Abstract
Obesity research progresses in understanding neuronal circuits and adipocyte biology to regulate metabolism. However, the interface of neuro-adipocyte interaction is less studied. We summarize the current knowledge of adipose tissue innervation and interaction with adipocytes and emphasize adipocyte transitions from white to brown adipocytes and vice versa. We further highlight emerging concepts for the differential neuronal regulation of brown/beige versus white adipocyte and the interdependence of both for metabolic regulation.
Keywords: differential innervation, preganglionic, sympathetic chain, thermogenesis, varicosities
Introduction
Adipose tissue biology made huge progress in the last decades, largely driven by the raging obesity epidemic and the need for a better understanding of how and why adipose tissue is able to expand tremendously and why chronically increased fat mass is associated with metabolic diseases like type-2-diabetes (T2D), cardiovascular problems, and most recently increased infection vulnerability for Covid-19 symptoms. The historic view of adipose tissue as a mere energy storage site has been replaced with the understanding of adipose tissue as an important endocrine organ, with leptin and adiponectin as the most prominent hormones, as well as many other relevant adipokines (1, 2). The obesity research field is now keenly aware that adipose tissue plays a central role in metabolic disease, which is promoted by excess fat accumulation in overweight and obese individuals, but also by the lack of adipose tissue in individuals with lipodystrophy (3, 4).
It is well accepted that adipose tissue is under neuronal control from the sympathetic nervous system (SNS) and sensory nerves provide feedback from the adipose tissue to the central nervous system (CNS) (5), and despite initial controversy (6), it is generally accepted that adipose tissue does not receive any parasympathetic innervation. Functionally, the sympathetic tone in adipose tissue promotes lipolysis and induces thermogenesis in brown adipocytes via activation of uncoupling protein 1 (UCP1). Another prominent sympathetic function is the induction of thermogenic features in white adipose tissue (WAT). This switch of white to brown adipocytes (termed “beige” or “brite” adipocytes) is known as “beiging” or “browning” (7–9). The interface of sympathetic tone and adipocyte function is mediated by adrenergic receptors (AR) where the sympathetic transmitter norepinephrine binds and mediates lipolysis, thermogenesis, and WAT beiging.
There is little doubt that WAT beiging is present in humans and rodents, even though the functionality and capacity of beige fat for body weight regulation in humans are debated (10). This review will focus on emerging concepts that address specific aspects of the sympathetic innervation and regulation of adipocyte function. We will discuss existing discrepancies in the literature and highlight gaps in the understanding of the neuro-adipocyte interface with regards to adipose tissue beiging and thermogenic coupling and the importance within the larger context of physiological adaptations in health and disease.
CNS Sympathetic Circuits and Adipose Tissue Innervation
The anatomy of adipose tissue innervation circuits involves hypothalamic and brainstem neurons, preganglionic spinal cord neurons, and postganglionic sympathetic chain ganglia neurons (FIGURE 1). These circuits have been mapped from their sympathetic endings in interscapular brown adipose tissue (iBAT) and inguinal WAT (iWAT) to higher order neurons in the brain with the use of retrograde, transsynaptic viral tracers (11–14). Several hypothalamic sites (preoptic area (POA), dorsomedial hypothalamus (DMH), paraventricular hypothalamus (PVH), and pro-opiomelancortin (POMC) neurons were identified with multisynaptic connection to iBAT or iWAT and play an important role in different aspects of thermoregulation (body temperature, fever, metabolism, and body weight) (13, 15–22). Some sites and neurons, like the ventromedial hypothalamus (VMH) or agouti-related-peptide (AgRP) neurons, may be too far upstream in the circuit to be detected by viral tracing methods, but their involvement in energy expenditure regulation is well established (23–27). The VMH has received particular attention for its role in sex-specific metabolic control (24). However, we currently lack a clear understanding if and how these hypothalamic sites interplay during a given physiological challenge like cold temperature, fasting, overfeeding/obesity, or fever (as recently reviewed Ref. 22) and if they also modulate iWAT beiging. Although select neuronal populations are known for their robust impact on iWAT beiging (28–31), if and how these neurons connect with thermoregulatory circuits remains unclear.
FIGURE 1.
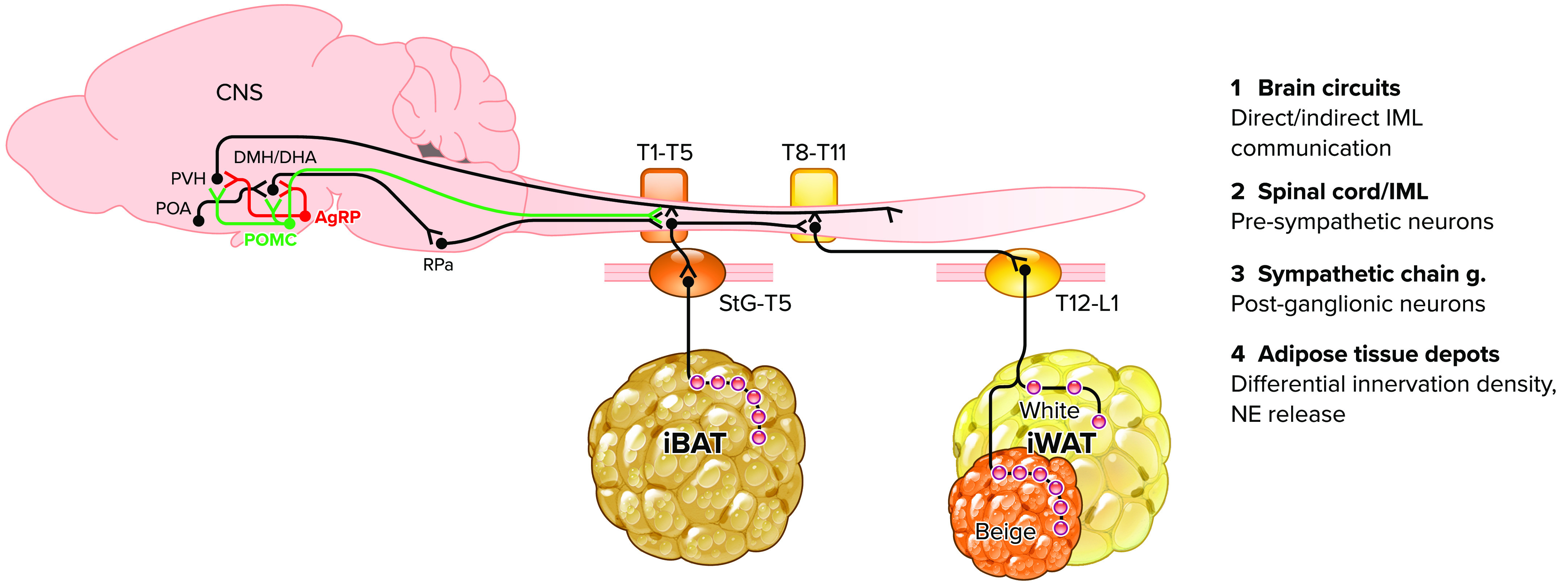
Adipose tissue innervation scheme This scheme highlights the emerging concept of differential sympathetic tone and innervation density in select adipose tissue depots [e.g., interscapular brown adipose tissue (iBAT) and inguinal white adipose tissue (iWAT) and beige islands]. Brain circuits connect neurons either directly (e.g., POMC, PVN, and RPa neurons) or indirectly (e.g., POA, DMH/DHA, POMC, and AgRP neurons) with presympathetic neurons in the spinal cord (1 and 2). The differential location of iBAT vs. iWAT innervating presympathetic neurons is a promising interface for differential regulation of postganglionic sympathetic tone to select adipose tissue depots (3 and 4). POA, preoptic area; PVH, paraventricular hypothalamus; DMH/DHA, dorsomedial hypothalamus/dorsal hypothalamic area; POMC, pro-opiomelanocortin; AgRP, agouti-related peptide; RPa, raphe pallidus; StG, stellate ganglion; T, thoracic level; IML, intermediolateral bundle; NE, norepinephrine.
Furthermore, in contrast to detailed studies on hypothalamic circuits, very limited work has addressed how hypothalamic neurons communicate with adipose tissue innervating preganglionic neurons in the spinal cord and if they may differentially regulate iBAT and iWAT. We recently performed a detailed mapping study to identify the full extent of the preganglionic inputs to iBAT and iWAT (32, 33) and highlighted the stark anatomical dissociation of pre- and postganglionic inputs to iBAT versus iWAT (FIGURE 1). Hypothalamic neurons can connect directly or indirectly (via premotor neurons) and innervate preganglionic, cholinergic neurons in the intermediolateral column (IML) of the spinal cord. A premotor site for sympathetic iBAT innervation is the raphe pallidus (RPa) in the mediobasal brainstem (FIGURE 1), and hypothalamic neurons in the PVN, POA, and DMH regulate iBAT thermogenesis via the Rpa (16, 18, 34). Anatomical evidence also indicates the RPa as premotor input to iWAT sympathetic circuits (21). Hypothalamic neurons that project directly to the spinal cord are found in the parvocellular PVN (e.g., oxytocine and vasopressin expressing neurons) (35), select POMC-expressing neurons (11, 36), and orexin neurons in the lateral hypothalamus (37), even though their importance for adipose tissue regulation is unclear. However, projections of these hypothalamic neurons are consistent with the spinal cord levels where iWAT and/or iBAT innervating preganglionic cell bodies are located (11, 36).
The melanocortin system is an important component of sympathetic adipose tissue innervation and includes POMC- and AgRP-expressing neurons with opposing effects at the melaconortin-4-receptors (MC4R). MC4R-expressing neurons are found in the spinal cord-projecting hypothalamic neurons that connect to iWAT (13) and iBAT (11) sympathetic circuits. Bartness and colleagues (38) first introduced the concept of differential sympathetic activation of iBAT versus iWAT and demonstrated that physiological adaptation to energy deficiency states (fasting, glucoprivation) increased norepinephrine turnover (NETO) only in iWAT but not iBAT. Similarly, energy deficiency causes differential activation of AgRP neurons, while inhibiting POMC neurons (22), which causes an overall inhibition of MC4R signaling and decreased thermogenesis. Conversely, MC4R stimulation increased NETO in both iWAT and iBAT (39) and is well known to increase thermogenesis.
MC4R is expressed in hypothalamic and IML neurons (13, 14, 40–42), but the relative impact of these neurons for adipose tissue innervation and beiging has not been explored. However, IML-specific MC4R signaling significantly impacts body weight, energy expenditure, and glucose homeostasis and is uniquely innervated by POMC neurons and lacks AgRP neuronal inputs. Melanocortin neurons are also highly responsive to the adipose tissue-derived hormone leptin, and circulating leptin levels are controlled by the sympathetic tone to adipose tissue (43–47). Furthermore, leptin levels impact other hormones like adiponectin, glucocorticoids, and insulin (2, 48–50). Recent work further suggests that leptin action on POMC and AgRP neurons differentially affects iBAT versus iWAT sympathetic tone (51). These data suggest a disconnect of POMC leptin action from sympathetic iWAT, but not iBAT, tone and contribute to an emerging concept that the relative sympathetic tone in iBAT versus iWAT might be an important determinant of adipose tissue thermogenic function.
Adipose Depot Sympathetic Innervation, Proliferation, and Beiging
Adipose tissue depots have enormous flexibility with adipocytes that can expand in size (hypertrophy), increase in cell numbers (hyperplasia/proliferation), transition their metabolic features from white to brown/beige adipocytes (beiging/trans-differentiation), and generate de novo beige adipocytes (52–55). These phenomena are of great interest, as they are largely associated with increasing (hyperplasia/proliferation) or decreasing (beiging/trans-differentiation) body weight and as such are important mechanisms to understand illnesses associated with body weight changes such as obesity or cancer- and other illness-associated weight loss (for detailed reviews see Refs. 55–58). However, it is important to point out that changes in adipose tissue size and metabolic efficiency are part of normal physiology and adaptations to daily or seasonal changes (e.g., ambient temperature, food availability, stress, sleep) and robust seasonal changes in body adiposity and insulin sensitivity are not inherently associated with illness (59–62).
Differential Innervation within Adipose Tissue Depots
An emerging concept is that overall innervation density is tightly associated with adipocyte status (FIGURE 2), and high innervation density goes together with high brown/beige adipocyte density. This is true when comparing iBAT to adipose tissue depots that are most prone (iWAT) or least prone (epididymal WAT) to beiging (63, 64) or when comparing select regions within iWAT (dorsolumbar versus inguinal portion) (63, 65), with the differential appearance of beige islands within the iWAT (33, 65).
FIGURE 2.
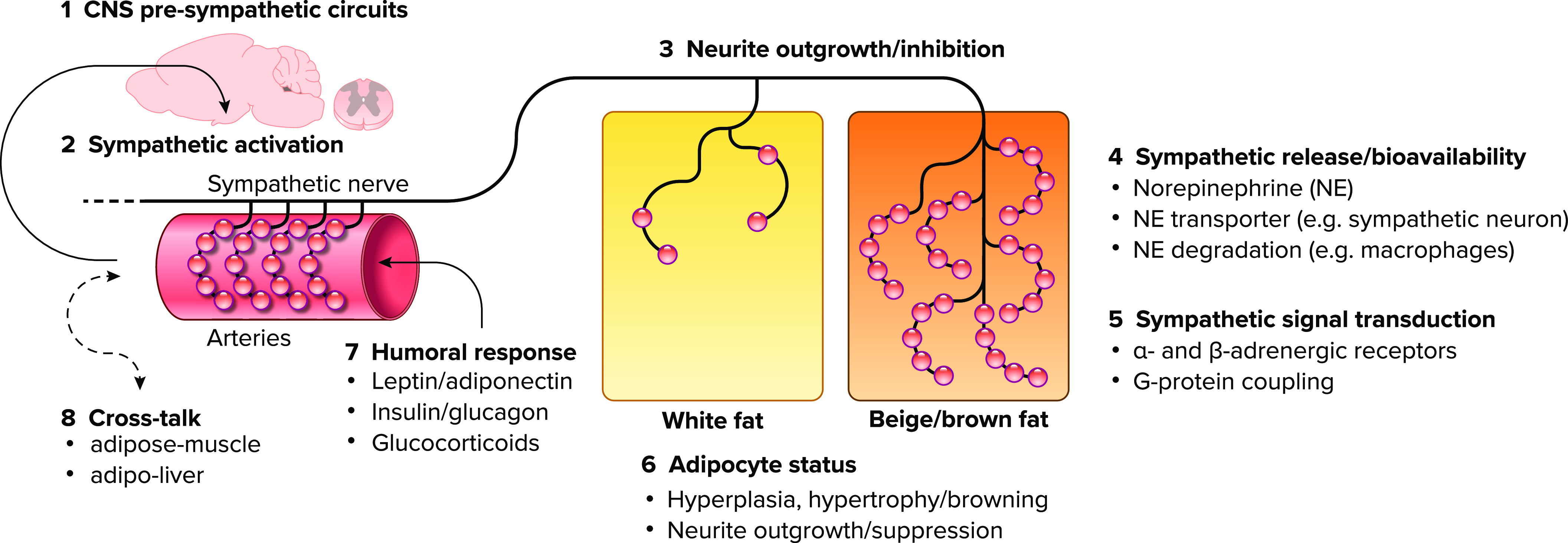
Complex communication at the adipocyte/innervation interface Schematic diagram showing the neuronal axis from central nervous system (1) via sympathetic nerve (2) to the differential innervation density (3) of adipocytes. Sympathetic activity and adrenergic signal transduction (4 and 5) are actively involved to modulate innervation density and adipocyte status (6) from brown adipocytes (either in interscapular brown adipose tissue or beige islands of interscapular white adipose tissue) to white adipocytes. Vice versa innervation density is dependent on adipocyte status. Thus the highlighted complex communication interface for neuron/adipocyte shows an emerging concept that modulations at any level in this axis will impact innervation density and adipocyte status accordingly [7: humoral response; 8: cross talk].
Recent technological advances with adipose tissue clearing have given us unprecedented insight into the sympathetic innervation network of iWAT and the rich innervation of iWAT has been emphasized (63). However, only axonal varicosities, but not synaptic branches, have norepinephrine-filled vesicles that are released upon neuronal activation and are the functional unit of the sympathetic nerve. These axonal varicosities densely innervate adipose tissue arteries and are found in close proximity to adipocytes and even make close synaptic contacts (20 nm, no basal lamina), at least among brown adipocytes (66). Axonal varicosities are associated with increased branching toward beige adipocytes and dense varicosities are found close to UCP1-expressing beige adipocytes, while adjacent white adipocytes are sharply distinguished by sparse varicose endings within the same adipose depot (33) (FIGURE 2). Thus the interaction of adipocyte and sympathetic varicosities seems highly relevant to maintain adipocytes in their beige/brown state, while denervation initiates hyperplasia/proliferation and whitening of beige and brown adipocytes (67, 68).
A helpful working model is shown in FIGURE 2, depicting the brain and preganglionic circuits as modulator of sympathetic activation (bullets 1 and 2). The innervation density (bullet 3) and thus sympathetic norepinephrine release and signal transduction via adrenergic receptors (α- and β-AR, bullets 4 and 5, respectively) is an important component to recruit beige adipocytes, to maintain brown/beige adipocyte status (bullet 6), and to prevent adipocyte hyperplasia/proliferation. This is supported by surgical or chemical denervation of either iBAT or iWAT, resulting in reduced expression of the brown adipocyte marker UCP1(68) as an indicator of de-differentiation, while promoting adipocyte hyperplasia/proliferation (13, 69, 70). Although adipose tissue denervation does not cause obesity, a disruption of adipose tissue innervation dynamics may likely contribute to obesity development with predominant adipocyte hypertrophy and proliferation, and it is critical to understand the mechanisms that regulate the dynamic changes in adipose tissue innervation, activation and adipocyte function.
Components of Dynamic Innervation Patterns
During development, the SNS establishes functional circuits with peripheral target organs. This process is distinguished into specific growth stages: 1) axonal growth initiation; 2) proximal axon extension (for long-distance growth along the vasculature); 3) distal axon extension (for short-distance growth within peripheral target organs, not bound to vasculature); 4) final target innervation and neuronal survival (formation of axonal varicosities and neuronal survival signals); and 5) growth inhibition (growth-restricting and apoptotic signals) (71, 72). Importantly, these steps in axonal growth require interaction of growth factors and their receptors to guide axonal growth toward their targets. Final target innervation, neuronal survival, and growth inhibition seem to fit well with the observed adipocyte innervation patterns and dynamic changes. Several growth factors, including the nerve growth factor (NGF), and their interaction with according receptors, e.g., tyrosine kinase A (TrkA), are involved to regulated neuronal growth and survival and have been studied in adipose tissue. NGF is expressed in target tissues, and NGF is enriched in the adipose tissue stromal vascular fraction (73) that contains endothelial cells and preadipocytes. Similarly, NGF expression is highest in undifferentiated preadipocytes and decreased in mature white adipocytes (74), in line with the lack of sympathetic varicosities close to mature white adipocytes. Conversely, TrkA is expressed in sympathetic neurons, and genetic manipulation of this neurotrophic system at the target cell or in sympathetic neurons profoundly diminishes innervation density (71).
Dynamic innervation changes in peripheral organs of adult organisms are not well studied but are notable features in WAT exposed to physiological challenges like chronic cold exposure, which can be mimicked by chronic β3-AR stimulation (63, 73, 75). Several mouse models exist with decreased adipose tissue innervation and obesity that have genetic deletions in common with a disruption of the neuro-adipocyte communication axis and highlight the importance of cross talk between sympathetic neurons and adipocytes to maintain proper innervation and body weight homeostasis [TrkA deletion in sympathetic neurons (76), impaired secretion of growth factors NGF (77), S100B (78) from adipocytes, impaired beige adipogenesis by BMP8b (79), PRDM16 (63), and sarcolipin (80) deletion].
Importantly, growth factors that are secreted by adipose tissue can also act centrally to influence adipose tissue innervation density. This was demonstrated for the adipokine leptin. Leptin’s neurotrophic function had been recognized earlier for central circuits (81, 82), but the relevance of leptin to maintain normal sympathetic innervation in iBAT and iWAT was just recently revealed. Wang et al. (29) showed that leptin signaling in the arcuate nucleus is critical to maintain proper innervation of iBAT and iWAT. Lack of leptin signaling in either POMC or AgRP neurons resulted in a strong decrease of sympathetic fibers in both iBAT and iWAT depots. Importantly, decreased hypothalamic leptin signaling (aka leptin resistance) of diet-induced obese mice causes reduced POMC and AgRP innervation of the PVN (81), as well as reduced sympathetic innervation of iBAT and iWAT and contributed to obesity by reducing thermogenesis (29). The central neurotrophic effects are mediated by brain-derived-neurotrophic-factor (BDNF) expression in PVN neurons (29) and are in line with its role to maintain POMC and AgRP innervation of the PVN (81). However, BDNF is unlikely to act anterogradely on adipose tissue innervation density, and sympathetic innervation levels of iBAT and iWAT might instead involve classic transmitter release to maintain proper stimulation levels of pre- and postganglionic sympathetic circuits.
Regulatory Components of Sympathetic Transmission and Metabolic Effects
In contrast to long-term neurotrophic effects (several days), postganglionic sympathetic activity concerns immediate (seconds) changes in norepinephrine release to adipose tissue. As we highlighted in FIGURE 1, the activity of sympathetic neurons depends on central circuits within the hypothalamus and brainstem that either directly or indirectly change the activity level of pre-and postganglionic neurons to control the release of norepinephrine. Yet, several additional layers of regulation have been recognized with significant impact on adipose tissue beiging, metabolism, and finally body weight regulation.
Norepinephrine Bioavailability
The amount of norepinephrine that is bioavailable for adipocytes depends on the norepinephrine release at axonal varicosities, but is counteracted by reuptake transporters (Slc6a2) in presynaptic membranes of the sympathetic neuron (FIGURE 2, bullet 4). Pharmacological inhibition of these transporters, e.g., by fenfluramine, are powerful antiobesity drugs, despite their cardiovascular risk (83). An emerging concept is that the harmful cardiovascular and hyperthermic effects are mediated by the CNS, while the beneficial antiobesity effects were fully preserved by peripheral actions that involved increased excitability of sympathetic neurons (84). The critical role of peripheral norepinephrine bioavailability is further supported by the norepinephrine reuptake and degradation in adipose tissue immune cells with significant effects on adipose tissue beiging, metabolism and body weight regulation (85–87). This has also relevance in humans, even though the genetic machinery to degrade norepinephrine was also enriched in mature adipocytes and is also relevant for metabolic changes with aging (88).
Adrenergic Signal Transduction
The interface for sympathetic signaling via norepinephrine are adrenergic receptors, specifically, β3-adrenergic receptors (β3-AR) that are enriched in brown and white adipocytes of mice (89–91) (FIGURE 2, bullet 5). Functional evidence comes from studies with the highly selective β3-AR agonist CL-316,243 (CL), which robustly increases energy expenditure, fat oxidation, pancreatic insulin release, and insulin-dependent and -independent glucose uptake (92, 93) and suppresses food intake. These metabolic CL effects are highly specific to β3-AR action in brown and white adipocytes (94, 95). Such data clearly link sympathetic activity in adipose tissue with whole body metabolism, even though it is still unclear how β3-AR signaling results in regulation of pancreatic insulin release or modulation of food intake. These data have led to extensive use of CL as a thermogenic agent and a surrogate to induce iBAT thermogenesis, iWAT lipolysis, and beiging (96–100).
Despite these well-accepted concepts, select β3-AR reexpression in brown adipocytes in otherwise β3-AR knockout mice is insufficient to reinstate CL effects on metabolism, food intake, and glucose homeostasis. However, β3-AR reexpression in brown and white adipocytes fully reinstated CL effects (95), suggesting that β3-AR signaling in brown adipocytes might be necessary but not sufficient to induce thermogenesis in vivo. Similarly, genetic impairment of lipolysis in brown adipocytes (considered as critical step for BAT thermogenesis) was surprisingly without effect on β3-AR-or cold-induced iBAT thermogenesis, while deletion in brown and white adipocytes abolished β3-AR-induced BAT thermogenesis and cold-induced thermogenesis in fasted animals (101, 102). Together these data support the idea that sympathetic input to brown and white adipocytes has to work in synchrony for proper thermoregulatory control.
These data are in line with earlier findings of differential sympathetic tone in BAT and WAT during select physiological challenges (38, 39), where increased metabolism is associated with iBAT and iWAT sympathetic activation (cold-exposure, MC4R activation), while decreased metabolism is associated with iWAT, but not iBAT, sympathetic activation. Taken together these data support the idea that combined sympathetic β3-AR stimulation of WAT lipolysis and BAT thermogenic pathways is required to enable iBAT thermogenesis and that differential regulation of sympathetic activity in iBAT versus iWAT is involved to control diverse physiological adaptations.
In this context it is particularly paradoxical how the sympathetic activation of iWAT lipolysis is functionally aligned with the dramatic innervation difference among white and brown adipocytes. Another peculiar phenomenon is that denervation of iBAT greatly increases iWAT beiging but lacks metabolic effects (21, 68, 103). De novo lipogenesis (DNL) via fatty acid synthase in adipocytes is tightly coupled to lipolysis, and lipolysis robustly increases DNL (99). Interestingly, genetic perturbation of DNL has profound effects on adipocyte beiging, innervation and sympathetic activity in iBAT and iWAT, even though cold-induced thermogenesis was not affected (104). Thus an overall picture emerges where adipose tissue lipid fluxes have a key role to couple iBAT and iWAT innervation and thermogenic function, even though the exact nature of this cross talk, e.g., via circulating nutrient or adipokines remains unclear.
Signaling via β3-AR has remained the focus for obesity research until today, even though it is known that genetic deletion of β3-AR is insufficient to cause obesity and only genetic deletion of all three β-adrenergic receptors (β-less mice) results in enhanced weight gain on a calorie rich high-fat diet (105). Also, the translation of β3-AR-induced thermogenesis to in humans has been difficult, even though the newly developed drug mirabegron has recently shown promising results (106). Thus reexamining the importance of other adrenergic receptors for body weight homeostasis and their interaction with β3-AR signaling is an upcoming and important future task.
Cold-exposed β-less mice or treatment with β-blockers recently revealed that adipocyte beiging occurs even in the absence of β-AR signaling and recruits beige adipocytes from muscle-like cells that contribute to thermogenesis and glucose uptake (107). It remains unclear how cold-induced sympathetic tone could induce this beiging event in the absence of β-AR signaling. Another recent finding highlighted the importance of α2-AR signaling for the regulation of adipocyte released circulating leptin levels (108, 109). Signaling via α2-AR involves Gi-coupling and is best known to counteract Gq-coupled β-AR signaling via adenylate cyclase. However, all β-AR receptors can also switch to an alternative Gi-coupling and engage an alternative MAPK signaling pathway that paradoxically results in enhanced thermogenic function (110).
Another recent study found that β2-AR are the main thermogenic receptor in human adipocytes, with comparable function to β3-AR in mouse adipocytes (111). Similarly, a recent study showed the importance of β2-AR signaling to increase sympathetic excitability and its role for thermogenesis and adipose tissue beiging (84). Future studies need to re-address tissue-specific functions of adrenergic receptor subtypes and address possible confounding effects of germline deletion in contrast to inducible deletion systems.
Adipokine Feedback and Cross Talk
Adipokines (including leptin and adiponectin) effect other hormones like glucocorticoids and insulin/glucagon levels provides feedback signals to the hypothalamus (29, 31, 48, 49, 112) and cross talk with other tissues (2, 113, 114). (FIGURE 2, bullets 7 and 8). The sympathetic regulation of adipose tissue is required for leptin gene expression and secretion and similarly leptin regulates sympathetic innervation density in iBAT and iWAT and iWAT beiging (29–31). The implementation of optogenetic and chemogenetic technologies to directly stimulate and inhibit sympathetic nerves to select adipose tissue depots could greatly facilitate our understanding of the neuro-adipocyte interface and adipokine mobilization. First studies used optogenetic activation of sympathetic nerve endings in iBAT and showed the expected increase in BAT temperature and glucose uptake (115, 116), in contrast to brown adipocyte specific β3-AR signaling, which was unable to increase thermogenesis (95). Also, select optogenetic activation of iWAT was able to induce lipolysis (117). However, effects on adipokines, iWAT beiging, and innervation changes were not investigated in this study. It should be cautioned that conditional strategies to target the peripheral nervous system require thorough characterization of marker genes to ensure proper expression profiles. For example, tyrosine hydroxylase is an excellent marker to distinguish sympathetic from parasympathetic neurons in adult mice; however, its transient expression in parasympathetic precursors during development (118) makes it unsuitable to distinguish these peripheral neurons by germline procedures (33) and will require manipulation strategies in adult animals for proper targeting. Further progress of these technologies is also a prospective avenue to further understand the importance of sensory nerves in the communication of adipocytes and CNS as suggested earlier (119–121).
Adipose Tissue Innervation and Sex Differences
The central nervous system is well acknowledged to harbor several sites and neuronal populations with pronounced sex differences and differential expression of estrogen and androgen receptors (26, 122, 123). POMC neurons, which have profound effects on adipose tissue beiging (29, 31), are also regulated by sex hormones during early life and regulate the number and innervation density of POMC neurons (124), metabolism, insulin sensitivity, and body weight in adult mice (122, 125), even though adipose tissue beiging and innervation were not directly assessed in these experiments. Similarly, sex-specific obesity phenotypes are also mediated by adipokine feedback via vagal afferents where effects on adipose tissue must be mediated indirectly by peripheral tissue cross talk (114, 126).
In line with this, sex differences are well known for adipose tissue distribution in humans with more subcutaneous, but less visceral, adipose tissue in women and increased genetic markers for beiging were found in visceral abdominal fat in women compared to men (127), even though another study using PET scan did not show differences in beige/BAT based on gender (128). These studies did not look for sympathetic innervation and the role of melanocortins in human adipose tissue, and beiging has not been confirmed so far. Furthermore, in mice sex hormones act directly on adipocytes to enhance adipose tissue beiging (129), further supporting that sex-dependent effects on adipose tissue beiging are mediated via multiple axes, including central and peripheral routes.
Summary and Outlook
Despite these clear interactions of innervation, adipose tissue function, and body weight, it is notable that the mere denervation of BAT or WAT does not result in frank obesity (68), even though adipose tissue weight increases (130). Robust states of adipose tissue beiging, do not necessarily result in measurable metabolic enhancement in mice (103, 131) or weight loss in humans (106, 132). The mixed outcomes for brown/beige adipose tissue and weight loss have reengaged the discussion if adipose tissue is a viable target for body weight control (10, 133).
In this review we highlighted the many layers that contribute to proper and dynamic regulation of adipose tissue innervation, regulation of sympathetic tone, and bioavailability of norepinephrine for adipocyte function. The provided model for the interaction of neuronal and peripheral signaling emphasizes that manipulations at any level will cause similar changes in body weight and that future studies should address the interface of neuron/adipocyte interaction in more detail. Given the historic difficulty to treat obesity safely and over the long term, targeting the peripheral interface will likely be a meaningful and safer target for pharmacological intervention.
Furthermore, we have outlined that the differential sympathetic activation of iBAT versus iWAT and the importance of acute and chronic changes in energy expenditure and beiging is not well characterized. Further progress using viral molecular-genetic tools in the peripheral nervous system will hopefully move the field forward to address these important questions.
Finally, we note that the role of sex hormones in the neuron-adipocyte interface can be integrated in the reviewed model. However, sex differences in adipose tissue distribution are not fully explained, but working toward a common model of a neuro-adipose interface to capture experimental modulations at the level of sex hormones, immune function, as well as obesity and cachexia is an important step for meaningful strategies to treat human illness.
Acknowledgments
This work was in part supported by National Institutes of Health Grants 2R01-DK-092587-06 and 4OT2-OD-23864-01 (to H. M.), P50-AT-002776 (to Z.E.F.), and 1R01-DK-104748 (to J.S.C.).
No conflicts of interest, financial or otherwise, are declared by the authors.
H.M. prepared figures; H.M., E.F., and J.S.C. drafted manuscript; H.M., E.F., and J.S.C. edited and revised manuscript; H.M., E.F., and J.S.C. approved final version of manuscript.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Funcke JB, Scherer PE. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res 60: 1648–1684, 2019. doi: 10.1194/jlr.R094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruder-Nascimento T, Kress TC, Belin de Chantemele EJ. Recent advances in understanding lipodystrophy: a focus on lipodystrophy-associated cardiovascular disease and potential effects of leptin therapy on cardiovascular function. F1000Res 8: 1756, 2019. doi: 10.12688/f1000research.20150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann JP, Savage DB. What lipodystrophies teach us about the metabolic syndrome. J Clin Invest 129: 4009–4021, 2019. doi: 10.1172/JCI129190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL. The importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology (Basel) 8: 10, 2019. doi: 10.3390/biology8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthoud HR, Fox EA, Neuhuber WL. Vagaries of adipose tissue innervation. Am J Physiol Regul Integr Comp Physiol 291: R1240–R1242, 2006. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- 7.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931–942, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auffret J, Viengchareun S, Carre N, Denis RG, Magnan C, Marie PY, Muscat A, Feve B, Lombes M, Binart N. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J 26: 3728–3737, 2012. doi: 10.1096/fj.12-204958. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Verdejo R, Marlatt KL, Ravussin E, Galgani JE. Contribution of brown adipose tissue to human energy metabolism. Mol Aspects Med 68: 82–89, 2019. doi: 10.1016/j.mam.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110: 515–526, 2002. doi: 10.1016/S0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 12.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 13.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 289: R1467–R1476, 2005. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 14.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295: R417–R428, 2008. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster MT, Song CK, Bartness TJ. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 18: 682–689, 2010. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Yanagawa Y, Morrison SF, Nakamura K. Medullary reticular neurons mediate neuropeptide Y-induced metabolic inhibition and mastication. Cell Metab 25: 322–334, 2017. doi: 10.1016/j.cmet.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu S, Francois M, Huesing C, Munzberg H. The hypothalamic preoptic area and body weight control. Neuroendocrinology 106: 187–194, 2018. doi: 10.1159/000479875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden CJ, Morrison SF. Central nervous system circuits that control body temperature. Neurosci Lett 696: 225–232, 2019. doi: 10.1016/j.neulet.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Bi S. Effects of physical exercise on food intake and body weight: role of dorsomedial hypothalamic signaling. Physiol Behav 192: 59–63, 2018. doi: 10.1016/j.physbeh.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Machado NL, Bandaru SS, Abbott SB, Saper CB. EP3R-expressing glutamatergic preoptic neurons mediate inflammatory fever. J Neurosci 40: 2573–2588, 2020. doi: 10.1523/JNEUROSCI.2887-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol 312: R132–R145, 2017. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munzberg H, Singh P, Heymsfield SB, Yu S, Morrison CD. Recent advances in understanding the role of leptin in energy homeostasis. F1000Res 9: 451, 2020. doi: 10.12688/f1000research.24260.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, Hnasko TS, Ingraham HA. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol Metab 4: 857–866, 2015. doi: 10.1016/j.molmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause WC, Ingraham HA. Origins and functions of the ventrolateral VMH: a complex neuronal cluster orchestrating sex differences in metabolism and behavior. Adv Exp Med Biol 1043: 199–213, 2017. doi: 10.1007/978-3-319-70178-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10: 62–74, 2015. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Veen JE, Kammel LG, Bunda PC, Shum M, Reid MS, Massa MG, Arneson D, Park JW, Zhang Z, Joseph AM, Hrncir H, Liesa M, Arnold AP, Yang X, Correa SM. Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat Metab 2: 351–363, 2020. doi: 10.1038/s42255-020-0189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab 13: 573–583, 2011. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Loh KH, Wu M, Morgan DA, Schneeberger M, Yu X, Chi J, Kosse C, Kim D, Rahmouni K, Cohen P, Friedman J. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 583: 839–844, 2020. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- 30.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6: 431–445, 2007. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160: 88–104, 2015. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francois M, Torres H, Huesing C, Zhang R, Saurage C, Lee N, Qualls-Creekmore E, Yu S, Morrison CD, Burk D, Berthoud HR, Munzberg H. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann N Y Acad Sci 1454: 3–13, 2019. doi: 10.1111/nyas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huesing C, Qualls-Creekmore E, Lee N, Francois M, Torres H, Zhang R, Burk DH, Yu S, Morrison CD, Berthoud HR, Neuhuber W, Munzberg H. Sympathetic innervation of inguinal white adipose tissue in the mouse. J Comp Neurol 529: 1465–1485, 2021. doi: 10.1002/cne.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151: 645–657, 2012. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern JE. Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides. J Neuroendocrinol 27: 487–497, 2015. doi: 10.1111/jne.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998. doi: 10.1016/S0896-6273(00)80656-X. doi:. [DOI] [PubMed] [Google Scholar]
- 37.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 19: 3171–3182, 1999. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 39.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 40.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 41.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 612–619, 2013. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berglund ED, Liu T, Kong X, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, Scherer PE, Lowell BB, Elmquist JK. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci 17: 911–913, 2014. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trayhurn P, Duncan JS, Rayner DV. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J 311: 729–733, 1995. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (oblob) mice. FEBS Lett 368: 488–490, 1995. doi: 10.1016/0014-5793(95)00719-P. doi:. [DOI] [PubMed] [Google Scholar]
- 45.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter R, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology 140: 4772–4778, 1999. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 46.Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J Biol Chem 275: 33059–33067, 2000. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- 47.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci 26: 241–245, 2006. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry RJ, Resch JM, Douglass AM, Madara JC, Rabin-Court A, Kucukdereli H, Wu C, Song JD, Lowell BB, Shulman GI. Leptin's hunger-suppressing effects are mediated by the hypothalamic-pituitary-adrenocortical axis in rodents. Proc Natl Acad Sci U S A 116: 13670–13679, 2019. doi: 10.1073/pnas.1901795116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry RJ, Lyu K, Rabin-Court A, Dong J, Li X, Yang Y, Qing H, Wang A, Yang X, Shulman GI. Leptin mediates postprandial increases in body temperature through hypothalamus-adrenal medulla-adipose tissue crosstalk. J Clin Invest 130: 2001–2016, 2020. doi: 10.1172/JCI134699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab 16: 144–152, 2012. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Bell BB, Harlan SM, Morgan DA, Guo DF, Cui H, Rahmouni K. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol Metab 8: 1–12, 2018. doi: 10.1016/j.molmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344, 2013. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15: 480–491, 2012. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29: 286–299, 2015. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab 1: 189–200, 2019. doi: 10.1038/s42255-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kir S, Spiegelman BM. Cachexia & brown fat: a burning issue in cancer. Trends Cancer 2: 461–463, 2016. doi: 10.1016/j.trecan.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alipoor E, Hosseinzadeh-Attar MJ, Rezaei M, Jazayeri S, Chapman M. White adipose tissue browning in critical illness: a review of the evidence, mechanisms and future perspectives. Obes Rev 21: e13085, 2020. doi: 10.1111/obr.13085. [DOI] [PubMed] [Google Scholar]
- 58.Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, Gupta RK. Cellular origins of beige fat cells revisited. Diabetes 68: 1874–1885, 2019. doi: 10.2337/db19-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol 15: 507–524, 2019. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 60.Martin SL. Mammalian hibernation: a naturally reversible model for insulin resistance in man? Diab Vasc Dis Res 5: 76–81, 2008. doi: 10.3132/dvdr.2008.013. [DOI] [PubMed] [Google Scholar]
- 61.Rigano KS, Gehring JL, Evans HB, Chen AV, Nelson OL, Vella CA, Robbins CT, Jansen HT. Life in the fat lane: seasonal regulation of insulin sensitivity, food intake, and adipose biology in brown bears. J Comp Physiol B 187: 649–676, 2017. doi: 10.1007/s00360-016-1050-9. [DOI] [PubMed] [Google Scholar]
- 62.Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr Rev 39: 261–273, 2018. doi: 10.1210/er.2017-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chi J, Wu Z, Choi CH, Nguyen L, Tegegne S, Ackerman SE, Crane A, Marchildon F, Tessier-Lavigne M, Cohen P. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab 27: 226–236, 2018.doi: 10.1016/j.cmet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 214: 171–178, 2009. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barreau C, Labit E, Guissard C, Rouquette J, Boizeau ML, Gani Koumassi S, Carriere A, Jeanson Y, Berger-Muller S, Dromard C, Plouraboue F, Casteilla L, Lorsignol A. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity (Silver Spring) 24: 1081–1089, 2016. doi: 10.1002/oby.21455. [DOI] [PubMed] [Google Scholar]
- 66.Umahara Y. Light and electron microscopic studies on the brown adipose tissue in the bat. Arch Histol Jpn 29: 459–509, 1968. doi: 10.1679/aohc1950.29.459. [DOI] [PubMed] [Google Scholar]
- 67.Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15: 659–667, 2013. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 68.Cao Q, Jing J, Cui X, Shi H, Xue B. Sympathetic nerve innervation is required for beigeing in white fat. Physiol Rep 7: e14031, 2019. doi: 10.14814/phy2.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF, Penicaud L. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 133: 2255–2262, 1993. doi: 10.1210/endo.133.5.8404678. [DOI] [PubMed] [Google Scholar]
- 70.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol 291: R1630–R1637, 2006. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 71.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28: 191–222, 2005. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 72.Thiede-Stan NK, Schwab ME. Attractive and repulsive factors act through multi-subunit receptor complexes to regulate nerve fiber growth. J Cell Sci 128: 2403–2414, 2015. doi: 10.1242/jcs.165555. [DOI] [PubMed] [Google Scholar]
- 73.Kim SN, Jung YS, Kwon HJ, Seong JK, Granneman JG, Lee YH. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol Sex Differ 7: 67, 2016. doi: 10.1186/s13293-016-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peeraully MR, Jenkins JR, Trayhurn P. NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab 287: E331–E339, 2004. doi: 10.1152/ajpendo.00076.2004. [DOI] [PubMed] [Google Scholar]
- 75.Cao Y, Wang H, Zeng W. Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 9: 527–539, 2018. doi: 10.1007/s13238-018-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang H, Ding X, Cao Y, Wang H, Zeng W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab 26: 686–692, 2017. doi: 10.1016/j.cmet.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci 2: 699–705, 1999. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 78.Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM. Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature 569: 229–235, 2019. doi: 10.1038/s41586-019-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellegrinelli V, Peirce VJ, Howard L, Virtue S, Turei D, Senzacqua M, Frontini A, Dalley JW, Horton AR, Bidault G, Severi I, Whittle A, Rahmouni K, Saez-Rodriguez J, Cinti S, Davies AM, Vidal-Puig A. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat Commun 9: 4974, 2018. doi: 10.1038/s41467-018-07453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bal NC, Singh S, Reis FC, Maurya SK, Pani S, Rowland LA, Periasamy M. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem 292: 16616–16625, 2017. doi: 10.1074/jbc.M117.790451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110, 2004. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 82.Bouret SG, Simerly RB. Development of leptin-sensitive circuits. J Neuroendocrinol 19: 575–582, 2007. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 83.Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol 27: 479–496, 2013. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahu I, Barateiro A, Rial-Pensado E, Martinez-Sanchez N, Vaz SH, Cal P, Jenkins B, Rodrigues T, Cordeiro C, Costa MF, Mendes R, Seixas E, Pereira MM, Kubasova N, Gres V, Morris I, Temporao C, Olivares M, Sanz Y, Koulman A, Corzana F, Sebastiao AM, Lopez M, Bernardes GJ, Domingos AI. Brain-sparing sympathofacilitators mitigate obesity without adverse cardiovascular effects. Cell Metab 31: 1120–1135, 2020. doi: 10.1016/j.cmet.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, Rodeheffer MS, Schultze JL, Dixit VD. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550: 119–123, 2017. doi: 10.1038/nature24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, Mendes R, Gres V, Kubasova N, Morris I, Arus BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23: 1309–1318, 2017. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camell CD, Gunther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, Schultze J, Dixit VD. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab 30: 1024–1039, 2019. doi: 10.1016/j.cmet.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao H, Arner P, Beauchef G, Guere C, Vie K, Dahlman I, Mejhert N, Ryden M. Age-induced reduction in human lipolysis: a potential role for adipocyte noradrenaline degradation. Cell Metab 32: 1–3, 2020. doi: 10.1016/j.cmet.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Granneman JG, Lahners KN, Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol 40: 895–899, 1991. [PubMed] [Google Scholar]
- 90.Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem 266: 24053–24058, 1991. doi: 10.1016/S0021-9258(18)54391-X. [DOI] [PubMed] [Google Scholar]
- 91.Nahmias C, Blin N, Elalouf JM, Mattei MG, Strosberg AD, Emorine LJ. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J 10: 3721–3727, 1991. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Albert V, Svensson K, Shimobayashi M, Colombi M, Munoz S, Jimenez V, Handschin C, Bosch F, Hall MN. mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol Med 8: 232–246, 2016. doi: 10.15252/emmm.201505610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olsen JM, Aslund A, Bokhari MH, Hutchinson DS, Bengtsson T. Acute beta-adrenoceptor mediated glucose clearance in brown adipose tissue; a distinct pathway independent of functional insulin signaling. Mol Metab 30: 240–249, 2019. doi: 10.1016/j.molmet.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem 270: 29483–29492, 1995. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 95.Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem 272: 17686–17693, 1997. doi: 10.1074/jbc.272.28.17686. [DOI] [PubMed] [Google Scholar]
- 96.Himms-Hagen J, Cui J, Danforth E Jr., Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol 266: R1371–1382, 1994. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 97.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab 289: E608–E616, 2005. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 98.Chang JS, Fernand V, Zhang Y, Shin J, Jun HJ, Joshi Y, Gettys TW. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. J Biol Chem 287: 9100–9111, 2012. doi: 10.1074/jbc.M111.320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. J Lipid Res 55: 2276–2286, 2014. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J, Park MS, Ha K, Park C, Lee J, Mynatt RL, Chang JS. NT-PGC-1alpha deficiency decreases mitochondrial FA oxidation in brown adipose tissue and alters substrate utilization in vivo. J Lipid Res 59: 1660–1670, 2018. doi: 10.1194/jlr.M085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, Kolb D, Hoeks J, Kershaw EE, Sedej S, Schrauwen P, Haemmerle G, Zechner R. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26: 753–763, 2017. doi: 10.1016/j.cmet.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AK, Jackson R, Rumore D, Xue B, Shi H, Gavrilova O, Yu L. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab 26: 764–777, 2017. doi: 10.1016/j.cmet.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Labbe SM, Caron A, Festuccia WT, Lecomte R, Richard D. Interscapular brown adipose tissue denervation does not promote the oxidative activity of inguinal white adipose tissue in male mice. Am J Physiol Endocrinol Metab 315: E815–E824, 2018. doi: 10.1152/ajpendo.00210.2018. [DOI] [PubMed] [Google Scholar]
- 104.Guilherme A, Pedersen DJ, Henriques F, Bedard AH, Henchey E, Kelly M, Morgan DA, Rahmouni K, Czech MP. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Mol Metab 16: 116–125, 2018. doi: 10.1016/j.molmet.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 106.O'Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, Cero C, Sater ZA, Piccinini F, Baskin AS, Leitner BP, Cai H, Millo CM, Dieckmann W, Walter M, Javitt NB, Rotman Y, Walter PJ, Ader M, Bergman RN, Herscovitch P, Chen KY, Cypess AM. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest 130: 2209–2219, 2020. doi: 10.1172/JCI131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Ikeda K, Yoneshiro T, Scaramozza A, Tajima K, Wang Q, Kim K, Shinoda K, Sponton CH, Brown Z, Brack A, Kajimura S. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565: 180–185, 2019. doi: 10.1038/s41586-018-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caron A, Dungan Lemko HM, Castorena CM, Fujikawa T, Lee S, Lord CC, Ahmed N, Lee CE, Holland WL, Liu C, Elmquist JK. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife 7: e33710, 2018. doi: 10.7554/eLife.33710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caron A, Reynolds RP, Castorena CM, Michael NJ, Lee CE, Lee S, Berdeaux R, Scherer PE, Elmquist JK. Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Mol Metab 27: 11–21, 2019. doi: 10.1016/j.molmet.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soeder KJ, Snedden SK, Cao W, Della Rocca GJ, Daniel KW, Luttrell LM, Collins S. The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J Biol Chem 274: 12017–12022, 1999. doi: 10.1074/jbc.274.17.12017. [DOI] [PubMed] [Google Scholar]
- 111.Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, Phoenix S, Frisch F, Guerin B, Turcotte EE, Haman F, Richard D, Picard F, Rensen PC, Scheele C, Carpentier AC. Human brown adipocyte thermogenesis is driven by beta2-AR stimulation. Cell Metab 32: 287–300, 2020. doi: 10.1016/j.cmet.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Kleinert M, Sachs S, Habegger KM, Hofmann SM, Muller TD. Glucagon regulation of energy expenditure. Int J Mol Sci 20: 5407, 2019. doi: 10.3390/ijms20215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou P, Santoro A, Peroni OD, Nelson AT, Saghatelian A, Siegel D, Kahn BB. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J Clin Invest 129: 4138–4150, 2019. doi: 10.1172/JCI127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang KP, Goodson ML, Vang W, Li H, Page AJ, Raybould HE. Leptin signaling in vagal afferent neurons supports the absorption and storage of nutrients from high-fat diet. Int J Obes 45: 348–357, 2021. doi: 10.1038/s41366-020-00678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeong JH, Chang JS, Jo YH. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci Rep 8: 6672, 2018. doi: 10.1038/s41598-018-25265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lyons CE, Razzoli M, Larson E, Svedberg D, Frontini A, Cinti S, Vulchanova L, Sanders M, Thomas M, Bartolomucci A. Optogenetic-induced sympathetic neuromodulation of brown adipose tissue thermogenesis. FASEB J 34: 2765–2773, 2020. doi: 10.1096/fj.201901361RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163: 84–94, 2015. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol 277: 271–286, 2005. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 119.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 537: 199–225, 2014. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Navarro G, Allard C, Morford JJ, Xu W, Liu S, Molinas AJ, Butcher SM, Fine NH, Blandino-Rosano M, Sure VN, Yu S, Zhang R, Munzberg H, Jacobson DA, Katakam PV, Hodson DJ, Bernal-Mizrachi E, Zsombok A, Mauvais-Jarvis F. Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3: e98607, 2018. doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morford JJ, Wu S, Mauvais-Jarvis F. The impact of androgen actions in neurons on metabolic health and disease. Mol Cell Endocrinol 465: 92–102, 2018. doi: 10.1016/j.mce.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H, Mauvais-Jarvis F. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology 152: 1661–1669, 2011. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nohara K, Laque A, Allard C, Munzberg H, Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring) 22: 1477–1484, 2014. doi: 10.1002/oby.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang KP, Ronveaux CC, de Lartigue G, Geary N, Asarian L, Raybould HE. Deletion of leptin receptors in vagal afferent neurons disrupts estrogen signaling, body weight, food intake and hormonal controls of feeding in female mice. Am J Physiol Endocrinol Metab 316: E568–E577, 2019. doi: 10.1152/ajpendo.00296.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barquissau V, Leger B, Beuzelin D, Martins F, Amri EZ, Pisani DF, Saris WH, Astrup A, Maoret JJ, Iacovoni J, Dejean S, Moro C, Viguerie N, Langin D. Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Rep 22: 1079–1089, 2018. doi: 10.1016/j.celrep.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 128.Fletcher LA, Kim K, Leitner BP, Cassimatis TM, O'Mara AE, Johnson JW, Halprin MS, McGehee SM, Brychta RJ, Cypess AM, Chen KY. Sexual dimorphisms in adult human brown adipose tissue. Obesity (Silver Spring) 28: 241–246, 2020. doi: 10.1002/oby.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos RS, Frank AP, Fatima LA, Palmer BF, Oz OK, Clegg DJ. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol Metab 18: 51–59, 2018. doi: 10.1016/j.molmet.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Demas GE, Bartness TJ. Novel method for localized, functional sympathetic nervous system denervation of peripheral tissue using guanethidine. J Neurosci Methods 112: 21–28, 2001. doi: 10.1016/S0165-0270(01)00452-6. doi:. [DOI] [PubMed] [Google Scholar]
- 131.Labbe SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058, 2015. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 132.Chen KY, Brychta RJ, Abdul Sater Z, Cassimatis TM, Cero C, Fletcher LA, Israni NS, Johnson JW, Lea HJ, Linderman JD, O'Mara AE, Zhu KY, Cypess AM. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J Biol Chem 295: 1926–1942, 2020. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marlatt KL, Ravussin E. Brown adipose tissue: an update on recent findings. Curr Obes Rep 6: 389–396, 2017. doi: 10.1007/s13679-017-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


