Abstract
Fibroblast growth factor 23 (FGF23) is a phosphate regulating protein hormone released by osteocytes. FGF23 becomes markedly elevated in chronic kidney disease (CKD), for which the leading cause of death is cardiovascular disease, particularly sudden cardiac death. Previously, we found that FGF23 increases intracellular Ca2+ in cardiomyocytes and alters contractility in mouse ventricles ex vivo via FGF receptor 4 (FGFR4). In the present study, we demonstrate that FGF23 induces cardiac arrhythmias and prolongs QTc interval in mice, and we tested whether these effects are mediated through FGFR4. In isolated Langendorff perfused hearts, FGF23 perfusion increased mechanical arrhythmias in the form of premature ventricular beats (PVBs), and induced runs of ventricular tachycardia in 6 of 11 animals, which were attenuated with pretreatment of an anti-FGFR4 blocking antibody. Ex vivo ECG analysis of isolated intact hearts showed increased ventricular arrhythmias and QTc prolongation after FGF23 infusion compared with vehicle. In vivo, injection of FGF23 into the jugular vein led to the emergence of premature ventricular contractions (PVCs) in 5 out of 11 experiments. FGF23 also produced a significant lengthening effect upon QTc interval in vivo. In vivo FGFR4 blockade ameliorated the arrhythmogenic and QTc prolonging effects of FGF23. Finally, FGF23 increased cardiomyocyte Ca2+ levels in intact left ventricular muscle which was inhibited by FGR4 blockade. We conclude that FGF23/FGFR4 signaling in the heart may contribute to ventricular arrhythmogenesis and repolarization disturbances commonly observed in patients with CKD via Ca2+ overload and may be an important therapeutic target to reduce cardiac mortality in CKD.
NEW & NOTEWORTHY Here we provide direct evidence that fibroblast growth factor 23 (FGF23), a phosphaturic hormone elevated in chronic kidney disease, is proarrhythmic. FGF23 acutely triggered ventricular arrhythmias and prolonged corrected QT interval (QTc) in isolated mouse hearts and in vivo. FGF23 also increased Ca2+ levels in ventricular muscle tissue. Blockade of the FGF receptor 4 signaling pathway using a monoclonal antibody ameliorated ventricular arrhythmias, QTc prolongation, and elevated ventricular Ca2+ induced by FGF23, and may represent a potential therapeutic target in chronic kidney disease.
Keywords: calcium, cardiac function, chronic kidney disease, electrocardiogram, sudden cardiac death
INTRODUCTION
Mortality related to chronic kidney disease (CKD) has grown in significance, increasing by 82% between 1990 and 2010 (1, 2). The cause of death in those with CKD is multifactorial, owing to worsening multiorgan dysfunction with chronic uremia, metabolic derangements, and entangled comorbidities, particularly cardiovascular disease (CVD) (3). The risk of sudden cardiac death, in particular, is increased in patients with CKD, and is the leading cause of cardiovascular-related death in end-stage renal disease (ESRD) (4, 5). Patients with ESRD are predisposed to arrhythmic disorders, including ventricular arrhythmias, and they experience a high burden of premature ventricular contractions (PVCs) (6, 7). It has additionally been observed that electrocardiogram (ECG) abnormalities, namely, prolongation of the corrected QT interval (QTc), a risk factor for cardiac-related mortality, occurs with CKD progression (8, 9).
The connection between CKD and cardiac disease is likely multifaceted but may be in part due to direct interorgan-related adaptation. Fibroblast growth factor 23 (FGF23) is one hormone known to interact with multiple organ systems. FGF23 is a bone-derived, phosphaturic hormone that targets the kidney via FGF receptors (FGFR) and the coreceptor klotho. FGF23 induces renal excretion of phosphate by downregulating sodium-phosphate cotransporters in the proximal renal tubule and reducing gut absorption of phosphate, indirectly, through reduction of active vitamin D (10, 11). Serum FGF23 levels rise in the setting of hyperphosphatemia, which occurs during CKD. FGF23 levels continue to rise throughout the progression of declining renal function and are at their highest in patients with ESRD receiving dialysis, who can have levels up to 1,000-fold higher than normal (12). Given that patients with CKD carry such a high burden of CVD and sudden cardiac death, it is noteworthy that FGF23 has been found to serve as an independent marker of cardiovascular morbidity and mortality (13–15).
A direct interaction between the heart and FGF23 was observed in 2011, when we found that FGF23 induces hypertrophic growth of isolated ventricular myocytes and cardiac hypertrophy in rodents (16). We further demonstrated that FGF23 induces an acute increase in cardiomyocyte intracellular Ca2+ and increases cardiac muscle contractility (17). All of these effects were found to be mediated specifically through cardiac FGFR isoform 4 (FGFR4) in an α-klotho-independent manner (18–20).
There have been clinical associations with FGF23, atrial fibrillation, and ventricular arrhythmias (21, 22). Given that we previously found FGF23 alters intracellular Ca2+ (17), we hypothesized that FGF23 can directly induce cardiac arrhythmias and give rise to ECG disturbances, mediated through activation of FGFR4. As such, cardiac FGF23/FGFR4 signaling may play a role in the proarrhythmogenic state of CKD.
METHODS
Animals and Tissue Harvesting
All animal procedures were approved by the University of Missouri-Kansas City Institutional Animal Care and Use Committee (IACUC). CD1 male mice of 12 and 16 wk old (Harland Laboratories, Madison, WI) were housed in a temperature-controlled facility with a 12-h:12-h light-dark cycle, and ad libitum access to food and water. Before organ harvesting, isoflurane (3%; 3 L/min) was administered via a Fortec vaporizer for induction of anesthesia and heparin (200 U) was administered intraperitoneally. For ex vivo experiments, hearts were harvested via thoracotomy and mediastinal tissue was dissected away under a stereomicroscope in a petri dish containing oxygenated Ringer’s solution (NaCl 140 mM, CaCl2 2.5 mM, KCl 2.0 mM, K2HPO4 1.5 mM, MgSO4 1 mM, HEPES 10 mM, glucose 10 mM, and pH 7.4). The aorta was cannulated and perfused with oxygenated Ringer’s solution, using the Langendorff technique.
Ex Vivo Contractility
For the measurement of ex vivo contractility, atria were removed from isolated, Langendorff-perfused hearts to reduce spontaneous beating and allow for electrical pacing as previously described (23). Hearts were suspended vertically from an isometric force transducer between bipolar platinum stimulating electrodes (Radnoti, Monrovia, CA). Hearts were submerged in a 25-mL glass tissue chamber (Radnoti) with Ringer’s solution, bubbled with 100% oxygen at room temperature. Tension was applied to the hearts until maximum force development during stimulation was achieved. Hearts were then paced at 1.2–1.8 Hz with an SD9 stimulator (Grass Technologies) and contractile data were recorded and analyzed via a PowerLab system with LabChart 8 software (ADInstruments, Colorado Springs, CO). Hearts were allowed to stabilize for 30 min before the addition of recombinant mouse FGF23 (9 ng/mL; R&D Systems) with and without pretreatment with FGFR4-specific antibody (anti-FGFR4, 10.5 µg/mL; human monoclonal, U3-11 (Lead Discovery Center GmbH, Germany) (18), or Ringer’s solution (vehicle) for 30 min. FGF23 dissolved in 200 µL of Ringer’s solution was added directly to the organ bath and allowed to recirculate through the coronary circulation via the Langendorff apparatus. Ectopic cardiac activity and contractile parameters were recorded and averaged over the last 5 min of baseline and over the 30 min following the addition of vehicle or FGF23 with and without anti-FGFR4 to the organ bath.
For the measurement of ex vivo contractility at 37°C, intact Langendorff perfused hearts were suspended vertically from an isometric force transducer between bipolar platinum stimulating electrodes. Hearts were submerged in a 25-mL glass tissue chamber with Krebs–Henseleit solution (NaCl 118.5 mM, CaCl2 1.8 mM, KCl 4.7 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25.0 mM, glucose 10 mM, and pH 7.4) bubbled with 95%/5% oxygen/carbon dioxide and heated to 37°C. Tension was applied to the hearts until maximum force development during stimulation was achieved. Hearts were then paced at 6–7.75 Hz with an SD9 stimulator and contractile data was recorded and analyzed. Hearts were allowed to stabilize for 10 min at which point baseline contractile parameters and rhythm were averaged in the last 5 min before the addition of recombinant mouse FGF23 (9 ng/mL) or Krebs–Henseleit solution (vehicle). FGF23 dissolved in 200 µL of vehicle was added directly to the organ bath and allowed to recirculate through the coronary circulation via the Langendorff apparatus. Ectopic cardiac activity was recorded and averaged over the last 5 min of baseline and for 30 min following the addition of vehicle or FGF23 to the organ bath.
Ectopic activity was defined as any transient variability in the contractile waveform in comparison with its baseline pattern. Contractile waveform changes were categorized as outlined in an adjunct on the Lambeth conventions for animal arrhythmias (24, 25), similar to our previous studies (26, 27). Ectopic activity was further categorized as premature ventricular beats (PVB), defined as any early contraction before relaxation; PVB in bigeminy, defined as normal and abnormal paired beats occurring in repetition; and ventricular tachycardia, defined as four or more consecutive PVB.
Ex Vivo ECG and Left Ventricular Pressure
Langendorff-perfused hearts with atria intact were placed in a dish, submerged in oxygenated Ringer’s solution, and Langendorff perfused. Positive and negative 29-gauge ECG needle electrodes (ADInstruments) were placed adjacent to the apex and base of the heart, respectively, and the ground electrode was placed next to the right ventricle. In a subset of experiments, a small incision was made in the left atrial appendage under a stereomicroscope and a pressure balloon catheter was inserted into the left ventricle through the mitral valve (28). Synchronized electrical and left ventricular (LV) pressure waveforms were observed over 30 min to establish a baseline rhythm, defined as the average number of ectopic events per minute. FGF23 (9 ng/mL) or an equal volume of Ringer’s (vehicle) solution was added to the dish to recirculate into the coronary vasculature. Cardiac activity was subsequently monitored for 30 min. Arrhythmic activity was defined by traditional ECG parameters, defined in the Lambeth conventions (25).
In Vivo ECG
Mice were anesthetized with isoflurane (3% induction, 1.5% maintenance), placed in the supine position and 29-gauge ECG needle electrodes (ADInstruments) were inserted subcutaneously in each of the limbs to obtain a six-lead ECG. For venous access, a small incision was made in the skin of the neck and cleared away to expose the jugular vein. The distal jugular vein was ligated, and a small incision was made in the proximal jugular vein, which was then cannulated. After a baseline period of 20–30 min, FGF23 dissolved in 200 µL of Hank’s balanced salt solution (HBSS) at 9 ng/mL of total blood volume or 200 µL vehicle (HBSS) was then infused slowly for 2 min. Some mice were pretreated with anti-FGFR4 by injecting at 10.5 µg/mL of total blood volume 15 min before FGF23 administration. Total blood volume was calculated using established reference values for mice (0.078 mL/g body wt) (29, 30). ECG signals were recorded using a PowerLab system with LabChart 8 software (ADInstruments) for the immediate 30 min following infusion. After 30 min of FGF23 or vehicle, 0.1 mg/kg isoproterenol was administered intravenously for 15 min. Ectopic ECG waveforms were categorized as outlined in the Lambeth conventions (25) and lead II ECG intervals were measured manually using LabChart 8 software (31).
Left Ventricular Muscle Ca2+ Imaging
Mouse hearts were isolated as described in Ex Vivo Contractility and placed in a petri dish containing oxygenated Ringer’s solution containing 2,3-butanedione monoxime (BDM) (NaCl 140 mM, CaCl2 2.5 mM, KCl 2.0 mM, K2HPO4 1.5 mM, MgSO4 1 mM, HEPES 10 mM, glucose 10 mM, BDM 20 mM, and pH 7.4). The left ventricle was cut into small strips of 2–3 mm wide and placed in Ringer’s solution cooled on ice. The ventricular muscle strips were then loaded with Fluo-8 AM (2.5 µM) (AAT Bioquest, Inc., Sunnyvale, CA) for 30 min and then placed in 35-mm optical dishes containing 2 mL Ringer’s solution with BDM. The muscle strips were then secured in the center of the dish via a small metal clamp and perfused with Ringer’s solution with BDM to allow for dye deesterification while preparing the imaging setup. During the imaging protocol, the muscle strips were first perfused with Ca2+ imaging Ringer’s solution for 5 min, followed by 7 min of perfusion with Ca2+ imaging Ringer’s solution (vehicle), FGF23 (9 ng/mL), or FGF23 following pretreatment with an FGFR4 blocking antibody. At the end of each experiment a high KCl Ringer’s solution (NaCl 60 mM, CaCl2 2.5 mM, KCl 80 mM, K2HPO4 1.5 mM, MgSO4 1 mM, HEPES 10 mM, glucose 10 mM, BDM 20 mM, and pH 7.4) was perfused for 7 min to fully depolarize the muscle and induce increased intracellular Ca2+ as a positive control. Hearts were stimulated at 1 Hz (50 V; 5-ms duration) via flanking platinum electrodes for the duration of imaging and fluorescence was captured at 1-s intervals. Imaging was performed using an Olympus IX51 (Olympus, Melville, NY) inverted microscope on ×20 magnification, and intracellular Ca2+ levels were monitored at room temperature in response to treatments. Fluo-8 was excited using an EXFO X-cite metal halide light source (Mississauga, ON, Canada) and FITC Semrock Bright Line (Rochester, NY) filter set, and the emission signal was captured with a high-resolution charge-coupled device camera (Hammamatsu Photonics, Bridgewater, NJ). Fluorescence data were recorded into Slidebook version 6.0 software (Intelligent Imaging Innovations, Denver, CO). Fluorescence measurements from four regions of interest were analyzed and averaged for each left ventricular muscle sample. The change in Fluo-8 fluorescence after vehicle or FGF23 perfusion is reported as a percentage of the KCl response in the same sample. Muscle strips that produced ≤5 units of fluorescence change in response to perfusion with high KCl Ringer’s were not included in the analysis.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism V.5 (GraphPad, La Jolla, CA). Comparisons in the rates of ectopic beats and contractile parameters in ex vivo contractility experiments were made using either a one-way ANOVA with a Dunnett’s multiple comparisons post hoc analysis or a two-tailed t test. Comparisons in ECG intervals were made using a two-way ANOVA with a Bonferroni post hoc analysis. Rate and incidence of PVCs in live mice were compared with one-way ANOVA with a Dunnett’s multiple comparisons post hoc analysis or individual Fisher’s exact tests, respectively. Ca2+ imaging data were statistically analyzed using a one-way ANOVA with a Dunnett’s multiple comparisons post hoc analysis. In all cases, P < 0.05 was established a priori as the threshold for significance.
RESULTS
Ex Vivo Contractility
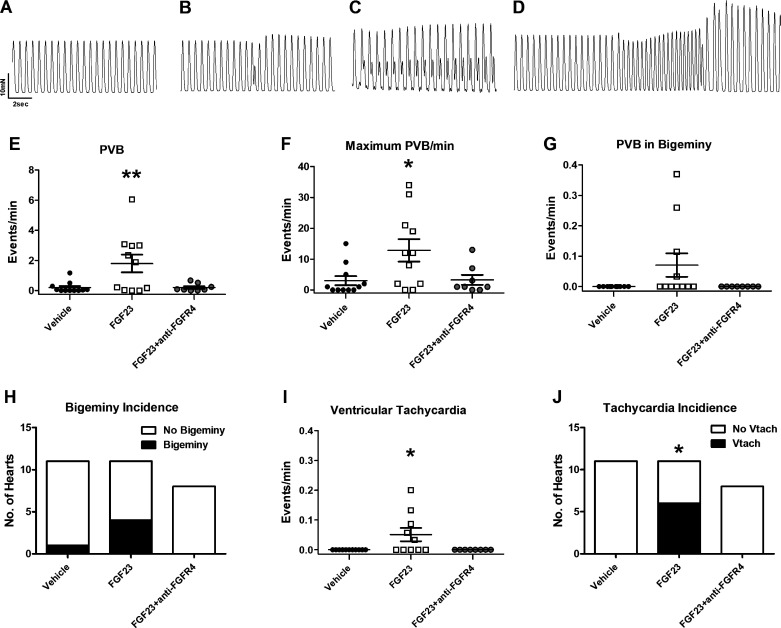
We found that perfusion of FGF23 (9 ng/mL) in isolated paced mouse hearts induces PVBs and ventricular tachycardia (Fig. 1, A and D). At baseline, there were no differences in the numbers of PVBs with vehicle control or FGF23 with and without anti-FGFR4 treatment (vehicle: 0.22 ± 0.15; FGF23: 0.20 ± 0.11; FGF23 + anti-FGFR4: 0.050 ± 0.050 PVB/min; P > 0.05). After the addition of vehicle control, we observed an average of 0.19 PVBs per minute. We observed one occurrence of PVB in bigeminy in one vehicle experiment and no occurrences of ventricular tachycardia during baseline or after vehicle administration. After 30 min of FGF23 treatment, we observed an average of 1.8 PVBs per minute (Fig. 1, E and F; n = 11, P < 0.01 compared with vehicle). We observed PVBs in bigeminy (Fig. 1, G and H) after administration of FGF23 in four of 11 animals (P > 0.05 compared with vehicle) with an average incidence of bigeminy of 0.8 per minute (n = 11, P > 0.05 compared with vehicle), although not statistically significant. We observed runs of ventricular tachycardia (Fig. 1, I and J) after administration of FGF23 in 6 of 11 animals (P < 0.05 compared with vehicle) which was statistically significant, with an average incidence of 0.05 events per minute (n = 11, P < 0.05 compared with vehicle). Pretreatment with an FGFR4-specific blocking antibody (anti-FGFR4) attenuated the generation of arrhythmic beats after FGF23 administration to 0.21 PVBs per minute with no instances of ventricular tachycardia (n = 8, P > 0.05 compared with vehicle) (Fig. 1, E–J). Pretreatment with the FGFR4-specific antibody alone did not induce arrhythmias or affect contractile parameters of our isolated hearts (data not shown). Assessment of contractility parameters revealed that force of contraction and the rate of force development were significantly increased during FGF23 treatment compared with vehicle, however the rate of relaxation was not affected (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.14340134). FGFR4 antibody pretreatment blocked the increases in contractile force and rate of force development elicited by FGF23 (Supplemental Fig. S1), similar to our previous findings (17, 18).
Figure 1.
A: baseline contractile pattern of paced Langendorff-perfused hearts. Fibroblast growth factor 23 (FGF23) induced abnormal contractile patterns as shown in (B). Premature ventricular beats (PVBs), defined as a new contraction before complete relaxation. C: PVB in bigeminy defined as at least two PVBs separated by one normal contraction. D: ventricular tachycardia defined as at least four PVBs without an intervening normal contraction. Average number of PVB per minute (E) and maximum number of PVBs per minute in vehicle, FGF23, and FGF23 + anti-FGFR4 groups (F). Average number of instances of bigeminy per minute (G) and incidence of bigeminy in hearts treated with vehicle, FGF23, or FGF23 + anti-FGFR4 (H). Average number of instances of ventricular tachycardia (Vtach) per minute (I) and incidence of Vtach in hearts treated with vehicle, FGF23, or FGF23 + anti-FGFR4 (J). n = 11 vehicle, n = 11 FGF23, n = 8 FGF23 + anti-FGFR4; **P < 0.01, *P < 0.05 compared with vehicle.
In a separate series of experiments, we analyzed the effects of FGF23 on contractile rhythm at high-beating frequencies (360–460 beats/min) obtained at 37°C and using a lower concentration of Ca2+. Under these conditions, FGF23 also led to an increase in the number of PVBs during 30 min of treatment compared with vehicle-treated hearts (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.14340170).
Ex Vivo Electrocardiography and Left Ventricular Pressure
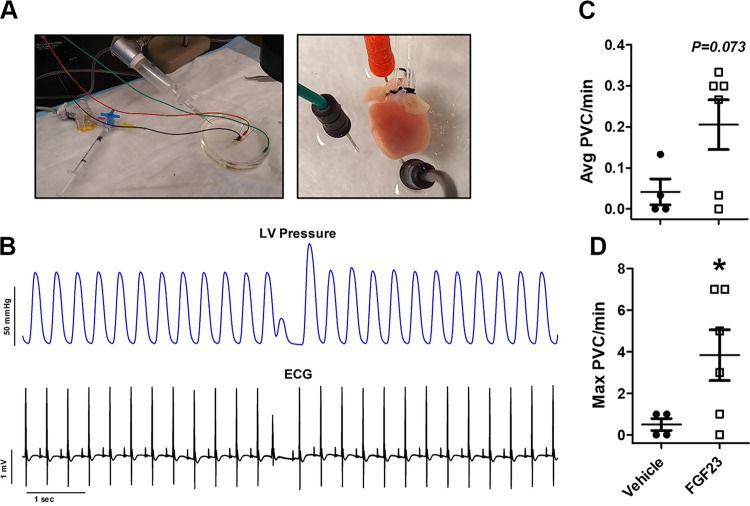
We next wanted to measure electrical activity in the heart during perfusion of FGF23 in the coronary circulation of isolated spontaneously beating mouse hearts while monitoring ECG and LV pressure ex vivo (Fig. 2A). We observed a statistically significant rise in the average number of PVBs in this model after FGF23 perfusion, which corresponded to premature ventricular depolarization in the ECG record (Fig. 2B). At baseline, there was an average of 0.006 PVCs per minute. After addition of vehicle, we observed an average of 0.05 PVC per minute and a maximal amount of 0.5 PVCs per minute (n = 4). After administration of FGF23, we observed an average of 0.21 PVCs per minute (n = 6, P = 0.073, compared with vehicle) and a maximal amount of 4 PVCs per minute (n = 6, P = 0.038, compared with vehicle).
Figure 2.
A: images of the ex vivo electrocardiogram (ECG) and left ventricular (LV) pressure setup. Electrodes were placed at the base and apex of the heart (along with a ground wire) in the dish to measure ECG during Langendorff perfusion while a balloon cannula was inserted into the LV via the left atria to measure pressure changes. B: ventricular dysrhythmias after fibroblast growth factor 23 (FGF23) (9 ng/mL) perfusion were confirmed via ex vivo ECG synchronized with intraventricular pressure via the LV balloon catheter. Average premature ventricular contractions (PVCs) per min (C) and maximal number of PVCs per minute by ECG after 30 min of perfusion with vehicle (n = 4) or FGF23 (n = 6) (D). *P < 0.05 compared with vehicle.
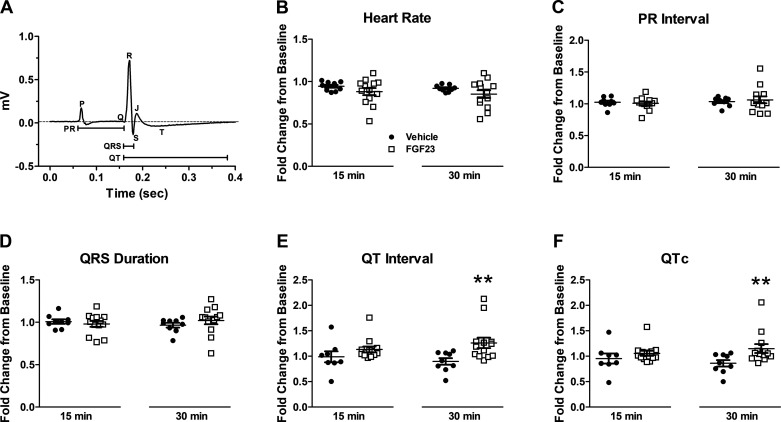
Next, we measured ECG wave parameters in a larger cohort of isolated, spontaneously beating hearts and observed a statistically significant elongation in QT and QTc interval within 30 min of addition of FGF23 (n = 13, P < 0.01 compared with vehicle) (Fig. 3, E and F). No increase in QT or QTc interval was noted in vehicle trials (n = 10). Among experimental and control trials, no difference was observed in heart rate, PR interval, or QRS duration (Fig. 3, A–D).
Figure 3.
A: representative ex vivo murine electrocardiogram (ECG) waveform showing interval measurements. Average fold change of heart rate (B), PR interval (C), QRS duration (D), QT interval (E), and QTc (Bazett correction) (F) after 15 min and 30 min of perfusion with vehicle (n = 10) or fibroblast growth factor 23 (FGF23) (9 ng/mL; n = 13). **P < 0.01 compared with vehicle.
In Vivo ECG
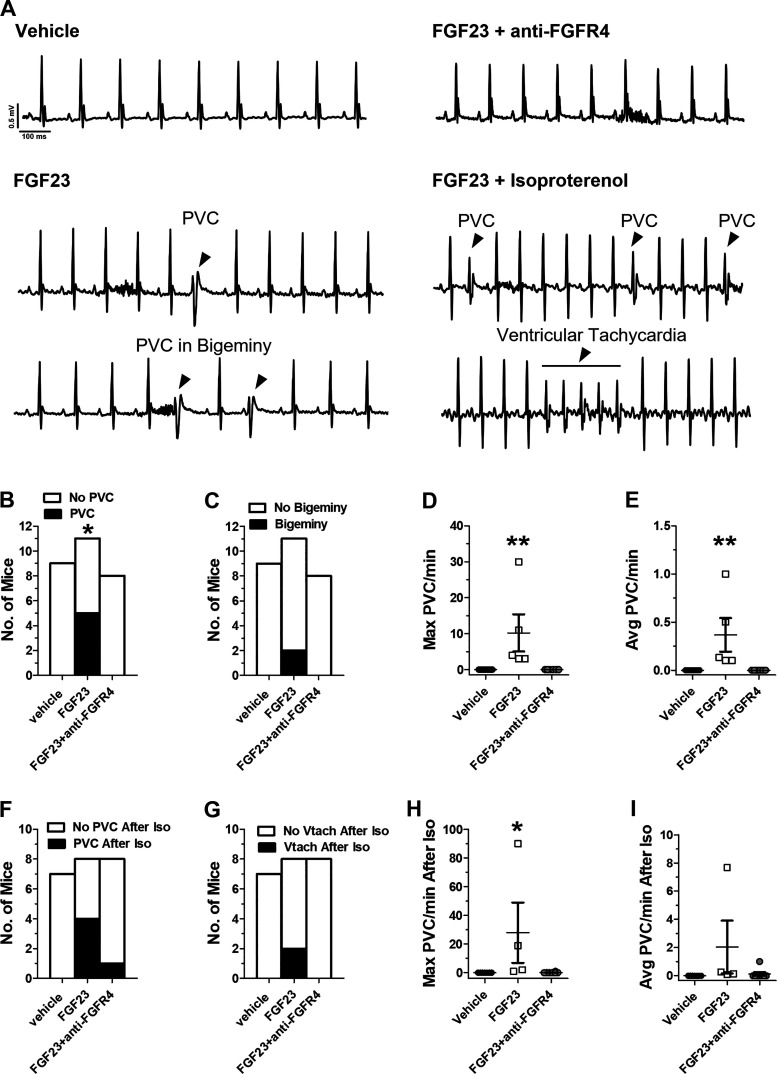
We next determined if FGF23 produced similar effects in anesthetized mice. After intravenous infusion of FGF23 (9 ng/mL total blood volume), we observed the emergence of PVCs in 5 out of 11 animals (Fig. 4D; P < 0.05 compared with vehicle) with a maximum rate of 10 PVCs per minute (Fig. 4B). We did not observe any PVCs after infusion of vehicle. Injection of isoproterenol increased the maximal rate of PVCs/min to 28 (Fig. 4F) and produced instances of ventricular tachycardia in 2 out of 8 mice in the FGF23 group (Fig. 4I) (P > 0.05). Isoproterenol did not induce arrhythmias in the vehicle-treated mice. Next, to determine if the arrhythmogenic effects of FGF23 were mediated by FGFR4, we pretreated mice by injecting anti-FGFR4 before FGF23 injection. Blockade of FGFR4 eliminated the occurrence of PVCs after FGF23 treatment in all eight mice tested (Fig. 4, B and C). One mouse exhibited a single PVC after isoproterenol administration in the FGF23 group pretreated with anti-FGFR4 (Fig. 4H).
Figure 4.
A: in vivo electrocardiogram (ECG) traces showing emergence of PVCs and PVCs in bigeminy after injection with FGF23 along with increased premature ventricular contractions (PVCs) and instances of ventricular tachycardia after fibroblast growth factor 23 (FGF23) + isoproterenol but not in mice treated with vehicle or FGF23 + anti-FGFR4. Proportion of mice exhibiting PVCs (B) and proportion of mice with PVCs in bigeminy after injection of vehicle, FGF23 (9 ng/mL total blood volume), or FGF23 + anti-FGFR4 (C). Maximal PVC rate in mice that exhibited PVCs (D), average PVC rate in mice that exhibited PVCs (E). *P < 0.05, compared to vehicle; n = 9 vehicle, n = 11 FGF23 and n = 8 FGF23 + anti-FGFR4. Proportion of mice exhibiting PVCs (F) and proportion of mice with ventricular tachycardia (Vtach) upon isoproterenol treatment after vehicle, FGF23, or FGF23 + anti-FGFR4 injection (G). Maximal PVC rate in mice with PVCs upon isoproterenol treatment (H) and average PVC rate in mice with PVCs upon isoproterenol treatment after vehicle, FGF23, or FGF23 + anti-FGFR4 injection (I). Arrowheads denote individual PVCs. **P < 0.01 compared with vehicle; n = 7 vehicle, n = 8 FGF23, and n = 8 FGF23 + anti-FGFR4.
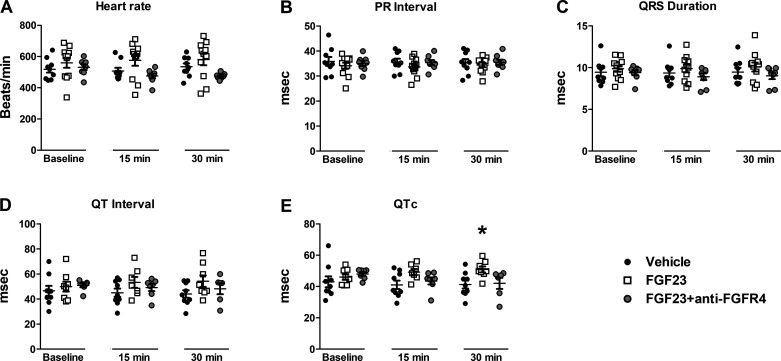
Similar to our ex vivo studies, we noted an increase in QTc after FGF23 administration (n = 8) compared with vehicle (n = 9), over 30 min of observation (P < 0.05) (Fig. 5, D and E). There was no statistically significant difference in resting heart rate, PR interval, or QRS duration after FGF23 injection (Fig. 5, A–C). The injection of anti-FGFR4 (n = 8) before FGF23 restored the QTc interval to that of vehicle values (Fig. 5E).
Figure 5.
Average heart rate (A), PR interval (B), QRS duration (C), QT interval (D), and QTc (Mitchell correction) (E) during 30 min of in vivo electrocardiogram (ECG) monitoring after venous injection of vehicle, FGF23 (9 ng/mL total blood volume), or FGF23 + anti-FGFR4 (10.5 µg/mL total blood volume). *P < 0.05 compared with vehicle; n = 9 vehicle, n = 11 FGF23, and n = 8 FGF23 + anti-FGFR4.
Left Ventricular Muscle Ca2+ Imaging
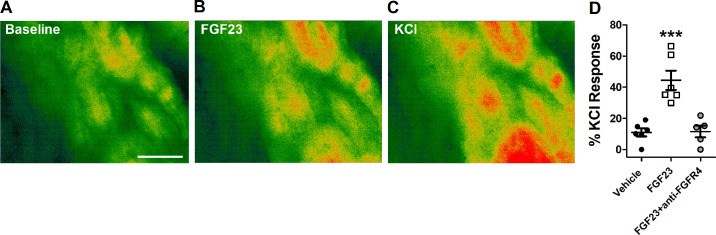
Imbalances in cardiomyocyte intracellular Ca2+ are known to lead to ectopic excitation of ventricular muscle and the generation of cardiac arrhythmias (32, 33). In order to assess whether FGF23 is inducing alterations to intracellular Ca2+ in the ventricular muscle of mouse hearts in our arrhythmia models, we measured intracellular Ca2+ using Fluo-8 in left ventricular muscle strips. As displayed in Fig. 6, perfusion of FGF23 (9 ng/mL) induced a statistically significant increase in Fluo-8 fluorescence compared with vehicle perfusion, indicating that FGF23 increased intracellular Ca2+ levels. Next, to determine the contribution of FGFR4 to the augmentation of intracellular Ca2+ by FG23, we pretreated left ventricular muscles with FGFR4-specific blocking antibody and found that this abolished the Ca2+ response following FGF23 treatment (Fig. 6D).
Figure 6.
Representative pseudocolored Fluo-8 fluorescent images of left ventricular muscle strips at baseline (A), following perfusion of FGF23 (9 ng/mL) (B), and following perfusion of high KCl buffer (60 mM) (C). Warmer colors indicate higher Ca2+ levels. D: Fluo-8 fluorescence measurements relative to KCl response after perfusion of vehicle (n = 6), 9 ng/mL FGF23 (n = 6), or FGF23 + anti-FGFR4 (n = 5). Scale bar represents 100 µm. ***P < 0.001 compared with vehicle.
DISCUSSION
The pathophysiology of CKD and sudden cardiac death is complex and involves a number of overlapping physiological alterations and underlying disease conditions. Sudden cardiac death may account for as much as 60% of mortality in individuals undergoing dialysis treatment (34) and incidence is greatly increased in those with lower glomerular filtration rates (35). In recent years, blood levels of the hormone FGF23 has become accepted as a nontraditional risk factor for the development of CVD and cardiac-related mortality during renal failure (36, 37). Although numerous association studies in humans and in rodent models of CKD show a link between FGF23 and cardiac function, relatively few studies have attempted to isolate the direct effects of pathological levels of FGF23 on cardiac muscle function and in vivo electrocardiography properties. To that end, we sought to delineate potential involvement of elevated FGF23 in cardiac arrhythmogenesis. We found that pathological concentrations of FGF23 induced contractile and ECG disturbances in isolated mouse hearts including PVCs, episodes of ventricular tachycardia, and prolonged QTc interval. FGF23 increased intracellular Ca2+ in myocytes of intact ventricular muscle, indicating a potential mechanism for the ventricular arrhythmias. Similarly, in vivo injection of FGF23 led to the emergence of PVCs in the ECG record and significantly prolonged QTc interval within 30 min after administration. Blockade of FGFR4 using an FGFR4-specific antibody abrogated the Ca2+ changes and arrhythmogenic properties of FGF23 ex vivo and in vivo.
Ventricular Arrhythmias
Patients with CKD present with varying degrees of cardiac arrhythmias including atrial disturbances (e.g., atrial fibrillation and premature atrial contractions) (38), supraventricular events, ventricular arrhythmias (e.g., PVCs, ventricular tachycardia, ventricular fibrillation), or some combination thereof (39). In our studies, somewhat surprisingly, we did not observe any instances of atrial disturbances induced by FGF23 treatment during live mouse experiments. Rather, the FGF23-induced premature arrhythmias lacked discernable P waves and contained misshapen QRS complexes indicative of spontaneous and heterogenic excitation originating within the ventricular myocardium. Injections of FGF23 induced ventricular arrhythmias in the form of PVCs occurring as individual complexes, in instances of bigeminy or as nonsustained episodes of ventricular tachycardia. Interestingly, in our experiments the average incidence of PVCs and ventricular tachycardia after FGF23 treatment was higher in our electrically paced, isolated heart experiments compared with the spontaneously beating isolated heart and live mouse studies. This could be due to the influence of the autonomic nervous system in vivo or difference in pacing rate.
Our findings are similar to arrhythmias documented in rodent models of CKD. The Cy/+ rat model for slowly progressive and spontaneous CKD displays frequent ventricular arrhythmias, spontaneous death, and elevated serum FGF23 (40). Based on this arrhythmic profile and the combined lack of an effect of FGF23 on intrinsic heart rate and PR interval in our experimental set up in vivo, it is unlikely that FGF23 is generating the arrhythmic response via directly activating nodal cell populations, influencing atrioventricular conduction or altering autonomic nervous input.
QTc Prolongation
We also measured other ECG waveform parameters and found that FGF23 had no effect on QRS duration; however, FGF23 treatment produced a significant lengthening of QTc compared with vehicle control.
The QTc interval represents the total depolarization and repolarization time in the ventricle corrected for heart rate. Prolongation of QTc is associated with increased risk of ventricular arrhythmias and sudden cardiac death in both the CKD and the general populations (8). The etiology of long QTc in CKD has been suggested to stem from various comorbidities like type II diabetes and CVD (8, 41), electrolyte imbalances during hemodialysis (42) or hypertrophy-related changes to cardiac ultrastructure, and potassium channel expression (43). Here, we present a novel mechanism in which increased levels of FGF23 may induce changes in QTc.
Interestingly, our laboratories have previously shown that FGF23 can induce cardiac hypertrophy with chronic exposure (16, 17, 19) and acutely induce vascular dysfunction in rodents (44). In more chronic conditions, FGF23 may thus contribute in multiple ways to cardiac remodeling, excitability, and sudden cardiac death. Based on our results, we hypothesize that elevated FGF23/FGFR4 signaling during CKD contributes to cardiac remodeling and prolongs QTc, which further predispose the heart to ventricular arrhythmias during various stages of CKD.
Potential Mechanism for Arrhythmias
Aberrant cardiac Ca2+ cycling is known to promote the generation of arrhythmias (33). A spontaneous increase in intracellular Ca2+ concentration can have downstream effects on cellular membrane potential leading to premature action potential propagation. At the level of the cardiomyocyte, Ca2+ overload plays an integral role in the formation of delayed after-depolarizations which can culminate into ventricular arrhythmias in the heart (45). In our original study in 2013, we demonstrated that FGF23 increased intracellular Ca2+ in cardiomyocytes by a mechanism that involved L-type Ca2+ channels (17). Subsequently, it was demonstrated via patch clamp analysis that FGF23 increased L-type Ca2+ current in pulmonary vein cardiomyocytes and HL-1 atrial cells (46, 47). Finally, there have been recent reports in isolated ventricular cardiomyocytes showing spontaneous cytosolic Ca2+ wave propagation during electrical pacing and increased Ca2+ spark frequency from the sarcoplasmic reticulum after FGF23 treatment (48, 49). In accordance with our laboratory and other previous studies, we have found that FGF23 perturbs Ca2+ homeostasis, specifically by stimulating an increase in the overall intracellular Ca2+ levels of resident cardiomyocytes inside whole left ventricular muscle tissue. Moreover, we found that this rise in ventricular muscle Ca2+ by FGF23 is largely mediated by the FGFR4 pathway. These findings provide mechanistic insight into the processes underlying the arrhythmogenic properties of pathological concentrations of FGF23, suggesting a link between augmented ventricular cardiomyocyte Ca2+ levels and the formation of arrhythmias, namely PVCs and ventricular tachyarrhythmias in the heart.
Intracellular Ca2+ balance also plays an integral role in the cardiac repolarization process. The normal myocardial repolarization mechanism primarily relies on reduction of inward Na+ and Ca2+ currents and an increase in outward voltage-gated K+ currents (50). Circumstances leading to sustained Ca2+ current via L-type channels can prolong the plateau phase of the cardiomyocyte action potential thereby delaying action potential duration, which displays as prolonged QTc interval on ECG (51–53). Although the exact mechanism behind the QTc prolonging and arrhythmogenic effects of FGF23 requires further exploration, our findings suggests that increased cardiomyocyte intracellular Ca2+ concentration likely plays a significant role.
FGFR4
Our group has previously demonstrated in the heart that FGF23 signals through activation of FGFR4 independently of coreceptor α-Klotho to augment cardiac muscle contractility and induce left ventricular hypertrophy (19). FGF23/FGFR4-mediated signaling leads to specific downstream induction of phospholipase Cγ (PLCγ) and subsequently the Ca2+/calcineurin/NFAT pathway (16, 18, 19). Activation of the PLC/IP3 pathway leads to changes in Ca2+ handling and arrhythmias as we and others have observed in previous studies (27, 54). In a study utilizing patch clamp recordings, FGF23 increased incidence of delayed afterdepolarizations in atrial HL-1 cells whereas a PLC inhibitor eliminated the Ca2+-dependent signaling induced by FGF23 (47).
In this study, treatment with an FGFR4-specific blocking antibody inhibited the increase in cardiomyocyte Ca2+, the generation of arrhythmias, and attenuated the lengthening of QTc induced by FGF23, suggesting a potential therapeutic application for FGFR4 blockade treatment in CKD. Intriguingly, we have found that FGF23 does not have an excitatory effect on skeletal muscle as it does in cardiac muscle despite the presence of FGFR4 in both slow and fast twitch skeletal muscle groups (55), indicating that FGF23 has unique excitatory effects on cardiac muscle. The current therapeutic options available to treat and prevent cardiovascular complications during CKD address traditional risk factors (i.e., hypertension, low-density lipoprotein cholesterol) and are not sufficient in preventing mortality, especially sudden cardiac death (56). This highlights the need to develop novel treatment options for patients with CKD especially since increased expression of FGFR4 has been observed in cardiac tissues from rodent models of CKD and in human patients with CKD (57, 58).
The potential additional benefits of specific FGFR4 blockade during CKD could include alleviating cardiac hypertrophy and hepatic inflammation which has been shown in a 5/6 nephrectomy rat model for CKD (19, 59). FGFR4 signaling does not contribute to serum phosphate regulation via FGF23 in a global FGFR4 deletion model of CKD and in mice with X-linked hypophosphatemic rickets (60, 61). This is important, as removal of phosphate by FGF23 is critical for survival during CKD as application of an FGF23-neutralizing antibody in CKD rats led to higher mortality rates (62). Furthermore, genetic deletion of Fgf23 in bone increased blood urea nitrogen and phosphate levels and aggravated cardiac hypertrophy in an adenine diet-induced CKD mouse model (63). Thus, specific anti-FGFR4 treatment could potentially serve as a viable cardio-protective therapy during CKD to counter the development of cardiovascular disease without affecting the normal physiological functions of FGF23 in the kidney.
Nevertheless, it will be important to consider the potential challenges associated with FGFR4 blocking treatment. Specifically, for liver toxicity and metabolic changes associated with interruption in FGFR4 signaling as seen in global FGFR4-deficient mice (64, 65). A recent phase I clinical trial investigating FGFR4 inhibitor, fisogatinib, for treating hepatocellular carcinoma reported a majority of side effects related to transaminitis and gastrointestinal issues, which would be expected since FGFR4 is also deeply involved in bile acid synthesis (66, 67). Finally, it is worthy to note that elevated FGF23 levels may provide some compensatory or beneficial cardiac effects during the early stages of CKD. Since we have shown that FGF23 can increase cardiac contractility through FGFR4 (19), these effects may be beneficial in the short-term for increasing cardiac output and glomerular filtration during CKD.
Conclusion and Limitations
Our study contained limitations, which should be regarded before translating these findings to human conditions with elevated FGF23. The hearts in our study were from young and disease-free mice. In addition, we delivered FGF23 in a pulse-dose and our animals did not receive elevated FGF23 over months or years like patients with CKD and ESRD. Although we used concentrations of FGF23 that are found in end-stage renal disease, it is difficult to know the exact concentration that was reaching myocytes in the ex vivo or in vivo studies. In light of these differences, it is impressive that we still observed significant numbers of arrhythmias demonstrating that FGF23 may be a significant proarrhythmic factor on its own. Next, our in vivo studies were carried out in anesthetized mice, which may have altered the autonomic nervous response to FGF23 and thus susceptibility to arrhythmias. In the future, it will be important to combine our findings in CKD models as well as in diseased hearts, as there may be synergistic factors with these comorbidities that enhance the arrhythmogenicity of FGF23 in vivo. Interestingly, our group and others have studied the Dmp1 null mouse model of elevated FGF23 that develops autosomal recessive hypophosphatemic rickets (68, 69). These animals did not demonstrate changes in cardiac remodeling. Although the levels of FGF23 in this disease are not as high as during CKD, this may indicate that additional factors may augment the effects of FGF23. It will also be important to study the downstream signaling from FGFR4 that plays a role in the arrhythmogenesis in various conditions. In summary, our data support a role of FGF23/FGFR4 signaling in aberrant cardiac depolarization/repolarization in CKD and may serve as a target for the development of treatments to reduce cardiac mortality in CKD, especially sudden cardiac death.
SUPPLEMENTAL DATA
Supplemental Fig. S1 (https://doi.org/10.6084/m9.figshare.14340134).
Supplemental Fig. S2 (https://doi.org/10.6084/m9.figshare.14340170).
Supplemental Figure Legends (https://doi.org/10.6084/m9.figshare.14340209).
GRANTS
This work was funded by National Institutes of Health (NIH) Grant P01AG039355 (to M.J.W.), NIH Minority Supplement 3P01AG039355-08S2 (to J.V. and M.J.W.), UMKC Sarah Morrison Medical Student Research Award (to J.M.G., C.S.H., D.W., R.A., S.P.), and NIH Grants R01HL128714 and R01HL145528 (to C.F.). C.F. was also supported by the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research, the AMC21 program of the Department of Medicine at UAB, and the Tolwani Innovation Award from the Division of Nephrology at UAB.
DISCLOSURES
C.F. has served as a consultant for Bayer and Calico Labs. C.F. is an inventor on two pending patents (PCT/US2019/049211; PCT/US19/49161) aimed to identify novel FGF23/FGFR4 inhibitors, and he is the cofounder of a startup biotech company (Alpha Young LLC) that has the goal to identify and commercialize these inhibitors. C.F. is currently the CSO and was previously the acting CEO of Alpha Young LLC. C.F. also has a patent on FGFR inhibition (European Patent No. 2723391).
AUTHOR CONTRIBUTIONS
J.M.G., J.A.V., C.S.H., and M.J.W. conceived and designed research; J.M.G., J.A.V., C.S.H., D.W., R.A., and S.P. performed experiments; J.M.G., J.A.V., C.S.H., D.W., R.A., S.P., and M.J.W. analyzed data; J.M.G., J.A.V., C.S.H., C.F., and M.J.W. interpreted results of experiments; J.M.G., J.A.V., R.A., S.P., and M.J.W. prepared figures; J.M.G. and J.A.V. drafted manuscript; J.M.G., J.A.V., C.S.H., D.W., R.A., S.P., C.F., and M.J.W. edited and revised manuscript; J.M.G., J.A.V., C.S.H., D.W., R.A., S.P., C.F., and M.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The anti-FGFR4 blocking antibody was a gift from Drs. Reimar Abraham and Johannes Bange from Lead Discovery Center, GmbH.
REFERENCES
- 1.Heron M. Deaths: leading causes for 2016. Natl Vital Stat Syst 67: 1–77, 2018. [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, , et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 26: 2504–2511, 2015. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 5: 2–7, 2015. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 23: 1929–1939, 2012. doi: 10.1681/asn.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozbas H, Atar I, Yildirir A, Ozgul A, Uyar M, Ozdemir N, Muderrisoglu H, Ozin B. Prevalence and predictors of arrhythmia in end stage renal disease patients on hemodialysis. Ren Fail 29: 331–339, 2007. doi: 10.1080/08860220701191237. [DOI] [PubMed] [Google Scholar]
- 7.Samanta R, Chan C, Chauhan VS. Arrhythmias and sudden cardiac death in end stage renal disease: epidemiology, risk factors, and management. Can J Cardiol 35: 1228–1240, 2019. doi: 10.1016/j.cjca.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Wang L, Han D, Sun C, Xue X, Li G. Acquired long QT syndrome in chronic kidney disease patients. Ren Fail 42: 54–65, 2020. doi: 10.1080/0886022X.2019.1707098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sato H, Nagata K, Shimada Y, Nojima Y, Iguchi K, Sugiyama T. Changes in QTc interval in long-term hemodialysis patients. PLoS One 14: e0209297, 2019. doi: 10.1371/journal.pone.0209297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2003. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. doi: 10.1172/JCI200419081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 13.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 83: 160–166, 2013. doi: 10.1038/ki.2012.327. [DOI] [PubMed] [Google Scholar]
- 14.Dalal M, Sun K, Cappola AR, Ferrucci L, Crasto C, Fried LP, Semba RD. Relationship of serum fibroblast growth factor 23 with cardiovascular disease in older community-dwelling women. Eur J Endocrinol 165: 797–803, 2011. doi: 10.1530/EJE-11-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano C, Hamano T, Fujii N, Obi Y, Matsui I, Tomida K, Mikami S, Inoue K, Shimomura A, Nagasawa Y, Okada N, Tsubakihara Y, Rakugi H, Isaka Y. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 50: 1266–1274, 2012. doi: 10.1016/j.bone.2012.02.634. [DOI] [PubMed] [Google Scholar]
- 16.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab 304: E863–E873, 2013. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstadt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22: 1020–1032, 2015. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, Di MG, Brand M, Wacker MJ, Faul C. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep 7: 1993, 2017. doi: 10.1038/s41598-017-02068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Cai C, Xiao Z, Quarles LD. FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol 138: 66–74, 2020. doi: 10.1016/j.yjmcc.2019.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol 1: 548–556, 2016. doi: 10.1001/jamacardio.2016.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamura M, Fujita S, Morita H, Sakane K, Okamoto Y, Sohmiya K, Hoshiga M, Ishizaka N. Circulating fibroblast growth factor 23 has a U-shaped association with atrial fibrillation prevalence. Circ J 79: 1742–1748, 2015. doi: 10.1253/circj.CJ-15-0413. [DOI] [PubMed] [Google Scholar]
- 23.Oakley CI, Vallejo JA, Wang D, Gray MA, Tiede-Lewis LM, Shawgo T, Daon E, Zorn G 3rd, Stubbs JR, Wacker MJ. Trimethylamine-N-oxide acutely increases cardiac muscle contractility. Am J Physiol Heart Circ Physiol 318: H1272–H1282, 2020. doi: 10.1152/ajpheart.00507.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huggins CE, Bell JR, Pepe S, Delbridge LM. Benchmarking ventricular arrhythmias in the mouse—revisiting the ‘Lambeth Conventions’ 20 years on. Heart Lung Circ 17: 445–450, 2008. doi: 10.1016/j.hlc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res 22: 447–455, 1988. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 26.Wacker MJ, Best SR, Kosloski LM, Stachura CJ, Smoot RL, Porter CB, Orr JA. Thromboxane A2-induced arrhythmias in the anesthetized rabbit. Am J Physiol Heart Circ Physiol 290: H1353–H1361, 2006. doi: 10.1152/ajpheart.00930.2005. [DOI] [PubMed] [Google Scholar]
- 27.Wacker MJ, Kosloski LM, Gilbert WJ, Touchberry CD, Moore DS, Kelly JK, Brotto M, Orr JA. Inhibition of thromboxane A2-induced arrhythmias and intracellular calcium changes in cardiac myocytes by blockade of the inositol trisphosphate pathway. J Pharmacol Exp Ther 331: 917–924, 2009. doi: 10.1124/jpet.109.157677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol 30: 867–878, 2003. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 29.Harkness JE, Turner PV, VandeWoude S, Wheler CL. Harkness and Wagner’s Biology and Medicine of Rabbits and Rodents. Ames, Iowa: Wiley-Blackwell, 1989. [Google Scholar]
- 30.Mitruka BM, Rawnsley HM. Clinical Biochemical and Hematological Reference Values in Normal Experimental Animals and Normal Humans. New York: Masson Pub, 1981. [Google Scholar]
- 31.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747–H751, 1998. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 32.Campos FO, Shiferaw Y, Prassl AJ, Boyle PM, Vigmond EJ, Plank G. Stochastic spontaneous calcium release events trigger premature ventricular complexes by overcoming electrotonic load. Cardiovasc Res 107: 175–183, 2015. doi: 10.1093/cvr/cvv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res 120: 1969–1993, 2017. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog CA. Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int Suppl S197–S200, 2003. doi: 10.1046/j.1523-1755.63.s84.17.x. [DOI] [PubMed] [Google Scholar]
- 35.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes 3: 159–164, 2010. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharaf El Din UA, Salem MM, Abdulazim DO. Is Fibroblast growth factor 23 the leading cause of increased mortality among chronic kidney disease patients? A narrative review. J Adv Res 8: 271–278, 2017. doi: 10.1016/j.jare.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Luo H, Tang X, Zeng X, Yu Y, Ma L, Fu P. Prognostic value of FGF23 among patients with end-stage renal disease: a systematic review and meta-analysis. Biomark Med 10: 547–556, 2016. doi: 10.2217/bmm.16.11. [DOI] [PubMed] [Google Scholar]
- 38.Bansal N, Fan D, Hsu CY, Ordonez JD, Go AS. Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc 3: e001303, 2014. doi: 10.1161/JAHA.114.001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapira OM, Bar-Khayim Y. ECG changes and cardiac arrhythmias in chronic renal failure patients on hemodialysis. J Electrocardiol 25: 273–279, 1992. doi: 10.1016/0022-0736(92)90032-U. [DOI] [PubMed] [Google Scholar]
- 40.Hsueh CH, Chen NX, Lin SF, Chen PS, Gattone VH 2nd, Allen MR, Fishbein MC, Moe SM. Pathogenesis of arrhythmias in a model of CKD. J Am Soc Nephrol 25: 2812–2821, 2014. doi: 10.1681/ASN.2013121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ninkovic VM, Ninkovic SM, Miloradovic V, Stanojevic D, Babic M, Giga V, Dobric M, Trenell MI, Lalic N, Seferovic PM, Jakovljevic DG. Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. Acta Diabetol 53: 737–744, 2016. doi: 10.1007/s00592-016-0864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosoosi NM, Saravi M, Oliaee F, Akbari R, Noorkhomami S, Bozorgi Rad SH, Fallahpoor K, Ramezani MS. Changes in QT interval before and after hemodialysis. Caspian J Intern Med 4: 590–594, 2013. [PMC free article] [PubMed] [Google Scholar]
- 43.De Ambroggi L, Francia P, De Ambroggi D. Repolarization abnormalities and arrhythmogenesis in hypertrophic myocardium. Anadolu Kardiyol Derg 7, Suppl1: 71–72, 2007. [PubMed] [Google Scholar]
- 44.Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang S, Andresen J, Stubbs JR, Wacker MJ. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 307: E426–E436, 2014. doi: 10.1152/ajpendo.00264.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res 87: 774–780, 2000. doi: 10.1161/01.RES.87.9.774. [DOI] [PubMed] [Google Scholar]
- 46.Huang SY, Chen YC, Kao YH, Hsieh MH, Lin YK, Chung CC, Lee TI, Tsai WC, Chen SA, Chen YJ. Fibroblast growth factor 23 dysregulates late sodium current and calcium homeostasis with enhanced arrhythmogenesis in pulmonary vein cardiomyocytes. Oncotarget 7: 69231–69242, 2016. doi: 10.18632/oncotarget.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kao YH, Chen YC, Lin YK, Shiu RJ, Chao TF, Chen SA, Chen YJ. FGF-23 dysregulates calcium homeostasis and electrophysiological properties in HL-1 atrial cells. Eur J Clin Invest 44: 795–801, 2014. doi: 10.1111/eci.12296. [DOI] [PubMed] [Google Scholar]
- 48.Navarro-García JA, Delgado C, Fernández-Velasco M, Val-Blasco A, Rodríguez-Sánchez E, Aceves-Ripoll J, Gómez-Hurtado N, Bada-Bosch T, Mérida-Herrero E, Hernández E, Praga M, Salguero R, Solís J, Arribas F, Delgado JF, Bueno H, Kuro OM, Ruilope LM, Ruiz-Hurtado G. Fibroblast growth factor-23 promotes rhythm alterations and contractile dysfunction in adult ventricular cardiomyocytes. Nephrol Dial Transplant 34: 1864–1875, 2019. doi: 10.1093/ndt/gfy392. [DOI] [PubMed] [Google Scholar]
- 49.Navarro-García JA, Rueda A, Romero-García T, Aceves-Ripoll J, Rodríguez-Sánchez E, González-Lafuente L, Zaragoza C, Fernández-Velasco M, Kuro OM, Ruilope LM, Ruiz-Hurtado G. Enhanced Klotho availability protects against cardiac dysfunction induced by uraemic cardiomyopathy by regulating Ca2+ handling. Br J Pharmacol 177: 4701–4719, 2020. doi: 10.1111/bph.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nerbonne JM. Studying cardiac arrhythmias in the mouse—a reasonable model for probing mechanisms? Trends Cardiovasc Med 14: 83–93, 2004. doi: 10.1016/j.tcm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu W, Ohe T, Kurita T, Kawade M, Arakaki Y, Aihara N, Kamakura S, Kamiya T, Shimomura K. Effects of verapamil and propranolol on early afterdepolarizations and ventricular arrhythmias induced by epinephrine in congenital long QT syndrome. J Am Coll Cardiol 26: 1299–1309, 1995. doi: 10.1016/0735-1097(95)00313-4. [DOI] [PubMed] [Google Scholar]
- 52.Thomas G, Chung M, Cohen CJ. A dihydropyridine (Bay k 8644) that enhances calcium currents in guinea pig and calf myocardial cells. A new type of positive inotropic agent. Circ Res 56: 87–96, 1985. doi: 10.1161/01.RES.56.1.87. [DOI] [PubMed] [Google Scholar]
- 53.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Murata M, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res 101: 69–77, 2007. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 54.Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147, 2008. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, Dallas SL, Moe SM, Wacker MJ. Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab 315: E594–E604, 2018. doi: 10.1152/ajpendo.00343.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomey MI, Winston JA. Cardiovascular pathophysiology in chronic kidney disease: opportunities to transition from disease to health. Ann Glob Health 80: 69–76, 2014. doi: 10.1016/j.aogh.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Leifheit-Nestler M, Grabner A, Hermann L, Richter B, Schmitz K, Fischer DC, Yanucil C, Faul C, Haffner D. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant 32: 1493–1503, 2017. doi: 10.1093/ndt/gfw454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, Klintschar M, Becker JU, Erbersdobler A, Aufricht C, Seeman T, Fischer DC, Faul C, Haffner D. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial transplant 31: 1088–1099, 2016. doi: 10.1093/ndt/gfv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985–996, 2016. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol 19: 2342–2350, 2008. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor A, Yanucil C, Musgrove J, Shi M, Ide S, Souma T, Faul C, Wolf M, Grabner A. FGFR4 does not contribute to progression of chronic kidney disease. Sci Rep 9: 14023, 2019. doi: 10.1038/s41598-019-50669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122: 2543–2553, 2012. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clinkenbeard EL, Noonan ML, Thomas JC, Ni P, Hum JM, Aref M, Swallow EA, Moe SM, Allen MR, White KE. Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD. JCI Insight 4: e123817, 2019. doi: 10.1172/jci.insight.123817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 56: 2501–2510, 2007. doi: 10.2337/db07-0648. [DOI] [PubMed] [Google Scholar]
- 65.Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol 161: 2003–2010, 2002. doi: 10.1016/S0002-9440(10)64478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim RD, Sarker D, Meyer T, Yau T, Macarulla T, Park JW, , et al. First-in-human phase i study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov 9: 1696–1707, 2019. doi: 10.1158/2159-8290.CD-19-0555. [DOI] [PubMed] [Google Scholar]
- 67.Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275: 15482–15489, 2000. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 68.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wacker MJ, Touchberry CD, Silswal N, Brotto L, Elmore CJ, Bonewald LF, Andresen J, Brotto M. Skeletal muscle, but not cardiovascular function, is altered in a mouse model of autosomal recessive hypophosphatemic rickets. Front Physiol 7: 173, 2016. doi: 10.3389/fphys.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]