Abstract
This cross-sectional study quantifies the number of randomized clinical trials in the literature for each indication for stem cell transplantation by disease type and status.
Hematopoietic stem cell transplantation (HCT) is a common procedure, with 22 000 cases performed in the US per year at a total cost of 1.3 billion dollars per year.1 To our knowledge, an evaluation of the quality and level of evidence of society guidelines in HCT has not been performed. In the 1980s, oncologists adopted autologous stem cell transplant for metastatic breast cancer following high-dose chemotherapy based on uncontrolled studies only to find no clinical benefit in randomized clinical trials (RCTs).2,3 In this study, we seek to quantify the number of RCTs in the literature for each indication by disease type and status. We then describe the published literature of the past 5 years.
Methods
We reviewed evidence according to the American Society for Transplantation and Cellular Therapy (ASTCT) 2020 guidelines4 for indications with S (standard of care) or C (clinical evidence available, standard of care) level recommendations for hematologic malignant diseases. Randomized clinical trials published in peer-reviewed journals were identified using the Ovid MEDLINE database. All publications under relevant medical subject headings (MeSH) were combined with results from keyword “transplantation” using the Boolean operator “AND.” We limited the search to RCTs. We reviewed all articles from 2016 to present using Google Scholar by searching disease type and keyword “transplantation” (eTables 1 and 2 in the Supplement). We reviewed the first 100 results of each search. This study was exempt from institutional review board approval because it involved publicly available data and did not involve individual patient data.
Results
In total there are 103 recommendations in the ASTCT 2020 guidelines for allogeneic transplant and autologous transplant.4 For allogeneic transplant, there are 43 S indications and 27 C indications. For autologous transplant, there are 23 S indications and 18 C indications. There were 4 RCTs for allogeneic transplant and 24 RCTs for autologous transplant corresponding to 3 and 11 S indications and 1 and 6 C indications for allogeneic and autologous transplant, respectively (Table and Figure).
Table. S and C Indications for Allogeneic and Autologous Transplant With vs Without Published RCTs.
| Indication | No. (%) | |||
|---|---|---|---|---|
| RCT | No RCTs | Total | RCT participants, No. (range) | |
| Allogeneic | ||||
| S | 3 (7) | 40 (93) | 43 | 96 (44-161) |
| C | 1 (4) | 26 (96) | 27 | 138 |
| Autologous | ||||
| S | 11 (48) | 12 (52) | 23 | 75 (4-1197) |
| C | 6 (33) | 12 (67) | 18 | 40 (3-425) |
Abbreviations: C, clinical evidence available, standard of care; S, standard of care; RCT, randomized clinical trial.
Figure. Evidence Map of Randomized Clininical Trials for Autologous Transplant Standard of Care Recommendations.
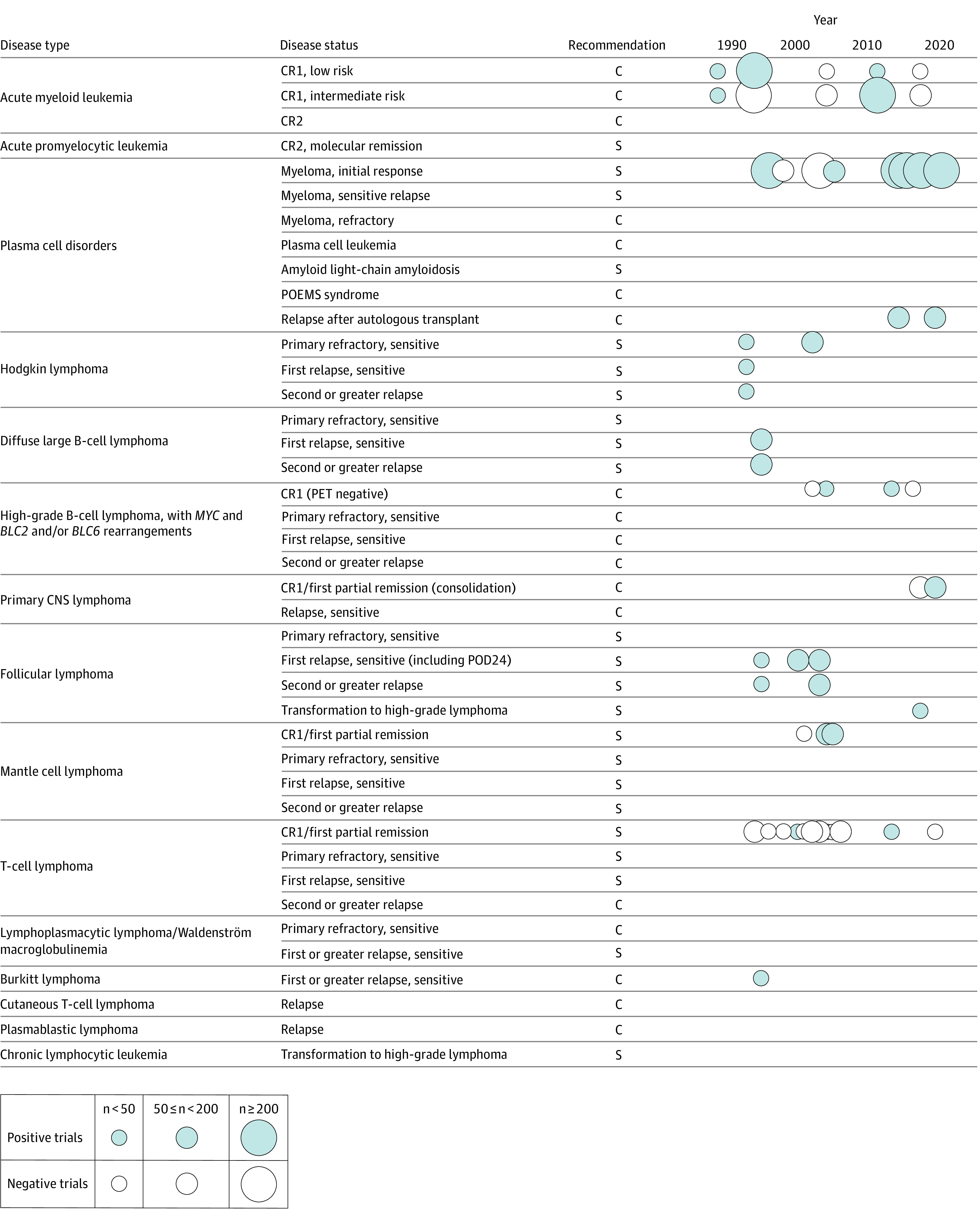
C, Indicates clinical evidence available, standard of care; CNS, central nervous system; PET, positron emission tomography; S, standard of care. Positive and negative trials for standard of care recommendations (S and C) in autologous transplantation are represented as circles. Size represents number of participants and are organized by date of publication and indication by disease type and status.
In the published literature since 2016 in allogeneic transplant, we found 299 observation or nonrandomized interventional studies, of which 208 (70%) were single-arm studies. For autologous transplant, there were 156 observational or nonrandomized interventional studies, of which 87 (56%) were single-arm studies. The number of RCTs were none for allogeneic transplant and 4 for autologous transplant.
Discussion
In this review of the literature, we found that only 4 of 70 (6%) standard-of-care recommendations for allogeneic transplantation and 17 of 41 (41%) for autologous transplantation were supported by randomized clinical trials. Yet of 103 ASTCT indications there were 70 S and C recommendations for allogeneic transplant and 41 for autologous transplant. Taken together, our results demonstrate that there has been widespread adoption of HCT, especially allogeneic transplant, based on low levels of evidence.
Allogeneic transplant has now become standard of care by historical precedent for hematologic malignant diseases with poor prognosis, such as high-risk acute myeloid leukemia. Ethics and feasibility are raised regarding RCTs in this setting. However, offering an unproven aggressive therapy with high treatment-related mortality merely on the basis of poor predicted outcome is also questionable. Physicians may underestimate the burden of treatment and treatment complications and equate higher response rates or feasibility with longer survival or higher cure rates. These components should be explored formally in RCTs. This study is limited as it is not a comprehensive review of evidence for specific disease types. Instead, we aimed to provide an overview of the broad literature behind stem cell transplant.
The benefits of allogeneic transplantation are unknown in both highly lethal conditions such as plasma cell leukemia and less dismal conditions such as peripheral T-cell lymphoma. However, it would be more feasible to do pragmatic RCTs when there is clinical equipoise. Intermediate-risk acute myeloid leukemia is an example where retrospective studies are conflicting, and a pragmatic RCT is feasible. Randomizing transplant-eligible patients at time of diagnosis with minimal restrictions on donor choice or induction regimens in both transplant and nontransplant cohorts would provide crucial information for informed decisions in patient care.
eTable 1. MeSH terms and subheadings in Ovid MEDLINE search
eTable 2. Search terms in Google Scholar search
References
- 1.Center for International Blood and Marrow Transplant Research Transplant Activity Report Covering 2013-2017. Health Resources & Services Administration website. Updated October, 2020. Accessed April 7, 2021. https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report
- 2.Eddy DM. High-dose chemotherapy with autologous bone marrow transplantation for the treatment of metastatic breast cancer. J Clin Oncol. 1992;10(4):657-670. doi: 10.1200/JCO.1992.10.4.657 [DOI] [PubMed] [Google Scholar]
- 3.Stadtmauer EA, O’Neill A, Goldstein LJ, et al. ; Philadelphia Bone Marrow Transplant Group . Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. N Engl J Med. 2000;342(15):1069-1076. doi: 10.1056/NEJM200004133421501 [DOI] [PubMed] [Google Scholar]
- 4.Kanate AS, Majhail NS, Savani BN, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247-1256. doi: 10.1016/j.bbmt.2020.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. MeSH terms and subheadings in Ovid MEDLINE search
eTable 2. Search terms in Google Scholar search


