Key Points
Question
Does respiratory support using a new system that has low imposed work of breathing and an interface with short binasal prongs decrease delivery room intubation or death during stabilization of extremely preterm infants?
Findings
In this randomized clinical trial involving 250 extremely preterm infants, 41 infants (33.1%) receiving the new respiratory support system were intubated or died in the delivery room compared with 55 infants (45.1%) receiving standard care (T-piece system with face mask). The adjusted odds ratio of intubation or death was statistically significant.
Meaning
In this study, using a respiratory support system with low imposed work of breathing and short binasal prongs during stabilization of extremely preterm infants decreased delivery room intubation and was safe and feasible.
Abstract
Importance
Establishing stable breathing is a key event for preterm infants after birth. Delivery of pressure-stable continuous positive airway pressure and avoiding face mask use could be of importance in the delivery room.
Objective
To determine whether using a new respiratory support system with low imposed work of breathing and short binasal prongs decreases delivery room intubations or death compared with a standard T-piece system with a face mask.
Design, Setting, and Participants
In this unblinded randomized clinical trial, mothers threatening preterm delivery before week 28 of gestation were screened. A total of 365 mothers were enrolled, and 250 infants were randomized before birth and 246 liveborn infants were treated. The trial was conducted in 7 neonatal intensive care units in 5 European countries from March 2016 to May 2020. The follow-up period was 72 hours after intervention.
Interventions
Infants were randomized to either the new respiratory support system with short binasal prongs (n = 124 infants) or the standard T-piece system with face mask (n = 122 infants). The intervention was providing continuous positive airway pressure for 10 to 30 minutes and positive pressure ventilation, if needed, with the randomized system.
Main Outcomes and Measures
The primary outcome was delivery room intubation or death within 30 minutes of birth. Secondary outcomes included respiratory and safety variables.
Results
Of 246 liveborn infants treated, the mean (SD) gestational age was 25.9 (1.3) weeks, and 127 (51.6%) were female. A total of 41 infants (33.1%) receiving the new respiratory support system were intubated or died in the delivery room compared with 55 infants (45.1%) receiving standard care. The adjusted odds ratio was statistically significant after adjusting for stratification variables (adjusted odds ratio, 0.53; 95% CI, 0.30-0.94; P = .03). No significant differences were seen in secondary outcomes or safety variables.
Conclusions and Relevance
In this study, using the new respiratory support system reduced delivery room intubation in extremely preterm infants. Stabilizing preterm infants with a system that has low imposed work of breathing and binasal prongs as interface is safe and feasible.
Trial Registration
ClinicalTrials.gov Identifier: NCT02563717
This randomized clinical trial investigates whether using a new respiratory support system with low imposed work of breathing and short binasal prongs decreases delivery room intubations or death compared with a standard T-piece system with a face mask.
Introduction
In the last 2 decades, the initial stabilization of premature infants has moved toward a less invasive approach. A Cochrane meta-analysis of randomized trials comparing noninvasive respiratory support in the delivery room (DR) with continuous positive airway pressure (CPAP) instead of intubation and mechanical ventilation showed that using CPAP was associated with a reduced risk of bronchopulmonary dysplasia (BPD) or death.1 Therefore, international guidelines recommend using CPAP rather than intubation and mechanical ventilation for initial stabilization of spontaneous breathing in preterm infants with respiratory distress.2,3 However, there is little evidence on which devices and interfaces are best suited to aid the transition to stable breathing after birth.
When supporting a breathing infant with CPAP, the infant will be challenged with additional workload that is needed to breathe through the system. This extra work is called imposed work of breathing (iWOB).4 The most commonly used system for respiratory support in the DR, the T-piece, was noted to have high iWOB during simulated breathing in a bench trial in 2012.5 This led to a hypothesis that providing CPAP support with lower iWOB might increase the number of infants who establish stable breathing after birth.
The use of face masks to deliver CPAP and positive pressure ventilation (PPV) with T-piece systems has been the standard of care for decades. Studies have shown that both mask leakage and airway obstruction are common when using a face mask, often undetected by the user.6,7,8,9 There is also a concern that pressure from the mask can cause apnea or bradycardia due to the trigeminocardiac reflex.10,11 Nasal prongs or nasopharyngeal tube have been suggested as alternatives12,13 and even mentioned in international guidelines.2
A new system for respiratory support has been designed and tested by a research group at the Karolinska Institute. The system delivers CPAP and allows PPV by occlusion similar to a T-piece system and can be used with nasal prongs. A bench study showed that the system had low iWOB compared with the T-piece, and a feasibility trial reveled no problems with usability or safety.14 We hypothesized that for infants born before 28 weeks of gestation, respiratory support after birth with a new system could reduce DR intubations or death compared with the standard T-piece system. The aim of this study was to evaluate if a new system with low iWOB and nasal prongs as interface could reduce the need for intubation of extremely preterm infants in the DR compared with the standard T-piece system with face mask.
Methods
Study Design
The Comparison of Respiratory Support After Delivery on Infants Born Before 28 Weeks Gestational Age (CORSAD) trial was a 2-armed, nonblinded, randomized clinical trial in 7 neonatal intensive care units (NICUs) in 5 countries in northern Europe. Independent review boards in each country approved the study. If eligible, parents signed informed consent after receiving both oral and written information about the study. All consents were prospective and deferred consent was not allowed. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
The trial was performed according to Good Clinical Practice following the principles of the Declaration of Helsinki.15 The trial protocol can be found in Supplement 1, and the statistical analysis plan can be found in Supplement 2. The trial was registered at ClinicalTrials.gov before the start of the trial. Site education, including DR training and web-based training modules, was implemented before each study site initiation.
Participants
Screening for eligibility and consent was performed by the site investigator for mothers threatening delivery of an extremely premature infant. All infants less than 28 weeks of gestational age were included if no exclusion criteria were fulfilled. Exclusion criteria were treatment limitations or decision to intubate prior to birth, known major congenital anomalies, or no study neonatologist available at time of birth.
Randomization
Randomization was performed by a site investigator when birth was imminent. Participants were randomly assigned in a 1:1 ratio to each group using a computer-generated randomization within a secure web-based electronic case report form. Randomization was concealed from researchers and stratified on center, gestational age (less than 24, 24 to 25, and 25 weeks or more), and antenatal steroid treatment (none, partial, and complete course) using dynamic allocation. Infants of mothers with multiple births were assigned to the same system.
DR Intervention
The intervention was respiratory support with the randomized system for the first 10 to 30 minutes of life started when the infant was placed on the resuscitation table. The T-piece group used the standard equipment available at each site with a round face mask. The new respiratory support system group was treated with rPAP (Inspiration Healthcare) with prongs of the original Infant Flow design (Inspiration Healthcare; Intersurgical) using resuscitation table (GE; Dräger) or designated rPAP driver (Inspiration Healthcare). Centers had an option of using heated humidification on the condition that both arms would receive the same treatment. Recommended starting pressure for positive inflating pressure was 20 to 25 cm H2O and 5 to 8 cm H2O for positive end-expiratory pressure (PEEP). Sustained inflation was not allowed. Routine care, including plastic wrapping, stimulation, heart rate assessment, and oxygen saturation monitoring, followed international guidelines.2,3 The intervention ended (1) when an infant was intubated, (2) if the patient was stable and breathing adequately after a minimum of 10 minutes of support with the randomized system, or (3) at 30 minutes when the respiratory support could continue as decided by the clinicians (crossover not allowed). The postintervention period in DR and NICU treatments followed local protocols and international guidelines.2,3 Final end points for secondary outcomes were collected at 72 hours of age. The clinical management protocol can be found in eAppendix 1 in Supplement 3.
Outcomes
The primary outcome was intubation or death in the DR (time frame, 0 to 30 minutes). Criteria for DR intubation were no or inadequate response to PPV and CPAP defined by one of the following: (1) bradycardia heart rate less than 100 beats per minute despite 60 seconds of effective PPV, (2) persistent apnea, (3) poor respiratory effort during the intervention, and (4) inadequate oxygenation and respiratory distress. The secondary outcomes included time to first intubation, use of surfactant, use of PPV, mechanical ventilation within 72 hours, and respiratory support at 72 hours. Safety variables included air leaks, intraventricular hemorrhage, and problems with ventilation or equipment. A complete list of secondary outcomes can be found in Supplement 1.
Good Clinical Practice and Data Monitoring Committee
The trial was performed according to Good Clinical Practice and monitored by independent monitors at each site. Monitoring was used to increase data quality and not required by regulatory bodies, since both systems had regulatory approval for use in infants.
Prior to trial commencement, an independent data monitoring committee (DMC) was appointed, consisting of 2 senior neonatologists with extensive research experience. The primary responsibilities of the DMC were to (1) periodically review and evaluate the accumulated study data for participant safety, study conduct and progress, and, when appropriate, efficacy and (2) make recommendations to study investigators concerning the continuation, modification, or termination of the trial. The DMC had full access to nonblinded reports of deaths, adverse events, and summary of outcomes. They reviewed all early deaths to assess a possible relationship to the allocation group. DMC guidelines are found in eAppendix 2 in Supplement 3.
Statistical Analysis
We calculated the number of patients needed from a baseline intubation rate of 60% in extremely preterm infants born in Sweden prior to the trial.16 Estimated treatment effect was not known, and a 20% absolute reduction was judged to be clinically important. The calculated number of infants was 195 with a binary outcome superiority trial design at a significance level (α) of 5% and power (1 − β) of 80%. This was increased to 250 patients to accommodate stillbirths, protocol violations, baseline intubation rate changes, and center differences.
Binary outcomes and demographic variables were compared by χ2 or Fisher exact test, as appropriate. The primary outcome was analyzed using a logistic regression model adjusted for stratification variables. The model was also used for estimating an adjusted risk difference. A multivariate generalized estimating equation logistic regression model adjusting for multiple births was also performed and is available in Supplement 2. Continuous data were compared using t tests or nonparametric test after tests of normality. Risk ratios and risk differences for secondary outcomes with 95% CIs were calculated using cross-tabulation risk function or in Excel version 16 (Microsoft). Kaplan-Meier method was used for cumulative incidences of DR primary outcome followed by NICU mechanical ventilation or death up to 72 hours. For further analytic methods, see Supplement 2. Statistical analysis was performed with SPSS version 27 (IBM Corporation) and SAS version 9.4 (SAS Institute). All analyses were 2-sided and based on intention-to-treat, and P values less than .05 were considered statistically significant. No adjustments for multiple comparisons were made, and secondary outcome results should be interpreted as exploratory.
Results
Participants
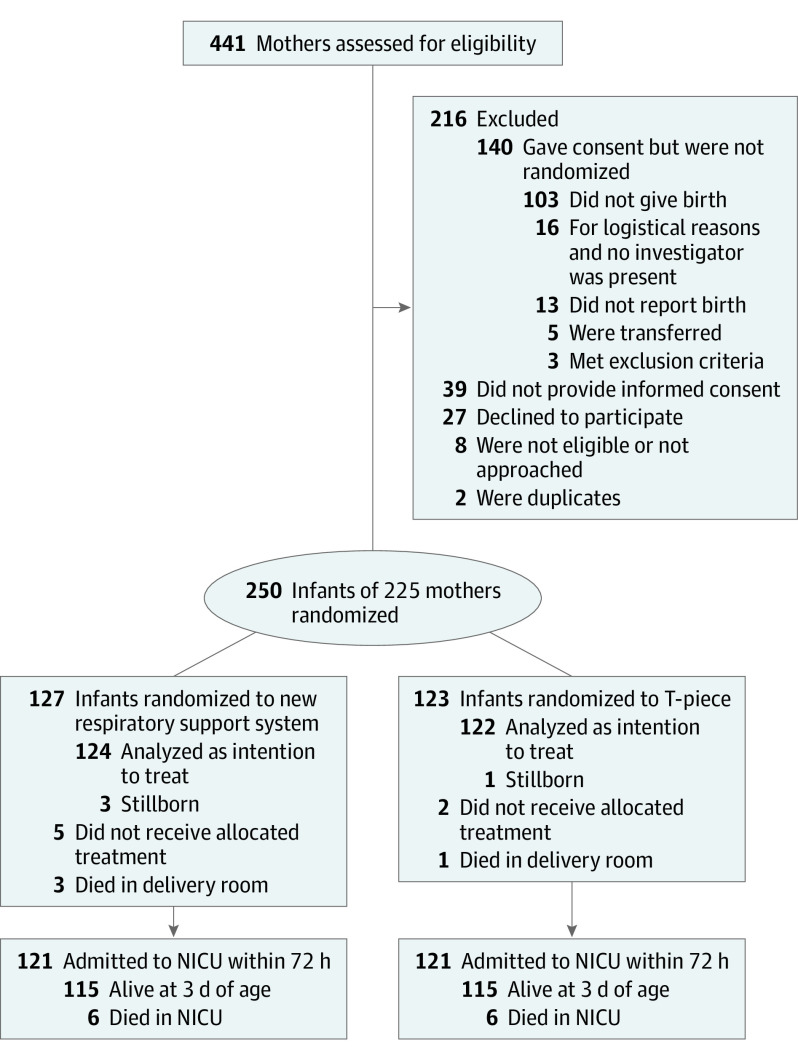
From March 23, 2016, to May 14, 2020, 250 infants were randomized; 127 were assigned to the new respiratory support system and 123 to the T-piece. There were 3 stillbirths in the new respiratory support system group and 1 stillbirth in the T-piece group, resulting in 124 and 122 liveborn infants, respectively, in each arm (Figure 1). The 2 groups were balanced with respect to demographic and clinical characteristics (Table 1). There was high exposure to any antenatal corticosteroids (more than 98% in both groups), with more than 80% receiving a full course. The overall mean (SD) gestational age was 25.8 (1.3) weeks, and the median birth weight was similar between groups. Compared with T-piece group, the new respiratory support system group had more caesarean deliveries (87 of 124 [70.2%] vs 77 of 122 [63.1%]), general anesthesia compared with spinal or epidural anesthesia during caesarean delivery (14 [16.1%] vs 6 [7.8%]), intrauterine growth retardation (31 [25.0%] vs 22 [18.0%]), and multiple births (26 [21.0%] vs 23 [18.9%]). Humidification use during resuscitation was similar in both groups (38 of 124 [30.6%] vs 44 of 122 [36.1%]).
Figure 1. CONSORT Diagram of Patient Recruitment, Randomization, and Retention.
NICU indicates neonatal intensive care unit.
Table 1. Infant and Pregnancy Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| New system (n = 124) | T-piece (n = 122) | |
| GA, mean (SD; range), wk:d | 25:6 (1:2; 23:0-27:6) | 25:5 (1:2; 22:4-27:6) |
| GA group, wk | ||
| <24 | 12 (9.7) | 16 (13.1) |
| 24-25 | 49 (39.5) | 45 (36.9) |
| >25 | 63 (50.8) | 61 (50.0) |
| Multiple pregnancy | ||
| Singleton | 98 (79.0) | 99 (81.1) |
| Multiple | 26 (21.0) | 23 (18.9) |
| Female | 61 (49.2) | 66 (54.1) |
| Infant weight, mean (SD; range), g | 800 (192; 445-1355) | 800 (188; 411-1420) |
| Antenatal steroids | ||
| Complete course | 100 (80.6) | 100 (82.0) |
| Incomplete course | 22 (17.7) | 21 (17.2) |
| None | 2 (1.6) | 1 (0.8) |
| Caesarean delivery | 87 (70.2) | 77 (63.1) |
| General anesthesia | 14 (16.1) | 6 (7.8) |
| Caesarean delivery for fetal concern | 55 (63.2) | 52 (67.5) |
| Mother in active labor | 76 (61.3) | 80 (65.6) |
| IUGR | 31 (25.0) | 22 (18.0) |
| Tocolytic therapy | 65 (52.4) | 67 (54.9) |
| Mother received antibiotics | 94 (75.8) | 87 (71.3) |
| Clinical chorioamnionitis | 31 (25.0) | 34 (27.9) |
| Preeclampsia or eclampsia | 28 (22.6) | 20 (16.4) |
| PROM | 40 (32.3) | 40 (32.8) |
| Ablation | 15 (12.1) | 17 (13.9) |
Abbreviations: GA, gestational age; IUGR, intrauterine growth retardation; PROM, premature rupture of membranes.
Primary and Secondary Outcomes
The primary outcome, intubation or death in the DR (time frame, 0 to 30 minutes of life), occurred in 41 of 124 infants (33.1%) in the new respiratory support system group and in 55 of 122 infants (45.1%) in the T-piece group. The adjusted odds ratio was statistically significant after adjusting for stratification variables (adjusted odds ratio, 0.53; 95% CI, 0.30-0.94; P = .03; risk difference, −14.6%; 95% CI, −26.5 to −2.6). Two infants in the new respiratory support system group died or were intubated in the DR after the time frame—one was intubated at 45 minutes and one was not intubated and died at 66 minutes with treatment limitations (Figure 2) (eAppendix 3 in Supplement 3).
Figure 2. Kaplan-Meier Plot of Cumulative Events for the First 72 Hours.
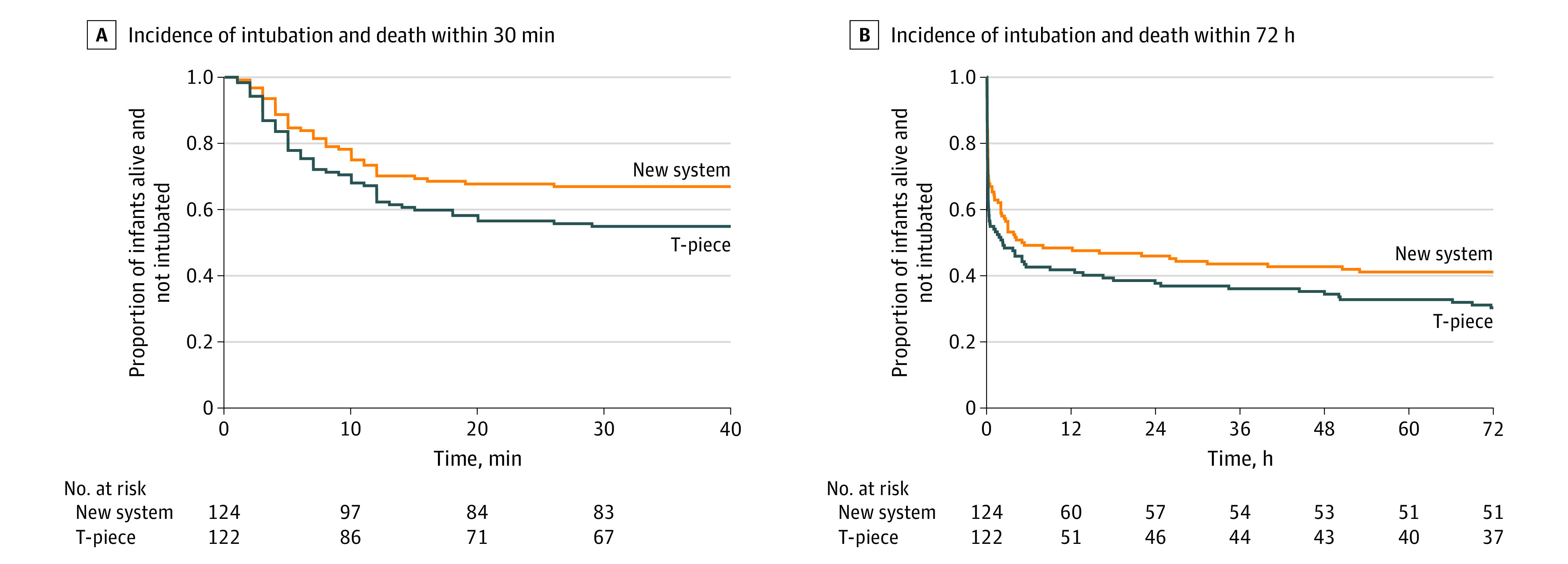
Events are defined as delivery room intubations and deaths followed by mechanical ventilation or deaths in the neonatal intensive care unit. Intubations for the INSURE method or catheter delivery of surfactant in the neonatal intensive care unit (without mechanical ventilation) were not included. All infants who died in the neonatal intensive care unit were intubated. There were no missing values and no infants lost to follow-up. Log-rank test at 30 minutes and 72 hours were not statistically significant.
None of the secondary outcomes were statistically significant after correction for multiple comparisons (Table 2 and Table 3; Figure 2).
Table 2. Secondary Outcomes in Delivery Room (DR).
| Outcome | No. (%) | Risk difference, % (95% CI) | |
|---|---|---|---|
| New system (n = 124) | T-piece (n = 122) | ||
| Died in DR | 3 (2.4) | 1 (0.8) | 1.6 (−1.5 to 4.7) |
| Intubated before transfer from DR | 40 (32.3) | 54 (44.3) | −12.0 (−24.1 to 0.1) |
| Time from birth to intubation in DR, median (IQR), mina | 7 (4-11) | 6 (3-12) | NA |
| Main reason for DR intubation | |||
| Bradycardia | 17 (40.5) | 15 (27.3) | NA |
| Inability to ventilate | 3 (7.1) | 5 (9.1) | NA |
| Inadequate breathing, apnea | 22 (52.3) | 35 (63.6) | NA |
| Breathing on arrival to table | 77 (62.1) | 72 (59.0) | 3.1 (−9.1 to 15.3) |
| Apgar score, median (IQR) | |||
| 1 min | 5 (4-7) | 5 (4-7) | NA |
| 5 min | 7 (6-9) | 7 (6-8) | NA |
| 10 min | 9 (7-10) | 8 (7-10) | NA |
| PPV used in DR | 102 (82.3) | 103 (84.4) | −2.2 (−11.5 to 7.1) |
| Surfactant in DRb | 44 (35.5) | 56 (45.9) | −10.4 (−22.6 to 1.8) |
Abbreviations: IQR, interquartile range; NA, not applicable; PPV, positive pressure ventilation.
A total of 42 infants in the new system group and 55 infants in the T-piece group were intubated.
Surfactant use in the DR included the LISA and MIST methods (12 infants in the new system group and 14 infants in the T-piece group) and INSURE method (1 infant in the new system group and 1 infant in the T-piece group). Infants receiving the INSURE method were counted as being intubated.
Table 3. Secondary Outcomes in the Neonatal Intensive Care Unit (NICU).
| Outcome | No. (%) | Risk difference, % (95% CI) | |
|---|---|---|---|
| New system (n = 121) | T-piece (n = 121) | ||
| Died in NICUa | 6 (5.0) | 6 (5.0) | 0 (−5.5 to 5.5) |
| Any mechanical ventilation before 72 hb | 68 (56.2) | 83 (68.6) | −12.4 (−24.5 to −0.3) |
| Mechanical ventilation at 72 h | 51 (44.3) | 59 (51.3) | −6.6 (−19.1 to 5.9) |
| Surfactant in NICU | 47 (38.8) | 36 (29.8) | 9.1 (−2.8 to 21.0) |
| Temperature on admission, mean (SD), °Cc | 36.7 (0.7) | 36.5 (0.6) | NA |
| IVH anyd | 29 (24.6) | 32 (26.7) | −2.5 (−13.4 to 8.5) |
| IVH grade III or mored | 5 (4.2) | 10 (8.3) | −4.1 (−10.2 to 1.9) |
| Lung hemorrhage | 6 (5.0) | 9 (7.4) | −2.5 (−8.5 to 3.6) |
| Air leaks in NICU | 2 (1.7) | 6 (5.0) | −3.3 (−7.8 to 1.2) |
| Early-onset septicemia | 28 (23.1) | 21 (17.4) | 5.8 (−4.3 to 15.9) |
Abbreviation: NA, not applicable.
All infants who died in NICU received mechanical ventilation.
Intubations leading to mechanical ventilation excluded NICU intubations for surfactant (INSURE method).
A total of 102 infants in the new system group and 90 in the T-piece group had their temperature measured.
IVH ultrasonography was not performed within 72 hours for 3 infants in the new system group and and 1 in the T-piece group.
Safety
There were 4 deaths in the DR (3 infants in the new respiratory support system group and 1 in the T-piece group) followed by 12 deaths in the NICU (6 infants in each group) before 72 hours (eAppendix 3 in Supplement 3). Infants not receiving the allocated treatment occurred more often in the new respiratory support system group (5 infants vs 2 infants). Reports of problems with protocol adherence, adverse events, and technical issues were also more common among infants in the new respiratory support system (7 infants vs 1 infants). There were no deaths or serious adverse events related to the trial protocol or devices used according to DMC review. All deaths and problems are listed in eAppendix 3 in Supplement 3. The interface was changed from prongs to face mask in 9 infants treated with the new respiratory support system as rescue and according to protocol.
Discussion
In our trial, using the new respiratory support system decreased DR intubation or death compared with using the standard T-piece system. In recent large clinical trials of DR stabilization in this population of infants, a treatment effect of 10% to 12% for reducing BPD has been judged as clinically significant.17,18,19 In our trial that compares intubations in the DR, the difference between groups is in the same range. We chose DR intubation rather than BPD as our primary outcome. This was based on the strong link between early ventilator-induced lung injury and the pathogenesis of BPD.20 Our primary outcome and several of our secondary outcomes are graded as important outcomes for respiratory support in the DR according to the newly revised International Liaison Committee on Resuscitation (ILCOR) guidelines.21
We believe that the statistically significant decrease in intubations in our trial is important for this population of extremely preterm infants. Intubation and mechanical ventilation are major risk factors for later pulmonary morbidity, and delaying or eliminating these interventions is crucial. A delayed start of mechanical ventilation is beneficial by reducing the duration of mechanical ventilation.22 Further, establishing spontaneous breathing and avoiding DR intubations creates an extended window for less invasive surfactant therapy.
The size of our sample was estimated on a baseline intubation rate of 60% in extremely preterm infants in Sweden prior to the trial.16 However, the observed intubation rate in the control group of our trial was only 45%, and the intubation rates in Sweden decreased to approximately 40% in 2019.16 For comparison, the Sustained Aeration for Infant Lungs (SAIL) trial,23 which compared sustained inflation with intermittent PPV, had a DR intubation rate of 51.6% in the sustained inflation group and 56.4% in the standard group; the CORSAD trial had a DR rate of intubation or death of 33% in the new respiratory support system group and 45% in the standard group. However, the infants were on average 4 days younger in the SAIL trial, and deferred consent was used in 45% of cases assessed for eligibility at delivery in the SAIL trial in contrast to none in the CORSAD trial.
With studies and national databases showing decreasing intubation rates in the DR and difficulties with defining strict intubation criteria, it is likely that DR intubation will not be suitable as a primary outcome in future DR studies of preterm infants in similar settings. A composite outcome that reflects transition to stable breathing and avoiding intraventricular hemorrhage, air leaks, and other complications is an attractive alternative.
Our trial investigated 2 different features of resuscitation systems. The new respiratory support system has low iWOB with prongs as interface. The standard T-piece treatment has high iWOB and a face mask. It was not possible to differentiate separate specific effects related to iWOB or to the interface.
The clinical importance of iWOB during stabilization and CPAP treatment of infants is not known. Resistance and iWOB when breathing through endotracheal tubes have been well described, and resistance of nasal interfaces has been thoroughly examined.24,25 A study by Green et al26 showed large resistance differences between nasal interfaces. The authors concluded that use of interfaces with high resistance may result in a greater drop in delivered airway pressure compared with set circuit pressure, which may have implications for clinical efficacy. The T-piece has high resistance, is pressure unstable, and has high iWOB.5 Compared with the new respiratory support system, the difference is large.14 For infants with spontaneous breathing in the DR, this difference can theoretically favor the system with lower resistance and iWOB.
To our knowledge, this is the first randomized clinical trial using short binasal prongs to provide both PPV and CPAP in the DR. Capasso et al12 showed a reduction in DR intubations when they compared Argyle prongs with triangular face mask, but a self-inflating bag without PEEP was used in both groups. Two trials have used nasopharyngeal tubes and T-piece systems. te Pas and Walther27 showed a reduction in intubations during the first 72 hours when using a nasopharyngeal tube, but the interface was one of several differences between the treatment groups. Kamlin et al28 found no differences in intubations for the first 24 hours when comparing nasopharyngeal tubes with face mask connected to a T-piece. A problem with the use of unilateral nasopharyngeal tube is high leakage,29 and if leakage is successfully reduced, there will be high resistance to breathing through the single narrow tube.30 In our study, 9 infants were switched from prongs to face mask. A noted problem was finding a suitable prong size for the smallest infants and proper fitting of prongs into nostrils. Switching from prongs to face mask could also be related to the introduction of a new and unfamiliar device in a stressful situation. Although it has been shown that short binasal prongs are superior to nasopharyngeal tubes and long binasal prongs during CPAP treatment,26,30 it remains unclear which nasal interface is best suited for resuscitation and stabilization. The use of face mask or nasal prongs during DR stabilization is reported in 2 recent systematic reviews. The overall results seem to favor a nasal interface but are inconclusive, with both reports stating that more studies are needed.31,32 Animal studies have shown convincing evidence that PEEP is beneficial in creating functional residual capacity.13,33,34,35,36 However, clinical studies have failed to show marked clinical significance with the devices commonly used.3 The reason for this discrepancy remains unclear, but resistance, pressure stability, and leakage of the devices and interfaces used could be part of the explanation. More research on the importance of pressure stability, iWOB and interfaces during transition to breathing after birth is needed.
Limitations
This study had limitations. The intervention was not blinded, leaving a potential for bias, despite clear guidelines for clinical management both in the DR and the NICU. To reveal possible bias, we included secondary outcomes, such as time to intubation in the DR and time to mechanical ventilation in the NICU. There were no statistical significances in these outcomes, and the Kaplan-Meier graphs did not show that intubations for the new system were delayed to the NICU.
The study used antenatal consent only. This risks exclusion of infants born precipitously. These infants often have a poorer prognosis and often are not exposed to antenatal steroids. This potentially affects the generalizability of our results.
Conclusions
In our study, using the new respiratory support system when stabilizing extremely preterm infants decreased intubation or death in the DR. Stabilization with a system that has low iWOB and short binasal prongs is safe and feasible.
Trial protocol.
Statistical analysis plan.
eAppendix 1. Clinical management appendix.
eAppendix 2. Data management committee instructions.
eAppendix 3. Summary of stillborn, infants not receiving allocated treatment, deaths, and miscellaneous.
Nonauthor Collaborators. CORSAD Trial Investigators.
Data sharing statement.
References
- 1.Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;(6):CD001243. doi: 10.1002/14651858.CD001243.pub3 [DOI] [PubMed] [Google Scholar]
- 2.Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 update. Neonatology. 2019;115(4):432-450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlman JM, Wyllie J, Kattwinkel J, et al. ; Neonatal Resuscitation Chapter Collaborators . Part 7: neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16)(suppl 1):S204-S241. doi: 10.1161/CIR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 4.Banner MJ, Kirby RR, Blanch PB. Differentiating total work of breathing into its component parts. essential for appropriate interpretation. Chest. 1996;109(5):1141-1143. doi: 10.1378/chest.109.5.1141 [DOI] [PubMed] [Google Scholar]
- 5.Drevhammar T, Nilsson K, Zetterström H, Jonsson B. Comparison of seven infant continuous positive airway pressure systems using simulated neonatal breathing. Pediatr Crit Care Med. 2012;13(2):e113-e119. doi: 10.1097/PCC.0b013e31822f1b79 [DOI] [PubMed] [Google Scholar]
- 6.Schilleman K, Witlox RS, Lopriore E, Morley CJ, Walther FJ, te Pas AB. Leak and obstruction with mask ventilation during simulated neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F398-F402. doi: 10.1136/adc.2009.182162 [DOI] [PubMed] [Google Scholar]
- 7.Schmölzer GM, Dawson JA, Kamlin COF, O’Donnell CPF, Morley CJ, Davis PG. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F254-F257. doi: 10.1136/adc.2010.191171 [DOI] [PubMed] [Google Scholar]
- 8.Schmölzer GM, Morley CJ, Wong C, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr. 2012;160(3):377-381.e2. doi: 10.1016/j.jpeds.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 9.Kaufman J, Schmölzer GM, Kamlin COF, Davis PG. Mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F405-F410. doi: 10.1136/archdischild-2012-303313 [DOI] [PubMed] [Google Scholar]
- 10.Kuypers K, Martherus T, Lamberska T, Dekker J, Hooper SB, Te Pas AB. Reflexes that impact spontaneous breathing of preterm infants at birth: a narrative review. Arch Dis Child Fetal Neonatal Ed. 2020;105(6):675-679. doi: 10.1136/archdischild-2020-318915 [DOI] [PubMed] [Google Scholar]
- 11.Kuypers KLAM, Lamberska T, Martherus T, et al. The effect of a face mask for respiratory support on breathing in preterm infants at birth. Resuscitation. 2019;144:178-184. doi: 10.1016/j.resuscitation.2019.08.043 [DOI] [PubMed] [Google Scholar]
- 12.Capasso L, Capasso A, Raimondi F, Vendemmia M, Araimo G, Paludetto R. A randomized trial comparing oxygen delivery on intermittent positive pressure with nasal cannulae versus facial mask in neonatal primary resuscitation. Acta Paediatr. 2005;94(2):197-200. doi: 10.1080/08035250410025113 [DOI] [PubMed] [Google Scholar]
- 13.Martherus T, Oberthuer A, Dekker J, et al. Supporting breathing of preterm infants at birth: a narrative review. Arch Dis Child Fetal Neonatal Ed. 2019;104(1):F102-F107. doi: 10.1136/archdischild-2018-314898 [DOI] [PubMed] [Google Scholar]
- 14.Donaldsson S, Drevhammar T, Taittonen L, Klemming S, Jonsson B. Initial stabilisation of preterm infants: a new resuscitation system with low imposed work of breathing for use with face mask or nasal prongs. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F203-F207. doi: 10.1136/archdischild-2016-310577 [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Norman M, Källén K, Wahlström E, Håkansson S; SNQ Collaboration . The Swedish Neonatal Quality Register—contents, completeness and validity. Acta Paediatr. 2019;108(8):1411-1418. doi: 10.1111/apa.14823 [DOI] [PubMed] [Google Scholar]
- 17.Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group . Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069-e1076. doi: 10.1542/peds.2010-3848 [DOI] [PubMed] [Google Scholar]
- 18.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet J-M, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700-708. doi: 10.1056/NEJMoa072788 [DOI] [PubMed] [Google Scholar]
- 19.Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970-1979. doi: 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Marter LJ, Allred EN, Pagano M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? the Neonatology Committee for the Developmental Network. Pediatrics. 2000;105(6):1194-1201. doi: 10.1542/peds.105.6.1194 [DOI] [PubMed] [Google Scholar]
- 21.Wyckoff MH, Wyllie J, Aziz K, et al. ; Neonatal Life Support Collaborators . Neonatal life support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142(16)(suppl 1):S185-S221. doi: 10.1161/CIR.0000000000000895 [DOI] [PubMed] [Google Scholar]
- 22.Carlo WA. Gentle ventilation: the new evidence from the SUPPORT, COIN, VON, CURPAP, Colombian Network, and Neocosur Network trials. Early Hum Dev. 2012;88(suppl 2):S81-S83. doi: 10.1016/S0378-3782(12)70022-1 [DOI] [PubMed] [Google Scholar]
- 23.Kirpalani H, Ratcliffe SJ, Keszler M, et al. ; SAIL Site Investigators . Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: the SAIL randomized clinical trial. JAMA. 2019;321(12):1165-1175. doi: 10.1001/jama.2019.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall MA. Infant endotracheal tube resistance: effects of changing length, diameter, and gas density. Crit Care Med. 1980;8(1):38-40. doi: 10.1097/00003246-198001000-00007 [DOI] [PubMed] [Google Scholar]
- 25.Jarreau PH, Louis B, Dassieu G, et al. Estimation of inspiratory pressure drop in neonatal and pediatric endotracheal tubes. J Appl Physiol (1985). 1999;87(1):36-46. doi: 10.1152/jappl.1999.87.1.36 [DOI] [PubMed] [Google Scholar]
- 26.Green EA, Dawson JA, Davis PG, De Paoli AG, Roberts CT. Assessment of resistance of nasal continuous positive airway pressure interfaces. Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F535-F539. doi: 10.1136/archdischild-2018-315838 [DOI] [PubMed] [Google Scholar]
- 27.te Pas AB, Walther FJA. A randomized, controlled trial of delivery-room respiratory management in very preterm infants. Pediatrics. 2007;120(2):322-329. doi: 10.1542/peds.2007-0114 [DOI] [PubMed] [Google Scholar]
- 28.Kamlin COF, Schilleman K, Dawson JA, et al. Mask versus nasal tube for stabilization of preterm infants at birth: a randomized controlled trial. Pediatrics. 2013;132(2):e381-e388. doi: 10.1542/peds.2013-0361 [DOI] [PubMed] [Google Scholar]
- 29.van Vonderen JJ, Kamlin CO, Dawson JA, Walther FJ, Davis PG, te Pas AB. Mask versus nasal tube for stabilization of preterm infants at birth: respiratory function measurements. J Pediatr. 2015;167(1):81-85.e1. doi: 10.1016/j.jpeds.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 30.De Paoli AG, Morley CJ, Davis PG, Lau R, Hingeley E. In vitro comparison of nasal continuous positive airway pressure devices for neonates. Arch Dis Child Fetal Neonatal Ed. 2002;87(1):F42-F45. doi: 10.1136/fn.87.1.F42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machumpurath S, O’Currain E, Dawson JA, Davis PG. Interfaces for non-invasive neonatal resuscitation in the delivery room: a systematic review and meta-analysis. Resuscitation. 2020;156:244-250. doi: 10.1016/j.resuscitation.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 32.Mangat A, Bruckner M, Schmölzer GM. Face mask versus nasal prong or nasopharyngeal tube for neonatal resuscitation in the delivery room: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2021;fetalneonatal-2020-319460. doi: 10.1136/archdischild-2020-319460 [DOI] [PubMed] [Google Scholar]
- 33.Probyn ME, Hooper SB, Dargaville PA, et al. Positive end expiratory pressure during resuscitation of premature lambs rapidly improves blood gases without adversely affecting arterial pressure. Pediatr Res. 2004;56(2):198-204. doi: 10.1203/01.PDR.0000132752.94155.13 [DOI] [PubMed] [Google Scholar]
- 34.Polglase GR, Morley CJ, Crossley KJ, et al. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J Appl Physiol (1985). 2005;99(4):1453-1461. doi: 10.1152/japplphysiol.00055.2005 [DOI] [PubMed] [Google Scholar]
- 35.Kitchen MJ, Siew ML, Wallace MJ, et al. Changes in positive end-expiratory pressure alter the distribution of ventilation within the lung immediately after birth in newborn rabbits. PLoS One. 2014;9(4):e93391-e93391. doi: 10.1371/journal.pone.0093391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crossley KJ, Morley CJ, Allison BJ, et al. Blood gases and pulmonary blood flow during resuscitation of very preterm lambs treated with antenatal betamethasone and/or Curosurf: effect of positive end-expiratory pressure. Pediatr Res. 2007;62(1):37-42. doi: 10.1203/PDR.0b013e31806790ed [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
Statistical analysis plan.
eAppendix 1. Clinical management appendix.
eAppendix 2. Data management committee instructions.
eAppendix 3. Summary of stillborn, infants not receiving allocated treatment, deaths, and miscellaneous.
Nonauthor Collaborators. CORSAD Trial Investigators.
Data sharing statement.