Abstract
Polycystic ovary syndrome (PCOS) affects up to 15% of women and is associated with increased risk of obesity and cardiovascular disease. Repeated passive heat exposure [termed “heat therapy” (HT)] is a lifestyle intervention with the potential to reduce cardiovascular risk in obesity and PCOS. Women with obesity (n = 18) with PCOS [age 27 ± 4 yr, body mass index (BMI) 41.3 ± 4.7 kg/m2] were matched for age and BMI, then assigned to HT (n = 9) or time control (CON; n = 9). HT subjects underwent 30 one-hour hot tub sessions over 8–10 wk, whereas CON subjects did not undergo HT. Muscle sympathetic nerve activity (MSNA), blood pressure, cholesterol, C-reactive protein, and markers of vascular function were assessed at the start (Pre) and end (Post) of 8–10 wk. These measures included carotid and femoral artery wall thickness and flow-mediated dilation (FMD), measured both before and after 20 min of ischemia-20 min of reperfusion (I/R) stress. HT subjects exhibited reduced MSNA burst frequency (Pre: 20 ± 8 bursts/min, Post: 13 ± 5 bursts/min, P = 0.012), systolic (Pre: 124 ± 5 mmHg, Post: 114 ± 6 mmHg; P < 0.001) and diastolic blood pressure (Pre: 77 ± 6 mmHg, Post: 68 ± 3 mmHg; P < 0.001), C-reactive protein (Pre: 19.4 ± 13.7 nmol/L, Post: 15.2 ± 12.3 nmol/L; P = 0.018), total cholesterol (Pre: 5.4 ± 1.1 mmol/L, Post: 5.0 ± 0.8 mmol/L; P = 0.028), carotid wall thickness (Pre: 0.054 ± 0.005 cm, Post: 0.044 ± 0.005 cm; P = 0.010), and femoral wall thickness (Pre: 0.056 ± 0.009 cm, Post: 0.042 ± 0.005 cm; P = 0.003). FMD significantly improved in HT subjects over time following I/R (Pre: 5.6 ± 2.5%, Post: 9.5 ± 1.7%; P < 0.001). No parameters changed over time in CON, and BMI did not change in either group. These findings indicate that HT reduces sympathetic nerve activity, provides protection from I/R stress, and substantially improves cardiovascular risk profiles in women who are obese with PCOS.
Keywords: heat acclimation, ischemia reperfusion injury, muscle sympathetic nerve activity, thermal therapy, vascular function
INTRODUCTION
Heat exposure has been used for centuries in various populations for purported therapeutic benefit, including Scandinavian saunas, Japanese Waon therapy, Turkish baths, and Native American sweat lodges. Heat is a complex, global stressor with the potential to impact a variety of systems both within an acute exposure and through the physiological acclimation that occurs with repeated bouts of heat exposure. Repeated passive heat exposure [termed “heat therapy” (HT)] has recently received renewed interest for improving cardiovascular risk profile in healthy populations (3, 4) and those with overt cardiovascular disease (23, 26, 52). However, a large spectrum of cardiovascular risk exists between health and disease, and populations at an elevated risk of developing cardiovascular disease may have the greatest potential to benefit from such an intervention. In addition, the majority of cardiovascular treatment guidelines and pharmaceutical interventions are based on research done in men, and women with elevated cardiovascular risk deserve focused interventions aimed at improving cardiovascular health.
Polycystic ovary syndrome (PCOS), an endocrine disorder characterized by menstrual dysfunction, clinical hyperandrogenism, and polycystic ovarian morphology (2), affects 6%–15% of women of child-bearing age and is often accompanied by extremely high rates of obesity (56), insulin resistance (14), autonomic dysfunction (29), and elevated markers of inflammation (17). In combination, these factors substantially impair cardiovascular health in women with PCOS (57). Few pharmaceutical therapies or lifestyle interventions for PCOS are specifically aimed at improving cardiovascular health in this population. Although regular exercise training can improve elements of health, including body mass index (BMI), endocrine function, and insulin resistance, in women who are obese with PCOS, effects of exercise training on cardiovascular health in this population are less consistent (19), and high dropout/noncompliance rates in exercise interventions further reduce efficacy in women who are obese with PCOS (19).
In obesity and PCOS (49), sympathetic nervous system overactivity is implicated in elevated blood pressure and cardiometabolic dysfunction (10a). Although the impact of HT on muscle sympathetic nerve activity (MSNA) has not been examined, rodent heat acclimation models have observed alterations in cardiac autonomic influence (22), and seasonal variation studies in humans observed a decrease in MSNA in summer months (39). Examining MSNA after a long-term heat intervention is particularly important in a clinical population with high sympathetic activity and associated cardiometabolic dysfunction, such as women who are obese with PCOS.
As these changes in vascular function with HT collectively reduce cardiovascular risk profile, emerging evidence from large prospective studies also indicates that increased frequency and duration of heat (sauna) exposure reduces cardiovascular morbidity [risk of incident hypertension (61)] and mortality (33). Much of the damage incurred during fatal cardiovascular events involving blockages to coronary (myocardial infarction) or cerebral vasculature (stroke) is due to ischemia-reperfusion (I/R) tissue injury. In murine models, 30-day heat acclimation affords protection from I/R injury such that cardiac myocytes are better able to survive I/R stress (34). In humans, acute hot tub use appears to temporarily protect tissue from I/R stress (5), but this effect has not been examined in a chronic heat intervention. This protection would be particularly powerful in women who are obese with PCOS, a population with increased risk of cardiovascular or cerebrovascular death.
Therefore, the purpose of this study was to examine the effect of a 30-session HT intervention on blood pressure, MSNA, arterial stiffness, arterial wall thickness, endothelial function, and vascular tolerance to I/R in women who are obese with PCOS. We hypothesized that HT in women who are obese with PCOS would decrease blood pressure, MSNA, and arterial wall thickness and stiffness and would increase endothelial function [assessed by flow-mediated dilation (FMD)] and vascular tolerance to I/R stress. This study was part of a larger investigation of the impact of HT on cardiometabolic health in women who are obese (clinical trial registration: NCT03644524), and the same subjects participated in an oral glucose tolerance test (OGTT), subcutaneous adipose tissue biopsy, and other biomarkers of metabolic health over the course of HT that are reported elsewhere (16). Demographic data, thermoregulatory responses to heating sessions, and relevant fasting blood data (insulin, glucose, cholesterol, and total testosterone) are included in both manuscripts, as they are relevant to both cardiovascular and metabolic outcome variables.
METHODS
Subjects.
Women with obesity (n = 18; defined as a BMI ≥30 and ≤45 kg/m2) volunteered to participate in this study. All subjects provided oral and written informed consent before participation in accordance with the Declaration of Helsinki, and all experimental procedures were approved by the Institutional Review Board at the University of Oregon. Subjects were nonsmokers, had not been diagnosed with overt cardiovascular or metabolic disease, and were diagnosed with PCOS by a physician based on the Rotterdam criteria (43). This diagnosis is made through the presence of two of the three following criteria: biochemical or clinical hyperandrogenism (elevated testosterone or free androgen index; presence of hirsutism or male pattern baldness), oligo- or anovulation (menstrual cycles >35 days apart), and polycystic ovarian morphology upon ultrasound examination. Women were matched for age and BMI and placed in either the HT intervention or time control (CON; no HT) group. The goal was to recruit subjects who were not taking any medications; however, the extremely high prescription rates in women with PCOS made this difficult. In total, one subject in each group was taking oral contraceptives, and two subjects in each group were taking selective serotonin reuptake inhibitors (SSRIs) for treatment of depression or anxiety, both common in women with PCOS (10). Although these medications can potentially influence autonomic outflow, medication rates were matched between groups, and subjects were taking the medication at a consistent dose and time of day throughout the study. A summary of physical characteristics is listed in Table 1. No significant differences in any parameter were observed between HT and CON in Pre.
Table 1.
Summary of cardiovascular risk variables over time in HT and CON subjects
| Group | Pre | Mid | Post |
|---|---|---|---|
| BMI, kg/m2 | |||
| HT | 41.8 ± 4.0 | 41.9 ± 4.2 | 41.8 ± 4.3 |
| CON | 39.9 ± 5.4 | 39.8 ± 5.4 | 39.5 ± 5.2 |
| Sum of skinfolds, mm | |||
| HT | 137 ± 6 | 138 ± 8 | 135 ± 7 |
| CON | 132 ± 8 | 134 ± 7 | 138 ± 8 |
| Systolic blood pressure, mmHg | |||
| HT | 124 ± 5 | 119 ± 6 | 114 ± 6*† |
| CON | 122 ± 5 | 124 ± 6 | 120 ± 6 |
| Diastolic blood pressure, mmHg | |||
| HT | 77 ± 6 | 69 ± 9* | 68 ± 3*† |
| CON | 74 ± 6 | 73 ± 6 | 75 ± 6 |
| Mean arterial pressure, mmHg | |||
| HT | 93 ± 5 | 86 ± 7*† | 83 ± 3*† |
| CON | 90 ± 6 | 90 ± 6 | 90 ± 6 |
| Total cholesterol, mmol/L | |||
| HT | 5.43 ± 1.10 | 5.17 ± 0.76 | 5.01 ± 0.81* |
| CON | 4.77 ± 0.58 | 4.68 ± 0.58 | 4.77 ± 0.72 |
| HDL, mmol/L | |||
| HT | 1.17 ± 0.26 | 1.19 ± 0.24 | 1.16 ± 0.24 |
| CON | 1.10 ± 0.21 | 1.09 ± 0.29 | 1.14 ± 0.25 |
| LDL, mmol/L | |||
| HT | 3.41 ± 0.88 | 3.22 ± 0.52 | 2.92 ± 0.49* |
| CON | 2.78 ± 0.53 | 2.78 ± 0.52 | 2.89 ± 0.62 |
| VLDL, mmol/L | |||
| HT | 0.87 ± 0.35 | 0.76 ± 0.31 | 0.94 ± 0.48 |
| CON | 0.89 ± 0.35 | 0.81 ± 0.44 | 0.74 ± 0.21 |
| Fasting glucose, mg/dL | |||
| HT | 105 ± 3 | 100 ± 5 | 89 ± 5*† |
| CON | 106 ± 3 | 102 ± 4 | 108 ± 3 |
| Fasting insulin, mIU/L | |||
| HT | 24 ± 3 | 21 ± 4 | 24 ± 4 |
| CON | 26 ± 2 | 25 ± 2 | 22 ± 2 |
| Total testosterone, nmol/L | |||
| HT | 1.78 ± 0.78 | 1.58 ± 0.49 | 1.18 ± 0.46*† |
| CON | 1.44 ± 0.40 | 1.56 ± 0.62 | 1.61 ± 0.61 |
| C-reactive protein, nmol/L | |||
| HT | 19.4 ± 13.9 | 14.7 ± 10.2* | 15.2 ± 12.6*† |
| CON | 18.3 ± 14.4 | 20.7 ± 17.7 | 20.3 ± 18.1 |
Values are means ± SD. CON, control; HT, heat therapy.
Significant (P < 0.05) difference from Pre within group;
Significant difference (P < 0.05) from CON in Post.
HT intervention.
HT occurred over an 8- to 10-wk period, with a total of 30 one-hour sessions scheduled 3–4 times per week in all subjects enrolled in HT. Passive hot water immersion was selected as the method of heat stress because it is capable of increasing core temperature at a rate similar to moderate-intensity exercise (25) while also producing high skin temperature and sweating rate, all requisite components for adaptation to heat. The exposure duration and total number of sessions was selected because it is similar in time commitment to exercise training interventions in women with PCOS (19).
For each session, HT subjects completed 60 min of supervised water immersion in a 40.5°C bath. Subjects immersed to shoulder level until core temperature (rectal thermistor; Yellow Springs Instruments) rose to 38.5°C (mean time to 38.5°C for all sessions = 32 ± 9 min), then sat upright (immersed to waist) for the remainder of the session to maintain core temperature between 38.5 and 39.0°C. After 60 min of exposure, subjects were seated next to the tub until core temperature fell below 38.5°C (10–15 min) for safety monitoring. The mean time spent with core temperature over 38.5°C for all sessions was 37 ± 10 min. Subjects were also weighed pre- and postheat exposure (nude, towel-dried, behind a privacy screen), and ad libitum water intake during heating was recorded to calculate sweat losses. Total sweat loss (accounting for fluid intake and any urine losses) was calculated (∆body mass/time and expressed in L/h).
Time control.
Control subjects were matched for age, BMI, and study timeline but did not undergo HT. Both subject groups were instructed to maintain all diet and lifestyle factors, and the time control group was communicated with on a weekly basis. Although we recognize that the control group did not include a sham intervention, this is an established model for nonpharmaceutical interventions (exercise, electroacupuncture) in PCOS literature (1, 47, 55). In addition, in previous work in our laboratory in sedentary men and women, we employed a similar protocol (36 sessions over ~8 wk) with both an HT and thermoneutral sham water immersion group. We did not observe any changes in cardiovascular health or function in the sham group over the course of the study (4).
Cardiovascular health assessment.
Study days to assess MSNA and vascular function took place at the beginning (Pre; 0 heating sessions), midpoint (Mid; after 14–16 heating sessions, or a similar 4-to 5-wk time interval in CON subjects), and end (Post; after 30 heat sessions or equivalent time control). All subjects reported to a temperature-controlled (18°C–21°C) laboratory environment, having refrained from food for a minimum of 4 h; caffeine and alcohol for 12 h; vitamin supplementation, medications (other than oral contraceptive), and exercise for 24 h; and heat exposure for at least 36 h. Upon arrival, BMI (weight in kg/height in m2) and 3-site skinfold thickness (tricep, suprailiac, thigh) were assessed using established techniques. All testing took place in the morning, and time of day was held constant (within 1 h) for each subject over time to minimize circadian influence. Subjects rested on a padded exam table for a minimum of 20 min before beginning testing, and during this time, they were instrumented with a 3-lead ECG, automated brachial blood pressure cuff, and beat-by-beat blood pressure monitor (Nexfin, Edwards Life Sciences, Irvine, CA).
Muscle sympathetic nerve activity.
MSNA was recorded via microneurography of the fibular nerve. The nerve was located using external stimulation in the region behind the knee and below the fibular head, and sites that showed strong muscle twitches were marked for reference. Once a site was selected, postganglionic MSNA was recorded through a tungsten microelectrode inserted percutaneously into the common fibular nerve. Nerve traffic was recorded continuously using WinDaq data acquisition software during quiet supine resting, with and without paced breathing to a metronome, and analyzed using standard techniques. Sympathetic nerve bursts were identified offline by the primary investigator and confirmed (±1 burst/min) by a second blinded investigator with a minimum 3:1 signal-to-noise ratio and confirmed by measuring pulse synchronicity. Primary variables of interest for MSNA were burst frequency (bursts/min; measured over a minimum of 5 min) and burst incidence (bursts/100 heartbeats; measured over a minimum of 5 min). Additionally, sympathetic baroreflex sensitivity was assessed by having subjects perform a Valsalva maneuver during MSNA. This was accomplished using an expiratory pressure gauge with an on-screen display of pressure. Subjects were encouraged to maintain pressure at 40 mmHg for 20 s while contracting their abdominals. Sympathetic baroreflex sensitivity was examined by locating the diastolic pressure nadir during Phase II and relating all sympathetic burst activity during the 20-s Valsalva maneuver to this maximal fall in diastolic pressure. By plotting change in diastolic pressure and burst incidence at rest compared with during Phase II nadir, a slope (∆burst incidence/∆diastolic blood pressure) was calculated as a measure of baroreflex sensitivity in response to blood pressure decrease. Although this method does not include blood pressure hysteresis, a similar technique has been used to assess sympathetic baroreflex sensitivity during Valsalva straining and matched well with measures of spontaneous baroreflex sensitivity (18, 48, 60).
Vascular function.
Testing order was held constant, beginning with assessment of resting brachial blood pressure, common carotid wall thickness, and dynamic arterial compliance (DAC), followed by superficial femoral wall thickness and DAC, carotid-femoral and brachial-ankle pulse wave velocity (PWV), and FMD before and after I/R (described in FMD with I/R).
Blood pressure.
Subjects rested supine on a padded exam table in a dark, quiet, room-temperature environment for 20 min before assessment. Brachial blood pressure was measured in triplicate on the left arm (Accutorr Plus, DataScope, Marlborough, MA), and the median value was used. Brachial blood pressure was also periodically monitored throughout vascular function testing, and beat-by-beat blood pressure was continuously monitored (Nexfin).
Wall thickness.
Wall thickness of the common carotid and superficial femoral artery was imaged using high-resolution Doppler ultrasound (Terason t3000cv; Teratech, Burlington, MA) in B mode with 10.0-MHz linear array ultrasound transducer probe artery. The carotid artery was imaged 2 cm distal to the carotid bulb at three angles: anterior, lateral, and posterior. The superficial femoral artery was imaged 2–4 cm distal to the femoral bifurcation in two planes: anterior and lateral. Clearly distinguished intimal-medial boundaries were obtained on the far wall. Images were frozen in diastole and enlarged, and calipers were used to make three repeat measurements of the wall thickness from the lumen-intima interface to the media-adventitia interface. Video recording of these measurements was later reviewed offline by a blinded investigator to confirm accuracy of caliper measurement, and three measurements from each angle were averaged.
Arterial stiffness.
Arterial stiffness was assessed using several different methods, including carotid and superficial femoral DAC and carotid-femoral and brachial-ankle PWV. Carotid and femoral DAC were measured using high-resolution Doppler ultrasound (Terason t3000cv; Teratech) with a 10.0-MHz linear array ultrasound transducer probe with concurrent applanation tonometry (PCU-2000; Millar, Houston, TX) on the same artery on the contralateral side of the body. Ultrasound probe placement on the body, including angle of approach and internal anchors such as distance from bifurcation, were recorded on the first trial day and repeated to ensure consistency between repeated measurements over the course of the study. All ultrasound recordings were performed on the right side of the body. Ultrasound images were recorded at 20 Hz using video recording software (Camtasia) and then analyzed for diameter and blood velocity using custom-designed edge detection and wall tracking software (DICOM; Perth, Australia). Pulse pressure using applanation tonometry was simultaneously recoded via WinDaq data acquisition (Dataq) at 250 Hz and analyzed using the trough-to-peak pressure differential (∆P). This ∆P was then analyzed relative to change in diameter (∆D) by a blinded investigator for a minimum of 50 cardiac cycles to calculate cross-sectional compliance and β stiffness using the following equations: DAC = [(∆D/D)/2∆P] × πD2 and β stiffness index = Ln (SBP/DBP) × D/∆D, where SBP is systolic blood pressure and DBP is diastolic blood pressure.
Pulse-wave velocity was assessed using tonometry probes placed on the carotid and femoral arteries (central or aortic PWV), as well as the brachial and dorsal pedal arteries (peripheral PWV). The pulse upswings of a minimum of 30 simultaneously recorded pressure tracings were identified offline by a blinded investigator to calculate the time differential. Velocity was calculated as distance over time, where distance was calculated as the differential of the linear measurements from the carotid probe to the sternal notch and the sternal notch to the femoral probe, or the distance differential between the brachial probe to sternal notch and the ankle probe to sternal notch.
FMD with I/R.
Endothelial function was assessed using gold-standard FMD at the brachial artery using Doppler ultrasonography in accordance with expert consensus guidelines (51). On each study day, FMD was measured at baseline and again after a 20-min occlusion/20-min reperfusion as a model of endothelial function in response to an acute I/R episode. FMD consists of imaging of the brachial artery by Doppler ultrasound to obtain baseline diameter and blood velocity measurement, then inflating an occlusion cuff (set to 250 mmHg) just below the elbow for a period of 5 min. Velocity and diameter were captured using Doppler ultrasound, and the ultrasound images were recorded and analyzed by a blinded investigator for changes in brachial artery diameter and blood velocity after cuff release. In addition to standard FMD analysis (% dilation), shear-corrected FMD and postocclusive reactive hyperemia (PORH) were calculated as previously described (4, 41). FMD at the brachial artery has been shown to parallel coronary artery endothelial function (50), is a well-established predictor of cardiovascular risk and future cardiovascular events (44), and has improved with HT in healthy humans (4). In addition, FMD is impaired by I/R (5). I/R was performed by placing an occlusion cuff on the upper arm (above the point where the brachial artery was imaged for FMD) and inflating it to 250 mmHg for a period of 20 min. Twenty minutes after release of the cuff, FMD was reassessed. This protocol was selected, as it has been used in our laboratory with an acute heat intervention (5), has been well tolerated by subjects, and shows a short-term impairment of endothelial and microvascular function.
Fasting blood samples.
On a separate day at each time point (Pre-Mid-Post), subjects reported to the laboratory after a 12-h overnight fast and having refrained from medications, exercise, and heat exposure for ≥24 h, for a venous blood draw. Blood was drawn from the antecubital space into serum separator tubes, then allowed to clot at room temperature for 30 min before centrifugation (10 min at 1,500 g at 4°C). Serum was frozen at −80°C and later thawed for analysis of cholesterol panels (Oregon Health & Science University lipid laboratory; Roche Diagnostics COBAS 311), high-sensitivity C-reactive protein (Enzo Life Sciences ELISA), glucose (YSI 2300 Stat Plus), and total testosterone (Enzo Life Sciences ELISA). Insulin samples were batch analyzed by the Oregon Clinical and Translational Research Institute.
Statistics.
All data are presented as means ± SD, except as noted in Figs. 3 and 4 (means ± SE). Results were analyzed using mixed-model ANOVA in GraphPad Prism 6, with repeated measures within HT or CON groups for each subject and a nonrepeated measures comparison between groups of subjects. If a significant main effect was observed, a Holm-Sidak post hoc analysis was utilized to examine within- or between-group effects. In addition, correlation analyses were performed using Graphpad Prism 6 to examine potential relationships between changes in MSNA and blood variables. Significance was accepted when a calculated slope was determined to be significantly different from zero, and r values are reported as appropriate. A power analysis for our primary variable of interest (mean arterial pressure) using conventional α = 0.05 and β = 0.80 determined a minimum sample size of 6 subjects/group.
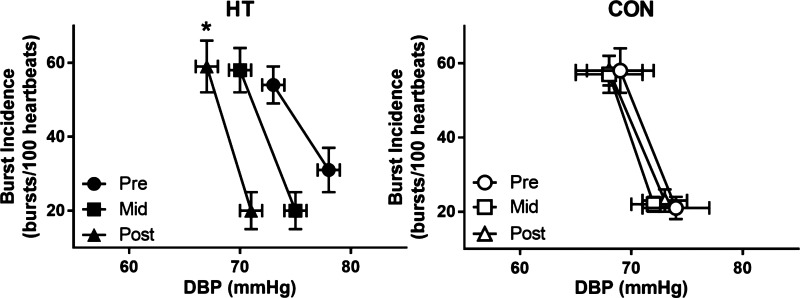
Fig. 3.
Sympathetic baroreflex sensitivity at Pre, Mid, and Post in heat therapy (HT) and control (CON) subjects, displayed as mean ± SE. *Significant (P < 0.05) difference from Pre within group. DBP, diastolic blood pressure.
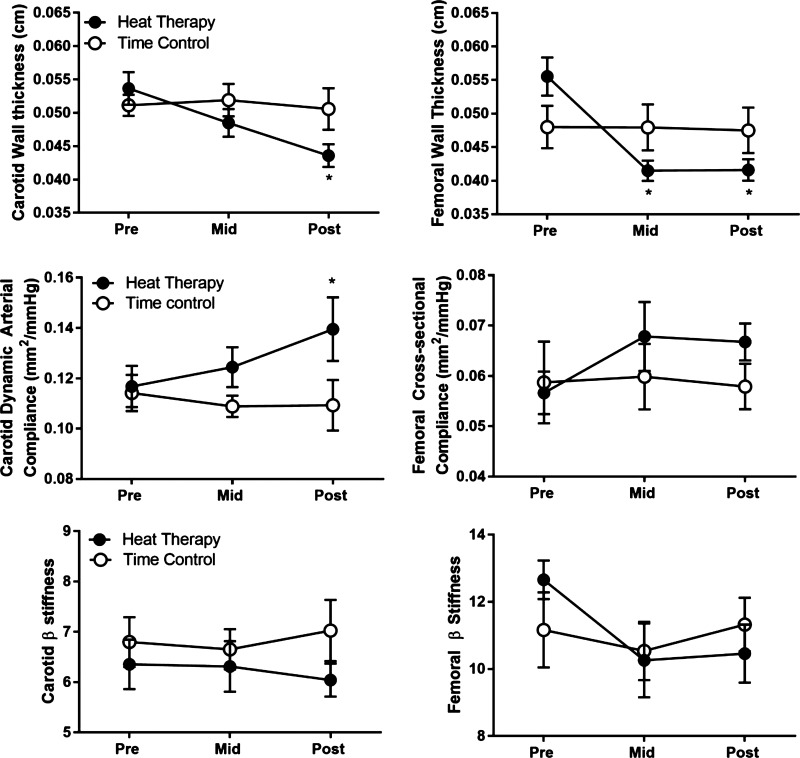
Fig. 4.
Common carotid and superficial femoral wall thickness, compliance, and β stiffness over time in heat therapy (HT) and control (CON) subjects, displayed as means ± SE. *Significant (P < 0.05) difference from Pre within group.
RESULTS
Subjects were well matched for age (HT: 26 ± 6 yr; CON: 27 ± 6 yr) and BMI (HT: 41.8 ± 3.9 kg/m2; CON: 40.7 ± 5.4 kg/m2). Nine HT subjects completed the HT intervention and exhibited classic signs of heat adaptation, including a reduced basal body temperature (session 1: 37.6 ± 0.6°C, session 30: 37.2 ± 0.6°C; P < 0.001) and increased sweating rate during heating (session 1: 0.71 ± 0.57 L/h, session 30: 1.21 ± 0.60 L/h; P < 0.001). HT subjects tolerated the heat sessions well, with only 3 instances of light-headedness (resolved by using a fan, fluids, and removal from the hot tub) in over 270 sessions administered. One CON subject withdrew before Post testing and was therefore not included in analyses. CON and HT subjects did not exhibit any changes in BMI over the course of 8–10 wk. Cardiovascular risk markers (BMI, blood pressure, cholesterol, glucose, insulin, C-reactive protein) over time are summarized in Table 1. Both HT and CON groups exhibited elevated cholesterol, glucose, insulin, testosterone, and C-reactive protein levels at Pre, with no significant differences between groups. Total cholesterol, LDL cholesterol, fasting glucose, total testosterone, and C-reactive protein significantly decreased in HT subjects over time, with no change in CON (Table 1). Fasting insulin, HDL, and VLDL did not change in either group.
Blood pressure.
With the use of 2017 American Heart Association guidelines, 7 subjects (3 HT, 4 CON) were classified as “elevated blood pressure” and 7 were classified as “stage one hypertensive” (4 HT, 3 CON). Two subjects in each group were classified as normotensive (<120 mmHg SBP and <80 mmHg DBP). HT subjects experienced a decrease in systolic (group × time interaction, P = 0.003), diastolic (group × time interaction, P = 0.0001), and mean arterial pressure over time (group × time interaction, P = 0.0004), with 7 of 9 subjects changing risk classification and no change in CON subjects (Fig. 1, mean values summarized in Table 1).
Fig. 1.
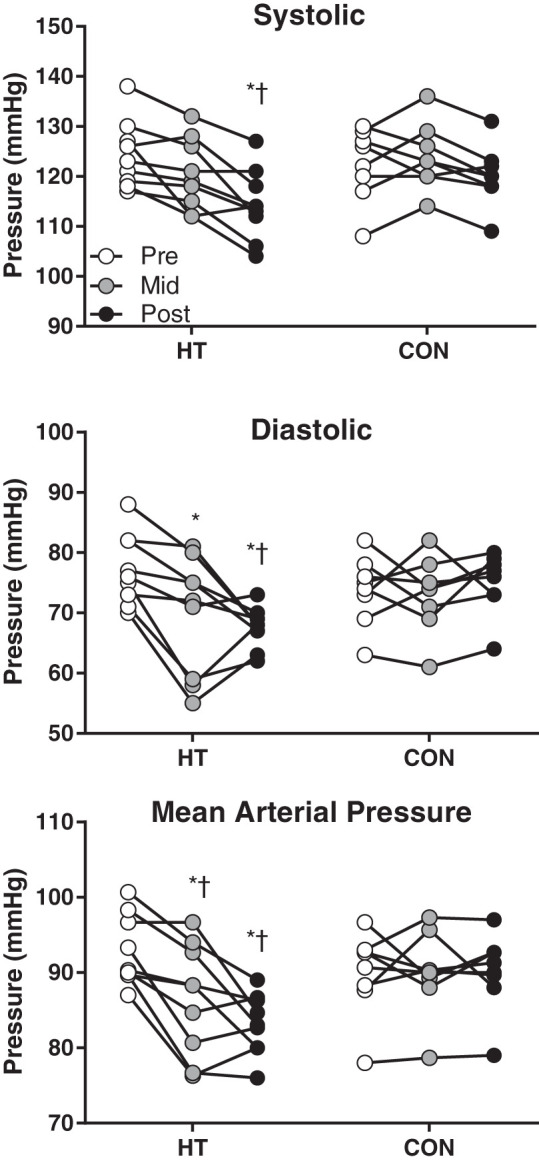
Individual systolic, diastolic, and mean arterial pressure over time in heat therapy (HT) and control (CON) subjects. *Significant (P < 0.05) difference from Pre within group. †Significant difference (P < 0.05) from CON at matched time point.
Muscle sympathetic nerve activity.
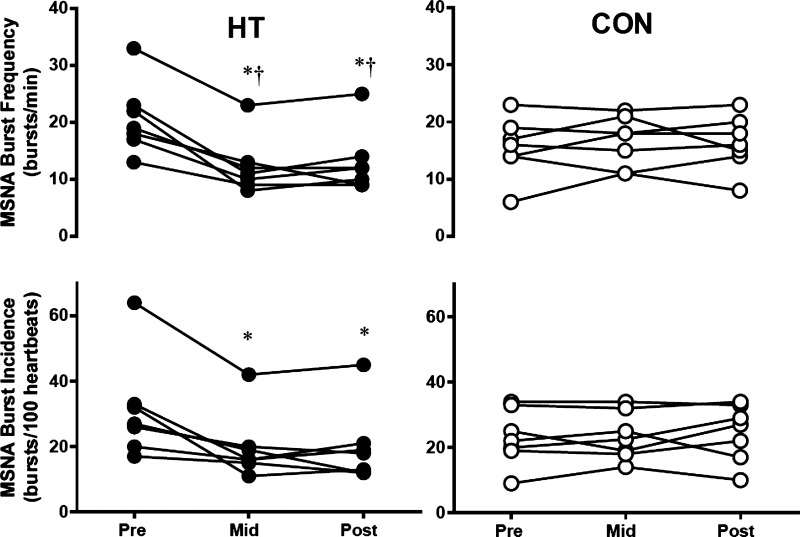
Successful nerve recordings were obtained on 14 individuals (7 HT, 7 CON), with one HT subject missing midpoint MSNA. Burst frequency declined over the course of HT (group × time interaction P < 0.0001), with an approximately ~40% decline in burst frequency evident at Mid testing. Individual data for all subjects for both burst frequency and incidence are shown in Fig. 2, and mean values are summarized in Table 2. Each group contained one subject taking SSRIs and one subject taking oral contraceptives; however, these subjects displayed a similar baseline, time course, and magnitude of change with HT as unmedicated subjects. All HT subjects exhibited a decrease in baseline MSNA by the Mid time point, which was maintained in Post testing, and no changes over time were observed in CON subjects. In addition, baroreflex sensitivity increased over time in HT, with no change in CON (Table 2 and Fig. 3; group × time interaction, P = 0.048). HT subjects also experienced a decrease in resting heart rate over time (group × time interaction, P = 0.025), whereas heart rate in CON subjects did not change (Table 2).
Fig. 2.
Individual muscle sympathetic nerve activity (MSNA) burst frequency and incidence in heat therapy (HT) and control (CON) subjects. *Significant (P < 0.05) difference from Pre within group. †Significant difference (P < 0.05) from CON at matched time point.
Table 2.
Summary of MSNA, sympathetic baroreflex sensitivity, and heart rate over time in HT and CON subjects
| Group | Pre | Mid | Post |
|---|---|---|---|
| Burst frequency, bursts/min | |||
| HT | 20 ± 8 | 12 ± 5*† | 13 ± 5*† |
| CON | 16 ± 5 | 17 ± 5 | 16 ± 5 |
| Burst incidence, bursts/100 heartbeats | |||
| HT | 31 ± 16 | 20 ± 13* | 20 ± 11* |
| CON | 23 ± 8 | 24 ± 8 | 25 ± 8 |
| sBRS, ∆BI/∆DBP | |||
| HT | −6.4 ± 2.9 | −8.1 ± 2.3 | −12.6 ± 6.8* |
| CON | −8.8 ± 4.2 | −9.5 ± 3.1 | −8.2 ± 3.2 |
| Heart rate, beats/min | |||
| HT | 73 ± 12 | 68 ± 9* | 64 ± 9*† |
| CON | 74 ± 14 | 74 ± 11 | 72 ± 11 |
Values are means ± SD. BI, burst incidence; CON, control; DBP, diastolic blood pressure; HT, heat therapy; sBRS, sympathetic baroreceptor reflex sensitivity.
Significant (P < 0.05) difference from Pre within group;
Significant difference (P < 0.05) from CON in Post.
Change in total testosterone (calculated as Mid − Pre and Post − Pre in HT and CON) was significantly correlated with change in MSNA burst frequency (P = 0.001, r = 0.64), incidence (P < 0.001, r = 0.65), and baroreflex slope (P = 0.017, r = 0.54). In contrast, change in C-reactive protein was not significantly correlated with change in MNSA burst frequency (P = 0.109, r = 0.11), incidence (P = 0.394 and r = 0.04), or baroreflex slope (P = 0.29, r = 0.27).
Wall thickness.
As seen in Fig. 4, both common carotid (group × time interaction, P = 0.0138) and superficial femoral wall thickness (group × time interaction, P = 0.0013) decreased in HT subjects, with femoral artery wall thickness decreasing by the Mid time point, and carotid decreasing in Post. Carotid and femoral wall thickness did not change in CON.
Arterial stiffness.
Cross-sectional compliance and β-stiffness for the common carotid and superficial femoral artery are shown in Fig. 4. β-Stiffness did not change in either the carotid or femoral artery; however; a decrease in carotid compliance occurred in Post testing for HT subjects (group × time interaction, P = 0.037). Brachial-ankle PWV decreased at the Post time point in HT (group × time interaction, P = 0.046), whereas carotid-femoral PWV did not change (Table 3).
Table 3.
FMD, PORH, and arterial stiffness measures in HT and CON
| Group | Pre | Mid | Post |
|---|---|---|---|
| Pre-IR FMD, % | |||
| HT | 7.6 ± 2.8 | 9.5 ± 1.7 | 9.2 ± 2.2 |
| CON | 8.7 ± 1.7 | 8.6 ± 2.8 | 8.1 ± 2.9 |
| Post-IR FMD, % | |||
| HT | 5.6 ± 2.7# | 8.7 ± 2.4* | 9.5 ± 1.8*† |
| CON | 7.7 ± 3.6# | 6.7 ± 1.7# | 6.3 ± 2.8# |
| Brachial artery diameter, cm | |||
| HT | 0.315 ± 0.075 | 0.326 ± 0.072* | 0.337 ± 0.066*† |
| CON | 0.302 ± 0.081 | 0.304 ± 0.084 | 0.306 ± 0.073 |
| Pre-IR FMD, shear-corrected | |||
| HT | 3.7 ± 0.9 | 6.3 ± 2.1* | 6.5 ± 2.5*† |
| CON | 4.7 ± 1.5 | 4.2 ± 1.1 | 4.0 ± 0.8 |
| Post-IR FMD, shear-corrected | |||
| HT | 3.4 ± 0.9 | 5.2 ± 1.3* | 5.7 ± 2.6*† |
| CON | 3.9 ± 2.5 | 3.2 ± 1.1 | 3.0 ± 1.2 |
| Pre-IR peak PORH, fold ∆ | |||
| HT | 1.0 ± 0 | 1.0 ± 0.1 | 1.2 ± 0.2 |
| CON | 1.0 ± 0 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Post-IR peak PORH, fold ∆ | |||
| HT | 0.6 ± 0.2# | 0.9 ± 0.3* | 1.1 ± 0.4*† |
| CON | 0.7 ± 0.2# | 0.6 ± 0.2# | 0.7 ± 0.2# |
| Pre-IR AUC PORH (fold ∆) | |||
| HT | 1.0 ± 0 | 1.3 ± 0.4* | 1.3 ± 0.2* |
| CON | 1.0 ± 0 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Post-IR AUC PORH, fold ∆ | |||
| HT | 0.7 ± 0.2# | 0.9 ± 0.4 | 1.2 ± 0.4*† |
| CON | 0.8 ± 0.3# | 0.7 ± 0.3# | 0.7 ± 0.3# |
| Carotid-femoral PWV, cm/s | |||
| HT | 698 ± 69 | 711 ± 79 | 689 ± 45 |
| CON | 697 ± 59 | 690 ± 72 | 680 ± 68 |
| Brachial-ankle PWV, cm/s | |||
| HT | 870 ± 81 | 844 ± 95 | 798 ± 72* |
| CON | 869 ± 86 | 841 ± 84 | 845 ± 82 |
Values are means ± SD. AUC, area under the curve; CON, control; FMD, flow-mediated dilation; HT, heat therapy; IR, ischemia-reperfusion; PORH, postocclusive reactive hyperemia; PWV, pulse-wave velocity.
Significant (P < 0.05) difference from Pre within group;
Significant difference (P < 0.05) from Pre-IR;
Significant difference (P < 0.05) from CON in Post.
Pre-I/R FMD.
FMD, expressed as a percent change in brachial artery diameter, did not significantly change in HT or CON subjects over time (Table 3). However, HT subjects experienced a significant increase in baseline diameter at Mid and Post (Table 3; group × time interaction, P = 0.014) so that when FMD was corrected for shear rate, an increase in FMD was seen at the Mid and Post time point (Table 3 and Fig. 5; group × time interaction, P = 0.018). Area under the curve for PORH increased over time in HT (main effect of time, P = 0.003), but no change was observed in peak PORH (P = 0.12). No changes in FMD were seen in CON subjects for PORH area under the curve, PORH peak, absolute % dilation, or shear-corrected FMD (Table 3).
Fig. 5.
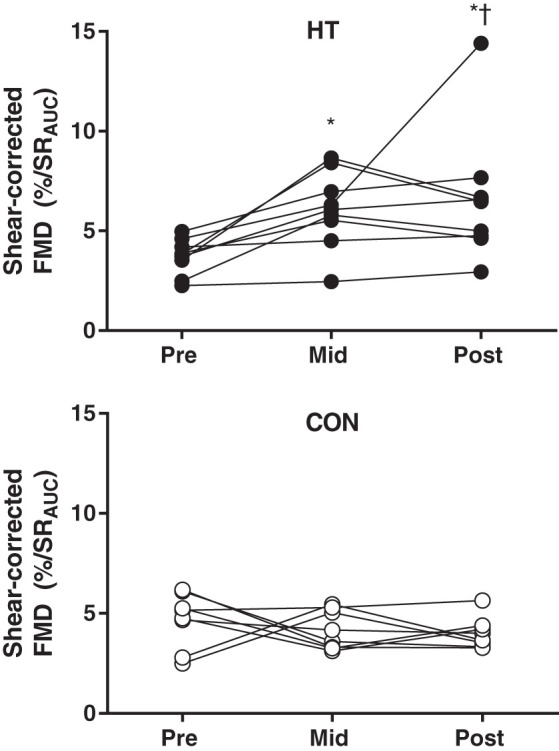
Individual shear-corrected flow-mediated dilation (FMD) over time in heat therapy (HT; top) and control (CON; bottom) subjects. *Significant (P < 0.05) difference from Pre within group. †Significant difference (P < 0.05) from CON at matched time point.
Post-I/R FMD.
FMD significantly decreased, albeit variably, following I/R at the Pre time point in both groups (Table 3). However, in HT subjects, Post-I/R FMD increased at the Mid and Post time points (group × time interaction, P = 0.024) so that Pre-I/R and Post-I/R FMD were not significantly different after 8–10 wk of HT, suggesting improved resistance to ischemic stress following HT. I/R resulted in a ~30% decrease in both peak (P = 0.001) and area under the curve PORH (P = 0.002) in all subjects. Both post-I/R PORH peak (P = 0.009) and area under the curve (P = 0.047) increased over time in HT, with no change in CON (Table 3).
DISCUSSION
The cardiovascular risk profile of women who are obese with PCOS has been well described; however, relatively few pharmaceutical or lifestyle interventions for women with PCOS substantially mitigate cardiovascular risk. Based on our results, HT appears to be a promising intervention to reduce cardiovascular risk in this population. Specifically, our primary findings indicate that a 30-session HT intervention in women who are obese with PCOS 1) reduced MSNA; 2) improved blood pressure, arterial compliance, and baroreflex sensitivity; 3) promoted improvements in wall thickness in carotid and femoral arteries; and 4) improved endothelial function and protected the endothelium from I/R-related impairments. These changes occurred in HT subjects along with reductions in total cholesterol, C-reactive protein, fasting glucose, and total testosterone, whereas fasting insulin and BMI did not change. As elevated MSNA and impaired vascular function in PCOS is related to dyslipidemia, meta-inflammation, androgen excess, and hyperinsulinemia, the relationships between the changes in cardiovascular variables and circulating factors illuminate several mechanisms through which HT can impact cardiovascular risk in women who are obese with PCOS.
A clinically important, novel finding of this investigation was the profound decrease in MSNA in HT subjects. The ~40% reduction in burst frequency and incidence after 4–5 wk of HT in this study is similar in magnitude to changes observed in women who are overweight with PCOS in response to 16 wk of exercise training or electroacupuncture (47). In women who are obese with PCOS, there are multiple factors associated with elevated MSNA, including obesity (38), hyperinsulinemia (37), dyslipidemia (28), elevated inflammatory cytokines (20), and androgen excess (49). BMI and fasting insulin did not change over the course of the study and are therefore unlikely responsible for changes in MSNA; however, HT subjects experienced a decrease in fasting glucose and a reduction in both glucose and insulin area under the curve during an OGTT (16) during Post testing. The timing of these changes (no change in OGTT at Mid, decreased glucose and insulin during OGTT in Post) did not coincide with changes in MSNA (substantial decrease in Mid, maintained in Post); however, it is likely that the change in metabolic function, coupled with decreases in total cholesterol (−0.4 mmol/L), C-reactive protein (−4.2 nmol/L), and testosterone (−0.6 nmol/L), all may have contributed to decreased MSNA. Decreases in total and LDL cholesterol have been observed in response to acute heat exposure in young men (59), and increased cholesterol content in cell membranes is associated with increased thermal tolerance (9). It is therefore plausible that the decrease in cholesterol is due to increased uptake by cell membranes to increase stress resistance to repeated heat exposure. C-reactive protein is released by the liver in response to proinflammatory cytokines and adipokines and is considered a global inflammatory marker that is highly associated with various markers of cardiovascular health (58). C-reactive protein has not previously been examined in HT interventions; however; frequency of sauna use was inversely associated with C-reactive protein in a prospective cohort study in men (32). In addition, animal models have observed reductions in inflammatory cytokines, including IL-6 and TNF-α, in response to heat (27), which may be mediated by increased heat shock protein 70 abundance (12). The relationship between HT and reduced total testosterone in PCOS is less clear. Elevated serum testosterone has been associated with sympathetic overactivity in PCOS, although there is some debate whether testosterone drives sympathetic activity (49) or if high sympathetic activity to the ovary drives androgen production (30). The moderate correlation between changes in MSNA and testosterone, as well as the timeline of changes in MSNA (decreased at Mid in HT) compared with changes in testosterone (unchanged at Mid, decreased in Post) indicate that changes in sympathetic activity preceded changes in androgen production with HT, lending support to the theory that sympathetic activity drives ovarian androgen production. Further supporting this, the HT subject taking oral contraceptives, who did not experience a change in testosterone, still exhibited similar decreases in MSNA compared with unmedicated subjects.
In addition to decreased MSNA, HT subjects displayed decreased blood pressure (SBP: −10 mmHg; DBP: −9 mmHg), resting heart rate, carotid compliance, and baroreflex sensitivity during the Valsalva maneuver. The change in DBP aligned with the timeline and magnitude of change in MSNA in HT subjects, as well as the improvement in endothelial function (shear-corrected FMD). The combination of reduced sympathetic activity and enhanced nitric oxide bioavailability, a consequence of repeated heat exposure (3), likely contributed to a substantial decrease in blood pressure and alteration of risk classification for 7/9 HT subjects. This change in transmural pressure may also have contributed to the small changes in carotid compliance and baroreflex sensitivity, both of which increased over time in HT subjects. Decreased arterial compliance is considered a primary factor in age-related decline in baroreflex sensitivity (36), so the increased sympathetic baroreflex sensitivity observed in HT in the present study was possibly due to increased arterial compliance, secondary to decreased blood pressure and wall thickness.
Changes in both carotid and superficial femoral wall thickness were evident in HT subjects (Fig. 4), similar in magnitude to exercise training protocols in women who are obese with PCOS (40), and HT interventions in healthy, sedentary humans (4). PCOS, especially when accompanied by obesity, is associated with a meta-inflammatory state (45) and dyslipidemia (11). In combination, these factors can lead to an accelerated plaque deposition on arterial walls and increased wall thickness observed in obesity and PCOS. Furthermore, this increased plaque deposition and arterial wall thickening is often exacerbated by the hyperinsulinemia, elevated blood pressure, and high serum testosterone that accompany PCOS (35). Although fasting insulin did not change, glucose, C-reactive protein, total cholesterol, LDL cholesterol, and blood pressure were all reduced in HT subjects and likely contributed to reduced arterial wall thickening, as all are associated with increased endothelial damage and lipid deposition. In addition, acute hot water immersion is associated with redistribution of blood flow because of cutaneous vasodilation, creating beneficial vascular shear patterns (52) similar to those experienced during exercise (42, 53), which can promote endothelial cell streaming (54) and reduce plaque deposition (8). These beneficial vascular shear patterns during heat exposure can additionally improve endothelial function (7, 31).
Clinically meaningful changes in endothelial function (shear-corrected FMD) were present by the midpoint of HT in experimental subjects and further increased by the end of HT. FMD is a well-described measure of endothelial function and strong clinical correlate of cardiovascular risk (44), with a 2% improvement in FMD representing a 15% improvement in cardiovascular risk (44). The ~3% change in shear-corrected FMD over the HT intervention is smaller than observed in healthy humans undergoing a similar protocol (4) but more robust than the change in FMD observed in women who are overweight with PCOS following 10–26 wk of exercise training (1, 40). Although FMD is not exclusively dependent upon nitric oxide, HT-mediated increases in nitric oxide bioavailability as observed in previous work (3) are likely predominantly responsible for increased FMD in women who are obese with PCOS over the course of HT. The effect of HT on endothelial function after I/R stress was even more pronounced than the improvement in baseline (Pre-I/R) FMD, approaching a 4% increase in FMD. Additionally, this improvement resulted in increased post-I/R PORH peak and area under the curve by the end of 8–10 wk. Although this experimental model may be difficult to translate to true I/R tissue injury, these data support the protective effect observed in isolated rat hearts following a 30-day heat acclimation protocol (34) and offer a potential explanation for the reduction in fatal cardiovascular events seen with increased sauna use in men (33). Additionally, recent cell culture models of I/R (hypoxia/reoxygenation) with serum exposure from humans who underwent HT demonstrate an increase in circulating factors that reduce oxidative and inflammatory markers following HT are involved in this protection from I/R stress (6). Although the observed reduction in MSNA and improvement in multiple cardiovascular risk factors show great promise for women who are obese with PCOS, this experiment had several limiting factors to consider. First, in regard to experimental design, our time control group was not a true sham treatment, and it was not possible to blind subjects to the treatment group. However, previous work in our laboratory that used a thermoneutral water immersion sham treatment did not result in any health improvements in sedentary control subjects (4), and similar untreated time control groups have been used in other lifestyle (exercise intervention) studies in women with PCOS (1, 46, 55) and in men with obesity and women undergoing HT (21). The lack of change in BMI in both HT and CON subjects suggests that lifestyle (diet and exercise) changes beyond the study intervention did not explain the changes in autonomic and cardiovascular function. Our subjects were matched for age and BMI rather than randomly assigned, which may be viewed as a limitation, but we felt it was necessary given the range of BMI and spectrum of metabolic dysfunction evident in PCOS. We additionally enrolled several women taking medications, who were also matched between groups. Our initial intent was to exclude all medications from this study, but women with PCOS have extremely high prescription rates, with oral contraceptives considered a first-line treatment (24), and high rates of depression and anxiety (10) often treated with SSRIs. Other than lower total testosterone levels in women taking oral contraceptives, we did not observe any differences in response between medicated and unmedicated subjects. MSNA burst incidence, magnitude, and time course of changes were not different in the subject in each group taking oral contraceptives or the one subject in each group taking SSRIs (successful nerve recordings were not obtained on one subject taking SSRIs in each HT and CON).
The overlap in several key variables (blood pressure, arterial compliance, wall thickness, and FMD) with previous work from our laboratory in healthy, inactive humans (4) undergoing a similar HT protocol allows for comparison between relatively healthy individuals and a cohort of women with elevated cardiovascular risk. Although some changes were much larger in magnitude in women who are obese with PCOS (i.e., blood pressure, heart rate, femoral wall thickness), other variables (carotid-femoral PWV, FMD, femoral arterial stiffness) either did not change or changes were of much smaller magnitude. For example, change in mean arterial pressure (−10 mmHg in obesity/PCOS; −4 mmHg in sedentary) and resting heart rate (−9 beats/min in obesity/PCOS, no change in sedentary) were larger in women who are obese with PCOS and indicated a clinically important reduction in cardiovascular risk. In contrast, other markers of cardiovascular risk such as carotid-femoral PWV (no change in obesity/PCOS, −100 cm/s in sedentary) and femoral β stiffness (no change in obesity/PCOS, −2 arbitrary units in sedentary) exhibited clinically important changes in relatively healthy subjects but did not significantly change in women who are obese with PCOS. In some cases, changes also appeared to take longer (changes in shear-corrected FMD evident by 2 wk in healthy subjects but took >4 wk to manifest in women who are obese with PCOS). Thus, it is conceivable that a longer period of HT would be required to achieve significant and clinically relevant changes in certain vascular measures in a patient population. Although it is difficult to determine which differences may be due to subject population and which may be due to a reduced time commitment for heating sessions, these data indicate that those with elevated cardiometabolic risk still benefit substantially from HT and provide promise for other clinical populations.
Perspectives and Significance
In this study, we report that a 30-session HT intervention lead to decreased blood pressure, decreased MSNA, and robust improvements in cardiovascular risk in women who are obese with PCOS. The reductions in blood pressure, wall thickness, arterial stiffness, and improved endothelial function are similar to or greater than those seen with exercise training and/or diet interventions in women with PCOS. As described in previous work (4) and recent reviews (15), there are multiple possible mechanisms for the observed decrease in MSNA and improvements in vascular function, but this requires further mechanistically designed investigation. The HT intervention was well tolerated by subjects (100% compliance; no dropouts), with most subjects expressing a desire to continue regular hot tub use after completion. Moreover, the changes we observed occurred without any change in BMI, indicating that HT used in combination with diet or exercise interventions that lead to weight loss may provide an additive benefit through mechanisms unrelated to changes in body mass. These data support previous work examining HT and cardiovascular health (4, 23) and additionally indicate that HT can reduce sympathetic nerve activity and inflammation. Notably, we also observed protection from I/R vascular injury in a population with elevated cardiovascular morbidity and mortality. This demonstrates that HT not only results in a healthier vascular profile at rest but also that the vasculature is more resilient to significant and damaging stress. The extent to which HT provides protection from other stressors known to contribute to cardiovascular and metabolic diseases is an area for future studies. Lastly, whether the changes observed with HT on autonomic function and vascular health in our study would be additive to benefits obtained through exercise training remain to be explored.
GRANTS
This project was funded by American Heart Association Fellowship no. 16PRE27780085, the Eugene and Clarissa Evonuk Memorial Fellowship, and the Kenneth and Kenda Singer Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.R.E., J.R.H., V.E.B., and C.T.M. conceived and designed research; B.R.E., M.A.F., S.D.B., L.N.C., E.A.L., and C.T.M. performed experiments; B.R.E., M.A.F., S.D.B., and L.N.C. analyzed data; B.R.E., M.A.F., J.R.H., and C.T.M. interpreted results of experiments; B.R.E. prepared figures; B.R.E. drafted manuscript; B.R.E., M.A.F., J.R.H., S.D.B., E.A.L., V.E.B., and C.T.M. edited and revised manuscript; B.R.E., M.A.F., J.R.H., S.D.B., L.N.C., E.A.L., V.E.B., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Elise Wright, Karen Wiedenfeld Needham, Matthew Howard, Jared Steele, Amber Stock, Alysia Lovemark, Taylor Eymann, and Elizabeth Bartlett for assistance with data collection. We also thank the subjects who volunteered time for this study.
Current address for B. R. Ely: Dept. of Sport and Movement Science, Salem State University, 352 Lafayette St., Salem, MA 01970.
Current address for V. E. Brunt: Dept. of Integrative Physiology, University of Colorado, 354 UCB, Boulder, CO 80309.
REFERENCES
- 1.Almenning I, Rieber-Mohn A, Lundgren KM, Shetelig Løvvik T, Garnæs KK, Moholdt T. Effects of high intensity interval training and strength training on metabolic, cardiovascular and hormonal outcomes in women with polycystic ovary syndrome: A pilot study. PLoS One 10: e0138793, 2015. doi: 10.1371/journal.pone.0138793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society . Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91: 4237–4245, 2006. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 3.Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol (1985) 121: 716–723, 2016. doi: 10.1152/japplphysiol.00424.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt VE, Jeckell AT, Ely BR, Howard MJ, Thijssen DH, Minson CT. Acute hot water immersion is protective against impaired vascular function following forearm ischemia-reperfusion in young healthy humans. Am J Physiol Regul Integr Comp Physiol 311: R1060–R1067, 2016. doi: 10.1152/ajpregu.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt VE, Wiedenfeld-Needham K, Comrada LN, Minson CT. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol 596: 4831–4845, 2018. doi: 10.1113/JP276559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 8.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998. doi: 10.1161/01.RES.82.5.532. [DOI] [PubMed] [Google Scholar]
- 9.Cress AE, Gerner EW. Cholesterol levels inversely reflect the thermal sensitivity of mammalian cells in culture. Nature 283: 677–679, 1980. doi: 10.1038/283677a0. [DOI] [PubMed] [Google Scholar]
- 10.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril 93: 2421–2423, 2010. doi: 10.1016/j.fertnstert.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 10a.Di Domenico K, Wiltgen D, Nickel FJ, Magalhães JA, Moraes RS, Spritzer PM. Cardiac autonomic modulation in polycystic ovary syndrome: does the phenotype matter? Fertil Steril 99: 286–292, 2013. doi: 10.1016/j.fertnstert.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab 18: 280–285, 2007. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones 15: 153–163, 2010. doi: 10.1007/s12192-009-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18: 774–800, 1997. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 15.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Minson CT. Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help? Temperature (Austin) 5: 9–21, 2018. doi: 10.1080/23328940.2017.1384089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Needham KW, Comrada LN, Minson CT. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 317: E172–E182, 2019. doi: 10.1152/ajpendo.00549.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 95: 1048–1058.e2, 2011. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 17: 171–183, 2011. doi: 10.1093/humupd/dmq045. [DOI] [PubMed] [Google Scholar]
- 20.Helwig BG, Craig RA, Fels RJ, Blecha F, Kenney MJ. Central nervous system administration of interleukin-6 produces splenic sympathoexcitation. Auton Neurosci 141: 104–111, 2008. doi: 10.1016/j.autneu.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoekstra SP, Bishop NC, Faulkner SH, Bailey SJ, Leicht CA. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol (1985) 125: 2008–2018, 2018. doi: 10.1152/japplphysiol.00407.2018. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M, Meiri U. Central and peripheral contributions to control of heart rate during heat acclimation. Pflugers Arch 422: 386–392, 1993. doi: 10.1007/BF00374295. [DOI] [PubMed] [Google Scholar]
- 23.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. doi: 10.1016/S0735-1097(01)01467-X. [DOI] [PubMed] [Google Scholar]
- 24.Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol 34: 272–277, 2018. doi: 10.1080/09513590.2017.1395841. [DOI] [PubMed] [Google Scholar]
- 25.Kenny GP, Giesbrecht GG, Thoden JS. A comparison of human thermoregulatory response following dynamic exercise and warm-water immersion Eur J Appl Physiol Occup Physiol 74: 336–341, 1996. doi: 10.1007/BF02226930. [DOI] [PubMed] [Google Scholar]
- 26.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/S0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Shin HM, Baek W. Heat-shock response is associated with decreased production of interleukin-6 in murine aortic vascular smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol 371: 27–33, 2005. doi: 10.1007/s00210-004-1007-5. [DOI] [PubMed] [Google Scholar]
- 28.Lambert E, Straznicky N, Sari CI, Eikelis N, Hering D, Head G, Dixon J, Esler M, Schlaich M, Lambert G. Dyslipidemia is associated with sympathetic nervous activation and impaired endothelial function in young females. Am J Hypertens 26: 250–256, 2013. doi: 10.1093/ajh/hps016. [DOI] [PubMed] [Google Scholar]
- 29.Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf) 77: 791–801, 2012. doi: 10.1111/cen.12003. [DOI] [PubMed] [Google Scholar]
- 30.Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 133: 2690–2695, 1993. doi: 10.1210/endo.133.6.7902268. [DOI] [PubMed] [Google Scholar]
- 31.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol (1985) 104: 588–600, 2008. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laukkanen JA, Laukkanen T. Sauna bathing and systemic inflammation. Eur J Epidemiol 33: 351–353, 2018. doi: 10.1007/s10654-017-0335-y. [DOI] [PubMed] [Google Scholar]
- 33.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 34.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 23: 79–88, 2005. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- 35.Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 18: 112–126, 2012. doi: 10.1093/humupd/dmr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 37.Monroe MB, Van Pelt RE, Schiller BC, Seals DR, Jones PP. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans Int J Obes Relat Metab Disord 24: 1183–1187, 2000. doi: 10.1038/sj.ijo.0801364. [DOI] [PubMed] [Google Scholar]
- 38.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea Circulation 98: 772–776, 1998. doi: 10.1161/01.CIR.98.8.772. [DOI] [PubMed] [Google Scholar]
- 39.Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Mano T. Comparison of sympathetic nerve response to head-up tilt in summer and winter J Gravit Physiol 6: 43–44, 1999. [PubMed] [Google Scholar]
- 40.Orio F, Muscogiuri G, Giallauria F, Savastano S, Bottiglieri P, Tafuri D, Predotti P, Colarieti G, Colao A, Palomba S. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: a randomized controlled trial. Clin Endocrinol (Oxf) 85: 764–771, 2016. doi: 10.1111/cen.13112. [DOI] [PubMed] [Google Scholar]
- 41.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41–47, 2004. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 44.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Shorakae S, Teede H, de Courten B, Lambert G, Boyle J, Moran LJ. The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med 33: 257–269, 2015. doi: 10.1055/s-0035-1556568. [DOI] [PubMed] [Google Scholar]
- 46.Sprung VS, Cuthbertson DJ, Pugh CJ, Aziz N, Kemp GJ, Daousi C, Green DJ, Cable NT, Jones H. Exercise training in polycystic ovarian syndrome enhances flow-mediated dilation in the absence of changes in fatness. Med Sci Sports Exerc 45: 2234–2242, 2013. doi: 10.1249/MSS.0b013e31829ba9a1. [DOI] [PubMed] [Google Scholar]
- 47.Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 297: R387–R395, 2009. doi: 10.1152/ajpregu.00197.2009. [DOI] [PubMed] [Google Scholar]
- 48.Stickford AS, VanGundy TB, Levine BD, Fu Q. Menstrual cycle phase does not affect sympathetic neural activity in women with postural orthostatic tachycardia syndrome. J Physiol 593: 2131–2143, 2015. doi: 10.1113/JP270088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sverrisdóttir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 50.Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries Clin Cardiol 28: 460–466, 2005. doi: 10.1002/clc.4960281004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas KN, van Rij AM, Lucas SJ, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312: R281–R291, 2017. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 53.Thomas KN, van Rij AM, Lucas SJ, Gray AR, Cotter JD. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature (Austin) 3: 286–297, 2016. doi: 10.1080/23328940.2016.1156215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VanBavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications. Prog Biophys Mol Biol 93: 374–383, 2007. doi: 10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Vigorito C, Giallauria F, Palomba S, Cascella T, Manguso F, Lucci R, De Lorenzo A, Tafuri D, Lombardi G, Colao A, Orio F. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 92: 1379–1384, 2007. doi: 10.1210/jc.2006-2794. [DOI] [PubMed] [Google Scholar]
- 56.Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obes Facts 2: 26–35, 2009. doi: 10.1159/000194971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 95: 2038–2049, 2010. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 58.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes 1: 92–97, 2008. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto H, Zheng KC, Ariizumi M. Influence of heat exposure on serum lipid and lipoprotein cholesterol in young male subjects Ind Health 41: 1–7, 2003. doi: 10.2486/indhealth.41.1. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Carter JR. Baroreflex sensitivity analysis: spontaneous methodology vs. Valsalva’s maneuver. Clin Auton Res 23: 133–139, 2013. doi: 10.1007/s10286-013-0195-9. [DOI] [PubMed] [Google Scholar]
- 61.Zaccardi F, Laukkanen T, Willeit P, Kunutsor SK, Kauhanen J, Laukkanen JA. Sauna bathing and incident hypertension: A prospective cohort study. Am J Hypertens 30: 1120–1125, 2017. doi: 10.1093/ajh/hpx102. [DOI] [PubMed] [Google Scholar]