Abstract
Metal-organic frameworks (MOFs), comprised of organic ligands and metal ions/metal clusters via coordinative bonds are highly porous, crystalline materials. Their tunable porosity, chemical composition, size and shape, and easy surface functionalization make this large family more and more popular for drug delivery. There is a growing interest over the last decades in the design of engineered MOFs with controlled sizes for a variety of biomedical applications. This article presents an overall review and perspectives of MOFs-based drug delivery systems (DDSs), starting with the MOFs classification adapted for DDSs based on the types of constituting metals and ligands. Then, the synthesis and characterization of MOFs for DDSs are developed, followed by the drug loading strategies, applications, biopharmaceutics and quality control. Importantly, a variety of representative applications of MOFs are detailed from a point of view of applications in pharmaceutics, diseases therapy and advanced DDSs. In particular, the biopharmaceutics and quality control of MOFs-based DDSs are summarized with critical issues to be addressed. Finally, challenges in MOFs development for DDSs are discussed, such as biostability, biosafety, biopharmaceutics and nomenclature.
Keywords: Metal-organic frameworks, Drug loading, Drug delivery systems, Synthesis and characterization, Diseases therapy, Pharmaceutics, Biopharmaceutics, Biosafety
Graphical abstract
This review focuses on metal–organic frameworks (MOFs)-based drug delivery systems (DDSs), including classification, synthesis, characterization, applications and biopharmaceutics of MOFs for DDSs.
1. Introduction
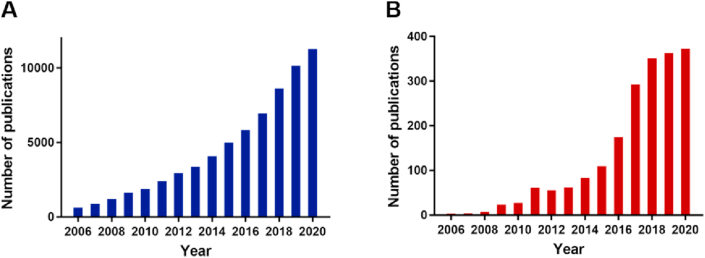
With the cracking growth of materials chemistry, a great deal of efforts have been dedicated to build original micro or nano-platforms for controlled and intelligent drug release systems in the interest of maximizing therapeutic efficacy and minimizing side effects. Metal-organic frameworks (MOFs), comprised of organic ligands and metal ions/metal clusters via coordinative bonds into two-dimensional or three-dimensional network are highly porous and crystalline materials, offering structural control at the molecular level, and have appealed to widespread attention since they were first reported in 1989 by Hoskins and Robson1. Nowadays, there are more than 20,000 diverse frameworks of MOFs reported in Cambridge database2. The publications tendency revealed an exponential increase relevant to MOFs in the period 2006–2020 (Fig. 1A). This trend highlights that MOFs are appealing new systems of great potential in drug delivery.
Figure 1.
Publications in the period of 2006–2020 from Web of Science (A) “Metal organic framework” and (B) “metal organic framework and drug delivery”.
By choosing judicious linkers and metal clusters, a variety of MOFs with tuned physicochemical properties (e.g., surface area, pore diameter, morphology, hydrophilicity or hydrophobicity) can be engineered for specific applications, such as gas storage3 and separations4,5, imaging6, sensing7, catalysis8, energy9, analytical chemistry10 and biomedicine11. Specially, the modulable porosity, tunable size and structure, and facile surface functionalization of MOFs make them become one of the most popular materials in biomedical field, of which drug delivery is promising with fast development.
Over the last decades, MOFs have found appliances in drug delivery systems (DDSs) and some novel designs and remarkable achievements using MOFs for drug delivery have been actualized. Similarly, articles about MOFs-based DDSs portray prevalent tendency over the past ten years (Fig. 1B). The well-defined porous crystalline MOFs showed additional advantages as compared to more conventional nanocarriers including liposomes12, polymers13, quantum dots14, inorganic nanoparticles15, allowing circumventing issues related to low drug loading, instability, systemic side effects and toxicities16, 17, 18. First and most importantly, as porous materials with large Brunauer–Emmett–Teller (BET) surface19, MOFs have remarkable cargo loading capacities, a variety of cargos with various physico-chemical properties such as small drug molecules20, peptides21, and even biomacromolecules22, can be loaded with efficiencies sometimes close to 100%23. Secondly, the porosities and compositions of MOFs can be adjusted by appropriately selecting the building blocks and metal ions to possess specific physical and chemical properties such as biodegradability, efficient drug loading and controlled release. In addition, MOFs can be conveniently surface-modified by using predesigning or post-synthetic strategies to achieve smart delivery using microrobots24 or surface functionalities25,26. Moreover, it is noteworthy that the modification of MOFs’ surfaces leads in most cases to no significant alteration of their physicochemical properties. Indeed, coating materials can be simply adsorbed from aqueous media onto the MOFs surface with high yields and good stabilities due to cooperative interactions27 or polymerized onto the surfaces28. Alternatively, MOFs can be coated with lipid29 or silica shells30.
Thirdly, as the coordinative bonds in the structures of MOFs are weak interactions, the MOFs are prone to readily degrade in biological media, releasing their constitutive ligands. This leads to excellent biodegradability and biocompatibility after accomplishment of the intended mission31,32. Last but not least, several MOFs showed intrinsic properties favorable to combat cancer or infections. For example, specific Fe-based nanoMOFs showed: i) antibacterial properties, contributing together with their drug cargo, to fight intracellular infections33 and ii) participations in improving radiation efficacy23. These studies lead the way for designing engineered nanoparticles (NPs) wherein each constituent plays a part in tumor remedy by radiotherapy or in the treatment of severe infections. In simple words, all these properties endow MOFs with promising potential in the field of DDSs.
In light of the expanding number of studies about MOFs over the past decades, several relevant reviews about the application of MOFs in biomedicine have appeared in the last few years on different aspects such as DDSs for cancer treatment34 and cancer theranostics35. Owing to the tunability and facile functionalization of MOFs, efforts have been dedicated to developing MOFs-based stimuli-responsive systems and MOFs-composite materials for biomedical applications. Wang et al.36 reviewed the growth of MOF families and members, then summarized the mechanisms of drug release in accordance with the endogenous stimuli (e.g., pH, glutathione, enzyme) and exogenous (e.g., temperature, light). Similarly, Cai et al.37 classified the MOFs-based stimuli-responsive systems into single or multiple stimuli. Differently, Giliopoulos et al.38 introduced various types of polymer/MOF nanocomposites for drug delivery and imaging. What's more, MOF composites and surface functionalization for nanomedicine in cancer therapy and diagnostics were summarized as well39,40. Specially, biological MOFs (BioMOFs) composed of metal ions and biomolecular likers including nucleobases, cyclodextrin, amino acids, polypeptides and others for bio-applications were also summarized and discussed41. On the other side, a comparison between MOFs and other nanocarriers such as mesoporous silica nanoparticles and dendrimers as drug delivery systems was addressed17.
By contrast, this review focuses on MOFs-based DDSs, instead of the bio-applications of MOFs. Firstly, a classification of MOFs for DDSs will be presented based on the type of both constitutive metals and ligands. Then, the methods for synthesis and characterizations of MOFs and MOFs-based DDSs are summarized. Next, the applications of MOFs-based DDSs are catalogued from three aspects. On the one hand, a perspective is given about the functions of MOFs-based DDS in pharmaceutics, including solubilization, increased stability, sustained release, etc. On the other side, practical applications of MOFs-based DDSs in the field of diseases are listed, including the treatment of infections, cancer, pulmonary and ocular diseases, etc. At the same time, the functionalization of MOFs-based DDSs for advanced applications is illustrated as well. Furthermore, the biopharmaceutics of MOFs and MOFs-based DDSs are stressed, which is rarely referred in previous reviews. Finally, the quality control and biosafety of MOFs-based DDS are presented, which are critical for clinical and industrial applications (Fig. 2).
Figure 2.
The graphical representation of MOFs in drug delivery.
2. Classification of MOFs
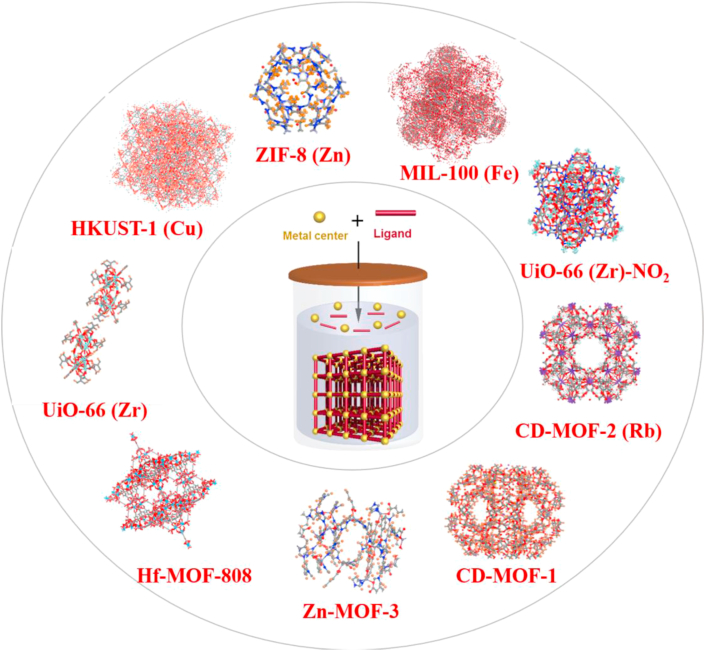
MOFs are built using diverse linkers and metals, which determine their polytropic structures and characteristics (Fig. 3). Besides, the linker and the metal cluster are congregated by frail coordination bonds, thereby enhancing biodegradability. However, a subtle balance between degradability and sufficient stability in biological media should be ensured. Specific applications can be reached by appropriately selecting both the metal ion and the organic linker, such as stimuli-responsiveness in drug delivery34, toward pH-responsive42, molecular-responsive43, thermo-responsive44, and pressure-responsive MOFs45. In this review, MOFs researched mostly for DDSs are classified based on the presence of specific components and features in their formulation.
Figure 3.
Representative MOFs crystal structures.
2.1. Classification by metal ions
Since the choice of ligands and metal ions is practically infinite, varieties of metal ions and organic linkers have been designed and selected to synthesize thousands of MOFs. When MOFs are used in the field of DDSs, they must comprehensively premeditate the biocompatibility and toxicity properly, which are closely related to their compositions46. Both linkers and metals should be nontoxic.
The median lethal dose (LD50) is usually used to assess the toxicity of metals47. Moreover, the recommended metals for DDSs with MOFs are, potassium, zinc, zirconium and iron with oral LD50 of 0.215, 0.35, 4.1 and 0.45 g/kg, respectively48,49. Nowadays, iron50,51, zirconium52,53, potassium20,54 and zinc-based55,56 MOFs remain the most employed for DDSs48 and a variety of anticancer57, 58, 59, antibiotics33 and antiviral drugs60,61 have been loaded in their porosities. Of note, drugs can be co-loaded in the MOF cages affording synergies to fight diseases33,62. Many other examples of MOFs as drug carriers have been reported in the pharmaceutical field, enabling reaching high drug payloads, increased drug solubility, improved stability, targeting abilities and better bioavailability. The MOFs for DDSs reported mostly so far are classified here according to their metal ions composition, as shown in Table 120,50,53,57,62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80.
Table 1.
Classification of MOFs by metal ions and their molecular pore size for drug loading.
| Genre | Naming | Organic linker | Pore size (Å) | Drug loading | Ref. |
|---|---|---|---|---|---|
| Fe-MOFs | MIL-89 (Fe) | Muconic acid | 11 | Ibuprofen, azidothymidine triphosphate | 57 |
| MIL-88A (Fe) | Fumaric acid | 6 | Ibuprofen, cidofovir | 57 | |
| MIL-100 (Fe) | 1,3,5-Benzenetricarboxylic acid | 25, 29 | Gemcitabine-monophosphate, topotecan, isoniazid, doxycycline, tetracycline, docetaxel, azidothymidine triphosphate, lamivudine triphosphate | 57,62,68,70‒72 | |
| MIL-101_NH2 (Fe) | Amino 1,4-benzenedicarboxylic acid | 29, 34 | Azidothymidine triphosphate, cidofovir, ethoxysuccinato-cisplatin | 57,73 | |
| MIL-53 (Fe) | 1,4-Benzenedicarboxylic acid | 8.6 | Ibuprofen, oridonin | 50,63 | |
| MIL-101 (Fe) | 2-Amino 1,4-benzenedicarboxylic acid | 25–30 | Ibuprofen, azidothymidine triphosphate | 57 | |
| MIL-127 | 3,3ʹ,5,5ʹ-Azobenzenetetracarboxylate | 4 | Caffeine | 74 | |
| Zn-MOFs | Zn (TATAT)2/3 ·3DMF·H2O | TATAT = 5,5ʹ,5ʹʹ-(1,3,5-Triazine-2,4,6-triyl) Tris (azanediyl)triisophthalate | 17, 21 | 5-Fluorouracil | 75 |
| ZnBDP_X | 1,4-Bis(1H-pyrazol-4-yl)-2-X-benzene (H2BDP_X; X = H, NO2, NH2, OH) | 11 | Mitoxantrone | 64 | |
| Bio-MOFs | Azobenzene-4,4ʹ-dicarboxylic acid and biphenyl-4,4ʹ-dicarboxylic acid | 8.3–26 | Etilefrine hydrochloride | 76 | |
| Zn8(O)2(CDDB)6 (DMF)4(H2O) | 4,4ʹ-(9-H-Carbazole-3,6-diyl) dibenzoic acid | 28.1 × 23.17 | 5-Fluorouracil | 65 | |
| ZIF-8 | 2-Methylimidazolate | 11.6 | 5-Fluorouracil, doxorubicin | 77,78 | |
| Zr-MOFs | UiO-66 | 1,4-Benzenedicarboxylic acid | 8 | Caffeine, dichloroacetate | 53,74 |
| UiO NMOFs | Amino-triphenyldicarboxylic acid | – | Cisplatin prodrug, siRNAs | 79 | |
| UiO-66-NH2/NO2 | 1,4-Benzenedicarboxylic acid | – | Ketoprofen | 66 | |
| K-MOFs | CD-MOF-1 | Cyclodextrins | 4–17 | Lansoprazole, azilsartan, budesonide, valsartan | 20,67,68,80 |
| Cu-MOFs | HKUST-1 | 1,3,5-Benzenetricarboxylic acid | 14.67 | Ibuprofen | 69 |
| MOF-2/MOF-3 | 1,3,5-Benzenetricarboxylic acid and isophthalic acid | 21.2/20.9 | Ibuprofen, doxorubicin hydrochloride | 69 |
‒, not applicable.
2.1.1. Cr-MOFs
The testification of drug loading into MOFs has been given in 2006, using two model systems, chromium-based MOFs (Cr-MOFs), named MIL-100 (Cr) and MIL-101 (Cr) (MIL stands for Materials of Institut Lavoisier), which made up of trimers of metal octahedra and di- or tricarboxylic acids. MIL-100 (Cr) consisted of Cr (III) ions and 1,3,5-benzenetricarboxylic acid (BTC) or trimesic acid while MIL-101(Cr) consisted of 1,4-benzenedicarboxylic acid (BDC) or terephthalic acid and Cr (III)81,82. Ibuprofen (IBU), a common model drug, was loaded in Cr-MOFs, showing high drug loading capacity, reaching up to 1.4 g IBU of per gram of dehydrated MIL-101 (Cr), while MIL-100 (Cr) only adsorbed 0.35 g IBU.
2.1.2. Fe-MOFs
Due to the toxicity of Cr, the above-mentioned MOFs are not compatible for biomedical uses. Iron-based MOFs (Fe-MOFs) named MIL-53 (Fe) based on Fe (III) octahedra and terephthalate anions were synthesized soon after63. Because of low toxicity, configuration flexibility and biodegradability, they appeared as prospective candidates for drug delivery spontaneously. The first nanosized Fe-MOFs57 were successfully utilized for loading anti-tumor or retroviral drugs and characterized in vitro and in vivo proving their degradability, biosafety and imaging properties. Owing to the flexibility of Fe-MOFs, Leng et al.50 chose the non-toxic and biocompatible MIL-53 (Fe) to load anti-cancer drug oridonin, and the drug loading capacity could reach up to 56.25% (w/w), with a sustained release time more than 7 days. In addition, Fe-MOFs were researched for drug loading and magnetic/fluorescence imaging simultaneously51. The hollow Fe-MOFs-5-NH2 with drug loading capacity as high as 35% (w/w) exhibited pH-controlled drug release property. Due to the existence of Fe (III) ions, the obtained MOFs displayed outstanding magnetic resonance imaging (MRI) performance. And after modification with folic acid (FA) and fluorescent reagent, targeted drug delivery and fluorescence imaging were realized.
Besides, MIL-100 (Fe) were used to co-encapsulate two active triphosphorylated nucleoside reverse transcriptase inhibitors, azidothymidine triphosphate and lamivudine triphosphate concomitantly for improving the efficacy of anti-human immunodeficiency virus (HIV)62. The azidothymidine triphosphate/lamivudine triphosphate ratio in MIL-100 (Fe) was equal to that among the currently commercialized triple therapy based on HIV prodrugs the overall drug loading was 9.6% (w/w). After freeze-drying, particles carried drugs could be preserved for 2 months, retaining similar physico-chemical properties.
2.1.3. Zn-MOFs
Zinc-based MOFs (Zn-MOFs) was developed by Rojas et al.64 in 2016 as four zinc pyrazolate isoreticular MOFs ZnBDP_X composed of Zn (II) and functionalized organic linkers 1,4-bis(1H-pyrazol-4-yl)-2-X-benzene (H2BDP_X; X = H, NO2, NH2, OH) for intravenous and oral administrations (<200 nm). This ZnBDP_X family presented tetragonal shape and square channels with a free pore aperture 11 Å. In vitro experiments demonstrated favorable structural and sticky durability in relevant biological conditions. Then, two kinds of antitumor drugs, namely, mitoxantrone and Ru (p-cymene) Cl2 (1,3,5-triaza-7-phospaadamantane) (RAPTA-c) were enveloped within the pores of the ZnBDP_X, for the sake of investigating the effects of the different functional structure on the drug packaging and delivery.
For the purpose of increasing the aqueous stability and therapeutic activity of MOFs, Bag et al.65 fabricated a sturdy bi-carboxylate ligand 4,4′-(9-H carbazole-3,6-diyl) dibenzoic acid (H2CDDB) for constructing Zn-MOFs. Through the reaction of Zn (NO3)2·6H2O and H2CDDB in dimethylformamide (DMF), a porous MOF [Zn8(O)2(CDDB)6 (DMF)4(H2O)] was fabricated, with an excellent loading ability of 53.3% (w/w) for 5-fluorouracil (5-FU). Furthermore, this MOF can keep stable up to three weeks in water and MTT assay against human hepatoblatoma cell line (HepG2) and human breast ductal carcinoma cell line (MDA-MB-435S) for 12 h incubation suggested the biosafety of this MOF.
Another Zn-MOFs named Zn-cpon-1 with 3D topological framework was prepared via employing ClO4– anion as template and 5-(4′-carboxyphenoxy) nicotinic acid (H2cpon) as organic linker83. This Zn-cpon-1 presenting a pH-responsive double stimulation behavior was an admirable drug delivery vessel, and the loading capacity of 5-FU in Zn-cpon-1 could reach 44.75% (w/w). Specially, the drug release behavior fitted well with the Weibull distribution model, which could be dual-irritated by pH and heating.
Zeolitic imidazolate frameworks (ZIFs), a sub-family of Zn-MOFs, connected by Zn(II) and imidazolate or its derivatives are widely used in DDSs84, 85, 86. Sun et al.86 exploited ZIF-8 to pack the volatile and hydrophobic d-α-tocopherol succinate by one-pot process, and the drug loading ratio reached to 43.03% (w/w). The obtained d-α-tocopherol succinate@ZIF-8 would swiftly degrade in acidic environment on account of the pH-responsiveness of ZIF-8, resulting in on-demand drug release for tumor chemotherapy.
2.1.4. Zr-MOFs
Since the discovery of Zr6 (μ3-O)4 (μ3-OH)4(BDC)6 (UiO-66, UiO stands for the University of Oslo) with Zr6(μ3-O)4 (μ3-OH)4(CO2)12 clusters and 1,4-benzene-dicarboxylate (BDC) by Cavka et al.87 in 2008, zirconium-based MOFs (Zr-MOFs), mostly Zr(IV) carboxylates, have received increasing attentions. Owing to the exorbitant oxidation status of Zr(IV) in Zr-MOFs and intense coordination bonds between Zr(IV) and carboxylate ligands in the great majority of carboxylate-based Zr-MOFs, they own unparalleled stability, particularly hydrothermal stability52. Hence, a great many Zr-MOFs maintain stability in organic solvents and in water, even in acidic media. Moreover, Zr is considered suitable for biomedicine given its wide distribution in nature and low toxicity in vivo (oral lethal dose LD50 ∼4.1 g/kg)88.
Zr-MOFs are proverbially used in the realm of biomedicine. For example, Abánades et al.53 found that synergistical delivery of dichloroacetate and 5-FU from Zr-MOFs to cancer cells can strengthen cytotoxicity in vitro. Adjusting the particle size, and most importantly, the surface chemistry can improve cytotoxicity by accelerating pit-mediated endocytosis and cytoplasmic drug delivery. In addition, Li et al.66 introduced UiO-66 with –NH2 and –NO2 functional groups to study the difference of drug loading capacity and release behavior between them. Interestingly, results revealed that UiO-66‒NH2 had the maximal loading of ketoprofen and exhibited lowest release rate on account of the strong hydrogen bond ability and alkaline characteristics of –NH2.
Another type of Zr-MOF, called Zr-fum, consists of an endogenous fumarate linker, and its structure is similar to UiO-66. Zr-fum could keep stable in aqueous solutions, with great potential as a DDS89. An anticancer molecule dichloroacetate was introduced into Zr-fum as a size-controlled modulator during fabrication process, with payloads of 20% (w/w)90. Contrast with UiO-66, Zr-fum shown enhanced biocompatibility in virtue of the endogenous fumarate linker, and transported the drug imitator calcein into HeLa cells more efficiently.
2.1.5. K-MOFs
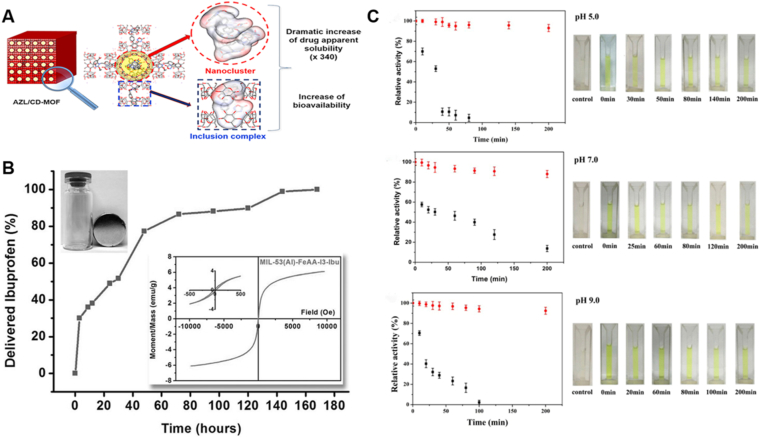
Smaldone et al.91 firstly reported a renewable, highly symmetric and porous, ultrahigh surface area, edible MOF, which was prepared only with edible ingredients: potassium (K) ions, alcohol (ethanol), and cyclodextrin, termed cyclodextrin-based metal-organic frameworks (CD-MOFs). Due to their inherently porous characteristics except for their water-soluble and non-toxic nature of CD-MOFs, it has extensive usages in the biomedical field34,54, making this hotspot attracting a great deal of attentions and developing very rapidly. To date, by utilizing impregnation, grinding and co-crystallization, drugs have been successfully loaded into CD-MOFs92. For example, lansoprazole imbedded CD-MOFs were synthesized using an optimized co-crystallization method by the assemblage with γ-CD in the existence of K(I) ions with the drug loading up to 23.2% (w/w)67. Moreover, CD-MOFs can dramatically ameliorate the bioavailability and solubility of insoluble drugs. He et al.20 reported that azilsartan (AZL) was loaded into CD-MOFs, and the bioavailability of AZL in Sprague–Dawley (SD) rats was increased by 9.7 times. Besides, compared with pure drugs, the apparent solubility of AZL/CD-MOF was increased by 340 times. Moreover, it is noteworthy that CD-MOFs have been proverbially used in oral, intravenous and even pulmonary DDSs68,93,94.
2.1.6. Cu-MOFs
Copper-based MOFs (Cu-MOFs) have been explored as viable hosts for bio-oriented guest@MOF composite systems owing to the high accessibility of their coordinatively unsaturated metal sites in the structures, creating strong binding sites for guest cargos95, 96, 97. Sun et al.69 devised mixed ligands Cu-MOFs, MOF-2 and MOF-3 for the delivery of IBU and doxorubicin hydrochloride (DOX), which were prepared by hydro-thermal method with reasonable alteration in the ratio of two ligands (BTC and isophthalic acid). They were nontoxic towards human normal cells, human embryonic kidney 293A cells (HEK 293A) and could load drug. Drug loading test showed that mixed ligand MOFs displayed better capacity in DDSs than single ligand MOFs, and MOF-2 with 40% BTC and 60% isophthalic acid had the best performance in drug delivery capability. By using the characteristics of amino-functionalized Cu-MOFs, a smartphone-based strategy for visual detection of alkaline phosphatase was designed by Hou et al.98 which possessed oxidase mimic and fluorescence virtue. This fluorescent-based technique could be used for detecting alkaline phosphatase in serum samples, which opened up a wild prospect for the diagnosis of other biomarkers in clinical serum samples on the basis of alkaline phosphatase mediated enzyme-linked immunosorbent assay. Moreover, Cu-MOFs could be utilized for antibacterial therapy. For example, Cu-MOFs composed of glutaric acid and pyridine derivative performed superb antibacterial activities against different kinds of bacteria with very low minimal bactericidal concentration99.
2.2. Classification by organic ligands
One of the primary merits of the MOFs is their variability in terms of compositions of both constitutive metals and organic linkers. In particular, the organic linkers perform a main part in the 3D supramolecular organization of the MOFs as well as their physicochemical properties. Carboxylates and other organic anions, including phosphonate, sulfonate, and heterocyclic compounds are the most common organic linkers. Actually, it has been highlighted that the choice of possible linkers is extremely wide100,101. Among them, MOFs composed of carboxylate ligands account for nearly half of all synthesized materials. In the case of MOFs for DDSs, the choice of the linker not only has a determinant part in the physical and chemical natures of the resulting MOFs, but also on their stability in biological media, degradability, bioavailability and toxicity.
Linker choice results in unique properties and MOF applications. For example, polycarboxylic acid or imidazole-based linkers are widely considered for MOFs preparation in reason of their relatively low toxicities, mainly on account of their strong polarity and metabolic clearance in physiological conditions48. Homologous iron carboxylates can be synthesized bio-safely102. Similarly, the drug payloads in MOFs and release patterns are affected by different organic linkers and functional groups48,57,103. Interestingly, active molecules were used as linkers to synthesize so-called BioMOFs. This strategy not only confers high drug payloads due to intrinsic self-assembly of active molecules, but also allows good biocompatibilities.
Indeed, a variety of biomolecules, including amino acid, nucleobases or sugars readily or naturally available can be used as building blocks104. As far as we know, Gramaccioli et al.105 synthesized the first amino acid-based biocompatible 3D MOFs in 1966, by mixing Zn(II) and glutamate, an important neurotransmitter. However, the biomedical applications of BioMOFs have not been fully explored mainly because of the lack of studies to the stabilities in biological media of these systems. Only few examples report BioMOFs that can adsorb and release drugs106. Considering that many therapeutic molecules have multiple complex groups in their structures, there are many reports on the use of active ingredients to construct BioMOF. The first example of a drug-based BioMOF study was reported in 2,010,107, which was composed of endogenous iron and therapeutically active vitamin B3, with pellagra treatment, vasodilation and anti-lipid properties. Similarly, olsalazine, a generally employed agent in the therapy of ulcerative colitis and other gastrointestinal disorders, could be used as a ligand for fabrication of a series of new mesoporous MOFs, and exhibited the same coordinating functionality as the dihydroxyterephthalic acid used in the composite of the CPO-27/MOF-74 family108. Here, MOFs are catalogued according to their organic linkers, as shown in Table 243,57,67,69,74, 75, 76, 77,82,107,109.
Table 2.
Classification of MOFs by organic linkers.
| Family of linkers | Organic linker | Drug loading | Ref. |
|---|---|---|---|
| Carboxylate ligands | 1,3,5-Benzenetricarboxylate acid | Doxorubicin | 69 |
| 4,4ʹ,4ʹʹ-Benzene-1,3,5-triyl-tribenzoate | Ibuprofen | 82 | |
| 5,5ʹ,5ʹʹ-(1,3,5-Triazine-2,4,6-triyl) tris(azanediyl)triisophthalate | 5-Fluorouracil | 75 | |
| Biphenyl-4,4ʹ-dicarboxylic acid | Hydrochloride | 76 | |
| Azobenzene-4,4ʹ-dicarboxylic acid | Etilefrine | 76 | |
| Pyrazolate ligands | Bis (pyrazolate) ligand (1,4-bis(1H-pyrazol-4-yl)-2-X-benzene (H2BDP_X; X = H, NO2, NH2, OH) | Mitoxantrone | 74 |
| Imidazolate | 2-Methylimidazolate | 5-Fluorouracil | 77 |
| Polysaccharide | Cyclodextrin | Lansoprazole | 67 |
| Other polysaccharides (agar, dextran) | Procainamide | 43 | |
| BioMOFs | Nicotinic acid | Vitamin B3 | 107 |
| Succinic acid | Cisplatin | 109 | |
| Fumarate ligands | Doxorubicin | 57 |
3. Synthesis of MOFs
3.1. Synthesis methods
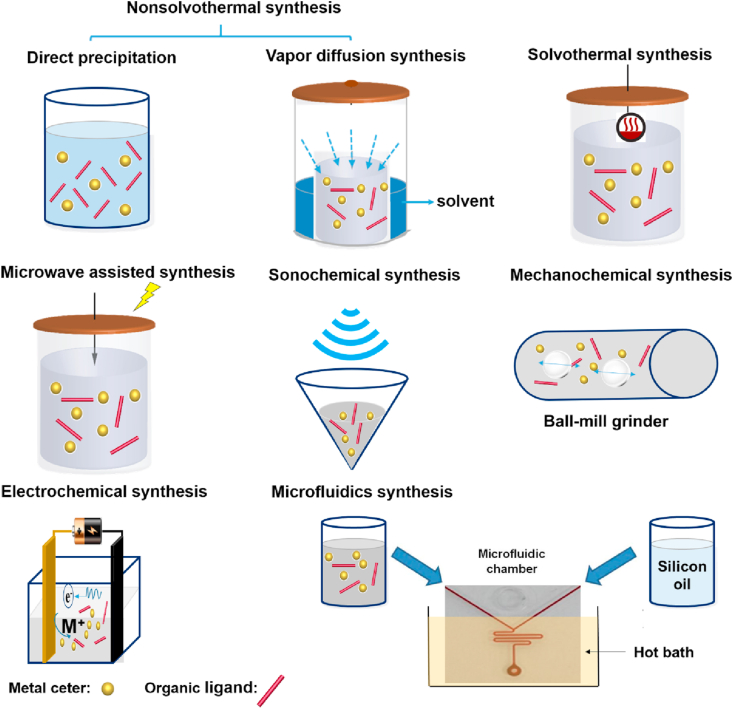
Several methods have been exploited to synthesize all kinds of MOFs110,111 (Fig. 4) for drug delivery, such as non-solvothermal synthesis, including direct precipitation and vapor diffusion synthesis91, and solvothermal methods, microwave-assisted solvothermal synthesis112,113, synthesis by reverse-phase microemulsions114, 115, 116, electrochemical synthesis117, dry-gel conversion methods118, preparation using a microfluidics device119, mechanochemical120, 121, 122 or sonochemical synthesis123, step-by-step synthesis124 and high-throughput synthesis methods125. The advantages and disadvantages of various synthesis methods are summarized in Table 3.
Figure 4.
Overview of MOFs synthesis methods.
Table 3.
Synthesis methods of MOFs.
| Method | Advantage | Disadvantage |
|---|---|---|
| Nonsolvothermal synthesis |
|
|
| Solvothermal synthesis |
|
|
| Microwave assisted solvothermal synthesis |
|
|
| Sonochemical synthesis |
|
|
| Mechanochemical synthesis |
|
|
| Electrochemical synthesis |
|
|
3.1.1. Conventional synthesis
Conventional synthesis of MOF usually refers to reactions performed by heating at different temperatures, which is one of the most important parameters in MOFs synthesis. According to the temperature used, conventional synthesis is normally distinguished as nonsolvothermal synthesis (temperature below or at the boiling point was used) and solvothermal (where reactions taking place above the boiling point).
3.1.1.1. Nonsolvothermal synthesis
It can be further categorized as the ones taking place at room temperature or elevated temperatures. For example, precipitation reactions followed by recrystallization or vapor diffusion synthesis were reported. Some MOFs have been synthesized via direct precipitation method by simply mixing the starting materials at room temperature, such as HKUST-1 (HKUST stands for Hong Kong University of Science and Technology), MOF-5, MOF-177, MOF-74, or ZIF-8126,127. Some of these MOFs, such as ZIF-8, exhibited fine chemical and thermal stabilities.
The vapor diffusion method is the earliest synthetic method for CD-MOFs synthesis91. A variety of γ-CD-MOFs were synthesized combining γ-CD and K+, Rb+, Cs+, Na+ or Sr+ by Stoddart's group91,128. Higher reaction temperature is also required in some CD-MOF synthesis to obtain good crystallinity and high yields. It was reported that the reaction time of γ-CD-MOFs synthesis was significantly reduced from days to 6 h129 by raising the temperature from room temperature to 50 °C.
3.1.1.2. Solvothermal synthesis
A β-CD-MOF was synthesized by heating a mixture of methanol and water containing β-CD and sodium oxalate (Na2C2O4) at 160 °C for 3 days130. Sha et al.131 obtained a novel α-CD-MOF with a mixture of α-CD and KOH after heating at 160 °C for 4 days. MOF-5 was synthesized at 105 °C to have much higher yield than the ones obtained at room temperature132.
Nonsolvothermal and solvothermal syntheses are limited to obtain MOFs in the laboratory. It is important to develop new synthetic routes towards MOFs production at industrial scale. For CD-MOFs, Ding et al.133 reported a novel production strategy based on crystal transformation to industrialization, which helped the productivity yield increase dozen times in comparison of reported synthesis methods.
3.1.2. Microwave assisted solvothermal synthesis
It has been widely applied for MOFs synthesis under microwave assisted hydrothermal conditions, due to many potential advantages such as environment-friendly rapid synthesis, high yield134, and morphology135 and size control136. The first reported MOFs obtained by microwave assisted solvothermal synthesis was MIL-100137. MIL-100 (Cr) synthesized at 220 °C in 4 h by microwave was reported to have similar physicochemical properties to the ones synthesized at 220 °C for 4 days using conventional heating. MIL-100 (Fe) synthesized at 130 °C for 6 min was shown to be well crystalized nanoparticles (NPs) with narrow particle size distribution, faceted morphology, and high porosity138.
Among different MOF preparation methods, microwave assisted synthesis allowed to achieve the best results in terms of high yields, small sizes (<100 nm) and monodispersed NPs139. “Green” synthesis allowed produced fluorine-free MIL-100 (Fe) within minutes opening the way to synthesize large-scale nano-MOFs for biomedical applications138. Microwave assisted solvothermal synthesis were also applied recently to synthesize Hf, Zr, Zn and Ca based nanoMOFs for drug delivery140, 141, 142, 143.
Liu et al.144 obtained γ-CD-MOFs within 10 min using the microwave-assisted method. By optimizing the reaction temperature, time, and solvent ratios, both micro- and nanometer-sized crystals were prepared. In addition, it was found to be a method of choice for fast crystallization of HKUST-1145, MIL-53 (Fe)73, MIL-101-NH2 (Fe)136, ZIF-8146 and MIL-100147.
3.1.3. Sonochemical synthesis
Sonochemical method is an easy and environmentally friendly pathway for rapid synthesis of MOFs. When high-energy ultrasound interacts with liquids, bubbles form and collapse in the solution, which is the so-called acoustic cavitation, leading to the production of very high local temperatures up to 5000 K and the pressures could reach 1000 bar148. This contributes to terrifically fast heating and cooling rates (>1010 K/s), favorable to fine crystal growth110. HKUST-1 was obtained using a mixture of DMF/ethanol/water in an ultrasonic bath149. The nanocrystalline NPs (10–40 nm) formed after only 5 min sonication. Increasing the sonication time resulted in larger crystals (50–200 nm), but further sonication led to the decomposition of the crystals. Sonochemical method was also employed to prepare well crystallized MOF-5 (5–25 μm)150, MOF-74 (Mn) (0.6 μm)151, PCN-6 (4.5–6.0 μm), IRMOF-9 (5–20 μm), PCN-6 (1.5–2.0 μm) and IRMOF-10 (5–20 μm)152. Ultrasound led to very tiny monodisperse MIL 88A nanoMOFs139, however, the yields were low.
3.1.4. Mechanochemical synthesis
Mechanochemistry, known as using mechanical force to induce and conduct chemical transformations, demonstrates the most evident benefit of providing a solvent-free or very small amounts of solvents synthetic path compared to conventional synthesis based on solution or microwave. Additionally, mechanochemical method has been affirmed to be a practical and environmentally friendly way to achieve high-throughput and low-cost production of MOFs. Different research groups recently reported that mechanochemical synthesis by planetary mill possessed substantial improvements in energy efficiency153,154. For MOFs mechanosynthesis, the oxide-based chemistry at room temperature with low-solvent and low-energy routes developed by Friščić et al.154 could avoid large energy and materials expenses. This facile method was also reported to the irreversible ball milling-155, pressure-156 and thermal-induced157 amorphous ZIFs synthesis.
3.1.5. Other methods
Other than the methods mentioned above, electrochemical synthesis was first reported in 2005 using metal ions continuously supplied through anodic dissolution, which reacted with the dissolved linker molecules158. This route is possible to prepare a higher solids content compared with batch reactions110. A variety of MOFs were synthesized by this method, including HKUST-1158, MIL-53 (Al), MIL-100 (Al), ZIF-8, and MIL-53-NH2 (Al)159. In addition, dry-gel conversion methods118, and preparation using a microfluidics device119 were also reported.
3.2. Particle size and shape control
Control of particle size and shape is of importance for biomedical applications, because the particle size dictates the interactions of the particles with cells, organs and biomolecules. It also plays a major role in several chemical and physical properties including rheology, surface reactivity towards biomolecules, external surface properties, packing, stability, etc.48,160, 161, 162, 163, 164, which are essential for drug loading capacity, surface modification for targeting and the administration routes. Consequently, it is very important to prepare homogeneous, monodispersed, and stable NPs. Whatever the synthesis method is, it remains challenging to control the crystallization process with controlled shapes and monodispersed size distribution. Primary strategies to actualize this goal composed of controlling composition and process parameters, adding additives, applying nanoscale templates, and downsizing the particles employing mechanical or mechano-chemical methods.
3.2.1. Compositional and process parameters
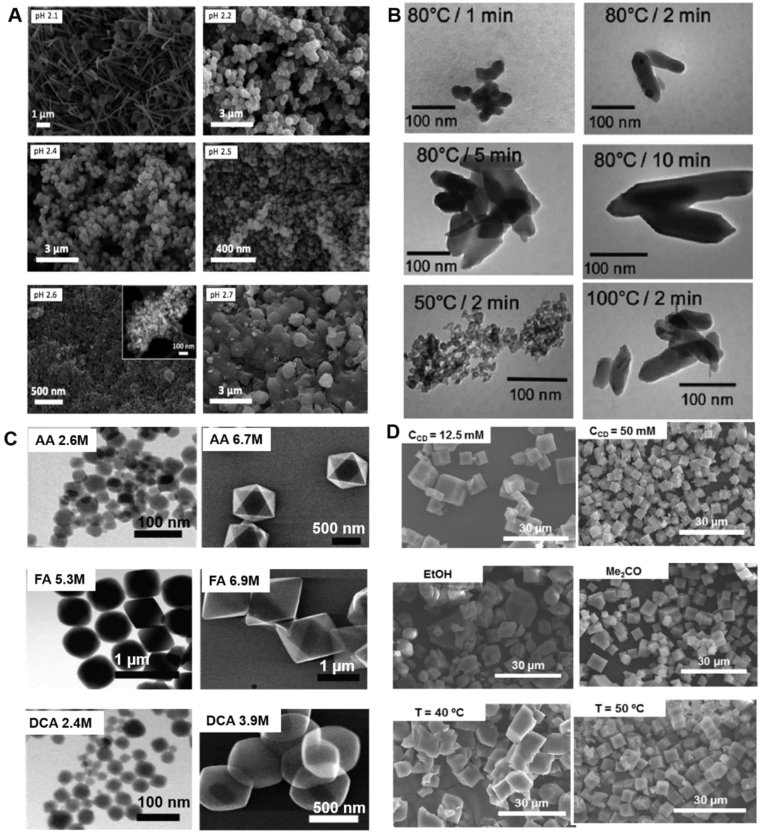
Compositional parameters include the nature of solvents, pH, metal source, reactant concentration and molar ratios. As an example, the fabrication of aluminium-based MOFs utilizing trimesic acid as an organic liker is dependent on the pH (Fig. 5A)165. The pH of the reactant [Al(NO3)3·9H2O, BTC and cetyltrimethyl ammonium bromide (CTAB)] was reported as 2.1, where MIL-100 formed after heating at 120 °C for 12 h using a mixture of ethanol and water as solvent. While keeping the reactant composition, MIL-100 started to form at higher pH values (2.3–2.5). At pH 2.6, a mixture of both MIL-96 and MIL-100 was produced, with the relative amount of the former growing when the pH was 2.7. When water alone was used as solvent, MOFs were obtained at different pH values: MIL-110 at acidic pH (both the range of 0–0.3, and 4), MIL-100 in a narrow acidic pH range (0.5–0.7), MIL-96 at pH 1–3. The topologies of the particles were significantly different with or without ethanol.
Figure 5.
SEM images of MOFs with different sizes and morphologies synthesized by synthetic parameters optimization (A) pH (B) reaction temperature and time (C) role of modulators (D) compositional and process parameters (A) Different aluminium trimesate MOFs synthesized at different pH: the particles obtained were MIL-110 (at pH of 2.1), MIL-100 (pH 2.2), MIL-100 (pH 2.4), mixture of MIL-100 and MIL-110 (pH 2.5) and mixture of MIL-100 and MIL-96 (pH of 2.6 and 2.7). Inset: TEM image acquired for the MIL-100 sample obtained at pH 2.6. Reprinted with permission from Ref. 165. Copyright © 2015, The Royal Society of Chemistry (B) Synthesis of MIL-88 by microwave assisted hydrothermal methods at different heating temperature and time. Reprinted with permission from Ref. 139. Copyright © 2011, The Royal Society of Chemistry (C) Role of modulators on the UiO-66 synthesis (AA: acetic acid; FA: formic acid; DCA: dichloroacetic acid. Reprinted with permission from Ref. 167. Copyright © 2017, American Chemical Society (D) Synthesis of CD-MOFs at different CD concentrations, using different solvents, and at different temperature. Reprinted with permission from Ref. 129. Copyright © 2016 Elsevier.
Process parameters consist of temperature, heating source, reaction time, and pressure. Generally, both microwave-assisted and sonochemical synthesis produce smaller and more homogeneous crystals than conventional synthesis. For instance, various methods139 were used to synthesize nano-MIL-88A, including conventional synthesis, microwave-assisted hydrothermal synthesis, and sonochemical method. Optimized reaction conditions yielded crystals with varied size using different methods: around 250 nm by conventional hydrothermal synthesis; 100 nm by sonochemical synthesis; smaller than 100 nm with good monodispersity for microwave assisted synthesis. Even using the same synthesis methods, the reaction temperature and time played important roles on both the crystal size and shape (Fig. 5B)139. Similarly, the size of Zn3(BTC)2·12H2O MOFs decreased from 900 nm to less than 100 nm by reducing the reaction time from 90 to 10 min166. Among many influencing factors, solvents, pH, temperature and reaction time are four crucial parameters mostly reported in terms of crystal size and shape.
3.2.2. Additives
Modulators are the linkers that can coordinate with the metal ions, therefore competing with the organic linkers during crystal growth. Therefore, the diameters and morphology of the crystals are highly affected by modulators. Modulators, e.g., acetic acid, formic acid, dichloroacetic acid, and trifluoroacetic acid, were systematically investigated in the synthesis of UiO-66 MOFs (Fig. 5C)167, showing a size difference from 20 nm to 1 μm with various modulators. In addition, other molecules such as amino acid168, polyoxometalates169, benzoic acid170, polyacids171 and water172 have been used. Recently, multivariate modulation of the UiO-66 for defect-controlled combination anticancer drug delivery was reported173, which was developed as a new strategy to incorporate up to three drugs in UiO-66 nanoMOFs by defect-loading.
Blocking agents or inhibiting additives, such as acetic acid, hydroxybenzoic acid and pyridine, have been shown to reduce the crystal growth. For example, the diameter of MIL-89 (Fe) was decreased to 30 nm by adding acetate ions in the synthesis process174. Luminescent terbium benzenedicarboxylate nanoMOFs with sizes of only 4–5 nm formed by adaption of a previous method175 utilizing a poly (vinylpyrrolidone) as inhibitors176. Besides, pyridine was used as inhibitors for synthetizing indium terephthalate MOF particles177, which was hexagonal rods of around 16.3 μm length and 1.75 μm width. After adding pyridine, the width and length were reduced to 0.43 and 0.97 μm, respectively. Not only the size but the morphology of the particles would be influenced by the inhibitor.
3.2.3. Nanoscale templates
The template approach is also a promising strategy to synthesize MOFs with controlled size distribution. For instance, MOF-66 (Gd) NPs formed in reverse microemulsions obtained from a mixture of 1-hexanol, isooctane, CTAB, and water. The size and shape of nanoscale templates were easily controlled by altering the constituents of the system178,179.
3.2.4. Downsizing
The size of nano-MOFs could also be controlled by mechanochemical synthesis as reported in 2006121. It induced a mechanical disruption of intramolecular bonds leading to a chemical transformation. Advantageously, this reaction occurred at room temperature180 under solvent-free conditions120 in the case of HKUST-1 NPs. Mechanochemical synthesis of nano-MOFs was also reviewed181.
In some cases, strategies can be combined to synthesize MOFs with controlled size distribution and morphologies. For instance, intense efforts have been made to prepare γ-CD-MOFs with narrow size distributions. Using conventional vapor diffusion method, only large crystals of γ-CD-MOFs were obtained in the range of 200–400 μm. After addition of CTAB182 to decrease the crystal growth, CD-MOFs were formed with uniform size of around 1–10 μm. Interestingly, Furukawa et al.182 successfully achieved to obtain nano γ-CD-MOFs (200–300 nm) by adding both CTAB and methanol. However, it still took more than one day for the synthesis. Liu et al.129 shortened the reaction time to 6 h using increased reaction temperature of 50 °C. The size of the resulting CD-MOFs was well controlled by addition of CTAB and/or methanol (about 6 μm with CTAB and around 600 nm with CTAB and methanol, Fig. 5D)129. However, the disadvantage of CTAB lies in its potential toxicity and it is difficult to be removed from the MOF particles. Recently, a seed-mediated method was employed to produce this CD-MOF using short-chain starch NPs instead of CTAB to have crystals with monodispersed size about 2 μm183. However, this method was not able to scale-down the particles to the nano-regime. Finally, the most efficient method was reported as microwave assisted method144. CD-MOF particles were obtained in 10 min, with monodispersed size distribution for different sizes (200 nm–300 μm) by adjusting the compositional and process parameters.
3.3. Physicochemical characterizations of MOFs
The synthesized MOFs need to be fully characterized with a set of complementary techniques to determine their physicochemical properties, including MOFs’ crystallinity, morphology, porosity, size distribution, colloidal stability, degradation patterns, drug loading capacity and drug release patterns. Various characterization techniques of MOFs are summarized in Table 4.
Table 4.
MOF characterization techniques.
| Technique | Property |
|---|---|
| Powder/single-crystal X-ray diffraction (XRD) | Crystallinity: crystal structure, crystalline parameters |
| Small- and wide-angle scattering (SAXS/WAXS) | Monitor the crystallization of MOFs |
| X-ray photoelectron spectroscopy (XPS) | Surface characterization: electronic structure, oxidation states, elemental composition, coating material binding |
| X-ray absorption spectroscopy (XAS) | Element-specifical investigation of chemical state and interatomic distances of species |
| Energy dispersive X-ray spectroscopy/spectroscopy (EDX/EDS) | Quantitatively determine elemental composition of MOFs |
| Porosimetry | Porosity: BET surface area and deep insight of pore size, volume and distribution of MOFs |
| Scanning electron microscopy (SEM) | Size and morphology |
| Transmission electron microscopy (TEM) | Size and morphology |
| Scanning transmission electron microscope (STEM) | Combined with high-angle annular dark field (HAADF) detector and EDX for investigation of morphology, elemental composition, and crystal structure |
| Raman microscopy | Homogeneity on composition and morphology |
| Atomic force microscope (AFM) | Size and shape in 3D mode, evaluate surface modification, localization of MOFs in cells or other matrices |
| Dynamic light scattering (DLS) | Hydrodynamic diameter |
| Nanoparticle tracking analysis (NTA) | Hydrodynamic diameter, particle concentration, individual particle tracking |
| Zeta potential | Surface charge |
| Fourier-transform infrared spectroscopy (FT-IR) | MOF degradation, drug loading, surface modification, structural defects |
| Ultrafast 2D IR spectroscopy | Structural dynamics inside a functionalized MOF |
| Nuclear magnetic resonance spectroscopy (NMR) | Interaction between the drug and MOF crystals, the precise drug localization, as well as catechizing the metal center and probing the linker molecules |
| Mössbauer spectroscopy | Investigation of chemical environment of Mössbauer nuclei (such as Fe atoms) in the sample, including the oxidation state, surface spins, and symmetry, etc. |
| Ultraviolet–visible spectroscopy (UV–Vis) | Optical properties, size, concentration |
| Inductively coupled plasma mass spectrometry (ICP-MS) | Elemental composition, intracellular MOFs quantification |
| Thermogravimetric analysis (TGA) | Thermal decomposition of MOFs |
4. Drug loading and characterizations
4.1. Methods for drug loading
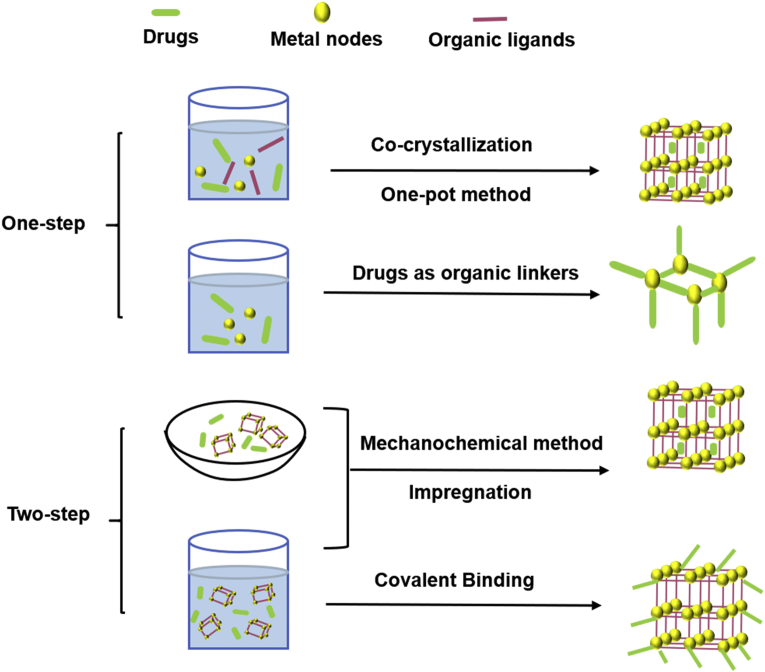
MOFs possess the unique characteristics of highly ordered structure and large surface area. Drugs can be embedded on the outer surface, or encapsulated into inter pores via diverse loading methods. Two kinds of widely applied loading strategies, namely, one-step method and two-step method are summarized here (Fig. 6).
Figure 6.
Two kinds of drug-loading strategies for MOFs.
4.1.1. One-step
One-step method is the most convenient loading strategy to incorporate drugs into MOFs, which is achieved either during the MOFs synthesis or by directly using drug molecules as MOF linkers.
4.1.1.1. Co-crystallization
It is widely used for drug loading in laboratory research or manufacturing. Under mild reaction conditions, drug could co-crystallize with MOFs and form a 3D supramolecular structure integrating the active molecules. More importantly, co-crystallization does not alter the physicochemical properties of the drug, which can be advantageously exploited to improve the loading efficiency and enhance the drugs solubility. As an example, poorly soluble drugs, such as IBU184, lansoprazole67, leflunomide93 and methotrexate (MTX)185 were successfully embedded in γ-CD-MOFs using the co-crystallization method and the drug loading was equal to or higher than that by an impregnation method.
4.1.1.2. One-pot method
It is an economic method for loading drug during MOFs synthesis, which not only shortens reaction time and reduces the waste, but also overcomes the limitations of MOFs' small pore window. In this method, drug molecules could be loaded in MOFs matrix by insertion inside MOFs crystal or coating onto the surface. In addition, the pore sizes of some MOFs are too small for the drugs to penetrate inside the crystalline matrix. For example, the diameter of the pore cavity of ZIF-8 is 11.6 Å, many bulky molecules including high-molecular weight drugs, nucleic acid or proteins cannot diffuse into the MOFs’ porosity. Nevertheless, successful encapsulations had been achieved for drugs as DOX84, camptothecin186, and 3-methyladenine187 via mixing with reactants through one-pot method. Similarly, 5-FU188 also could be loaded on zinc glutamate MOF coated cotton fabric using one-pot synthesis method. Besides, enzymes embedded in MOFs by this method were prevented from degradation. As a in situ loading strategy, organic linkers and inorganic metal ions simultaneously contributed to the efficient incorporation of the enzyme in one-pot method. The protein molecules induced the formation of MOFs and facilitated their crystallization. Cytochrome c (Cyt c) was directly immobilized in ZIF-8 utilizing one-pot method189. The peroxidase activity of Cyt c@ZIF-8 was 10-fold better than free enzyme. Similarly, Wu et al.190 also designed a ZIF-8 based multi-enzymatic system utilizing the same approach. Glucose oxidase (GOx) and horseradish peroxidase (HRP) were mixed with the zinc nitrate solution to prepare GOx&HRP@ZIF-8. Catalytic tests demonstrated that GOx&HRP@ZIF-8 composite displayed better catalytic efficiency than GOx@ZIF-8 or HRP@ZIF-8.
4.1.1.3. Drugs as organic linkers for MOFs
In addition to MOFs as reservoirs, drug or their prodrug can be as organic linkers to form MOFs, by coordination between the available coordinated functions of drugs and specific metal nodes. Employing biologically-acceptable ions Ca (II) and Mg (II) as metal ions and anti-osteoporosis bisphosphonate model drugs (e.g., etidronate, pamidronate, alendronate and neridronate) as organic linkers, a phosphonate MOF-based DDS was prepared that exhibited variable release rate pattern191.
4.1.2. Two-step
The two-step strategy immerses loading drugs with the MOFs in a drug-solution or in grinding MOFs together with the drugs.
4.1.2.1. Impregnation
The MOFs were immersed in drug solution to allow drug molecules to diffuse into MOFs through the porosity. Usually, interactions between drugs and MOFs include van der Waals interaction, π-π interaction and hydrogen bonding. The pore size, window dimension, chemical composition and flexibility of MOFs were key parameters to ensure a successful drug incorporation57. MOFs were applied for caffeine loading via impregnation192. Similarly, Javanbakht et al.193 immersed porous Cu-MOFs into an IBU solution to embed the drug into the 2D channels. The supercritical carbon dioxide (scCO2) assisted impregnation method could introduce poor-soluble drug honokiol into the CD-MOFs cavity with a high drug loading around 40.78% (w/w)194. In addition, this strategy reached almost 100% efficiency in the case of phosphate drug owing to the intense coordination between phosphates groups and Fe ions. Gemcitabine monophosphate (GEM) was encapsulated into MIL-100(Fe) with satisfied drug loading capacity of 30% (w/w) and exceptional encapsulation efficacy rates of >98%195.
4.1.2.2. Mechanochemical method
It is a solvent-free, green, and economical drug loading technique, mechanically mixing drug powder and MOFs together in solid status. Drugs such as 5-FU, caffeine, p-aminobenzoic acid and benzocaine were caged into MOFs by grinding method, reaching high drug loading amount and sustained release196.
4.1.2.3. Covalent binding
Although the appeal method incorporates various drugs into MOFs, the relatively weak interaction force between the cargos and MOFs usually leads to slow leaching problems of the drugs. Thus, it is necessary to adopt a solution of covalent bonding and immobilization. Generally, the surface of MOFs presents various active groups (e.g., carboxyl, amino, and hydroxyl) which can be used to mold covalent bond with reactive groups of active drugs197. Morris et al.198 exhibited the DNA-MOF conjugate, which was formed by a click reaction between azide-functionalized UiO-66 and dibenzylcyclooctyne-functionalized DNA. The DNA@UiO-66 conjugate was reported to have increased colloidal stability and improved cellular transfection capabilities in comparison to nonfunctionalized UiO-66. Additionally, enzymes also can be successfully immobilized onto MOFs by covalent binding. Cao et al.199 reported efficiently immobilization of soybean epoxide hydrolase onto the prepared surface of UiO-66-NH2 MOF via cross-linking approach. Compared with free soybean epoxide hydrolase, the synthesized soybean epoxide hydrolase@UiO-66-NH2 conjugates manifested high loading capacity, excellent enzyme–substrate binding affinity and enhanced catalytic efficiency.
4.2. Characterizations of drug-loaded MOFs
Drug loading efficiency following in Eq. (1) and encapsulation efficiency following in Eq. (2) are significant factors that are closely related to the pore size and surface property of MOFs. The drug can be physically adsorbed on the surface of the MOFs by the impregnation method when the size of the loaded drug is larger than the pore diameter of the MOFs. Typically, applying the one-step strategy can effectively encapsulate the large sized drugs to the inside of the MOFs for improving the drug loading and encapsulation efficiency84. In addition, changing the structure of MOFs may favor a higher drug loading level via improving the interactions (hydrogen bonding or van der Waals) between drugs and MOFs65. The determination of drug loading and encapsulation efficiency mostly adopts direct or indirect methods, that is, dissolving drug loaded MOFs in a solvent to determine the drug content, and calculating the loaded drug content by measuring the content of unloaded drugs supernatant. Moreover, the determination of the content of adsorbed drugs in MOFs also can be indirectly analyzed by TGA, since a mass loss step within a certain temperature range200.
| (1) |
| (2) |
Various strategies were developed to characterize drug@MOFs particle. The morphology of drug@MOFs was usually studied by SEM and TEM. Zeta potential might indicate the drug location in MOF, while the crystalline structure of drug@MOFs can be identified by Powder XRD for the crystallinity might be altered by drug loading. The reduced nitrogen adsorption of drug@MOFs was used to verify the occupation by drug in the pore and cavity of the MOFs. Lastly, adsorption isotherm was employed to evaluate loading capabilities of MOFs, and TGA to give information of drug loading and stability in the MOF matrix.
In addition, the state and interaction between drugs and MOFs may help to deepen knowledge of drug loading mechanism. DSC measurement for the melting point of the binary system of MOFs and drug was applied to explore the form of the cargo in MOFs and evaluate the interaction between them. FTIR spectroscopy was applied to investigate the interaction between host and guest. Differences in absorption frequency and chemical bond type were strong evidence to prove the relationship between drug and MOFs. The host/drug interactions were indicated by ultrafast magic-angle spinning (MAS) and NMR. Dielectric relaxation spectroscopy (DRS) was used to understand the molecular mobility of MOFs skeleton caused by the presence of drugs. Molecular simulation was helpful to analyze the interaction between drug and carrier, and the drug loading mechanism. For example, molecular simulation was applied to investigate how γ-CD-MOFs enhanced the solubility of insoluble drugs and a dual-molecule loading mechanism of complexation and clusterization was established20.
5. Applications of MOFs in therapy and drug delivery
5.1. MOF systems for disease therapies
On account of their advantageous features in drug delivery, MOFs have been explored as favorable DDSs for plenty of diseases, including infections, lung disease, diabetes mellitus, ocular disease and tumors, which have made prominent advance in the past few years.
5.1.1. Anti-bacterial applications
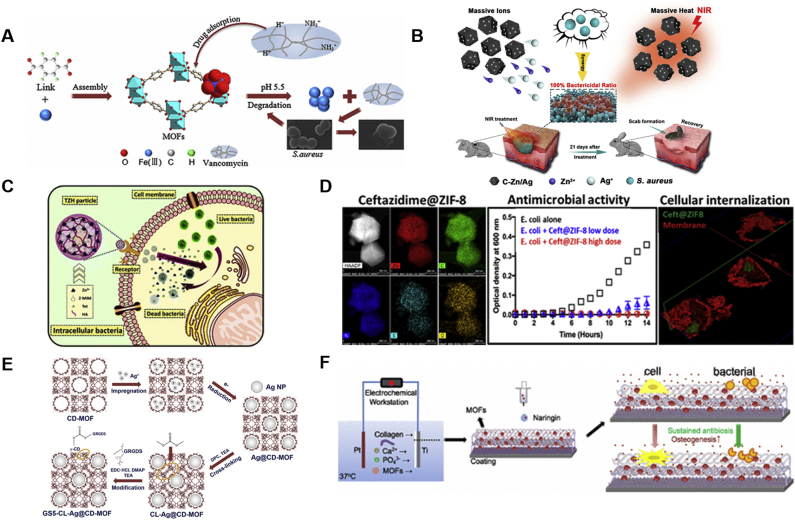
Smart delivery of antibacterial agent is of great interest in light of the bacteria resistance and bacterial infection in injury and surgical process. Inorganic carriers201 and organic polymer carriers202 have been proposed for targeting antibacterial DDSs, but their instability, poor biocompatibility and uncontrolled release behaviors limit their applications in anti-bacteria. Recently, MOFs combining the hybrid organic and inorganic framework have been studied for anti-bacteria therapy. Owing to good chemical stability in acidic environment, MOF-53(Fe) constituted of iron ions and BDC was explored as a carrier for antibacterial agents by Lin et al.203 (Fig. 7A). The glycopeptide macromolecule antibiotics, vancomycin was incorporated into MOF-53(Fe) NPs by physical absorption. And the drug loading of vancomycin could reach up to 20% (w/w). Moreover, MOF-53(Fe) showed a slower and controllable drug release profile with 99.3% antibacterial efficiency against Staphylococcus aureus under bacterial infection condition (pH = 5.5). Meanwhile, MOF-53 (Fe)@vancomycin exhibited an ideal chemical stability with excellent biocompatibility in vitro.
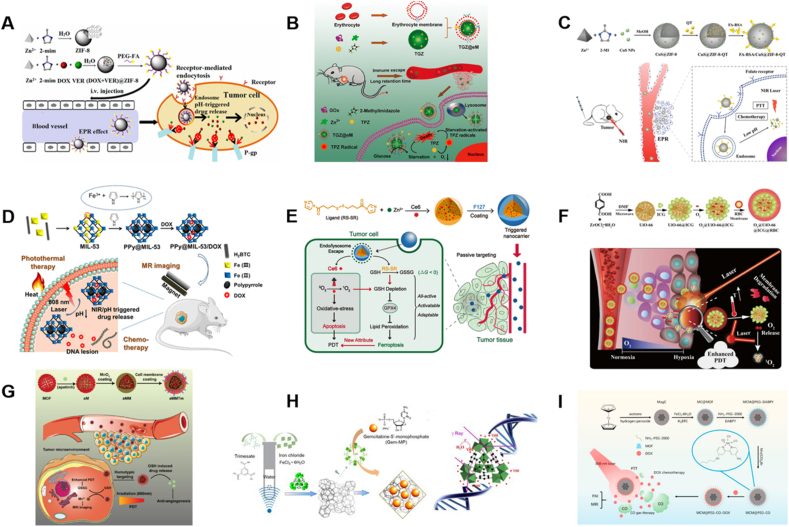
Figure 7.
Applications of MOFs in anti-bacteria (A) MOF-53 (Fe) structure and the process of MOF packing vancomycin for killing bacteria. Reprinted with permission from Ref. 203. Copyright © 2017, American Chemical Society (B) MOF/Ag-derived nanocomposite with forceful photothermal conversion capability and metal-ion-releasing ability for synergistic sterilization. Reprinted with permission from Ref. 211. Copyright © 2020, American Chemical Society (C) Elimination of intracellular bacteria by TZH particles. Reprinted with permission from Ref. 205. Copyright © 2019, The Royal Society of Chemistry (D) The antibacterial properties and internalization of ceftazidime@ZIF-8 particles. Reprinted with permission from Ref. 204. Copyright © 2019, American Chemical Society (E) Schematic diagram of CD-MOF template guided synthesis of Ag NPs for anti-bacteria and hemostasis. Reprinted with permission from Ref. 210. Copyright © 2019, WILEY-VCH (F) Naringin-loaded MOF NPs for coating mineralized collagen to promote osseointegration and prevent bacterial infection. Reprinted with permission from Ref. 207. Copyright © 2017, American Chemical Society.
Intracellularly bacterial infections are hard to treat in particular, as pathogens can hide inside the cell components, thereby avoiding the surveillance of the immune system. Gallis et al.204 utilized ZIF-8 to load ceftazidime, a third-generation broad-spectrum cephalosporin (Fig. 7D). And successful loading of ceftazidime in ZIF-8 was probed by high resolution STEM-EDS elemental mapping. Drug release test in PBS revealed that about 70% of the ceftazidime released at pH 5.0, while 50% for pH 7.0 during the first day. Antibacterial capabilities of pristine ZIF-8 and ceftazidime@ZIF-8 particles were measured against Gram-negative E. coli. Interestingly, no difference was noted between them after 24 h, but ceftazidime@ZIF-8 displayed nearly entire growth inhibition of E. coli at 50 μg/mL while the pristine ZIF-8 didn't present any antibacterial effect after 72 h incubation, suggesting the antibacterial effectiveness was relied on the degradation of ceftazidime@ZIF-8. In addition, cell internalization of particles was visualized directly by confocal microscopy, which qualified that this system can be applied for intracellular bacterial killing.
Another ZIF-8 application for anti-bacteria was reported by Zhang et al205. During the synthesis of ZIF-8, tetracycline was encapsulated in MOF through one-step. Moreover, hyaluronic acid (HA) was decorated on tetracycline@ZIF-8 via coordination for active-targeting of bacteria within the cell (Fig. 7C)205. This tetracycline@ZIF-8@HA nanocomposite (TZH) could realize a pH-responsive antibiotic release. More importantly, Zn(II) and antibacterials released from ZIF-8 could reach a synergistic antibacterial effect. In addition, with the help of HA, TZH could eliminate intracellular bacteria more efficiently and decrease the antibiotic dosage remarkably. Finally, clearance rate of intracellular bacteria by TZH was over 98%.
Hitherto, the mainly investigated MOFs in anti-bacterial field as drug carriers, few studies discussed that diameter, shape and surface modification of MOFs affected their cellular internalization. Guo et al.206 explores MIL-88 (A) and MIL-100 (Fe) particles both incorporated with mannose for active targeting, which were designed with rod-like and spherical shapes respectively, as a promising bacteria-mimicking delivery strategy for intra-macrophagic-based infections. The shape of MIL-88 (Fe) was rod with a long-axis size of 3628 ± 573 nm and the aspect ratio was 1:5, while the diameter of spherical MIL-100 (Fe) was 103.9 ± 7.2 nm. Cell internalization test displayed that MIL-100 (Fe) NPs were internalized quicker, however, the mannosylation did not enhance the uptake of MIL-100 (Fe), because its rate and extent of uptake were high enough, whereas cellular uptake of MIL-88A (Fe) were highly increased. Moreover, micropinocytosis/phagocytosis proved to be the primary internalized pathway in MOF particle uptake.
Prevention of infection and promotion of osseointegration are two important goals in orthopedics. Yu et al.207 designed naringin-loaded MOF NPs for coating mineralized collagen. With the help of MOF NPs, the release kinetics of naringin could be controlled to boost osseointegration and avoid bacterial infection (Fig. 7F)207. Results showed an outstanding performance of the coating for mesenchymal stem cells including the attachment, proliferation, osteogenic differentiation, and mineralization. Meanwhile, the antibacterial effectiveness against S. aureus was also enhanced which confirmed the potential application of this orthopedic coating for implants.
Numerous studies have revealed that ultrafine silver (Ag) NPs are promising antimicrobial agent because the release of Ag(I) ions and generation of reactive oxygen species (ROS)208. However, ultrafine Ag NPs are usually unstable and easily to aggregate209. Shakya et al.210 reported a template-assisted synthesis of ultrafine Ag NPs with size about 2 nm by utilizing the meso-porosity of γ-CD-MOFs in one pot, achieving stability enhancement of Ag NPs. Further, the Ag@CD-MOFs were cross-linked and surface modified with GRGDS peptide for improvement of hemostasis in the wound area. The anti-bacteria test as well as the hemostasis test proved that the obtained GS5-CL-Ag@CD-MOFs were a fine strategy to combine hemostasis with antibiosis (Fig. 7E)210.
Owing to the limited antibacterial efficiency, high-dose use and slow sterilization rate of single-model bactericidal method are usually used, and it's urgent to develop a dual bactericidal system. Yang et al.211 synthesized an MOF/Ag-derived nanocomposite consisting of metallic Zn and a cgraphitic-like carbon framework with preeminent metal-ion-releasing ability and forceful photothermal conversion capability for synergistic sterilization (Fig. 7B)211. When exposed to near-infrared irradiation, massive heat was generated to destroy bacterial membranes, at the same time, bacterial intracellular substances would be damaged by abundant released Zn(II) and Ag(I) ions from MOF-derived nanocarbon. Moreover, the nanocomposite exhibited less cytotoxicity with an excellent antibacterial effect in systematic antibacterial experiments.
5.1.2. Lung disease therapy
Pulmonary delivery of drugs can achieve high efficiently targeted drug therapy for lung disease, like asthma, respiratory tract infections, chronic obstructive pulmonary disease and lung cancer. Owing to the tunable structure and porosity, inhalable size, MOFs can be used as carriers for pulmonary drug delivery. Recently, Hu et al.68 utilized γ-CD-MOF to load budesonide which were modified with cholesterol (CHO) and leucine poloxamer for dry power inhaler (Fig. 8A)68. Particle size distribution showed that more than 90% particles were within the suitable diameter of 1–5 μm for the inhalation administration. Moreover, modification with CHO could successfully improve the flowability and aerodynamic properties of CD-MOF in vitro. Most importantly, in vivo animal experiments demonstrated that the CHO-CD-MOF based dry power inhaler may be a promising pulmonary delivery carrier.
Figure 8.
Applications of MOFs in lung disease (A) Cholesterol modification of CD-MOF to improve aerodynamic properties for pulmonary delivery of budesonide. Reprinted with permission from Ref. 68. Copyright © 2019, Elsevier (B) INH-loaded MOF combined with PLGA and LC microparticle for pulmonary delivery of anti-tuberculosis drug. Reprinted with permission from Ref. 214. Copyright © 2019, MDPI(OA) (C) Graphical mechanism of nano-MOF-based particle lung retention after i.v. administration. Reprinted with permission from Ref. 195. Copyright © 2017, WILEY-VCH.
Another research reported by Mohamed et al.212 utilized MIL-89 and PEGylated MIL-89 (MIL-89 PEG) as carriers for pulmonary arterial hypertension delivery. Both of them had particulate sizes, within 50–150 nm, with a majority particle size of 100 ± 38 nm. After PEGylation, MIL-89 PEG expressed enhanced homogeneity of shape and improved stability. Cell experiments demonstrated that both MIL-89 and MIL-89 PEG was nontoxic to endothelial cells. Moreover, both of them showed anti-inflammatory effect in macrophages. Besides, in vivo experiments revealed that MIL-89 was well-tolerated in short period and accumulated in lungs, which may be suitable carriers for pulmonary arterial hypertension drugs.
The treatment of tuberculosis needs the combination of drug delivery and imaging to achieve a better control and personalized therapy. For preventing massive systemic exposure and side effects, pulmonary delivery of anti-tuberculosis drug is an ideal strategy to maintain local therapeutically effective concentrations. Thus, Fe–MIL-101–NH2 NPs were used as carriers for controlling release of isoniazid (INH) and MRI contrast agent by Wyszogrodzka et al.213,214 (Fig. 8B). In vitro cytotoxicity experiments confirmed the safety of Fe-MIL-101-NH2 and cell internalized studies supported the potential application in tuberculosis treatment. For improving the aerodynamic properties of INH-loaded MOF (INH-MOF), hydrophobic poly (lactide-co-glycolide) (PLGA) microparticles were utilized to load INH-MOF via spray-drying, then mixed with spray-dried INH-MOF-loaded d-leucine (LC) microparticles. Finally, the obtained INH-MOF-loaded PLGA/LC expressed excellent aerodynamic properties, controlled release of INH and good internalization into the macrophages. Moreover, the Fe ions in INH-MOF could be MRI contrast agent for tracking particles after inhalation.
In addition, Simon-Yarza et al.195 discovered that Fe(III) polycarboxylate based nanoMOFs were capable of targeting lung tissue, due to their unique pH- sensitiveness and reversible aggregation properties (Fig. 8C)195. After intravenous administration, under the neutral pH in blood, the nanoMOFs formed micro-aggregates which were detained in the lung capillaries. During 24 h, the agglomerates disaggregated and started to degrade, leading to drug release with improved therapeutic effect as compared to free drug and metastasis reduction. Particularly, the appropriate timing of reversible aggregation/disaggregation was compatible with tissue physiology, avoiding of toxicity issues.
5.1.3. Diabetes therapy
Diabetes mellitus still remains a serious health problem around the world. The management of type 1 diabetes needs continual insulin injection to maintain euglycemia. Developing an efficacious and advanced insulin delivery system is of great significance. The most studied insulin delivery systems are based on GOx for realizing glucose responsiveness, which are usually modified with pH-responsive materials. Duan et al.215 investigated a simple one-pot approach for construction an advanced glucose-responsive insulin delivery system, where insulin-GOx/ZIF-8 was self-assembled by the mixture of Zn(II), 2-methylimidazole, GOx and insulin (Fig. 9A)215. When the blood glucose level was higher than normal, GOx catalyzed glucose to gluconic acid, then the local pH change led ZIF-8 decompose and thus caused insulin release. Moreover, when glycemic level reached to normoglycemic conditions, insulin release from the ZIF-8 decreased, avoiding hypoglycemia.
Figure 9.
Applications of MOFs in diabetes therapy (A) glucose-responsive insulin release from the MOF-based nanosystem. Reprinted with permission from Ref. 215. Copyright © 2018, The Royal Society of Chemistry (B) Microneedles containing Co-ZIF-8 embodied insulin and GOx for transdermal insulin delivery. Reprinted with permission from Ref. 216. Copyright © 2020, American Chemical Society (C) FA modified Cu-MOFs (F-HKUST-1) for the therapy of chronic nonhealing wounds. Reprinted with permission from Ref. 97. Copyright © 2018, American Chemical Society.
Recently, a novel strategy was designed by Yang et al.216, using multi-enzyme Co-ZIF-8 embodied insulin and GOx as repertories to integrate with microneedles for transdermal insulin delivery (Fig. 9B)216. Co(I) ions in ZIF-8 MOFs were designed as a biomimetic catalase to decompose the excessive H2O2 for avoiding the conceivable damage to normal tissue. Meanwhile, free Co(I) ions can be chelated by ethylene diamine tetraacetic acid modified SiO2 NPs in microneedles, then removed by peeling microneedles off. Results showed that the obtained MOF-based microneedles exhibited good insulin release performance depending on glucose concentration without leakage of H2O2 and Co(I).
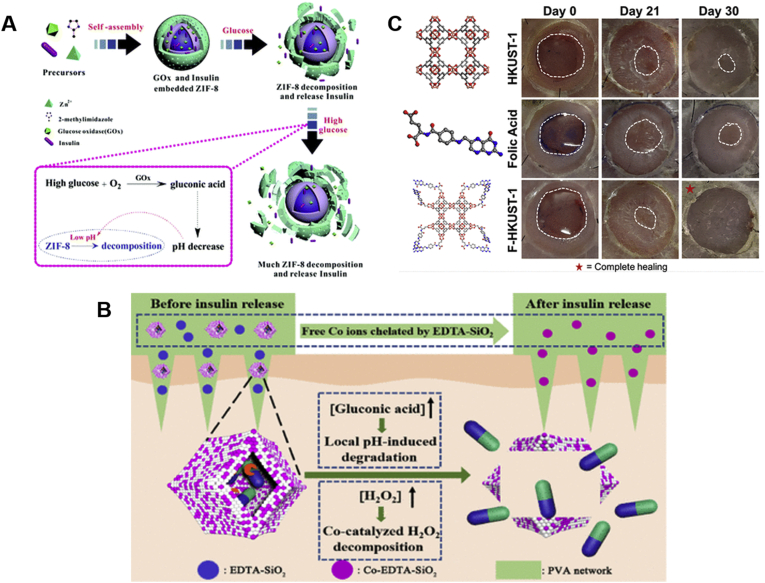
Diabetic foot ulcers are a serious problem for people with diabetes, and there are no effective therapies. Xiao et al.97 developed FA modified Cu-MOFs (F-HKUST-1) for the therapy of chronic nonhealing wounds (Fig. 9C)97. FA was added when synthesizing HKUST-1 to slow the release of Cu(II) ions, resulting in improved wound healing rates and reduced toxicity. In vivo experiments revealed that F-HKUST-1 could induce angiogenesis, promote collagen deposition and re-epithelialization and increase wound healing speed.
5.1.4. Ocular disease therapy
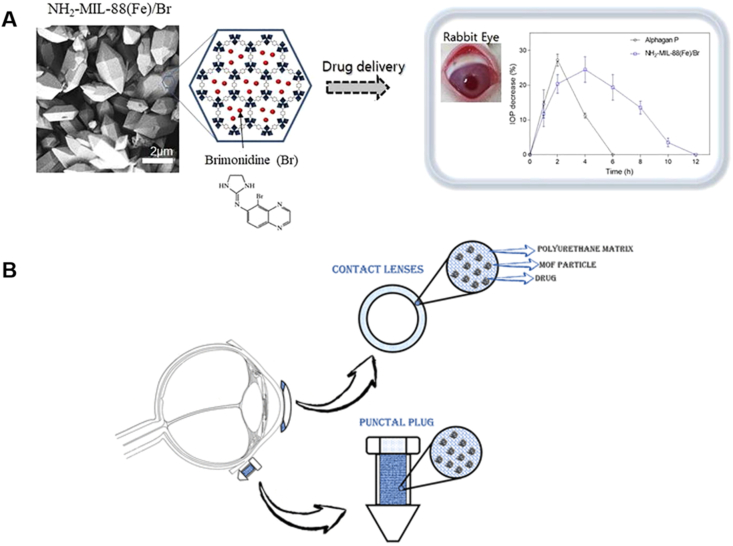
The ophthalmic drugs formulated as eye drops are usually removed fast from the eyes, thus limiting their ocular bioavailability, which to usually less than 5%. Therefore, Kim et al.217 utilized NH2–MIL-88 (Fe) for sustained release of brimonidine to enhance its ocular bioavailability (Fig. 10A)217. Brimonidine was incorporated via physical absorption into the large internal pores of NH2–MIL-88 (Fe). Besides, due to the amino groups and hydrogen-rich organic ligands in this MOF, hydrogen bonds were formed with the hydroxyl groups and intrinsic carboxyl of mucin chains, then generating mucoadhesive properties and thus increasing the retention of drugs in preocular. In vitro test showed a sustained release manner of encapsulated drug in NH2–MIL-88 (Fe). Moreover, in comparison with the marketed product of brimonidine (Alphagan-P), the bioavailability of brimonidine was enhanced in NH2–MIL-88 (Fe) in the in vivo test. Worthily, although this MOF can be fully degraded into Fe(III) and 2-amino BDC in physiological fluid during half a day, the long-term existence of Fe(III) may be threat for eye safety.
Figure 10.
Applications of MOFs in ocular disease therapy (A) NH2–MIL-88 (Fe) for sustained release of brimonidine to enhance its ocular bioavailability. Reprinted with permission from Ref. 217. Copyright © 2018, Elsevier (B) Zr-based UiO-67 loaded brimonidine tartrate and polyurethane nanocomposite films as novel ocular therapeutics. Reprinted with permission from Ref. 218. Copyright © 2020, American Chemical Society.
Another research utilizing Zr-based UiO-67 loaded brimonidine tartrate and polyurethane nanocomposite films as novel ocular therapeutics was developed by Gandara-Loe et al218. This functional MOFs-based ocular polymeric device exhibited excellent adsorption and release performance for brimonidine tartrate owing to large tetrahedral and octahedral cages of UiO-67 and realized a controllable and extended drug release in glaucoma therapy (Fig. 10B)218.
5.1.5. Anti-tumor applications
Developing novel DDSs to improve the effectiveness of chemotherapy and diminish the adverse reactions is of significant sense for the treatment of tumors. As nanocarriers with high drug loadings and easy modification, MOFs have been exploited to enhance the accumulation of drugs into tumors. In order to conquer the multidrug resistance effect, Zhang et al.219 utilized ZIF-8 to co-deliver chemotherapeutic drugs DOX and P-glycoprotein (P-gp) inhibitor verapamil hydrochloride (VER), realizing increased drug concentrations in multidrug resistance tumor cells (Fig. 11A)219. DOX and VER were co-incorporated into ZIF-8 through one-pot strategy, then (DOX + VER)@ZIF-8 was functionalized with poly (ethylene glycol)-folate (PEG-FA) by coordination for realizing prolong circulations and active targeting, which displayed increased therapeutic efficiencies and much safer properties than free DOX.
Figure 11.
Applications of MOFs in anti-tumor therapy (A) pH-responsive ZIF-8 as codelivery vehicles of DOX/VER for efficient anticancer effect. Reprinted with permission from Ref. 219. Copyright © 2017, American Chemical Society (B) Preparation of erythrocyte membrane cloaked MOF biomimetic nanoreactor for starvation therapy. Reprinted with permission from Ref. 220. Copyright © 2018, American Chemical Society (C) Illustration of ZIF-8 to co-deliver quercetin as an anticancer agent and CuS NPs as a photothermal therapy agent for enhanced synergistic tumor treatment. Reprinted with permission from Ref. 221. Copyright © 2018, American Chemical Society (D) Fabrication of nanocomposites made of MIL-53 (Fe) for chemotherapy-photothermal and MRI of tumors. Reprinted with permission from Ref. 222. Copyright © 2018, American Chemical Society (E) Illustration of MOF based nanocarriers for integrating PDT with ferroptosis. Reprinted with permission from Ref. 224. Copyright © 2019, American Chemical Society (F) Illustration of a biomimetic O2-evolving PDT nanoplatform O2@UiO-66@ICG@RBC using UiO-66 for NIR-triggered O2 releasing and improved PDT therapy. Reprinted with permission from Ref. 223. Copyright © 2018, Elsevier (G) Preparation of aMMTm for combination therapy of antiangiogenesis and PDT. Reprinted with permission from Ref. 225. Copyright © 2019, WILEY-VCH (H) Illustration of nano MOF preparation and Gem-MP encapsulation for enhanced radiotherapy. Reprinted with permission from Ref. 23. Copyright © 2019, WILEY-VCH (I) Preparation of Mn carbonyl modified PEGylated Fe (III)-based nanoMOFs for NIR-responded DOX-CO combination therapy. Reprinted with permission from Ref. 227. Copyright © 2019, Elsevier.
GOx-based cancer starving therapy has been regarded as a promising strategy for tumor therapy. However, the low GOx delivery efficacy and self-limiting curative effect have restricted its application. Therefore, Zhang et al.220 exploited ZIF-8 as the carriers to embody GOx and prodrug tirapazamine, then packaged with an erythrocyte membrane to develop biomimetic nano-reactor tirapazamine-GOx-ZIF-8@erythrocyte membrane (Fig. 11B)220. The large cavities of ZIF-8 would reach to a high loading efficacy of GOx, protecting GOx from leaching, aggregation and loss of catalytic activity. Erythrocyte membrane could help escape immunity and prolong blood circulation, then assisting the delivery of GOx to tumor cells for exhausting intracellular glucose and O2. The resulting tumor hypoxia further initiated the activation of tirapazamine for strengthened colon tumor therapy. In another study reported by Jiang et al.221, ZIF-8 was used to co-deliver quercetin as an antitumor drug and CuS NPs as a photothermal therapy (PTT) agent for enhanced synergistic tumor treatment (Fig. 11C)221. Using ZIF-8 to incorporate quercetin which is a promising anticancer agent with poor water solubility and chemical instability could greatly overcome the drawback of quercetin.
Owing to its minimal invasiveness and high selectivity, PTT has attracted tremendous attention. In this field, Huang et al.222 explored in situ fabrication of nanocomposites made of MIL-53 (Fe) taking advantage of the oxidation of the pyrrole monomer in the cage to generate polypyrrole NPs (Fig. 11D)222. After polymerization, the large surface area, porosities and initial structure of MIL-53 were unchanged, which allowed it to load DOX for chemotherapy. Meanwhile, the Fe ions in MIL-53 would act as a T2 MRI contrast agent for tracking the distribution of the composites. Results showed that the obtained PPy@MIL-53/DOX with high drug loading capability and photothermal effect exhibited good therapeutic synergism.
In addition to chemotherapy and PTT, photodynamic therapy (PDT) with the ability to generating cytotoxic singlet oxygen (1O2) by photosensitizers (PS) is also a promising strategy for management of malignant tumor. However, oxygen consumption induced by PDT treatment would lead to irreversible tumor metastasis and drug resistance. Therefore, developing novel O2-generating materials to supply O2 in tumor microenvironment would greatly enhance the efficiency of PDT against hypoxia tumor. The tunable MOFs pore sizes, high distinctive surface area and porous framework endow them with good capacity for gas storage. Inspired by these, Gao et al.223 developed a biomimetic O2-evolving PDT nanoplatform (O2@UiO-66@ICG@RBC), using UiO-66 as a depot for O2 storage, then conjugating with indocyanine green (ICG) (Fig. 11F)223. Finally, a coating with red blood cell (RBC) membranes was achieved to allow immunologic escape. When exposed to 808 nm laser irradiation, 1O2 generated from ICG degraded the RBC membrane and facilitated O2 release from UiO-66, and thus improved the PDT effects. In response to the intracellular damage induced by 1O2, the concentration of GSH, a vital intracellular antioxidant, was decreased for maintaining the redox homeostasis. It was noteworthy that GSH consumption was relevant to ferroptosis which is an Fe-dependent cell death, generally different from other cell death. In another study, Meng et al.224 designed an MOF-based nanocarrier for integrating PDT with ferroptosis, by using disulfide-containing imidazole as organic ligand and Zn(II) as corresponding coordination metal ions, then encapsulating a photosensitizer chlorin e6 (Fig. 11E)224. Upon light irradiation, GSH depletion caused by chlorin e6-loaded MOF through the disulfide-thiol reaction resulted in the inactivation of glutathione peroxide 4 which was attributed to ferroptosis, finally, obtaining an enhancement of antitumor PDT by the MOF nanocarrier.
Another study combining PDT with anti-angiogenesis therapy, utilizing porphyrinic MOF nanostructure as antiangiogenic drug delivery carrier and PDT agent was reported by Min et al.225, which was decorated with MnO2 layer to neutralize excessive GSH in tumor for enhancement of PDT therapy, then endowed by mouse breast cancer cells (4T1) membrane to achieve tumor-targeting ability (Fig. 11G)225. After intravenous administration, the multi-functionalized porphyrin Zr-MOF would selectively accumulate in tumor through homologous targeting mediated by tumor cell membrane camouflage, then, enhanced PDT by GSH consumption and release of antiangiogenic drug apatinib cooperatively improved tumor therapy. Besides, the released Mn(II) from MnO2 could be used for in vivo tumor imaging as its MRI contrast property.
Very recently, Haddad et al.226 developed a mitochondria targeting DDS based on MOF for cancer therapy, using UiO-66 as carriers to load anti-cancer drug dichloroacetate, then, conjugating with triphenylphosphonium for mitochondria targeting. Super-resolution microscopy experiments directly displayed that human breast carcinoma cell lines MCF-7 cells treated with the obtained MOF revealed mitochondrial morphological transformation followed with autophagy and cell death. Moreover, whole transcriptome analysis showed that cellular gene expression was widely changed when treated with the targeted MOF, indicating the valuable strategy of this targeting MOFs towards mitochondria.
As it is well known, carbon monoxide (CO) is harmful to people because of its greater affinity towards hemoglobin than oxygen, which can be used for malignant tumor treatment in combination with chemotherapy and PTT. It is noteworthy that intracellular delivery of CO and on-demand release are vital owing to its toxicity. For realizing near infrared spectroscopy light-responded CO gas therapy and chemotherapy combination, a novel protocol was proposed by Yao et al.227 by utilizing Mn carbonyl modified PEGylated Fe(III)-based nanoMOFs (MIL-100) enwrapped magnetic carbon NPs (MCM@PEG-CO) (Fig. 11I)227. In this strategy, Mn(CO)5Br was selected as the CO source whereas MIL-100 nano MOFs allowed high drug loading capacity of DOX. In the meantime, MIL-100 modified with 4,4ʹ-diamino-2,2ʹ-bipyridine (DABPY) would immobilize Mn(CO)5Br firmly for controlled release of CO. Moreover, the appearance of magnetic carbon core not only could execute PTT therapy by near infrared spectroscopy irradiation, but also enhanced T2 MRI which endued MCM@PEG-CO with capability of tumor dual-mode imaging, including photoacoustic imaging and MRI.
More recently, a study showed the first application of radioenhancers made of MIL-100 carried with a radiosensitizing anticancer drug, GEM23. The MIL-100 regular porous framework with oxocentered Fe trimers detached by around 5 Å (trimesate linkers) was able to disperse the electrons emitted from nanoMOFs owing to activation of γ radiation. In turn, this provoked water radiolysis and emergence of hydroxyl radicals, creating harm to cancer cells. Moreover, MIL-100 carried their GEM inside cancer cells. By exhibiting different mechanisms of action, both MIL-100 and GEM contributed to enhance radiation effect synergistically (Fig. 11H)23. This study opened new avenues toward the project of engineered NPs wherein each constituent played a part in tumor therapy by radiotherapy.
5.2. Applications in pharmaceutics
5.2.1. Enhancement of drug solubility and bioavailability
In drug discovery, almost 70% of new drug candidates show poor water solubility, which may limit their clinical applications for poor absorption228. In virtue of the highly porous structure, MOFs have boundlessly potential loading capacity to poorly soluble drugs, proving to be effective carriers for aqueous solubility and bioavailability improvement of certain hydrophobic drugs. A drug might immediately release if the particles quickly collapse in aqueous environment. Compared with leflunomide, the use of porous γ-CD-MOFs as a drug container to load leflunomide could significantly increase the solubility of leflunomide by 80 times93. The γ-CD-MOFs could load poorly soluble drugs including AZL (Fig. 12A)20, valsartan80, and FA229, increasing the solubility up to 340, 39.5 and 1453 times respectively, and for bioavailability 9.7, 1.9 and 1.5 times were reached, respectively. Similarly, the pharmacokinetics results exhibited that the IBU@CD-MOF can increase the uptake rate of IBU with a maximal plasma concentration within 20 min, and had a longer half-life in blood when compared to potassium salt of IBU230.
Figure 12.
Applications of MOFs in pharmaceutics (A) CD-MOFs significantly improve the solubility and bioavailability of Azilsartan. Reprinted with permission from Ref. 20. Copyright © 2019, Elsevier (B) Fe2O3@MIL-53(Al) exhibits excellent controlled release behavior of IBU. Reprinted with permission from Ref. 236. Copyright © 2014, WILEY-VCH (C) PH tolerance of lipase@ZIF-8 (red) and free lipase QLM (black) at pH 5.0, 7.0, and 9.0 for different time. Reprinted with permission from Ref. 233. Copyright © 2016, American Chemical Society.
5.2.2. Improvements of drug stability
Poor stability restricts the development, storage, and application of drugs. Many drugs are sensitive to acids, alkalis, heat, light, oxygen, and moisture, and cause oxidation, polymerization, degradation and crystallization. A number of studies have shown that MOFs can be used to improve the stability of drugs.
It is well known that curcumin is unstable under neutral and alkaline conditions. In order to solve this problem, curcumin was incorporated into the cavity of γ-CD-MOFs. Contrast to free curcumin and curcumin/γ-CD inclusion complex, the stability of curcumin at pH 11.5 was significantly improved by γ-CD-MOFs at least 3 times231. On the other hand, γ-CD-MOFs could also be used as vehicles to prevent drug degradation and crystallization during long-term storage. Lansoprazole degrades rapidly in humid environments and has a strong tendency to crystallize. Li et al.67 encapsulated lansoprazole during the synthesis of γ-CD-MOFs, which could be stored for up to two years and retained its spectral characteristics. γ-CD-MOFs also could be employed as carrier for the unstable vitamin A palmitate. Stability tests showed that γ-CD-MOFs improved the stability of encapsulated vitamin A palmitate without any antioxidant, the stability was better than the commercially available BASF vitamin A powder, and the half-life of vitamin A palmitate was extended up to 1.6 times232.
The pharmaceutical applications of enzymes are hampered because of their poor stability at high temperature and extreme pH conditions. Recently, MOFs for immobilization of enzymes have great attention that extremely improve chemical/thermal stability of enzymes. He et al.233 had encapsulated thermophilic lipase within ZIF-8 by a self-assembly approach which improved the chemical stability and preserve catalytic activity of lipase (Fig. 12C)233. Compared to lipase@ZIF-8 with no trypsin treatment, lipase@ZIF-8 could still preserve 91% of catalytic activity after trypsin treatment. Higher stability of lipase also be detected in the pH tolerance test, wherein lipase@ZIF-8 could maintain 90% of catalytic activity after incubation at 5.0, 7.0 and 9.0 for 200 min. Liang et al.234 studied the stability of urease embedded in ZIF-8 by one-pot strategy. Results showed that over a range of 23–80 °C, urease@ZIF-8 offered significant improvement of the initial reaction rate by comparing free enzyme.
5.2.3. Control of drug release
MOFs control drug release by avoiding the “burst effect” and prolong drug retention time. Diclofenac sodium (DS) encapsulated in MOFs allowed a sustained release behavior. DS@ZJU-800 compacted with different pressures were suitable candidates for drug delivery. Compared with the release kinetics of DS@ZJU-800 lasting 2 days in PBS (pH = 7.4), the drug release could be extended to 5 and 8 days when the pressure was increased to 10 and 30 MPa, respectively45. What's more, γ-CD-MOFs can also control DS release under simulated physiological conditions of the gastrointestinal tract (pH = 1.2). DS@γ-CD-MOF released about 20% of DS in the first 2 h. In contrast, free DS showed burst release, with more than 70% of the drug released within the first 0.5 h235.
In addition, MOFs were also excellent carriers for controlled release of IBU (Fig. 12B)236. After Fe2O3@MIL-53(Al) encapsulated IBU, the release behavior in saline was divided into three stages: first, around 30% of IBU was released fast in the first 3 h; then, 50% of IBU was released within 2 days; last, the remainder 20% of drug was released slowly over 5 days. MIL-100, MIL-101 and MIL-53 also presented better controlled release to IBU236. The release kinetics indicated that an entire release of IBU from MIL-100 happened after 3 days, from MIL-101 after 6 days82, and from MIL-53 after 21 days63.
The Fe(III) based non-toxic MOFs have the potential for controlled drug release61,62. The phosphorylated drug, azidothymidine triphosphate, was encapsulated into the biocompatible MIL-100. The gradual release of azidothymidine triphosphate from MIL-100(Fe) NPs was mainly triggered by the phosphate ion in the incubation medium. The release kinetics showed three different steps: a few percent of drug was released in 4 h; then, about 90% azidothymidine triphosphate was released to the PBS (pH = 7.4) with 10% FBS within 3 days; last, the remaining 10% of drug was slowly released in 3 days62. Interestingly, topotecan can aggregate into the cages of MIL-100 by stacking interactions following a “ship in a bottle” strategy, the drug was not released until the system was submitted to a two-photon excitation62. Besides, porous MIL-100 was utilized as a nanocarrier of DOX, with payload of 9% (w/w) and entire release within 14 days62.
5.3. Functionalization of MOFs and design of advanced systems using MOFs
5.3.1. Surface functionalization of MOFs
Some MOFs are excellent drug carriers without any surface modification, whilst some MOFs need to be modified to perform special functions. Surface or interior functionalization of MOFs can doubtlessly promote the applications of MOFs in biomedicine. Herein, the functionalizations of MOFs were summarized as targeted therapy of cancer, preparation of stimulus-responsive carriers, hemostatic systems, antibacterial systems and improvement of the encapsulation capability (Fig. 13).
Figure 13.
Overview the functionalization of MOFs and design of advanced systems using MOFs.
5.3.1.1. Targeted therapy of cancer
FA is often used for tumor targeting because of the ability to specifically recognize tumor cells. Hollow Fe–MOFs (MIL-53(Fe)-5–NH2) was grafted with FA to increase drug loading and confer targeting abilities for specific drug delivery51. Meanwhile, NH2–MIL-101(Fe) modified with pegylated folate significantly enhanced the validity of drug delivery to tumor cells237. By PEGulation on the appearance of the NH2–MIL-101(Fe), the circulation time of the carrier in vivo was prolonged, meanwhile the phagocytosis of the carrier by the cells was reduced. Furthermore, RGD-grafted camptothecin@zeolitic imidazolate framework-8 (RGD@CPT@ZIF-8) was constructed as a neoteric particle on the basis of hydrophobic DDS for targeted and improved cancer treatment238. In addition to linear peptides, cyclic peptides were also frequently used in the functionalization of MOFs. NMOF@SiO2 nanoparticles conjugated with a tumor-targeting peptide c (RGDyK) was prepared with a high binding affinity toward integrin αvβ3. Therefore, targeted therapy of tumor was achieved239.
5.3.1.2. Stimulus-responsive carriers
Stimulus-responsive carriers realize the thermal, optical and pH responsiveness via modification of functional groups to MOFs. Azobenzene is a typical photosensitive material, which could couple with the surface of MOFs to form a supramolecular complex with β-CD named UiO-68-azo. The UiO-68-azo compound showed favorable stimuli-responsive without any premature drug release240. Based on the low pH of micro-environment in tumor, the pH responsive poly dimethacrylate was grafted by surface reaction to manufacture the PEGMA@GQDs@γ-CD-MOF which provided a platform for valid DOX delivery and neoplasm growth suppression both in vitro and in vivo241. Furthermore, high temperatures facilitate drug released242. However, it's unrealistic to release drugs in the body at a high temperature, which will cause tissue burns. A thermal sensitive drug carrier (ZIF-8, EuxTby)@AuNP, was synthesized successfully to control the drug release at temperatures close to 37 °C243.
5.3.1.3. Hemostatic or antibacterial systems
In addition, MOFs coupled with polypeptide GRGDS have been shown to be effective in hematischesis and angiogenesis. CD-MOF by crosslinking and surface decoration with GRGDS became a particle with hemostatic function which realized the clustering of activated platelets and highly targeting to impaired vessels94. Furthermore, crosslinking CD-MOF grafted with GRGDs and loaded with Ag NPs possessed hemostatic and antibacterial functions210.
5.3.1.4. Improvement of the encapsulation capability
Modification of MOFs can enhance their encapsulation ability. The hydrophilic‒lipophilic balance value of organic linker had a huge influence on drug loading. For example, caffeine loading in UiO-66 was increased because the organic ligand had a high octanol‒water partition coefficient and possessed limited hydrogen bond acceptors. Nevertheless, the IBU entrapment was improved while the organic ligand contained active groups with a bulky solvent surface and free volume, and lesser degree hydrogen bond acceptor capabilities200.
5.3.2. Advanced systems made of MOFs
In addition to functional modification, MOFs can be combined with other carriers and materials to prepare nanoparticles-MOF, polymer-MOF, non-biological-MOF composites and biomolecule-MOF composites39, greatly expanding the application space of MOF as composites. Herein, the nanoparticles-MOF and polymer-MOF composites are discussed.
5.3.2.1. Nanoparticles-MOF composites
There were numerous combinations of NPs and MOFs244,245. Fe-MOFs and nanofibrous polycaprolactone were used to fabricate nanofiber composites by electrospinning technique. Polycaprolactone-MIL-53(Fe) offered proper surface and mechanical performance such as cellular biocompatibility, high porosity, stability, cell viability, and proliferation244. Bimetallic organic framework NP was prepared via parceling the inner MOFs with an outer MIL-100(Fe) via a layer-by-layer approach. The d-MOFs NPs regarded as a T1‒T2 dual-modal MRI contrast and fluorescence imaging agent as a result of the being of inner MOFs and outer MIL-100(Fe) MOFs, which showed a great potential in the treatment of tumors245. Furthermore, the magnetic Fe3O4@silica@MIL-100Fe/β-CD as a drug nanocarrier was studied for the drug loading and controlled release of cefalexin. It was found that the carrier could achieve zero-order release on specific locations246.
5.3.2.2. Polymer-MOF composites
Polymers modified with MOFs were engineered for applications as antibacterial gels, microspheres dry powder inhalers and so on. As a semisolid dosage form, medical gel is of great significance in antibacterial activities. Cu-MOFs embedded hydrogels were fabricated by UV light-mediated thiolene photopolymerization. The activities of hydrogels were tested against the gram-negative bacterium E. coli and the gram-positive bacterium S. aureus showing good antibacterial activities247. The niflumic acid and 2-methylimidazole were incorporated to the sodium alginate composite material as a ligand for Zn(II) which had strong antibacterial property and processed sustained release behavior of Zn(II) and high drug loading effect248. Furthermore, the mixture of CD-MOFs and polyacrylic acid (PAA) were fabricated by a solid in oil-in-oil (s/o/o) emulsifying solvent evaporation method, which presented a favorable dispersion of drug loaded CD-MOF nanocrystals (IBU and lansoprazole) mixed with PAA matrices and the uniform distribution of the drug molecules within these crystals. Meanwhile, the composite microspheres could significantly reduce the cytotoxicity and prolong the drug release time184.
Polymer@MOFs were also employed in pulmonary inhaled DDS. CHO and leucine were applied to embellish CD-MOF particles with loading of budesonide for pulmonary inhalation. To have enhanced the fluidity and optimize aerodynamics deposition by the modifications to the carrier using cholesterol, the distribution abundance in the lungs was significantly improved in rats compared with that of CD-MOF68.
6. Biopharmaceutics of MOFs and MOFs-based DDSs
It is of great significance for the pharmacokinetics investigation of MOFs for the sake of design and development of MOFs as pharmaceutical excipients. The pore sizes (micro- or mesopores), pore shapes (cages, channels, etc.), rigid or flexible frameworks, and the functional groups allow MOFs carriers to carry drugs with high capacities, controlled release kinetics, enhanced amphiphilic internal microenvironment, modulated biological interactions and penetration to overcome the gastrointestinal or the blood–brain barrier in a controllable manner40,101. MOFs carriers exhibited various merits such as increasing drug concentration at a local position, minimizing drug degradation and clearance, targeting specific cell, and creating drug-delivery formulation249. These all converge towards goals to improve the efficacy and decrease the toxicity of the therapy250.
Currently, improving the pharmacokinetic profile of MOFs carriers is a primary drug delivery strategy to optimize drug targeting toward diseased tissues or cells. A great number of studies20,185 focused on the capability to improve drug bioavailability and biodistribution. The absorption, distribution, metabolism and excretion (ADME) processes differ in MOFs and then have consequences on the pharmacokinetic profile of the loaded drugs57,251, 252, 253. An understanding of these pharmacokinetic characteristics is elemental to ensure effective therapy in MOF nanomedicine as an integral step to clinical application. However, very little is known about the pharmacokinetics of MOFs as relatively new drug carriers. The purpose of this part is to summarize what we can learn from the available data so far on the ADME of MOFs and the pharmacokinetic characteristics of drugs loaded in MOFs in vivo or in cells.
6.1. Pharmacokinetic characteristics of MOFs as drug carriers
In consideration of the hybrid nature of MOFs as drug carriers, their pharmacokinetic profiles were assessed by quantifying the components, the organic ligand and the ion cation of the MOFs in simulated physiological conditions, in cells and in vivo.
6.1.1. Studies in simulated physiological conditions
The physicochemical properties such as colloidal and chemical stability of nanomaterials strongly impact their efficacy and biodistribution254. Some researchers explored the biodegradability of MOFs under physiological conditions48,255,256. For instance, nanoMOFs of the MIL family progressively degraded in PBS after incubation at 37 °C57,257 as well as in cell culture media61. In all these studies, it was highlighted that the specific interaction between the MOFs and the phosphate groups in PBS that governed the progressive degradation of the NPs. Interestingly, it was discovered that degradation led to an amorphous material without significant changes of the initial size of the crystals258. In particular, the biodegradability of MIL-100(Fe) potentially relied on their physicochemical surface and the media composition. Furthermore, the excess phosphate ions in buffer decomposed the Zr-MOFs quickly leading to a rapid drug release74. In contrast, the NU-1000 degraded slowly and released its cargo gradually when exposed to simulated blood solution with a high concentration of phosphate ions259. Differently, the procainamide HCl released from the bio-MOF-1 in PBS showing a steady profile over 20 h while completed release within 72 h43.
6.1.2. In vitro experiments
Recently, studies have focused on the interaction between MOF particles and cells, which were assessed by cell viability, metabolism, penetration, and excretion57,253,260. For instance, the cytotoxicity of MIL-100(Fe), MIL-101(Cr), and Zr-fum MOF NPs of different sizes on primary gingiva fibroblasts as effector cells for dental implants were accessed249. Additionally, the cell uptake of the MIL-100(Fe) was pivotal to deliver cargos261. NanoMOFs like other drug carriers efficiently internalized in macrophages and degraded in their inner compartments33. Orellana-Tavra et al.262 revealed the internalization processes of UiO-66 MOF particles by the caveolae-mediated pathway, which could avoid to deliver the cargo in other intracellular location without the lysosomal acidic degradation. Besides, cellular internalization of UiO-66 into HeLa cells was depended on the particle size of the solids, and the uptake of larger UiO-66 (260 nm) particles were more efficient compared with smaller UiO-66 (150 nm). This difference is critical when lysosome activity may prevent the MOF action from pre-degradation for MOFs-based DDSs.
6.1.3. In vivo evaluations
One of the major issues with administration of the MOFs as drug carriers in vivo is their pharmacokinetic properties. The efficient drug delivery of MOFs is mainly depended on host–guest interactions, diffusion and their degradation in the body fluid. As an example, nano MIL-100(Fe) degraded to be compatible with biomedical applications in vivo31,32. In this study, the pharmacokinetics of MIL-100 were evaluated by intravenous administration in mice in short term (up to 24 h) (Fig. 14B)32. When MIL-100 arrived into the blood, phosphates replaced the carboxylate linkers, releasing the metal ions and the organic linker. Another criterion is closely related to their solubility, the release of solubilized metal ions, crystal parameters such as particle size and subsequent cellular uptake.
Figure 14.
Pharmacokinetic characteristic of MOFs (A/B) and drugs loaded in MOFs(C/D) (A) Assessment of salicylate elimination (black) and MIL-127 matrix degradation (red) under simulated GI environment. Different acetylsalicylic acid: MIL-127 ratios were studied: 3:1 (triangles) and 1.5:1 (circles). Reprinted with permission from Ref. 263. Copyright © 2018, American Chemical Society (B) Biodistribution. (a) Levels of BTC in major organs and urine (b) mg of BTC per g of major organ. Reprinted with permission from Ref. 32. Copyright © 2016, Elsevier (C) Plasma concentration–time curves of AZL in rats after oral administration. Reprinted with permission from Ref. 20. Copyright © 2019, Elsevier (D) Biodistribution of RhoB-labeled insulin in the bodies of the mice (a), livers (b), kidneys (c) after the oral administration at different time points. Reprinted with permission from Ref. 273. Copyright © 2020, American Chemical Society.
Besides, the biocompatible MIL-127 was used for oral drugs detoxification in vivo. Except for slight absorption in the intestine, the intact MIL-127 was remained along the gastrointestinal (GI) tract and then excreted in the feces after oral administration in rats (Fig. 14A)263. The large particle size, high structural and chemical stability, and low intestinal permeation of both MIL-127 and its constitutive ligand potentially led to the lack of intestinal absorption. Moreover, the multimodal cancer therapy nanocomposite, Fe3O4/ZIF-8-Au25264 and Fe3O4@PAA/AuNCs/ZIF-8 NPs265 were administrated by injection into the tumor-bearing mice for accessing the biocompatibility and synergistic therapeutic effect. More efforts should be made to fabricate good biocompatible MOFs to ensure the biosafety of MOFs in vivo. The accumulation took place mainly in the liver and spleen and then degraded into their constituent of ions and ligands, and excreted through urine and feces without any metabolization.
6.2. Pharmacokinetic characteristics of drug-loaded MOFs
Bioavailability is a combinative result of physicochemical properties, mucosal penetration, gastric pH, drug-metabolizing enzymes, and drug transporters, whilst the extensive first pass metabolism is a major impediment to clinical application of a therapeutic agent266,267. Plenty of efforts have been dedicated to the improvement of drug solubility using CD-MOF230. Kritskiy et al.185 reported enhanced bioavailability of MTX loaded in γ-CD-MOF with increased dissolution of MTX and the decreased drug permeability through the lipophilic membrane. At the same time, the γ-CD-MOF was suitable for long-term administration due to its elimination with a longer time (T1/2 = 4.5 h) than that of MTX (T1/2=1.5 h). However, the unknown biodistribution and aqueous instability might limit their practical use. Hence, it is of sense to understand the mechanism of bioavailability enhancement to the insoluble drug in CD-MOFs. He et al.20 fabricated CD-MOF with molecular cages confined with nano cluster of AZL and its CD complexation. The AZL/CD-MOF and raw AZL were orally administrated to SD rats at a dose of 1 mg/kg. The plasma drug level of AZL in CD-MOF increased quickly and achieved maximal concentration at 1.5 h after oral administration, and the AUC0–24h of 30.65 ± 14.24 μg/mL·h was almost nearly 9.7 times higher than that of free AZL, which intensively displayed that AZL/CD-MOF enhanced the absorption and bioavailability of AZL (Fig. 14C)20. The mechanism of the AZL distribution inside the CD-MOF by molecular docking suggested that the drug nanoclusters with uniform and tiny size immediately released from CD-MOF could temporarily improve the apparent solubility of AZL in water. Additionally, further studies should be paid to the mechanism of distribution, metabolism and excretion.
Overcoming the barriers of the GI system to provide sufficient bioavailability following oral administration is vital for MOFs potential clinical usages. A number of research groups have explored the bioavailability with controlled and pH specific release behaviors without burst effects84,193,268,269. For instance, PCN-221 with MTX loading demonstrated that about 40% of MTX was released at the pH of stomach after 3 days, whereas 100% was released at the intestinal270. Besides, the challenges to protect MOFs from enzymatic degradation in the GI condition and low permeation across the intestinal epithelium remain to be solved. Hidalgo et al.271 showed an increased penetration of chitosan coated MIL-100(Fe) NPs across the intestinal barrier as compared to uncoated NPs. Chen et al.272 found that acid stable MOF capsules named NU-1000 could effectively prevent insulin from degrading under simulated physiological conditions.
What's more, Zhou et al.273 studied insulin loaded microspheres named Ins@MIL100/SDS@MS, which showed resistance to the stomach acid environment while absorption and transportation at intestinal after oral administration in vivo. The distribution of insulin in major organs suggested that the microsphere potentially circulated through the portal veins to the liver then into the heart, finally metabolized via the kidneys (Fig. 14D)273. From pharmacokinetic parameter analysis, the peak level of BALB/c nude mice plasma insulin was emerged at 4 h and remained over 8 h. Moreover, contrast to the subcutaneous insulin injection, the relatively lagged absorption of oral system was likely due to the absorption process of GI tract.
Until now, the intravenous injection is the most preferred administration route for MOF nanomedicines to prevent the nanomedicines from being degraded in advance36. Intravenously administered the porous Fe(III) nanoMOFs in rats were quickly captured by the liver and spleen, then further biodegraded and directly removed in urine or feces. The BTC ligands were gradually eliminated in urine31. Pan et al.274 explored relatively comprehensive pharmacokinetic characteristics of 5-FU enveloped in Mn-ZIF-8. The Mn-ZIF-8 (30 mg/kg) was injected intravenously into U87-MG tumor bearing mice were, which indicated the accumulation of Mn-ZIF-8 from peaking at 12 h to slightly decrease at 24 h. For in vivo biodistribution and metabolism, the tumor bearing mice were administered intravenously with Mn-ZIF-8 (30 mg/kg) solution, the biological distribution of the NPs in the major organs and tumor were observed. The amount of Mn(II) ions exhibited high levels of accumulation in the liver and spleen. And the retained Mn(II) ions in major organs reduced quickly over time, suggesting that Mn-ZIF-8 may efficiently be removed from mice after gradual decomposition into small molecules and ions in a relatively rapid way, to have minor side effects on the functions of vital organs.
The bone targeting immunostimulatory MOF (BT-is MOF) NPs can effectively target tumor bone tissues through blood circulation and enhanced tumor penetration275. The specific deposition and accumulation of BT-is MOF on bone tissues were detected after sequential injection to C57BL/6 mice. Also, the in vivo distribution was largely deposited in bone and liver tissues but not in the kidney after vein injection.
Moreover, latest study show that the bioavailability and therapeutic efficacy of GEM encapsulated in the MIL-100(Fe) were improved in comparation to the GEM276. In this study, the Wistar rats were given i.v. dose as 40 mg/kg of GEM for MIL100-GEM and GEM solution. The plasma drug level of MIL100-GEM was 5 times higher than the GEM, while the GEM had a wider distribution in major organs, which indicated that encapsulating of GEM into MIL-100 restricted drug distribution in systemic circulation while elongating its distribution half-life. Further, MIL100-GEM exhibited a much slower removal (t1/2β = 173.25 min) compared to GEM (t1/2β = 11.17 min). Namely, MIL-100-GEM showed enhanced stability and durable release profile, leading to improved therapeutic effect at target site.
Zn is an essential trace element in human body, which was revealed to gather the highest content in brain compared with other organs277. Du et al.277 constructed a coordination strategy by ion-exchange for controlling release of drug molecules, and the topological types of MOF-In1 and MOF-In2 were selected to illustrate the strategy. With the increase of node connectivity and the capture of guest OH anions, 5-Fu is preferentially loaded into MOF-In1. Meanwhile, the Zn(II) concentration was also elevated along with the enhanced controlled release of 5-FU.
6.3. Future prospects
The complex in vivo fate of MOFs as well as their passengers loaded inside should be clarified. Currently, MOFs biodistribution (bio-adhesion, stealth, targeting, etc.) and their stability can be both improved by surface modification and functionalization. However, plenty of challenges remain to be exploited before these materials can reach to the market as drug carriers. First of all, very little data of the MOFs in vivo are available. The degradation mechanism of the MOF particles is difficult to be mimicked in vitro, as body fluids contain huge number of different ions, proteins, and cells which all potentially interact with MOF particles. Optimizing the route and dosage of administration is necessary to identify the appropriate animal model such as orthotopic tumor models, syngeneic mouse tumor models for the in vivo test or the utilization of 3D in vitro preclinical evaluation101. Indeed, potentially interesting pharmaceutical preparations such as pellets, thin films, gels, ocular, etc. have been developed using MOFs. Nevertheless, most studies mainly focused on bioavailability and biodistribution of drugs after intravenous/oral administration and there is a lack of a comprehensive MOFs pharmacokinetics mechanism as the whole ADME process. For the case of bioavailability, it is indispensable to pay more attention to enzymatic degradation (e.g., CYP450) and the efflux transporters (e.g., P-gp), which hinder the absorption and permeation of therapeutics from the body278.
7. Quality control of MOFs-based DDSs
For pharmaceutical applications, not only the toxicological compatibility but also the quality, especially the purity of MOFs should be well controlled by established methodologies. Powder XRD is the most commonly used technique to measure the crystalline purity of MOFs, such as the crystallinity, phase purity and crystal phases inside materials279,280. Pure-phase crystalline or multiphase MOFs materials could be identified by powder XRD. However, this technique is limited to analyze detect weakly crystalline and even amorphous impurities. Through the elements of MOFs by EDS, nanosized MOF-5 was confirmed to be homogeneous and highly pure281. But for the starting materials as impurities in MOFs which share the same elements as MOFs, they could not be distinguished by techniques like EDS. The surface area analysis was also used to the phase purity confirmation of MOFs. The expected surface area will not be reached if MOFs have not been thoroughly made free of solvent, starting materials or by-products from the synthesis. In more specific cases, other technique like photoluminescence spectroscopy can complement these characterizations282. Nevertheless, the above measurements are limited to the qualitative evaluation on MOFs, lack of quantitative analysis. As the starting materials and structural compositions of MOFs, quantitative determination of the free or residual uncoordinated organic linkers as impurities is of difficulty, such as γ-CD and free ions in CD-MOFs. It is estimated that quantitative quality control strategies for contents of MOF and limit of starting materials as impurities are the primary concerns in the aspect of developing MOFs as pharmaceutical excipients or drug delivery carriers.
8. Conclusions and perspectives
Combining molecular functional building blocks into an advanced porous multifunctional system, it is expected that the MOFs will find increasing conceivable utilizations in drug delivery. In last decades, significant advance has been realized in MOF-based DDSs, though most of the researches remain in the proof-of-concept phase, partially due to the difficulty to achieve the consistency of the drug release under in vitro and in vivo conditions spatiotemporally. Moreover, developing MOFs with optimized pore/channel size, surface properties (charge, hydrophilicity and presence of grafted ligands for targeting purposes) for therapeutic cargos delivery without disrupting their activity still has a long way to go. Furthermore, the underlying mechanisms of drug release in vivo in MOFs-based DDSs are still to be fully unraveled whilst extremely vital, and most of the studies contain hypothesis to be confirmed283, 284, 285. In addition, the majority of MOFs-based DDSs were administrated intravenously, the design of MOFs-based DDSs given by other administration routes should be more thoroughly investigated. Similarly, the applications of MOFs-based DDSs are expected to be broadened, close attention should be paid to other diseases except for cancer to fully take advantages of the MOFs.
In simple words, an overall perspective of MOFs-based DDSs has been reviewed, from the classification, synthesis, characterization to application. In particular, the biopharmaceutical characteristics and biosafety of MOFs-based DDSs were highlighted. To develop an efficient DDSs using MOFs, toxicity is the first and most important consideration in exploring their applications. Besides, the quality control of MOFs-based DDSs was put forward as well. The requirements of MOFs for DDSs are much stricter than non-biomedical applications, and biomedical applications of MOFs are still confronted with many challenges. Thus, the particle size, morphology, drug loading efficacy, stability and toxicology of the MOFs must be comprehensively characterized. Future continuative efforts should be endeavored to the biostability, biosafety, biopharmaceutics and manufacture to achieve the application of MOFs-based DDSs in clinic (Table 5).
Table 5.
Current challenges and potential suggestions for MOFs-based DDS.
| Challenge | Suggestion |
|---|---|
| In vivo fates of MOFs and drugs loaded inside are unclear |
|
| Current researches are limited to qualitative evaluation of MOFs |
|
| Some MOFs are unstable in physiological conditions |
|
| Lack of systematic biosafety evaluation to MOFs |
|
| No established standard for MOFs classification and nomenclature |
|
8.1. Balance between degradability and stability of MOFs in vivo
Some MOFs are unstable in physiological conditions, which may cause serious issues, such as decomposition of MOFs, short circulation time and premature drug release, limiting their biomedical application significantly. The stability of MOFs depends on the intensity of the coordination bond between the metal ions and the ligands, which is a significant factor to be taken into consideration during the fabrication of MOFs for application in aqueous environments. The main reason why some MOFs are unstable in biological media is that water molecules, ions and other molecules in the environment compete with the linkers for the interaction with metal ions or clusters, resulting in the collapse or the degradation of the framework. Other factors such as high hydrophobicity and poor crystallinity can affect the stability of MOFs in water as well. In addition, the pH and temperature of the environment are also key factors. Surface functionalization of the MOFs or their combination with other more stable materials may improve the colloidal stability for biomedical applications. On one hand, the stability and biodegradability of MOFs are vital factors that should be emphasized. One the other hand, the collapse of MOFs at a targeted organ or tissue is preferable to avoid endogenous accumulation and toxicity.
8.2. Systematic biosafety evaluation to MOFs
The biosafety of MOFs carriers is pivotal to determine whether an MOF carrier can enter clinical studies or not. However, the evaluation of the in vivo long-term toxicity for MOFs-based DDSs needs further studies. Currently, while numerous studies deal with the in vitro toxicity of MOFs, there is still a lack on in vivo studies. Moreover, it is well known that in vitro cell experiments can hardly simulate the in vivo environments. Only very few researches have reported the in vivo toxicity of MOFs. Thus far, MOFs-based DDSs for in vivo therapy have emerged, but far away to clinical studies. Hence, selecting metal ions and organic linkers with favorable biocompatibility needs careful deliberation and more efforts should be made to evaluate the acute and chronic toxicities of MOFs. Nevertheless, toxicity is a complex topic to deal with, as not only the MOF composition, but their size distribution, degradation behaviors, aggregation, interaction with biomolecules etc. can make a great difference to their biosafety.
8.3. Comprehension of the biopharmaceutics of MOFs in vivo
To unveil the in vivo fate of MOFs which is essential to reach the clinical stage, further efforts should be dedicated to exploring the metabolic mechanisms and metabolites of MOFs in vivo. What's more, a comprehensive investigation of the mechanism of ADME is indispensable. Although most of the synthesized MOFs for drug delivery have been tested via in vitro experiments for their biosafety, the ADME mechanism of MOFs-based DDSs has not been well-established. Particle size and polydispersity strongly influences the ADME of MOFs-based DDSs, as NPs with sizes less than 30 nm tend to be eliminated by renal excretion while larger ones are prone to accumulate in liver, spleen and lungs. Therefore, the kinetics of MOFs-based DDSs in vivo is of great importance to deeply understand their biopharmaceutics in vivo and promote clinical translation of MOFs-based DDSs.
8.4. Nomenclature
Series of MOFs have been reported, but there is no established standard for MOFs classification and nomenclature until now. MOFs are named based on the following four sides: (1) material composition; (2) structure; (3) laboratory/institution; (4) function. In most cases, the members in the same MOF families are named with identical letters in the front, while disparate in the last numbers for distinguishing diverse individuals. Hence, it's of urgency to unify the naming rules of MOFs.
Author contributions
Siyu He, Li Wu, Xue Li, Hongyu Sun, Ting Xiong, Jie Liu, Chengxi Huang and Huipeng Xu wrote the manuscript. Huimin Sun, Weidong Chen, Ruxandra Gref and Jiwen Zhang revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (No. 2020YFE0201700), National Nature Science Foundation of China (No. 81773645) and a public grant overseen by the French National Research Agency (ANR), France as part of the “Investissements d’Avenir” program (Labex NanoSaclay: ANR-10-LABX-0035, France).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Weidong Chen, Email: anzhongdong@126.com.
Ruxandra Gref, Email: ruxandra.gref@u-psud.fr.
Jiwen Zhang, Email: jwzhang@simm.ac.cn.
References
- 1.Hoskins B.F., Robson R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J Am Chem Soc. 1989;111:5962–5964. [Google Scholar]
- 2.Furukawa H., Cordova K.E., O'Keeffe M., Yaghi O.M. The chemistry and applications of metal-organic frameworks. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- 3.Connolly B.M., Madden D.G., Wheatley A.E.H., Fairen-Jimenez D. Shaping the future of fuel: monolithic metal-organic frameworks for high-density gas storage. J Am Chem Soc. 2020;142:8541–8549. doi: 10.1021/jacs.0c00270. [DOI] [PubMed] [Google Scholar]
- 4.Ding N., Li H., Feng X., Wang Q., Wang S., Ma L. Partitioning MOF-5 into confined and hydrophobic compartments for carbon capture under humid conditions. J Am Chem Soc. 2016;138:10100–10103. doi: 10.1021/jacs.6b06051. [DOI] [PubMed] [Google Scholar]
- 5.Bloch E.D., Queen W.L., Krishna R., Zadrozny J.M., Brown C.M., Long J.R. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science. 2012;335:1606–1610. doi: 10.1126/science.1217544. [DOI] [PubMed] [Google Scholar]
- 6.Wang D., Zhao C., Gao G., Xu L., Wang G., Zhu P. Multifunctional NaLnF4@MOF-in nanocomposites with dual-mode luminescence for drug delivery and cell imaging. Nanomaterials. 2019;9:1274. doi: 10.3390/nano9091274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar P., Deep A., Kim K.H. Metal organic frameworks for sensing applications. Trends Anal Chem. 2015;73:39–53. [Google Scholar]
- 8.Li G.D., Zhao S.L., Zhang Y., Tang Z.Y. Metal-organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: recent progress and perspectives. Adv Mater. 2018;30 doi: 10.1002/adma.201800702. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Yu J., Gosztola D.J., Fry H.C., Deria P. Wavelength-dependent energy and charge transfer in MOF: a step toward artificial porous light-harvesting system. J Am Chem Soc. 2019;141:16849–16857. doi: 10.1021/jacs.9b08078. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Ye N. Recent advances in metal-organic frameworks and covalent organic frameworks for sample preparation and chromatographic analysis. Electrophoresis. 2017;38:3059–3078. doi: 10.1002/elps.201700248. [DOI] [PubMed] [Google Scholar]
- 11.Luo Z., Fan S., Gu C., Liu W., Chen J., Li B. Metal-organic framework (MOF)-based nanomaterials for biomedical applications. Curr Med Chem. 2019;26:3341–3369. doi: 10.2174/0929867325666180214123500. [DOI] [PubMed] [Google Scholar]
- 12.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Ulbrich K., Holá K., Šubr V., Bakandritsos A., Tuček J., Zbořil R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116:5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 14.Matea C.T., Mocan T., Tabaran F., Pop T., Mosteanu O., Puia C. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed. 2017;12:5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayed E., Haj-Ahmad R., Ruparelia K., Arshad M.S., Chang M.W., Ahmad Z. Porous inorganic drug delivery systems—a review. AAPS PharmSciTech. 2017;18:1507–1525. doi: 10.1208/s12249-017-0740-2. [DOI] [PubMed] [Google Scholar]
- 16.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Wuttke S., Lismont M., Escudero A., Rungtaweevoranit B., Parak W.J. Positioning metal-organic framework nanoparticles within the context of drug delivery—a comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials. 2017;123:172–183. doi: 10.1016/j.biomaterials.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Ye E., David Lakshminarayanan R., Loh X.J. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small. 2016;12:4782–4806. doi: 10.1002/smll.201601129. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H.C., Kitagawa S. Metal-organic frameworks (MOFs) Chem Soc Rev. 2014;43:5415–5418. doi: 10.1039/c4cs90059f. [DOI] [PubMed] [Google Scholar]
- 20.He Y., Zhang W., Guo T., Zhang G., Qin W., Zhang L. Drug nanoclusters formed in confined nano-cages of CD-MOF: dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm Sin B. 2019;9:97–106. doi: 10.1016/j.apsb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnefoy J., Legrand A., Quadrelli E.A., Canivet J., Farrusseng D. Enantiopure peptide-functionalized metal-organic frameworks. J Am Chem Soc. 2015;137:9409–9416. doi: 10.1021/jacs.5b05327. [DOI] [PubMed] [Google Scholar]
- 22.Chu C., Su M., Zhu J., Li D., Cheng H., Chen X. Metal-organic framework nanoparticle-based biomineralization: a new strategy toward cancer treatment. Theranostics. 2019;9:3134–3149. doi: 10.7150/thno.33539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Porcel E., Menendez-Miranda M., Qiu J., Yang X., Serre C. Highly porous hybrid metal-organic nanoparticles loaded with gemcitabine monophosphate: a multimodal approach to improve chemo- and radiotherapy. ChemMedChem. 2020;15:274–283. doi: 10.1002/cmdc.201900596. [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Chen X.Z., Alcântara C.C.J., Sevim S., Hoop M., Terzopoulou A. Mofbots: metal-organic-framework-based biomedical microrobots. Adv Mater. 2019;31 doi: 10.1002/adma.201901592. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., McGuirk C.M., d'Aquino A., Mason J.A., Mirkin C.A. Metal-organic framework nanoparticles. Adv Mater. 2018;30 doi: 10.1002/adma.201800202. [DOI] [PubMed] [Google Scholar]
- 26.Agostoni V., Horcajada P., Noiray M., Malanga M., Aykaç A., Jicsinszky L. A "green" strategy to construct non-covalent, stable and bioactive coatings on porous mof nanoparticles. Sci Rep. 2015;5:7925. doi: 10.1038/srep07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aykaç A., Noiray M., Malanga M., Agostoni V., Casas-Solvas J.M., É Fenyvesi. A non-covalent "click chemistry" strategy to efficiently coat highly porous mof nanoparticles with a stable polymeric shell. Biochim Biophys Acta Gen Subj. 2017;1861:1606–1616. doi: 10.1016/j.bbagen.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Giménez-Marqués M., Bellido E., Berthelot T., Simón-Yarza T., Hidalgo T., Simón-Vázquez R. Graftfast surface engineering to improve MOF nanoparticles furtiveness. Small. 2018;14 doi: 10.1002/smll.201801900. [DOI] [PubMed] [Google Scholar]
- 29.Wuttke S., Braig S., Preiß T., Zimpel A., Sicklinger J., Bellomo C. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem Commun. 2015;51:15752–15755. doi: 10.1039/c5cc06767g. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Martínez A., Pérez-Juste J., Liz-Marzán L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv Mater. 2010;22:1182–1195. doi: 10.1002/adma.200901263. [DOI] [PubMed] [Google Scholar]
- 31.Baati T., Njim L., Neffati F., Kerkeni A., Bouttemi M., Gref R. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem Sci. 2013;4:1597–1607. [Google Scholar]
- 32.Simon-Yarza T., Baati T., Neffati F., Njim L., Couvreur P., Serre C. In vivo behavior of MIL-100 nanoparticles at early times after intravenous administration. Int J Pharm. 2016;511:1042–1047. doi: 10.1016/j.ijpharm.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Semiramoth N., Hall S., Tafani V., Josse J., Laurent F. Compartmentalized encapsulation of two antibiotics in porous nanoparticles: an efficient strategy to treat intracellular infections. Part Part Syst Char. 2019;36:180360. [Google Scholar]
- 34.Wu M.X., Yang Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017;29:1606134. doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 35.He L., Liu Y., Lau J., Fan W., Li Q., Zhang C. Recent progress in nanoscale metal-organic frameworks for drug release and cancer therapy. Nanomedicine. 2019;14:1343–1365. doi: 10.2217/nnm-2018-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Yan J., Wen N., Xiong H., Cai S., He Q. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials. 2020;230:119619. doi: 10.1016/j.biomaterials.2019.119619. [DOI] [PubMed] [Google Scholar]
- 37.Cai W., Wang J., Chu C., Chen W., Wu C., Liu G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv Sci. 2019;6:1801526. doi: 10.1002/advs.201801526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giliopoulos D., Zamboulis A., Giannakoudakis D., Bikiaris D., Triantafyllidis K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules. 2020;25:185. doi: 10.3390/molecules25010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterrieth J.W.M., Fairen-Jimenez D. Metal-organic framework composites for theragnostic and drug delivery applications. Biotechnol J. 2020 doi: 10.1002/biot.202000005. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Zhao Y., Chen X. Bioengineering of metal-organic frameworks for nanomedicine. Theranostics. 2019;9:3122–3133. doi: 10.7150/thno.31918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai H., Huang Y.L., Li D. Biological metal-organic frameworks: structures, host–guest chemistry and bio-applications. Coord Chem Rev. 2019;378:207–221. [Google Scholar]
- 42.Gao P.F., Zheng L.L., Liang L.J., Yang X.X., Li Y.F., Huang C.Z. A new type of pH-responsive coordination polymer sphere as a vehicle for targeted anticancer drug delivery and sustained release. J Mater Chem B. 2013;1:3202–3208. doi: 10.1039/c3tb00026e. [DOI] [PubMed] [Google Scholar]
- 43.An J., Geib S.J., Rosi N.L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J Am Chem Soc. 2009;131:8376–8377. doi: 10.1021/ja902972w. [DOI] [PubMed] [Google Scholar]
- 44.Nagata S., Kokado K., Sada K. Metal-organic framework tethering pnipam for on-off controlled release in solution. Chem Commun. 2015;51:8614–8617. doi: 10.1039/c5cc02339d. [DOI] [PubMed] [Google Scholar]
- 45.Jiang K., Zhang L., Hu Q., Zhao D., Xia T., Lin W. Pressure controlled drug release in a Zr-cluster-based MOF. J Mater Chem B. 2016;4:6398–6401. doi: 10.1039/c6tb01756h. [DOI] [PubMed] [Google Scholar]
- 46.Nel A.E., Parak W.J., Chan W.C., Xia T., Hersam M.C., Brinker C.J. Where are we heading in nanotechnology environmental health and safety and materials characterization? ACS Nano. 2015;9:5627–5630. doi: 10.1021/acsnano.5b03496. [DOI] [PubMed] [Google Scholar]
- 47.Rojas S., Devic T., Horcajada P. Metal organic frameworks based on bioactive components. J Mater Chem B. 2017;5:2560–2573. doi: 10.1039/c6tb03217f. [DOI] [PubMed] [Google Scholar]
- 48.Horcajada P., Gref R., Baati T., Allan P.K., Maurin G., Couvreur P. Metal-organic frameworks in biomedicine. Chem Rev. 2012;112:1232–1268. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- 49.Dongmei L., Zhiwei W., Qi Z., Fuyi C., Yujuan S., Xiaodong L. Drinking water toxicity study of the environmental contaminant—bromate. Regul Toxicol Pharmacol. 2015;73:802–810. doi: 10.1016/j.yrtph.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Leng X., Dong X., Wang W., Sai N., Yang C., You L. Biocompatible Fe-based micropore metal-organic frameworks as sustained-release anticancer drug carriers. Molecules. 2018;23:2490. doi: 10.3390/molecules23102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao X., Cui R., Song L., Liu Z. Hollow structural metal-organic frameworks exhibit high drug loading capacity, targeted delivery and magnetic resonance/optical multimodal imaging. Dalton Trans. 2019;48:17291–17297. doi: 10.1039/c9dt03287h. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M., Chen Y.P., Bosch M., Gentle T., Wang K., Feng D. Symmetry-guided synthesis of highly porous metal-organic frameworks with fluorite topology. Angew Chem Int Ed Engl. 2014;53:815–818. doi: 10.1002/anie.201307340. [DOI] [PubMed] [Google Scholar]
- 53.Abánades Lázaro I., Abánades Lázaro S., Forgan R.S. Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles. Chem Commun. 2018;54:2792–2795. doi: 10.1039/c7cc09739e. [DOI] [PubMed] [Google Scholar]
- 54.Inoue Y., Nanri A., Murata I., Kanamoto I. Characterization of inclusion complex of coenzyme Q10 with the new carrier CD-MOF-1 prepared by solvent evaporation. AAPS PharmSciTech. 2018;19:3048–3056. doi: 10.1208/s12249-018-1136-7. [DOI] [PubMed] [Google Scholar]
- 55.Schnabel J., Ettlinger R., Bunzen H. Zn-MOF-74 as pH-responsive drug-delivery system of arsenic trioxide. ChemNanoMat. 2020;6:1229–1236. [Google Scholar]
- 56.Alsaiari S.K., Patil S., Alyami M., Alamoudi K.O., Aleisa F.A., Merzaban J.S. Endosomal escape and delivery of CRISPR/CAS9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J Am Chem Soc. 2018;140:143–146. doi: 10.1021/jacs.7b11754. [DOI] [PubMed] [Google Scholar]
- 57.Horcajada P., Chalati T., Serre C., Gillet B., Sebrie C., Baati T. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172–178. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 58.Chalati T., Horcajada P., Couvreur P., Serre C., Ben Yahia M., Maurin G. Porous metal organic framework nanoparticles to address the challenges related to busulfan encapsulation. Nanomedicine. 2011;6:1683–1695. doi: 10.2217/nnm.11.69. [DOI] [PubMed] [Google Scholar]
- 59.Anand R., Borghi F., Manoli F., Manet I., Agostoni V., Reschiglian P. Host-guest interactions in Fe(III)-trimesate MOF nanoparticles loaded with doxorubicin. J Phys Chem B. 2014;118:8532–8539. doi: 10.1021/jp503809w. [DOI] [PubMed] [Google Scholar]
- 60.Agostoni V., Chalati T., Horcajada P., Willaime H., Anand R., Semiramoth N. Towards an improved anti-HIV activity of NRTI via metal-organic frameworks nanoparticles. Adv Healthc Mater. 2013;2:1630–1637. doi: 10.1002/adhm.201200454. [DOI] [PubMed] [Google Scholar]
- 61.Agostoni V., Anand R., Monti S., Hall S., Maurin G., Horcajada P. Impact of phosphorylation on the encapsulation of nucleoside analogues within porous iron(III) metal-organic framework MIL-100(Fe) nanoparticles. J Mater Chem B. 2013;1:4231–4242. doi: 10.1039/c3tb20653j. [DOI] [PubMed] [Google Scholar]
- 62.Marcos-Almaraz M.T., Gref R., Agostoni V., Kreuz C., Clayette P., Serre C. Towards improved HIV-microbicide activity through the co-encapsulation of NRTI drugs in biocompatible metal organic framework nanocarriers. J Mater Chem B. 2017;5:8563–8569. doi: 10.1039/c7tb01933e. [DOI] [PubMed] [Google Scholar]
- 63.Horcajada P., Serre C., Maurin G., Ramsahye N.A., Balas F., Vallet-Regí M. Flexible porous metal-organic frameworks for a controlled drug delivery. J Am Chem Soc. 2008;130:6774–6780. doi: 10.1021/ja710973k. [DOI] [PubMed] [Google Scholar]
- 64.Rojas S., Carmona F.J., Maldonado C.R., Horcajada P., Hidalgo T., Serre C. Nanoscaled zinc pyrazolate metal-organic frameworks as drug-delivery systems. Inorg Chem. 2016;55:2650–2663. doi: 10.1021/acs.inorgchem.6b00045. [DOI] [PubMed] [Google Scholar]
- 65.Bag P.P., Wang D., Chen Z., Cao R. Outstanding drug loading capacity by water stable microporous MOF: a potential drug carrier. Chem Commun. 2016;52:3669–3672. doi: 10.1039/c5cc09925k. [DOI] [PubMed] [Google Scholar]
- 66.Li Z., Zhao S., Wang H., Peng Y., Tan Z., Tang B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf B Biointerfaces. 2019;178:1–7. doi: 10.1016/j.colsurfb.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Li X., Guo T., Lachmanski L., Manoli F., Menendez-Miranda M., Manet I. Cyclodextrin-based metal-organic frameworks particles as efficient carriers for lansoprazole: study of morphology and chemical composition of individual particles. Int J Pharm. 2017;531:424–432. doi: 10.1016/j.ijpharm.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 68.Hu X., Wang C., Wang L., Liu Z., Wu L., Zhang G. Nanoporous CD-MOF particles with uniform and inhalable size for pulmonary delivery of budesonide. Int J Pharm. 2019;564:153–161. doi: 10.1016/j.ijpharm.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 69.Sun K., Li L., Yu X., Liu L., Meng Q., Wang F. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J Colloid Interface Sci. 2017;486:128–135. doi: 10.1016/j.jcis.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 70.Simon M.A., Anggraeni E., Soetaredjo F.E., Santoso S.P., Irawaty W., Thanh T.C. Hydrothermal synthesize of hf-free MIL-100(Fe) for isoniazid-drug delivery. Sci Rep. 2019;9:16907. doi: 10.1038/s41598-019-53436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taherzade S.D., Soleimannejad J., Tarlani A. Application of metal-organic framework nano-MIL-100(Fe) for sustainable release of doxycycline and tetracycline. Nanomaterials. 2017;7:215. doi: 10.3390/nano7080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rezaei M., Abbasi A., Varshochian R., Dinarvand R., Jeddi-Tehrani M. NanoMIL-100(Fe) containing docetaxel for breast cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46:1390–1401. doi: 10.1080/21691401.2017.1369425. [DOI] [PubMed] [Google Scholar]
- 73.Taylor-Pashow K.M., Della Rocca J., Xie Z., Tran S., Lin W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J Am Chem Soc. 2009;131:14261–14263. doi: 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cunha D., Ben Yahia M., Hall S., Miller S.R., Chevreau H., Elkaim E. Rationale of drug encapsulation and release from biocompatible porous metal-organic frameworks. Chem Mater. 2013;25:2767–2776. [Google Scholar]
- 75.Sun C.Y., Qin C., Wang C.G., Su Z.M., Wang S., Wang X.L. Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Adv Mater. 2011;23:5629–5632. doi: 10.1002/adma.201102538. [DOI] [PubMed] [Google Scholar]
- 76.Oh H., Li T., An J. Drug release properties of a series of adenine-based metal-organic frameworks. Chemistry. 2015;21:17010–17015. doi: 10.1002/chem.201501560. [DOI] [PubMed] [Google Scholar]
- 77.Sun C.Y., Qin C., Wang X.L., Yang G.S., Shao K.Z., Lan Y.Q. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012;41:6906–6909. doi: 10.1039/c2dt30357d. [DOI] [PubMed] [Google Scholar]
- 78.Gao H., Zhang Y., Chi B., Lin C., Tian F., Xu M. Synthesis of 'dual-key-and-lock' drug carriers for imaging and improved drug release. Nanotechnology. 2020;31:445102. doi: 10.1088/1361-6528/aba65a. [DOI] [PubMed] [Google Scholar]
- 79.He C., Lu K., Liu D., Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled sirnas to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136:5181–5184. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W., Guo T., Wang C., He Y., Zhang X., Li G. MOF capacitates cyclodextrin to mega-load mode for high-efficient delivery of valsartan. Pharm Res (N Y) 2019;36:117. doi: 10.1007/s11095-019-2650-3. [DOI] [PubMed] [Google Scholar]
- 81.Férey G., Mellot-Draznieks C., Serre C., Millange F. Crystallized frameworks with giant pores: are there limits to the possible? Acc Chem Res. 2005;38:217–225. doi: 10.1021/ar040163i. [DOI] [PubMed] [Google Scholar]
- 82.Horcajada P., Serre C., Vallet-Regí M., Sebban M., Taulelle F., Férey G. Metal-organic frameworks as efficient materials for drug delivery. Angew Chem Int Ed Engl. 2006;45:5974–5978. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- 83.Xing K., Fan R., Wang F., Nie H., Du X., Gai S. Dual-stimulus-triggered programmable drug release and luminescent ratiometric pH sensing from chemically stable biocompatible zinc metal-organic framework. ACS Appl Mater Interfaces. 2018;10:22746–22756. doi: 10.1021/acsami.8b06270. [DOI] [PubMed] [Google Scholar]
- 84.Zheng H., Zhang Y., Liu L., Wan W., Guo P., Nyström A.M. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138:962–968. doi: 10.1021/jacs.5b11720. [DOI] [PubMed] [Google Scholar]
- 85.Wu Q., Niu M., Chen X., Tan L., Fu C., Ren X. Biocompatible and biodegradable zeolitic imidazolate framework/polydopamine nanocarriers for dual stimulus triggered tumor thermo-chemotherapy. Biomaterials. 2018;162:132–143. doi: 10.1016/j.biomaterials.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Sun Q., Bi H., Wang Z., Li C., Wang X., Xu J. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials. 2019;223:119473. doi: 10.1016/j.biomaterials.2019.119473. [DOI] [PubMed] [Google Scholar]
- 87.Cavka J.H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc. 2008;130:13850–13851. doi: 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- 88.Lee D.B., Roberts M., Bluchel C.G., Odell R.A. Zirconium: biomedical and nephrological applications. Asaio J. 2010;56:550–556. doi: 10.1097/MAT.0b013e3181e73f20. [DOI] [PubMed] [Google Scholar]
- 89.Röder R., Preiß T., Hirschle P., Steinborn B., Zimpel A., Höhn M. Multifunctional nanoparticles by coordinative self-assembly of his-tagged units with metal-organic frameworks. J Am Chem Soc. 2017;139:2359–2368. doi: 10.1021/jacs.6b11934. [DOI] [PubMed] [Google Scholar]
- 90.Abánades Lázaro I., Haddad S., Rodrigo-Muñoz J.M., Marshall R.J., Sastre B., Del Pozo V. Surface-functionalization of Zr-fumarate MOF for selective cytotoxicity and immune system compatibility in nanoscale drug delivery. ACS Appl Mater Interfaces. 2018;10:31146–31157. doi: 10.1021/acsami.8b11652. [DOI] [PubMed] [Google Scholar]
- 91.Smaldone R.A., Forgan R.S., Furukawa H., Gassensmith J.J., Slawin A.M., Yaghi O.M. Metal-organic frameworks from edible natural products. Angew Chem Int Ed Engl. 2010;49:8630–8634. doi: 10.1002/anie.201002343. [DOI] [PubMed] [Google Scholar]
- 92.Han Y., Liu W., Huang J., Qiu S., Zhong H., Liu D. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics. 2018;10:271. doi: 10.3390/pharmaceutics10040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kritskiy I., Volkova T., Surov A., Terekhova I. γ-Cyclodextrin-metal organic frameworks as efficient microcontainers for encapsulation of leflunomide and acceleration of its transformation into teriflunomide. Carbohydr Polym. 2019;216:224–230. doi: 10.1016/j.carbpol.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 94.He Y., Xu J., Sun X., Ren X., Maharjan A., York P. Cuboidal tethered cyclodextrin frameworks tailored for hemostasis and injured vessel targeting. Theranostics. 2019;9:2489–2504. doi: 10.7150/thno.31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKinlay A.C., Morris R.E., Horcajada P., Férey G., Gref R., Couvreur P. BioMOFs: metal-organic frameworks for biological and medical applications. Angew Chem Int Ed Engl. 2010;49:6260–6266. doi: 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]
- 96.Li Y., Li X., Guan Q., Zhang C., Xu T., Dong Y. Strategy for chemotherapeutic delivery using a nanosized porous metal-organic framework with a central composite design. Int J Nanomed. 2017;12:1465–1474. doi: 10.2147/IJN.S119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao J., Zhu Y., Huddleston S., Li P., Xiao B., Farha O.K. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano. 2018;12:1023–1032. doi: 10.1021/acsnano.7b01850. [DOI] [PubMed] [Google Scholar]
- 98.Hou L., Qin Y., Li J., Qin S., Huang Y., Lin T. A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens Bioelectron. 2019;143:111605. doi: 10.1016/j.bios.2019.111605. [DOI] [PubMed] [Google Scholar]
- 99.Jo J.H., Kim H.C., Huh S., Kim Y., Lee D.N. Antibacterial activities of Cu-MOFs containing glutarate and bipyridyl ligands. Dalton Trans. 2019;48:8084–8093. doi: 10.1039/c9dt00791a. [DOI] [PubMed] [Google Scholar]
- 100.Lu W., Wei Z., Gu Z.Y., Liu T.F., Park J., Park J. Tuning the structure and function of metal-organic frameworks via linker design. Chem Soc Rev. 2014;43:5561–5593. doi: 10.1039/c4cs00003j. [DOI] [PubMed] [Google Scholar]
- 101.Simon-Yarza T., Mielcarek A., Couvreur P., Serre C. Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv Mater. 2018;30 doi: 10.1002/adma.201707365. [DOI] [PubMed] [Google Scholar]
- 102.Bala S., Bhattacharya S., Goswami A., Adhikary A., Konar S., Mondal R. Designing functional metal-organic frameworks by imparting a hexanuclear copper based secondary building unit (sbu) specific properties: structural correlation with magnetic and photocatalytic activity. Cryst Growth Des. 2014;14:6391–6398. [Google Scholar]
- 103.Gimenez-Marques M., Hidalgo T., Serre C., Horcajada P. Nanostructured metal-organic frameworks and their bio-related applications. Coord Chem Rev. 2016;307:342–360. [Google Scholar]
- 104.Xu H., Cai J., Xiang S., Zhang Z., Wu C., Rao X. A cationic microporous metal-organic framework for highly selective separation of small hydrocarbons at room temperature. J Mater Chem. 2013;1:9916–9921. [Google Scholar]
- 105.Gramaccioli C.M. The crystal structure of zinc glutamate dihydrate. Acta Crystallogr. 1966;21:600–605. doi: 10.1107/s0365110x66003529. [DOI] [PubMed] [Google Scholar]
- 106.Imaz I., Rubio-Martínez M., An J., Solé-Font I., Rosi N.L., Maspoch D. Metal-biomolecule frameworks (MBioFs) Chem Commun. 2011;47:7287–7302. doi: 10.1039/c1cc11202c. [DOI] [PubMed] [Google Scholar]
- 107.Miller S.R., Heurtaux D., Baati T., Horcajada P., Grenèche J.M., Serre C. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. Chem Commun. 2010;46:4526–4528. doi: 10.1039/c001181a. [DOI] [PubMed] [Google Scholar]
- 108.Levine D.J., Runčevski T., Kapelewski M.T., Keitz B.K., Oktawiec J., Reed D.A. Olsalazine-based metal-organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. J Am Chem Soc. 2016;138:10143–10150. doi: 10.1021/jacs.6b03523. [DOI] [PubMed] [Google Scholar]
- 109.Rieter W.J., Pott K.M., Taylor K.M., Lin W. Nanoscale coordination polymers for platinum-based anticancer drug delivery. J Am Chem Soc. 2008;130:11584–11585. doi: 10.1021/ja803383k. [DOI] [PubMed] [Google Scholar]
- 110.Stock N., Biswas S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev. 2012;112:933–969. doi: 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- 111.Kumar S., Jain S., Nehra M., Dilbaghi N., Kim K.H. Green synthesis of metal-organic frameworks: a state-of-the-art review of potential environmental and medical applications. Coord Chem Rev. 2020;420:213407. [Google Scholar]
- 112.Jhung S.H., Lee J.H., Forster P.M., Férey G., Cheetham A.K., Chang J.S. Microwave synthesis of hybrid inorganic-organic porous materials: phase-selective and rapid crystallization. Chemistry. 2006;12:7899–7905. doi: 10.1002/chem.200600270. [DOI] [PubMed] [Google Scholar]
- 113.Mendes R.F., Rocha J., Paz F.A.A. Elsevier; Amsterdam: 2020. Microwave synthesis of metal-organic frameworks. Metal-organic frameworks for biomedical applications; pp. 159–176. [Google Scholar]
- 114.Rieter W.J., Taylor K.M., An H., Lin W., Lin W. Nanoscale metal-organic frameworks as potential multimodal contrast enhancing agents. J Am Chem Soc. 2006;128:9024–9025. doi: 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun C.Y., Qin C., Wang X.L., Su Z.M. Metal-organic frameworks as potential drug delivery systems. Expet Opin Drug Deliv. 2013;10:89–101. doi: 10.1517/17425247.2013.741583. [DOI] [PubMed] [Google Scholar]
- 116.Taylor K.M., Jin A., Lin W. Surfactant-assisted synthesis of nanoscale gadolinium metal-organic frameworks for potential multimodal imaging. Angew Chem Int Ed Engl. 2008;47:7722–7725. doi: 10.1002/anie.200802911. [DOI] [PubMed] [Google Scholar]
- 117.Sun Y., Zhou H.C. Recent progress in the synthesis of metal-organic frameworks. Sci Technol Adv Mater. 2015;16 doi: 10.1088/1468-6996/16/5/054202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Das A.K., Vemuri R.S., Kutnyakov I., McGrail B.P., Motkuri R.K. An efficient synthesis strategy for metal-organic frameworks: dry-gel synthesis of MOF-74 framework with high yield and improved performance. Sci Rep. 2016;6:28050. doi: 10.1038/srep28050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juan-Alcaiz J., Gascon J., Kapteijn F. Metal-organic frameworks as scaffolds for the encapsulation of active species: state of the art and future perspectives. ChemInform. 2012;22:10102–10118. [Google Scholar]
- 120.Pichon A., James S.L. An array-based study of reactivity under solvent-free mechanochemical conditions—insights and trends. CrystEngComm. 2008;10:1839–1847. [Google Scholar]
- 121.Pichon A., Lazuen-Garay A., James S.L. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm. 2006;8:211–214. [Google Scholar]
- 122.Lee Y.R., Kim J., Ahn W.S. Synthesis of metal-organic frameworks: a mini review. Kor J Chem Eng. 2013;30:1667–1680. [Google Scholar]
- 123.Carné A., Carbonell C., Imaz I., Maspoch D. Nanoscale metal-organic materials. Chem Soc Rev. 2011;40:291–305. doi: 10.1039/c0cs00042f. [DOI] [PubMed] [Google Scholar]
- 124.Shekhah O., Wang H., Kowarik S., Schreiber F., Paulus M., Tolan M. Step-by-step route for the synthesis of metal-organic frameworks. J Am Chem Soc. 2007;129:15118–15119. doi: 10.1021/ja076210u. [DOI] [PubMed] [Google Scholar]
- 125.Banerjee R., Phan A., Wang B., Knobler C., Furukawa H., O'Keeffe M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science. 2008;319:939–943. doi: 10.1126/science.1152516. [DOI] [PubMed] [Google Scholar]
- 126.Tranchemontagne D.J., Hunt J.R., Yaghi O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron. 2008;64:8553–8557. [Google Scholar]
- 127.Cravillon J., MüNzer S., Lohmeier S.J., Feldhoff A., Huber K., Wiebcke M. Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical zeolitic imidazolate framework. Chem Mater. 2009;21:1410–1412. [Google Scholar]
- 128.Forgan R.S., Smaldone R.A., Gassensmith J.J., Furukawa H., Cordes D.B., Li Q. Nanoporous carbohydrate metal-organic frameworks. J Am Chem Soc. 2012;134:406–417. doi: 10.1021/ja208224f. [DOI] [PubMed] [Google Scholar]
- 129.Liu B., Li H., Xu X., Li X., Lv N., Singh V. Optimized synthesis and crystalline stability of γ-cyclodextrin metal-organic frameworks for drug adsorption. Int J Pharm. 2016;514:212–219. doi: 10.1016/j.ijpharm.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 130.Lu H.J., Yang X.N., Li S.X., Zhang Y., Sha J.Q., Li C.D. Study on a new cyclodextrin based metal-organic framework with chiral helices. Inorg Chem Commun. 2015;61:48–52. [Google Scholar]
- 131.Sha J., Yang X., Sun L., Zhang X., Li S., Li J. Unprecedented α-cyclodextrin metal-organic frameworks with chirality: structure and drug adsorptions. Polyhedron. 2017;127:396–402. [Google Scholar]
- 132.Eddaoudi M., Kim J., Rosi N., Vodak D., Wachter J., O'Keeffe M. Systematic design of pore size and functionality in isoreticular mofs and their application in methane storage. Science. 2002;295:469–472. doi: 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- 133.Ding H.Y., Wu L., Guo T., Zhang Z.Y., Garba B.M., Gao G. CD-MOFs crystal transformation from dense to highly porous form for efficient drug loading. Cryst Growth Des. 2019;19:3888–3894. [Google Scholar]
- 134.John N.S., Scherb C., Shöâeè M., Anderson M.W., Attfield M.P., Bein T. Single layer growth of sub-micron metal-organic framework crystals observed by in situ atomic force microscopy. Chem Commun. 2009:6294–6296. doi: 10.1039/b908299a. [DOI] [PubMed] [Google Scholar]
- 135.Bang J.H., Suslick K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater. 2010;22:1039–1059. doi: 10.1002/adma.200904093. [DOI] [PubMed] [Google Scholar]
- 136.Ni Z., Masel R.I. Rapid production of metal-organic frameworks via microwave-assisted solvothermal synthesis. J Am Chem Soc. 2006;128:12394–12395. doi: 10.1021/ja0635231. [DOI] [PubMed] [Google Scholar]
- 137.Jhung S.H., Lee J.H., Chang J.S. Microwave synthesis of a nanoporous hybrid material, chromium trimesate. Bull Kor Chem Soc. 2005;26:880–881. [Google Scholar]
- 138.Agostoni V., Horcajada P., Rodriguez-Ruiz V., Willaime H., Couvreur P., Serre C. ‘Green’ fluorine-free mesoporous iron(III) trimesate nanoparticles for drug delivery. Green Mater. 2013;1:209–217. [Google Scholar]
- 139.Chalati T., Horcajada P., Gref R., Couvreur P., Serre C. Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A. J Mater Chem. 2011;21:2220–2227. [Google Scholar]
- 140.Thi Dang Y., Hoang H.T., Dong H.C., Bui K.-B.T., Nguyen L.H.T., Phan T.B. Microwave-assisted synthesis of nano Hf- and Zr-based metal-organic frameworks for enhancement of curcumin adsorption. Microporous Mesoporous Mater. 2020;298:110064. [Google Scholar]
- 141.Fu C., Zhou H., Tan L., Huang Z., Wu Q., Ren X. Microwave-activated mn-doped zirconium metal-organic framework nanocubes for highly effective combination of microwave dynamic and thermal therapies against cancer. ACS Nano. 2018;12:2201–2210. doi: 10.1021/acsnano.7b08868. [DOI] [PubMed] [Google Scholar]
- 142.Ranjbar M., Pardakhty A., Amanatfard A., Asadipour A. Efficient drug delivery of β-estradiol encapsulated in Zn-metal-organic framework nanostructures by microwave-assisted coprecipitation method. Drug Des Dev Ther. 2018;12:2635–2643. doi: 10.2147/DDDT.S173324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.George P., Das R.K., Chowdhury P. Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of curcumin. Microporous Mesoporous Mater. 2019;281:161–171. [Google Scholar]
- 144.Liu B.T., He Y.P., Han L.P., Singh V., Xu X.N., Guo T. Microwave-assisted rapid synthesis of gamma-cyclodextrin metal-organic frameworks for size control and efficient drug loading. Cryst Growth Des. 2017;17:1654–1660. [Google Scholar]
- 145.Schlesinger M., Schulze S., Hietschold M., Mehring M. Evaluation of synthetic methods for microporous metal-organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H2O)3] and [Cu2(btc)(OH)(H2O)] Microporous Mesoporous Mater. 2010;132:121–127. [Google Scholar]
- 146.Park H.J., Jeong D.H., Noh S.G., Lim H.J., Park J.Y. Identification of secondary chemistry teachers' ability to carry-out experimentation. J Kor Chem Soc. 2009;53:765–773. [Google Scholar]
- 147.Aguiar L.W., Otto G.P., Kupfer V.L., Fávaro S.L., Silva C.T.P., Moisés M.P. Simple, fast, and low-cost synthesis of MIL-100 and MIL-88B in a modified domestic microwave oven. Mater Lett. 2020;276:128127. [Google Scholar]
- 148.Jones W.D., Kosar W.P. Carbon-hydrogen bond activation by ruthenium for the catalytic synthesis of indoles. J Am Chem Soc. 1986;108:5640–5641. [Google Scholar]
- 149.Li Z.Q., Qiu L.G., Xu T., Wu Y., Wang W., Wu Z.Y. Ultrasonic synthesis of the microporous metal-organic framework Cu3(BTC)2 at ambient temperature and pressure: an efficient and environmentally friendly method. Mater Lett. 2009;63:78–80. [Google Scholar]
- 150.Son W.J., Kim J., Kim J., Ahn W.S. Sonochemical synthesis of MOF-5. Chem Commun. 2008:6336–6338. doi: 10.1039/b814740j. [DOI] [PubMed] [Google Scholar]
- 151.Yang D.A., Cho H.Y., Kim J., Yang S.T., Ann W.S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ Sci. 2012;5:6465–6473. [Google Scholar]
- 152.Kim J., Yang S.T., Choi S.B., Sim J., Kim J., Ahn W.S. Control of catenation in CuTATB-n metal-organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J Mater Chem. 2011;21:3070–3076. [Google Scholar]
- 153.Hernández J.G., Friscic T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015;56:4253–4265. [Google Scholar]
- 154.Friscic T., Halasz I., Strukil V., Eckert-Maksic M., Dinnebier R.E. Clean and efficient synthesis using mechanochemistry: coordination polymers, metal-organic frameworks and metallodrugs. Croat Chem Acta. 2012;85:367–378. [Google Scholar]
- 155.Bennett T.D., Cao S., Tan J.C., Keen D.A., Bithell E.G., Beldon P.J. Facile mechanosynthesis of amorphous zeolitic imidazolate frameworks. J Am Chem Soc. 2011;133:14546–14549. doi: 10.1021/ja206082s. [DOI] [PubMed] [Google Scholar]
- 156.Bennett T.D., Simoncic P., Moggach S.A., Gozzo F., Macchi P., Keen D.A. Reversible pressure-induced amorphization of a zeolitic imidazolate framework (ZIF-4) Chem Commun. 2011;47:7983–7985. doi: 10.1039/c1cc11985k. [DOI] [PubMed] [Google Scholar]
- 157.Bennett T.D., Keen D.A., Tan J.C., Barney E.R., Goodwin A.L., Cheetham A.K. Thermal amorphization of zeolitic imidazolate frameworks. Angew Chem Int Ed Engl. 2011;50:3067–3071. doi: 10.1002/anie.201007303. [DOI] [PubMed] [Google Scholar]
- 158.Mueller U, Puetter H, Hesse M, Wessel H, Schubert M, Huff J, et al., inventors; BASF Aktiengesellschaft, BASF AG, assignees. Method for electrochemical production of a crystalline porous metal organic skeleton material. United States patent US No. 2011105776. 2011 May 5.
- 159.Joaristi A.M., Juan-Alcaiz J., Serra-Crespo P., Kapteijn F., Gascon J. Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ metal organic frameworks. Cryst Growth Des. 2012;12:3489–3498. [Google Scholar]
- 160.Sun S., Murray C.B., Weller D., Folks L., Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 161.Mokari T., Zhang M., Yang P. Shape, size, and assembly control of PbTe nanocrystals. J Am Chem Soc. 2007;129:9864–9865. doi: 10.1021/ja074145i. [DOI] [PubMed] [Google Scholar]
- 162.Peng X., Manna L., Yang W., Wickham J., Scher E., Kadavanich A. Shape control of CdSe nanocrystals. Nature. 2000;404:59–61. doi: 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- 163.Manna L., Milliron D.J., Meisel A., Scher E.C., Alivisatos A.P. Controlled growth of tetrapod-branched inorganic nanocrystals. Nat Mater. 2003;2:382–385. doi: 10.1038/nmat902. [DOI] [PubMed] [Google Scholar]
- 164.Truong N.P., Whittaker M.R., Mak C.W., Davis T.P. The importance of nanoparticle shape in cancer drug delivery. Expet Opin Drug Deliv. 2015;12:129–142. doi: 10.1517/17425247.2014.950564. [DOI] [PubMed] [Google Scholar]
- 165.Seoane B., Dikhtiarenko A., Mayoral A., Tellez C., Coronas J., Kapteijn F. Metal organic framework synthesis in the presence of surfactants: towards hierarchical MOFs? CrystEngComm. 2015;17:1693–1700. doi: 10.1039/c4ce02324b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qiu L.G., Li Z.Q., Wu Y., Wang W., Xu T., Jiang X. Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines. Chem Commun. 2008:3642–3644. doi: 10.1039/b804126a. [DOI] [PubMed] [Google Scholar]
- 167.Morris W., Wang S., Cho D., Auyeung E., Li P., Farha O.K. Role of modulators in controlling the colloidal stability and polydispersity of the UiO-66 metal-organic framework. ACS Appl Mater Interfaces. 2017;9:33413–33418. doi: 10.1021/acsami.7b01040. [DOI] [PubMed] [Google Scholar]
- 168.Gutov O.V., Molina S., Escudero-Adán E.C., Shafir A. Modulation by amino acids: toward superior control in the synthesis of zirconium metal-organic frameworks. Chemistry. 2016;22:13582–13587. doi: 10.1002/chem.201600898. [DOI] [PubMed] [Google Scholar]
- 169.Xu X., Lu Y., Yang Y., Nosheen F., Wang X. Tuning the growth of metal-organic framework nanocrystals by using polyoxometalates as coordination modulators. Sci China Mater. 2015;58:370–377. [Google Scholar]
- 170.Rosnes M.H., Nesse F.S., Opitz M., Dietzel P.D.C. Morphology control in modulated synthesis of metal-organic framework CPO-27. Microporous Mesoporous Mater. 2019;275:207–213. [Google Scholar]
- 171.Bentz K.C., Ayala S., Kalaj M., Cohen S.M. Polyacids as modulators for the synthesis of UiO-66. Aust J Chem. 2019;72:848–851. [Google Scholar]
- 172.Müller K., Singh Malhi J., Wohlgemuth J., Fischer R.A., Wöll C., Gliemann H. Water as a modulator in the synthesis of surface-mounted metal-organic framework films of type HKUST-1. Dalton Trans. 2018;47:16474–16479. doi: 10.1039/c8dt03310b. [DOI] [PubMed] [Google Scholar]
- 173.Abánades Lázaro I., Wells C.J.R., Forgan R.S. Multivariate modulation of the Zr MOF UiO-66 for defect-controlled combination anticancer drug delivery. Angew Chem Int Ed Engl. 2020;59:5211–5217. doi: 10.1002/anie.201915848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Horcajada P., Serre C., Grosso D., Boissiere C., Perruchas S., Sanchez C. Colloidal route for preparing optical thin films of nanoporous metal-organic frameworks. Adv Mater. 2010;21:1931–1935. [Google Scholar]
- 175.Reineke T.M., Eddaoudi M., Fehr M., Kelley D., Yaghi O.M. From condensed lanthanide coordination solids to microporous frameworks having accessible metal sites. J Am Chem Soc. 2015;121:1651–1657. [Google Scholar]
- 176.Kerbellec N., Catala L., Daiguebonne C., Gloter A., Stephan O., Bünzli J.C. Luminescent coordination nanoparticles. New J Chem. 2008;32:584–587. [Google Scholar]
- 177.Cho W., Lee H.J., Oh M. Growth-controlled formation of porous coordination polymer particles. J Am Chem Soc. 2008;130:16943–16946. doi: 10.1021/ja8039794. [DOI] [PubMed] [Google Scholar]
- 178.Hatakeyama W., Sanchez T.J., Rowe M.D., Serkova N.J., Liberatore M.W., Boyes S.G. Synthesis of gadolinium nanoscale metal-organic framework with hydrotropes: manipulation of particle size and magnetic resonance imaging capability. ACS Appl Mater Interfaces. 2011;3:1502–1510. doi: 10.1021/am200075q. [DOI] [PubMed] [Google Scholar]
- 179.Bhat M., Gaikar V.G. Characterization of interaction between butylbenzene sulfonates and cetyl pyridinium chloride in a mixed aggregate system. Langmuir. 2000;16:1580–1592. [Google Scholar]
- 180.Garay A.L., Pichon A., James S.L. Solvent-free synthesis of metal complexes. Chem Soc Rev. 2007;36:846–855. doi: 10.1039/b600363j. [DOI] [PubMed] [Google Scholar]
- 181.Friscic Tomislav. New opportunities for materials synthesis using mechanochemistry. J Mater Chem. 2010;20:7599–7605. [Google Scholar]
- 182.Furukawa Y., Ishiwata T., Sugikawa K., Kokado K., Sada K. Nano- and microsized cubic gel particles from cyclodextrin metal-organic frameworks. Angew Chem Int Ed Engl. 2012;51:10566–10569. doi: 10.1002/anie.201204919. [DOI] [PubMed] [Google Scholar]
- 183.Qiu C., Wang J., Qin Y., Fan H., Xu X., Jin Z. Green synthesis of cyclodextrin-based metal-organic frameworks through the seed-mediated method for the encapsulation of hydrophobic molecules. J Agric Food Chem. 2018;66:4244–4250. doi: 10.1021/acs.jafc.8b00400. [DOI] [PubMed] [Google Scholar]
- 184.Li H., Lv N., Li X., Liu B., Feng J., Ren X. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale. 2017;9:7454–7463. doi: 10.1039/c6nr07593b. [DOI] [PubMed] [Google Scholar]
- 185.Kritskiy I., Volkova T., Sapozhnikova T., Mazur A., Tolstoy P., Terekhova I. Methotrexate-loaded metal-organic frameworks on the basis of γ-cyclodextrin: design, characterization, in vitro and in vivo investigation. Mater Sci Eng C Mater Biol Appl. 2020;111:110774. doi: 10.1016/j.msec.2020.110774. [DOI] [PubMed] [Google Scholar]
- 186.Zhuang J., Kuo C.H., Chou L.Y., Liu D.Y., Weerapana E., Tsung C.K. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8:2812–2819. doi: 10.1021/nn406590q. [DOI] [PubMed] [Google Scholar]
- 187.Chen X., Tong R., Shi Z., Yang B., Liu H., Ding S. MOF nanoparticles with encapsulated autophagy inhibitor in controlled drug delivery system for antitumor. ACS Appl Mater Interfaces. 2018;10:2328–2337. doi: 10.1021/acsami.7b16522. [DOI] [PubMed] [Google Scholar]
- 188.Noorian S.A., Hemmatinejad N., Navarro J.A.R. BioMOF@cellulose fabric composites for bioactive molecule delivery. J Inorg Biochem. 2019;201:110818. doi: 10.1016/j.jinorgbio.2019.110818. [DOI] [PubMed] [Google Scholar]
- 189.Lyu F., Zhang Y., Zare R.N., Ge J., Liu Z. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett. 2014;14:5761–5765. doi: 10.1021/nl5026419. [DOI] [PubMed] [Google Scholar]
- 190.Wu X., Ge J., Yang C., Hou M., Liu Z. Facile synthesis of multiple enzyme-containing metal-organic frameworks in a biomolecule-friendly environment. Chem Commun. 2015;51:13408–13411. doi: 10.1039/c5cc05136c. [DOI] [PubMed] [Google Scholar]
- 191.Vassaki M., Papathanasiou K.E., Hadjicharalambous C., Chandrinou D., Turhanen P., Choquesillo-Lazarte D. Self-sacrificial MOFs for ultra-long controlled release of bisphosphonate anti-osteoporotic drugs. Chem Commun. 2020;56:5166–5169. doi: 10.1039/d0cc00439a. [DOI] [PubMed] [Google Scholar]
- 192.Devautour-Vinot S., Martineau C., Diaby S., Ben-Yahia M., Miller S., Serre C. Caffeine confinement into a series of functionalized porous zirconium MOFs: a joint experimental/modeling exploration. J Phys Chem C. 2013;117:11694–11704. [Google Scholar]
- 193.Javanbakht S., Nezhad-Mokhtari P., Shaabani A., Arsalani N., Ghorbani M. Incorporating Cu-based metal-organic framework/drug nanohybrids into gelatin microsphere for ibuprofen oral delivery. Mater Sci Eng C Mater Biol Appl. 2019;96:302–309. doi: 10.1016/j.msec.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 194.He Y., Hou X., Guo J., He Z., Guo T., Liu Y. Activation of a gamma-cyclodextrin-based metal-organic framework using supercritical carbon dioxide for high-efficient delivery of honokiol. Carbohydr Polym. 2020;235:115935. doi: 10.1016/j.carbpol.2020.115935. [DOI] [PubMed] [Google Scholar]
- 195.Simon-Yarza T., Giménez-Marqués M., Mrimi R., Mielcarek A., Gref R., Horcajada P. A smart metal-organic framework nanomaterial for lung targeting. Angew Chem Int Ed Engl. 2017;56:15565–15569. doi: 10.1002/anie.201707346. [DOI] [PubMed] [Google Scholar]
- 196.Noorian S.A., Hemmatinejad N., Navarro J.A.R. Bioactive molecule encapsulation on metal-organic framework via simple mechanochemical method for controlled topical drug delivery systems. Microporous Mesoporous Mater. 2020;302:110199. [Google Scholar]
- 197.Wang Z., Cohen S.M. Postsynthetic modification of metal-organic frameworks. Chem Soc Rev. 2009;38:1315–1329. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]
- 198.Morris W., Briley W.E., Auyeung E., Cabezas M.D., Mirkin C.A. Nucleic acid–metal organic framework (MOF) nanoparticle conjugates. J Am Chem Soc. 2014;136:7261–7264. doi: 10.1021/ja503215w. [DOI] [PubMed] [Google Scholar]
- 199.Cao S.L., Yue D.M., Li X.H., Smith T.J., Li N., Zong M.H. Novel nano-/micro-biocatalyst: soybean epoxide hydrolase immobilized on UiO-66-NH2 MOF for efficient biosynthesis of enantiopure (R)-1,2-octanediol in deep eutectic solvents. ACS Sustain Chem Eng. 2016;4:3586–3595. [Google Scholar]
- 200.Cunha D., Gaudin C., Colinet I., Horcajada P., Maurin G., Serre C. Rationalization of the entrapping of bioactive molecules into a series of functionalized porous zirconium terephthalate MOFs. J Mater Chem B. 2013;1:1101–1108. doi: 10.1039/c2tb00366j. [DOI] [PubMed] [Google Scholar]
- 201.Xu Z., Li M., Li X., Liu X., Ma F., Wu S. Antibacterial activity of silver doped titanate nanowires on Ti implants. ACS Appl Mater Interfaces. 2016;8:16584–16594. doi: 10.1021/acsami.6b04161. [DOI] [PubMed] [Google Scholar]
- 202.Xu H., Zhang G., Xu K., Wang L., Yu L., Xing M.M.Q. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater Sci Eng C Mater Biol Appl. 2018;90:379–386. doi: 10.1016/j.msec.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 203.Lin S., Liu X., Tan L., Cui Z., Yang X., Yeung K.W.K. Porous iron-carboxylate metal-organic framework: a novel bioplatform with sustained antibacterial efficacy and nontoxicity. ACS Appl Mater Interfaces. 2017;9:19248–19257. doi: 10.1021/acsami.7b04810. [DOI] [PubMed] [Google Scholar]
- 204.Gallis D.F.S., Butler K.S., Agola J.O., Pearce C.J., McBride A.A. Antibacterial countermeasures via metal-organic framework-supported sustained therapeutic release. ACS Appl Mater Interfaces. 2019;11:7782–7791. doi: 10.1021/acsami.8b21698. [DOI] [PubMed] [Google Scholar]
- 205.Zhang X., Liu L., Huang L., Zhang W., Wang R., Yue T. The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale. 2019;11:9468–9477. doi: 10.1039/c9nr01284b. [DOI] [PubMed] [Google Scholar]
- 206.Guo A., Durymanov M., Permyakova A., Sene S., Serre C., Reineke J. Metal organic framework (MOF) particles as potential bacteria-mimicking delivery systems for infectious diseases: characterization and cellular internalization in alveolar macrophages. Pharm Res (N Y) 2019;36:53. doi: 10.1007/s11095-019-2589-4. [DOI] [PubMed] [Google Scholar]
- 207.Yu M., You D., Zhuang J., Lin S., Dong L., Weng S. Controlled release of naringin in metal-organic framework-loaded mineralized collagen coating to simultaneously enhance osseointegration and antibacterial activity. ACS Appl Mater Interfaces. 2017;9:19698–19705. doi: 10.1021/acsami.7b05296. [DOI] [PubMed] [Google Scholar]
- 208.Wang S., Wang Y., Peng Y., Yang X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS Appl Mater Interfaces. 2019;11:8461–8469. doi: 10.1021/acsami.8b22143. [DOI] [PubMed] [Google Scholar]
- 209.Tran C.D., Prosenc F., Franko M., Benzi G. One-pot synthesis of biocompatible silver nanoparticle composites from cellulose and keratin: characterization and antimicrobial activity. ACS Appl Mater Interfaces. 2016;8:34791–34801. doi: 10.1021/acsami.6b14347. [DOI] [PubMed] [Google Scholar]
- 210.Shakya S., He Y., Ren X., Guo T., Maharjan A., Luo T. Ultrafine silver nanoparticles embedded in cyclodextrin metal-organic frameworks with GRGDS functionalization to promote antibacterial and wound healing application. Small. 2019;15 doi: 10.1002/smll.201901065. [DOI] [PubMed] [Google Scholar]
- 211.Yang Y., Wu X., He C., Huang J., Yin S., Zhou M. Metal-organic framework/Ag-based hybrid nanoagents for rapid and synergistic bacterial eradication. ACS Appl Mater Interfaces. 2020;12:13698–13708. doi: 10.1021/acsami.0c01666. [DOI] [PubMed] [Google Scholar]
- 212.Mohamed N.A., Davies R.P., Lickiss P.D., Ahmetaj-Shala B., Reed D.M., Gashaw H.H. Chemical and biological assessment of metal organic frameworks (MOFs) in pulmonary cells and in an acute in vivo model: relevance to pulmonary arterial hypertension therapy. Pulm Circ. 2017;7:643–653. doi: 10.1177/2045893217710224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wyszogrodzka-Gaweł G., Dorożyński P., Giovagnoli S., Strzempek W., Pesta E., Węglarz W.P. An inhalable theranostic system for local tuberculosis treatment containing an isoniazid loaded metal organic framework Fe-MIL-101-NH2-from raw MOF to drug delivery system. Pharmaceutics. 2019;11:687. doi: 10.3390/pharmaceutics11120687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wyszogrodzka G., Dorożyński P., Gil B., Roth W.J., Strzempek M., Marszałek B. Iron-based metal-organic frameworks as a theranostic carrier for local tuberculosis therapy. Pharm Res (N Y) 2018;35:144. doi: 10.1007/s11095-018-2425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Duan Y., Ye F., Huang Y., Qin Y., He C., Zhao S. One-pot synthesis of a metal-organic framework-based drug carrier for intelligent glucose-responsive insulin delivery. Chem Commun. 2018;54:5377–5380. doi: 10.1039/c8cc02708k. [DOI] [PubMed] [Google Scholar]
- 216.Yang X.X., Feng P., Cao J., Liu W., Tang Y. Composition-engineered metal-organic framework-based microneedles for glucose-mediated transdermal insulin delivery. ACS Appl Mater Interfaces. 2020;12:13613–13621. doi: 10.1021/acsami.9b20774. [DOI] [PubMed] [Google Scholar]
- 217.Kim S.N., Park C.G., Huh B.K., Lee S.H., Min C.H., Lee Y.Y. Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018;79:344–353. doi: 10.1016/j.actbio.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 218.Gandara-Loe J., Souza B.E., Missyul A., Giraldo G., Tan J.C., Silvestre-Albero J. MOF-based polymeric nanocomposite films as potential materials for drug delivery devices in ocular therapeutics. ACS Appl Mater Interfaces. 2020;12:30189–30197. doi: 10.1021/acsami.0c07517. [DOI] [PubMed] [Google Scholar]
- 219.Zhang H., Jiang W., Liu R., Zhang J., Zhang D., Li Z. Rational design of metal organic framework nanocarrier-based codelivery system of doxorubicin hydrochloride/verapamil hydrochloride for overcoming multidrug resistance with efficient targeted cancer therapy. ACS Appl Mater Interfaces. 2017;9:19687–19697. doi: 10.1021/acsami.7b05142. [DOI] [PubMed] [Google Scholar]
- 220.Zhang L., Wang Z., Zhang Y., Cao F., Dong K., Ren J. Erythrocyte membrane cloaked metal-organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12:10201–10211. doi: 10.1021/acsnano.8b05200. [DOI] [PubMed] [Google Scholar]
- 221.Jiang W., Zhang H., Wu J., Zhai G., Li Z., Luan Y. Cus@MOF-based well-designed quercetin delivery system for chemo-photothermal therapy. ACS Appl Mater Interfaces. 2018;10:34513–34523. doi: 10.1021/acsami.8b13487. [DOI] [PubMed] [Google Scholar]
- 222.Huang J., Li N., Zhang C., Meng Z. Metal-organic framework as a microreactor for in situ fabrication of multifunctional nanocomposites for photothermal-chemotherapy of tumors in vivo. ACS Appl Mater Interfaces. 2018;10:38729–38738. doi: 10.1021/acsami.8b12394. [DOI] [PubMed] [Google Scholar]
- 223.Gao S., Zheng P., Li Z., Feng X., Yan W., Chen S. Biomimetic O2-evolving metal-organic framework nanoplatform for highly efficient photodynamic therapy against hypoxic tumor. Biomaterials. 2018;178:83–94. doi: 10.1016/j.biomaterials.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 224.Meng X., Deng J., Liu F., Guo T., Liu M., Dai P. Triggered all-active metal organic framework: ferroptosis machinery contributes to the apoptotic photodynamic antitumor therapy. Nano Lett. 2019;19:7866–7876. doi: 10.1021/acs.nanolett.9b02904. [DOI] [PubMed] [Google Scholar]
- 225.Min H., Wang J., Qi Y., Zhang Y., Han X., Xu Y. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv Mater. 2019;31 doi: 10.1002/adma.201808200. [DOI] [PubMed] [Google Scholar]
- 226.Haddad S., Abánades Lázaro I., Fantham M., Mishra A., Silvestre-Albero J., Osterrieth J.W.M. Design of a functionalized metal-organic framework system for enhanced targeted delivery to mitochondria. J Am Chem Soc. 2020;142:6661–6674. doi: 10.1021/jacs.0c00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yao J., Liu Y., Wang J., Jiang Q., She D., Guo H. On-demand CO release for amplification of chemotherapy by MOF functionalized magnetic carbon nanoparticles with NIR irradiation. Biomaterials. 2019;195:51–62. doi: 10.1016/j.biomaterials.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 228.Ku M.S., Dulin W. A biopharmaceutical classification-based right-first-time formulation approach to reduce human pharmacokinetic variability and project cycle time from first-in-human to clinical proof-of-concept. Pharm Dev Technol. 2012;17:285–302. doi: 10.3109/10837450.2010.535826. [DOI] [PubMed] [Google Scholar]
- 229.Xu J., Wu L., Guo T., Zhang G., Wang C., Li H. A "ship-in-a-bottle" strategy to create folic acid nanoclusters inside the nanocages of γ-cyclodextrin metal-organic frameworks. Int J Pharm. 2019;556:89–96. doi: 10.1016/j.ijpharm.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 230.Hartlieb K.J., Ferris D.P., Holcroft J.M., Kandela I., Stern C.L., Nassar M.S. Encapsulation of ibuprofen in CD-MOF and related bioavailability studies. Mol Pharm. 2017;14:1831–1839. doi: 10.1021/acs.molpharmaceut.7b00168. [DOI] [PubMed] [Google Scholar]
- 231.Moussa Z., Hmadeh M., Abiad M.G., Dib O.H., Patra D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016;212:485–494. doi: 10.1016/j.foodchem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 232.Zhang G., Meng F., Guo Z., Guo T., Peng H., Xiao J. Enhanced stability of vitamin a palmitate microencapsulated by γ-cyclodextrin metal-organic frameworks. J Microencapsul. 2018;35:249–258. doi: 10.1080/02652048.2018.1462417. [DOI] [PubMed] [Google Scholar]
- 233.He H., Han H., Shi H., Tian Y., Sun F., Song Y. Construction of thermophilic lipase-embedded metal-organic frameworks via biomimetic mineralization: a biocatalyst for ester hydrolysis and kinetic resolution. ACS Appl Mater Interfaces. 2016;8:24517–24524. doi: 10.1021/acsami.6b05538. [DOI] [PubMed] [Google Scholar]
- 234.Liang K., Coghlan C.J., Bell S.G., Doonan C., Falcaro P. Enzyme encapsulation in zeolitic imidazolate frameworks: a comparison between controlled co-precipitation and biomimetic mineralisation. Chem Commun. 2016;52:473–476. doi: 10.1039/c5cc07577g. [DOI] [PubMed] [Google Scholar]
- 235.Abuçafy M.P., Caetano B.L., Chiari-Andréo B.G., Fonseca-Santos B., do Santos A.M., Chorilli M. Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs. Eur J Pharm Biopharm. 2018;127:112–119. doi: 10.1016/j.ejpb.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 236.Wu Y.N., Zhou M., Li S., Li Z., Li J., Wu B. Magnetic metal-organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small. 2014;10:2927–2936. doi: 10.1002/smll.201400362. [DOI] [PubMed] [Google Scholar]
- 237.Yin X., Yang B., Chen B., He M., Hu B. Multifunctional gold nanocluster decorated metal-organic framework for real-time monitoring of targeted drug delivery and quantitative evaluation of cellular therapeutic response. Anal Chem. 2019;91:10596–10603. doi: 10.1021/acs.analchem.9b01721. [DOI] [PubMed] [Google Scholar]
- 238.Dong K., Zhang Y., Zhang L., Wang Z., Ren J., Qu X. Facile preparation of metal-organic frameworks-based hydrophobic anticancer drug delivery nanoplatform for targeted and enhanced cancer treatment. Talanta. 2019;194:703–708. doi: 10.1016/j.talanta.2018.10.101. [DOI] [PubMed] [Google Scholar]
- 239.Wang G.D., Chen H., Tang W., Lee D., Xie J. Gd and Eu co-doped nanoscale metal-organic framework as a t1–t2 dual-modal contrast agent for magnetic resonance imaging. Tomography. 2016;2:179–187. doi: 10.18383/j.tom.2016.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Meng X., Gui B., Yuan D., Zeller M., Wang C. Mechanized azobenzene-functionalized zirconium metal-organic framework for on-command cargo release. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jia Q., Li Z., Guo C., Huang X., Song Y., Zhou N. A γ-cyclodextrin-based metal-organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale. 2019;11:20956–20967. doi: 10.1039/c9nr06195a. [DOI] [PubMed] [Google Scholar]
- 242.Wu M.X., Gao J., Wang F., Yang J., Song N., Jin X. Multistimuli responsive core-shell nanoplatform constructed from Fe3O4@MOF equipped with pillar[6]arene nanovalves. Small. 2018;14 doi: 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- 243.Silva J.Y.R., Proenza Y.G., da Luz L.L., de Sousa Araújo S., Filho M.A.G., Junior S.A. A thermo-responsive adsorbent-heater-thermometer nanomaterial for controlled drug release: (ZIF-8,EuxTby)@AuNP core–shell. Mater Sci Eng C Mater Biol Appl. 2019;102:578–588. doi: 10.1016/j.msec.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 244.Ramezani M.R., Ansari-Asl Z., Hoveizi E., Kiasat A.R. Fabrication and characterization of Fe(III) metal-organic frameworks incorporating polycaprolactone nanofibers: potential scaffolds for tissue engineering. Fibers Polym. 2020;21:1013–1022. [Google Scholar]
- 245.Wang D., Zhou J., Chen R., Shi R., Zhao G., Xia G. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials. 2016;100:27–40. doi: 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 246.Lajevardi A., Sadr M.H., Yaraki M.T., Badiei A., Armaghan M. A pH-responsive and magnetic Fe3O4@silica@MIL-100 (Fe)/β-CD nanocomposite as a drug nanocarrier: loading and release study of cephalexin. New J Chem. 2018;42:9690–9701. [Google Scholar]
- 247.Gwon K., Han I., Lee S., Kim Y., Lee D.N. Novel metal-organic framework-based photocrosslinked hydrogel system for efficient antibacterial applications. ACS Appl Mater Interfaces. 2020;12:20234–20242. doi: 10.1021/acsami.0c03187. [DOI] [PubMed] [Google Scholar]
- 248.Luo D., Wang C., Tong Y., Liu C., Xiao Y., Zhu Z. An NIF-doped ZIF-8 hybrid membrane for continuous antimicrobial treatment. RSC Adv. 2020;10:7360–7367. doi: 10.1039/d0ra00108b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wuttke S., Zimpel A., Bein T., Braig S., Stoiber K., Vollmar A. Validating metal-organic framework nanoparticles for their nanosafety in diverse biomedical applications. Adv Healthc Mater. 2017;6:1600818. doi: 10.1002/adhm.201600818. [DOI] [PubMed] [Google Scholar]
- 250.Schütz C.A., Juillerat-Jeanneret L., Mueller H., Lynch I., Riediker M. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine. 2013;8:449–467. doi: 10.2217/nnm.13.8. [DOI] [PubMed] [Google Scholar]
- 251.Lucena F.R., de Araújo L.C., Rodrigues Mdo D., da Silva T.G., Pereira V.R., Militão G.C. Induction of cancer cell death by apoptosis and slow release of 5-fluoracil from metal-organic frameworks Cu-BTC. Biomed Pharmacother. 2013;67:707–713. doi: 10.1016/j.biopha.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 252.Lu K., He C., Lin W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J Am Chem Soc. 2014;136:16712–16715. doi: 10.1021/ja508679h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ruyra À., Yazdi A., Espín J., Carné-Sánchez A., Roher N., Lorenzo J. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal-organic framework nanoparticles. Chemistry. 2015;21:2508–2518. doi: 10.1002/chem.201405380. [DOI] [PubMed] [Google Scholar]
- 254.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M., Somasundaran P. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 255.Huxford R.C., Dekrafft K.E., Boyle W.S., Liu D., Lin W. Lipid-coated nanoscale coordination polymers for targeted delivery of antifolates to cancer cells. Chem Sci. 2012;3:198–201. doi: 10.1039/C1SC00499A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lu K., Aung T., Guo N., Weichselbaum R., Lin W. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv Mater. 2018;30 doi: 10.1002/adma.201707634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bellido E., Guillevic M., Hidalgo T., Santander-Ortega M.J., Serre C., Horcajada P. Understanding the colloidal stability of the mesoporous MIL-100(Fe) nanoparticles in physiological media. Langmuir. 2014;30:5911–5920. doi: 10.1021/la5012555. [DOI] [PubMed] [Google Scholar]
- 258.Li X., Lachmanski L., Safi S., Sene S., Serre C., Grenèche J.M. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci Rep. 2017;7:13142. doi: 10.1038/s41598-017-13323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu W.G., Truhlar D.G. Computational linker design for highly crystalline metal-organic framework nu-1000. Chem Mater. 2017;29:8073–8081. [Google Scholar]
- 260.Kundu T., Mitra S., Patra P., Goswami A., Díaz Díaz D., Banerjee R. Mechanical downsizing of a gadolinium(III)-based metal-organic framework for anticancer drug delivery. Chemistry. 2014;20:10514–10518. doi: 10.1002/chem.201402244. [DOI] [PubMed] [Google Scholar]
- 261.Grall R., Hidalgo T., Delic J., Garcia-Marquez A., Chevillard S., Horcajada P. In vitro biocompatibility of mesoporous metal (III; Fe, Al, Cr) trimesate MOF nanocarriers. J Mater Chem B. 2015;3:8279–8292. doi: 10.1039/c5tb01223f. [DOI] [PubMed] [Google Scholar]
- 262.Orellana-Tavra C., Mercado S.A., Fairen-Jimenez D. Endocytosis mechanism of nano metal-organic frameworks for drug delivery. Adv Healthc Mater. 2016;5:2261–2270. doi: 10.1002/adhm.201600296. [DOI] [PubMed] [Google Scholar]
- 263.Rojas S., Baati T., Njim L., Manchego L., Neffati F., Abdeljelil N. Metal-organic frameworks as efficient oral detoxifying agents. J Am Chem Soc. 2018;140:9581–9586. doi: 10.1021/jacs.8b04435. [DOI] [PubMed] [Google Scholar]
- 264.Yang D., Yang G., Gai S., He F., An G., Dai Y. Au25 cluster functionalized metal-organic nanostructures for magnetically targeted photodynamic/photothermal therapy triggered by single wavelength 808 nm near-infrared light. Nanoscale. 2015;7:19568–19578. doi: 10.1039/c5nr06192j. [DOI] [PubMed] [Google Scholar]
- 265.Bian R., Wang T., Zhang L., Li L., Wang C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal-organic framework based composite nanoparticles. Biomater Sci. 2015;3:1270–1278. doi: 10.1039/c5bm00186b. [DOI] [PubMed] [Google Scholar]
- 266.Parayath N.N., Nehoff H., Taurin S., Greish K. Prospects of nanocarriers for oral delivery of bioactives using targeting strategies. Curr Pharm Biotechnol. 2016;17:683–699. doi: 10.2174/1389201017666160401145608. [DOI] [PubMed] [Google Scholar]
- 267.Mrsny R.J. Oral drug delivery research in Europe. J Control Release. 2012;161:247–253. doi: 10.1016/j.jconrel.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 268.Javanbakht S., Pooresmaeil M., Hashemi H., Namazi H. Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int J Biol Macromol. 2018;119:588–596. doi: 10.1016/j.ijbiomac.2018.07.181. [DOI] [PubMed] [Google Scholar]
- 269.Chowdhuri A.R., Laha D., Pal S., Karmakar P., Sahu S.K. One-pot synthesis of folic acid encapsulated upconversion nanoscale metal organic frameworks for targeting, imaging and pH responsive drug release. Dalton Trans. 2016;45:18120–18132. doi: 10.1039/c6dt03237k. [DOI] [PubMed] [Google Scholar]
- 270.Lin W., Hu Q., Jiang K., Yang Y., Yang Y., Cui Y. A porphyrin-based metal-organic framework as a pH-responsive drug carrier. J Solid State Chem. 2016;237:307–312. [Google Scholar]
- 271.Hidalgo T., Giménez-Marqués M., Bellido E., Avila J., Asensio M.C., Salles F. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci Rep. 2017;7:43099. doi: 10.1038/srep43099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen Y., Li P., Modica J.A., Drout R.J., Farha O.K. Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: protein encapsulation, protection, and release. J Am Chem Soc. 2018;140:5678–5681. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]
- 273.Zhou Y., Liu L., Cao Y., Yu S., He C., Chen X. A nanocomposite vehicle based on metal-organic framework nanoparticle incorporated biodegradable microspheres for enhanced oral insulin delivery. ACS Appl Mater Interfaces. 2020;12:22581–22592. doi: 10.1021/acsami.0c04303. [DOI] [PubMed] [Google Scholar]
- 274.Pan Y.B., Wang S., He X., Tang W., Wang J., Shao A. A combination of glioma in vivo imaging and in vivo drug delivery by metal-organic framework based composite nanoparticles. J Mater Chem B. 2019;7:7683–7689. doi: 10.1039/c9tb01651a. [DOI] [PubMed] [Google Scholar]
- 275.Pang Y., Fu Y., Li C., Wu Z., Cao W., Hu X. Metal-organic framework nanoparticles for ameliorating breast cancer-associated osteolysis. Nano Lett. 2020;20:829–840. doi: 10.1021/acs.nanolett.9b02916. [DOI] [PubMed] [Google Scholar]
- 276.Kush P., Bajaj T., Kaur M., Madan J., Jain U.K., Kumar P. Biodistribution and pharmacokinetic study of gemcitabine hydrochloride loaded biocompatible iron-based metal organic framework. J Inorg Organomet Polym Mater. 2019;30:2827–2841. [Google Scholar]
- 277.Du X., Fan R., Qiang L., Xing K., Ye H., Ran X. Controlled Zn2+-triggered drug release by preferred coordination of open active sites within functionalization indium metal organic frameworks. ACS Appl Mater Interfaces. 2017;9:28939–28948. doi: 10.1021/acsami.7b09227. [DOI] [PubMed] [Google Scholar]
- 278.Mei L., Zhang Z., Zhao L., Huang L., Yang X.L., Tang J. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–890. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 279.Castillo-Blas C., de la Peña-O'Shea V.A., Puente-Orench I., de Paz J.R., Sáez-Puche R., Gutiérrez-Puebla E. Addressed realization of multication complex arrangements in metal-organic frameworks. Sci Adv. 2017;3 doi: 10.1126/sciadv.1700773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Smith R., Vitórica-Yrezábal I.J., Hill A., Brammer L. Arene guest selectivity and pore flexibility in a metal-organic framework with semi-fluorinated channel walls. Philos Trans A Math Phys Eng Sci. 2017;375:20160031. doi: 10.1098/rsta.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Burgaz E., Erciyes A., Andac M., Andac O. Synthesis and characterization of nano-sized metal organic framework-5 (MOF-5) by using consecutive combination of ultrasound and microwave irradiation methods. Inorg Chim Acta. 2019;485:118–124. [Google Scholar]
- 282.Feng P.L., Perry JJt, Nikodemski S., Jacobs B.W., Meek S.T., Allendorf M.D. Assessing the purity of metal-organic frameworks using photoluminescence: MOF-5, ZnO quantum dots, and framework decomposition. J Am Chem Soc. 2010;132:15487–15489. doi: 10.1021/ja1065625. [DOI] [PubMed] [Google Scholar]
- 283.Su F., Jia Q., Li Z., Wang M., He L., Peng D. Aptamer-templated silver nanoclusters embedded in zirconium metal-organic framework for targeted antitumor drug delivery. Microporous Mesoporous Mater. 2018;275:152–162. [Google Scholar]
- 284.Wang Z.C., Zhang Y., Li Z.Y. A low cytotoxic metal-organic framework carrier: pH-responsive 5-fluorouracil delivery and anti-cervical cancer activity evaluation. J Clust Sci. 2018;29:1285–1290. [Google Scholar]
- 285.Lei Z., Yan C., Rui S., Tingguo K., Guangsheng P., Boran W. Synthesis of hollow nanocages MOF-5 as drug delivery vehicle to solve the load-bearing problem of insoluble antitumor drug oleanolic acid. Inorg Chem Commun. 2018;96:20–23. [Google Scholar]