Abstract
The aim of this study was to test the antimicrobial properties of dental cements modified with magnesium oxide (MgO) nanoparticles. Zein-modified MgO nanoparticles (zMgO) in concentrations (0.0, 0.3, 0.5, and 1.0%) were mixed with dental cements (Fuji II, Rely X Temp E, Ionoglass Cem, Es Temp NE, and System P link). Eight discs were fabricated from each zMgO-cement pair for a total of 32 specimens for each cement. Characterization of the dental cements incorporating zMgO was done by X-ray Diffraction (XRD) and Field Emission Scanning Electron Microscopy (FESEM). The antimicrobial properties of the mixtures were tested using direct contact and agar diffusion assays against Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans. Data was analyzed using two-way analysis of variance and LSD post hoc test at 0.05 significance level. XRD spectra showed sharp peaks of zMgO indicating its high crystalline nature, while the amorphous dental cements with zMgO had broad peaks. FESEM analysis showed a uniform distribution of the zMgO nanoparticles in the cement. There were significant inhibition zone values associated with all concentrations of zMgO-cement mixtures tested compared to controls (p < 0.001) with a dose-response recorded only with Fuji II. Optical density values were significantly lower in zMgO groups compared to controls for all microorganisms. The effect was most prominent with Rely X against C. albicans and S. aureus. Dental cements containing zMgO showed significant antimicrobial properties that were dependent on the specific initial cement substrate.
Keywords: Magnesium oxide nanoparticles, Antimicrobial biomaterials, Dental cement, Nanocoatings
Graphical abstract
Highlights
-
•
Antimicrobial nanoparticles (NPs) are widely used in dental materials to improve their biological properties.
-
•
Magnesium Oxide (MgO) NPs are novel antimicrobial agents.
-
•
Incorporation of MgO NPs in dental cements aids in minimizing bacterial colonization at the restoration margin.
-
•
Zein polymer facilitates the dispersion of MgO NPs and avoid its agglomeration.
-
•
Zein polymer effectively enhances the performance of MgO NPs.
1. Introduction
The success of indirect restorations needs a well organized approach to dental cements, restorative materials, and tooth preparation design [1]. Open margins of restorations occur due to defects in the prosthesis or disintegration of dental cements and is usually accompanied by bacterial microleakage leading to recurrent caries [2]. Recurrent caries formed in restoration margins, is a biofilm containing different bacteria such as Streptococcus mutans that produce acids leading to the dissolution of the mineral content of tooth structure [3]. Similarly, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans play major roles in the pathogenesis of some oral diseases [4]. Elimination of bacterial colonization at the restoration margin can interfere with the pathogenesis of dental caries and periodontal disease at tooth-restoration interface [5]. Recurrent decay around dental restorations is still considered a major problem reaching between 50 and 60% of all restorations placed according to some reports [[6], [7], [8]]. Placing an antimicrobial agent at the restoration margin could potentially decrease microbial load and consequently recurrent caries [9].
Multiple active ingredients were previously added to dental cements in order to prevent bacterial colonization such as nhexametaphosphate microparticles, calcium phosphate, and silver ions [10]. These agents showed improvement in the antimicrobial effect, amount of fluoride delivered, and rate of demineralization, but caused a decrease in the mechanical properties of the cements [11,12]. Maintaining adequate mechanical properties of dental cements is paramount for the long-term service of dental restorations.
The use of nanotechnology in dentistry attained many researchers’ interest in the last few years. Metal oxide nanoparticles have been broadly used in mechanical and biomedical fields [13] since they produce reactive oxygen species that target bacterial metabolism in a variety of mechanisms decreasing the probability of bacterial resistance [14]. However, the promising aspect of nanoparticles like ZnO, AgO and TiO nanoparticles was hindered by biosafety and decrease in materials properties [[15], [16], [17], [18], [19]]. However, nanoparticulate MgO is a novel potent antibacterial agent that has been recently incorporated in dental materials to enhance their antibacterial properties. Nonetheless, MgO nanoparticles tend to aggregate and form clusters that can affect its utilization in dental applications [[20], [21], [22]]. A workaround is required in order to maintain an amorphous phase of the active nanocompound; thus, producing a consistent and prolonged antimicrobial effect.
The addition of zein polymer to the MgO nanoparticle formulation creates a coating that prevents the agglomeration of MgO particles. Zein is a naturally-occurring protein polymer that has a wide range of pharmaceutical applications [23]. The inherent properties of this polymer help it stabilizes particles against aggregation by decreasing the hydrophobic properties of these particles [24]. As reported previously, scanning and transmission electron microscopy images indicated significantly less aggregation of zMgO nanoparticles, while maintaining a nano-sized structure, when compared to pure MgO nanoparticles. Also, there was a 20% more sustained release of the active ingredient detected with zMgO nanoparticles in comparison to pure MgO [[25], [26], [27]]. Karimi and collaborators have reported that MgO nanowires have good mechanical properties allowing them to be used in dental cements [21,28,29]. Also, Noori and Kareem showed that the addition of MgO nanoparticles to glass ionomer cement enhanced its antimicrobial activity establishing a biocompatible antibacterial dental restorative cement [30]. Similarly, the antibacterial and mechanical properties of denture base materials were tested after the incorporation of MgO nanoparticles showing a significant reduction in the growth of Staphylococcus aureus [31].
Cements are an integral part of the materials utilized in dentistry. They have various applications such as being used as liners, bases, and affixing indirect restorations [28]. The chemistry of these cements is different and can range between glass ionomer-based, resin-based, and oxide-based materials [28]. The addition of antimicrobial agents to dental cements is a viable method to counteract colonization of oral microbes and potentially decreasing the side effect of microleakage. However, this effect could be dependent on the type of the dental cement.
To the authors’ knowledge, the effect of zMgO nanoparticles formulation on oral microbes has not been studied previously. Thus, the main objective of this study was to investigate the antimicrobial properties of common dental cements after incorporating zMgO nanoparticles. The null hypothesis of this study was that there will be no difference between the zMgO cements across the tested dental cements in terms of antimicrobial properties. The significance of this study is that enhancing the antimicrobial activities of dental cements will result in cements with superior biological properties that can enhance the longevity of the restorations and minimize recurrent caries.
2. Materials and methods
2.1. Experimental design
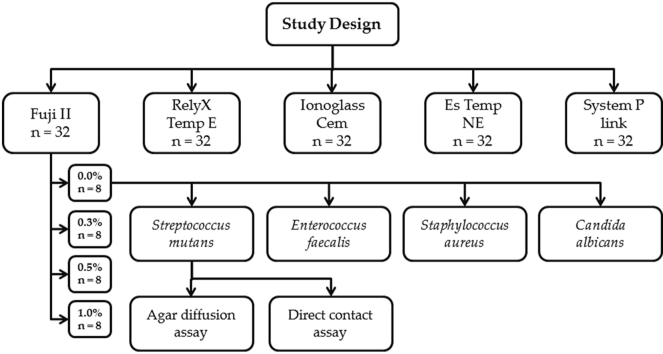
Five dental cements were used for the study from different manufacturers. Four concentrations of zMgO nanoparticles (0.0, 0.3, 0.5, and 1.0%) were mixed with five dental cements. Eight specimens from each cement concentration were fabricated for a total of 32 specimens per dental cement. Kirby-Bauer agar diffusion and modified direct contact tests were utilized to determine the antimicrobial properties of the resultant cements on four common microbial strains (Fig. 1).
Fig. 1.
Schematic outline of the design of the study showing an example of one group's workflow.
2. 2 Coating of MgO nanoparticles
MgO nanoparticles were coated with zein polymer using pH-controlled nanoprecipitation as described previously by Naguib et al. [22]. A mixture of ethanol and 0.1 NaOH solution (93.7% (v/v)) was used to dissolve 0.02 g of zein polymer (Sigma-Aldrich, St. Louis, MO, USA). Using ultrasonic shear of 750 W, a frequency of 20 kHz, and a temperature of 10 °C, 15 ml of dissolved mix of 0.02 g of MgO and polyvinyl alcohol (PVA) at 0.9% (w/v) were infused by droplets of zein solution. In order to evaporate the ethanol, the mixture of zein was stirred magnetically at a speed of 500 rpm at room temperature. After that, nanoparticles were purified to remove excess PVA using a pair of different centrifugal cycles of 3000 rpm for 45 min. Afterwards, the pellet was dissolved in 5 mL of buffer following the elimination of the supernatant [32]. Then, 2% (w/v) of trehalose was added to the mixture and lyophilized (VirTis Bench Top Lyophilizer, SP Industries, Stone Ridge, NY, USA) [27,32].
2.3. Preparation of the cement specimens
A total of 32 discs were prepared using a Teflon mold (4 mm diameter and 6 mm thickness as used in previous studies) from each of the five types dental cements (Table 1): Fuji II (FII; GC America Inc.), Rely X Temp E (RX; 3 M ESPE, Dental Products), Ionoglass Cem (IC; Harvard Dental International GmbH), Es Temp NE (NE; Spident), System P link (SP; Ivoclar Vivadent). Different weights of zMgO nanoparticles were calculated, weighed by a high precision electronic balance accurate to 0.0001 g (Mettler Toledo™, Fischer Scientific, USA), and added to each cement at 0.0, 0.3, 0.5, and 1.0% for a total of eight discs per group [27,33]. zMgO nanoparticles were added to the powder or paste of the cement. When added to the powder it was vortexed at 2000 rpm for 1 min then mixed with the liquid. The paste/paste or powder/liquid ratio was maintained per the manufacturer's instructions. Mixing was performed manually with a plastic spatula until we obtain a homogenous consistency. The materials were placed into the Teflon mold pressed between polyester matrix (Proben, Catanduva, SP, Brazil) and glass plates to remove the excess. The material was left for 5 min to ensure that complete setting was achieved [34].
Table 1.
Summary of the cements used in the study.
| Cement | Category | Matrix | Manufacturer |
|---|---|---|---|
| Fuji II (FII) | Self-cure glass ionomer | Copolymer of acrylic acid itaconic acid with acid reactive fluoroaluminosilicate glass, strontium and lanthanum | GC America Inc., Alsip, IL, USA |
| Rely X Temp E (RX) | Zinc oxide eugenol | Hydrogenated rosin, eugenol, modified rosin, silane-treated silica, oleic acid | 3 M ESPE, Dental Products, Saint Paul, MN, USA |
| Ionoglass Cem (IC) | Self-cure glass ionomer | Copolymer of acrylic, itaconic, maleic and tricarboxylic acids with calcium fluoro aluminosilicate glass | Harvard Dental International GmbH, Hoppegarten, Germany |
| Es Temp NE (NE) | Zinc oxide non-eugenol | Zinc oxide, white mineral oil, petrolatum | Spident, Namdong-gu, Incheon, South Korea |
| System P link (SP) | Dual-cure resin | Dimethacrylates, inorganic fillers, catalysts, stabilizers and pigments | Ivoclar Vivadent, Zurich, Switzerland |
2.4. Bacteria preparation
The following bacteria and fungi strains were obtained from the American Type Culture Collection (ATCC) and used to test the antimicrobial activity of dental cements after incorporation of zMgO nanoparticles in the study: Streptococcus mutans: 10,449 (ATCC 25175), Staphylococcus aureus: Seattle 1945 (ATCC 25923), Enterococcus faecalis: Portland (ATCC 29212) and Candida albicans: 3147 (ATCC 10231). The antimicrobial effect of the five cements with their four different concentrations was tested in triplicates against each microbial specimen.
2.5. Agar diffusion assay
Sterile agar plates were cultured with the different freshly prepared bacterial and fungal inoculum. Cement discs of various groups of control and 0.3%, 0.5% and 1% of zMgO cements were placed in the inoculated agar plates. The plates were placed for 24 h in an incubator (Thermo Fischer Scientific, Waltham, MA, USA) for 24 h at a temperature of 37 °C. Inhibition zones were measured around each disc and every experiment was performed in triplicates.
2.6. Direct contact assay
Solutions of 0.5 McFarland in broth were prepared from the bacteria and fungi and mixed with 3 mL of thioglycolate 3028 broth (Splm, Riyadh Saudi Arabia). Cement discs of control and those mixed with different concentrations of zMgO nanoparticles were added to the tubes with the previously-prepared solution and incubated at 37 °C for 24 h. After incubation, the optical density (OD) of each bacterial solution was read at 490 nm using a spectrophotometer (SpectraMax Plus, Molecular devices Inc. Sunnyvale, CA, USA) with isopropanol as the blank. Each experiment was repeated in triplicates and an average of the three OD readings for each solution was reported.
2.7. Characterization of the tested dental cements modified with zein modified MgO
2.7.1. X ray diffraction analysis
The crystalline nature of the five dental cements after incorporation of zMgO nanoparticles and also for the zMgO nanoparticles alone was verified by X-ray diffraction patterns conducted through an X-ray diffractometer (XRD; Rigaku, Ultima IV, Japan) fitted with Cu-Kα X-ray radiation (λ = 1.5418 A°). The XRD spectra were scanned in the 2θ range of 30–80°.
2.7.2. Field emission scanning electron microscopy
The surface morphology and the distribution of zMgO nanoparticles in the five tested dental cements were investigated by field emission scanning electron microscopy (FESEM) (JEOL JSM-7600F, JEOL Ltd. Tokyo, Japan), under ultra-high vacuum (~10−6 mbar). Images were taken at magnification 15,000× at an acceleration voltage of 5 kV with LEI detector.
2.8. Statistical tests
Inhibition zone and OD values were analyzed using two-way analysis of variance (ANOVA) followed by least square difference (LSD) post hoc test at a 0.05 significance level. All tests were conducted using Statistical Package for the Social Sciences (SPSS Version 23, IBM Inc. Armonk, NY, USA).
3. Results
3.1. Agar diffusion assay
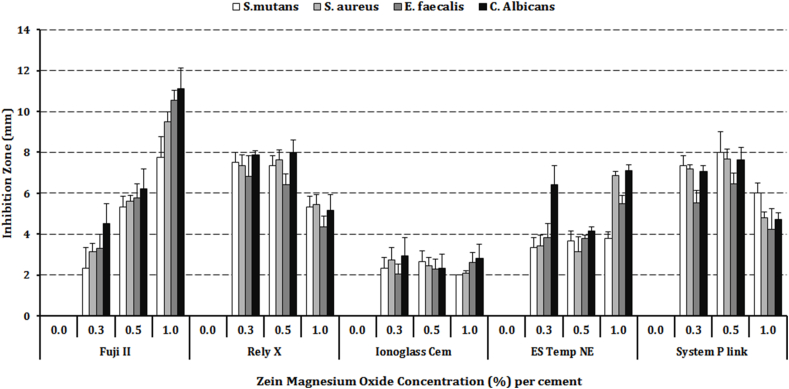
Inhibition zone values are reported in Fig. 1 and Table 2. Both the zMgO concentration and the cement type were significant (p < 0.001). Clear inhibition zones were reported with all cements at all zMgO concentrations compared to controls. The maximum inhibition zones were reported with FII at 1.0%; which were between 7.7 and 11.1 mm depending on the microorganism. A direct association between the zMgO concentration and the width of the inhibition zone was observed with FII but not with other cements.
Table 2.
Means (mm) and standard deviations of inhibition zones of different microorganisms around different cements mixed with different Zein modified magnesium oxide (zMgO) concentrations. FII: GC Fuji II, RX: 3 M ESPE Rely X Temp, IC: Harvard Ionoglass Cem, NE: Spident Es Temp NE, and SP: Ivoclar Vivadent System P link.
| Microorganism | Cement | Inhibition zone (mm) at zMgO concentration |
|||
|---|---|---|---|---|---|
| 0% n = 8 | 0.3% n = 8 | 0.5% n = 8 | 1.0% n = 8 | ||
| S. mutans | FII | 0A/a | 2.3 (1.0)B/a | 5.3 (0.5)C/a | 7.7 (1.0)D/a |
| RX | 0A/a | 7.5 (0.5)B/b | 7.3 (0.5)B/b | 5.3 (0.5)C/b | |
| IC | 0A/a | 2.3 (0.5)B/a | 2.6 (0.5)B/c | 2.0 (0.0)C/c | |
| NE | 0A/a | 3.3 (0.5)B/a | 3.6 (0.5)B/d | 3.8 (0.3)C/d | |
| SP | 0A/a | 7.3 (0.5)B/b | 8.0 (1.0)C/b | 6.0 (0.5)D/b | |
| S. aureus | FII | 0A/a | 3.1 (0.4)B/a | 5.6 (0.3)C/a | 9.5 (0.5)D/a |
| RX | 0A/a | 7.4 (0.5)B/b | 7.6 (0.5)B/b | 5.4 (0.5)C/b | |
| IC | 0A/a | 2.7 (0.6)B/a | 2.4 (0.4)B/c | 2.1 (0.1)C/c | |
| NE | 0A/a | 3.4 (0.5)B/c | 3.1 (0.7)B/d | 6.8 (0.2)C/d | |
| SP | 0A/a | 7.2 (0.2)B/b | 7.6 (0.5)B/b | 4.8 (0.3)C/b | |
| E. faecalis | FII | 0A/a | 3.3 (0.7)B/a | 5.7 (0.7)C/a | 10.5 (0.5)D/a |
| RX | 0A/a | 6.8 (1.0)B/b | 6.4 (0.5)B/b | 4.3 (0.5)C/b | |
| IC | 0A/a | 2.0 (0.5)B/c | 2.2 (0.5)B/c | 2.6 (0.5)C/c | |
| NE | 0A/a | 3.8 (0.7)B/a | 3.7 (0.2)B/d | 5.5 (0.4)C/d | |
| SP | 0A/a | 5.5 (0.6)B/d | 6.4 (0.5)C/b | 4.2 (1.0)D/b | |
| C. albicans | FII | 0A/a | 4.5 (1.0)B/a | 6.2 (1.0)C/a | 11.1 (1.0)D/a |
| RX | 0A/a | 7.9 (0.2)B/b | 8.0 (0.6)B/b | 5.1 (0.8)C/b | |
| IC | 0A/a | 2.9 (0.9)B/c | 2.3 (0.7)C/c | 2.8 (0.7)B/c | |
| NE | 0A/a | 6.4 (0.9)B/d | 4.1 (0.2)C/d | 7.1 (0.3)D/d | |
| SP | 0A/a | 7.0 (0.3)B/d | 7.6 (0.6)C/b | 4.7 (0.3)D/b | |
*Upper case letters indicate significant differences (p < 0.05) between different zMgO concentrations for the same microorganism and cement type. Lower case letters indicate significant differences (p < 0.05) across different cements within the same zMgO concentration and microorganism.
3.2. Direct contact assay
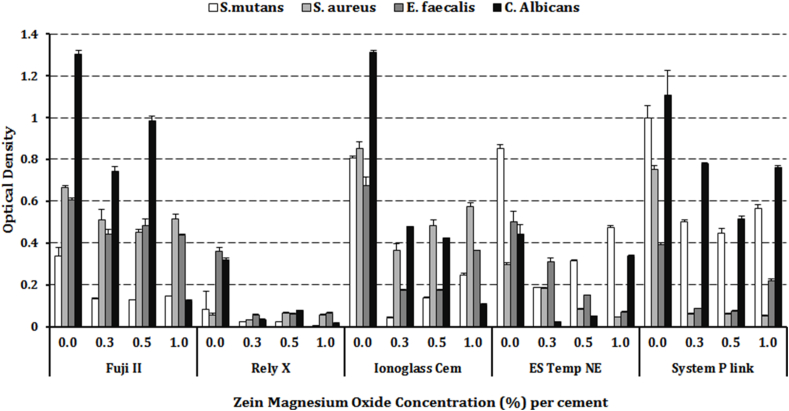
Optical density values are illustrated in Fig. 2 and Table 3. Both the zMgO concentration and the cement type had significant (p < 0.001) effect on OD values. For S. mutans, there was a significant decrease in OD values with FII and RX (p < 0.001) with a clear dose-response effect for RX. There was a decrease with other cements as well. For S. aureus and E. faecalis, there was a significant (p < 0.001) decrease in OD values with all cements mixtures. Values for 0.3 and 0.5% were similar and there was an increase in OD values with 1.0% zMgO concentration. A dose-response was recorded for ES with S. aureus. For C. albicans, there was a significant decrease in OD values for all cement mixtures (p < 0.001) with a dose-response only with ES. An increase in OD values was observed in 1.0% groups of SP and NE cements.
Fig. 2.
Bar graph showing statistical comparison of inhibition zones of different microorganisms around different cements mixed with different zein modified magnesium oxide (zMgO) nanoparticles concentrations.
Table 3.
Means and standard deviations of optical density values of different microorganisms around different cements mixed with different zein modified magnesium oxide (zMgO) nanoparticles concentrations. FII: GC Fuji II, RX: 3 M ESPE Rely X Temp, IC: Harvard Ionoglass Cem, NE: Spident Es Temp NE, and SP: Ivoclar Vivadent System P link.
| Microorganism | Cement | Optical Density (nm) at MgO concentration |
|||
|---|---|---|---|---|---|
| 0% | 0.3% | 0.5% | 1.0% | ||
| S. mutans | FII | 0.33 (0.04)A/a | 0.13 (0.003)B/a | 0.12 (0.002)B/a | 0.14 (0.003)B/a |
| RX | 0.80 (0.09)A/a | 0.02 (0.001)B/b | 0.02 (0.002)B/b | 0.00 (0.000)B/b | |
| IC | 0.80 (0.01)A/c | 0.04 (0.002)B/b | 0.13 (0.002)C/a | 0.24 (0.01)D/c | |
| NE | 0.85 (0.02)A/c | 0.18 (0.004)B/a | 0.31 (0.008)C/c | 0.47 (0.01)D/d | |
| SP | 0.99 (0.06)A/d | 0.50 (0.007)B/c | 0.44 (0.02)C/d | 0.56 (0.02)B/e | |
| S. aureus | FII | 0.66 (0.01)A/a | 0.51 (0.05)B/a | 0.45 (0.01)C/a | 0.51 (0.02)B/a |
| RX | 0.54 (0.01)A/b | 0.03 (0.003)B/b | 0.06 (0.004)C/b | 0.05 (0.004)C/b | |
| IC | 0.85 (0.03)A/c | 0.36 (0.03)B/c | 0.48 (0.03)C/a | 0.57 (0.02)D/a | |
| NE | 0.29 (0.01)A/d | 0.18 (0.004)B/d | 0.08 (0.002)C/b | 0.04 (0.003)D/b | |
| SP | 0.75 (0.02)A/e | 0.06 (0.004)B/b | 0.06 (0.003)B/b | 0.05 (0.004)B/b | |
| E .faecalis | FII | 0.60 (0.01)A/a | 0.44 (0.02)B/a | 0.48 (0.03)C/a | 0.43 (0.006)B/a |
| RX | 0.35 (0.02)A/b | 0.05 (0.003)B/b | 0.06 (0.002)B/b | 0.06 (0.003)B/b | |
| IC | 0.67 (0.04)A/a | 0.17 (0.003)B/c | 0.17 (0.003)B/c | 0.36 (0.000)C/c | |
| NE | 0.50 (0.05)A/c | 0.31 (0.02)B/d | 0.15 (0.001)C/c | 0.07 (0.002)D/b | |
| SP | 0.39 (0.01)A/b | 0.08 (0.002)B/b | 0.07 (0.003)B/b | 0.21 (0.01)C/d | |
| C. albicans | FII | 1.30 (0.02)A/a | 0.74 (0.02)B/a | 0.98 (0.02)C/a | 0.12 (0.002)D/a |
| RX | 0.31 (0.01)A/b | 0.03 (0.002)B/b | 0.07 (0.002)B/b | 0.01 (0.002)C/b | |
| IC | 1.31 (0.01)A/a | 0.47 (0.003)B/c | 0.42 (0.003)B/c | 0.10 (0.008)C/a | |
| NE | 0.44 (0.05)A/c | 0.02 (0.001)B/b | 0.05 (0.001)B/b | 0.33 (0.005)C/c | |
| SP | 1.10 (0.12)A/a | 0.77 (0.006)B/a | 0.51 (0.01)C/d | 0.76 (0.01)B/d | |
*Upper case letters indicate significant differences (p < 0.05) between different zMgO concentrations for the same microorganism and cement type. Lower case letters indicate significant differences (p < 0.05) across different cements within the same zMgO concentration and microorganism.
3.3. X ray diffraction analysis
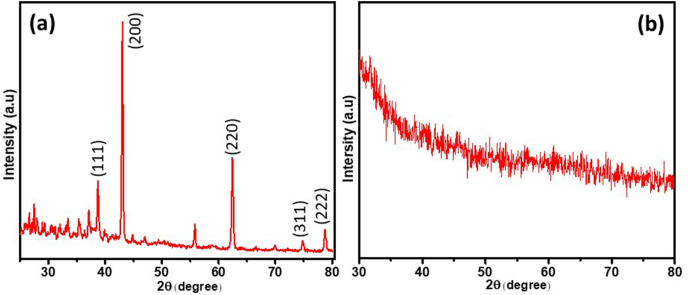
The structural characteristics and crystallinity of zMgO nanoparticles and dental cements modified with zMgO nanoparticles are illustrated in Fig. 3 [35]. The high intensity and sharp peaks in the XRD spectra show that zMgO is highly crystalline in nature with the observance of some extra peaks which may arise due to the presence of zein with MgO. MgO shows its respective peaks at 36.98°, 42.95°, 62.40°, 74.80° and 78.75° 2θ due to the (111), (200), (220), (311), and (222) planes respectively, which confirms the presence of cubic MgO [36]. Fig. 4(b) demonstrates the XRD spectrum of cement with zMgO nanoparticles. No diffraction peaks were observed in this sample in comparison to the zMgO nanoparticles alone. This could be due to the minute amount of the zMgO nanoparticles mixed in the cement [37,38].
Fig. 3.
Bar graph showing statistical comparison of the microbial absorbance values (Optical density) of different microorganisms around different cements mixed with different zein modified magnesium oxide (zMgO) nanoparticles concentrations.
Fig. 4.
Diagrammatic representation of XRD spectra, (a) zMgO nanoparticles and (b) Cement containing 0.5% zMgO nanoparticles.
3.4. Field emission scanning electron microscopy
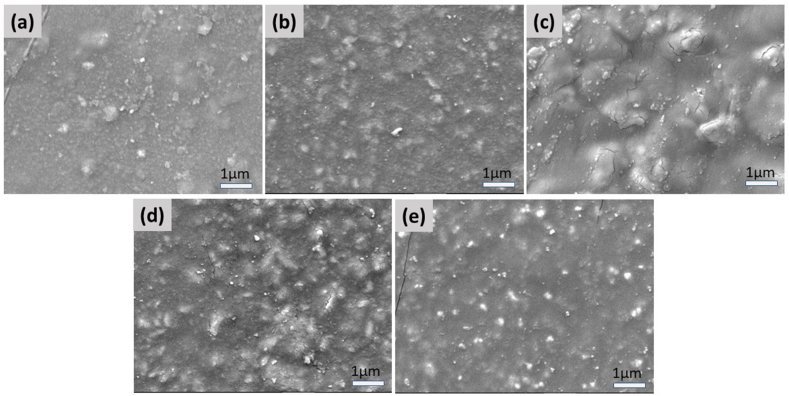
Fig. 5 shows the morphological characteristics of five cement samples FII, RX, IC, NE, and SP. Micrographs show that the zMgO nanoparticles are uniformly distributed all over the surface of the cement samples. It can be seen clearly from the FESEM micrographs that the particle size is less than 100 nm in all samples. However, due to non-conducting nature of the cement material very high magnification images could not be obtained.
Fig. 5.
FESEM micrographs of cement FII (a), RX (b), IC (c), NE (d), and SP (e) containing 0.5% zein modified MgO. Markers equal 1 μm.
4. Discussion
With the increase in demand for indirect restorations owing to the increase in esthetic demand in the society, cementation techniques are used more widely [39]. Decreasing the growth of microorganisms and improving marginal seal in relation to dental materials are very critical in order to decrease bacterial microleakage. This phenomenon has been associated with postoperative hypersensitivity, pulpal irritation, and recurrent caries [[40], [41], [42]]. Among these complications, recurrent caries poses a major problem and is considered in many investigations as the primary cause for restorations replacement [39,43]. The use of antimicrobial agents in dental cements, therefore, is warranted. In 1985, the US Food and Drug administration approved the admission of use of MgO (21CFR184.1431) and encouraged its biomedical applications [44]. MgO has well-documented antimicrobial properties which do not affect mammalian cells [[18], [19], [20]]. The alkaline nature of MgO nanoparticles potentially decrease the aggregation of microbes at the restoration interface and can neutralize the acid produced by cariogenic bacteria [45].
However, due to an agglomeration problem of MgO nanoparticles changing its nanoproperties into micro ones, its application in dental materials is affected [21]. When MgO nanoparticles were coated with the natural zein polymer, agglomeration was avoided, and the particles were adequately dispersed in a nanoform [27]. In our previous study [46] of testing the antimicrobial activity of zMgO nanoparticles, we investigated different concentrations of zein polymer (0.5%, 1% and 2%) on various microorganisms but all zein samples tested did not show any antimicrobial effect. Both MgO nanoparticles and the zein coated ones showed antimicrobial activity against different microorganisms. Zein coating reduced the nanoparticles agglomeration and enhanced their antimicrobial effect. Analysis of zMgO nanoparticles were investigated by Transmission Electron microscope (TEM), XRD and Scanning Electron microscope (SEM) in our previous paper indicating that: pure MgO are nanowires of 30 nm wide and 150 nm long while the zMgO nanoparticles were 30–60 nm wide and 230 nm long [27].
This was also verified and confirmed by X-ray crystallography of zMgO nanoparticles that were highly pure and crystalline in nature, and also by Fourier Transform Infrared (FTIR) spectra distinct peaks that showed no possible interaction or formation of new products but only a coating of zein around the MgO nanoparticles [27]. The well-distributed MgO nanoparticles into the substrate could enhance the antimicrobial properties of commercially-available cement formulations; hence, the objective of this investigation was to test antimicrobial effects of cements-modified with zein-coated MgO nanoparticles against four common oral microorganisms.
The characterization of zMgO alone using XRD showed high intensity and sharp peaks indicating its' highly crystalline nature. No diffraction peaks were observed with the dental cements incorporating the zMgO nanoparticles, which can be attributed to the very small wt.% of the zMgO nanoparticles mixed in the cement. This can be in harmony with Laven's explanation that XRD analysis of nanoparticles depends on several factors; the elastic and inelastic scattering contributions that involve information regarding the nano-size character of the crystals in the sample, the amorphous region, and the background [37]. Also that is in agreement with Upadhyay et al. who reported that the broadening of XRD peaks occurs due to the minuscule size of the nanoparticles and their small amount in some cases following laboratory-scale production [38].
Furthermore, as the cements are highly amorphous in nature, they suppress the crystallinity of the zMgO. The disappearance of the diffraction pattern revealed the amorphous transformation. This transformation could be attributed to the hydrophilic interaction among zMgO and the cements [47,48]. In the FESEM analysis, the micrographs of the different cements showed a uniform distribution of the zMgO nanoparticles in the cement samples. Some cracks developed in the images because the FESEM scanning was performed in ultra-high vacuum (~10−6 mbar). However, the absence of aggregation of zMgO nanoparticles ensures that there is good antimicrobial activity even with the low concentration of nanoparticles.
In our prior investigation of assessment of the antimicrobial activity of zMgO nanoparticles, the minimal inhibitory concentration (MIC) was evaluated using the broth dilution method and detected as 0.1% of zMgO concentration. Accordingly, zMgO nanoparticles were added in the dental cements in the concentration of 0.3, 0.5, and 1.0%, since these concentrations have shown potent antimicrobial properties based on previous data from Naguib et al. [46]. These concentrations produced clear antimicrobial effect that was seen both in the direct contact and agar diffusion assay results. The microbial strains tested in this study represent some of the most common causative microorganisms in the oral cavity. S. mutans is the main causative agent for dental caries which is still considered as one of the most widely spread disease affecting humans [49]. Removal of infected tooth structure and placing a restorative material are required when the tooth structure is compromised. C. albicans is one of the most widely spread fungus species in the oral cavity and is linked with many opportunistic infections such as geographic tongue and angular chelitis as well as contaminating denture bases by penetrating into the microporosities of polymethyl methacrylate materials [50]. E. faecalis is associated with endodontic infections; especially antibiotic resistant conditions in endodontic disease. This species in particular is an excellent target for metal-oxides since these active agents produce reactive oxygen species that target bacterial metabolism in a variety of mechanisms decreasing the chance for bacterial resistance [51]. Last, S. aureus is linked with many oral diseases, such as oral mucositis, periodontitis, peri-implantitis, and endodontic infections [52].
MgO has been tested previously and showed it can affect food bacteria in a dose-response manner [53]. However, in the present investigation, this dose-response pattern was dependent on the type of the cement and the pathogen it was tested against. For example in the case of FII, this was apparent in inhibition of microorganisms in a dose-dependent response in the agar diffusion assay showing a clear superior effect for FII-mixed with zMgO nanoparticles. This could be due to the active ingredients within the original cement that has polycarboxylic acid and fluoride. Fluoride in particular has been reported previously to have antibacterial effect especially against different strains of S. mutans [54]. However, active ingredients in the other cements could have played a role in the observed behavior including zinc oxides and methacrylates. The five tested cements were chosen due to the expanded amount of literature available for them as well as their frequent use in the dental field.
This study showed that the concentrations of 0.3% and 0.5% of zMgO nanoparticles had consistently shown similar antimicrobial values as the 1% except in FII. Thus, the inhibitory effect does not seem to be dose-dependent possibly indicating that zMgO nanoparticles levels could have reached their maximum antimicrobial potential at 0.5% without the need for further addition of the active molecule. These findings are in harmony with other studies that indicated that the increase in concentration can lead to agglomeration of the nanoparticles affecting the properties of the material [46]. In a study on the effect of zMgO nanoparticles incorporated in composite resin, results showed that concentrations above 1% affected the surface roughness and wettability of the composite resin [55].
Zein coating has been shown to improve the dispersion of the MgO nanoparticles, enhancing their ability to bind to bacterial cell walls and interfere with bacterial metabolism and improving their antimicrobial property [46]. However, in the present investigation, the antimicrobial activity was dependent on the type of dental cements. The five cements used in this research have different compositions that might affect their interaction with the zMgO nanoparticles, resulting in different antimicrobial effects. A similar inhibition effect was reported for FII with other tested microorganisms which could also be related to the porous structure of the cement and to its relatively weaker mechanical properties that affect the release of the zMgO nanoparticles. Further, IC produced the widest inhibition zones in relation to all tested microbial species.
Also, some decrease in OD values were recorded in some control groups especially with FII and RX with S. mutans and RX and ES in relation with C. albicans and S. aureus. This could indicate some leaching of the cement ingredients into the solutions [56]. It was reported previously that unreacted monomer leaching from light-cured resin materials could affect bacterial growth [57]; however, all cements were self-cured or dual cured in the present investigation. Results from direct contact assay confirm findings from diffusion test with clear advantage for FII and RX cements compared to other three cements. It should be noted; however, that fresh FII and RX did not show the same inhibitory effect as in groups with zMgO groups. In addition, C. albicans was very sensitive to FII, RX, and IC when 1.0% zMgO concentration was used. S. aureus was more sensitive to NE and SP as reported in the direct contact assay.
There are some limitations with this study, as with other in vitro investigations. Study conditions do not fully simulate the oral environment which has polyclonal population of microorganisms as well as different humidity conditions. However, we have decided not to use a multispecies biofilm in the current investigation in order to isolate the effect of each microorganism. Furthermore, saliva was not utilized due to infection control considerations. Still, testing the proposed effect of cements in a well-controlled environment would help depict efficiency and general trends in relation to the nanomaterial. Second, the study duration was relatively short; however, based on the tested parameters from the current investigation we are aiming for future in-vitro and clinical studies with longer durations and comparative focus on the difference in effect between coated and uncoated MgO Nanoparticles.
5. Conclusion
It can be concluded that zMgO nanoparticles significantly enhanced the antibacterial property of dental cements improving their ability to resist bacterial microleakage and potential secondary caries. The use of zMgO nanoparticles is recommended in order to decrease the probability of recurrent caries formation and gingival infections owing to the antimicrobial properties of the zMgO-infused cements. Still, more studies are required to test these effects in clinical settings based on levels depicted from the current investigation.
The addition of zein polymer to metal oxides can lead to the formation of hybrid metal polymers that can have distinct chemical features, such as cross linking, which can affect the optical and physical properties of these new substances [58]. Future studies of these new hybrids (including zMgO) can have appealing purposes in the medical field such as biological applications and physical properties optimizations [59].
CRediT authorship contribution statement
Ghada H. Naguib: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Hani M. Nassar: Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Writing – review & editing, Visualization. Mohamed T. Hamed: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Funding acquisition.
Acknowledgments
This research was funded by Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia, under grant No.(DF-044-165-1441). The authors gratefully acknowledge DSR technical and financial support. The authors would like to thank Dr. Hadeel Mohammad and Dr. Heba Alsoufi for their valuable contribution to the study.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ghada H. Naguib, Email: gnagieb@kau.edu.sa.
Hani M. Nassar, Email: hnassar@kau.edu.sa.
Mohamed T. Hamed, Email: mthamed@kau.edu.sa.
References
- 1.Alptekin T., Ozer F., Unlu N., Cobanoglu N., Blatz M. Invitro evaluations of microleakage around class I amalgam and composite restorations. Operat. Dent. 2010;35:641–648. doi: 10.2341/10-065-L. [DOI] [PubMed] [Google Scholar]
- 2.Kanika V., Pradhuman V., Ashwaryan T. Evaluation of microleakage of various restorative materials: an invitro study. J. Life Sci. 2011;3:29–33. [Google Scholar]
- 3.Dennison J., Sarrett D. Prediction and diagnosis of clinical outcomes affecting restoration margins. J. Oral Rehabil. 2012;39:301–318. doi: 10.1111/j.1365-2842.2011.02267.x. [DOI] [PubMed] [Google Scholar]
- 4.Nirupama D., Nainan M., Ramaswamy R., Muralidharan S., Usha H., Sharma R., Gupta S. Vitro evaluation of the antimicrobial efficacy of four endodontic biomaterials against Enterococcus faecalis, Candida albicans, and Staphylococcus aureus. Int. J. Biomater. Res. Eng. 2014:1–6. doi: 10.1155/2014/383756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazar V., Ditu L., Curutiu C., Gheorghe I., Holban A., Popaand M., Chifiriuc C. Impact of dental plaque biofilms in periodontal disease: management and future therapy. Peridontitis: a useful reference. Pachiappan Arjunan. 2017 doi: 10.5772/intechopen.69959. [DOI] [Google Scholar]
- 6.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 7.Fan C., Chu L., Rawls H., Norling B., Cardenas H., Whang K. Development of an antimicrobial resin. Dent. Mater. 2011;27:322–328. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Melo M., Cheng L., Weir M., Hsia R., Rodrigues L., Xu H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101:620–629. doi: 10.1002/jbm.b.32864. (b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie D., Wang Y., Gua X., Zhao J., Gregory R., Zheng C. Preparation and evaluation of a novel glass-ionomercement with antibacterial functions. Dent. Mater. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K., Tanagawa M., Matsumoto S., Yamada T., Atsuta M. Antibacterial activity of resin composites with silver-containing materials. Eur. J. Oral Sci. 1999;107:290–296. doi: 10.1046/j.0909-8836.1999.eos107409.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosida T., Delbem A., Morais L., Moraes J., Duque C., Souza J., Pedrini D. Ion release, antimicrobial and physio-mechanical properties of glass ionomer cement containing micro or nanosized hexametaphosphate, and their effect on enamel demineralization. Clin. Oral Invest. 2019;23:2345–2354. doi: 10.1007/s00784-018-2674-9. [DOI] [PubMed] [Google Scholar]
- 12.Shieh T., Hsu S., Chang K., Chen W., Lin D. Calcium phosphate cement with antimicrobial properties and radiopacity as an. Endodontic Material. Materials (Basel). 2017;10:1256. doi: 10.3390/ma10111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ficai D., Oprea O., Ficai A., Holban A. Metal oxide nanoparticles: potential uses in biomedical applications. Curr. Proteonomics. 2014;11:139–149. [Google Scholar]
- 14.Gordon T., Perlstein B., Houbara O., Felner I., Banin E., Margel S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloid. Surface. Physicochem. Eng. Aspect. 2011;374:1–8. [Google Scholar]
- 15.Ahmadian E., Shahi S., Yazdani J., Dizaj S., Sharifi S. Local treatment of the dental caries using nanomaterials. Biomed. Pharmacother. 2018;108:443–447. doi: 10.1016/j.biopha.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 16.Chunxu D., John C., Qunhui S., Lee M., Gaohong H., Yulin D. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J. Nanoparticle Res. 2010;12:2101–2109. 101007/s11051-009-9769-9D. [Google Scholar]
- 17.Sawai J., Kojima H., Igarashi H., Hashimoto A., Shoji S., Sawaki T. Antibacterial characteristics of magnesium oxide powder. World J. Microbiol. Biotechnol. 2000;16:187–194. [Google Scholar]
- 18.Sharma D., Sharma S., Kaith B., Rajput J., Kaur M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011;257:9661–9672. [Google Scholar]
- 19.Mirhosseini M., Afzali M. Investigation into the antibacterial behavior of suspensions of magnesium oxide nanoparticles in combination with nisin and heat against Escherichia coli and Staphylococcus aureus in milk. Food Contr. 2016;68:208–215. [Google Scholar]
- 20.Huang L., Li D., Lin Y., Wei M., Evans D., Duan X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. J. Inorg. Biochem. 2005;99:986–993. doi: 10.1016/j.jinorgbio.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Makhluf S., Dror R., Nitzan Y., Abramovich Y., Jelinek R., Gedanken A. Microwave‐assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005;15:1708–1715. [Google Scholar]
- 22.Krishnamoorthy K., Mannivannan G., Kim S., Jeyasubramanian K., Premanathan M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nanoparticle Res. 2012;14:1063. [Google Scholar]
- 23.Anderson T., Lamsal B. Zein extraction from corn, corn products, and coproducts and modifications for various applications: a review. Cereal Chem. 2011;88:159–173. [Google Scholar]
- 24.Podaralla S., Perumal O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS PharmSciTech. 2012;13:919–927. doi: 10.1208/s12249-012-9816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regier M., Taylor J., Borcyk T., Yang Y., Pannier A. Fabrication and characterization of DNA-loaded zein nanospheres. J. Nanobiotechnol. 2012;10:44. doi: 10.1186/1477-3155-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karimi A., Haghdar R., Aadina R., Hatefi M., Mashhadizadeh M., Behjatmanesh A. Synthesis and characterization of nanoparticles and nanocomposite of ZnO and MgO by sonochemical method and their application for zinc polycarboxylate dental cement preparation. Int. Nano Lett. 2011;1:43–51. [Google Scholar]
- 27.Naguib G., Hassan A., Al-Hazmi F., Kurakula M., Al-Dharrabh A., Alkhalidi H., Hamed M. Zein based magnesium oxide nanowires: effect of anionic charge on size, release and stability. Digest Journal of Nanomaterials and Biostructures. 2017;12:741–749. [Google Scholar]
- 28.Sakaguchi R., Ferracane J., Powers J. 2018. Craig's Restorative Dental Materials. [Google Scholar]
- 29.Saruta J., To M., Sakaguchi W., Kondo Y., Tsukinoki K. Brain-derived neurotrophic factor is related to stress and chewing in saliva and salivary glands. Japanese Dental Science Review. 2020;56:43–49. doi: 10.1016/j.jdsr.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noori A., Kareem F. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakir T., Abbas S. The effect of magnesium oxide (MgO) nano-fillers on the antibacterial activity and Some Properties of heat cured acrylic. Int. J. Sci. Res. 2018;7:1381–1387. [Google Scholar]
- 32.Anhorn M., Mahler H., Langer K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int. J. Pharm. 2008;363:162–169. doi: 10.1016/j.ijpharm.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Panahandeh N., Torabzadeh H., Aghaee M., Hasani E., Safa S. Effect of incorporation of zinc oxide nanoparticles on mechanical properties of conventional glass ionomer cements. J. Conserv. Dent. 2018;21:130–135. doi: 10.4103/JCD.JCD_170_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magalhaes A., Santos L., Lopes L., Estrela C., Estrela C., Torres E., Bakuzis A., Cardoso P., Carriao M. Nanosilver application in dental cements. International Scholarly Research Network ISRN Nanotechnology. 2012:1–7. [Google Scholar]
- 35.Retrievals I.J.P. 1999. Level- 2 PDF; pp. 1–54. [Google Scholar]
- 36.Kumar R., Sharma A., Kishore N. Preparation and characterization of MgO nanoparticles by Co-precipitation method precipitation. International Journal of Engineering, Applied and Management Sciences Paradigms. 2013;7:66–70. [Google Scholar]
- 37.Laven P. Separating diffraction from scattering: the million-dollar challenge. J. Nanophotonics. 2010;4:1–18. [Google Scholar]
- 38.Upadhyay S., Parech K., Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J. Alloys Compd. 2016;678:478–485. [Google Scholar]
- 39.Demarco F., Correa M., Cenci M., Moraes R., Opdam N. Longevity of posterior composite restorations: not only a matter of materials. Dent. Mater. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Jia S., Chen D., Wang D., Bao X., Tian X. Comparing marginal microleakage of three different dental materials in veneer restoration using a stereomicroscope: an in vitro study. BDJ Open. 2017;3:16010. doi: 10.1038/bdjopen.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray P., Hafez A., Smith A., Cox C. vol. 18. Official Publication of the Academy of Dental Materials; 2002. Bacterial microleakage and pulp inflammation associated with various restorative materials; pp. 470–478. (Dental Materials). [DOI] [PubMed] [Google Scholar]
- 42.Cox C., Keall C., Keall H., Ostro E., Bergenholtz G. Biocompatibility of surface-sealed dental materials against exposed pulps. J. Prosthet. Dent. 1987;57:1–8. doi: 10.1016/0022-3913(87)90104-1. [DOI] [PubMed] [Google Scholar]
- 43.Wilson N., Lynch C., Brunton P., Hickel R., Meyer-Lueckel H., Gurgan S., Pallesen U., Shearer A., Tarle Z., Cotti E., Vanherle G., Opdam N. Criteria for the replacement of restorations: academy of operative dentistry European section. Operat. Dent. 2016;41:S48–S57. doi: 10.2341/15-058-O. [DOI] [PubMed] [Google Scholar]
- 44.Fda . US Food& Drug administration; 1985. CFR - Code of Federal Regulations Title 21. [Google Scholar]
- 45.Tang Z., Lv B. MgO nanoparticles as antibacterial agent: preparation and activity. Braz. J. Chem. Eng. 2014;31:591–601. [Google Scholar]
- 46.Naguib G., Hosny K., Hassan A., Al Hazmi F., Al Dharrab A., Alkhalidi H. Hamed M. Zein based magnesium oxide nanoparticles: assessment of antimicrobial activity for dental implications. Pak. J. Pharm. Sci. 2018;31:245–250. [PubMed] [Google Scholar]
- 47.Alshehri S., Tiwari R., Alsulays B., Ashour E., Alshetaili A., Almutairy B., Park J., Morott J., Sandhu B., Majumdar S., Repka M. Investigation of the combined effect of MgO and PEG on the release profile of mefenamic acid prepared via hot-melt extrusion techniques. Pharmaceut. Dev. Technol. 2017;22:740–753. doi: 10.3109/10837450.2016.1138129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Igawa K., Yoshinari N., Okumura M., Ohtsu H., Kawano M., Konno T. Crystalline-amorphous-crystalline transformation in a highly brilliant luminescent System with trigonal-planar gold(I) centers. Sci. Rep. 2016;6:26002. doi: 10.1038/srep26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman J. The role of Streptococcus mutans in the formation of dental caries: an ecological perspective. The Sci J Lander College Arts Sci. 2011;5:40–46. [Google Scholar]
- 50.Odonkor S., Addo K. Bacteria resistance to antibiotics: recent trends and challenges. Int. J. Biol. Med. Res. 2011;2:1204–1210. [Google Scholar]
- 51.Wang Q., Zhang C., Chu C., Zhu X. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int. J. Oral Sci. 2012;4:19–23. doi: 10.1038/ijos.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akwa A., Zabara A., Al-Shamahy H., Al-labani M., Al-Ghaffari K., Al-Mortada A., Al-Haddad A., Al-Sharani A. Prevalence of Staphylococcus aureus in dental infections and the occurrence of MRSA in isolates. Univ J Pharma Res. 2020;5:23–27. [Google Scholar]
- 53.Vicentini D., Smania A., Laranjeira M. Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater. Sci. Eng. C. 2010;30:503–508. [Google Scholar]
- 54.Nassar H., Gregory R. Biofilm sensitivity of seven Streptococcus mutans strains to different fluoride levels. J. Oral Microbiol. 2017;9:1–7. doi: 10.1080/20002297.2017.1328265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naguib G., Nasser M., Mirdad L., Mirdad F., Merdad Y., Alturki B., Bakhsh T., Turkistani A., Hamed M. Surface characteristics of composite resin enhanced by new antibacterial nanofillers. Int J Current Adv Res. 2018;7:15965–15969. [Google Scholar]
- 56.Arora S., Arora A., Upadhyaya V., Jain S. Comparative evaluation of marginal leakage of provisional crowns cemented with different temporary luting cements: in vitro study. J. Indian Prosthodont. Soc. 2015;16:42–48. doi: 10.4103/0972-4052.164911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Souza ArauÂjo A., De Paula A., Alonso R., Taparelli J., Mei L., Stipp R., Puppin-Rontani R. A novel Triclosan Methacrylate-based composite reduces the virulence of Streptococcus mutans biofilm. PloS One. 2018;13 doi: 10.1371/journal.pone.0195244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran R., Jung D., Spokoyny A. Cross-linking dots on metal oxides. NPG Asia Mater. 2019;11:1–4. [Google Scholar]
- 59.Dizaj S., Lotfipour F., Barzegar-Jalali M., Zarrintan M., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]