Keywords: acute kidney injury, acute renal failure, core, kidney disease, O'Brien Center
Abstract
Acute kidney injury (AKI) remains a significant clinical problem through its diverse etiologies, the challenges of robust measurements of injury and recovery, and its progression to chronic kidney disease (CKD). Bridging the gap in our knowledge of this disorder requires bringing together not only the technical resources for research but also the investigators currently endeavoring to expand our knowledge and those who might bring novel ideas and expertise to this important challenge. The University of Alabama at Birmingham-University of California-San Diego O’Brien Center for Acute Kidney Injury Research brings together technical expertise and programmatic and educational efforts to advance our knowledge in these diverse issues and the required infrastructure to develop areas of novel exploration. Since its inception in 2008, this O’Brien Center has grown its impact by providing state-of-the-art resources in clinical and preclinical modeling of AKI, a bioanalytical core that facilitates measurement of critical biomarkers, including serum creatinine via LC-MS/MS among others, and a biostatistical resource that assists from design to analysis. Through these core resources and with additional educational efforts, our center has grown its investigator base to include >200 members from 51 institutions. Importantly, this center has translated its pilot and catalyst funding program with a $37 return per dollar invested. Over 500 publications have resulted from the support provided with a relative citation ratio of 2.18 ± 0.12 (iCite). Through its efforts, this disease-centric O’Brien Center is providing the infrastructure and focus to help the development of the next generation of researchers in the basic and clinical science of AKI. This center creates the promise of the application at the bedside of the advances in AKI made by current and future investigators.
INTRODUCTION
Established in 2008, the University of Alabama at Birmingham (UAB)-University of California-San Diego (UCSD) O’Brien Center for Acute Kidney Injury (AKI) Research (UAB-UCSD O’Brien Center) is an interdisciplinary center of excellence in AKI-related research. It is one of eight National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded O’Brien Centers in the nation and the only O’Brien Center that connects two distinct universities under a common and integrated framework with a singular, disease-centric research focus (1). Preexisting strengths in research at each institution have been brought together to reciprocally enhance each other but also provide this breadth and depth of expertise to the larger research community in the United States and beyond. The natural collaborative spirit at each institution has enhanced the ability of the UAB-UCSD O’Brien Center to sustain and integrate seamlessly as a unit, leveraging institutional commitments at both academic medical centers to further propel the development of a preeminent center of excellence for AKI-related research. Our mission is as follows:
“To provide shared core facilities to enhance collaborations and further investigations in kidney-related research, foster interactions among investigators from different backgrounds and disciplines, and provide intellectual resources and infrastructure to attract new and established investigators to AKI research.”
HISTORICAL BACKGROUND
The UAB-UCSD O’Brien Center began from an idea to combine the individual strengths of UCSD in clinical research and UAB in basic science research in AKI, thereby enhancing the research capabilities of both institutions. As this collaboration grew and the UAB-UCSD O’Brien Center became a reality, our vision matured to not only include enhancement of the research depth of our respective institutions but especially to foster national and international research in AKI beyond these institutions. By supporting new young investigators and facilitating incorporation of established investigators with new research efforts in AKI, the impact of the center was amplified. The UAB-UCSD O’Brien Center uses biomedical research cores to facilitate conduct of unique experimental techniques and to train investigators in their use. In addition, a resource for biostatistical analysis provides early and continuing statistical support to investigators doing AKI research. As an added benefit, these resources have assisted research efforts beyond AKI to include studies in chronic kidney disease (CKD), a known long-term consequence of AKI. Collaborative efforts with institutional centers at UAB, funded by the National Institutes of Health (NIH)/NIDDK, in hereditary and genetic causes of kidney disease, such as polycystic kidney disease, have also broadened the reach of the UAB-UCSD O’Brien Center. As the center has matured, a continued focused effort to facilitate AKI-related research and efforts to allow for translational studies to be accomplished by the investigator base has been directed in the key thematic areas of 1) AKI in the intensive care unit and transplant setting, 2) renal vascular dysfunction and hemodynamic alterations, 3) oxidative stress and metabolism, and 4) genetic susceptibility. The aims illustrate the integrated approach to enhancing AKI research (Table 1).
Table 1.
Mission and aims of the UAB-UCSD O’Brien Center
| Mission: We support shared core facilities to enhance collaborations and further investigations in kidney-related research, foster interactions among investigators from different backgrounds and disciplines, and provide intellectual resources and infrastructure to attract new and established investigators to AKI research. |
|---|
Aims
|
AKI, acute kidney injury; UAB, University of Alabama at Birmingham; UCSD, University of California-San Diego.
CENTER STRUCTURE
This center uniquely has a disease-oriented approach and provides both preclinical and clinical core resources for investigators pursuing AKI-related research. However, although the program resides under one AKI “umbrella,” there is research latitude to assist investigators with other kidney-related research activities. This is in recognition that AKI is on a continuum between normal kidney function, progressive CKD, and kidney failure and transplantation. A strength of the UAB-UCSD O’Brien Center is the translational nature of the contribution across the lifecycle of an investigation by the three major cores, providing expertise in design and implementation of technical approaches for the NIH nomenclature of T0–T4 research (Fig. 1). For T4 research, we leverage resources within the Centers for Clinical and Translational Science (CTSA) at our respective institutions. In addition, the incorporation of training opportunities for the entire research investigator base, including graduate and medical students, research fellows, and faculty through the Enrichment Program (discussed below), provide opportunities to accomplish research on AKI at any level.
Figure 1.
Diagram of the integration of resources across the translational science spectrum. The core resources of the University of Alabama at Birmingham (UAB)-University of California-San Diego (UCSD) O’Brien Center for Acute Kidney Injury Research provide infrastructure across the spectrum of research from basic science investigations (T0) to translation to patient care (T4). These resources include a variety of approaches to facilitate research in each realm and leverage additional available resources at each academic medical center, including those of the Centers for Clinical and Translational Science (CTSAs) of each institution. AKI, acute kidney injury. [Modified from Zarjou et al. (81).]
The administrative core of the UAB-UCSD O’Brien Center is guided by Dr. Anupam Agarwal at UAB and codirected by Dr. Ravindra Mehta at UCSD and Dr. Paul W. Sanders at UAB. The administrative core provides overarching resources to the center, maintains a robust Enrichment Program, Pilot and Feasibility Grants Program, and Biostatistical Resource, which provides statistical support to users of the facilities. The administrative core coordinates the diverse activities of the center, facilitates interactions and collaborations among the researchers, maintains communications through our website, video conferences, and newsletters, and promotes scientific development through pilot grant funding and education initiatives. It also links cross-cutting activities of the three Biomedical Cores: the Clinical Studies of AKI (Core A), the Preclinical Studies of AKI Core (Core B), and the Bioanalytical Core (Core C). Investigators can access the services of the cores through our website by completing a core usage request (https://www.uab.edu/medicine/obriencenter/core-usage-request-form) with a response from the core personnel within 1−2 days.
The Clinical Studies of AKI Core (Core A) is directed by Dr. Ravindra Mehta at UCSD and codirected by Dr. Michael Seifert at UAB. This core offers support in the design and conduct of clinical research in AKI, access to comprehensive data sets of well-characterized patients with and without AKI from multiple sources for epidemiological studies evaluating risk factors and consequences of AKI and outcomes research (Table 2). A biorepository that includes human kidney biopsy tissue, blood, DNA, and urine is linked to an accessible deidentified clinical database of patients with and without AKI and enables translational research studies (Table 3). The kidney biopsy repository includes 196 biopsies from patients with a top-line diagnosis of AKI. The repository also has >15,000 archived kidney biopsies from various kidney diseases and is managed by renal pathologist Dr. Frida Rosenblum at UAB. In response to the coronavirus disease 2019 (COVID-19) pandemic, this core is also operating a global registry to understand the natural history of COVID-19-related AKI, identify risk factors that could lead to modification of therapy in high-risk individuals, and understand the course and outcomes of patients with kidney dysfunction from COVID-19 (Clinical Trials Registration No. NCT04491227). At UAB, in collaboration with our CTSA, we have an ongoing collection of urine samples (>450 samples as of November 15, 2020 ) from hospitalized patients with COVID-19 with and without AKI.
Table 2.
Comprehensive databases accessible for AKI research (Core A)
| Database | Data Source | Data Content |
|---|---|---|
| International ICU AKI Registry | 33 centers worldwide | Comorbidities, risk factors for AKI, clinical and laboratory data, fluid balance status, severity of illness scores, dialysis requirements and outcomes through hospital discharge. Currently, 17,444 patients have been screened and 3,179 patients are enrolled. |
| Drug-Induced Renal Injury Consortium | 42 centers worldwide | 634 patients (adult, n = 493; pediatric, n = 141) with drug-induced kidney disease. Blood, urine, and DNA samples have been collected as well as the clinical course of injury and outcomes up to 90 days post event. |
| Global Snapshot of AKI | International Society of Nephrology 0by25 project | Phenotypic information on 4,018 patients (adult, n = 3,664; pediatric, n = 354) enrolled from 289 centers in 92 countries. Presenting symptoms, risk factors, course, and outcome over 7 days of patients with AKI. |
| UAB and UCSD Clinical Data Warehouse from EHR | UCSD Epic with Clarity and UAB Cerner Datasets | Clinical laboratory, medication, and administrative data for outpatient and inpatient encounters at UAB and UCSD. |
| UC Accrual to Clinical Trials | Administrative and EHR data from 5 UC medical centers | Detailed data on demographics, diagnosis, procedures, laboratory, medication, and vital signs. |
| COVID-19 Kidney Disease Global Study | Data from 74 centers across 25 countries | Clinical data on course and outcomes of patients with COVID-19 with AKI, chronic kidney disease, end-stage kidney disease, and transplant (>3,000 patients as of March 1, 2021). |
AKI, acute kidney injury; ICU, intensive care unit; UAB, University Of Alabama at Birmingham; UCSD, University of California-San Diego; EHR, electronic health records; UC, University of California.
Table 3.
Samples available in the Core A Biorepository
| Patient Group | Sample Size | Blood | DNA | Urine |
|---|---|---|---|---|
| AKI Biorepository | ||||
| ICU AKI registry (UAB/UCSD sites) | 87 | 211 | 127 | 1,275 |
| Drug-Induced Renal Injury Consortium Study | 631 | 733 | 276 | 973 |
| Post-AKI followup | 93 | 273 | 1,180 | |
| Normal healthy subjects | 126 | 298 | 204 | 871 |
| Kidney Tissue Biorepository | ||||
| Donor kidneys | 68 | 34 | 34 | |
| Normal kidney tissues from nephrectomies | 25 | |||
| Native and transplant kidney biopsies* | 196† |
AKI, acute kidney injury; ICU, intensive care unit; UAB, University of Alabama at Birmingham; UCSD, University of California-San Diego. *Ongoing and includes >15,000 biopsies; †biopsies with a diagnosis of AKI.
As part of the educational mission, the Clinical Studies of AKI Core sponsors the Workshop on Healthcare Data Analytics. This new workshop began in 2020 and provides a hands-on learning opportunity to 10 people/year to grow and enhance skills in large data set analysis. This program has been well received by the kidney community.
The Preclinical Studies of AKI Core (Core B) is directed by Dr. Paul W. Sanders at UAB, with codirectorship by Dr. James George at UAB and Dr. Volker Vallon at UCSD. This core provides three subcores that focus on individual robust experimental modalities for animal studies of AKI. The Small Animal Microsurgical Core Facility, directed by Dr. James George, provides surgical expertise in a variety of surgical models of AKI in rodents (Table 4). Assistance in the development of additional, novel surgical models applicable for AKI research are available on an as-requested basis and leverage the significant precision of dedicated microsurgeons. Assistance with nonsurgical models is also provided to individual investigators, as is access to state-of-the-art surgical facilities. The Small Animal Imaging Facility, codirected by Dr. Anna Sorace and Dr. Jason Warram at UAB, provides state-of-the-art molecular imaging of functional, structural, and metabolic parameters, including molecular ultrasound, microcomputed tomography (CT), single-photon emission CT/CT, magnetic resonance imaging, positron emission tomography/CT, γ-camera, and bioluminescence and fluorescence imaging in rodents. The Renal Physiology subcore, directed by Dr. Volker Vallon at UCSD and supported by Dr. Prabhleen Singh and Dr. Scott Thomson, provides expertise and training for studying function of the whole kidney, including renal oxygenation evaluation in preclinical models and at the single nephron level, and renal micropuncture. In addition, technical expertise in preclinical models of AKI and CKD as well as the isolation of primary renal and vascular cells in culture is available through this core. The innovation of Core B centers on provision of requisite skills and knowledge in the field and flexibility in the preclinical procedures that are made available to hone and test an investigator’s hypothesis.
Table 4.
Surgical procedures in mice and rats (Core B)
| Surgical Models for the Study of Kidney Disease |
|---|
|
|
|
|
|
| Other microsurgical techniques |
|
|
The Preclinical Studies of AKI Core provides hands-on education through workshops annually. The Rodent Kidney Physiology/Injury Workshop, now in its 11th year, is held at UCSD and offers a comprehensive demonstration and hands-on engagement in a variety of animal handling and models as well as phenotype techniques to evaluate renal function. The Flow Cytometry Workshop has been held at UAB since 2017 as a 3-day hands-on workshop covering the basics of use of antibodies, preparation of kidney samples, mechanics, and scientific theory of FACS analysis as well as the analysis of data. These workshops bring together small groups each year (6–9 individuals/course) so that focused attention is provided to enhance skill development in the investigator base.
The Bioanalytical Core (Core C) is directed by Dr. Stephen Barnes at UAB and codirected by Dr. Victor Darley-Usmar at UAB and Dr. Sucheta Vaingankar at UCSD. This core provides state-of-the-art bioenergetics, oxidative stress analysis, metabolite analysis, and biomarker assays for AKI research as well as consultation, training, and experimental design support. The core also offers assays for posttranslational modification of proteins and quantitative analysis of tricarboxylic acid cycle and bioenergetic metabolic intermediates by liquid chromatography with tandem mass spectrometry (LC-MS/MS). The techniques available through this core include LC-MS/MS-based assays for serum and urine creatinine as well as the quantitation of a variety of other biomarkers of renal injury and repair (Table 5). Nano LC-MS for targeted and untargeted metabolomics and associated statistical and network analyses for clinical and animal model samples are available through this Core. This technical expertise builds on a funding investment provided by the NIH for a week-long Workshop on Metabolomics, held annually from 2013 to 2018. Determination of mitochondrial bioenergetics using the Seahorse platform provides an important technical resource for individual users as well as training in the use of this instrument. Innovations in the use of novel starting material for these measurements allow new opportunities to explore these measurements in archival material (2). As part of its educational mission, this core has offered a Mitochondrial Bioenergetics Workshop that provided real-world examples of the use of this technology as well as sessions dedicated to instruction on the use of the Seahorse instrument. The Biostatistical Resource is directed by Dr. Gary R. Cutter and codirected by Dr. Orlando Gutierrez, both at UAB. It provides statistical support to the research projects, pilot projects, and other Cores in the UAB-UCSD O’Brien Center with expertise for the planning, design, data management, and analysis of their research projects and specific expertise in clinical trials and epidemiological studies. This resource is available to the entire research investigator base, but it is particularly helpful to new investigators who are initiating projects. Pilot project applicants are particularly encouraged to discuss their projects with the biostatistical resource before submission for competitive funding. The support of this core from development to completion of research ensures a robust approach and analysis to all these studies of AKI.
Table 5.
Selected biomarker assays (Core C)
| Biomarker |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bio-ADM, bioactive adrenomedullin; BSAP, B cell-specific activator protein; BUN, blood urea nitrogen; CRP, C-reactive protein; FGF, fibroblast growth factor; GST, glutathione S-transferase; IGFBP, insulin-like growth factor-binding protein; IL, interleukin; KIM-1, kidney injury molecule-1; L-FABP, L-type fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; penKid, proenkephalin A; TGF-β1, transforming growth factor-β1; TIMP-2, tissue inhibitor of metalloproteinase-2; TNF-α, tumor necrosis factor-α; TRAP-5, tartrate-resistant acid phosphatase-5.
The Pilot and Feasibility Grants Program is overseen by Dr. Paul W. Sanders at UAB and is offered across the investigator base to elicit novel research in AKI by new investigators around the nation. This program provides the intellectual resources and the research infrastructure to attract new and established investigators to AKI research. A Request For Applications is announced annually on our website and to members of the investigator base. Three to four pilot projects are provided seed funding to develop novel projects in AKI research (Table 6). All pilot awards are considered mentored projects, and even the senior investigators who are transitioning into AKI research are assigned an appropriate advisor who has interests in AKI. Pilot award recipients present their findings at quarterly video conferences. As part of this program, access to the biostatistical resource ensures well-designed and robust studies. In addition to this funding, a catalyst program provides vouchers to highly meritorious applications that were not selected for full funding. This voucher program, which has supported 50 unique investigators to date, provides seed funding specifically for the use of core facilities, with the overall goal of catalyzing and encouraging continued advancement of these projects to facilitate garnering additional external funding in the future. Since its inception, the Pilot and Feasibility Grants program has supported 27 investigators (14 physician scientists and 13 PhD scientists) that include promising young investigators. Of these, 21 investigators (78%) are also new to AKI research and 83% have remained in kidney-related research. These pilot awards are given to investigators at UAB and UCSD as well as those in the extended research base. Since 2015, ∼1/3 of investigators have been from outside UAB/UCSD, with the remaining approximately split across these primary institutions. For every dollar invested, the return on investment of these funds has been estimated at $37.
Table 6.
Pilot and feasibility grant proposal submissions (2007−present)*
| Type | Junior Investigator | Established Investigator | Total |
|---|---|---|---|
| Clinical research | 96 | 25 | 121 |
| Preclinical research | 103 | 27 | 130 |
| Total | 198 | 52 | 251 |
Although the O’Brien Center was funded by the National Institutes of Health in 2008, two pilots were funded in 2007 using institutional funds.
The Enrichment Program, directed by Dr. Lisa M. Curtis at UAB with oversight by Dr. Joachim H. Ix at UCSD, includes cross-cutting initiatives to enhance collaborations, educate the research investigator base regarding unique technical approaches, and provide training for investigators throughout the workforce pipeline (Fig. 2). In addition to independent initiatives by each core (see Table 7), the Enrichment Program leverages offerings at each institution to provide cohesive value to the investigator base and integrates the center through cross-center offerings. Weekly Research Series/Lectures at both UCSD and UAB are used to incorporate discussions of AKI research and clinical care. These include traditional renal grand rounds as well as stand-alone seminar series that routinely serve 50−85 participants at each seminar and receive high satisfaction scores. In addition, journal clubs focused on kidney physiology and pathophysiology are conducted to enhance the training of students. The Core Concepts in Kidney Research, initiated and developed by Dr. Lisa M. Curtis at UAB, is an 8-wk seminar series that is conducted in late spring and cross-links clinical care and research initiatives in a broad discussion, often using tandem presentations by clinical and research faculty. Routinely, this latter offering reaches 80−90 participants at each session and routinely receives very positive evaluations (>90% positive measures). Each of these entities uses evaluations to rate the success of the programs and to improve and revise them. The recently started National O’Brien Centers Kidney Seminars (NOCKS) provide an additional opportunity for dissemination of research findings and core capabilities across each of the O’Brien Centers around the United States. These seminars are advertised to our investigator base to encourage a greater understanding of resources available to them.
Figure 2.

Enrichment program integration between the University of Alabama at Birmingham (UAB) and University of California-San Diego (UCSD). Training opportunities are provided across the range of levels from undergraduate students to postgraduate trainees to junior faculty at both UCSD and UAB as indicated by the coloring. These offerings are available to all research base investigators to foster development of renal scientists and enhance the retention of these individuals in the workforce pipeline, thereby increasing the numbers of individuals performing cutting-edge research in acute kidney injury (AKI). KURE, Kidney Undergraduate Research Experience; PROmoTE, Predoctoral PhD and MD Research Training in Teams; PRIME, Predoctoral Interdisciplinary Training in Renal Physiology and Medicine.
Table 7.
Core workshops and symposia
| Workshop/Symposium | Description | Details |
|---|---|---|
| Centerwide | ||
| Annual Renal Symposium | Held in collaboration with the UAB Childhood Cystic Kidney Disease Core Center at UAB, this symposium presents basic and translational research on AKI and polycystic kidney disease. This symposium also includes the Dr. James Schaffer Lecture which honors his contribution to enhancing understanding of renal physiology. | Held on World Kidney Day. The 2020 and 2021 symposia were conducted virtually. |
| Annual AKI Symposium | Held in conjunction with the annual International CRRT meeting in San Diego, CA, this symposium covers several topics in basic science, translational, and clinical aspects of AKI. Video recordings of presentations are freely available on our website. Proceedings are published each year in Nephron. | Usually held in February with registration in concert with the International CRRT meeting. The 2021 symposium was held virtually. |
| Core A | ||
| Workshop on Healthcare Data Analytics | This hands-on workshop helps investigators gain new knowledge and experience in conducting research using the increasingly available public and private biomedical data resources using robust methodologies. | Registration is limited to 10 participants. The workshop is usually held in January. |
| Core B | ||
| Rodent Kidney Physiology/Injury Workshop | Held at UCSD, this program includes faculty from UCSD and UAB and is designed to provide hands-on practice in animal handling and phenotyping techniques for commonly used methods to study kidney function in rodents. | Limited to 9 participants for the maximum interaction and time for training. This workshop is usually held in the spring. |
| Flow Cytometry Workshop | Held at UAB, this program provides hands-on instruction on the use of flow cytometry to analyze cells from tissues and cell culture and includes instruction on the preparation of samples, selection of fluorophores, and analysis using FlowJo software. | Registration is limited to 6 individuals to allow for hands-on training with the FACS machine. Held as needed, this workshop has been held in early summer. |
| Core C | ||
| Workshop on Metabolomics | Held in conjunction with the Targeted Metabolomics and Proteomics Laboratory led by Dr. Barnes, this workshop provides instruction and hands-on experience with metabolomic investigation. | Offered as needed |
| Mitochondrial Bioenergetics Workshop | Held in collaboration with Seahorse Bioscience and led by Dr. Daley-Usmar, this workshop provides real-world examples and instruction on the use of Seahorse technology. | Offered as needed |
AKI, acute kidney injury; ICU, intensive care unit; UAB, University of Alabama at Birmingham; UCSD, University of California-San Diego; CRRT, Continuous Renal Replacement Therapy.
To enhance education center-wide, two symposia are held annually (Table 7). The annual AKI Symposium, now in its 11th year, is held in San Diego, CA, in conjunction with the annual International Continuous Renal Replacement Therapy (CRRT) meeting and focuses on advances in AKI research and clinical care. An annual Renal Symposium is held at UAB in conjunction with the Childhood Cystic Kidney Disease Core Center at UAB (Dr. Brad Yoder, Principal Investigator), a NIDDK-funded center that has a strong focus on cystic kidney disease. This symposium draws the investigator base together with onsite or virtual interfaces and allows for the dissemination of findings obtained through the resources provided by the center. Both opportunities are widely advertised and promoted among our investigator base.
A unique offering that can be used for any of the core activities is the Sabbatical Program, which allows investigators to focus on enhancing expertise by engaging in an on-site short-term instruction. These are offered as nanosabbaticals (1−2 days) and microsabbaticals (1−2 wk) depending on the time allotted to this training. Formal NIH-funded training programs in AKI research are available at all levels of the workforce pipeline, from undergraduate, graduate, and medical students to established investigators at both primary institutions. These include summer research programs for graduate and medical students and training grant opportunities for graduate students and postdoctoral fellows. Although these programs are not housed within our O’Brien Center, they nonetheless leverage offerings at UCSD and UAB and are used to expand the reach of our O’Brien Center. A summer student supplemental program supports six to eight first-year medical students each summer, with three to four students at UCSD and UAB, respectively. In addition, a biannual 1-day retreat and 1-half-day research retreat are held between our O’Brien Center and the Vanderbilt O’Brien Center allowing trainees and junior investigators to present their work.
ACCOMPLISHMENTS
The accomplishments of the UAB-UCSD O’Brien Center include 1) serving as a vital catalyst in AKI research nationally, 2) disseminating research accomplishments of the investigator base, and 3) sharing data and resources to advance the understanding and management of AKI.
Serve as a Vital Catalyst in AKI Research Nationally
The UAB-UCSD O’Brien Center has galvanized UAB and UCSD investigators around the study of AKI, raising the prominence of kidney-related research at our institutions, garnering the support and enthusiasm of the universities’ leadership, fostering new collaborative interactions, and developing junior investigators via the pilot grants program (Table 8). The center has also catalyzed recruitment efforts and promoted research progress through its Biomedical Core Resources. These efforts have attracted new investigators to AKI research. The use of the UAB-UCSD O’Brien Center cores has grown extensively (Fig. 3) and, in turn, has facilitated new extramural funding. Over the 12+ years since the inception of the UAB-UCSD O’Brien Center, the leadership structure has been stable providing continuity and consistency while continually being responsive to the evolving needs of the investigator base. Importantly, the investigator base in 2020 has grown to include a total of more than 200 investigators, including an extended investigator base that provides support to nearly 100 investigators around the United States.
Table 8.
Pilot and Feasibility Program accomplishments (2007−present)*
| 2007 − 2012 | 2013 − 2017 | 2018−Present | |
|---|---|---|---|
| Pilot and feasibility investigators | 12† (16 awards) |
9† (12 awards) |
7† (11 awards) |
| Young investigators, % | 75 | 83.3 | 85.7 |
| Investigators remaining in kidney-related research(since 2008), % | 66.7 | 91.3 | 91.3 |
| Subsequent extramural funding success (since 2008), % | 63.2 | 66.7 | 66.7 |
| O’Brien catalyst awards | 9 (8 investigators) |
35 (31 investigators) |
21 (21 investigators) |
A list of the awardees and their project titles is provided at our website (https://www.uab.edu/medicine/obriencenter/pilots/grant-recipients). †Some recipients received a second year of support.
Figure 3.
Utilization of the University of Alabama at Birmingham-University of California-San Diego O’Brien Center for Acute Kidney Injury Research Core Facilities. Core utilization has increased since 2008, demonstrating the relevance of these resources as well as the innovative approach to meeting the needs of the research base. The bars on the left and middle represent activity over 5-yr spans, whereas the bars on the right reflect usage in 3 yr, including 2020, when the COVID-19 pandemic resulted in shutdown at our institutions during the months of March to June. Core A, Clinical Studies of AKI Core; Core B, Preclinical Studies of AKI Core; Core C, Bioanalytical Core; BR, Biostatistical Resource.
Disseminate Research Accomplishments of the Investigator Base
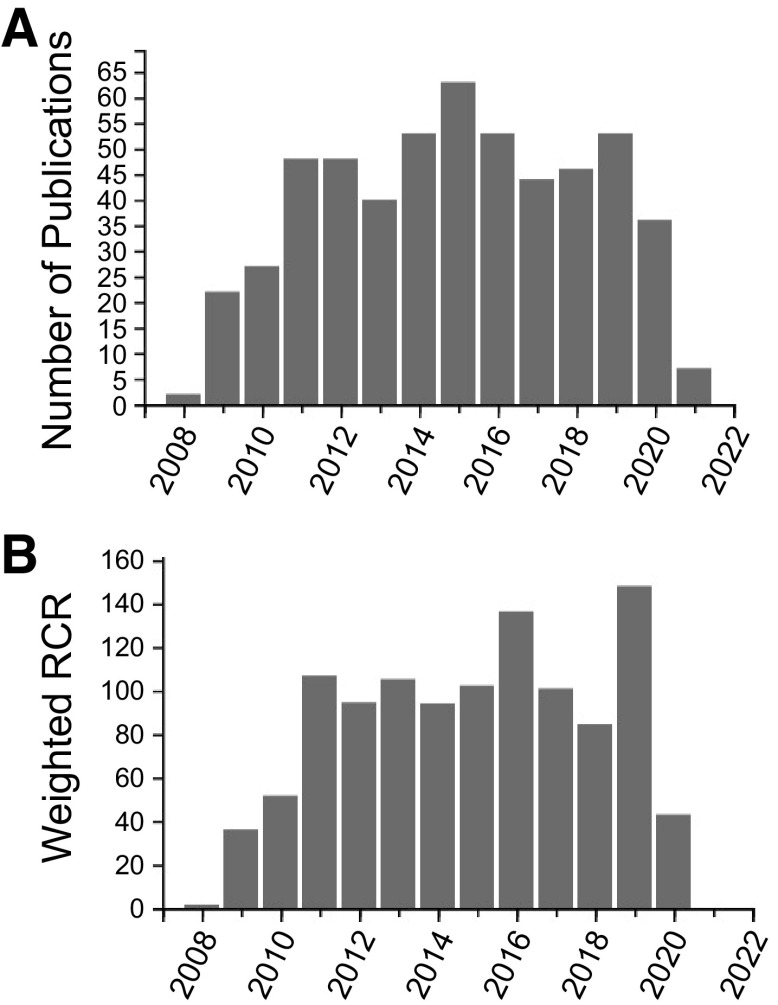
Investigators have used the core services and published several high-impact papers, including in the American Journal of Physiology-Renal Physiology among others (Nature Medicine, Journal of Clinical Investigation, Kidney International, and Journal of the American Society of Nephrology) (Table 9); a few are highlighted in the references (3–85), and a listing of all publications from our center is available on our website (https://www.uab.edu/medicine/obriencenter/publications). Research facilitated by the UAB-UCSD O’Brien Center has been presented at multiple scientific meetings, including at the annual meetings of the American Society of Nephrology and Experimental Biology. Notably, since 2008, over 500 papers have been published using the UAB-UCSD O’Brien Center Cores and/or Pilot/Enrichment support as of March 1, 2021. According to iCite (https://icite.od.nih.gov/), the average relative citation ratio (RCR) for our O’Brien Center publications is 2.18 ± 0.12, with a median of 1.29 and a maximum of 27.86 (as of March 1, 2021) for our O’Brien Center publications (Fig. 4). This RCR indicates a significant impact compared with other NIH-funded papers in the field. A paper with an RCR of 1.0 has the same number of cites/year as the average NIH-funded paper in its field, whereas a paper with an RCR of 2.0 has twice as many cites/year as the average NIH-funded paper in its field. The weighted RCR, which represents the sum of the RCRs for the articles in the group, was 1,104.33 for papers citing our O’Brien Center (as of March 1, 2021). A highly influential set of articles will have a higher weighted RCR than total publications, whereas a set of articles with below average influence will have a lower weighted RCR than total publications (https://icite.od.nih.gov/).
Table 9.
Major advances by our O’Brien Center
| AKI in the ICU/Transplant Setting | |
|---|---|
| AKI Registry | Center members (Drs. Mehta, Tolwani, Cerda, Bouchard, and others) used Core A to develop a unique resource for well-phenotyped patients with AKI in a prospective multicenter, international registry of patients with AKI in the ICU. Biological samples are being collected on all patients developing AKI daily until recovery or discharge/death at UAB and UCSD sites. |
| CRRT | Center investigator Dr. Tolwani at UAB has provided new insights into the development and standardization of citrate as an anticoagulant in CRRT. This work was awarded a United States patent (US patent no. 8147698 B2) and has been licensed to Baxter worldwide. |
| Biorepository of human kidney tissues with AKI and healthy controls | Center investigator Dr. Roslyn Mannon and Dr. Michael Seifert have obtained >60 human kidney biopsies from heart beating deceased donors before implantation in addition to blood and urine samples from these donors. >65% of these biopsies display features of AKI and represent an innovative approach for obtaining human tissue to study AKI. |
| Global Snapshot of Kidney Disease in SARS-COV2 patients | Center members (Drs. Mehta, Macedo, and others) developed a new database customizing the CDC EPI-Info app for an ongoing international multicenter cohort of patients with AKI, chronic kidney disease, transplant, and end-stage kidney disease hospitalized with COVID-19 infection. This global registry will provide investigators with a unique resource for benchmarking epidemiology and outcomes of kidney disease in COVID-19. |
| Renal vascular dysfunction and hemodynamic alterations | |
| Molecular ultrasound imaging | Center investigators Drs. Hoyt, Warram, and Agarwal developed a novel ultrasound imaging technique for monitoring early inflammatory changes in AKI using P-selectin and VCAM-1 targeted microbubbles for Core B. |
| Intravital imaging | Center investigators Drs. Yoder, George, and Mrug, with the assistance of Core B, developed an intravital imaging system using an optical window to study vascular and tubular changes after AKI in live mice. |
| Computational modeling | Center investigators Dr. Vallon and Dr. Anita Layton (Duke University) are using computational modeling techniques and animal experiments to gain new insights into novel pathways in the pathogenesis of AKI and other kidney diseases (5R01DK106102, Principal Investigator: A. Layton). |
| Renal inflammation in ventilator-induced lung injury | Center investigators Dr. Singh and Dr. Crotty-Alexander at UCSD used Core B and demonstrated that ventilator-induced lung injury alters renal expression of vascular endothelial growth factor, VCAM-1, and angiopoietin-2 in sepsis models. |
| Biomarkers | |
| Neonatal AKI | Center investigator Dr. Askenazi has demonstrated the significant negative outcomes in neonates with AKI using biomarkers analyzed by Core C. He has a National Institutes of Health multicenter planning grant to study sequelae of AKI in the neonatal ICU (U34DK117435). |
| Mass spectrometry- based imaging | Center investigator Dr. Kabarowski has optimized mass spectrometry imaging in kidney sections coupled with sequential window acquisition of all theoretical spectra-lipidomics to identify the role of lipids in AKI for Core C investigators. |
| Genetic susceptibility | |
| Drug-Induced Renal Injury Consortium | Center members (Drs. Awdishu, Mehta, Tolwani, and others) studied the genetics of drug-induced AKI and identified a rare variant, rs 117992092, on the MHC class 2 region of northern Europeans that associates with vancomycin nephrotoxicity. |
AKI, acute kidney injury; ICU, intensive care unit; UAB, University of Alabama at Birmingham; UCSD, University of California-San Diego; VCAM-1, vascular cell adhesion molecule-1; MHC, major histocompatibility complex.
Figure 4.
Publication metrics for the University of Alabama at Birmingham-University of California-San Diego O’Brien Center for Acute Kidney Injury Research (UAB-UCSD O’Brien Center). A: publications citing the UAB-UCSD O’Brien Center. These absolute numbers of publications indicate the productive impact of the Center. These publications have appeared in high-impact journals, including the American Journal of Physiology-Renal Physiology, Nature Medicine, Science, Journal of Clinical Investigation, Journal of the American Society of Nephrology, Clinical Journal of the American Society of Nephrology, American Journal of Pathology, Journal of Biological Chemistry, Kidney International, and others. B: weighted relative citation ratio (RCR) for center publications. Data were obtained from iCite [National Institutes of Health (NIH) Office of Portfolio Analysis]. To measure the impact of the publications from our center, we used the iCite tool from the NIH Office of Portfolio Analysis. This tool uses the RCR, which represents a citation-based measure of the scientific influence of articles. It is calculated as the cites/year of each paper, normalized to the citations per year received by NIH-funded papers in the same field and year. A paper with an RCR of 1.0 has received the same number of cites/year as the average NIH-funded paper in its field, whereas a paper with an RCR of 2.0 has received twice as many cites/year as the average NIH-funded paper in its field.
Share Data and Resources to Advance the Understanding and Management of AKI
Research data generated by the UAB-UCSD O’Brien Center are available upon request to any member of our existing and any newly added investigator for noncommercial research uses. For investigators outside the research investigator base of the UAB-UCSD O’Brien Center, data are shared as requested with any investigator or entity after appropriate assurances from the investigator and their institution that data will be used in a compliant manner as required by the NIH and federal entities. For human or animal subjects, all federally required assurances are requested and documented. Material transfers are made with no more restrictive terms than the Simple Letter Agreement (MTA) and any patented IP is widely available to the community in accordance with NIH guidelines. New techniques and methods are disseminated through the center’s website and newsletters as well as in publications. After the conclusion of funding, we will adhere to the NIH Grants Policy on Sharing of Unique Research Resources, including making biological samples available to the NIDDK Biorepository, which may include urine, serum/plasma, tissue, or DNA.
INNOVATION
Innovations continue to be developed in technical and infrastructural aspects and frequently occur in response to the needs of the research investigator base.
Technical Innovations
Using competitive institutional funding at UAB, the UAB-UCSD O’Brien Center was able to renovate space and install a Bubble Room, a biological isolation room that is designed to permit surgical procedures on animals without the need to quarantine. The Bubble Room allows animals to be directly received for surgical procedures with a seamless return after recovery to the requesting investigator’s institution for subsequent studies. Thus, investigators can send their unique animals for surgeries at the UAB Small Animal Microsurgical Facility of Core B. For experiments that involve clamping of the renal pedicle, we have found that the force applied by the clamp is an important cause of experimental variability in ischemia-reperfusion injury, particularly if the clamp is reused. We have therefore developed a quick and reliable method to determine the force applied by the clamp. This information is documented and tracked within the Small Animal Microsurgical Core Facility.
To enhance rigor and reproducibility and as a result of our quality control efforts, we have identified that different brands of plastics used can impact outcomes as protein adherence to the plastics can vary, particularly when analyzing small amounts of biological samples. In addition, not all antibodies to the same protein have the same affinity or specificity. Once an immunochemical reagent is used, the reagent is not changed without strong rationale and sufficient testing to ensure validity. For any set of samples that will require multiple gels or tubes, care is taken to use the same batch or lot to minimize variation at that level of analysis. All relevant information that is used in quality control, including vendors, brands, type of plastic, etc., is documented in the facility, and the information is available to investigators. To improve consistency in experimentation, the cores recommend and offer processing of samples or dissecting out the organ or tissue within the facility.
We have developed a web-based database that is accessible worldwide and is modular in nature to enable investigators to pursue AKI studies in different settings. The database provides a systematic set of variables covering different aspects of AKI, for example, postcardiac surgery, intensive care unit, transplant, or contrast, that can be customized for individual research protocols and can additionally track biological samples. More recently, we have developed tools for direct extraction of data variables from the Epic electronic health record to populate case report forms, thereby reducing time spent by research coordinators to enter data. With the COVID-19 registry project, we have leveraged the CDC EPI Info database for a customized AKI data set for cloud-based data capture using cell phone technology. These enhancements further facilitate data acquisition for observational and interventional studies in AKI.
Infrastructural Innovations
In furtherance of a focus on patient care, a new patient advisory group was initiated to create opportunities for thoughtful communication and education strategies for patient groups. Likewise, a desire to broaden integration in research areas resulted in increased partnerships with industry, who have reached out to elicit our significant expertise in animal model generation as well as our bioanalytical methods. Such collaboration with industry has resulted in Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) grants. The combined thematic approach continues to facilitate AKI-related research at UAB and UCSD and beyond and allows for translational studies to be accomplished.
The intent of the UAB-UCSD O’Brien Center is to provide comprehensive support to investigators at every level, to foster development of entry-level investigators to support novel and innovative research programs, and to encourage the development of new lines of investigation by more experienced investigators, all in the support of AKI research and closely related diseases. The technical expertise and education infrastructure provide the tools, knowledge, and experienced technical support to advance AKI research in all facets from basic science exploration to clinical research in traditional and underserved populations and to innovations in the practice of clinical care of patients with AKI. The UAB-UCSD O’Brien Center has used the technology and tools of the cores along with the Pilot Grants Program and Enrichment Program to build a vibrant, interactive, interdisciplinary community of investigators facilitating fundamental and translational sciences related to AKI research. Unanticipated synergies and collaborations have emerged from the interactive and interdisciplinary faculty, which has been fostered by our center through its resources. Through this synergy of exploration, leveraging the strengths of UAB and UCSD, this O’Brien Center stands ready to push the frontiers of AKI research.
GRANTS
The UAB-UCSD Center is funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK079337. R.L.M. was supported by grants from Fresenius-Kabi and Fresenius.
DISCLOSURES
A.A. serves on the medical advisory boards of Akebia, Alpha Young LLC, Angion, Reata, and Dynamed; he also serves on the medical advisory board and has stock options for Goldilocks Therapeutics. R.L.M. has provided the following disclosures: consulting for Baxter, AM Pharma, Sanofi, Akebia, Intercept, Mallinckrodt, Biomerieux, Sphingotec, GE Healthcare, Indalo, and CHF solutions.
AUTHOR CONTRIBUTIONS
L.M.C. conceived and designed research; L.M.C. prepared figures; L.M.C. drafted manuscript; L.M.C., J.G., V.V., S.B., V.D.-U., S.V., G.R.C., O.M.G., M.S., J.H.I., R.L.M., P.W.S., and A.A. edited and revised manuscript; L.M.C., J.G., V.V., S.B., V.D.-U., S.V., G.R.C., O.M.G., M.S., J.H.I., R.L.M., P.W.S., and A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The UAB-UCSD O’Brien Center is ably administered by Program Director Dr. Kelly Andringa.
REFERENCES
- 1.Staruschenko A, Brooks HL. O'Brien kidney research centers. Am J Physiol Renal Physiol 319: F1042, 2020. doi: 10.1152/ajprenal.00580.2020. [DOI] [PubMed] [Google Scholar]
- 2.Acin-Perez R, Benador IY, Petcherski A, Veliova M, Benavides GA, Lagarrigue S, Caudal A, Vergnes L, Murphy AN, Karamanlidis G, Tian R, Reue K, Wanagat J, Sacks H, Amati F, Darley-Usmar VM, Liesa M, Divakaruni AS, Stiles L, Shirihai OS. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J 39: e104073, 2020. doi: 10.15252/embj.2019104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF, Agarwal A. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol 314: F702–F714, 2018. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andringa KK. Proceedings of the 10th Annual UAB-UCSD O'Brien Center Symposium: changing paradigms in acute kidney injury: from mechanisms to management. Nephron 144: 607–608, 2020. doi: 10.1159/000512339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askenazi DJ, Halloran B, Patil N, Keeling S, Saeidi B, Koralkar R, Ambalavanan N. Genetic polymorphisms of heme-oxygenase 1 (HO-1) may impact on acute kidney injury, bronchopulmonary dysplasia, and mortality in premature infants. Pediatr Res 77: 793–798, 2015. doi: 10.1038/pr.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, Kinsey GR, Stevens BK, Jobin K, Kenwood BM, Hoehn KL, Lynch KR, Okusa MD. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol 26: 908–925, 2015. doi: 10.1681/ASN.2013121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balena-Borneman J, Ambalavanan N, Tiwari HK, Griffin RL, Halloran B, Askenazi D. Biomarkers associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Res 81: 519–525, 2017. doi: 10.1038/pr.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkawade RS, Chen C, Crowley MR, Crossman DK, Clapp WL, Verlander JW, Marshall CB. Podocyte-specific expression of Cre recombinase promotes glomerular basement membrane thickening. Am J Physiol Renal Physiol 316: F1026–F1040, 2019. doi: 10.1152/ajprenal.00359.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes S, Benton HP, Casazza K, Cooper SJ, Cui X, Du X, Engler J, Kabarowski JH, Li S, Pathmasiri W, Prasain JK, Renfrow MB, Tiwari HK. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J Mass Spectrom 51: 461–475, 2016. doi: 10.1002/jms.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes S, Benton HP, Casazza K, Cooper SJ, Cui X, Du X, Engler J, Kabarowski JH, Li S, Pathmasiri W, Prasain JK, Renfrow MB, Tiwari HK. Training in metabolomics research. II. Processing and statistical analysis of metabolomics data, metabolite identification, pathway analysis, applications of metabolomics and its future. J Mass Spectrom 51: 535–548, 2016. doi: 10.1002/jms.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benyamin B, Maihofer AX, Schork AJ, Hamilton BA, Rao F, Schmid-Schönbein GW, Zhang K, Mahata M, Stridsberg M, Schork NJ, Biswas N, Hook VY, Wei Z, Montgomery GW, Martin NG, Nievergelt CM, Whitfield JB, O'Connor DT. Identification of novel loci affecting circulating chromogranins and related peptides. Hum Mol Genet 26: 233–242, 2017. doi: 10.1093/hmg/ddw380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, Jiang Y, Cutter GR, Boddu R, George JF, Agarwal A. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol 315: F1107–F1118, 2018. doi: 10.1152/ajprenal.00179.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blantz RC, Steiner RW. Benign hyperfiltration after living kidney donation. J Clin Invest 125: 972–974, 2015. doi: 10.1172/JCI80818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313: F740–F755, 2017. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boddu R, Hull TD, Bolisetty S, Hu X, Moehle MS, Daher JP, Kamal AI, Joseph R, George JF, Agarwal A, Curtis LM, West AB. Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Hum Mol Genet 24: 4078–4093, 2015. doi: 10.1093/hmg/ddv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 310: F385–F394, 2016. doi: 10.1152/ajprenal.00335.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolisetty S, Traylor A, Zarjou A, Johnson MS, Benavides GA, Ricart K, Boddu R, Moore RD, Landar A, Barnes S, Darley-Usmar V, Agarwal A. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am J Physiol Renal Physiol 305: F255–F264, 2013. doi: 10.1152/ajprenal.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolisetty S, Zarjou A, Hull TD, Traylor AM, Perianayagam A, Joseph R, Kamal AI, Arosio P, Soares MP, Jeney V, Balla J, George JF, Agarwal A. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int 88: 95–108, 2015. doi: 10.1038/ki.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol 10: 1324–1331, 2015. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush KT, Singh P, Nigam SK. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight 5: e133817, 2020. doi: 10.1172/jci.insight.133817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacko BK, Zhi D, Darley-Usmar VM, Mitchell T. The bioenergetic health index is a sensitive measure of oxidative stress in human monocytes. Redox Biol 8: 43–50, 2016. doi: 10.1016/j.redox.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med 20: 37–46, 2014. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, Notbohm J, Benjamin A, He S, Wang M, Ang LH, Bantawa M, Bouzid M, Del Gado E, Krishnan R, Pollak MR. Disease-causing mutation in α-actinin-4 promotes podocyte detachment through maladaptation to periodic stretch. Proc Natl Acad Sci USA 115: 1517–1522, 2018. doi: 10.1073/pnas.1717870115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng W, Guan Z, Xing D, Li X, Ying WZ, Remedies CE, Inscho EW, Sanders PW. Avian erythroblastosis virus E26 oncogene homolog-1 (ETS-1) plays a role in renal microvascular pathophysiology in the Dahl salt-sensitive rat. Kidney Int 97: 528–537, 2020. doi: 10.1016/j.kint.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg AX, Devereaux PJ, Hill A, Sood M, Aggarwal B, Dubois L, Hiremath S, Guzman R, Iyer V, James M, McArthur E, Moist L, Ouellet G, Parikh CR, Schumann V, Sharan S, Thiessen-Philbrook H, Tobe S, Wald R, Walsh M, Weir M, Pannu N; Curcumin AAA AKI Investigators. Oral curcumin in elective abdominal aortic aneurysm repair: a multicentre randomized controlled trial. CMAJ 190: E1273–E1280, 2018. doi: 10.1503/cmaj.180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetsch MR, Tamhane A, Varshney M, Kapil A, Overton ET, Towns GC, Franco RA. Direct-acting antivirals in kidney transplant patients: successful hepatitis C treatment and short-term reduction in urinary protein/creatinine ratios. Pathog Immun 2: 366–375, 2017. doi: 10.20411/pai.v2i3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo L, Agarwal A, George JF. Orthotopic aortic transplantation in mice for the study of vascular disease. J Vis Exp 69: e4338, 2012. doi: 10.3791/4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hepokoski M, Englert JA, Baron RM, Crotty-Alexander LE, Fuster MM, Beitler JR, Malhotra A, Singh P. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am J Physiol Renal Physiol 312: F654–F660, 2017. doi: 10.1152/ajprenal.00523.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyt K, Warram JM, Wang D, Ratnayaka S, Traylor A, Agarwal A. Molecular ultrasound imaging of tissue inflammation using an animal model of acute kidney injury. Mol Imaging Biol 17: 786–792, 2015. doi: 10.1007/s11307-015-0860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hull TD, Agarwal A, Hoyt K. New ultrasound techniques promise further advances in AKI and CKD. J Am Soc Nephrol 28: 3452–3460, 2017. doi: 10.1681/ASN.2017060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Tisher CC, Rangarajan S, Chen B, Curtis LM, George JF, Agarwal A. Heme oxygenase-1 regulates myeloid cell trafficking in AKI. J Am Soc Nephrol 26: 2139–2151, 2015. doi: 10.1681/ASN.2014080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyndman KA, Kasztan M, Mendoza LD, Monteiro-Pai S. Dynamic changes in histone deacetylases following kidney ischemia-reperfusion injury are critical for promoting proximal tubule proliferation. Am J Physiol Renal Physiol 316: F875–F888, 2019. doi: 10.1152/ajprenal.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyndman KA, Speed JS, Mendoza LD, Allan JM, Colson J, Sedaka R, Jin C, Jung HJ, El-Dahr S, Pollock DM, Pollock JS. Fluid-electrolyte homeostasis requires histone deacetylase function. JCI Insight 5: e137792, 2020. doi: 10.1172/jci.insight.137792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest 126: 1939–1952, 2016. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson JL, Judd SE, Panwar B, Howard VJ, Wadley VG, Jenny NS, Gutiérrez OM. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J Clin Transl Endocrinol 5: 21–25, 2016. doi: 10.1016/j.jcte.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasztan M, Aban I, Hande SP, Pollock DM, Lebensburger JD. Sex differences in the trajectory of glomerular filtration rate in pediatric and murine sickle cell anemia. Blood Adv 4: 263–265, 2020. doi: 10.1182/bloodadvances.2019001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasztan M, Fox BM, Speed JS, De Miguel C, Gohar EY, Townes TM, Kutlar A, Pollock JS, Pollock DM. Long-term endothelin-A receptor antagonism provides robust renal protection in humanized sickle cell disease mice. J Am Soc Nephrol 28: 2443–2458, 2017. doi: 10.1681/ASN.2016070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kefaloyianni E, Muthu ML, Kaeppler J, Sun X, Sabbisetti V, Chalaris A, Rose-John S, Wong E, Sagi I, Waikar SS, Rennke H, Humphreys BD, Bonventre JV, Herrlich A. ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1: e87023, 2016. doi: 10.1172/jci.insight.87023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1(+) pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J Vis Exp 334: 51301, 2014. [2474733] doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2: 206–210, 2014. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFavers KA, Macedo E, Garimella PS, Lima C, Khan S, Myslinski J, McClintick J, Witzmann FA, Winfree S, Phillips CL, Hato T, Dagher PC, Wu XR, El-Achkar TM, Micanovic R. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med 11: eaaw3639, 2019. [31578243] doi: 10.1126/scitranslmed.aaw3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layton AT, Edwards A, Vallon V. Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. Am J Physiol Renal Physiol 313: F199–F209, 2017. doi: 10.1152/ajprenal.00018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layton AT, Edwards A, Vallon V. Renal potassium handling in rats with subtotal nephrectomy: modeling and analysis. Am J Physiol Renal Physiol 314: F643–F657, 2018. doi: 10.1152/ajprenal.00460.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 314: F969–F984, 2018. doi: 10.1152/ajprenal.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leaf DE, Jacob KA, Srivastava A, Chen ME, Christov M, Jüppner H, Sabbisetti VS, Martin A, Wolf M, Waikar SS. Fibroblast growth factor 23 levels associate with AKI and death in critical illness. J Am Soc Nephrol 28: 1877–1885, 2017. doi: 10.1681/ASN.2016080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Boerger EAS, Mc Causland FR, Eisenga MF, Singh K, Babitt JL, Kellum JA, Palevsky PM, Christov M, Waikar SS. Iron, hepcidin, and death in human AKI. J Am Soc Nephrol 30: 493–504, 2019. doi: 10.1681/ASN.2018100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leaf DE, Siew ED, Eisenga MF, Singh K, Mc Causland FR, Srivastava A, Ikizler TA, Ware LB, Ginde AA, Kellum JA, Palevsky PM, Wolf M, Waikar SS. Fibroblast growth factor 23 associates with death in critically Ill patients. Clin J Am Soc Nephrol 13: 531–541, 2018. doi: 10.2215/CJN.10810917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lebensburger JD, Cutter GR, Howard TH, Muntner P, Feig DI. Evaluating risk factors for chronic kidney disease in pediatric patients with sickle cell anemia. Pediatr Nephrol 32: 1565–1573, 2017. doi: 10.1007/s00467-017-3658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, Yang Z, Traylor AM, Jiang Y, Li Z, Peabody JE, Eckenrode HE, Crossman DK, Crowley MR, Bolisetty S, Zimmerman KA, Wende AR, Mrug M, Yoder BK, Agarwal A, George JF. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4: e125503, 2019. doi: 10.1172/jci.insight.125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lever JM, Yang Z, Boddu R, Adedoyin OO, Guo L, Joseph R, Traylor AM, Agarwal A, George JF. Parabiosis reveals leukocyte dynamics in the kidney. Lab Invest 98: 391–402, 2018. doi: 10.1038/labinvest.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Boddeda SR, Chen B, Zeng Q, Schoeb TR, Velazquez VM, Shimamura M. NK cell and Th17 responses are differentially induced in murine cytomegalovirus infected renal allografts and vary according to recipient virus dose and strain. Am J Transplant 18: 2647–2662, 2018. doi: 10.1111/ajt.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Nourbakhsh N, Pham H, Tham R, Zuckerman JE, Singh P. Evolution of altered tubular metabolism and mitochondrial function in sepsis-associated acute kidney injury. Am J Physiol Renal Physiol 319: F229–F244, 2020. doi: 10.1152/ajprenal.00390.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liebow A, Li X, Racie T, Hettinger J, Bettencourt BR, Najafian N, Haslett P, Fitzgerald K, Holmes RP, Erbe D, Querbes W, Knight J. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol 28: 494–503, 2017. doi: 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27: 781–790, 2016. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maarouf OH, Uehara M, Kasinath V, Solhjou Z, Banouni N, Bahmani B, Jiang L, Yilmam OA, Guleria I, Lovitch SB, Grogan JL, Fiorina P, Sage PT, Bromberg JS, McGrath MM, Abdi R. Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing. JCI Insight 3: e120546, 2018. doi: 10.1172/jci.insight.120546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, Vallon V, Nagata D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep 8: e14360, 2020. doi: 10.14814/phy2.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta RL. Renal-replacement therapy in the critically Ill–does timing matter? N Engl J Med 375: 175–176, 2016. doi: 10.1056/NEJMe1606125. [DOI] [PubMed] [Google Scholar]
- 60.Mustian MN, Kumar V, Stegner K, Mompoint-Williams D, Hanaway M, Deierhoi MH, Young C, Orandi BJ, Anderson D, MacLennan PA, Reed RD, Shelton BA, Eckhoff D, Locke JE. Mitigating racial and sex disparities in access to living donor kidney transplantation: impact of the nation's longest single-center kidney chain. Ann Surg 270: 639–646, 2019. doi: 10.1097/SLA.0000000000003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nespoux J, Patel R, Hudkins KL, Huang W, Freeman B, Kim YC, Koepsell H, Alpers CE, Vallon V. Gene deletion of the Na(+)-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol 316: F1201–F1210, 2019. doi: 10.1152/ajprenal.00111.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, Sharma K, Thomson SC, Vallon V. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 319: F712–F728, 2020. doi: 10.1152/ajprenal.00264.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang P, Abbott M, Chang SL, Abdi M, Chauhan N, Mistri M, Ghofrani J, Fucci QA, Walker C, Leonardi C, Grady S, Halim A, Hoffman R, Lu T, Cao H, Tullius SG, Malek S, Kumar S, Steele G, Kibel A, Freedman BS, Waikar SS, Siedlecki AM. Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney Int 91: 129–143, 2017. doi: 10.1016/j.kint.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramos S, Carlos AR, Sundaram B, Jeney V, Ribeiro A, Gozzelino R, Bank C, Gjini E, Braza F, Martins R, Ademolue TW, Blankenhaus B, Gouveia Z, Faísca P, Trujillo D, Cardoso S, Rebelo S, Del Barrio L, Zarjou A, Bolisetty S, Agarwal A, Soares MP. Renal control of disease tolerance to malaria. Proc Natl Acad Sci USA 116: 5681–5686, 2019. doi: 10.1073/pnas.1822024116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rangarajan S, Sunil B, Fan C, Wang PX, Cutter G, Sanders PW, Curtis LM. Distinct populations of label-retaining cells in the adult kidney are defined temporally and exhibit divergent regional distributions. Am J Physiol Renal Physiol 307: F1274–F1282, 2014. doi: 10.1152/ajprenal.00213.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shan D, Rezonzew G, Mullen S, Roye R, Zhou J, Chumley P, Revell DZ, Challa A, Kim H, Lockhart ME, Schoeb TR, Croyle MJ, Kesterson RA, Yoder BK, Guay-Woodford LM, Mrug M. Heterozygous Pkhd1(C642*) mice develop cystic liver disease and proximal tubule ectasia that mimics radiographic signs of medullary sponge kidney. Am J Physiol Renal Physiol 316: F463–F472, 2019. doi: 10.1152/ajprenal.00181.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith MR, Chacko BK, Johnson MS, Benavides GA, Uppal K, Go YM, Jones DP, Darley-Usmar VM. A precision medicine approach to defining the impact of doxorubicin on the bioenergetic-metabolite interactome in human platelets. Redox Biol 28: 101311, 2020. doi: 10.1016/j.redox.2019.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, Liu R, Vallon V. Knockout of Na(+)-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol 317: F207–F217, 2019. doi: 10.1152/ajprenal.00120.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srivastava RK, Traylor AM, Li C, Feng W, Guo L, Antony VB, Schoeb TR, Agarwal A, Athar M. Cutaneous exposure to lewisite causes acute kidney injury by invoking DNA damage and autophagic response. Am J Physiol Renal Physiol 314: F1166–F1176, 2018. doi: 10.1152/ajprenal.00277.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Y, Byon CH, Yang Y, Bradley WE, Dell’Italia LJ, Sanders PW, Agarwal A, Wu H, Chen Y. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight 2: e94920, 2017. doi: 10.1172/jci.insight.94920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med 367: 2505–2514, 2012. doi: 10.1056/NEJMct1206045. [DOI] [PubMed] [Google Scholar]
- 72.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uchida M, Maier B, Waghwani HK, Selivanovitch E, Pay SL, Avera J, Yun E, Sandoval RM, Molitoris BA, Zollman A, Douglas T, Hato T. The archaeal Dps nanocage targets kidney proximal tubules via glomerular filtration. J Clin Invest 129: 3941–3951, 2019. doi: 10.1172/JCI127511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Liu J, Yin W, Abdi F, Pang PD, Fucci QA, Abbott M, Chang SL, Steele G, Patel A, Mori Y, Zhang A, Zhu S, Lu TS, Kibel AS, Wang B, Lim K, Siedlecki AM. miR-218 Expressed in endothelial progenitor cells contributes to the development and repair of the kidney microvasculature. Am J Pathol 190: 642–659, 2020. doi: 10.1016/j.ajpath.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warnock DG, Powell TC, Donnelly JP, Wang HE. Categories of hospital-associated acute kidney injury: time course of changes in serum creatinine values. Nephron 131: 227–236, 2015. doi: 10.1159/000441956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Widmeier E, Yu S, Nag A, Chung YW, Nakayama M, Fernández-Del-Río L, Hugo H, Schapiro D, Buerger F, Choi WI, Helmstädter M, Kim JW, Ryu JH, Lee MG, Clarke CF, Hildebrandt F, Gee HY. ADCK4 deficiency destabilizes the coenzyme Q complex, which Is rescued by 2,4-dihydroxybenzoic acid treatment. J Am Soc Nephrol 31: 1191–1211, 2020. doi: 10.1681/ASN.2019070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, Sanders PW. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest 129: 2792–2806, 2019. doi: 10.1172/JCI125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarjou A, Black LM, Bolisetty S, Traylor AM, Bowhay SA, Zhang MZ, Harris RC, Agarwal A. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab Invest 99: 1376–1388, 2019. doi: 10.1038/s41374-019-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013. doi: 10.1172/JCI67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zarjou A, Guo L, Sanders PW, Mannon RB, Agarwal A, George JF. A reproducible mouse model of chronic allograft nephropathy with vasculopathy. Kidney Int 82: 1231–1235, 2012. doi: 10.1038/ki.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zarjou A, Sanders PW, Mehta RL, Agarwal A. Enabling innovative translational research in acute kidney injury. Clin Transl Sci 5: 93–101, 2012. doi: 10.1111/j.1752-8062.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Ouyang X, Schoeb TR, Bolisetty S, Cui X, Mrug S, Yoder BK, Johnson MR, Szalai AJ, Mrug M. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol 23: 1161–1171, 2012. doi: 10.1681/ASN.2011050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimmerman KA, Bentley MR, Lever JM, Li Z, Crossman DK, Song CJ, Liu S, Crowley MR, George JF, Mrug M, Yoder BK. Single-cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J Am Soc Nephrol 30: 767–781, 2019. doi: 10.1681/ASN.2018090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zimmerman KA, Song CJ, Li Z, Lever JM, Crossman DK, Rains A, Aloria EJ, Gonzalez NM, Bassler JR, Zhou J, Crowley MR, Revell DZ, Yan Z, Shan D, Benveniste EN, George JF, Mrug M, Yoder BK. Tissue-resident macrophages promote renal cystic disease. J Am Soc Nephrol 30: 1841–1856, 2019. doi: 10.1681/ASN.2018080810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, Rosal-Vela A, Botta D, Bradley JE, Wojciechowski W, Ptacek T, Danila MI, Edberg JC, Bridges SL Jr, Kimberly RP, Chatham WW, Schoeb TR, Rosenberg AF, Boss JM, Sanz I, Lund FE. IFNγ induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. eLife 8: e41641, 2019. doi: 10.7554/eLife.41641. [DOI] [PMC free article] [PubMed] [Google Scholar]