Keywords: breathing, neuromodulation, OIRD, opioid, respiration
Abstract
Opioid-induced respiratory depression (OIRD) represents the primary cause of death associated with therapeutic and recreational opioid use. Within the United States, the rate of death from opioid abuse since the early 1990s has grown disproportionally, prompting the classification as a nationwide “epidemic.” Since this time, we have begun to unravel many fundamental cellular and systems-level mechanisms associated with opioid-related death. However, factors such as individual vulnerability, neuromodulatory compensation, and redundancy of opioid effects across central and peripheral nervous systems have created a barrier to a concise, integrative view of OIRD. Within this review, we bring together multiple perspectives in the field of OIRD to create an overarching viewpoint of what we know, and where we view this essential topic of research going forward into the future.
INTRODUCTION
In the late 1990s, a large increase in prescriptions for opioid-based medications spurred by misunderstanding and a lack of transparency between pharmaceutical companies and the medical community led to a rapid expansion of opioid abuse. From 2011 to 2018, the rate of overdoses involving prescription opioids began to plateau (https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates); however, the rate of heroin overdose more than quadrupled, whereas the overdose rate from synthetic opioids (other than methadone), such as fentanyl, increased ∼×9 during the same time frame (1–3). In 2016, two-thirds of all drug overdoses involved opioids (4), and in 2017, the “opioid epidemic” was deemed a public health emergency in the United States. The opioid epidemic continues to claim the lives of an estimated 130 people per day in the United States (https://www.hhs.gov/opioids/about-the-epidemic/index.html), and new, relatively easy to synthesize, opioid drugs are reaching the streets with ever increasing potencies.
The primary cause of death associated with opioid-based analgesics and drugs of abuse is opioid-induced respiratory depression (OIRD). Although on average the risk of mortality associated with opioid use increases in a dose-dependent manner, no amount of opioid is without risk (5–7). Within and among individuals, variability in the response to opioids makes these drugs particularly dangerous and is a major contributor to overdose. One factor underlying variability in OIRD is that the respiratory response to opioids is state dependent. Opioid sensitivity is heightened during different neurological and physiological states including concurrent drug administration, such as anesthesia (8–10) and benzodiazepines (11), as well as sleep disordered breathing (SDB, Ref. 12). Indeed, SDB is prevalent among opioid users (13–15), and this clinical population is particularly vulnerable to OIRD since hypercapnic (16, 17) and hypoxic (18, 19) conditions present during SDB exaggerate opioid responses. Although numerous studies using animal models including mice, rats, dogs, cats, rabbits, and goats (5, 20–28) suggest that variability is a hallmark of OIRD, this variability is often overlooked, poorly understood, and has proven to be a significant barrier to forming a consensus on the specific causes and prevention of OIRD.
This review brings together multiple research teams to discuss the current state of knowledge regarding key brain regions and mechanisms responsible for OIRD. Breathing is a highly complex and integrated behavior (29–32), and there is growing evidence that its underlying rhythmogenic and modulatory networks are highly dynamic and plastic (30, 33–36). As a result, unraveling the underlying cause of OIRD is not a simple task. Although there is general consensus that the breathing rhythm itself is generated within the brainstem (32), numerous regions and modulatory systems distributed throughout the central nervous system modulate breathing (37), and breathing in turn affects many areas distributed throughout the brain (31, 38). Breathing is also highly regulated by chemo- and mechano-feedback mechanisms that are critical for maintaining homeostasis. The respiratory response to opioids is similarly complex since OIRD ultimately emerges from the combined effects of opioids on mechanisms of respiratory rhythm generation, modulation, and sensory feedback (Fig. 1) at sites distributed throughout the nervous system. Here, we highlight key respiratory centers in the brainstem (Fig. 1), as well as cortical regions (Fig. 6) and components of the peripheral nervous system (PNS), that are strongly implicated in the failure or impairment of breathing due to OIRD. In addition, we emphasize that the variability and state-dependence of OIRD likely reflects the dynamic nature of the mechanisms that generate and regulate breathing (30). By embracing this variability, we hope to better understand how opioids affect these dynamic mechanisms to more effectively identify risk factors for, and develop treatments and countermeasures against, OIRD.
Figure 1.
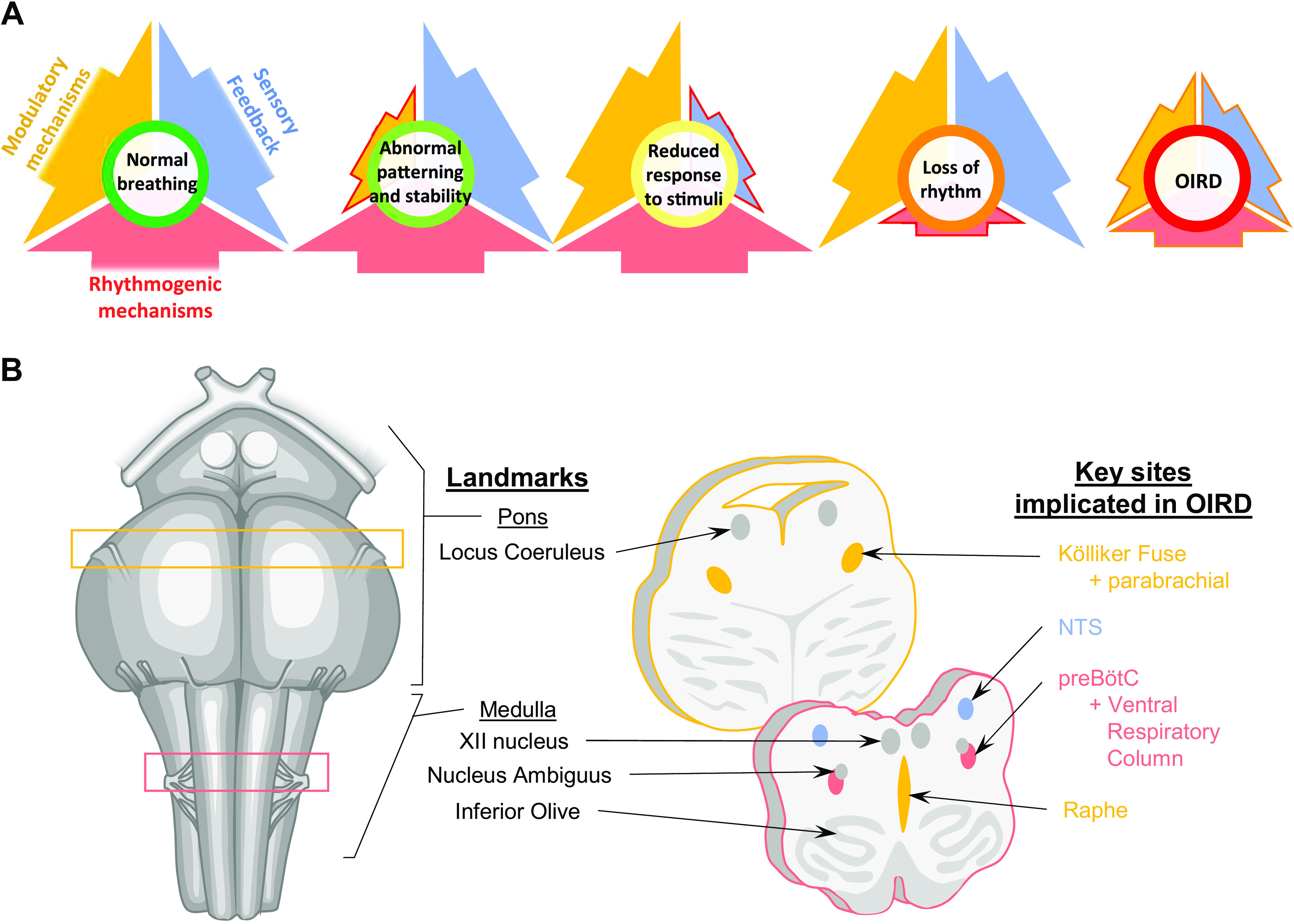
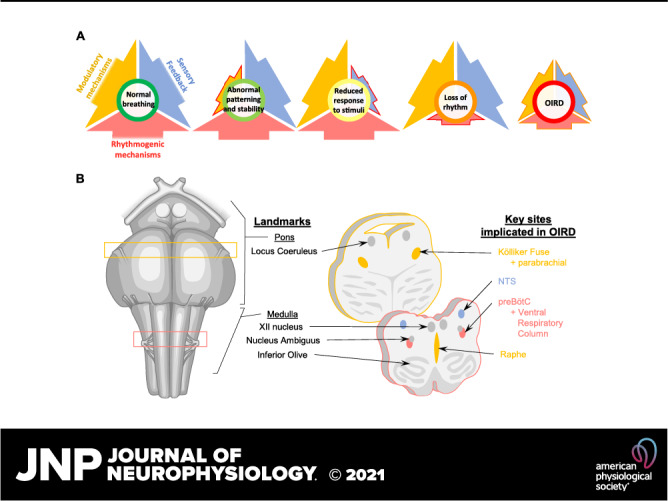
A: normal breathing requires the integration of rhythmogenic, modulatory, and sensory feedback mechanisms. Opioid overdose can suppress all of these important mechanisms of respiratory control, leading to OIRD. B: human brainstem schematic illustrating key sites in the pons and medulla that mediate OIRD. Ventral view is shown on the left, and transverse sections are shown on the right. Colors in B correspond to mechanisms depicted in A. NTS, nucleus tractus solitarius; OIRD, opioid-induced respiratory depression; preBOTc, preBӧtzinger complex.
MEDULLARY NETWORKS
It is widely accepted that the respiratory rhythm emerges from a highly interconnected brainstem network located within the medulla (39–52). Neurons within this wider respiratory network are synchronized to generate the specific phases and patterns of breathing (42, 45, 46, 49, 50, 53–55). For example, synchrony critically contributes to network stability, rhythmogenesis, and transmission of motor drive to inspiratory muscles (56–58). One manifestation of synchrony is high frequency oscillations (HFOs) (50–100 Hz) (59) (i.e., periodicities) present in neurograms and neuron firing rates (60). Morphine administration depressed HFOs to lower frequencies (61), consistent with disruption of inspiratory network synchrony. However, HFOs can be nonspecific metrics of overall circuit behavior. Lalley and Mifflin (62) showed similar effects of the opioid fentanyl on autocorrelations of respiratory neurons (61), which can show oscillations due to HFOs These studies support the concept that synchrony in the brainstem inspiratory circuit is sensitive to opioids. Interestingly, the magnitude of coherence between right and left phrenic nerves was not changed by morphine (61), suggesting that crossed pathways at the brainstem and spinal level were not altered by this drug.
Opioids can change discharge identity (respiratory phenotype) of neurons that participate in the respiratory network. Existing evidence is consistent with significant alterations in discharge patterns of breathing- and nonbreathing-modulated (NBM) neurons in the ventral respiratory column (VRC). This is illustrated in the respiratory cycle-triggered histograms before and after intra-arterial administration of the µ-opioid receptor agonist, codeine, as shown in Fig. 2. Seven examples of NBM, expiratory-tonic (E-T), and inspiratory-tonic (I-T) neurons are shown. In the first three examples, NBM neurons that were not modulated during breathing became respiratory-modulated (Fig. 2, 1–3). In two cases, inspiratory or expiratory neurons changed their main phase of activation during breathing (Fig. 2, 4 and 5). In most cases, the baseline discharge rate of these neurons increased or decreased. These changes are consistent with altered discharge identity of these neurons. Discharge identity is the activity pattern of a given neuron relative to the breathing cycle (63).
Figure 2.
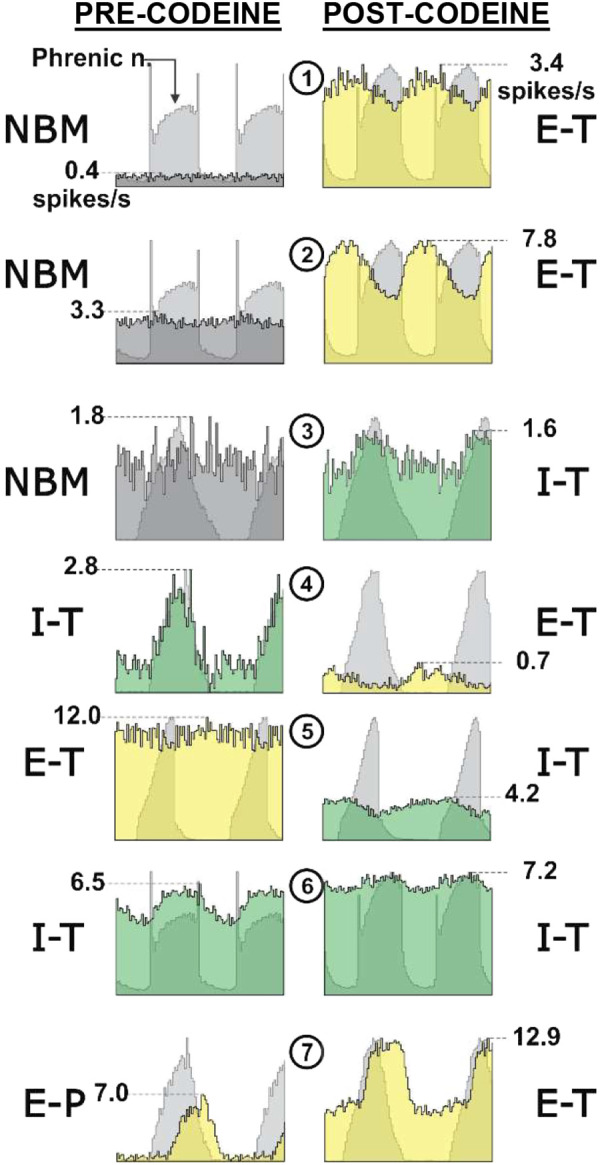
Examples of neurons with altered discharge identity or discharge rate after codeine administration from several animals. Several of the neurons shown became breathing modulated (examples 1–3). In others, the time of maximum discharge rate during the respiratory cycle changed (4 and 5). Discharge rates of most of the neurons shown increased (1, 2, and 7) or decreased (4 and 5) after codeine. Cycle-triggered histograms of neuronal activity (colors) overlying phrenic discharge (gray). E-P, expiratory phasic; E-T, expiratory tonic; I-T, inspiratory tonic; n, neuron; NBM, nonbreathing modulated. Anesthetized, paralyzed, and artificially ventilated cats. Codeine (1–3,000 µg/kg) was administered via the vertebral artery.
Alterations in discharge identity in the respiratory network may affect respiratory rhythmogenesis and pattern formation, since these alterations can result in differing temporal delivery of postsynaptic potentials to their targets. The timing of arrival of synaptic volleys has an important role in excitability and phase switching in the respiratory network (64). Even subtle changes to these volleys from multiple presynaptic terminals would alter the spatial and temporal summation of PSPs and thus could greatly alter the dynamic membrane potential of the target neuron. Some neurons in the breathing network can change discharge identity during non-breathing behaviors, such as cough, and this collective process has been termed reconfiguration (39, 52, 65, 66). Reconfiguration is hypothesized to contribute to large changes in motor patterns of respiratory muscles that are necessary for execution of ballistic-like behaviors (65–67). It is conceivable that opioids, in addition to altering the firing rates of respiratory neurons, induce far reaching changes in the organization of the respiratory network through reconfiguration. This proposed opioid-induced reconfiguration represents a higher scale effect of these drugs than at individual cells and integrates alterations in the excitability of individual members of the entire network into an aggregate response that determines behavior. Given that the network is a higher-level scale than that of individual cells, it can be hypothesized that unique opioid-sensitive mechanisms exist that are not easily detectable at the cellular scale (67). As noted above, there is evidence that network synchrony, a mechanism that is a feature of network behavior, is sensitive to opioids (61). Unraveling the mechanisms underlying synchrony is an evolving research field and further investigation will reveal additional manifestations of the effects of opioids at the network scale. Discovery of these proposed additional mechanisms will lead to a more comprehensive, multiscale hypothesis for the mechanisms of opioid-induced respiratory depression.
Rhythmogenetic Networks within the Medulla
Although neurons within the medullary network are highly connected and interacting, these neurons are not evenly distributed, and anatomically distinct, yet interconnected rhythmogenic brainstem microcircuits can be identified. These microcircuits are capable of maintaining respiratory rhythmic activity even when isolated from the remaining respiratory network (68, 69). So far, three such microcircuits have been identified: the preBӧtzinger Complex (preBӧtC), which generates inspiration (70); the retrotrapezoid/parafacial nucleus (RTN/pFRG) critical for active expiration (71–73) and sensing hypercapnia (74–76); and the postinspiratory complex (PiCo) (77) associated with postinspiratory activity. These networks receive descending inputs from various areas including the Kölliker-Fuse/Parabrachial (KF) nuclei which exert significant additional regulatory control. As will be discussed later in this review, lesions of this region result in apneustic breathing characterized by prolonged inspirations and extended expiratory pauses (78). Respiratory activity in each of these brainstem centers, with the exception of RTN/pFRG, is depressed by opioids. However, so far only the preBӧtC has been demonstrated to be essential for in vivo breathing and survival in mouse models (79–82), but see Lalley et al. (83), making this region a critical focus for understanding the depressive action of opioids on respiratory motor generation.
The PreBötzinger Complex
Although multiple brainstem sites are involved in OIRD (11), the preBötzinger Complex (preBötC) is particularly important, because inhibiting this medullary region in vivo leads to respiratory failure (84, 85). The mouse preBötC microcircuit is composed of ∼800–1,000 glutamatergic neurons bilaterally distributed to two ventrolateral nuclei (32, 69), which are part of the larger ventral respiratory group (VRG) column composed of the Bӧtzinger complex (BC) (rostral to the preBӧtC), the rostral ventral respiratory group (rVRG), and the caudal ventral respiratory group (cVRG) (progressively more caudal to the preBӧtC). The transiently expressed embryonic homeobox transcription factor Dbx1 provides a useful molecular marker for a significant fraction of these glutamatergic neurons in Cre/loxP transgenic mouse lines (86). However, additional molecular markers may also define other nonoverlapping subsets of these excitatory neurons (87–89). Based on computational models, these neurons are thought to be connected in a sparse network composed of multiple hubs, each enriched in local short-range synaptic interactions (90–93). Because rhythmic activity persists following pharmacological blockade of inhibitory transmission (GABAergic and glycinergic), it has been concluded that inspiratory rhythmogenesis primarily depends upon excitatory glutamatergic transmission (94, 95). However, inhibitory transmission during both inspiratory and expiratory phases of the rhythm plays a critical role in controlling respiratory frequency, and shaping the amplitude and response dynamics of the respiratory rhythm (36, 96).
Inspiratory bursts emanating from the preBötC are highly synchronous rhythmic bursts generated over a substantial fraction of neurons in the network, apparently arising from a threshold assembly of less completely synchronized “burstlets” generated by small subsets of neurons within the network (97, 98). Only fully synchronous inspiratory bursts are transmitted in part through a pool of somatostatin-positive (Sst+) preBötC interneurons, to distinct secondary pools of premotor interneurons interposed between the preBӧtC and respiratory-driven motoneuronal pools in the brainstem and spinal cord (57, 99). Inspiratory bursts result from the dynamic interactions of several functional classes of preBӧtC neurons, defined by their firing patterns from single cell recordings including: 1) quiescent cells, whose activity is recruited during strong respiratory bursts, 2) tonic cells with firing frequencies modulated by respiratory phase, 3) conditional bursters with firing timed with inspiration, but which fail to burst upon pharmacological blockade of synaptic transmission, and 4) intrinsic bursters or “pacemaker” neurons which fire bursts during inspiration, and which persist in isolation from all synaptic transmission. The topology of this network is thought to allow for the flexibility needed to make rapid and reversible adaptive changes in cycle-to-cycle burst phasing or scaling of inspiratory output, in response to acute sensory and neuromodulatory input (90, 91). Synaptic dynamics may play an important role in shaping and constraining the oscillatory properties of this network (100). Recurrent excitatory connections are likely another important general feature of this network, since simultaneous photoactivation of a small randomly-selected subset (<1%) of preBӧtC neurons suffices to initiate full inspiratory bursts, measured by propagation to the XII hypoglossal nucleus (101). This nonlinear dynamic behavior is remarkably reminiscent of similar findings in the cortex (102, 103). Conversely, random photoablation of 10%–17% of neurons in this network is enough to silence rhythmic activity (92, 104–106). These findings highlight the critical role and the unique vulnerability of glutamatergic transmission, in the context of this network architecture for inspiratory burst generation. Overlaid on this requirement for glutamatergic transmission is the contribution from intrinsic bursters, although the relative importance of these neurons to network activity remains unresolved (90, 107, 108). Intrinsic bursters possess specific ion channels required for autonomous bursting, many of which may be modulated by opioid receptor activation. Thus, both the molecular components underlying glutamatergic synaptic transmission and ion channels necessary for pacemaker activity present plausible molecular targets for modulation by opioids, with relevance for understanding and reversing opioid-mediated depression of respiratory output from the preBӧtC.
Opioid Action on the PreBötC—Cellular Mechanisms
Opioids act through binding to a family of four G-protein coupled receptors (GPCRs) (mu-, delta-, kappa-, and nociceptin/orphanin FQ), which signal through the canonical Gαi/o intracellular signaling pathway (Fig. 3). Mouse KO studies have implicated the mu-opioid receptor (encoded by Oprm1, hereafter referred to as MOR) as the primary receptor type underlying both antinociceptive and adverse nonanalgesic side effects, including opioid-induced respiratory depression (OIRD) and constipation due to suppression of gastric motility (109, 110). In addition to canonical signaling via Gαi/o signaling, MOR is also capable of noncanonical signaling by the recruitment of β-arrestin2 to its phospho-C-terminal tail, following activation. Recruited β-arrestin2 mediates a well-characterized role for recycling ligand-bound receptors from the plasma membrane, via clathrin-mediated endocytosis. However, β-arrestin2 may also serve as a scaffolding platform for a variety of signaling molecules, including kinases, phosphatases, and phosphodiesterases (111, 112). These assembled signaling complexes provide the basis for non-canonical intracellular signaling events, independent and exclusive of G-proteins (113, 114). Early reports using β-arrestin2 KO mice suggested that beneficial opioid-mediated analgesia could be dissociated from adverse OIRD and constipation side effects in the absence of β-arrestin2, implicating noncanonical intracellular signaling as a key mechanism underlying these adverse side effects (115). This led to substantial interest in the development of “biased agonist” drugs to promote receptor signaling exclusively through the canonical G-protein pathway, with the aim of providing analgesia while minimizing adverse side effects (116, 117). However, the validity of this concept has been questioned by recent studies, using transgenic mice engineered to express phosphorylation-defective MOR, and by a multilaboratory re-examination of OIRD and constipation phenotypes in β-arrestin2 KO mice, both of which failed to show any significant difference between WT and genetically altered mouse lines (118, 119). Presently, it remains unclear how much non-canonical signaling contributes to OIRD, or whether unique effectors of non-canonical signaling may be identified and harnessed to provide analgesia without adverse side effects. However, these conflicting recent studies re-emphasize the importance of more fully examining well-established mechanisms and molecular effectors of canonical Gαi/o signaling as potential mediators of OIRD.
Figure 3.
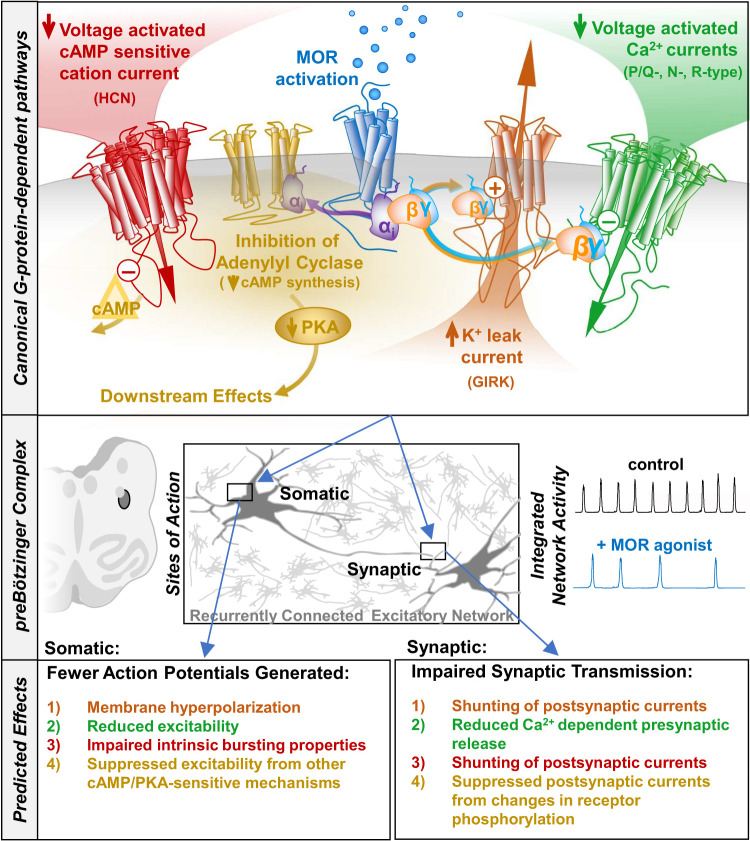
Schematic illustrating the canonical MOR signaling pathways via Gi/o-protein coupling. Gβγ can directly activate GIRK currents and directly inhibit voltage-gated Ca2+ currents. Gαi inhibits adenylyl cyclase, reducing the intracellular concentration of cyclic AMP. Reduced cAMP concentrations inhibit voltage-activated cation currents, such as HCN and can also indirectly influence the activity of numerous downstream effectors through inhibition of PKA. The predicted effects of these mechanisms on preBötC network function and the inspiratory rhythm depend on their somatic and/or synaptic sites of action. Predicted effects are color coded to the canonical pathways outlined above. GIRK, G-protein-activated inward rectifier K+ channels; MOR, mu-opioid receptor; preBOTc, preBӧtzinger complex.
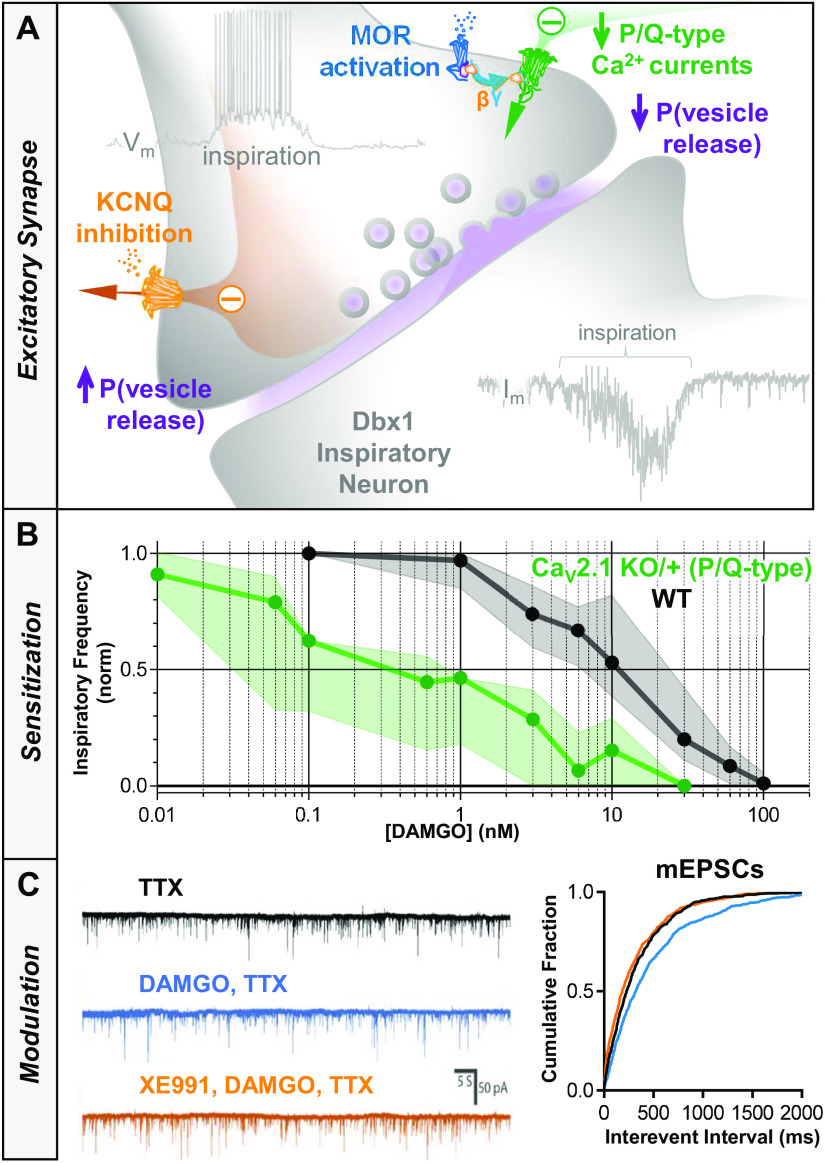
The two best characterized molecular effectors downstream of MOR activation are ion channels: 1) G-protein-activated inward rectifier K+ channels (GIRK), opened by direct binding of Gβ/γ released from activated MOR (120–124), and 2) Presynaptic voltage-gated calcium channels (N-, P/Q- and R-type) whose voltage-dependent gating is suppressed by the binding of Gβ/γ to the second intracellular loop between homology domains I and II (125–130). Canonical opioid-mediated Gαi/o intracellular signaling to either of these ion channels would be expected to suppress excitability (Fig. 3). A role for GIRK potassium channels activated through MOR signaling was examined by Montandon and colleagues, and proposed as the primary mechanism for OIRD (131–133). However, this may be an oversimplification, and there is also increasing evidence for a significant role for presynaptic voltage-gated calcium channel inhibition in OIRD. A recent study suggests that MOR-mediated suppression of presynaptic calcium channels, and the integrity of glutamatergic transmission more generally, is a major mechanism underlying OIRD in the preBötC (Fig. 4). This conclusion is based on the following observations: 1) Heterozygous loss of a single copy of Cacna1a (encoding the P/Q-type calcium channel) sensitized preBötC slices to OIRD by DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin, a MOR-specific agonist), 2) DAMGO reduces the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) recorded from Dbx1+ respiratory neurons in the preBӧtC, consistent with reduced presynaptic calcium channel activity and lower intracellular Ca2+ concentrations (20). Moreover, this study also demonstrated that at the presynaptic terminal, KCNQ potassium channels play a critical role in modulating the depressive effect of DAMGO on synaptic transmission. Application of XE991, a blocker of KCNQ channels, increased the frequency of mEPSCs after suppression by DAMGO, in a large fraction of recorded neurons (86%, 6/7). This effect was corroborated at the network level by population recordings of inspiratory bursts from isolated in vitro preBӧtC slice preparations. Blockers of KCNQ channels (Chromanol 293B, XE991) reversed DAMGO-mediated OIRD in slices, and KCNQ activators (ICA69673, Retigabine) suppressed inspiratory rhythms, mimicking OIRD in the absence of MOR activation (20).
Figure 4.
A: schematic illustrating the proposed effect of opioids at excitatory synapses on rhythmogenic Dbx1 neurons. In response to MOR activation, Gβγ-mediated inhibition of voltage-gated Ca2+ currents reduces the probability of vesicular release in response to bouts of action potentials that occur during inspiratory bursts. B: PreBötC slices from mice with a heterozygous deletion of CaV 2.1 encoding P/Q-type Ca2+ channels are ∼10-fold more sensitive to the MOR agonist DAMGO than WT controls (data shown as median ± interquartile range). C: representative voltage clamp recordings of mEPSCs from a Dbx1 neuron following blockade of action potentials with TTX (1 µM). The frequency of mEPSCs is reduced in the presence of DAMGO (100 nM), and subsequent blockade KCNQ channels with XE991 (20 µM) restores mEPSC frequency to baseline levels. Data adapted with permission from Wei and Ramirez (20). KO, knockout; mEPSCs, miniature excitatory postsynaptic currents; MOR, mu-opioid receptor; P, probability; preBOTc, preBӧtzinger complex; WT, wild type.
Other potential OIRD-related molecular effectors are suggested by studies which report reversal of OIRD by pharmacological manipulations related by their actions to increase intracellular cAMP. These include: 1) agonists of Gαs-coupled GPCRs (5-HT4a receptors, Ref. 24; D1 dopamine receptors, Refs. 134–136), 2) Methylxanthines, caffeine, and theophylline given to counter apneas of prematurity (137–139), and 3) Phosphodiesterase 4 (PDE4) inhibitors (rolipram) (140–142). Each of these manipulations would be expected to counter cAMP decreases due canonical Gαi/o signaling downstream of activated MOR (143). We speculate that molecular machinery affecting glutamatergic synaptic transmission or electrical excitability, modulated directly by cAMP or indirectly by PKA phosphorylation, could thus be implicated as possible effectors and contributors to OIRD. Among potential ion channels, HCN (hyperpolarization-activated cyclic nucleotide-gated) cation channels are plausible OIRD-related targets because they are modulated by directly binding cAMP and play an important role in controlling neuronal excitability (144–146). These ion channels underlie slow depolarizing Ih currents triggered upon after-hyperpolarization during interburst intervals, implicated in pacemaker activity (147). They also regulate dendritic input resistance and thus synaptic efficacy, by shunting ligand-gated postsynaptic currents (148, 149). Another attractive class of ion channels is TWK-type two-pore potassium channels (KCNK) which mediate K+ leak conductance through weakly voltage-dependent, outwardly-rectifying K+ currents, many of which are modulated by both volatile anesthetics that depress respiration, and PKA phosphorylation (150). The respiratory stimulant Doxapram acts through blocking TASK1 and TASK3 channels in peripheral carotid bodies, increasing the gain of chemosensory input to the brainstem (151, 152). Although these channels are expressed in the preBӧtC (153), a central role for TASK channels in OIRD has not been examined.
G-protein modulation of voltage-gated sodium channels is another well-characterized general phenomenon within the nervous system, mediated in part by PKA phosphorylation of sites between homology domains I and II, affecting both peak transient and persistent components of sodium conductance (154, 155). A possible role for opioid modulation of voltage-gated sodium channels in OIRD has yet to be investigated. However, a critical role for persistent sodium currents has been demonstrated for sustaining the rhythmicity of perinatal brainstem respiratory circuits, carried by the NALCN “sodium leak” channel (156). These channels were first described to be modulated by CaSR, a Gαq-coupled GPCR which senses extracellular Ca2+(157), but recent reports also suggest that NALCN channels are susceptible to modulation by Gαi/o-coupled D2 dopamine receptors, suggesting a similar possible susceptibility to MOR (158, 159).
Additionally, G-protein signaling has been shown to control synaptic efficacy by modifying both presynaptic and postsynaptic molecular targets. At some presynaptic terminals, vesicular release is suppressed by phosphorylation-independent binding of Gβ/γ to SNAP-25 (160–164). Postsynaptically, constitutive PKA-dependent phosphorylation maintains AMPA glutamate receptors at postsynaptic densities, underlying long-term potentiation (LTP) and long-term depression (LTD) (165–168). These observations suggest additional molecular targets for investigation as mediators of OIRD, consistent with the demonstrated ability of ampakines such as CX1942 to reverse centrally derived OIRD (21, 169, 170).
In summary, multiple molecular and cellular mechanisms likely contribute to OIRD in the preBӧtC, constrained by the network architecture and specific electrical properties of its constituent neurons. Current evidence suggests that MOR-mediated inhibition of presynaptic calcium channels is a significant component of OIRD, which is consistent with the demonstrated critical role for glutamatergic transmission in sustaining rhythmic network activity. However, other mechanisms likely contribute to OIRD. New molecular components contributing to OIRD may emerge from future studies, focusing on candidate ion channels and effectors affecting glutamatergic transmission known to be modulated downstream of canonical Gαi/o signal transduction.
Opioid Effect on the PreBötC In Vivo
Multiple studies in vitro have shown that administration of the synthetic opioid, DAMGO into the isolated brainstem slice preparation generally results in a reduction in fictive respiratory frequency (20, 21, 171), suggesting the importance of this medullary subregion in OIRD. However, when opioids are administered locally into this region in vivo, effects on ventilation have been shown to be variable, resulting in either a decrease, no-change, or an increase in respiratory activity. For example, Montandon et al. (131) showed that dialysis of DAMGO into the preBötC of rats during wakefulness and sleep resulted in depression of respiratory activity. By contrast, Langer et al. (172) and Krause et al. (173) demonstrated that injection or dialysis of DAMGO into the preBötC of awake and alert goats resulted in either no change or an increase in respiratory activity. Furthermore, Mustapic et al. (174) demonstrated that similar DAMGO dialysis into the preBötC of decerebrate dogs led to an increase in overall respiratory activity. If the opioid-sensitive preBötC is indeed necessary for eupneic ventilation in the intact in vivo system, this raises the question of why there is such discordance in the effects of opioid administration into this medullary region in intact physiological preparations.
One hypothesis proposed for the variable effect of in vivo opioid administration into the preBötzinger complex is a concept termed “interdependence of neuromodulators.” This concept, as proposed by Doi and Ramirez (175) states that, “A modulator’s action is determined by the concurrent modulation and interaction with other neuromodulators.” In other words, the action of a single neuromodulator, such as a therapeutic opioid may be compensated for, or influenced by the action of other endogenous neuromodulators within the network. This hypothesis was based on studies comparing the effects of Substance P receptor (NK-1) antagonism on respiratory network activity, when administered in vitro on rhythmic brainstem slice preparations and in vivo locally into the preBötC of intact animal preparations. These studies demonstrated that NK1 antagonism in the isolated in vitro brainstem preparation resulted in a reduction in fictive respiratory frequency, while in vivo local NK1 receptor antagonism within the intact preBötC urethane-anesthetized preparation failed to reduce spontaneous breathing rate. However, in vivo respiratory activity could be reduced following concurrent antagonism of NK1, α1-noradrenergic, and 5-HT2A receptors within the preBötC (175). This suggested that changes in one neuromodulator within the preBötC are likely influenced by compensatory changes in one or more neuromodulatory systems in order to maintain stable eupneic ventilation. Similar observations have been suggested in earlier studies of neural networks in the crustacean stomatogastric ganglion (176), and the neuromuscular junction of Aplasia by Marder and Brezina, et al., who described this phenomenon as “redundancy” or “degeneracy” of neural control networks (177–179).
The presence of “redundancy,” “degeneracy,” or “interdependence of neuromodulators” within the preBötC creates a barrier for direct interpretation of studies examining the effects of opioids on the preBötC in vivo, because direct agonism of opioid-receptors may be partially or completely compensated by changes in other neuromodulators. Indeed, this was shown to be the case when Langer et al. (172) observed a paradoxical increase in ventilation when DAMGO was dialyzed into the preBötC of awake and alert goats. In these studies, goats were given 10, 50, and 100 μM of DAMGO into the preBötC through microdialysis. When dialyzed unilaterally, DAMGO resulted in no-change in ventilation. However, it was found that DAMGO dialysis led to a dose-dependent reduction of the inhibitory neuromodulator GABA, suggesting that a DAMGO-dependent reduction in GABA-mediated inhibition offset the inhibitory effects of DAMGO within the preBötC. Similarly, bilateral dialysis of DAMGO into the preBötC of awake and alert goats resulted in an overall increase in ventilation, however with no change of any neuromodulators measured in effluent dialysate. Additionally, despite no-change or an increase in ventilatory rate, it was shown that respiratory rhythm was destabilized following both unilateral and bilateral dialysis of DAMGO into the preBötC. These studies are important because they: 1) Further highlight the possible variability of OIRD following opioid administration directly into the preBötC of awake and alert animals, 2) Provide evidence of neuromodulatory mechanisms within the preBötC, capable of compensating for the depressant effects of opioids, and 3) Suggest the need to understand how these compensatory neuromodulator systems may be harnessed across different physiological conditions to prevent or reverse OIRD.
If indeed there are intrinsic mechanisms within the preBötC capable of compensating for, or reversing OIRD, then understanding the conditions and states of the respiratory network that lead to resistance or reversal of OIRD will be key in understanding the specific mechanisms leading to OIRD, and for developing strategies to protect against OIRD. For example, neuromodulator compensation, or more generally, neuromodulation has been shown to be state-dependent across various brain states such as sleep and wakefulness (180). Additionally, within the preBötC, chronic intermittent hypoxia has been shown to destabilize rhythm generation, within the isolated brainstem (84). These studies strongly suggest that mechanisms of respiratory rhythmogenesis within the preBötC may be dynamically set across varying physiological conditions. Further, it is likely that the sensitivity of the respiratory network to perturbations such as opioid exposure likely changes under different “states” of rhythmogenesis. Thus, future studies aimed at understanding the relationship between state-dependency, neuromodulation, and rhythm generation will be necessary for unraveling the specific action of opioids within the brainstem and for creating strategies to prevent or reverse OIRD.
PONS
The pontine respiratory group in the dorsolateral pons was shown long ago to be important for normal breathing and opioid-induced respiratory depression (181, 182). Key structures that comprise the pontine respiratory group are the lateral parabrachial nucleus (LPB), medial parabrachial nucleus (MPB), and Kölliker-Fuse (KF). The KF/PB significantly contributes to the control of respiratory rate (183–186), regularity (187), eupneic pattern formation (188–191), and chemosensory reflexes (192–196). In addition, the KF plays a major role in coordination of the upper airways in breathing and swallowing (189, 197–199).
During opioid-induced respiratory depression, the majority of these functions are compromised (200–202), including impairment of the upper airways at therapeutically relevant doses (203–205). The correlation between the clinically observed effects of opioids and the known functions of the KF/PB is striking. However, due to the abundance of mu opioid receptors in the majority of the respiratory control areas of the brainstem, it has proven challenging to resolve the neural substrates of respiratory malfunctions during overdose. Further complications arise when one considers the numerous reciprocal projections and feedback loops present in the respiratory network.
Opioid Effect on the KF/PB In Vivo
Direct administration of opioid agonists locally into the KF/PB results in suppression of respiratory rate (Fig. 5), due to increases in the duration of both inspiration and expiration (182, 184, 206, 207). This reduction in respiratory rate is consistently observed across species, including cat, dog, rabbit and rat. This is in contrast to the conflicting results observed following local opioid administration within the ventral respiratory column, particularly the preBötC, as described above (131, 172–174, 208, 209).
Figure 5.
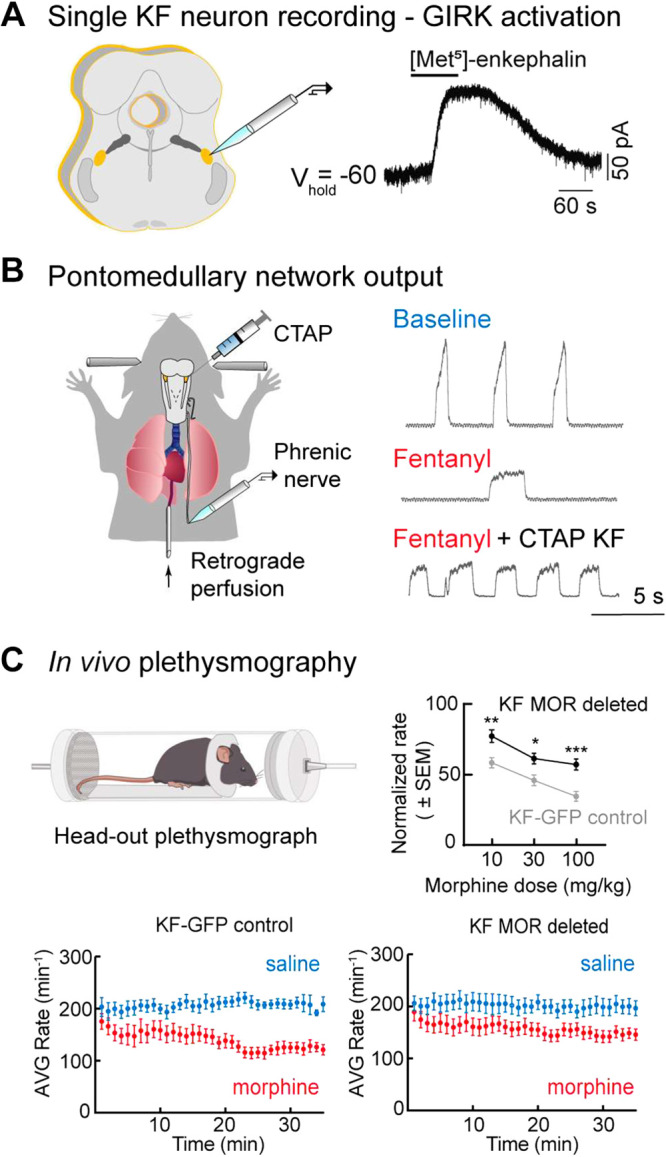
Opioid modulation of pontine networks. A: whole cell voltage-clamp recording from a KF neuron in brain slice illustrating activation of GIRK current by opioid agonist Met-enkephalin. Modified from Levitt et al. (184). B: schematic of the in situ working heart-brainstem preparation of the rat. On the right, phrenic bursts indicating inspiratory activity during baseline, following systemic fentanyl administration, and following systemic fentanyl with opioid antagonist, CTAP microinjection into the KF. Antagonism of KF MORs eliminates rate depression by fentanyl; however, the apneustic pattern characteristic of opioid effects persists. Modified from Saunders and Levitt, 2020 (211). C: schematic of in vivo plethysmograph. Graph on the top right demonstrates significant attenuation of morphine-induced respiratory rate depression in vivo following bilateral MOR deletion from KF neurons compared to control animals. Below, example time course of average respiratory rate following saline and morphine (10 mg/kg, i.p.) in control mice (left) vs. mice following MOR deletion from KF neurons (right). Modified from Varga et al. (212). GFP, green fluorescent protein; GIRK, G-protein-activated inward rectifier K+ channels; KF, Kölliker-Fuse; MOR, mu-opioid receptor. *,**,***Statistically significant (P < 0.05) in order of least to most significant.
Systemic administration of opioids causes reductions in respiratory rate due to small increases in inspiration and large increases in expiration (11, 169, 210). In the presence of systemic opioid administration, antagonism of MORs (both pre- and postsynaptic receptors) located in the KF/PB at least partially restores respiratory rate, mostly due to reductions in expiratory duration (206, 207, 211). Antagonism of opioid receptors in the KF/PB also prevents and reverses sustained apnea following systemic fentanyl or remifentanil (206, 211). Similarly, deletion of MORs from a large portion of KF neurons reduces the number of apneas induced by morphine in awake mice (212). Together, these studies suggest that opioids in the KF/PB enhance tonic expiratory drive leading to prolonged expiration and apnea (211). Expiratory drive from KF has also been implicated in generating apnea in Rett Syndrome and nasotrigeminal reflex (213, 214). The mechanisms within the KF generating apnea are likely different because enhanced postinspiratory discharge accompanies the apneas of RTT and nasotrigeminal reflex, whereas postinspiratory discharge is abolished by opioids.
In awake mice, removal of MORs from KF neurons significantly rescues morphine-induced respiratory rate depression (87, 212). This occurs at a therapeutically relevant analgesic dose and, importantly, also at a very high dose that is near overdose (212). The improvement in rate is due to shortening of both inspiration and expiration. By comparison, removal of mu opioid receptors from preBötzinger complex neurons only rescues morphine-induced rate depression at analgesic doses, but not at a high dose (212).
The rate changes caused by opioid modulation come hand-in-hand with alterations in respiratory pattern, which are easily observed and analyzed in the in situ working heart brainstem preparation of the rat (215). The in situ preparation produces a eupneic respiratory cycle with a clear readout of three respiratory phases in nerve recordings in a nonanesthetized, vagotomized setting. Local injection of the opioid agonist DAMGO into the KF of the in situ preparation leads to the emergence of an apneustic 2-phase respiratory pattern, exhibiting loss of post-inspiration and lengthened, low amplitude inspiratory bursts (184). This pattern is very similar to bilateral pontine lesion or pharmacological inhibition of the KF in the in situ preparation (189, 216) and has implications for both the quality of breathing and patency of the upper airways. This two-phase respiratory pattern is also present, though at a higher frequency, in preparations with systemic fentanyl and antagonism of KF/PB opioid receptors (211), indicating that blockade of receptors in the KF/PB is not sufficient, on its own, to restore a normal breathing pattern. Local injection of DAMGO into the KF also increased the variability of phrenic nerve bursting (36), but deletion of MORs from KF neurons did not improve the increased variability of breathing rate caused by systemic morphine in awake mice (69).
Opioids also decrease tidal volume—an effect often attributed to muscle rigidity and decreased chest wall compliance (217–219). Opioids, especially fentanyl, cause muscle rigidity, which is blocked by opioid receptor antagonist and alpha-2 adrenergic receptor agonist, and proposed to be due to central opioid receptor-mediated increase in adrenergic activity (220–223). However, in paralyzed preparations fentanyl causes a decrease in the amplitude of phrenic motor output, indicating that there is also a central component to the reduction of tidal volume (169, 211). Antagonism of opioid receptors in the KF/PB does not restore the amplitude of phrenic motor output (211), indicating that the reduction in tidal volume does not occur in the dorsolateral pons.
Opioid Action on the KF/PB—Cellular Mechanisms
The effects of opioid administration into the KF/PB largely mimic the effects of global inhibition of the KF/PB by pharmacological inhibition or ablation (186, 188, 189, 193, 216, 224, 225), indicating that opioids have a strong inhibitory action in the KF/PB. The KF/PB express an abundance of MORs (226–228). A proportion of KF neurons (∼60%) contain somatodendritic MORs that directly hyperpolarize KF neurons by activation of GIRK conductance (184, 212). Opioid-sensitive and nonsensitive KF neurons have different intrinsic properties (firing rate, action potential duration, and afterhyperpolarization), indicating that they are indeed separate populations of neurons serving distinct functions (184). Lateral parabrachial complex neurons also contain somatodendritic MORs that activate GIRK conductance (229). The sensitivity of the somatodendritic opioid receptors on KF/PB neurons is similar to somatodendritic opioid receptors on other neuronal populations involved in pain or reward (184, 229).
MORs are also expressed on neuronal processes in the KF (226–228). These processes could include reciprocal projections to the KF from medullary respiratory structures, including preBötzinger complex, Bötzinger complex, or NTS (82, 230–232). The functional contribution of presynaptic opioid receptors in the KF/PB is yet to be determined.
The KF/PB contains a heterogeneous population of respiratory patterned neurons with various projection targets. Glutamatergic KF neurons send dense projections to the majority of the ventral respiratory column (198, 233–237), as well as the nucleus tractus solitarius (NTS) and hypoglossal motor nucleus (197, 236, 237). Many KF neurons fire phasically relative to the respiratory cycle, including neurons with phasic firing (discharge identity) in inspiration, postinspiration and expiration (189, 233, 238–240). The discharge identity of some KF neurons changes following exposure to mu opioid agonist DAMGO (238), but a full understanding of the discharge identity and projection target of opioid-sensitive KF neurons is lacking. This understanding would help predict the effect of modulating KF circuits in respiratory depression.
Chemosensory and Arousal Pathways in the Pons
In addition to rate and pattern changes, opioids reduce hypercapnic and hypoxic ventilatory responses (11), due to activation of mu opioid receptors (109). In opioid overdose, limited, or even lack of hypoxic/hypercapnic arousal responses can occur when respiratory cessation is abrupt, such as with high-dose fentanyls (11). Under normal conditions, changes in blood gases lead to shifts in behavioral state toward arousal to increase ventilatory drive and stabilize blood gas concentrations (241). Respiratory chemosensory nuclei in the pons can either serve as relay centers for blood gas information, or serve as arousal centers that directly or indirectly feed-back to respiratory control centers to increase respiratory drive.
The PB/KF contains a population of glutamatergic neurons that are part of a chemosensory relay circuit projecting to higher order arousal centers (194, 236, 242–245). Stimulation of PB, especially external lateral PB neurons that express CGRP (calcitonin gene-related peptide), causes cortical arousal, meanwhile lesions of the glutamatergic relay population leads to increased amount of sleep and decreased hypercapnic arousal responses (194, 243, 246, 247). These CGRP-expressing external lateral PB neurons contain mu opioid receptors (227), thus opioid-induced inhibition of these neurons likely contributes to the sedative effects of opioids.
The locus coeruleus (LC) is another pontine region critical for a wide range of homeostatic functions, including arousal, which is studied mainly in the context of sleep apnea and sleep-wake states (248–250). The LC is directly involved in inducing arousal when blood CO2 levels rise (251–255). Since all LC neurons contain mu opioid receptors and are hyperpolarized by opioids (256), this region is also a prime candidate for investigating opioid-induced loss of hypercapnia reflexes.
Endogenous Opioid Expression in Pons
A large population of KF/PB neurons express pre-pro-enkephalin mRNA and produce enkephalin, an endogenous opioid peptide that activates mu and delta opioid receptors (257–260). KF neurons containing pre-pro-enkephalin project to the ventral medulla and the spinal cord (257). Enkephalin-producing KF neurons may be one source of endogenous opioids important in regulating respiration. The endogenous opioid peptide endomorphin-2 is also expressed in neuronal fibers of the KF/PB and overlaps with MOR-expressing fibers (227). Global MOR knockout mice not only lack respiratory depression in response to exogenously administered opioids, but also breathe at a substantially higher rate at baseline than littermate controls, indicating an endogenous opioid tone in the respiratory network (109). However, MOR removal from KF and/or preBötzinger complex neurons does not increase baseline respiratory rate, indicating that the KF and preBötzinger complex may not play a significant role in maintaining this baseline inhibitory tone (212).
Pons—Conclusions
Given the high proportion of mu opioid receptor expression and the wide range of respiratory-related roles played by the pontine respiratory group, it is predictable that the pons has a substantial contribution to OIRD. In particular, opioid actions in the pons contribute to reduction in respiratory rate, loss of upper airway patency, and degenerate pattern formation. The KF appears to be especially important for apnea generation following high-dose opioids. We infer that countering opioid effects in the KF is especially important in overdose. Some countermeasure targets with potential activity in the KF/PB include orexin and 5-HT1A receptors (261–264). Further work into understanding the mechanisms of opioids in pontine circuits will hopefully produce additional countermeasure targets.
PREFRONTAL CORTEX
Although breathing is known to be generated by the pontomedullary brain stem (33, 232), the goal of this section is to highlight evidence that the prefrontal cortex (PFC) is a brain region mediating OIRD (265). The PFC also contributes to pain processing (266) and addiction (267). Functional studies reviewed elsewhere propose that PFC dysfunction underlies loss of inhibitory control over appetitively motivated behaviors, such as drug abuse (268). In support of this concept, recent studies show that transcranial, direct current stimulation over human dorsolateral PFC causes a significant decrease in drug craving and reduces deficits in cognitive control (269).
The forgoing findings and scientific constructs raise many questions regarding the mechanisms through which the PFC modulates a wide range of complex phenotypes. Partial answers include the fact that the PFC comprises multiple subregions (270) containing cells that project to and receive projections from widely distributed neuronal networks (271). Anatomical connections between PFC and respiratory nuclei in the pontine and medullary brain stem have been documented (272). One example of the diverse connectivity through which the PFC might exert effects on breathing is the discovery of monosynaptic projections from medial regions of rat PFC to the thoracic spinal cord (273). Although the PFC contains no respiratory neurons, it has been known since the 19th century that breathing frequency can be altered by electrically stimulating the PFC (see Lépine, 1875 in Ref. 274).
Contemporary pharmacological data show that microinjecting the GABA-A receptor antagonist bicuculline into rat PFC causes site-specific, apneic breathing (275). Medial regions of the rodent PFC are organized into four layers comprised of up to 90% excitatory pyramidal neurons and 10 to 20% inhibitory GABAergic interneurons (276). Systematic reviews of PFC neurochemistry (277) indicate the contribution of at least eight neurotransmitter systems to PFC function. Pharmacological studies confirm the presence of opioid receptors in PFC of rodent (278) and human (279). Mu, kappa, and delta subtypes of opioid receptors are widely and differentially distributed throughout the rodent (278) and human (280) brain. Thus, connectivity and opioid receptor data provide ample substrates through which systemically administered opioids can simultaneously inhibit and disinhibit widely distributed neuronal networks. The functional significance of these receptor and network data is clear from the observation that systemically administered opioids alter the cortical electroencephalogram (EEG) and corresponding states of consciousness (281). Figure 6 illustrates that in mice, cortical EEG power and frequency are differentially altered by antinociceptive doses of morphine, fentanyl, and buprenorphine (282).
Figure 6.
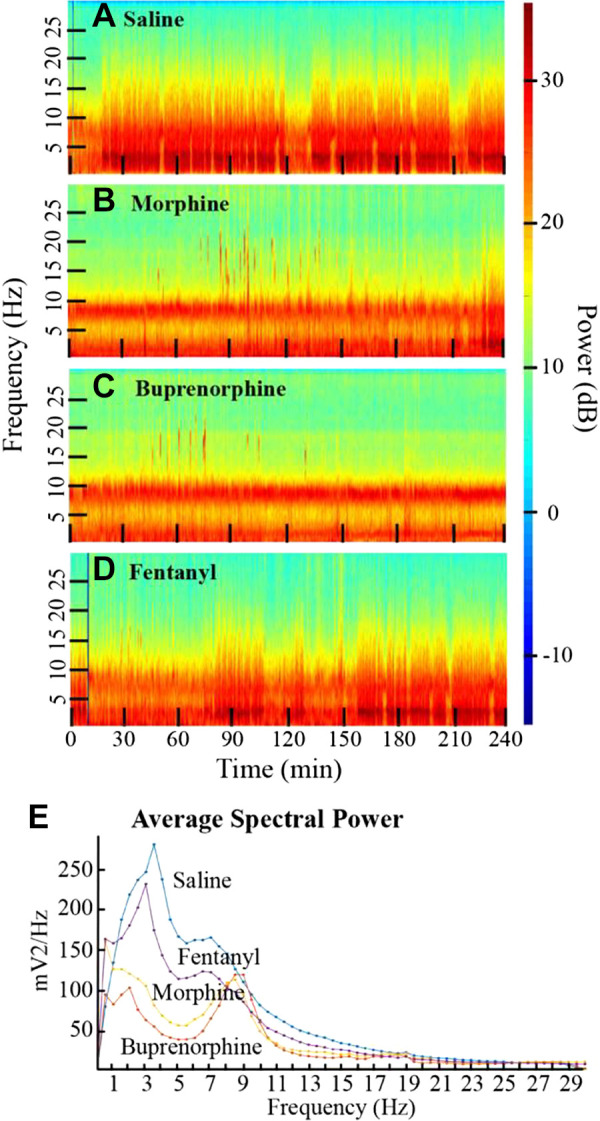
Examples of how tapered spectrograms reveal that administering saline (A), morphine, (B), buprenorphine (C), or fentanyl (D) differentially changes cortical EEG power and frequency in mice. E illustrates how EEG power can be quantified to show opioid-specific alterations. These spectrograms demonstrate the importance of understanding nuanced effects of opioids on cortical excitability. Modified from O’Brien et al. (282).
The cortical EEG data and recent studies of PFC (283) encourage evaluation of the hypothesis that administering opioids directly into mouse PFC causes respiratory depression. Figure 7 shows that microdialysis delivery of fentanyl into the PFC of intact, behaving mice depresses breathing (284). Additional preliminary data show that microinjection of morphine into PFC of awake mice decreases minute ventilation (285).
Figure 7.
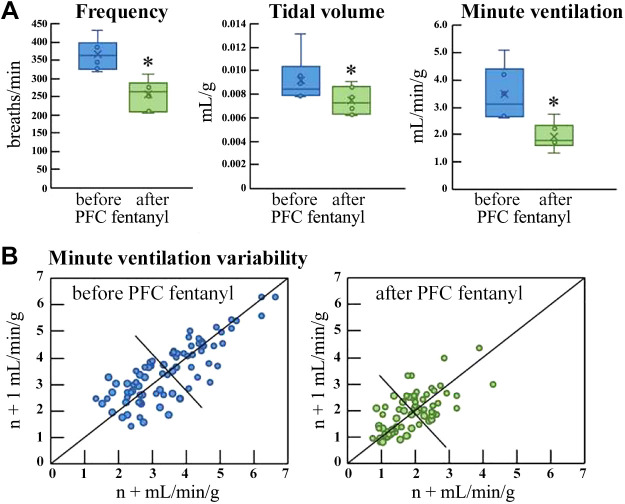
An example of how breathing in mice is depressed by direct administration of fentanyl into the prefrontal cortex A: adequate respiratory control includes the ability to vary breathing in response to environmental challenges. B shows how Poincaré analyses can be used to visualize the decrease in breathing variability caused by delivering fentanyl into mouse prefrontal cortex. Modified from Zhang et al. 2019 (284). PFC, prefrontal cortex. *P < 0.05.
In summary, the PFC has long been known to modulate breathing (274), but evidence that the PFC may contribute to addiction (268, 269) and OIRD (265) has appeared only recently. The preliminary data of Figs. 6 and 7 encourage future studies designed to specify which regions of the PFC modulate breathing and OIRD. Such studies should also clarify the mechanisms through which the PFC interacts with other neural networks involved in respiratory control. The pontine parabrachial nuclei modulate the timing of breathing and the PFC projects to (286) and receives projections from (272) the parabrachial respiratory nuclei. Considered together, these data support the view that future studies of the interaction between the PFC and parabrachial nuclei will enhance understanding of OIRD.
PERIPHERAL ACTIONS OF OPIOIDS
Although central nervous system (CNS) mechanisms are certainly critically involved in OIRD, it is also important to consider the action of opioids on the peripheral nervous system. To our knowledge, the first evidence that opioids can have peripheral actions to alter breathing was published by Gruhzit (287) in the late 1950s. This investigator showed that bolus i.v. administration of codeine, morphine or the nonopioid drug, dextromethorphan could induce rapid shallow breathing and apnea in dogs or cats (287). Vagotomy or vagal cooling sufficient to block c-fibers eliminated this effect (287). Since that time, a wealth of evidence has emerged that strongly supports the concept that peripherally administered opioid drugs, especially µ-opioid agonists, can induce apnea and rapid shallow breathing (288–296).
Pulmonary Opioid Receptors
MORs are found in lung tissue, on airway sensory nerves, and in vagal and spinal ganglia (297–299). Utilizing autoradiography, it was shown that the highest density of binding sites for [3H] morphine was in the alveolar walls (300) with lower densities in the larger airways. In lung tissue preparations, the binding affinities of tritiated morphine and naloxone were lower than in the brain, but the binding site densities were up to two orders of magnitude higher (299). These data suggest a high level of importance for opioid receptor-mediated mechanisms in the lung.
The mechanism by which MOR agonists are thought to alter the breathing pattern is through excitation of pulmonary c-fibers (290). Excitation of these c-fibers with peripheral vascular administration of TRPV-1 agonists and other drugs (e.g., capsaicin, phenylbiguanide) is well-known to elicit apnea and rapid shallow breathing in a variety of species (301). It is presumed that MOR agonists act on opioid receptors located on sensory terminals of c-fibers. However, local application of MOR agonists in the nodose ganglion also can elicit apnea and/or tachypnea consistent with stimulation of airway c-fibers (289). The functional relevance of opioid receptor modulation of vagal sensory traffic at the level of the nodose ganglion is not well understood.
Opioids elicit local effects in the airways that may alter the pattern of breathing through changes in pulmonary mechanics. The efferent function of c-fibers to release the pro-inflammatory neuropeptide, substance P, can be inhibited by a MOR agonist (302). Both mucus secretion and cholinergic-mediated contraction of tracheal smooth muscle are inhibited by MOR agonists as well (303, 304).
Carotid Body Opioid Receptors
Endogenous opioids (leu- and met-enkephalin) are present in carotid body glomus cells (305, 306). Intracarotid injections of MOR agonists inhibit spontaneous chemoreceptor discharge but have smaller depressive effects on acetylcholine-evoked discharge of these sensory receptors (307). However, an in vivo rank order potency study showed that carotid chemoreceptor discharge was more affected by delta opioid receptor agonists than those with high affinity for the MOR (308).
In anesthetized cats, the peripheral influence of morphine on the isocapnic hypoxic ventilatory response may be limited to depression of CO2 sensitivity during hyperoxia (309). In conscious rats, there is evidence that morphine more strongly depresses the hypoxic and hypercapnic ventilatory responses after section of the carotid sinus nerve (310), suggesting that MOR-related mechanisms in the carotid body may ameliorate opioid-related respiratory depression. Hyperoxia in spontaneously breathing humans enhances opioid-induced respiratory depression, presumably via profound reductions in carotid body afferent feedback (311, 312). It is possible that this straightforward hypothesis needs revision. The effect of opioids on carotid body sensory afferents in concert with recent preclinical in vivo results strongly suggest that the peripheral effects on hypoxic and hypercapnic ventilatory mechanisms are likely conditional and more complex that previously appreciated.
Opioid Receptors and Actions in the Nucleus of the Tractus Solitarius, as the Integration/Relay Station for Peripheral Inputs
NTS interneurons mediating airway sensory information include pump cells (313, 314), Iβ cells (313), laryngeal interneurons (315, 316), neurons mediating pulmonary c-fiber reflexes (313, 316), and neurons mediating rapidly adapting receptor information (317). Extra-NTS targets of these second-order populations include the pons, VRC, and cervical spinal cord (313). Within the NTS, slowly adapting receptor (SAR) second-order interneurons monosynaptically inhibit their rapidly adapting receptor (RAR) counterparts in the rat (318). Pump cells can project contralaterally to excite or inhibit Iβ cells (313). A recent study by McGovern et al. (319) using herpes simplex virus mapped central pathways from the extrathoracic trachea and identified a large neuronal network in the NTS and surrounding areas that may exceed the substrate of second-order NTS interneurons by an order of magnitude. Results from these studies strongly support complex processing of sensory feedback from the lungs and airways by NTS circuits.
Opioid receptors are found in the NTS (320). Application of opioids to the caudal NTS can mimic the respiratory effects of peripheral administration of these drugs (321). Relatively little is known regarding the distribution of MOR receptor afferent projections to the caudal NTS, which processes airway vagal c-fiber sensory feedback. Interneurons in the caudal NTS also process sensory feedback from the carotid sinus nerve related to hypoxic responses (322), indicating that this region of the NTS represents a critical area in understanding the anatomical and functional elements that are responsible for the peripheral actions of these drugs on breathing.
OVERALL CONCLUSIONS
Currently there are limited treatment options available for acute opioid overdose and long-term opioid addiction. Following acute overdose, administration of the competitive opioid receptor antagonist, naloxone, remains the standard of care (323). This treatment has proved effective in rapidly reversing the fatal respiratory effects of an opioid overdose (323–325). However, the effects are not limited to the respiratory system and simultaneously cause reversal of analgesia (326), resulting in unpredictable effects and acute withdrawal symptoms (327–329) that may become life threatening. Conversely, there are currently three available medication assisted therapies available for long-term opioid addiction (330). These include the use of controlled opioid-based replacements such as methadone and buprenorphine, or opioid receptor antagonists such as naltrexone. Although these therapies have proven to be effective in a small proportion of patients, there remain major hurdles in the use of these therapies due to poor patient adherence and abuse of opioid-based replacement drugs. Accordingly, alternative therapies targeted at preventing overdose, specifically reversing respiratory suppression, and mitigating long-term addiction are desperately needed.
The NIH initiative to help end addiction over the long-term (HEAL) has called for an “all hands on deck” effort to improve treatments for addiction and to enhance strategies for pain management (331). The HEAL initiative is a logical extension of earlier calls (332) for the development of pharmacological interventions to counter opioid-induced respiratory depression (OIRD). The “all hands” call can be extended to “all brain regions” for research on OIRD. In this review, we provided an overview of the different CNS and PNS regions involved in the neuronal control of breathing and their potential contribution to OIRD. From this overview it is clear, that opioids inhibit breathing at multiple, and highly distributed sites. Inhibition of the different regions is manifested in a variety of differential effects on rhythmogenesis, pattern formation, and neuromodulation. The opioid action on these sites is highly dependent on the metabolic and modulatory state of the organism, thus explaining why OIRD is so variable and unpredictable. Although OIRD is on average dose dependent, no dose is safe. Even small opioid doses under certain metabolic conditions can lead to an overdose. Unraveling these state-dependent mechanisms will be critical to find effective countermeasures to cope with the opioid crisis.
GRANTS
This work was supported by R01 HL144801, R01 HL126523, and P01 HL090554 to J-M. Ramirez; and R01 DA047978, R00 DA038069, and Grant 3608 from IRSF (International Rett Syndrome Foundation) to E. S. Levitt. A. V. Varga was supported by T32 HL134621.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J-M.R., E.S.L., N.J.B., A.D.W., N.A.B., A.G.V., H.A.B., R.L., K.F.M., and D.C.B. drafted manuscript; J-M.R., E.S.L., N.J.B., A.D.W., N.A.B., A.G.V., H.A.B., R.L., K.F.M., and D.C.B. edited and revised manuscript; J-M.R., E.S.L., N.J.B., A.D.W., N.A.B., A.G.V., H.A.B., R.L., K.F.M., and D.C.B. approved final version of manuscript.
REFERENCES
- 1.Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. Hyattsville, MD: National Center for Health Statistics, 2020. [Google Scholar]
- 2.National Academies of Sciences Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use, edited byPhillips JK, Ford MA, Bonnie RJ.. Washington, DC: National Academic Press, 2017. [PubMed] [Google Scholar]
- 3.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452, 2016. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 4.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep 67: 1419–1427, 2018. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 16: E85–E94, 2013. [PubMed] [Google Scholar]
- 6.Weak” opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine. Prescrire Int 25: 45–50, 2016. [PubMed] [Google Scholar]
- 7.Coyle DT, Pratt CY, Ocran-Appiah J, Secora A, Kornegay C, Staffa J. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf 27: 464–472, 2018. doi: 10.1002/pds.4366. [DOI] [PubMed] [Google Scholar]
- 8.Gueye PN, Borron SW, Risède P, Monier C, Buneaux F, Debray M, Baud FJ. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol Sci 65: 107–114, 2002. doi: 10.1093/toxsci/65.1.107. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwenhuijs DJ, Olofsen E, Romberg RR, Sarton E, Ward D, Engbers F, Vuyk J, Mooren R, Teppema LJ, Dahan A. Response surface modeling of remifentanil-propofol interaction on cardiorespiratory control and bispectral index. Anesthesiology 98: 312–322, 2003. doi: 10.1097/00000542-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Dahan A, Nieuwenhuijs D, Olofsen E, Sarton E, Romberg R, Teppema L. Response surface modeling of alfentanil-sevoflurane interaction on cardiorespiratory control and bispectral index. Anesthesiology 94: 982–991, 2001. doi: 10.1097/00000542-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 12.Lam KK, Kunder S, Wong J, Doufas AG, Chung F. Obstructive sleep apnea, pain, and opioids: is the riddle solved? Curr Opin Anaesthesiol 29: 134–140, 2016. doi: 10.1097/ACO.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, Shilling KC. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 03: 455–461, 2007. doi: 10.5664/jcsm.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath 13: 49–57, 2009. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 15.Rose AR, Catcheside PG, McEvoy RD, Paul D, Kapur D, Peak E, Vakulin A, Antic NA. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med 10: 847–852, 2014. doi: 10.5664/jcsm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey PL, Lu JK, Pace NL, Orr JA, White JL, Hamber EA, Slawson MH, Crouch DJ, Rollins DE. Effects of intrathecal morphine on the ventilatory response to hypoxia. N Engl J Med 343: 1228–1234, 2000. doi: 10.1056/NEJM200010263431705. [DOI] [PubMed] [Google Scholar]
- 17.Potter JV, Moon RE. Commentaries on viewpoint: why do some patients stop breathing after taking narcotics? Ventilatory chemosensitivity as a predictor of opioid-induced respiratory depression. J Appl Physiol (1985) 119: 420–422, 2015. doi: 10.1152/japplphysiol.00034.2015. [DOI] [PubMed] [Google Scholar]
- 18.Emery MJ, Groves CC, Kruse TN, Shi C, Terman GW. Ventilation and the response to hypercapnia after morphine in opioid-naive and opioid-tolerant rats. Anesthesiology 124: 945–957, 2016. doi: 10.1097/ALN.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 19.Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med 292: 1103–1106, 1975. doi: 10.1056/NEJM197505222922106. [DOI] [PubMed] [Google Scholar]
- 20.Wei AD, Ramirez JM. Presynaptic mechanisms and KCNQ potassium channels modulate opioid depression of respiratory drive. Front Physiol 10: 1407, 2019. doi: 10.3389/fphys.2019.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med 174: 1384–1391, 2006. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 22.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 23.Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci 78: 682–688, 2006. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 25.Bouillon T, Bruhn J, Roepcke H, Hoeft A. Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol 20: 127–133, 2003. doi: 10.1017/s0265021503000243. [DOI] [PubMed] [Google Scholar]
- 26.Leino K, Mildh L, Lertola K, Seppälä T, Kirvelä O. Time course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depression. Anaesthesia 54: 835–840, 1999. doi: 10.1046/j.1365-2044.1999.00946.x. [DOI] [PubMed] [Google Scholar]
- 27.Teppema LJ, Nieuwenhuijs D, Olievier CN, Dahan A. Respiratory depression by tramadol in the cat: involvement of opioid receptors. Anesthesiology 98: 420–427, 2003. doi: 10.1097/00000542-200302000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Weinstock M, Roll D, Erez E, Bahar M. Physostigmine antagonizes morphine-induced respiratory depression but not analgesia in dogs and rabbits. Br J Anaesth 52: 1171–1176, 1980. doi: 10.1093/bja/52.12.1171. [DOI] [PubMed] [Google Scholar]
- 29.Maric V, Ramanathan D, Mishra J. Respiratory regulation & interactions with neuro-cognitive circuitry. Neurosci Biobehav Rev 112: 95–106, 2020. doi: 10.1016/j.neubiorev.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez JM, Baertsch NA. The dynamic basis of respiratory rhythm generation: one breath at a time. Annu Rev Neurosci 41: 475–499, 2018. doi: 10.1146/annurev-neuro-080317-061756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez JM. The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Prog Brain Res 209: 91–129, 2014. doi: 10.1016/B978-0-444-63274-6.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez J-M, Baertsch N. Defining the rhythmogenic elements of mammalian breathing. Physiology 33: 302–316, 2018. doi: 10.1152/physiol.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgraff NJ, Neumueller SE, Buchholz KJ, Hodges MR, Pan L, Forster HV. Glutamate receptor plasticity in brainstem respiratory nuclei following chronic hypercapnia in goats. Physiol Rep 7: e14035, 2019. doi: 10.14814/phy2.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baertsch NA, Ramirez JM. Insights into the dynamic control of breathing revealed through cell-type-specific responses to substance P. eLife 8: e51350, 2019. doi: 10.7554/eLife.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baertsch NA, Severs LJ, Anderson TM, Ramirez JM. A spatially dynamic network underlies the generation of inspiratory behaviors. Proc Natl Acad Sci USA 116: 7493–7502, 2019. doi: 10.1073/pnas.1900523116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA. Breathing control center neurons that promote arousal in mice. Science 355: 1411–1415, 2017. doi: 10.1126/science.aai7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol 534: 565–581, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J Appl Physiol 96: 2057–2072, 2004. doi: 10.1152/japplphysiol.00778.2003. [DOI] [PubMed] [Google Scholar]
- 41.Lindsey B, Hernandez Y, Morris K, Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol 67: 890–904, 1992. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey B, Ott M, Nuding S, Segers L, O'Connor R, Morris K. Central chemoreceptors modulate breathing via multipath tuning in ventrolateral respiratory column (VRC) circuits. J Neurophysiol 107: 603–617, 2012. doi: 10.1152/jn.00808.2011.[21994272] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey BG, Morris KF, Segers LS, Shannon R. Respiratory neuronal assemblies. Respir Physiol 122: 183–196, 2000. doi: 10.1016/s0034-5687(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 44.Morris KF, Arata A, Shannon R, Lindsey B. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neuron correlations. J Physiol 491: 241–259, 1996. doi: 10.1113/jphysiol.1996.sp021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris K, Shannon R, Lindsey B. Changes in cat medullary neuron firing rates and synchrony following induction of respiratory long‐term facilitation. J Physiol 532: 483–497, 2001. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuding SC, Segers LS, Iceman KE, O'Connor R, Dean JB, Bolser DC, Baekey DM, Dick TE, Shannon R, Morris KF, Lindsey BG. Functional connectivity in raphe-pontomedullary circuits supports active suppression of breathing during hypocapnic apnea. J Neurophysiol 114: 2162–2186, 2015. doi: 10.1152/jn.00608.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, Davenport PW, Lindsey BG. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol 3: 264, 2012. doi: 10.3389/fphys.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. J Neurophysiol 105: 2960–2975, 2011. doi: 10.1152/jn.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O'Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol 57: 1078–1100, 1987. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- 52.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol 84: 2020–2035, 1998. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 53.Lindsey B, Hernandez Y, Morris K, Shannon R, Gerstein G. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol 67: 923–930, 1992. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- 54.Lindsey B, Morris K, Shannon R, Gerstein G. Repeated patterns of distributed synchrony in neuronal assemblies. J Neurophysiol 78: 1714–1719, 1997. doi: 10.1152/jn.1997.78.3.1714. [DOI] [PubMed] [Google Scholar]
- 55.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurons and responses during fictive cough in the cat. J Physiol 525: 207–224, 2000. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci 23: 8152–8158, 2003. doi: 10.1523/JNEUROSCI.23-22-08152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X, Thorn PC, Halemani DN, Shao XM, Greenwood M, Heath S, Feldman JL, Kam K. Opioids modulate an emergent rhythmogenic process to depress breathing. eLife 8: 50613, 2019. doi: 10.7554/eLife.50613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice A, Fuglevand AJ, Laine CM, Fregosi RF. Synchronization of presynaptic input to motor units of tongue, inspiratory intercostal, and diaphragm muscles. J Neurophysiol 105: 2330–2336, 2011. doi: 10.1152/jn.01078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen MI. Central determinants of respiratory rhythm. Ann Rev Physiol 43: 91–104, 1981. doi: 10.1146/annurev.ph.43.030181.000515. [DOI] [PubMed] [Google Scholar]
- 60.Cohen MI, See WR, Christakos CN, Sica AL. High-frequency and medium-frequency components of different inspiratory nerve discharges and their modification by various inputs. Brain Res 417: 148–152, 1987. doi: 10.1016/0006-8993(87)90190-9. [DOI] [PubMed] [Google Scholar]
- 61.Kato F. Suppression of inspiratory fast rhythm, but not bilateral short-term synchronization, by morphine in anesthetized rabbit. Neurosci Lett 258: 89–92, 1998. doi: 10.1016/S0304-3940(98)00861-1. [DOI] [PubMed] [Google Scholar]
- 62.Lalley PM, Mifflin SW. Oscillation patterns are enhanced and firing threshold is lowered in medullary respiratory neuron discharges by threshold doses of a mu-opioid receptor agonist. Am J Physiol Regul Integr Comp Physiol 312: R727–R738, 2017. doi: 10.1152/ajpregu.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segers L, Nuding S, Vovk A, Pitts T, Baekey D, O’Connor R, Morris K, Lindsey B, Shannon R, Bolser DC. Discharge identity of medullary inspiratory neurons is altered during repetitive fictive cough. Front Physiol 3: 1–15, 2012. doi: 10.3389/fphys.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982. [DOI] [PubMed] [Google Scholar]
- 65.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am 46: 957–964, 2013. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shannon R, Baekey D, Morris K, Lindsey B. Brainstem respiratory networks and cough. Pulm Pharmacol 9: 343–347, 1996. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 67.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol 152: 255–265, 2006. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson TM, Ramirez JM. Respiratory rhythm generation: triple oscillator hypothesis. F1000Res 6: 139, 2017. doi: 10.12688/f1000research.10193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Ann Rev Physiology 75: 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28: 12845–12850, 2008. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thoby-Brisson M, Karlén M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat Neurosci 12: 1028–1035, 2009. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- 73.Huckstepp RT, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: 14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. PHYSIOLOGY. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348: 1255–1260, 2015. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33: 7756–7761, 2013. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. A novel excitatory network for the control of breathing. Nature 536: 76–80, 2016. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W, Fung ML, St John WM. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol (1985) 74: 2801–2811, 1993. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- 79.Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol 507: 895–907, 1998. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286: 1566–1568, 1999. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBötzinger complex neurons in adult rats. J Comp Neurol 518: 1862–1878, 2010. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. Crosstalk opposing view: The pre-Botzinger complex is not essential for respiratory depression following systemic administration of opioid analgesics. J Physiol 592: 1163–1166, 2014. doi: 10.1113/jphysiol.2013.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia AJ, Dashevskiy T, Khuu MA, Ramirez JM. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol 8: 571, 2017. doi: 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol (1985) 97: 1629–1636, 2004. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 86.Kottick A, Martin CA, Del Negro CA. Fate mapping neurons and glia derived from Dbx1-expressing progenitors in mouse preBötzinger complex. Physiol Rep 5: e13300, 2017. doi: 10.14814/phy2.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. eLife 9: 52694, 2020. doi: 10.7554/eLife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayes JA, Kottick A, Picardo MCD, Halleran AD, Smith RD, Smith GD, Saha MS, Del Negro CA. Transcriptome of neonatal preBötzinger complex neurones in Dbx1 reporter mice. Sci Rep 7: 8669, 2017. doi: 10.1038/s41598-017-09418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenín J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci 30: 14883–14895, 2010. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carroll MS, Ramirez JM. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J Neurophysiol 109: 296–305, 2013. doi: 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carroll MS, Viemari JC, Ramirez JM. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J Neurophysiol 109: 285–295, 2013. doi: 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song H, Hayes JA, Vann NC, Wang X, LaMar MD, Del Negro CA. Functional interactions between mammalian respiratory rhythmogenic and premotor circuitry. J Neurosci 36: 7223–7233, 2016. doi: 10.1523/JNEUROSCI.0296-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaiteri C, Rubin JE. The interaction of intrinsic dynamics and network topology in determining network burst synchrony. Front Comput Neurosci 5: 10, 2011. doi: 10.3389/fncom.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci 26: 3721–3730, 2006. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci 12: 2975–2984, 2000. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 96.Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat Commun 9: 843, 2018. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci 33: 9235–9245, 2013. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kallurkar PS, Grover C, Picardo MCD, Del Negro CA. Evaluating the Burstlet theory of inspiratory rhythm and pattern generation. eNeuro 7: ENEURO.0314-19.2019, 2020. doi: 10.1523/ENEURO.0314-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerrier C, Hayes JA, Fortin G, Holcman D. Robust network oscillations during mammalian respiratory rhythm generation driven by synaptic dynamics. Proc Natl Acad Sci USA 112: 9728–9733, 2015. doi: 10.1073/pnas.1421997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kam K, Worrell JW, Ventalon C, Emiliani V, Feldman JL. Emergence of population bursts from simultaneous activation of small subsets of preBötzinger complex inspiratory neurons. J Neurosci 33: 3332–3338, 2013. doi: 10.1523/JNEUROSCI.4574-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carrillo-Reid L, Yang W, Bando Y, Peterka DS, Yuste R. Imprinting and recalling cortical ensembles. Science 353: 691–694, 2016. doi: 10.1126/science.aaf7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrillo-Reid L, Han S, Yang W, Akrouh A, Yuste R. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178: 447–457, 2019. doi: 10.1016/j.cell.2019.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayes JA, Wang X, Del Negro CA. Cumulative lesioning of respiratory interneurons disrupts and precludes motor rhythms in vitro. Proc Natl Acad Sci USA 109: 8286–8291, 2012. doi: 10.1073/pnas.1200912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Hayes JA, Revill AL, Song H, Kottick A, Vann NC, LaMar MD, Picardo MC, Akins VT, Funk GD, Del Negro CA. Laser ablation of Dbx1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. eLife 3: e03427, 2014. doi: 10.7554/eLife.03427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song H, Hayes JA, Vann NC, Drew LaMar M, Del Negro CA. Mechanisms leading to rhythm cessation in the respiratory PreBötzinger complex due to piecewise cumulative neuronal deletions. eNeuro 2: ENEURO.0031-15.2015, 2015. doi: 10.1523/ENEURO.0031-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harris KD, Dashevskiy T, Mendoza J, Garcia AJ, Ramirez JM, Shea-Brown E. Different roles for inhibition in the rhythm-generating respiratory network. J Neurophysiol 118: 2070–2088, 2017. doi: 10.1152/jn.00174.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramirez JM, Garcia A. Point: medullary pacemaker neurons are essential for both eupnea and gasping in mammals. J Appl Physiol (1985) 103: 717–718, 2007. doi: 10.1152/japplphysiol.00003.2007. [DOI] [PubMed] [Google Scholar]
- 109.Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL. Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology 94: 824–832, 2001. doi: 10.1097/00000542-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 110.Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr Opin Pharmacol 6: 559–563, 2006. doi: 10.1016/j.coph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Jean-Charles PY, Kaur S, Shenoy SK. G Protein-coupled receptor signaling through β-arrestin-dependent mechanisms. J Cardiovasc Pharmacol 70: 142–158, 2017. doi: 10.1097/FJC.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith JS, Rajagopal S. The β-arrestins: multifunctional regulators of G protein-coupled receptors. J Biol Chem 291: 8969–8977, 2016. doi: 10.1074/jbc.R115.713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52: 179–197, 2012. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luttrell LM, Miller WE. Arrestins as regulators of kinases and phosphatases. Prog Mol Biol Transl Sci 118: 115–147, 2013. doi: 10.1016/B978-0-12-394440-5.00005-X. [DOI] [PubMed] [Google Scholar]
- 115.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201, 2005. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 116.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537: 185–190, 2016. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 17: 243–260, 2018. doi: 10.1038/nrd.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10: 367, 2019. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol 177: 2923–2931, 2020. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326, 1987. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 121.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature 370: 143–146, 1994. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 122.Krapivinsky G, Krapivinsky L, Wickman K, Clapham DE. Gβγ binds directly to the G protein-gated K+ channel, IKACh. J Biol Chem 270: 29059–29062, 1995. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 123.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147: 199–208, 2011. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498: 190–197, 2013. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neuron action potentials. Nature 276: 837–839, 1978. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- 126.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153–156, 1989. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 127.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380: 258–262, 1996. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 128.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 129.Colecraft HM, Patil PG, Yue DT. Differential occurrence of reluctant openings in G-protein-inhibited N- and P/Q-type calcium channels. J Gen Physiol 115: 175–192, 2000. doi: 10.1085/jgp.115.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zamponi GW, Currie KP. Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim Biophys Acta 1828: 1629–1643, 2013. doi: 10.1016/j.bbamem.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31: 1292–1301, 2011. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Montandon G, Liu H, Horner RL. Contribution of the respiratory network to rhythm and motor output revealed by modulation of GIRK channels, somatostatin and neurokinin-1 receptors. Sci Rep 6: 32707, 2016. doi: 10.1038/srep32707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124: 641–650, 2016. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lalley PM. D1-dopamine receptor blockade slows respiratory rhythm and enhances opioid-mediated depression. Respir Physiol Neurobiol 145: 13–22, 2005. doi: 10.1016/j.resp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lalley PM. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. Am J Physiol Regul Integr Comp Physiol 296: R1829–R1836, 2009. doi: 10.1152/ajpregu.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mosca EV, Ciechanski P, Roy A, Scheibli EC, Ballanyi K, Wilson RJ. Methylxanthine reversal of opioid-induced respiratory depression in the neonatal rat: mechanism and location of action. Respir Physiol Neurobiol 200: 80–89, 2014. doi: 10.1016/j.resp.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 138.Ruangkittisakul A, Panaitescu B, Kuribayashi J, Ballanyi K. Caffeine reversal of opioid-evoked and endogenous inspiratory depression in perinatal rat en bloc medullas and slices. Adv Exp Med Biol 669: 123–127, 2010. doi: 10.1007/978-1-4419-5692-7_25. [DOI] [PubMed] [Google Scholar]
- 139.Ruangkittisakul A, Ballanyi K. Methylxanthine reversal of opioid-evoked inspiratory depression via phosphodiesterase-4 blockade. Respir Physiol Neurobiol 172: 94–105, 2010. doi: 10.1016/j.resp.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 140.Ruangkittisakul A, Ballanyi K. Reversal by phosphodiesterase-4 blockers of in vitro apnea in the isolated brainstem-spinal cord preparation from newborn rats. Neurosci Lett 401: 194–198, 2006. doi: 10.1016/j.neulet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 141.Kimura S, Haji A. Pharmacological strategy for overcoming opioid-induced ventilatory disturbances. Eur J Pharmacol 725: 87–90, 2014. doi: 10.1016/j.ejphar.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 142.Kimura S, Ohi Y, Haji A. Blockade of phosphodiesterase 4 reverses morphine-induced ventilatory disturbance without loss of analgesia. Life Sci 127: 32–38, 2015. doi: 10.1016/j.lfs.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 143.Kramer IM. Signal Transduction. London: Elsevier/Academic Press, 2016, p. 1. [Google Scholar]
- 144.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 145.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Ann Rev Physiol 65: 453–480, 2003. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 146.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci 19: 235–263, 1996. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 147.Baruscotti M, Bottelli G, Milanesi R, DiFrancesco JC, DiFrancesco D. HCN-related channelopathies. Pflugers Arch 460: 405–415, 2010. doi: 10.1007/s00424-010-0810-8. [DOI] [PubMed] [Google Scholar]
- 148.Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56: 1076–1089, 2007. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of K2P channels: salient structural and functional properties. J Physiol 593: 2587–2603, 2015. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cotten JF, Keshavaprasad B, Laster MJ, Eger EI, Yost CS. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth Analg 102: 779–785, 2006. doi: 10.1213/01.ane.0000194289.34345.63. [DOI] [PubMed] [Google Scholar]
- 152.Cotten JF. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth Analg 116: 810–816, 2013. doi: 10.1213/ANE.0b013e318284469d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci 30: 4273–4284, 2010. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheuer T. Regulation of sodium channel activity by phosphorylation. Semin Cell Dev Biol 22: 160–165, 2011. doi: 10.1016/j.semcdb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mattheisen GB, Tsintsadze T, Smith SM. Strong G-protein-mediated inhibition of sodium channels. Cell Rep 23: 2770–2781, 2018. doi: 10.1016/j.celrep.2018.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yeh SY, Huang WH, Wang W, Ward CS, Chao ES, Wu Z, Tang B, Tang J, Sun JJ, Esther van der Heijden M, Gray PA, Xue M, Ray RS, Ren D, Zoghbi HY. Respiratory network stability and modulatory response to substance P require Nalcn. Neuron 94: 294–303, 2017.doi: 10.1016/j.neuron.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ren D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 72: 899–911, 2011. doi: 10.1016/j.neuron.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Philippart F, Khaliq ZG. Gi/o protein-coupled receptors in dopamine neurons inhibit the sodium leak channel NALCN. eLife 7: e40984, 2018. doi: 10.7554/eLife.40984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Topalidou I, Cooper K, Pereira L, Ailion M. Dopamine negatively modulates the NCA ion channels in C. elegans. PLoS Genet 13: e1007032, 2017. doi: 10.1371/journal.pgen.1007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292: 293–297, 2001. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 161.Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci 8: 421–425, 2005. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- 162.Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci 8: 597–605, 2005. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- 163.Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. Gbetagamma interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol Pharmacol 72: 1210–1219, 2007. doi: 10.1124/mol.107.039446. [DOI] [PubMed] [Google Scholar]
- 164.Heinke B, Gingl E, Sandkühler J. Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers. J Neurosci 31: 1313–1322, 2011. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBötzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol 547: 543–553, 2003. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143, 2003. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 167.Hell JW. How Ca2+-permeable AMPA receptors, the kinase PKA, and the phosphatase PP2B are intertwined in synaptic LTP and LTD. Sci Signal 9: e2, 2016. doi: 10.1126/scisignal.aaf7067. [DOI] [PubMed] [Google Scholar]
- 168.Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron 100: 314–329, 2018. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110: 1364–1370, 2009. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 170.Ren J, Ding X, Greer JJ. Ampakines enhance weak endogenous respiratory drive and alleviate apnea in perinatal rats. Am J Respir Crit Care Med 191: 704–710, 2015. doi: 10.1164/rccm.201410-1898OC. [DOI] [PubMed] [Google Scholar]
- 171.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Langer TM, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV. Effects on breathing of agonists to μ-opioid or GABA. Respir Physiol Neurobiol 239: 10–25, 2017. doi: 10.1016/j.resp.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol (1985) 107: 1591–1599, 2009. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol 103: 409–418, 2010. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Doi A, Ramirez J-M. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci 7: 1345–1352, 2004. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 177.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci 365: 2363–2374, 2010. doi: 10.1098/rstb.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Forster HV, Julius H. Comroe distinguished lecture: interdependence of neuromodulators in the control of breathing. J Appl Physiol (1985) 125: 1511–1525, 2018. doi: 10.1152/japplphysiol.00477.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Langer TM, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan L, Forster HV. State-dependent and -independent effects of dialyzing excitatory neuromodulator receptor antagonists into the ventral respiratory column. J Appl Physiol (1985) 122: 327–338, 2017. doi: 10.1152/japplphysiol.00619.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hurlé MA, Mediavilla A, Flórez J. Participation of pontine structures in the respiratory action of opioids. Life Sci 33: 571–574, 1983. doi: 10.1016/0024-3205(83)90567-2. [DOI] [PubMed] [Google Scholar]
- 182.Hurlé MA, Mediavilla A, Flórez J. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology 24: 597–606, 1985. doi: 10.1016/0028-3908(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 183.Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 14: 6500–6510, 1994. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Levitt ES, Abdala AP, Paton JF, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol 593: 4453–4469, 2015. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zuperku EJ, Stucke AG, Hopp FA, Stuth EA. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J Neurophysiol 117: 1030–1042, 2017. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Navarrete-Opazo AA, Cook-Snyder DR, Miller JR, Callison JJ, McCarthy N, Palkovic B, Stuth EAE, Zuperku EJ, Stucke AG. Endogenous glutamatergic inputs to the parabrachial nucleus/Kölliker-fuse complex determine respiratory rate. Respir Physiol Neurobiol 277: 103401, 2020. doi: 10.1016/j.resp.2020.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dhingra RR, Dutschmann M, Galán RF, Dick TE. Kölliker-fuse nuclei regulate respiratory rhythm variability via a gain-control mechanism. Am J Physiol Regul Integr Comp Physiol 312: R172–R188, 2017. doi: 10.1152/ajpregu.00238.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fung ML, St John WM. The functional expression of a pontine pneumotaxic centre in neonatal rats. J Physiol 489: 579–591, 1995. doi: 10.1113/jphysiol.1995.sp021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 190.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dhingra RR, Furuya WI, Bautista TG, Dick TE, Galán RF, Dutschmann M. Increasing local excitability of brainstem respiratory nuclei reveals a distributed network underlying respiratory motor pattern formation. Front Physiol 10: 887, 2019. doi: 10.3389/fphys.2019.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol 489: 877–884, 1995. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol 307: R57–R67, 2014. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- 194.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, Wellman A, Arrigoni E, Fuller PM, Saper CB. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron 96: 1153–1167, 2017. doi: 10.1016/j.neuron.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB. The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J Neurophysiol 119: 401–412, 2018. doi: 10.1152/jn.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Heijden ME, Zoghbi HY. Loss of Atoh1 from neurons regulating hypoxic and hypercapnic chemoresponses causes neonatal respiratory failure in mice. eLife 7: e38455, 2018. doi: 10.7554/eLife.38455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y. Glutamatergic Kölliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Res 1404: 10–20, 2011. doi: 10.1016/j.brainres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 198.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bautista TG, Dutschmann M. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience 267: 22–29, 2014. doi: 10.1016/j.neuroscience.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 200.Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 4: 317–325, 2014. doi: 10.2217/pmt.14.19. [DOI] [PubMed] [Google Scholar]
- 201.Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL. The effects of anesthesia and opioids on the upper airway: a systematic review. Laryngoscope 126: 270–284, 2016. doi: 10.1002/lary.25399. [DOI] [PubMed] [Google Scholar]
- 202.Pattinson KTS, Rowland MJ, Nickol AH, Quinlan J. Adverse respiratory effects of opioids for chronic breathlessness: learning lessons from chronic pain. Eur Respir J 51: 1702531, 2018. doi: 10.1183/13993003.02531-2017. [DOI] [PubMed] [Google Scholar]
- 203.Christ A, Arranto CA, Schindler C, Klima T, Hunziker PR, Siegemund M, Marsch SC, Eriksson U, Mueller C. Incidence, risk factors, and outcome of aspiration pneumonitis in ICU overdose patients. Intensive Care Med 32: 1423–1427, 2006. doi: 10.1007/s00134-006-0277-4. [DOI] [PubMed] [Google Scholar]
- 204.Savilampi J, Ahlstrand R, Magnuson A, Geijer H, Wattwil M. Aspiration induced by remifentanil: a double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 121: 52–58, 2014. doi: 10.1097/ALN.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 205.Savilampi J, Omari T, Magnuson A, Ahlstrand R. Effects of remifentanil on pharyngeal swallowing: a double blind randomised cross-over study in healthy volunteers. Eur J Anaesthesiol 33: 622–630, 2016. doi: 10.1097/EJA.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 206.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol 108: 2430–2441, 2012. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG. A subregion of the parabrachial nucleus partially mediates respiratory rate depression from intravenous remifentanil in young and adult rabbits. Anesthesiology 127: 502–514, 2017. doi: 10.1097/ALN.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol 138: 165–178, 2003. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- 209.Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, Stuth EA. Opioid-induced respiratory depression is only partially mediated by the preBötzinger complex in young and adult rabbits in vivo. Anesthesiology 122: 1288–1298, 2015. doi: 10.1097/ALN.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schmidt CF, Harer WB. The action of drugs on respiration: I. The Morphine series. J Exp Med 37: 47–67, 1923. doi: 10.1084/jem.37.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Saunders SE, Levitt ES. Kölliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respir Physiol Neurobiol 275: 103388, 2020. doi: 10.1016/j.resp.2020.103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice. J Physiol 598: 189–205, 2020. doi: 10.1113/JP278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dutschmann M, Herbert H. NMDA and GABAA receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J Physiol 510: 793–804, 1998. doi: 10.1111/j.1469-7793.1998.793bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JF. Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. J Physiol 594: 223–237, 2016. doi: 10.1113/JP270966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol 123: 201–213, 2000. doi: 10.1016/S0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 216.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Comstock MK, Carter JG, Moyers JR, Stevens WC. Rigidity and hypercarbia associated with high dose fentanyl induction of anesthesia. Anesth Analg 60: 362–363, 1981. [PubMed] [Google Scholar]
- 218.Çoruh B, Tonelli MR, Park DR. Fentanyl-induced chest wall rigidity. Chest 143: 1145–1146, 2013. doi: 10.1378/chest.12-2131. [DOI] [PubMed] [Google Scholar]
- 219.Burns G, DeRienz RT, Baker DD, Casavant M, Spiller HA. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin Toxicol (Phila) 54: 420–423, 2016. doi: 10.3109/15563650.2016.1157722. [DOI] [PubMed] [Google Scholar]
- 220.Jerussi TP, Capacchione JF, Benvenga MJ. Reversal of opioid-induced muscular rigidity in rats: evidence for alpha-2 adrenergic involvement. Pharmacol Biochem Behav 28: 283–289, 1987. doi: 10.1016/0091-3057(87)90226-7. [DOI] [PubMed] [Google Scholar]
- 221.Negus SS, Pasternak GW, Koob GF, Weinger MB. Antagonist effects of beta-funaltrexamine and naloxonazine on alfentanil-induced antinociception and muscle rigidity in the rat. J Pharmacol Exp Ther 264: 739–745, 1993. [PubMed] [Google Scholar]
- 222.Weinger MB, Chen DY, Lin T, Lau C, Koob GF, Smith NT. A role for CNS alpha-2 adrenergic receptors in opiate-induced muscle rigidity in the rat. Brain Res 669: 10–18, 1995. doi: 10.1016/0006-8993(94)01216-5. [DOI] [PubMed] [Google Scholar]
- 223.Campbell C, Weinger MB, Quinn M. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respir Physiol 100: 107–117, 1995. doi: 10.1016/0034-5687(94)00119-k. [DOI] [PubMed] [Google Scholar]
- 224.Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol 56: 97–117, 1998. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 226.Chamberlin NL, Mansour A, Watson SJ, Saper CB. Localization of mu-opioid receptors on amygdaloid projection neurons in the parabrachial nucleus of the rat. Brain Res 827: 198–204, 1999. doi: 10.1016/s0006-8993(99)01168-3. [DOI] [PubMed] [Google Scholar]
- 227.Greenwell TN, Martin-Schild S, Inglis FM, Zadina JE. Colocalization and shared distribution of endomorphins with substance P, calcitonin gene-related peptide, gamma-aminobutyric acid, and the mu opioid receptor. J Comp Neurol 503: 319–333, 2007. doi: 10.1002/cne.21374. [DOI] [PubMed] [Google Scholar]
- 228.Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 220: 677–702, 2015. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Christie MJ, North RA. Agonists at mu-opioid, M2-muscarinic and GABAB-receptors increase the same potassium conductance in rat lateral parabrachial neurones. Br J Pharmacol 95: 896–902, 1988. doi: 10.1111/j.1476-5381.1988.tb11719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol 143: 167–175, 2004. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 231.Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 175: 145–153, 2011. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang CF, Feldman JL. Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J Comp Neurol 526: 1389–1402, 2018. doi: 10.1002/cne.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141: 1011–1023, 2006. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 234.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 235.Song G, Wang H, Xu H, Poon CS. Kölliker–Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct Funct 217: 835–858, 2012. doi: 10.1007/s00429-012-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J Comp Neurol 523: 907–920, 2015. doi: 10.1002/cne.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL. Kölliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol 525: 1844–1860, 2017. doi: 10.1002/cne.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kobayashi S, Onimaru H, Inoue M, Inoue T, Sasa R. Localization and properties of respiratory neurons in the rostral pons of the newborn rat. Neuroscience 134: 317–325, 2005. doi: 10.1016/j.neuroscience.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 239.Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci 26: 300–310, 2006. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mörschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain research 197: 291–317, 1980. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 243.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci 33: 7627–7640, 2013. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Martelli D, Stanić D, Dutschmann M. The emerging role of the parabrachial complex in the generation of wakefulness drive and its implication for respiratory control. Respir Physiol Neurobiol 188: 318–323, 2013. doi: 10.1016/j.resp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 245.Kaur S, Saper CB. Neural circuitry underlying waking up to hypercapnia. Front Neurosci 13: 401, 2019. doi: 10.3389/fnins.2019.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hayashi Y, Kashiwagi M, Yasuda K, Ando R, Kanuka M, Sakai K, Itohara S. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science 350: 957–961, 2015. doi: 10.1126/science.aad1023. [DOI] [PubMed] [Google Scholar]
- 247.Qiu MH, Chen MC, Fuller PM, Lu J. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra-thalamic forebrain circuit nodes. Curr Biol 26: 2301–2312, 2016. doi: 10.1016/j.cub.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493: 99–110, 2005. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 249.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13: 1526–1533, 2010. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Berridge CW, Schmeichel BE, España RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev 16: 187–197, 2012. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol 513: 381–398, 1998. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.de Carvalho D, Patrone LG, Taxini CL, Biancardi V, Vicente MC, Gargaglioni LH. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2drive to breathe. Front Physiol 5: 288, 2014. doi: 10.3389/fphys.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vicente MC, Dias MB, Fonseca EM, Bícego KC, Gargaglioni LH. Orexinergic system in the locus coeruleus modulates the CO2 ventilatory response. Pflugers Arch 468: 763–774, 2016. doi: 10.1007/s00424-016-1793-x. [DOI] [PubMed] [Google Scholar]
- 254.Oliveira LM, Tuppy M, Moreira TS, Takakura AC. Role of the locus coeruleus catecholaminergic neurons in the chemosensory control of breathing in a Parkinson's disease model. Exp Neurol 293: 172–180, 2017. doi: 10.1016/j.expneurol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 255.Magalhães KS, Spiller PF, da Silva MP, Kuntze LB, Paton JFR, Machado BH, Moraes DJA. Locus coeruleus as a vigilance centre for active inspiration and expiration in rats. Sci Rep 8: 15654, 2018. doi: 10.1038/s41598-018-34047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.North RA, Williams JT. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol 364: 265–280, 1985. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Blomqvist A, Hermanson O, Ericson H, Larhammar D. Activation of a bulbospinal opioidergic projection by pain stimuli in the awake rat. Neuroreport 5: 461–464, 1994. doi: 10.1097/00001756-199401120-00023. [DOI] [PubMed] [Google Scholar]
- 258.Hermanson O, Blomqvist A. Enkephalinergic and catecholaminergic neurons constitute separate populations in the rat Kölliker-Fuse/A7 region. Neurosci Lett 190: 57–60, 1995. doi: 10.1016/0304-3940(95)11499-m. [DOI] [PubMed] [Google Scholar]
- 259.Hermanson O, Blomqvist A. Preproenkephalin messenger RNA-expressing neurons in the rat parabrachial nucleus: subnuclear organization and projections to the intralaminar thalamus. Neuroscience 81: 803–812, 1997. doi: 10.1016/s0306-4522(97)00241-8. [DOI] [PubMed] [Google Scholar]
- 260.Engström L, Engblom D, Ortegren U, Mackerlova L, Paues J, Blomqvist A. Preproenkephalin mRNA expression in rat parabrachial neurons: relation to cells activated by systemic immune challenge. Neurosci Lett 316: 165–168, 2001. doi: 10.1016/s0304-3940(01)02393-x. [DOI] [PubMed] [Google Scholar]
- 261.Dutschmann M, Kron M, Mörschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol 159: 232–235, 2007. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 262.Abdala AP, Lioy DT, Garg SK, Knopp SJ, Paton JF, Bissonnette JM. Effect of Sarizotan, a 5-HT1a and D2-like receptor agonist, on respiration in three mouse models of Rett syndrome. Am J Respir Cell Mol Biol 50: 1031–1039, 2014. doi: 10.1165/rcmb.2013-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dhingra RR, Dutschmann M, Dick TE. Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Respir Physiol Neurobiol 226: 110–114, 2016. doi: 10.1016/j.resp.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Toyama S, Shimoyama N, Tagaito Y, Nagase H, Saitoh T, Yanagisawa M, Shimoyama M. Nonpeptide orexin-2 receptor agonist attenuates morphine-induced sedative effects in rats. Anesthesiology 128: 992–1003, 2018. doi: 10.1097/ALN.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 265.Pattinson KTS, Wise RG. Imaging the respiratory effects of opioids in the human brain. In: Hypoxia, edited byRoach R, Hackett P, and Wagner P.. Boston, MA: Springer, 2016, p. 145–156. [DOI] [PubMed] [Google Scholar]
- 266.Ong W-Y, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol 56: 1137–1166, 2019. doi: 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL. Opioid use disorder. Nat Rev Dis Primers 6: 1–28, 2020. doi: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- 268.Baldino BA. Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci 39: 366–377, 2016. doi: 10.1016/j.tins.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Alizadehgoradel J, Nejati V, Sadeghi Movahed F, Imani S, Taherifard M, Mosayebi-Samani M, Vicario CM, Nitsche MA, Salehinejad MA. Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: a randomized, double-blind, parallel-group study. Brain Stimul 13: 582–593, 2020. doi: 10.1016/j.brs.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 270.Carlén M. What constitutes the prefrontal cortex? Science 358: 478–482, 2017. doi: 10.1126/science.aan8868. [DOI] [PubMed] [Google Scholar]
- 271.Furster J. Chemical neurotransmission. In: The Prefrontal Cortex. New York, NY: Academic Press, 2015, p. 9–62. [Google Scholar]
- 272.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177, 2005. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 273.Bacon SJ, Smith AD. A monosynaptic pathway from an identified vasomotor centre in the medial prefrontal cortex to an autonomic area in the thoracic spinal cord. Neuroscience 54: 719–728, 1993. doi: 10.1016/0306-4522(93)90242-8. [DOI] [PubMed] [Google Scholar]
- 274.Smith WK. The representation of respiratory movements in the cerebral cortex. J Neurophysiol 1: 55–68, 1938. doi: 10.1152/jn.1938.1.1.55. [DOI] [Google Scholar]
- 275.Hassan SF, Cornish JL, Goodchild AK. Respiratory, metabolic and cardiac functions are altered by disinhibition of subregions of the medial prefrontal cortex. J Physiol 591: 6069–6088, 2013. doi: 10.1113/jphysiol.2013.262071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Xu P, Chen A, Li Y, Xing X, Lu H. Medial prefrontal cortex in neurological diseases. Physiol Genomics 51: 432–442, 2019. doi: 10.1152/physiolgenomics.00006.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Furster J. Chemical neurotransmission. In: The Prefrontal Cortex. New York: Academic Press, 2015, p. 63–131. [Google Scholar]
- 278.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18: 22–29, 1995. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 279.Light SN, Bieliauskas LA, Zubieta J-K. “Top-down” mu-opioid system function in humans: mu-opioid receptors in ventrolateral prefrontal cortex mediate the relationship between hedonic tone and executive function in major depressive disorder. J Neuropsychiatry Clin Neurosci 29: 357–364, 2017. doi: 10.1176/appi.neuropsych.16090171. [DOI] [PubMed] [Google Scholar]
- 280.Peciña M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, Zubieta J-K. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry 24: 576–587, 2019. doi: 10.1038/s41380-018-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69: 1061–1068, 2011. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.O’Brien CB, Baghdoyan HA, Lydic R. Computer-based multitaper spectrogram program for electroencephalographic data. J Vis Exp: 1–7, 2019.doi: 10.3791/60333. [DOI] [PubMed] [Google Scholar]
- 283.Bourdon AK, Spano GM, Marshall W, Bellesi M, Tononi G, Serra PA, Baghdoyan HA, Lydic R, Campagna SR, Cirelli C. Metabolomic analysis of mouse prefrontal cortex reveals upregulated analytes during wakefulness compared to sleep. Sci Rep 8: 11225, 2018. doi: 10.1038/s41598-018-29511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhang X, Baer A, Ferraro K, Hargrove B, Mi W, Lydic R, Baghdoyan HA. Morphine and fentanyl delivered to prefrontal cortex of behaving mice depress breathing and alter neurotransmitter concentrations. Anesth Analg 128: 422, 2019. https://journals.lww.com/anesthesia-analgesia/Citation/2019/05002/Abstracts_of_Posters_presented_at_the.1.aspx. [Google Scholar]
- 285.Glovak ZT, O’Brien CB, Baghdoyan HA, Lydic R. Delivery of morphine to mouse prefrontal cortex alters minute ventilation. Soc Neurosci Meeting Planner, 2019. Program No. 231.204. https://www.abstractsonline.com/pp8/#!/7883/presentation/61336. [Google Scholar]
- 286.Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull 19: 639–649, 1987. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- 287.Gruhzit CC. Chemoreflex activity of dextromethorphan (romilar), dextrorphan, codeine and morphine in the cat and the dog. J Pharmacol Exp Ther 120: 399–407, 1957. [PubMed] [Google Scholar]
- 288.Wojciechowski P, Szereda-Przestaszewska M, Lipkowski AW. Supranodose vagotomy eliminates respiratory depression evoked by dermorphin in anaesthetized rats. Eur J Pharmacol 563: 209–212, 2007. doi: 10.1016/j.ejphar.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 289.Zhang Z, Zhang C, Zhou M, Xu F. Activation of opioid mu-receptors, but not delta- or kappa-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats. Respir Physiol Neurobiol 183: 211–217, 2012. doi: 10.1016/j.resp.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Willette RN, Sapru HN. Pulmonary opiate receptor activation evokes a cardiorespiratory reflex. Eur J Pharmacol 78: 61–70, 1982. doi: 10.1016/0014-2999(82)90372-7. [DOI] [PubMed] [Google Scholar]
- 291.Kaczynska K, Szereda-Przestaszewska M. Involvement of vagal opioid receptors in respiratory effects of morphine in anaesthetized rats. J Physiol Pharmacol 56: 195–203, 2005. [PubMed] [Google Scholar]
- 292.Henderson F, May WJ, Gruber RB, Young AP, Palmer LA, Gaston B, Lewis SJ. Low-dose morphine elicits ventilatory excitant and depressant responses in conscious rats: Role of peripheral mu-opioid receptors. Open J Mol Integr Physiol 3: 111–124, 2013. doi: 10.4236/ojmip.2013.33017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhuang J, Zhang Z, Zhang C, Xu F. 8-OH-DPAT abolishes the pulmonary C-fiber-mediated apneic response to fentanyl largely via acting on 5HT1A receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 303: R449–R458, 2012. doi: 10.1152/ajpregu.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wojciechowski P, Szereda-Przestaszewska M, Lipkowski AW. Respiratory and cardiovascular effects of biphalin in anaesthetized rats. Eur J Pharmacol 602: 50–53, 2009. doi: 10.1016/j.ejphar.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 295.Sapru HN, Willette RN, Krieger AJ. Stimulation of pulmonary J receptors by an enkephalin-analog. J Pharmacol Exp Ther 217: 228–234, 1981. [PubMed] [Google Scholar]
- 296.Willette RN, Gatti P, Gertner SB, Sapru HN. Pulmonary vagal afferent stimulants in the conscious rat: opioids and phenyldiguanide. Pharmacol Biochem Behav 17: 19–23, 1982. doi: 10.1016/0091-3057(82)90256-8. [DOI] [PubMed] [Google Scholar]
- 297.Bhargava HN, Villar VM, Cortijo J, Morcillo EJ. Binding of [3H][D-Ala2, MePhe4, Gly-ol5] enkephalin, [3H][D-Pen2, D-Pen5]enkephalin, and [3H]U-69,593 to airway and pulmonary tissues of normal and sensitized rats. Peptides 18: 1603–1608, 1997. doi: 10.1016/S0196-9781(97)00247-7. [DOI] [PubMed] [Google Scholar]
- 298.Li JL, Kaneko T, Mizuno N. Effects of peripheral nerve ligation on expression of mu-opioid receptor in sensory ganglion neurons: an immunohistochemical study in dorsal root and nodose ganglion neurons of the rat. Neurosci Lett 214: 91–94, 1996. doi: 10.1016/0304-3940(96)12894-9. [DOI] [PubMed] [Google Scholar]
- 299.Cabot PJ, Dodd PR, Cramond T, Smith MT. Characterization of non-conventional opioid binding sites in rat and human lung. Eur J Pharmacol 268: 247–255, 1994. doi: 10.1016/0922-4106(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 300.Cabot PJ, Cramond T, Smith MT. Quantitative autoradiography of peripheral opioid binding sites in rat lung. Eur J Pharmacol 310: 47–53, 1996. doi: 10.1016/0014-2999(96)00363-9. [DOI] [PubMed] [Google Scholar]
- 301.Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol 56: 69–91, 1994. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 302.Cabot PJ, Cramond T, Smith MT. Morphine has a dual concentration-dependent effect on K(+)-evoked substance P release from rat peripheral airways. Pulm Pharmacol Ther 10: 215–221, 1997. doi: 10.1006/pupt.1997.0094. [DOI] [PubMed] [Google Scholar]
- 303.Kuo HP, Rohde JA, Barnes PJ, Rogers DF. Differential inhibitory effects of opioids on cigarette smoke, capsaicin and electrically-induced goblet cell secretion in guinea-pig trachea. Br J Pharmacol 105: 361–366, 1992. doi: 10.1111/j.1476-5381.1992.tb14259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Belvisi MG, Stretton CD, Barnes PJ. Modulation of cholinergic neurotransmission in guinea-pig airways by opioids. Br J Pharmacol 100: 131–137, 1990. doi: 10.1111/j.1476-5381.1990.tb12064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hansen JT, Brokaw J, Christie D, Karasek M. Localization of enkephalin-like immunoreactivity in the cat carotid and aortic body chemoreceptors. Anat Rec 203: 405–410, 1982. doi: 10.1002/ar.1092030310. [DOI] [PubMed] [Google Scholar]
- 306.Wang ZZ, Stensaas LJ, Dinger B, Fidone SJ. The co-existence of biogenic amines and neuropeptides in the type I cells of the cat carotid body. Neuroscience 47: 473–480, 1992. doi: 10.1016/0306-4522(92)90261-y. [DOI] [PubMed] [Google Scholar]
- 307.McQueen DS, Ribeiro JA. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Br J Pharmacol 71: 297–305, 1980. doi: 10.1111/j.1476-5381.1980.tb10939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Br J Pharmacol 88: 889–898, 1986. doi: 10.1111/j.1476-5381.1986.tb16263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Berkenbosch A, Teppema LJ, Olievier CN, Dahan A. Influences of morphine on the ventilatory response to isocapnic hypoxia. Anesthesiology 86: 1342–1349, 1997. doi: 10.1097/00000542-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 310.Baby SM, Gruber RB, Young AP, MacFarlane PM, Teppema LJ, Lewis SJ. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur J Pharmacol 834: 17–29, 2018. doi: 10.1016/j.ejphar.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 110: 837–841, 2013. doi: 10.1093/bja/aes494. [DOI] [PubMed] [Google Scholar]
- 312.Dahan A, DeGoede J, Berkenbosch A, Olievier IC. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol 428: 485–499, 1990. doi: 10.1113/jphysiol.1990.sp018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985) 101: 618–627, 2006. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res 135: 231–254, 1977. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- 315.Mifflin SW. Laryngeal afferent inputs to the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 265: R269–R276, 1993. doi: 10.1152/ajpregu.1993.265.2.R269. [DOI] [PubMed] [Google Scholar]
- 316.Bonham AC, Joad JP. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol 441: 95–112, 1991. doi: 10.1113/jphysiol.1991.sp018740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. Neuroreport 11: 1709–1712, 2000. doi: 10.1097/00001756-200006050-00023. [DOI] [PubMed] [Google Scholar]
- 319.McGovern AE, Davis-Poynter N, Farrell MJ, Mazzone SB. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 207: 148–166, 2012. doi: 10.1016/j.neuroscience.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 320.Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res 549: 181–193, 1991. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- 321.Zhuang J, Gao X, Gao F, Xu F. Mu-opioid receptors in the caudomedial NTS are critical for respiratory responses to stimulation of bronchopulmonary C-fibers and carotid body in conscious rats. Respir Physiol Neurobiol 235: 71–78, 2017. doi: 10.1016/j.resp.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 322.Favero MT, Takakura AC, de Paula PM, Colombari E, Menani JV, Moreira TS. Chemosensory control by commissural nucleus of the solitary tract in rats. Respir Physiol Neurobiol 179: 227–234, 2011. doi: 10.1016/j.resp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 323.Handal KA, Schauben JL, Salamone FR. Naloxone. Ann Emerg Med 12: 438–445, 1983. doi: 10.1016/s0196-0644(83)80343-6. [DOI] [PubMed] [Google Scholar]
- 324.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112: 226–238, 2010. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 325.van Dorp E, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf 6: 125–132, 2007. doi: 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- 326.Martin WR. Drugs five years later: naloxone. Ann Intern Med 85: 765–768, 1976.doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 327.Frenois F, Cador M, Caillé S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci 16: 1377–1389, 2002. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- 328.Loimer N, Schmid R, Lenz K, Presslich O, Grünberger J. Acute blocking of naloxone-precipitated opiate withdrawal symptoms by methohexitone. Br J Psychiatry 157: 748–752, 1990. doi: 10.1192/bjp.157.5.748. [DOI] [PubMed] [Google Scholar]
- 329.Jain K, Jain R, Dhawan A. A double-blind, double-dummy, randomized controlled study of memantine versus buprenorphine in naloxone-precipitated acute withdrawal in heroin addicts. J Opioid Manag 7: 11–20, 2011. doi: 10.5055/jom.2011.0044. [DOI] [PubMed] [Google Scholar]
- 330.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies–tackling the opioid-overdose epidemic. N Engl J Med 370: 2063–2066, 2014. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 331.Collins FS, Koroshetz WJ, Volkow ND. Helping to end addiction over the long-term. The research plan for the NIH HEAL initiative. JAMA 320: 129–130, 2018. doi: 10.1001/jama.2018.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med 377: 391–394, 2017. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]