Abstract
Heart failure (HF) results in a myriad of central and peripheral abnormalities that impair the ability to sustain skeletal muscle contractions and, therefore, limit tolerance to exercise. Chief among these abnormalities is the lowered maximal oxygen uptake, which is brought about by reduced cardiac output and exacerbated by O2 delivery-utilization mismatch within the active skeletal muscle. Impaired nitric oxide (NO) bioavailability is considered to play a vital role in the vascular dysfunction of both reduced and preserved ejection fraction HF (HFrEF and HFpEF, respectively), leading to the pursuit of therapies aimed at restoring NO levels in these patient populations. Considering the complementary role of the nitrate-nitrite-NO pathway in the regulation of enzymatic NO signaling, this review explores the potential utility of inorganic nitrate interventions to increase NO bioavailability in the HFrEF and HFpEF patient population. Although many preclinical investigations have suggested that enhanced reduction of nitrite to NO in low Po2 and pH environments may make a nitrate-based therapy especially efficacious in patients with HF, inconsistent results have been found thus far in clinical settings. This brief review provides a summary of the effectiveness (or lack thereof) of inorganic nitrate interventions on exercise tolerance in patients with HFrEF and HFpEF. Focus is also given to practical considerations and current gaps in the literature to facilitate the development of effective nitrate-based interventions to improve exercise tolerance in patients with HF.
Keywords: beetroot juice, fatigue, nitric oxide, nitrite, skeletal muscle
INTRODUCTION
Our view of the biological significance of nitric oxide (NO) has expanded greatly since the initial discovery as an endothelium-derived vasodilator to now being appreciated as a pluripotent signaling molecule involved in numerous cardiovascular, metabolic, renal, and immunological processes. Conversely, reduced NO bioavailability has been coupled to the pathophysiology of cardiometabolic and renal disorders. Generation of this small molecule is interestingly complex and is carried out by specific NO synthases (NOS) in a five-step oxidation process requiring l-arginine, molecular oxygen, and several crucial cofactors (Fig. 1) (1). In blood and tissues, NO is very short lived due to rapid oxidation to the more stable anions nitrate (NO3−) and nitrite (NO2−), which have been widely used as surrogate measures of NOS activity (2). Canonical NO signaling, such as in the vasculature, involves activation of soluble guanylyl cyclase (sGC) that increases the levels of the second messenger cyclic guanosine monophosphate (cGMP), activating cGMP-dependent protein kinases that induce relaxation of vascular smooth muscle. Modulation of protein functions also exists via cGMP-independent mechanisms that contribute to the regulatory effects of NO signaling (3). Considering the wide array of effects controlled by NO in health and disease, the purpose of this mini-review is to shed light on the potential therapeutic applications of the nitrate-nitrite-NO pathway for the treatment of heart failure (HF). Attention has been given to both reduced and preserved ejection fraction HF (HFrEF and HFpEF, respectively), with a special emphasis on inorganic nitrate supplementation and exercise tolerance.
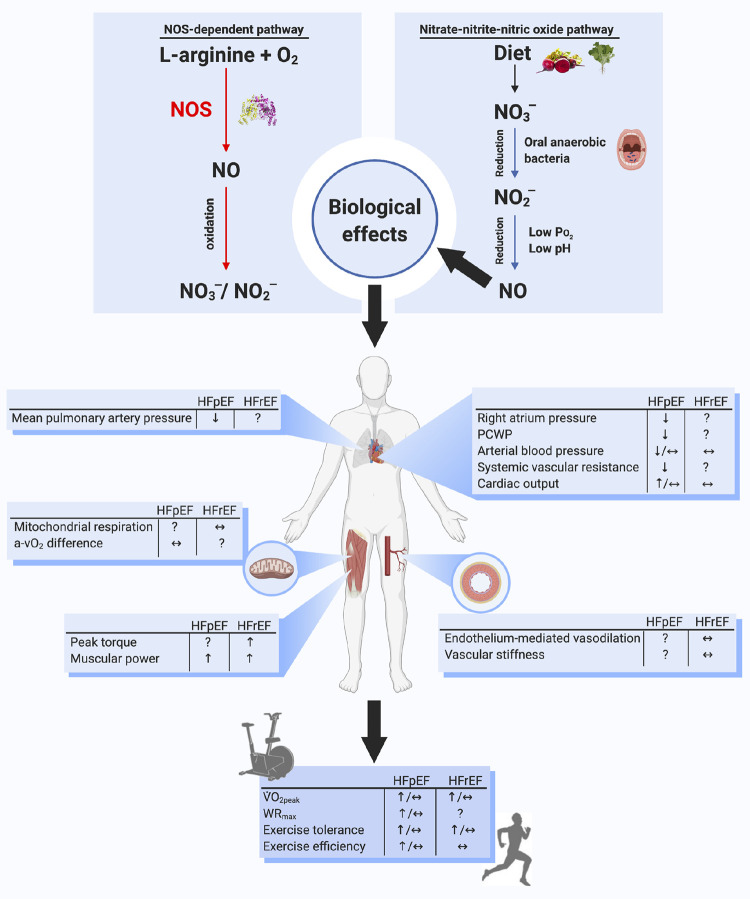
Figure 1.
Top: schematic of the two parallel pathways for NO formation: the NOS-dependent and nitrate-nitrite-NO pathways. Middle: impact of inorganic nitrate and nitrite supplementation on many of the dysfunctional cardiorespiratory and skeletal muscle elements found in patients with HFpEF and HFrEF. Bottom: impact of inorganic nitrate and nitrite supplementation on exercise efficiency and tolerance in patients with HFpEF and HFrEF. See text for additional information. a-Vo2 difference, arteriovenous O2 content difference; NO, nitric oxide; NO3−, nitrate; NO2−, nitrite; NOS, nitric oxide synthase; PCWP, pulmonary capillary wedge pressure; V̇o2peak, peak oxygen uptake; WRmax, maximal power output.
THE NITRATE-NITRITE-NITRIC OXIDE PATHWAY
Ten years after the discovery of NO as a signaling molecule, an alternative pathway for NO generation was found wherein nitrate and nitrite are serially reduced to NO and other bioactive nitrogen species (Fig. 1) (4, 5). As mentioned earlier, circulating nitrate originates from oxidation of NOS-generated NO but also to a large extent from dietary sources, such as green leafy vegetables and beetroot (6). Regardless of source, circulating nitrate is actively taken up by the salivary glands and secreted in the saliva (7). In the oral cavity, several bacterial species belonging to the commensal flora contain nitrate reductases that can catalyze the first reduction of salivary nitrate to nitrite (8, 9). In humans, but not to the same extent in rodents, the salivary levels of these anions normally exceed plasma levels by several orders of magnitude (9, 10). The crucial role of the oral microflora in nitrate bioactivation is evident from experiments where antibacterial mouthwash products abrogate the effects of both endogenous and dietary nitrate (11, 12). Under normal conditions, swallowed nitrite is rapidly protonated due to the acidic conditions in the stomach, instantly and nonenzymatically giving rise to NO and several other nitrogen intermediates with seemingly protective effects on the gastric mucosa (13). However, in the presence of dietary amines, formation of carcinogenic nitrosamines from nitrite can also occur, which has led to very strict regulations of nitrate levels in our food and drinking water (14). These measures are debatable since the relationship between dietary nitrate and cancer has not been demonstrated in humans (15, 16). After passage from the stomach, remaining nitrite is rapidly and very efficiently absorbed in the gut and enters the systemic circulation. There are several enzymatic and nonenzymatic pathways in blood and tissues that reduce nitrite to NO and other nitrogen intermediates, including those involving deoxyhemoglobin, xanthine oxidoreductase, and mitochondrial complexes (17). Interestingly, these pathways for NO generation from nitrite are potentiated during hypoxia and low pH, conditions where NOS activity is markedly reduced.
Accumulating data from experimental and human studies show that dietary stimulation of the nitrate-nitrite-NO pathway can provide beneficial cardiovascular (18) and metabolic (19) effects as well as renoprotection (20). These include lowering of blood pressure, increased aerobic efficiency during exercise, protection against ischemia-reperfusion injury and inflammation, and improved glucose control in models of diabetes. In view of these promising data, it is noteworthy that despite extensive NO research over the past 30 years, which indeed has accumulated considerable knowledge on the biological role of NO, there is unfortunately to date only a limited number of effective clinical applications. Inhaled NO gas is given to treat pulmonary hypertension in newborns (21), and exhaled NO is measured to monitor airway inflammation in asthma (22). Apart from nitroglycerin, known long before the discovery of NO in physiology, few novel NO-related drugs have emerged into clinical practice. Intravenous nitroprusside is recommended in the management of acute decompensated HF and typically requires close hemodynamic monitoring for marked hypotension (23). Phosphodiesterase inhibitors that block cGMP breakdown are used to treat pulmonary hypertension and erectile dysfunction (24). Novel sGC stimulators are prescribed for pulmonary hypertension, but promising studies may indicate a wider use of this class of drugs (25). In addition to therapies that target downstream signaling of NO, stimulation of the nitrate-nitrite-NO pathway (either by the diet or by administration of nitrate or nitrite) may represent an alternative way to enhance NO bioavailability, especially in HF where NOS function is impaired.
CONTEXT FOR THE DEVELOPMENT OF NITRATE-BASED HEART FAILURE THERAPIES
HF is a complex, multifactorial clinical syndrome with high morbidity and mortality. The disease is broadly defined by the inability to provide adequate cardiac output to meet peripheral metabolic demand at normal intracardiac filling pressures. HFpEF and HFrEF are the two main distinct phenotypes of HF identified among patients. Although patients with HFpEF demonstrate a similar poor prognosis as those with HFrEF, the pathophysiology of HFpEF is poorly understood, thus limiting the development of effective pharmacological therapies. Neurohumoral activation dominates HFrEF pathophysiology, whereas variable extracardiac mechanisms (e.g., arterial hypertension, metabolic risk, and renal insufficiency) seem to promote chronic systemic inflammation and result in the heterogeneous clinical presentation of HFpEF (26–28). Common to both HF classifications is the hallmark symptom of severe exercise intolerance, which constitutes a critical determinant of poor prognosis and reduced quality of life (27).
Recent advances indicate that noncardiac, peripheral skeletal muscle abnormalities contribute significantly to exercise intolerance in both HFpEF and HFrEF. Such muscle abnormalities include reductions in capillary density, vasodilatory capacity, oxygen transport (i.e., impaired perfusive and diffusive O2 fluxes), percentage of type I muscle fibers, and mitochondrial content and function (27, 29–31). These HF-induced alterations culminate in muscle oxygen delivery-utilization (Q̇o2/V̇o2) mismatch during contractions predisposing to reduced exercise tolerance and maximal oxygen uptake (V̇o2max) (27). Pursuant to the scope of this mini-review, several lines of evidence suggest that reduced NO bioavailability plays a crucial role in exercise intolerance in HFpEF and HFrEF (15, 27, 32).
Although the notion that nitrite reduction to NO is potentiated in relatively low Po2 and pH environments is well enshrined in studies dating before the discovery of NO’s signaling properties, the holistic and applied effects of nitrate bioactivation have only become evident within the past two decades. For example, seminal work from Cosby et al. (33) revealed that relaxation of isolated vessels was potentiated largely at a Po2 <30 mmHg when nitrite was combined with red blood cells. These data suggest that microvascular Po2 (PO2mv) values often observed in contracting muscle could facilitate the reduction of nitrite to NO in vivo and thus promote a local increase in blood flow. About a decade later, Ferguson et al. (34) demonstrated in rats that short periods (5 days) of nitrate supplementation enhanced blood flow during treadmill running in muscles composed predominantly of fast-twitch fibers, thereby showcasing the integrated aspects of low Po2-induced nitrite reduction to NO during exercise. It was around this time that other investigations showed preferential effects of nitrate supplementation on fast-twitch muscles and athletic events where these types of muscle fibers are relied heavily upon (35).
The fiber-type specific effects of nitrate supplementation are likely facilitated by the large degree of blood flow heterogeneity across the spectrum of fast- and slow-twitch muscle phenotypes, such that at rest and during contractions, the PO2mv of fast-twitch muscles is significantly lower than that of their slow-twitch compatriots (35–37). In HF, peripheral vascular derangements decrease PO2mv predominantly in slow- but not fast-twitch muscles (38), bringing the contracting PO2mv of the soleus (primarily slow-twitch) to ∼15 mmHg, which is well below the suggested Po2 required to reduce nitrite to NO optimally in vivo (33). In this sense, HF may “level the playing field” for slow-twitch fibers to also benefit from nitrate supplementation, as it creates an environment ripe for nitrite bioactivation in muscles that may not usually reach the “optimal” PO2mv range for those effects during submaximal contractions (26).
Considering the impact of inorganic nitrate supplementation in healthy humans and animal models and the muscle fiber-type specific effects mentioned above many have postulated that a nitrate-based therapy could improve contracting muscle Q̇o2/V̇o2 matching and ameliorate exercise intolerance in HF. These latter improvements could be achieved by enhanced local perfusion and oxygenation, increased muscle contractility and resistance to fatigue, reduced O2 cost of muscle contractions, faster O2 uptake kinetics, and/or lowered arterial blood pressure (26, 27, 32, 39, 40). In this context, inorganic nitrate and nitrite supplementation has recently emerged as a potential clinical intervention for both patients with HFpEF and HFrEF. Inorganic nitrate is further attractive because it can be supplemented orally via natural products (e.g., beetroot juice and leafy green vegetables) with no evidence of tachyphylaxis or significant adverse effects during chronic dosing, which is not the case with organic nitrates (15). The following sections will summarize the effects of inorganic nitrate and nitrite supplementation on peripheral responses to exercise and exercise tolerance in patients with HFpEF and HFrEF.
INORGANIC NITRATE AND NITRITE SUPPLEMENTATION IN HFPEF
Despite the potential for nitrate and nitrite reduction to NO in HF and the increased NO bioavailability afforded by nitrates, the pathway to an effective NO-based clinical intervention is not without complexity. For example, initial multicenter trials aiming at improving NO bioavailability and exercise tolerance in patients with HFpEF used organic nitrate (isosorbide mononitrate; NEAT-HFpEF trial) (41) and phosphodiesterase-5 inhibition (sildenafil; RELAX trial) (42). However, neither intervention improved exercise performance or the clinical status of patients with HFpEF. This prompted the evaluation of inorganic nitrate and nitrite as a potential therapy for HFpEF. While organic nitrates promote the tonic release of NO, stimulation of the nitrate-nitrite-NO pathway is potentiated by hypoxic and acidic conditions likely to be found in the contracting skeletal muscle of patients with HF, as noted previously (15, 38). As such, preferential vasodilation and increased perfusion to tissues of high metabolic demand form the basis for improved peripheral Q̇o2/V̇o2 matching and enhanced exercise tolerance with inorganic nitrates.
To date, only five studies have examined the effects of inorganic nitrate and nitrite on cardiorespiratory function and exercise tolerance in patients with HFpEF (Table 1). Most studies have used oral nitrate supplementation (via beetroot juice or potassium nitrate; KNO3), whereas one multicenter trial used inhaled (nebulized) inorganic nitrite. These different oral nitrate sources (i.e., beetroot juice vs. KNO3) produce similar increases in dose-normalized plasma nitrate and nitrite concentrations in healthy individuals (52). In terms of administration routes, inhaled and intravenous nitrite administration have resulted in similar pharmacokinetic and pharmacodynamic effects in patients with HFpEF (53, 54). Targeting the pulmonary alveolar-capillary interface with nebulized nitrite circumvents first-pass metabolism inherent to dietary nitrate supplementation, thus avoiding potential impairments in oral bacterial nitrate reductase activity. Nonetheless, the efficiency of pulmonary drug delivery depends on particle size and velocity of inspiratory flow produced by the nebulizer device (45, 55). Inorganic nitrate and nitrite supplementation are generally well tolerated among patients with HFpEF, with few adverse effects but mixed results with respect to exercise tolerance.
Table 1.
Effect of inorganic nitrate and nitrite supplementation on select physiological responses to exercise and exercise tolerance in patients with HFpEF and HFrEF
| HF Phenotype and Study | Duration | Design | Participants/Groups | Dose and Administration Route | Exercise Protocol | Main Exercise Outcomes |
|---|---|---|---|---|---|---|
| Heart failure with preserved ejection fraction (HFpEF) | ||||||
| Eggebeen et al. (43) | Acute | Randomized, double-blind, placebo-controlled, crossover | 18 patients with HFpEF (85% female) 69 ± 7 yr | Oral (beetroot juice, 6.1 mmol nitrate, single dose) | Upright submaximal cycling exercise (∼75% WRmax) | ↔V̇o2peak, ↔tlim, ↔BP |
| Chronic | Randomized, double-blind, placebo-controlled | Oral (beetroot juice, 6.1 mmol nitrate/day for 1 wk) | ↔V̇o2peak, ↑tlim, ↔BP | |||
| Shaltout et al. (44)* | Chronic | Randomized, double-blind, placebo-controlled | Placebo and exercise training: 9 patients with HFpEF (89% female) 71 ± 8 yrBeetroot juice and exercise training: 10 HFpEF patients (80% female) 68 ± 6 yr | Oral (beetroot juice, 6.1 mmol nitrate 3× per wk for 4 wks) | Upright maximal and submaximal cycling exercise (∼75% WRmax) | ↔V̇o2peak, ↔WRmax, ↔tlim, ↔BP |
| Borlaug et al. (45)† | Chronic | Multicenter, randomized, double-blind, placebo-controlled, crossover | 105 patients with HFpEF (56% female) 68 yr | Inhaled (nebulized inorganic nitrite, 46 mg 3× per day for 1 wk + 80 mg 3× per day for 3 wks) | Upright maximal cycling exercise | ↔V̇o2peak, ↔tlim |
| Zamani et al. (46) | Chronic | Randomized, double-blind, placebo-controlled | Placebo: 3 patients with HFpEF (67% female) 63 ± 8 yrPotassium nitrate: 9 patients with HFpEF (67% female) 62 ± 5 yr | Oral (potassium nitrate, 6 mmol 2× per day for 1 wk + 6 mmol 3× per day for 1 wk) | Supine maximal cycling exercise | ↔V̇o2peak, ↑WRmax, ↑tlim, ↔BP |
| Zamani et al. (31) | Acute | Randomized, double-blind, placebo-controlled, crossover | 17 patients of HFpEF (12% female) 65 ± 9 yr | Oral (beetroot juice, 12.9 mmol nitrate, single dose) | Supine maximal cycling exercise | ↑V̇o2peak, ↑WRmax, ↑tlim, ↔BP |
| Heart failure with reduced ejection fraction (HFrEF) | ||||||
| Kerley et al. (47) | Acute | Randomized, double-blind, placebo-controlled, crossover | 10 patients with HFrEF (64% male), 56 ± 11 yr | Oral (beetroot juice 12.9 mmol nitrate, single dose) | Incremental shuttle walk test | ↑distance walked, ↔BP |
| Coggan et al. (48)‡ | Acute | Randomized, double-blind, placebo-controlled, crossover | 9 patients with HFrEF (56% male) 57 ± 10 yr | Oral (beetroot juice, 11.2 mmol nitrate, single dose) | 6-min walk test and single leg isokinetic dynamometry | ↔distance walked,↑maximal knee extensor power & velocity, ↔BP |
| Hirai et al. (49) | Chronic | Randomized, double-blind, placebo-controlled, crossover | 10 patients with HFrEF (100% male) 63 ± 5 yr | Oral (beetroot juice, 12.9 mmol nitrate per day for 9 days) | Upright low- and high-intensity cycling exercise | ↔V̇o2peak, ↔tlim, ↔BP |
| Coggan et al. (50) | Acute | Randomized, double-blind, placebo-controlled, crossover | 8 patients with HFrEF (75% male) 52 ± 5 yr | Oral (beetroot juice, 11.2 mmol nitrate, single dose) | Semirecumbent submaximal and maximal cycling exercise | ↑V̇o2peak, ↑tlim, ↔BP |
| Woessner et al. (51) | Chronic | Randomized, double-blind, placebo-controlled, crossover | 16 patients with HFrEF (93.75% male) 63 ± 4 yr | Oral (beetroot juice, 16 mmol nitrate per day for 5 days) | Treadmill maximal exercise | ↔V̇o2peak, ↔tlim, ↔BP |
BP, blood pressure (i.e., mean arterial pressure and/or systolic blood pressure); tlim, time to exhaustion; V̇o2peak, peak oxygen uptake; WRmax, maximal power output.
Protocol includes combined exercise training and nitrate intervention.
Protocol utilized nebulized nitrite intervention.
Protocol includes resistance strength outcomes.
Two acute, single-dose studies have reported contrasting effects of beetroot juice supplementation on exercise tolerance in HFpEF. The higher nitrate dose (12.9 mmol nitrate) used by Zamani et al. (31) resulted in a modest increase in V̇o2peak (i.e., ∼1 mL/kg/min) and exercise tolerance during supine cycling exercise partly due to reductions in systemic vascular resistance. Conversely, the lower dose (6.1 mmol nitrate) used by Eggebeen et al. (43) had no significant effects on V̇o2peak or exercise tolerance during upright submaximal cycling. Apart from differences in experimental protocols (i.e., exercise mode), these findings suggest that relatively high inorganic nitrate doses may improve clinical status and exercise tolerance in patients with HFpEF.
Chronic inorganic nitrate and nitrite supplementation studies in patients with HFpEF have also produced mixed results. Short-term (1–2 wk) oral administration of beetroot juice (43) or KNO3 (46) elevated exercise tolerance with no changes in V̇o2peak. Of note, lower hemoglobin concentration with serial blood draws likely constrained improvements in V̇o2peak in the latter study by Zamani et al. (46). Conversely, a long-term (4-wk) study using beetroot juice supplementation combined with exercise training (44) and another using inhaled nitrite (45) failed to improve V̇o2peak or exercise tolerance. It is important to note, however, the following when interpreting these long-term interventional studies: 1) the relatively low nitrate dose supplementation in the aforementioned training study increased plasma nitrate but not nitrite concentration, and 2) plasma nitrite concentration was not measured following inhaled nitrite.
A recent meta-analysis evaluating the existing HFpEF studies (as listed in Table 1) suggested that inorganic nitrate and nitrite supplementation may not be associated with improvements in exercise performance (as evaluated by time to exhaustion and V̇O2peak) in patients with HFpEF (56). However, as rightfully noted by the authors, interpretation of those results must be made with caution, given the small number of studies and sample sizes, differences in experimental protocols, and other potential confounding factors (e.g., clinical heterogeneity, polypharmacy, potential variability in oral bacteria, and unknown optimal supplementation doses). Compounding these technical challenges is the highly heterogeneous nature of the disease encompassing hypertension, diabetes/metabolic syndrome, obesity, and/or chronic kidney disease, among others, all under the same HFpEF diagnosis (28).
Despite the inconsistent effects on exercise tolerance described above, inorganic nitrate and nitrite have the potential to improve central hemodynamics in patients with HFpEF. Invasive cardiac catheterization studies by Borlaug et al. (53, 54) revealed that infused and inhaled inorganic nitrite lowered pulmonary capillary wedge pressure, mean pulmonary artery pressure, and right atrial pressures at rest and during low-intensity cycling exercise. It is interesting that dietary nitrate supplementation (with beetroot juice) seems to lower resting systolic blood pressure in patients with HFpEF but not in patients with HFrEF (Fig. 2), suggesting greater systemic activation of the nitrate-nitrite-NO pathway in HFpEF and/or fundamental differences in response to vasodilatory therapy between HF phenotypes (58).
Figure 2.
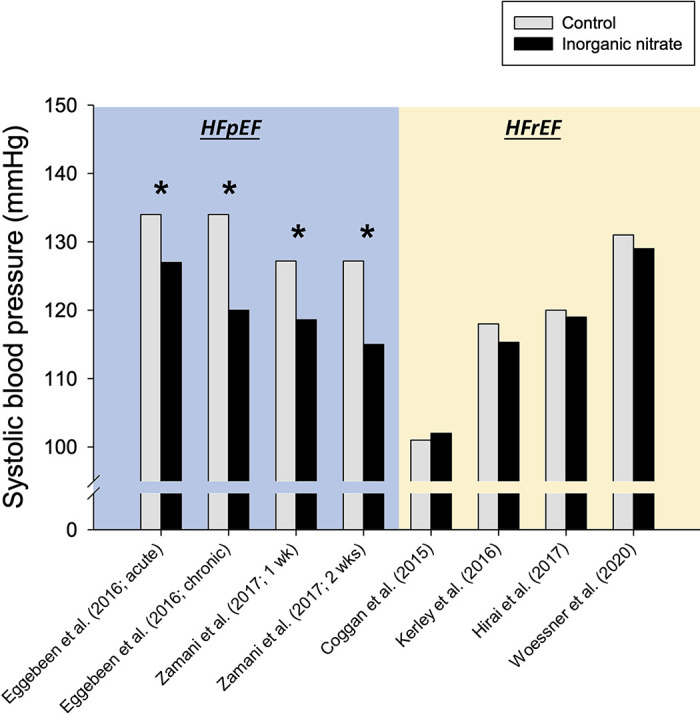
Effect of dietary inorganic nitrate supplementation (with beetroot juice) on mean resting systolic blood pressure from HFpEF and HFrEF studies published previously. See Table 1 and text for additional information. The following HFpEF studies were not included: Zamani et al. (31), no available resting systolic blood pressure data; Shaltout et al. (44), no within-subject data comparison (i.e., control vs. inorganic nitrate) preexercise training; and Borlaug et al. (45), protocol used inhaled (nebulized) nitrite. The following HFrEF study was not included: Coggan et al. (50), no available resting systolic blood pressure data. Standard deviation/error bars omitted for clarity. *P < 0.05 vs. control.
In summary, it is clear that additional studies with larger cohorts are critical to further understand the physiological responses to and potential clinical value of these interventions in patients with HFpEF. Encouraging results in some studies provide a compelling rationale to continue pursuing inorganic nitrate and nitrite clinical trials in HFpEF (see Fig. 1 for a summary of previous data). The nitrate-nitrite-NO pathway may constitute an important target to enhance exercise tolerance and, consequently, clinical outcomes in patients with HFpEF.
INORGANIC NITRATE SUPPLEMENTATION IN HFREF
Despite evidence of reduced NO bioavailability in individuals with HFrEF, inorganic nitrate supplementation studies have failed to show consistent benefits on cardiac hemodynamics and skeletal muscle vascular, metabolic, or contractile function in patients with HFrEF (Fig. 1) (31, 47, 49–51). As illustrated in Fig. 2, dietary nitrate supplementation with beetroot juice may lower resting systolic blood pressure in patients with HFpEF, but a similar, robust effect appears to be absent in patients with HFrEF. The limited body of research to date has provided mixed results on the effects of nitrate supplementation on exercise tolerance in patients with HFrEF (Table 1).
Dietary nitrate supplementation via beetroot juice in the studies by Hirai et al. (49) and Woessner et al. (51) did not improve central or peripheral vascular function, skeletal muscle microvascular oxygenation, mitochondrial respiration, or exercise tolerance (as evaluated by time to exhaustion and V̇o2peak during treadmill and upright cycling protocols) in patients with HFrEF. In contrast, two other studies found modest improvements in aerobic exercise performance following dietary nitrate supplementation in patients with HFrEF. Coggan et al. (50) reported improved time to exhaustion and V̇o2peak during semirecumbent cycling exercise, and Kerley et al. (47) showed improved walking performance to exhaustion as assessed via an incremental shuttle walk test. It is interesting that, in addition to methodological differences among studies (e.g., upright vs. semirecumbent cycling and related effects of gravity on hemodynamics), the heterogeneity of patients under the umbrella of HFrEF may also play a significant role in these mixed outcomes. In the two studies that did not show an improvement in aerobic performance with dietary nitrate (49, 51), most of the patients had ischemic cardiomyopathy. Conversely, the two studies reporting beneficial effects of dietary nitrate only included patients with nonischemic cardiomyopathy (47, 50).
A recent pilot study (59) found that chronic (∼13 days) nitrate supplementation may enhance cardiac output (assessed noninvasively via Doppler ultrasound) during submaximal exercise on a recumbent cycle ergometer in patients with HFrEF. However, that nitrate supplementation with beetroot juice (9 days) did not improve cardiac output (evaluated noninvasively via impedance cardiography) during upright cycling exercise in patients with HFrEF (49) further highlights the impact of exercise mode on these cardiovascular outcomes.
To our knowledge, only a single study by Coggan et al. (48) has reported that nitrate supplementation confers muscle strength benefits (i.e., increased peak torque and power during knee extension) in patients with HFrEF. Although these improvements in muscle contractile function were not translated into greater aerobic exercise performance (as assessed via the 6-min walk test), it is noteworthy that nitrate supplementation may retain important clinical implications for HFrEF. Diminished muscle power with HFrEF can result in functional limitations highly predictive of disability and dependence (i.e., reduced ability to perform activities of daily living) and mortality in this patient population (60, 61). Future studies are thus needed to determine whether nitrate-induced increases in muscle power translate into improved habitual physical activity, quality of life, and/or survival in HFrEF.
Based on the evidence summarized above and similar to the HFpEF condition, factors that likely influence the clinical effectiveness of nitrate supplementation in HFrEF include disease etiology and severity, associated comorbidities, prescribed medications, and nitrate/nitrite supplementation regimens. It is also feasible that unique aspects of HFrEF pathophysiology could explain, at least in part, the inconsistent effects of inorganic nitrate supplementation on exercise tolerance. Examination of plasma nitrite concentrations following oral inorganic nitrate consumption suggests a potential disruption within the nitrate-nitrite reduction pathway compared with HFpEF populations and healthy controls (51). Such derangements could drive the apparently distinct effects of dietary inorganic nitrate on resting systolic blood pressure in HFrEF versus HFpEF (Fig. 2). Moreover, central dysfunction in HFrEF could abrogate potential improvements in peripheral (skeletal muscle) Q̇o2/V̇o2 matching with inorganic nitrate supplementation. Additional work in these areas may provide important clues for therapeutic exploitation in the future. It is also worth noting that sex differences in cardiovascular (dys)regulation may contribute to the disparity in findings between HFrEF and HFpEF, as most investigations in HFrEF included predominantly male participants (with the opposite being true for HFpEF studies; vide Table 1). The studies investigating nitrate supplementation in HFrEF are in their infancy, with relatively small sample sizes and short dosing periods. The initial findings suggest that nitrate supplementation in the HFrEF population may not consistently improve exercise tolerance when used as an acute intervention (i.e., a single dose or up to 9 days). Future studies are needed to 1) uncover the mechanistic limitations of this intervention to maximize potential beneficial outcomes and 2) explore the combined effect of inorganic nitrate with exercise training to unveil potential additive or synergistic effects.
KEY CONSIDERATIONS AND FUTURE DIRECTIONS
Although a host of early investigations in healthy humans and animals suggested that a nitrate-based therapy may benefit patients with HF, the successful translation of these findings into the clinical population has been challenging. The specific reasons for these mixed findings are currently unclear but likely include differences in study methods and HF pathophysiology (i.e., HFpEF vs. HFrEF), as mentioned earlier. What is clear is that the underpinnings of nitrate therapy in HF remain to be fully elucidated and that the confounding effects of differing HF severity, pharmaceutical treatment strategies, and other clinical/environmental factors likely preclude consistent improvements in exercise tolerance following nitrate supplementation. Development of direct in vivo NO evaluation methods could provide valuable insights particularly in the context of elevated oxidative stress as seen in HF (57, 62). The central role of symbiotic bacteria in the bioactivation of nitrate must also not be overlooked, as alterations in bacterial loads and/or species could modulate physiological effects and, therefore, exercise tolerance (42). The acute blood pressure-lowering effects of orally ingested nitrite have been shown to be dependent on the acidic gastric environment in healthy individuals (63). Moreover, it is intriguing that infused nitrite has failed to reduce resting blood pressure in both healthy and HFpEF populations despite reaching similar plasma levels as when administered orally (53, 63). The variety of HF animal models and differences in rodent versus human physiology (e.g., higher distribution of type II muscles in rodents) further challenges our ability to directly translate inorganic nitrate and nitrite interventions into the clinical HF population.
Some of the critical questions that remain to be answered include 1) whether HF alters the oral microbiome and/or gastrointestinal environment, thereby reducing the performance-enhancing properties of nitrate treatment, and, if so, 2) whether the buccal flora and/or gastric milieu of HF patients can be modulated to use the nitrate-nitrite-NO pathway better and improve exercise tolerance. Considering the fiber-type specific effects of nitrate supplementation (35), investigations into fiber-type characteristics of patients with HF included in clinical trials may shed light on potential mechanisms and therapeutic applications of inorganic nitrate in HFpEF and HFrEF.
GRANTS
This work was supported in part by a PRF Summer Faculty Grant from Purdue University (to D.M.H.) and pilot funding (to S.K.F) as part of the IDeA Networks of Biomedical Research Excellence (INBRE), award P20GM103466.
DISCLOSURES
E.W. is a co-applicant on patents related to the therapeutic use of inorganic nitrate and nitrite.
AUTHOR CONTRIBUTIONS
S.K.F., M.N.W., M.J.H., M.D.B., M.C., E.W., J.D.A., and D.M.H. prepared figures; S.K.F., M.N.W., M.J.H., M.D.B., M.C., E.W., J.D.A., and D.M.H. drafted manuscript; S.K.F., M.N.W., M.J.H., M.D.B., M.C., E.W., J.D.A., and D.M.H. edited and revised manuscript; S.K.F., M.N.W., M.J.H., M.D.B., M.C., E.W., J.D.A., and D.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joseph Yao and Christopher Kinnick for graphical assistance.
REFERENCES
- 1.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 2.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40: 295–302, 2006. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell 106: 675–683, 2001. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature 368: 502, 1994. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut 35: 1543–1546, 1994. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr 33: 129–159, 2013. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 7.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol 14: 545–548, 1976. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 8.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1: 546–551, 1995. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 9.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis 21: 7–16, 2015. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 10.Montenegro MF, Sundqvist ML, Nihlén C, Hezel M, Carlström M, Weitzberg E, Lundberg JO. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: implications for translational research. Redox Biol 10: 206–210, 2016. doi: 10.1016/j.redox.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93–100, 2013. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med 36: 1177–1185, 2015. doi: 10.1055/s-0035-1554700. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg JO, Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut 62: 616–629, 2013. doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 14.Powlson DS, Addiscott TM, Benjamin N, Cassman KG, de Kok TM, van Grinsven H, L’Hirondel J-L, Avery AA, van Kessel C. When does nitrate become a risk for humans? J Environ Qual 37: 291–295, 2008. doi: 10.2134/jeq2007.0177. [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Zamani P. The nitrate-nitrite-NO pathway and its implications for heart failure and preserved ejection fraction. Curr Heart Fail Rep 13: 47–59, 2016. doi: 10.1007/s11897-016-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Food Safety Authority. Nitrate in vegetables - scientific opinion of the panel on contaminants in the food chain. Food Saf. Auth 2008. Eur (Online) https://www.efsa.europa.eu/en/efsajournal/pub/689 [19 Dec. 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg JO, Carlström M, Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab 28: 9–22, 2018. doi: 10.1016/j.cmet.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Carlstrom M, Montenegro MF. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J Intern Med 285: 2–18, 2019. doi: 10.1111/joim.12818. [DOI] [PubMed] [Google Scholar]
- 21.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83: 2038–2047, 1991[Erratum inCirculation84: 2212, 1991]doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 22.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 6: 1368–1370, 1993. [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147–239, 2013. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13: 290–314, 2014. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM, O’Connor CM, VICTORIA Study Group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 382: 1883–1893, 2020. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 26.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309: H1419–H1439, 2015. doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124: 208–224, 2018. doi: 10.1152/japplphysiol.00747.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah SJ. Precision medicine for heart failure with preserved ejection fraction: an overview. J Cardiovasc Transl Res 10: 233–244, 2017. doi: 10.1007/s12265-017-9756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 139: 1435–1450, 2019. doi: 10.1161/CIRCULATIONAHA.118.036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JF, Barrett-O'Keefe Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, Richardson RS, Wray DW. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 211: 14–21, 2016. doi: 10.1016/j.ijcard.2016.02.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias P-T, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131: 371–380, 2015. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woessner MN, McIlvenna LC, Ortiz de Zevallos J, Neil CJ, Allen JD. Dietary nitrate supplementation in cardiovascular health: an ergogenic aid or exercise therapeutic? Am J Physiol Heart Circ Physiol 314: H195–H212, 2018. doi: 10.1152/ajpheart.00414.2017. [DOI] [PubMed] [Google Scholar]
- 33.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC. Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev 44: 53–60, 2016. doi: 10.1249/JES.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC. Microvascular oxygen pressures in muscles comprised of different fiber types: Impact of dietary nitrate supplementation. Nitric Oxide Biol Chem 48: 38–43, 2015. doi: 10.1016/j.niox.2014.09.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res 61: 325–332, 2004. doi: 10.1016/j.cardiores.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 40.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 41.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WHW, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 373: 2314–2324, 2015. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 4: 428–437, 2016. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaltout HA, Eggebeen J, Marsh AP, Brubaker PH, Laurienti PJ, Burdette JH, Basu S, Morgan A, Dos Santos PC, Norris JL, Morgan TM, Miller GD, Rejeski WJ, Hawfield AT, Diz DI, Becton JT, Kim-Shapiro DB, Kitzman DW. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide Biol Chem 69: 78–90, 2017. doi: 10.1016/j.niox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM, National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network. Effect of inorganic nitrite vs placebo on exercise capacity among patients With heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 320: 1764–1773, 2018. doi: 10.1001/jama.2018.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamani P, Tan V, Soto-Calderon H, Beraun M, Brandimarto JA, Trieu L, Varakantam S, Doulias P-T, Townsend RR, Chittams J, Margulies KB, Cappola TP, Poole DC, Ischiropoulos H, Chirinos JA. Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Circ Res 120: 1151–1161, 2017. doi: 10.1161/CIRCRESAHA.116.309832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerley CP, O'Neill JO, Reddy Bijjam V, Blaine C, James PE, Cormican L. Dietary nitrate increases exercise tolerance in patients with non-ischemic, dilated cardiomyopathy-a double-blind, randomized, placebo-controlled, crossover trial. J Heart Lung Transplant 35: 922–926, 2016. doi: 10.1016/j.healun.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled. Circ Heart Fail 8: 914–920, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirai DM, Zelt JT, Jones JH, Castanhas LG, Bentley RF, Earle W, Staples P, Tschakovsky ME, McCans J, O’Donnell DE, Neder JA. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am J Physiol Regul Integr Comp Physiol 312: R13–R22, 2017. doi: 10.1152/ajpregu.00263.2016. [DOI] [PubMed] [Google Scholar]
- 50.Coggan AR, Broadstreet SR, Mahmood K, Mikhalkova D, Madigan M, Bole I, Park S, Leibowitz JL, Kadkhodayan A, Thomas DP, Thies D, Peterson LR. Dietary nitrate increases V̇o2peak and performance but does not alter ventilation or efficiency in patients with heart failure with reduced ejection fraction. J Card Fail 24: 65–73, 2018. doi: 10.1016/j.cardfail.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woessner MN, Neil C, Saner NJ, Goodman CA, McIlvenna LC, Ortiz de Zevallos J, Garnham A, Levinger I, Allen JD. Effect of inorganic nitrate on exercise capacity, mitochondria respiration, and vascular function in heart failure with reduced ejection fraction. J Appl Physiol 128: 1355–1364, 2020. doi: 10.1152/japplphysiol.00850.2019. [DOI] [PubMed] [Google Scholar]
- 52.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010[Erratum inHypertension56: e37-9, 2010]. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 53.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 66: 1672–1682, 2015. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 54.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure With preserved ejection fraction. Circ Res 119: 880–886, 2016. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 56: 588–599, 2003. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gui Y, Chen J, Hu J, Ouyang M, Deng L, Liu L, Sun K, Tang Y, Xiang Q, Xu J, Zhu L, Peng Z, Zou P, Li B, Zheng Z, Xu D. Efficacy and safety of inorganic nitrate versus placebo treatment in heart failure with preserved ejection fraction. Cardiovasc Drugs Ther 34: 503–513, 2020. doi: 10.1007/s10557-020-06980-4. [DOI] [PubMed] [Google Scholar]
- 57.Pfeiffer S, Gorren AC, Schmidt K, Werner ER, Hansert B, Bohle DS, Mayer B. Metabolic fate of peroxynitrite in aqueous solution. Reaction with nitric oxide and pH-dependent decomposition to nitrite and oxygen in a 2:1 stoichiometry. J Biol Chem 272: 3465–3470, 1997. doi: 10.1074/jbc.272.6.3465. [DOI] [PubMed] [Google Scholar]
- 58.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 59: 442–451, 2012. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 59.Woessner MN, Levinger I, Allen JD, McIlvenna LC, Neil C. The effect of dietary inorganic nitrate supplementation on cardiac function during submaximal exercise in men with heart failure with reduced ejection fraction (HFrEF): a pilot study. Nutrients 12: 2132, 2020. doi: 10.3390/nu12072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coggan AR, Hoffman RL, Gray DA, Moorthi RN, Thomas DP, Leibowitz JL, Thies D, Peterson LR. A single dose of dietary nitrate increases maximal knee extensor angular velocity and power in healthy older men and women. J Gerontol A Biol Sci Med Sci 75: 1154–1160, 2020. doi: 10.1093/gerona/glz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hülsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, Mörtl D, Moser P, Pacher R. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail 6: 101–107, 2004. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montenegro MF, Sundqvist ML, Larsen FJ, Zhuge Z, Carlström M, Weitzberg E, Lundberg JO. Blood pressure-lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension 69: 23–31, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]