Abstract
The considerable development of carrier-free nanodrugs has been achieved due to their high drug-loading capability, simple preparation method, and offering “all-in-one” functional platform features. However, the native defects of carrier-free nanodrugs limit their delivery and release behavior throughout the in vivo journey, which significantly compromise the therapeutic efficacy and hinder their further development in cancer treatment. In this review, we summarized and discussed the recent strategies to enhance drug delivery and release of carrier-free nanodrugs for improved cancer therapy, including optimizing the intrinsic physicochemical properties and external modification. Finally, the corresponding challenges that carrier-free nanodrugs faced are discussed and the future perspectives for its application are presented. We hope this review will provide constructive information for the rational design of more effective carrier-free nanodrugs to advance therapeutic treatment.
Keywords: Carrier-free nanodrugs, Drug delivery and release, Intrinsic physicochemical properties, External modification, Therapeutic efficacy
Graphical abstract
Great enhancement of carrier-free nanodrugs has been achieved to overcome the defects during the in vivo journey. This review has systemically summarized the recent strategies to enhance drug delivery and release of carrier-free nanodrugs, including optimizing the intrinsic physicochemical properties and external modification.
Highlights
-
•
Recent progresses on carrier-free nanodrugs for enhancing drug delivery and release are summarized.
-
•
Strategies of optimizing the intrinsic physicochemical properties and external modification are summarized.
-
•
Future perspectives and challenges of carrier-free nanodrugs are discussed.
1. Introduction
Carrier-assistant drug delivery systems (CDDS) have been widely used to treat cancer due to their enhancement to biological stability and bioavailability of therapeutic drugs. In particular, CDDS can significantly reduce the toxicity to the body by achieving site-specific delivery of chemotherapeutics through the enhanced permeability and retention effect [[1], [2], [3], [4], [5], [6]]. Despite the clinical success of some CDDS (e.g. Doxil® and Genexol-PM®) for cancer treatments [7,8], their respective efficacy has not significantly improved due to challenges such as undesirable drug loading capacity [9,10] and potential systematic toxicity owing to the carriers as vehicles, which limit their clinical translation.
Carrier-free nanodrugs that are used as a form of drug delivery are mainly self-assembled by prodrugs, pure drugs (e.g. single drug, or multi drugs), or amphiphilic drug-drug conjugates without the use of inert materials. They generally possess low systemic toxicity, high drug loading capability, stimulus sensitive features, and synergistic therapeutic efficacy. The advantages of carrier-free nanodrugs compared to CDDS have aroused significant attention for cancer therapy, as summarized in Table 1. For example, pure drugs without chemical modification, such as curcumin, 10-hydroxycamptothecin (HCPT), and paclitaxel (PTX) [[11], [12], [13]], can directly self-assemble into carrier-free nanodrugs through non-covalent forces such as hydrophobic interaction, electrostatic interaction, or hydrogen bonding. The obtained nanodrugs usually present high drug loading efficiency, reduced systemic toxicity, and promising antitumor activity. Furthermore, the incorporation of stimulus-sensitive bonds (such as acid-, redox-, or enzyme-sensitive bonds) into drugs to fabricate prodrugs can achieve on-demand drug release to corresponding tumor sites [[14], [15], [16]]. Additionally, the co-assembly of two or more chemotherapeutic drugs into carrier-free nanodrugs can greatly enhance the therapeutic efficacy and overcome multi-drug resistance (MDR) owing to the synergistic effects of their distinct anti-tumor mechanisms [17,18]. Different therapeutic agents, such as photosensitizers, photothermal agents, immunological agents, or imaging agents can also self-assemble into carrier-free nanodrugs through free combination, based on the different requirements of antitumor therapy, such as therapeutic or diagnostic abilities [[19], [20], [21], [22]]. Apart from the above-mentioned advantages of carrier-free nanodrugs, they also show excellent flexibility and changeability with varying design properties, such as size, morphology, and surface chemistry, resulting in extended blood circulation and efficient internalization.
Table 1.
Summary of the main preparation methods, similarities, and differences between carrier-free nanodrugs and carrier-assistant drug delivery systems.
| Carrier-free nanodrugs | Carrier-assistant drug delivery systems | |
|---|---|---|
| Preparation methods | ·High-pressure homogenization method [23] | ·Dialysis method [24,25] |
| ·Medium milling method [26] | ·Emulsification method [27] | |
| ·Reverse solvent precipitation method [28] | ·Solvent evaporation method [29] | |
| ·Template-assisted method [30,31] | ·Freeze drying method [32] | |
| ·In-vivo self-assembly method [33] | ·Film dispersion [34] | |
| Similarities | Improved drug stability and biocompatibility, prolonged the blood circulation time and enhanced drug accumulation within tumor tissue | |
| Differences | ·Avoiding tedious steps for preparing | ·Sophisticated preparation procedures |
| ·No carrier-induced toxicity and immunogenicity | ·potential systematic toxicity caused by inner carrier | |
| ·High drug loading capacity (could reach 100%) | ·Low drug capacity (≤10%, w/w) | |
The aforementioned advantages of carrier-free nanodrugs have led to several Phase II clinical trial, including Theralux®, Panzem®-NCD, etc. [35]. Panzem®-NCD is the nanocrystal of 2-methoxyestradiol, a naturally-occurring metabolite of estradiol that exhibits anti-angiogenic and anti-tumor effects in preclinical models, and has improved tolerance and bioavailability over the oral capsule formulation in clinical epithelial ovarian cancer trials [36]. However, the clinical translation of carrier-free nanodrugs from bench-to-bedside is far from desirable due to their respective efficacy not offering an obvious improvement over established formulations. Even so, there are many studies devoted to the design of more reasonable and effective carrier-free nanodrugs to achieve efficient tumor treatment. For instance, the strategies of adding a small number of additives and modifying carrier-free nanodrugs with polymers, small molecules, or cell membranes have been developed to improve the stability of carrier-free nanodrugs and subsequently prolong the blood circulation time during in vivo transportation. Introducing active targeting agents into drugs or the combination of some therapeutic agents possessing targeting abilities (e.g. methotrexate, MTX [37]) is conducive towards enhancing the effective concentration of therapeutic agents at the tumor site and also beneficial towards enhancing the internalization efficiency after guiding the carrier-free nanodrugs to the tumor sites. Undoubtedly, the rapid intracellular release of therapeutic agents plays a paramount role in determining the therapeutic efficacy. Incorporation of responsive linkers, such as amide bonds, ester bonds, or disulfide bonds between the different drugs to achieve responsive disintegration of carrier-free nanodrugs or the utilization of extracellular stimuli, such as heat, light, or ultrasound to provide site-specific release of drugs both are both effective strategies to accelerate the drug release at designated tumor sites. Apart from the above-mentioned chemical modifications, the physicochemical properties of the carrier-free nanodrugs, such as size, charge, or surface chemistry, can be modified for favorable blood circulation, tumor-homing, and intratumoral penetration delivery. For example, Truong et al. demonstrated that changing the morphology of carrier-free nanodrugs from spherical to rod-shaped through alternating the assembling force can improve their internalization efficiency [38].
Collectively, the development of approaches aimed at effectively targeting specific intracellular release and delivering the payload in its active status directly to its intracellular site of action is a fundamental step in the development of more effective therapeutics. Therefore, gaining an in-depth understanding of the entire drug delivery and release process and improving each process efficiency are of paramount importance for the design of carrier-free nanodrugs and the successful translation from fundamental and preclinical studies to clinics.
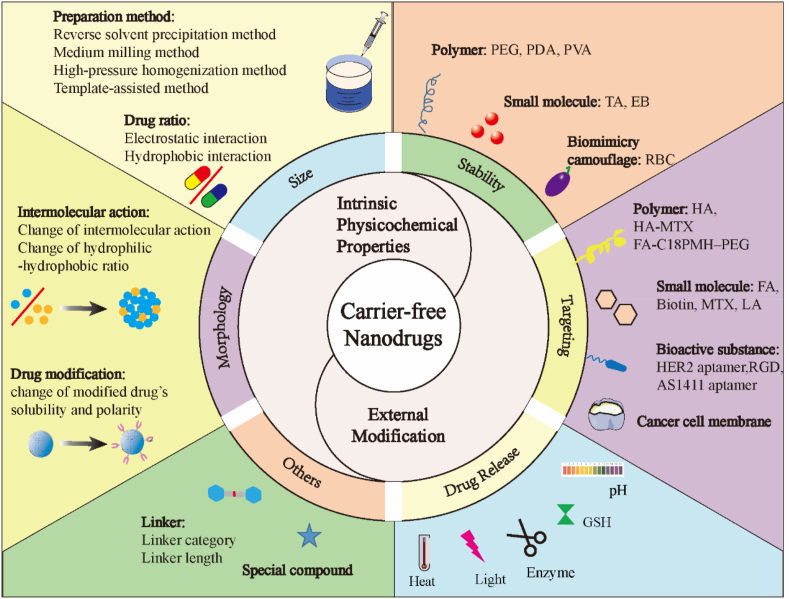
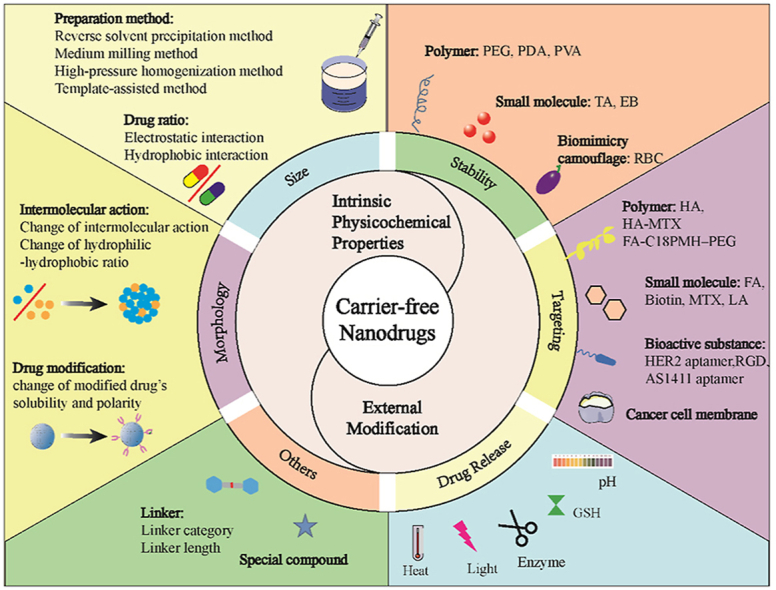
Based on the considerable progress of carrier-free nanodrugs in cancer treatment, most of the excellent recent reviews have summarized the preparation and application of carrier-free nanodrugs and emphasized the prominent antitumor efficiency of carrier-free nanodrugs through combinations of therapeutic agents with different antitumor mechanisms [35,[39], [40], [41]], while little attention was given to the improvement of the delivery and release efficiency to maximize the therapeutic efficacy. In this review, we aim to provide an in-depth understanding of the delivery and release behavior of carrier-free nanodrugs by summarizing the corresponding feasible strategies to improve the delivery and release efficiency, with an emphasis on the optimization of their intrinsic physicochemical properties and their external modifications (Fig. 1). Subsequently, the future perspectives on the opportunities and challenges for the clinical translation of carrier-free nanodrugs are provided. Collectively, it is hoped that this review will provide a cohesive overview and clear directions for the rational design of carrier-free nanodrugs to achieve maximum therapeutic efficacy through efficient drug delivery and release.
Fig. 1.
Schematic illustration of the intrinsic physicochemical property optimization strategies and external modification strategies for drug delivery and release of carrier-free nanodrugs during the in vivo transportation from the blood to the targeted tumor sites.
2. Optimized physicochemical properties of carrier-free nanodrugs
2.1. Preparation methods of carrier-free nanodrugs
Compared to the traditional CDDS, the preparation method of the carrier-free nanodrugs is a simpler process, uses fewer or no organic solvents, and is generally divided into in-vitro and in-vivo self-assembly strategies. The in-vitro self-assembly strategies includes the top-down method (e.g. the high-pressure homogenization method and the medium milling method), reverse solvent precipitation method, and template-assisted method. Through accurate and reasonable design, the carrier-free nanodrugs produced through in-vitro self-assembly strategies can have higher drug loading, longer blood circulation time, or more efficient uptake. In-vivo self-assembly strategies utilize small molecules that reach the target site triggered by tumor specific stimuli, which is much more facile and does not need any high energy-consuming machines or other assembly process compared to in-vitro self-assembly strategies.
The top-down method, including the high-pressure homogenization method and the media milling method, involves crushing the drug powder with larger particle size into nano-sized particles by mechanical force to prepare drug nanocrystals. The preparation process of the top-down method is simple, thus it provides an effective technical method for improving the solubility and bioavailability of insoluble drugs, and has promising industrial applications and development potential. Currently, some drug nanocrystals prepared by this method (Rapamune®, Emend®, Triglide™) have already been industrialized [42]. The high-pressure homogenization method utilizes typical technologies such as Microfluidizer technology (IDD-P™ technology) and Piston-gap homogenization (Dissocubes® technology, Nanopure® technology) [43] to prepare carrier-free nanodrugs, such as drug nanocrystals. The main procedures of this method are as follow: large-particle drugs were dispersed in an aqueous solution or other solvent to form a crude suspension. Under high-pressure conditions, impact force, shear force, and cavity action are used to reduce the particle size of drugs. Although this method is beneficial for the scale of production and the prepared nanocrystals have a narrow size distribution, the raw materials need to be powdered and the drug suspension needs to be pretreated with high-speed stirring and dispersion before high-pressure homogenization. The media milling method aims to gradually reduce the particle size of the solid particles to the nano level through strong collision and shear forces generated between the drug particles, the grinding medium, and the machine wall [44]. Water, drugs, and stabilizers are mixed together in a specific ratio before being poured into a grinding chamber equipped with grinding media (glass beads, ceramic beads, steel balls, etc.), followed by repeated grinding for narrowly distributed nanoparticles. However, the wear and erosion of the grinding media during the preparation process may cause product contamination. Therefore, it is encouraged to use a combination of different top-down method technologies with the application of some pre-treatment processes to reduce the particle size of the powders, overcome the shortcomings of the individual technologies, and further improve the efficiency of preparing drug nanocrystals.
Commonly, the reverse solvent precipitation method involves dissolving hydrophobic drugs or amphiphilic compounds (e.g. prodrugs, multi drugs, amphiphilic drug-drug conjugates [39]) in a small amount of solvents (usually organic solvents), and then slowly dropped into deionized water with stirring and/or ultrasound [45]. After using dialysis, ultrafiltration, freeze-drying, centrifugation, or evaporation in vacuum for purification, the carrier-free nanodrugs with high drug loading content are obtained. The biggest advantage of this method is the simple operation, which does not require any chemical modifications. More importantly, this method is suitable for most drugs and the ratio of different drugs can be controlled to obtain satisfactory particle size and morphology to achieve longer blood circulation time and more effective cell uptake. Despite the simplicity of the reverse solvent precipitation method, the method faces some key challenges, including the batch-to-batch variations, low productivity, and relatively large particle sizes. To address these limitations, a template-assisted method has been widely developed, which involves dropping drug solution into a template and obtaining the aqueous dispersions or solid powder of carrier-free nanodrugs after removing the template, namely an anodized aluminum oxide (AAO) [30] or the ice template-assisted method [31]. The prominent advantages for the method is that the particle size can be precisely controlled, varying from 100 nm to 20 nm. For example, Zhang et al. uses the ice-template method to produce curcumin carrier-free nanodrugs with a size ranging from 20 nm to 200 nm. Notably, this method can also increase the yield of carrier-free nanodrugs and can be easily applied to various hydrophobic anticancer drugs, thus demonstrating its potential for conversion of laboratory products to industrial commodities.
Apart from the in-vitro self-assembly strategies mentioned above, many in-vivo self-assembly strategies have been proposed in recent years. The primary in-vivo self-assembly strategy utilizes predesigned molecules triggered by specific stimuli in the tumor microenvironment to self-assemble in situ into nanostructures after intravenous injection [46,47]. The in-vivo self-assembly strategy could greatly reduce the low drug delivery efficiency and unfavorable side effects from off-target drug delivery compared to other nanoscale-delivery systems by taking advantage of the high penetrability of small molecular drugs and the high retention of nanoassemblies. For example, Wang et al. conjugated the cytotoxic peptide KLAK with a pH-sensitive group (CAA) and a cell-penetrating peptide (TAT) to a poly (β-thioester) backbone to produce PT-K-CAA [33]. In the physiological environment, PT-K-CAA had good hydrophilicity due to the exposed carboxyl groups of CAA and existed in a small single chain form. Upon reaching the slightly acidic tumor microenvironment, the CAA of PT-K-CAA hydrolyzed, thus increasing the hydrophobicity and promoting the self-assembly of polymer-peptide conjugates into nanoparticles though hydrophobic interactions, resulting in high internalization efficiency and improved therapeutic activity.
2.2. Strategies to optimize particle size of carrier-free nanodrugs
As mentioned previously, nanoparticle size critically affects the in vivo delivery fate of nanoparticles after intravenous injection, such as blood circulation, accumulation, penetration, and retention in tumor. Commonly, particle sizes larger than 100 nm will be phagocytized by the liver, kidney, spleen, or other tissues with large blood flow and rich in mononuclear macrophages, followed by removal from the body through metabolism. Particle sizes lower than 7 nm are directly filtered out of the kidney and excreted out of the body [48]. Based on this, the particle sizes within the 10–100 nm range are the most optimal for prolonged blood circulation time and subsequent accumulation at the tumor site. Furthermore, the penetration of nanoparticles into the tumor is highly dependent on the particle size, with larger nanoparticles staying near the vasculature and smaller nanoparticles rapidly spreading throughout the tumor stroma [49]. Although smaller particle sizes have the ability to increase the permeability effect, it generally suffers from poor tumor retention. In this section, we summarize the influence factors on particle size of carrier-free nanodrugs, providing possible suggestions on the rational design of carrier-free nanodrugs for improving the therapeutic efficacy.
2.2.1. Influence of drug ratio on particle size
As previously mentioned, the carrier-free nanodrugs can be self-assembled by the drug itself (one or more drugs together) through noncovalent interactions such as electrostatic interactions, hydrophobic interactions, and hydrogen bonds. Generally, the drug ratios have some effects on their interactions during the self-assembly process, which can result in the formation of carrier-free nanodrugs with different particle sizes. Changing the drug ratio indicates modifications to the total amount of hydrophobic or charged groups, which can influence the intermolecular hydrophobic effects or electrostatic effects, leading to the variation of particle sizes.
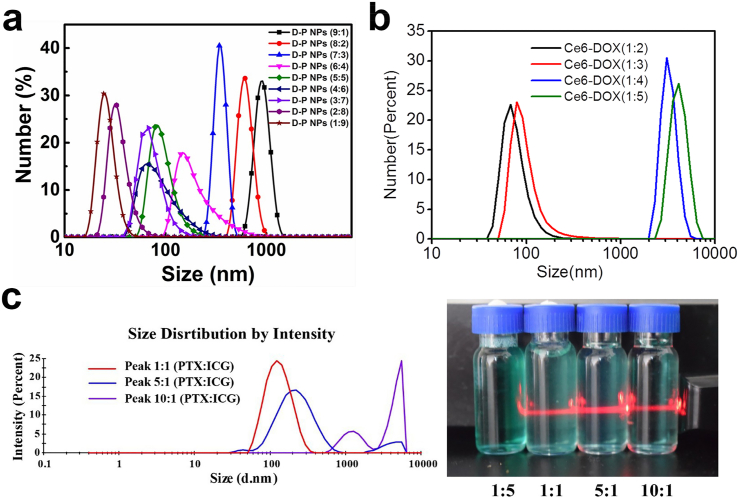
Electrostatic interaction is one of the noncovalent interactions to construct carrier-free nanodrugs. The self-assembly of carrier-free nanodrugs relies on the interaction between drugs which contains charged groups, such as -NH3+ and -COO–. However, the electrostatic interactions among the carrier-free nanodrugs are easily affected by factors such as pH and the amount of charge. Its action energy is directly proportional to the amount of charge between the interacting groups and inversely proportional to the distance between the charge centers of the groups [50]. As for drugs with charged groups, altering drug ratios usually trigger the variation of activation energy for the electrostatic interaction, thereby obtaining carrier-free nanodrugs with different particle sizes. For example, Xiao et al. constructed carrier-free nanodrugs due to electrostatic interactions from Doxorubicin (DOX) with positively charged –NH2 groups and the photosensitizer, pheophorbide A (PhA), with negatively charged –COOH groups. The obtained nanodrugs exhibited a homogeneous nanoscale particle size distribution [51]. More interestingly, carrier-free nanodrugs with different particle sizes were obtained after the variation of DOX and PhA ratios (1:9, 2:8, 3:7, 3.5:6.5, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1). The mechanism driving the formation of different particle size was mainly attributed to the varying amount of charged groups, which ultimately alternated the electrostatic interactions (Fig. 2a). Similarly, Zhang et al. also demonstrated that the increase of DOX to Chlorine e6 (Ce6) ratio can trigger the formation of larger particle sizes. Surprisingly, when the ratio of Ce6 to DOX increased from 1:3 to 1:4, the particle size suddenly increased from 100 nm to 1000 nm [52] (Fig. 2b). This was attributed to the rapid decrease in zeta potential from −20 mV to 0 mV, which lead to the aggregation of nanoparticles.
Fig. 2.
a) The size distribution of the different ratios of DOX to PhA. Reproduced with permission from Ref. [51]. Copyright 2018, Royal Society of Chemistry. b) The size distribution of the different ratios of Ce6 to DOX. Reproduced with permission from Ref. [52]. Copyright 2016, American Chemical Society. c) Size distribution and the Tyndall effect of different ratios of PTX to ICG. Reproduced with permission from Ref. [53]. Copyright 2019, Royal Society of Chemistry.
Besides electrostatic interactions, the hydrophobic interactions between drugs are also commonly used to drive the formation of carrier-free nanodrugs due to most anticancer therapeutic agents (e.g. DOX, paclitaxel (PTX), indocyanine Green (ICG)) containing hydrophobic benzene rings or long-chain alkanes. Similarly, different particle sizes of carrier-free nanodrugs were obtained when the ratio of drugs varied, owing to the changes in the strength of hydrophobic interactions. For instance, Shao's group fabricated carrier-free nanodrugs composed of PTX and ICG through electrostatic interaction and π-π stacking for chemo-photothermal therapy [22]. When the ratio of PTX and ICG changed from 1:1 to 5:1, the particle sizes were still less than 200 nm, which are beneficial for the EPR effect. However, when the drug ratio of PTX to ICG reached 10:1, the particle sizes were over 1000 nm, and the Tyndall effect even disappeared after the molar ratio of PTX to ICG decreased to 1:5 (Fig. 2c). The above results indicated that when PTX was excessive, PTX and ICG can be easily aggregated due to the large combined tricycloalkane taxane skeleton of PTX. Conversely, when ICG was excessive, it can increase the solubility of PTX and completely dissolve PTX to form a solution. It is worth noting that carrier-free nanodrugs self-assembled by dual-drug or multi-drugs via hydrophobic interaction currently have no general rule to adjust the particle size via the variation of drug ratio. Therefore, based on the previously discussed results, it is urgent to develop related rules to fabricate carrier-free nanodrugs with optimized particle sizes for effective in vivo delivery.
2.2.2. Influence of preparation method on particle size
As discussed before, several preparation methods are widely used to construct the carrier-free nanodrugs. Studies have demonstrated that preparation methods affect particle sizes. For example, the reverse solvent precipitation method involves nanoemulsion droplets forming after the drug dissolved in organic solvent was dropped into water. Subsequently, the nanoemulsion becomes supersaturated as the solvent continues to diffuse and dissolve, followed by the formation of crystal nucleus, and then nanoparticles grow within the droplets [54]. The formation process, type of solvent, and initial drug concentration all play a key role in determining their diffuse rate and the size of the droplet, thereby controlling the particle size of the carrier-free nanodrugs. Currently, research efforts have been concentrated on adjusting the solvent and the initial drug concentration to obtain a satisfactory size. Generally, if the drug concentration is high, more drugs will be aggregated in droplets, resulting in a larger particle size. Similarly, if the viscosity of a good solvent is greater, it tends to form larger droplets and form larger nanoparticles.
In addition to the reverse solvent precipitation method, the medium milling method is another radical strategy to prepare carrier-free nanodrugs. During the grinding process, particles breaking into smaller particles and the secondary growth of particles occur simultaneously. In order to obtain nano-scale particles, the probability of collision between particles, which is greatly impacted by drug concentration and grinding time, should be minimized. Excessive drug concentration and long-term grinding will increase the chance of excessive drug collision, resulting in secondary aggregation of broken particles. It is necessary to rationally select a suitable grinding time and speed to avoid aggregation. Moreover, smaller particle sizes of the grinding medium lead to more contact points with the drug particles, which increases the collision frequency between the drug and the grinding beads, thereby resulting in more uniform and smaller particle sizes of drug nanocrystals. Notably, studies have shown that low temperature grinding can slow down the particle aggregation process and improve grinding efficiency. Low temperature can also increase the fragility of the particles, reduce the heat of the system, and obtain smaller and more uniform particle sizes [55].
As for the high-pressure homogenization method, the particle size is greatly influenced by homogenization pressure and the number of homogeneous cycles. Evidently, the greater the homogenization pressure is, the smaller and more uniform the particle sizes are. Although the high pressure is beneficial to reduce the particle size, more heat is generated with the increased homogenization pressure, which may not be suitable for temperature-sensitive drugs. In addition, the number of homogeneous cycles also affects the particle size distribution. The prospect of crushing all large drug particles into uniform nanocrystals in a single cycle is unfulfillable. Obtaining uniform size requires increasing the number of homogeneous cycles and providing more energy to fully crush the drug to obtain nanoscale particles. Furthermore, the flow velocity in different areas is different due to the gradually decreasing flow velocity from the center of the pipe to the pipe wall, resulting in uneven energy distribution in the homogenous cracks, which ultimately leads to uneven particle size distribution. Increasing the number of cycles will increase the chance of large particles passing through the high-energy region, further breaking the large particles into smaller particles.
The template-assisted method also has the potential to precisely control the particle size. The basic process of this method to prepare carrier-free nanodrugs is as follows: when the drug dissolved in organic solvent fills the holes or gaps in the template, the organic solvent is first removed to enable the drug to self-assemble, and then the template is removed to get the pure nanodrug. Therefore, the size of the nanoparticles is limited by drug concentration and the template pore size. As the concentration of drug increases, the local concentration in the pore increases, leading to a larger particle size. However, when the concentration is too high, the particle size will be limited by the size of the pore instead of the drug concentration. Notably, the size of the nanoparticles prepared by the ice template-assisted method is also affected by the preparation temperature. In the ice template method, the drug solution on ice may melt the surface of ice and widen the grain boundary, resulting in larger particle sizes [31]. Therefore, preparation of small size nanoparticles by the ice template-assisted method requires low drug concentration, small template holes, and low temperature. Compared to the reverse solvent precipitation method, the template-assisted method has higher production rates and relatively smaller particle size, which are conducive to clinical transformation.
2.3. Strategies to optimize particle morphology of carrier-free nanodrugs
In general, CDDS or carrier-free nanodrugs are prone to self-assemble into spherical nanoparticles with low-energy. Studies have demonstrated that nanoparticles with spherical shapes displayed prolonged blood circulation time [56]. However, whether sphericity or non-sphericity is more favorable for the delivery of nanoparticles in vivo has been controversial [57]. Some studies demonstrated that nanoparticles with different length-width ratios are better at accumulation, retention at the tumor site, and cellular uptake by cancer cells compared to spherical nanoparticles [37,58]. Compared to spherical nanoparticles, elongated particles are more likely to pass through some curved and narrow tissues, thus having a longer blood circulation time. More importantly, the flat end of non-spherical nanoparticles with larger surface area can increase the probability of binding to receptors on the cell membrane, thus improving the targeting efficiency. In addition, the shapes of the nanoparticle contact with the membrane have a very important influence on the uptake of nanoparticles. For instance, Champion et al. found that phagocytosis occurs in a relatively short time if the cell first touches the tip of the elliptical particle, as compared to a sphere [59]. Considering the morphology influence on the in vivo delivery behavior, this section discusses the influence of intermolecular interaction of drugs and drug modification on the morphology of carrier-free nanodrugs. This section aims to provide references for the design of carrier-free nanodrugs with high delivery capacity and improved anti-tumor effect.
2.3.1. Influence of intermolecular interaction on morphology
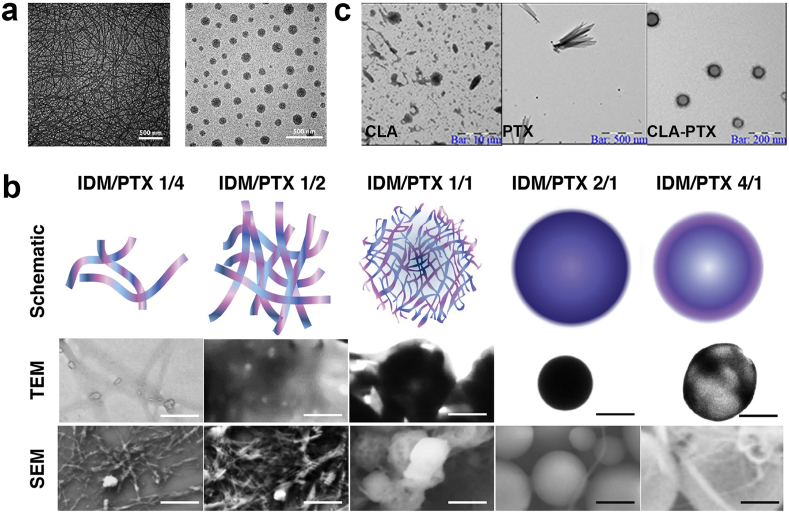
Currently, most drugs can be self-assembled into carrier-free nanodrugs with a spherical shape, however, some drugs, such as PTX [60,61] and camptothecin (CPT) [62], tend to self-assemble into needle-shaped nanocrystals due to their own special planar ring structure. Unfortunately, these needle-like nanocrystals tend to aggregate and are unstable in aqueous solutions. Although some strategies like surface modification have been reported to prevent the undesired aggregation [13,37,63,64], there is also widespread interest in improving the stability by changing the morphology into a spherical shape. One of the effective strategies is through the addition of other drugs to break the original trend of planar assembly, resulting in the formation of spherical nanoparticles rather than needle-like or rod-like nanoparticles. For instance, Zhao and coworkers found that the HCPT nanorods undergoes a morphological transformation from rod-like to spherical after adding DOX into the self-assembly system [65]. The morphology change can be attributed to DOX molecules interacting with the HCPT nanorods, resulting in the gradual co-assembly of DOX and the original HCPT nanorods into spherical HCPT/DOX particles. Another interesting study showed that the π-π interaction in redox responsive camptothecin-gemcitabine amphiphilic prodrug (CPT-ss-GEM) can induce the formation of carrier-free nanodrugs with a nanowire structure. However, the nanostructure transformed from nanowires to uniform spherical nanoparticles when the hydrophobic NPAPF (a near-infrared (NIR) emission AIEgens) was added [66] (Fig. 3a). The primary reason for this conversion is that the hydrophobic interaction becomes the main force for the self-assembly instead of the π-π interaction. Compared to carrier-free nanodrugs with the nanowire structure, the spherical nanoparticles showed improved cellular uptake and tumor tissue penetration abilities. A possible explanation is that the 1 μm long nanowires make them easily capturable by FBS protein molecules to hinder their interaction with cancer cells, thus resulting in the poor cellular uptake performance.
Fig. 3.
a) The TEM images of CPT-ss-GEM nanowires and CPT-ss-GEM nanoparticles. Reproduced with permission from Ref. [66]. Copyright 2019, Elsevier. b) Schematic, TEM, and SEM images of IDM/PTX assemblies with different drug ratio. Reproduced with permission from Ref. [67]. Copyright 2019, American Chemical Society. c) The TEM images of CLA, PTX and CLA-PTX nanoparticles. Reproduced with permission from Ref. [68]. Copyright 2016, Springer Nature.
Adjusting the hydrophilic-to-hydrophobic ratio of therapeutic agents to change the intermolecular interactions is another radical strategy to change the morphology of carrier-free nanodrugs. One example is that the morphologies of nanodrugs gradually change from wire-like to net-like, honeycomb-like, sphere-like, or capsule-like when the ratio of Indomethacin (IDM), a hydrophilic nonsteroidal anti-inflammatory drug, to PTX changed from 4:1 to 1:4 (Fig. 3b) [67]. In this case, the morphological change is due to the modification of the hydrophilic-to-hydrophobic ratio. PTX formed a crystal nucleus in aqueous solution and then IDM, acting like cements, tended to gather around the PTX nanocrystal through strong interactions, such as π-π stacking, H-bonding, and hydrophobic interactions rather than forming IDM crystals.
2.3.2. Influence of drug modification on morphology
Despite the widely used strategies to change the morphology of nanoparticles through adding other therapeutic agents or varying the hydrophilic to hydrophobic ratio, they are not suitable for most therapeutic agents because they are greatly reliant on the structures of drugs. Therefore, the modification of therapeutic agents is another attractive strategy to influence their own interactions. Generally, the solubility and polarity of therapeutic agents can be modified, which can influence their self-assembly process and form carrier-free nanodrugs with different morphologies. For example, the solubility and polarity of PTX was greatly improved after being modified with linoleic acid (CLA) through chemical conjugation due to a longer carbon chain in the CLA molecules [68]. More importantly, the carbon chains in the CLA prevent the growth of PTX along the edge, thus resulting in the formation of spherical rather than needle-like structures (Fig. 3c). However, it is interesting that PTX modified by Vitamin E (VE) contains a longer carbon chain, but they have not been able to successfully self-assemble into particles [69]. One possible explanation is that the hydrophobicity of PTX-VE increases sharply leading to aggregation in the self-assembly process. Moreover, the introduction of some special linkers, such as disulfide bonds, can also have an effect on their self-assembly behavior due to its unique properties such as bond angles. Wang and coworkers demonstrated that the insertion of disulfide-bonds between PTX and VE can easily trigger the formation of regular spheres and no crystals were detected [69]. Conversely, PTX-VE (without disulfide bond) only produces crystal aggregates. It is well known that the dihedral angles of the disulfide bond tend to 90°, which is favorable to generate stable nanoparticles in an irregular and non-periodic manner rather than forming crystals, thus the crystallization is blocked and the stability of nanoparticles is significantly improved with the existence of disulfide bonds.
2.4. Other properties of carrier-free nanodrugs
Apart from the effect of particle size and morphology on the delivery and release of carrier-free nanodrugs, other factors such as the length and type of linker connecting the therapeutic agents also have some impact on their delivery and release fate in vivo. As mentioned above, the introduction of disulfide bonds to connect different therapeutic agents is beneficial to enhance the stability of carrier-free nanodrugs due to its dihedral angles approaching 90°, which is essential for balancing intermolecular forces and establishing a stable conformation [69]. Compared with alkane bonds (C–C) and single thioether bonds, disulfide bonds are more flexible and favorable for self-assembly. Furthermore, shorter length linkers in the existence of therapeutic agents will hinder their self-assembly ability owing to their poor flexibility [70]. Therefore, the chain length of disulfide bonds should be taken into account when introducing them as a linker to enhance the stability of carrier-free nanodrugs. Alternatively, ester bonds and amide bonds, as tumor microenvironment sensitive linkers, are commonly utilized as linkers between drugs for improved release rate. In general, therapeutic agents connected with ester linkers are more beneficial for drug release owing to its easily hydrolyzed nature compared to that of amide linkers [71]. The further combination of hydrophilic disulfide bonds allows for easier absorption of water molecules to hydrolyze the ester or amide bonds, resulting in accelerated drug release.
Aside from the influence of the linker, some special components will also have a great impact on the delivery and release of carrier-free nanodrugs. For example, tannic acid (TA) contains a large number of hydroxyl groups to reduce the nonspecific binding of carrier-free nanodrugs with conditioning components in the plasma and avoid being cleaned by the Reticuloendothelial System (RES). In addition, TA can significantly improve the drug loading content though π−π interactions from the benzene ring in their structure. More importantly, the pKa of TA is 6.33, so it primarily exists in the form of ions when pH > 6, coexists in the form of molecules and ions when pH ≈ 6, and exists in the form of molecules when pH < 6 [72]. Based on this, Xiong and coworkers used this property to design a carrier-free nanodrugs consisting of DOX, ICG and TA (DTIG) [73]. Under the normal physiological condition, the particle size is 74 nm. However, the particle size rapidly increases to 737 nm upon accumulating at the tumor site due to the gradual weakening of the electrostatic effect and the gradual enhancement of π−π interactions in nanoparticles conducted by the pH changes. Subsequently, the particle size of DTIG consciously increased to 1.5 μm after being internalized by cells, indicating that the nanoparticles have the capability of escaping lysosomes. After reaching the neutrophil cytoplasm, the nanoparticles reassemble themselves into small particles (~152 nm). Therefore, TA is able to guide the whole delivery process, including long circulation time in blood, effective cellular uptake, favorable retention, and fast drug release. In the future, it is necessary to develop similar intelligent responsive compounds that can respond to the environment in the delivery process to achieve intelligent changes, such as the transformation of particle size, zeta potential, and morphology to improve their delivery efficiency and enhance anti-tumor effects.
3. Modification strategies on improving the carrier-free nanodrugs delivery and release behavior
The optimization of physicochemical properties of carrier-free nanodrugs through various strategies described above has made significant progress towards improving the delivery and release behavior of carrier-free nanodrugs. However, the current poor clinical transformation of those carrier-free nanodrugs reminds us that prior to delivering the drug to the site of action, the complex in vivo microenvironment and multiple biological barriers are key factors to determining their therapeutic efficacy. Therefore, a detailed discussion of the delivery process has emerged to guide the development of corresponding strategies for improving their delivery and release behaviors. The primary obstacles for carrier-free nanodrug's in vivo delivery journey can be briefly summarized as follows. Carrier-free nanodrugs have no carrier and they rely on noncovalent interactions to drive their self-assembly. These weak interactions result in poor stability of the carrier-free nanodrugs, thus making it difficult to guarantee sufficient blood circulation time. In addition, carrier-free nanodrugs made up by therapeutic agents commonly accumulate at the tumor site through EPR effects due to their lack of tumor-specific targeting ability, resulting in subpar cellular uptake. Finally, the lack of timely intracellular drug release hinders the therapeutic efficacy of the drugs after being internalized by the cells. To address these problems, it is advisable to modify the carrier-free nanodrugs to possess different capabilities for tumor management, including excellent stability for prolonged the blood circulation, active-targeting for efficient cellular internalization, and rapid intracellular drug release. Possible strategies to circumvent these obstacles are reviewed in detail below to provide insight towards rational design of carrier-free nanodrugs for prominent therapeutic efficiency.
3.1. Strategies to prolong blood circulation
Carrier-free nanodrugs are mostly self-assembled through weak noncovalent interactions, which are usually unstable and may be rapidly recognized and sequestered by the mononuclear phagocyte system (MPS). The large amounts of proteins in the blood can easily absorb on their surface to form a protein corona. Moreover, the carrier-free nanodrugs with a protein corona are easily aggregated and have a lower stability. This major biological limitation for carrier-free nanodrugs can result in short systemic circulation time, ultimately causing limited therapeutic levels of drugs at the tumor sites. Despite numerous innovations and modifications to alternate the physicochemical properties to improve the circulation time of carrier-free nanodrugs, there is still a major shortfall when it comes to rapid clearance by the RES. Many studies have focused on the surface modification of carrier-free nanodrugs, which has been accepted as a primary strategy to prolong the blood circulation time and avoid the undesirable aggregation, because it provides a higher probability of circulating carrier-free nanodrugs to encounter targets of interest. Strategies to prolong blood circulation time and avoid undesirable aggregation can be achieved through inserting polymers, small molecules, biomolecules, or some adjuvants into the carrier-free nanodrugs, as summarized in Table 2.
Table 2.
Summary of stability improvement strategies for prolonged blood circulation of carrier-free nanodrugs.
| Molecules type | Stabilizer | Therapeutic agent | Functions | Refs |
|---|---|---|---|---|
| Polymers | C18PMH-PEG | TPP | Photodynamic therapy | [63] |
| C18PMH-PEG | MTX, PTX, HCPT | Synergistic chemotherapy | [74] | |
| C18PMH-SS-PEG | HCPT, Fe3O4 | Chemo-photothermal therapy, MR imaging |
[75] | |
| C18PMH-PEG-FA | Bis(4-(N-(2-naphthyl)phenylamino) phenyl)-fumaronitrile (NPAPF) | Bioimaging | [76] | |
| DPSE-PEG2000 | MTX, PPa | Chemo-photodynamic therapy | [77] | |
| DPSE-PEG2000 | CPT, fluoropyrimidine derivative floxuridine | Synergistic chemotherapy | [78] | |
| DPSE-PEG2000 | PTX | Chemotherapy | [79] | |
| DPSE-PEG2000 | PTX, PPa | Chemo-photodynamic therapy | [80] | |
| DPSE-PEG2000 | 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) | Photothermal therapy, Imaging | [81] | |
| DPSE-PEG2000 | DOX, α-TOS | Chemotherapy | [82] | |
| DPSE-MPEG | EPI, ICG | Chemo-photothermal therapy, Imaging | [83] | |
| DPSE-MPEG, | PTX, 4-chloro-7-nitro-1, 2, 3-benzoxadiazole (NBD-Cl) | Chemotherapy, Imaging | [84] | |
| DSPE-PEG-MTX | CPT | Synergistic chemotherapy | [37] | |
| MeO-PEG3000-NH2 | PTX, lapatinib (LAPA) | Synergistic chemotherapy | [85] | |
| PEG-LA | HCPT, Au | Chemo-photothermal therapy | [12] | |
| PEG methyl ether methacrylate | CPT | Chemotherapy | [86] | |
| PEG-b-PPOH | epigallocatechin-3-gallate (EGCG), phenolic platinum(IV) prodrug | Chemodynamic therapy | [87] | |
| PEG-EGCG | oligomerized EGCG, Herceptin | Chemotherapy | [88] | |
| RGDyc-PEG3500-NH2 | PTX | Chemotherapy | [89] | |
| FA-PEG-PMHC18 | PTX | Chemotherapy | [13,90] | |
| FA-PEG-PMHC18 | 2-tert-butyl-9,10-di(naphthalen-2yl)anthracene (TBADN) | Imaging | [64] | |
| MTX–chitosan | MTX, HCPT | Synergistic chemotherapy, diagnosis | [91] | |
| PDA | DOX, Gn | Synergistic chemotherapy | [92] | |
| PVA | DOX, Gn | Synergistic chemotherapy | [93] | |
| Small molecules | Tannic acid (TA) and FeIII | PTX | Chemotherapy | [94] |
| Evans blue (EB) | CPT | Chemotherapy | [95] | |
| Cell membranes | Red blood cell (RBC) membrane | ICG, HSA, PFC | Phototherapy | [96] |
| Red blood cell (RBC) membrane | ICG, HCPT | Chemo-photothermal therapy, Imaging | [97] | |
| RGD modification RBC membrane | Docetaxel (DTX) | Chemotherapy | [98] |
3.1.1. Inserting polymers to prolong blood circulation
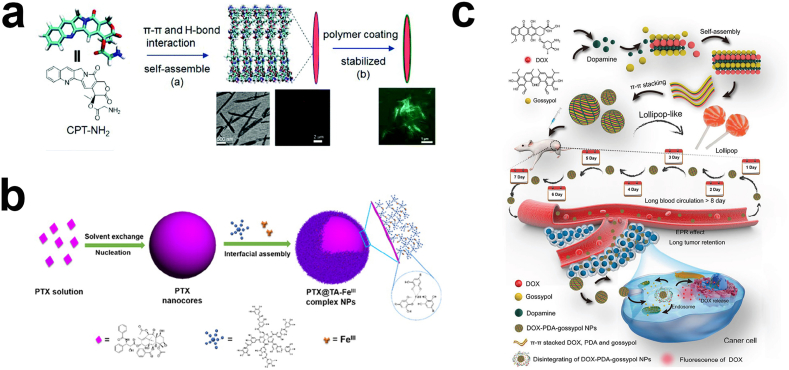
To date, the imparting of a hydrophilic polymer coating on the surface of carrier-free nanodrugs is the most widely used strategy to improve the stability and reduce the non-specific adsorption of proteins. A large number of studies have been carried out to modify the carrier-free nanodrugs with biocompatible synthetic or natural polymers to prolong their blood circulation time, like polyethylene glycol (PEG) [81,89,99], polydopamine (PDA) [92], and polyvinyl alcohol (PVA) [93]. For example, PEG, as a typical hydrophilic macromolecule, on the surface of carrier-free nanodrugs can tightly bind water molecules to create a hydrated coating that can sterically block carrier-free nanodrugs from interacting with proteins to avoid clearance by the MPS [100]. Furthermore, the flexibility of PEG chains is not conducive to the mutual penetration of blood components, thereby beneficial for prolonging the circulation time in the body. Aside from PEG, PEG derivatives modified with different functional groups can also immobilize on the surface of carrier-free nanodrugs through different forces like electrostatic interactions and hydrophobic interactions. For instance, Zhou et al. utilized PEG-block-poly(l-lysine), a pH-sensitive negative-to-positive charge reversal PEG derivative, to decorate the surface of carrier-free nanodrugs self-assembled by camptothecin (CPT) through electrostatic adsorption [86]. The study showed that the charge of the carrier-free nanodrugs changed from positive to negative, resulting in longer blood circulation because negatively charged surfaces tend to avoid aggregation and reduce nonspecific interactions (Fig. 4a). Another example is concentrated on fabricating PEG-grafted poly (maleic anhydride-alt-1-octadecene) (C18PMH–PEG) macromolecules, which can decorate on the surface of meso-tetraphenylporphyrin (TPP) nanocrystals via hydrophobic interactions. The PEG-coated TPP nanocrystals showed no obvious aggregation in saline after 48h incubation, while uncoated TPP nanocrystals had visible precipitation [63]. Although pioneering studies indicated that the improved systemic circulation through PEGylation lead to the rapid development of carrier-free nanodrugs, frequently using these PEG-modified carrier-free nanodrugs triggered the emergence of accelerated blood clearance after significantly inducing the humoral immune response. Moreover, the PEG-modified carrier-free nanodrugs will be more easily recognized and cleared by phagocytes after the neutralizing antibody secreted by B cells binds to PEG, resulting in a significantly shortened blood half-life time. Additionally, carrier-free nanodrugs with PEG coatings can reduce their cancer cell internalization due to steric hindrance and decrease intracellular drug delivery owing to hindered endosomal escape, known as the “PEG dilemma’’ [[101], [102], [103], [104]].
Fig. 4.
The strategies to enhance carrier-free nanodrugs' stability. a) PEG-modified CPT nanocrystal through electrostatic interaction. Reproduced with permission from Ref. [86]. Copyright 2019, Royal Society of Chemistry. b) PDA fills the gap between DOX and Gn to form lollipop-like nanoparticles. Reproduced with permission from Ref. [92]. Copyright 2016, American Chemical Society. c) Cooperation between TA and FeIII to form a coating that prevents Ostwald's maturation. Reproduced with permission from Ref. [94]. Copyright 2018, John Wiley and Sons.
Although the stability of carrier-free nanodrugs can be improved through physical modifications, it is difficult to withstand long term stability when encountering the complex delivery environment. The most effective strategy to further improve the blood circulation time is focused on chemical modifications. For instance, Wang and coworkers designed PTX-Lapatinib nanocrystals with polydopamine, and took advantage of the active catechol hydroxyl groups on polydopamine to react with the amine groups of MeO-PEG3000-NH2 on the surface of nanocrystals. Results showed that the modified nanocrystals with a PEG coating were stable in PBS and 10% FBS for a long duration, while the bare PTX-Lapatinib nanocrystals aggregated quickly within 2h [85]. The improved stability can be attributed to the PDA on the surface of nanocrystals providing a reaction site for chemical modification. Based on these results, it is suggested that the utilization of covalent bonds rather than non-covalent bonds for carrier-free nanodrugs show great advantages in improving their stability and prolonging the blood circulation time.
In addition to hydrophilic macromolecule coatings, another strategy focuses on adding a small amount of adjuvant to increase their blood circulation time. Compared to the traditional method of wrapping the outer layer of carrier-free nanodrugs with a shell, adding adjuvants can fill in the gaps in the carrier-free nanodrugs, which can not only prolong their blood circulation time, but also maintain their original penetration and internalization capabilities due to no significant particle size increase. Nanoparticles functionalized with polymers possessed larger hydrodynamic sizes compared to unfunctionalized particles, which hindered the nanoparticles from penetrating deep targets [105]. For example, Wu's group prepared lollipop-like dual-drug-loaded carrier-free nanodrugs (DOX–PDA–gossypol NPs) [92] (Fig. 4b). Due to the difference of hydrophobicity and structure of gossypol (hydrophobic) and DOX (hydrophilic), it is not favorable for them to form stable nanoparticles by π–π stacking, thus the system composed with only dual drugs had loose structure and exhibited poor stability. After the addition of polydopamine that exhibit benzene rings, to fill the gap between gossypol (Gn) and DOX like “cement”, the strong π–π stacking between Gn and DOX made the nanoparticle structure more compact, thus improving their bio-environmental stability without obvious size increase and prolonging its circulation time in the body (8 days), whose elimination half-life times were 458-fold and 258-fold longer than that of free DOX and free gossypol, respectively. Moreover, Liu and co-workers reported that PVA could also be used to fill the gaps in DOX and Gn [93]. PVA contains a large number of hydroxyl groups that can form hydrogen bonds with the hydroxyl groups of Gn and DOX to increase the nanoparticle's tightness. This strategy to enhance the stability through the utilization of macromolecules filling in the “gaps” between different drugs by strong noncovalent interactions, like π–π stacking and hydrogen bonds, is quite different from the traditional outer coating modification strategy, which opens up a new direction to achieve the prolonged blood circulation time of carrier-free nanodrugs. Most importantly, the macromolecules that fill the gaps from the inside make the nanoparticles more compact and smaller, which is more favorable for deep penetration of the nanoparticles.
3.1.2. Small molecules-enabled prolonged blood circulation
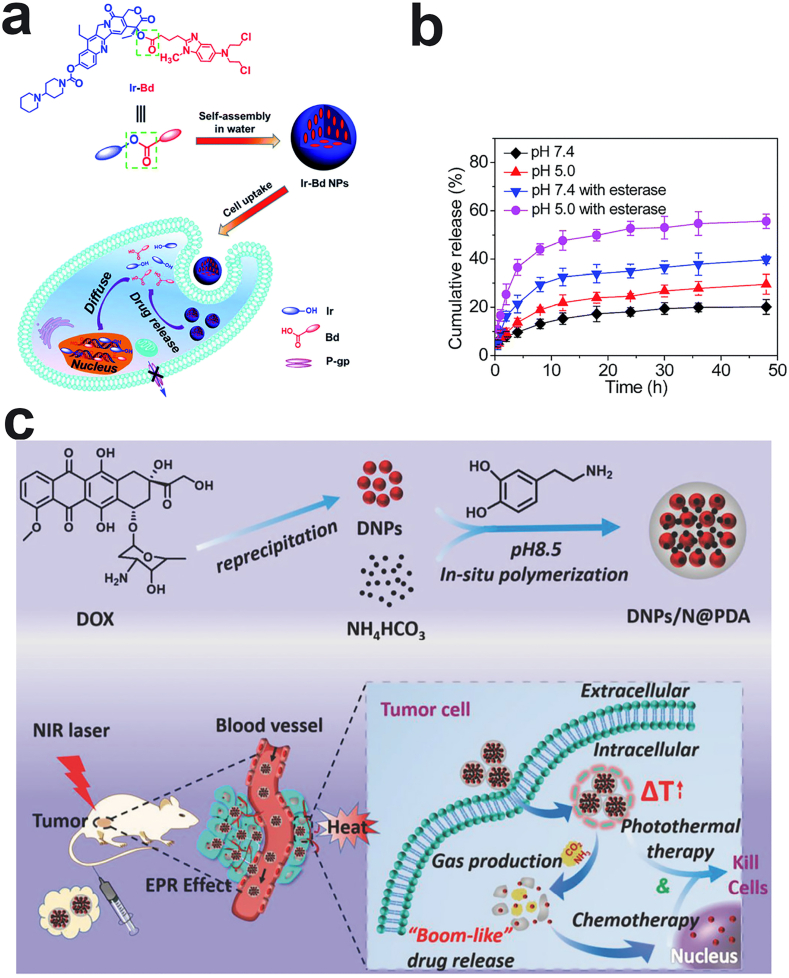
Aside from the hydrophilic polymer coatings, small molecules coordinated with metal ions, like FeIII, on the surface of carrier-free nanodrugs is another radical strategy to improve their stability [87,106]. For example, TA is rich in catechol and pyrogallol parts, so it has a high binding affinity for FeIII and other metal ions. Shen et al. stabilized PTX nanocrystals with a TA and FeIII complex coating [94] (Fig. 4c). Based on this characteristic of TA, it can form a dense layer around the drug core, which can effectively inhibit and prevent Ostwald's maturation, resulting in small and uniform particle sizes and long-term colloidal stability. In addition to the incorporation of small molecules on the surface of carrier-free nanodrugs to form a shell, it is a good strategy to construct prodrugs through chemical modification of drugs, which can be self-assembled into nanoparticles with good stability. As we know, CPT has limited clinical use due to its hydrophobicity and strong side effects, as well as its lactone rings were rapidly hydrolyzed in PBS with a half-life of 24 min. Chen's group conjugated CPT with Evans blue (EB) through a disulfide bond to form stable carrier-free nanodrugs [95]. The conjugated nanodrugs can be stable in PBS for over 6 days and exhibit remarkably long blood circulation time in mice (130-fold greater than CPT). The remarkable in vivo stability can be attributed to the albumin/CPT-SS-EB nanocomplex. EB has been documented to bind with albumin through hydrogen bonds, so the nanoparticle quickly transformed into albumin/CPT-SS-EB nanocomplexes after injection. The serum albumin has a long blood half-life (~20 days), thus its incorporation is beneficial for enhancing their blood circulation time. Although small molecules present several advantages to improve the stability of carrier-free nanodrugs, limited attention is concentrated on their functions. In the future, we hope that more efforts will be concentrated on developing new and suitable small molecules that can not only prolong the blood circulation time, but also impart some other functions, such as deep penetration and active targeting.
3.1.3. Biomimicry camouflage-benefited prolonged blood circulation
Biomimicry camouflage is another attractive strategy to increase nanoparticle circulation time by utilizing endogenous components. A major endogenous mimicry target utilized by researchers is red blood cells (RBCs) [[107], [108], [109], [110]], which has been reported that the addition of RBC membrane coatings can help nanoparticles escape from macrophage uptake and systemic clearance [111,112]. Ye et al. have developed carrier-free nanodrugs self-assembled by HCPT-ICG coated with RBC membranes. Results showed that the modified carrier-free nanodrugs could be stable in PBS and FBS enrichment medium for 120 h [97]. Ren and co-workers have also developed a novel oxygen self-enriched carrier-free nanodrug by coating albumin nanoparticles that contain ICG and perfluorocarbon (PFC) with RBC membranes to significantly reduce immune clearance in macrophage cells (RAW264.7) and notably achieve both prolonged blood circulation time and high accumulation at the tumor [81]. Meanwhile, cancer cell membranes (CCMs) as another kind of biomimicry camouflage, also have the potential to prolong the blood circulation due to its biogenetic derivation and biodegradability, as well as actively target tumors due to homotypic targeting [113]. It seems that coating carrier-free nanodrugs with cell membranes is a promising strategy to improve their blood circulation time. Recently, platelet membranes [114], dendritic cell (DC) membranes [115] and macrophage membranes [116,117] have been applied to drug delivery systems to escape the clearance of the immune system, prolong blood circulation time, and target the tumor site. For the future, it is suggested that more efforts will concentrate on the development of multiple natural cellular membranes to form a hybrid biomimetic coating for carrier-free nanodrugs to prolong the blood circulation time. Besides natural cellular membranes, it is anticipated that studies develop new endogenous mimicry targets, such as albumin and RBC components (e.g. CD47), to improve the blood circulation time. For example, bovine serum albumin was widely used to modify CDSS to improve the circulation time through the binding of the Fc receptors during recycling as a form of protection for albumin from degradation in the endosomes and lysosomes. It also has the ability to prompt binding of previously mentioned hydrophilic polymers for a synergistic effect to prolong the blood circulation due to the presence of functional carboxyl and amine groups on the CDSS surface.
3.2. Strategies to improve tumor targeting ability
As discussed previously, carrier-free nanodrugs with good stability can prolong the circulation time in blood and accumulate at the tumor site through the EPR effect. However, most of the carrier-free nanodrugs are trapped on the tumor vasculature periphery and could not be retained at the tumor site, resulting in insufficient internalization by cancer cells [35]. Moreover, this passive targeting type has its limitation such as depending on the degree of tumor angiogenesis/vascularization, the low cellular uptake capacity of drugs, and heterogeneous drug distribution within the tumor microenvironment. In addition, the tumor's lymphatic drainage is poorly developed, which increases intratumor pressure making it difficult for nanodrugs to penetrate deeply into the tumor site [118,119]. Targeted drug delivery has been widely recognized as having the potential to overcome the bottleneck associated with systemic administration of drugs due to its ability to reduce off target toxicity and lower the required dosage for therapeutic efficacy. Although the optimization of their physicochemical properties, including particle size, charge, and morphology can improve their internalization efficiency, the lack of selective targeting towards cancer cells can compromise their cellular uptake capability. Currently, researchers are investigating active-targeting strategy, including tumor-targeting peptides, small molecule non-peptide ligands, vitamins, aptamers, monoclonal antibodies, and protein scaffolds, to improve their internalization efficiency. It is widely accepted that active targeting can assist with selective amassing of nanoparticles at a target site due to overexpressed receptors or surface membrane proteins expressed on target cells, ideally to exclusively interact with only the target cells and avoid off-target toxicity. Additionally, the active targeting strategy can be considered in rational combination with physicochemical property modifications for further enhancing cellular uptake. Some active targeting molecules, including polymers, small molecules, and biomimetic cell membranes, used to improve the internalization efficiency are summarized in Table 3.
Table 3.
Summary of tumor targeting strategies for improved tumor cellular uptake.
| Targeting agent | Targeting receptor | Therapeutic agent | Functions | Refs | |
|---|---|---|---|---|---|
| Polymers | HA | CD44 | DOX, PTX | Synergistic chemotherapy | [120] |
| DOX, Gn | Synergistic chemotherapy | [93] | |||
| DOX, proapoptotic peptide KLA | Chemotherapy | [33] | |||
| MTX, DOX ICG | Chemo-phototherapy, dual tumor targeting | [121] | |||
| Curcumin | Chemotherapy | [122] | |||
| MTX | Chemotherapy, dual tumor targeting | [123] | |||
| Small molecule | FA | Folate receptor | PTX | Chemotherapy | [13,90] |
| 2-tert-butyl-9,10-di(naphthalen-2yl)anthracene (TBADN) | Imaging | [64] | |||
| NPAPF | Bioimaging | [76] | |||
| Biotin | Biotin receptor | IR780, quercetin | Chemo-phototherapy | [124] | |
| MTX | Folate receptor | MTX, HCPT | Synergistic chemotherapy, diagnosis | [37] | |
| MTX, PTX, HCPT | Synergistic chemotherapy | [74] | |||
| MTX, CPT, DiR | Synergistic chemotherapy, imaging | [125] | |||
| MTX, UA | Synergistic chemotherapy | [126] | |||
| LA | ASGPR | DOX | Chemotherapy | [127] | |
| UA, ICG | Chemo-phototherapy, Imaging | [128] | |||
| Biomolecules | HER2 aptamer | HER2 receptor | UA, DOX | Synergistic chemotherapy | [129] |
| RGD tetrapeptide | Integrin receptor | CPT | Chemotherapy | [130] | |
| PTX | Chemotherapy | [89] | |||
| CPT, ICy5 | Chemo-photodynamic therapy | [131] | |||
| metallized Au(III) tetra-(4-pyridyl) porphine | Chemo-photothermal therapy | [132] | |||
| RGD-FA | Integrin receptor Folate receptor |
PTX | Chemotherapy, dual tumor targeting | [133] | |
| AS1411 aptamer | Nucleolin | 3′,5′- dioleoyl clofarabine (DOC) | Chemotherapy | [134] | |
| Cancer cell membranes | CCCM | DOX, ICG | Chemo-photothermal therapy | [135] | |
| HCPT, ICG | Chemo-photothermal therapy | [136] |
3.2.1. Polymer-prompted tumor targeting
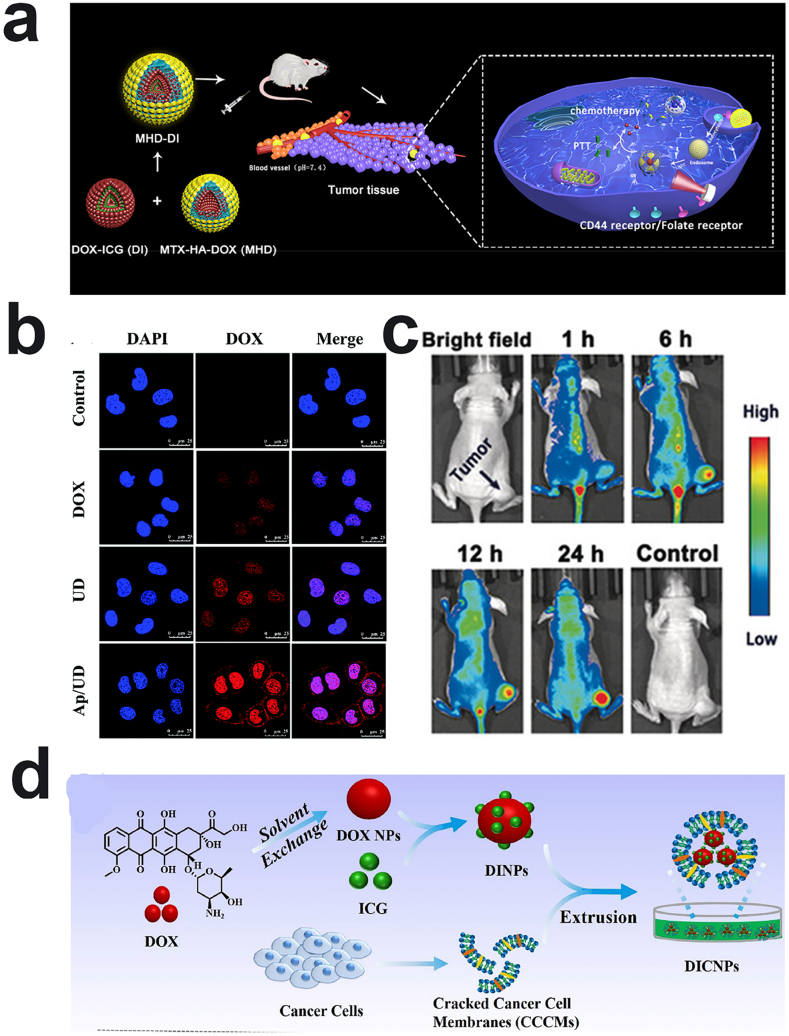
Cancer cells express a large number of cell surface receptors, such as CD44, folate receptors, or integrin receptors, to meet the needs of tumor growth, migration, invasion, and metastasis. Therefore, these receptors can be used as suitable candidates for ligand-targeted cancer therapy. For the highly expressed receptors of tumor cells, introducing targeting ligands into carrier-free drugs can improve the ability of specific uptake and enhance cellular internalization. For example, hyaluronic acid (HA) has good biocompatibility and possesses four specific binding sites with various cancer cells (e.g. CD44, RHAMM, IVd4, LEC), such as human breast adenocarcinoma cells and human lung cancer cells. Among them, CD44 is the most widely used receptor on the cell surface to bind with HA [137,138]. More importantly, after internalization by cancer cells via the CD44-mediated pathway, the enrichment of hyaluronidase (HAase) in the endosome/lysosome can promote the degradation of hyaluronic acid (HA) shell-based nanoparticles, thereby allowing de-shielding of the coating and consequently exposing the core to accelerate tumor penetration and lysosomal escape [24,139]. Shen and coworkers fabricated positively charged nanoparticles composed of amphiphilic proapoptotic peptide and DOX, and then the negatively charged HA was coated on their surface through electrostatic interactions [33]. According to the in vitro uptake experiments, their internalization efficiency was enhanced with an HA coating. More importantly, the tendency of cellular internalization was selective between tumor cells and normal cells, leading to excellent tumor inhibition efficiency with insignificant side effects. Although the expression level of cancer cell surface receptors has been reported to show heterogeneity, most of the targeted receptors expressed on both cancer cells and normal cells is the primary contributor for undesired drug uptake. Therefore, it is a very attractive strategy to achieve multiple targeting functions through the combination of HA and other targeting molecules. Zhu's group coupled MTX and DOX to the HA backbone, resulting in self-targetable prodrug conjugates [121]. According to the cellular uptake experiment results, the intracellular DOX intensity decreases significantly in HeLa cells (overexpressed with folate/CD44 receptors) in the presence of folic acid (FA) or HA, whereas significant fluorescence of DOX and ICG could be observed in the HeLa cells in the absence of FA or HA. Significantly less DOX fluorescence could also be observed in A549 cells, which express low levels of folate receptors (Fig. 5a). The above results revealed that the imparting of multiple targeting functions to carrier-free nanodrugs showed prominent advantages to enhance their internalization efficacy.
Fig. 5.
a) Schematic illustration of MHD−DI NPs for dual-targeting drug delivery and imaging-guided combinational chemo-PTT therapy. Reproduced with permission from Ref. [121]. Copyright 2019, American Chemical Society. b) CLSM images of cells co-incubated with DOX, UD NPs, and Ap/UD NPs for 2 h. Reproduced with permission from Ref. [129]. Copyright 2017, Royal Society of Chemistry. c) In vivo fluorescence imaging of PTN at different times after intravenous injection. Reproduced with permission from Ref. [131]. Copyright 2018, John Wiley and Sons. d) Schematic illustration of the preparation of carrier-free nanosystems based on packing the DOX and ICG coassembly nanoparticles with CCCMs. Reproduced with permission from Ref. [135]. Copyright 2018, Elsevier.
Although there are relatively few polymers that can be directly used as targeting ligands, other natural or synthetic polymers such as PEG modified with corresponding targeting ligands also have the potential to enhance the tumor-targeting ability of carrier-free drugs (e.g. folic acid conjugated C18PMH-PEG (FA-C18PMH–PEG)). For example, Li and coworkers attempted to conjugate folic acid with PEG as a shell to improve the targeting ability of PTX nanorods [13]. Similar studies were also applied to bis(4-(N-(2-naphthyl) phenylamino) phenyl)-fumaronitrile (NPAPF) nanoparticles [76] and (2-tert-butyl-9,10-di(naphthalen-2-yl)anthracene (TBADN) nanoparticles [64]. Although the uptake efficiency can be improved through targeting polymers, very few polymers with targeting abilities have been found. In the future, nanodrug systems combining more diverse targeting polymers and targeting ligands should be developed to achieve active targeting and high cellular uptake efficiency.
3.2.2. Small molecules-enabled tumor targeting
Small molecules, such as FA, biotin, and lactobionic acid (LA), are the most widely used targeting ligands to improve specific targeting ability through receptor mediated endocytosis due to their small size, high affinity, low immunogenicity, and ease of modification. This strategy shows high avidity and specificity properties, as well as ideally a high rate of endocytosis. Among them, the most widely studied marker overexpressed on tumor cells is folate receptors, which are glycoproteins that are essential for the biosynthesis of nucleotide bases [140]. The most direct targeting folate receptor interaction involves the conjugation of folic acid or other folate conjugates on nanoparticles [141,142]. DOX intensity decreases significantly in HeLa cells (overexpressed with folate/CD44 receptors) in the presence of folic acid (FA) or HA, for folate receptors showed rapid internalization, nonimmunogenicity, and high stability. Moreover, the method of conjugation of folic acid on nanoparticles is very simple. For example, Yang et al. developed pure Bis(4-(N-(2-naphthyl) phenylamino) phenyl)-fumaronitrile (NPAPF) with near-infrared (NIR) dye nanoparticles coated with C18PMH-PEG-FA through stirring. According to the cellular uptake results, more C18PMH-PEG-FA-modified nanoparticles were internalized by the folate-receptor-expressed KB cells compared to C18PMH-PEG-modified nanoparticles. Moreover, weaker fluorescence was observed after the KB cells were preincubated with free FA.
Besides FA, biotin, also known as vitamin B7 or vitamin H, is another promising small targeting molecule candidate. Biotin receptors such as avidin and streptavidin are overexpressed in many malignant cancer cells, such as L1210FR, Ov2008, Colo-26, M109, and 4T1 cells lines [143,144]. Due to the water solubility and terminal carboxyl of biotin, it can combine with hydrophobic therapeutic agents to obtain amphiphilic prodrugs. Luan's group linked biotin and a hydrophobic photosensitizer, IR780, to form amphiphilic IR780 derivatives (B780) [124]. Quercetin (Qu), an anticancer drug and a typical HSP-70 inhibitor, and B780 could form stable nanoparticles (B780/Qu NPs) through π-π stacking and hydrophobic interactions. Due to the excellent targeting ability of biotin, more B780/Qu NPs were internalized by 4T1 cells expressing high levels of biotin receptors compared with that of L929, normal cells expressing low levels of biotin receptors. Interestingly, MTX as an anticancer drug possesses prominent therapeutic efficacy through inhibiting intracellular dihydrofolate reductase activity [145]. Besides the antitumor function, MTX as an FA analog, also has a specific ability to bind with overexpressed folate receptors in various cancer cells [146]. Nanodrugs that integrate MTX can introduce a tumor dual-self-recognition ability into carrier-free nanodrugs, as well as provide a synergistic therapeutic function. For instance, Hou et al. self-assembled MTX with DOX or CPT and in vitro cellular uptake results showed that stronger fluorescence was observed in KB cells (FR positive) than A549 cells (FR negative), which can be attributed to the active targeting ability of MTX [147].
LA with high selectivity for tumor cells such as human hepatocellular carcinoma (HepG2) cells, MCF-7 cells, and A549 cells overexpressed with asialoglycoprotein receptors (ASGPR) was also chosen as a potential targeting ligand [148,149]. Mou et al. created an active targeting nanodrug delivery system by connecting LA, rich in hydroxyl groups, with hydrophobic DOX via a pH-responsive hydrazone group [127]. Self-assembled LA-DOX nanoparticles make use of both passive targeting through the EPR effect and active targeting to greatly improve treatment efficiency. In addition to chemical bonding, LA can also self-assemble with hydrophobic ursolic acid (UA) and ICG into nanoparticles via non-covalent interactions for chemo-phototherapy [128].
Although the small molecule targeting ligand can improve the uptake efficiency, there are only a few types of designed targeting molecules and no specific properties were found in those small molecules. In the future, it is suggested that small molecule targeting ligands can be developed from two ways: finding more tumor related targets on the cell membrane or seeking more specific ligands to achieve precise targeting and tumor cell uptake.
3.2.3. Bioactive substance-prompted tumor targeting
A structurally alternative targeting ligand involves the utilization of proteins [150], peptides [[151], [152], [153]], nucleic acids [154], and antibodies [155]. Current studies for prompting tumor targeting ability through bioactive substances mostly focus on aptamers, peptides, and nucleic acids. The HER2 aptamer, oligonucleotides or short polypeptides, are favorable candidates for biological targeting molecules due to their small molecular size, reproducible synthesis, and low immunity [156]. For example, Jiang et al. developed a HER2 aptamer modified carrier-free nanodrug (Ap/UD) self-assembled by UA and DOX through electrostatic interactions [129]. The confocal laser scanning microscope (CLSM) results showed that the presence of HER2 aptamer could significantly increase the uptake of Ap/UD nanoparticles (Fig. 5b) owing to the active targeting ability [89]. RGD has also been widely used to conjugate with anticancer drugs. Yin's group synthesized an amphiphilic pentamethine indocyanine (ICy5)-CPT-RGD conjugate which can self-assemble into nanodrugs (PTN) with RGD-rich hydrophilic surface and hydrophobic core [131]. Owing to the RGD moiety in ICy5-CPT-RGD, the nanodrugs exhibited remarkable in vitro cellular uptake efficiency and the strong fluorescence observed at the tumor site demonstrated potential for imaging-guided cancer therapy (Fig. 5c). When using RGD-modified RBC coated carrier-free nanodrugs, the modified carrier-free nanodrugs not only show excellent stability, but can also actively target cancer cells and enhance drug accumulation within the cell. Chai et al. fabricated nanocrystals with RBC membrane modified with c(RGDyK) [98]. The biodistribution results showed that the RGD-RBC-NC (RGD-modified RBC membrane-coated nanocrystals) had even higher accumulation in tumors than RBC-NC and NC due to its excellent active-targeting capability. More interestingly, RGD-modified RBC vesicles had higher binding affinity than the c(RGDyK) peptide alone. The high binding affinity of RGD-modified RBC vesicles might be attributed to the membrane fluidity and the high surface density of the peptide on the RBC membrane. In addition to the single targeting ability, when RGD is covalently bounded to other targeting ligands such as folic acid [133], dual targeting can be achieved and the internalization efficiency can be further improved.
Some nucleic acids can also serve as targeting ligands. AS1411 aptamer, containing 26 bases, can specifically target nucleolin [157,158]. Nucleolin plays an important role in cell proliferation activities, and when the cell is in the proliferation state, it will migrate to the cytoplasm and membrane. Due to the intense proliferation of tumor cells, the overexpressed nucleolin on the tumor surface has become an important target for targeted therapy [159,160]. Wang and coworkers designed an amphiphilic prodrug 3′,5′-dioleoyl clofarabine (DOC) and modified it with AS1411 via the molecular recognition between an adenosine (A) analog and thymidine (T) [134]. The results showed that nanoparticles equipped with aptamers can achieve more effective cellular uptake and significantly improved therapeutic efficacy.
3.2.4. Cancer cell membrane-prompted tumor targeting
As previously mentioned, a cancer cell membrane (CCCM) coating on the surface of carrier-free nanodrugs can prolong their blood circulation time. In addition, it can also be used to improve the targeting of nanoparticles. They can actively aggregate to the homologous tumors with the same recognition mechanism. Currently, there have been some studies that have introduced cancer cell membranes into nanodrug delivery systems [[161], [162], [163]], but there are fewer studies on carrier-free nanodrugs. Zhang's group co-assembled DOX and ICG into nanoparticles in water, and then used a simple extrusion method to coat the cracked cancer cell membrane derived from HeLa cells on the surface of the nanoparticles [135] (Fig. 5d). The outcome of in vitro targeting experiments showed that the fluorescence intensity of DOX and ICG from nanodrugs was 3.3–5 fold higher in the HeLa cells than that in other cell lines, like COS7 cells, L929 cells, and HepG2 cells. The nanoparticles exhibited excellent stability and achieved a highly tumor-selective accumulation of nanodrugs due to their unique homologous targeting ability. In addition to cancer cell membranes, there are other biological membranes that have the potential to modify nanodrugs, such as bacterial membranes, platelet membranes, and endoplasmic reticulum membranes. These biomimetic strategies can effectively modify and deliver nanodrugs, avoid immune clearance, improve the targeting ability of nanodrugs, and achieve the suppression of tumors.
3.3. Strategies to accelerate drug release behavior
Upon the optimization of the physicochemical properties and functional modifications of carrier-free nanodrugs for prolonged blood circulation and enhanced cellular uptake, the timely intracellular drug release is paramount for drugs to exert their functions. The drug release mechanism can be broadly categorized into endogenous stimuli or exogenous stimuli. Endogenous stimuli that trigger the drug release includes pH, oxidation, and enzyme degradation. The incorporation of responsive linkers into carrier-free nanodrugs by taking advantage of the characteristics of the tumor microenvironment is a widely used strategy to accelerate drug release. The nanostructure could be broken through the cleavage of bonds or the reduction of assembly forces upon exposure to the tumor microenvironment. Exogenous stimuli that trigger drug release include heat, light, ultrasound, or magnetic fields. They are usually used to weaken drug intermolecular forces or to destroy the nanodrugs’ structure for release purposes [131,135]. In this section, we summarize current approaches to the utilization of intracellular or extracellular stimuli to accelerate drug release behavior.
3.3.1. Drug release-stimulated by intracellular signals
Rapid release of drugs in tumor tissues or cells is one of the key conditions to ensure an effective treatment. As we know, the tumor microenvironment (TME) has a unique tissue structure and metabolic characteristics, such as high reactive oxygen levels, high GSH concentration, low pH, and high expression of specific enzymes. Therefore, using the specific characteristics of the tumor microenvironment to design stimulus-responsive carrier-free nanodrugs is a widely accepted strategy to accelerate drug release, as shown in Table 4. Compared to normal tissues, the TME is weakly acidic (pH = 5.5), which is beneficial for breaking ester bonds, amide bonds, and hydrazone bonds. For instance, Zhang and coworkers linked a hydrophilic anticancer drug, floxuridine (FdU), and the hydrophobic anticancer drug, bendamustine (BdM), through ester bonds to form an amphiphilic drug conjugate to overcome MDR [164]. In vitro studies showed that FdU and BdM drugs can rapidly be released from carrier-free nanodrugs because the ester bond is being cleaved by hydrolysis in the acidic environment.
Table 4.
Summary of carrier-free nanodrugs containing responsive linkers.
| Trigger | Linker | Antitumor drugs | Functions | Refs |
|---|---|---|---|---|
| pH | Carbamate bond | Taxol, Tyroservatide (YSV) | Synergistic chemotherapy and overcome MDR | [174] |
| Carbamate bond | Irinotecan (Ir), DOX | Synergistic chemotherapy and real-time self-tracking | [17] | |
| Ester bond, multivalent pentaerythritol | CPT, Floxuridine (FUDR) | Synergistic chemotherapy | [78] | |
| Ester bond, 4-(dimethylamino) pyridine (DMAP) | CPT, FUDR | Synergistic chemotherapy | [175] | |
| Ester bond | Floxuridine (FdU), Bendamustine (BdM) | Synergistic chemotherapy and overcome MDR | [164] | |
| Ester bond | Ir, Chlorambucil (Cb) | Synergistic chemotherapy | [176] | |
| Ester bond | 5,6-dimethylxanthenone-4-acetic acid(DMXAA), Diethylaminophenyl (DEAP) | Chemo-phototherapy | [177] | |
| Ester bond | Floxuridine (FdU), Bendamustine (BdM) | Synergistic chemotherapy and overcome MDR | [164] | |
| P–O bonds | DOX | Chemotherapy | [178] | |
| GSH | Disulfide bond | CPT, Gemcitabine | Synergistic chemotherapy | [66,179] |
| Disulfide bond | DOX, PTX | Synergistic chemotherapy | [180] | |
| Disulfide bond | MTX, Podophyllotoxin (PPT) | Synergistic chemotherapy and targeting | [170] | |
| Disulfide bond | MTX | Chemotherapy, dual tumor targeting | [123] | |
| Disulfide bond | CPT, Gemcitabine triazole derivatives | Synergistic chemotherapy | [181] | |
| Disulfide bond | CPT, Cytarabine (Ara-C) | Synergistic chemotherapy | [182] | |
| Disulfide bond | CPT, Boron dipyrromethene (BODIPY) | Chemotherapy, Imaging | [183] | |
| Di-selenium bond | Vitamin E succinate, MTX | Chemotherapy | [99] | |
| Enzyme | Ester bond | CPT, DTPA | Chemotherapy, MRI | [184] |
| Ester bond | Ir, Bendamustine (Bd) | Synergistic chemotherapy and overcome MDR | [171] | |
| ROS | single thioether bond | Cabazitaxel (CTX), PPa | Chemo-photodynamic therapy | [172] |
| pH and enzyme | Ester bond | Ir, Vitamin E succinate (VES) | Synergistic chemotherapy | [15] |
| Ester bond | MTX, CPT, DiR | Synergistic chemotherapy, Imaging | [125] | |
| Ester bond | CPT, FdU | Synergistic chemotherapy | [175] | |
| pH and GSH | Hydrazone bond, disulfide bond | MTX, CPT, DOX | Synergistic chemotherapy and targeting; MTX-SS-CPT, MTX-hydrazone-DOX | [147] |
| Carbamate bond, ɑ-ethylenic bond, | Cisaconitic anhydride-modified DOX, PTX | Synergistic chemotherapy and targeting | [120] | |
| P–O bonds | CUR, Ce6 | Chemo-photodynamic therapy, FL and MDR imaging | [173] |
Aside from the lower pH in the endosome/lysosome, a significantly high intracellular concentration of glutathione (GSH) as a reducing agent is often utilized to cleave various reductive-sensitive linkers, such as diselenide bonds [165,166], disulfide bonds [167,168], and succinimide-sulfide bonds [169] to accelerate drug release. Li's group has fabricated GSH-sensitive carrier-free nanodrugs self-assembled by amphiphilic prodrugs composed of MTX and podophyllotoxin (PPT) with a disulfide linker (MTX-SS-PPT). Upon internalization into the tumor cells from FA active targeting, the high intracellular GSH concentration can induce the breakage of disulfide bonds in carrier-free nanodrugs and subsequently accelerate drug release, resulting in improved antitumor efficacy [170].
The TME also contains a plethora of enzymes in the cytoplasm, such as esterase. Zhu et al. used ester bonds to link a hydrophobic anticancer drug, bendamustine (Bd), and hydrophilic irinotecan (Ir) [171] (Fig. 6a). Although the ester bond can be hydrolyzed under acidic conditions, the experimental results showed that only about 30% of the Ir was released after 48 h incubation at pH 5.0. Interestingly, the drug release increased up to 56% after 30 UI mL−1of esterase was added, indicating that the esterase can accelerate drug release (Fig. 6b).
Fig. 6.
a) The formation of Ir-Bd and its mechanism in vivo. b) Drug release curve of Ir-Bd. Reproduced with permission from Ref. [171]. Copyright 2015, Royal Society of Chemistry. c) The preparation process and “bomb-like” drug release of DNPs/N@PDA and enhanced chemo-/photothermal therapy triggered by NIR irradiation. Reproduced with permission from Ref. [187]. Copyright 2018, John Wiley and Sons.
Apart from lower pH, enzymes, and GSH, using intracellular signals such as ROS, to break the structure of carrier-free nanodrugs could also be beneficial for the rapid release of drugs. Sun and coworkers used PPa to self-assemble oxidation-responsive cabazitaxel homodimer (CTX-S-CTX) into nanoparticles (pCTX-S-CTX/PPa NPs) [172]. After pCTX-S-CTX/PPa NPs enter the tumor cells, the endogenous ROS in tumor cells, like H2O2, break the single thioether bond in CTX-S-CTX, resulting in the activation of most dimer prodrugs and disassembly of the pCTX-S-CTX/PPa NPs.
As discussed above, it has been speculated that the combination of two or more stimuli response cues have promising potential to trigger timely drug release. For example, Jing et al. designed multiple-sensitive carrier-free nanodrugs composed of superparamagnetic Fe3O4 nanoclusters coated with polyphosphazene containing disulfide cross-linkers, curcumin, and Ce6 [173]. These carrier-free nanodrugs can achieve pH and GSH dual-sensitive response in TME. In vitro drug release results showed that almost 90% of curcumin and Ce6 were released after incubation at pH 5.5 in the presence of GSH (10 mM), which is much higher than that in the presence of GSH at higher pH or in the absence of GSH. Moreover, Li and coworkers have constructed pH/esterase dual responsive carrier-free nanodrugs that self-assembled by CPT connected with MTX through an ester bond [125]. In vitro drug release results showed that both CPT and MTX drugs can be released in an acidic condition, and the release rate of two drugs is remarkably promoted after adding esterase into the release medium.
Notably, another attractive strategy is focused on weakening the non-covalent interactions of carrier-free nanodrugs when encountering the TME, thereby destroying the nanostructures and fabricating drug release. The most common TME trigger for drug release from carrier-free nanodrugs is the low pH because some hydrophobic drugs, such as DOX, MTX and Epirubicin (EPI) have amino groups that are prone to protonate under low pH and increase their solubility, which can significantly weaken their noncovalent interactions, such as hydrophobic and π–π stacking interactions. For example, Sun's group recently developed carrier-free nanodrugs composed of MTX and pyropheophorbide a (PPa) via hydrophobic interactions, π–π stacking, intermolecular hydrogen bonding, and electrostatic interactions (PPa@MTX) [77]. Under neutral conditions (pH = 7.4), only 4% of the loaded MTX released within 24 h, but about 15% and 23% of the loaded MTX was released from PPa@MTX at pH 6.5 and pH 5.0, respectively. The increased drug release in acidic conditions can be attributed to protonation of the amino groups of MTX molecules leading to weakened interactions between MTX and PPa in the nanodrugs.
3.3.2. Drug release stimulated by extracellular signals
Although promising results have been achieved using endogenous triggers for drug release and the therapeutic efficiency was greatly enhanced, the in vivo release is far less than that of in vitro experiments. The human physiological environment is complex and changeable. Even if drug release based on physiological environment stimulus responses can be regulated according to the distinction of stimulus signal in different environments, it is difficult to have precise spatiotemporal control of the drug release behavior. External stimuli can achieve precise control over the release of the drug at the tumor site due to the ability to artificially control the location and duration of the applied stimuli. The extracellular stimuli can be classified into physical stimuli and chemical stimuli. Compared to physical stimuli that directly destroys the structure of nanoparticles to release drugs, chemical stimuli generate new substances, such as gases, to disintegrate nanoparticles to achieve responsive drug release.
3.3.2.1. Physical stimuli
The most commonly used physical stimuli include light and heat, where the effect of temperature on drug release is most evident in PTT. Under NIR irradiation, the temperature of the carrier-free nanodrugs containing photothermal agents rises rapidly and the thermal vibration increases, which weaken the interactions of carrier-free nanodrugs and destroy the nanostructure to facilitate their drug release [185]. For example, Hou and coworkers developed a self-assembled carrier-free nanodrug through noncovalent interactions containing EPI, as a broad-spectrum chemotherapy drug, and ICG, as a photothermal agent [83]. After NIR irradiation, the drug release rate was almost 1.5 times higher than that of no irradiation, demonstrating that the production of heat from ICG under NIR irradiation disrupted the noncovalent interactions to accelerate EPI release.
In addition to prompting drug release through breaking down the internal forces of carrier-free nanodrugs, the degradation or detachment of the shell is another strategy to facilitate drug release. As discussed above, some carrier-free nanodrugs are not very stable and usually modified with a polymer or cell membrane coating to improve their stability, which might also hinder the smooth release of the drug. For example, Zhang's group designed CCCM-coated nanoparticles self-assembled by DOX and ICG [135]. The temperature could reach 73.3 °C under NIR laser irradiation of 808 nm at a power density of 3 W/cm2 for 6 min. The generated heat leads to the disruption of the membrane [162,186] allowing the DOX release to dramatically accelerate.
3.3.2.2. Chemical stimuli
The utilization of gas generated by chemical stimuli to trigger drug release is another interesting strategy. Recently, NIR light, X-rays, magnetic force, ultrasound, and microwaves are increasingly being explored as exogenous stimuli to stimulate stimuli-responsive gas releasing molecules to release gaseous moieties at the targeted site, due to recent studies on gas therapy or synergistic cancer therapy [[165], [166], [167]]. Generating gas under the exogenous stimulus can not only enhance the therapeutic efficiency (such as generate O2 to enhance the effect of PDT), but can also accelerate the drug release. Li et al. introduced NH4HCO3 into their nanodrugs, which can be thermally triggered to generate carbon dioxide (CO2) and ammonia (NH3) gases [187] (Fig. 6c). The nanodrugs covered with a PDA layer could produce heat under the irradiation of NIR and NH4HCO3 decomposes into CO2 and NH3 gases when the temperature reaches a specific threshold, causing DOX to “bomb-like” release. Currently, there are few studies on this type of method to promote drug release. In the future, ultrasound or microwaves could be suggested to trigger released gas to promote drug release. It is also possible to develop nanodrug systems that generate gas to promote both drug release and therapeutic function.
4. Challenges of carrier-free nanodrugs
4.1. Drug early leakage
Currently, most of the carrier-free nanodrugs are formed through weak interaction forces. However, the nanostructure of carrier-free nanodrugs can be significantly affected by the complex in vivo environment, resulting drug leakage before it reaches the target site. This phenomenon reduces the content of the drug reaching the target site and could cause systemic toxicity to the body. Various strategies are currently being adopted to handle these problems, such as modifying the outer layer of the nanoparticles with polymers, small molecules, or biomolecules to increase its stability under the condition of not hindering the drug loading capacity. Current strategies can limit early release to an extent, but some obstacles still exist. Strategies that utilize stabilizers as a coating are mediated by weak forces, which can still cause instability of the nanoparticles and drug leakage. Strategies that use chemical bonding for stabilization might affect the activity of the drug through these chemical modifications. Therefore, it is extremely urgent to find a suitable strategy to prevent the premature leakage of drugs. Hydrogen bonding is a strong force in non-covalent interactions, and the binding energy of hydrogen bonds is about 20–40 kJ/mol, which is slightly weaker than electrostatic interaction, but stronger than other intermolecular forces. Hydrogen bonds can form between carboxy-pyridine, carboxy-amino, and carboxy-imidazole, which are very common in tumor therapeutics [188]. Double or triple hydrogen bonds can also be formed under certain conditions, as evidenced in DNA, which is an ideal driving force for the construction of stable self-assembly systems. Thus, it is desirable to screen and develop drugs or stabilizers with the potential to form hydrogen bonds in the future.
4.2. Precise control of drug ratio
As previously discussed, the optimization of drug ratio in carrier-free nanodrugs can precisely control their morphology, particle size, and surface charge of nanoparticles, which play a key role in determining the in vivo fate, such as blood circulation time, internalization efficiency, and drug release. Based on the current research, although we feed the materials according to a certain ratio during the preparation process, different drugs with different structures would affect their interaction force during self-assembly, resulting in the actual obtained nanoparticle being quite different from the original feed ratio. To ensure the precise proportion of drugs, the usage of the drug structure itself to enhance intermolecular force through base pairing or host-guest pairing could become the future direction. Since two or more drugs are assembled through strong and specific interaction forces, the drug ratio is difficult to change. The precise drug ratio can also be adjusted according to the number of drug functional groups and the material ratio. This strategy makes it possible to achieve 100% drug loading due to no additional adjuvants added.
4.3. Difficulties in industrialization
Despite the research of carrier-free nanodrugs still being in its infancy, there have been a large number of related studies in recent years. It is unfortunate that very few carrier-free nanodrugs have truly achieved clinical transformation and industrialization. The industrialization of carrier-free nanodrug mainly faces the following problems: Firstly, in industrial large-scale production, it is difficult to ensure that physicochemical properties of nanoparticles are consistent with laboratory preparation due to the high drug concentration. Secondly, since there is no carrier as a stabilizer, most carrier-free nanodrugs are prone to coagulation during long-term storage and sometimes lose their therapeutic effect. Finally, if the carrier-free nanodrug is powered by lyophilization for long-term storage, its redispersibility and efficacy need to be carefully evaluated. With these limitations for industrialization, the template preparation method introduced above has the potential for large-scale industrial production, but this method is currently only used for the preparation of single drugs, and further research is required for the preparation of dual and multi drugs.
5. Conclusion and future perspectives
In this review, we illustrated advances to carrier-free nanodrugs by discussing strategies related to drug proportion, preparation method, and intermolecular interactions to optimize their physicochemical properties for improving their delivery efficiency. Although great progress has been achieved, the native obstacles that carrier-free nanodrugs face during in vivo delivery after administration result in unsatisfactory delivery efficiency. Subsequently, several key drawbacks facing carrier-free nanodrugs have been discussed and the related strategies to overcome those problems are presented, including prolonged blood circulation time, enhanced internalization efficiency, and augmented intracellular drug release. Carrier-free nanodrugs have made substantial improvements in cancer treatment and shown the potential for further development and rational design. In the future, more effort should be concentrated on the following aspects to significantly improve their antitumor efficacy.
Firstly, most of the carrier-free nanodrugs have difficulty achieving size or surface charge changeability, which would not satisfy both excellent retention and penetration capabilities. Generally, within a certain particle size range, the smaller the particle size of the carrier-free nanodrugs, the more favorable they are for deep penetration into the tumor. However, the retention capacity of carrier-free nanodrugs at the tumor site is positively correlated with the particle size, indicating that carrier-free nanodrugs with large particle sizes can more effectively stay at the tumor site. Therefore, it should be an important research focus to design carrier-free nanodrugs whose particle size changes along with the environment.
Secondly, although carrier-free nanodrugs provide a good platform for multimodal therapy, the current studies are mainly focused on the combination of chemotherapy and PDT/PTT, however, these treatments have difficulty overcoming tumor metastasis and recurrence. Immunotherapy is one of the most promising therapies in cancer treatment, which not only inhibits the growth of tumors, but also stimulates the body's immune system to inhibit tumor metastasis and recurrence. Unfortunately, the current research efforts on carrier-free nanodrugs for immunotherapy mainly focuses on combining immunosuppressive drugs to achieve the effect of combined therapy, but the low therapeutic response (~20%) of using anti-PD-L1 or anti-PD-1 limits its clinical applications. Directly assembling the immunosuppressive agents with therapeutic agents such as chemotherapy drugs into carrier-free nanodrugs to be simultaneously delivered to the tumor site is a promising strategy. Currently, many hydrophobic small molecule immunosuppressants, such as Indoximod, have been combined with other therapies to achieve a synergistic effect [189,190]. Indoximod, a methylated tryptophan, acts as an IDO (indolamine- (2,3) -dioxygenase) pathway inhibitor and can reverse IDO-mediated immunosuppression. Indoximod contains rigid plane structures such as benzene rings and aromatic heterocycles and has the potential to self-assemble with chemotherapy drugs through non-covalent forces, such as hydrophobic interactions and π-π stacks. It also has amino and carboxyl groups on its side chains, which have the potential to form pH-sensitive bonds with other therapeutic agents to form amphiphilic molecules. These ideas provide a promising foundation to construct carrier-free nanodrugs for combined immunotherapy, which not only improves the therapeutic effect, but also inhibits tumor metastasis and recurrence.
Additionally, tumor metabolic dysregulation is an emerging sign of cancer. It can also become a new method of cancer treatment by adjusting the metabolism of tumor cells by interfering with the metabolic pathways of tumor cells, including energy expenditure, reduced antioxidant capacity, and dysfunctional regulation. Before chemotherapy or phototherapy, weakening the protective mechanism of tumor cell adaptation and survival makes the tumor cells more likely to be destroyed. Intervention tumor cell metabolism can use specific drugs, such as arginine imine enzyme, to transform arginine to citrulline and run out of ornithine to arginine in the serum, cutting the tumor needs a source of arginine) [191], 5 - (furan - 2 - formamide) - 3 - methyl - 4 - thiophene thiocyanate - 2 - carboxylic acid ethyl ester (CBR - 5884, regulate metabolism by inhibiting serine from to inhibit tumor growth) and so on [192]. Additionally, special structures such as sulfide linkers oxidizing GSH to break the redox balance of tumor cells can be designed to adjust the metabolism of tumor cells. It can be a new direction of future development to design carrier-free nanodrugs composed of metabolism-interfering drugs and other therapeutic drugs or modifying the drug structure for metabolism interference without affecting the activity of the drugs.
Collectively, carrier-free nanodrug development is still in its infancy and provide a broader view of drug delivery system, which holds great potential for superior clinical outcomes. Although there is still a long way to go before clinical conversion, we still firmly believe that the carrier-free nanodrugs will develop rapidly in the future and become a new and widely used method for cancer treatment.
Author statement
Heng Mei, Huile Gao and Jun Cao presented the outline of this review. Heng Mei, Shengsheng Cai and Dennis Huang collected literature and wrote the review. Huile Gao, Jun Cao and Bin He guided manuscript modification.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by grants from the National Key Research and Development Program of China (No. 2018YFC1106103) and the National Natural Science Foundation of China (Grant No. 51973135).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Huile Gao, Email: gaohuile@scu.edu.cn.
Jun Cao, Email: caojun@scu.edu.cn.
References
- 1.Shi J.J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Canc. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Contr. Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y., He Y., Su T., Li C., Cai S., Wu Z., Huang D., Zhang X., Cao J., He B. Exogenous vitamin c triggered structural changes of redox-activated dual core-crosslinked biodegradable nanogels for boosting the antitumor efficiency. J. Mater. Chem. B. 2020;8:5109–5116. doi: 10.1039/d0tb00356e. [DOI] [PubMed] [Google Scholar]
- 4.Cao J., Huang D., Peppas N.A. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv. Drug Deliv. Rev. 2020;167:170–188. doi: 10.1016/j.addr.2020.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Cao J., Xie X., Lu A., He B., Chen Y., Gu Z., Luo X. Cellular internalization of doxorubicin loaded star-shaped micelles with hydrophilic zwitterionic sulfobetaine segments. Biomaterials. 2014;35:4517–4524. doi: 10.1016/j.biomaterials.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 6.He Y., Lei L., Cao J., Yang X., Cai S., Tong F., Huang D., Mei H., Luo K., Gao H., He B., Peppas N.A. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci. Adv. 2021;7 doi: 10.1126/sciadv.aba0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y., Jin E., Zhang B., Murphy C.J., Sui M., Zhao J., Wang J., Tang J., Fan M., Van Kirk E., Murdoch W.J. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J. Am. Chem. Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J.F., Li S.L., An F.F., Liu J., Jin S.B., Zhang J.C., Wang P.C., Zhang X.H., Lee C.S., Liang X.J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale. 2015;7:13503–13510. doi: 10.1039/c5nr03259h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun M., Zhang Y., He Y., Xiong M., Huang H., Pei S., Liao J., Wang Y., Shao D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf., B. 2019;180:313–318. doi: 10.1016/j.colsurfb.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Zhang X.J., Zhou M.J., Tian B.S., Yu C.T., Jie J.S., Hao X.J., Zhang X.H. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv. Healthcare Mater. 2014;3:1475–1485. doi: 10.1002/adhm.201300577. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.A., Yang Y.L., An F.F., Liu Z., Zhang X.J., Zhang X.H. Carrier-free, functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/1/015103. 015103. [DOI] [PubMed] [Google Scholar]
- 14.Zuo W.B., Chen D.Y., Fan Z.X., Chen L.P., Zhu Z.Y., Zhu Q.X., Zhu X. Design of light/ros cascade-responsive tumor-recognizing nanotheranostics for spatiotemporally controlled drug release in locoregional photo-chemotherapy. Acta Biomater. 2020;111:327–340. doi: 10.1016/j.actbio.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 15.Ling L.B., Ismail M., Shang Z.H., Hu Y.H., Li B.H. Vitamin e-based prodrug self-delivery for nanoformulated irinotecan with synergistic antitumor therapeutics. Int. J. Pharm. 2020;577:119049. doi: 10.1016/j.ijpharm.2020.119049. [DOI] [PubMed] [Google Scholar]
- 16.Cheng C., Sui B., Wang M., Hu X., Shi S., Xu P. Carrier-free nanoassembly of curcumin-erlotinib conjugate for cancer targeted therapy. Adv. Healthcare Mater. 2020;9:2001128. doi: 10.1002/adhm.202001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S., Deng H., Huang P., Sun P., Huang X., Su Y., Zhu X., Shen J., Yan D. Real-time self-tracking of an anticancer small molecule nanodrug based on colorful fluorescence variations. RSC Adv. 2016;6:12472–12478. doi: 10.1039/c5ra24273h. [DOI] [Google Scholar]
- 18.Xiao Y., Liu J., Guo M., Zhou H., Jin J., Liu J., Liu Y., Zhang Z., Chen C. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale. 2018;10:12639–12649. doi: 10.1039/c8nr02700e. [DOI] [PubMed] [Google Scholar]
- 19.Liu P., Xie X., Shi X., Peng Y., Ding J., Zhou W. Oxygen-self-supplying and hif-1 alpha-inhibiting core-shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:48261–48270. doi: 10.1021/acsami.9b18112. [DOI] [PubMed] [Google Scholar]
- 20.Feng B., Niu Z., Hou B., Zhou L., Li Y., Yu H. Enhancing triple negative breast cancer immunotherapy by icg-templated self-assembly of paclitaxel nanoparticles. Adv. Funct. Mater. 2020;30:1906605. doi: 10.1002/adfm.201906605. [DOI] [Google Scholar]
- 21.Zhang J.F., Liang Y.C., Lin X.D., Zhu X.Y., Yan L., Li S.L., Yang X., Zhu G.Y., Rogach A.L., Yu P.K.N., Shi P., Tu L.C., Chang C.C., Zhang X.H., Chen X.F., Zhang W.J., Lee C.S. Self-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in vitro and in vivo. ACS Nano. 2015;9:9741–9756. doi: 10.1021/acsnano.5b02513. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y., Jiang K., Shen Z., Zheng G., Fan L., Zhao R., Shao J. A small molecule nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-infrared cancer theranostics. ACS Appl. Mater. Interfaces. 2017;9:43508–43519. doi: 10.1021/acsami.7b14755. [DOI] [PubMed] [Google Scholar]
- 23.Kakran M., Shegokar R., Sahoo N.G., Gohla S., Li L., Mueller R.H. Long-term stability of quercetin nanocrystals prepared by different methods. J. Pharm. Pharmacol. 2012;64:1394–1402. doi: 10.1111/j.2042-7158.2012.01515.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X.Q., Cai S.S., He Y.M., Zhang M., Cao J., Mei H., Li S., He B. Enzyme-triggered deshielding of nanoparticles and positive-charge mediated lysosomal escape for chemo/photo-combination therapy. J. Mater. Chem. B. 2019;7:4758–4762. doi: 10.1039/c9tb00685k. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M., Zhang X., Cai S., Mei H., He Y., Huang D., Shi W., Li S., Cao J., He B. Photo-induced specific intracellular release egfr inhibitor from enzyme/ros-dual sensitive nano-platforms for molecular targeted-photodynamic combinational therapy of non-small cell lung cancer. J. Mater. Chem. B. 2020;8:7931–7940. doi: 10.1039/d0tb01053g. [DOI] [PubMed] [Google Scholar]
- 26.Colombo M., Staufenbiel S., Ruehl E., Bodmeier R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int. J. Pharm. 2017;521:156–166. doi: 10.1016/j.ijpharm.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Leroux J.C., Allemann E., Doelker E., Gurny R. New approach for the preparation of nanoparticles by an emulsification-diffusion method. Eur. J. Pharm. Biopharm. 1995;41:14–18. Go to ISI, //WOS:A1995QK26800003. [Google Scholar]
- 28.Cheng J.J., Zhao H.T., Wang J.C., Han Y., Yang X. Bioactive natural small molecule-tuned coassembly of photosensitive drugs for highly efficient synergistic and enhanced type i photochemotherapy. ACS Appl. Mater. Interfaces. 2020;12:43488–43500. doi: 10.1021/acsami.0c13164. [DOI] [PubMed] [Google Scholar]
- 29.Odonnell P.B., McGinity J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Li Y., An F.-F., Zhang X., Chen X., Lee C.-S. Preparation and size control of sub-100 nm pure nanodrugs. Nano Lett. 2015;15:313–318. doi: 10.1021/nl503598u. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Nie W., Chen R., Chelora J., Wan Y., Cui X., Zhang X., Zhang W., Chen X., Xie H.-Y., Lee C.-S. Green mass production of pure nanodrugs via an ice-template-assisted strategy. Nano Lett. 2019;19:658–665. doi: 10.1021/acs.nanolett.8b03043. [DOI] [PubMed] [Google Scholar]
- 32.Fournier E., Dufresne M.H., Smith D.C., Ranger M., Leroux J.C. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm. Res. (N. Y.) 2004;21:962–968. doi: 10.1023/b:Pham.0000029284.40637.69. [DOI] [PubMed] [Google Scholar]
- 33.Chen S., Fan J.X., Liu X.H., Zhang M.K., Liu F., Zeng X., Yan G.P., Zhang X.Z. A self-delivery system based on an amphiphilic proapoptotic peptide for tumor targeting therapy. J. Mater. Chem. B. 2019;7:778–785. doi: 10.1039/c8tb02945h. [DOI] [PubMed] [Google Scholar]
- 34.Li X.M., Ding L.Y., Xu Y.L., Wang Y.L., Ping Q.N. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int. J. Pharm. 2009;373:116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Yang P., Zhao X., Gao D., Sun N., Tian Z., Ma T., Yang Z. Multifunctional cargo-free nanomedicine for cancer therapy. Int. J. Mol. Sci. 2018;19:2963. doi: 10.3390/ijms19102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matei D., Schilder J., Sutton G., Perkins S., Breen T., Quon C., Sidor C. Activity of 2 methoxyestradiol (panzem (r) ncd) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a hoosier oncology group trial. Gynecol. Oncol. 2009;115:90–96. doi: 10.1016/j.ygyno.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Lin J., Huang Y., Li Y., Yang X., Wu H., Wu S., Xie L., Dai L., Hou Z. Self-targeted, shape-assisted, and controlled-release self-delivery nanodrug for synergistic targeting/anticancer effect of cytoplasm and nucleus of cancer cells. ACS Appl. Mater. Interfaces. 2015;7:25553–25559. doi: 10.1021/acsami.5b07348. [DOI] [PubMed] [Google Scholar]
- 38.Truong N.P., Whittaker M.R., Mak C.W., Davis T.P. The importance of nanoparticle shape in cancer drug delivery. Expet Opin. Drug Deliv. 2015;12:129–142. doi: 10.1517/17425247.2014.950564. [DOI] [PubMed] [Google Scholar]
- 39.Yang M.-Y., Zhao R.-R., Fang Y.-F., Jiang J.-L., Yuan X.-T., Shao J.-W. Carrier-free nanodrug: a novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019;570:118663. doi: 10.1016/j.ijpharm.2019.118663. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Li N., Zhang S., Sun B., Chen Q., He Z., Luo C., Sun J. Emerging carrier-free nanosystems based on molecular self-assembly of pure drugs for cancer therapy. Med. Res. Rev. 2020;40:1754–1775. doi: 10.1002/med.21669. [DOI] [PubMed] [Google Scholar]
- 41.Huang L., Zhao S., Fang F., Xu T., Lan M., Zhang J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials. 2021:268. doi: 10.1016/j.biomaterials.2020.120557. [DOI] [PubMed] [Google Scholar]
- 42.Gigliobianco M.R., Casadidio C., Censi R., Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10:134. doi: 10.3390/pharmaceutics10030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junyaprasert V.B., Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015;10:13–23. doi: 10.1016/j.ajps.2014.08.005. [DOI] [Google Scholar]
- 44.Merisko-Liversidge E., Liversidge G.G., Cooper E.R. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur. J. Pharmaceut. Sci. 2003;18:113–120. doi: 10.1016/s0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 45.Karaosmanoglu S., Zhou M.J., Shi B.Y., Zhang X.J., Williams G.R., Chen X.F. Carrier-free nanodrugs for safe and effective cancer treatment. J. Contr. Release. 2021;329:805–832. doi: 10.1016/j.jconrel.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Cong Y., Ji L., Gao Y.-J., Liu F.-H., Cheng D.-B., Hu Z., Qiao Z.-Y., Wang H. Microenvironment-induced in situ self-assembly of polymer-peptide conjugates that attack solid tumors deeply. Angew Chem. Int. Ed. Engl. 2019;58:4632–4637. doi: 10.1002/anie.201900135. [DOI] [PubMed] [Google Scholar]
- 47.Hu X.-X., He P.-P., Qi G.-B., Gao Y.-J., Lin Y.-X., Yang C., Yang P.-P., Hao H., Wang L., Wang H. Transformable nanomaterials as an artificial extracellular matrix for inhibiting tumor invasion and metastasis. ACS Nano. 2017;11:4086–4096. doi: 10.1021/acsnano.7b00781. [DOI] [PubMed] [Google Scholar]
- 48.Jo D.H., Kim J.H., Lee T.G., Kim J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015;11:1603–1611. doi: 10.1016/j.nano.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Yu W., Shevtsov M., Chen X., Gao H. Advances in aggregatable nanoparticles for tumor-targeted drug delivery. Chin. Chem. Lett. 2020;31:1366–1374. doi: 10.1016/j.cclet.2020.02.036. [DOI] [Google Scholar]
- 50.Murray J.S., Politzer P. The electrostatic potential: an overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011;1:153–163. doi: 10.1002/wcms.19. [DOI] [Google Scholar]
- 51.Xiao Y., An F.-F., Chen J., Xiong S., Zhang X.-H. The impact of light irradiation timing on the efficacy of nanoformula-based photo/chemo combination therapy. J. Mater. Chem. B. 2018;6:3692–3702. doi: 10.1039/c8tb00427g. [DOI] [PubMed] [Google Scholar]
- 52.Zhang R., Xing R., Jiao T., Ma K., Chen C., Ma G., Yan X. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces. 2016;8:13262–13269. doi: 10.1021/acsami.6b02416. [DOI] [PubMed] [Google Scholar]
- 53.Lin J., Li C., Guo Y., Zou J., Wu P., Liao Y., Zhang B., Le J., Zhao R., Shao J.-W. Carrier-free nanodrugs for in vivo nir bioimaging and chemo-photothermal synergistic therapy. J. Mater. Chem. B. 2019;7:6914–6923. doi: 10.1039/c9tb00687g. [DOI] [PubMed] [Google Scholar]
- 54.Tagawa N., Masuhara A., Kasai H., Nakanishi H., Oikawa H. Monodispersed and size-controlled diarylethene nanoparticles fabricated by the reprecipitation method. Mol. Cryst. Liq. Cryst. 2010;520:521–526. doi: 10.1080/15421400903584549. [DOI] [Google Scholar]
- 55.Patravale V.B., Date A.A., Kulkarni R.M. Nanosuspensions: a promising drug delivery strategy. J. Pharm. Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 56.Zhu X.J., Vo C., Taylor M., Smith B.R. Non-spherical micro- and nanoparticles in nanomedicine. Mater. Horiz. 2019;6:1094–1121. doi: 10.1039/c8mh01527a. [DOI] [Google Scholar]
- 57.Yang Y.W., Nie D., Liu Y., Yu M.R., Gan Y. Advances in particle shape engineering for improved drug delivery. Drug Discov. Today. 2019;24:575–583. doi: 10.1016/j.drudis.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Chauhan V.P., Popovic Z., Chen O., Cui J., Fukumura D., Bawendi M.G., Jain R.K. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem. Int. Ed. Engl. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Champion J.A., Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Huang L., Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol. Pharm. 2010;7:863–869. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollis C.P., Weiss H.L., Leggas M., Evers B.M., Gemeinhart R.A., Li T.L. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the epr effect and image-guided drug delivery. J. Contr. Release. 2013;172:12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H., Hollis C.P., Zhang Q., Li T.L. Preparation and antitumor study of camptothecin nanocrystals. Int. J. Pharm. 2011;415:293–300. doi: 10.1016/j.ijpharm.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 63.An F.F., Li Y.A., Zhang J.F. Carrier-free photosensitizer nanocrystal for photodynamic therapy. Mater. Lett. 2014;122:323–326. doi: 10.1016/j.matlet.2014.02.067. [DOI] [Google Scholar]
- 64.Diao X.J., Li W., Yu J., Wang X.J., Zhang X.J., Yang Y.L., An F.F., Liu Z., Zhang X.H. Carrier-free, water dispersible and highly luminescent dye nanoparticles for targeted cell imaging. Nanoscale. 2012;4:5373–5377. doi: 10.1039/c2nr31153d. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Chen F., Pan Y., Li Z., Xue X., Okeke C.I., Wang Y., Li C., Peng L., Wang P.C., Ma X., Liang X.-J. Nanodrug formed by coassembly of dual anticancer drugs to inhibit cancer cell drug resistance. ACS Appl. Mater. Interfaces. 2015;7:19295–19305. doi: 10.1021/acsami.5b05347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W., Wen Y., He D.-X., Wang Y.-F., Liu X.-L., Li C., Liang X.-J. Near-infrared aiegens as transformers to enhance tumor treatment efficacy with controllable self-assembled redox-responsive carrier-free nanodrug. Biomaterials. 2019;193:12–21. doi: 10.1016/j.biomaterials.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C., Long L., Xiong Y., Wang C., Peng C., Yuan Y., Liu Z., Lin Y., Jia Y., Zhou X., Li X. Facile engineering of indomethacin-induced paclitaxel nanocrystal aggregates as carrier-free nanomedicine with improved synergetic antitumor activity. ACS Appl. Mater. Interfaces. 2019;11:9872–9883. doi: 10.1021/acsami.8b22336. [DOI] [PubMed] [Google Scholar]
- 68.Zhong T., Yao X., Zhang S., Guo Y., Duan X.-C., Ren W., Huang D., Yin Y.-F., Zhang X. A self-assembling nanomedicine of conjugated linoleic acid-paclitaxel conjugate (cla-ptx) with higher drug loading and carrier-free characteristic. Sci. Rep. 2016;6:36614. doi: 10.1038/srep36614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., Liu D., Zheng Q., Zhao Q., Zhang H., Ma Y., Fallon J.K., Fu Q., Haynes M.T., Lin G., Zhang R., Wang D., Yang X., Zhao L., He Z., Liu F. Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett. 2014;14:5577–5583. doi: 10.1021/nl502044x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y.Q., Wang X., Deng F.Y., Zheng N., Liang Y.Q., Zhang H., He B., Dai W.B., Wang X.Q., Zhang Q. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J. Contr. Release. 2018;279:136–146. doi: 10.1016/j.jconrel.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 71.Soudy R., Chen C., Kaur K. Novel peptide-doxorubucin conjugates for targeting breast cancer cells including the multidrug resistant cells. J. Med. Chem. 2013;56:7564–7573. doi: 10.1021/jm400647r. [DOI] [PubMed] [Google Scholar]
- 72.Liu F., Kozlovskaya V., Zavgorodnya O., Martinez-Lopez C., Catledge S., Kharlampieva E. Encapsulation of anticancer drug by hydrogen-bonded multilayers of tannic acid. Soft Matter. 2014;10:9237–9247. doi: 10.1039/c4sm01813c. [DOI] [PubMed] [Google Scholar]
- 73.Xiong H., Wang Z., Wang C., Yao J. Transforming complexity to simplicity: protein-like nanotransformer for improving tumor drug delivery programmatically. Nano Lett. 2020;20:1781–1790. doi: 10.1021/acs.nanolett.9b05008. [DOI] [PubMed] [Google Scholar]
- 74.Zhou M.J., Zhang X.J., Yang Y.L., Liu Z., Tian B.S., Jie J.S., Zhang X.H. Carrier-free functionalized multidrug nanorods for synergistic cancer therapy. Biomaterials. 2013;34:8960–8967. doi: 10.1016/j.biomaterials.2013.07.080. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y.L., Zhang X.J., Yu C.T., Hao X.J., Jie J.S., Zhou M.J., Zhang X.H. Smart nanorods for highly effective cancer theranostic applications. Adv. Healthcare Mater. 2014;3:906–915. doi: 10.1002/adhm.201300463. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y.L., An F.F., Liu Z., Zhang X.J., Zhou M.J., Li W., Hao X.J., Lee C.S., Zhang X.H. Ultrabright and ultrastable near-infrared dye nanoparticles for in vitro and in vivo bioimaging. Biomaterials. 2012;33:7803–7809. doi: 10.1016/j.biomaterials.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z., Sun M., Liu T., Tan X., Zhang H., Zhang X., He Z., Sun J. A surfactant-like chemotherapeutic agent as a nanocarrier for delivering photosensitizers against cancer: a facile drug-delivering-drug strategy. Int. J. Pharm. 2019;562:313–320. doi: 10.1016/j.ijpharm.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 78.Liang X., Gao C., Cui L., Wang S., Wang J., Dai Z. Self-assembly of an amphiphilic janus camptothecin-floxuridine conjugate into liposome-like nanocapsules for more efficacious combination chemotherapy in cancer. Adv. Mater. 2017;29:1703135. doi: 10.1002/adma.201703135. [DOI] [PubMed] [Google Scholar]
- 79.Wang D.D., Wang T., Zhao G.C., Zhuang J., Wu W. Improving systemic circulation of paclitaxel nanocrystals by surface hybridization of dspe-peg2000. Colloids Surf., B. 2019;182:110337. doi: 10.1016/j.colsurfb.2019.06.066. [DOI] [PubMed] [Google Scholar]
- 80.Luo C., Sun B., Wang C., Zhang X., Chen Y., Chen Q., Yu H., Zhao H., Sun M., Li Z., Zhang H., Kan Q., Wang Y., He Z., Sun J. Self-facilitated ros-responsive nanoassembly of heterotypic dimer for synergistic chemo-photodynamic therapy. J. Contr. Release. 2019;302:79–89. doi: 10.1016/j.jconrel.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X.B., Sun B.J., Zuo S.Y., Chen Q., Gao Y.L., Zhao H.Q., Sun M.C., Chen P.Y., Yu H., Zhang W.J., Wang K.Y., Zhang R.S., Kan A.M., Zhang H.T., He Z.G., Luo C., Sun J. Self-assembly of a pure photosensitizer as a versatile theragnostic nanoplatform for imaging-guided antitumor photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10:30155–30162. doi: 10.1021/acsami.8b10421. [DOI] [PubMed] [Google Scholar]
- 82.Mallick A., More P., Ghosh S., Chippalkatti R., Chopade B.A., Lahiri M., Basu S. Dual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cells. ACS Appl. Mater. Interfaces. 2015;7:7584–7598. doi: 10.1021/am5090226. [DOI] [PubMed] [Google Scholar]
- 83.Li Y., Liu G., Ma J., Lin J., Lin H., Su G., Chen D., Ye S., Chen X., Zhu X., Hou Z. Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. J. Contr. Release. 2017;258:95–107. doi: 10.1016/j.jconrel.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Guo F.Q., Shang J.J., Zhao H., Lai K.R., Li Y., Fan Z.X., Hou Z.Q., Su G.H. Cube-shaped theranostic paclitaxel prodrug nanocrystals with surface functionalization of spc and mpeg-dspe for imaging and chemotherapy. Colloids Surf., B. 2017;160:649–660. doi: 10.1016/j.colsurfb.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Wang J., Lv F.M., Wang D.L., Du J.L., Guo H.Y., Chen H.N., Zhao S.J., Liu Z.P., Liu Y. Synergistic antitumor effects on drug-resistant breast cancer of paclitaxel/lapatinib composite nanocrystals. Molecules. 2020;25:604. doi: 10.3390/molecules25030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Z., Piao Y., Hao L., Wang G., Zhou Z., Shen Y. Acidity-responsive shell-sheddable camptothecin-based nanofibers for carrier-free cancer drug delivery. Nanoscale. 2019;11:15907–15916. doi: 10.1039/c9nr03872h. [DOI] [PubMed] [Google Scholar]
- 87.Ren Z.G., Sun S.C., Sun R.R., Cui G.Y., Hong L.J., Rao B.C., Li A., Yu Z.J., Kan Q.C., Mao Z.W. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv. Mater. 2020;32:1906024. doi: 10.1002/adma.201906024. [DOI] [PubMed] [Google Scholar]
- 88.Chung J.E., Tan S., Gao S.J., Yongvongsoontorn N., Kim S.H., Lee J.H., Choi H.S., Yano H., Zhuo L., Kurisawa M., Ying J.Y. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang Z.G., Lv F.M., Wang J., Ca S.J., Liu Z.P., Liu Y., Lu W.Y. Rgd-modified pegylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int. J. Pharm. 2019;556:217–225. doi: 10.1016/j.ijpharm.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 90.Li W., Yang Y.L., Wang C., Liu Z., Zhang X.J., An F.F., Diao X.J., Hao X.J., Zhang X.H. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem. Commun. 2012;48:8120–8122. doi: 10.1039/c2cc33214k. [DOI] [PubMed] [Google Scholar]
- 91.Wu S., Yang X., Lu Y., Fan Z., Li Y., Jiang Y., Hou Z. A green approach to dual-drug nanoformulations with targeting and synergistic effects for cancer therapy. Drug Deliv. 2017;24:51–60. doi: 10.1080/10717544.2016.1228716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Wu Y., Li K., Shen S., Liu Z., Wu D. Ultralong circulating lollipop-like nanoparticles assembled with gossypol, doxorubicin, and polydopamine via pi-pi stacking for synergistic tumor therapy. Adv. Funct. Mater. 2019;29:1805582. doi: 10.1002/adfm.201805582. [DOI] [Google Scholar]
- 93.Liu Y., Li K., Wu Y., Ma J., Tang P., Liu Y., Wu D. Pva reinforced gossypolone and doxorubicin pi-pi stacking nanoparticles towards tumor targeting and ultralow dose synergistic chemotherapy. Biomater. Sci. 2019;7:3662–3674. doi: 10.1039/c9bm00674e. [DOI] [PubMed] [Google Scholar]
- 94.Shen G., Xing R., Zhang N., Chen C., Ma G., Yan X. Interfacial cohesion and assembly of bioadhesive molecules for design of long term stable hydrophobic nanodrugs toward effective anticancer therapy. ACS Nano. 2016;10:5720–5729. doi: 10.1021/acsnano.5b07276. [DOI] [PubMed] [Google Scholar]
- 95.Zhang F., Zhu G., Jacobson O., Liu Y., Chen K., Yu G., Ni Q., Fan J., Yang Z., Xu F., Fu X., Wang Z., Ma Y., Niu G., Zhao X., Chen X. Transformative nanomedicine of an amphiphilic camptothecin prodrug for long circulation and high tumor uptake in cancer therapy. ACS Nano. 2017;11:8838–8848. doi: 10.1021/acsnano.7b03003. [DOI] [PubMed] [Google Scholar]
- 96.Ren H., Liu J., Li Y., Wang H., Ge S., Yuan A., Hu Y., Wu J. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy. Acta Biomater. 2017;59:269–282. doi: 10.1016/j.actbio.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 97.Ye S., Wang F., Fan Z., Zhu Q., Tian H., Zhang Y., Jiang B., Hou Z., Li Y., Su G. Light/ph-triggered biomimetic red blood cell membranes camouflaged small molecular drug assemblies for imaging-guided combinational chemo-photothermal therapy. ACS Appl. Mater. Interfaces. 2019;11:15262–15275. doi: 10.1021/acsami.9b00897. [DOI] [PubMed] [Google Scholar]
- 98.Chai Z., Ran D., Lu L., Zhan C., Ruan H., Hu X., Xie C., Jiang K., Li J., Zhou J., Wang J., Zhang Y., Fang R.H., Zhang L., Lu W. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13:5591–5601. doi: 10.1021/acsnano.9b00661. [DOI] [PubMed] [Google Scholar]
- 99.Li Y., Lin J.Y., Wang P.Y., Luo Q., Zhu F.K., Zhang Y., Hou Z.Q., Liu X.L., Liu J.F. Tumor microenvironment cascade-responsive nanodrug with self-targeting activation and ros regeneration for synergistic oxidation-chemotherapy. Nano-Micro Lett. 2020;12:182. doi: 10.1007/s40820-020-00492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walkey C.D., Olsen J.B., Guo H., Emili A., Chan W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 101.Li Y., Liu R., Yang J., Shi Y., Ma G., Zhang Z., Zhang X. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials. 2015;41:1–14. doi: 10.1016/j.biomaterials.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Hatakeyama H., Akita H., Harashima H. The polyethyleneglycol dilemma: advantage and disadvantage of pegylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013;36:892–899. doi: 10.1248/bpb.b13-00059. [DOI] [PubMed] [Google Scholar]
- 103.Hatakeyama H., Ito E., Akita H., Oishi M., Nagasaki Y., Futaki S., Harashima H. A ph-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of sirna-containing nanoparticles in vitro and in vivo. J. Contr. Release. 2009;139:127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Remaut K., Lucas B., Braeckmans K., Demeester J., De Smedt S.C. Pegylation of liposomes favours the endosornal degradation of the delivered phosphodiester oligonucleotides. J. Contr. Release. 2007;117:256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 105.Xue K.H., Tian H.N., Zhu F.K., Wang F.F., Fan Z.X., Zhao Q.L., Hou Z.Q., Li Y. Ultralong-circulating and self-targeting "watson-crick a = t"-inspired supramolecular nanotheranostics for nir-ii imaging-guided photochemotherapy. ACS Appl. Mater. Interfaces. 2020;12:32477–32492. doi: 10.1021/acsami.0c09090. [DOI] [PubMed] [Google Scholar]
- 106.Zhang P., Wang J., Chen H., Zhao L., Chen B., Chu C., Liu H., Qin Z., Liu J., Tan Y., Chen X., Liu G. Tumor microenvironment-responsive ultrasmall nanodrug generators with enhanced tumor delivery and penetration. J. Am. Chem. Soc. 2018;140:14980–14989. doi: 10.1021/jacs.8b09396. [DOI] [PubMed] [Google Scholar]
- 107.Wan G., Chen B., Li L., Wang D., Shi S., Zhang T., Wang Y., Zhang L., Wang Y. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials. 2018;155:25–40. doi: 10.1016/j.biomaterials.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Wu Z., Li T., Gao W., Xu T., Jurado-Sanchez B., Li J., Gao W., He Q., Zhang L., Wang J. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv. Funct. Mater. 2015;25:3881–3887. doi: 10.1002/adfm.201501050. [DOI] [Google Scholar]
- 109.Rao L., Bu L.-L., Xu J.-H., Cai B., Yu G.-T., Yu X., He Z., Huang Q., Li A., Guo S.-S., Zhang W.-F., Liu W., Sun Z.-J., Wang H., Wang T.-H., Zhao X.-Z. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11:6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 110.Luk B.T., Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Contr. Release. 2015;220:600–607. doi: 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia Q., Zhang Y., Li Z., Hou X., Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm. Sin. B. 2019;9:675–689. doi: 10.1016/j.apsb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu C.M.J., Fang R.H., Zhang L.F. Erythrocyte-inspired delivery systems. Adv. Healthcare Mater. 2012;1:537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 113.Fang R.H., Hu C.-M.J., Luk B.T., Gao W., Copp J.A., Tai Y., O'Connor D.E., Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Z., Wang Y., Ding Y., Repp L., Kwon G.S., Hu Q. Cell-based delivery systems: emerging carriers for immunotherapy. Adv. Funct. Mater. 2021:2100088. doi: 10.1002/adfm.202100088. [DOI] [Google Scholar]
- 115.Cheng S., Xu C., Jin Y., Li Y., Zhong C., Ma J., Yang J., Zhang N., Li Y., Wang C., Yang Z., Wang Y. Artificial mini dendritic cells boost t cell-based immunotherapy for ovarian cancer. Adv. Sci. 2020;7:1903301. doi: 10.1002/advs.201903301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu R., An Y., Jia W., Wang Y., Wu Y., Zhen Y., Cao J., Gao H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Contr. Release. 2020;321:589–601. doi: 10.1016/j.jconrel.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 117.Zhang W., Wang M., Tang W., Wen R., Zhou S., Lee C., Wang H., Jiang W., Delahunty I.M., Zhen Z., Chen H., Chapman M., Wu Z., Howerth E.W., Cai H., Li Z., Xie J. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv. Mater. 2018;30:1805557. doi: 10.1002/adma.201805557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Netti P.A., Baxter L.T., Boucher Y., Skalak R., Jain R.K. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Canc. Res. 1995;55:5451–5458. Go to ISI, //WOS:A1995TD88700057. [PubMed] [Google Scholar]
- 119.Zhou Y., Chen X., Cao J., Gao H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B. 2020;8:6765–6781. doi: 10.1039/d0tb00649a. [DOI] [PubMed] [Google Scholar]
- 120.Zhou M., Wei W., Chen X., Xu X., Zhang X., Zhang X. Ph and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomedicine. 2019;20:102008. doi: 10.1016/j.nano.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y., Chen Q., Zhu Q., Liu J., Li Y., Gao X., Chen D., Zhu X. Small molecular theranostic assemblies functionalized by doxorubicin-hyaluronic acid-methotrexate prodrug for multiple tumor targeting and imaging-guided combined chemo-photothermal therapy. Mol. Pharm. 2019;16:2470–2480. doi: 10.1021/acs.molpharmaceut.9b00072. [DOI] [PubMed] [Google Scholar]
- 122.Ji P., Wang L., Chen Y.W., Wang S.Q., Wu Z.H., Qi X.L. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater. Sci. 2020;8:462–472. doi: 10.1039/c9bm01605h. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y.B., Li Y., Tian H.N., Zhu Q.X., Wang F.F., Fan Z.X., Zhou S., Wang X.W., Xie L.Y., Hou Z.Q. Redox-responsive and dual-targeting hyaluronic acid-methotrexate prodrug self-assembling nanoparticles for enhancing intracellular drug self-delivery. Mol. Pharm. 2019;16:3133–3144. doi: 10.1021/acs.molpharmaceut.9b00359. [DOI] [PubMed] [Google Scholar]
- 124.Tian H., Zhang J., Zhang H., Jiang Y., Song A., Luan Y. Low side-effect and heat-shock protein-inhibited chemo-phototherapy nanoplatform via co-assembling strategy of biotin-tailored ir780 and quercetin. Chem. Eng. J. 2020;382:123043. doi: 10.1016/j.cej.2019.123043. [DOI] [Google Scholar]
- 125.Li Y., Lin J., Ma J., Song L., Lin H., Tang B., Chen D., Su G., Ye S., Zhu X., Luo F., Hou Z. Methotrexate-camptothecin prodrug nanoassemblies as a versatile nanoplatform for biomodal imaging-guided self-active targeted and synergistic chemotherapy. ACS Appl. Mater. Interfaces. 2017;9:34650–34665. doi: 10.1021/acsami.7b10027. [DOI] [PubMed] [Google Scholar]
- 126.Lan J.-S., Qin Y.-H., Liu L., Zeng R.-F., Yang Y., Wang K., Ding Y., Zhang T., Ho R.J.Y. A carrier-free folate receptor-targeted ursolic acid/methotrexate nanodelivery system for synergetic anticancer therapy. Int. J. Nanomed. 2021;16:1775–1787. doi: 10.2147/ijn.S287806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mou Q., Ma Y., Zhu X., Yan D. A small molecule nanodrug consisting of amphiphilic targeting ligand-chemotherapy drug conjugate for targeted cancer therapy. J. Contr. Release. 2016;230:34–44. doi: 10.1016/j.jconrel.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 128.Zhao R., Zheng G., Fan L., Shen Z., Jiang K., Guo Y., Shao J.-W. Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy. Acta Biomater. 2018;70:197–210. doi: 10.1016/j.actbio.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 129.Jiang K., Han L., Guo Y., Zheng G., Fan L., Shen Z., Zhao R., Shao J. A carrier-free dual-drug nanodelivery system functionalized with aptamer specific targeting her2-overexpressing cancer cells. J. Mater. Chem. B. 2017;5:9121–9129. doi: 10.1039/c7tb02562a. [DOI] [PubMed] [Google Scholar]
- 130.Peng M.Y., Qin S.Y., Jia H.Z., Zheng D.W., Rong L., Zhang X.Z. Self-delivery of a peptide-based prodrug for tumor-targeting therapy. Nano Res. 2016;9:663–673. doi: 10.1007/s12274-015-0945-1. [DOI] [Google Scholar]
- 131.Ji C.D., Gao Q., Dong X.H., Yin W.Y., Gu Z.J., Gan Z.H., Zhao Y.L., Yin M.Z. A size-reducible nanodrug with an aggregation-enhanced photodynamic effect for deep chemo-photodynamic therapy. Angew Chem. Int. Ed. Engl. 2018;57:11384–11388. doi: 10.1002/anie.201807602. [DOI] [PubMed] [Google Scholar]
- 132.Wang X., Wang J., Wang J., Zhong Y., Han L., Yan J., Duan P., Shi B., Bai F. Noncovalent self-assembled smart gold(iii) porphyrin nanodrug for synergistic chemo-photothermal therapy. Nano Lett. 2021;21:3418–3425. doi: 10.1021/acs.nanolett.0c04915. [DOI] [PubMed] [Google Scholar]
- 133.Shu C., Sabi-mouka E.M.B., Wang X.L., Ding L. Self-assembly hydrogels as multifunctional drug delivery of paclitaxel for synergistic tumour-targeting and biocompatibility in vitro and in vivo. J. Pharm. Pharmacol. 2017;69:967–977. doi: 10.1111/jphp.12732. [DOI] [PubMed] [Google Scholar]
- 134.Liu B., Ma Y., Wu C.W., Mou Q.B., Deng H.P., Wang R.B., Yan D.Y., Zhang C., Zhu X.Y. A molecular recognition approach to synthesize nucleoside analogue based multifunctional nanoparticles for targeted cancer therapy. J. Am. Chem. Soc. 2017;139:14021–14024. doi: 10.1021/jacs.7b08303. [DOI] [PubMed] [Google Scholar]
- 135.Zhang N., Li M., Sun X., Jia H., Liu W. Nir-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials. 2018;159:25–36. doi: 10.1016/j.biomaterials.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L.J., Zhang X., Lu G.H., Li F., Bao W.E., Song C., Wei W., Ma G.H. Cell membrane camouflaged hydrophobic drug nanoflake sandwiched with photosensitizer for orchestration of chemo-photothermal combination therapy. Small. 2019;15:1805544. doi: 10.1002/smll.201805544. [DOI] [PubMed] [Google Scholar]
- 137.Misra S., Heldin P., Hascall V.C., Karamanos N.K., Skandalis S.S., Markwald R.R., Ghatak S. Hyaluronan-cd44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1429–1443. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luo Z., Dai Y., Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B. 2019;9:1099–1112. doi: 10.1016/j.apsb.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu R., Xiao W., Hu C., Xie R., Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Contr. Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Lu Y., Low P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2012;64:342–352. doi: 10.1016/j.addr.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 141.Huang S.N., Duan S.F., Wang J., Bao S.J., Qiu X.J., Li C.M., Liu Y., Yan L.J., Zhang Z.Z., Hu Y.R. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-mir-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv. Funct. Mater. 2016;26:2532–2544. doi: 10.1002/adfm.201504912. [DOI] [Google Scholar]
- 142.Sega E.I., Low P.S. Tumor detection using folate receptor-targeted imaging agents. Canc. Metastasis Rev. 2008;27:655–664. doi: 10.1007/s10555-008-9155-6. [DOI] [PubMed] [Google Scholar]
- 143.Ren W.X., Han J., Uhm S., Jang Y.J., Kang C., Kim J.-H., Kim J.S. Recent development of biotin conjugation in biological imaging, sensing, and target delivery. Chem. Commun. 2015;51:10403–10418. doi: 10.1039/c5cc03075g. [DOI] [PubMed] [Google Scholar]
- 144.Park S., Kim E., Kim W.Y., Kang C., Kim J.S. Biotin-guided anticancer drug delivery with acidity-triggered drug release. Chem. Commun. 2015;51:9343–9345. doi: 10.1039/c5cc03003j. [DOI] [PubMed] [Google Scholar]
- 145.Rajagopalan P.T.R., Zhang Z.Q., McCourt L., Dwyer M., Benkovic S.J., Hammes G.G. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99:13481–13486. doi: 10.1073/pnas.172501499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin J., Li Y., Li Y., Wu H., Yu F., Zhou S., Xie L., Luo F., Lin C., Hou Z. Drug/dye-loaded, multifunctional peg-chitosan-iron oxide nanocomposites for methotraxate synergistically self-targeted cancer therapy and dual model imaging. ACS Appl. Mater. Interfaces. 2015;7:11908–11920. doi: 10.1021/acsami.5b01685. [DOI] [PubMed] [Google Scholar]
- 147.Hou M., Gao Y.-E., Shi X., Bai S., Ma X., Li B., Xiao B., Xue P., Kang Y., Xu Z. Methotrexate-based amphiphilic prodrug nanoaggregates for co-administration of multiple therapeutics and synergistic cancer therapy. Acta Biomater. 2018;77:228–239. doi: 10.1016/j.actbio.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 148.Chen G., Li D., Li J., Cao X., Wang J., Shi X., Guo R. Targeted doxorubicin delivery to hepatocarcinoma cells by lactobionic acid-modified laponite nanodisks. New J. Chem. 2015;39:2847–2855. doi: 10.1039/c4nj01916d. [DOI] [PubMed] [Google Scholar]
- 149.Zhao R., Li T., Zheng G., Jiang K., Fan L., Shao J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and ph-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials. 2017;143:1–16. doi: 10.1016/j.biomaterials.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 150.Ke Y., Xiang C. Transferrin receptor-targeted hmsn for sorafenib delivery in refractory differentiated thyroid cancer therapy. Int. J. Nanomed. 2018;13:8339–8354. doi: 10.2147/ijn.S187240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jin H., Pi J., Zhao Y., Jiang J., Li T., Zeng X., Yang P., Evans C.E., Cai J. Egfr-targeting plga-peg nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. 2017;9:16365–16374. doi: 10.1039/c7nr06898k. [DOI] [PubMed] [Google Scholar]
- 152.Yin H., Shi X., Wang H., Jin W., Li Y., Fu Y. Photodynamic therapy targeting vcam-1-expressing human umbilical vein endothelial cells using a ppix-vcam-1 binding peptide-quantum dot conjugate. RSC Adv. 2017;7:50562–50570. doi: 10.1039/c7ra10648c. [DOI] [Google Scholar]
- 153.Babu A., Amreddy N., Muralidharan R., Pathuri G., Gali H., Chen A., Zhao Y.D., Munshi A., Ramesh R. Chemodrug delivery using integrin-targeted plga-chitosan nanoparticle for lung cancer therapy. Sci. Rep. 2017;7:14674. doi: 10.1038/s41598-017-15012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kwak G., Jo S.D., Kim D., Kim H., Kim M.G., Kim K., Kwon I.C., Kim S.H. Synergistic antitumor effects of combination treatment with metronomic doxorubicin and vegf-targeting rnai nanoparticles. J. Contr. Release. 2017;267:203–213. doi: 10.1016/j.jconrel.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 155.Yue P.-j., He L., Qiu S.-w., Li Y., Liao Y.-j., Li X.-p., Xie D., Peng Y. Ox26/ctx-conjugated pegylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Canc. 2014;13:191. doi: 10.1186/1476-4598-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tai W.Y., Mahato R., Cheng K. The role of her2 in cancer therapy and targeted drug delivery. J. Contr. Release. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hovanessian A.G., Soundaramourty C., El Khoury D., Nondier I., Svab J., Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PloS One. 2010;5 doi: 10.1371/journal.pone.0015787. e15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo J., Gao X., Su L., Xia H., Gu G., Pang Z., Jiang X., Yao L., Chen J., Chen H. Aptamer-functionalized peg-plga nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 159.Fogal V., Sugahara K.N., Ruoslahti E., Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 160.Christian S., Pilch J., Akerman M.E., Porkka K., Laakkonen P., Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li S.-Y., Xie B.-R., Cheng H., Li C.-X., Zhang M.-K., Qiu W.-X., Liu W.-L., Wang X.-S., Zhang X.-Z. A biomimetic theranostic o-2-meter for cancer targeted photodynamic therapy and phosphorescence imaging. Biomaterials. 2018;151:1–12. doi: 10.1016/j.biomaterials.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 162.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W., Zhang Z., Yu H., Zhang P., Wang S., Li Y. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv. Funct. Mater. 2017;27:1604300. doi: 10.1002/adfm.201604300. [DOI] [Google Scholar]
- 163.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P., Gao G., Pan H., Liu L., Ma A., Cui H., Ma Y., Cai L. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 164.Zhang T., Huang P., Shi L., Su Y., Zhou L., Zhu X., Yan D. Self-assembled nanoparticles of amphiphilic twin drug from floxuridine and bendamustine for cancer therapy. Mol. Pharm. 2015;12:2328–2336. doi: 10.1021/acs.molpharmaceut.5b00005. [DOI] [PubMed] [Google Scholar]
- 165.Ma N., Li Y., Xu H., Wang Z., Zhang X. Dual redox responsive assemblies formed from diselenide block copolymers. J. Am. Chem. Soc. 2010;132:442–443. doi: 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- 166.Zeng X., Zhou X., Li M., Wang C., Xu J., Ma D., Xue W. Redox poly(ethylene glycol)-b-poly(l-lactide) micelles containing diselenide bonds for effective drug delivery. J. Mater. Sci. Mater. Med. NLM. 2015;26:234. doi: 10.1007/s10856-015-5573-5. [DOI] [PubMed] [Google Scholar]
- 167.Chang B.S., Chen D., Wang Y., Chen Y.Z., Jiao Y.F., Sha X.Y., Yang W.L. Bioresponsive controlled drug release based on mesoporous silica nanoparticles coated with reductively sheddable polymer shell. Chem. Mater. 2013;25:574–585. doi: 10.1021/cm3037197. [DOI] [Google Scholar]
- 168.Kakizawa Y., Harada A., Kataoka K. Glutathione-sensitive stabilization of block copolymer micelles composed of antisense DNA and thiolated poly(ethylene glycol)-block-poly(l-lysine): a potential carrier for systemic delivery of antisense DNA. Biomacromolecules. 2001;2:491–497. doi: 10.1021/bm000142l. [DOI] [PubMed] [Google Scholar]
- 169.Baldwin A.D., Kiick K.L. Reversible maleimide-thiol adducts yield glutathione-sensitive poly(ethylene glycol)-heparin hydrogels. Polym. Chem. 2013;4:133–143. doi: 10.1039/c2py20576a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hou M.L., Li S.S., Xu Z.G., Li B.S. A reduction-responsive amphiphilic methotrexate-podophyllotoxin conjugate for targeted chemotherapy. Chem. Asian J. 2019;14:3840–3844. doi: 10.1002/asia.201901070. [DOI] [PubMed] [Google Scholar]
- 171.Huang P., Hu M., Zhou L., Wang Y., Pang Y., Tong G., Huang W., Su Y., Zhu X. Self-delivery nanoparticles from an amphiphilic covalent drug couple of irinotecan and bendamustine for cancer combination chemotherapy. RSC Adv. 2015;5:86254–86264. doi: 10.1039/c5ra16511c. [DOI] [Google Scholar]
- 172.Zhang S., Wang Z., Kong Z., Wang Y., Zhang X., Sun B., Zhang H., Kan Q., He Z., Luo C., Sun J. Photosensitizer-driven nanoassemblies of homodimeric prodrug for self-enhancing activation and synergistic chemo-photodynamic therapy. Theranostics. 2021;11:6019–6032. doi: 10.7150/thno.59065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jing X.N., Zhi Z., Jin L.M., Wang F., Wu Y.S., Wang D.Q., Yan K., Shao Y.P., Meng L.J. Ph/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale. 2019;11:9457–9467. doi: 10.1039/c9nr01194c. [DOI] [PubMed] [Google Scholar]
- 174.Ren C., Gao Y., Guan Y., Wang Z., Yang L., Gao J., Fan H., Liu J. Carrier-free supramolecular hydrogel composed of dual drugs for conquering drug resistance. ACS Appl. Mater. Interfaces. 2019;11:33706–33715. doi: 10.1021/acsami.9b12530. [DOI] [PubMed] [Google Scholar]
- 175.Hu M., Huang P., Wang Y., Su Y., Zhou L., Zhu X., Yan D. Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug-drug conjugate. Bioconjugate Chem. 2015;26:2497–2506. doi: 10.1021/acs.bioconjchem.5b00513. [DOI] [PubMed] [Google Scholar]
- 176.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., Zhu X., Yan D. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 177.Liang P., Huang X., Wang Y., Chen D., Ou C., Zhang Q., Shao J., Huang W., Dong X. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano. 2018;12:11446–11457. doi: 10.1021/acsnano.8b06478. [DOI] [PubMed] [Google Scholar]
- 178.Hou S.L., Chen S.S., Dong Y., Gao S., Zhu B.S., Lu Q.H. Biodegradable cyclomatrix polyphosphazene nanoparticles: a novel ph-responsive drug self-framed delivery system. ACS Appl. Mater. Interfaces. 2018;10:25983–25993. doi: 10.1021/acsami.8b06114. [DOI] [PubMed] [Google Scholar]
- 179.Hou M., Xue P., Gao Y.-E., Ma X., Bai S., Kang Y., Xu Z. Gemcitabine-camptothecin conjugates: a hybrid prodrug for controlled drug release and synergistic therapeutics. Biomater. Sci. 2017;5:1889–1897. doi: 10.1039/c7bm00382j. [DOI] [PubMed] [Google Scholar]
- 180.Ma X.D., Ozliseli E., Zhang Y.Z., Pan G.Q., Wang D.Q., Zhang H.B. Fabrication of redox-responsive doxorubicin and paclitaxel prodrug nanoparticles with microfluidics for selective cancer therapy. Biomater. Sci. 2019;7:634–644. doi: 10.1039/c8bm01333k. [DOI] [PubMed] [Google Scholar]
- 181.Dong S.X., He J.L., Sun Y., Li D., Li L., Zhang M.Z., Ni P.H. Efficient click synthesis of a protonized and reduction-sensitive amphiphilic small-molecule prodrug containing camptothecin and gemcitabine for a drug self-delivery system. Mol. Pharm. 2019;16:3770–3779. doi: 10.1021/acs.molpharmaceut.9b00349. [DOI] [PubMed] [Google Scholar]
- 182.He W., Hu X., Jiang W., Liu R., Zhang D., Zhang J., Li Z., Luan Y. Rational design of a new self-codelivery system from redox-sensitive camptothecin-cytarabine conjugate assembly for effectively synergistic anticancer therapy. Adv. Healthcare Mater. 2017;6:1703135. doi: 10.1002/adhm.201700829. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y., Pei Q., Chen L., Li Z., Xie Z. Reduction-responsive fluorescence off-on bodipy-camptothecin conjugates for self-reporting drug release. J. Mater. Chem. B. 2016;4:2332–2337. doi: 10.1039/c6tb00009f. [DOI] [PubMed] [Google Scholar]
- 184.Ma Y., Mou Q., Sun M., Yu C., Li J., Huang X., Zhu X., Yan D., Shen J. Cancer theranostic nanoparticles self-assembled from amphiphilic small molecules with equilibrium shift-induced renal clearance. Theranostics. 2016;6:1703–1716. doi: 10.7150/thno.15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang C., Xu H., Liang C., Liu Y., Li Z., Yang G., Cheng H., Li Y., Liu Z. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano. 2013;7:6782–6795. doi: 10.1021/nn4017179. [DOI] [PubMed] [Google Scholar]
- 186.Zheng M., Yue C., Ma Y., Gong P., Zhao P., Zheng C., Sheng Z., Zhang P., Wang Z., Cai L. Single-step assembly of dox/icg loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7:2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 187.Li M., Sun X., Zhang N., Wang W., Yang Y., Jia H., Liu W. Nir-activated polydopamine-coated carrier-free "nanobomb" for in situ on-demand drug release. Adv. Sci. 2018;5:1800155. doi: 10.1002/advs.201800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang Y., Dou D.D. Triply and quadruply hydrogen bonded systems:Design, structure and application. Prog. Chem. 2014;26:706–726. doi: 10.7536/pc131051. [DOI] [Google Scholar]
- 189.Lu J., Liu X., Liao Y.-P., Wang X., Ahmed A., Jiang W., Ji Y., Meng H., Nel A.E. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the Ido-1 pathway. ACS Nano. 2018;12:11041–11061. doi: 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 190.Chen Y., Xia R., Huang Y., Zhao W., Li J., Zhang X., Wang P., Venkataramanan R., Fan J., Xie W., Ma X., Lu B., Li S. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 2016;7:13443. doi: 10.1038/ncomms13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takaku H., Takase M., Abe S., Hayashi H., Miyazaki K. Invivo antitumor-activity of arginine deiminase purified from mycoplasma-arginini. Int. J. Canc. 1992;51:244–249. doi: 10.1002/ijc.2910510213. [DOI] [PubMed] [Google Scholar]
- 192.Mullarky E., Lucki N.C., Zavareh R.B., Anglin J.L., Gomes A.P., Nicolay B.N., Wong J.C.Y., Christen S., Takahashi H., Singh P.K., Blenis J., Warren J.D., Fendt S.-M., Asara J.M., DeNicola G.M., Lyssiotis C.A., Lairson L.L., Cantley L.C. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:1778–1783. doi: 10.1073/pnas.1521548113. [DOI] [PMC free article] [PubMed] [Google Scholar]