Abstract
Lignin is a versatile biomass that possesses many different desirable properties such as antioxidant, antibacterial, anti-UV, and good biocompatibility. Natural lignin can be processed through several chemical processes. The processed lignin can be modified into functionalized lignin through chemical modifications to develop and enhance biomaterials. Thus, lignin is one of the prime candidate for various biomaterial applications such as drug and gene delivery, biosensors, bioimaging, 3D printing, tissue engineering, and dietary supplement additive. This review presents the potential of developing and utilizing lignin in the outlook of new and sustainable biomaterials. Thereafter, we also discuss on the challenges and outlook of utilizing lignin as a biomaterial.
Keywords: Biomass, Biomedical applications, Antioxidants, Tissue engineering, 3D printing
Graphical abstract
Highlights
-
•
Lignin's antioxidant, antibacterial, anti-UV and biocompatibility properties can be harnessed.
-
•
Modification and functionalization of lignin allows synthesis of enhanced biomaterials.
-
•
Biomedical applications of lignin-derived biomaterials include drug and gene delivery, biosensors, and tissue engineering.
1. Introduction
Lignin constitutes about 15–35% of lignocellulosic biomass obtained from the processes of wood and paper processing industries, and it is the second most abundant natural material on earth [1]. It consists of cross-linked polyphenolic structures which provide structural support for plants and it plays an important role in the formation of cell walls [2]. As of today, merely less than 2% of the industrial lignin waste are being used commercially [3]. Most of the utilized lignins are in the form of lignosulfonates which are used as an additive to building materials, while the remaining are mostly used as a low-grade fuel or simply discarded as waste [4]. The worldwide production of lignin is presently produced on a large production volume with an estimated market size of USD 954.5 million in 2019 with a projected annual growth rate of 2% by 2027 [5].
With a combination of large-scale production and the rising awareness of environmental sustainability, there has been an increasing interest in the valorization of lignin in different fields, such as materials and chemicals. In the biomedical field, natural polymers such as cellulose, alginate, chitosan, and chitin are some of the most commonly used materials in the development of drug delivery and tissue engineering [[6], [7], [8]]. In particular, cellulose, another main component of lignocellulosic biomass, has been recognized as a biomedical material for decades, due to its unique mechanical properties, availability, biodegradability and biosafety [9]. With years of investigation, various cellulose subcategories, such as cellulose micro/nanofibrils, cellulose nanocrystals, bacterial cellulose and nanowood, have been developed and shown their advantages in the area.
Unfortunately, lignin is still rarely known in the biomedical field. The heterogeneity of natural lignin makes it rather difficult to further develop lignin for biomedical applications. The heterogeneity of lignin arises from the multiple resources and extraction methods that are used to obtain lignin. Also, current research and application works on lignin-based biomaterials lacked the focus on in vivo biocompatibility and biodegradability. The lack of the fundamental understanding on the mechanism of lignin breakdown by the human body remains one of the greatest challenges of utilizing lignin as a biomaterial today.
Despite these drawbacks, lignin possesses many unique properties (antioxidant and anti-UV properties, as well as good antimicrobial activities), which are lacking in other natural polymers [10]. With such advantages, lignin could be potentially used as a bioactive compound to complement commonly-used biomaterials, such as cellulose. With an increase of interest in utilizing lignin as a functional material and its biomedical applications over the past 5 years as shown in Fig. 1, this shows lignin potential as a strong candidate for the development of new and sustainable biomaterials. In the past five years, a few groups have written a review of lignin use in biomedical applications [[10], [11], [12], [13], [14]]. A recent review by Liu et al. focused on the utilization of lignin nanoparticles and hydrogels in biomedical applications such as drug and gene delivery, and bioimaging [14]. Figueiredo et al. have also provided an overview of the preparation of lignin-based nanomaterials with antioxidant, anti-UV and antimicrobial properties in nanocomposites, drug delivery and gene delivery applications [12].
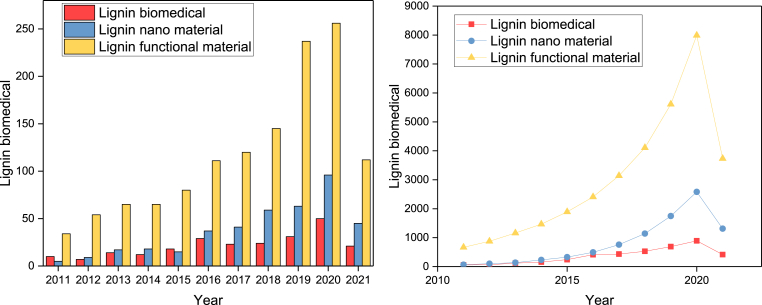
Fig. 1.
Total number of publications in the last 10 years. a) by number of publications; b) by number of citations per year. Search terms: “Lignin biomedical”, “Lignin functional material”, “Lignin nano material”. According to Web of Science, searched on 1 June 2021.
In contrast to previous reviews, this review provides a detailed overview on the current state and the progression of lignin as a biomaterial. We outlined advanced methods for lignin modification and highlighted unique benefits of lignin for biomedical applications, notably its antioxidant, antimicrobial, anti-UV, and biocompatibility. Moreover we provided an in-depth review of the potential applications of lignin in various biomedical fields, especially in tumor therapy, bioimaging/biosensors, tissue engineering and 3D printing (Fig. 2).
Fig. 2.
Overview of lignin as a biomaterial.
2. Background of lignin
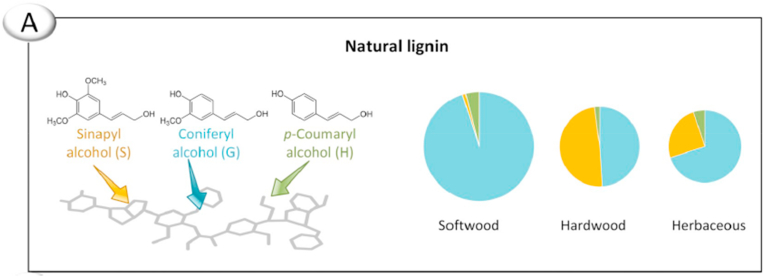
Lignin is biosynthesized from three hydroxycinnamyl alcohols or monolignols: p-coumaryl, coniferyl, and sinapyl. An enzyme-mediated dehydrogenative polymerization converts p-coumaryl, coniferyl, and sinapyl into p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) respectively [15]. Fig. 3 illustrates the conversion of monolignols into their corresponding polymer units.
Fig. 3.
Compositions of natural lignin in different types of plants. Reproduced from Ref. [13] with permission from Elsevier. Copyright 2019.
Lignins can be classified based on their origin. Lignins from softwood are composed almost entirely of G, have little to no S with low levels of H. Lignins from grass have roughly equal parts of G and S with more H units than softwood and hardwood. Lignins from hardwood is a mixture of G and S with traces of H. As hardwood lignins contain both G and S, it would have two or three methoxy groups per aromatic ring. The extra methoxy groups inhibit the aromatic rings from forming specific linkages, and hence hardwood lignins having more linear structures compared to softwood.
Monolignols are polymerized via oxidative coupling, catalyzed by laccases and peroxidases to generate monolignol radicals [16]. Electronic delocalization of these radicals propagates the polymerization reaction resulting in lignin having a high degree of heterogeneity. Due to its complex nature, an accurate characterization of lignins’ structure has eluded modern analytical techniques. Spectroscopic analysis by 31P NMR and fractionation of lignin into lignin-carbohydrate complexes alludes that even within the same sample, the structure of individual lignin molecules may vary widely, with some molecules displaying a high degree of linearity, others demonstrate extensive branching [17]. Hence its composition is normally illustrated by the abundance of H/G/S polymeric units and the types of inter-unit linkages in the biopolymer [18].
The inter-unit linkages of lignin comprise of ether bonds, methoxy, benzyl alcohol, phenolic hydroxyl, aliphatic hydroxyl, noncyclic benzyl ether, and carbonyl groups [19]. The numerous functional groups impart lignin with a distinctive reactivity to various chemical reactions and therefore, a large potential to chemically alter lignin.
2.1. Types of lignins
There exist several different types of lignin that are produced via different processing methods [20]. These include kraft, organosolv, soda lignins, as well as lignosulphonates. The most common of which is kraft lignin that accounts for about 85% of all lignin produced in the world.
Kraft lignin is created from kraft (sulfate) pulping, a process in which wood chips are dissolved in white liquor, a solution of sodium hydroxide and sodium sulfide, with an initial pH between 13 and 14 [21]. Sodium hydroxide deprotonates the phenolic hydroxyl groups of lignin while causing a secondary reaction that cleaves the α-O-4-ether and ß-O-4-ether links, the most frequent bonds in lignin. The mixture is cooked at about 170 °C, causing dissolution of lignin and hemicellulose (used by pulp and paper making industry), to produce black liquor. The lignin particles are then precipitated by acidifying the black liquor to pH 9 [22]. The precipitate can then be separated with filtration. Dark-colored and generally insoluble, kraft lignins can dissolve in alkali as they contain many phenolic hydroxyl groups. The molecular weight of kraft lignin ranges from 3700 to 19800 Da [23].
Organosolv lignins are produced from solvent pulping – an alternative to kraft and sulfite pulping – that has been reported to be more environmentally friendly. Most solvent pulping processes employ sulfur-free chemicals, such as water, acetone, ethylene glycol, formic, and acetic acid for delignification [24]. Temperatures for organosolv pulping typically range from 140 to 220 °C, hence solvents with high boiling points are used for pulping to be carried out under atmospheric pressure, although recovery of the spent liquor would be more laborious. Water is almost always used in conjunction with organic solvents to reduce vapor pressure and solubilize hemicellulose. Organosolv lignins are generated from precipitation, normally by adjusting concentration, pH, or temperature. Organosolv lignins are normally of high purity, low molecular weight, and narrow molecular weight distribution [20]. The molecular weight of organosolv lignin is between 4100 and 10800 Da [23].
Soda lignins are produced from soda pulping, by cooking pulp residue with sodium hydroxide at 170 °C, for about 1.5 h. The black liquor is then cooled, sieved to remove fibrous material, and slowly acidified to pH 5.5 which induces precipitation. After stirring for about 15 min, the mixture was further acidified to pH 3. Lignin was recovered by filtration [25]. Often derived from non-wood sources like straw, grass, and bagasse, soda lignins are characterized by their high phenolic hydroxy content and relatively low (but variable) glass transition temperature, and low molecular weight [20]. The molecular weight of soda lignin is between 1300 and 10400 Da [21]. Despite having a lower lignin recovery efficiency compared to kraft pulping, lignin produced from soda pulping is essentially sulfur-free [21]. Sulfur-free lignin is desirable as the presence of sulfur is an obstacle in downstream catalytic processes.
As the most widely available form of commercial lignin [26], lignosulphonates are produced via the sulfite process that extracts lignin using various salt of sulfurous acid. Sulfonate groups are incorporated into lignin, thus making lignosulphonates water-soluble and anionically charged [27]. Ultrafiltration is the most common method to separate lignosulphonates as it is relatively easy to upscale and the process is not particularly sensitive to pH or temperature. The molecular weight of lignosulphonates can also be selected by controlling membrane size. The average molecular weight of lignosulfonate from hardwood is around 12000 Da, while lignosulfonate from softwood is around 60000 Da [21]. Table 1 shows a summary of the different types of lignin.
Table 1.
Summary of different lignin types.
| Type of Lignin | Source of Lignina | Solubilitya | Mw (Da) | PDIa | Tg (°C)a | Aliphatic OH (%)b | Phenolic OH (%)b | N (%)b | S (%)c | Characteristicsb | Application |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kraft lignin | Softwood, Hardwood | Alkali, organic solvents | 3700–19,800 | 2.5–3.5 | 140–150 | 9.8–10.1 | 4.5 ± 0.3 | 0.05 | 1–3 | Lower S content, high phenolic OH groups | High antioxidant, antimicrobial, anti-UV activities [28]. Easy to modify for biomedical application |
| Lignosulfonate | Softwood, Hardwood | Water | 12,000–60,000 | 6–8 | 130–140 | – | 2.0 ± 0.2 | 0.02 | 3.5–8 | High S content, water soluble, high MW and PDI, self-association and agglomeration in aqueous solution | Antioxidant, antiviral, anticoagulant, antiulcerogenic, and antitumor [27]. Difficult to use for biomedical application due to high sulfur content. |
| Soda lignin | Annual plants | Alkali | 1300–10,400 | 2.5–3.5 | 130–140 | 2.5–3.1 | 4.4 ± 0.3 | 0.17 | 0 | Sulfur-free, more p-hydroxyl units and carboxyl groups, high silicate and Ni content | Antioxidant, antimicrobial [29]. May not be suitable due to low yield |
| Organosolv lignin | Softwood, Hardwood, Annual plants | Wide range of organic solvents | 4100–10,800 | 1.5–2.5 | 90–110 | 3.2–3.5 | 2.7 ± 0.3 | 0.02 | 0 | Sulfur-free, higher chemical purity, lower Mw, very hydrophobic | High antioxidant, antimicrobial, anti-UV activities [28]. Easy to modify for biomedical application |
3. Lignin properties for biomedical applications
3.1. Antioxidant
Molecules with the ability to scavenge or inhibit free radicals in living systems and foods in order to prevent oxidation are classified as antioxidants. To prolong their shelf-life and to preserve their inherent properties, antioxidants are normally added to many products, such as food, beverages, cosmetics, and pharmaceutical products. Some of the commonly used synthetic antioxidants are butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate (PG), and tert-butylhydroquinone (TBHQ) [33]. Unfortunately, despite their effectiveness as antioxidants, BHT and BHA are also known for their cytotoxicity and carcinogenicity, even in low concentrations [[34], [35], [36], [37], [38]]. Therefore, natural antioxidants which possess high biocompatibility are in great need to replace the currently used harmful antioxidants.
The intriguing free-radical scavenging (antioxidant) activity of lignin has long been studied by many scientists [39]. Faustino et al. and Azadfar et al. reported surprisingly high antioxidant activity possessed by lignin [[40], [41], [42]]. Discovery by Azadfar et al. showed that lignin's antioxidant activity is comparable to the commercially used antioxidants, guaiacol, and butylated hydroxytoluene. DPPH (2,2-diphenyl-1-picrylhydrazyl) antioxidant assay results suggested a percentage of inhibition of the DPPH radicals in the following order: guaiacol (103.6 ± 1.36) > butylated hydroxytoluene (103.3 ± 1.0) > ferulic acid (102.6 ± 0.8) > pretreated lignin (86.9 ± 0.3). Several studies have been conducted to investigate the free radical scavenging activity of lignin extracted from different sources, such as mulberry juice [43], pine cone extract [44,45], and cacao husk [46].
The antioxidant property of lignin is often attributed to the abundant amount of phenolic group and oxygen-containing functional groups which exist in lignin [[47], [48], [49], [50]]. These functional groups in lignin are able to terminate oxidative chain reactions by reacting with free radicals that are present in the system efficiently [51]. The phenolic hydroxy groups (ArO–H) in lignin allow it to scavenge the free radicals (R •) through hydrogen transfer and single electron transfer reactions according to this scheme: ArO–H + R • → ArO • + R–H [48]. The process is as illustrated in Scheme 1. The antioxidant capacity of lignin is dependent on the availability of the phenolic hydroxy groups and hence it varies according to the number of phenolic functional groups available.
Scheme 1.
Free radical scavenging mechanism by lignin which is stabilized by the resonance structure.
Additionally, several studies have reported that the antioxidant activity of lignin is dependent on its biomass source, extraction method, and post-treatment reactions [48,49,52]. For example, Kaur et al. found that unmodified lignin from sugarcane bagasse had higher antioxidant activity than lignin that was chemically modified via acetylation and epoxidation [49]. Also, in a study by Wang et al. that obtained three lignin fractions through successive fractionation of enzymatic hydrolysis lignin (EHL) which is a byproduct of ethanol production, lignin fractions with lower molecular weight were found to have higher antioxidant activity [52]. This is due to the presence of more phenolic groups present in lignin fractions with smaller molecular weights. As the scavenging activity of lignin is related to its polyphenolic structure, Wang et al. proposed that the antioxidant activity increases as the molecular weight of lignin fractions decrease in the case of their research.
Despite the antioxidant property of lignin, its application was once limited due to its poor miscibility with numerous polymer matrices [53]. However, various strategies have since been developed to chemically graft polymer chains onto lignin [[53], [54], [55], [56]]. It was also reported that the antioxidant property remains intact in lignin-based copolymers, such as lignin– poly(ε-caprolactone-co-lactide) (lignin-PCLLA) [57]. This further expands the possibility for lignin copolymers to be used in the development of novel materials for applications where the antioxidant property is required.
3.2. Antibacterial
A bacterial infection is caused by pathogenic bacteria. It leads to many infectious diseases, such as strep throat, pneumonia, tuberculosis, bronchitis, meningitis, etc. Although some of these diseases are treatable using antibiotics, they remain a major health problem in the world. Since the discovery of penicillin in 1928, many conventional antibacterial agents have been developed in the drug pipeline and have played a pivotal role in saving millions of lives [[58], [59], [60]]. However, its cost-effectiveness and easy accessibility have caused overuse leading to the development of antibiotic resistance [61]. Therefore, there is a need for the continuous development of novel alternative antibacterial agents. These include metal-based, polymer-based, and plant-based antimicrobial agents [62]. Even though silver nanoparticles (AgNPs) often display huge potentials as antibacterial, antifungal [63], and antiviral [64] agents, their low degradability is often of concern. Thus, an environmentally friendly antibacterial alternative is needed.
Lignin is a natural biopolymer which is a promising candidate for the synthesis of green materials that are non-toxic to the environment [62,65]. Moreover, the antibacterial property of lignin has been quite intensively studied over the years [66,67]. Its activity against bacteria is attributed to its phenolic fragment which possesses a double bond in the Cα = Cβ position of the side chain and a methyl group in the γ position [56,62,67]. In general, it was suggested that the antibacterial activity is attained by the contact of phenolic compounds with bacteria that leads to the damage of cell membrane and lysis of the bacteria [[68], [69], [70]]. Additionally, the antimicrobial property of lignin may function as a form of resistance to bacterial adherence. In a study by Larraneta et al., lignin hydrogels synthesized form dealkalinated lignin developed for biomedical application were reported to resist bacterial adherence of S. aureus and P.mirabilis up to five times compared to the typical medical material poly(vinyl chloride) (PVC) [71].
While antioxidant properties are detected in all types of lignin, its antimicrobial performances are dependent on the type of lignin and the microbial species [72]. Investigations conducted by Dong et al. on lignin extracted from residue of corn stover to ethanol production, the extracts exhibited antimicrobial activities, with specificity against Gram-positive bacteria and yeast, but not against Gram-negative bacteria or bacteriophage MS2 [73]. Similarly, Nada et al. reported that lignins precipitated from pulping liquor have specific efficacy towards Gram-positive bacteria, such as B. subtilis and B. mycoides, but not towards Gram-negative bacteria such as E. coli [66].
Due to the heterogeneity of lignin, its antimicrobial properties can also be influenced by the extraction conditions and botanical source [62,[73], [74], [75]]. Alzagameem et al. found that different kinds of lignin followed a trend in antimicrobial activity: organosolv of softwood > kraft of softwood > organosolv of grass. Kraft lignin generally has a reduced antimicrobial activity against Gram-positive bacteria due to the presence of carbohydrate content and aliphatic OH groups. On the other hand, Organosolv lignin has higher methoxyl content in general, which may act as secondary antiradical source [48]. Additionally, the study by Klapiszewski et al. also showed that the antimicrobial effectiveness of lignin is affected by extraction and preparation parameters such as pH and temperature [75].
3.3. Anti-ultraviolet
Ultraviolet (UV) light is the segment of the electromagnetic spectrum with wavelengths between 200 and 400 nm. UV radiation can be further divided into UV-C (200–280 nm), UV-B (280–320 nm), and UV-A (320–400 nm). A distinguishing feature of UV compared to the visible light spectrum is its ability to ionize molecules and hence induce chemical reactions [76]. Apart from its harmful effects on the human body such as damaging DNA molecules, the ability of UV light to photodegrade organic materials or polymers is well established [77,78]. Thus, there has been growing interest in the development of UV-shielding materials, particularly in bio-based and sustainable materials that are environmentally friendly [79]. Among other natural materials such as melanin and cellulose, scientists have also explored the use of lignin as a UV filter [[80], [81], [82]].
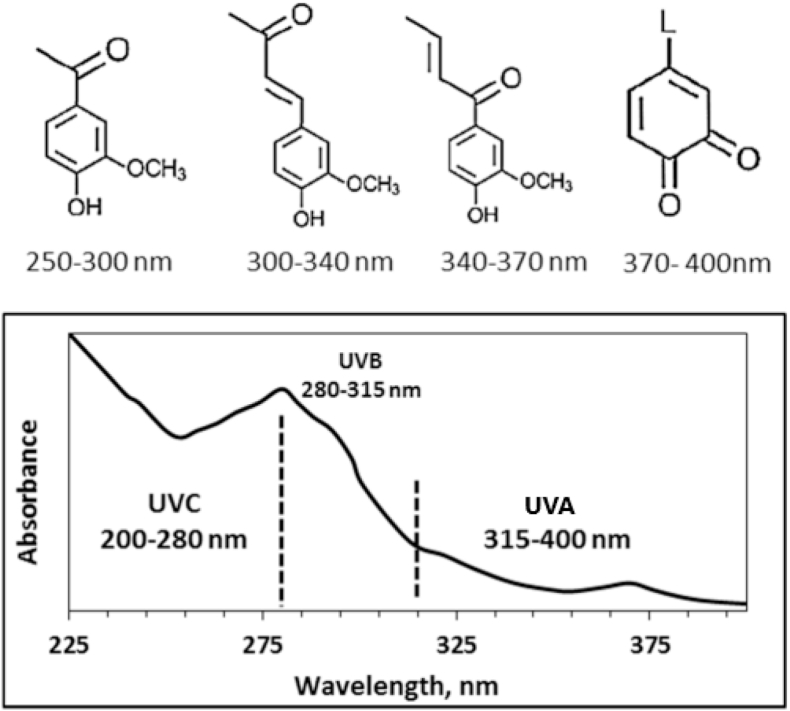
Lignin is well-known for its UV-absorbent properties [[82], [83], [84]]. In nature, lignin protects the cellulose fibers of plants that are vulnerable to UV [85]. The anti-UV properties of lignin are attributed to the UV-absorbing functional groups such as phenolic, ketones, and other chromophores [83,84,86] as shown in Fig. 4.
Fig. 4.
Chromophore groups in lignin and their absorption spectra in the UV light range. Reproduced and modified from Ref. [87] with permission from MDPI. Copyright 2020.
The use of lignin as a UV-shield has been thoroughly reviewed recently by Sadeghifar and Raauskas. [87]. They have reviewed the use of lignin in applications such as sunscreen products, packaging films, varnish, paint, and microorganism protection. Sadeghifar and Raauskas reported in their review that both modified and non-modified lignin with a blend of smaller than 10% with other material increases the UV blocking effect of commercial products such as lotion and creams [87]. It is noteworthy to point out that lignin also demonstrated a synergistic effect and increased the total UV protection when used in mixtures containing other synthetic anti-UV materials as compared to using the anti-UV synthetic material or lignin alone [87].
3.4. Biocompatibility/biosafety
Biocompatible polymers, such as polysaccharides and proteins have attracted a lot of attention from many researchers for biomedical purposes such as drug and gene nanocarriers [[88], [89], [90], [91], [92], [93]]. The key distinguishing feature of a biomaterial compared to any other materials is its ability to co-exist with the human body with an appropriate host response in a specific situation [94]. Biomaterials prepared from natural sources such as plants typically have good biocompatibility. Unlike other common plant-based natural polymers such as cellulose and pectin, there have not been as many studies investigating the biocompatibility of lignin [95].
An important concept that is closely related to biocompatibility is cytotoxicity. A study that evaluated the cytotoxicity of lignins from different sources using immortalized human keratinocyte cell line, HaCaT, and mouse fibroblast cell line was done using colorimetric assay which measures the ability of live cells to take up neutral red dye, the study revealed that the tested lignins (lignosulfonate, bagasse, steam explosion, and Curan 100) have cytotoxic effects only at very high concentrations based on their IC50 values at above 400 μg/mL [96]. Lignosulfonate was discovered to be the least cytotoxic in the two cell lines tested among the four lignins studied and the most cytotoxic lignin was found to be kraft lignin on HaCaT cells and Bagasse on 3T3 cells.
However, it is also important to note that native lignin and extracted lignin are different as the different chemical extraction processes alters both the physical and chemical properties of lignin, this includes the molecular weight which affects its solubility, chemical modification, varying aromatic content, and presence of impurities such as sulfur and ash [97]. High temperatures and harsh chemical processes are often used in the extraction of lignin, such as kraft process, often results in irreversible degradation of highly condensed lignin due to the reduction of ether bond linkages, especially β-O-4 bonds that causes the processed lignin to be chemically less reactive as compared to native lignin [98]. Therefore, it is crucial that studies are conducted for each different types of lignin for the biocompatibility and biodegradability.
4. Modification of functional groups
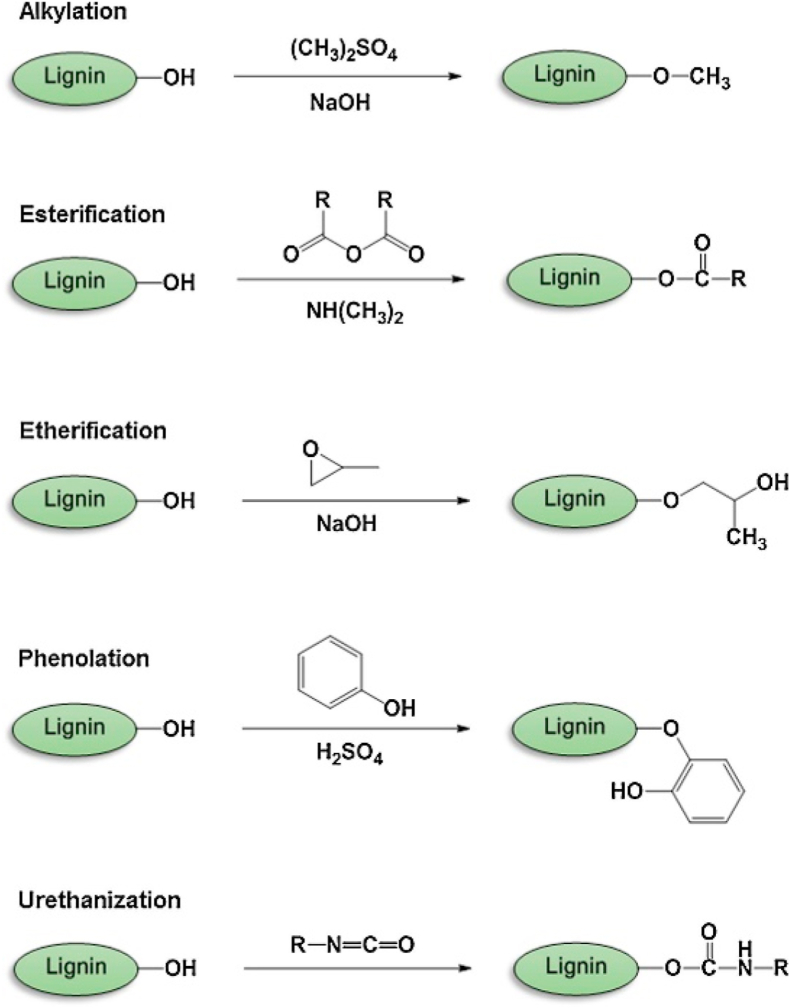
Modification of lignin can also take place without depolymerizing its innate structure. A variety of chemical modifications have been proposed, to improve the solubility in organic solvents, increase reactivity and decrease brittleness of lignin-based materials. More importantly, modified lignins allows incorporation of necessary functional groups which enhances the blending of lignin with other polymers in the development of new biomaterials and also enhance the biocompatibility of biopolymers [14]. Fig. 5 shows some of the common modifications to add new chemical sites to lignin.
Fig. 5.
Overview of chemical modifications of lignin. Reproduced from Ref. [12] with permission from Elsevier. Copyright 2017.
4.1. Functionalization of hydroxyl groups
Lignin possesses phenolic hydroxyl groups, and aliphatic hydroxyl groups at C-α and C-γ positions of the side chain. Altering these reactive phenolic hydroxyl groups can significantly affect the properties of the resulting material. One particularly useful change is the enhanced solubility of lignin when the hydroxyl groups form lignin polyol derivatives. As unmodified lignin exhibits poor solubility in many common organic solvents, dissolution of lignin is vital for structural characterization and valorization.
Modifications to lignin explore ways to incorporate new chemical sites onto lignin via pathways such as, alkylation/dealkylation [99,100], esterification, etherification [101], and phenolation [102] with significant focus being placed on functionalizing hydroxyl groups. The modification of lignin showed an important role in the development of new biomedical materials.
Esterification is amongst the most straightforward ways to alter lignins' hydroxyl groups. This reaction has been accomplished by a variety of reagents, including acid chlorides, acid anhydrides, and other acidic compounds [[103], [104], [105]]. The mechanical properties of esterified lignin were found to deteriorate less compared to alkylated lignin [106]. Esterification may also be carried out with anhydrides to enhance solubility in nonpolar solvents, by reducing polarity as the length of alkyl moiety increases [107,108]. Some esterified lignin had shown favorable characteristics of biomaterials. Hajirahimkhan et al. performed esterification of kraft Lignin and successfully synthesized methacrylated lignin (ML), up to 70% of hydroxyl group conversion, to form UV-curable lignin formulation. Their work showed that incorporation of ML improved hydrophobicity, thermal stability, increased cure percentage, and surface adhesion as compared to the control without ML [109]. Hydrogels that was synthesized through the esterification of lignin's hydroxyl groups, together with polyethylene glycol and carboxylic acid polymer was investigated by Larrañeta et al. The formed hydrogels incorporate up to 40% of lignin into a novel hydrogel which possesses antibacterial properties and ability to deliver hydrophobic drugs, in comparison to the control GANPEG hydrogel, lignin-based hydrogel had shown significantly greater resistance to two pathogens [71].
4.2. Graft copolymers
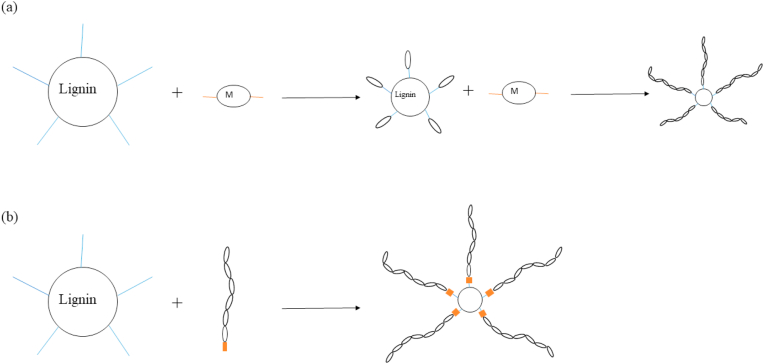
Another approach for the chemical modification of lignin is to bind polymeric chains to it through its reactive, predominantly hydroxyl groups. This results in a star-like branched copolymer, with lignin at its core. Fabrication of lignin graft copolymers can be accomplished via two distinct routes, the “grafting from” and the “grafting to” methods as shown in Fig. 6. The first approach is normally favored as it yields a larger graft density compared to the latter.
Fig. 6.
Schematic of two grafting approach: (a) “Grafting to” approach; (b) “Grafting from” approach.
“Grafting from” approach can be done via two methods, atom transfer radical polymerization (ATRP) [110,111] and ring-opening polymerization (ROP) [101,[112], [113], [114]]. Such polymerization approaches are able to endow lignin with more features suitable for biomedical applications. For example Liu et al. [111]polymerized 2-(dimethylamino)ethyl methacrylate (DMAEMA) with lignin. The copolymer, lignin-g-PDMAEMA could efficiently compact plasmid DNA, while grafts with short-chain PDMAEMA demonstrated high gene transfection efficiency. On the other hand, “Grafting to” is a relatively more difficult and unpopular method of producing lignin-graft copolymers, often requiring multiple step reactions [[115], [116], [117], [118]]. A study done by Kai et al. showed the potential of using kraft Lignin grafted with Poly(ethylene glycol) methacrylate (PEGMA) via ATRP was used as an antioxidant which can also enhance UV protection [119]. Also, Kim et al. showed how thermoresponsive lignin-based biomaterial formed via ATRP grafting of N‐isopropylacrylamide (NIPAM) on kraft lignin could have an interesting biomedical, biosensor and hydrogel applications, due to the unique thermal sensitivity of polyNIPAM lower critical solution temperature (LCST) behavior [110].
5. Applications of lignin-based materials
5.1. Lignin as drug and gene delivery vehicle
Drug and gene deliveries are used in both targeted delivery and controlled release of therapeutic agents which can improve the efficacy of the therapeutic agents. There is a need to discover enhanced natural and biocompatible materials to safely deliver the drugs to targeted areas. One of the important aspects in the development of drug and gene delivery systems is the search for better delivery vehicles which include the use of nanoparticles, encapsulation, and microspheres. In recent years, lignin-derived biomaterials have some promising potential as a drug and gene delivery vehicle due to their desirable properties such as anti-microbial, antioxidant, and biocompatibility.
Engineered nanoparticles are particularly useful in the area of biomedicine due to the larger surface to mass ratio as compared to other particles. This unique feature enables nanoparticles to have enhanced capability to bind, adsorb and carry useful compounds such as drugs, genes, and proteins [120]. In recent years, there has been an increasing interest in lignin-based nanoparticles for the development of drug and gene delivery.
5.1.1. Drug encapsulation
Lignin has tremendous potential in drug encapsulations due to its ability to encapsulate lipophilic drugs, which allows for controlled and/or targeted drug release which are applicable in cancer and tumor therapy. The versatility of lignin's unique characteristics and modification in the development of functional materials, such as its ability to self-assemble into nanoparticles as well as synthesis of grafted lignin via ATRP and ROP, further value adds lignin's potential as a biomaterial. For example, Alqahtani et al. shown the potential viability of utilizing cross-linked organosolv lignin nanoparticles (LNP) for oral drug delivery. In their work, they have successfully encapsulated a lipophilic drug model using curcumin in LNP with an average size of 104 nm with an encapsulation efficiency of 92%. The encapsulated curcumin was also found to be stable under storage conditions. In addition, in vitro release assays showed the high stability of the LNP in simulated gastric conditions and desirable slow release under intestinal conditions. The absorption and bioavailability study also showed that the curcumin-loaded LNP showed no toxicity to Caco-2 cells and it also enhanced curcumin cellular uptake and intestinal permeation compared to free curcumin. Incubation of LNPs on a Caco-2 cell monolayer led to a fivefold increase in apparent permeability compared to free curcumin. It was also showed that using LNP increases the bioavailability of curcumin by 10-folds as compared to unformulated curcumin. Their work showed the potential of LNP as a non-toxic enhancement for the absorption of lipophilic oral drugs [121].
Tortora et al. also investigated the ultrasound-assisted formation of oil-filled Kraft lignin microcapsules. The group showed that lignin microcapsules were viable for both drug release study of coumarin-6 in different mediums and biocompatibility for in vitro study of Chinese hamster ovary (CHO) cells. They have proven the non-cytotoxicity and successfully incorporated lignin microcapsules into the CHO cells. This work showed a promising outlook of the potential of using bio-based renewable lignin sources in the biomedical field [122].
Li et al. developed pH-responsive lignin-based complex micelles using purified AL. Self-assembly of quaternized AL and sodium dodecyl benzenesulfonate (SDBS) into complex micelles in ethanol and water mixture. The complex micelles were able to form uniform nanoparticles that were able to encapsulate more than 74% of the model drug, ibuprofen. These micelles were also able to exhibit pH-responsive behavior through phase changes in different pH. An in vitro release study of ibuprofen showed controlled release of the drug in different pH environment which resulted in more than 75% of ibuprofen drug being preserved in simulated gastric fluid (pH 1.2), and over 90% of the drug was released in simulated intestinal fluid (pH 7.4). This suggests the capability of lignin to be utilized in oral drug delivery systems [123].
Recently, Siddiqui et al. also studied the potential of self-assembling nanoparticles. In their work, they used solvent displacement and flash pH change to control and optimize the size of the blank Kraft lignin nanoparticle (BLNP). They further studied the viability of BLNP as a drug carried by studying the hemocompatibility, cytotoxicity, and genotoxicity studies on Drosophila melanogaster model organism which showed that BLNPs were biocompatible as compared to metallic and inorganic nanoparticles. Irinotecan was used as a model drug which was incorporated in the synthesis of drug-loaded lignin nanoparticles (DLNP). It was found that DLNP was able to have a good drug loading capacity and high encapsulation efficiency. This demonstrated the sustained release capability of DLNP with high drug loading, which was able to reduce the IC50 value of irinotecan by almost 3 folds. This showed the promising capability of lignin nanoparticles as a biomaterial of the future [124].
Bryne et al. proposed using novel AL conjugated with poly(lactic-co-glycolic acid) (L-PLGA) as a nanoparticle drug delivery system to enhance the efficacy of targeted therapies. L-PLGA nanoparticles were loaded with MEK1/2 inhibitor GDC-0623 and were tested in vitro on MDA-MB-231 TNBC cell line, loaded PLGA nanoparticles were used as a control. L-PLGA nanoparticles were found to be much smaller than PLGA nanoparticles and also had slower drug release with improved efficacy [125]. Their work showed the positive impact of grafted lignin nanoparticles which could support the importance of lignin as a biomaterial in drug encapsulations.
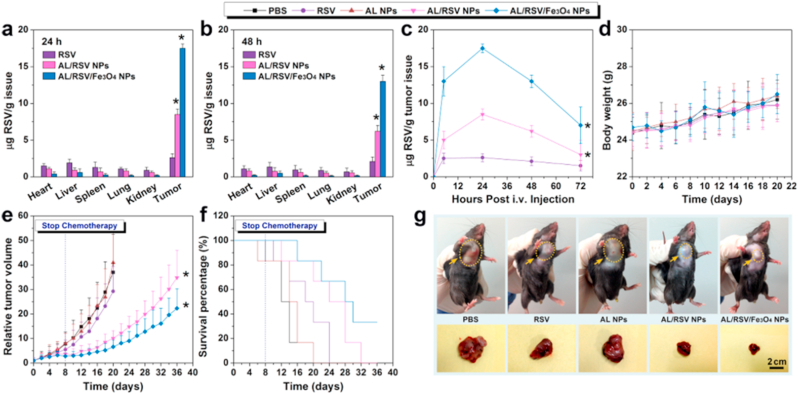
Lignin has been widely studied in cancer and tumor therapy due to its potential to act as a targeted drug carrier with its biocompatible properties as mentioned earlier. Dai et al. developed a novel green lignin nanoparticle without modification by using alkali lignin (AL) with a simple and scalable preparation technique. Their group formed perfect AL spheres via the self-assembly method and showed that self-assembly of AL loaded with bioactive resveratrol (RSV) and Fe3O4 magnetic nanoparticles were able to form a stable nano-drug carrier with proven efficacy of the drug with sustained release, stability, and drug accumulation as shown in Fig. 7 [126]. In another work, Figueiredo et al. developed lignin-derived nanoparticles using corn cob derived alkali lignin, which are round with a narrow size distribution. These nanoparticles have shown good stability at pH 7.4 and also low cytotoxicity in all the tested cell lines with a hemolytic rate below 12% after 12 h of incubation. On the other hand, pure lignin nanoparticles were shown to efficiently load hydrophobic and cytotoxic drugs and enhance the sustained drug release profile at both pH 5.5. and pH 7.4. They also reported that Fe3O4 infused lignin nanoparticles showed promising results in cancer therapy and diagnosis in magnetic targeting and MRI [127].
Fig. 7.
Tissue distribution with the different RSV formulations for 24 h (a) and 48 h (b). RSV content in tumor issues post-IV injection (c). Bodyweight (d), tumor inhibition (e), and survival rates (f) of tumor-bearing mice with different formulations. Tumor photographs from different groups on day 20 (g). Reproduced from Ref. [126] with permission from ACS Publications. Copyright 2017.
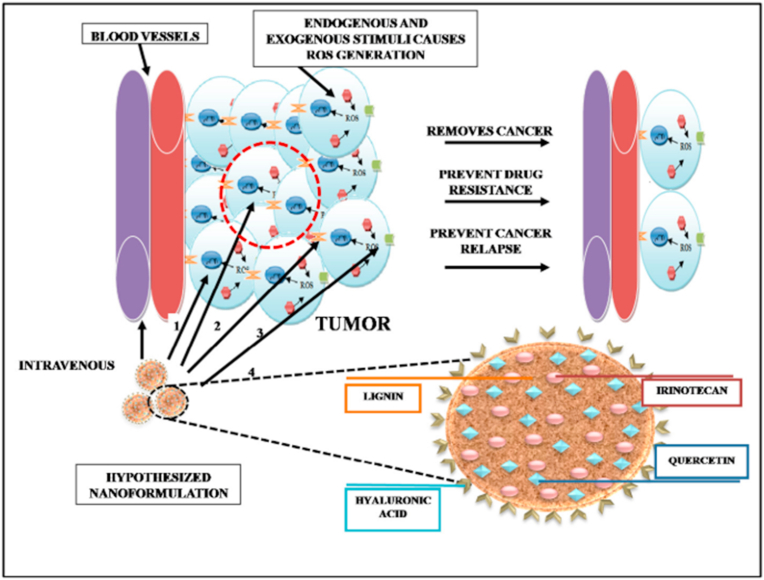
Further, Siddiqui et al. did some work on irinotecan and P-gp modulator loaded lignin nanoparticles that are functionalized with CD44 receptors for the treatment of colon cancer. In this work, they have successfully demonstrated the use of lignin's antioxidant properties to control the oxidative stress that can potentially prevent cancer relapse as part of the 4-way approach as shown in Fig. 8 [128]. This work has shown lignin's crucial potential as a viable nano-vehicle for therapeutic drugs along with its innate property as an antioxidant for its potential in treating cancer and also prevention of cancer relapse.
Fig. 8.
Novel 4-in-1 strategy to combat colon cancer, drug resistance, and cancer relapse. Hypothesized functionalized lignin nanoparticles can achieve this through the following 4 approaches. 1. Chemotherapeutic drug acts on cancer cells. 2. Antioxidants (lignin and quercetin) act on cells under high oxidative stress and prevent cancer relapse. 3. Quercetin modulates P-gp and overcomes drug efflux. 4. Hyaluronic acid actively targets nanoparticles to cancer cell through CD44 receptor and also makes them long-circulating. Reproduced from Ref. [128] with permission from Elsevier, Copyright 2018.
Zhou et al. grafted β-cyclodextrin (β-CD) into enzymatic-hydrolysis lignin (EHL) which self-assembled to form hollow nanoparticles to encapsulate an antitumor drug, hydroxycamptothecin (HCPT). The formed lignin nanoparticles were shown to have low toxicity. Their study showed good drug loading and encapsulation efficiency, along with a good sustained-release capability [129].
5.1.2. Gene delivery
Recent advances in gene delivery that utilizes lignin low cytotoxic nature as potential carriers. Presently, polyethylenimine (PEI) is commonly used for gene transfection, however it also contributes to the dose-dependent cytotoxicity [130]. To address this challenge, lignin was discovered to have the potential to be a viable better alternative to PEI due to its low cytotoxicity. However, lignin on its own does not have a binding site to the negatively charged DNA. In order to solve this, the use of lignin-based nanotubes and functionalization of lignin with potential DNA binding sites were investigated. Ten et al. synthesized lignin nanotubes (LNTs) that were shown to have low cytotoxicity to human HeLa cells up to concentrations of 90 mg/mL, which is 10 times higher than the tolerable concentration of carbon nanotubes (CNTs). This work showed the potential of using LNTs as a gene delivery vehicle. Formulated LNTs were able to enter HeLa cells without the need for auxiliary agents, AL-based LNTs were showed to be able to enter the cell nucleus. AL-based LNTs were also well capable of binding to DNA and delivering DNA into the nucleus. Making LNTs particularly useful in smart gene delivery systems. However, the immunogenicity of using LNTs needs to be further investigated, along with the effect of different lignin sources on the immune response [131].
Liu et al. formed kraft lignin graft copolymers using lignin macroinitiator and 2-(dimethylamino)ethyl methacrylate (DMAEMA) via atom transfer radical polymerization (ATRP) to form hyperbranched copolymers. They studied the copolymer's DNA binding capability, the formation of nanoparticles with plasmid DNA (pDNA), cytotoxicity, and gene transfection in cultured cells with reasonable success. The newly formed lignin-based copolymer was able to inhibit the migration of pDNA through the formation of nanoparticle complexes with sizes ranging from 100 to 200 nm at N/P of at least 5. They have also reported that the lignin copolymer with PDMAEMA showed much lower cytotoxicity as compared to PEI (25 kDa). Lignin copolymers with shorter PDMAEMA grafts have proven surprisingly good gene transfection efficiency in cell lines of Cos-7, Hela, and MDA-MB-231 [111].
In another work, Jiang et al. used kraft lignin to synthesize multi-armed cationic lignin–PGEA–PEGMA copolymer via ATRP. Their work showed that the multi-armed copolymers were able to efficiently compact pDNA into nanoparticles with sizes ranging from 150 to 250 nm at an N/P ratio of at least 10. The lignin-based copolymer showed high transfection efficiency which is comparable or much higher as compared to PEI control when tested in HEK 293T and Hep G2 cell lines. In addition, the copolymer also showed excellent antioxidant activity which made the cationic lignin-based copolymer a suitable candidate for gene delivery [132].
5.2. Biosensors and bioimaging
A biosensor can be defined as a sensor that consists of a bioreceptor that identifies and communicates with the analyte to give off a biological signal [133]. This signal is then transformed into an electrical signal via a transducer element. The obtained electrical signal signifies the proportionate amount of biochemical analyte present in the tested sample [134]. Biosensors are classified into electrochemical, optical, thermal, and piezoelectric biosensors depending on the transducer [133]. Due to the abundance of aromatic subunits, lignin exhibits strong compatibility with carbon-based materials and effective adsorption onto sp2-hybridized carbon surfaces [135,136]. Lignin is also capable of acting as a stabilizing agent for nanoparticles including silver [137]. In combination with its biocompatible property, lignin presents itself to be a suitable material for biosensing and bioimaging applications.
Biosensors based on oxides and lignin have been extensively studied by Jedrzak et al. for glucose detection via the catalytic oxidation mechanism of glucose. They presented the assembly of silica/lignin (SiO2/Lig) hybrid material that is suited for surface adsorption of glucose oxidase (GOx) [135]. It was reported that the hybrid material functionalized with lignin was able to immobilize 25.89 mg/g of GOx, two times more amount compared to SiO2 itself. The GOx-SiO2/Lig system acted as a biocatalyst with carbon paste electrode (CPE) and ferrocene (Fc) as redox mediators to form a second-generation glucose biosensor. Following this, the group fabricated a magnetite/lignin (Fe3O4/Lig) and magnetite/lignin/polydopamine (Fe3O4/Lig/PDA) material to immobilize GOx with favorable affinity [138]. Similarly, the two materials were combined with CPE and Fc for glucose detection in the concentration range of 0.5–9.0 mM. The CPE/Fe3O4/Lig/PDA/GOx/Fc biosensor was further studied and compared with other available commercial glucose biosensors. It was concluded that the CPE/Fe3O4/Lig/PDA/GOx/Fc biosensor has high sensing ability and good accuracy because of the high GOx enzyme loading of 29.44 mg/g of support. The group also tried modifying gallium oxide (Ga2O3) and zirconium (IV) oxide (ZrO2) with kraft lignin to produce supports capable of GOx adsorption [136]. 24.7 and 27.1 mg/g of GOx were successfully immobilized onto the surface of Ga2O3/lignin and ZrO2/lignin supports respectively. Afterward, Ga2O3/lignin/GOx and ZrO2/lignin/GOx systems were coupled with CPE and Fc, as shown in Fig. 9, to construct an electrochemical biosensor used for glucose examination.
Fig. 9.
Biosensor construction based on Ga2O3/lignin/GOx or ZrO2/lignin/GOx material. Reproduced from Ref. [136] with permission from MDPI. Copyright 2019.
Carbon quantum dots (CQDs) are biocompatible and non-toxic carbon core particles of less than 10 nm. They are capable of emitting radiation in the blue to red visible range. These minimally cytotoxic CQDs offer a prospective method for in vitro or in vivo biological imaging of cells. Lignin-derived CQDs with fluorescent ability were functionalized with amino-acids by Janus et al. [139]. Furthermore, when tested in DPPH assay, they displayed radical scavenging behavior and are non-cytotoxic in the presence of human dermal fibroblasts cells. Wang et al. demonstrated the conversion of alkali lignin into graphene quantum dots (GQDs) using a bottom-up synthesis approach [140]. O-aminobenzenesulfonic acid (A-acid) is a recyclable solid aromatic acid acting as a hydrotrope to dissolve lignin into lignin nanoparticles (LNPs) and subsequently transform them into lignin-based GQDs (LGQDs) via hydrothermal re-fusion. LGQDs display excellent fluorescence, water solubility, and prolonged photostability. Biocompatibility determination was conducted with mouse fibroblast L929 and 3T3 cell lines. The cell viability results indicated acceptable biocompatibility. As such, LGQDs were adapted for the detection of hydrogen peroxide (H2O2). Outcome from the experiments indicated exceptional detection ability even at low concentration of 0.13 nM, with initially high excitonic fluorescence followed by significant fluorescence quenching when H2O2 is present. Fluorescent carbon dots (CDs) produced via the hydrothermal treatment of lignin under H2O2 condition were synthesized by Chen et al. [141]. The CDs exhibited photostable blue photoluminescence and are water-soluble. Further investigation with A549 human lung adenocarcinoma cells demonstrated low cytotoxicity. Their imaging capabilities were also evaluated using HeLa cells through in vitro experiments and results revealed good photoluminescence. Therefore, the CDs are deemed suitable for bio-imaging.
Aadil et al. studied the usage of silver nanoparticles (AGNPs) stabilized by Acacia alkali lignin for hydrogen peroxide (H2O2) detection [142]. It was proclaimed that silver ions (Ag+) were reduced by active functional groups on lignin to silver metal (Ag0). The lignin stabilized AGNPs appear to have good sensitivity and a linear relation across a wide range of H2O2 concentration, from 10−1 to 10−6 M. When tested in vitro for their toxicity, they exhibit cytotoxic behavior in breast cancer MCF-7 and melanoma A375 cell lines. Lignin nanoparticles (LNPs) were proved to be suitable for immobilization of horseradish peroxidase (HRP) and GOX by Capecchi et al. [143]. Mediated by Concanavalin A (Con A), HRP and GOX were oriented and adsorbed onto LNPs in the right position. Cationic lignin (CATLIG) was determined to be the best polyelectrolyte. The Con A/HRP-GOX system was subsequently tested via ABTS and dopamine chromogenic substrates for the oxidation of glucose. Nitrogen-doped laser-scribed graphene (N-LSG) fabricated from lignin precursor via CO2 laser scribing process was reported by Lei et al. [144]. N-LSG boasted enhanced electrochemical activity and heterogeneous electron transfer rate which are the result of highly conductive porous N-LSG and enriched active edge-plane sites. N-LSG spray coated with MXene/Prussian blue (Ti3C2Tx/PB) to form Ti3C2Tx/PB-modified N-LSG electrodes for H2O2 detection. The electrodes are further functionalized with catalytic enzymes for glucose, lactate, and alcohol distinguishing purposes. Improved electrocatalytic activity for detection of the compounds was illustrated over a broad concentration range.
Yuan et al. first synthesized Fe3O4-lignin clusters through facile hydrogen bonding, following which the clusters were carbonized to form Fe3C@C composite core-shell magnetic nanoparticles [145]. Modified with an aptamer, the Fe3C@C-aptamer can be adopted for the detection of prion protein (PrPSc). Due to the distinct affinity of Fe3C@C-aptamer with PrPSc, the detection sensitivity of PrPSc was improved approximately 10 times alongside a good linear relationship. A layer-by-layer (LBL) film immunosensor was fabricated from antigenic peptide (p17-1) sequence and lignin onto gold electrodes by Cerrutti et al. [146]. The immunosensor was reported to be suitable for the specific detection of anti-p17 human immune deficiency virus (HIV) antibodies (Ab) at a concentration as low as 0.1 ng/mL. This is attributed to the lignin matrix maintaining the secondary α-helix structure of the peptide, which is essential for anti-p17 HIV AB detection.
In essence, lignin coupled with various compounds and different processing methods for functional biosensors can possibly be a developing research area in the biomedical field. These biosensing systems could be a promising alternative for more accurate and sensitive diagnostic applications. Nonetheless, more intensive testing needs to be conducted using these biosensors on biological fluids. In addition to in vitro applications, the in vivo function has yet to be explored. Table 2 summarises the key findings of lignin-related biosensors and bioimaging.
Table 2.
Summary and key findings of lignin-related biosensors and bioimaging applications.
| Type of Lignin | Matrix Material | Form | In-vitro Study | Key Findings | References |
|---|---|---|---|---|---|
| Kraft lignin | Silica Glucose oxidase |
Biosensor | – | Two-fold increase in glucose oxidase immobilization (25.28 mg/g) Successful linear response glucose detection (0.5–9 mM) with detection limit (145 μM) and high sensitivity (0.78 μA/mM) |
[135] |
| Kraft lignin | Magnetite Polydopamine Glucose oxidase |
Biosensor | – | High glucose oxidase loading (29.44 ± 2.39 mg/g) Comparable glucose detection sensitivity and accuracy with commercial biosensors |
[138] |
| Kraft lignin | Gallium oxide Zirconium (IV) oxide Glucose oxidase |
Biosensor | – | 24.7 and 27.1 mg/g glucose oxidase immobilization Successful glucose detection |
[136] |
| Lignin from wood | – | Bioimaging | Human dermal fibroblasts | Good fluorescence ability and photostable for 30 days About 10–30% of radical scavenging behavior Less than 20% decrease in fibroblasts, hence considered non-cytotoxic |
[139] |
| Alkali lignin | – | Biosensor | Mouse L929 fibroblasts Mouse 3T3 fibroblasts |
Excellent sensitivity for hydrogen peroxide detection as low as 0.13 nM Low cytotoxicity (≥90.32% cell viability) |
[140] |
| Lignin | – | Bioimaging | Human lung A549 adenocarcinoma cells HeLa cells |
Biocompatible and low cytotoxicity (≥80% cell viability) Good photoluminescence for imaging of HeLa cells |
[141] |
| Alkali lignin | Silver | Biosensor | Breast cancer MCF-7 cells Melanoma A375 cells |
Hydrogen peroxide detection across 10−1 – 10−6 M with high sensitivity and linear relationship Cytotoxic characteristics (≤30% cell viability) |
[142] |
| Organosolv lignin | Concanavalin A Horseradish peroxidase Glucose oxidase |
Biosensor | – | Suitable for glucose detection using chromogenic substrates Better or comparable limit of detection at 0.85 μM |
[143] |
| Lignosulfonate | Nitrogen MXene/Prussian blue |
Biosensor | – | Successful detection behavior for hydrogen peroxide (0–10 mM), glucose (10 μM–5.3 mM), lactate (0–20 mM) and alcohol (0–50 mM) across broad concentrations | [144] |
| Corn stover lignin | Magnetite Anti-prion protein aptamer |
Biosensor | – | 10-fold sensitivity improvement and successful detection of prion protein across 0.1–200 ng/mL | [145] |
| Organosolv lignin | Antigenic p17-1 peptide sequence | Biosensor | – | Successful detection of specific anti-p17 human immune deficiency virus antibodies as low as 0.1 ng/mL | [146] |
5.3. Tissue engineering
In the field of regenerative medicine, tissue engineering encompasses the development of biocompatible functional materials through the combination of multidisciplinary principles. Tissue engineering functions as an ingenious way for the recovery, replacement, improvement, or preservation of specific biological tissue or organ. Owing to its non-toxic, ultraviolet protective, antioxidant and antibacterial properties of the complex lignin macromolecule, multiple studies have been conducted on the probable use of lignin in different types of materials and processing methods for tissue engineering applications.
5.3.1. Hydrogel
Hydrogels are three-dimensional polymeric networks that are able to absorb a large amount of fluid and retain them within itself [147]. Hydrogels have been one of the promising candidates in tissue engineering as hydrogels are able to trap a great quantity of water within its extended polymer network to mimic the surrounding tissue environment [148]. There have been many developments in recent years to shift the use of synthetic polymers to natural polymers for their biocompatibility and low cytotoxicity which are suitable for use in a variety of biomedical applications [149]. Lignin renders itself as a suitable choice of natural biocompatible polymer and a versatile choice in the development of hydrogels for tissue engineering. In comparison to commonly used materials for hydrogel such as polyethylene glycol, lignin is able to bring special properties such as increased mechanical strength, adhesiveness, as well as antioxidant and antimicrobial properties [150] which are desirable for biomaterial applications.
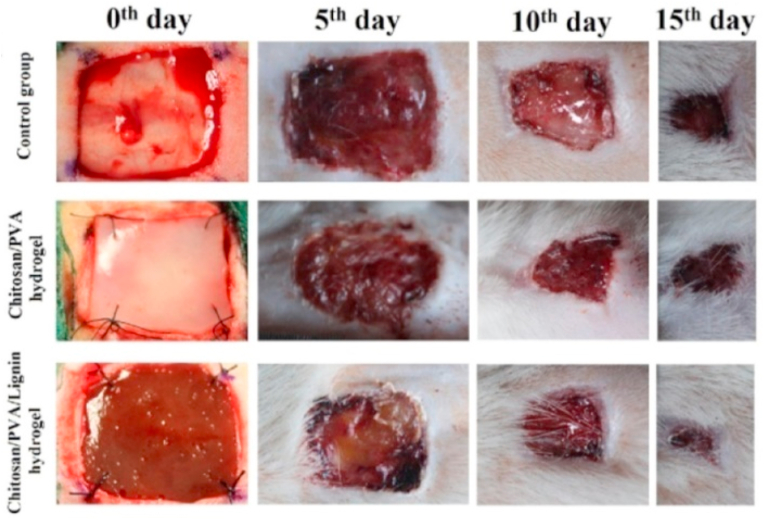
To reduce infection and promote wound healing, hydrogel consisting of lignin has been widely investigated. Zhang et al. used developed lignin-chitosan-PVA composite hydrogel for wound healing [151]. While PVA-chitosan has been previously studied to have excellent biocompatibility and antibacterial properties, it has poor mechanical strength. By incorporating lignin into the hydrogel system, his team was able to improve the mechanical strength to around 47 MPa with good protein adsorption capacity and wound compatibility. Wound healing studies shown in the mouse model showed faster healing as compared to the control and PVA-chitosan hydrogel as illustrated in Fig. 10.
Fig. 10.
Photographs of wounds with control group (no dressing), LCPH0 dressing, and LCPH1 dressing on the 0th, 5th, 10th, and 15th day. Reproduced from Ref. [151] with permission from Elsevier. Copyright 2019.
Correspondingly, Ravishankar et al. established the use of alkali lignin as an ionotropic cross-linker with chitosan to fabricate chitosan-alkali lignin hydrogels [152]. The hydrogels were proven to be non-cytotoxic when tested in vitro against Mesenchymal stem cells and to zebrafish at a maximum concentration of 100 μg/mL. Moreover, the hydrogel surface is suited for cell adhesion and proliferation. Scratch wound healing assay with mouse fibroblast NIH 3T3 cells displayed cell migration attributes. In another study, Mahata et al. grafted hydrophilic polyoxazoline copolymer onto lignin and further altered it with triazole moiety to strengthen antimicrobial and antibiofilm capabilities [153]. The resultant compound was developed into a hydrogel and investigated for its potential as an anti-inflammatory material. Hydrogel with 20 wt% lignin was selected for in vitro and in vivo studies. In in vitro experiment, it displayed anti-inflammation ability via reducing gene expression of iNOS and IL-1β of inflamed mouse macrophage cells induced by lipopolysaccharide (LPS), a component of outer membrane of gram-negative bacteria, often used to establish an inflammatory model to stimulate the release of inflammatory cytokines including IL-1β [154]. In addition, the hydrogel exhibits antioxidant capability. Further in vivo investigations were conducted on burnt and infected wounds of rats. Results revealed promising abilities such as infection prevention and wound healing capabilities. Similarly, Xu et al. extracted lignin from coconut husks and integrated it into thermoresponsive polyurethane-based nanogel for wound-dressing application [155]. The incorporation of lignin did not significantly affect gelation and rheological properties, however critical micelle concentration slightly increased. Through in vitro study on LO2 cells, the nanogel exhibited excellent biocompatibility and nontoxicity. It was also evidenced to be capable of possessing radical scavenging behavior. In vivo studies using mouse burn wound models demonstrated the wound healing potential of the nanogel.
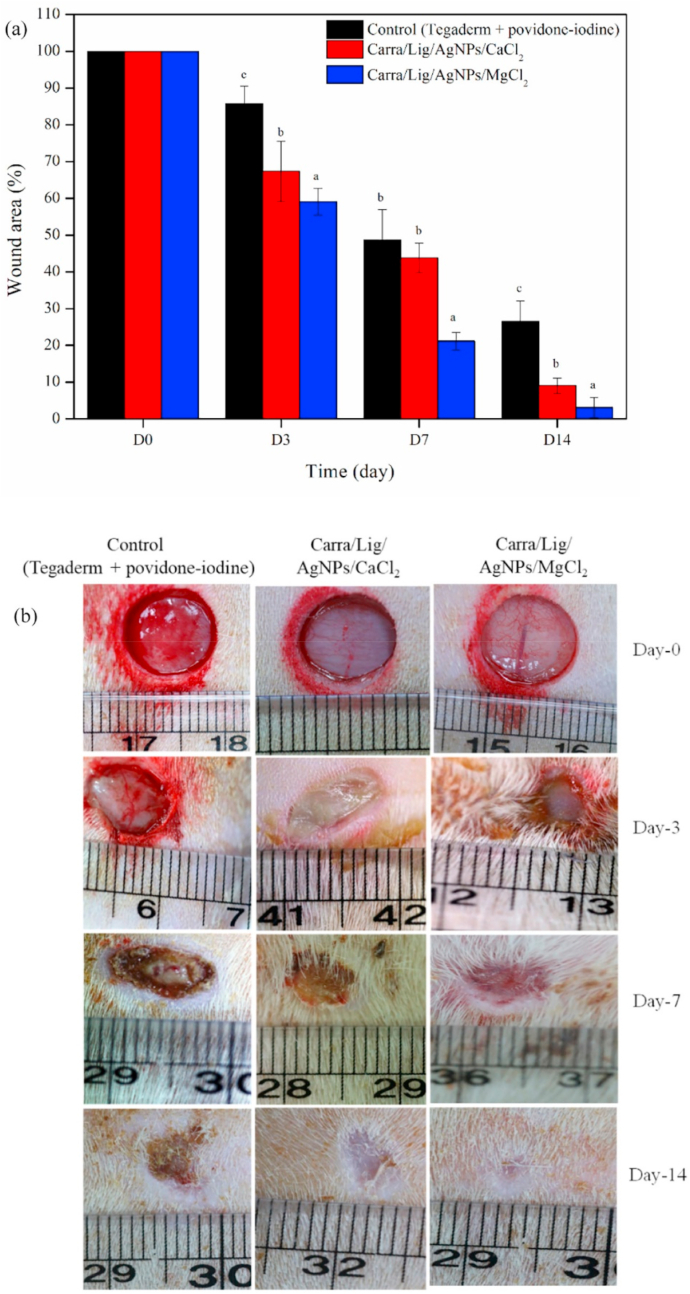
Silver nanoparticles are known to have remarkable antibacterial characteristics which could aid in healing injuries [156]. The potential of coupling these silver nanoparticles along with the properties of lignin in hydrogels was evaluated in some research. Li et al. modified sodium lignin sulfonate by grafting amino group to attain lignin amine (LA) [157]. Afterward, LA was cross-linked with poly(vinyl alcohol) PVA to form a hydrogel. Subsequent combination with silver nitrate solution led to in situ reduction and formation of silver nanoparticles. As such, the hydrogel exhibits improved antibacterial properties and was proven in-vitro against S. aureus and E. coli bacteria. The hydrogel also displayed biocompatibility in toxicity tests with L929 cells. Jaiswal et al. demonstrated the use of lignin as a reducing and capping agent for the synthesis of silver nanoparticles (AgNPs) in carrageenan hydrogel matrix cross-linked with divalent cations [158]. The physical properties of the hydrogel were also improved as a result of divalent cation cross-linking. In vitro study with mouse fibroblast L929 cell line proved acceptable biocompatibility. In vivo investigation detailed in Fig. 11 using Carra/Lig/AgNPs/MgCl2 hydrogel illustrated superior wound healing capabilities on incision-induced Sprague-Dawley rats.
Fig. 11.
Wound healing effect of carrageenan-based nanocomposite hydrogels evaluated by (a) the wound area and (b) the apparent photographs are showing wound healing progress. Reproduced from Ref. [158] with permission from Elsevier. Copyright 2020.
The addition of water-soluble glycinated kraft lignin (WS/KL) into hyaluronan (NaHy) hydrogels were presented by Musilova et al. [159]. Both WS/KL and NaHy were found to be compatible with each other. Hydrogel with lignin content up to 3% (w/w) enhanced creep resistance and creep recovery. However, the swelling ability was reduced. Cytotoxicity studies evaluated NaHy hydrogel with incorporated WS/KL to be non-toxic as cells can migrate and proliferate within the hydrogel structure. Chandna et al. designed nanocomposite pH-stimulated poly(acrylic acid) hydrogels ingrained with photodynamic nanoconjugates derived from lignin [160]. The nanoconjugates were formed by binding rose Bengal photosensitizer with silver and gold-based lignin nanocomplexes. They possess good physiological stability and photophysical ability. They reported that the hydrogel doped with lignin-based nanoconjugates are favorable against Candida tropicalis, providing maximum laser-assisted antifungal inhabitation at low concentration. Polar lignin-carbohydrate complexes (LCCs), LCC-48 and LCC-72, were prepared by Zhao et al. and subsequently form hydrogel carriers [161]. LCC-48 and LCC-72 were cross-linked with polyethylene glycol diglycidyl ether for human hepatocyte (L-02) cell culture application. The outcome from the cell culture study showed significant adhesion of hepatocytes onto LCCs porous structure. Additionally, there was improved liver cell proliferation and metabolic activity on the LCCs carriers, thus suitable for liver cell culture.
To sum up, lignin was proven to be a viable ingredient in the formation of lignin-based hydrogels. The hydrogels presented in the various studies were capable of wound healing with no cytotoxic effects. Hence, making them a potential biocompatible material in the field of regenerative medicine.
5.3.2. Nanofibers
To fabricate nanofibers of uniform and controlled diameters, electrospinning is a relatively simple and versatile technique widely available [162]. The porous morphology and nanoscale fibers make electrospun matrixes potentially suited for tissue engineering applications such as cell culturing scaffolds [163].
Salami et al. electrospun PCL with 0–15 wt% lignin to produce a PCL/Lig nanocomposite scaffold [164]. The scaffold containing 10 wt% lignin has suitable physical, morphological, and mechanical properties for fibroblast cell proliferation. Utilizing atom transfer radical polymerization, Kai et al. grafted poly(methyl methacrylate) (PMMA) onto lignin to form lignin-PMMA copolymers [165]. Molecular weights and thermal properties can be modified by adjusting the ratio of lignin to PMMA ratio. Afterward, the lignin-PMMA copolymers were mixed with poly(ε-caprolactone) (PCL) and electrospun into PCL/lignin-PMMA nanofibers. The biocompatibility of these nanofibers was investigated with human dermal fibroblasts (HDFs). The HDFs cells exhibited favorable interactions, proliferation, and attachment with PCL/lignin-PMMA nanofibers.
Wang et al. synthesized lignin-polycaprolactone (PCL) copolymer through solvent-free ring-opening polymerization of alkali lignin and ε-caprolactone [166]. The copolymer was integrated into the PCL matrix and electrospun to form PCL/lignin-PCL nanofibers for potential nerve tissue regeneration. Mechanical properties were improved and the nanofibers displayed good antioxidant properties upon the inclusion of lignin-PCL. Bone marrow mesenchymal stem cells were used to evaluate the biocompatibility of PCL/lignin-PCL nanofibers and results indicated enhanced cell viability with respect to PCL. Furthermore, Schwann cells and dorsal root ganglion (DRG) neurons were cultured on the nanofibers. The outcome was positive with stimulated Schwann cell proliferation, boosted myelin protein expressions of Schwann cells, and promoted neurite outgrowth of DRG neurons. Likewise, through ring-opening polymerization, Kai et al. demonstrated the combination of 10–50 wt% alkylated lignin and poly(lactic acid) (PLA) [167]. Following which the PLA-lignin copolymers were mixed with poly(l-lactide) (PLLA) to produce PLLA/PLA-lignin nanofibers using the electrospinning technique. The nanofibers exhibited positive antioxidant behavior across 72 h. Additionally, rat PC12, human dermal fibroblasts, and human mesenchymal stem cells were cultured s onto the nanofibers to investigate the biocompatibility of PLLA/PLA-lignin nanofibers. It was concluded that the nanofibers incorporated with lignin offer better cell proliferation and are suitable for tissue engineering scaffolds.
Saudi et al. incorporated 0–5 wt% lignin into poly(vinyl alcohol) (PVA)-poly(glycerol sebacate) (PGS) to create nanofibers via electrospinning for nerve tissue engineering application [168]. The higher amount of lignin led to decreased fiber diameter and increased elastic modulus. Also, the presence of lignin improved cell proliferation and exhibited the ability for neural cell differentiation. Wang et al. reported on the use of electrospun lignin and PCL to form lignin/PCL nanofibrous films for hydroxyapatite (HAp) cultivation [169]. Since lignin contains numerous hydroxyl groups, these reactive sites can accommodate Ca+ ions. After which, successive Ca+ and PO43− coprecipitation promoted HAp nucleation on lignin/PCL fibrous substrate. As observed in Fig. 12, HAp nucleation has occurred. The following surface biomineralized lignin/PCL-HAp composites were investigated for their oesteoconductivity capability. Improved osteoblast cell viability and proliferation were observed, signifying its suitability as an interface for bone treatment.
Fig. 12.
Formation of HAp minerals facilitated by lignin. (a) SEM images showing the absence and presence of lignin for the formation of HAp after a two-day culturing. (b) SEM images of lignin/PCL nanofibrous film showing that the entire surface of the film was covered with HAp after incubation for 5 days. (c) High-magnification SEM image showing a platelet-like structure, which is typically found in natural HAp. (d–f) Results from EDX elemental analysis showing the distribution of Ca and P. Reproduced from Ref. [169] with permission from ACS Publications. Copyright 2019.
Polycaprolactone (PCL) fibers loaded with 0–15 wt% lignin nanoparticles were electrospun by Amini et al. for nerve regeneration purposes [170]. In vitro preliminary study of the PCL/lignin fibers was conducted with rat pheochromocytoma (P12) cell line and human adipose-derived stem cells (hADSCs). Results indicated improved cell viability and differentiation with increasing lignin content. In vivo study using adult male Wistar rats to evaluate sciatic nerve regeneration was conducted. It was concluded that PCL fiber implants containing 15 wt% lignin were beneficial for peripheral nerve regeneration by promoting axonal sprouting. β-butyrolactone was grafted onto alkali lignin through ring-opening polymerization to form lignin-poly(3-hydroxybutyrate) (PHB) copolymer as validated by Kai et al. [171]. The copolymer was then blended with PHB and electrospun into nanofibers. By incorporating lignin-PHB, the mechanical properties of the resultant nanofiber were enhanced. Furthermore, the antioxidant behavior of PHB/lignin nanofibers can be altered accordingly. NIH-3T3 fibroblast cells were cultured in vitro onto the nanofibers and displayed acceptable confluence and biocompatibility. In vivo investigation on rat models also suggest the implanted PHB/lignin nanofibers were non-irritant and biocompatible. Liang et al. determined the use of electrospun nanofibers comprised of poly(ε-caprolactone) (PCL) combined with poly(ε-caprolactone)-grafted lignin (PCL-g-lignin) obtained via solvent-free ring-opening polymerization for osteoarthritis (OA) therapy [172]. Antioxidant and mechanical properties proved to be tunable by adjusting the ratio of PCL-g-lignin and PCL respectively. The nanofibers exhibited minimal cytotoxicity and improved cell viability, alongside superior antioxidant and anti-inflammatory properties as evidenced from in vitro oxidative stress stimulated human chondrocytes and in vivo OA rabbit model studies. Antioxidant enzymes were triggered through autophagy mechanism resulting in inhabitation of reactive oxygen species (ROS) formation. Also, in vivo macroscopic and histological analysis indicated successful suppression of OA development and cartilage degradation.
Morganti et al. fabricated natural beauty masks from electrospinning of chitin nanofibril-lignin block co-polymeric nanoparticles and other natural biocompatible polymers [173]. The fibers are capable of trapping and releasing various active ingredients such as sodium ascorbyl phosphate, melatonin, beta-glucan, and nicotinamide. Cytotoxicity was studied and was deduced in-vitro to be non-toxic to keratinocytes and fibroblasts cells. Cytokine expression was also reduced. Anti-aging and restorative properties were established as evidenced by the release of Metallo Proteinase I and elevated production of collagen type I. The effectiveness of the beauty masks was examined on 30 women volunteers with evident photoaging. The outcome from the study showed protective and rejuvenating activity thus making these masks suitable for mitigating skin premature aging.
In general, electrospinning is an easy, flexible, and cost-effective processing technique for the fabrication of nanofibers with consistent physical and chemical properties. As discussed earlier, nanofibers incorporated with lignin were proved to be biocompatible and non-cytotoxic in widely tested in-vitro and in-vivo experiments. Therefore, electrospun lignin nanofibers are potentially suitable for tissue engineering scaffolds in the biomedical industry. Although many of the lignin combined nanofibers were determined to be biocompatible, insufficient in vivo research hinders the application as implants in humans.
5.3.3. Additive manufacturing
The popularity of additive manufacturing technology, also known as 3-dimensional (3D) printing, has been on the rise due to its adaptability and promising approach for tissue engineering [174]. 3D printing allows healthcare professionals to produce customizable and personalized forms of treatment for injured individuals. Furthermore, increasing demands for sustainable materials have driven the incorporation of natural polymers such as lignin in 3D printing technologies. Fused deposition modeling (FDM), direct ink writing (DIW), stereolithography (SLA), and digital light processing (DLP) are some examples of commonly employed 3D printing techniques.
Polylactic acid (PLA) is an excellent thermoplastic biomaterial for biomedical applications owing to its biodegradability and biocompatibility. Tanase-Opedal et al. incorporated 20 and 40 wt% sulfur-free soda lignin into PLA matrix and showed that the PLA/lignin blends are suitable for FDM 3D printing [175]. The biocomposites with lignin integrated led to significant improvement in antioxidant activity compared to neat PLA. In a separate study, Dominguez-Robles et al. successfully extruded PLA filament containing kraft lignin, as evidenced in Fig. 13, which displayed localized and prolonged antioxidant capabilities for wound healing applications [176]. 0–3 wt% lignin was coated onto PLA pellets with the aid of biocompatible castor oil and extruded at 200 °C. The addition of antibiotics, tetracycline, for wound infection prevention was also demonstrated and effectively reduced S. aureus adhesion. The FDM-based filament can be 3D printed into meshes of customizable geometry catered to a patient's wound. Curcumin, a model drug, was applied onto the mesh to study diffusion characteristics and results indicated permeation rate was influenced by mesh dimensions.
Fig. 13.
Photographs of (A) PLA and PLA coated pellets; (B) LIG and TC containing PLA filaments; (C) LIG and TC containing 1 cm × 1 cm squares prepared using 3D printing; (D) different shapes printed using the filament containing 2%(w/w) LIG. Reproduced from Ref. [176] with permission from MDPI. Copyright 2019.
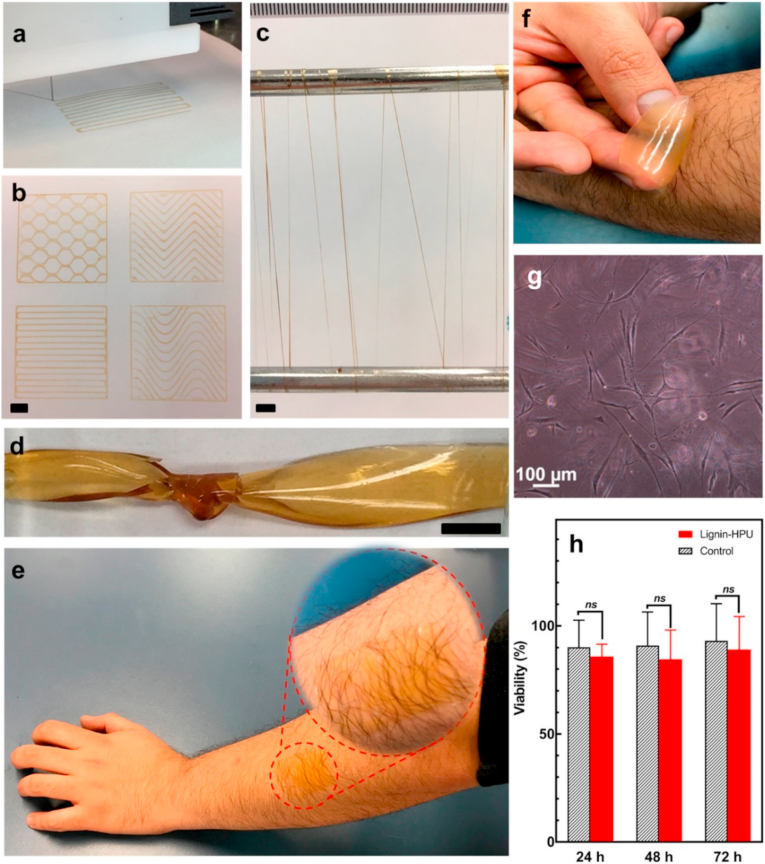
With the advancement in 3D printing technologies, soft materials such as hydrogels can also be printed with the use of DIW 3D printing technique. 0–2.5 wt% lignin was introduced as a physical cross-linker in hydrophilic polyether-based polyurethane (HPU) hydrogels by Oveissi et al. [177]. The addition of 2.5 wt% lignin increased fracture energy, Young's modulus, and lap shear adhesiveness of the hydrogel, which is primarily affiliated to increased hydrogen bonding within the lignin-HPU network. In addition, it also displays load recovery performance of 95% in loading/unloading cycles. Cell viability was examined by culturing human dermal fibroblast onto the lignin-HPU film. Outcome up to 72 h imply no significant changes in cell viability, thus illustrates the film's biocompatibility and supportive cell growth ability. The group established that the lignin-HPU hydrogel has suitable and adjustable rheological properties that can be extruded using direct ink writing (DIW) 3D printing technique. Fig. 14 showcases the 3D printability and biocompatibility of the lignin-HPU hydrogel.
Fig. 14.
Different forms of lignin-HPU hydrogel: (a) extruding the lignin-HPU ink from the 3D printer nozzle, (b) various patterns printed from lignin-HPU hydrogels, (c) dry-spun hydrogel fibers from lignin-HPU, and (d) a knot formed with a fully swollen lignin-HPU hydrogel films. Scale bars: 5 mm. (e) Lignin-HPU patch on arm, (f) peeling off the hydrogel film without any attached hair and without any pain, (g) human dermal fibroblast cells on lignin-HPU film, (h) viability of human dermal fibroblast cells on lignin-HPU film versus controls (no material) at over 1, 2 and 3 days Reproduced from Ref. [177] with permission from ACS Publications. Copyright 2018.
A more recent hydrogel DIW-based bioink made from cellulose nanofibril (CNF), alginate, and colloidal lignin particles (CLP) was prepared by Zhang et al. [178]. Antioxidant properties were brought about when CLP was incorporated into the CNF-alginate-CLP nanocomposite scaffolds. The antioxidant behavior is dependent on the concentration of CLP added. Additionally, raising CLP concentration increased the hydrogel viscosity which allows better shape accuracy and 3D printing resolution. After printing, the scaffolds displayed shape stability and storage up to 7 days in Dulbecco's phosphate buffer. The rehydration ratio was above 80%, illustrating its high water-retention capability. No negative effect of CLPs on hepatocellular carcinoma cell line HepG2 was evidenced. HepG2 cells were proliferated on the interior and exterior of the porous scaffolds, implying good biocompatibility.
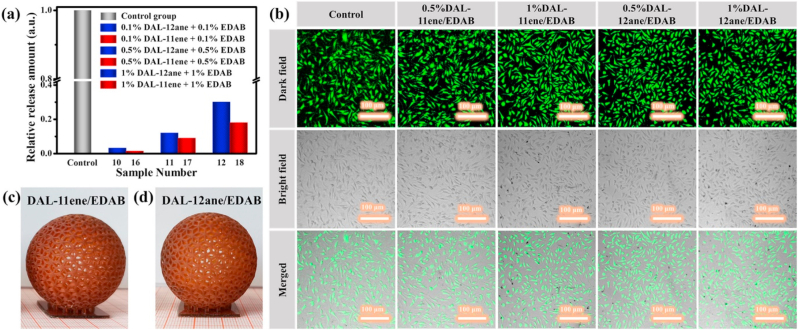
DLP 3D printing technique relies on the photopolymerization of a photopolymer matrix in the presence of photoinitiators. As such, Zhang et al. synthesized two alkylated dealkaline lignin, DAL-11ene, and DAL-12ane, via one-step esterification reaction between dealkaline lignin (DAL) and undecanoyl chloride or dodecanoyl chloride [179]. Both DAL-11ene and DAL-12ane exhibited better photoinitating efficiencies compared to DAL. Co-initiator, ethyl 4-(dimethylamino)benzoate (EDAB) was combined as an additional photoinitiator for DLP 3D printing. Biosafety of 0.5–1 wt% DAL-11ene/EDAB or DAL-12ane/EDAB as photoinitiators were evaluated by photopolymerization with polyethylene glycol diacrylate (PEGDA) monomer. The tablets printed were incubated with L929 mouse fibroblasts followed by live/dead staining. L929 cells were well proliferated on both tablets indicating excellent biosafety and cytocompatibility. Successful DLP 3D printing capability was demonstrated by adding 1 wt% of either photoinitiators into 1,6-hexanediol diacrylate (HDDA) monomer. Cytocompatibility and 3D printed structures are shown in Fig. 15.
Fig. 15.
(a) Relative release amount of PIs from samples using DAL-12ane/EDAB and DAL-11ene/EDAB as PIs. (b) Confocal laser scanning microscope images of L929 cells incubated on poly PEGDA tablet fabricated by using different PIs. Hollow spheres fabricated by DLP 3D printed using HDDA as a monomer and (c) DAL-11ene/EDAB or (d) DAL-12ane/EDAB as PIs. Reproduced from Ref. [179] with permission from ACS Publications. Copyright 2020.
To summarize, lignin was demonstrated to be a possible additive into a polymeric matrix and suitable for 3D printing applications. Moreover, the blended materials exhibit desirable bioactive properties. These properties together with the customizability of 3D printing are ideal for patient-specific treatment. However, limited studies have been conducted on the biocompatibility of using lignin as a 3D printing additive especially for other types of 3D printing techniques other than extrusion based methods. Future research should explore the use of lignin with different 3D printing techniques and in-vivo studies to understand the interactions between the lignin additive biomaterial and the biological system. Table 3 summarises the key findings of lignin-related hydrogels, nanofibers, and 3D printing applications.
Table 3.
Summary and key findings of lignin-related hydrogels, nanofibers, and 3D printing applications.
| Type of Lignin | Matrix Material | Form | In-vitro Study | In-vivo Study | Key Findings | References |
|---|---|---|---|---|---|---|
| Lignosulfonate | Chitosan Polyvinyl alcohol |
Hydrogel |
Staphylococcus aureus Bovine serum albumin Mouse osteoblasts |
Female Kunming mice | 70% reduce in free radicals with 0.2 mg/mL lignin solution Increased cell viability with increasing lignin concentration (0.2–3.2 mg/mL) Good antibacterial abilities at 10 wt% lignin Faster and complete wound healing over 15 days |
[151] |
| Alkali lignin | Chitosan | Hydrogel | Mesenchymal stem cells Mouse NIH3T3 fibroblast cells |
Zebrafish embryo | Biocompatible in-vitro (99 ± 3% cell viability) Positive wound healing potential after 24 h Low cytotoxicity up to 100 μg/mL Suitable cell attachment, proliferation, and migration surface |
[152] |
| Lignosulfonate | Polyoxazoline conjugated triazole | Hydrogel | Mouse RAW 264.7 macrophage cells Escherichia coli Pseudomonas aeruginosa Salmonella typhi Klebsiella pneumonia Staphylococcus aureus Staphylococcus epidermidis Candida albicans Candida tropicalis |
Sprague Dawley rats | Exhibited antioxidant, cellular anti-inflammatory, and antimicrobial activities Capable of preventing infection, promote healing, and reduced inflammation on burn wound within 14 days |
[153] |
| Coconut husk extracted lignin | Polyethylene glycol Polypropylene glycol Polydimethylsiloxane |
Hydrogel | Human LO2 liver cells | SPF mice | Excellent biocompatibility (>90% cell viability) Cell survival rate of 64–83% in the oxidative stress cell model Rapid and complete wound healing within 25 days |
[155] |
| Sodium lignosulfonate | Polyvinyl alcohol Silver |
Hydrogel |
Staphylococcus aureus Escherichia coli Mouse L929 fibroblasts |
– | Improved antibacterial properties Non-cytotoxic and biocompatible (≈60% cell viability) |
[157] |
| Alkali lignin | Carrageenan Calcium chloride Copper chloride Magnesium chloride Silver |
Hydrogel |
Staphylococcus aureus Escherichia coli Mouse L929 fibroblasts |
Sprague Dawley rats | Antibacterial behavior between 3 and 6 h Biocompatible properties (>95% cell viability after 48 h) Wound healing effect within 14 days |
[158] |
| Kraft lignin | Hyaluronan | Hydrogel | Mouse embryonic fibroblasts | – | Non-cytotoxic (>90% cell viability) Positive cell migration and cell growth over 2–7 days |
[159] |
| Kraft lignin | Rose Bengal Gold Silver Polyacrylic acid |
Hydrogel | Candida tropicalis | – | Antifungal and antimicrobial properties at very low IC50 value (0.1 μg/mL) | [160] |
| Lignin-carbohydrate complex | Polyethylene glycol diglycidyl ether | Hydrogel | Human L-02 hepatocytes | – | Positive hepatocytes adhesion Biocompatible with cell proliferation and metabolic activity (highest after 5 days) |
[161] |
| Kraft lignin | Polycaprolactone | Nanofibers | Fibroblasts | – | Biocompatible (>98% cell viability across 8 days) Improved biological cell response and mechanical signals |
[164] |
| Kraft lignin | Polymethyl methacrylate Polycaprolactone |
Nanofibers | Human dermal fibroblasts | – | Biocompatible Compatible cell attachment, proliferation, and interaction across 9 days |
[165] |
| Alkali lignin | Polycaprolactone | Nanofibers | Rat Schwann cells Human bone marrow mesenchymal stem cells |
– | Biocompatible with antioxidant properties (up to 98.3 ± 1.9% free radical inhabitation) Stimulated cell proliferation, improved myelin protein expression of Schwann cells, and induced neurite outgrowth of DRG neurons |
[166] |
| Alkali lignin | Polylactic acid Poly-l-lactide |
Nanofibers | Rat PC12 cells Human dermal fibroblasts Human mesenchymal stem cells |
– | Positive antioxidant ability over 72 h Biocompatible with increased cell proliferation |
[167] |
| Kraft lignin | Polyvinyl alcohol Polyglycerol sebacate |
Nanofibers | Rat PC12 cells | – | Non-cytotoxic Induced cell proliferation and differentiation across 7 days |
[168] |
| Kraft lignin | Polycaprolactone Hydroxyapatite |
Nanofibers | Mouse MC3T3-E1 osteoblasts | – | Suitable biocompatibility after 2 days Osteoconductive with positive cell adhesion and proliferation across 5–7 days |
[169] |
| Kraft lignin | Polycaprolactone | Nanofibers | Rat PC12 cells Human adipose-derived stem cells |
Male Wistar rats | Enhanced cell viability and differentiation across 7–14 days Supportive nerve regeneration characteristics |
[170] |
| Alkali lignin | Poly-3-hydroxybutyrate | Nanofibers | Mouse NIH3T3 fibroblasts | Female Sprague Dawley rats | Adjustable antioxidant property with the highest inhabitation at 98.8 ± 0.9% in 4 h Non-cytotoxic and biocompatible across 35 days |
[171] |
| Kraft lignin | Polycaprolactone | Nanofibers | Human TC28a2 chondrocyte cells | Male New Zealand rabbits | Best antioxidant behavior at 97.50 ± 1.30% inhabitation after 48 h Highest cell viability at 82.95% and evidence of cell proliferation from day 1–3 Biocompatible with the capability of reducing OARSI score by 40.49% |
[172] |
| Bio-ligninTM | Chitin nanofibril Sodium ascorbyl phosphate, Melatonin Beta-glucan Nicotinamide |
Nanofibers | Keratinocytes Fibroblasts |
Women volunteers | Non-cytotoxic behavior Reduced cytokine expression and promoted cell proliferation (>100% cell viability) Positive protective, reparative, and antiaging properties |
[173] |
| Soda lignin | Polylactic acid | 3D Printing (FDM) | – | – | Improved antioxidant activity (≈45%) | [175] |
| Kraft lignin | Polylactic acid | 3D Printing (FDM) | – | – | Highest antioxidant behavior at 80% in 5 h Localized and prolonged antioxidant capabilities |
[176] |
| Alkali lignin | Polyether-based hydrophilic polyurethane | 3D Printing (DIW) | Human dermal fibroblasts | – | Biocompatible and supports cell growth (>80% cell viability across 3 days) | [177] |
| Kraft lignin | Cellulose nanofibrils Alginate |
3D Printing (DIW) | Hepatocellular carcinoma HepG2 cells | – | Concentration-dependent antioxidant behavior Shape stability and storage up to 7 days Proliferation of HepG2 cells internally and externally over 5 days |
[178] |
| Alkylated dealkaline lignin | Polyethylene glycol diacrylate 1,6-hexanediol diacrylate |
3D Printing (DLP) | Mouse L929 fibroblasts | – | Biosafe and non-cytotoxic across 2 days | [179] |
6. Challenges and perspective
Another potential area for the valorization of lignin is the application of lignin in health dietary supplements. Lignin extracted from the natural resource is commonly employed as a highly insoluble fiber in dietary supplement which may be helpful for digestion. Nevertheless, its rich content in phenolic chemicals could find many other potential benefits in human health. This includes anti-diabetic [[180], [181], [182], [183]], anti-obesity [184,185], anti-tyrosine [186,187] and gut-microbiota balancing [188] which are beneficial to the human dietary health.
Processing and utilization of lignin as a biomaterial remains to be a huge challenge today. There are many different types of lignins that originate from a variety of natural sources, and there are also many different processing methods as highlighted in this review. Each different type of lignin possesses different properties due to its heterogeneity, which makes it particularly challenging to characterize lignin. There is a need to further investigate the structure of lignin and discover a way to obtain a more uniform lignin structure in order to complete the picture of lignin as a biomaterial.
Today's lignin source mainly comes from the processes of the pulp and paper industries. Extensive mechanical and chemical processes have resulted in lignin that is contaminated with salts and other toxic chemicals. At the moment, there is no immediate clean source of lignin that can be directly utilized as a biomaterial. One potential candidate is organosolv lignin which uses green organic solvents such as acetone and methanol for lignin extraction which reduces its environmental effects and producing comparatively cleaner lignin as compared to other processing methods. Even though there are some strong candidate that uses lignin as a biomaterial such as lignin-based hydrogels, smart lignin materials need to be further developed.
In addition, lignin is only used at very low concentrations and is primarily used as an additive. In most cases, the lignin content in the whole material system is <5%, and the benefit of adding lignin seems to be limited. However, it is worth noting that even at low concentrations, lignin-based materials are able to exhibit high anti-UV and antioxidant properties, which could be potentially applied in functional wound dressing or anti-inflammatory biomaterials. Of course, the ultimate challenge is to be able to use more of lignin that would eventually progress towards using lignin as a main component in biomaterials while harnessing the benefits of lignin. There will be a strong need to develop new lignin modification/processing methods. Chemical modifications to lignin have shown to be helpful to improve the compatibility and miscibility of lignin to integrate it with other polymeric matrixes, which can effectively increase lignin content without scarifying the mechanical strength of materials.
Despite the extensive studies done on the biocompatibility of lignin, the mechanisms of the biocompatibility of lignin have yet to be fully understood. More studies would be needed to obtain the fundamental knowledge of lignin's impact on cells, proteins, and genes. Moreover, although lignin is naturally degraded by some bacteria and fungi, the biodegradability of lignin in the human body remains to be a debatable topic. Even though lignin is biodegradable lignin in environment (by certain enzymes, fungi and bacterial species), in the context of biomaterials for medical applications, the concept of biodegradation focuses on the biological processes inside human body which causes the breakdown of the material [189]. Presently, lignin has been reported to remain undigested in the small and large intestines in humans [190]. Nevertheless, the degradability of lignin in human body is still unclear and future studies exploring this area will also be needed.
As lignin is a new material, there is limited data on its properties to effectively compare it to other biomaterials. With a better understanding of the important underlying mechanisms of lignin, it would be possible to propel the development of lignin as a potential new alternative of a biomaterial through the potential exploitation of lignin's biocompatibility, antimicrobial, anti-UV, and antioxidant properties.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the financial support (Project No. CDA 202D800033) from Agency for Science, Technology and Research (A*STAR), Singapore.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Steinbüchel A., Hofrichter M. Wiley-VCH; Weinheim: 2001. Biopolymers : Biology, Chemistry, Biotechnology, Applications Volume 1, Volume 1. [Google Scholar]
- 2.Tsegaye B., Balomajumder C., Roy P. Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bulletin of the National Research Centre 43(1) 2019 [Google Scholar]
- 3.S. Schoenherr, M. Ebrahimi, P. Czermak, Lignin degradation processes and the purification of valuable products, Lignin - Trends and Applications2018.
- 4.Cao L., Yu I.K.M., Liu Y., Ruan X., Tsang D.C.W., Hunt A.J., Ok Y.S., Song H., Zhang S. Lignin valorization for the production of renewable chemicals: state-of-the-art review and future prospects. Bioresour. Technol. 2018;269:465–475. doi: 10.1016/j.biortech.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 5.Research G.V. Lignin market size, share & trends analysis report by product (Ligno-Sulphonates, kraft, organosolv), by application (macromolecule, aromatic) By Region, And Segment Forecasts, 2020-2027. 2020 https://www.grandviewresearch.com/industry-analysis/lignin-market [Google Scholar]
- 6.Gheorghita Puscaselu R., Lobiuc A., Dimian M., Covasa M. Polymers; Basel: 2020. Alginate: from Food Industry to Biomedical Applications and Management of Metabolic Disorders. 12(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seddiqi H., Oliaei E., Honarkar H., Jin J., Geonzon L.C., Bacabac R.G., Klein-Nulend J. Cellulose and its derivatives: towards biomedical applications. Cellulose 28(4) 2021:1893–1931. [Google Scholar]
- 8.Anitha A., Sowmya S., Kumar P.T.S., Deepthi S., Chennazhi K.P., Ehrlich H., Tsurkan M., Jayakumar R. Chitin and chitosan in selected biomedical applications, Progress in Polymer. Science 39(9) 2014:1644–1667. [Google Scholar]
- 9.Hickey R.J., Pelling A.E. Cellulose biomaterials for tissue engineering. Frontiers in Bioengineering and Biotechnology. 2019;7:45. doi: 10.3389/fbioe.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzler M., Alzagameem A., Bergs M., Khaldi-Hansen B.E., Klein S.E., Hielscher D., Kamm B., Kreyenschmidt J., Tobiasch E., Schulze M. Lignin-derived biomaterials for drug release and tissue engineering. molecules 23(8) 2018 doi: 10.3390/molecules23081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinardell M.P., Mitjans M., Lignins, Derivatives Their. With beneficial effects on human health. Int J Mol Sci 18(6) 2017 doi: 10.3390/ijms18061219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiredo P., Lintinen K., Hirvonen J.T., Kostiainen M.A., Santos H.A. Properties and chemical modifications of lignin: towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018;93:233–269. [Google Scholar]
- 13.Becker J., Wittmann C. A field of dreams: lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv 37(6) 2019 doi: 10.1016/j.biotechadv.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Liu R., Dai L., Xu C., Wang K., Zheng C., Si C. Lignin-based micro- and nanomaterials and their composites in biomedical applications. ChemSusChem 13(17) 2020:4266–4283. doi: 10.1002/cssc.202000783. [DOI] [PubMed] [Google Scholar]
- 15.Fisher A.B., Fong S.S. Lignin biodegradation and industrial implications. AIMS Bioengineering 1(2) 2014:92–112. [Google Scholar]
- 16.Ten E., Vermerris W. Recent developments in polymers derived from industrial lignin. Journal of Applied Polymer Science 132(24) 2015 (n/a-n/a) [Google Scholar]
- 17.Duval A., Lawoko M. A review on lignin-based polymeric, micro- and nano-structured materials, React. Funct. Polym. 2014;85:78–96. [Google Scholar]
- 18.Sette M., Wechselberger R., Crestini C. Elucidation of lignin structure by quantitative 2D NMR. chemistry – a European journal 17(34) 2011:9529–9535. doi: 10.1002/chem.201003045. [DOI] [PubMed] [Google Scholar]
- 19.Tamilarasan K., Sellamuthu P.S., Gurunathan B. In: Bioremediation: Applications for Environmental Protection and Management. Varjani S.J., Agarwal A.K., Gnansounou E., Gurunathan B., editors. Springer Singapore; Singapore: 2018. Integration of lignin removal from black liquor and biotransformation process; pp. 77–97. [Google Scholar]
- 20.Lora J.H., Glasser W.G. Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. Journal of Polymers and the Environment 10(1) 2002:39–48. [Google Scholar]
- 21.Azadi P., Inderwildi O.R., Farnood R., King D.A. Liquid fuels, hydrogen and chemicals from lignin: a critical review. Renew. Sustain. Energy Rev. 2013;21:506–523. [Google Scholar]
- 22.Fatehi P., Chen J. In: Production of Biofuels and Chemicals from Lignin. Fang Z., Smith J.R.L., editors. Springer Singapore; Singapore: 2016. Extraction of technical lignins from pulping spent liquors, challenges and opportunities; pp. 35–54. [Google Scholar]
- 23.Glasser W.G., Davé V., Frazier C.E. Molecular weight distribution of (semi-) commercial lignin derivatives. J. Wood Chem. Technol. 1993;13(4):545–559. [Google Scholar]
- 24.Thring R.W., Chornet E., Overend R.P. Recovery of a solvolytic lignin: effects of spent liquor/acid volume ratio, acid concentration and temperature. Biomass 23(4) 1990:289–305. [Google Scholar]
- 25.Mousavioun P., Doherty W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crop. Prod. 2010;31(1):52–58. [Google Scholar]
- 26.Mansouri N.-E.E., Salvadó J. Structural characterization of technical lignins for the production of adhesives: application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crop. Prod. 2006;24(1):8–16. [Google Scholar]
- 27.Aro T., Fatehi P. Production and application of lignosulfonates and sulfonated lignin. ChemSusChem 10(9) 2017:1861–1877. doi: 10.1002/cssc.201700082. [DOI] [PubMed] [Google Scholar]
- 28.Gordobil O., Herrera R., Yahyaoui M., İlk S., Kaya M., Labidi J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Advances 8(43) 2018:24525–24533. doi: 10.1039/c8ra02255k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W., Fortunati E., Bertoglio F., Owczarek J.S., Bruni G., Kozanecki M., Kenny J.M., Torre L., Visai L., Puglia D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018;181:275–284. doi: 10.1016/j.carbpol.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 30.Laurichesse S., Avérous L. Chemical modification of lignins: towards biobased polymers. Progress in Polymer Science 39(7) 2014:1266–1290. [Google Scholar]
- 31.El Mansouri N.-E., Salvadó J. Analytical methods for determining functional groups in various technical lignins. Industrial Crops and Products 26(2) 2007:116–124. [Google Scholar]
- 32.Bhattacharyya S., Matsakas L., Rova U., Christakopoulos P., Stable Functionalized Organosolv Melt, Thermoplastic Kraft Lignin. Processes 8(9) 2020 [Google Scholar]
- 33.Gülçin İ. Antioxidant activity of food constituents: an overview. Arch. Toxicol. 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 34.Altmann H.J., Grunow W., Mohr U., Richter-Reichhelm H.B., Wester P.W. Effects of BHA and related phenols on the forestomach of rats. Food Chem. Toxicol. : an international journal published for the British Industrial Biological Research Association 24(10-11) 1986:1183–1188. doi: 10.1016/0278-6915(86)90306-6. [DOI] [PubMed] [Google Scholar]
- 35.Altmann H.J., Wester P.W., Matthiaschk G., Grunow W., van der Heijden C.A. Induction of early lesions in the forestomach of rats by 3-tert-butyl-4-hydroxyanisole (BHA) Food Chem. Toxicol. 1985;23(8):723–731. doi: 10.1016/0278-6915(85)90265-0. [DOI] [PubMed] [Google Scholar]
- 36.Altmann H.J., Grunow W., Wester P.W., Mohr U. In: Receptors and Other Targets for Toxic Substances. Chambers P.L., Cholnoky E., Chambers C.M., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 1985. Induction of forestomach lesions by butylhydroxyanisole and structurally related substances; pp. 114–116. [Google Scholar]
- 37.Grice H.C. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem. Toxicol. 1988;26(8):717–723. doi: 10.1016/0278-6915(88)90072-5. [DOI] [PubMed] [Google Scholar]
- 38.Thompson D., Moldéus P. Cytotoxicity of butylated hydroxyanisole and butylated hydroxytoluene in isolated rat hepatocytes. Biochem. Pharmacol. 1988;37(11):2201–2207. doi: 10.1016/0006-2952(88)90582-5. [DOI] [PubMed] [Google Scholar]
- 39.Dizhbite T., Telysheva G., Jurkjane V., Viesturs U. Characterization of the radical scavenging activity of lignins––natural antioxidants. Bioresour. Technol. 2004;95(3):309–317. doi: 10.1016/j.biortech.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Azadfar M., Gao A.H., Bule M.V., Chen S. Structural characterization of lignin: a potential source of antioxidants guaiacol and 4-vinylguaiacol, Int. J. Biol. Macromol. 2015;75:58–66. doi: 10.1016/j.ijbiomac.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 41.Faustino H., Gil N., Baptista C., Duarte A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 15(12) 2010 doi: 10.3390/molecules15129308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu F.J., Chu L.H., Gau R.J. Free radical-scavenging properties of lignin. Nutr Cancer 30(1) 1998:31–38. doi: 10.1080/01635589809514637. [DOI] [PubMed] [Google Scholar]
- 43.Sakagami H., Asano K., Satoh K., Takahashi K., Kobayashi M., Koga N., Takahashi H., Tachikawa R., Tashiro T., Hasegawa A., Kurihara K., Ikarashi T., Kanamoto T., Terakubo S., Nakashima H., Watanabe S., Nakamura W. Anti-stress, anti-HIV and vitamin C-synergized radical scavenging activity of mulberry juice fractions. In Vivo 21(3) 2007:499–505. [PubMed] [Google Scholar]
- 44.Sakagami H., Satoh K., Ida Y., Koyama N., Premanathan M., Arakaki R., Nakashima H., Hatano T., Okuda T., Yoshida T. In: Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology. Gross G.G., Hemingway R.W., Yoshida T., Branham S.J., editors. Springer US; Boston, MA: 1999. Induction of apoptosis and anti-HIV activity by tannin- and lignin-related substances; pp. 595–611. [DOI] [PubMed] [Google Scholar]
- 45.Sakagami H., Oh-Hara T., Kaiya T., Kawazoe Y., Nonoyama M., Konno K. Molecular species of the antitumor and antiviral fraction from pine cone extract. Anticancer Res. 1989;9(6):1593–1598. [PubMed] [Google Scholar]
- 46.Sakagami H., Satoh K., Fukamachi H., Ikarashi T., Shimizu A.I., Yano K., Kanamoto T., Terakubo S., Nakashima H., Hasegawa H., Nomura A., Utsumi K., Yamamoto M., Maeda Y., Osawa K. Anti-HIV and vitamin C-synergized radical scavenging activity of cacao husk lignin fractions. In Vivo 22(3) 2008:327–332. [PubMed] [Google Scholar]
- 47.Ogan A., Yüce-Dursun B., Abdullah D., Beyler-Çiğil A., Kahraman M.V., Çağlayan P., Birbir M., Mutlu Ö., Gülsoy N. Preparation and antimicrobial properties of LL-37 peptide immobilized lignin/caprolactone polymer film, Polym. Adv. Technol. 2020 [Google Scholar]
- 48.Alzagameem A., Klein S.E., Bergs M., Do X.T., Korte I., Dohlen S., Huwe C., Kreyenschmidt J., Kamm B., Larkins M., Schulze M. Polymers; Basel: 2019. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. 11(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur R., Uppal S.K., Sharma P., Antioxidant, Activities Antibacterial. Of sugarcane bagasse lignin and chemically modified lignins. Sugar Tech 19(6) 2017:675–680. [Google Scholar]
- 50.Guilhen A., Gadioli R., Fernandes F.C., Waldman W.R., Aurelio De Paoli M. High-density green polyethylene biocomposite reinforced with cellulose fibers and using lignin as antioxidant. J. Appl. Polym. Sci. 2017;134(35) [Google Scholar]
- 51.Majira A., Godon B., Foulon L., van der Putten J.C., Cezard L., Thierry M., Pion F., Bado-Nilles A., Pandard P., Jayabalan T., Aguie-Beghin V., Ducrot P.H., Lapierre C., Marlair G., Gosselink R.J.A., Baumberger S., Cottyn B. Enhancing the antioxidant activity of technical lignins by combining solvent fractionation and ionic-liquid treatment. ChemSusChem 12(21) 2019:4799–4809. doi: 10.1002/cssc.201901916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G., Xia Y., Liang B., Sui W., Si C. Successive ethanol-water fractionation of enzymatic hydrolysis lignin to concentrate its antimicrobial activity. Journal of Chemical Technology & Biotechnology 93(10) 2018:2977–2987. [Google Scholar]
- 53.Chee P.L., Yew P.Y.M., Kai D., Loh X.J. Reinforcement of aligned cellulose fibers by lignin-polyester copolymers. Materials Today Chemistry. 2020;18 [Google Scholar]
- 54.Fang C., Liu W., Qiu X. Preparation of polyetheramine-grafted lignin and its application in UV-resistant polyurea coatings. Macromol. Mater. Eng. 2019;304(10) [Google Scholar]
- 55.Liu X., Zong E., Jiang J., Fu S., Wang J., Xu B., Li W., Lin X., Xu Y., Wang C., Chu F. Preparation and characterization of Lignin-graft-poly (varepsilon-caprolactone) copolymers based on lignocellulosic butanol residue. Int. J. Biol. Macromol. 2015;81:521–529. doi: 10.1016/j.ijbiomac.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 56.Kai D., Tan M.J., Chee P.L., Chua Y.K., Yap Y.L., Loh X.J. Towards lignin-based functional materials in a sustainable world. Green Chemistry 18(5) 2016:1175–1200. [Google Scholar]
- 57.Kai D., Zhang K., Jiang L., Wong H.Z., Li Z., Zhang Z., Loh X.J., Sustainable, Lignin–Polyester Antioxidant. Copolymers and nanofibers for potential healthcare applications. ACS Sustain. Chem. Eng. 2017;5(7):6016–6025. [Google Scholar]
- 58.Spellberg B., Gilbert D.N. The future of antibiotics and resistance: a tribute to a career of leadership by john bartlett, clinical infectious diseases. An Official Publication of the Infectious Diseases Society of America 59(Suppl 2. 2014:S71–S75. doi: 10.1093/cid/ciu392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gould I.M., Bal A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 4(2) 2013:185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright G.D. Something old, something new: revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014;60(3):147–154. doi: 10.1139/cjm-2014-0063. [DOI] [PubMed] [Google Scholar]
- 61.Grenni P., Ancona V., Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: a review, Microchem. J. 2018;136:25–39. [Google Scholar]
- 62.Ndaba B., Roopnarain A., Daramola M.O., Adeleke R. Influence of extraction methods on antimicrobial activities of lignin-based materials: a review, Sustainable Chemistry and Pharmacy. 2020;18 [Google Scholar]
- 63.Panáček A., Kolář M., Večeřová R., Prucek R., Soukupová J., Kryštof V., Hamal P., Zbořil R., Kvítek L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 30(31) 2009:6333–6340. doi: 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 64.Lara H.H., Garza-Treviño E.N., Ixtepan-Turrent L., Singh D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol 9(1) 2011:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong S.-D., Huang Y., Cao H.-J., Lin Y.-H., Li Y., Tang S.-H., Wang M.-S., Li X. A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin, J. Power Sources. 2016;307:624–633. [Google Scholar]
- 66.Nada A.M.A., El-Diwany A.I., Elshafei A.M. Infrared and antimicrobial studies on different lignins. Acta Biotechnol 9(3) 2004:295–298. [Google Scholar]
- 67.Zemek J., Košíková B., Augustín J., Joniak D. Antibiotic properties of lignin components. Folia Microbiologica 24(6) 1979:483–486. doi: 10.1007/BF02927180. [DOI] [PubMed] [Google Scholar]
- 68.Espinoza-Acosta J.L., Torres-Chávez P.I., Ramírez-Wong B., López-Saiz C.M., Montaño-Leyva B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioResources 11(2) 2016:5452–5481. [Google Scholar]
- 69.Shweta K., Manupati K., Das A., Jha H. Novel nanocomposites with selective antibacterial action and low cytotoxic effect on eukaryotic cells. Int. J. Biol. Macromol. 2016;92:988–997. doi: 10.1016/j.ijbiomac.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 70.Sriroth K., Sunthornvarabhas J. Lignin from Sugar Process as Natural Antimicrobial Agent, Biochemistry & Pharmacology: Open Access 07(01) 2018 [Google Scholar]
- 71.Larraneta E., Imizcoz M., Toh J.X., Irwin N.J., Ripolin A., Perminova A., Dominguez-Robles J., Rodriguez A., Donnelly R.F. Synthesis and characterization of lignin hydrogels for potential applications as drug eluting antimicrobial coatings for medical materials. ACS Sustain Chem Eng 6(7) 2018:9037–9046. doi: 10.1021/acssuschemeng.8b01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee E., Song Y., Lee S. Crosslinking of lignin/poly(vinyl alcohol) nanocomposite fiber webs and their antimicrobial and ultraviolet-protective properties. Textil. Res. J. 2017;89(1):3–12. [Google Scholar]
- 73.Dong X., Dong M., Lu Y., Turley A., Jin T., Wu C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crop. Prod. 2011;34(3):1629–1634. [Google Scholar]
- 74.Wang G., Pang T., Xia Y., Liu X., Li S., Parvez A.M., Kong F., Si C. Subdivision of bamboo kraft lignin by one-step ethanol fractionation to enhance its water-solubility and antibacterial performance. Int. J. Biol. Macromol. 2019;133:156–164. doi: 10.1016/j.ijbiomac.2019.04.093. [DOI] [PubMed] [Google Scholar]
- 75.Klapiszewski L., Rzemieniecki T., Krawczyk M., Malina D., Norman M., Zdarta J., Majchrzak I., Dobrowolska A., Czaczyk K., Jesionowski T. Kraft lignin/silica-AgNPs as a functional material with antibacterial activity. Colloids Surf. B Biointerfaces. 2015;134:220–228. doi: 10.1016/j.colsurfb.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 76.Maverakis E., Miyamura Y., Bowen M.P., Correa G., Ono Y., Goodarzi H. Light, including ultraviolet. J. Autoimmun. 2010;34(3):J247–J257. doi: 10.1016/j.jaut.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yousif E., Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus. 2013;2:398. doi: 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nowicki M., Richter A., Wolf B., Kaczmarek H. Nanoscale mechanical properties of polymers irradiated by UV. Polymer 44(21) 2003:6599–6606. [Google Scholar]
- 79.Lyu Y., Gu X., Mao Y. Green composite of instant coffee and poly(vinyl alcohol): an excellent transparent UV-shielding material with superior thermal-oxidative stability. Ind. Eng. Chem. Res. 2020;59(18):8640–8648. [Google Scholar]
- 80.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin†, photochem. Photobiol. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solano F., Photoprotection, Pigmentation Skin. Melanin-related molecules and some other new agents obtained from natural sources. molecules 25(7) 2020 doi: 10.3390/molecules25071537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong F., Wu Y., Li G., Han Y., Chu F. Transparent nanocomposite films of lignin nanospheres and poly(vinyl alcohol) for UV-absorbing. Ind. Eng. Chem. Res. 2018;57(4):1207–1212. [Google Scholar]
- 83.Mehta M.J., Kumar A. Ionic liquid stabilized gelatin-lignin films: a potential UV-shielding material with excellent mechanical and antimicrobial properties. Chemistry 25(5) 2019:1269–1274. doi: 10.1002/chem.201803763. [DOI] [PubMed] [Google Scholar]
- 84.Park S.Y., Kim J.Y., Youn H.J., Choi J.W. Utilization of lignin fractions in UV resistant lignin-PLA biocomposites via lignin-lactide grafting. Int. J. Biol. Macromol. 2019;138:1029–1034. doi: 10.1016/j.ijbiomac.2019.07.157. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y., Suhr J., Seo H.-W., Sun H., Kim S., Park I.-K., Kim S.-H., Lee Y., Kim K.-J., Nam J.-D. All biomass and UV protective composite composed of compatibilized lignin and poly (Lactic-acid) Sci Rep 7(1) 2017 [Google Scholar]
- 86.Qian Y., Qiu X., Zhu S. Lignin: a nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015;17(1):320–324. [Google Scholar]
- 87.Sadeghifar H., Ragauskas A. Polymers; Basel: 2020. Lignin as a UV Light Blocker-A Review. 12(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang B., Opatz T., Landfester K., Wurm F.R. Carbohydrate nanocarriers in biomedical applications: functionalization and construction. Chem. Soc. Rev. 2015;44(22):8301–8325. doi: 10.1039/c5cs00092k. [DOI] [PubMed] [Google Scholar]
- 89.Kang B., Okwieka P., Schöttler S., Winzen S., Langhanki J., Mohr K., Opatz T., Mailänder V., Landfester K., Wurm Frederik R. Carbohydrate-based nanocarriers exhibiting specific cell targeting with minimum influence from the protein corona. Angewandte Chemie International Edition 54(25) 2015:7436–7440. doi: 10.1002/anie.201502398. [DOI] [PubMed] [Google Scholar]
- 90.Piradashvili K., Fichter M., Mohr K., Gehring S., Wurm F.R., Landfester K. Biodegradable protein nanocontainers. Biomacromolecules 16(3) 2015:815–821. doi: 10.1021/bm5016915. [DOI] [PubMed] [Google Scholar]
- 91.Wurm F.R., Weiss C.K. Nanoparticles from renewable polymers. Frontiers in Chemistry. 2014;2:49. doi: 10.3389/fchem.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain A., Barve A., Zhao Z., Jin W., Cheng K. Comparison of avidin, neutravidin, and streptavidin as nanocarriers for efficient siRNA delivery, mol. Pharm. Times. 2017;14(5):1517–1527. doi: 10.1021/acs.molpharmaceut.6b00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams D.F. On the mechanisms of biocompatibility. Biomaterials 29(20) 2008:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 95.Iravani S., Varma R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019;21(18):4839–4867. [Google Scholar]
- 96.Ugartondo V., Mitjans M., Vinardell M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour Technol 99(14) 2008:6683–6687. doi: 10.1016/j.biortech.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 97.Melro E., Filipe A., Sousa D., Medronho B., Romano A. Revisiting lignin: a tour through its structural features, characterization methods and applications. New J. Chem. 2021 [Google Scholar]
- 98.Cheng J., Hirth K., Ma Q., Zhu J., Wang Z., Zhu J.Y. Toward sustainable and complete wood valorization by fractionating lignin with low condensation using an acid hydrotrope at low temperatures (≤80 °C) Industrial & Engineering Chemistry Research 58(17) 2019:7063–7073. [Google Scholar]
- 99.Sen S., Patil S., Argyropoulos D.S. Thermal properties of lignin in copolymers, blends, and composites: a review. Green Chemistry 17(11) 2015:4862–4887. [Google Scholar]
- 100.Diao B., Zhang Z., Zhu J., Li J. Biomass-based thermogelling copolymers consisting of lignin and grafted poly(N-isopropylacrylamide), poly(ethylene glycol), and poly(propylene glycol) RSC Adv. 2014;4(81):42996–43003. [Google Scholar]
- 101.Wu L.C.F., Glasser W.G. Engineering plastics from lignin. I. Synthesis of hydroxypropyl lignin. J. Appl. Polym. Sci. 1984;29(4):1111–1123. [Google Scholar]
- 102.Alonso M.V., Oliet M., Rodriguez F., Garcia J., Gilarranz M.A., Rodriguez J.J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005;96(9):1013–1018. doi: 10.1016/j.biortech.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Sailaja R.R.N. Low density polyethylene and grafted lignin polyblends using epoxy-functionalized compatibilizer: mechanical and thermal properties. Polym. Int. 2005;54(12):1589–1598. [Google Scholar]
- 104.Thiebaud S., Borredon M.E. Solvent-free wood esterification with fatty acid chlorides. Bioresour. Technol. 1995;52(2):169–173. [Google Scholar]
- 105.Maldhure A.V., Ekhe J.D., Deenadayalan E. Mechanical properties of polypropylene blended with esterified and alkylated lignin. Journal of Applied Polymer Science 125(3) 2012:1701–1712. [Google Scholar]
- 106.Dehne L., Vila Babarro C., Saake B., Schwarz K.U. Influence of lignin source and esterification on properties of lignin-polyethylene blends. Ind. Crop. Prod. 2016;86:320–328. [Google Scholar]
- 107.Thielemans W., Wool R.P. Lignin esters for use in unsaturated Thermosets: lignin modification and solubility modeling. Biomacromolecules 6(4) 2005:1895–1905. doi: 10.1021/bm0500345. [DOI] [PubMed] [Google Scholar]
- 108.Dehne L., Vila C., Saake B., Schwarz K.U. Esterification of Kraft lignin as a method to improve structural and mechanical properties of lignin-polyethylene blends. J. Appl. Polym. Sci. 2017;134(11) [Google Scholar]
- 109.Hajirahimkhan S., Xu C.C., Ragogna P.J. Ultraviolet curable coatings of modified lignin. ACS Sustainable Chemistry & Engineering 6(11) 2018:14685–14694. [Google Scholar]
- 110.Kim Y.S., Kadla J.F. Preparation of a thermoresponsive lignin-based biomaterial through atom transfer radical polymerization. Biomacromolecules 11(4) 2010:981–988. doi: 10.1021/bm901455p. [DOI] [PubMed] [Google Scholar]
- 111.Liu X., Yin H., Zhang Z., Diao B., Li J. Functionalization of lignin through ATRP grafting of poly(2-dimethylaminoethyl methacrylate) for gene delivery. Colloids Surf. B Biointerfaces. 2015;125:230–237. doi: 10.1016/j.colsurfb.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 112.Sadeghifar H., Cui C., Argyropoulos D.S. Toward thermoplastic lignin polymers. Part 1. Selective masking of phenolic hydroxyl groups in kraft lignins via methylation and oxypropylation chemistries. Ind. Eng. Chem. Res. 2012;51(51):16713–16720. [Google Scholar]
- 113.Li Y., Ragauskas A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012;32(3):210–224. [Google Scholar]
- 114.Hofmann K., Glasser W.G. Engineering plastics from lignin. 21.1Synthesis and properties of epoxidized lignin-poly (propylene oxide) copolymers. J. Wood Chem. Technol. 1993;13(1):73–95. [Google Scholar]
- 115.de Oliveira W., Glasser W.G. Multiphase materials with lignin. 11. Starlike copolymers with caprolactone. Macromolecules 27(1) 1994:5–11. [Google Scholar]
- 116.de Oliveira W., Glasser W.G. Multiphase materials with lignin. XIV. Star-like copolymers with styrene. J. Wood Chem. Technol. 1994;14(1):119–126. [Google Scholar]
- 117.Korich A.L., Clarke K.M., Wallace D., Iovine P.M. Chemical modification of a lignin model polymer via arylboronate ester formation under mild reaction conditions. Macromolecules 42(16) 2009:5906–5908. [Google Scholar]
- 118.Korich A.L., Fleming A.B., Walker A.R., Wang J., Tang C., Iovine P.M. Chemical modification of organosolv lignin using boronic acid-containing reagents. Polymer 53(1) 2012:87–93. [Google Scholar]
- 119.Kai D., Chua Y.K., Jiang L., Owh C., Chan S.Y., Loh X.J. Dual functional anti-oxidant and SPF enhancing lignin-based copolymers as additives for personal and healthcare products. RSC Adv. 2016;6(89):86420–86427. [Google Scholar]
- 120.De Jong W.H., Borm P.J. Drug delivery and nanoparticles:applications and hazards, Int. J. Nanomedicine 3(2) 2008:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alqahtani M.S., Alqahtani A., Al-Thabit A., Roni M., Syed R. Novel lignin nanoparticles for oral drug delivery. Journal of Materials Chemistry B 7(28) 2019:4461–4473. [Google Scholar]
- 122.Tortora M., Cavalieri F., Mosesso P., Ciaffardini F., Melone F., Crestini C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 15(5) 2014:1634–1643. doi: 10.1021/bm500015j. [DOI] [PubMed] [Google Scholar]
- 123.Li Y., Qiu X., Qian Y., Xiong W., Yang D. pH-responsive lignin-based complex micelles: preparation, characterization and application in oral drug delivery. Chem. Eng. J. 2017;327:1176–1183. [Google Scholar]
- 124.Siddiqui L., Bag J. Seetha, D. Mittal, A. Leekha, H. Mishra, M. Mishra, A.K. Verma, P.K. Mishra, A. Ekielski, Z. Iqbal, S. Talegaonkar, Assessing the potential of lignin nanoparticles as drug carrier: synthesis, cytotoxicity and genotoxicity studies. Int. J. Biol. Macromol. 2020;152:786–802. doi: 10.1016/j.ijbiomac.2020.02.311. [DOI] [PubMed] [Google Scholar]
- 125.Byrne C.E., Astete C.E., Vaithiyanathan M., Melvin A.T., Moradipour M., Rankin S.E., Knutson B.L., Sabliov C.M., Martin E.C. Lignin-graft-PLGA drug-delivery system improves efficacy of MEK1/2 inhibitors in triple-negative breast cancer cell line. Nanomedicine 15(10) 2020:981–1000. doi: 10.2217/nnm-2020-0010. [DOI] [PubMed] [Google Scholar]
- 126.Dai L., Liu R., Hu L.-Q., Zou Z.-F., Si C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017;5(9):8241–8249. [Google Scholar]
- 127.Figueiredo P., Lintinen K., Kiriazis A., Hynninen V., Liu Z., Bauleth-Ramos T., Rahikkala A., Correia A., Kohout T., Sarmento B., Yli-Kauhaluoma J., Hirvonen J., Ikkala O., Kostiainen M.A., Santos H.A. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials. 2017;121:97–108. doi: 10.1016/j.biomaterials.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 128.Siddiqui L., Mishra H., Mishra P.K., Iqbal Z., Talegaonkar S. Novel 4-in-1 strategy to combat colon cancer, drug resistance and cancer relapse utilizing functionalized bioinspiring lignin nanoparticle. Med. Hypotheses. 2018;121:10–14. doi: 10.1016/j.mehy.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 129.Zhou Y., Han Y., Li G., Yang S., Chu F. Lignin-based hollow nanoparticles for controlled drug delivery: grafting preparation using beta-cyclodextrin/enzymatic-hydrolysis lignin. Nanomaterials (Basel) 9(7) 2019 doi: 10.3390/nano9070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ho Y.K., Kai D., Tu G.X.E., Deen G.R., Too H.P., Loh X.J. Enhanced transfection of a macromolecular lignin-based DNA complex with low cellular toxicity. Biosci Rep 38(6) 2018 doi: 10.1042/BSR20181021. BSR20181021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ten E., Ling C., Wang Y., Srivastava A., Dempere L.A., Vermerris W. Lignin nanotubes as vehicles for gene delivery into human cells. Biomacromolecules 15(1) 2014:327–338. doi: 10.1021/bm401555p. [DOI] [PubMed] [Google Scholar]
- 132.Jiang S., Kai D., Dou Q.Q., Loh X.J. Multi-arm carriers composed of an antioxidant lignin core and poly(glycidyl methacrylate-co-poly(ethylene glycol)methacrylate) derivative arms for highly efficient gene delivery. Journal of Materials Chemistry B 3(34) 2015:6897–6904. doi: 10.1039/c5tb01202c. [DOI] [PubMed] [Google Scholar]
- 133.S.N. Sawant, 13-Development of biosensors from biopolymer composites, in: K.K. Sadasivuni, D. Ponnamma, J. Kim, J.J. Cabibihan, M.A. AlMaadeed (Eds.), Biopolymer Composites in Electronics, Elsevier2017, pp. 353–383.
- 134.Muguruma H. In: Encyclopedia of Interfacial Chemistry. Wandelt K., editor. Elsevier; Oxford: 2018. Biosensors: enzyme immobilization chemistry; pp. 64–71. [Google Scholar]
- 135.Jędrzak A., Rębiś T., Klapiszewski Ł., Zdarta J., Milczarek G., Jesionowski T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sensor. Actuator. B Chem. 2018;256:176–185. [Google Scholar]
- 136.Jędrzak A., Rębiś T., Kuznowicz M., Kołodziejczak-Radzimska A., Zdarta J., Piasecki A., Jesionowski T. Advanced Ga2O3/lignin and ZrO2/lignin hybrid microplatforms for glucose oxidase immobilization: evaluation of biosensing properties by catalytic glucose oxidation. Catalysts 9(12) 2019 [Google Scholar]
- 137.Milczarek G., Rebis T., Fabianska J. One-step synthesis of lignosulfonate-stabilized silver nanoparticles. Colloids Surf. B Biointerfaces. 2013;105:335–341. doi: 10.1016/j.colsurfb.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 138.Jędrzak A., Rębiś T., Kuznowicz M., Jesionowski T. Bio-inspired magnetite/lignin/polydopamine-glucose oxidase biosensing nanoplatform. From synthesis, via sensing assays to comparison with others glucose testing techniques. Int. J. Biol. Macromol. 2019;127:677–682. doi: 10.1016/j.ijbiomac.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 139.Janus Ł., PiĄTkowski M., Radwan-PragŁOwska J., Sierakowska A. Kropki kwantowe węgla (CQD) przygotowane z biomasy odpadowej jako nowa klasa biomateriałów o właściwościach luminescencyjnych. Inżynieria Mineralna 2(1) 2020 [Google Scholar]
- 140.Wang R., Xia G., Zhong W., Chen L., Chen L., Wang Y., Min Y., Li K. Direct transformation of lignin into fluorescence-switchable graphene quantum dots and their application in ultrasensitive profiling of a physiological oxidant. Green Chem. 2019;21(12):3343–3352. [Google Scholar]
- 141.Chen W., Hu C., Yang Y., Cui J., Liu Y. Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials (Basel) 9(3) 2016:184. doi: 10.3390/ma9030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aadil K.R., Barapatre A., Meena A.S., Jha H. Hydrogen peroxide sensing and cytotoxicity activity of Acacia lignin stabilized silver nanoparticles. Int. J. Biol. Macromol. 2016;82:39–47. doi: 10.1016/j.ijbiomac.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 143.Capecchi E., Piccinino D., Tomaino E., Bizzarri B.M., Polli F., Antiochia R., Mazzei F., Saladino R. Lignin nanoparticles are renewable and functional platforms for the concanavalin a oriented immobilization of glucose oxidase–peroxidase in cascade bio-sensing. RSC Adv. 2020;10(48):29031–29042. doi: 10.1039/d0ra04485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lei Y., Alshareef A.H., Zhao W., Inal S. Laser-scribed graphene electrodes derived from lignin for biochemical sensing. ACS Applied Nano Materials 3(2) 2020:1166–1174. [Google Scholar]
- 145.Yuan C., Lou Z., Wang W., Yang L., Li Y. Synthesis of Fe₃C@C from pyrolysis of Fe₃O₄-Lignin clusters and its application for quick and sensitive detection of PrP(Sc) through a sandwich SPR detection assay, int. J. Mol. Sci. 2019;20(3):741. doi: 10.3390/ijms20030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cerrutti B.M., Moraes M.L., Pulcinelli S.H., Santilli C.V. Lignin as immobilization matrix for HIV p17 peptide used in immunosensing. Biosens. Bioelectron. 2015;71:420–426. doi: 10.1016/j.bios.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 147.B. Choudhary, S.R. Paul, S.K. Nayak, D. Qureshi, K. Pal, 11-Synthesis and biomedical applications of filled hydrogels, in: K. Pal, I. Banerjee (Eds.), Polymeric Gels, Woodhead Publishing2018, pp. 283–302.
- 148.Lee J.-H., Kim H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018;9 doi: 10.1177/2041731418768285. 2041731418768285–2041731418768285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.S.A. Varghese, S.M. Rangappa, S. Siengchin, J. Parameswaranpillai, Chapter 2-Natural polymers and the hydrogels prepared from them, in: Y. Chen (Ed.), Hydrogels Based on Natural Polymers, Elsevier2020, pp. 17–47.
- 150.Rico-García D., Ruiz-Rubio L., Pérez-Alvarez L., Hernández-Olmos S.L., Guerrero-Ramírez G.L., Vilas-Vilela J.L. Lignin-based hydrogels: synthesis and applications. Polymers. 2020;12(1) doi: 10.3390/polym12010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Y., Jiang M., Zhang Y., Cao Q., Wang X., Han Y., Sun G., Li Y., Zhou J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C. 2019;104 doi: 10.1016/j.msec.2019.110002. [DOI] [PubMed] [Google Scholar]
- 152.Ravishankar K., Venkatesan M., Desingh R.P., Mahalingam A., Sadhasivam B., Subramaniyam R., Dhamodharan R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C. 2019;102:447–457. doi: 10.1016/j.msec.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 153.Mahata D., Jana M., Jana A., Mukherjee A., Mondal N., Saha T., Sen S., Nando G.B., Mukhopadhyay C.K., Chakraborty R., Mandal S.M. Lignin-graft-Polyoxazoline conjugated triazole a novel anti-infective ointment to control persistent inflammation. Scientific Reports 7(1) 2017 doi: 10.1038/srep46412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tong W., Chen X., Song X., Chen Y., Jia R., Zou Y., Li L., Yin L., He C., Liang X., Ye G., Lv C., Lin J., Yin Z. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp Ther Med 19(3) 2020:1824–1834. doi: 10.3892/etm.2019.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu J., Xu J.J., Lin Q., Jiang L., Zhang D., Li Z., Ma B., Zhang C., Li L., Kai D., Yu H.-D., Loh X.J. Lignin-incorporated nanogel serving as an antioxidant biomaterial for wound healing. ACS Applied Bio Materials 4(1) 2021:3–13. doi: 10.1021/acsabm.0c00858. [DOI] [PubMed] [Google Scholar]
- 156.Yin I.X., Zhang J., Zhao I.S., Mei M.L., Li Q., Chu C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry, int. J. Nanomedicine. 2020;15:2555–2562. doi: 10.2147/IJN.S246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li M., Jiang X., Wang D., Xu Z., Yang M. In situ reduction of silver nanoparticles in the lignin based hydrogel for enhanced antibacterial application. Colloids Surf. B Biointerfaces. 2019;177:370–376. doi: 10.1016/j.colsurfb.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 158.Jaiswal L., Shankar S., Rhim J.-W., Hahm D.-H. Lignin-mediated green synthesis of AgNPs in carrageenan matrix for wound dressing applications. Int. J. Biol. Macromol. 2020;159:859–869. doi: 10.1016/j.ijbiomac.2020.05.145. [DOI] [PubMed] [Google Scholar]
- 159.Musilová L., Mráček A., Kovalcik A., Smolka P., Minařík A., Humpolíček P., Vícha R., Ponížil P. Hyaluronan hydrogels modified by glycinated Kraft lignin: morphology, swelling, viscoelastic properties and biocompatibility. Carbohydr. Polym. 2018;181:394–403. doi: 10.1016/j.carbpol.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 160.Chandna S., Thakur N.S., Kaur R., Bhaumik J. Lignin–bimetallic nanoconjugate doped pH-responsive hydrogels for laser-assisted antimicrobial photodynamic therapy. Biomacromolecules 21(8) 2020:3216–3230. doi: 10.1021/acs.biomac.0c00695. [DOI] [PubMed] [Google Scholar]
- 161.Zhao H., Feng Q., Xie Y., Li J., Chen X. Preparation of Biocompatible Hydrogel from Lignin-Carbohydrate Complex (LCC) as Cell Carriers. 2017;12(4):15. 2017. [Google Scholar]
- 162.Haider A., Haider S., Kang I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian Journal of Chemistry 11(8) 2018:1165–1188. [Google Scholar]
- 163.F.K. Mwiiri, R. Daniels, Chapter 3-Electrospun nanofibers for biomedical applications, in: R. Shegokar (Ed.), Delivery of Drugs, Elsevier2020, pp. 53–74.
- 164.Salami M.A., Kaveian F., Rafienia M., Saber-Samandari S., Khandan A., Naeimi M. Electrospun polycaprolactone/lignin-based nanocomposite as a novel tissue scaffold for biomedical applications. J Med Signals Sens 7(4) 2017:228–238. [PMC free article] [PubMed] [Google Scholar]
- 165.Kai D., Jiang S., Low Z.W., Loh X.J. Engineering highly stretchable lignin-based electrospun nanofibers for potential biomedical applications. Journal of Materials Chemistry B 3(30) 2015:6194–6204. doi: 10.1039/c5tb00765h. [DOI] [PubMed] [Google Scholar]
- 166.Wang J., Tian L., Luo B., Ramakrishna S., Kai D., Loh X.J., Yang I.H., Deen G.R., Mo X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B Biointerfaces. 2018;169:356–365. doi: 10.1016/j.colsurfb.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 167.Kai D., Ren W., Tian L., Chee P.L., Liu Y., Ramakrishna S., Loh X.J. Engineering poly(lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016;4(10):5268–5276. [Google Scholar]
- 168.Saudi A., Amini S., Amirpour N., Kazemi M., Zargar Kharazi A., Salehi H., Rafienia M. Promoting neural cell proliferation and differentiation by incorporating lignin into electrospun poly(vinyl alcohol) and poly(glycerol sebacate) fibers. Mater. Sci. Eng. C. 2019;104 doi: 10.1016/j.msec.2019.110005. [DOI] [PubMed] [Google Scholar]
- 169.Wang D., Jang J., Kim K., Kim J., Park C.B. “Tree to bone”: lignin/polycaprolactone nanofibers for hydroxyapatite biomineralization. Biomacromolecules 20(7) 2019:2684–2693. doi: 10.1021/acs.biomac.9b00451. [DOI] [PubMed] [Google Scholar]
- 170.Amini S., Saudi A., Amirpour N., Jahromi M., Najafabadi S.S., Kazemi M., Rafienia M., Salehi H. Application of electrospun polycaprolactone fibers embedding lignin nanoparticle for peripheral nerve regeneration: in vitro and in vivo study. Int. J. Biol. Macromol. 2020;159:154–173. doi: 10.1016/j.ijbiomac.2020.05.073. [DOI] [PubMed] [Google Scholar]
- 171.Kai D., Zhang K., Liow S.S., Loh X.J. New dual functional PHB-grafted lignin copolymer: synthesis, mechanical properties, and biocompatibility studies. ACS Applied Bio Materials 2(1) 2019:127–134. doi: 10.1021/acsabm.8b00445. [DOI] [PubMed] [Google Scholar]
- 172.Liang R., Zhao J., Li B., Cai P., Loh X.J., Xu C., Chen P., Kai D., Zheng L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119601. [DOI] [PubMed] [Google Scholar]
- 173.Morganti P., Palombo M., Carezzi F., Nunziata M., Morganti G., Cardillo M., Chianese A. Green nanotechnology serving the bioeconomy: natural beauty masks to save the environment. Cosmetics 3(4) 2016 [Google Scholar]
- 174.Yan Q., Dong H., Su J., Han J., Song B., Wei Q., Shi Y. A review of 3D printing technology for medical applications. Engineering 4(5) 2018:729–742. [Google Scholar]
- 175.Tanase-Opedal M., Espinosa E., Rodríguez A., Chinga-Carrasco G. Lignin: a biopolymer from forestry biomass for biocomposites and 3D printing. Materials (Basel) 12(18) 2019:3006. doi: 10.3390/ma12183006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Domínguez-Robles J., Martin N.K., Fong M.L., Stewart S.A., Irwin N.J., Rial-Hermida M.I., Donnelly R.F., Larrañeta E. Antioxidant PLA composites containing lignin for 3D printing applications: a potential material for healthcare applications. Pharmaceutics 11(4) 2019 doi: 10.3390/pharmaceutics11040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oveissi F., Naficy S., Le T.Y.L., Fletcher D.F., Dehghani F. Tough and processable hydrogels based on lignin and hydrophilic polyurethane. ACS Applied Bio Materials 1(6) 2018:2073–2081. doi: 10.1021/acsabm.8b00546. [DOI] [PubMed] [Google Scholar]
- 178.Zhang X., Morits M., Jonkergouw C., Ora A., Valle-Delgado J.J., Farooq M., Ajdary R., Huan S., Linder M., Rojas O., Sipponen M.H., Österberg M. Three-dimensional printed cell culture model based on spherical colloidal lignin particles and cellulose nanofibril-alginate hydrogel. Biomacromolecules 21(5) 2020:1875–1885. doi: 10.1021/acs.biomac.9b01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang X., Keck S., Qi Y., Baudis S., Zhao Y. Study on modified dealkaline lignin as visible light macromolecular photoinitiator for 3D printing. ACS Sustain. Chem. Eng. 2020;8(29):10959–10970. [Google Scholar]
- 180.Hasegawa Y., Kadota Y., Hasegawa C., Kawaminami S. Lignosulfonic acid-induced inhibition of intestinal glucose absorption. J. Nutr. Sci. Vitaminol. 2015;61(6):449–454. doi: 10.3177/jnsv.61.449. [DOI] [PubMed] [Google Scholar]
- 181.Xie F., Gong S., Zhang W., Wu J., Wang Z. Potential of lignin from Canna edulis ker residue in the inhibition of alpha-d-glucosidase: kinetics and interaction mechanism merging with docking simulation. Int. J. Biol. Macromol. 2017;95:592–602. doi: 10.1016/j.ijbiomac.2016.11.100. [DOI] [PubMed] [Google Scholar]
- 182.Coral Medina J.D., Woiciechowski A.L., Zandona Filho A., Bissoqui L., Noseda M.D., de Souza Vandenberghe L.P., Zawadzki S.F., Soccol C.R. Biological activities and thermal behavior of lignin from oil palm empty fruit bunches as potential source of chemicals of added value. Ind. Crop. Prod. 2016;94:630–637. [Google Scholar]
- 183.Barapatre A., Aadil K.R., Tiwary B.N., Jha H. In vitro antioxidant and antidiabetic activities of biomodified lignin from Acacia nilotica wood. Int. J. Biol. Macromol. 2015;75:81–89. doi: 10.1016/j.ijbiomac.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 184.Raza G.S., Maukonen J., Makinen M., Niemi P., Niiranen L., Hibberd A.A., Poutanen K., Buchert J., Herzig K.H. Hypocholesterolemic effect of the lignin-rich insoluble residue of brewer's spent grain in mice fed a high-fat diet. J Agric Food Chem 67(4) 2019:1104–1114. doi: 10.1021/acs.jafc.8b05770. [DOI] [PubMed] [Google Scholar]
- 185.Sato S., Mukai Y., Tokuoka Y., Mikame K., Funaoka M., Fujita S. Effect of lignin-derived lignophenols on hepatic lipid metabolism in rats fed a high-fat diet. Environ Toxicol Pharmacol 34(2) 2012:228–234. doi: 10.1016/j.etap.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 186.Wang G., Xia Y., Sui W., Si C. Lignin as a novel tyrosinase inhibitor: effects of sources and isolation processes, ACS. Sustain. Chem. Eng. 2018;6(7):9510–9518. [Google Scholar]
- 187.Lu F.J., Chu L.H., Gau R.J. Free radical-scavenging properties of lignin. Nutr Cancer 30(1) 1998:31–38. doi: 10.1080/01635589809514637. [DOI] [PubMed] [Google Scholar]
- 188.Wang B., Kong Q., Li X., Zhao J., Zhang H., Chen W., Wang G. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients 12(10) 2020:3197. doi: 10.3390/nu12103197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.E. Tamariz, A. Rios-Ramrez, Biodegradation of medical purpose polymeric materials and their impact on biocompatibility, Biodegradation - Life of Science2013.
- 190.Merritt R.J. Digestion of certain fractions of dietary fiber in humans: W. D. Halloway, C. Tasman-Jones, and S. P. Lee. Am J Clin Nutr. 1979;31:927–930. doi: 10.1093/ajcn/31.6.927. (June), 1978, J. Pediatr. Surg. 14(1) 97. [DOI] [PubMed] [Google Scholar]