Keywords: autonomous firing, axon collaterals, globus pallidus, network activity, unitary synaptic currents
Abstract
Neurons in the external globus pallidus (GPe) are autonomous pacemakers, but their spontaneous firing is continually perturbed by synaptic input. Because GPe neurons fire rhythmically in slices, spontaneous inhibitory synaptic currents (IPSCs) should be evident there. We identified periodic series of IPSCs in slices, each corresponding to unitary synaptic currents from one presynaptic cell. Optogenetic stimulation of the striatal indirect pathway axons caused a pause and temporal resetting of the periodic input, confirming that it arose from local neurons subject to striatal inhibition. We determined the firing statistics of the presynaptic neurons from the unitary IPSC statistics and estimated their frequencies, peak amplitudes, and reliabilities. To determine what types of GPe neurons received the spontaneous inhibition, we recorded from genetically labeled parvalbumin (PV) and Npas1-expressing neurons. Both cell types received periodic spontaneous IPSCs with similar frequencies. Optogenetic inhibition of PV neurons reduced the spontaneous IPSC rate in almost all neurons with active unitary inputs, whereas inhibition of Npas1 neurons rarely affected the spontaneous IPSC rate in any neurons. These results suggest that PV neurons provided most of the active unitary inputs to both cell types. Optogenetic pulse stimulation of PV neurons at light levels that can activate cut axons yielded an estimate of connectivity in the fully connected network. The local network is a powerful source of inhibition to both PV and Npas1 neurons, which contributes to irregular firing and may influence the responses to external synaptic inputs.
NEW & NOTEWORTHY Brain circuits are often quiet in slices. In the globus pallidus, network activity continues because of the neurons’ rhythmic autonomous firing. In this study, synaptic currents generated by the network barrage were measured in single neurons. Unitary synaptic currents arising from single presynaptic neurons were identified by their unique periodicity. Periodic synaptic currents were large and reliable, even at the cell’s natural firing rates, but arose from a small number of other globus pallidus neurons.
INTRODUCTION
The external segment of the globus pallidus (GPe) is an interconnected network of neurons whose axons innervate most of the other nuclei of the basal ganglia (for review, see 1). GPe neurons are autonomous pacemakers that fire rapidly and rhythmically in the absence of synaptic input (2, 3), and equally rapidly but very irregularly in vivo (e.g., 4). The irregularity of firing in vivo is caused by ongoing synaptic perturbation of their intrinsic oscillation, and the neurons revert to very regular firing with little change in rate when synaptic excitation and inhibition are blocked by local application of GABA and glutamate receptor antagonists (4). Glutamatergic synaptic input derives from axons of neurons located outside the GPe, most famously from the subthalamic nucleus (STN) (3, 5–7), but also from the cerebral cortex (8–10) and the intralaminar thalamic nuclei (11). External sources also provide GABAergic synaptic inhibition to the GPe. By far the largest afferent system is from the striatal spiny projection neurons, which make thousands of inhibitory synaptic connections on each GPe cell (1, 12). A smaller inhibitory innervation arises internally from the synaptic interconnections among GPe neurons (13–18). The GPe contains two major cell types: prototypic neurons projecting to the STN, internal segment of the globus pallidus, and/or substantia nigra pars reticulata (SNr) and a group of other neurons lacking those projections but with prominent axonal arborizations in the striatum (19, 20), the cerebral cortex, or the thalamic reticular nucleus (8). All of these cell types form local axon collaterals in the GPe (21).
Because of the GPe neurons’ high rates of firing, these local connections are expected to produce a continuous barrage of synaptic inhibition, possibly contributing to the irregularity of ongoing firing, whereas striatal neurons’ firing is sparser and more phasic. Moreover, inhibitory synapses formed by striatal axons are located everywhere on the GPe cell, whereas local collaterals, while fewer, are located on average more proximally on the somata and proximal dendrites (18). This suggests that the intrinsic connections of GPe neurons might be very influential as a background on which phasic inputs are imposed, providing an ongoing mutual inhibition in the nucleus (e.g., 22, 23). Consistent with this, electrical or optogenetic stimulation of GPe local axons produces brief, powerful inhibitory synaptic currents (IPSCs) and inhibitory synaptic potentials (23–25).
Despite the existence of lateral interconnections, there is little evidence for spike time correlations among GPe neurons recorded in vivo, although there has been a very systematic search (26–28). One reason may be a very low pairwise connectivity. The local axon collaterals of GPe neurons span a large portion of the nucleus (15, 16, 21), but each axon contacts only a small number of other GPe neurons. A paired recording study in slices found connections between nearby GPe neurons in only five out of 173 pairs of neighboring neurons (22).
To understand the importance of the local connections within GPe, it is useful to know the strength of the unitary responses during natural firing activity. Estimation of synaptic strength using the responses of neurons to single synchronous volleys in axon stimulation experiments may produce overestimates because of frequency-dependent synaptic depression. For example, during repetitive axon stimulation at rates comparable with their spontaneous firing rates, the synaptic connections made by GPe neurons on their target neurons in the STN and SNr undergo severe depression (29, 30). When stimulated at the spontaneous firing rate of GPe neurons, these synapses depress to a few percent of the size seen after an isolated stimulus. Depression has been reported in GPe-to-GPe connections during brief trains of repetitive, synchronous stimulation (23, 24). GPe neurons fire at these rates continuously, in sleep and wakefulness, throughout the entire course of life. Synaptic depression may normally place a limit on their effectiveness, raising the possibility that they become relevant only after periods of prolonged inhibition.
An appreciation for the effective strength and connectivity of the intrinsic pallidal axon collaterals during ongoing activity requires direct measurement of the synaptic currents evoked in connected GPe neurons firing at their normal rates. Because GPe neurons fire rhythmically in slices, each at a rate different from the others, it should be possible to identify individual unitary synaptic currents generated in any one cell by intact presynaptic neurons, each one identifiable by its firing rate. In this report, we used the autocorrelation method developed by Higgs and Wilson (31) to isolate periodic components in the synaptic conductance barrage in single neurons in slices of mouse GPe. We compared these measurements in prototypic GPe neurons (identified by expression of parvalbumin) and nonprototypic neurons (identified by expression of Npas1). We used optogenetic manipulation of firing rate in each cell type to identify the source of ongoing unitary IPSCs in individual neurons. By suppressing firing in presynaptic neurons for a period of several seconds, we estimated the degree of frequency-dependent synaptic depression at GPe intrinsic synapses at normal firing rates in the absence of stimulation. We further showed that inhibition of the GPe by activation of striato-pallidal axons produces a profound pause in the background local barrage, followed by a reset of firing in each of the presynaptic neurons.
MATERIALS AND METHODS
All experimental procedures followed National Institutes of Health guidelines, and all animal experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas at San Antonio.
Animals
Experiments were performed using brain slices from male and female mice at least 4 wk of age. The transgenic strains used are listed in Table 1.
Table 1.
Transgenic mice used
| Applications | Mouse | Breeders |
|---|---|---|
| Stimulate indirect pathway | A2A-ChR2-tdTomato | A2A-cre1Ai27D2 |
| Transduce PV neurons | PV-cre3 | |
| Identify PV neurons | PV-tdTomato | PV-cre3Ai144 |
| Identify and transduce Npas1 neurons | Npas1-cre-tdTomato5 | |
| Stimulate PV neurons and axons | PV-ChR2-tdTomato | PV-cre3Ai27D2 |
| Silence PV neurons | PV-Arch-GFP | PV-cre3Ai40D6 |
ChR2, channelrhodopsin 2; PV, parvalbumin.
MMRRC UC Davis stock # 036158, B6.FVB(Cg)-Tg(Adora2a-cre)KG139Gsat/Mmcd.
Jackson Labs, B6.Cg-Gt(ROSA)26Sortm27.1(CAG-COP4*H134R/tdTomato)Hze/J.
Jackson Labs, B6.129P2-Pvalbtm1(cre)Arbr/J.
Jackson Labs, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J.
C57BL/6-Tg(Npas1-icre,-tdTomato)1Cschn.
Jackson Labs, B6.Cg-Gt(ROSA)26Sortm40.1(CAG-aop3/EGFP)Hze/J.
Viral Constructs and Injections for Optogenetics
To achieve high levels of channelrhodopsin 2 (ChR2) expression in PV neurons for axonal stimulation, AAV9-Flex-ChR2-tdTomato (AAV9.CAGGS.Flex.ChR2.tdTomato-WPRE.SV40, University of Pennsylvania Vector Core) was stereotaxically injected into GPe of PV-cre mice at least 6 wk old. For silencing using the inhibitory opsin archaerhodopsin (Arch), AAV9-Flex-Arch-GFP (AAV9.Flex.CBA.Arch-GFP.WPRE.SV.40, University of Pennsylvania Vector Core) was injected into GPe of PV-cre or Npas1-cre-tdTomato mice. The injection sites were (relative to Bregma) 0 mm anterior, 1.75 mm lateral, and 3.0 mm ventral. Examples of the AAV injections in PV-cre and Npas1-cre-tdTomato mice are shown in Fig. 1. Recording experiments were performed at least 2 wk after injection.
Figure 1.
AAV injections in mouse GPe, examined in sagittal sections. A: AAV9-Flex-ChR2-tdTomato in PV-cre mouse, showing widespread expression throughout the nucleus. B: AAV9-Flex-Arch-GFP in Npas1-cre mouse. AAV9-Flex-Arch-GFP, AAV9.Flex.CBA.Arch-GFP.WPRE.SV.40, University of Pennsylvania Vector Core; AAV9-Flex-ChR2-tdTomato, AAV9.CAGGS. Flex.ChR2.tdTomato-WPRE.SV40, University of Pennsylvania Vector Core; C, caudal; D, dorsal; GPe, external globus pallidus; PV, parvalbumin; R, rostral; V, ventral.
Brain Slice Preparation
Mice were deeply anesthetized with isoflurane and euthanized by decapitation. Coronal brain slices (300 µm) containing GPe were prepared by standard methods using a Vibratome (Leica), in ice-cold cutting solution containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgSO4, 25 glucose, 11.6 Na ascorbate, 3.1 Na pyruvate, and 26 NaHCO3, bubbled with 95% O2, 5% CO2. Slices were collected in artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, and 10 glucose, bubbled with 95% O2, 5% CO2. The slice storage ACSF (but not the recording ACSF) also contained (in mM) 0.005 l-glutathione, 1 Na pyruvate, and 1 Na ascorbate. The slices were heated to 34°C for 30 min and then allowed to cool to room temperature until use.
Recording
A slice was superfused with ACSF and maintained at 34°C. In optogenetic stimulation experiments, glutamatergic transmission was blocked with 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX, 5 µM) and 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 5 µM). Neurons were visualized with an Olympus BX50WI microscope with a ×40 water-immersion objective and Dodt gradient contrast optics. Fluorescent-labeled neurons were identified by epifluorescence. Whole cell voltage-clamp recordings were obtained using a MultiClamp 700B amplifier (Molecular Devices, San Jose, CA) connected to an ITC-18 A/D converter (HEKA Instruments, New York, NY) and a Macintosh computer running custom software written in Igor Pro (WaveMetrics, Portland, OR). Data were low pass filtered online at 10 kHz and sampled at 20 kHz. The recording pipettes were pulled from borosilicate glass and had resistances of ∼3–5 MΩ. The pipette solution contained (in mM) 140.5 KCl, 7.5 NaCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP, and 0.21 Na-GTP, or, where indicated in the results, an otherwise identical solution with CsCl substituted for KCl.
Optical Stimulation
Light stimuli were produced by an LED and directed at GPe through the microscope objective via an appropriate filter cube. ChR2 was activated using blue (475 nm) light (∼5 mW maximum power), and Arch was activated using green (560 nm) light (∼3 mW). In experiments where high levels of Arch expression were obtained by AAV injection, the light was defocused or delivered through a low-power objective to avoid overstimulation.
Detection of Spontaneous Postsynaptic Currents
Data were analyzed using custom routines in Mathematica (Wolfram, Champaign, IL). The raw current was differentiated twice and smoothed by convolution with a Gaussian filter (0.4 ms SD). The detection level was set to −5 times the noise level, determined as the median absolute value of the processed signal. The time of each synaptic current was taken at the minimum of the smoothed second derivative, or maximum downward kink, which was located near the onset of each synaptic current.
Analysis of Unitary IPSC Timing
Unitary inputs from rhythmically firing neurons were identified by periodic components in the autocorrelation of IPSC times (31). The autocorrelation, AC(Δt), was obtained for time differences of 10–500 ms, with a bin width of 2 ms, and was expressed as the expected value of the IPSC rate given a reference IPSC at time tref.
| (1) |
The initial portion of the autocorrelation (Δt < 10 ms) was not analyzed, because small IPSCs on the decaying phase of another IPSC might not be detected, producing a shadowing artifact.
Using the model described below, we estimated four parameters for each unitary input: the mean presynaptic firing frequency (f), the rate of synaptic successes (r), the standard deviation of presynaptic ISIs (σ), and a power describing the growth of standard deviation for higher-order ISIs (p). Our model can be understood as a sum of component autocorrelations for each input i, where each IPSC time can be the reference but the flanking IPSCs whose rates make up the autocorrelation come from input i. Input 0 represents all aperiodic input, including miniature IPSCs and any other aperiodic spontaneous IPSCs, and inputs 1 to n are the periodic unitary inputs.
| (2) |
| (3) |
Because input 0 is aperiodic, its expected contribution to the autocorrelation is time invariant, with a value equal to its mean rate (r0).
| (4) |
At large Δt, the expected value of each other autocorrelation component, ACi, is equal to the mean rate of that component, ri. However, at small Δt, flanking events produced by periodic input i are seen only when the reference event was not from input i. The probability of this is 1 − ri/rtotal, and so
| (5) |
The other component of ACi is a harmonic series of peaks representing the first order and higher order intervals between all reference IPSCs and flanking IPSCs from input i. Different inputs are assumed to be uncorrelated, and thus do not contribute to the peaks. If only input i were present and the unitary synaptic connection were reliable, each peak area (ai) would be unity, as in the autocorrelation of a periodic spike train. However, the peak area for component i of the IPSC time series is less, because input i provides only a fraction of the reference events (ri/rtotal), and the unitary synaptic success probability (ri/fi) may be less than 1. Thus,
| (6) |
Each peak (peaki,h), where h is the harmonic peak number, is modeled as a Gaussian distribution centered at a mean value (µi,h) equal to a multiple of the mean ISI, with a standard deviation (σi,h) that grows as h raised to a power pi:
| (7) |
| (8) |
| (9) |
| (10) |
Although in principle each unitary input produces an infinite series of harmonic peaks, the autocorrelation for a finite time range is approximated by a finite series of peaks. For a 500-ms window and the input frequencies encountered, this was achieved with 100 peaks.
The waveform of AC(t) was fitted to each autocorrelation with periodic components, with free parameters r0 for the aperiodic input and fi, ri, CVi (i.e., fi × σI), and pi for each periodic input. The number of unitary inputs and approximate values of each fi were initially chosen by inspection of the autocorrelation. Most of the autocorrelations showing periodic components were successfully fitted with one or two harmonic series of peaks, suggesting that the neurons received one or two unitary inputs. The autocorrelations from a few neurons with dense IPSC input could not be fitted accurately with physiologically plausible parameter values, suggesting that two inputs may be the practical limit for this method of analysis, given the degree of variability in GPe neurons’ interspike intervals. However, this limit was only occasionally exceeded in the present study.
Analysis of Unitary IPSC Amplitudes
The raw current trace was smoothed by convolution with a Gaussian filter (0.2 ms SD), and the amplitude of each IPSC was measured. The amplitude distribution for each input was estimated based on contributions to each component of the autocorrelation (ACi, see Eq. 10). For this analysis, the IPSCs were sorted by amplitude and divided into 20 bins, each containing 5% of the IPSCs. The IPSC time autocorrelation was computed for flanking events from each amplitude group, taking all IPSC times as reference events, and each amplitude-selected autocorrelation was fitted with a weighted sum of the input component autocorrelations, providing a weight, wi,b, for each input component i and amplitude bin b. The mean and standard deviation of the IPSC amplitudes for each input were then calculated from the bin means (µb) and the weights:
| (11) |
| (12) |
Note that these values are for synaptic successes, where the presynaptic action potential produced a detected IPSC.
Statistical Comparisons
Except as stated otherwise, statistical comparisons were made using the Mann–Whitney U test, and differences were considered significant when P < 0.05.
RESULTS
Action Potential-Dependent Spontaneous IPSCs
To investigate the local synaptic inhibition produced by GPe neurons’ spontaneous firing, we recorded spontaneous postsynaptic currents (PSCs) from GPe neurons in coronal slices from male and female wild-type mice. Most experiments were performed in slices containing the rostral portion of GPe. It has been reported that many of the dendrites and local axon collaterals of GPe neurons lie in a plane parallel to the striatum-GPe border (15, 16). Because the border curves into the coronal plane at the rostral end of GP, these slices were expected to preserve some of the local connections. Recordings were obtained using high-chloride pipette solution at a holding potential of −60 mV, providing a calculated Cl− driving force of 66 mV. Most of the data were obtained in control ACSF without synaptic blockers. The experiments described in this section were done using a CsCl pipette solution; those in the remainder of the study were done with an otherwise identical KCl solution, which was expected to preserve some of the natural attenuation of dendritic synaptic currents by potassium channels.
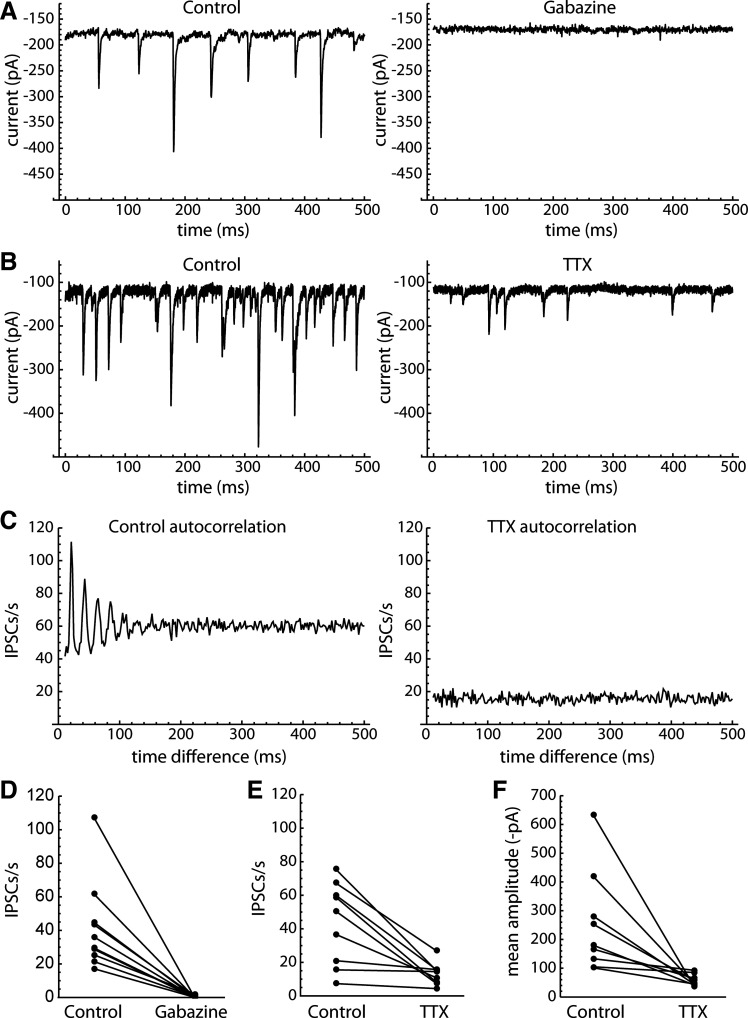
We first investigated whether the spontaneous PSCs were primarily excitatory or inhibitory, using gabazine (10 µM) to block GABAA receptor-mediated currents. Although presence of excitatory inputs to GPe neurons is well known (15, 32, 33), almost all of the PSCs were blocked by gabazine (n = 11; Fig. 2, A and D). Thus, from here on we refer to the spontaneous synaptic currents as IPSCs.
Figure 2.
Action potential-dependent, periodic spontaneous IPSCs in GPe neurons. A: effect of gabazine (10 µM) on spontaneous PSCs in an example neuron. B: effect of TTX (1 µM) in another example neuron. C: autocorrelation of the IPSC time series (same neuron as B). The control autocorrelation (left) shows a series of periodic peaks, suggesting that the cell received IPSCs from a rhythmically firing presynaptic neuron. The autocorrelation obtained in the presence of TTX (right) does not show the periodic peaks, confirming that they arose from presynaptic action potential firing. D: effect of gabazine on spontaneous PSC rates (n = 11 cells). Glutamate receptors were not blocked in these recordings, so the effect of gabazine indicates that almost all of the spontaneous PSCs were GABAA receptor-mediated IPSCs. E: effect of TTX on spontaneous IPSC rates (n = 9 cells). F: effect of TTX on the mean spontaneous IPSC amplitudes. The IPSCs remaining in the presence of TTX were generally smaller than the TTX-sensitive IPSCs, although the TTX-sensitive events showed a wide range of amplitudes. GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; PSCs, synaptic currents; TTX, tetrodotoxin.
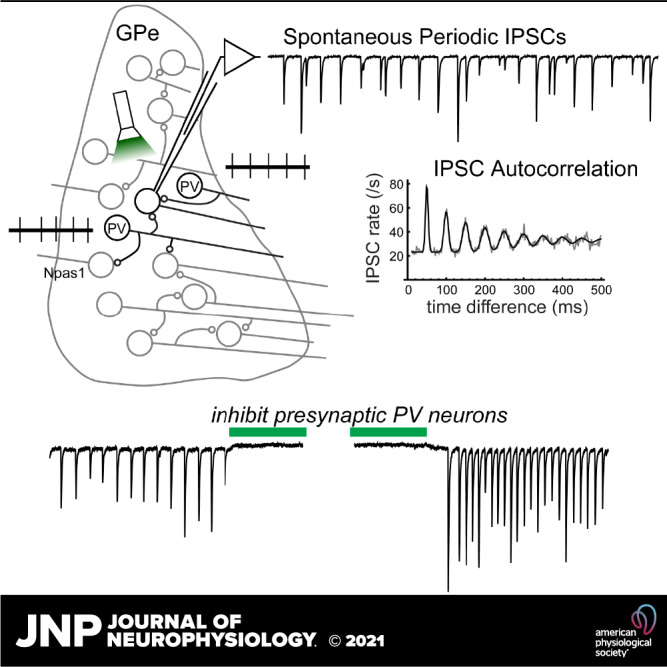
Because of the spontaneous firing and local connections of GPe neurons, we expected to see a high rate of action potential-dependent IPSCs. To examine this, we measured the effect of tetrodotoxin (TTX, 1 µM) on spontaneous IPSCs in nine GPe neurons. An example is shown in Fig. 2B. In control solution, the example cell appeared to receive runs of periodic IPSCs, which were interrupted by gaps and intermixed with some other IPSCs. The rhythmicity of the synaptic input was evident in the autocorrelation of spontaneous IPSC times (Fig. 2C), which showed a harmonic series of peaks. Application of TTX reduced the IPSC frequency and the number of large IPSCs and also eliminated the periodic pattern in the autocorrelation, indicating that the periodic input was action potential dependent. Similar periodic patterns were observed in the IPSC time autocorrelations of six of nine cells, and in each case TTX eliminated the periodic input. The effects of TTX on the mean IPSC rates and amplitudes are shown in Fig. 2, E and F. These data indicate that a majority of GPe neurons in the slice preparation received action potential-dependent IPSCs from rhythmically firing local neurons.
Identifying Unitary Synaptic Inputs
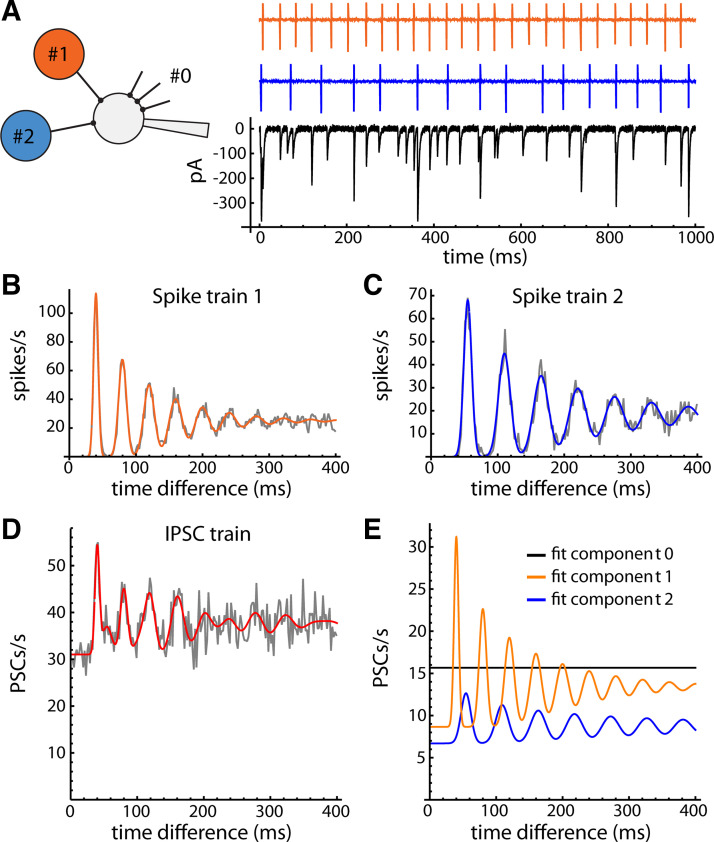
To identify components of the spontaneous synaptic input that came from individual presynaptic neurons, we applied a previously developed method based on the autocorrelation of the IPSC times (31). The idea behind the method is that the postsynaptic neuron receives synaptic input from one or more rhythmically firing presynaptic neurons (Fig. 3A). Each presynaptic spike train has a unique firing rate, resulting in an autocorrelation with a regular series of peaks, which progressively broaden and sum together at long time delays (Fig. 3, B and C). These peaks represent the first-order and higher order ISIs (one-spike time differences, two-spike time differences, and so on). If the interspike interval (ISI) variability is small, as we typically observe in brain slices, the ISI distribution can be approximated by a Gaussian function. The higher order ISIs also have Gaussian distributions, but with increasing standard deviation. If the ISIs are independent, the standard deviation of the ISI distribution increases as the square root of the ISI order, or σh = σ1 × h0.5, where σ1 is the standard deviation of first-order ISIs and h is the ISI order. Because sequential ISIs might not be independent, we generalized this expression by allowing the standard deviation to grow as a power: σh = σ1 × hp. Spike train autocorrelations of GPe neurons could then be fitted with a sum of Gaussian peaks centered at multiples of the mean ISI, each peak having unit area (Fig. 3, B and C). The autocorrelation of IPSC times (Fig. 3D) has periodic components that closely match the individual spike train autocorrelations, demonstrating the ability of our method to recover the presynaptic firing patterns from the synaptic current data.
Figure 3.
Simulation showing the origin of the IPSC time autocorrelation. A: simulated synaptic arrangement: two presynaptic neurons (#1 and #2) connect to a postsynaptic neuron, which also receives aperiodic synaptic input from other axons (input #0, truncated black lines). Top traces show the spike trains of two GPe neurons recorded in the cell-attached configuration. Bottom trace shows simulated postsynaptic currents in a cell receiving the two presynaptic spike trains, each producing IPSCs with 50% success probability and variable amplitudes, and also receiving aperiodic synaptic currents (Poisson process, mean rate = 15 s−1). B and C: autocorrelation of each presynaptic spike train (gray line) fitted with the sum-of-Gaussians model described in the text (colored line). D: autocorrelation of the IPSC time series, fitted with the IPSC autocorrelation model described in the text (red line). E: components of the fitted curve produced by each input, where the first, reference IPSC of each pair could come from any input. The waveforms of the two periodic components closely match the two spike train autocorrelations. Fit component 0 (flat black line) provides an estimate of the rate of aperiodic synaptic currents. GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; PSCs, synaptic currents.
Perturbation of Spontaneous Inhibition by Indirect Pathway Input
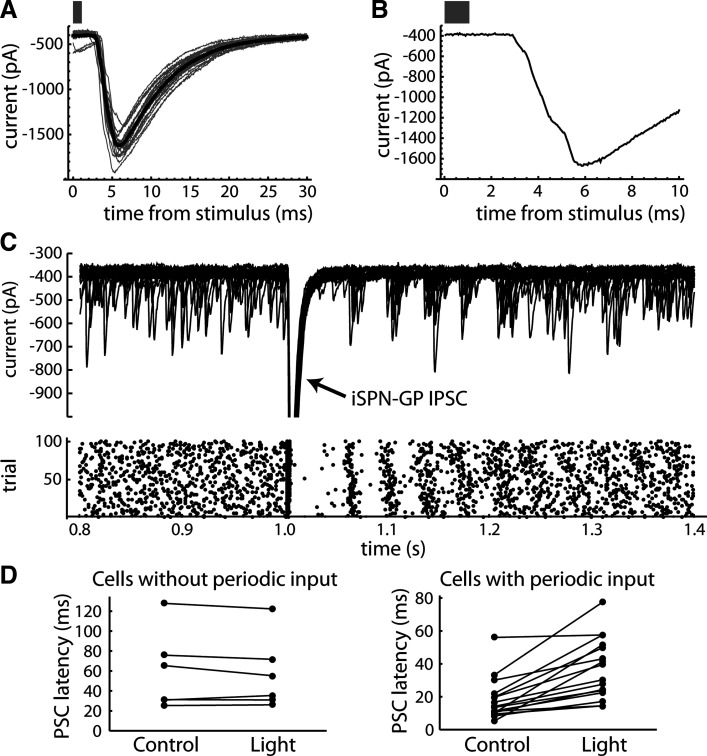
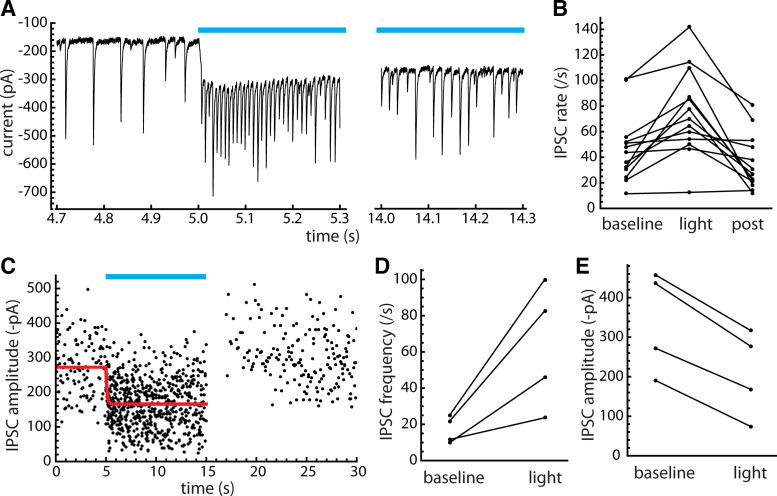
If the periodic spontaneous IPSCs come from other GPe neurons, their timing should be perturbed by an external input to GPe. The largest source of inhibitory input to GPe is the indirect pathway striatal projection neurons (iSPNs), which can be targeted based on their expression of the adenosine 2 A (A2A) receptor (34). To test the local origin of periodic spontaneous IPSCs, we stimulated iSPN axons in GPe optogenetically using brain slices from A2A-ChR2-tdTomato mice (see materials and methods). Brief flashes of blue light produced large IPSCs in the recorded neurons (Fig. 4A), which presumably arise from multiple iSPN axons. Consistent with this, we usually observed multiple components in the rising phase of the light-evoked IPSC (Fig. 4B), which likely result from latency differences among these inputs.
Figure 4.
Indirect pathway input paused and reset periodic spontaneous IPSCs. A: IPSCs in a GPe neuron produced by optogenetic stimulation of iSPN axons. Gray traces are individual IPSCs, and thick black trace is the average. Bar indicates the duration of the light stimulus. B: expanded trace from one trial, showing multiple components in the rising phase of the iSPN → GPe IPSC. C: effect of the iSPN → GPe IPSC on spontaneous IPSCs. Top: current traces from 20 consecutive trials of light stimulation. Bottom: raster of IPSC times for 100 trials. Note the pause in spontaneous IPSCs after the stimulus, followed by temporal resetting of the spontaneous IPSC train. The periodic pattern seen after the pause was typical for neurons with one unitary input. D: summary data comparing the pause (IPSC latency) after the light stimulus to the same measurement at another time point (0.5 s before the light stimulus). To control for possible shadowing of IPSC detection by the large evoked IPSC, the mean evoked IPSC was added to the data at the control time point before detecting IPSCs. The IPSC latency was measured starting 20 ms after the light stimulus, to avoid including any component of the light-evoked IPSC. The left graph is for neurons without detected periodic input, and the right graph is for neurons with periodic input. None of the neurons without periodic input showed a significant difference between the control IPSC latencies and the IPSC latencies after the light stimulus, whereas 15 of 16 neurons with periodic input showed significantly longer spontaneous IPSC latencies after the light-evoked IPSC (Mann–Whitney U test, P < 0.05). GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; iSPN, indirect pathway striatal projection neuron; PSC, synaptic current.
In GPe neurons with spontaneous periodic input, the iSPN → GPe IPSC produced a pause in spontaneous IPSCs that generally outlasted the light-evoked IPSC (Fig. 4C). Significant pauses were observed only in neurons with periodic spontaneous IPSCs (Fig. 4D). Neurons without periodic IPSCs showed no such pause, even when the response to striatal axon stimulation was large. In addition to the pause, the iSPN → GPe IPSC caused a temporal resetting of the spontaneous IPSCs, which produced a clear periodic pattern in neurons with one active unitary input. These results confirm that the periodic spontaneous IPSCs arise from other GPe neurons that also receive indirect pathway inhibition. Further, they indicate that external input to a group of connected GPe neurons will produce a complex response, as the primary input alters the ongoing local inhibition.
Unitary IPSCs in PV and Npas1 Neurons
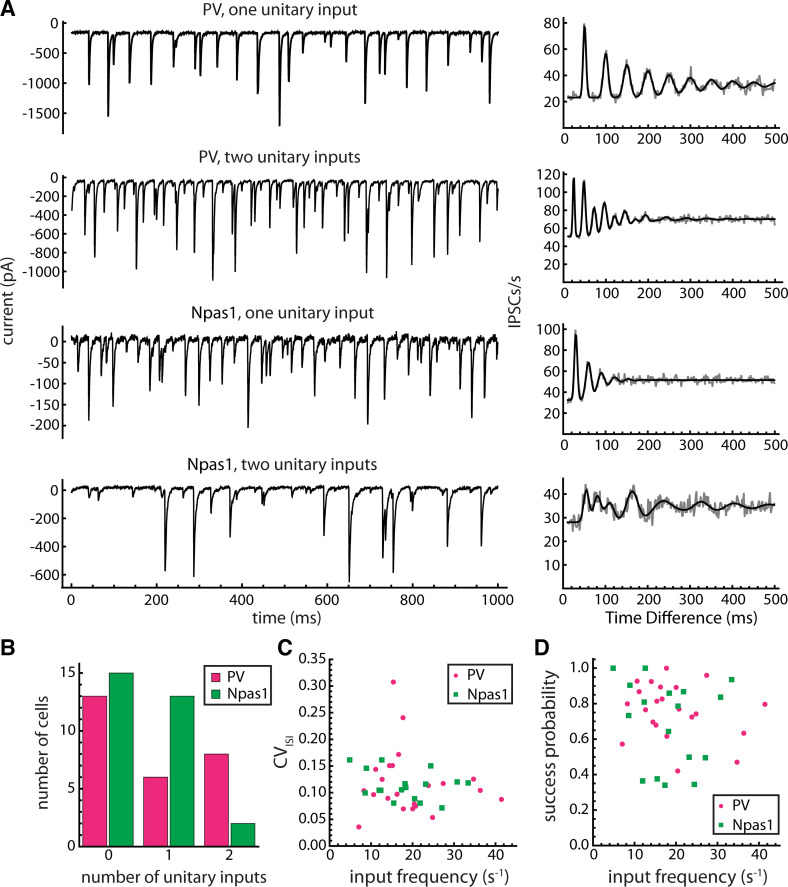
To determine whether the spontaneous periodic IPSCs differ between neuron types, we recorded from genetically labeled PV and Npas1 neurons. PV and Npas1 expression identify two separate supersets of GPe neuron types (6, 8, 19, 35). PV neurons contribute to the classical axonal projections to the GPi, SNr, and STN, whereas Npas1 neurons do not target those structures but include cell types projecting primarily to the striatum, cerebral cortex, and thalamic reticular nucleus (8). PV neurons were recorded in slices from PV-tdTomato, PV-ChR2-tdTomato, and PV-Arch-GFP mice, and Npas1 neurons were recorded in slices from Npas1-cre-tdTomato mice (see materials and methods).
PV and Npas1 neurons both received periodic spontaneous IPSCs. Examples from both cell types are shown in Fig. 5A. Overall, we detected at least one periodic input in 14 of 27 PV neurons and 15 of 29 Npas1 neurons. Each autocorrelation from these datasets that showed periodic components was successfully fitted with our model, with either one or two unitary inputs. The numbers of neurons with 0, 1, or 2 unitary inputs are shown in Fig. 5B. The difference in number of inputs between PV and Npas1 neurons was not statistically significant (P = 0.40).
Figure 5.
PV and Npas1 neurons received similar unitary inputs. A: examples of spontaneous IPSCs (left) and the corresponding IPSC time autocorrelations (right) in two PV neurons and two Npas1 neurons. Gray trace is the raw autocorrelation, and black line is the fitted model curve. B: distributions of number of unitary inputs detected in PV and Npas1 neurons. The number of inputs did not differ significantly between the two cell types. C: estimated coefficient of variation of presynaptic interspike intervals (CVISI) versus estimated presynaptic firing frequency (“input frequency”) for unitary inputs to PV neurons (n = 22 unitary inputs) and Npas1 neurons (n = 17 unitary inputs). The input frequency and CVISI did not differ significantly between PV and Npas1 neurons. D: unitary synaptic success probability vs. input frequency. The success probabilities did not differ significantly between PV and Npas1 neurons. CVISI, coefficient of variation of presynaptic interspike intervals; IPSCs, inhibitory synaptic currents; PV, parvalbumin.
The estimated presynaptic firing frequency and coefficient of variation of interspike intervals (CVISI) for each unitary input are shown in Fig. 5C. The input frequencies were 19.4 ± 9.0 s−1 for postsynaptic PV neurons (n = 22 unitary inputs, range: 7.1 to 41.4 s−1) and 18.2 ± 8.0 s−1 for postsynaptic Npas1 neurons (n = 17 unitary inputs, range: 4.8 to 33.4 s−1), and the difference was not significant (P = 0.90). CVISI was 0.118 ± 0.061 for postsynaptic PV neurons and 0.114 ± 0.027 for postsynaptic Npas1 neurons (P = 0.56). Because PV neurons have been reported to fire faster and more regularly than Npas1 neurons (19), these data suggest that the two postsynaptic cell types received synaptic input from the same presynaptic cell types. To examine the differences between PV and Npas1 neuron firing ourselves, we recorded spiking in the cell-attached configuration before establishing whole cell access. On average, we found mean firing rates of 26.6 ± 10.2 spikes/s in PV neurons (n = 25) and 15.9 ± 8.4 spikes/s in Npas1 neurons (n = 14), and CVISI of 0.112 ± 0.034 in PV neurons and 0.155 ± 0.126 in Npas1 neurons. The difference in mean rates was statistically significant (P = 0.005), but the difference in CVISI was not (P = 0.51). These data suggest that inputs from PV versus Npas1 neurons might be statistically distinguishable based on their frequency if each presynaptic cell type consistently provided the input to a different postsynaptic cell type. However, the large range and overlap in the distributions of firing frequencies in neurons of each type did not allow us to identify the source of each unitary input based on its frequency. Thus, additional experiments were required to identify the presynaptic cell type(s).
The autocorrelation analysis also provided estimates of the synaptic success probabilities, based on the areas of the autocorrelation peaks (see materials and methods; Fig. 5D). The success probabilities varied widely among individual unitary inputs, but they were similar in PV and Npas1 neurons (PV: 0.76 ± 0.15, Npas1: 0.69 ± 0.24, P = 0.58). These values were consistent with observations of variable numbers of apparent synaptic failures interrupting runs of periodic IPSCs. The success probabilities were not significantly correlated with the estimated presynaptic firing frequency in either postsynaptic cell type, suggesting that frequency-dependent synaptic depression was not the major source of variability in success probability.
Heterogeneity and Variation of Unitary IPSC Amplitudes
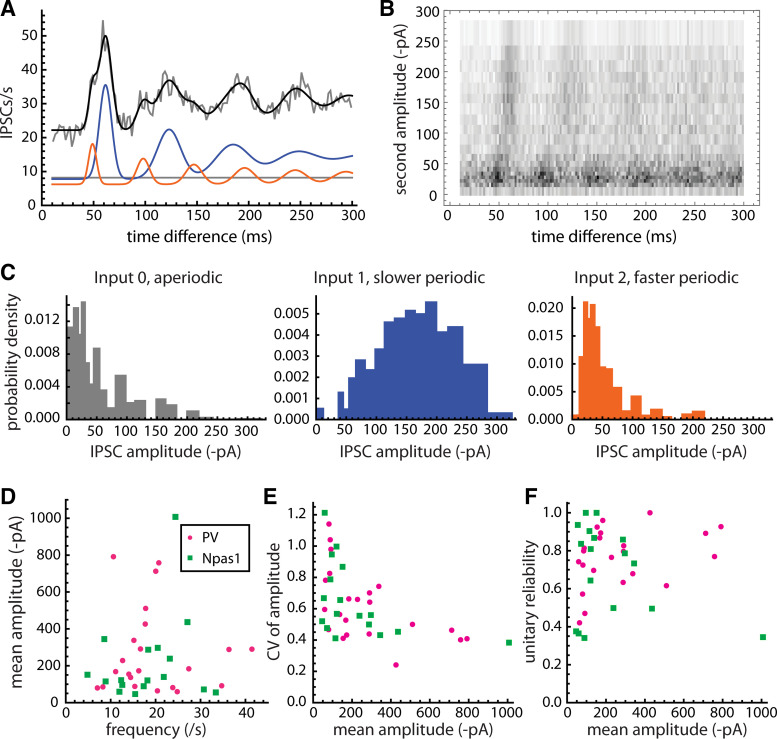
The amplitudes of periodic spontaneous IPSCs varied widely among neurons, and in some cases an individual GPe cell received two periodic inputs with distinctly different amplitudes. To estimate the amplitude distribution of each unitary input, we performed an additional analysis based on the components of the autocorrelation. The method is illustrated in Fig. 6, A–C, for an example cell with two unitary inputs. The autocorrelation and its components are shown in Fig. 6A. To decompose the autocorrelation by IPSC amplitudes, we obtained the individual inter-IPSC time differences making up the autocorrelation and plotted a density histogram with the time difference between pairs of IPSCs on the horizontal axis and the amplitude of the second IPSC on the vertical axis (Fig. 6B). The density histogram reveals that the neuron received two unitary inputs with different frequencies and amplitude distributions. To quantify these data, the IPSC amplitudes were divided into 20 bins, each containing 5% of the total events. For each bin, we computed the autocorrelation for pairs of IPSCs where the amplitude of the second IPSC fell within the bin. Each amplitude-component autocorrelation was fitted with a weighted sum of the input-component autocorrelations making up the original fitted curve (the blue and red curves in Fig. 5A). Thus, we obtained a weight for each input for each amplitude bin. These weights give the area of each bar in the component amplitude distributions in Fig. 6C. From these data, we estimated the mean and standard deviation of the IPSC amplitudes for each unitary input, and for the aperiodic input (input 0). Note that for the unitary inputs, these amplitudes are for the synaptic successes, which were mixed with some failures as described above.
Figure 6.
Unitary IPSC amplitudes. A–C illustrate the analysis for an example GPe neuron with two active unitary inputs. A: autocorrelation showing two periodic components. Gray line is the raw autocorrelation, black line is the fitted model curve, and blue and orange lines are the autocorrelation components arising from the slower and faster unitary inputs, respectively. Gray line is the baseline component from the aperiodic input. B: density histogram of IPSC amplitude versus time difference for pairs of IPSCs. As in the autocorrelation, both first-order and higher order intervals were collected, and the amplitudes were taken for the second IPSC in each pair. It is apparent that the slower periodic input produced IPSCs with larger amplitudes. C: IPSC amplitude distributions for the aperiodic input and the two periodic inputs, estimated based on the contribution of each amplitude bin (each row of pixels in B) to the autocorrelation components shown in A (see materials and methods). D: mean amplitude versus frequency for each unitary input, including data from 22 PV and 17 Npas1 neurons. The IPSC amplitudes and frequencies both showed a large heterogeneity among unitary inputs, but there was no significant correlation between mean amplitude and frequency in either cell type. E: CV of IPSC amplitude versus mean amplitude for each unitary input. F: unitary success probability (reliability) versus mean amplitude. CV, coefficient of variation; GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; PV, parvalbumin.
The mean unitary IPSC amplitudes were highly variable among individual inputs (PV, range 59–791 pA or 0.98 to 13.18 nS, mean 273 ± 230 pA; Npas1, range 46–1,007 pA, mean 216 ± 233 pA) and did not differ significantly between the two postsynaptic cell types (P = 0.31). It was expected that the unitary IPSC amplitudes might vary as a function of the presynaptic firing frequency, if synaptic depression were present as reported at GPe output synapses in the STN and SNr (29, 30). To examine this possibility, we plotted the mean IPSC amplitude for each unitary input versus the estimated presynaptic frequency of that input (Fig. 6D). However, the correlation of mean amplitude and frequency was not significant for either cell type (PV, r = −0.04, P = 0.81; Npas1, r = 0.20, P = 0.76), indicating that frequency-dependent depression was not the major source of variation among the mean unitary IPSC amplitudes. Individual presynaptic neurons also produced unitary IPSCs with highly variable amplitudes. On average, the coefficient of variation (CV) of unitary IPSC amplitudes (not including synaptic failures) was 0.62 ± 0.23 in PV neurons and 0.65 ± 0.24 in Npas1 neurons. The CV of amplitude was negatively correlated with the mean amplitude in both cell types (Fig. 6E; PV, r = −0.53, P = 0.007; Npas1, r = −0.47, P = 0.047), as expected if the quantal content were higher for larger unitary IPSCs (e.g., 36). However, the unitary success probability estimated from the autocorrelation fit parameters was not significantly correlated with the mean success amplitude in either cell type (Fig. 6F). The large variability of amplitude CV and success probability among neurons with similar mean IPSC amplitudes suggests that differences in quantal size, rather than the number of quanta released, may contribute to the heterogeneity of unitary input strengths.
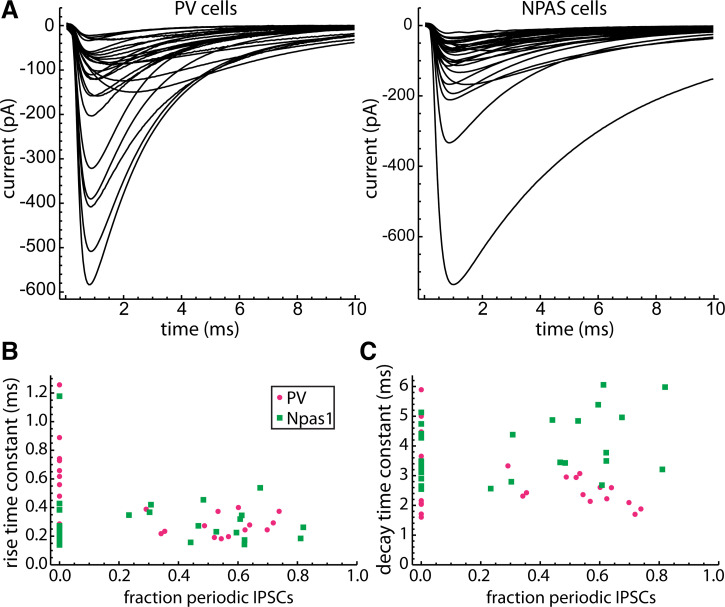
Spontaneous IPSC Kinetics
To estimate the rise and decay time constants of aperiodic and periodic IPSCs, we collected and averaged all of the well separated IPSCs in each neuron. The average IPSCs from each PV neuron and each Npas1 neuron are shown in Fig. 7A. Because individual IPSCs usually could not be identified as periodic or aperiodic, we compared average IPSC kinetics between neurons having no periodic IPSCs and cells with periodic IPSCs. We fitted each average IPSC with a sum of rising and falling exponentials to measure the rise and decay time constants, and then plotted each time constant versus the fraction of IPSCs accounted for by the periodic unitary inputs (“fraction periodic”), based on the parameter values obtained by analysis of the IPSC time autocorrelation (Fig. 7, B and C). The points along the left axis are from neurons with no detected unitary input, and the other points are from neurons with one or two unitary inputs, as summarized above. For statistical tests, we compared PV versus Npas1 neurons with no periodic input and PV versus Npas1 neurons with >50% periodic input (Table 2).
Figure 7.
Spontaneous IPSC kinetics in 27 unitary IPSCs from PV and 29 unitary IPSCs from Npas1 neurons. A: average of the well separated IPSCs in each PV neuron (left) and each Npas1 neuron (right). Well separated IPSCs were defined by the absence of other detected IPSCs within 10 ms on either side of the detection point. B: rise time constant of the average IPSC versus fraction of periodic IPSCs (i.e., sum of the unitary IPSC rates/total IPSC rate). A fraction of zero indicates neurons with no detected unitary input. Any difference between periodic and nonperiodic IPSC kinetics should be most evident in the comparison of cells with no periodic IPSCs and those with a large fraction of periodic IPSCs. C: decay time constant of the average IPSC versus fraction periodic IPSCs. Npas1 neurons with active unitary inputs had longer decay time constants compared with PV neurons with active unitary inputs (see Table 2), indicating that the unitary IPSCs decay slower in Npas1 neurons. IPSCs, inhibitory synaptic currents; PV, parvalbumin.
Table 2.
Time constants of the average well-separated IPSCs in PV and Npas1 neurons without periodic IPSCs and in neurons with more than 50% periodic IPSCs
| PV, n = 27 IPSCs | Npas1, n = 29 IPSCs | P | |
|---|---|---|---|
| Rise, no periodic input, ms | 0.55 ± 0.31 | 0.30 ± 0.27 | 0.006 |
| Decay, no periodic input, ms | 3.34 ± 1.42 | 3.64 ± 0.80 | 0.45 |
| Rise, >50% periodic input, ms | 0.28 ± 0.08 | 0.27 ± 0.12 | 0.49 |
| Decay, >50% periodic input, ms | 2.36 ± 0.44 | 4.49 ± 1.24 | 0.0004 |
IPSCs, inhibitory synaptic currents; PV, parvalbumin.
In GPe neurons with no periodic input, the major source of spontaneous synaptic currents is probably miniature IPSCs from the abundant striatal afferents. In contrast, in neurons with >50% periodic input, IPSCs from the local unitary inputs are more frequent and usually larger, and thus contribute more to the average waveform. Thus, our results suggest that miniature IPSCs had shorter rise times in Npas1 neurons, possibly indicating a difference in the makeup of nonpallidal afferents to the two neuron types. Local unitary IPSCs had longer decay times in Npas1 neurons than in PV neurons. Because the rise times were not different, the slower decay times in Npas1 neurons most likely do not result from electrotonic filtering. A possible explanation for the difference would be expression of different GABAA receptor subtypes in PV and Npas1 neurons. To our knowledge, this has not yet been investigated.
Unitary IPSCs from Presynaptic PV Neurons
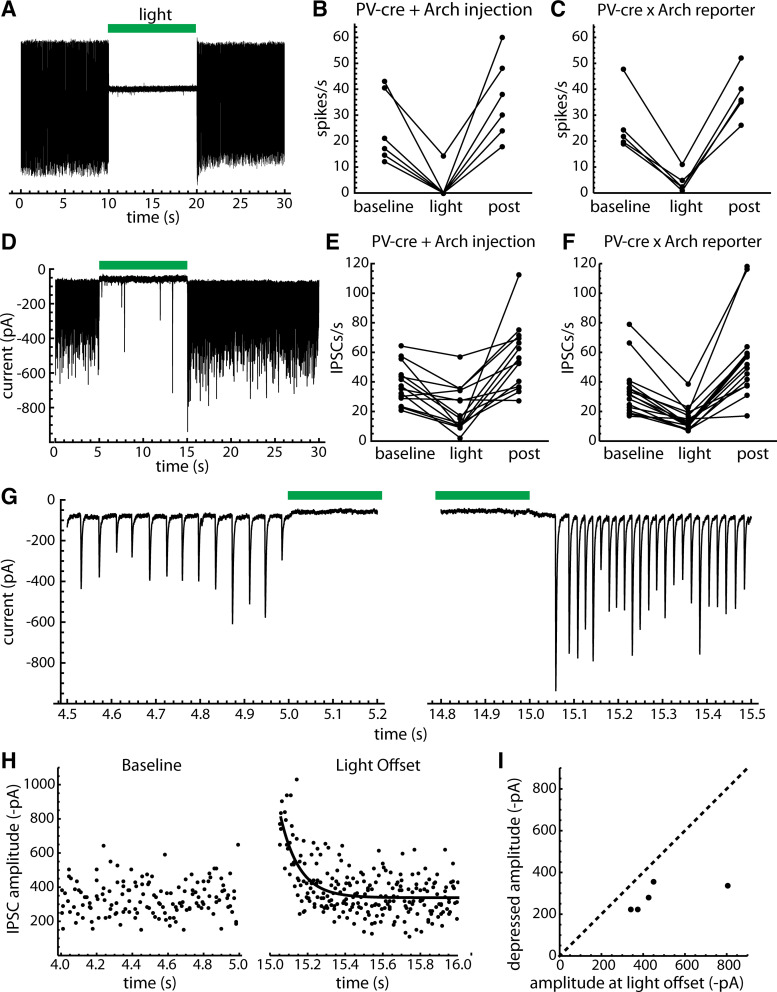
To investigate whether presynaptic PV neurons produce the unitary IPSCs, we performed silencing experiments using the inhibitory opsin archaerhodopsin (Arch), first by injection of AAV9-Flex-Arch-GFP in PV-cre mice, and then by using PV-Arch-GFP mice (see materials and methods). Arch was activated using 10-s green light stimuli delivered to the GPe through the microscope objective. Recorded PV neurons were selected by their GFP fluorescence, and their identity was confirmed by a sustained outward current (direct response) produced by Arch activation and/or a slowing or silencing of firing recorded in the cell-attached configuration before breaking in. In both preparations, Arch activation silenced or greatly slowed the firing of PV neurons recorded in the cell-attached configuration (Fig. 8, A–C). After light offset, we generally observed a transient increase in firing rate, which was presumably caused by removal of spike frequency adaptation.
Figure 8.
Effects of optogenetic inhibition of PV neurons. Archaerhodopsin (Arch) was expressed in PV GPe neurons by injection of AAV9-Flex-Arch-GFP and by creating PV-Arch-GFP mice (see materials and methods). Arch was activated by green light delivered to GPe through the microscope objective. A: cell-attached recording from a GPe neuron in an AAV9-Flex-Arch-GFP injected preparation, showing sustained silencing of spontaneous firing. Green bar indicates the light stimulus. B: group data showing the efficacy of spike silencing in six PV GPe neurons from AAV9-Flex-Arch-GFP-injected mice. Baseline indicates the entire period before illumination, light indicates the entire 10-s light stimulus, and post indicates the first 1 s after light offset. Note the transient increases in firing rate at light offset. C: efficacy of spike silencing in five PV GPe neurons from PV-Arch-GFP mice. D: whole cell recording in the AAV9-Flex-Arch-GFP-injected preparation. The upward baseline shift is the Arch current, which identifies the recorded neuron as PV+. Arch activation greatly reduced the IPSC rate, indicating that most of the IPSCs resulted from firing of one or more local PV neurons. E: group data showing the effect of Arch activation on IPSC rates in PV GPe neurons from AAV9-Flex-Arch-GFP-injected mice, including only neurons with spontaneous periodic IPSCs. F: same as E, but for PV-Arch-GFP mice. G: expanded trace showing the baseline IPSCs before the light and their resumption at light offset. This cell received one periodic unitary input with high reliability, producing a comb-like IPSC train. At light offset the frequency of the IPSC train was increased, consistent with relief from spike frequency adaptation in the presynaptic neuron. The IPSC amplitudes also increased transiently, suggesting that synaptic depression was present during the baseline activity. H: the decay of IPSC amplitude at light offset was fitted with a single-exponential function. I: mean IPSC amplitudes at light offset and at steady state, taken from exponential fits as shown in H. The data were obtained from five PV GPe neurons that each had one clearly distinguishable periodic input. AAV9-Flex-Arch-GFP, AAV9.Flex.CBA.Arch-GFP.WPRE.SV.40, University of Pennsylvania Vector Core; GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; PV, parvalbumin.
In whole cell recordings from PV neurons with periodic spontaneous IPSCs (n = 14 from AAV9-Flex-Arch-GFP-injected mice, n = 16 from PV-Arch-GFP mice), Arch suppression of presynaptic PV neurons consistently reduced the spontaneous IPSC rate during illumination (Fig. 8, D–F). To quantify this effect, we counted IPSCs in 1-s time bins during each repetition of the stimulus and compared the pooled counts from multiple trials before, during, and 0–1 s after the light. Overall, combining the data from the cross and the virus injections, 29 of 30 neurons with periodic input showed a significant, reversible slowing of the IPSC rate (P < 0.05 for baseline vs. light and light vs. postlight). The mean IPSC rates were 36.1 ± 15.4 s−1 before, 17.9 ± 12.1 s−1 during, and 56.4 ± 24.4 s−1 after the light. A total of 18 of the 26 neurons that lacked periodic unitary input also lacked an effect of Arch activation on IPSC rates. The remaining eight of 26 neurons did show a small but significant, reversible reduction of the IPSC rate by Arch, suggesting they received some previously undetected input from local PV neurons. In these eight neurons, the mean IPSC rates were 15.0 ± 6.8 s−1 before, 10.1 ± 3.1 s−1 during, and 33.0 ± 23.7 s−1 after the light. On average, for 26 cells lacking detectable periodic IPSCs, the IPSC frequencies were 16.8 ± 6.8 s−1 before, 14.2 ± 5.6 s−1 during, and 22.0 ± 15.1 s−1 after the light. Overall, the results of this experiment indicate that most of the identified periodic inputs to PV neurons came from other PV neurons. In addition, some inputs that did not produce detectable periodic components in the autocorrelation also came from presynaptic PV neurons. The relatively small changes in IPSC rate caused by suppressing these inputs suggest they may have gone undetected because of low presynaptic firing frequency and/or low synaptic success probability.
Analysis of IPSC amplitudes in this experiment suggests that synaptic depression was present during the baseline activity, as amplitudes were transiently increased when the periodic IPSCs resumed at light offset (Fig. 8G). To quantify the depression, we measured the amplitudes of periodic IPSCs for 1 s after light offset in five PV GPe neurons that each received one clearly distinguishable periodic IPSC train, and then fitted each cell’s IPSC amplitude-time relationship with an exponential function (example in Fig. 8H). From the fits, we estimated the mean periodic IPSC amplitude of each cell at light offset and during steady-state depression (Fig. 8I). On average, the steady-state amplitudes of the periodic IPSCs were 62 ± 13% of the values immediately after light offset. These data indicate that local synaptic connections from presynaptic PV neurons undergo depression during spontaneous firing activity. However, this depression was not severe, allowing robust transmission at the natural firing frequencies.
We also recorded from a smaller number of Arch-negative neurons (n = 8 with AAV9-Flex-Arch-GFP injection, n = 10 from PV-Arch-GFP mice) that did not show a light-induced reduction in cell-attached firing rate or an Arch current during whole cell recording. Of the 18 neurons, 11 had spontaneous periodic input based on the autocorrelation, and Arch activation reduced the IPSC rate significantly in eight of 11 of these. Although these neurons were not positively identified as Npas1 neurons, the data suggest that spontaneous periodic inputs from presynaptic PV neurons may not be restricted to postsynaptic PV neurons.
To confirm the results obtained with Arch, we recorded IPSCs from PV neurons in preparations with channelrhodopsin (ChR2) expressed in PV neurons, including 18 neurons from PV-cre mice injected with AAV9-Flex-ChR2-tdTomato and nine neurons from PV-ChR2-tdTomato mice. ChR2 was activated by sustained blue light stimuli (0.5–10 s) delivered to GPe through the microscope objective and produced a sustained inward current in ChR2-expressing PV neurons (Fig. 9A). The results were qualitatively similar in the two preparations and were combined into one group. Of the 27 neurons, 13 had spontaneous periodic input, and of these, 10 had a significant increase in IPSC rate during ChR2 activation (Fig. 9, A and B). These data are consistent with our other results obtained with Arch, suggesting that local PV neurons were the major source of spontaneous periodic IPSCs.
Figure 9.
Effects of optogenetic excitation of PV neurons. A: effect of sustained ChR2 stimulation (blue bar) on periodic IPSCs. The example cell is identified as a PV neuron by the sustained ChR2 current. On top of this, the light stimulus caused a large increase in the rate of periodic IPSCs. B: changes in overall IPSC rate in PV neurons with periodic IPSCs observed before stimulation. IPSC rates were measured for the entire baseline period, the entire light stimulation period, and the first 1 s after light offset (post). C: example of changes in periodic IPSC amplitudes before, during, and after the ChR2 stimulus, for the cell illustrated in A. Periodic IPSCs were selected visually, eliminating other interspersed IPSCs. Red line is a piecewise fit to the amplitudes before and during the light, with a constant component for the baseline followed by a single-exponential component for the stimulation period. The average periodic IPSC amplitudes for the baseline and at steady state during the light were taken as the initial and final values of the fit. After light offset there was a brief pause in IPSCs followed by a resumption with amplitudes relaxing to prelight levels. D: frequency of periodic IPSCs at baseline and at steady state during the light, in four PV neurons that each had one clearly identifiable periodic input. E: average amplitude of periodic IPSCs at baseline and at steady state during the light. ChR2, channelrhodopsin 2; IPSCs, inhibitory synaptic currents; PV, parvalbumin.
We also examined the synaptic depression produced by the ChR2-induced speedup of presynaptic firing, analyzing data from four neurons that each had a single, clearly identifiable periodic input. The periodic IPSCs were selected by visual inspection, skipping any events that fell between the periodic ones or were uncertain. An example of the periodic IPSC amplitude result is shown in Fig. 9C. The baseline presynaptic firing frequency and the steady-state firing frequency during the light were estimated as the reciprocal of the median inter-IPSC interval for the selected IPSCs in each condition. The effects of ChR2 stimulation on the steady-state frequencies and amplitudes of periodic IPSCs are summarized in Fig. 9, D and E. On average, the frequencies were 17.0 ± 7.4 Hz at baseline and 63.0 ± 34.6 Hz during the light, and the relative frequencies during the light were 3.6 ± 1.1 times the respective baseline frequencies. The mean periodic IPSC amplitudes were −340 ± 129 pA at baseline and −209 ± 110 pA during the light, and the relative amplitudes during the light were 58 ± 13% of the baseline amplitudes. These data confirm that the local unitary inputs undergo frequency-dependent synaptic depression. However, the depression was not severe, considering the large increases in presynaptic firing frequency produced by ChR2 activation. The results suggest that the local connections do not operate in a limiting-frequency regime where the steady-state IPSC amplitudes vary inversely with the presynaptic firing frequency, at least for the stimulus durations examined. Thus, the overall strength of local inhibition is expected to vary with changes in GPe neurons’ firing frequencies.
Unitary IPSCs from Presynaptic Npas1 Neurons?
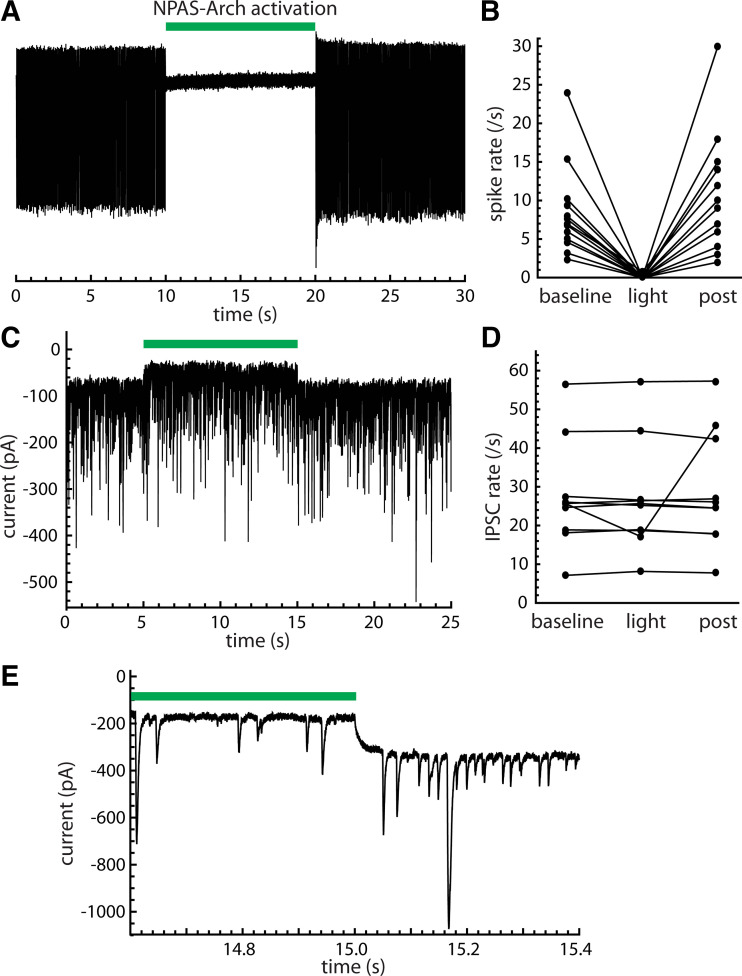
The experiments described in the previous section were limited by the inability to identify Npas1 neurons definitively while stimulating presynaptic PV neurons. Thus, another experiment was required to determine whether Npas1 neurons receive unitary IPSCs from other Npas1 neurons. For this purpose, we expressed Arch in Npas1 neurons by injecting AAV9-Flex-Arch-GFP into GPe in Npas1-cre mice.
Activating Arch in Npas1 neurons consistently suppressed the firing of these neurons (Fig. 10, A and B) but seldom altered the spontaneous synaptic currents (Fig. 10, C–E). In cell-attached recordings, 14 of 15 Npas1 neurons showed complete silencing by Arch, and the remaining cell was slowed to a small fraction of its baseline rate. Spontaneous IPSCs were recorded in 25 Npas1 neurons, including 10 neurons with spontaneous periodic inputs. In each Npas1 neuron, Arch activation produced a sustained outward current (example in Fig. 10C). However, Arch only reduced the IPSC rate significantly and reversibly (P < 0.05 for baseline vs. light and light vs. postlight) in one cell (Fig. 10D). In this cell, the periodic input affected by Arch was most evident at light offset, where a high-frequency train of small IPSCs was observed (Fig. 10E). This observation indicates that some Npas1-to-Npas1 cell connections do exist, and our experimental protocol could reveal them. However, overall, our data indicate that Npas1 neurons were not the major source of spontaneous periodic input to other Npas1 neurons.
Figure 10.
Npas1 neurons were not the major source of local input to other Npas1 neurons. Data were obtained from GPe neurons in slices from Npas1-cre mice injected with AAV9-Flex-Arch-GFP. A: on-cell spike recording from an Npas1 neuron, showing the silencing by Arch. The green bar indicates the timing of the light stimulus. B: effect of Arch activation on spike rate in 15 Npas1 neurons. C: effect of Arch activation on spontaneous IPSCs in an Npas1 neuron. The Arch current (the outward shift of the baseline current observed for the duration of the light stimulus) identifies this cell as Npas1+. The spontaneous synaptic currents were not affected by Arch activation, suggesting there was no contribution from presynaptic Npas1 neurons. D: IPSC rates of 10 Npas1 neurons that each had periodic spontaneous input. Only one of these neurons showed a significant, reversible effect of Arch activation on IPSC rate. E: periodic IPSCs observed at light offset in the one cell showing an effect of Arch activation on the spontaneous IPSCs. AAV9-Flex-Arch-GFP, AAV9.Flex.CBA.Arch-GFP.WPRE.SV.40, University of Pennsylvania Vector Core; Arch, archaerhodopsin; GPe, external globus pallidus; IPSCs, inhibitory synaptic currents.
Our Npas1-cre/AAV9-Flex-Arch-GFP preparation did not provide the means to examine Npas1-to-PV cell connections directly. However, we did record from 13 unlabeled neurons (which also lacked an Arch current or an inhibition of spiking in the cell-attached configuration). Four of these neurons had periodic spontaneous input, but none showed a significant reduction of the spontaneous IPSC rate during illumination. Although these neurons cannot be identified definitively, it is likely that at least some were PV neurons, because these are the most abundant neurons in GPe. Taken together, our data obtained using optogenetic manipulations of presynaptic PV and Npas1 neurons suggest that presynaptic PV neurons were the major source of spontaneous, periodic IPSCs in both postsynaptic cell types.
Evoked IPSCs from PV Inputs
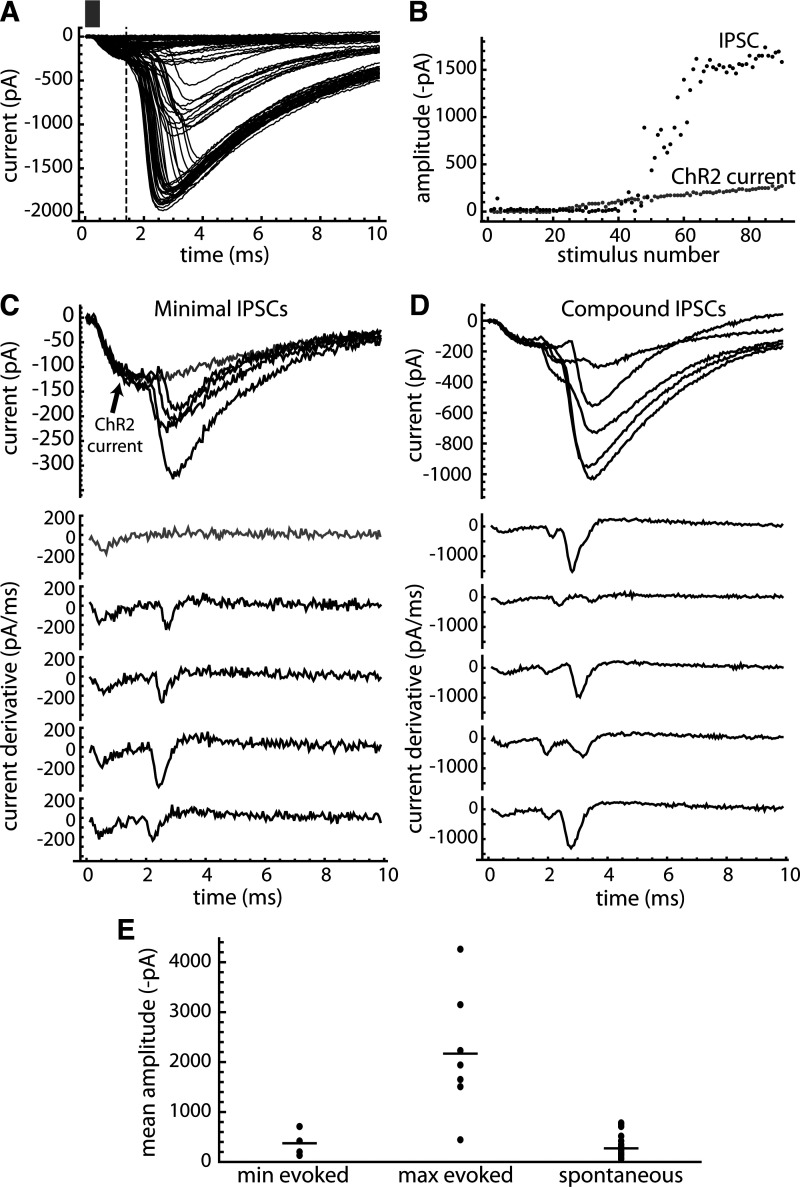
The results described so far all relied on spontaneous periodic IPSCs produced by intact connections from presynaptic to postsynaptic neurons. Although the numbers of active unitary inputs detected were low, usually 0, 1, or 2 per cell, it was expected that more connections were present before slicing, and it would be possible to activate the severed axons with appropriate stimuli. To examine this, we applied low-frequency (0.25 Hz), brief (0.5-2 ms) flashes of blue light in slices from PV-cre mice injected with AAV9-Flex-ChR2-tdTomato, increasing the pulse intensity gradually to identify the minimal and maximal IPSCs.
An example of the responses obtained is shown in Fig. 11A. In six of seven neurons, the ChR2 stimulus produced a low-threshold, short-latency current that increased in a graded manner with light intensity, identifying these neurons as ChR2-expressing PV neurons. At slightly higher stimulus intensities, an IPSC was evoked, usually starting near the peak of the ChR2 current. The amplitudes of the ChR2 currents and the evoked IPSCs in the example cell are plotted in Fig. 11B. Unlike the ChR2 current, the evoked IPSCs appeared to increase in steps, suggesting that the neuron received several unitary inputs from axons with different stimulus thresholds.
Figure 11.
IPSCs evoked by optogenetic pulse stimulation of local PV inputs. PV neurons and axons were stimulated in slices from PV-cre mice injected with AAV9-Flex-ChR2-tdTomato. A: example from a neuron with no spontaneous periodic input, showing the baseline-subtracted responses to brief ChR2 activation (0.5 ms light pulse, bar) at a range of stimulus intensities. The short-latency response is the ChR2 current of the postsynaptic neuron, and the longer-latency responses are the evoked IPSCs. The ChR2 current was measured at the time point indicated by the dashed line and was subtracted from the measured IPSC amplitudes. B: amplitudes of the ChR2 currents and IPSCs produced as the stimulus intensity was increased. The ChR2 currents grew in a graded manner, whereas the IPSCs appeared to increase in steps, suggesting that the recorded neuron received several unitary inputs, each with a different stimulus threshold. C, top: minimal IPSCs in the example neuron (black traces) and the last ChR2 current elicited before reaching the minimal IPSC threshold (gray trace). Bottom: derivative of the ChR2 current (gray trace) and derivative of each minimal IPSC (black traces). The derivative of each minimal IPSC had a single peak corresponding to the rising phase of the current, suggesting these may be unitary IPSCs. D: same as C, but for IPSCs evoked at slightly higher stimulus intensities, showing at least two components in the rising phase. E: mean amplitudes of the identified minimal ChR2-evoked IPSCs (n = 5 neurons), the maximal ChR2-evoked IPSCs (n = 7), and the spontaneous unitary IPSCs measured in PV GPe neurons (n = 22). Bars indicate the overall mean for each group. AAV9-Flex-ChR2-tdTomato, AAV9.CAGGS. Flex.ChR2.tdTomato-WPRE.SV40, University of Pennsylvania Vector Core; ChR2, channelrhodopsin 2; GPe, external globus pallidus; IPSCs, inhibitory synaptic currents; PV, parvalbumin.
In five of seven neurons, a minimal evoked IPSC was observed with a clear threshold, whereas in the other two neurons the minimal IPSC was not clearly distinguished from the ongoing spontaneous synaptic activity. The minimal IPSCs in the example neuron are shown in Fig. 11C, along with their derivatives. The minimal IPSCs rose rapidly without obvious inflections, producing a single peak in the derivative, suggesting they might represent one unitary input. When the stimulus intensity was increased, larger IPSCs were evoked (Fig. 11D), and these typically showed two or more peaks in the derivative, suggesting that more than one unitary input was activated.
The minimal and maximal evoked IPSC amplitudes are collected in Fig. 11E, comparing these to the spontaneous unitary IPSCs measured in PV GPe neurons. The minimal ChR2-evoked IPSCs (measuring from near the IPSC onset on the ChR2 current) were 375 ± 231 pA, which was comparable with the spontaneous unitary IPSCs in PV neurons (273 ± 230 pA). The difference between the evoked minimal and spontaneous unitary IPSC amplitudes was not significant. The maximal ChR2-evoked IPSC amplitudes were 2,171 ± 1,227 pA (n = 7), or almost six times the average minimal IPSC. These data indicate that, in our slice preparations, GPe neurons received excitable inputs from multiple local PV neurons, the majority of which were severed from their parent cell body and thus did not produce periodic spontaneous IPSCs. Because most of the likely errors in these measurements would lead to underestimation of the maximal IPSCs (see discussion), the total number of local inputs to each neuron in the intact GPe is likely to be somewhat larger than the observed sixfold ratio of maximal to minimal IPSC amplitudes.
DISCUSSION
The results of our study provide a detailed description of the unitary synaptic currents produced by connections among mouse GPe neurons during spontaneous firing. Almost all of the active connections were made by presynaptic PV neurons, which appeared to target postsynaptic PV and Npas1 neurons similarly. We found that the local unitary IPSCs had fast kinetics in both cell types but decayed faster in postsynaptic PV neurons than in postsynaptic Npas1 neurons. The IPSC amplitudes were heterogeneous among different unitary inputs, and largely independent of the input frequencies. Thus, the amplitude heterogeneity appeared to be primarily a property of the synaptic connections, rather than a consequence of frequency-dependent synaptic depression.
How Connected Are They?
The impact of the local inhibitory network in the GPe depends critically on the number of unitary local connections made and received by each GPe neuron. In the rat, GPe neurons have been reported to form hundreds of local collateral boutons. Sadek et al. (16) reported an average of 413 axonal boutons within GPe, and Fujiyama et al. (21) found an average of 475 local collateral boutons for prototypic neurons and 327 for arkypallidal neurons. Statistics on the number of synapses each axon forms with each postsynaptic cell are not available, but Sadek et al. (16) observed as many as 14 boutons from an individual axon contacting the soma of a single GPe cell. Although mostly proximal, with 60%–79% on processes >1.6 µm in diameter, somatic synapses were a minority of the contacts made by local axons. Up to 80% of them were dendritic. The existence of local collateral synapses on the dendrites as well as the soma is consistent with the wide range of unitary IPSC sizes seen in our results. If the average number of boutons were on the order of 10 per connection, this would suggest that each GPe neuron, including the arkypallidal neurons, might make and receive ∼30–50 local connections. On the other hand, it is possible that unitary synaptic connections routinely consist of more than the 14 boutons seen by Sadek et al. (16), and that connectivity among GPe neurons is much sparser than that, and each connection is correspondingly stronger.
Our spontaneous IPSC data revealed only small numbers of active unitary inputs to each neuron—usually zero, one, or two. Often, these produced very large unitary IPSCs, consistent with multiple synaptic connections formed by each collateral axon. Might the local collateral connectivity actually be that sparse? It is possible that some unitary inputs were not detected by our autocorrelation-based analysis because of insufficient reliability or periodicity, but on average, the rates of spontaneous IPSCs not accounted for by periodic inputs were only slightly greater than the rates of miniature IPSCs detected in the presence of TTX. Consistent with this, optogenetic silencing of PV neurons produced only small decreases in IPSC frequency or had no effect in neurons without identified periodic spontaneous input. Thus, we conclude that undetected, intact local connections accounted for a very small fraction of the synaptic input. Possibly, there are local synaptic connections so distal (and small) that they went undetected. However, the incidence of periodic unitary connections was not increased in neurons recorded using CsCl-filled electrodes.
The limiting factor for our measurement is the requirement for preserved connectivity in our slices. In the slice preparation, many pairs of presynaptic and postsynaptic neurons are likely to be disconnected, perhaps even when both somata are present in the same slice. Our use of coronal sections was intended to maximize the connectivity among neurons with disk-shaped local axonal fields, as described from intracellular staining (15, 16, 21). In those studies, the local axonal fields of many neurons consisted of two parts: one near the dendritic field, and one more distant, usually located caudal and medial to the cell of origin. These more distant connections were certainly severed in our experiments.
One estimate of the complete local collateral innervation of a GPe neuron is the maximal response to synchronous axonal stimulation with a large, brief light pulse. For PV neurons, the maximal currents evoked this way averaged almost six times the minimal currents, and the minimal currents were not significantly different in amplitude from the spontaneous unitary responses in PV neurons. This provides a lower estimate of the total number of local unitary inputs to each GPe neuron. Consistent with this, the large IPSCs were evoked even in neurons having zero spontaneous periodic inputs, indicating that all neurons receive local connections, but some were disconnected more, and some less. Given these sources of error, our data suggest that, on average, each GPe neuron receives on the order of 10 unitary inputs from other GPe PV neurons, and that all GPe neurons receive synaptic input from the local collateral network.
Connectivity of Npas1 Neurons
All GPe neuron types exhibit local axon collaterals, and pulse optogenetic stimulation of Npas1 neurons evokes IPSCs in Npas1 neurons in slices (14), so it was surprising that in our results there was only minimal evidence for intact local connections from presynaptic Npas1 neurons (only one observation). No systematic comparison of the axonal fields of PV and Npas1 neurons is available. It is possible that the lower yield of periodic unitary connections originating from presynaptic Npas1 versus PV neurons is a result of a difference in the geometry of their local axonal arborizations, and that they were disconnected in our slices. However, this cannot account for the results of Ketzef and Silberberg (37), who optogenetically stimulated nonprototypic neurons (expressing FoxP2) in vivo as well as in slices and did not observe inhibitory synaptic potentials in other FoxP2 expressing neurons, whereas activation of prototypic neurons inhibited neurons of both kinds.
Synaptic Success and Failure
The relatively high proportion of synaptic failures (average of 0.24 for synapses on PV neurons and 0.31 for Npas1) combined with the large number of synapses at each connection (16) suggests that the probability of release at individual synapses might be very low. If this were true, the synaptic currents we recorded might be composed of very few quanta and fluctuations in amplitude might occur in discrete steps. We could not discern any such stepwise fluctuations in amplitude (e.g., see Fig. 6). Possibly, release probability at each bouton might be so low that the synaptic currents we measured, even the very large ones with amplitudes approaching 1 nA, were nearly always composed of single quanta. We also cannot rule out conduction failure in GPe collaterals as a contributor to the synaptic failure rate. Failure in spike generation in presynaptic neurons was ruled out by direct observations of spiking in cell attached recordings of GPe neurons.
Connection Strength
It is well established that striatal inhibition to each GPe neuron consists of striato-pallidal synapses that are independently weak but undergo frequency-dependent facilitation, and local pallido-pallidal synapses that are strong but undergo frequency-dependent depression (23, 24). Because of the high tonic rate of firing in GPe, the importance of local collaterals in the network hinges on the level of short-term synaptic depression. Our results demonstrate that synaptic currents evoked by single action potentials in another GPe neuron can be very large and reliable, even when continuously activated at the normal firing rates seen in GPe. This is in agreement with the results on a small sample of unitary synaptic potentials reported in paired recordings by Bugaysen et al. (22). Estimates of synaptic depression based on repetitive electrical stimulation (23–25) suggested that depression may be more severe. Synaptic depression is also very severe at synapses formed by the same GPe neurons at their targets in the SNr (30) and STN (29). In contrast, using optogenetic slowing or silencing of presynaptic GPe PV neurons for as long as 10 s, we saw that during normal tonic activity synapses were on average depressed only to ∼60% of their undepressed amplitude. Likewise, optogenetically increasing presynaptic rate beyond the spontaneous rate produced only small decreases in IPSC amplitude. Moreover, there was no relationship between spontaneous presynaptic firing rate and IPSC amplitude. It is possible that electrical stimuli usually used to assess short-term synaptic plasticity may release neuromodulators like enkephalin or dynorphin, both of which have been shown to reduce the probability of transmitter release at GABAergic synapses in the GPe (38, 39).
Functional Impact of Local Connections
Local inhibition is often considered as a mechanism of signal competition or contrast enhancement, analogous to surround inhibition in the retina (40). However, this kind of function is not readily supported by the sparse organization of intra-GPe connections. If each neuron inhibits ∼10 other local neurons, and these are distributed over a large region of GPe, extending hundreds of microns from the presynaptic cell body, the probability of contacting each neighboring cell is very low, consistent with the paired recordings and cross-correlation studies (e.g., 22, 27). Thus, the local connections might inhibit very select subsets of neurons with competing functions, but they cannot exert a broad suppressive effect on a large group of neighboring neurons.
The local connections do appear sufficient to account in large part for the irregularity of GPe neurons’ firing in vivo. Based on previous studies of synaptic inhibition in substantia nigra pacemaker neurons with similar firing properties (41, 42), a single local IPSC of typical amplitude (accounting for the difference in electromotive force between our voltage clamp conditions and natural firing) is predicted to lengthen the ISI on the order of 10%, with the exact amount depending on when the input arrives within the interval, as well as the input size. With a connectivity ratio of 10 to 1 and similar presynaptic and postsynaptic firing frequencies, ∼10 IPSCs are expected to arrive during each ISI. This local inhibitory barrage is expected to be highly irregular, because of the largely uncorrelated firing of individual GPe neurons (26, 43), the IPSC amplitude heterogeneity among different unitary inputs, and the amplitude variation and synaptic failures of each input. Thus, the total inhibition will vary greatly among ISIs, producing a high CV.
In addition to altering the baseline firing statistics of GPe neurons, the local inhibition may alter the responses to external inputs, by at least two mechanisms. First, if connected neurons receive common inputs, the local network is expected to produce a sequence of inhibition followed by disinhibition (for striatal input; see Fig. 4) or excitation followed by inhibition (for STN input), sharpening the time course of the response. Second, the background barrage of local inhibition (along with the ongoing excitation from the STN) may alter the oscillation dynamics of each GPe neuron. This effect was demonstrated in SNr neurons, where inhibitory barrages prolonged the time each cell spent in a near-firing state, resulting in a disinhibition response when the barrage was terminated (42). The effect of local inhibition on the autonomous oscillation could alter the sensitivity or time course of GPe neurons’ responses to external inputs arriving from the striatum, STN, cerebral cortex, or other sources.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS097185 (to C. J. Wilson) and NS069777 (to C. S. Chan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.H., J.A.J., C.S.C., and C.J.W. conceived and designed research; M.H.H., J.A.J., and C.J.W. performed experiments; M.H.H., J.A.J., and C.J.W. analyzed data; M.H.H., J.A.J., C.S.C., and C.J.W. interpreted results of experiments; M.H.H., J.A.J., and C.J.W. prepared figures; M.H.H., J.A.J., and C.J.W. drafted manuscript; M.H.H., J.A.J., C.S.C., and C.J.W. edited and revised manuscript; M.H.H., J.A.J., C.S.C., and C.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sharmon Lebby for excellent technical assistance.
REFERENCES
- 1.Kita H, Jaeger D. Organization of the globus pallidus. In: Handbook of Basal Ganglia Structure and Function (2nd ed.), edited bySteiner H, Tseng K.. Amsterdam: Elsevier, 2017, p. 259–276. [Google Scholar]
- 2.Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci 24: 9921–9932, 2004. doi: 10.1523/JNEUROSCI.2162-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res 564: 296–305, 1991. doi: 10.1016/0006-8993(91)91466-e. [DOI] [PubMed] [Google Scholar]
- 4.Kita H, Nambu A, Kaneda K, Tachibana Y, Takada M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J Neurophysiol 92: 3069–3084, 2004. doi: 10.1152/jn.00346.2004. [DOI] [PubMed] [Google Scholar]
- 5.Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol 260: 435–452, 1987. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- 6.Pamukcu A, Cui Q, Xenias HS, Berceau BL, Augustine EC, Fan I, Chalasani S, Hantman AW, Lerner TN, Boca SM, Chan CS. Parvalbumin+ and Npas1+ pallidal neurons have distinct circuit topology and function. J Neurosci 40: 7855–7876, 2020. doi: 10.1523/JNEUROSCI.0361-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith Y, Hazrati LN, Parent A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J Comp Neurol 294: 306–323, 1990. doi: 10.1002/cne.902940213. [DOI] [PubMed] [Google Scholar]
- 8.Abecassis ZA, Berceau BL, Win PH, Garcia D, Xenias H, Cui Q, Pamuku A, Cherian S, Hernández VM, Chon U, Lim BK, Kim Y, Justice NJ, Awatramani R, Hooks BM, Gerfen CR, Boca SM, Chan CS. Npas1+-Kkx2.1+ neurons are an integral part of the cortico-pallido-cortical loop. J Neurosci 40: 743–768, 2020. doi: 10.1523/JNEUROSCI.1199-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karube F, Takahashi S, Kobayashi K, Fujiyama M. Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat. eLife 8: e49511, 2019. doi: 10.7554/eLife.49511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito A, Kita H. The cortico-pallidal projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res 653: 251–257, 1994. doi: 10.1016/0006-8993(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa T, Kita T, Xue Y, Kita H. Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J Comp Neurol 471: 153–167, 2004. doi: 10.1002/cne.20029. [DOI] [PubMed] [Google Scholar]
- 12.Cui Q, Du X, Chang IYM, Pamukcu A, Lilascharoen V, Berceau BL, Garcia D, Hong D, Chon U, Naravanan A, Kim Y, Lim BK, Chan CS. Striatal direct pathway targets Npas1+ pallidal neurons. J Neurosci JN-RM-2306-20, 2021. doi: 10.1523/JNEUROSCI.2306-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci 18: 9438–9452, 1998. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Q, Pamukcu A, Cherian S, Chang IYM, Berceau B, Xenias HS, Higgs MH, Rajamanickam S, Chen Y, Du X, Zhang Y, McMorrow H, Abecassis ZA, Boca SM, Justice NJ, Wilson CJ, Chan CS. Dissociable roles of pallidal neuron subtypes in regulating motor patterns. J Neurosci JN-RM-2210-20, 2021. doi: 10.1523/JNEUROSCI.2210-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita H, Kitai ST. The morphology of globus pallidus projection neurons in the rat: an intracellular staining study. Brain Res 636: 308–319, 1994. doi: 10.1016/0006-8993(94)91030-8. [DOI] [PubMed] [Google Scholar]
- 16.Sadek AR, Magill PJ, Bolam JP. A single-cell analysis of intrinsic connectivity in the rat globus pallidus. J Neurosci 27: 6352–6362, 2007. doi: 10.1523/JNEUROSCI.0953-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol 417: 17–31, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol 358: 119–141, 1995. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- 19.Hernández VM, Hegeman DJ, Cui Q, Kelver DA, Fiske MP, Glajch KE, Pitt JE, Huang TY, Justice NJ, Chan CS. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus. J Neurosci 35: 11830–11847, 2015. doi: 10.1523/JNEUROSCI.4672-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. Dichotomous organization of the external globus pallidus. Neuron 74: 1075–1086, 2012. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiyama F, Nakano T, Matsuda W, Furuta T, Udagawa J, Kaneko T. A single-neuron tracing study of arkypallidal and prototypic neurons in healthy rats. Brain Struct Funct 221: 4733–4740, 2016. doi: 10.1007/s00429-015-1152-2. [DOI] [PubMed] [Google Scholar]
- 22.Bugaysen J, Bar-Gad I, Korngreen A. Continuous modulation of action potential firing by a unitary GABAergic connection in the globus pallidus in vitro. J Neurosci 33: 12805–12809, 2013. doi: 10.1523/JNEUROSCI.1970-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sims RE, Woodhall GL, Wilson CL, Stanford IM. Functional characterization of GABAergic pallidopallidal and striatopallidal synapses in the rat globus pallidus in vitro. Eur J Neurosci 28: 2401–2408, 2008. doi: 10.1111/j.1460-9568.2008.06546.x. [DOI] [PubMed] [Google Scholar]
- 24.Miguelez C, Morin S, Martinez A, Goillandeau M, Bezard E, Bioulac B, Baufreton J. Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson’s disease. J Physiol 590: 5861–5875, 2012. doi: 10.1113/jphysiol.2012.241331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rav-Acha M, Sagiv N, Segev I, Bergman H, Yarom Y. Dynamic and spatial features of the inhibitory pallidal GABAergic synapses. Neuroscience 135: 791–802, 2005. doi: 10.1016/j.neuroscience.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Bar-Gad I, Heimer G, Ritov Y, Bergman H. Functional correlations between neighboring neurons in the primate globus pallidus are weak or nonexistent. J Neurosci 23: 4012–4016, 2003. doi: 10.1523/jneurosci.23-10-04012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol 74: 1800–1805, 1995. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- 28.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neuron in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci 20: 8559–8571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atherton JF, Menard A, Urbain N, Bevan MD. Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. J Neurosci 33: 7130–7144, 2013. doi: 10.1523/JNEUROSCI.3576-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connelly WM, Schulz JM, Lees G, Reynolds JNR. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci 30: 14854–14861, 2010. doi: 10.1523/JNEUROSCI.3895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgs MH, Wilson CJ. Unitary synaptic connections among substantia nigra pars reticulata neurons. J Neurophysiol 115: 2814–2829, 2016. doi: 10.1152/jn.00094.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouroux M, Hassani OK, Féger J. Electrophysiological and Fos immunohistochemical evidence for the excitatory nature of the parafascicular projection to the globus pallidus. Neuroscience 81: 387–397, 1997. doi: 10.1016/s0306-4522(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 33.Robledo P, Féger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Res 518: 47–54, 1990. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- 34.Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Targeting neuronal populations of the striatum. Front Neuroanat 5: 40, 2011. doi: 10.3389/fnana.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174: 1015–1030.e16, 2018. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AR. Junctional transmission II. Presynaptic mechanisms. In: Handbook of Physiology, Section 1: The Nervous System, Cellular Biology of Neurons, edited by Kandel ER.Bethesda, MD: American Physiological Society, 1977, vol. 1 part 1, p. 329–355. 10.1002/cphy.cp010110. [DOI] [Google Scholar]
- 37.Ketzef M, Silberberg G. Differential synaptic input to external globus pallidus neuronal subpopulations in vivo. Neuron 109: 516–514.e4, 2021. doi: 10.1016/j.neuron.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Ogura M, Kita H. Dynorphin exerts both postsynaptic and presynaptic effects in the globus pallidus of the rat. J Neurophysiol 83: 3366–3376, 2000. doi: 10.1152/jn.2000.83.6.3366. [DOI] [PubMed] [Google Scholar]
- 39.Stanford IM, Cooper AJ. Presynaptic µ and δ opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro. J Neurosci 19: 4796–4803, 1999. doi: 10.1523/JNEUROSCI.19-12-04796.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16: 37–68, 1953. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 41.Simmons DV, Higgs MH, Lebby S, Wilson CJ. Predicting responses to inhibitory synaptic input in substantia nigra pars reticulata neurons. J Neurophysiol 120: 2679–2693, 2018. doi: 10.1152/jn.00535.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simmons DV, Higgs MH, Lebby S, Wilson CJ. Indirect pathway control of firing rate and pattern in the substantia nigra pars reticulata. J Neurophysiol 123: 800–814, 2020. doi: 10.1152/jn.00678.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deister CA, Dodla R, Barraza D, Kita H, Wilson CJ. Firing rate and pattern heterogeneity in the globus pallidus arise from a single neuronal population. J Neurophysiol 109: 497–506, 2013. doi: 10.1152/jn.00677.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]