Abstract
Abnormal tendons are rarely ever repaired to the natural structure and morphology of normal tendons. To better guide the repair and regeneration of injured tendons through a tissue engineering method, it is necessary to have insights into the internal morphology, organization, and composition of natural tendons. This review summarized recent researches on the structure and function of the extracellular matrix (ECM) components of tendons and highlight the application of multiple detection methodologies concerning the structure of ECMs. In addition, we look forward to the future of multi-dimensional biomaterial design methods and the potential of structural repair for tendon ECM components. In addition, focus is placed on the macro to micro detection methods for tendons, and current techniques for evaluating the extracellular matrix of tendons at the micro level are introduced in detail. Finally, emphasis is given to future extracellular matrix detection methods, as well as to how future efforts could concentrate on fabricating the biomimetic tendons.
Keywords: Tendon, Collagen fibrillogenesis, Extracellular matrix, FIB-SEM, Biomimetic
Graphical abstract
Highlights
-
•
Summarize recent research on the structure and function of the extracellular matrix (ECM) components of tendons.
-
•
Comments on current research methods concerning the structure of ECMs.
-
•
Perspective on the future of multi-dimensional detection techniques and structural repair of tendon ECM components.
1. Introduction
Tendon is a uniaxial connective tissue component of the musculoskeletal system, a unique form of connective tissue that links muscle to bone, which is capable of withstanding tension [1,2]. The physiological function of tendons is to transmit force between muscles and bones, which is mediated by the tenocyte extracellular matrix (ECM) components within the tissue ultrastructure [3]. According to clinical data, primary disorders of tendons (tendinopathies), due to overuse, age related degeneration and inflammation [4,5]. Injured tendons are rarely ever repaired to the natural structure and morphology of normal tendons, but instead often result in the formation of scar tissue [6].
In recent years, great strides have been made in the area of tendinogenesis and differentiation towards tendon cells due to a greater understanding of the tendon stem cell niche, development of advanced materials, improved scaffold fabrication techniques, and delineation of the phenotype development process [7]. However, the putative repaired tendon loss the mechanical function to a large extent. Collagen fibrils diameter stops increasing to form a heterogeneous feature but remain the same size as the immature tendon tissue consisted of small homogeneous collagen fibrils [8]. The reason that adult tendon regeneration does not recapitulate embryonic tendon tissue development is not well understood. Hence, understanding intrinsic morphology, organization, and composition of tendon is essential to comprehending the architectural phenomena and the biomechanical cues of the tendon after damage occurs. This, in turn, is important for optimizing the method of tissue engineering and stem cell differentiation [3,9,10].
Analyzed using different kinds of measurement and imaging techniques, the structure and function of adult tendon ECM components under health and normal conditions are summarized here. Additionally, the structure of different components of the ECM of tendons during embryonic and postnatal tendon development are displayed, paying particular attention to the collagen fibrillogenesis that forms a functional and mature tendon. The ultrastructure of abnormal tendons, as in the case of tendinopathies and aging, are also elaborated. These efforts are intended to enhance the understanding of tendon structure and to provide assistance when designing regenerative strategies.
2. Architecture of the mature tendon
A tendon is a fibrous soft connective tissue structure that acts passively and is relatively inelastic with high tensile strength [11].
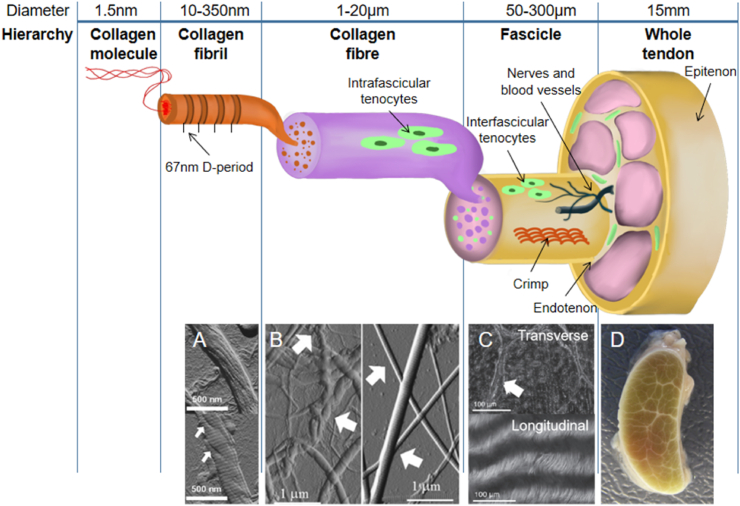
Tendons have a hierarchical organization (Fig. 1). A connective tissue sheath called the epitenon encloses the periphery of the tendon. The subunits of the tendon are the fascicles, which consist of numerous collagen fibers that are bound together. Fascicles are irregular in shape and vary in diameter, ranging from 150 to 500 μm [11], having a complex interweave arrangement [12]. Surrounding the fascicles is a connective tissue compartment termed the interfascicular matrix (IFM), also called endotendinous connective tissue (endotenon), which contains blood vessels and nerves [13]. The endotendon divides the tendon into the fascicle subunits and connects with the epitenon.
Fig. 1.
Schematic diagram of the structural hierarchy of tendons showing the organization of collagen bundles [13,20,21]: collagen molecules aggregate into collagen fibrils; collagen fibrils gather to form collagen fibers; collagen fibers combine to form fascicles. Below the schematic diagram, images corresponding to different levels are also displayed. (A) The fibril shown in the upper part is unwound, resembling a rope, and the D-period is almost invisible; whereas the fibril shown in the lower part is less unwound (arrowhead) with a clear D-period. Scale bars = 500 nm [22]. (B) Part of the fibrils in the left image show a significant crimp (spiral morphology) (arrowhead) and are tangled together. The image on the right shows that the collagen in the mouse tail tendon is straight. Scale bars = 1 μm [22]. (C) The upper part is the transverse section of the tendon, showing the endotenon sheath enveloping the tendon fascicles; the lower part is the longitudinal section of the tendon, and the collagen crimp is clearly presented. Scale bars = 100 μm. (D) Transverse section of a whole tendon [11]. Reproduced and modified with permission of Elsevier and John Wiley and Sons, respectively. Fig.1 A and B were taken by atomic force microscopy, C was taken by upright light microscope.
Tenocytes secrete the ECM and growth factors to maintain tendon homeostasis. According to the location distribution of fibroblasts, the cells distributed in the IFM area are termed interfascicular tenocytes, while the cells distributed in the fascicle area are termed intrafascicular tenocytes. Fluorescence staining against connexins 32 and 43 showed positive results in mature tendon cell-cell junctions [14].
The subunits of collagen fibers are fibrils, the diameter of which ranges from a few nanometers to about 500 nm. In mature tendons, the diameter of collagen fibrils exhibits a bimodal distribution [6], which is intended to provide tensile strength and counteract fibril slip [15]. Specific inter-molecular cross-links bind adjacent microfibrils together, thereby keeping collagen fibrils stable [16,17]. Fibrils are densely packed, and individual glycosaminoglycan chains usually run orthogonal to the fibril axis, passing from one fibril to another [17]. Collagen molecules align longitudinally and interlace with an adjacent molecule. This arrangement results in the periodicity of fibrils, showing a nearly 67 nm-wide band of D-spacing (also termed D-period) [18,19]. The value of D-spacing is considered to be independent of species and to exhibit a 10-nm wide distribution, with the difference of D-spacing being less than 1 nm in a single fascicle [19].
3. Distribution and structure of mature extracellular matrix components
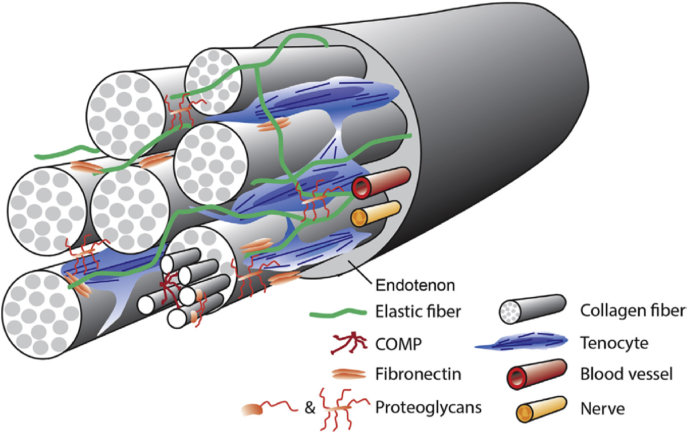
The ECM of the tendon includes collagen and non-collagenous components. Collagen is the most abundant component of the ECM of the tendon, accounting for 60–85% of its dry weight [8,23]. A highly aligned collagen component enables the tendon to bear a certain degree of tension and has sufficient tensile strength, while the non-collagen component, scattered in the collagen-rich hierarchical structure, makes the tendon viscoid, and the expression of the non-collagenous component changes with time (Fig. 2). For a certain tendon, the internal force is not uniform when it is subjected to longitudinal force, which leads to the tendon composition varying longitudinally within a tendon. In addition, the component content of the tendon differs due to different types of stress. For example, the tendon region mainly subjected to tensile force has a highly aligned arrangement of collagen fibrils and low-level proteoglycan expression. Non-collagen components increase the water content of tissues to some extent, and studies have shown that non-collagen components almost exclusively account for the biological, structural, and composite changes that occur after birth to adapt to functional changes [24]. Hence, the basic importance of the non-collagen matrix has been emphasized [25].
Fig. 2.
Schematic of tendon architecture. Collagen fibers, proteoglycans, and glycoproteins (COMP and elastic fiber) distribute in the tenocyte ECM and interact with adjacent components. Reprinted from Ref. [27] with permission.
In general, the expression levels of ECM components in the tendon differ with respect to maturity changes (embryo, young, mature, old); different types of tendons, such as flexor tendons, achilles tendons, rotator cuffs, and patellar tendons, have different expression levels of ECM components due to different mechanical stimulations. Liquid chromatography tandem mass spectrometry (LC-MS/MS) is used to measure the quantitative expression of different components [26]. Here, the main components of the ECM of the tendon are introduced, but it is worth noting that in addition to these components, the proteome data provided by Clegg et al. appear to demonstrate that there are many infrequent types of collagen that also exist in the tendon. This finding likewise applies to glycoproteins and proteoglycan families [25].
3.1. Collagens
There is some controversy about the fibril length in adult tendons. Direct structural investigations of fibrils in mature tissue favor continuity [[28], [29], [30]]. These fibrils have been observed to bend back on themselves to form a hairpin ring, curving back on themselves twice or branching (one fibril separates into two fibrils) [30]. However, according to the results of mechanical tests on rat tail tendons, collagen fibers have been demonstrated to be discontinuous [31,32], and long discontinuous fibers have been shown to be composed of 5–10 mm-long short fibrils [30,33].
The collagen network follows circadian rhythms. While tendon fibrils are divided into three groups according to diameter (<75 nm; 75–150 nm; > 150 nm), the trimodal distribution of tendon collagen changes with time and follows a periodicity of 24 h. The relative position of collagen fibrils with a diameter greater than 75 nm remains unchanged, while the position of those with a diameter less than 75 nm constantly changes. The smaller diameter (<75 nm) fibrils wrap around the larger diameter (>75 nm) fibrils and make contact with different larger diameter fibrils [24]. The synthesis and degradation of collagen via circadian rhythms affect the whole collagen pool and maintain the homeostasis of collagen.
The main collagen types in the tendon can be divided into several classes: fibrillar fibril-forming collagen, non-fibrillar fibril-associated collagen with interrupted triple helices (FACIT) collagen, and beaded filament-forming collagen (Table 1) [[34], [35], [36], [37]].
Table 1.
| Classification | Collagen types | Supramolecular structure |
|---|---|---|
| Fibril-forming collagen | I, III | Striated fibrils (67-nm periodicity) |
| V, XI | Striated fibrils, retain N-terminal regulatory domains | |
| FACIT collagen | XII, XIV | Associated with fibrils, other interactions |
| Beaded filament-forming collagen | VI | Beaded filaments, networks |
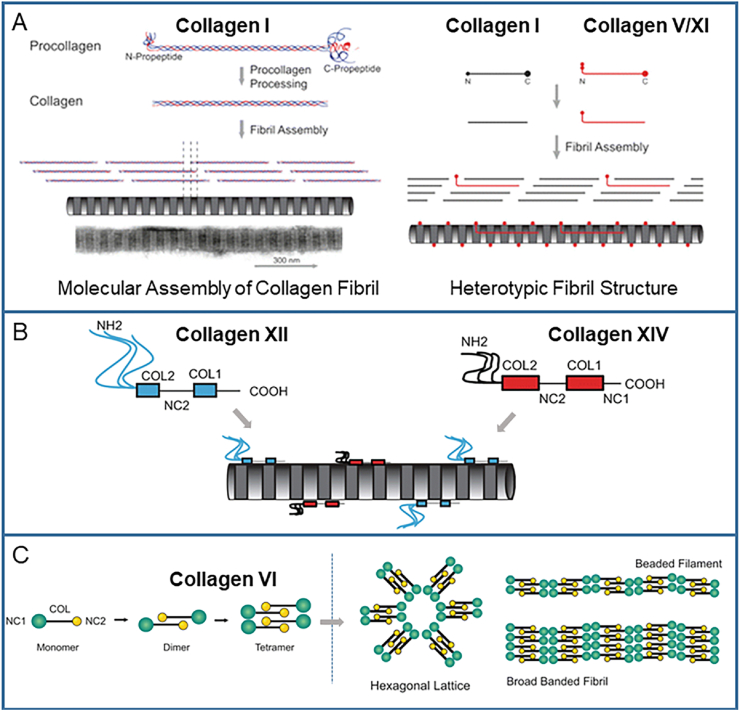
Collagen type I is the most abundant, accounting for 97–98% of the total collagen content in the tendon, while collagen type III accounts for only 1–1.5% of the total collagen content [13,[39], [40], [41]]. Type I collagen is heterotrimeric and comprises two α1 chains and one α2 chain, determined by Col1A1 and Col1A2 independently, nearly all parallel to the tendon axis. The crystallographic determination of the supramolecular structure of type I collagen has revealed the stacking topological structure of collagen molecules: hypertwisted and discontinuous right-handed quasi-hexagonal microfibrils crossed with adjacent microfibrils to form a crystal superlattice [42,43]. Type III collagen is highly expressed during embryonic development [44,45] and is thought to play an important role in regulating the diameter of collagen I fibrils [34,46].
Type V and XI collagen are located in the core of collagen fibrils [47]. While aggregating with type I collagen to form the heterotypic collagen fibril, the partially processed N-terminal propeptide of collagen V and XI protrudes in the gap region, leading to the surface irregularity of the fibril [35] (Fig. 3A). The expression of COL11A1 results in an abnormal fibril structure with an obvious smaller diameter in the tendon and to the disruption of the parallel arrangement of fibers, indicating that collagen XI mainly plays a role in the development process [48].
Fig. 3.
Schematic of the collagen structure. (A) The homotypic and heterotypic collagen fibrils are synthesized by fibril-forming collagens. (B) Short collagenous (COL) domains of FACIT collagens are interrupted by non-collagenous (NC) domains, and FACIT collagens adhere to the surface of collagen fibrils. (C) Beaded filament-forming monomers are polymerized into dimers, after which two dimers are polymerized to form tetramers, and the tetramers subsequently form different polymers. Reproduced and modified from Ref. [38] with permission.
Type VI collagen is enriched in pericellular regions. This type of collagen can be assembled into different polymers: beaded micro-filaments, broad-banded structures, and hexagonal lattices [34] (Fig. 3C). The absence of collagen VI leads to the abnormal arrangement of fibrils, a decrease of the cross sectional area, and a decrease of mechanical properties [[49], [50], [51]]. This indicates that when the pericellular environment is interrupted, the sequestering of small leucine-rich proteoglycans will be affected and the formation of fibrils will be disturbed [36,50].
Collagen XII and XIV are also considered to have a synergistic effect on collagen fibril formation, with the former alleged to stabilize the collagen fibers, and the latter assumed to limit the diameter of collagen fibrils. Both types are classed as FACIT collagens and adhere to the surface of the collagen fibrils (Fig. 3B). They also provide a molecular bridge facilitating the interaction of collagen fibrils and other molecules in the ECM, such as the proteoglycans decorin and fibromodulin [52]. Collagen XII can be covalently linked to glycosaminoglycan chains [38], and the abnormally reduced expression of Col12a1 influences tenocyte organization, destroys the connection between cells, and leads to the deformation of the tendon hierarchy and to the decrease of mechanical properties [53].
3.2. Proteoglycans
Proteoglycans (PGs) are also important components of the tendon ECM. The core of a proteoglycan is a protein, and more than one type of glycosaminoglycan (GAG) side chains are connected to this core protein [11]. GAGs are highly negatively charged unbranched polysaccharides, small amounts of which absorbs water into a gel. PGs in the tendon include two classes: small leucine-rich proteoglycans (SLRPs) and large aggregating PGs.
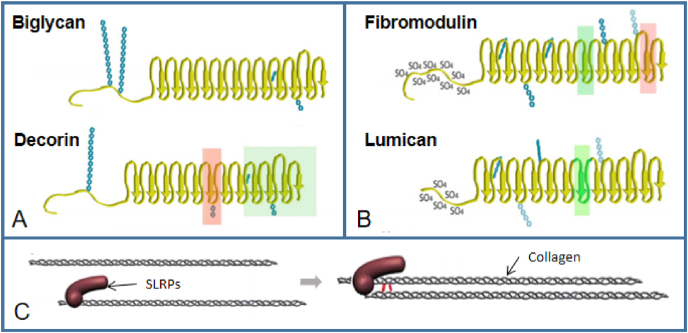
SLRPs, classified as class I (includes biglycan and decorin, Fig. 4A) and class II (includes fibromodulin and lumican, Fig. 4B), are most abundant in tendons, with decorin accounts for roughly 80% of the total proteoglycan content of the tissue [54]. And these content can be improved through resistance training [55]. These proteoglycans are known to regulate collagen fibril assembly and are postulacted to transfer mechanical force between fibrils [56]. Via steric hindrance, directly connecting two collagen monomers or creating interfibrillar links during fibril growth, SLRP–collagen interactions lead to the juxtaposition of collagen molecules [57] (Fig. 4C). Decorin (DCN, PGS2), a horseshoe-shaped protein that non-covalently binds to specific regions of collagen fibers (I, VI, XII, XIV) [55], is the most abundant SLRP in the tendon. The expression of decorin is rhythmic, corresponding to the circadian rhythm of collagen fibers synthesis [24]. The GAG side chain of two decorins respectively attached to two collagen fibrils can interact and form an interfibrillar bridge to connect the neighboring fibrils. Decorin exists in the IFM and in the fascicles [58], and is supposed to limit the lateral growth of collagen fibrils [57,59]. Biglycan (BGN, PGS1) and decorin compete for the binding site of collagen fibrils and are necessary for maintaining the fibril structure of collagen [60]. Similarly, lumican (LUM) and fibromodulin (FMOD) share binding sites different from the class I SLRPs [58]. Decorin and lumican have been demonstrated to lead to larger interfibrillar spaces and decreased fibril diameters in vitro [61]. Hence, it is proposed that these four SLRPs can inhibit the lateral fusion of collagen fibrils.
Fig. 4.
Schematic of SLRP in the ECM of tendons and SLRP–collagen interaction. (A) Class I SLRPs. (B) Class II SLRPs. Red rectangles show the identified collagen-binding regions with high affinity, while the green rectangles correspond to collagen-binding regions with low affinity. Blue chains show the glycosylations. (C) Possible SLRP regulation of collagen fibrillogenesis. Reprinted from Ref. [57] with permission.
Aggrecan, lubricin, and versican are large aggregating PGs. Their large core protein contains a number of domains that can generate many specific interactions [62,63]. In the compressive regions of the tendon, the expression level of aggrecan and lubricin increases [64]. Aggrecan slows down collagen gelation as well as collagen fibrillogenesis [63]. Lubricin (also termed superficial zone PG, PRG4) is preferentially expressed in the epitenon and endotenon. It has a role in lubricating the tendon surface while tendon gliding with each other and preventing cellular adhesion to the tendon surface [58,65]. Versican localizes in the IFM and predominantly distributes pericellularly supporting cell-shape changes that required for cell proliferating and migrating [66]. Versican mainly exits in the dermis of skin and in the media of the aorta that interacting with elastic fibers increasing viscoelasticity of pericellular matrix [67,68]. And lower level appears in tendon which G3 domain containing a C-type lectin region is associated with fibulins 1 and 2 [69]. It has been demonstrated that versican promotes collagen fibrillogenesis as well as upregulates collagen compaction and reorganization mediated by cells [63].
3.3. Glycoproteins
Carbohydrates covalently link to proteins to form glycoproteins. Cartilage oligomeric matrix protein (COMP), also termed thrombospondin-5 (TSP-5), is an abundant component in the tendon [70]. Knockout or mutation of COMP genes are associated with cartilage abnormalities but no tendon abnormalities [71,72]. The five subunit “arms” of COMP are arranged around a central cylinder [73]. This multi-domain modular structure of COMP contribute to its interaction with ECM proteins [74,75]. For example, COMP brings free collagen I closer to each other and promotes its further assembly [74]. COMP has also been shown to bind with lubricin both non-covalently and covalently by in vitro experiments [76]. In addition, COMP also interacts with growth factors and acts as a “lattice” to facilitate their use by cells [77].
Tenascin-C (TNC) is a hexameric, multimodular ECM glycoprotein. Tenascin-C binds to fibronectin, collagen, and fibrillin-2, as well as to proteoglycans, although the binding sites for these molecules have not yet been fully mapped [78]. Tenascin-C is widely distributed and highly expressed in embryonic tissues, but its distribution in mature tissues is limited and its expression is relatively low [79]. Under the conditions of wound healing, pathology, or high mechanical force, tenascin-C has relatively high expression [80,81]. Therefore, it is assumed that tenascin-C may have an effect on tissue elasticity [79]. Additionally, tenascin-C has also been described as a modulator of cell adhesion.
Fibronectin (FN) consists of a dimer bound by a disulfide bond. Each of its subunits comprises 29 to 31 modules, the connections of which have been likened to “beads on a string.” The well-organized secondary and tertiary structures of these modules can be defined by nuclear magnetic resonance and crystallography, and the module–module interactions are likely to maintain a compact quaternary structure [82]. Alternative splicing produces many different forms of fibronectin so that fibronectin regions can be exposed for binding to collagen, heparan sulfate, and integrins on the cell surface [81,83].
Elastic fibers have anti-fatigue elastic properties and serve the function of energy storage [58]. It could associate with molecules such as decorin and biglycan. The thin sheath of elastic fibers is localized between and surrounds the fascicles in rat tail tendons [84]; while in bovine flexor tendons, elastic fibers are widely distributed between and within the fascicles (specifically localized around cells) both longitudinally and perpendicular to the tendon fibers [22]. When paralleling to the tendon fibrils, the elastic fibers form the same crimp conforming to the collagen fibers of the tendon. Elastic fibers consist of a central cylinder of elastin (ELN) enveloped by scattered fibrillin-1 and fibrillin-2. Elastin represents approximately 1–10% of the dry weight of the tendon, as well as 10% of the tendon's volume [84]. Elastin has a wide range of intermolecular cross-links and endows tissues with elasticity. Although fibrillin-1 and fibrillin-2 are co-located with elastin most of the time, fibrillin-1 is occasionally found independently [22], whereas fibrillin-2 has been found to be more distributed in the interior of the fascicle [67].
4. Distribution of ECM components varies in different regions of mature tendon
This section discusses the differences in the content and distribution of ECM components in mature tendons. According to function, tendons can be classified into energy-storing tendons and positional tendons, both of which are discussed in detail in 4.1. According to the internal structure of the tendon, the composition of the ECM in the IFM and in the fascicle is different, which is discussed in detail in 4.2. Further, the tendons that connect bone and muscle are called the enthesis and the myotendinous junction (MTJ), respectively. These tendons have an ECM composition that is more varied than in ordinary tendons, a feature that is discussed in detail in 4.3 and 4.4.
4.1. Different compositions of collagen fibrils in energy-storing tendons and positional tendons
Although all tendons transfer muscle-generated force to bones, specific tendons can also reduce the energy consumption of exercise by stretching and recoiling. According to different functions, tendons can be divided into two categories: the positional tendon, and the energy-storing tendon. Positional tendons, which are more stiff, are relatively inextensive under physiological loads, whereas energy-storing tendons are characterized as more extensive [41,85]. Therefore, the two types of tendons show different stress–strain curves [86]. Relevant data have shown that energy-storing tendons can withstand pressure of up to 90 MPa and can stretch as much as 16% under strain, whereas positional tendons can only bear pressure of 20–30 MPa and cannot stretch more than 3% under strain [85,87].
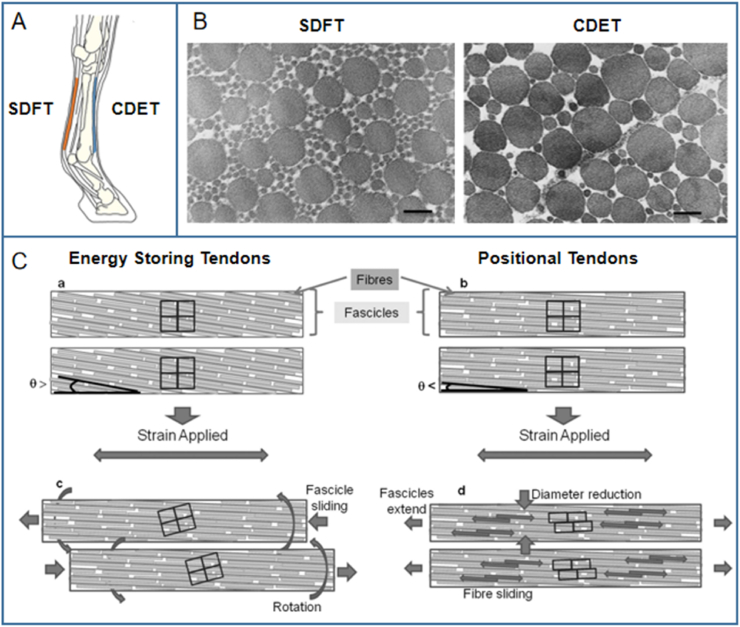
The results of confocal microscopy and scanning electron microscopy have demonstrated that in the positional common digital extensor tendon (CDET), the extension of fascicles occurs via sliding between the fibers inside the fascicles; in the energy-storing superficial digital flexor tendon (SDFT), on the other hand, fascicles have a large degree of rotation (Fig. 5) [[88], [89], [90]]. This rotation indicates that the spring-like helical component in the energy-storing SDFT provides superior recoverability and fatigue resistance to tendon fascicles [90]. The fibril diameters of positional fascicles are often larger than in energy-storing fascicles, but the mature pyridinium-type cross-links are always relatively fewer in positional tendons [41,91,92]. Although the total collagen content of the two types of tendons are similar, the level of collagen type III in energy-storing tendons is higher. Hence, it is suggested that the matrix turnover ability of cells varies in energy-storing tendons and positional tendons [41]. This highlights the need to distinguish the composition of the ECM from a functional perspective in the study and reconstruction of different tendons.
Fig. 5.
The difference between the energy-storing SDFT and the positional CDET. (A) Schematic of SDFT and CDET in an equine forelimb.[26] (B) Electron micrograph of the transverse section of SDFT (left) and CDET (right). Scale bar = 200 nm[86] (C) The different extension mechanisms of energy-storing and positional tendons.[90] Reproduced with permission.
4.2. Different expression levels of tendon ECM components in the IFM and fascicles
Fascicles and the IFM contain different kinds of matrix components, and many proteins exhibit different abundances between matrix phases. The IFM has a more complex proteome than the fascicle matrix, an observation supported by the results of laser-capture microdissection and mass spectrometry [93]. Clegg et al. separated the tendon into the IFM and the fascicle and evaluated them respectively. The mechanical properties of the isolated IFM and the individual fascicle indicate that mechanical adaptation is localized to the IFM. The immunohistology of decorin (DCN), fibromodulin (FMOD), lubricin (PRG4), and tenascin-C (TNC) of the fascicle and the IFM in the SDFT and the CDET shows that PRG4 and TNC are mainly present in the IFM of the tendon, but are rarely found in the fascicle. Elastin is also preferentially located in the IFM. During postnatal development, the proteome of the fascicle remains unchanged under loading, while the IFM proteome begins to change. In conclusion, it is suggested that adaptation depends only on the evolution of IFM components [25].
4.3. The transition from collagen type I to collagen type II in the enthesis
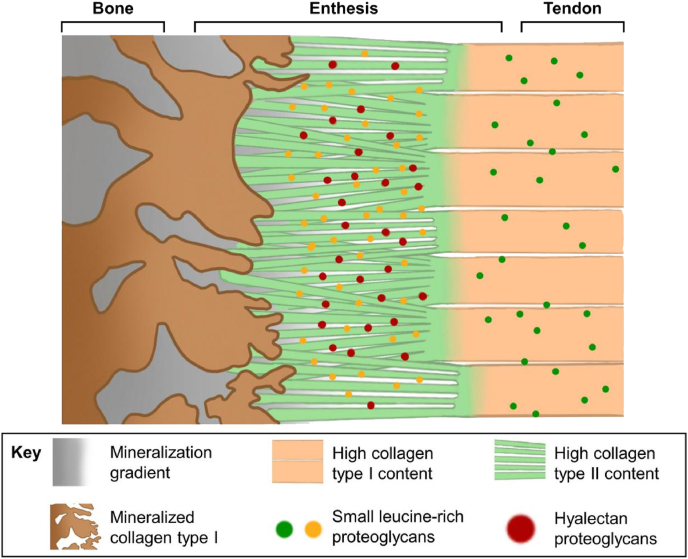
Tendon-to-bone interface is a functionally graded tissue which means transitioning from about 200 MPa tensile modulus at the tendon end to about 20 GPa tensile modulus at the bone [5], across just a few hundred micrometers [26]. As a transitional tissue, the enthesis has a complex hierarchical structure—four distinct zones along the longitudinal direction: tendon proper, unmineralized fibrocartilaginous tissue, mineralized fibrocartilage, and bone [94]. From tendon to bone, the collagen fibril component of the enthesis gradually changes from type I to type II [26] (Fig. 6). The unmineralized fibrocartilaginous tissue zone contains abundant proteoglycans, while the mineralized fibrocartilage zone contains varying amounts of bone minerals and the osteogenic collagen type X [95].
Fig. 6.
The schematic of the enthesis, with the fundamental structure and the molecular components highlighted. Reprinted with permission from [26].
4.4. ECM components vary in the finger-like processes of the myotendinous junction
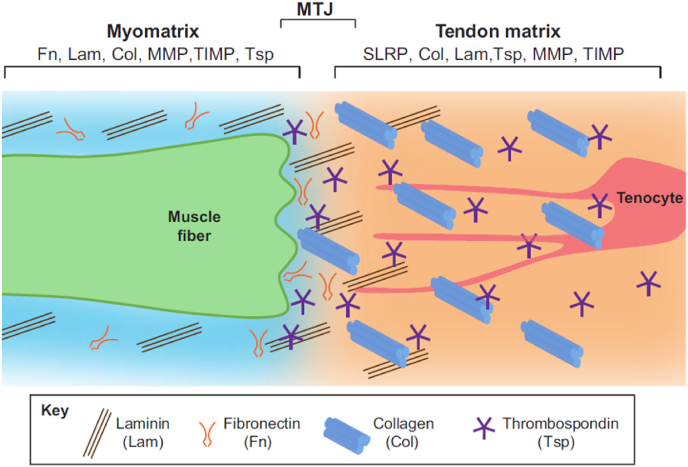
The myotendinous junction (MTJ), where tenocytes connect to muscles through the ECM, is easily injured. Muscle and tendon fibers interdigitate through finger-like processes in the narrow region of the MTJ. This finger-like structure increases the contact area, facilitating the transfer of muscle contraction to the tendon [96]. The MTJ has a composition similar to the ECM in tendons and is mainly composed of collagen and many other non-collagen ECM components [54]. Components in the ECM guide the connection between muscle fibers and tendon collagen fibers, regulate the signal of both tendon progenitor cells and muscles, and regulate the maturation of the MTJ [96]. However, the different proportions of the various components lead to different intensities of the MTJ and tendons (Fig. 7). An abnormal MTJ can lead to muscle dysfunction, indicating that this connection plays a key role in muscle function [97]. Swimming training has been shown to promote tissue reorganization and to increase the length of sarcoplasmatic invaginations and evaginations [98].
Fig. 7.
The schematic of the myotendinous junction (MTJ). The composition of the ECM is gradually different in the myomatrix, the MTJ matrix, and the tendon matrix. Reprinted with permission from [97].
According to the current findings, these tendon components do have differences among different positions, but data varies from different species. In order to provide better strategies for better recovery of tendinopathy patients, further study should be needed to find out whether there are similar situations in human tendon.
5. Characteristics of tendon ECM components during growth and development
The results of polarized light microscopy and transmission electron microscopy (TEM) showed that the diameter of collagen fibers in newly born tendon tissue was small and uniform. On the 7th day after birth, the tendon tissue structure was determined and showed a trend of difference in terms of collagen fibril diameter. In a rat post-natal Achilles tendon model, on day 14, two different ranges of collagen fibril diameters were clearly observed. On the 56th day, the parallel arrangement of collagen fibers and the bimodal distribution of collagen fibril diameters could be observed [6]. In general, with the development of tendons and the deposition of new fibers, the tissue structure becomes more dense and regular [99].
The expression of collagen type I, III, and V and TNC are higher in the immature tendons of horses than in mature tendons, while decorin expression increases with age.[99] During development, the mechanical properties of mouse tail tendons exhibit obvious changes, with their ultimate stretching ability becoming greater and the occurrence of inelastic deformation becoming less frequent. However, the expression change ratio of collagen type I and elastin, related to mechanical properties to a great extent, cannot keep up with the changes in mechanical properties [84].
Collagen fibrillogenesis model indicates the mature structure of fibrillar collagen. From the embryonic stage to the neonatal stage to the adult stage of the collagen structure, the change and distribution of the diameter of tendon collagen fibers, as well as the change of tendon length, has caused researchers to reconsider the process of collagen fiber formation. The force exerted by muscles on one end of the tendon micro-filament is transferred through the macro length in the whole tendon by some unknown mechanism. Therefore, it is necessary to determine the length change of collagen fibrils. From infancy to maturity, how the collagen fibrils become thicker and longer and whether the fibers are continuous are still unsolved problems. Many molecules are involved in collagen fibrillogenesis in the developing tendon, such as collagen type III, XII, and XIV, SLRPs, COMP, cytoskeleton actin, and scleroaxis [13].
The secretion process of type I collagen is in focus since it is the main component of the tendon [13]. Procollagen monomers go through many post-translational modifications after been translated. After C- and N-propeptide folding and C-propeptide disulfide bonding, monomers are folded and assembled to form the procollagens. Procollagens possess a triple helical collagenous conformation containing non-collagenous C- and N-terminal propeptides [34]. Procollagen molecules are transported from the rough ER to the Golgi apparatus by vesicular tubular clusters (VTCs). Due to the long length of procollagen, its accumulation in the Golgi apparatus will cause morphological changes and volume expansion of the Golgi body. However, as procollagen is discharged from the vesicles on the Golgi membrane, secretory vesicles, called Golgi-to-plasma membrane carriers (GPCs), are formed [100], resulting in the contraction of the Golgi membrane area, which is suggested to increase the concentration and accumulation of procollagen and to facilitate longitudinal alignment. Typical non-collagen-related vacuoles are spherical, with a diameter varying from 50 to 100 nm [101], while the length of GPCs ranges from 300 to 1700 nm [100].
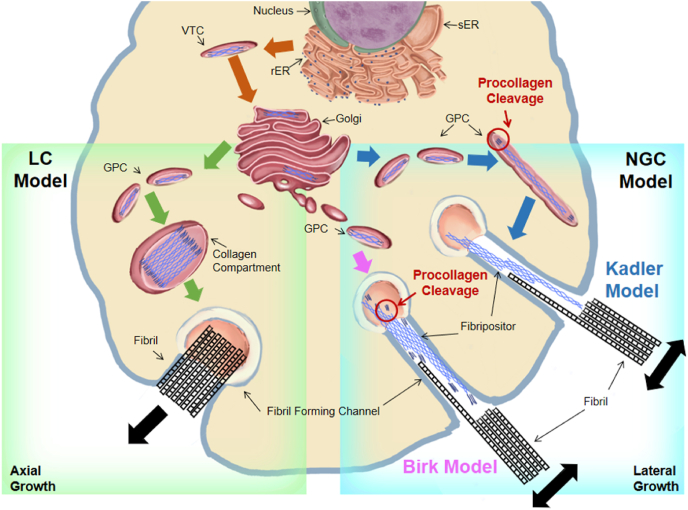
Procollagens can be cleaved from the C- and N-terminals to form rod-like tropocollagen proteins, 300 nm in length and 1.5 nm in diameter, which could form the collagen fibrils with D-spacing spontaneously [13]. Birk and Kadler proposed different models for the time and place of procollagen cleavage (Fig. 8). According to Birk's model, the procollagen wrapped in GPCs moves directly to the cell membrane, fuses with the fibril-forming channel, and excretes procollagen out of the cell. Then, in the fibril-forming channel, procollagen is cleaved by extracellular proteases to form tropocollagen [102]. In Kadler's model, the cleavage of procollagen occurs in GPCs, after which the tropocollagen proteins are secreted from the cell through the fibril-forming channel and form fibripositors [100,103,104].
Fig. 8.
A scheme of NGC model and LC model. In the NGC model, the fibroblast forms ruffled protrusions or depressions called fibripositor. Collagen fibril nucleation and growth starts within fibripositor. Individual fibrils then coalesce into bundles when they are excreted into the ECM. In the LC model collagen precursors pre-align in secretory compartments or the ECM and form a bundle simultaneously.
Both Birk's and Kadler's models support the nucleation, growth, and coalescence (NGC) model and the liquid crystalline (LC) model, which are theories about the arrangement of collagen fibrils after cellular secretion [19]. In the NGC model, the tropocollagens are secreted from the cell and targeted to the fibripositors. Fibripositors' tip act as the core to guide the deposition of nearby tropocollagen and to make the tropocollagen fuse laterally and vertically in the ECM [105]. Therefore, the collagen fibrils can gradually become laterally thicker and longitudinally longer. The D-spacing of collagen fibrils also comes from the visual display of the fusion gaps of the tropocollagen. The NGC model has been verified by some experimental results. Starborg et al. observed a single collagen fibril being secreted from the cell plasma membrane recess [106]. The lateral fusion of fibrils and the tip-to-tip fusion of fibrils have also been observed [100,107]. In addition, in the NGC model, the formation of D-spacing is affected by extracellular factors. Therefore, according to the different extracellular environments, D-spacing is suggested to possess a large difference between bundles and a small difference within bundles, which is supported by Ming Fang's experimental data [19]. The LC model was proposed and supported by Giraud-Guille et al. [[108], [109], [110]] LC model suggest another mechanism for the generation of liquid crystalline-like assemblies in extracellular matrices. Giraud-Guille's group assumed that procollagen processing occurs with molecules already in highly ordered liquid crystalline arrays, and fibril assembly is a process of compaction and sliding of prealigned molecules which might be responsible for the D-spacing of collagen fibrils [110].
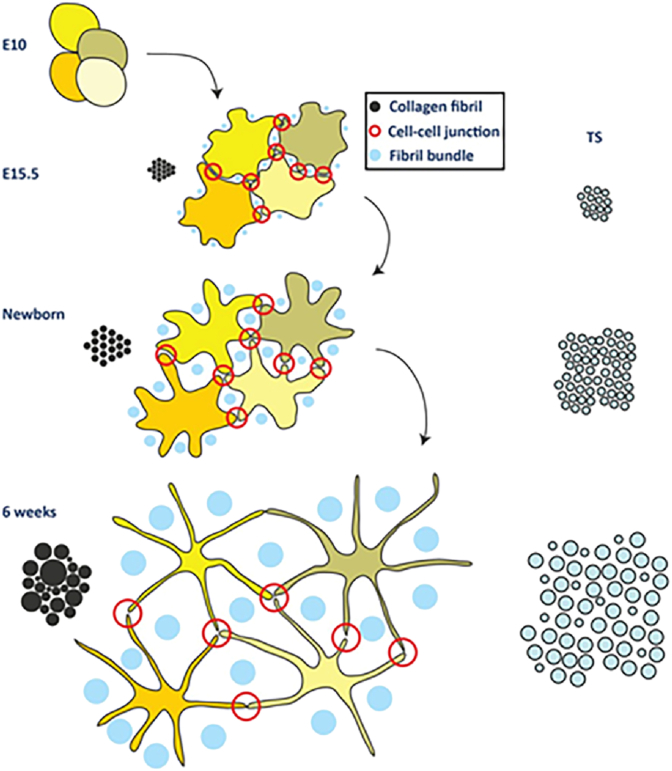
In the process of new fibril formation, such as embryo development or wound healing, the end of the fibril often appears in the plasma membrane recesses [106]. Then, collagen fibrillogenesis continues in a series of extracellular compartments defined by the fibroblasts [52], and the fibrils are enveloped by the cell membrane. After the formation of collagen fibrils, for collagen fibril growth, linear and lateral fusions will lead to the bulk of fiber bundles. Kalson et al. presented a model of fibrous tissue growth (Fig. 9). On the one hand, during the embryonic period, the number and length of collagen fibrils increases, and their diameter slightly expands. Collagen fibrils form a helical crimp structure during embryogenesis and continue to exist postnatally. After birth, growth in the volume of tendons mainly occurs due to the increase in the length and diameter of collagen fibrils. On the other hand, the number of fibroblasts increases in the embryo, after which the number remains constant. The location of cells is relatively fixed during the growth and development process after birth. As a result, existing cells play a role in positioning, and collagen fibrils grow in the position (coarsening or lengthening) delineated by the cells [14,52]. Due to the lateral binding of fiber intermediates, in chick embryos, the diameter and length of fibrils can increase by 10 times or more until the 17th day [13]. Enzymatic fibril cross-link, a trivalent mature bond, is generated from enzyme-catalyzed lysine reactions on telopeptides. Except for lysines, fibril cross-links can also be formed from allysines and histidines [92]. Cross-linking maturity of the tissues affects mechanical behavior of tendon [27]. For example, crosslinks within energy storing fibrils appears to limit molecular sliding, making the fibril structure resistant [111].
Fig. 9.
Schematic of collagen fibril growth. Adapted with permission from Ref. [14].
6. Characteristics of ECM components in abnormal tendons
Age-related changes occur at specific substructure locations and might be ignored by measuring the performance of the entire tendon [112]. With the increase of age, the number of fascicles have different trends in different regions of tendon [12]. In aged tendons, the structure and composition of ECM remained normal in morphological analysis, and the gene expression related to collagen turnover was similar to that of normal tendon, whereas the proliferation of tenocytes decrease and the migration potential of tenocytes is impaired [113]. Advanced glycation end-products (AGEs) accumulate in the aged tissue, while the formation of cross-links may influence the normal function of tendons [92,112]. The mRNA expression of a variety of types I, III, and V and proteoglycan decreases in old animals. Besides, the expression of aggrecan and biglycan mRNA in painful tendons is higher than that in normal tendon tissues, but the mRNA levels of versican and decorin do not change significantly. Tendinitis tendons are characterized by disordered collagen distribution, fiber dispersion, increased mucinous matter and abnormal angiogenesis and neurogenesis [[114], [115], [116]]. In detail, the fibril diameter shift from large to small in the tendinopathic regions and the ratio of small-diameter: total collagen fibrils increase [117]. Besides, compared with normal tendons, tendinopathy leads to an increased cellularity and abnormal buckling round cellular morphology [117,118], an increase in the ratio of collagen type III to collagen type I (might lead to low resistance of tendons) [117,119], and an increase in the expression of fibronectin, tenascin C, aggrecan, versican and biglycan [115,120,121]. The high-level expression of decorin is detected in calcified tendinopathy [119]. In the ruptured tendon, there are no significant changes in aggrecan, biglycan, and versican mRNA levels, but decorin mRNA is significantly decreased [122]. High levels of COMP are present in tendons after physical exercise [81], whereas the relative abundance of COMP in torn tendons is lower than in normal tendons [123]. Although researchers have long been concerned about the huge differences in the expression of ECM components between abnormal tendons and normal tendons, the research on the microstructure of ECM components in abnormal tendons is still limited to the number and diameter of collagen fibers [12,117], and there are few studies on the microstructure of non-collagenous matrix in abnormal tendons.
Tendon overuse and age-related intrinsic tissue degeneration can lead to tendinopathy. Exercise can cause tendon tears or ruptures. Repair of abnormal tendons follows a typical wound-healing process, including the early inflammatory stage, followed by cell proliferation and tendon structural remodeling [124]. The healing process of tendons leads to the formation of fibrotic scars. In addition, the structure and mechanical properties of the healing tissue are not as good as those of normal tendons [8]. After rotator cuff repair, the ultrastructure of tendon–bone interface cells is different from that of normal cells [125]. Normally, scar-tendon formation provides morphological and mechanical support so that tendons can perform the role of transmitting force through muscle and bone. However, excessive scars will lead to tendon adhesion and limit tendon slipping, which further reduce tendon function and lead to chronic complications. The ECM is essential for tendon healing because it provides chemotactic signals to fibroblasts, leukocytes, and endothelial cells and acts as a reservoir of cytokines. It is worth noting that injured adult tendons do not show optimal healing through regeneration, while fetal tendons can heal without scar formation [126]. Mice fetal fibroblasts and adult fibroblasts have been used as seed cells to promote tendon regeneration. The results showed that the expression level of tendon-related genes in fetal fibroblasts was higher, and that the fetal fibroblasts led to higher levels of collagen deposition and better microstructure repair [127].
7. The interaction between tendon stem cells and ECM components
A cluster of stem cells known as tendon stem/progenitor cells (TSPCs) exists in the tendon [128]. TSPCs differentiate into tenocytes to regulating the tendon homeostasis [129]. In the process of growth and development in vivo, TSPCs gradually renew into tenocytes, accompanied by changes in cell characters. Embryonic TSPCs are rounded and assembled in a crowd, while cells in newborn tendons show an obvious wavy orientation corresponding to crimped collagen fiber bundles. Adult tenocytes exhibit a star shape in the transverse section. Cell overlap was observed in embryonic and newborn stage cells in the longitudinal axis, while the cell membrane of adult tenocytes contacted each other and almost no longer overlapped. The distance between cells becomes larger and the cell per unit volume of tissues becomes smaller—that is, the cell density becomes smaller. Besides, the cell volume remains relatively unchanged, but the cell surface area becomes larger. Additionally, the cell membrane extends out to form processing and connects with adjacent cells to form cell–cell contacts. The mature cell processes are longer than in the previous period, but the cell–cell contacts in mature tendon are the same as those in embryo. The interspace between one star-shaped cells and several other star-shaped cells forms channels and encapsulates the fiber bundles (Fig. 9), guiding the position of fascicles [14]. When isolated and cultured in vitro, TSPCs can not only exhibit stem properties like mesenchymal stem cells (MSCs), but also highly express collagen type I, TNC, and FN [128].
The interaction between TSPCs and ECM is very complex, and the influence between them is dynamic and cyclical [130]. The mechanical stimulation of TSPCs leads to the gene expression level fluctuation of ECM components, thus affecting the structure and characteristics of ECM. In vivo, mechanical stimulation results in deformation of ECM, and the force is transferred to tenocytes through the hierarchical structure of ECM, leading to the gene expression changes of tenocytes [131]. This process involves quantitative and qualitative changes in the ECM, mediated by specific enzymes that are responsible for ECM degradation, such as metalloproteinases [132]. When TSPCs are stimulated by mechanical stimulation in vitro, the expression of ECM components fibromodulin, lumican and versican are up-regulated significantly and convincing, the expression of decorin and biglycan is unaltered, while whether the protein levels of collagen type I and III is influenced by mechanical stimulation need to be further clarified [133].
In turn, TSPCs are also regulated by the composition and structure of the ECM niche. biglycan and fibromodulin, PGs highly expressed in the ECM matrix, have been demonstrated to serve as the niche of tendon stem cells, affecting the self-renewal and differentiation of TSPCS. The TSPCs from biglycan and fibromodulin double-knockout mice have a faster proliferation rate and the possibility of forming bone-like tissue, the arrangement of collagen fibers secreted by these TSPCs were more disordered as well. In the ECM absence of biglycan and fibromodulin, TSPCs are speculated more sensitive to BMP-2, thus the fate of TSPCs changes [128]. COLase and ELNase were used to incubate intact tendon tissue in vitro, respectively. Incubation with COLase showed a tendency to inhibit the expression of collagen I in tenocytes, while ELNase may induce COL synthesis after ELN degradation [134]. Changes in the structure and composition of ECM may also interfere with the local release of growth factors and cytokines, thereby affecting cell proliferation, differentiation, migration and adhesion [129,130]. Tendon derived acellular matrix can promote the tenogenesis of TSPCs by regulating specific transcription factors, scleroaxis and Runx 2 [9]. Therefore, maintaining the integrity of ECM is conducive to maintaining the stem cell function of TSPCs. Besides, under artificial intervention, tendon stem cell differentiation is modulated by the topography of the microenvironment constituted by biomaterials [3]. The artificial scaffolds induce and regulate cell adhesion, proliferation, migration, and support cell implantation, and influence the mechanical bearing capacity of cells. The differentiation of the stem cell to the tenocyte lineage could be promoted by native tendon sections [135], the mimicking topography of the natural tendon environment [136], or the decellularized tendon matrix [9]. Study had proved that the microfibers' diameter, stiffness, and alignment can regulate 3D cell shape and enable control of mesenchymal stem cells differentiation and tissue formation.
8. Current evaluation techniques of tendon components
In the process of research on the ECM of tendons, researchers use a variety of methods to detect different component organizations (Table 2). Detection methods can be either macro-scale or micro-scale.
Table 2.
Burgeoning detection methods of the structure of ECM components of tendons.
| Detection methods | Scale rank (as the indicator of resolution) | Objects of study | Special research direction |
|---|---|---|---|
| Fluorescence labeled collagen hybridizing peptides (CHPs) | Optical scale | Collagen fibril | Measuring fluorescence to quantify denatured collagen |
| Birefringence Measurements | 0.2 mm | Collagen, elastin | Elastin distribution [84] |
| high-resolution microcomputed tomography (μCT) | 1.9 μm | Enthesis, tendon | the interface of enthesis [26], the structure of normally mineralizing avian leg tendon [148] |
| Immunofluorescence and laser confocal imaging | 1 μm-100 μm | Cell-cell contacts of tenocytes, enthesis | Common method, the interface of enthesis [26] |
| Synchrotron X-ray diffraction | 100 nm-1 0 μm | Collagen fibril | sub-structural mechanisms occurring during stress relaxation [140] |
| Serial sectioning transmission electron microscopy | 500 nm | Collagen fibril, tenocytes | Secretion of the fibril [106], tenocytes and matrix buckling in tendinopathic region [117] |
| Serial block face-scanning electron microscopy (SBF-SEM) | 100 nm-1 μm | Tenocytes, collagen fibril | 3D reconstruction of cell [14], circularity of fibrils [24] |
| Focused ion beam-scanning electron microscopy (FIB-SEM) | 50 nm-1 μm | Collagen fibril | Fibrils can be extremely long and likely continuous [30], mineralized collagen fibril [148] |
| Time-series TEM | 200 nm | Collagen fibril | Circadian regulation of the tendon collagen ECM [24] |
| SHG microscopy | 20 nm | Collagen fibril | Do not require staining and also can be coupled directly with fluorescence labeling of other components [149] |
| Atomic force microscopy (AFM) | 1 nm-1 μm | Collagen fibril | Examining fibril D-spacing distribution [19], the binding of immunoglobulin receptors and collagen I [143], abundance of collagen-bound proteoglycans [17], mechanical measure of collagen fibrils loaded to failure [92] |
| Cryo-TEM | 1 nm | Collagen VI | Nanostructure of collagen VI microfibrils [150] |
| X-ray and X‐ray crystallography | 3.15 Å-40.0 Å | Collagen fibril, COMP | Crystallographic super lattice of collagen fibrils [42], the structure of a recombinant COMP with growth factors [75] |
At the macro-scale, photography, videography, and ultrasound images have been used to record the fascicle-level structure, showing sliding inside the tendon in response to strain [137]. Tendons are evaluated by computerized MRI 3D seed-growing techniques [138].
At the micro-scale, the common structural and organizational detection method are histological techniques, as well as the combined techniques of confocal and multiphoton microscopy with fluorescence staining, allowing the stretching process of the tendon to be observed [139,140]. High-resolution microcomputed tomography (μCT), polarizing light microscopy, and other methods are also used [26]. A fluorescently labeled collagen hybridizing peptide (CHP) microplate assay could be used to measure the amount of denatured collagen on a series of specimens [86]. Second harmonic generation (SHG) enables in situ imaging of fibrillar collagen architecture in connective tissues. Recently, Circular Dichroism SHG (CD-SHG) microscopy has been implemented to take advantage of collagen chirality to improve 3D visualization. It measures the normalized difference in the SHG signal obtained upon excitation by left versus right circular polarizations [141].
Since the evolution of micro-scale detection methods in recent years, more details are discussed. Atomic force microscopy (AFM) measures both tissue structure and mechanics. The differences of collagen fibril topology from SDFT and CDET have been investigated [21]. Entanglement and distortion of collagen fibrils and the periodicity of the D-period can be clearly observed using AFM [17,19,21]. AFM can also be used to detect the fracture mechanics of collagen fibrils [92]. Fibril adheres to the AFM cantilever by a thin layer of epoxy and can be sandwiched between the glue pad and the cantilever. Then, a mechanical test can be carried out after the equilibration time of the specimen [142]. Interactions between the immunoglobulin and collagen type I have also been detected by AFM to study cellular immunity [143].
In recent years, a burgeoning method called “volume electron microscopy” has been applied to make continuous slices of tendon tissue, record the images of each slice by scanning electron microscopy, and then reconstruct the images of serial slices by Amira or other software [144]. The ultrastructure and morphology of the ECM of tendons can clearly be observed [32]. Individual mature fibrils are too long to be recorded by traditional volume electron microscopy, serial sectioning transmission electron microscopy [30], while the secretion of a 5.1-μm-long single collagen fibril from the cell plasma membrane recess can only roughly be observed. Serial block face-scanning electron microscopy (SBF-SEM) is a practical method of serial section reconstruction. This technique can usually obtain ~100000 μm3 volume data on tendon tissues, and the resolution can reach ~10 nm on the slice plane. Therefore, the number, shape, and cell–cell interactions of cells in tendon tissues can be analyzed by this method, as well as the reconstruction analysis of collagen fibers [101]. The measurement scale of this method is limited such that the full length of a single mature collagen fibril in a human tendon cannot be traced [30]. Compared with SBF-SEM, the measuring scale of focused ion beam scanning electron microscopy (FIB-SEM) tomography is smaller, in which a sample is milled layer by layer with a focused ion beam (FIB), and each newly produced block face is imaged by the scanning electron microscope (SEM) [30,145,146]. FIB-SEM could be used to detect and analyze a large volume of fibrils in shorter image stacks, increasing the understanding of fibril alignment [147]. However, due to current technique limitations, both the integrity of the collagen fibers and the morphological structure of the fiber surface lacks precision. First, it is worth noting that this method is time-consuming. The collection time increases with the increase of sample volume so that unexpected situations might interfere with the data collection. Second, the number of milled layers is limited, since artifacts are created as the stack gets deeper. Third, the irregular orientation of a lump of collagen fibrils makes it difficult to be traced on FIB-SEM scale [30].
Besides, synchrotron X-ray diffraction characterizes tendon mechanics at the Å- and nm-levels [140]. In another study, X-ray fiber diffraction data—that is, an electron density map— characterized a crystallographic super lattice of collagen fibrils [42]. Time series TEM refers to the extraction of samples at different time points, the imaging of the samples by TEM, and the analysis of the samples as they change with time according to the time sequence. However, the sampling can only be done once in an in vivo study because of the imbalance of the extraction site and body [24].
9. Concluding remarks and future perspectives
For a long time, our tendon reconstruction strategy was based on our limited understanding of tendon morphology. The limitations of our understanding of normal tendon, juvenile tendon, and abnormal tendon structures are likely to affect our reconstruction strategy, thus affecting the effectiveness of regenerated tendons. Until now, tendons repaired by artificial intervention could not reach the same level of functionality as that of healthy tendons. In natural mature tendons, the diameter of collagen shows a bimodal distribution; while in repaired or artificially reconstructed tendons, the diameter of collagen is still similar to that of embryonic tendons, with a small diameter and a single peak distribution. How fibril thickens in tendons and how to establish interactions are still unknown. The mechanisms of tendon collagen fibril formation need to be further studied. Of equal importance is the fundamental role of the non-collagenous matrix in tendon development and regenerative medicine strategies [25]. Besides, the intrinsic 24-h rhythmic pattern of tendon ECM homeostasis is worthy of noting and being applied in strategies. Tendon adhesion formation describes the development of fibrotic tissue between the tendon and its surrounding tissues, which commonly occurs as a reaction to injury or surgery. A recent review summarized the application of fibrous membranes, which ability to act as drug delivery vehicles as well as the combination with other therapeutic structures to prevent adhesion formation [151].
The prospects for the future are mainly based on two aspects: one is how to use more novel detection technologies to further analyze the components of the ECM of tendons; and the other is how to regenerate tissue according to the existing recognition of tendon structure.
9.1. Update of evaluation techniques: multi-dimensional detection and functional evaluation
Some high-resolution evaluation techniques for cells and other tissues are discussed here. To some extent, these methods can be used for further decoding of tendon tissue structure (Table 3). Cryo-FIB-ET is a kind of electron cryotomography, one of the most popular forms of cryo-electron microscopy [152]. Freezing technology is introduced into focused ion beam scanning electron microscopy (FIB-SEM) to generate in situ imaging of macromolecular complexes at near-atomic-resolution [153]. That is, the structure of the ECM of tendons could be analyzed with higher precision. However, the maximum thickness of cryo-electron tomography (cryo-ET) volume is 200–300 nm, which limits the 3D information to a specific structure [154]. Hoffman et al. developed a platform for three-dimensional cryogenic super-resolution and focused ion beam-milled block-face EM across entire vitreously frozen cells [155], and this technique could be used for a large volume tendon specimen. In the study of tendon ultrastructure, this technique can be used to label the ECM components of tendons by multicolor three-dimensional structured illumination (3D SIM) and single-molecule localization microscopy (SMLM), and to reconstruct the tendon structure by FIB-SEM. The two microscopy detection methods of tendons can be correlated through component landmarks (such as elastic fibers), after which the whole three-dimensional reconstruction of tendons can be carried out. This method enables fluorescence labeling of components in the context of whole tenocytes and tendon ECM niches, which can help us to further understand the secretion, localization, and variation of ECM components in tendons. In another kind of cryo-electron microscopy, single-particle cryo-EM, the structure is determined by computationally calculating a large number of side projections of proteins within the ice layer [156]. Due to the variability of collagen morphology, however, this method is difficult to be applied [152]. FIB-SEM technology is constantly updated and iterated to improve the resolution and meet the imaging needs of large-scale specimens [157]. In most cases, the lateral resolution of FIB-SEM is better than the depth resolution, so that the methods based on image processing or depth learning are used in image analysis to improve the depth resolution [158].
Table 3.
Predicted detection methods of the structure of ECM components of tendons.
| Detection methods | Scale bar rank (as the indicator of resolution) | Studies already exist | Future research direction |
|---|---|---|---|
| Combination of 3D cryogenic super resolution and FIB–milled block-face EM | 0.5 μm–1 μm | Whole-cell correlative imaging [155] | large volume tendon reconstruction and component co-localization |
| Immunoelectron microscopy | 100 nm–200 nm | Exosome labeling [160,161], labeling mitochondria marker [162], cell activity of tenocytes [163] | Label the component in tendon ECM |
| cryo-FIB-ET | 10 nm | Cell [153], mammalian cells [154] | Improve the accuracy of FIB-SEM |
| single-particle Cryo-EM | 10 Å | human IAPP (amylin) fibrils [156] |
The ultrastructure of collagen fibrils |
In order to better study the in situ structure of ECM, a method termed in situ decellularization of tissues (ISDoT) was developed and could be used to prepare acellular tendon tissue without affecting the three-dimensional spatial distribution of ECM networks. Combined with confocal and multiphoton microscopy methods, the in situ distribution of ECM specific proteins in tendon cells can be visualized by this method after immunostaining [159]. For further characterization of the ultrastructure of the ECM of tendons, especially collagen fibrils, immunolabeling with antibodies against the components is recommended before electron microscopy. Immunoelectron microscopy (IEM), which is based on the principle of specific binding between antigen and antibody, can localize, quantify, and semi-quantify antigen at the ultrastructural level. Immunogold labeling is widely used to locate proteins on exosomes [160,161], mitochondria in muscle tissue [162], and interchanges of vesicles in tenocytes [163]. This technique can be used to label the ECM components of tendons, locate the distribution of these components, and display the structure by electron microscopy simultaneously.
9.2. Regenerative strategies for 3D reconstruction of tendons
A study explored that a kind of cell surface proteoglycan, glypican‐1 (GPC1), specifically enriched on exosomes derived from cancer cells which can serve as a potential noninvasive diagnostic and screening tool for the early stage of pancreatic cancer detection, benefiting possible curative surgical therapy [164]. It is believed that these extrasellar vesicles derived from tenocytes would provide another strategy by identifying subtypes of tendinopathy, so that making accurate treatment. By accumulating and recording the images and data of the ECM, a database can be constructed. Peter et al. constructed a dataset of mice tail tendons. The mechanical part of the dataset includes the tension and tensile properties of the tendon; the structural part of this dataset includes the TEM of the collagen fiber and the derived parameters. This dataset can provide data support for researchers [165]. With the development of detection methods and the data acquired, more datasets can be constructed, and the information stored in them can be used as templates in the 3D reconstruction of biomimetic tendons in the future.
Series of tissue engineering studies have been used in the treatment of tendon diseases. However, after tendon injury, cell density and general synthetic activity are gradually decreased and the repaired tissue appears scar-like, leading to incompletely regains the biomechanical properties [5]. These methods can indeed promote tendon regeneration in different aspects, but the complete regeneration of the whole tendon still needs further optimization of the tendon tissue engineering strategy. In situ injection of formable biodegradable hydrogel has been shown to treat tendon damage in a minimally invasive way and reduce the pain of patients. Through an in-depth understanding of tendon tissue structure, an injectable hydrogel to alleviate symptoms was designed [166]. Li et al. contributed to the field of tendon-to-bone interface engineering through the generation of a heterogeneous 3D-printed porous multiphasic scaffold. Three phases of the scaffold, replicating the native interface, were constructed by imitating different regions of the tendon-to-bone interface. In this way, cell implantation and matrix deposition were achieved [167]. Many scaffolds are fabricated by simulating the topology of tendon cell niche in nano or micro scale. Stem cells are cultured on these scaffolds to promote tenogenic differentiation [10,168,169]. Parallel fiber scaffolds with micro grooves can induce tendon-specific gene expression and tenogenesis of TSPC [170,171]. Wang et al. reported a 3D scaffold with tendon-like mechanical properties and an anisotropic microstructure. When tendon cells were cultured on the scaffolds, type-I collagen and decorin were highly expressed. When implanted into animals, the new tissue formed on the scaffold was similar to the natural tendon tissue in composition and structure [172]. It is speculated that ECM components of tendons can also induce stem cells into tenocytes, but such research is rarely seen. The mimicry of in situ ECM topography may be a direction for tissue engineering of tenogenic differentiation in the future.
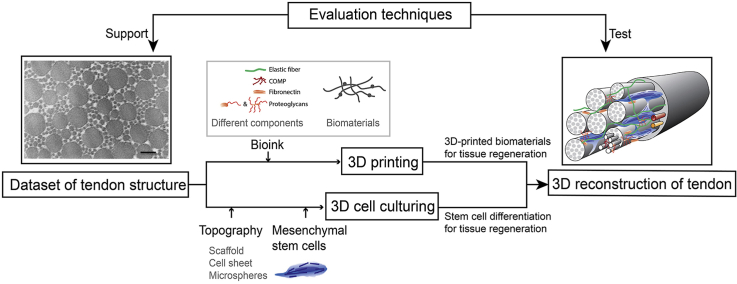
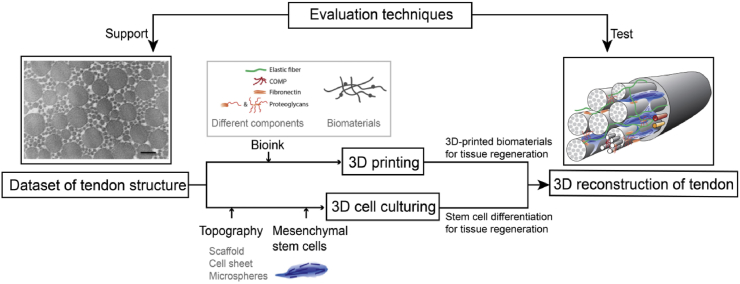
Study has showed that the ECM constructed by the 3D regeneration strategy based on 1D fiber-shaped materials, 2D layers and 3D bulk scaffold materials is more integrated [173]. With the in-depth understanding of tendon structure and further innovation in technology, combining 3D printing and 3D cell culture technology to simulate tendon in situ structure is the trend of tissue engineering research in the near future. The workflow of the 3D reconstruction of tendons could be put into effect (Fig. 10): 1. Determining the tendon structure under different age, races and living habits can serve as a reference template for mimic therapy, while the microstructure in the process of growth and development can be imitated to modify the morphology and structure of the material when making biomimetic scaffolds in order to expect more effective results, mimic the cell niche, and facilitate cell regeneration. 2. Tissue-specific hybrid biolinks include various ECM components derived from decellularized tissue or are artificially produced, as well as a variety of kinds of biomaterials, maintaining their rheological and gelation properties while retaining their biologically inducible properties, while being conducive to 3D biological printing and in vivo functional properties [174]. 3. Different topography, coating of topography, and the multiple culture medium can serve as different cell niches, inducing MSCs or TSPC differentiation into mature tenocytes. 4. The biomimetic tissue is implanted to promote tendon-to-bone repair, tendon repair, and MTJ repair. This kind of biomimetic tissue cans also serve as a substitute to analyze diverse damaging and/or issues of applied medicine.
Fig. 10.
Overview of tendon reconstruction strategy. Based on various advanced detection methods, the dataset of the tendon structure was obtained [90]. By imitating the original composition of the tendon, different biomaterials and matrix components can be used for 3D printing to simulate the state of the tendon ECM. Different types of scaffolds and microspheres can also be used to simulate the microenvironment of tendon cells in vivo so as to induce mesenchymal stem cells to differentiate into tendon cells [27]. As a result, the three-dimensional reconstruction of the whole tendon can be completed by combining the differentiated tendon cells and the ECM of the tendon. Abundant evaluation techniques can be further used to detect the tendon structure after reconstruction. Reprinted with permission.
Although advanced research is still needed to study the microstructure of tendon involvement in different situation and verify their application for tendon repair, it is undeniable that decoding the niche have promising prospects in the subjects of treatment for tendinopathy.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFC1105100), NSFC grants (81772418, 81972099, 81871764, 82072463), Zhejiang Provincial Natural Science Foundation of China (LR20H060001), Fundamental Research Funds for the Central Universities.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zi Yin, Email: yinzi@zju.edu.cn.
Xiao Chen, Email: chenxiao-610@zju.edu.cn.
References
- 1.Gaut L., Duprez D. Tendon development and diseases. Wiley Interdiscip. Rev. Dev. Biol. 2016;5:5–23. doi: 10.1002/wdev.201. [DOI] [PubMed] [Google Scholar]
- 2.Nourissat G., Berenbaum F., Duprez D. Tendon injury: from biology to tendon repair. Nat. Rev. Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 3.Chen J.L. Physical regulation of stem cells differentiation into teno-lineage: current strategies and future direction. Cell Tissue Res. 2015;360:195–207. doi: 10.1007/s00441-014-2077-4. [DOI] [PubMed] [Google Scholar]
- 4.Docheva D., Müller S.A., Majewski M., Evans C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leadbetter W.B. Cell-matrix response in tendon injury. Clin. Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- 6.Chen J. Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Sci. Rep. 2016;6:22946. doi: 10.1038/srep22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ms P. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PloS One. 2017;12 doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin T.W., Cardenas L., Soslowsky L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004;37:865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z., Chen X., Zhu T. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–9329. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H. Physical microenvironment-based inducible scaffold for stem cell differentiation and tendon regeneration. Tissue Eng. B Rev. 2018;24:443–453. doi: 10.1089/ten.TEB.2018.0018. [DOI] [PubMed] [Google Scholar]
- 11.CTTLBDCRC, S Tendon physiology and mechanical behavior. Tendon Regen. 2015:3–39. [Google Scholar]
- 12.Ali O.J., Comerford E.J., Clegg P.D., Canty-Laird E.G. Variations during ageing in the three-dimensional anatomical arrangement of fascicles within the equine superficial digital flexor tendon. Eur. Cell. Mater. 2018;35:87–102. doi: 10.22203/eCM.v035a07. [DOI] [PubMed] [Google Scholar]
- 13.Banos C.C., Thomas A.H., Kuo C.K. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- 14.Kalson N.S. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. Elife. 2015;4 doi: 10.7554/eLife.05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birch H.L., Thorpe C.T., Rumian A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 2013;3:12–22. doi: 10.11138/mltj/2013.3.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard K., Light N.D., Sims T.J., Bailey A.J. Chemistry of the collagen cross-links. Origin and partial characterization of a putative mature cross-link of collagen. Biochem. J. 1987;244:303–309. doi: 10.1042/bj2440303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raspanti M., Congiu T., Guizzardi S. Structural aspects of the extracellular matrix of the tendon: an atomic force and scanning electron microscopy study. Arch. Histol. Cytol. 2002;65:37–43. doi: 10.1679/aohc.65.37. [DOI] [PubMed] [Google Scholar]
- 18.Franchi M., Trirè A., Quaranta M., Orsini E., Ottani V. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang M., Goldstein E.L., Turner A.S. Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. ACS Nano. 2012;6:9503–9514. doi: 10.1021/nn302483x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver F.H., Freeman J.W., Seehra G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 21.Bozec L., Heijden G., Horton M. Collagen fibrils: nanoscale ropes. Biophys. J. 2007;92:70–75. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant T.M., Thompson M.S., Urban J., Yu J. Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J. Anat. 2013;222:573–579. doi: 10.1111/joa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 24.Chang J., Garva R., Pickard A. Circadian control of the secretory pathway maintains collagen homeostasis. Nat. Cell Biol. 2020;22:74–86. doi: 10.1038/s41556-019-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamboulis D.E. Postnatal mechanical loading drives adaptation of tissues primarily through modulation of the non-collagenous matrix. Elife. 2020;9 doi: 10.7554/eLife.58075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetti L., Kuntz L.A., Kunold E. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017;16:664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 27.Rothrauff B.B., Yang G., Tuan R.S. 2015. Tendon Resident Cells—Functions and Features in Section I—Developmental Biology and Physiology of Tendons. [Google Scholar]
- 28.Provenzano P.P., R V., Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Craig A.S., Birtles M.J., Conway J.F., Parry D.A.D. An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect. Tissue Res. Tissue Res. 1989;19:51. doi: 10.3109/03008208909016814. [DOI] [PubMed] [Google Scholar]
- 30.Svensson R.B. Evidence of structurally continuous collagen fibrils in tendons. Acta Biomater. 2017;50:293–301. doi: 10.1016/j.actbio.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Silver F.H., Christiansen D.L., Snowhill P.B., Chen Y. Role of storage on changes in the mechanical properties of tendon and self-assembled collagen fibers. Connect. Tissue Res. Tissue Res. 2000;41:155. doi: 10.3109/03008200009067667. [DOI] [PubMed] [Google Scholar]
- 32.Szczesny S.E., Caplan J.L., Pedersen P., Elliott D.M. Quantification of interfibrillar shear stress in aligned soft collagenous tissues via notch tension testing. Sci. Rep. 2015;5:14649. doi: 10.1038/srep14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson B.E., Szczesny S.E. Dependence of tendon multiscale mechanics on sample gauge length is consistent with discontinuous collagen fibrils. Acta Biomater. 2020;117:302–309. doi: 10.1016/j.actbio.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mienaltowski M.J., Birk D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014;802:5–29. doi: 10.1007/978-94-007-7893-1_2. [DOI] [PubMed] [Google Scholar]
- 35.Wenstrup R.J., Smith S.M., Florer J.B. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J. Biol. Chem. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izu Y., Ansorge H.L., Zhang G. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 2011;30:53–61. doi: 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen P.K., Pan X.S., Li J., Kuo C.K. Roadmap of molecular, compositional, and functional markers during embryonic tendon development. Connect. Tissue Res. 2018;59:495–508. doi: 10.1080/03008207.2018.1511710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birk D.E., Brückner P. The Extracellular Matrix: an Overview. Springer; 2011. Collagens, suprastructures, and collagen fibril assembly; pp. 77–115. [Google Scholar]
- 39.Riley G.P. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjaer M., Langberg H., Heinemeier K. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 41.Birch H.L. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 2007;88:241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orgel J.P., Irving T.C., Miller A., Wess T.J. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silver F.H., Landis W.J. Deposition of apatite in mineralizing vertebrate extracellular matrices: a model of possible nucleation sites on type I collagen. Connect. Tissue Res. 2011;52:242–254. doi: 10.3109/03008207.2010.551567. [DOI] [PubMed] [Google Scholar]
- 44.Tozer S., Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 45.Birk D.E., Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- 46.Kadler K.E., Hojima Y., Prockop D.J. Collagen fibrils in vitro grow from pointed tips in the C- to N-terminal direction. Biochem. J. 1990;268:339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatol. Oxf. Engl. 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 48.Sun M., Luo E.Y., Adams S.M. Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon. Matrix Biol. 2020;94:77–94. doi: 10.1016/j.matbio.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniel M., Traina F., Merlini L. Tendon extracellular matrix remodeling and defective cell polarization in the presence of collagen VI mutations. Cells. 2020;9 doi: 10.3390/cells9020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sardone F., Santi S., Tagliavini F. Collagen VI–NG2 axis in human tendon fibroblasts under conditions mimicking injury response. Matrix Biol. 2016;55:90. doi: 10.1016/j.matbio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Lamandé B., R S., F J. Collagen VI disorders: insights on form and function in the extracellular matrix and beyond. Matrix Biol. 2018;71–72:348–367. doi: 10.1016/j.matbio.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Zhang G., Young B.B., Ezura Y. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- 53.Izu Y., Adams S.M., Connizzo B.K. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2020;95:52–67. doi: 10.1016/j.matbio.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narayanan N., Calve S. Extracellular matrix at the muscle - tendon interface: functional roles, techniques to explore and implications for regenerative medicine. Connect. Tissue Res. 2021;62:53–71. doi: 10.1080/03008207.2020.1814263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marqueti R.C. Effects of aging and resistance training in rat tendon remodeling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018;32:353–368. doi: 10.1096/fj.201700543R. [DOI] [PubMed] [Google Scholar]
- 56.Connizzo B.K., Yannascoli S.M., Soslowsky L.J. Structure-function relationships of postnatal tendon development: a parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalamajski S., Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Thorpe C.T., Birch H.L., Clegg P.D., Screen H.R. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013;94:248–259. doi: 10.1111/iep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang G., Ezura Y., Chervoneva I. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 60.Robinson K.A., Sun M., Barnum C.E. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017;64:81–93. doi: 10.1016/j.matbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamov D.R., Müller A., Wegrowski Y., Brezillon S., Franz C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013;183:394–403. doi: 10.1016/j.jsb.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Samiric T., Ilic M.Z., Handley C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Chen D., Smith L.R., Khandekar G. Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization. Sci. Rep. 2020;10:19065. doi: 10.1038/s41598-020-76107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rees S.G. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem. J. 2000;350 Pt 1:181–188. [PMC free article] [PubMed] [Google Scholar]
- 65.Rees S.G., Davies T., R J., D Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- 66.Corps A.N. The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol. 2008;27:393–401. doi: 10.1016/j.matbio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritty T.M., Ditsios K., Starcher B.C. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat. Rec. 2002;268:430–440. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- 68.Isogai Z. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- 69.Olin A.I. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding *. J. Biol. Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- 70.DiCesare P., Hauser N., Lehman D., Pasumarti S., Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354:237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 71.Svensson L., Aszódi A., Heinegård D. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol. Cell Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maddox B.K., Mokashi A., Keene D.R., Bächinger H.P. A cartilage oligomeric matrix protein mutation associated with pseudoachondroplasia changes the structural and functional properties of the type 3 domain. J. Biol. Chem. 2000;275:11412–11417. doi: 10.1074/jbc.275.15.11412. [DOI] [PubMed] [Google Scholar]
- 73.Smith R.K., Zunino L., Webbon P.M., Heinegård D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16:255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 74.Acharya C. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Tan K., Duquette M., Joachimiak A., Lawler J. The crystal structure of the signature domain of cartilage oligomeric matrix protein: implications for collagen, glycosaminoglycan and integrin binding. Faseb. J. 2009;23:2490–2501. doi: 10.1096/fj.08-128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flowers S.A., Kalamajski S., Ali L. Cartilage oligomeric matrix protein forms protein complexes with synovial lubricin via non-covalent and covalent interactions. Osteoarthritis Cartilage. 2017;25:1496–1504. doi: 10.1016/j.joca.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Ishida K. Vol. 55. 2013. (Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2). [DOI] [PubMed] [Google Scholar]
- 78.Orend G., Saupe F., Schwenzer A., Midwood K. Iconceptpress Com; 2014. The Extracellular Matrix and Cancer: Regulation of Tumor Cell Biology by Tenasc. [Google Scholar]
- 79.Midwood K.S., Chiquet M., Tucker R.P., Orend G. Tenascin-C at a glance. J. Cell Sci. Cell Sci. 2016;129:4321. doi: 10.1242/jcs.190546. [DOI] [PubMed] [Google Scholar]
- 80.Järvinen T.A., Józsa L., Kannus P. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J. Cell Sci. 2003;116:857–866. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- 81.Halper J., Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 82.Maurer L.M., Ma W., Mosher D.F. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem. Mol. Biol. 2015;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh P., Schwarzbauer J.E. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J. Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korol R.M., Finlay H.M., Josseau M.J., Lucas A.R., Canham P.B. Fluorescence spectroscopy and birefringence of molecular changes in maturing rat tail tendon. J. Biomed. Opt. 2007;12 doi: 10.1117/1.2714055. [DOI] [PubMed] [Google Scholar]
- 85.Lichtwark G.A., Wilson A.M. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 2005;208:4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- 86.Lin A.H. Collagen denaturation is initiated upon tissue yield in both positional and energy-storing tendons. Acta Biomater. 2020;118:153–160. doi: 10.1016/j.actbio.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stephens P.R., Nunamaker D.M., Butterweck D.M. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 1989;50:1089–1095. [PubMed] [Google Scholar]
- 88.Thorpe C.T., Spiesz E.M., Chaudhry S., Screen H.R., Clegg P.D. Science in brief: recent advances into understanding tendon function and injury risk. Equine Vet. J. 2015;47:137–140. doi: 10.1111/evj.12346. [DOI] [PubMed] [Google Scholar]
- 89.Thorpe C.T. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater. 2013;9:7948–7956. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Smith T.J. 2006. The Relationship between Tendon Morphology and Function. [Google Scholar]
- 91.Hansen P., Haraldsson B.T., Aagaard P. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J. Appl. Physiol. 1985;108:47–52. doi: 10.1152/japplphysiol.00944.2009. [DOI] [PubMed] [Google Scholar]
- 92.Svensson R.B., Mulder H., Kovanen V., Magnusson S.P. Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys. J. 2013;104:2476–2484. doi: 10.1016/j.bpj.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thorpe C.T. Anatomical heterogeneity of tendon: fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci. Rep. 2016;6:20455. doi: 10.1038/srep20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benjamin M., McGonagle D. Entheses: tendon and ligament attachment sites. Scand. J. Med. Sci. Sports. 2009;19:520–527. doi: 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 95.Wopenka B., Kent A., Pasteris J.D., Yoon Y., Thomopoulos S. The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl. Spectrosc. 2008;62:1285–1294. doi: 10.1366/000370208786822179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charvet B., Ruggiero F., Le G.D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J. 2012;2:53–63. [PMC free article] [PubMed] [Google Scholar]
- 97.Subramanian A., Schilling T.F. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development. 2015;142:4191–4204. doi: 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacob C., Rocha L.C., Neto J.P., Watanabe I.S., Ciena A.P. Effects of physical training on sarcomere lengths and muscle-tendon interface of the cervical region in an experimental model of menopause. Eur. J. Histochem. 2019;63 doi: 10.4081/ejh.2019.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu W. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell Biol. 2010;30:4797–4807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 101.Schekman R., Mellman I. Does COPI go both ways. Cell. 1997;90:197–200. doi: 10.1016/s0092-8674(00)80326-8. [DOI] [PubMed] [Google Scholar]
- 102.Birk D.E., Trelstad R.L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muir K.K.E.F. Lecture: collagen fibril formation in vitro and in vivo. Int. J. Exp. Pathol. 2017;98:4–16. doi: 10.1111/iep.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kalson N.S. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:E4743–E4752. doi: 10.1073/pnas.1314348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Canty E.G. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Starborg T., Kalson N.S., Lu Y. Using transmission electron microscopy and 3 View to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013;8:1433–1448. doi: 10.1038/nprot.2013.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graham H.K., Holmes D.F., Watson R.B., Kadler K.E. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J. Mol. Biol. 2000;295:891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- 108.Gobeaux F., Mosser G., Anglo A. Fibrillogenesis in dense collagen solutions: a physicochemical study. J. Mol. Biol. 2008;376:1509–1522. doi: 10.1016/j.jmb.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 109.Giraud-Guille M. Liquid crystalline properties of type I collagen: perspectives in tissue morphogenesis. Compt. Rendus Chem. 2008;11:245. [Google Scholar]
- 110.Martin R. Liquid crystalline ordering of procollagen as a determinant of three-dimensional extracellular matrix architecture11Edited by M. F. Moody. J. Mol. Biol. 2000;301:11–17. doi: 10.1006/jmbi.2000.3855. [DOI] [PubMed] [Google Scholar]
- 111.Quigley A.S. In tendons, differing physiological requirements lead to functionally distinct nanostructures. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-22741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Birch H.L., Peffers M.J., Clegg P.D. Influence of ageing on tendon homeostasis. Adv. Exp. Med. Biol. 2016;920:247–260. doi: 10.1007/978-3-319-33943-6_24. [DOI] [PubMed] [Google Scholar]
- 113.Gagliano N., Menon A., Cabitza F., Compagnoni R., Randelli P. Morphological and molecular characterization of human hamstrings shows that tendon features are not influenced by donor age. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:343–352. doi: 10.1007/s00167-017-4661-0. [DOI] [PubMed] [Google Scholar]
- 114.Hoksrud A., Ohberg L., Alfredson H., Bahr R. Ultrasound-guided sclerosis of neovessels in painful chronic patellar tendinopathy: a randomized controlled trial. Am. J. Sports Med. 2006;34:1738–1746. doi: 10.1177/0363546506289168. [DOI] [PubMed] [Google Scholar]
- 115.Riley G. Tendinopathy—from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 116.Lian Ø. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am. J. Sports Med. 2006;34:1801–1808. doi: 10.1177/0363546506289169. [DOI] [PubMed] [Google Scholar]
- 117.Pingel J. 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. J. Anat. 2014;224:548–555. doi: 10.1111/joa.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thankam F.G., Dilisio M.F., Gross R.M., Agrawal D.K. Collagen I: a kingpin for rotator cuff tendon pathology. Am. J. Transl. Res. 2018;10:3291–3309. [PMC free article] [PubMed] [Google Scholar]
- 119.Lui P.P.-Y., Chan L.-S., Lee Y.-W., Fu S.C., Chan K.-M. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatol. Oxf. Engl. 2010;49:231–239. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 120.Docking S., Samiric T., Scase E., Purdam C., Cook J. Relationship between compressive loading and ECM changes in tendons. Muscles Ligaments Tendons J. 2013;3:7–11. doi: 10.11138/mltj/2013.3.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samiric T. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. J. Int. Soc. Matrix Biol. 2009;28:230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Corps A.N. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatol. Oxf. 2006;45:291–294. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- 123.Hakimi O., Ternette N., Murphy R., Kessler B.M., Carr A. A quantitative label-free analysis of the extracellular proteome of human supraspinatus tendon reveals damage to the pericellular and elastic fibre niches in torn and aged tissue. PloS One. 2017;12 doi: 10.1371/journal.pone.0177656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomopoulos S., Parks W.C., Rifkin D.B., Derwin K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015;33:832–839. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanazawa T., Gotoh M., Ohta K. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. Sci. Rep. 2016;6:33800. doi: 10.1038/srep33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beredjiklian P.K. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 127.Tang Q.M., Chen J.L., Shen W.L. Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration. Sci. Rep. 2014;4:5515. doi: 10.1038/srep05515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bi Y., Ehirchiou D., Kilts T.M. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 129.Zhang C., Zhu J., Zhou Y., Thampatty B.P., Wang J.H. Tendon stem/progenitor cells and their interactions with extracellular matrix and mechanical loading. Stem Cell. Int. 2019;2019:3674647. doi: 10.1155/2019/3674647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Screen H.R., Berk D.E., Kadler K.E., Ramirez F., Young M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015;33:793–799. doi: 10.1002/jor.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gracey E. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020;16:193–207. doi: 10.1038/s41584-019-0364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi H.J. Enhanced tendon restoration effects of anti-inflammatory, lactoferrin-immobilized, heparin-polymeric nanoparticles in an Achilles tendinitis rat model. Carbohydr. Polym. 2020;241:116284. doi: 10.1016/j.carbpol.2020.116284. [DOI] [PubMed] [Google Scholar]
- 133.Popov C. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol. 2015;16:6. doi: 10.1186/s12867-015-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Y.T. Sequential inflammation model for Achilles tendinopathy by elastin degradation with treadmill exercise. J Orthop Transl. 2020;23:113–121. doi: 10.1016/j.jot.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Omae H., Zhao C., Sun Y.L., An K.N., Amadio P.C. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J. Orthop. Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tong W.Y., Shen W., Yeung C.W. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33:7686–7698. doi: 10.1016/j.biomaterials.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 137.Franz S.J.R., LC R., K T., D G. Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture. 2015;41:192–197. doi: 10.1016/j.gaitpost.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shalabi A., Movin T., Kristoffersen-Wiberg M., Aspelin P., Svensson L. Reliability in the assessment of tendon volume and intratendinous signal of the Achilles tendon on MRI: a methodological description. Knee Surg. Sports Traumatol. Arthrosc. 2005;13:492. doi: 10.1007/s00167-004-0546-0. [DOI] [PubMed] [Google Scholar]
- 139.Screen H.R., Lee D.A., Bader D.L., Shelton J.C. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc. Inst. Mech. Eng. H. 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 140.Gupta H.S., Seto J., Krauss S., Boesecke P., Screen H.R.C. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J. Struct. Biol. 2010;169:183–191. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Schmeltz M. Implementation of artifact-free circular dichroism SHG imaging of collagen. Opt Express. 2019;27:22685–22699. doi: 10.1364/OE.27.022685. [DOI] [PubMed] [Google Scholar]
- 142.Svensson R.B., Hassenkam T., Grant C.A., Magnusson S.P. Tensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys. J. 2010;99:4020–4027. doi: 10.1016/j.bpj.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu J., Madhurapantula R.S., Kalyanasundaram A. Ultrastructural location and interactions of the immunoglobulin receptor binding sequence within fibrillar type I collagen. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schneider J.P., Hegermann J., Wrede C. Volume electron microscopy: analyzing the lung. Histochem Cell Biol. 2020 doi: 10.1007/s00418-020-01916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Villinger C., Schauflinger M., Gregorius H. Three-dimensional imaging of adherent cells using FIB/SEM and STEM. Methods Mol. Biol. 2014;1117:617–638. doi: 10.1007/978-1-62703-776-1_27. [DOI] [PubMed] [Google Scholar]
- 146.Bushby A.J. Imaging three-dimensional tissue architectures by focused ion beam scanning electron microscopy. Nat. Protoc. 2011;6:845–858. doi: 10.1038/nprot.2011.332. [DOI] [PubMed] [Google Scholar]
- 147.Reese S.P., Farhang N., Poulson R., Parkman G., Weiss J.A. Nanoscale imaging of collagen gels with focused ion beam milling and scanning electron microscopy. Biophys. J. 2016;111:1797–1804. doi: 10.1016/j.bpj.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zou Z. Three-dimensional structural interrelations between cells, extracellular matrix, and mineral in normally mineralizing avian leg tendon. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:14102–14109. doi: 10.1073/pnas.1917932117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tokarz D. Characterization of pancreatic cancer tissue using multiphoton excitation fluorescence and polarization-sensitive harmonic generation microscopy. Front. Oncol. 2019;9:272. doi: 10.3389/fonc.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Godwin A.R., Starborg T., Sherratt M.J., Roseman A.M., Baldock C. Defining the hierarchical organisation of collagen VI microfibrils at nanometre to micrometre length scales. Acta Biomater. 2017;52:21–32. doi: 10.1016/j.actbio.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Q., Yang Y., Yildirimer L., Xu T., Zhao X. Advanced technology-driven therapeutic interventions for prevention of tendon adhesion: design, intrinsic and extrinsic factor considerations. Acta Biomater. 2021;124:15–32. doi: 10.1016/j.actbio.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 152.Cheng Y. Single-particle cryo-EM-How did it get here and where will it go. Science. 2018;361:876–880. doi: 10.1126/science.aat4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wagner F.R., Watanabe R., Schampers R. Preparing samples from whole cells using focused-ion-beam milling for cryo-electron tomography. Nat. Protoc. 2020;15:2041–2070. doi: 10.1038/s41596-020-0320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayles M.F., Winter D.E., D An introduction to cryo-FIB-SEM cross-sectioning of frozen, hydrated Life Science samples. J. Microsc. 2020;281(2):138–156. doi: 10.1111/jmi.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hoffman D.P., Shtengel G., Xu C.S. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science. 2020;367:6457. doi: 10.1126/science.aaz5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cao Q., Boyer D.R., Sawaya M.R., Ge P., Eisenberg D.S. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat. Struct. Mol. Biol. 2020;27:653–659. doi: 10.1038/s41594-020-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu C.S., Hayworth K.J., Lu Z. Enhanced FIB-SEM systems for large-volume 3D imaging. Elife. 2017;6 doi: 10.7554/eLife.25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hagita K., Higuchi T., Jinnai H. Super-resolution for asymmetric resolution of FIB-SEM 3D imaging using AI with deep learning. Sci. Rep. 2018;8:5877. doi: 10.1038/s41598-018-24330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mayorca-Guiliani A.E., Willacy O., Madsen C.D. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 2019;14:3395–3425. doi: 10.1038/s41596-019-0225-8. [DOI] [PubMed] [Google Scholar]
- 160.Kamerkar S. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 162.Polimeno L., Rossi R., Mastrodonato M. Augmenter of liver regeneration, a protective factor against ROS-induced oxidative damage in muscle tissue of mitochondrial myopathy affected patients. Int. J. Biochem. Cell Biol. 2013;45:2410–2419. doi: 10.1016/j.biocel.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 163.Schneider P.R., Buhrmann C., Mobasheri A., Matis U., Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J. Orthop. Res. 2011;29:1351–1360. doi: 10.1002/jor.21400. [DOI] [PubMed] [Google Scholar]
- 164.Melo S.A. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goh K.L., Holmes D.F., Lu Y.H., Kadler K.E., Purslow P.P. Age-related dataset on the mechanical properties and collagen fibril structure of tendons from a murine model. Sci Data. 2018;5:180140. doi: 10.1038/sdata.2018.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu R., Zhang S., Chen X. Injectable hydrogels for tendon and ligament tissue engineering. J Tissue Eng Regen Med. 2020;14:1333–1348. doi: 10.1002/term.3078. [DOI] [PubMed] [Google Scholar]
- 167.Cao Y., Yang S., Zhao D. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J Orthop Transl. 2020;23:89–100. doi: 10.1016/j.jot.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang J., Chen Y., Tang C. The relationship between substrate topography and stem cell differentiation in the musculoskeletal system. Cell. Mol. Life Sci. 2019;76:505–521. doi: 10.1007/s00018-018-2945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin J., Zhou W., Han S. Cell-material interactions in tendon tissue engineering. Acta Biomater. 2018;70:1–11. doi: 10.1016/j.actbio.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 170.Shi Y., Zhou K., Zhang W. Microgrooved topographical surface directs tenogenic lineage specific differentiation of mouse tendon derived stem cells. Biomed. Mater. 2017;12(1) doi: 10.1088/1748-605X/12/1/015013. [DOI] [PubMed] [Google Scholar]
- 171.Zheng Z., Ran J., Chen W. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017;51:317–329. doi: 10.1016/j.actbio.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 172.Wang Z., Lee W.J., Koh B. Functional regeneration of tendons using scaffolds with physical anisotropy engineered via microarchitectural manipulation. Sci Adv. 2018;4:4537. doi: 10.1126/sciadv.aat4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee J. Organ-level functional 3D tissue constructs with complex compartments and their preclinical applications. Adv. Mater. 2020;32(51) doi: 10.1002/adma.202002096. [DOI] [PubMed] [Google Scholar]
- 174.De Santis M.M. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mater. Deerfield Beach Fla. 2021;33(3) doi: 10.1002/adma.202005476. [DOI] [PMC free article] [PubMed] [Google Scholar]