. 2018;125:151–162. doi: 10.1016/j.ejps.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Pastor M., Moreno-Sastre M., Esquisabel A., Sans E., Viñas M., Bachiller D. Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2014;477:485–494. doi: 10.1016/j.ijpharm.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 141.Andrade F., Videira M., Ferreira D., Sarmento B. Micelle-based systems for pulmonary drug delivery and targeting. Drug Deliv Lett. 2011;1:171–185. [Google Scholar]
- 142.Torchilin V.P. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 143.Mishra B., Patel B.B., Tiwari S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine. 2010;6:9–24. doi: 10.1016/j.nano.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 144.Mansour H.M., Rhee Y.S., Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lukyanov A.N., Torchilin V.P. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 146.Craparo E.F., Teresi G., Bondi M.L., Licciardi M., Cavallaro G. Phospholipid-polyaspartamide micelles for pulmonary delivery of corticosteroids. Int J Pharm. 2011;406:135–144. doi: 10.1016/j.ijpharm.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 147.Rabinow B.E. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 148.Fu T.T., Cong Z.Q., Zhao Y., Chen W.Y., Liu C.Y., Zheng Y. Fluticasone propionate nanosuspensions for sustained nebulization delivery: an in vitro and in vivo evaluation. Int J Pharm. 2019;572:118839. doi: 10.1016/j.ijpharm.2019.118839. [DOI] [PubMed] [Google Scholar]
- 149.Osman N.M., Sexton D.W., Saleem I.Y. Toxicological assessment of nanoparticle interactions with the pulmonary system. Nanotoxicology. 2020;14:21–58. doi: 10.1080/17435390.2019.1661043. [DOI] [PubMed] [Google Scholar]
- 150.Kuzmov A., Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 151.Li J.Q., Zheng H.L., Qin L., Xu E.Y., Yang L.L., Zhang L. In vitro‒in vivo correlation of inhalable budesonide-loaded large porous particles for sustained treatment regimen of asthma. Acta Biomater. 2019;96:505–516. doi: 10.1016/j.actbio.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 152.Popov A., Schopf L., Bourassa J., Chen H.M. Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles. Int J Pharm. 2016;502:188–197. doi: 10.1016/j.ijpharm.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 153.Amore E., Ferraro M., Manca M.L., Gjomarkaj M., Giammona G., Pace E. Mucoadhesive solid lipid microparticles for controlled release of a corticosteroid in the chronic obstructive pulmonary disease treatment. Nanomedicine. 2017;12:2287–2302. doi: 10.2217/nnm-2017-0072. (Lond) [DOI] [PubMed] [Google Scholar]
- 154.Garbuzenko O.B., Kbah N., Kuzmov A., Pogrebnyak N., Pozharov V., Minko T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J Control Release. 2019;296:225–231. doi: 10.1016/j.jconrel.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haworth C.S., Bilton D., Chalmers J.D., Davis A.M., Froehlich J., Gonda I. Inhaled liposomal ciprofoxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med. 2019;7:213–226. doi: 10.1016/S2213-2600(18)30427-2. [DOI] [PubMed] [Google Scholar]
- 156.Moreno-Sastre M., Pastor M., Esquisabel A., Sans E., Viñas M., Fleischer A. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016;498:263–273. doi: 10.1016/j.ijpharm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 157.Verschraegen C.F., Gilbert B.E., Evelyne Loyer, Huaringa A., Walsh G., Newman R.A. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 158.Hanna L.A., Basalious E.B., ELGazayerly O.N. Respirable controlled release polymeric colloid (RCRPC) of bosentan for the management of pulmonary hypertension: in vitro aerosolization, histological examination and in vivo pulmonary absorption. Drug Deliv. 2016;24:188–198. doi: 10.1080/10717544.2016.1239661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gupta V., Gupta N., Shaik I.H., Mehvar R., McMurtry I.F., Oka M. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167:189–199. doi: 10.1016/j.jconrel.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamamoto H., Kuno Y., Sugimoto S., Takeuchi H., Kawashima Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J Control Release. 2005;102:373–381. doi: 10.1016/j.jconrel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 161.Al-Qadi S., Grenha A., Carrión-Recio D., Seijo B., Remuñán-López C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Control Release. 2012;157:383–390. doi: 10.1016/j.jconrel.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 162.Shadab M., Haque S., Sheshala R., Meng L.W., Meka V.S., Ali J. Recent advances in non-invasive delivery of macromolecules using nanoparticulate carriers system. Curr Pharmaceut Des. 2017;23:440–453. doi: 10.2174/1381612822666161026163201. [DOI] [PubMed] [Google Scholar]
- 163.Healy A.M., Amaro M.I., Paluch K.J., Tajber L. Dry powders for oral inhalation free of lactose carrier particles. Adv Drug Deliv Rev. 2014;75:32–52. doi: 10.1016/j.addr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 164.Yoo N.Y., Youn Y.S., Oh N.M., Oh K.T., Lee D.K., Cha K.H. Antioxidant encapsulated porous poly(lactide-co-glycolide) microparticles for developing long acting inhalation system. Colloids Surf B Biointerfaces. 2011;88:419–424. doi: 10.1016/j.colsurfb.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 165.Ungaro F., d'Angelo I., Miro A., La Rotonda M.I., Quaglia F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: challenges and promises. J Pharm Pharmacol. 2012;64:1217–1235. doi: 10.1111/j.2042-7158.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 166.Yeh T.H., Hsu L.W., Tseng M.T., Lee P.L., Sonjae K., Ho Y.C. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32:6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 167.Jaafar-Maalej C., Elaissari A., Fessi H. Lipid-based carriers: manufacturing and applications for pulmonary route. Expet Opin Drug Deliv. 2012;9:1111–1127. doi: 10.1517/17425247.2012.702751. [DOI] [PubMed] [Google Scholar]
- 168.Scalia S., Haghi M., Losi V., Trotta V., Young P.M., Traini D. Quercetin solid lipid microparticles: a flavonoid for inhalation lung delivery. Eur J Pharmaceut Sci. 2013;49:278–285. doi: 10.1016/j.ejps.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chono S., Fukuchi R., Seki T., Morimoto K. Aerosolized liposomes with dipalmitoyl phosphatidylcholine enhance pulmonary insulin delivery. J Control Release. 2009;137:104–119. doi: 10.1016/j.jconrel.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 170.Miller D.P., Tan T., Tarara T.E., Nakamura J., Malcolmson R.J., Weers J.G. Physical characterization of tobramycin inhalation powder: I. Rational design of a stable engineered-particle formulation for delivery to the lungs. Mol Pharm. 2015;12:2582–2593. doi: 10.1021/acs.molpharmaceut.5b00147. [DOI] [PubMed] [Google Scholar]
- 171.Florence A.T., Attwood D. 5th ed. Pharmaceutical Press; London: 2011. Physiochemical principles of pharmacy. [Google Scholar]
- 172.Zhu M.T., Feng W.Y., Wang B., Wang T.C., Gu Y.Q., Wang M. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247:102–111. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 173.Rogueda P.G., Traini D. The nanoscale in pulmonary delivery. Part 1: deposition, fate, toxicology and effects. Expet Opin Drug Deliv. 2007;4:595–606. doi: 10.1517/17425247.4.6.595. [DOI] [PubMed] [Google Scholar]
- 174.Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 175.Gill S., Löbenberg R., Ku T., Azarmi S., Roa W., Prenner E.J. Nanoparticles: characteristics, mechanisms of action, and toxicity in pulmonary drug delivery—a review. J Biol Nanotechnol. 2007;3:107–119. [Google Scholar]
- 176.Ferin J., Oberdörster G., Soderholm S.C., Gelein R. Pulmonary tissue access of ultrafine particles. J Aerosol Med. 1991;4:57–68. [Google Scholar]
- 177.Gosens I., Post J.A., de la Fonteyne L.J.J., Jansen E.H.J.M., Geus J.W., Cassee F.R. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol. 2010;7:37. doi: 10.1186/1743-8977-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Truong N.P., Whittaker M.R., Mak C.W., Davis T.P. The importance of nanoparticle shape in cancer drug delivery. Expet Opin Drug Deliv. 2015;12:129–142. doi: 10.1517/17425247.2014.950564. [DOI] [PubMed] [Google Scholar]
- 179.Bhattacharjee S., de Haan L.H., Evers N.M., Jiang X., Marcelis A.T.M., Zuilhof H. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol. 2010;7:25. doi: 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhattacharjee S., Rietjens I.M.C.M., Singh M.P., Atkins T.M., Purkait T.K., Xu Z.J. Cytotoxicity of surface-functionalized silicon and germanium nanoparticles: the dominant role of surface charges. Nanoscale. 2013;5:4870–4883. doi: 10.1039/c3nr34266b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernandez L., Bustos R.H., Zapata C., Garcia J., Jauregui E., Ashraf G.M. Immunogenicity in protein and peptide based-therapeutics: an overview. Curr Protein Pept Sci. 2018;19:958–971. doi: 10.2174/1389203718666170828123449. [DOI] [PubMed] [Google Scholar]
- 182.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Therapeut. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- 183.Sperinde G., Montgomery D., Mytych D.T. Clinical immunogenicity risk assessment for a fusion protein. AAPS J. 2020;22:64. doi: 10.1208/s12248-020-00447-y. [DOI] [PubMed] [Google Scholar]
- 184.Stephen T.L., Groneck L., Kalka-Moll W.M. The modulation of adaptive immune responses by bacterial zwitterionic polysaccharides. Internet J Microbiol. 2010;2010:917075. doi: 10.1155/2010/917075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dowds C.M., Kornell S.C., Blumberg R.S., Zeissig S. Lipid antigens in immunity. Biol Chem. 2014;395:61–81. doi: 10.1515/hsz-2013-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Onoue S., Suzuki H., Seto Y. Formulation approaches to overcome biopharmaceutical limitations of inhaled peptides/proteins. Curr Pharmaceut Des. 2015;21:3867–3874. doi: 10.2174/1381612821666150820110826. [DOI] [PubMed] [Google Scholar]
- 187.Andrade F., Videira M., Ferreira D., Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine. 2011;6:123–141. doi: 10.2217/nnm.10.143. (Lond) [DOI] [PubMed] [Google Scholar]
- 188.Zaman R., Othman I., Chowdhury E.H. Carrier mediated systemic delivery of protein and peptide therapeutics. Curr Pharmaceut Des. 2016;22:6167–6191. doi: 10.2174/1381612822666160720145328. [DOI] [PubMed] [Google Scholar]
- 189.Fineberg S.E., Kawabata T.T., Krasner A.S., Fineberg N.S. Insulin antibodies with pulmonary delivery of insulin. Diabetes Technol Therapeut. 2007;9:S102–S110. doi: 10.1089/dia.2007.0207. [DOI] [PubMed] [Google Scholar]
- 190.Kraus T., Winter G., Engert J. Test models for the evaluation of immunogenicity of protein aggregates. Int J Pharm. 2019;559:192–200. doi: 10.1016/j.ijpharm.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 191.Deehan M., Garcês S., Kramer D., Baker M.P., Rat D., Roettger Y. Managing unwanted immunogenicity of biologicals. Autoimmun Rev. 2015;14:569–574. doi: 10.1016/j.autrev.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 192.Saraf S.K., Semalty A., Semalty M., Singh R., Saraf S.A. Properties and formulation of oral drug delivery systems of protein and peptides. Indian J Pharmaceut Sci. 2007;69:741–747. [Google Scholar]
- 193.Antosova Z., Mackova M., Kral V., Macek T. Therapeutic application of peptides and proteins: parenteral forever?. Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 194.Dingman R., Balu-Iyer S.V. Immunogenicity of protein pharmaceuticals. J Pharm Sci. 2019;108:1637–1654. doi: 10.1016/j.xphs.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ross C., Clemmesen K.M., Svenson M., Sørensen P.S., Koch-Henriksen N., Skovgaard G.L. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Ann Neurol. 2000;48:706–712. [PubMed] [Google Scholar]
- 196.Chen J., Tang Y., Liu Y., Dou Y.S. Nucleic acid-based therapeutics for pulmonary diseases. AAPS PharmSciTech. 2018;19:3670–3680. doi: 10.1208/s12249-018-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maeda M., Kojima T., Song Y., Takayama S. DNA-based biomaterials for immunoengineering. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201801243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guo S.J., Li H., Ma M.S., Fu J., Dong Y.Z., Guo P.X. Size, shape, and sequence-dependent immunogenicity of RNA nanoparticles. Mol Ther Nucleic Acids. 2017;9:399–408. doi: 10.1016/j.omtn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]